- 1Shandong Analysis and Test Center, Laboratory of Immunology for Environment and Health, Qilu University of Technology (Shandong Academy of Sciences), Jinan, China
- 2School of Pharmaceutical Sciences, Qilu University of Technology (Shandong Academy of Sciences), Jinan, China
- 3School of Pharmaceutical Science, Shandong University, Jinan, China
- 4The University of Queensland Diamantina Institute, Translational Research Institute, Brisbane, QLD, Australia
- 5School of Food Science and Engineering, Qilu University of Technology (Shandong Academy of Sciences), Jinan, China
- 6Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 7Drug Discovery Biology, Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia
Atopic dermatitis (AD), also known as atopic eczema, is one of the most common skin diseases and is characterized by allergic skin inflammation, redness, and itchiness and is associated with a hyperactivated type 2 immune response. The leading causes of AD include an imbalance in the immune system, genetic predisposition, or environmental factors, making the development of effective pharmacotherapies complex. Steroids are widely used to treat AD; however, they provide limited efficacy in the long term and can lead to adverse effects. Thus, novel treatments that offer durable efficacy and fewer side effects are urgently needed. Here, we investigated the therapeutic potential of Huangbai Liniment (HB), a traditional Chinese medicine, using an experimental AD mouse model, following our clinical observations of AD patients. In both AD patient and the mouse disease model, HB significantly improved the disease condition. Specifically, patients who received HB treatment on local skin lesions (3–4 times/day) showed improved resolution of inflammation. Using the 1-Chloro-2,4-dinitrobenzene (DNCB)-induced AD model in BALB/c mice, we observed that HB profoundly alleviated severe skin inflammation and relieved the itching. The dermatopathological results showed markedly reversed skin inflammation with decreased epidermal thickness and overall cellularity. Correspondingly, HB treatment largely decreased the mRNA expression of proinflammatory cytokines, including IL-1β, TNF-α, IL-17, IL-4, and IL-13, associated with declined gene expression of IL-33, ST2, and GATA3, which are connected to the type 2 immune response. In addition, HB restored immune tolerance by promoting regulatory T (TREG) cells and inhibiting the generation of TH1, TH2, and TH17 cells in vitro and in the DNCB-induced AD mouse model. For the first time, we demonstrate that HB markedly mitigates skin inflammation in AD patients and the DNCB-induced AD mouse model by reinvigorating the T cell immune balance, shedding light on the future development and application of novel HB-based therapeutics for AD.
Introduction
As the physical barrier of the host, the skin is the organ where dynamic environmental-host interactions occur to drive the host’s defense against stimuli, including microbial antigens and environmental chemicals, while abnormities in this response can lead to atopic reactions (Weidinger and Novak, 2016). Atopic dermatitis (AD), also known as atopic eczema, is a chronic allergic inflammatory skin disease that affects millions of people worldwide. Patients with AD exhibit mild to severe symptoms and often display eczematous lesions, erythema, pruritus, and skin inflammation (Leung et al., 2004; Weidinger and Novak, 2016). A proportion of AD patients also show comorbidities, including food allergies, asthma, allergic rhinitis, and other immune-mediated inflammatory diseases (Weidinger and Novak, 2016). A key pathophysiological mechanism of AD is the inappropriate immune response to antigens in the skin, which results in abnormalities of the epidermal structure and function and serious skin inflammation (Leung et al., 2004; Biedermann et al., 2015; Weidinger and Novak, 2016). Immune cells largely contribute to the development and regulation of AD, where the imbalance of CD4 T helper cell subsets is considered to be one of the major causes of the disease (Biedermann et al., 2015). Therefore, targeting the immune system imbalance is a promising approach to treat AD patients (Leung et al., 2004; Kim et al., 2019).
CD4 T helper cells play a vital role in the pathogenesis of AD. Emerging studies have shown that increased frequencies of TH1, TH2, TH17, and TH22 together with an excessive accumulation of inflammatory cytokines indispensably contribute to the onset of AD (Grewe et al., 1998; Weidinger and Novak, 2016). Specifically, the type 2 immune response and enhanced IgE response to allergens are the major factors that induce AD (Grewe et al., 1998; Weidinger and Novak, 2016). Antigen-primed TH2 cells and their associated cytokines, including IL-4, IL-5, and IL-13 induce immune dysfunction and subsequently damage cutaneous tissue integrity, leading to AD initiation (Suárez-Fariñas et al., 2011; Dainichi et al., 2018). In chronic lesions of AD, both TH2 and TH1 cells induce further cellular infiltration, while TH17 and TH22 cells play important roles in acute lesions (Nograles et al., 2009; Gittler et al., 2012). It is thus critical to inhibit the excess of these pro-AD T cell subsets to improve AD in patients. Regulatory T (TREG) cells regulate inflammation by substantially inhibiting proinflammatory T cells (Lei et al., 2015). By producing IL-10 and TGF-β, TREG cells induce immune tolerance suppressing the severity of AD (Biedermann et al., 2015). Moreover, TREG effector memory cells are reported to display the highest suppressive function (Rostaher et al., 2018; Looman et al., 2020). Furthermore, TH2-cytokine-producing type 2 innate lymphoid cells are also found in AD lesions and contribute to IL-17 dependent inflammation (Imai et al., 2013).
Although significant progress has been made in understanding AD pathogenesis, the lack of durable and well-tolerated treatments has led to significant health and economic burdens in managing AD in patients. Immunosuppressive drugs, such as steroids, are most commonly used to treat AD; however, side effects include severe immunosuppression, increased risk of opportunistic infection, and osteoporosis (Nguyen et al., 2019). Thus, novel therapeutics that can provide long-term benefits and are well tolerated in AD patients are urgently needed.
Emerging studies have demonstrated that traditional Chinese medicine provides desirable therapeutic effects in inflammatory diseases. Huangbai Liniment (HB) is a standardized medicinal product clinically prescribed for use in dermatology and wound management (Liu et al., 2020; Zhang et al., 2020). It is a compound traditional Chinese medicine that includes forsythia, honeysuckle, phellodendron, dandelion, and centipede. In clinical trials, HB has been reported to exert profound anti-inflammatory effects during wound healing in several chronic inflammatory diseases, such as diabetic foot ulcers (Liu et al., 2020; Zhang et al., 2020), ulcerative colitis (Xiao et al., 2009), and traumatic infection (Li-yun, 2016). Mechanistically, experimental and clinical data indicate that HB accelerates diabetic wound healing via activation of Nrf2 and its downstream antioxidant genes (Zhang et al., 2020). Although the protective effects of HB are reported in many mucosal damage and inflammatory diseases in clinical therapy, the therapeutic effects and mechanism of HB in ameliorating dermatitis remains unclear. In this study, we investigated the therapeutic potential of HB in controlling inflammatory dermatoses in a DNCB-induced AD mouse model following clinical observations in AD patients and found that HB markedly alleviated skin inflammation and reinvigorated TREG-induced immune balance in AD.
Materials and Methods
Patient
Using the Hanifin and Rajka AD diagnostic guidelines and the eczema area and severity index (EASI) AD guidelines as standard, patient was selected from the diagnostic clinic of Dongzhimen Hospital for AD diagnosis. AD patient had no history or family history of allergic disease. Patient had not received steroid or immunosuppressive therapy and had no history of parasitic infection 2 weeks before the examination. The study design was approved by the Ethics committee of Beijing University of Chinese Medicine (NO. ECSL-BDY-2012-45-01). Written informed consent was obtained from patient included in the study.
Animals
All experimental 6–8 week-old BALB/c female mice were purchased from Vital River Laboratories (VRL, Beijing, China), and maintained at the Shandong Analysis and Test Center at a controlled temperature of 23 °C ± 2°C, and a humidity of 50 ± 10%. All animal work was in accordance with protocols approved by the Animal Experimentation Ethics committee of Shandong Analysis and Test Center (NO. ECAESDATC-2018-010) and followed the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Reagents
The HB (lot number: 18010111) used in this study was supplied by Shandong Hanfang Pharmaceutical Co., Ltd (Chinese medicine character: Z10950097). Hydrocortisone butyrate cream and 2, 4-Dinitrochlorobenzene (DNCB) were purchased from Jinyao Medical & Pharmaceutical Company Ltd. and Shanghai Chemical Reagent Company Ltd., respectively. The mouse antibodies against specific antigens used in flow cytometry analysis were purchased from BD Biosciences (San Jose, CA, United States) or BioLegend (San Diego, CA, United States). Recombinant murine IL-1β, IL-2, IL-4, IL-6, TGF-β, and IL-12 were purchased from PeproTech (Rocky Hill, NJ, United States). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, United States).
Experimental 1-Chloro-2,4-Dinitrobenzene-Induced AD Mouse Model
For induction of AD-like skin lesions in an experimental mouse model, mice were topically sensitized with 100 μl of 1% DNCB, diluted in a 4:1 mixture of acetone and olive oil, on shaved dorsal skin three times a week. From day five, mice were challenged with 0.4% DNCB on the dorsal skin (100 μl) and right ear (10 μl) every 3 days. The HB or hydrocortisone was applied by hydropathic compress on treatment group mice twice a day for 20 days (400 μl/day), and the control animals received the same volume of phosphate-buffered saline (PBS). At the end of the model construction, ear thickness measurements were performed with dial calipers as previously described (KimKim et al., 2013; Kim et al., 2014). For behavioral assessments, mice were acclimated to the recording cage and then evaluated for their scratching behavior. Total numbers of scratching bout per 10 min were record manually by an assessor who was blind to the experimental design. One scratching bout was defined as one instance of lifting the forepaw from the floor, scratching, and returning the paw to the floor or placing the paw around the animal’s mouth (Oetjen et al., 2017). The severity of dermatitis was evaluated according to symptoms, including scaling, erythema, erosion, and edema. Each symptom was scored as 0 (no symptom), 1 (mild), 2 (moderate), or 3 (severe). After 20 days, at the end of the DNCB treatment, murine ear skin tissues and dorsal skin tissues were fixed in 4% paraformaldehyde (PFA) and subjected to histopathological analysis and other related experiments. Representative data are from one of three independent experiments (n ≥ 5 in each group).
Skin Histology
Dorsal skin tissues were fully fixed with 4% paraformaldehyde at the endpoint before embedding in paraffin, sectioning, and staining with hematoxylin and eosin (H&E). Morphological evaluation of inflammatory infiltrates, and structural alterations were determined at a magnification of ×200 or ×50. To score the skin histopathology, inflammatory cell infiltration, epidermal thickness, and hair follicles were assessed blind-based using a five-point grading system and evaluated by blind scoring from 1 to 5, resulting in an average pathology score for each section. The infiltrated cells and the thickness of the epidermis or dermis were measured. Five randomized fields were counted for each section slide and calculated from five animals. All histology results were measured by blind evaluation.
Quantitative Real-Time Polymerase Chain Reaction
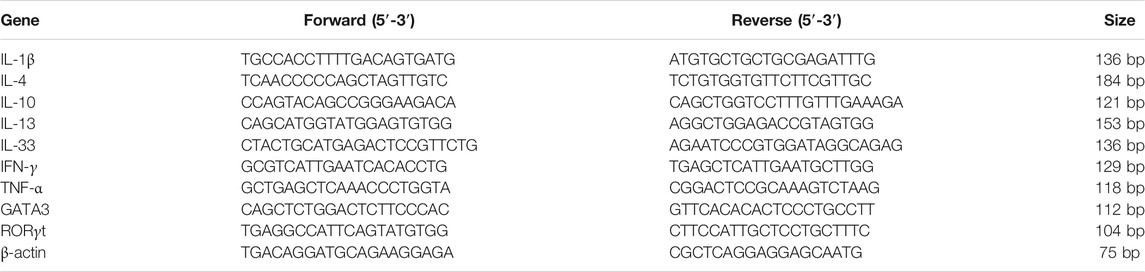
Total RNA was purified from skin tissue using TRIzol reagent (Invitrogen), and cDNA was synthesized from a total of 5 μg of RNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific) and amplified using qPCR according to the manufacturer’s instructions. All qPCRs were run on the Roche real-time system using SYBR Green Master Mix (Roche) and normalized with β-Actin. The sequences of the primers used for qPCR are shown in Table 1.
In vitro Polarization
Naïve CD4+ T cells were sorted from the spleens of healthy female 8 week-old BALB/c mice using a flow cytometer (BD FACS Aria III, BD Biosciences). The purity of the naïve CD4+ T cells was >98%. Sorted naïve CD4+ T cells (50,000 cells per well) were cultured in a 96-well culture plate pre-coated with anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) and polarized under TH1 (IL-2 20 ng/ml, anti-IL-4 10 μg/ml and IL-12 20 ng/ml), TH2 (IL-2 20 ng/ml, anti-IFN-γ 10 μg/ml, anti-IL-12 10 μg/ml, and IL-4 20 ng/ml), TH17 (IL-2 20 ng/ml, IL-6 50 ng/ml, TGF-β 1ng/ml, IL-1β 10 ng/ml, anti-IL-4 10 μg/ml, and anti-IFN-γ 10 μg/ml), or TREG cell (IL-2 20 ng/ml and TGF-β 2 ng/ml) differentiation conditions with or without 1/1000 H B for 4 days.
MTT Assay
Sorted naïve CD4+ T cells were cultured with 20 ng/ml IL-2 with or without 1/1000 HB for 2 days, MTT solution was then added, and the sorted cells were incubated in the dark. After a 6 h incubation, solubilization buffer (10% SDS in 0.01 M HCl) was added, and the cells were cultured overnight. Cell proliferation was measured at an OD value of 570 nm.
Flow Cytometry
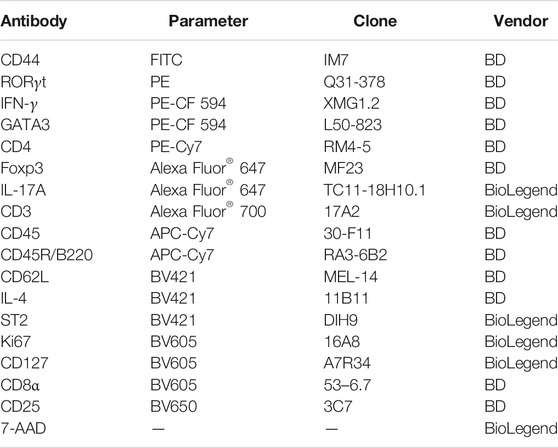
Mouse splenocytes were stained with Fc-receptor blocking antibodies (clone 2.4G2, 1:100 dilution, BD Biosciences) for 10 min on ice to block non-specific staining. For surface staining, cells were washed once with PBS containing 2% heat-inactivated fetal bovine serum (FBS, Gibco) and stained in an appropriately diluted antibody solution for 40 min at 4 C. Dead cells were excluded after staining with 7-amino-actinomycin D (7-AAD, BioLegend). For intracellular staining of cytokines, PMA and ionomycin stimulated cells were washed once after surface staining and permeabilized using Cytofix/Cytoperm (BD Biosciences) for 40 min on ice. The antibodies were diluted in Perm/Wash Buffer (BD), and stained in an appropriately diluted antibody solution for 1 h at 4 C. For intranuclear staining of transcription factors, cells were washed once after surface staining and permeabilized using Foxp3/Transcription Factor Staining Buffer Set (eBioscience) for 40 min on ice. The specific antibodies were diluted in Fixation/Permeabilization buffer (eBioscience) and incubated for 1 h at 4 C. Data were collected on a BD FACS Aria III flow cytometer (BD Biosciences) and analyzed using FlowJo (BD Biosciences) software. Antibody information is presented in Table 2.
Statistical Analysis
All data are expressed as Standard Error of the Mean (SEM) and are representative of three independent experiments. In the heatmap diagram, Z-score is measured in terms of standard deviations from the mean. The z-score is calculated by subtracting the population mean from the raw score, then dividing the difference by the population standard deviation. Groups were compared using unpaired Student’s t-tests and one-way analysis of variance (ANOVA). Differences were considered to be statistically significant at p < 0.05.
Results
Huangbai Liniment Ameliorates Dermatitis in AD Patient and a 1-Chloro-2,4-Dinitrobenzene-Induced AD Mouse Model
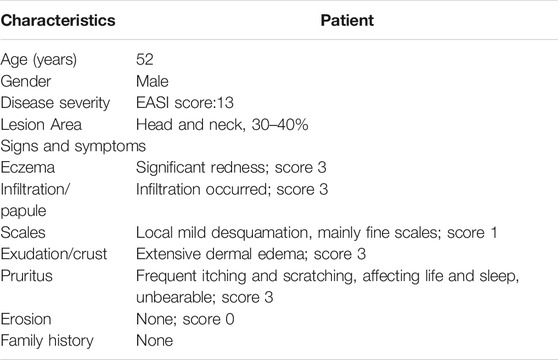
HB is a common dermatological medicinal product clinically prescribed to treat allergic contact dermatitis and eczema (Li and Gong, 2014; Yao and Zhao, 2014); however, its mechanism remains unclear. To evaluate the clinical efficacy and safety of HB in the treatment of AD, we locally applied hydropathic compresses of HB on lesioned skin of an AD patient. The AD patient presented with features of skin inflammation, including pruritus, erythema exudativum, and a mixed dermal inflammatory infiltrate around the head and neck region. The EASI score was around 13, and the lesion area was approximately 30–40% (Table 3). Several treatments had previously failed, and the patient had not received steroid or immunosuppressive therapy. The hydropathic compress of HB was administered to the lesioned skin region of the patient 3 times a day for 15 days. After the treatment, the AD patient showed marked improvement in AD clinical characteristics, including lessened random redness and limited itch symptoms (Figure 1A). These findings suggested that HB could be beneficial to patients with chronic skin inflammation who might be resistant to conventional treatments.
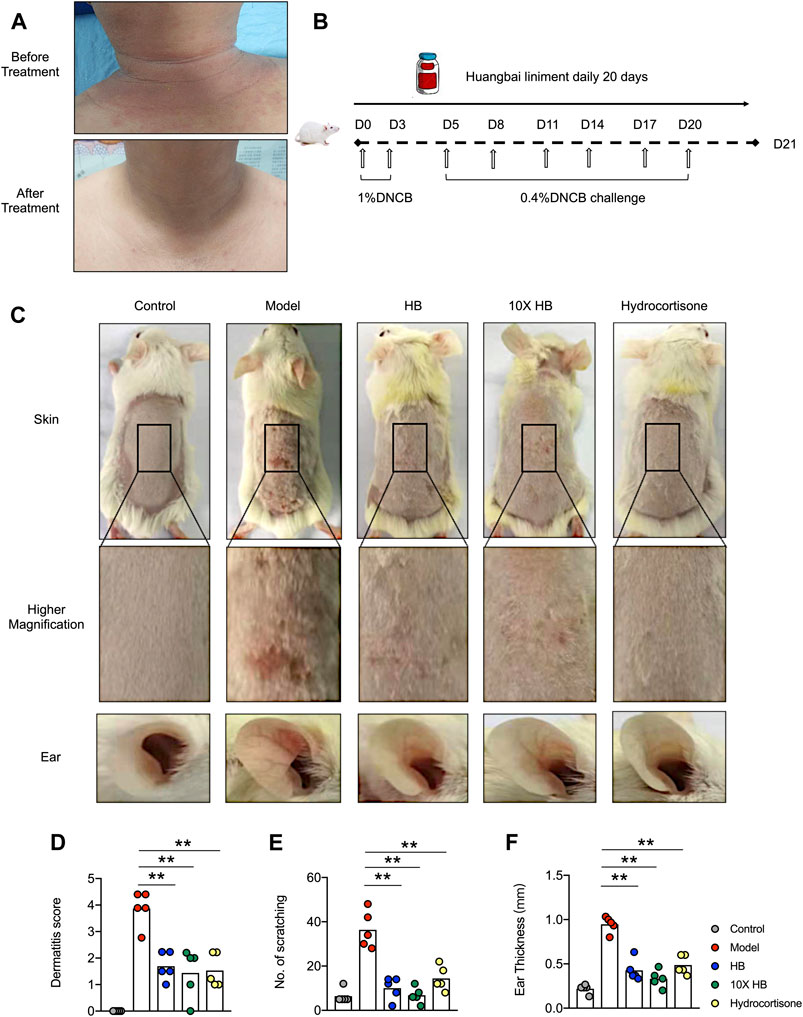
FIGURE 1. HB ameliorates the symptoms of AD in clinical AD patient and mice. (A) HB was topically applied to a patient with AD 3 times a day for a continuous 15 days. Representative image of dermatitis on the patient before and after treatment with HB. After HB application, the eczema on the skin of the AD patient’s neck was obviously ameliorated and the skin condition altered. (B) Schematic for (C–F). Mice were sensitized with 1% DNCB on dorsal skin at days 0 and 3 and then challenged with 0.4% DNCB on the dorsum of ears at 3 day intervals from days 5 to 20. HB, 10X HB (a 10-fold concentration of HB), hydrocortisone, or PBS was topically applied daily to the dorsal skin of the DNCB-induced mice. (C) Representative images of dorsal skins and ears in each group. The severity of AD was evaluated, and statistics are shown in (D). (E,F) The amount of scratching and ear thicknesses were recorded at the endpoint, and statistics are shown. Each dot represents one mouse from one of three independent experiments, and bars indicate mean values. Statistical significance was determined by one-way ANOVA, *p < 0.05, **p < 0.01.
To investigate the underlying mechanism of HB on AD, a DNCB-induced AD mouse model was used (Figure 1B). Following DNCB treatment, the ears of the mice became red and swollen, and the dorsal skin showed severe erosion, erythema, and dryness. A variety of skin inflammation parameters and pathology were compared at the endpoint of the DNCB-induced AD mouse model. Interestingly, we found these atopic skin lesions were markedly attenuated by HB and 10-fold concentrated HB. The efficiency of HB was comparable with hydrocortisone, a common steroid medicine, which is widely used in the treatment of different dermatitis types (Figure 1C). Consistent with the photographic images of the skin lesions of the AD patient, the dorsal skin of the mice had obviously relieved edema, redness, and erosion following HB treatment (Figure 1D). HB also suppressed scratching behavior due to defused itching and skin lesions in the DNCB-induced AD mouse model group (Figure 1E, Supplementary Figure S1A) and ear thickness was also significantly thinner than in the DNCB-induced AD mouse model group (Figure 1F, Supplementary Figure S1B). Together, these results demonstrate that HB profoundly ameliorated the clinical symptoms of AD in mice.
Huangbai Liniment Improves Symptoms of AD Through the Inhibition of Local Inflammatory Cytokines and Allergic Reactions
The improvements in the atopic symptoms of mice in the DNCB-induced AD mouse model group following HB and conventional hydrocortisone treatments were confirmed by morphological analysis of H&E-stained sections from damaged dorsal skin at the endpoint (Figure 2A). The DNCB-induced AD mouse model group exhibited the typical microscopic characteristics of dermatitis, such as hyperkeratosis, thickened epidermal tissue, and immune cell infiltration. However, in skin tissue from HB and 10-fold concentrated HB treated mice, the epidermal and dermal tissues were markedly thinner than tissue from untreated mice. Furthermore, the infiltration of inflammatory cells was also significantly reduced by HB treatment (Figures 2B–E). Accordingly, the pathology score, mRNA expression of inflammation, and the type 2 cytokine-related genes, including IL-1β, TNF-α, IL-17A, IFN-γ, IL-4, IL-13, and IL-33, were largely downregulated by HB treatment. In contrast, HB increased the expression of anti-inflammatory cytokine IL-10 (Figure 2F). Moreover, the mRNA expression of TH2 and TH17 classical transcription factors, GATA3 and RORγt, were suppressed in HB-treated mice compared with untreated mice (Figures 2F,G). Collectively, these data suggest that HB improves AD and largely rescues the excessive expression of cytokines critical for skin inflammation and the atopic response.
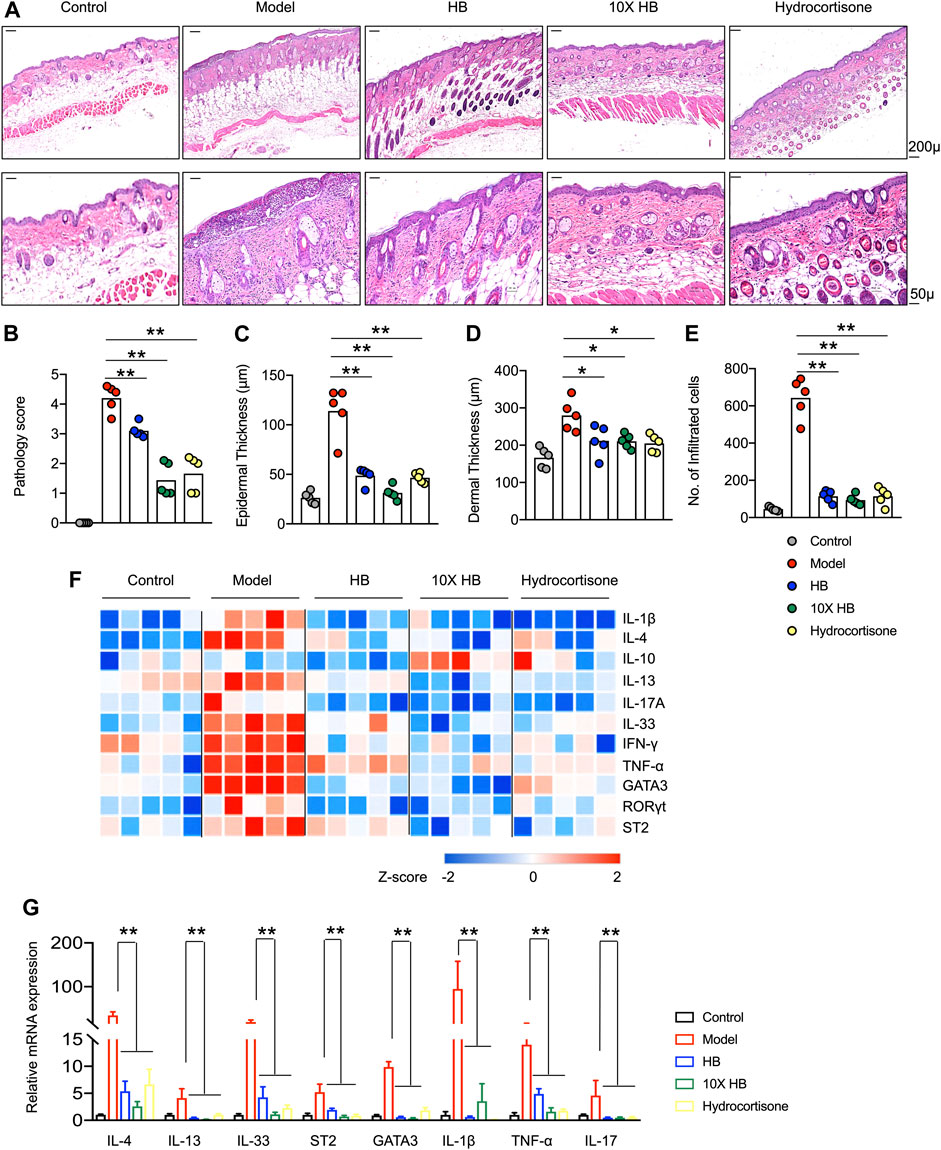
FIGURE 2. HB reduces AD-like skin inflammation and the type 2 immune response induced by DNCB in mice. Mice were sensitized with 1% DNCB on dorsal skin at days 0 and 3 and then challenged with 0.4% DNCB on the dorsum of both ears at 3 day intervals from days 5 to 20. HB, 10X HB (a 10-fold concentration of HB), hydrocortisone, or PBS was topically applied daily to the dorsal skin of these DNCB-induced mice. (A) Histopathology of mouse dorsal skin tissue was evaluated by H&E staining (scale bars, 50 or 200 μM). Representative images in each group are shown. Pathology scores were blindly evaluated, and statistics are shown in (B). (C–E) ImagePro10 software was used to measure and calculate epidermal thickness (C), dermal thickness (D), and number of infiltrated cells (E). (F) Gene expression in skin tissue from five individual mice in each group, measured by qPCR and shown in a heatmap. Blue represents low expression, and red represents high expression of each inflammatory gene compared between three groups, with Row Z-Score (−2–2). (G) Type 2 cytokines and transcription factors in skin tissues were measured by qPCR. Each dot represents one mouse from one of three independent experiments, and bars indicate mean values. Statistical significance was determined by one-way ANOVA, *p < 0.05, **p < 0.01.
Huangbai Liniment Relieves Skin Inflammation by Modulating the Immune Balance of TH17/TREG Cells
To clarify the underlying mechanism of HB-based immunomodulation, we analyzed mouse splenocytes in different treatment groups using multi-color flow cytometry to characterize the phenotype of CD4+ T cell subsets in DNCB-induced skin inflammation. We established gating strategies based on the key cytokines produced by each cell type to evaluate the different CD4+ T cell subsets (Figure 3A). Intriguingly, we found altered frequencies of CD4+ T cells in the different groups. Specifically, HB treatment reduced the frequency of CD4+ T cells that were substantially reduced in the DNCB-induced AD mouse model group (Figure 3B), while no difference was observed in the frequencies of CD8+ T cells (Figure 3C), which led to an increased CD4/CD8 ratio in the DNCB-induced AD mouse model group after HB treatment (Figure 3D). Furthermore, using the gating strategy of naïve (CD25−CD62L+CD44low), effector memory (CD25−CD62LlowCD44+), TREG cells (CD25+Foxp3+) CD4+T cells (Figure 3A), we noted a comparable frequency of naïve CD4 T cells (Figure 3E), but a significant reduction in effector memory CD4 T cells in HB-treated mice compared with untreated mice (Figure 3F). TREG cells have been shown to control excessive T cell responses in inflammation, atopic responses, and AD-like inflammation (Ou et al., 2004; Fyhrquist et al., 2012). Interestingly, we also noticed that HB increased the frequency of TREG cells in the DNCB-induced AD mouse model group, which partially constituted the underlying mechanisms of HB-induced improvement of skin inflammation in our DNCB-induced AD mouse model (Figure 3H).
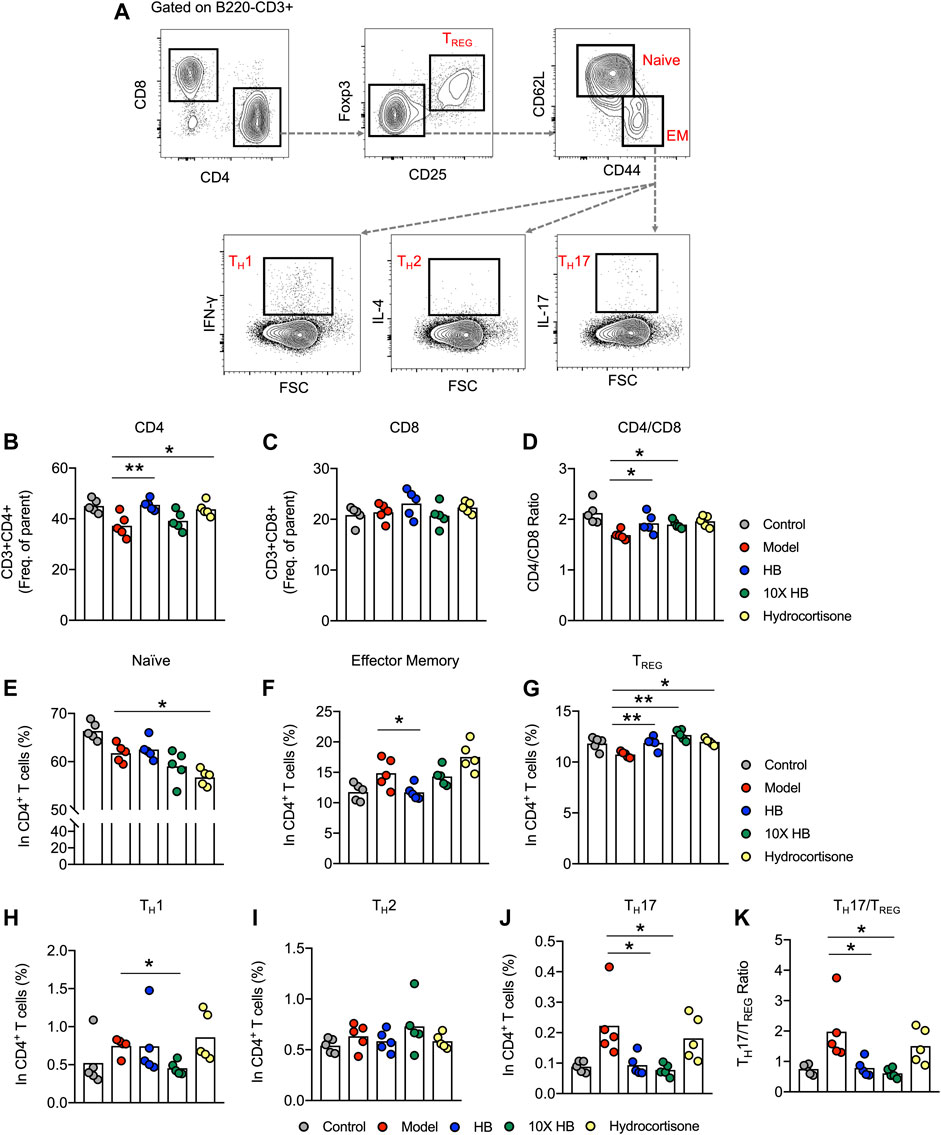
FIGURE 3. HB relieves skin inflammation by downregulation of the TH17/TREG ratio. Mice were sensitized with 1% DNCB on dorsal skin at days 0 and 3, and then challenged with 0.4% DNCB on the dorsum of both ears at 3 day intervals from days 5 to 20. HB, 10X HB (a 10-fold concentration of HB), hydrocortisone, or PBS, was topically applied daily to the dorsal skin of the DNCB-induced mice. Splenocytes of AD model mice treated with PBS, HB, 10X HB, and hydrocortisone were isolated and stained using immunofluorescence antibodies. (A) Gating strategy for different T cell subsets. (B,C) Statistical analysis of the frequencies of B220−CD3+CD4+ T cells (B) and B220−CD3+CD8+ T cells (C) in different groups. (D) CD4/CD8 ratio. (E–J) Statistical analysis showing the differences in the frequencies of naïve (E) and effector memory (F) CD4 T cells and TREG(G), TH1 (H), TH2 (I), and TH17 (J) cells. (K) The TH17/TREG ratio was calculated to show the differences in different groups. Each dot represents one mouse from one of three independent experiments, and bars indicate mean values. Statistical significance was determined by one-way ANOVA, *p < 0.05, **p < 0.01.
Effector CD4+ T cells are known to contribute to the pathology of AD. TH1, TH2, and TH17 are examined by gating on CD25−CD4+CD44+ T cells and through the expression of their signature cytokines (TH1: IFN-γ+CD44+; TH2: IL-4+CD44+; TH17: IL-17A+CD44+). We found that HB treatment reduced the frequency of TH17 and slightly suppressed the TH1 subset in the DNCB-induced AD mouse model group (Figures 3I,K). Comparable frequencies were observed for TH2 cells in untreated and HB-treated mice (Figure 3J). TH17/TREG imbalance is widely reported in autoimmune, inflammatory, and allergic diseases (Ma et al., 2014). We next analyzed the percentage of TH17 and TREG cells in different mouse groups. The ratio of TH17/TREG was compared between untreated and HB-treated mice, and a significant reduction in the ratio was noticed in HB-treated mice (Figure 3L). Taken together, we reveal that HB treatment plays an important role in controlling AD-like inflammation by potentiating TREG cells while reducing TH17 cells in vivo.
Huangbai Liniment Directly Promotes TREG Cells and Inhibits Effector T Cell Subsets in vitro
To further evaluate whether HB can directly affect the differentiation of different T cell subsets, we in vitro polarized purified naïve CD4+ T cells into TH1, TH2, TH17, and TREG cells and added HB to the cell culture (Figure 4A). Based on the cell survival data, HB did not affect the survival of naive CD4+ T cells when cultured in TH0 conditions for 48 h (Figure 4B). In line with the in vivo data, we found that HB directly suppressed the generation of TH1, TH2, and TH17 cells and promoted TREG differentiation in cell culture with altered cell proliferation indicated by Ki67 (Figures 4C–F). This mechanistic evidence indicates that HB directly promotes TREG cells while largely inhibiting the generation of TH1 and TH17 cells.
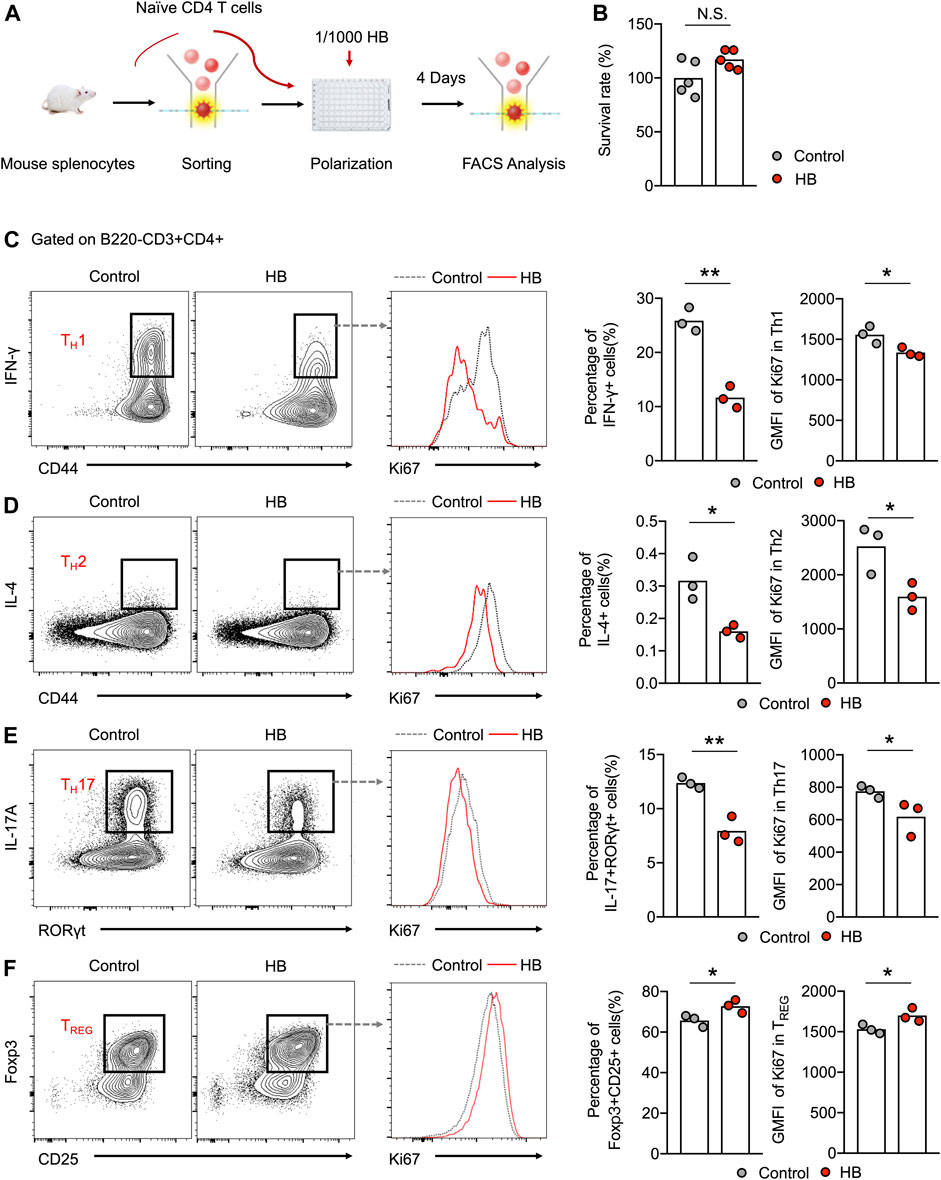
FIGURE 4. HB directly potentiates regulatory T cell activation and depresses inflammatory cells in vitro. (A) Schematic in vitro experiments. Naïve CD4 T cells, derived from mouse splenocytes, were sorted by flow cytometry and polarized under different culture conditions with or without 1/1000 HB for 4 days. (B) Survival analysis of HB for naïve CD4 cells was performed using an MTT assay after 48 h of culture with or without 1/1000 H B. (C–F) Representative FACS plots and statistical analysis of in vitro polarized TH1 (C), TH2 (D), TH17 (E), and TREG(F) cells with or without HB treatment. Histogram plots and statistical analysis indicated the changes in Ki67 in different CD4 subsets after HB treatment. Each dot represents one mouse from one of three independent experiments, and bars indicate mean values. Statistical significance was determined by Student’s t-test, *p < 0.05, **p < 0.01.
Discussion
AD is a chronic relapsing inflammatory skin disease characterized by an impaired immune response (Auriemma et al., 2013). The relapsing and persistent itch triggers a self-perpetuating itch-scratch cycle, which can have a significant impact on the patient’s quality of life, and effective treatments are limited (Oetjen et al., 2017). HB was reported to have therapeutic effects on different types of inflammation in patients, especially in those patients with eczema (Li and Gong, 2014; Yao and Zhao, 2014; Li-yun, 2016). However, it is unknown whether HB could be used to treat skin inflammation, such as AD, and if so, what are the underlying mechanisms of HB-mediated anti-inflammatory effects. In this study, we first examined the therapeutic potential of HB in a patient with AD and found that HB profoundly mitigated skin inflammation in the patient. Further, we investigated the therapeutic effects of HB on DNCB-induced AD in mice and examined its immune-modulative effects on CD4+ T cell subsets. In mice with DNCB-induced AD, HB markedly alleviated skin inflammation and inhibited proinflammatory immune responses mediated by Th1 and Th17 cells. This evidence suggests that HB effectively mitigates skin inflammation in mice with DNCB-induced AD by restoring the balance of the CD4+ T cell response. Our study also provides insights for the future development and application of HB-based immune-modulation of AD.
Type 2 cytokines, such as IL-4, IL-5, and IL-13 coordinate inflammatory and atopic responses in AD (Bieber, 2010). In our study, we found that HB markedly suppressed mRNA expression of IL-4, IL-13, and the transcription factor GATA3 known to drive immune cells to produce a type 2 immune response in dermatitis skin tissue. IL-33 and its receptor ST2 are strongly associated with the development of AD disease triggered by allergen exposure, irritants, scratching, and the bacterial and viral infections seen in this condition (Savinko et al., 2012). We noticed that that HB inhibits DNCB-induced IL-33 and ST2 mRNA levels in dermatitis skin tissue. Because ST2 is also expressed on innate lymphoid cells, such as ILC2 cells that are known to synergize with TH2 cells to induce type 2 inflammation (Artis and Spits, 2015; Eberl et al., 2015; Zhou et al., 2021), it will be interesting in future studies to know whether HB could directly regulate ILC2s to control skin inflammation in AD mouse models and patients. IFN-γ mRNA and protein were highly expressed in eczematous skin in the vast percentage of AD patients (Leung et al., 2011; Weidinger and Novak, 2016). More importantly, the in situ expression of IFN-γ decreased with the successful treatment of AD patients (Grewe et al., 1998). We also found that HB could largely reduce the mRNA expression of IFN-γ in skin tissue from mice with DNCB-induced AD. Together, our data indicate that HB suppresses the overall production of inflammatory cytokines in the skin of mice with DNCB-induced AD-like inflammation.
An imbalanced immune response is one of the major causes of AD. An excessive accumulation of activated T lymphocytes in the skin drives severe inflammation and induces destruction of the skin tissue integrity. Several effector subsets of CD4 T cells have been identified as potent regulators of AD pathogenesis, including TH1, TH2, TH17, and TREG cells (Grewe et al., 1998; Martel et al., 2016). We found that HB treatment restored the CD4/CD8 T cell ratio in mice with DNCB-induced AD-like inflammation. Our data also suggested that DNCB-induced AD-like inflammation in mice was associated with reduced naïve cells and increased effector memory CD4+ T cells, and this imbalance was largely rescued after HB treatment. Activated TH2 cells prominently mediated the development of AD-like skin inflammation, manifesting as enhanced IgE-mediated sensitization and eosinophil infiltration (Bieber, 2010). Other effector T cell subsets, such as proinflammatory cells TH1, TH9, TH17, and TH22, produced a large number of proinflammatory cytokines, including IFN-γ, IL-9, IL-17, and IL-22, driving the development of AD. This promotion of effector CD4+ T cells was often associated with a decline in the presence of TREG cells that are essential for immune tolerance and control of inflammation in AD (Auriemma et al., 2013). Our study further revealed that HB could potentiate TREG cells while suppressing other effector CD4+ T cell subsets both in vivo and in vitro, which at least partially constituted the mechanisms of HB-mediated improvement in AD.
Th17 cells are critical for the development of AD (Koga et al., 2008). The frequency of IL-17-producing CD4+T cells in AD patients is increased and associated with AD severity (Esaki et al., 2016). In addition, IL-17 was reported to trigger the production of IL-4 by TH2 cells (Milovanovic et al., 2010). Mice lacking IL-17A exhibited reduced dermatitis together with less IL-4 and IgE production (Milovanovic et al., 2010). In line with these reports, our study showed that the frequency of Th17 cells was drastically reduced by HB in vitro and in vivo. The local expression of Th17-related cytokines and transcription factors were also shown to be suppressed by qPCR. These findings support the notion that HB directly regulates the differentiation of TH17 cells, could have immune-modulative effects in other inflammatory diseases and might be beneficial in controlling lesioned skin of sclerosis (Ahmed et al., 2019) or psoriasis, which are largely IL-17-driven diseases (Noda et al., 2015).
Interestingly, TH17 and T follicular helper (TFH) cells produce IL-21, which promotes TH17 cell differentiation (Ouyang et al., 2008; Gong et al., 2019), and IL-21 is increased in AD lesions in humans and mice (Jin et al., 2009). Moreover, IL-21 suppresses the production of IgE and possesses anti-inflammatory effects (Ozaki et al., 2002). The administration of IL-21 not only reduces the frequency of TH2 cells but also suppresses the secretion of TH2-associated cytokines, such as IL-4, IL-5, and IL-13 (Lin et al., 2015). Additionally, TFH cells predominantly produce IL-2, IL-4 and IL-21 in B cell follicles and closely regulate antibody class-switching in severe inflammatory and allergic diseases, including AD, asthma, and COVID-19-induced airway inflammation (Yao et al., 2018; Crotty, 2019; Gong et al., 2019; Papillion et al., 2019; Yao et al., 2019; Zhou et al., 2019; Gong et al., 2020). Further, circulating TFH cells are associated with disease severity in children with AD (Gong et al., 2019; Marschall et al., 2021). These accumulating findings indicate the importance of TFH cells in the context of AD and other inflammatory diseases. It is thus of interest to know whether HB treatment also regulates TFH cells in the context of AD in the DNCB-induced AD mouse model and patients.
In contrast to the reduction in proinflammatory CD4+ T cell subsets, HB enhanced TREG cells in vivo and in vitro. In addition to the TH1/TH2 paradigm, the imbalance of TREG and TH17 cells is another cardinal mechanism in AD pathogenesis (Ma et al., 2014). Our data importantly showed that HB potently restored this imbalance in mice with DNCB-induced AD. Future studies should further demonstrate the potential molecular insights underlying HB-mediated direct regulation of TREG cells and other effector CD4+ T cell subsets, including but not limited to transcriptional regulation, epigenetic changes, and metabolic alterations.
In summary, our findings indicate that HB treatment improves AD in patients and can ameliorate skin inflammation in mice with DNCB-induced AD. HB profoundly reduced the expression of proinflammatory cytokines, including IL-1β, IL-4, IFN-γ, IL-13, and IL-17, and increased the expression of anti-inflammatory cytokine IL-10. Furthermore, we demonstrated that HB reduced skin inflammation and inhibited TH17 cells while promoting TREG cells. In line with in vivo data, HB directly inhibited TH1, TH2, and TH17 differentiation and promoted TREG polarization in vitro. Altogether, this study provides mechanistic insights into HB-mediated amelioration of AD through the restoration of CD4+ T cells and sheds light on the broad use of HB in treating inflammatory diseases.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics committee of Beijing University of Chinese Medicine (NO. ECSL-BDY-2012-45-01). The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by the Animal Experimentation Ethics committee of Shandong Analysis and Test Center (NO. ECAESDATC-2018-010). Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
TZ conceived and oversaw the study. TZ and PZ designed the experiments. TZ, MF, and AX performed the experiments. TZ, YW, JF, PZ, and XS analyzed the data. CQ and DY participated in scientific discussions. TZ and PZ wrote the manuscript and prepared the figures. PZ revised the manuscript. TZ led the submission.
Funding
This project was supported by the National Natural Science Foundation of China (81970759, 82101920, 81803539), Key Technology Research and Development Program of Shandong (2018YYSP022).
Conflict of Interest
The authors disclose that this project is partially funded by Shandong Hanfang Pharmaceutical Co. Ltd., who also provided Huangbai Liniment (Chinese Medicine Character: Z910950097) for this research. The authors promise that the authenticity and conclusions of this study are not affected by the funding source. CQ was also a paid consultant for Shandong Hanfang Pharmaceutical Co. Ltd.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Xuding Sun and Jizhen Zhao from Shandong Hanfang Pharmaceutical Co. Ltd. for supplying Huangbai Liniment.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.726035/full#supplementary-material
References
Ahmed, S., Misra, D. P., and Agarwal, V. (2019). Interleukin-17 Pathways in Systemic Sclerosis-Associated Fibrosis. Rheumatol. Int. 39 (7), 1135–1143. doi:10.1007/s00296-019-04317-5
Artis, D., and Spits, H. (2015). The Biology of Innate Lymphoid Cells. Nature 517 (7534), 293–301. doi:10.1038/nature14189
Auriemma, M., Vianale, G., Amerio, P., and Reale, M. (2013). Cytokines and T Cells in Atopic Dermatitis. Eur. Cytokine Netw. 24 (1), 37–44. doi:10.1684/ecn.2013.0333
Biedermann, T., Skabytska, Y., Kaesler, S., and Volz, T. (2015). Regulation of T Cell Immunity in Atopic Dermatitis by Microbes: The Yin and Yang of Cutaneous Inflammation. Front. Immunol. 6, 353. doi:10.3389/fimmu.2015.00353
Crotty, S. (2019). T Follicular Helper Cell Biology: a Decade of Discovery and Diseases. Immunity 50 (5), 1132–1148. doi:10.1016/j.immuni.2019.04.011
Dainichi, T., Kitoh, A., Otsuka, A., Nakajima, S., Nomura, T., Kaplan, D. H., et al. (2018). The Epithelial Immune Microenvironment (EIME) in Atopic Dermatitis and Psoriasis. Nat. Immunol. 19 (12), 1286–1298. doi:10.1038/s41590-018-0256-2
Eberl, G., Colonna, M., Di Santo, J. P., and McKenzie, A. N. (2015). Innate Lymphoid Cells. Innate Lymphoid Cells: a New Paradigm in Immunology. Science 348 (6237), aaa6566. doi:10.1126/science.aaa6566
Esaki, H., Brunner, P. M., Renert-Yuval, Y., Czarnowicki, T., Huynh, T., Tran, G., et al. (2016). Early-onset Pediatric Atopic Dermatitis Is TH2 but Also TH17 Polarized in Skin. J. Allergy Clin. Immunol. 138 (6), 1639–1651. doi:10.1016/j.jaci.2016.07.013
Fyhrquist, N., Lehtimäki, S., Lahl, K., Savinko, T., Lappeteläinen, A. M., Sparwasser, T., et al. (2012). Foxp3+ Cells Control Th2 Responses in a Murine Model of Atopic Dermatitis. J. Invest. Dermatol. 132 (6), 1672–1680. doi:10.1038/jid.2012.40
Gittler, J. K., Shemer, A., Suárez-Fariñas, M., Fuentes-Duculan, J., Gulewicz, K. J., Wang, C. Q., et al. (2012). Progressive Activation of T(H)2/T(H)22 Cytokines and Selective Epidermal Proteins Characterizes Acute and Chronic Atopic Dermatitis. J. Allergy Clin. Immunol. 130 (6), 1344–1354. doi:10.1016/j.jaci.2012.07.012
Gong, F., Zheng, T., Zhou, P., et al. (2019). T Follicular Helper Cell Subsets and the Associated Cytokine IL-21 in the Pathogenesis and Therapy of Asthma. Front. Immunol. 10, 2918. doi:10.3389/fimmu.2019.02918
Gong, F., Dai, Y., Zheng, T., Cheng, L., Zhao, D., and Wang, H. (2020). Peripheral CD4+ T Cell Subsets and Antibody Response in COVID-19 Convalescent Individuals. J. Clin. Invest. 130 (12), 6588–6599. doi:10.1172/JCI141054
Grewe, M., Schopf, E., Bruijnzeel-Koomen, C. A., Thepen, T., Langeveld-Wildschut, A. G., Ruzicka, T., et al. (1998). A Role for Th1 and Th2 Cells in the Immunopathogenesis of Atopic Dermatitis. Immunol. Today 19 (8), 359–361. doi:10.1016/s0167-5699(98)01285-7
Imai, Y., Yasuda, K., Sakaguchi, Y., Haneda, T., Mizutani, H., Yoshimoto, T., et al. (2013). Skin-specific Expression of IL-33 Activates Group 2 Innate Lymphoid Cells and Elicits Atopic Dermatitis-like Inflammation in Mice. Proc. Natl. Acad. Sci. U S A. 110 (34), 13921–13926. doi:10.1073/pnas.1307321110
Jin, H., Oyoshi, M. K., Le, Y., Bianchi, T., Koduru, S., Mathias, C. B., et al. (2009). IL-21R Is Essential for Epicutaneous Sensitization and Allergic Skin Inflammation in Humans and Mice. J. Clin. Invest. 119 (1), 47–60. doi:10.1172/JCI32310
Li, H., and Gong, H. (2014). Clinical Study of Fufang Huangbai Liquid in Treating Acute and Sub-acute Aczema. J. Nanjing University TCM 30 (5), 492–494.
Kim, B. S., Wang, K., Siracusa, M. C., Saenz, S. A., Brestoff, J. R., Monticelli, L. A., et al. (2014). Basophils Promote Innate Lymphoid Cell Responses in Inflamed Skin. J. Immunol. 193 (7), 3717–3725. doi:10.4049/jimmunol.1401307
Kim, I. S., Lee, S. H., Kwon, Y. M., Adhikari, B., Kim, J. A., Yu, D. Y., et al. (2019). Oral Administration of β-Glucan and Lactobacillus Plantarum Alleviates Atopic Dermatitis-like Symptoms. J. Microbiol. Biotechnol. 29 (11), 1693–1706. doi:10.4014/jmb.1907.07011
KimKim, B. S., Siracusa, M. C., Siracusa, S. A., Noti, M., Monticelli, L. A., Sonnenberg, G. F., et al. (2013). TSLP Elicits IL-33-independent Innate Lymphoid Cell Responses to Promote Skin Inflammation. Sci. Transl Med. 5 (170), 170ra16. doi:10.1126/scitranslmed.3005374
Koga, C., Kabashima, K., Shiraishi, N., Kobayashi, M., and Tokura, Y. (2008). Possible Pathogenic Role of Th17 Cells for Atopic Dermatitis. J. Invest. Dermatol. 128 (11), 2625–2630. doi:10.1038/jid.2008.111
Lei, H., Schmidt-Bleek, K., Dienelt, A., Reinke, P., and Volk, H. D. (2015). Regulatory T Cell-Mediated Anti-inflammatory Effects Promote Successful Tissue Repair in Both Indirect and Direct Manners. Front. Pharmacol. 6, 184. doi:10.3389/fphar.2015.00184
Leung, D. Y., Boguniewicz, M., Howell, M. D., Nomura, I., and Hamid, Q. A. (2004). New Insights into Atopic Dermatitis. J. Clin. Invest. 113 (5), 651–657. doi:10.1172/JCI21060
Leung, D. Y., Gao, P. S., Grigoryev, D. N., Rafaels, N. M., Streib, J. E., Howell, M. D., et al. (2011). Human Atopic Dermatitis Complicated by Eczema Herpeticum Is Associated with Abnormalities in IFN-γ Response. J. Allergy Clin. Immunol. 127 (4), 965–5. doi:10.1016/j.jaci.2011.02.010
Li-yun, X. X. (2016). Clinical Efficacy of Compound Fluid of Cortex Phellodendri in the Treatment of Traumatic Infections. Chin. J. New Drugs 25 (20), 2349–2352.
Lin, P. Y., Jen, H. Y., Chiang, B. L., Sheu, F., and Chuang, Y. H. (2015). Interleukin-21 Suppresses the Differentiation and Functions of T Helper 2 Cells. Immunology 144 (4), 668–676. doi:10.1111/imm.12419
Liu, Y., Li, Y., Du, Y., Huang, T., and Zhu, C. (2020). Multicenter Clinical Trials Analyzing Efficacy and Safety of Topical Cortex Phellodendri Compound Fluid in Treatment of Diabetic Foot Ulcers. Med. Sci. Monit. 26, e923424. doi:10.12659/MSM.923424
Looman, K. I. M., van Meel, E. R., Grosserichter-Wagener, C., Vissers, F. J. M., Klingenberg, J. H., de Jong, N. W., et al. (2020). Associations of Th2, Th17, Treg Cells, and IgA+ Memory B Cells with Atopic Disease in Children: The Generation R Study. Allergy 75 (1), 178–187. doi:10.1111/all.14010
Ma, L., Xue, H. B., Guan, X. H., Shu, C. M., Wang, F., Zhang, J. H., et al. (2014). The Imbalance of Th17 Cells and CD4(+) CD25(high) Foxp3(+) Treg Cells in Patients with Atopic Dermatitis. J. Eur. Acad. Dermatol. Venereol. 28 (8), 1079–1086. doi:10.1111/jdv.12288
Marschall, P., Wei, R., Segaud, J., Yao, W., Hener, P., German, B. F., et al. (2021). Dual function of Langerhans cells in skin TSLP-promoted TFH differentiation in mouse atopic dermatitis. J Allergy Clin. Immunol. 147 (5), 1778–1794. doi:10.1016/j.jaci.2020.10.006
Martel, B. C., Dyring-Andersen, B., Skov, L., Thestrup-Pedersen, K., Skov, S., Skak, K., et al. (2016). Different Cytokine Profiles of Skin-Derived T Cell Cultures from Patients with Atopic Dermatitis and Psoriasis. Inflamm. Res. 65 (4), 265–272. doi:10.1007/s00011-015-0912-z
Milovanovic, M., Drozdenko, G., Weise, C., Babina, M., and Worm, M. (2010). Interleukin-17A Promotes IgE Production in Human B Cells. J. Invest. Dermatol. 130 (11), 2621–2628. doi:10.1038/jid.2010.175
Nguyen, H. L., Anderson, K. R., and Tollefson, M. M. (2019). New and Emerging Therapies for Pediatric Atopic Dermatitis. Paediatr. Drugs 21 (4), 239–260. doi:10.1007/s40272-019-00342-w
Noda, S., Suárez-Fariñas, M., Ungar, B., Kim, S. J., de Guzman Strong, C., Xu, H., et al. (2015). The Asian Atopic Dermatitis Phenotype Combines Features of Atopic Dermatitis and Psoriasis with Increased TH17 Polarization. J. Allergy Clin. Immunol. 136 (5), 1254–1264. doi:10.1016/j.jaci.2015.08.015
Nograles, K. E., Zaba, L. C., Shemer, A., Fuentes-Duculan, J., Cardinale, I., Kikuchi, T., et al. (2009). IL-22-producing "T22" T Cells Account for Upregulated IL-22 in Atopic Dermatitis Despite Reduced IL-17-producing TH17 T Cells. J. Allergy Clin. Immunol. 123 (6), 1244–e2. doi:10.1016/j.jaci.2009.03.041
Oetjen, L. K., Mack, M. R., Feng, J., Whelan, T. M., Niu, H., Guo, C. J., et al. (2017). Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 171 (1), 217–e13. doi:10.1016/j.cell.2017.08.006
Ou, L. S., Goleva, E., Hall, C., and Leung, D. Y. (2004). T Regulatory Cells in Atopic Dermatitis and Subversion of Their Activity by Superantigens. J. Allergy Clin. Immunol. 113 (4), 756–763. doi:10.1016/j.jaci.2004.01.772
Ouyang, W., Kolls, J. K., and Zheng, Y. (2008). The Biological Functions of T Helper 17 Cell Effector Cytokines in Inflammation. Immunity 28 (4), 454–467. doi:10.1016/j.immuni.2008.03.004
Ozaki, K., Spolski, R., Feng, C. G., Qi, C. F., Cheng, J., Sher, A., et al. (2002). A Critical Role for IL-21 in Regulating Immunoglobulin Production. Science 298 (5598), 1630–1634. doi:10.1126/science.1077002
Papillion, A., Powell, M. D., Chisolm, D. A., Bachus, H., Fuller, M. J., Weinmann, A. S., et al. (2019). Inhibition of IL-2 Responsiveness by IL-6 Is Required for the Generation of GC-TFH Cells. Sci. Immunol. 4 (39). doi:10.1126/sciimmunol.aaw7636
Rostaher, A., Fischer, N. M., Urwyler, A., and Favrot, C. (2018). Circulating CD4(+)CD25(+)Foxp3(+) T Regulatory Cell Levels in an Experimental Model of Canine Atopic Dermatitis. Vet. Dermatol. 29 (6), 511–e171. doi:10.1111/vde.12693
Savinko, T., Matikainen, S., Saarialho-Kere, U., Lehto, M., Wang, G., Lehtimäki, S., et al. (2012). IL-33 and ST2 in Atopic Dermatitis: Expression Profiles and Modulation by Triggering Factors. J. Invest. Dermatol. 132 (5), 1392–1400. doi:10.1038/jid.2011.446
Suárez-Fariñas, M., Tintle, S. J., Shemer, A., Chiricozzi, A., Nograles, K., Cardinale, I., et al. (2011). Nonlesional Atopic Dermatitis Skin Is Characterized by Broad Terminal Differentiation Defects and Variable Immune Abnormalities. J. Allergy Clin. Immunol. 127 (4), 954–964 e14. doi:10.1016/j.jaci.2010.12.1124
Weidinger, S., and Novak, N. (2016). Atopic Dermatitis. Lancet 387 (10023), 1109–1122. doi:10.1016/S0140-6736(15)00149-X
Xiao, J., He, W., Li, J., and Xia, B. (2009). Investigation on Therapeutic Mechanism of Compound Huangbai Solution in TNBS-Induced Colitis in Rats. Gastroenterology 14 (8), 473–477. doi:10.3969/j.issn.1008-7125.2009.08.007
Yao, J., and Zhao, X. (2014). Advance in Clinical Application of Compound Phellodendron Liquid. Chin. J. New Drugs 23 (3), 308–337.
Yao, Y., Chen, C. L., Wang, N., Wang, Z. C., Ma, J., Zhu, R. F., et al. (2018). Correlation of Allergen-specific T Follicular Helper Cell Counts with Specific IgE Levels and Efficacy of Allergen Immunotherapy. J. Allergy Clin. Immunol. 142 (1), 321–e10. doi:10.1016/j.jaci.2018.03.008
Yao, Y., Wang, Z. C., Wang, N., Zhou, P. C., Chen, C. L., Song, J., et al. (2019). Allergen Immunotherapy Improves Defective Follicular Regulatory T Cells in Patients with Allergic Rhinitis. J. Allergy Clin. Immunol. 144 (1), 118–128. doi:10.1016/j.jaci.2019.02.008
Zhang, J., Zhou, R., Xiang, C., Jia, Q., Wu, H., and Yang, H. (2020). Huangbai Liniment Accelerated Wound Healing by Activating Nrf2 Signaling in Diabetes. Oxid Med. Cel Longev 2020, 4951820. doi:10.1155/2020/4951820
Zhou, P., Liang, K., and Yu, D. (2019). Germinal center TFH Cells: T(w)o Be or Not T(w)o Be, IL-6 Is the Answer. Sci. Immunol. 4 (39). doi:10.1126/sciimmunol.aay7668
Keywords: atopic dermatitis, huangbai liniment (HB), CD4 T cells, regulatory T (treg) cell, immunomodulation
Citation: Zheng T, Fan M, Wei Y, Feng J, Zhou P, Sun X, Xue A, Qin CX and Yu D (2021) Huangbai Liniment Ameliorates Skin Inflammation in Atopic Dermatitis. Front. Pharmacol. 12:726035. doi: 10.3389/fphar.2021.726035
Received: 16 June 2021; Accepted: 19 August 2021;
Published: 31 August 2021.
Edited by:
Zhaocheng Ma, Huazhong Agricultural University, ChinaReviewed by:
Masanori Fujii, Kyoto Pharmaceutical University, JapanMarc Christophe Karam, University of Balamand, Lebanon
Copyright © 2021 Zheng, Fan, Wei, Feng, Zhou, Sun, Xue, Qin and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Zheng, dGluZzA4MTIyNkBob3RtYWlsLmNvbQ==
 Ting Zheng
Ting Zheng Miao Fan
Miao Fan Yunbo Wei
Yunbo Wei Jinhong Feng1,2
Jinhong Feng1,2 Pengcheng Zhou
Pengcheng Zhou Cheng Xue Qin
Cheng Xue Qin Di Yu
Di Yu