- 1Shenyang the Tenth People’s Hospital, Shenyang, China
- 2Shunde Hospital of Guangzhou University of Chinese Medicine, Foshan, China
- 3The Second Clinical School of Medicine, Guangzhou University of Chinese Medicine, Guangdong Provincial Hospital of Chinese Medicine-Zhuhai Hospital, Zhuhai, China
Heart failure (HF) is the terminal stage of multifarious heart diseases and is responsible for high hospitalization rates and mortality. Pathophysiological mechanisms of HF include cardiac hypertrophy, remodeling and fibrosis resulting from cell death, inflammation and oxidative stress. Heat shock proteins (HSPs) can ameliorate folding of proteins, maintain protein structure and stability upon stress, protect the heart from cardiac dysfunction and ameliorate apoptosis. Traditional Chinese medicine (TCM) regulates expression of HSPs and has beneficial therapeutic effect in HF. In this review, we summarized the function of HSPs in HF and the role of TCM in regulating expression of HSPs. Studying the regulation of HSPs by TCM will provide novel ideas for the study of the mechanism and treatment of HF.
Introduction
Heart failure (HF) is a clinical syndrome that is characterized by impaired myocardial structure or ventricular contraction/diastolic function and it causes insufficient cardiac output (Yancy et al., 20132013). HF is a critical health problem that affects 26 million people worldwide, and an estimated 17–45% of patients with HF admitted to hospital die within 1 year of admission. Most patients die within 5 years after admission (Ambrosy et al., 2014; Ponikowski et al., 2014). The recommended pharmacological treatments for HF include angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), angiotensin receptor neprilysin inhibitor (ARNI), If channel inhibitor, β-adrenergic blockers and diuretics. Recommended treatments are able to reduce hospitalizations, morbidity and mortality, but can have severe side effects like angioedema, electrolyte depletion and fluid depletion (Yancy et al., 20172017). Therefore, developing new therapeutic methods and medicine will be of great significance in the treatment of HF.
Heat shock proteins (HSPs) are a group of conserved proteins with multiple biological activities (Stetler et al., 2010). Previous studies reveal the vital role played by HSPs in HF (Ranek et al., 2018). Therefore, it would be imperative to focus on regulation of HSPs in the treatment of HF. Traditional Chinese Medicine (TCM) contains numerous chemical components and active ingredients, which can regulate expression of HSPs in various diseases (Yang et al., 2017a; Kunde et al., 2017; Zhou et al., 2018; Zhao et al., 2020). Furthermore, TCM can improve cardiac function and ameliorate damage caused by HF (Wang et al., 2017a). Recent studies suggest that TCM can alter expression of HSPs in HF (Wang et al., 2014; Zhang et al., 2018a; Nie et al., 20192019). Consequently, TCM may regulate expression of HSPs to treat HF. We therefore summarized the role of HSPs in the pathogenesis of HF, effects of TCM in regulating HSPs and action of TCM targeting HSPs in treating HF to enhance our understanding of the mechanisms in HF, and provide novel ideas for its application as a therapeutic strategy of HF.
HSPs Family
HSPs widely occur in eukaryotic cells and can respond to multiple stimuli, high temperature, lack of nutrients, energy depletion, aging, oxidative stress, acute and chronic inflammatory reactions, viral and bacterial infections, ischemia, heavy metals and excessive exercise (Kregel, 19852002). HSPs have a variety of biological functions. The most crucial role is they act as molecular chaperones which ensure correct folding of newly synthesized proteins, facilitating refolding of misfolded proteins upon stress, and maintaining protein structure and stability (Stetler et al., 2010). HSPs is divided into the following six families according to their relative molecular masses; HSP110, HSP90, HSP70, HSP60, HSP40, and small HSPs (HSPs).
HSP110 is a high molecular weight HSP belonging to HSP70 superfamily. Its expression is also induced by stress, and it cooperates with other HSPs to facilitate refolding of proteins and cell survival (Zuo et al., 2016).
HSP90 is a highly conserved ATP-dependent molecular chaperone that is involved in homeostasis and folding of proteins (Wu et al., 2017). The HSP90 family has two isoforms which occur in the cytoplasm: 1) stress-inducible HSP90α and 2) a constitutively expressed HSP90β.
HSP70 family is by far the most widely studied group of HSPs which generally occur in the cytoplasm and nucleus (Shrestha and Young, 2016). HSP70 acts in an ATP-dependent manner, and its family includes inducible HSP70, constitutively expressed HSP70 and glucose-regulated protein 78 (GRP78). The chaperone protein, HSP70 is principally dedicated to the degradation of unstable and misfolded proteins and refolding of proteins, preventing and dissolving protein complexes, and stabilizing cellular homeostasis (Daugaard et al., 2007). GRP78 belongs to the HSP70 family and plays an essential role in attenuating endoplasmic reticulum (ER) stress. ER is a cellular organelle responsible for storage of calcium, protein synthesis and folding, and lipid metabolism (Schwarz and Blower, 2016). Ischemia, hypoxia, disruption of calcium homeostasis, ATP depletion, and oxidative stress result in accumulation of unfolded proteins in the ER subsequently causing endoplasmic reticulum (ER) stress. This initiates unfolded protein response (UPR) to maintain homeostasis in the ER (Minamino et al., 2010). However, sustained UPR can cause cell death. Consequently, expression of GRP78 is increased acting as a quality control system.
HSP60 is a chaperone protein that forms a complex with the chaperone protein, HSP10 to promote protein folding. HSP60 mainly exists in the mitochondria, but can also be distributed within the cytoplasm, cell membrane and extracellular matrix (Rizzo et al., 2011).
Small HSPs are a group of proteins which are small size (12–42 kDa) and are present in the cytoplasm and nucleus. Small HSPs include HSP20, HSP27, heme oxygenase-1 (HO-1), and αB-crystallin (CRYAB). HSPs are involved in the regulation of anti-oxidants, anti-apoptosis, muscle contraction and cell motility, which can prevent irreversible aggregation of damaged proteins in an ATP-independent manner and protect cells under unfavorable conditions (Mymrikov et al., 2011).
Function of HSPs in HF
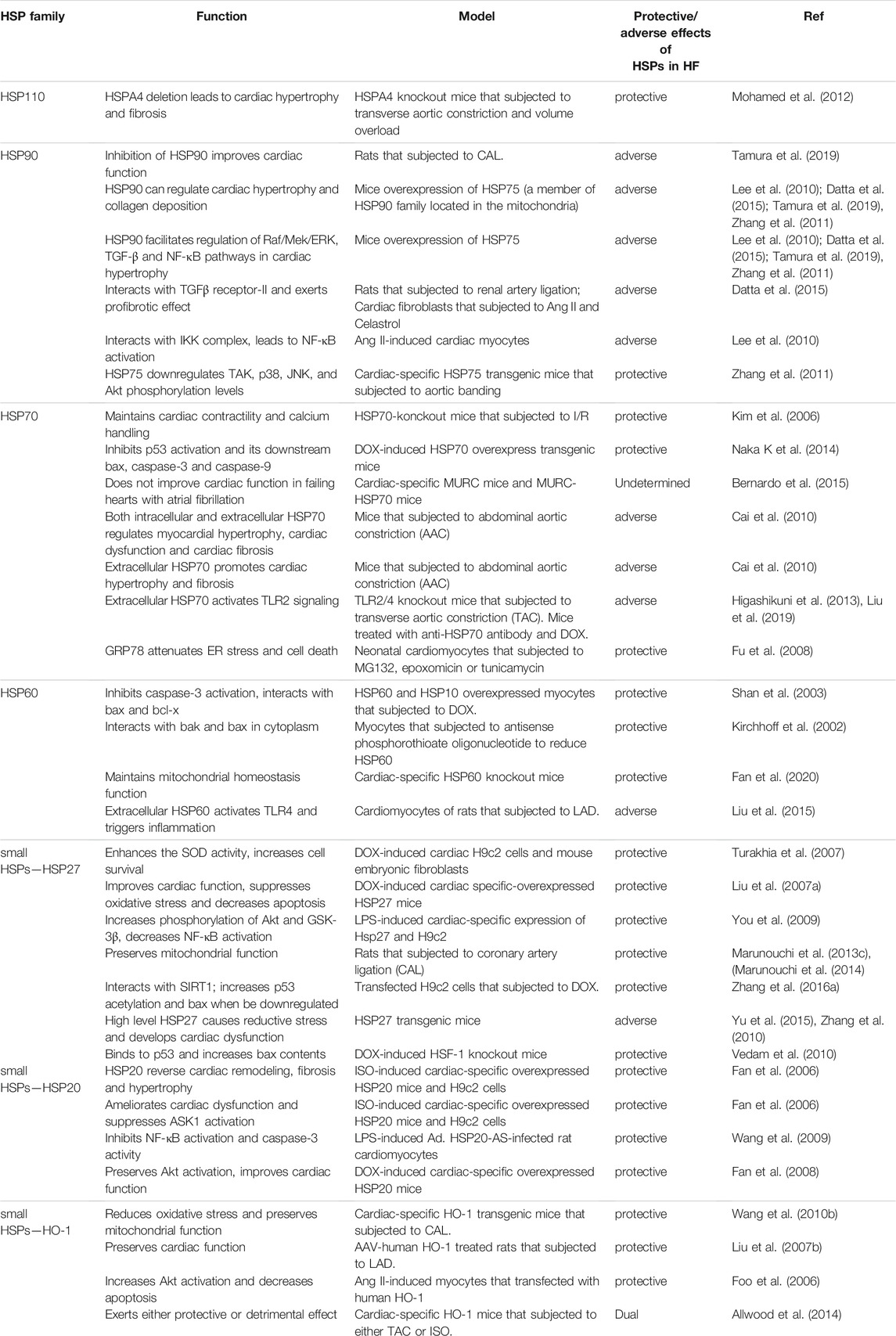
HSPs participate in a wide range of biological activities, can contribute to intracellular homeostasis in cells and counteract pathological factors. Previous studies have investigated changes in the expression of HSPs in HF and the effects of overexpressed/deficient HSPs in HF. In this review, we have summarized recent advances in functions of HSPs in HF (Figure 1; Table 1).
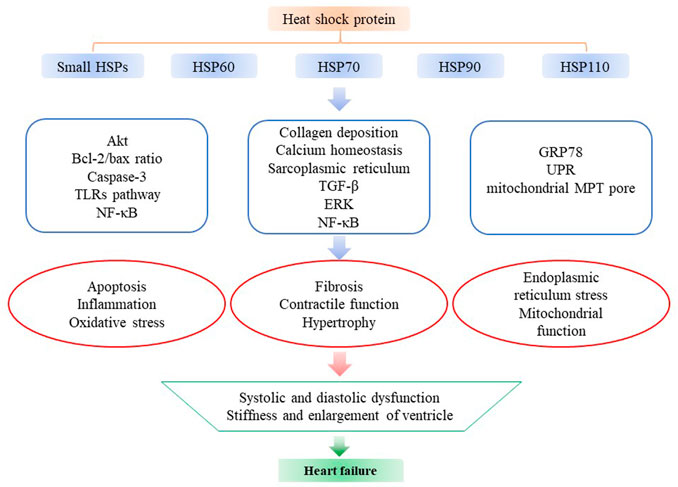
FIGURE 1. The functions of heat shock proteins in HF. Small HSPs (HSP27, HSP20, HO-1), HSP60, HSP70, HSP90, and HSP110 are the most studied HSPs in HF. They can affect apoptosis, inflammation, oxidative stress, fibrosis, contractile function, hypertrophy, ER stress and mitochondrial function by regulating multiple pathways like Akt, caspase-3, ERK and various cellular functions like ER and mitochondria in the progression of HF, including modulating the systolic and diastolic function and the stiffness and enlargement of ventricle.
HSP110
HSPA4 is a member of the HSP110 family that acts as a nucleotide exchange factor for HSP70 chaperones. Expression of HSPA4 was significantly elevated in hearts of mice subjected to TAC (Mohamed et al., 2012). HSPA4 is essential in ensuring proper folding of proteins and maintaining homeostasis in cardiomyocytes. Deletion of HSPA4 accelerates cardiac hypertrophy and fibrosis (Mohamed et al., 2012).
HSP90
Expression of HSP90 was decreased in animals treated with fluoride (Panneerselvam et al., 2017), and no significant change was observed after CAL in comparison with control group (Tanonaka et al., 2001a), whereas expression of HSP90 increased in patients with DCM (Kapustian et al., 2013). DCM alters distribution of HSP90 in cells: mitochondrial HSP90 content was increased in the left ventricular myocardium of individuals with DCM (Kapustian et al., 2013). HSP90 can have a detrimental effect on HF and cardiac hypertrophy. Inhibiting functional expression of HSP90 can attenuate cardiac hypertrophy and reduce collagen deposition. HSP90 facilitates regulation of Raf/Mek/ERK, transformation of growth factor-β (TGF-β) and NF-κB pathways in cardiac hypertrophy which are either induced by MI or pressure overload (Lee et al., 2010; Datta et al., 2015; Tamura et al., 2019). Mice with cardiac-specific overexpressed HSP75 (a member of HSP90 family located in the mitochondria) may attenuate hypertrophy and fibrosis in response to pressure overload. Protection depends on the inhibitory effect of HSP75 in regulating MAPK and Akt pathways by reducing phosphorylation of p38, JNK and Akt (Zhang et al., 2011).
HSP70
Previous studies have proven the protective function of HSP70s in HF. Expression of HSP70 in HF varies with models. Levels of intracellular HSP70 were elevated in patients with HF of arrhythmogenic right ventricular cardiomyopathy (ARVC), ischemic cardiomyopathy (ICM) and DCM (Wei et al., 2009). Nonetheless, expression of HSP70 remained unchanged at 8 w after CAL in rats in comparison with the control group, accompanied by decreased cardiac contractility and function. HSP70 was not induced even under heat stress (Tanonaka et al., 2001b; Tanonaka et al., 2001c). Myocardial dysfunction of CAL-induced HF was partially due to impaired induction of HSP70 and the mechanisms can be elucidated as follows: 1) total expression of HSF-1 is enhanced in CAL-induced HF rat model, whereas phosphorylated HSF-1 at ser303 is accumulated in the cytoplasm and fails to translocate to the nucleus thereby becoming incapable of inducing HSP70 (Marunouchi et al., 2013a), 2) interaction of HSP90 and HSF-1 is enhanced in the cytoplasm hindering nuclear translocation of HSF-1 (Marunouchi et al., 2013b), 3) downregulated mitochondrial aldehyde dehydrogenase2 (ALDH2) and the upregulated 4-hydroxy-2-nonenol (4-HNE) suppress expression of HSP70 in response to hypoxia, and this process is independent of HSF-1 (Sun et al., 2014). HSP70 can inhibit apoptosis and enhance tolerance to harmful stimuli to protect the heart from further damage. HSP70 knockout mice are more susceptible to ischemia/reperfusion (I/R) injury and more likely to develop myocardial hypertrophy resulting in decreased Ca2+ in the sarcoplasmic reticulum, damaged myocardial contractility, activation of JNK, p38, Raf-1 and extracellular regulated protein kinases (ERKs) pathways (Kim et al., 2006). Overexpressed HSP70 can protect mice from HF induced by DOX by inactivating p53 and its downstream bax, caspase-3 and caspase-9 (Naka K et al., 2014). However, long-term overexpression of HSP70 does not mitigate cardiac dysfunction and reverses remodeling in failing hearts with atrial fibrillation (AF). This indicates that HSP70 can be beneficial during acute cardiac condition but it cannot adequately inhibit chronic stimuli (Bernardo et al., 2015; Bernardo et al., 2016).
Intracellular HSP70 and extracellular HSP70 have differential effects on pressure overload-induced HF. Inhibition of HSP70 expression (both intracellular and extracellular) through inactivation of HSF-1 can promote myocardial hypertrophy and cardiac dysfunction but ameliorate cardiac fibrosis; functional inhibition of extracellular HSP70 using anti-HSP70 attenuates cardiac hypertrophy and fibrosis (Cai et al., 2010). Results of a study indicated the protective effect of intracellular HSP70 in cardiac function, and that the potential mechanism of anti-HSP70 lies in its inhibitory effect on ERK and p38 pathway through neutralization of extracellular HSP70. Concentration of plasma HSP70 was increased in both TAC-induced pressure overload and DOX-induced HF mice models. Extracellular HSP70 activates TLR2/NF-κB pathway, triggers inflammation and causes cardiac hypertrophy and fibrosis (Higashikuni et al., 2013; Liu et al., 2019). Furthermore, anti-HSP70 antibodies attenuate cardiac dysfunction induced by TAC or DOX by blocking extracellular HSP70-mediated activation of TLR2 pathway (Higashikuni et al., 2013; Liu et al., 2019). Plasma HSP70 was significantly increased in patients with HF and ARVC, ICM or DCM (Genth-Zotz et al., 2004; Gombos et al., 2008; Wei et al., 2009). Plasma HSP70 can be an independent prognostic biomarker for early diagnosis and is suitable for predicting long-term survival of patients with HF (Li et al., 2013a; Jenei et al., 2013).
Stress-induced UPR in the endoplasmic reticulum plays crucial role in the development and progression of HF (Minamino et al., 2010). Increased expression levels of GRP78, a marker of ER stress can also be an indicator of impaired UPR during progression of HF (Okada et al., 2004; Dally et al., 2009; Sawada et al., 2010). However, overexpressed GRP78 has a protective function in myocytes (Fu et al., 2008).
HSP60
Unlike other HSPs, expression of HSP60 was elevated at 8w after CAL, and elevated HSP60 expression was driven by loss in the transcriptional activity of NF-κB for heat shock factor-1 (HSF-1) and failure to induce HSP72 in CAL-induced HF (Tanonaka et al., 2001a; Toga et al., 2007; Wang et al., 2010a). In addition, HF and DCM induced mitochondrial translocation of HSP60 (Sidorik et al., 2005; Lin et al., 2007). The potential protective mechanisms of HSP60 in the myocardium are involvement in anti-apoptosis and preservation of mitochondrial function. HSP60 can increase b-cell lymphoma-2 (bcl-2)/bcl-2-associated x (bax) ratio, inhibit caspase-3 and poly (ADP-ribose) polymerase (PARP) (Kirchhoff et al., 2002; Shan et al., 2003). HSP60 deletion causes HF in mice and impairs mitochondrial protein homeostasis (Fan et al., 2020). HSP60 transfers to the plasma and plasma membrane in HF, and its surface translocation is highly associated with apoptosis (Lin et al., 2007). Extracellular HSP60 can trigger toll-like receptor4 (TLR4) pathway and induce inflammatory response (Liu et al., 2015). The plasma HSP60 is positively correlated with occurrence of adverse cardiac events in both acute and chronic HF, implicating its potential of being a biomarker of HF (Niizeki et al., 2008; Zhang et al., 2008; Bonanad et al., 2013).
Small HSPs—HSP27
HSP27 (also called HSP25 in murine) is involved in numerous cellular functions; it can counteract apoptosis and oxidative stress, and inhibit cardiac remodeling and dysfunction of a failing heart (Liu et al., 2007a; Turakhia et al., 2007; You et al., 2009; Marunouchi et al., 2013c; Marunouchi et al., 2014). Expression levels of HSP27 are increased in failing hearts, and this is induced by doxorubicin (DOX) and fluoride (Vedam et al., 2010; Panneerselvam et al., 2017) as a response to harmful stimuli. HSP27 may possibly have a dual effect on HF; it not only acts as an antioxidant to protect the heart from damages and improve cardiac function (Liu et al., 2007a; Turakhia et al., 2007; You et al., 2009), but also augments injury in a failing heart (Vedam et al., 2010; Zhang et al., 2010; Yu et al., 2015). Overexpression and phosphorylation of HSP27 counteracts the cardiotoxic effect of DOX, mitigates cardiac dysfunction in dilated cardiomyopathy (DCM) and congestive HF (Liu et al., 2007a; Turakhia et al., 2007). Cardiac-specific overexpressed HSP27 enhances phosphorylation of serine/threonine kinase (Akt), attenuates activation of glycogen synthase kinase-3β (GSK-3β) and nuclear factor kappa-B (NF-κB) to ameliorate cardiac dysfunction induced by lipopolysaccharide (LPS) (You et al., 2009). Expression and phosphorylation of HSP27 in the cytoplasm and mitochondria increased at 2w after coronary artery ligation (CAL) but decreased in the mitochondria at 8 w. This indicates that mitochondrial HSP27 and phosphorylated HSP27 significantly contribute to mitochondrial function in HF (Marunouchi et al., 2013c; Marunouchi et al., 2014). The co-chaperones of HSP27 alter its function. Downregulation of HSP27 hinders interaction of silent information regulator1 (SIRT1)-p53 and endowed p53 acetylation, augmenting apoptosis in DOX-induced H9c2 cells (Zhang et al., 2016a). However, inducible HSP27 can be pro-apoptotic by binding to and transactivating p53 resulting in loss of cardiomyocytes in HF (Vedam et al., 2010). Moderate level of HSP27 is beneficial, whereas higher levels of HSP27 can induce reductive stress and aggravate cardiomyopathy (Zhang et al., 2010; Yu et al., 2015). Plasma HSP27 is regarded as a novel candidate biomarker for diagnosing chronic HF and an independent predictor of HF- related mortality (Liu et al., 2016a; Traxler et al., 2017).
Other Small HSPs
Other HSPs are also involved in the pathophysiology of HF. HSP20 has anti-apoptotic and anti-oxidative effects in cardiomyocytes which improve cardiac function. HSP20 can reverse cardiac remodeling, fibrosis and hypertrophy induced by isoproterenol (ISO) by inhibiting apoptosis signal regulating kinase1 (ASK1)/Jun N-terminal kinase (JNK)/p38 pathways (Fan et al., 2006). HSP20 decreases activity of NF-κB to attenuate apoptosis and myocardial dysfunction induced by LPS (Wang et al., 2009). HSP20 maintains activity of Akt signaling pathway and suppresses oxidative stress to alleviate damage of DOX (Fan et al., 2008). Expression of HO-1 was elevated at both protein and mRNA levels in the right-sided HF and post-myocardial infarction (MI) HF (Raju et al., 1999; Wang et al., 2010b). HO-1 induces anti-oxidant and anti-apoptotic effects, and enhances tolerance to HF. HO-1 can attenuate cardiac hypertrophy, fibrosis, oxidative stress, mitochondrial MPT pore (mPTP) opening and promote angiogenesis to preserve left ventricular function and attenuates remodeling of post-MI HF (Liu et al., 2007b; Wang et al., 2010b). Overexpressed HO-1 activates Akt pathway to reduce apoptosis in myocytes which is induced by angiotensin II (Ang II) (Foo et al., 2006). However, the protective role of HO-1 seems to depend on the type of stimulation. HO-1 significantly attenuated ISO-induced cardiac dysfunction, fibrosis and hypertrophy, but was detrimental in aging and transverse aortic constriction (TAC) models (Allwood et al., 2014).
In conclusion, HSPs make significant contributions in HF and most HSPs can exhibit protective effects whereas a few HSPs may accelerate damage based on a specific condition. Functions of HSPs seem to vary with their location: intracellular HSPs exhibit anti-apoptotic, anti-inflammatory and anti-oxidative effects, whereas extracellular HSPs are on the contrary. Moreover, HSPs modulate several signaling pathways to initiate biological effects. Consequently, regulation of the expression of HSPs is a promising treatment for HF.
TCM Regulates Expression of HSPs
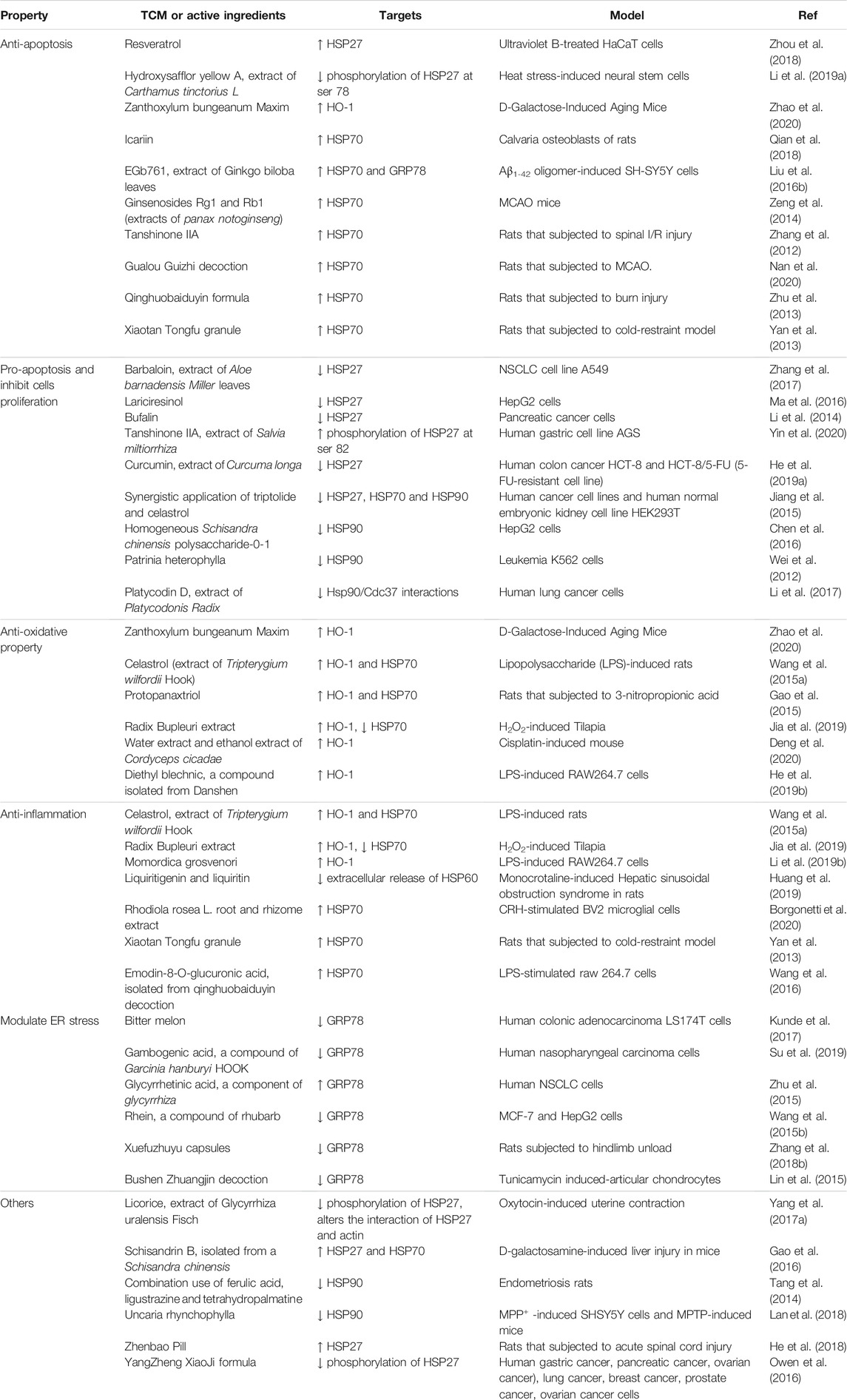
TCM can regulate expression of HSPs to initiate anti-apoptotic, pro-apoptotic and anti-inflammatory responses. TCM can be used as anti-oxidants and for modulating ER stress in cancer, diseases of the nervous system, ischemic diseases, hepatopathy, gastroenteropathy and uterine diseases. Regulatory effects of TCM on HSPs are summarized and listed in Figure 2 and Table 2.
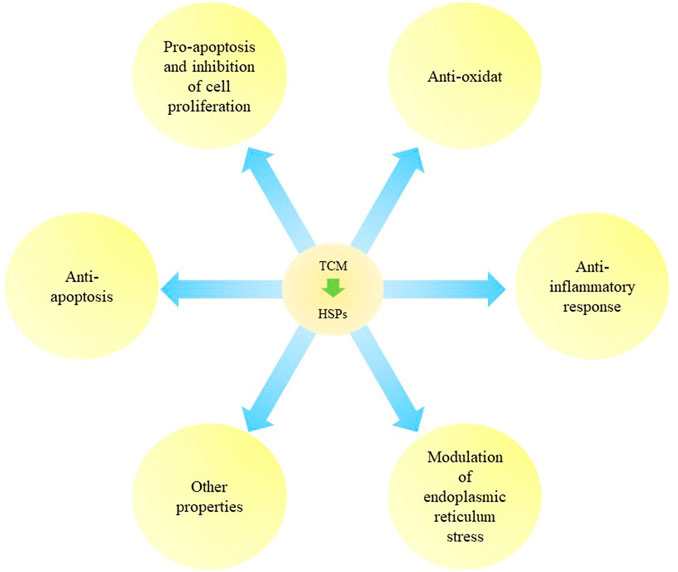
FIGURE 2. The regulation of Traditional Chinese Medicine on heat shock proteins. Traditional Chinese medicine (TCM) can exert various biological functions like anti-apoptosis, pro-apoptosis and inhibition of cell proliferation, anti-oxidant, anti-inflammatory response, modulation of ER stress and other properties via regulating HSPs.
Anti-Apoptosis
Resveratrol inhibits apoptosis in ultraviolet B-treated HaCaT cells, and can upregulate HSP27 expression, increase bcl-2/bax ratio, and inhibit caspase-3 activity and p65 expression (Zhou et al., 2018). Hydroxysafflor yellow A is extracted from the flowers of Carthamus tinctorius L.; it can inhibit phosphorylation of p38 and HSP27 Ser78, and prevent apoptosis in heat stress-induced neural stem cells (NSCs) (Li et al., 2019a). Icariin upregulates HSP70 and serpin family F-1 (PEDF-1) to promote proliferation, calcium deposition and inhibits osteoblast apoptosis (Qian et al., 2018). Pretreatment with EGb761, an extract of Ginkgo biloba leaves can increase levels of HSP70 and GRP78 to reduce apoptosis and neurotoxicity in Aβ1-42 oligomer-induced SH-SY5Y cells (Liu et al., 2016b). Ginsenosides Rg1 and Rb1, extracts of Panax notoginseng increased HSP70 levels and restored the Akt/NF-κB signaling pathway in the hippocampus, causing neuroprotective effects against cerebral I/R (Zeng et al., 2014). Tanshinone IIA can attenuate spinal I/R injury and promote expression of HSP70 and bcl-2 (Zhang et al., 2012). Some formula can also be anti-apoptotic. Gualou Guizhi decoction increases expression of HSP70 in middle cerebral artery occlusion (MCAO) rat model and alleviates neuronal apoptosis by inhibiting PARP-1/apoptosis inducing factor (AIF) signaling pathway (Nan et al., 2020). Qinghuobaiduyin formula (contains extracts of Astragalus membranaceus, Lonicera japonica, Scutellaria baicalenis Georgi, Ophiopogon japonicus and Rheum rhabarbarum) increases HSP70 levels and induces anti-apoptotic effects on the intestinal mucosa following burn injury (Zhu et al., 2013). Granules of Xiaotan Tongfu promote cell proliferation, inhibit gastric mucosal cell apoptosis and local inflammation, and increase expression of HSP70 in rats with stress ulceration (Yan et al., 2013).
Pro-Apoptosis and Inhibition of Cell Proliferation
Induction of apoptosis in cancer cells is vital and certain types of TCM can inactivate HSPs resulting in increased cell death. Barbaloin which is extracted from leaves of Aloe barbadensis Miller, inactivates p38 mitogen-activated protein kinase (MAPK)/HSP27 pathway, induces apoptosis and inhibits growth of human non-small cell lung cancer (NSCLC) cell line, A549 (Zhang et al., 2017). Lariciresinol downregulates HSP27 and initiates apoptosis in HepG2 cells (Ma et al., 2016). Bufalin induces apoptosis by partially targeting HSP27, eliminates anti-apoptotic effect of HSP27 in pancreatic cancer cells, and induces caspase-3 and caspase-9 (Li et al., 2014). Temporal treatment with tanshinone IIA (a diterpene quinone extract from Salvia miltiorrhiza) increases phosphorylation of HSP27 at Ser 82, and subsequent overexpression of HSP27 limits tanshinone IIA-induced cell death in gastric cells (Yin et al., 2020). Curcumin is a hydrophobic polyphenol derived from the rhizomes of Curcuma longa, which can inhibit cell proliferation and decrease expression of HSP27 at mRNA levels in human colon cancer (HCT)-8 and HCT-8/5-FU (5-FU-resistant cell line) (He et al., 2019a). Triptolide reduces protein levels of HSP27, HSP70 and HSP90 whereas celastrol increases protein levels of HSP27 and HSP70. Synergistic application of triptolide and celastrol can mitigate effect of increased HSP27 and HSP70, inhibit growth of cancer cells, and induce apoptosis in cancer cells (Jiang et al., 2015). Homogeneous polysaccharide-0-1 (SCP-0-1) from Schisandra chinensis induces mitochondrial apoptosis in human hepatocellular liver carcinoma, a mechanism involved in the downregulation of HSP90 and inhibition of Akt pathway (Chen et al., 2016). Patrinia heterophylla, a member of Valerianaceae family, inhibits expression of HSP90α to induce apoptosis in leukemia K562 cells (Wei et al., 2012). Platycodin D is a saponin isolated from Platycodonis radix, which can disrupt Hsp90/Cdc37 co-chaperone interactions without affecting ATPase activity of HSP90 and reduces Akt phosphorylation in human lung cancer cells (Li et al., 2017).
Anti-Oxidant
Zanthoxylum bungeanum Maxim is a plant that can be used both as a condiment and as medicine. Its extracts in water and volatile oil can activate Akt/nuclear factor E2-related factor 2 (Nrf2)/HO-1 pathway to prevent cognitive dysfunction and hippocampal neuronal cell damage which are induced by D-galactose (Zhao et al., 2020). Celastrol is extracted from the root of Tripterygium wilfordii Hook, and it possesses anti-oxidant and anti-inflammatory effects which can attenuate cardiac iNOS, tumor necrosis factor-α (TNF-α), NF-κB and activity of caspase-3. Celastrol can also increase contents of HO-1 and HSP70 in the heart and aorta to prevent circulatory failure in sepsis (Wang et al., 2015a). Protopanaxtriol increases expression of HO-1 to induce anti-oxidative effect, relatively increases reactive oxygen species (ROS) and HSP70, and alleviates behavior disorders in 3-nitropropionic acid-induced rat model of Huntington’s disease (Gao et al., 2015). Pretreatment with extracts from Radix bupleuri can reverse increased HSP70 at mRNA levels in liver injury induced by H2O2. The primary beneficial effects of Radix bupleuri extracts of inhibiting oxidative stress is due to its role in enhancing Nrf2/HO-1 signaling pathway and inhibiting TLRs/MyD88/NF-κB signaling pathway (Jia et al., 2019). Water and t and ethanol extracts of Cordyceps cicadae increase production of Nrf2, HO-1 and other antioxidants, inhibit activation of NF-κB, attenuates oxidative stress and inflammation to prevent cisplatin-induced kidney injury (Deng et al., 2020). Diethyl blechnic, a compound isolated from Salvia miltiorrhiza, increases expression of Nrf2/HO-1 and inhibits TLR4/MyD88 signaling pathway to ameliorate oxidative stress in LPS-induced RAW264.7 cells (He et al., 2019b).
Anti-Inflammatory Response
Momordica grosvenori attenuates phosphorylation of Akt1 pathway, increases expression of HO-1 to initiate anti-inflammatory effect on LPS-induced RAW264.7 cells (Li et al., 2019b). Liquiritigenin and liquiritin are two key compounds in Glycyrrhizae radix et Rhizoma, which have the ability to alleviate liver inflammatory injury. These compounds can prevent release of HSP60 to the extracellular matrix in monocrotaline-induced rat models and block exogenous HSP60-activated NF-κB in RAW264.7 cells (Huang et al., 2019). Root and rhizome extracts of Rhodiola rosea L. increase expression of HSP70 in corticotropin releasing hormone (CRH)-stimulated BV2 microglial cells, counteract neuroinflammatory effect and enhance cell survival (Borgonetti et al., 2020). Emodin-8-O-glucuronic acid, a compound isolated from qinghuobaiduyin decoction (TCM), increases expression of HSP70 to inhibit inflammatory cytokines in the LPS-stimulated Raw 264.7 cells (Wang et al., 2016).
Modulation of Endoplasmic Reticulum Stress
The chaperone heat shock protein GRP78, together with C/-EBP homologous protein (CHOP) are commonly used as markers of endoplasmic reticulum (ER) stress. As an ER chaperone, GRP78 functions as a potent anti-apoptotic factor and confers drug resistance, whereas CHOP is a key initiating factor of ER stress-related cell death. Moreover, as a master of UPR in ER of normal cells, GRP78 force the unfolded proteins to refold or degrade by cellular degradation mechanisms. While under stress, the overexpression of GRP78 on the cell membrane mediates the vast amount of disordered proteins (Ibrahim et al., 2019).
Gambogenic acid is a component of Gamboge, a dry resin obtained from Garcinia hanburyi HOOK. f. (Guttiferae), which downregulates GRP78 and upregulates CHOP to induce apoptosis in poorly differentiated human nasopharyngeal carcinoma cells (Su et al., 2019). Glycyrrhetinic acid, a bioactive component of glycyrrhiza, upregulates GRP78 and CHOP to modulate ER stress and suppresses proliferation of human NSCLC cells (Zhu et al., 2015). Rhein, a compound of rhubarb can adequately induce GRP78 and inhibit expression of GRP78 induced by ER stress, disrupting the anti-apoptotic pathway in cancer cells (Wang et al., 2015b). Bitter melon ameliorates ER stress in epithelial cells of the colon thus decreasing expression of GRP78 and CHOP (Kunde et al., 2017). Capsules of Xuefu Zhuyu decrease expression of GRP78 and CHOP to alleviate ER stress. Capsules also attenuate loss of muscle mass and cross-sectional areas induced by hindlimb unloading (Zhang et al., 2018b). A decoction of Bushen Zhuangjin downregulates expression of GRP78 and inhibits ER stress to suppress tunicamycin induced-chondrocyte apoptosis (Lin et al., 2015).
Other Properties
Licorice is derived from the roots and rhizomes of Glycyrrhiza uralensis Fisch, and it reduces levels of phosphorylated HSP27 at Ser15, altering interaction of HSP27 and actin, and it decreases actin polymerization to enhance spasmolytic effects in oxytocin-stimulated uterus (Yang et al., 2017a). Schisandrin B is isolated from Schisandra chinensis and it attenuates D-galactosamine-induced liver injury in mice. Hepatoprotective effect of schisandrin B is partially attributed to increased levels of HSP27 and HSP70 (Gao et al., 2016). Combined use of ferulic acid, ligustrazine and tetrahydropalmatine enhance downregulation of hypothalamus–pituitary–ovarian axis (HPOA), estrogen response element (ERE) pathway and expression of HSP90 in rat model of endometriosis (Tang et al., 2014). Uncaria rhynchophylla inhibits expression of HSP90 and activates Akt pathway to induce neuroprotective effect in mouse model of Parkinson’s disease (Lan et al., 2018). Zhenbao pills promote expression of HSP27, affect Treg cell differentiation and ameliorate acute spinal cord injury in rats (He et al., 2018). YangZheng XiaoJi formula is able to inhibit phosphorylation of HSP27 and reduce migration of cancer cells (Owen et al., 2016).
Therapeutic Functions of TCM in HF
TCM is widely distributed in nature and the various forms of TCM include signal herbs, formula, decoctions, capsules and others. Discovery and application of TCM is based on TCM theories. TCM with particular therapeutic effects have been applied in the treatment of HF in China for thousands of years. A systematic review has revealed that Shengmai (comprising herbs from Panax ginseng, Ophiopogon japonicus and Schisandra chinensis) improves ejection fraction, cardiac output, cardiac index, left ventricular end-systolic volume and myocardial contractility (Zhou et al., 2014). Clinical studies have mostly been conducted by the Chinese and recent studies come to emphasize a uniform standard.
Studies have summarized the commonly prescribed herbs for treating different HF syndromes are as follows: Radix aconiti carmichaeli (Fuzi), Atractylodes (Baizhu), Cassia twig (Guizhi), Dried ginger (Ganjiang), Radix pseudostellariae (Taizishen), Radix astragali (Huangqi), Codonopsis pilosula (Dangshen), Ginseng (Renshen), Panax notoginseng (Sanqi), Chinese angelica (Danggui), Safflower (Honghua), Ligusticum wallichii (Chuanxiong), Salvia miltiorrhiza (Danshen), Red paeony root (Chishao), Peach kernel (Taoren), Hawthorn (Shanzha), Semen lepidii (Tinglizi), Alisma (Xieze), Poria cocos (Fuling); Radix Ophiopogonis (Maidong), Fructus schisandrae (Wuweizi), Radix rehmanniae (Shengdi), Pinellia (Banxia), Trichosanthes Kirilowii (Gualou), Dried tangerine or orange peel (Chenpi), and Scallions white (Xiebai), etc (Wang et al., 2017a). Moreover, there are several most commonly prescribed formulae that have been proven effective clinically for the treatment of HF. These decoctions prescribed by physicians include: Zhenwu tang, Shengmai san, Baoyuan tang, Xuefuzhuyu tang, Tinglidazaoxiefei tang, Danshen yin, and Taohongsiwu tang etc. Meanwhile, several Chinese patent drugs have been successfully produced by standardized procedures and are widely used in health care industry. Drugs in the form of capsules or pills include: Qishenyiqi dripping pill (QSYQ), Fufang danshen dripping pill, Danqi pill (DQP), Qili qiangxin capsule, and Shengmai capsule, etc. The produced injections include: Shenmai injection, Shengmai injection, Huangqi injection, Shenfu injection, and Danhong injection, etc (Jian, 2002). Among these patent medicine above, a randomized clinical trial indicates QSYQ could promote left ventricular function, increase exercise capacity and reduce re-admission rate (Hou et al., 2013; Shang et al., 2013). A clinical trial of Qili qiangxin capsule demonstrated superior performance in comparison to the placebo in terms of NYHA functional classification, 6-min walking distance, LVEF and quality of life (Li et al., 2013b). The underlying mechanisms includes regulating TGF-β1 in the progression of fibrosis (Zhang et al., 2016b), or modulates the expressions of collagen I (Col I), collagen III (Col III), matrix metalloproteinase-2 (MMP-2), and MMP-9, which are the main contributors to extracellular matrix remodeling (Zhang et al., 2015).
I/R injury in myocardial infarction is an important inducing or exacerbating factor for acute HF. The underlying mechanisms of TCM in the treatment of HF include anti-fibrosis, anti-inflammation, anti-oxidant, anti-apoptosis, pro-angiogenesis effects and regulation of metabolism, thus directly mitigate the I/R injury or indirectly reducing the adverse cardiac remodeling which could induce or exacerbate HF. For example, dioscin attenuates apoptosis and oxidative stress by regulating bcl-2/bax ratio and SOD (). Shensong Yangxin and Sini Tang (comprising Aconitum carmichaelii Debeaux, Cinnamomum cassia (L.) J. Presl, Zingiber officinale Roscoe and Glycyrrhiza uralensis Fisch. ex DC.) can enhance cardiac function by suppressing cardiac collagen hyperplasia in rabbits and TGF-β1 expression in MI-induced rat models (Liu et al., 2014; Dang et al., 2016).
TCM is usually used together with western medicine to treat HF. The multiple effects of TCM can counteract adverse effects of pharmacological treatment, making it a potential therapeutic option. However, application of TCM is limited because of lack of large-scale multi-center clinical trials and experiments. Therefore, further research on the mechanism of TCM in treating HF is necessary to enhance its applicability worldwide.
TCM Regulates Expression of HSPs in HF
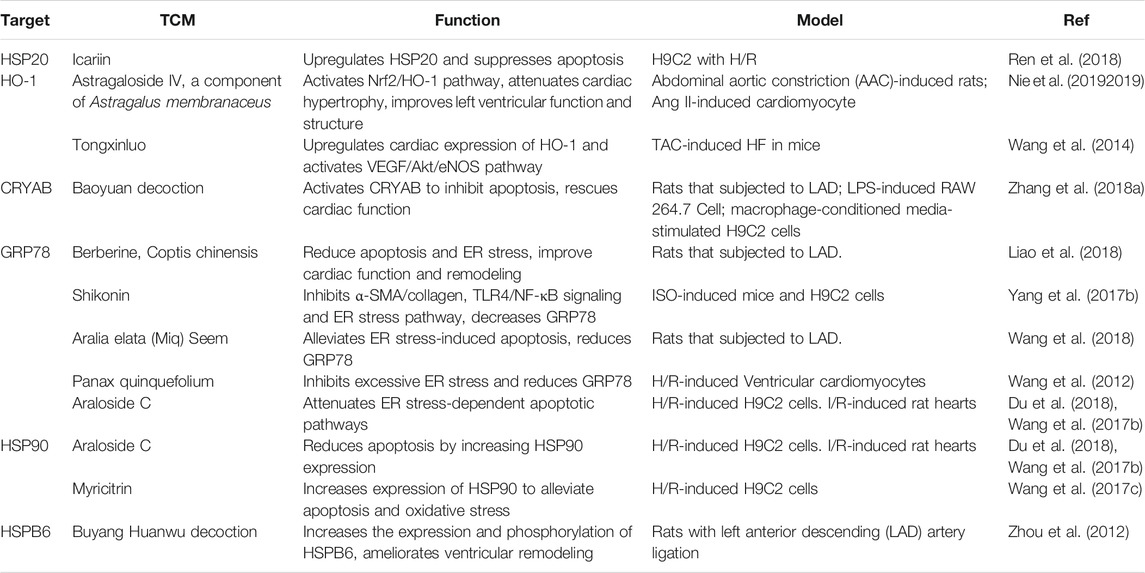
Based on the functions of HSPs in HF, regulation of HSPs and the protective effects of TCM in treating HF, it can be hypothesized that TCM regulate HSPs to enhance therapeutic effects on HF. A fraction of TCM has been proven to regulate HSPs in the myocardium and protect the heart from fibrosis, remodeling and hypertrophy. Functions of TCM which target HSPs in myocardial injuries are summarized in Figure 3 and Table 3.
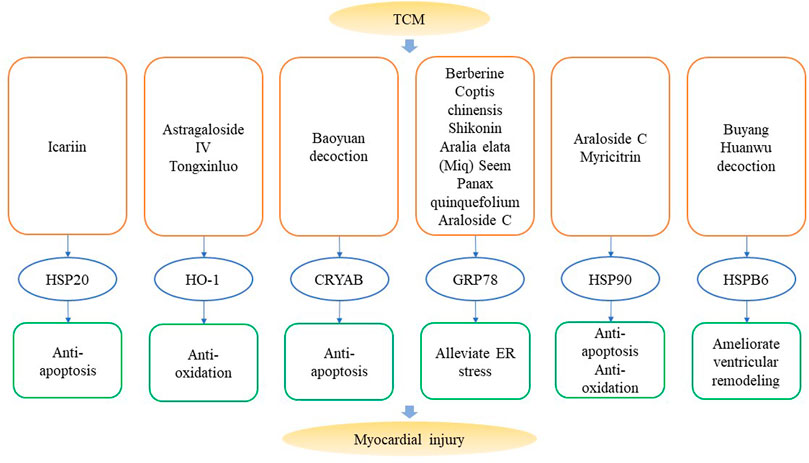
FIGURE 3. Traditional Chinese Medicine that target heat shock proteins in myocardial injuries. Components like icariin, astragaloside IV, berberine and decoctions like Baoyuan decoction and Buying Huanwu decoction can alleviate myocardial injury via anti-apoptosis, anti-oxidation, reducing ER stress and cardiac remodeling by regulating the expression of HSPs.
On the one side, TCM could directly relieve HF by regulating HSPs and HSPs-mediated ER stress. Astragaloside IV is an active component of Astragalus membranaceus, can activate Nrf2/HO-1 pathway to protect the heart from hypertrophy and fibrosis (Nie et al., 20192019). Shikonin is extracted from the red-root gromwell, and it ameliorates ISO-induced myocardial damage, and cardiac hypertrophy by inhibiting α-smooth muscle actin (α-SMA)/collagen, TLR4/NF-κB signaling and ER stress pathways. Suppression of ER stress is reflected as decreased expression of GRP78 (Yang et al., 2017b). Tongxinluo is a TCM compound, which can increase cardiac expression of HO-1 and activate vascular endothelial growth factor (VEGF)/Akt/eNOS pathway to prevent TAC-induced HF in mice (Wang et al., 2014).
On the other side, as I/R injury in myocardial infarction is an important inducing or exacerbating factor for acute HF, TCM could also indirectly prevent HF pathogenesis by decreasing I/R injury and impeding fibrosis and cardiac remodeling in myocardial infarction. Berberine, a key active ingredient of Coptis chinensis can improve cardiac function and remodeling, reduce apoptosis and ER stress (marked as decreased GRP78 and CHOP) in post-MI HF (Liao et al., 2018). Icariin suppresses apoptosis by reversing downregulation of HSP20 in H9c2 cells induced by hypoxia/reoxygenation (H/R) injury (Ren et al., 2018). Araloside C, a compound isolated from Aralia elata (Miq) Seem, icariin and Panax quinquefolius L. can ameliorate apoptosis and ER stress, reduce expression of GRP78 in myocytes induced by either I/R or tunicamycin (Wang et al., 2012; Zhang et al., 2013; Wang et al., 2017b; Du et al., 2018; Wang et al., 2018). In addition, Araloside C can increase expression of HSP90 and alleviate apoptosis in either H9c2 with H/R injury or rat with I/R injury (Wang et al., 2017b; Du et al., 2018). Myricitrin can also alleviate apoptosis and oxidative stress induced by H/R injury by increasing expression of HSP90, and the protective function of myricitrin partially depends on phosphatidylinositol 3-kinase (PI3K)/Akt pathway (Wang et al., 2017c). Buyang Huanwu decoction ameliorates I/R-induced ventricular remodeling by upregulating expression of HSPB6 and peroxiredoxin-6 (PRDX6), and downregulating atrial natriuretic factor (ANF) thereby decreasing activities of bax and caspase-3 (Zhou et al., 2012). Baoyuan decoction is a TCM formula composed of astragalus, ginseng, liquorice and cinnamon. It can activate CRYAB to inhibit apoptosis and enhance cardiac function in post-MI-induced HF (Zhang et al., 2018a). Scutellarin can alleviate apoptosis in H/R induced human cardiac microvascular endothelial cells (HCMECs) and increased expression of HSP60 might be a crucial factor for its protective effect (Shi et al., 20152015). Emodin restores activity of peroxisome proliferators-activated receptor-γ (PPAR-γ), eNOS phosphorylation, and interaction of HSP90/eNOS to alleviate H/R-induced injury in HAECs (Shou et al., 2018).
Other Cardio-Protective Effects of TCM by Regulating HSPs
Besides HF and myocardial infarction, studies indicates TCM could also prohibit pathological process of atherosclerosis by regulating HSPs. Decoctions like Xiaoyaosan can inhibit expression of HSP27, HSP60 and HSP90, and promote interaction of HSP90 with glucocorticoid receptor (GR) and CD36 to prevent development of atherosclerotic vulnerable plaque in mouse model of atherosclerosis induced by high-fat food coupled with chronic stress (Fu et al., 2019). Ligustrazine increases NO production in human umbilical vein endothelial cells (HUVECs), downregulates intercellular cell adhesion molecule-1 (ICAM-1) and HSP60 expression levels to induce immunomodulatory effect on TNF-α-stimulated HUVECs (Wu et al., 2012). Baicalin increases HSP72 expression at both mRNA and protein levels in a cow’s mammary epithelial cells (CMECs) and inactivates NF-κB pathway to alleviate LPS-induced apoptosis (Yang et al., 2016). Catalpol, an extract of Radix rehmannia, inhibits homocysteine-induced apoptosis in the human aorta endothelial cells (HAECs) by suppressing Nox4/ROS/NF-κB pathway and GRP78/dsRNA-activated protein kinase–like endoplasmic reticulum kinase (PERK) pathway to alleviate ER stress (Hu et al., 2019).
Conclusion and Perspectives
HF describes the terminal stage of multifarious heart diseases such as dilated cardiomyopathy, myocardial infarction and myocarditis. Pathogenesis of HF is characterized by cardiomyocyte apoptosis, oxidative stress, inflammation and mitochondrial dysfunction, all of which cause myocardial fibrosis and remodeling. HSPs have various functions, including regulation of apoptosis, anti-oxidant and anti-inflammation effects, and are capable of ameliorating cardiac dysfunction in HF. However, not all the HSPs are protective in HF, some HSPs exerts detrimental effects in HF progressive. Even some HSPs can modulate HF pathogenesis with dual effects. Thus, further studies are still required to explore accurate functions of HSPs in HF with different cell and molecular microenvironment. New treatment methods that focuses on the regulation of HSPs would have a promising application prospect in the prevention and treatment of HF.
TCM has been applied in the treatment of HF in China for thousands of years. Small sample clinical trials indicate the single compounds extracted from herbal medicine and formula, as well as patent medicine, are able to regulate HSPs in HF. Consequently, TCM is a potential therapeutic medium for modulating HSPs in HF and improving cardiac function. Studies on effects of various forms of TCM have confirmed the hypothesis that TCM alters expression of HSPs in HF but such studies are few. Thus, the application of TCM is limited in clinic because of lack of large-scale multi-center and randomized clinical trials. Therefore, further investigations on the effects of TCM in reliving HF by targeting HSPs are needed, and the underlying mechanisms involved in TCM regulating HSPs are also encouraged to be explored in future.
Author Contributions
QL designed the study. YW, JW and DW drafted the manuscript. QL finalized the manuscript. Critical comments and typesetting correction on the final version were made by RY and QL. All authors read, revised and approved the final manuscript.
Funding
This study is supported by Guangdong Provincial Bureau of Traditional Chinese Medicine Fund Project (No. 202106082257336500, to Q.L.), Guangdong Medical Science and Technology Research Fund Project (No. B2020155, to Q.L.), Zhuhai Medical Science and Technology Research Fund Project (No. ZH24013310210002PWC, to Q.L.). National Natural Science Foundation of China (82174156), Guangzhou Science and Technology Plan Project (202002030432).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allwood, M. A., Kinobe, R. T., Ballantyne, L., Romanova, N., Melo, L. G., Ward, C. A., et al. (2014). Heme Oxygenase-1 Overexpression Exacerbates Heart Failure with Aging and Pressure Overload but Is Protective against Isoproterenol-Induced Cardiomyopathy in Mice. Cardiovasc. Pathol. 23, 231–237. doi:10.1016/j.carpath.2014.03.007
Ambrosy, A. P., Fonarow, G. C., Butler, J., Chioncel, O., Greene, S. J., Vaduganathan, M., et al. (2014). The Global Health and Economic burden of Hospitalizations for Heart Failure: Lessons Learned from Hospitalized Heart Failure Registries. J. Am. Coll. Cardiol. 63, 1123–1133. doi:10.1016/j.jacc.2013.11.053
Bernardo, B. C., Sapra, G., Patterson, N. L., Cemerlang, N., Kiriazis, H., Ueyama, T., et al. (2015). Long-Term Overexpression of Hsp70 Does Not Protect against Cardiac Dysfunction and Adverse Remodeling in a MURC Transgenic Mouse Model with Chronic Heart Failure and Atrial Fibrillation. PloS one 10, e0145173. doi:10.1371/journal.pone.0145173
Bernardo, B. C., Weeks, K. L., Patterson, N. L., and McMullen, J. R. (2016). HSP70: Therapeutic Potential in Acute and Chronic Cardiac Disease Settings. Future Med. Chem. 8, 2177–2183. doi:10.4155/fmc-2016-0192
Bonanad, C., Núñez, J., Sanchis, J., Bodi, V., Chaustre, F., Chillet, M., et al. (2013). Serum Heat Shock Protein 60 in Acute Heart Failure: a New Biomarker? Congest. Heart Fail. 19, 6–10. doi:10.1111/j.1751-7133.2012.00299.x
Borgonetti, V., Governa, P., Biagi, M., Dalia, P., and Corsi, L. (2020). Rhodiola Rosea L. Modulates Inflammatory Processes in a CRH-Activated BV2 Cell Model. Phytomedicine 68, 153143. doi:10.1016/j.phymed.2019.153143
Cai, W. F., Zhang, X. W., Yan, H. M., Ma, Y. G., Wang, X. X., Yan, J., et al. (2010). Intracellular or Extracellular Heat Shock Protein 70 Differentially Regulates Cardiac Remodelling in Pressure Overload Mice. Cardiovasc. Res. 88, 140–149. doi:10.1093/cvr/cvq182
Chen, Y., Shi, S., Wang, H., Li, N., Su, J., Chou, G., et al. (2016). A Homogeneous Polysaccharide from Fructus Schisandra Chinensis (Turz.) Baill Induces Mitochondrial Apoptosis through the Hsp90/AKT Signalling Pathway in HepG2 Cells. Int. J. Mol. Sci. 17. doi:10.3390/ijms17071015
Dally, S., Monceau, V., Corvazier, E., Bredoux, R., Raies, A., Bobe, R., et al. (2009). Compartmentalized Expression of Three Novel Sarco/endoplasmic Reticulum Ca2+ATPase 3 Isoforms Including the Switch to ER Stress, SERCA3f, in Non-failing and Failing Human Heart. Cell Calcium 45, 144–154. doi:10.1016/j.ceca.2008.08.002
Dang, S., Huang, C. X., Wang, X., Wang, X., Hu, J., and Huang, H. (2016). Shensong Yangxin (SSYX) Ameliorates Disordered Excitation Transmission by Suppressing Cardiac Collagen Hyperplasia in Rabbits with Chronic Myocardial Infarction. J. Huazhong Univ. Sci. Technolog Med. Sci. 36, 162–167. doi:10.1007/s11596-016-1560-4
Datta, R., Bansal, T., Rana, S., Datta, K., Chattopadhyay, S., Chawla-Sarkar, M., et al. (2015). Hsp90/Cdc37 Assembly Modulates TGFβ Receptor-II to Act as a Profibrotic Regulator of TGFβ Signaling during Cardiac Hypertrophy. Cell Signal. 27, 2410–2424. doi:10.1016/j.cellsig.2015.09.005
Daugaard, M., Rohde, M., and Jäättelä, M. (2007). The Heat Shock Protein 70 Family: Highly Homologous Proteins with Overlapping and Distinct Functions. FEBS Lett. 581, 3702–3710. doi:10.1016/j.febslet.2007.05.039
Deng, J. S., Jiang, W. P., Chen, C. C., Lee, L. Y., Li, P. Y., Huang, W. C., et al. (2020). Cordyceps Cicadae Mycelia Ameliorate Cisplatin-Induced Acute Kidney Injury by Suppressing the TLR4/NF-Κb/MAPK and Activating the HO-1/Nrf2 and Sirt-1/AMPK Pathways in Mice. Oxid Med. Cel Longev 2020, 7912763. doi:10.1155/2020/7912763
Du, Y., Wang, M., Liu, X., Zhang, J., Xu, X., Xu, H., et al. (2018). Araloside C Prevents Hypoxia/Reoxygenation-Induced Endoplasmic Reticulum Stress via Increasing Heat Shock Protein 90 in H9c2 Cardiomyocytes. Front. Pharmacol. 9, 180. doi:10.3389/fphar.2018.00180
Fan, F., Duan, Y., Yang, F., Trexler, C., Wang, H., Huang, L., et al. (2020). Deletion of Heat Shock Protein 60 in Adult Mouse Cardiomyocytes Perturbs Mitochondrial Protein Homeostasis and Causes Heart Failure. Cell Death Differ 27, 587–600. doi:10.1038/s41418-019-0374-x
Fan, G. C., Yuan, Q., Song, G., Wang, Y., Chen, G., Qian, J., et al. (2006). Small Heat-Shock Protein Hsp20 Attenuates Beta-Agonist-Mediated Cardiac Remodeling through Apoptosis Signal-Regulating Kinase 1. Circ. Res. 99, 1233–1242. doi:10.1161/01.RES.0000251074.19348.af
Fan, G. C., Zhou, X., Wang, X., Song, G., Qian, J., Nicolaou, P., et al. (2008). Heat Shock Protein 20 Interacting with Phosphorylated Akt Reduces Doxorubicin-Triggered Oxidative Stress and Cardiotoxicity. Circ. Res. 103, 1270–1279. doi:10.1161/CIRCRESAHA.108.182832
Foo, R. S., Siow, R. C., Brown, M. J., and Bennett, M. R. (2006). Heme Oxygenase-1 Gene Transfer Inhibits Angiotensin II-Mediated Rat Cardiac Myocyte Apoptosis but Not Hypertrophy. J. Cel Physiol 209, 1–7. doi:10.1002/jcp.20723
Fu, H. Y., Minamino, T., Tsukamoto, O., Sawada, T., Asai, M., Kato, H., et al. (2008). Overexpression of Endoplasmic Reticulum-Resident Chaperone Attenuates Cardiomyocyte Death Induced by Proteasome Inhibition. Cardiovasc. Res. 79, 600–610. doi:10.1093/cvr/cvn128
Fu, W., Chen, M., Ou, L., Li, T., Chang, X., Huang, R., et al. (2019). Xiaoyaosan Prevents Atherosclerotic Vulnerable Plaque Formation through Heat Shock Protein/glucocorticoid Receptor axis-mediated Mechanism. Am. J. Transl Res. 11, 5531–5545.
Gao, Y., Chu, S. F., Li, J. P., Zhang, Z., Yan, J. Q., Wen, Z. L., et al. (2015). Protopanaxtriol Protects against 3-nitropropionic Acid-Induced Oxidative Stress in a Rat Model of Huntington's Disease. Acta Pharmacol. Sin 36, 311–322. doi:10.1038/aps.2014.107
Gao, Z., Zhang, J., Li, L., Shen, L., Li, Q., Zou, Y., et al. (2016). Heat Shock Proteins 27 and 70 Contribute to the protection of Schisandrin B against D-Galactosamine-Induced Liver Injury in Mice. Can. J. Physiol. Pharmacol. 94, 373–378. doi:10.1139/cjpp-2015-0419
Genth-Zotz, S., Bolger, A. P., Kalra, P. R., von Haehling, S., Doehner, W., Coats, A. J., et al. (2004). Heat Shock Protein 70 in Patients with Chronic Heart Failure: Relation to Disease Severity and Survival. Int. J. Cardiol. 96, 397–401. doi:10.1016/j.ijcard.2003.08.008
Gombos, T., Förhécz, Z., Pozsonyi, Z., Jánoskuti, L., and Prohászka, Z. (2008). Interaction of Serum 70-kDa Heat Shock Protein Levels and HspA1B (+1267) Gene Polymorphism with Disease Severity in Patients with Chronic Heart Failure. Cell Stress Chaperones 13, 199–206. doi:10.1007/s12192-007-0001-5
He, J., Han, S., Li, X. X., Wang, Q. Q., Cui, Y., Chen, Y., et al. (2019). Diethyl Blechnic Exhibits Anti-inflammatory and Antioxidative Activity via the TLR4/MyD88 Signaling Pathway in LPS-Stimulated RAW264.7 Cells. Molecules 24. doi:10.3390/molecules24244502
He, W. T., Zhu, Y. H., Zhang, T., Abulimiti, P., Zeng, F. Y., Zhang, L. P., et al. (2019). Curcumin Reverses 5-Fluorouracil Resistance by Promoting Human Colon Cancer HCT-8/5-FU Cell Apoptosis and Down-Regulating Heat Shock Protein 27 and P-Glycoprotein. Chin. J. Integr. Med. 25, 416–424. doi:10.1007/s11655-018-2997-z
He, Y., Li, M., Leng, W., Lv, B., Huan, Y., Liu, B., et al. (2018). Zhenbao Pill Reduces Treg Cell Proportion in Acute Spinal Cord Injury Rats by Regulating TUG1/miR-214/HSP27 axis. Biosci. Rep. 38. doi:10.1042/BSR20180895
Higashikuni, Y., Tanaka, K., Kato, M., Nureki, O., Hirata, Y., Nagai, R., et al. (2013). Toll-like Receptor-2 Mediates Adaptive Cardiac Hypertrophy in Response to Pressure Overload through Interleukin-1β Upregulation via Nuclear Factor κB Activation. J. Am. Heart Assoc. 2, e000267. doi:10.1161/Jaha.113.000267
Hou, Y. Z., Wang, S., Zhao, Z. Q., Wang, X. L., Li, B., Soh, S. B., et al. (2013). Clinical Assessment of Complementary Treatment with Qishen Yiqi Dripping Pills on Ischemic Heart Failure: Study Protocol for a Randomized, Double-Blind, Multicenter, Placebo-Controlled Trial (CACT-IHF). Trials 14, 138. doi:10.1186/1745-6215-14-138
Hu, H., Wang, C., Jin, Y., Meng, Q., Liu, Q., Liu, Z., et al. (2019). Catalpol Inhibits Homocysteine-Induced Oxidation and Inflammation via Inhibiting Nox4/NF-Κb and GRP78/PERK Pathways in Human Aorta Endothelial Cells. Inflammation 42, 64–80. doi:10.1007/s10753-018-0873-9
Huang, Z., Zhao, Q., Chen, M., Zhang, J., and Ji, L. (2019). Liquiritigenin and Liquiritin Alleviated Monocrotaline-Induced Hepatic Sinusoidal Obstruction Syndrome via Inhibiting HSP60-Induced Inflammatory Injury. Toxicology 428, 152307. doi:10.1016/j.tox.2019.152307
Ibrahim, I. M., Abdelmalek, D. H., and Elfiky, A. A. (2019). GRP78: A Cell's Response to Stress. Life Sci. 226, 156–163. doi:10.1016/j.lfs.2019.04.022
Jenei, Z. M., Gombos, T., Förhécz, Z., Pozsonyi, Z., Karádi, I., Jánoskuti, L., et al. (2013). Elevated Extracellular HSP70 (HSPA1A) Level as an Independent Prognostic Marker of Mortality in Patients with Heart Failure. Cell Stress Chaperones 18, 809–813. doi:10.1007/s12192-013-0425-z
Jia, R., Gu, Z., He, Q., Du, J., Cao, L., Jeney, G., et al. (2019). Anti-oxidative, Anti-inflammatory and Hepatoprotective Effects of Radix Bupleuri Extract against Oxidative Damage in tilapia (Oreochromis niloticus) via Nrf2 and TLRs Signaling Pathway. Fish. Shellfish Immunol. 93, 395–405. doi:10.1016/j.fsi.2019.07.080
Jian, M. (2002). Clinical Observation of Congestive Heart Failure Treated by Integrated Traditional Chinese and Western Medicine. Zhongguo Zhong Xi Yi Jie He Za Zhi 22, 542–544.
Jiang, Q. W., Cheng, K. J., Mei, X. L., Qiu, J. G., Zhang, W. J., Xue, Y. Q., et al. (2015). Synergistic Anticancer Effects of Triptolide and Celastrol, Two Main Compounds from Thunder God Vine. Oncotarget 6, 32790–32804. doi:10.18632/oncotarget.5411
Kapustian, L. L., Vigontina, O. A., Rozhko, O. T., Ryabenko, D. V., Michowski, W., Lesniak, W., et al. (2013). Hsp90 and its Co-chaperone, Sgt1, as Autoantigens in Dilated Cardiomyopathy. Heart Vessels 28, 114–119. doi:10.1007/s00380-011-0226-1
Kim, Y. K., Suarez, J., Hu, Y., McDonough, P. M., Boer, C., Dix, D. J., et al. (2006). Deletion of the Inducible 70-kDa Heat Shock Protein Genes in Mice Impairs Cardiac Contractile Function and Calcium Handling Associated with Hypertrophy. Circulation 113, 2589–2597. doi:10.1161/Circulationaha.105.598409
Kirchhoff, S. R., Gupta, S., and Knowlton, A. A. (2002). Cytosolic Heat Shock Protein 60, Apoptosis, and Myocardial Injury. Circulation 105, 2899–2904. doi:10.1161/01.cir.0000019403.35847.23
Kregel, K. C. (19852002). Heat Shock Proteins: Modifying Factors in Physiological Stress Responses and Acquired Thermotolerance. J. Appl. Physiol. (1985) 92, 2177–2186. doi:10.1152/japplphysiol.01267.2001
Kunde, D. A., Chong, W. C., Nerurkar, P. V., Ahuja, K. D., Just, J., Smith, J. A., et al. (2017). Bitter Melon Protects against ER Stress in LS174T Colonic Epithelial Cells. BMC Complement. Altern. Med. 17, 2. doi:10.1186/s12906-016-1522-1
Lan, Y. L., Zhou, J. J., Liu, J., Huo, X. K., Wang, Y. L., Liang, J. H., et al. (2018). Uncaria Rhynchophylla Ameliorates Parkinson's Disease by Inhibiting HSP90 Expression: Insights from Quantitative Proteomics. Cell Physiol Biochem 47, 1453–1464. doi:10.1159/000490837
Lee, K. H., Jang, Y., and Chung, J. H. (2010). Heat Shock Protein 90 Regulates IκB Kinase Complex and NF-Κb Activation in Angiotensin II-Induced Cardiac Cell Hypertrophy. Exp. Mol. Med. 42, 703–711. doi:10.3858/emm.2010.42.10.069
Li, H., Liu, Y., Wen, M., Zhao, F., Zhao, Z., Liu, Y., et al. (2019). Hydroxysafflor Yellow A (HSYA) Alleviates Apoptosis and Autophagy of Neural Stem Cells Induced by Heat Stress via P38 MAPK/MK2/Hsp27-78 Signaling Pathway. Biomed. Pharmacother. 114, 108815. doi:10.1016/j.biopha.2019.108815
Li, M., Yu, X., Guo, H., Sun, L., Wang, A., Liu, Q., et al. (2014). Bufalin Exerts Antitumor Effects by Inducing Cell Cycle Arrest and Triggering Apoptosis in Pancreatic Cancer Cells. Tumour Biol. 35, 2461–2471. doi:10.1007/s13277-013-1326-6
Li, T., Chen, X., Dai, X. Y., Wei, B., Weng, Q. J., Chen, X., et al. (2017). Novel Hsp90 Inhibitor Platycodin D Disrupts Hsp90/Cdc37 Complex and Enhances the Anticancer Effect of mTOR Inhibitor. Toxicol. Appl. Pharmacol. 330, 65–73. doi:10.1016/j.taap.2017.07.006
Li, X., Zhang, J., Huang, J., Ma, A., Yang, J., Li, W., et al. (2013). A Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study of the Effects of Qili Qiangxin Capsules in Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 62, 1065–1072. doi:10.1016/j.jacc.2013.05.035
Li, Y., Zou, L., Li, T., Lai, D., Wu, Y., and Qin, S. (2019). Mogroside V Inhibits LPS-Induced COX-2 Expression/ROS Production and Overexpression of HO-1 by Blocking Phosphorylation of AKT1 in RAW264.7 Cells. Acta Biochim. Biophys. Sin (Shanghai) 51, 365–374. doi:10.1093/abbs/gmz014
Li, Z., Song, Y., Xing, R., Yu, H., Zhang, Y., Li, Z., et al. (2013). Heat Shock Protein 70 Acts as a Potential Biomarker for Early Diagnosis of Heart Failure. PLoS One 8, e67964. doi:10.1371/journal.pone.0067964
Liao, Y., Chen, K., Dong, X., Li, W., Li, G., Huang, G., et al. (2018). Berberine Inhibits Cardiac Remodeling of Heart Failure after Myocardial Infarction by Reducing Myocardial Cell Apoptosis in Rats. Exp. Ther. Med. 16, 2499–2505. doi:10.3892/etm.2018.6438
Lin, L., Kim, S. C., Wang, Y., Gupta, S., Davis, B., Simon, S. I., et al. (2007). HSP60 in Heart Failure: Abnormal Distribution and Role in Cardiac Myocyte Apoptosis. Am. J. Physiol. Heart Circ. Physiol. 293, H2238–H2247. doi:10.1152/ajpheart.00740.2007
Lin, P., Weng, X., Liu, F., Ma, Y., Chen, H., Shao, X., et al. (2015). Bushen Zhuangjin Decoction Inhibits TM-Induced Chondrocyte Apoptosis Mediated by Endoplasmic Reticulum Stress. Int. J. Mol. Med. 36, 1519–1528. doi:10.3892/ijmm.2015.2387
Liu, J., Peter, K., Shi, D., Zhang, L., Dong, G., Zhang, D., et al. (2014). Traditional Formula, Modern Application: Chinese Medicine Formula Sini Tang Improves Early Ventricular Remodeling and Cardiac Function after Myocardial Infarction in Rats. Evid. Based Complement. Alternat Med. 2014, 141938. doi:10.1155/2014/141938
Liu, L., Wang, Y., Cao, Z. Y., Wang, M. M., Liu, X. M., Gao, T., et al. (2015). Up-regulated TLR4 in Cardiomyocytes Exacerbates Heart Failure after Long-Term Myocardial Infarction. J. Cel Mol Med 19, 2728–2740. doi:10.1111/jcmm.12659
Liu, L., Zhang, C., Kalionis, B., Wan, W., Murthi, P., Chen, C., et al. (2016). EGb761 Protects against Aβ1-42 Oligomer-Induced Cell Damage via Endoplasmic Reticulum Stress Activation and Hsp70 Protein Expression Increase in SH-Sy5y Cells. Exp. Gerontol. 75, 56–63. doi:10.1016/j.exger.2016.01.003
Liu, L., Zhang, X., Qian, B., Min, X., Gao, X., Li, C., et al. (2007). Over-expression of Heat Shock Protein 27 Attenuates Doxorubicin-Induced Cardiac Dysfunction in Mice. Eur. J. Heart Fail. 9, 762–769. doi:10.1016/j.ejheart.2007.03.007
Liu, P., Bao, H. Y., Jin, C. C., Zhou, J. C., Hua, F., Li, K., et al. (2019). Targeting Extracellular Heat Shock Protein 70 Ameliorates Doxorubicin-Induced Heart Failure through Resolution of Toll-like Receptor 2-Mediated Myocardial Inflammation. J. Am. Heart Assoc. 8, e012338. doi:10.1161/Jaha.119.012338
Liu, S., Iskandar, R., Chen, W., Zhang, J., Wang, Y., Chen, X., et al. (2016). Soluble Glycoprotein 130 and Heat Shock Protein 27 as Novel Candidate Biomarkers of Chronic Heart Failure with Preserved Ejection Fraction. Heart Lung Circ. 25, 1000–1006. doi:10.1016/j.hlc.2016.02.011
Liu, X., Simpson, J. A., Brunt, K. R., Ward, C. A., Hall, S. R., Kinobe, R. T., et al. (2007). Preemptive Heme Oxygenase-1 Gene Delivery Reveals Reduced Mortality and Preservation of Left Ventricular Function 1 Yr after Acute Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 293, H48–H59. doi:10.1152/ajpheart.00741.2006
Ma, Z. J., Wang, X. X., Su, G., Yang, J. J., Zhu, Y. J., Wu, Y. W., et al. (2016). Proteomic Analysis of Apoptosis Induction by Lariciresinol in Human HepG2 Cells. Chem. Biol. Interact 256, 209–219. doi:10.1016/j.cbi.2016.07.011
Marunouchi, T., Abe, Y., Murata, M., Inomata, S., Sanbe, A., Takagi, N., et al. (2013). Changes in Small Heat Shock Proteins HSPB1, HSPB5 and HSPB8 in Mitochondria of the Failing Heart Following Myocardial Infarction in Rats. Biol. Pharm. Bull. 36, 529–539. doi:10.1248/bpb.b12-00796
Marunouchi, T., Araki, M., Murata, M., Takagi, N., and Tanonaka, K. (2013). Possible Involvement of HSP90-HSF1 Multichaperone Complex in Impairment of HSP72 Induction in the Failing Heart Following Myocardial Infarction in Rats. J. Pharmacol. Sci. 123, 336–346. doi:10.1254/jphs.13109fp
Marunouchi, T., Inomata, S., Sanbe, A., Takagi, N., and Tanonaka, K. (2014). Protective Effect of Geranylgeranylacetone via Enhanced Induction of HSPB1 and HSPB8 in Mitochondria of the Failing Heart Following Myocardial Infarction in Rats. Eur. J. Pharmacol. 730, 140–147. doi:10.1016/j.ejphar.2014.02.037
Marunouchi, T., Murata, M., Takagi, N., and Tanonaka, K. (2013). Possible Involvement of Phosphorylated Heat-Shock Factor-1 in Changes in Heat Shock Protein 72 Induction in the Failing Rat Heart Following Myocardial Infarction. Biol. Pharm. Bull. 36, 1332–1340. doi:10.1248/bpb.b13-00196
Minamino, T., Komuro, I., and Kitakaze, M. (2010). Endoplasmic Reticulum Stress as a Therapeutic Target in Cardiovascular Disease. Circ. Res. 107, 1071–1082. doi:10.1161/CIRCRESAHA.110.227819
Mohamed, B. A., Barakat, A. Z., Zimmermann, W. H., Bittner, R. E., Mühlfeld, C., Hünlich, M., et al. (2012). Targeted Disruption of Hspa4 Gene Leads to Cardiac Hypertrophy and Fibrosis. J. Mol. Cel Cardiol 53, 459–468. doi:10.1016/j.yjmcc.2012.07.014
Mymrikov, E. V., Seit-Nebi, A. S., and Gusev, N. B. (2011). Large Potentials of Small Heat Shock Proteins. Physiol. Rev. 91, 1123–1159. doi:10.1152/physrev.00023.2010
Naka K, K., Vezyraki, P., Kalaitzakis, A., Zerikiotis, S., Michalis, L., and Angelidis, C. (2014). Hsp70 Regulates the Doxorubicin-Mediated Heart Failure in Hsp70-Transgenic Mice. Cell Stress Chaperones 19, 853–864. doi:10.1007/s12192-014-0509-4
Nan, L., Xie, Q., Chen, Z., Zhang, Y., Chen, Y., Li, H., et al. (2020). Involvement of PARP-1/AIF Signaling Pathway in Protective Effects of Gualou Guizhi Decoction against Ischemia-Reperfusion Injury-Induced Apoptosis. Neurochem. Res. 45, 278–294. doi:10.1007/s11064-019-02912-3
Nie, P., Meng, F., Zhang, J., Wei, X., and Shen, C. (20192019). Astragaloside IV Exerts a Myocardial Protective Effect against Cardiac Hypertrophy in Rats, Partially via Activating the Nrf2/HO-1 Signaling Pathway. Oxid Med. Cel Longev 2019, 4625912. doi:10.1155/2019/4625912
Niizeki, T., Takeishi, Y., Watanabe, T., Nitobe, J., Miyashita, T., Miyamoto, T., et al. (2008). Relation of Serum Heat Shock Protein 60 Level to Severity and Prognosis in Chronic Heart Failure Secondary to Ischemic or Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 102, 606–610. doi:10.1016/j.amjcard.2008.04.030
Okada, K., Minamino, T., Tsukamoto, Y., Liao, Y., Tsukamoto, O., Takashima, S., et al. (2004). Prolonged Endoplasmic Reticulum Stress in Hypertrophic and Failing Heart after Aortic Constriction: Possible Contribution of Endoplasmic Reticulum Stress to Cardiac Myocyte Apoptosis. Circulation 110, 705–712. doi:10.1161/01.Cir.0000137836.95625.D4
Owen, S., Zhao, H., Dart, A., Wang, Y., Ruge, F., Gao, Y., et al. (2016). Heat Shock Protein 27 Is a Potential Indicator for Response to YangZheng XiaoJi and Chemotherapy Agents in Cancer Cells. Int. J. Oncol. 49, 1839–1847. doi:10.3892/ijo.2016.3685
Panneerselvam, L., Raghunath, A., and Perumal, E. (2017). Differential Expression of Myocardial Heat Shock Proteins in Rats Acutely Exposed to Fluoride. Cell Stress Chaperones 22, 743–750. doi:10.1007/s12192-017-0801-1
Ponikowski, P., Anker, S. D., AlHabib, K. F., Cowie, M. R., Force, T. L., Hu, S., et al. (2014). Heart Failure: Preventing Disease and Death Worldwide. ESC Heart Fail. 1, 4–25. doi:10.1002/ehf2.12005
Qian, W., Su, Y., Zhang, Y., Yao, N., Gu, N., Zhang, X., et al. (2018). Secretome Analysis of Rat Osteoblasts during Icariin Treatment Induced Osteogenesis. Mol. Med. Rep. 17, 6515–6525. doi:10.3892/mmr.2018.8715
Raju, V. S., Imai, N., and Liang, C. S. (1999). Chamber-specific Regulation of Heme Oxygenase-1 (Heat Shock Protein 32) in Right-Sided Congestive Heart Failure. J. Mol. Cel Cardiol 31, 1581–1589. doi:10.1006/jmcc.1999.0995
Ranek, M. J., Stachowski, M. J., Kirk, J. A., and Willis, M. S. (2018). The Role of Heat Shock Proteins and Co-chaperones in Heart Failure. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373. doi:10.1098/rstb.2016.0530
Ren, Z. H., Ke, Z. P., Luo, M., and Shi, Y. (2018). Icariin Protects against Ischemia-reperfusion I-njury in H9C2 C-ells by U-pregulating H-eat S-hock P-rotein 20. Mol. Med. Rep. 17, 3336–3343. doi:10.3892/mmr.2017.8251
Rizzo, M., Macario, A. J., de Macario, E. C., Gouni-Berthold, I., Berthold, H. K., Rini, G. B., et al. (2011). Heat Shock Protein-60 and Risk for Cardiovascular Disease. Curr. Pharm. Des. 17, 3662–3668. doi:10.2174/138161211798220981
Sawada, T., Minamino, T., Fu, H. Y., Asai, M., Okuda, K., Isomura, T., et al. (2010). X-box Binding Protein 1 Regulates Brain Natriuretic Peptide through a Novel AP1/CRE-like Element in Cardiomyocytes. J. Mol. Cel Cardiol 48, 1280–1289. doi:10.1016/j.yjmcc.2010.02.004
Schwarz, D. S., and Blower, M. D. (2016). The Endoplasmic Reticulum: Structure, Function and Response to Cellular Signaling. Cell Mol Life Sci 73, 79–94. doi:10.1007/s00018-015-2052-6
Shan, Y. X., Liu, T. J., Su, H. F., Samsamshariat, A., Mestril, R., and Wang, P. H. (2003). Hsp10 and Hsp60 Modulate Bcl-2 Family and Mitochondria Apoptosis Signaling Induced by Doxorubicin in Cardiac Muscle Cells. J. Mol. Cel Cardiol 35, 1135–1143. doi:10.1016/s0022-2828(03)00229-3
Shang, H., Zhang, J., Yao, C., Liu, B., Gao, X., Ren, M., et al. (2013). Qi-shen-yi-qi Dripping Pills for the Secondary Prevention of Myocardial Infarction: a Randomised Clinical Trial. Evid. Based Complement. Alternat Med. 2013, 738391. doi:10.1155/2013/738391
Shi, M., Liu, Y., Feng, L., Cui, Y., Chen, Y., Wang, P., et al. (20152015). Protective Effects of Scutellarin on Human Cardiac Microvascular Endothelial Cells against Hypoxia-Reoxygenation Injury and its Possible Target-Related Proteins. Evid. Based Complement. Alternat Med. 2015, 278014. doi:10.1155/2015/278014
Shou, X., Zhou, R., Zhu, L., Ren, A., Wang, L., Wang, Y., et al. (2018). Emodin, A Chinese Herbal Medicine, Inhibits Reoxygenation-Induced Injury in Cultured Human Aortic Endothelial Cells by Regulating the Peroxisome Proliferator-Activated Receptor-γ (PPAR-γ) and Endothelial Nitric Oxide Synthase (eNOS) Signaling Pathway. Med. Sci. Monit. 24, 643–651. doi:10.12659/msm.908237
Shrestha, L., and Young, J. C. (2016). Function and Chemotypes of Human Hsp70 Chaperones. Curr. Top. Med. Chem. 16, 2812–2828. doi:10.2174/1568026616666160413142028
Sidorik, L., Kyyamova, R., Bobyk, V., Kapustian, L., Rozhko, O., Vigontina, O., et al. (2005). Molecular Chaperone, HSP60, and Cytochrome P450 2E1 Co-expression in Dilated Cardiomyopathy. Cell Biol Int 29, 51–55. doi:10.1016/j.cellbi.2004.11.011
Stetler, R. A., Gan, Y., Zhang, W., Liou, A. K., Gao, Y., Cao, G., et al. (2010). Heat Shock Proteins: Cellular and Molecular Mechanisms in the central Nervous System. Prog. Neurobiol. 92, 184–211. doi:10.1016/j.pneurobio.2010.05.002
Su, J., Xu, T., Jiang, G., Hou, M., Liang, M., Cheng, H., et al. (2019). Gambogenic Acid Triggers Apoptosis in Human Nasopharyngeal Carcinoma CNE-2Z Cells by Activating Volume-Sensitive Outwardly Rectifying Chloride Channel. Fitoterapia 133, 150–158. doi:10.1016/j.fitote.2019.01.002
Sun, A., Zou, Y., Wang, P., Xu, D., Gong, H., Wang, S., et al. (2014). Mitochondrial Aldehyde Dehydrogenase 2 Plays Protective Roles in Heart Failure after Myocardial Infarction via Suppression of the Cytosolic JNK/p53 Pathway in Mice. J. Am. Heart Assoc. 3, e000779. doi:10.1161/JAHA.113.000779
Tamura, S., Marunouchi, T., and Tanonaka, K. (2019). Heat-shock Protein 90 Modulates Cardiac Ventricular Hypertrophy via Activation of MAPK Pathway. J. Mol. Cel Cardiol 127, 134–142. doi:10.1016/j.yjmcc.2018.12.010
Tang, Q., Shang, F., Wang, X., Yang, Y., Chen, G., Chen, Y., et al. (2014). Combination Use of Ferulic Acid, Ligustrazine and Tetrahydropalmatine Inhibits the Growth of Ectopic Endometrial Tissue: a Multi-Target Therapy for Endometriosis Rats. J. Ethnopharmacol 151, 1218–1225. doi:10.1016/j.jep.2013.12.047
Tanonaka, K., Furuhama, K. I., Yoshida, H., Kakuta, K., Miyamoto, Y., Toga, W., et al. (2001). Protective Effect of Heat Shock Protein 72 on Contractile Function of Perfused Failing Heart. Am. J. Physiol. Heart Circ. Physiol. 281, H215–H222. doi:10.1152/ajpheart.2001.281.1.H215
Tanonaka, K., Toga, W., Yoshida, H., Furuhama, K., and Takeo, S. (2001). Effect of Long-Term Treatment with Trandolapril on Hsp72 and Hsp73 Induction of the Failing Heart Following Myocardial Infarction. Br. J. Pharmacol. 134, 969–976. doi:10.1038/sj.bjp.0704323
Tanonaka, K., Yoshida, H., Toga, W., Furuhama, K., and Takeo, S. (2001). Myocardial Heat Shock Proteins during the Development of Heart Failure. Biochem. Biophys. Res. Commun. 283, 520–525. doi:10.1006/bbrc.2001.4801
Toga, W., Tanonaka, K., and Takeo, S. (2007). Changes in Hsp60 Level of the Failing Heart Following Acute Myocardial Infarction and the Effect of Long-Term Treatment with Trandolapril. Biol. Pharm. Bull. 30, 105–110. doi:10.1248/bpb.30.105
Traxler, D., Lainscak, M., Simader, E., Ankersmit, H. J., and Jug, B. (2017). Heat Shock Protein 27 Acts as a Predictor of Prognosis in Chronic Heart Failure Patients. Clin. Chim. Acta 473, 127–132. doi:10.1016/j.cca.2017.08.028
Turakhia, S., Venkatakrishnan, C. D., Dunsmore, K., Wong, H., Kuppusamy, P., Zweier, J. L., et al. (2007). Doxorubicin-induced Cardiotoxicity: Direct Correlation of Cardiac Fibroblast and H9c2 Cell Survival and Aconitase Activity with Heat Shock Protein 27. Am. J. Physiol. Heart Circ. Physiol. 293, H3111–H3121. doi:10.1152/ajpheart.00328.2007
Vedam, K., Nishijima, Y., Druhan, L. J., Khan, M., Moldovan, N. I., Zweier, J. L., et al. (2010). Role of Heat Shock Factor-1 Activation in the Doxorubicin-Induced Heart Failure in Mice. Am. J. Physiol. Heart Circ. Physiol. 298, H1832–H1841. doi:10.1152/ajpheart.01047.2009
Wang, B., Yang, Q., Bai, W. W., Xing, Y. F., Lu, X. T., Sun, Y. Y., et al. (2014). Tongxinluo Protects against Pressure Overload-Induced Heart Failure in Mice Involving VEGF/Akt/eNOS Pathway Activation. PLoS One 9, e98047. doi:10.1371/journal.pone.0098047
Wang, C., Li, Y. Z., Wang, X. R., Lu, Z. R., Shi, D. Z., and Liu, X. H. (2012). Panax Quinquefolium Saponins Reduce Myocardial Hypoxia-Reoxygenation Injury by Inhibiting Excessive Endoplasmic Reticulum Stress. Shock 37, 228–233. doi:10.1097/SHK.0b013e31823f15c4
Wang, G., Hamid, T., Keith, R. J., Zhou, G., Partridge, C. R., Xiang, X., et al. (2010). Cardioprotective and Antiapoptotic Effects of Heme Oxygenase-1 in the Failing Heart. Circulation 121, 1912–1925. doi:10.1161/CIRCULATIONAHA.109.905471
Wang, J., Liu, S., Yin, Y., Li, M., Wang, B., Yang, L., et al. (2015). FOXO3-mediated Up-Regulation of Bim Contributes to Rhein-Induced Cancer Cell Apoptosis. Apoptosis 20, 399–409. doi:10.1007/s10495-014-1071-3
Wang, M., Sun, G. B., Du, Y. Y., Tian, Y., Liao, P., Liu, X. S., et al. (2017). Myricitrin Protects Cardiomyocytes from Hypoxia/Reoxygenation Injury: Involvement of Heat Shock Protein 90. Front. Pharmacol. 8, 353. doi:10.3389/fphar.2017.00353
Wang, M., Tian, Y., Du, Y. Y., Sun, G. B., Xu, X. D., Jiang, H., et al. (2017). Protective Effects of Araloside C against Myocardial Ischaemia/reperfusion Injury: Potential Involvement of Heat Shock Protein 90. J. Cel Mol Med 21, 1870–1880. doi:10.1111/jcmm.13107
Wang, P., He, Q., and Zhu, J. (2016). Emodin-8-O-glucuronic A-cid, from the T-raditional Chinese M-edicine Q-inghuobaiduyin, A-ffects the S-ecretion of I-nflammatory C-ytokines in LPS-stimulated R-aw 264.7 C-ells via HSP70. Mol. Med. Rep. 14, 2368–2372. doi:10.3892/mmr.2016.5512
Wang, R., Yang, M., Wang, M., Liu, X., Xu, H., Xu, X., et al. (2018). Total Saponins of Aralia Elata (Miq) Seem Alleviate Calcium Homeostasis Imbalance and Endoplasmic Reticulum Stress-Related Apoptosis Induced by Myocardial Ischemia/Reperfusion Injury. Cel Physiol Biochem 50, 28–40. doi:10.1159/000493954
Wang, X., Zingarelli, B., O'Connor, M., Zhang, P., Adeyemo, A., Kranias, E. G., et al. (2009). Overexpression of Hsp20 Prevents Endotoxin-Induced Myocardial Dysfunction and Apoptosis via Inhibition of NF-kappaB Activation. J. Mol. Cel Cardiol 47, 382–390. doi:10.1016/j.yjmcc.2009.05.016
Wang, Y., Chen, L., Hagiwara, N., and Knowlton, A. A. (2010). Regulation of Heat Shock Protein 60 and 72 Expression in the Failing Heart. J. Mol. Cel Cardiol 48, 360–366. doi:10.1016/j.yjmcc.2009.11.009
Wang, Y., Wang, Q., Li, C., Lu, L., Zhang, Q., Zhu, R., et al. (2017). A Review of Chinese Herbal Medicine for the Treatment of Chronic Heart Failure. Curr. Pharm. Des. 23, 5115–5124. doi:10.2174/1381612823666170925163427
Wang, Y. L., Lam, K. K., Cheng, P. Y., and Lee, Y. M. (2015). Celastrol Prevents Circulatory Failure via Induction of Heme Oxygenase-1 and Heat Shock Protein 70 in Endotoxemic Rats. J. Ethnopharmacol 162, 168–175. doi:10.1016/j.jep.2014.12.062
Wei, D. F., Wei, Y. X., Cheng, W. D., Yan, M. F., Su, G., Hu, Y., et al. (2012). Proteomic Analysis of the Effect of Triterpenes from Patrinia Heterophylla on Leukemia K562 Cells. J. Ethnopharmacol 144, 576–583. doi:10.1016/j.jep.2012.09.043
Wei, Y. J., Huang, Y. X., Shen, Y., Cui, C. J., Zhang, X. L., Zhang, H., et al. (2009). Proteomic Analysis Reveals Significant Elevation of Heat Shock Protein 70 in Patients with Chronic Heart Failure Due to Arrhythmogenic Right Ventricular Cardiomyopathy. Mol. Cel Biochem 332, 103–111. doi:10.1007/s11010-009-0179-1
Wu, H. J., Hao, J., Wang, S. Q., Jin, B. L., and Chen, X. B. (2012). Protective Effects of Ligustrazine on TNF-α-Induced Endothelial Dysfunction. Eur. J. Pharmacol. 674, 365–369. doi:10.1016/j.ejphar.2011.10.046
Wu, J., Liu, T., Rios, Z., Mei, Q., Lin, X., and Cao, S. (2017). Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 38, 226–256. doi:10.1016/j.tips.2016.11.009
Yan, B., Shi, J., Xiu, L. J., Liu, X., Zhou, Y. Q., Feng, S. H., et al. (2013). Xiaotan Tongfu Granules Contribute to the Prevention of Stress Ulcers. World J. Gastroenterol. 19, 5473–5484. doi:10.3748/wjg.v19.i33.5473
Yancy, C. W., Jessup, M., Bozkurt, B., Butler, J., Casey, D. E., Colvin, M. M., et al. (20172017). 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 136, e137–e161. doi:10.1161/CIR.0000000000000509
Yancy, C. W., Jessup, M., Bozkurt, B., Butler, J., Casey, D. E., Drazner, M. H., et al. (20132013). 2013 ACCF/AHA Guideline for the Management of Heart Failure: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 62, e147–239. doi:10.1016/j.jacc.2013.05.019
Yang, J., Wang, Z., and Chen, D. L. (2017). Shikonin Ameliorates Isoproterenol (ISO)-induced Myocardial Damage through Suppressing Fibrosis, Inflammation, Apoptosis and ER Stress. Biomed. Pharmacother. 93, 1343–1357. doi:10.1016/j.biopha.2017.06.086
Yang, L., Chai, C. Z., Yan, Y., Duan, Y. D., Henz, A., Zhang, B. L., et al. (2017). Spasmolytic Mechanism of Aqueous Licorice Extract on Oxytocin-Induced Uterine Contraction through Inhibiting the Phosphorylation of Heat Shock Protein 27. Molecules 22. doi:10.3390/molecules22091392
Yang, W., Li, H., Cong, X., Wang, X., Jiang, Z., Zhang, Q., et al. (2016). Baicalin Attenuates Lipopolysaccharide Induced Inflammation and Apoptosis of Cow Mammary Epithelial Cells by Regulating NF-Κb and HSP72. Int. Immunopharmacol 40, 139–145. doi:10.1016/j.intimp.2016.08.032
Yin, C. F., Kao, S. C., Hsu, C. L., Chang, Y. W., Cheung, C. H. Y., Huang, H. C., et al. (2020). Phosphoproteome Analysis Reveals Dynamic Heat Shock Protein 27 Phosphorylation in Tanshinone IIA-Induced Cell Death. J. Proteome Res. 19, 1620–1634. doi:10.1021/acs.jproteome.9b00836
You, W., Min, X., Zhang, X., Qian, B., Pang, S., Ding, Z., et al. (2009). Cardiac-specific Expression of Heat Shock Protein 27 Attenuated Endotoxin-Induced Cardiac Dysfunction and Mortality in Mice through a PI3K/Akt-dependent Mechanism. Shock 32, 108–117. doi:10.1097/SHK.0b013e318199165d
Yu, P., Zhang, Y., Li, C., Li, Y., Jiang, S., Zhang, X., et al. (2015). Class III PI3K-Mediated Prolonged Activation of Autophagy Plays a Critical Role in the Transition of Cardiac Hypertrophy to Heart Failure. J. Cel Mol Med 19, 1710–1719. doi:10.1111/jcmm.12547
Zeng, X. S., Zhou, X. S., Luo, F. C., Jia, J. J., Qi, L., Yang, Z. X., et al. (2014). Comparative Analysis of the Neuroprotective Effects of Ginsenosides Rg1 and Rb1 Extracted from Panax Notoginseng against Cerebral Ischemia. Can. J. Physiol. Pharmacol. 92, 102–108. doi:10.1139/cjpp-2013-0274
Zhang, C., Qu, S., Wei, X., Feng, Y., Zhu, H., Deng, J., et al. (2016). HSP25 Down-Regulation Enhanced P53 Acetylation by Dissociation of SIRT1 from P53 in Doxorubicin-Induced H9c2 Cell Apoptosis. Cell Stress Chaperones 21, 251–260. doi:10.1007/s12192-015-0655-3
Zhang, G., Yang, G., Deng, Y., Zhao, X., Yang, Y., Rao, J., et al. (2016). Ameliorative Effects of Xue-Fu-Zhu-Yu Decoction, Tian-Ma-Gou-Teng-Yin and Wen-Dan Decoction on Myocardial Fibrosis in a Hypertensive Rat Mode. BMC Complement. Altern. Med. 16, 56. doi:10.1186/s12906-016-1030-3
Zhang, L., Gan, W., and An, G. (2012). Influence of Tanshinone IIa on Heat Shock Protein 70, Bcl-2 and Bax Expression in Rats with Spinal Ischemia/reperfusion Injury. Neural Regen. Res. 7, 2882–2888. doi:10.3969/j.issn.1673-5374.2012.36.005
Zhang, Q., Li, H., Wang, S., Liu, M., Feng, Y., and Wang, X. (2013). Icariin Protects Rat Cardiac H9c2 Cells from Apoptosis by Inhibiting Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 14, 17845–17860. doi:10.3390/ijms140917845
Zhang, S., Yuan, M., Cheng, C., Xia, D. H., and Wu, S. W. (2018). Chinese Herbal Medicine Effects on Muscle Atrophy Induced by Simulated Microgravity. Aerosp Med. Hum. Perform. 89, 883–888. doi:10.3357/AMHP.5079.2018
Zhang, X., He, M., Cheng, L., Chen, Y., Zhou, L., Zeng, H., et al. (2008). Elevated Heat Shock Protein 60 Levels Are Associated with Higher Risk of Coronary Heart Disease in Chinese. Circulation 118, 2687–2693. doi:10.1161/CIRCULATIONAHA.108.781856
Zhang, X., Min, X., Li, C., Benjamin, I. J., Qian, B., Zhang, X., et al. (2010). Involvement of Reductive Stress in the Cardiomyopathy in Transgenic Mice with Cardiac-specific Overexpression of Heat Shock Protein 27. Hypertension 55, 1412–1417. doi:10.1161/HYPERTENSIONAHA.109.147066
Zhang, Y., Jiang, D. S., Yan, L., Cheng, K. J., Bian, Z. Y., and Lin, G. S. (2011). HSP75 Protects against Cardiac Hypertrophy and Fibrosis. J. Cel Biochem 112, 1787–1794. doi:10.1002/jcb.23091
Zhang, Y., Li, C., Meng, H., Guo, D., Zhang, Q., Lu, W., et al. (2018). BYD Ameliorates Oxidative Stress-Induced Myocardial Apoptosis in Heart Failure Post-Acute Myocardial Infarction via the P38 MAPK-CRYAB Signaling Pathway. Front. Physiol. 9, 505. doi:10.3389/fphys.2018.00505
Zhang, Y., Wang, H., Cui, L., Zhang, Y., Liu, Y., Chu, X., et al. (2015). Continuing Treatment with Salvia Miltiorrhiza Injection Attenuates Myocardial Fibrosis in Chronic Iron-Overloaded Mice. PloS one 10, e0124061. doi:10.1371/journal.pone.0124061
Zhang, Z., Rui, W., Wang, Z. C., Liu, D. X., and Du, L. (2017). Anti-proliferation and Anti-metastasis Effect of Barbaloin in Non-small Cell Lung Cancer via Inactivating p38MAPK/Cdc25B/Hsp27 Pathway. Oncol. Rep. 38, 1172–1180. doi:10.3892/or.2017.5760
Zhao, M., Tang, X., Gong, D., Xia, P., Wang, F., and Xu, S. (2020). Bungeanum Improves Cognitive Dysfunction and Neurological Deficits in D-Galactose-Induced Aging Mice via Activating PI3K/Akt/Nrf2 Signaling Pathway. Front. Pharmacol. 11, 71. doi:10.3389/fphar.2020.00071
Zhou, F., Huang, X., Pan, Y., Cao, D., Liu, C., Liu, Y., et al. (2018). Resveratrol Protects HaCaT Cells from Ultraviolet B-Induced Photoaging via Upregulation of HSP27 and Modulation of Mitochondrial Caspase-dependent Apoptotic Pathway. Biochem. Biophys. Res. Commun. 499, 662–668. doi:10.1016/j.bbrc.2018.03.207
Zhou, Q., Qin, W. Z., Liu, S. B., Kwong, J. S., Zhou, J., and Chen, J. (2014). Shengmai (A Traditional Chinese Herbal Medicine) for Heart Failure. Oxford, UK: The Cochrane database of systematic reviews, Cd005052.
Zhou, Y. C., Liu, B., Li, Y. J., Jing, L. L., Wen, G., Tang, J., et al. (2012). Effects of Buyang Huanwu Decoction on Ventricular Remodeling and Differential Protein Profile in a Rat Model of Myocardial Infarction. Evid. Based Complement. Alternat Med. 2012, 385247. doi:10.1155/2012/385247
Zhu, J., Chen, M., Chen, N., Ma, A., Zhu, C., Zhao, R., et al. (2015). Glycyrrhetinic Acid Induces G1-phase C-ell C-ycle A-rrest in H-uman N-on-small C-ell L-ung C-ancer C-ells through E-ndoplasmic R-eticulum S-tress P-athway. Int. J. Oncol. 46, 981–988. doi:10.3892/ijo.2015.2819
Zhu, J., Wang, P., He, Q., Zhou, J., and Luo, C. (2013). Evidence of an Anti-apoptotic Effect of Qinghuobaiduyin on Intestinal Mucosa Following Burn Injury. Exp. Ther. Med. 6, 1390–1396. doi:10.3892/etm.2013.1314
Zuo, D., Subjeck, J., and Wang, X. Y. (2016). Unfolding the Role of Large Heat Shock Proteins: New Insights and Therapeutic Implications. Front. Immunol. 7, 75. doi:10.3389/fimmu.2016.00075
Glossary
4-HNE 4-hydroxy-2-nonenol
AAC Abdominal aortic constriction;
ACEI Angiotensin-converting enzyme inhibitors
AF Atrial fibrillation
AIF Apoptosis inducing factor
Akt Serine/threonine kinase
ALDH2 Aldehyde dehydrogenase 2
ANF Atrial natriuretic factor
Ang II Angiotensin II
ARB Angiotensin receptor blockers
ARNI Angiotensin receptor neprilysin inhibitor
ARVC Arrhythmogenic right ventricular cardiomyopathy
ASK1 Apoptosis signal regulating kinase1
bax Bcl-2-associated x
bcl-2 B-cell lymphoma-2
CAL Coronary artery ligation
CHOP C/EBP-homologous protein
CMECs Cow mammary epithelial cells
CRH Corticotropin releasing hormone
CRYAB αB-crystallin
DCM dilated cardiomyopathy
DOX Doxorubicin
ER Endoplasmic reticulum
ERE Estrogen response element
ERKs Extracellular regulated protein kinases
GR Glucocorticoid receptor
GRP78 Glucose regulated protein 78
GSK-3β Glycogen synthase kinase-3β
H/R Hypoxia/reoxygenation
HAECs Human aorta endothelial cells
HCMECs Human cardiac microvascular endothelial cells
HF Heart failure
HO-1 Heme oxygenase-1
HPOA Hypothalamus-pituitary–ovarian axis
HSF-1 Heat shock factor-1
HSPs Heat shock proteins Small heat shock proteins
HUVECs Human umbilical vein endothelial cells
I/R Ischemia/reperfusion
ICAM-1 Intercellular cell adhesion molecule-1
ICM Ischemic cardiomyopathy
ISO Isoproterenol
JNK Jun N-terminal kinase
LAD Left anterior descending
LPS Lipopolysaccharide
MAPK Mitogen-activated protein kinase
MCAO Middle cerebral artery occlusion
MI Myocardial infarction
mPTP Mitochondrial MPT pore
NF-κB Nuclear factor kappa-B
Nrf2 Nuclear factor E2-related factor2
NSCLC Non-small cell lung carcinoma
NSCs Neural stem cells
PARP Poly (ADP-ribose) polymerase
PEDF-1 Serpin family F-1
PERK dsRNA-activated protein kinase–like endoplasmic reticulum kinase
PI3K Phosphatidylinositol 3-kinase
PPAR-γ Peroxisome proliferators-activated receptor-γ
PRDX6 Peroxiredoxin 6
ROS Reactive oxygen species
SCP-0-1 Homogeneous Schisandra chinensis polysaccharide-0-1
HSPs Heat shock proteins Small heat shock proteins
SIRT1 Silent information regulator1
TAC Transverse aortic constriction
TCM Traditional Chinese Medicine
TGF-β Transforming growth factor-β
TLR4 Toll-like receptor 4
TNF-α Tumor necrosis factor-α
UPR Unfolded protein response
VEGF Vascular endothelial growth factor
α-SMA α-smooth muscle actin.
Keywords: heat shock proteins, heart failure, traditional Chinese medicine, myocardial injury, therapeutic targets
Citation: Wang Y, Wu J, Wang D, Yang R and Liu Q (2022) Traditional Chinese Medicine Targeting Heat Shock Proteins as Therapeutic Strategy for Heart Failure. Front. Pharmacol. 12:814243. doi: 10.3389/fphar.2021.814243
Received: 13 November 2021; Accepted: 21 December 2021;
Published: 18 January 2022.
Edited by:
Xianwei Wang, Xinxiang Medical University, ChinaReviewed by:
Yusuf Tutar, University of Health Sciences, TurkeyMin Wu, China Academy of Chinese Medical Sciences, China
Hongcai Shang, Beijing University of Chinese Medicine, China
Copyright © 2022 Wang, Wu, Wang, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Liu, ODUxNzU3NjI2QHFxLmNvbQ==; Dawei Wang, ZGF2aWR3YW5nMzNAMTM5LmNvbQ==; Rongyuan Yang, eWFuZ3Jvbmd5dWFuQDE2My5jb20=
 Yanchun Wang1
Yanchun Wang1 Qing Liu
Qing Liu