- 1Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China
- 2Department of Orthopedics, People’s Hospital of Ningxiang City, Ningxiang, China
- 3Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 4Institute of Material Medical: Chinese Academy of Medical Sciences & Peking Union Medical College Institute of Materia Medica, Beijing, China
Background: Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease with inflammatory synovitis. Iguratimod (IGU) combined with methotrexate (MTX) therapy may have better efficacy and safety.
Methods: First, we searched randomized controlled trials (RCTs) of IGU + MTX in the treatment of RA through literature databases (such as PubMed, Corkland Library, CNKI, etc.) and then conducted RCT quality assessment and data extraction. Finally, we used RevMan 5.3 for meta-analysis, STATA 15.0 for publication bias assessment, and GRADE tool for the evidence quality assessment of primary outcomes. This systematic review and meta-analysis were registered in PROSPERO (CRD42021220780).
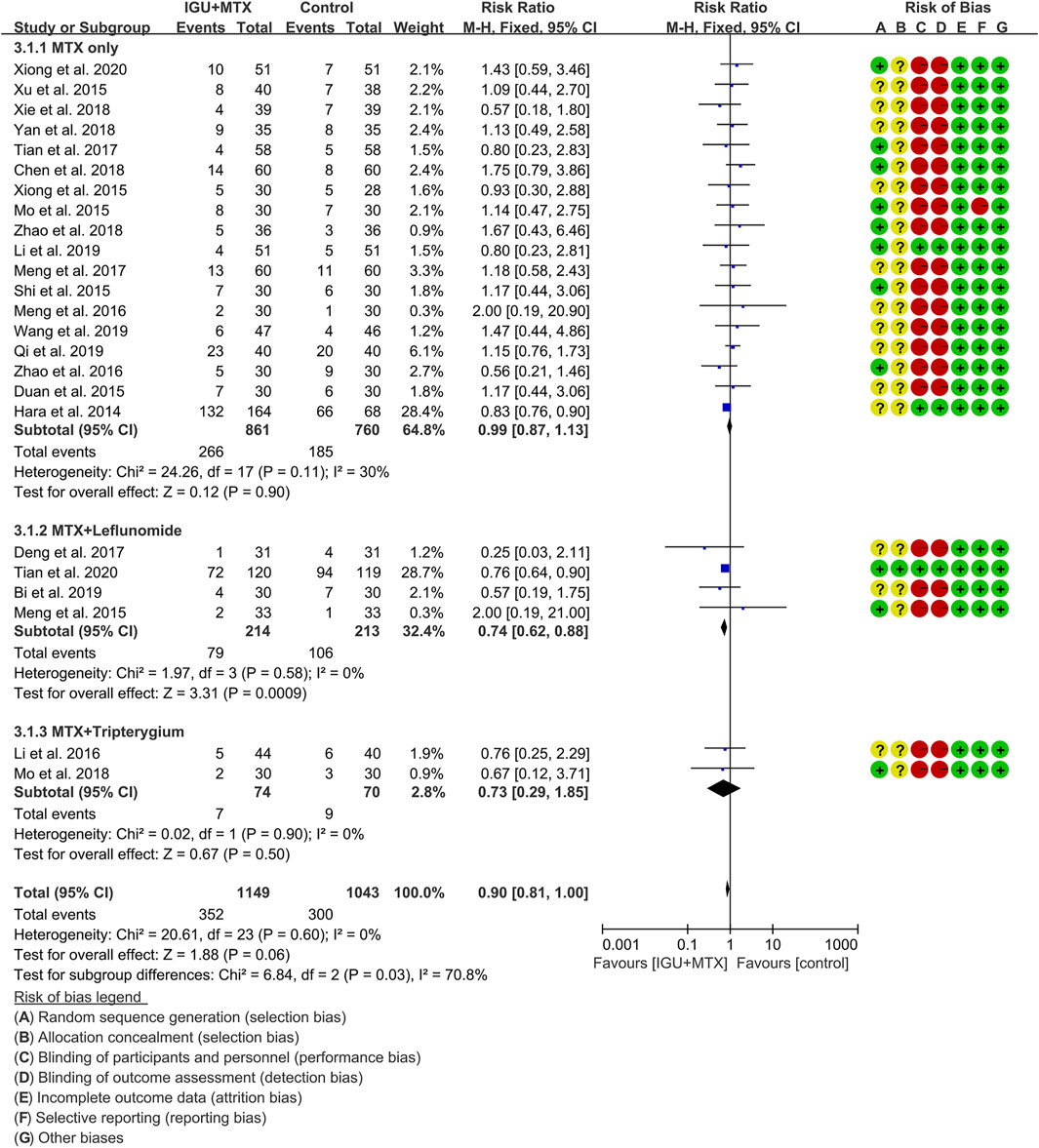
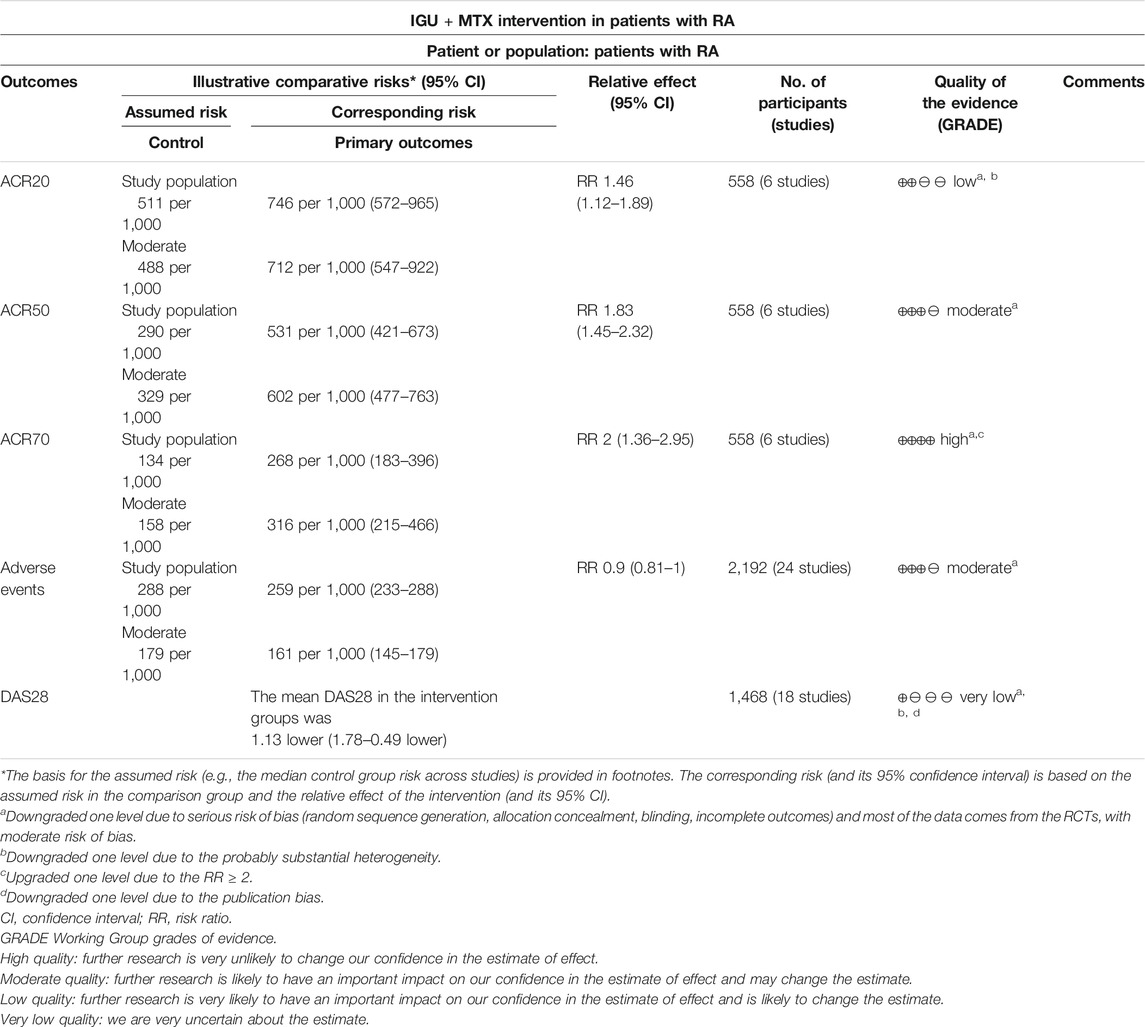
Results: This systematic review and meta-analysis included 31 RCTs involving 2,776 patients. Compared with MTX alone, the ACR20, ACR50, and ACR70 of IGU + MTX are higher, while DAS28 is lower [ACR20: (RR 1.55, 95% CI 1.14–2.13, p = 0.006); ACR50: (RR 2.04, 95% CI 1.57–2.65, p < 0.00001); ACR70: (RR 2.19, 95% CI 1.44–3.34, p = 0.00003); DAS28: (weighted mean difference (WMD) −1.65, 95% CI −2.39 to −0.91, p < 0.0001)]. Compared with MTX + leflunomide, IGU + MTX has no significant difference in improving ACR20, ACR50, ACR70, but IGU + MTX improves DAS28 more significantly [ACR20: (RR 1.09, 95% CI 0.79–1.89, p = 0.59); ACR50: (RR 1.07, 95% CI 0.64–1.78, p = 0.81); ACR70: (RR 1.17, 95% CI 0.44–3.10, p = 0.76); DAS28: (WMD −0.40, 95% CI −0.42 to −0.38, p < 0.0001)]. Compared with the MTX + tripterygium subgroup and MTX-only subgroup, the incidence of adverse events of the IGU + MTX group is of no statistical significance [MTX only: (RR 0.99, 95% CI 0.87–1.13, p = 0.90); MTX + Tripterygium: (RR 0.73, 95% CI 0.29–1.85, p = 0.50)]. However, compared with MTX + leflunomide, the incidence of adverse events in the IGU + MTX group was lower (RR 0.74, 95% CI 0.62–0.88, p = 0.0009). The quality of ACR70 was high; the quality of adverse events and ACR50 test was moderate.
Conclusion: Compared with conventional therapy, IGU + MTX may be a safer and more effective therapy for RA patients. When the intervention method is (IGU 25 mg Bid, MTX 10–25 mg once a week), and the intervention lasts for at least 12 weeks, the curative effect may be achieved without obvious adverse events.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease with inflammatory synovitis, the cause of which is not yet clear, and it mainly damages the synovial tissue of the joints. It is characterized by the increase of interleukin (IL) and tumor necrosis factor (TNF), and the activation of T lymphocytes, which may lead to severe chronic inflammation of joints, and even erosion and destruction of cartilage, bone, and tendons (Scott et al., 2010; Wasserman 2011; Wasserman 2018). Epidemiological studies show that the incidence of RA worldwide is 0.5%–1% (Wasserman 2018; Otón and Carmona 2019). Although the incidence is not high, the number of patients is very large due to the long survival time of patients. In particular, many patients with RA have joint deformities in the late stage, causing paralysis, complete loss of labor, and occupation of a large number of medical and social resources (Hunter et al., 2017; Otón and Carmona 2019). The current goal of RA treatment is to alleviate patients’ clinical symptoms, reduce or prevent patients’ joint damage, and emphasize the early use of disease-modifying anti-rheumatic drugs (DMARDs), such as methotrexate and leflunomide (Burmester and Pope 2017; Ferro et al., 2017). In addition, some biological agents such as anti-TNF-α blockers, anti-IL antibodies, and CD20 monoclonal antibodies are also used in the treatment of RA (Burmester and Pope 2017; Liu et al., 2018).
In view of the complex pathogenesis of RA, a single drug often fails to effectively achieve the treatment goals. Therefore, major international and domestic guidelines recommend the use of combination therapy when a single DMARD treatment fails to meet the standard (Singh et al., 2016; Smolen et al., 2017; Luo et al., 2019). Combination therapy can improve the efficacy and reduce the incidence of adverse reactions, which is the main trend of RA treatment. The 2018 Chinese Rheumatoid Arthritis Diagnosis and Treatment Guidelines also mention that those who have not reached the standard after MTX single-agent standard treatment are recommended to be combined with another synthetic DMARD for treatment (Chinese Rheumatology Association, 2018). Iguratimod (IGU) was considered to be the preferred drug for combination therapy. A number of studies have confirmed that after the treatment of patients with poor efficacy of methotrexate (MTX) combined with IGU treatment, the efficacy indicators such as disease activity and bone metabolism have been significantly improved, and the incidence of adverse reactions is low (Ishiguro et al., 2013; Hara et al., 2014; Zhang et al., 2014; Ding et al., 2019; Suto et al., 2019). Recently, we conducted a multicenter, large-scale randomized controlled trial to investigate the efficacy and safety of the IGU combined with MTX group (group A) and the leflunomide combined with MTX group (group B) on the two groups at 12, 24, and 52 weeks after treatment and compared the ACR20 compliance rate, the improvement range of DAS28, and the incidence of adverse reactions after 52 weeks (Tian et al., 2020). The results show that IGU combined with MTX might be as effective as leflunomide combined with MTX in the treatment of patients with active RA, and the incidence of adverse events is low. At present, Chen et al. (2021) conducted a systematic review and meta-analysis of IGU + MTX treatment of RA, but only seven randomized controlled trials (RCTs) were included. A number of RCTs from other clinical research centers have also reported the effectiveness and safety of IGU combined with MTX in the treatment of RA (Lu 2014; Duan et al., 2015; Xia et al., 2016). However, the previous RCTs are often small-scale single-center clinical trials (Lu 2014; Duan et al., 2015; Shi et al., 2015; Xia et al., 2016). Therefore, this study would conduct a systematic review and meta-analysis of the effectiveness and safety of IGU combined with MTX in the treatment of RA for the first time, in order to provide solid evidence and new directions for clinical use and also provide new experience and direction for future RCTs.
Materials and Methods
Protocol
This meta-analysis were conducted strictly in accordance with the protocol registered in PROSPERO (CRD42021220780) and PRISMA guidelines (see Supplementary Materials).
Literature Search Strategy
We searched Embase, PubMed, The ClinicalTrials.gov, VIP Database for Chinese Technical Periodicals, Wanfang Database on Academic Institutions in China, China National Knowledge Infrastructure (CNKI), Cochrane Library, China Biology Medicine (CBM), MEDLINE Complete, and Web of Science with the retrieval time up to December 2020. The search strategy is shown in Supplementary Table S1.
Selection Criteria
Participants
The participants were patients with RA. The age, gender, and nationality of patients are not limited. The literature needs to mention clear diagnostic criteria for RA, with a balanced baseline and comparability.
Intervention
The treatment of the experimental group is IGU + MTX. The treatment of the control group was MTX alone or MTX combined with other therapies that did not contain IGU.
Outcomes
Primary outcomes are ACR20, ACR50, ACR70, 28 joint disease activity score (DAS28), and adverse events. Secondary outcomes are: (1) symptom-related outcomes: Health Assessment Questionnaire (HAQ), morning stiffness time (min), number of swollen joints, and number of tender joints; (2) bone protection-related outcomes: N-terminal osteocalcin (N-MID), total T-type procollagen amino terminal propeptide (T-PINP) levels, 25-hydroxy vitamin D [25(OH)D], and β-I collagen carboxy terminal peptide (β-CTX); (3) inflammation and immune response-related outcomes: rheumatoid factor (RF), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), anti-cyclic citrullinated peptides (CCP), and TNF-α; and (4) angiogenesis-related outcomes: VEGF.
Study Design
The study design was RCTs, with no limitations to publication time, language, quality, and publication status.
Literature Screening and Data Extraction
In the first stage of literature screening, two reviewers independently conducted manual screening of the initially included clinical research literature. First, two reviewers read the title, abstract, and keywords and then exclude non-RCT, duplicate or identical papers, papers with obscure data, and papers whose full text cannot be obtained. In the second stage, after reading the full text of the literature, two reviewers further screened the literature that could eventually be included in the meta-analysis based on selection criteria. Finally, two reviewers independently extracted data from the literature. The differences between the two reviewers in the selection of literature and data extraction were resolved through consultation with the third reviewer.
Risk of Bias Assessment
The “risk of bias” assessment tool recommended by the Cochrane Collaboration (Deeks et al., 2021a) is used to assess the risk of bias. The tools include 1) generation of random sequence; 2) allocation concealment; 3) blinding of subjects and intervention providers; 4) blinding of outcome evaluators; 5) incomplete outcome data; 6) selective outcome report; and 7) other sources of bias.
Statistical Analysis
RevMan 5.3 software was used for risk-of-bias assessment and meta-analysis. First, the heterogeneity test was performed. The subgroup analysis was carried out based on the intervention measures of the control group. When the heterogeneity among the included studies was low (p > 0.1 and I2 ≤ 50%), the fixed-effect model was used; otherwise, the random-effect model was used (Deeks et al., 2021b). For continuous variables, when the measurement data units are different, the values differ greatly, or the measurement methods are different between different studies, the standardized mean difference (SMD) is used as the effect size indicator, and the other uses the mean difference (MD) as the effect size indicator. For dichotomous variables, the risk ratio (RR) is used as the effect size indicator. Each effect size is expressed with a 95% confidence interval (95% CI). The publication bias was detected by STATA 15 with the Egger method (continuous variable) and Harbord method (dichotomous variable) for primary outcomes. p > 0.1 is considered to have no publication bias. The GRADE tool was utilized to rate the quality of the evidence (GRADEpro 2015) according to the GRADE handbook (Schünemann et al., 2013).
Results
Results of the Search
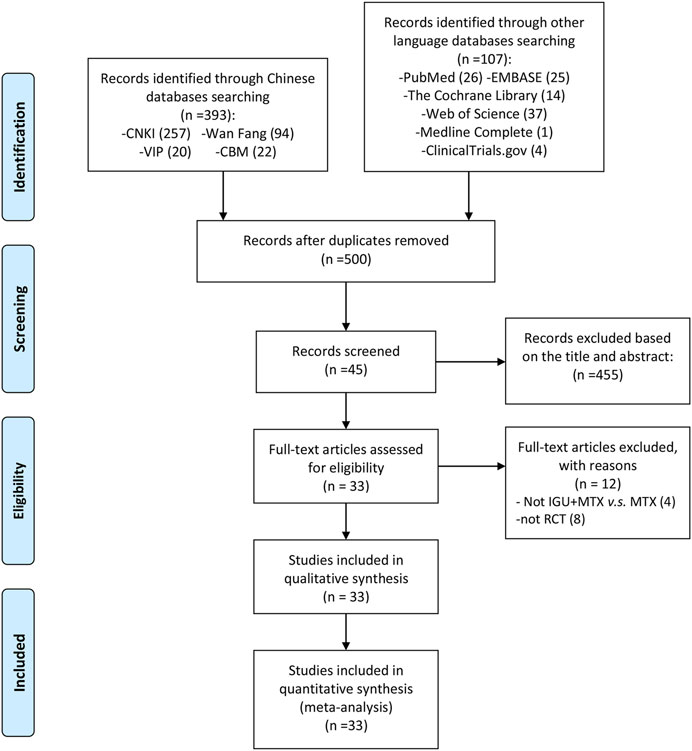
The total records identified through database searching and other sources were 500. According to the search strategy, a total of 45 articles were obtained through preliminary search. By eliminating duplicate documents, carefully reading the title and abstract, a total of 456 articles were excluded. After carefully reading the full text and comparing the selection criteria, 33 RCTs were screened out and finally included (Shi et al., 2015; Lu 2014; Duan et al., 2015; Xiong et al., 2020; Xu et al., 2015; Ding et al., 2019; Deng et al., 2017; Tian et al., 2020; Xie et al., 2018; Xu et al., 2017; Bi et al., 2019; Yan et al., 2018; Tian et al., 2017; Chen et al., 2018; Xiong et al., 2015; Mo et al., 2015; Fan et al., 2020; Zhao et al., 2018; Li et al., 2020; Li et al., 2019; Meng et al., 2017; Li et al., 2016; Xia et al., 2020; Meng et al., 2015; Mo et al., 2018; Meng et al., 2016; Wang et al., 2019; Qi et al., 2019; Ju et al., 2020; Zhao et al., 2016; Hara et al., 2014; Ishiguro et al., 2013; Xia et al., 2016) (Figure 1). The main reason why 12 studies were excluded is that eight studies are not RCTs (Yoshioka et al., 2016; Wang et al., 2017; Suto et al., 2019; Okamura et al., 2015a,b; Wang 2017; Meng et al., 2016; Gu et al., 2020), and the other four studies are not comparing IGU + MTX with MTX (Liu 2016; Xu et al., 2017; Lu and Liu 2018; Zhu 2019).
Description of Included Trials
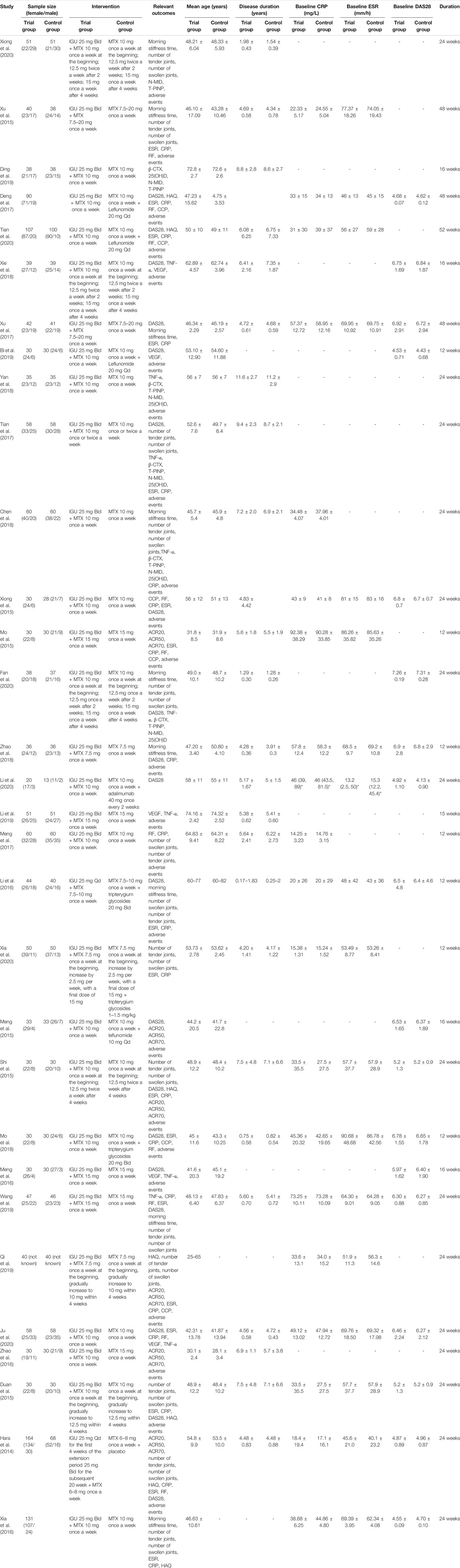
The study of Hara et al. (2014) (Ishiguro et al., 2013; Hara et al., 2014) was conducted in Japan. The participants in the other RCTs were from China. There are 10 RCTs with more than 100 participants (Hara et al., 2014; Xia et al., 2016; Meng et al., 2017; Tian et al., 2017; Chen et al., 2018; Li et al., 2019; Ju et al., 2020; Tian et al., 2020; Xia et al., 2020; Xiong et al., 2020); there are 20 RCTs ranging from 50 to 100 participants (Duan et al., 2015; Meng et al., 2015; Mo et al., 2015; Shi et al., 2015; Xu et al., 2015; Li et al., 2016; Meng et al., 2016; Zhao et al., 2016; Deng et al., 2017; Xu et al., 2017; Mo et al., 2018; Xie et al., 2018; Yan et al., 2018; Xiong et al., 2015; Zhao et al., 2018; Bi et al., 2019; Ding et al., 2019; Qi et al., 2019; Wang et al., 2019; Fan et al., 2020). The interventions in the control group were mostly MTX alone. The control group of Deng et al. (2017), Tian et al. (2020), Bi et al. (2019), and Meng et al. (2015) used MTX + leflunomide; the control group of Li et al. (2019), Xia et al. (2020), and Mo et al. (2018) used MTX + tripterygium; and the control group of Li et al. (2020) used MTX + adalimumab. Subgroup analysis would be based on the treatment of the control group. The details of study characteristics are presented in Table 1.
Risk of Bias of Included Studies
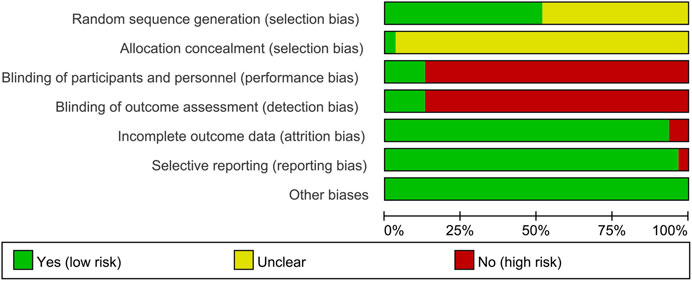
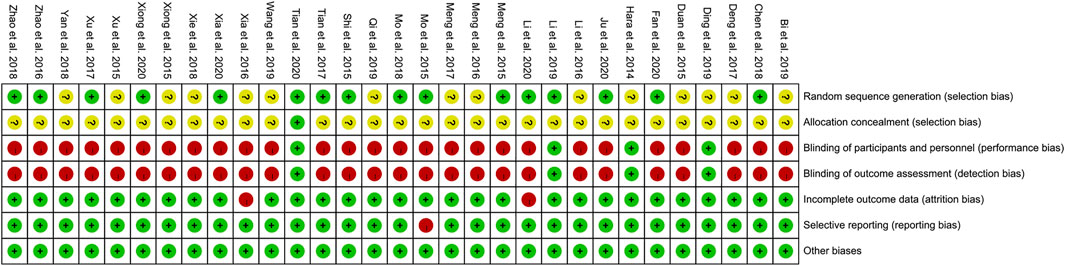
Sequence Generation and Allocation Concealment
Fifteen RCTs describe the random sequence generating method: 10 RCTs utilized random number table (Meng et al., 2015; Mo et al., 2015; Shi et al., 2015; Zhao et al., 2016; Tian et al., 2017; Chen et al., 2018; Mo et al., 2018; Ju et al., 2020; Xia et al., 2020; Xiong et al., 2020); two RCT utilized the two-color ball method (Zhao et al., 2018; Li et al., 2020); Fan et al. utilized the odd and even number random method; Xu et al. utilized random lottery; Li et al. (2019) utilized the envelope random method; and Tian et al. utilized centralized randomization. We rate these studies as low risk of bias. The other RCTs did not describe the random sequence generating methods and were rated as unclear risk of bias. Tian et al. (2020) used the “double dummy” method to make the number and appearance of the tablets in the two groups similar. We consider this to be allocation concealment and rated it as low risk of bias. The other RCTs did not state whether allocation concealment was carried out, so we evaluated the risk of bias as unclear.
Blinding
Tian et al. (2020) and Hara et al. (2014) used a double-blind method, so they were considered to be a low risk of bias. Although Ding et al. (2019) and Meng et al. (2017) did not state whether blinding was used, but because its outcomes are objective indicators (β-CTX, N-MID, T-PINP), which is less affected by blinding, we assessed the risk of bias as low. Other RCTs did not mention whether to use blinding, and their main outcome indicators are subjective evaluation indicators (such as DAS28, ACR20, ACR50, ACR70), which are easily affected by not implementing the blind method. Therefore, we evaluate them as a high risk of bias.
Incomplete Outcome Data and Selective Reporting
Li et al. (2020) and Xia et al. (2016) have incomplete outcome, and there is an imbalance between the number of missing persons and the reasons for the missing between groups. Hence, they were rated as having a high risk of bias. The other RCTs do not have incomplete outcome data. Mo et al. (2015) mentioned the morning stiffness time, number of tender joints, and number of swollen joints but did not report in the article. Therefore, we thought that they have selective reporting and assess the risk of bias as high. No selective reports were found in other RCTs, so they were considered low risk of bias.
Other Potential Bias
Other sources of bias were not observed in 13 RCTs; therefore, the risks of other bias of the RCTs were low (Figures 2, 3).
Primary Outcomes
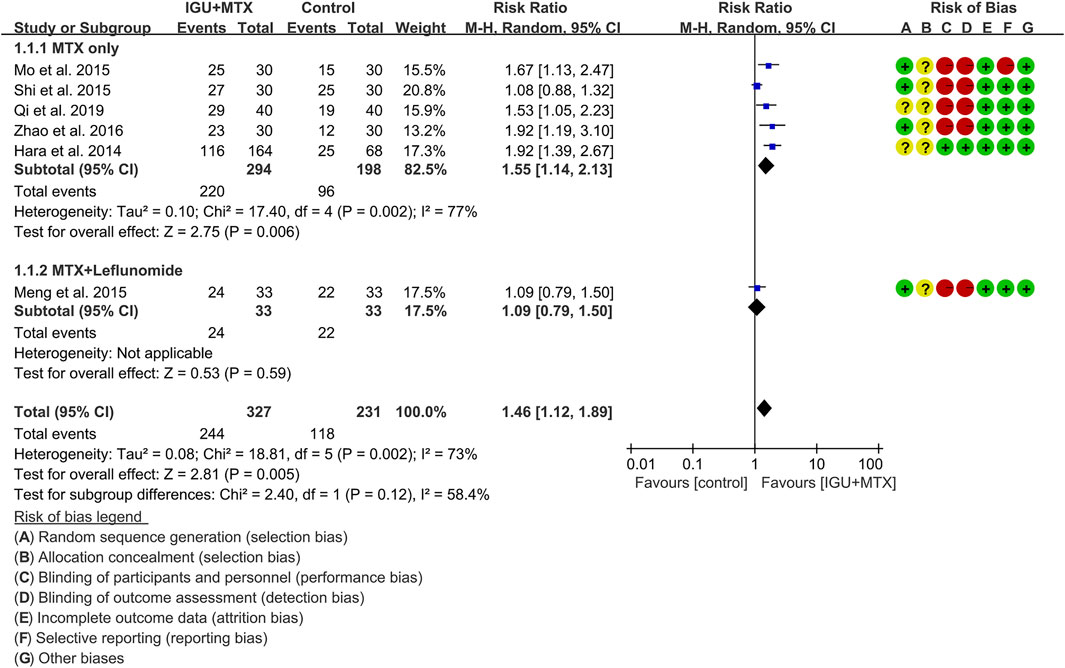
ACR20
Six RCTs assessed the ACR20 of patients, which involves 327 patients in the IGU + MTX group and 231 patients in the control group. According to the intervention characteristics, six RCTs were subdivided into two subgroups (MTX-only subgroup and MTX + leflunomide subgroup). There was high heterogeneity in each subgroup (MTX only: I2 = 77%, p = 0.002; MTX + leflunomide: not applicable) among the RCTs. The random-effect model was used. According to Figure 4, the ACR20 in the IGU + MTX group was higher than that in the control group in the MTX-only subgroup (RR 1.55, 95% CI 1.14–2.13, p = 0.006; random-effect model), but its difference is of no statistical significance in the MTX + leflunomide subgroup (RR 1.09, 95% CI 0.79–1.89, p = 0.59; random-effect model). The summary result also showed that the ACR20 in the IGU + MTX group was higher (RR 1.46, 95% CI 1.12–1.89, p = 0.005; random-effect model) (Figure 4).
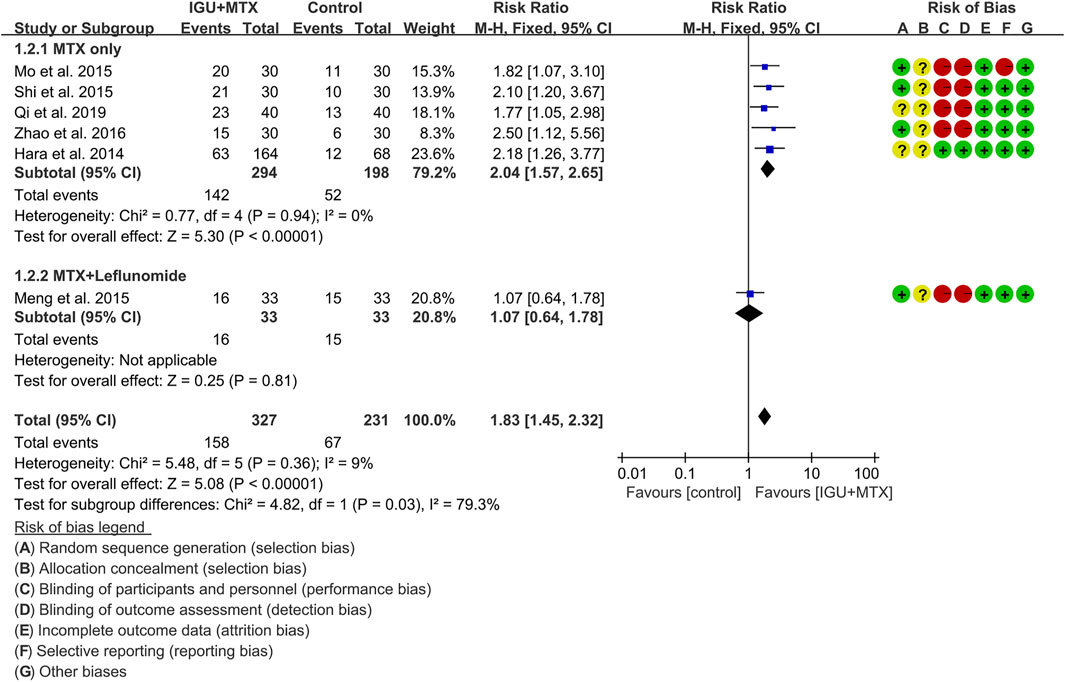
ACR50
Six RCTs assessed the ACR50 of patients, which involves 327 patients in the IGU + MTX group and 231 patients in the control group. According to the intervention characteristics, they were subdivided into two subgroups (MTX-only subgroup and MTX + leflunomide subgroup). There was low heterogeneity in each subgroup (MTX only: I2 = 0%, p = 0.94; MTX + leflunomide: not applicable) among the RCTs. The fixed-effect model was used. According to Figure 5, the ACR50 in the IGU + MTX group was higher than that in the control group in the MTX-only subgroup (RR 2.04, 95% CI 1.57–2.65, p < 0.00001; fixed-effect model), but its difference is of no statistical significance in the MTX + leflunomide subgroup (RR 1.07, 95% CI 0.64–1.78, p = 0.81; fixed-effect model). The summary result also showed that the ACR50 in the IGU + MTX group was higher (RR 1.83, 95% CI 1.45–2.32, p < 0.00001; fixed-effect model) (Figure 5).
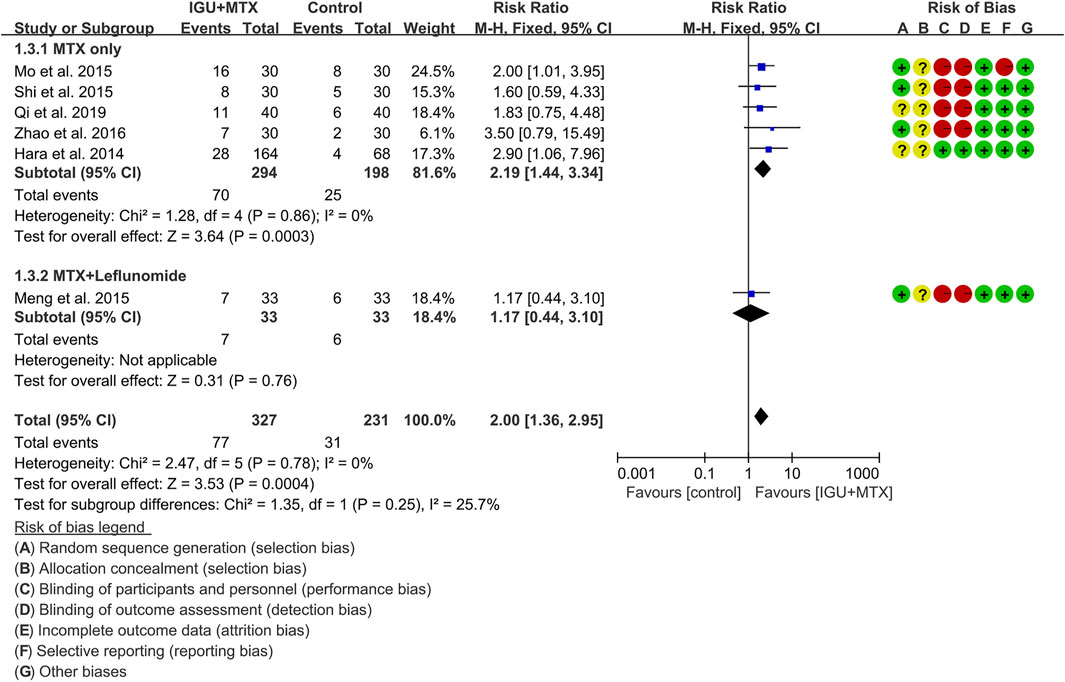
ACR70
Six RCTs assessed the ACR70 of patients, which involves 327 patients in the IGU + MTX group and 231 patients in the control group. According to the intervention characteristics, they were subdivided into two subgroups (MTX-only subgroup and MTX + leflunomide subgroup). There was low heterogeneity in each subgroup (MTX only: I2 = 0%, p = 0.86; MTX + leflunomide: not applicable) among the RCTs. The fixed-effect model was used. According to Figure 6, the ACR70 in the IGU + MTX group was higher than that in the control group in the MTX-only subgroup (RR 2.19, 95% CI 1.44–3.34, p = 0.00003; fixed-effect model), but its difference is of no statistical significance in the MTX + leflunomide subgroup (RR 1.17, 95% CI 0.44–3.10, p = 0.76; fixed-effect model). The summary result also showed that the ACR70 in the IGU + MTX group was higher (RR 2.00, 95% CI 1.36–2.95, p = 0.00004; fixed-effect model) (Figure 6).
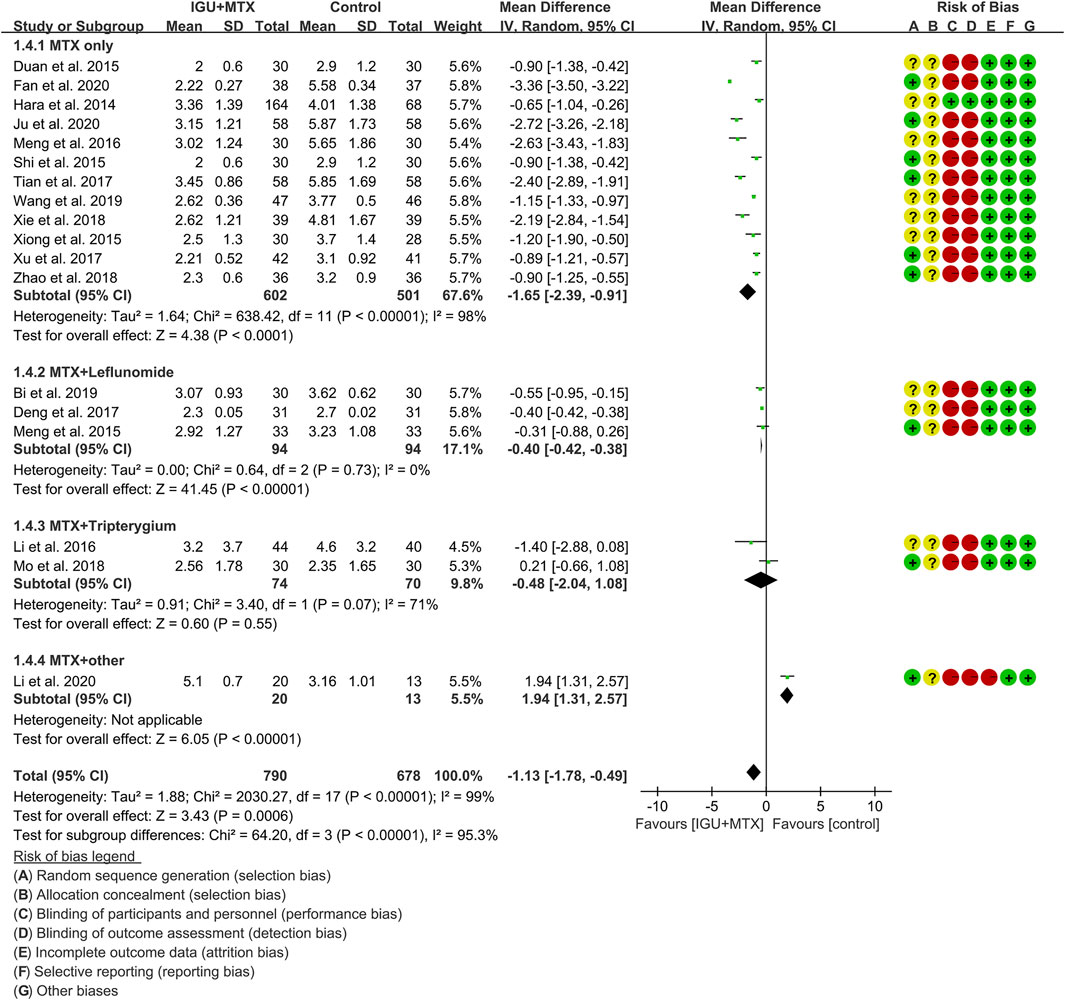
DAS28
Eighteen RCTs assessed the DAS28 of patients, which involves 790 patients in the IGU + MTX group and 678 patients in the control group. According to the intervention characteristics, they were subdivided into four subgroups (MTX-only subgroup, MTX + leflunomide subgroup, MTX + tripterygium subgroup, MTX + other subgroup). There was high heterogeneity in the most subgroup (MTX only: I2 = 98%, p < 0.00001; MTX + leflunomide: I2 = 0%, p = 0.73; MTX + tripterygium: I2 = 71%, p = 0.07; MTX + other: not applicable) among the RCTs. The random-effect model was used. According to Figure 7, the DAS28 in the IGU + MTX group was lower than that in the control group in the MTX-only subgroup (WMD -1.65, 95% CI −2.39 to −0.91, p < 0.0001; random-effect model) and MTX + leflunomide subgroup (WMD −0.40, 95% CI −0.42 to −0.38, p < 0.0001; random-effect model), but its difference is of no statistical significance in the MTX + tripterygium subgroup (WMD −0.48, 95% CI −2.04 to 1.08, p = 0.55; random-effect model). However, in the MTX + other subgroup, the DAS28 in IGU + MTX group is higher than that of the control group (WMD 1.94, 95% CI 1.31–2.57, p < 0.00001; random-effect model). The summary result also showed that the DAS28 in the IGU + MTX group was lower (WMD −1.13, 95% CI −1.78 to −0.49, p = 0.0006; random-effect model).
Secondary Outcomes
The secondary outcomes include 1) symptom-related outcomes: HAQ, morning stiffness time (min), number of swollen joints, and number of tender joints; 2) bone protection-related outcomes: N-MID, total T-PINP levels, 25(OH)D, and β-CTX; 3) inflammation and immune response-related outcomes: RF, ESR, CRP, anti-CCP, and TNF-α; and 4) angiogenesis-related outcomes: VEGF. The results are shown in Table 2.
Adverse Events
Twenty-four RCTs reported the adverse events. According to the intervention characteristics, they were subdivided into three subgroups (MTX-only subgroup, MTX + leflunomide subgroup, MTX + tripterygium subgroup). There was low heterogeneity in the most subgroup (MTX only: I2 = 30%, p = 0.11; MTX + leflunomide: I2 = 0%, p = 0.58; MTX + tripterygium: I2 = 0%, p = 0.90) among the RCTs. The fixed-effect model was used. According to Figure 8, the adverse events in the IGU + MTX group were lower than those in the control in the MTX + leflunomide subgroup (RR 0.74, 95% CI 0.62–0.88, p = 0.0009; fixed-effect model). However, the difference is of no statistical significance in the MTX-only subgroup (RR 0.99, 95% CI 0.87–1.13, p = 0.90; fixed-effect model) and MTX + tripterygium subgroup (RR 0.73, 95% CI 0.29–1.85, p = 0.50; fixed-effect model). The summary result also showed that the difference is of no statistical significance between the two groups (RR 0.90, 95% CI 0.81–1.00, p = 0.06; fixed-effect model).
Other Subgroup Analysis Results
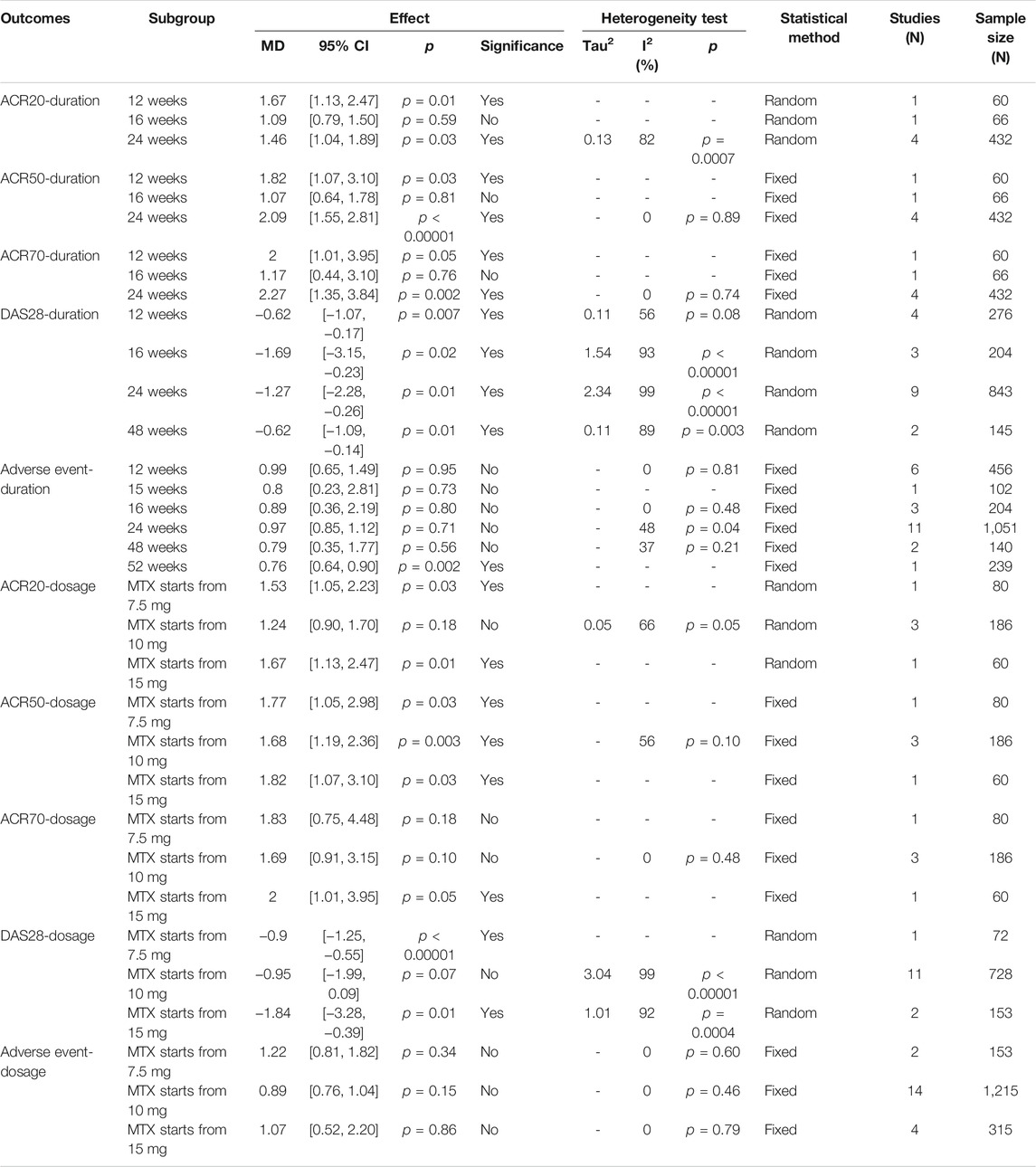
This study also conducted a subgroup analysis of primary outcomes based on duration and drug dose (Table 3). For the drug dose, since the dose of IGU is basically the same, the subgroup analysis is performed based on the starting dose of MTX. The results show that there is no obvious rule for the outcomes of different starting doses. For the duration of the intervention, the results showed that DAS28 improved after the intervention lasted at least 12 weeks.
Publication Bias Detection
The publication bias of the primary outcomes was detected by STATA 15.0. 1) ACR20: the publication bias detection suggests that the possibility of publication bias was small (p = 0.355) (Figure 9A). 2) ACR50: the publication bias detection suggests that the possibility of publication bias was small (p = 0.837) (Figure 9B). 3) ACR70: the publication bias detection suggests that the possibility of publication bias was small (p = 0.699) (Figure 9C). 4) DAS28: the publication bias detection suggests that there may be publication bias (p = 0.097) (Figure 9D). 4) Adverse events: the publication bias detection suggests that the possibility of publication bias was small (p = 0.234) (Figure 9E).
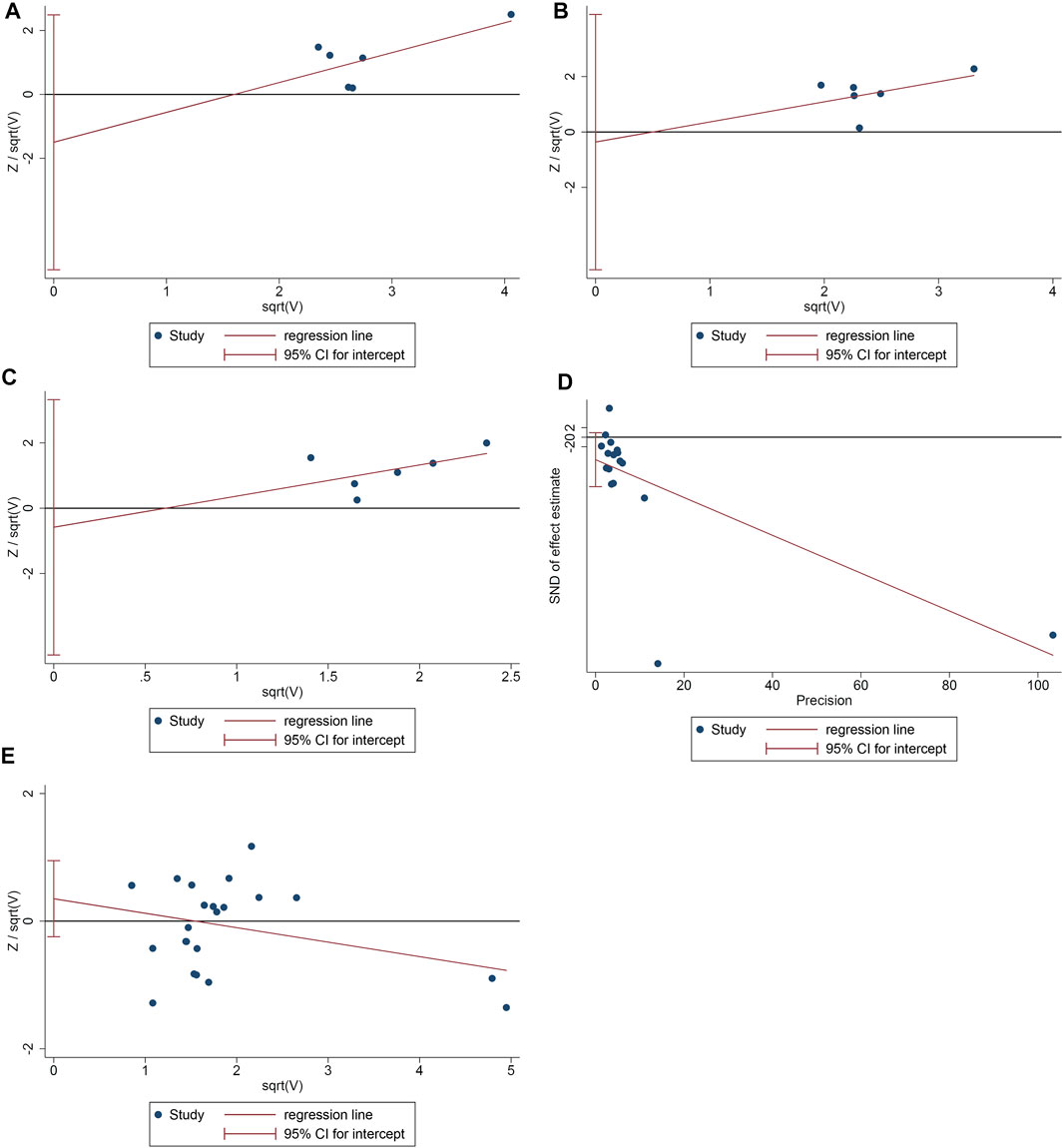
FIGURE 9. The results of publication bias detection (A: ACR20; B: ACR50; C: ACR70; D: DAS28; E: adverse events).
Evidence Quality Assessment
The evidence was judged to be high to very low (Table 2). The quality of ACR70 was high; the quality of adverse events and ACR50 test was moderate; the quality of ACR20 was low; and the quality of DAS28 was very low (Table 4).
Discussion
Main Outcome Summary
This systematic review and meta-analysis included 31 RCTs involving 2,776 participants. The number of study participants was mostly between 30 and 240, and the interventions in the control group were mostly MTX alone. The control group of Deng et al. (2017), Tian et al. (2020), Bi et al. (2019), and Meng et al. (2015) used MTX + leflunomide; the control group of Li et al. (2019), Xia et al. (2020), and Mo et al. (2018) used MTX + tripterygium; and the control group of Li et al. (2020) used MTX + adalimumab. Sixteen RCTs failed to describe their random sequence generating method. Only Tian et al. (2020) described the allocation concealment methods. Twenty-nine RCTs failed to utilize blinding. Li et al. (2020) and Xia et al. (2016) have incomplete outcome, and Mo et al. (2015) mentioned the morning stiffness time, number of tender joints, and number of swollen joints but did not report in the article. The overall risk of bias is higher.
The main findings of this study can be summarized as the following: 1) compared with MTX alone, the ACR20, ACR50, and ACR70 of IGU + MTX are higher, while DAS28 is lower; and the symptom-related outcomes (HAQ, morning stiffness time, number of tender joints, number of swollen joints), bone protection-related outcomes [N-MID, T-PINP, β-CTX, 25(OH)D], inflammatory and immune response-related outcomes (RF, ESR, CRP, anti-CCP, TNF-α), and angiogenesis outcomes (VEGF) in the IGU + MTX group improved better. In terms of safety, there was no significant difference in the incidence of adverse events between the IGU + MTX group and the MTX-only group. 2) Compared with MTX + leflunomide, IGU + MTX has no significant difference in improving ACR20, ACR50, and ACR70, but IGU + MTX improves DAS28, HAQ, and anti-CCP more significantly. In terms of improving number of tender joints, number of swollen joints, RF, ESR, and CRP, the difference between the two groups was not statistically significant. However, the incidence of adverse events in the IGU + MTX group was lower. Our previous RCT also showed that compared with MTX + leflunomide, IGU + MTX has no statistical significance in improving ACR20, ACR50, ACR70, ESR, and CRP (Tian et al., 2020). Our RCT also shows that IGU + MTX is safer than MTX + leflunomide (Tian et al., 2020). 3) Compared with MTX + tripterygium, IGU + MTX has no significant difference in improving DAS28 and anti-CCP, but IGU + MTX improves morning stiffness time, number of tender joints, number of swollen joints, RF, ESR, and CRP more significantly. In terms of safety, there was no significant difference in the incidence of adverse events between the IGU + MTX group and the MTX + tripterygium group. 4) Compared with the IGU + adalimumab group, the DAS28 in the IGU + MTX group is higher. 5) For the duration of the intervention, DAS28 improved after the intervention lasted at least 12 weeks. 6) The publication bias test of the primary outcomes showed that there was no publication bias in ACR20, ACR50, ACR70, and adverse events, while DAS28 may have publication bias.
In summary, this systematic review and meta-analysis provide a new treatment strategy for the combination of IGU and MTX in the treatment of RA. When the intervention method is (IGU 25 mg Bid, MTX 10–25 mg once a week), and the intervention lasts for at least 12 weeks, the curative effect may be achieved without obvious adverse events.
Applicability of Evidences
RA is a common autoimmune disease characterized by painful and swollen joints, which severely impairs the patient’s physical function and quality of life. The treatment of RA has made great progress in the past 10 years. Relevant therapeutic drugs have been continuously introduced, which has improved the treatment and management of RA patients. In recent years, standard treatment has been used in the management of many chronic diseases (Jin et al., 2020). Especially considering the complex pathogenesis of RA, a single drug often fails to effectively achieve the treatment goal. Therefore, international and domestic guidelines recommend the use of combination therapy when a single DMARD treatment fails to meet the standard (Jiang et al., 2020; Jin et al., 2020). Combination therapy can improve the efficacy and reduce the incidence of adverse reactions, which is the main trend of RA treatment (Xie et al., 2020).
MTX is a first-line drug for the treatment of RA. Its main mechanism of action is as follows: inhibiting the formation of tetrahydrofolate by inhibiting dihydrofolate reductase, blocking DNA synthesis, and achieving anti-inflammatory and anti-immune effects (Lu et al., 2009; Mimori et al., 2019). Recent studies have shown that MTX is the “anchoring drug” in the treatment of most RA patients. The European Federation for the Prevention and Treatment of Rheumatism (EULAR) recommends that the efficacy of MTX be evaluated after 3 months of treatment. If there is no improvement within 3 months of the first treatment or the treatment goal is not reached within 6 months, the treatment plan should be reconsidered (GRADEpro 2015). The 2018 Chinese Rheumatoid Arthritis Diagnosis and Treatment Guidelines also mentioned that if the standard treatment of MTX still fails to reach the target, it is recommended to combine another synthetic DMARD for treatment (Schünemann et al., 2013). However, in clinical practice, there are a variety of prescriptions for combination drugs for the treatment of RA, such as IGU + MTX, MTX + leflunomide, and islammod + diacerein (Hao and Li 2014; Yoshikawa et al., 2018; Dai et al., 2019).
IGU is a new type of immunosuppressant used in RA in recent years. It can not only inhibit intracellular inflammatory factors and suppress immunity but also significantly inhibit bone resorption and bone destruction (Jiang et al., 2020; Xie et al., 2020). Joint damage is one of the important characteristics of RA, caused by abnormal bone metabolism. The balance of bone metabolism is controlled by bone resorption and bone formation, and they can be regulated by a variety of cytokines and signaling pathways. With regard to the combination of IGU and MTX, recent studies have also shown the complementarity of the two. Research by Wang et al. showed that both IGU and MTX can significantly inhibit the high expression of RANKL mRNA (p < 0.01), and the combination of the two drugs showed a stronger inhibitory effect (compared with MTX, p < 0.01; compared with IGU, p < 0.05). In addition, the combined drug group showed a more significant difference in inhibiting the ratio of RANKL mRNA/OPG mRNA than the single drug (p < 0.05) (Wang 2017). Yan and Wang (2018) also found that IGU combined with MTX can synergistically reduce the inflammatory response in patients with RA, better play the role of bone formation, and antagonize bone resorption.
This study found that the ACR20, ACR50, and ACR70 of IGU + MTX were not statistically different from that of MTX + leflunomide. However, the results of DAS28 show that the effect of IGU + MTX is better than that of MTX + leflunomide. This may be due to the fact that the MTX + leflunomide subgroup in ACR20, ACR50, and ACR70 only contains one RCT with extractable data, and the conclusion is unstable. In the future, more relevant research is needed to revise or verify this result.
Discussion of the Source of Heterogeneity
The summary results show that the heterogeneity of ACR20 and DAS28 is high, while the heterogeneity of ACR50 and ACR70 is low. After subgroup analysis (based on the control group interventions, drug dosage and intervention duration), the heterogeneity of ACR50 and ACR70 is still low, while the heterogeneity of DAS28 and ACR20 is still high. As for the heterogeneity after subgroup analysis, we considered that the heterogeneity may be related to the following points: 1) it may be related to the patient’s baseline state of illness activity, and the baseline patient’s illness activity is not clearly stated in each study, so it cannot be further analyzed; 2) the heterogeneity of DAS28 may also be related to publication bias. In DAS28, IGU + MTX compared with IGU + adalimumab showed the opposite result from the comprehensive result. However, since only one study reported this, no definite conclusion can be drawn, and more relevant RCTs are needed to modify or verify this result. Most RCTs reported adverse reactions, but no deaths were reported. There was no difference in adverse reactions between the IGU combined with MTX and MTX alone in the blood system (leukopenia), liver function abnormalities, and gastrointestinal adverse reactions (malignant, vomiting). This shows that IGU combined with MTX will not cause additional infection or gastrointestinal adverse reactions to patients.
Safety of IGU + MTX
Safety analysis showed that compared with MTX + tripterygium and MTX only, there was no statistically significant difference in the incidence of adverse events in IGU + MTX. Compared with MTX + leflunomide, the incidence of adverse events in IGU + MTX was lower. Current research also shows that compared with other DMARDs, IGU is safe and suitable for long-term use. For example, a multicenter, randomized, double-blind, controlled trial showed that the IGU group had a lower incidence of adverse reactions than the MTX group after 24 weeks of treatment (Lu et al., 2009). The long-term application of IGU has relatively mild adverse reactions and a low incidence. The most common adverse reactions are gastrointestinal reactions and elevated liver enzymes. Tsuneyo Mimori et al. conducted a 52-week multicenter, prospective observational, phase IV clinical study in Japan, which proved that the incidence of IGU adverse reactions reached a peak after about 4 weeks of treatment. Subsequently, the incidence of various adverse reactions did not increase over time (Mimori et al., 2019). Our previous RCT showed that during 52 weeks of treatment, IGU combined with MTX therapy is safer than traditional leflunomide combined with MTX therapy. The incidence of overall adverse events, alanine aminotransferase (ALT)/aspartate aminotransferase (AST) that reflect liver function increased, and the rate of leukopenia was lower (Tian et al., 2020). Our previous RCT showed that during 52 weeks of treatment, IGU combined with MTX therapy is safer than MTX + leflunomide therapy. The incidence of overall adverse events, the incidence of ALT/AST elevation, and leukopenia decrease are lower (Tian et al., 2020).
A recently published multicenter, prospective, real-world study of IGU in the treatment of RA also showed its better safety. The data come from the participation of 48 hospitals in China, and a total of 1,759 patients with active RA were enrolled. It showed that the incidence of ≥ grade 3 adverse events was 3.4%, and only 0.7% (13/1751) of serious adverse events were related to IGU. Compared with previous studies, no new adverse reactions were seen in this large sample study. Meanwhile, there is no obvious correlation between the patient’s age and the occurrence of adverse reactions, so it can be considered that IGU has good safety in the treatment of elderly RA patients (Mu et al., 2021). In the non-RCT of IGU combined with MTX in the treatment of RA, Okamura et al. (2015) found that the adverse reactions of this combination were mainly interstitial pneumonia and liver dysfunction (alp increase). Ishiguro et al. (2013) found that the adverse reactions were elevated liver enzymes (elevated ALS), stomatitis, pharyngitis, decreased lymphocyte count, decreased white blood cell count, and decreased red blood cell count. It can be seen that the adverse reactions of IGU combined with MTX in the treatment of RA still exist, and more clinical trial studies are needed to improve the efficacy and safety. It is necessary to expand the sample size and prolong the observation time to further evaluate its clinical efficacy and safety.
The Strengths and Limitation of this Review
Compared with a previous review (Chen et al., 2021), the strength of this systematic review and meta-analysis is that this is the newest evaluation of the efficacy and safety of IGU + MTX for RA patients. This study also used subgroup analysis to compare the efficacy and safety of IGU + MTX versus different control groups (MTX only, MTX + leflunomide, MTX + tripterygium, MTX + adalimumab). This study also conducted an evidence quality assessment to provide clinical information on the use of IGU + MTX. This study also adopted a more rigorous risk of bias assessment and a comprehensive analysis of 31 RCTs.
The limitation of this study is that most studies have high or unclear risk of bias in random sequence generation, allocation concealment, blinding, and incomplete outcomes, which affects the accuracy of the results and the evidence’s grade. Meanwhile, most of the outcomes have high heterogeneity, and the heterogeneity does not decrease after subgroup analysis (such as DAS28). This may be related to the fact that the dosage of the medication is not exactly the same; the basic treatment, the treatment process, and the observation time are different; and the baseline data of the patients in different RCTs are different. In the future, more RCTs from different regions and nationalities with clear random sequence generation methods, allocation concealment, and blinding are needed to modify or verify the results. In addition, the country distribution of RCTs will affect the spread of evidence. The RCTs included in the meta-analysis of this study are mainly from China and Japan. The main reason is that as a new drug, IGU has recently been approved for marketing in China and Japan, and clinical researchers and patients have more convenient access to drugs. Especially in 2017, IGU entered the new version of China’s National Medical Insurance Catalogue (Category B) (Jin and Luo 2020), and its reach was further expanded, reducing the personal burden of RA patients. Since 2017, the RCT of IGU combined with MTX in the treatment of RA has increased rapidly, and it mainly comes from China, and the applicability of the evidence is mainly extrapolated to Asian countries.
Implications for Future Research
In future clinical practice, in addition to combining MTX, RCT of IGU combined with other conventional synthetic DMARDs (csDMARDs) can also be carried out, which also shows good clinical efficacy advantages. Dai et al. found that compared with leflunomide treatment, the improvement of DAS28, joint function indexes, serum inflammation indexes, and bone metabolism indexes of combined treatment with leflunomide and IGU was more significant (p < 0.05) (Dai et al., 2019). A retrospective analysis by Li et al. also showed that after 12 weeks of treatment with other csDMARDs (such as sulfasalazine, hydroxychloroquine sulfate, leflunomide) combined with IGU, the DAS28, RF, CRP, ESR, and morning stiffness of RA patients with chronic interstitial pneumonia were significantly improved compared to those before treatment, and the incidence of adverse reactions was low (Hao and Li 2014). In addition, IGU can also be combined with biological disease-improving antirheumatic drugs (bDMARDs) for the treatment of patients with poor efficacy of biological agents (Yoshikawa et al., 2018). For example, in patients with poor response to tocilizumab, combined IGU treatment can significantly improve their disease activity (DAS28, CDAI, and EULAR response criteria), thereby effectively controlling the disease (Ebina et al., 2019). In summary, future studies can explore the efficacy and safety of IGU combined with various DMARDs (such as ABC) or biological agents and provide new reference information for clinical treatment.
Conclusion
1) Compared with the MTX alone subgroup, IGU + MTX has obvious advantages in improving the compliance rate of patients with ACR20, ACR50, and ACR70. 2) In terms of secondary outcomes such as the number of tender joints, the number of swollen joints, ESR, and CRP, IGU + MTX is more effective. 3) In terms of adverse reactions, compared with the MTX alone subgroup and MTX + MTX + tripterygium, IGU + MTX does not increase the risk of infection, abnormal liver function, nausea, and vomiting in RA patients. IGU + MTX is safer than IGU + MTX + leflunomide. In the future, especially for some RA patients with poor efficacy or poor tolerance of MTX, tripterygium or leflunomide, IGU + MTX can be used as an alternative treatment. 4) When the intervention method is (IGU 25 mg Bid, MTX 10–25 mg once a week), and the intervention lasts for at least 12 weeks, the curative effect may be achieved without obvious adverse events (Shrestha et al., 2020).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author Contributions
LZ, GY, and HC are responsible for the study concept and design. LZ, KY, WH, GY, and HC are responsible for the data collection, data analysis and interpretation. LZ and KY drafted the paper. HC and GY supervised the study. All authors participated in the analysis and interpretation of data and approved the final paper.
Funding
This research is supported by Scientific Research Fund of Hunan Provincial Education Department (2019A1710).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.780154/full#supplementary-material
References
Bi, W. H. (2019). The Effect of Iguratimod Combined with Methotrexate on Serum VEGF Levels in Patients with Rheumatoid Arthritis and Evaluation of the efficacy. Inner Mongolia Medical University (Thesis).
Burmester, G. R., and Pope, J. E. (2017). Novel Treatment Strategies in Rheumatoid Arthritis. Lancet 389, 2338–2348. doi:10.1016/S0140-6736(17)31491-5
Chen, J., Ding, Z. H., and Liu, J. (2018). Effects of Iguratimod Combined with Methotrexate on Serum Inflammatory Factors and Bone Metabolism in Patients with Rheumatoid Arthritis. Zhejiang J. Integrated Traditional Chin. West. Med. 28, 552–555. doi:10.3969/j.issn.1005-4561.2018.07.010 (in chinese).
Chen, L. J., Zhou, Y. J., Wen, Z. H., Tian, F., and Li, J. Y. (2021). Efficacy and Safety of Iguratimod Combined with Methotrexate vs. Methotrexate Alone in Rheumatoid Arthritis : A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Z. Rheumatol. 80 (5), 432–446. Epub 2020 Dec 21. PMID: 33346891; PMCID: PMC8189982. doi:10.1007/s00393-020-00944-7
Chinese Rheumatology Association (2018). 2018 Chinese Guideline for the Diagnosis and Treatment of Rheumatoid Arthritis. Zhonghua Nei Ke Za Zhi 57, 242–251. doi:10.3760/cma.j.issn.0578-1426.2018.04.004
Dai, L., Song, X. L., Qiu, X. M., Zhang, W., Shi, Y., Lian, Y., et al. (2019). Observation on the Effect of Iguratimod Combined with Leflunomide in the Treatment of Moderate and Severe Elderly Active Rheumatoid Arthritis. J. Clin. Med. Pract. 23, 73–77. doi:10.7619/jcmp.201903020 (in chinese).
Deeks, J. J., Higgins, J. P., and Altman, D. G. (2021a). “Chapter 8: Assessing Risk of Bias in Included Studies,” in Cochrane Handbook or Systematic Reviews of Interventions Version 6.2.0. Editors J. P. Higgins, and S. Green (UK: The Cochrane Collaboration), 2021.
Deeks, J. J., Higgins, J. P., and Altman, D. G. (2021b). “Chapter 9: Analyzing Data and Undertaking Meta-Analyses,” in Cochrane Handbook for Systematic Reviews of Interventions. Editors J P. Higgins, and S. Green (UK: The Cochrane Collaboration), 2021.
Deng, J. X. (2017). The Effect of Iguratimod on the Proliferation and Migration of Fibroblast-like Synovial Cells in Rheumatoid Arthritis and the Clinical observation. Southern Medical University. (Thesis) (in chinese).
Ding, L., He, S., Wang, M., Wang, M., Zou, C., and Pan, Y. (2019). Efficacy and Bone Metabolism Analysis of Iguratimod on Senile Rheumatoid Arthritis. Jilin Med. 40, 1269–1270. doi:10.3969/j.issn.1004-0412.2019.06.041 (in chinese).
Duan, X. W., Zhang, X. L., Mao, S. Y., Shang, J. J., and Shi, X. D. (2015). Efficacy and Safety Evaluation of a Combination of Iguratimod and Methotrexate Therapy for Active Rheumatoid Arthritis Patients: a Randomized Controlled Trial. Clin. Rheumatol. 34, 1513–1519. doi:10.1007/s10067-015-2999-6
Ebina, K., Miyama, A., Tsuboi, H., Kaneshiro, S., Nishikawa, M., Owaki, H., et al. (2019). The Add-On Effectiveness and Safety of Iguratimod in Patients with Rheumatoid Arthritis Who Showed an Inadequate Response to Tocilizumab. Mod. Rheumatol. 29, 581–588. doi:10.1080/14397595.2018.1486939
Fan, Z. X., Wu, P. C., Song, M. H., and Tang, J. (2020). The Clinical Efficacy of Iguratimod Combined with Methotrexate in the Treatment of Rheumatoid Arthritis. J. Clin. Rational Use 13, 81–83. doi:10.15887/j.cnki.13-1389/r.2020.33.031 (in chinese).
Ferro, F., Elefante, E., Luciano, N., Talarico, R., and Todoerti, M. (2017). One Year in Review 2017: Novelties in the Treatment of Rheumatoid Arthritis. Clin. Exp. Rheumatol. 35, 721–734.
GRADEpro, G. D. T. (2015). GRADEpro Guideline Development Tool [Software]. Germany: McMaster University. (developed by Evidence Prime, Inc.). Available from gradepro.org.
Gu, X. J., Chen, H., Li, R. P., Gan, F. Y., and Guo, D. B. (2020). The Clinical Efficacy of Ilamud Combined with Methotrexate in the Treatment of Early Rheumatoid Arthritis (RA). Contemp. Med. 26, 138–139. doi:10.3969/j.issn.1009-4393.2020.25.057 (in chinese).
H. Schünemann, J. Brożek, G. Guyatt, and A. Oxman (Editors) (2013). GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013 (Germany: The GRADE Working Group). Available from guidelinedevelopment.org/handbook.
Hao, G. F., and Li, Y. S. (2014). Observation on the Short-Term Clinical Efficacy of Iguratimod on Rheumatoid Arthritis Complicated with Chronic Interstitial Pneumonia. China Mod. Appl. Pharm. 31, 1275–1278. doi:10.13748/j.cnki.issn1007-7693.2014.10.029 (in chinese).
Hara, M., Ishiguro, N., Katayama, K., Kondo, M., Sumida, T., Mimori, T., et al. (2014). Safety and Efficacy of Combination Therapy of Iguratimod with Methotrexate for Patients with Active Rheumatoid Arthritis with an Inadequate Response to Methotrexate: an Open-Label Extension of a Randomized, Double-Blind, Placebo-Controlled Trial. Mod. Rheumatol. 24, 410–418. doi:10.3109/14397595.2013.843756
Hunter, T. M., Boytsov, N. N., Zhang, X., Schroeder, K., Michaud, K., and Araujo, A. B. (2017). Prevalence of Rheumatoid Arthritis in the United States Adult Population in Healthcare Claims Databases, 2004-2014. Rheumatol. Int. 37, 1551–1557. doi:10.1007/s00296-017-3726-1
Ishiguro, N., Yamamoto, K., Katayama, K., Kondo, M., Sumida, T., Mimori, T., et al. (2013). Concomitant Iguratimod Therapy in Patients with Active Rheumatoid Arthritis Despite Stable Doses of Methotrexate: a Randomized, Double-Blind, Placebo-Controlled Trial. Mod. Rheumatol. 23, 430–439. doi:10.1007/s10165-012-0724-8
Jiang, H., Gao, H., Wang, Q., Wang, M., and Wu, B. (2020). Molecular Mechanisms and Clinical Application of Iguratimod: A Review. Biomed. Pharmacother. 122, 109704. doi:10.1016/j.biopha.2019.109704
Jin, Q., Luo, W. B., and Z, N. (2020). The Latest Research Progress in the Clinical Application and Mechanism of Iramud. Rheum. Arthritis 9, 67–71. doi:10.3969/j.issn.2095-4174.2020.09.018 (in chinese).
Ju, Y. J., Guo, D. B., Chen, H., and Li, W. (2020). Evaluation of the Clinical Efficacy of Methotrexate and Ilamod in the Treatment of Refractory Rheumatoid Arthritis. China Mod. Doctor 058, 106–109. (in chinese).
Lau, C. S., Chia, F., Dans, L., Harrison, A., Hsieh, T. Y., Jain, R., et al. (2019). 2018 Update of the APLAR Recommendations for Treatment of Rheumatoid Arthritis. Int. J. Rheum. Dis. 22, 357–375. doi:10.1111/1756-185X.13513
Li Ch, H. W. H. (2020). The Effect of Irammod Combined with Methotrexate in the Treatment of Rheumatoid Arthritis with Peripheral Blood Nuclear Factor Kappa B Receptor Activator Ligand and Bone Protective Factor. Chin. Medicines Clin. 20, 2981–2983. (in chinese).
Li, J., Chen, Q. P., Liu, J. Y., Wang, X., and Liu, D. (2016). The Treatment of 84 Cases of Senile Rheumatoid Arthritis with the Combination of Iguratimod and Methotrexate. Shaanxi Med. J. 45, 120–121. doi:10.3969/j.issn.1000-7377.2016.01.049 (in chinese).
Li, L., Wang, J., and Li, X. (2019). A Clinical Study on the Treatment of Early Rheumatoid Arthritis by Iguratimod Combined with Methotrexate. China Pharmaceuticals 28, 63–65. doi:10.3969/j.issn.1006-4931.2019.07.020 (in chinese).
Liu, R., Zhao, P., Tan, W., and Zhang, M. (2018). Cell Therapies for Refractory Rheumatoid Arthritis. Clin. Exp. Rheumatol. 36, 911–919.
Liu, Y. Q. (2016). A Comparative Study on the Curative Effect of Methotrexate Combined with Ilamod in the Treatment of Rheumatoid Arthritis with Different Syndromes of Traditional Chinese medicine. Inner Mongolia Medical University. (Thesis) (in chinese).
Lu, J. (2014). The Therapeutic Effect of Combined Application of Iguratimod and Methotrexate on Active Rheumatoid arthritis. Shandong University. (Thesis) (in chinese).
Lu, L.-j., Bao, C.-d., Dai, M., Teng, J.-l., Fan, W., Du, F., et al. (2009). Multicenter, Randomized, Double-Blind, Controlled Trial of Treatment of Active Rheumatoid Arthritis with T-614 Compared with Methotrexate. Arthritis Rheum. 61, 979–987. doi:10.1002/art.24643
Lu, X. L., and Liu, Y. Q. (2018). A Comparative Study on the Efficacy of Methotrexate Combined with Ilamud in the Treatment of Rheumatoid Arthritis with Different Syndromes of Traditional Chinese Medicine. Rheum. Arthritis 7, 15–19. doi:10.3969/j.issn.1000-744X.2018.07.025 (in chinese).
Meng, D. Y., Pan, W. Y., Li, J., Li, H., Li, F., Liu, S. S., et al. (2016). The Effect of Methotrexate Combined with Ilamud in the Treatment of Refractory Rheumatoid Arthritis. China Med. Herald 13, 137–141. (in chinese).
Meng, D. Y., Wang, G. R., Pan, W. Y., et al. (2015). Short-term Clinical Efficacy of Methotrexate Combined with Iguratimod on Refractory Rheumatoid Arthritis. Chin. J. Clin. Res. 28, 40–42. doi:10.13429/j.cnki.cjcr.2015.12.010 (in chinese).
Meng, Y., Li, M. Y., Rode, M., Zhang, X. Y., and Luo, L. (2017). Clinical Study on the Treatment of Senile Rheumatoid Arthritis with Iguratimod Tablets Combined with Methotrexate Tablets. Chin. J. Clin. Pharmacol. 33, 1098–1101. doi:10.13699/j.cnki.1001-6821.2017.12.008
Mimori, T., Harigai, M., Atsumi, T., Fujii, T., Kuwana, M., Matsuno, H., et al. (2019). Safety and Effectiveness of Iguratimod in Patients with Rheumatoid Arthritis: Final Report of a 52-week, Multicenter Postmarketing Surveillance Study. Mod. Rheumatol. 29, 314–323. doi:10.1080/14397595.2018.1460230
Mo, H., and Ma, S. B. (2015). Clinical Study on the Treatment of Active Rheumatoid Arthritis with Iguratimod Combined with Methotrexate. Intern. Med. 10, 156–159. doi:10.16121/j.cnki.cn45-1347/r.2015.02.06 (in chinese).
Mo, M. L., Tang, D. X., Zhang, J., Liu, Y., and Xie, L. (2018). A Randomized Controlled Trial of Methotrexate Combined with Iguratimod in the Treatment of Active Rheumatoid Arthritis. J. Fujian Med. Univ. 52, 40–43. (in chinese).
Mu, R., Li, C., Li, X., Ke, Y., Zhao, L., Chen, L., et al. (2021). Effectiveness and Safety of Iguratimod Treatment in Patients with Active Rheumatoid Arthritis in Chinese: A Nationwide, Prospective Real-World Study. Lancet Reg. Health West. Pac. 10, 100128. doi:10.1016/j.lanwpc.2021.100128
Okamura, K., Yonemoto, Y., Suto, T., Okura, C., and Takagishi, K. (2015a). Efficacy at 52 Weeks of Daily Clinical Use of Iguratimod in Patients with Rheumatoid Arthritis. Mod. Rheumatol. 25, 534–539. doi:10.3109/14397595.2014.998361
Okamura, K., Yonemoto, Y., Suto, T., Okura, C., and Takagishi, K. (2015b). Efficacy at 52 Weeks of Daily Clinical Use of Iguratimod in Patients with Rheumatoid Arthritis. Mod. Rheumatol. 25 (4), 534–539. doi:10.3109/14397595.2014.998361
Otón, T., and Carmona, L. (2019). The Epidemiology of Established Rheumatoid Arthritis. Best Pract. Res. Clin. Rheumatol. 33, 101477. doi:10.1016/j.berh.2019.101477
Qi, D. X., Liu, Y., and Huang, D. H. (2019). Study on the Efficacy and Safety of Methotrexate and Isilamod in the Treatment of Rheumatoid Arthritis. Chin. J. Drug Eval. 036, 217–220. doi:10.3969/j.issn.2095-3593.2019.03.015 (in chinese).
Scott, D. L., Wolfe, F., and Huizinga, T. W. (2010). Rheumatoid Arthritis. Lancet 376, 1094–1108. doi:10.1016/S0140-6736(10)60826-4
Shi, X. D., Zhang, X. L., and Duan, X. W. (2015). Efficacy and Safety of Methotrexate Combined with Iguratimod in the Treatment of Active Rheumatoid Arthritis. J. Nanchang Univ. (Medical Edition) 55, 33–36+47. doi:10.13764/j.cnki.ncdm.2015.01.010 (in chinese).
Shrestha, S., Zhao, J., Yang, C., and Zhang, J. (2020). Relative Efficacy and Safety of Iguratimod Monotherapy for the Treatment of Patients with Rheumatoid Arthritis: a Systematic Review and Meta-Analysis. Clin. Rheumatol. 39, 2139–2150. doi:10.1007/s10067-020-04986-9
Singh, J. A., Saag, K. G., Bridges, S. L., Akl, E. A., Bannuru, R. R., Sullivan, M. C., et al. (2016). 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. (Hoboken) 68, 1–25. doi:10.1002/acr.22783
Smolen, J. S., Landewé, R., Bijlsma, J., Burmester, G., Chatzidionysiou, K., Dougados, M., et al. (2017). EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2016 Update. Ann. Rheum. Dis. 76, 960–977. doi:10.1136/annrheumdis-2016-210715
Suto, T., Yonemoto, Y., Okamura, K., Sakane, H., Takeuchi, K., Tamura, Y., et al. (2019). The Three-Year Efficacy of Iguratimod in Clinical Daily Practice in Patients with Rheumatoid Arthritis. Mod. Rheumatol. 29, 775–781. doi:10.1080/14397595.2018.1510879
Tian, J. W., and Tao, P. F. (2017). The Effect of Iguratimod Combined with Methotrexate on Serum M-CSF, IL-6, IL-8 and Bone Metabolism in Patients with Rheumatoid Arthritis. Hainan Med. 28, 391–394. doi:10.3969/j.issn.1003-6350.2017.03.015 (in chinese).
Tian, X. P., Liu, S. Y., Li, Q., Bi, L. Q., Kong, X. D., Zhao, D. B., et al. (2020). Efficacy and Safety of Ellamod or Leflunomide Combined with Methotrexate in the Treatment of Active Rheumatoid Arthritis Sexual Comparison: a Multicenter Randomized Double-Blind Double-Simulation Controlled Clinical Study. Chin. J. Rheumatol. 24, 148–158. doi:10.3760/cma.j.issn.1007-7480.2020.03.002 (in chinese).
Wang, L., Liu, S., Rong, C., and Fu, K. (2019). Clinical Study on the Treatment of Rheumatoid Arthritis with Methotrexate Tablets Combined with Iguratimod Tablets. Chin. J. Clin. Pharmacol. 35, 231–234. (in chinese).
Wang, X., Ma, C., Li, P., Zhao, F., and Bi, L. (2017). Effects of Iguratimod on the Levels of Circulating Regulators of Bone Remodeling and Bone Remodeling Markers in Patients with Rheumatoid Arthritis. Clin. Rheumatol. 36, 1369–1377. doi:10.1007/s10067-017-3668-8
Wang, X. T. (2017). Study on the Effect of Iguratimod on Bone Metabolism in Patients with Rheumatoid arthritis. Jilin, China: Jilin University. (Thesis) (in chinese).
Wasserman, A. M. (2011). Diagnosis and Management of Rheumatoid Arthritis. Am. Fam. Physician 84, 1245–1252.
Wasserman, A. (2018). Rheumatoid Arthritis: Common Questions about Diagnosis and Management. Am. Fam. Physician 97, 455–462.
Xia, N. N., Chen, Z. F., and Zhang, W. F. (2020). Observation on the Effects of Iguratimod and Tripterygium Glycosides in the Treatment of Rheumatoid Arthritis. Pract. Integrated Traditional Chin. West. Med. 20, 76–77. doi:10.13638/j.issn.1671-4040.2020.11.037 (in chinese).
Xia, Z., Lyu, J., Hou, N., Song, L., Li, X., and Liu, H. (2016). Iguratimod in Combination with Methotrexate in Active Rheumatoid Arthritis : Therapeutic Effects. Z. Rheumatol. 75, 828–833. English. doi:10.1007/s00393-015-1641-y
Xie, Li., Zou, Q. H., Shi, Y., Cheng, X., and Fang, Y. F. (2018). The Effect of Iguratimod Combined with MTX on IL-1, Serum TNF-α and VEGF Levels in Patients with Refractory Rheumatoid Arthritis. Guizhou Med. 42, 831–832. doi:10.3969/j.issn.1000-744X.2018.07.025 (in chinese).
Xie, S., Li, S., Tian, J., and Li, F. (2020). Iguratimod as a New Drug for Rheumatoid Arthritis: Current Landscape. Front. Pharmacol. 11, 73. doi:10.3389/fphar.2020.00073
Xiong, M., and Geng, G. (2020). The Clinical Efficacy of Methotrexate Combined with Iguratimod on Active Rheumatoid Arthritis. Henan Med. Res. 29 (2020), 93–95. doi:10.3969/j.issn.1004-437X.2020.01.044 (in chinese).
Xu, B., Mo, S., and Xue, X. (2015a). Clinical Study of Methotrexate Combined with Ilamud in the Treatment of Rheumatoid Arthritis. J. Clin. Med. Pract. 19, 120–122. doi:10.7619/jcmp.201501038 (in chinese).
Xu, J. P., Guo, F. E., Yue, X. L., Wang, P., Zhou, H. M., Li, J. F., et al. (2017a). The Curative Effect of Methotrexate, Isilamod Combined with Physical Therapy on Patients with Rheumatoid Arthritis and the Effects of RF, ESR and CRP. China Med. Guide 19, 1026–1030. doi:10.3969/j.issn.1009-0959.2017.10.015 (in chinese).
Xu, L. M., Yuan, M., Liu, Y. F., Sun, H. X., Liu, L. N., Shi, Y. J., et al. (2017b). Clinical Observation on the Treatment of Rheumatoid Arthritis with Iguratimod Combined with Methotrexate. Guide Chin. Med. 15, 47–48. doi:10.15912/j.cnki.gocm.2017.22.031 (in chinese).
Xu, Y. M., Fan, S. N., and Zou, L. (2015b). Study on the Effect of Iguratimod Combined with Methotrexate Treatment on Anti-cyclic Guanidine Peptide Antibodies and Other Indicators in Patients with Rheumatoid Arthritis. J. Clin. Intern. Med. 32, 833–835. doi:10.3969/j.issn.1001-9057.2015.12.014 (in chinese).
Yan, X. Z., and Wang, F. L. (2018). Effect of Iguratimod Combined with Methotrexate on Serum-Related Cytokines and Bone Metabolism in Patients with Rheumatoid Arthritis. J. Changchun Univ. Traditional Chin. Med. 34, 369–372. doi:10.13463/j.cnki.cczyy.2018.02.054 (in chinese).
Yoshikawa, A., Yoshida, S., Kimura, Y., Tokai, N., Fujiki, Y., Kotani, T., et al. (2018). Add-on Iguratimod as a Therapeutic Strategy to Achieve Remission in Patients with Rheumatoid Arthritis Inadequately Responding to Biological DMARDs: A Retrospective Study. Mod. Rheumatol. 28, 227–234. doi:10.1080/14397595.2017.1336865
Yoshioka, Y., Takahashi, N., Kaneko, A., Hirano, Y., Kanayama, Y., Kanda, H., et al. (2016). Disease Activity Early in Treatment as a Predictor of Future Low Disease Activity in RA Patients Treated with Iguratimod. Mod. Rheumatol. 26, 169–174. doi:10.3109/14397595.2015.1069475
Zhang, J. Y., Guo, J., Shen, J., Ruan, X., and Chen, P. (2014). Clinical Observation of MTX Combined with Iguratimod in the Treatment of Refractory Rheumatoid Arthritis. Chin. Med. Guide 1, 98–99. doi:10.15912/j.cnki.gocm.2014.01.111 (in chinese).
Zhao, H. N., and Hao, X. J. (2018). The Clinical Effect of Iguratimod Combined with Methotrexate in the Treatment of Rheumatoid Arthritis. Clin. Med. Res. Pract. 003, 44–45. doi:10.19347/j.cnki.2096-1413.201815020 (in chinese).
Zhao, W. M., Yao, D. Y., Huo, H. S., Qin, C. M., Wei, Q. J., and Sun, K. (2016). Clinical Study of Iguratimod in the Treatment of Active Rheumatoid Arthritis. Chin. J. Postgraduates Med. 39, 450–452. doi:10.3760/cma.j.jssn.1673-4904.2016.05.017 (in chinese).
Keywords: methotrexate, rheumatoid arthritis, systematic review, meta-analysis, iguratimod
Citation: Zeng L, Yu G, Yang K, Hao W and Chen H (2022) The Effect and Safety of Iguratimod Combined With Methotrexate on Rheumatoid Arthritis: A Systematic Review and Meta-Analysis Based on a Randomized Controlled Trial. Front. Pharmacol. 12:780154. doi: 10.3389/fphar.2021.780154
Received: 20 September 2021; Accepted: 01 November 2021;
Published: 18 January 2022.
Edited by:
Gerard Bannenberg, Global Organization for EPA and DHA Omega-3s (GOED), United StatesReviewed by:
Yuan-Hao Wu, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, ChinaAbdallah El-Sayed Allam, Tanta University, Egypt
Copyright © 2022 Zeng, Yu, Yang, Hao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Chen, Y2hodWFhMzMyMjExQDE2My5jb20=; Liuting Zeng, emx0YWIyMDE2QGhvdG1haWwuY29t; Kailin Yang, eWFuZy5jYXJkaW9sb2d5QGNjbXUuZWR1LmNu; Ganpeng Yu, eXVnYW5wZW5nLmd1a2VAaG90bWFpbC5jb20=
†These authors share first authorship
 Liuting Zeng1*†
Liuting Zeng1*† Kailin Yang
Kailin Yang