
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 14 January 2022
Sec. Pharmacoepidemiology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.789872
This article is part of the Research Topic Shaping with Data: Using Pharmacoepidemiology to Shape Pharmaceutical Policy and Clinical Decision-Making View all 16 articles
 Lisiane Freitas Leal1
Lisiane Freitas Leal1 Claudia Garcia Serpa Osorio-de-Castro2
Claudia Garcia Serpa Osorio-de-Castro2 Luiz Júpiter Carneiro de Souza3
Luiz Júpiter Carneiro de Souza3 Felipe Ferre4
Felipe Ferre4 Daniel Marques Mota5
Daniel Marques Mota5 Marcia Ito6
Marcia Ito6 Monique Elseviers7
Monique Elseviers7 Elisangela da Costa Lima8
Elisangela da Costa Lima8 Ivan Ricardo Zimmernan9
Ivan Ricardo Zimmernan9 Izabela Fulone10
Izabela Fulone10 Monica Da Luz Carvalho-Soares5
Monica Da Luz Carvalho-Soares5 Luciane Cruz Lopes10*
Luciane Cruz Lopes10*Background: In Brazil, studies that map electronic healthcare databases in order to assess their suitability for use in pharmacoepidemiologic research are lacking. We aimed to identify, catalogue, and characterize Brazilian data sources for Drug Utilization Research (DUR).
Methods: The present study is part of the project entitled, “Publicly Available Data Sources for Drug Utilization Research in Latin American (LatAm) Countries.” A network of Brazilian health experts was assembled to map secondary administrative data from healthcare organizations that might provide information related to medication use. A multi-phase approach including internet search of institutional government websites, traditional bibliographic databases, and experts’ input was used for mapping the data sources. The reviewers searched, screened and selected the data sources independently; disagreements were resolved by consensus. Data sources were grouped into the following categories: 1) automated databases; 2) Electronic Medical Records (EMR); 3) national surveys or datasets; 4) adverse event reporting systems; and 5) others. Each data source was characterized by accessibility, geographic granularity, setting, type of data (aggregate or individual-level), and years of coverage. We also searched for publications related to each data source.
Results: A total of 62 data sources were identified and screened; 38 met the eligibility criteria for inclusion and were fully characterized. We grouped 23 (60%) as automated databases, four (11%) as adverse event reporting systems, four (11%) as EMRs, three (8%) as national surveys or datasets, and four (11%) as other types. Eighteen (47%) were classified as publicly and conveniently accessible online; providing information at national level. Most of them offered more than 5 years of comprehensive data coverage, and presented data at both the individual and aggregated levels. No information about population coverage was found. Drug coding is not uniform; each data source has its own coding system, depending on the purpose of the data. At least one scientific publication was found for each publicly available data source.
Conclusions: There are several types of data sources for DUR in Brazil, but a uniform system for drug classification and data quality evaluation does not exist. The extent of population covered by year is unknown. Our comprehensive and structured inventory reveals a need for full characterization of these data sources.
Drug utilization research (DUR) aims to examine patterns of medication use and adherence to treatments and to assess determinants of utilization (Godman et al., 2016; Wettermark et al., 2016) The history of DUR is described elsewhere (World Health Organization, 1993; World Health Organization, 2003b; Wettermark, 2013; Wettermark et al., 2016). Over the years, the scope of DUR has expanded; methods have improved, and the use of secondary data has increased. Nonetheless, additional work is required, particularly with regard to the quality of available data (Evans, 2012; Schneeweiss, 2019).
Secondary data that are used for pharmacoepidemiology research are usually derived from information routinely collected for administrative purposes and as part of patient care (Eriksson and Ibáñez, 2016), such as drug sales, medical billing, and prescriptions (Shalini et al., 2010). Given the cost and difficulty of primary data collection, electronic healthcare databases (EHD) are commonly used in many countries to study drug safety (Pacurariu et al., 2018). Linkage of data on medication use with diagnostic, mortality, and other health databases has become routine in Europe, North America, and Asian countries (Wettermark, 2013), but not in low- and middle-income countries, notably, in Latin America (de Castro, 1999; de Castro, 2000; World Health Organization, 2003a; Baldoni, 2011; Coelho and Santos, 2012).
While high-income countries are leveraging the use of Real-World Evidence to inform regulatory decision-making (European Medicines Agency (EMA), 2018; Health Canada, 2019; Food and Drug Administration (FDA), 2020), in Latin America initiatives are incipient and limited to few settings (Durán et al., 2016; Salas et al., 2018). In Brazil, efforts related to “open data” have improved the prospects for creating systematic approaches to the use of secondary data, not only for decision-making but also for research (Controladoria Geral da União, 2020).
Despite awareness of the value of existing databases, and observed expansion of DUR in Brazil using secondary data, a mapping of databases to evaluate their potential, as well as their characteristics and applications, has not been undertaken.
The present work aimed, therefore, to identify, catalogue, and characterize secondary data sources for DUR in Brazil.
This project was derived from the “Publicly Available Data Sources for Drug Utilization Research in Latin American (LatAm) Countries—DASDURLATAM study,” which is an initiative supported by the International Society for Pharmacoepidemiology (ISPE) to make an inventory for all LatAm countries (Lopes et al., 2021).
We employed a multi-phase approach to map Brazilian data sources. A network of national health experts was assembled to prepare an initial inventory of data sources for DUR. A multidisciplinary network was established. Fourteen Brazilian researchers experts in pharmacoepidemiology and health professionals working in both academia and the government sector were invited and accepted to participate. A pharmacoepidemiology expert in European data sources for DUR joined the Brazilian team (ME). A literature review was conducted to retrieve drug utilization studies conducted in Brazil using secondary data. Finally, data sources were selected and characterized.
The eligibility criteria for inclusion in the inventory specified Brazilian data sources generated by healthcare organizations that provide information related to medication use. Data sources from health insurance companies or other commercial providers (e.g., IQVIA) were not eligible. The Brazilian health care system consists of public and private components. Population access depends on several factors, including the ability to pay for health care. We, therefore, focused on data sources generated by the public health system because:
1) The public system provides national data with municipality granularity.
2) Almost 80% of the Brazilian population is covered by the public system; private health care insurance companies are spread across the country and comprise many small companies, not representative of the general population (Paim et al., 2011; Massuda et al., 2018).
3) It is not possible to map data with no payment requests or ethical approval.
We excluded data sources in which information about medicines (names or codes) was not recorded.
We conducted an internet search of institutional government websites up to January 2021. To retrieve studies, we reviewed the literature available on traditional bibliographic databases (MEDLINE/PubMed, LILACS, Google Scholar) from inception to August 2020, with no limits on publication type, status, or language. The concept terms were freely combined, using Boolean operators (AND/OR): “pharmacoepidemiology,” “drug utilization,” “BRAZIL,” and the acronym of the data source first identified. The Systems and Products Catalog of the Informatics Department of the Unified Health System–DataSUS (Ministério da Saúde, 2020) was also reviewed. This was done to assess the description of all systems already available through the Ministry of Health interface, and the availability of medication data recorded by the Ministry of Health, and not previously identified by the network of specialists or through the literature review.
Working in pairs and independently, the expert network (DMM, CGSOC, LCL, FF, LFL and LJCS) conducted in-depth screening and reviewed potentially eligible data sources. Disagreements on whether specific data sources contained drug information and whether they should remain on the list to be mapped as potential data source for DUR were discussed in online meetings. A consensus was achieved on the inclusion or exclusion of data sources.
The data sources were classified and grouped into the following categories: 1) automated databases (subclassified as administrative claims data and other transactional and operational data); 2) Electronic Medical Records (EMR); 3) national surveys or datasets; 4) adverse event reporting systems; and 5) other sources, according to Harpe et al.‘s classification for secondary data (Harpe, 2010) (Supplemental Table S1). For a general description of each data source, we used a seven-criteria checklist (Box 1). Additional information for characterizing the data sources was collected: custodian; data retrieval pathway, corresponding to the Uniform Resource Locator (URL) where the data source may be found; file format in which data are provided, that is, the way in which information is encoded for storage (comma-separated values—CSV, XLSX, ZIP, Plain Text-txt, or another format); and type of tables used for medication coding—European Article Number-EAN, Brazilian Non-proprietary Names (in Portuguese, Denominação Comum Brasileira—DCB), or other). Additional information was completed according to the provider’s definitions and specialist consultation (FF and LJCS). Each national DUR expert was responsible for reviewing the descriptions of the data sources and their final characterization.
The expert network identified 62 data sources. After application of the exclusion criteria, 39 sources were included. Two of them (SIASG-Sistema Integrado de Administração de Serviços Gerais and SISME-Sistema de Minuta de Empenho) were related to the same drug-purchasing system and were grouped as one data source. Thus, the final selection consisted of 38 data sources, which underwent further characterization (Figure 1). Six rounds of discussion took place among the national health experts in order to achieve consensus and define the final list (Supplementary Table S2).
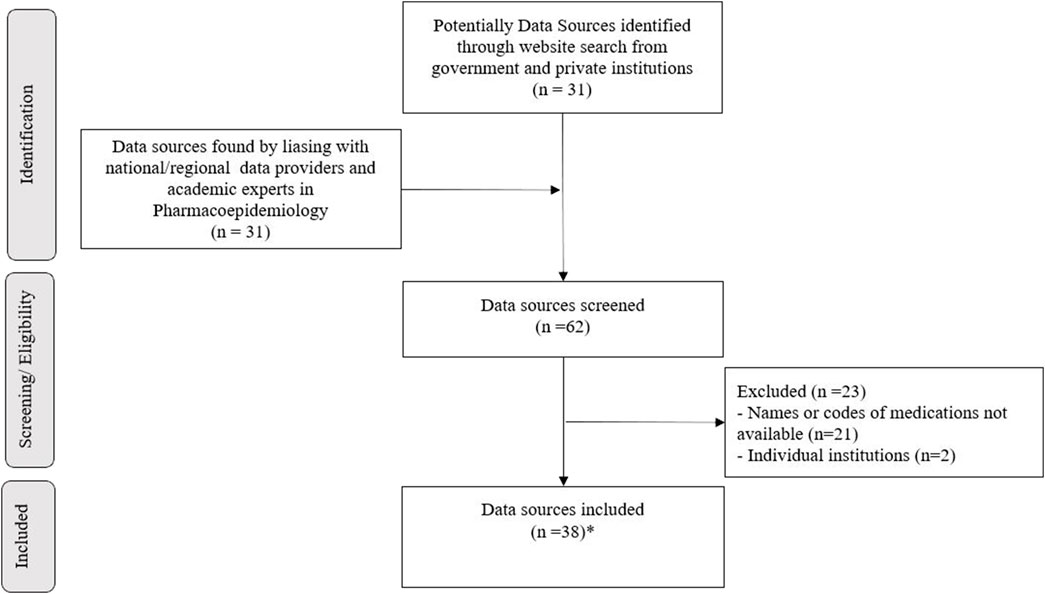
FIGURE 1. Data sources for DUR in Brazil selection flowchart. * means third-nine data sources were selected. When characterized, two were related to the same drug-purchasing system and were grouped into one data source (SIASG and SISME).
Figure 2 shows how the data sources were grouped. Twenty-three (60%) were classified as automated health care databases; four (11%) as EMRs; four (11%) as adverse event reporting systems; three (8%) as national surveys or datasets; and four (11%) as other types. The description of each data source, as well as the rationale for grouping it in a particular category, is provided in the supplementary material (Supplementary Table S3).
Based on the analysis of each data source, 18 (47%) were classified as “publicly and conveniently accessible online,” 15 of which (88%) were accessible through the DataSUS, with the Brazilian Ministry of Health as custodian. All publicly available online data sources provided national information; most of them had more than 5 years of coverage and both individual- and aggregate-level information. Twenty data sources (53%) were known to collect individual-level data, and three (PNAUM, SIA-SUS, and SIVEP-Gripe) were available for download. Table 1 displays the data sources, grouped by accessibility, geographic granularity, type, setting, and initial year of release. The detailed classification, which allows comparability among the data sources is provided in the supplementary material (Supplementary Table S4).
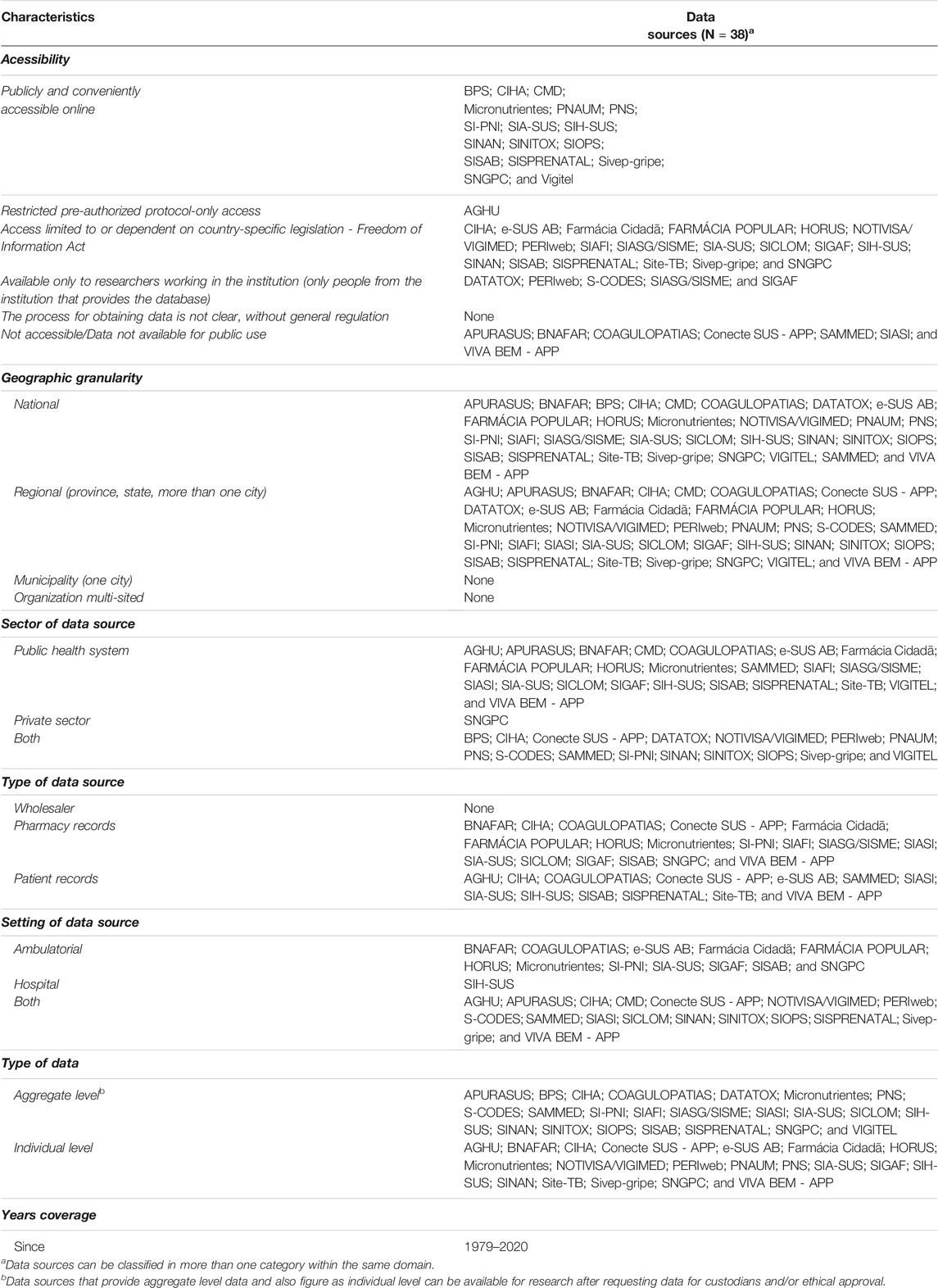
TABLE 1. Selected data sources, grouped by accessibility, geographic granularity, sector, setting, and type of data.
URLs for the “publicly and conveniently accessible online” data sources are shown in Table 2, as well as the file formats. The URLs for all data sources selected are provided in the supplemental material (Supplementary Table S5). Access through the FTP directory is provided for limited data sources and is also provided in the supplemental material (Supplementary Table S6).
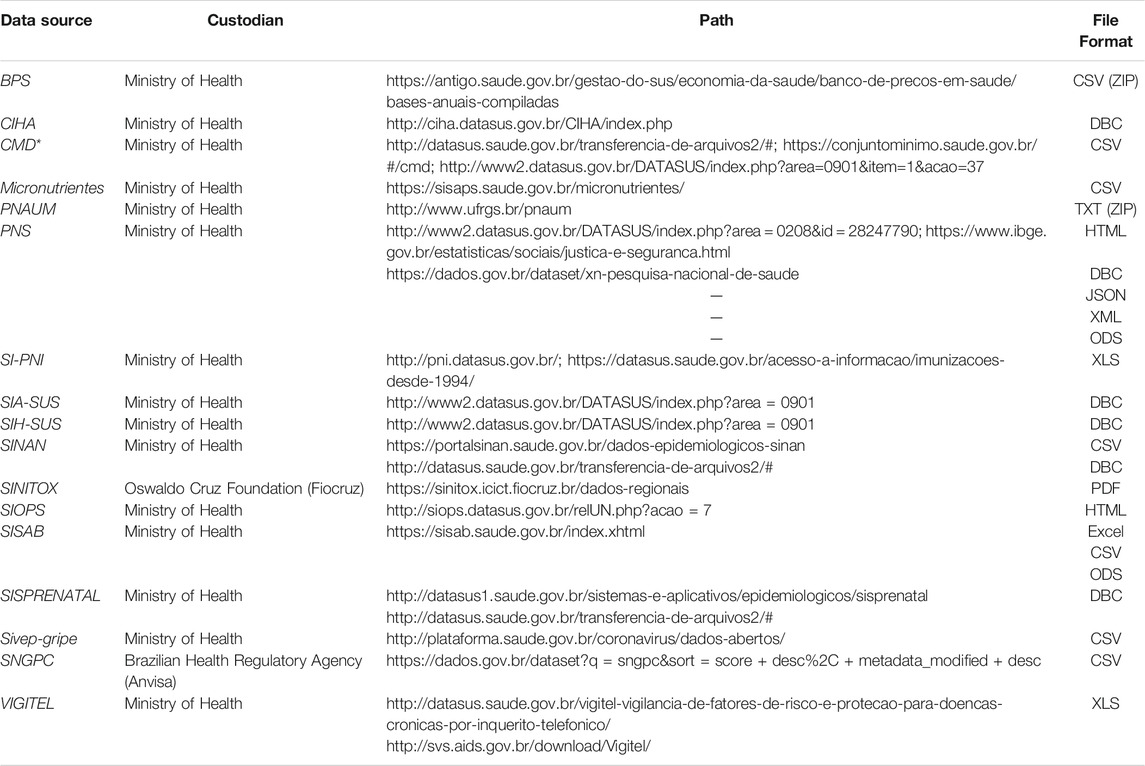
TABLE 2. Additional characteristics: path and file format available among data sources freely available online.
In Brazil, six different ways of assigning codes to medicines were found. Drug coding is not uniform; each data source has its own coding system, depending on the purpose of the data. The drug coding systems employed in Brazil are shown in Table 3, with examples of data sources that use each system. This information was not available for all data sources, an indication of the need for further work on characterization.
The literature review was part of the initial process for mapping Brazilian databases. We found publications related to 23 of the 38 data sources, including reports, manuals, and other documents available online. Scientific articles had been published in national and in international journals. Examples of studies that used some of the selected data sources are presented in Table 4.
This study provides an overview of data sources that are used or have the potential to be used for DUR in Brazil. A total of 38 sources were identified, almost half of which are publicly available and provide national information. Nineteen sources collect individual-level data, but few provide it for download. Those classified as “other sources” were generally related to Ministry of Health administrative processes, as medicines purchases and prices. Further characterization to determine the types of research questions they might address is needed. In Brazil, six different ways of assigning codes to medicines are employed, none of which is recognized internationally. Brazilian data sources have the potential to answer research questions related to medication use, adherence to treatments, purchases, and safety. However, currently mapped sources comprise a mix of databases, of unknown quality, centralized by the national government, but decentralized in terms of research and their usability and purposes for decision-making and post-market surveillance.
Some of the data sources presented here had been used by researchers. Ali et al. have described the linkable databases currently available for evaluating health technology assessment in Brazil (Ali et al., 2019). For example, the CIDACS initiative uses SINAN, SIH-SUS, SINASC (Live Births Information System), and SIM (Mortality Information System) to assess outcomes of major social programs (Barreto et al., 2019). SIM (not included in our inventory because it presents only data related to ICD-10 codes for drug poisoning mortality (Mota et al., 2012)) and SINASC are important sources of data for evaluating health outcomes and indicators. The quality of data in both systems has improved over time (Szwarcwald et al., 2019; França et al., 2020); however, health outcomes of medication exposure (not related to poisoning) remain unexplored for most classes of medicines.
Junior et al. linked SIH, SIA, SIM, SINASC and SINAN (Guerra Junior et al., 2018) and created a National Database of Health for longitudinal studies. Freire et al. linked SIM and SIH, including information from APACs (Authorization of High Complexity Procedures of the Outpatient), provided by the SIA-SUS system, and were able to describe the trajectory of patients in the health care network, and cancer-related hospital admissions (Freire et al., 2015). In fact, the APAC reports are among the most important sources of information on medication dispensing in Brazil. However, the information pertains only to drugs dispensed free of charge; that is, only medications supplied by SUS under the APACs are recorded and available through DataSus systems. Moreover, the generation and consolidation of APACs to make the data available for DUR are complex. Few research groups have the expertise required to link the different data sources and prepare the data for longitudinal analysis (Soares and Silva, 2013).
Exposure to medications among the Brazilian population is complicated by the structure of health care delivery, where a private system co-exists with a public system, and no overall control is in place for dispensing most medicines. Consequently, only studies using data from APACs for biological agents, chemotherapy, and other high-cost medicines have the potential to correctly ascertain exposure (Prestes, 2017; Junior et al., 2018).
Other automated health care databases, some of which were identified by Ali et al. (Ali et al., 2019), could be valuable for DUR, but not without an extensive evaluation of the quality of the data they contain. Notable examples are Horus, Farmacia Popular and BNAFAR. Interfaces among the systems that generate these databases are known, but nothing is known about their quality, coverage, and completeness. These data sources, specifically the BNAFAR and the Horus, were not available for research (Ministério da Saúde, 2018). Infrastructure issues are familiar limitations, and at least partially explain why data on drug dispensing are so difficult to obtain in our country (Herrett et al., 2015; Hallas et al., 2017). Pharmacoepidemiology research perspectives in Brazil suffer constraints not due to lack of data, but to lack of linked data and cross-validated secondary data (de Castro, 1999; Junior et al., 2018; da Saúde, 2018).
The Sistema Nacional de Gerenciamento de Produtos Controlados (SNGPC) (Agência Nacional de Vigilância Sanitária, 2019), which monitors dispensing of narcotic and psychotropic medications, and since 2013, antibiotics, is an important data source for controlling the purchase and dispensing of medicines. An “open data” initiative launched by ANVISA has yielded data for DUR. The expectation is that data provided by ANVISA might allow assessing, for example, policy impact of medicines regulation. However, a complete characterization of these data sources for understanding the quality of provided data, and what research questions would be answered using the open data are still lacking.
SIVEP-Gripe and SI-PNI, among other automated health care databases (Table 1), record information on medication use, but the quality, temporality and feasibility for linkage of these data have not been adequately explored for DUR. SIVEP-Gripe is available and provides individual-level data, but the incompleteness of certain variables and lack of temporality in recording medication use, render the information useless for examining, for instance, the effectiveness of medication use. SIVEP-Gripe is an epidemiologic surveillance system that was designed for other purposes, but with properly recorded information, it could help answer important research questions and support other voluntary reporting systems in evaluating adverse drug effects (Melo et al., 2021). As well, non-prescription drugs recorded in surveillance systems such as SINAN, and SIVEP-Gripe are often taken during the onset of a disease—an upturn in sales may serve as an early indicator of an outbreak or epidemic (Das et al., 2005; Edge et al., 2006).
The Electronic Medical Record (EMR) of the Management Application for University Hospitals–AGHU currently covers 30 hospitals across the country (Ministério da Educação, 2019). It is the standard management system for all federal university hospitals provided by the Empresa Brasileira de Serviços Hospitalares (Ebserh) network and is a potential data source for DUR. University hospitals treat both in- and outpatients. The creation of a large cohort of patients receiving different levels of care would allow for follow-up of short- and long-term effects of medication on several outcomes. e-SUS AB might be used for the same purpose. However, no single DUR study was found to have used the Ebserh data.
We classified four data sources as adverse event report systems: NOTIVISA/VIGIMED, SINAN, SINITOX, and DATATOX. Recently, ANVISA published implementation of the VigiFlow (named Vigimed in Brazil) (Vogler et al., 2020) as a substitute for the NOTIVISA in an effort to enhance the usability of the national system. But no information is available about how different pharmacovigilance systems across the country could be integrated. In 2021, part of Vigimed aggregated data was available on the Anvisa website by drug, adverse reaction (MedDRA SOC/Preferred Term), severity, age group, gender, state of the case report, for example. Clinical trial reports are also recorded in the same database (Notivisa EC) but are not available given the need for data confidentiality.
Spontaneous reporting systems constitute a major resource for detecting adverse drug effects and have made important contributions to pharmacoepidemiology (Strom and Carson, 1990). Systems for active surveillance and projects for detecting signals and monitoring recently approved medications (Racoosin et al., 2012) have been established in other countries. Recent studies involving disproportionality analysis for safety signal screening in children (Vieira et al., 2020) and breast cancer patients (Barcelos et al., 2019) using Notivisa were conducted, demonstrating the potential of this data source. However, Brazil lags behind in terms of research initiatives and decision-making using automated administrative data.
The only national-level drug utilization study that has been conducted in Brazil was based on primary data (Mengue et al., 2016a). The National Survey on Access, Use and Promotion of Rational Use of Medicines (PNAUM) was a cross-sectional, population-based study focusing on urban households. Fieldwork was carried out between September 2013 and February 2014. In total, 41,433 interviews were carried out. The survey examined medication use for chronic health conditions. However, the PNAUM has not been repeated, and the cross-sectional data do not allow evaluation of outcomes. Also, this was the only study to collect population-level data about over-the-counter medication use. Currently, no information about over-the-counter is available in any of the automated databases (Arrais et al., 2016). Other important surveys (cross-sectional) were included in our inventory—PNAD and Vigitel—although their purpose is to assess other characteristics of the Brazilian population and do not provide medication details.
Brazil has no formal policy on setting priorities and using administrative data to evaluate the effectiveness and safety of medications. However, many systems contain information for managing logistics and drug expenditures. APURASUS, SIGAF and SIASG are used by different levels of government to control costs and transmit information from local systems to the national level to plan acquisition and distribution. For example, SIASG made it possible to explore expenditures, pricing and judicial demands for a variety of drugs and drug classes, and it has been important for decision-making about the incorporation of drugs in the national list and the sustainability of provision programs (Luo et al., 2014; Chaves et al., 2017; Chama Borges Luz et al., 2017; Magarinos-Torres et al., 2017; dos Santos Teodoro et al., 2017; Alves et al., 2018; Caetano et al., 2020; dos Santos Dias et al., 2020, 2009–2017; Matos et al., 2020). However, the safety profile of medicines and outcomes in the population cannot be examined with these data.
Despite efforts made by the Ministry of Health to harmonize the recording of information, health institutions’ data collection processes differ considerably. Because of the structure of the healthcare system, patients typically seek care from a variety of providers at several institutions with nonlinked electronic health record systems. Combining data from these systems is a challenge. One of the most important issues to emerge from this study is the lack of unique key identifiers for individuals. These factors, in addition to technological infrastructure and skilled human resource constraints, limit the usefulness of routinely collected data in generating evidence to support clinical and policy decisions and in answering epidemiological questions (Ali et al., 2019).
Another important finding is the heterogeneity of drug-coding systems in Brazil. Federal Law No. 9,787/99 requires that, within the scope of the SUS, purchases of medicines, under any type of acquisition, as well as medical and dental prescriptions for medicines, adopt the DCB (Brazilian Non-proprietary name) or, in their absence, the International Non-proprietary Name (INN). However, this does not apply to administrative databases. For each data source, it is necessary to know the types of codes that are employed, how they are constructed, and why they are used, but no clear definitions are provided.
The limitations of this inventory of Brazilian databases that contain medication-related information are mainly related to the design of the study and the difficulty of assembling a group of experts with an in-depth knowledge of each data source. We may have missed data sources and relevant studies. The literature search was conducted using the names of the data sources, but if a name was unknown, studies could not be found, and the data source was not included. Moreover, this is only an inventory; full characterization of each database has yet to be done.
The main value of this study is to provide an overview with a focus on data sources for DUR. The methodology used by the LatAm project may be highlighted as one of the main strengths of our study, an original multi-phase approach allowing to map national data sources for DUR. The next step is to fully characterize each database using pre-established checklists (Hall et al., 2012), and thereby provide information that will help researchers determine which sources may be may be useful for specific types of studies; what research questions can feasibly be addressed; how the data can be accessed; and what quality may be expected from the data.
Based on this comprehensive and structured inventory, we provided an overview of the several types of data sources for DUR in Brazil. Our findings demonstrated that a uniform system for drug classification, data quality evaluation, and the extent of population covered by year are lacking in the mapped data sources. National administrative health databases are provided mainly through the DataSus and contain information about the population covered by the SUS. Further work is required to assess the reliability of Brazilian data for DUR.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
CGSOC, LCL, ME, and LFL contributed to the conception and design of the study. LFL organized the data and wrote the first draft of the manuscript. LJCS, FF, DMM, MI, ME, ECL, IRZ, IF, and MLCS performed the review and critical analysis of the data sources. All authors contributed to manuscript revision, read, and approved the submitted version.
This study is sponsored by the International Society of Pharmacoepidemiology ISPE/2018. LFL is supported by a postdoctoral scholarship award from the Fonds de recherche du Québec–Santé (FRQS; Quebec Foundation for Health Research) in parternership with Société québécoise d’hypertension artérielle (SQHA) — Dossier:290908 (2020–2021); and by a CIHR Drug Safety and Effectiveness Cross-Disciplinary Training Program (DSECT)— trainee funding Stream 1 (2021–2022).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors acknowledge the International Society for Pharmacoepidemiology for supporting the Publicly Available Data Sources for Drug Utilization Research in Latin American (LatAm) Countries—DASDURLATAM study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.789872/full#supplementary-material
Agência Nacional de Vigilância Sanitária (2019). Sistema Nacional de Gerenciamento de Produtos Controlados (SNGPC). Available at: http://portal.anvisa.gov.br/produtos_controlados (Accessed June 24, 2019).
Ali, M. S., Ichihara, M. Y., Lopes, L. C., Barbosa, G. C. G., Pita, R., Carreiro, R. P., et al. (2019). Administrative Data Linkage in Brazil: Potentials for Health Technology Assessment. Front. Pharmacol. 10, 984. doi:10.3389/fphar.2019.00984
Alves, J. C., Osorio-de-Castro, C. G. S., Wettermark, B., and Luz, T. C. B. (2018). Immunosuppressants in Brazil: Underlying Drivers of Spending Trends, 2010-2015. Expert Rev. Pharmacoecon Outcomes Res. 18, 565–572. doi:10.1080/14737167.2018.1485098
Alves, J. M., Albino, D. B. L., Resener, M. C., Zannin, M., Savaris, A., von Wangenheim, C. G., et al. (2016). “Quality Evaluation of Poison Control Information Systems: A Case Study of the DATATOX System,” in 2016 IEEE 29th International Symposium on Computer-Based Medical Systems (CBMS), Belfast and Dublin, Ireland, 20-24 June 2016 (IEEE), 30–35. doi:10.1109/CBMS.2016.53
Arrais, P. S. D., Fernandes, M. E., Pizzol, T., Ramos, L. R., Mengue, S. S., Luiza, V. L., et al. (2016). Prevalence of Self-Medication in Brazil and Associated Factors. Rev. Saude Publica 50, 13s. doi:10.1590/s1518-8787.2016050006117
Baldoni, A. (2011). A Farmacoepidemiologia No Brasil: Estado Da Arte Da Produção Científica. Ruvrv 9, 78–88. doi:10.5892/RUVRV.91.7888
Barbosa, W. B., Costa, J. O., de Lemos, L. L. P., Gomes, R. M., de Oliveira, H. N., Ruas, C. M., et al. (2018). Costs in the Treatment of Schizophrenia in Adults Receiving Atypical Antipsychotics: An 11-Year Cohort in Brazil. Appl. Health Econ. Health Pol. 16, 697–709. doi:10.1007/s40258-018-0408-4
Barcelos, F. C., de Matos, G. C., de Silva, M. J. S., da Silva, F. A. B., and da Silva, E. D. C. (2019). Suspected Adverse Drug Reactions Related to Breast Cancer Chemotherapy: Disproportionality Analysis of the Brazilian Spontaneous Reporting System. Front. Pharmacol. 10, 498. doi:10.3389/fphar.2019.00498
Barreto, M. L., Ichihara, M. Y., Almeida, B. A., Barreto, M. E., Cabral, L., Fiaccone, R. L., et al. (2019). The Centre for Data and Knowledge Integration for Health (CIDACS): Linking Health and Social Data in Brazil. Int. J. Popul. Data Sci. 4, 1140. doi:10.23889/ijpds.v4i2.1140
Bortoletto, M. É., and Bochner, R. (1999). Impacto Dos Medicamentos Nas Intoxicações Humanas No Brasil. Cad. Saúde Pública 15, 859–869. doi:10.1590/S0102-311X1999000400020
Caetano, R., Rodrigues, P. H. A., Corrêa, M. C. V., Villardi, P., and Osorio-de-Castro, C. G. S. (2020). The Case of Eculizumab: Litigation and Purchases by the Brazilian Ministry of Health. Rev. Saude Publica 54, 22. doi:10.11606/s1518-8787.2020054001693
Chama Borges Luz, T., Garcia Serpa Osorio-de-Castro, C., Magarinos-Torres, R., and Wettermark, B. (2017). Trends in Medicines Procurement by the Brazilian Federal Government from 2006 to 2013. PLoS ONE 12, e0174616. doi:10.1371/journal.pone.0174616
Chaves, G. C., Osorio-de-Castro, C. G. S., and Oliveira, M. A. (2017). Compras públicas de medicamentos para hepatite C no Brasil no período de 2005 a 2015. Ciênc. Saúde Coletiva 22, 2527–2538. doi:10.1590/1413-81232017228.05602017
Chieffi, A. L., and Barata, R. d. C. B. (2010). Ações judiciais: estratégia da indústria farmacêutica para introdução de novos medicamentos. Rev. Saúde Pública 44, 421–429. doi:10.1590/S0034-89102010000300005
Coelho, H. L. L., and Santos, D. B. (2012). “Farmacoepidemiologia,” in Epidemiologia & Saúde - Fundamentos, Métodos e Aplicações (Rio de Janeiro: Guanabara Koogan), 670–677.
Controladoria Geral da União (2020). Portal Brasileiro de Dados Abertos. Available at: https://dados.gov.br/(Accessed August 11, 2021).
Das, D., Metzger, K., Heffernan, R., Balter, S., Weiss, D., Mostashari, F., et al. (2005). Monitoring Over-the-counter Medication Sales for Early Detection of Disease Outbreaks--New York City. MMWR Suppl. 54, 41–46.
de Castro, C. G. S. O. (2000). Estudos de utilização de medicamentos: noções básicas. Rio de Janeiro, RJ: Editora Fiocruz.
de Castro, L. L. C. (1999). Farmacoepidemiologia no Brasil: evolução e perspectivas. Ciênc. Saúde Coletiva 4, 405–410. doi:10.1590/S1413-81231999000200014
dos Santos Dias, L. L., Santos, M. A. B. D., and Osorio-de-Castro, C. G. S. (2020). Public Financing of Human Insulins in Brazil: 2009-2017. Rev. Bras. Epidemiol. 23, e200075. doi:10.1590/1980-549720200075
dos Santos Teodoro, C. R., Caetano, R., Godman, B., dos Reis, A. L. A., Maia, A. A., Ramos, M. C. B., et al. (2017). Federal Procurement of Unlicensed Medicines in Brazil; Findings and Implications. Expert Rev. Pharmacoecon Outcomes Res. 17, 607–613. doi:10.1080/14737167.2017.1311209
Durán, C. E., Christiaens, T., Acosta, Á., and Vander Stichele, R. (2016). Systematic Review of Cross-National Drug Utilization Studies in Latin America: Methods and Comparability. Pharmacoepidemiol. Drug Saf. 25, 16–25. doi:10.1002/pds.3896
Edge, V. L., Pollari, F., Ng, L. K., Michel, P., McEwen, S. A., Wilson, J. B., et al. (2006). Syndromic Surveillance of Norovirus Using Over-the-counter Sales of Medications Related to Gastrointestinal Illness. Can. J. Infect. Dis. Med. Microbiol. 17, 235–241. doi:10.1155/2006/958191
Eriksson, I., and Ibáñez, L. (2016). “Secondary Data Sources for Drug Utilization Research,” in Drug Utilization Research. Editors M. Elseviers, B. Wettermark, A. B. Almarsdóttir, M. Andersen, R. Benko, M. Bennieet al. (Chichester, UK: John Wiley & Sons), 39–48. doi:10.1002/9781118949740.ch4
European Medicines Agency (EMA) (2018). Scientific Guidance on post-authorisation Efficacy Studies. Eur. Medicines Agency. Available at: https://www.ema.europa.eu/en/scientific-guidance-post-authorisation-efficacy-studies (Accessed January 26, 2021).
Evans, S. J. (2012). An Agenda For UK Clinical Pharmacology: Pharmacoepidemiology: Commentary. Br. J. Clin. Pharmacol. 73, 973–978. doi:10.1111/j.1365-2125.2012.04248.x
Food and Drug Administration (FDA) (2020). 21st Century Cures Act. FDA. Available at: https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act (Accessed January 26, 2021).
França, E., Ishitani, L. H., Teixeira, R., Duncan, B. B., Marinho, F., and Naghavi, M. (2020). Changes in the Quality of Cause-Of-Death Statistics in Brazil: Garbage Codes Among Registered Deaths in 1996-2016. Popul. Health Metr 18, 20. doi:10.1186/s12963-020-00221-4
Freire, S. M., Souza, R. C. d., and Almeida, R. T. d. (2015). Integrating Brazilian Health Information Systems in Order to Support the Building of Data Warehouses. Res. Biomed. Eng. 31, 196–207. doi:10.1590/2446-4740.0666
Godman, B., Kwon, H. Y., Bennie, M., and Almarsdóttir, A. B. (2016). “Drug Utilization and Health Policy,” in Drug Utilization Research. Editors M. Elseviers, B. Wettermark, A. B. Almarsdóttir, M. Andersen, R. Benko, M. Bennieet al. (Chichester, UK: John Wiley & Sons), 203–209. doi:10.1002/9781118949740.ch19
Gomes, R. M., Guerra Júnior, A. A., Lemos, L. L., Costa, J. O., Almeida, A. M., Alvares, J., et al. (2016). Ten-year Kidney Transplant Survival of Cyclosporine- or Tacrolimus-Treated Patients in Brazil. Expert Rev. Clin. Pharmacol. 9, 991–999. doi:10.1080/17512433.2016.1190270
Guerra Junior, A. A., Pereira, R. G., Gurgel, E. I., Cherchiglia, M., Dias, L. V., Ávila, J., et al. (2018). Building the National Database of Health Centred on the Individual: Administrative and Epidemiological Record Linkage - Brazil, 2000-2015. Int. J. Popul. Data Sci. 3 (1), 446. doi:10.23889/ijpds.v3i1.446
Hall, G. C., Sauer, B., Bourke, A., Brown, J. S., Reynolds, M. W., LoCasale, R., et al. (2012). Guidelines for Good Database Selection and Use in Pharmacoepidemiology Research. Pharmacoepidemiol. Drug Saf. 21, 1–10. doi:10.1002/pds.2229
Hallas, J., Hellfritzsch, M., Rix, M., Olesen, M., Reilev, M., and Pottegård, A. (2017). Odense Pharmacoepidemiological Database: A Review of Use and Content. Basic Clin. Pharmacol. Toxicol. 120, 419–425. doi:10.1111/bcpt.12764
Harpe, S. E. (2010). “4 Using Secondary Data in Pharmacoepidemiology,” in Understanding Pharmacoepidemiology (New York, USA: McGraw-Hill Professional Publishing), 55–78.
Health Canada (2019). Optimizing the Use of Real World Evidence to Inform Regulatory Decision-Making. Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/optimizing-real-world-evidence-regulatory-decisions.html (Accessed January 26, 2021).
Herrett, E., Gallagher, A. M., Bhaskaran, K., Forbes, H., Mathur, R., van Staa, T., et al. (2015). Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int. J. Epidemiol. 44, 827–836. doi:10.1093/ije/dyv098
Lopes, L. C., Salas, M., Osorio-de-Castro, C. G. S., Leal, L. F., Doubova, S. V., Cañás, M., et al. (2021). Data Sources for Drug Utilization Research in Latin American countries - A Cross-National study: DASDUR-LATAM Study. Pharmacoepidemiol Drug Saf. doi:10.1002/pds.5404
Luo, J., Oliveira, M. A., Ramos, M. B., Maia, A., and Osorio-de-Castro, C. G. (2014). Antiretroviral Drug Expenditure, Pricing and Judicial Demand: an Analysis of Federal Procurement Data in Brazil from 2004-2011. BMC Public Health 14, 367. doi:10.1186/1471-2458-14-367
Machado-dos-Santos, S. C. (2014). “Farmácia cidadã: integralidade, humanização e racionalidade na atenção ao paciente,” in Assistência farmacêutica: gestão e prática para profissionais da saúde (Rio de Janeiro: Editora Fiocruz), 407–416.
Madruga, L. G. d. S. L., da Silva, G. V. V., Alves, V. A. R., Velarde, L. G. C., Azeredo, T. B., Setúbal, S., et al. (2018). Aspectos relacionados à utilização de antirretrovirais em pacientes de alta complexidade no estado Do Rio de Janeiro, Brasil. Ciênc. Saúde Coletiva 23, 3649–3662. doi:10.1590/1413-812320182311.24742016
Magarinos-Torres, R., Lynd, L. D., Luz, T. C. B., Marques, P. E. P. C., and Osorio-de-Castro, C. G. S. (2017). Essential Medicines List Implementation Dynamics: A Case Study Using Brazilian Federal Medicines Expenditures. Basic Clin. Pharmacol. Toxicol. 121, 181–188. doi:10.1111/bcpt.12783
Maior, M. d. C. L. S., Osorio-de-Castro, C. G. S., and de Andrade, C. L. T. (2020). Demografia, óbitos e indicadores de agravamento nas internações por intoxicações medicamentosas entre menores de 5 anos no Brasil. Rev. Bras. Epidemiol. 23, e200016. doi:10.1590/1980-549720200016
Massuda, A., Hone, T., Leles, F. A. G., de Castro, M. C., and Atun, R. (2018). The Brazilian Health System at Crossroads: Progress, Crisis and Resilience. BMJ Glob. Health 3, e000829. doi:10.1136/bmjgh-2018-000829
Matos, C. A., Osorio-de-Castro, C. G. S., Coimbra Jr., C. E. A., and Silva, M. J. S. d. (2020). Perfil de utilização de medicamentos antineoplásicos entre indígenas atendidos pelo Sistema Único de Saúde. Cad. Saúde Pública 36, e00100520. doi:10.1590/0102-311x00100520
Melo, J. R. R., Duarte, E. C., de Moraes, M. V., Fleck, K., do Nascimento e Silva, A. S., and Arrais, P. S. D. (2021). Reações adversas a medicamentos em pacientes com COVID-19 no Brasil: análise das notificações espontâneas Do sistema de farmacovigilância brasileiro. Cad. Saúde Pública 37, e00245820. doi:10.1590/0102-311x00245820
Mengue, S. S., Bertoldi, A. D., Boing, A. C., Tavares, N. U., Pizzol, T. D., Oliveira, M. A., et al. (2016a). National Survey on Access, Use and Promotion of Rational Use of Medicines (PNAUM): Household Survey Component Methods. Rev. Saude Publica 50, 4s. doi:10.1590/s1518-8787.2016050006156
Mengue, S. S., Bertoldi, A. D., Ramos, L. R., Farias, M. R., Oliveira, M. A., Tavares, N. U., et al. (2016b). Access to and Use of High Blood Pressure Medications in Brazil. Rev. Saude Publica 50, 8s. doi:10.1590/s1518-8787.2016050006154
Ministério da Educação (2019). Empresa Brasileira de Serviços Hospitalares (Ebserh). Available at: https://www.ebserh.gov.br/inicio (Accessed June 24, 2019).
Ministério da Saúde (2020). Sistemas – DATASUS. Available at: https://datasus.saude.gov.br/sistemas/(Accessed January 26, 2021).
Ministério da Saúde (2017). Vigitel Brasil 2015 Saúde Suplementar: vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônicoAvailable at: http://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2015.pdf(Accessed December 9, 2021).
Ministério da Saúde (2018). Assistência Farmacêutica no SUS: 20 anos de políticas e propostas para o desenvolvimento e qualificação - Relatório com análise e recomendações de gestores, especialistas e representantes sa sociedade civil organizada. Available at: http://bvsms.saude.gov.br/publicacoes/assistencia_farmaceutica_sus_relatorio_recomendacoes.pdf (Accessed February 4, 2019).
Monteiro, C. N., Lima, M. G., Szwarcwald, C. L., Bastos, T. F., and Barros, M. B. d. A. (2019). Utilização de anti-hipertensivos e antidiabéticos no Brasil: análise das diferenças socioeconômicas. Pesquisa Nacional de Saúde 2013. Rev. Bras. Epidemiol. 22, E190014. doi:10.1590/1980-549720190014.supl.2
Mota, D. M., Melo, J. R. R., Freitas, D. R. C. d., and Machado, M. (2012). Perfil da mortalidade por intoxicação com medicamentos no Brasil, 1996-2005: retrato de uma década. Ciênc. Saúde Coletiva 17, 61–70. doi:10.1590/S1413-81232012000100009
Mota, D. M., and Silva, G. G. (2012). Evidências advindas do consumo de medicamentos moduladores Do apetite no Brasil: um estudo farmacoeconométrico. Revista da Associação Médica Brasileira 58, 88–94. doi:10.1590/S0104-42302012000100020
Mota, D. M., Vigo, Á., and Kuchenbecker, R. d. S. (2019). Reações adversas a medicamentos no sistema de farmacovigilância do Brasil, 2008 a 2013: estudo descritivo. Cad. Saúde Pública 35, e00148818. doi:10.1590/0102-311x00148818
Oliveira, J. R. d., Xavier, R. M. F., and Santos Júnior, A. d. F. (2013). Eventos adversos notificados ao Sistema Nacional de Notificações para a Vigilância Sanitária (NOTIVISA): Brasil, estudo descritivo no período 2006 a 2011. Epidemiol. Serv. Saúde 22, 671–678. doi:10.5123/S1679-49742013000400013
Pacurariu, A., Plueschke, K., McGettigan, P., Morales, D. R., Slattery, J., Vogl, D., et al. (2018). Electronic Healthcare Databases in Europe: Descriptive Analysis of Characteristics and Potential for Use in Medicines Regulation. BMJ Open 8, e023090. doi:10.1136/bmjopen-2018-023090
Paim, J., Travassos, C., Almeida, C., Bahia, L., and Macinko, J. (2011). The Brazilian Health System: History, Advances, and Challenges. Lancet 377, 1778–1797. doi:10.1016/S0140-6736(11)60054-8
Paula, T. C. d., Bochner, R., and Montilla, D. E. R. (2012). Análise clínica e epidemiológica das internações hospitalares de idosos decorrentes de intoxicações e efeitos adversos de medicamentos, Brasil, de 2004 a 2008. Rev. Bras. Epidemiol. 15, 828–844. doi:10.1590/S1415-790X2012000400014
Pizzol, T. D., Tavares, N. U., Bertoldi, A. D., Farias, M. R., Arrais, P. S., Ramos, L. R., et al. (2016). Use of Medicines and Other Products for Therapeutic Purposes Among Children in Brazil. Rev. Saude Publica 50, 12s. doi:10.1590/s1518-8787.2016050006115
Prestes, I. V. (2017). Pareamento de registros das grandes bases Do SUS para permitir análises longitudinais de pacientes com câncer. Available at: http://hdl.handle.net/10183/179058 (Accessed May 13, 2019).
Racoosin, J. A., Robb, M. A., Sherman, R. E., and Woodcock, J. (2012). “FDA's Sentinel Initiative: Active Surveillance to Identify Safety Signals,” in Pharmacoepidemiology. Editors B. L. Strom, S. E. Kimmel, and S. Hennessy (Hoboken, NJ: Wiley-Blackwell), 534–554.
Rezende, S. M., Rodrigues, S. H., Brito, K. N., da Silva, D. L., Santo, M. L., Simões, B. J., et al. (2017). Evaluation of a Web-Based Registry of Inherited Bleeding Disorders: a Descriptive Study of the Brazilian Experience with HEMOVIDAweb Coagulopatias. Orphanet J. Rare Dis. 12, 27. doi:10.1186/s13023-016-0560-6
Rodrigues, F. d. A., and Bertoldi, A. D. (2010). Perfil da utilização de antimicrobianos em um hospital privado. Ciênc. Saúde Coletiva 15, 1239–1247. doi:10.1590/S1413-81232010000700033
Rodrigues-Neto, A. J., Biazoni-Albaricci, C., Ribeiro, A., Lima, S., and Figueras, A. (2018). Towards Preventive Pharmacovigilance through Medicine Misuse Identification: an Example with Recombinant Human Growth Hormone for Aesthetic Purposes. Eur. J. Clin. Pharmacol. 74, 1471–1474. doi:10.1007/s00228-018-2510-9
Salas, M., Lopes, L. C., Godman, B., Truter, I., Hartzema, A. G., Fadare, J., et al. (2018). “Challenges and Issues in Drug Utilization Research Identified from the Latin American and African Regions,” in 34th International Conference on Pharmacoepidemiology and Therapeutic Risk Management 2018 (Prague, Czech Republic: Prague Congress Centre). Available at: https://strathprints.strath.ac.uk/63989/(Accessed May 10, 2019).
Santana, R. A. L. d., Bochner, R., and Guimarães, M. C. S. (2011). Sistema nacional de informações tóxico-farmacológicas: o desafio da padronização dos dados. Ciênc. Saúde Coletiva 16, 1191–1200. doi:10.1590/S1413-81232011000700051
Schneeweiss, S. (2019). Real‐World Evidence of Treatment Effects: The Useful and the Misleading. Clin. Pharmacol. Ther. 106, 43–44. doi:10.1002/cpt.1405
Shalini, S., Ravichandran, V., Saraswathi, R., Mohanty, B. K., and Dhanaraj, S. K. (2010). Drug Utilization Studies – an Overview. PCI- Approved-IJPSN 3. Available at: https://ijpsnonline.com/index.php/ijpsn/article/view/470 (Accessed August 6, 2020).
Silva, B. S., Coelho, H. V., Cavalcante, R. B., Oliveira, V. C., and Guimarães, E. A. A. (2018). Evaluation Study of the National Immunization Program Information System. Rev. Bras. Enferm. 71, 615–624. doi:10.1590/0034-7167-2017-0601
Silva, R. M. d., and Caetano, R. (2018). Gastos com pagamentos no Programa Aqui Tem Farmácia Popular: evolução entre 2006-2014. Physis: Revista de Saúde Coletiva 28 (1), e280105. doi:10.1590/s0103-73312018280105
Silva, R. M. d., and Caetano, R. (2015). Programa "Farmácia Popular Do Brasil": caracterização e evolução entre 2004-2012. Ciênc. Saúde Coletiva 20, 2943–2956. doi:10.1590/1413-812320152010.17352014
Soares, C., and Silva, G. A. (2013). Uso de registros de assistência farmacêutica Do Sistema de Informações Ambulatorial para avaliação longitudinal de utilização e adesão a medicamentos. Cad. Saúde Colet. 21, 245–252. doi:10.1590/S1414-462X2013000300003
Strom, B. L., and Carson, J. L. (1990). Use Of Automated Databases For Pharmacoepidemiology Research. Epidemiol. Rev. 12, 87–107. doi:10.1093/oxfordjournals.epirev.a036064
Szwarcwald, C. L., do Carmo Leal, M., Esteves-Pereira, A. P., da Silva de Almeida, W., de Frias, P. G., Damacena, G. N., et al. (2019). Avaliação das informações Do Sistema de Informações sobre Nascidos Vivos (SINASC), Brasil. Cad. Saúde Pública 35, e00214918. doi:10.1590/0102-311x00214918
Thum, M. A., Baldisserotto, J., and Celeste, R. K. (2019). Utilização Do e-SUS AB e fatores associados ao registro de procedimentos e consultas da atenção básica nos municípios brasileiros. Cad. Saúde Pública 35, e00029418. doi:10.1590/0102-311x00029418
Vieira, J. M. L., de Matos, G. C., da Silva, F. A. B., Bracken, L. E., Peak, M., and Lima, E. D. C. (2020). Serious Adverse Drug Reactions and Safety Signals in Children: A Nationwide Database Study. Front. Pharmacol. 11, 964. doi:10.3389/fphar.2020.00964
Vogler, M., Ricci Conesa, H., de Araújo Ferreira, K., Moreira Cruz, F., Simioni Gasparotto, F., Fleck, K., et al. (2020). Electronic Reporting Systems in Pharmacovigilance: The Implementation of VigiFlow in Brazil. Pharmaceut Med. 34, 327–334. doi:10.1007/s40290-020-00349-6
Wettermark, B. (2013). The Intriguing Future of Pharmacoepidemiology. Eur. J. Clin. Pharmacol. 69 (Suppl. 1), 43–51. doi:10.1007/s00228-013-1496-6
Wettermark, B., Elseviers, M., Almarsdóttir, A. B., Andersen, M., Benko, R., Bennie, M., et al. (2016). “Introduction to Drug Utilization Research,” in Drug Utilization Research. Editors M. Elseviers, B. Wettermark, A. B. Almarsdóttir, M. Andersen, R. Benko, M. Bennieet al. (Chichester, UK: John Wiley & Sons), 1–12. doi:10.1002/9781118949740.ch1
World Health Organization (1993). Drug Utilization Studies: Methods and Uses. Copenhagen: World Health Organization, Regional Office for Europe. Available at: https://apps.who.int/iris/handle/10665/260517.
World Health Organization (2003a). Essential Drugs Monitor No. 032, Drug Utilization in Latin America - the Example of DURG-LA. Available at: https://digicollections.net/medicinedocs/#d/s4940e (Accessed January 26, 2021).
World Health Organization (2003b). Introduction to Drug Utilization Research. Geneva: World Health Organization.
Zorzanelli, R. T., Giordani, F., Guaraldo, L., Matos, G. C. d., Brito Junior, A. G. d., Oliveira, M. G. d., et al. (2019). Consumo do benzodiazepínico clonazepam (Rivotril) no estado Do Rio de Janeiro, Brasil, 2009-2013: estudo ecológico. Ciênc. Saúde Coletiva 24, 3129–3140. doi:10.1590/1413-81232018248.23232017
(alphabetic order, Portuguese acronyms and names) AGHU: Aplicativo de Gestão para Hospitais Universitários
APURASUS: Sistema de Apuração e Gestão de Custos do SUS
BNAFAR: Base Nacional de Dados de Ações e Serviços da Assistência Farmacêutica no SUS
BPS: Banco de Preços em Saúde
CIHA: Sistema de Comunicação de Informação Hospitalar e Ambulatorial
CMD: Conjunto Mínimo de Dados
Datatox: Sistema Brasileiro de Dados de Intoxicações
e-SUS AB: e-SUS Atenção Básica
HÓRUS: Sistema Nacional de Gestão da Assistência Farmacêutica
NOTIVISA/VIGIMED: Sistema de Notificação em Vigilância Sanitária
PERIweb Sistema de Notificação Espontânea de Suspeita de Reação Adversa a Medicamento ou Desvio da Qualidade de Medicamento do Estado de São Paulo
PNAU Pesquisa Nacional sobre Acesso, Utilização e Promoção do Uso Racional de Medicamentos no Brasil
PNS Pesquisa Nacional de Saúde
S-CODES Sistema de Coordenação de Demandas Estratégicas–SP
SAMMED Sistema de Acompanhamento do Mercado de Medicamentos
SIAFI WebService Sistema Integrado de Administração Financeira
SIASG/SISME Sistema Integrado de Administração de Serviços Gerais/Sistema de Minuta de Empenho
SIASI Sistema de Informação da Atenção da Saúde Indígena
SIA-SUS Sistema de Informações Ambulatoriais do SUS
SICLOM Sistema Gerencial de Controle Logístico de Medicamentos
SIGAF Sistema Integrado de Gerenciamento da Assistência Farmacêutica
SIH-SUS Sistema de Informação Hospitalar
SINAN Sistema de Informação de Agravos de Notificação
SINITOX Sistema Nacional de Informações Tóxico-Farmacológicas
SIOPS Sistema de Informações sobre Orçamentos Públicos em saúde
SI-PNI Sistema de Informações do Programa Nacional de Imunizações
SISAB Sistema de Informação em Saúde para a Atenção Básica
SISPRENATAL Sistema de acompanhamento do programa de humanização no pré natal e nascimento
SITE-TB Sistema de Informação de Tratamentos Especiais de Tuberculose
SIVEP-Gripe Sistema de informação de vigilância epidemiológica da gripe
SNGPC Sistema Nacional de Gerenciamento de Produtos Controlados
VIGITEL Vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico
Keywords: pharmacoepidemiology, health information systems, databases (all types), Brazil, database management systems, pharmaceutical preparations, data sources, drug utilisation research
Citation: Leal LF, Osorio-de-Castro CGS, Souza LJCd, Ferre F, Mota DM, Ito M, Elseviers M, Lima EdC, Zimmernan IR, Fulone I, Carvalho-Soares MDL and Lopes LC (2022) Data Sources for Drug Utilization Research in Brazil—DUR-BRA Study. Front. Pharmacol. 12:789872. doi: 10.3389/fphar.2021.789872
Received: 05 October 2021; Accepted: 20 December 2021;
Published: 14 January 2022.
Edited by:
Mina Tadrous, Women’s College Hospital, CanadaReviewed by:
Talita Duarte-Salles, Fundació Institut Universitari per a la recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), SpainCopyright © 2022 Leal, Osorio-de-Castro, Souza, Ferre, Mota, Ito, Elseviers, Lima, Zimmernan, Fulone, Carvalho-Soares and Lopes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luciane Cruz Lopes, bHVjaWFuZS5sb3Blc0Bwcm9mLnVuaXNvLmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.