- Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Background: Poststroke cognitive impairment (PSCI) is a common complication observed after stroke. Current pharmacologic therapies have no definitive evidence for cognitive recovery or disease progression. Recent studies have verified the positive effect of DL-3-n-butylphthalide (NBP). However, the clinical efficacy and safety are still unclear. The aim of this study was to assess the efficacy of NBP and its harmful effect in the treatment of PSCI.
Method: Eligible randomized controlled trials (RCTs) were retrieved from inception to June 2021 from seven medical databases and two clinical registries. The revised Cochrane risk of bias tool (RoB 2.0) was used for methodological quality. RevMan v5.4.1 from Cochrane Collaboration was used for statistical analysis, and Hartung-Knapp-Sidik-Jonkman (HKSJ) method was used for post hoc testing depend on the number of studies. This study has been submitted to PROSPERO with registration number is CRD42021274123.
Result: We identified 26 studies with a total sample size of 2,571 patients. The results of this study showed that NBP as monotherapy or combination therapy had better performance in increasing the MoCA (monotherapy: SMDN = 1.05, 95% CI [0.69, 1.42], p < 0.00001; SMDP = 1.06, 95% CI [0.59, 1.52], p < 0.00001. combination: SMDO = 0.81, 95% CI [0.62, 1.01], p < 0.00001; SMDN = 0.90, 95% CI [0.46, 1.33], p < 0.0001; SMDD = 1.04, 95% CI [0.71, 1.38], p < 0.00001), MMSE (monotherapy: MDN = 4.89, 95% CI [4.14, 5.63]), p < 0.00001). combination: SMDO = 1.26, 95% CI [0.97, 1.56], p < 0.00001; SMDC = 1.63, 95% CI [1.28, 1.98], p < 0.00001; SMDN = 2.13, 95% CI [1.52, 2.75], p < 0.00001) and BI (monotherapy: MDN = 13.53, HKSJ 95% CI [9.84, 17.22], p = 0.014. combination: SMDO = 2.24, HKSJ 95%CI [0.37, 4.11], p = 0.032; SMDC = 3.36, 95%CI [2.80, 3.93], p < 0.00001; SMDD = 1.48, 95%CI [1.13, 1.83], p < 0.00001); and decreasing the NIHSS (monotherapy: MDN = −3.86, 95% CI [−5.22, −2.50], p < 0.00001. combination: SMDO = −1.15, 95% CI [−1.31, −0.98], p < 0.00001; SMDC = −1.82, 95% CI [−2.25, −1.40], p < 0.00001) and CSS (combination: MDO = −7.11, 95% CI [−8.42, −5.80], p < 0.00001), with no serious adverse reactions observed. The funnel plot verified the possibility of publication bias.
Conclusion: NBP maintains a stable pattern in promoting the recovery of cognitive function and abilities of daily living, as well as reducing the symptoms of neurological deficits. However, there is still a need for more high-quality RCTs to verify its efficacy and safety.
1 Introduction
Poststroke cognitive impairment (PSCI) is a common complication after stroke (Zhang and Bi, 2020). Approximately 9–30% of patients develop dementia within 1 year (Kim et al., 2020), and 35.6% of patients develop cognitive impairment within 5 years after an event (Rohde et al., 2019). The impairment of cognitive function after stroke involves multiple cognitive fields, with attention and executive dysfunction as the core symptoms (Carlson et al., 2009; Aam et al., 2020), which not only affects the quality of daily life and the ability to live independently (Shi et al., 2018; Ding et al., 2019) but also brings a heavy burden to medical institutions and nursing staff (Black et al., 2018). Brain plasticity refers to the ability of the brain to spontaneously repair nerves based on structural and functional changes after stroke (Mcewen, 2016; Ossenkoppele et al., 2020; Regenhardt et al., 2020). Drug therapy can promote the recovery of neurological function and reduce cognitive impairment. Although the consensus of experts recommends the use of cholinesterase inhibitors, memantine, nimodipine and oxiracetam for the treatment of PSCI (Sun et al., 2014; Dong et al., 2017; Wang et al., 2021), there is insufficient evidence to support drug therapy in this process (Quinn et al., 2021). Moreover, current evidence is based on clinical studies of Alzheimer’s disease (AD) and vascular cognitive impairment (VCI), and there is no convincing clinical evidence that current pharmacologic therapies can promote cognitive recovery or prevent disease progression in the treatment of PSCI (Brainin et al., 2015).
DL-3-n-butylphthalide (NBP) is a new drug developed independently in China that acts on multiple pathological links of acute ischemic stroke (Wang et al., 2018). In addition to promoting the recovery of cognitive function after stroke, recent clinical studies have demonstrated that NBP can improve the oxidative stress response of the nervous system (Lv et al., 2018), inhibit neuronal apoptosis and autophagy (Xu J et al., 2017), regulate central cholinergic function (Zhao et al., 2014; Sun et al., 2020) and promote neuroplasticity (Yang et al., 2015). A randomized controlled study involving 281 patients with subcortical nondementia VCI also showed that 6 months of NBP treatment effectively improved the cognitive and overall function of patients (Jia et al., 2016). The expert Consensus on the Management of Cognitive Impairment after Stroke (2021) recommends the use of NBP as a drug therapy for PSCI (Class Ⅱ, Level B evidence) (Wang et al., 2021), but current clinical studies are mostly small sample randomized controlled trials, and the clinical efficacy and safety of NBP are still uncertain. In 2018, Feng Zhiguo et al. (Feng et al., 2018) systematically evaluated the clinical effectiveness of NBP, and the results showed that NBP can significantly improve cognitive function and activities of daily living of patients after stroke. However, because of the simplicity of the search strategy, the possibility of incomplete search and publication bias, and the publication of new clinical studies, it is necessary to update the systematic review of the clinical efficacy of NBP in the treatment of PSCI to provide new evidence references for the clinical treatment of this disease.
2 Materials and Methods
2.1 Protocol Registration
This systematic review has been registered on the PROSPERO International prospective register of systematic reviews (ID = CRD42021274123).
2.2 Search Methods
Seven major medical databases were comprehensively retrieved, including the China National Knowledge Infrastructure (CNKI), Wanfang Database, China Science and Technology Journal Database (VIP) and Chinese Biomedical literature Service System (SinoMed) in Chinese and PubMed, EMBASE, and Cochrane Library in English. Ongoing or unpublished studies registered on clinical registration websites, such as the Chinese Clinical Trial Registry and Clinical Trials.gov, were also searched to collect overall information. The retrieval time was limited from database establishment to June 2021, with the combination of medical subject heading and free-text terms.
2.3 Inclusion Criteria
2.3.1 Types of Studies
Eligible randomized controlled trials (RCTs) evaluating the clinical efficacy and safety of NBP in the treatment of PSCI were included. All studies were full-text literature in English and Chinese, regardless of study site, publication date or research status.
2.3.2 Types of Participants
We included studies in patients definitively diagnosed with PSCI (Wang et al., 2021). In addition, RCTs that met the first diagnosis of stroke and the second diagnosis of cognitive impairment, emphasizing that cognitive impairment occurred after stroke, were also included.
2.3.3 Types of Interventions
The intervention group was treated with oral NBP alone or in combination with other positive control drugs (drug dosage, dosage form and course of treatment were consistent with the control group). The control group contained the same positive control drugs recommended by the expert consensus (Wang et al., 2021), such as cholinesterase inhibitors, memantine, nimodipine and oxiracetam. Both groups were treated continuously with no limitation of treatment course.
2.3.4 Types of Outcomes
The primary outcome was cognitive function evaluation, and the eligible RCTs contained at least one of the following cognitive scales: Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), Vascular Dementia Assessment Scale-Cognitive Subscale (VADAS-Cog), Wechsler Memory Scale (WMS), and Hastgawa Dementia Scale (HDS).
The secondary outcome included abilities of daily living, evaluated by Barthel index (BI) and Activities of Daily Living scale (ADL); neurological impairment, assessed by National Institutes of Health Stroke Scale (NIHSS) and China Stroke Scale (CSS).
Safety indicators, such as the occurrence of adverse events or adverse reactions, were used to analyze the clinical safety of NBP.
2.4 Exclusion Criteria
We excluded studies with incorrect or incomplete data. For duplicated studies, we included only the most recent and comprehensive information.
2.5 Study Selection and Data Extraction
Endnote X9 was used for literature management. One reviewer (Xueming Fan) downloaded the literature information identified from the search strategy and eliminated duplicates according to the titles and authors. Then, two researchers (Xueming Fan and Liuding Wang) independently screened and extracted data information according to the eligibility criteria, and a third researcher (Wei Shen) adjudicated any disagreement. Through reading the titles, abstracts and full text of the literature, the final number of studies that might be included was determined, and the reasons for exclusion were recorded. According to the predesigned standardized information extraction table, the relevant data of the qualified literature were extracted, including the first author, study title, publication date, sample size, gender, age, intervention measures, drug dose, comorbid conditions, course of treatment, follow-ups, outcome measures, adverse reactions.
2.6 Risk of Bias Assessment of Included Studies
The methodological quality of the included studies was evaluated by two researchers (Xueming Fan and Liuding Wang) using the revised Cochrane risk of bias tool (RoB 2.0) (Sterne et al., 2019). Specific evaluation contents included randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Through reading the full texts of the studies, the risk of bias for each domain was judged as high risk, low risk and some concerns. If all domains showed low risk, the overall risk of bias was considered low; If any of the above domains showed some concerns with no “high risk” field, the overall risk of bias was determined as some concerns; If high risk was reached for at least one domain, or judgement in multiple domains included some concerns, the overall risk of bias was considered high. A third researcher (Wei Shen) convened discussions or meetings to set rules when a disagreement arose between two reviewers.
2.7 Data Synthesis and Statistical Analysis
RevMan v5.4.1 provided by the Cochrane Collaboration was used for statistical analysis of the extracted clinical information. Relative risk (RR) analysis statistics for dichotomous outcomes and mean difference (MD) or standard mean difference (SMD) analysis statistics for continuous outcomes were applied according to different data types, both of which were calculated by 95% confidence intervals (CIs). Cochrane’s X2 and I2 tests were used to determine heterogeneity. Considering that the intervention measures of the control group had a certain influence on the results of the study, we conducted subgroup analysis according to different measures, and carried out SMD analysis statistics in these studies with continuous outcomes. Results were pooled when there were two or more trials with a common outcome. In view of the model selection based on heterogeneity results should be avoided, for studies that could not obtain complete information, we selected random effects model for analysis. Hartung-Knapp-Sidik-Jonkman (HKSJ) method was used for post hoc testing with a predesigned program (Inthout et al., 2014), for HKSJ has more sufficient error rate compared with DerSimonian and Laird’s (DL) applied in RevMan software, especially when the number of studies is small (Inthout et al., 2014). In addition, if the number of studies was ≥ 10, funnel plot analysis was used to detect publication bias.
2.8 Certainty Assessment of Evidence
Two reviewers (Xueming Fan and Liuding Wang) evaluated the certainty of evidence using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system (Guyatt et al., 2008), which identified the quality of outcome evidence as high, moderate, low, or very low. The certainty can be downgraded for five reasons (risk of bias, imprecision, inconsistency, indirectness, and publication bias) and upgraded for three reasons (large magnitude of an effect, dose-response gradient, and effect of plausible residual confounding). Besides, any disagreements were resolved through a third reviewer (Wei Shen).
3 Result
3.1 Study Selection
A total of 877 studies were retrieved in our search strategy (Supplementary Table S1), and 491 duplicate studies were excluded. After screening the retrieved titles and abstracts, 341 unrelated studies, 10 reviewed studies and 2 clinical research protocols were removed. Thirty-three full-text studies remained and were evaluated for eligibility. Seven studies were excluded because they did not meet the intervention or outcome criteria, including duplicate or unclear data (Supplementary Table S2). Finally, 26 relevant studies (Yang, 2011; Xia, 2013; Fu, 2014; You, 2014; Su et al., 2015; Xing and Abudushalamu, 2015; Zhao, 2015; Cheng et al., 2016; Ji et al., 2016; Qi et al., 2016; Zhu et al., 2016; Li, 2017; Wang, 2017; Xu H et al., 2017; Li et al., 2018; Liu et al., 2018; Sun, 2018; Wang, 2018; Yang, 2018; Bai, 2019; Li X et al., 2019; Liu, 2019; Liu et al., 2019; Luo, 2019; Chen et al., 2020; Fang, 2021) were included in our study. The specific literature screening process is shown in Figure 1.
3.2 Study Inclusion Characteristics
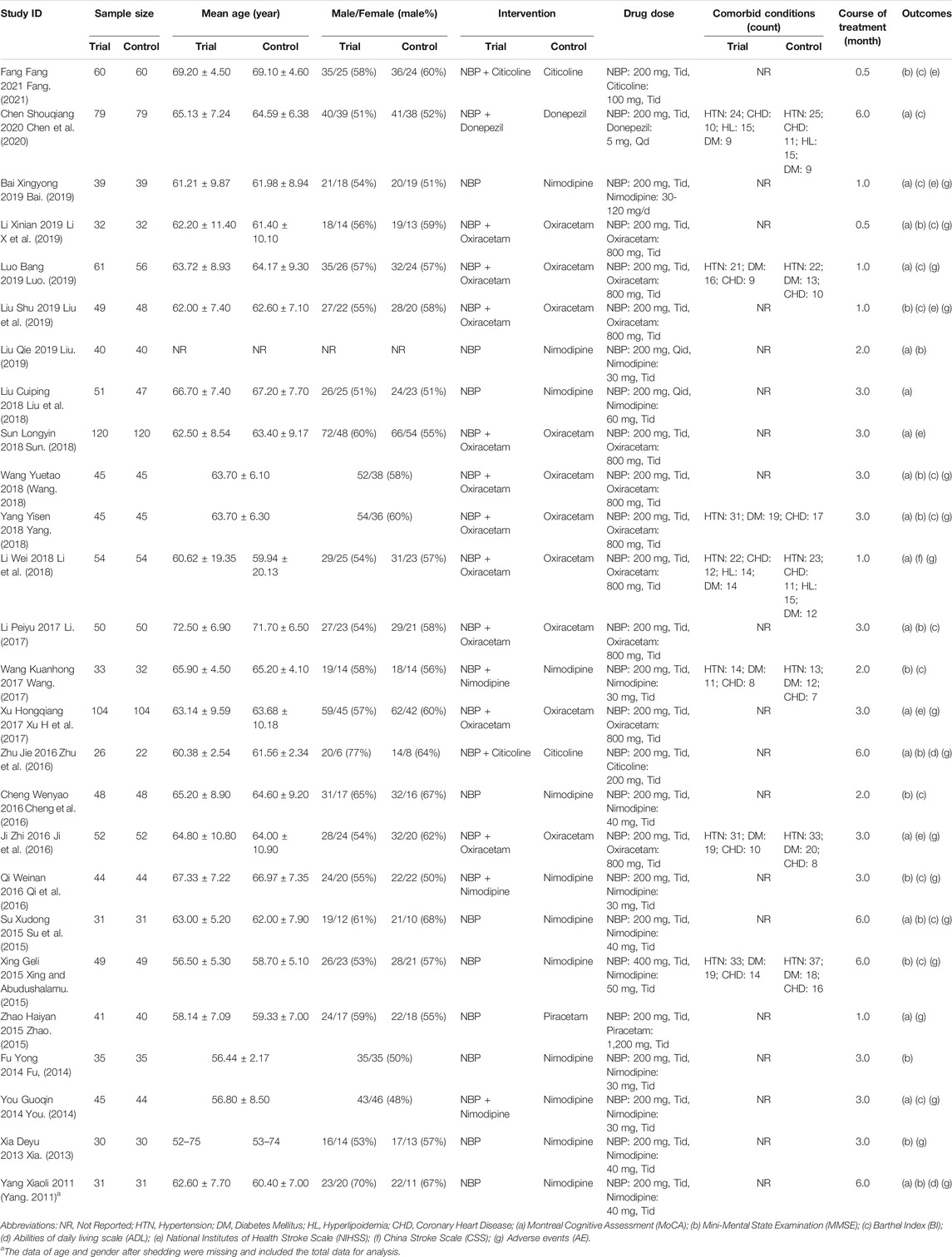
We included 26 randomized controlled trials, with a total sample size of 2,571 patients. The mean age of participants in each study ranged from 50 to 80 years. All the studies were conducted in Chinese and published between 2011 and 2021. The comorbid conditions of participants were mostly hypertension, diabetes mellitus, hyperlipoidemia and coronary heart disease. The course of treatment ranged from 0.5 to 6 months. Ten studies reported comparisons of NBP with other positive control drugs, and 16 studies reported combination therapy with NBP. Cognitive function was assessed by Montreal Cognitive Assessment (MoCA) in 18 studies and Mini-Mental State Examination (MMSE) in 16 studies. The basic characteristics of the 26 included studies are summarized in detail in Table 1.
3.3 Risk of Bias Assessment
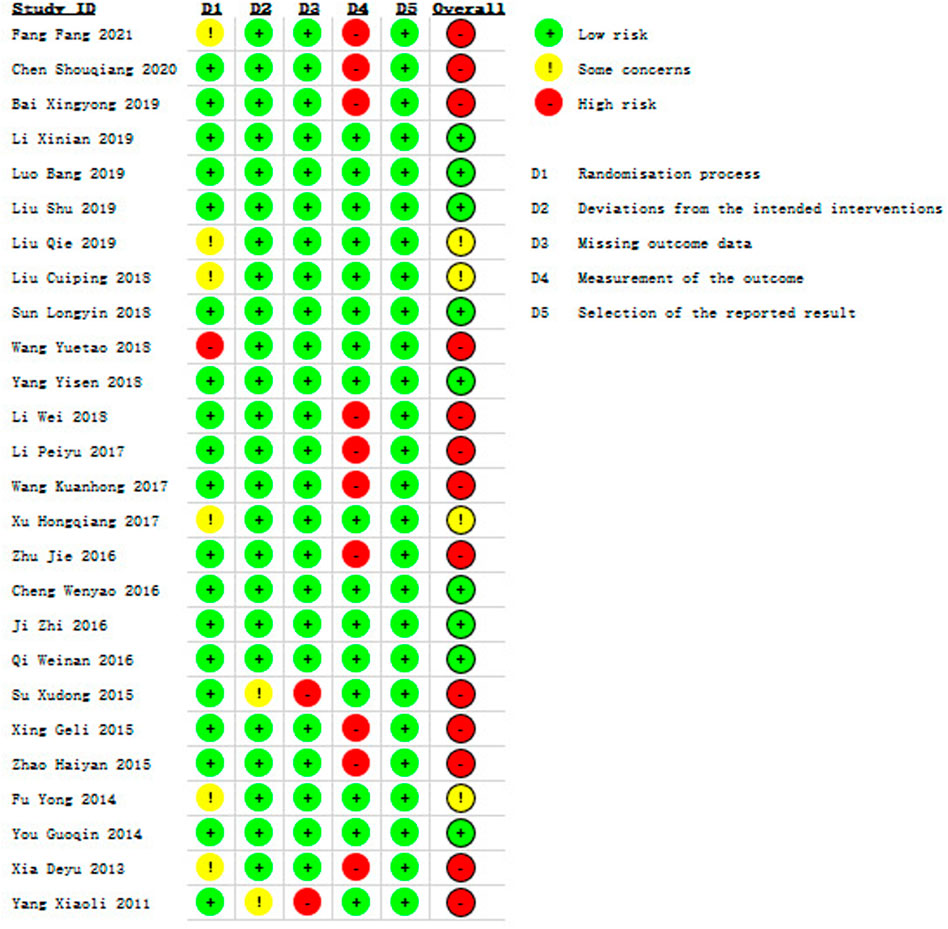
In terms of bias, the overall risk of bias was identified high in thirteen studies (Fang, 2021; Chen et al., 2020; Bai, 2019; Wang, 2018; Li et al., 2018; Li, 2017; Wang, 2017; Zhu et al., 2016; Su et al., 2015; Xing and Abudushalamu, 2015; Zhao, 2015; Xia, 2013; Yang, 2011), moderate in four studies (Liu, 2019; Liu et al., 2018; Xu H et al., 2017; Fu, 2014) and low in nine studies (Li X et al., 2019; Luo, 2019; Liu et al., 2019; Sun, 2018; Yang, 2018; Cheng et al., 2016; Ji et al., 2016; Qi et al., 2016; You, 2014), which is shown in Figure 2 and Supplementary Figure S1. We presented some concerns in the randomization process, as 6 studies (Xia, 2013; Fu, 2014; Xu H et al., 2017; Liu et al., 2018; Liu, 2019; Fang, 2021) provided insufficient information in the method of random allocation, and 1 study (Wang, 2018) showed high risk due to the use of a semirandomization method. The risk of bias in the deviations from intended interventions of two studies (Yang, 2011; Su et al., 2015) were considered as some concerns due to the use of per-protocol analysis for their descriptions of shedding cases were not included in statistics, and these two studies were rated as high risk for missing outcome data because the proportions of losing participants were directly linked to the subsequent symptoms in stroke patients. The risk of bias from measurement of the outcome in ten studies (Xia, 2013; Xing and Abudushalamu, 2015; Zhao, 2015; Zhu et al., 2016; Li, 2017; Wang, 2017; Li et al., 2018; Bai, 2019; Chen et al., 2020; Fang, 2021) were evaluated as high risk owing to the use of self-design methods to measure clinical efficacy rate.
3.4 Cognitive Function Assessment
3.4.1 Montreal Cognitive Assessment
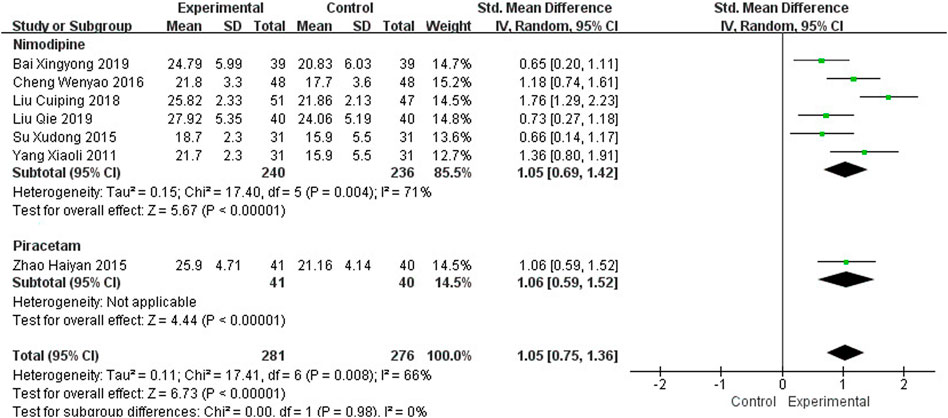
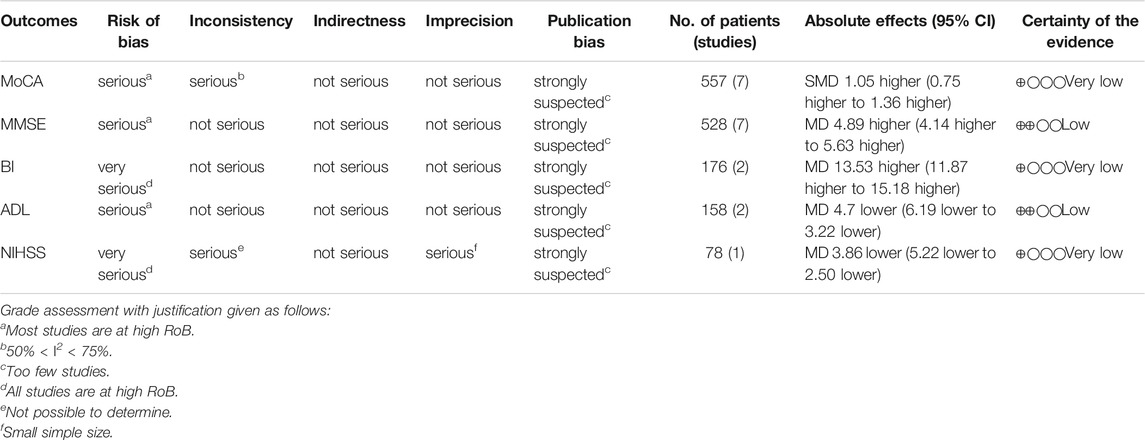
Seven studies, including 557 participants, reported the clinical efficacy of NBP as monotherapy, which is shown in Figure 3. Subgroup analysis was performed according to different intervention measures in the control group. Six studies (Yang, 2011; Su et al., 2015; Cheng et al., 2016; Liu et al., 2018; Bai, 2019; Liu, 2019) were treated with Nimodipine (I2 = 71%, p = 0.004), and the control group in 1 study (Zhao, 2015) was treated with Piracetam. A random effects model was used for the meta-analysis, showing a significant benefit in favor of NBP as monotherapy in MoCA scores (SMDN = 1.05, 95% CI [0.69, 1.42], p < 0.00001; SMDP = 1.06, 95% CI [0.59, 1.52], p < 0.00001).
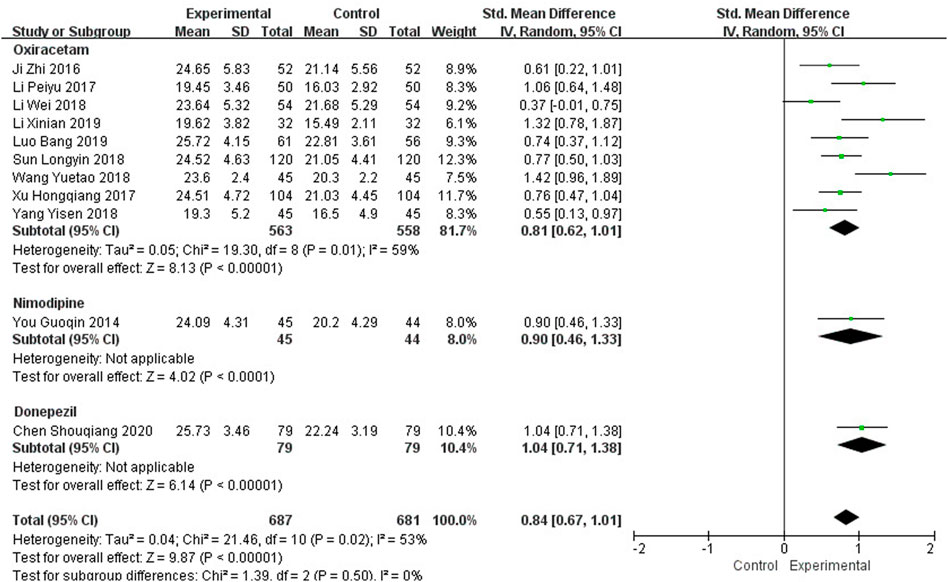
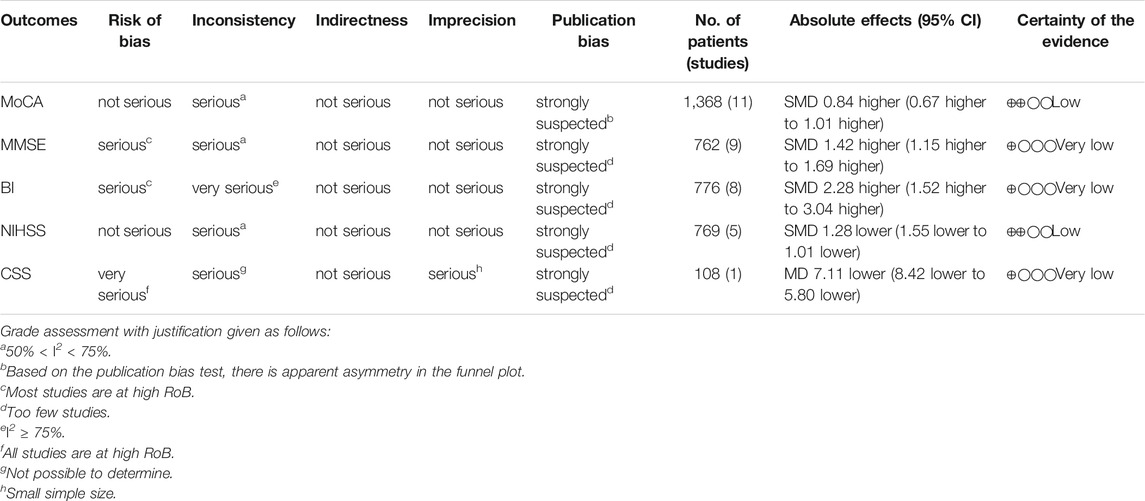
Eleven studies, including 1,368 participants, reported the clinical efficacy of NBP as combination therapy, which is shown in Figure 4. Subgroup analysis was conducted according to different intervention measures. The control group in 9 studies (Ji et al., 2016; Li, 2017; Xu H et al., 2017; Li et al., 2018; Sun, 2018; Wang, 2018; Yang, 2018; Li X et al., 2019; Luo, 2019) was treated with Oxiracetam (I2 = 59%, p = 0.01), and 2 studies were treated with Nimodipine (You, 2014) and Donepezil (Chen et al., 2020). We used a random effects model for the meta-analysis and found a statistically significant benefit of NBP as combination therapy in MoCA scores (SMDO = 0.81, 95% CI [0.62, 1.01], p < 0.00001; SMDN = 0.90, 95% CI [0.46, 1.33], p < 0.0001; SMDD = 1.04, 95% CI [0.71, 1.38], p < 0.00001).
3.4.2 Mini-Mental State Examination
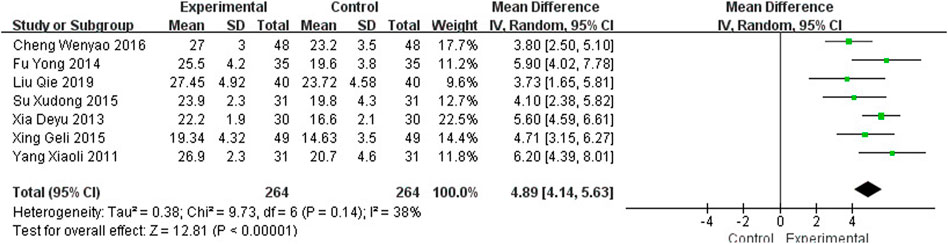
Seven studies (Yang, 2011; Xia, 2013; Fu, 2014; Su et al., 2015; Xing and Abudushalamu, 2015; Cheng et al., 2016; Liu, 2019), including 528 participants, reported the clinical efficacy of NBP as monotherapy, which is shown in Figure 5. The control groups were all treated with Nimodipine, with no obvious heterogeneity (I2 = 38%, p = 0.14). The meta-analysis using a random effects model showed an important benefit in favor of NBP as monotherapy in MMSE scores (MDN = 4.89, 95% CI [4.14, 5.63], p < 0.00001).
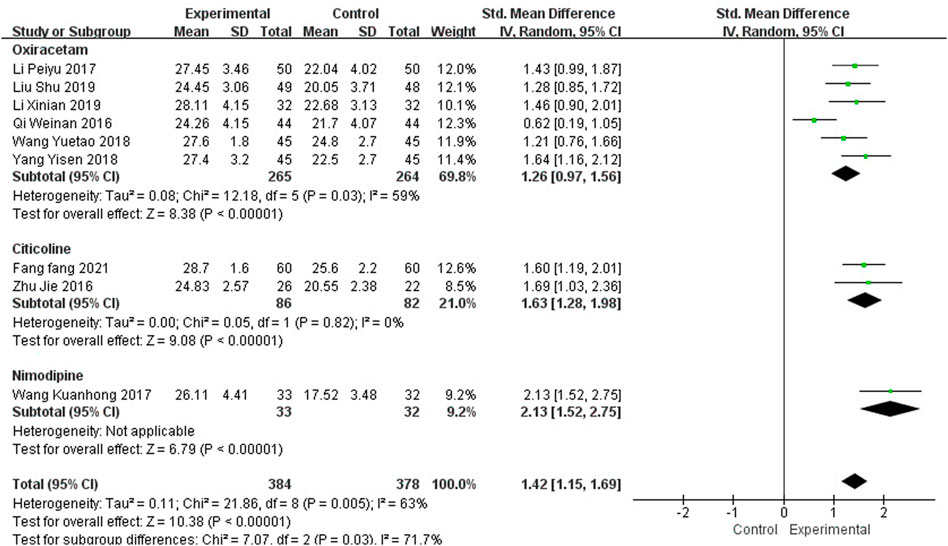
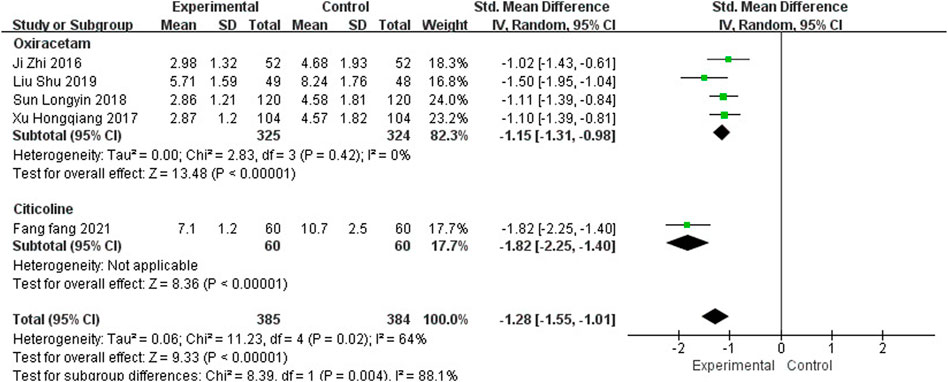
Nine studies, including 762 participants, reported the clinical efficacy of NBP as combination therapy, which is shown in Figure 6. Subgroup analysis was performed according to different intervention measures. The control group in 6 studies (Qi et al., 2016; Li, 2017; Wang, 2018; Yang, 2018; Li X et al., 2019; Liu et al., 2019) was treated with Oxiracetam (I2 = 59%, p = 0.03), 2 studies (Zhu et al., 2016; Fang, 2021) was treated with Citicoline (I2 = 0%, p = 0.82), and 1 study (Wang, 2017) was treated with Nimodipine. A random effects model was used for the meta-analysis, the results indicated significant benefit in favor of NBP as combination therapy in MMSE scores (SMDO = 1.26, 95% CI [0.97, 1.56], p < 0.00001; SMDC = 1.63, 95% CI [1.28, 1.98], p < 0.00001; SMDN = 2.13, 95% CI [1.52, 2.75], p < 0.00001).
3.5 Abilities of Daily Living
3.5.1 Barthel Index
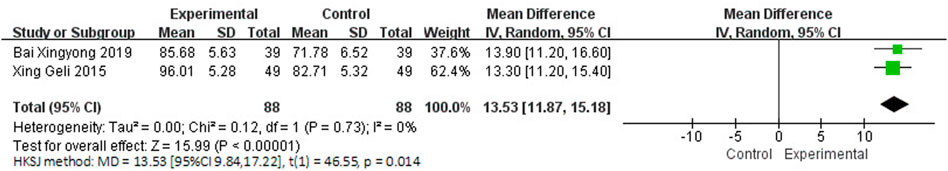
Three studies involving 238 participants reported the clinical efficacy of NBP as monotherapy. Among them, the score criteria of 1 study (Su et al., 2015) were not clearly described, so it was excluded from data analysis. The control groups of the remaining 2 studies (Xing and Abudushalamu, 2015; Bai, 2019) were all treated with Nimodipine, with no obvious heterogeneity (I2 = 0%, p = 0.73), which is shown in Figure 7. We used a random effects model for the meta-analysis, and the results showed a statistically significant benefit of NBP as monotherapy in BI scores (MDN = 13.53, HKSJ 95% CI [9.84, 17.22], p = 0.014).
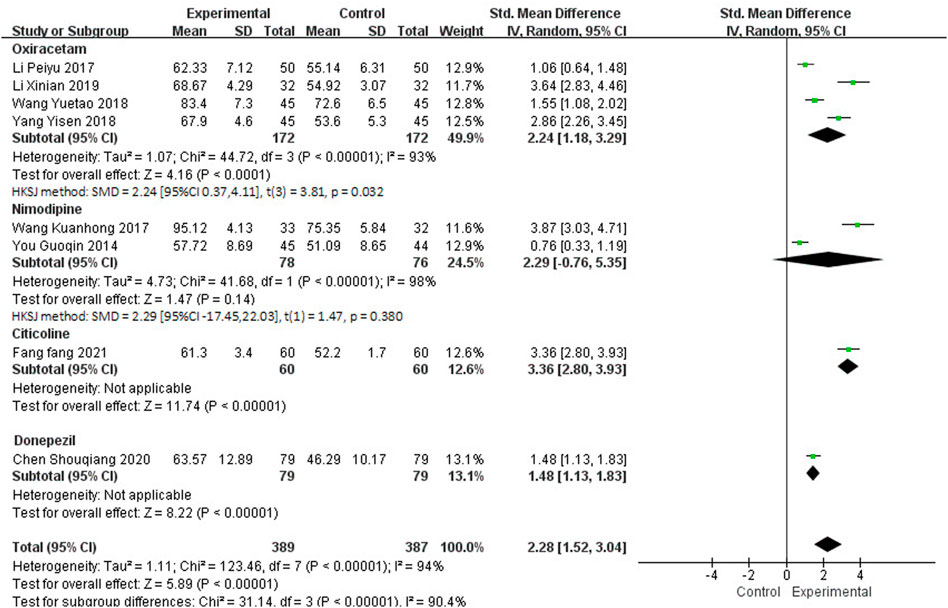
Twelve studies, including 1,126 participants, reported the clinical efficacy of NBP as combination therapy. Among them, the score criteria of 4 studies (Luo, 2019; Liu et al., 2019; Zhu et al., 2016; Qi et al., 2016) were not clearly described, so they were excluded from data analysis. Subgroup analysis was performed according to the different intervention measures of the remaining 8 studies, which is shown in Figure 8. The control group in 4 studies (Li, 2017; Wang, 2018; Yang, 2018; Li X et al., 2019) was treated with Oxiracetam (I2 = 93%, p < 0.00001), 2 studies (You, 2014; Wang, 2017) was treated with Nimodipine (I2 = 98%, p < 0.00001), and 2 studies was treated with Citicoline (Fang, 2021) and Donepezil (Chen et al., 2020). A random effects model was used for meta-analysis, showing an important benefit in favor of NBP as combination therapy in BI scores in Oxiracetam, Citicoline and Donepezil group (SMDO = 2.24, HKSJ 95%CI [0.37, 4.11], p = 0.032; SMDC = 3.36, 95%CI [2.80, 3.93], p < 0.00001; SMDD = 1.48, 95%CI [1.13, 1.83], p < 0.00001), and no significant benefit in Nimodipine group (SMDN = 2.29, HKSJ 95%CI [−17.45, 22.03], p = 0.380).
3.5.2 Activities of Daily Living Scale
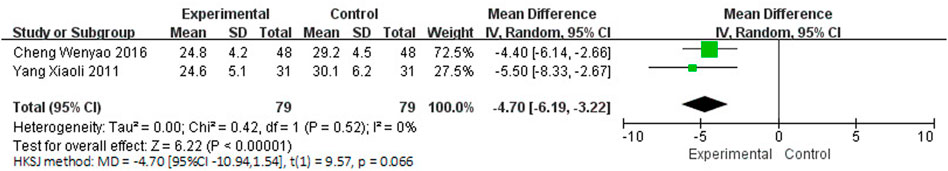
Two studies (Yang, 2011; Cheng et al., 2016), involving 158 participants, reported the clinical efficacy of NBP as monotherapy, which is shown in Figure 9. The control groups were all treated with Nimodipine, with no obvious heterogeneity (I2 = 0%, p = 0.52). The meta-analysis using a random effects model suggested no significant benefit in favor of NBP as monotherapy in ADL scores (MDN = −4.70, HKSJ 95% CI [−10.94, 1.54], p = 0.066).
3.6 Neurological Deficit
3.6.1 National Institutes of Health Stroke Scale
One study (Bai, 2019) reported the clinical efficacy of NBP as monotherapy, including 78 participants. The control group was treated with Nimodipin. The results of the meta-analysis showed a beneficial effect of NBP as monotherapy on NIHSS scores (MDN = −3.86, 95% CI [−5.22, −2.50], p < 0.00001).
Five studies, including 769 participants, reported the clinical efficacy of NBP as combination therapy, which is shown in Figure 10. Subgroup analysis was performed according to different intervention measures. Four studies (Ji et al., 2016; Xu H et al., 2017; Sun, 2018; Liu et al., 2019) were treated with Oxiracetam (I2 = 0%, p = 0.42), and the control group in 1 study (Fang, 2021) was treated with Citicoline. We used a random effects model for the meta-analysis and found that NBP as combination therapy had a statistically significant effect on improving NIHSS scores (SMDO = −1.15, 95% CI [−1.31, −0.98], p < 0.00001; SMDC = −1.82, 95% CI [−2.25, −1.40], p < 0.00001).
3.6.2 China Stroke Scale
One study (Li et al., 2018) reported the clinical efficacy of NBP as combination therapy, including 108 participants. The control group was treated with Oxiracetam, and the results of the meta-analysis showed a significant effect of NBP as combination therapy on CSS scores (MDO = −7.11, 95% CI [−8.42, −5.80], p < 0.00001).
3.7 Adverse Events
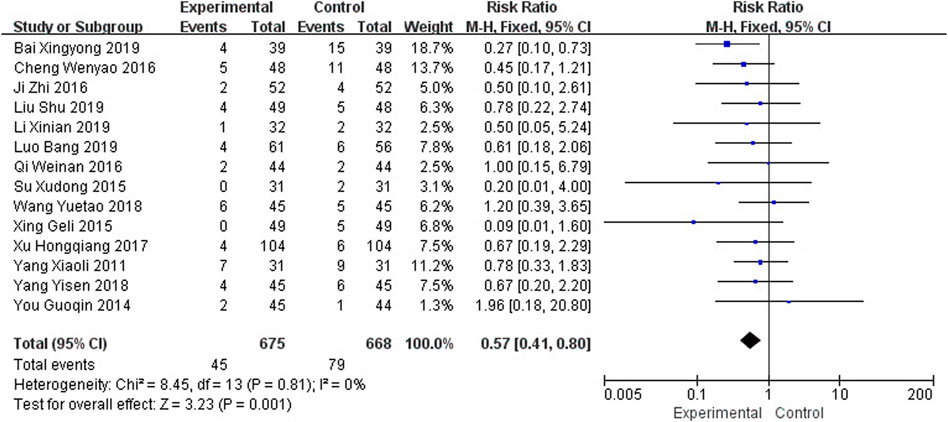
Seventeen studies reported the occurrence of adverse events, among which 3 studies (Xia, 2013; Zhao, 2015; Li et al., 2018) reported no adverse reactions in the intervention and control groups, while the other 14 studies (Yang, 2011; You, 2014; Su et al., 2015; Xing and Abudushalamu, 2015; Cheng et al., 2016; Ji et al., 2016; Qi et al., 2016; Xu H et al., 2017; Wang, 2018; Yang, 2018; Bai, 2019; Li X et al., 2019; Liu et al., 2019; Luo, 2019) reported that most adverse reactions were elevated transaminase, gastrointestinal discomfort, nausea and vomiting, anorexia, dizziness, insomnia, and decreased blood pressure. The heterogeneity was not obvious (I2 = 0%, p = 0.81), and a fixed effects model was used for meta-analysis, as shown in Figure 11, and the results showed that the number of adverse events in the intervention group was lower than that in the control group (RR = 0.57, 95% CI [0.41, 0.80], p = 0.001).
3.8 Publication Bias
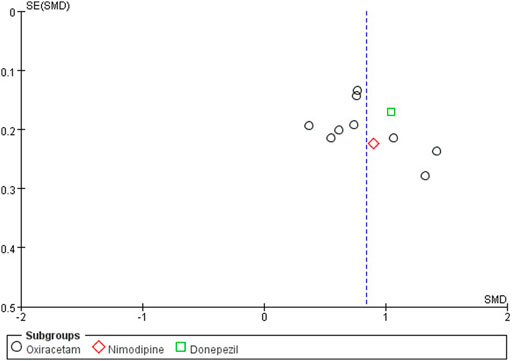
The funnel plot was drawn on the MoCA score of NBP as combination therapy, as shown in Figure 12. It was found that the graph was slightly biased due to the asymmetry of left and right scatter points, suggesting the possibility of publication bias.
3.9 GRADE Assessment
GRADE system was used to evaluate the overall evidence of the above six outcomes, as is shown in Tables 2, 3. The certainty of the evidence indicated low or very low with serious methodological problems, obvious inter-study heterogeneity and significant publication bias.
4 Discussion
4.1 Summary of Evidence
A total of 26 randomized controlled trials were included in our systematic review, including 2,571 patients suffering from PSCI. Through subgroup analysis of different intervention measures in the control group, we found that NBP as monotherapy could effectively improve the recovery of cognitive function, promote the ability of daily living, and reduce neurological deficits. In addition, the combination therapy also had a corresponding synergistic effect on PSCI patients, which is basically consistent with previous research conclusions (Feng et al., 2018). Fourteen out of 26 studies reported adverse reactions during the trial, most of which were elevated transaminase and gastrointestinal discomfort, suggesting that NBP was better tolerated in patients with PSCI.
4.2 Implications for Practice
The use of NBP for PSCI patients has increased in the past few decades. However, there is still limited evidence on the use of drug therapy for the treatment of PSCI. The available evidence from the present study indicated, to a certain extent, that NBP has a positive effect on the cognitive function of patients with PSCI, which can be considered for clinical application in the future.
4.3 Implications for Research
At present, there is still a lack of consistent guideline recommendations for PSCI therapeutic schedules; thus, drug treatment projects for cognitive function after stroke are a significant and difficult point in clinical practice. In our systematic review, we evaluated the efficacy and safety of NBP in PSCI patients and proposed some recommendations for future researchers.
First, current studies have indicated that PSCI generally affects complex cognitive function in multiple domains (Middleton et al., 2014), and assessment scales used only for cognitive screening tests such as MoCA and MMSE are insensitive in detecting cognitive function after stroke (Jokinen et al., 2015). Some studies demonstrated that ADAS-Cog has certain clinical value for cognitive recovery of vascular factors (Rockwood et al., 2017), but it still lacks specificity (Kueper et al., 2018). Future studies should pay more attention to the score results of VADAS-Cog to provide better detection of vascular conditions (Ylikoski et al., 2007).
Second, cognitive impairment has a continuous dynamic change trend after stroke (Levine et al., 2015). Previous studies confirmed that even during adequate drug treatment, cognitive dysfunction was highly associated with stroke recurrence (Kwon et al., 2020); therefore, the recovery of cognitive function may help control the progression of ischemic stroke. However, only one of the included studies (Yang, 2011) described the recurrence of cerebral infarction after the trial. Endpoint criteria, such as dementia transformation, stroke recurrence and death, deserve further investigation to clarify the clinical efficacy of NBP.
Third, our systematic review suggested a better safety of NBP. However, some eligible studies reported transient elevation of transaminase after NBP administration, which was demonstrated in a systematic review of ischemic stroke (Xu et al., 2019). Therefore, we recommend that future researchers regularly monitor the liver function of patients during treatment and weigh whether NBP can be used in patients with pre-existing liver function damage.
Fourth, effective therapies for ischemic stroke depend on timely restoration of blood supply to brain tissue (Wu et al., 2019); therefore, early identification of symptoms may be beneficial to rehabilitation. The specification of NBP included patients with acute stroke less than 72 h after onset, while none of the included studies stated the intervention time of NBP, and whether timely treatment is beneficial to early recovery of cognitive function deserves further study.
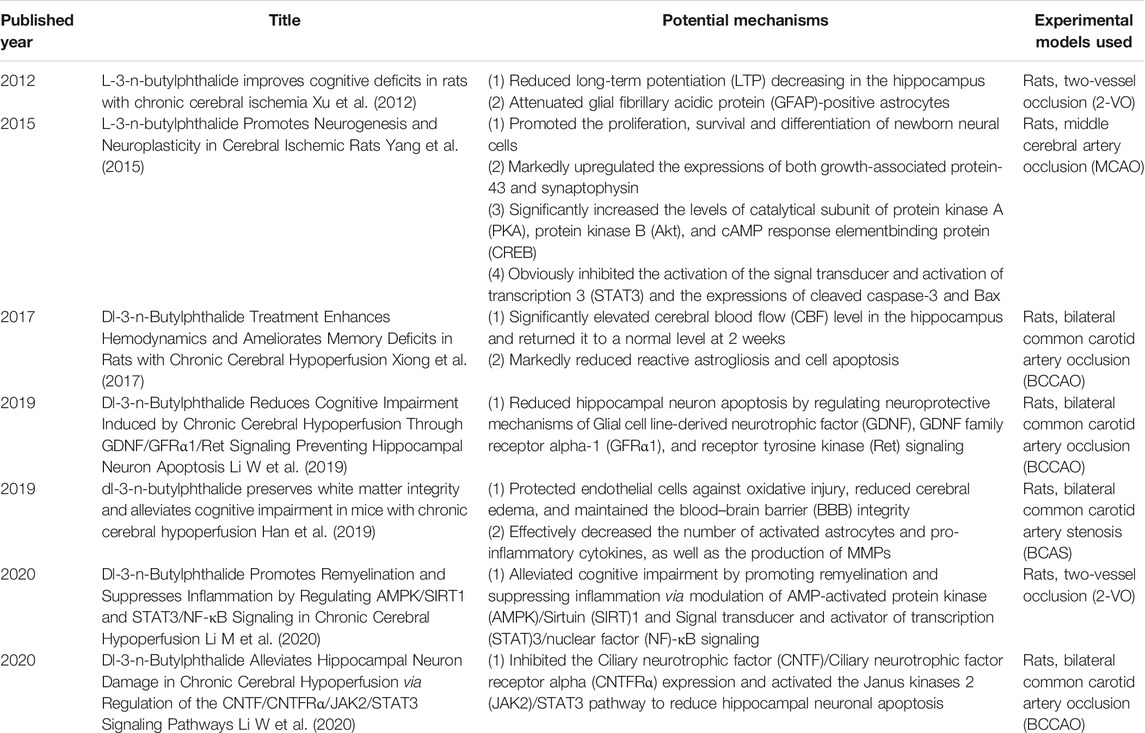
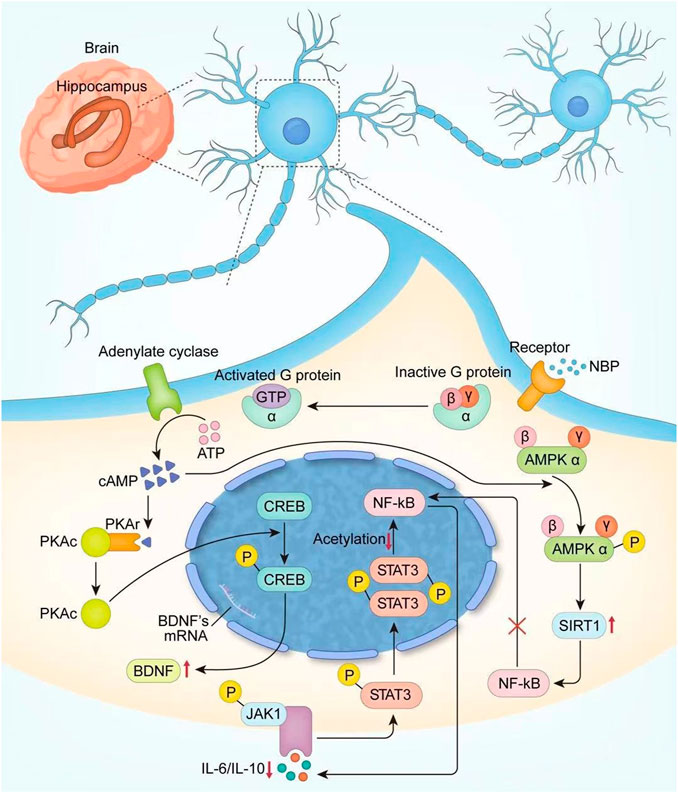
Finally, NBP is a synthetic compound isolated from the seeds of Apium graveolens (Wang et al., 2018), and a series of clinical trials confirmed the positive effect of it in PSCI patients. We systematically retrieved the possible mechanisms of NBP and summarized them according to the timeline in Table 4, and conducted a more detailed analysis and localization of NBP in the light of neuroplasticity (On the one hand, NBP can increase the newborn neuron survival rate by increasing CREB activity and upregulating BDNF expression; on the other hand, it can promote regeneration and repair of myelin sheath by upregulating AMPK/SIRT1 and downregulating the STAT3/NF-κB pathway), which is shown in Figure 13. Although neuroplasticity is a hot spot in current research, the mechanism of NBP has not been completely clarified. We believe that the clinical treatment of NBP is critical in terms of neuroplasticity and suggest that future studies explore the relationship between them to obtain a clear understanding of the potential therapeutic mechanism. In addition, the specification of NBP only focuses on the use of ischemic stroke, and further research is needed to determine whether NBP should be used more widely.
4.4 Limitations
The findings of our meta-analysis provided evidence to support the clinical efficacy of NBP, but there are some limitations that can be discussed in future studies. First, the methodological quality of the eligible studies is generally low. Although we adopted a rigorous and reproducible method to retrieve and screen the literature, all studies included in this research were conducted in China, raising the question of generalizability to Western patients. Most studies lack information on random allocation, blind design and complete study data, which, to a certain extent, affect the results of intervention effectiveness (Bilandzic et al., 2016; Di Girolamo et al., 2018). Second, clinical heterogeneity is inevitable. The outcome indicators of our meta-analysis were mainly evaluated by assessment scales. However, because of the scale version and subjectivity of the scale scores, heterogeneity between studies could not be further reduced by sensitivity or subgroup analysis. Third, the treatment intervention time was unclear. NBP is generally used for 1–3 months in clinical practice depending on the severity of the disease. However, some high-quality clinical studies set the treatment time as 6 months (Jia et al., 2016), and 5 out of 26 included studies concluded that a treatment time up to 6 months can have a better clinical effect, while none of them described the follow-ups. Fourth, the comorbid conditions of patients was unclear. Stroke usually associated with multiple risk factors and causes. Primary stroke prevention with emphasis on underlying diseases improvement plays an important role in reducing the burden of stroke patients (Caprio and Sorond, 2019). However, most of the included studies provided incomplete information, the treatment measures of other diseases was undefined. Fifth, the result of ADL showed no statistically significant difference, and the effect of NBP on the improvement of this respect was unclear. However, due to the small number of included studies and the general lack of description of evaluation criteria, effects analysis could not be carried out, which impaired the value of research.
5 Conclusion
NBP maintains a stable pattern in promoting the recovery of cognitive function and abilities of daily living, as well as reducing neurological deficits in the treatment of PSCI. However, due to the low quality of the methodology, large samples, multicenter and high-quality randomized controlled trials are still needed to verify the clinical efficacy and safety of NBP.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
XF and WS conceived and designed this study. XF retrieved the literature, analyzed the data, and drafted the manuscript. XF and LW selected studies, extracted data, and assessed the risk of bias. WS and YZ directed the research. All authors contributed to the article and approved the final manuscript.
Funding
This research was supported by the National TCM Leading Personnel Support Program (NATCM Personnel and Education Department (2018)) (No. 12), the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-C-202007), and the China Academy of Chinese Medical Sciences Innovation Fund (No. CI2021A01310).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our appreciation to all authors of the primary studies included in the current systematic review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.810297/full#supplementary-material
References
Aam, S., Einstad, M. S., Munthe-Kaas, R., Lydersen, S., Ihle-Hansen, H., Knapskog, A. B., et al. (2020). Post-stroke Cognitive Impairment-Impact of Follow-Up Time and Stroke Subtype on Severity and Cognitive Profile: The Nor-COAST Study. Front. Neurol. 11, 699. doi:10.3389/fneur.2020.00699
Bai, X. (2019). Effect and Cognitive Function of Dl-3-N-Butylphthalide in the Treatment of Vascular Dementia after Cerebral Infarction. China Foreign Med. Treat. 38 (32), 91–93+96. doi:10.16662/j.cnki.1674-0742.2019.32.091
Bilandzic, A., Fitzpatrick, T., Rosella, L., and Henry, D. (2016). Risk of Bias in Systematic Reviews of Non-randomized Studies of Adverse Cardiovascular Effects of Thiazolidinediones and Cyclooxygenase-2 Inhibitors: Application of a New Cochrane Risk of Bias Tool. Plos Med. 13 (4), e1001987. doi:10.1371/journal.pmed.1001987
Black, C. M., Ritchie, C. W., Khandker, R. K., Wood, R., Jones, E., Hu, X., et al. (2018). Non-professional Caregiver burden Is Associated with the Severity of Patients' Cognitive Impairment. PLoS One 13 (12), e0204110. doi:10.1371/journal.pone.0204110
Brainin, M., Tuomilehto, J., Heiss, W. D., Bornstein, N. M., Bath, P. M., Teuschl, Y., et al. (2015). Post-stroke Cognitive Decline: an Update and Perspectives for Clinical Research. Eur. J. Neurol. 22 (2), 229–e16. doi:10.1111/ene.12626
Caprio, F. Z., and Sorond, F. A. (2019). Cerebrovascular Disease: Primary and Secondary Stroke Prevention. Med. Clin. North. Am. 103 (2), 295–308. doi:10.1016/j.mcna.2018.10.001
Carlson, M. C., Xue, Q. L., Zhou, J., and Fried, L. P. (2009). Executive Decline and Dysfunction Precedes Declines in Memory: the Women's Health and Aging Study II. J. Gerontol. A. Biol. Sci. Med. Sci. 64 (1), 110–117. doi:10.1093/gerona/gln008
Chen, S., Li, B., Su, F., Liu, F., and Xu, X. (2020). Effect of Donepezil Hydrochloride Combined with Butylphthalide in the Treatment of Patients with Cognitive Impairment after Cerebral Stroke. Clin. Res. Pract. 5 (22), 29–31+34. doi:10.19347/j.cnki.2096-1413.202022011
Cheng, W., Yang, J., and Yu, X. (2016). Effect of Butylphthalide Soft Capsules and Nimodipine on Patients with Vascular Dementia after Stroke. J. Prev. Med. Chin. PLA 34 (4), 516–518. doi:10.13704/j.cnki.jyyx.2016.04.020
Di Girolamo, N., Winter, A., and Meursinge Reynders, R. (2018). High and Unclear Risk of Bias Assessments Are Predominant in Diagnostic Accuracy Studies Included in Cochrane Reviews. J. Clin. Epidemiol. 101, 73–78. doi:10.1016/j.jclinepi.2018.05.001
Ding, M. Y., Xu, Y., Wang, Y. Z., Li, P. X., Mao, Y. T., Yu, J. T., et al. (2019). Predictors of Cognitive Impairment after Stroke: A Prospective Stroke Cohort Study. J. Alzheimers Dis. 71 (4), 1139–1151. doi:10.3233/JAD-190382
Dong, Q., Guo, Q., Luo, B., and Xu, Y. (2017). Expert Consensus on the Management of Poststroke Cognitive Impairment. Chin. J. Stroke 12 (6), 519–531. doi:10.3969/j.issn.1673-5765.2017.06.011
Fang, F. (2021). Effect of Butylphthalide Combined with Citicoline in the Treatment of Poststroke Cognitive Impairment. China Prac Med. 16 (9), 106–108. doi:10.14163/j.cnki.11-5547/r.2021.09.043
Feng, Z., Zhang, J., and Hou, Y. (2018). Butylphthalide in the Treatment of Post-stroke Cognitive Impairment: a Systematic Review. Chin. J. Integr. Med. Cardio-/Cerebrovascular Dis. 16 (10), 1341–1346. doi:10.12102/j.issn.1672-1349.2018.10.009
Fu, Y. (2014). Comparative Observation of Butylphthalide and Nimodipine in the Treatment of Vascular Dementia after Stroke. ShiYong ZhenLiao 4 (9), 14–1415. doi:10.3969/j.issn.1006-9488.2013.09.012
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). GRADE: an Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 336 (7650), 924–926. doi:10.1136/bmj.39489.470347.AD
Han, Q. Y., Zhang, H., Zhang, X., He, D. S., Wang, S. W., Cao, X., et al. (2019). dl-3-n-butylphthalide Preserves white Matter Integrity and Alleviates Cognitive Impairment in Mice with Chronic Cerebral Hypoperfusion. CNS Neurosci. Ther. 25 (9), 1042–1053. doi:10.1111/cns.13189
Inthout, J., Ioannidis, J. P., and Borm, G. F. (2014). The Hartung-Knapp-Sidik-Jonkman Method for Random Effects Meta-Analysis Is Straightforward and Considerably Outperforms the Standard DerSimonian-Laird Method. BMC Med. Res. Methodol. 14, 25. doi:10.1186/1471-2288-14-25
Ji, Z., Fan, X., and He, Y. (2016). Impact of Butylphthalide Combined with Oxiracetam on Neurological Function, Cognitive Function and Quality of Life of Patients with Cerebral Infarction-Induced Cognitive Disorders. Pract. J. Card. Cereb. Pneumal Vasc. Dis. 24 (5), 43–46. doi:10.3969/j.issn.1008-5971.2016.05.010
Jia, J., Wei, C., Liang, J., Zhou, A., Zuo, X., Song, H., et al. (2016). The Effects of DL-3-N-Butylphthalide in Patients with Vascular Cognitive Impairment without Dementia Caused by Subcortical Ischemic Small Vessel Disease: A Multicentre, Randomized, Double-Blind, Placebo-Controlled Trial. Alzheimers Dement 12 (2), 89–99. doi:10.1016/j.jalz.2015.04.010
Jokinen, H., Melkas, S., Ylikoski, R., Pohjasvaara, T., Kaste, M., Erkinjuntti, T., et al. (2015). Post-stroke Cognitive Impairment Is Common Even after Successful Clinical Recovery. Eur. J. Neurol. 22 (9), 1288–1294. doi:10.1111/ene.12743
Kim, J. O., Lee, S. J., and Pyo, J. S. (2020). Effect of Acetylcholinesterase Inhibitors on post-stroke Cognitive Impairment and Vascular Dementia: A Meta-Analysis. PLoS One 15 (2), e0227820. doi:10.1371/journal.pone.0227820
Kueper, J. K., Speechley, M., and Montero-Odasso, M. (2018). The Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog): Modifications and Responsiveness in Pre-dementia Populations. A Narrative Review. J. Alzheimers Dis. 63 (2), 423–444. doi:10.3233/JAD-170991
Kwon, H. S., Lee, D., Lee, M. H., Yu, S., Lim, J. S., Yu, K. H., et al. (2020). Post-stroke Cognitive Impairment as an Independent Predictor of Ischemic Stroke Recurrence: PICASSO Sub-study. J. Neurol. 267 (3), 688–693. doi:10.1007/s00415-019-09630-4
Levine, D. A., Galecki, A. T., Langa, K. M., Unverzagt, F. W., Kabeto, M. U., Giordani, B., et al. (2015). Trajectory of Cognitive Decline after Incident Stroke. JAMA 314 (1), 41–51. doi:10.1001/jama.2015.6968
Li, M., Meng, N., Guo, X., Niu, X., Zhao, Z., Wang, W., et al. (2020). Dl-3-n-Butylphthalide Promotes Remyelination and Suppresses Inflammation by Regulating AMPK/SIRT1 and STAT3/NF-Κb Signaling in Chronic Cerebral Hypoperfusion. Front. Aging Neurosci. 12, 137. doi:10.3389/fnagi.2020.00137
Li, P. (2017). Effect of Oxiracetam Combined with Butylphthalide on Cognitive Function and Quality of Life in Elderly Patients with Cognitive Impairment after Cerebral Infarction. Med. Inf. 30 (5), 127–128. doi:10.3969/j.issn.1006-1959.2017.05.082
Li, W., Wei, D., Lin, J., Liang, J., Xie, X., Song, K., et al. (2019). Dl-3-n-Butylphthalide Reduces Cognitive Impairment Induced by Chronic Cerebral Hypoperfusion through GDNF/GFRα1/Ret Signaling Preventing Hippocampal Neuron Apoptosis. Front. Cel. Neurosci. 13, 351. doi:10.3389/fncel.2019.00351
Li, W., Wei, D., Zhu, Z., Xie, X., Zhan, S., Zhang, R., et al. (2020). Dl-3-n-Butylphthalide Alleviates Hippocampal Neuron Damage in Chronic Cerebral Hypoperfusion via Regulation of the CNTF/CNTFRα/JAK2/STAT3 Signaling Pathways. Front. Aging Neurosci. 12, 587403. doi:10.3389/fnagi.2020.587403
Li, W., Chen, Y., Zhu, X., Yang, J., and Chen, Z. (2018). Effect of Butylphthalide Combined with Oxiracetam on Cognitive Impairment in Patients with Acute Cerebral Infarction. China Pharmacist 21 (7), 1202–12041213. doi:10.3969/j.issn.1008-049X.2018.07.018
Li, X., Hong, R., Zeng, J., Zhang, Y., and Luo, C. (2019). Effect of Butylphthalide Combined with Oxiracetam in the Treatment of Acute Cerebral Infarction with Cognitive Dysfunction. China Med. Pharm. 9 (3), 66–68. doi:10.3969/j.issn.2095-0616.2019.03.019
Liu, C., Liu, Y., Zhang, Y., Xu, Y., and Xu, Y. (2018). Effect of Dl-3-N-Butylphthalide on Mild Cognitive Impairment after Acute Cerebral Infarction. XinLi YiSheng 24 (14), 75–76.
Liu, Q. (2019). Effect of Butylphthalide on Cognitive Function and Quality of Life after Acute Cerebral Infarction. Chin. J. Mod. Drug Appl. 13 (9), 96–97. doi:10.14164/j.cnki.cn11-5581/r.2019.09.055
Liu, S., Li, Y., Liu, G., Zhao, M., Xu, M., and Guo, N. (2019). Effect of Oxiracetam Combined with Butylphthalide on Neurological Function and Brain-Derived Neurotrophic Factor in Patients with Vascular Dementia after Stroke. Chin. J. Prev. Contr Chron. Dis. 27 (7), 538–541. doi:10.16386/j.cjpccd.issn.1004-6194.2019.07.013
Luo, B. (2019). Effect and Mechanism of Butylphthalide Combined with Oxiracetam on Cognitive Function in Convalescent Patients with Ischemic Stroke. Chronic Pathematology J. 20 (12), 1838–1839. doi:10.16440/j.cnki.1674-8166.2019.12.024
Lv, C., Ma, Q., Han, B., Li, J., Geng, Y., Zhang, X., et al. (2018). Long-Term DL-3-N-Butylphthalide Treatment Alleviates Cognitive Impairment Correlate with Improving Synaptic Plasticity in SAMP8 Mice. Front. Aging Neurosci. 10, 200. doi:10.3389/fnagi.2018.00200
Mcewen, B. S. (2016). In Pursuit of Resilience: Stress, Epigenetics, and Brain Plasticity. Ann. N. Y Acad. Sci. 1373 (1), 56–64. doi:10.1111/nyas.13020
Middleton, L. E., Lam, B., Fahmi, H., Black, S. E., Mcilroy, W. E., Stuss, D. T., et al. (2014). Frequency of Domain-specific Cognitive Impairment in Sub-acute and Chronic Stroke. Neurorehabilitation 34 (2), 305–312. doi:10.3233/NRE-131030
Ossenkoppele, R., Lyoo, C. H., Jester-Broms, J., Sudre, C. H., Cho, H., Ryu, Y. H., et al. (2020). Assessment of Demographic, Genetic, and Imaging Variables Associated with Brain Resilience and Cognitive Resilience to Pathological Tau in Patients with Alzheimer Disease. JAMA Neurol. 77 (5), 632–642. doi:10.1001/jamaneurol.2019.5154
Qi, W., Xu, P., and Wang, S. (2016). Clinical Evaluation of Butylphthalide Combined with Nimodipine in Vascular Dementia after Stroke. Med. Forum 20 (4), 460–461. doi:10.19435/j.1672-1721.2016.04.016
Quinn, T. J., Richard, E., Teuschl, Y., Gattringer, T., Hafdi, M., O'Brien, J. T., et al. (2021). European Stroke Organisation and European Academy of Neurology Joint Guidelines on post‐stroke Cognitive Impairment. Eur. J. Neurol. 28, 3883–3920. doi:10.1111/ene.15068
Regenhardt, R. W., Takase, H., Lo, E. H., and Lin, D. J. (2020). Translating Concepts of Neural Repair after Stroke: Structural and Functional Targets for Recovery. Restor Neurol. Neurosci. 38 (1), 67–92. doi:10.3233/RNN-190978
Rockwood, K., Howlett, S. E., Hoffman, D., Schindler, R., and Mitnitski, A. (2017). Clinical Meaningfulness of Alzheimer's Disease Assessment Scale-Cognitive Subscale Change in Relation to Goal Attainment in Patients on Cholinesterase Inhibitors. Alzheimers Dement 13 (10), 1098–1106. doi:10.1016/j.jalz.2017.02.005
Rohde, D., Gaynor, E., Large, M., Mellon, L., Bennett, K., Williams, D. J., et al. (2019). Cognitive Impairment and Medication Adherence post-stroke: A Five-Year Follow-Up of the ASPIRE-S Cohort. PLoS One 14 (10), e0223997. doi:10.1371/journal.pone.0223997
Shi, D., Chen, X., and Li, Z. (2018). Diagnostic Test Accuracy of the Montreal Cognitive Assessment in the Detection of post-stroke Cognitive Impairment under Different Stages and Cutoffs: a Systematic Review and Meta-Analysis. Neurol. Sci. 39 (4), 705–716. doi:10.1007/s10072-018-3254-0
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Su, X., Yang, X., and Tai, L. (2015). Effect of Butylphthalide and Nimodipine on Vascular Dementia after Stroke. Clin. Focus 30 (5), 569–572. doi:10.3969/j.issn.1004-583X.2015.05.024
Sun, J. H., Tan, L., and Yu, J. T. (2014). Post-stroke Cognitive Impairment: Epidemiology, Mechanisms and Management. Ann. Transl Med. 2 (8), 80. doi:10.3978/j.issn.2305-5839.2014.08.05
Sun, L. (2018). Effect of Butylphthalide Combined with Oxiracetam on Acute Cerebral Infarction with Non-dementia Cognitive Impairment. J. Imaging Res. Med. Appl. 2 (13), 232–233. doi:10.3969/j.issn.2096-3807.2018.13.156
Sun, Y., Zhao, Z., Li, Q., Wang, C., Ge, X., Wang, X., et al. (2020). Dl-3-n-butylphthalide Regulates Cholinergic Dysfunction in Chronic Cerebral Hypoperfusion Rats. J. Int. Med. Res. 48 (7), 300060520936177. doi:10.1177/0300060520936177
Wang, K., Dong, Q., Yu, J., and Hu, P. (2021). Expert Consensus on the Management of Poststroke Cognitive Impairment 2021. Chin. J. Stroke 16 (4), 376–389. doi:10.3969/j.issn.1673-5765.2021.04.011
Wang, K. (2017). Effect of Butylphthalide Combined with Nimodipine on Mild Cognitive Impairment after Acute Cerebral Infarction. Henan Med. Res. 26 (15), 2760–2761. doi:10.3969/j.issn.1004-437X.2017.15.042
Wang, S., Ma, F., Huang, L., Zhang, Y., Peng, Y., Xing, C., et al. (2018). Dl-3-n-Butylphthalide (NBP): A Promising Therapeutic Agent for Ischemic Stroke. CNS Neurol. Disord. Drug Targets 17 (5), 338–347. doi:10.2174/1871527317666180612125843
Wang, Y. (2018). Effect of Oxiracetam Combined with Butylphthalide on Cognitive Impairment after Cerebral Infarction. Chin J. Clin. Rational Drug Use 11 (31), 45–47. doi:10.15887/j.cnki.13-1389/r.2018.31.023
Wu, S., Wu, B., Liu, M., Chen, Z., Wang, W., Anderson, C. S., et al. (2019). Stroke in China: Advances and Challenges in Epidemiology, Prevention, and Management. Lancet Neurol. 18 (4), 394–405. doi:10.1016/S1474-4422(18)30500-3
Xia, D. (2013). Comparative Observation of Butylphthalide and Nimodipine in the Treatment of Vascular Dementia after Stroke. J. Clin. Exp. Med. 12 (12), 978–979. doi:10.3969/j.issn.1671-4695.2013.12.035
Xing, G., and Abudushalamu, A. (2015). Comparative Study for Clinical Effect on Vascular Dementia after Stroke between Butylphthalide Soft Capsules and Nimodipine. Pract. J. Card. Cereb. Pneumal Vasc. Dis. 23 (9), 48–50. doi:10.3969/j.issn.1008-5971.2015.09.014
Xiong, Z., Lu, W., Zhu, L., Zeng, L., Shi, C., Jing, Z., et al. (2017). Dl-3-n-Butylphthalide Treatment Enhances Hemodynamics and Ameliorates Memory Deficits in Rats with Chronic Cerebral Hypoperfusion. Front. Aging Neurosci. 9, 238. doi:10.3389/fnagi.2017.00238
Xu, H., Wang, Y., and Sun, L. (2017). Effects of Butylphthalide Combined with Oxiracetam on Treating Acute Cerebral Infarction with Cognitive Impairment of None Dementia. Chin. J. Pract. Nervous Dis. 20 (4), 19–21. doi:10.3969/j.issn.1673-5110.2017.04.008
Xu, J., Huai, Y., Meng, N., Dong, Y., Liu, Z., Qi, Q., et al. (2017). L-3-n-Butylphthalide Activates Akt/mTOR Signaling, Inhibits Neuronal Apoptosis and Autophagy and Improves Cognitive Impairment in Mice with Repeated Cerebral Ischemia-Reperfusion Injury. Neurochem. Res. 42 (10), 2968–2981. doi:10.1007/s11064-017-2328-3
Xu, J., Wang, Y., Li, N., Xu, L., Yang, H., and Yang, Z. (2012). L-3-n-butylphthalide Improves Cognitive Deficits in Rats with Chronic Cerebral Ischemia. Neuropharmacology 62 (7), 2424–2429. doi:10.1016/j.neuropharm.2012.02.014
Xu, Z. Q., Zhou, Y., Shao, B. Z., Zhang, J. J., and Liu, C. (2019). A Systematic Review of Neuroprotective Efficacy and Safety of DL-3-N-Butylphthalide in Ischemic Stroke. Am. J. Chin. Med. 47 (3), 507–525. doi:10.1142/S0192415X19500265
Yang, L. C., Li, J., Xu, S. F., Cai, J., Lei, H., Liu, D. M., et al. (2015). L-3-n-butylphthalide Promotes Neurogenesis and Neuroplasticity in Cerebral Ischemic Rats. CNS Neurosci. Ther. 21 (9), 733–741. doi:10.1111/cns.12438
Yang, X. (2011). Butylphthalide Soft Capsules and Nimodipine Treatment of Vascular Dementia after Stroke Effect Assessment. Hebei, China: Hebei Medical University. doi:10.7666/d.y1900465
Yang, Y. (2018). Effect of Oxiracetam Combined with Butylphthalide on Cognitive Impairment after Acute Cerebral Infarction. J. Med. Theor. Pract. 31 (12), 1770–1772. doi:10.19381/j.issn.1001-7585.2018.12.026
Ylikoski, R., Jokinen, H., Andersen, P., Salonen, O., Madureira, S., Ferro, J., et al. (2007). Comparison of the Alzheimer's Disease Assessment Scale Cognitive Subscale and the Vascular Dementia Assessment Scale in Differentiating Elderly Individuals with Different Degrees of white Matter Changes. The LADIS Study. Dement Geriatr. Cogn. Disord. 24 (2), 73–81. doi:10.1159/000103865
You, G. (2014). Effect of Butylphthalide Combined with Nimodipine on 48 Cases of Vascular Dementia after Stroke. China Prac Med. 9 (4), 141–142. doi:10.14163/j.cnki.11-5547/r.2014.04.190
Zhang, X., and Bi, X. (2020). Post-stroke Cognitive Impairment: A Review Focusing on Molecular Biomarkers. J. Mol. Neurosci. 70 (8), 1244–1254. doi:10.1007/s12031-020-01533-8
Zhao, H. (2015). Effect of Butylphthalide on Cognitive Impairment in Convalescent Patients with Acute Cerebral Infarction. World Notes Antibiot. 36 (3), S1–S3. doi:10.13461/j.cnki.wna.004871
Zhao, W., Luo, C., Wang, J., Gong, J., Li, B., Gong, Y., et al. (2014). 3-N-butylphthalide Improves Neuronal Morphology after Chronic Cerebral Ischemia. Neural Regen. Res. 9 (7), 719–726. doi:10.4103/1673-5374.131576
Keywords: Dl-3-n-butylphthalide, poststroke cognitive impairment, systematic review, cognitive function, potential mechanism
Citation: Fan X, Shen W, Wang L and Zhang Y (2022) Efficacy and Safety of DL-3-n-Butylphthalide in the Treatment of Poststroke Cognitive Impairment: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12:810297. doi: 10.3389/fphar.2021.810297
Received: 06 November 2021; Accepted: 08 December 2021;
Published: 25 January 2022.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Shivshankar Thanigaimani, James Cook University, AustraliaSergey Viktorovich Kotov, Regional Research Clinical Institute, Russia
Copyright © 2022 Fan, Shen, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Shen, Njc2NjY1NzA5QHFxLmNvbQ==; Yunling Zhang, eXVubGluZ3poYW5nMjAwNEAxNjMuY29t
 Xueming Fan
Xueming Fan Wei Shen
Wei Shen Liuding Wang
Liuding Wang Yunling Zhang
Yunling Zhang