
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 28 July 2021
Sec. Pharmacology of Anti-Cancer Drugs
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.724923
A correction has been applied to this article in:
Corrigendum: NLRP3 Overexpression Associated With Poor Prognosis and Presented as an Effective Therapeutic Target in Osteosarcoma
 Zhen Huang1,2,3
Zhen Huang1,2,3 Hui Chen4
Hui Chen4 Shenglin Wang3
Shenglin Wang3 Hongxiang Wei3
Hongxiang Wei3 Xinwen Wang5
Xinwen Wang5 Rongkai Shen3
Rongkai Shen3 Yunqing Wang3
Yunqing Wang3 Rongjin Lin6
Rongjin Lin6 Jianhua Lin2,3*
Jianhua Lin2,3*Despite the development of diagnostic and treatment strategies, the survival outcome of patients with osteosarcoma remains poor. Nod-like receptor protein 3 (NLRP3) plays a crucial role in the inflammasome pathway, which is related to the progression of various tumors. However, the effect of NLRP3 on osteosarcoma has not yet been well explored. Our study aimed to investigate the role of NLRP3 in the malignant biological behavior of osteosarcoma as well as its therapeutic value. Immunohistochemistry was applied to investigate the NLRP3 expression in osteosarcoma and osteochondroma specimens. Cell Counting Kit-8, colony formation, wound healing, transwell, and flow cytometry assays were used to explore the contribution of NLRP3 to the proliferation, migration, invasion, apoptosis and cell cycle distribution of osteosarcoma cells in vitro. Western blot was performed to evaluate the expression of NLRP3 and the related proteins in osteosarcoma cell lines after the blockade of NLRP3 using CY-09 and lentivirus intervention. Furthermore, tumor formation assay was used to analyze the effect of NLRP3 on the growth of osteosarcoma in vivo. The results showed that the NLRP3 protein was overexpressed in osteosarcoma, which was independently correlated with the poor prognosis of patients. Moreover, NLRP3 suppression by the inhibitor of CY-09 or lentivirus-induced gene knockdown inhibited the cell proliferation, migration, invasion and promoted the cell apoptosis and G1 cell cycle arrest in osteosarcoma via targeting the inflammasome pathway. Our in vivo results confirmed that the inhibition of NLRP3 suppressed the tumor formation of osteosarcoma. In conclusion, NLRP3 may be regarded as an independent prognostic biomarker and a potential therapeutic target for osteosarcoma.
Osteosarcoma is the most common primary aggressive bone tumor that usually occurs during childhood and adolescence (Chow et al., 2020). With the development of novel therapies, the five-year survival rate of patients with osteosarcoma has improved from 20 to 70%. However, nearly 30% of osteosarcoma patients are prone to metastasis or recurrence, with an unfavorable prognosis, due to the limited efficacy of current treatment strategies (Dai et al., 2011). Therefore, exploring novel biomarkers and therapeutic targets for the treatment of osteosarcoma is critical.
Nod-like receptor protein 3 (NLRP3) has been intensively investigated for its potential role in various human diseases (Yang et al., 2019; Lin et al., 2020). The NLRP3 protein was also reported to play a vital role in activating and promoting of human cancers, including oral squamous cell carcinoma Wang et al. (2018), endometrial cancer Liu et al. (2019), and breast cancer (Li et al., 2020). According to Xue et al. (2019) patients with NLRP3 overexpression was found to have a poorer overall survival and disease-free survival five years after surgery in laryngeal squamous cell carcinoma, indicating NLRP3 serving as an auxiliary indicator for the long-term prognosis in patients with laryngeal squamous cell carcinoma. However, the prognostic and therapeutic values of NLRP3 protein have not been fully analyzed in osteosarcoma. Therefore, our study aimed to evaluate the expression of NLRP3 in osteosarcoma and its association with clinicopathological parameters of patients, and further explore the in vitro and in vivo effects of NLRP3 on the biological behaviors of osteosarcoma.
In total, 55 patients with primary osteosarcoma and 30 patients with osteochondroma who have undergone surgical resection at our hospital were selected between January 1, 2012, and December 31, 2015. The clinicopathological parameters, including sex, age, tumor size, tumor location, histological subtype, Enneking stage, response to chemotherapy, and distant metastasis status, were recorded. The date of diagnosis to the date of first tumor progression was defined as progression-free survival (PFS), and the date of diagnosis to the date of death was defined as overall survival (OS). Patients were censored if tumor progression or death did not occur in the last follow-up. All patients participating in our study signed an informed consent form, and the study was conformed with the ethical guidelines of the Declaration of Helsinki.
NLRP3 protein expression in osteosarcoma and osteochondroma specimens was detected by immunohistochemical staining using a PV9000 immunohistochemical kit (OriGene Technologies, Inc., Beijing, China). Following deparaffinization, hydration, and blockage of endogenous peroxidase activity by 3% hydrogen peroxide, the specimen sections underwent antigen retrieval process in citrate buffer (pH 6.0) and were incubated overnight at 4°C with antibodies against NLRP3 (Affinity, United States) and Ki-67 (Affinity). Then, the sections were incubated with poly-peroxidase-anti-mouse/rabbit IgG and stained with diaminobenzidine (OriGene Technologies), followed by counterstaining with hematoxylin and dehydration. The results of IHC were evaluated by two pathologists independently. The grade of NLRP3 protein expression was scored from 0 to 3 according to the staining intensity of osteosarcoma cells (0, negative; 1, weakly positive; 2, moderately positive; 3, strongly positive). The mean percentage of positive osteosarcoma cells was also scored from 1 to 3 (1, <25%; 2, 25%–75%; 3, >75%) (Wang et al., 2018). The final scores were the grade multiplied by the percentage score of tumor cells, giving final scores of 0, 1, 2, 3, 4, 6, 9. Scores greater than 2 (3, 4, 6, 9) were considered as high expression, whereas those below or equal to 2 (0, 1, 2) were defined as low expression (Srirajaskanthan et al., 2010).
The human fetal osteoblastic cells hFOB 1.19 and human osteosarcoma cells MNNG/HOS and Saos2 were purchased from the Typical Culture Preservation Committee of the Chinese Academy of Sciences (Shanghai, China). 143B, MG63, and U2OS cell lines were obtained from the American Type Culture Collection (Manassas, VA, United States). The 143B, U2OS, MNNG/HOS and MG63 cells were cultured in Dulbecco’s modified Eagle’s medium (Biological Industries, Israel), and the Saos2 cells were was cultured in Roswell Park Memorial Institute 1,640 medium (Biological Industries). All medium were supplemented with 10% fetal bovine serum (FBS; Biological Industries) and 1% penicillin/streptomycin (Biological Industries) in an atmosphere of 5% CO2 at 34°C (hFOB 1.19) or 37°C (MNNG/HOS, MG63, 143B, U2OS and Saos2 cells).
Negative control vector sequences (sh-NC: TTCTCCGAACGTGTCACGT) and NLRP3 lentivirus sequences (sh-NLRP3: GTTAGAAACACTTCAAGAA) were obtained from GeneChem (Shanghai, China). Approximately 2 × 105 cells/well were seeded into six-well plates. Then, the medium was replaced with 1 °ml fresh medium containing an appropriate amount of lentiviral suspension with an infection multiplicity of 50. After 24-h incubation, lentivirus-containing medium were replaced with fresh complete medium. The cells were incubated for another 48 h and green fluorescence was observed. Then, the cells were treated with puromycin for 2°weeks to screen stably transfected clones. The infection efficiency was further evaluated using western blot analysis.
The cell proteins were extracted by using a Membrane and Cytosol Protein Extraction Kit (Beyotime Biotechnology, Shanghai, China). Then the proteins were then quantified using a Pierce™ Microplate BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, United States). A total of 20 μg proteins were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and then transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA, United States). All membranes were cultured overnight at 4°C with primary antibodies against NLRP3 (Affinity), caspase-1 (Affinity), gasdermin d (GSDMD, Affinity), IL-1β (Affinity), IL-18 (Affinity) and tubulin (Affinity) after blockage by 5% skim milk for 1 h. Then the membranes were incubated with appropriate secondary antibodies (Affinity) at room temperature for 1 h. Finally, the blots were detected by using an ECL Western Blotting Substrate (Affinity) and FluorChem R detection system (ProteinSimple, United States).
Cell Counting Kit-8 (CCK-8; New Cell & Molecular Biotech, China) was used to measure the cell viability of osteosarcoma. Briefly, the 143B, MG63, MNNG/HOS, Saos2, and U2OS cell lines were seeded into 96-well plates at a density of 1.5 × 103 cells/well and cultured at 37°C for 24°h, followed by incubation in a medium containing different concentrations of CY-09 for 24, 48, and 72 h. Cells with lentivirus infection were cultured at a density of 1.5 × 103 cells/well on a 96-well plate for 24, 48, 72, and 96°h, followed by the supplement of 100 μL medium which containing 10 μL of CCK-8 solution to each well and then incubation at 37°C for 2 h. Absorbance (OD450) was detected at a wavelength of 450 nm using a microplate reader (ELx800; BioTek Instruments, United States). The experiment was carried out in triplicate.
Colony formation assay was applied to detect the effect of NLRP3 inhibition on cell proliferation. 143B and U2OS cells were seeded into six-well plates at a density of 200 cells/well and treated with different concentrations of CY-09 (0, 5, 10, and 20 μM). Simultaneously, 143B and U2OS cells in sh-NLRP3 and sh-NC groups were seeded into six-well plates at a density of 200 cells/well. After incubation for seven days, the cells were fixed with ice-cold methanol for 20 min and stained with crystal violet (Solarbio, Beijing, China). Colonies including more than 50 cells were counted using an optical microscope (Carl Zeiss, Germany). The experiment was carried out in triplicate.
Both 143B and U2OS cells were incubated at a density of 5 × 105 cells/well in six-well plates for 24 h. Then, a 200 μL sterile pipette was used to make scratches, and cells were then cultured with CY-09 at concentrations of 0, 5, 10, and 20 μM in FBS-free medium. Simultaneously, 143B and U2OS cells in sh-NLRP3 and sh-NC groups (5 × 105 cells/well) were seeded into six-well plates and scratched with a 200 μL sterile pipette, and cultured in FBS-free medium. Images of wound healing were then acquired at the time of wounding and, 12, 24, and 36 h later. The experiment was carried out in triplicate.
Transwell assay was conducted to explore the cell migration and invasion of osteosarcoma using chamber with 8-μm pores (Corning Inc., Corning, NY, United States). For invasion assay, the upper chamber was precoated with Matrigel solution for 2 h (BD Biosciences, Franklin Lakes, NJ, United States). Cells (1 × 105 cells/well) pretreated with CY-09 or lentivirus infection were cultured in the upper chamber in 100 μl of FBS-free medium, with 600 μL of complete medium adding to the lower chamber in 24-well plates. After incubation for 24°h, the cells remaining in the upper side of chamber (non-migrating or non-invading cells) were removed. The migrated or invaded cells in the lower side were fixed with methanol for 20°min, stained with 0.1% crystal violet (Solarbio) for 30 min and counted under the inverted microscope. The experiment was performed in triplicate.
143B and U2OS cells were seeded with approximately 2 × 105 cells/well into six-well plates and incubated with different concentrations of CY-09 (0, 5, 10 and 20 μM). sh-NLRP3 and sh-NC cells were cultured on six-well plates at the same density as before (2 × 105 cells/well). After incubation for 48°h, a Cell Cycle and Apoptosis Analysis Kit (Beyotime Biotechnology) was applied to detect cell apoptosis and cell cycle changes in 143B and U2OS cells. Then the samples were assessed through a flow cytometer (BD Biosciences), and evaluated using the Accuri C6 software (BD Biosciences). The experiment was conducted in triplicate.
The animal experiments were conducted according to the Committee of Animal Ethics of our hospital. Nude BALB/c mice (four to six weeks old) were purchased from the Charles River (Beijing, China). To investigate the effect of CY-09 on the tumor growth, 143B cells (2 × 106/mouse) were injected into the left subcutaneous region of the mice. After the tumor volume reached 200 mm3, CY-09 (10 mg/kg) or DMSO was injected into the tail vein every day for the first 3°days and then every second day for 20 consecutive days. Tumor size was measured every week after administration. To investigate the effect of NLRP3 knockdown on the tumor growth, sh-NLRP3 and sh-NC cells (2 × 106 cells/mouse) were injected into the left subcutaneous region of the mice. Tumor size was measured every week after transplantation. Then, the mice were sacrificed and tumor tissues underwent H&E staining, and Ki-67 immunohistochemical staining to examine the progression viability in different groups.
The statistical analysis was conducted using SPSS software version 19.0 (SPSS, Chicago, United States). Student’s t-test or One-way ANOVA was applied for two-group comparisons. The survival status was investigated using log-rank test and Kaplan-Meier survival plots. Cox proportional hazards regression was performed for multivariate analysis of parameters which were significant in univariate analysis. Proportional hazards assumption was validated via the log-minus-log-survival curves and survival times against cumulative survival using SPSS software version 19.0. The Harrell concordance index (C-index) was applied to evaluate the accuracy of the model. The optimal cutoff value of the NLRP3 expression scores for PFS and OS was determined by receiver operating characteristic (ROC) curve and Youden Index (Schisterman et al., 2005). p-values less than 0.05 were considered statistically significant.
IHC was first used to investigate the expression of NLRP3 in 55 osteosarcoma and 30 osteochondroma tissues. In total, high expression of NLRP3 protein was found in 45.45% (25/55) of the osteosarcoma tissues whereas not in the osteochondroma tissues. The difference in the NLRP3 expression between osteosarcoma and osteochondroma specimens was significant (Figure 1A). The western blotting assay showed that NLRP3 protein was upregulated in MG63, MNNG/HOS, 143B, U2OS and Saos2 cells compared to the hFOB 1.19 cells, with 143B and U2OS cells exhibiting the highest level (Figure 1B). Furthermore, high expression of NLRP3 was significantly related to poor response to chemotherapy (Table 1, p = 0.021) and distant metastasis (Table 1, p = 0.013). Univariate and multivariate analyses were performed to assess the contribution of NLRP3 high expression to PFS and OS in osteosarcoma. According to univariate analysis, NLRP3 high expression (PFS, p < 0.001; OS, p < 0.001; Figure 1C), response to chemotherapy (PFS, p = 0.047; OS, p = 0.002), and distant metastasis (PFS, p = 0.002; OS, p = 0.003) were closely related to PFS and OS (Table 2). The multivariate analysis further confirmed the independent prognostic value of NLRP3 protein overexpression for PFS (hazard ratio (HR) = 2.92, p = 0.002, C-index = 0.70) and OS (HR = 4.09, p = 0.017, C-index = 0.79) in osteosarcoma patients (Table 3). The ROC curve and Youden index revealed that the optimal cutoff value of NLRP3 protein expression for PFS was 3.0 (area under the curve = 0.712, p = 0.026, sensitivity = 55.8%, specificity = 91.7%, Youden’s index = 0.475), and OS was 3.0 (area under the curve = 0.817, p < 0.001, sensitivity = 78.9%, specificity = 72.2%, Youden’s index = 0.515) (Figure 1D; Table 4). Our definition of NLRP3 expression level according to immunohistochemical scores was consistent with the optimal cutoff value for PFS and OS. Therefore, high expression of NLRP3 protein may be considered as an independent prognostic biomarker for osteosarcoma.
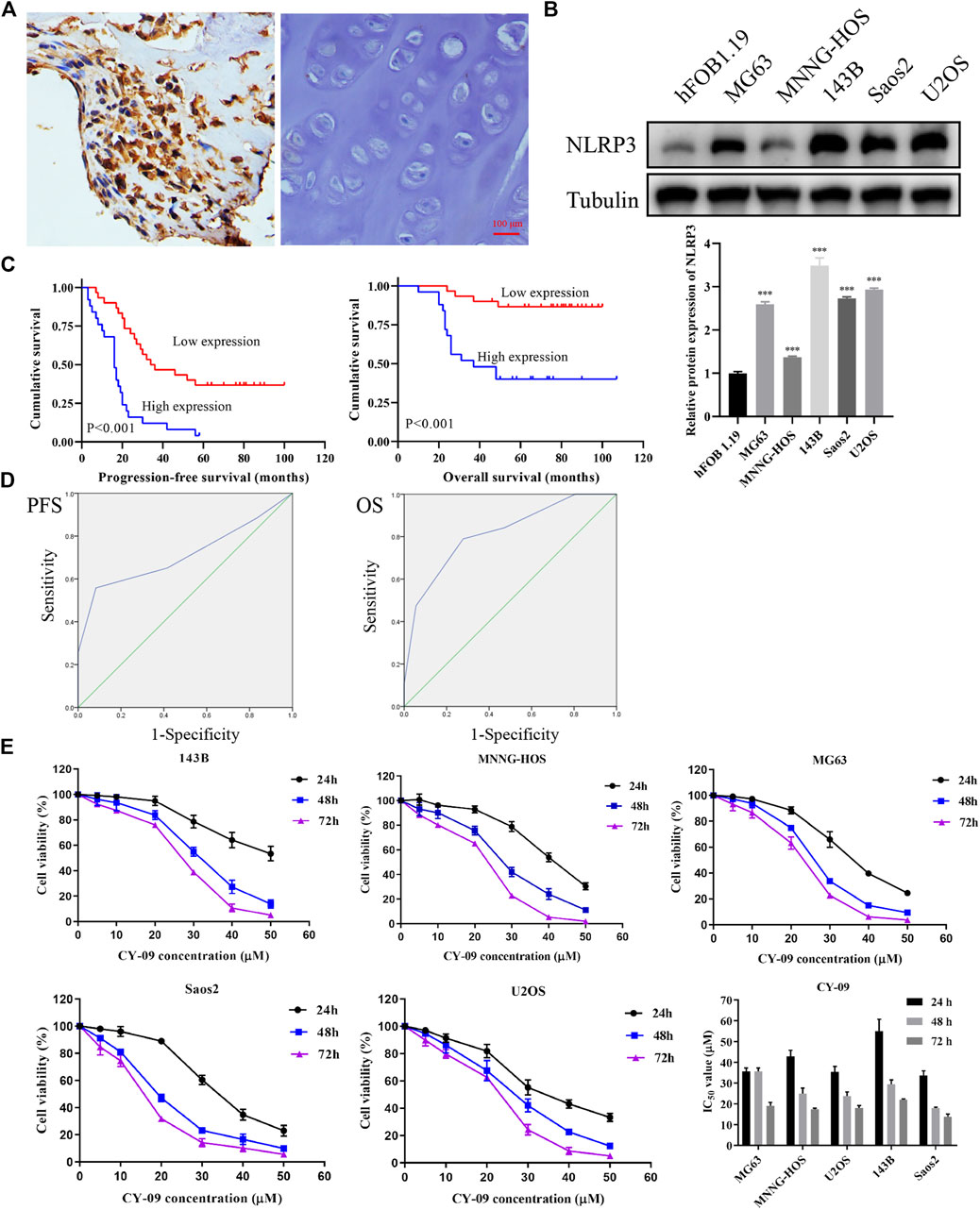
FIGURE 1. NLRP3 expression in osteosarcoma and cell viability changes after CY-09 administration (A) NLRP3 expression in osteosarcoma and osteochondroma tissues (B) NLRP3 expression in hFOB 1.19 and osteosarcoma cell lines (C) Prognostic value of NLRP3 for patients with osteosarcoma (D) ROC curves for PFS and OS in osteosarcoma with different expression scores of NLRP3 protein (E) Cell viability changes in osteosarcoma cells after treatment with different concentrations of CY-09. The NLRP3 expression in cells and the cell viability were measured in triplicate.
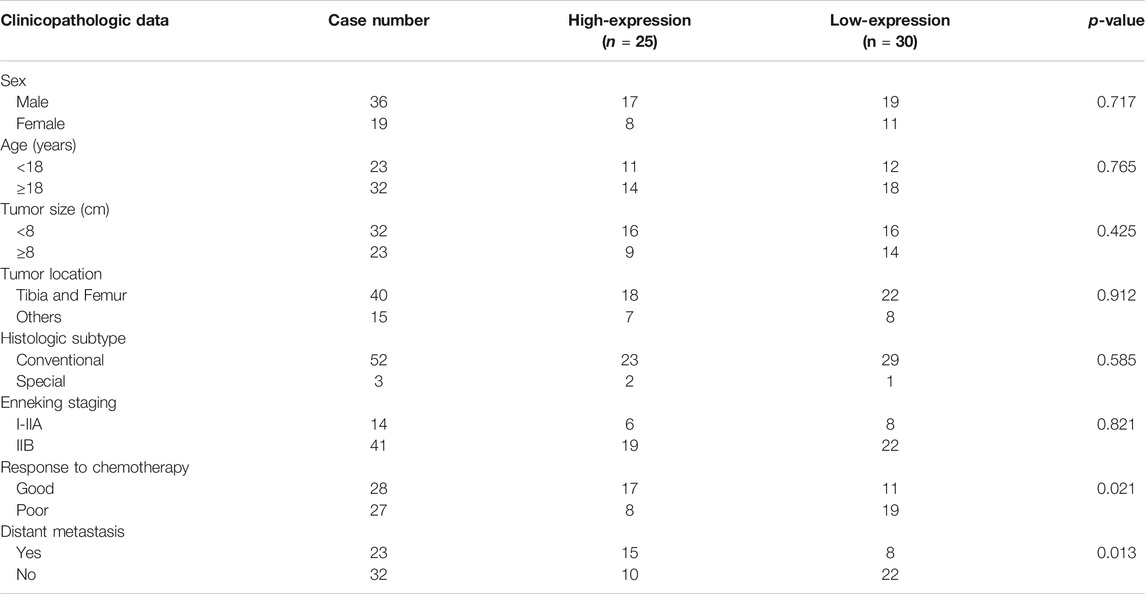
TABLE 1. Association between NLRP3 expression and clinicopathological characteristics in osteosarcoma.

TABLE 4. ROC curve analysis for PFS and OS in osteosarcoma with different expression scores of NLRP3 protein.
According to the CCK-8, the osteosarcoma cell viability gradually decreased with the increase of CY-09 (NLRP3 inhibitor) concentration and the treatment time (Figure 1D). Colony formation assay revealed that the proliferation ability was suppressed with the increase of the CY-09 concentration (Figure 2A). The transwell assay demonstrated that the migration and invasion abilities of 143B and U2OS cells were also inhibited by increasing the CY-09 concentration (Figure 2B). The suppression of migration ability by CY-09 was also validated by the wound healing assay (Figure 2C).
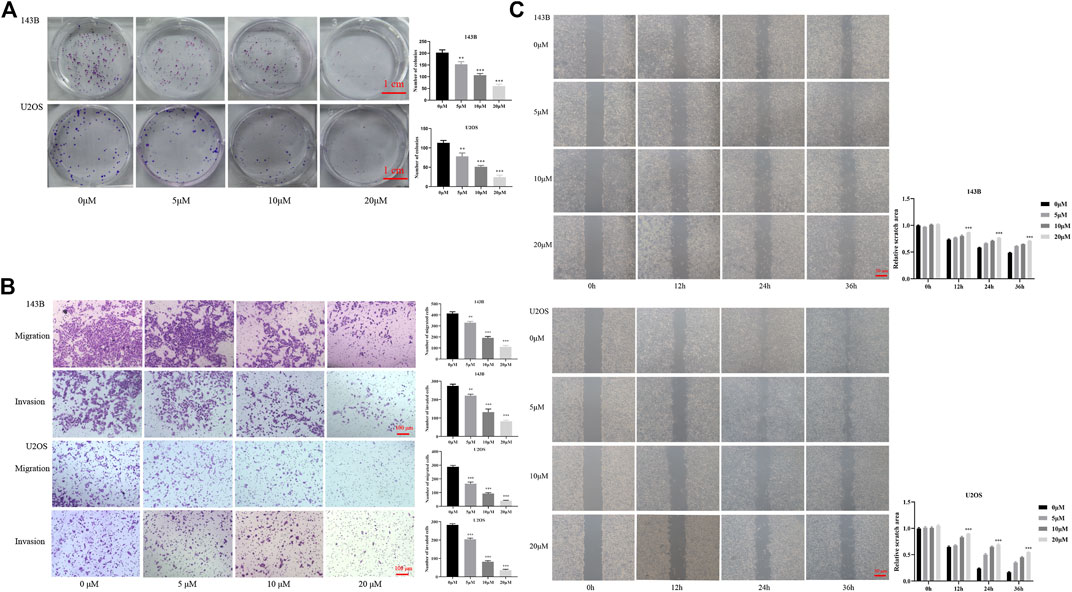
FIGURE 2. Effect of CY-09 on cell proliferation, migration, and invasion in osteosarcoma (A) Cell proliferation of 143B and U2OS cells detected by colony formation assay after treatment with different concentrations of CY-09 for seven days (B) Cell migration and invasion of 143B and U2OS cells were examined using the transwell assay after treatment with different concentrations of CY-09 (C) Cell migration of 143B and U2OS cells was evaluated using the wound healing assay after treatment with different concentrations of CY-09 for 0, 12, 24, and 36 h. The experiments were conducted in triplicate.
To further confirm the target therapy value of NLRP3 for osteosarcoma, lentivirus infection was performed to reduce the expression of the NLRP3 gene in 143B and U2OS cells. Green fluorescence showed the high infection efficiency of lentivirus in 143B and U2OS cells (Figure 3A). Western blot analysis demonstrated decreased NLRP3 protein in 143B and U2OS cells in the NLRP3 knockdown groups (sh-NLRP3) compared to their respective control groups (sh-NC) (Figure 3B). The cell viability was significantly reduced in NLRP3-knockdown 143B and U2OS cells (Figure 3C). Similarly, colony formation assay showed decreased proliferation ability in 143B and U2OS cells after the knockdown of NLRP3 gene (Figure 4A). The transwell assay demonstrated that the migration and invasion abilities was significantly reduced in 143B and U2OS cells undergoing NLRP3 knockdown (Figure 4B). The reduced migration ability of osteosarcoma cells owing to NLRP3 knockdown was also validated via the wound healing assay (Figure 4C).
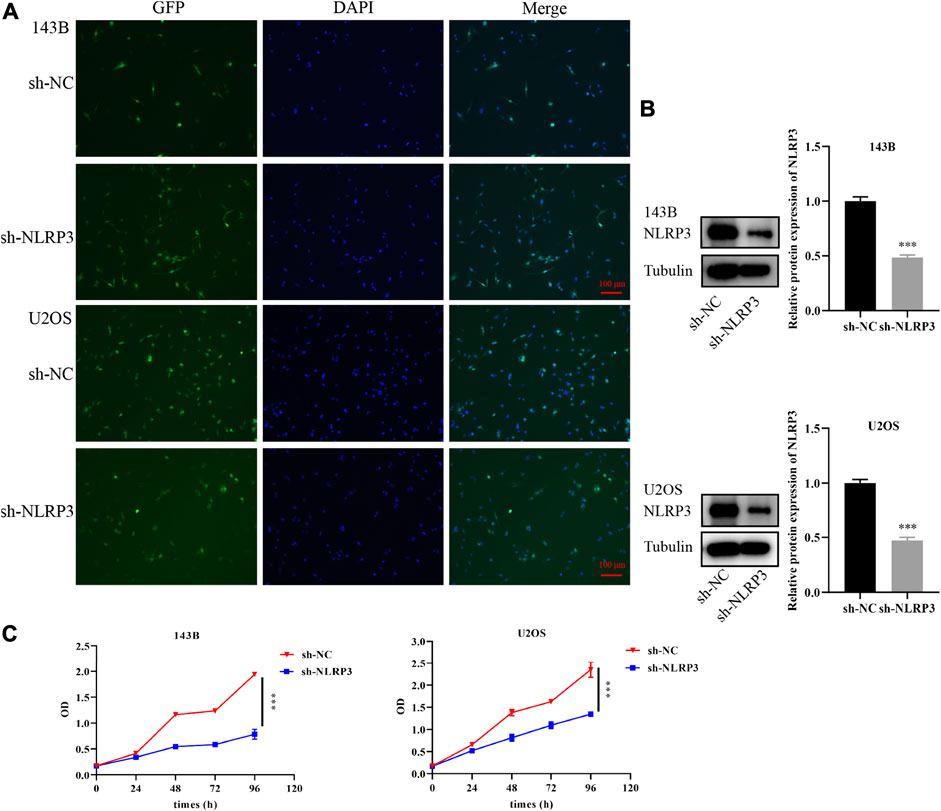
FIGURE 3. Knockdown of NLRP3 gene and cell viability changes in 143B and U2OS cells (A) Infection efficiency of lentivirus in 143B and U2OS cells (B) Interference effect of 143B and U2OS cells verified by western blot analysis (C) Th changes of cell viability in 143B and U2OS cells after NLRP3 knockdown detected by CCK-8. The experiments were conducted in triplicate.

FIGURE 4. Effect of NLRP3 knockdown on the proliferation, migration, and invasion of 143B and U2OS cells (A) Cell proliferation of lentivirus-transfected 143B and U2OS cells detected by colony formation assay after incubation for seven days (B) Cell migration and invasion of lentivirus-transfected 143B and U2OS cells examined using the transwell assay (C) Cell migration of lentivirus-transfected 143B and U2OS cells detected using the wound healing assay after incubation for 0, 12, 24, and 36 h. The experiments were carried out in triplicate.
To examine the role of NLRP3 in cell apoptosis and cell cycle distribution, Annexin V-FITC and PI staining was applied to evaluate the changes of cell apoptosis and cell cycle after CY-09 treatment or the incubation of cells undergoing lentivirus infection for 48 h. The results showed that cell apoptosis increased after the CY-09 intervention and lentivirus knockdown in 143B and U2OS cells via inhibiting cell cycle progression from G0/G1 phase to S and G2/M phases (Figures 5A, B). Similarly, NLRP3 knockdown also induced cell apoptosis and G0/G1 cell cycle arrest in 143B and U2OS cells (Figures 5C, D).
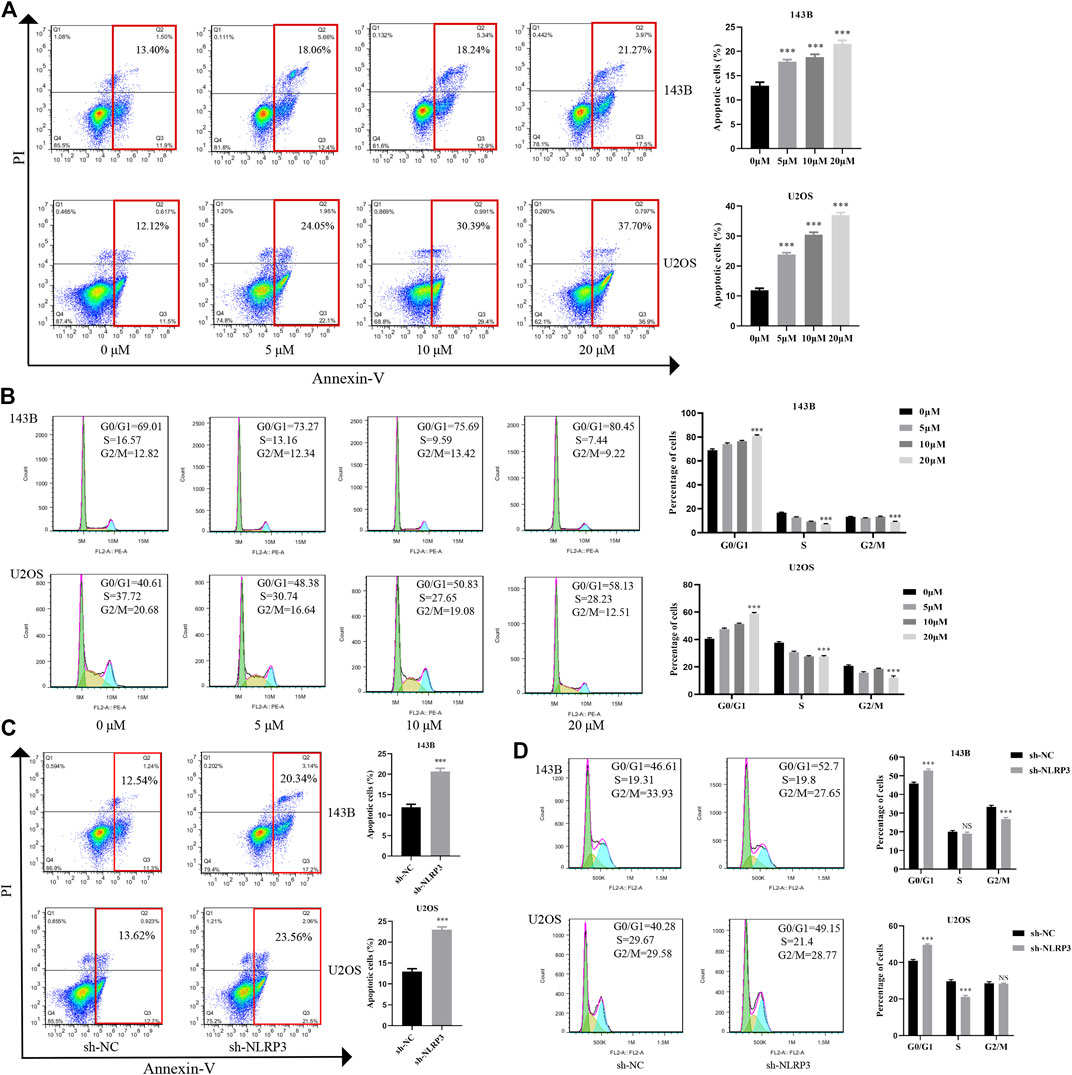
FIGURE 5. Effect of NLRP3 inhibition on cell apoptosis and cell cycle changes in osteosarcoma (A) The cell apoptosis rate of 143B and U2OS cells treated with different concentrations of CY-09 detected using the flow cytometry (B) Cell cycle distribution of 143B and U2OS cells treated with different concentrations of CY-09 was detected using the flow cytometry (C) The rate of cell apoptosis of lentivirus-transfected 143B and U2OS cells assessed using the flow cytometry (D) The cell cycle distribution of lentivirus-transfected 143B and U2OS cells was assessed using the flow cytometry. The experiments were conducted in triplicate.
NLRP3 inhibition influences the biological behaviors of osteosarcoma via the NLRP3/Caspaseaspase-1/GSDMD pathway.
The NLRP3/Caspaseaspase-1/GSDMD pathway has been reported to be crucial for NLRP3 induced tumor progression (Yang et al., 2020). The expression changes of proteins in this signaling pathway were observed in osteosarcoma cells undergoing CY-09 treatment and gene knockdown of NLRP3. Our study found that the expressions of NLRP3 and the downstream proteins including GSDMD, IL-1β, caspase-1 and IL-18 were notably reduced in 143B and U2OS cells after CY-09 treatment or NLRP3 knockdown (Figure 6). These results suggested that NLRP3/Caspase-1/GSDMD pathway may contribute to the effect of NLRP3 on the malignant progression of osteosarcoma.
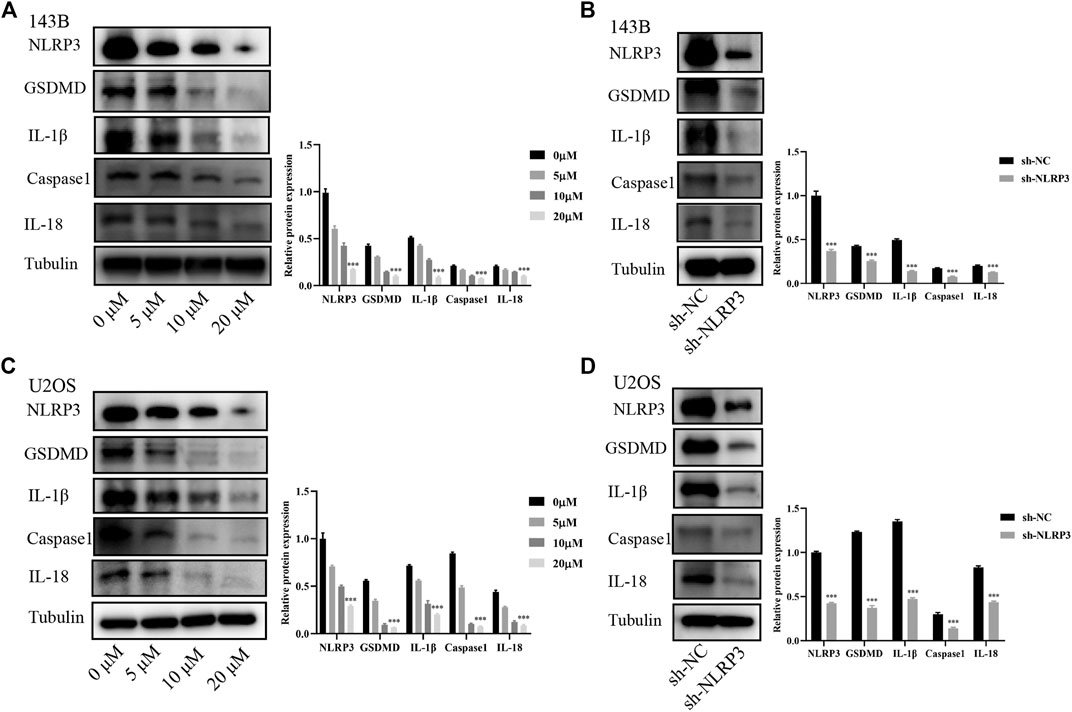
FIGURE 6. Involvement of the NLRP3/caspase-1/GSDMD pathway in the effect of NLRP3 on osteosarcoma. Expressions of NLRP3/caspase-1/GSDMD and the related proteins IL-1β and IL-18 in 143B cells treated with CY-09 (A) or lentivirus infection (B) Expressions of NLRP3, caspase-1, GSDMD and the related proteins IL-1β and IL-18 in U2OS cells treated with CY-09 (C) or lentivirus infection (D) The protein levels were measured in triplicate.
The in vitro experiment was species-specific, simpler, more convenient and more detailed, whereas it was at risk of over-interpretation of the results. Therefore, we performed the in vivo experiment where the condition recapitulates the reality. We established an osteosarcoma xenograft model using nude mice to evaluate the effects of NLRP3 inhibitor and gene knockdown on tumor formation in osteosarcoma in vivo. The results demonstrated that the tumor weight and volume clearly decreased with the CY-09 treatment compared to the control group, which was treated with dimethyl sulfoxide (DMSO) (Figures 7A, B). The weaker expression of Ki-67 of tumors in CY-09 group compared to the DMSO group validated the proliferation inhibition of CY-09 in osteosarcoma (Figure 7C). Similarly, NLRP3 knockdown also reduced the tumor weight and volume of osteosarcoma, as well as the Ki-67 expression in tumor (Figures 7D–F).
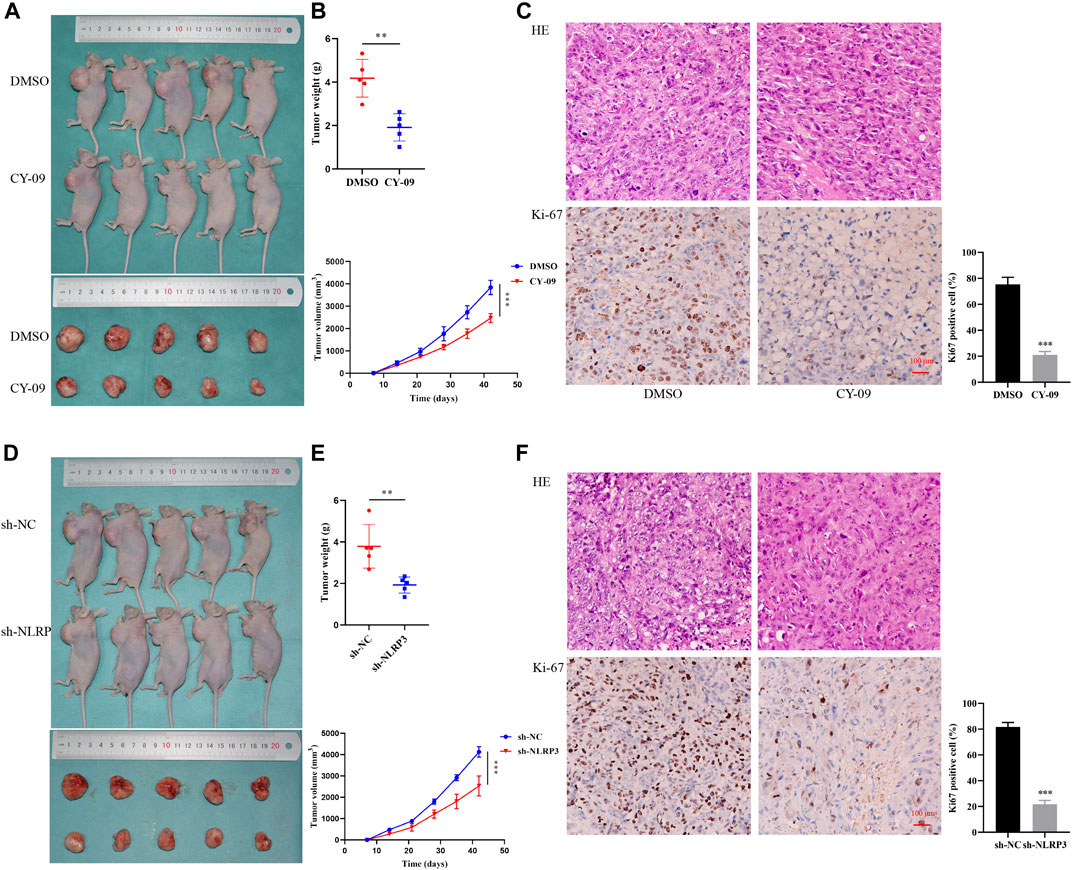
FIGURE 7. Effect of NLRP3 on tumor formation in osteosarcoma in vivo(A,B) Images of the xenografted tumor as well as the tumor weight and volume after CY-09 treatment (C) H&E staining and immunohistochemical staining of Ki-67 of the osteosarcoma tissues after treatment with CY-09 (D, E) Images of the xenografted tumor as well as the tumor weight and volume after NLRP3 lentivirus infection (F) H&E staining, immunohistochemical and statistic staining of Ki-67 of the osteosarcoma tissues after NLRP3 lentivirus infection.
Chronic inflammation plays crucial roles in carcinogenesis, tumor progression, and tumor-related inflammation, which have been defined as the seventh hallmark of cancer (Grivennikov et al., 2010; Landskron et al., 2014; Suarez-Carmona et al., 2017). Dysregulation of NLRP3 inflammasome is involved in tumor pathogenesis (Mcallister and Weinberg 2010; Moossavi et al., 2018). However, the real status of NLRP3 in self-antigen modification and upregulation in osteosarcoma carcinogenesis remains unclear. The results obtained in this study observed overexpression of the NLRP3 protein in osteosarcoma tissues, which was closely associated with clinicopathological characteristics including poor response to chemotherapy and distant metastasis. Furthermore, NLRP3 protein was found to be independently related to short PFS and OS in osteosarcoma patients. Suppression of NLRP3 protein by its targeted inhibitor CY-09 or knockdown of the NLRP3 gene could inhibit the cell proliferation, migration, invasion, promote apoptosis and G0/G1 cell cycle arrest in osteosarcoma cells in vitro via NLRP3/Caspase-1/GSDMD pathway. Our in vivo results further confirmed that the blockage of NLRP3 could suppress the formation of xenografted tumors of osteosarcoma.
Several inflammasomes, including NLRP3, may play a pathogenic role in carcinogenesis through regulating differentiation, adaptive immunity, apoptosis, and gut microbiota (Chen et al., 2020). Some studies have observed significantly high expression of NLRP3 in paraffin-embedded oral squamous cell carcinoma tissues, which was associated with lymph nodes, tumor size, and metastatic status. Moreover, the authors found that overexpression of the NLRP3 protein contributed to the malignant biological behaviors of oral squamous cell carcinoma (Huang et al., 2017; Feng et al., 2018). The present study showed that the NLRP3 protein was overexpressed in osteosarcoma specimens and could be an independent biomarker for the poor clinical outcomes of osteosarcoma patients.
Several types of NLRP3 inhibitors, including those that either directly or indirectly inhibiting the NLRP3 protein, have been reported to date. Directly targeting NLRP3 by small molecules is less invasive whereas more specific and cost-effective than cytokine blockade (Swanson et al., 2019). CY-09 is a selective and direct NLRP3 inhibitor (Jiang et al., 2017). Our results showed that CY-09 suppressed the proliferation, migration, invasion and promoted cell apoptosis of osteosarcoma cells in vitro. To investigate the mechanism of NLRP3 blockage on the cell viability, changes of the cell cycle were further examined. The results demonstrated that NLRP3 inhibitor prevented the progression of the cell cycle from G0/G1 to S phases, which may further inhibit the repair process of DNA damage in osteosarcoma cells. Moreover, the in vivo results demonstrated that CY-09 regressed the xenografted tumor growth of osteosarcoma. The therapeutic value of CY-09 has been mainly reported in inflammatory diseases, including inflammation after spinal cord injuries Fan et al. (2020) and cerebral ischemia/reperfusion injuries (Sun et al., 2020). Recently, researchers also focused on contributing chronic inflammation created by NLRP3 activation to carcinogenesis, including proliferation, angiogenesis, immunosuppression, and metastasis Liu et al. (2021), Huang et al. (2017), and Chen et al. (2018) showed that blockage of the NLRP3 inflammasome could enhance the antitumor immune responses in head and neck squamous cell carcinoma, suggesting that the NLRP3 inflammasome pathway may be regarded as a novel approach for the treatment of head and neck squamous cell carcinoma. Inhibition of the NLRP3 inflammasome through an NLRP3-specific antagonist could also reduce the cell viability in pancreatic adenocarcinoma (Yaw et al., 2020). Moreover, knockdown of NLRP3 was found to diminish the migration and invasion abilities of colorectal carcinoma cells (Wang et al., 2016). Yin et al. (2018) confirmed that downregulation of NLRP3 could significantly affect glioma cell progression in terms of cellular proliferation, apoptosis, and metastasis. In line with those former studies, our results demonstrated that knockdown of the NLRP3 gene could suppress the malignant progression of osteosarcoma. Therefore, NLRP3 plays a crucial pathogenic role for the tumorigenesis of osteosarcoma.
Activation of the NLRP3 leads to the release of proinflammatory cytokines IL-1β and IL-18 which are mediated by caspase-1, as well as pyroptotic cell death which is mediated by GSDMD (Riestra et al., 2019). Moreover, inhibitor or knockdown of NLRP3 expression can interfere with the formation of the NLRP3 inflammasome, which inhibits the expressions of GSDMD, Caspase-1, IL-1β, and IL-18 protein expression (He et al., 2019; Sanchez-Lopez et al., 2019). The present results of the western blot revealed that the NLRP3 as well as caspase-1, GSDMD, IL-1β, and IL-18 proteins decreased after NLRP3 blockage in osteosarcoma. One previous study by Jin et al. have also observed higher expressions of caspase-1 and its downstream target IL-1β are higher in osteosarcoma cells than in normal cells both in vitro and in vivo (Jin et al., 2017). Furthermore, a reduced expression of caspase-1 and IL-1β via the administration of berberine could significantly suppress the cell viability in osteosarcoma (Jin et al., 2017). Therefore, NLRP3/Caspase-1/GSDMD pathway may be involved in NLRP3-induced carcinogenesis of osteosarcoma.
There were some limitations to this study. Firstly, selection bias should be considered due to the retrospective nature of the study. In addition, the HR with wide CI may be inflated due to monotone likelihood phenomenon (Tzeng 2021). Further large-scale studies with a longer follow-up time should be performed for more convincing evidence.
To sum up, our results showed that overexpression of the NLRP3 protein was closely associated with the adverse clinicopathological characteristics of osteosarcoma. Additionally, NLRP3 may be regarded as an independent prognostic biomarker for osteosarcoma. The inhibition of NLRP3 by CY-09 or gene knockdown significantly suppressed the cell proliferation, tumor formation, migration, invasion, induced cell apoptosis and G0/G1 cell cycle arrest in osteosarcoma. Individualized therapy targeting NLRP3 may serve as a modality to prevent tumor progression of osteosarcoma. The findings obtained in this study may be promising for osteosarcoma patients because of the limitations of current therapies and relative stagnation of patient outcomes over the past decades. Nevertheless, further investigations are required to confirm the therapeutic value of NLRP3 in osteosarcoma.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
All experiments were carried out in accordance with the guidelines of the Ethics Committee of Fujian Medical University and approved by the Research Ethics Committee of the First Affiliated Hospital of Fujian Medical University, China. The studies involving human participants were reviewed and approved by The first affiliated hospital of fujian medical university. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. The animal study was reviewed and approved by Fujian medical university.
ZH, CH and SW performed the experiments, wrote and reviewed the manuscript; HW, XW, RS and YW analyzed the data; RL and JL designed the research study. All authors listed have approved the work for publication.
Our research was supported by the Startup Fund for Scientific Research of Fujian Medical University (Grant no. 2020QH1042), the National Natural Science Foundation of China (Grant no. 31571292), the Natural Science Foundation of Fujian Province.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Chen, L., Huang, C.-F., Li, Y.-C., Deng, W.-W., Mao, L., Wu, L., et al. (2018). Blockage of the NLRP3 Inflammasome by MCC950 Improves Anti-tumor Immune Responses in Head and Neck Squamous Cell Carcinoma. Cell. Mol. Life Sci. 75 (11), 2045–2058. doi:10.1007/s00018-017-2720-9
Chen, Y., Yang, Q., Lv, C., Chen, Y., Zhao, W., Li, W., et al. (2020). NLRP3 Regulates Alveolar Bone Loss in Ligature-Induced Periodontitis by Promoting Osteoclastic Differentiation. Cell Prolif 54, e12973. doi:10.1111/cpr.12973
Chow, T., Wutami, I., Lucarelli, E., Choong, P. F., Duchi, S., and Di Bella, C. (2020). Creating In Vitro Three-Dimensional Tumor Models: A Guide for the Biofabrication of a Primary Osteosarcoma Model. Tissue Eng. Part. B Rev. doi:10.1089/ten.teb.2020.0254
Dai, X., Ma, W., He, X., and Jha, R. K. (2011). Review of Therapeutic Strategies for Osteosarcoma, Chondrosarcoma, and Ewing's Sarcoma. Med. Sci. Monit. 17 (8), RA177–RA190. doi:10.12659/msm.881893
Fan, X., Ma, W., Zhang, Y., and Zhang, L. (2020). P2X7 Receptor (P2X7R) of Microglia Mediates Neuroinflammation by Regulating (NOD)-Like Receptor Protein 3 (NLRP3) Inflammasome-dependent Inflammation after Spinal Cord. Inj. Med Sci Monit 26, e925491. doi:10.12659/msm.925491
Feng, X., Luo, Q., Wang, H., Zhang, H., and Chen, F. (2018). MicroRNA‐22 Suppresses Cell Proliferation, Migration and Invasion in Oral Squamous Cell Carcinoma by Targeting NLRP3. J. Cel Physiol 233 (9), 6705–6713. doi:10.1002/jcp.26331
Grivennikov, S. I., Greten, F. R., and Karin, M. (2010). Immunity, Inflammation, and Cancer. Cell 140 (6), 883–899. doi:10.1016/j.cell.2010.01.025
He, W., Long, T., Pan, Q., Zhang, Y., Zhang, S., Zhang, D., et al. (2019). Microglial NLRP3 Inflammasome Activation Mediates IL-1beta Release and Contributes to central Sensitization in a Recurrent Nitroglycerin-Induced Migraine Model. J. Neuroinflammation 16 (1), 78. doi:10.1186/s12974-019-1459-7
Huang, C. F., Chen, L., Li, Y. C., Wu, L., Yu, G. T., Zhang, W. F., et al. (2017). NLRP3 Inflammasome Activation Promotes Inflammation-Induced Carcinogenesis in Head and Neck Squamous Cell Carcinoma. J. Exp. Clin. Cancer Res. 36 (1), 116. doi:10.1186/s13046-017-0589-y
Jiang, H., He, H., Chen, Y., Huang, W., Cheng, J., Ye, J., et al. (2017). Identification of a Selective and Direct NLRP3 Inhibitor to Treat Inflammatory Disorders. J. Exp. Med. 214 (11), 3219–3238. doi:10.1084/jem.20171419
Jin, H., Jin, X., Cao, B., and Wang, W. (2017). Berberine Affects Osteosarcoma via Downregulating the caspase-1/IL-1β Signaling axis. Oncol. Rep. 37 (2), 729–736. doi:10.3892/or.2016.5327
Landskron, G., De la Fuente, M., Thuwajit, P., Thuwajit, C., and Hermoso, M. A. (2014). Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 149185. doi:10.1155/2014/149185
Li, A. Y., Atenafu, E. G., Bernard, R. S., Masih-Khan, E., Reece, D., Franke, N., et al. (2020). Toxicity and Survival Outcomes of Autologous Stem Cell Transplant in Multiple Myeloma Patients with Renal Insufficiency: an Institutional Comparison between Two Eras. Bone Marrow Transpl. 55 (3), 578–585. doi:10.1038/s41409-019-0697-8
Lin, Y., Luo, T., Weng, A., Huang, X., Yao, Y., Fu, Z., et al. (2020). Gallic Acid Alleviates Gouty Arthritis by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis through Enhancing Nrf2 Signaling. Front. Immunol. 11, 580593. doi:10.3389/fimmu.2020.580593
Liu, C.-C., Miao, Y., Chen, R.-L., Zhang, Y.-Q., Wu, H., Yang, S.-M., et al. (2021). STIM1 Mediates IAV-Induced Inflammation of Lung Epithelial Cells by Regulating NLRP3 and Inflammasome Activation via Targeting miR-223. Life Sci. 266, 118845. doi:10.1016/j.lfs.2020.118845
Liu, S.-G., Wu, X.-X., Hua, T., Xin, X.-y., Feng, D.-L., Chi, S.-Q., et al. (2019). NLRP3 Inflammasome Activation by Estrogen Promotes the Progression of Human Endometrial Cancer. Ott 12, 6927–6936. doi:10.2147/ott.s218240
McAllister, S. S., and Weinberg, R. A. (2010). Tumor-host Interactions: a Far-Reaching Relationship. Jco 28 (26), 4022–4028. doi:10.1200/jco.2010.28.4257
Moossavi, M., Parsamanesh, N., Bahrami, A., Atkin, S. L., and Sahebkar, A. (2018). Role of the NLRP3 Inflammasome in Cancer. Mol. Cancer 17 (1), 158. doi:10.1186/s12943-018-0900-3
Riestra, A. M., Valderrama, J. A., Patras, K. A., Booth, S. D., Quek, X. Y., Tsai, C.-M., et al. (2019). Trichomonas Vaginalis Induces NLRP3 Inflammasome Activation and Pyroptotic Cell Death in Human Macrophages. J. Innate Immun. 11 (1), 86–98. doi:10.1159/000493585
Sanchez-Lopez, E., Zhong, Z. A. Stubelius., Stubelius, A., Sweeney, S. R., Booshehri, L. M., Antonucci, L., et al. (2019). Choline Uptake and Metabolism Modulate Macrophage IL-1β and IL-18 Production. Cel Metab. 29 (6), 1350–1362. doi:10.1016/j.cmet.2019.03.011
Schisterman, E. F., Perkins, N. J., Liu, A., and Bondell, H. (2005). Optimal Cut-point and its Corresponding Youden Index to Discriminate Individuals Using Pooled Blood Samples. Epidemiology 16 (1), 73–81. doi:10.1097/01.ede.0000147512.81966.ba
Srirajaskanthan, R., Shah, T., Watkins, J., Marelli, L., Khan, K., and Caplin, M. E. (2010). Expression of the HER-1-4 Family of Receptor Tyrosine Kinases in Neuroendocrine Tumours. Oncol. Rep. 23 (4), 909–915. doi:10.3892/or_00000714
Suarez-Carmona, M., Lesage, J., Cataldo, D., and Gilles, C. (2017). EMT and Inflammation: Inseparable Actors of Cancer Progression. Mol. Oncol. 11 (7), 805–823. doi:10.1002/1878-0261.12095
Sun, R., Peng, M., Xu, P., Huang, F., Xie, Y., Li, J., et al. (2020). Low-density Lipoprotein Receptor (LDLR) Regulates NLRP3-Mediated Neuronal Pyroptosis Following Cerebral Ischemia/reperfusion Injury. J. Neuroinflammation 17 (1), 330. doi:10.1186/s12974-020-01988-x
Swanson, K. V., Deng, M., and Ting, J. P.-Y. (2019). The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 19 (8), 477–489. doi:10.1038/s41577-019-0165-0
Tzeng, I. S. (2021). To Handle the Inflation of Odds Ratios in a Retrospective Study with a Profile Penalized Log-Likelihood Approach. J. Clin. Lab. Anal. 35, e23849. doi:10.1002/jcla.23849
Wang, H., Luo, Q., Feng, X., Zhang, R., Li, J., and Chen, F. (2018). NLRP3 Promotes Tumor Growth and Metastasis in Human Oral Squamous Cell Carcinoma. BMC Cancer 18 (1), 500. doi:10.1186/s12885-018-4403-9
Wang, H., Wang, Y., Du, Q., Lu, P., Fan, H., Lu, J., et al. (2016). Inflammasome-independent NLRP3 Is Required for Epithelial-Mesenchymal Transition in colon Cancer Cells. Exp. Cel Res. 342 (2), 184–192. doi:10.1016/j.yexcr.2016.03.009
Wang, S. L., Zhong, G. X., Wang, X. W., Yu, F. Q., Weng, D. F., Wang, X. X., et al. (2018). Prognostic Significance of the Expression of HER Family Members in Primary Osteosarcoma. Oncol. Lett. 16 (2), 2185–2194. doi:10.3892/ol.2018.8931
Xue, Y., Du, H. D., Tang, D., Zhang, D., Zhou, J., Zhai, C. W., et al. (2019). Correlation between the NLRP3 Inflammasome and the Prognosis of Patients with LSCC. Front. Oncol. 9, 588. doi:10.3389/fonc.2019.00588
Yang, Y., Liu, P. Y., Bao, W., Chen, S. J., Wu, F. S., and Zhu, P. Y. (2020). Hydrogen Inhibits Endometrial Cancer Growth via a ROS/NLRP3/caspase-1/GSDMD-mediated Pyroptotic Pathway. BMC Cancer 20 (1), 28. doi:10.1186/s12885-019-6491-6
Yang, Y., Wang, H., Kouadir, M., Song, H., and Shi, F. (2019). Recent Advances in the Mechanisms of NLRP3 Inflammasome Activation and its Inhibitors. Cell Death Dis 10 (2), 128. doi:10.1038/s41419-019-1413-8
Yaw, A. C. K., Chan, E. W. L., Yap, J. K. Y., and Mai, C. W. (2020). The Effects of NLRP3 Inflammasome Inhibition by MCC950 on LPS-Induced Pancreatic Adenocarcinoma Inflammation. J. Cancer Res. Clin. Oncol. 146 (9), 2219–2229. doi:10.1007/s00432-020-03274-y
Yin, X. F., Zhang, Q., Chen, Z. Y., Wang, H. F., Li, X., Wang, H. X., et al. (2018). NLRP3 in Human Glioma Is Correlated with Increased WHO Grade, and Regulates Cellular Proliferation, Apoptosis and Metastasis via Epithelial-Mesenchymal Transition and the PTEN/AKT Signaling Pathway. Int. J. Oncol. 53 (3), 973–986. doi:10.3892/ijo.2018.4480
Keywords: osteosarcoma, prognosis, NLRP3, CY-09, lentivirus
Citation: Huang Z, Chen H, Wang S, Wei H, Wang X, Shen R, Wang Y, Lin R and Lin J (2021) NLRP3 Overexpression Associated With Poor Prognosis and Presented as an Effective Therapeutic Target in Osteosarcoma. Front. Pharmacol. 12:724923. doi: 10.3389/fphar.2021.724923
Received: 14 June 2021; Accepted: 16 July 2021;
Published: 28 July 2021.
Edited by:
Tong-Chuan He, University of Chicago Medicine, United StatesReviewed by:
I-Shiang Tzeng, Chinese Culture University, TaiwanCopyright © 2021 Huang, Chen, Wang, Wei, Wang, Shen, Wang, Lin and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Lin, Smlhbmh1YWxAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.