- 1Department of Obstetrics and Gynecology, Second Affiliated Hospital of Harbin Medical University, Harbin, China
- 2Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China
- 3Department of Obstetrics and Gynecology, First Hospital of Qiqihar, Qiqihar, China
Ovarian cancer is the second most common gynecological malignancy, and one of the most deadly. The bottleneck restricting the treatment of ovarian cancer is its multi-drug resistance to chemotherapy. Cajanol is an isoflavone from pigeon pea (Cajanus cajan) that has been reported to have anti-tumor activity. In this work, we evaluate the effect of cajanol in reversing paclitaxel resistance of the A2780/Taxol ovarian cancer cell line in vitro and in vivo, and we discuss its mechanism of action. We found that 8 μM cajanol significantly restored the sensitivity of A2780/Taxol cells to paclitaxel, and in vivo experiments demonstrated that the combination of 0.5 mM/kg paclitaxel and 2 mM/kg cajanol significantly inhibited the growth of A2780/Taxol metastatic tumors in mice. Flow cytometry, fluorescence quantitative PCR, western blotting and immunohistochemical staining methods were used to study the mechanism of reversing paclitaxel resistance with cajanol. First, we determined that cajanol inhibits paclitaxel efflux in A2780/Taxol cells by down-regulating permeability glycoprotein (P-gp) expression, and further found that cajanol can inhibit P-gp transcription and translation through the PI3K/Akt/NF-κB pathway. The results of this work are expected to provide a new candidate compound for the development of paclitaxel sensitizers.
Introduction
Ovarian cancer is one of the three major malignant tumors of the female reproductive system and has the highest death rate among all the gynecological malignancies (Deb et al., 2018). Because ovarian cancer is difficult to diagnose, most ovarian cancer patients are recognized at an advanced stage, leading to a decreased survival rate (Grunewald and Ledermann, 2017). At present, the combination of paclitaxel and platinum chemotherapy is the primary regimen for ovarian cancer patients. At the beginning of the chemotherapy regimen, these drugs are effective for more than 80% of patients, but cancer cells develop resistance to the drugs, which results in cancer recurrence, leading to a five-year survival rate of 45% for ovarian cancer patients. Moreover, the survival rate of cancer patients diagnosed as terminal is less than 30% (Webb and Jordan, 2017).
Studies have shown that drug resistance to paclitaxel involves various mechanisms, including the increase of multi-drug resistance (MDR) proteins (Mihanfar et al., 2019), and alterations in expression of vascular endothelial growth factor (Akiyama et al., 2012), matrix metalloproteinases (Hu et al., 2012; Kato et al., 2012) or microtubule associated proteins (Ren et al., 2016). MDR occurs in many human cancers, including colon cancer, breast cancer and kidney cancer (Amawi et al., 2019) and causes resistance to chemotherapy. Overexpression of ABC transporters such as MDR1/P-gp is considered to be the classical mechanism of drug resistance (Waghray and Zhang, 2018). P-gp is encoded by the ABCB1 gene, and the P-gp promoter sequence contains a κB site, which can be recognized and activated by nuclear factor kappa B (NF-κB) (Loaiza et al., 2016). There have been reports that the PI3K/Akt signal transduction pathway is involved in NF-κB-mediated MDR (Solt and May 2008). It has also been reported that ivermectin and ferulic acid can reverse drug resistance by inhibiting the EGFR/ERK/Akt/NF-κB pathway, down-regulating the expression of P-gp (Muthusamy et al., 2019). This suggests the use of suitable inhibitors to inhibit NF-κB-mediated P-gp overexpression as a feasible method to reverse MDR.
Natural products from plants are effective sources of anti-tumor drugs, and some have been shown to modulate MDR. Many types of natural products, such as flavonoids, alkaloids and terpenes, have been demonstrated to inhibit P-gp. Cajanol is an isoflavanone compound derived from the root of C. cajan, and has a variety of pharmacological actions, including antibacterial, antifungal, antimalarial and antitumor activities (Zhao et al., 2013). In this study we demonstrate for the first time the inhibition of P-gp by cajanol, using cell proliferation, rhodamine accumulation, fluorescence quantitative PCR, western blot, and in vivo assays. We investigated the effects of cajanol on the expression and function of P-gp, and we also examined the regulatory effects of cajanol through the PI3K/Akt/NF-κB signaling pathway to mediate P-gp proteins. The objective was to further develop cajanol for the reversal of ovarian cancer drug resistance and to provide data for the potential clinical application of cajanol.
Materials and Methods
Cell Lines, Antibodies and Reagents
The A2780 human ovarian cancer cell line and the A2780/Taxol paclitaxel-resistant ovarian cancer cell line were purchased from Keygen Biotech (Jiangsu, China). The human non-small cell lung cancer cell line A549 and the paclitaxel-resistant cell line A549/Taxol were donated by Prof. Yang from Qiqihar Medical College. All the cells were cultured in RPMI-1640 cell culture medium (Solarbio Science and Technology Co., Ltd., Beijing, China) at 37°C and 5% CO2. The culture medium was supplemented with 10% inactivated fetal bovine serum (Gibco, NY, United States) and 1% penicillin and streptomycin. The A2780/Taxol cells were cultured in medium containing 800 ng/mL paclitaxel and then switched to a non-drug medium 2 weeks before the experiment. An antibody against P-gp (0.1 μg/m; ab170904), NF-κB/p65 (1/2000, ab32536), p-NF-κB/p65 (1/1000, ab239882) and VEGF (1/1000, ab32152) were purchased from Abcam (Cambridge, United Kingdom); antibodies against PI3K (1/1000, #4292), Akt (1/1000, #9272), p-Akt (1/1000, #9271), α-Tubulin (1/2000, #2144), MMP-9 (1/1000, #3852), MRP1 (1/1000, ab233383), MRP2 (1/2000, ab172630), LRP (1/1000, #64099), lamin B (1/1000, #17416) and ß-actin (1/1000, #4970) were purchased from Cell Signaling Technology (CST, MA, United States).
Cajanol was extracted by our laboratory with purity greater than 98%. The structural formula is shown in Figure 1A. The extraction method of cajanol refers to the published extraction method with slightly modified (Liu et al., 2011). Specifically, the crushed pigeon pea root was impregnated with ethanol-water (80:20, V/V) solution for 24 h with a solid-liquid ratio of 1:10, repeated 3 times. The filtrate was concentrated using a rotary evaporator to obtain a crude extract. And the crude extract was extracted with ethyl acetate/distilled water (3/1, v/v), the ethyl acetate was separated and concentrated to obtain brown product. The brown product was separated by a resin column, water and ethanol were used as mobile phases, and the 50% ethanol eluted part was retained for further purification. Using a silica gel column with chloroform-methanol as the mobile phase, cajanol was separated from the chloroform-methanol (12:1, v/v) fraction. After crystallization and recrystallization, white crystals were obtained. The HPLC and negative ion mode mass spectra of Cajanol are shown in Supplementary Figure S1. All other reagents were purchased from Sigma (St. Louis, MO, United States).

FIGURE 1. Effect of cajanol on ABCB1 mRNA and P-gp protein expression in ovarian cancer cells. Cells were treated with cajanol (0, 2, 4 or 8 μM) for 48 h. (A) Chemical formula of cajanol. (B) Effect of cajanol on ABCB1, MMP-9, VEGF, Tubα1a and Tubβ3 mRNA expression in A2780/Taxol cells as determined by RT-qPCR assay. (C) Expression of α-tubulin, βIII-tubulin, VEGF, MMP-9, P-gp, MRP1, MRP2 and LRP protein in A2780/Taxol cells after treated with cajanol. (D) Expression was normalized with ß-actin, respectively. Each column shows the mean ± S.D. *p < 0.05, **p < 0.01 vs. control group.
Methylthiazolyldiphenyl-Tetrazolium Bromide Assay
Cells to be tested (100 µL; 5 × 103 cells/well) were seeded in a 96-well plate and cultured overnight. Paclitaxel and cajanol were prepared as stock solutions in dimethyl sulfoxide (DMSO) and diluted (1000, 500, 250, 125, 62.5, 31.25, 15.63, 7.81, 3.91, 1.96 and 0.98 μM). Seeded cells were treated in quadruplicate with the diluted solutions for 72 h. MTT was added, and the cells were incubated for 4 h. The culture medium was then removed, and DMSO (150 µL) was added. The plate was placed in a shaker to fully dissolve the purple formazan crystals. The OD was measured at 570 nm, and the growth inhibition rate was calculated. All the experiments were repeated in triplicate. GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, United States) was used to calculate the IC50 values. Fold resistance (FR) is calculated by dividing the IC50 obtained in the resistant cancer cells (with or without the reversal compounds) by the IC50 of the non-resistant, parental cancer cells (Hussein et al., 2017).
qRT-PCR
Medium containing 2, 4 or 8 µM cajanol was prepared and incubated with A2780/Taxol cells to determine the effect of cajanol on ABCB1, MMP-9, VEGF, Tubα1a and Tubβ3 mRNA expression. After 48 h of co-incubation, total RNA was isolated using a RNA Extraction Kit (Takara Bio Inc., Dalian, China), and total RNA reverse transcription was performed using a Reverse Transcription Kit (Takara). Real-time quantitative PCR primers were designed using Primer Premier 5.0 (Supplementary Table S1), and SYBR® Premix Ex Taq™ II kit (Takara) was used for the RT-qPCR assay. β-actin was selected as the internal reference gene; the reaction conditions have been previously described (Huang et al., 2017). Relative gene expression was calculated by the 2−ΔΔ Ct method.
Rhodamine Accumulation
Logarithmic cells (4 × 105/mL density) were seeded in a 12-well plate. Positive control verapamil (8 µM) and appropriate concentrations of cajanol were added, and the cells were incubated for 2 h at 37°C. Then, rhodamine-123 at a final concentration of (1 μg/mL) was added, and the cells were incubated at 37°C, 5% CO2 for 1 h. After the reaction was completed, the supernatant was discarded after centrifugation at 4°C, and the cells were washed twice with pre-chilled PBS buffer (4°C), terminating the rhodamine efflux. The cells were then re-suspended in cold PBS and the intracellular drug fluorescence was determined by flow cytometry (Beckman Coulter, Fullerton, CA, United States), with an excitation wavelength of 466 nm and an emission wavelength of 535 nm. The results were analyzed by Expo32ADC software (Beckman Coulter).
Western Blot
To determine whether cajanol affects the expression of P-gp through the PI3K/Akt signaling pathway, we measured the expression of P-gp, t-Akt, p-Akt, NF-κB/p65 and p-NF-κB/p65 in A2780/Taxol cells. The A2780/Taxol cells were treated with cajanol (2, 4, or 8 μM) for 48 h before the total cell protein was extracted. Cell lysis buffer for Western and IP (P0013, Beyotime Biotechnology, Shanghai, China) is selected as the whole cell protein extraction lysate. The nucleoprotein separation method is strictly implemented in accordance with the instructions. Specifically, the cells are scraped off with a cell scraper, and the cell pellet is collected after centrifugation. Add 200 µl of cytoplasmic protein extraction reagent A supplemented with PMSF for every 20 µl of cell pellet. Vortex for 5 s to completely suspend and disperse the cell pellet. After ice bath for 10 min, add 10 µl of cytoplasmic protein extraction reagent B and Vortex for 5 s. After the sample was ice bathed for 1 min, Vortex again for 5 s, and then placed in a 4°C centrifuge at 12,000 g for 5 min. Immediately draw the supernatant into a pre-cooled plastic tube, which is the cytoplasmic protein extracted. Equal amounts of proteins were separated by SDS-PAGE and transferred to a polyvinylidene fluoride membrane. After blocking, the membrane-bound proteins were probed with the relevant primary antibodies. The membranes were washed and then incubated with the secondary antibodies; finally antibody-bound proteins were detected using enhanced chemiluminescence reagents.
In Vivo Experiments
Six-week-old healthy female pure BABL/c nude mice (weighing 18–20 g) were purchased from Vital River Experimental Animal Co. Ltd. (Beijing, China). The mice were placed in a thermostatted laminar flow box and kept in a specific pathogen-free environment with a 12 h/12 h light/dark cycle. The animal experiments and use of tumor cells were approved by the Animal Care Welfare Committee of the First hospital of Qiqihar according to the ethical guidelines of the animals (scientific procedures) act 1986 amendment regulations (SI 2012/3039); Approval number: QAEC20190043.
A2780/Taxol cells were cultured in RPMI-1640 culture medium containing 10% fetal bovine serum. Logarithmic growth phase cells were collected to prepare a suspension with a concentration of living cells of 1×105/mL. Live cells accounted for more than 95% of the population. The cell suspension was inoculated subcutaneously in the right armpit near the back of each nude mouse (approximately 105 cells). After 1 week of inoculation, 16 mice with successful tumor formation were randomly divided into four groups, ensuring adequate numbers for statistical analysis: normal saline group, Taxol group (0.5 mM/kg), cajanol group (2 mM/kg) and paclitaxel (0.5 mM/kg) + cajanol (2 mM/kg) combined group. The agents were administered through the caudal vein on the 1st, 8th and 15th day after successful tumor implantation. The body weights and condition of the mice and growth of the transplanted tumors were observed every 3 days for 24 days. The tumor volume was calculated with the following formula: V = (length × width2)/2. When the tumor diameter was over 2 cm or the ulcer area was greater than 4 mm, the mice were euthanized by cervical dislocation after inducing a coma by inhalation of 2.5% isoflurane vapor. Remaining mice were euthanized after 24 days. The tumor tissue was collected for immunohistochemistry, fluorescence quantitative PCR and western blot analyses.
Immunohistochemical Staining
Immunohistochemistry was performed on paraformaldehyde-fixed paraffin-embedded sections. The detailed staining procedure has been described previously (Xu et al., 2017).
Statistical Analysis
Statistical analyses were carried out using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA). One-way ANOVA was performed between groups. For ANOVA, the observed variance was partitioned into components according to different explanatory variables. *p < 0.05 was considered to be significant.
Results
Cajanol was Able to Reverse the Resistance of A2780/Taxol and A549/Taxol Cells to Paclitaxel.
The paclitaxel MTT IC50 values after treatment of A2780 and A2780/Taxol cells for 72 h were 1.23 ± 0.10 µM and 35.85 ± 1.23 µM, respectively (Table 1and Figure 2). The IC50 values in A2780/Taxol cells for paclitaxel treatment combined with 2, 4, 8 or 16 μM cajanol were 25.67 ± 0.94 µM, 16.25 ± 0.54 µM, 6.54 ± 0.37 µM and 6.05 ± 0.33 µM, respectively. When cajanol concentration was 0 µM, 2 µM, 4 µM, 8 µM or 16 μM, the fold resistance of A2780/Taxol cells on A2780 cells were 29.15, 22.52, 14.51, 5.64 and 5.50, respectively. For A549/Taxol cells, cajanol also showed a good drug resistance reversal effect. The IC50 of paclitaxel on A549 cells and A549/Taxol cells were 7.52 ± 0.46 µM and 289.34 ± 11.46 µM, respectively. After treatment with 2 µM, 4 µM, 8 µM or 16 μM cajanol, the IC50 of paclitaxel for A549 cells was almost unchanged, but the IC50 for A549/Taxol cells decreased to 189.43 ± 10.87 µM, 68.95 ± 4.87 µM, 27.9 ± 1.23 µM and 23.76 ± 1.12 µM, respectively. And the corresponding fold resistance is also reduced from 38.48 to 3.15. The results showed that co-treatment of cajanol with paclitaxel substantially inhibits A2780/Taxol cells and that the IC50 value is decreased by more than 4-fold at a cajanol concentration of 8 µM. These results suggest that cajanol effectively reverses the resistance of A2780/Taxol cells to paclitaxel and that the effect is concentration dependent at levels up to 8 µM.
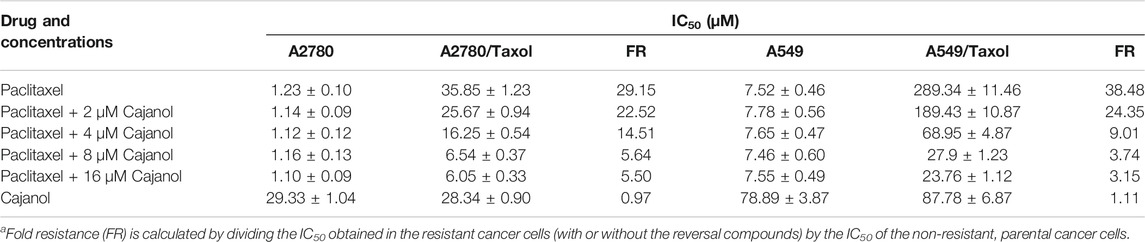
TABLE 1. Effect of cajanol on the cytotoxicity of paclitaxel in A2780/Taxol and A2780 cells by MTT assay.
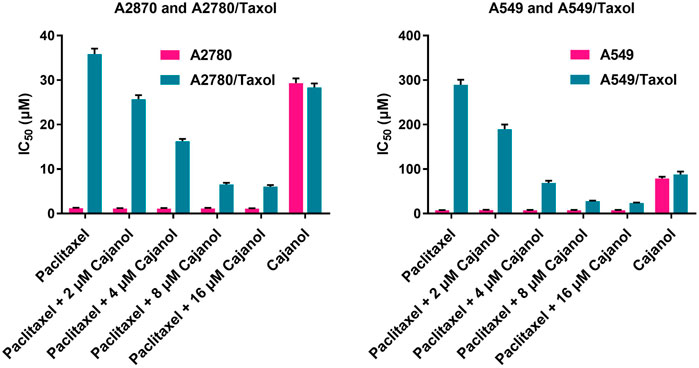
FIGURE 2. Effect of cajanol on intracellular Rhodamine-123 accumulation in A2780 and A2780/Taxol cells. Rhodamine-123 fluorescence intensity was measured using a flow cytometer and expressed as the mean ± SD. *p < 0.05, **p < 0.01 vs. A2780.
Cajanol Restores the Sensitivity of Paclitaxel to A2780/Taxol Cells by Inhibiting the Expression of P-gp Protein
In order to study the mechanism by which cajanol restores the sensitivity to paclitaxel of A2780/Taxol cells, the expression of resistance-related genes in A2780/Taxol cells was determined after treatment with different concentrations of cajanol (2, 4 or 8 μM). The expression of ABCB1 gene was significantly down-regulated by treatment with cajanol in a concentration-dependent manner (Figure 1B). When the concentration of cajanol reached 8 μM, the expression of ABCB1 gene was about 10% of the control group. The expression levels of VEGF, MMP-9, Tubα1a and Tubβ3 did not change. Further, western blots were used to determine the protein expressions of P-gp, VEGF, MMP-9, α-Tubulin and βIII-Tubulin in A2780/Taxol cells after cajanol treatment, and the results were consistent with the results of fluorescence quantitative PCR (Figures 1C,D). The expression levels of VEGF, MMP-9, α-Tubulin and βIII-Tubulin were not significantly changed, while expression levels of P-gp were significantly reduced. These results suggest that cajanol can reverse paclitaxel sensitivity by inhibiting this MDR protein in A2780/Taxol cells.
In order to further determine whether cajanol has the same inhibitory effect on other MDR proteins, we determined the expression of MRP1, MRP2 and LRP proteins in A2780/Taxol cells treated with cajanol. Western blot results showed that the expression levels of MRP1, MRP2 and LRP were not significantly changed (Figures 1C,D). These results all indicate that cajanol specifically reduces expression of ABCB1 and P-gp.
Cajanol Inhibits the Transport of P-gp Protein in A2780/Taxol Cells
Flow cytometry was used to assess the effect of cajanol on P-gp protein transport. Rhodamine-123 is transported by P-gp and can be detected by its emission of yellow-green fluorescence. By monitoring Rhodamine-123 accumulation in cells, we evaluated the transport capacity of P-gp protein in A2780/Taxol cells treated with cajanol at different concentrations (Figure 3). The intracellular fluorescence intensity of control cells A2780 (24.5%) was found to be significantly higher than that of untreated A2780/Taxol cells (3.7%). After treatment with 2, 4 or 8 μM cajanol, the fluorescence intensity in A2780/Taxol cells increased to 14.8, 18.5 and 21.3% respectively. The P-gp inhibitor verapamil also increased the fluorescence intensity of A2780/Taxol cells to 23.8% at 8 μM. This result further confirms the utility of cajanol in reversing drug resistance by inhibiting the expression of P-gp, thus reducing the efflux of paclitaxel.

FIGURE 3. Cajanol treatment down-regulates P-gp expression via inhibition of the PI3K/Akt/NF-κB signaling pathway. Cajanol inhibits the phosphorylation of IκB and the translocation of NF-κB from the cytoplasm to the nucleus by inhibiting the expression of PI3K and the phosphorylation of Akt, and ultimately inhibits the transcription and translation of the P-gp protein.
Cajanol Regulates MDR Via the Akt/NF-κB/P-gp Signaling Pathway in A2780/Taxol Cells
The above results show that the expression of ABCB1 and P-gp in A2780/Taxol cells is inhibited by cajanol, suggesting that cajanol could inhibit P-gp transcription and/or translation. Since Akt and NF-κB signal transduction are highly correlated with P-gp expression, it is suggested that cajanol may regulate P-gp through the Akt/NF-κB/P-gp signaling pathway. The expression of each of PI3K, Akt and phosphorylated Akt in A2780/Taxol cells was detected by western blot. Notably, cajanol reduced PI3k and p-Akt in A2780/Taxol cells, but did not significantly affect the expression of Akt itself (Figures 4A,B). Further, we examined the effect of cajanol on the expression of NF-κB/p65 and phosphorylated NF-κB/p65 in cells and in the nucleus. Similar to Akt and P-Akt, down-regulation of p-NF-κB/p65 was observed in 2780/Taxol cells treated with cajanol. The expression levels of NF-κB/p65 and p-NF-κB/p65 in the nucleus were down-regulated with the increase of cajanol concentration. To verify the effect of inhibiting the PI3K/Akt/NF-κB pathway on P-gp protein expression, the PI3K inhibitor LY294002 was used to treat A2780/Taxol cells. Treatment with LY294002 had effects on the expression of Akt, p-Akt8, NF-κB/p65, p-NF-κB/p65 and P-gp similar to those found after exposure to 8 μM cajanol. These results fully demonstrate that cajanol can inhibit PI3K and phosphorylation of Akt in A2780/Taxol cells, thereby preventing the phosphorylation and nuclear translocation of NF-κB/p65, and ultimately inhibiting the transcription and translation of P-gp.
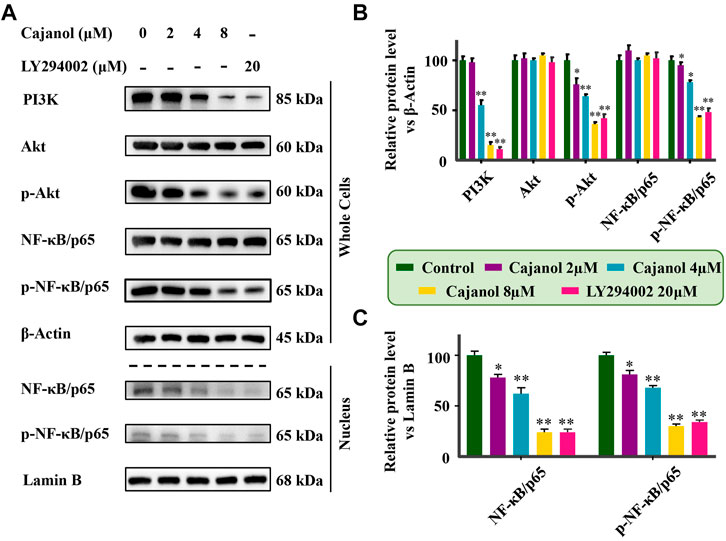
FIGURE 4. Inhibition of cajanol on PI3K/Akt/NF-κB pathway in A2780/Taxol cells. (A) Expression of PI3k, Akt, P-Akt, NF-κB/p65 (whole cells and nucleus) and p-F-κB/p65 (whole cells and nucleus) in A2780/Taxol cells after treatment with cajanol and LY294002. (B,C) Expression was normalized with ß-actin or lamin B, respectively. Each column shows the mean ± S.D. *p < 0.05, **p < 0.01 vs. control group.
Cajanol Restores Taxol-Resistant Metastatic Tumor Sensitivity in Mice
To verify whether cajanol can restore Taxol sensitivity in mice, we established a BABL/c nude mouse tumor model using A2780/Taxol cells with the following four groups: control, cajanol, paclitaxel and cajanol + paclitaxel, each group consisted of 4 nude mice that had been successfully modeled. After treatment for 24 days, the tumor volumes of the mice in the combined group were significantly smaller than those of the other three groups (Figures 5A–C); the final tumor volume of the cajanol + paclitaxel group was 182.4 ± 20.4 mm3, while tumors in the paclitaxel, cajanol and control groups had volumes of 758 ± 154 mm3, 680 ± 176 mm3 and 981 ± 215 respectively. After treatment, only the mice in the combined treatment group could maintain a body weight of approximately 25 g, while the mice in the other three groups weighed less than 20 g (Figure 5D). These results show that cajanol (2 mM/kg) combined with paclitaxel (0.5 mM/kg) in vivo has a significant inhibitory effect on paclitaxel-resistant cancer.
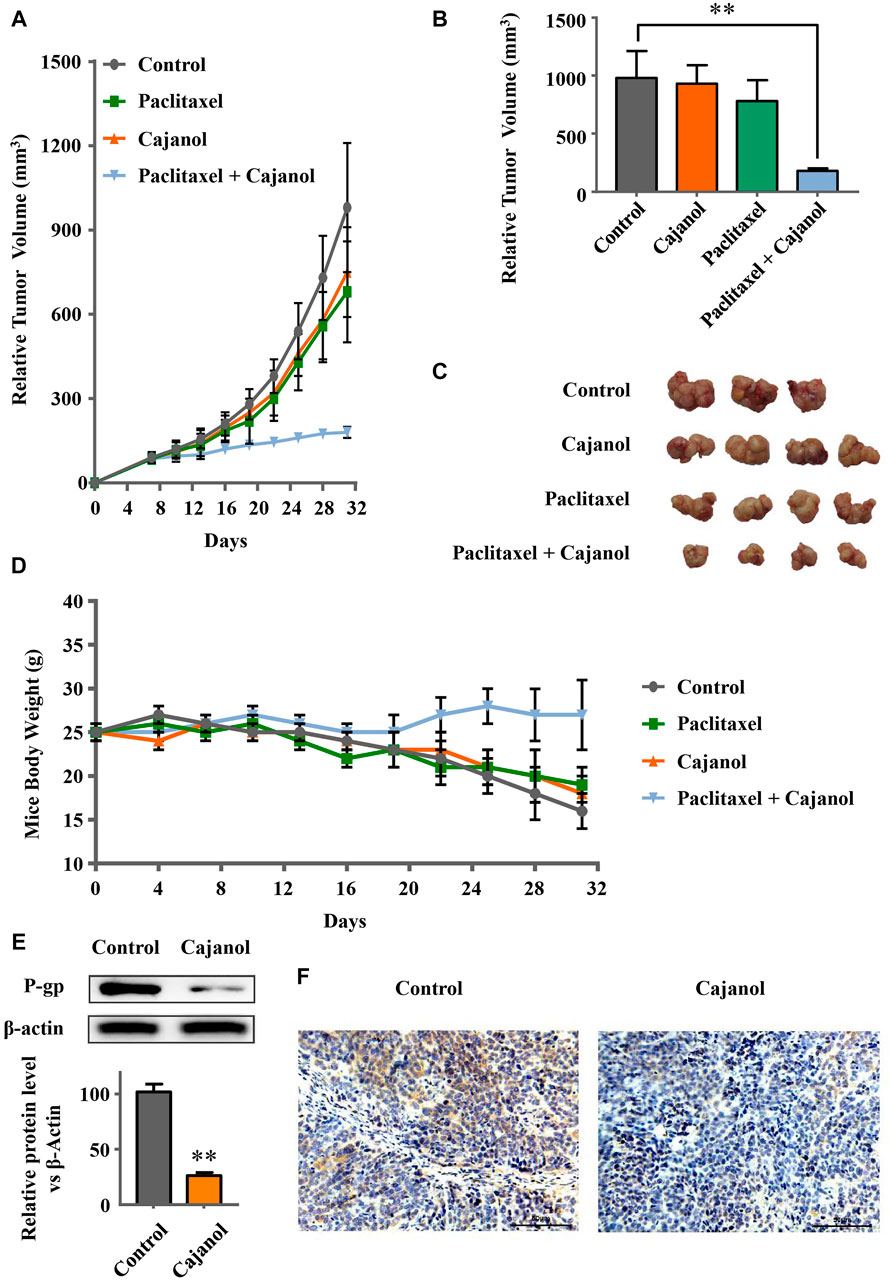
FIGURE 5. Sensitization of mice to paclitaxel by cajanol. Healthy six-week-old female mice were selected and inoculated with A2780/Taxol cells to form solid tumors. After solid tumor formation, the agents were administered once a week. Changes in the tumor volume and body weight were observed during the treatment. (A) Changes in the tumor volume during treatment. (B) Tumor growth of the mice in each group after 24 days of treatment. *p < 0.05 vs control group. (C) Tumor images of the mice after 24 days of treatment. (d) Changes in the body weight of the mice during treatment. (E,F) Effect of cajanol on P-gp expression in tumor tissue as determined by western blot assay and immunohistochemistry. *p < 0.05 vs. control group.
We also measured the expressions of ABCB1 mRNA and of P-gp protein in the tumor tissues. Western blots showed that cajanol inhibits the expression of P-gp in tumor tissues, similar to the results in vitro (Figure 5E). The immunohistochemistry results confirm that expression of P-gp in the cajanol treatment group was significantly inhibited (Figure 5F).
Discussion
For patients with advanced ovarian cancer, paclitaxel-based chemotherapy is currently the mainstay of treatment. However, drug resistance usually emergences, leading to treatment failure and tumor recurrence. Therefore there is a need for more research and development of new therapeutic drugs that can reverse this resistance.
It has been found that more than 90% of cancer-related deaths are due to MDR, and the main cause of MDR is the overexpression of the ABC transporters in tumors (Goldman, 2003). Among these, multidrug resistance gene ABCB1 is considered to be one of the most important mechanisms that cause paclitaxel resistance. Overexpression of ABCB1 leads to the overexpression of membrane P-gp, with a consequent reduction in the concentration of intracellular paclitaxel, reducing the inhibitory effect of paclitaxel on tumor cells (Schondorf et al., 2002; Lukyanova, 2010; Shen et al., 2016). The change in microtubule structure is another reason for paclitaxel resistance; the increased expression of α-tubulin and ß-III-tubulin will disrupt the internal stability of microtubules and reduce the efficacy of paclitaxel (Li J. et al., 2009; Kobayashi et al., 2011). In addition, matrix metalloproteinase (MMP-9) promotes tumor angiogenesis by increasing the expression of vascular endothelial growth factor (VEGFA) and its receptor (VEGFR) (Bergers et al., 2000). Abnormal expression of VEGF may be related to paclitaxel resistance. Tumor tissues release VEGF through up-regulation of P-gp (Li L. et al., 2009; Akiyama et al., 2012). In this work, we found that cajanol can inhibit the expression of ABCB1 gene and P-gp protein, but has no effect on the expression of VEGF, MMP-9, α-tubulin or βIII-tubulin. Combined with the results of flow cytometry, we confirmed that cajanol can reduce the efflux of paclitaxel from A2780/Taxol cells by modulating the expression of P-gp protein. In addition to P-gp, the MDR of some tumor cells is caused by MDR-associated protein (MRP) (Cole et al., 1992; Sodani et al., 2012) or lung resistance-related protein (LRP) (De Figueiredo-Pontes et al., 2008). As shown in the results, the expression levels of these three proteins were low in A2780/Taxol cells, and there was no significant change after cajanol treatment.
Studies have shown that the PI3K/Akt signaling pathway is closely related to MDR, and the PI3K/Akt pathway activates the NF-κB system, which may lead to an increase in MDR1 transcription (Xi et al., 2016). When the PI3K/Akt pathway is activated, the Akt protein is phosphorylated, leading to the phosphorylation of downstream IκB-α and its dissociation from NF-κB. After nuclear translocation of NF-κB and binding to its recognition site, the promoter of the MDR1 gene is activated and gene expression is induced (Guo et al., 2016; Wu et al., 2016). Blocking the PI3K/Akt pathway can lead to down-regulation of MDR1/P-gp protein expression, thereby reversing the MDR. The results of this study show that cajanol can inhibit the expression of PI3K and the phosphorylation of Akt, thereby inhibiting the phosphorylation of IκB. It also prevents the translocation of NF-κB from the cytoplasm to the nucleus. Therefore, cajanol can inhibit P-gp expression through the PI3K/Akt/NF-κB pathway (Figure 6).
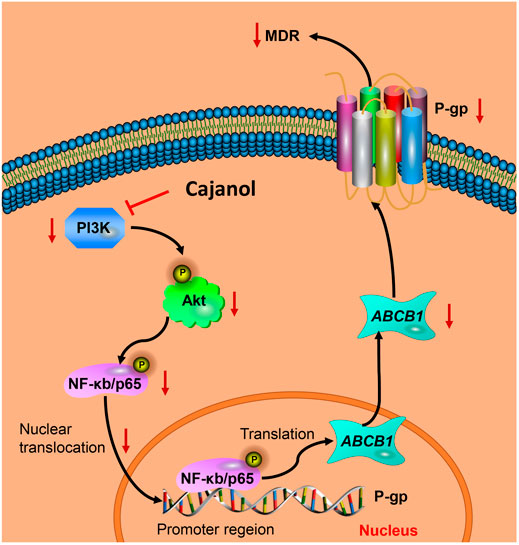
FIGURE 6. Cajanol treatment down-regulates P-gp expression via inhibition of the PI3K/Akt/NF-κB signaling pathway. Cajanol inhibits the phosphorylation of IκB and the translocation of NF-κB from the cytoplasm to the nucleus by inhibiting the expression of PI3K and the phosphorylation of Akt, and ultimately inhibits the transcription and translation of the P-gp protein.
Researchers have been developing and synthesizing P-gp inhibitors to reverse MDR for the last 30 years. Verapamil, nifedipine, quinidine and cyclosporine A are substrates of P-gp that have been developed as first generation inhibitors, but they have unacceptable side effects. The second generation inhibitors, including dexverapamil and PSC833, were modified on the basis of the first-generation drugs to reduce toxicity (Boesch et al., 1991; Martin et al., 1999). These inhibitors are substrates of cytochrome P450, and they interfere with the pharmacokinetics of chemotherapeutic agents. No synthetic inhibitors have yet been approved for clinical applications, prompting researchers to explore new reversal agents from natural products. It has been reported that ferulic acid can down-regulate P-gp expression through NF-κB (Muthusamy et al., 2019). In this study, 8 μM cajanol significantly restored the sensitivity of A2780/Taxol cells to paclitaxel, and 2 mM/kg cajanol and 0.5 mM/kg paclitaxel significantly inhibited the growth of metastatic tumors in mice. The results of this study will provide new ideas for the development of effective inhibitors of MDR in ovarian cancer.
Conclusion
In this work, cajanol inhibited phosphorylation and nuclear ectopia of NF-κB by inhibiting PI3K expression and Akt phosphorylation, thereby reducing transcription and translation of P-gp protein, and ultimately reducing MDR induced by paclitaxel efflux. This report may contribute to further explore the clinical application of cajanol in the treatment of ovarian cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by The animal experiments and use of tumor cells were approved by the Animal Care Welfare Committee of the First hospital of Qiqihar according to the ethical guidelines of the animals (scientific procedures) act 1986 amendment regulations (SI 2012/3039); Approval number: QAEC20190043.
Author Contributions
PL and JJ designed research. MS, HY, MG, and WL analyzed data. MS and ZG performed research. MS wrote the paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.783317/full#supplementary-material
References
Akiyama, K., Ohga, N., Hida, Y., Kawamoto, T., Sadamoto, Y., Ishikawa, S., et al. (2012). Tumor Endothelial Cells Acquire Drug Resistance by MDR1 Up-Regulation via VEGF Signaling in Tumor Microenvironment. Am. J. Pathol. 180, 1283–1293. doi:10.1016/j.ajpath.2011.11.029
Amawi, H., Sim, H. M., Tiwari, A. K., Ambudkar, S. V., and Shukla, S. (2019). ABC Transporter-Mediated Multidrug-Resistant Cancer. Adv. Exp. Med. Biol. 1141, 549–580. doi:10.1007/978-981-13-7647-4_12
Bergers, G., Brekken, R., Mcmahon, G., Vu, T. H., Itoh, T., Tamaki, K., et al. (2000). Matrix Metalloproteinase-9 Triggers the Angiogenic Switch during Carcinogenesis. Nat. Cel Biol 2, 737–744. doi:10.1038/35036374
Boesch, D., Muller, K., Pourtier-Manzanedo, A., and Loor, F. (1991). Restoration of Daunomycin Retention in Multidrug-Resistant P388 Cells by Submicromolar Concentrations of SDZ PSC 833, a Nonimmunosuppressive Cyclosporin Derivative. Exp. Cel Res 196, 26–32. doi:10.1016/0014-4827(91)90452-z
Cole, S. P., Bhardwaj, G., Gerlach, J. H., Mackie, J. E., Grant, C. E., Almquist, K. C., et al. (1992). Overexpression of a Transporter Gene in a Multidrug-Resistant Human Lung Cancer Cell Line. Science 258, 1650–1654. doi:10.1126/science.1360704
De Figueiredo-Pontes, L. L., Pintão, M. C., Oliveira, L. C., Dalmazzo, L. F., Jácomo, R. H., Garcia, A. B., et al. (2008). Determination of P-Glycoprotein, MDR-Related Protein 1, Breast Cancer Resistance Protein, and Lung-Resistance Protein Expression in Leukemic Stem Cells of Acute Myeloid Leukemia. Cytometry B Clin. Cytom 74, 163–168. doi:10.1002/cyto.b.20403
Deb, B., Uddin, A., and Chakraborty, S. (2018). miRNAs and Ovarian Cancer: An Overview. J. Cel Physiol 233, 3846–3854. doi:10.1002/jcp.26095
Goldman, B. (2003). Multidrug Resistance: Can New Drugs Help Chemotherapy Score against Cancer? J. Natl. Cancer Inst. 95, 255–257. doi:10.1093/jnci/95.4.255
Grunewald, T., and Ledermann, J. A. (2017). Targeted Therapies for Ovarian Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 41, 139–152. doi:10.1016/j.bpobgyn.2016.12.001
Guo, Y., Ding, Y., Zhang, T., and An, H. (2016). Sinapine Reverses Multi-Drug Resistance in MCF-7/dox Cancer Cells by Downregulating FGFR4/FRS2α-Erk1/2 Pathway-Mediated NF-Κb Activation. Phytomedicine 23, 267–273. doi:10.1016/j.phymed.2015.12.017
Hu, X., Li, D., Zhang, W., Zhou, J., Tang, B., and Li, L. (2012). Matrix Metalloproteinase-9 Expression Correlates with Prognosis and Involved in Ovarian Cancer Cell Invasion. Arch. Gynecol. Obstet. 286, 1537–1543. doi:10.1007/s00404-012-2456-6
Huang, C. Z., Wang, Y. F., Zhang, Y., Peng, Y. M., Liu, Y. X., Ma, F., et al. (2017). Cepharanthine Hydrochloride Reverses P-glycoprotein-M-ediated M-ultidrug R-esistance in H-uman O-varian C-arcinoma A2780/Taxol C-ells by I-nhibiting the PI3K/Akt S-ignaling P-athway. Oncol. Rep. 38, 2558–2564. doi:10.3892/or.2017.5879
Hussein, N., Amawi, H., Karthikeyan, C., Hall, F. S., Mittal, R., Trivedi, P., et al. (2017). The Dopamine D3 Receptor Antagonists PG01037, NGB2904, SB277011A, and U99194 Reverse ABCG2 Transporter-Mediated Drug Resistance in Cancer Cell Lines. Cancer Lett. 396, 167–180. doi:10.1016/j.canlet.2017.03.015
Kato, T., Fujita, Y., Nakane, K., Kojima, T., Nozawa, Y., Deguchi, T., et al. (2012). ETS1 Promotes Chemoresistance and Invasion of Paclitaxel-Resistant, Hormone-Refractory PC3 Prostate Cancer Cells by Up-Regulating MDR1 and MMP9 Expression. Biochem. Biophys. Res. Commun. 417, 966–971. doi:10.1016/j.bbrc.2011.12.047
Kobayashi, Y., Seino, K., Hosonuma, S., Ohara, T., Itamochi, H., Isonishi, S., et al. (2011). Side Population Is Increased in Paclitaxel-Resistant Ovarian Cancer Cell Lines Regardless of Resistance to Cisplatin. Gynecol. Oncol. 121, 390–394. doi:10.1016/j.ygyno.2010.12.366
Li, J., Liu, P., Mao, H., Wanga, A., and Zhang, X. (2009a). Emodin Sensitizes Paclitaxel-Resistant Human Ovarian Cancer Cells to Paclitaxel-Induced Apoptosis In Vitro. Oncol. Rep. 21, 1605–1610. doi:10.3892/or_00000394
Li, L., Jiang, A. C., Dong, P., Wang, H., Xu, W., and Xu, C. (2009b). MDR1/P-gp and VEGF Synergistically Enhance the Invasion of Hep-2 Cells with Multidrug Resistance Induced by Taxol. Ann. Surg. Oncol. 16, 1421–1428. doi:10.1245/s10434-009-0395-7
Liu, X. L., Zhang, X. J., Fu, Y. J., Zu, Y. G., Wu, N., Liang, L., et al. (2011). Cajanol Inhibits the Growth of Escherichia coli and Staphylococcus aureus by Acting on Membrane and DNA Damage. Planta Med. 77, 158–163. doi:10.1055/s-0030-1250146
Loaiza, B., Hernández-Gutierrez, S., Montesinos, J. J., Valverde, M., and Rojas, E. (2016). Nuclear Transcription Factor Kappa B Downregulation Reduces Chemoresistance in Bone Marrow-Derived Cells through P-Glycoprotein Modulation. Arch. Med. Res. 47, 78–88. doi:10.1016/j.arcmed.2016.05.004
Lukyanova, N. Y. (2010). Characteristics of Homocysteine-Induced Multidrug Resistance of Human MCF-7 Breast Cancer Cells and Human A2780 Ovarian Cancer Cells. Exp. Oncol. 32, 10–14.
Martin, C., Berridge, G., Mistry, P., Higgins, C., Charlton, P., and Callaghan, R. (1999). The Molecular Interaction of the High Affinity Reversal Agent XR9576 with P-Glycoprotein. Br. J. Pharmacol. 128, 403–411. doi:10.1038/sj.bjp.0702807
Mihanfar, A., Aghazadeh Attari, J., Mohebbi, I., Majidinia, M., Kaviani, M., Yousefi, M., et al. (2019). Ovarian Cancer Stem Cell: A Potential Therapeutic Target for Overcoming Multidrug Resistance. J. Cel Physiol 234, 3238–3253. doi:10.1002/jcp.26768
Muthusamy, G., Gunaseelan, S., and Prasad, N. R. (2019). Ferulic Acid Reverses P-Glycoprotein-Mediated Multidrug Resistance via Inhibition of PI3K/Akt/NF-Κb Signaling Pathway. J. Nutr. Biochem. 63, 62–71. doi:10.1016/j.jnutbio.2018.09.022
Ren, F., Shen, J., Shi, H., Hornicek, F. J., Kan, Q., and Duan, Z. (2016). Novel Mechanisms and Approaches to Overcome Multidrug Resistance in the Treatment of Ovarian Cancer. Biochim. Biophys. Acta 1866, 266–275. doi:10.1016/j.bbcan.2016.10.001
Schöndorf, T., Kurbacher, C. M., Göhring, U. J., Benz, C., Becker, M., Sartorius, J., et al. (2002). Induction of MDR1-Gene Expression by Antineoplastic Agents in Ovarian Cancer Cell Lines. Anticancer Res. 22, 2199–2203.
Shen, Y., Zhang, X. Y., Chen, X., Ren, M. L., and Cai, Y. L. (2016). Octreotide Reverses the Resistance of A2780/Pacliaxel Ovarian Cancer Cell Line to Paclitaxel Chemotherapy In Vitro. J. Cancer Res. Ther. 12, 657–662. doi:10.4103/0973-1482.151861
Sodani, K., Patel, A., Kathawala, R. J., and Chen, Z. S. (2012). Multidrug Resistance Associated Proteins in Multidrug Resistance. Chin. J. Cancer 31, 58–72. doi:10.5732/cjc.011.10329
Solt, L. A., and May, M. J. (2008). The IkappaB Kinase Complex: Master Regulator of NF-kappaB Signaling. Immunol. Res. 42, 3–18. doi:10.1007/s12026-008-8025-1
Waghray, D., and Zhang, Q. (2018). Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 61, 5108–5121. doi:10.1021/acs.jmedchem.7b01457
Webb, P. M., and Jordan, S. J. (2017). Epidemiology of Epithelial Ovarian Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 41, 3–14. doi:10.1016/j.bpobgyn.2016.08.006
Wu, W., Yang, J. L., Wang, Y. L., Wang, H., Yao, M., Wang, L., et al. (2016). Reversal of Multidrug Resistance of Hepatocellular Carcinoma Cells by Metformin through Inhibiting NF-Κb Gene Transcription. World J. Hepatol. 8, 985–993. doi:10.4254/wjh.v8.i23.985
Xi, G., Hayes, E., Lewis, R., Ichi, S., Mania-Farnell, B., Shim, K., et al. (2016). CD133 and DNA-PK Regulate MDR1 via the PI3K- or Akt-NF-Κb Pathway in Multidrug-Resistant Glioblastoma Cells In Vitro. Oncogene 35, 241–250. doi:10.1038/onc.2016.64
Xu, J., Liu, D., Niu, H., Zhu, G., Xu, Y., Ye, D., et al. (2017). Resveratrol Reverses Doxorubicin Resistance by Inhibiting Epithelial-Mesenchymal Transition (EMT) through Modulating PTEN/Akt Signaling Pathway in Gastric Cancer. J. Exp. Clin. Cancer Res. 36, 19. doi:10.1186/s13046-016-0487-8
Keywords: cajanol, MDR, paclitaxel resistance ovarian cancer, P-glycoprotein, PI3K/AKT/NF-κB pathway
Citation: Sui M, Yang H, Guo M, Li W, Gong Z, Jiang J and Li P (2021) Cajanol Sensitizes A2780/Taxol Cells to Paclitaxel by Inhibiting the PI3K/Akt/NF-κB Signaling Pathway. Front. Pharmacol. 12:783317. doi: 10.3389/fphar.2021.783317
Received: 26 September 2021; Accepted: 22 November 2021;
Published: 08 December 2021.
Edited by:
Jianbin Zhang, Zhejiang Provincial People’s Hospital, ChinaReviewed by:
Noor Ayad Hussein, Stanford University, United StatesRania Harati, University of Sharjah, United Arab Emirates
Copyright © 2021 Sui, Yang, Guo, Li, Gong, Jiang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peiling Li, cGVpbHlAMTI2LmNvbQ==; Jing Jiang, amo4MTA3MDRAMTYzLmNvbQ==
 Ming Sui
Ming Sui Hairong Yang3
Hairong Yang3 Jing Jiang
Jing Jiang