
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 08 November 2021
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.708522
 Tao Zhong1*
Tao Zhong1* Yuzheng Fan1
Yuzheng Fan1 Xiao-Li Dong2,3
Xiao-Li Dong2,3 Xujun Guo1
Xujun Guo1 Ka Hing Wong2,3
Ka Hing Wong2,3 Wing-tak Wong2,3
Wing-tak Wong2,3 Daihai He4,5*
Daihai He4,5* Shengyuan Liu1*
Shengyuan Liu1*Objectives: To identify the risk factors associated with anti-tuberculosis drug-induced liver injury (AT-DILI) or abnormal living functioning from 757 patients with pulmonary tuberculosis (TB) registered at Nanshan Center for Chronic Disease Control (Nanshan CCDC), Shenzhen, Guangdong Province, China.
Design and methods: We identified 757 TB patients who met our inclusion criteria by screening the Hospital Information System (HIS) at Nanshan CCDC. Next, we identified positive cases of AT-DILI or abnormal liver functioning based on results of the first-time liver function tests (LFTs) after taking anti-TB drugs. The χ2 test was used to relate the positive rate with a variety of factors. A logistic regression model was also used to identify statistically significant risk factors.
Results: Of the 757 patients, the positive rate of AT-DILI or abnormal liver functioning was 37.9% (287/757). Univariate analysis revealed that the positive rate was 42.91% (212/494) for males and 28.52% (75/263) for females. The positive rate was significantly higher in males (p <0.001). Patients with an annual income of 9,231–13,845 USD had a significantly higher positive rate (67.35%; 33/49) than those with an income of 1,540–4616 USD (37.97%; 30/79) (p = 0.022). The most frequent prescription regime among positive cases was a 2 months supply of fixed dose combination Ethambutol Hydrochloride, Pyrazinamide, Rifampicin and Isoniazid Tablets (Ⅱ) 450 mg) followed by a 4 months supply of fixed dose combination Rifampin and Isoniazid Capsules (2FDC-HRZE half/4FDC-HR) at 56.03% (144/257). The least frequent prescription regime was a 2 months supply of fixed dose combination Rifampin, Isoniazid and Pyrazinamide Capsules with Ethambutol independently followed by a 4 months supply of fixed dose combination Rifampin and Isoniazid Capsules (2FDC-HRZ + EMB/4FDC-HR) at 24.27% (25/103). The difference between these two different regimes was significant (p = 0.022). With an increase in the duration of medication, patients under various prescription regimes all showed a gradual increase in the positive rate of AT-DILI or abnormal liver functioning.
Conclusion: We identified several risk factors for the occurrence of AT-DILI or abnormal liver functioning, including gender, annual income, prescription regime, dosage, and treatment time.
Pulmonary Tuberculosis (TB) is a chronic infectious disease that predominantly attacks the lungs and seriously endangers human health. TB is caused by Mycobacterium tuberculosis, a bacterium that is spread throughout populations via coughs and sneezes. It is critical that we treat TB in an effective manner if we are to reduce morbidity, mortality, and to control epidemics. The treatment of TB includes the oral administration of four different drugs over a period of 6 months. However, both the International Union Against Tuberculosis and Lung Diseases (IUATLD) and the World Health Organization (WHO) recommend prescribers to use fixed-dose combinations (FDCs) in which the four drugs are combined in one tablet (Girling et al., 1988). The application of FDCs can simplify prescriptions, reduce drug abuse, improve patient compliance, and effectively prevent the rising trend of TB drug resistance caused by irrational and irregular treatment (World Health Organization, 1994). The WHO also recommends short courses of FDCs lasting 6–8 months; this strategy has also been proven to be effective for the treatment of TB. However, in practice, studies also showed that some patients could not complete the entire course of treatment, thus leading to a series of adverse effects on epidemic control and even the emergence of multidrug-resistance against TB (The Global Plan to Stop T, 2006; Zigonl et al., 2006).
Many factors may cause treatment interruptions in patients with TB; one of the most important is anti-tuberculosis drug-induced liver injury (AT-DILI). A previous study reported that 11.90% of all liver injuries were found to be attributed to the use of anti-TB drugs (Xia and Zhan, 2007). Another study, involving 4,577 patients taking FDCs across four cities in China, showed that 10.47% of all patients stopped FDC therapy due to liver injury (Kong and Qiao, 2014). Kabbara et al. (2016) reported that the application of isoniazid as a single drug also caused severe liver injury. Research data from the Chronic Disease Prevention and Control Hospital of Nanshan (Nanshan CDC), Shenzhen, China showed that 27% of TB patients registered for FDC treatment from October 2013 to September 2014 stopped TB medications due to drug-induced liver injury (Zhong, 2016).
Nanshan Center for Chronic Disease Control (Nanshan CCDC) is the only public TB-designated medical institution in Nanshan District, Shenzhen, Guangdong Province of China. The functions of this Institute include the prevention, control, diagnosis, and treatment of TB. However, the Institute only provides outpatient treatment for patients with TB and does not provide treatment for more serious underlying diseases or comorbidities such as liver cirrhosis and immunodeficiency. When patients with TB undergo treatment in this Institute, the medical staff provide consistent follow-up over the entire course of treatment. They also observe and record side effects and urge patients to adhere to their daily medications. Some patients experience discomfort due to abnormal liver function or liver injury, and then proceed to stop the medication or refuse treatment on their own initiative, thus leading to treatment failure. In this study, we investigated the risk factors associated with drug-induced liver injury during the treatment of TB. Providing early interventions to address key risk factors will help to prevent further deterioration in liver function and improve treatment compliance. During this study, we acquired and analyzed a series of data from our Hospital Information System (HIS), including individual patient information, prescription regimes, and liver function tests (LFTs).
We used our HIS system to identify all patients with TB who were registered and treated in our hospital between 2014 and 2019. For each patient, we acquired a range of information, including age, gender (biological sex), education background, income, body mass index (BMI), prescription regimen, duration and dosage of medication, and liver function results. We also considered comorbidities in any patients receiving treatment for TB, including hepatitis B and diabetes. Cases with confirmed hepatitis B, diabetes, and abnormal liver function prior to treatment were excluded. Finally, 757 patients with TB patients who underwent their first round of liver function tests during treatment with anti-TB drugs were recruited. The data collected in this study were not sufficiently detailed to allow scoring on the Roussel Uclaf Causality Assessment Method (RUCAM) scale. Manual for Diagnosis and Treatment of Adverse Tuberculosis Drug Reactions (2009 Edition) is routinely used for diagnosis and treatment at designated TB medical institutions in China and can be used to classify liver function in patients and define abnormal liver function (
Between 2014 and 2019, eight types of first-line anti-TB drugs were prescribed in Nanshan District. Of these, there were four types of FDCs, including 1) FDC-HRZ (Rifampin, Isoniazid and Pyrazinamide Capsules), 2) FDC-HRZE (Ethambutol Hydrochloride, Pyrazinamide, Rifampicin and Isoniazid Tablets (Ⅱ) 900 mg), 3) FDC-HRZE half (Ethambutol Hydrochloride, Pyrazinamide, Rifampicin and Isoniazid Tablets (Ⅱ) 450 mg), and 4) FDC-HR (Rifampin and Isoniazid Capsules). There were four drugs that were prescribed independently, including 1) Isoniazid (INH), 2) Rifampicin (RFP), 3) Ethambutol (EMB), and 4) Pyrazinamide (PZA). The dosage and usage of these drugs are described in the “Guidelines for the Implementation of China’s Tuberculosis Prevention and Control Plan” (The Disease Control and P, 2008). Four prescription regimes were commonly used in our institute: 1) 2 months of FDC-HRZE-half followed by 4 months of FDC-HR (2 FDC-HRZE-half/4FDC-HR; 2) 2 months of FDC-HRZE followed by 4 months of FDC-HR (2FDC-HRZE/4FDC-HR); 3) 2 months of FDC-HRZ with Ethambutol independently (FDC-HRZ + EMB) followed by 4 months of FDC-HR (2FDC-HRZ + EMB/4FDC-HR); 4) 2 months of HRZE followed by 4 months of HR (2HRZE/4HR). Further details are given in Table 1.
To remove invalid and defective data, we performed data cleaning on personal information, some of the physiological testing data, and prescription information for TB patients. Valid data were then encoded. TB patients who met the inclusion criteria were then identified by R software. Component proportion ratios, based on the different demographic characteristics of patients, were analyzed with incidence descriptions, and the
A total of TB patients taking anti-TB drugs were included in this study, including 494 males (65.26%) and 263 females (34.74%). The age distribution was as follows: 21–30 years (43.86%), 31–40 years (20.48%), >50 years (17.97%), 41–50 years (11.49%), ≤20 years (6.21%). In total, 636 patients provided information relating to their educational background: college degree or above (43.4%), junior high school or below (32.55%), or high school or technical secondary school (24.06%). In total, 425 patients provided their annual income (USD): 22.59% had an income >13,845 USD, 11.53% had an income between 9,231 and 13,845 USD, 27.76% of patients had an income between 4,615 and 9230 USD, 18.59% had an income between 1540 USD and 4614 USD, and 19.53% had an income of ≤1539 USD (which is the minimum standard of living in Shenzhen). We cut the income into these categories such that each category has a substantial proportion of cases. We obtained height and weight measurements from 627 patients; this allowed us to calculate body mass index. Based on BMI values, the 627 patients were classified as normal (63.32%), thin (25.04%), overweight (10.21%), or obese (1.44%). Further details are shown in Table 2.
The positive cases were diagnosed based on the first-round liver function test results among TB patients after taking anti-TB drugs. We identified a total of 287 positive patients with AT-DILI or abnormal liver functioning, including 212 males (42.91%) and 75 females (28.52%). The proportion of positive cases was significantly higher in males than in females (
Tests showed that TBIL was normal in all positive cases of AT-DILI or abnormal live functioning and that the highest concentration of ALT was
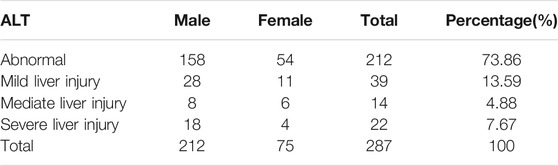
TABLE 3. First-time liver function test results of all subjects after the start of anti-TB drug treatment.
The initial anti-TB treatment scheme generally included an intensive 2 month period followed by another 4 months continuation period. Based on the first-time liver function test results after taking anti-TB drugs, the distribution of positive cases in TB patients under different treatment schemes was as follows in decreasing order: 2FDC-HRZE half/4FDC-HR (56.03%), 2FDC-HRZE/4FDC-HR (31.11%), single-drug combination (2HRZE/4HR) (29.32%), and 2FDC-HRZ + EMB/4FDC-HR (24.27%); there were significant differences between these different drug regimens (
We divided the number of medication days into four 15 days intervals in the first 2 months intensive period, but four 30 days intervals in the following 4 months continuation period. As shown in Table 5; Figure 1, the positive incidence when taking 2HRZE/4HR for ≤15 days was the lowest (14.84%); the highest incidence was after taking drugs for 61–90 days (62.5%); this difference was significant (
A logistic regression model was established to perform multi-factor analysis with positive status as the dependent variable; the independent variables were those that had been found to be significantly related to positive status: gender, annual income, and medication scheme. The assignment of dependent and independent variables is shown in Table 6. Our analysis showed that male gender (
Anti-TB drugs or their metabolites may induce allergic reactions in the liver and lead to hepatitis (Qiu, 2012). The occurrence of AT-DILI or abnormal liver functioning may be attributed to drugs and the physiological condition of patients. Risk factors of AT-DILI or abnormal liver functioning include the type and dosage of drugs, the duration of medication, and the combination of medications. The occurrence of AT-DILI or abnormal liver functioning is also associated with age, gender, genetics, the nutritional condition of patients, and the presence of comorbidities. The combination of INH, RFP, EMB, and PZA, also referred to as an FDC, is the standard first line treatment for TB (Huai et al., 2019). However, 0.8–40% of patients worldwide are known to suffer from AT-DILI induced by anti-TB drugs (Chen et al., 2015).
In the present study, our subjects had normal liver function before taking anti-TB drugs but went on to develop abnormal liver function or AT-DILI after taking drugs. Although we did not grade our patients with the RUCAM score, the risk factors used herein (e.g., prescription regimes) were reconfirmed by clinicians during the diagnosis and treatment of patients in designated TB medical institutions in China. Accordingly, clinicians stopped anti-TB drug treatment in most of the AT-DILI patients and placed them on liver protection treatments until their liver function returned to normal. Consequently, we were unable to relate our findings to the RUCAM scale.
Since 2010, patients with TB have been provided with a free FDC therapy plan in Guangdong Province of China. Furthermore, self-paid single-drug treatment is also administered to patients who were not able to take FDCs. In general, we prefer to prescribe adult patients with FDCs if they do not have any other form of complication. However, those with liver dysfunction or other diseases are provided with a single drug. In China, the standard FDC scheme involves 2HRZE/4HR (The Disease Control and P, 2008).
It is well known that hepatitis B and diabetes are significant risk factors for AT-DILI. Consequently, our institute operates enhanced observation and follow-up of TB patients if they are diagnosed with hepatitis B and/or diabetes. Prior to carrying out this study, we compared the positive rate of AT-DILI or abnormal liver functioning between patients with and without these comorbidities and found no statistically significant difference (Table 8). Therefore, we excluded patients with hepatitis B and diabetes.
Our study cohort featured more males than females; furthermore, 82% of our cohort were under 50 years-of-age. The prevalence of TB across all age groups was similar to that reported by our institute for TB epidemic data (Zhong et al., 2018). In this study, age was not identified as a significant risk factor for AT-DILI. This might be because older TB patients are most likely to have complications and were therefore excluded from our study. Furthermore, there is a current trend for TB to affect the younger population. In our study, we found that male TB patients had a higher positive incidence of AT-DILI than female patients following the administration of anti-TB drugs. This finding may be related to the higher incidence of TB in male populations. However, the precise relationship between gender and AT-DILI is still the source of much debate (Chang et al., 2008). Our present results were consistent with those reported previously by Kwok et al. (Chang et al., 2008); however, other studies failed to find any significant differences when comparing the incidence rate of AT-DILI between male and females (Yee et al., 2003; Gülbay et al., 2006). In addition, some studies have reported that the risk of AT-DILI in females is greater than that in men (Døssing et al., 1996; Devoto et al., 1997; Teleman et al., 2002) and that women may be more susceptible to certain drugs, such as minocycline and methyldopa, and are therefore more prone to chronic autoimmune hepatitis (Yu et al., 2017). Furthermore, our analysis also indicated that TB patients with higher incomes are more likely to develop AT-DILI. This may be due to a potential decline of immunity induced by the daily pressure/workload experienced by patients earning a high-income. We are the first to investigate the relationship between income and DILI; consequently, we are unable to compare our results with previous studies. Over recent years, the incidence of TB amongst the population of white-collar workers and high-income groups has increased in Nanshan District. Furthermore, Nanshan District included the employees of large and local enterprises in TB monitoring schemes in 2021; this data may provide useful information in future analyses.
Next, we investigated the relationships between TB drug prescriptions and the positive rate of AT-DILI. We found that patients taking the 2FDC-HRZE half/4FDC-HR regimen had the highest positive incidence of AT-DILI (56.03%); this was followed by those taking the 2FDC-HRZE/4FDC-HR regimen (31.11%); the lowest rate was observed in patients taking the 2FDC-HRZ + EMB/4FDC-HR regimen (24.27%). Importantly, the dosage of PZA in the first two schemes showing high positivity was 1,600 mg; this was higher than the usual dosage of 1,500 mg. Our current results suggest that the FDC regimen for anti-TB treatment has a higher risk of abnormal liver functioning or AT-DILI; it is likely that this finding was related to the high dosage of PZA. Studies have also shown that INH and PZA can cause serious damage to liver function; even after these drugs are terminated, AT-DILI can still develop (Ichai et al., 2010). The combined use of RFP and PZA for the treatment of TB can also cause severe AT-DILI; PZA appears to be the major contributor to such injury (Ridzon et al., 1997). In a survey of elderly TB patients in France, the use of PZA was found to be the only independent risk factor for adverse drug reactions (Rousset et al., 2020). Similar studies, conducted in Japan, found that the mortality rate of TB patients over 80 years-of-age was also positively associated with use of PZA (Hagiwara et al., 2019). Therefore, an excessive dosage of PZA in FDCs may be an important risk factor for the development of AT-DILI. Consequently, it is important that clinicians remain vigilant in clinical practice with regards to the development of AT-DILI in patients undergoing treatment for TB. Furthermore, murine studies have found that combined treatments, featuring INH/RFP, can cause the accumulation of endogenous hepatic toxin protoporphyrin IX (PPIX) or bile acids by downregulating the bile acid transporter; this process destroyed liver function and led to the development of AT-DILI (Guo et al., 2015). Because the INH/RFP combination is generally applied during the entire course of initial TB treatment as a standard strategy, the prospect of AT-DILI is unavoidable.
In the present study, we also found that as medication increased, there was a gradual increase in the positive incidence of AT-DILI or abnormal liver functioning in patients under various medication schemes. A previous study of 926 patients with TB who were followed-up for 4,122.9 persons × months (pm) in Taiwan showed that 12.0% of patients developed AT-DILI in the first 38 days after commencing treatment and that the positive incidences of AT-DILI in response to INH, RFP, and PZA, were 0.59, 0.69, and 3.71 per 100 pm, respectively (Shu et al., 2013). These results indicated that longer periods of medication use will lead to the gradual accumulation of drug metabolites in the liver, thus increasing the likelihood of more severe AT-DILI.
There are some limitations to this study that need to be considered. First, this was a retrospective study. The data were acquired from our hospital information system and were collected in real time. However, the data items were relatively limited. Some of the data required for the determination of RUCAM scores were not available. Consequently, we cannot confirm the specific causality of AT-DILI; furthermore, these our finding were only based on 287 positive patients. However, the purpose of this study was to investigate the risk factors for AT-DILI or abnormal liver functioning. Our team has developed an early warning model for AT-DILI or abnormal liver functioning during the first-line treatment of patients with TB and can be used to facilitate clinical practice (Zhong et al., 2021). We assess the risk of abnormal liver functioning or AT-DILI for all patient before anti-tuberculosis treatment, and intervene in advance for patients who may have a higher risk, such as adjusting the FDC program to a single drug combination regime, increasing liver protection drugs, strengthening the frequency of follow-up during treatment. We collect relevant data to compare the positive rates before and after implementation of our model. In the future, we will continue to study the causal relationship between risk factors and AT-DILI to facilitate clinical diagnosis and treatment.
We investigated patients receiving first-line drug treatment for TB and identified male gender, high annual income, prescription regime, drug dosage, and treatment time, as significant risk factors for the development of AT-DILI or abnormal liver functioning. In addition to the development of new anti-TB drugs with less side effects, it is important that we establish an effective and evidence-based scheme and/or predictive model for AT-DILI based on existing prevalence data. We hope that our findings provide a strong body of evidence for clinicians to select appropriate anti-TB prescription regimes to reduce the risk of AT-DILI in patients with TB (Zhang, 2019).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were approved by Ethics Committee of Shenzhen Nanshan Center for Chronic Disease Control and the participants provided their written informed consent in this study.
SL and TZ conceived the study and carried out the analysis. All authors discussed the results, drafted the first article, critically read, and revised the article, and gave their final approval for publication.
DH was supported by the General Research Fund (15205119) of the Research Grants Council of Hong Kong. TZ and SL were supported by the Shenzhen Science and Technology Innovation Commission (JCYJ20190809153201668) and Sanming Project of Medicine in Shenzhen (SZSM201603029).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Chang, K. C., Leung, C. C., Yew, W. W., Lau, T. Y., and Tam, C. M. (2008). Hepatotoxicity of Pyrazinamide: Cohort and Case-Control Analyses. Am. J. Respir. Crit. Care Med. 177 (12), 1391–1396. doi:10.1164/rccm.200802-355OC
Chen, R., Wang, J., Zhang, Y., Tang, S., and Zhan, S. (2015). Key Factors of Susceptibility to Anti-tuberculosis Drug-Induced Hepatotoxicity. Arch. Toxicol. 89 (6), 883–897. doi:10.1007/s00204-015-1473-1
Devoto, F. M., González, C., Iannantuono, R., Serra, H. A., González, C. D., and Sáenz, C. (1997). Risk Factors for Hepatotoxicity Induced by Antituberculosis Drugs. Acta Physiol. Pharmacol. Ther. Latinoam. 47 (4), 197–202.
Døssing, M., Wilcke, J. T., Askgaard, D. S., and Nybo, B. (1996). Liver Injury during Antituberculosis Treatment: an 11-year Study [J]. Tubercle Lung Dis. 77 (4), 335–340. the official journal of the International Union against Tuberculosis and Lung Disease. doi:10.1016/s0962-8479(96)90098-2
Girling, D., Caulet, P., Pamra, S. P., and Pilheu, J. A. (1988). Anti-tuberculosis Regimens of Chemotherapy. Recommendations from the Committee on Treatment of the International Union against Tuberculosis and Lung Disease. Indian J. Chest Dis. Allied Sci. 30 (4), 296–304.
Gülbay, B. E., Gürkan, O. U., Yildiz, O. A., Onen, Z. P., Erkekol, F. O., Baççioğlu, A., et al. (2006). Side Effects Due to Primary Antituberculosis Drugs during the Initial Phase of Therapy in 1149 Hospitalized Patients for Tuberculosis. Respir. Med. 100 (10), 1834–1842. doi:10.1016/j.rmed.2006.01.014
Guo, Y. X., Xu, X. F., Zhang, Q. Z., Li, C., Deng, Y., Jiang, P., et al. (2015). The Inhibition of Hepatic Bile Acids Transporters Ntcp and Bsep Is Involved in the Pathogenesis of Isoniazid/rifampicin-Induced Hepatotoxicity. Toxicol. Mech. Methods 25 (5), 382–387. doi:10.3109/15376516.2015.1033074
Hagiwara, E., Suido, Y., Asaoka, M., Katano, T., Okuda, R., Sekine, A., et al. (2019). Safety of Pyrazinamide-Including Regimen in Late Elderly Patients with Pulmonary Tuberculosis: A Prospective Randomized Open-Label Study. J. Infect. Chemother. 25 (12), 1026–1030. doi:10.1016/j.jiac.2019.05.030
Huai, C., Wei, Y., Li, M., Zhang, X., Wu, H., Qiu, X., et al. (2019). Genome-Wide Analysis of DNA Methylation and Antituberculosis Drug-Induced Liver Injury in the Han Chinese Population. Clin. Pharmacol. Ther. 106 (6), 1389–1397. doi:10.1002/cpt.1563
Ichai, P., Saliba, F., Antoun, F., Azoulay, D., Sebagh, M., Antonini, T. M., et al. (2010). Acute Liver Failure Due to Antitubercular Therapy: Strategy for Antitubercular Treatment before and after Liver Transplantation. Liver Transpl. 16 (10), 1136–1146. the Study of Liver Diseases and the International Liver Transplantation Society. doi:10.1002/lt.22125
Kabbara, W. K., Sarkis, A. T., and Saroufim, P. G. (2016). Acute and Fatal Isoniazid-Induced Hepatotoxicity: A Case Report and Review of the Literature. Case Rep. Infect. Dis. 2016, 1–6. doi:10.1155/2016/3617408
Kong, W., and Qiao, L. (2014). Analysis of the Use of Fixed-Dose Anti-tuberculosis Compound Preparations in Our Province[J]. J. Clin. Pulm. Med. 19 (7), 1275–1277. doi:10.3969/j.issn.1009-6663.2014.07.039
Qiu, L. (2012). Study on Clinical Characteristics and Prognostic Factors of Drug-Induced Liver Injury [D]. Changchun: Jilin University.
Ridzon, R., Meador, J., Maxwell, R., Higgins, K., Weismuller, P., and Onorato, I. M. (1997). Asymptomatic Hepatitis in Persons Who Received Alternative Preventive Therapy with Pyrazinamide and Ofloxacin. Clin. Infect. Dis. 24 (6), 1264–1265. doi:10.1093/clinids/24.6.1264
Rousset, S., Lafaurie, M., Guet-revillet, H., Protin, C., Le Grusse, J., Derumeaux, H., et al. (2020). Safety of Pyrazinamide for the Treatment of Tuberculosis in Older Patients over 75 Years of Age: A Retrospective Monocentric Cohort Study [J]. Drugs & aging 38 (1), 43–52. doi:10.1007/s40266-020-00811-9
Shu, C. C., Lee, C. H., Lee, M. C., Wang, J. Y., Yu, C. J., and Lee, L. N. (2013). Hepatotoxicity Due to First-Line Anti-tuberculosis Drugs: a Five-Year Experience in a Taiwan Medical centre. Int. J. Tuberc. Lung Dis. 17 (7), 934–939. doi:10.5588/ijtld.12.0782
Teleman, M. D., Chee, C. B., Earnest, A., and Wang, Y. T. (2002). Hepatotoxicity of Tuberculosis Chemotherapy under General Programme Conditions in Singapore. Int. J. Tuberc. Lung Dis. 6 (8), 699–705.
The Disease Control and Prevention Bureau of the Ministry of Health, the Medical Administration Department of the Ministry of Health, and the Chinese Center for Disease Control and Prevention (2008). Guidelines for the Implementation of China's Tuberculosis Prevention and Control Program. Peking Union Medical College Press, 56–57.
The Global Plan to Stop TB, 2006-2015 (2006). Actions for Life: towards a World Free of Tuberculosis [J]. Int. J. Tuberc. Lung Dis. 10 (3), 240–241. doi:10.1258/095646206775809277
World Health Organization (1994). The Promise and Reality of Fixed-Dose Combinations with Rifampicin. A Joint Statement of the International Union against Tuberculosis and Lung Disease and the Tuberculosis Programme of the World Health Organization. Tuber Lung Dis. 75, 180–181. doi:10.1016/0962-8479(94)90004-3
Xia, Y., and Zhan, S. (2007). Comprehensive Analysis of the Incidence of Adverse Reactions to Anti-tuberculosis Drugs in China. Chin. J. Tuberculosis Respir. Med. [J] 30 (6), 419–423. doi:10.3760/j.issn:1001-0939.2007.06.007
Xiao, D. L. (2009). TB Diagnosis and Treatment Manual of Adverse Drug Reactions. Chinese Edition. China: People’s health press.
Yee, D., Valiquette, C., Pelletier, M., Parisien, I., Rocher, I., and Menzies, D. (2003). Incidence of Serious Side Effects from First-Line Antituberculosis Drugs Among Patients Treated for Active Tuberculosis. Am. J. Respir. Crit. Care Med. 167 (11), 1472–1477. doi:10.1164/rccm.200206-626OC
Yu, Y. C., Mao, Y. M., Chen, C. W., Chen, J. J., Chen, J., Cong, W. M., et al. (2017). CSH Guidelines for the Diagnosis and Treatment of Drug-Induced Liver Injury. Hepatol. Int. 11 (02), 221–241. doi:10.1007/s12072-017-9793-2
Zhang, J. (2019). Interpretation of Health Security Data for Metropolitan Population[J]. Finance Weekly 931 (41), 50–51.
Zhong, T., Liu, S. Y., Zhan, G. X., Han, G., Huang, Y., He, J., et al. (2018). Analysis of Epidemiological Characteristics of Tuberculosis in Nanshan District, Shenzhen from 2005 to 2016[J]. Dis. Surveill. 033 (009), 724–727. doi:10.3784/j.issn.1003-9961.2018.09.006
Zhong, T. (2016). Analysis of Supervision and Medication Administration under the Management of Electronic Medication System for Registered Tuberculosis Patients in Nanshan District, Shenzhen[J]. Chin. Prev. Control. Chronic Dis. 24 (5), 332–336. doi:10.16386/j.cjpccd.issn.1004-6194.2016.05.004
Zhong, T., Zhuang, Z., Dong, X., Wong, K. H., Wong, W. T., Wang, J., et al. (2021). Predicting Antituberculosis Drug-Induced Liver Injury Using an Interpretable Machine Learning Method: Model Development and Validation Study. JMIR Med. Inform. 9 (7), e29226. doi:10.2196/29226
Keywords: prescription scheme, risk factor, anti-tuberculosis drug-induced liver injury, pulmonary tuberculosis, China
Citation: Zhong T, Fan Y, Dong X-L, Guo X, Wong KH, Wong W-t, He D and Liu S (2021) An Investigation of the Risk Factors Associated With Anti-Tuberculosis Drug-Induced Liver Injury or Abnormal Liver Functioning in 757 Patients With Pulmonary Tuberculosis. Front. Pharmacol. 12:708522. doi: 10.3389/fphar.2021.708522
Received: 12 May 2021; Accepted: 19 October 2021;
Published: 08 November 2021.
Edited by:
David Sacerdoti, University of Verona, ItalyReviewed by:
Amelia Filippelli, University of Salerno, ItalyCopyright © 2021 Zhong, Fan, Dong, Guo, Wong, Wong, He and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengyuan Liu, bGl1c2hlbmdsYkAxMjYuY29t; Daihai He, ZGFpaGFpLmhlQHBvbHl1LmVkdS5oaw==; Tao Zhong, NDc2ODk3NzVAcXEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.