
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 19 January 2022
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.759741
 Yue Lu1,2,3
Yue Lu1,2,3 Yao Qi4,5
Yao Qi4,5 Li Li1,2,3
Li Li1,2,3 Yuhong Yan1,2,3
Yuhong Yan1,2,3 Jianan Wei1,2,3
Jianan Wei1,2,3 Danni Yao1,2,3
Danni Yao1,2,3 Jingjing Wu1,2,3
Jingjing Wu1,2,3 Hao Deng1,2,3
Hao Deng1,2,3 Jingwen Deng1,2,3
Jingwen Deng1,2,3 Shuyan Ye1,2,3
Shuyan Ye1,2,3 Haiming Chen1,2,3
Haiming Chen1,2,3 Qubo Chen1,2,3
Qubo Chen1,2,3 Hengjun Gao4,5*
Hengjun Gao4,5* Ling Han1,2,3*
Ling Han1,2,3* Chuanjian Lu1,2,3*
Chuanjian Lu1,2,3*Psoriasis is chronic skin disease and an important health concern. Traditional Chinese Medicine (TCM) has shown great promise in the treatment of psoriasis. However, the correlation between TCM Syndromes and genomics of psoriasis has not been evaluated. Here, we analyzed gene expression profiling of monocytes from psoriasis vulgaris patients with different TCM syndrome types to reveal the molecular basis of different psoriasis syndromes. Of the 62 cases of psoriasis vulgaris recruited, 16, 23, and 23 cases were of blood-heat syndrome, blood stasis syndrome, and blood-dryness syndrome, respectively; 10 healthy controls were recruited as controls. Affymertix’s Gene Chip ®clariom D gene chip was used to detect the gene expression profile of peripheral blood monocytes collected from recruited individuals. Compared with the healthy control group, 1570 genes were up-regulated and 977 genes were down-regulated in the psoriasis vulgaris patients group; 798 genes and 108 genes were up- and down-regulated in the blood-heat syndrome group respectively; 319 and 433 genes were up- and down-regulated in the blood-dryness syndrome group, respectively; and 502 and 179 genes were up-and down-regulated in the blood-stasis syndrome group. Our analyses indicated not only common differential genes and pathways between psoriasis syndrome groups and healthy controls, but also syndrome-specific genes and pathways. The results of this study link the three syndromes at the gene level and will be useful for clarifying the molecular basis of TCM syndromes of psoriasis.
Clinical Trial Registration: (http://www.chictr.org.cn/showproj.aspx?proj=4390), identifier (ChiCTR-TRC-14005185).
Psoriasis is a chronic proliferative skin disease mediated by abnormal immune system, which is determined by polygenic inheritance and stimulated by multiple environmental factors. Globally, 2–3% of the population is affected by psoriasis; currently, there are approximately 125 million psoriasis patients in the world. In China, the total incidence rate is 0.72%, with the current estimation being 10 million patients (Langley et al., 2005; Menter et al., 2008; Perera et al., 2012). Traditional Chinese Medicine (TCM) has great advantages and broad prospects in the treatment of psoriasis, and has accumulated rich treatment experience over a long time (Yu et al., 2013; Zhang et al., 2014; Zhang et al., 2016; Yu et al., 2017). PSORI-CM02 and FZHFZY has been used for decades to treat blood stasis and blood dryness syndromes of psoriasis vulgaris at the Guangdong Provincial Hospital of Chinese Medicine (Lu et al., 2019; Lu et al., 2021a; Lu et al., 2021b). PSORI-CM02 consists of Rhizoma Curcumae (Curcuma longa L.), Radix Paeoniae rubra (Paeonia lactiflora Pall.), Sarcandra glabra [Sarcandra glabra (Thunb.) Nakai], Rhizoma Smilacis glabrae (Smilax glabra Roxb.), and Fructus mume [Prunus mume (Siebold) Siebold & Zucc.] (the ratio was 2: 3: 5: 5: 2). FZHFZY includes Radix seu Herba Cynanchi Paniculati (Vincetoxicum mukdenense Kitag.), Densefruit Pittany Root-bark (Dictamnus dasycarpus Turcz.), Fructus Cnidii [Cnidium monnieri (L.) Cusson], Rhizoma Smilacis Glabrae (Smilax glabra Roxb.), Radix Rehmanniae Preparata [Rehmannia glutinosa (Gaertn.) DC.], Radix Angelicae Sinensis [Angelica sinensis (Oliv.) Diels], and Pericarpium Punicae Granati (Punica granatum L.) (the ratio was 3: 3: 2: 3: 3: 2: 3).
We have analyzed the distribution of syndromes in psoriasis vulgaris cases from 1979 to 2010 reported in literature, and found that among all syndromes in these patients, the top three syndromes, namely blood heat, blood dryness, and blood stasis syndromes accounted for 75.85%. Moreover, blood stasis syndrome was related to the stable phase, while blood dryness syndrome was related to regression phase (Lu CJ. et al., 2012; Yan et al., 2012). At present, there is no research on the correlation between TCM syndromes and genomics of psoriasis. Therefore, we carried out gene expression profiling to analyze the expression levels of genes associated with blood heat, blood dryness, and blood stasis syndromes, which are common TCM syndromes of psoriasis. We further examined the differences in gene expression among the three syndromes in order to reveal the molecular basis of the different syndromes of psoriasis at the gene level.
All recruited patients in this study were in the clinic and wards of the Department of Dermatology of Guangdong Provincial Hospital of Chinese Medicine. This study was approved by the Ethics Committee of our hospital, and all patients and healthy volunteers provided informed signed consent. The psoriasis vulgaris group comprised of 62 cases in total, including 16 cases of blood heat syndrome, 23 cases of blood stasis syndrome, and 23 cases of blood dryness syndrome. Of these, 44 were males and 18 were females; the average age was 45.5 years (range, 19–60 years). The disease course was 1 month–10 years. The diagnostic criteria were in line with those described for psoriasis vulgaris (Boehncke and Schön, 2015); the TCM diagnosis was consistent with that of bai bi (TCM disease name for psoriasis) (Lu C. J. et al., 2012). The diagnostic criteria of TCM syndrome types refer to the local standard of Guangdong Province–Psoriasis vulgar syndrome differentiation standard of traditional Chinese medicine (2018 Edition), as follows. Blood heat syndrome: in the progressive stage, the skin lesions are bright red, consciously hot, dry mouth and like drinking, upset and irritable, dry stool, yellow urine, red tongue or yellow tongue coating, and rolling and rapid pulse; blood stasis syndrome: in the static stage, the skin lesions are dark red or purple in color, thick lesion plaques, obvious infiltration, the scales are attached tightly, hard to peel off, dark purple tongue or ecchymosis, ecchymosis, astringent or slow pulse; blood dryness syndrome: in the static or regression stage, the skin color is light red, consciously dry, with many scales and easy to fall off, conscious fatigue, dry mouth and throat, light tongue, little or no moss, and the pulse is heavy or slow. The healthy control group comprised of 10 individuals that were proven to be healthy by examinations at the outpatient clinic; their average gender and age distributions matched that of the psoriasis group.
Pregnant or lactating women, those orally administered with steroid drugs and/or retinoic acids or biological agents within the past 6 months, those given topical application of steroid preparations or retinoic acid cream within the past 1 month, patients with hyperthyroidism, cardiovascular diseases, cerebrovascular diseases, liver diseases, kidney diseases, hematopoietic system diseases and other primary diseases, patients with mental diseases, patients on long-term medications, and patients with mixed TCM syndromes were all excluded.
The TRI Reagent (Sigma, Germany, Item No. T9424), miRNeasy Micro Kit (QIAGEN, Germany, Item No. 217084), RNase-Free DNase Set (QIAGEN, Germany, Item No. 79254), Affymetrix gene expression profiling kit GeneChip® WT PLUS Reagent Kit (Affymetrix, US, Item No. 902280), GeneChip® Hybridization, Wash and Stain Kit (Affymetrix, US, Item No. 900720), shaking hybridization oven (Affymetrix, US, Item No. 00-0331-220 V), washing workstation 450DX2 [Affymetrix, US, Command Console Software 3.1 (Affymetrix, Santa Clara, CA, US), Item No. 00-0335], data acquisition software Command Console Software 3.1 (Affymetrix, US), data analysis software Transcriptome Analysis Console Software (Affymetrix, US); Agilent Bioanalyzer 2100 Electrophoresis Instrument (Agilent, US); chip scanner GeneChip® Scanner 3000DX2 (Affymetrix, US, Item No. 00-0334); and GeneChip® Clariom D (Affymertix, US) were obtained from the indicated manufacturers.
Venous blood (10 ml) was collected from the median cubital vein into an anticoagulant tube containing heparin. Peripheral blood monocytes (PBMC) were isolated by Ficoll density gradient centrifugation method (Mendez-David et al., 2013) with lymphocyte separation solution (absin, China, item No. abs930b). First, suck the lymphocyte layer to a clean centrifuge tube and add 6 ml PBS to it, gently blow to fully mix the lymphocyte layer with PBS, then centrifuge the centrifuge tube at 60–100 g at 18–20°C for 10 min. Remove the upper separation solution, add 6–8 ml PBS to the centrifuge tube, gently blow with a Pasteur pipette to fully mix the lymphocytes, centrifuge for 10 min at 18–20°C and 100 × g, and remove the upper separation solution to obtain PBMC and stored in a refrigerator at −80°C.
The total RNA was extracted from the samples using the TRI Reagent according to the manufacturer’s instructions. Then, miRNeasy Micro kit and RNase-Free DNase Set were used to purify total RNA. After examining the quality, the purified total RNA was evaluated for RNA integrity using the Agilent Bioanalyzer 2100 electrophoresis apparatus.
Affymetrix gene expression profiling kit GeneChip® WT PLUS Reagent Kit was used to carry out in vitro reverse transcription amplification of the mRNA from the samples according to the manufacturer’s instructions. At the same time, biotin was used to label cRNA.
According to Affymetrix’s chip hybridization procedure, the biotin-labeled cRNA was added to the cartridge gene chip, the matching reagent kit was used, and the chip was subjected to hybridization for 16 h in the shaking hybridization oven at 45°C. After hybridization was completed, the chip was washed in the washing workstation according to the standard operating procedure.
The chip results were scanned using GeneChip® Scanner 3000DX2, the raw data were read by Command Console Software 3.1, and the data that passed quality control were normalized by Command Console Software 3.1 software. The Gene level_SST_RMA algorithm was used to perform quality control on the data and issue the data report, and the Transcriptome Analysis Console software was used to perform preliminary analysis on gene differential expression. The differentially expressed genes with p < 0.05 and fold change ≥2 were screened out and enriched using DAVID, and the pathways with p < 0.05 were selected for analysis. To determine the biological functions or signalling pathways affected by the differentially expressed genes, the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was performed using the online metascape tool (https://metascape.org/gp/index.html#/main/step1).
The isolated RNA from the PBMC samples from different groups was reverse transcribed directly into cDNA using a SuperScript IV Reverse Transcriptase (Thermo Fisher, Dalian, China) according to the manufacturer’s instructions. PCR amplification conditions were as follows: initial denaturation at 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 5 s and 60°C for 30 s, then annealing at 95°C for 5 s and 60°C for 1 min. USP17L15, JUND, OR1J1, FAM98B, HNRNPA2B1, NDUFA6, NDUFS8, UQCRC1, FAM208A, METTL15, USP17L19, CTLA4, HEMGN, SLC24A4, SLC30A6, CD9, S100A6, ITGB3, UPF3B, and MRPS27 gene expression were examined using qRT-PCR by a SYBR Green PCR kit (TaKaRa, Dalian, China).
Statistical analyses were performed with SPSS software 19.0 and visualized using GraphPad Prism 5.0 (La Jolla, CA, United States). The results are expressed as means ± standard errors of the means. Student’s t-test was used for the statistical analyses. p values <0.05 were considered significant.
Preliminary analysis of the gene expression profiles from psoriasis patients compared with those from the healthy control group showed that 1570 and 977 differentially expressed genes were up-regulated and down-regulated, respectively. Comparison of the psoriasis blood heat syndrome group with the healthy control group showed that 798 and 108 differentially expressed genes were up-regulated and down-regulated, respectively. In case of the psoriasis blood dryness syndrome group, 319 and 433 differentially expressed genes were up-regulated and down-regulated, respectively, compared with the healthy control group. Comparing the gene expression profiles from the psoriasis blood stasis syndrome group with those from the healthy control group, we screened out 502 up-regulated and 179 down-regulated differentially expressed genes (Table 1).
Principal component analysis was carried out on the expression profile chip data of the three syndromes of psoriasis, i.e., blood heat, blood dryness, and blood stasis, and it was found that the three syndromes could be clearly distinguished (Figures 1, 2). This indicates that the TCM syndrome differentiation theory of psoriasis is consistent with the differences in gene expression levels among the syndromes.
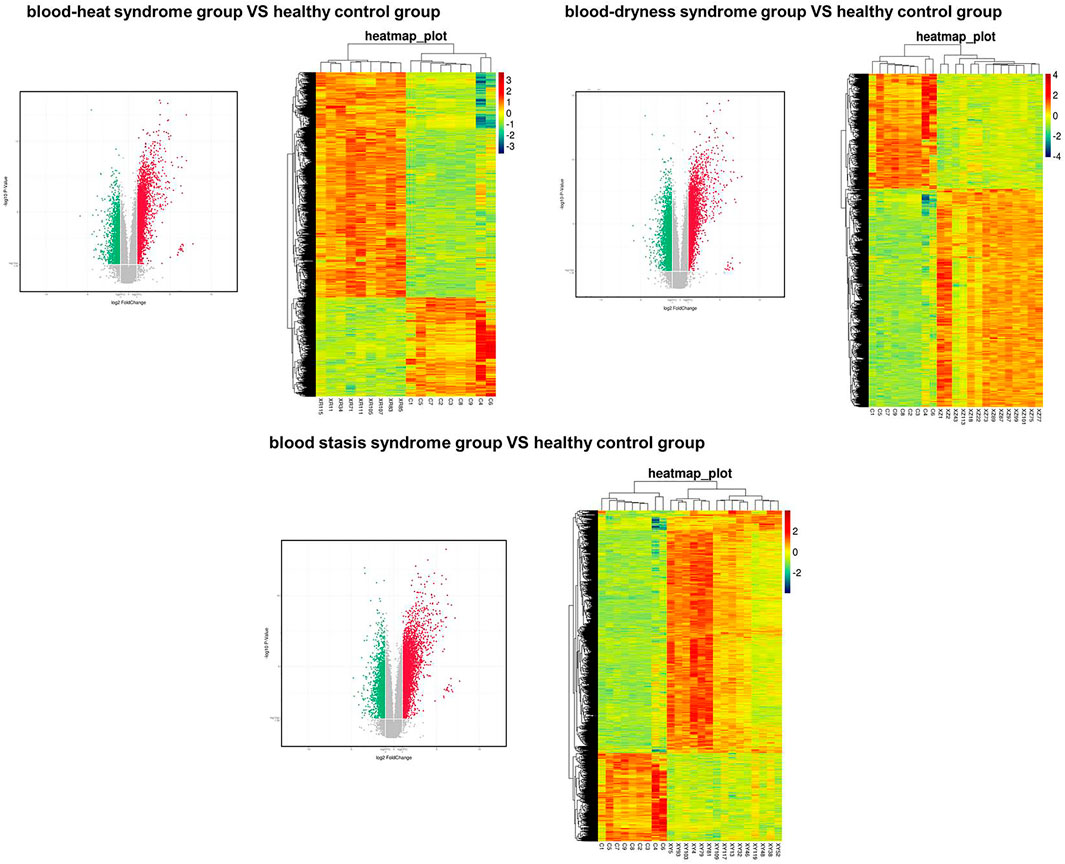
FIGURE 1. Volcanic map (left panel) and heat map (right) showed the distributions of genes in different syndromes.
The enrichment analysis showed that the top five major signalling pathways in the psoriasis blood heat syndrome group included detection of chemical stimulus involved in sensory perception of smell, cellular responses to stress, assembly of the pre-replicative complex, viral carcinogenesis, olfactory signaling pathway (Figures 3A,B). The top five major signalling pathways in the psoriasis blood dryness group included interferon signaling, GAB1 signalosome, negative regulators of DDX58/IFIH1 signaling, negative regulation of binding, ubiquinone and other terpenoid-quinone biosynthesis (Figures 3C,D). The top five major signalling pathways in the psoriasis blood stasis syndrome group included detection of chemical stimulus involved in sensory perception of smell, ribosome, olfactory signaling, alcoholism, ribosome, and cytoplasmic (Figures 3E,F). Venn diagram analysis of the differential genes was carried out by comparing the expression profile chip data of the three respective psoriasis syndromes with those of the healthy control group. It was found that the three syndromes showed specific differential genes as well as some common differential genes (Figure 3G).
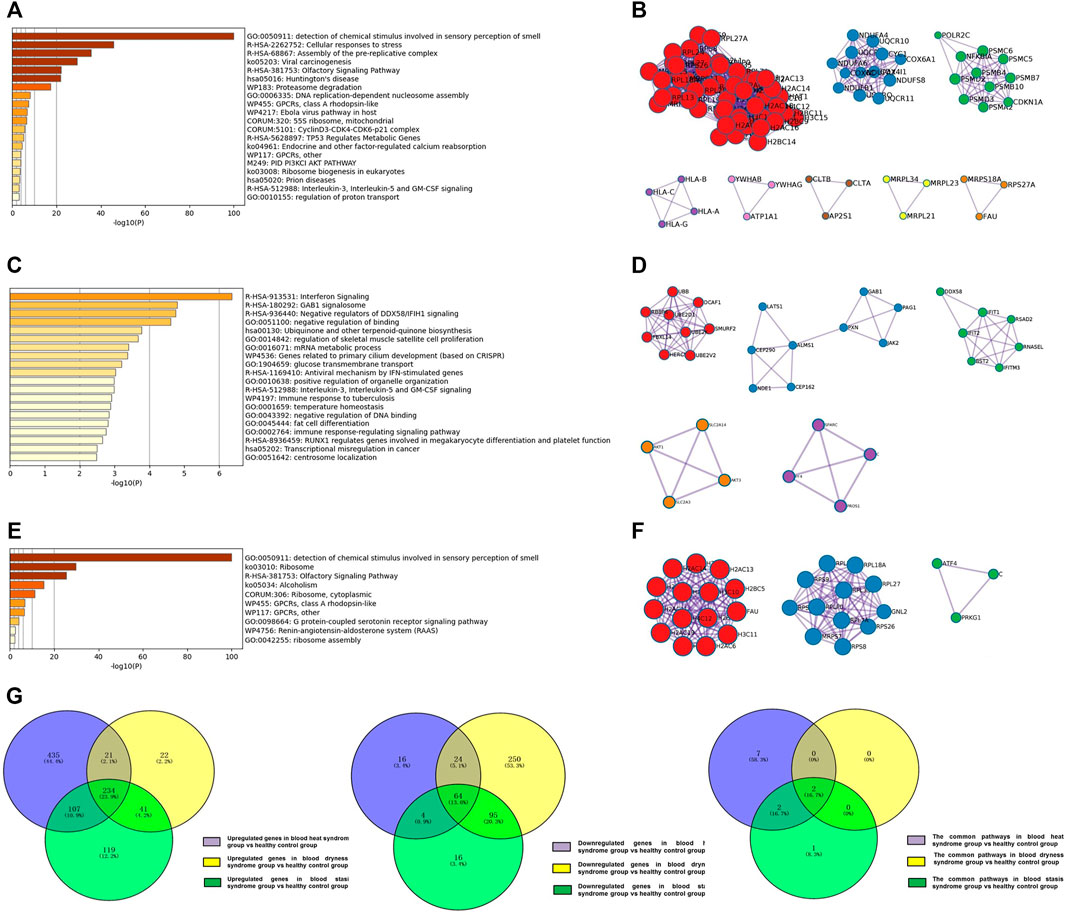
FIGURE 3. Analysis of regulated differential genes and pathways between three syndromes of psoriasis and healthy control group. (A) Analysis of regulated differential pathways between the psoriasis blood heat syndrome group and the control group; (B) Analysis of differentially expressed genes between the psoriasis blood heat syndrome group and the control groups; (C) Analysis of regulated differential pathways between the psoriasis blood dryness group and the control group; (D) Analysis of differentially expressed genes between the psoriasis blood dryness group and the control groups; (E) Analysis of regulated differential pathways between the psoriasis blood stasis syndrome group and the control group; (F) Analysis of differentially expressed genes between the psoriasis blood stasis syndrome and the control groups; (G) Venn analysis of differentially expressed genes and pathways between three syndromes of psoriasis and healthy control group.
Venn diagram analysis on the pathways associated with the three syndromes showed that blood heat and blood stasis syndromes were associated with their own specific pathways (Table 2). Specific abnormalities (mainly up-regulated) existed in the proteasome and oxidative phosphorylation pathways in the psoriasis blood heat syndrome group, and mitochondrial related ribosome pathway abnormalities (down-regulated) existed in the psoriasis blood stasis syndrome group. The result of KEGG enrichment indicated that the three psoriasis syndromes showed some common pathways (Table 2), including common abnormalities in ribosome and olfactory transduction.
Using qRT-PCR, we verified the twenty differentially expressed genes obtained from microarray, which was in accordance with microarray results (Figure 4). The genes USP17L15, OR1J1, and JUND were significantly up-regulated in all three syndromes. FAM98B and HNRNPA2B1 were significantly down-regulated in all three syndromes. In addition, we found that several oxidative phosphorylation pathway-related genes, such as NDUFS8, NDUFA6, and UQCRC1, showed syndrome-specific abnormal up-regulation in the blood heat syndrome group. FAM208A and METTL15 were significantly down-regulated in the blood heat syndrome group. USP17L19, CTLA4, and HEMGN were significantly up-regulated while SLC24A4 and SLC30A6 were significantly down-regulated in the blood dryness syndrome group. CD9, S100A6, and ITGB3 were significantly up-regulated while UPF3B and MRPS27 were significantly down-regulated in the blood stasis syndrome group.
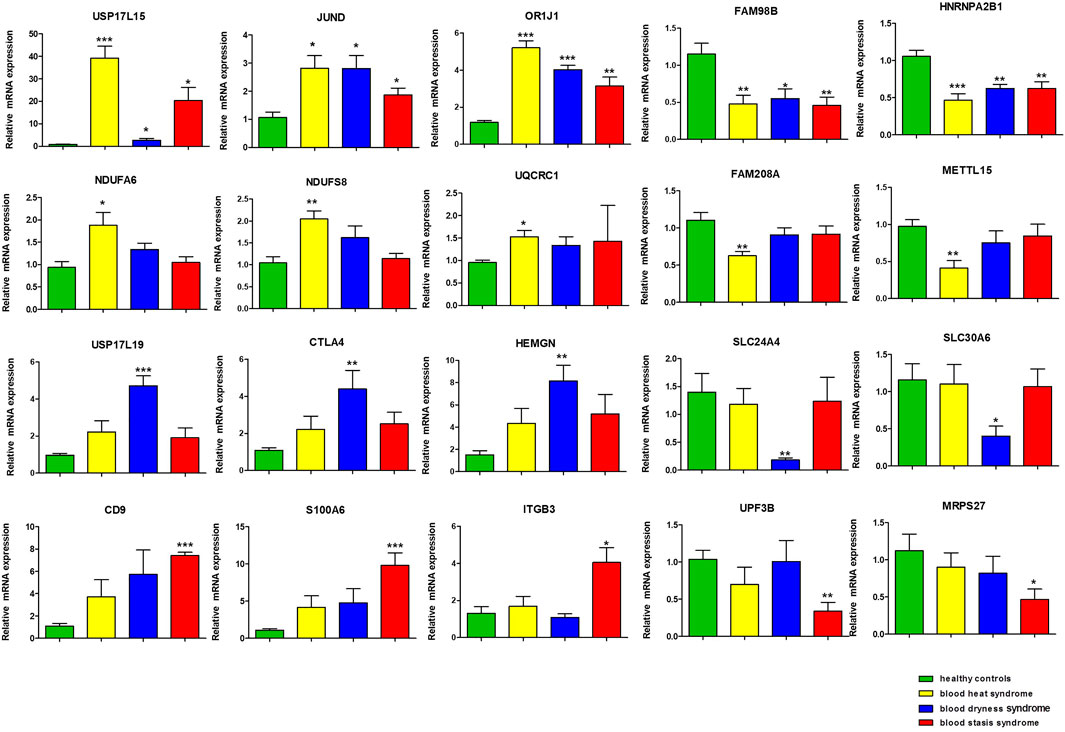
FIGURE 4. The gene validation by qRT-PCR. (*p < 0.05, **p < 0.01, ***p < 0.001 in qRT-PCR verification when comparing data between different syndrome groups and healthy control group).
Psoriasis is a common clinical chronic inflammatory skin disease, and its pathogenesis is still unclear. In this study, the three most common TCM syndromes of psoriasis were analyzed at gene expression level using gene expression profiling chip. There were some common as well as some specific differential genes and pathways between the three TCM psoriasis syndromes and the healthy control group.
In blood heat syndrome, a common syndrome of psoriasis, we found that a large number of oxidative phosphorylation pathway-related genes, such as NDUFS8, NDUFA6, and UQCRC1, showed syndrome-specific expression abnormalities. The literatures showed that these gene abnormalities were associated with mitochondrial metabolic activities (Murray et al., 2003; Angerer et al., 2014; Unni et al., 2019), suggesting a relationship between psoriasis and cellular metabolism. In recent years, epidemiological studies in China and other countries have shown that psoriasis and metabolic syndrome are correlated (Kim et al., 2012; Danielsen et al., 2015). It is generally believed that the common metabolic pathways in the pathogenesis of the two diseases contribute to the correlation. A study has shown that moderate to severe psoriasis often presents clinical features of metabolic disorders (Armstrong et al., 2013).
Ubiquitination is a reversible biological process and participates in the regulation of inflammatory signaling pathways (Farshi et al., 2015; Young et al., 2019). USP17 is one of the members of the deubiquitinase family. It has been reported that it promotes the growth of lung cancer and the expression of inflammatory genes in cancer (Lu et al., 2018). Our results show that the abnormal up-regulation of USP17L19 in blood dryness syndrome. It suggested that the induction of inflammation by USP17 may be involved in the immune dysfunction of psoriasis. Solute carrier (SLC) transporter is a special protein that can transport substrates across cell membranes (Rives et al., 2017). It is responsible for many basic physiological functions, including nutrient absorption, ion transport and waste treatment (Hediger et al., 2004). Some studies believe that it is the gatekeeper of immune cells and an important participant in regulating the metabolic function of immune cells (Song et al., 2020). We found the abnormal expression of solute carrier transporter (SLC24A4 and SLC30A6), which may be an important part of mediating metabolic and immune dysfunction in blood dryness syndrome of psoriasis.
Our result showed that UPF3B and MRPS27 were significantly down-regulated in the blood stasis syndrome group. Mitochondrial Ribosomal protein S27-like (MRPS27) is reported as a physiological regulator of p53, which can suppress genomic instability and tumorigenesis (Xiong et al., 2014). At present, lots of studies on ribosomal proteins are related to cancer, and there is no study related to psoriasis. Ribosomal protein dysfunction is related to cancer, metabolic disorder and inflammation and ribosomal protein ubiquitination is a feedback mechanism of the body (Wang W. et al., 2015; Lin et al., 2021). Our results show that the level of ubiquitinase decreases in psoriasis blood dryness syndrome, while ribosomal protein decreases in blood stasis syndrome, which may suggest that there are some connections and differences between the two syndrome types in inflammation and metabolism.
From the results of pathway analysis, the oxidative phosphorylation pathway-specific abnormalities were associated only with blood heat syndrome. According to literature, skin oxidative phosphorylation, ATP level and ATP enzyme activity level vary in the different stages of psoriasis (Cannavo et al., 2019; Xu et al., 2019). Genetic susceptibility and oxidative stress caused by exogenous and endogenous factors can lead to abnormal differentiation and proliferation of keratinocytes, thus leading to the development and persistence of psoriasis (Zhou et al., 2009; Lin and Huang, 2016). The European S3-Guidelines on the systemic treatment of psoriasis vulgaris--Update 2015 (Nast et al., 2015) clearly point out that skin lesions of psoriasis patients show a lack of cyclic adenosine monophosphate (cAMP), which can suppress epidermal cell division and maintain the balance between cell growth and apoptosis. In addition, as cAMP facilitates glycogenolysis by activating phosphorylase, the lack of cAMP can affect glycogen metabolism and lead to metabolic disorders (Ravnskjaer et al., 2016). The progressive stage of psoriasis is accompanied by a large number of inflammatory cell infiltration (Al-Harbi et al., 2020) and overactivated oxidative inflammation signals (Greb et al., 2016). Studies have shown that inhibiting oxidative inflammation signal transduction can reduce the inflammatory symptoms of psoriasis (Al-Harbi et al., 2020). The main manifestations of blood heat syndrome are related to the progressive stage of psoriasis, which may explain the abnormal expression of oxidative phosphorylation pathway in blood heat syndrome.
Furthermore, it was found that there were common differential genes between the three syndromes of psoriasis and the normal control group, which also suggests that the three syndromes, differentiated based on the dialectical perspective of TCM, have common pathogenesis. The USP17L15 gene was significantly up-regulated in all three syndromes. This gene is related to deubiquitination, and the functions of its encoded protein have rarely been studied. However, the correlation between deubiquitination and the development and progression of skin diseases is an important direction in the current research on psoriasis (Layman and Oliver, 2016). There are numerous members in the deubiquitinase family that are important components of the ubiquitin-proteasome pathway, and can participate in skin diseases such as psoriasis and skin squamous cell carcinoma by interacting with ectodysplasin receptor, Fas-associated death domain protein, B cell-stimulating factor-3, nuclear factor kappa-B protein kinase inhibitor, and other factors (Zilberman-Rudenko et al., 2016). Therefore, USP17L15 may be involved in the pathogenesis of psoriasis (Lippens et al., 2011). JUND was also significantly up-regulated in all three syndromes of psoriasis, and current research shows that its encoded protein can inhibit cell apoptosis (Wang T. et al., 2015). Moreover, this gene participates in the osteoclast differentiation pathway (Beranger et al., 2007). Studies have reported that inflammatory factors of psoriasis can promote the differentiation of human monocytes to active osteoclasts, thus promoting bone injury (Raimondo et al., 2017). Therefore, it is speculated that JUND may be involved in the pathogenesis of psoriasis and psoriatic arthritis. As a multifunctional protein, HNRNP is involved in various cellular processes, including RNA splicing (Chaudhury et al., 2010), transcriptional regulation (Miau et al., 1998) and immunoglobulin gene recombination (Dempsey et al., 1999). Our results showed that compared with the normal control group, HNRNPA2B1 was significantly down regulated in the three syndrome groups of psoriasis. Some studies have pointed out that HNRNP is down regulated in damaged skin of patients with psoriasis (Moldovan et al., 2019), which is consistent with our findings. This suggests that HNRNP is involved in the occurrence of psoriasis and may be related to the regulation of immune function related genes.
In the analysis of the common pathways between the three syndromes of psoriasis and healthy controls, it was found that there were abnormalities in the ribosome and olfactory transduction pathways in all three TCM syndromes of psoriasis. Ribosomal proteins in mitochondria participate in different cellular processes, such as cell cycle, apoptosis and mitochondrial homeostasis regulation. Mutation of MRPS genes in mitochondrial ribosome is related to mitochondrial dysfunction and diseases (Menezes et al., 2015; Richman et al., 2015). For example, MRPS16 (Bs16m) mutation can cause mitochondrial respiratory chain disorder and abnormal ATP level (Miller et al., 2004). A study has shown that there is a correlation between cytoplasmic metabolic processes and mitochondrial ribosomes (Suhm et al., 2018). Busse et al. (2014) reported that the olfactory receptor gene OR2AT4 is expressed in keratinocytes, and its exposure to artificial odors activates calcium signal transduction pathways, leading to wound healing. Jabbari et al. (2012), Li et al. (2014) found that the expression of OR2T10, OR2T11, OR52B6, OR9Q1, OR10V1, OR1L8, OR2A1, OR2A20P, OR2A42, and OR2A9P was down-regulated, while the expression of OR1J1 and ORMDL2 was up-regulated. Li et al. also found that a module closely associated with psoriasis detected by WGCNA was significantly enriched due to “olfactory receptor activity” (Li et al., 2014).
In summary, the three TCM syndromes of psoriasis showed their own specific as well as some common differential genes and signaling pathways. This study organically links the three common TCM syndromes of psoriasis and provides information for clarifying the molecular basis of TCM syndromes of psoriasis.
The data presented in the study are deposited in the GEO repository (https://www.ncbi.nlm.nih.gov/geo/), accession number GSE192867.
The studies involving human participants were reviewed and approved by the all procedures performed in studies involving human participants were in accordance with the ethical standards of Guangdong Provincial Hospital of Chinese Medicine Clinical Research Ethics Committee (Ethical approval No. B2012-53-03) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all eligible participants. The patients/participants provided their written informed consent to participate in this study.
All authors have participated extensively in the study and had proofread the final manuscript. CL, LH, and HG conceived and designed the research. YL and YQ conducted the experiments. YY, DY, HD, JD, and SY collected patients. QC helped sample preservation and transportation. YL, YQ, LL, JaW, JnW, and HC analyzed the data. YL wrote the manuscript. CL, LH, and HG approved and reviewed the final manuscript. All authors have read and agreed with the final manuscript.
This research was financially supported by the National Natural Science Foundation of China (82004363 and 81803804), the Guangdong Province Science and Technology Planning Project (2017A030310124, 2017A050506041, 2017B030314166, 2020B1111100006, and 2020B1212030006), Guangdong Basic and Applied Basic Research Foundation (2019A1515010636 and 2020A1515010607), Guangzhou Basic and Applied Basic Research Project (202102020545), State Key Laboratory of Dampness Syndrome of Chinese Medicine Special Fund (SZ2021ZZ29) and Guangdong Provincial Hospital of Chinese Medicine Special Fund (YN2018HK01, YN2018ZD08, YN2018RBA02, YN2016XP02, YN2019QJ04, and YN2019QJ08).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al-Harbi, N. O., Nadeem, A., Ahmad, S. F., Bakheet, S. A., El-Sherbeeny, A. M., Ibrahim, K. E., et al. (2020). Therapeutic Treatment with Ibrutinib Attenuates Imiquimod-Induced Psoriasis-like Inflammation in Mice through Downregulation of Oxidative and Inflammatory Mediators in Neutrophils and Dendritic Cells. Eur. J. Pharmacol. 877, 173088. doi:10.1016/j.ejphar.2020.173088
Angerer, H., Radermacher, M., Mańkowska, M., Steger, M., Zwicker, K., Heide, H., et al. (2014). The LYR Protein Subunit NB4M/NDUFA6 of Mitochondrial Complex I Anchors an Acyl Carrier Protein and Is Essential for Catalytic Activity. Proc. Natl. Acad. Sci. U S A. 111 (14), 5207–5212. doi:10.1073/pnas.1322438111
Armstrong, A. W., Harskamp, C. T., and Armstrong, E. J. (2013). Psoriasis and Metabolic Syndrome: a Systematic Review and Meta-Analysis of Observational Studies. J. Am. Acad. Dermatol. 68 (4), 654–662. doi:10.1016/j.jaad.2012.08.015
Beranger, G. E., Momier, D., Guigonis, J. M., Samson, M., Carle, G. F., and Scimeca, J. C. (2007). Differential Binding of poly(ADP-Ribose) Polymerase-1 and JunD/Fra2 Accounts for RANKL-Induced Tcirg1 Gene Expression during Osteoclastogenesis. J. Bone Miner. Res. 22 (7), 975–983. doi:10.1359/jbmr.070406
Boehncke, W. H., and Schön, M. P. (2015). Psoriasis. Lancet 386 (9997), 983–994. doi:10.1016/s0140-6736(14)61909-7
Busse, D., Kudella, P., Grüning, N. M., Gisselmann, G., Ständer, S., Luger, T., et al. (2014). A Synthetic Sandalwood Odorant Induces Wound-Healing Processes in Human Keratinocytes via the Olfactory Receptor OR2AT4. J. Invest. Dermatol. 134 (11), 2823–2832. doi:10.1038/jid.2014.273
Cannavò, S. P., Riso, G., Casciaro, M., Di Salvo, E., and Gangemi, S. (2019). Oxidative Stress Involvement in Psoriasis: a Systematic Review. Free Radic. Res. 53 (8), 829–840. doi:10.1080/10715762.2019.1648800
Chaudhury, A., Chander, P., and Howe, P. H. (2010). Heterogeneous Nuclear Ribonucleoproteins (hnRNPs) in Cellular Processes: Focus on hnRNP E1's Multifunctional Regulatory Roles. RNA 16 (8), 1449–1462. doi:10.1261/rna.2254110
Danielsen, K., Wilsgaard, T., Olsen, A. O., Eggen, A. E., Olsen, K., Cassano, P. A., et al. (2015). Elevated Odds of Metabolic Syndrome in Psoriasis: a Population-Based Study of Age and Sex Differences. Br. J. Dermatol. 172 (2), 419–427. doi:10.1111/bjd.13288
Dempsey, L. A., Sun, H., Hanakahi, L. A., and Maizels, N. (1999). G4 DNA Binding by LR1 and its Subunits, Nucleolin and hnRNP D, A Role for G-G Pairing in Immunoglobulin Switch Recombination. J. Biol. Chem. 274 (2), 1066–1071. doi:10.1074/jbc.274.2.1066
Farshi, P., Deshmukh, R. R., Nwankwo, J. O., Arkwright, R. T., Cvek, B., Liu, J., et al. (2015). Deubiquitinases (DUBs) and DUB Inhibitors: a Patent Review. Expert Opin. Ther. Pat. 25 (10), 1191–1208. doi:10.1517/13543776.2015.1056737
Greb, J. E., Goldminz, A. M., Elder, J. T., Lebwohl, M. G., Gladman, D. D., Wu, J. J., et al. (2016). Psoriasis. Nat. Rev. Dis. Primers 2, 16082. doi:10.1038/nrdp.2016.82
Hediger, M. A., Romero, M. F., Peng, J. B., Rolfs, A., Takanaga, H., and Bruford, E. A. (2004). The ABCs of Solute Carriers: Physiological, Pathological and Therapeutic Implications of Human Membrane Transport proteinsIntroduction. Pflugers Arch. 447 (5), 465–468. doi:10.1007/s00424-003-1192-y
Jabbari, A., Suárez-Fariñas, M., Dewell, S., and Krueger, J. G. (2012). Transcriptional Profiling of Psoriasis Using RNA-Seq Reveals Previously Unidentified Differentially Expressed Genes. J. Invest. Dermatol. 132 (1), 246–249. doi:10.1038/jid.2011.267
Kim, G. W., Park, H. J., Kim, H. S., Kim, S. H., Ko, H. C., Kim, B. S., et al. (2012). Analysis of Cardiovascular Risk Factors and Metabolic Syndrome in Korean Patients with Psoriasis. Ann. Dermatol. 24 (1), 11–15. doi:10.5021/ad.2012.24.1.11
Langley, R. G., Krueger, G. G., and Griffiths, C. E. (2005). Psoriasis: Epidemiology, Clinical Features, and Quality of Life. Ann. Rheum. Dis. 64 (Suppl. 2), ii18–5. doi:10.1136/ard.2004.033217
Layman, A. A., and Oliver, P. M. (2016). Ubiquitin Ligases and Deubiquitinating Enzymes in CD4+ T Cell Effector Fate Choice and Function. J. Immunol. 196 (10), 3975–3982. doi:10.4049/jimmunol.1502660
Li, B., Tsoi, L. C., Swindell, W. R., Gudjonsson, J. E., Tejasvi, T., Johnston, A., et al. (2014). Transcriptome Analysis of Psoriasis in a Large Case-Control Sample: RNA-Seq Provides Insights into Disease Mechanisms. J. Invest. Dermatol. 134 (7), 1828–1838. doi:10.1038/jid.2014.28
Lin, X., and Huang, T. (2016). Oxidative Stress in Psoriasis and Potential Therapeutic Use of Antioxidants. Free Radic. Res. 50 (6), 585–595. doi:10.3109/10715762.2016.1162301
Lin, Z., Peng, R., Sun, Y., Zhang, L., and Zhang, Z. (2021). Identification of Ribosomal Protein Family in Triple-Negative Breast Cancer by Bioinformatics Analysis. Biosci. Rep. 41 (1), BSR20200869. doi:10.1042/bsr20200869
Lippens, S., Lefebvre, S., Gilbert, B., Sze, M., Devos, M., Verhelst, K., et al. (2011). Keratinocyte-specific Ablation of the NF-Κb Regulatory Protein A20 (TNFAIP3) Reveals a Role in the Control of Epidermal Homeostasis. Cell Death Differ 18 (12), 1845–1853. doi:10.1038/cdd.2011.55
Lu, C. H., Yeh, D. W., Lai, C. Y., Liu, Y. L., Huang, L. R., Lee, A. Y., et al. (2018). USP17 Mediates Macrophage-Promoted Inflammation and Stemness in Lung Cancer Cells by Regulating TRAF2/TRAF3 Complex Formation. Oncogene 37 (49), 6327–6340. doi:10.1038/s41388-018-0411-0
Lu, C. J., Yu, J. J., and Deng, J. W. (2012a). Disease-syndrome Combination Clinical Study of Psoriasis: Present Status, Advantages, and Prospects. Chin. J. Integr. Med. 18 (3), 166–171. doi:10.1007/s11655-012-1006-1
Lu, C. J., Zeng, Z., Xie, X. L., and Ning, J. (2012b). Distribution of Chinese Medical Syndrome in Ordinary Psoriasis: Literature from 1979 to 2010. J. Trad. Chin. Med. 11 (53), 64–66. doi:10.13359/j.cnki.gzxbtcm.2012.04.013
Lu, Y., Chen, H., Zhang, J., Tang, B., Zhang, H., Ma, C., et al. (2021b). Fuzhenghefuzhiyang Formula (FZHFZY) Improves Epidermal Differentiation via Suppression of the Akt/mTORC1/S6K1 Signalling Pathway in Psoriatic Models. Front. Pharmacol. 12, 650816. doi:10.3389/fphar.2021.650816
Lu, Y., Yang, Y., Zhang, J., Zhang, H., Ma, C., Tang, X., et al. (2021a). Anti-angiogenic Efficacy of PSORI-CM02 and the Associated Mechanism in Psoriasis In Vitro and In Vivo. Front. Immunol. 12, 649591. doi:10.3389/fimmu.2021.649591
Mendez-David, I., El-Ali, Z., Hen, R., Falissard, B., Corruble, E., Gardier, A. M., et al. (2013). A Method for Biomarker Measurements in Peripheral Blood Mononuclear Cells Isolated from Anxious and Depressed Mice: β-arrestin 1 Protein Levels in Depression and Treatment. Front. Pharmacol. 4, 124. doi:10.3389/fphar.2013.00124
Menezes, M. J., Guo, Y., Zhang, J., Riley, L. G., Cooper, S. T., Thorburn, D. R., et al. (2015). Mutation in Mitochondrial Ribosomal Protein S7 (MRPS7) Causes Congenital Sensorineural Deafness, Progressive Hepatic and Renal Failure and Lactic Acidemia. Hum. Mol. Genet. 24 (8), 2297–2307. doi:10.1093/hmg/ddu747
Menter, A., Gottlieb, A., Feldman, S. R., Van Voorhees, A. S., Leonardi, C. L., Gordon, K. B., et al. (2008). Guidelines of Care for the Management of Psoriasis and Psoriatic Arthritis: Section 1. Overview of Psoriasis and Guidelines of Care for the Treatment of Psoriasis with Biologics. J. Am. Acad. Dermatol. 58 (5), 826–850. doi:10.1016/j.jaad.2008.02.039
Miau, L. H., Chang, C. J., Shen, B. J., Tsai, W. H., and Lee, S. C. (1998). Identification of Heterogeneous Nuclear Ribonucleoprotein K (hnRNP K) as a Repressor of C/EBPbeta-mediated Gene Activation. J. Biol. Chem. 273 (17), 10784–10791. doi:10.1074/jbc.273.17.10784
Miller, C., Saada, A., Shaul, N., Shabtai, N., Ben-Shalom, E., Shaag, A., et al. (2004). Defective Mitochondrial Translation Caused by a Ribosomal Protein (MRPS16) Mutation. Ann. Neurol. 56 (5), 734–738. doi:10.1002/ana.20282
Moldovan, L. I., Hansen, T. B., Venø, M. T., Okholm, T. L. H., Andersen, T. L., Hager, H., et al. (2019). High-throughput RNA Sequencing from Paired Lesional- and Non-lesional Skin Reveals Major Alterations in the Psoriasis circRNAome. BMC Med. Genomics. 12 (1), 174. doi:10.1186/s12920-019-0616-2
Murray, J., Taylor, S. W., Zhang, B., Ghosh, S. S., and Capaldi, R. A. (2003). Oxidative Damage to Mitochondrial Complex I Due to Peroxynitrite: Identification of Reactive Tyrosines by Mass Spectrometry. J. Biol. Chem. 278 (39), 37223–37230. doi:10.1074/jbc.M305694200
Nast, A., Gisondi, P., Ormerod, A. D., Saiag, P., Smith, C., Spuls, P. I., et al. (2015). European S3-Guidelines on the Systemic Treatment of Psoriasis Vulgaris--Update 2015--Short Version--EDF in Cooperation with EADV and IPC. J. Eur. Acad. Dermatol. Venereol. 29 (12), 2277–2294. doi:10.1111/jdv.13354
Perera, G. K., Di Meglio, P., and Nestle, F. O. (2012). Psoriasis. Annu. Rev. Pathol. 7, 385–422. doi:10.1146/annurev-pathol-011811-132448
Raimondo, A., Lembo, S., Di Caprio, R., Donnarumma, G., Monfrecola, G., Balato, N., et al. (2017). Psoriatic Cutaneous Inflammation Promotes Human Monocyte Differentiation into Active Osteoclasts, Facilitating Bone Damage. Eur. J. Immunol. 47 (6), 1062–1074. doi:10.1002/eji.201646774
Ravnskjaer, K., Madiraju, A., and Montminy, M. (2016). Role of the cAMP Pathway in Glucose and Lipid Metabolism. Handb. Exp. Pharmacol. 233, 29–49. doi:10.1007/164_2015_32
Richman, T. R., Ermer, J. A., Davies, S. M., Perks, K. L., Viola, H. M., Shearwood, A. M., et al. (2015). Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction. Plos Genet. 11 (3), e1005089. doi:10.1371/journal.pgen.1005089
Rives, M. L., Javitch, J. A., and Wickenden, A. D. (2017). Potentiating SLC Transporter Activity: Emerging Drug Discovery Opportunities. Biochem. Pharmacol. 135, 1–11. doi:10.1016/j.bcp.2017.02.010
Song, W., Li, D., Tao, L., Luo, Q., and Chen, L. (2020). Solute Carrier Transporters: the Metabolic Gatekeepers of Immune Cells. Acta Pharm. Sin B 10 (1), 61–78. doi:10.1016/j.apsb.2019.12.006
Suhm, T., Kaimal, J. M., Dawitz, H., Peselj, C., Masser, A. E., Hanzén, S., et al. (2018). Mitochondrial Translation Efficiency Controls Cytoplasmic Protein Homeostasis. Cell Metab 27 (6), 1309–e6. doi:10.1016/j.cmet.2018.04.011
Unni, S., Thiyagarajan, S., Srinivas Bharath, M. M., and Padmanabhan, B. (2019). Tryptophan Oxidation in the UQCRC1 Subunit of Mitochondrial Complex III (Ubiquinol-Cytochrome C Reductase) in a Mouse Model of Myodegeneration Causes Large Structural Changes in the Complex: A Molecular Dynamics Simulation Study. Sci. Rep. 9 (1), 10694. doi:10.1038/s41598-019-47018-6
Wang, T., Li, P., Ma, X., Tian, P., Han, C., Zang, J., et al. (2015a). MicroRNA-494 Inhibition Protects Nucleus Pulposus Cells from TNF-α-Induced Apoptosis by Targeting JunD. Biochimie 115, 1–7. doi:10.1016/j.biochi.2015.04.011
Wang, W., Nag, S., Zhang, X., Wang, M. H., Wang, H., Zhou, J., et al. (2015b). Ribosomal Proteins and Human Diseases: Pathogenesis, Molecular Mechanisms, and Therapeutic Implications. Med. Res. Rev. 35 (2), 225–285. doi:10.1002/med.21327
Xiong, X., Zhao, Y., Tang, F., Wei, D., Thomas, D., Wang, X., et al. (2014). Ribosomal Protein S27-like Is a Physiological Regulator of P53 that Suppresses Genomic Instability and Tumorigenesis. Elife 3, e02236. doi:10.7554/eLife.02236
Xu, F., Xu, J., Xiong, X., and Deng, Y. (2019). Salidroside Inhibits MAPK, NF-Κb, and STAT3 Pathways in Psoriasis-Associated Oxidative Stress via SIRT1 Activation. Redox Rep. 24 (1), 70–74. doi:10.1080/13510002.2019.1658377
Yan, Y. H., Lu, C. J., Yao, D. N., Du, H., and Li, H. L. (2012). Study on the Correlation between the Basic Syndrome of Traditional Chinese Medicine and the Stage and Condition of Psoriasis Vulgaris. J. Guangzhou Univ. Trad. Chin. Med. 29 (04), 358–362. doi:10.13359/j.cnki.gzxbtcm.2012.04.013
Young, M. J., Hsu, K. C., Lin, T. E., Chang, W. C., and Hung, J. J. (2019). The Role of Ubiquitin-specific Peptidases in Cancer Progression. J. Biomed. Sci. 26 (1), 42. doi:10.1186/s12929-019-0522-0
Yu, J. J., Zhang, C. S., Coyle, M. E., Du, Y., Zhang, A. L., Guo, X., et al. (2017). Compound Glycyrrhizin Plus Conventional Therapy for Psoriasis Vulgaris: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Med. Res. Opin. 33 (2), 279–287. doi:10.1080/03007995.2016.1254605
Yu, J. J., Zhang, C. S., Zhang, A. L., May, B., Xue, C. C., and Lu, C. (2013). Add-on Effect of Chinese Herbal Medicine bath to Phototherapy for Psoriasis Vulgaris: a Systematic Review. Evid. Based Complement. Alternat Med. 2013, 673078. doi:10.1155/2013/673078
Yue, L., Ailin, W., Jinwei, Z., Leng, L., Jianan, W., Li, L., et al. (2019). PSORI-CM02 Ameliorates Psoriasis In Vivo and In Vitro by Inducing Autophagy via Inhibition of the PI3K/Akt/mTOR Pathway. Phytomedicine 64, 153054. doi:10.1016/j.phymed.2019.153054
Zhang, C. S., May, B., Yan, Y., Yu, J. J., Yao, D., Chang, S., et al. (2016). Terms Referring to Psoriasis Vulgaris in the Classical Chinese Medicine Literature: a Systematic Analysis. Complement. Ther. Med. 25, 55–60. doi:10.1016/j.ctim.2015.12.014
Zhang, C. S., Yu, J. J., Parker, S., Zhang, A. L., May, B., Lu, C., et al. (2014). Oral Chinese Herbal Medicine Combined with Pharmacotherapy for Psoriasis Vulgaris: a Systematic Review. Int. J. Dermatol. 53 (11), 1305–1318. doi:10.1111/ijd.12607
Zhou, Q., Mrowietz, U., and Rostami-Yazdi, M. (2009). Oxidative Stress in the Pathogenesis of Psoriasis. Free Radic. Biol. Med. 47 (7), 891–905. doi:10.1016/j.freeradbiomed.2009.06.033
Keywords: gene chip, gene expression, psoriasis vulgaris, TCM syndrome type, peripheral blood monocytes
Citation: Lu Y, Qi Y, Li L, Yan Y, Wei J, Yao D, Wu J, Deng H, Deng J, Ye S, Chen H, Chen Q, Gao H, Han L and Lu C (2022) The Gene Expression Analysis of Peripheral Blood Monocytes From Psoriasis Vulgaris Patients With Different Traditional Chinese Medicine Syndromes. Front. Pharmacol. 12:759741. doi: 10.3389/fphar.2021.759741
Received: 17 August 2021; Accepted: 13 December 2021;
Published: 19 January 2022.
Edited by:
Karl Tsim, Hong Kong University of Science and Technology, Hong Kong SAR, ChinaReviewed by:
Yuan-Hao Wu, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, ChinaCopyright © 2022 Lu, Qi, Li, Yan, Wei, Yao, Wu, Deng, Deng, Ye, Chen, Chen, Gao, Han and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hengjun Gao, aGVuZ2p1bl9nYW9Ac2hiaW9jaGlwLmNvbQ==; Ling Han, bGluZ2hhbjk5QGd6dWNtLmVkdS5jbg==; Chuanjian Lu, bGNqQGd6dWNtLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.