
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 27 September 2021
Sec. Obstetric and Pediatric Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.755075
This article is part of the Research Topic Therapeutic Drug Monitoring (TDM): A Useful Tool for Pediatric Pharmacology Applied to Routine Clinical Practice View all 18 articles
 Milo Gatti1,2
Milo Gatti1,2 Pier Giorgio Cojutti2
Pier Giorgio Cojutti2 Caterina Campoli3
Caterina Campoli3 Fabio Caramelli4
Fabio Caramelli4 Luigi Tommaso Corvaglia1,5
Luigi Tommaso Corvaglia1,5 Marcello Lanari1,6
Marcello Lanari1,6 Andrea Pession1,7
Andrea Pession1,7 Stefania Ramirez8
Stefania Ramirez8 Pierluigi Viale1,3
Pierluigi Viale1,3 Federico Pea1,2*
Federico Pea1,2*Introduction: Antimicrobial treatment is quite common among hospitalized children. The dynamic age-associated physiological variations coupled with the pathophysiological alterations caused by underlying illness and potential drug-drug interactions makes the implementation of appropriate antimicrobial dosing extremely challenging among paediatrics. Therapeutic drug monitoring (TDM) may represent a valuable tool for assisting clinicians in optimizing antimicrobial exposure. Clinical pharmacological advice (CPA) is an approach based on the correct interpretation of the TDM result by the MD Clinical Pharmacologist in relation to specific underlying conditions, namely the antimicrobial susceptibility of the clinical isolate, the site of infection, the pathophysiological characteristics of the patient and/or the drug-drug interactions of cotreatments. The aim of this study was to assess the role of TDM-based CPAs in providing useful recommendations for the real-time personalization of antimicrobial dosing regimens in various paediatric settings.
Materials and methods: Paediatric patients who were admitted to different settings of the IRCCS Azienda Ospedaliero-Universitaria of Bologna, Italy (paediatric intensive care unit [ICU], paediatric onco-haematology, neonatology, and emergency paediatric ward), between January 2021 and June 2021 and who received TDM-based CPAs on real-time for personalization of antimicrobial therapy were retrospectively assessed. Demographic and clinical features, CPAs delivered in relation to different settings and antimicrobials, and type of dosing adjustments were extracted. Two indicators of performance were identified. The number of dosing adjustments provided over the total number of delivered CPAs. The turnaround time (TAT) of CPAs according to a predefined scale (optimal, <12 h; quasi-optimal, between 12–24 h; acceptable, between 24–48 h; suboptimal, >48 h).
Results: Overall, 247 CPAs were delivered to 53 paediatric patients (mean 4.7 ± 3.7 CPAs/patient). Most were delivered to onco-haematological patients (39.6%) and to ICU patients (35.8%), and concerned mainly isavuconazole (19.0%) and voriconazole (17.8%). Overall, CPAs suggested dosing adjustments in 37.7% of cases (24.3% increases and 13.4% decreases). Median TAT was 7.5 h (IQR 6.1–8.8 h). Overall, CPAs TAT was optimal in 91.5% of cases, and suboptimal in only 0.8% of cases.
Discussion: Our study provides a proof of concept of the helpful role that TDM-based real-time CPAs may have in optimizing antimicrobial exposure in different challenging paediatric scenarios.
Severe bacterial infections are a growing problem in the pediatric population and the rise of multidrug-resistant (MDR) pathogens may seriously challenge optimal treatment (Hsu and Tamma, 2014). Antimicrobial use is quite common among hospitalized children, possibly exceeding 50% in different paediatric settings, but unfortunately most antimicrobials do not have specific paediatric posology based on pharmacokinetic and/or pharmacokinetic/pharmacodynamic studies that were carried out in this patient population (Korth-Bradley, 2018). Although the paradigm shift that states “children are not small adults” is widely accepted nowadays (Moore, 1998), in most cases dose scaling in pediatrics is still based on allometric scaling (Le and Bradley, 2018). This approach selects drug dosage on the basis of the non-proportional relationship that exists between the pharmacokinetic (PK) parameters, such as drug clearance and/or volume of distribution, and the body size descriptors, such as body surface area and/or lean body weight (Le and Bradley, 2018). Unfortunately, allometric scaling is far from being optimal, as it has some intrinsic limitations. Importantly, it does not take into account the process of organ maturation that occurs in the first years of life. It should not be overlooked that drug disposition in newborns, infants and toddlers may be affected by age-related factors both concerning organ development and maturation, and body composition, thus potentially rendering drug exposure unpredictable (Kearns et al., 2003). Renal function normalized to body weight (in terms of creatinine clearance in mL/min/kg) may be 2-3 fold higher during the first year of life than in adults (Hayton, 2000), and in the subsequent years progressively decreases reaching values similar to those of adults within 10 years of age (Funk et al., 2012). Additionally, allometric scaling does not consider the influence that some pathophysiological conditions may have in altering the pharmacokinetic behavior of some drugs. Interindividual pharmacokinetic variability, namely a well-known issue that may affect drug exposure and treatment outcomes (Collins and Varmus, 2015), may be especially relevant in the case of the critically ill and/or of the onco-hematological pediatric patients. The underlying presence of sepsis and/or septic shock, and/or of hematological malignancies like acute myeloid leukemia and/or acute lymphoblastic leukemia may frequently lead to the so-called augmented renal clearance, namely a pathophysiological condition that may significantly increase the renal clearance of hydrophilic drugs, as for example beta-lactams and/or aminoglycosides. Last, but not least, drug-drug interactions may furtherly make the implementation of appropriate antimicrobial dosing extremely challenging, as in the case of antifungal triazoles.
Therapeutic drug monitoring (TDM) may represent a valuable tool for assisting clinicians in optimizing antimicrobial dosing. However, for providing clinicians with optimal TDM-based dosing adjustments in each single patient, it is necessary that the results could be provided on real-time and that they are interpreted correctly. The clinical pharmacological advice (CPA) is an advice for optimizing drug exposure in each single patient that is delivered by the MD Clinical Pharmacologist who interprets on real-time the TDM results of antimicrobials in relation to some specific underlying conditions, namely the antimicrobial susceptibility of the clinical isolate, the site of infection, the pathophysiological characteristics of the patient and/or the potential drug-drug interactions of co-treatments.
The aim of this study was to provide a proof of concept of the role that TDM-based CPAs may have for real-time personalization of antimicrobial exposure in different paediatric settings.
This is a proof-of-concept study that has the purposes of describing the organizational procedures of a newly established Clinical Pharmacology Unit focused at providing real-time CPAs for individualizing antimicrobial exposure in different specific paediatric settings, and of assessing the clinical impact of the CPAs in the first 6 months of activity.
The IRCCS Azienda Ospedaliero-Universitaria of Bologna, Italy is a 1362-bed tertiary care teaching hospital which is currently organized in nine integrated activity Departments including 87 different operating units. The Clinical Pharmacology Unit was activated on November 2020, and since December 2020 started in providing educational webinars concerning the role of CPAs for real-time personalization of antimicrobial exposure that are based on TDM samples that are analyzed at the Unique Metropolitan Laboratory (LUM).
The CPA is an approach based on the correct interpretation of the TDM result by the MD Clinical Pharmacologist in relation to specific underlying conditions, namely the antimicrobial susceptibility of the clinical isolate according to the MIC provided by the Clinical Microbiologist, the site of infection, the pathophysiological characteristics of the patient (e.g., body mass index, renal function, sepsis, requirement for continuous renal replacement therapy or intermittent haemodialysis) and/or the influence of concomitant therapies. Clinical features of each patient (i.e., weight, height, diagnosis, concomitant therapies, date of starting antimicrobial therapy, drug dose and frequency of administration, time of blood sample collection) were supplied by the physician who requested the CPA and had the patient in charge. This approach allowed to provide dosing adjustment recommendations useful at optimizing drug exposure in each single patient.
The CPAs were provided five times weekly (from Monday to Friday) for 18 different antimicrobials: 13 antibiotics (piperacillin-tazobactam, ampicillin, meropenem, ceftazidime, cefepime, vancomycin, teicoplanin, amikacin, gentamicin, linezolid, levofloxacin, ciprofloxacin, and rifampicin), four antifungals (fluconazole, voriconazole, posaconazole, and isavuconazole), and one antiviral (ganciclovir) as detailed in Table 1. Optimal pharmacodynamic targets both for maximizing clinical efficacy and for minimizing resistance occurrence were considered a plasma steady-state concentration 4-fold above the minimum inhibitory concentration (MIC) (Css/MIC >4) for time-dependent antimicrobials, and a peak concentration to MIC ratio (Cmax/MIC) ≥ 8–12 for concentration-dependent antimicrobials (Abdul-Aziz et al., 2020) (Table 1). Blood samples for first TDM assessment were routinely collected at 48- and 72-h after the start of the treatment for beta-lactams and for other agents (e.g., linezolid, azoles), respectively, in order to ensure that steady state concentrations have been achieved. Re-assessments were commonly performed at least after 48-h the implementation of dosing adjustments recommended in CPAs.
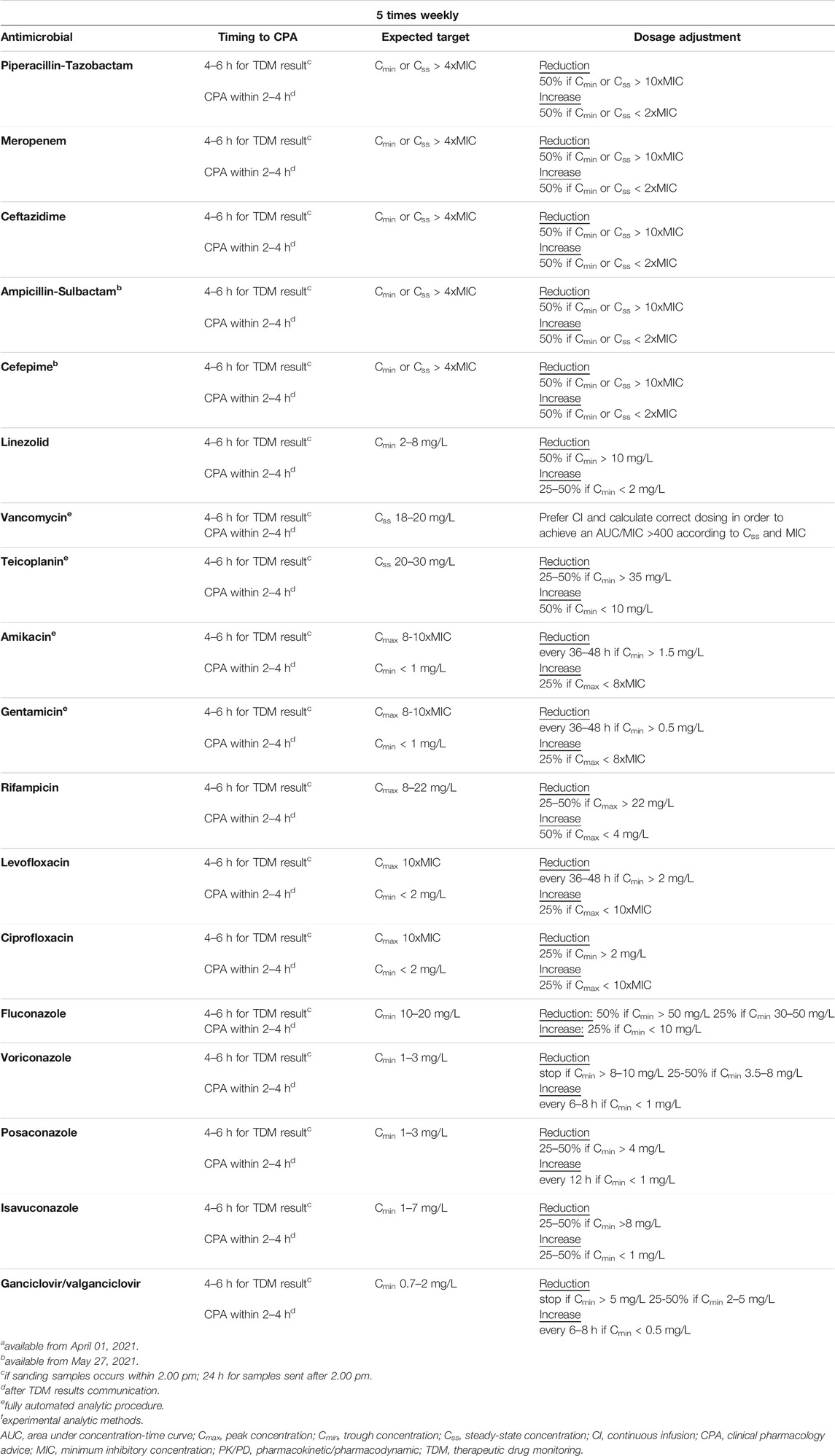
TABLE 1. Scheduled timing, expected PK/PD target, and TDM-guided dosage adjustments of antimicrobials for which clinical pharmacological advice is available five-times weekly.
The CPAs were provided usually within 4 h after that the TDM results were made available in the hospital intranet system by the LUM. Blood samples for TDM were processed by the LUM in the same day if they were delivered within 2.00 p.m., otherwise they were processed in the subsequent day.
The CPAs had been adopted by four specific paediatric settings, namely paediatric intensive care unit [ICU], paediatric onco-haematology/transplant unit, neonatology, and emergency paediatric ward. Antimicrobial treatment and relative dosage were initially selected on the basis of dedicated national and local guidelines with the support of the infectious disease consultant, and successively adjusted according to the TDM-based CPAs. The relationship between antimicrobial exposure and clinical outcome in terms of efficacy and safety was regularly assessed during the delivery of CPAs.
Paediatric patients who were admitted to the four different specific paediatric settings of the IRCCS Azienda Ospedaliero-Universitaria of Bologna, Italy and who required TDM-based CPAs for real-time personalization of antimicrobials between January 2021 and June 2021 were retrospectively assessed. Demographic and clinical features (age, gender, diagnosis), and CPAs were retrieved for each patient.
Two indicators of performance were defined in order to assess the usefulness of the CPAs for real-time optimization of antimicrobial dosing in the paediatric settings. First, the proportion of dosing adjustments (increase or reduction) recommended in the CPAs and applied by the attending clinicians over the total number of delivered CPAs in relation to the different settings and antimicrobials. Second, the turnaround time (TAT) of the antimicrobial CPA, defined as the timeframe elapsing between the delivery of the TDM sample to the LUM and the publication in the hospital intranet of the definitive TDM-guided CPA. The TAT of the antimicrobial CPA was defined as optimal, if < 12 h; quasi-optimal, if between 12–24 h; acceptable, if between 24–48 h and suboptimal, if > 48 h. The scale was predefined according to the feasibility in promptly providing paediatricians a real-time CPA, so that the recommended dosing adjustments could be implemented as quickly as possible after the delivery of TDM sample to the laboratory.
Data were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) according to data distribution, while categorical variables were expressed as count and percentage.
The study was approved by the local Ethic Committee (No. 443/2021/Oss/AOUBo).
The Clinical Pharmacology Unit is included in the Department for the Integrated Management of Infective Risk of the IRCCS Azienda Ospedaliero-Universitaria of Bologna. The priority goal consists in providing personalized CPAs for both inpatients and outpatients. An example of CPA performed in a specific paediatric scenario is shown in Figure 1. In the early period (Jan-Mar 2021), the main activity was to provide educational interventions in order to increase the awareness of clinicians regarding the importance that a real-time and integrated personalization of antimicrobial therapy may have in challenging paediatric scenarios. These interventions were based on dedicated webinars and on a specific on field work at the bedside aimed to identify which paediatric patients could have benefit more by a personalized CPAs, and to stress the importance of correct timing for TDM blood sampling in relation to the time of drug administration. The individualization of antimicrobial therapy in the paediatric population was made possible thanks to the implementation of a multidisciplinary taskforce composed by the pediatrician, the infectious disease consultant, the clinical microbiologist and the MD clinical pharmacologist. The main features of members involved in the multidisciplinary team and their coordinated and synergistic activities is shown in Figure 2.

FIGURE 1. Example of a TDM-guided clinical pharmacological advice for personalizing antibiotic treatment with meropenem in a critically ill paediatric patient affected by augmented renal clearance (estimated creatinine clearance according to bedside revised Schwartz equation) and concomitant bloodstream infection due to Pseudomonas aeruginosa exhibiting a minimum inhibitory concentration for meropenem of 8 mg/L. SSD: departmental structure.

FIGURE 2. Features of multidisciplinary taskforce involved in the management of empirical and targeted antimicrobial therapy in paediatric patients. CPA, clinical pharmacological advice; MALDI-TOF, Matrix Assisted Laser Desorption Ionization–Time of Flight; PK/PD, pharmacokinetic/pharmacodynamic; TDM, therapeutic drug monitoring.
Overall, during the 6-month period study, 247 CPAs were delivered to 53 paediatric patients (mean 4.7 ± 3.7 CPAs per patient). Median age of patients was 6 years (IQR 1–16 years), with no gender preponderance (male 49.1%). Leukemia and pneumonia were the most frequent underlying diseases, accounting for 39.6 and 20.8% of paediatric patients having CPAs, respectively (Table 2).
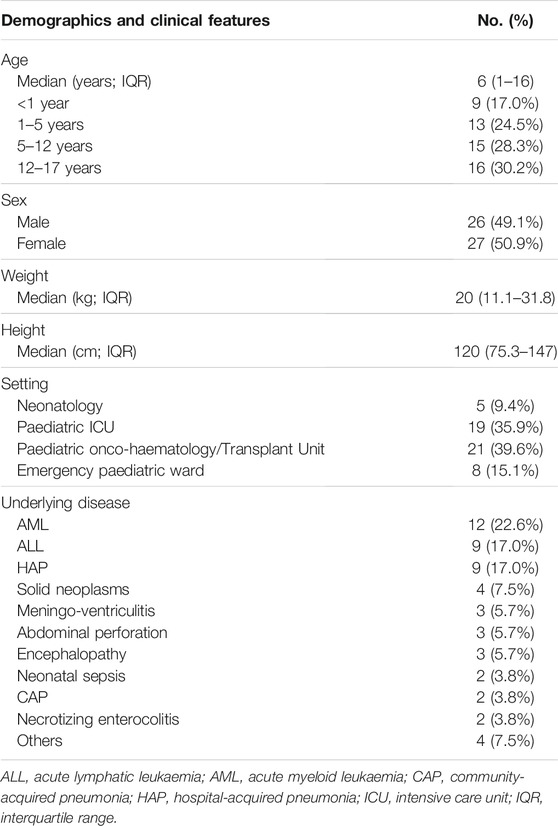
TABLE 2. Clinical and demographics features of paediatric patients in which at least one CPA for antimicrobial dosing adjustment was performed.
Most of the patients who received the CPAs were admitted to the paediatric onco-haematology/transplant unit (21; 39.6%) and to the paediatric ICU (19; 35.8%), and overall received more than 80% of the total CPAs. The mean (±SD) number of CPAs per patient was higher in the paediatric onco-haematology/transplant unit and in the emergency paediatric ward, and amounted respectively to 6.2 ± 4.2 and 4.4 ± 4.9 (Table 3).
The CPAs concerned 17 out of the 18 antimicrobials for which the TDM was available, and were provided mainly for antibiotics (121; 49.0%) and for antifungals (112; 45.3%). Overall, 84.0% of the CPAs delivered to the paediatric ICU concerned antibiotics, and 78.5% of those delivered to the paediatric onco-haematology/transplant unit regarded azole antifungals. The total number of CPAs requested for each antimicrobial were ≥10 for four antibiotics (piperacillin-tazobactam, meropenem, linezolid, and teicoplanin), four antifungals (isavuconazole, voriconazole, posaconazole, and fluconazole), and for the antiviral ganciclovir (Table 4). The most frequently requested CPAs concerned isavuconazole (47; 19.0%) and voriconazole (44; 17.8%).
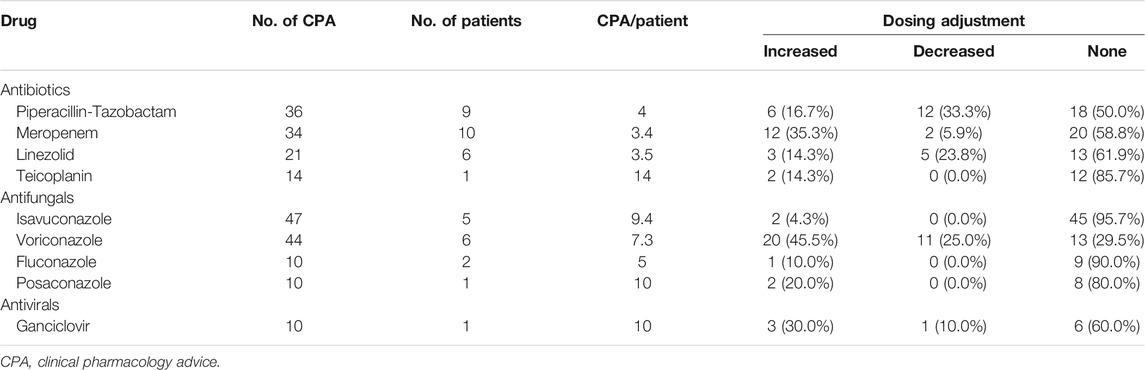
TABLE 4. Antimicrobials with ≥10 CPAs delivered in the study period and the proportion of recommended dosing adjustments.
Overall, the CPAs recommended dosing adjustments in 37.7% of cases, and suggested increases in 24.3% and decreases in the other 13.4% (Figure 3). Most of the dosing adjustments were needed in the paediatric ICU patients (48%), with increases and decreases almost equally distributed.
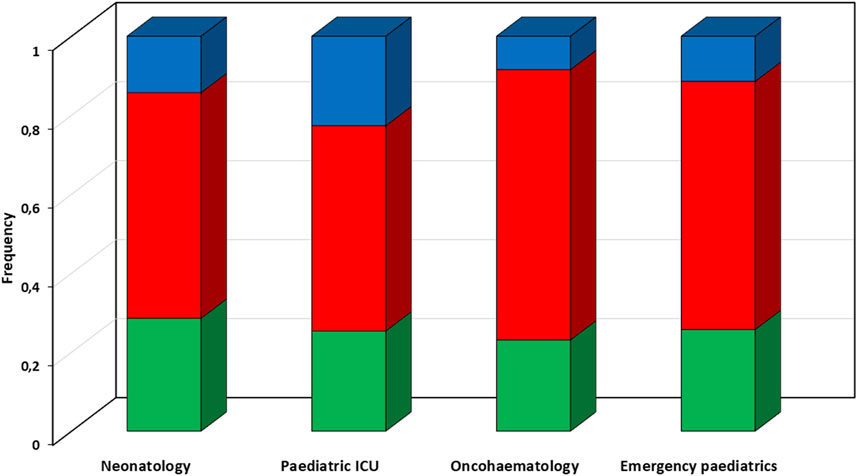
FIGURE 3. Proportion of types of recommended dosing adjustments according to different paediatric settings. Red box, suggested dosing increase; green box, suggested dosing confirm; blue box, suggested dosing reduction. ICU, intensive care unit.
In regard to each single antimicrobial agent, dose adjustments were recommended in 70.5 and 50.0% of CPAs concerning voriconazole and piperacillin-tazobactam, respectively. Dose increases concerned mainly voriconazole (20 cases; 45.5%) and meropenem (12 cases; 35.3%), whereas dose reductions were needed mainly for piperacillin-tazobactam (12 cases; 33.3%) and voriconazole (11 cases; 25.0%). Conversely, dose adjustments were considered unnecessary in most of the CPAs delivered for isavuconazole (95.7%), fluconazole (90.0%), teicoplanin (85.7%), and posaconazole (80.0%).
Median CPA turnaround time (TAT) was 7.5 h (IQR 6.1–8.8 h). No significant differences in median TAT was observed among the different paediatric setting (ranging from 6.4 h for neonatology to 8.2 h for paediatric onco-haematology/transplant unit) (Figure 4). Overall, CAP TAT was optimal in 91.5% of cases, and quasi-optimal in 96.0% of cases. Among the antimicrobials accounting for most of the delivered CPAs, the median TAT ranged between 6.4 h (IQR 4.1–7.5 h) for teicoplanin and 8.8 h (IQR 8.7–16.3 h) for posaconazole.
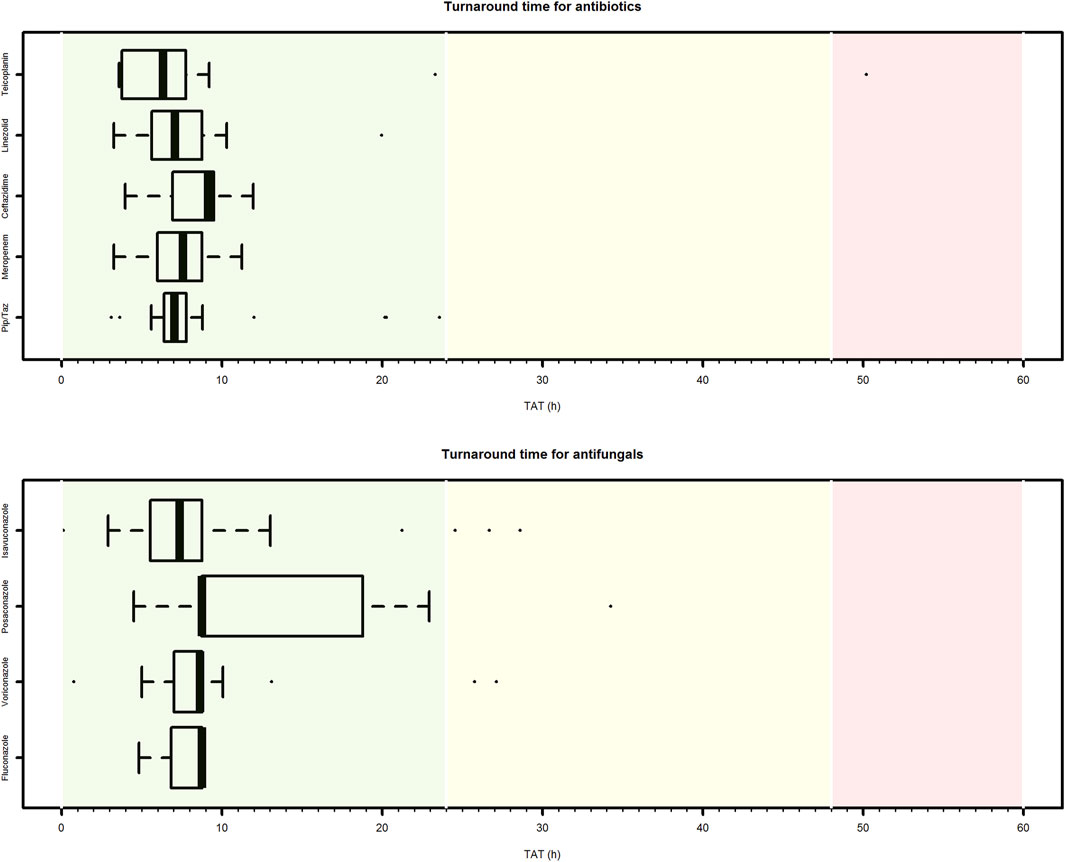
FIGURE 4. Box plot of turnaround time of clinical pharmacological advices (CPAs) for antimicrobial dosing regimen individualization in paediatric settings. Only antibiotic or antifungal agents for which at least ten CPAs were delivered are showed. Pip/Taz, piperacillin/tazobactam.
Our study provides a proof of concept of the helpful role that the CPAs based on TDM may have in optimizing antimicrobial exposure on real time in different populations and setting of hospitalized paediatric patient.
Both the indicators of performance of the CPAs may support the clinical utility of this approach. Dosing adjustments were needed in more than one third of cases, and the TAT of the CPAs was optimal in the vast majority of cases. This allowed clinicians to promptly implement recommended antimicrobial dosing adjustments in different challenging scenarios, and involved agents characterized by wide inter- and intraindividual variability according to specific pharmacokinetic-pharmacogenetic issues (e.g., voriconazole) (Bartelink et al., 2013) or underlying conditions (e.g., beta-lactams in critically ill children) (Marsot, 2018; Dhont et al., 2020), as found in our analysis. The prompt dosing adaptation may minimize either the risk of antimicrobial underexposure potentially associated with therapeutic failure, or those of overexposure potentially associated with toxicity.
In the last 10 years, TDM emerged as a valuable tool for assisting clinicians in optimizing antimicrobial dosing in different paediatric settings (Jager et al., 2016; Pauwels and Allegaert, 2016; John et al., 2019; De Rose et al., 2020; Hartman et al., 2020). The real added value of the CPA consists in the possibility of personalizing drug exposure on real-time in each single patient according to the antimicrobial susceptibility of the clinical isolate, the site of infection, the pathophysiological underlying conditions, and/or the drug-drug interactions of cotreatments. The innovative feature of this approach represents a paradigm shift in the TDM era. It is based on a multidisciplinary taskforce that may optimally handle complex hospitalized paediatric patients affected by severe infections, similarly to what just retrieved in other challenging scenarios (Viale et al., 2017; Gatti et al., 2019).
Antimicrobial use is quite frequent among hospitalized children, possibly exceeding 50% of cases in some paediatric settings (van Houten et al., 1998). Unfortunately, for most antimicrobials well-defined paediatric posology based on specific pharmacokinetic and/or pharmacokinetic/pharmacodynamic studies carried out in this patient population are currently lacking (Korth-Bradley, 2018) (Kearns et al., 2003; Ferro, 2015; De Rose et al., 2020). Consequently, our approach may represent the best way for dealing with the issue of appropriate treatment of infections in the paediatric setting. In this regard, we are confident that the implementation of TDM-guided CPAs for personalizing antimicrobial treatment could be of benefit especially in four challenging paediatric scenarios of infection.
Sepsis and septic shock represent a major cause of ICU admission and mortality among critically ill paediatric patients (Garcia et al., 2020). The American College of Critical Medicine/Pediatric Advanced Life Support protocol recommends the administration of antibiotic therapy within the first hours of sepsis diagnosis (Davis et al., 2017). Although the adherence to this approach has been associated with improved patient care quality and reduced mortality (Weiss et al., 2014; Balamuth et al., 2016), the choice of appropriate antimicrobial dosing may result extremely challenging in this scenario. Consequently, the failure in achieving optimal PK/PD target may lead to an increased risk of antimicrobial inefficacy or toxicity.
Pathophysiological alterations in volume of distribution, plasma protein binding, and drug clearance, coupled with dynamic age-associated physiological variations in glomerular filtration rate and/or in drug metabolizing enzymes, may lead to non-attainment of the PK/PD targets of antimicrobials in the critically ill paediatric patients (Kearns et al., 2003; Marsot, 2018; Dhont et al., 2020; Hartman et al., 2020).
Our findings showing that dosing adjustments were needed in almost 50% of the CPAs delivered for ICU paediatric patients support this hypothesis. It has been shown that the prevalence of augmented renal clearance among critically ill children may be higher than 25% (Dhont et al., 2020). This means that the need for personalized TDM-guided CPAs could be remarkable especially for antimicrobials that are eliminated by the renal route, like beta-lactams, for which dosage increases are frequently required in this setting.
Neonatal sepsis is a systemic condition of bacterial, viral, or fungal etiology associated with haemodynamic changes and other clinical manifestations that may result in remarkable morbidity and mortality (Shane et al., 2017). Different causative agents may be identified in early-compared to late-onset sepsis, leading to different therapeutic strategies (Shane et al., 2017). Empirical therapy of early-onset neonatal sepsis is based on the combination of ampicillin plus an aminoglycoside, whereas that of late-onset neonatal sepsis is based on the combination of vancomycin plus an aminoglycoside (Shane et al., 2017). Additionally, linezolid could represent a valuable alternative in neonatal ICUs with high prevalence of vancomycin-resistant Enterococci (Subramanya et al., 2019).
Antimicrobial TDM may be very helpful in this challenging scenario, considering that in the newborns the relationship between drug dose and exposure is quite unpredictable, especially in the pre-terms, due to the abrupt developmental physiological changes that occur in this age period (Pauwels and Allegaert, 2016; De Rose et al., 2020). In this regard, several evidences support the potential role of TDM in the management of neonatal sepsis (Hoff et al., 2009; Touw et al., 2009; Sicard et al., 2015; Sosnin et al., 2019; Tauzin et al., 2019).
In our study CPAs in neonatal septic patients were performed only in seven cases, but it is worth noting that dosing adjustments were need in almost half of these. This may support the need for implementing personalized TDM-guided CPAs in this challenging scenario. Indeed, the low number of CPAs delivered in this setting is justified strictly by ethical and clinical restrictions. Venipuncture is distressing and painful in this fragile population (although in unstable neonates the umbilical vein is commonly catheterized), and extensive sampling should be avoided because of the risk of iatrogenic anemia possibly resulting in requirement for blood transfusions (De Rose et al., 2020). We are now dealing with this issue by implementing a new rapid mass spectrometry method based on capillary microsampling (50 µL) that will make feasible to quantify 14 different antibiotics in each microsample, as showed previously by Bacco et al. (Barco et al., 2020). This would greatly contribute in increasing the feasibility of TDM-based CPAs in the neonatal setting.
Invasive fungal infections represent a major cause of morbidity and mortality in children who are affected by onco-haematological malignancies or who need allogeneic haemopoietic stem-cell transplantation (Groll and Tragiannidis, 2010; Dvorak et al., 2012; Pana et al., 2017; Arad-Cohen et al., 2020; Groll et al., 2021). The breakdown in natural barriers due to mucositis and the need for indwelling catheters coupled with immunosuppression caused by underlying onco-haematological disease and/or myelosuppressive chemotherapy may represent major determinants of the increased risk of invasive fungal infections in this scenario (Dvorak et al., 2012).
Aspergillus spp are responsible for a large proportion of invasive fungal infections, followed by Candida species. Triazoles represent the most important class of antifungal agents for prophylaxis and treatment of invasive fungal infections (Groll and Tragiannidis, 2010). Unfortunately, the use of azoles is challenged by several pharmacokinetic and pharmacogenetic issues in the paediatric scenario (Bury et al., 2021). Large interindividual and intraindividual variability in azole exposure is frequently present among oncohaematologic paediatric patients, especially for voriconazole. Additionally, the remarkable risk of clinically relevant drug-drug interactions with immunosuppressants and/or chemotherapeutic agents may make the achievement of therapeutic targets of exposure even more difficult. TDM-guided approach has just been considered to play a relevant role in the optimization of antifungal prophylaxis/treatment among onco-haematological paediatric patients (Groll et al., 2017; Allegra et al., 2018; Lempers et al., 2019).
Our data support this, as in almost 30% of the CPAs delivered in onco-haematological paediatric dosing adjustments of voriconazole were needed. This approach could minimize the risk of antifungal underexposure that may cause breakthrough invasive fungal infections, and/or that of overexposure, possibly causing toxicity (e.g., hepatotoxicity), in relation to drug-drug interactions, pharmacogenetic issues, or disease-related organ failure (Kyriakidis et al., 2017; Bury et al., 2021).
CAP is a common and potentially severe infection, with high incidence and relevant morbidity and mortality among children (McIntosh, 2002; Gupta et al., 2018), and is one of the major causes of admission to the emergency paediatric wards (Nascimento-Carvalho, 2020). Although respiratory viruses are the major causative pathogens in children under 5 years, also bacteria may play a relevant role. Streptococcus pneumoniae, Haemophilus influenzae, and Mycoplasma pneumoniae represent the most frequent bacterial pathogens (Gupta et al., 2018; Nascimento-Carvalho and Nascimento-Carvalho, 2019; Nascimento-Carvalho, 2020). Ampicillin represents the first-line antibiotic therapy in hospitalized children with CAP. Vancomycin or linezolid should be considered in presence of CA-MRSA (Gupta et al., 2018; Nascimento-Carvalho, 2020).
TDM could be a valuable tool in optimizing antibiotic therapy in paediatric patients with severe CAP admitted in the emergency ward, as suggested by the fact that in our study approximately 20% of CPAs were performed in this setting. Both ampicillin and vancomycin are hydrophilic antimicrobials whose pharmacokinetic variability could be considered high and unpredictable in this scenario. In this regard, antibiotic dosing adjustments were recommended in more than 60% of cases, even if we recognize that the total number of delivered CPAs was quite low. These findings may justify the need for implementing personalized TDM-guided CPAs in paediatric patients affected by severe pneumonia, similarly to that has been suggested for adults (Pea and Viale, 2006).
In conclusion, our study provides a proof of concept of the helpful role that CPAs based on real-time TDM may have in optimizing antimicrobial exposure in different challenging paediatric scenarios. Although we recognize that the short duration of the study period and the limited sample size may be potential limitations, the non-negligible proportion of recommended dosing adjustments coupled with the optimal performance of TAT support the feasibility and usefulness of this approach. The forthcoming implementation of innovative methods based on capillary microsampling will make sample collection even more feasible in the paediatric setting, and this may hopefully lead to a relevant growth in the use of personalized TDM-guided CPAs for optimizing antimicrobial treatment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Azienda Ospedaliero Universitaria di Bologna Ethic Committee (No. 443/2021/Oss/AOUBo). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
MG, PC and FP made substantial contribution to study conception and design. MG and PC made substantial contribution to acquisition and analysis of data. MG, PC and FP made substantial contribution to interpretation of data. MG and PC were involved in drafting the article. All authors revised the article critically for important intellectual content. All authors approved the final version of the article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdul-Aziz, M. H., Alffenaar, J. C., Bassetti, M., Bracht, H., Dimopoulos, G., Marriott, D., et al. (2020). Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: a Position Paper. Intensive Care Med. 46, 1127–1153. doi:10.1007/s00134-020-06050-1
Allegra, S., Fatiguso, G., De Francia, S., Favata, F., Pirro, E., Carcieri, C., et al. (2018). Therapeutic Drug Monitoring of Voriconazole for Treatment and Prophylaxis of Invasive Fungal Infection in Children. Br. J. Clin. Pharmacol. 84, 197–203. doi:10.1111/bcp.13401
Arad-Cohen, N., Rowe, J. M., and Shachor-Meyouhas, Y. (2020). Pharmacological Prophylaxis of Infection in Pediatric Acute Myeloid Leukemia Patients. Expert Opin. Pharmacother. 21, 193–205. doi:10.1080/14656566.2019.1701654
Balamuth, F., Weiss, S. L., Fitzgerald, J. C., Hayes, K., Centkowski, S., Chilutti, M., et al. (2016). Protocolized Treatment Is Associated with Decreased Organ Dysfunction in Pediatric Severe Sepsis. Pediatr. Crit. Care Med. 17, 817–822. doi:10.1097/PCC.0000000000000858
Barco, S., Mesini, A., Barbagallo, L., Maffia, A., Tripodi, G., Pea, F., et al. (2020). A Liquid Chromatography-Tandem Mass Spectrometry Platform for the Routine Therapeutic Drug Monitoring of 14 Antibiotics: Application to Critically Ill Pediatric Patients. J. Pharm. Biomed. Anal. 186, 113273. doi:10.1016/j.jpba.2020.113273
Bartelink, I. H., Wolfs, T., Jonker, M., de Waal, M., Egberts, T. C., Ververs, T. T., et al. (2013). Highly Variable Plasma Concentrations of Voriconazole in Pediatric Hematopoietic Stem Cell Transplantation Patients. Antimicrob. Agents Chemother. 57, 235–240. doi:10.1128/AAC.01540-12
Bury, D., Tissing, W. J. E., Muilwijk, E. W., Wolfs, T. F. W., and Brüggemann, R. J. (2021). Clinical Pharmacokinetics of Triazoles in Pediatric Patients. Clin. Pharmacokinet. 60, 1103–1147. doi:10.1007/s40262-021-00994-3
Collins, F. S., and Varmus, H. (2015). A New Initiative on Precision Medicine. N. Engl. J. Med. 372, 793–795. doi:10.1056/NEJMp1500523
Davis, A. L., Carcillo, J. A., Aneja, R. K., Deymann, A. J., Lin, J. C., Nguyen, T. C., et al. (2017). American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock. Crit. Care Med. 45, 1061–1093. doi:10.1097/CCM.0000000000002425
De Rose, D. U., Cairoli, S., Dionisi, M., Santisi, A., Massenzi, L., Goffredo, B. M., et al. (2020). Therapeutic Drug Monitoring Is a Feasible Tool to Personalize Drug Administration in Neonates Using New Techniques: An Overview on the Pharmacokinetics and Pharmacodynamics in Neonatal Age. Int. J. Mol. Sci. 21, E5898. doi:10.3390/ijms21165898
Dhont, E., Van Der Heggen, T., De Jaeger, A., Vande Walle, J., De Paepe, P., and De Cock, P. A. (2020). Augmented Renal Clearance in Pediatric Intensive Care: Are We Undertreating Our Sickest Patients? Pediatr. Nephrol. 35, 25–39. doi:10.1007/s00467-018-4120-2
Dvorak, C. C., Fisher, B. T., Sung, L., Steinbach, W. J., Nieder, M., Alexander, S., et al. (2012). Antifungal Prophylaxis in Pediatric Hematology/oncology: New Choices & New Data. Pediatr. Blood Cancer 59, 21–26. doi:10.1002/pbc.23415
Ferro, A. (2015). Paediatric Prescribing: Why Children Are Not Small Adults. Br. J. Clin. Pharmacol. 79, 351–353. doi:10.1111/bcp.12540
Funk, R. S., Brown, J. T., and Abdel-Rahman, S. M. (2012). Pediatric Pharmacokinetics: Human Development and Drug Disposition. Pediatr. Clin. North. Am. 59, 1001–1016. doi:10.1016/j.pcl.2012.07.003
Garcia, P. C. R., Tonial, C. T., and Piva, J. P. (2020). Septic Shock in Pediatrics: the State-Of-The-Art. J. Pediatr. (Rio J. 96 Suppl 1 (Suppl. 1), 87–98. doi:10.1016/j.jped.2019.10.007
Gatti, M., Gasparini, L. E., Laratta, M., Sigurtà, A., Rossi, A., Brioschi, P., et al. (2019). Intensive Multidisciplinary Management in Critical Care Patients Affected by Severe Necrotizing Soft Tissue Infections: a Cooperative Method to Improve the Efficacy of Treatment. Eur. J. Clin. Microbiol. Infect. Dis. 38, 1153–1162. doi:10.1007/s10096-019-03521-2
Groll, A. H., Pana, D., Lanternier, F., Mesini, A., Ammann, R. A., Averbuch, D., et al. (2021). 8th European Conference on Infections in Leukaemia: 2020 Guidelines for the Diagnosis, Prevention, and Treatment of Invasive Fungal Diseases in Paediatric Patients with Cancer or post-haematopoietic Cell Transplantation. Lancet Oncol. 22, e254–e269. doi:10.1016/S1470-2045(20)30723-3
Groll, A. H., Townsend, R., Desai, A., Azie, N., Jones, M., Engelhardt, M., et al. (2017). Drug-drug Interactions between Triazole Antifungal Agents Used to Treat Invasive Aspergillosis and Immunosuppressants Metabolized by Cytochrome P450 3A4. Transpl. Infect. Dis. 19. doi:10.1111/tid.12751
Groll, A. H., and Tragiannidis, A. (2010). Update on Antifungal Agents for Paediatric Patients. Clin. Microbiol. Infect. 16, 1343–1353. doi:10.1111/j.1469-0691.2010.03334.x
Gupta, S., Lodha, R., and Kabra, S. K. (2018). Antimicrobial Therapy in Community-Acquired Pneumonia in Children. Curr. Infect. Dis. Rep. 20, 47. doi:10.1007/s11908-018-0653-6
Hartman, S. J. F., Brüggemann, R. J., Orriëns, L., Dia, N., Schreuder, M. F., and de Wildt, S. N. (2020). Pharmacokinetics and Target Attainment of Antibiotics in Critically Ill Children: A Systematic Review of Current Literature. Clin. Pharmacokinet. 59, 173–205. doi:10.1007/s40262-019-00813-w
Hayton, W. L. (2000). Maturation and Growth of Renal Function: Dosing Renally Cleared Drugs in Children. AAPS PharmSci. 2, E3. doi:10.1208/ps020103
Hoff, D. S., Wilcox, R. A., Tollefson, L. M., Lipnik, P. G., Commers, A. R., and Liu, M. (2009). Pharmacokinetic Outcomes of a Simplified, Weight-Based, Extended-Interval Gentamicin Dosing Protocol in Critically Ill Neonates. Pharmacotherapy 29, 1297–1305. doi:10.1592/phco.29.11.1297
Hsu, A. J., and Tamma, P. D. (2014). Treatment of Multidrug-Resistant Gram-Negative Infections in Children. Clin. Infect. Dis. 58, 1439–1448. doi:10.1093/cid/ciu069
Jager, N. G., van Hest, R. M., Lipman, J., Taccone, F. S., and Roberts, J. A. (2016). Therapeutic Drug Monitoring of Anti-infective Agents in Critically Ill Patients. Expert Rev. Clin. Pharmacol. 9, 961–979. doi:10.1586/17512433.2016.1172209
John, J., Loo, A., Mazur, S., and Walsh, T. J. (2019). Therapeutic Drug Monitoring of Systemic Antifungal Agents: a Pragmatic Approach for Adult and Pediatric Patients. Expert Opin. Drug Metab. Toxicol. 15, 881–895. doi:10.1080/17425255.2019.1671971
Kearns, G. L., Abdel-Rahman, S. M., Alander, S. W., Blowey, D. L., Leeder, J. S., and Kauffman, R. E. (2003). Developmental Pharmacology-Ddrug Disposition, Action, and Therapy in Infants and Children. N. Engl. J. Med. 349, 1157–1167. doi:10.1056/NEJMra035092
Korth-Bradley, J. M. (2018). The Path to Perfect Pediatric Posology - Drug Development in Pediatrics. J. Clin. Pharmacol. 58 Suppl 10 (Suppl. 10), S48–S57. doi:10.1002/jcph.1081
Kyriakidis, I., Tragiannidis, A., Munchen, S., and Groll, A. H. (2017). Clinical Hepatotoxicity Associated with Antifungal Agents. Expert Opin. Drug Saf. 16, 149–165. doi:10.1080/14740338.2017.1270264
Le, J., and Bradley, J. S. (2018). Optimizing Antibiotic Drug Therapy in Pediatrics: Current State and Future Needs. J. Clin. Pharmacol. 58 Suppl 10 (Suppl. 10), S108–S122. doi:10.1002/jcph.1128
Lempers, V. J., Meuwese, E., Mavinkurve-Groothuis, A. M., Henriet, S., van der Sluis, I. M., Hanff, L. M., et al. (2019). Impact of Dose Adaptations Following Voriconazole Therapeutic Drug Monitoring in Pediatric Patients. Med. Mycol. 57, 937–943. doi:10.1093/mmy/myz006
Marsot, A. (2018). Pharmacokinetic Variability in Pediatrics and Intensive Care: Toward a Personalized Dosing Approach. J. Pharm. Pharm. Sci. 21, 354–362. doi:10.18433/jpps30082
McIntosh, K. (2002). Community-acquired Pneumonia in Children. N. Engl. J. Med. 346, 429–437. doi:10.1056/NEJMra011994
Nascimento-Carvalho, A. C., and Nascimento-Carvalho, C. M. (2019). Clinical Management of Community-Acquired Pneumonia in Young Children. Expert Opin. Pharmacother. 20, 435–442. doi:10.1080/14656566.2018.1552257
Nascimento-Carvalho, C. M. (2020). Community-acquired Pneumonia Among Children: the Latest Evidence for an Updated Management. J. Pediatr. (Rio J. 96 Suppl 1 (Suppl. 1), 29–38. doi:10.1016/j.jped.2019.08.003
Pana, Z. D., Roilides, E., Warris, A., Groll, A. H., and Zaoutis, T. (2017). Epidemiology of Invasive Fungal Disease in Children. J. Pediatr. Infect Dis Soc 6 (S3), S3–S11. doi:10.1093/jpids/pix046
Pauwels, S., and Allegaert, K. (2016). Therapeutic Drug Monitoring in Neonates. Arch. Dis. Child. 101, 377–381. doi:10.1136/archdischild-2013-305309
Pea, F., and Viale, P. (2006). The Antimicrobial Therapy Puzzle: Could Pharmacokinetic-Pharmacodynamic Relationships Be Helpful in Addressing the Issue of Appropriate Pneumonia Treatment in Critically Ill Patients? Clin. Infect. Dis. 42, 1764–1771. doi:10.1086/504383
Shane, A. L., Sánchez, P. J., and Stoll, B. J. (2017). Neonatal Sepsis. Lancet 390, 1770–1780. doi:10.1016/S0140-6736(17)31002-4
Sicard, M., Launay, E., Caillon, J., Jacqueline, C., Legrand, A., Deslandes, G., et al. (2015). Pharmacokinetics of Linezolid Treatment Using Intravenous and Oral Administrations in Extremely Premature Infants. Eur. J. Clin. Pharmacol. 71, 611–615. doi:10.1007/s00228-015-1813-3
Sosnin, N., Curtis, N., Cranswick, N., Chiletti, R., and Gwee, A. (2019). Vancomycin Is Commonly Under-dosed in Critically Ill Children and Neonates. Br. J. Clin. Pharmacol. 85, 2591–2598. doi:10.1111/bcp.14084
Subramanya, S. H., Amberpet, R., Chaudhary, D., Nayak, N., Padukone, S., Bairy, I., et al. (2019). Neonatal Sepsis Due to Glycopeptide Resistant Enterococcus Faecium from Colonized Maternal Gut- Rare Case Evidence. Antimicrob. Resist. Infect. Control. 8, 29. doi:10.1186/s13756-019-0490-x
Tauzin, M., Cohen, R., Durrmeyer, X., Dassieu, G., Barre, J., and Caeymaex, L. (2019). Continuous-Infusion Vancomycin in Neonates: Assessment of a Dosing Regimen and Therapeutic Proposal. Front. Pediatr. 7, 188. doi:10.3389/fped.2019.00188
Touw, D. J., Westerman, E. M., and Sprij, A. J. (2009). Therapeutic Drug Monitoring of Aminoglycosides in Neonates. Clin. Pharmacokinet. 48, 71–88. doi:10.2165/00003088-200948020-00001
van Houten, M. A., Luinge, K., Laseur, M., and Kimpen, J. L. (1998). Antibiotic Utilisation for Hospitalised Paediatric Patients. Int. J. Antimicrob. Agents 10, 161–164. doi:10.1016/s0924-8579(98)00022-3
Viale, P., Tedeschi, S., Scudeller, L., Attard, L., Badia, L., Bartoletti, M., et al. (2017). Infectious Diseases Team for the Early Management of Severe Sepsis and Septic Shock in the Emergency Department. Clin. Infect. Dis. 65, 1253–1259. doi:10.1093/cid/cix548
Keywords: clinical pharmacology advice, personalized antimicrobial therapy, neonatology, paediatric intensive care unit, paediatric emergency, paediatric onco-haematology
Citation: Gatti M, Cojutti PG, Campoli C, Caramelli F, Corvaglia LT, Lanari M, Pession A, Ramirez S, Viale P and Pea F (2021) A Proof of Concept of the Role of TDM-Based Clinical Pharmacological Advices in Optimizing Antimicrobial Therapy on Real-Time in Different Paediatric Settings. Front. Pharmacol. 12:755075. doi: 10.3389/fphar.2021.755075
Received: 07 August 2021; Accepted: 13 September 2021;
Published: 27 September 2021.
Edited by:
Raffaele Simeoli, Bambino Gesù Children’s Hospital (IRCCS), ItalyCopyright © 2021 Gatti, Cojutti, Campoli, Caramelli, Corvaglia, Lanari, Pession, Ramirez, Viale and Pea. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federico Pea, ZmVkZXJpY28ucGVhQHVuaWJvLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.