- 1Comprehensive Department, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Graduate School, Beijing University of Chinese Medicine, Beijing, China
Atherosclerotic thrombotic disease continues to maintain a high morbidity and mortality rate worldwide at present. Aspirin, which is reckoned as the cornerstone of primary and secondary prevention of atherosclerotic cardiovascular diseases (ASCVDs), has been applied in clinics extensively. However, cardiovascular events continue to occur even though people utilize aspirin appropriately. Therefore, the concept of aspirin resistance (AR) was put forward by scholars, which is of great significance for the prediction of the clinical outcome of diseases. The pathogenesis of AR may be incorporated with low patient compliance, insufficient dose, genetic polymorphism, increased platelet transformation, inflammation, and the degenerative changes and calcification of platelets. The improvement of AR in the treatment of ASCVDs has gradually become a research hot spot in recent years. Traditional Chinese medicine (TCM) regards individuals as a whole and treats them from a holistic view, which has been found to have advantages in clinical studies on the treatment of AR. Many kinds of blood-activating TCM have the effect of improving AR. The potential mechanism for the improvement of AR by blood-activating herbs combined with aspirin was explored. The combination of blood-activating herbs and aspirin to improve AR is likely to turn into a hot topic of research in the future.
Introduction
Atherosclerotic cardiovascular diseases (ASCVDs) have a high incidence in the world and are the main cause of morbidity and mortality in China (The interpretation of, 2017). The increase in platelet reactivity, platelet activation, aggregation, and interaction with surface molecules is closely related to ischemic stroke, thrombosis, and cardiovascular diseases (CVDs) (Kannan et al., 2019; Khodadi, 2020; Wiśniewski et al., 2020). Aspirin, an acetylated salicylate, can irreversibly inhibit the conversion of arachidonic acid (AA) to thromboxane A2 (TXA2) by acetylating the serine 529 site of platelet cyclooxygenase-1 (COX-1), and then inhibit platelet production and play an antithrombotic role. Aspirin is widely used in clinics as the cornerstone of primary and secondary prevention of CVDs (Welsh et al., 2019; Nudy et al., 2020; Rocca and Patrono, 2020; Mogul et al., 2021). Nevertheless, a large number of studies have shown that about 2–4% of cardiovascular ischemic events, such as myocardial infarction, stroke, and death still reoccur every year after the rational use of drugs (Eikelboom et al., 2017; Sabatine et al., 2017; Schwartz et al., 2018). Patients with CVD who are at high risk of aspirin-induced bleeding were challenged by novel evidence of aspirin tolerance poses (Santos-Gallego and Badimon, 2021). The conception of aspirin resistance (AR) has been proposed, and the improvement of AR in the treatment of ASCVDs has gradually become a research hot spot in recent years (Locāne et al., 2019).
Conception of AR and Current Research Studies
Conception of AR
Aspirin is the representative medicine of antiplatelet aggregation (Ornelas et al., 2017). However, thrombosis events still occur after patients take aspirin in clinical practice, which is called AR (Paven et al., 2020). Henry et al. (2010) evaluated the 2- to 24-h peak and trough biological efficacy of daily low-dose aspirin in 150 patients with stable coronary heart disease (CHD). Light transmittance concentration (LTA) induced by 0.5 mg/ml AA was measured. It was found that AR appeared in one quarter of the patients. At present, AR is generally defined as the expected effect of antiplatelet aggregation which does not appear after patients regularly take conventional dose of aspirin, and laboratory indicators show that platelet activity or accumulation rate is not ideal, resulting in increased risk of cardiovascular events (Paseban et al., 2020). A study conducted on 126 Asian Indian patients with stable CHD found that 36% of patients had no response to aspirin (Chadha et al., 2016). In a systematic review and meta-analysis of 65 studies involving 10,729 patients, the overall prevalence of AR in CVD patients defined by the laboratory was 24.7% (95% CI 21.4–28.4). The risk of it was higher in women than in men, with a ratio of 1.16 (95% CI 0.87–1.54) (Ebrahimi et al., 2020). These pieces of evidence suggest that AR is common clinically and may affect the therapeutic efficacy.
Detection and Significance of AR
There are no official diagnostic or regulatory guidelines for the AR concept (Ferreira et al., 2020). The determination of platelet function is a significant method to estimate the clinical prognosis of patients with AR. The in vivo platelet function test is prothrombin time. The in vitro platelet function test included the determination of the thromboxane and aspirin metabolite thromboxane B2 (TXB2) (Yassin et al., 2019). However, the AR standard most commonly used and accepted by researchers is that the average aggregation of 10 μmol/L adenosine diphosphate (ADP) is greater than 70%, and the average aggregation of 0.5 mg/ml AA is greater than 20% as proposed by Gum et al. (2001). Mohring et al. (2017) detected the formation of thromboxane induced by AA through enzyme-linked immunosorbent assay (ELISA) and the AA-induced antiplatelet effect of aspirin by the LTA method. The results showed that there was a non-linear correlation between the formation of thromboxane and the maximum value of AA-induced LTA aggregation (Spearman’s rho R = 0.7396; 95% CI 0.7208–0.7573, p < 0.0001). Receiver characteristics analysis and Youden’s J statistics showed that 209.8 ng/ml was the optimal cutoff value for thromboxane ELISA to detect high on-treatment platelet reactivity to aspirin (area under the curve: 0.92, p < 0.0001, sensitivity: 82.7%, specificity: 90.3%). This study showed that the thromboxane formation examined by ELISA is reliable for detecting high on-treatment platelet reactivity to aspirin.
The detection of AR has important implications to predict the clinical outcome of the disease. A 5-year follow-up study of 465 patients treated with aspirin found that multivariate logistic regression analysis showed a high association between AR and cardiovascular events (adjusted odds ratio, 4.28; 95% CI: 1.64 11.20; p = 0.03) (Chen and Chou, 2017). Wang et al. (2019) conducted a systematic review and meta-analysis of 35 clinical trials involving 19,025 patients with CHD to explore the relationship between the laboratory test AR and endpoint events. They found that the risk of all-cause death [7.9 vs. 2.5%, OR = 2.42 (95% CI 1.86–3.15, p < 0.00001)] and target vessel reconstruction [4.5 vs. 1.7%, OR = 2.20 (95% CI 1.19–4.08, p = 0.01)] in aspirin non-responders was higher than that in aspirin responders, indicating that AR has a good predictive effect on clinical outcomes. Li et al. conducted a systematic review of nine prospective studies including 1889 confirmed adherence patients with CHD who insisted on taking aspirin to explore the relationship between AR and the risk of major adverse cardiovascular events (MACEs). The results illustrated that the incidence of MACEs in patients with AR was significantly higher than that in patients with aspirin sensitivity (odds ratio 2.44, 95% CI 1.81 to 3.30; p < 0.000001). The risk of MACEs in the laboratory AR patients with CHD was 2.4 times higher than that in aspirin-sensitive patients (Li et al., 2014).
Mechanism of AR Formation
AR may be caused by many factors and involves multiple complex mechanisms. AR was found to be related to patients’ low compliance and insufficient drug dosage in clinics. Molecular studies have shown that the mechanisms of AR involves platelet gene polymorphism, increased platelet conversion rate, inflammation, and other mechanisms (Figure 1) (Floyd and Ferro, 2014; Du et al., 2016).
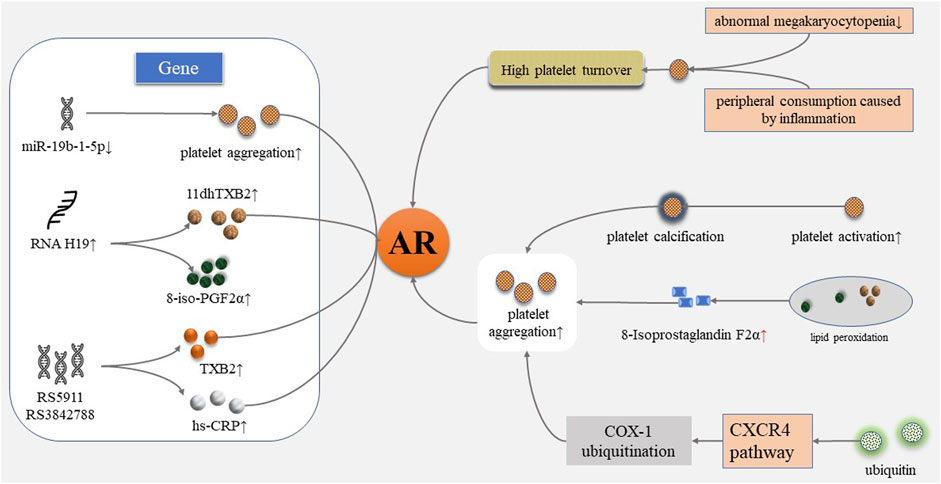
FIGURE 1. Mechanism of aspirin resistance. AR, aspirin resistance; 11dhTXB2, 11-dehydrogenation thromboxane B2; 8-iso-PGF2α, 8-iso-prostaglandin F2α; hs-CRP, high-sensitivity C-reactive protein; CXCR4, CXC chemokine receptor 4; COX-1, cyclooxygenase-1.
Patients’ Incompliance to Aspirin
Grinstein and Cannon (2012) considered that low patient adherence to aspirin was the main reason for the failure of aspirin treatment and thus affected the analysis of AR. Dawson et al. (2011) used aspirin to investigate compliance in 90 ischemic stroke patients and 90 control subjects. Platelet function was assessed using standard definitions of resistance using platelet function analyzer (PFA)-100 and rapid platelet function analyzer (RPFA) devices. Urine levels of aspirin metabolites were measured by high-performance liquid chromatography to confirm treatment adherence. The results indicated that poor compliance accounted for nearly half of the cases of aspirin failure. The key point of AR research is to evaluate the compliance objectively. Cuisset et al. (2009) recruited 136 patients who underwent coronary stenting to explore the compliance of aspirin. The maximum intensity of AA-induced aggregation (AA-Ag) during hospitalization and a month after discharge was detected; aspirin non-responders were defined as AA-Ag > 30%.19 patients (14%) were identified as non-responsive 1 month after discharge, and AA-Ag in the population was significantly higher than that during hospitalization (15.3 ± 23 vs. 7.5 ± 10%, p = 0.0004). Only one person did not respond after receiving aspirin, suggesting that these changes were due to non-adherence. Schwartz, K. A. defines this class of patients as “inadequately responsive to aspirin” and believes that such patients are at increased risk of vascular events, and suggests that future studies should focus on improving compliance and reducing the risk of future cardiovascular events (Schwartz, 2011).
Inadequate Dose of Aspirin
Doses of aspirin for primary and secondary prophylaxis of thrombosis in ASCVD vary from country to country and are usually aimed at achieving analgesic effects rather than achieving antithrombotic effects by complete acetylation of COX-1 (Linden and Tran, 2012).
Low-dose aspirin (75–150 mg) is a long-term effective antiplatelet aggregation therapy (Collaboration, 2002). Quinn, M. J. et al. compared the effects of low-dose (<150 mg) and medium-dose (≥150 mg) aspirin on the incidence of patients with unstable angina pectoris or acute myocardial infarction (AMI) within 6 months in the Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIb and Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrelin Therapy (PURSUIT) trials. It was found that high-dose aspirin was associated with a reduced incidence of MI [HR 0.79 (95% CI 0.64 to 0.98), p = 0.03] (Quinn et al., 2004). Lee, P.Y. et al. observed 468 patients with stable CHD who regularly took aspirin 80–325 mg for 4 weeks and found that the incidence of AR was higher with daily aspirin dose ≤100 mg than with daily aspirin dose of 150 and 300 mg (30.2 vs. 16.7% vs. 0%, p = 0.0062) (Lee et al., 2005). Vivas, D. et al. researched the effect of an additional dose of 100 mg aspirin on platelet function and the proportion of aspirin non-responders in 141 patients with CHD who took 100 mg aspirin on a long-term treatment using PFA-100. The incidence of aspirin non-response decreased from 50.7% (95% CI 42.4–59) to 35.0% (95% CI 27.3–43.2), illustrating that the additional dose of aspirin had a significant effect on aspirin non-response (Vivas et al., 2011). Perrier-Cornet, A. et al. observed that the AR rate decreased from 94 to 47% in patients with myeloid proliferative tumor after changing the aspirin dose from 75 mg once daily to twice daily (Perrier-Cornet et al., 2018).
The dosage of aspirin is still controversial. Haley, S.P. et al. reviewed 12 randomized controlled trials (RCTs) and found that aspirin was not dose-dependent on the incidence of bleeding or ASCVD events, indicating that the dose might not be critical (Haley and Chessman, 2020).
Platelet Receptor Gene Polymorphism
Platelet receptor gene polymorphism is considered to be closely related to platelet activation, adhesion, aggregation, and the development of thrombotic diseases (Haybar et al., 2018). Singh, S. et al. explored the expression of MiR-19b-1-5p from the buffy coat of 945 patients with acute coronary syndrome (ACS) through reverse transcription quantitative polymerase chain reaction (PCR). Platelet function was detected by multiplate aggregometry testing. The results showed that after adjusting for age, gender, race, and history of previous stroke, platelet aggregation continues in the presence of aspirin [-Log-MiR-19b-1-5p (unstandardized beta, 44.50; 95% CI, 2.20–86.80; p < 0.05)], illustrating that AR is related to the lower expression of MiR-19b-1-5p (Singh et al., 2021). Wang, J. et al. evaluated the expression of long chain non-coding RNA H19 in ischemic stroke patients in order to explore the relationship between H19 and AR. 150 acute ischemic stroke patients were recruited, and urine 11-dehydrogenation thromboxane B2 (11dhTXB2) level, plasma H19, and 8-iso-prostaglandin F2α (8-iso-PGF2α) levels were determined. The results showed that plasma H19 levels were elevated significantly in patients with AR (p = 0.0203). The H19 level was positively related with urine 11dhTXB2/creatinine (R = 0.04364, p = 0.0106) and 8-iso-PGF2α (R = 0.04561, p = 0.0089). It was considered that H19 might induce AR by increasing the production of 8-iso-PGF2α (Wang et al., 2020). Xue, M. et al. explored genetic markers in Chinese patients with chronic stable angina pectoris (SAP) after percutaneous coronary intervention (PCI). 207 subjects were recruited to receive 100 mg aspirin daily. The inhibitory effect of platelets was evaluated by LTA. TXB2 and hypersensitive C-reactive protein (hs-CRP) were determined by radioimmunoassay. Genotyping was performed using Taqmanprobe technology (rs5787 and rs5911) and gene sequencing technology (rs3842788). The results showed that rs5911 A/C, C/C and A/A genotype (OR = 5.546, 95% CI = 1.812–11.404), and rs3842788 A/G and G/G genotype (OR = 8.358, 95% CI = 2.470–28.286) were correlated with AR. TXB2 and hs-CRP were significantly increased in the AR group, and the plasma TXB2 level was significantly increased in rs3842788a/G genotype carriers. This suggested that rs5911 and rs3842788 were specific genetic markers for AR in Chinese patients with chronic SAP (Xue et al., 2017).
Increased Turnover of Platelet
The regeneration of platelet COX-1 improves the ability of circulating platelet pools to produce TXA2 and then promotes platelet aggregation (Floyd and Ferro, 2014). High platelet turnover can be triggered by reduction of abnormal megakaryocytopenia from primary thrombocythemia or peripheral consumption caused by inflammation (Coccheri, 2012). Restoration of thromboxane synthesis capacity in circulating blood reflects the number of platelets with uninhibited COX activity by aspirin. Data had shown that the time during which platelets with normal COX activity entered the circulation in diabetic patients was shorter than that of healthy individuals (Di Minno, 2011). Grove, E. L. et al. investigated the effect of platelet turnover on the antiplatelet effect of aspirin in 177 patients with stable CHD. Platelet turnover was measured by immature platelets and thrombopoietin. Platelet aggregation was measured using the VerifyNow aspirin test and MEA. The results showed that the level of immature platelets was closely related to MEA (r = 0.31–0.36, p < 0.0001), and sP-selector which is the marker of platelet activation (r = 0.19, p = 0.014). The antiplatelet effect of aspirin decreased within the increase of the platelet turnover rate, and AR was obvious consequently (Grove et al., 2011). The clinical association between accelerated platelet turnover and AR increased the risk of thrombotic disease events (Di Minno et al., 2012).
Inflammation
Chronic inflammation is the underlying pathological mechanism of atherosclerosis-induced CVDs. The complex process involves the interaction of vascular endothelial cells, immune cells, and molecular transmitters, and promotes the development of atherosclerotic thrombotic disease by activating the rupture of atherosclerotic plaque (Shah, 2019). Inflammatory cytokines contribute to the formation of AR through platelet transformation, activation, and adhesion processes (Du et al., 2016). The pre-thrombotic state can be generated by the production and release of thromboxane A2 due to the increased expression of cyclooxygenase-2, which is associated with inflammation (Yalcinkaya and Celik, 2014). Reactivity to aspirin therapy may be reduced by inflammation-induced AR (Shan et al., 2010).
Other Mechanisms
Kyyak, Y. H. et al. observed the functional status and ultrastructure of platelets in 36 patients with ACS under electron microscopy. The majority of platelets were found to be under an activated state, with pseudopedence, partial platelet aggregation, osmiophilism, vacuolation, and even calcification and apoptosis. Therefore, the researchers considered that AR may be caused by the degenerative changes and calcification of platelets (Kyyak et al., 2019).
Recent studies have shown that 8-isoprostaglandin F2α induced by oxidative stress can mediate the occurrence of AR. 8-Isoprostaglandin F2α as an agonist binding to the thromboxane platelet receptor and promoting vasoconstriction and platelet activation. 8-Isoprostaglandin F2α produced by the lipid peroxidation pathway is independent of COX activity, which means it is not affected by aspirin (Bauer et al., 2014). Therefore, platelet aggregation still occurs after TXA2 is inhibited by aspirin and leads to AR (Guo et al., 2019).
Tan, C. et al. observed platelet function and serum ubiquitin levels in 250 patients with AMI before and after taking aspirin to explore the possible mechanism of AR. They found that AR occurred in 47 patients, and serum ubiquitin levels were higher in AR patients than in healthy patients. Patients with high serum ubiquitin levels showed high levels of platelet ubiquitination, ubiquitinated proteins, and ubiquitinated cyclooxygenase-1. Serum ubiquitin promoted COX-1 ubiquitination through the CXC chemokine receptor 4 (CXCR4) pathway and prevented aspirin from acetylation of its target in vitro studies, thereby reducing the antiplatelet effect of aspirin, which might be involved in the mechanism of AR (Tan et al., 2015). In addition, there are also drug interactions and the influence of patient comorbidities in clinical practice which deserve more exploration.
Current Clinical Researches on AR
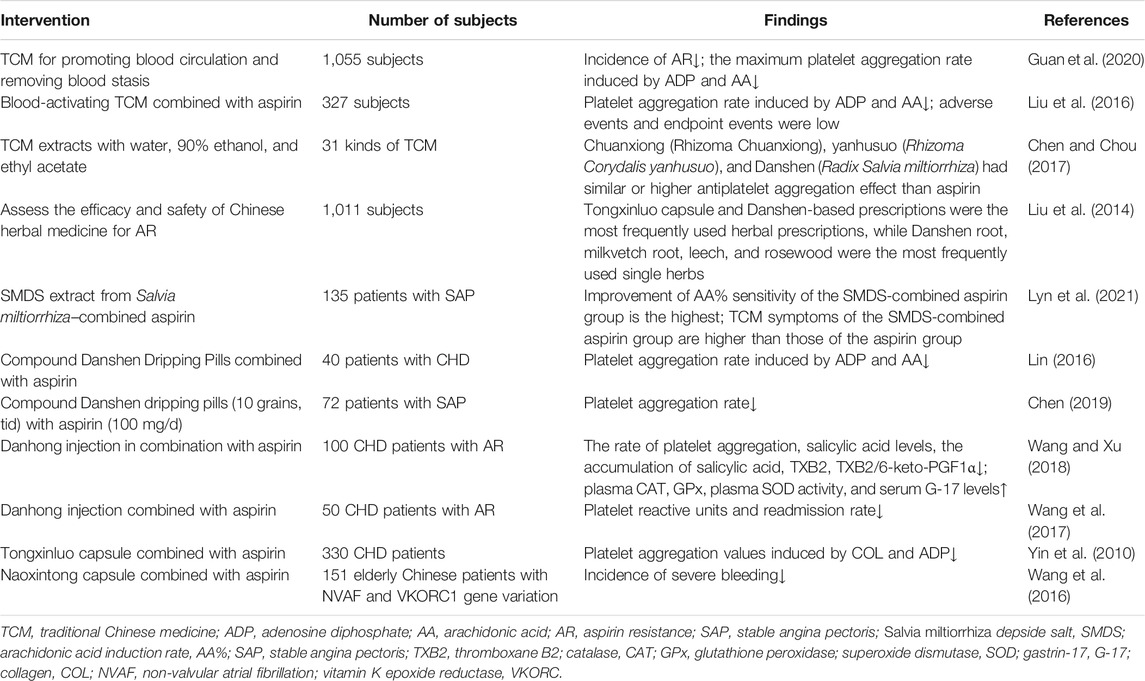
Olas, B. investigated the antiplatelet effects of fish and vegetable oils and their constituent fatty acids. Studies in patients with a variety of CVDs have shown that both fish and vegetable oils contain protective components with antiplatelet activity. And, vegetable oils contain compounds known as phytosterols that protect the heart from hypercholesterolemia. Therefore, the author considered that vegetable oils might play a key role in the prevention and treatment of CVDs associated with platelet overactivation (Olas, 2020). Al-Azzam, S. I. et al. conducted a cross-sectional study of 418 patients taking aspirin and found that the use of statin was associated with the improvement of AR (Al-Azzam et al., 2012). Flavonoids have been found to have antiplatelet activity. In recent years, they have been focused on research and considered favorable drug candidates in the future (Khan et al., 2018). Some researchers considered that lifestyle changes such as smoking cessation, exercise, and weight loss might improve aspirin response, and prevention and treatment of complications related to AR such as hyperlipidemia, diabetes, and hypertension might also help improve AR (Kasmeridis et al., 2013; Ardeshna et al., 2019). In addition, other antiplatelet drugs, such as clopidogrel and P2Y12 inhibitor ticagrelor in combination with aspirin have also been studied (Divani et al., 2013). TCM treatment is based on a holistic perspective and treats the patient as a whole rather than just targeting a certain disease. In recent years, studies on the improvement of AR by TCM have been paid more and more attention, and the advantages of TCM have been reflected in clinical studies (Table 1) (Chen, 2019).
Researches on Improving AR by Blood-Activating Herbs Combined With Aspirin
Low compliance, insufficient dose, and the interaction between non-steroidal inflammatory drugs (NSAIDs) and platelets are important factors leading to AR and the risk of thrombus. Blood-activating herbs have good effects on improving microcirculation, antiplatelet aggregation, and anti-thrombosis in CVDs (Wang et al., 2014).
A systematic review of 1,055 subjects studying the improvement of AR by TCM for promoting blood circulation and removing blood stasis showed that the incidence of AR [RR = 0.41, 95% CI (0.32,0.52), p < 0.00001], the maximum platelet aggregation rate induced by ADP [MD = −6.20, 95% CI (−7.83, −4.57),p < 0.0001], and the maximum platelet aggregation rate induced by AA [MD = −4.8,95% CI (−8.16, −1.44), p = 0.005] were significantly decreased after treatment, indicating a very positive efficacy of blood-activating herbs in improving AR (Guan et al., 2020). Liu et al. conducted a meta-analysis of the RCTs of blood-activating TCM combined with aspirin versus aspirin alone in the treatment of AR and found that the combined therapy significantly reduced the ADP-induced platelet aggregation rate [SMD = −1.78, 95% CI (−2.95, 0.61), p < 0.003] and AA-induced platelet aggregation rate [SMD = −2.31, 95% CI (−3.41, −1.21), p < 0.0001], and there was no increase in endpoint events [R = 0.26, 95% CI (0.05, 1.35), p > 0.05], indicating the good safety of blood-activating TCM (Liu et al., 2016). Chen Cen. et al. prepared 31 kinds of TCM extracts with water, 90% ethanol, and ethyl acetate. The antiplatelet aggregation effects of various TCM for blood activation and stasis removal were measured on the platelet aggregator in vitro using 5′-ADP, bovine thrombin, and AA as inducers, respectively, and aspirin was taken as the positive control. It was found that Chuanxiong (Rhizoma Chuanxiong), yanhusuo (Rhizoma Corydalis yanhusuo), and Danshen (Radix Salvia miltiorrhiza) had at least similar or higher antiplatelet aggregation effect than the aspirin group (Cen et al., 2017). Liu et al. conducted a systematic review of 16 RCTs with a total of 1,011 subjects to evaluate the therapeutic effect of Chinese herbal medicine on AR. The results indicated that Tongxinluo capsules and prescriptions based on Danshen (Radix Salvia miltiorrhiza) were the most commonly used TCM prescriptions, and the most commonly used single TCM included Danshen (Radix Salvia miltiorrhiza), Leech (Whitmania pigra Whitman), and Rosewood (Pterocarpus indicus Willd). It was suggested that Chinese herbal medicines as potential agents for improving AR merit further more rigorous designs of RCTs to provide further evidence (Liu et al., 2014).
Lyu, J. et al. explored the curative effect of the Salvia Miltiorrhiza Depside Salt (SMDS) extract from Danshen (Radix Salvia miltiorrhiza) for SAP. A total of 135 subjects were randomly assigned to the SMDS group, aspirin group, and SMDS-combined aspirin group. Thromboelastography, visual analog scale score of TCM symptoms, and platelet aggregation detected by light transmittance aggregometry were determined at baseline and after 10-day treatment, respectively. The results showed that the improvement of arachidonic acid induction rate (AA%) sensitivity of the SMDS-combined aspirin group was the highest among the three groups [p < 0.001, 95% CI (0.00–0.00)], and TCM symptoms of the SMDS-combined aspirin group was higher than those of the aspirin group [MD = 1.71, 95% CI (0.15–3.27), p = 0.032]. Those findings indicated that SMDS combined with aspirin is an effective intervention for SAP (Lyu et al., 2021).
Research on compound Danshen dripping pills combined with aspirin in treating patients with CHD showed that the platelet aggregation rate induced by ADP and AA was lower than that of aspirin alone (74.2 ± 1.4 vs. 70.1 ± 1.3, p < 0.05; 26.4 ± 5.3 vs. 24.3 ± 3.1, p < 0.05), illustrating the high clinical application value (Lin, 2016). Chen et al. recruited 72 patients with SAP who took aspirin (100 mg/d) continuously for more than 4 weeks and were confirmed as AR by thrombelastography. The control group took 100 mg/d orally according to the original dose, and the experimental group took compound Danshen dripping pills (10 grains, tid) additionally. After 1-month treatment, the platelet aggregation rate of the experimental group was significantly lower than that of the control group [(69 ± 6)% vs. (44 ± 5)%, p < 0.05], suggesting that the compound Danshen dripping pill has a preferable effect on reducing platelet aggregation (Si-rui et al., 2016).
Wang et al. observed the effect of Danhong injection on the antiplatelet effect of aspirin and gastric mucosa damage in patients with CHD. Danhong injection in combination with aspirin decreased the rate of platelet aggregation, the salicylic acid levels, and the accumulation of salicylic acid, TXB2, and TXB2/6-keto-PGF1α. The activity of plasma catalase (CAT), glutathione peroxidase (GPx), and plasma superoxide dismutase (SOD), and the serum gastrin-17 (G-17) level were higher than those of the control group (p < 0.05), suggesting that the combination of aspirin strengthened the inhibition of COX-1, promoted gastric mucus secretion, and enhanced the body’s antioxidant capacity (Wang and Xu, 2018). In addition, Danhong injection combined with aspirin was also found to reduce platelet reactive units (540.39 ± 54.39 vs. 654.49 ± 39.48, p < 0.01.) and readmission rate (20 vs. 40%, p = 0.029) in CHD patients with AR (Wang et al., 2017).
Yin et al. observed the Tongxinluo capsule on platelet aggregation in AR patients with CHD. Platelet aggregation values were determined by collagen (COL) and ADP as inducers. Results showed that after 1-month treatment, platelet aggregation values of the Tongxinluo capsule group and Tongxinluo capsule–combined aspirin group were significantly decreased, while those of the aspirin group did not, suggesting that Tongxinluo capsule inhibits the function of platelets and prevented the progress of the disease (Yin et al., 2010).
Wang et al. compared the effect of Naoxintong capsule combined with aspirin with warfarin at adjusted doses on thrombus risk in elderly Chinese patients with non-valvular atrial fibrillation (NVAF) and vitamin K epoxide reductase (VKORC1) gene variation. It was observed that combination therapy reduced the incidence of severe bleeding (0 vs. 7.9%, OR: 0.921, 95% CI: 0.862–0.984, p = 0.028), demonstrating the antithrombotic effect of blood-activating herbs (Wang et al., 2016).
Potential Mechanisms on Improving AR by Blood-Activating Herbs Combined With Aspirin
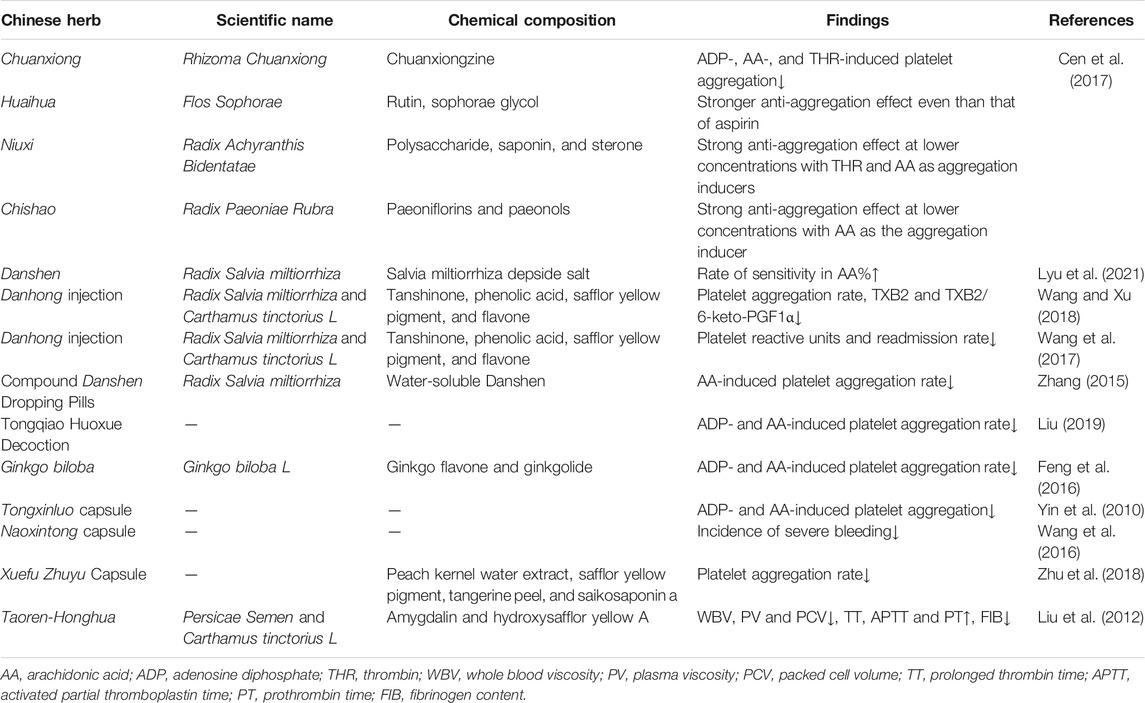
The mechanism of traditional Chinese medicine to improve aspirin resistance is not clear. The studies of Chinese herbal medicine that have been tested for aspirin resistance are shown in Table 2.
Lai et al. explored the potential active ingredients and mechanism of Danhong injection in improving AR based on network pharmacology. The Traditional Chinese Medicine Database and Analysis Platform was used to collect the main active components and action targets of Danshen (Salviae miltiorrhizae radix et rhizome) and Honghua (Carthamus tinctorius L.). The main active ingredients were screened, and 60 active ingredients were obtained, including 51 components of Danshen (Salviae miltiorrhizae radix et rhizome), 11 components of Honghua (Carthamus tinctorius L.), two components of Danshen (Salviae miltiorrhizae radix et rhizome) and Honghua (Carthamus tinctorius L.), and 159 target genes. The results of gene enrichment analysis suggested that Danhong injection mainly improved AR through multicomponent, multi-target, and multichannel pathways involving coagulation process, inflammatory response, and metabolic regulation (Lai et al., 2019).
Xue et al. observed the effect of Xuefu Zhuyu Capsule on AR and explored its mechanism. Patients with AR were randomly divided into three groups: aspirin high-dose group (300 mg/d, qd), Xuefu Zhuyu Capsule group (six grains each time, bid), and Xuefu Zhuyu Capsule (six grains each time, bid) in combination with aspirin (100 mg/d, qd). AA and platelet aggregation rate, TXB2, 6-Keto-prostaglandin F 1 alpha (6-Keto-PGF1α), and hs-CRP induced by ADP were measured. The platelet aggregation rate in the combination group (AA-induced: 23.57 ± 4.1 vs. 25.76 ± 3.76; ADP-induced: 72.18 ± 7.57 vs. 77.01 ± 9.83), TXB2 (279.81 ± 52.49 vs. 304.53 ± 47.3), and Hs-CRP (3.69 ± 0.99 vs. 4.3 ± 1.24) was significantly lower than that in the aspirin group. The effect of increasing 6-Keto-PGF1α in the combination group was better than that of the high-dose aspirin group. The mechanism of Xuefu Zhuyu Capsule to improve AR was considered to be associated with TXB2, hs-CRP, and 6-Keto-PGF1α (Zhu et al., 2018).
Taoren (Persicae Semen) and Honghua (Carthamus tinctorius L.), which is also called Taoren-Honghua (TH), is a commonly used herb group for promoting blood circulation and removing blood stasis in TCM. Liu et al. explored the effects of its main components amygdalin and hydroxysafflor yellow A (HSYA) on hemorheology in rats. The intervention methods include TH, amygdalin, HSYA, amygdalin combined with HSYA, and aspirin. Rats were administered every 12 h. After the fifth administration, during the interval between the two injections of adrenaline hydrochloride, the rats except the control group with blood stasis syndrome were placed in ice water to establish the model; blood samples were collected 30 min after the last administration the next day. The results indicated that TH significantly reduced whole blood viscosity (WBV), plasma viscosity (PV), and packed cell volume (PCV); prolonged thrombin time (TT), activated partial thromboplastin time (APTT), and increased prothrombin time (PT); and decreased fibrinogen content (FIB). Amygdalin mainly reduced PV and FIB and prolonged APTT while HSYA mainly reduced WBV and PV. This reveals that TH plays a synergistic role in reducing blood viscosity and improving coagulation parameters (Liu et al., 2012).
Conclusion and Prospective
Platelet activation and coagulation regulation play an important role in vascular injury and atherosclerotic thrombus events (Tomaiuolo et al., 2017). Aspirin as an antiplatelet drug is an important part of the secondary prevention of atherosclerotic thrombosis; however, the antiplatelet aggregation effect of aspirin is different among the crowd. AR is defined as the state in which platelet reactivity does not reach the ideal reduction after aspirin treatment (Ping-ping et al., 2019). Research had shown that AR is an independent predictor of cardiovascular adverse risk (Pasala et al., 2016). In recent years, more and more studies have been conducted on AR, and the improvement of AR may become a research hot spot in the future (Xue et al., 2014; Al-Jabi, 2017; AJin et al., 2019; Baş et al., 2020).
It has been found that the combination of blood-activating herbs and aspirin can improve AR and reduce the rate of platelet aggregation (Youlong and Zhongjun, 2014). Monomers including Danshen (Radix Salvia miltiorrhiza) and Honghua (Carthamus tinctorius L.) and compounds including Danshen Dropping Pills, Danhong injection, Tongxinluo capsule, Naoxintong capsule, and Xuefu Zhuyu capsule have advantages in antiplatelet aggregation, which were the research hot spots in recent years. However, the effectiveness of blood-activating TCM in CVDs still needs further rigorous large-scale clinical trials to provide further strong evidence. There are many studies on the role of blood-activating traditional Chinese medicine, but scattered studies have not formed a unified therapeutic target network. Researchers have used different computational approaches to construct network models of Chinese herbal medicine to explore the interaction of ingredients of disease pathways (Jafari et al., 2020; Wang et al., 2021). The network model construction of Chinese herbal medicine may further reveal the intervention targets and potential treatment orientation of diseases. The mechanism of blood-activating herbs on improving AR remains unclear, and there is still a lack of relevant research at home and abroad. Studies have found that blood-activating herbs may improve AR through multicomponent, multi-target, and multichannel pathways such as coagulation process, inflammatory reaction, and metabolic regulation, which are worthy of further exploration and may become a potential target for future treatment.
Author Contributions
MW designed and directed the manuscript. YZ wrote the manuscript. SY revised the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81202805 and 82074254), the Beijing Natural Science Foundation (No.7172185).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
AJin, C., Jin, C. M., Young-Ki, L., Hoon, H. C., Koo, J. R., Yoon, J. W., et al. (2019). Effects of Aspirin Resistance and Mean Platelet Volume on Vascular Access Failure in Hemodialysis Patients. Korean J. Intern. Med. 34, 1304–1312. doi:10.3904/kjim.2018.111
Al-Azzam, S. I., Alzoubi, K. H., Khabour, O., Alowidi, A., and Tawalbeh, D. (2012). The Prevalence and Factors Associated with Aspirin Resistance in Patients Premedicated with Aspirin. Acta Cardiol. 67, 445–448. doi:10.1080/ac.67.4.2170686
Al-Jabi, S. W. (2017). Global Trends in Aspirin Resistance-Related Research from 1990 to 2015: A Bibliometric Analysis. Basic Clin. Pharmacol. Toxicol. 5, 1–19. doi:10.1111/bcpt.12840
Ardeshna, D., Khare, S., Jagadish, P. S., Bhattad, V., Cave, B., and Khouzam, R. N. (2019). The Dilemma of Aspirin Resistance in Obese Patients. Ann. Transl Med. 7, 404. doi:10.21037/atm.2019.07.52
Baş, H. A., Aksoy, F., Bağcı, A., Varol, E., and Altınbaş, A. (2020). Incidence of Aspirin Resistance Is Higher in Patients with Acute Coronary Syndrome and Atrial Fibrillation Than without Atrial Fibrillation. Rev. Assoc. Med. Bras 66, 800–805. doi:10.1590/1806-9282.66.6.800
Bauer, J., Ripperger, A., Frantz, S., Ergün, S., Schwedhelm, E., and Benndorf, R. A. (2014). Pathophysiology of Isoprostanes in the Cardiovascular System: Implications of Isoprostane-Mediated Thromboxane A2 Receptor Activation. Br. J. Pharmacol. 171, 3115–3131. doi:10.1111/bph.12677
Cen, C., Fengqin, W., Wen, X., Zhining, X., Guang, H., Jianbo, W., et al. (2017). Effect on Platelet Aggregation Activity: Extracts from 31 Traditional Chinese Medicines with the Property of Activating Blood and Resolving Stasis. J. Traditional Chin. Med. 37, 64–75. doi:10.1016/s0254-6272(17)30028-6
Chadha, D. S., Sumana, B., Karthikeyan, G., Jayaprasad, V., and Arun, S. S. (2016). Prevalence of Aspirin Resistance in Asian-Indian Patients with Stable Coronary Artery Disease. Catheter Cardiovasc. Interv. 88, E126–E131. doi:10.1002/ccd.25420
Chen, H. (2019). Integrative Medicine on Optimizing Clopidogrel and Aspirin Therapy. Chin. J. Integr. Med. 25, 395–400. doi:10.1007/s11655-017-2551-4
Chen, H. Y., and Chou, P. (2017). Pfa-100-measured Aspirin Resistance Is the Predominant Risk Factor for Hospitalized Cardiovascular Events in Aspirin-Treated Patients: A 5-year Cohort Study. J. Clin. Pharm. Ther., 1–7.
Coccheri, S. (2012). Antiplatelet Therapy: Controversial Aspects. Thromb. Res. 129, 225–229. doi:10.1016/j.thromres.2011.10.036
Collaboration, A. T. (2002). Collaborative Meta-Analysis of Randomised Trials of Antiplatelet Therapy for Prevention of Death, Myocardial Infarction, and Stroke in High Risk Patients. BMJ 324, 71–86. doi:10.1136/bmj.324.7329.71
Cuisset, T., Frere, C., Quilici, J., Gaborit, B., Bali, L., Poyet, R., et al. (2009). Aspirin Noncompliance Is the Major Cause of "aspirin Resistance" in Patients Undergoing Coronary Stenting. Am. Heart J. 157, 889–893. doi:10.1016/j.ahj.2009.02.013
Dawson, J., Quinn, T., Rafferty, M., Higgins, P., Ray, G., Lees, K. R., et al. (2011). Aspirin Resistance and Compliance with Therapy. Cardiovasc. Ther. 29, 301–307. doi:10.1111/j.1755-5922.2010.00188.x
Di Minno, G. (2011). Aspirin Resistance and Platelet Turnover: A 25-year Old Issue. Nutr. Metab. Cardiovasc. Dis. 21, 542–545. doi:10.1016/j.numecd.2011.04.002
Di Minno, M. N., Lupoli, R., Palmieri, N. M., Russolillo, A., Buonauro, A., and Di Minno, G. (2012). Aspirin Resistance, Platelet Turnover, and Diabetic Angiopathy: A 2011 Update. Thromb. Res. 129, 341–344. doi:10.1016/j.thromres.2011.11.020
Divani, A. A., Zantek, N. D., Borhani-Haghighi, A., and Rao, G. H. (2013). Antiplatelet Therapy: Aspirin Resistance and All that Jazz!. Clin. Appl. Thromb. Hemost. 19, 5–18. doi:10.1177/1076029612449197
Du, G., Lin, Q., and Wang, J. (2016). A Brief Review on the Mechanisms of Aspirin Resistance. Int. J. Cardiol. 220, 21–26. doi:10.1016/j.ijcard.2016.06.104
Ebrahimi, P., Farhadi, Z., Behzadifar, M., Shabaninejad, H., Abolghasem Gorji, H., Taheri Mirghaed, M., et al. (2020). Prevalence Rate of Laboratory Defined Aspirin Resistance in Cardiovascular Disease Patients: A Systematic Review and Meta-Analysis. Caspian J. Intern. Med. 11, 124–134. doi:10.22088/cjim.11.2.124
Eikelboom, J. W., Connolly, S. J., Bosch, J., Dagenais, G. R., Hart, R. G., Shestakovska, O., et al. (2017). Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 377, 1319–1330. doi:10.1056/NEJMoa1709118
Feng, Y., Yao, M. H., and Wu, L. J. (2016). Effect of Ginkgo Biloba on Platlete Aggregation Rate and Aspirin Resistance in Patients with Acute Cerebral Infractions. Chin. J. Coal Industry Med. 19, 1476–1479.
Ferreira, M., Freitas-Silva, M., Assis, J., Pinto, R., Nunes, J. P., and Medeiros, R. (2020). The Emergent Phenomenon of Aspirin Resistance: Insights from Genetic Association Studies. Pharmacogenomics 21, 125–140. doi:10.2217/pgs-2019-0133
Floyd, C. N., and Ferro, A. (2014). Mechanisms of Aspirin Resistance. Pharmacol. Ther. 141, 69–78. doi:10.1016/j.pharmthera.2013.08.005
Grinstein, J., and Cannon, C. P. (2012). Aspirin Resistance: Current Status and Role of Tailored Therapy. Clin. Cardiol. 35, 673–681. doi:10.1002/clc.22031
Grove, E. L., Hvas, A. M., Mortensen, S. B., Larsen, S. B., and Kristensen, S. D. (2011). Effect of Platelet Turnover on Whole Blood Platelet Aggregation in Patients with Coronary Artery Disease. J. Thromb. Haemost. 9, 185–191. doi:10.1111/j.1538-7836.2010.04115.x
Guan, B. Y., Gao, J., and Ma, X. J. (2020). Clinical Efficacy of Blood-Activating and Stasis-Removing Chinese Medicines on Aspirin Resistance: A Meta-Analysis Chinese. J. Integr. Med. Cardio-cerebrovascular Dis. 18, 6–12.
Gum, P. A., Kottke-Marchant, K., Poggio, E. D., Gurm, H., Welsh, P. A., Brooks, L., et al. (2001). Profile and Prevalence of Aspirin Resistance in Patients with Cardiovascular Disease. Am. J. Cardiol. 88, 230–235. doi:10.1016/s0002-9149(01)01631-9
Guo, J., Wang, J., and Feng, J. (2019). Aspirin Resistance Mediated by Oxidative Stress-Induced 8-isoprostaglandin F2. J. Clin. Pharm. Ther. 44, 823–828. doi:10.1111/jcpt.12838
Haley, S. P., and Chessman, A. (2020). Treatment Effect of Aspirin for Primary Prevention Does Not Differ According to Baseline Ascvd Risk. Ann. Intern. Med. 173, JC39–39. doi:10.7326/ACPJ202010200-039
Haybar, H., Khodadi, E., Zibara, K., and Saki, N. (2018). Platelet Activation Polymorphisms in Ischemia. Cardiovasc. Hematol. Disord. Drug Targets 18, 153–161. doi:10.2174/1871529X18666180326121239
Henry, P., Vermillet, A., Boval, B., Guyetand, C., Petroni, T., Dillinger, J. G., et al. (2010). 24-hour Time-dependent Aspirin Efficacy in Patients with Stable Coronary Artery Disease. Thromb. Haemost. 105, 336–344. doi:10.1160/TH10-02-0082
Jafari, M., Wang, Y., Amiryousefi, A., and Tang, J. (2020). Unsupervised Learning and Multipartite Network Models: A Promising Approach for Understanding Traditional Medicine. Front. Pharmacol. 11, 1319. doi:10.3389/fphar.2020.01319
Kannan, M., Ahmad, F., and Saxena, R. (2019). Platelet Activation Markers in Evaluation of Thrombotic Risk Factors in Various Clinical Settings. Blood Rev. 37, 1–9. doi:10.1016/j.blre.2019.05.007
Kasmeridis, C., Apostolakis, S., and Lip, G. Y. (2013). Aspirin and Aspirin Resistance in Coronary Artery Disease. Curr. Opin. Pharmacol. 13, 242–250. doi:10.1016/j.coph.2012.12.004
Khan, H., Jawad, M., Kamal, M. A., Baldi, A., Xiao, J., Nabavi, S. M., et al. (2018). Evidence and Prospective of Plant Derived Flavonoids as Antiplatelet Agents: Strong Candidates to Be Drugs of Future. Food Chem. Toxicol. 119, 355–367. doi:10.1016/j.fct.2018.02.014
Khodadi, E. (2020). Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules. Cardiovasc. Toxicol. 20, 1–10. doi:10.1007/s12012-019-09555-4
Kyyak, Y. H., Barnett, O. Y., Halkevych, M. P., Labinska, O. Y., Kyyak, H. Y., Kysil, O. Y., et al. (2019). Impact of Risk Factors of Ischemic Heart Disease on the Development of Acute Coronary Syndrome, Platelet Ultrastructure, and Aspirin Resistance. Wiad Lek 72, 1–9.
Lai, R. M., Ju, J. Q., Zhao, Y. H., and Xu, H. (2019). Network Pharmacology-Based Study on Mechanisms of Danhong Injection in Treatment of Aspirin Resistance. Zhongguo Zhong Yao Za Zhi 44, 2719–2726. doi:10.19540/j.cnki.cjcmm.20190215.001
Lee, P. Y., Chen, W. H., Ng, W., Cheng, X., Kwok, J. Y., Tse, H. F., et al. (2005). Low-dose Aspirin Increases Aspirin Resistance in Patients with Coronary Artery Disease. Am. J. Med. 118, 723–727. doi:10.1016/j.amjmed.2005.03.041
Li, J., Song, M., Jian, Z., Guo, W., Chen, G., Jiang, G., et al. (2014). Laboratory Aspirin Resistance and the Risk of Major Adverse Cardiovascular Events in Patients with Coronary Heart Disease on Confirmed Aspirin Adherence. J. Atheroscler. Thromb. 21, 239–247. doi:10.5551/jat.19521
Lin, B. (2016). Clinical Effect of Compound Salvia Miltiorrhiza Dropping Pills on Aspirin Resistance of Coronary Heart Disease. World Latest Med. Inf. (Electronic Version) 16, 122–123.
Linden, M. D., and Tran, H. A. (2012). Overcoming Aspirin Treatment Failure in Diabetes. Crit. Rev. Clin. Lab. Sci. 49, 183–198. doi:10.3109/10408363.2012.731377
Liu, A. J., Li, H. Q., Li, J. H., Wang, Y. Y., Chen, D., Wang, Y., et al. (2014). Chinese Herbal Medicine for Aspirin Resistance: A Systematic Review of Randomized Controlled Trials. Evid. Based Complement. Alternat Med. 2014, 1–16. doi:10.1155/2014/890950
Liu, L., Duan, J. A., Tang, Y., Guo, J., Yang, N., Ma, H., et al. (2012). Taoren-honghua Herb Pair and its Main Components Promoting Blood Circulation through Influencing on Hemorheology, Plasma Coagulation and Platelet Aggregation. J. Ethnopharmacol 139, 381–387. doi:10.1016/j.jep.2011.11.016
Liu, Q. Q., Jiang, K., and Chen, X. H. (2016). Meta-analysis of Aspirin Combined with Traditional Chinese Medicine to Promote Blood Circulation and Remove Blood Stasis on Aspirin Resistance. J. Yunnan Univ. Traditional Chin. Med. 39, 54–58.
Liu, W. C. (2019). Preventive Effect of Tongqiao Huoxue Decoction on Aspirin Resistance in Elderly Patients with Coronary Heart Disease. Chin. Med. Sci. Technol. 26, 106–107.
Locāne, S., Pūcīte, E., Miglāne, E., Millers, A., Novasa, A., Levina, R., et al. (2019). Antiplatelet Resistance in Patients with Atherosclerosis. Proc. Latvian Acad. Sci. Section B Nat. Exact, Appl. Sci. 73, 373–378. doi:10.2478/prolas-2019-0058
Lyu, J., Xue, M., Li, J., Lyu, W., Wen, Z., Yao, P., et al. (2021). Clinical Effectiveness and Safety of Salvia Miltiorrhiza Depside Salt Combined with Aspirin in Patients with Stable Angina Pectoris: A Multicenter, Pragmatic, Randomized Controlled Trial. Phytomedicine 81, 153419–153512. doi:10.1016/j.phymed.2020.153419
Mogul, A., Leppien, E. E., Laughlin, E., and Spinler, S. A. (2021). Aspirin for Primary Prevention of Cardiovascular Disease: A Review of Recent Literature and Updated Guideline Recommendations. Expert Opin. Pharmacother. 22, 83–91. doi:10.1080/14656566.2020.1817389
Mohring, A., Piayda, K., Dannenberg, L., Zako, S., Schneider, T., Bartkowski, K., et al. (2017). Thromboxane Formation Assay to Identify High On-Treatment Platelet Reactivity to Aspirin. Pharmacology 100, 127–130. doi:10.1159/000477303
Nudy, M., Cooper, J., Ghahramani, M., Ruzieh, M., Mandrola, J., and Foy, A. J. (2020). Aspirin for Primary Atherosclerotic Cardiovascular Disease Prevention as Baseline Risk Increases: A Meta-Regression Analysis. Am. J. Med. 133, 1056–1064. doi:10.1016/j.amjmed.2020.04.028
Olas, B. (2020). Biochemistry of Blood Platelet Activation and the Beneficial Role of Plant Oils in Cardiovascular Diseases. Adv. Clin. Chem. 95, 219–243. doi:10.1016/bs.acc.2019.08.006
Ornelas, A., Zacharias-Millward, N., Menter, D. G., Davis, J. S., Lichtenberger, L., Hawke, D., et al. (2017). Beyond Cox-1: The Effects of Aspirin on Platelet Biology and Potential Mechanisms of Chemoprevention. Cancer Metastasis Rev. 36, 289–303. doi:10.1007/s10555-017-9675-z
Pasala, T., Hoo, J. S., Lockhart, M. K., Waheed, R., Sengodan, P., Alexander, J., et al. (2016). Aspirin Resistance Predicts Adverse Cardiovascular Events in Patients with Symptomatic Peripheral Artery Disease. Tex. Heart Inst. J. 43, 482–487. doi:10.14503/THIJ-14-4986
Paseban, M., Marjaneh, R. M., Banach, M., Riahi, M. M., Bo, S., and Sahebkar, A. (2020). Modulation of Micrornas by Aspirin in Cardiovascular Disease. Trends Cardiovasc. Med. 30, 249–254. doi:10.1016/j.tcm.2019.08.005
Paven, E., Dillinger, J. G., Bal Dit Sollier, C., Vidal-Trecan, T., Berge, N., Dautry, R., et al. (2020). Determinants of Aspirin Resistance in Patients with Type 2 Diabetes. Diabetes Metab. 46, 370–376. doi:10.1016/j.diabet.2019.11.002
Perrier-Cornet, A., Ianotto, J. C., Mingant, F., Perrot, M., Lippert, E., and Galinat, H. (2018). Decreased Turnover Aspirin Resistance by Bidaily Aspirin Intake and Efficient Cytoreduction in Myeloproliferative Neoplasms. Platelets 29, 723–728. doi:10.1080/09537104.2017.1361018
Ping-ping, G., Xiao-xia, C., and Xiao-rong, W. (2019). Progress in the Mechanism and Clinical Treatment of Antiplatelet Resistance of Aspirin and Clopidogrel. Chin. J. Clin. Neurosci. 27, 321–328.
Quinn, M. J., Aronow, H. D., Califf, R. M., Bhatt, D. L., Sapp, S., Kleiman, N. S., et al. (2004). Aspirin Dose and Six-Month Outcome after an Acute Coronary Syndrome. J. Am. Coll. Cardiol. 43, 972–978. doi:10.1016/j.jacc.2003.09.059
Rocca, B., and Patrono, C. (2020). Aspirin in the Primary Prevention of Cardiovascular Disease in Diabetes Mellitus: A New Perspective. Diabetes Res. Clin. Pract. 160, 108008. doi:10.1016/j.diabres.2020.108008
Sabatine, M. S., Giugliano, R. P., Keech, A. C., Honarpour, N., Wiviott, S. D., Murphy, S. A., et al. (2017). Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 3766, 1713–1722. doi:10.1056/NEJMoa1615664
Santos-Gallego, C. G., and Badimon, J. (2021). Overview of Aspirin and Platelet Biology. Am. J. Cardiol. 144 (Suppl. 1), 2–9. doi:10.1016/j.amjcard.2020.12.018
Schwartz, G. G., Steg, P. G., Szarek, M., Bhatt, D. L., Bittner, V. A., Diaz, R., et al. (2018). Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 379, 2097–2107. doi:10.1056/NEJMoa1801174
Schwartz, K. A. (2011). Aspirin Resistance: A Clinical Review Focused on the Most Common Cause, Noncompliance. Neurohospitalist 1, 94–103. doi:10.1177/1941875210395776
Shah, P. K. (2019). Inflammation, Infection and Atherosclerosis. Trends Cardiovasc. Med. 29, 468–472. doi:10.1016/j.tcm.2019.01.004
Shan, Y., Zhao, R., Geng, W., Lin, N., Wang, X., Du, X., et al. (2010). Protective Effect of Sulforaphane on Human Vascular Endothelial Cells against Lipopolysaccharide-Induced Inflammatory Damage. Cardiovasc. Toxicol. 10, 139–145. doi:10.1007/s12012-010-9072-0
Si-rui, C., Li-hua, Z., and Jun1, L. (2016). Risk Factors and Impact of Danshen Compound Dripping Pill on Aspirin Resistance in Patients with Stable Angina Pectoris. Chin. Heart J. 28, 435–438.
Singh, S., de Ronde, M. W. J., Creemers, E. E., Van der Made, I., Meijering, R., Chan, M. Y., et al. (2021). Low Mir-19b-1-5p Expression Is Related to Aspirin Resistance and Major Adverse Cardio- Cerebrovascular Events in Patients with Acute Coronary Syndrome. J. Am. Heart Assoc. 10, e017120–9. doi:10.1161/JAHA.120.017120
Tan, C., Lu, X., Chen, W., and Chen, S. (2015). Serum Ubiquitin via Cxc Chemokine Receptor 4 Triggered Cyclooxygenase-1 Ubiquitination Possibly Involved in the Pathogenesis of Aspirin Resistance. Clin. Hemorheol. Microcirc. 61, 59–81. doi:10.3233/CH-141900
The Interpretation of, (2017). The Interpretation of “Aspirin Use in Patients with Atherosclerotic Cardiovascular Disease: the 2016 Chinese Expert Consensus Statement”. Zhonghua Nei Ke Za Zhi, 56, 4–6. doi:10.3760/cma.j.issn.0578-1426.2017.01.002
Tomaiuolo, M., Brass, L. F., and Stalker, T. J. (2017). Regulation of Platelet Activation and Coagulation and its Role in Vascular Injury and Arterial Thrombosis. Interv. Cardiol. Clin. 6, 1–12. doi:10.1016/j.iccl.2016.08.001
Vivas, D., Bernardo, E., García-Rubira, J. C., Azcona, L., Núñez-Gil, I., González-Ferrer, J. J., et al. (2011). Can Resistance to Aspirin Be Reversed after an Additional Dose? J. Thromb. Thrombolysis 32, 356–361. doi:10.1007/s11239-011-0596-3
Wang, H., Zhou, Xk., Zheng, L. F., Wu, X. Y., and Chen, H. (2016). Comparison of Aspirin and Naoxintong Capsule with Adjusted-Dose Warfarin in Elderly Patients with High-Risk of Non-valvular Atrial Fibrillation and Genetic Variants of Vitamin K Epoxide Reductase. Chin. J. Integr. Med. 24, 247–253. doi:10.1007/s11655-015-2443-4
Wang, J., Cao, B., Gao, Y., Han, D., Zhao, H., Chen, Y., et al. (2020). Long Non-coding Rna H19 Positively Associates with Aspirin Resistance in the Patients of Cerebral Ischemic Stroke. Front. Pharmacol. 11, 1–7. doi:10.3389/fphar.2020.580783
Wang, J., Liu, J., Zhou, Y., Wang, F., Xu, K., Kong, D., et al. (2019). Association Among Pla1/a2 Gene Polymorphism, Laboratory Aspirin Resistance and Clinical Outcomes in Patients with Coronary Artery Disease: An Updated Meta-Analysis. Sci. Rep. 9, 13177–13179. doi:10.1038/s41598-019-49123-y
Wang, J., Xiong, X., and Feng, B. (2014). Aspirin Resistance and Promoting Blood Circulation and Removing Blood Stasis: Current Situation and Prospectives. Evid. Based Complement. Alternat Med. 2014, 1–11. doi:10.1155/2014/954863
Wang, Y., Kong, L. J., and Wang, S. H. (2017). Effect of Danhong Injection on Aspirin Reaction Units and Clinical Prognosis in Patients with Coronary Heart Disease. CHINESE ARCHIVES TRADITIONAL CHINESE MEDICINE 35, 1243–1246.
Wang, Y., Yang, H., Chen, L., Jafari, M., and Tang, J. (2021). Network-based Modeling of Herb Combinations in Traditional Chinese Medicine. Brief Bioinform 22. doi:10.1093/bib/bbab106
Wang, Y. Y., and Xu, Q. S. (2018). Effect of Danhong Injection on Aspirin Antiplatelet and Gastric Mucosa Injury in Patients with Coronary Heart Disease. Shandong Med. J. 58, 44–47.
Welsh, R. C., Peterson, E. D., De Caterina, R., Bode, C., Gersh, B., and Eikelboom, J. W. (2019). Applying Contemporary Antithrombotic Therapy in the Secondary Prevention of Chronic Atherosclerotic Cardiovascular Disease. Am. Heart J. 218, 100–109. doi:10.1016/j.ahj.2019.09.006
Wiśniewski, A., Sikora, J., Sławińska, A., Filipska, K., Karczmarska-Wódzka, A., Serafin, Z., et al. (2020). High On-Treatment Platelet Reactivity Affects the Extent of Ischemic Lesions in Stroke Patients Due to Large-Vessel Disease. J. Clin. Med. 9, 251–264.
Xue, M., Xue, L. N., and Shi, D. Z. (2014). Progress and Prospective of Prevention and Treating Aspirin Resistance by Integrative Medicine. Zhongguo Zhong Xi Yi Jie He Za Zhi 34, 245–249.
Xue, M., Yang, X., Yang, L., Kou, N., Miao, Y., Wang, M., et al. (2017). Rs5911 and Rs3842788 Genetic Polymorphism, Blood Stasis Syndrome, and Plasma Txb2 and Hs-Crp Levels Are Associated with Aspirin Resistance in Chinese Chronic Stable Angina Patients. Evid. Based Complement. Alternat Med. 2017, 9037094–9037098. doi:10.1155/2017/9037094
Yalcinkaya, E., and Celik, M. (2014). Evaluation of Inflammatory Conditions Associated with Aspirin Resistance. Ups J. Med. Sci. 119, 292–293. doi:10.3109/03009734.2014.918679
Yassin, A. S., Abubakar, H., Mishra, T., Subahi, A., Hartman, M., Ahmed, A., et al. (2019). Aspirin Resistance: Cardiovascular Risk Game Changer. Am. J. Ther. 26, 593–599. doi:10.1097/MJT.0000000000000780
Yin, C. H., Bi, D. P., and Du, M. (2010). Effect of Tongxinluo Capsule on Platelet Aggregation Function in Patients with Aspirin Resistance. Zhongguo Zhong Xi Yi Jie He Za Zhi 30, 380–382.
Youlong, X., and Zhongjun, L. (2014). Application of Different Traditional Chinese Medicine in Antiplatelet Resistance. J. Changchun Univ. Traditional Chin. Med. 30, 34–36.
Zhang, J. C. (2015). Effect of Compound Danshen Dropping Pills on Aspirin Resistance in Patients with Coronary Heart Disease. Med. Inf. 28, 337–338.
Keywords: blood-activating, traditional Chinese medicine, aspirin resistance, atherosclerotic cardiovascular diseases, mechanism
Citation: Zhao Y, Yang S and Wu M (2021) Mechanism of Improving Aspirin Resistance: Blood-Activating Herbs Combined With Aspirin in Treating Atherosclerotic Cardiovascular Diseases. Front. Pharmacol. 12:794417. doi: 10.3389/fphar.2021.794417
Received: 13 October 2021; Accepted: 29 November 2021;
Published: 17 December 2021.
Edited by:
Xianwei Wang, Xinxiang Medical University, ChinaReviewed by:
Gao Zhu Ye, China Academy of Chinese Medical Sciences, ChinaJing Tang, University of Helsinki, Finland
Copyright © 2021 Zhao, Yang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Wu, d3VtaW4xOTc2MjAwMEAxMjYuY29t
 Yixi Zhao
Yixi Zhao Shengjie Yang
Shengjie Yang Min Wu
Min Wu