- 1Primary Care Research Centre, Aldermoor Health Centre, University of Southampton, Southampton, United Kingdom
- 2Medicines Unit, Antenna Foundation, Geneva, Switzerland
Aims: To rank the effectiveness of medicinal plants for glycaemic control in Type 2 Diabetes (T2DM).
Methods: MEDLINE, EMBASE, CINAHL and Cochrane Central were searched in October 2020. We included meta-analyses of randomised controlled clinical trials measuring the effectiveness of medicinal plants on HbA1c and/or Fasting Plasma Glucose (FPG) in patients with T2DM.
Results: Twenty five meta-analyses reported the effects of 18 plant-based remedies. Aloe vera leaf gel, Psyllium fibre and Fenugreek seeds had the largest effects on HbA1c: mean difference –0.99% [95% CI−1.75, −0.23], −0.97% [95% CI −1.94, −0.01] and −0.85% [95% CI −1.49, −0.22] respectively. Four other remedies reduced HbA1c by at least 0.5%: Nigella sativa, Astragalus membranaceus, and the traditional Chinese formulae Jinqi Jiangtang and Gegen Qinlian. No serious adverse effects were reported. Several other herbal medicines significantly reduced FPG. Tea and tea extracts (Camellia sinensis) were ineffective. However, in some trials duration of follow-up was insufficient to measure the full effect on HbA1c (<8 weeks). Many herbal remedies had not been evaluated in a meta-analysis.
Conclusion: Several medicinal plants appear to be as effective as conventional antidiabetic treatments for reducing HbA1c. Rigorous trials with at least 3 months’ follow-up are needed to ascertain the effects of promising plant-based preparations on diabetes.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=220291, PROSPERO.
Highlights
- Aloe vera, Psyllium fibre and Fenugreek seeds had the largest effects on HbA1c: −0.99, −0.97, and −0.85% respectively.
- Four other remedies reduced HbA1c by >0.5%, including Nigella sativa and Astragalus membranaceus.
- Tea (Camellia sinensis) and tea extracts were ineffective.
- No serious adverse effects were reported.
Introduction
Type 2 Diabetes Mellitus (T2DM) is a major, growing health problem. It is estimated that 9.3% of the world’s population (463 million people) were living with diabetes in 2019 and this is projected to increase to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2,045 (Saeedi et al., 2019). Over 90% of these have T2DM and over 1 million deaths per year are attributable to diabetes (Khan et al., 2020). The costs are huge: the USA alone spends $294 billion per year on management of diabetes in the population aged 20–79 (International Diabetes Federation, 2019).
Initial treatment of diabetes involves lifestyle modifications including changes to the diet and increasing physical activity, but dietary advice does not usually extend to herbs and phytomedicines. On average, compared to normal diets, low carbohydrate diets reduce HbA1c by only 0.09% (1 mmol/mol) (Korsmo-Haugen et al., 2019). Individualised dietary advice is recommended alongside a personalised management plan that aims to reduce and maintain HbA1c to below 6.5% (National Institute for Health and Care Excellence, 2020). Pharmacotherapy is initiated if patients fail to maintain HbA1c levels below this threshold.
Among adults with T2DM, 45% had not achieved adequate glycaemic control, in a national cross-sectional survey in the USA (Wong et al., 2013); poor adherence to medications is a major reason (Polonsky and Henry, 2016). Less than 50% of patients prescribed metformin were adherent and a third discontinued within 12 months, in a retrospective study in the UK Clinical Practice Research Datalink database (CPRD) (Tang et al., 2020). Side-effects of medication are the commonest reason for non-adherence (Grant et al., 2003). As many as 62% of patients taking metformin complain of diarrhoea (Florez et al., 2010).
Diabetes mellitus has been recognised for thousands of years and treated by traditional systems of medicine in Egypt, China, India, and Africa (Simmonds et al., 2006). Many patients with diabetes still use complementary therapies, ranging from 17% in the UK to 72% in the USA (Chang et al., 2007). Herbal medicines are among the most popular: they are used by 68% of diabetic patients in Saudi Arabia (Alqathama et al., 2020), 62% in Mexico (Chang et al., 2007), 62% in Ethiopia (Mekuria et al., 2018) and 58% in Sudan (Ali and Mahfouz, 2014). In India, 67% of diabetic patients use naturopathy or Ayurveda (Chang et al., 2007). However, the majority do not inform their doctors about their use of herbal medicine (Mekuria et al., 2018; Alqathama et al., 2020). In a qualitative study of members of the Pakistani community in Bradford (UK), two-thirds preferred using herbal medicine compared to conventional medicine and many believed that the vegetable “Karela” (Momordica charantia) could cure diabetes (Pieroni et al., 2008). Worldwide, about 1,200 plant species are reportedly used for the treatment of diabetes (Simmonds et al., 2006).
Although there has been a wealth of laboratory and clinical research on herbal medicines for diabetes, this has not been translated into user-friendly evidence-based information to guide patients or clinicians. Most patients base their choice of herbal medicines on advice from family and friends (Ali and Mahfouz, 2014; Mekuria et al., 2018). Although there have been several systematic reviews about herbal medicines for diabetes (Yeh et al., 2003; Wang et al., 2013; Gupta et al., 2017; Governa et al., 2018), none has yet provided a ranking of remedies for their effectiveness on glycaemic control in patients with T2DM. We aim to determine the relative effectiveness of common herbal remedies for treatment of type 2 diabetes through a systematic overview of meta-analyses of controlled clinical trials.
Methods
The protocol for this study was registered on PROSPERO prior to starting data extraction: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=220291.
The protocol included the research question, search strategy, inclusion criteria and quality appraisal.
Data Sources and Searches
We searched the following databases on October 7, 2020 for systematic reviews of randomised controlled clinical trials: EMBASE via OVID (from 1947), MEDLINE via OVID (from 1946), CINAHL (Cumulated Index to Nursing and Allied Health Literature from 1977) and the Cochrane Library including the Cochrane Central Register of Controlled Trials (CENTRAL). Each search strategy was adapted to take into account differences in controlled vocabulary and syntax rules. An example search strategy is given in Supplementary Material. We also contacted experts in the field to identify any relevant studies which had not been found by the search.
Study Selection
Two reviewers independently screened titles and abstracts to select articles for full-text screening. Two reviewers then independently screened the full-text articles. We selected articles which met the following inclusion criteria:
- Study type: Systematic reviews of randomised controlled clinical trials with a reported systematic search strategy and with the intention to perform a meta-analysis.
- Participants: human subjects diagnosed with Type 2 diabetes, both diet-controlled and those on oral hypoglycaemic medications.
- Interventions: one specific herb or standardised herbal remedy
- Comparison: An inactive treatment (placebo) or standard care (oral hypoglycaemic medications, conventional diets)
-Outcomes: quantified change in HbA1c and/or fasting plasma glucose (FPG), reported as a numerical effect size.
We excluded reviews which only presented results in a narrative format and did not attempt to meta-analyse the outcomes. We did however include systematic reviews which found only a single relevant trial and presented its results in the correct format–where a meta-analysis had been intended but included only a single trial. Some reviews included trials both on T2DM and also on prediabetes. If results for T2DM were presented separately, and/or if trials in T2DM were the majority of included trials, we included these. We excluded reviews where the majority of included trials were not on patients with T2DM and where it was not possible to separate out the results for T2DM patients. We also excluded reviews where results for type 1 diabetes (T1DM) were not presented separately. We excluded reviews of multiple different herbal remedies and of pure compounds extracted from herbs, because none of these presented meta-analyses of individual medicinal plants. We did not apply any language restrictions.
Data Extraction and Quality Assessment
Two reviewers independently extracted relevant data using a data extraction form created on Microsoft Excel, and any discrepancies were checked by a third reviewer. Where a review reported several patient groups and/or outcomes, we extracted the number of trials and participants which matched our inclusion criteria (type 2 diabetes) and which reported each relevant outcome (HbA1c and FPG). When results were presented separately for different types of control, we preferentially chose the comparison against placebo (rather than comparison against standard treatment), in order to gauge the effect size of the medicinal plant itself. Where HbA1c results were reported in mmol/mol, they were multiplied by the conversion factor 0.09148 to give the equivalent as a percentage (National Glycohemoglobin Standardization Program, 2010). Where FPG results were presented as mg/dL, they were divided by 18 to convert to mmol/L. For each review we extracted the number of trials which had reported on adverse effects, and the number of these which reported any specific adverse effects.
Two reviewers independently appraised the quality of the studies using the AMSTAR-2 tool (Shea et al., 2017) and discrepancies were resolved by discussion with a third reviewer.
Data Synthesis and Analysis
Results from meta-analyses of HbA1c and FPG were ranked in order of effect size and presented on a Forest plot. A clinically significant reduction in HbA1c has been defined by clinicians as a reduction of at least ≥0.5% (Lenters-Westra et al., 2014); we defined a clinically significant reduction in FPG as a change of 0.5 mmol/l or more. We conducted a narrative synthesis of the other results. We calculated the Spearman’s rank correlation coefficient for the correlation between rank of effect on HbA1c and FPG. In this analysis we only included remedies for which both measures were reported. Where a remedy had differing results from several reviews, we took the rank of the best result for each of HbA1c and FPG.
Results
Included Studies
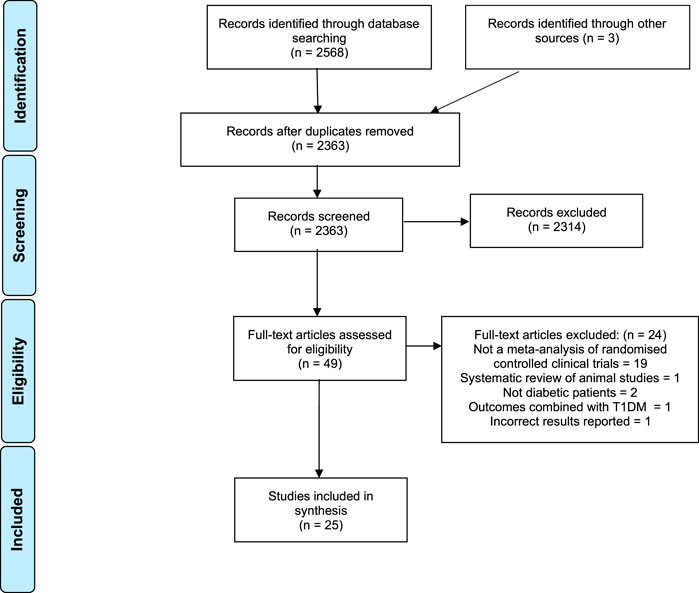
Our initial search identified 2,363 articles after removing duplicates (Figure 1). Forty-nine full texts were screened and of these, 25 met all our inclusion criteria (Davis and Yokoyama, 2011; Kim et al., 2011; Leach and Kumar, 2012; Ooi et al., 2012; Allen et al., 2013; Ooi and Loke, 2013; Neelakantan et al., 2014; Gibb et al., 2015; Gui et al., 2016; Li et al., 2016; Shin et al., 2016; Suksomboon et al., 2016; Tian et al., 2016; Zhang et al., 2016; Daryabeygi-Khotbehsara et al., 2017; Poolsup et al., 2017; Schwingshackl et al., 2017; Gu et al., 2018; Deyno et al., 2019; Gao et al., 2019; Huang et al., 2019; Namazi et al., 2019; Peter et al., 2019; Yang et al., 2019; Ziaei et al., 2020). The commonest reason for exclusion was that the review did not attempt a quantitative meta-analysis of randomised controlled trials. One of the meta-analyses was excluded because it had incorrectly reported underlying data from included studies and its results were inaccurate (Gong et al., 2016).
There were reviews on 18 different medicinal plants (Table 1). Some herbal remedies had more than one review: cinnamon (Davis and Yokoyama, 2011; Leach and Kumar, 2012; Allen et al., 2013; Deyno et al., 2019; Namazi et al., 2019), ginseng (Kim et al., 2011; Gui et al., 2016), Aloe vera (Suksomboon et al., 2016; Zhang et al., 2016) and karela (Momordica charantia) (Ooi et al., 2012; Peter et al., 2019). Three reviews evaluated the effect of a standard traditional Chinese herbal formula which contained a mixture of several herbs. Gegen formulae contained Pueraria lobata root as their main constituent alongside other ingredients such as Salvia miltiorrhiza root, liquorice root and Dioscorea opposita rhizome (Yang et al., 2019). Jinqi Jiangtang contains Astragalus membranaceus root, Coptis spp rhizome and lonicera japonica (Gao et al., 2019). Tianmai Xiaoke contains Trichosanthes root, Ophiopogon japonicus root, Schisandra chinensis fruit and chromium picolinate (Gu et al., 2018). Some reviews studied the effect of specific plant products which are also used as foods: olive oil (Schwingshackl et al., 2017), sweet potato (Ooi and Loke, 2013), dragon fruit (Poolsup et al., 2017), and fenugreek powder incorporated into chapatis (Neelakantan et al., 2014).
All the reviews included mainly clinical trials in patients with T2DM (see Table 1) but four also included a few trials in patients with pre-diabetes. One included a single trial in patients with T1DM, but its results were presented separately and excluded from this review. Five reviews included only trials of patients with diet-controlled diabetes, not taking any conventional antidiabetic medications. Fourteen reviews included trials in which both intervention and control groups received concomitant conventional treatment with oral hypoglycaemic agents (OHA). Five reviews did not specify whether concomitant treatment was given. In 19 reviews, the control groups received a placebo, in four reviews they received only the conventional care (diet and/or medications) and in one, some control groups received a fish oil supplement. In three reviews, some studies gave a conventional OHA to the control group only, not to the treatment group (Ooi et al., 2012; Shin et al., 2016; Peter et al., 2019) but for this review we only extracted the outcomes from the studies using a placebo control.
Duration of follow-up was most often 4–12 weeks, but there was a wide range with a few included studies following up for as little as 1 week or for as long as 4 years. All the reviews included randomised controlled trials but two also included a few non-randomised controlled trials. The reviews included a median of eight trials and 390 participants but the smallest included only a single trial and the largest review included 25 studies (1724 participants).
Quality Assessment
The AMSTAR-2 scores for each study are shown in Supplementary Material. Only three reviews scored “yes” on all the criteria–all of them Cochrane reviews (Leach and Kumar, 2012; Ooi et al., 2012; Ooi and Loke, 2013). Several quality issues were identified with the other reviews. Most did not report that there was a pre-established published protocol. Most did not have a fully comprehensive search strategy including the grey literature. Most did not list all excluded studies and most did not report on the sources of funding for the studies included in the review. Seven did not adequately investigate publication bias. Six did not report conflicts of interest, including the review on Psyllium which was led by a company marketing a Psyllium product (Gibb et al., 2015)—this review is at high risk of bias.
Effect Size on HbA1c
Twenty-one studies on 16 remedies attempted to conduct a meta-analysis quantifying the reduction in HbA1c (Figure 2). The most effective remedy appeared to be Aloe vera (freshly extracted juice) (Suksomboon et al., 2016). Psyllium fibre (Gibb et al., 2015) and Fenugreek seeds (Neelakantan et al., 2014) also led to similar reductions in HbA1c although with wider confidence intervals. Nigella sativa seeds (Daryabeygi-Khotbehsara et al., 2017), Astragalus membranaceus root (Tian et al., 2016), and two complex traditional Chinese formulae (Gegen Qinlian (Yang et al., 2019) and Jinqi Jiantang (Gao et al., 2019)) also led to clinically and statistically significant reductions in HbA1c. Nettle (Urtica dioica) appeared to lead to a clinically significant reduction but this was not statistically significant because of very wide confidence intervals (Ziaei et al., 2020).
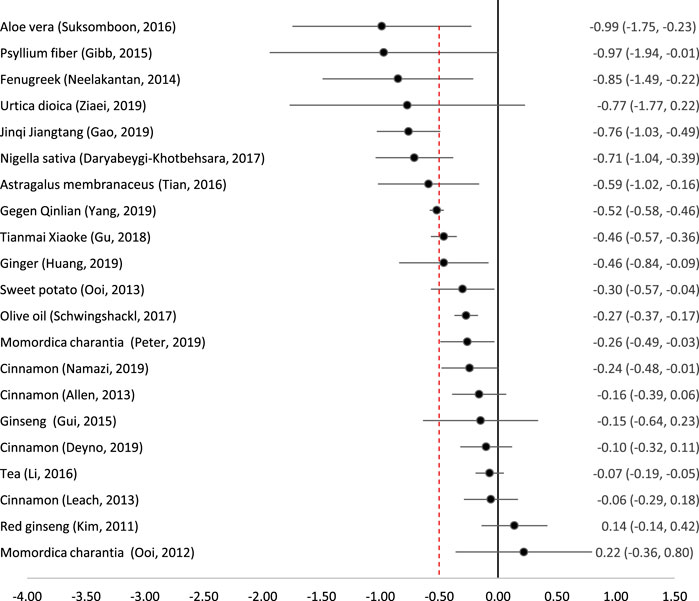
FIGURE 2. Effect of medicinal plants on HbA1c (%). The red dotted line indicates the threshold for a clinically significant effect (reduction by 0.5%). Point indicates the effect size, and the line (and figures to the right) indicate the 95% confidence interval.
Several remedies produced a statistically significant reduction in HbA1c but the standard mean difference fell below the pre-determined threshold of 0.5%. These were the patent traditional Chinese formula Tianmai Xiaoke (Gu et al., 2018), ginger (Huang et al., 2019), sweet potato tablets (Ooi and Loke, 2013), olive oil (Schwingshackl et al., 2017), karela (Momordica charantia) (Peter et al., 2019) and cinnamon (Namazi et al., 2019). Momordica charantia was studied by two reviews which came to differing conclusions; an early Cochrane review found only a single small RCT with 40 participants, which concluded that Karela dried powder in capsules appeared to be ineffective (Ooi et al., 2012). However, a more recent and comprehensive review including five RCTs (243 participants) found that there was a statistically significant reduction in HbA1c by 0.26% (Peter et al., 2019). Similarly, the four reviews on cinnamon which reported HbA1c came to slightly different conclusions; only one found a statistically significant reduction and none of them reported a clinically significant reduction.
Two meta-analyses of ginseng (Kim et al., 2011; Gui et al., 2016) and one of tea (Camellia sinensis) and tea extracts (Li et al., 2016) all showed that these remedies had no clinically or statistically significant effect on HbA1c.
Effect Size on Fasting Plasma Glucose (FPG)
Twenty-five reviews meta-analysed the reduction in FPG (Figure 3). All the remedies which produced clinically significant reductions in HbA1c also produced clinically and statistically significant reductions in FPG, with the exception of Astragalus membranaceus, which reduced FPG slightly less than the predetermined clinically significant threshold of 0.5 mmol/l. Nettle (Urtica dioica), Momordica charantia and sweet potato also all produced clinically significant reductions in FPG.
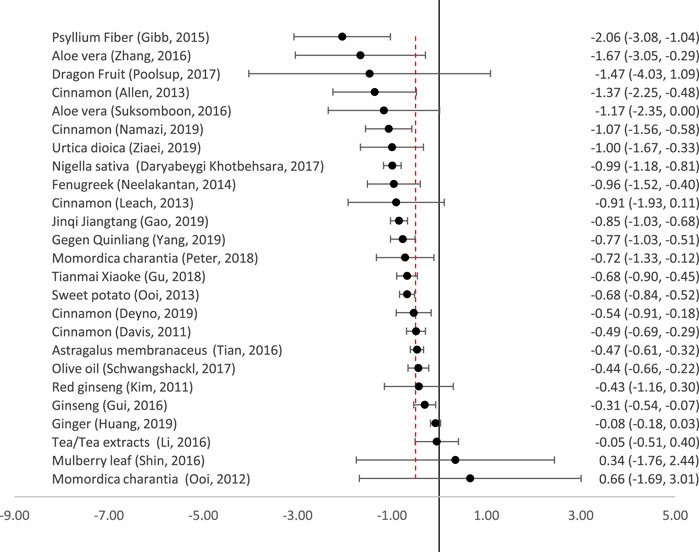
FIGURE 3. Effect of medicinal plants on Fasting Plasma Glucose (mmol/l). The red dotted line indicates the threshold for a clinically significant effect (reduction by 0.5mmol/l). The point indicates the effect size, and the line (and figures to the right) indicate the 95% confidence interval.
There were varying results in the five meta-analyses of cinnamon, but the largest and most recent (which only included patients with T2DM) showed a clinically significant reduction in FPG of −1.07 mmol/l (95% CI-1.56 to −0.58) (Namazi et al., 2019). Other reviews also included patients with T1DM (Leach and Kumar, 2012) or pre-diabetes (Davis and Yokoyama, 2011; Deyno et al., 2019).
For several remedies, there was a wide degree of uncertainty regarding their effectiveness in reducing FPG. Dragon fruit appeared to have a large effect but this was not statistically significant as there were very wide confidence intervals (Poolsup et al., 2017). There was also a large degree of uncertainty about the effect of Mulberry leaf–there was a wide confidence interval, and a second trial (not included in the meta-analysis) reported that it was more effective than glibenclamide (Shin et al., 2016). Of the two reviews on Ginseng, that by Kim et al. (2011) was the only one to focus purely on T2DM; it showed a non-significant reduction in FPG. Another meta-analysis did report a significant reduction in FPG but also included pre-diabetic patients (Gui et al., 2016).
It can be stated with some certainty that ginger and tea (Camellia sinensis) extracts were ineffective for reducing FPG. Neither had a significant effect, and confidence intervals were tight.
Correlation Between Effect on HbA1c and FPG
Spearman’s rank correlation coefficient was 0.70, indicating that there was a moderate correlation between effect on HbA1c and FPG.
Adverse Effects
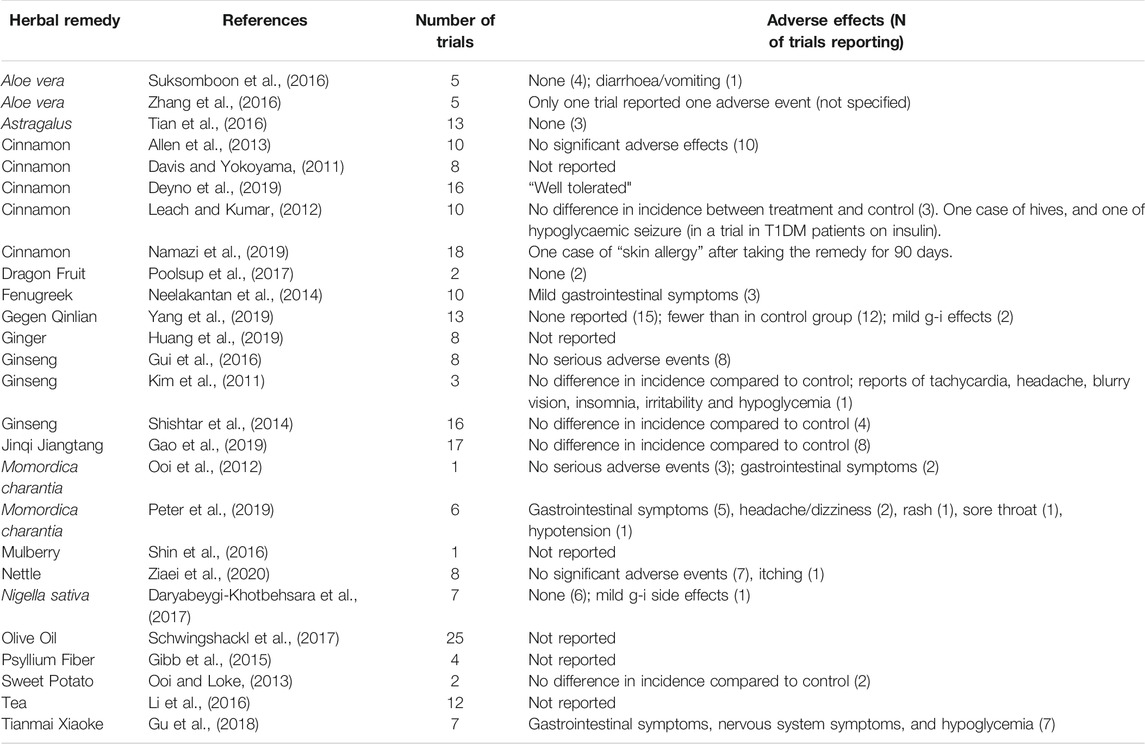
None of the included reviews reported any serious adverse events. In most cases there was no significant difference in the incidence of adverse events between the treatment and control groups (Table 2). Mild gastrointestinal symptoms such as diarrhoea, vomiting and abdominal discomfort were reported in a few cases for certain herbal remedies, in particular Momordica charantia (three participants) and Fenugreek seeds (three participants). There was no specific mention of drug interactions although 14 of the reviews included trials in which the herbal medicine was given in addition to conventional oral hypoglycaemic agents. Only three of these reviews mentioned cases of hypoglycaemia, including only one reported case of a hypoglycaemic seizure in a clinical trial of cinnamon given to adolescent T1DM patients on insulin (Leach and Kumar, 2012).
Discussion
Summary of Main Findings
There have been many RCTs on different phytomedicines and herbal medicines for T2DM, and 25 published meta-analyses on 18 different medicinal plants. Of these, seven have a clinically and statistically significant effect on HbA1c and 12 on FPG (Figures 2, 3). The most effective on both measures appear to be Aloe vera, Psyllium fibre, Fenugreek seeds, Nigella sativa seeds, and the complex traditional Chinese formula Jinqi Jiangtang. Tea and tea extracts were ineffective. The 12 other remedies showed some degree of effectiveness on either HbA1c or FPG, but in some cases with a wide degree of uncertainty. All of the medicinal plants evaluated in this review appeared to be safe, with no serious adverse effects reported. However, some were associated with minor side-effects, in particular gastrointestinal disturbances.
Strengths and Limitations
This the first study to provide a systematic, evidence-based overview of meta-analyses of the effectiveness of medicinal plants for glycaemic control in type 2 diabetes. Our systematic approach with broad search terms ensured that we probably found most relevant articles. One limitation is that we did not have the time to search the grey literature or databases in foreign languages such as Chinese. Another limitation is that we were not able to include medicinal plants for which there had been no systematic review with a meta-analysis. For example there was a systematic review of the Ayurvedic remedy Gymnema sylvestre (Leach, 2007) but this found no clinical trials which met its inclusion criteria. It is also likely that there are other potentially effective medicinal plants which have been evaluated in RCTs but not reviewed in a meta-analysis, and others which have not been evaluated in an RCT although lower-level evidence suggests they could be effective (Sissoko et al., 2020).
Our results are also limited by the quality of the trials included in the meta-analyses. Although most only included RCTs, in some cases the preparation or dosage of the phytomedicine may have been suboptimal; in some reviews both herbal remedies and standardised phytomedicines were included. The clinical condition of the patients may have been different between trials where patients were taking concomitant oral antidiabetics and those who were purely diet controlled. In some trials, the duration of follow-up was insufficient to measure the effect on HbA1c, which should be measured at least 3 months after the start of treatment to reveal its full effect. Follow-up duration was generally short: only three reviews included studies with follow-up of 1 year or more, so there is little information on long-term adherence to herbal remedies.
Comparison With the Existing Literature
The effect of the most promising medicinal plants was similar to that of standard oral hypoglycaemic agents. In a meta-analysis, metformin monotherapy lowered HbA1c by 1.12% (95% CI 0.92–1.32) versus placebo. Metformin added to oral therapy lowered HbA1c by 0.95% (0.77–1.13) versus placebo added to oral therapy (Hirst et al., 2012). In another meta-analysis, metformin reduced FPG by −2.0 mmol/l (95% CI: −2.4, −1.7) (Johansen, 1999). Other conventional hypoglycaemic medications have a smaller effect, for example sitagliptin lowers HbA1c by −0.94% and FPG by 1.2 mmol/l (Aschner et al., 2006).
Several mechanisms of action explain the effect of medicinal plants. Firstly, many plant products contain gel-forming fibres which delay gastric emptying and interfere with glucose absorption from the intestines–for example Aloe vera (Suksomboon et al., 2016), Fenugreek (Madar and Shomer, 1990) and Psyllium (Gibb et al., 2015). Secondly, some medicinal plants contain substances which inhibit enzymes involved in digestion of carbohydrates (eg α-amylase, α-glucosidase), such as nettle (Ziaei et al., 2020) and the Chinese formula Jinqui Jiangtan (Gao et al., 2019). Third, others stimulate release of insulin; these include Fenugreek seeds (Neelakantan et al., 2014) and Nigella sativa seeds (Daryabeygi-Khotbehsara et al., 2017). Fourth, some medicinal plants inhibit gluconeogenesis, including Nigella sativa (Daryabeygi-Khotbehsara et al., 2017). Fifth, some, such as nettle (Ziaei et al., 2020), mimic the effect of insulin by increasing peripheral uptake of glucose, while others such as Nigella sativa induce insulin sensitivity (Daryabeygi-Khotbehsara et al., 2017).
Implications for Policy and Practice
Dietary and lifestyle advice for patients with diabetes rarely includes information on natural remedies, herbs and spices that can help with glycaemic control. The results presented here can guide patients who wish to try herbal supplements and foods as part of their self-care and diet, and clinicians who wish to advise them. Several of the remedies tested are effective and safe. Many of these herbs and spices with clinically assessed hypoglycemic properties are common food products, and as such generally considered very safe. Some can easily be incorporated into the diet–for example in some studies fenugreek seed powder was mixed with flour for baking chapatis, to reach a total daily dose of 100 g (Neelakantan et al., 2014); but the most effective preparation appeared to be a standardised extract of Fenugreek seed total saponins given in six capsules three times daily after meals (Lu et al., 2008). Other herbs can easily be purchased without a prescription (for example Aloe vera, Psyllium fibre, and Nigella seeds). However, it would be necessary to ensure that an adequate dosage is taken of the most effective preparations. The most effective preparation of Aloe vera appeared to be freshly extracted juice, followed by powdered gel in capsules (Suksomboon et al., 2016). In the case of Psyllium, the most effective preparation appeared to be the seed husk of Plantago ovata Forssk (Ziai et al., 2005). For Nigella sativa, the seed powder (at a dose of 2 g daily) was more effective than the oil (Daryabeygi-Khotbehsara et al., 2017). It is equally important to inform patients and clinicians about remedies which appear to be ineffective–such as tea extracts–and those for which there is insufficient evidence of effectiveness–for example cinnamon and ginseng.
Priorities for Future Research
Firstly, some of the meta-analyses were performed more than 5 years ago and need to be updated to include the most recent trials. Some reviews were not performed to the highest standards and could be improved. In particular we recommend that the meta-analysis on Fenugreek should be updated because this appears to be one of the most effective remedies but the systematic review was done in 2014 (Neelakantan et al., 2014). A later systematic review suggested an even greater effect but incorrectly reported some of the underlying data (Gong et al., 2016). It would also be useful to perform a network meta-analysis to estimate the relative effects between the different herbal interventions.
Secondly, it would be interesting to evaluate the impact on glycaemic control of including information on effective medicinal plants and herbal remedies within dietary and lifestyle advice for patients with type 2 diabetes. These may have an additional benefit, and for some patients may be more acceptable, so may be a useful addition to the “menu” of options. This information would need to include clear instructions on the most effective preparations and dosages, and to warn patients about potential side-effects.
Thirdly, this review found a large number of potentially effective medicinal plants for which there is insufficient evidence of effectiveness. For example, Nettle (Urtica dioica) appears to have a significant effect on HbA1c and FPG (Ziaei et al., 2020) but the confidence intervals are very wide. Larger trials are needed to provide a more precise estimate of efficacy. Although it appears effective, the results on Psyllium were at high risk of bias because the review was undertaken by a company selling it–a higher quality review, with low risk of bias, would be helpful. In some studies, cinnamon appears to significantly reduce FPG, but not HbA1c. However, there is a wide variety of cinnamon species, preparations and doses–it is likely that some are more effective than others. Further research is needed to identify the most effective preparations and dosages, and to conduct high-quality clinical trials of these.
Fourth, for the majority of the 1,200 remedies which have been traditionally used in the treatment of diabetes (Simmonds et al., 2006), no meta-analyses and/or no RCTs have been conducted. Some of these have preliminary evidence of effectiveness, for example on post-prandial glucose; these include the Ayurvedic remedy Gymnema sylvestre (Leach, 2007) and the West African tree Moringa oleifera (Sissoko et al., 2020). It is important to conduct high-quality clinical trials of these (at low risk of bias, using a standardised, replicable dosage and preparation, and measuring HbA1c after at least 12 weeks).
Conclusion
Several medicinal plants have the potential to lower HbA1c and could be effective as an adjunct to other lifestyle measures and current treatment, in particular Aloe vera, Psyllium fibre, Fenugreek seeds, Nigella sativa seeds and the Chinese formula Jinqi Jiangtang. It is also clear that tea and tea extracts are ineffective. Rigorous trials with at least 3 months follow-up are needed to ascertain the safety and effectiveness of promising plant-based preparations on diabetes. Practical information on safe plant-based preparations with hypo-glycaemic effects should be made widely available to clinicians and patients with diabetes.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
MW, ML, and BG conceived and designed the study. CE and MA-A conducted the literature searches, screening, quality appraisal and data extraction. MW checked quality appraisal and data extraction and wrote the first draft of the manuscript. All authors contributed to revising the manuscript and approved the final version.
Funding
MLW’s and ML’s salaries were funded by the National Institute of Health Research (grants CL-2016-26-005, NIHR301108). MA-A’s and BG’s salaries were funded by the Antenna Foundation (Geneva, Switzerland).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Paula Sands for her assistance in developing and refining the search strategy and her guidance throughout the search process.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.777561/full#supplementary-material
References
Ali, B., and Mahfouz, M. (2014). Herbal Medicine Use Among Patients with Type 2 Diabetes in North Sudan. Annual Research and Review in Biology 4, 1827–1838. doi:10.9734/arrb/2014/8015
Allen, R. W., Schwartzman, E., Baker, W. L., Coleman, C. I., and Phung, O. J. (2013). Cinnamon Use in Type 2 Diabetes: an Updated Systematic Review and Meta-Analysis. Ann. Fam. Med. 11, 452–459. doi:10.1370/afm.1517
Alqathama, A., Alluhiabi, G., Baghdadi, H., Aljahani, L., Khan, O., Jabal, S., et al. (2020). Herbal Medicine from the Perspective of Type II Diabetic Patients and Physicians: what Is the Relationship? BMC Complement. Med. Ther. 20, 65. doi:10.1186/s12906-020-2854-4
Aschner, P., Kipnes, M. S., Lunceford, J. K., Sanchez, M., Mickel, C., and Williams-Herman, D. E. (2006). Effect of the Dipeptidyl Peptidase-4 Inhibitor Sitagliptin as Monotherapy on Glycemic Control in Patients with Type 2 Diabetes. Diabetes Care 29, 2632–2637. doi:10.2337/dc06-0703
Chang, H. Y., Wallis, M., and Tiralongo, E. (2007). Use of Complementary and Alternative Medicine Among People Living with Diabetes: Literature Review. J. Adv. Nurs. 58, 307–319. doi:10.1111/j.1365-2648.2007.04291.x
Daryabeygi-Khotbehsara, R., Golzarand, M., Ghaffari, M. P., and Djafarian, K. (2017). Nigella Sativa Improves Glucose Homeostasis and Serum Lipids in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 35, 6–13. doi:10.1016/j.ctim.2017.08.016
Davis, P. A., and Yokoyama, W. (2011). Cinnamon Intake Lowers Fasting Blood Glucose: Meta-Analysis. J. Med. Food 14, 884–889. doi:10.1089/jmf.2010.0180
Deyno, S., Eneyew, K., Seyfe, S., Tuyiringire, N., Peter, E. L., Muluye, R. A., et al. (2019). Efficacy and Safety of Cinnamon in Type 2 Diabetes Mellitus and Pre-diabetes Patients: A Meta-Analysis and Meta-Regression. Diabetes Res. Clin. Pract. 156, 107815. doi:10.1016/j.diabres.2019.107815
Florez, H., Luo, J., Castillo-Florez, S., Mitsi, G., Hanna, J., Tamariz, L., et al. (2010). Impact of Metformin-Induced Gastrointestinal Symptoms on Quality of Life and Adherence in Patients with Type 2 Diabetes. Postgrad. Med. 122, 112–120. doi:10.3810/pgm.2010.03.2128
Gao, H., Yang, Y., Deng, J., Liang, J., Zhang, W., and Feng, X. (2019). A Systematic Review and Meta-Analysis on the Efficacy and Safety of Traditional Chinese Patent Medicine Jinqi Jiangtang Tablet in the Treatment of Type 2 Diabetes. Complement. Ther. Med. 47, 102021. doi:10.1016/j.ctim.2019.01.016
Gibb, R. D., McRorie, J. W., Russell, D. A., Hasselblad, V., and D'Alessio, D. A. (2015). Psyllium Fiber Improves Glycemic Control Proportional to Loss of Glycemic Control: a Meta-Analysis of Data in Euglycemic Subjects, Patients at Risk of Type 2 Diabetes Mellitus, and Patients Being Treated for Type 2 Diabetes Mellitus. Am. J. Clin. Nutr. 102, 1604–1614. doi:10.3945/ajcn.115.106989
Gong, J., Fang, K., Dong, H., Wang, D., Hu, M., and Lu, F. (2016). Effect of Fenugreek on Hyperglycaemia and Hyperlipidemia in Diabetes and Prediabetes: A Meta-Analysis. J. Ethnopharmacol 194, 260–268. doi:10.1016/j.jep.2016.08.003
Governa, P., Baini, G., Borgonetti, V., Cettolin, G., Giachetti, D., Magnano, A. R., et al. (2018). Phytotherapy in the Management of Diabetes: A Review. Molecules 23, 105. doi:10.3390/molecules23010105
Grant, R. W., Devita, N. G., Singer, D. E., and Meigs, J. B. (2003). Polypharmacy and Medication Adherence in Patients with Type 2 Diabetes. Diabetes Care 26, 1408–1412. doi:10.2337/diacare.26.5.1408
Gu, Y., Xu, X., Wang, Z., Xu, Y., Liu, X., Cao, L., et al. (2018). Chromium-Containing Traditional Chinese Medicine, Tianmai Xiaoke Tablet, for Newly Diagnosed Type 2 Diabetes Mellitus: A Meta-Analysis and Systematic Review of Randomized Clinical Trials. Evid. Based Complement. Alternat Med. 2018, 3708637. doi:10.1155/2018/3708637
Gui, Q. F., Xu, Z. R., Xu, K. Y., and Yang, Y. M. (2016). The Efficacy of Ginseng-Related Therapies in Type 2 Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis. Medicine (Baltimore) 95, e2584. doi:10.1097/MD.0000000000002584
Gupta, R. C., Chang, D., Nammi, S., Bensoussan, A., Bilinski, K., and Roufogalis, B. D. (2017). Interactions between Antidiabetic Drugs and Herbs: an Overview of Mechanisms of Action and Clinical Implications. Diabetol. Metab. Syndr. 9, 59. doi:10.1186/s13098-017-0254-9
Hirst, J. A., Farmer, A. J., Ali, R., Roberts, N. W., and Stevens, R. J. (2012). Quantifying the Effect of Metformin Treatment and Dose on Glycemic Control. Diabetes Care 35, 446–454. doi:10.2337/dc11-1465
Huang, F. Y., Deng, T., Meng, L. X., and Ma, X. L. (2019). Dietary Ginger as a Traditional Therapy for Blood Sugar Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 98, e15054. doi:10.1097/MD.0000000000015054
International Diabetes Federation (2019). IDF Diabetes Atlas. 9th edn ed. Brussels, Belgium: International Diabetes Federation.
Johansen, K. (1999). Efficacy of Metformin in the Treatment of NIDDM. Meta-Analysis. Diabetes Care 22, 33–37. doi:10.2337/diacare.22.1.33
Khan, M. A. B., Hashim, M. J., King, J. K., Govender, R. D., Mustafa, H., and Al Kaabi, J. (2020). Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 10, 107–111. doi:10.2991/jegh.k.191028.001
Kim, S., Shin, B. C., Lee, M. S., Lee, H., and Ernst, E. (2011). Red Ginseng for Type 2 Diabetes Mellitus: A Systematic Review of Randomized Controlled Trials. Chin. J. Integr. Med. 17, 937–944. doi:10.1007/s11655-011-0937-2
Korsmo-Haugen, H. K., Brurberg, K. G., Mann, J., and Aas, A. M. (2019). Carbohydrate Quantity in the Dietary Management of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Obes. Metab. 21, 15–27. doi:10.1111/dom.13499
Leach, M. J. (2007). Gymnema Sylvestre for Diabetes Mellitus: a Systematic Review. J. Altern. Complement. Med. 13, 977–983. doi:10.1089/acm.2006.6387
Leach, M. J., and Kumar, S. (2012). Cinnamon for Diabetes Mellitus. Cochrane Database Syst. Rev. 2012, CD007170. doi:10.1002/14651858.cd007170.pub2
Lenters-Westra, E., Schindhelm, R. K., Bilo, H. J., Groenier, K. H., and Slingerland, R. J. (2014). Differences in Interpretation of Haemoglobin A1c Values Among Diabetes Care Professionals. Neth. J. Med. 72, 462–466.
Li, Y., Wang, C., Huai, Q., Guo, F., Liu, L., Feng, R., et al. (2016). Effects of tea or tea Extract on Metabolic Profiles in Patients with Type 2 Diabetes Mellitus: a Meta-Analysis of Ten Randomized Controlled Trials. Diabetes Metab. Res. Rev. 32, 2–10. doi:10.1002/dmrr.2641
Lu, F. R., Shen, L., Qin, Y., Gao, L., Li, H., and Dai, Y. (2008). Clinical Observation on Trigonella Foenum-Graecum L. Total Saponins in Combination with Sulfonylureas in the Treatment of Type 2 Diabetes Mellitus. Chin. J. Integr. Med. 14, 56–60. doi:10.1007/s11655-007-9005-3
Madar, Z., and Shomer, I. (1990). Polysaccharide Composition of a Gel Fraction Derived from Fenugreek and its Effect on Starch Digestion and Bile Acid Absorption in Rats. J. Agric. Food Chem. 38, 1535–1539. doi:10.1021/jf00097a023
Mekuria, A. B., Belachew, S. A., Tegegn, H. G., Ali, D. S., Netere, A. K., Lemlemu, E., et al. (2018). Prevalence and Correlates of Herbal Medicine Use Among Type 2 Diabetic Patients in Teaching Hospital in Ethiopia: a Cross-Sectional Study. BMC Complement. Altern. Med. 18, 85. doi:10.1186/s12906-018-2147-3
Namazi, N., Khodamoradi, K., Khamechi, S. P., Heshmati, J., Ayati, M. H., and Larijani, B. (2019). The Impact of Cinnamon on Anthropometric Indices and Glycemic Status in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Clinical Trials. Complement. Ther. Med. 43, 92–101. doi:10.1016/j.ctim.2019.01.002
National Glycohemoglobin Standardization Program (2010). IFCC Standardization of HbA1c. Available at http://www.ngsp.org/ifccngsp.asp.
National Institute for Health and Care Excellence (2020). Type 2 Diabetes in Adults: Management. London: NICE.
Neelakantan, N., Narayanan, M., De Souza, R. J., and Van Dam, R. M. (2014). Effect of Fenugreek (Trigonella Foenum-Graecum L.) Intake on Glycemia: A Meta-Analysis of Clinical Trials. Nutr. J. 13, 7. doi:10.1186/1475-2891-13-7
Ooi, C. P., and Loke, S. C. (2013). Sweet Potato for Type 2 Diabetes Mellitus. Cochrane Database Syst. Rev. 2017, CD009128. doi:10.1002/14651858.CD009128.pub3
Ooi, C. P., Yassin, Z., and Hamid, T. A. (2012). Momordica Charantia for Type 2 Diabetes Mellitus. Cochrane Database Syst. Rev. 2012, CD007845. doi:10.1002/14651858.CD007845.pub2
Peter, E. L., Kasali, F. M., Deyno, S., Mtewa, A., Nagendrappa, P. B., Tolo, C. U., et al. (2019). Momordica Charantia L. Lowers Elevated Glycaemia in Type 2 Diabetes Mellitus Patients: Systematic Review and Meta-Analysis. J. Ethnopharmacol 231, 311–324. doi:10.1016/j.jep.2018.10.033
Pieroni, A., Sheikh, Q. Z., Ali, W., and Torry, B. (2008). Traditional Medicines Used by Pakistani Migrants from Mirpur Living in Bradford, Northern England. Complement. Ther. Med. 16, 81–86. doi:10.1016/j.ctim.2007.03.005
Polonsky, W. H., and Henry, R. R. (2016). Poor Medication Adherence in Type 2 Diabetes: Recognizing the Scope of the Problem and its Key Contributors. Patient Prefer Adherence 10, 1299–1307. doi:10.2147/PPA.S106821
Poolsup, N., Suksomboon, N., and Paw, N. J. (2017). Effect of Dragon Fruit on Glycemic Control in Prediabetes and Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLoS ONE 12, e0184577. doi:10.1371/journal.pone.0184577
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., et al. (2019). Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 157, 107843. doi:10.1016/j.diabres.2019.107843
Schwingshackl, L., Lampousi, A. M., Portillo, M. P., Romaguera, D., Hoffmann, G., and Boeing, H. (2017). Olive Oil in the Prevention and Management of Type 2 Diabetes Mellitus: a Systematic Review and Meta-Analysis of Cohort Studies and Intervention Trials. Nutr. Diabetes 7, e262. doi:10.1038/nutd.2017.12
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). AMSTAR 2: a Critical Appraisal Tool for Systematic Reviews that Include Randomised or Non-randomised Studies of Healthcare Interventions, or Both. BMJ 358, j4008. doi:10.1136/bmj.j4008
Shin, S.-O., Seo, H.-J., Park, H., and Song, H. J. (2016). Effects of mulberry Leaf Extract on Blood Glucose and Serum Lipid Profiles in Patients with Type 2 Diabetes Mellitus: A Systematic Review. Eur. J. Integr. Med. 8, 602–608. doi:10.1016/j.eujim.2016.06.008
Simmonds, M. S. J., and Howes, M-J. R. (2006). “Plants Used in the Treatment of Diabetes,” in Antidiabetic Plants. Editor A. Soumyanath (Boca Raton: CRC Press).
Sissoko, L., Diarra, N., Nientao, I., Stuart, B., Togola, A., Diallo, D., et al. (2020). Moringa Oleifera Leaf Powder for Type 2 Diabetes: a Pilot Clinical Trial. Afr. J. Traditional, Complement. Altern. Medicines 17, 29–36. doi:10.21010/ajtcam.v17i2.3
Suksomboon, N., Poolsup, N., and Punthanitisarn, S. (2016). Effect of Aloe Vera on Glycaemic Control in Prediabetes and Type 2 Diabetes: a Systematic Review and Meta-Analysis. J. Clin. Pharm. Ther. 41, 180–188. doi:10.1111/jcpt.12382
Tang, Y., Weiss, T., Liu, J., Rajpathak, S., and Khunti, K. (2020). Metformin Adherence and Discontinuation Among Patients with Type 2 Diabetes: A Retrospective Cohort Study. J. Clin. Transl Endocrinol. 20, 100225. doi:10.1016/j.jcte.2020.100225
Tian, H., Lu, J., He, H., Zhang, L., Dong, Y., Yao, H., et al. (2016). The Effect of Astragalus as an Adjuvant Treatment in Type 2 Diabetes Mellitus: A (Preliminary) Meta-Analysis. J. Ethnopharmacol 191, 206–215. doi:10.1016/j.jep.2016.05.062
Wang, Z., Wang, J., and Chan, P. (2013). Treating Type 2 Diabetes Mellitus with Traditional Chinese and Indian Medicinal Herbs. Evid. Based Complement. Alternat Med. 2013, 343594. doi:10.1155/2013/343594
Wong, N. D., Patao, C., Wong, K., Malik, S., Franklin, S. S., and Iloeje, U. (2013). Trends in Control of Cardiovascular Risk Factors Among US Adults with Type 2 Diabetes from 1999 to 2010: Comparison by Prevalent Cardiovascular Disease Status. Diab Vasc. Dis. Res. 10, 505–513. doi:10.1177/1479164113496828
Yang, L., Chen, J., Lu, H., Lai, J., He, Y., Liu, S., et al. (2019). Pueraria Lobata for Diabetes Mellitus: Past, Present and Future. Am. J. Chin. Med. 47, 1419–1444. doi:10.1142/S0192415X19500733
Yeh, G. Y., Eisenberg, D. M., Kaptchuk, T. J., Phillips, R. S., Yeh, G. Y., Eisenberg, D. M., et al. (2003). Systematic Review of Herbs and Dietary Supplements for Glycemic Control in Diabetes. Diabetes Care 26, 1277–1294. doi:10.2337/diacare.26.4.1277
Zhang, Y., Liu, W., Liu, D., Zhao, T., and Tian, H. (2016). Efficacy of Aloe Vera Supplementation on Prediabetes and Early Non-treated Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 8, 388. doi:10.3390/nu8070388
Ziaei, R., Foshati, S., Hadi, A., Kermani, M. A. H., Ghavami, A., Clark, C. C. T., et al. (2020). The Effect of Nettle (Urtica Dioica) Supplementation on the Glycemic Control of Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Phytother Res. 34, 282–294. doi:10.1002/ptr.6535
Keywords: type 2 diabetes mellitus, phytomedicines, medicinal plants, herbal preparations, metaanalysis, randomised controlled clinical trials, glycaemic control, HbA1c
Citation: Willcox ML, Elugbaju C, Al-Anbaki M, Lown M and Graz B (2021) Effectiveness of Medicinal Plants for Glycaemic Control in Type 2 Diabetes: An Overview of Meta-Analyses of Clinical Trials. Front. Pharmacol. 12:777561. doi: 10.3389/fphar.2021.777561
Received: 15 September 2021; Accepted: 09 November 2021;
Published: 26 November 2021.
Edited by:
Massimo Lucarini, Council for Agricultural Research and Economics, ItalyReviewed by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoRachael Frost, University College London, United Kingdom
Copyright © 2021 Willcox, Elugbaju, Al-Anbaki, Lown and Graz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Merlin L. Willcox, TS5MLldpbGxjb3hAc290b24uYWMudWs=
 Merlin L. Willcox
Merlin L. Willcox Christina Elugbaju
Christina Elugbaju Marwah Al-Anbaki
Marwah Al-Anbaki Mark Lown
Mark Lown Bertrand Graz
Bertrand Graz