
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol., 09 November 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1008946
This article is part of the Research TopicEvidence-Based Drug Treatment of Infectious Diseases - Volume IIView all 8 articles
 Shin Takayama1*
Shin Takayama1* Takao Namiki2
Takao Namiki2 Ryutaro Arita1
Ryutaro Arita1 Rie Ono1
Rie Ono1 Akiko Kikuchi1
Akiko Kikuchi1 Minoru Ohsawa1
Minoru Ohsawa1 Natsumi Saito1
Natsumi Saito1 Satoko Suzuki1
Satoko Suzuki1 Hajime Nakae3
Hajime Nakae3 Seiichi Kobayashi4
Seiichi Kobayashi4 Tetsuhiro Yoshino5
Tetsuhiro Yoshino5 Tomoaki Ishigami6
Tomoaki Ishigami6 Koichiro Tanaka7
Koichiro Tanaka7 Kotaro Nochioka8
Kotaro Nochioka8 Airi Takagi9
Airi Takagi9 Masaru Mimura5,10
Masaru Mimura5,10 Takuhiro Yamaguchi11
Takuhiro Yamaguchi11 Tadashi Ishii1
Tadashi Ishii1 Akito Hisanaga12
Akito Hisanaga12 Kazuo Mitani13
Kazuo Mitani13 Takashi Ito14
Takashi Ito14The traditional Japanese (Kampo) medicine, kakkonto with shosaikotokakikyosekko, has antiviral and anti-inflammatory effects. In this randomized trial, patients with mild and moderate coronavirus disease (COVID-19) were randomly allocated to the control group receiving conventional treatment for symptom relief such as antipyretics and antitussives or the Kampo group receiving mixed extract granules of kakkonto (2.5 g) and shosaikotokakikyosekko (2.5 g) three times a day for 14 days in addition to conventional treatment. The main outcome was the number of days until total symptom relief. The secondary outcome was the number of days until each symptom’s relief and whether the disease progressed to respiratory failure. We enrolled a total of 161 patients (Kampo group, n = 81; control group, n = 80). The results from Kaplan–Meier estimates of symptom relief showed that there are no significant differences between the groups. However, covariate-adjusted cumulative incidence of fever relief considering competitive risk showed that the recovery was significantly faster in the Kampo group than in the control group (HR 1.76, 95% CI 1.03–3.01). Additionally, the risk of disease progression to moderate COVID-19 requiring oxygen inhalation was lower in the Kampo group than in the control group (Risk Difference −0.13, 95% CI −0.27–0.01). No significant drug-related side effects were observed. Kakkonto with shosaikotokakikyosekko is effective for fever relief with suppression of disease progression in COVID-19 patients.
Clinical Trial Registration: https://jrct.niph.go.jp/en-latest-detail/jRCTs021200020, identifier [jRCTs021200020]
Coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a serious life-threatening viral disease affecting millions of people worldwide. As of 1 June 2022, there had been over 500 million cases and over 6 million deaths (The Center for Systems Science and Engineering at Johns Hopkins University, 2022). The clinical manifestations of COVID-19 include fever, chills, cough, dyspnea, fatigue, body aches, headache, loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, and diarrhea (US Centers for Disease Control and Prevention, 2022a), and the condition of approximately 20% of patients progresses to respiratory failure (Wu and McGoogan, 2020).
Many clinical trials have been conducted to demonstrate the efficacy and safety of drug repurposing, such as lopinavir/ritonavir, hydroxychloroquine, nafamostat mesilate, azithromycin, steroid inhalation, metformin, ivermectin, and fluvoxamine; however, the efficacy and safety of these drugs for clinical use remain unclear (Furtado et al., 2020; Patel et al., 2021; Bramante et al., 2022; Gupta A. et al., 2022; Clemency et al., 2022; Quinn et al., 2022). The clinical efficacy of anti-SARS-CoV-2 monoclonal antibodies (Gupta T. et al., 2022; O’Brien et al., 2022) and ribonucleoside analogs (Jayk Bernal et al., 2022) has been demonstrated in COVID-19 patients with mild-to-moderate disease, but these clinical applications have limited applicability in patients with risk factors (Ministry of Health, Labor and Welfare, 2022). Although there are several treatment approaches used, there is no established treatment protocol for the disease, and the drugs used have some reported safety and efficacy issues.
Traditional Japanese medicine, Kampo medicine, which traditionally practiced in Japan based on ancient Chinese medicine, has been applied for acute viral infectious disease. In particular, saikatsugekito (SKGT) reconstructed by Sohaku Asada in the 19th century based on the original Shokan un’yo (Shanghan yunyao in Chinese) has been widely used for acute viral infection. It was used for the symptoms in acute viral infectious diseases, such as headache, chill, fever, malaise, arthralgia, thirst, dry nose, nausea, appetite loss, and even dysphoria. SKGT was also used to treat the patients in Spanish flu. Spread more than 100 years ago and recently it was used applies as the mixture of the extract Kampo medicines with kakkonto (KT) and shosaikotokakikyosekko (SSKKS) (Division of Pharmacognosy, Phytochemistry and Narcotics, 2022). KT has proven antiviral effects (Saito et al., 2021) and pro-inflammatory cytokine modulation (Geng et al., 2019; Arita et al., 2020). SSKKS has proven anti-inflammatory effects, lung tissue protective effects, and inflammatory cytokine modulating effects (Ohtake et al., 2000; Arita et al., 2020). Thus, the combined use of KT and SSKKS can alleviate symptoms of the common cold, pharyngitis, bronchitis, and pneumonia, with multifunctional antiviral and anti-inflammatory effects (Arita et al., 2020). In a multicenter, retrospective, observational study showed that early treatment with Kampo medicines including KT and SSKKS may suppress illness worsening risk in COVID-19 cases without steroid use (Takayama et al., 2022).
This study intended to evaluate the clinical effects of two Kampo medicines on alleviating the symptoms and preventing disease progression using a randomized controlled study approach not previously completed. The main hypothesis of this study was that, compared to conventional treatment alone, the combination of the Kampo medicines KT and SSKKS with conventional treatment would significantly improve patients’ symptoms during the first 14 days of treatment for SARS-CoV-2 infection.
The study protocol was published in Trials on 2 October 2020, with the title “A multicenter, randomized controlled trial by the Integrative Management in Japan for Epidemic Disease (IMJEDI study-RCT) on the use of Kampo medicine, kakkonto with shosaikotokakikyosekko, in mild-to-moderate COVID-19 patients for symptomatic relief and prevention of severe stage: a structured summary of a study protocol for a randomized controlled trial” (Takayama et al., 2020).
Protocol version 1.6 as of 3 March 2022.
Start of application 1 June 2020.
Actual date of first enrollment, 22 February 2021.
Last follow-up date, 16 February 2022.
The trial was registered in the Japan Registry of Clinical Trials (jRCT) jRCTs021200020. Registered on 25 August 2020 (https://jrct.niph.go.jp/latest-detail/jRCTs021200020).
This protocol was approved by the Ministry of Health, Labour and Welfare Certified Clinical Research Review Board of Tohoku University, Sendai, Miyagi, Japan, on 4 August 2020 (certification no. CRB2180001). The authors certify that this trial received ethical approval from the appropriate ethics committee, as described above. Before inclusion in this study, conscious patients were informed of the purpose and clinical procedures required by the study protocol. The investigators in each hospital or clinic explained the purpose, risks, and benefits of study participation. Patients were also informed of their right to withdraw from the study at any time without explanation and without losing their right to future medical care. Patients were free to leave the study protocol at any stage of the study, withdraw their consent, and, consequently, ask for the elimination of their personal data from the database.
This was a multicenter, interventional, parallel-group, randomized (1:1 ratio), investigator-sponsored, two-arm study. It was performed in collaboration with seven medical facilities: Tohoku University Hospital, Chiba University Hospital, Keio University Hospital, Akita University Hospital, Toho University Hospital, Yokohama City University Hospital, and Japanese Red Cross Ishinomaki Hospital.
Patients were recruited from outpatient clinics, isolation facilities, and the seven hospitals. The inclusion criteria were as follows: 1) diagnosis of SARS-CoV-2 infection with positive nasopharyngeal reverse transcription polymerase chain reaction detecting SARS-CoV-2 RNA; 2) COVID-19 clinical stages mild and moderate stage I; 3) symptomatic; 4) ≥20 years of age; 5) male or female; 6) ability to communicate in Japanese; 7) outpatients and inpatients; and 8) provision of informed consent.
The exclusion criteria were as follows: 1) difficulty providing informed consent due to dementia, psychosis, or psychiatric symptoms; 2) allergy to the Kampo or Western medicines used in this study; 3) pregnancy and lactation; 4) inability to follow-up; 5) participation in another clinical trial or interventional study; 6) hypokalemia or use of oral furosemide or steroids; and 7) determined unsuitable for this study by the attending physician.
The following criteria for symptomatic COVID-19 are used to determine staging in Japan (Ministry of Health, Labor and Welfare, 2022): mild stage, oxygen saturation (SpO2) ≥96% with cough without dyspnea; moderate stage I, SpO2 93%–96% with dyspnea and pneumonia findings; moderate stage II, SpO2 ≤93% and requiring oxygen administration and treatment; and severe stage, requiring intensive care or mechanical ventilation. Thus, the clinical stage of COVID-19 was determined by physicians according to respiratory symptoms, SpO2, and the findings of chest radiography or computed tomography (CT).
Patients were randomized (1:1 ratio) to each group using the minimization method, with balancing of the arms based on disease stage severity (mild, moderate stage I) and patient age (<65, 65 to <75, or ≥75 years). Computer-generated random numbers were used for minimization.
Blinding (masking): Open-label with no blinding.
Patients in the control group received conventional treatment with some combination of antipyretics, antitussives, or expectorants for symptoms that occurred after SARS-CoV-2 infection; acetaminophen 500 mg was used as needed for a fever of 38°C or higher, up to a maximum of three times a day. Dimemorfan phosphate 30 mg/day was used when the cough was numerical rating scale (NRS: ranging from 0 with no symptoms to 10 with severe symptoms) two or higher. If the cough worsened, tulobuterol 2 mg/day was also used. L-Carbocisteine 750 mg/day was used if the sputum was more than NRS 2. Anti-SARS-CoV-2 monoclonal antibodies, antiviral agents for SARS-CoV-2, and steroid drugs were not used for the treatment.
Patients in the Kampo group received mixed extract granules of 2.5 g of KT (@TSUMURA and Co., Tokyo, Japan) and 2.5 g of SSKKS (@TSUMURA and Co., Tokyo, Japan) orally, three times a day, for 14 days in addition to the conventional treatment, as mentioned above. KT extract granules approved for use in Japan contain a dried extract of seven crude drugs: Japanese Pharmacopoeia (JP) Pueraria root, JP Jujube, JP Ephedra herb, JP Glycyrrhiza, JP Cinnamon bark, JP Peony root, and JP Ginger. The results of high-performance liquid chromatography (HPLC) analysis of KT are shown in Figure 1A. SSKKS extract granules contain dried extracts of nine crude drugs: JP Bupleurum root, JP Scutellaria root, JP Ginseng, JP Pinellia tuber, JP Jujube, JP Ginger, JP Glycyrrhiza, JP Platycodon root, and JP Gypsum. The HPLC results for SSKKS are shown in Figure 1B. Detailed information of crude drugs included in KT and SSKKS is shown in Supplementary Tables S1, S2.
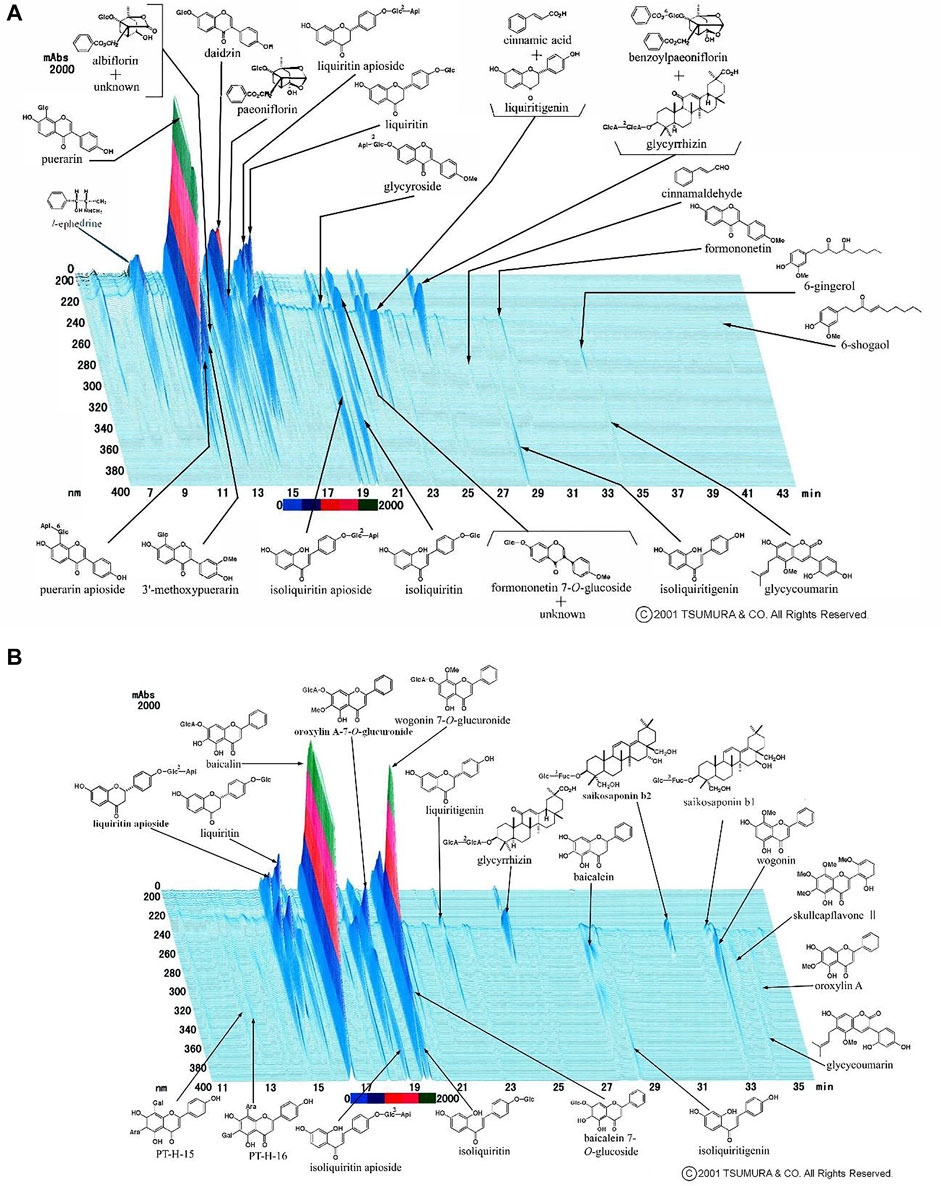
FIGURE 1. (A)High-performance liquid chromatography of kakkonto. (B) High-performance liquid chromatography of shosaikotokakikyosekko.
The primary outcome was the symptomatic relief of at least one common cold-like symptom, such as fever, cough, sputum, fatigue, and shortness of breath, within the first 14 days of treatment. Cough, sputum, fatigue, and shortness of breath were assessed using NRS ranging from 0 (no symptoms) to 10 (severe symptoms). Symptom relief was defined as two-point reduction from the value at the start of treatment on the NRS scale, for at least 2 days in a row. Fever improvement was defined as lowering of body temperature less than 37°C for at least 2 days in a row.
The secondary outcomes were the incidence of progression to severe respiratory failure (at least one of the following features: SpO2 ≤93%, respiratory rate ≥30/min, or need for oxygen administration), which was categorized as moderate stage II COVID-19 during the first 14 days of treatment and relief of each symptom (fever, cough, sputum, fatigue, and shortness of breath) in the first 14 days of treatment. Symptom relief was the same definition as Primary endpoint.
The incidence of adverse events judged to be unrelated to primary disease was assessed. The number of participants in each group with numbness in the hands and feet, edema, skin rash, other allergic symptoms, and gastric discomfort were also calculated.
The following history and examinations were completed upon registration: co-morbid diseases, history of vaccination for SARS-CoV-2, body height, body weight, body temperature, blood pressure, pulse rate, SpO2, chest X-ray or CT scan of the chest, blood sampling, leukocyte count (Ly), C-reactive protein (CRP), and lactate dehydrogenase (LDH). We also monitored clinical symptoms, such as SpO2, fever, and NRS of cough, sputum, fatigue, and shortness of breath.
The main research hypothesis of this study was that, compared to conventional treatment alone, the combination of Kampo medicine and conventional treatment would significantly improve patients’ symptoms (i.e., fever, fatigue, cough, sputum, and shortness of breath) during the first 14 days of treatment for SARS-CoV-2 infection. To analyze the primary endpoint, the duration of time before the improvement of at least one symptom (e.g., fever, fatigue, cough, sputum, or shortness of breath) was estimated using the Kaplan-Meier method. Survival curves were compared between groups using the log-rank test. Assuming this method of analysis and based on previous studies reporting the efficacy of Kampo medicine for patients with COVID-19 and H1N1 influenza, the median survival time in the Kampo medicine group was estimated to be 3 days, which was 1.5 times longer in the control group. Assuming a one-sided significance level of 5%, power of 70%, and allocation ratio of 1:1, the required sample size was calculated as 126 cases. To compensate for loss during follow-up and patient exclusion, we planned to include 160 patients in both groups (Kampo group = 80, control group = 80).
The main research hypothesis of this study was that symptom relief would significantly improve during the first 14 days of treatment in the Kampo group compared to that in the control group. To estimate the duration before the improvement of at least one symptom, survival curves were estimated for each group using the Kaplan-Meier method. Point estimates and confidence intervals for median time to symptom improvement were calculated. Comparisons between groups were conducted using the log-rank test. The Cox regression model was also used to estimate hazard ratios. The significance level was set at 5% on one side. Symptom relief was defined as two-point reduction in common cold-like symptoms (e.g., cough, sputum, fatigue, shortness of breath) on the NRS, compared to the value at the start of treatment, for at least two consecutive days. Fever improvement was defined as lowering of body temperature to <37°C, for at least two consecutive days.
In the incidence of progression to severe respiratory failure, which needed supplemental oxygen during the first 14 days of treatment, if the incidence proportion in the intervention group is significantly lower than that in the control group, the conclusion was that the combination of Kampo medicine prevented disease progression to severe respiratory failure. The groups were compared by calculating the point estimates of the proportion of progression to severe respiratory failure in the first 14 days of treatment for each group, the point estimates of the differences between the groups, and their confidence intervals. Two-tailed test was used with significance set at 5%. Additionally, the time duration before the relief of each symptom (i.e., fever, fatigue, cough, sputum, and shortness of breath) in the first 14 days of treatment was estimated, and between-group comparisons were conducted like those for the primary outcomes. As a supplemental analysis, we compared the two groups in terms of covariate-adjusted cumulative incidence of symptom relief when drop out due to worsening of symptoms was treated as a competing risk. The adjusted covariates included age, severity, duration from onset to enrollment, vaccination, and baseline of each symptom. Baseline was defined as the value of the NRS or the initial body temperature at the start of the treatment. Two-tailed test was used with significance set at 5%.
A flowchart of the clinical trial is shown in Figure 2. A total of 161 patients were enrolled, and after confirmation of eligibility, they were randomly assigned to the Kampo (n = 81) or control (n = 80) groups; 80 patients in the Kampo group and 79 patients in the control group received interventions. In total, 70 patients in Kampo group and 73 patients in control group were included in the primary analysis due to their availability to collect analyzable symptom data on the starting date. The demographic characteristics of the patients at baseline are shown in Table 1. Age, sex, risk factors, symptoms, and clinical staging of COVID-19 matched closely in both groups. Regarding age, there were only two patients over 65 years of age. Most enrollment of patients who had been vaccinated coincided with the Omicron variant epidemic period. There were few cases with co-morbidities (such as diabetes mellitus, hypertension, dyslipidemia, cardiovascular disease, respiratory disease, renal dysfunction, and cancer), as shown in Table 1.
The Kaplan–Meier estimates for relief of at least one of the symptoms, including fever, cough, sputum, fatigue, and shortness of breath, are shown in Figure 3, and the statistical summary is shown in Table 2. The median number of days until symptomatic relief in the control group (median, 3.0; 90% confidence interval [CI], 3.0–4.0) was similar to that in the Kampo group (median, 3.0; 90% CI, 2.0–4.0) and there were not significant differences between the two groups (p = 0.4343 by log-rank test; hazard ratio, 1.02; 90% CI, 0.76–1.38).
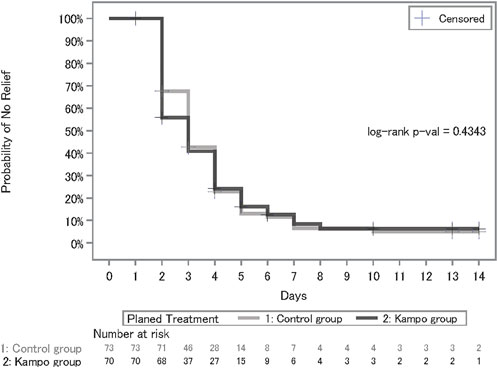
FIGURE 3. Kaplan–Meier curves of the days till symptomatic relief of at least one of the symptoms, including fever, cough, sputum, fatigue, and shortness of breath.
The median number of days until the symptomatic relief and hazard ratio for each outcome, including fever, cough, sputum, fatigue, and shortness of breath, are shown in Table 2 and the Kaplan–Meier estimates are shown in Figure 4. Patients with body temperature at the start of treatment of <37°C were excluded from the analysis population of fever. Regarding cough, sputum, fatigue, and breath shortness, patients with NRS scores at the start of treatment of <2 were excluded from applicable analyses. In the Kampo group, fever decreased relatively faster (median, 3.0; 95% CI, 2.0–4.0) than in the control group (median, 4.0; 95% CI, 3.0–5.0); however, there were no significant differences between the treatment groups (p = 0.1563 by log-rank test; hazard ratio, 1.44; 95% CI, 0.77–2.70). There were neither relative nor statistically significant differences between the treatment groups with regards to relief of cough, sputum, fatigue, and shortness of breath as shown in Table 2.
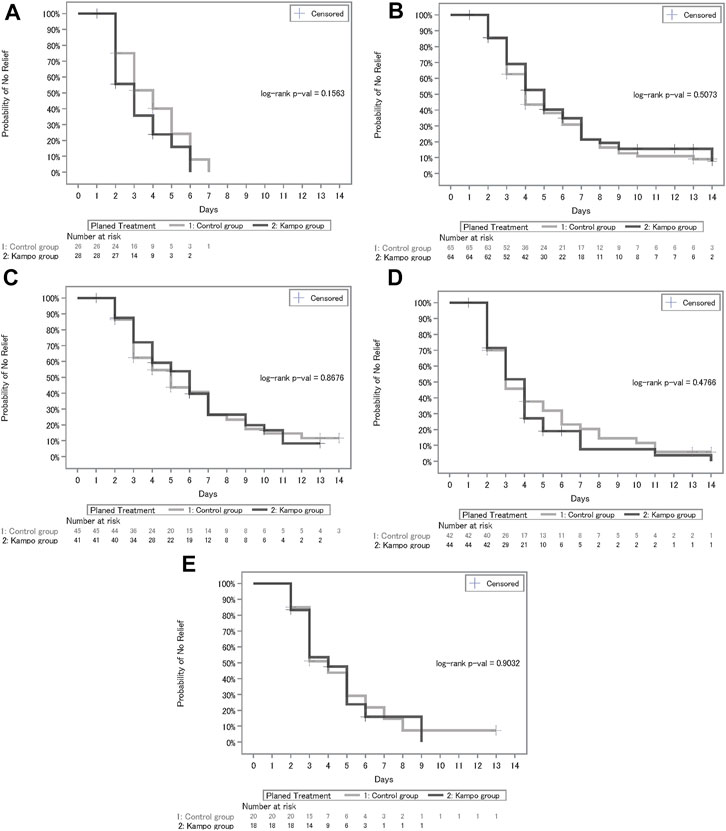
FIGURE 4. (A) Kaplan–Meier curves of days until recovery of fever. (B)Kaplan–Meier curves of days until recovery of cough. (C) Kaplan–Meier curves of days until recovery of sputum. (D) Kaplan–Meier curves of days until recovery of fatigue. (E) Kaplan–Meier curves of days until recovery of shortness of breath.
Furthermore, we compared the two groups based on covariate-adjusted cumulative incidence of symptom relief wherein drop out due to primary disease worsening was treated as a competing risk. Age (<45 or ≥45 years), severity (mild or moderate stage I), duration from onset to enrollment (≤7 or >7 days), vaccination, and each baseline symptom were included as adjusted covariates (Table 3). Since the trial unexpectedly included only few elderly patients (aged >65 years), the threshold for age classification was defined as 45 years with reference to higher hospitalization rate in the age class of ≥45 years (Harrs et al., 2021). The threshold for classification of duration from onset to enrollment was defined as 7 days with relation to the high-risk period for disease worsening. The results of covariate-adjusted cumulative incidence analysis showed that recovery of fever was significantly faster in the Kampo group than in the control group (HR, 1.76; 95% CI, 1.03–3.01; p = 0.0385), and the relationship between age and duration until fever relief was significant (HR, 2.56; 95% CI, 1.37–4.78; p = 0.0031). In regard to relief of cough, sputum, and shortness of breath, there were no significant difference between the treatment groups, whereas the covariates of age and vaccination were significantly associated with duration until symptom relief.

TABLE 3. Covariate-adjusted cumulative incidence of symptom relief when drop out due to worsening of symptoms was treated as a competing risk.
In all cases, the number of patients that progressed to respiratory failure was 10 and 6 in the control and Kampo groups, respectively, with a smaller risk in the Kampo group than in the control group. However, there was no significant differences between the groups, as shown in Table 2 (risk difference, −0.05; 95% CI, −0.15–0.04; p = 0.2786). Patients in the mild stage and who were vaccinated were thought to be less likely to progress to severe respiratory failure because unexpectedly few elderly patients were included in this trial.
Thus, we focused additional assessment only on patients who were unvaccinated and in moderate stage I at the baseline. In this subgroup, the number of patients who progressed to severe respiratory failure in the Kampo group (n = 3) was less than half of that in the control group (n = 8). This suggests that the Kampo treatment substantially decreased the risk of progression to severe disease (risk difference, −0.13; 95% CI, −0.27–0.01; p = 0.0752). None of the patients who progressed to respiratory failure had received a SARS-CoV-2 vaccine.
Adverse events included epigastric disconfort (Kampo group, n = 1), gout (Kampo group, n = 1), and hand eczema (Kampo group, n = 1) (Table 4). No significant differences were observed between the groups.
Numbness in the hands and feet, edema, skin rash and other allergic symptoms, and gastric discomfort were prespecified as events of interest, and the incidence of these events was monitored (Table 5). Their incidence was less in the control group (n = 19) than in the Kampo group (n = 27); however, there were no significant differences between the groups.
The clinical efficacy of Kampo medicine, KT with SSKKS, and conventional treatments was evaluated in a multicenter randomized controlled trial. KT with SSKKS did not significantly shorten the days to symptom relief in patients with mild-to-moderate COVID-19; however, the number of days of fever was shorter in the Kampo group than in the control group. Further, the disease progression in moderate stage I COVID-19 in patients without vaccination for SARS-CoV-2 also tended to be lower in the Kampo group than in the control group. Drug safety was also demonstrated. To the best of our knowledge, this is the first randomized controlled study reporting the efficacy and safety of Kampo medicines for mild-to-moderate COVID-19 patients.
SARS-CoV-2 invades human epithelial cells via the angiotensin-converting enzyme 2 (ACE2) receptor and transmembrane protease serine 2 (Hoffmann et al., 2020). After capture in the endosome, the virus is released into the cytoplasm, processed by proteases, and replicated (Jin et al., 2020). In severe cases, the virus also causes cytokine storms (US Centers for Disease Control and Prevention, 2022b). Therefore, multifunctional drugs with both antiviral and immune modulation effects may contribute to symptom relief and suppress disease worsening in COVID-19. Alleviation of the symptoms and improvement of the disease course require multifunctional drugs that have antiviral, anti-inflammatory, and immunomodulatory effects; however, identifying such medications is challenging due to the frequent new variants of the virus.
KT and SSKKS are used for common cold, pharyngitis, bronchitis, and pneumonia (Division of Pharmacognosy, Phytochemistry and Narcotics, 2022). KT is used for the symptoms of acute viral infection and has shown antiviral effects on single-stranded RNA viruses via the inhibition of acidification of endosomes (Saito et al., 2021) and pro-inflammatory cytokine modulation, such as interleukin (IL)-1α, IL-6, tumor necrosis factor alpha, interferon gamma, and IL-4 (Geng et al., 2019; Arita et al., 2020) In contrast, SSKKS which is used for the symptoms of subacute viral infection, has shown anti-inflammatory effects, regulating IL-6 levels, lung tissue-protective effects, and inflammatory cytokine-modulating effects (Ohtake et al., 2000; Arita et al., 2020). Thus, the combined use of KT and SSKKS can alleviate symptoms of common cold, pharyngitis, bronchitis, and pneumonia, with multifunctional antiviral and anti-inflammatory effects (Arita et al., 2020). A case series of mild and moderate COVID-19 patients treated with KT and SSKKS has already been reported (Irie et al., 2021a; 2021b). We have reported that early Kampo medicine treatment including KT and SSKKS may suppress illness worsening risk in COVID-19 cases without steroid use in a multicenter, retrospective, observational study (Takayama et al., 2022). The present study was conducted based on these results.
KT and SSKKS included 13 crude drugs: JP Pueraria root, JP Jujube, JP Ephedra herb, JP Glycyrrhiza, JP Cinnamon bark, JP Peony root, JP Ginger, JP Bupleurum root, JP Scutellaria root, JP Ginseng, JP Pinellia tuber, JP Platycodin root, and JP Gypsum. Some crude drugs have demonstrated antiviral effects against SARS-CoV-2. Platycodin D, an ingredient of JP Platycodon root, inhibits both the lysosome- and TMPRSS2-mediated SARS-CoV-2 entry pathways, inhibits exocytosis-mediated membrane fusion, and blocks SARS-CoV-2 entry by preventing cholesterol-dependent membrane fusion (Kim et al., 2021). Ephedrine and pseudoephedrine, which are ingredients of JP Ephedra herb, inhibit the SARS-CoV-2 spike with the ACE2 receptor (Lv et al., 2021). Glycyrrhizin, an ingredient of JP Glycyrrhiza, has been shown to inhibit SARS-CoV-2 replication by inhibiting the main viral protease (van de Sand et al., 2021). Baicalein, an ingredient of JP Scutellaria root, inhibits the 3C-like (3CL) protease in SARS-CoV-2 (Liu et al., 2021). In silico, multiple components containing in KT and SSKKS were also identified as candidates to have potential inhibitory activities against SARS-CoV-2. The crude drugs with such potential activities include Bupleurum root, Scutellaria root, Glycyrrhiza (Hejazi et al., 2021; Shakhsi-Niaei et al., 2021; Yu et al., 2021), Ginger (Teng et al., 2021; Zubair et al., 2021.), Pueraria root (Pan et al., 2020), Ephedra herb (Lv et al., 2021), and Cinnamon bark (Prasanth et al., 2021; Husain et al., 2022.). The problem of mutations in the SARS-CoV-2 spike gene have also resulted in the global spread of variants, which decreased vaccine and drug efficacy (Yongbing et al., 2022). Kampo medicines have multifunction with antiviral, anti-inflammatory, and immunomodulatory effects; they may be resistant to viral mutations.
The Kampo medicine maoto includes common pharmacological components with KT, such as ephedrine. Ephedrine has been shown to ameliorate respiratory symptoms by distributing ephedrine in the lung (Matsumoto et al., 2017). Maoto has also been shown to suppress SARS-CoV-2 infection in vitro experiments (Kakimoto et al., 2022). Post-exposure prophylaxis with maoto has also shown efficacy in healthcare workers exposed to COVID-19 (Nabeshima et al., 2022). Other Kampo medicines have also been shown to relieve the symptoms of the olfactory disorder (Takayama et al., 2021a; Ono et al., 2022) and refractory chest pain in COVID-19 patients (Arita et al., 2022). Many case reports and case series of patients successfully treated with Kampo medicines have been reported in acute, subacute, and post-COVID-19 conditions (Takayama et al., 2021b).
Several oral antiviral agents have been developed and used in patients with mild and moderate disease. Molnupiravir, a ribonucleoside analog with antiviral activity against RNA viruses, was used to treat COVID-19 with risk factors in a double-blind, randomized, placebo-controlled trial (Jayk Bernal et al., 2022). Early treatment with molnupiravir reduced the risk of hospitalization or death due to risk factors in unvaccinated adults with COVID-19. In a further analysis, molnupiravir showed faster normalization of CRP and SpO2, with improvements observed on day 3 of therapy (Johnson et al., 2022). Nirmatrelvir-ritonavir is also used as oral medicine for COVID-19 in patients with risk factors. Nirmatrelvir inhibits the 3CL protease, resulting in antiviral efficacy against SARS-CoV-2. In non-hospitalized COVID-19 patients with a high risk of progression to severe disease, nirmatrelvir-ritonavir reduced the risk of COVID-19-related hospitalization or death. A retrospective cohort study reported the clinical benefits of oral antiviral treatments with molnupiravir or nirmatrelvir-ritonavir in patients not requiring oxygen therapy (Wong et al., 2022). Remdesivir is also a ribonucleoside analog prodrug with antiviral activity against RNA viruses. Early remdesivir infusion for non-hospitalized patients with a high risk of COVID-19 progression led to a lower risk of hospitalization or death than the placebo group in a randomized, double-blind, placebo-controlled trial (Gottlieb et al., 2022). However, in animal studies, molnupiravir has also been shown to be a risk factor for fetal malformations; therefore, contraception use is required for women of reproductive age, and it is contraindicated in pregnant women (Government of Western Australia Department of Health, 2022). Nirmatrelvir-ritonavir includes ritonavir, which requires caution due to the numerous drug interactions (Hammond et al., 2022), and detailed confirmation of medications is required. Remdesivir is inconvenient to use in an outpatient setting because remdesivir is an intravenous formulation. Monoclonal antibody drugs against the SARS-CoV-2, such as a combination of casirivimab and imdevimab and sotrovimab, have been used worldwide. A combination drug of casirivimab and imdevimab (Deeks, 2021) reduced the risk of COVID-19-related hospitalization or death and shortened the median time to resolution of COVID-19 symptoms (Weinrech et al., 2021). Sotrovimab is also a monoclonal antibody directed against the SARS-CoV-2 (Pinto et al., 2020), and it was effective in reducing the risk of hospitalization or death in patients with mild-to-moderate COVID-19 with high risk (Gupta et al., 2022). However, casirivimab or sotrovimab has not shown effective treatment against Omicron BA.2.12.1, BA.4, or BA.5 in vitro (Takashita et al., 2022). The safety and efficacy of KT and SSKKS as oral medications for COVID-19 patients are shown in the present study. KT with SSKKS is also inexpensive and already available for use in clinical settings in Japan. Early application for COVID-19 patients may contribute to treating most patients with mild-to-moderate illness who do not require hospitalization by reducing medical expenses and providing economic and medical benefits.
Regarding traditional medicine, a prospective multicenter open-label RCT with a Chinese medicine, the Lianhuaqingwen (LH) capsule, was conducted in COVID-19 patients (Hu et al., 2021). Compared to conventional treatment alone, additional treatment with LH capsules for 14 days significantly increased the symptom recovery rate. However, no significant difference in the conversion rate to the severe stage was observed between the groups. LH is composed of 13 crude drugs, including ephedra herb, glycyrrhiza, and gypsum, which are also commonly included in KT with SSKKS. However, the total components and amounts differed, and the outcome of conversion to the severe stage may differ from that in the present study.
This study has some limitations. First, not all subtypes of SARS-CoV-2 were identified during the registration period, and the influence of subtypes could not be excluded. Second, sample size estimation was performed according to the RCT on H1N1 influenza using Kampo medicines; the sample size may be too small to prove the hypothesis in the present study. Third, it required days for treatment to begin after confirming the positive SARS-CoV-2 Polymerase Chain Reaction test result because delays occurred due to instructions from the municipal health department. The mean number of days before the first visit was six in the present study, which was influenced by this delay, particularly in 2020. Fourth, in Japan, COVID-19 vaccination in elderly individuals began in April 2021 and subsequently, gradually included middle-aged and young adults. There were very few vaccinated patients in the study to make any predictions regarding the use of Kampo medications with vaccinated patients. Finally, this study was an open-label, randomized controlled trial, which cannot exclude placebo bias. To further evidence, double blinded randomized controlled trials should be performed in the future.
The days of fever were shortened, and the disease progression to respiratory failure in the moderate stage tended to decrease with the addition of KT with SSKKS to conventional treatment. These oral drugs may be an effective treatment choice for fever relief in patients with mild-to-moderate COVID-19. Early application of these Kampo medicines for mild-to-moderate COVID-19 patients may contribute to treating most patients by reducing medical care burden and medical expenses and providing economic and medical benefits.
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
This protocol was approved by the Ministry of Health, Labour and Welfare Certified Clinical Research Review Board of Tohoku University, Sendai, Miyagi, Japan, on August 4, 2020 (certification no. CRB2180001). The authors certify that this trial received ethical approval from the appropriate ethics committee, as described above.
ST, TN, KN, TI, AH, KM, and TI designed the study and provided administrative support. ST, TN, RA, RO, AK, MO, NS, SS, HN, SK, TY, TI, KT, and MM treated the patients or collected patient information. AT analyzed and interpreted the patient data. TY confirmed the patient data. ST, RA, RO, and AK were major contributors with regards to manuscript writing. All authors have read and approved the final manuscript.
This study was supported by a research grant from TSUMURA and Co., Tokyo, Japan. The funding body had no role in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
The Japan Society for Oriental Medicine supported this project as an integrative management study in Japan for an epidemic disease (IMJEDI). We would like to thank Akiko Kuwabara and Emiko Yoshida for their coordination regarding the clinical research.
ST, RA, AK, MO, and TI belong to the Department of Kampo and Integrative Medicine, Tohoku University Graduate School of Medicine, which is a joint research course with TSUMURA and Co.. TSUMURA and Co. is a pharmaceutical company that produces Kampo medicine in Japan. MO received lecture fees from TSUMURA and Co. MM received research grant support from TSUMURA & Co., and TY was employed in the joint research program at Keio University.The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1008946/full#supplementary-material
SUPPLEMENTARY TABLE S1 | Crude drugs included in kakkonto and shosaikotokakikyosekko.
SUPPLEMENTARY TABLE S2 | Plant names and part of each ingredient of kakkonto and shosaikotokakikyosekko.
CRP, C-reactive protein; CSSE, Center for Systems Science and Engineering; CT, Computed tomography; DPPN, Division of Pharmacognosy, Phytochemistry, and Narcotics; HPLC, High-performance liquid chromatography; IMJEDI, Integrative Management in Japan for Epidemic Disease; JHU, Johns Hopkins University; JP, japanese pharmacopoeia; NRS, Numerical rating scale.
Arita, R., Ono, R., Saito, N., Suzuki, S., Kikuchi, A., Ohsawa, M., et al. (2022). Refractory chest pain in mild to moderate coronavirus disease 2019 successfully treated with saikanto, a Japanese traditional medicine. Tohoku J. Exp. Med. 257, 241–249. doi:10.1620/tjem.2022.J040
Arita, R., Ono, R., Saito, N., Takayama, S., Namiki, T., Ito, T., et al. (2020). Kakkonto, shosaikoto, Platycodon grandiflorum root, and gypsum (a Japanese original combination drug known as saikatsugekito): Pharmacological review of its activity against viral infections and respiratory inflammatory conditions and a discussion of its applications to COVID ‐19. Traditional Kampo Med. 7, 115–127. doi:10.1002/tkm2.1258
Bramante, C. T., Huling, J. D., Tignanelli, C. J., Buse, J. B., Liebovitz, D. M., Niclias, J. M., et al. (2022). Randomized trial of metformin, ivermectin, and fluvoxamine for covid-19. N. Engl. J. Med. 387 (7), 599–610. doi:10.1056/NEJMoa2201662
Clemency, B. M., Varughese, R., Gonzalez-Rojas, Y., Morse, C. G., Phipatanakul, W., Koster, D. J., et al. (2022). Efficacy of inhaled Ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: A randomized clinical trial. JAMA Intern. Med. 182, 42–49. doi:10.1001/jamainternmed.2021.6759
Deeks, E. D. (2021). Casirivimab/Imdevimab: First approval. Drugs 81, 2047–2055. doi:10.1007/s40265-021-01620-z
Division of Pharmacognosy, Phytochemistry and Narcotics (DPPN), National Institute of Health Sciences (NIHS) of Japan and National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN) (2022). STORK. Available at: http://mpdb.nibiohn.go.jp/stork/ (Accessed June 1, 2022).
Furtado, R. H. M., Berwanger, O., Fonseca, H. A., Corrêa, T. D., Ferraz, L. R., Lapa, M. G., et al. (2020). Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (coalition II): A randomised clinical trial. Lancet 396, 959–967. doi:10.1016/S0140-6736(20)31862-6
Geng, Z. K., Li, Y. Q., Cui, Q. H., Du, R. K., and Tian, J. Z. (2019). Exploration of the mechanisms of Ge gen decoction against influenza A virus infection. Chin. J. Nat. Med. 17, 650–662. doi:10.1016/S1875-5364(19)30079-2
Gottlieb, R. L., Vaca, C. E., Paredes, R., Mera, J., Webb, B. J., Perez, G., et al. (2022). Early remdesivir to prevent progression to severe Covid-19 in outpatients. N. Engl. J. Med. 386, 305–315. doi:10.1056/NEJMoa2116846
Government of Western Australia Department of Health (2022). Drug guideline - Molnupiravir (LAGEVRIO®). Available at: https://ww2.health.wa.gov.au/∼/media/Corp/Documents/Health-for/Infectious-disease/COVID19/WA-guidelines-for-use-of-molnupiravir.pdf (Accessed June 1, 2022).
Gupta, A., Gonzalez-Rojas, Y., Juarez, E., Crespo Casal, M., Moya, J., Rodrigues Falci, D., et al. (2022a). Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: A randomized clinical trial. JAMA 327, 1236–1246. doi:10.1001/jama.2022.2832
Gupta, A., Gonzalez-Rojas, Y., Juarez, E., Manuel, C., Jaynier, M., Diego, R., et al. (2021). Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N. Engl. J. Med. 385, 1941–1950. doi:10.1056/NEJMoa2107934
Gupta, T., Thakkar, P., Kalra, B., and Kannan, S. (2022b). Hydroxychloroquine in the treatment of coronavirus disease 2019: Rapid updated systematic review and meta-analysis. Rev. Med. Virol. 32, e2276. doi:10.1002/rmv.2276
Hammond, J., Leister-Tebbe, H., Gardner, A., Abreu, P., Bao, W., Wisemandle, W., et al. (2022). Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 386, 1397–1408. doi:10.1056/NEJMoa2118542
Harrs, E. J., Angulo, F. J., McLaughlin, J. M., Anis, E., Singer, S. R., Khan, F., et al. (2021). Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 397, 1819–1829. doi:10.1016/S0140-6736(21)00947-8
Hejazi, I. I., Beg, M. A., Imam, M. A., Athar, F., and Islam, A. (2021). Glossary of phytoconstituents: Can these be repurposed against sars CoV-2? A quick in silico screening of various phytoconstituents from plant Glycyrrhiza glabra with sars CoV-2 main protease. Food Chem. Toxicol. 150, 112057. doi:10.1016/j.fct.2021.112057
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280. e8-. doi:10.1016/j.cell.2020.02.052
Hu, K., Guan, W. J., Bi, Y., Zhang, W., Li, L., Zhang, B., et al. (2021). Efficacy and safety of lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine. 85, 153242. doi:10.1016/j.phymed.2020.153242
Husain, I., Ahmad, R., Siddiqui, S., Chandra, A., Misra, A., Srivastava, A., et al. (2022). Structural interactions of phytoconstituent(s) from cinnamon, bay leaf, oregano, and parsley with SARS-CoV-2 nucleocapsid protein: A comparative assessment for development of potential antiviral nutraceuticals. J. Food Biochem., e14262. doi:10.1111/jfbc.14262
Irie, Y., Nakae, H., and Fukui, S. (2021a). Three mild cases of coronavirus disease 2019 treated with saikatsugekito, a Japanese herbal medicine. Traditional Kampo Med. 8, 111–114. doi:10.1002/tkm2.1261
Irie, Y., Nakae, H., and Fukui, S. (2021b). Treatment of coronavirus disease 2019 with saikatsugekito: A case series. Traditional Kampo Med. 8, 211–220. doi:10.1002/tkm2.1290
Jayk Bernal, A., Gomes da Silva, M. M., Musungaie, D. B., Kovalchuk, E., Gonzalez, A., Delos Reyes, V., et al. (2022). Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N. Engl. J. Med. 386, 509–520. doi:10.1056/NEJMoa2116044
Jin, Z., Du, X., Xu, Y., Deng, Y., Liu, M., Zhao, Y., et al. (2020). Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 582, 289–293. doi:10.1038/s41586-020-2223-y
Johnson, M. G., Puenpatom, A., Moncada, P. A., Burgess, L., Duke, E. R., Ohmagari, N., et al. (2022). Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19 : A randomized, placebo-controlled trial. Ann. Intern. Med. 175 (8), 1126–1134. doi:10.7326/M22-0729
Kakimoto, M., Nomura, T., Nazmul, T., Kitagawa, H., Kanno, K., Ogawa-Ochiai, K., et al. (2022). In vitro suppression of SARS-CoV-2 infection by existing Kampo formulas and crude constituent drugs used for treatment of common cold respiratory symptoms. Front. Pharmacol. 13, 804103. doi:10.3389/fphar.2022.804103
Kim, T. Y., Jeon, S., Jang, Y., Gotina, L., Won, J., Ju, Y. H., et al. (2021). Platycodin D, a natural component of Platycodon grandiflorum, prevents both lysosome- and TMPRSS2-driven SARS-CoV-2 infection by hindering membrane fusion. Exp. Mol. Med. 53, 956–972. doi:10.1038/s12276-021-00624-9
Liu, H., Ye, F., Sun, Q., Liang, H., Li, C., Li, S., et al. (2021). Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Enzyme Inhib. Med. Chem. 36, 497–503. doi:10.1080/14756366.2021.1873977
Lv, Y., Wang, S., Liang, P., Wang, Y., Zhang, X., Jia, Q., et al. (2021). Screening and evaluation of anti-SARS-CoV-2 components from Ephedra sinica by ACE2/CMC-HPLC-IT-TOF-MS approach. Anal. Bioanal. Chem. 413, 2995–3004. [Epub 2021 February 19]. doi:10.1007/s00216-021-03233-7
Matsumoto, T., Kushida, H., Matsushita, S., Oyama, Y., Suda, T., Watanabe, J., et al. (2017). Distribution analysis via mass spectrometry imaging of ephedrine in the lungs of rats orally administered the Japanese kampo medicine maoto. Sci. Rep. 7, 44098. doi:10.1038/srep44098
Ministry of Health, Labor and Welfare (2022). Guide to medical treatment for COVID-19 version 7.2. Available at: https://www.mhlw.go.jp/content/000936655.pdf (Accessed June 1, 2022).
Nabeshima, A., Sakamoto, A., Iwata, K., Kitamura, Y., Masui, S., Inomata, S., et al. (2022). Maoto, a traditional herbal medicine, for post-exposure prophylaxis for Japanese healthcare workers exposed to COVID-19: A single center study. J. Infect. Chemother. 28, 907–911. doi:10.1016/j.jiac.2022.03.014
O’Brien, M. P., Forleo-Neto, E., Sarkar, N., Isa, F., Hou, P., Chan, K. C., et al. (2022). Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: A randomized clinical trial. JAMA 327, 432–441. doi:10.1001/jama.2021.24939
Ohtake, N., Suzuki, R., Daikuhara, H., Nakai, Y., Yamamoto, M., Amagaya, S., et al. (2000). Modulation of lung local immune responses by oral administration of a herbal medicine Sho-saiko-to. Int. J. Immunopharmacol. 22, 419–430. doi:10.1016/s0192-0561(00)00007-2
Ono, R., Arita, R., Takayama, S., Kikuchi, A., Ohsawa, M., Saito, N., et al. (2022). Kampo medicine promotes early recovery from coronavirus disease 2019-related olfactory dysfunction: A retrospective observational study. Front. Pharmacol. 13, 844072. doi:10.3389/fphar.2022.844072
Pan, B., Fang, S., Zhang, J., Pan, Y., Liu, H., Wang, Y., et al. (2020). Chinese herbal compounds against SARS-CoV-2: Puerarin and quercetin impair the binding of viral S-protein to ACE2 receptor. Comput. Struct. Biotechnol. J. 18, 3518–3527. doi:10.1016/j.csbj.2020.11.010
Patel, T. K., Patel, P. B., Barvaliya, M., Saurabh, M. K., Bhalla, H. L., and Khosla, P. P. (2021). Efficacy and safety of lopinavir-ritonavir in COVID-19: A systematic review of randomized controlled trials. J. Infect. Public Health 14, 740–748. doi:10.1016/j.jiph.2021.03.015
Pinto, D., Park, Y. J., Beltramello, M., Walls, A. C., Tortorici, M. A., BianChi, S., et al. (2020). Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583, 290–295. doi:10.1038/s41586-020-2349-y
Prasanth, D. S. N. B. K., Murahari, M., Chandramohan, V., Panda, S. P., Atmakuri, L. R., and Guntupalli, C. (2021). In silico identification of potential inhibitors from Cinnamon against main protease and spike glycoprotein of SARS CoV-2. J. Biomol. Struct. Dyn. 39 (13), 4618–4632. doi:10.1080/07391102.2020.1779129
Quinn, T. M., Gaughan, E. E., Bruce, A., Antonelli, J., O’Connor, R., Li, F., et al. (2022). Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: Phase 1b/2a experimental study to investigate safety, Pharmacokinetics and Pharmacodynamics. EBiomedicine 76, 103856. doi:10.1016/j.ebiom.2022.103856
Saito, N., Kikuchi, A., Yamaya, M., Deng, X., Sugawara, M., Takayama, S., et al. (2021). Kakkonto inhibits cytokine production induced by rhinovirus infection in primary cultures of human nasal epithelial cells. Front. Pharmacol. 12, 687818. doi:10.3389/fphar.2021.687818
Shakhsi-Niaei, M., Soureshjani, E. H., and Babaheydari, A. K. (2021). In silico comparison of separate or combinatorial effects of potential inhibitors of the SARS-CoV-2 binding site of ACE2. Iran. J. Public Health 50 (5), 1028–1036. doi:10.18502/ijph.v50i5.6120
Takashita, E., Yamayoshi, S., Simon, V., van Bakel, H., Sordillo, E. M., Pekosz, A., et al. (2022). Efficacy of antibodies and antiviral drugs against omicron BA.2.12.1, BA.4, and BA.5 subvariants. N. Engl. J. Med. 387 (5), 468–470. doi:10.1056/NEJMc2207519
Takayama, S., Arita, R., Ono, R., Saito, N., Suzuki, S., Kikuchi, A., et al. (2021a). Treatment of COVID-19-related olfactory disorder promoted by kakkontokasenkyushin’i: A case series. Tohoku J. Exp. Med. 254, 71–80. doi:10.1620/tjem.254.71
Takayama, S., Namiki, T., Ito, T., Arita, R., Nakae, H., Kobayashi, S., et al. (2020). A multi-center, randomized controlled trial by the integrative management in Japan for epidemic disease (IMJEDI study-RCT) on the use of kampo medicine, kakkonto with shosaikotokakikyosekko, in mild-to-moderate COVID-19 patients for symptomatic relief and prevention of severe stage: A structured summary of a study protocol for a randomized controlled trial. Trials 21, 827. doi:10.1186/s13063-020-04746-9
Takayama, S., Namiki, T., Odaguchi, H., Arita, R., Hisanaga, A., Mitani, K., et al. (2021b). Prevention and recovery of COVID-19 patients with Kampo medicine: Review of case reports and ongoing clinical trials. Front. Pharmacol. 12, 656246. doi:10.3389/fphar.2021.656246
Takayama, S., Yoshino, T., Koizumi, S., Irie, Y., Suzuki, T., Fujii, S., et al. (2022). Conventional and kampo medicine treatment for mild-to-moderate COVID-19: A multicenter, retrospective, observational study by the integrative management in Japan for epidemic disease (IMJEDI study-observation). Intern Med. in press.
Teng, Y., Xu, F., Zhang, X., Mu, J., Sayed, M., Hu, X., et al. (2021). Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 29 (8), 2424–2440. doi:10.1016/j.ymthe.2021.05.005
The Center for Systems Science and Engineering (SCCE) at Johns Hopkins University (JHU) (2022). COVID-19 dashboard. Available at: https://gisanddata.maps.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6 (Accessed June 1, 2022).
US Centers for Disease Control and Prevention (2022b). SARS-CoV-2 variant classifications and definitions. Available at: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html (Accessed June 1, 2022).
US Centers for Disease Control and Prevention (2022a). Symptoms of COVID-19. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (Accessed June 1, 2022).
van de Sand, L., Bormann, M., Alt, M., Schipper, L., Heilingloh, C. S., Steinmann, E., et al. (2021). Glycyrrhizin Effectively Inhibits SARS-CoV-2 Replication by inhibiting the viral main protease. Viruses 13, 609. doi:10.3390/v13040609
Weinrech, D. M., Sivapalasingam, S., Norton, T., Ali, S., Gao, H., Bhore, R., et al. (2021). REGEN-COV antibody combination and outcomes in outpatients with covid-19. N. Engl. J. Med. Overseas. Ed. 385, e81. doi:10.1056/nejmoa2108163
Wong, C. K. H., Au, I. C. H., Lau, K. T. K., Lau, E. H. Y., Cowling, B. J., and Leung, G. M. (2022). Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: A retrospective cohort study. Lancet Infect. Dis [Epub ahead of print]. doi:10.1016/S1473-3099(22)00507-2
Wu, Z., and McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 323, 1239–1242. doi:10.1001/jama.2020.2648
Yongbing, Z., Huilin, Z., and Yong, T. (2022). The outbreak of SARS-CoV-2 Omicron lineages, immune escape and vaccine effectivity. J. Med. Virol. 2022. First publishedOnline ahead of print. doi:10.1002/jmv.28138
Yu, S., Zhu, Y., Xu, J., Yao, G., Zhang, P., Wang, M., et al. (2021). Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine. 85, 153364. doi:10.1016/j.phymed.2020.153364
Zubair, M. S., Maulana, S., Widodo, A., Pitopang, R., Arba, M., Hariono, M. G. C-M. S., et al. (2021). GC-MS, LC-MS/MS, docking and molecular dynamics approaches to identify potential SARS-CoV-2 3-chymotrypsin-like protease inhibitors from Zingiber officinale roscoe. Molecules 26 (17), 5230. doi:10.3390/molecules26175230
Keywords: kakkonto, shosaikotokakikyosekko, Kampo medicines, COVID-19, herbal medicine
Citation: Takayama S, Namiki T, Arita R, Ono R, Kikuchi A, Ohsawa M, Saito N, Suzuki S, Nakae H, Kobayashi S, Yoshino T, Ishigami T, Tanaka K, Nochioka K, Takagi A, Mimura M, Yamaguchi T, Ishii T, Hisanaga A, Mitani K and Ito T (2022) Multicenter, randomized controlled trial of traditional Japanese medicine, kakkonto with shosaikotokakikyosekko, for mild and moderate coronavirus disease patients. Front. Pharmacol. 13:1008946. doi: 10.3389/fphar.2022.1008946
Received: 01 August 2022; Accepted: 07 October 2022;
Published: 09 November 2022.
Edited by:
Yonggang Zhang, Sichuan University, ChinaReviewed by:
Abdelsamed I. Elshamy, National Research Centre, EgyptCopyright © 2022 Takayama, Namiki, Arita, Ono, Kikuchi, Ohsawa, Saito, Suzuki, Nakae, Kobayashi, Yoshino, Ishigami, Tanaka, Nochioka, Takagi, Mimura, Yamaguchi, Ishii, Hisanaga, Mitani and Ito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shin Takayama, dGFrYXlhbWFAbWVkLnRvaG9rdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.