- 1Fujian Provincial Key Laboratory of Innovative Drug Target Research and State Key Laboratory of Cellular Stress Biology, School of Pharmaceutical Sciences, Xiamen University, Xiamen, China
- 2Department of Cardiology, Xiamen Key Laboratory of Cardiac Electrophysiology, Xiamen Institute of Cardiovascular Diseases, The First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, China
- 3Department of Breast Surgical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 4Clinical Laboratory, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 5State Key Laboratory of Coal Resources and Safe Mining, School of Chemical and Environmental Engineering, China University of Mining and Technology, Beijing, China
- 6School of Population Medicine and Public Health, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 7Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC, Australia
Background: Nowadays, due to the limitation of single therapy, combination therapy for cancer treatments has become important strategy. With the advancement of research on cardiotoxicities induced by anti-cancer treatment, among which cancer treatment-induced hypertension is the most frequent case. However, due to the small sample size and the absence of comparison (single-arm study alone), these studies have limitations to produce a feasible conclusion. Therefore, it is necessary to carry out a meta-analysis focusing on hypertension caused by cancer combination therapy.
Methods: We systematically searched PubMed, Embase, Cochrane Library, Web of Science, and CNKI, from database inception to November 31, 2020, with randomized controlled trials (RCTs) associated with hypertension induced by cancer combination drugs. The main endpoint of which was to assess the difference in the incidence of hypertension in cancer patients with monotherapy or combination therapy. We calculated the corresponding 95% confidence interval (95% CIs) according to the random effect model and evaluated the heterogeneity between different groups.
Results: According to the preset specific inclusion and exclusion criteria, a total of 23 eligible RCTs have been included in the present meta-analysis, including 6,241 patients (Among them, 2872 patients were the control group and 3369 patients were the experimental group). The results showed that cancer patients with combination therapy led to a higher risk of hypertension (All-grade: RR 2.85, 95% CI 2.52∼3.22; 1∼2 grade: RR 2.43, 95% CI 2.10∼2.81; 3∼4 grade: RR 4.37, 95% CI 3.33∼5.72). Furthermore, compared with the control group who received or did not receive a placebo, there was a higher risk of grade 3-4 hypertension caused by cancer combination treatment.
Conclusion: The present meta-analysis carries out a comprehensive analysis on the risk of patients suffering from hypertension in the process of multiple cancer combination therapies. Findings in our study support that the risk of hypertension may increase significantly in cancer patients with multiple cancer combination therapies. The outcomes of this meta-analysis may provide a reference value for clinical practice and may supply insights in reducing the incidence of hypertension caused by cancer combined treatment.
Introduction
Hypertension has been recognized as the most common comorbidity among various types of cancers, which directly affects the prognosis of cancer patients, and is one of the high-risk factors for cancer survivors suffering from the comorbidity of heart diseases (Jain and Townsend, 2007). In the early stage of diagnosis, there is generally a similar probability of developing hypertension. However, with different cancer treatment patterns, patients may experience significantly altered incidence of hypertension, especially those receiving chemotherapy, which can reach 38% (Piccirillo et al., 2004; Maitland et al., 2010). In addition, novel cancer therapies, such as targeted therapy, which is a type of cancer treatment that targets proteins controlling cancer cells’ growth, division, and spreading, are also associated with the incidence of hypertension. Cardio-Oncology is an evolving discipline which aims to analyze the relationship between cancer treatment and cardiotoxicity (Lenneman et al., 2016; Barac, 2020). Cardiovascular toxicity in cancer treatment refers to the occurrence of cardiovascular disease during the disturbance or elimination of cancer cells in patients in vivo. Significantly, cardiovascular disease is the second leading cause of the morbidity and mortality of cancer survivors. According to previous studies, the probability of all-grade hypertension is between 15 and 67% during the treatment by using small molecule vascular endothelial growth factor tyrosinase inhibitors (e.g., sunitinib, sorafenib, pazopanib, etc.), and the rate would be higher with the use of inhibitors with higher efficiency (e.g., axitinib) (Brinda et al., 2016). The incidence of hypertension induced by tyrosinase inhibitors ranges from 5 to 80% in a dose-dependent manner (Agarwal et al., 2018). In addition, some patients may have a history of hypertension before the diagnosis of cancer. However, some patients develop hypertension due to anti-cancer treatment, and hypertension may be the direct result of cancer treatment under this circumstance.
The progress of cancer treatment has promoted the development of multiple new treatment strategies. Combination therapies means combining two or more therapies for cancer patients and the effectiveness may be excellent than single therapy. However, most programs will be accompanied by a series of cardiovascular adverse reactions, especially the existed high correlation of some new drugs with hypertension. In addition, the use of some chemotherapy drugs can also induce hypertension.
Generally, angiogenesis is a necessary process of tumorigenesis, growth, and metastasis. Vascular endothelial growth factor (VEGF) is an angiogenic growth factor. Angiogenesis inhibitor is a classic drug highly associated with the occurrence of hypertension (Hamnvik et al., 2015), primarily including monoclonal antibodies and small-molecule drugs. It has been documented that the proposed highly specific drugs are important inhibitors of angiogenesis, which play a role by blocking the signaling pathways necessary for angiogenesis, such as blocking Vascular Endothelial Growth Factor Receptor (VEGFR), Epidermal Growth Factor Receptor (EGFR), basic Fibroblast Growth Factor (bFGF), Platelet-derived Growth Factor Receptor (PDGFR), etc. (Folkman, 2007). To be specific, VEGF is the main growth factor that controls angiogenesis. Epidermal growth factor (EGF) is responsible for differentiation and apoptosis. bFGF can regulate the proliferation and differentiation of specific types of cells and has an effective effect on angiogenesis. Platelet-derived growth factor (PDGF) involves significantly cell growth, cell division, and angiogenesis (Wilkins et al., 2014; Agarwal et al., 2018).
With the emergence of various novel approaches to cancer treatment, the survival of cancer patients is becoming higher, which, however, is accompanied by an increasingly more obvious change in cardiotoxicity. Given the differences in cancer tissue types, therapeutic drugs, and drug doses, a systematic review and meta-analysis were carried out on hypertension caused by cancer treatment (Said et al., 2017), which aimed to clarify the incidence and risk of hypertension in cancer patients treated with combination therapy. At present, there is incomplete knowledge of hypertension caused by cancer combination therapy. Besides, there is few systematic reviews or meta-analyses in this aspect based on the comprehensive analysis of previous literature. Accordingly, through comprehensive literature analysis, it is expected to analyze and elaborate the risk factors of hypertension caused by cancer combination therapy, to provide a certain reference value for clinical treatment.
Methods
The present systematic review and meta-analysis were conducted following PRISMA guidelines (Moher et al., 2009). The protocol has been registered in PROSPERO with the registration number CRD42021220923.
Data Sources and Searches
A comprehensive literature search was made in databases [PubMed, embase, Cochrane Library, Web of Science, and CNKI] since November 31, 2020, to identify all articles related to the subject. In addition to the above databases, the clinical trial registration website (https://clinicaltrials.gov/) was searched to obtain information about registered prospective trials.
The keywords used in PubMed were listed as follows:
1) randomized controlled trial [pt]
2) controlled clinical trial [pt]
3) randomized [tiab]
4) placebo [tiab]
5) clinical trials as topic [mesh: noexp]
6) randomly [tiab]
7) trial [ti]
8) (1) OR (2) OR (3) OR (4) OR (5) OR (6) OR (7)
9) animals [mh] NOT humans [mh]
10) (8) NOT (9)
The final selected literatures were checked and reviewed separately to include the latest and most complete clinical trial reports in the case of repeated publications. All the search results were incorporated into the management tool of Endnote.
Study Selection and Data Extraction
The major objective of our study was to determine the incidence of hypertension associated with combination therapy for cancer and to establish a relationship between combination therapy and the risk of hypertension. Therefore, eligible studies were those evaluating the combination of drugs with hypertension induced in cancer patients. Phase I trial was excluded considering the multi-dose level and limited sample size. In addition, phase II, III, and IV randomized controlled trials (RCTs) in combination therapy were enrolled in the analysis compared with those without combination therapy.
The eligible studies met the inclusion criteria:
1) Phase II, III, and IV trials involving cancer patients;
2) RCTs for cancer treatment;
3) Intervention group: combination therapy (including targeted therapy and chemotherapy);
4) Control group: monotherapy or placebo treatment;
5) Studies with available data on hypertension events or incidence and sample size.
The exclusion criteria:
1) Review articles
2) Not randomized control trial
3) Reports from same study sample
4) Not report associate with hypertension
5) Not report associate with cancer combination therapy
6) No usable data
7) No comparable trial
8) Republished literature
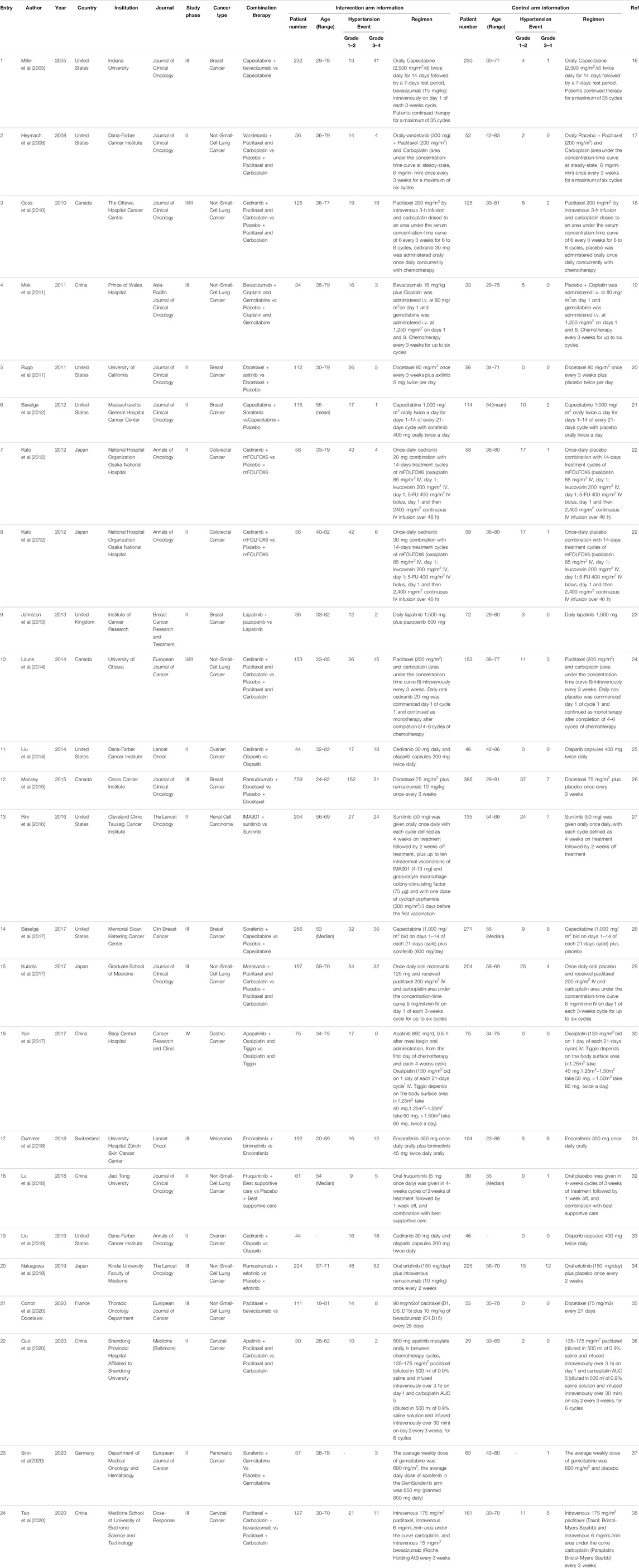
Two investigators (G.X and Q.X) extracted data independently, and any disagreements between the two reviewers were resolved by consensus. Online studies before publication were also eligible, but not including reviews, Conference reviews, studies published only in abstract form, quality of life research, non-randomized trials, and studies that could not determine the toxicity of combination therapy. Data extraction covered author, year of publication, research institution, journal name, trial phase, cancer tissue type, combination therapy, number of patients, age of patients, administration schedule and drug dose, size of control group, number of patients with hypertension, with the data of hypertension at all grades extracted.
Data Synthesis and Analysis
Statistical analysis of this study was performed by using the Cochrane Review Manager (RevMan 5.3) software provided by the Cochrane Library Collaboration Network.
The proportion of patients with hypertension in each study was calculated by dividing the number of patients with hypertension caused by combination therapy extracted from eligible clinical trials by the total number of patients receiving combination therapy in each study. We refer to all levels of hypertension events as “All-grade,” “1–2 grade” is combined the grade of 1 or 2 hypertension events, and “3–4 grade” which is the sum of the level of 3 or 4 hypertension events.
For each study enrolled in this analysis, the relative risk (RR) and 95% confidence interval (95% CI) of the incidence of events between the intervention group and the control group were calculated according to the number of reported events and sample size. The I2 index and Q-statistics were used to evaluate the heterogeneity among studies, among which the Q-test is widely used at present (Zintzaras and Ioannidis, 2005). p < 0.05 of the Q-test indicated the existence of heterogeneity (Zhang et al., 2019), and p < 0.05 meant the existence of statistical significance. If p > 0.05, the results of the independent studies might be homogeneous, suggesting the use of the fixed-effect model; On the contrary, the random-effect model should be used and/or consider the clinical suitability of combination therapy when there was heterogeneity with p < 0.05. I2 can quantify the heterogeneity among studies, which is calculated generally based on χ2 test. It describes the percentage of variation among studies in total variation, which may indicate a higher heterogeneity with the increase of the value of I2 (Huedo-Medina et al., 2006). I2 > 25, 50, and 75% suggest that there may be low, moderate, and high heterogeneity among studies. Besides, it is generally believed that there is substantial heterogeneity when I2 > 50%.
Results
Search Results
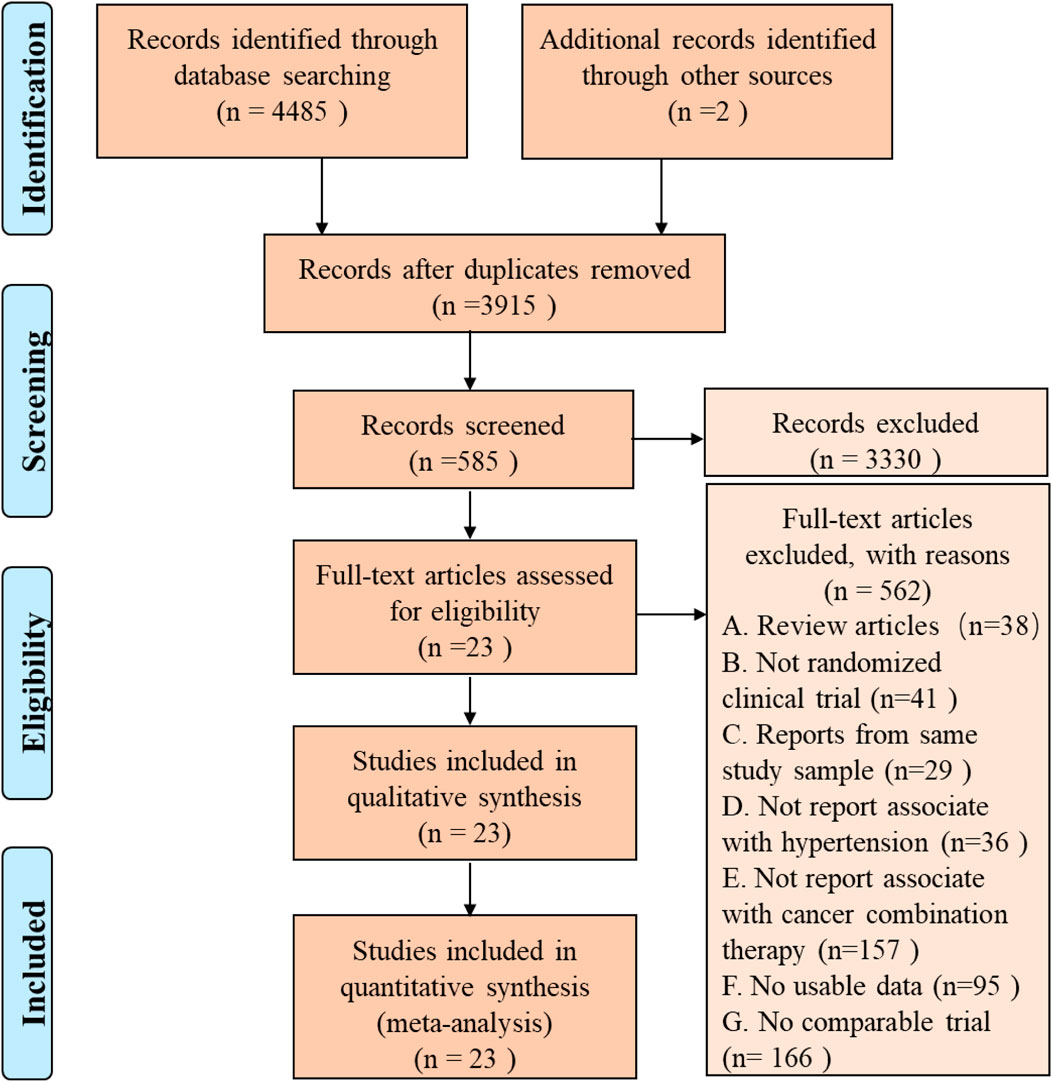
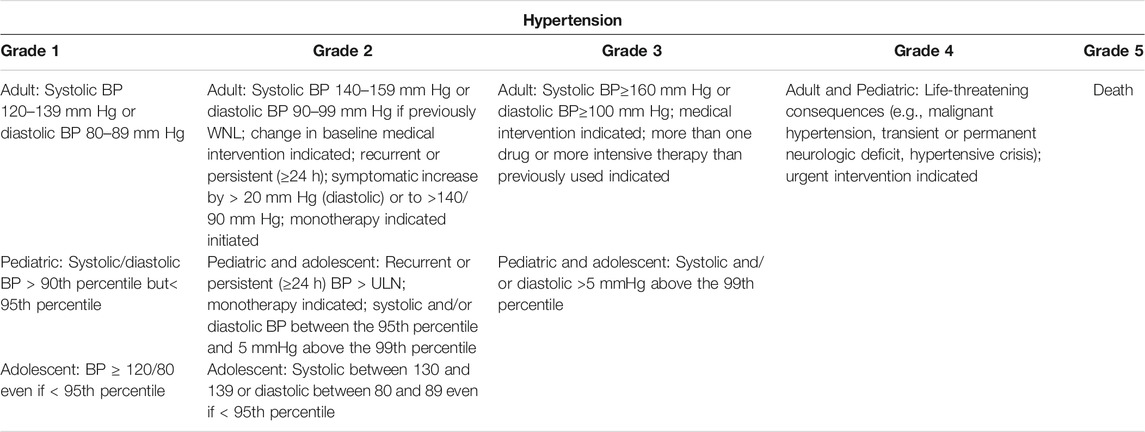
A total of 3,915 articles were identified by literature search and reference list review. After screening and qualification evaluation, 23 clinical trials involving 6,241 patients were finally included after excluding review articles, case reports, and meta-analysis articles, with the flow chart of literature selection shown in Figure 1. Of the 23 studies, there were 12 phase II, 11 phase III, and 1 phase IV trials, with the year of publication ranging from 2005 to 2020 (Table 1) (Miller et al., 2005; Heymach et al., 2008; Goss et al., 2010; Mok et al., 2011; Rugo et al., 2011; Baselga et al., 2012; Kato et al., 2012; Johnston et al., 2013; Laurie et al., 2014; Liu et al., 2014; Mackey et al., 2015; Rini et al., 2016; Baselga et al., 2017; Kubota et al., 2017; Yan et al., 2017; Dummer et al., 2018; Lu et al., 2018; Liu et al., 2019; Nakagawa et al., 2019; Cortot et al., 2020; Guo et al., 2020; Sinn et al., 2020; Tao et al., 2020). According to the published Common Terminology Criteria for Adverse Events (CTCAE) by the National Cancer Institute (NCI), hypertension caused by anti-cancer treatment includes 5 grades of grade 1–5 (Table 2) (National Cancer Institute, 2017). Among them, grade 5 hypertension includes fatal elevated blood pressure. There were no patients with grade 5 hypertension in the included literatures. Consequently, only grade 1–4 hypertension was enrolled in the data extraction. After research, there is no discovery showing that the patients enrolled in the reviewed RCTs were taking anti-hypertensive drugs.
In this study, cancer types were Breast Cancer (n = 6), Cervical Cancer (n = 2), Colorectal Cancer (n = 1), Gastric Cancer (n = 1), Melanoma (n = 1), Non-Small-Cell Lung Cancer (n = 8), Ovarian Cancer (n = 2), Pancreatic Cancer (n = 1), and Renal Cell Carcinoma (n = 1). As for cancer combination therapy regimens, there was the combination of 2 drugs (n = 14), 3 drugs (n = 8), and >3 drugs (n = 1). Among the 23 therapeutic regimens, there were targeted therapy combined with chemotherapy (n = 17), two targeted therapies combined with chemotherapy (n = 5), and targeted therapy combined with other treatments (n = 1). In the control group, 10 studies adopted monotherapy, and 13 studies used placebo combined with monotherapy.
In all eligible studies, the average age of patients ranged from 18 to 89 years old. Among the eligible research articles, papers published in the United States accounted for the majority, with 8 articles, followed by China with 5 articles, Canada with 3 articles, Japan with 3 articles, Britain with 1 article, France with 1 article, Germany with 1 article and Switzerland with 1 article. Meanwhile, 8 articles were published in “Journal of Clinical Oncology,”, 4 in “The Lancet Oncology,”, and 3 in “European Journal of Cancer.”
Evaluation of Included Studies
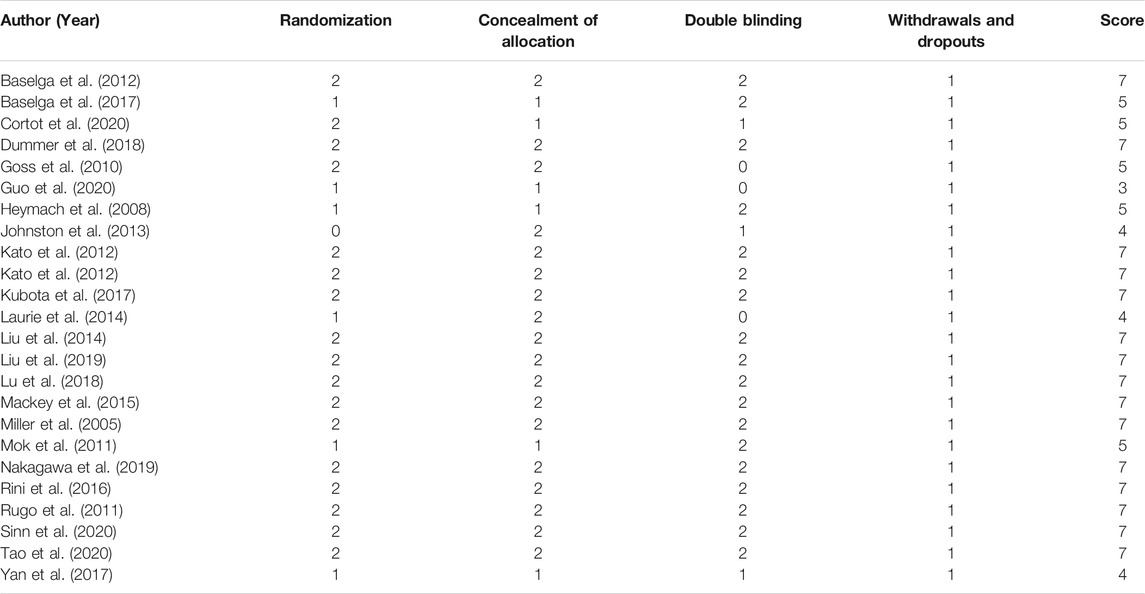
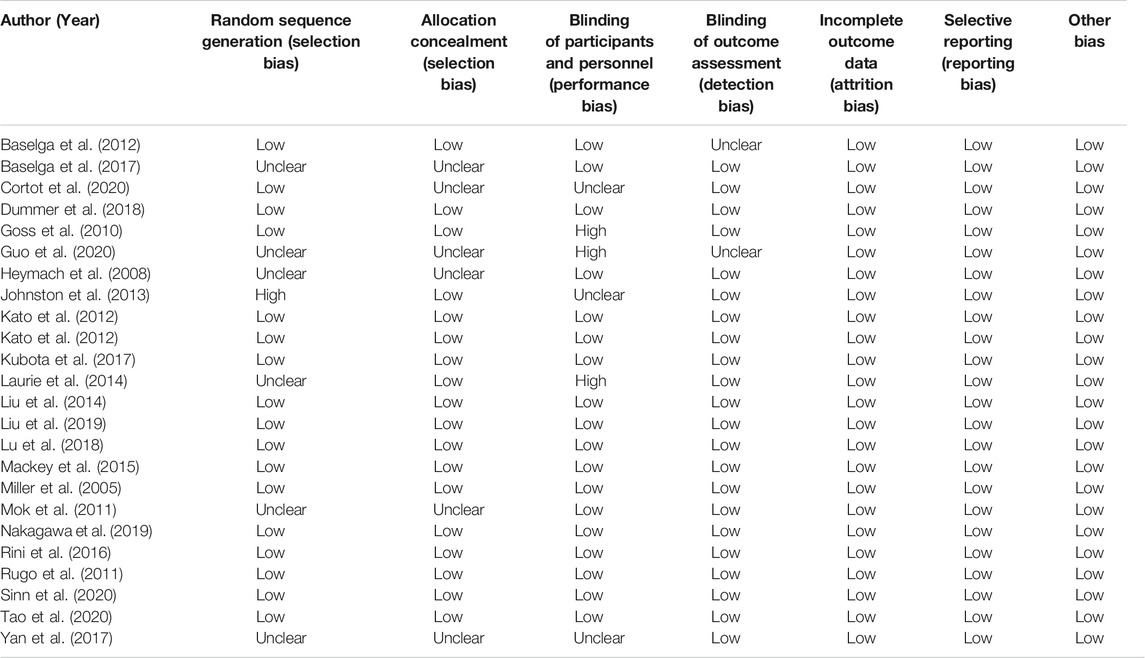
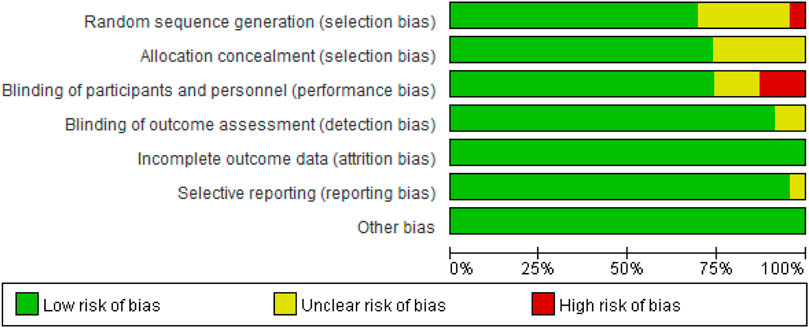
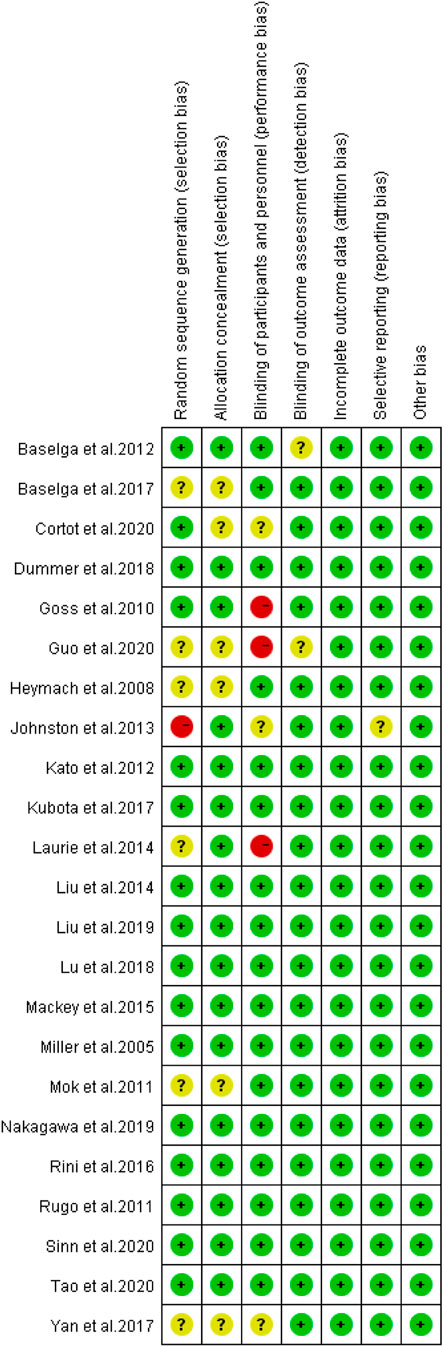
The Modified Jadad Scores scale (Jadad et al., 1996) was used to evaluate the quality of the 23 eligible articles. Following the evaluation based on the Randomization, Concealment of Allocation, Double Blinding, Withdrawals, and Dropouts, etc., there were 15 articles in 7 points, 5 articles in 5 points, 3 articles in 4 points, and 1 article in 3 points, as shown in Table 3.
Relative Risk of Hypertension
A total of 3,369 patients received cancer combination therapy, as well as 2,872 patients received cancer single therapy and/or placebo, which was available for comparative analysis. The incidence of grade 1–2 hypertension events ranged from 0 to 75%, and cediranib combined with mFOLFOX6 for the treatment of Colorectal Cancer had the highest probability of inducing hypertension (Kato et al., 2012). However, no events were observed in grade 1–2 hypertension in one trial (Sinn et al., 2020). Using the random-effect model, the RR in all patients developing grade 1–2 hypertension was 2.43 [95% CI 2.10–2.81, p < 0.001, Figure 2A]. Furthermore, the probability of grade 3–4 hypertension in all patients ranged from 0 to 40.9%, among which cediranib combined with Olaparib in treating Ovarian Cancer showed the highest probability of developing hypertension events (Liu et al., 2014; Liu et al., 2019). However, no grade 3–4 hypertension events were observed in the use of Oxaliplatin combined with oxaliplatin and Tiggio in the treatment of Gastric Cancer (Yan et al., 2017). Based on the random-effect model, the RR in all patients developing grade 3–4 hypertension was 4.37 [95% CI 3.33–5.72, p < 0.001, Figure 2B]. In addition, the incidence of all-grade hypertension ranged from 5.26 to 85.71%, and the highest incidence of hypertension was observed in the use of cediranib combined with mFOLFOX6 for the treatment of Colorectal Cancer (Kato et al., 2012). In the random-effect model, the RR in all patients developing grade 3–4 hypertension was 2.85 [95% CI 2.52–3.22, p < 0.001, Figure 2C].
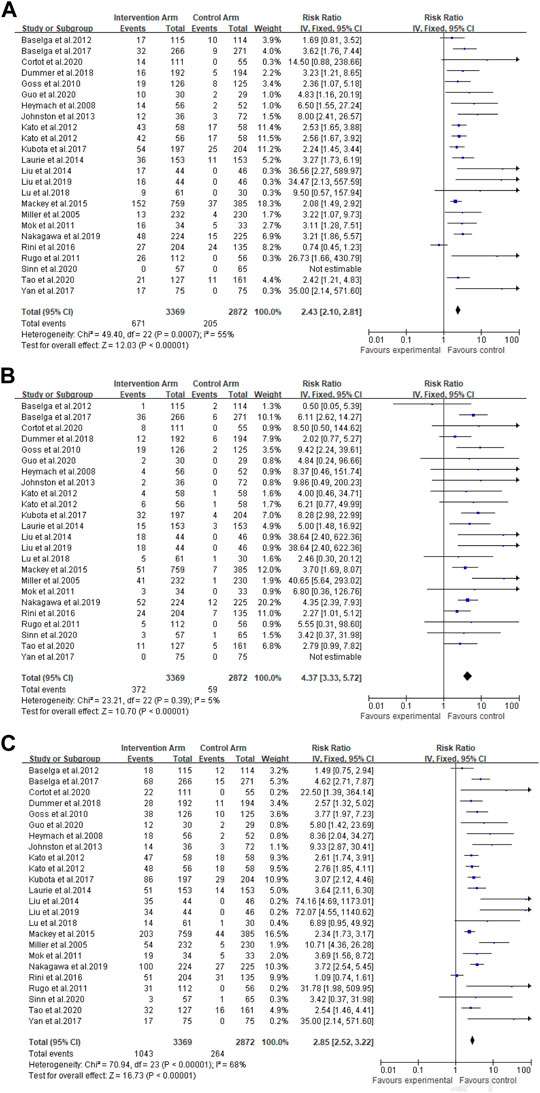
FIGURE 2. (A) Forest Plots for the Overall Comparison of Hypertension caused by cancer combination therapy. (B) Summary Relative Risks for Hypertension at Grade 3–4. (C) Summary Relative Risks for Hypertension at All Grade.
Overall Comparison of Hypertension
For all grades of hypertension, cancer patients receiving combination therapy had a relatively higher probability of developing hypertension (All-grade: RR 2.85, 95% CI 2.52–3.22; 1–2 grade: RR 2.43, 95% CI 2.10–2.81; 3–4 grade: RR 4.37, 95% CI 3.33–5.72) (Figure 2). In terms of all grades of hypertension caused by targeted drugs combined with chemotherapy, schemes with a relatively higher risk of developing hypertension included Paclitaxel combined with bevacizumab (RR 22.50, 95%CI 1.39–364.14) (Cortot et al., 2020), cediranib combined with Olaparib (RR 74.16, 95%CI 4.69–1,173.01; RR 72.07, 95%CI 4.55–1,140.62) (Liu et al., 2014; Liu et al., 2019), Docetaxel combined with axitinib (RR 31.78, 95%CI 1.98–509.95) (Johnston et al., 2013), as well as apapatinib combined with Oxaliplatin and Tiggio (RR 35.00, 95%CI 2.14–571.60) (Yan et al., 2017).
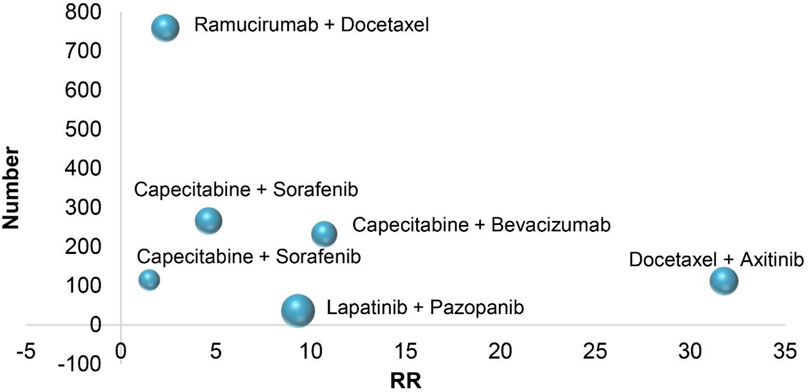
In six RCTs on the treatment of breast cancer, combination therapies included Capecitabine combined with bevacizumab (All-grade: RR 10.71, 95% CI 4.36–26.28; 1–2 grade: RR 3.22, 95% CI 1.07–9.73; 3–4 grade: RR 40.65, 95% CI 5.64–293.02) (Miller et al., 2005), Docetaxel combined with axitinib (All-grade: RR 31.78, 95% CI 1.98–509.95; 1–2 grade: RR 26.73, 95% CI 1.66–430.79; 3–4 grade: RR 5.55, 95% CI 0.31–98.60) (Rugo et al., 2011), Capecitabine combined with Sorafenib (All-grade: RR 1.49, 95% CI 0.75–2.94; 1–2 grade: RR 1.69, 95% CI 0.81–3.52; 3–4 grade: RR 0.50, 95% CI 0.05–5.39) (Baselga et al., 2012), lapatinib combined with pazopanib (All-grade: RR 9.33, 95% CI 2.87–30.41; 1–2 grade: RR 8.00, 95% CI 2.41–26.57; 3–4 grade: RR 9.86, 95% CI 0.49–200.23) (Johnston et al., 2013), ramucirumab combined with Docetaxel (All-grade: RR 2.34, 95% CI 1.73–3.17; 1–2 grade: RR 2.08, 95% CI 1.49–2.92; 3–4 grade: RR 3.70, 95% CI 1.69–8.07) (Mackey et al., 2015), Sorafenib combined with Capecitabine (All-grade: RR 4.62, 95% CI 2.71–7.87; 1–2 grade: RR 3.62, 95% CI 1.76–7.44; 3–4 grade: RR 6.11, 95% CI 2.62–14.27) (Baselga et al., 2017). According to the treatment of breast cancer, the RR of combination therapies induced hypertension is different, and the RR of Docetaxel combined with axitinib is higher than that of other treatments. In the combined treatment of breast cancer patients, Figure 3, it is not difficult to see that the RR of hypertension caused by ramucirumab combined with Docetaxel is small when the number of patients is gradually increasing, which indicates that ramucirumab combined with Docetaxel is the best treatment for low risk of hypertension caused by breast cancer in 6 RCTs of this research.
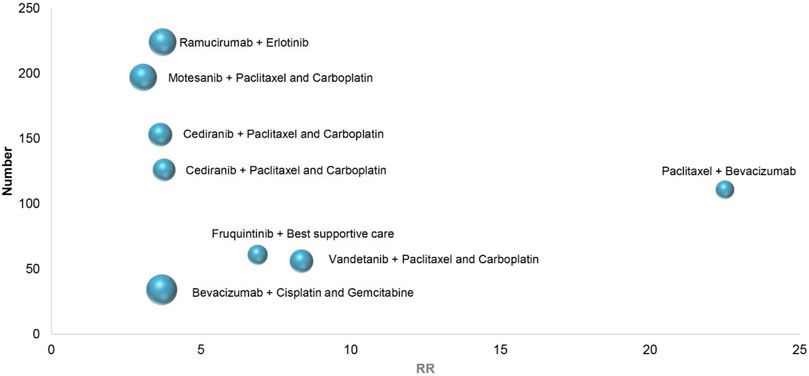
In eight RCTs on the treatment of non-small cell lung cancer, combination therapies included vandetanib combined with Paclitaxel and Carboplatin (All-grade: RR 8.36, 95% CI 2.04–32.27; 1–2 grade: RR 6.50, 95% CI 1.55–27.24; 3–4 grade: RR 8.37, 95% CI 0.46–151.74) (Heymach et al., 2008), cediranib combined with Paclitaxel and Carboplatin (All-grade: RR 3.77, 95% CI 1.97–7.23; 1–2 grade: RR 2.36, 95% CI 1.07–5.18; 3–4 grade: RR 9.42, 95% CI 2.24–39.61) (Goss et al., 2010), bevacizumab combined with Cisplatin and Gemcitabine (All-grade: RR 3.69, 95% CI 1.56–8.72; 1–2 grade: RR 3.11, 95% CI 1.28–7.51; 3–4 grade: RR 6.80, 95% CI 0.36–126.76) (Mok et al., 2011), cediranib combined with Paclitaxel and Carboplatin (All-grade: RR 3.64, 95% CI 2.11–6.30; 1–2 grade: RR 3.27, 95% CI 1.73–6.19; 3–4 grade: RR 5.00, 95% CI 1.48–16.92) (Laurie et al., 2014), motesanib combined with Paclitaxel and Carboplatin (All-grade: RR 3.07, 95% CI 2.12–4.46; 1–2 grade: RR 2.24, 95% CI 1.45–3.44; 3–4 grade: RR 8.28, 95% CI 2.98–22.99) (Kubota et al., 2017), fruquintinib combined with Best supportive care (All-grade: RR 6.89, 95% CI 0.95–49.92; 1–2 grade: RR 9.50, 95% CI 0.57–157.94; 3–4 grade: RR 2.46, 95% CI 0.30–20.12) (Lu et al., 2018), ramucirumab combined with erlotinib (All-grade: RR 3.72, 95% CI 2.54–5.45; 1–2 grade: RR 3.21, 95% CI 1.86–5.57; 3–4 grade: RR 4.35, 95% CI 2.39–7.93) (Nakagawa et al., 2019), Paclitaxel combined with bevacizumab (All-grade: RR 22.50, 95% CI 1.39–364.14; 1–2 grade: RR 14.50, 95% CI 0.88–238.66; 3–4 grade: RR 8.50, 95% CI 0.50–144.62) (Cortot et al., 2020). Depending on the above data, Figure 4, in 8 RCTs of non-small cell lung cancer, the highest RR of hypertension caused by Paclitaxel combined with bevacizumab, With the increase of the number of patients, ramucirumab combined with erlotinib has a relatively small and better chance of inducing hypertension in the treatment of non-small cell lung cancer.
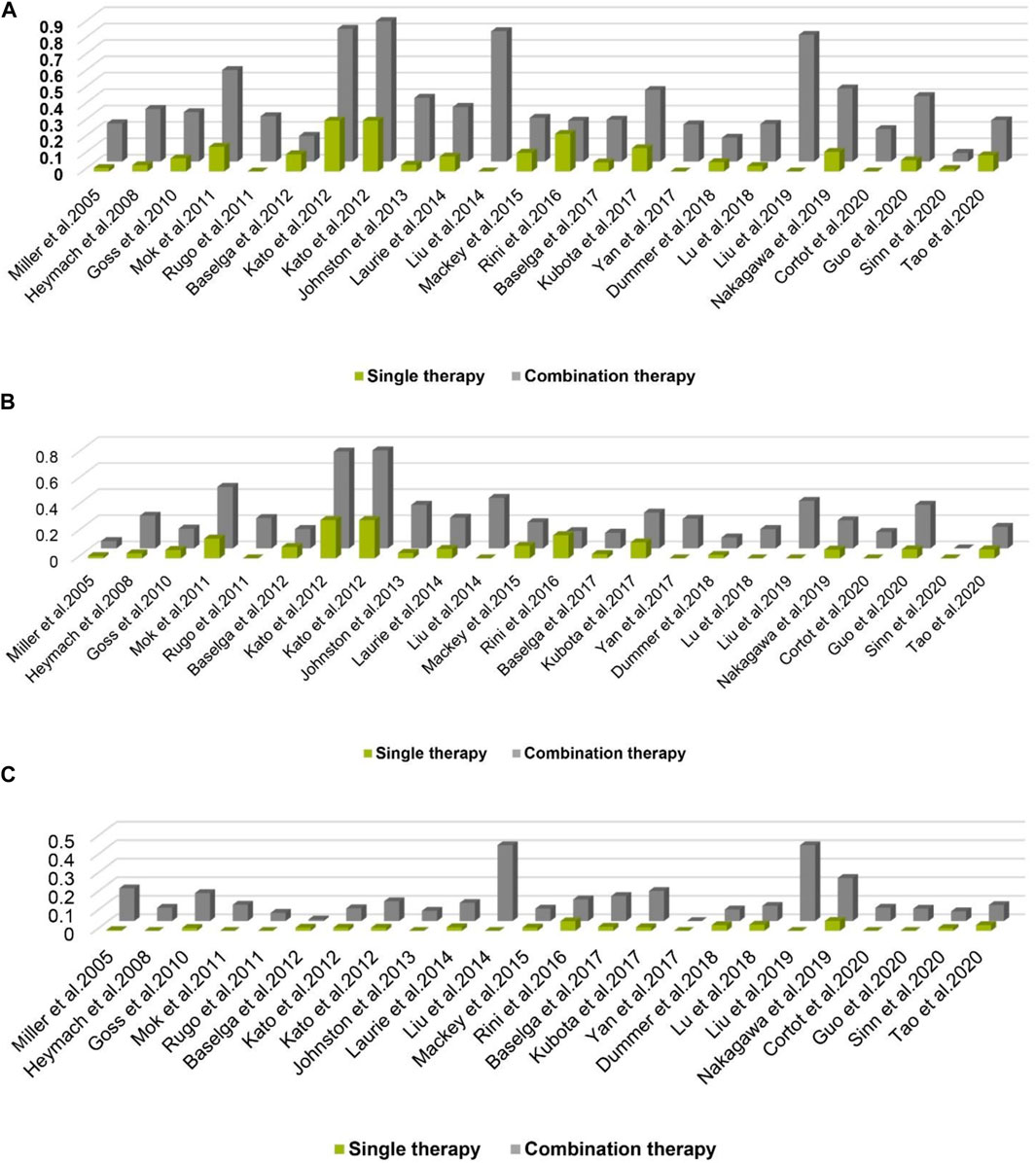
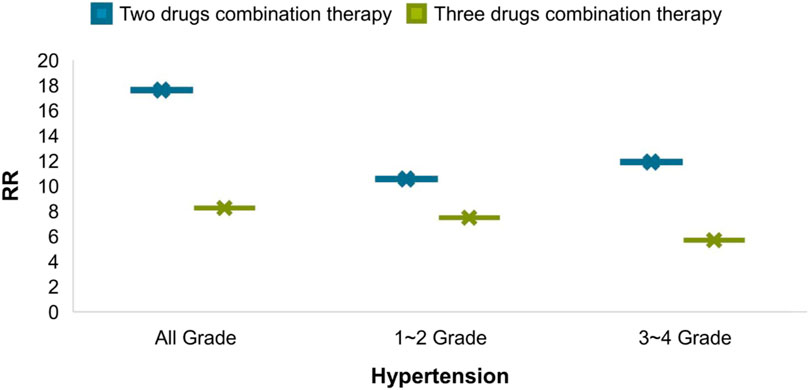
From an intuitive point of view, the incidence of hypertension caused by combination therapy of cancer is higher than that of single therapy, whether it is at all-grade, 1–2 grade or 3–4 grade hypertension, the results shown in Figure 5A–C. As cancer combination therapy regimens, Figure 6, the result of analyze show that the RR of hypertension caused by two drugs combination therapy is higher than three drugs combination therapy, because there are very few plans of multi-drug (n > 3) combination therapy, it is not included as a comparison. For more details of the other schemes, please refer to Table 1.
Heterogeneity and Bias of Included Studies
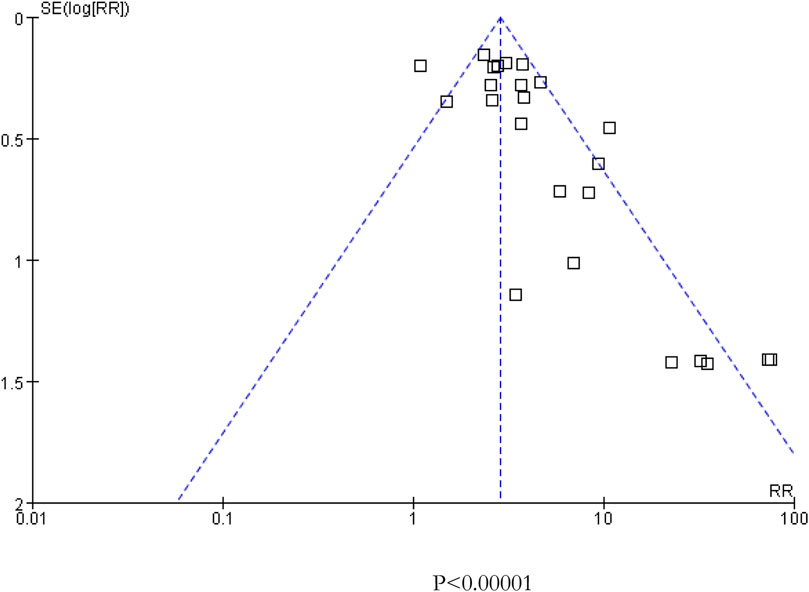
As presented in Figure 2, there was moderate heterogeneity in grade 1–2 hypertension (I2 = 55%, p < 0.001), low heterogeneity in grade 3–4 hypertension (I2 = 5%, p = 0.39), and moderate heterogeneity in all grades of hypertension (I2 = 68%, p < 0.001) caused by cancer combination therapy, with the presence of statistical significance. Using the risk-of-bias assessment tool (Higgins et al., 2011), the results of the Cochrane risk-of-bias assessment of the enrolled 23 RCTs are shown in Table 4 and Figures 7–9 showed that the funnel plot indicated evidence of heterogeneities and publication bias in the studies included in the meta-analysis with scatters beyond 95% CI and asymmetry display (p < 0.00001).
Discussion
To our knowledge, the present meta-analysis for the first time evaluated the potential risk of hypertension in cancer patients treated with combination therapy. As a “silent killer,”, hypertension has been reported to have a doubled prevalence in the past 40 years, with 7.6 million people dying of hypertension annually in the world (Arima et al., 2011). Despite no significant direct influence, long-term hypertension may result in damage of the heart and blood vessels, and cerebral artery vasospasm as well.
In the field of Cardio-Oncology, cancer combination therapy may produce the effective outcome in killing cancer cells and controlling the deterioration of cancer. Nevertheless, there is an inevitable adverse effect of heart disease, especially the occurrence of hypertension. In this regard, there is an urgent need for medical staff to adjust the therapeutic schemes of patients, timely prevent and alleviate side effects during and after cancer treatment, to ensure the life safety of patients.
Current anti-hypertensive therapeutics included Selective α1 adrenoceptor antagonist, non-selective α1 and α2-antagonists, β-adrenoceptor antagonists, angiotensin II receptor blockers, calcium channel blockers, ACE inhibitors, renin inhibitors, direct vasodilators, loop diuretics (Kumar et al., 2020). However, we should pay more attention to the related complications which they are accompanied, such as organ damage, hypotension and so on (Kumar et al., 2020).
In our meta-analysis, based on the collection of all relevant data from retrospective clinical trials, the final objects of study were a total of 23 clinical trials involving 6,241 patients. The combination therapy of cancer patients resulted in a higher risk of developing hypertension (All-grade: RR 2.85, 95% CI 2.52–3.22; 1–2 grade: RR 2.43, 95% CI 2.10–2.81; 3–4 grade: RR 4.37, 95% CI 3.33–5.72). According to the results, the risk of grade 3–4 hypertension induced by cancer combination therapy was higher than that of the control group with or without placebo therapy.
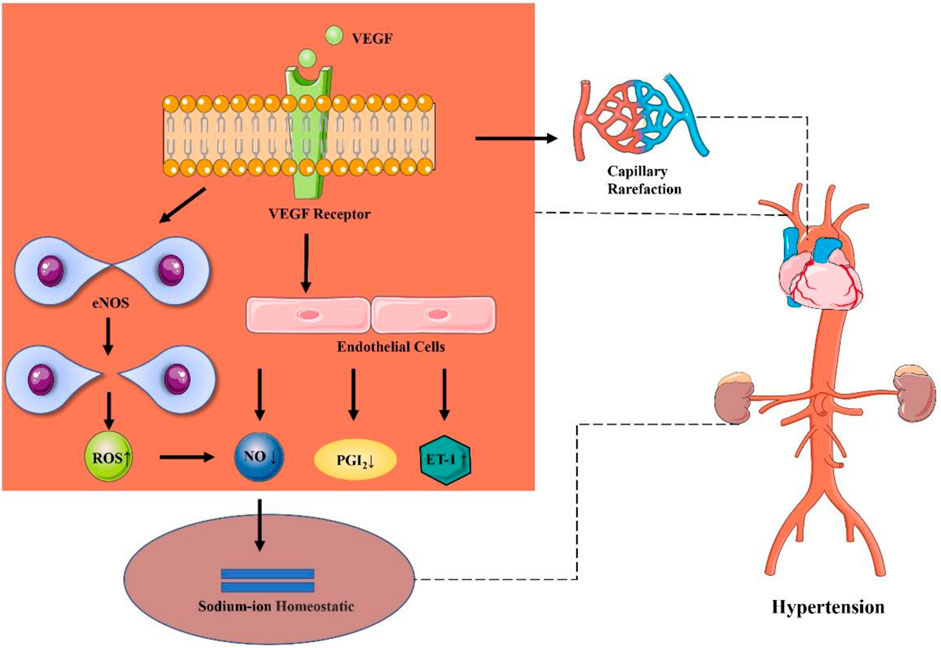
There may exist different mechanisms of increase in blood pressure under different anti-cancer therapeutic schemes. The mechanism of elevated blood pressure by using anti-cancer drugs may exhibit a direct association with its anti-cancer mechanism. The mechanism of hypertension induced by cancer combination therapy may be explained by the following reasons. To be specific, monoclonal antibodies (for example, bevacizumab) may reduce the number of capillaries in microcirculation, competitively inhibit the binding of EGFR with other ligands, and block the interaction between VEGF and endothelial cell surface receptors, resulting in inhibit the signal pathway of VEGF, reduce the activity of endothelial nitric oxide synthase and the production of NO and PGI2 by vascular endothelial cells, decrease vascular permeability and vasodilation, increased peripheral vascular resistance and blood flow, and finally lead to hypertension (Chen et al., 2011; Mayer et al., 2011; Mourad and Levy, 2011; Campia et al., 2019). Besides, it has been reported that reducing the activity of eNOS will lead to expression of uncoupling protein of eNOS, produces a large amount of reactive oxygen species and then decrease the level of NO (Kumar et al., 2020). Meanwhile, NO is involved in maintaining the steady state of sodium ions and participating in tubuloglomerular feedback to regulate renal blood flow and glomerular filtration, which can increase systemic blood pressure (Lankhorst et al., 2017). Another possible mechanism of hypertension caused by inhibiting other VEGF pathways is that angiogenesis inhibitors may reduce the number of blood vessels and lead to hypertension owing to the thinning of peripheral microvessels (Aparicio-Gallego et al., 2011). In addition, additional research also reveals that the increase in blood pressure may be related to the inhibition of VEGFR-2 (Kamba and McDonald, 2007). Also, Small molecular targeted drugs (such as sunitinib) can upregulate endothelin-1, increase salt sensitivity, and further increase in blood pressure owing to thrombotic glomerular injury (Kidoguchi et al., 2021). In addition, some novel targeted drugs (e.g., brutinib) may increase the risk of hypertension by inhibiting PI3K/Akt or reducing the level of NO (Dickerson et al., 2019). (Figure 10)
With respect to the above, there is necessary to adopt targeted treatment of hypertension. Before the treatment of cancer patients, it is recommended to adopt a comprehensive risk assessment, including blood pressure measurement and examination of known risk factors. For cancer patients with existed cardiovascular diseases, it is necessary to consider carefully whether to use anti-cancer drugs that may lead to cardiotoxicity or not. In the field of Cardio-Oncology, further consideration of the overall health status of patients is required for doctors to make a prudent decision in patients with a high risk of hypertension and those with hypertension prior to the use of anti-cancer drugs. Moreover, in case of poor control of cancer development by monotherapy, the better therapeutic outcome may be produced by combination therapy, However, it should be noted that combination therapy may also lead to a higher risk of hypertension.
So far, there is still no systematic analysis of hypertension caused by cancer combination therapy. Data in our study fully supports that cancer combination therapy has a high risk of inducing hypertension. Findings in this meta-analysis suggest that much attention shall be paid constantly to the adverse reactions of combined use of drugs, with in-time prevention required simultaneously. However, there are limitations in this study. For example, due to the absence of experimental data, relevant experiments are needed in the future to fully clarify the pathophysiological basis of hypertension caused by the combination of drugs and to increase the credibility of the results of our study.
Conclusion
The accuracy of meta-analysis research is high, but there is also a certain degree of publication bias, and risk of bias is low. It is worth mentioned that the reliability of meta-analysis results as well as the suitability in clinical practice might still requires critical thinking and objective judgments.
To sum up, the present meta-analysis carries out a comprehensive analysis on the risk of patients suffering from hypertension in the process of multiple cancer combination therapies. Findings in our study support that the risk of hypertension may increase significantly in cancer patients with multiple cancer combination therapies. The outcomes of this meta-analysis may provide a reference value for clinical practice and may supply insights in reducing the incidence of hypertension caused by cancer combined treatment.
Author Contributions
XG, XQ, YJ, XK, ZQ, TC, LZ, CW, WL: Study concept and design; acquisition of data; statistical analysis; interpretation of data; drafting and critical review of the manuscript for important intellectual content; approval of the final version of the manuscript.
Funding
This review was supported by the National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2020ZX09201005); National Natural Science Foundation of China (81803621 and U1903119); National Key R&D Program of China (2018YFC1315000 and 2018YFC1315003). Fundamental Research Funds for the Central Universities (20720200052 and 2020XJHH05).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, M., Thareja, N., Benjamin, M., Akhondi, A., and Mitchell, G. D. (2018). Tyrosine Kinase Inhibitor-Induced Hypertension. Curr. Oncol. Rep. 20 (8), 65. doi:10.1007/s11912-018-0708-8
Aparicio-Gallego, G., Afonso-Afonso, F. J., León-Mateos, L., Fírvida-Pérez, J. L., Vázquez-Estévez, S., Lázaro-Quintela, M., et al. (2011). Molecular Basis of Hypertension Side Effects Induced by Sunitinib. Anticancer Drugs 22 (1), 1–8. doi:10.1097/CAD.0b013e3283403806
Arima, H., Barzi, F., and Chalmers, J. (2011). Mortality Patterns in Hypertension. J. Hypertens. 29 Suppl 1 (Suppl. 1), S3–S7. doi:10.1097/01.hjh.0000410246.59221.b1
Barac, A. (2020). Cardio-Oncology in 2020: Prime for Translation. J. Cardiovasc. Transl Res. 13 (3), 345–346. doi:10.1007/s12265-020-10036-1
Baselga, J., Segalla, J. G., Roché, H., Del Giglio, A., Pinczowski, H., Ciruelos, E. M., et al. (2012). Sorafenib in Combination with Capecitabine: An Oral Regimen for Patients with HER2-Negative Locally Advanced or Metastatic Breast Cancer. J. Clin. Oncol. 30 (13), 1484–1491. doi:10.1200/JCO.2011.36.7771
Baselga, J., Zamagni, C., Gómez, P., Bermejo, B., Nagai, S. E., Melichar, B., et al. (2017). RESILIENCE: Phase III Randomized, Double-Blind Trial Comparing Sorafenib with Capecitabine versus Placebo with Capecitabine in Locally Advanced or Metastatic HER2-Negative Breast Cancer. Clin. Breast Cancer 17 (8), 585–e4. doi:10.1016/j.clbc.2017.05.006
Brinda, B. J., Viganego, F., Vo, T., Dolan, D., and Fradley, M. G. (2016). Anti-VEGF-Induced Hypertension: a Review of Pathophysiology and Treatment Options. Curr. Treat. Options. Cardiovasc. Med. 18 (5), 33. doi:10.1007/s11936-016-0452-z
Campia, U., Moslehi, J. J., Amiri-Kordestani, L., Barac, A., Beckman, J. A., Chism, D. D., et al. (2019). Cardio-Oncology: Vascular and Metabolic Perspectives: A Scientific Statement from the American Heart Association. Circulation 139, e579–e602. doi:10.1161/CIR.0000000000000641
Chen, T., Xu, T., Li, Y., Liang, C., Chen, J., Lu, Y., et al. (2011). Risk of Cardiac Dysfunction with Trastuzumab in Breast Cancer Patients: a Meta-Analysis. Cancer Treat. Rev. 37, 312–320. doi:10.1016/j.ctrv.2010.09.001
Cortot, A. B., Audigier-Valette, C., Molinier, O., Le Moulec, S., Barlesi, F., Zalcman, G., et al. (2020). Weekly Paclitaxel Plus Bevacizumab versus Docetaxel as Second- or Third-Line Treatment in Advanced Non-squamous Non-small-cell Lung Cancer: Results of the IFCT-1103 ULTIMATE Study. Eur. J. Cancer 131, 27–36. doi:10.1016/j.ejca.2020.02.022
Dickerson, T., Wiczer, T., Waller, A., Philippon, J., Porter, K., Haddad, D., et al. (2019). Hypertension and Incident Cardiovascular Events Following Ibrutinib Initiation. Blood 134 (22), 1919–1928. doi:10.1182/blood.2019000840
Dummer, R., Ascierto, P. A., Gogas, H. J., Arance, A., Mandala, M., Liszkay, G., et al. (2018). Overall Survival in Patients with BRAF-Mutant Melanoma Receiving Encorafenib Plus Binimetinib versus Vemurafenib or Encorafenib (COLUMBUS): a Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 19 (10), 1315–1327. doi:10.1016/S1470-2045(18)30497-2
Folkman, J. (2007). Angiogenesis: an Organizing Principle for Drug Discovery? Nat. Rev. Drug Discov. 6 (4), 273–286. doi:10.1038/nrd2115
Goss, G. D., Arnold, A., Shepherd, F. A., Dediu, M., Ciuleanu, T. E., Fenton, D., et al. (2010). Randomized, Double-Blind Trial of Carboplatin and Paclitaxel with Either Daily Oral Cediranib or Placebo in Advanced Non-small-cell Lung Cancer: NCIC Clinical Trials Group BR24 Study. J. Clin. Oncol. 28 (1), 49–55. doi:10.1200/JCO.2009.22.9427
Guo, Q., Sun, Y., Kong, E., Rao, L., Chen, J., Wu, Q., et al. (2020). Apatinib Combined with Chemotherapy or Concurrent Chemo-Brachytherapy in Patients with Recurrent or Advanced Cervical Cancer: A Phase 2, Randomized Controlled, Prospective Study. Medicine (Baltimore) 99 (11), e19372. doi:10.1097/MD.0000000000019372
Hamnvik, O. P., Choueiri, T. K., Turchin, A., McKay, R. R., Goyal, L., Davis, M., et al. (2015). Clinical Risk Factors for the Development of Hypertension in Patients Treated with Inhibitors of the VEGF Signaling Pathway. Cancer 121 (2), 311–319. doi:10.1002/cncr.28972
Heymach, J. V., Paz-Ares, L., De Braud, F., Sebastian, M., Stewart, D. J., Eberhardt, W. E., et al. (2008). Randomized Phase II Study of Vandetanib Alone or with Paclitaxel and Carboplatin as First-Line Treatment for Advanced Non-small-cell Lung Cancer. J. Clin. Oncol. 26 (33), 5407–5415. doi:10.1200/JCO.2008.17.3138
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Huedo-Medina, T. B., Sánchez-Meca, J., Marín-Martínez, F., and Botella, J. (2006). Assessing Heterogeneity in Meta-Analysis: Q Statistic or I2 index? Psychol. Methods 11 (2), 193–206. doi:10.1037/1082-989X.11.2.193
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., Gavaghan, D. J., et al. (1996). Assessing the Quality of Reports of Randomized Control Trials: Is Blinding Necessary? Control. Clin. Trials 17 (1), 1–12. doi:10.1016/0197-2456(95)00134-4
Jain, M., and Townsend, R. R. (2007). Chemotherapy Agents and Hypertension: a Focus on Angiogenesis Blockade. Curr. Hypertens. Rep. 9 (4), 320–328. doi:10.1007/s11906-007-0058-7
Johnston, S. R., Gómez, H., Stemmer, S. M., Richie, M., Durante, M., Pandite, L., et al. (2013). A Randomized and Open-Label Trial Evaluating the Addition of Pazopanib to Lapatinib as First-Line Therapy in Patients with HER2-Positive Advanced Breast Cancer. Breast Cancer Res. Treat. 137 (3), 755–766. doi:10.1007/s10549-012-2399-4
Kamba, T., and McDonald, D. M. (2007). Mechanisms of Adverse Effects of Anti-VEGF Therapy for Cancer. Br. J. Cancer 96 (12), 1788–1795. doi:10.1038/sj.bjc.6603813
Kato, T., Muro, K., Yamaguchi, K., Bando, H., Hazama, S., Amagai, K., et al. (2012). Cediranib in Combination with mFOLFOX6 in Japanese Patients with Metastatic Colorectal Cancer: Results from the Randomised Phase II Part of a Phase I/II Study. Ann. Oncol. 23 (4), 933–941. doi:10.1093/annonc/mdr359
Kidoguchi, S., Sugano, N., Tokudome, G., Yokoo, T., Yano, Y., Hatake, K., et al. (2021). New Concept of Onco-Hypertension and Future Perspectives. Hypertension 77 (1), 16–27. doi:10.1161/HYPERTENSIONAHA.120.16044
Kubota, K., Yoshioka, H., Oshita, F., Hida, T., Yoh, K., Hayashi, H., et al. (2017). Phase III, Randomized, Placebo-Controlled, Double-Blind Trial of Motesanib (AMG-706) in Combination with Paclitaxel and Carboplatin in East Asian Patients with Advanced Nonsquamous Non-small-cell Lung Cancer. J. Clin. Oncol. 35 (32), 3662–3670. doi:10.1200/JCO.2017.72.7297
Kumar, G., Dey, S. K., and Kundu, S. (2020). Functional Implications of Vascular Endothelium in Regulation of Endothelial Nitric Oxide Synthesis to Control Blood Pressure and Cardiac Functions. Life Sci. 259, 118377. doi:10.1016/j.lfs.2020.118377
Lankhorst, S., Severs, D., Markó, L., Rakova, N., Titze, J., Müller, D. N., et al. (2017). Salt Sensitivity of Angiogenesis Inhibition-Induced Blood Pressure Rise: Role of Interstitial Sodium Accumulation? Hypertension 69 (5), 919–926. doi:10.1161/HYPERTENSIONAHA.116.08565
Laurie, S. A., Solomon, B. J., Seymour, L., Ellis, P. M., Goss, G. D., Shepherd, F. A., et al. (2014). Randomised, Double-Blind Trial of Carboplatin and Paclitaxel with Daily Oral Cediranib or Placebo in Patients with Advanced Non-small Cell Lung Cancer: NCIC Clinical Trials Group Study BR29. Eur. J. Cancer 50 (4), 706–712. doi:10.1016/j.ejca.2013.11.032
Lenneman, C. G., and Sawyer, D. B. (2016). Cardio-Oncology: An Update on Cardiotoxicity of Cancer-Related Treatment. Circ. Res. 118 (6), 1008–1020. doi:10.1161/CIRCRESAHA.115.303633
Liu, J. F., Barry, W. T., Birrer, M., Lee, J. M., Buckanovich, R. J., Fleming, G. F., et al. (2014). Combination Cediranib and Olaparib versus Olaparib Alone for Women with Recurrent Platinum-Sensitive Ovarian Cancer: a Randomised Phase 2 Study. Lancet Oncol. 15 (11), 1207–1214. doi:10.1016/S1470-2045(14)70391-2
Liu, J. F., Barry, W. T., Birrer, M., Lee, J. M., Buckanovich, R. J., Fleming, G. F., et al. (2019). Overall Survival and Updated Progression-free Survival Outcomes in a Randomized Phase II Study of Combination Cediranib and Olaparib versus Olaparib in Relapsed Platinum-Sensitive Ovarian Cancer. Ann. Oncol. 30 (4), 551–557. doi:10.1093/annonc/mdz018
Lu, S., Chang, J., Liu, X., Shi, J., Lu, Y., Li, W., et al. (2018). Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Fruquintinib after Two Prior Chemotherapy Regimens in Chinese Patients with Advanced Nonsquamous Non‒Small-Cell Lung Cancer. J. Clin. Oncol. 36 (12), 1207–1217. doi:10.1200/JCO.2017.76.7145
Mackey, J. R., Ramos-Vazquez, M., Lipatov, O., McCarthy, N., Krasnozhon, D., Semiglazov, V., et al. (2015). Primary Results of ROSE/TRIO-12, a Randomized Placebo-Controlled Phase III Trial Evaluating the Addition of Ramucirumab to First-Line Docetaxel Chemotherapy in Metastatic Breast Cancer. J. Clin. Oncol. 33 (2), 141–148. doi:10.1200/JCO.2014.57.1513
Maitland, M. L., Bakris, G. L., Black, H. R., Chen, H. X., Durand, J. B., Elliott, W. J., et al. (2010). Initial Assessment, Surveillance, and Management of Blood Pressure in Patients Receiving Vascular Endothelial Growth Factor Signaling Pathway Inhibitors. J. Natl. Cancer Inst. 102 (9), 596–604. doi:10.1093/jnci/djq091
Mayer, E. L., Dallabrida, S. M., Rupnick, M. A., Redline, W. M., Hannagan, K., Ismail, N. S., et al. (2011). Contrary Effects of the Receptor Tyrosine Kinase Inhibitor Vandetanib on Constitutive and Flow-Stimulated Nitric Oxide Elaboration in Humans. Hypertension 58 (1), 85–92. doi:10.1161/HYPERTENSIONAHA.110.168120
Miller, K. D., Chap, L. I., Holmes, F. A., Cobleigh, M. A., Marcom, P. K., Fehrenbacher, L., et al. (2005). Randomized Phase III Trial of Capecitabine Compared with Bevacizumab Plus Capecitabine in Patients with Previously Treated Metastatic Breast Cancer. J. Clin. Oncol. 23 (4), 792–799. doi:10.1200/JCO.2005.05.098
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. Plos Med. 6 (7), e1000097. doi:10.1371/journal.pmed.1000097
Mok, T. S., Hsia, T. C., Tsai, C. M., Tsang, K., Chang, G. C., Chang, J. W., et al. (2011). Efficacy of Bevacizumab with Cisplatin and Gemcitabine in Asian Patients with Advanced or Recurrent Non-squamous Non-small Cell Lung Cancer Who Have Not Received Prior Chemotherapy: A Substudy of the Avastin in Lung Trial. Asia Pac. J. Clin. Oncol. 7 Suppl 2 (Suppl. 2), 4–12. doi:10.1111/j.1743-7563.2011.01397.x
Mourad, J. J., and Levy, B. I. (2011). Mechanisms of Antiangiogenic-Induced Arterial Hypertension. Curr. Hypertens. Rep. 13 (4), 289–293. doi:10.1007/s11906-011-0206-y
Nakagawa, K., Garon, E. B., Seto, T., Nishio, M., Ponce Aix, S., Paz-Ares, L., et al. (2019). Ramucirumab Plus Erlotinib in Patients with Untreated, EGFR-Mutated, Advanced Non-small-cell Lung Cancer (RELAY): a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 20 (12), 1655–1669. doi:10.1016/S1470-2045(19)30634-5
National Cancer Institute (U.S) (2017). Common Terminology Criteria for Adverse Events (CTCAE). Bethesda, MD: U.S. Department of Health and Human Services,National Institutes of Health, National Cancer Institute, 153.
Piccirillo, J. F., Tierney, R. M., Costas, I., Grove, L., and Spitznagel, E. L. (2004). Prognostic Importance of Comorbidity in a Hospital-Based Cancer Registry. JAMA 291 (20), 2441–2447. doi:10.1001/jama.291.20.2441
Rini, B. I., Stenzl, A., Zdrojowy, R., Kogan, M., Shkolnik, M., Oudard, S., et al. (2016). IMA901, a Multipeptide Cancer Vaccine, Plus Sunitinib versus Sunitinib Alone, as First-Line Therapy for Advanced or Metastatic Renal Cell Carcinoma (IMPRINT): a Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 17 (11), 1599–1611. doi:10.1016/S1470-2045(16)30408-9
Rugo, H. S., Stopeck, A. T., Joy, A. A., Chan, S., Verma, S., Lluch, A., et al. (2011). Randomized, Placebo-Controlled, Double-Blind, Phase II Study of Axitinib Plus Docetaxel versus Docetaxel Plus Placebo in Patients with Metastatic Breast Cancer. J. Clin. Oncol. 29 (18), 2459–2465. doi:10.1200/JCO.2010.31.2975
Said, R., Nickolich, M., Lenihan, D. J., and Tsimberidou, A. M. (2017). Cardio-Oncology, 15–42. doi:10.1007/978-3-319-43096-6_2Cardiotoxicity of Anticancer Therapies
Sinn, M., Liersch, T., Riess, H., Gellert, K., Stübs, P., Waldschmidt, D., et al. (2020). CONKO-006: A Randomised Double-Blinded Phase IIb-Study of Additive Therapy with Gemcitabine + Sorafenib/placebo in Patients with R1 Resection of Pancreatic Cancer - Final Results. Eur. J. Cancer 138, 172–181. doi:10.1016/j.ejca.2020.06.032
Tao, W., Yang, J., Jiang, Y., Chen, W., and Wang, Y. (2020). Paclitaxel, Carboplatin, and Bevacizumab in Advanced Cervical Cancer: A Treatment Response and Safety Analysis. Dose Response 18 (3), 1559325820941351. doi:10.1177/1559325820941351
Wilkins, J. R., Pike, D. B., Gibson, C. C., Kubota, A., and Shiu, Y. T. (2014). Differential Effects of Cyclic Stretch on bFGF- and VEGF-Induced Sprouting Angiogenesis. Biotechnol. Prog. 30 (4), 879–888. doi:10.1002/btpr.1883
Yan, X., Zhao, Y., Wang, H., Geng, Y., Yang, Q., Dong, L., et al. (2017). Observation of the Effect of Apatinib Combined with Oxaliplatin and Tiogio in the Treatment of Advanced Gastric Cancer. Cancer Res. Clinic 29 (11), 761–764. doi:10.3760/cma.j.issn.1006-9801.2017.11.010
Zhang, L., Zou, H., Zhao, Y., Hu, C., Atanda, A., Qin, X., et al. (2019). Association between Blood Circulating Vitamin D and Colorectal Cancer Risk in Asian Countries: a Systematic Review and Dose-Response Meta-Analysis. BMJ Open 9 (12), e030513. doi:10.1136/bmjopen-2019-030513
Keywords: hypertension, combination therapy, angiogenesis inhibitors, meta-analysis, randomized controlled trial
Citation: Guo X, Qian X, Jin Y, Kong X, Qi Z, Cai T, Zhang L, Wu C and Li W (2021) Hypertension Induced by Combination Therapy of Cancer: A Systematic Review and Meta-Analysis of Global Clinical Trials. Front. Pharmacol. 12:712995. doi: 10.3389/fphar.2021.712995
Received: 25 May 2021; Accepted: 20 August 2021;
Published: 06 September 2021.
Edited by:
Robert Clarke, University of Minnesota Twin Cities, United StatesReviewed by:
Sonam Mittal, Medical College of Wisconsin, United StatesFrancesca Maffei, University of Bologna, Italy
Copyright © 2021 Guo, Qian, Jin, Kong, Qi, Cai, Zhang, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhang, dG9ueTE5ODIxMTBAZ21haWwuY29t; Caisheng Wu, d3Vjc2hAeG11LmVkdS5jbg==; Weihua Li, bGl3ZWlodWFAeG11LmVkdS5jbg==
†These authors have contributed equally to this article.
 Xiaodan Guo1†
Xiaodan Guo1† Lin Zhang
Lin Zhang Caisheng Wu
Caisheng Wu