- 1Chinese-German Joint Laboratory for Natural Product Research, Qinling-Bashan Mountains Bioresources Comprehensive Development C.I.C., College of Biological Science and Engineering, Shaanxi University of Technology, Hanzhong, China
- 2Department of Agriculture Nutrition and Food Systems, University of New Hampshire, Durham, NH, United States
- 3Centre of Molecular and Environmental Biology (CBMA), Department of Biology, University of Minho, Campus de Gualtar, Braga, Portugal
- 4Jinhuifang Traditional Chinese Medicine Technology Co., Ltd, Hanzhong, China
- 5Centre of Biological Engineering (CEB), University of Minho, Campus de Gualtar, Braga, Portugal
- 6Department of Biomedical Sciences, Ontario Veterinary College, University of Guelph, Guelph, ON, Canada
The growth location and plant variety may influence the active components and biological activities of plants used in phytomedicine. In this study, nine sets of different Epimedii Folium, from different representative cultivation locations and Epimedium species, were collected for comparison, using HPLC-DAD combined with multivariate analysis. The objective was to investigate the influence of geographical origin and Epimedium species on the quality of Epimedii Folium, and provide applicable guidance for cultivation and quality control of Epimedii Folium. Several Epimedium spp. sets were used to establish the HPLC-DAD fingerprints and 91 peaks (compounds) were selected for the multivariate analysis. Major compounds were analyzed by HPLC-DAD combined with principal component analysis (PCA). HPLC quantitative analysis of known bioactive compounds was performed. Application of PCA to HPLC data showed that Epimedium samples sharing the same geographical origin or species clustered together, indicating that both species and geographical origin have impacts on the quality of Epimedii Folium. The major bioactive flavonoid compounds, epimedin C, icariin and baohuoside I, were identified and quantified. The concentration of bioactive compounds was significantly influenced both by species and geographical origin. E. sagittatum from Sichuan showed the highest content of bioactive compounds. The results showed that both Epimedium species and geographical origin have strong impact into quality of Epimedii Folium. HPLC data combined with multivariate analysis is a suitable approach to inform the selection of cultivation areas and choose Epimedium spp. most suitable for different geographical areas, resulting in improved quality of Epimedii Folium.
Introduction
Epimedii Folium, “淫羊藿 (Yin Yang Huo)” in Chinese - also known as Herba Epimedii, barrenwort, bishop’s hat, fairy wings, horny goat weed, and rowdy lamb herb - is an important medicinal herb ingredient used in traditional Chinese medicine (TCM) to treat osteoporosis and sexual dysfunction, among other conditions (Ma et al., 2011). Epimedii Folium has been used for more than 2000 years with the major functions of “tonifying kidney Yang, strengthening muscles and bones, dispelling wind and dampness” (Chen et al., 2015b). Epimedium sp. improved osteoporosis condition and strengthening bones in human studies (Indran et al., 2016), and has been used to treat sexual dysfunction (Shindel et al., 2010) and cardiovascular diseases (Li et al., 2015b). Nowadays, Chinese Pharmacopeia accepts four Epimedium species as a source of Epimedii Folium, including Epimedium brevicornum Maxim, Epimedium sagittatum (Siebold and Zucc.) Maxim, Epimedium pubescens Maxim, and Epimedium koreanum Nakai. Its dried leaves have spicy and sweet tastes, and have been used for further dosage preparations (Chinese Pharmacopoeia, 2020).
Many active compounds, including epimedin A, epimedin B, epimedin C, icariin and baohuoside I, have been identified from Epimedium (Wu et al., 2012). Among them, the prenylflavonoids flavonoids icariin, epimedin C and baohuoside I, are considered as the major bioactive components and used as marker compounds for quality control (Zhao et al., 2010). Icariin, a flavonol glycoside obtained from the aerial part of the plant (Indran et al., 2016), could enhance the osteogenic effect of bone morphogenetic protein 2 (BMP2) which induces osteoblast differentiation and stimulate bone or cartilage formation and cyclic adenosine monophosphate (cAMP) signaling pathway which regulates osteogenic differentiation and mineralization (Chen et al., 2019). Additionally, icariin has been reported to have anti-tumorigenic activity. Icariin significantly inhibited the proliferation of several cancer cells, like ovarian cancer cells (Li et al., 2015a), medulloblastoma cells (Sun et al., 2016), and human neural cells (Yang et al., 2016).
Epimedium brevicornum Maxim is widely distributed in northwest China, including Gansu, Shaanxi, Ningxia and He’nan provinces, whilst Epimedium pubescens Maxim grows in the south provinces of Sichuan, Guizhou and Anhui (Guo and Xiao, 2003). These two species have been regarded having higher quality with consistent higher levels of major active components (He et al., 2019). Quality of commercial Epimedii Folium is mainly controlled by its icariin content, with the minimal content of 0.5% (g/g DW) in dried products, according to Chinese Pharmacopoeia (Chinese Pharmacopoeia, 2015). However, icariin contents of Epimedium on the markets remain uneven, even undetectable in some batches of commercial Epimedium, possibly due to the regional and varietal differences. According to a survey performed in 2014, the ranges of icariin contents in 104 batches from different species were 0.01–0.17% (g/g DW) and all of them were substandard (Ma et al., 2014). Other studies support such observation (Pei et al., 2007; Polat and Coskun, 2016).
This study aimed to investigate the influence of the cultivation location (province) and Epimedium species on the phytocomposition and quality of Epimedii Folium, namely the major relevant bioactive components, using HPLC-DAD and multivariate statistical analysis, since these issues are highly relevant for cultivation and quality control of Epimedii Folium.
Materials and Methods
Chemicals
HPLC-grade ethanol, acetonitrile and formic acid were purchased from Chron chemicals (Chengdu, Sichuan, China), Damao chemical (Tianjin, China) and Kermel Chemical (Tianjin, China), respectively. Ultrapure water with a resistivity of 18 MΩ.cm at 25°C was generated with Microporous system (Ulu pure, Xian, Shaanxi, China). The analytical standards were purchased from Desite (Chengdu, Sichuan, China): Epimedin C (purity >98%), icariin (purity >98%) and Baohuoside I (purity >99%).
Collection and Preparation of Epimedium sp. Samples
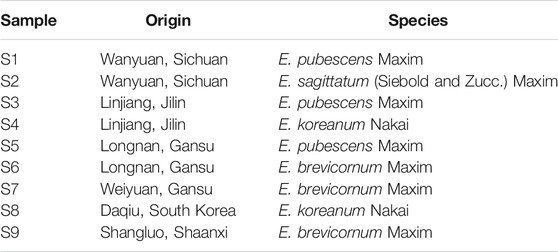
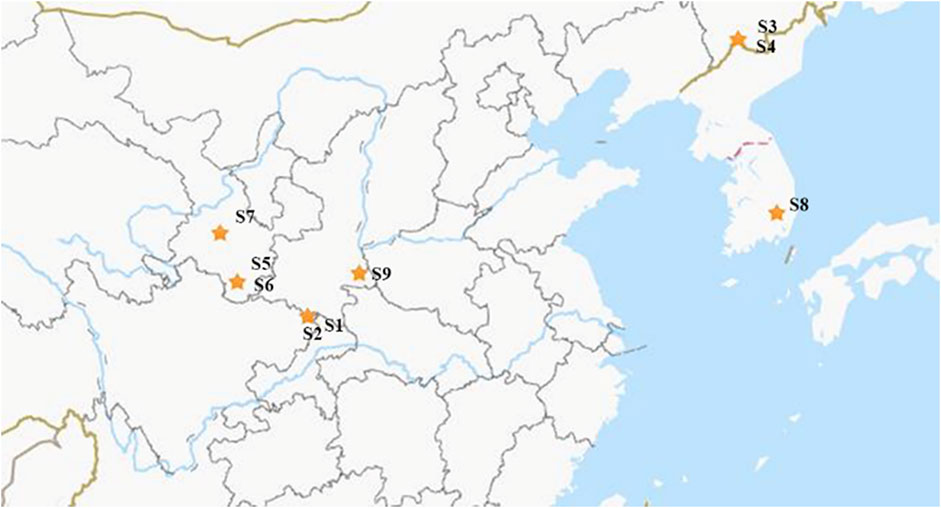
Leaves of E. pubescens and E. sagittatum were collected at a cultivation field located at Wanyuan (Sichuan) (S1 and S2 samples, Table 1). Other Epimedium samples were purchased directly from local certified TCM markets, with a valid and clear certificate of origin, provided by Chinese official regulators (State Administration for Market Regulation). All the samples were further verified and confirmed by experts and voucher specimens were deposited in the herbarium collection of College of Biological Science and Engineering, Shaanxi University of Technology, Hanzhong, China. The species and respective origin are listed in Table 1 and geographical locations are shown in Figure 1. From each location/species, five independent samples were obtained based on batch leaves from individual plants, to account for normal in vivo variability.
The leaves were dried by lyophilization to constant weight, milled into powder, and stored in the dark at room temperature until use. Aliquots (0.2 g) of powder samples of Epimedium were weighed and added to 8 ml of 70% aqueous ethanol. Extraction was done using sonication for 2 min × 30 min. After this, solutions were centrifuged at 13,500 g for 5 min, the supernatant was filtered through 0.22 μm Nylon six microporous filter membrane, and the filtrate was collected in amber borosilicate glass vials for HPLC-DAD analysis.
HPLC-DAD Analysis
Samples were injected into a liquid chromatograph system UltiMate 3000 (Thermo, Waltham, MA, United States). Chromatographic separations were achieved using gradient elution on an Inertsil ODS-3 column (150 mm × 4.6 mm, 4 μm). Mobile phase A was acetonitrile containing 0.1% formic acid and mobile phase B was ultrapure water containing 0.1% formic acid. The gradient elution program was set as follows: 80% (B) for 0–3 min, 80%–70% (B) for 3–15 min, 70% (B) for 10–15 min, 70%–10% (B) for 15–30 min, 10% (B) for 30–35 min, 10%–80% (B) for 35–40 min. The flow rate was 0.75 ml/min. The column was maintained at 30°C and the sample injection volume was 10 μL. The detection wavelength was recorded between 230 and 600 nm, and chromatograms were recorded at 274 nm. Quantification of epimedin C, icariin and baohuoside I was made at 274 nm based on the external standard method using standard curves of commercial pure compounds.
The HPLC chromatograms were exported as txt ASCII files and the chromatographic fingerprint process was drew using Origin Lab Pro version 9.4 (Origin Lab software, Northampton, MA, United States).
Statistical Analysis
A total of 91 peaks in the HPLC chromatograms of the nine Epimedium sets (45 independent samples in total) were selected for multivariate statistical analysis. Peaks were manually aligned based on their retention time and UV spectra, to assure common identity, and named 1 to 91. Peak areas (274 nm) were corrected by the amount of biomass extracted. The resulting table was imported into GraphPad Prism version 9.1.1 for Windows (GraphPad Software, San Diego, CA, United States, www.graphpad.com). Data was standardized prior to principal component analysis (PCA). The R-statistical software version 4.1.0 (R Core Team, 2021), ggplot2 version 3.3.5 (Wickham, 2016), and ggrepel version 0.9.1 (https://cran.r-project.org/web/packages/ggrepel/index.html) packages were used to display the corresponding plots. The amounts of epimedin C, icariin and baohuoside I from the different Epimedium species cultivated in different regions were plotted and compared in GraphPad Prism using one-way ANOVA followed Tuckey’s test or t-test, to compare three or two groups, respectively. One outlier of E. sagittatum, one of E. pubescens from Sichuan, and two outliers of E. brevicornum from Weiyuan Gansu were removed prior to comparison. Data normality was assessed using the Kolmogorov-Smirnov test. Statistical significance was considered at p < 0.05. All matrices were also imported into the SIMCA14.0 software (Umetrics, Umea, Västerbotten, Sweden). The obtained quantification data were scaled with unit variance scaling, and sample subgroups (E. koreanum and Sichuan) were subjected to PCA.
Results and Discussion
Epimedii Folium HPLC-DAD Analysis
The chemical quality of plants is influenced by both biotic and abiotic environmental factors and known to exhibit extensive geographic variation (Chen et al., 2013). Epimedium is native to China with wide distribution in He’nan, Shanxi, Shaanxi, Gansu, and Ningxia Provinces (although Epimedium spp. can be found in other regions of East Asia such as E. koreanum in Japan and North Korea), and has abundant pharmacological functions (Xu et al., 2013; Li et al., 2018). However, safe and effective use of Epimedium has been limited by variation of Epimedium quality, and identification of plant location and variation (Han et al., 2012).
Typical chromatograms resulting from the HPLC analysis of nine sets of Epimedium from different geographical origin and species are shown in Figure 2. There were good chromatogram resolutions in the fingerprint of all Epimedium samples, namely for the standards epimedin C, icariin and baohuoside I, considered quality marker compounds for Epimedii Folium, and the other major compounds found. Differences between the different Epimedium samples could not be easily detected in the chromatograms by simple visual inspection. Hence, HPLC data was subjected to PCA, with the purpose of uncovering an effect related to geographical origin and/or species on the quality of Epimedium.
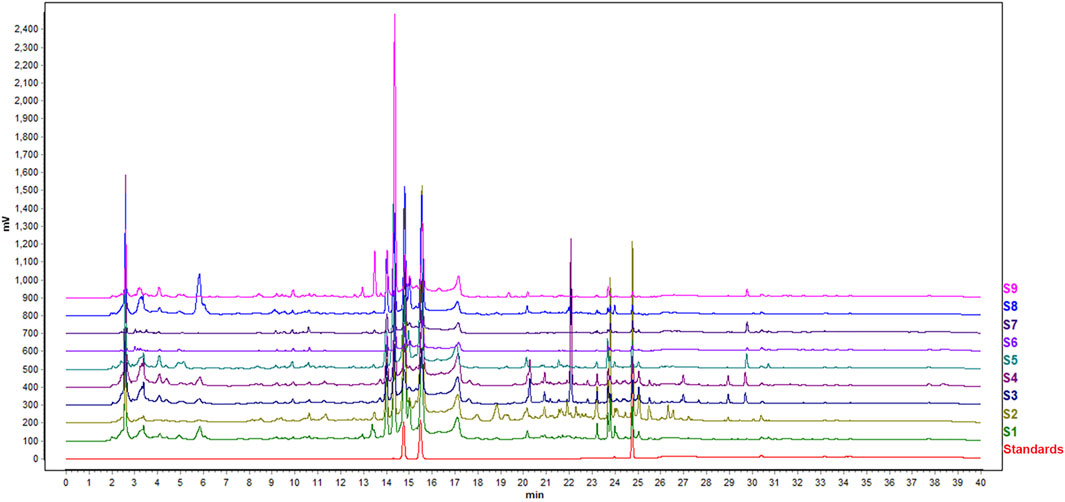
FIGURE 2. HPLC fingerprints of nine sets of Epimedium samples studied and standards (epimedin C, icariin and baohuoside I, from left to right).
PCA Analysis
The use of Multivariate Analysis, like Principal Component Analysis (PCA), is nowadays commonly used for better understanding metabolite diversity, namely of phenolics, and link it with adulterations (Windarsih et al., 2019), biotic stress (Lima et al., 2010), and different geographical and species variation (Chen et al., 2015a).
In this work, PCA was used to investigate how different species and geographical origin are relevant (or not) for differences and quality of Epimedii Folium. The best discriminating principal components (PCs), PC1 and PC2, cumulatively accounted to the explanation of 42.01% of the total variance in the data. The PC1 and PC2 scores scatter plot (Figure 3A) clearly shows the separation of E. sagittatum species from the other Epimedium species along PC1, with E. sagittatum samples clustering towards higher positive values of PC1 (orange ellipse in Figure 3A), and all other species grouping towards lower and negative values of PC1.
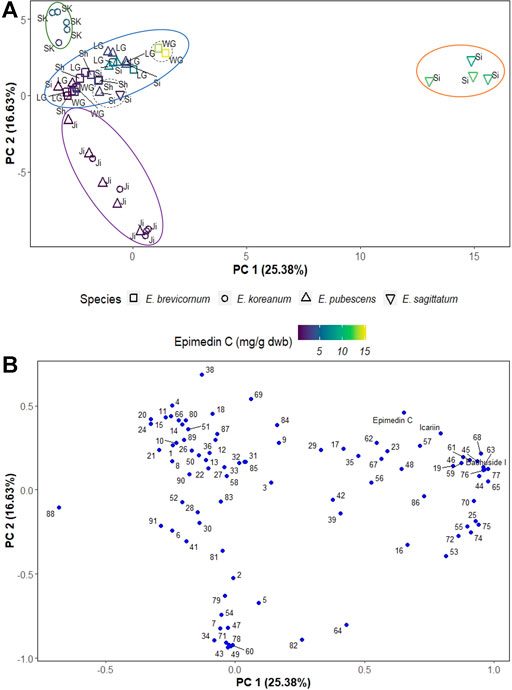
FIGURE 3. PCA scores plot (A) and loadings plot (B) of Epimedium samples. In the scores plot, Epimedium species are represented by different shapes, and each data point is colored according to its amount of epimedin C and labeled with its geographical origin (SK—South Korea, LG—Longnan Gansu, WG—Weiyuan Gansu, Ji—Jilin, Si—Sichuan, Sh—Shaanxi). Ellipses indicate sample groups based on species or geographical origin; dashed-line circles indicate outliers (two E. brevicornum outliers from Weiyuan Gansu, one E. sagittatum outlier and another E. pubescens both from Sichuan). The loadings plot (B) shows the compounds responsible for the group separation; for clarity, the peak numbers corresponding to epimedin C, icariin and baohuoside I were substituted by their name.
Additionally, PC2 clearly separates Epimedium samples based on geographical origin. Samples from South Korea clustered towards the highest values of PC2 (green ellipse in Figure 3A), the samples from central China provinces (Gansu, Sichuan, and Shaanxi) clustered at lower values of PC2 (blue ellipse in Figure 3A), and the samples from the Jilin province, in northeast China, clustered towards negative values of PC2 (purple ellipse in Figure 3A).
The PC1 and PC2 loadings plot (Figure 3B) shows the compounds contributing to the separation of Epimedium samples into different groups. The pharmacologically active compounds epimedin C, icariin and baohuoside I, were among the compounds that most contribute to sample separation, because they are associated with higher PC1 values and positive PC2 values. To further confirm the importance of these bioactive compounds in separating Epimedium samples, the data points in the scores scatter plot were colored according to a gradient based on epimedin C concentration (Figure 3A). The E. sagittatum samples contained higher amounts of Epidemium C (orange ellipse), followed by the E. koreanum samples from South Korea with medium-high amounts (green ellipse), then the E. pubescens and E. brevicornum samples from central China with medium-low concentration of epimedin C (blue ellipse), and finally the E. koreanum and E. pubescens samples from the Jilin province containing the lowest amounts of epimedin C (purple ellipse).
Some isolated studies indicated that the compounds of Epimedium from neighboring locations were similar (Huang et al., 2007; Xu et al., 2013; Xu et al., 2017). This study included samples from a wide geographical area and from different species (Table 1; Figure 1), as a way to offer a more comprehensive view of how location and species may affect biomass quality. In fact, using PCA analysis, it was easy to distinguish Epimedii Folium from the same species but from different provenience (Figure 4A), and from different species grown in the same cultivated field, under the same abiotic and biotic stressors (Figure 4B). It is clear that, both Epimedium species and provenience have strong impact on the phenolic contents and quality of Epimedii Folium.
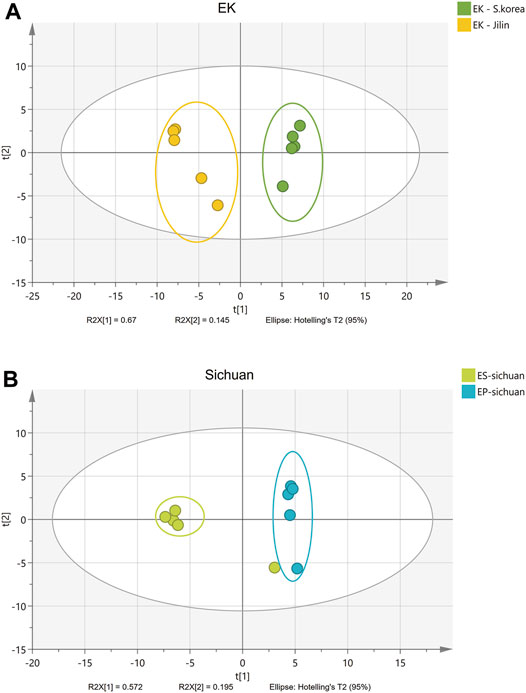
FIGURE 4. PCA scores plot (A) for same Epimedium species (E. koreanum, EK) collected in different locations (Daqiu, South Korea and Jilin, China)—A; and PCA scores plot (B) for different species (E. sagittatum, EK, and E. pubescens, EP) cultivated in the same field (Wanyuan, Sichuan).
Content Differences of Bioactive Components in Epimedium Sets
The relevant bioactive compounds to Epimedii Folium (or Herba Epimedii) used in TCM, epimedin C, icariin, and baohuoside I, were quantified in the samples studied (Figures 5, 6). E. sagittatum contained the highest amounts of epimedin C (10.88 ± 0.83 mg/g dwb), icariin (11.21 ± 1.12 mg/g dwb), and baohuoside I (3.23 ± 0.24 mg/g dwb). The bioactive amounts in the other Epimedium species were lower and varied according to geographical origin (Figure 5). For E. pubescens (Figure 5A), average epimedin C concentration was significantly higher when cultivated in Sichuan (5.43 ± 1.87 mg/g dwb) compared to E. pubescens samples from Longnan Gansu (2.54 ± 1.05 mg/g dwb) and Jilin (0.65 ± 0.36 mg/g dwb). Average E. pubescens icariin concentration was significantly higher in samples from Longnan Gansu (4.42 ± 1.79 mg/g dwb) when compared to samples from Jilin (1.28 ± 0.73 mg/g dwb), but not significantly different from Sichuan samples (3.07 ± 1.22 mg/g dwb). Average E. pubescens baohuoside I concentration was significantly higher in samples from Sichuan (0.63 ± 0.07 mg/g dwb) when compared to samples from Jilin (0.30 ± 0.14 mg/g dwb), but not significantly different from Longnan Gansu samples (0.51 ± 0.17 mg/g dwb). It is noteworthy to mention that some of the species indicated by producers as having the highest contents in bioactive contents might be different. As an example, E. pubescens was considered to have the highest contents in bioactives (He et al., 2019). Nevertheless, for E. sagittatum and E. pubescens plants cultivated in the same field location, under the same abiotic and biotic environment, the bioactive contents (icariin, epimedin C, baohuoside I) were significantly higher for E. sagittatum (Figure 6).
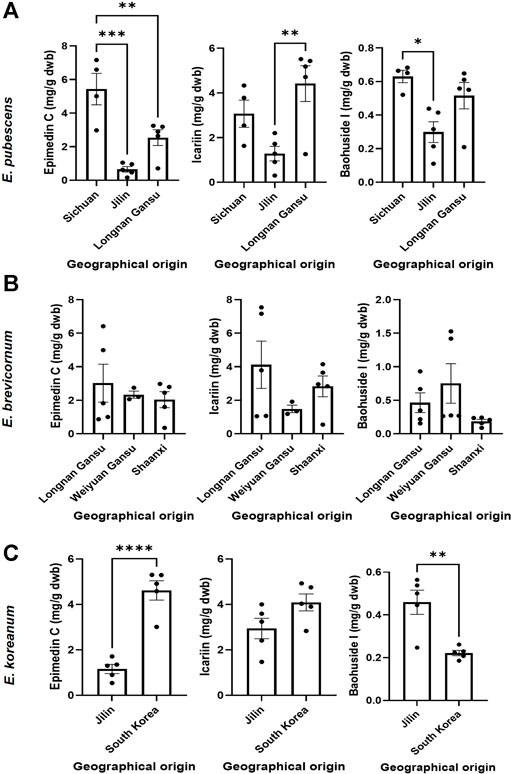
FIGURE 5. Amount (mg/g dry weight biomass) of epimedin C, icariin and baohuoside I in E. pubescens (A), E. brevicornum (B) and E. koreanum (C) cultivated in different geographical locations. Bars represent average ±SEM; dots represent individual data points. Statistically significant differences are represented by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
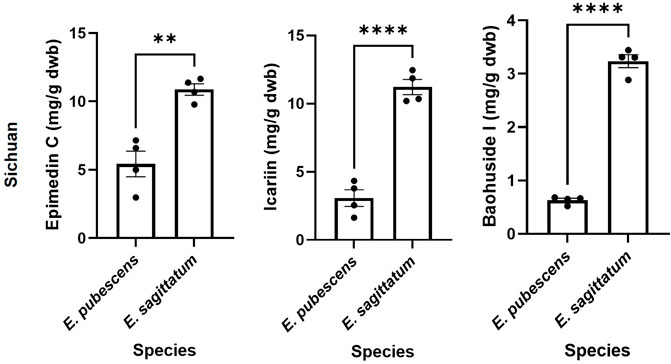
FIGURE 6. Amount (mg/g dry weight biomass) of epimedin C, icariin and baohuoside I of different plant species cultivated in same place (Wanyuan, Sichuan). Bars represent average ±SEM; dots represent individual data points. Statistically significant differences are represented by asterisks (**p < 0.01, ****p < 0.0001).
For E. brevicornum (Figure 5B) the amount of the identified bioactive components varied, on average, between 2.04 and 3.02 mg/g dwb for epimidium C, between 1.49 and 4.12 mg/g dwb for icariin, and between 0.19 and 0.75 mg/g dwb for baohuoside I. No significant differences were detected among the different geographical regions, likely because all the E. brevicornum samples analyzed in this study were cultivated in regions exclusively located to central China. For E. koreanum (Figure 5C), average epimedin C concentration was significantly higher when cultivated in South Korea (4.62 ± 0.95 mg/g dwb) compared to E. koreanum samples from Jilin (1.16 ± 0.45 mg/g dwb). Average E. koreanum icariin content was not significantly different in samples from South Korea (4.10 ± 0.83 mg/g dwb) compared to samples from Jilin (2.94 ± 1.01 mg/g dwb). Average E. koreanum baohuoside I concentration was significantly higher in samples from Jilin (0.46 ± 0.13 mg/g dwb) compared to samples from South Korea (0.22 ± 0.03 mg/g dwb).
In our current study, the contents of icariin in E. sagittatum from Sichuan and E. brevicornum from Gansu Wanyuan were above standard according to the 2015 Chinese Pharmacopoeia (Chinese Pharmacopoeia, 2015). However, in the current Chinese Pharmacopoeia released in 2020 (Chinese Pharmacopoeia, 2020), the standard for Epimedium quality control has been changed to the analysis of the icariin content and calculation of the total amount of Epimedium A, B, C and icariin based on the correction factor, that means, E. pubescens from Jilin and E. brevicornum from Shaanxi were below standard. There is clear difference between the two editions of the pharmacopoeia standards, despite the content of icariin has been the major quality consideration in both editions. It is noteworthy that, according to the results of the recent studies, the contents of icariin in Epimedium were easily affected by external factors (Chen et al., 2015b; Deng et al., 2018; Li et al., 2020). Therefore, finding the variation patterns of the content of various components in Epimedium herbs of different origins and varieties and screening the appropriate content determination index are the keys to solve the current Epimedium quality control issue.
Overall, both species variant and geographical location influence the contents of bioactive components in Epimedii Folium, and so the pharmacology quality of the biomass (Wei et al., 2017; Yuan et al., 2017). Therefore, it is necessary to explore the geo-herbalism of Herba epimedii by the characteristic component variation and chromatographic fingerprint among different sets. E. koreanum belongs to large-flowered taxa and E. pubescens, E. sagittatum and E. brevicornum all belong to small-flowered taxa (Xu and He, 2005). However, the icariin content of E. sagittatum was significantly higher compared with E. pubescens cultivated in the same region (Figure 6). E. sagittatum also showed relatively independent from E. pubescens, E. koreanum and E. brevicornum through PCA scores plot compared with E. wushanense (Xie et al., 2010), which proved that Epimedium species variation is a factor in the interspecific differences, and indicated that the differences between different species of Epimedium should be explored.
As a conclusion, the use of HPLC-DAD combined with multivariate analysis (PCA) is an effective methodology to discriminate different Epimedii Folium samples from different epimedium species and geographical origins. Our results provide applicable guidance to the geographical location and plant species selection of GAP (Good Agricultural Practices) production for Epimedii Folium. Both species and geographical location variations have impacts on the quality and composition of Epimedii Folium. However, the components of herbal products are diverse and complex, and their pharmacological activities are always affected by unique component constituents as well as their combinations, instead of a single component (Zhang et al., 2013). Therefore, associations between the variation of plant species and geographical locations with pharmacological activity of Epimedii Folium need to be further explored for providing better evaluation criteria for geo-herbalism of Epimedii Folium.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
AD and XZ contributed to conception and design of the study. BL and ML performed the statistical analysis. BL and ML wrote the first draft of the manuscript. HN, LX, HY, CC, AD, and XZ wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by Incubation Project on State Key Laboratory of Biological Resources and Ecological Environment of Qinba Areas (SLGPT2019KF04-04), China, and the ERDF through the COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI), Portugal.
Conflict of Interest
Author HY was employed by the Jinhuifang Traditional Chinese Medicine Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Chen, J., Li, Z., Maiwulanjiang, M., Zhang, W. L., Zhan, J. Y., Lam, C. T., et al. (2013). Chemical and Biological Assessment of Ziziphus Jujuba Fruits from China: Different Geographical Sources and Developmental Stages. J. Agric. Food Chem. 61 (30), 7315–7324. doi:10.1021/jf402379u
Chen, J., Xu, Y., Wei, G., Liao, S., Zhang, Y., Huang, W., et al. (2015a). Chemotypic and Genetic Diversity in Epimedium Sagittatum from Different Geographical Regions of China. Phytochemistry 116, 180–187. doi:10.1016/j.phytochem.2015.04.005
Chen, M., Cui, Y., Li, H., Luan, J., Zhou, X., and Han, J. (2019). Icariin Promotes the Osteogenic Action of BMP2 by Activating the cAMP Signaling Pathway. Molecules 24 (21), 3875. doi:10.3390/molecules24213875
Chen, X. J., Tang, Z. H., Li, X. W., Xie, C. X., Lu, J. J., and Wang, Y. T. (2015b). Chemical Constituents, Quality Control, and Bioactivity of Epimedii Folium (Yinyanghuo). Am. J. Chin. Med. 43 (5), 783–834. doi:10.1142/s0192415x15500494
Chinese Pharmacopoeia (2015). Pharmacopoeia of the People's Republic of China. Beijing: China Medical Science Press.
Chinese Pharmacopoeia (2020). Pharmacopoeia of the People's Republic of China. Beijing: China Medical Science Press.
Deng, A. P., Fang, W. T., Zhou, Q. G., Yang, H. J., Wang, L., Nan, T. G., et al. (2018). Research Actuality and Quality-Influencing Factor of Epimedii Folium. Zhongguo Zhong Yao Za Zhi 43 (5), 1062–1070. doi:10.19540/j.cnki.cjcmm.2018.0037
Guo, B., and Xiao, P. (2003). Review on Main Species of Herba Epimedii. Zhongguo Zhong Yao Za Zhi 04, 18–22.
Han, S., Xie, Y. Y., Wang, Y. M., Liang, Q. L., and Luo, G. A. (2012). Comparative Study on Chemical Quality of Main Species of Epimedium. Yao Xue Xue Bao 47 (4), 502–507. doi:10.16438/j.0513-4870.2012.04.007
He, L., Huang, N., Yan, Y., Lin, W., Gao, L., and Lin, X. (2019). Identification and Determination of Components in Five Kinds of Yinyanghuo. Clin. J. Chin. Med. 11 (22), 139–144.
Huang, H., Liang, M., Zhang, X., Zhang, C., Shen, Z., and Zhang, W. (2007). Simultaneous Determination of Nine Flavonoids and Qualitative Evaluation of Herba Epimedii by High Performance Liquid Chromatography with Ultraviolet Detection. J. Sep. Sci. 30 (18), 3207–3213. doi:10.1002/jssc.200700262
Indran, I. R., Liang, R. L., Min, T. E., and Yong, E. L. (2016). Preclinical Studies and Clinical Evaluation of Compounds from the Genus Epimedium for Osteoporosis and Bone Health. Pharmacol. Ther. 162, 188–205. doi:10.1016/j.pharmthera.2016.01.015
Li, J., Jiang, K., and Zhao, F. (2015a). Icariin Regulates the Proliferation and Apoptosis of Human Ovarian Cancer Cells through microRNA-21 by Targeting PTEN, RECK and Bcl-2. Oncol. Rep. 33 (6), 2829–2836. doi:10.3892/or.2015.3891
Li, R., Guo, M., and Pang, X. (2018). Identification and Classification of Medicinal Plants in Epimedium. Chin. Herbal Medicines 10 (03), 249–254. CNKI:SUN:CHME.0.2018-03-004. doi:10.1016/j.chmed.2018.07.001
Li, W. X., Deng, Y. Y., Li, F., Liu, B., Liu, H. Y., Shi, J. S., et al. (2015b). Icariin, a Major Constituent of Flavonoids from Epimedium Brevicornum, Protects against Cognitive Deficits Induced by Chronic Brain Hypoperfusion via its Anti-amyloidogenic Effect in Rats. Pharmacol. Biochem. Behav. 138, 40–48. doi:10.1016/j.pbb.2015.09.001
Li, X. M., Pan, J. Q., Luo, Y. J., Yang, Q. R., Xu, C. Q., Shen, G. A., et al. (2020). Effects of Light Quality on Growth and Icariin Flavonoid Content of Epimedium Pseudowushanense under Different Light Intensity. Zhongguo Zhong Yao Za Zhi 45 (11), 2502–2508. doi:10.19540/j.cnki.cjcmm.20200329.113
Lima, M. R., Felgueiras, M. L., Graça, G., Rodrigues, J. E., Barros, A., Gil, A. M., et al. (2010). NMR Metabolomics of Esca Disease-Affected Vitis vinifera Cv. Alvarinho Leaves. J. Exp. Bot. 61 (14), 4033–4042. doi:10.1093/jxb/erq214
Ma, H., He, X., Yang, Y., Li, M., Hao, D., and Jia, Z. (2011). The Genus Epimedium: an Ethnopharmacological and Phytochemical Review. J. Ethnopharmacol. 134 (3), 519–541. doi:10.1016/j.jep.2011.01.001
Ma, Q., Wang, J., Han, L., Lv, C., Jia, L., and Lu, J. (2014). Simultaneous Determination of 8 Flavonoids in Epimedium by HPLC. Shenyang Pharm. Univ. 31 (12), 970–978. doi:10.14066/j.cnki.cn21-1349/r.2014.12.008
Pei, L. K., Huang, W. H., He, T. G., and Guo, B. L. (2007). Systematic Studies on Quality of Main Species of Herba Epimedii. Zhongguo Zhong Yao Za Zhi 32 (21), 2217–2222.
Polat, D. C., and Coskun, M. (2016). Quantitative Determination by HPLC-DAD of Icariin, Epimedin A, Epimedin B, and Epimedin C in Epimedium (Berberidaceae) Species Growing in Turkey. Nat. Prod. Commun. 11 (11), 1665–1666. doi:10.1177/1934578x1601101110
R Core Team (2021). R: A Language and Environment for Statistical Computing. Vienna, Austria): R Foundation for Statistical Computing.
Shindel, A. W., Xin, Z. C., Lin, G., Fandel, T. M., Huang, Y. C., Banie, L., et al. (2010). Erectogenic and Neurotrophic Effects of Icariin, a Purified Extract of Horny Goat weed (Epimedium spp.) In Vitro and In Vivo. J. Sex. Med. 7 (4 Pt 1), 1518–1528. doi:10.1111/j.1743-6109.2009.01699.x
Sun, Y., Sun, X. H., Fan, W. J., Jiang, X. M., and Li, A. W. (2016). Icariin Induces S-phase Arrest and Apoptosis in Medulloblastoma Cells. Cell. Mol. biol. 62 (4), 123–129. doi:10.14715/cmb/2016.62.4.21
Wei, Q., He, M., Chen, M., Chen, Z., Yang, F., Wang, H., et al. (2017). Icariin Stimulates Osteogenic Differentiation of Rat Bone Marrow Stromal Stem Cells by Increasing TAZ Expression. Biomed. Pharmacother. 91, 581–589. doi:10.1016/j.biopha.2017.04.019
Windarsih, A., Rohman, A., and Swasono, R. T. (2019). Application of 1H-NMR Based Metabolite Fingerprinting and Chemometrics for Authentication of Curcuma Longa Adulterated with C. Heyneana. J. Appl. Res. Med. Aromatic Plants 13, 100203. doi:10.1016/j.jarmap.2019.100203
Wu, B., Chen, Y., Huang, J., Ning, Y., Bian, Q., Shan, Y., et al. (2012). Icariin Improves Cognitive Deficits and Activates Quiescent Neural Stem Cells in Aging Rats. J. Ethnopharmacol 142 (3), 746–753. doi:10.1016/j.jep.2012.05.056
Xie, P. S., Yan, Y. Z., Guo, B. L., Lam, C. W., Chui, S. H., and Yu, Q. X. (2010). Chemical Pattern-Aided Classification to Simplify the Intricacy of Morphological Taxonomy of Epimedium Species Using Chromatographic Fingerprinting. J. Pharm. Biomed. Anal. 52 (4), 452–460. doi:10.1016/j.jpba.2010.01.025
Xu, N., Zhou, G., Li, X., Lu, H., Meng, F., and Zhai, H. (2017). Geographical Classification of Epimedium Based on HPLC Fingerprint Analysis Combined with Multi-Ingredients Quantitative Analysis. Biomed. Chromatogr. 31 (5), e3871. doi:10.1002/bmc.3871
Xu, W., and He, S. (2005). Species and Geographic Distribution of Large-Flowered Taxa of Epimedium in China. Zhong Yao Cai 28 (4), 267–271. doi:10.13863/j.issn1001-4454.2005.04.005
Xu, Y., Li, Z., Yuan, L., Zhang, X., Lu, D., Huang, H., et al. (2013). Variation of Epimedins A - C and Icariin in Ten Representative Populations of Epimedium Brevicornu Maxim., and Implications for Utilization. Chem. Biodivers 10 (4), 711–721. doi:10.1002/cbdv.201100424
Yang, P., Guan, Y. Q., Li, Y. L., Zhang, L., Zhang, L., and Li, L. (2016). Icariin Promotes Cell Proliferation and Regulates Gene Expression in Human Neural Stem Cells In Vitro. Mol. Med. Rep. 14 (2), 1316–1322. doi:10.3892/mmr.2016.5377
Yuan, X. Y., Wang, M., Lei, S., Yang, Q. X., and Liu, Y. Q. (2017). Rapid Screening of Active Components with an Osteoclastic Inhibitory Effect in Herba Epimedii Using Quantitative Pattern-Activity Relationships Based on Joint-Action Models. Molecules 22 (10), 1767. doi:10.3390/molecules22101767
Zhang, M. H., Feng, L., Hu, S. Y., and Jia, X. B. (2013). Essence of Material Base in Geoherbs: Specificality of Constituent Structure. Zhongguo Zhong Yao Za Zhi 38 (1), 136–140.
Keywords: Epimedium sp, high performance liquid chromatography (HPLC), principal component analysis (PCA), epimedin C, icariin, baohuoside I
Citation: Li B, Lima MRM, Nie Y, Xu L, Liu X, Yuan H, Chen C, Dias ACP and Zhang X (2021) HPLC-DAD Fingerprints Combined With Multivariate Analysis of Epimedii Folium From Major Producing Areas in Eastern Asia: Effect of Geographical Origin and Species. Front. Pharmacol. 12:761551. doi: 10.3389/fphar.2021.761551
Received: 20 August 2021; Accepted: 09 November 2021;
Published: 26 November 2021.
Edited by:
George Qian Li, Western Sydney University, AustraliaReviewed by:
Hossein Hashempour, Azarbaijan Shahid Madani University, IranAbuzar Kabir, Florida International University, United States
Copyright © 2021 Li, Lima, Nie, Xu, Liu, Yuan, Chen, Dias and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto CP Dias, YWNwZGlhc0BiaW8udW1pbmhvLnB0; Xiaoying Zhang, emhhbmdAYmlvLnVtaW5oby5wdA==
†Present address: Marta R. M. Lima, School of Plant and Environmental Sciences, Virginia Tech, Blacksburg, VA, United States
 Ben Li1
Ben Li1 Marta R. M. Lima
Marta R. M. Lima Yuhao Nie
Yuhao Nie Long Xu
Long Xu Xiang Liu
Xiang Liu Xiaoying Zhang
Xiaoying Zhang