- 1Department of Pharmacy, Peking University People’s Hospital, Beijing, China
- 2Department of Pharmaceutical Administration and Clinical Pharmacy, School of Pharmaceutical Sciences, Peking University Health Science Center, Beijing, China
- 3Department of Pharmacy, Peking University Third Hospital, Beijing, China
Background: Hepatic inflow occlusion proceeded to reduce blood loss during hepatectomy induces ischemia-reperfusion (IR) injury in the remnant liver. Dexmedetomidine, a selective α2-adrenoceptor agonist used as an anesthetic adjuvant, has been shown to attenuate IR injury in preclinical and clinical studies. However, a meta-analysis is needed to systematically evaluate the protective effect of perioperative dexmedetomidine use on IR injury induced by hepatectomy.
Methods: A prospectively registered meta-analysis following Cochrane and PRISMA guidelines concerning perioperative dexmedetomidine use on IR injury after hepatectomy was performed via searching Cochrane Library, PubMed, EMBASE, ClinicalTrials.gov, Web of Science, CNKI, WanFang, and Sinomed for eligible randomized controlled trials up to 2021.3.31. The main outcome is postoperative liver function. Risk of bias was assessed by the Cochrane Risk of Bias tool. Review Manager 5.3 and Stata12.0 were applied to perform data analyses.
Results: Eight RCTs enrolling 468 participants were included. Compared with 0.9% sodium chloride, dexmedetomidine decreased serum concentration of ALT (WMD = −66.54, 95% CI: −92.10–−40.98), AST (WMD= −82.96, 95% CI: −106.74–−59.17), TBIL (WMD = −4.51, 95% CI: −7.32–−1.71), MDA (WMD = −3.09, 95% CI: −5.17–−1.01), TNF-α (WMD = −36.54, 95% CI: −61.33–−11.95) and IL-6 (WMD = −165.05, 95% CI: −225.76–−104.34), increased SOD activity (WMD = 24.70, 95% CI: 18.09–31.30) within postoperative one day. There was no significant difference in intraoperative or postoperative recovery parameters between groups.
Conclusions: Perioperative administration of dexmedetomidine can exert a protective effect on liver IR injury after hepatectomy. Additional studies are needed to further evaluate postoperative recovery outcomes of dexmedetomidine with different dosing regimens.
Introduction
Hepatic inflow occlusion by clamping the portal triad (Pringle maneuver), which is traditionally performed to reduce blood loss during hepatectomy, would cause ischemia-reperfusion (IR) injury of the remnant liver after the release of blood flow (Jarnagin et al., 2002; Gurusamy et al., 2009). IR injury is a common but dangerous complication of hepatectomy, especially in patients with underlying liver disease. It can induce local and systemic release of inflammatory mediators and oxygen free radicals, leading to liver or remote organ dysfunction. Numerous strategies have been attempted to reduce IR injury after liver resection, including surgical manipulations, e.g., ischemic preconditioning (Rodríguez et al., 2015) and intermittent hepatic inflow occlusion (Petrowsky et al., 2006), as well as pharmacological intervention, such as anesthetic or sedative agents (Abu-Amara et al., 2009; Li et al., 2016). Although many of them exhibit a protective effect in laboratory and/or clinical researches, agents that commonly used in anesthesia are more preferred as they don’t require additional surgical procedures and won’t prolong the overall time of operation.
Dexmedetomidine, a highly selective α2-adrenoceptor agonist, is generally used as an anesthetic adjuvant during surgery and a sedative agent in intensive care unit (ICU). It can offer satisfactory sedation without respiratory depression or hemodynamic instability and exhibit intraoperative anesthetic-sparing effect. It has a theoretical advantage in organ protection, which has been studied in multiple organ systems (Dahmani et al., 2005; Okada et al., 2007; Kundra et al., 2018; Liu et al., 2018; Si et al., 2018; Peng et al., 2020). Animal experiments showed that dexmedetomidine decreased hepatic IR induced oxidative stress and inflammatory responses in liver, serum, and other remote organs (Sahin et al., 2013; Tüfek et al., 2013; Kucuk et al., 2014). Several clinical studies in recent years also proved the hepatoprotective properties of dexmedetomidine against IR injury in patients receiving hepatectomy (Wang et al., 2014; Taman and Elhefnawy, 2019; Zhang et al., 2020). Although most of these researches yield a positive outcome, a comprehensive meta-analysis is still needed to fully evaluate the protective effect of dexmedetomidine on hepatic IR injury.
Therefore, in this study, we conducted a meta-analysis of available randomized controlled trials (RCTs) to systematically discuss the efficacy and safety of perioperative dexmedetomidine use in reducing IR injuries associated with hepatectomy, in order to provide evidence for new strategy of preventive treatment.
Methods
This meta-analysis was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (Moher et al., 2015). It was prospectively registered on PROSPERO (CRD42020212072). The PRISMA checklist was exhibited in Supplementary File S1.
Literature Search Strategy
We searched Cochrane Library (Cochrane Center Register of Controlled Trials), MEDLINE, EMBASE, ClinicalTrials.gov, Web of Science, China National Knowledge Infrastructure (CNKI), WanFang, and SinoMed for eligible studies from inception to March 31, 2021. The search keywords were “dexmedetomidine”, “hepatectomy”, “hepatic”, “liver” and “resection”. The search query is given in Supplementary Table S1. Manual searches of references in systematic reviews and included studies were conducted to identify additional qualified researches.
Inclusion Criteria
We included studies meeting the following criteria: 1) Study design: RCTs; 2) Participants: patients who were scheduled for hepatic surgery irrespective of original liver diseases or Child-Pugh Score; 3) Interventions and Comparisons: comparing dexmedetomidine treatment versus another pharmacological intervention, irrespective of the dose, time, or pharmacological class of the administered drug; 4) Outcomes: perioperative liver function is included as an outcome with available data.
Exclusion Criteria
RCTs without English abstracts were excluded.
Outcome Measures
The primary outcome was postoperative liver function as measured by serum concentration of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL).
Secondary outcomes included biomarkers of systematic oxidative stress and inflammatory response, postoperative function of remote organs, intraoperative and postoperative recovery parameters. Oxidative stress was indicated by serum malondialdehyde (MDA) level and superoxide dismutase (SOD) activity. Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were used for evaluation of inflammatory response. Renal function was assessed by urea nitrogen (BUN) and creatinine (Cr); intestinal injury was evaluated by serum diamine oxidase (DAO) activity. Intraoperative parameters included operation time, blood loss and number of patients transfused. Postoperative recovery variables included ventilator support time and length of hospital stay (LOS).
Data Extraction and Quality Assessment
Two investigators (YH and RW) independently performed the literature retrieval, data extraction and quality assessment. Disagreements were resolved through consensus or by consulting a third author (XL). Extracted data included general study information (e.g., year of publication, country, details regarding interventions and outcomes), baseline data of study participants (e.g., age, gender, original diseases, Child-Pugh score, ASA-status), primary and secondary outcomes. We used the Cochrane risk of bias tool (Higgins et al., 2011) to assess the quality of included studies.
Statistical Analysis
Continuous data were entered into REVMAN 5.3 (Cochrane Collaboration, Oxford, United Kingdom) for meta-analysis and analyzed using weighted mean difference (WMD) with 95% confidence interval (CI). Data unsuitable for meta-analysis were reported narratively. Heterogeneity across studies was assessed by the Cochrane χ2 test and I2 statistic. The random effect model was applied for pooled results with significant statistical heterogeneity (I2 > 50%); otherwise, the fixed effect model would be used. Sensitivity analysis was performed in Stata (version 12.0; StataCorp LP) to test the stability of this meta-analysis and detect the source of heterogeneity (Chen et al., 2021).
Results
Search Results and Study Characteristics
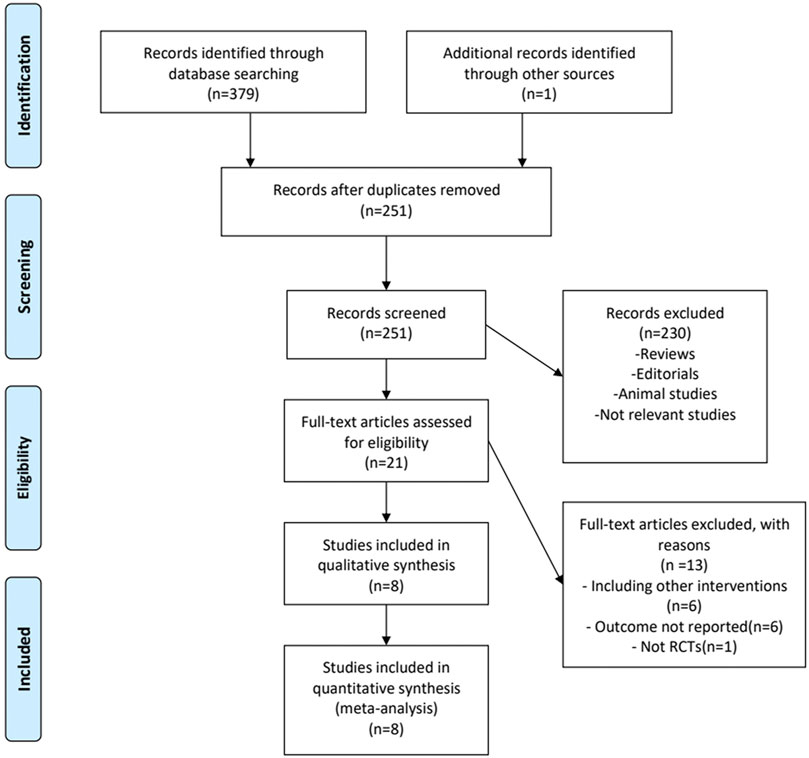
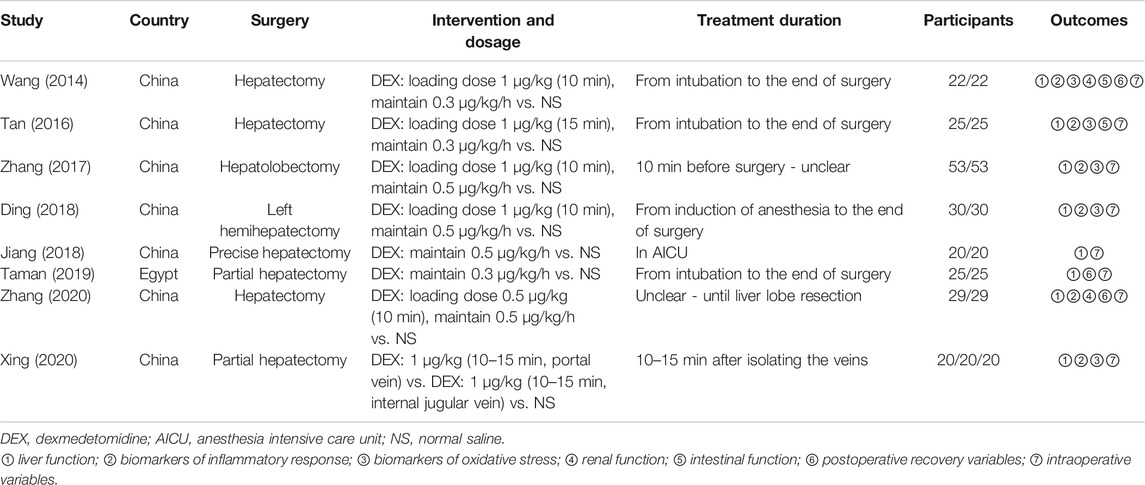
A detailed overview of PRISMA flow chart for database searching and study identification is presented in Figure 1. Eight studies enrolling 468 participants were included in this meta-analysis (Wang et al., 2014; Tan et al., 2016; Zhang et al., 2017; Ding et al., 2018; Jiang et al., 2018; Taman and Elhefnawy, 2019; Xing et al., 2020; Zhang et al., 2020). Characteristics of included RCTs and study participants were summarized in Tables 1, 2.
Three RCTs were published in English (Wang et al., 2014; Taman and Elhefnawy, 2019; Zhang et al., 2020), while the other five were published in Chinese with English abstracts (Tan et al., 2016; Zhang et al., 2017; Ding et al., 2018; Jiang et al., 2018; Xing et al., 2020). These studies all involved one control and one dexmedetomidine intervention group, except one trial which set one control and two intervention groups (same dose of dexmedetomidine given through portal vein and internal jugular vein, respectively) (Xing et al., 2020). In subsequent analysis, this three-arm trial was considered as two independent double-arm trials to facilitate data merging. Dexmedetomidine administration was given intravenously by using a loading dose plus continuous infusion in five studies (Wang et al., 2014; Tan et al., 2016; Zhang et al., 2017; Ding et al., 2018; Zhang et al., 2020), a constant administration rate ranging from 0.3 to 0.5 μg/kg/h in two studies (Jiang et al., 2018; Taman and Elhefnawy, 2019), and a single dose injection in one study (Xing et al., 2020). Dexmedetomidine and control were given before or during the operation except for one study in which they were given during postoperative stay in anesthesia intensive care unit (AICU) (Jiang et al., 2018).
Study Quality
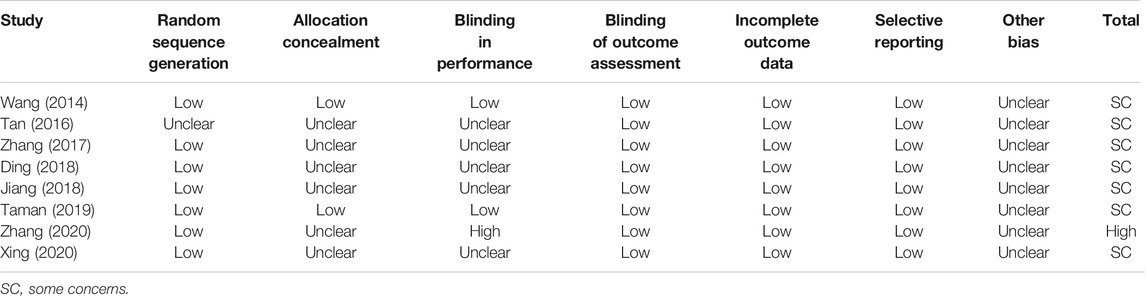
One study was deemed to be of high risk of bias for inadequate blinding of investigators (single-blind) (Zhang et al., 2020). The other studies were assessed to be of some concerns. The details of quality assessment for included studies are listed in TABLE 3.
Outcome Measures
Primary Outcomes
Postoperative Liver Function
ALT and AST levels at different time points were reported in all included studies. Compared with baseline (before surgery), postoperative ALT and AST levels were significantly elevated after surgery in control group, indicating the occurrence of hepatic IR injury. ALT and AST levels within 2 h after surgery were markedly decreased in dexmedetomidine group (ALT: WMD = −23.59, 95% CI: −32.60 to −14.57, p < 0.00001, I2 = 95%; AST: WMD = −39.54, 95% CI: −50.33–−28.75, p < 0.00001, I2 = 88%) (Figures 2A,D). Similarly, ALT and AST levels at 6–24 h (ALT: WMD = −66.54, 95% CI: −92.10–−40.98, p < 0.00001, I2 = 98%; AST: WMD = −82.96, 95% CI: −106.74–−59.17, p < 0.00001, I2 = 97%) (Figures 2B,E) and 48–72 h (ALT: WMD = −63.55, 95% CI: −124.01–−3.09, p = 0.04, I2 = 98%; AST: WMD = −56.61, 95% CI: −105.84–−7.38, p = 0.02, I2 = 91%) (Figures 2C,F) after surgery was also reduced by dexmedetomidine. Sensitivity analysis by excluding each included RCT at one time revealed that Taman 2019 was inconsistent with the size of the overall hepaprotective effect of dexmedetomidine (ALT: WMD = −87.57, 95% CI: −98.40–−76.74, p = 0.58, I2 = 0%; AST: WMD = −77.90, 95% CI: −99.55–−56.26, p = 0.73, I2 = 0%) (Supplementary Figure S1), while the other studies were consistent.
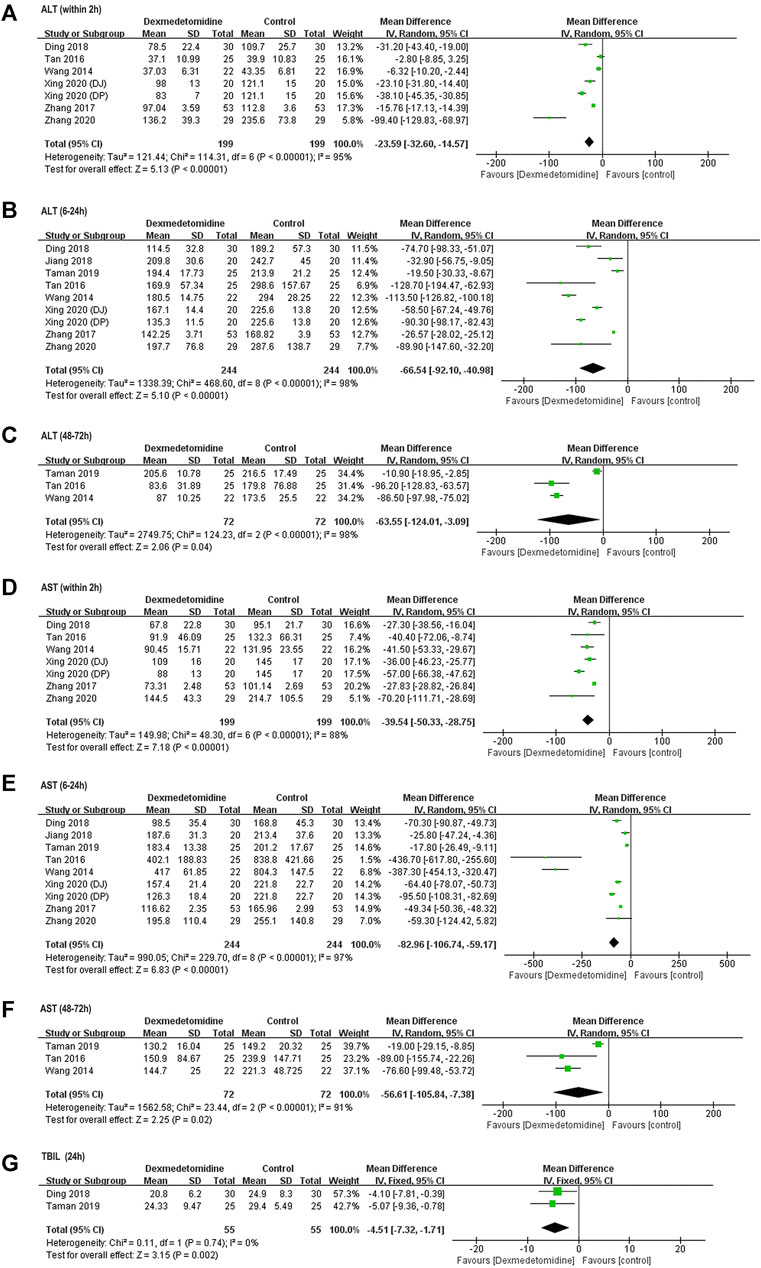
FIGURE 2. Forest plots for meta-analyses comparing postoperative liver function between dexmedetomidine and control group of participants undergoing hepatectomy. (A) ALT (within 2 h after surgery); (B) ALT (6–24 h after surgery); (C) ALT (48–72 h after surgery); (D) AST (with 2 h after surgery); (E) AST (6–24 h after surgery); (F) AST (48–72 h after surgery); (G) TBIL (24 h after surgery).
TBIL content was reported in two studies (Ding et al., 2018; Taman and Elhefnawy, 2019). Pooled results with a fixed effect model revealed that dexmedetomidine inhibited the increase in TBIL level at 24 h after surgery compared with control (WMD = −4.51, 95% CI: −7.32–−1.71, p = 0.002, I2 = 0%) (Figure 2G).
Secondary Outcomes
Oxidative Stress
Perioperative serum MDA and SOD concentration was reported in four studies involving 260 (Wang et al., 2014; Tan et al., 2016; Zhang et al., 2017; Ding et al., 2018) and 270 (Wang et al., 2014; Zhang et al., 2017; Xing et al., 2020) participants, respectively. Significantly elevated MDA level and reduced SOD activity were observed in control group after surgery among all these studies. Postoperative MDA level was markedly reduced (1 h: WMD = −3.93, 95% CI: −7.35–−0.51, p = 0.02, I2 = 98%; 6–24 h: WMD = −3.09, 95% CI: −5.17–−1.01, p = 0.004, I2 = 92%) (Figures 3A,B), while SOD activity was significantly elevated (1 h: WMD = 36.40, 95% CI: 23.80–49.00, p < 0.00001, I2 = 98%; 6–24 h: WMD = 24.70, 95% CI: 18.09–31.30, p < 0.00001, I2 = 95%) in dexmedetomidine group (Figures 3C,D).
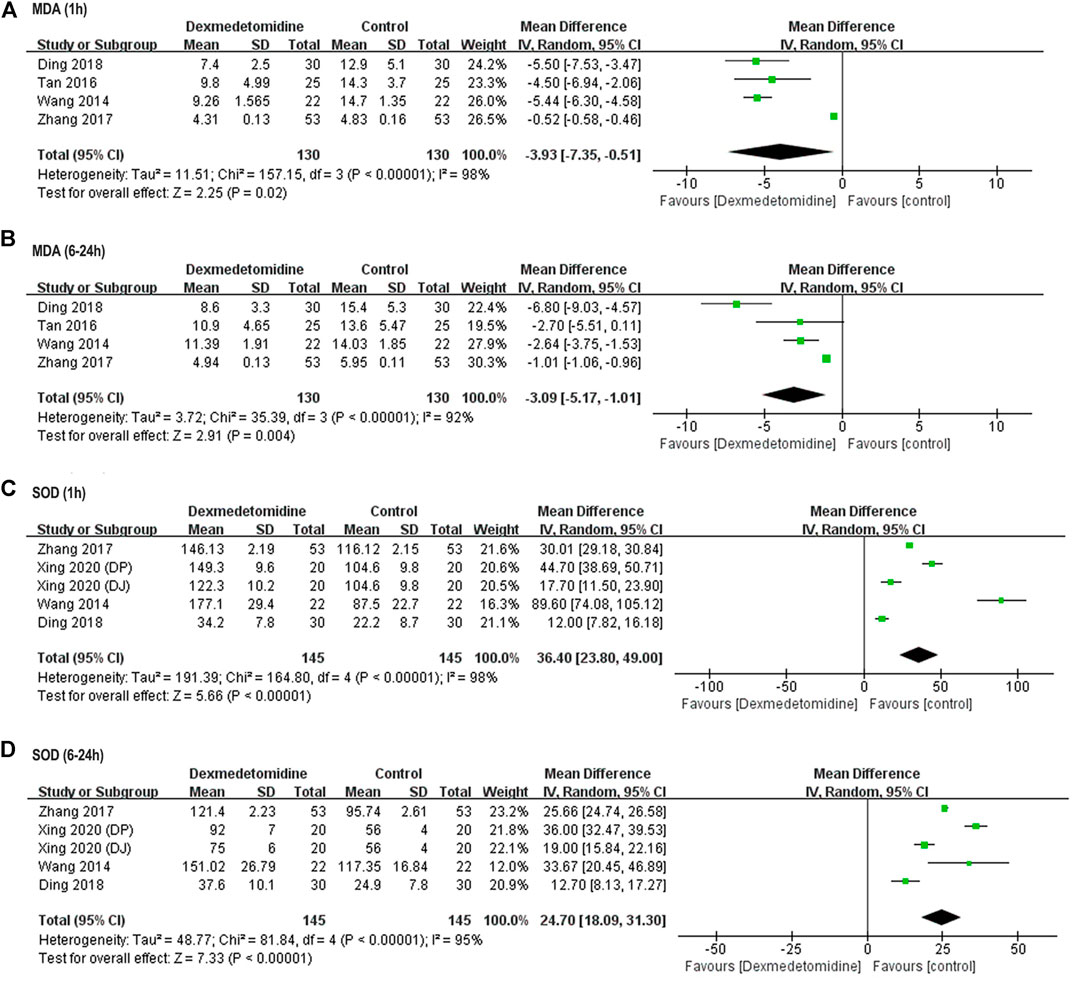
FIGURE 3. Forest plots for meta-analyses comparing postoperative oxidative stress between dexmedetomidine and control group of participants undergoing hepatectomy. (A) MDA (1 h after surgery); (B) MDA (6–24 h after surgery); (C) SOD (1 h after surgery); (D) SOD (6–24 h after surgery).
Inflammatory Response
TNF-α and IL-6 concentration at different time points was reported in five (Wang et al., 2014; Tan et al., 2016; Zhang et al., 2017; Xing et al., 2020; Zhang et al., 2020) and four (Wang et al., 2014; Tan et al., 2016; Ding et al., 2018; Zhang et al., 2020) studies enrolling 318 and 212 participants, respectively. In all these studies, TNF-α and IL-6 level was significantly increased after surgery in control group. However, dexmedetomidine administration decreased TNF-α release from 1 to 6–24 h after surgery (1 h: WMD = −21.77, 95% CI: −36.25–−7.29, p = 0.003, I2 = 99%; 6–24 h: WMD = −36.54, 95% CI: −61.33–−11.95, p = 0.004, I2 = 99%) (Figures 4A,B). Similarly, IL-6 level was also reduced at the same time (1 h: WMD = −24.3, 95% CI: −35.23–−13.36, p < 0.0001, I2 = 85%; 6–24 h: WMD = −165.05, 95% CI: −225.76–−104.34, p < 0.00001, I2 = 94%) (Figures 4C,D).
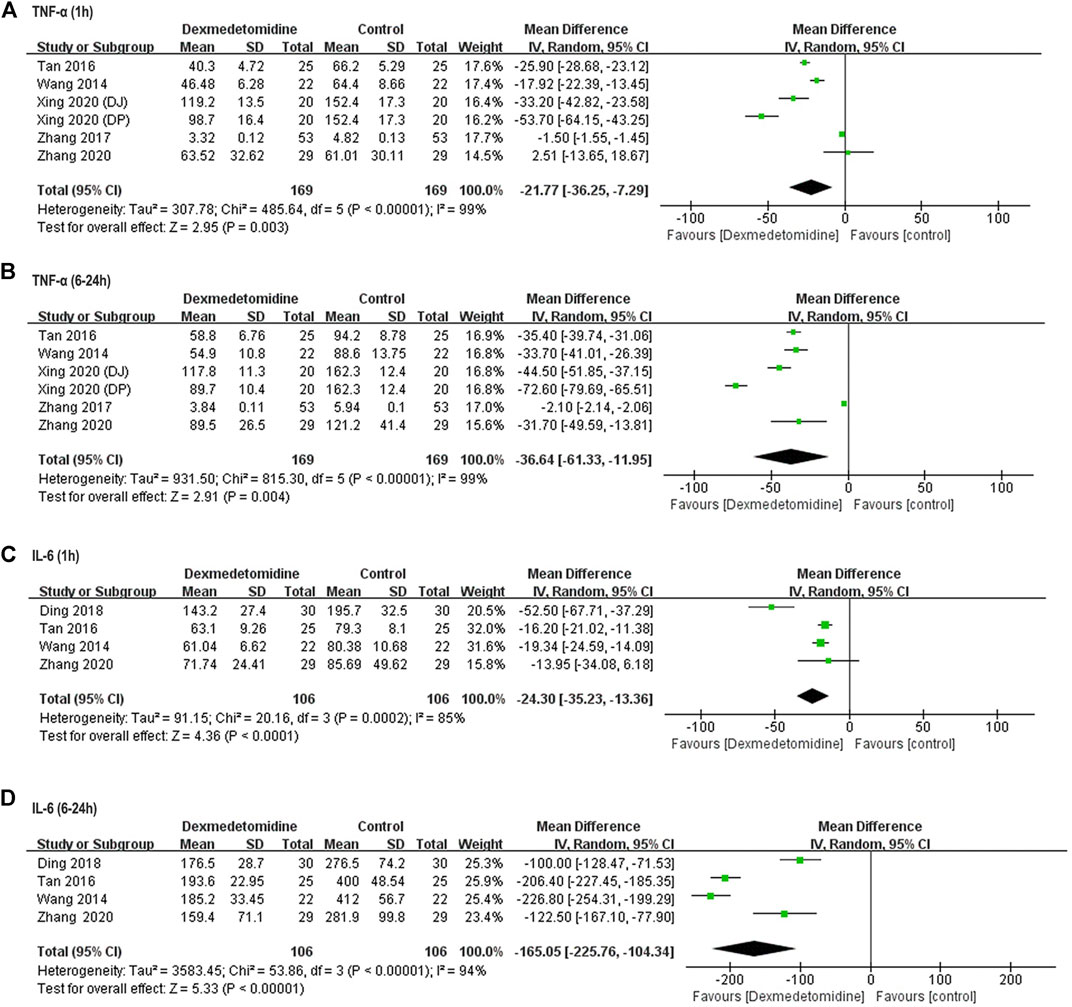
FIGURE 4. Forest plots for meta-analyses comparing postoperative biomarkers of the inflammatory response between dexmedetomidine and control group of participants undergoing hepatectomy. (A) TNF-α (1 h after surgery); (B) TNF-α (6–24 h after surgery); (C) IL-6 (1 h after surgery); (D) IL-6 (6–24 h after surgery).
Postoperative Function of Remote Organs
Two studies including 102 participants (Wang et al., 2014; Zhang et al., 2020) reported perioperative BUN and Cr concentration. In one study, BUN and Cr level at 24 h after surgery was significantly higher compared with baseline in control group, and dexmedetomidine reversed this elevation; in the other study, postoperative BUN and Cr level remained unaffected in both control and dexmedetomidine group. Taken together, no significant difference was detected in postoperative BUN and Cr level between two groups (BUN: WMD = 0.16, 95% CI: −1.12–1.45, p = 0.80, I2 = 86%; Cr: WMD = −1.76, 95% CI: −13.17–9.65, p = 0.76, I2 = 76%) (Figures 5A,B).
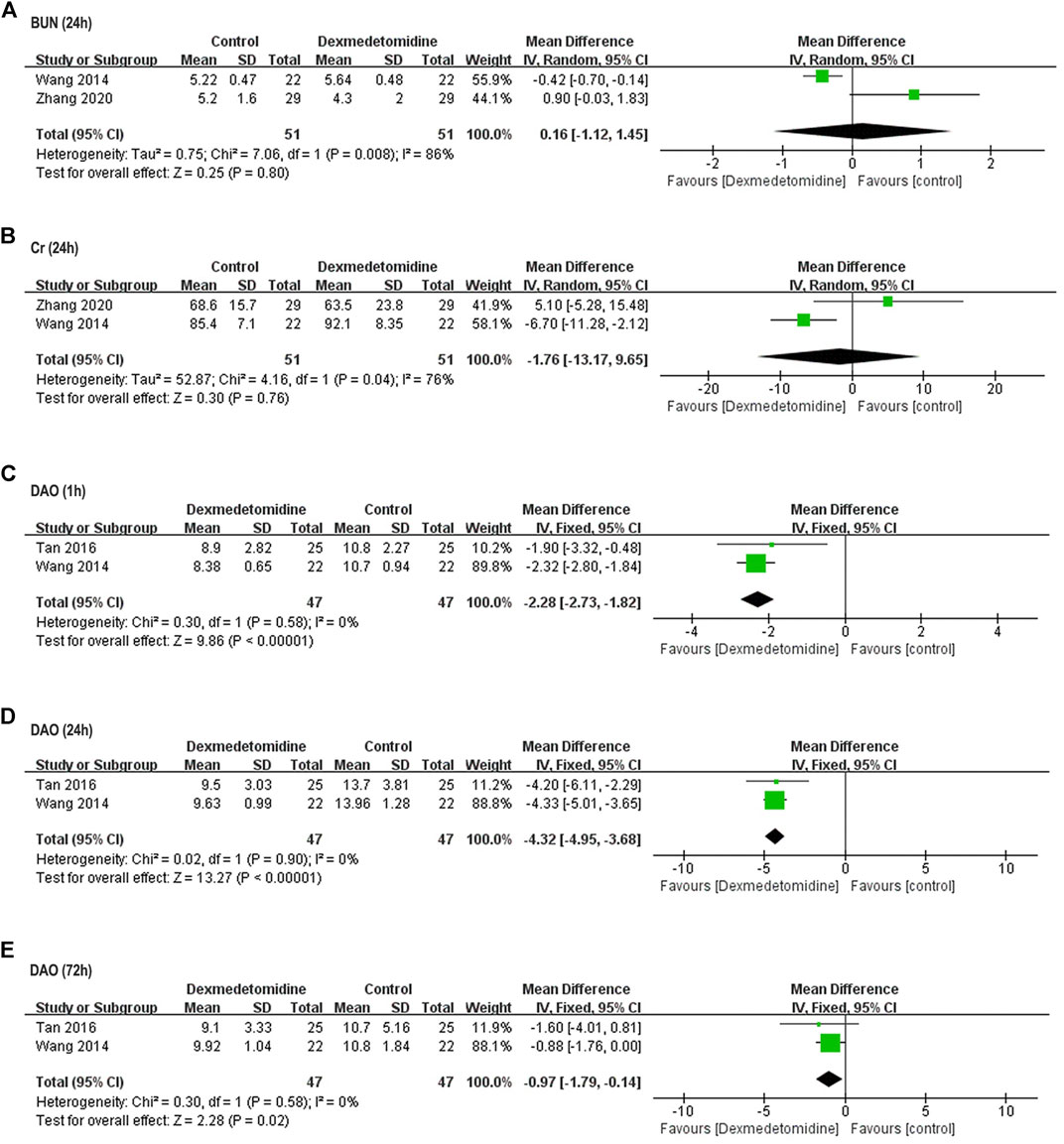
FIGURE 5. Forest plots for meta-analyses comparing postoperative renal function and intestinal injury. (A) BUN (24 h after surgery); (B) Cr (24 h after surgery); (C) DAO (1 h after surgery); (D) DAO (24 h after surgery); (E) DAO (72 h after surgery).
Two studies including 94 participants reported perioperative DAO activity (Wang et al., 2014; Tan et al., 2016). Postoperative DAO activity was significantly elevated in control group of both studies, while dexmedetomidine reversed this elevation within 72 h after surgery (1 h: WMD = −2.28, 95% CI: −2.73–−1.82, p < 0.00001, I2 = 0%; 24 h: WMD = −4.32, 95% CI: −4.95–-3.68, p < 0.00001, I2 = 0%; 72 h: WMD = −0.97, 95% CI: −1.79–−0.14, p = 0.02, I2 = 0%) (Figures 5C–E).
One study reported significant increased CK-MB level in control group compared with that in dexmedetomidine group at 24 h after surgery, but this value was in normal range at baseline and all the postoperative time points in both groups (Wang et al., 2014). Furthermore, there was no declaration of renal failure, myocardial infarction, congestive heart failure, or death after surgery in either group among all studies.
Intraoperative Parameters
Operation time was reported in all the eight studies. Blood loss and number of patients transfused were reported in four studies involving participants (Ding et al., 2018; Taman and Elhefnawy, 2019; Xing et al., 2020; Zhang et al., 2020), and two studies enrolling 104 participants (Wang et al., 2014; Ding et al., 2018). For these three parameters, no significant difference was detected between dexmedetomidine and control group (operation time: WMD = 0.07, 95% CI: −4.50–4.63, p = 0.98, I2 = 29%; blood loss: WMD = 10.35, 95% CI: −25.67–46.37, p = 0.57, I2 = 87%; number of patients transfused: OR = 1, 95% CI: 0.41–2.46, p = 1, I2 = 0%) (Figure 6).
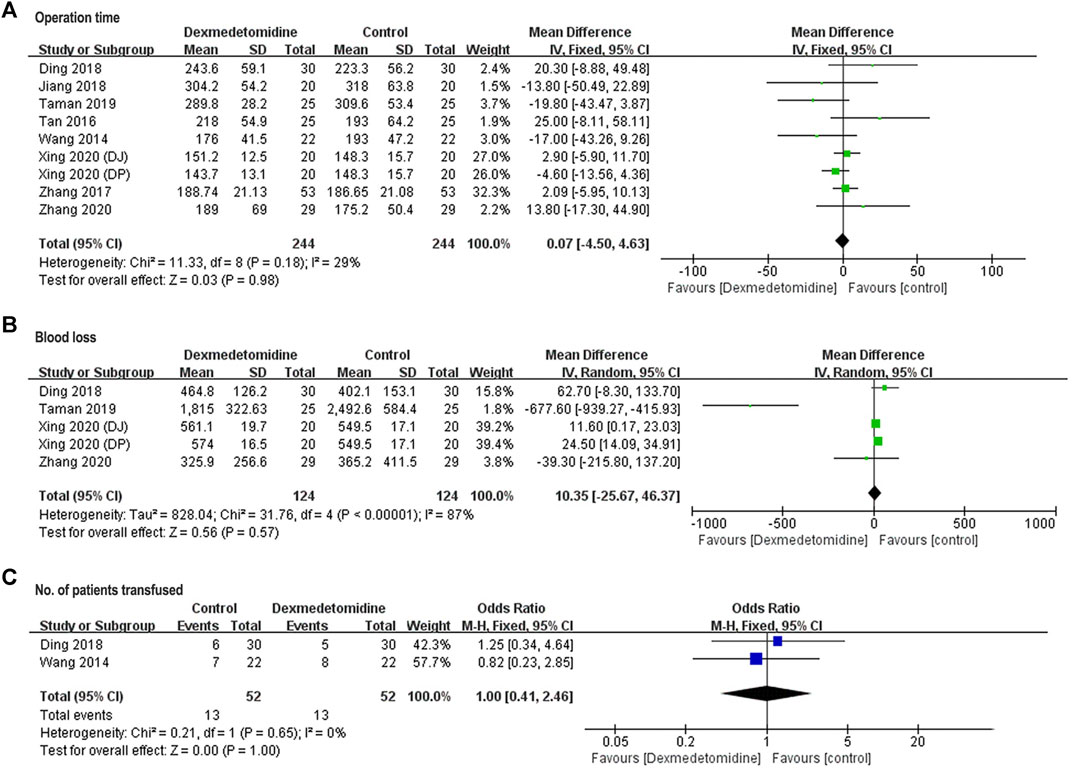
FIGURE 6. Forest plots for meta-analyses comparing operation time (A), blood loss (B), and No. of patients transfused (C).
Postoperative Recovery Variables
Pooled results of postoperative ventilator support time in two studies (Wang et al., 2014; Zhang et al., 2020) (Figure 7A) and LOS in three studies (Wang et al., 2014; Taman and Elhefnawy, 2019; Zhang et al., 2020) (Figure 7B) revealed no significant difference between control and dexmedetomidine group (ventilator support time: WMD = −4.51, 95% CI: −12.37–3.35, p = 0.26, I2 = 0%; LOS: WMD = −0.16, 95% CI: −0.45–0.12, p = 0.26, I2 = 0%).
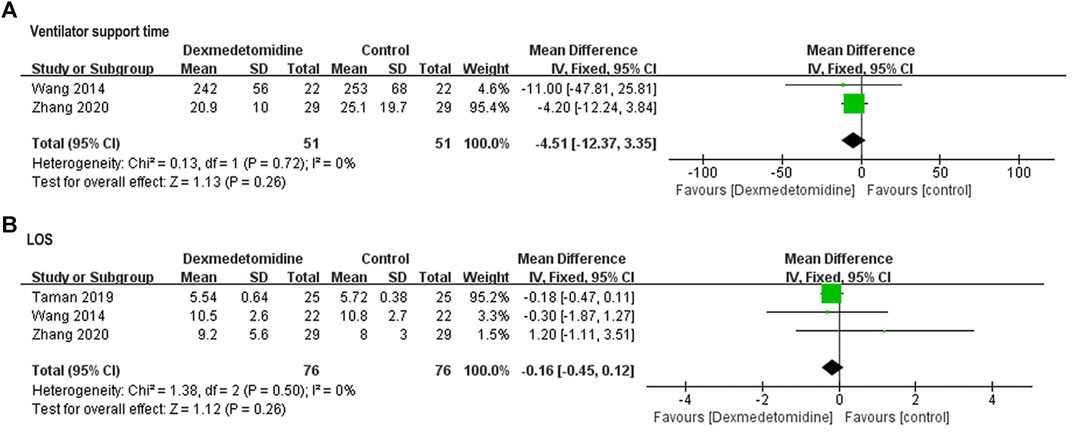
FIGURE 7. Forest plots for meta-analyses comparing postoperative ventilator support time (A) and LOS (B).
Discussion
The present meta-analysis of RCTs demonstrates that perioperative use of dexmedetomidine can produce a protective effect against hepatic IR injury induced by hepatectomy with inflow occlusion. This effect is associated with a systematic decrease in inflammatory response and oxidative stress. Meanwhile, dexmedetomidine also shows a tendency in inhibiting hepatic IR-induced renal and intestinal injury, but has no significant effect on operation time, intraoperative blood loss, and postoperative ventilator support time and LOS. To the best of our knowledge, this is the first meta-analysis that evaluates the efficacy and safety of perioperative dexmedetomidine administration in preventing hepatic IR injury.
Intraoperative blood loss and transfusion is proven to correlate closely with morbidity and mortality after hepatic resection (Jarnagin et al., 2002). Inflow occlusion proceeded to decrease blood loss can induce hepatic IR injury, which may also affect postoperative outcome by activating systemic inflammatory and oxidative stress responses, leading to dysfunction of liver and other remote organs. It has been generally accepted that hepatic IR injury consists of two distinct phases. The initial phase occurs within 2 h postreperfusion and is characterized by Kupffer cell-induced oxidant stress and inflammatory response, resulting in acute hepatocellular injury. The secondary phase appears 6 h or longer after reperfusion and is mainly attributed to neutrophil recruitment induced oxidants and protease release, causing the progression of hepatocyte damage. Therefore, we summarized the observation time points among all studies into three intervals: within 2, 6–24, and 48–72 h after surgery, to evaluate dexmedetomidine effect on IR injury.
In many clinical trials, ALT and AST level have been commonly used as indicators to detect hepatic IR injury and evaluate liver function. In this meta-analysis, all the included studies showed positive hepaprotective effect of dexmedetomidine by reducing postoperative ALT and AST level. TBIL level was also decreased in two studies. Although the elimination half-life of dexmedetomidine is only 2 h, decrease in ALT, AST and TBIL levels can be observed for 24–72 h postoperatively. However, one included study used α-glutathione S-transferase (α-GST) as the primary outcome to detect hepatic IR injury, for its sole distribution in cytosol of centrilobular hepatocytes and rapid response to hepatic IR injury (Zhang et al., 2020). Serum concentration of α-GST was elevated rapidly at 0.5 h postoperatively and returned to normal at 24 h after liver resection, while dexmedetomidine inhibited this elevation at postoperative 0.5 h. Due to relatively high sensitivity and specificity, α-GST may be applied as a parameter to detect hepatic IR injury more rapidly in future clinical trials.
The hepaprotective effect of dexmedetomidine appears to involve attenuation in oxidative stress and inhibition of inflammatory response, which play crucial roles in hepatic IR injury. During the IR process, many chemical substances cause the release of reactive oxygen species (ROS), inducing lipid peroxidation, membrane injury, alterations in ion permeability and enzyme activity, which ultimately leads to cell death. Simultaneously, the inflammatory process begins, significantly increasing the severity of IR injury. In preclinical studies, dexmedetomidine was shown to reduce oxidative activity and increase antioxidant capacity by preventing the elevation of MDA level and increasing paraoxonase activity in the liver and serum of hepatic IR rat model, leading to amelioration of histopathological liver damage (Arslan et al., 2012; Sahin et al., 2013; Tüfek et al., 2013). Meanwhile, dexmedetomidine can suppress the activation of inflammatory pathways, e.g., TLR4-NFκB signaling, and decrease inflammatory mediator level (Wang et al., 2016). Although dexmedetomidine has remarkable binding capacity for all the three subtypes (α2A, α2B, and α2C) of the human α2-adrenoceptors, the protective effect against hepatic IR injury may be probably mediated by the activation of α2A-adrenoceptor subtype (Wang et al., 2016). Hepatic IR injury induces a systemic response and releases of harmful substances that may affect remote organs. Previous researches showed that the Pringle manoeuvre had a close relationship with intestinal mucosal epithelial injury and represented a predisposing factor for bacterial translocation after liver resection (Erenoglu et al., 2011; Dello et al., 2012). DAO, a degradative enzyme of polyamines highly expressed in the upper villus cells of intestinal mucosa, is generally considered as a specific biomarker of small intestinal mucosal lesions (Luk and Baylin, 1983; Tsujikawa et al., 1999). In our analyses, dexmedetomidine was showed to alleviate postoperative intestinal injury by reducing serum DAO activity. On the other hand, postoperative renal injury appeared only in one study but was not observed in the other. Correspondingly, renoprotective effect of dexmedetomidine was only apparent in one study. Thus, the overall renoprotective effect was not evident according to this meta-analysis. However, previous meta-analysis on the renoprotective effect of dexmedetomidine showed that perioperative use of dexmedetomidine could significantly reduce the incidence of acute kidney injury (AKI) in patients undergoing cardiac surgery (Liu et al., 2018). Thus, additional researches involving renal function as an outcome are needed to further evaluate the protective effect of dexmedetomidine in renal injury induced by hepatic IR. In preclinical studies, the protective effect of dexmedetomidine was also observed in the lungs, kidneys and brain (Tüfek et al., 2013; Yu et al., 2020). Thus, further clinical trials may involve the evaluation of dexmedetomidine effect on more remote organs.
For safety aspects, dexmedetomidine showed no significant alteration on operation time, intraoperative blood loss and number of patients transfused compared to the control group, indicating it is unlikely to cause adverse side effect during operation. Postoperative recovery variables, such as ventilator support time and LOS, were not affect by dexmedetomidine, either. The probable reason may be that dexmedetomidine was administered only during operation or postoperative stay in AICU. The relatively short treatment duration and half-life of dexmedetomidine may prevent its effect from lasting long after surgery.
Among all the included RCTs, dexmedetomidine was administered with different dose regimen and treatment duration. For the limited number of included studies, subgroup analyses were not able to be performed. Previous studies showed that the renoprotective effect of dexmedetomidine differed among dosing groups, but the results are controversial (Balkanay et al., 2015; Liu et al., 2018). Thus, further RCTs with larger sample size and different dexmedetomidine administration regimen will be needed to find the optimal dose of dexmedetomidine treatment.
Our study has several limitations. First, high heterogeneity (I2 > 50%) existed in most outcomes. It may be attributed to different dose regimen of dexmedetomidine (constant administration rate or loading dose plus maintenance dosage) and sample size of included studies. Due to the limited number of included studies and participants, subgroup analyses were not able to be performed, which made it difficult to detect the source of heterogeneity. Second, included studies were only reported in China and Egypt. Additional studies conducted in other regions with more included participants are needed for systematic evaluation. Third, only two included studies enrolled postoperative parameters as study outcomes, which limited the evaluation of long-term effect of dexmedetomidine.
Conclusion
In summary, available evidence in the present meta-analysis shows that perioperative use of dexmedetomidine can exhibit a protective effect against hepatic IR injury in patients undergoing hepatectomy. Additional RCTs with larger sample size, different region sites, multiple dosing regimen, and more postoperative outcomes are needed to further evaluate the hepaprotective property of dexmedetomidine against IR injury associated with hepatectomy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
Study design: Y-QH and R-TW; data acquisition: Y-QH and R-TW; methodology: X-TL; data analysis/curation: Y-QH, X-TL, and JZ; manuscript draft: Y-QH and R-TW; manuscript review and edit: Z-YY and Y-FF. Supervision: Y-FF.
Funding
This work was supported by research grant from National Nature Science Foundation of China (Grant No. 81503050). The Foundation had no involvement on study design, collection, analysis and interpretation of data, writing of the report and the decision to submit the article for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.747911/full#supplementary-material.
References
Abu-Amara, M., Gurusamy, K. S., Hori, S., Glantzounis, G., Fuller, B., and Davidson, B. R. (2009). Pharmacological Interventions versus No Pharmacological Intervention for Ischaemia Reperfusion Injury in Liver Resection Surgery Performed under Vascular Control. Cochrane Database Syst. Rev. (4), CD007472. doi:10.1002/14651858.CD007472.pub2 Available at https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008154/full
Arslan, M., Metin Çomu, F., Küçük, A., Oztürk, L., and Yaylak, F. (2012). Dexmedetomidine Protects against Lipid Peroxidation and Erythrocyte Deformability Alterations in Experimental Hepatic Ischemia Reperfusion Injury. Libyan J. Med. 7 (1), 18185. doi:10.3402/ljm.v7i0.18185
Balkanay, O. O., Goksedef, D., Omeroglu, S. N., and Ipek, G. (2015). The Dose-Related Effects of Dexmedetomidine on Renal Functions and Serum Neutrophil Gelatinase-Associated Lipocalin Values after Coronary Artery Bypass Grafting: a Randomized, Triple-Blind, Placebo-Controlled Study. Interact Cardiovasc. Thorac. Surg. 20 (2), 209–214. doi:10.1093/icvts/ivu367
Chen, X., Liang, R., Lai, L., Chen, K., and Zhu, X. (2021). Prognostic Role of EGFR/p-EGFR in Patients With Nasopharyngeal Carcinoma: A Meta-Analysis. Front. Oncol. 11, 697369. doi:10.3389/fonc.2021.697369
Dahmani, S., Rouelle, D., Gressens, P., and Mantz, J. (2005). Effects of Dexmedetomidine on Hippocampal Focal Adhesion Kinase Tyrosine Phosphorylation in Physiologic and Ischemic Conditions. Anesthesiology 103 (5), 969–977. doi:10.1097/00000542-200511000-00011
Dello, S. A., Reisinger, K. W., van Dam, R. M., Bemelmans, M. H., van Kuppevelt, T. H., van den Broek, M. A., et al. (2012). Total Intermittent Pringle Maneuver during Liver Resection Can Induce Intestinal Epithelial Cell Damage and Endotoxemia. PloS one 7 (1), e30539. doi:10.1371/journal.pone.0030539
Ding, H. T., Liu, Z. Q., Jia, X. P., and Hao, J. H. (2018). Effect of Dexmedetomidin on Hepatic Ischemia-Reperfusion Injury in Left Hemihepatectomy. Acad. J. Chin. PLA Med. Sch. 39 (08), 695–698. doi:10.3969/j.issn.2095-5227.2018.08.014
Erenoglu, B., Gokturk, H. S., Kucukkartallar, T., Sahin, M., Tekin, A., Tatkan, Y., et al. (2011). Mechanical Intestinal Cleansing and Antibiotic Prophylaxis for Preventing Bacterial Translocation during the Pringle Maneuver in Rabbits. Surg. Today 41 (6), 824–828. doi:10.1007/s00595-010-4363-4
Gurusamy, K. S., Sheth, H., Kumar, Y., Sharma, D., and Davidson, B. R. (2009). Methods of Vascular Occlusion for Elective Liver Resections. Cochrane Database Syst. Rev. (1), CD007632. doi:10.1002/14651858.CD007632
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Jarnagin, W. R., Gonen, M., Fong, Y., DeMatteo, R. P., Ben-Porat, L., Little, S., et al. (2002). Improvement in Perioperative Outcome after Hepatic Resection: Analysis of 1,803 Consecutive Cases over the Past Decade. Ann. Surg. 236 (4), 397–7. doi:10.1097/01.SLA.0000029003.66466.B3
Jiang, M., Xu, H. Y., Feng, D. D., and Zhou, L. Y. (2018). Effect of Dexmedetomidine for Patients Undergoing Precisehepatectomy in Anesthesia Intensive Care Unit. J. Clin. Anesthesiology 34 (12), 1209–1212. doi:10.12089/jca.2018.12.016
Kucuk, A., Yaylak, F., Cavunt-Bayraktar, A., Tosun, M., Arslan, M., Comu, F. M., et al. (2014). The Protective Effects of Dexmedetomidine on Hepatic Ischemia Reperfusion Injury. Bratisl Lek Listy 115 (11), 680–684. doi:10.4149/bll_2014_132
Kundra, T. S., Thimmarayappa, A., Dhananjaya, M., and Manjunatha, N. (2018). Dexmedetomidine for Prevention of Skeletal Muscle Ischaemia-Reperfusion Injury in Patients with Chronic Limb Ischaemia Undergoing Aortobifemoral Bypass Surgery: A Prospective Double-Blind Randomized Controlled Study. Ann. Card. Anaesth. 21 (1), 22–25. doi:10.4103/aca.ACA_113_17
Li, J., Yuan, T., Zhao, X., Lv, G. Y., and Liu, H. Q. (2016). Protective Effects of Sevoflurane in Hepatic Ischemia-Reperfusion Injury. Int. J. Immunopathol Pharmacol. 29 (2), 300–307. doi:10.1177/0394632016638346
Liu, Y., Sheng, B., Wang, S., Lu, F., Zhen, J., and Chen, W. (2018). Dexmedetomidine Prevents Acute Kidney Injury after Adult Cardiac Surgery: a Meta-Analysis of Randomized Controlled Trials. BMC Anesthesiol. 18 (1), 7. doi:10.1186/s12871-018-0472-1
Luk, G. D., and Baylin, S. B. (1983). Polyamines and Intestinal Growth-Iincreased Polyamine Biosynthesis after Jejunectomy. Am. J. Physiol. 245 (5 Pt 1), G656–G660. doi:10.1152/ajpgi.1983.245.5.G656
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 4 (1), 1. doi:10.1186/2046-4053-4-1
Okada, H., Kurita, T., Mochizuki, T., Morita, K., and Sato, S. (2007). The Cardioprotective Effect of Dexmedetomidine on Global Ischaemia in Isolated Rat Hearts. Resuscitation 74 (3), 538–545. doi:10.1016/j.resuscitation.2007.01.032
Peng, K., Chen, W. R., Xia, F., Liu, H., Meng, X. W., Zhang, J., et al. (2020). Dexmedetomidine post-treatment Attenuates Cardiac Ischaemia/reperfusion Injury by Inhibiting Apoptosis through HIF-1α Signalling. J. Cel. Mol. Med. 24 (1), 850–861. doi:10.1111/jcmm.14795
Petrowsky, H., McCormack, L., Trujillo, M., Selzner, M., Jochum, W., and Clavien, P. A. (2006). A Prospective, Randomized, Controlled Trial Comparing Intermittent portal Triad Clamping versus Ischemic Preconditioning with Continuous Clamping for Major Liver Resection. Ann. Surg. 244 (6), 921–930. doi:10.1097/01.sla.0000246834.07130.5d
Rodríguez, A., Taurà, P., García Domingo, M. I., Herrero, E., Camps, J., Forcada, P., et al. (2015). Hepatic Cytoprotective Effect of Ischemic and Anesthetic Preconditioning before Liver Resection when Using Intermittent Vascular Inflow Occlusion: a Randomized Clinical Trial. Surgery 157 (2), 249–259. doi:10.1016/j.surg.2014.09.005
Sahin, T., Begeç, Z., Toprak, H. İ., Polat, A., Vardi, N., Yücel, A., et al. (2013). The Effects of Dexmedetomidine on Liver Ischemia-Reperfusion Injury in Rats. J. Surg. Res. 183 (1), 385–390. doi:10.1016/j.jss.2012.11.034
Si, Y., Bao, H., Han, L., Chen, L., Zeng, L., Jing, L., et al. (2018). Dexmedetomidine Attenuation of Renal Ischaemia-Reperfusion Injury Requires Sirtuin 3 Activation. Br. J. Anaesth. 121 (6), 1260–1271. doi:10.1016/j.bja.2018.07.007
Taman, H. I., and Elhefnawy, E. (2019). Hepatic Protective Effect of Dexmedetomidine after Partial Hepatectomy Surgery: A Prospective Controlled Study. Anesth. Essays Res. 13 (1), 132–137. doi:10.4103/aer.AER_106_18
Tan, W. L., Hua, Y. P., Huang, C. Y., and Liu, W. F. (2016). Protective Effects of Dexmedetomidine on Perioperative Digestive Function of Patients with Liver Cirrhosis. Chin. Arch. Gen. Surg. (Electronic Edition) 10 (03), 200–204. doi:10.3877/cma.j.issn.1674-0793.2016.03.009
Tsujikawa, T., Uda, K., Ihara, T., Inoue, T., Andoh, A., Fujiyama, Y., et al. (1999). Changes in Serum Diamine Oxidase Activity during Chemotherapy in Patients with Hematological Malignancies. Cancer Lett. 147 (1-2), 195–198. doi:10.1016/s0304-3835(99)00307-9
Tüfek, A., Tokgöz, O., Aliosmanoglu, I., Alabalik, U., Evliyaoglu, O., Çiftçi, T., et al. (2013). The Protective Effects of Dexmedetomidine on the Liver and Remote Organs against Hepatic Ischemia Reperfusion Injury in Rats. Int. J. Surg. 11 (1), 96–100. doi:10.1016/j.ijsu.2012.12.003
Wang, Y., Wu, S., Yu, X., Zhou, S., Ge, M., Chi, X., et al. (2016). Dexmedetomidine Protects Rat Liver against Ischemia-Reperfusion Injury Partly by the α2A-Adrenoceptor Subtype and the Mechanism Is Associated with the TLR4/NF-Κb Pathway. Int. J. Mol. Sci. 17 (7). doi:10.3390/ijms17070995
Wang, Z. X., Huang, C. Y., Hua, Y. P., Huang, W. Q., Deng, L. H., and Liu, K. X. (2014). Dexmedetomidine Reduces Intestinal and Hepatic Injury after Hepatectomy with Inflow Occlusion under General Anaesthesia: a Randomized Controlled Trial. Br. J. Anaesth. 112 (6), 1055–1064. doi:10.1093/bja/aeu132
Xing, X. L., Zhu, Y. M., Tang, B. Q., Deng, H. L., Deng, F. M., and Hu, Y. H. (2020). Effects of Dexmedetomidine via portal Vein Preconditioning on Inflammatory Response and Oxidative Stress in Hepatic Ischemia- Reperfusion Injury. J. Clin. Anesthesiology 36 (04), 317–321. doi:10.12089/jca.2020.04.001
Yu, W., Lyu, J., Jia, L., Sheng, M., Yu, H., and Du, H. (2020). Dexmedetomidine Ameliorates Hippocampus Injury and Cognitive Dysfunction Induced by Hepatic Ischemia/Reperfusion by Activating SIRT3-Mediated Mitophagy and Inhibiting Activation of the NLRP3 Inflammasome in Young Rats. Oxid Med. Cel. Longev. 2020, 7385458. doi:10.1155/2020/7385458
Zhang, N., Guo, Z. Z., and Cheng, Y. (2017). Effect of Dexmedetomidine on Liver Function, Cytokine Levels, and Oxidative Stress in Patients Undergoing Hepatolobectomy. J. Clin. Hepatol. 33 (03), 507–511. doi:10.3969/j.issn.1001-5256.2017.03.022
Keywords: hepatectomy, ischemia-reperfusion injury, dexmedetomidine, meta-analysis, randomized controlled trials
Citation: Huang Y-Q, Wen R-T, Li X-T, Zhang J, Yu Z-Y and Feng Y-F (2021) The Protective Effect of Dexmedetomidine Against Ischemia-Reperfusion Injury after Hepatectomy: A Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 12:747911. doi: 10.3389/fphar.2021.747911
Received: 27 July 2021; Accepted: 27 September 2021;
Published: 12 October 2021.
Edited by:
Ralf Weiskirchen, RWTH Aachen University, GermanyReviewed by:
Yansong Ge, Heilongjiang Bayi Agricultural University, ChinaWei Liu, University of Science and Technology of China, China
Copyright © 2021 Huang, Wen, Li, Zhang, Yu and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui-Ting Wen, dHp3eG1AYmptdS5lZHUuY24=; Yu-Fei Feng, ZmVueXVmZWlAMTI2LmNvbQ==
†These authors contributed equally to this work.
 Ya-Qun Huang
Ya-Qun Huang Rui-Ting Wen1,2*†
Rui-Ting Wen1,2*†