- 1Department of Pharmacy, The Fifth Medical Center of PLA General Hospital, Beijing, China
- 2College of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Objective: This study was intended to provide data to support the effect of Shashen Maidong Decoction in improving mycoplasma pneumonia in pediatric patients through systematic evaluation.
Methods: PubMed, the Web of Science, EMbase, CNKI, CQVIP, Wan-Fang, and CBM databases were comprehensively searched from established in June 2021. Randomized controlled trials of TRQI were selected by screening the literature and extracting information. The Cochrane RCT Evaluation Manual was used to evaluate the methodological quality of all included studies, and Meta-analysis was performed using Stata 14.0 and Review Manager 5.4 software.
Results: A total of 1,127 patients from 12 clinical studies met the inclusion criteria. Meta-analysis results showed that the treatment group of Shashen Maidong Decoction was able to significantly increase the overall efficiency level and significantly reduce the incidence of adverse reactions, time for disappearance of cough, time for relief of cough, time for defervescence, time for disappearance of lung rales, time for return to normal of chest X-ray, T lymphocyte subpopulation (CD3+) and tumor necrosis factor-α (TNF-α) and other index levels (p < 0.05).
Conclusion: Shashen Maidong Decoction has a significant improvement in the levels of relevant indexes in pediatric mycoplasma pneumonia, which provides a basis for the safety and efficacy of pediatric mycoplasma pneumonia. However, due to the small sample size included in the study, the study quality was not high, and more randomized controlled trials of high quality are required for further validation.
Introduction
Mycoplasma pneumoniae (MP) is one of the most common causes of community-acquired pneumonia (CAP) in children, and studies have shown that the incidence of pediatric mycoplasma pneumonia (MPP) accounts for approximately 25% of all CAP (Lee et al., 2018; Jain et al., 2015; Wang, 2017). MP is a highly evolved and polymorphic bacterial pathogen without a cell wall (Guo et al., 2019). It has a wide range of clinical manifestations, including upper respiratory tract infections, pneumonia, and extrapulmonary manifestations (such as encephalitis) (Kutty et al., 2019). The most common pathological manifestation of MPP is characterized by mononuclear cell infiltration in the bronchi and perivascular areas and thickening of the bronchovascular bundles (Tanaka, 2016). Currently, macrolide antibiotics (MA) such as azithromycin (AZM) are preferred in Western medicine for the treatment of pediatric MMP (Ni, 2019), however, epidemiological results in recent years have shown that resistant MP strains of macrolides are increasing year by year, leading to an increase in the number of critically ill and refractory patients or a prolonged course of disease (Wu and Wu, 2016). Therefore, there is an urgent need to find alternative drugs under the theoretical system of TCM. In the theoretical system of TCM, there is no concept of MP, which is considered by TCM to be related to the delicate lungs, lack of strengthening of the external guard, and sensation of wind evils (Jiang et al., 2017). Chinese medicine is gradually showing the unique advantages of motherland medicine in the treatment of MPP, and MPP can achieve better efficacy after the diagnosis and treatment of Chinese medicine, especially in relieving symptoms, reducing cough, and shortening the course of the disease (Tan and Jiang, 2018). The effectiveness of TCM in the treatment of mycoplasma pneumonia has been shown (Shi and Yang, 2019), such as Yangyin Qingfei Decoction (YYQFD) (Lin, 2020) and Shaoyao Gancao Decoction (SGD) (Liu et al., 2020) for chronic cough following mycoplasma pneumonia in pediatric patients. The main effects of Shashen Maidong Decoction (SMD), which is derived from the “Article of Warming Diseases” written by Jutong Wu, a famous doctor in the Qing Dynasty, is mainly composed of 7 kinds of Chinese medicinal materials (Table 1), are to generate fluid and moisten dryness and to clear the lung and stomach and is clinically applied to symptoms of warming evil injuring the lung and deficiency of lung yin and heat (Zhou, 2017). Xiaoxia Shi (Shi, 2011) and Jing Xu (Xu, 2012) et al. found the effectiveness of SMD with addition and subtraction in the treatment of mycoplasma pneumonia-related conditions. Thus suggesting that SMD could be used as a new treatment for MP in pediatric patients.
Therefore, this study used the method of Meta-analysis, aiming to systematically analyze the therapeutic effect of SMD on pediatric MPP in clinical practice based on clinical studies and to provide a reference basis for its related subsequent clinical studies.
Materials and Methods
Literature Source and Search Strategy
PubMed, Web of Science, EMbase, CNKI, CQVIP, Wan-Fang, and CBM databases were comprehensively searched from established to June 2021. The clinical experimental studies related to SMD, Supplemented of SMD, pediatric mycoplasma pneumonia, and Mycoplasma pneumonia were searched using a combination of subject terms and free words. The English search terms included: Shashen Maidong Decoction, Shashen Maidong Decoctionrosis, SMD, MMP, Mycoplasma pneumonia, MP; the Chinese search terms included: Shashen Maidong Decoction, Supplemented of Shashen Maidong Decoction, pediatric mycoplasma pneumonia, Mycoplasma pneumonia.
Inclusion and Exclusion Criteria
Study Type
Randomized controlled trial (RCT).
Research Subjects
Children with mycoplasma pneumonia.
Interventions
The experimental group used SMD alone or combined with SMD (such as ACU or SGD); the control group used conventional treatment (such as azithromycin) or conventional treatment + YYQFD.
Observation Index
1) total efficiency: If the clinical symptoms and objective indexes of the child disappeared and returned to normal, it was regarded as a cure; if the clinical symptoms and objective indexes of the child improved, it was regarded as an improvement. If the clinical symptoms and objective indexes do not change or are aggravated or there are other side effects, the children can be judged to be in an ineffective state. Total effective rate = (number of cured patients + number of improved patients)/total number of patients× 100%;
2) incidence of adverse reactions;
3) time for relief or disappearance of clinical symptoms: time for disappearance of cough, time for relief of cough, time for disappearance of lung rales, time for defervescence, time for return to normal of chest X-ray;
4) other outcome indicators: T lymphocyte subpopulation (CD3+), tumor necrosis factor-α (TNF-α).
Exclusion Criteria
The exclusion criteria are as follows: 1) studies with systematic evaluation or Meta-analysis; 2) primary and secondary outcome indicators not included in the full text or insufficient data; 3) incomplete articles and duplicate publications; 4) literature not in Chinese or English; 5) reviews, conference abstracts, and animal experiments.
Data Extraction
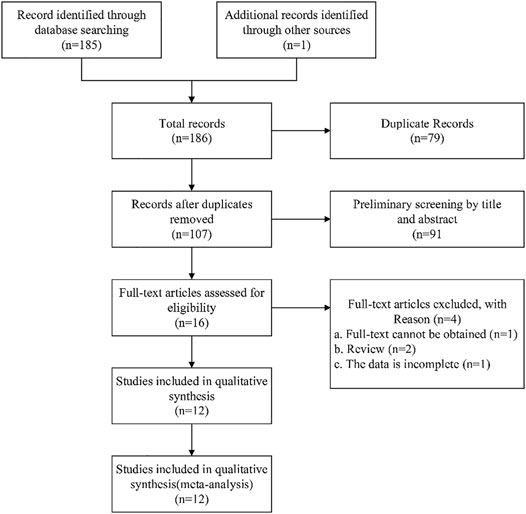
More complete data extraction and collection were performed for all included clinical studies. The following basic data were extracted from all included clinical studies: 1) year of publication and first author’s name; 2) sample size of experimental and control groups; 3) age of children; 4) overall sex ratio; 5) interventions; 6) treatment duration; (g) main outcome indicators. Details of all studies are shown in Table 2.
Quality Assessment
The two researchers who assessed the quality of the literature did so according to the Cochrane Collaboration’s risk of bias criteria (Higgins et al., 2011): random sequence generation (selection bias), allocation concealment (selection bias), investigator and subject blinding (implementation bias), outcome evaluator blinding (measurement bias), incomplete outcome data (follow-up bias), selective outcome reporting (reporting bias), and other biases. Both investigators independently reviewed each study, and the final results were expressed as “yes,” “no,” and “inconclusive,” with “yes " represents low-risk bias, “No” represents high-risk bias, and “Uncertain” represents uncertain risk bias.
Statistical Analysis
The full Meta-analysis was performed using STATA 14.0 and Revman 5.4. For dichotomous variables, we used 95% confidence intervals (95% CI) to calculate the risk ratio (RR) and 95% CI to calculate the mean difference (MD) for continuous outcomes. The χ2 test and I2 test were used to evaluate whether the data were heterogeneous. If p < 0.05 or I2 > 50%, the combined data were considered heterogeneous and a random-effects model was used; otherwise, a fixed-effects model was used. We examined the effect of different sample sizes and dosing regimens on the total effective rate by subgroup analysis. In addition, sensitivity analyses were used to investigate the effect of a high-risk study on the overall Mate analysis. Publication bias was analyzed for all included studies using funnel plots and Egger’s test.
Result
Literature Selection Process and Results
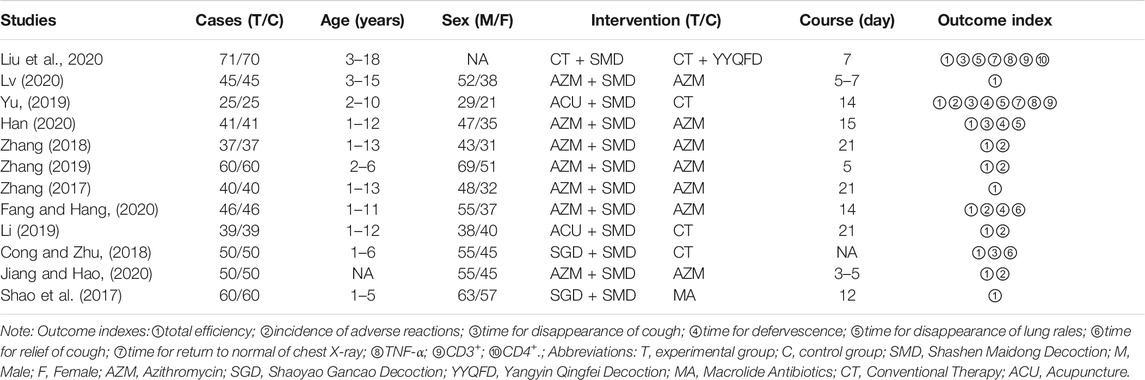
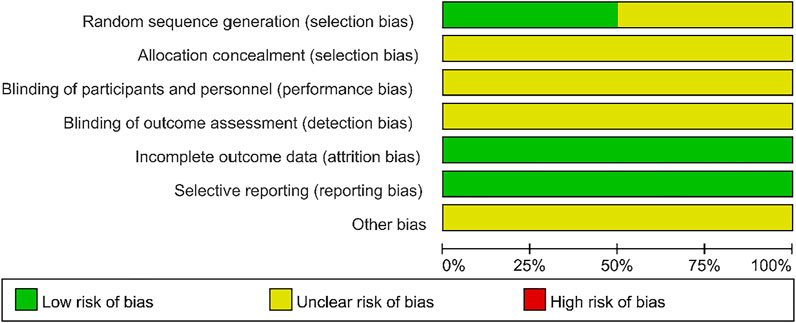
The selection process for selecting eligible studies according to the flow chart is shown in Figure 1. A total of 186 articles were searched through the database, and 107 studies were recorded after removing 79 duplicates. The remaining 16 full-text articles were assessed for eligibility by excluding 91 articles. Among them, one full-text article was not available, two review articles, and one article with incomplete data. Finally, 12 studies that met the inclusion criteria were included (Figure 1).
Basic Characteristics of the Included Studies
The baseline characteristics of all included studies are shown in Table 2. All studies are randomized controlled trials. A total of 1,127 children participated in 12 studies (Shao et al., 2017; Zhang, 2017; Cong and Zhu, 2018; Zhang, 2018; Li, 2019; Yu, 2019; Zhang, 2019; Fang and Hang, 2020; Han, 2020; Jiang and Hao, 2020; Liu et al., 2020; Lv, 2020), including the experimental group (n = 564) and the control group (n = 563). The age range of patients is 1–18 years old. The subjects of the study were children suffering from mycoplasma pneumonia. A total of 12 studies (Shao et al., 2017; Zhang, 2017; Cong and Zhu, 2018; Zhang, 2018; Li, 2019; Yu, 2019; Zhang, 2019; Fang and Hang, 2020; Han, 2020; Jiang and Hao, 2020; Liu et al., 2020; Lv, 2020) reported the results of clinical efficacy; six studies (Zhang, 2018; Li, 2019; Yu, 2019; Zhang, 2019; Fang and Hang, 2020; Jiang and Hao, 2020) involved adverse reactions; four studies (Cong and Zhu, 2018; Yu, 2019; Han, 2020; Liu et al., 2020) reported the measurement results of the cough disappearance time; three studies (Yu, 2019; Fang and Hang, 2020; Han, 2020) reported the measurement results of the defervescence time; three studies (Yu, 2019; Han, 2020; Liu et al., 2020) reported the measurement results of the disappearance time of rales in the lungs; two studies (Cong and Zhu, 2018; Fang and Hang, 2020) reported the measurement results of the cough relief time; two studies (Yu, 2019; Liu et al., 2020) reported the measurement results of the return to normal of chest X ray time; two studies (Yu, 2019; Liu et al., 2020) Reported the results of the determination of TNF-α; two studies (Yu, 2019; Liu et al., 2020) reported the results of the determination of CD3+.
Risk of Bias of Included Trials
All 12 studies were randomized controlled trials. Six studies (Liu et al., 2020; Zhang, 2018; Zhang, 2019; Zhang, 2017; Fang and Hang, 2020 et al., 2020; Li, 2019) reported the generation of random sequences: four studies (Zhang, 2017; Li, 2019; Fang and Hang, 2020; Liu et al., 2020) used the random number table method; one study (Zhang, 2018) used the parity number method; one study (Zhang, 2019) used the lottery method. All studies had no incomplete outcome data and no selective outcomes were reported. However, the following four aspects were unclear: allocation concealment; whether investigators and subjects were blinded; whether blinding was imposed by outcome evaluators; and whether other biases were present. The results of the risk of bias assessment for the included experiments are shown in Figure 2.
Meta-Analysis of SMD in the Treatment of Pediatric Mycoplasma Pneumonia
Ending Measurement Indicators
Effectiveness: Total Efficiency
Twelve clinical studies (Shao et al., 2017; Zhang, 2017; Cong and Zhu, 2018; Zhang, 2018; Li, 2019; Yu, 2019; Zhang, 2019; Fang and Hang, 2020; Han, 2020; Jiang and Hao, 2020; Liu et al., 2020; Lv, 2020) reported the total efficiency of SMD in the treatment of mycoplasma pneumonia in children. Meta-analysis showed that there was no obvious heterogeneity in the index level of total effective rate (p = 0.162, I2 = 28.9%). Therefore, the fixed effects model is selected for Meta-analysis. The results showed that the total effective rate of the experimental group in the treatment of mycoplasma pneumonia in children was compared with that of the control group (RR = 1.22, 95%CI 1.16–1.28, p ≤ 0.001), the difference was statistically significant (Figure 3).
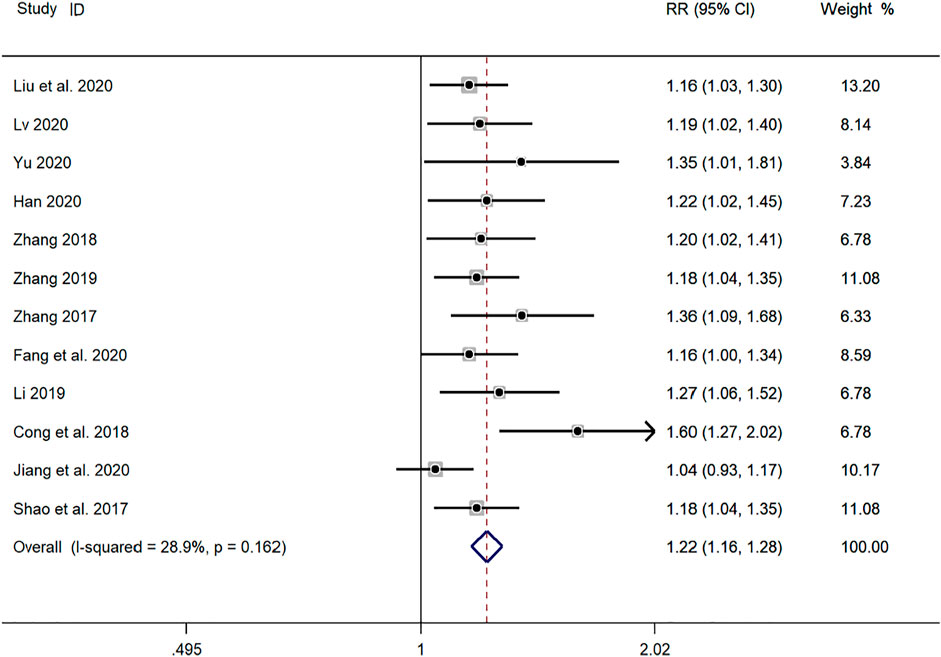
FIGURE 3. Meta-analysis forest plot comparing the total efficiency level of the experimental group and the control group.
Indicators of Relief or Disappearance of Different Symptoms of the Disease
Time for Relief of Cough
Two clinical studies (Cong and Zhu, 2018; Fang and Hang, 2020) reported the cough relief time of SMD in the treatment of mycoplasma pneumonia in children. Meta-analysis showed that the index level of cough remission time is obviously heterogeneous (p ≤ 0.001, I2 = 96.9%). Therefore, the random-effects model is selected for Meta-analysis. The results showed that the cough relief time of the experimental group in the treatment of children with mycoplasma pneumonia was compared with that of the control group (SMD = −2.25, 95%CI −4.39 to −0.10, p = 0.040), and the difference was statistically significant (Figure 4).
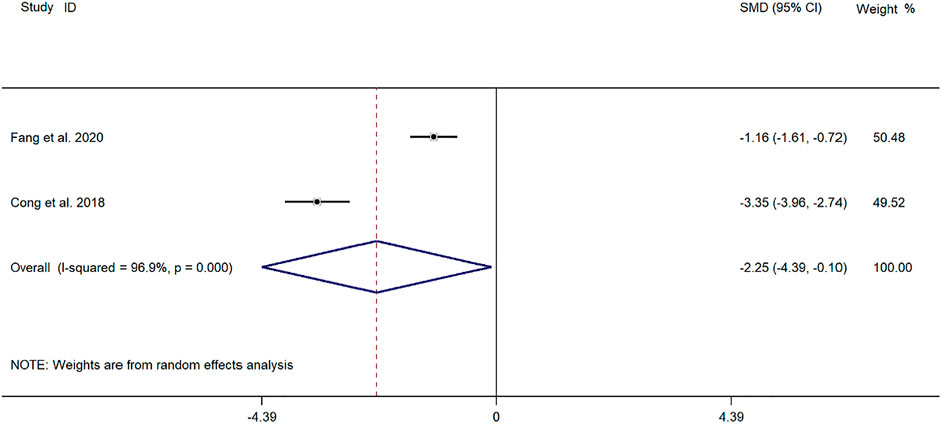
FIGURE 4. Meta-analysis forest plot comparing the time for relief of cough in the experimental and control group.
Time for Disappearance of Cough
Four clinical studies (Cong and Zhu, 2018; Yu, 2019; Han, 2020; Liu et al., 2020) reported the cough disappearance time of SMD in the treatment of children with mycoplasma pneumonia. Meta-analysis showed that the index level of cough remission time is obviously heterogeneous (p ≤ 0.001, I2 = 86.4%). Therefore, the random-effects model is selected for Meta-analysis. The results showed that the cough disappearance time of the experimental group in the treatment of children with mycoplasma pneumonia was compared with that of the control group (SMD = −2.02, 95%CI −2.72 to −1.32, p ≤ 0.001), and the difference was statistically significant (Figure 5).
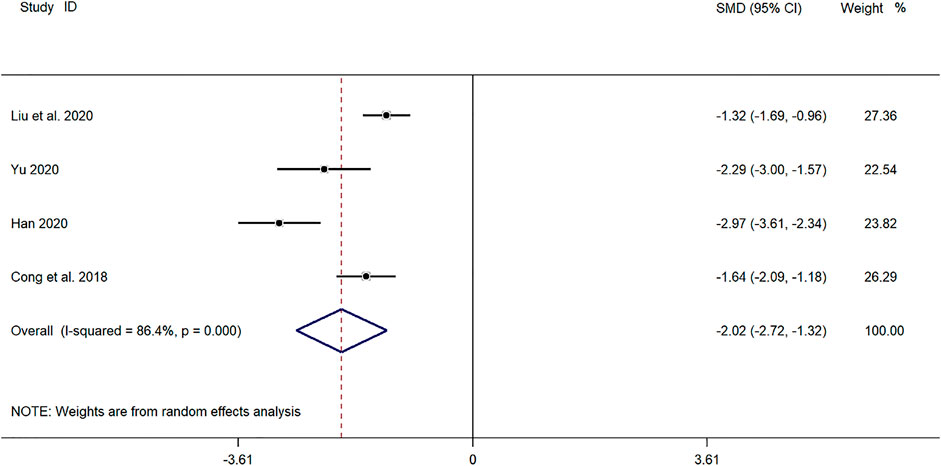
FIGURE 5. Meta-analysis of forest plots comparing the time for disappearance of cough in the experimental and control group.
Time for Disappearance of Lung Rales
Three clinical studies (Yu, 2019; Han, 2020; Liu et al., 2020) reported the disappearance time of lung rales in the treatment of Mycoplasma pneumonia in children with SMD. Meta-analysis showed that the index level of cough remission time is obviously heterogeneous (p ≤ 0.001, I2 = 91.8%). Therefore, the random-effects model is selected for Meta-analysis. The results showed that the disappearance time of pulmonary rales in the treatment of children with mycoplasma pneumonia in the experimental group was compared with that in the control group (SMD = −2.21, 95%CI −3.35 to −1.07, p ≤ 0.001), and the difference was statistically significant (Figure 6).
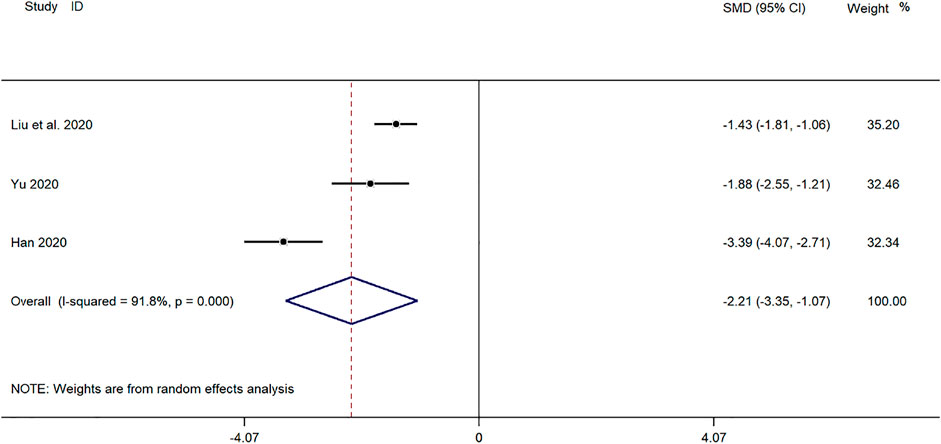
FIGURE 6. Meta-analysis of forest plots comparing the time for disappearance of lung rales in the experimental and control group.
Time for Return to Normal of Chest X-Ray
Two clinical studies (Yu, 2019; Liu et al., 2020) reported the time for return to normal of chest X-ray in the treatment of mycoplasma pneumonia in children with SMD. Meta-analysis showed that there was no obvious heterogeneity in the index level of chest X-ray recovery time (p = 0.253, I2 = 23.5%). Choose a fixed-effects model for Meta-analysis. The results showed that the disappearance time of pulmonary rales in the treatment of children with mycoplasma pneumonia in the experimental group was compared with that in the control group (SMD = −1.93, 95%CI −2.28 to −1.59, p ≤ 0.001), and the difference was statistically significant (Figure 7).
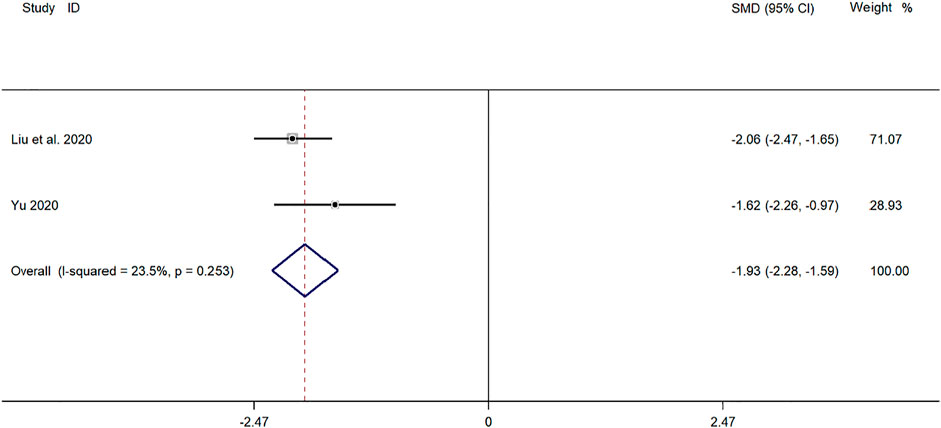
FIGURE 7. Meta-analysis of forest plots comparing the time for return to normal of chest X-ray in the experimental and control group.
Time for Defervescence
Three clinical studies (Yu, 2019; Fang and Hang, 2020; Han, 2020) reported the time for defervescence in the treatment of pediatric mycoplasma pneumonia with SMD. Meta-analysis showed significant heterogeneity in the level of defervescence time indicators (p ≤ 0.001, I2 = 88.3%). A random-effects model was selected for Meta-analysis. The results showed a statistically significant difference in the time for defervescence in the treatment of pediatric mycoplasma pneumonia in the experimental group was compared with that in the control group (SMD = −1.58, 95% CI −2.49 to −0.27, p ≤ 0.001), and the difference was statistically significant (Figure 8).
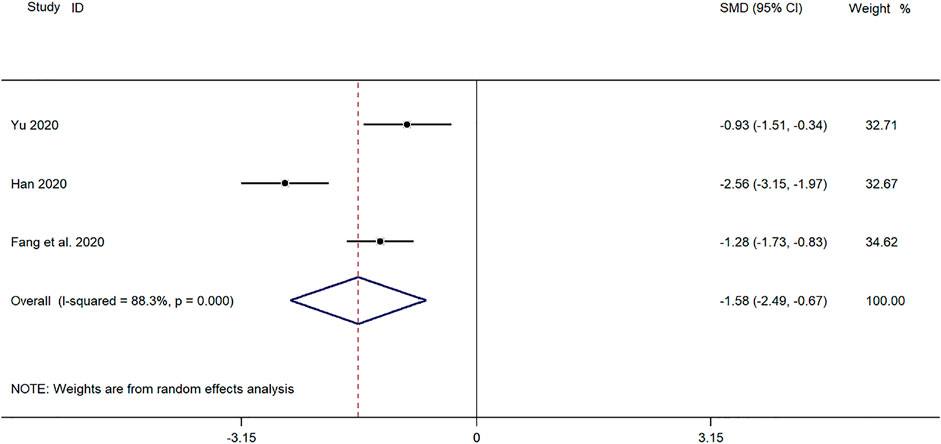
FIGURE 8. Meta-analysis forest plot comparing the time for defervescence in the experimental and control group.
CD3+ Cell Levels
Two clinical studies (Yu, 2019; Liu et al., 2020) reported CD3+ levels in the treatment of pediatric mycoplasma pneumonia with SMD. Meta-analysis showed significant heterogeneity in CD3+ index levels (p = 0.001, I2 = 90.4%). A random-effects model was selected for Meta-analysis. The results showed a statistically significant difference in CD3+ cell levels in the treatment of pediatric mycoplasma pneumonia in the experimental group was compared with that in the control group (SMD = 1.71, 95% CI 0.40 to 3.03, p ≤ 0.001), and the difference was statistically significant (Figure 9).
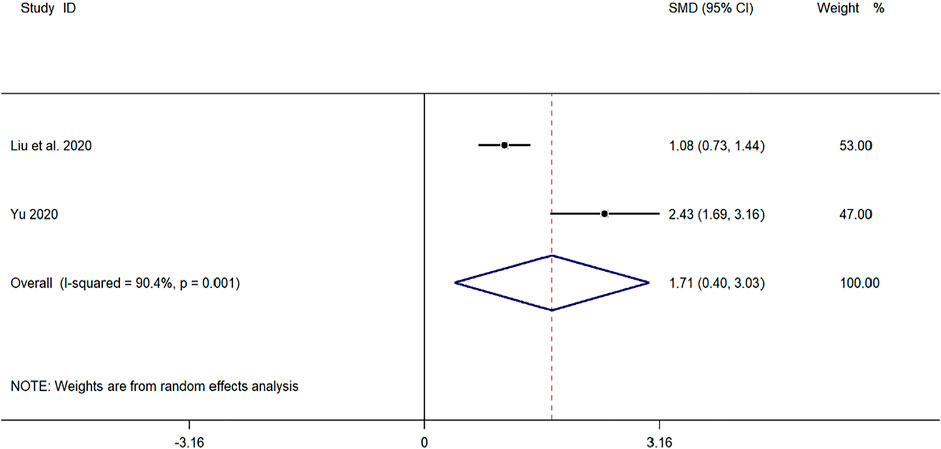
FIGURE 9. Meta-analysis of forest plots comparing CD4+ cell levels in the experimental and control group.
TNF-α Levels
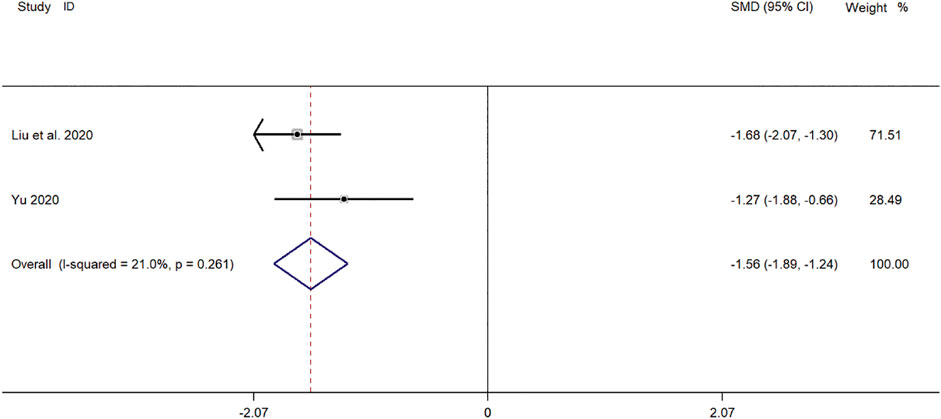
Two clinical studies (Yu, 2019; Liu et al., 2020) reported TNF-α levels in SMD for pediatric mycoplasma pneumonia. Meta-analysis showed significant heterogeneity in TNF-α index levels (p = 0.261, I2 = 21.0%). A fixed-effect model was selected for Meta-analysis. The results showed a statistically significant difference in TNF-α levels in the treatment of pediatric mycoplasma pneumonia in the experimental group was compared with that in the control group (SMD = −1.58, 95% CI −1.89 to −1.24, p ≤ 0.001), and the difference was statistically significant (Figure 10).
Safety: Incidence of Adverse Reactions
Six clinical studies (Zhang, 2018; Li, 2019; Yu, 2019; Zhang, 2019; Fang and Hang, 2020; Jiang and Hao, 2020) reported the incidence of adverse reactions in the treatment of pediatric mycoplasma pneumonia with SMD. Meta-analysis showed no significant heterogeneity in the level of indicators of adverse reaction incidence (p = 0.908, I2 = 0.0%). Therefore, a fixed-effect model was selected for Meta-analysis. The results showed a statistically significant difference in the incidence of adverse reactions in the treatment of pediatric mycoplasma pneumonia in the experimental group was compared with that in the control group (RR = 0.18, 95% CI 0.10–0.35, p ≤ 0.001), and the difference was statistically significant (Figure 11).
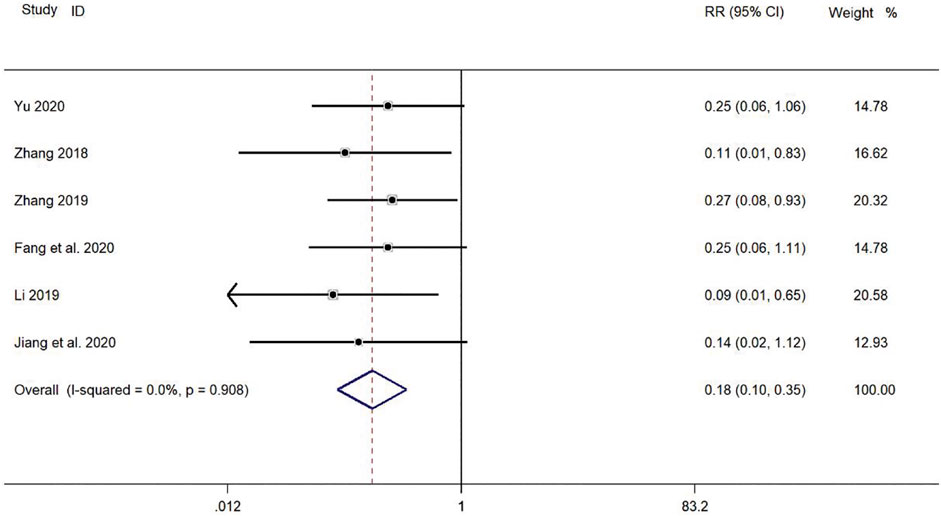
FIGURE 11. Meta-analysis forest plot comparing the level of incidence of adverse reactions in the experimental and control group.
Subgroup Analysis of Relevant Indicators
Subgroup Analysis of Different Treatment Duration
Five clinical studies (Shao et al., 2017; Zhang, 2019; Jiang and Hao, 2020; Liu et al., 2020; Lv, 2020) with a duration of <14 days and six clinical studies (Zhang, 2017; Zhang, 2018; Li, 2019; Yu, 2019; Fang and Hang, 2020; Han, 2020) with a duration of ≥14 days reported the total efficiency level of SMD in the treatment of pediatric mycoplasma pneumonia. Meta-analysis showed no significant heterogeneity in the level of the total efficiency index (p = 0.624, I2 = 0.0%). A fixed-effects model was selected for Meta-analysis. The results showed that the total efficiency level of both courses of treatment in the experimental group was significantly higher than that of both courses of treatment in the control group (RR = 1.19, 95% CI 1.14–1.25, p ≤ 0.001), and the difference was statistically significant (Figure 12).
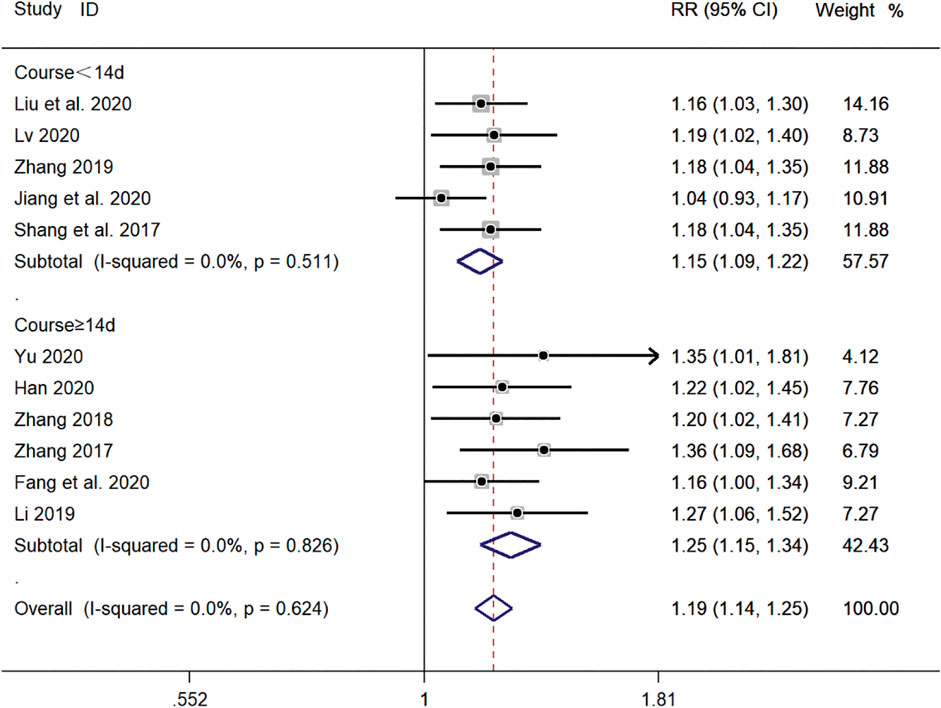
FIGURE 12. Meta-analysis forest plot comparing the total efficiency level of the experimental group with the control group for subgroup analysis of different treatment duration.
Subgroup Analysis of Different Treatment Methods
Eight clinical studies (Zhang, 2017; Zhang, 2018; Zhang, 2019; Fang and Hang, 2020; Han, 2020; Jiang and Hao, 2020; Liu et al., 2020; Lv, 2020) using WM + SMD and four clinical studies (Shao et al., 2017; Cong and Zhu, 2018; Li, 2019; Yu, 2019) using TCM + SMD, for a total of 12 publications, reported the total efficiency level of SMD in the treatment of pediatric mycoplasma pneumonia. Meta-analysis showed no significant heterogeneity in the level of the total efficiency index (p = 0.162, I2 = 28.9%). A fixed-effects model was selected for Meta-analysis. The results showed that the total efficiency of WM + SMD in the experimental group was significantly higher than that of TCM + SMD in the control group (RR = 1.22, 95% CI 1.16–1.28, p ≤ 0.001), and the difference was statistically significant (Figure 13).
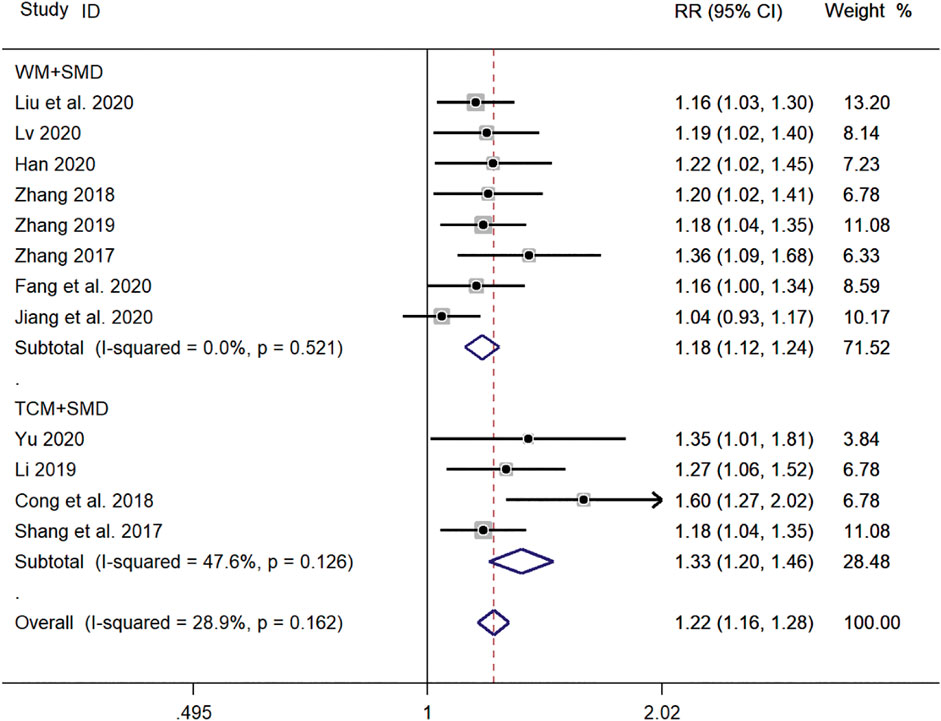
FIGURE 13. Meta-analysis forest plot comparing the total efficiency levels of the experimental group with the control group for subgroup analysis of different treatment methods. Note: SMD, Shashen Maidong Decoction; WM, Western Medicine; TCM, Traditional Chinese Medicine.
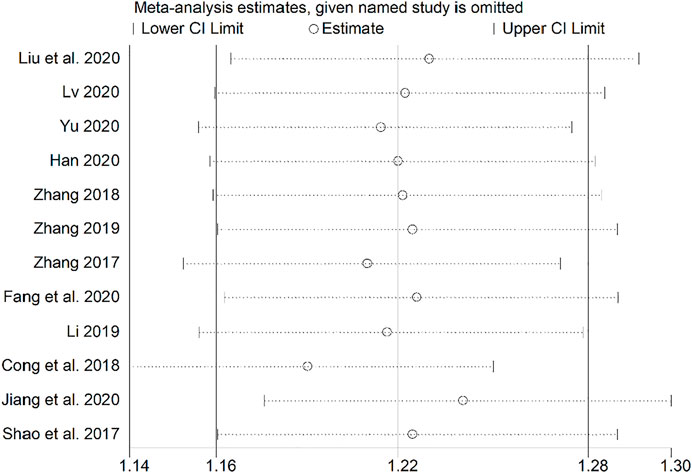
Sensitivity Analysis
Sensitivity analysis was performed using a study-by-study exclusion method, and none of the results changed significantly, suggesting more stable results (Figure 14).
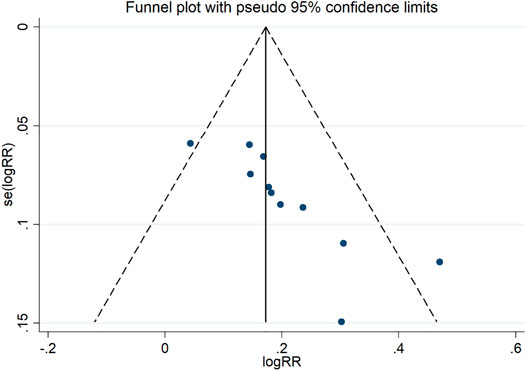
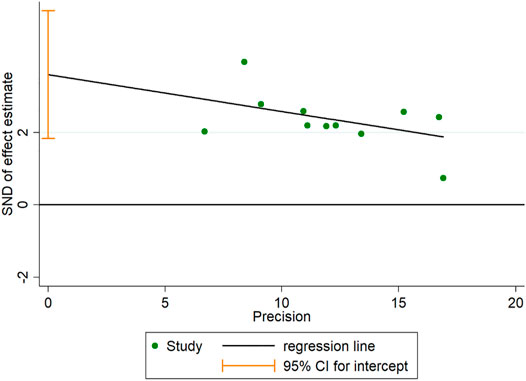
Publication Bias
A funnel plot for publication bias test for the outcome indicator of the total efficiency showed an asymmetric left-right distribution across study sites (Figure 15), with an Egger’s test result of p = 0.001 (Figure 16), suggesting the existence of publication bias.
Discussion
Mycoplasma pneumoniae (MP) infections occur frequently in different countries and regions, and bronchitis and pneumonia are the most common clinical diagnoses associated with MP infections (Defilippi et al., 2008). MP is the main pathogen of MPP, and modern medical studies have confirmed that macrolide antibiotics can significantly improve the cure rate of this disease (Qiu et al., 2020). However, over the past decade, macrolide resistant Mycoplasma pneumoniae has shown epidemic trends worldwide, the most severe of which is in Asia, where prevalence rates range from 13.3 to 100%, and can even reach 90% in Japan at certain times of the year (Chen et al., 2020), and nearly 80% in Korea (Lee et al., 2021). In China, more than 85% of Mycoplasma pneumoniae (MP) strains in pediatric patients have been reported as macrolide-resistant Mycoplasma pneumoniae (Guo et al., 2019). This bacterium is also one of the most common causes of CAP infection (Atkinson et al., 2008). The clinical features of patients are mainly headache, cough, and sore throat, and the short onset and worse condition are the characteristics of their disease, which may manifest as dyspnea, wheezing, and coughing when it worsens, and if left untreated, can lead to substantial organ lesions that can recur (Han et al., 2018), so the prevention and treatment of this disease is necessary and urgent.
SMD is mainly composed of Glycyrrhiza uralensis Fisch. ex DC (Fabaceae; Glycyrrhiza uralensis radix & rhizome), Polygonatum odoratum (Mill.) Druce (Asparagaceae; Polygonatum odoratum rhizome), Adenophora stricta Miq. (Zingiberaceae; Alpinia oxyphylla fruit), Vicia lens subsp. Lens (Fabaceae; Vicia lens subsp. Lens seed), Ophiopogon japonicus (Thunb.) Ker Gawl. (Asparagaceae; Ophiopogon japonicus radix), Trichosanthes kirilowii Maxim. (Cucurbitaceae; Trichosanthes kirilowii radix) and Morus alba L. (Moraceae; Morus alba leaf). We have finally determined the preparation method (Yan et al., 2021) and administration method (He et al., 2021) of SMD by consulting the literature. Preparation method: Weigh 11.19 g each of Sha Shen and Mai Dong, 5.60 g each of Sang Ye, Tian Hua Fen, and Sheng Bian Dou, 7.46 g of Yu Zhu, 3.73 g of Gan Cao, add 1 L of water, decoct until the remaining 400 ml, filter the medicinal residues, and get the SMD. Method of administration: Take 200 ml of decoction, take it warmly, 2 times a day, once in the morning and once in the evening, 1 dose a day. The specific stopping or reducing time depends on the condition of the disease (He et al., 2021). It is mainly used in traditional Chinese medicine to treat “yin deficiency, dryness, heat and lung injury, fever and cough” (Yang and Zhou, 2019). By studying the HPLC fingerprint of SMD, the significance of the quality evaluation of SMD has been improved (Yan et al., 2021). Modern research shows that the pharmacological effects of SMD are mainly reflected in anti-inflammatory, immune enhancement, gastric mucosal protection, inhibition of gastric hyperactivity, anti-oxidation, anti-tumor, etc., (Gao et al., 2020), and its main components such as Adenophora polysaccharides (Zheng et al., 2012), Ophiopogon japonicus polysaccharide (Sun et al., 2021), Mulberry leaf polysaccharide (Wang and Xiao, 2020) and Trichosanthin (Ouyang and Wu, 2021) can regulate the inflammatory environment. Therefore, it can provide reliable preclinical evidence for the treatment of mycoplasma pneumonia in children with SMD.
Systematic review and meta-analysis is a widely accepted research method and is at the top of the hierarchy of clinical evidence (Izzo et al., 2016). However, there is no clinical evidence evaluating the safety and efficacy of SMD in the treatment of pediatric mycoplasma pneumonia in current evidence-based medical studies.
According to the analysis of the overall research results of mate analysis, there is an interesting phenomenon: when the experimental group contains SMD, the relevant index levels are better than those of the control group; when both the experimental group and the control group have SMD, The observation group treated with traditional Chinese medicine has better-related index levels than the control group treated with western medicine. This can at least explain that SMD and traditional Chinese medicine are more effective than western medicine in the treatment of childhood mycoplasma pneumonia.
In this study, a total of 12 clinical studies were included, and a total of 1,127 patients with mycoplasma pneumonia were treated with SMD, and the levels of total effective rate, time to disappearance of cough, time to relief of cough, time to fever reduction, time to chest film normalization, T lymphocyte subpopulation (CD3+) and tumor necrosis factor-α (TNF-α) were analyzed, and the results showed that the clinical efficacy of the observation group treated with SMD was significantly The results showed that the clinical efficacy of the observation group treated with SMD was significantly better than that of the control group, and the levels of all indexes analyzed were statistically significant. The results of the subgroup analysis of the different treatment courses showed that the treatment duration could be extended to maximize the effect when treating mycoplasma pneumonia, and the subgroup analysis of the different treatment methods showed that the use of TCM and SMD in combination was more effective than the use of Western medicine and SMD in combination, which highlights the important role of TCM in the prevention and treatment of the disease.
Among all 12 studies, six studies reported adverse reactions. Meta-analysis was performed on the observation group and the control group. The results showed that the use of SMD in the treatment of mycoplasma pneumonia can significantly reduce the incidence of adverse reactions after treatment. Modern pharmacological studies have found that Mai Dong (Ophiopogon japonicus (Thund.) Ker Gawl.) (Fan et al., 2020), Yu Zhu (Polygonatum odoratum (Mill.) Druce) (Meng et al., 2020), Tian Hua Fen (Trichosanthes kirilowii Maxim.) (Cao, 2017), Gan Cao (Glycyrrhiza uralensis Fisch. ex.DC) (Deng et al., 2021), etc., have immunomodulatory functions, which can improve disease resistance and treatment tolerance of children, and reduce the treatment Toxic and side effects reduce the incidence of adverse reactions.
Limitations
This study has the following limitations: first, the number of studies included in this study is small, and there is an uncertainty in the assessment of clinical efficacy of SMD in the treatment of pediatric mycoplasma pneumonia. Second, the search text type of this study was limited to Chinese and English databases, and did not include Japanese and Korean databases, which may result in the exclusion of some high-quality articles. In addition, for the risk of bias assessment of the included studies, only six papers specifically described the randomization method, and the risk of bias for the others was not known, which also led to some risk of bias in this study. Finally, the quality of the included clinical studies was not high, which also suggests that the next more in-depth studies need to include higher-quality literature to provide more reliable clinical evidence to support the rational clinical application of SMD.
Conclusion
The results of the systematic evaluation showed that SMD significantly reduced the level of outcome indicators such as time to symptom relief. The analysis of different subgroups can further illustrate the characteristics of SMD in the treatment of pediatric mycoplasma pneumonia. SMD can improve the clinical symptoms of pediatric mycoplasma pneumonia. The efficiency of SMD in the treatment of mycoplasma pneumonia in pediatric patients was high, and the incidence of adverse effects was low. In general, random-effects models yield conservative conclusions, and the final combined results of random-effects and fixed-effects models do not differ significantly when heterogeneity is small, and are more effective when used with large heterogeneity. Therefore, random-effects model analysis can be used to reduce the variation for clinical indicators with fewer included studies. In general, the results of the meta-analysis are sufficient to show that it is effective at least in the treatment of mycoplasma pneumonia in children, whether combined with WM or alone. The quality assessment of the included literature is risky to a certain extent, because some factors of the assessment are uncertain. SMD is a traditional Chinese prescription and is widely used and researched in China. Therefore, most of the included literature is in Chinese and the original research data are from the clinic. This can provide reliable evidence for the conclusion of this study. The effectiveness of SMD in treating mycoplasma pneumonia in children is credible, however, the main components and specific mechanism of the efficacy of SMD are unknown, which will be a major research in the future, not only SMD, but also the mechanism of action of more herbal compounds need to be explored, which is also the core direction of the development of Motherland Medicine. For future clinical studies on mycoplasma pneumonia, experimental protocols should be designed more scientifically and rationally to reduce the risk of bias and improve the quality of evidence, to further evaluate the efficacy of SMD in the treatment of pediatric mycoplasma pneumonia and its scientific and feasibility in clinical studies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
JW and XM designed the search strategy; YT and TY conducted the search of the studies; JW and MJ screened the studies; JW evaluated the studies; JW and SW conducted the statistical analysis; JW, YT and SW wrote the article; YZ was mainly responsible for the final check of the article.
Funding
This work was supported by grants from the National Key R&D Program of China (No. 2018YFC1704500) and China Medical Education Association 2020 major scientific problems and medical technical problems (No.2020KTZ002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACU, acupuncture, AZM, azithromycin, CAP, community-acquired pneumonia, C, control group; CT, conventional therapy, T, experimental group; F, female; M, male; MA, macrolide antibiotics; MP, mycoplasma pneumoniae; MPP, mycoplasma pneumonia; MA, macrolide antibiotics; TCM, traditional chinese medicine; WM, western medicine; SMD, Shashen Maidong decoction; SGD, Shaoyao Gancao decoction; YYQFD, Yangyin Qingfei decoction.
References
Atkinson, T. P., Balish, M. F., and Waites, K. B. (2008). Epidemiology, Clinical Manifestations, Pathogenesis and Laboratory Detection of Mycoplasma Pneumoniae Infections. FEMS Microbiol. Rev. 32 (6), 956–973. doi:10.1111/j.1574-6976.2008.00129.x
Cao, C. G. (2017). Pharmacological Characteristics and Clinical Application of Trichosanthes. Smart Healthc. 3 (21), 19–21. doi:10.3969/j.issn.2096-1219.2017.21.009
Chen, Y. C., Hsu, W. Y., and Chang, T. H. (2020). Macrolide-Resistant Mycoplasma Pneumoniae Infections in Pediatric Community-Acquired Pneumonia. Emerg. Infect. Dis. 26 (7), 1382–1391. doi:10.3201/eid2607.200017
Cong, F. F., and Zhu, J. L. (2018). Sha Shen Maidong Decoction and Shaoyao Gancao Decoction in the Treatment of Chronic Cough in Children after Mycoplasma Pneumoniae Infection Analysis of. Health Way 17 (1), 199–200.
Defilippi, A., Silvestri, M., Tacchella, A., Giacchino, R., Melioli, G., Di Marco, E., et al. (2008). Epidemiology and Clinical Features of Mycoplasma Pneumoniae Infection in Children. Respir. Med. 102, 1762–1768. doi:10.1016/j.rmed.2008.06.022
Deng, T. M., Peng, C., Peng, D. Y., Yu, N. J., Chen, W. D., and Wang, L. (2021). Research Progress on Chemical Constituents and Pharmacological Effects of Licorice and Discussion of Quality Markers. China J. Chin. Materia Med. 46 (11), 2660–2676.
Fan, M. M., Zhang, J. Y., Zhang, X. L., and Wang, S. (2020). Research Progress on the Chemical Constituents and Pharmacological Effects of Ophiopogon Japonicus[J]. Inf. Traditional Chin. Med. 37 (04), 130–134.
Fang, Y. J., and Hang, J. Y. (2020). Mycoplasma Pneumonia Analysis of the Clinical Efficacy and Safety of Erxing Shashen Maidong Decoction with Modified Treatment. J. Med. Theor. Pract. 33 (19), 3250–3252.
Gao, S., Li, J., Huang, F. B., Xie, Q. L., Yi, P., Qu, J. Y., et al. (2020). Research Progress on the Pharmacological Effects and Clinical Application of Shashen Maidong Decoction. Guiding J. Traditional Chin. Med. Pharm. 26 (02), 115–118+123. doi:10.13862/j.cnki.cn43-1446/r.2020.02.032
Guo, D. X., Hu, W. J., Wei, R., Wang, H., Xu, B. P., Zhou, W., et al. (2019). Epidemiology and Mechanism of Drug Resistance of Mycoplasma Pneumoniae in Beijing, China: A Multicenter Study. Bosn J. Basic Med. Sci. 19, 288–296. doi:10.17305/bjbms.2019.4053
Han, X. L. (2020). Observation on the Clinical Efficacy of Shashen Maidong Decoction in the Treatment of Mycoplasma Pneumonia in Children. Heilongjiang J. Traditional Chin. Med. 49 (02), 25–26.
He, M., Luo, Y., Chen, L., Zeng, M., Liao, Q., Zhang, W., et al. (2021). Shashen Maidong Decoction: the Effect of TNF-α and IL-6 on Lung Cancer Cachexia Based on Cancer Toxicity Theory. Am. J. Transl Res. 13 (6), 6752–6758.
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Izzo, A. A., Hoon-Kim, S., Radhakrishnan, R., and Williamson, E. M. (2016). A Critical Approach to Evaluating Clinical Efficacy, Adverse Events and Drug Interactions of Herbal Remedies. Phytother Res. 30 (5), 691–700. doi:10.1002/ptr.5591
Jain, S., Williams, D. J., Arnold, S. R., Ampofo, K., Bramley, A. M., Reed, C., et al. (2015). Community-acquired Pneumonia Requiring Hospitalization Among U.S. Children. N. Engl. J. Med. 372 (9), 835–845. doi:10.1056/NEJMoa1405870
Jiang, Y. L., and Hao, C. Y. (2020). The Influence of Shashen Maidong Decoction on the Probability of Complications in the Treatment of Children with Mycoplasma Pneumonia. Electron. J. Clin. Med. Lit. 7 (71), 132–133.
Jiang, Z. Y., Wang, X. F., Wang, L. N., Qin, Y. H., Xu, H., Bai, X. H., et al. (2017). Multi-center Curative Effect Evaluation Study on the Treatment of Mycoplasma Pneumonia in Children with Integrated Traditional Chinese and Western Medicine. World Chin. Med. 12 (3), 536. doi:10.3969/j.issn.1673-7202.2017.03.014
Kutty, P. K., Jain, S., Taylor, T. H., Bramley, A. M., Diaz, M. H., Ampofo, K., et al. (2019). Mycoplasma Pneumoniae Among Children Hospitalized with Community-Acquired Pneumonia. Clin. Infect. Dis. 68 (1), 5–12. doi:10.1093/cid/ciy419
Lee, H., Yun, K. W., Lee, H. J., and Choi, E. H. (2018). Antimicrobial Therapy of Macrolide-Resistant Mycoplasma Pneumoniae Pneumonia in Children. Expert Rev. Anti Infect. Ther. 16 (1), 23–34. doi:10.1080/14787210.2018.1414599
Lee, J. K., Choi, Y. Y., Sohn, Y. J., Kim, K.-M., Kim, Y. K., Han, M. S., et al. (2021). Persistent High Macrolide Resistance Rate and Increase of Macrolide-Resistant ST14 Strains Among Mycoplasma Pneumoniae in South Korea, 2019-2020. J. Microbiol. Immunol. Infect. 26 (21), S1684–S1182. doi:10.1016/j.jmii.2021.07.011
Li, T. L., Pu, F. L., Wu, Y., Zhao, G. Z., Li, H. Z., Ma, Q. X., et al. (2018). Meta-analysis of Xiaoer Xiaoji Zhike Oral Liqud Combined with Azithromycin in Treatment of Mycoplasma Pneumonia in Children. Zhongguo Zhong Yao Za Zhi 45 (3), 2193–2202. doi:10.19540/j.cnki.cjcmm.20200211.503
Li, Y. (2019). Shashen Maidong Decoction Plus Acupuncture on Mycoplasma Study on the Therapeutic Effect of Children with Pneumonia. Health for Everyone 12, 112.
Lin, L. T. (2020). The Effect of Treatment of Chronic Cough after Mycoplasma Pneumonia in Children with Yangyin Qingfei Decoction Combined with Montelukast. Health for Everyone 8, 103.
Liu, Y., Liu, X. Y., and Chen, A. M. (2020). Professor Ni Zhuying's Experience in Treating Pediatric Diseases with Shaoyao Gancao Decoction. World Chin. Med. 15 (01), 99–103. doi:10.3969/j.issn.1673-7202.2020.01.019
Liu, Y. S., Wang, J. J., and Zhang, H. G. (2020). The Effect of Shashen Maidong Decoction on the Immune Inflammatory Response in Patients with Yin Deficiency and Lung Heat Syndrome during Recovery of Mycoplasma Pneumoniae Pneumonia. Chin. J. Exp. Traditional Med. Formulae 26 (21), 149–154. doi:10.13422/j.cnki.syfjx.20200632
Lv, K. F. (2020). The Clinical Observation of the Adjuvant Treatment of Mycoplasma Pneumonia in Children with Shashen Maidong Decoction. J. Pract. Traditional Chin. Med. 36 (10), 1284–1285.
Meng, Q. L., Cui, W. Y., Liu, Y. J., Jin, S., and Pan, J. Z. (2020). Research Progress on the Chemical Constituents and Pharmacological Effects of Polygonatum Odoratum. Shanghai J. Traditional Chin. Med. 54 (09), 93–98.
Ni, X. (2019). Guidelines for Diagnosis and Treatment of Community-Acquired Pneumonia in Children (2019 Edition). Clin. Edu. Gen. Pract. 17 (09), 771–777. doi:10.13558/j.cnki.issn1672-3686.2019.09.002
Ouyang, L. S., and Wu, Y. Y. (2021). Effects of Different Parts of Jialou on the Proliferation of Human Bronchial Epithelial Cells and the Expression of Inflammatory Factors Induced by Total Particulate Matter in Cigarette Smoke. Traditional Chin. Drug Res. Clin. Pharmacol. 32 (02), 219–225. doi:10.19378/j.issn.1003-9783.2021.02.010
Qiu, J. L., Huang, L., Shao, M. Y., Chai, Y. N., Zhang, H. J., Li, X. F., et al. (2020). Efficacy and Safety of Azithromycin Combined with Glucocorticoid on Refractory Mycoplasma Pneumoniae Pneumonia in Children: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine (Baltimore) 99 (22), e20121. doi:10.1097/MD.0000000000020121
Shao, L., Liang, Y. J., and Xie, J. (2017). Therapeutic Effect of Sha Shen Maidong Decoction and Shaoyao Gancao Decoction on Children's Chronic Cough after Mycoplasma Pneumoniae Infection Observation. Chin. J. Clin. Rational Drug Use 10 (17), 70–71.
Shi, J. Q., and Yang, H. D. (2019). Observation on the Effect of Qingfei Jianpi Decoction in the Auxiliary Treatment of Mycoplasma Pneumonia in Children. Chin. J. Traditional Med. Sci. Tech. 26 (5), 758–759.
Shi, X. X. (2011). Traditional Chinese Medicine for High Airway Response during Recovery from Mycoplasma Pneumonia Treatment. J. Zhejiang Chin. Med. Univ. 35 (2), 155–156. doi:10.3969/j.issn.1005-5509.2011.02.011
Sun, Y., Wang, J., Hou, Z., Qi, F., and Wang, W. M. (2021). Extraction of Effective Parts of Ophiopogon Japonicus and its Immunomodulatory Effect on Lung Tissue of Mice Infected with Mycoplasma. Jilin J. Chin. Med. 41 (04), 510–515. doi:10.13463/j.cnki.jlzyy.2021.04.025
Tan, D., and Jiang, Z. Y. (2018). Research Progress on the Mechanism of Traditional Chinese Medicine in the Treatment of Childhood Mycoplasma Pneumoniae Pneumonia. Chin. Arch. Traditional Chin. Med. 36 (06), 1403–1406.
Tanaka, H. (2016). Correlation between Radiological and Pathological Findings in Patients with Mycoplasma Pneumoniae Pneumonia. Front. Microbiol. 7 (07), 695. doi:10.3389/fmicb.2016.00695
Wang, J. (2017). Study on 435 Children with Mycoplasma Pneumoniae Infection and Drug Resistance[J]. J. Qiqihar Med. Univ. 38 (07), 791–792. doi:10.3969/j.issn.1002-1256.2017.07.021
Wang, X., and Xiao, D. F. (2020). Research Progress of mulberry Leaf Extract on Animal Immune Function and its Mechanism. Feed Rev. 12, 9–13+19.
Wu, Q., and Wu, Q. W. (2016). Childhood Pneumonia Research Progress on the Epidemiology of Mycoplasma Infection. Matern. Child Health Care China 31 (5), 1111–1114. doi:10.1177/0883073816639378
Xu, J. (2012). Shashen Maidong Decoction and Shaoyao Gancao Decoction in the Treatment of Chronic Cough in Children after Mycoplasma Pneumoniae Infection. Chin. J. Traditional Med. Sci. Tech. 19 (2), 101. doi:10.3969/j.issn.1005-7072.2012.02.004
Yan, H., Peng, Z. R., Gao, S. Q., Luo, H. T., Zhou, Y. L., and Liu, S. L. (2021). Study on the HPLC Fingerprint of Adenophora Adenophora Decoction. West China J. Pharm. Sci 36 (04), 433–438. doi:10.13375/j.cnki.wcjps.2021.04.014
Yang, Y., and Zhou, Y. (2019). Shashen-Maidong Decoction-Mediated IFN-γ and IL-4 on the Regulation of Th1/Th2 Imbalance in RP Rats. Biomed. Res. Int. 2019, 6012473. doi:10.1155/2019/6012473
Yu, X. P. (2019). Shashen Maidong Decoction Analysis of the Application Effect of Combined Acupuncture and Moxibustion in the Treatment of Mycoplasma Pneumonia in Children and the Incidence of Adverse Reactions. Biped and Health 28 (05), 118–119.
Zhang, J. (2019). Analysis of the Clinical Effect of Shashen Maidong Decoction in the Treatment of Mycoplasma Pneumonia in Children. Renowned Doctor 1, 239.
Zhang, L. (2017). Clinical Observation of Shashen Maidong Decoction in the Treatment of Mycoplasma Pneumonia in Children[J]. Inner Mongolia J. Traditional Chin. Med. 36 (02), 44. doi:10.3969/j.issn.1006-0979.2017.02.045
Zhang, S. P. (2018). The Treatment of Shashen Maidong Decoction Clinical Effect of Mycoplasma Pneumonia in Children. Chin. Community Doctors 34 (05), 104–105. doi:10.3969/j.issn.1007-614x.2018.5.63
Zheng, Y., Xiong, S., Jiang, P., Liu, R., Liu, X., Qian, J., et al. (2012). Glucocorticoids Inhibit Lipopolysaccharide-Mediated Inflammatory Response by Downregulating microRNA-155: a Novel Anti-inflammation Mechanism. Free Radic. Biol. Med. 52 (8), 1307–1317. doi:10.1016/j.freeradbiomed.2012.01.031
Keywords: shashen maidong decoction, pediatric mycoplasma pneumonia, systematic review, meta-analysis, clinical research
Citation: Wang J, Ma X, Wei S, Yang T, Tong Y, Jing M, Wen J and Zhao Y (2021) Clinical Efficacy and Safety of Shashen Maidong Decoction in the Treatment of Pediatric Mycoplasma Pneumonia: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12:765656. doi: 10.3389/fphar.2021.765656
Received: 27 August 2021; Accepted: 27 September 2021;
Published: 12 October 2021.
Edited by:
Luca Rastrelli, University of Salerno, ItalyReviewed by:
Imma Pagano, University of Salerno, ItalyPriyia Pusparajah, Monash University Malaysia, Malaysia
Copyright © 2021 Wang, Ma, Wei, Yang, Tong, Jing, Wen and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanling Zhao, emhhb3lsMjg1NUAxMjYuY29t
 Jiawei Wang
Jiawei Wang Xiao Ma
Xiao Ma Shizhang Wei
Shizhang Wei Tao Yang
Tao Yang Yuling Tong1,2
Yuling Tong1,2 Manyi Jing
Manyi Jing Jianxia Wen
Jianxia Wen