
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 02 November 2021
Sec. Respiratory Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.754268
This article is part of the Research TopicProgresses in the Drug Treatment of Chronic Cardiopulmonary DiseasesView all 20 articles
Background: Although the predominant airway inflammation in chronic obstructive pulmonary disease (COPD) is neutrophilic, approximately 20–40% of COPD patients present with eosinophilic airway inflammation. Compared with non-eosinophilic COPD patients, eosinophilic COPD patients are characterized by a greater number of total exacerbations and higher hospitalization rates. Furthermore, anti-interleukin-5 (IL-5) therapy, consisting of monoclonal antibodies (mAbs) targeting IL-5 or IL-5 receptor α (IL-5Rα), has been proven to be effective in severe eosinophilic asthma. This meta-analysis aimed to determine the efficacy and safety of anti-IL-5 therapy in eosinophilic COPD.
Methods: We searched the PubMed, Web of Science, Embase, and Cochrane Library databases from inception to August 2020 (updated in June 2021) to identify studies comparing anti-IL-5 therapy (including mepolizumab, benralizumab, and reslizumab) with placebo in eosinophilic COPD patients.
Results: Anti-IL-5 therapy was associated with a decrease in acute exacerbation rate (RR 0.89; 95% CI 0.84 to 0.95, I2 = 0%) and the severe adverse events (RR 0.90; 95% CI 0.84 to 0.97, I2 = 0%). However, no significant improvement was observed in pre-bronchodilator forced expiratory volume in 1 s (FEV1) (WMD 0.01; 95% CI −0.01 to 0.03, I2 = 25.9%), SGRQ score (WMD −1.17; 95% CI −2.05 to −0.29, I2 = 0%), and hospital admission rate (RR 0.91; 95% CI 0.78 to 1.07, I2 = 20.8%).
Conclusion: Anti-IL-5 therapy significantly reduced the annual acute exacerbation rate and severe adverse events in eosinophilic COPD patients. However, it did not improve lung function, quality of life, and hospitalization rate.
Chronic obstructive pulmonary disease (COPD) is characterized by progressive and irreversible airflow limitation that is triggered by the response of the airways and the lungs to noxious particles or fumes (Dave and Arjun, 2021). It is a leading cause of chronic morbidity and mortality worldwide (Dave and Arjun, 2021). COPD is a heterogeneous disease with different underlying pathobiological mechanisms (endotypes) and includes pulmonary and extra-pulmonary symptoms (phenotypes) (Han et al., 2010; Lange et al., 2016; Balkissoon, 2018; Dave and Arjun, 2021). Furthermore, as of May 2015, 99.9 million individuals suffering from COPD have been identified in China (Wang et al., 2018a). With continued exposure to COPD risk factors and an aging population, the prevalence of COPD is expected to increase over the next 40 years, and by 2060, more than 5.4 million may die from COPD and related conditions annually (Mathers and Loncar, 2006; Dave and Arjun, 2021).
Moreover, the exacerbation of COPD is associated with increased healthcare costs (Hilleman et al., 2000; Toy et al., 2010), progressive loss of lung function, subsequent cardiovascular events, and decline in quality of life (Dransfield et al., 2017; Kunisaki et al., 2018). Currently, Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines have recommended triple inhaled therapy (inhaled glucocorticoids, long-acting β2-agonists, and long-acting muscarinic-receptor antagonists) as maintenance treatment for patients with frequent exacerbations, which was proven to decrease acute exacerbation rates in COPD patients (Calzetta et al., 2019; Dave and Arjun, 2021). Despite this, approximately 30–40% of patients continue to have moderate or severe exacerbations even after receiving triple inhaled therapy (Vestbo et al., 2017). Thus, it is essential to explore new treatment options for COPD patients with acute exacerbation.
Compared with non-eosinophilic COPD patients, eosinophilic COPD patients are associated with a higher number of total exacerbations and higher hospitalization rates (Couillard et al., 2017). Saha et al. have reported that 20–40% of COPD patients presented with airway eosinophilic inflammation (peripheral blood eosinophil count of 3% or more or >150 cells per cubic millimeter) (Dasgupta et al., 2013; Singh et al., 2014), although the predominant airway inflammation in COPD is neutrophilic (Hogg et al., 2004; Dasgupta et al., 2013). Interleukin-5 (IL-5) regulates the differentiation, proliferation, survival, and activation of eosinophils via the IL-5 receptor (Takatsu et al., 1994). Anti-IL-5 therapy includes monoclonal antibodies (mAbs) targeting IL-5 or IL-5R α (including mepolizumab, benralizumab, and reslizumab), which have been proven to be effective in severe eosinophilic asthma (Farne et al., 2017). Given the similarity between asthma and COPD in terms of eosinophilic airway inflammation, several randomized controlled trials (RCTs) have studied the efficacy and safety of anti-IL-5 treatment in eosinophilic COPD patients (Brightling et al., 2014; Dasgupta et al., 2017; Sciurba et al., 2018; Criner et al., 2019).
However, contrasting results on the efficacy of anti-IL-5 therapy to reduce annual exacerbation rates of eosinophilic COPD have been reported. Pavord et al. have found that treatment with mepolizumab was associated with a lower incidence of moderate and severe exacerbations than placebo (Sciurba et al., 2018). In contrast, Brightling et al. and Criner et al. have noted that benralizumab did not reduce the annual exacerbation rates compared with the placebo (Brightling et al., 2014; Criner et al., 2019). Takudzwa et al. have conducted a meta-analysis and demonstrated that mepolizumab decreased the exacerbation rate by 23% in COPD patients with eosinophil counts of 300 cells/μL or greater than controls. (Mkorombindo and Dransfield, 2019). The efficacy of anti-IL-5 therapy in eosinophilic COPD is therefore not consistent.
Although the meta-analysis on anti-IL-5 in COPD patients already existed (Donovan et al., 2020; Lan et al., 2020), study participants were not limited to eosinophilic COPD patients. To provide more accurate and stronger evidence for the efficacy of anti-IL-5 therapy in eosinophilic COPD patients, the current study differs in two ways from the previous meta-analysis (Dave and Arjun, 2021): we only included eosinophilic COPD patients (peripheral blood eosinophil count of 3% or more or >150 cells per cubic millimeter) (Balkissoon, 2018); we compared anti-IL-5 therapy in eosinophilic COPD and in asthma, which enabled a more robust assessment of the effect of anti-IL-5 therapy in eosinophilic COPD patients.
This meta-analysis followed the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions. Furthermore, we conducted this meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Moher et al., 2009). The protocol for this meta-analysis is available in PROSPERO (CRD42020156189) (Wang et al., 2018b; Ge et al., 2018).
We searched the PubMed, Web of Science, Embase, and Cochrane Library databases from inception to August 2020 (updated in June 2021) to identify studies comparing anti-IL-5 therapy (including mepolizumab, benralizumab, and reslizumab) with placebo in COPD patients. There was no language or population restriction. In addition, we searched the ClinicalTrials.gov database to identify completed studies. We used the following keywords to perform the search: monoclonal antibody (mepolizumab, benralizumab, and reslizumab) and chronic obstructive pulmonary disease. We have displayed the detailed search strategy in Supplementary Material.
Inclusion criteria were as follows:
1. RCTs included parallel group studies, had a controlled design, and compared anti-IL-5 therapies with placebo.
2. Studies were conducted in adult patients with eosinophilic COPD, defined as peripheral blood eosinophil count of 3% or more or >150 cells per cubic millimeter.
3. Intervention was restricted to anti-IL-5 therapy or placebo.
4. Study outcomes were required to be at least one of the following: annual exacerbations, hospital admission for acute exacerbation, improvement of pre-bronchodilator forced expiratory volume in 1 s (FEV1), quality of life as assessed using the St. George’s Respiratory Questionnaire (SGRQ) total score, and severe adverse events.
Exclusion criteria were as follows:
1. Studies including participants who suffered from clinically significant lung disease or asthma.
2. Conference abstracts, letters, comments, reviews, and meta-analyses.
3. Studies of animals or cells.
Author CZ screened all titles and assessed full-text eligibility and then excluded studies that did not meet the inclusion and exclusion criteria. Author YW reassessed the selection results; all discrepancies were resolved by discussing them with a third author MZ. Two authors (XS and TL) independently extracted the following data from all included studies: lead author or study title, year of publication, location and duration, demographic characteristics of participants, drug and dose of anti-IL-5 therapy, annual exacerbations, hospital admission for acute exacerbation, change of pre-bronchodilator FEV1 from baseline, SGRQ score, and severe adverse events. Disagreements were settled by cross-checking original papers and consensus was achieved. Author HY validated and sorted specific data in a tabular format. The primary outcome was annual exacerbations, as acute exacerbation is a major cause of hospitalization and poor prognosis in COPD. The secondary outcomes were hospital admission for acute exacerbation, pre-bronchodilator FEV1, SGRQ score, and severe adverse events.
Two authors (CZ and XS) independently evaluated the quality of the methodology of the eligible RCTs. They applied the Cochrane Collaboration tool following the Cochrane Handbook for Systematic Reviews of Interventions (Stovold et al., 2014). There were six perspectives used to assess the quality, including random sequence generation (selection bias), allocation concealment (selection bias), blinding (performance bias and detection bias), incomplete outcome data (attrition bias), selective outcome reporting (attrition bias), and other potential sources of bias. The criteria to grade the included studies were as follows: 1) low-quality trial: either randomization or allocation concealment was assessed to indicate a high risk of bias, regardless of other items; 2) high-quality trial: both randomization and allocation concealment were graded as low risk of bias, and all other items were assessed as low or unclear risk of bias; 3) moderate-quality trial: they did not meet the criteria for high or low risk. Any discrepancy was resolved by consulting an evidence-based medicine professor.
Stata/SE 15.0 was used to perform data analysis. We pooled the rate ratio (RR) and 95% confidence interval (CI) to analyze the overall annual exacerbation rates. Dichotomous data, including hospital admission rate, severe adverse events, and all-cause mortality, were analyzed by calculating risk ratios (RR) and the corresponding 95% CI. Continuous data (pre-bronchodilator FEV1 and SGRQ scores) were analyzed by calculating the weighted mean difference (WMD) or standardized mean difference (SMD) and 95% CI. We used P and I2 statistics to measure heterogeneity among trials in each analysis. Fixed-effects models were used without important heterogeneity (I2 ≤ 50%). Otherwise, random effects models were used. A funnel plot was generated for examining publication bias when there were >10 included trials (Lau et al., 2006; Stovold et al., 2014). A p-value <0.05 was considered statistically significant.
We obtained 1,227 articles from the four databases and five studies from the ClinicalTrials.gov database. After removing the duplicates, 1,048 articles remained. We excluded 1,015 articles after scanning the titles and abstracts. Finally, three articles, including five studies, were included in this meta-analysis after reading the full text (Criner et al., 2019; Sciurba et al., 2018; Brightling et al., 2014). The detailed selection process is shown in Figure 1, which was prepared based on the PRISMA guidelines (Moher et al., 1996). Three studies were rated as high quality based on the grade criteria, the six items of the Cochrane tool shown in Supplementary Figures S1, S2.
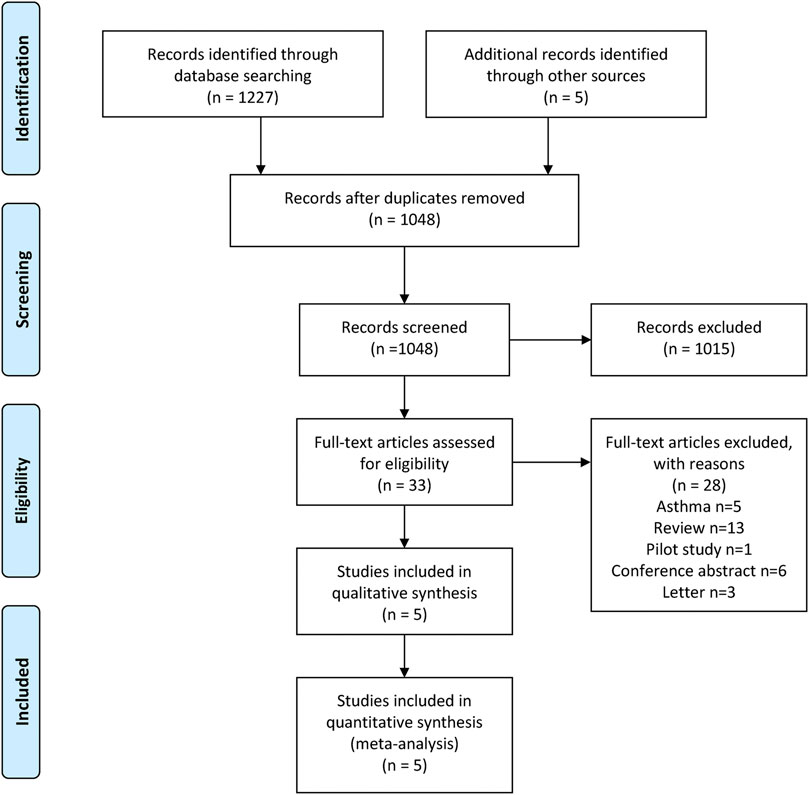
FIGURE 1. Study selection process: PRISMA flow diagram identifying studies included in the meta-analysis. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
All included studies were randomized, double-blinded, multicentered RCT, aiming to compare the clinical efficacy and safety of anti-IL-5 therapy with those of the placebo in adult patients with eosinophilic COPD. In the included five studies, the intervention was performed with benralizumab (10, 30, and 100 mg) targeting the IL-5 receptor α (20, 22) or mepolizumab (100 and 300 mg) targeting IL-5 (Brightling et al., 2014; Criner et al., 2019). Overall, there were 3902 COPD patients included in this meta-analysis. Current smoker status ranged from 25 to 42% among the study population and 58.0–70.7% of the patients were males. We have listed the detailed baseline characteristics in Table1.
All included studies reported the annual rate of exacerbations. There were five RCTs (Brightling et al., 2014; Sciurba et al., 2018; Criner et al., 2019) that compared anti-IL-5 therapy with placebo, showing that anti-IL-5 therapy was associated with a lower risk of acute exacerbation rate of eosinophilic COPD patients (RR 0.89; 95% CI 0.84 to 0.95, I2 = 0%; Figure 2).
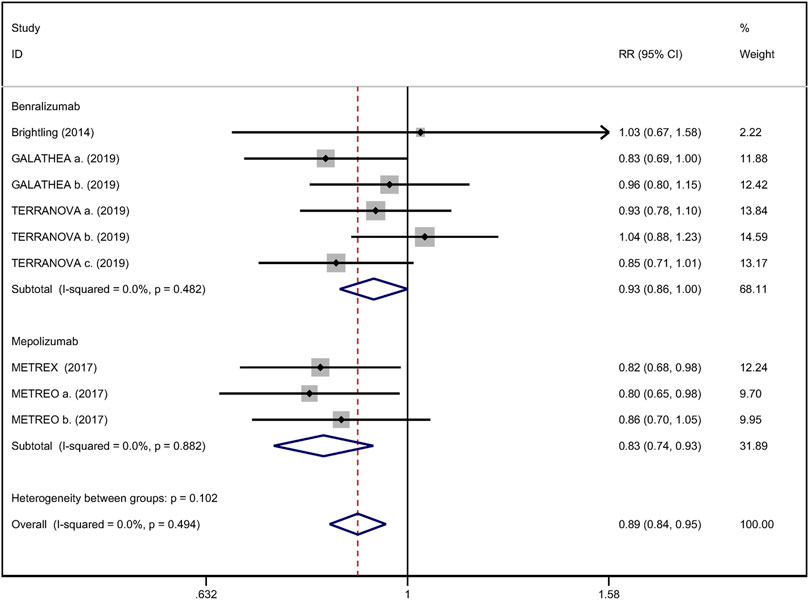
FIGURE 2. Forest plot of annual acute exacerbation rates in eosinophilic COPD patients with anti-IL-5 therapy vs. placebo.
Mean change from baseline of pre-bronchodilator FEV1 was used to assess lung function. Three RCTs reported an improvement in FEV1. However, no significant difference between anti-IL-5 therapy and placebo with regard to pre-bronchodilator FEV1 was observed (WMD 0.01; 95% CI −0.01 to 0.03, I2 = 25.9%; Figure 3) (Criner et al., 2019; Brightling et al., 2014). Improvement in quality of life was evaluated by the SGRQ total score, with a threshold of 4 units being considered clinically significant (Jones, 2005). Five RCTs reported changes in SGRQ total score. Anti-IL-5 was not associated with a significant improvement in the quality of life compared with placebo (WMD −1.17; 95% CI −2.05 to −0.29, I2 = 0%; Figure 4) (Brightling et al., 2014; Sciurba et al., 2018; Criner et al., 2019). In addition, we assessed the hospital admission for acute exacerbation (Brightling et al., 2014; Criner et al., 2019). There was no significant difference in hospitalization rate between the anti-IL-5 therapy group and the placebo group (RR 0.91; 95% CI 0.78 to 1.07, I2 = 20.8%; Figure 5). Regarding safety outcomes, the anti-IL-5 group demonstrated a significantly lower risk in the incidence of severe adverse events compared with the placebo group (RR 0.90; 95% CI 0.84 to 0.97, I2 = 0%; Figure 6) (Brightling et al., 2014; Sciurba et al., 2018; Criner et al., 2019).
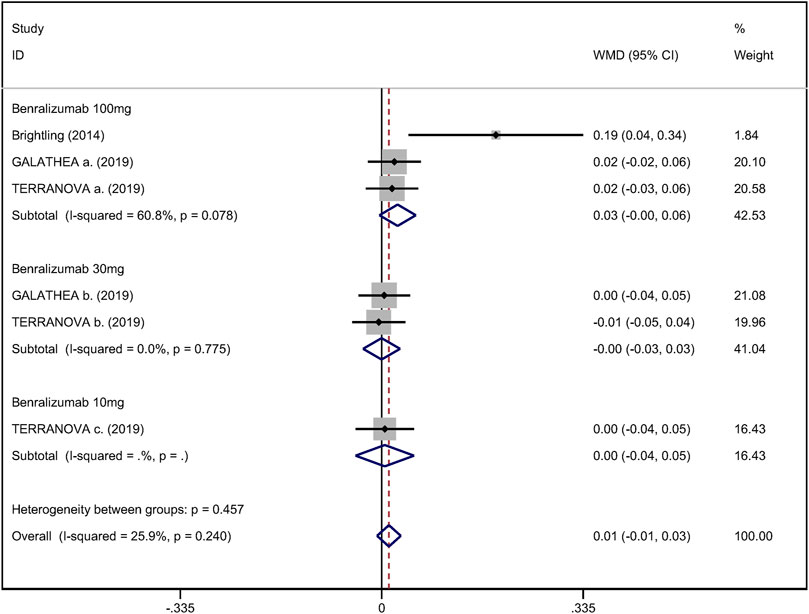
FIGURE 3. Forest plot of pre-bronchodilator FEV1 in eosinophilic COPD patients with anti-IL-5 therapy vs. placebo.
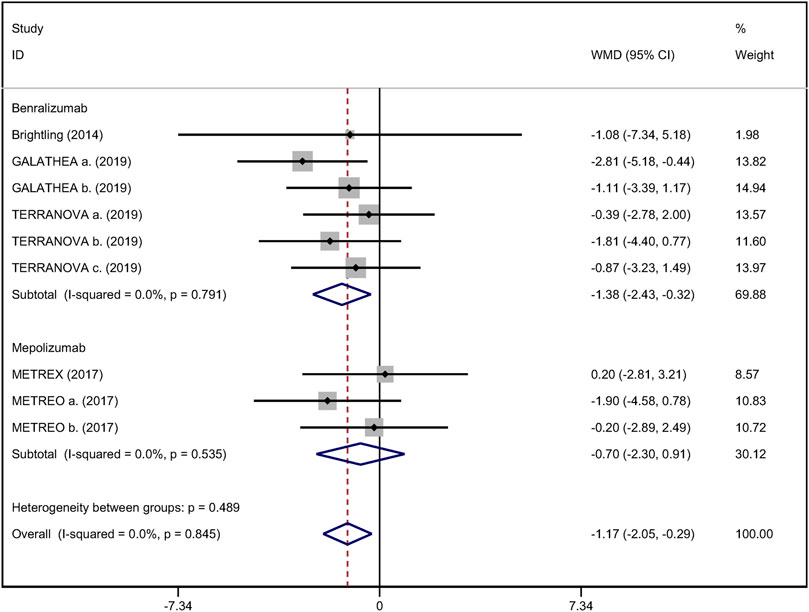
FIGURE 4. Forest plot of SGRQ score in eosinophilic COPD patients with anti-IL-5 therapy vs. placebo.
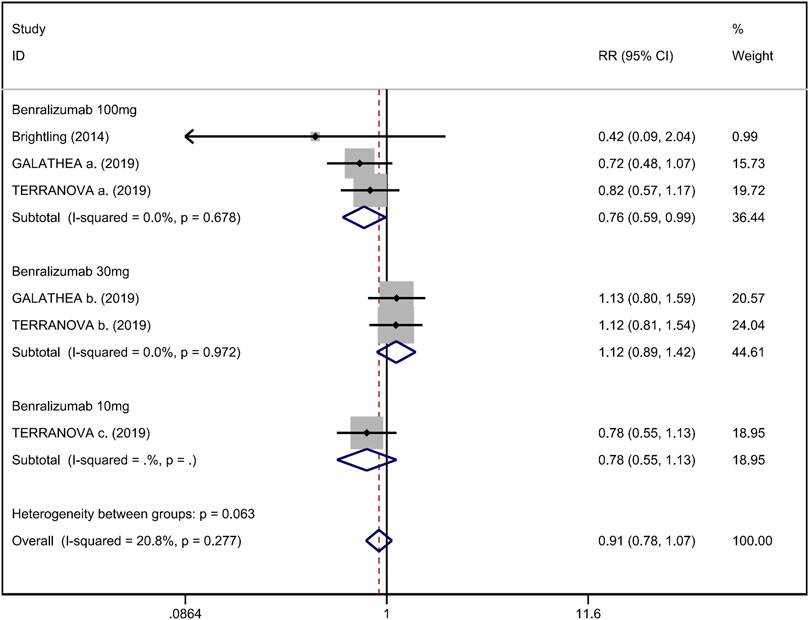
FIGURE 5. Forest plot of hospital admission rate for acute exacerbation in eosinophilic COPD patients with anti-IL-5 therapy vs. placebo.
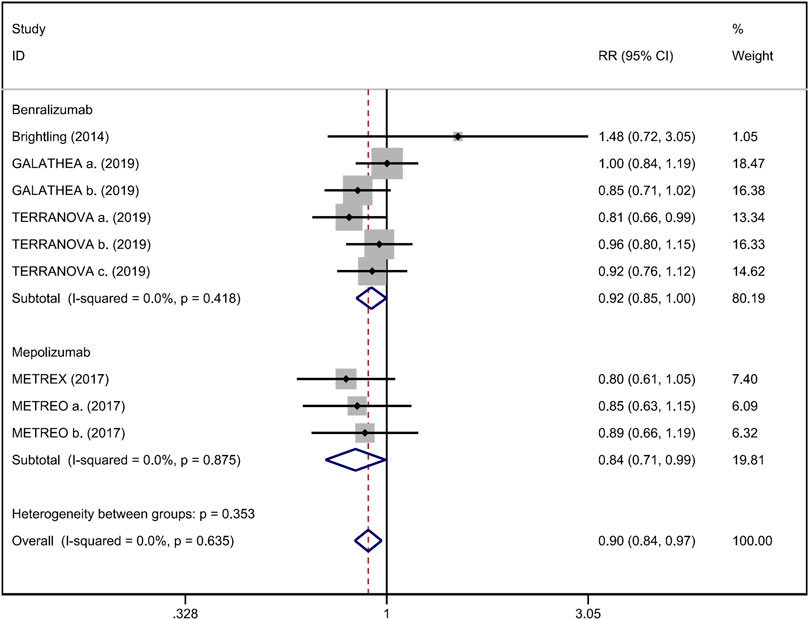
FIGURE 6. Forest plot of severe adverse event in eosinophilic COPD patients with anti-IL-5 therapy vs. placebo.
To enrich our study, we compared the efficacy of anti-IL-5 therapy in eosinophilic COPD and asthma (Farne et al., 2017; He et al., 2018). The outcomes (including annual exacerbation rate, the pre-bronchodilator FEV1, the health-related quality of life, and the severe adverse events) of anti-IL-5 therapy on eosinophilic COPD or asthma are listed in Table 2. Anti-IL-5 therapy was significantly more effective in reducing the annual exacerbation rate in asthma patients than in eosinophilic COPD patients. Similarly, anti-IL-5 therapy showed a more remarkable improvement of pre-bronchodilator FEV1 in asthma patients than in eosinophilic COPD. Furthermore, mepolizumab led to a significant enhancement of health-related quality of life (by SGRQ score) in asthma but not in eosinophilic COPD. Finally, mepolizumab caused a more significant reduction of severe adverse events in asthma than in eosinophilic COPD.
In this meta-analysis, we assessed the efficacy and safety of anti-IL-5 therapy in eosinophilic COPD patients. Several key findings were obtained: anti-IL-5 therapy significantly reduced the annual exacerbation rates without increasing the occurrence of severe adverse events (Brightling et al., 2014; Sciurba et al., 2018; Criner et al., 2019). However, the anti-IL-5 group did not show a significant improvement with regard to lung function, quality of life, and hospitalization (Brightling et al., 2014; Sciurba et al., 2018; Criner et al., 2019).
This meta-analysis demonstrated that anti-IL-5 therapy decreased the acute exacerbation rate in eosinophilic COPD patients. This result has physiological plausibility. IL-5 is a well-researched cytokine in eosinophilic inflammation, which is particularly vital for the differentiation, proliferation, and activation of eosinophils. It is released by the following 3 cells: CD4+ Th2 lymphocytes, eosinophils, and innate lymphoid cells. Both eosinophils and basophils express the IL-5R (Bagnasco et al., 2017; Yousuf et al., 2019). Mepolizumab reduces eosinophil counts in the blood and tissues by avidly binding to IL-5, preventing IL-5 from binding to eosinophil surface receptors (Hart et al., 2001; Varricchi et al., 2016). Benralizumab enhances antibody-dependent cell-mediated cytotoxic effects by binding to IL-5Rα, in turn reducing sputum and blood eosinophil count (Busse et al., 2010; Laviolette et al., 2013).
Furthermore, similar results were reported in severe asthma patients. Pavord et al. (2012), Ortega et al. (2014), and Chupp et al. (2017) have reported that mepolizumab treatment was associated with lower rates of exacerbations and symptoms and with greater improvements in health-related quality of life compared with placebo among patients with severe eosinophilic asthma. Similarly, a meta-analysis by Farne et al. has revealed that anti-IL-5 reduced asthma exacerbations roughly by half (Farne et al., 2017). In addition, Cabon et al. have conducted an RCT and reported that mAbs targeting IL-5 significantly reduced blood and sputum eosinophil counts and attenuated bronchial submucosal eosinophils by approximately 50% in patients with eosinophilic asthma (Cabon et al., 2017).
However, no significant improvement in lung function, quality of life, and hospitalization rate was observed in the anti-IL-5 group. Anti-IL-5 therapy was associated with a mean difference of −0.01–0.03 L in pre-bronchodilator FEV1 compared with placebo. A change of 0.1 L from baseline in FEV1 has been described as a difference that patients can perceive (Donohue, 2005). The mean difference in SGRQ reduction between the anti-IL-5 and placebo groups was 0.29–2.05, while a threshold of 4 units is considered clinically significant (Jones, 2005). Likewise, other anti-inflammatory therapies for COPD, including macrolide antibiotics, have been reported to show similar results, i.e., significant reductions in exacerbation rate that were not associated with significant improvements in pre-bronchodilator FEV1 or health-related quality of life (Herath et al., 2018). A major therapeutic goal in COPD patients is to prevent or reduce future exacerbations (Dave and Arjun, 2021). Therefore, anti-IL-5 therapy can be considered for use in eosinophilic COPD patients due to the decrease in acute exacerbation rate. Based on the GOLD guidelines, cornerstone treatments such as LAMA, LABA, and ICS greatly improve lung function and the quality of life (Dave and Arjun, 2021). Additionally, the anti-IL-5 group was associated with a lower risk of severe adverse events than the placebo group. This result was consistent with that noted in previous phase 3 trials of benralizumab for severe, uncontrolled eosinophilic asthma (Bleecker et al., 2016; FitzGerald et al., 2016).
There was heterogeneity in the SGRQ total score. We speculate that the main source of this heterogeneity was the subjectivity of the scorer’s perception of the scale. In addition, a single scoring scale does not accurately reflect the true status of the quality of life. Heterogeneity also existed in the change from baseline of pre-bronchodilator FEV1. One possible reason might be that the measurement device or the professional level of the implementer may be different. Another reason may be that the education and cooperation level of COPD patients could influence lung function test results.
There are several limitations to this meta-analysis. First, among the RCTs admitted included in this meta-analysis, benralizumab failed to reduce the annual rate of acute exacerbation, whereas mepolizumab showed opposite results. The differences observed between benralizumab and mepolizumab might be due to the differences in sample sizes of the studies. In addition, owing to the limited original research, we could not perform subgroup analysis and the reliability of the conclusions inevitably decreased. Therefore, additional large RCTs assessing the efficacy of anti-IL-5 therapy (including benralizumab, mepolizumab, and reslizumab) in eosinophilic COPD patients are urgently needed. Second, although we conducted the comparison between anti-IL-5 therapy in eosinophilic COPD and in asthma, further RCTs that compare the anti-IL-5 therapy with ICS in eosinophilic COPD are needed, which may allow us to better determine the efficacy of anti-IL-5 therapy in eosinophilic COPD. Finally, all RCTs included in this meta-analysis were sponsored by a biopharmaceutical company.
In this meta-analysis, we found that anti-IL-5 therapy significantly reduced the annual acute exacerbation rate and severe adverse events among eosinophilic COPD patients. In contrast, anti-IL-5 therapy did not improve lung function, quality of life, or hospitalization rate.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JL and CZ designed this systematic review. MZ and YW have been involved in the search strategy. CZ, MZ, and YW did the collection and the analysis of the data. XS, TL, and HY interpreted the data. CZ wrote the systematic review and all the other authors revised the manuscript. JL provided general advice on the manuscript. All the authors read and approved the final manuscript.
This study was supported by the Science and Technology Projects of Gansu Province (Grant no. 18JR3RA344). The authors remain independent of any funding influence.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The author gratefully acknowledges the support of the First Clinical Medical College of Lanzhou University, Lanzhou University First Affiliated Hospital, and all the authors who participated in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.754268/full#supplementary-material
Bagnasco, D., Ferrando, M., Varricchi, G., Puggioni, F., Passalacqua, G., and Canonica, G. W. (2017). Anti-Interleukin 5 (IL-5) and IL-5Ra Biological Drugs: Efficacy, Safety, and Future Perspectives in Severe Eosinophilic Asthma. Front. Med. (Lausanne) 4, 135. doi:10.3389/fmed.2017.00135
Balkissoon, R. (2018). New Treatment Options for COPD: How Do We Decide Phenotypes, Endotypes or Treatable Traits? Chronic Obstr Pulm. Dis. 5 (1), 72–80.0128 doi:10.15326/jcopdf.5.1.201810.15326/jcopdf.5.1.2018.0128
Bleecker, E. R., FitzGerald, J. M., Chanez, P., Papi, A., Weinstein, S. F., Barker, P., et al. (2016). Efficacy and Safety of Benralizumab for Patients with Severe Asthma Uncontrolled with High-Dosage Inhaled Corticosteroids and Long-Acting β2-agonists (SIROCCO): a Randomised, Multicentre, Placebo-Controlled Phase 3 Trial. Lancet 388 (10056), 2115–2127. doi:10.1016/s0140-6736(16)31324-1
Brightling, C. E., Bleecker, E. R., Panettieri, R. A., Bafadhel, M., She, D., Ward, C. K., et al. (2014). Benralizumab for Chronic Obstructive Pulmonary Disease and Sputum Eosinophilia: a Randomised, Double-Blind, Placebo-Controlled, Phase 2a Study. Lancet Respir. Med. 2 (11), 891–901. doi:10.1016/s2213-2600(14)70187-0
Busse, W. W., Katial, R., Gossage, D., Sari, S., Wang, B., Kolbeck, R., et al. (2010). Safety Profile, Pharmacokinetics, and Biologic Activity of MEDI-563, an anti-IL-5 Receptor Alpha Antibody, in a Phase I Study of Subjects with Mild Asthma. J. Allergy Clin. Immunol. 125 (6), 1237–e2. doi:10.1016/j.jaci.2010.04.005
Cabon, Y., Molinari, N., Marin, G., Vachier, I., Gamez, A. S., Chanez, P., et al. (2017). Comparison of Anti-interleukin-5 Therapies in Patients with Severe Asthma: Global and Indirect Meta-Analyses of Randomized Placebo-Controlled Trials. Clin. Exp. Allergy 47 (1), 129–138. doi:10.1111/cea.12853
Calzetta, L., Cazzola, M., Matera, M. G., and Rogliani, P. (2019). Adding a LAMA to ICS/LABA Therapy: A Meta-Analysis of Triple Combination Therapy in COPD. Chest 155 (4), 758–770. doi:10.1016/j.chest.2018.12.016
Chupp, G. L., Bradford, E. S., Albers, F. C., Bratton, D. J., Wang-Jairaj, J., Nelsen, L. M., et al. (2017). Efficacy of Mepolizumab Add-On Therapy on Health-Related Quality of Life and Markers of Asthma Control in Severe Eosinophilic Asthma (MUSCA): a Randomised, Double-Blind, Placebo-Controlled, Parallel-Group, Multicentre, Phase 3b Trial. Lancet Respir. Med. 5 (5), 390–400. doi:10.1016/s2213-2600(17)30125-x
Couillard, S., Larivée, P., Courteau, J., and Vanasse, A. (2017). Eosinophils in COPD Exacerbations Are Associated with Increased Readmissions. Chest 151 (2), 366–373. doi:10.1016/j.chest.2016.10.003
Criner, G. J., Celli, B. R., Brightling, C. E., Agusti, A., Papi, A., Singh, D., et al. (2019). Benralizumab for the Prevention of COPD Exacerbations. N. Engl. J. Med. 381 (11), 1023–1034. doi:10.1056/NEJMoa1905248
Dasgupta, A., Kjarsgaard, M., Capaldi, D., Radford, K., Aleman, F., Boylan, C., et al. (2017). A Pilot Randomised Clinical Trial of Mepolizumab in COPD with Eosinophilic Bronchitis. Eur. Respir. J. 49 (3). doi:10.1183/13993003.02486-2016
Dasgupta, A., Neighbour, H., and Nair, P. (2013). Targeted Therapy of Bronchitis in Obstructive Airway Diseases. Pharmacol. Ther. 140 (3), 213–222. doi:10.1016/j.pharmthera.2013.07.001
Dave, S., and Arjun, R., (2021). Global Initiative for Chronic Obstructive Lung Disease (GOLD) [database on Internet]. Glob. strategy Diagn. Manag. Prev. chronic obstructive Pulm. Dis..71(01): 9–14. Available at from: http://goldcopd.org. Accessed November 17, 2020. doi:10.1055/s-0042-121903
Donohue, J. F. (2005). Minimal Clinically Important Differences in COPD Lung Function. Copd 2 (1), 111–124. doi:10.1081/copd-200053377
Donovan, T., Milan, S. J., Wang, R., Banchoff, E., Bradley, P., and Crossingham, I. (2020). Anti-IL-5 Therapies for Chronic Obstructive Pulmonary Disease. Cochrane database Syst. Rev. Epub 2020/12/10. PubMed PMID: 33295032; PubMed Central PMCID: PMCPMC8106745 (respiratory medicine). EB: none known. PB: I work in a clinically relevant speciality (respiratory medicine). IC: I work in a clinically relevant speciality (respiratory medicine). I have been involved as a local investigator for a GSK‐sponsored drug trial of inhaled nemiralisib for COPD, but did not directly receive funding for this, 12. 12. Cd013432. doi:10.1002/14651858.CD013432.pub2
Dransfield, M. T., Kunisaki, K. M., Strand, M. J., Anzueto, A., Bhatt, S. P., Bowler, R. P., et al. (2017). Acute Exacerbations and Lung Function Loss in Smokers with and without Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 195 (3), 324–330. doi:10.1164/rccm.201605-1014OC
Farne, H. A., Wilson, A., Powell, C., Bax, L., and Milan, S. J. (2017). Anti-IL5 Therapies for Asthma. Cochrane Database Syst. Rev. 9 (9), Cd010834. doi:10.1002/14651858.CD010834.pub3
FitzGerald, J. M., Bleecker, E. R., Nair, P., Korn, S., Ohta, K., Lommatzsch, M., et al. (2016). Benralizumab, an Anti-interleukin-5 Receptor α Monoclonal Antibody, as Add-On Treatment for Patients with Severe, Uncontrolled, Eosinophilic Asthma (CALIMA): a Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet 388 (10056), 2128–2141. doi:10.1016/s0140-6736(16)31322-8
Ge, L., Tian, J. H., Li, Y. N., Pan, J. X., Li, G., Wei, D., et al. (2018). Association between Prospective Registration and Overall Reporting and Methodological Quality of Systematic Reviews: a Meta-Epidemiological Study. J. Clin. Epidemiol. 93, 45–55. doi:10.1016/j.jclinepi.2017.10.012
Han, M. K., Agusti, A., Calverley, P. M., Celli, B. R., Criner, G., Curtis, J. L., et al. (2010). Chronic Obstructive Pulmonary Disease Phenotypes: the Future of COPD. Am. J. Respir. Crit. Care Med. 182 (5), 598–604. doi:10.1164/rccm.200912-1843CC
Hart, T. K., Cook, R. M., Zia-Amirhosseini, P., Minthorn, E., Sellers, T. S., Maleeff, B. E., et al. (2001). Preclinical Efficacy and Safety of Mepolizumab (SB-240563), a Humanized Monoclonal Antibody to IL-5, in Cynomolgus Monkeys. J. Allergy Clin. Immunol. 108 (2), 250–257. doi:10.1067/mai.2001.116576
He, L. L., Zhang, L., Jiang, L., Xu, F., and Fei, D. S. (2018). Efficacy and Safety of Anti-interleukin-5 Therapy in Patients with Asthma: A Pairwise and Bayesian Network Meta-Analysis. Int. Immunopharmacol 64, 223–231. doi:10.1016/j.intimp.2018.08.031
Herath, S. C., Normansell, R., Maisey, S., and Poole, P. (2018). Prophylactic Antibiotic Therapy for Chronic Obstructive Pulmonary Disease (COPD), Cochrane database Syst. Rev., 2018. 10. Cd009764. doi:10.1002/14651858.CD009764.pub3
Hilleman, D. E., Dewan, N., Malesker, M., and Friedman, M. (2000). Pharmacoeconomic Evaluation of COPD. Chest 118 (5), 1278–1285. doi:10.1378/chest.118.5.1278
Hogg, J. C., Chu, F., Utokaparch, S., Woods, R., Elliott, W. M., Buzatu, L., et al. (2004). The Nature of Small-Airway Obstruction in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 350 (26), 2645–2653. doi:10.1056/NEJMoa032158
Jones, P. W. (2005). St. George's Respiratory Questionnaire: MCID. Copd 2 (1), 75–79. doi:10.1081/copd-200050513
Kunisaki, K. M., Dransfield, M. T., Anderson, J. A., Brook, R. D., Calverley, P. M. A., Celli, B. R., et al. (2018). Exacerbations of Chronic Obstructive Pulmonary Disease and Cardiac Events. A Post Hoc Cohort Analysis from the SUMMIT Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 198 (1), 51–57. doi:10.1164/rccm.201711-2239OC
Lan, S.-H., Lai, C.-C., Chang, S.-P., Hsu, C.-C., Chen, C.-H., Wang, Y.-H., et al. (2020). Efficacy and Safety of Anti-interleukin-5 Therapy in Patients with Chronic Obstructive Pulmonary Disease: A Meta-Analysis of Randomized, Controlled Trials, J. Microbiol. Immunol. Infect.. S1684-1182(20). 30253-X. doi:10.1016/j.jmii.2020.11.001
Lange, P., Halpin, D. M., O'Donnell, D. E., and MacNee, W. (2016). Diagnosis, Assessment, and Phenotyping of COPD: beyond FEV₁. Int. J. Chron. Obstruct Pulmon Dis. 11 Spec Iss, 3–12. doi:10.2147/copd.S85976
Lau, J., Ioannidis, J. P., Terrin, N., Schmid, C. H., and Olkin, I. (2006). The Case of the Misleading Funnel Plot. BMJ 333 (7568), 597–600. doi:10.1136/bmj.333.7568.597
Laviolette, M., Gossage, D. L., Gauvreau, G., Leigh, R., Olivenstein, R., Katial, R., et al. (2013). Effects of Benralizumab on Airway Eosinophils in Asthmatic Patients with Sputum Eosinophilia. J. Allergy Clin. Immunol. 132 (5), 1086–e5. doi:10.1016/j.jaci.2013.05.020
Mathers, C. D., and Loncar, D. (2006). Projections of Global Mortality and burden of Disease from 2002 to 2030. Plos Med. 3 (11), e442. doi:10.1371/journal.pmed.0030442
Mkorombindo, T., and Dransfield, M. T. (2019). Mepolizumab in the Treatment of Eosinophilic Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct Pulmon Dis. 14, 1779–1787. doi:10.2147/copd.S162781
Moher, D., Jadad, A. R., and Tugwell, P. (1996). Assessing the Quality of Randomized Controlled Trials. Current Issues and Future Directions. Int. J. Technol. Assess. Health Care 12 (2), 195–208. doi:10.1017/s0266462300009570
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. BMJ 339, b2535. doi:10.1136/bmj.b2535
Ortega, H. G., Liu, M. C., Pavord, I. D., Brusselle, G. G., FitzGerald, J. M., Chetta, A., et al. (2014). Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 371 (13), 1198–1207. doi:10.1056/NEJMoa1403290
Pavord, I. D., Korn, S., Howarth, P., Bleecker, E. R., Buhl, R., Keene, O. N., et al. (2012). Mepolizumab for Severe Eosinophilic Asthma (DREAM): a Multicentre, Double-Blind, Placebo-Controlled Trial. Lancet 380 (9842), 651–659. doi:10.1016/s0140-6736(12)60988-x
Sciurba, F. C., Bradford, E. S., and Pavord, I. D. (2018). Mepolizumab for Eosinophilic COPD. N. Engl. J. Med. 378 (7), 681–683. doi:10.1056/NEJMc1715454
Singh, D., Kolsum, U., Brightling, C. E., Locantore, N., Agusti, A., and Tal-Singer, R. (2014). Eosinophilic Inflammation in COPD: Prevalence and Clinical Characteristics. Eur. Respir. J. 44 (6), 1697–1700. doi:10.1183/09031936.00162414
Stovold, E., Beecher, D., Foxlee, R., and Noel-Storr, A. (2014). Study Flow Diagrams in Cochrane Systematic Review Updates: an Adapted PRISMA Flow Diagram. Syst. Rev. 3, 54. doi:10.1186/2046-4053-3-54
Takatsu, K., Takaki, S., and Hitoshi, Y. (1994). Interleukin-5 and its Receptor System: Implications in the Immune System and Inflammation. Adv. Immunol. 57, 145–190. doi:10.1016/s0065-2776(08)60673-2
Toy, E. L., Gallagher, K. F., Stanley, E. L., Swensen, A. R., and Duh, M. S. (2010). The Economic Impact of Exacerbations of Chronic Obstructive Pulmonary Disease and Exacerbation Definition: a Review. Copd 7 (3), 214–228. doi:10.3109/15412555.2010.481697
Varricchi, G., Bagnasco, D., Borriello, F., Heffler, E., and Canonica, G. W. (2016). Interleukin-5 Pathway Inhibition in the Treatment of Eosinophilic Respiratory Disorders: Evidence and Unmet Needs. Curr. Opin. Allergy Clin. Immunol. 16 (2), 186–200. doi:10.1097/aci.0000000000000251
Vestbo, J., Papi, A., Corradi, M., Blazhko, V., Montagna, I., Francisco, C., et al. (2017). Single Inhaler Extrafine Triple Therapy versus Long-Acting Muscarinic Antagonist Therapy for Chronic Obstructive Pulmonary Disease (TRINITY): a Double-Blind, Parallel Group, Randomised Controlled Trial. Lancet 389 (10082), 1919–1929. doi:10.1016/s0140-6736(17)30188-5
Wang, C., Xu, J., Yang, L., Xu, Y., Zhang, X., Bai, C., et al. (2018). Prevalence and Risk Factors of Chronic Obstructive Pulmonary Disease in China (The China Pulmonary Health [CPH] Study): a National Cross-Sectional Study. Lancet 391 (10131), 1706–1717. doi:10.1016/s0140-6736(18)30841-9
Wang, X., Chen, Y., Yao, L., Zhou, Q., Wu, Q., Estill, J., et al. (2018). Reporting of Declarations and Conflicts of Interest in WHO Guidelines Can Be Further Improved. J. Clin. Epidemiol. 98, 1–8. doi:10.1016/j.jclinepi.2017.12.021
Keywords: eosinophils, monoclonal antibodies, anti-IL-5, COPD, meta-analysis
Citation: Zhang C, Wang Y, Zhang M, Su X, Lei T, Yu H and Liu J (2021) Monoclonal Antibodies Targeting IL-5 or IL-5Rα in Eosinophilic Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12:754268. doi: 10.3389/fphar.2021.754268
Received: 06 August 2021; Accepted: 21 September 2021;
Published: 02 November 2021.
Edited by:
Djuro Kosanovic, I. M. Sechenov First Moscow State Medical University, RussiaReviewed by:
Corrado Pelaia, University of Catanzaro, ItalyCopyright © 2021 Zhang, Wang, Zhang, Su, Lei, Yu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Liu, bWVkZWNpbmxpdUBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.