- 1Department of Cardiology, The Second Medical Center and National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, Beijing, China
- 2Beijing Key Laboratory of Precision Medicine for Chronic Heart Failure, Chinese PLA General Hospital, Beijing, China
- 3Ping An Health Technology, Beijing, China
- 4Institute of Materia Medica, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 5Research Center of Medical Big Data, The Medical Innovation Research Division, Chinese PLA General Hospital, Beijing, China
Objective: Although guidelines have recommended standardized drug treatment for heart failure (HF), there are still many challenges in making the correct clinical decisions due to the complicated clinical situations of HF patients. Each patient would satisfy several recommendations, meaning the decision tree of HF treatment should be nonmutually exclusive, and the same patient would be allocated to several leaf nodes in the decision tree. In the current study, we aim to propose a way to ensemble a nonmutually exclusive decision tree for recommendation system for complicated diseases, such as HF.
Methods: The nonmutually exclusive decision tree was constructed via knowledge rules summarized from the HF clinical guidelines. Then similar patients were defined as those who followed the same pattern of leaf node allocation according to the decision tree. The frequent medication patterns for each similar patient were mined using the Apriori algorithms, and we also carried out the outcome prognosis analyses to show the capability for the evidence-based medication recommendations of our nonmutually exclusive decision tree.
Results: Based on a large database that included 29,689 patients with 84,705 admissions, we tested the framework for HF treatment recommendation. In the constructed decision tree, the HF treatment recommendations were grouped into two independent parts. The first part was recommendations for new cases, and the second part was recommendations when patients had different historical medication. There are 14 leaf nodes in our decision tree, and most of the leaf nodes had a guideline adherence of around 90%. We reported the top 10 popular similar patients, which accounted for 32.84% of the whole population. In addition, the multiple outcome prognosis analyses were carried out to assess the medications for one of the subgroups of similar patients. Our results showed even for the subgroup of the same similar patients that no one medication pattern would benefit all outcomes.
Conclusion: In the present study, the methodology to construct a nonmutually exclusive decision tree for medication recommendations for HF and its application in CDSS was proposed. Our framework is universal for most diseases and could be generally applied in developing the CDSS for treatment.
Introduction
Heart failure (HF) is a clinical syndrome that is a result of the abnormalities in the structure and function of the myocardium impairing cardiac output or decreasing the filling of the ventricles (Metra and Teerlink, 2017). The treatment of heart failure is guided by the stage of symptoms and signs as well as a robust literature on therapies proven to be beneficial by randomized trials (Yancy et al., 2017). Despite tangible advances in recent years, HF is still a leading cause of death worldwide (Conrad et al., 2018). As a terminal stage of patients, HF is complexed with multiple comorbidities, such as coronary heart disease, hypertension, and diabetes (Chamberlain et al., 2020), making the clinical decision process complicated. Although guidelines have recommended standardized drug treatment for HF, given the complexity of HF, there are still many challenges in making the correct clinical decisions. Artificial Intelligence-Clinical Decision Support Systems (AI-CDSSs) has the potential to assist physicians in the treatment decision process in HF.
CDSSs provide evidence for physicians in making clinical decisions, such as differential diagnosis and recommending medications (Bates et al., 2001; Office of the National Coordinator for Health Information Technology, Department of Health and Human Services, 2012). The key component for providing the evidence is finding similar patients. Patients who have similar clinical conditions are expected to suffer from similar diseases and be treated with similar medications (Downie et al., 2020). Within a similar patient group, the retrospective EHR (electronic health record) data can be used to rank all candidate medications that occurred in a similar patient group (Austin et al., 2020). The way of ranking those candidate suggestions is by calculating the conditional probability (such as the fraction of diagnoses) and the effectiveness (such as prognoses after treatment with certain medication) of the suggestions.
To maintain the clinical correctness and interpretability of the model used in CDSS, knowledge-based decision trees should be constructed. A decision tree is a model consisting of consecutive decisions, starting from the root node, and each sample would be allocated to different branches based on the condition it satisfied. Nodes without downstream branches are called the leaf node, and all samples would be classified into different leaf nodes according to the decision tree. In general, the construction of such a decision tree is based on the summary of the clinical rules for a specific disease (Zhao et al., 2020). The clinical rules are composed of the conditions and actions simultaneously, indicating the actions under certain conditions. Decision trees are constructed by integrating all clinical rules to partition patients into specific subgroups represented by the leaf nodes. The conditions and corresponding actions, suggested by the clinical guidelines, are denoted in the non-leaf nodes (Song and Lu, 2015).
The core challenge in the construction of the decision tree relies on the integration of clinical rules (Ehrhardt et al., 2021). For some diseases, such as type 2 diabetes and hypertension, the whole population should be partitioned systematically according to the clinical guidelines. Therefore, the mutually exclusive decision trees are easily constructed following the clinical guidelines. For instance, in our work in the AMIA 2019 Annual Symposium (Sun et al., 2019), the whole population of diabetes patients was grouped by the HbA1c (hemoglobin A1C) value and numbers of historical antidiabetic medications according to the clinical guidelines of type 2 diabetes. In this case, each patient only belonged to one unique group. However, in most other cases, the clinical guidelines have provided independent clinical condition rules, and there are no logical exclusiveness between different clinical rules (Keikes et al., 2021). For example, for heart failure patients, the current medication recommendations are based on the previous drugs (Ponikowski et al., 2016), and historically using one drug A is not mutually exclusive with using another drug B. Therefore, it is unrealistic to integrate those recommendations into a mutually exclusive decision tree.
It is challenging to convert clinical guidelines into a normal decision tree since those trees, where one patient can only be allocated in a unique leaf node, are designed to be mutually exclusive. To tackle this problem, we proposed a novel way to construct and leverage a nonmutually exclusive decision tree in CDSSs. The construction process was just listing all the clinical rules horizontally if they were independent. Therefore, each branch was not mutually exclusive with others in the decision tree. One patient could enter multiple branches and be allocated to several different leaf nodes simultaneously.
In the current study, we proposed a framework to construct the nonmutually exclusive decision tree and applied it in the HF treatment recommendation as shown in Figure 1. The three essential components for CDSS in application included the definition of similar patients, the medication patterns for each similar patient group, and evidence-based recommendation strategy. For the first component, similar patients were defined as patients following the exact same leaf node allocation patterns in the nonmutually exclusive tree. Second, to obtain the medication patterns for each similar patient group, the frequent mining algorithm was applied. Third, to provide real-world evidence, we analyzed the prognoses for each medication pattern, which were calibrated by the propensity score of a patient following a particular medication pattern. The multiple prognoses provided a multidimensional view for physicians when making decisions.
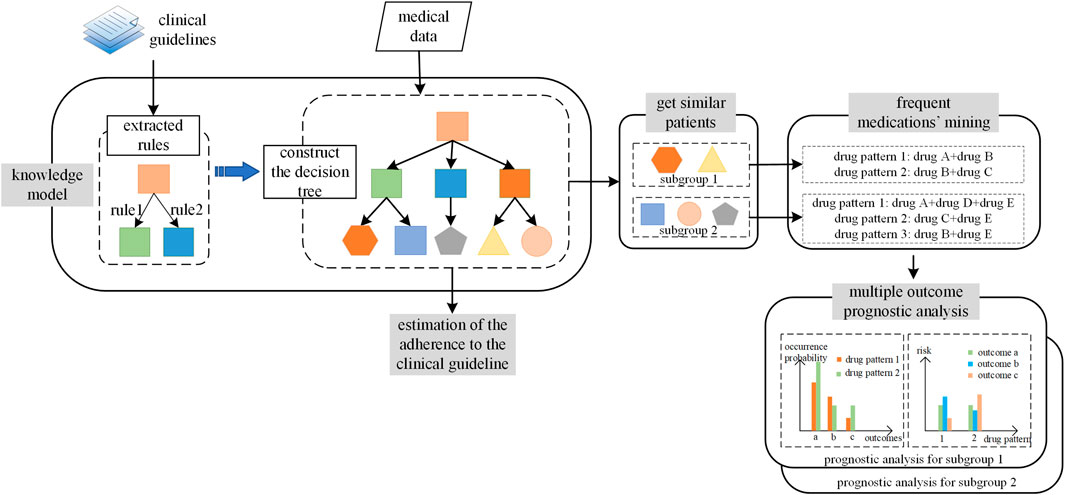
FIGURE 1. The workflow to construct and apply nonmutually exclusive decision tree. The workflow included two parts. The first part is the procedure of the construction of nonmutually exclusive decision tree, denoted as “knowledge model.” The second part is the application of the nonmutually exclusive decision tree, which included tree components: the fine group of the patients, the frequent medication patterns mining for each patients group, and the multiple outcomes prognoses analyses.
Methods
This study was approved by the ethics committee of the Chinese PLA General Hospital.
Datasets
The dataset we used was extracted from a heart failure database in the Chinese PLA General Hospital in Beijing, China. This database was initially constructed for all patients diagnosed with heart failure from 2008 to 2018. The database included 29,689 patients with 84,705 admissions in total. According to the classification and diagnostic criteria of heart failure in the guidelines (Ponikowski et al., 2016), all samples that satisfied the definition of HF with reduced ejection fraction (HFrEF) were included in the current study. A total of 9,414 patients with 16,063 visits were obtained. Each record consisted of demographic information, physical examinations, laboratory test results, medication history, and current medication.
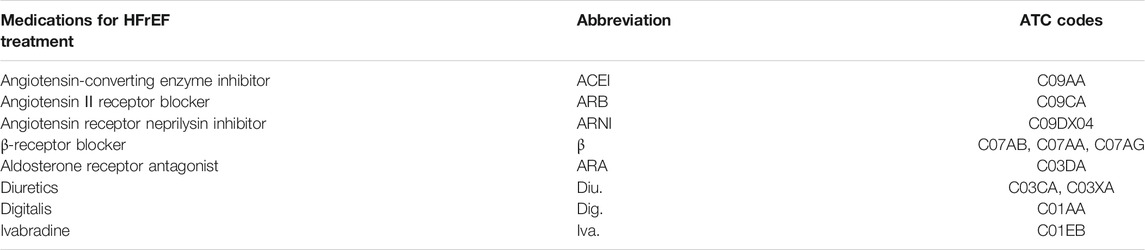
It should be noted that the quality of the data was critical in providing real-world evidence. We used the data of inpatients instead of those of outpatients, considering that the coverages of many features of the data of inpatients were much higher than the data of the outpatients. Such substitution hypothesized that the medication for inpatients and outpatients of HfrEF was similar in terms of medication categories, such as ACEIs (angiotensin-converting enzyme inhibitors) or β-receptor blocker, and our CDSS also provided real-world evidence in the granularity of medication categories. The medication considered in the current study is listed in Table 1. Another hypothesis was that the relative day of the admission for one patient was insignificantly related to the medication decisions, which we had checked in the current study by regressing the medication decisions with the relative day as the condition.
Data Preprocessing
The preprocessing of the data of inpatients contained three steps: data standardization, data segmentation, and missing value imputations.
First, the medications used in the current study were standardized by the ATC (Anatomical Therapeutic Chemical) five-digit code as listed in Table 1.
Second, the data of the inpatients were fragmented by the day. As shown in Figure 2, the records of the inpatients with a length of stay equal to n could be converted into n samples. For each sample, the history was referred to as the activities that happened in the previous day. It should be noted that by multivariable logistic regression to the prescriptions, the day indexes in the segmented daily records were independent of the medications in each day. A total of 132,158 samples were obtained after data fragmentation.
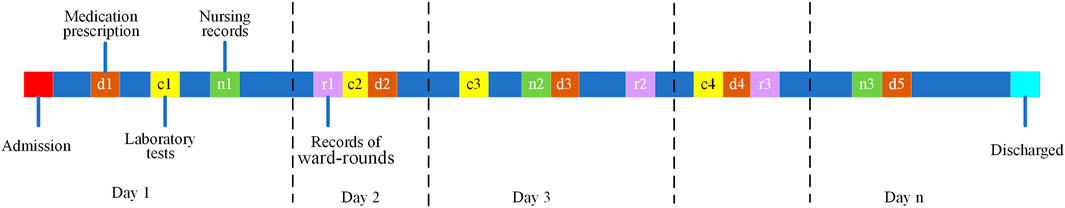
FIGURE 2. The schematic diagram of an n-day electronic health record (EHR) for inpatient. The data would be segmented into n fragments as indicated by the vertical dash lines, and the colorful boxes represent the different categories of information: d for the prescript medications, c for the results of the laboratory tests, n for the nursing records, and r for the records of the ward rounds.
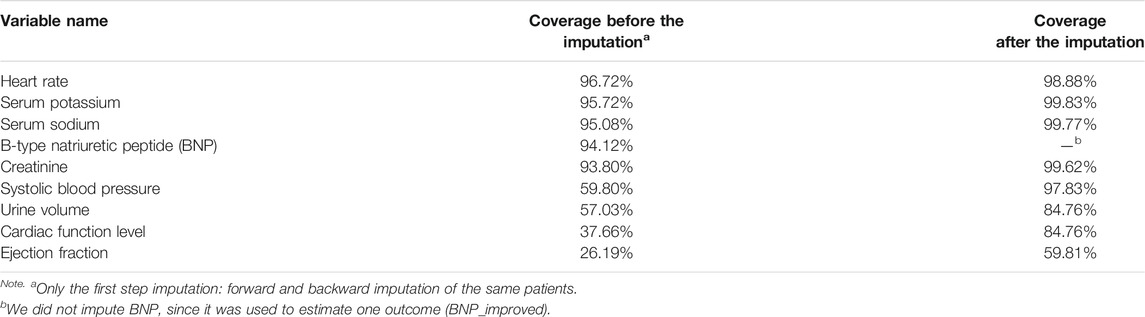
Third, a two-step strategy of missing value imputation was utilized in the current study. First, the missing values were imputed forward or backward for the same admission. Then the rest of the missing values were imputed with means for continuous variables and medoids for discrete variables. The coverage of each variable before and after the first step imputation is listed in Table2.
Construction of Knowledge-Based Non-mutually Exclusive Decision Tree
To construct the knowledge model, clinical recommendations were first summarized and extracted from the clinical guidelines. Then the clinical recommendations were organized in the form of a decision tree with each branch representing one recommendation in the clinical guidelines. The decision-tree-like knowledge model was constructed and reviewed by multiple clinical experts.
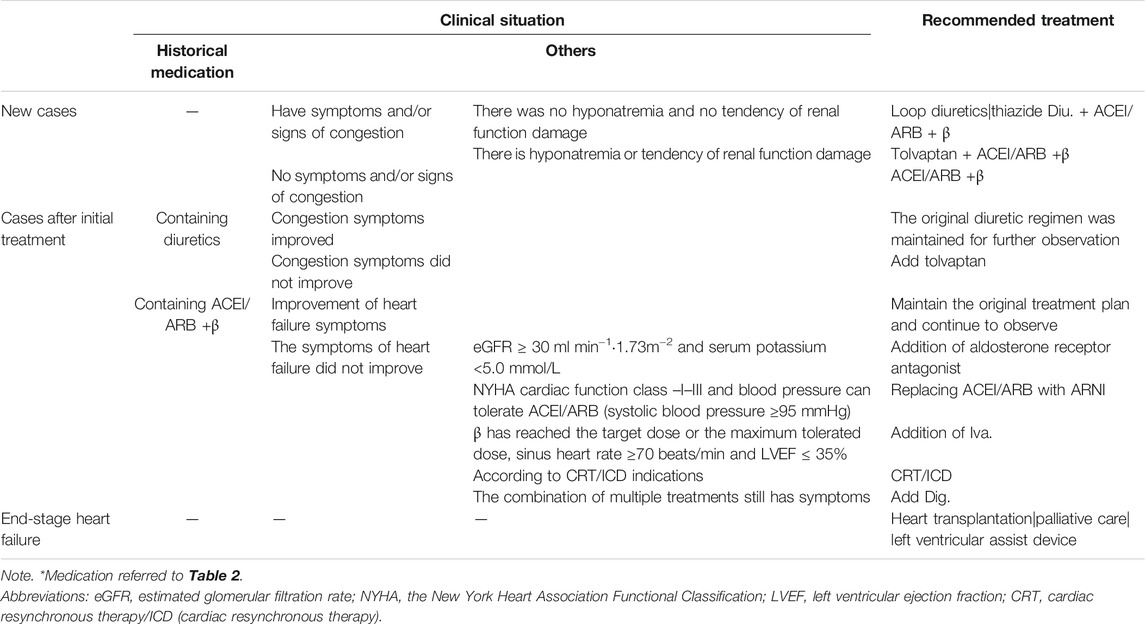
As shown in Table 3, the clinical recommendations for HfrEF were extracted from the 2016 ESC guidelines for the diagnosis and treatment of HF (Ponikowski et al., 2016). According to the clinical guidelines, the treatment of HfrEF was divided into three phases: the new cases without historical HF treatment, cases after the initial treatment, and end-stage heart failure cases. The last phase was excluded since there was no medication-related treatment. For each phase, the clinical conditions and the recommended treatments were listed horizontally. For the new cases, the prescriptions were made based on whether there were symptoms of congestion. Besides, for the ones who had congestion symptoms, patients were further divided according to the existence of hyponatremia and the tendency of renal function damage. For cases after the initial treatment, the medication decisions were determined by the previous treatment and the current symptoms.
To construct the decision tree, the extracted clinical rules were integrated in the following ways. If the clinical conditions were independent, the clinical rules were organized horizontally; otherwise, they were integrated following the logical hierarchy. The constructed decision tree for the new cases and the cases after initial treatment were shown, respectively, in Figures 3A, B. The leaf node represented the treatment strategies as listed in Table 3. Note that the leaf nodes indexed by 4, 8, 10, 12, and 14 stood for the same treatment as previous ones.

FIGURE 3. The integrated knowledge-based decision trees for heart failure (HF) with reduced ejection fraction (HFrEF): (A) the decision tree for new cases; (B) the decision tree for cases with initial treatment of HFrEF. The nonmutually exclusive branches were labeled with the red *. For (B), the medications were grouped into two independent parts as indicated by the dashed boxes. The leaf nodes were colored in green, indicating the medications for each branch. The details for decision condition 1 was the existence of congestion symptom; for decision condition 2, the existence of hyponatremia or the tendency of renal function impairment; for decision condition 3, the whether the congestion symptoms got improved; for decision condition 4, whether the heart failure symptoms got improved; for decision condition 5, whether the LVEF ≤40% and no symptom improvement with a combination of multiple medications; for decision condition 6, whether the eGFR ≥30 ml min−1,·1.73 m−2, and the blood potassium <5.0 mmol/L; for decision condition 7, whether the NYHA was between II and III and the tolerance of ACEI/ARB (the systolic blood pressure ≥95 mmHg); for decision condition 8, whether the LVEF ≤35%, the sinus heart rate ≥70 beats/min, and β-receptor blocker reached the target dose (or the maximum tolerated dose). Abbreviations: eGFR, estimated glomerular filtration rate; NYHA, the New York Heart Association Functional Classification; LVEF, left ventricular ejection fraction.
It should be noted that in Figure 3B, the decision tree had four branches, and the leftmost one (marked with a red star) was independent of the other three. Thus, patients allocated in the leftmost branch would not be mutually exclusive with the other three. The clinical records would be allocated to leaf node 4 and leaf node 6 at the same time. All the nonmutually exclusive branches were marked with a star in Figure 3B.
Similar patients and medication recommendation
Similar patients
After the knowledge tree was constructed, the clinical records would be allocated to a set of leaf nodes. In the current study, we proposed defining similar patients as those who followed the same pattern of leaf node allocation. For example, patients allocated to leaf node 4 and leaf node 6 were treated as the same patient group. Note that only the exact leaf allocated patterns were counted in the fine grouping of the patients.
Frequent Medications’ Mining for Each Subgroup and Each Non-Mutually Exclusive Part in the Decision Tree
To provide real-world clinical evidence, for each patient subgroup, the frequent drug patterns were mined using the Apriori algorithms (Ding et al., 2008). The Apriori algorithm was a powerful tool to mine the frequent patterns for transaction data. By iteratively adding candidate frequent items into the kth frequent itemsets, the k+1st frequent itemsets were generated. Then those generated candidates were excluded once they did not satisfy the frequency threshold. The remaining k+1st frequent itemsets were used for the next round of generation.
It should be noted that the nonmutually exclusive feature led to the independence of the frequent medication patterns between nonmutually exclusive parts in the decision tree. As shown in Figure 3B, the prescription for a particular patient would be the combination of the diuretics with other medicines related to heart functions, such as ACEI and β-receptor blocker. Therefore, the frequent medication patterns would be mined, respectively, for diuretics and heart function-related drugs.
Prognosis’s Analyses
Multiple Outcomes
The real-world evidence for each subgroup was provided based on the prognosis analyses. The real-world evidence played a critical role for leaf nodes with variable choice of medications. Therefore, multiple outcomes were considered in the current study to assist the physician to make decisions even for the same patient subgroup.
To assess each treatment effect of HfrEF, six outcomes were considered in the current study. They are hyponatremia, hypernatremia, hypokalemia, hyperkalemia, acute kidney injury, and the reduction of B-type natriuretic peptide. They could be grouped into the following three categories:
(1) Electrolyte disturbance
In the treatment of HfrEF, electrolyte disturbances mainly referred to the blood potassium and the blood sodium. The high or low blood potassium and the blood sodium were all considered as electrolyte disturbances. Hyponatremia is defined as blood sodium <135 mmol/L, while hypernatremia is defined as blood sodium >145 mmol/L. The lower limit for blood potassium was 3.5 mmol/L, and the upper limit was 7.0 mmol/L. Any values out of the range were defined as hypokalemia or hyperkalemia accordingly.
(2) Acute kidney injury
Heart failure and chronic kidney disease often coexist, and the existence of renal insufficiency could worsen the prognosis of HF (Bock and Gottlieb, 2010). The following standard was used to judge the occurrence of acute kidney injury (a severe case for renal insufficiency) (Khwaja, 2012): the creatinine rises 26.5 µmol/L within 48 h or 1.5 times of the baseline within 7 days (increase >50%), and urine output of <0.5 ml kg−1 h−1 (time >6 h).
(3) B-type natriuretic peptide
The B-type natriuretic peptide (BNP) was one of the most common heart failure biomarkers used in screening, diagnosis, severity assessment, and prognosis of heart failure. Also, it was an indicator of the risk of cardiovascular events in patients with heart failure after discharge. The lower the BNP values, the better the clinical conditions the patient had. We chose a 20% reduction of BNP as the indicator of improvement of clinical conditions for HF, denoted as BNP_ improved17. The 20% increase in BNP was labeled as the deterioration of HF conditions.
Medication Assessments Using Propensity Score
As aforementioned, the treatment using diuretic and other HF drugs were independent; the prognosis analyses were also carried out separately for diuretic and other HF drugs. For each group of similar patients with each nonmutually exclusive part, suppose there were n candidate medications, and there would be 6n prognosis analyses since there were six outcomes considered in the current study.
To calibrate the bias introduced by patients using the specific medication, the propensity score of the medications was included in the regression to the outcomes. To be specific, for the prognosis analysis with treatment T, other variables X, and the outcome Y, we first regressed T on X and obtained the propensity score of a patient using treatment T. Then the propensity score, as well as the variable X, was used to the regression of the outcome Y. The formula for the second regression was:
where
The
Results
Loading all Clinical Records to the Knowledge-Based Decision Tree for Heart Failure With Reduced Ejection Fraction
Historical Medication Patterns’ Mining and Expansion of the Knowledge-Based Decision Tree
According to the HfrEF knowledge decision tree, the first decision point was the history of medication of the patient. To understand the distribution of medication history, we first mined the history medication using the Apriori algorithms (Agrawal and Srikant, 1994) as introduced in the method section and identified the frequent sets of medications with a support threshold of 0.05%. The frequent set is shown in Figure 4. Only the top 10 medication strategies had their name listed in Figure 4 for the sake of clarity. The top 10 medication strategies accounted for 78.55% of the total sample of 132,158. The most popular medication strategy was no medication, which accounted for 30.28%. Those samples would be loaded into the decision tree for new cases (see Figure 3A), while the remaining 69.72% would be loaded into the decision tree shown in Figure 3B.

FIGURE 4. Distribution of the mined frequent historical medications for all HFrEF clinical records (132,158 in total). The top 10 medications were listed with the name and the proportions. Please see Table 1 for the abbreviations of the medicines. The medicines labeled in red were not considered as historical medication strategies in the decision trees for HFrEF.
Among all samples loaded for Figure 3B, 47,028 records were successfully loaded into the tree, which accounted for 35.58% (47,028/132,158) of the candidate samples. The main reasons came from the missing historical medication strategies in the knowledge-based decision tree. The other reason for records failed to be loaded into Figure 3B was the missing value for clinical conditions, such as the ejection fraction. It should be noted that the records were loaded before the second imputation step.
As shown in Figure 4, historically, only β-receptor blocker was used for heart function accounting for 15.85% of the records, which consisted of using β-receptor blocker (12.63%) only and using β-receptor blocker with diuretics (3.22%). The same reason for the other historical medication strategies was denoted in red in Figure 4. Considering that HF patients only had β-receptor blocker (15.85%), or ACEI/ARB (angiotensin II receptor blocker) (3.47%) were common in clinical practice, the knowledge decision tree for the cases after initial treatment got expanded as shown in Figure 5. As shown in Figure 5, there were two expanded branches: branch (6) and branch (7). Branch (6) was ACEI/ARB, and branch (7) was a β-receptor blocker. The corresponding decision condition 9 was defined as systolic blood pressure >90 mmHg or heart rate >60 beats/min, and decision condition 10 was systolic blood pressure >90 mmHg or heart rate >60 beats/min. Leaf node 15 represented adding β-receptor blocker based on ACEI/ARB medication, leaf node 16 represented maintaining the original medication ACEI/ARB, leaf node 17 represented adding ACEI/ARB based on β-receptor blocker, and leaf node 18 indicated maintaining the original medication β-receptor blocker.
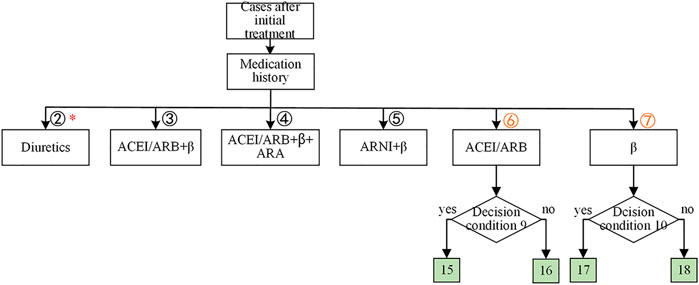
FIGURE 5. The expansion of the knowledge-based decision tree for cases with initial treatment of HFrEF. The nonmutually exclusive branches were labeled with red *. The leaf nodes were colored in green, indicating the medications for each branch. The decision condition 9 was whether the systolic blood pressure >90 mmHg or the heart rate >60 beats/min. The decision condition 10 was whether the systolic blood pressure >90 mmHg or the heart rate >60 beats/min.
Ambiguous clinical conditions
Since the existence of some subjective or hardly recorded clinical conditions, it may be difficult to run through the decision tree. Thus, we proposed their alternative estimations. For example, the decision condition 1/3/4 (the existence of congestion symptoms/the improvement of congestion symptoms/the improvement of heart failure symptoms) was difficult to assess directly by using our data. For the decision condition ¾, changes in the BNP values were used instead (Nassif et al., 2019). More specifically, if the BNP value decreased by more than 20%, the symptoms were defined as improved, and vice versa. For decision condition 1, there was no substitute for judgment, so we did not analyze the corresponding branch.
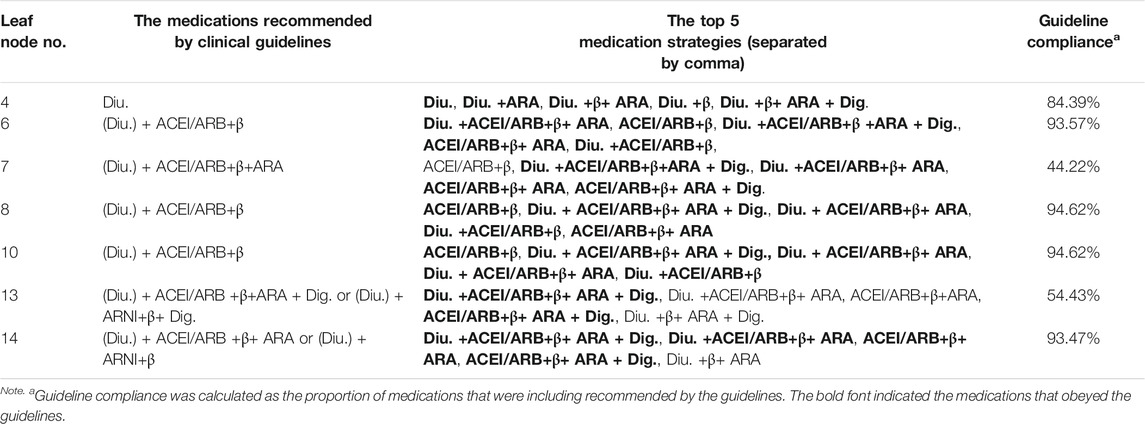
Estimation of the Adherence to the Clinical Guideline for Each Leaf Node
After loading the records to the decision tree, we estimated the adherence of each clinical node to the clinical guideline. By adherence, we meant the medication patterns were not in conflict with the clinical guidelines. To estimate the adherence, the frequent medication patterns were mined for each leaf node, and the top ones were used to be compared with the guideline-recommended medications. The top five frequent medications and the corresponding guideline adherence for each leaf node are listed in Table 4. By the time the dataset was generated, there was no usage of new drugs, such as tolvaptan, ARNI (angiotensin receptor neprilysin inhibitor), and ivabradine. Therefore, the leaf nodes involving the new drugs were excluded from the following analyses, such as leaf node 4. Most of the leaf nodes had a guideline adherence of around 90%, except leaf nodes 7 and 13.
Similar Patients for Non-Mutually Exclusive Decision Tree
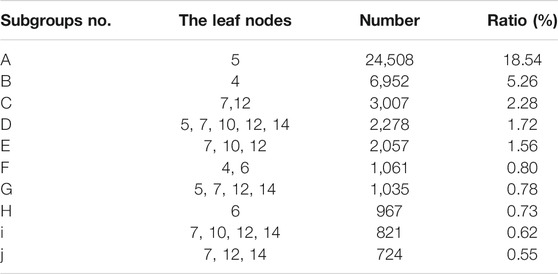
Once the clinical records were loaded into the decision tree, the patients were clustered into different subgroups according to our definition of similar patients. The leaf node patterns and sample size of the top 10 subgroups are listed in Table 5. The most popular pattern was allocated to leaf node 5 only, which accounted for 18.54% of all running through clinical examples. Subgroup c was the most popular pattern that contained multiple leaf nodes.
Prognostic Analyses for Similar Patients and Medication Recommendation Strategy
As introduced in Prognosis analyses, all combinations for the top five medications and the six outcomes were analyzed for each leaf node. As a running example, here we showed the prognosis analysis for subgroup d. For subgroup d, patients were allocated to five leaf nodes simultaneously. As the prescription of the diuretics and heart functional-related drugs was independent according to the clinical guidelines, the prognostic analyses were carried out separately. Shown in Figures 6A, B are the prognostic analyses on whether to use diuretics, while Figures 6C, D are the analyses for heart function-related medications. The occurrence ratios for each medication are shown in Figures 6A, C, while the calibrated coefficients of each medication for different outcomes are indicated in Figures 6B, D.
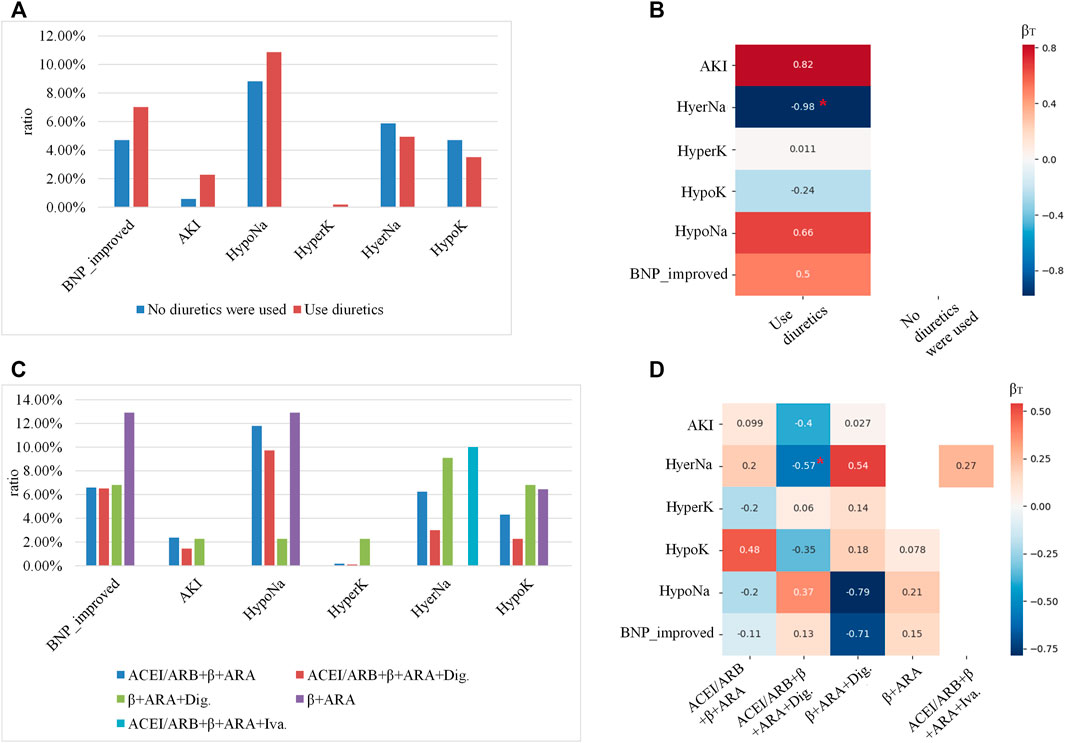
FIGURE 6. The prognosis’s analyses for the subgroup (allocated to leaf nodes 5, 7, 10, 12, and 14): (A,C) showed the occurrence ratios of each outcome for different medications, where the x-axis indicted the different outcomes, the y-axis indicated the occurrence ratio, and the color denoted different medications. (B,D) were 2D heatmaps indicating the calibrated coefficients of each medication for different outcomes, where the x-axis showed the medications, the y-axis indicated the outcomes, and the color was in proportion to the coefficients. Red colors denote the risk factors for the outcomes, and the blue ones denote the protective factors. The coefficients with p-value <0.05 were labeled with red *.
As shown in Figure 6A, using diuretics for subgroup c had a higher ratio of improving BNP level, and also the risk of AKI and HypoNa. According to Figure 6B, using diuretics only would significantly lower the risk of occurring HyperNa. As shown in Figure 6C, the medication of β+ ARA (aldosterone receptor antagonist) benefitted the improvement of the BNP level the most, but would also increase the risk of HypoNa. As indicated by Figure 6D, using ACEI/ARB+β+ARA + Dig. Would significantly lower the risk of occurring HyperNa.
Discussions
In the present study, we proposed a framework to construct a nonmutually exclusive decision tree and to combine the tree with real-world data for a treatment recommendation. Based on this framework, we successfully built the CDSS for chronic heart failure treatment with a large real-world inpatient dataset. In addition, the multiple outcome prognosis analyses were carried out to assess the medications for the subgroup of each similar patient, which facilitated the physicians in making decisions in a patient-specific way.
Although many achievements have been made in improving the model precision for medication recommendations (Liu et al., 2017; Shang et al., 2019; Chowdhury and Turin, 2020), it is still necessary to maintain the interpretability and ensure consistency with clinical knowledge in the real application of CDSS. Therefore, there is a trend in combining clinical guidelines and retrospective I data. Wei Zhao et al. proposed to construct a decision tree with clinical rules extracted from the clinical guidelines by the Gini impurity calculated by using the real data (Zhao et al., 2020). Sun et al. (2019) proposed to integrate the real-world evidence calculated using data with the knowledge-based decision trees. In the present study, we found data helped in expanding the knowledge-based decision tree for cases with the initial treatments for HFrEF. Also, the mining of the frequent medication patterns enriched the knowledge-based tree, especially when the prognosis analyses showed benefit for some outcomes.
The decision tree is composed of the clinical conditions mentioned in the clinical guidelines and the candidate medications in the leaf nodes. In the mutually exclusive settings, following the clinical conditions on the decision trees, one patient is allocated to a unique leaf node. Those required a systematic partition of the whole population, which was infeasible for most cases, especially for complicated diseases such as chronic heart failure.
As proposed in the current manuscript, the construction of the nonmutually exclusive decision tree simply organized the independent clinical rules horizontally. The similar patients were defined as the same patterns of leaf nodes allocated, and the preparation of the real-world evidence for each subgroup of similar patients relied on the two-step linear regression for the nonmutually exclusive parts separately. In summary, the three key components for nonmutually exclusive decision trees, the construction process, the similar patients, and the real-world evidence were nondisease specific; thus, the methodology would be a general solution for all diseases without a systemic partition of the whole population.
The limitation of the current work was that we only used single-center data and had not yet tested the construct CDSS with an external dataset. Besides, the effect of the substitution of the data of outpatients for the data of the inpatients was not carefully evaluated in the application of CDSS.
The novelty of our work relied on how to utilize such a nonmutually exclusive decision tree in CDSS. First, one key concept in CDSS was identifying similar patients, which were defined as patients assigned to the same set of leaf nodes. Second, to provide real-world evidence, we separated different types of medications and recommended independent medications for each similar patient group. To make precise medication recommendation for each patient, the prognoses evidence for each treatment should be calibrated by the propensity score of a particular treatment. In practice, the calibration included two steps. First, the propensity for patients in a particular patient group to choose one medication pattern were evaluated by regression to the medication patterns. Second, the effect of each medication pattern to the outcome should be calibrated by considering the propensity of medication chosen when regressed to the outcome. Therefore, our nonmutually exclusive decision tree would provide risks of different outcomes for each medication pattern of each patient group, which would assist physicians to make medication decisions for a specific patient.
In conclusion, in the present study, the methodology to construct a nonmutually exclusive decision tree for medication recommendations for HFrEF and its application in CDSS was proposed. Our framework is universal for most diseases and could be generally applied for developing the CDSS for treatment. This provides a promising solution for diseases that are infeasible to obtain a mutually exclusive decision tree for treatment.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of the Chinese PLA General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
KH, XJ, and LS conceptualized the study. HY, SB, and YB conducted the data preprocessing. HY performed the formal analysis. YB, LS, and KH acquired the funding. HY and JZ investigated the study. XJ, JZ, HY, and XS developed the methodology. HY, JZ, and XJ wrote the original draft. XJ, XS, LS, and YB wrote, reviewed, and edited the manuscript.
Funding
This work was supported by the grant from the National Key Technologies R&D Program for New Drugs of China (No. 2018ZX09J18109-004), the Project of the National Ministry of Industry and Information Technology (2020-0103-3-1), the Open Project of the National Clinical Research Center for Geriatric Diseases (NCRCG-PLAGH-2019025), and the National Natural Science Foundation (Grant Number: 82070056).
Conflict of Interest
Authors HY, XJ, JZ, XS, GH, and GX were employed by the company Ping An Health Technology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the participants in this work for their support especially Mingmin Feng for her contribution in the prognosis analyses and the proofreading of the manuscript.
References
Austin, J., Barras, M., and Sullivan, C. (2020). Interventions Designed to Improve the Safety and Quality of Therapeutic Anticoagulation in an Inpatient Electronic Medical Record. Int. J. Med. Inform. 135, 104066. doi:10.1016/j.ijmedinf.2019.104066
Agrawal, R., and Srikant, R. (1994). Fast algorithms for mining association rules. Proc. 20th int. conf. very large data bases, VLDB 1215, 487–499.
Bates, D. W., Cohen, M., Leape, L. L., Overhage, J. M., Sheridan, T., and Xie, G. (2001). Reducing the frequency of errors in medicine using information technology. J. Amer. Med. Inform. Assoc. 8(4), 299–308. doi:10.1136/jamia.2001.0080299
Bock, J. S., and Gottlieb, S. S. (2010). Cardiorenal Syndrome: New Perspectives. Circulation 121, 2592–2600. doi:10.1161/CIRCULATIONAHA.109.886473
Chamberlain, A. M., Boyd, C. M., Manemann, S. M., Dunlay, S. M., Gerber, Y., Killian, J. M., et al. (2020). Risk Factors for Heart Failure in the Community: Differences by Age and Ejection Fraction. Am. J. Med. 133, e237–e248. doi:10.1016/j.amjmed.2019.10.030
Chowdhury, M. Z. I., and Turin, T. C. (2020). Precision Health through Prediction Modelling: Factors to Consider before Implementing a Prediction Model in Clinical Practice. J. Prim. Health Care 12, 3–9. doi:10.1071/HC19087
Conrad, N., Judge, A., Tran, J., Mohseni, H., Hedgecott, D., Crespillo, A. P., et al. (2018). Temporal Trends and Patterns in Heart Failure Incidence: a Population-Based Study of 4 Million Individuals. Lancet 391, 572–580. doi:10.1016/S0140-6736(17)32520-5
Ding, Q., Ding, Q., and Perrizo, W. (2008). PARM--an Efficient Algorithm to Mine Association Rules from Spatial Data. IEEE Trans. Syst. Man. Cybern B Cybern 38, 1513–1524. doi:10.1109/TSMCB.2008.927730
Downie, A. S., Hancock, M., Abdel Shaheed, C., McLachlan, A. J., Kocaballi, A. B., Williams, C. M., et al. (2020). An Electronic Clinical Decision Support System for the Management of Low Back Pain in Community Pharmacy: Development and Mixed Methods Feasibility Study. JMIR Med. Inform. 8, e17203. doi:10.2196/17203
Ehrhardt, M. J., Flerlage, J. E., Armenian, S. H., Castellino, S. M., Hodgson, D. C., and Hudson, M. M. (2021). Integration of Pediatric Hodgkin Lymphoma Treatment and Late Effects Guidelines: Seeing the Forest beyond the Trees. J. Natl. Compr. Canc Netw. 19, 755–764. doi:10.6004/jnccn.2021.7042
Keikes, L., Kos, M., Verbeek, X., Van Vegchel, T., Nagtegaal, I. D., Lahaye, M. J., et al. (2021). Conversion of a Colorectal Cancer Guideline into Clinical Decision Trees with Assessment of Validity. Int. J. Qual. Health Care 33, mzab051. doi:10.1093/intqhc/mzab051
Khwaja, A. (2012). KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 120, c179–84. doi:10.1159/000339789
Liu, H., Li, X., Xie, G., Du, X., Zhang, P., Gu, C., et al. (2017). Precision Cohort Finding with Outcome-Driven Similarity Analytics: A Case Study of Patients with Atrial Fibrillation. Stud. Health Technol. Inform. 245, 491–495. doi:10.3233/978-1-61499-830-3-491
Metra, M., and Teerlink, J. R. (2017). Heart Failure. Lancet 390, 1981–1995. doi:10.1016/S0140-6736(17)31071-1
Nassif, M. E., Windsor, S. L., Tang, F., Khariton, Y., Husain, M., Inzucchi, S. E., et al. (2019). Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients with Heart Failure with Reduced Ejection Fraction: The DEFINE-HF Trial. Circulation 140, 1463–1476. doi:10.1161/CIRCULATIONAHA.119.042929
Office of the National Coordinator for Health Information Technology, Department of Health and Human Services (2012). Health Information Technology: Standards, Implementation Specifications, and Certification Criteria for Electronic Health Record Technology, 2014 Edition; Revisions to the Permanent Certification Program for Health Information Technology. Final Rule. Fed. Regist. 77, 54163–54292.
Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H., Cleland, J. G. F., Coats, A. J. S., et al. (2016). 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC)Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 37, 2129–2200. doi:10.1093/eurheartj/ehw128
Rubin, D. (2005). Causal Inference Using Potential Outcomes: Design, Modeling, Decisions. Journal of the American Statistical Association. J. Amer. Med. Inform. Assoc. 100(469), 322–331. doi:10.2307/27590541
Shang, J., Ma, T., Xiao, C., and Sun, J. (2019). Pre-training of graph augmented transformers for medication recommendation. CoRR. doi:10.24963/ijcai.2019/825
Song, Y. Y., and Lu, Y. (2015). Decision Tree Methods: Applications for Classification and Prediction. Shanghai Arch. Psychiatry 27, 130–135. doi:10.11919/j.issn.1002-0829.215044
Sun, X., Zhao, W., Zuo, L., Dumitriu, A., Lee, C. C., Cui, N., et al. (2019). Integrating Clinical Knowledge and Real-World Evidence for Type 2 Diabetes Treatment. AMIA Annu. Symp. Proc. 2019, 838–847.
Yancy, C. W., Jessup, M., Bozkurt, B., Butler, J., Casey, D. E., Colvin, M. M., et al. (2017). 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 70, 776–803. doi:10.1016/j.jacc.2017.04.025
Keywords: decision tree, medication recommendation, clinical decision support system (CDSS), chronic heart failure, treatment, machine learning
Citation: Bai Y, Yao H, Jiang X, Bian S, Zhou J, Sun X, Hu G, Sun L, Xie G and He K (2022) Construction of a Non-Mutually Exclusive Decision Tree for Medication Recommendation of Chronic Heart Failure. Front. Pharmacol. 12:758573. doi: 10.3389/fphar.2021.758573
Received: 16 August 2021; Accepted: 31 December 2021;
Published: 23 February 2022.
Edited by:
Abdulhamit Subasi, University of Turku, FinlandReviewed by:
Alexander E. Berezin, Zaporizhia State Medical University, UkraineM. Abdullah Canbaz, Indiana University, United States
Copyright © 2022 Bai, Yao, Jiang, Bian, Zhou, Sun, Hu, Sun, Xie and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Sun, c3VuaGFueGluZzIwMDVAaW1tLmFjLmNu; Guotong Xie, eGllZ3VvdG9uZ0BwaW5nYW4uY29tLmNu; Kunlun He, a3VubHVuaGVAcGxhZ2gub3Jn
†These authors have contributed equally to this work and share first authorship
 Yongyi Bai
Yongyi Bai Haishen Yao
Haishen Yao Xuehan Jiang
Xuehan Jiang Suyan Bian1,2
Suyan Bian1,2 Lan Sun
Lan Sun Kunlun He
Kunlun He