
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 02 December 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1004821
This article is part of the Research Topic Immune Checkpoint Inhibitor and Metabolites in the Tumor Microenvironment View all 9 articles
Background: Immune checkpoint inhibitors (ICIs) have been an emerging treatment strategy for advanced triple-negative breast cancer (TNBC). Some studies have shown that high expression of programmed death-ligand 1 (PD-L1) can achieve a better response of clinical efficacy. However, the efficacy of ICIs in advanced TNBC remains controversial. In this meta-analysis, we evaluated the correlation of PD-L1 expression with the efficacy of ICIs in patients with advanced TNBC.
Methods: We conducted a systematic search using four databases until March 2022 to obtain eligible randomized controlled trials (RCTs). The quality of the studies was assessed by the Cochrane risk of bias tool. Hazard ratio (HR) was extracted to evaluate the relationship between PD-L1 expression and progression-free survival (PFS) or overall survival (OS) in patients with advanced TNBC.
Results: Five randomized controlled clinical trials (RCTs) with 3104 patients were included in this meta-analysis. The results demonstrated that ICIs could significantly improve the OS (HR 0.77, 95% CI 0.60–0.98, p = 0.03) in PD-L1 positive TNBC group. In the subgroup analysis, longer OS was observed (HR: 0.70, 95% CI: 0.60–0.82, p = 0.00001) in PD-L1 positive TNBC patients receiving ICIs alone or ICIs combined with nab-paclitaxel. In terms of PFS, PFS was significantly improved (HR: 0.68, 95% CI: 0.58–0.79, p < 0.00001) in PD-L1 positive patients receiving first-line ICIs and chemotherapy compared to those with ICIs alone. No significant improvement was observed for OS or PFS in PD-L1 negative group.
Conclusion: Our study indicated significant improvement for OS in advanced TNBC with ICIs therapy in the PD-L1 positive status, and ICIs alone or ICIs combined with nab-paclitaxel might be a excellent choice in terms of OS. Although PFS has no significant benefit in PD-L1 positive patients, the subgroup analysis showed that ICIs combined with chemotherapy could achieve the PFS benefit in the first-line treatment. However, further clinical studies are needed to validate our conclusions due to limited relevant research.
Breast cancer has the highest incidence of all malignant tumors among women worldwide (Zhang et al., 2021). Triple-negative breast cancer (TNBC) accounts for approximately 15–20% of all breast cancers. Its pathological features are negative for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2), characterized by an early age of onset, strong invasiveness and high recurrence and metastasis rate (Li et al., 2016; Sharma et al., 2018). The clinical prognosis of TNBC patients is poor due to the lack of effective targets for endocrine therapy and targeted therapy. The 5-year survival rate of patients with advanced TNBC is less than 15% (Bagegni et al., 2019; Berger et al., 2021). Immunotherapy provides a new treatment strategy for the patients. Pathologically, TNBC has relatively abundant tumor-infiltrating lymphocytes and high expression level of programmed death-ligand 1 (PD-L1), providing a suitable immune microenvironment and target basis for the application of immune checkpoint inhibitors (ICIs) (Disis et al., 2015; García-Teijido et al., 2016; Huang et al., 2021). Clinical trials such as KEYNOTE-012, KEYNOTE-086, and IMpassion 031 have confirmed that the immune checkpoint programmed cell death-1 (PD-1)/PD-L1 inhibitors are effective treatment options for TNBC (Seiwert et al., 2016; Adams et al., 2019; Mittendorf et al., 2020). However, only a small proportion of patients showed a long-term sustained response (Keenan et al., 2020). Therefore, there is an urgent need for reliable biomarkers of immune therapy to screen out the optimal beneficiary population clinically (Emens et al., 2021). PD-L1 expression level is currently the most important and controversial predictor of immunotherapy efficacy. IMpassion130 trial indicated that atezolizumab in combination with nab-paclitaxel significantly increased the overall survival (OS) and the progression-free survival (PFS) in PD-L1 positive patients with metastatic TNBC (Schmid et al., 2020), but, KEYNOTE-119 trial demonstrated that pembrolizumab did not significantly improved the OS in PD-L1 positive patients with previously treated metastatic TNBC versus chemotherapy (Winer et al., 2021).
In recent years, several studies have investigated the association between PD-L1 and immunotherapy clinical outcomes in TNBC, but most of them focused on the early stage of TNBC. Two meta-analysis studied the prognostic role of PD-L1 in advanced or metastatic TNBC (Ji et al., 2021; Latif et al., 2022), they conducted the subgroup analysis stratifying the status of PD-L1 by including two studies, and the results suggested that better PFS or OS could be found in PD-L1 positive group. Notably, these meta-analyses were short of the latest relevant clinical trials and further subgroup analysis such as ICIs alone or ICIs combination with other chemotherapeutic drugs. In this study, we summarized recent and relevant clinical trials and conducted a meta-analysis of randomized controlled trials (RCTs) to assess the relationship between PD-L1 expression and the efficacy of ICIs in locally advanced or metastatic TNBC.
PubMed, Embase, Cochrane Library, and Web of Science databases were searched to collect and select clinical studies on PD-L1 expression and PD-1/PD-L1 inhibitors for the treatment of TNBC published before March 2022 and the following keywords were used: “immune checkpoint inhibitor” or “PD-L1” or “PD-1” or “durvalumab” or “pembrolizumab” or “atezolizumab” or “nivolumab” or “avelumab” or “ipilimumab” or “tremelimumab” and “triple-negative breast cancer”. We also manually screened the relevant studies in the references to retrieve other eligible literatures.
Eligible studies met the following inclusion criteria:1) studies should be randomized controlled trials (RCTs) in patients with locally advanced or metastatic TNBC in stage III-IV; 2) the expression of PD-L1 in patients was detected by immunohistochemistry (IHC); 3) study evaluated the efficacy of ICIs or ICIs combined with chemotherapy; 4) studies directly provided hazard ratio (HR) of OS or PFS and 95% confidence intervals (95% CIs) or they could be calculated indirectly in patients with different PD-L1 expression. 5) The language of the publication was English. Articles were excluded according to the following criteria: 1) patients with early stage TNBC; 2) conference abstracts, reviews, case reports, or non-RCTs; 3) the latest and most complete study was chosen between the same studies in different periods, others were excluded.
The studies were independently evaluated and extracted according to the inclusion and exclusion criteria by two reviewers, and the disagreements between them were resolved by discussion. The following data from the eligible study were retrieved: name of the trial, year of publication, study phase, study population, therapeutic regimen, rate of PD-L1 positive expression, cutoff value, HRs, and 95% CI of OS and PFS, if available. The latest and most complete study was chosen between the same studies in different periods.
The quality of the included studies was assessed using the assessment criteria provided by the Cochrane Collaboration bias assessment tool version 5.4. The criteria were based on seven aspects: parameters of details of random sequence generation, allocation concealment, blinding for participants and personnel, blinding for outcome assessment, incomplete outcome data and selective reporting. In addition, the risk of bias was divided into three levels: low risk, high risk and unclear.
All data were statistically analyzed using the Review Manager software (RevMan, version 5.3 for windows; Cochrane Collaboration, Oxford, United Kingdom) and STATA version 16.0 software. HR and 95% CIs of OS and PFS were directly extracted. HR < 1 indicated that the survival outcomes were better in immunotherapy group compared with chemotherapy group. HR > 1 indicated the opposite. The pooled HRs were considered statistically significant if p < 0.05 (two-sided). The heterogeneity between studies was assessed using Cochran Q-test and I2 statistics. A p ≤ 0.10 or I2 ≥ 50% indicated the existence of heterogeneity among studies, and a random-effects model was adopted; A p > 0.10 or I2 < 50% indicated the absence of heterogeneity among studies, and a fixed-effects model was used.
A total of 289 related literature were retrieved and 210 were retained after excluding 79 repeated studies. The authors browsed the titles and abstracts, 46 papers were screened by excluding reviews, conference abstracts, and non-anthropological studies. According to the inclusion criteria of this study, five eligible RCTs [KEYNOTE-119 (Winer et al., 2021), KEYNOTE-355 (Cortes et al., 2020), IMpassion 130 (Schmid et al., 2020), IMpassion131 (Miles et al., 2021), SAFIR02-BREAST IMMUNO(Bachelot et al., 2021)] studies were finally included after reading the full text. A flow chart of the study selection is presented in Figure 1.
A total of 3104 patients with TNBC were enrolled, of whom 1734 were PD-L1 positive. The five RCTs were published between 2019 and 2021, which included four phase III studies and one phase II study. The ICIs involved were atezolizumab, pembrolizumab, and durvalumab. In addition, three studies compared the efficacy of ICIs combined with chemotherapy versus chemotherapy and two studies compared the efficacy of ICIs alone versus chemotherapy. Table 1 presents the characteristics of the five RCTs. The results of the quality evaluation are shown in Figure 2.
Four studies, including 3022 patients, reported the data of PFS with 1702 patients in the PD-L1 positive group and 1320 patients in the PD-L1 negative group. In the PD-L1 positive group, we adopted a random-effects model for analysis due to considerable heterogeneity (I2 = 69%, p = 0.02). No statistically significant result was observed in the correlation between the efficacy of ICIs and PFS in the PD-L1 positive locally advanced or metastatic TNBC (HR: 0.77, 95% CI: 0.60–1.00, p = 0.05; Figure 3). The subgroup analysis was performed by stratifying the treatment project and treatment line to explore the sources of heterogeneity among studies. The subgroup analysis results showed that patients could achieve increased PFS in the PD-L1 positive group if they received ICIs combined with chemotherapy in first-line treatment (HR: 0.68, 95% CI: 0.58–0.79, p < 0.00001; Figure 4).

FIGURE 3. Forest plots of meta-analyses between ICIs combination with chemotherapy or not vs chemotherapy in PD-L1 positive TNBC (A) for progression-free survival and (B) for overall survival.
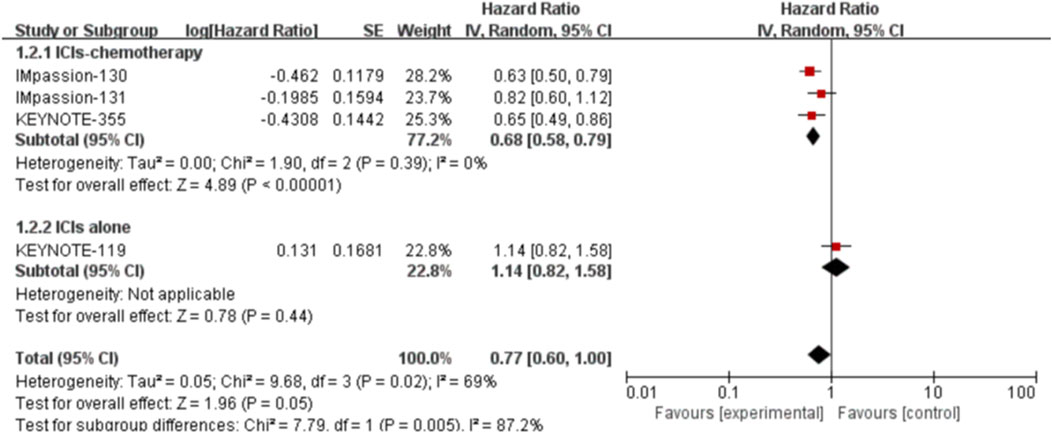
FIGURE 4. Subgroup analysis of the effect of ICIs combined with chemotherapy or not on PFSin PD-L1 positive TNBC patients.
In the PD-L1 negative group, two studies reported relevant data. We used a fixed-effects model for analysis due to the absence of heterogeneity (I2 = 0%, p = 0.39). The result also indicated no significant improvement in PFS in patients who received ICIs (HR: 0.93, 95% CI: 0.81–1.08, p = 0.35).
Five studies, including 3105 patients, reported the information of OS with a total of 1210 PD-L1 positive patients and employed a random-effects model for analysis because of obvious heterogeneity (I2 = 52%, p = 0.08). The benefit of OS was found in ICIs, and ICIs combined with chemotherapy compared to standard chemotherapy (HR: 0.77, 95% CI: 0.60–0.98, p = 0.03; Figure 3). The subgroup analysis based on ICIs with or without chemotherapy did not reduce heterogeneity (Figure 5). Notably, the results of the subgroup analysis showed that patients had decreased OS when ICIs were combined with solvent-based paclitaxel (Figure 6).
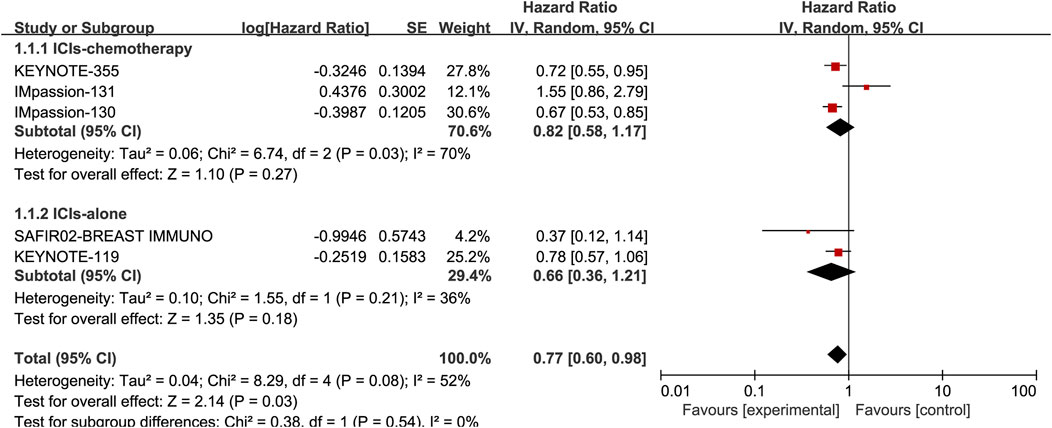
FIGURE 5. Subgroup analysis of the effect of ICIs combined with chemotherapy or not on OS in PD-L1 positive TNBC patients.
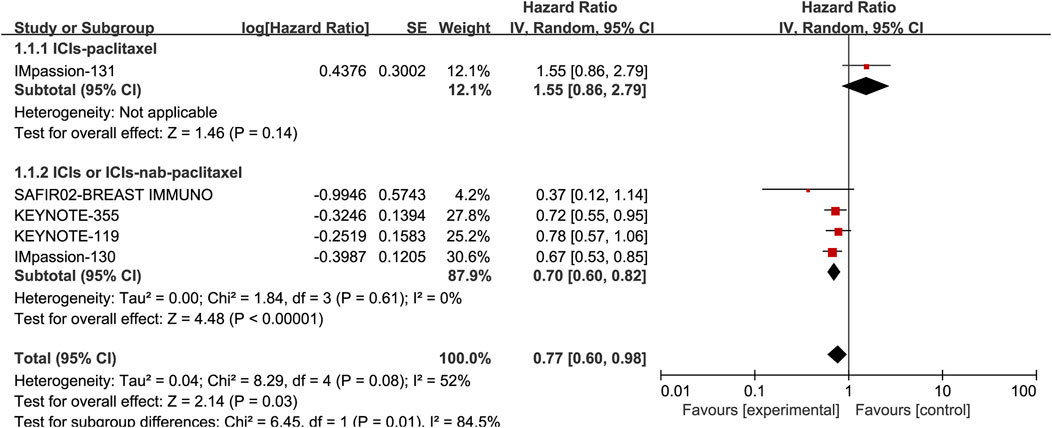
FIGURE 6. Subgroup analysis of the effect of ICIs combined with paclitaxel or not on OS in PD-L1 positive TNBC patients.
Two studies comprising 1349 PD-L1 negative patients provided the data of OS. We used a random-effects model for analysis due to potential heterogeneity (I2 = 50%, p = 0.16). The result revealed no significant OS benefit in patients who received ICIs therapy (HR: 0.84, 95% CI: 0.44–1.58, p = 0.59).
Sensitivity analysis was performed by excluding each study one by one to determine the reliability of the results. The results showed stable pooled HRs (Figure 7).
Publication Bias.
The publication bias of the included studies was assessed by Egger’s test, and the results showed that there was no publication bias (PFS: p = 0.116, OS: p = 0.231; Figure 8).

FIGURE 8. Egger’s test of publication bias (A) for progression-free survival and (B) for overall survival.
TNBC has become the mainstay in malignant tumor immunotherapy due to its high tumor mutational burden, increased infiltration of immune cells and high expression of PD-L1. Currently, the PD-1/PD-L1 pathway is the most prominen target of immunotherapy in locally advanced or metastatic TNBC (Lipson et al., 2015; Hartkopf et al., 2016; Pusztai et al., 2016). IMpassion130 was the first phase III clinical trial to demonstrate the efficacy of first-line immunotherapy in advanced TNBC, atezolizumab plus nab-paclitaxel improved both PFS and OS compared with placebo plus nab-paclitaxel in the PD-L1 positive TNBC. However, the subsequent study of IMpassion131 found that atezolizumab combined with paclitaxel had no benefit for PD-L1 positive metastatic TNBC. KEYNOTE-355 study explored the clinical efficacy of pembrolizumab plus chemotherapy versus placebo plus chemotherapy in the first-line treatment of metastatic TNBC, preliminary results confirmed that pembrolizumab combined with chemotherapy significantly improved PFS compared with placebo combined with chemotherapy for TNBC patients with a combined positive score ≥10. It is worth thinking deeply about these inconsistent findings. Therefore, searching for reliable biomarkers for ICI therapy to screen out the optimal beneficiary population is an important clinical problem that needs to be solved.
In terms of PFS, the effect of ICIs combination chemotherapy in first-line was better than that with ICIs alone in post-line. The main reasons for this result might be as follows: first, due to delayed onset and the pseudoprogress in immunotherapy (Wang et al., 2018; Failing et al., 2019), chemotherapy could reduce tumor burden in patients in the shortest time and cause the declination of tumor interstitial fluid pressure (TIFP). The difficulty in monoclonal antibodies’ entrance into the tumor is due to an increased TIFP. Consequently, the decrease of TIFP could promote the entry of macromolecular substances such as monoclonal antibodies into the tumor, thereby improving the effect of antitumor immunotherapy. Meanwhile, the hypoxic state of tumor microenvironment was improved by reducing TIFP, and then alleviated the immunosuppression of T-cells (Hofmann et al., 2013; Baronzio et al., 2015; Patel et al., 2021). Second, factors leading to immunosuppression include: PD-1/PD-L1, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), lymphocyte activation gene 3 (LAG-3), and overexpression of androgen receptor (Cimino-Mathews et al., 2015; Andrews et al., 2017; Pardoll et al., 2021; Guan et al., 2022). These factors made it impossible to fully activate T-cells activity by the inhibition of only the PD-1/PD-L1 signal pathway and led to insignificant effects of ICIs alone. Thus, a synergistic anti-tumor effect can result from the combination of PD-1/PD-L1 inhibitors and other drugs which could affect the activity of immune, including 1) Chemotherapy drugs. Chemotherapy drugs could directly and indirectly enhance immune activity. Paclitaxel and gemcitabine could enhance antitumor immune responses by eliminating immunosuppressive cells (myeloid-derived suppressor cells, regulatory T cells) and promoting the activation of immune cells such as the maturation of natural killer cells and the activation of cytotoxic lymphocytes. Chemotherapy could fully expose tumor antigens by killing tumor cells and improve anti-tumor immune recognition, which provide a good antigenic basis for the application of ICIs, thus improving the efficacy of immunotherapy (Lesterhuis et al., 2011; Roselli et al., 2013; Bracci et al., 2014; Wang et al., 2017). 2) Costimulatory molecule. 3) Androgen receptor antagonist. In addition, the lines of treatment might also be one of the factors affecting the efficiency of immunotherapy (first-line in the ICIs combination chemotherapy group versus post-line in the ICIs alone). The increased tumor burden and the decreased autoimmune potential of patients with post-line therapy were also a aspect that should be considered.
In terms of OS, with similarities to various baseline characteristics (age, sex, race, tumor stage, the status of PD-L1) among included clinical studies, it was speculated that paclitaxel might be the most likely source of heterogeneity given that the treatment regimen of this study were ICIs combined with paclitaxel, while the other three studies were either ICIs alone or ICIs combined with nab-paclitaxel. The subgroup analyses demonstrated significant OS benefit in PD-L1 positive TNBC with immunotherapy but without paclitaxel. The reasons for the absence of clinical benefits of atezolizumab combined with paclitaxel compared with atezolizumab combined nab-paclitaxel were as shown below: Different chemotherapy drugs for immune combination led to different synergistic effects. Because paclitaxel is highly lipophilic and insoluble in water, the vehicle of paclitaxel was polyoxyethylene castor oil and absolute ethanol, the vehicle system could easily cause severe allergic reactions. Therefore, glucocorticoids pretreatment is clinically required to prevent allergic reactions before administration. However the efficacy of immunotherapy might be impaired due to glucocorticoids pretreatment with paclitaxel. In the IMpassion131 trial, the effect of weekly high-dose glucocorticoids on the efficacy of immunotherapy was an important factor. Additionally, paclitaxel had high systemic toxicity due to the lack of specific distribution in tumor tissue, leading to poor tolerance after long-term application. Albumin-bound paclitaxel belonged to a nano-drug delivery system, which greatly improved the solubility of the drug and avoided the use of polyoxyethylene castor oil, thus the occurrence of allergic reactions was greatly reduced (Kundranda et al., 2015). Moreover, albumin-bound paclitaxel could passively target tumor tissue by utilizing the enhanced permeability and retention (EPR) effect of blood vessels of tumor tissue (Kouchakzadeh et al., 2015), thereby reducing systemic toxicity, improving patient tolerance and the effect of anti-tumor therapy. The differences in the dosage forms of paclitaxel and nab-paclitaxel might result in different therapeutic effects of the two drugs in combination with immunotherapy drugs, which were able to provide some guidance for the choice of clinical chemotherapy regimens.
Finally, the limitations of this study include; 1) Different antibodies, cutoffs, cell types and scoring criteria were used to assess PD-L1 expression. PD-L1 expression was assessed by IHC using SP142 antibody for atezolizumab (IC ≥ 1%), 22C3 antibody for pembrolizumab (CPS≥10%), and SP142 antibody for durvalumab (IC ≥ 1%). In the exploratory analysis of IMpassion130 study, SP142 antibody was used to detect the PD-L1 expression by IHC in advanced TNBC on tumor-infiltrating immune cells, and the IC positive population of PD-L1 accounted for 41% in TNBC, which included most tumor cell PD-L1 positive patients (8.7%). IC-positive population could achieve clinical benefit regardless of the expression of PD-L1 on tumor cell. Therefore, PD-L1 expression on IC was a biomarker for predicting the benefit of atezolizumab combined with nab-paclitaxel in metastatic TNBC. Besides, the exploratory analysis also assessed the conformity of 22C3 and SP142 in detecting PD-L1 expression, about 80% concordance was observed between IC ≥ 1% (SP142) and CPS≥10% (22C3) (Rugo et al., 2020; Schmid et al., 2020). However, it should be noted that these two approaches were not equivalent (Dill et al., 2017; Torlakovic et al., 2020; Noske et al., 2021). 2) Only five RCTs were included in our meta-analysis, and we could not performed a detailed subgroup analysis due to the small number of included studies, which might lead to bias. In addition, some clinical RCTs in TNBC patients are currently ongoing, and further analysis of clinical data was required to draw sound conclusions in the future. 3) The differences between PD-1 and PD-L1 inhibitors were also worth considering.
In our meta-analysis, we suggested OS benefit in PD-L1 positive advanced or metastatic TNBC, and the subgroup analysis showed that ICIs combination nab-paclitaxel or ICIs alone might be a better choice compared with ICIs combination paclitaxel in the PD-L1 positive TNBC group. In terms of PFS, no significant PFS benefit was found in PD-L1 positive patients, but the subgroup analysis indicated that a significant benefit of PFS was observed for ICIs combination chemotherapy compared to ICIs alone in the first-line treatment in PD-L1 positive TNBC. No significant improvement was observed for OS or PFS in PD-L1 negative group. The results of this meta-analysis may be beneficial to clinicians in forming better treatment strategies to manage TNBC patients. However, further research is needed, given the limited number of studies currently available for data analysis.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
YQ, XY, HC, and CW conceived and designed the study, YQ and XY performed the literature search, data extraction, quality assessment and data analysis. YQ and XY wrote the paper. CW and GL reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adams, S., Schmid, P., Rugo, H. S., Winer, E. P., Loirat, D., Awada, A., et al. (2019). Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 30, 397–404. doi:10.1093/annonc/mdy517
Andrews, L. P., Marciscano, A. E., Drake, C. G., and Vignali, D. A. (2017). LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 276, 80–96. doi:10.1111/imr.12519
Bachelot, T., Filleron, T., Bieche, T., Arnedos, M., Campone, M., Dalenc, F., et al. (2021). Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat. Med. 27, 250–255. doi:10.1038/s41591-020-01189-2
Bagegni, N. A., Tao, Y., and Ademuyiwa, F. O. (2019). Clinical outcomes with neoadjuvant versus adjuvant chemotherapy for triple negative breast cancer: A report from the national cancer database. PloS One 14, e0222358. doi:10.1371/journal.pone.0222358
Baronzio, G., Parmar, G., and Baronzio, M. (2015). Overview of methods for overcoming hindrance to drug delivery to tumors, with special attention to tumor interstitial fluid. Front. Oncol. 5, 165. doi:10.3389/fonc.2015.00165
Berger, E. R., Park, T., Saridakis, A., Mehra Golshan, M., Rachel A Greenup, R. A., and Nita Ahuja, N. (2021). Immunotherapy treatment for triple negative breast cancer. Pharm. (Basel) 14, 763. doi:10.3390/ph14080763
Bracci, L., Schiavoni, G., Sistigu, A., and Belardelli, F. (2014). Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 21, 15–25. doi:10.1038/cdd.2013.67
Cimino-Mathews, A., Foote, J. B., and Emens, L. A. (2015). Immune targeting in breast cancer. Oncol. Willist. Park) 29, 375–385.
Cortes, J., Cescon, D. W., Rugo, H. S., Nowecki, Z., Im, S. A., Yusof, M. M., et al. (2020). Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396, 1817–1828. doi:10.1016/S0140-6736(20)32531-9
Dill, E. A., Gru, A. A., Atkins, K. A., Friedman, L. A., Moore, M. E., Bullock, T. N., et al. (2017). PD-L1 expression and intratumoral heterogeneity across breast cancer subtypes and stages: An assessment of 245 primary and 40 metastatic tumors. Am. J. Surg. Pathol. 41, 334–342. doi:10.1097/PAS.0000000000000780
Disis, M. L., and Stanton, S. E. (2015). Triple-negative breast cancer: Immune modulation as the new treatment paradigm. Am. Soc. Clin. Oncol. Educ. Book, e25–e30. 35(1). doi:10.14694/EdBook_AM.2015.35.e25
Emens, L. A. (2021). Immunotherapy in triple-negative breast cancer. Cancer J. 27, 59–66. doi:10.1097/PPO.0000000000000497
Failing, J. J., Dudek, O. A., Acevedo, J. A., Chirila, R. M., Dong, H., Markovic, S. N., et al. (2019). Biomarkers of hyperprogression and pseudoprogression with immune checkpoint inhibitor therapy. Future Oncol. 15, 2645–2656. doi:10.2217/fon-2019-0183
García-Teijido, P., Cabal, M. L., Fernández, I. P., and Pérez, Y. F. (2016). Tumor-infiltrating lymphocytes in triple-negative breast cancer: The future of immune targeting. Clin. Med. Insights. Oncol. 10, 31–39. doi:10.4137/CMO.S34540
Guan, X. N., Polesso, F., Wang, C., Sehrawat, A., Hawkins, R. M., Murray, S. E., et al. (2022). Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 606, 791–796. doi:10.1038/s41586-022-04522-6
Hartkopf, A. D., Taran, F. A., Wallwiener, M., Walter, C. B., Krämer, B., Grischke, E. M., et al. (2016). PD-1 and PD-L1 immune checkpoint blockade to treat breast cancer. Breast Care 11, 385–390. doi:10.1159/000453569
Hofmann, M., Pflanzer, R., Zoller, N. N., Bernd, A., Kaufmann, R., Thaci, D., et al. (2013). Vascular endothelial growth factor C-induced lymphangiogenesis decreases tumor interstitial fluid pressure and tumor. Transl. Oncol. 6, 398–404. doi:10.1593/tlo.13274
Huang, X. H., Wang, X. Q., Qian, H. Y., Jin, X. X., and Jiang, G. Q. (2021). Expression of PD-L1 and BRCA1 in triple-negative breast cancer patients and relationship with clinicopathological characteristics. Evidence-Based Complementary Altern. Med. 22, 1–5. doi:10.1155/2021/5314016
Ji, Q., Ding, J., Hao, M., Luo, N., Huang, J., and Zhang, W. (2021). Immune checkpoint inhibitors combined with chemotherapy compared with chemotherapy alone for triple-negative breast cancer: A systematic review and meta-analysis. Front. Oncol. 11. doi:10.3389/fonc.2021.795650
Keenan, T. E., and Tolaney, S. M. (2020). Role of immunotherapy in triple-negative breast cancer. J. Natl. Compr. Canc. Netw. 18, 479–489. doi:10.6004/jnccn.2020.7554
Kouchakzadeh, H., Safavi, M. S., and Shojaosadati, S. A. (2015). Efficient delivery of therapeutic agents by using targeted albumin nanoparticles. Adv. Protein Chem. Struct. Biol. 98, 121–143. doi:10.1016/bs.apcsb.2014.11.002
Kundranda, M. N., and Niu, J. N. (2015). Albumin-bound paclitaxel in solid tumors: Clinical development and future directions. Drug Des. devel. Ther. 9, 3767–3777. doi:10.2147/DDDT.S88023
Latif, F, Jabbar, H.B., Malik, H., Sadaf, H., Sarfraz, A., et al. (2022). Atezolizumab and pembrolizumab in triple-negative breast cancer: A meta-analysis. Expert Rev. Anticancer Ther. 22, 229–235. doi:10.1080/14737140.2022.2023011
Lesterhuis, W. J., Punt, C. J., V Hato, S. V., Eleveld-Trancikova, D., Jansen, B. J., Nierkens, S., et al. (2011). Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J. Clin. Invest. 121, 3100–3108. doi:10.1172/JCI43656
Li, Z., Qiu, Y., Lu, W., Jiang, Y., and Wang, J. (2018). Immunotherapeutic interventions of triple negative breast cancer. J. Transl. Med. 16, 147. doi:10.1186/s12967-018-1514-7
Lipson, E. J., Forde, P. M., Hammers, H. J., Emens, L. A., Taube, J. M., and Topalian, S. L. (2015). Antagonists of PD-1 and PD-L1 in cancer treatment. Semin. Oncol. 42, 587–600. doi:10.1053/j.seminoncol.2015.05.013
Miles, D., Gligorov, J., André, F., Cameron, D., Schneeweiss, A., Barrios, C., et al. (2021). Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 32, 994–1004. doi:10.1016/j.annonc.2021.05.801
Mittendorf, E. A., Zhang, H., Barrios, C. H., Saji, S., Jung, K. H., Hegg, R., et al. (2020). Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 396, 1090–1100. doi:10.1016/S0140-6736(20)31953-X
Noske, A., Ammann, J. U., Wagner, D. C., Denkert, C., Lebeau, A., Sinn, P., et al. (2021). A multicentre analytical comparison study of inter-reader and inter-assay agreement of four programmed death-ligand 1 immunohistochemistry assays for scoring in triple-negative breast cancer. Histopathology 78, 567–577. doi:10.1111/his.14254
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264. doi:10.1038/nrc3239
Patel, J. P., Spiller, S. E., and Barker, E. D. (2021). Drug penetration in pediatric brain tumors: Challenges and opportunities. Pediatr. Blood Cancer 68, e28983. doi:10.1002/pbc.28983
Pusztai, L., Karn, T., Safonov, A., Abu-Khalaf, M. M., and Bianchini, G. (2016). New strategies in breast cancer: Immunotherapy. Clin. Cancer Res. 22, 2105–2110. doi:10.1158/1078-0432.CCR-15-1315
Roselli, M., Cereda, V., Bari, M. G., Formica, V., Spila, A., Jochems, C., et al. (2013). Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology 2, e27025. doi:10.4161/onci.27025
Rugo, H., Loi, S., Adams, S., Schmid, P., Schneeweiss, A., Barrios, C. H., et al. (2020). Abstract PD1-07: Exploratory analytical harmonization of PD-L1 immunohistochemistry assays in advanced triple-negative breast cancer: A retrospective substudy of IMpassion130. Cancer. Res. 80, PD1-07–14. doi:10.1158/1538-7445.SABCS19-PD1-07
Schmid, P., Rugo, H. S., Adams, S., Schneeweiss, A., Barrios, C. H., Iwata, H., et al. (2020). Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Oncol. 21, 44–59. doi:10.1016/S1470-2045(19)30689-8
Seiwert, T. Y., Burtness, B., Mehra, R., Weiss, J., Berger, R., Eder, J. P., et al. (2016). Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1B trial. Lancet. Oncol. 17, 956–965. doi:10.1016/S1470-2045(16)30066-3
Sharma, P. (2016). Biology and management of patients with triple-negative breast cancer. Oncologist 21, 1050–1062. doi:10.1634/theoncologist.2016-0067
Torlakovic, E., Lim, H. J., Adam, J., Barnes, P., Bigras, G., Chan, A. W., et al. (2020). Interchangeability" of PD-L1 immunohistochemistry assays: A meta-analysis of diagnostic accuracy. Mod. Pathol. 33, 4–17. doi:10.1038/s41379-019-0327-4
Wang, Q. H., Gao, J. Z., and Wu, X. (2018). Pseudoprogression and hyperprogression after checkpoint blockade. Int. Immunopharmacol. 58, 125–135. doi:10.1016/j.intimp.2018.03.018
Wang, Z. B., Till, B., and Gao, Q. L. (2017). Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology 6, e1331807. doi:10.1080/2162402X.2017.1331807
Winer, E. P., Lipatov, O., Im, S. A., Goncalves, A., Muñoz-Couselo, E., Lee, K. S., et al. (2021). Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 22, 499–511. doi:10.1016/S1470-2045(20)30754-3
Keywords: triple-negative breast cance, immune checkpoint inhibitors, PD-L1 expression, efficacy, meta-analysis
Citation: Qi Y, Yan X, Wang C, Cao H and Liu G (2022) Predictive value of PD-L1 expression to the efficacy of immune checkpoint inhibitors in advanced triple-negative breast cancer: A systematic review and meta-analysis. Front. Pharmacol. 13:1004821. doi: 10.3389/fphar.2022.1004821
Received: 27 July 2022; Accepted: 22 November 2022;
Published: 02 December 2022.
Edited by:
Yunjian Zhang, The First Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Ran Wang, Anhui Medical University, ChinaCopyright © 2022 Qi, Yan, Wang, Cao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingjie Qi, cHJpbmNlc3NxeWpAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.