- 1Department of Biomedical Science, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia
- 2School of Public Health, Kunming Medical University, Kunming, China
- 3Department of Epidemiology, School of Public Health, Fudan University, Shanghai, China
- 4Key Laboratory of Public Health Safety, Ministry of Education, Fudan University, Shanghai, China
- 5Genetics and Regenerative Medicine Research Group, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia
- 6Department of Medicine, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia
- 7Centre for Materials Engineering and Regenerative Medicine, Bharath Institute of Higher Education and Research, Chennai, India
The total number of cumulative cases and deaths from the COVID-19 pandemic caused by SARS-CoV-2 is still increasing worldwide. Although many countries have actively implemented vaccination strategies to curb the epidemic, there is no specific efficient therapeutic drug for this virus to effectively reduce deaths. Therefore, the underappreciated macromolecular compounds have become the spotlight of research. Furthermore, the medicinal compounds in plants that provide myriad possibilities to treat human diseases have become of utmost importance. Experience indicates that Traditional Chinese medicine effectively treats SARS and has been used for treating patients with COVID-19 in China. As one of the world’s oldest herbal remedies, licorice is used for treating patients with all stages of COVID-19. Glycyrrhizic acid (GA), the main active compound in licorice, has been proven effective in killing the SARS virus. Meanwhile, as a natural plant molecule, GA can also directly target important protein structures of the SARS-CoV-2 virus and inhibit the replication of SARS-CoV-2. In this review, we summarized the immune synergy of GA and its potential role in treating COVID-19 complications. Besides, we reviewed its anti-inflammatory effects on the immune system and its positive effects in cooperation with various drugs to fight against COVID-19 and its comorbidities. The purpose of this review is to elucidate and suggest that GA can be used as a potential drug during COVID-19 treatment.
Introduction
Coronavirus disease 2019 (COVID-19) pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Lai et al., 2020b). Based on previous experience in managing pandemics, a safe and effective vaccine could reduce virus transmission (Lipsitch and Dean, 2020). Therefore, several COVID-19 vaccines with high efficacy levels have been widely accepted worldwide, and most people are willing to get the vaccination (WHO, 2020a; Lazarus et al., 2021). However, the vaccines are not 100% effective (WHO, 2020a). As of May 9, 2021, 156 million cases of COVID-19 have been reported to the World Health Organization (WHO) in various countries worldwide, and more than 3.2 million people have died as a result (WHO, 2020b). Due to the lack of specific antiviral therapeutics, the primary treatment strategy for COVID-19 is supportive care, supplemented by broad-spectrum antiviral and antibiotics, drugs for preventing cytokine storm, corticosteroids as well as healing plasma from infected patients (Chen et al., 2020b). The corticosteroid drug dexamethasone has proven to improve patient's survival rates with severe COVID-19 (WHO, 2020c).
Interestingly, traditional Chinese medicine has been promoted as a treatment for COVID-19 in China and some other countries (Yang et al., 2020b; Cyranoski, 2020), although there is a lack of sufficient evidence. But in fact, statistical analysis has shown that integrated traditional Chinese medicine and Western medicine have effectively reduced the mortality rate during the SARS virus outbreak (Chen and Nakamura, 2004). According to the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia” issued by the National Health Commission of China (NHC) (Pei-Fang, 2020), licorice is the most frequently used herbal medicine among all recommended Chinese medicine formulas for COVID-19 treatment (Figure 1).
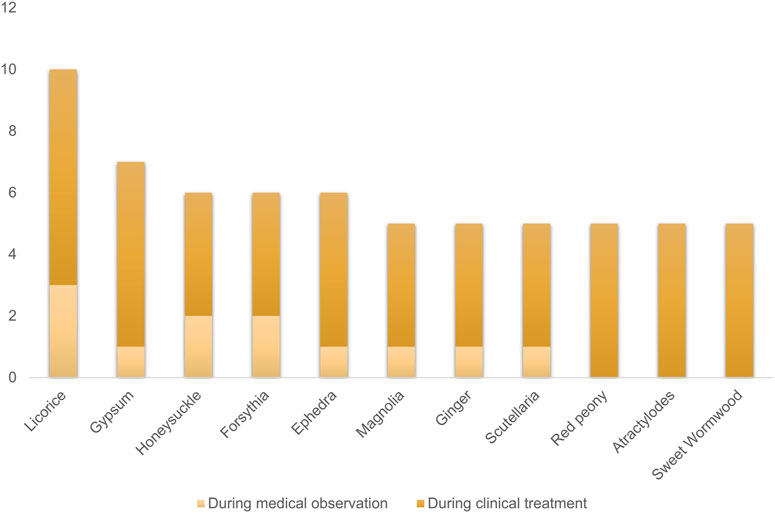
FIGURE 1. The top 10 most frequently used herbs among the recommended Chinese medicine formulas for COVID-19 treatment.
Licorice has a long history in traditional Chinese medicine, especially on its antiviral and antibacterial effects (Wang et al., 2015). The bibliometric analysis results show that glycyrrhizic acid (GA, also known as glycyrrhizin) is the most used molecule in licorice research for antiviral (Ram, 2015; Gomaa and Abdel-Wadood, 2021). As one of the main ingredients in licorice, GA is consumed as a natural sweetener due to its low toxicity and is also traditionally prescribed for treating asthma, dry cough, and other “pectoral diseases” (Ploeger et al., 2001; Banerjee and Giri, 2016). Through virtual screening and molecular dynamics simulations, multiple studies have found that GA has the potential to bind to multiple essential SARS-CoV-2 proteins (S protein, RBD, 3CLpro, Nsp15, RdRp, ACE2, furin, etc.), which can be used as candidate plant molecules for the treatment of COVID-19 infection (Mahdian et al., 2020; Sinha et al., 2020; Dharmendra Kumar, 2021). Although these molecular autodocking research results may be due to particular molecules’ non-specific interactions, the previous study has shown that GA makes it difficult for the SARS virus to attach and invade target cells and slows down the virus spread from one to another cell. It also has more substantial inhibitory power than ribavirin and other broad-spectrum antiviral drugs in inhibiting the SARS-associated virus’s replication (Cinatl et al., 2003). Furthermore, recent studies have shown that GA potently inhibits the replication of SARS-CoV-2 in vitro and relieves the excessive inflammation caused by SARS-CoV-2 in the surrogate mouse model (van de Sand et al., 2021; Zhao et al., 2021).
Based on the above, it seems that GA can be used as the first selective drug to treat COVID-19. But in fact, a high dose of GA is needed clinically to affect virus-infected cells and wipe out the virus (Pilcher, 2003). Nevertheless, we still believe that GA can be a supplement and adjuvant agent to treat COVID-19. This article aims to summarize the immune synergy of glycyrrhizic acid and describe its potential role in reducing COVID-19 complications. Furthermore, this review describes the synergistic phenomenon of glycyrrhizic acid and other drugs, intending to suggest its potential application during COVID-19 treatment.
Immune System Synergy of GA
The antiviral mechanism of GA against coronaviruses, mentioned before, is mainly on its activity of inhibiting virus replication, preventing virus attachment, or enhancing host cell activity. However, its role in the fight against coronavirus infection is multi-faceted.
Clinical studies have shown that SARS-CoV-2 activates CD4+ T lymphocytes and becomes Th1 helper cells after entering the human body. However, most COVID-19 patients have a significant decrease in lymphocytes and T cell subsets, especially CD4+ and CD8+ T cells (Chen et al., 2020a). The main reasons for this are the innate immune escape mechanism of SARS-CoV-2, and the delayed development of the adaptive immune response, and the prolonged virus clearance time (Grifoni et al., 2020). GA can promote the proliferation of T cells and has Th1 immunological adjuvant activity, thus enhancing the immune system’s resistance to SARS-CoV-2 early (Kim et al., 2013).
When SARS-CoV-2 infects T lymphocytes, it will remain latent in the infected host cells for 12–36 h (Bar-On et al., 2020; Diao et al., 2020). It has been reported that GA could induce apoptosis of host cells in G1 cell cycle arrest and latent virus infection (Cohen, 2005; Curreli et al., 2005), indicating that the roles of GA on apoptosis during the treatment of SARS-CoV-2 is worthy of further investigation.
Except for participating in the immune response, Th1 may secrete granulocyte-macrophage colony-stimulating factor (GM-CSF), which leads to the appearance of inflammatory factors CD14 and CD16 with high IL6 expression and accelerates the development of pneumonia (Tang et al., 2020). The activation of IL-6 is composed of multiple pathways, one of which cannot be ignored is that SARS-CoV-2 infection may activate nuclear factor-κB (NF-κB), and overactivation of NF-κB activates IL-6 amplifier (IL-6 Amp). Subsequently, IL-6 Amp induces various pro-inflammatory cytokines and chemokines, including IL-6, and ultimately enhances IL-6 Amp through positive feedback regulation (Hirano and Murakami, 2020). Inhibition of NF-κB could block the inflammatory response induced by a coronavirus and increase patient survival rate (Dediego et al., 2014b; Yang et al., 2017a). In addition, SARS-CoV-2 may bind to LPS to enhance NF-κB and cytokine responses and promote the development of inflammation and ARDS (Van Gucht et al., 2006; Petruk et al., 2021). GA has anti-inflammatory effects of inhibiting the NF-κB expression (Wang et al., 2011) and significantly inhibiting the production of a variety of cytokines secreted by macrophages, including IL-6 production (Liu et al., 2014).
High mobility group box-1 (HMGB1), as a biomarker of acute lung injury (Qu et al., 2019) and a key mediator of fatal systemic inflammation, could induce cell death (pyroptosis) and immunosuppression, thereby impairing the body’s ability to eradicate microbial infections and leading to death. It could also regulate autophagy and participate in the invasion and replication of SARS-CoV-2 (Street, 2020). Inhibiting the activity of HMGB1 or reducing its release could prevent fatal endotoxemia and sepsis (Bailly and Vergoten, 2020). GA can bind to HMGB1 to disrupt its protein activity, inhibit inflammation, and reduce acute respiratory distress syndrome (ARDS) through the HMGB1-TLR4 signaling pathway (Cohen, 2005).
The Role of GA in COVID-19 Comorbidities
Clinical investigations have shown that patients with diabetes have an increased risk of adverse consequences after being infected with COVID-19 (Eastin and Eastin, 2020). Obesity has been proven to be another critical risk factor for COVID-19 (Muscogiuri et al., 2020). Lipid raft is a cholesterol-rich microdomain on the cell's plasma membrane, which can be used as an attachment object by the coronavirus and assist it in invading host cells (Lu et al., 2008; Wang et al., 2008; Fantini et al., 2020). Diabetes can cause cholesterol to be loaded into tissues rich in macrophages, which may be one reason for the poor prognosis of COVID-19 patients with diabetes (Wang et al., 2020). When the cholesterol content in the lipid raft decreases, coronavirus invasion (including SARS-CoV-2) will be blocked (Guo et al., 2017; Wang, et al., 2020). There is evidence that GA can reduce the invasion of SARS-CoV-2 by down-regulating the size of the lipid raft domain (Sakamoto et al., 2013; Sakamoto et al., 2015; Baglivo et al., 2020).
In addition to diabetic patients, in some clinical investigations, hypertension is another risk factor that can lead to severe or fatal COVID-19 (Lippi et al., 2020). Patients with hypertension are often accompanied by hypercholesterolemia, and the accumulation of cholesterol in the vascular endothelium can lead to the formation of blood clots or thrombus (Rafieian-Kopaei et al., 2014; Ivanovic and Tadic, 2015). Cholesterol can help SARS-CoV-2 invade host cells, and the latter can promote platelet aggregation and thrombus formation. It may be one of the reasons for the poor prognosis of patients with COVID-19 with hypertension (Manne et al., 2020). There is evidence that GA can reduce the vascular endothelial cell membrane's cholesterol domain while inhibiting platelet aggregation and thrombus formation (Francischetti et al., 1997; Mendes-Silva et al., 2003; Mason et al., 2016). However, it cannot lower blood pressure and can lead to pseudoaldosteronism and hypertension by long-term treatment. The above symptoms can be reduced after treatment with spironolactone (Omar et al., 2012).
Metabolic syndrome (MetS), a clinical syndrome consists of obesity, hyperglycemia, hypertension, and dyslipidemia, is a risk factor for the development of severe COVID-19 and acute respiratory distress syndrome (ARDS) (Felsenstein et al., 2020). The lipopolysaccharide (LPS) in the blood often stays in a high level of MetS patients (Vors et al., 2015; Awoyemi et al., 2018). When SARS-CoV-2 is combined with LPS, it can enhance nuclear factor-κB (NF-κB) and cytokine response, promote inflammation, and develop ARDS (Van Gucht et al., 2006; Petruk et al., 2021). GA can inhibit NF-κB in regulating the inflammatory response induced by LPS (Yu et al., 2005; Wang et al., 2011; Zhao et al., 2016a).
Patients with COVID-19 often have gastrointestinal symptoms, and experiments have shown that SARS-CoV-2 can infect intestinal epithelial cells (Lamers et al., 2020). Clinical studies have shown that patients with inflammatory bowel disease (IBD) infected with COVID-19 can lead to poor recovery, so anti-TNF-α preparations are recommended (Tursi et al., 2020). GA can inhibit TNF activity, reduce intestinal inflammation, and improve IBD (Yang et al., 2017b; Zeeshan et al., 2019).
Brain nerve damage caused by SARS-CoV-2 infection seems very common and has nothing to do with the severity of COVID-19 (Helms et al., 2020). GA’s anti-apoptotic mechanism can adjust the ratio of mitochondrial Bax/Bcl-2 family, affect PI3K/Akt signaling and inhibit HMGB1 activity, resulting in a powerful neuroprotective effect (Kao et al., 2009). Furthermore, GA has been proven to exert powerful neuroprotective properties in neuroinflammation and ischemic brain damage (Kim et al., 2012; Liu et al., 2019).
Accompanying pain in COVID-19 patients is a relatively common phenomenon (Huang et al., 2020). Previous studies have shown that SARS-CoV can activate/increase the expression of activating transcription factor 2 (ATF2) (Dediego et al., 2014a). ATF2 not only can activate pro-inflammatory genes but also play an active role in regulating inflammatory pain. Inhibiting ATF2 can exert not only anti-inflammatory activity but also reduce inflammatory pain (Reimold et al., 2001; Fang et al., 2013). GA was reported likely to inhibit the activity of ATF2 by inhibiting the expression of P38 upstream of ATF2 (Zhao et al., 2016b; Wang and Du, 2016).
Combination of GA and Other Drugs
Therapeutic drugs for COVID-19 mainly include antiviral agents (remdesivir, ribavirin, hydroxychlo-roquine, chloroquine, etc.) and supporting agents (nitric oxide, sebaceous steroid, etc.) (Li and De Clercq, 2020; Wu et al., 2020). It has been shown that corticosteroid dexamethasone could reduce the mortality of patients with severe COVID-19 by limiting the destructive effects of cytokines. However, dexamethasone had also been reported to suppress the body’s immune system and lead to an increase in plasma viral load (Ledford, 2020; Theoharides and Conti, 2020). We noted that GA could affect the metabolism of steroids and increase its plasma concentration by inhibiting glucocorticoid metabolism (Chen et al., 1991). The combination of dexamethasone and GA effectively reduced the severity of shock in animal experiments (Yu et al., 2005). These data suggested that the combination of dexamethasone and GA could be a promising treatment strategy for patients with COVID-19.
There is a bi-directional relationship between diabetes and COVID-19. Not only patients with diabetes are more susceptible to SARS-CoV-2 infection, but also the prognosis is worse (Apicella et al., 2020; Rubino et al., 2020). Moreover, surveys show that 14% of recovered patients from COVID-19 will have new-onset diabetes (Sathish et al., 2021). One reason for this is that SARS-CoV-2 might directly or indirectly damage pancreatic islets and causes acute β-cell dysfunction followed by type II diabetes (Fignani et al., 2020; Mukherjee et al., 2020; Müller et al., 2021). Another reason is that COVID-19 therapeutic drugs can induce diabetes. For example, some antiviral agents can cause autoimmune damage to pancreatic islet cells. Therefore, after taking the drug, it will cause insulin resistance or abnormal insulin secretion. Ultimately, these antiviral agents will have a greater probability of inducing the occurrence of secondary diabetes (Nakamura et al., 2011). On the other hand, corticosteroids are the culprit of ketoacidosis during the treatment of COVID-19, and their chronic or high-dose use can eventually lead to the onset of diabetes (Alessi et al., 2020; Zhang and Zhang, 2020). Previous studies have shown that GA has an anti-diabetic activity that can improve drug-induced diabetes (Takii et al., 2001; Sen et al., 2011; Yang et al., 2020a). Based on these factors, we recommend that the combined use of GA in the treatment of COVID-19 can effectively improve or even inhibit the occurrence of diabetes.
As a rescue therapy, inhaled nitric oxide (NO) therapy has been used to improve the ARDS caused by COVID-19 (Kobayashi and Murata, 2020). NO could inhibit the replication of coronavirus and reduce lung injury mediated by inflammatory cells and effectively and selectively relax pulmonary blood vessels. These effects of NO could further reduce pulmonary vascular resistance, reduce alveolar cavity edema, and ultimately alleviate ARDS (Åkerström et al., 2005; Martel et al., 2020). It is possible that GA could stimulate and enhance the production of NO by macrophages through up-regulating the expression of the inducible NO synthase (iNOS) gene (Yi et al., 1996; Jeong and Kim, 2002).
Additionally, most of the drugs used in the clinic in treating COVID-19 patients may cause abnormal liver functions (Lai et al., 2020a; Wiersinga et al., 2020). GA has also been used as a hepatoprotective drug with minimal adverse effects, indicating that GA might be suitable for use in combination with various types of drugs to enhance their effects and reduce adverse effects (Cohen, 2005; Lee et al., 2007; Li et al., 2014). Therefore, the effects of GA on liver protection may play a critical role in cooperating with the drug treatment of COVID-19.
Conclusion
GA is a well-established botanical medicinal molecule used for a long time for antiviral and anti-inflammatory treatments. It has been proved that it can effectively inhibit the invasion and replication of SARS. It has been predicted that it can be combined with multiple proteins of SARS-CoV-2 in molecular docking studies. Moreover, it can also inhibit the replication of SARS-CoV-2 and the release of inflammatory factors in vitro and animal experiments. However, GA has been disregarded due to the large dose required as an independent antiviral drug. In this review, we have analyzed the mechanisms of action of GA in the following aspects and illustrated them in Figure 2.
• GA has adjuvant immune activity, which assists the body in the immune response to viruses in the incubation period.
• The anti-inflammatory activity of GA could reduce the cytokine storm caused by the virus.
• GA can reduce or prevent the invasion of SARS-CoV-2 by regulating steroid metabolism.
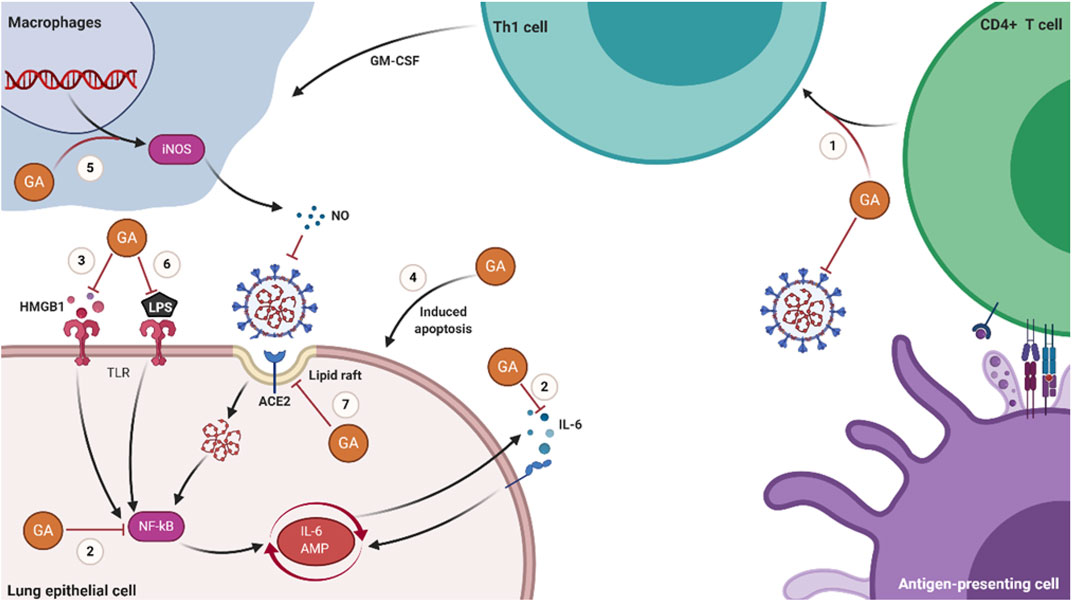
FIGURE 2. Schematic diagram of the molecular mechanisms of GA in treating COVID-19. (1) GA promotes the proliferation of T cells and has Th1 cell immune adjuvant activity. (2) GA inhibits the production of IL-6 and the activation of NF-κB. (3) GA inhibits the activity of HMGB1 and the signal transduction of the HMGB1-TLR4 pathway. (4) GA induces the apoptosis of host cells latent with SARS-CoV-2. (5) GA stimulates macrophages to produce NO. (6) GA inhibits the inflammatory response induced by LPS. (7) GA prevents the invasion of SARS-CoV-2 by reducing the domain of lipid rafts.
In conclusion, we believe that the role of GA in the comorbidities of COVID-19 is still worthy of attention. In particular, GA can be used in combination with various drugs to produce a synergistic effect and effectively reduce the mortality of virus-infected patients. Therefore, we regard that the compound can be used as a conventional adjuvant agent to treat COVID-19.
Author Contributions
All authors had made a contribution to this manuscript. ZS and GH designed, drafted, and edited the manuscript. NH, SK and JL edited the manuscript. KT and CX conceptualized and designed the framework of the manuscript. All authors had read and agreed to the published version of the manuscript.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Grant No. 71964020 and 81872673), and a grant from the National Key Research and Development Program of China (2017YFC1200203).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alessi, J., De Oliveira, G. B., Schaan, B. D., and Telo, G. H. (2020). Dexamethasone in the Era of Covid-19: Friend or Foe? an Essay on the Effects of Dexamethasone and the Potential Risks of its Inadvertent Use in Patients with Diabetes. Diabetology Metab. Syndr. 12 (1), 80. doi:10.1186/s13098-020-00583-7
Apicella, M., Campopiano, M. C., Mantuano, M., Mazoni, L., Coppelli, A., and Prato, S. D. (2020). Covid-19 in People with Diabetes: Understanding the Reasons for Worse Outcomes. Lancet Diabetes Endocrinol. 8 (9), 782–792. doi:10.1016/s2213-8587(20)30238-2
Awoyemi, A., Trøseid, M., Arnesen, H., Svein, S., and Ingebjørg, Se. (2018). Markers of Metabolic Endotoxemia as Related to Metabolic Syndrome in an Elderly Male Population at High Cardiovascular Risk: A Cross-Sectional Study. Diabetology Metab. Syndr. 10 (1), 59. doi:10.1186/s13098-018-0360-3
Åkerström, S., Mousavi-Jazi, M., Klingström, J., Leijon, M., Lundkvist, A., and Mirazimi, A. (2005). Nitric Oxide Inhibits the Replication Cycle of Severe Acute Respiratory Syndrome Coronavirus. J. Virol. 79 (3), 1966. doi:10.1128/JVI.79.3.1966-1969.2005
Baglivo, M., Baronio, M., Natalini, G., Beccari, T., Chiurazzi, P., and Fulcheri, E. (2020). Natural Small Molecules as Inhibitors of Coronavirus Lipid-dependent Attachment to Host Cells: A Possible Strategy for Reducing Sars-Cov-2 Infectivity? Acta Biomed. 91 (1), 161–164. doi:10.23750/abm.v91i1.9402
Bailly, C., and Vergoten, G., 2020. Glycyrrhizin: An Alternative Drug for the Treatment of Covid-19 Infection and the Associated Respiratory Syndrome? Pharmacol. Ther. 214, 107618. doi:10.1016/j.pharmthera.2020.107618
Banerjee, A., and Giri, R. (2016). Nutraceuticals in Gastrointestinal Disorders. Nutraceuticals: Efficacy, Safety And Toxicity 10, 109–122. doi:10.1016/B978-0-12-802147-7.00009-7
Bar-On, Y. M., Flamholz, A., and Phillips, R. (2020). Sars-cov-2 (Covid-19) by the Numbers. eLife 9, e57309. doi:10.7554/eLife.57309
Chen, G., Wu, D., Guo, W., Cao, Y., Huang, D., and Wang, H. (2020a). Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J. Clin. Invest. 130 (5), 2620–2629. doi:10.1172/jci137244
Chen, J., Lu, H., Melino, G., Boccia, S., Piacentini, M., and Ricciardi, W. (2020b). Covid-19 Infection: The china and italy Perspectives. Cell Death Dis 11 (6), 438. doi:10.1038/s41419-020-2603-0
Chen, M. F., Shimada, F., Kato, H., Yano, S., and Kanaoka, M. (1991). Effect of Oral Administration of Glycyrrhizin on the Pharmacokinetics of Prednisolone. Endocrinol. Jpn. 38 (2), 167–174. doi:10.1507/endocrj1954.38.167
Chen, Z., and Nakamura, T. (2004). Statistical Evidence for the Usefulness of Chinese Medicine in the Treatment of Sars. Phytother Res. 18 (7), 592–594. doi:10.1002/ptr.1485
Cinatl, J., Morgenstern, B., Bauer, G., Chandra, P., Rabenau, H., and Doerr, H. W. (2003). Glycyrrhizin, an Active Component of Liquorice Roots, and Replication of Sars-Associated Coronavirus. Lancet 361 (9374), 2045–2046. doi:10.1016/s0140-6736(03)13615-x
Cohen, J. I. (2005). Licking Latency with Licorice. J. Clin. Invest. 115 (3), 591–593. doi:10.1172/jci24507
Curreli, F., Friedman-Kien, A. E., and Flore, O. (2005). Glycyrrhizic Acid Alters Kaposi Sarcoma-Associated Herpesvirus Latency, Triggering P53-Mediated Apoptosis in Transformed B Lymphocytes. J. Clin. Invest. 115 (3), 642–652. doi:10.1172/jci23334
Cyranoski, D. (2020). China Is Promoting Coronavirus Treatments Based on Unproven Traditional Medicines. Nature. doi:10.1038/d41586-020-01284-x
Dediego, M. L., Nieto-Torres, J. L., Jimenez-Guardeño, J. M., Regla-Nava, J. A., Castaño-Rodriguez, C., and Fernandez-Delgado, R. (2014a). Coronavirus Virulence Genes with Main Focus on Sars-Cov Envelope Gene. Virus. Res. 194, 124–137. doi:10.1016/j.virusres.2014.07.024
Dediego, M. L., Nieto-Torres, J. L., Regla-Nava, J. A., Jimenez-Guardeño, J. M., Fernandez-Delgado, R., and Fett, C. (2014b). Inhibition of Nf-Κb-Mediated Inflammation in Severe Acute Respiratory Syndrome Coronavirus-Infected Mice Increases Survival. J. Virol. 88 (2), 913. doi:10.1128/JVI.02576-13
Dharmendra Kumar, M. (2021). Evaluation of Yashtimadhu (Glycyrrhiza glabra) Active Phytochemicals Against Novel Coronavirus (SARS-CoV-2). Res. Square. doi:10.21203/rs.3.rs-26480/v1
Diao, B., Wang, C., Tan, Y., ChenLiu, X. Y., and Ning, L. (2020). Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (Covid-19). Front. Immunol. 11, 827. doi:10.3389/fimmu.2020.00827
Eastin, C., and Eastin, T. (2020). Clinical characteristics of coronavirus disease 2019 in china: Guan w, ni z, hu y, et al. N engl j med. 2020 feb 28. J. Emerg. Med. 58 (4), 711–712. doi:10.1016/j.jemermed.2020.04.004
Fang, J. Q., Du, J. Y., Liang, Y., and Fang, J. F. (2013). Intervention of Electroacupuncture on Spinal P38 Mapk/atf-2/vr-1 Pathway in Treating Inflammatory Pain Induced by Cfa in Rats. Mol. Pain 9, 13. doi:10.1186/1744-8069-9-13
Fantini, J., Di Scala, C., Chahinian, H., and Nouara, Y. 2020. Structural and Molecular Modelling Studies Reveal a New Mechanism of Action of Chloroquine and Hydroxychloroquine against Sars-Cov-2 Infection. Int. J. Antimicrob. Agents. 55 (5), 105960. doi:10.1016/j.ijantimicag.2020.105960
Felsenstein, S., Herbert, J. A., Mcnamara, P. S., and Hedrich, C. M. (2020). Covid-19: Immunology and Treatment Options. Clin. Immunol. 215, 108448. doi:10.1016/j.clim.2020.108448
Fignani, D., Licata, G., Brusco, N., Nigi, L., Grieco, G. E., and Marselli, L. (2020). Sars-cov-2 Receptor Angiotensin I-Converting Enzyme Type 2 (Ace2) Is Expressed in Human Pancreatic β-cells and in the Human Pancreas Microvasculature. Front. Endocrinol. 11, 876. doi:10.3389/fendo.2020.596898
Francischetti, I. M., Monteiro, R. Q., and Guimarães, J. A. (1997). Identification of Glycyrrhizin as a Thrombin Inhibitor. Biochem. Biophys. Res. Commun. 235 (1), 259–263. doi:10.1006/bbrc.1997.6735
Gomaa, A. A., and Abdel-Wadood, Y. A., 2021. The Potential of Glycyrrhizin and Licorice Extract in Combating Covid-19 and Associated Conditions. Phytomedicine Plus. 1 (3), 100043. doi:10.1016/j.phyplu.2021.100043
Grifoni, A., Weiskopf, D., Ramirez, S. I., Mateus, J., Dan, J. M., and Moderbacher, C. R. (2020). Targets of T Cell Responses to Sars-Cov-2 Coronavirus in Humans with Covid-19 Disease and Unexposed Individuals. Cell 181 (7), 1489–1501. doi:10.1016/j.cell.2020.05.015
Guo, H., Huang, M., Yuan, Q., Yanquan, W., Yuan, G., and Lejiao, M. (2017). The Important Role of Lipid Raft-Mediated Attachment in the Infection of Cultured Cells by Coronavirus Infectious Bronchitis Virus Beaudette Strain. PLoS One 12 (1), e0170123. doi:10.1371/journal.pone.0170123
Helms, J., Kremer, S., Merdji, H., Clere-Jehl, R., Schenck, M., and Kummerlen, C. (2020). Neurologic Features in Severe Sars-Cov-2 Infection. N. Engl. J. Med. 382 (23), 2268–2270. doi:10.1056/NEJMc2008597
Hirano, T., and Murakami, M. (2020). Covid-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity 52 (5), 731–733. doi:10.1016/j.immuni.2020.04.003
Huang, C., Wang, Y., Li, X., Lili, R., Jianping, Z., and Yi, H. (2020). Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, china. Lancet 395 (10223), 497–506. doi:10.1016/s0140-6736(20)30183-5
Ivanovic, B., and Tadic, M. (2015). Hypercholesterolemia and Hypertension: Two Sides of the Same coin. Am. J. Cardiovasc. Drugs 15 (6), 403–414. doi:10.1007/s40256-015-0128-1
Jeong, H. G., and Kim, J. Y. (2002). Induction of Inducible Nitric Oxide Synthase Expression by 18beta-Glycyrrhetinic Acid in Macrophages. FEBS Lett. 513 (2-3), 208–212. doi:10.1016/s0014-5793(02)02311-6
Kao, T. C., Shyu, M. H., and Yen, G. C. (2009). Neuroprotective Effects of Glycyrrhizic Acid and 18beta-Glycyrrhetinic Acid in Pc12 Cells via Modulation of the Pi3k/akt Pathway. J. Agric. Food Chem. 57 (2), 754–761. doi:10.1021/jf802864k
Kim, J., Joo, I., Kim, H., and Han, Y. (2013). 18β-glycyrrhetinic Acid Induces Immunological Adjuvant Activity of Th1 against candida Albicans Surface Mannan Extract. Phytomedicine 20 (11), 951–955. doi:10.1016/j.phymed.2013.04.008
Kim, S. W., Jin, Y., Shin, J. H., Kim, Il-D., Lee, H. K., and Park, S. (2012). Glycyrrhizic Acid Affords Robust Neuroprotection in the Postischemic Brain via Anti-inflammatory Effect by Inhibiting Hmgb1 Phosphorylation and Secretion. Neurobiol. Dis. 46 (1), 147–156. doi:10.1016/j.nbd.2011.12.056
Kobayashi, J., and Murata, I. (2020). Nitric Oxide Inhalation as an Interventional rescue Therapy for Covid-19-Induced Acute Respiratory Distress Syndrome. Ann. Intensive Care 10 (1), 61. doi:10.1186/s13613-020-00681-9
Lai, C. C., Ko, W. C., Lee, P. I., Jean, S. S., and Hsueh, P. R. (2020a). Extra-respiratory Manifestations of Covid-19. Int. J. Antimicrob. Agents 56 (2), 106024. doi:10.1016/j.ijantimicag.2020.106024
Lai, C. C., Shih, T. P., Ko, W. C., Tang, H. J., and Hsueh, P. R. (2020b). Severe Acute Respiratory Syndrome Coronavirus 2 (Sars-cov-2) and Coronavirus Disease-2019 (Covid-19): The Epidemic and the Challenges. Int. J. Antimicrob. Agents 55 (3), 105924. doi:10.1016/j.ijantimicag.2020.105924
Lamers, M. M., Beumer, J., Van Der Vaart, J., Kèvin, K., Jens, P., and Tim, I. B. (2020). Sars-cov-2 Productively Infects Human Gut Enterocytes. Science 369 (6499), 50–54. doi:10.1126/science.abc1669
Lazarus, J. V., Ratzan, S. C., Palayew, A., Lawrence, O. G., Heidi, J. L., and Kenneth, R. (2021). A Global Survey of Potential Acceptance of a Covid-19 Vaccine. Nat. Med. 27 (2), 225–228. doi:10.1038/s41591-020-1124-9
Ledford, H. (2020). Coronavirus Breakthrough: Dexamethasone Is First Drug Shown to Save Lives. Nature 582 (7813), 469. doi:10.1038/d41586-020-01824-5
Lee, C. H., Park, S. W., Kim, Y. S., Kang, S. S., Kim, J. A., and Lee, S. H. (2007). Protective Mechanism of Glycyrrhizin on Acute Liver Injury Induced by Carbon Tetrachloride in Mice. Biol. Pharm. Bull. 30 (10), 1898–1904. doi:10.1248/bpb.30.1898
Li, G., and De Clercq, E. (2020). Therapeutic Options for the 2019 Novel Coronavirus (2019-ncov). Nat. Rev. Drug Discov. 19 (3), 149–150. doi:10.1038/d41573-020-00016-0
Li, J. Y., Cao, H. Y., Liu, P., Cheng, G. H., and Sun, M. Y. (2014). Glycyrrhizic Acid in the Treatment of Liver Diseases: Literature Review. Biomed. Res. Int. 2014, 872139. doi:10.1155/2014/872139
Lippi, G., Wong, J., and Henry, B. M. (2020). Hypertension in Patients with Coronavirus Disease 2019 (Covid-19): A Pooled Analysis. Pol. Arch. Intern. Med. 130 (4), 304–309. doi:10.20452/pamw.15272
Lipsitch, M., and Dean, N. E. (2020). Understanding Covid-19 Vaccine Efficacy. Science 370 (6518), 763–765. doi:10.1126/science.abe5938
Liu, W., Huang, S., and Li, Y. (2019). Suppressive Effect of Glycyrrhizic Acid against Lipopolysaccharide-Induced Neuroinflammation and Cognitive Impairment in C57 Mice via Toll-like Receptor 4 Signaling Pathway. Food Nutr. Res. 63. doi:10.29219/fnr.v63.1516
Liu, Z., Zhong, J. Y., Gao, E. N., and Yang, H. (2014). [Effects of Glycyrrhizin Acid and Licorice Flavonoids on Lps-Induced Cytokines Expression in Macrophage]. Zhongguo Zhong Yao Za Zhi 39 (19), 3841–3845. doi:10.4268/cjcmm20141935
Lu, Y., Liu, D. X., and Tam, J. P. (2008). Lipid rafts are involved in sars-cov entry into vero e6 cells. Biochem. Biophys. Res. Commun. 369 (2), 344–349. doi:10.1016/j.bbrc.2008.02.023
Mahdian, S., Ebrahim-Habibi, A., and Zarrabi, M. (2020). Drug Repurposing Using Computational Methods to Identify Therapeutic Options for Covid-19. J. Diabetes Metab. Disord. 19 (2), 1–9. doi:10.1007/s40200-020-00546-9
Manne, B. K., Denorme, F., Middleton, E. A., Portier, I., Rowley, J. W., and Stubben, C. (2020). Platelet Gene Expression and Function in Patients with Covid-19. Blood 136 (11), 1317–1329. doi:10.1182/blood.2020007214
Martel, J., Ko, Y. F., Young, J. D., and Ojcius, D. M. (2020). Could Nasal Nitric Oxide Help to Mitigate the Severity of Covid-19?. Microbes Infect. 22 (4-5), 168–171. doi:10.1016/j.micinf.2020.05.002
Mason, R. P., Jacob, R. F., Shrivastava, S., Sherratt, S. C. R., and Chattopadhyay, A. (2016). Eicosapentaenoic Acid Reduces Membrane Fluidity, Inhibits Cholesterol Domain Formation, and Normalizes Bilayer Width in Atherosclerotic-like Model Membranes. Biochim. Biophys. Acta 1858 (12), 3131–3140. doi:10.1016/j.bbamem.2016.10.002
Mendes-Silva, W., Assafim, M., and Ruta, B. (2003). Antithrombotic Effect of Glycyrrhizin, a Plant-Derived Thrombin Inhibitor. Thromb. Res. 112 (1-2), 93–98. doi:10.1016/j.thromres.2003.10.014
Mukherjee, S., Banerjee, O., and Singh, S. (2020). Covid 19 Could Trigger Global Diabetes burden - a Hypothesis. Diabetes Metab. Syndr. 14 (5), 963–964. doi:10.1016/j.dsx.2020.06.049
Müller, J. A., Groß, R., Conzelmann, C., Krüger, J., Merle, U., and Steinhart, J. (2021). Sars-cov-2 Infects and Replicates in Cells of the Human Endocrine and Exocrine Pancreas. Nat. Metab. 3 (2), 149–165. doi:10.1038/s42255-021-00347-1
Muscogiuri, G., Pugliese, G., Barrea, L., Savastano, S., and Colao, A. (2020). Commentary: Obesity: The "achilles Heel" for Covid-19? Metab. Clin. Exp. 108, 154251. doi:10.1016/j.metabol.2020.154251
Nakamura, K., Kawasaki, E., Imagawa, A., Awata, T., Ikegami, H., and Uchigata, Y. (2011). Type 1 Diabetes and Interferon Therapy: A Nationwide Survey in japan. Diabetes Care 34 (9), 2084–2089. doi:10.2337/dc10-2274
Omar, H. R., Komarova, I., El-Ghonemi, M., Fathy, A., Rashad, R., and Abdelmalak, H. D. (2012). Licorice Abuse: Time to Send a Warning Message. Ther. Adv. Endocrinol. Metab. 3 (4), 125–138. doi:10.1177/2042018812454322
Petruk, G., Puthia, M., Petrlova, J., Samsudin, F., Strömdahl, A.-C., and Cerps, S. (2021). Sars-cov-2 Spike Protein Binds to Bacterial Lipopolysaccharide and Boosts Proinflammatory Activity. J. Mol. Cel Biol 12 (12), 916–932. doi:10.1093/jmcb/mjaa067
Pei-Fang, W. (2020). Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (trial version 7). Chin. Med. J. 133, 1087-1095. doi:10.1097/CM9.0000000000000819
Ploeger, B., Mensinga, T., Sips, A., Seinen, W., Meulenbelt, J., and DeJongh, J. (2001). The Pharmacokinetics of Glycyrrhizic Acid Evaluated by Physiologically Based Pharmacokinetic Modeling. Drug Metab. Rev. 33, 125–147. doi:10.1081/DMR-100104400
Qu, L., Chen, C., Chen, Y., Li, Y., Tang, F., and Huang, H. (2019). High-mobility Group Box 1 (Hmgb1) and Autophagy in Acute Lung Injury (Ali): A Review. Med. Sci. monitor: Int. Med. J. Exp. Clin. Res. 25, 1828–1837. doi:10.12659/MSM.912867
Rafieian-Kopaei, M., Setorki, M., Doudi, M., Baradaran, A., and Nasri, H. (2014). Atherosclerosis: Process, Indicators, Risk Factors and New Hopes. Int. J. Prev. Med. 5 (8), 927–946.
Ram, S. (2015). A Bibliometric Assessment of Liquorice (Glycyrrhiza Glabra) Research Trends. Ann. Libr. Inf. Stud. 62 (1), 27–32. Available at:. http://hdl.handle.net/123456789/31613
Reimold, A. M., Kim, J., Finberg, R., and Glimcher, L. H. (2001). Decreased Immediate Inflammatory Gene Induction in Activating Transcription Factor-2 Mutant Mice. Int. Immunol. 13 (2), 241–248. doi:10.1093/intimm/13.2.241
Rubino, F., Amiel, S. A., Zimmet, P., Alberti, G., Bornstein, S., and Eckel, R. H. (2020). New-onset Diabetes in Covid-19. N. Engl. J. Med. 383 (8), 789–790. doi:10.1056/NEJMc2018688
Sakamoto, S., Nakahara, H., Uto, T., Shoyama, Y., and Shibata, O., 2013. Investigation of Interfacial Behavior of Glycyrrhizin with a Lipid Raft Model via a Langmuir Monolayer Study. Biochim. Biophys. Acta (Bba) - Biomembranes. 828 (4), 1271–1283. doi:10.1016/j.bbamem.2013.01.006
Sakamoto, S., Uto, T., and Shoyama, Y., 2015. Effect of Glycyrrhetinic Acid on Lipid Raft Model at the Air/water Interface. Biochim. Biophys. Acta (Bba) - Biomembranes. 1848 (2), 434–443. doi:10.1016/j.bbamem.2014.11.014
Sathish, T., Kapoor, N., Cao, Y., Tapp, R. J., and Zimmet, P. (2021). Proportion of Newly Diagnosed Diabetes in Covid-19 Patients: A Systematic Review and Meta-Analysis. Diabetes Obes. Metab. 23 (3), 870–874. doi:10.1111/dom.14269
Sen, S., Roy, M., and Chakraborti, A. S. (2011). Ameliorative Effects of Glycyrrhizin on Streptozotocin-Induced Diabetes in Rats. J. Pharm. Pharmacol. 63 (2), 287–296. doi:10.1111/j.2042-7158.2010.01217.x
Sinha, S. K., Prasad, S. K., and Islam, M. A. (2020). Identification of Bioactive Compounds from glycyrrhiza Glabra as Possible Inhibitor of Sars-Cov-2 Spike Glycoprotein and Non-structural Protein-15: A Pharmacoinformatics Study. J. Biomol. Struct. Dyn., 1–15. doi:10.1080/07391102.2020.1779132
Street, M. E. (2020). Hmgb1: A Possible Crucial Therapeutic Target for Covid-19? Horm. Res. paediatrics 93 (2), 73–75. doi:10.1159/000508291
Takii, H., Kometani, T., and Nishimura, T. (2001). Antidiabetic Effect of Glycyrrhizin in Genetically Diabetic Kk-Ay Mice. Biol. Pharm. Bull. 24 (5), 484–487. doi:10.1248/bpb.24.484
Tang, X., Wu, C., and Li, X. (2020). On the Origin and Continuing Evolution of Sars-Cov-2. Natl. Sci. Rev. 7, 1012–1023. doi:10.1093/nsr/nwaa036
Theoharides, T. C., and Conti, P. (2020). Dexamethasone for Covid-19? Not So Fast. J. Biol. Regul. Homeost Agents 34 (3), 1241–1243. doi:10.23812/20-editorial_1-5
Tursi, A., Vetrone, L. M., and Papa, A. (2020). Anti-tnf-α Agents in Inflammatory Bowel Disease and Course of Covid-19. Inflamm. Bowel Dis. 26 (7), e73. doi:10.1093/ibd/izaa114
van de Sand, L., Bormann, M., Alt, M., Schipper, L., Heilingloh, C. S., and Steinmann, E. (2021). Glycyrrhizin Effectively Inhibits Sars-Cov-2 Replication by Inhibiting the Viral Main Protease. Viruses 13, 609. doi:10.3390/v13040609
Van Gucht, S., Atanasova, K., and Barbé, F. (2006). Effect of Porcine Respiratory Coronavirus Infection on Lipopolysaccharide Recognition Proteins and Haptoglobin Levels in the Lungs. Microbes Infect. 8 (6), 1492–1501. doi:10.1016/j.micinf.2006.01.009
Vors, C., Pineau, G., Drai, J., Meugnier, E., Pesenti, S., Laville, M., et al. (2015). Postprandial Endotoxemia Linked with Chylomicrons and Lipopolysaccharides Handling in Obese versus Lean Men: A Lipid Dose-Effect Trial. J. Clin. Endocrinol. Metab. 100 (9), 3427–3435. doi:10.1210/jc.2015-2518
Wang, C. Y., Kao, T. C., Lo, W. H., and Yen, G. C. (2011). Glycyrrhizic Acid and 18β-Glycyrrhetinic Acid Modulate Lipopolysaccharide-Induced Inflammatory Response by Suppression of Nf-Κb through Pi3k P110δ and P110γ Inhibitions. J. Agric. Food Chem. 59 (14), 7726–7733. doi:10.1021/jf2013265
Wang, H., Yang, P., Liu, K., Guo, F., Zhang, Y., and Zhang, G. (2008). Sars Coronavirus Entry into Host Cells through a Novel Clathrin- and Caveolae-independent Endocytic Pathway. Cell Res 18 (2), 290–301. doi:10.1038/cr.2008.15
Wang, H., Yuan, Z., and Pavel, M. A. (2020). The Role of High Cholesterol in Age-Related Covid19 Lethality. bioRxiv, 086249. doi:10.1101/2020.05.09.086249
Wang, L., Yang, R., and Yuan, B. (2015). The Antiviral and Antimicrobial Activities of Licorice, a Widely-Used Chinese Herb. Acta pharmaceutica Sinica B 5 (4), 310–315. doi:10.1016/j.apsb.2015.05.005
Wang, Y. M., and Du, G. Q. (2016). Glycyrrhizic Acid Prevents Enteritis through Reduction of Nf-Κb P65 and P38mapk Expression in Rat. Mol. Med. Rep. 13 (4), 3639–3646. doi:10.3892/mmr.2016.4981
WHO (2020a). Coronavirus Disease (Covid-19): Vaccines. Available at: https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines (Accessed May 9, 2021).
WHO (2020b). WHO Coronavirus (Covid-19) Dashboard | WHO Coronavirus (Covid-19) Dashboard with Vaccination Data. Available at: https://covid19.who.int/ (Accessed May 9, 2021).
WHO (2020c). Coronavirus Disease (COVID-19): Dexamethasone. Available at: https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-dexamethasone (Accessed May 9, 2021).
Wiersinga, W. J., Rhodes, A., and Cheng, A. C. (2020). Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (Covid-19): A Review. Jama 324 (8), 782–793. doi:10.1001/jama.2020.12839
Wu, R., Wang, L., and Kuo, H. D. (2020). An Update on Current Therapeutic Drugs Treating Covid-19. Curr. Pharmacol. Rep. 6, 56–70. doi:10.1007/s40495-020-00216-7
Yang, C. W., Lee, Y. Z., and Hsu, H. Y. (2017a). Targeting Coronaviral Replication and Cellular Jak2 Mediated Dominant Nf-Κb Activation for Comprehensive and Ultimate Inhibition of Coronaviral Activity. Sci. Rep. 7 (1), 4105. doi:10.1038/s41598-017-04203-9
Yang, R., Yuan, B. C., and Ma, Y. S. (2017b). The Anti-inflammatory Activity of Licorice, a Widely Used Chinese Herb. Pharm. Biol. 55 (1), 5–18. doi:10.1080/13880209.2016.1225775
Yang, L., Jiang, Y., and Zhang, Z. (2020a). The Anti-diabetic Activity of Licorice, a Widely Used Chinese Herb. J. Ethnopharmacol 263, 113216. doi:10.1016/j.jep.2020.113216
Yang, Y., Islam, M. S., and Wang, J. (2020b). Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-new Coronavirus (Sars-cov-2): A Review and Perspective. Int. J. Biol. Sci. 16 (10), 1708–1717. doi:10.7150/ijbs.45538
Yi, H., Nakashima, I., and Isobe, K. (1996). Enhancement of Nitric Oxide Production from Activated Macrophages by Glycyrrhizin. Am. J. Chin. Med. 24 (3-4), 271–278. doi:10.1142/s0192415x96000335
Yu, Z., Ohtaki, Y., Kai, K., Sasano, T., Shimauchi, H., and Yokochi, T. (2005). Critical Roles of Platelets in Lipopolysaccharide-Induced Lethality: Effects of Glycyrrhizin and Possible Strategy for Acute Respiratory Distress Syndrome. Int. Immunopharmacol 5 (3), 571–580. doi:10.1016/j.intimp.2004.11.004
Zeeshan, M., Ali, H., Khan, S., Mukhtar, M., Khan, M. I., and Arshad, M. (2019). Glycyrrhizic Acid-Loaded Ph-Sensitive Poly-(lactic-Co-Glycolic Acid) Nanoparticles for the Amelioration of Inflammatory Bowel Disease. Nanomedicine (Lond) 14 (15), 1945–1969. doi:10.2217/nnm-2018-0415
Zhang, B., and Zhang, S. (2020). Letter to the Editor: "Our Response to Covid-19 as Endocrinologists and Diabetologists. J. Clin. Endocrinol. Metab. 105 (7). doi:10.1210/clinem/dgaa228
Zhao, H., Liu, Z., Shen, H., Jin, S., and Zhang, S. (2016a). Glycyrrhizic Acid Pretreatment Prevents Sepsis-Induced Acute Kidney Injury via Suppressing Inflammation, Apoptosis and Oxidative Stress. Eur. J. Pharmacol. 781, 92–99. doi:10.1016/j.ejphar.2016.04.006
Zhao, H., Zhao, M., Wang, Y., Li, F., and Zhang, Z. (2016b). Glycyrrhizic Acid Prevents Sepsis-Induced Acute Lung Injury and Mortality in Rats. J. Histochem. Cytochem. 64 (2), 125–137. doi:10.1369/0022155415610168
Keywords: COVID-19, SARS-CoV-2, glycyrrhizic acid, glycyrrhizin, immune synergy, steroid metabolism
Citation: Sun Z, He G, Huang N, Thilakavathy K, Lim JCW, Kumar SS and Xiong C (2021) Glycyrrhizic Acid: A Natural Plant Ingredient as a Drug Candidate to Treat COVID-19. Front. Pharmacol. 12:707205. doi: 10.3389/fphar.2021.707205
Received: 09 May 2021; Accepted: 28 June 2021;
Published: 09 July 2021.
Edited by:
Rownak Jahan, University of Development Alternative, BangladeshReviewed by:
Qi Wang, Harbin Medical University, ChinaJen-Tsung Chen, National University of Kaohsiung, Taiwan
Copyright © 2021 Sun, He, Huang, Thilakavathy, Lim, Kumar and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guozhong He, Z3VvemhvbmdfaGVAdmlwLnNpbmEuY29t; Karuppiah Thilakavathy, dGhpbGF0aHlAdXBtLmVkdS5teQ==; Chenglong Xiong, eGlvbmdjaGVuZ2xvbmdAZnVkYW4uZWR1LmNu
†These authors have contributed equally to this work
 Zhong Sun
Zhong Sun Guozhong He
Guozhong He Ninghao Huang
Ninghao Huang Karuppiah Thilakavathy
Karuppiah Thilakavathy Jonathan Chee Woei Lim
Jonathan Chee Woei Lim S. Suresh Kumar
S. Suresh Kumar Chenglong Xiong
Chenglong Xiong