- 1Department of Human Anatomy, Medical Institute of Qinghai University, Xining, China
- 2Department of Poultry and Animal Breeding, Faculty of Animal Production and Technology, The Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan
- 3Faculty of Pharmacy, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
- 4Department of Poultry, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 5School of Pharmacy, Nanjing Medical University, Nanjing, China
Health consciousness and increased knowledge about the side effects of synthetic drugs have enhanced interest in traditional medicines. Medicinal plants offer cures for various diseases, leading to improved living standards. This has brought ethnomedicinal studies into the spotlight and increased demand for herb-based medicines. Citrullus colocynthis is an herbaceous plant containing an abundance of nutrients that play a key role in the improvement of wellbeing. C. colocynthis has many biological properties, such as antioxidative, hypoglycemic, antibacterial, anti-cancerous, anti-inflammatory, analgesic, gastrointestinal tract, reproduction, protection, anti-microbial, antidiabetic, hypolipidemic, antineoplastic, profibrinolytic, anti-allergic, pesticidal, and immune-stimulatory. There are numerous bioactive compounds like cucurbitacin, flavonoids, and polyphenols in C. colocynthis that give it medicinal properties. Herein, we have extensively compiled, reviewed, and analyzed significant information on C. colocynthhis from the best published available evidence in PubMed, Scopus (Embase), Web of Science (Web of Knowledge), Cochrane Library, and Google Scholar, etc. Scientific literature evidenced that owing to the bioactive constituents, including cucurbitacin, polyphenols, flavonoids, and other potent molecules, C. colocynthis has many pharmacological and physiological functions. It possesses multi-beneficial applications in treating various disorders of humans and animals. So, the primary purpose of this comprehensive review is to provide an overview of the findings of positive impacts and risks of C. colocynthis consumption on human health, especially in poultry and veterinary fields. In the future, this narrative article will be aware of discoveries about the potential of this promising natural fruit and its bioactive compounds as the best nutraceuticals and therapeutic drugs in veterinary and human medicine.
1 Introduction
Plants have supplied many essential human needs, including a variety of therapeutic medications (Alagawany et al., 2020, 2021a, b; Dhama et al., 2021). Therefore, deliberate efforts towards cultivation are crucial for the continuous availability of those plant species. Medicinal plants have been used in healthcare for a long time, and their use to prevent and treat illness is expanding worldwide (Dhama et al., 2018; Bilal et al., 2021; Reda et al., 2021; Saeed et al., 2021). The medicinal properties of plants are due to the natural chemicals/compounds they contain (Saeed et al., 2019; Alagawany et al., 2021c; Garg et al., 2021; Zhang et al., 2021). Plants are a source of food and act as raw materials from which a variety of drugs are synthesized (Hassan, 2012). Citrullus colocynthis is a desert plant and a source of several bioactive compounds such as essential oils, glycosides, flavonoids, alkaloids, and fatty acids. Medicinal plants improve the immune system. The dried fruit pulp of C. colocynthis has been used to treat gastrointestinal disorders like indigestion, gastroenteritis, and intestinal parasites. C. colocynthis also has excellent pharmacological properties, such as being a laxative and purgative; it is anti-diabetic, anti-inflammatory, anthelmintic, and anti-cancerous. The fruit has been studied extensively for its antimicrobial, antioxidant, and anti-inflammatory activities (Hussain et al., 2014). C. colocynthis seed powder (CCSP) has been used as an emulsifier, fat binder, and flavoring (De Smet., 1997). C. colocynthis has also long been utilized in popular cuisine. Some of its medicinal characteristics include antioxidant, anti-inflammatory, anti-diabetic, and antibacterial activities (Kamran et al., 2018). Its pharmacological properties include antioxidative, hypoglycemic, antibacterial, anti-cancerous, anti-inflammatory, analgesic (Sanafi et al., 2006). C. colocynthis has antidiabetic, hypolipidemic, antineoplastic, profibrinolytic, antiallergic, antimicrobial, pesticidal, and immune-stimulatory effects. It also affects the reproductive system and fertility (Meybodi, 2020). C. colocynthis acts as an antioxidant and anesthetic in humans (Hyderi et al., 2015); its oil can be used to treat constipation (Qureshi et al., 2010), while an extract showed anti-tumor activity on cancerous cells (Abdulridha et al., 2020) and its leaves are anti-cancerous and anti-adipogenic (Perveen et al., 2020). Phytochemical screening of C. colocynthis fruit extract revealed anti-diarrheal properties (Dhakad, 2017). The irregular use of antimicrobials results in drug resistance in animals and humans, adversely affecting their health. Therefore, in 2006, the European Union prohibited antibiotics as growth promoters (Milanov et al., 2016). Due to this restriction, many alternative antimicrobials are being used, and preferences trend towards photogenic products extracted from herbs and spices with known antimicrobial properties (Bajagai et al., 2020). Many other products have been selected as alternatives to antibiotic growth promoters; these include probiotics, prebiotics, enzymes, organic acids, acidifiers, antioxidants, and phytogenic additives (Perić et al., 2009).
In Pakistan, the poultry industry is a key sub-sector of the livestock industry, with current investment of >750 billion and a growth rate of 7.5% per annum. Pakistan is the 11th largest poultry producer globally, with an estimated population of 64.01 million layers, 1,407.73 million broilers, and 14.34 million breeders (Pakistan economic survey, 2020). This indicates the strong growth and importance of, as well as prospects for broiler farming in Pakistan. The antimicrobial growth promoters boost feed conversion and body weight gain as they change the composition and activity of gut microflora (Al Dobaib and Mousa, 2009). The focus of broiler production is growth and performance, and the latter and health depend on the microflora present in the lower gastrointestinal tract (GIT) of broiler chicken (Rinttilä and Apajalahti, 2013). Change or imbalance in gut microbiota can adversely affect nutrient utilization and gut health (Choct., 2009). Phytobiotics are natural, less toxic, and residue-free. Growth promoters improve digestive capacity and growth, increase nutrient availability, and reduce potential pathogens in the GIT (Yitbarek, 2015). These additives also improve feed intake, thus improving the feed conversion and weight gain of broiler chickens (Ertas et al., 2005). Phytobiotics are added to poultry feed and are considered an antimicrobial substitute. These compounds can be used as replacements for antibiotic growth promoters because of their antibacterial, antifungal, antiparasitic, and immune stimulatory attributes, resulting in improved product performance of chickens (Abd El Ghany and Yazar Soyadı, 2020). Ten bioactive components were isolated from C. colocynthis seeds (CCS). CCS are anti-microbial, immune-stimulating, and enhance growth. CCSP improves production performance and alleviates immune suppression (Alzarah et al., 2021). CCS contains 13.5% protein, is rich in methionine and cysteine, and is limited in lysine. The in vitro digestibility of seed protein is 75.9% (Sawaya et al., 1986).
Previous research has reported multiple benefits of C. colocynthis for humans, livestock, and poultry. This literature, from various sources, has been reviewed. As the literature on the use of C. colocynthis in poultry and its importance in humans is limited, we recommend further research on it and its extract in manufacturing poultry and human medicine. We aim to broaden the scope of C. colocynthis use and increase the awareness of scientists and veterinarians regarding the benefits of this plant for human and poultry health.
2 Botanical Description
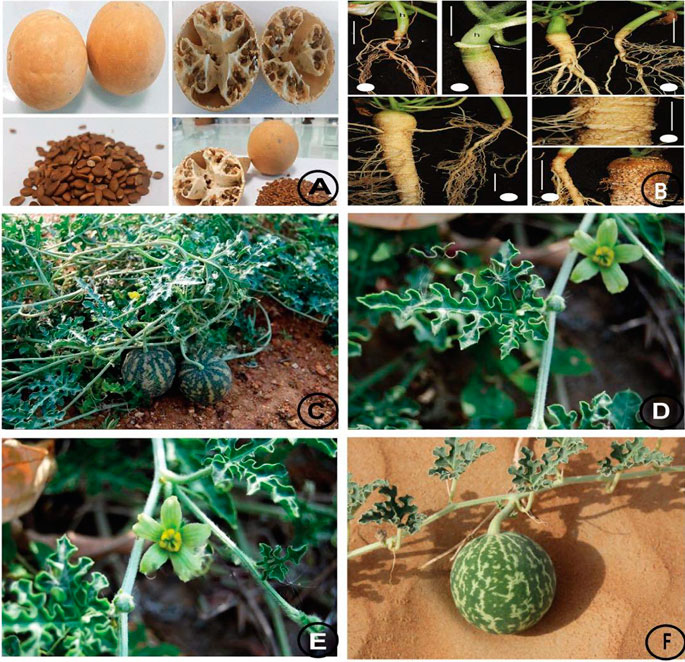
C. colocynthis is a perennial plant with perennial roots and angular, tough, rough, and vine-like stems that spread on the ground and may climb up from there. They produce a single yellow flower at leaf axils. They are monoecious and have long peduncles and tuberous rootstock sprouting long trailing or climbing stems (Pravin et al., 2013) (Table 1).

TABLE 1. Botanical description of C. colocynthis (Pravin et al., 2013).
3 Geographic Distribution
The plant is native to the arid sandy areas of West Asia, Arabia, tropical Africa, and the Mediterranean (Pravin et al., 2013). It is also widely distributed in the desert area of Pakistan (Kamran et al., 2018). C. colocynthis originated in Asia and the Mediterranean Basin, particularly Turkey and Nubia, to the western coastal regions of Africa, the Sahara, and Egypt in the east. It is also found in India and the northern coastal regions of the Caspian and Mediterranean seas. C. colocynthis belongs to the Cucurbitaceae family, and its common names are shown in Table 2.
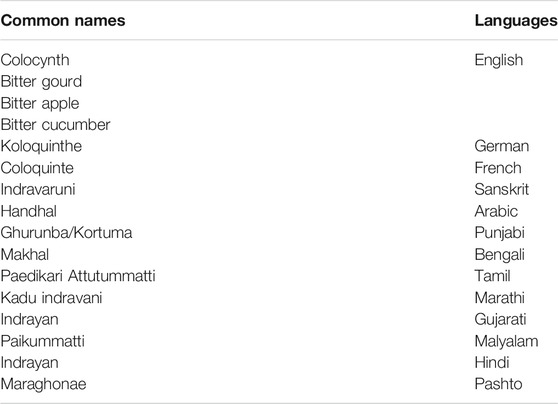
TABLE 2. Common names used for C. colocynthis (De Smet., 1997; Elltayeib et al., 2020; Pravin et al., 2013).
3.1 Proximate Composition
The proximate composition of C. colocynthis is given in Table 3. The proximate analysis of C. colocynthis revealed 24.37% protein, 1.91% fiber, 10.88% carbohydrate, 56.61% fat, 3.15% ash, and 3.08% moisture (Ogundele et al., 2012).

TABLE 3. Proximate composition of C. colocynthis (Akpambang et al., 2008).
4 Traditional Uses
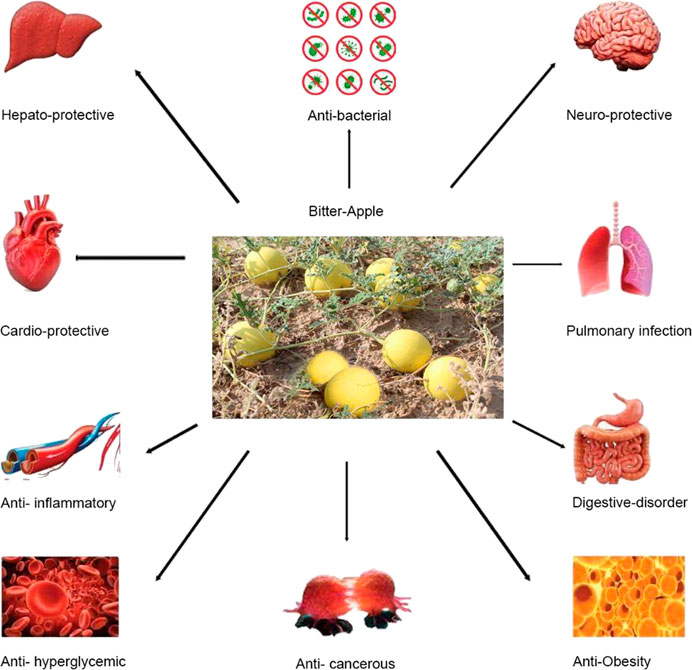
C. colocynthis can be used to treat gastrointestinal conditions and pulmonary, skin, and bacterial infections (Hameed et al., 2020); constipation; edema, cancer, and diabetes (Kumar et al., 2008). The dried pulp of the fruit of C. colocynthis is used as a remedy for gastrointestinal disorders like indigestion, gastroenteritis, and intestinal parasites (Hussain et al., 2014). The plant is also used to treat diabetes, liver problems, weak bowel movements, and obstruction or paralysis of the intestine (Rahimi et al., 2012). The fruit extract is used as an analgesic (Heydari et al., 2015). The vital pharmacological effects of C. colocynthis are shown in Figure 2.
5 Phytochemistry
C. colocynthis contains several bioactive compounds like cucurbitacin, flavonoids, and polyphenols, which impart medicinal properties (Bhasin et al., 2020). The phytochemical constituents of C. colocynthis are shown in Table 4.

TABLE 4. Chemical constituents of C. colocynthis (Al-Snafi, 2016).
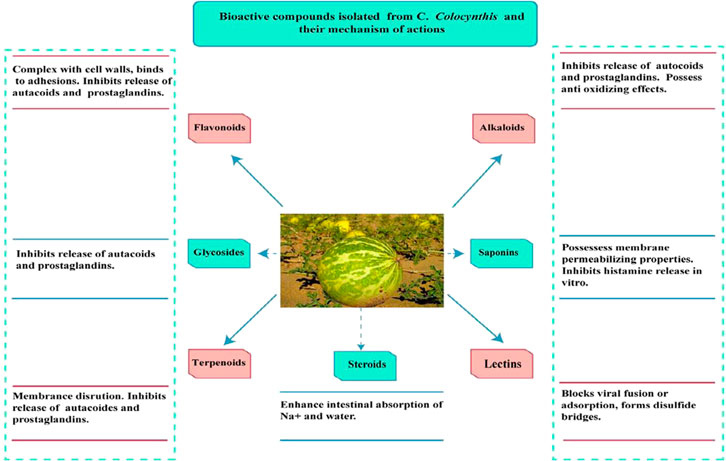
Three flavone glucosides–isovitexin, isosaponarin, isoorientin, and the two cucurbitacin glucosides 2-glucopyranosyl-cucurbitacin L and glucopyranosyl cucurbitacin were extracted from the fruits of the locally growing C. colocynthis and identified. The flavonoids were shown to have considerable antioxidant effects, which is a key characteristic for treating various disorders because reactive oxygen species play an important role in inflammation, cancer, tissue damage, and a variety of diseases (Delazar et al., 2006). Phytochemical screening also revealed the presence of tannins, flavonoids, alkaloids, saponins, and glycosides in C. colocynthis. The chemical components of the ethanolic extract of C. colocynthis, including alkaloids, glycosides, and flavonoids, could have a strong antibacterial effect (Najafi et al., 2010). Terpenoids, steroids, alkaloids, flavonoids, glycosides, phenols, tannins, flavones, and saponins were found in crude extracts of C. colocynthis (Ahmed et al., 2019). Carbohydrates, proteins, tannins, distinct amino acids, steroids, phenolic compounds, alkaloids, glycosides, terpenoids, and cucurbitacins A, B, C, D, E, J, and L were also all found in various preparations of C. colocynthis (Mazher et al., 2020).
5.1 Bioactive Compounds and their Structure-Activity Relationship
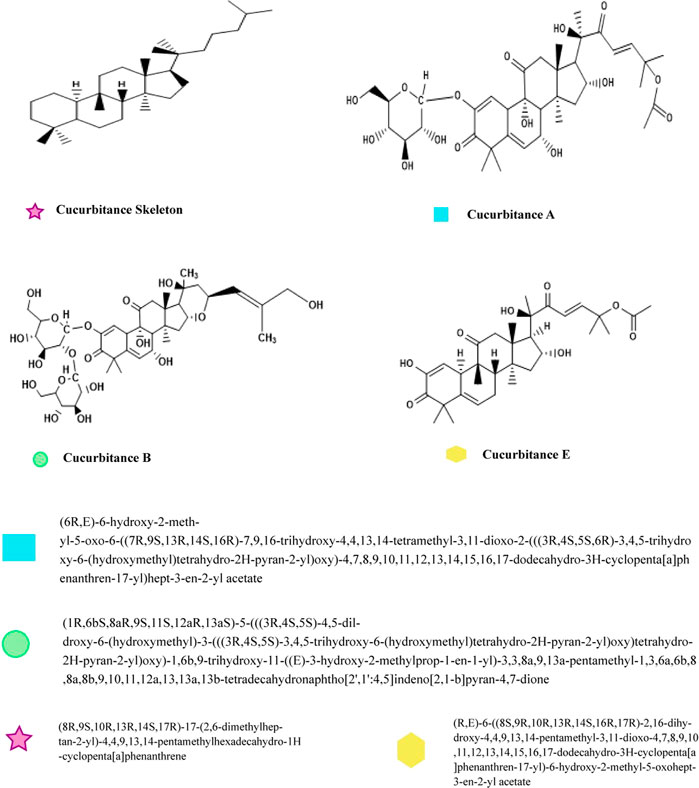
5.1.1. Cucurbitacin
Colocynthosides A, cucurbitacin L, and cucurbitacin B were isolated from the fruit of C. colocynthis. The main cucurbitane-type triterpene glycoside and its aglycon, Cucurbitacin E 2-O—D-glucopyranoside, and cucurbitacin E, showed anti-allergic properties (Yoshikawa et al., 2007). Natural cucurbitacins are triterpenoid chemicals famous for their bitter taste and toxicity. Due to their cytotoxic activities, cucurbitacins play an important role in drug discovery, particularly in anticancer drug development (Chen et al., 2005). Figure 3 shows the structure of various Cucurbitacin.
The structure-activity relationship of the anti-cancerous effects of cucurbitacin and their derivatives, which are capable of electrophilic attack on cellular structures or genetic material, have been studied. This could be used to derive new anti-cancerous agents (Lang et al., 2014).
5.2 Glycosides, Flavonoids, and Phenolic Acids
C. colocynthis fruit contained 2-O—D-glucopyranosyl-Cucurbitacin L, 2-O—D-glucopyranosyl-Cucurbitacin I and isosaponarin. Kaempferol, quercetin, myricetin, catechin, gallic acid, vanillic acid, p-hydroxybenzoic acid, p-coumaric acid, caffeic acid, sinapic acid, chlorogenic acid, and ferulic acid were also found in C. colocynthis (Delazar et al., 2006; Hussain et al., 2014). Flavonoid C-glycosides show considerable anticancer and antitumor action and antibacterial, antifungal, antioxidative, anti-diabetic, anti-inflammatory, antiviral, and hepatoprotective activities, among other biological benefits (Xiao et al., 2016). The structure-activity relationship for quercetin and structurally similar flavonoids has a strong tumor necrosis factor-alpha inhibitory effect and a positive chemical potential and negative electrophilicity index that was considered beneficial (Geoffrey et al., 2020).
5.3 Fatty Acids
Stearic, linolenic, oleic, linoleic, myristic, and palmitic acids were present in CCS (Gurudeeban et al., 2010). Figure 4 shows various phytochemicals along with their mechanism of action.
6 Medicinal Properties
The bitter and spicy C. colocynthis fruit is used to treat colds, diarrhea, parasitic worms, the expulsion of wind, tumors, ascites, leukoplakia, ulcers, asthma, bronchitis, diabetes insipidus, jaundice, splenomegaly, neck tuberculosis, constipation, anemia, throat diseases, elephantiasis, and joint pain; it is also used as an antipyretic. The root can be used to treat jaundice, ascites, urinary disorders, rheumatism; in children, and it can be used against enlarged abdomens, coughs, and asthma attacks. A root plaster can also be used to treat breast inflammation. An application of the fruit or root with a mixture of water and/or Nux vomica can treat papules and acne (Pravin et al., 2013). Different studies on C. colocynthis have been summarized in Table 5.
7 Pharmacological Effects of C. Colocynthis
C. colocynthis has many therapeutic uses and has also been studied for its various pharmacological effects. It is considered an excellent therapeutic agent for the trachea, gut, and cardiovascular system (Hussain et al., 2014).
7.1 Antimicrobial Properties
Previous studies report that aqueous and diluted acetone extracts (from the plant’s roots, stems, leaves), and three maturation stages of its fruit and seeds of C. colocynthis plant are active against Gram-positive and Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Enterococcus faecalis), but have a more substantial effect on newer bacteria. The broth dilution method measured the minimal inhibitory concentration (MIC) preventing visible bacterial growth. MIC was tested for concentrations ranging from 0.10 to 6.50 mg/ml. For aqueous extracts of immature fruits, the MIC was 0.20 mg/ml for Escherichia coli, Pseudomonas aeruginosa. The activity depends on the strains, plant organs, stage of maturity, and the nature of the extraction (Marzouk et al., 2009).
The effect of the ethanolic extract of the C. colocynthis fruit was studied by the well diffusion method and disc diffusion method, and results showed that it has a standard antibacterial effect on both Gram-positive bacteria (S. aureus and Bacillus subtilis) and Gram-negative bacteria like Klebsiella pneumoniae. However, the ethanolic extract from the pulp was more active against Gram-positive bacteria, while the seed extract was slightly less effective against both types of bacteria (Hameed et al., 2020). Both aqueous and diluted acetone extracts showed antibacterial effects against both Gram-positive and Gram-negative bacteria as evaluated by in vitro study; the best results came from an aqueous fruit extract and poorest from a root extract. Immature seeds and fruits showed the highest antibacterial activity. The highest MICs were obtained from the fruit aqueous extracts 0.20 mg/ml against E. coli and P. aeruginosa (Khatibi and Teymorri, 2011). The ethyl acetate extract of the leaves showed promising results against Gram-positive and Gram-negative bacteria (Salmonella enteritidis, B. cereus, Escherichia coli, S. aureus, Enterococcus faecalis, and P. aeruginosa) using the agar disc well-diffusion method. MIC values were obtained for the ethyl acetate extract 0.625 mg/ml against Bacillus cereus (Chawech et al.,. 2015).
7.2 Anti-Fungal Properties
The aqueous plant extract and a diluted acetone extract of C. colocynthis (roots, stems, leaves, and fruit and seeds at various stages of maturity) were selected for testing against several strains of Candida (Candida glabrata, C. albicans, C. parapsilosis, and C. kreusei). In a water extract, the mature fruit had the greatest anti-Candida effect among all strains (MIC 0.20 mg/ml); the immature fruit was the most active in an acetone extract against all strains (Marzouk et al., 2009). Using an ethanol extract of C. colocynthis fruit on various fungal species (Fusarium oxysporum, C. albicans, Aspergillus fumigatus, and A. niger) were assessed in vitro and produced good results against all strains, particularly C. albicans. The efficiency of the extracts was enhanced by increasing the concentration thereof. The results showed that all fungal strains were sensitive against the extracts of the fruit pulp, seeds, and roots of C. colocynthis (Hameed et al., 2020).
The anti-mycotic activity of the ethanol extract of C. colocynthis fruit was tested against pathogenic plant fungi using the agar dilution method and showed promising results. An organic extract of C. colocynthis fruit can thus be used as to alternative synthetic fungicide in agro-industries (Hadizadeh et al., 2009).
7.3 Antioxidant Effects
The methanolic fruit extract of C. colocynthis was found to be a good antioxidant. It exhibited good free radical scavenging activity due to the presence of gallic acid, a phenolic compound. The highest antioxidant and free radical scavenging ability of the fruit extract was observed at a concentration of 2,500 mg ml–1 (Kumar et al., 2008). Cucurbitacin is also an effective antioxidant. that can eliminate free radicals like hydroxyl radicals, superoxide anions, and singlet oxygen. It can also completely inhibit lipid peroxidation and oxidation (Bernard and Olayinka, 2010). Phytochemical screening of C. colocynthis extracts revealed that the natural compounds present therein make it an excellent antioxidant (Benariba et al., 2013). C. colocynthis oil can boost the function of antioxidant enzymes and protect the liver from injury (Amamou et al., 2015). An in-vitro study states that C. colocynthis can prevent the damage caused by free radicals to the body. Various biochemicals in C. colocynthis make it a good antioxidant (Rizvi et al., 2018).
7.4 Anti-Inflammatory and Analgesic Properties
C. colocynthis water extracts were found to possess anti-inflammatory and analgesic activities. All extracts displayed palliative and anti-inflammatory potential at unique doses despite causing acute toxicity. The outcomes of the problem were acquired from unripe fruits and seeds. Stem and root extracts reduced big inhibitory endeavors in analgesic and anti-inflammatory models (Marzouk et al., 2009). The main bioactive chemical components in the chloroform part of CCS extracts came from the separation and characterization of glycoside 11-deoxycucurbitacinI2ObD at the doses of 0.5 and 1 mg/kg body weight in two animal models. The compounds studied demonstrated strong analgesic and anti-inflammatory effects in two animal models (Marzouk et al., 2013). The in vivo analgesic and anti-inflammatory actions of organic extracts of unripe fruits and seeds of Tunisian melon were studied. All extracts showed marked analgesic and anti-inflammatory effects at different doses. C. colocynthis Schrad appeared to interfere with histamine and serotonin pathways and strongly interfered with prostaglandin and kinin-like pathways (Marzouk et al., 2011). The methanolic extract of C. colocynthis leaves was evaluated for anti-inflammatory activity using different in vivo screening models. It had an inhibitory effect on the edema of the paw caused by different inflammatory drugs at the doses of 250 and 500 mg/kg, the infiltration of leukocytes, and the formation of exudate caused by carrageenan, thus presenting an anti-inflammatory effect on the acute and subacute phases of inflammation (Rajamanikam et al., 2010).
7.5 Anti-Hyperglycemic Activity
Various extracts of C. colocynthis peel–aqueous, alkaloidal, saponin, and glycosidic–were examined for their effects on plasma glucose levels in rabbits. The activity of the saponin extract on fasting blood sugar levels of alloxan-induced diabetic rabbits was examined. Normal rabbits orally (300 mg/kg) administered with an aqueous extract of C. colocynthis showed noticeably low plasma glucose levels after 1 h; this increased to high levels after 2, 3, and 6 h. The saponin extract lowers the fasting glucose levels after 1 and 2 h and considerably after 3 and 6 h (Abdel Hassan et al., 2000). The ethanol extract of C. colocynthis at the dose rate of 300 mg/kg on the blood glucose attention within the alloxan brought about diabetes in rats. The results showed that C. colocynthis could lower blood glucose markedly in contrast to manipulating the diabetic team. CCS were also shown to have a marked anti-hyperglycemic effect, supporting the everyday use of C. colocynthis to treat diabetes mellitus (Oryan et al., 2014). Wistar rats and streptozotocin-diabetic rats were injected with various extracts of CCS (total alkaloids, aqueous, saponin, and glycosidic) intraperitoneally to examine their anti-hyperglycemic activity. The results showed that these extracts had a good anti-hyperglycemic effect on the diabetic rats and stabilized the blood glucose of the control rats to within a normal range. The aqueous extract 2.5 g/kg (BW) showed the highest activity by decreasing the blood glucose level (Lahfa et al., 2017). The hydro-ethanolic pulpy flesh of C. colocynthis also demonstrated an exceptional anti-hyperglycemic effect in a diabetic rat at the dose rate of 300 mg/kg by decreasing its blood glucose and triglyceride, and cholesterol levels. In vitro testing also showed that C. colocynthis inhibited glucosidase, which is responsible for postprandial hyperglycemia, strongly indicating that it is a potential candidate for a hyperglycemia treatment (Ghauri et al., 2020).
The C. colocynthis fruit possesses insulin-enhancing activity. This activity may explain in part its antidiabetic effects in traditional medicine. It also identifies the C. colocynthis as a source of a potential novel insulin enhancer that may prove to be useful to reduce hyperglycemia in type 2 diabetes. The ethyl acetate fractions of aqueous non-defatted seed and pulp extracts were used. Two extracts enhanced the insulin-induced translocation of glucose transporter (GLUT4) from intracellular storage sites towards the plasma membrane and accordingly increased insulin-induced glucose uptake. Several of our findings suggested that pulp extract, which increased glucose uptake more than its seed homolog, increased GLUT4 translocation and glucose uptake by acting on the same intracellular signaling cascade as the one employed by insulin (Drissi et al., 2021).
7.6 Anti-Obesity Activity
Results from the administration of 4%colocynth oil to the offspring of overweight rats suggest that it can aid in weight reduction, maintenance of a healthy lipid profile, and controlling glucose levels. This suggests that the oil has a remedial and regulating effect on obesity (Meziane et al., 2012). The effect of glycoside and alkaloid extracts of colocynth were studied on 26 adult male Wistar rats. Animals administered with alkaloids showed weight regression, while those given glycosides were appropriately sized, starting from the 6th week. It became a widespread give-up of treatment (Tabani et al., 2018). These results suggest that C. colocynthis seed oil has good potential for treating obesity and related problems (Sari et al., 2019).
7.7 Anti-Tumor Activity
The anti-tumor activity of C. colocynthis can be attributed to different pathways and properties, including apoptotic pathways, antioxidant and anti-inflammatory effects, inhibition of the Wnt/ß-catenin signaling pathway, and anti-metastatic effects. The cucurbic acid in C. colocynthis gives the plant its anti-cancerous properties (Abdulridha et al., 2020). The methanolic extract of C. colocynthis leaves and its two fractions, ethyl acetate and chloroform, possess notable anti-cancerous effects on the human breast cancer cell line. Bioassays showed a marked reduction in the multiplication and growth of cells treated with these extracts compared to untreated cells. The presence of cyclin-CDK inhibitors means that C. colocynthis extract can arrest human breast cancer cells (Perveen et al., 2021). Colocynth fruit pulp extracts can also block the proliferation and metastatic activity of breast cancer cells and prevent cell migration, the induction of cell apoptosis and cell proliferation, and inhibit cancer stemness properties in breast cancer cells (Chowdury et al., 2017). By modulating the metabolism of lipids, C. colocynthis leaves showed excellent potential as anti-cancerous agents in treating human breast cancer (Perveen et al., 2020). The extract of C. colocynthis fruit also showed anti-tumor activity on cancerous cell lines (Saeed et al., 2019).
7.8 Hepatoprotective Activity
The glycoside and alkaloid extract of colocynth (70 mg/kg single intraperitoneal injection) were analyzed for their effect on metabolic and histological liver disorders in Wistar rats. Treatments therewith showed hypoglycemic, lipid-lowering, and hepatoprotective effects. There was a marked increase in the levels of the liver function markers aspartate aminotransferase, ALT, and alkaline phosphatase (Tabani et al., 2018). The administration of ethanolic extracts of C. colocynthis (200 mg/kg BW), as opposed to paracetamol, resulted in hepatotoxicity in albino rats. The 90% ethanolic extract of C. colocynthis leaves exhibited in-vivo hepatoprotective effects that can be attributed to cell membrane stabilization and liver cell regeneration (Dar et al., 2012).
The hydro-alcoholic extract of C. colocynthis leaves (75 mg/kg body weight orally for 3 weeks) showed good anti-hyperglycemic and anti-hyperlipidemic effects. In addition, C. colocynthis leaf extract might also have a protective effect on the liver, as demonstrated by the markedly lower fasting blood sugar, low-density lipoprotein, cholesterol, alanine aminotransferase, creatinine, aspartate aminotransferase, urea, triglycerides, and bilirubin levels in diabetic rats to which it was administered (Ebrahimi et al., 2016).
7.9 Cardioprotective Activity
Experiments on male rabbits suggest that the administration of adrenaline prompted myocardial damage, as shown by the increased ranges of histomorphological adjustments in the myocardium associated with free radical manufacturing in cardiac tissue. C. colocynthis provided cardiac protection by decreasing oxidative stress caused by the experimental myocardial infarction, preventing the free radical-arbitrated damage of a catecholamine attack. The hydro-alcoholic extract of C. colocynthis peel also showed cardioprotective potential in experimentally induced myocardial infarction in rabbits, as shown by improvements in histological variations and the estimation of different biochemical and inflammatory markers in injured cardiac tissue. Rabbits pretreated with extract 300 mg/kg for 14 successive days significantly prevented the effect of adrenaline and maintained the biochemical parameters at a normal level (Manzoor et al., 2020).
7.10 Neuroprotective Activity
The neuroprotective efficacy of C. colocynthis was observed by estimating its effect on endogenous antioxidant molecules in brain samples of a rat with rotenone-induced Parkinson’s disease (Ahmed et al., 2019). The therapeutic impact of C. colocynthis and its protective mechanisms confirmed that it showed an excellent neuroprotective impact, lessening oxidative stress and inhibiting apoptotic cell death in both in-vitro and in-vivo model (Chen et al., 2019). Treatment with hydro-alcoholic C. colocynthis pulp extract also showed an anticonvulsant effect in rats. Injection of the C. colocynthis extract (25 and 50 mg/kg) exhibited protection against seizure, prolonged the onset of a seizure significantly, and decreased the duration of seizures (Mehrzadi et al., 2016).
All these studies, either in-vitro or in-vivo, are suggestive of promising effects of C. colocynthis and validate its use in traditional medicine as a treatment of gastrointestinal, pulmonary infection and skin infections, constipation, edema, bacterial infections, cancer, diabetes, gastrointestinal disorders, liver problems and as an analgesic.
7.11 Toxicity Assessment
The effect of methanolic extract of C. colocynthis fruit was evaluated on male albino Wister rats to assess its toxicity. The bone marrow, liver, and kidney functions of the animals were measured using preferred techniques. The acute median deadly dose of the extract was calculated to be 1,311, 45 mg/kg. Plasma AST, urea, ALT, and creatinine titers were affected to a notable extent, indicating that the extract was hepato-nephrotoxic. These findings confirmed that the consumption of the extract of ripe C. colocynthis fruit has some undesirable effects on the bone marrow, liver, and kidneys of rats (Soufane et al., 2013).
The membranolytic effect of some C. colocynthis components can cause intestinal damage (Javadzadeh et al., 2013). In a study of the subchronic hemotoxicity and cytotoxicity of C. colocynthis on albino rats, the oral LD50 for extraction of C. colocynthis flowers was found to be 162.4 mg/kg of bodyweight. Pathological adjustments to the lung, liver, kidney, spleen, stomach and intestine of the treated rats were also recorded (Elgerwi et al., 2013). The noxiousness of ingesting an extract with 10% C. colocynthis fruits was checked in the rats. The outcomes of C. colocynthis treatment were depression, ruffled hair, low body weight, feeding efficiency, and entero-hepato-nephropathy. Diarrhea is a clear sign of C. colocynthis poisoning. Lesions were observed on the organs in addition to leukopenia, anemia, modifications in serum enzyme (AST, ALT ALP, and ALT) levels, and concentrations of whole protein, urea, bilirubin, albumin, and one of a kind serum constituent (Al-Yahya et al., 2000). C. colocynthis is a strong laxative, with one case report suggesting that ingestion of the former causes inflammation of the colon with bloody diarrhea (Goldfain et al., 1989). High doses of C. colocynthis have detrimental effects on liver cells (Dehghan and Panjeh, 2006). High doses of its pulp extract, in particular, were deadly in rabbits, causing dehydration owing to severe diarrhea, heart failure due to cardio-stimulatory action, hepatorenal insults, or hypoglycemia; seed extract caused mild intestinal lesions (Shafaei et al., 2012). Hepatic damage, watery diarrhea, hypoglycemia, and hypotension were observed in a man who received high doses of a C. colocynthis fruit decoction to treat constipation (Rezvani et al., 2011). Chickens fed a diet of 10% Citrullus developed reversible lesions in their livers, small intestines, and kidneys (Bakhiet and Adam, 1995). Ten sheep fed fresh C. colocynthis fruits and leaves developed poisoning symptoms and died between 4 and 25 days of being dosed. Diarrhea, dyspnea, anorexia, and loss of condition are clinical symptoms (Elawad et al., 1984). Oral administration of C. colocynthis fruit fruits 0.25 g/kg/day with Rhazya stricta leaves resulted in dehydration, loss of condition, profuse diarrhea, ataxia, and recumbency prior to death within 26 days (Adam et al., 2000).
8 Applications in Poultry
CCS was fed to 144-day-old straight-run chicks as a potential source of protein in feed, in place of soybean meal. The feeding experiment revealed that including up to 15% of the whole seed in the feed resulted in the normal growth of the chicks. However, the inclusion of 15% unprocessed meals depressed growth and showed a poor feed conversion ratio (FCR) (Sawaya et al., 1986).
CCSP was fed to 360-day-old Ross strain broiler chickens as a 0, 2, 4, and 6% supplement in feed. The result of the study showed that the 6% supplement in feed improved live body weight and dressing percentage while decreasing feed intake and FCR (Ali et al., 2012).
C. colocynthis fruit powder was fed to 100 broiler chickens, among which 100 chicks were given this on the sixth day after inoculation with Eimeria tenella. The power was supplemented in feed at 0.05, 0.01, 0.15, and 0.00%. The result showed that the 0.15% C. colocynthis fruit powder supplement was the most efficient at preventing coccidiosis (ALamery and ALsaeq, 2011). The effects of CCS meal (CCSM) on 270-day-old male Cobb broiler chickens were studied. CCSM was supplemented through feed at 0, 2, and 4%, and results showed that supplementation at 4% improved carcass weight, dressing percentage, and live body weight. As the dietary level of CCSM increased, feed intake decreased, and FCR was impaired (Ali et al., 2012). In another study, C. colocynthis was fed to 240-day-old Ross broiler chicks to check the effects of the former on growth performance and intestinal morphology. Here, C. colocynthis was supplemented at 0, 0.2, 0.4, and 0.6% of bitter cucumber feed with 0 and 0.01% protein. The results showed that supplementation at 0.6% improved feed intake, body weight gain, breast meat, and carcass yield while reducing FCR. Villus height, crypt depth, and intestinal mucosal muscle also increased (Hashemi et al., 2016).
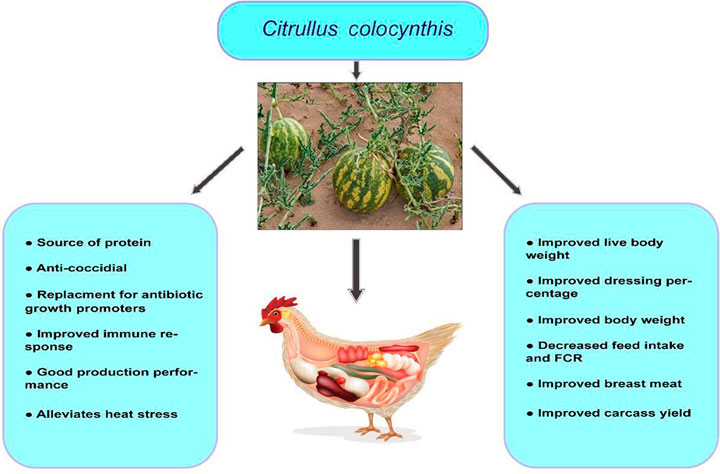
In a different study, C. colocynthis fruit pulp was fed to replace antibiotic growth promoters with 400-day-old Ross broiler chicken chicks. Here, C. colocynthis fruit pulp was supplemented at 1 g/kg feed and 1.5 g/kg. The result showed that supplementation of C. colocynthis fruit pulp at the latter rate could replace antibiotic growth promoters (Kamran et al., 2018). In a separate study, CCS was fed via feed to 300 Cobb 21-day-old broiler chickens subjected to chronic heat stress at the rate of 0.1%. The result showed that this supplementation rate improved immune response and production performance in the heat-stressed group but had no effect on a control (thermo-neutral) group (Alzarah et al., 2021). The promising effects of C. colocynthis for poultry nutrition are shown in Figure 5.
9 Veterinary Uses
At 4 g/day, a polyherbal combination including C. colocynthis modified gene expression to promote growth and health from the pre-ruminant to weaning phase. Some gene expression research indicates that polyherbal therapy enhanced lipid, protein, carbohydrate, and immune response metabolism. These findings support using plant chemicals in animal feed (Díaz Galván et al._, 2021). Supplementation with C. colocynthis fruit showed potential to minimize methanogenesis and improve rumen fermentation. However, in vivo testing on ruminants is required to evaluate the persistence of benefits as well as health issues (Hundal et al., 2020). De-oiled CCS cake was added to dairy cow feed and showed no effect on milk yield (Khatri et al., 1993).
Ten sheep were fed fresh C. colocynthis fruits and leaves. The sheep developed poisoning symptoms and died within 4–25 days after being dosed. They showed symptoms of dyspnea, diarrhea, loss of condition, and anorexia (Elawad et al., 1984). C. colocynthis fruits oral dosing with Rhazya stricta leaves proved deadly within 26 days, resulting in ataxia, profuse diarrhea, loss of condition, dehydration, and recumbency prior to death (Adam et al., 2000). A trial of the ingestion and metabolism of C. colocynthis was undertaken in 12 yearlings Najdi sheep to investigate the consumption of crude protein in CCS meal as this was shown to be a good partial substitute for soybean meal in sheep diets (Bhattacharya, 1990). After reviewing the literature, we found that there is currently a lack of research-based data on the use of C. colocynthis in veterinary science. More research is needed to determine its importance and note effective inclusion levels in the diet of animals.
10 Applications in Humans
The methanolic extract of C. colocynthis leaves and its two fractions, ethyl acetate, and chloroform possess notable anti-cancerous effects. Bioassays showed a significant reduction in the multiplication and growth of treated cells compared to untreated cells. Owing to the expression of cyclin-CDK inhibitors, C. colocynthis arrests the cell cycle in human breast tumor cells (Perveen et al., 2021). C. colocynthis is used for treating colorectal cancer in humans. Cucurbic acid present in C. colocynthis extract is believed to stop the multiplication of cancerous cells. The anti-tumor activity of C. colocynthis can be attributed to different pathways and effects, such as apoptotic pathways, antioxidant and anti-inflammatory effects, inhibition of Wnt/ß-catenin signaling pathway, and anti-metastatic effects (Abdulridha et al., 2020). CCSP lowers the cholesterol level in non-diabetic patients (Rahbar and Nabipour, 2010). The C. colocynthis plant acts as a good anti-diabetic agent in humans with type II diabetes as it reduces glucose and cholesterol levels (Youshan et al., 2015; Chenghe et al., 2014). C. colocynthis fruit pulp of mature seed can also be used to treat tuberculosis, and it was found to have active anti-bacterial properties against various strains of normal and drug-resistant mycobacterium (Archana et al., 2013). The methanolic extract of C. colocynthis fruit is also active against several food-borne bacteria hazardous to human health (Kim et al., 2014). C. colocynthis also shows excellent potential as an anti-cancerous agent for treating human breast cancer via the modulation of lipid metabolism (Perveen et al., 2020). Consumption of C. colocynthis for a long time could lead to anti-fertility issues in both males and females (Chaturvedi et al., 2003; Amal et al., 2016). C. colocynthis fruit extract shows anti-tumor activity on drug-resistant cancerous cell lines (Saeed et al., 2019). C. colocynthis oil can be used for treating constipation in humans (Qureshi et al., 2010). C. colocynthis fruit has shown promising results in treating diabetic patients. A dose of 300 mg/day given to patients showed no adverse effects on their health (Huseini et al., 2009). An extract of C. colocynthis leaves can be used to treat skin infections, and a paste of C. colocynthis roots can be applied to treat joint problems (Upadhyay et al., 2007). C. colocynthis has also been used to treat edema, cancer, constipation, bacterial infections, and diabetes and has been used as an abortifacient (Delazar et al., 2006). C. colocynthis is a desert shrub with a long history as a valuable oil source and medicinal plant. During the first 4 weeks of ripening, yield and size of fruit, seed output, and overall oil yield were all at their peak. C. colocynthis has the potential to be grown as a source of edible oil. The oil has a laxative effect and contains between 80 and 85 percent unsaturated oleic and linoleic fatty acids, making it a high-quality oil for human consumption (Schafferman et al., 1998).
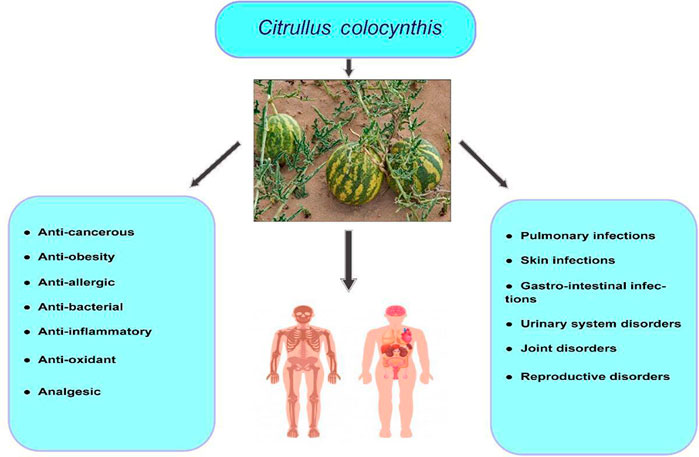
The health benefits of C. colocynthis are shown in Figure 6.
11 Limitation and Future Research
The chemical cucurbitacin, found in colocynth, irritates mucous membranes, especially those in the stomach and intestines. Colocynth is extremely dangerous to use. The Food and Drug Administration (FDA) prohibited it in 1991. Even tiny doses of colocynth can induce severe stomach and gut lining irritation, bloody diarrhea, kidney damage, bloody urine, and inability to pee. Convulsions, paralysis, and death are some of the other adverse effects. Colocynth dosage is determined by a number of factors, including the user’s age, health, and other circumstances. There is insufficient scientific evidence to define a suitable dosing range for colocynth. Keep in mind that natural products aren’t always safe and that doses are crucial. Before using, be sure to read the product label and consult your pharmacist, physician, or another healthcare expert. According to reports, ingestion of merely 1-1/2 tablespoons of the powder has resulted in death. Colocynth is unsuitable for use during pregnancy and breastfeeding. Ingestion of less than 2 gm of the powder has been reported to result in death. In clinical studies, as little as 300 mg of colocynth powder was found to cause moderate diarrhea.
Oral administration of C. colocynthis fruits 0.25 g/kg/day with Rhazya stricta leaves proved deadly within 26 days, resulting in dehydration, loss of condition, profuse diarrhea, ataxia, and recumbency before death (Adam et al., 2000). The treatment regimen having 10% of C. colocynthis fruits was once checked for rats. The outcomes characterized by C. colocynthis treatment were depression, ruffled hair, low physique weight, low feed efficiency, and entero-hepato-nephropathy (Al-Yahya et al., 2000). Chickens fed a 10% Citrullus diet developed reversible lesions in the liver, small intestine, and kidney (Bakhiet and Adam, 1995). 60 ml of decoction of the plant fruit taken by a 48-year old man to treat constipation resulted in watery diarrhea, hypotension and hypoglycemia, and hepatic injury (Rezvani et al., 2011). C. colocynthis being stimulant laxatives can cause the body’s potassium levels to drop. Low potassium levels might exacerbate digoxin side effects. Lanoxin: Colocynth can induce diarrhea in some patients who are taking Warfarin. Diarrhea can make Warfarin less effective and raise the risk of bleeding. Taking colocynth with water pills may cause the body’s potassium levels to drop too low.
The biological activity of the extracts and isolated compounds have been discovered, particularly in antidiabetic, anticancer, anti-inflammatory, antioxidant, insecticidal, and antibacterial areas. Interestingly, the plant has been demonstrated to have a high nutritional value since it is a strong source of protein, has edible seed oil, and contains certain vital minerals such as calcium, potassium, and magnesium, all of which are known to have medical benefits. Despite the fact that growing interest has driven greater research on C. colocynthis’ phytochemistry and pharmacology, there are still many areas where existing understanding might be improved. Furthermore, there is a scarcity of information concerning its mode of action and dosing rate. In recent pharmacological investigations, various traditional applications of the C. colocynthis fruit have been verified; however, some of these studies were only examined in vitro. As a result, in vivo experiments should be used to evaluate further the efficacy and safety of C. colocynthis fruit extracts and isolated chemicals. In previous studies, C. colocynthis has been shown to have many roles in people, cattle, and fowl. The aforementioned system’s literature has been evaluated from a variety of sources. Because there is limited research on the use of C. colocynthis in poultry, veterinary medicine, and human medicine, it is the forward reassessment to advocate C. colocynthis plant and extract for use in poultry and human medicine manufacturing.
12 Conclusion and Perspectives
In the present assessment, the nutrient composition and medicinal qualities of C. colocynthis have been evaluated based on various previous studies. This review strongly indicates that C. colocynthis is a fruit crop that could benefit the treatment of a range of diseases. Although C. colocynthis has high dietary value, it is not widely known. More investigations are required to spotlight the utility of such fruit crops as a dietary supplement that can enhance fitness. This review demonstrates that C. colocynthis is a medicinal plant with a wide variety of pharmacological properties that might make it useful and effective in numerous medical applications. To date, no review article has published comprehensive literature about its uses in poultry, veterinary and human areas. This review gives a thorough insight into its phytochemistry along with the structure-activity relationship of some bioactive compounds, pharmacology, beneficial effects, limitations, and drug interaction. The study compiles the most recent data present on C. colocynthis. So, the objective of this review is to provide comprehensive data about the benefits and limitations of C. colocynthis, as the data about inclusion levels and its use and possible side effect are still not precise and need to be validated by pharmacological investigations against various disorders in vivo. C. colocynthis has many vital health-promoting effects like neurological, physiological as well as biological functions, but still, their mechanism of action behind these properties in different species is not known and needs to be exploited. The future avenues for the veterinary and pharmaceutical researchers would be to identify more of these demanding areas and document reliable markers (bio and molecular) which are responsible for a vast array of C. colocynthis’s benefits.
Author Contributions
Q-YL, MM, and MS: Conceptualization. MK and J-QS: Review and editing. MA, MN, SN, and AM: Original draft, writing–review and editing and C-XL: Supervision.
Funding
This work was financed by the Qinghai University School of Medicine Young and Middle-aged Scientific Research Fund Project (Natural Science) (#. 2020-kyy-8) and the Qinghai Fundamental Scientific and Technological Research Plan (#. 2018-ZJ-730, 2019-SF-134) and CUVAS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
All the authors of the manuscript also thank and acknowledge their respective Universities and Institutes.
References
Abd El-Ghany, W. A., and Yazar Soyadı, Y. A. (2020). Phytobiotics in Poultry Industry as Growth Promoters, Antimicrobials and Immunomodulators - A Review. jwpr 10, 571–579. doi:10.36380/jwpr.2020.65
Abdel-Hassan, I. A., Abdel-Barry, J. A., and Tariq Mohammeda, S. (2000). The Hypoglycaemic and Antihyperglycaemic Effect of Citrullus colocynthis Fruit Aqueous Extract in normal and Alloxan Diabetic Rabbits. J. Ethnopharmacol 71 (1-2), 325–330. doi:10.1016/s0378-8741(99)00215-9
Abdulridha, M. K., Al-Marzoqi, A. H., and Ghasemian, A. (2020). The Anticancer Efficiency of Citrullus colocynthis toward the Colorectal Cancer Therapy. J. Gastrointest. Cancer 51 (2), 439–444. doi:10.1007/s12029-019-00299-6
Adam Sei, S. E. I., Al-Farhan, A. H., and Al-Yahya, A. (2000). Effect of Combined Citrullus colocynthis and Rhazya Stricta Use in Najdi Sheep. Am. J. Chin. Med. 28 (03n04), 385–390. doi:10.1142/S0192415X00000453
Ahmed, M., Ji, M., Qin, P., Gu, Z., Liu, Y., Sikandar, A., et al. (2019). Phytochemical Screening, Total Phenolic and Flavonoids Contents and Antioxidant Activities of Citrullus colocynthis L. And Cannabis Sativa L. Appl. Ecol. Environ. Res. 17, 6961–6979. doi:10.15666/aeer/1703_69616979
Akpambang, V. O. E., Amoo, I. A., and Izuagie, A. A. (2008). Comparative Compositional Analysis on Two Varieties of Melon (Colocynthis citrullus and Cucumeropsis Edulis) and a Variety of almond (Prunus Amygdalus). Res. J. Agric. Biol. Sci. 4 (6), 639–642.
Al-Dobaib, S. N., and Mousa, H. M. (2009). Benefits and Risks of Growth Promoters in Animal Production. J. Food Agric. Environ. 7 (2), 202–208.
Al-Snafi, P. D. A. E. (2016). Chemical Constituents and Pharmacological Effects of Cynodon Dactylon- A Review. Iosrphr 06 (7), 17–31. doi:10.9790/3013-06721731
Al-Yahya, M. A., Al-Farhan, A. H., and Adam, S. E. (2000). Preliminary Toxicity Study on the Individual and Combined Effects of Citrullus colocynthis and Nerium Oleander in Rats. Fitoterapia 71 (4), 385–391. doi:10.1016/s0367-326x(00)00135-0
Alagawany, M., Abd El-Hack, M. E., Saeed, M., Naveed, M., Arain, M. A., Arif, M., et al. (2020). Nutritional Applications and Beneficial Health Applications of green tea and L-Theanine in Some Animal Species: A Review. J. Anim. Physiol. Anim. Nutr. (Berl) 104, 245–256. doi:10.1111/jpn.13219
Alagawany, M., El-Saadony, M. T., Elnesr, S. S., Farahat, M., Attia, G., Madkour, M., et al. (2021c). Use of Lemongrass Essential Oil as a Feed Additive in Quail's Nutrition: its Effect on Growth, Carcass, Blood Biochemistry, Antioxidant and Immunological Indices, Digestive Enzymes and Intestinal Microbiota. Poult. Sci. 100 (6), 101172. doi:10.1016/j.psj.2021.101172
Alagawany, M., Elnesr, S. S., Farag, M. R., Tiwari, R., Iqbal Yatoo, M., Karthik, K., et al. (2021a). Nutritional Significance of Amino Acids, Vitamins and Minerals as Nutraceuticals in Poultry Production and Health - A Comprehensive Review. Vet. Q. 41 (1), 1–45. doi:10.1080/01652176.2020.1857887
Alagawany, M., Farag, M. R., Abdelnour, S. A., Dawood, M. A. O., Elnesr, S. S., and Dhama, K. (2021b). Curcumin and its Different Forms: A Review on Fish Nutrition. Aquaculture 532 (2021), 736030. doi:10.1016/j.aquaculture.2020.736030
ALamery, M. M., and ALsaeq, A. A. (2011). Addition Powder Citrullus Colocynthis Fruits to the Broiler Ratio for Treat’s Coccidiosis Type E. Tenella. AL-Qadisiyah J. Vet. Med. Sci. 10 (2), 131–137.
Ali, S. A., Abdalla, H. O., and Elamin, M. A. (2012). Citrullus colocynthis (Handal) Seed Meal as a Natural Feed Supplementation in Broiler Chickens' Diets. Egypt. Poult. Sci. J. 32 (2), 237–246.
Alzarah, M. I., Althobiati, F., Abbas, A. O., Mehaisen, G. M. K., and Kamel, N. N. (2021). Citrullus colocynthis Seeds: A Potential Natural Immune Modulator Source for Broiler Reared under Chronic Heat Stress. Animals (Basel) 11 (7), 1951. doi:10.3390/ani11071951
Amamou, F., Nemmiche, S., Meziane, R. K., Didi, A., Yazit, S. M., and Chabane-Sari, D. (2015). Protective Effect of Olive Oil and Colocynth Oil against Cadmium-Induced Oxidative Stress in the Liver of Wistar Rats. Food Chem. Toxicol. 78, 177–184. doi:10.1016/j.fct.2015.01.001
Bajagai, Y. S., Alsemgeest, J., Moore, R. J., Van, T. T. H., and Stanley, D. (2020). Phytogenic Products, Used as Alternatives to Antibiotic Growth Promoters, Modify the Intestinal Microbiota Derived from a Range of Production Systems: an In Vitro Model. Appl. Microbiol. Biotechnol. 104 (24), 10631–10640. doi:10.1007/s00253-020-10998-x
Bakhiet, A. O., and Adam, S. E. (1995). An Estimation of Citrullus colocynthis Toxicity for Chicks. Vet. Hum. Toxicol. 37 (4), 356–358.
Ben Geoffrey, A. S., Christian Prasana, J., and Muthu, S. (2020). Structure-activity Relationship of Quercetin and its Tumor Necrosis Factor Alpha Inhibition Activity by Computational and Machine Learning Methods. Mater. Today Proc. doi:10.1016/j.matpr.2020.07.464
Benariba, N., Djaziri, R., Bellakhdar, W., Belkacem, N., Kadiata, M., Malaisse, W. J., et al. (2013). Phytochemical Screening and Free Radical Scavenging Activity of Citrullus colocynthis Seeds Extracts. Asian Pac. J. Trop. Biomed. 3 (1), 35–40. doi:10.1016/S2221-1691(13)60020-9
Bernard, S. A., and Olayinka, O. A. (2010). Search for a Novel Antioxidant, Anti-inflammatory/analgesic or Anti-proliferative Drug: Cucurbitacins Hold the Ace. J. Med. Plants Res. 4 (25), 2821–2826. doi:10.5897/JMPR.9001120
Bhasin, A., Singh, S., and Garg, R. (2020). Nutritional and Medical Importance of Citrullus Colocynthis-A Review. Plant Arch. 20 (2), 3400–3406.
Bhattacharya, A. N. (1990). Citrullus colocynthis Seed Meal as a Protein Supplement for Najdi Sheep in Northern Saudi Arabia. Anim. feed Sci. Technol. 29 (1-2), 57–62. doi:10.1016/0377-8401(90)90093-N
Bilal, R. M., Liu, C., Zhao, H., Wang, Y., Farag, M. R., Alagawany, M., et al. (2021). Olive Oil: Nutritional Applications, Beneficial Health Aspects and its Prospective Application in Poultry Production. Front. Pharmacol. 12, 723040. doi:10.3389/fphar.2021.723040
Chabane Sari, M., Nemmiche, S., Benmehdi, H., Amrouche, A., Lazouni Hamadi, A., and Chabane Sari, D. (2019). Hypolipidemic and Antioxidant Effects ofCitrullus colocynthisSeeds Oil in High-Fat Diets Induced Obese Rats. Phytothérapie 17 (6), 310–320. doi:10.3166/phyto-2018-0066
Chaturvedi, M., Mali, P. C., and Ansari, A. S. (2003). Induction of Reversible Antifertility with a Crude Ethanol Extract of Citrullus colocynthis Schrad Fruit in Male Rats. Pharmacology 68 (1), 38–48. doi:10.1159/000068727
Chawech, R., Jarraya, R., Girardi, C., Vansteelandt, M., Marti, G., Nasri, I., et al. (2015). Cucurbitacins from the Leaves of Citrullus colocynthis (L.) Schrad. Molecules 20 (10), 18001–18015. doi:10.3390/molecules201018001
Chen, J. C., Chiu, M. H., Nie, R. L., Cordell, G. A., and Qiu, S. X. (2005). Cucurbitacins and Cucurbitane Glycosides: Structures and Biological Activities. Nat. Prod. Rep. 22 (3), 386–399. doi:10.1039/b418841c
Chen, Y., Sa, Y., Wang, G., Pan, X., Zhen, Y., Cheng, X., et al. (2019). The Protective Effects of citrullus Colocynthis on Inhibiting Oxidative Damage and Autophagy-Associated Cell Death in Parkinson's Disease. J. Taiwan Inst. Chem. Eng. 100, 18–25. doi:10.1016/j.jtice.2019.04.003
Chowdhury, K., Sharma, A., Kumar, S., Gunjan, G. K., Nag, A., and Mandal, C. C. (2017). Colocynth Extracts Prevent Epithelial to Mesenchymal Transition and Stemness of Breast Cancer Cells. Front. Pharmacol. 8, 593. doi:10.3389/fphar.2017.00593
Dar, A. I., Saxena, R. C., and Bansal, S. K. (20122012). Hepatoprotection: A Hallmark of Citrullus colocynthis L. Against Paracetamol Induced Hepatotoxicity in Swiss Albino Rats. Ajps 03, 1022–1027. doi:10.4236/ajps.2012.327121
De Smet, P. A. G. M. (1997). “Citrullus Colocynthis,” in Adverse Effects of Herbal Drugs (Berlin, Heidelberg: Springer), 29–36. doi:10.1007/978-3-642-60367-9_4
Dehghan, F., and Panjeh, S. M. (2006). The Toxic Effect of Alcoholic Extract of Citrullus colocynthis on Rat Liver.
Delazar, A., Gibbons, S., Kosari, A. R., Nazemiyeh, H., Modarresi, M. A. S. O. U. D., Nahar, L. U. T. F. U. N., et al. (2006). Flavone C-Glycosides and Cucurbitacin Glycosides from Citrullus colocynthis. Daru 14 (3), 109–114.
Dhakad, Pk. (2017). Phytochemical Investigation and Anti-diarrheal Activity of Hydroalcoholic Extract of Fruits of Citrullus colocynthis (L.) Schrad. (Cucurbitaceae). J. Mol. Genet. Med. 11 (305), 1747–0862. doi:10.4172/1747-0862.1000305
Dhama, K., Karthik, K., Khandia, R., Munjal, A., Tiwari, R., Rana, R., et al. (2018). Medicinal and Therapeutic Potential of Herbs and Plant Metabolites/Extracts Countering Viral Pathogens - Current Knowledge and Future Prospects. Curr. Drug Metab. 19 (3), 236–263. doi:10.2174/1389200219666180129145252
Dhama, K., Sharun, K., Gugjoo, M. B., Tiwari, R., Alagawany, M., Iqbal Yatoo, Yatoo., et al. (2021). A Comprehensive Review on Chemical Profile and Pharmacological Activities of Ocimum Basilicum. Food Rev. Int., 1–29. doi:10.1080/87559129.2021.1900230
Díaz Galván, C., Méndez Olvera, E. T., Martínez Gómez, D., Gloria Trujillo, A., Hernández García, P. A., Espinosa Ayala, E., et al. (2021). Influence of a Polyherbal Mixture in Dairy Calves: Growth Performance and Gene Expression. Front. Vet. Sci. 7. doi:10.3389/fvets.2020.623710
Ebrahimi, E., Mohammadzadeh, G., Mansouri, E., and Aberomand, M. (2016). Effects of Hydro-Alcoholic Leaf Extract of citrullus Colocynthis on Biochemical Factors and Histopathological Changes in Streptozotocin-Induced Diabetic Rats. Jundishapur J. Nat. Pharm. Prod. 11 (3). doi:10.17795/jjnpp-33214
Elawad, A. A., Abdel Bari, E. M., Mahmoud, O. M., and Adam, S. E. (1984). The Effect of Citrullus colocynthis on Sheep. Vet. Hum. Toxicol. 26 (6), 481–485.
Elgerwi, A., Benzekri, Z., Awaidat, S., El-Magdoub, A., Abusnina, A., and El-Mahmoudy, A. (2013). Subchronic Haemotoxicity and Histotoxicity of Citrullus colocynthis. J. Am. Sci. 9 (5).
Elltayeib, A. A. E., Mohamed, B. K., and Elshami, R. M. (2020). Chemical Properties and Antibacterial Effect of the Oil from Seeds of Citrullus colocynthis. Insi Chem. Biochem. doi:10.33552/icbc.2020.01.000512
Ertas, O. N., Guler, T., Çiftçi, M., DalkIlIç, B., and Simsek, U. G. (2005). The Effect of an Essential Oil Mix Derived from Oregano, Clove and Anise on Broiler Performance. Int. J. Poult. Sci. 4 (11), 879–884. doi:10.3923/ijps.2005.879.884
Garg, A. K., Faheem, M., and Singh, S. (2021). Role of Medicinal Plant in Human Health Disease. Asian J. Plant Sci. Res. 11 (1), 19–21.
Ghauri, A. O., Ahmad, S., and Rehman, T. (2020). In Vitro and In Vivo Anti-diabetic Activity of Citrullus colocynthis Pulpy Flesh with Seeds Hydro-Ethanolic Extract. J. Complement. Integr. Med. 17 (2). doi:10.1515/jcim-2018-0228
Goldfain, D., Lavergne, A., Galian, A., Chauveinc, L., and Prudhomme, F. (1989). Peculiar Acute Toxic Colitis after Ingestion of Colocynth: a Clinicopathological Study of Three Cases. Gut 30 (10), 1412–1418. doi:10.24941/ijcr.40170.11.202010.1136/gut.30.10.1412
Gurudeeban, S., Satyavani, K., and Ramanathan, T. (2010). Bitter Apple (Citrullus colocynthis): An Overview of Chemical Composition and Biomedical Potentials. Asian J. Plant Sci. 9 (7), 394–401. doi:10.3923/ajps.2010.394.401
Hadizadeh, I., Peivastegan, B., and Kolahi, M. (2009). Antifungal Activity of Nettle (Urtica Dioica L.), Colocynth (Citrullus colocynthis L. Schrad), Oleander (Nerium Oleander L.) and Konar (Ziziphus Spina-Christi L.) Extracts on Plants Pathogenic Fungi. Pak J. Biol. Sci. 12 (1), 58–63. doi:10.3923/pjbs.2009.58.63
Hameed, B., Ali, Q., Hafeez, M. M., and Malik, A. (2020). Antibacterial and Antifungal Activity of Fruit, Seed and Root Extracts of Citrullus colocynthis Plant. Biol. Clin. Sci. Res. J. 2020 (1). doi:10.47264/bcsrj010103310.54112/bcsrj.v2020i1.33
Hashemi, M., Jalali, S. M. A., and Kheiri, F. (2016). The Effect of Using of Bitter Cucumber Fruit Powder, Citrullus colocynthis, and Probiotic on Growth Performance and Intestinal Morphology of Broiler Chicks. J. Med. Herbs 7 (3), 193–200.
Heydari, M., Homayouni, K., Hashempur, M. H., and Shams, M. (2015). Topical Citrullus colocynthis (Bitter Apple) Extract Oil in Painful Diabetic Neuropathy: A Double-Blind Randomized Placebo-Controlled Clinical Trial. J. Diabetes 8, 246–252. doi:10.1111/1753-0407.12287
Hundal, J. S., Wadhwa, M., Singh, J., Dhanoa, J. K., and Kaur, H. (2020). Potential of Citrullus colocynthis as Herbal Feed Additive for Ruminants. Indian J. Anim. Sci. 90 (2), 244–248.
Huseini, H. F., Darvishzadeh, F., Heshmat, R., Jafariazar, Z., Raza, M., and Larijani, B. (2009). The Clinical Investigation of Citrullus colocynthis (L.) Schrad Fruit in Treatment of Type II Diabetic Patients: a Randomized, Double Blind, Placebo-Controlled Clinical Trial. Phytother Res. 23 (8), 1186–1189. doi:10.1002/ptr.2754
Hussain, A. I., Rathore, H. A., Sattar, M. Z., Chatha, S. A., Sarker, S. D., and Gilani, A. H. (2014). Citrullus colocynthis (L.) Schrad (Bitter Apple Fruit): A Review of its Phytochemistry, Pharmacology, Traditional Uses and Nutritional Potential. J. Ethnopharmacol 155 (1), 54–66. doi:10.1016/j.jep.2014.06.011
Javadzadeh, H. R., Davoudi, A., Davoudi, F., Valizadegan, G., Goodarzi, H., Mahmoodi, S., et al. (20132013). Citrullus colocynthis as the Cause of Acute Rectorrhagia. Case Rep. Emerg. Med. 2013, 1–5. doi:10.1155/2013/652192
Kamran, Z., Ruby, T., Hussain, M., Ali, S., Ahmad, S., Koutoulis, K. C., et al. (2018). Comparative Efficacy of Citrullus colocynthis Fruit Powder and Popular Antibiotic Growth Promoters in Broilers. 6th Mediterranean Poultry Summit.
Khatibi, R., and Teymorri, J. (2011). Anticandidal Screening and Antibacterial of Citrullus colocynthis in South East of Iran. J. Hortic. For. 3 (13), 392–398. doi:10.5897/JHF11.030
Khatri, L. M., Nasir, M. K. A., Saleem, Rubina., and Valhari, M. U. (1993). Pakistan J. Scientific Ind. Res. 36, 383. BCI:BCI199497409740.
Kim, M. G., Lee, S. E., Yang, J. Y., and Lee, H. S. (2014). Antimicrobial Potentials of Active Component Isolated from Citrullus colocynthis Fruits and Structure-Activity Relationships of its Analogues against Foodborne Bacteria. J. Sci. Food Agric. 94 (12), 2529–2533. doi:10.1002/jsfa.6590
Kumar, S., Kumar, D., Manjusha, K., Saroha, K., Singh, N., and Vashishta, B. (2008). Antioxidant and Free Radical Scavenging Potential of Citrullus colocynthis (L.) Schrad. Methanolic Fruit Extract. Acta Pharm. 58 (2), 215–220. doi:10.2478/v10007-008-0008-1
Lahfa, F. B., Azzi, R., Mezouar, D., and Djaziri, R. (2017). Hypoglycemic Effect of Citrullus colocynthis Extracts. Phytothérapie 15 (2), 50–56. doi:10.1007/s10298-015-0997-4
Lang, K. L., Silva, I. T., Machado, V. R., Zimmermann, L. A., Caro, M. S., Simões, C. M., et al. (2014). Multivariate SAR and QSAR of Cucurbitacin Derivatives as Cytotoxic Compounds in a Human Lung Adenocarcinoma Cell Line. J. Mol. Graph Model. 48, 70–79. doi:10.1016/j.jmgm.2013.12.004
Manzoor, A., Khan, I. A., Iqbal, M. O., Kousar, S., Munawar, s. H., Manzoor, Z., et al. (2020). Evaluation of Cardioprotective Potential of Hydroalcoholic Peel Extract of Citrullus Colocynthis. doi:10.21203/rs.3.rs-124440/v1
Marzouk, B., Marzouk, Z., Décor, R., Edziri, H., Haloui, E., Fenina, N., et al. (2009). Antibacterial and Anticandidal Screening of Tunisian Citrullus colocynthis Schrad. From Medenine. J. Ethnopharmacol 125 (2), 344–349. doi:10.1016/j.jep.2009.04.025
Marzouk, B., Marzouk, Z., Fenina, N., Bouraoui, A., and Aouni, M. (2011). Anti-inflammatory and Analgesic Activities of Tunisian Citrullus colocynthis Schrad. Immature Fruit and Seed Organic Extracts. Eur. Rev. Med. Pharmacol. Sci. 15 (6), 665–672. doi:10.1016/j.jep.2009.11.027
Marzouk, B., Mahjoub, M. A., Bouraoui, A., Fenina, N., Aouni, M., and Marzouk, Z. (2013). Anti-inflammatory and Analgesic Activities of a New Cucurbitacin Isolated from Citrullus colocynthis Seeds. Med. Chem. Res. 22 (8), 3984–3990. doi:10.1007/s00044-012-0406-2
Mazher, M., Ishtiaq, M., Mushtaq, W., Maqbool, M., Zahid, N., Husain, T., et al. (2020). Comprehensive Review of Phytochemistry and Bioactivities of Citrullus Colocynthis (L.) Schrad. Pharm. Res. 4 (4), 486. doi:10.33215/sjom.v4i1.486
Mehrzadi, S., Shojaii, A., Pur, S. A., and Motevalian, M. (2016). Anticonvulsant Activity of Hydroalcoholic Extract of Citrullus colocynthis Fruit: Involvement of Benzodiazepine and Opioid Receptors. J. Evid. Based Complement. Altern Med 21 (4), NP31–5. doi:10.1177/2156587215615455
Meybodi, M. S. K. (2020). A Review on Pharmacological Activities of Citrullus colocynthis (L.) Schrad. Asian J. Res. Rep. Endocrinol., 25–34.
Meziane, R. K., Khemmar, L., Amamou, F., Yazit, M., and Didi, A. (2012). Anti-obesity and Anti-hyperlipidemic Effect of Citrullus colocynthis Oil in the Offspring of Obese Rats. Ann. Biol. Res. 3 (5), 2486–2490. www.scholarsresearchlibrary.com.
Milanov, D., Ljubojevic, D., Cabarkapa, I., and Aleksic, N. (2016). Impact of Antibiotics Used as Growth Promoters on Bacterial Resistance. Food Feed Res. 43 (2), 83–92. doi:10.5937/FFR1602083M
Najafi, S., Sanadgol, N., Nejad, B. S., Beiragi, M. A., and Sanadgol, E. (2010). Phytochemical Screening and Antibacterial Activity of Citrullus colocynthis (Linn.) Schrad against Staphylococcus aureus. J. Med. Plants Res. 4 (22), 2321–2325. doi:10.5897/JMPR10.192
Ogundele, J. O., Oshodi, A. A., and Amoo, I. A. (2012). Comparative Study of Amino Acid and Proximate Composition of Citurllus Colocynthis and Citrullus vulgaris Seeds. Pakistan J. Nutr. 11 (3), 247.
Oryan, A., Hashemnia, M., Hamidi, A. R., and Mohammadalipour, A. (2014). Effects of Hydro-Ethanol Extract of Citrullus colocynthis on Blood Glucose Levels and Pathology of Organs in Alloxan-Induced Diabetic Rats. Asian Pac. J. Trop. Dis. 4 (2), 125–251. doi:10.1016/S2222-1808(14)60328-510.3923/pjn.2012.247.251
Pakistan economic survey (2020-2021). Accessed on https://www.finance.gov.pk/survey_2021.html.
Parveen, B., Upadhyay, B., Roy, S., and Kumar, A. (2007). Traditional Uses of Medicinal Plants Among the Rural Communities of Churu District in the Thar Desert, India. J. Ethnopharmacol 113 (3), 387–399. doi:10.1016/j.jep.2007.06.010
Peric, L., Zikic, D., and Lukic, M. (2009). Application of Alternative Growth Promoters in Broiler Production. Biotec Anim. Husb 25 (5-6-1), 387–397. doi:10.2298/BAH0906387P
Perveen, S., Ashfaq, H., Ambreen, S., Ashfaq, I., Kanwal, Z., and Tayyeb, A. (2021). Methanolic Extract of Citrullus colocynthis Suppresses Growth and Proliferation of Breast Cancer Cells through Regulation of Cell Cycle. Saudi J. Biol. Sci. 28 (1), 879–886. doi:10.1016/j.sjbs.2020.11.029
Perveen, S., Ashfaq, H., Shahjahan, M., Manzoor, A., and Tayyeb, A. (2020). Citrullus colocynthis Regulates De Novo Lipid Biosynthesis in Human Breast Cancer Cells. J. Cancer Res. Ther. 16 (6), 1294–1301. doi:10.4103/jcrt.JCRT_206_20
Pravin, B., Tushar, D., Vijay, P., and Kishanchnad, K. (2013). Review on Citrullus colocynthis. Int. J. Res. Pharm. Chem. 3 (1), 46–53. doi:10.1089/acm.2011.0297
Qureshi, R., Bhatti, G. R., and Memon, R. A. (2010). Ethnomedicinal Uses of Herbs from Northern Part of Nara Desert, Pakistan. Pak J. Bot. 42 (2), 839–851.
Rahbar, A. R., and Nabipour, I. (2010). The Hypolipidemic Effect of Citrullus colocynthis on Patients with Hyperlipidemia. Pak J. Biol. Sci. 13 (24), 1202–1207. doi:10.3923/pjbs.2010.1202.1207
Rahimi, R., Amin, G., and Ardekani, M. R. (2012). A Review on Citrullus colocynthis Schrad.: from Traditional Iranian Medicine to Modern Phytotherapy. J. Altern. Complement. Med. 18 (6), 551–554. doi:10.1089/acm.2011.0297
Rasool Hassan, B. A. (2012). Medicinal Plants (Importance and Uses). Pharm. Anal. Acta 03, 32153–32435. doi:10.4172/2153-2435.1000e139
Reda, F. M., El-Saadony, M. T., El-Rayes, T. K., Farahat, M., Attia, G., and Alagawany, M. (2021). Dietary Effect of Licorice (Glycyrrhiza Glabra) on Quail Performance, Carcass, Blood Metabolites and Intestinal Microbiota. Poult. Sci., 100, 101266, doi:10.1016/j.psj.2021.101266
Rezvani, M., Hassanpour, M., Khodashenas, M., Naseh, G., Abdollahi, M., and Mehrpour, O. (2011). Citrullus Colocynthis (Bitter Apple) Poisoning; A Case Report. Indian J. Forensic Med. Toxicol. 5 (2). http://bsid.bums.ac.ir/dspace/handle/bums/7901.
Rinttilä, T., and Apajalahti, J. (2013). Intestinal Microbiota and Metabolites-Implications for Broiler Chicken Health and Performance. J. Appl. Poult. Res. 22 (3), 647–658. doi:10.3382/japr.2013-00742
Rizvi, T. S., Khan, A. L., Ali, L., Al-Mawali, N., Mabood, F., Hussain, J., et al. (2018). In Vitro oxidative Stress Regulatory Potential of Citrullus colocynthis and Tephrosia Apollinea. Acta Pharm. 68 (2), 235–242. doi:10.2478/acph-2018-0012
Saeed, M., Boulos, J. C., Elhaboub, G., Rigano, D., Saab, A., Loizzo, M. R., et al. (2019). Cytotoxicity of Cucurbitacin E from Citrullus colocynthis against Multidrug-Resistant Cancer Cells. Phytomedicine 62, 152945. doi:10.1016/j.phymed.2019.152945
Saeed, M., Naveed, M., Arif, M., Kakar, M. U., Manzoor, R., Abd El-Hack, M. E., et al. (2021). Biomed. Pharmacother. 270, 113772. doi:10.1016/j.jep.2020.113772
Saeed, M., Naveed, M., BiBi, J., Ali Kamboh, A., Phil, L., and Chao, S. (2019). Potential Nutraceutical and Food Additive Properties and Risks of Coffee: a Comprehensive Overview. Crit. Rev. Food Sci. Nutr. 59 (20), 3293–3319. doi:10.1080/10408398.2018.1489368
Sawaya, W. N., Daghir, N. J., and Khalil, J. K. (1986). Citrullus colocynthis Seeds as a Potential Source of Protein for Food and Feed. J. Agric. Food Chem. 34 (2), 285–288. doi:10.1021/jf00068a035
Schafferman, D., Beharav, A., Shabelsky, E., and Yaniv, Z. (1998). Evaluation ofCitrullus Colocynthis, a Desert Plant Native in Israel, as a Potential Source of Edible Oil. J. Arid Environments 40 (4), 431–439. doi:10.1006/jare.1998.0454
Shafaei, H., Rad, J. S., Delazar, A., and Behjati, M. (2014). The Effect of Pulp and Seed Extract of Citrullus Colocynthis, as an Antidaibetic Medicinal Herb, on Hepatocytes Glycogen Stores in Diabetic Rabbits. Adv. Biomed. Res. 3, 258. doi:10.4103/2277-9175.148230
Shafaei, H., Esmaeili, A., Rad, J. S., Delazar, A., and Behjati, M. (2012). Citrullus colocynthis as a Medicinal or Poisonous Plant: a Revised Fact. J. Med. Plants Res. 6 (35), 4922–4927. doi:10.5897/JMPR11.264
Soufane, S., Bedda, A., Mahdeb, N., and Bouzidi, A. (2013). Acute Toxicity Study on Citrullus colocynthis Fruit Methanol Extract in Albino Rats. J. Appl. Pharm. Sci. 3 (6), 88. doi:10.7324/JAPS.2013.3614
Tabani, K., Birem, Z., Halzoune, H., Saiah, W., Lahfa, F., Koceir, E. A., et al. (2018). Therapeutic Effect of Alkaloids and Glycosides of Colocynth Seeds on Liver Injury, Associated with Metabolic Syndrome in Wistar Rats, Subject to Nutritional Stress. Pak J. Pharm. Sci. 31, 277–290.
Xiao, J., Capanoglu, E., Jassbi, A. R., and Miron, A. (2016). Advance on the Flavonoid C-Glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 56 Suppl 1 (Suppl. 1), S29–S45. doi:10.1080/10408398.2015.1067595
Yitbarek, M. B. (2015). Phytogenics as Feed Additives in Poultry Production: a Review. Int. J. Extensive Res. 3, 49–60.
Yoshikawa, M., Morikawa, T., Kobayashi, H., Nakamura, A., Matsuhira, K., Nakamura, S., et al. (2007). Bioactive Saponins and Glycosides. XXVII. Structures of New Cucurbitane-type Triterpene Glycosides and Antiallergic Constituents from Citrullus colocynthis. Chem. Pharm. Bull. (Tokyo) 55 (3), 428–434. doi:10.1248/cpb.55.428
Keywords: Citrullus colocynthis, traditional uses, health aspects, human, poultry
Citation: Li Q-Y, Munawar M, Saeed M, Shen J-Q, Khan MS, Noreen S, Alagawany M, Naveed M, Madni A and Li C-X (2022) Citrullus colocynthis (L.) Schrad (Bitter Apple Fruit): Promising Traditional Uses, Pharmacological Effects, Aspects, and Potential Applications. Front. Pharmacol. 12:791049. doi: 10.3389/fphar.2021.791049
Received: 07 October 2021; Accepted: 09 December 2021;
Published: 25 January 2022.
Edited by:
Massimo Lucarini, Council for Agricultural Research and Economics, ItalyReviewed by:
Syed Nasir Abbas Bukhari, Al Jouf University, Saudi ArabiaZohara Yaniv Bachrach, Agricultural Research Organization, Volcani Center, Israel
Copyright © 2022 Li, Munawar, Saeed, Shen, Khan, Noreen, Alagawany, Naveed, Madni and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Sajjad Khan, ZHJzYWpqYWQyQHlhaG9vLmNvbQ==; Chang-Xing Li, bGN4MTUzNUAxNjMuY29t
†These authors have contributed equally to this work
 Qin-Yuan Li1†
Qin-Yuan Li1† Mahzaib Munawar
Mahzaib Munawar Muhammad Saeed
Muhammad Saeed Mahmoud Alagawany
Mahmoud Alagawany Muhammad Naveed
Muhammad Naveed