- 1College of Veterinary Medicine, South China Agricultural University, Guangzhou, China
- 2Institute of Animal Health, Guangdong Academy of Agricultural Sciences, Guangzhou, China
- 3Guangdong Research Center for Veterinary Traditional Chinese Medicine and Natural Medicine Engineering Technology, Guangzhou, China
- 4Guangdong Laboratory for Lingnan Modern Agriculture, Guangzhou, China
Forsythiae Fructus (FF), the dry fruit of Forsythia suspensa (Thunb.) Vahl, has a long history of use in traditional Chinese Medicine for its heat-clearing and detoxifying properties. It possesses clinical therapeutic effects and biological functions showing efficacy in handling different diseases. To investigate the FF differences in Henan, Shanxi, and Shaanxi in August and October, the surface morphology, mid-infrared and near-infrared spectrums, and HPLC were analyzed. Concurrently, the anti-inflammatory and antioxidant effects on LPS-induced J774A.1 cells were evaluated by western blot and RT-qPCR. The results showed that FF from different Harvest Seasons and Regions are provided with different microstructures and mid-infrared and near-infrared spectrums, and the levels of forsythiaside A and phillyrin of FF from Shanxi in August and phillygenin of FF from Shaanxi in August were the highest. Meanwhile, FF from Shanxi and Shaanxi in August markedly reduced the levels of inflammatory cytokines and mediators (TNF-α, IL-1β, NF-κB, and iNOS) and the protein expression levels of phosphorylated total IKKα/β and nuclear NF-κB. In August, SXFF and SAXFF also promoted the mRNA expression levels of HO-1 and NQO1 and the protein expression levels of HO-1 and nuclear Nrf2 and suppressed the protein expression levels of KEAP1. Spearman correlation analysis showed that phillygenin had a strong correlation with the protein expression on LPS-induced J774A.1 cells. In summary, our results showed that FF from harvest seasons and regions contributed to the distinct differences in microstructure, the mid-infrared and near-infrared spectrums, and compound content. More importantly, FF from Shanxi and Shaanxi in August showed marked anti-inflammatory and antioxidant activities, but with some differences, which may be because of different contents of phillygenin and phillyrin of lignans in FF.
Introduction
Forsythiae Fructus (FF) is the dry fruit of Forsythia suspensa (Thunb.) Vahl in the family Oleaceae, which has been widely used as an antipyretic and antidotal herb in Traditional Chinese Medicine (TCM) (named Lianqiao in Chinese) for thousands of years. The TCM characteristics of FF are summarized as a bitter flavor with a mildly cold nature and lung, heart, or intestinal meridian distribution; these characteristics are parallel to the characterization of anti-inflammatory TCM (Lee et al., 2018). FF is used to treat pyrexia, gonorrhea, carbuncles, and erysipelas in Shennong’s Herbal, and it is also included in many TCM prescriptions, which are used to treat influenza, hyperlipidemia, cardiovascular, pneumonia, and so on. Indeed, more than 40 TCM prescriptions containing FF are listed in the Chinese pharmacopoeia (Bao et al., 2017; Tsai et al., 2018; Zhang et al., 2021).
According to the harvest period, two kinds of FF are selected: one is a green, indehiscent, and nearly ripe fruit that is harvested in August and September and named Qingqiao in Chinese; the other is a yellow, dehiscent, and fully ripe fruit that is harvested in October and named Laoqiao (Wang et al., 2018). Both of them serve as official sources of FF; however, Qingqiao is used more frequently in TCM prescriptions (Jia et al., 2015; Fang et al., 2018). FF is predominantly produced in the Hebei, Shaanxi, Shanxi, Shandong, Anhui, Henan, Hubei, Jiangsu, and Sichuan Provinces (Wang et al., 2018). In TCM, genuine Chinese medicine has better efficacy and disease treatment effects, which may be due to their different origins, resulting in different chemical substances even though they are the same kind of herbs (Zhu et al., 2019; Luo et al., 2021). FF contains many chemical components, such as forsythoside A and B (phenylethanolamine), phillyrin, and phillygenin (lignans), which have been reported to exhibit multiple biological activities (Zhang et al., 2020b; Hu et al., 2020; Jiang et al., 2020; Wang et al., 2021b). What are the distinctions between FF from different producing areas and harvest time? What kinds of changes happen to the function of FF caused by different producing areas and harvest time? Previous studies have shown that the obvious distinctions in compounds between green and ripe FF may be the main reason for their different anticancer activity (Bao et al., 2017). Additionally, the Jia showed that green and ripe FF have distinct chemical compositions based on NMR metabolic profiling (Jia et al., 2015).
Near-infrared (NIR) spectroscopy is a promising method that has been widely used as a rapid and non-destructive technique for qualitative and quantitative analysis of traditional Chinese medicine (TCM) (Wang et al., 2017; Wang et al., 2019). A variety of methods have been applied for the identification of TCM, such as mid-infrared (MIR) spectroscopy, high-performance liquid chromatography, mass spectrometry, chemometrics, and so on (Chen et al., 2016; Qu et al., 2017; Zhao et al., 2020). For instance, Sun et al. identified the genuine and adulterated Pinellia ternate by MIR and NIR spectroscopy, and Chen et al. evaluated the decoction pieces of Rhizoma Atractylodis Macrocephalae by near-infrared spectroscopy coupled with chemometrics (Chen et al., 2019; Sun et al., 2019).
Previous studies have shown that FF is widely used to treat lipopolysaccharide (LPS)-induced inflammation and oxidation (Lee et al., 2018; Shao et al., 2021). In response to LPS, macrophages produce NO, which is synthesized by iNOS, increasing the level of nuclear NF-κB as well as pro-inflammatory cytokines such as TNF-α, IL-1β (Heo et al., 2018; Hung et al., 2019). It also activates the Nrf2 signaling pathway, resulting in the secretion of antioxidant enzymes such as HO-1 and NQO1 (Lim et al., 2020).
In the present study, the differences in the microstructure and mid-infrared and near-infrared spectrum were observed in three chemical compositions of Forsythia Fructus collected from Henan, Shanxi, and Shaanxi in August and October. We, therefore, aimed to further explore whether these differences lead to changes in their anti-inflammatory and antioxidant effects and to provide a certain chemical basis for the clinical use of FF in August and October from Henan, Shanxi, and Shaanxi.
Materials and Methods
Reagents and Antibodies
Forsythoside A (purity>98%), phillyrin (purity>98%), and phillygenin (purity>97%) were purchased from Dalian Meilun Biotechnology (China), and methanol (purity>99%) and acetonitrile (purity>99%) were obtained from Thermo Fisher Scientific—CN. Antibodies specific to α-tubulin, β-Actin, TBP, HO-1, Nrf2, KEAP1, P-NF-κB p65, p-IKKα/β were purchased from Cell Signaling Technology and Lipolysaccharide (LPS) and Penicillin-Streptomycin from Sigma-Aldrich.
Preparation of the FF Methanol Extract
In total, 180 FF samples (30 g) were acquired from apiculture producers from the Shanxi, Shaanxi, and Henan Provinces in China in August and October. In total, 180 samples were from different times and different provinces [FF in August from Shanxi Province (SXFF-A), October from Shanxi Province (SXFF-O), August from Shaanxi Province (SAXFF-A), October from Shaanxi Province (SAXFF-O), August from Henan Province (HNFF-A), and October from Henan Province (HNFF-O)]. FF samples were broken to pieces and sieved through 60 pieces of mesh, dissolved in methanol, and extracted by ultrasound for 30 min. On the next day, samples were extracted again by ultrasound for 30 min and then centrifuged at 3,000 rpm. The upper methanol layer was filtered using a 0.22 μm PVDF membrane and stored at −80°C.
Surface Morphology of FF
The FF were powdered and screened through 60 meshs, and evenly poured on to tape. After gold-plating, a scanning electron microscope (SEM) (SUPRA 55 VP, Zeiss) was used to collect samples at an accelerating voltage of 5.00 kV with a magnification of 4,500 times.
Mid-infrared of FF
After 60 mesh screening, the 1 mg FF samples were mixed with 100 mg KBr powder, and the mixture was ground to less than 2 μm and put into an infrared tablet press with 20–24 MPa for approximately 1 min form potassium bromide tablets. The analysis was performed in the frequency range of 4,000 cm−1 to 400 cm−1 using a Vertex 70 FT-IR spectrometer (Brooke Biotechnology, Germany).
HPLC Analysis of FF
Analysis of constituents in FF was performed on an 1525-2707-2489 series HPLC system (Waters, United States) equipped with a binary solvent manager, sample manager, column compartment, UV detector with 280 nm, and LC-Solution software. The separation was performed on an Agilent TC-C18 column (5 μm, 4.6 × 250 mm). The mobile phase consisted of water containing 0.1% acetic acid (A) and acetonitrile (B). The linear gradient was as follows: 0–30 min, 10–25% B; 30–45 min, 25–75% B; and 45–50 min, 75% B at a flow rate of 1.0 ml/min. The column temperature was maintained at 28°C and the injection volume was 10 μL (Lee et al., 2018). Chemicals used as a standard are Forsythoside A, phillyrin, and phillygenin. The constituents were identified by comparison of their retention times to those of standard compounds under identical analysis conditions and the UV spectra with our in-house DAD library.
Acquisition of Near-Infrared Spectrum
The NIR spectrum of FF was measured by an Antaris II FT-NIR spectrometer (Thermo Fisher Scientific, Verona, United States), with a wavenumber range from 10,000 to 4,000 cm−1, scanning times of 64, and a resolution of 8 cm−1. The signals were generated in reflectance (%R) mode and showed using log 1/R. The NIR spectrum was recorded in triplicate for each sample, and the average spectrum was used in the data analysis. Analysis of NIR spectrum was performed with TQ Analyst 9.0 software.
Cell Culture
J774A.1 cells purchased from the American Type Culture Collection were cultured according to a previously described method (Alizadeh et al., 2021). Cells were cultured in high glucose DMEM containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a humidified incubator at 37°C with 5% CO2.
Measurement of Cell Viability
Cell viability was measured by the MTT assay, which is described in the previous research (Hung et al., 2019). Briefly, the cells were seeded in a 96-well plate at a density of 2 × 104 cells/well, treated with 1, 0.5, 0.2, 0.1 and 0.05 mg/ml SXFF-A and 1, 0.5, 0.2, 0.1 and 0.05 mg/ml SAXFF-A for 24 h. After incubation, the supernatant was removed, and then 100 μL of MTT solution (0.5 mg/ml) was added to each well and incubated for 4 h at 37°C. Next, the cell culture supernatant was removed and the resulting formazan crystals were dissolved in 100 μL DMSO. The absorbance was evaluated at 540 nm using a Multi-Mode Microplate Reader (Thermo Fisher Scientific, Inc.).
Measurement of NO Production
The levels of NO in the J774A.1 cells and the supernatant were performed with Griess Reagent I and II (Beyotime Co., Ltd.). The cells were seeded in a 96-well plate at a density of 2 × 104 cells/well, treated with or without 0.5, 0.2, and 0.1 mg/ml SXFF-A and 0.5, 0.2, and 0.1 mg/ml SAXFF-A for 0.5 h followed by the addition of LPS (1 μg/ml) for 24 h. After incubation, the cell culture supernatant was transferred to 96-well, and the remaining cells were cleaved with Cell lysis buffer for Western and IP (Beyotime Co., Ltd.) to measure NO levels in supernatant and cells according to the manufacturer’s instructions. Briefly, 50 μL cell culture supernatant and 50 μL centrifuged cell lysis supernatant was mixed with 50 μL Griess Reagent I and II respectively for 10 min, and the absorbance was evaluated at 540 nm using a Multi-Mode Microplate Reader.
Isolation of Total RNA and RT-qPCR
J744A.1 cells were plated onto a 12-well plate at a density of 4 × 105 cells/well, treated with or without 0.5, 0.2, and 0.1 mg/ml SXFF-A and 0.5, 0.2 and 0.1 mg/ml SAXFF-A for 0.5 h, and then had LPS (1 μg/ml) added to them for 12 h. Total RNA was extracted from collected cells using FastPure® Cell/Tissue Total RNA Isolation Kit (Vazyme Biotech Co., Ltd.) according to the manufacturer’s instructions. cDNA was synthesized with total RNA (1 μg) using HiScript III RT SuperMix for qPCR (+gDNA wiper) (Vazyme) and subjected to real-time quantitative PCR (RT-qPCR) amplification using the ChamQ universal SYBR qPCR Master Mix (Vazyme) in a QuantStudio®5 (Thermo Fisher Scientific, Inc.). The primer sequences are shown in Table 1, and the data were analyzed and expressed as relative gene expression to β-Action using the 2−△△CT method.
Western Blot Analysis
J744A.1 cells were plated onto a 6-well plate at a density of 6 × 105 cells/well, treated with or without 0.5, 0.2, and 0.1 mg/ml SXFF-A and 0.5, 0.2, and 0.1 mg/ml SAXFF-A for 0.5 h, then had LPS (1 μg/ml) added to them for 12 h. The total protein and nuclear protein were extracted using Enhanced RIPA Lysis buffer with a 1 mM PMSF and phosphatase inhibitor cocktail (Beyotime) and a Nuclear Extract kit (EMD Millipore Corp), respectively, according to the manufacturer’s instructions. The concentrations of protein were determined by a BCA protein assay kit (Beyotime) according to the manufacturer’s instructions. Then, briefly, the protein (10 μg/lane) was separated with SDS-PAGE and transferred to a PVDF membrane, followed by blocking with 5% non-fat milk. After blocking, they were incubated with primary antibodies at 1:1,000 dilution in primary antibody dilution buffer overnight at 4°C and then incubated with the following HRP-conjugated secondary antibodies (1:10,000) in TBST at room temperature for 1 h, and immune-reacted bands were detected with enhanced chemiluminescence reagents. The relative density of the western blot bands was determined using the BLT GelView 6,000 Pro software.
Statistical Analysis
SPSS software (version 26.0), Origin 2021, and GraphPad (version 8.0) were used for statistical analysis. Statistical comparisons were assessed by one-way analysis (ANOVA), followed by Least significant difference (LSD) test. All experiments were performed at least three times independently.
Results
Surface Morphology of FF by SEM
The obtained SEM micrographs of FF from different harvest seasons and regions showed obvious differences in the surface morphologies, as shown in Figure 1A. The SEM images indicated that the surface structure of FF from the same regions in October was looser and had a more net-like structure than that in August, whereas no significant differences were observed among the three regions in August and October. There were also more pore-like structures on the surface of SXFF-A compared with that in SAXFF-A and HNFF-A (Figure 1A a–c).
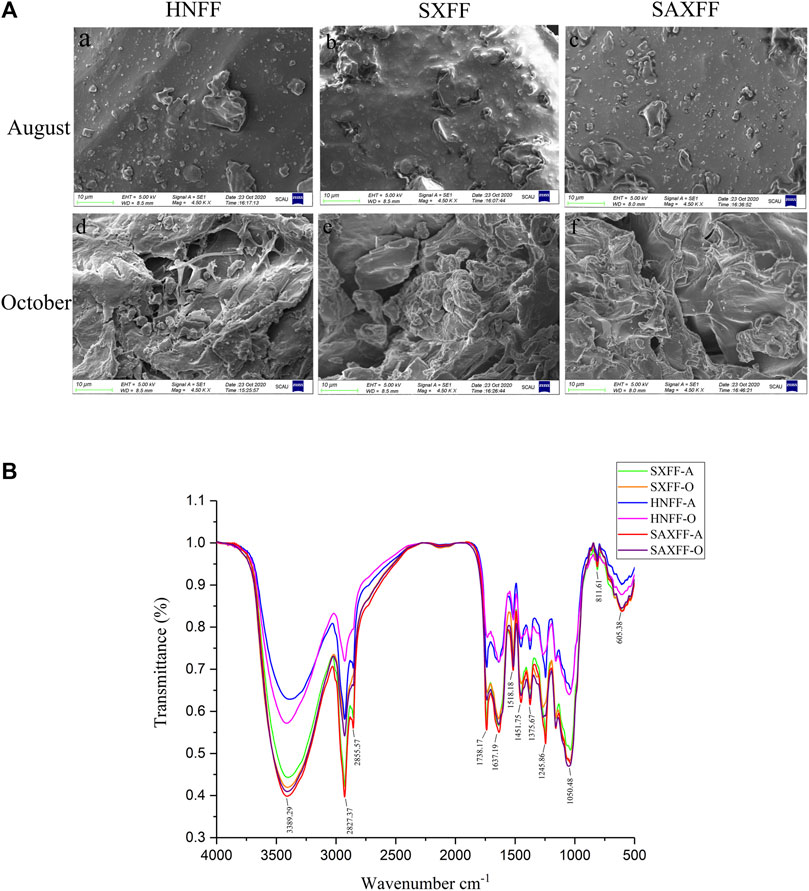
FIGURE 1. Scanning electron microscope and Mid-infrared spectra of FF. (A) Scanning electron microscope of FF from Henan Province (HNFF), Shanxi Province (SXFF), and Shaanxi Province (SAXFF) in August and October. (B) Mid-infrared (MIR) spectra of FF from Shanxi Province in August (SXFF-A), Shanxi Province in October (SXFF-O), Henan Province in August (HNFF-A), Henan Province in October (HNFF-O), Shaanxi Province in August (SAXFF-A), and Shaanxi Province in October (SAXFF-O).
MIR Spectra of FF
The MIR spectra revealed that FF from different harvest seasons and regions had the same functional groups through the absorption bands of the phytocompounds (Figure 1B). Bands for FF were obtained at peak 3,389 cm−1, 2,927.37 cm−1,1738.17 cm−1, 1,637.19 cm−1, 1,245.86 cm−1, 1,032.27 cm−1, and so on. The results showed that FF in different harvest times and regions have the same absorption peaks and different transmittance. This suggested that FF were provided with identical major functional groups, but different MIR spectra transmittance, indicating that FF from different regions and harvest times had similar compounds, yet may have varied in the content of the compound.
NIR Spectra of FF
The NIR spectrum of 180 FF samples from different gathering times and localities is shown in Figure 2, which shows that there is a significant distinction among them. FF samples were recorded by NIR spectra from 10,000 to 4,000 cm−1 with a resolution of 8 cm−1 (Figure 2A). Cluster analysis is used to analyze the similarity of the NIR spectrum of 180 FF samples by clustering samples based on their intimacy. K-means clustering analysis is an iterative clustering analysis method. Firstly, all data are pre-divided into K groups, and K objects are randomly selected as the initial cluster center. Then, the distance between each object and each seed cluster center is calculated, and each object is assigned to the nearest cluster center. According to the results of K-means cluster analysis, 180 FF samples were divided into two clusters: FF from Hehan, Shanxi, and Shaanxi in August and in October. The different producing areas of FF were not distinguished, indicating that the effect of harvest time on FF was more obvious than that of regions (Figure 2B).
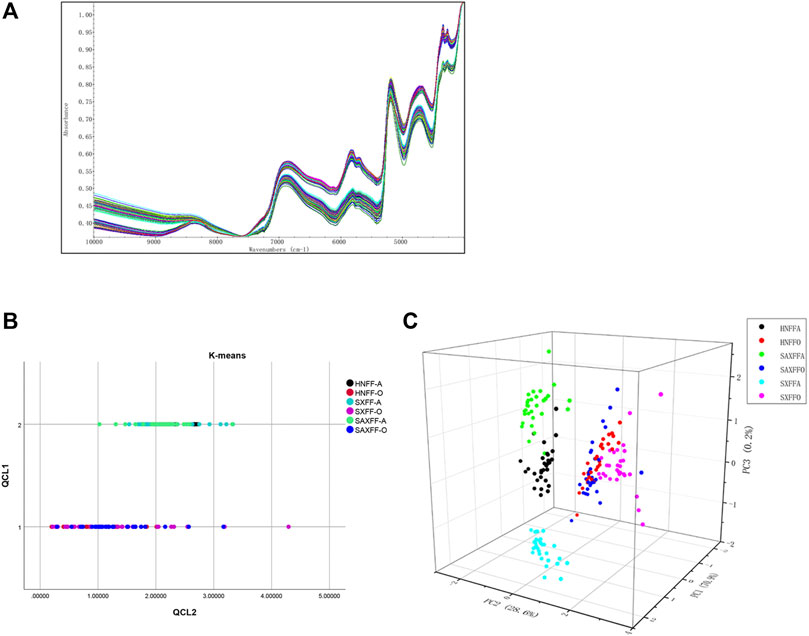
FIGURE 2. Analysis of NIR spectrum of FF from different harvest seasons and regions. (A) NIR spectrum images of 180 FF in the frequency range of 10,000 to 4,000 cm-1 with 8 cm-1 increments. (B) The K-means cluster analysis of NIR spectrum of 180 FF from different harvest seasons and regions by SPSS software (version 26.0). QCL1 is the number of clusters and QCL2 is the distance from the sample to the cluster center. (C) 3-dimensional graph of NIR spectrum of 180 FF based on PCA. PC1, PC2, and PC3 are the three main components after dimension reduction.
Principal component analysis (PCA), which reduces its dimensionality into several main components, is applied to analyze the NIR spectrum of the 180 FF samples. PCA is a dimensionality reduction method that converts a large quantity of data into a few comprehensive indicators, reducing the dimension of observation and obtaining the most important information. In this study, the NIR spectrum of PCA based on variance decomposition was projected into the new coordinate system, and the principal components (PC1, PC2, and PC3) take the eigenvalues that can reflect the maximum variance value. Figure 2C showed the PC1, PC2, and PC3 loading plots of different FF PCA models that corresponded to 70.9, 28.6, and 0.2% of the variance. The three-dimensional scores of PCA showed there was a significant difference in the distribution of the FF between August and October; Henan, Shanxi, and Shaanxi in August were also distinguished, whereas there was no significant difference in October of the FF from Henan, Shanxi, and Shaanxi (Figure 2C).
Taken together, the K-means clustering and PCA of NIR spectrum showed that FF from different producing areas and harvest seasons had obvious characteristics of the harvest season. Meanwhile, FF in August showed obvious characteristics of producing area, while FF from different producing areas in October is not distinguished. These results indicated that the impact of harvest season on FF was significantly greater than that of producing areas.
The Forsythiaside A, Phillyrin, and Phillygenin of FF
To investigate the differences in the surface morphology, MIR spectra transmittance, and the NIR spectra of FF from different harvest seasons and regions, the contents of forsythiaside A, phillyrin, and phillygenin were determined by HPLC. The chromatograms of the forsythiaside A, phillyrin, and phillygenin from standards and samples were shown in Figures 3A,B. And the contents of the three components showed that different producing areas and harvest time played obvious effects on the FF (Figure 3C). Compared with HNFF, the forsythiaside A, phillyrin, and phillygenin of SXFF and SAXFF in August was markedly improved, while that of HNFF and SAXFF was significantly higher than that of SXFF in October. The level of forsythiaside A and phillyrin in SXFF-A was the highest and the phillygenin in SAXFF-A was the highest, which indicated that the SXFF-A and SAXFF-A perhaps had the highest constituents basis.
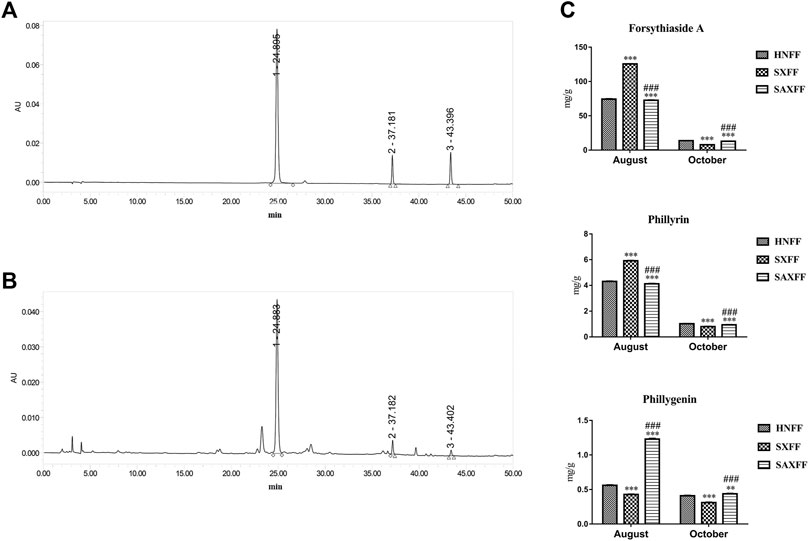
FIGURE 3. The contents of forsythiaside A, phillyrin, and phillygenin of FF by HPLC. (A) Standard chemicals of forsythiaside A, phillyrin, and phillygenin, (B) the methanol extract of Forsythia suspense by HPLC, and peak numbers indicate the following compounds, peak 1 is Forsythiaside A, peak 2 is phillyrin and peak 3 is phillygenin. (C) The levels of forsythiaside A, phillyrin, and phillygenin of FF from Henan, Shanxi, and Shaanxi in August and October.
Anti-inflammatory Activity of FF on LPS-Induced J774A.1 Cells
Based on the high levels of forsythiaside A, phillyrin, and phillygenin by HPLC, SXFF, and SAXFF in August were selected to treat J774A.1 cells. The results of the MTT assay suggested that 0.5 mg/ml, 0.2 mg/ml, and 0.1 mg/ml of SXFF and SAXFF in August were not toxic to J774A.1 cells (Figure 4A), which indicated that the effects of SXFF and SAXFF on J774A.1 cells were not due to their cytotoxicity. The NO levels in supernatant and cells of LPS-induced J774A.1 cells were significantly reduced by SXFF and SAXFF (Figure 4B). In this study, the anti-inflammatory activity of FF on LPS-induced J774A.1 cells was investigated. As shown in Figure 5A, the relative expression levels of inflammatory cytokines and mediators, namely, TNF-α, IL-1β, NF-κB, and iNOS were markedly downregulated by SXFF and SAXFF (August) in LPS-induced J774A.1 cells. Moreover, SXFF obviously downregulated the relative expression levels of IL-1β and iNOS mRNA, and SAXFF significantly attenuated the expression of IL-1β and NF-κB in a dose-dependent manner.
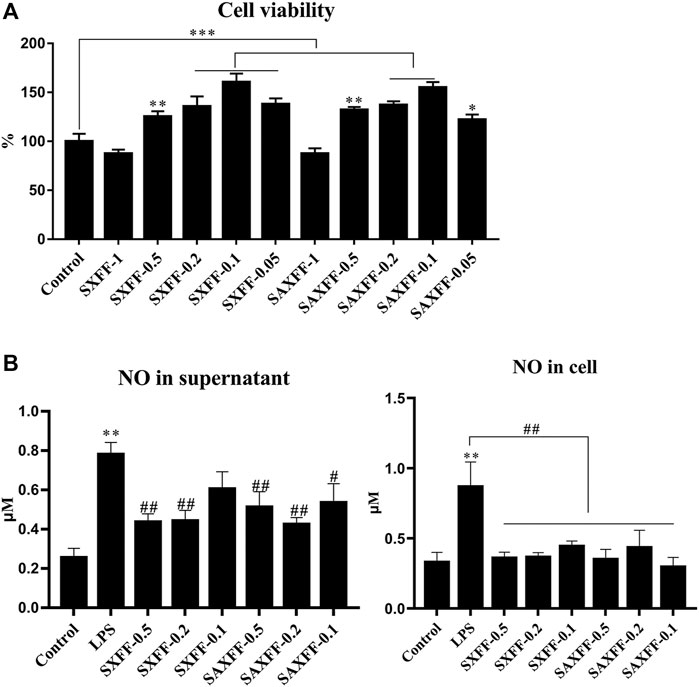
FIGURE 4. The cell viability and NO levels of FF on LPS-induced J744A.1 cells. (A) LPS-induced J744A.1 cells were treated with FF from Shanxi and Shaanxi in August for 24 h, cell viability was detected by MTT. The concentration of FF from Shanxi and Shaanxi were 1, 0.5, 0.2, 0.1, and 0.05 mg/ml, respectively. (B) The NO levels in supernatant and cells of LPS-induced J744A.1 cells treating with FF were measured by Griess reaction. SXFF-0.5, SXFF-0.2, and SXFF-0.1 is FF from Shanxi in August with concentration of 0.5, 0.2, and 0.1 mg/ml, and SAXFF-0.5, SAXFF-0.2, and SAXFF-0.1 is FF from Shaanxi with concentration of 0.5, 0.2, and 0.1 mg/ml, respectively. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the Control group; #p < 0.05, ##p < 0.01, and ###p < 0.001 compared with the LPS group.
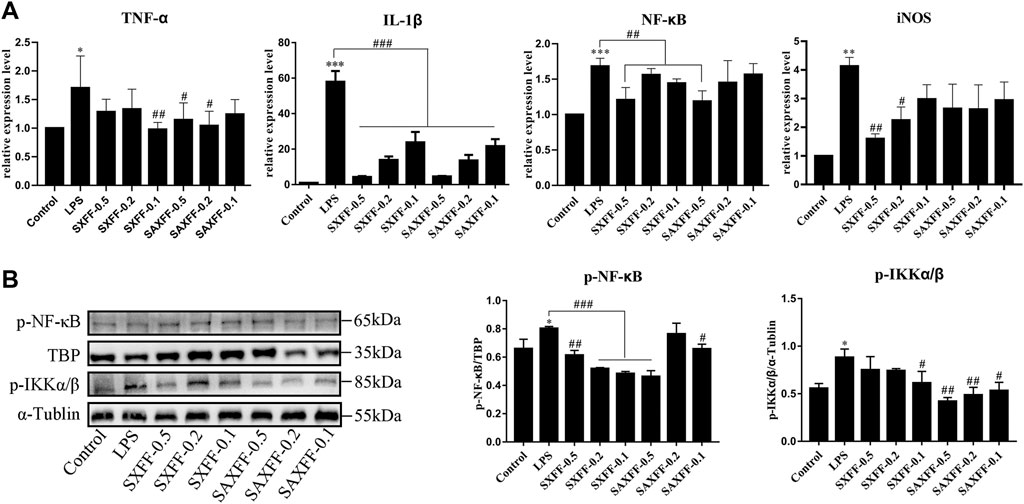
FIGURE 5. Anti-inflammatory activity of FF from Shanxi and Shaanxi in August on LPS-induced J774A.1 cells. (A) The mRNA expression levels of TNF-α, IL-1β, NF-κB, and iNOS were measured by RT-qPCR. (B) the protein expression levels of phosphorylated total IKKα/β and nuclear NF-κB were detected by Western Blot. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the Control group; #p < 0.05, ##p < 0.01, and ###p < 0.001 compared with the LPS group.
NF-κB is a transcription factor in promoting inflammation, so we investigate whether the anti-inflammatory effect of SXFF and SAXFF is related to the suppression of NF-κB. Western blot analysis was performed to analyze the protein expression levels of phosphorylated total IKKα/β and nuclear NF-κB. As shown in Figure 5B, the levels of p-IKKα/β and nuclear p-NF-κB were markedly increased by LPS and remarkably decreased by SXFF and SAXFF in LPS-induced J774A.1 cells, and the levels of p-IKKα/β were decreased by SAXFF in a dose-dependent manner.
Taken together, these results showed that SXFF and SAXFF suppressed the relative expression levels of TNF-α, IL-1β, NF-κB, and iNOS mRNA and the protein expression levels of p-IKKα/β and nuclear p-NF-κB, suggesting that SXFF and SAXFF have evident anti-inflammatory function in vitro, and the anti-inflammatory effects may be related to suppressing the NF-κB signaling pathway.
Antioxidant Activity of FF on LPS-Induced J774A.1 Cells
Nrf2 is an important transcription factor regulating the cellular oxidative stress response, and also a central regulator maintaining intracellular redox homeostasis. Therefore, we investigated how SXFF and SAXFF regulate Nrf2 signaling to exert an antioxidant role, and whether there is a difference in antioxidant effect between SXFF and SAXFF. As shown in Figure 6A, the mRNA expression levels of HO-1 were significantly increased by SXFF and SAXFF with a dose of 0.5 mg/ml, and the NQO1 were markedly increased by SAXFF with a dose of 0.5 mg/ml in LPS-induced J774A.1 cells.
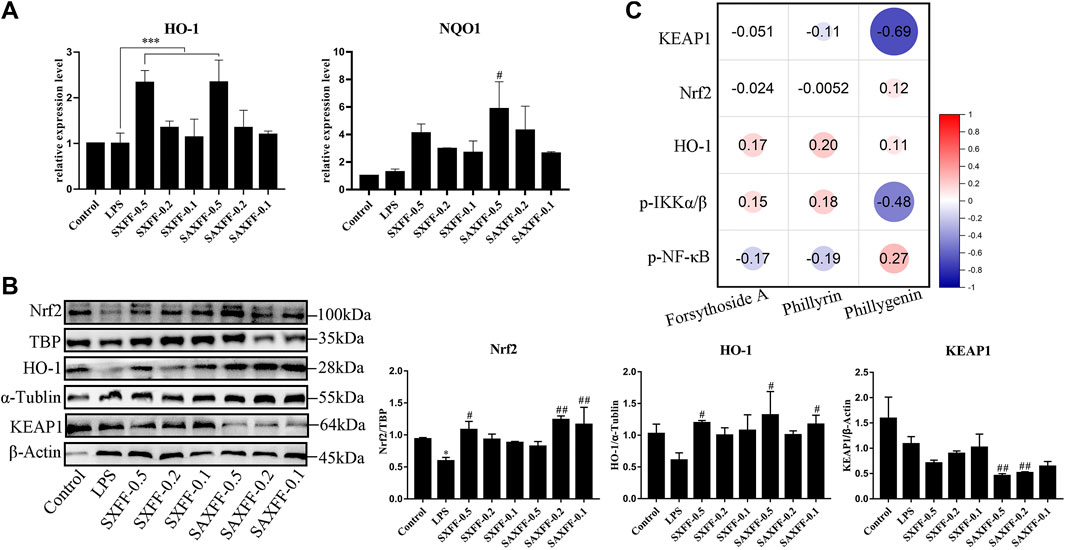
FIGURE 6. Antioxidant activity of FF from Shanxi and Shaanxi in August on LPS-induced J774A.1 cells. (A) The mRNA expression levels of HO-1 and NQO1 by LPS-induced J774A.1 cells treated with FF were measured by RT-qPCR. (B) The protein expression levels of total HO-1, KEAP1, and nuclear Nrf2 were assayed via Western Blot. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the Control group; #p < 0.05, ##p < 0.01, and ###p < 0.001 compared with the LPS group. (C) Spearman correlation analysis between the levels of forsythoside A, phillyrin, and phillygenin and the protein expression levels of KEAP1, Nrf2, HO-1, p-IKKα/β, and p-NF-κB by Origin software. The size of the circle represents the correlation, red represents a positive correlation, and blue represents a negative correlation.
To further determine the antioxidant effect of SXFF and SAXFF, Western blot analysis was performed to analyze the protein expression levels of total HO-1, KEAP1, and nuclear Nrf2. As shown in Figure 6B, SXFF and SAXFF significantly improved the protein expression levels of total HO-1 and nuclear Nrf2. Meanwhile, the protein expression levels of total KEAP1were obviously reduced by SXFF and SAXFF in a dose-dependent manner, and the levels of KEAP1 in SAXFF were lower than that in SXFF.
Collectively, these results showed that SXFF and SAXFF promoted the mRNA expression levels of HO-1 and NQO1 and the protein expression levels of HO-1 and nuclear Nrf2 and suppressed the protein expression levels of KEAP1, suggesting that the antioxidant activity of SXFF and SAXFF on LPS-induced J774A.1 cells is correlated with the Nrf2 signaling, and the antioxidant activity in SAXFF was stronger than that in SXFF.
The Correlation Analysis
In view of the different contents of forsythoside A, phillyrin, and phillygenin in FF from different localities, we analyzed the correlation between them and the anti-inflammatory and antioxidant effects of FF based on Spearman correlation. As shown in Figure 6C, the results showed that the correlation between the levels of phillygenin and the protein expression levels of KEAP1, Nrf2, and p-IKKα/β was higher than that of forsythoside A and phillyrin, whereas the correlation between the phillyrin and the protein of HO-1 and p-NF-κB was higher than that of forsythoside A and phillygenin. Phillyrin and phillygenin belong to bicyclo lignans, which indicates that the anti-inflammatory and antioxidant effects of FF may be mainly exerted by the lignans.
Discussion
Forsythiae fructus (FF), which includes forsythoside A, forsythoside B, phillyrin, phillygenin, etc., is widely used as Chinese herbal medicine for heat-clearing and detoxification purposes and appears in more than 100 kinds of TCM (Chen et al., 2016; Zhang et al., 2020b; Wang et al., 2021a). In fact, previous studies have shown that FF has anti-inflammatory, antiviral, anticancer, and other therapeutic effects (Ko et al., 2005; Long et al., 2020; Wang et al., 2021b). In TCM, there are many distinctions between geo-authentic and non-authentic producing areas, and the picking time also has marked impacts on herbs. FF has many producing areas, including Henan, Hebei, Shanxi, Shaanxi, and so on. In August or September, FF is harvested when it is green and immature, which is called Qingqiao, and in October, the yellow and mature FF is called LaoQiao. In the present study, FF collected in August and October from Henan, Shanxi, and Shaanxi was examined to identify the distinctions in the surface morphology, compound content, NIR and MIR spectrum, and the anti-inflammatory and anti-oxidation effects of FF.
Scanning electron microscope (SEM) is a momentous technique for the determination of morphological parameters and is performed to analyze the surface morphology of FF from different harvest seasons and regions, providing visual evidence (Yao et al., 2019; Mitsuwan et al., 2020). In the present study, the SEM images showed that FF in October has more loose and reticular structure than that in August. And the data of MIR spectra demonstrated that FF from different picking times and areas had similar compounds, but different contents of some components. Therefore, our results suggest that the surface morphology and compound contents are obviously different between FF in August and October. In agreement with our study, Qu et al. showed that phytochemical profiles were similar among different Qingqiao and Laoqiao samples, while contents of major components are significantly different (Qu et al., 2017).
Near-infrared (NIR) spectroscopy is used to identify the authenticity of traditional Chinese Medicine (TCM), with faster speed, non-destructive properties, and no sample preparation (Balabin et al., 2011; Wang et al., 2012; Sarraguca et al., 2014; Borraz-Martinez et al., 2019; Sun et al., 2019). In our results, the cluster analysis based on the K-mean of the NIR spectrum suggested that FF was divided into two categories—FF in August and October—indicating that the main reason for the distinction of FF was the picking time. Furthermore, based on PCA and discriminant analysis, our results also showed that FF was significantly different in August and October, and FF from Henan, Shanxi, and Shaanxi in August was also different, whereas FF from different regions in October was not. Our results were in agreement with those of other studies, which reported that the green and ripe FF were clearly separated by HPLC-ESI-MS/MS and NMR-based analysis, and the FF from Henan, Shanxi, and Anhui were clearly different (Jia et al., 2015; Qu et al., 2017).
Furthermore, the levels of forsythiaside A, phillyrin, and phillygenin, the main components of FF, were analyzed by HPLC. We found that the contents of them in FF were significantly increased in August, compared with October. At the same time, the levels of forsythiaside A and phillyrin of FF from Shanxi in August and phillygenin of FF from Shaanxi in August were the highest. Previous studies have found that forsythiaside A and phillyrin exhibited anti-oxidative, anti-inflammatory, antiviral (Wei et al., 2014; Qu et al., 2016; Dong et al., 2017; Zhang et al., 2020a; Gong et al., 2020; Jiang et al., 2020; Fu et al., 2021; Zhao et al., 2021), and phillygenin showed anti-oxidative, anti-inflammatory, anti-cancer (Chang et al., 2008; He et al., 2019; Hu et al., 2020; Zhou et al., 2021b). Consequently, FF from Shanxi and Shaanxi in August was selected to study which one has better anti-oxidative and anti-inflammatory effects based on the high levels of forsythiaside A, phillyrin, and phillygenin of FF.
Excessive secretion of inflammatory cytokines, such as TNF-α, IL-1β, and inflammatory mediators, for example, NO, iNOS have been proved to lead to inflammation, and NF-κB, the key transcription factor of inflammation, regulates the secretion of inflammatory cytokines (An et al., 2020; Chen et al., 2021). In our study, our results showed that FF markedly reduced the levels of inflammatory cytokines, inflammatory mediators, and the protein expression levels of phosphorylated total IKKα/β and nuclear NF-κB, and the inhibitory effects of FF from Shaanxi on IKKα/β and NF-κB were higher than those of FF from Shanxi, indicating that the anti-inflammatory of FF from Shanxi and Shaanxi in August may be related to NF-κB signaling, and the activity of FF from Shaanxi is higher than that of FF from Shanxi. FF has been proven by many studies to alleviate diseases or LPS-induced inflammation, which is consistent with our results, but there are few studies on the comparison of anti-inflammatory effects of FF from different regions.
Keap1 negatively regulates the activation of Nrf2, and activated Nrf2 regulates the release of HO-1, NQO1, and antioxidant enzymes to play an antioxidant role, which is conducive to down-regulating inflammation (Wang et al., 2021c; Li et al., 2021; Osman et al., 2021). In the present study, the activity of Nrf2 was activated by FF, and the mRNA expression of HO-1 and NQO1 was increased, which indicated that FF may perform antioxidant action by activating the Nrf2 pathway. Consistent with the anti-inflammatory action of FF, FF from Shaanxi has a more obvious antioxidant effect than FF from Shanxi.
Forsythiaside A, phillyrin, and phillygenin have been shown to have significant anti-inflammatory and antioxidant activities in previous studies, but there are few studies on their comparison of effects (Yuan et al., 2014; Ma et al., 2019; Du et al., 2020; Zhou et al., 2021a; Tian et al., 2021). In this study, correlation analysis demonstrated that phillygenin and phillyrin, which belong to lignans, may be the main anti-inflammatory and antioxidant compounds of FF.
In conclusion, our results indicated that FF from Henan, Shanxi, and Shaanxi in August and October have different microstructures and mid-infrared and near-infrared spectrums. Our findings also shown that the levels of forsythiaside A and phillyrin of FF from Shanxi in August and phillygenin of FF from Shaanxi in August were higher than that of other regions and harvest seasons, especially FF in October, which indicated that the content of the compounds of FF in August (named Qingqiao) is higher than that of FF in October (named Laoqiao). What is more, FF from Shanxi and Shaanxi in August showed marked anti-inflammatory and antioxidant activities, but with some differences, which may be mainly due to the different content of phillygenin and phillyrin. Our finding provides a new perspective for FF research, whereas further in-depth research will be needed to prove whether phillygenin or phillyrin is the main anti-inflammatory and antioxidant component in FF.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
QQ and YL designed the work, performed the research study, and drafted the manuscript. QD, SL, HD, and ZW, participated in the experimental work. XG and WZ analyzed the database. WL, ML and LC revised the manuscript. SG designed and supervised the research study. All authors read and approved the final manuscript. QQ and YL contributed equally.
Funding
This work was supported by R and D Projects in important areas of Guangdong Province, studies and applications about key technology biosynthesis in antibiotic-free feeds, 2019B020218003, and research and application of African swine fever vaccine creation and control agent, 20190211.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
FF, Forsythiae Fructus; TCM, Traditional Chinese Medicine; SEM, scanning electron microscope; MIR, Mid-infrared; NIR, Near-infrared; RT-qPCR, real-time quantitative PCR; PCA, Principal component analysis.
References
Alizadeh, Z., Farimani, M. M., Parisi, V., Marzocco, S., Ebrahimi, S. N., and De Tommasi, N. (2021). Nor-Abietane Diterpenoids from Perovskia Abrotanoides Roots with Anti-Inflammatory Potential. J. Nat. Prod. 84 (4), 1185–1197. doi:10.1021/acs.jnatprod.0c01256
An, M. Y., Eo, H. J., Son, H. J., Geum, N. G., Park, G. H., and Jeong, J. B. (2020). Anti-Inflammatory Effects of Leaf and Branch Extracts of Honeyberry (Lonicera Caerulea) on Lipopolysaccharide Stimulated RAW264.7 Cells through ATF3 and Nrf2/HO1 Activation. Mol. Med. Rep. 22 (6), 5219–5230. doi:10.3892/mmr.2020.11638
Balabin, R. M., Lomakina, E. I., and Safieva, R. Z. (2011). Neural Network (ANN) Approach to Biodiesel Analysis: Analysis of Biodiesel Density, Kinematic Viscosity, Methanol and Water Contents Using Near Infrared (NIR) Spectroscopy. Fuel 90 (5), 2007–2015. doi:10.1016/j.fuel.2010.11.038
Bao, J., Ding, R. B., Liang, Y., Liu, F., Wang, K., Jia, X., et al. (2017). Differences in Chemical Component and Anticancer Activity of Green and Ripe Forsythiae Fructus. Am. J. Chin. Med. 45 (7), 1513–1536. doi:10.1142/S0192415X17500823
Borraz-Martínez, S., Boqué, R., Simó, J., Mestre, M., and Gras, A. (2019). Development of a Methodology to Analyze Leaves from Prunus Dulcis Varieties Using Near Infrared Spectroscopy. Talanta 204, 320–328. doi:10.1016/j.talanta.2019.05.105
Chang, M. J., Hung, T. M., Min, B. S., Kim, J. C., Woo, M. H., Choi, J. S., et al. (2008). Lignans from the Fruits of Forsythia Suspensa (Thunb.) Vahl Protect High-Density Lipoprotein During Oxidative Stress. Biosci. Biotechnol. Biochem. 72 (10), 2750–2755. doi:10.1271/bbb.80392
Chen, J., Chen, Q., Yu, F., Huang, H., Li, P., Zhu, J., et al. (2016). Comprehensive Characterization and Quantification of Phillyrin in the Fruits ofForsythia Suspensaand its Medicinal Preparations by Liquid Chromatography-Ion Trap Mass Spectrometry. Acta Chromatogr. 28 (1), 145–157. doi:10.1556/AChrom.28.2016.1.11
Chen, X., Sun, X., Hua, H., Yi, Y., Li, H., and Chen, C. (2019). Quality Evaluation of Decoction Pieces of Rhizoma Atractylodis Macrocephalae by Near Infrared Spectroscopy Coupled with Chemometrics. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 221, 117169. doi:10.1016/j.saa.2019.117169
Chen, Y., Pan, R., Zhang, J., Liang, T., Guo, J., Sun, T., et al. (2021). Pinoresinol Diglucoside (PDG) Attenuates Cardiac Hypertrophy via AKT/mTOR/NF-κB Signaling in Pressure Overload-Induced Rats. J. Ethnopharmacol. 272, 113920. doi:10.1016/j.jep.2021.113920
Dong, Z., Lu, X., Tong, X., Dong, Y., Tang, L., and Liu, M. (2017). Forsythiae Fructus: A Review on its Phytochemistry, Quality Control, Pharmacology and Pharmacokinetics. Molecules 22 (9), 1466. doi:10.3390/molecules22091466
Du, Y., You, L., Ni, B., Sai, N., Wang, W., Sun, M., et al. (2020). Phillyrin Mitigates Apoptosis and Oxidative Stress in Hydrogen Peroxide-Treated RPE Cells through Activation of the Nrf2 Signaling Pathway. Oxid. Med. Cell Longev. 2020, 1–16. doi:10.1155/2020/2684672
Fang, X., Gu, S., Jin, Z., Hao, M., Yin, Z., and Wang, J. (2018). Optimization of Ultrasonic-Assisted Simultaneous Extraction of Three Active Compounds from the Fruits of Forsythia Suspensa and Comparison with Conventional Extraction Methods. Molecules 23 (9), 2115. doi:10.3390/molecules23092115
Fu, L., Lu, M., Fu, Q., Wang, K., Hui, M., Liu, Z., et al. (2021). Application of a Phillyrin/Phillygeninin Composition in Preparing a Medicine or Health Care Product for Alleviating Or/and Treating Viral Diseases, and Medicine or Health Care Product for Treating Viral Diseases. U.S. Patent No 10,881,679. Washington, DC: U.S. Official Gazette of the United States Patent and Trademark Office Patents.
Gong, L., Yu, L., Gong, X., Wang, C., Hu, N., Dai, X., et al. (2020). Exploration of Anti-Inflammatory Mechanism of Forsythiaside A and Forsythiaside B in CuSO4-Induced Inflammation in Zebrafish by Metabolomic and Proteomic Analyses. J. Neuroinflamm. 17 (1), 173. doi:10.1186/s12974-020-01855-9
He, J., Wei, W., Yang, Q., and Wang, Y. (2019). Phillygenin Exerts In Vitro and In Vivo Antitumor Effects in Drug-Resistant Human Esophageal Cancer Cells by Inducing Mitochondrial-Mediated Apoptosis, ROS Generation, and Inhibition of the Nuclear Factor Kappa B NF-Κb Signalling Pathway. Med. Sci. Monit. 25, 739–745. doi:10.12659/msm.913138
Heo, S.-Y., Ko, S.-C., and Jung, W.-K. (2018). The Pepsinolytic Hydrolysate from Johnius Belengerii Frame Inhibited LPS-Stimulated Production of Pro-inflammatory Mediators via the Inactivating of JNK and NF-κB Pathways in RAW 264.7 Macrophages. Fish Aquat. Sci 21 (1), 1–8. doi:10.1186/s41240-018-0091-2
Hu, N., Wang, C., Dai, X., Zhou, M., Gong, L., Yu, L., et al. (2020). Phillygenin Inhibits LPS-Induced Activation and Inflammation of LX2 Cells by TLR4/MyD88/NF-Κb Signaling Pathway. J. Ethnopharmacol. 248, 112361. doi:10.1016/j.jep.2019.112361
Hung, Y. L., Wang, S. C., Suzuki, K., Fang, S. H., Chen, C. S., Cheng, W. C., et al. (2019). Bavachin Attenuates LPS-Induced Inflammatory Response and Inhibits the Activation of NLRP3 Inflammasome in Macrophages. Phytomedicine 59, 152785. doi:10.1016/j.phymed.2018.12.008
Jia, J., Zhang, F., Li, Z., Qin, X., and Zhang, L. (2015). Comparison of Fruits of Forsythia Suspensa at Two Different Maturation Stages by NMR-Based Metabolomics. Molecules 20 (6), 10065–10081. doi:10.3390/molecules200610065
Jiang, Q., Chen, J., Long, X., Yao, X., Zou, X., Yang, Y., et al. (2020). Phillyrin Protects Mice from Traumatic Brain Injury by Inhibiting the Inflammation of Microglia via PPARγ Signaling Pathway. Int. Immunopharmacol. 79, 106083. doi:10.1016/j.intimp.2019.106083
Ko, H. C., Wei, B. L., and Chiou, W. F. (2005). Dual Regulatory Effect of Plant Extracts of Forsythia Suspense on RANTES and MCP-1 Secretion in Influenza A Virus-Infected Human Bronchial Epithelial Cells. J. Ethnopharmacol. 102 (3), 418–423. doi:10.1016/j.jep.2005.06.029
Lee, J. J., Kim, K. H., Kim, E. J., Choi, J. Y., Kim, S. J., Jeong, S. I., et al. (2018). Anti-inflammatory Activity of the Decoction of Forsythia Suspensa (Thunb.) Vahl Is Related to Nrf2 and A20. J. Ethnopharmacol. 227, 97–104. doi:10.1016/j.jep.2018.08.027
Li, X., He, S., Zhou, J., Yu, X., Li, L., Liu, Y., et al. (2021). Cr (VI) Induces Abnormalities in Glucose and Lipid Metabolism through ROS/Nrf2 Signaling. Ecotoxicol Environ. Saf. 219, 112320. doi:10.1016/j.ecoenv.2021.112320
Lim, D. W., Choi, H. J., Park, S. D., Kim, H., Yu, G. R., Kim, J. E., et al. (2020). Activation of the Nrf2/HO-1 Pathway by Amomum Villosum Extract Suppresses LPS-Induced Oxidative Stress In Vitro and Ex Vivo. Evid. Based Complement. Alternat Med. 2020, 2837853. doi:10.1155/2020/2837853
Long, S. F., He, T. F., Wu, D., Yang, M., and Piao, X. S. (2020). Forsythia Suspensa Extract Enhances Performance via the Improvement of Nutrient Digestibility, Antioxidant Status, Anti-inflammatory Function, and Gut Morphology in Broilers. Poult. Sci. 99 (9), 4217–4226. doi:10.1016/j.psj.2020.05.011
Luo, H., Zhao, Y., Hua, H., Zhang, Y., Zhang, X., Fang, Q., et al. (2021). Research Progress on Quality Assurance of Genuine Chinese Medicinal in Sichuan. Chin. Med. 16 (1), 19. doi:10.1186/s13020-021-00428-z
Ma, T., Shi, Y. L., and Wang, Y. L. (2019). Forsythiaside A Protects against Focal Cerebral Ischemic Injury by Mediating the Activation of the Nrf2 and Endoplasmic Reticulum Stress Pathways. Mol. Med. Rep. 20 (2), 1313–1320. doi:10.3892/mmr.2019.10312
Mitsuwan, W., Sangkanu, S., Romyasamit, C., Kaewjai, C., Jimoh, T. O., de Lourdes Pereira, M., et al. (2020). Curcuma Longa Rhizome Extract and Curcumin Reduce the Adhesion of Acanthamoeba Triangularis Trophozoites and Cysts in Polystyrene Plastic Surface and Contact Lens. Int. J. Parasitol. Drugs Drug Resist. 14, 218–229. doi:10.1016/j.ijpddr.2020.11.001
Osman, A. T., Sharkawi, S. M. Z., Hassan, M. I. A., Abo-Youssef, A. M., and Hemeida, R. A. M. (2021). Empagliflozin and Neohesperidin Mitigate Methotrexate Hepatotoxicity via Nrf2/PPARγ/HO-1 Signalling Initiation and Suppression of NF-κB/Keap1/HSP70/caspase-3 Axis in Rats. Life Sci. 278, 119638. doi:10.1016/j.lfs.2021.119638
Qu, X. Y., Li, Q. J., Zhang, H. M., Zhang, X. J., Shi, P. H., Zhang, X. J., et al. (2016). Protective Effects of Phillyrin against Influenza A Virus In Vivo. Arch. Pharm. Res. 39 (7), 998–1005. doi:10.1007/s12272-016-0775-z
Qu, J., Yan, X., Li, C., Wen, J., Lu, C., Ren, J., et al. (2017). Comparative Evaluation of Raw and Ripe Fruits of Forsythia Suspensa by HPLC-ESI-MS/MS Analysis and Anti-microbial Assay. J. Chromatogr. Sci. 55 (4), 451–458. doi:10.1093/chromsci/bmw203
Sarraguça, M. C., Ribeiro, P. R., Santos, A. O., Silva, M. C., and Lopes, J. A. (2014). A PAT Approach for the On-Line Monitoring of Pharmaceutical Co-Crystals Formation with Near Infrared Spectroscopy. Int. J. Pharm. 471 (1-2), 478–484. doi:10.1016/j.ijpharm.2014.06.003
Shao, S. Y., Zhang, F., Yang, Y. N., Feng, Z. M., Jiang, J. S., and Zhang, P. C. (2021). Neuroprotective and Anti-inflammatory Phenylethanoidglycosides from the Fruits of Forsythia Suspensa. Bioorg. Chem. 113, 105025. doi:10.1016/j.bioorg.2021.105025
Sun, F., Chen, Y., Wang, K.-Y., Wang, S.-M., and Liang, S.-W. (2019). Identification of Genuine and Adulterated Pinellia Ternata by Mid-infrared (MIR) and Near-Infrared (NIR) Spectroscopy with Partial Least Squares - Discriminant Analysis (PLS-DA). Anal. Lett. 53 (6), 937–959. doi:10.1080/00032719.2019.1687507
Tian, J., Xu, X., and Tian, D. (2021). Forsythiaside-A Alleviates Traumatic Brain Injury by Regulating Toll-like Receptor 4/Myeloid Differentiation Factor 88/Nuclear Factor-Kappa B Signaling Pathway. Curr. Top. Nutraceutical Res. 19 (3), 326–332. doi:10.37290/ctnr2641-452X.19:326-332
Tsai, F. J., Li, T. M., Cheng, C. F., Wu, Y. C., Lai, C. H., Ho, T. J., et al. (2018). Effects of Chinese Herbal Medicine on Hyperlipidemia and the Risk of Cardiovascular Disease in HIV-Infected Patients in Taiwan. J. Ethnopharmacol. 219, 71–80. doi:10.1016/j.jep.2018.03.006
Wang, X., Bai, Y., Zhang, Y., and Qiao, Y. (2012). “Rapid Analysis of Fructus Forsythiae by Near-Infrared Spectroscopy,” in 2012 International Conference on Biomedical Engineering and Biotechnology, Macau, Macao, May 28-30, 2012. doi:10.1109/icbeb.2012.326
Wang, J., Li, T., Yang, H., Hu, T., Nie, L., Wang, F., et al. (2017). Geographical Origin Discrimination and Polysaccharides Quantitative Analysis of Radix Codonopsis with Micro Near-Infrared Spectrometer Engine. J. Innov. Opt. Health Sci. 11 (01), 1850004. doi:10.1142/s1793545818500049
Wang, Z., Xia, Q., Liu, X., Liu, W., Huang, W., Mei, X., et al. (2018). Phytochemistry, Pharmacology, Quality Control and Future Research of Forsythia Suspensa (Thunb.) Vahl: A Review. J. Ethnopharmacol 210, 318–339. doi:10.1016/j.jep.2017.08.040
Wang, A., Yang, P., Chen, J., Wu, Z., Jia, Y., Ma, C., et al. (2019). A New Calibration Model Transferring Strategy Maintaining the Predictive Abilities of NIR Multivariate Calibration Model Applied in Different Batches Process of Extraction. Infrared Phys. Techn. 103, 103046. doi:10.1016/j.infrared.2019.103046
Wang, D. H., Wang, M. Y., Shen, W. H., and Yuan, J. F. (2021a). Analysis of Chemical Compounds and Toxicological Evaluation of Forsythia Suspensa Leaves tea. Food Sci. Biotechnol. 30 (2), 305–314. doi:10.1007/s10068-020-00855-y
Wang, X., Li, X., Wang, X., Chen, L., Ning, E., Fan, Y., et al. (2021b). Experimental Study of Forsythoside A on Prevention and Treatment of Avian Infectious Bronchitis. Res. Vet. Sci. 135, 523–531. doi:10.1016/j.rvsc.2020.11.009
Wang, Z., Zheng, N., Liang, J., Wang, Q., Zu, X., Wang, H., et al. (2021c). Emodin Resists to Cyprinid Herpesvirus 3 Replication via the Pathways of Nrf2/Keap1-ARE and NF-Κb in the Ornamental Koi Carp (Cyprinus carpio Haematopterus). Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 246, 109023. doi:10.1016/j.cbpc.2021.109023
Wei, T., Tian, W., Yan, H., Shao, G., and Xie, G. (2014). Protective Effects of Phillyrin on H2O 2-induced Oxidative Stress and Apoptosis in PC12 Cells. Cell Mol Neurobiol 34 (8), 1165–1173. doi:10.1007/s10571-014-0091-4
Yao, L., Bai, L., Tan, Y., Sun, J., Qu, Q., Shi, D., et al. (2019). The Immunoregulatory Effect of Sulfated Echinacea Purpurea Polysaccharide on Chicken Bone Marrow-Derived Dendritic Cells. Int. J. Biol. Macromol 139, 1123–1132. doi:10.1016/j.ijbiomac.2019.08.028
Yuan, J.-F., Liu, X.-Q., Yang, J.-X., and Cui, X.-Q. (2014). Forsythia Suspense Leaves, a Plant: Extraction, Purification and Antioxidant Activity of Main Active Compounds. Eur. Food Res. Technol. 238 (4), 527–533. doi:10.1007/s00217-014-2179-y
Zhang, D., Qi, B., Li, D., Feng, J., Huang, X., Ma, X., et al. (2020a). Phillyrin Relieves Lipopolysaccharide-Induced AKI by Protecting against Glycocalyx Damage and Inhibiting Inflammatory Responses. Inflammation 43 (2), 540–551. doi:10.1007/s10753-019-01136-5
Zhang, F. X., Li, Z. T., Li, C., Li, M., Yao, Z. H., Yao, X. S., et al. (2020b). Characterization of Lignans in Forsythiae Fructus and Their Metabolites in Rats by Ultra-performance Liquid Chromatography Coupled Time-Of-Flight Mass Spectrometry. J. Pharm. Pharmacol. 72 (12), 1879–1892. doi:10.1111/jphp.13346
Zhang, F. X., Li, Z. T., Yang, X., Xie, Z. N., Chen, M. H., Yao, Z. H., et al. (2021). Discovery of Anti-flu Substances and Mechanism of Shuang-Huang-Lian Water Extract Based on Serum Pharmaco-Chemistry and Network Pharmacology. J. Ethnopharmacol. 268, 113660. doi:10.1016/j.jep.2020.113660
Zhao, J., Cui, P., Liu, H., Wang, C., liu, M., Li, G., et al. (2020). Rapid Screening and Quantitative Analysis of Adulterant Lonicerae Flos in Lonicerae Japonicae Flos by Fourier-Transform Near Infrared Spectroscopy. Infrared Phys. Techn. 104, 103139. doi:10.1016/j.infrared.2019.103139
Zhao, L., Li, W., Dai, S. J., Liu, R. X., Xie, Z. P., Zhang, S. M., et al. (2021). Alkaloids Bearing Rare Skeletons from Forsythia Suspensa with Anti-inflammatory and Anti-viral Activities In Vitro. Phytochemistry 186, 112739. doi:10.1016/j.phytochem.2021.112739
Zhou, M., Tang, Y., Liao, L., Liu, M., Deng, Y., Zhao, X., et al. (2021a). Phillygenin Inhibited LPS-Induced RAW 264.7 Cell Inflammation by NF-Κb Pathway. Eur. J. Pharmacol. 899, 174043. doi:10.1016/j.ejphar.2021.174043
Zhou, S., Wen, H., Han, X., and Li, H. (2021b). Phillygenin Protects against Osteoarthritis by Repressing Inflammation via PI3K/Akt/NF-Κb Signaling: In Vitro and Vivo Studies. J. Funct. Foods 80, 104456. doi:10.1016/j.jff.2021.104456
Zhu, S., Guo, L., Cui, Y., Xiao, R., Yu, Z., Jin, Y., et al. (2019). Quality Suitability Modeling of Volatile Oil in Chinese Materia Medica - Based on Maximum Entropy and Independent Weight Coefficient Method: Case Studies of Atractylodes Lancea, Angelica Sinensis, Curcuma Longa and Atractylodes Macrocephala. Ind. Crops Prod. 142, 111807. doi:10.1016/j.indcrop.2019.111807
Keywords: forsythiae fructus, harvest seasons, regions, anti-inflammatory, antioxidant
Citation: Qu Q, Li Y, Dong Q, Li S, Du H, Wang Z, Gong X, Zhang W, Lv W, Chao L, Liu M, Tang X and Guo S (2021) Comparative Evaluation of Forsythiae Fructus From Different Harvest Seasons and Regions by HPLC/NIR Analysis and Anti-inflammatory and Antioxidant Assays. Front. Pharmacol. 12:737576. doi: 10.3389/fphar.2021.737576
Received: 17 July 2021; Accepted: 01 November 2021;
Published: 24 November 2021.
Edited by:
Rudolf Bauer, University of Graz, AustriaReviewed by:
Krzysztof Bernard Bec, University of Innsbruck, AustriaHuihui Cao, Southern Medical University, China
Copyright © 2021 Qu, Li, Dong, Li, Du, Wang, Gong, Zhang, Lv, Chao, Liu, Tang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinggang Tang, OTU4OTA5NzIyQHFxLmNvbQ==; Shining Guo, c2hpbmluZ0BzY2F1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Qian Qu1†
Qian Qu1† Shining Guo
Shining Guo