- 1Laboratory on the Biology of Addictive Diseases, The Rockefeller University, New York, NY, United States
- 2Department of Pharmaceutical Sciences, College of Pharmacy, University of Kentucky, Lexington, KY, United States
The kappa-opioid receptor (KOR) / dynorphin system is implicated with behavioral and neurobiological effects of stress exposure (including heavy exposure to drugs of abuse) in translational animal models. Thus some KOR-antagonists can decrease the aversive, depressant-like and anxiety-like effects caused by stress exposure. The first generation of selective KOR-antagonists have slow onsets (hours) and extremely long durations of action (days-weeks), in vivo. A new generation of KOR antagonists with rapid onset and shorter duration of action can potentially decrease the effects of stress exposure in translational models, and may be of interest for medication development. This study examined the rapid onset anti-stress effects of one of the shorter acting novel KOR-antagonists (LY2795050, (3-chloro-4-(4-(((2S)-2-pyridin-3-ylpyrrolidin-1-yl)methyl) phenoxy)benzamide)) in a single-session open space swim (OSS) stress paradigm (15 min duration), in adult male and female C57BL/6 J mice. LY2795050 (0.32 mg/kg, i.p.) had rapid onset (within 15 min) and short duration (<3 h) of KOR-antagonist effects, based on its blockade of the locomotor depressant effects of the KOR-agonist U50,488 (10 mg/kg). LY2795050 (0.32 mg/kg), when administered only 1 min prior to the OSS stress paradigm, decreased immobility in males, but not females. With a slightly longer pretreatment time (15 min), this dose of LY2795050 decreased immobility in both males and females. A 10-fold smaller dose of LY2795050 (0.032 mg/kg) was inactive in the OSS, showing dose-dependence of this anti-stress effect. Overall, these studies show that a novel KOR-antagonist can produce very rapid onset anti-immobility effects in this model of acute stress exposure.
Introduction
Acute stress exposure can cause behavioral and neurobiological changes (Reed et al., 2012; Carter et al., 2020; Varlinskaya et al., 2020; Becker et al., 2021), and can result in psychiatric sequeleae in humans, including acute stress disorder. The κ(kappa)-opioid receptors (KOR) and their endogenous ligands, the dynorphins, are one of the systems implicated in behavioral and neurobiological effects of stress exposure (Shirayama et al., 2004; Knoll and Carlezon, 2010; Reed et al., 2012; Chavkin, 2018). Thus, several studies have shown that the earliest known selective KOR-antagonists, including nor-binaltorphimine (nor-BNI), decreased immobility in the standard forced swim test (FST), as well as other behavioral consequences of stress exposure (Portoghese et al., 1987; Zhang et al., 2007; Carroll and Carlezon, 2013; Falcon et al., 2014). However, some of these early selective KOR-antagonists, such as nor-BNI, have slow onsets of action (e.g., several hours), and extremely long-lasting profiles in vivo (days-weeks, depending on the assay) (Horan et al., 1992; Broadbear et al., 1994; Butelman et al., 1998; Carroll and Carlezon, 2013). This may complicate their use in translational models of acute stress exposure and its consequences, and eventual medication development (Carroll and Carlezon, 2013; Page et al., 2019).
Several chemically unrelated shorter-acting KOR-antagonists have been developed more recently, and some have reached the clinical investigation stage (Aldrich et al., 2009; Chang et al., 2011; Rorick-Kehn et al., 2014; Huang et al., 2016; Valenza et al., 2017; Guerrero et al., 2019; Margolis et al., 2020; Valenza et al., 2020). We recently reported that one of the shorter acting KOR-antagonists, LY2795050, produced anti-immobility effects in the forced swim test (FST) in male, but not female, mice (Butelman et al., 2021). This is consistent with a sex difference observed with nor-BNI in the same assay (Laman-Maharg et al., 2018). Of translational relevance, 11C-LY2795050 has been used as a KOR positron emission tomography radiotracer in humans (Naganawa et al., 2015; de Laat et al., 2019), and is also structurally related to LY2456302 (aticaprant, CERC-501), which has reached the clinical investigation stage (Lowe et al., 2014; Reed et al., 2017). LY2795050 exhibits selectivity for KOR over mu-opioid and delta-opioid receptors (MOR and DOR) (Zheng et al., 2013). Thus, in cloned human receptors, the affinity (Ki) of LY2795050 for KOR, MOR and DOR is 0.72, 25.8 and 153 nM respectively (Zheng et al., 2013). The antagonist potency of LY2795050 in vitro (Kb) at KOR, MOR and DOR is 0.63, 6.8 and 83.3 nM respectively (Zheng et al., 2013).
The FST was originally developed as a screening test for anti-depressants, and its mechanistic underpinnings and interpretation have been extensively discussed (Porsolt et al., 1977; Porsolt et al.,1978; Chen et al., 2015; Molendijk and de Kloet, 2019; Armario, 2021). Recent reviews conclude that increased immobility in swim stress assays is reflective of a transition from active to passive coping, in the face of an inescapable stressor (Lanius et al., 2018; Molendijk and de Kloet, 2019; Armario, 2021). Several authors have also found that greater swim area allows more adaptive swimming behaviors for study (Jacobson M. L. et al., 2020; Armario, 2021). One modification of the FST assay is the open space swim (OSS) stress model, which due to its substantially greater available swim area, allows observation of more adaptive swim behaviors (Stone et al., 2008; Lin et al., 2011; Cates et al., 2013). In this study, we used a 1-day OSS procedure to examine in greater detail the rapid onset anti-stress effects of the KOR-antagonist LY2795050, as well as a follow-up to previously reported KOR-antagonist sex differences for the FST test (Laman-Maharg et al., 2018; Butelman et al., 2021).
Methods
Subjects: Mouse studies were approved by the Rockefeller University Animal Care and Use Committee, consistent with the Guide for the Care and Use of Animals (National Academy Press; Washington, DC). Adult (9–10 weeks of age upon arrival to the facility), gonadally intact C57BL/6 J male and female mice were purchased from the Jackson Laboratory. The facility was approved by the American Association for Accreditation of Laboratory Animal Care (AAALAC). Mice were housed in one room at least 7 days prior to experiments. Mice were housed in same-sex groups (two to four per cage), in transparent plastic shoebox cages (19.6 cm wide X 30.9 cm long X 13.3 cm tall; Thoren Caging Systems, Hazleton, PA, United States). A n = 8 was the standard group size; this could vary slightly due to the availability of mice per cage. The cages had corncob bedding (“Bed-o’Cobs”, the Andersons, Maumee, OH, United States); shredded paper was added for nesting. The room was maintained at controlled temperature (≈20–22°C) and humidity, and had a 12:12 light:dark cycle (lights on at 0700), with overhead lighting. Experimental procedures occurred in the same room as housing. Procedures took place between 09:00 and 13:00 h.
Apparatus
Locomotor activity: Locomotor activity was examined in mice placed individually in rectangular transparent plastic cages (19.7 cm width × 41.3 cm length × 20.3 cm height). Each bin had bedding identical to home cages and was placed in a photocell frame, which had an array of beams in perpendicular directions (SmartFrame; Kinder Scientific, Poway, CA). Beam breaks caused by the mouse were cumulated for analysis, on a computer interface.
Open Space Swim (OSS): This assay has been previously described (Stone et al., 2008) and was used with minor modifications, as a single session stress exposure. Mice were individually placed in a standard rectangular shoebox transparent plastic cage (19.7 cm width × 41.3 cm length × 20.3 cm height), with water at 31 ± 1°C, at a depth of approximately 13 cm. The swim surface area of the cage for OSS used here was therefore ≈814 cm2. For comparison, the standard FST test (not used here) provides an approximately 6-fold smaller swim surface area (typically 13 cm diameter; ≈133 cm2) (Porsolt et al., 1977; Castagne et al., 2011).
Sessions (15-min long) were recorded with digital videocameras, and analyzed by a trained observer, for “blind” scoring of duration of immobility. The camera was pointed horizontally, at the surface level of the water. This allowed visualization of movements above and below the surface.
Procedures
Locomotor activity: One to three days prior to experiments, mice were habituated individually to locomotor cages, for a 60-min session. Consecutive experimental sessions in the same mice (90 min in duration) were separated by at least 72 h. Beam breaks were quantified as a measure of locomotor activity, over 15-min bins in the session.
Open Space Swim (OSS): A 1-day OSS procedure was carried out (Stone et al., 2008). Each mouse was used only once, in a single 15-min swim period. Immobility was defined as having only movements necessary to stay afloat, for ⩾2 s. After the test, the animal was returned to home cage. The water was changed after 8 mice had swum, or as needed to maintain water temperature. Immobility time was timed cumulatively in 1-min bins, by a trained observer who was “blinded” as to the treatment (i.e., vehicle or LY2795050). After training, intra-rater correlation for repeated scoring of 8 separate 1-min bins was high (Pearson r = 0.97; p < 0.0001), as was inter-rater correlation (Pearson r = 0.93; p = 0.001).
Design
Locomotor activity: Locomotor experiments were performed to define basic KOR-antagonist effects of LY2795050, to be further examined in the OSS. Experiments were carried out with a within-subjects design, balanced for order. We determined the time course of the antagonist effects of LY2795050 (0.32 mg/kg, or vehicle) in preventing locomotor deficits caused by U50,488 (10 mg/kg), in males only. These dosing conditions were based on prior dose-effect studies, including determinations in both males and females (Butelman et al., 2019; Butelman et al., 2021). LY2795050 (0.32 mg/kg or vehicle) was therefore injected 15 min, 3 h, or 24 h prior to U50,488 (10 mg/kg). Mice were placed into locomotor cages immediately following U50,488 injection, for a 90-min session. In a second study, a 10-fold smaller dose of LY2795050 (0.032 mg/kg or vehicle) was injected as a 15 min pretreatment to U50,488 (10 mg/kg); this smaller LY2795050 was found to be inactive in other assays (Butelman et al., 2019; Butelman et al., 2021).
Open space swim (OSS): OSS studies were performed with a between-groups design; each mouse was used for a single session. In the first experiment, LY2795050 (0.32 mg/kg, or vehicle) was administered as a 1-min pretreatment to OSS in males and females. The second study examined the effects of the same dose of LY2795050 (0.32 mg/kg, or vehicle), but with a longer pretreatment time (15 min). Lastly, the effects of a 10-fold smaller dose of LY2795050 (0.032 mg/kg, or vehicle) was studied, with a 15-min pretreatment before OSS.
Statistics
Graphpad Prism (version 9) software was used for statistical analyses. Data for locomotor activity and immobility were analyzed with repeated measures ANOVAs and Sidak’s or Tukey’s post-hoc tests (including planned comparisons), or with t-tests (p ≤ 0.05 level used as the criterion for significance). Mixed effects ANOVAs were used if there were missing data. Data for each 15-min OSS session were also analyzed as 1-min bins, in order to examine in greater detail the emergence of immobility over time. This was done with a non-linear regression with an exponential plateau growth curve (Graphpad Prism), with the following parameters: Y=Ymax -(Ymax-Ymin) * exp(-k*x), where Ymin and Ymax are the minimum and maximum value of the curve, respectively, “k” is the rate constant, and “x” is the time (1-min bins). Since these were 1-min bins measuring immobility, Ymin was constrained at 0 s, and the largest possible Ymax value was 60 s. A lack of overlap in the 95%CI for the maximum plateau value (Ymax) between LY2795050 and vehicle treatment was used as an indicator of a significant difference.
Drugs
As mentioned above, doses of the KOR-antagonist LY2795050 and the KOR-agonist U50,488 were based on recent reports (Butelman et al., 2019; Butelman et al., 2021). Thus, 0.32 mg/kg LY2795050 causes KOR-antagonist effects in different assays (including a locomotor assay) in both male and female C57BL/6 J mice (Butelman et al., 2021). By contrast, a 10-fold smaller LY2795050 dose (0.032 mg/kg) is inactive (Butelman et al., 2019; Butelman et al., 2021); see also (Placzek et al., 2018). U50,488 (10 mg/kg) produces robust decreases in locomotor activity in both male and female mice (Paris et al., 2011; Butelman et al., 2021). Therefore controls for these conditions were not carried out here, in order to limit the total number of mouse experiments. Non-radiolabeled LY2795050 (3-chloro-4-(4-(((2S)-2-pyridin-3-ylpyrrolidin-1-yl)methyl) phenoxy)benzamide)) (Naganawa et al., 2015; 2015b; Placzek et al., 2018) was purchased from BOC Sciences (Shirley, NY). LY2795050 was dissolved in 5% ethanol/10% Tween 80/85% sterile water, by volume. U50,488 was (Sigma-Aldrich) was dissolved in sterile water. LY2795050 and U50,488H were used within approximately 2 h of being in solution. Each experiment had its own appropriate vehicle condition. Mice were injected i.p. (volume of 10 ml/kg).
Results
Locomotor Activity
LY2795050, at a dose that is devoid of direct locomotor effects (0.32 mg/kg) (Butelman et al., 2021), prevented the locomotor depressant effects of U50,488 (10 mg/kg) when given as a 15 min, but not 3 h or 24 h, pretreatment (only males studied; Figure 1A). A 2-way ANOVA [pretreatment (LY2795050 or vehicle) X pretreatment time] found a main effect of both pretreatment (F (1, 7) = 21.73; p = 0.0023) and pretreatment time [F (2, 14) = 20.85; p < 0.0001] but no interaction [F (2, 14) = 2.572; NS]. A Sidak’s multiple comparisons test determined that LY2795050 pretreatment significantly reduced U50,488-induced locomotor deficits compared to vehicle pretreatment, only at the shortest PT time (15 min).
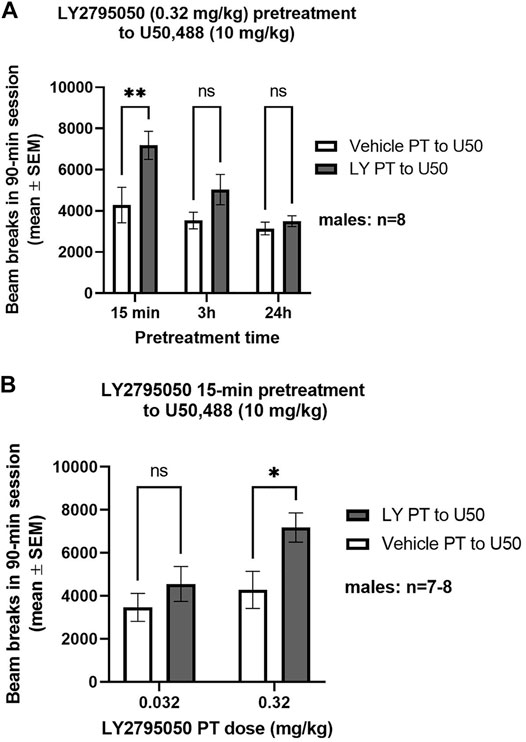
FIGURE 1. KOR-antagonist effects of LY2795050 pretreatment to U50,488 in the locomotor activity assay (in males). Total locomotor counts are shown for a 90-min session, starting immediately after injection of U50,488. (A): Effect of LY2795050 (0.32 mg/kg or vehicle) given at different pretreatment times (15 min, 3 h, or 24 h) before U50,488 (10 mg/kg). (B): Effect of different doses of LY2795050 (0.032 and 0.32 mg/kg, or vehicle) given 15 min before U50,488 (10 mg/kg).
The KOR-antagonist effect of LY2795050 in the locomotor assay was dose-dependent, as a 10-fold smaller dose (0.032 mg/kg), given as a 15 min pretreatment to U50,488, did not prevent U50,488-induced locomotor deficits (Figure 1B). A 2-way ANOVA (pretreatment X LY2795050 dose) revealed main effects of pretreatment [F (1, 13) = 7.351; p = 0.0178] and of LY2795050 dose [F (1, 13) = 4.79; p = 0.047]. There was no pretreatment X dose interaction [F (1, 13) = 1.509; NS]. Planned comparisons with Sidak’s tests revealed significant differences between the larger dose of LY2795050 (0.32 mg/kg) pretreatment and its vehicle condition, but not for the smaller dose (0.032 mg/kg) (Figure 1).
Open Space Swim (OSS)
1-min pretreatment with LY2795050 (0.32 mg/kg): A 1-min pretreatment with LY2795050 (0.32 mg/kg) decreased immobility in the OSS session (15-min in duration) compared to vehicle, in males but not in females (Figure 2). In order to examine sex differences, data for 3 consecutive 5-min bins were analyzed with a 3-way mixed model ANOVA (treatment X sex X time bin, with repeated measures on the last variable) (Figure 2A). In this 3-way ANOVA, there was a main effect of treatment [F (1, 27) = 6.10; p = 0.02], of sex [F (1, 27) = 21.00; p < 0.0001] and of time bin [F (1.67,44.29) = 9.92; p = 0.0006]. There was also a significant interaction between sex and time bin [F (2, 53) = 6.20; p = 0.0038], but no other interaction was significant (not shown). Tukey’s post-hoc tests were used to examine sex differences. In the first two time bins (i.e., 1-5 and 6–10 min), there were no differences between males and females, either for the vehicle or LY2795050 conditions. However, in the third time bin (11–15 min), males had significantly greater immobility scores under the vehicle condition, compared to females (Tukey test; p = 0.0043). There were no sex differences in the LY2795050 condition.
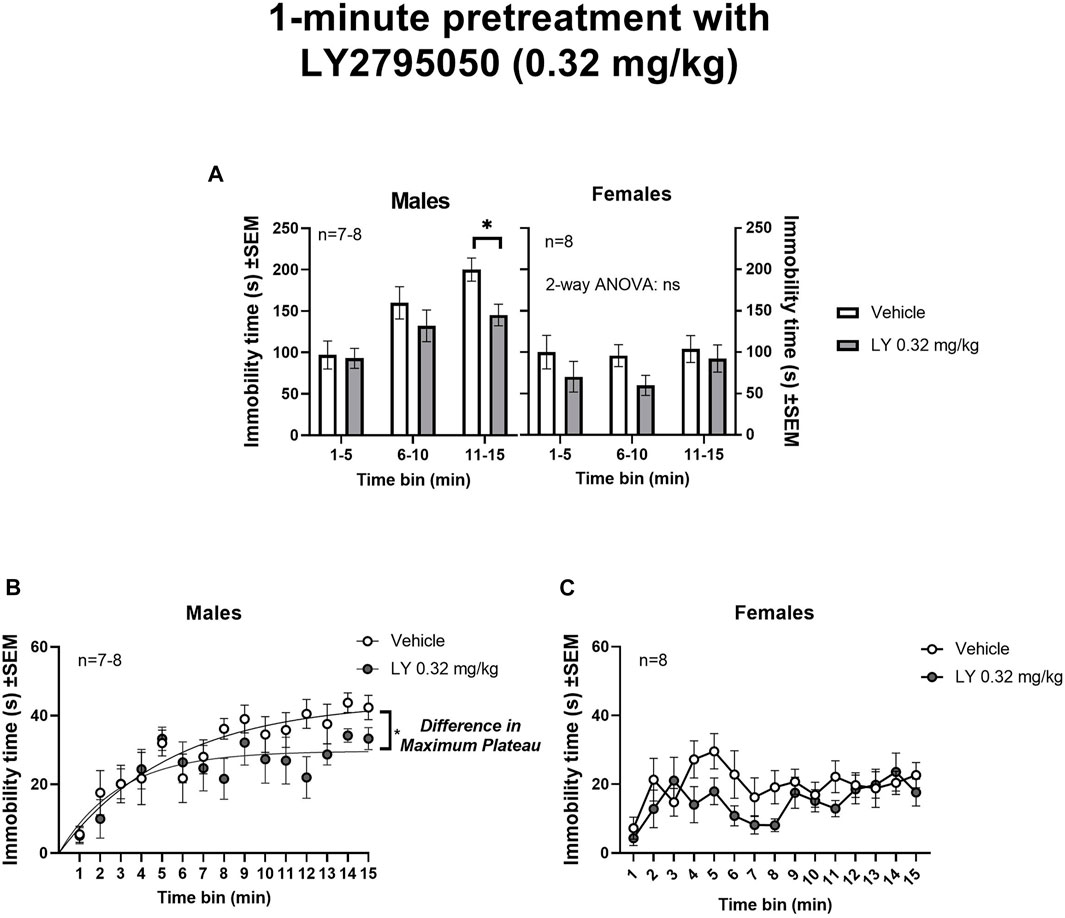
FIGURE 2. Effect of 1-min pretreatment of LY2795050 (0.32 mg/kg, or vehicle) on immobility in the open space swim (15-min duration). (A) shows data in three consecutive 5-min bins, analyzed by mixed model 2-way ANOVA, in males and females (the data were also analyzed in a 3-Way ANOVA; see Results). (B,C) show data re-plotted as 1-min bins, in males and females respectively. The regression lines in (B) (males) shows exponential plateau growth curves (see Methods). The fitted maximum plateau for immobility in males was significantly lower for LY2795050 versus vehicle (based on non-overlapping 95%CI). This regression could not be fit for panel C (females).
In order to further explore the effects of LY2795050, we then analyzed the data separately for males and females. In males, a mixed-effects 2-way ANOVA of three consecutive 5-min time bins (treatment X time bin) detected a main effect of time bin [F (1.58, 19.76) = 14.50; p = 0.0003] but not of treatment [F (1, 13) = 3.87; ns] or their interaction [F (2,25) = 1.50; ns] (Figure 2A). A planned comparison with Sidak’s test showed a significant decrease in immobility only in the last bin (11–15 min), in the group pretreated with LY2795050 compared to vehicle. A non-linear regression was carried out to examine maximum immobility in greater detail across the OSS session (consecutive 1-min time bins; see Methods) (Figure 2B). This regression indicated a significantly lower maximum plateau value (Ymax) for immobility in the LY2795050 versus the vehicle group, as denoted by a lack of overlap in 95%CI. Thus, the best fit value for maximum immobility after LY2795050 (29.7 s; 95%CI: 25.8–35.6) was lower than after vehicle (44.2 s; 95%CI: 37.4–58.3).
In females, no effect of LY2795050 was detected, in a similar 2-way ANOVA for 5-min bins, neither for treatment [F (1,14) = 2.45; ns], nor time bin [F (2,28) = 1.06; ns], or their interaction [F(2,28) = 0.4; ns] (Figure 2A). A non-linear fit could not be adequately achieved with the 1-min bin data in females (Figure 2C).
15-min pretreatment with LY2795050 (0.32 mg/kg): When administered with a slightly longer (15 min) pretreatment, LY2795050 decreased immobility in both males and females (Figure 2). As above, data for 3 consecutive 5-min bins were initially analyzed with a 3-way mixed model ANOVA (treatment X sex X time bin) (Figure 3A). In this 3-way ANOVA, there was a main effect of treatment [F (1, 33) = 26.46; p < 0.0001] and of time bin [F (1.73, 55.30) = 9.86; p = 0.0004], but no main effect of sex. There no significant interactions between these three variables (not shown). Likewise, there were no significant differences across sex, either in vehicle or LY2795050 conditions (Tukey’s tests).
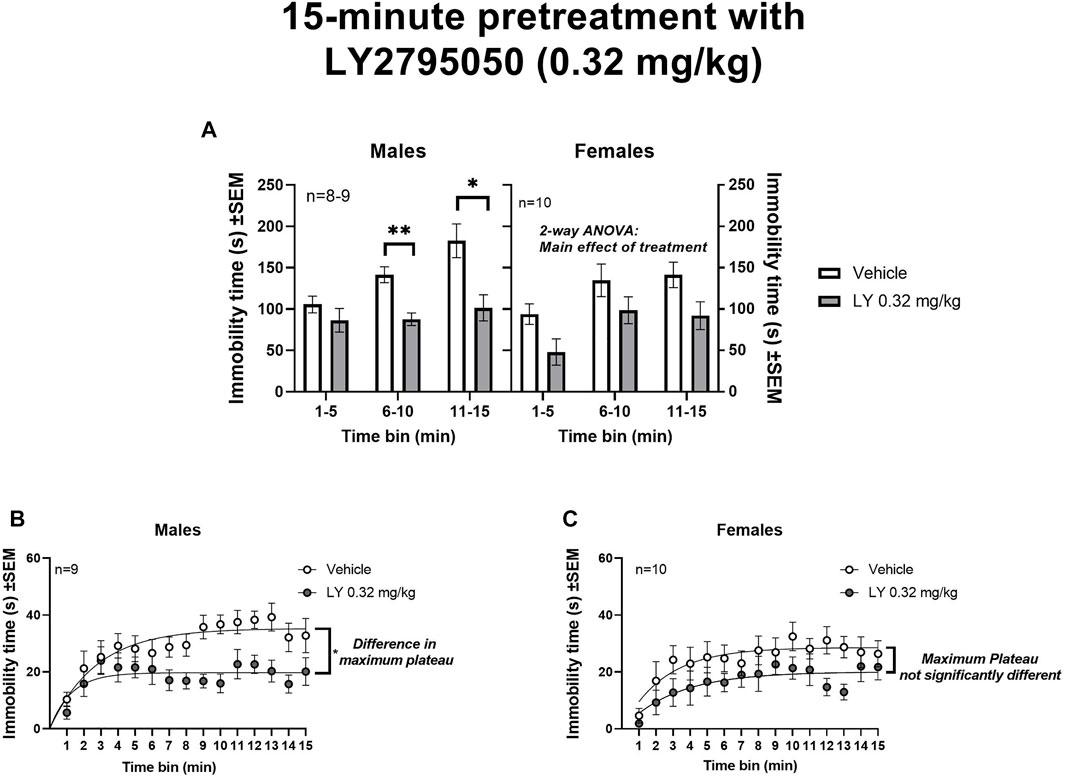
FIGURE 3. Effect of 15-min pretreatment of LY2795050 (0.32 mg/kg, or vehicle) on immobility in the open space swim (15-min duration). (A) shows data in three consecutive 5-min bins, analyzed by mixed model 2-way ANOVA, in males and females (the data were also analyzed in a 3-Way ANOVA; see Results). (B,C) show data re-plotted as 1-min bins, in males and females respectively. The regression lines in (B) (males) show exponential plateau growth curves; the fitted maximum plateau for immobility is significantly lower for LY2795050 versus vehicle. In (C) (females), the fitted maximum plateau is not significantly different for LY2795050 versus vehicle.
We then further examined effects of LY2795050 in males and females separately, as above. In males, a 2-way mixed-effects ANOVA in three consecutive 5-min time bins (treatment X time bin) detected significant main effect of treatment [F (1, 44) = 21.97; p < 0.0001] and time bin [F (1.50, 33.07) = 5.87; p = 0.012], but no interaction [F (2, 44) = 2.65; ns] (Figure 3A). A planned comparison with Sidak’s test showed a significant decrease in immobility in the LY2795050-pretreated group compared to vehicle, in the last two time bins of the OSS (i.e., 6–10 min and 11–15 min bins). As above, a non-linear regression was used to examine immobility data in greater detail (1-min time bins) (Figure 3B, in males). This regression indicated a significantly lower maximum plateau value (Ymax) for immobility in the LY2795050 versus the vehicle group. Thus, the best fit value for maximum immobility after LY2795050 (19.7 s; 95%CI: 17.5–22.1) was lower than after vehicle (35.3 s; 95%CI: 31.8–39.8).
In the females, a similar mixed-effects 2-way ANOVA over 5-min time bins (treatment X time bin) showed a main effect of treatment [F (1, 18) = 8.72; p = 0.0085] and time bin [F (1.76, 30.81) = 5.61; p = 0.011], but no interaction [F (2, 35) = 0.095; ns] (Figure 3A). Planned comparisons with Sidak’s test did not detect that any specific time bin was significantly different in LY2795050 versus vehicle conditions. A non-linear regression on the 1-min time bins in the females did not detect a difference in the maximum immobility in the LY2795050- versus the vehicle-treated mice (as shown by overlapping 95%CI) (Figure 3C). Thus, the best fit value for maximum immobility after LY2795050 (20.1 s; 95%CI: 16.8–25.6) was not different than after vehicle (28.6 s; 95%CI: 25.3–32.9).
15-min pretreatment with a 10-fold smaller dose of LY2795050 (0.032 mg/kg): At a 10-fold smaller dose (0.032 mg/kg) and with a 15-min pretreatment time, LY2795050 did not decrease immobility in the OSS, in either males or females. For example, in the last 5-min time bin of the session (i.e., 11–16 min), LY2795050- and vehicle-treated groups were not different, either in males or females (Table 1).

TABLE 1. Immobility in the last 5-min bin (i.e., 11–15 min) of the open space swim session after a 10-fold smaller dose of LY27950590 (0.032 mg/kg).
Discussion
This study shows that the short-acting KOR-antagonist LY2795050 can produce very rapid-onset anti-immobility effects in a model of acute stress exposure. The study also shows that apparent sex differences in the effects of KOR-antagonists may be due to the specific stress paradigm used (Laman-Maharg et al., 2018; Williams et al., 2018; Diester et al., 2019; Butelman et al., 2021).
Initial examinations in the locomotor activity assay demonstrated that LY2795050 (0.32 mg/kg) prevented U50,488-induced locomotor deficits when administered as a 15-min pretreatment, but was ineffective at longer pretreatments (3 and 24 h, determined only in males). This is consistent with the effectiveness of LY2795050 (0.32 mg/kg), previously determined in locomotor activity studies in both male and female mice (Butelman et al., 2021). The effectiveness of this dose of LY2795050, its rapid onset and relatively short duration of action are also consistent with prior data in rodents, in other assays (Placzek et al., 2018; Butelman et al., 2019). Other novel KOR antagonists, such as the congener LY2444296 (also known as FP3FBZ) (Melief et al., 2011; Valenza et al., 2017) have slightly longer durations of action than LY2795050 (Butelman et al., 2019). Duration of KOR antagonist action has been correlated to activation of c-Jun-N-terminal kinase-1 (JNK1) (Melief et al., 2011). The effects of LY2795050 on JNK1 activity have not been reported, to our knowledge.
We found for the first time that a very short (1 min) pretreatment with LY2795050 (0.32 mg/kg) was able to decrease immobility in the OSS assay (15-min duration), but only in male, but not female mice. Growth curve analysis over 1-min bins revealed that LY2795050 was able to decrease maximal immobility, which was observed typically in the last 5 min of the swim exposure.
Since KOR-antagonist effects of LY2795050 (0.32 mg/kg) were detected here in the locomotor assay with a 15-min pretreatment in males (and previously in females also) (Butelman et al., 2021), this pretreatment time was then studied in the OSS assay. With this slightly longer pretreatment, LY2795050 (0.32 mg/kg) caused a decrease in immobility in both male and female mice, when examined over the three consecutive 5-min bins of OSS stress exposure. Overall, these results indicate that short-acting KOR-antagonists such as LY2795050 can also produce anti-stress effects in females, depending on the assay, consistent with other reports (Williams et al., 2018; Jacobson et al., 2020c). Intriguingly, pretreatment with a different short-acting KOR antagonist prevented the effects of social defeat stress in both male and female California mice, whereas administration after the stress was ineffective (Williams et al., 2018). Potential sex differences in the behavioral effects of novel KOR-antagonists have been studied under a limited set of conditions (Williams et al., 2018; Jacobson et al., 2020a; Jacobson et al., 2020c; Butelman et al., 2021), but sexual dimorphism in the KOR/dynorphin system has been previously reported (Chartoff and Mavrikaki, 2015; Becker and Chartoff, 2019; Jacobson et al., 2020c).
The effects of LY2795050 in the OSS were dose-dependent, since a 10-fold smaller dose (0.032 mg/kg) did not affect immobility. This is consistent previous findings that this smaller dose of LY2795050 does not exhibit KOR-antagonist effects in other assays mice (Butelman et al., 2019; Butelman et al., 2021).
Limitations and methodological considerations: The largest dose of LY2795050 alone (0.32 mg/kg) does not have locomotor effects in either male or female C57BL/6 J mice (Butelman et al., 2021). Therefore the anti-immobility effects of LY2795050 in the OSS are not simply due to a confound with locomotor effects.
The time-dependent increase of immobility within a session of swim stress has been examined in the standard FST, previously (Castagne et al., 2009; Chen et al., 2015; Molendijk and de Kloet, 2019). In the standard FST in mice, immobility is typically reported for the last 4 min of a 6-min swim (Porsolt et al., 1977; Castagne et al., 2011). In order to examine the increase of immobility across the full 15-min OSS herein, we selected an exponential plateau growth curve, for nonlinear regression analysis. This regression model is appropriate for analysis of time series data where a maximum plateau is observed (as occurs in the OSS session). Using these regression analyses, we detected a LY2795050-induced decrease in the maximum plateau of immobility in males, but not in females. It cannot be excluded that a different regression model, or an increase in number of mice per group may have yielded slightly different results. Likewise, it cannot be excluded that there was a “floor” effect on immobility scores in the OSS in female mice.
In this study, male versus female differences in the effects of LY2795050 were examined in gonadally intact adult mice. Larger doses of LY2795050 (i.e., >0.32 mg/kg) were not studied, because they also exhibit MOR-antagonist effects (Butelman et al., 2019). Future studies could determine whether the subtle sex differences observed in the OSS were due to hormonal or organizational effects (Craft and Bernal, 2001; Land et al., 2009; Chartoff and Mavrikaki, 2015).
KOR and endogenous dynorphin function can be regulated by stress exposure in several brain areas and pathways (Shirayama et al., 2004; Carlezon et al., 2006; Rosa et al., 2018). Future studies could determine which brain pathway(s) mediate the rapid anti-stress effects of a systemically administered KOR-antagonist, such as LY2795050.
Conclusion: This is the first study to examine the effects of a KOR-antagonist in the open space swim stress (OSS) procedure. Very rapid onset anti-stress effects of LY2795050 were detected, more robustly in males than in females. This profile may be of potential interest in the modulation of the behavioral and neurobiological consequences of acute stress exposure, in translational models, and possibly for medication development.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by The Rockefeller University Animal Care and Use Committee.
Author Contributions
All authors contributed to the rationale of the project. EB and CB designed and anaylzed the experiments.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We gratefully acknowledge funding from NIH-NIDA grant RO1 DA018151.
Abbreviations
95%CI, 95%confidence interval; FST, Forced swim test; DOR, delta-opioid receptor; KOR, kappa-opioid receptor; MOR, mu-opioid receptor; LY, LY2795050 (short-acting KOR antagonist); NS, non-significant; OSS, Open space swim; PT, pretreatment.
References
Aldrich, J. V., Patkar, K. A., and Mclaughlin, J. P. (2009). Zyklophin, A Systemically Active Selective Kappa Opioid Receptor Peptide Antagonist with Short Duration of Action. Proc. Natl. Acad. Sci. USA. 106, 18396–18401. doi:10.1073/pnas.0910180106
Armario, A. (2021). The Forced Swim Test: Historical, Conceptual and Methodological Considerations and its Relationship with Individual Behavioral Traits. Neurosci. Biobehavioral Rev. 128, 74–86. doi:10.1016/j.neubiorev.2021.06.014
Becker, J. B., and Chartoff, E. (2019). Sex Differences in Neural Mechanisms Mediating Reward and Addiction. Neuropsychopharmacology 44, 166–183. doi:10.1038/s41386-018-0125-6
Becker, M., Pinhasov, A., and Ornoy, A. (2021). Animal Models of Depression: What Can They Teach Us about the Human Disease. Diagnostics (Basel) 11. 123. doi:10.3390/diagnostics11010123
Broadbear, J. H., Negus, S. S., Butelman, E. R., De Costa, B. R., and Woods, J. H. (1994). Differential Effects of Systemically Administered Nor-Binaltorphimine (Nor-BNI) on Kappa-Opioid Agonists in the Mouse Writhing Assay. Psychopharmacology (Berl) 115, 311–319. doi:10.1007/BF02245071
Butelman, E. R., Baynard, C., Mcelroy, B. D., Prisinzano, T. E., and Kreek, M. J. (2021). Profile of a Short-Acting κ-antagonist, LY2795050, on Self-Grooming Behaviors, Forced Swim Test and Locomotor Activity: Sex Comparison in Mice. J. Psychopharmacol. 35, 579–590. doi:10.1177/0269881121996883
Butelman, E. R., Ko, M. C., Sobczyk-Kojiro, K., Mosberg, H. I., Van Bemmel, B., Zernig, G., et al. (1998). Kappa-Opioid Receptor Binding Populations in Rhesus Monkey Brain: Relationship to an Assay of thermal Antinociception. J. Pharmacol. Exp. Ther. 285, 595–601.
Butelman, E. R., Mcelroy, B. D., Prisinzano, T. E., and Kreek, M. J. (2019). Impact of Pharmacological Manipulation of the κ-Opioid Receptor System on Self-Grooming and Anhedonic-like Behaviors in Male Mice. J. Pharmacol. Exp. Ther. 370, 1–8. doi:10.1124/jpet.119.256354
Carlezon, W. A., Béguin, C., Dinieri, J. A., Baumann, M. H., Richards, M. R., Todtenkopf, M. S., et al. (2006). Depressive-like Effects of the Kappa-Opioid Receptor Agonist Salvinorin A on Behavior and Neurochemistry in Rats. J. Pharmacol. Exp. Ther. 316, 440–447. doi:10.1124/jpet.105.092304
Carroll, F. I., and Carlezon, W. A. (2013). Development of κ Opioid Receptor Antagonists. J. Med. Chem. 56, 2178–2195. doi:10.1021/jm301783x
Carter, J. S., Kearns, A. M., Vollmer, K. M., Garcia-Keller, C., Weber, R. A., Baker, N. L., et al. (2020). Long-term Impact of Acute Restraint Stress on Heroin Self-Administration, Reinstatement, and Stress Reactivity. Psychopharmacology (Berl) 237, 1709–1721. doi:10.1007/s00213-020-05486-z
Castagné, V., Moser, P., Roux, S., and Porsolt, R. D. (2011). Rodent Models of Depression: Forced Swim and Tail Suspension Behavioral Despair Tests in Rats and Mice. Curr. Protoc. Neurosci. 8, Unit 8 10A. doi:10.1002/0471142301.ns0810as55
Castagné, V., Porsolt, R. D., and Moser, P. (2009). Use of Latency to Immobility Improves Detection of Antidepressant-like Activity in the Behavioral Despair Test in the Mouse. Eur. J. Pharmacol. 616, 128–133. doi:10.1016/j.ejphar.2009.06.018
Cates, L. N., Roberts, A. J., Huitron-Resendiz, S., and Hedlund, P. B. (2013). Effects of Lurasidone in Behavioral Models of Depression. Role of the 5-HT₇ Receptor Subtype. Neuropharmacology 70, 211–217. doi:10.1016/j.neuropharm.2013.01.023
Chang, C., Byon, W., Lu, Y., Jacobsen, L. K., Badura, L. L., Sawant-Basak, A., et al. (2011). Quantitative PK-PD Model-Based Translational Pharmacology of a Novel Kappa Opioid Receptor Antagonist between Rats and Humans. AAPS J. 13, 565–575. doi:10.1208/s12248-011-9296-3
Chartoff, E. H., and Mavrikaki, M. (2015). Sex Differences in Kappa Opioid Receptor Function and Their Potential Impact on Addiction. Front. Neurosci. 9, 466. doi:10.3389/fnins.2015.00466
Chavkin, C. (2018). Kappa-opioid Antagonists as Stress Resilience Medications for the Treatment of Alcohol Use Disorders. Neuropsychopharmacology 43, 1803–1804. doi:10.1038/s41386-018-0046-4
Chen, L., Faas, G. C., Ferando, I., and Mody, I. (2015). Novel Insights into the Behavioral Analysis of Mice Subjected to the Forced-Swim Test. Transl Psychiatry 5, e551. doi:10.1038/tp.2015.44
Craft, R. M., and Bernal, S. A. (2001). Sex Differences in Opioid Antinociception: Kappa and 'mixed Action' Agonists. Drug Alcohol Depend 63, 215–228. doi:10.1016/s0376-8716(00)00209-x
De Laat, B., Goldberg, A., Shi, J., Tetrault, J. M., Nabulsi, N., Zheng, M. Q., et al. (2019). The Kappa Opioid Receptor Is Associated with Naltrexone-Induced Reduction of Drinking and Craving. Biol. Psychiatry 86, 864–871. doi:10.1016/j.biopsych.2019.05.021
Diester, C. M., Banks, M. L., Neigh, G. N., and Negus, S. S. (2019). Experimental Design and Analysis for Consideration of Sex as a Biological Variable. Neuropsychopharmacology 44, 2159–2162. doi:10.1038/s41386-019-0458-9
Falcon, E., Maier, K., Robinson, S. A., Hill-Smith, T. E., and Lucki, I. (2014). Effects of Buprenorphine on Behavioral Tests for Antidepressant and Anxiolytic Drugs in Mice. Psychopharmacology (Berl) 232, 907–915. doi:10.1007/s00213-014-3723-y
Guerrero, M., Urbano, M., Kim, E. K., Gamo, A. M., Riley, S., Abgaryan, L., et al. (2019). Design and Synthesis of a Novel and Selective Kappa Opioid Receptor (KOR) Antagonist (BTRX-335140). J. Med. Chem. 62, 1761–1780. doi:10.1021/acs.jmedchem.8b01679
Horan, P., Taylor, J., Yamamura, H. I., and Porreca, F. (1992). Extremely Long-Lasting Antagonistic Actions of Nor-Binaltorphimine (Nor-BNI) in the Mouse Tail-Flick Test. J. Pharmacol. Exp. Ther. 260, 1237–1243.
Huang, P., Tunis, J., Parry, C., Tallarida, R., and Liu-Chen, L. Y. (2016). Synergistic Antidepressant-like Effects between a Kappa Opioid Antagonist (LY2444296) and a delta Opioid Agonist (ADL5859) in the Mouse Forced Swim Test. Eur. J. Pharmacol. 781, 53–59. doi:10.1016/j.ejphar.2016.03.061
Jacobson, M. L., Browne, C. A., and Lucki, I. (2020a). Kappa Opioid Receptor Antagonists as Potential Therapeutics for Stress-Related Disorders. Annu. Rev. Pharmacol. Toxicol. 60, 615–636. doi:10.1146/annurev-pharmtox-010919-023317
Jacobson, M. L., Wulf, H. A., Browne, C. A., and Lucki, I. (2020b). The Kappa Opioid Receptor Antagonist Aticaprant Reverses Behavioral Effects from Unpredictable Chronic Mild Stress in Male Mice. Psychopharmacology (Berl) 237, 3715–3728. doi:10.1007/s00213-020-05649-y
Jacobson, M. L., Wulf, H. A., Tsuda, M. C., Browne, C. A., and Lucki, I. (2020c). Sex Differences in the Modulation of Mouse Nest Building Behavior by Kappa Opioid Receptor Signaling. Neuropharmacology 177, 108254. doi:10.1016/j.neuropharm.2020.108254
Knoll, A. T., and Carlezon, W. A. (2010). Dynorphin, Stress, and Depression. Brain Res. 1314, 56–73. doi:10.1016/j.brainres.2009.09.074
Laman-Maharg, A., Williams, A. V., Zufelt, M. D., Minie, V. A., Ramos-Maciel, S., Hao, R., et al. (2018). Sex Differences in the Effects of a Kappa Opioid Receptor Antagonist in the Forced Swim Test. Front. Pharmacol. 9, 93. doi:10.3389/fphar.2018.00093
Land, B. B., Bruchas, M. R., Schattauer, S., Giardino, W. J., Aita, M., Messinger, D., et al. (2009). Activation of the Kappa Opioid Receptor in the Dorsal Raphe Nucleus Mediates the Aversive Effects of Stress and Reinstates Drug Seeking. Proc. Natl. Acad. Sci. U S A. 106, 19168–19173. doi:10.1073/pnas.0910705106
Lanius, R. A., Boyd, J. E., Mckinnon, M. C., Nicholson, A. A., Frewen, P., Vermetten, E., et al. (2018). A Review of the Neurobiological Basis of Trauma-Related Dissociation and its Relation to Cannabinoid- and Opioid-Mediated Stress Response: a Transdiagnostic, Translational Approach. Curr. Psychiatry Rep. 20, 118. doi:10.1007/s11920-018-0983-y
Lin, Y., Suckow, R. F., Sarfraz, Y., and Stone, E. A. (2011). Further Evidence for an Immediate Antidepressant Action of Intracerebral Drug Administration in a Model of Chronic Depression. Int. J. Neuropsychopharmacol. 14, 691–696. doi:10.1017/S1461145710001161
Lowe, S. L., Wong, C. J., Witcher, J., Gonzales, C. R., Dickinson, G. L., Bell, R. L., et al. (2014). Safety, Tolerability, and Pharmacokinetic Evaluation of Single- and Multiple-Ascending Doses of a Novel Kappa Opioid Receptor Antagonist LY2456302 and Drug Interaction with Ethanol in Healthy Subjects. J. Clin. Pharmacol. 54, 968–978. doi:10.1002/jcph.286
Margolis, E. B., Wallace, T. L., Van Orden, L. J., and Martin, W. J. (2020). Differential Effects of Novel Kappa Opioid Receptor Antagonists on Dopamine Neurons Using Acute Brain Slice Electrophysiology. PLoS One 15, e0232864. doi:10.1371/journal.pone.0232864
Melief, E. J., Miyatake, M., Carroll, F. I., Béguin, C., Carlezon, W. A., Cohen, B. M., et al. (2011). Duration of Action of a Broad Range of Selective κ-opioid Receptor Antagonists Is Positively Correlated with C-Jun N-Terminal Kinase-1 Activation. Mol. Pharmacol. 80, 920–929. doi:10.1124/mol.111.074195
Molendijk, M. L., and De Kloet, E. R. (2019). Coping with the Forced Swim Stressor: Current State-Of-The-Art. Behav. Brain Res. 364, 1–10. doi:10.1016/j.bbr.2019.02.005
Naganawa, M., Zheng, M. Q., Henry, S., Nabulsi, N., Lin, S. F., Ropchan, J., et al. (2015). Test-retest Reproducibility of Binding Parameters in Humans with 11C-LY2795050, an Antagonist PET Radiotracer for the κ Opioid Receptor. J. Nucl. Med. 56, 243–248. doi:10.2967/jnumed.114.147975
Page, S., Mavrikaki, M. M., Lintz, T., Puttick, D., Roberts, E., Rosen, H., et al. (2019). Behavioral Pharmacology of Novel Kappa Opioid Receptor Antagonists in Rats. Int. J. Neuropsychopharmacol. 22, 735–745. doi:10.1093/ijnp/pyz054
Paris, J. J., Reilley, K. J., and Mclaughlin, J. P. (2011). Kappa Opioid Receptor-Mediated Disruption of Novel Object Recognition: Relevance for Psychostimulant Treatment. J. Addict. Res. Ther. S4, 007. doi:10.4172/2155-6105.s4174-400710.4172/2155-6105.S4-007
Placzek, M. S., Schroeder, F. A., Che, T., Wey, H. Y., Neelamegam, R., Wang, C., et al. (2018). Discrepancies in Kappa Opioid Agonist Binding Revealed through PET Imaging. ACS Chem. Neurosci. 10, 384–395. doi:10.1021/acschemneuro.8b00293
Porsolt, R. D., Bertin, A., and Jalfre, M. (1978). "Behavioural Despair" in Rats and Mice: Strain Differences and the Effects of Imipramine. Eur. J. Pharmacol. 51, 291–294. doi:10.1016/0014-2999(78)90414-4
Porsolt, R. D., Bertin, A., and Jalfre, M. (1977). Behavioral Despair in Mice: a Primary Screening Test for Antidepressants. Arch. Int. Pharmacodyn Ther. 229, 327–336.
Portoghese, P. S., Lipkowski, A. W., and Takemori, A. E. (1987). Binaltorphimine and Nor-Binaltorphimine, Potent and Selective Kappa-Opioid Receptor Antagonists. Life Sci. 40, 1287–1292. doi:10.1016/0024-3205(87)90585-6
Reed, B., Butelman, E. R., Fry, R. S., Kimani, R., and Kreek, M. J. (2017). Repeated Administration of Opra Kappa (LY2456302), a Novel, Short-Acting, Selective KOP-R Antagonist, in Persons with and without Cocaine Dependence. Neuropsychopharmacology 43, 739–750. doi:10.1038/npp.2017.205
Reed, B., Fang, N., Mayer-Blackwell, B., Chen, S., Yuferov, V., Zhou, Y., et al. (2012). Chromatin Alterations in Response to Forced Swimming Underlie Increased Prodynorphin Transcription. Neuroscience 220, 109–118. doi:10.1016/j.neuroscience.2012.06.006
Rorick-Kehn, L. M., Witkin, J. M., Statnick, M. A., Eberle, E. L., Mckinzie, J. H., Kahl, S. D., et al. (2014). LY2456302 Is a Novel, Potent, Orally-Bioavailable Small Molecule Kappa-Selective Antagonist with Activity in Animal Models Predictive of Efficacy in Mood and Addictive Disorders. Neuropharmacology 77, 131–144. doi:10.1016/j.neuropharm.2013.09.021
Rosa, S. G., Pesarico, A. P., Martini, F., and Nogueira, C. W. (2018). m-Trifluoromethyl-diphenyl Diselenide Regulates Prefrontal Cortical MOR and KOR Protein Levels and Abolishes the Phenotype Induced by Repeated Forced Swim Stress in Mice. Mol. Neurobiol. 55:8991–9000. doi:10.1007/s12035-018-1024-x
Shirayama, Y., Ishida, H., Iwata, M., Hazama, G. I., Kawahara, R., and Duman, R. S. (2004). Stress Increases Dynorphin Immunoreactivity in Limbic Brain Regions and Dynorphin Antagonism Produces Antidepressant-like Effects. J. Neurochem. 90, 1258–1268. doi:10.1111/j.1471-4159.2004.02589.x
Stone, E. A., Lin, Y., and Quartermain, D. (2008). Evaluation of the Repeated Open-Space Swim Model of Depression in the Mouse. Pharmacol. Biochem. Behav. 91, 190–195. doi:10.1016/j.pbb.2008.07.006
Valenza, M., Butelman, E. R., and Kreek, M. J. (2017). "Effects of the Novel Relatively Short-Acting Kappa Opioid Receptor Antagonist LY2444296 in Behaviors Observed after Chronic Extended-Access Cocaine Self-Administration in Rats". Psychopharmacology (Berl) 234, 2219–2231. doi:10.1007/s00213-017-4647-0
Valenza, M., Windisch, K. A., Butelman, E. R., Reed, B., and Kreek, M. J. (2020). Effects of Kappa Opioid Receptor Blockade by LY2444296 HCl, a Selective Short-Acting Antagonist, during Chronic Extended Access Cocaine Self-Administration and Re-exposure in Rat. Psychopharmacology (Berl) 237, 1147–1160. doi:10.1007/s00213-019-05444-4
Varlinskaya, E. I., Johnson, J. M., Przybysz, K. R., Deak, T., and Diaz, M. R. (2020). Adolescent Forced Swim Stress Increases Social Anxiety-like Behaviors and Alters Kappa Opioid Receptor Function in the Basolateral Amygdala of Male Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 98, 109812. doi:10.1016/j.pnpbp.2019.109812
Williams, A. V., Laman-Maharg, A., Armstrong, C. V., Ramos-Maciel, S., Minie, V. A., and Trainor, B. C. (2018). Acute Inhibition of Kappa Opioid Receptors before Stress Blocks Depression-like Behaviors in California Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 86, 166–174. doi:10.1016/j.pnpbp.2018.06.001
Zhang, H., Shi, Y. G., Woods, J. H., Watson, S. J., and Ko, M. C. (2007). Central Kappa-Opioid Receptor-Mediated Antidepressant-like Effects of Nor-Binaltorphimine: Behavioral and BDNF mRNA Expression Studies. Eur. J. Pharmacol. 570, 89–96. doi:10.1016/j.ejphar.2007.05.045
Keywords: open space swim, kappa-opioid receptor, stress, acute stress disorder, dynorphin
Citation: Baynard C, Prisinzano TE and Butelman ER (2021) Rapid-Onset Anti-Stress Effects of a Kappa-Opioid Receptor Antagonist, LY2795050, Against Immobility in an Open Space Swim Paradigm in Male and Female Mice. Front. Pharmacol. 12:775317. doi: 10.3389/fphar.2021.775317
Received: 13 September 2021; Accepted: 08 November 2021;
Published: 22 November 2021.
Edited by:
Wendy Margaret Walwyn, University of California, Los Angeles, United StatesReviewed by:
Brian Trainor, University of California, Davis, United StatesAnushree N Karkhanis, Binghamton University, United States
Glenn Valdez, Grand Valley State University, United States
Copyright © 2021 Baynard, Prisinzano and Butelman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eduardo R. Butelman, YnV0ZWxtZUByb2NrZWZlbGxlci5lZHU=
In memory of Mary Jeanne Kreek, MD
 Caroline Baynard1
Caroline Baynard1 Thomas E. Prisinzano
Thomas E. Prisinzano Eduardo R. Butelman
Eduardo R. Butelman