
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 20 July 2021
Sec. Pharmacology of Anti-Cancer Drugs
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.699044
This article is part of the Research Topic Role of Phytochemicals and Structural Analogs in Cancer Chemoprevention and Therapeutics View all 14 articles
 Nikita Aggarwal
Nikita Aggarwal Joni Yadav
Joni Yadav Suhail Chhakara
Suhail Chhakara Divya Janjua
Divya Janjua Tanya Tripathi
Tanya Tripathi Apoorva Chaudhary
Apoorva Chaudhary Arun Chhokar
Arun Chhokar Kulbhushan Thakur
Kulbhushan Thakur Tejveer Singh
Tejveer Singh Alok Chandra Bharti*
Alok Chandra Bharti*Head and neck cancer (HNC) usually arises from squamous cells of the upper aerodigestive tract that line the mucosal surface in the head and neck region. In India, HNC is common in males, and it is the sixth most common cancer globally. Conventionally, HNC attributes to the use of alcohol or chewing tobacco. Over the past four decades, portions of human papillomavirus (HPV)-positive HNC are increasing at an alarming rate. Identification based on the etiological factors and molecular signatures demonstrates that these neoplastic lesions belong to a distinct category that differs in pathological characteristics and therapeutic response. Slow development in HNC therapeutics has resulted in a low 5-year survival rate in the last two decades. Interestingly, HPV-positive HNC has shown better outcomes following conservative treatments and immunotherapies. This raises demand to have a pre-therapy assessment of HPV status to decide the treatment strategy. Moreover, there is no HPV-specific treatment for HPV-positive HNC patients. Accumulating evidence suggests that phytochemicals are promising leads against HNC and show potential as adjuvants to chemoradiotherapy in HNC. However, only a few of these phytochemicals target HPV. The aim of the present article was to collate data on various leading phytochemicals that have shown promising results in the prevention and treatment of HNC in general and HPV-driven HNC. The review explores the possibility of using these leads against HPV-positive tumors as some of the signaling pathways are common. The review also addresses various challenges in the field that prevent their use in clinical settings.
Head and neck cancer (HNC) constitutes a large group of cancers arising in different anatomical sites of the head and neck (HN) region, comprising the lip and oral cavity, larynx, nasopharynx, hypopharynx, oropharynx, nasal cavity, paranasal sinuses, and salivary glands. Over 90% of these neoplastic tissues are squamous cell carcinomas (SCCs). According to WHO estimates for 2019, HNC was one of the leading forms of cancer with 931,931 new cases, representing 4.9% of all cancer cases (Globocan, 2020). Lip and oral cavity cancer made up nearly 40% of the total HNC cases followed by the cancer of the larynx region. Mortality statistics reported by GLOBOCAN estimate 467,125 deaths due to head and neck cancers, representing 4.7% of all cancer deaths. Prevalence data for 2020 point to India as carrying the highest burden of head and neck cancer, with 143,242 cases, followed by China (100,871), the United States of America (51,533), and the Russian Federation (23,772). These numbers are alarming and draw attention to immediate action against this highly preventable cancer as the etiological agents are well known.
Tobacco use, excessive alcohol consumption, and lately, infection of human papillomavirus (HPV) are the established risk factors for HNC (Marur and Forastiere, 2016). The risk of HNC is 10-fold higher in smokers than that of HNC in nonsmokers (IARC, 2004). Although excessive alcohol consumption is an independent risk factor, it also increases the risk for smokers (Smith et al., 2004; Chaturvedi et al., 2015). In the past decade, however, there has been a shift in the anatomic distribution of HNC with an increasing occurrence of neoplastic lesions in the oropharynx (Sturgis and Cinciripini, 2007). A concordant decrease in smoking prevalence and increase in HPV prevalence has been noted, especially in the younger age-group. The review of clinical manifestations of HNC based on their anatomical, histological, and etiological factors revealed a dichotomy in treatment response (Aggarwal et al., 2020). The data strongly point toward existence of two distinct types of HNC, namely, one that is caused by tobacco and alcohol abuse or occupational exposure to various carcinogens, and the other which is caused by biological agents like infection of HPV and possibly the EBV. The evidence presented in the present manuscript suggests discrete differences among the two disease groups, with each requiring separate clinical management.
Most patients with HNC seek clinical intervention at advanced stages of the disease (Haddad and Shin, 2008). This trend is quite common in individuals of low socioeconomic status, who cannot afford expensive medical/surgical treatments. Despite a well-standardized treatment regimen, current therapy has a very low success rate as 30–60% of patients diagnosed develop recurrent locoregional cancer or second primary cancers even after complete remission (Hashim et al., 2019). A major underlying factor is onset of chemo/radioresistance and treatment failure (Nikolaou et al., 2018). Thus, better therapeutic options are needed to mitigate this challenge. Moreover, prevention of HNC at an early precancer/cancer stage could be another window of opportunity by which disease burden and mortality due to HNC could be reduced. Currently, prevention focuses on risk behavior reduction like cessation of tobacco and early diagnosis of the disease. However, there is an unmet need for new therapeutics that could effectively eliminate HNC cells, reduce the onset of chemo/radioresistance, and could prevent the progression of the disease.
Recently, there has been a renewed interest in phytochemicals and herbal derivatives with therapeutic correlates from traditional medicine in the treatment and prevention of HNC due to their safety, availability, efficacy, and low cost. A number of studies carried out to investigate screening of phytochemicals using different HNC cell lines, animal models, and clinical evaluation in patients showed potent anticancer activities in a small set of phytochemicals. However, very limited number of studies addressed the impact of these herbal derivatives on HPV infection and HPV-positive HNC. In this article, we have systematically reviewed the existing data on various phytochemicals demonstrating chemotherapeutic and chemopreventive activities against HNC with a special emphasis on phytochemicals/herbal derivatives that showed anticancer effects against HPV-positive HNC. Further, major deficiencies and actionable leads in this field have been highlighted.
HNC is a group of neoplastic diseases that can be broadly classified based on their anatomical site, histological origin, and etiological factors (Figure 1A).
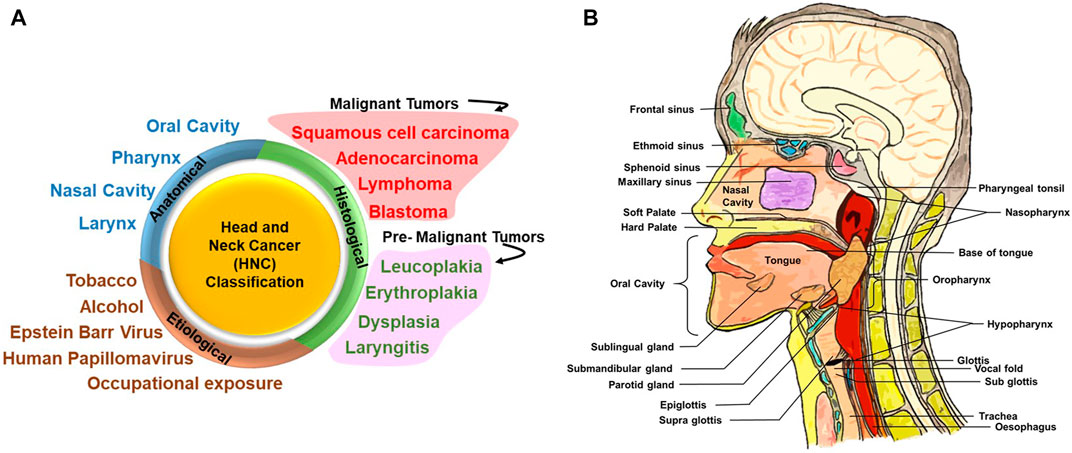
FIGURE 1. Head and neck cancer (HNC) classification and different anatomical sites involved. (A) HNC can be broadly classified on the basis of its anatomical site, histological origin, and etiological factors. Upon histological evaluation, tumors in the head and neck region can be broadly classified into malignant and premalignant lesions. Premalignant lesions that have been indicated here are not cancer but are precursors of malignant lesions. (B) Detailed anatomical architecture of the head and neck region illustrating the location of the oral cavity, nasal cavity, tongue, salivary glands (sublingual gland, submaxillary gland, and parotid gland), larynx and pharynx (including oropharynx, nasopharynx, and hypopharynx), and site of primary tumors.
Figure 1B illustrates the anatomic sites of the HN region. Broadly, the HN area is classified into four regions, namely, the oral cavity, pharynx, nasal cavity, and larynx. The oral cavity consists of the vestibule (the area between the teeth and mucosa of the lips and cheeks) and the oral cavity proper. The oral cavity proper is the interior region of the mouth: the region between the two dental arches and majorly occupied by the tongue (Akintoye and Mupparapu, 2020). Soft palate and hard palate separates the oral cavity from the nasal cavity.
Oral SCC (OSCC) arises from mucosal areas of the lips, front 2/3rd of the tongue, gums, internal lining of cheeks and lips, floor of mouth below the tongue, hard palate, and the area behind the wisdom teeth (Gartner, 1994), and constitutes a major proportion of cancers of the HN region. Globally, lip and oral cavity cancer prevalence is 34.7% among the overall cases of HNC. Lip and oral cavity cancer has the highest incidence in South-Central Asia (Globocan, 2020). The prevalence of lip and oral cavity cancer in the past 5 years is the highest in India, with a total burden of 300,413 cases. In the oral cavity proper, the tongue accounts for 40% of intraoral carcinomas (Neville and Day, 2002).
The pharynx is a channel located in the region of the neck midline. The pharynx is majorly classified into three regions: the nasopharynx (located posterior to nasal cavity), oropharynx (posterior end of oral cavity), and hypopharynx (behind the opening of larynx) (Albahout and Lopez, 2021). Globally, among HNC, the prevalence of the nasopharynx is 15.8%, which is the highest among three regions, followed by oropharynx 10.7%, which is higher than hypopharynx 5.5%. The incidence rate of the nasopharynx is high in Southeastern Asia, whereas the incidence rate of the hypopharynx is high in Central and Eastern Europe (Globocan, 2020). Incidence rates for the oropharynx are high in Europe, which is linked with alcohol consumption, tobacco smoking, and HPV. Incidence of HPV infection in the oropharyngeal region is rising at an alarming rate (Wierzbicka et al., 2021).
The nasal cavity is the upper most part of the respiratory tract. The nasal cavity is surrounded by four types of paranasal sinuses: frontal sinuses, sphenoid sinuses, paired maxillary sinuses, and ethmoid sinuses. Paranasal sinus malignancies are rare, accounting for less than 3–5% of the total HNC (Patel, 2017). The nasal cavity and paranasal sinuses disease burden are not covered by (Globocan, 2020) under HNC.
The internal space of the larynx is a pyramid shaped about 5 cm long, connecting the pharynx to the trachea and is a part of the respiratory system. According to Globocan (2020), the incidence of larynx cancer is highest in Central and Eastern Europe. Laryngeal cancer constitutes around 21.4% among HNC (Globocan, 2020).
Exocrine glands and salivary glands function to secrete saliva in the oral cavity. Three type of salivary glands are present: parotid gland (situated front of both ears), submandibular gland (posterior of the mandible), and sublingual gland (floor of the oral cavity) (Ghannam and Singh, 2021). In the salivary gland, majority tumors are benign, whereas malignant tumors are generally mucoepidermoid carcinoma and adenocarcinoma. Primary SCC is rare and aggressive in salivary glands, specifically in the parotid gland (Flynn et al., 1999). The incidence of the salivary gland cancer has been reported to be the highest in Middle Africa. Salivary gland cancer constitutes 6.6% of total HNC.
In the oral cavity, the mucosa is of masticatory, specialized, and mobile type. It covers around 25% of the oral cavity. In order to understand mechanical forces caused by mastication, it is covered by specialized, orthokeratinized, stratified squamous epithelium. Depending on the anatomic site, over 60% of the mucosa in the oral cavity is lined by the stratified squamous epithelium. The upper surface of the tongue is lined by specialized mucosa, with numerous lingual papillae (Winning and Townsend, 2000).
Histologically, the tumors of the HN region are classified as carcinoma, adenocarcinoma, lymphoma, and blastoma depending upon the tissue from where they are originating (Ologe et al., 2005). For instance, cancer originating in squamous cells in the HN region is collectively termed as HNSCC, and the one originating in salivary glands is of glandular origin and classified as an adenocarcinoma. The most common cancer affecting the HN region is epithelial carcinoma, which constitutes 80–90% of total cases, followed by lymphomas and blastomas accounting for the rest (Ologe et al., 2005; Gilyoma et al., 2015). Among carcinomas, squamous cell carcinoma constitutes 67.7% of total carcinoma cases, whereas other carcinomas like follicular carcinoma, adenocarcinoma, adenoid cystic carcinoma, clear cell carcinoma, mucoepidermoid carcinoma, and malignant melanoma cover the remaining carcinoma cases (Adeyemi et al., 2008).
Carcinomas mostly spread in the regions of the larynx, nasopharynx, and least in maxillofacial bones and oral cavity regions, whereas predominant anatomical sites for lymphomas were lymph nodes, followed by the maxillofacial bones. In contrast, sarcomas occurred most frequently in the maxillofacial bones, face/scalp, and the nose area (Adisa et al., 2011). The distribution of these tumors varies among the age-group of the patients. Most of the carcinomas are detected in the age-group of 45–64 years in contrast to sarcomas frequently occurring in the age-group of 25–44 years (Adeyemi et al., 2008; Adisa et al., 2011).
In the oral cavity, leukoplakia (white plaque) and its variants, erythroplakia (fiery red patch) and submucous fibrosis (most prevalent in India), are three conditions that are highly associated with the development of oral epithelial dysplasia (OED) and oral squamous cell carcinoma (OSCC). Malignant transformation rates of leukoplakia range from 8.9 to 17.5 percent (Silverman et al., 1984; Lind, 1987). The buccal mucosa had the highest incidence of leukoplakia, with 18% of lesions, but had the lowest rate of malignant transformation (3%). The tongue accounted for 16% of lesions but had the highest rate of transformation at 24% (Warnakulasuriya and Ariyawardana, 2016). Erythroplakia occurs mainly in the middle aged and the elderly and has the prevalence ranging from 0.02 to 1%. Soft palate, floor of the mouth, and buccal mucosa have their highest rate of incidence. The reason for etiopathogenesis has not been determined, but chewing tobacco and consuming alcohol have been implicated as factors for the development erythroplakia. The malignant transformation rate in erythroplakia is very high (14–50%) (Reichart and Philipsen, 2005). Oral submucous fibrosis is another chronic and potentially malignant disorder characterized by juxtaepithelial fibrosis of the oral cavity. This lesion has been reported to have a malignant transformation rate of 7–30%. Its incidence is highly associated with the chewing of betel quid containing areca nut (Ranganathan et al., 2004).
Dysplasia can be categorized as mild (architectural disturbance and cytological atypia in lower third of the epithelium), moderate (architectural disturbance and cytological atypia in middle third of the epithelium), and severe (architectural disturbance and cytological atypia in greater than two-third of the epithelium). This classification of dysplasia by the WHO is referred to as the gold standard for histological diagnosis of oral potentially malignant disorders (OPMDs). The WHO defines OPMDs as “clinical presentation that carry a risk of cancer development in the oral cavity, whether in a clinically definable precursor lesion or in clinically normal mucosa” (Muller, 2018). Epithelial dysplasia, an important precursor of malignant transformation in the HN region, can be defined as a change in morphological characteristics of the epithelium, including architectural and cytotoxic changes and loss of differentiation of keratinocytes toward the surface. It involves replacement of a part or the entire epithelium by cells showing cellular atypia (Tilakaratne et al., 2019; Wils et al., 2020).
The stratified squamous epithelium lines the pharynx to protect it from mechanical stress. The pharynx and larynx both are lined with the ciliated pseudostratified columnar epithelium with goblet cells. A study suggests that lesions such as erythroplakia at high-risk sites in the oropharynx should be considered as invasive carcinoma or carcinoma in situ at high-risk sites unless a biopsy proves otherwise (Mashberg and Samit, 1995). However, the vocal cords are lined with the stratified squamous epithelium (Stiblar-Martincic, 1997). Although there is no consensus, premalignant lesions of the larynx are usually classified as chronic laryngitis, erythroplakia, leukoplakia, and erythroleukoplakia (Gale et al., 2009). In the premalignant and malignant lesions of the larynx, severe dysplasia and carcinoma in situ occur at the rate of 10–20% (Hellquist et al., 1982). The nasal mucous membrane is lined with the sensory epithelium with olfactory cells and the respiratory epithelium. The mucosa is rich in mucus-producing goblet cell. Nasal drainage is facilitated by the ciliated epithelium. Premalignant lesions of paranasal sinuses differ from other lesions of the HN region and are present as inverted papillomas. This cancer goes undiagnosed before the onset of symptoms. Malignant tumors of paranasal sinus are diagnosed at stages T3–T4 in two-thirds of cases. Additionally, in paranasal cancer, 10% of total SCCs and 4% of all adenocarcinomas have some degree of cervical lymph node involvement (Jegoux et al., 2013). Salivary glands constitute three cell types, namely, acinar cells, myoepithelial cells, and ductal cells (Brazen and Dyer, 2020). In the parotid gland, 70% of the tumors detected are benign. In the submandibular gland, adenoid cystic carcinoma is the common malignancy (16%). Sublingual gland tumors are rare but have the highest frequency of malignancy, ranging from 70 to 90% (Carlson and Schlieve, 2019).
Tobacco-associated HNC: Association of tobacco and alcohol use with the onset of HNC is well established (IARC, 2004). Tobacco use is the leading cause of preventable death in the world. Tobacco smoking alone is the leading cause of cancer and cancer-related deaths worldwide. Nearly 85% of HNC are linked with tobacco use. Within the HN region, it has been conclusively shown to directly cause oral cavity, laryngeal, and pharyngeal cancer (Centers for Disease Control and Prevention, 2004). The International Agency for Research on Cancer (IARC) has classified carcinogens in groups, group 1: tobacco smoking, secondhand smoking, and smokeless tobacco for HNCs, which are sufficient for evident carcinogenicity in human (IARC, 2004). In developed countries, most inhaled or “mainstream” tobacco smoke comes from the use of manufactured cigarettes. Cigarettes burn at very high temperature and produce smoke that includes toxins and carcinogens. Similar drawbacks are with cigars, pipes, and water pipes (IARC, 2004).
Tobacco smoke contains a variety of group 1 carcinogens, namely, arsenic and benzene, but research is more focused on tobacco-specific N-nitrosamines, especially N-nitrosonornicotine and 4-(N-nitrosomethylamino)-1-(3-pyridyl)-1-butanone, as they are established carcinogens. In HNC of HNSCC type, the latter one is more associated with increasing the risk of cancer development (Oreggia et al., 1991). Tar is another compound which is linked with an increased risk of HNC (Franceschi et al., 1992).
Studies have shown that development of HNC is strongly related with dose-dependent tobacco smoking but can also occur with low daily usage (Berthiller et al., 2016). Moreover, the duration of exposure also significantly affects the risk of HNC. The risk of daily smoking for more than 30 years was found to be more carcinogenic (Cohen et al., 2018).
Alcohol-associated HNC: HNC is also associated with alcohol abuse. Studies suggest that alcohol consumption and cigarette smoking are differentially associated with the risk of HNSCC subtypes (Bagnardi et al., 2001). A large prospective study has confirmed that alcohol consumption is strongly linked to HNSCC (Freedman et al., 2007). Among all, oropharyngeal SCC (OPSCC) is the most associated, while laryngeal SCC (LSCC) is the least associated with heavy alcohol consumption (Zeka et al., 2003; Lubin et al., 2009; Toporcov et al., 2015). Clinically, there is no distinction between alcohol- and tobacco-associated HNC.
Occupational exposure–associated HNC: Apart from smoking of tobacco products, occupational exposure to dusts from wood, textiles, leather industries, flour, nickel, chromium, fumes from rubbing alcohol (also called isopropyl alcohol), radium, glue, formaldehyde as well as solvent fumes used in furniture and shoe production, and asbestos are the main risk factors for sinonasal carcinomas. Hypopharygeal and laryngeal carcinoma are associated with the use of coal for heating or cooking (IARC, 2012). These tumors have an aggressive clinical behavior and resemble tobacco-associated tumors in progression and therapeutic response.
Epstein–Barr virus–associated HNC: The etiology and natural history of nasopharyngeal SCC (NPSCC) is closely linked to that of Epstein–Barr virus (EBV) infection. This neoplasm is an uncommon disease with very low prevalence in most countries (Wei and Sham, 2005). Although EBV infection is pervasive, NPSCC incidence differs considerably around the world (Chang and Adami, 2006). In most geographical regions where NPSCC is endemic, the onset of EBV infection occurs at an early age. The estimated latency period of this virus is around a decade, so other factors also contribute for NPSCC development. Evidences indicate that this cancer is predominant in individuals of Southeast Asian descent due to genetic differences (Chang and Adami, 2006; Bei et al., 2016; Liu et al., 2017).
HPV-associated HNC: HPV is a DNA virus with oncogenic potential associated with over a dozen genotypes referred to as high-risk HPV. Persistent HPV infection is chiefly associated with the development of anogenital and cervical carcinomas. HPV16 and HPV18 genotypes are the most prevalent carcinogenic types and act via action of two major oncogenes, E6 and E7. These oncogenes target cell cycle and promote tumor growth by targeting and downregulating p53 and pRb, respectively. Many molecular and epidemiological studies support association of HPV with HNC, especially with OPSCC (Franceschi et al., 1996). Over the last 125 years, observations speculating the presence of a virus transmitting oral tumors have matured and led to the identification of a subset of HNC with distinct clinical presentation that show an early onset (Table 1). Approximately 35% of all HNC and 77% of tonsillar cancers harbor HPV, with greater than 60% of cases being the HPV16 subtype (McKaig et al., 1998). A significant variation in HPV prevalence in HNC types is recorded within different studies and from different geographical regions (Gillison et al., 2015).
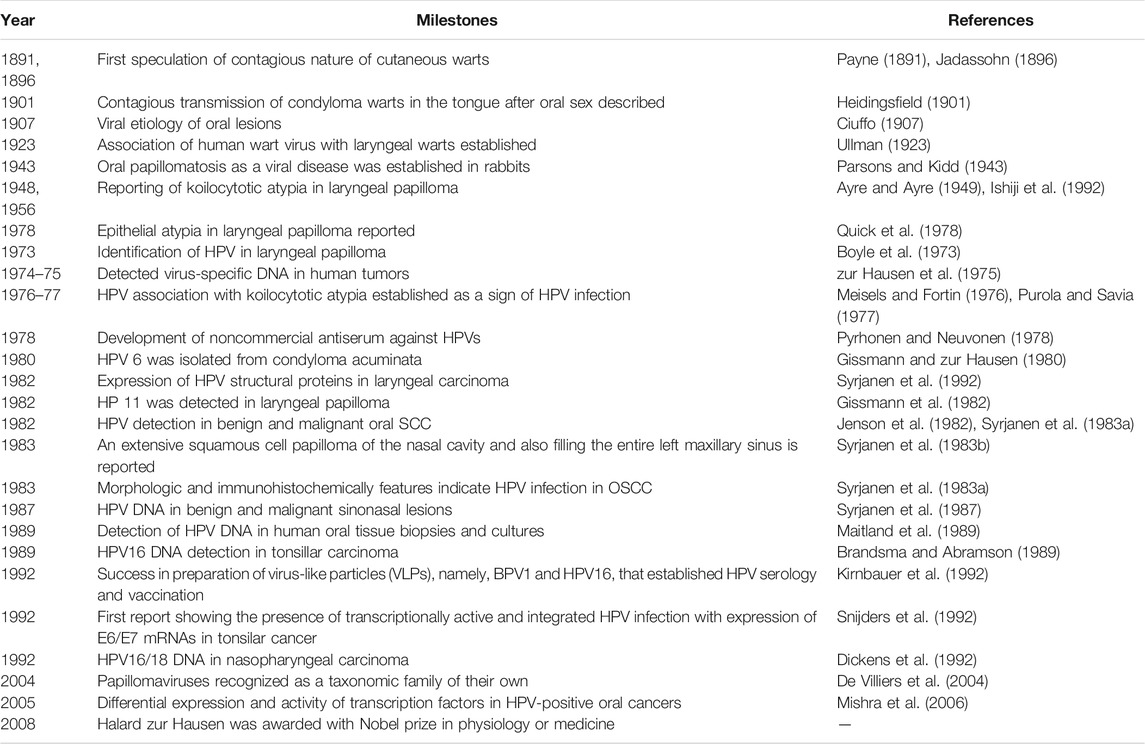
TABLE 1. Major historical milestone events in the description of HPV infection in the head and neck region (adapted from Syrjanen et al. (2017)).
Finding HPV in the HN region is paradoxical. However, a sexual mode of transmission has been suggested. Due to muco-epithelial tropicity of these viruses, if the virus gets access to these tissues via opportunistic contact with infected genital organs, it can result in the establishment of HPV infection in the HN region (Figure 2). Patients with other HPV-associated neoplasms or premalignant conditions are presumed to be at a higher risk of HNC development. Among spouses, women having a history of cervical dysplasia showed higher incidence of HPV-related oropharyngeal cancer (Hemminki et al., 2000). Patients with a history of anogenital cancer have shown a higher risk of tonsillar cancer (Frisch and Biggar, 1999). These HPV-positive cancers are primarily SCCs in their histological manifestations.
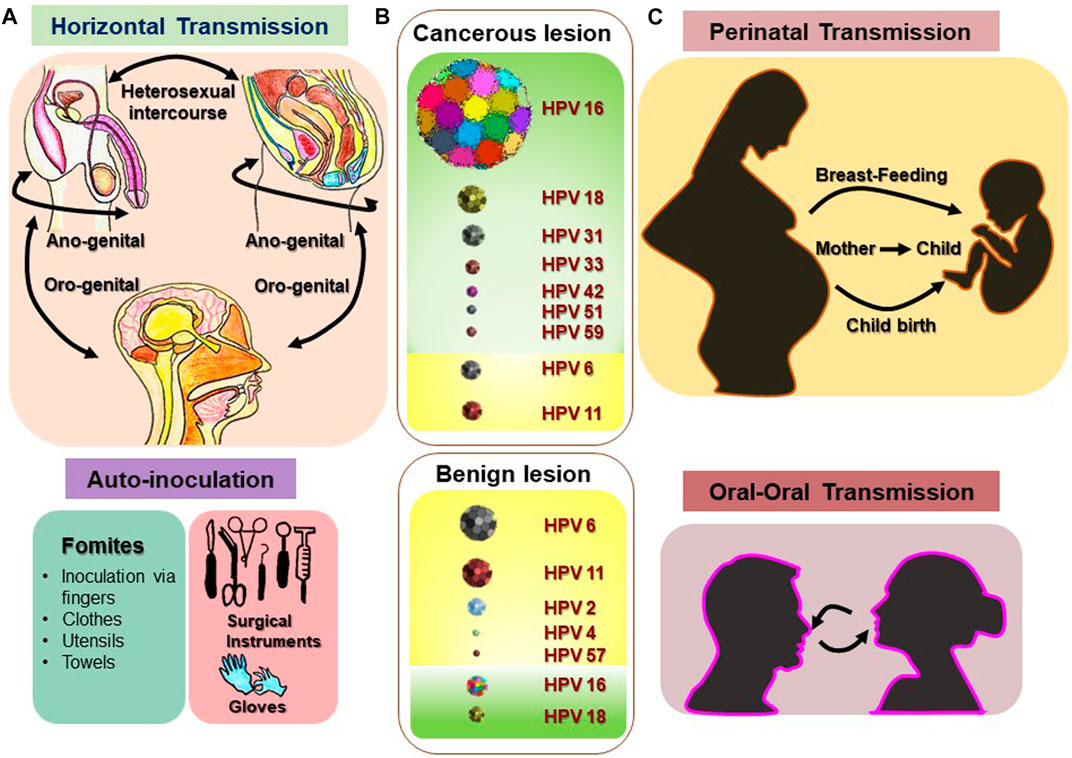
FIGURE 2. Various routes and sites of HPV transmission. HPV is a sexually transmitted infection that can be received through horizontal transmission (heterosexual intercourse, anogenital, and oro-genital) and in some rare cases through various fomites, and inoculation via fingers, clothes, utensils, towels, and surgical instruments. (A) Vertical transmission (perinatal transmission: mother to baby) and oral–oral transmission of HPV could be the main source of nonsexual transmission of HPV in oral sites. (C) Most prevalent high-risk and low-risk HPV types reported in benign and malignant lesions of the HN region are depicted with representative figures where the relative frequency of the each type is indicated with corresponding sizes. (B) [Disclaimer: Pictures used to make composite diagram and to represent HPV types are derived from different internet sources and does not claim to be the original representation of the indicated HPV type] (Sarkola et al., 2008; Chaturvedi et al., 2015; Fu et al., 2015; Visalli et al., 2016; Louvanto et al., 2017; Sabeena et al., 2017; Syrjanen, 2018; Houlihan et al., 2019).
Data emerged in last 2 decades strongly support the recognition of HPV-positive HNSCC as a distinct disease with a well-defined clinical and molecular pattern and unique risk factors (Table 2). These HPV-positive tumors were reported in early stage (Pintos et al., 1999; Smith et al., 2004; Hammarstedt et al., 2006), well differentiated histology (Pintos et al., 1999; Gupta et al., 2015), basaloid morphology (Gillison et al., 2000), larger tumors (Gletsou et al., 2018), and either no lymph node involvement (Pintos et al., 1999) or with cystic cervical lymph node positivity (Goldenberg et al., 2008). These tumors showed low risk of second primary malignant neoplasm (Adjei Boakye et al., 2018) with a better overall and disease-free survival (Ragin and Taioli, 2007; Fakhry et al., 2008; Ang et al., 2010; Rischin et al., 2010; Posner et al., 2011; Fakhry et al., 2014). Irrespective of the tissue subtype involved, HPV positivity in HNSCC emerged as a strong biomarker associated with better prognosis (Gillison et al., 2000; Wookey et al., 2019).
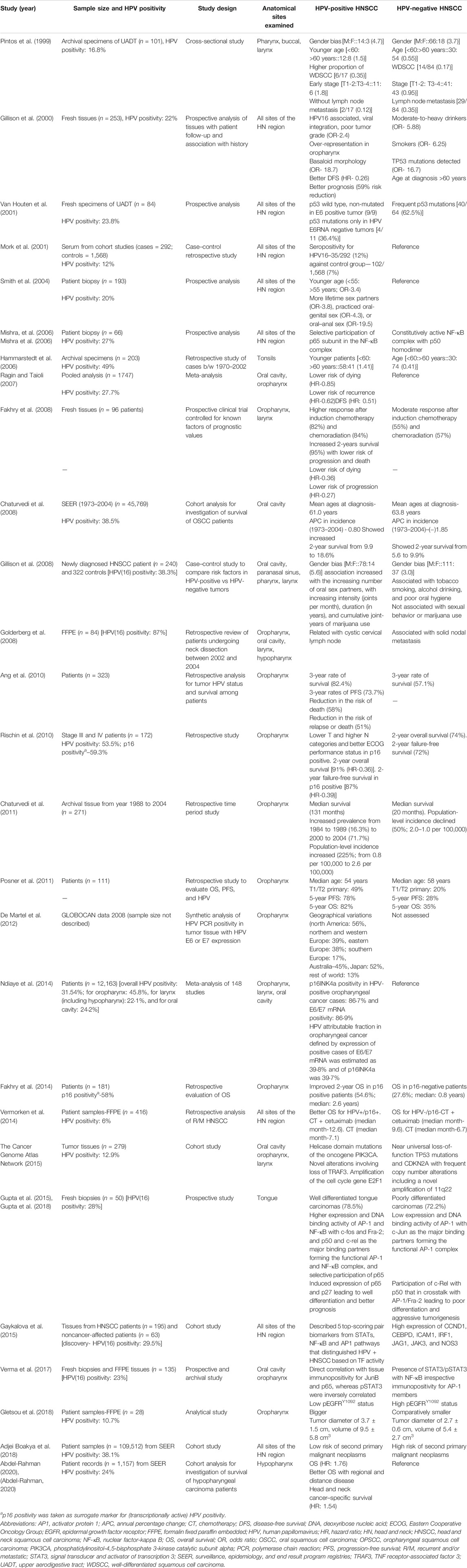
TABLE 2. Representative studies demonstrating the existence of HPV-positive HNC as a distinct disease group.
HNSCC is overrepresented in males (Pintos et al., 1999; Gillison et al., 2008). The gender bias increases further in HPV-positive tumors (Pintos et al., 1999). Gender-specific data derived from HPV-positive oropharyngeal cancer (OPC) patients showed a higher risk of premalignant lesions in men (Ryerson et al., 2008). These observations are indicative of a tumor-promoting role of either male-specific hormones leading to differences in clearance of HPV infections due to the endocrine-immune interactions (Klein, 2000), or a distinct cellular environment in oral mucosal cells of men that promote transcriptional activation of viral oncogenes and HPV-mediated HNC. An increased anal HPV16/18 prevalence has been noticed, which correlated with high free testosterone levels in men having sex with men (Hsu et al., 2015).
Time trend studies carried out in different cohorts and registries particularly in North America and Europe revealed an interesting disease dynamics among all the HN sites (Chaturvedi et al., 2008; Chaturvedi et al., 2011). OPC showed a characteristic change in incidence (Hammarstedt et al., 2006; Mehanna et al., 2013). During the 30 year period, HPV-negative OPC declined steeply with a simultaneous and more prominent emergence of HPV-positive OPC (Chaturvedi et al., 2011). HPV-negative OPC and non-OPC that included all other HN sites are HPV-unrelated and traditionally linked to smoking and alcohol abuse. On the contrary, the studies showed a definitive and strong link of HPV-positive tumors with the oral–genital sexual contact (Gillison et al., 2008). The HPV-positive HNSCC shows large variations in prevalence among different geographical regions (De Martel et al., 2019) and may be associated with prevailing sociocultural and sexual practices, whereas genetic predispositions that may also play a sizable role in this phenomenon cannot be ruled out. In line with these observations, a higher incidence of HPV-positive tumors in Hispanic population has been reported (Gillison et al., 2008).
Early studies repeatedly pointed to a lower median age of HPV-positive HNSCC (Chaturvedi et al., 2008; Posner et al., 2011). However, a recent study demonstrated increased HPV positivity even in older age-group (Windon et al., 2018), thus indicating that early onset of HNSCC was merely circumstantial. Reviewing the factors contributing to the changing pattern of HNSCC over last 50 years revealed a major shift in societal practices with respect to depiction of sexuality (Syrjanen et al., 1982). Surprisingly, in 1969, Denmark legitimized display of explicit content, which was followed by the Netherlands and Sweden, and by 1972, the United States observed a peak in the films displaying oral sexual acts. Therefore, the shift in the HNSCC from HPV-negative to HPV-positive tumors observed in the western population is possibly associated with two independent phenomena that occurred simultaneously. First, establishment of tobacco’s carcinogenic potential (Vizcaino et al., 2015) and consequent implementation of anti-tobacco policies; and second, display of oral sex on motion pictures that promoted indulgence in high-risk behavior leading to increased exposure of oral mucosa to genital HPV infections. Treatment efficacy can be maintained by evaluating the HPV-positivity in OPSCC; as they have better prognosis, they can be treated with less aggressive treatment to avoid serious side effects to reduce treatment-associated toxicities in relatively younger patients (Boscolo-Rizzo et al., 2016).
During first 2 decades, research was emphasized on the detection of HPV and its distribution in the HN region. Subsequent studies revealed a series of distinctive molecular features in HPV-positive HNC (Table 3). In HPV-positive tumors, wild-type p53 was functionally active and was downregulated by E6 oncoprotein. Reduced p53 transcript was associated with the activation of many oncongenic pathway genes, which contributes to genetic instability in the development of cancer (van Houten et al., 2001; Licitra et al., 2006). HPV-positive HNC lesions show characteristically high expression of p16INK4a, which serves as a surrogate marker for HPV (Licitra et al., 2006). In contrast, HPV-negative tumors showed inactivating p53 and p16INK4a mutation in HNSCC.
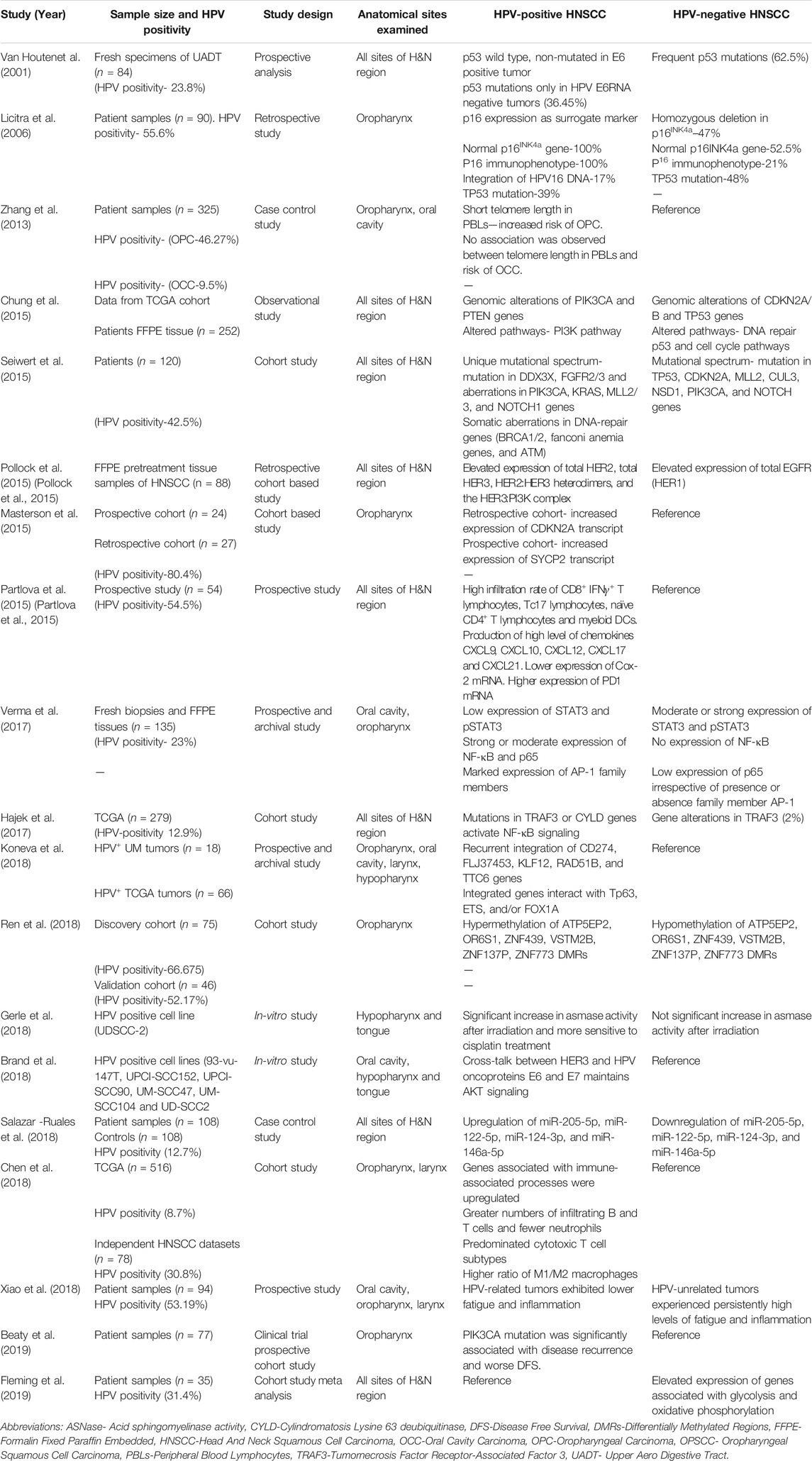
TABLE 3. Representative studies showing specific molecular differences in HPV negative vs HPV positive HNC.
In the proliferative cell signaling pathway, HPV-positive HNC showed elevated expression of HER2, HER3, and HER2:HER3, and HER3:PI3K complex. In contrast, HPV-negative HNC showed higher expression of EGFR (HER1), which is responsible for resistance to EGFR inhibitors (Pollock et al., 2015). HPV-positive HNC was PI3K inhibitor resistant due to abundance of E6 and E7 oncoproteins. A crosstalk among PI3K, HER3, and E6/E7 oncogenes was reported (Brand et al., 2018). Differential regulation of several microRNAs was observed in HNC, miR-205-5p, miR-122-5p, miR-124-3p, and miR-146a-5p that were upregulated in HPV-positive HNC. In contrast, these miRNAs were downregulated in HPV-negative HNC (Salazar-Ruales et al., 2018).
Based on transcription milieu, HNC showed constitutively active nuclear factor-κB (NF-κB) irrespective of their HPV status. However, a detailed molecular dissection of the constitutively active NF-κB complex showed the presence of p50:p65 heterodimer in HPV-positive tumors, whereas homodimer of p50:p50 was found in HPV-negative tumors (Mishra et al., 2006; Gupta et al., 2018). Similarly, in HNC tumors for constitutively active AP-1, JunB and JunD were involved with c-Fos and Fra-2 in HPV-positive HNC, whereas in HPV-negative HNC, c-Jun was the major binding partner (Gupta et al., 2015).
STAT3, another transcription factor that is linked with carcinogenic outcome, was strongly associated with HPV-negative HNC and was characteristically low in HPV-positive tumors (Gaykalova et al., 2015; Verma et al., 2017). SOX2 amplification was observed in HPV-negative HNC, while there was no amplification in HPV-positive HNC (Schrock et al., 2014). HPV-positive HNCs were immunologically more active with high infiltration of T and B lymphocytes and myeloid dendritic cells, and had higher M1-type macrophages along with high chemokine production and PD1 expression (Partlova et al., 2015; Chen et al., 2018). A detailed discussion of various differentially expressed carcinogenically relevent genes in HPV-positive and HPV-negative HNC that contribute to better prognosis was described earlier (Aggarwal et al., 2020).
Treatment of HNC requires a multi-modality approach depending on the stage and site of the tumor (Marur and Forastiere, 2008). Early tumors are treated with surgery or radiation, whereas intermediate- and late-stage tumors benefit from a combined modality approach. Due to essential requirement of clear margins in surgery, it is an option only for early tumors; still it carries a risk of cosmetic deformity and impaired function (Kofler et al., 2014). A study on the quality of life after oropharyngeal surgery reports high incidence of fatigue, reduced sexuality, difficulty in swallowing and other teeth, salivary gland, and mouth-opening–related problems (Bozec et al., 2018). Surgery also requires additional treatment to reduce the risk of locoregional and distant failure in advanced-stage HNC (Porceddu et al., 2004). Platinum-based chemotherapy has been central in treating HNC. Combinatorial therapies with or without platinum drugs have been proven superior in terms of the response rate and the ability to tackle drug resistance than platinum-based chemotherapy treatment. Targeted therapies using monoclonal antibodies such as cetuximab, against epidermal growth factor receptor (EGFR) either in combination with a standard chemotherapy regimen or as a single agent, have also proven effective to some extent to treat HNC. But these approaches also bear side effects apart from the development of chemoresistance in a short period of time (Price and Cohen, 2012). These therapies have a myriad of debilitating toxic effects such as nephrotoxicity, hepatotoxicity, and cardiotoxicity. Also, various cardiac events have been reported, like arrhythmias, myocarditis, and cardiomyopathy, to congestive heart failure (Hartmann and Lipp, 2003).
Radiation therapy (RT) is often performed as an adjunct to surgery or in concurrence with chemotherapy (Marur and Forastiere, 2016). Wendt et al. reported a 3-year overall survival rate of 24% in RT arm vs. 48% in RT plus CT arm in stage III/IV HNC, whereas the 3-year locoregional control rate was 17% in RT arm and 36% in RT plus CT arm (Wendt et al., 1998). However, a long-term toxicity risk to the salivary glands, pharyngeal constrictor muscles, and thyroid gland, leading to xerostomia, dysphagia, percutaneous endoscopic gastrostomy tube dependence, chronic aspiration, and hypothyroidism, had been observed (Langendijk et al., 2008).
Despite a clear prognostic advantage and better response to therapy, therapeutic management for HPV-positive HNC is almost the same as that of any HPV-negative HNC. Considering the younger age of the patients, there have been efforts to reduce the long-term toxicity of anticancer treatment without risking the survival benefits (Kofler et al., 2014). Reduction in dose of radiotherapy, use of cetuximab (Marur et al., 2017) instead of cisplatin for chemoradiation, and transoral robotic surgery (TORS) are a few efforts to mention that are specifically directed to HPV-positive HNC. Considering HPV-positive tumors to be immunologically active, in recent past, attempts have been made to design PD1-PDL1 immunotherapeutic strategies (Qiao et al., 2020). New cancer immune-prevention treatments include FDA-approved inhibitory antibodies such as pembrolizumab (anti-PD1 mAb), nivolumab (anti-PD1), and ipilimumab (anti–CTLA-4 mAb) (Bauman and Ferris, 2014; Ferris et al., 2018; Mehra et al., 2018; Havel et al., 2019); co-stimulation and co-inhibition pathways (Kuss et al., 2003; Tsukishiro et al., 2003; Baruah et al., 2012; Pardoll, 2012); and check-point blockade therapy (Davis et al., 2016; Muzaffar et al., 2021). A systematic assessment of the cost effectiveness of ICIs showed nivolumab was not cost-effective over chemotherapy for HNC (Verma et al., 2018). Moreover, none of these approaches target HPV. A study attempted to develop Trojan vaccine against HPV could not show significant benefit of therapeutic vaccines against HPV in HNC (Voskens et al., 2012). A recent study showed a chimeric HPV16 E7 DNA vaccine induced prophylactic and therapeutic efficacy in a cervical cancer mouse model, but its effect on HPV-positive HNC remains to be examined (Garza-Morales et al., 2019).
Despite aggressive treatment and organ preservation with current clinically administered curative therapies, the overall 5-year survival is less than 50% (Forastiere et al., 2013). With existing heterogeneity in the origin, poor response rates and substantial systemic toxicity associated with current standard-of-care treatment of advanced HNC remain a significant challenge (De Lartigue, 2015). As molecular targeted therapies come into clinical use, the great interindividual variability in the efficacy of these compounds highlights the absolute need to determine predictive factors of tumor and toxic responses to these new therapeutic agents (Bozec et al., 2009). Further, patients with locally advanced or recurrent HNC present a separate therapeutic challenge. Treatment options are limited, and morbidity can be substantial. Surgical intervention has debilitating effect on normal daily routine and patient psychology. Recurrent HNC is difficult to treat for multiple reasons, including the effects of prior treatment on tumor cells and normal tissues, as well as the infiltrative and multifocal nature that typically characterizes recurrent disease in this area (Ho et al., 2014).
Limitations of these therapies have prompted clinical and translational research for better chemotherapeutics with less treatment-associated toxicities. Many studies are focusing on biologically active compounds from herbal origin to develop chemotherapeutic agents with fewer side effects and higher efficacy (Seo et al., 2015; Kunnumakkara et al., 2017). Many of these phytochemicals can serve as alternatives for chemotherapy sensitizers (Bharti and Aggarwal, 2017; Huang and Yu, 2017).
Phytochemicals have found relevance in HNC therapy because natural compounds provide a cost-effective, safe, and less toxic alternative to synthetic drugs currently in wide use. Effectiveness of various phytochemicals as therapeutic agents has been well documented in the literature, and they are now widely being studied as potential agents to treat and prevent HNC. Many preclinical studies have successfully demonstrated the anticancer activity of pure and well-characterized phytochemicals and herbal derivatives on cells obtained from different HN regions using in vitro and in vivo experimental systems (Table 4). However, a majority of these studies employed cell lines derived from the oral cavity, so the data may be slightly skewed.
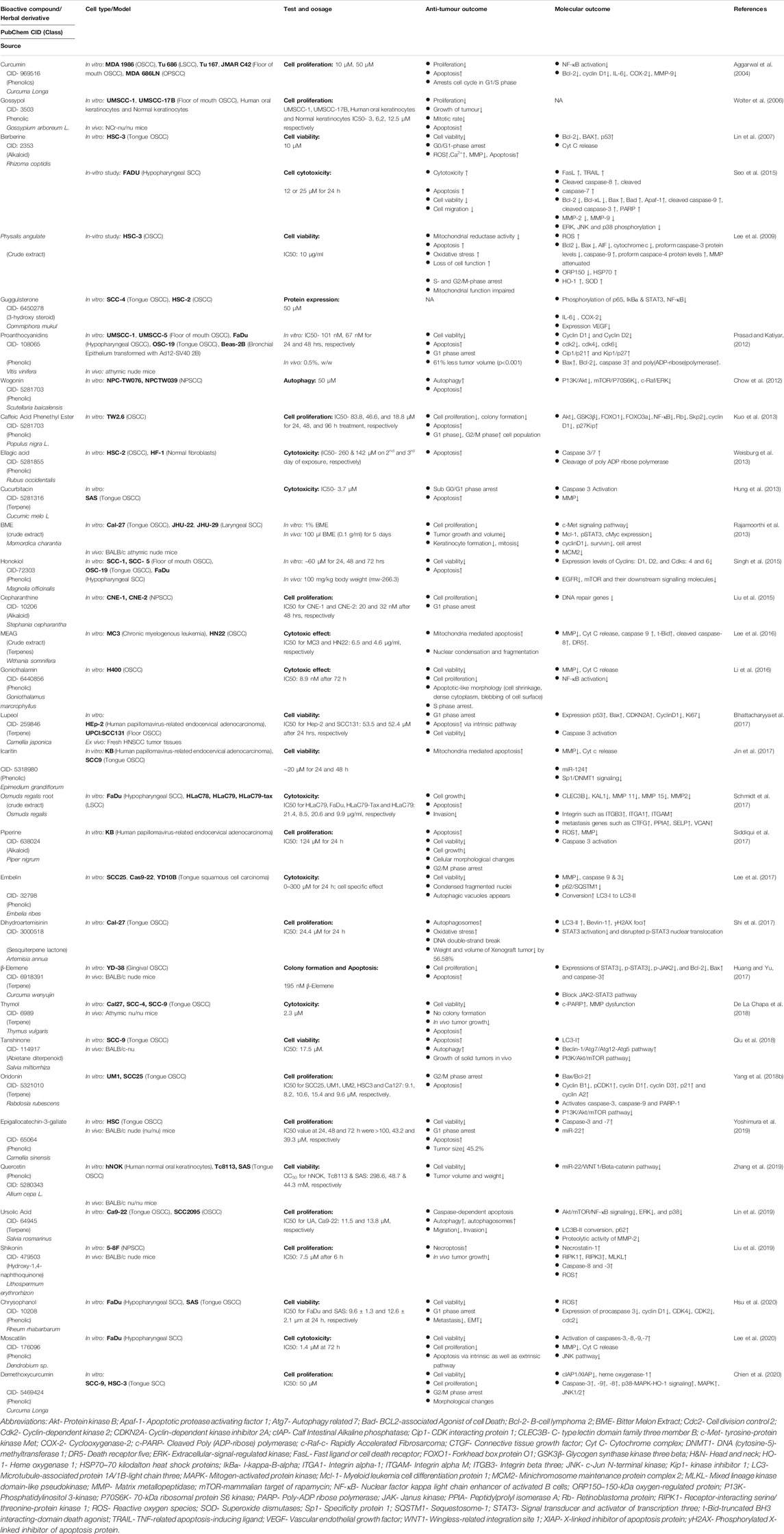
TABLE 4. Preclinical studies showing therapeutic phytochemicals/herbal derivatives against tobacco/alcohol-associated HNC.
A range of phytochemicals showed anticancer activity against different HNC cells over 2 decades (Figure 3). Phytochemicals like thymol, oridonin, shikonin, and moscatilin with potent dose-dependent antiproliferative activity showed IC50 values lower than 10 μM over a wide range of HNC cell types. A detailed investigation of molecular mechanisms revealed targeting of key cellular carcinogenic pathways, namely, MAPK/JNK/p38 (role of ROS), NF-κB, EGFR/JAK2/STAT3, P13K/Akt, mTOR/P70S6K, c-Raf/ERK, GSK3β, FOXO1, FOXO3a, and p53, that concurrently operate in HNC and contribute to cancer progression and treatment resistance.
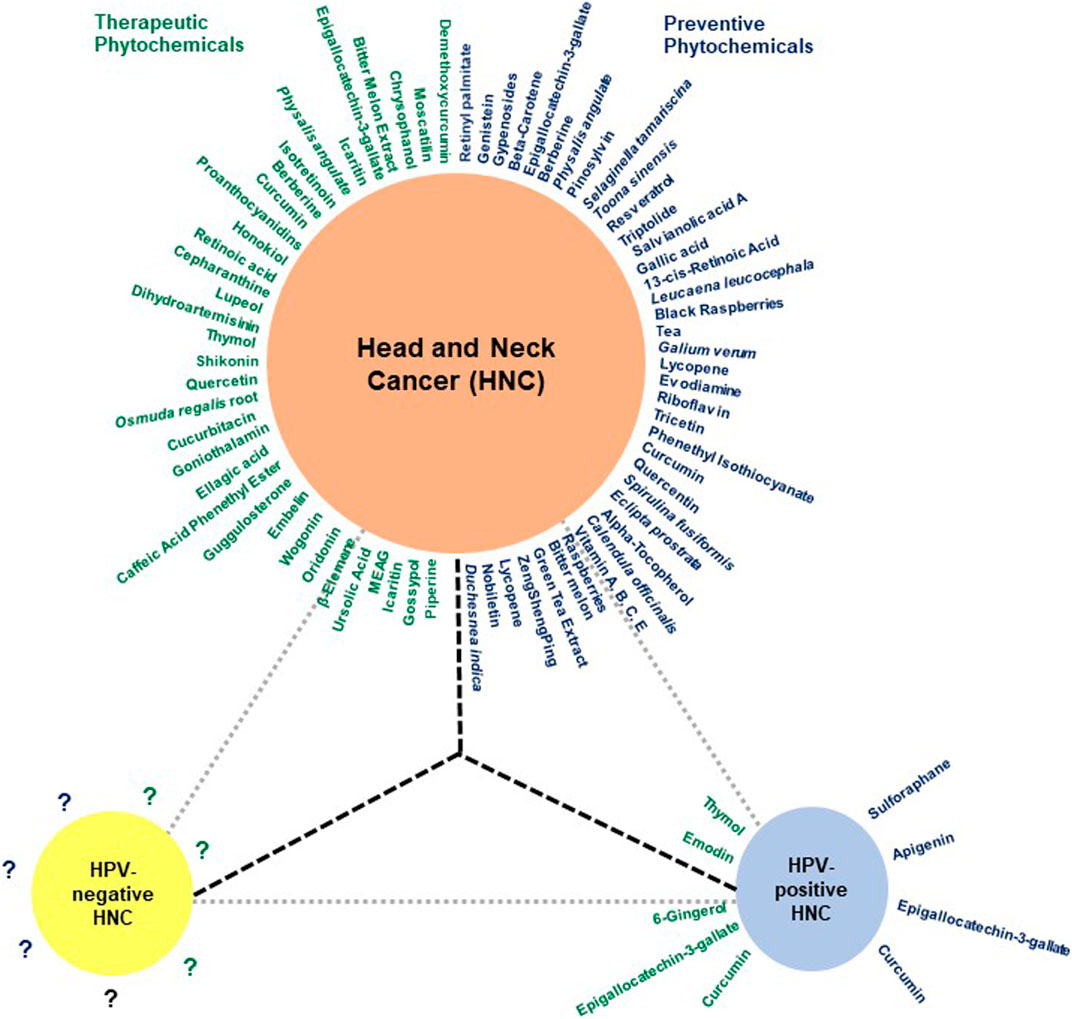
FIGURE 3. The huddle of chemopreventive and chemotherapeutic phytochemicals in HNC. Schematic diagram showing phytochemicals with chemopreventive and chemotherapeutic properties in blue and green, respectively. Since most reports addressing the effect of herbal derivatives on HNC lacked HPV-related information, the data may not be directly applicable to HPV-negative HNC and require prior validation in HPV-negative HNC cells. Additionally, the HPV-positive HNC remains a poorly explored area.
NF-κB is a family of transcription factors (TFs) comprising c-Rel, RelA (p65), RelB, NF-κB1 (p50 and p105), and NF-κB2 (p52), which plays important roles in immunity, inflammation, cell proliferation, survival, and differentiation (Oeckinghaus and Ghosh, 2009). Many basic and clinical studies demonstrated aberrantly expressed and constitutively expressed NF-κB in HNC with its contribution to cancer cell survival and proliferation, and poor survival of patients (Mishra et al., 2006; Monisha et al., 2017; Verma et al., 2017). Cigarette smoke phosphorylates IκBα, which in turn activates NF-κB (Anto et al., 2002). Early evidence of phytochemicals like curcumin showing anticancer action on HNC came from abrogated IκBα kinase (IKK) which inhibited NF-κB activation and cell survival/proliferation genes such as cyclin D1, Bcl-2, IL-6, COX-2, and MMP-9 (Aggarwal et al., 2004). Based on a similar approach, blocking activity of NF-κB, or its downstream molecules, therapies were designed to downregulate cell growth and metastasis. Caffeic acid phenethyl ester (CAPE) and goniothalamin inhibited NF-κB-p65 activity in a potential primary and metastatic OSCC (Kuo et al., 2013; Li et al., 2016).
STAT3, a central transcription factor and known oncogene, works downstream of EGFR, and TGFα signaling also plays a key carcinogenic role in HNC (Song and Grandis, 2000). Guggulsterone, a biosafe nutraceutical, phosphorylated p65 and inhibited tobacco smoke and nicotine-induced NF-κB and pSTAT3 proteins and their downstream targets COX-2 and VEGF (Macha et al., 2011). Dihydroartemisinin is a known phytochemical, which is effective as an antimalarial agent, induces DNA double-strand break and promoted oxidative stress, and decreases pSTAT3 nuclear localization which successively increases autophagic cell death (Shi et al., 2017).
In 90% HNC, the PI3K/AKT/mTOR pathway is upregulated (Marquard and Jucker, 2020). Whenever ligand-like growth factors bind with RTKs, they dimerize and lead to the activation of intercellular tyrosine kinase. PI3K partially activates Akt through PIP3 and PIP2. Then to stimulate full activity of Akt, mTORC2 phosphorylates its carboxy-terminal. Akt functions by phosphorylation that leads to the activation or suppression of many proteins involved in cell proliferation, growth, and cell motility (Brazil and Hemmings, 2001; Chaisuparat et al., 2016). Wogonin, a flavonoid compound, has anticancer activity which induces autophagy by LC3 I/II cleavage and inhibits mTOR/P70S6K and Raf/ERK, which in turn inactivates PI3K/Akt and induces apoptosis in NPC cells (Chow et al., 2012). Urosolic acid downregulated Akt/mTOR signaling and expression of NF-κB, which further downregulates ERK and MMP-2 in OSCC cells (Lin et al., 2019).
Loss of carcinogenic signaling was associated with reduced cell survival mechanisms. Honokiol, a phytochemical from Magnolia plant, reduced the level of Bcl-xL protein, while Bax expression in xenograft HNC tumors increased. It also reduces the expression of mTOR and its downstream p70S6K (Singh et al., 2015). Similarly, (-)-gossypol, a polyphenol, was reported to bind to Bcl-xL that inhibited HNC proliferation (Wolter et al., 2006).
Antiproliferative activity of phytochemicals was associated with various degrees of cell cycle arrest in most of these studies. Cell cycle–regulating molecules such as cyclins and cdks were downregulated by oridonin, chrysophanol, lupeol, honokiol, and proanthocyadins. Piperine, a nitrogenous pungent substance, induced cell cycle arrest in the G2/M phase and induced apoptosis by changing mitochondrial membrane potential and by activating caspase-3 (Siddiqui et al., 2017). Chrysophanol, a secondary metabolite, downregulated the expression of cyclinD1, CDK4, cdc2, and CDK2, and arrested cell cycle at the G1 phase. It also induced cell death by ROS production (Hsu et al., 2020). Similarly, lupeol induced cell cycle arrest in the G1 phase by increasing the expression of p53, Bax, and CDKN2A, and downregulating cyclin D1 (Bhattacharyya et al., 2017). Oridonin, a bioactive diterpenoid, induced apoptosis by regulating Bax/Bcl-2 and activating caspases. It also decreased cell proliferation by downregulating PI3K/Akt/mTOR pathways. By regulating cyclins, it arrested cells in the G2/M phase (Yang et al., 2018b). Even though the end effect was antiproliferative, the mechanism of action of these phytochemicals differed significantly.
A family of cysteine proteases known as caspases regulates apoptosis. Targeting these caspases can induce apoptosis in OSCC. Demethoxycurcumin, a curcumin analog, induced apoptosis in tongue SCCs by upregulating caspase-3, -9, and -8. It also regulated p38-MAPK-HO1 signaling, MAPK, and JNK1/2 (Chien et al., 2020). Shikonin induced necroptosis in NPC via upregulating the expression of RIPK1/RIPK3/MLKL, caspase-3, and -8, and increasing ROS production (Liu et al., 2019). Ellagic acid induced apoptosis by upregulating caspase-3 and -7 (Weisburg et al., 2013). Curcurbitacin, embelin, and proanthacyadins induced apoptosis by attenuating mitochondrial membrane potential and by regulating the activity of Bcl-2, Bcl-xL, and Bax in cells (Prasad and Katiyar, 2012; Hung et al., 2013; Lee et al., 2017).
In in vivo studies, the phytochemicals were tested in murine models, where nude mice were implanted with OSCC cell lines. These mice were used to measure the effect of phytochemical on tumor growth. Tumors from euthanized mice were examined for their size and volume. ECGC (Yoshimura et al., 2019), gossypol (Wolter et al., 2006), quercentin (Zhang et al., 2019), proanthocyadins (Prasad and Katiyar, 2012), tanshinomes (Qiu et al., 2018), shikonin (Liu et al., 2019), β elemene (Huang and Yu, 2017), and bitter melon extract (Rajamoorthi et al., 2013) depicted reduction in size and volume of tumor xenografts, and inhibition of xenograft growth. Inhibition of growth was also observed in the ex vivo study with lupeol.
Some of the phytochemicals were also tested in clinical trials; however, these studies are very limited (Table 5) and emphasize an urgent unmet need in this area to harness the translational potential of emerging phytochemicals. Lippman et al., 1988 conducted a phase II randomized study with 13-cis-retinoic acid (isotretinoin) (3 mg/kg/day) and methotrexate (15 mg/m2 on the first three days in a 3-week cycle) among 40 patients with advanced SCCs. They achieved a response rate of 16% with isotretinoin, which included a complete response, a partial response, and a minor response. In the methotrexate-treated group, however, the response rate was 5%. The median survival rate from the start of treatment was also lower in the methotrexate group (4 months) than that in the isotretinoin group (4.5 months) (Lippman et al., 1988). Another phase I study with isotretinoin by Weisman et al., 1998 reported its strong synergetic relationship with cisplatin. The maximum tolerated dosage as determined by the study (20 mg/day) was able to attain a complete response at the primary site in all of the 10 evaluable patients (Weisman et al., 1998). There are very few clinical trials on therapeutic potential of phytochemicals in HNC because of lack of interest from pharma industry due to low cost of the molecules, and clinical trial requires a lot of investments. Also, HNC patients with advance stage tumor do not participate in therapeutic clinical trials as it may risk the available therapeutic benefits of existing therapies; however, use of phytochemicals as adjunct therapies may prove beneficial in long run as they will not compromise the benefits of participating patients. Nevertheless, more in vivo studies are needed to screen promising leads into clinical trials.
Cancer chemoprevention refers to the use of agents to retard the progression of carcinogenesis, reverse, or inhibit it. The aim of chemoprevention is to lower the risk of developing invasive or clinically significant disease. Chemopreventive phytochemicals thus seek to occasion a chemopreventive response when the primary tumors have not reached a critical size, or seek to block and reverse development of a diagnosed premalignant tumor, or prevent metastasis and growth of metastatic tumors (Tosetti et al., 2002). Angiogenesis, which refers to the biological process of vessel formation, also plays a crucial role in cancer progression. Angiogenesis is also responsible for transition of a dormant tumor to a malignant state (Sogno et al., 2009). An early intervention could possibly prevent cancer formation by regulating “angiogenic switch,” the point at which the tumor induces angiogenesis. Thus, angiogenesis is a critical target for chemoprevention (Tosetti et al., 2002).
A battery of phytochemicals reportedly possess cell invasion, migration, angiogenesis, and metastasis inhibitory activities (Table 6). These phytochemicals exhibit these antitumor activities by regulating the expression of various molecules such as metalloproteinases (MMPs), especially MMP-2 and MMP-9, which affect cancer migration and invasion. Some MMPs also exhibit proangiogenic properties as they can activate proangiogenic factors such as VEGF, and angiopoietin (Folgueras et al., 2004). These phytochemicals were also observed to regulate the MAPK/ERK pathway, which plays a crucial role in cell proliferation (Chen et al., 2019).
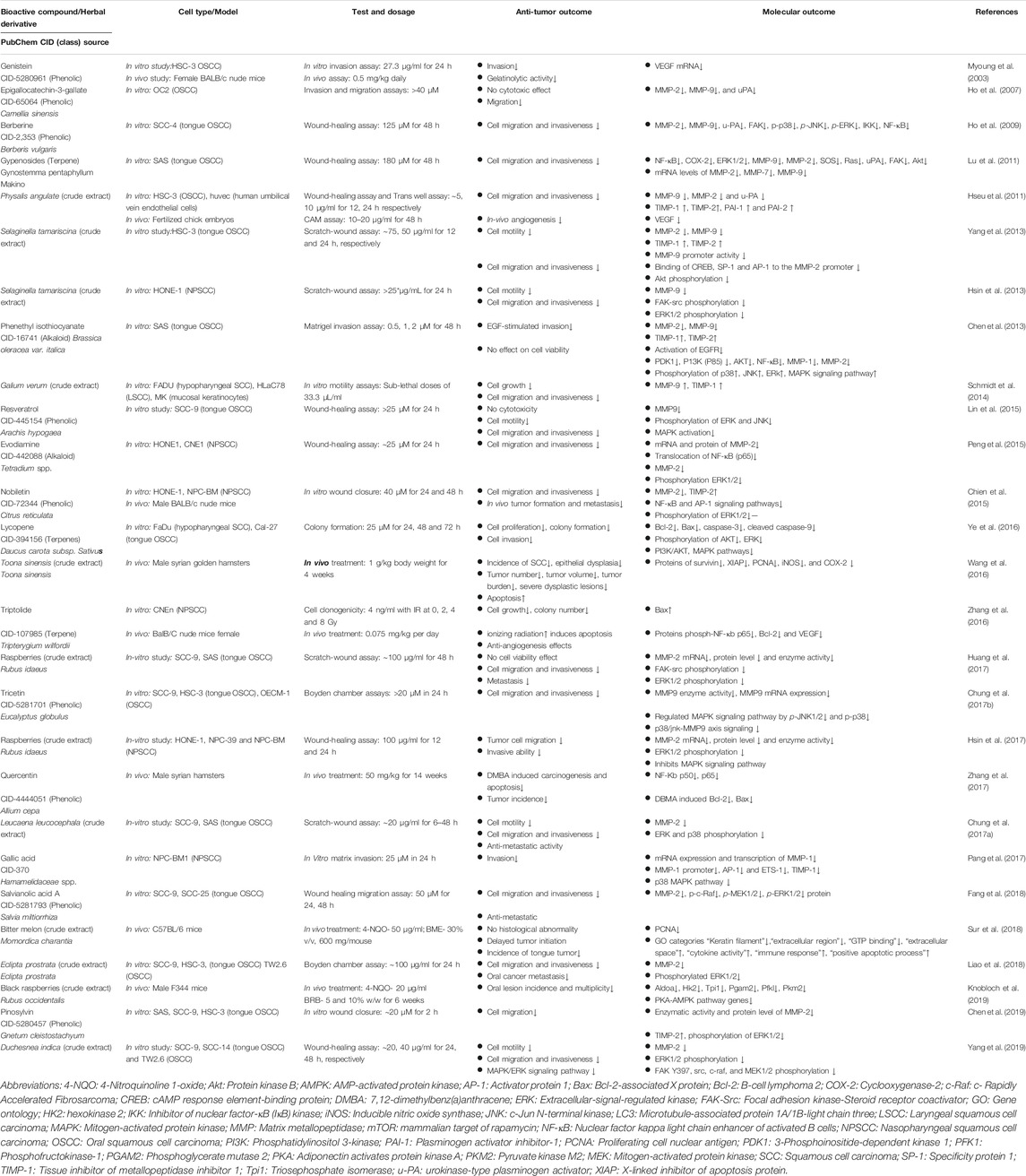
TABLE 6. Pre-clinical studies in emerging chemopreventive phytochemicals/herbal derivatives against HNC.
In vitro studies conducted with epigallocatechin-3-gallate, berberine, gypenosides, phenethyl isothiocyanate, resveratrol, tricetin, nobiletin, evodiamine, salvianolic acid A, gallic acid, pimosylvin, and extracts of Eclipta prostrata, Physalis angulata, Selaginella tamariscina, Leucaena leucocephala, Duchesnea indica, rasberries (Rubus idaeus), and Galium verum downregulated the expression of MMPs (Thomas et al., 1999; Ho et al., 2007; Ho et al., 2009; Hseu et al., 2011; Lu et al., 2011; Chen et al., 2013; Chien et al., 2015; Lin et al., 2015; Peng et al., 2015; Chung HH. et al., 2017; Chung TT. et al., 2017; Huang et al., 2017; Pang et al., 2017; Fang et al., 2018; Liao et al., 2018; Chen et al., 2019; Yang et al., 2019). The increase in MMPs is generally associated with invasive and metastatic phenotype of oral carcinoma (Thomas et al., 1999). Tissue inhibitor of metalloproteinases (TIMPs) are endogenous inhibitors of MMPs, and play a role in cell migration and wound healing. TIMPs were found to be up-regulated in phenethyl isothiocyanate, nobiletin, gallic acid, Physalis angulata, Selaginella tamariscina, and Galium verum (Chen et al., 2013; Hsin et al., 2013; Chien et al., 2015; Yu et al., 2016; Pang et al., 2017).
Berberine, phenethyl isothiocyanate, resveratrol, gypenosides, lycopene, evodiamine, gallic acid, nobiletin, tricetin salvianolic acid A, pinosylvin, and extracts of Eclipta prostrata, Selaginella tamariscina, Leucaena leucocephala, Duchesnea indica, and Rubus idaeus inhibited the MAPK/ERK pathway (Ho et al., 2009; Hseu et al., 2011; Chen et al., 2013; Hsin et al., 2013; Schmidt et al., 2014; Chien et al., 2015; Ye et al., 2016; Yu et al., 2016; Chung HH. et al., 2017; Huang et al., 2017; Pang et al., 2017; Fang et al., 2018; Yang et al., 2019). Additionally, genistein, triptolide, and Physalis angulata extract downregulated VEGF expression (Myoung et al., 2003; Hseu et al., 2011; Zhang et al., 2016).
In vivo studies with nobiletin on male BALB/c nude mice suppressed tumor formation and metastasis by downregulating NF-κB translocation, MMP-2, and TIMP-2 proteins, and decreased phosphorylation of ERK1/2 (Chien et al., 2015). Toona sinensis crude extract decreased the incidences of SCCs, tumor number, tumor volume, and tumor burden in male Syrian golden hamsters by downregulating protein levels of survivin, XIAP, PCNA, iNOS, and COX-2 (Wang et al., 2016). Delayed tumor initiation incidence was reported in bitter melon extract–fed mice (Sur et al., 2018). Oral lesion incidence decreased in 4NQO exposed mice after being fed a black raspberry diet by downregulating PKA-AMPK pathway genes, which regulates mitochondrial functions (Knobloch et al., 2019).
Most of the reported clinical trials focusing on chemoprevention in HNC have been conducted on oral premalignant lesions (Table 7). Historically, clinical studies conducted on HNC chemoprevention with natural agents have centered on the use of retinoid. Bichler et al. (1983) reported that serum levels of retinol, RBP, and PACB were significantly lower in patients with carcinomas of the HN region (Bichler et al., 1983). This was considered to be of significance in tumor development studies, and since then, it has been corroborated by various research groups such as by Kapil et al. (2003). One of the initial studies conducted with retinoids was by Hong et al., 1986. The double-blind study demonstrated the effectiveness of 13-cRA in reducing the size of oral premalignant lesions in 44 patients. In a study conducted by Stich et al. (1988a) on 65 patients having well-developed oral leukoplakia, a complete remission in the lesions was observed in 57.1% of patients in the vitamin A group as compared to 3% of patients in the placebo group (Stich et al., 1988a). An interesting study was also conducted by Mathew et al., (1995) using lyophilized Spirulina fusiformis, an effective source of dietary vitamin A and other micronutrients (Mathew et al., 1995). A 1 g/day dose of oral Spirulina fusiformis powder demonstrated an effective chemoprevention activity by producing a complete response in 20/44 subjects in the treatment group as compared to 3/43 subjects in the placebo group. A partial response was observed in five patients in the Spirulina fusiformis treatment group as compared to zero in the placebo group.
A study by Stich et al. (1988b) reported on the combined effect of beta-carotene and vitamin A on betel quid chewers in India with well-established leukoplakias. Remission in the group receiving combined treatment was 27.5% as compared to 14.8 and 3% in groups receiving just beta-carotene and the placebo, respectively. The rate of new leukoplakia occurrence was also found to be higher in the beta-carotene (14.8%) and placebo groups (21.2%) that that of new leukoplakia occurrence in the group treated with both beta-carotene and vitamin A (7.8%) (Stich et al., 1988a). The effectiveness of beta-carotene as a chemopreventive agent was also established by Garewal et al., (1990), who in study with 25 patients achieved a response rate of 71% in the group treated with 30 mg/day beta-carotene (Garewal et al., 1990). A comparative study conducted in two phases with beta-carotene and isotretinoin by Lippman et al. (1993) reported that low-dose isotretinoin therapy was significantly more active against leukoplakia than beta carotene when preceded by high-dose induction therapy (Lippman et al., 1993). In another three-arm double-blind study conducted with 160 patients by Sankaranarayana et al. (1997), the vitamin A and beta-carotene arms attained a complete regression of leukoplakia lesions in 52 and 32% of the subjects, respectively, as compared to just 10% in the placebo arm (Sankaranarayanan et al., 1997).
Two clinical studies conducted with retinyl palmitate by Jyothimayi et al. (1996) and Issing et al. (1996) reported a complete inhibition of the formation of secondary primary tumors (SPTs) and a complete remission of leukoplakia lesions in 75% of participants in drug-receiving arms, respectively (Issing et al., 1996; Jyothirmayi et al., 1996). Significant decrease in the prevalence odds ratio of oral leukoplakia was observed by Zaridze et al. (1993) in a double-blind trial conducted among 532 subjects with various combinations of riboflavin, retinol, vitamin E, and beta-carotene (Zaridze et al., 1993). Another combinatorial study with beta-carotene, ascorbic acid, and alpha-tocopherol by Kaugars et al. (1994) noted a clinical improvement in 55.7% of the participants; 48.8% of people who continued their pre-study levels of risk factor exposure showed improvement (Kaugars et al., 1994).
A 24-week study by Benner et al. (1993) using alpha-tocopherol as a single agent to treat patients with oral leukoplakia attained a clinical response in 20 patients from the 43 patients who had signed up for it (Benner et al., 1993). Alpha-tocopherol was part of yet another study by Shin et al. (2001), when delivered with IFN-α and 13-cis-retinoic acid; among 44 patients evaluable at a median 24-month follow-up, 9% had locoregional recurrence, 5% had both locoregional recurrence and distant metastases, and 2% developed an SPT. The overall survival rate at the 24-month follow-up was noted to be 91% (Shin et al., 2001).
Green tea, a widely consumed beverage, has been previously reported to exhibit chemopreventive properties against cancer (Imai et al., 1997). Since it inhibits tumor development, is nontoxic, and is easily available to the general population, it has been a subject of interest in cancer studies. Two clinical studies where green tea was used as an agent to treat precancerous lesions like leukoplakia were included. Li et al. (1999) reported a decrease in lesions in 37.9% patients in the tea-receiving arm as compared to improvement in lesions of only 10% patients in the placebo arm (Li et al., 1999). Tsao et al. (2009) reported a dose-dependent clinical response by randomizing 41 patients in three green tea extract–receiving arms (dosage: 500 mg/m2, 750 mg/m2, 1,000 mg/m2) and 1 placebo arm, with a clinical response in 50% of patients in the three combined arms and a 58% clinical response rate in the two combined higher dose arms (Tsao et al., 2009). They also reported a histological improvement in lesions after treatment.
Lycopene is a carotenoid that is abundant in a human diet and has been associated with a reduced risk of cancer of the upper digestive tract (De Stefani et al., 2000). Singh et al. (2004) reported a dose-dependent response of oral leukoplakia for administration of lycopene, with clinical improvement observed in 80% of patients receiving 8 mg/day lycopene; 66.3% patients receiving 4 mg/day dose showed a clinical response (Singh et al., 2004). A clinical study with lycopene and Calendula officinalis by Singh and Bagewadi (2017) reported a reduction in the average size of lesions posttreatment. The mean difference in the reduction in size before and after treatment for Group I was 2% ± 1.0 cm, while for the Group II, it was 1.6% ± 0.9 cm (Singh and Bagewadi, 2017).
Curcumin, a flavonoid derived from Curcuma longa, has been extensively investigated for its pharmacological properties. It is known to have antioxidant, anti-inflammatory, and anticancer properties, and thus is a promising phytochemical for HN region chemoprevention. A randomized double-blind phase IIB study by Kuriakose et al. (2016) on 223 patients with oral leukoplakia reported a clinical response in 67.5% of patients in the curcumin arm (dosage: 3.6 g/day for 6 months) and a histological response in 22.5% of patients (Kuriakose et al., 2016).
Sun et al. (2010) conducted a randomized placebo-controlled study with ZengShengPing; a mixture of six medicinal herbs was known to have pharmacological effects. 3.6 g of ZSP administered daily for 8–12 months was observed to produce a positive response in 67.8% of patients in the treatment arm as compared to 17% in the placebo group (Sun et al., 2010).
Mallery et al. (2014) conducted a placebo-controlled clinical trial using topically applied 10% w/w black raspberry (BRB) gel among 40 patients with oral premalignant lesions. The study reported an average decrease of 26% in the size of BRB-treated lesions as compared to an increase in size by 18% in the placebo gel–applied lesions. Two patients in the BRB arm exhibited a complete lesional resolution as compared to zero in the placebo gel group (Mallery et al., 2014).
Although a large volume of data reflects targeting of key pro-carcinogenic signaling pathways by various phytochemicals, none of them directly address their possible impact on HPV infection or in HPV-positive HNC lesions. Therefore, we specifically looked for evidences where phytochemicals have been tested against HNC cells with HPV-positive background.
Most of the studies described earlier lack specificity against HPV infection. The natural derivatives having both anti-HPV and anti-HNC activity hold great potential as chemotherapeutic and chemopreventive agents for HNC caused by HPV. However, there are only limited resources in terms of HPV-related HNC model systems. Unlike many other infections, HPV cannot be propagated in in vitro cultures or in animal models. Unfortunately, suitable animal models that mimic HPV-driven HNC do not exist. In such a scenario, HPV-positive HNSCC cell lines serve as a suitable in vitro system. There are currently only a limited set of HPV-driven HN cancer cell lines developed by different investigators (Table 8). As of now, we could identify only 11 cell lines that have been described as HPV positive, and their HPV genotype has been confirmed. A majority of them have HPV16 positivity, and the genome was found to be integrated (Steenbergen et al., 1995; Ballo et al., 1999; White et al., 2007; Brenner et al., 2010; Ye et al., 2011; Tang et al., 2012; Kalu et al., 2017). Similarly, one cell line each of HPV18 and HPV33 has been reported (Owen et al., 2016; Kalu et al., 2017). Although there are various HNSCC cell lines described so far, their HPV status must be ascertained. These cells lines proved to be useful model systems as they showed p16 positivity and demonstrated higher radiosensitivity (Rieckmann et al., 2013). In these cell line integration of HPV from E1, E2, L1, L2, and LCR have been observed which recapitulate observation in primary tumors by whole genome sequence which suggests various hotspots for HPV integration events in HPV-positive tumors and that may play varied role in the development of HNC (Gao et al., 2019). These cell lines and tumor tissues showed the presence of the viral infection by the presence of viral DNA and transcripts which emerged as valuable tools (Steenbergen et al., 1995; Ballo et al., 1999; White et al., 2007; Brenner et al., 2010; Ye et al., 2011; Tang et al., 2012; Kalu et al., 2017).
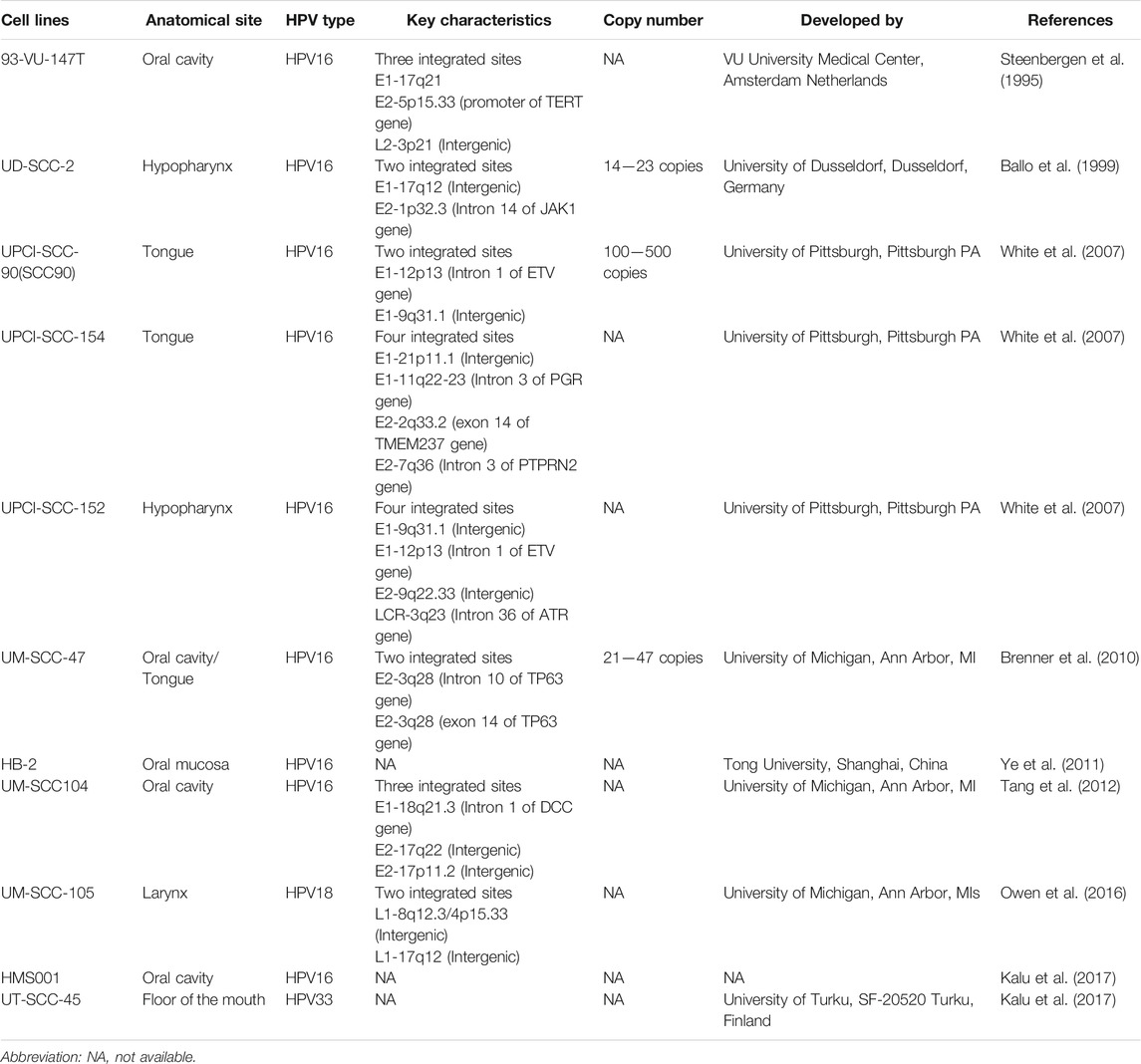
TABLE 8. List of HPV positive Head and neck cancer cell lines developed and described with their key characteristics.
A limited set of studies have been conducted to examine anti-HPV and anticancer activities in HNC (Table 9). The evidence suggests that HPV-positive cells can serve as suitable tools for screening of anti-HPV and anti-HNC. Green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG), a green tea derivative, exhibits various chemopreventive effects, including inhibition of growth factor–mediated proliferation (Liang et al., 1997; Liang et al., 1999a), induction of G1 arrest (Khafif et al., 1998; Liang et al., 1999b; Liberto and Cobrinik, 2000), and apoptosis (Ahmad et al., 1997; Paschka et al., 1998; Yang et al., 1998; Li et al., 2000). In this study, it induced apoptosis via the mitochondrial pathway through decreasing the expression level of Bcl2 and Bcl-xL and simultaneously increasing the Bax expression level that in turn activates caspase-9 in HNC cell lines YCU-N861 and HPV18 transformant YCU-H891 cell line. Treatment with EGCG inhibited the phosphorylation of EGFR, STAT3, and ERK proteins. It also inhibited the basal and transforming growth factor α-stimulated c-fos and cyclin D1 promoter activity. It decreased the level of cyclin D1 and pRB, accounting for the cellular arrest in the G1 phase (Masuda et al., 2001). The efficacy of the therapies used for the treatment of HNC can be enhanced by the incorporation of EGCG in current therapeutic regimens. Currently, anti-EGFR antibodies or specific tyrosine kinase inhibitors are being used in combination with radiation and certain chemotherapy agents in clinical trials for various types of cancer, as inhibition of the EGFR-related signal transduction pathway enhances the cytotoxic effects of radiation or various chemotherapy agents (Wu et al., 1995; Dent et al., 1999; Bonner et al., 2000). Hence, EGCG may have certain advantages over EGFR antibodies or selected tyrosine kinase inhibitors, as it is relatively inexpensive, natural, and nontoxic, and hence might be useful in administering for a longer period without any adverse effects. Clinical efficacy of EGCG still needs to be determined, and the direct correlation between chemopreventive effect of EGCG and HPV activity is yet to be established by further in vitro and in vivo studies. Also, the p53 status during EGCG administration needs to be determined as 50% of HNC carry mutations in the p53 gene, which in turn can modulate effects of EGCG (Wang et al., 2020).
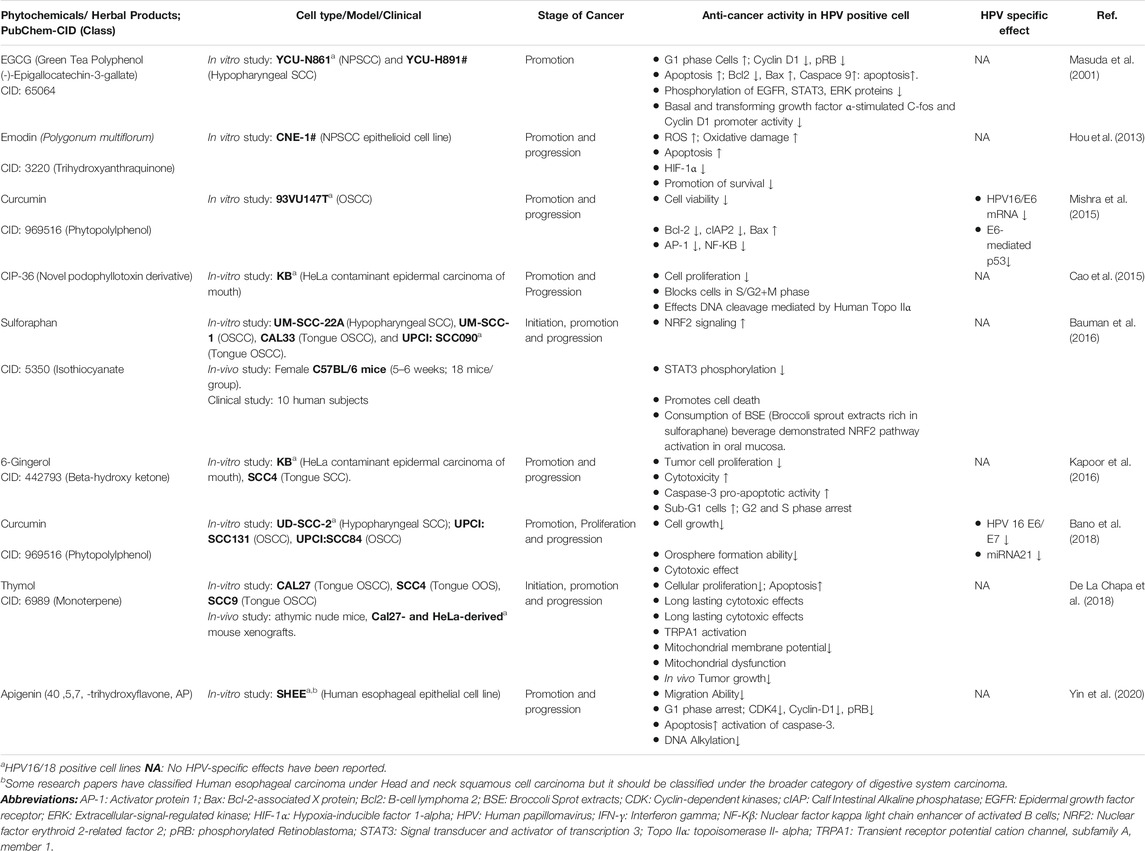
TABLE 9. Chemotherapeutic and Chemopreventive phytochemicals/Herbal derivative with anti-cancer and anti-HPV activity in HNC.
Emodin, a natural trihydroxyanthraquinone, has lower oxidation–reduction potential than that of oxygen; hence, under hypoxic conditions, it can be reduced to cytotoxic agent, sensitizing the cells to irradiation (Zhu et al., 2005; Zou et al., 2010; Schwartz et al., 2011). It affected the NPC cell (CNE1, a HeLa contaminated cell line) promotion and progression by inducing oxidative damage by significantly increasing the expression level of ROS, which induces apoptosis and downregulates mRNA and protein levels of HIF-1α. It also reduces the promotion of survival of carcinoma cells and induces cell cycle arrest at the G2/M phase. Hence, exposure of NPC cells in vitro and xenografts in vivo to emodin enhanced their radiosensitivity (Hou et al., 2013). Therefore, incorporation of emodin, a bioreductive agent, represents a viable therapeutic strategy targeting HIF-1α, by enhancing cytotoxicity of chemotherapeutic drugs via modulation of redox status of cancer cells and multidrug resistance reversal (Yi et al., 2004; Brown et al., 2007; Cai et al., 2008; Huang et al., 2008). It may also serve as an effective radiosensor, thereby improving efficacy of radiation therapy in radiation-resistant cancer cells. Moreover, since emodin can effectively enhance the radiosensitivity in vivo, it holds a potential as a radiosensitizing drug for NPC patients in future. Still a direct correlation between emodin and HPV-activity needs to be established.
Curcumin (diferulolylmethane), an active component of turmeric and a perennial herb, has been shown to suppress the expression of HPV oncogenes mediated by downregulation and reduced transactivation of AP-1 and NF-kB superfamily members, representing a novel mechanism regulating HPV-induced oral carcinogenesis (Li et al., 1993; Prusty and Das, 2005). Its effect was also observed in HPV-positive 93VU147T cells. The cell viability is reduced significantly. It also induces apoptosis by decreasing the expression level of antiapoptotic factors such as Bcl-2 and cIAP2, and inducing proapoptotic factors like Bax. It downregulated the protein expression of AP-1 members: c-Jun, JunD, and JunB along with NF-kB members, p50 and p65. Curcumin also tends to show HPV-specific effects like reducing the mRNA levels of HPV16/E6, which in turn inhibits E6-mediated p53 degradation. Hence, curcumin exhibits therapeutic potential for HPV16-mediated oral oncogenesis suppression (Mishra et al., 2015). Similar result was observed in a later study on curcumin as phytochemical having both anti-HNSCC and anti-HPV activities, which was carried out on UD-SCC-2, UPCI:SCC131, and UPCI:SCC84 cell lines. It affected cancer promotion, cellular proliferation, and progression. Curcumin inhibited cancer cell growth and orosphere formation ability. Also, it induced cytotoxic effect along with HPV-specific effects like decreasing the expression level of HPV16 E6/E7 oncoproteins, and downregulated mi-RNA21 expression significantly in HPV-positive oral CSCs. Hence, curcumin can sensitize the HPV-positive oral CSCs, thus making the cancer treatment more effective when used in combination with standard anticancer drugs or radiation, depicting its potential as a therapeutic agent. Further studies are required for deciphering the therapeutic effects of curcumin by determining its solubility and bioavailability, mechanism(s) of action, and potential molecular targets (Bano et al., 2018).
Sulforaphane, an isothiocyanate, derived from broccoli sprout extracts; treatment of HPV-negative HNC cell lines–UM-SCC-22A, UM-SSC-1, and CAL33–and HPV-positive cell line SSC090 led to dose- and time-dependent stimulation of NRF2 signaling for carcinogen detoxication. It also dephosphorylated inhibitedSTAT3 and promoted cell death. Similar effects were also observed in in vivo and clinical study including female C57BL/6 mice (5–6 weeks; 18 mice/group) and 10 human subjects, respectively. The pilot clinical trial demonstrated consistent bioavailability of sulforaphane, promising sustainable chronic administration. Although it is a cost-effective and natural product, further studies planned with encapsulated broccoli extract are required to enhance the ease of acceptability and dispensing. Also, HPV-specific chemopreventive effects are yet to be determined (Bauman et al., 2016).
6-Gingerol, a β-hydroxy ketone, derived from ginger rhizome, inhibited tumor cell proliferation and induces cellular toxicity, cell cycle arrest, apoptosis, and caspase 3/7 activation, as observed in KB and SCC4 cells. Also, the caspase-3–dependent proapoptotic activity was stimulated. It also inhibited cell cycle progression arresting the cells in G2 and M phases. Hence, 6-gingerol can be considered as a safe and potent chemotherapeutic/chemopreventive compound acting via cell cycle arrest and induction of apoptosis (Kapoor et al., 2016). Further studies should be directed toward determination of the chemopreventive effects of 6-gingerol in in vivo conditions and clinical trials along with direct correlation with HPV activity.
Thymol, a monoterpene derivative phenol, is a TRPA1 agonist found in thyme and oregano. It inhibited cellular proliferation and exhibited long-lasting cytotoxic effects as observed in CAL27, SSC4, and SSC9 cell lines. It also inhibited tumor growth in vivo as observed in CAL27 and HeLa-derived mouse xenografts. It induces the activation of TRPA1 and apoptosis via the mitochondria-dependent pathway. It promoted mitochondrial dysfunction via reducing mitochondrial membrane potential significantly (De La Chapa et al., 2018). Its HPV-specific effects still need to be determined along with the determination of bioavailability and tolerability to understand its therapeutic effects for future incorporation into cancer treatment.
Apigenin, a flavonoid, found abundantly in flowers of plants, vegetables, and fruits, exerts anticarcinogenic effects via preventing malignant transformation of cells, regulating cell signal transduction pathways, increasing apoptosis, and modulating cell cycle (Fang et al., 2007; Zhao et al., 2011; Zhu et al., 2013; Salmani et al., 2017; Yang et al., 2018a). It inhibited cancerous cell migration ability and arrested them in the G1 phase as observed in SHEE cells induced by HPV-18 and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). It downregulated the expression of CDK4, cyclin D1, and pRB, affecting cell cycle. Apigenin also induced cellular apoptosis via caspase-3 activation and inhibits DNA alkylation. With low toxicity and various beneficial bioactivities, apigenin can be considered as a potential chemopreventive agent against cancers, particularly, in smokers with HR-HPV coinfection (Yin et al., 2020).
Hence, most of the phytochemicals mentioned above showed anticancer activity in HPV-positive cells, where only a limited studies focused on HPV-specific effects. Thus, considerable attention should be paid to analyze the correlation between anti-HNC and anti-HPV activity of the phytochemicals as a chemopreventive and chemotherapeutic measure to prevent HPV-HNC.
Despite their encouraging pharmacological activities, there are bottlenecks in the translation of phytochemical-based therapies applicable in clinical settings.
Low bioavailability: Many phytochemicals suffer from having poor aqueous solubility and low retention in blood circulation. Pharmacological concentration of these phytochemicals in blood and tumor tissues is low because of poor absorption, high rate of metabolism, chemical degradation, and speedy clearance. It has been reported that serum levels of curcumin were quite low, reaching a maximum of 0.06 ± 0.01 μg/ml after oral administration of 500 mg/kg in rats (Yang et al., 2007). Ravindranath and Chandrasekhara (1980) also demonstrated that 40% of curcumin gets excreted unchanged in feces when orally administered to rats (Ravindranath and Chandrasekhara, 1980). A pilot study conducted among 10 healthy patients also reported poor bioavailability of sulforaphane with a regimen of topical exposure to sulforaphane-rich broccoli sprout extracts (Bauman et al., 2016). Chen et al. (1997) investigated the plasma pharmacokinetics of EGCG in rats and found the oral bioavailability of only 1.6% after a 75 mg/kg oral dose and a 10 mg/kg intravenous dose (Chen et al., 1997). Similarly, circulation half-life of resveratrol when administered through i. v. was few minutes and showed rapid elimination (Marier et al., 2002), whereas EGCG and quercetin attain low concentrations in blood, which is inadequate for antitumor activity (Lagoa et al., 2017).
Obstacles associated with the use of phytochemicals for treating and preventing cancer can be overcome with advances in the field of nanotechnology. A 10-fold dose advantage was achieved without any loss of effectiveness by encapsulating ECGC in polylactic acid–polyethylene glycol nanoparticles (Siddiqui et al., 2009). Increased absorption was also reported by nanoparticle encapsulation of curcumin despite its low solubility in water. Additionally, curcumin loaded poly lactic-co-glycolic acid nanoparticles increased the oral bioavailability to nine times that of the native form, with piperine as absorption enhancer (Shaikh et al., 2009). Further advancements in this field should be encouraged.
Toxicity: Although phytochemicals may show toxicity when administered in high doses, they exhibit less adverse effects than conventional therapies. In a clinical trial with 50 oral leucoplakia patients, significant toxicity, severe enough to cause withdrawal of 6 patients, was observed with the use of isoretinoin (Garewal et al., 1999). Additionally, not all phytochemicals are safe for consumption. It has been found that a few natural compounds such as capsaicin (chilli pepper), cycasin, and cycas seed are tumor-promoting and must be avoided (Bode and Dong, 2015). Moreover, unregulated use of phytochemicals may have a danger of contamination by potential carcinogens.
Pharmaceutical industry challenges: Pharma-research into phytochemicals and herbal derivatives has experienced a slow decline during the recent times (Koehn and Carter, 2005; Katiyar et al., 2012). This can be attributed to advancements in high-throughput screening technology against defined molecular targets, advances in genomics, molecular and cellular biology, development of combinatorial chemistry, and a declining importance among large pharma-companies on the commercial considerations of phytochemicals that are often associated with poor financial returns and nearly absent IPR protection. Unique features of natural compounds such as a greater number of chiral centers, higher number of oxygen atoms, and greater molecular rigidity pose further challenges for medicinal chemists as they develop analogs to reduce toxicity, improve absorption, or to improve the efficacy, which is often achieved by adding or deleting selected functional groups.
Poor independent agents: While phytochemicals may not be efficient as standalone chemotherapeutic agents, many groups have established their efficacy as adjuvants to traditional therapies. A study demonstrated the benefits of combining sulforaphane with cisplatin and 5-fluorouracil (Elkashty et al., 2018). Sulforaphane increased the cytotoxicity of cisplatin and 5-fluorouracil by two-fold and ten-fold, respectively. It did not alter the viability and functions of noncancerous stem cells. Sulforaphane combined treatments successfully inhibited cancer stem cell colony formation, sphere formation, and tumor progression in vivo. In an Italian study conducted among 23 patients undergoing treatment with 5-fluorouracil and cisplatin, prolonged responses were reported with the use of retinol palmitate in chemotherapy intervals. Toxicity levels were acceptable, and treatment did not interfere with the quality of life (Recchia et al., 1993). A study also observed significant growth inhibition and enhanced apoptosis in HNC cells with the use of curcumin along with 5-fluorouracil or doxorubicin. The study thus demonstrated the significant potential of combining curcumin with 5-fluorouracil or doxorubicin as a treatment modality for HNC management (Sivanantham et al., 2016).
Preclinical efficacy vs. clinical response: The cause for discrepancy in effectiveness of phytochemical agents in preclinical and human clinical trials has been conjectured to arise because of differences in dosage, metabolic differences, bioavailability, differences in circulating tissue levels of chemopreventive agents in humans and animals, exposure conditions to damaged tissue vs normal tissue, follow-up time, and the assessed ends. Second, high doses are often administered to animals in contrast to low doses admisible to humans in clinical trials. Although animal models have significantly helped in the identification of carcinogens, and chemopreventive and chemotherapeutic agents, they are not available for every HNC organ site. Furthermore, existing models cannot mimic human exposure complexities of carcinogens, metabolic competence, turnover of cells, and their repair capacity.
Phytochemicals show immense potential in the field of HNC chemotherapy and chemoprevention agents. In this evolving landscape, the success of employability of phytochemicals depends on our ability to decipher their molecular mechanics. Using phytochemicals in combination with another or in conjunction with existing chemotherapeutic practices or an alternate therapy is an area worth exploring.
We have also observed that there has not been much phytochemical-related research on HPV-induced HNC. However, numerous phytochemicals that are effective against HPV-induced cervical cancer have been reported in the literature (Bharti et al., 2018). In today’s era, therapies to distinguish HPV-positive HNC from HPV-negative HNC are required. As HPV-positive HNC has better outcomes, the tumors can be treated with well-established phytochemicals targeting the HPV-mediated carcinogenic mechanisms. Thus, it might be valuable to study whether these phytochemicals can find application in HNC treatment and prevention. Activity of these phytochemicals can be checked on HNC cell lines or in vivo in laboratory conditions and can also be screened by using bioinformatic tools. There is a strong requirement to develop HPV-based concurrent therapies so that HNC can be treated more effectively. There are many associated challenges with the use of natural compounds. In pharmacological doses, the adverse effects of these natural compounds such as increased toxicity and low bioavailability are amplified. For chemoprevention to be feasible in treating premalignant lesions, the compound must be well tolerated and have long-lasting benefit. Moreover, the various signaling pathways contributing to HNC tumorigenesis mandate the use of compounds with multiple molecular targets. It is noteworthy that molecular targets of many such phytochemicals in active HNC are now well known (Figure 4). It is also worthy to note that not many clinical studies have been conducted despite discovery of numerous phytochemicals with multiple molecular targets. In order to determine the safety and efficacy of phytochemicals, it is imperative that more of such clinical studies, with different phytochemicals, are funded and conducted. Challenges associated with the use of phytochemicals such as low bioavailibilty, and toxicity can be possibly overcome with the use of chemical analogs, adjuvant therapies, and nanoparticle delivery mechanisms. Hence, a number of studies on phytochemicals against HPV-driven HNC are now accumulating; a comparative account on their relative efficacy is needed and should be addressed to harness the potential of phytochemicals in clinical studies. Research in these areas needs encouragement for effective management of HPV-positive HNC in future.
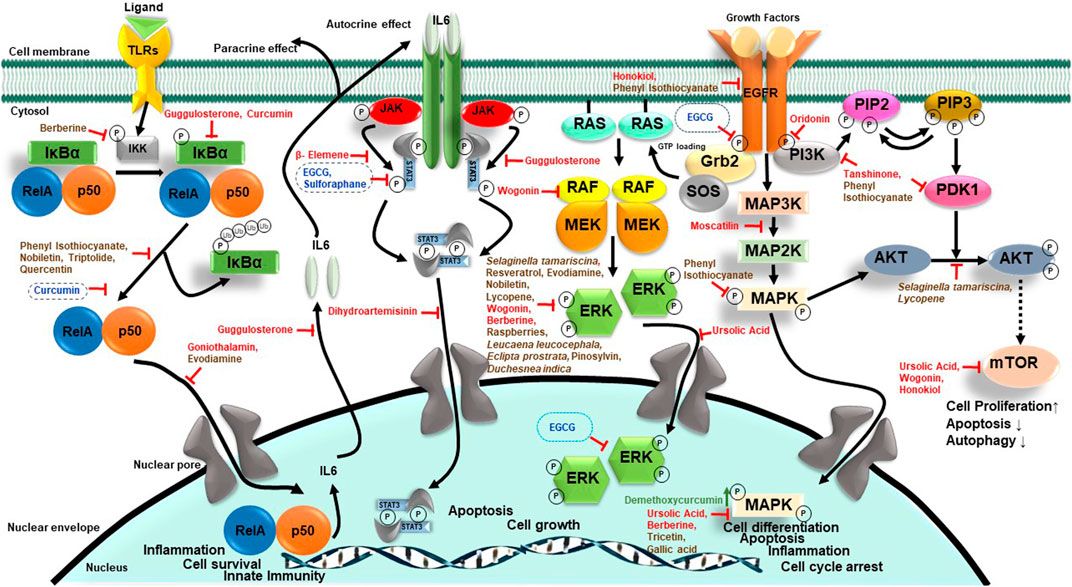
FIGURE 4. Effect of phytochemicals on different oncogenic signaling pathways of HNC. Summarized here are chemotherapeutic (red) and chemopreventive (brown) phytochemicals that target different signaling pathways along with specific phytochemicals (blue) demonstrating anti-HPV activity in HPV-positive HNC.
NA participated in study writing and manuscript preparation, JY, SC, DJ, TT, and A Chaudhary contributed to manuscript preparation. A Chhokar, KT, and TS: advisory in manuscript preparation. AB conceived the presented idea and designed the manuscript, and critically reviewed, drafted, and communicated the final manuscript. All authors have read and approved the final manuscript.
The study was supported by research funds to ACB from the Central Council for Research in Homeopathy, Department of AYUSH, Government of India (17-51/2016-17/CCRH/Tech/Coll./DU-Cervical Cancer.4850), the Department of Science and Technology-SERB, India (EMR/2017/004018/BBM), and the Indian Council of Medical Research (5/13/38/2014 NCDIII-Eoffice73143). Senior Research Fellowship to NA (09/045 (1,622)/2018-EMR-I), JY (09/045 (1,629)/2019-EMR-I), and Junior Research Fellowship to DJ (15/12/2019 (ii) EU-V) by the Council of Scientific and Industrial Research (CSIR); Senior Research Fellowship to A Chhokar (573/(CSIR-UGC NET JUNE 2017)); and Junior Research Fellowship to TT (764/(CSIR-UGC NET JUNE 2019)) by University Grants Commission (UGC). Senior Research Fellowship to KT (5/13/38/2014 NCDIII-Eoffice73143) by the ICMR, India. Senior Research Fellowship to TS, and Technical Assistantship to SC by CCRH, India (17-51/2016-17/CCRH/Tech/Coll./DU-Cervical Cancer.4850) Junior Research Fellowship to A Chaudhary by DST-SERB, India (EMR/2017/004018/BBM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abdel-Rahman, O. (2020). Prognostic Value of HPV Status Among Patients with Hypopharyngeal Carcinoma: a Population-Based Study. Clin. Transl Oncol.
Adeyemi, B. F., Adekunle, L. V., Kolude, B. M., Akang, E. E., and Lawoyin, J. O. (2008). Head and Neck Cancer-Aa Clinicopathological Study in a Tertiary Care center. J. Natl. Med. Assoc. 100, 690–697. doi:10.1016/s0027-9684(15)31343-2
Adisa, A. O., Adeyemi, B. F., Oluwasola, A. O., Kolude, B., Akang, E. E., and Lawoyin, J. O. (2011). Clinico-pathological Profile of Head and Neck Malignancies at University College Hospital, Ibadan, Nigeria. Head Face Med. 7, 9. doi:10.1186/1746-160X-7-9
Adjei Boakye, E., Buchanan, P., Hinyard, L., Osazuwa-Peters, N., Schootman, M., and Piccirillo, J. F. (2018). Incidence and Risk of Second Primary Malignant Neoplasm after a First Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol. Head Neck Surg. 144, 727–737. doi:10.1001/jamaoto.2018.0993
Aggarwal, N., Yadav, J., Thakur, K., Bibban, R., Chhokar, A., Tripathi, T., et al. (2020). Human Papillomavirus Infection in Head and Neck Squamous Cell Carcinomas: Transcriptional Triggers and Changed Disease Patterns. Front Cel Infect Microbiol 10, 537650. doi:10.3389/fcimb.2020.537650
Aggarwal, S., Takada, Y., Singh, S., Myers, J. N., and Aggarwal, B. B. (2004). Inhibition of Growth and Survival of Human Head and Neck Squamous Cell Carcinoma Cells by Curcumin via Modulation of Nuclear Factor-kappaB Signaling. Int. J. Cancer 111, 679–692. doi:10.1002/ijc.20333
Ahmad, N., Feyes, D. K., Nieminen, A. L., Agarwal, R., and Mukhtar, H. (1997). Green tea Constituent Epigallocatechin-3-Gallate and Induction of Apoptosis and Cell Cycle Arrest in Human Carcinoma Cells. J. Natl. Cancer Inst. 89, 1881–1886. doi:10.1093/jnci/89.24.1881
Akintoye, S. O., and Mupparapu, M. (2020). Clinical Evaluation and Anatomic Variation of the Oral Cavity. Dermatol. Clin. 38, 399–411. doi:10.1016/j.det.2020.05.001
Albahout, K. S., and Lopez, R. A. (2021). "Anatomy, Head and Neck, Pharynx," in StatPearls. (Treasure Island (FL).
Ang, K. K., Harris, J., Wheeler, R., Weber, R., Rosenthal, D. I., Nguyen-Tân, P. F., et al. (2010). Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 363, 24–35. doi:10.1056/NEJMoa0912217
Anto, R. J., Mukhopadhyay, A., Shishodia, S., Gairola, C. G., and Aggarwal, B. B. (2002). Cigarette Smoke Condensate Activates Nuclear Transcription Factor-kappaB through Phosphorylation and Degradation of IkappaB(alpha): Correlation with Induction of Cyclooxygenase-2. Carcinogenesis 23, 1511–1518. doi:10.1093/carcin/23.9.1511
Ayre, J. E., and Ayre, W. B. (1949). Progression from "Precancer" Stage to Early Carcinoma of Cervix within One Year: Combined Cytologic and Histologic Study with Report of a Case. Am. J. Clin. Pathol. 19, 770–778. doi:10.1093/ajcp/19.8.770
Bagnardi, V., Blangiardo, M., La Vecchia, C., and Corrao, G. (2001). A Meta-Analysis of Alcohol Drinking and Cancer Risk. Br. J. Cancer 85, 1700–1705. doi:10.1054/bjoc.2001.2140
Ballo, H., Koldovsky, P., Hoffmann, T., Balz, V., Hildebrandt, B., Gerharz, C. D., et al. (1999). Establishment and Characterization of Four Cell Lines Derived from Human Head and Neck Squamous Cell Carcinomas for an Autologous Tumor-Fibroblast In Vitro Model. Anticancer Res. 19, 3827–3836.
Bano, N., Yadav, M., and Das, B. C. (2018). Differential Inhibitory Effects of Curcumin between HPV+ve and HPV-Ve Oral Cancer Stem Cells. Front. Oncol. 8, 412. doi:10.3389/fonc.2018.00412
Baruah, P., Lee, M., Odutoye, T., Williamson, P., Hyde, N., Kaski, J. C., et al. (2012). Decreased Levels of Alternative Co-stimulatory Receptors OX40 and 4-1BB Characterise T Cells from Head and Neck Cancer Patients. Immunobiology 217, 669–675. doi:10.1016/j.imbio.2011.11.005
Bauman, J. E., and Ferris, R. L. (2014). Integrating Novel Therapeutic Monoclonal Antibodies into the Management of Head and Neck Cancer. Cancer 120, 624–632. doi:10.1002/cncr.28380
Bauman, J. E., Zang, Y., Sen, M., Li, C., Wang, L., Egner, P. A., et al. (2016). Prevention of Carcinogen-Induced Oral Cancer by Sulforaphane. Cancer Prev. Res. (Phila) 9, 547–557. doi:10.1158/1940-6207.CAPR-15-0290
Beaty, B. T., Moon, D. H., Shen, C. J., Amdur, R. J., Weiss, J., Grilley-Olson, J., et al. (2019). PIK3CA Mutation in HPV-Associated OPSCC Patients Receiving Deintensified Chemoradiation. J Natl Cancer Inst.112(8):855-858. doi:10.1093/jnci/djz224
Bei, J. X., Su, W. H., Ng, C. C., Yu, K., Chin, Y. M., Lou, P. J., et al. (2016). A GWAS Meta-Analysis and Replication Study Identifies a Novel Locus within CLPTM1L/TERT Associated with Nasopharyngeal Carcinoma in Individuals of Chinese Ancestry. Cancer Epidemiol. Biomarkers Prev. 25, 188–192. doi:10.1158/1055-9965.EPI-15-0144
Benner, S. E., Winn, R. J., Lippman, S. M., Poland, J., Hansen, K. S., Luna, M. A., et al. (1993). Regression of Oral Leukoplakia with Alpha-Tocopherol: a Community Clinical Oncology Program Chemoprevention Study. J. Natl. Cancer Inst. 85, 44–47. doi:10.1093/jnci/85.1.44
Berthiller, J., Straif, K., Agudo, A., Ahrens, W., Bezerra Dos Santos, A., Boccia, S., et al. (2016). Low Frequency of Cigarette Smoking and the Risk of Head and Neck Cancer in the INHANCE Consortium Pooled Analysis. Int. J. Epidemiol. 45, 835–845. doi:10.1093/ije/dyv146
Bharti, A. C., Singh, T., Bhat, A., Pande, D., and Jadli, M. (2018). Therapeutic Startegies for Human Papillomavirus Infection and Associated Cancers. Front. Biosci. (Elite Ed. 10, 15–73. doi:10.2741/e808
Bharti, A. C., and Aggarwal, B. B. (2017). Role of Nutraceuticals in Cancer Chemosensitization. 1st Edn. Cambridge, MA: Academic Press.
Bhattacharyya, S., Sekar, V., Majumder, B., Mehrotra, D. G., Banerjee, S., Bhowmick, A. K., et al. (2017). CDKN2A-p53 Mediated Antitumor Effect of Lupeol in Head and Neck Cancer. Cel Oncol (Dordr) 40, 145–155. doi:10.1007/s13402-016-0311-7
Bichler, E., Daxenbichler, G., and Marth, C. (1983). Vitamin A Status and Retinoid-Binding Proteins in Carcinomas of the Head and Neck Region. Oncology 40, 336–339. doi:10.1159/000225757
Bode, A. M., and Dong, Z. (2015). Toxic Phytochemicals and Their Potential Risks for Human Cancer. Cancer Prev. Res. 8, 1–8. doi:10.1158/1940-6207.capr-14-0160
Bonner, J. A., Raisch, K. P., Trummell, H. Q., Robert, F., Meredith, R. F., Spencer, S. A., et al. (2000). Enhanced Apoptosis with Combination C225/radiation Treatment Serves as the Impetus for Clinical Investigation in Head and Neck Cancers. J. Clin. Oncol. 18, 47S–53S.
Boscolo-Rizzo, P., Pawlita, M., and Holzinger, D. (2016). From HPV-Positive towards HPV-Driven Oropharyngeal Squamous Cell Carcinomas. Cancer Treat. Rev. 42, 24–29. doi:10.1016/j.ctrv.2015.10.009
Boyle, W. F., Riggs, J. L., Oshiro, L. S., and Lennette, E. H. (1973). Electron Microscopic Identification of Papova Virus in Laryngeal Papilloma. Laryngoscope 83, 1102–1108. doi:10.1288/00005537-197307000-00013
Bozec, A., Demez, P., Gal, J., Chamorey, E., Louis, M. Y., Blanchard, D., et al. (2018). Long-term Quality of Life and Psycho-Social Outcomes after Oropharyngeal Cancer Surgery and Radial Forearm Free-Flap Reconstruction: A GETTEC Prospective Multicentric Study. Surg. Oncol. 27, 23–30. doi:10.1016/j.suronc.2017.11.005
Bozec, A., Peyrade, F., Fischel, J. L., and Milano, G. (2009). Emerging Molecular Targeted Therapies in the Treatment of Head and Neck Cancer. Expert Opin. Emerg. Drugs 14, 299–310. doi:10.1517/14728210902997947
Brand, T. M., Hartmann, S., Bhola, N. E., Li, H., Zeng, Y., O'keefe, R. A., et al. (2018). Cross-talk Signaling between HER3 and HPV16 E6 and E7 Mediates Resistance to PI3K Inhibitors in Head and Neck Cancer. Cancer Res. 78, 2383–2395. doi:10.1158/0008-5472.CAN-17-1672
Brandsma, J. L., and Abramson, A. L. (1989). Association of Papillomavirus with Cancers of the Head and Neck. Arch. Otolaryngol. Head Neck Surg. 115, 621–625. doi:10.1001/archotol.1989.01860290079018
Brazil, D. P., and Hemmings, B. A. (2001). Ten Years of Protein Kinase B Signalling: a Hard Akt to Follow. Trends Biochem. Sci. 26, 657–664. doi:10.1016/s0968-0004(01)01958-2
Brenner, J. C., Graham, M. P., Kumar, B., Saunders, L. M., Kupfer, R., Lyons, R. H., et al. (2010). Genotyping of 73 UM-SCC Head and Neck Squamous Cell Carcinoma Cell Lines. Head Neck 32, 417–426. doi:10.1002/hed.21198
Brown, M., Bellon, M., and Nicot, C. (2007). Emodin and DHA Potently Increase Arsenic Trioxide Interferon-Alpha-Induced Cell Death of HTLV-I-Transformed Cells by Generation of Reactive Oxygen Species and Inhibition of Akt and AP-1. Blood 109, 1653–1659. doi:10.1182/blood-2006-04-015537
Cai, J., Niu, X., Chen, Y., Hu, Q., Shi, G., Wu, H., et al. (2008). Emodin-induced Generation of Reactive Oxygen Species Inhibits RhoA Activation to Sensitize Gastric Carcinoma Cells to Anoikis. Neoplasia 10, 41-IN19. doi:10.1593/neo.07754
Cancer Genome Atlas, N. (2015). Comprehensive Genomic Characterization of Head and Neck Squamous Cell Carcinomas. Nature 517, 576–582. doi:10.1038/nature14129
Cao, B., Chen, H., Gao, Y., Niu, C., Zhang, Y., and Li, L. (2015). CIP-36, a Novel Topoisomerase II-Targeting Agent, Induces the Apoptosis of Multidrug-Resistant Cancer Cells In Vitro. Int. J. Mol. Med. 35, 771–776. doi:10.3892/ijmm.2015.2068
Carlson, E. R., and Schlieve, T. (2019). Salivary Gland Malignancies. Oral Maxillofac. Surg. Clin. North. Am. 31, 125–144. doi:10.1016/j.coms.2018.08.007
Centers for Disease Control and Prevention, U. (2004). "The Health Consequences of Smoking: A Report of the Surgeon General," in The Health Consequences of Smoking: A Report of the Surgeon General. (Atlanta (GA)).
Chaisuparat, R., Limpiwatana, S., Kongpanitkul, S., Yodsanga, S., and Jham, B. C. (2016). The Akt/mTOR Pathway Is Activated in Verrucous Carcinoma of the Oral Cavity. J. Oral Pathol. Med. 45, 581–585. doi:10.1111/jop.12422
Chang, E. T., and Adami, H. O. (2006). The Enigmatic Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiol. Biomarkers Prev. 15, 1765–1777. doi:10.1158/1055-9965.EPI-06-0353
Chaturvedi, A. K., Engels, E. A., Anderson, W. F., and Gillison, M. L. (2008). Incidence Trends for Human Papillomavirus-Related and -unrelated Oral Squamous Cell Carcinomas in the United States. J. Clin. Oncol. 26, 612–619. doi:10.1200/JCO.2007.14.1713
Chaturvedi, A. K., Engels, E. A., Pfeiffer, R. M., Hernandez, B. Y., Xiao, W., Kim, E., et al. (2011). Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 29, 4294–4301. doi:10.1200/JCO.2011.36.4596
Chaturvedi, A. K., Graubard, B. I., Broutian, T., Pickard, R. K., Tong, Z. Y., Xiao, W., et al. (2015). NHANES 2009-2012 Findings: Association of Sexual Behaviors with Higher Prevalence of Oral Oncogenic Human Papillomavirus Infections in U.S. Men. Cancer Res. 75, 2468–2477. doi:10.1158/0008-5472.CAN-14-2843
Chen, H. J., Lin, C. M., Lee, C. Y., Shih, N. C., Amagaya, S., Lin, Y. C., et al. (2013). Phenethyl Isothiocyanate Suppresses EGF-Stimulated SAS Human Oral Squamous Carcinoma Cell Invasion by Targeting EGF Receptor Signaling. Int. J. Oncol. 43, 629–637. doi:10.3892/ijo.2013.1977
Chen, L., Lee, M. J., Li, H., and Yang, C. S. (1997). Absorption, Distribution, Elimination of tea Polyphenols in Rats. Drug Metab. Dispos 25, 1045–1050.
Chen, M. K., Liu, Y. T., Lin, J. T., Lin, C. C., Chuang, Y. C., Lo, Y. S., et al. (2019). Pinosylvin Reduced Migration and Invasion of Oral Cancer Carcinoma by Regulating Matrix Metalloproteinase-2 Expression and Extracellular Signal-Regulated Kinase Pathway. Biomed. Pharmacother. 117, 109160. doi:10.1016/j.biopha.2019.109160
Chen, X., Yan, B., Lou, H., Shen, Z., Tong, F., Zhai, A., et al. (2018). Immunological Network Analysis in HPV Associated Head and Neck Squamous Cancer and Implications for Disease Prognosis. Mol. Immunol. 96, 28–36. doi:10.1016/j.molimm.2018.02.005
Chien, M. H., Yang, W. E., Yang, Y. C., Ku, C. C., Lee, W. J., Tsai, M. Y., et al. (2020). Dual Targeting of the P38 MAPK-HO-1 Axis and cIAP1/XIAP by Demethoxycurcumin Triggers Caspase-Mediated Apoptotic Cell Death in Oral Squamous Cell Carcinoma Cells. Cancers (Basel) 12. doi:10.3390/cancers12030703
Chien, S.-Y., Hsieh, M.-J., Chen, C.-J., Yang, S.-F., and Chen, M.-K. (2015). Nobiletin Inhibits Invasion and Migration of Human Nasopharyngeal Carcinoma Cell Lines by Involving ERK1/2 and Transcriptional Inhibition of MMP-2. Expert Opin. Ther. Targets 19, 307–320. doi:10.1517/14728222.2014.992875
Chow, S. E., Chen, Y. W., Liang, C. A., Huang, Y. K., and Wang, J. S. (2012). Wogonin Induces Cross-Regulation between Autophagy and Apoptosis via a Variety of Akt Pathway in Human Nasopharyngeal Carcinoma Cells. J. Cel Biochem 113, 3476–3485. doi:10.1002/jcb.24224
Chung, C. H., Guthrie, V. B., Masica, D. L., Tokheim, C., Kang, H., Richmon, J., et al. (2015). Genomic Alterations in Head and Neck Squamous Cell Carcinoma Determined by Cancer Gene-Targeted Sequencing. Ann. Oncol. 26, 1216–1223. doi:10.1093/annonc/mdv109
Chung, H. H., Chen, M. K., Chang, Y. C., Yang, S. F., Lin, C. C., and Lin, C. W. (2017a). Inhibitory Effects of Leucaena Leucocephala on the Metastasis and Invasion of Human Oral Cancer Cells. Environ. Toxicol. 32, 1765–1774. doi:10.1002/tox.22399
Chung, T. T., Chuang, C. Y., Teng, Y. H., Hsieh, M. J., Lai, J. C., Chuang, Y. T., et al. (2017b). Tricetin Suppresses Human Oral Cancer Cell Migration by Reducing Matrix Metalloproteinase-9 Expression through the Mitogen-Activated Protein Kinase Signaling Pathway. Environ. Toxicol. 32, 2392–2399. doi:10.1002/tox.22452
Ciuffo, G. (1907). Innesto postiveo con filtrado di verrucae volgare. Gior Ital. D Mal Ven 48, 12–17.
Cohen, N., Fedewa, S., and Chen, A. Y. (2018). Epidemiology and Demographics of the Head and Neck Cancer Population. Oral Maxillofac. Surg. Clin. North. Am. 30, 381–395. doi:10.1016/j.coms.2018.06.001
Davis, R. J., Ferris, R. L., and Schmitt, N. C. (2016). Costimulatory and Coinhibitory Immune Checkpoint Receptors in Head and Neck Cancer: Unleashing Immune Responses through Therapeutic Combinations. Cancers Head Neck 1, 12. doi:10.1186/s41199-016-0013-x
De La Chapa, J. J., Singha, P. K., Lee, D. R., and Gonzales, C. B. (2018). Thymol Inhibits Oral Squamous Cell Carcinoma Growth via Mitochondria-Mediated Apoptosis. J. Oral Pathol. Med. 47, 674–682. doi:10.1111/jop.12735
De Lartigue, J. (2015). Rising to the Therapeutic challenge of Head and Neck Cancer. J. Community Support. Oncol. 13, 73–80. doi:10.12788/jcso.0111
De Martel, C., Ferlay, J., Franceschi, S., Vignat, J., Bray, F., Forman, D., et al. (2012). Global burden of Cancers Attributable to Infections in 2008: a Review and Synthetic Analysis. Lancet Oncol. 13, 607–615. doi:10.1016/S1470-2045(12)70137-7
De Martel, C., Georges, D., Bray, F., Ferlay, J., and Clifford, G. M. (2019). Global burden of Cancer Attributable to Infections in 2018: a Worldwide Incidence Analysis. Lancet Glob. Health.
De Stefani, E., Oreggia, F., Boffetta, P., Deneo-Pellegrini, H., Ronco, A., and Mendilaharsu, M. (2000). Tomatoes, Tomato-Rich Foods, Lycopene and Cancer of the Upper Aerodigestive Tract: a Case-Control in Uruguay. Oral Oncol. 36, 47–53. doi:10.1016/s1368-8375(99)00050-0
De Villiers, E. M., Fauquet, C., Broker, T. R., Bernard, H. U., and Zur Hausen, H. (2004). Classification of Papillomaviruses. Virology 324, 17–27. doi:10.1016/j.virol.2004.03.033
Dent, P., Reardon, D. B., Park, J. S., Bowers, G., Logsdon, C., Valerie, K., et al. (1999). Radiation-induced Release of Transforming Growth Factor Alpha Activates the Epidermal Growth Factor Receptor and Mitogen-Activated Protein Kinase Pathway in Carcinoma Cells, Leading to Increased Proliferation and protection from Radiation-Induced Cell Death. Mol. Biol. Cel 10, 2493–2506. doi:10.1091/mbc.10.8.2493
Dickens, P., Srivastava, G., and Liu, Y. T. (1992). Human Papillomavirus 16/18 and Nasopharyngeal Carcinoma. J. Clin. Pathol. 45, 81–82. doi:10.1136/jcp.45.1.81
Elkashty, O. A., Ashry, R., Elghanam, G. A., Pham, H. M., Su, X., Stegen, C., et al. (2018). Broccoli Extract Improves Chemotherapeutic Drug Efficacy against Head-Neck Squamous Cell Carcinomas. Med. Oncol. 35, 124. doi:10.1007/s12032-018-1186-4
Fakhry, C., Westra, W. H., Li, S., Cmelak, A., Ridge, J. A., Pinto, H., et al. (2008). Improved Survival of Patients with Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J. Natl. Cancer Inst. 100, 261–269. doi:10.1093/jnci/djn011
Fakhry, C., Zhang, Q., Nguyen-Tan, P. F., Rosenthal, D., El-Naggar, A., Garden, A. S., et al. (2014). Human Papillomavirus and Overall Survival after Progression of Oropharyngeal Squamous Cell Carcinoma. J. Clin. Oncol. 32, 3365–3373. doi:10.1200/JCO.2014.55.1937
Fang, C. Y., Wu, C. Z., Chen, P. N., Chang, Y. C., Chuang, C. Y., Lai, C. T., et al. (2018). Antimetastatic Potentials of Salvianolic Acid A on Oral Squamous Cell Carcinoma by Targeting MMP-2 and the C-Raf/MEK/ERK Pathway. Environ. Toxicol. 33, 545–554. doi:10.1002/tox.22542
Fang, J., Zhou, Q., Liu, L. Z., Xia, C., Hu, X., Shi, X., et al. (2007). Apigenin Inhibits Tumor Angiogenesis through Decreasing HIF-1alpha and VEGF Expression. Carcinogenesis 28, 858–864. doi:10.1093/carcin/bgl205
Ferris, R. L., Blumenschein, G., Fayette, J., Guigay, J., Colevas, A. D., Licitra, L., et al. (2018). Nivolumab vs Investigator's Choice in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: 2-year Long-Term Survival Update of CheckMate 141 with Analyses by Tumor PD-L1 Expression. Oral Oncol. 81, 45–51. doi:10.1016/j.oraloncology.2018.04.008
Fleming, J. C., Woo, J., Moutasim, K., Mellone, M., Frampton, S. J., Mead, A., et al. (2019). HPV, Tumour Metabolism and Novel Target Identification in Head and Neck Squamous Cell Carcinoma. Br. J. Cancer 120, 356–367. doi:10.1038/s41416-018-0364-7
Flynn, M. B., Maguire, S., Martinez, S., and Tesmer, T. (1999). Primary Squamous Cell Carcinoma of the Parotid Gland: the Importance of Correct Histological Diagnosis. Ann. Surg. Oncol. 6, 768–770. doi:10.1007/s10434-999-0768-y
Folgueras, A. R., Pendas, A. M., Sanchez, L. M., and Lopez-Otin, C. (2004). Matrix Metalloproteinases in Cancer: From New Functions to Improved Inhibition Strategies. Int. J. Dev. Biol. 48, 411–424.
Forastiere, A. A., Zhang, Q., Weber, R. S., Maor, M. H., Goepfert, H., Pajak, T. F., et al. (2013). Long-term Results of RTOG 91-11: a Comparison of Three Nonsurgical Treatment Strategies to Preserve the Larynx in Patients with Locally Advanced Larynx Cancer. J. Clin. Oncol. 31, 845–852. doi:10.1200/JCO.2012.43.6097
Franceschi, S., Barra, S., La Vecchia, C., Bidoli, E., Negri, E., and Talamini, R. (1992). Risk Factors for Cancer of the Tongue and the Mouth. A Case-Control Study from Northern Italy. Cancer 70, 2227–2233. doi:10.1002/1097-0142(19921101)70:9<2227::aid-cncr2820700902>3.0.co;2-z
Franceschi, S., Muñoz, N., Bosch, X. F., Snijders, P. J., and Walboomers, J. M. (1996). Human Papillomavirus and Cancers of the Upper Aerodigestive Tract: a Review of Epidemiological and Experimental Evidence. Cancer Epidemiol. Biomarkers Prev. 5, 567–575.
Freedman, N. D., Schatzkin, A., Leitzmann, M. F., Hollenbeck, A. R., and Abnet, C. C. (2007). Alcohol and Head and Neck Cancer Risk in a Prospective Study. Br. J. Cancer 96, 1469–1474. doi:10.1038/sj.bjc.6603713
Frisch, M., and Biggar, R. J. (1999). Aetiological Parallel between Tonsillar and Anogenital Squamous-Cell Carcinomas. Lancet 354, 1442–1443. doi:10.1016/S0140-6736(99)92824-6
Fu, T. C., Hughes, J. P., Feng, Q., Hulbert, A., Hawes, S. E., Xi, L. F., et al. (2015). Epidemiology of Human Papillomavirus Detected in the Oral Cavity and Fingernails of Mid-adult Women. Sex. Transm. Dis. 42, 677–685. doi:10.1097/OLQ.0000000000000362
Gale, N., Michaels, L., Luzar, B., Poljak, M., Zidar, N., Fischinger, J., et al. (2009). Current Review on Squamous Intraepithelial Lesions of the Larynx. Histopathology 54, 639–656. doi:10.1111/j.1365-2559.2008.03111.x
Gao, G., Wang, J., Kasperbauer, J. L., Tombers, N. M., Teng, F., Gou, H., et al. (2019). Whole Genome Sequencing Reveals Complexity in Both HPV Sequences Present and HPV Integrations in HPV-Positive Oropharyngeal Squamous Cell Carcinomas. BMC Cancer 19, 352. doi:10.1186/s12885-019-5536-1
Garewal, H. S., Katz, R. V., Meyskens, F., Pitcock, J., Morse, D., Friedman, S., et al. (1999). Beta-carotene Produces Sustained Remissions in Patients with Oral Leukoplakia: Results of a Multicenter Prospective Trial. Arch. Otolaryngol. Head Neck Surg. 125, 1305–1310. doi:10.1001/archotol.125.12.1305
Garewal, H. S., Meyskens, F. L., Killen, D., Reeves, D., Kiersch, T. A., Elletson, H., et al. (1990). Response of Oral Leukoplakia to Beta-Carotene. J. Clin. Oncol. 8, 1715–1720. doi:10.1200/JCO.1990.8.10.1715
Garza-Morales, R., Perez-Trujillo, J. J., Martinez-Jaramillo, E., Saucedo-Cardenas, O., Loera-Arias, M. J., Garcia-Garcia, A., et al. (2019). A DNA Vaccine Encoding SA-4-1BBL Fused to HPV-16 E7 Antigen Has Prophylactic and Therapeutic Efficacy in a Cervical Cancer Mouse Model. Cancers (Basel) 11. doi:10.3390/cancers11010096
Gaykalova, D. A., Manola, J. B., Ozawa, H., Zizkova, V., Morton, K., Bishop, J. A., et al. (2015). NF-κB and Stat3 Transcription Factor Signatures Differentiate HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinoma. Int. J. Cancer 137, 1879–1889. doi:10.1002/ijc.29558
Gerle, M., Medina, T. P., Gülses, A., Chu, H., Naujokat, H., Wiltfang, J., et al. (2018). Acid Sphingomyelinase Activity as an Indicator of the Cell Stress in HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinoma. Med. Oncol. 35, 58. doi:10.1007/s12032-018-1117-4
Ghannam, M. G., and Singh, P. (2021). "Anatomy, Head and Neck, Salivary Glands," in StatPearls. (Treasure Island (FL)).
Gillison, M. L., Chaturvedi, A. K., Anderson, W. F., and Fakhry, C. (2015). Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 33, 3235–3242. doi:10.1200/JCO.2015.61.6995
Gillison, M. L., D'souza, G., Westra, W., Sugar, E., Xiao, W., Begum, S., et al. (2008). Distinct Risk Factor Profiles for Human Papillomavirus Type 16-positive and Human Papillomavirus Type 16-negative Head and Neck Cancers. J. Natl. Cancer Inst. 100, 407–420. doi:10.1093/jnci/djn025
Gillison, M. L., Koch, W. M., Capone, R. B., Spafford, M., Westra, W. H., Wu, L., et al. (2000). Evidence for a Causal Association between Human Papillomavirus and a Subset of Head and Neck Cancers. J. Natl. Cancer Inst. 92, 709–720. doi:10.1093/jnci/92.9.709
Gilyoma, J. M., Rambau, P. F., Masalu, N., Kayange, N. M., and Chalya, P. L. (2015). Head and Neck Cancers: a Clinico-Pathological Profile and Management Challenges in a Resource-Limited Setting. BMC Res. Notes 8, 772. doi:10.1186/s13104-015-1773-9
Gissmann, L., Diehl, V., Schultz-Coulon, H. J., and Zur Hausen, H. (1982). Molecular Cloning and Characterization of Human Papilloma Virus DNA Derived from a Laryngeal Papilloma. J. Virol. 44, 393–400. doi:10.1128/JVI.44.1.393-400.1982
Gissmann, L., and Zur Hausen, H. (1980). Partial Characterization of Viral DNA from Human Genital Warts (Condylomata Acuminata). Int. J. Cancer 25, 605–609. doi:10.1002/ijc.2910250509
Gletsou, E., Papadas, T. A., Baliou, E., Tsiambas, E., Ragos, V., Armata, I. E., et al. (2018). HPV Infection in Oropharyngeal Squamous Cell Carcinomas: Correlation with Tumor Size. J. BUON 23, 433–438.
Goldenberg, D., Begum, S., Westra, W. H., Khan, Z., Sciubba, J., Pai, S. I., et al. (2008). Cystic Lymph Node Metastasis in Patients with Head and Neck Cancer: An HPV-Associated Phenomenon. Head Neck 30, 898–903. doi:10.1002/hed.20796
Gupta, S., Kumar, P., Kaur, H., Sharma, N., Gupta, S., Saluja, D., et al. (2018). Constitutive Activation and Overexpression of NF-κB/c-Rel in Conjunction with P50 Contribute to Aggressive Tongue Tumorigenesis. Oncotarget 9, 33011–33029. doi:10.18632/oncotarget.26041
Gupta, S., Kumar, P., Kaur, H., Sharma, N., Saluja, D., Bharti, A. C., et al. (2015). Selective Participation of C-Jun with Fra-2/c-Fos Promotes Aggressive Tumor Phenotypes and Poor Prognosis in Tongue Cancer. Sci. Rep. 5, 16811. doi:10.1038/srep16811
Haddad, R. I., and Shin, D. M. (2008). Recent Advances in Head and Neck Cancer. N. Engl. J. Med. 359, 1143–1154. doi:10.1056/NEJMra0707975
Hajek, M., Sewell, A., Kaech, S., Burtness, B., Yarbrough, W. G., and Issaeva, N. (2017). TRAF3/CYLD Mutations Identify a Distinct Subset of Human Papillomavirus-Associated Head and Neck Squamous Cell Carcinoma. Cancer 123, 1778–1790. doi:10.1002/cncr.30570
Hammarstedt, L., Lindquist, D., Dahlstrand, H., Romanitan, M., Dahlgren, L. O., Joneberg, J., et al. (2006). Human Papillomavirus as a Risk Factor for the Increase in Incidence of Tonsillar Cancer. Int. J. Cancer 119, 2620–2623. doi:10.1002/ijc.22177
Hartmann, J. T., and Lipp, H. P. (2003). Toxicity of Platinum Compounds. Expert Opin. Pharmacother. 4, 889–901. doi:10.1517/14656566.4.6.889
Hashim, D., Genden, E., Posner, M., Hashibe, M., and Boffetta, P. (2019). Head and Neck Cancer Prevention: from Primary Prevention to Impact of Clinicians on Reducing burden. Ann. Oncol. 30, 744–756. doi:10.1093/annonc/mdz084
Havel, J. J., Chowell, D., and Chan, T. A. (2019). The Evolving Landscape of Biomarkers for Checkpoint Inhibitor Immunotherapy. Nat. Rev. Cancer 19, 133–150. doi:10.1038/s41568-019-0116-x
Hellquist, H., Lundgren, J., and Olofsson, J. (1982). Hyperplasia, Keratosis, Dysplasia and Carcinoma In Situ of the Vocal Cords-Aa Follow-Up Study. Clin. Otolaryngol. Allied Sci. 7, 11–27. doi:10.1111/j.1365-2273.1982.tb01557.x
Hemminki, K., Dong, C., and Frisch, M. (2000). Tonsillar and Other Upper Aerodigestive Tract Cancers Among Cervical Cancer Patients and Their Husbands. Eur. J. Cancer Prev. 9, 433–437. doi:10.1097/00008469-200012000-00010
Ho, A. S., Kraus, D. H., Ganly, I., Lee, N. Y., Shah, J. P., and Morris, L. G. (2014). Decision Making in the Management of Recurrent Head and Neck Cancer. Head Neck 36, 144–151. doi:10.1002/hed.23227
Ho, Y. C., Yang, S. F., Peng, C. Y., Chou, M. Y., and Chang, Y. C. (2007). Epigallocatechin-3-gallate Inhibits the Invasion of Human Oral Cancer Cells and Decreases the Productions of Matrix Metalloproteinases and Urokinase-Plasminogen Activator. J. Oral Pathol. Med. 36, 588–593. doi:10.1111/j.1600-0714.2007.00588.x
Ho, Y. T., Yang, J. S., Li, T. C., Lin, J. J., Lin, J. G., Lai, K. C., et al. (2009). Berberine Suppresses In Vitro Migration and Invasion of Human SCC-4 Tongue Squamous Cancer Cells through the Inhibitions of FAK, IKK, NF-kappaB, U-PA and MMP-2 and -9. Cancer Lett. 279, 155–162. doi:10.1016/j.canlet.2009.01.033
Hong, W. K., Endicott, J., Itri, L. M., Doos, W., Batsakis, J. G., Bell, R., et al. (1986). 13-cis-retinoic Acid in the Treatment of Oral Leukoplakia. N. Engl. J. Med. 315, 1501–1505. doi:10.1056/NEJM198612113152401
Hong, W. K., Lippman, S. M., Itri, L. M., Karp, D. D., Lee, J. S., Byers, R. M., et al. (1990). Prevention of Second Primary Tumors with Isotretinoin in Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 323, 795–801. doi:10.1056/NEJM199009203231205
Hou, H., Li, D., Cheng, D., Li, L., Liu, Y., and Zhou, Y. (2013). Cellular Redox Status Regulates Emodin-Induced Radiosensitization of Nasopharyngeal Carcinoma Cells In Vitro and In Vivo. J. Pharm. (Cairo) 2013, 218297.
Houlihan, C. F., Baisley, K., Bravo, I. G., Pavón, M. A., Changalucha, J., Kapiga, S., et al. (2019). Human Papillomavirus DNA Detected in Fingertip, Oral and Bathroom Samples from Unvaccinated Adolescent Girls in Tanzania. Sex. Transm. Infect. 95, 374–379. doi:10.1136/sextrans-2018-053756
Hseu, Y. C., Wu, C. R., Chang, H. W., Kumar, K. J., Lin, M. K., Chen, C. S., et al. (2011). Inhibitory Effects of Physalis Angulata on Tumor Metastasis and Angiogenesis. J. Ethnopharmacol 135, 762–771. doi:10.1016/j.jep.2011.04.016
Hsin, C. H., Huang, C. C., Chen, P. N., Hsieh, Y. S., Yang, S. F., Ho, Y. T., et al. (2017). Rubus Idaeus Inhibits Migration and Invasion of Human Nasopharyngeal Carcinoma Cells by Suppression of MMP-2 through Modulation of the ERK1/2 Pathway. Am. J. Chin. Med. 45, 1557–1572. doi:10.1142/S0192415X17500847
Hsin, C. H., Wu, B. C., Chuang, C. Y., Yang, S. F., Hsieh, Y. H., Ho, H. Y., et al. (2013). Selaginella Tamariscina Extract Suppresses TPA-Induced Invasion and Metastasis through Inhibition of MMP-9 in Human Nasopharyngeal Carcinoma HONE-1 Cells. BMC Complement. Altern. Med. 13, 234. doi:10.1186/1472-6882-13-234
Hsu, H. K., Brown, T. T., Li, X., Young, S., Cranston, R. D., D'souza, G., et al. (2015). Association between Free Testosterone Levels and Anal Human Papillomavirus Types 16/18 Infections in a Cohort of Men Who Have Sex with Men. PLoS One 10, e0119447. doi:10.1371/journal.pone.0119447
Hsu, P. C., Cheng, C. F., Hsieh, P. C., Chen, Y. H., Kuo, C. Y., and Sytwu, H. K. (2020). Chrysophanol Regulates Cell Death, Metastasis, and Reactive Oxygen Species Production in Oral Cancer Cell Lines. Evid. Based Complement. Alternat Med. 2020, 5867064. doi:10.1155/2020/5867064
Huang, C., and Yu, Y. (2017). Synergistic Cytotoxicity of β-Elemene and Cisplatin in Gingival Squamous Cell Carcinoma by Inhibition of STAT3 Signaling Pathway. Med. Sci. Monit. 23, 1507–1513. doi:10.12659/msm.903783
Huang, X. Z., Wang, J., Huang, C., Chen, Y. Y., Shi, G. Y., Hu, Q. S., et al. (2008). Emodin Enhances Cytotoxicity of Chemotherapeutic Drugs in Prostate Cancer Cells: the Mechanisms Involve ROS-Mediated Suppression of Multidrug Resistance and Hypoxia Inducible Factor-1. Cancer Biol. Ther. 7, 468–475. doi:10.4161/cbt.7.3.5457
Huang, Y. W., Chuang, C. Y., Hsieh, Y. S., Chen, P. N., Yang, S. F., Shih-Hsuan-Lin, L., et al. (2017). Rubus Idaeus Extract Suppresses Migration and Invasion of Human Oral Cancer by Inhibiting MMP-2 through Modulation of the Erk1/2 Signaling Pathway. Environ. Toxicol. 32, 1037–1046. doi:10.1002/tox.22302
Hung, C. M., Chang, C. C., Lin, C. W., Ko, S. Y., and Hsu, Y. C. (2013). Cucurbitacin E as Inducer of Cell Death and Apoptosis in Human Oral Squamous Cell Carcinoma Cell Line SAS. Int. J. Mol. Sci. 14, 17147–17156. doi:10.3390/ijms140817147
Iarc, W. G. O. T. E. O. C. R. T. H. (2012). Personal Habits and Indoor Combustions. Volume 100 E. A Review of Human Carcinogens. IARC Monogr. Eval. Carcinog Risks Hum. 100, 1–538.
Iarc, W. G. O. T. E. O. C. R. T. H. (2004). Tobacco Smoke and Involuntary Smoking. IARC Monogr. Eval. Carcinog Risks Hum. 83, 1–1438.
Imai, K., Suga, K., and Nakachi, K. (1997). Cancer-preventive Effects of Drinking green tea Among a Japanese Population. Prev. Med. 26, 769–775. doi:10.1006/pmed.1997.0242
Ishiji, T., Lace, M. J., Parkkinen, S., Anderson, R. D., Haugen, T. H., Cripe, T. P., et al. (1992). Transcriptional Enhancer Factor (TEF)-1 and its Cell-specific Co-activator Activate Human Papillomavirus-16 E6 and E7 Oncogene Transcription in Keratinocytes and Cervical Carcinoma Cells. EMBO J. 11, 2271–2281. doi:10.1002/j.1460-2075.1992.tb05286.x
Issing, W. J., Struck, R., and Naumann, A. (1996). Long-term Follow-Up of Larynx Leukoplakia under Treatment with Retinyl Palmitate. Head Neck 18, 560–565. doi:10.1002/(sici)1097-0347(199611/12)18:6<560::aid-hed11>3.0.co;2-c
Jadassohn (1896). Sind die verrucae vulgares ubertragbar? Vehandel D Deutsch Dem Gesellsch 5, 497–512.
Jégoux, F., Métreau, A., Louvel, G., and Bedfert, C. (2013). Paranasal Sinus Cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 130, 327–335. doi:10.1016/j.anorl.2012.07.007
Jenson, A. B., Sommer, S., Payling-Wright, C., Pass, F., Link, C. C., and Lancaster, W. D. (1982). Human Papillomavirus. Frequency and Distribution in Plantar and Common Warts. Lab. Invest. 47, 491–497.
Jin, L., Miao, J., Liu, Y., Li, X., Jie, Y., Niu, Q., et al. (2017). Icaritin Induces Mitochondrial Apoptosis by Up-Regulating miR-124 in Human Oral Squamous Cell Carcinoma Cells. Biomed. Pharmacother. 85, 287–295. doi:10.1016/j.biopha.2016.11.023
Jyothirmayi, R., Ramadas, K., Varghese, C., Jacob, R., Nair, M. K., and Sankaranarayanan, R. (1996). Efficacy of Vitamin A in the Prevention of Loco-Regional Recurrence and Second Primaries in Head and Neck Cancer. Eur. J. Cancer B Oral Oncol. 32B, 373–376. doi:10.1016/s0964-1955(96)00010-3
Kalu, N. N., Mazumdar, T., Peng, S., Shen, L., Sambandam, V., Rao, X., et al. (2017). Genomic Characterization of Human Papillomavirus-Positive and -negative Human Squamous Cell Cancer Cell Lines. Oncotarget 8, 86369–86383. doi:10.18632/oncotarget.21174
Kapil, U., Singh, P., Bahadur, S., Shukla, N. K., Dwivedi, S., Pathak, P., et al. (2003). Association of Vitamin A, Vitamin C and Zinc with Laryngeal Cancer. Indian J. Cancer 40, 67–70.
Kapoor, V., Aggarwal, S., and Das, S. N. (2016). 6-Gingerol Mediates its Anti Tumor Activities in Human Oral and Cervical Cancer Cell Lines through Apoptosis and Cell Cycle Arrest. Phytother Res. 30, 588–595. doi:10.1002/ptr.5561
Katiyar, C., Gupta, A., Kanjilal, S., and Katiyar, S. (2012). Drug Discovery from Plant Sources: An Integrated Approach. Ayu 33, 10–19. doi:10.4103/0974-8520.100295
Kaugars, G. E., Silverman, S., Lovas, J. G., Brandt, R. B., Riley, W. T., Dao, Q., et al. (1994). A Clinical Trial of Antioxidant Supplements in the Treatment of Oral Leukoplakia. Oral Surg. Oral Med. Oral Pathol. 78, 462–468. doi:10.1016/0030-4220(94)90039-6
Khafif, A., Schantz, S. P., Al-Rawi, M., Edelstein, D., and Sacks, P. G. (1998). Green tea Regulates Cell Cycle Progression in Oral Leukoplakia. Head Neck 20, 528–534. doi:10.1002/(sici)1097-0347(199809)20:6<528::aid-hed7>3.0.co;2-3
Kirnbauer, R., Booy, F., Cheng, N., Lowy, D. R., and Schiller, J. T. (1992). Papillomavirus L1 Major Capsid Protein Self-Assembles into Virus-like Particles that Are Highly Immunogenic. Proc. Natl. Acad. Sci. U S A. 89, 12180–12184. doi:10.1073/pnas.89.24.12180
Klein, S. L. (2000). The Effects of Hormones on Sex Differences in Infection: from Genes to Behavior. Neurosci. Biobehav Rev. 24, 627–638. doi:10.1016/s0149-7634(00)00027-0
Knobloch, T. J., Ryan, N. M., Bruschweiler-Li, L., Wang, C., Bernier, M. C., Somogyi, A., et al. (2019). Metabolic Regulation of Glycolysis and AMP Activated Protein Kinase Pathways during Black Raspberry-Mediated Oral Cancer Chemoprevention. Metabolites 9. doi:10.3390/metabo9070140
Koehn, F. E., and Carter, G. T. (2005). The Evolving Role of Natural Products in Drug Discovery. Nat. Rev. Drug Discov. 4, 206–220. doi:10.1038/nrd1657
Kofler, B., Laban, S., Busch, C. J., Lörincz, B., and Knecht, R. (2014). New Treatment Strategies for HPV-Positive Head and Neck Cancer. Eur. Arch. Otorhinolaryngol. 271, 1861–1867. doi:10.1007/s00405-013-2603-0
Koneva, L. A., Zhang, Y., Virani, S., Hall, P. B., Mchugh, J. B., Chepeha, D. B., et al. (2018). HPV Integration in HNSCC Correlates with Survival Outcomes, Immune Response Signatures, and Candidate Drivers. Mol. Cancer Res. 16, 90–102. doi:10.1158/1541-7786.MCR-17-0153
Kunnumakkara, A. B., Bordoloi, D., Harsha, C., Banik, K., Gupta, S. C., and Aggarwal, B. B. (2017). Curcumin Mediates Anticancer Effects by Modulating Multiple Cell Signaling Pathways. Clin. Sci. (Lond) 131, 1781–1799. doi:10.1042/CS20160935
Kuo, Y. Y., Lin, H. P., Huo, C., Su, L. C., Yang, J., Hsiao, P. H., et al. (2013). Caffeic Acid Phenethyl Ester Suppresses Proliferation and Survival of TW2.6 Human Oral Cancer Cells via Inhibition of Akt Signaling. Int. J. Mol. Sci. 14, 8801–8817. doi:10.3390/ijms14058801
Kuriakose, M. A., Ramdas, K., Dey, B., Iyer, S., Rajan, G., Elango, K. K., et al. (2016). A Randomized Double-Blind Placebo-Controlled Phase IIB Trial of Curcumin in Oral Leukoplakia. Cancer Prev. Res. (Phila) 9, 683–691. doi:10.1158/1940-6207.CAPR-15-0390
Kuss, I., Donnenberg, A. D., Gooding, W., and Whiteside, T. L. (2003). Effector CD8+CD45RO-CD27-T Cells Have Signalling Defects in Patients with Squamous Cell Carcinoma of the Head and Neck. Br. J. Cancer 88, 223–230. doi:10.1038/sj.bjc.6600694
Lagoa, R., Samhan-Arias, A. K., and Gutierrez-Merino, C. (2017). Correlation between the Potency of Flavonoids for Cytochrome C Reduction and Inhibition of Cardiolipin-Induced Peroxidase Activity. Biofactors 43, 451–468. doi:10.1002/biof.1357
Langendijk, J. A., Doornaert, P., Verdonck-De Leeuw, I. M., Leemans, C. R., Aaronson, N. K., and Slotman, B. J. (2008). Impact of Late Treatment-Related Toxicity on Quality of Life Among Patients with Head and Neck Cancer Treated with Radiotherapy. J. Clin. Oncol. 26, 3770–3776. doi:10.1200/JCO.2007.14.6647
Lee, E., Han, A. R., Nam, B., Kim, Y. R., Jin, C. H., Kim, J. B., et al. (2020). Moscatilin Induces Apoptosis in Human Head and Neck Squamous Cell Carcinoma Cells via JNK Signaling Pathway. Molecules 25. doi:10.3390/molecules25040901
Lee, H. E., Shin, J. A., Jeong, J. H., Jeon, J. G., Lee, M. H., and Cho, S. D. (2016). Anticancer Activity of Ashwagandha against Human Head and Neck Cancer Cell Lines. J. Oral Pathol. Med. 45, 193–201. doi:10.1111/jop.12353
Lee, H. Z., Liu, W. Z., Hsieh, W. T., Tang, F. Y., Chung, J. G., and Leung, H. W. (2009). Oxidative Stress Involvement in Physalis Angulata-Induced Apoptosis in Human Oral Cancer Cells. Food Chem. Toxicol. 47, 561–570. doi:10.1016/j.fct.2008.12.013
Lee, Y. J., Park, B. S., Park, H. R., Yu, S. B., Kang, H. M., and Kim, I. R. (2017). XIAP Inhibitor Embelin Induces Autophagic and Apoptotic Cell Death in Human Oral Squamous Cell Carcinoma Cells. Environ. Toxicol. 32, 2371–2378. doi:10.1002/tox.22450
Li, C. J., Zhang, L. J., Dezube, B. J., Crumpacker, C. S., and Pardee, A. B. (1993). Three Inhibitors of Type 1 Human Immunodeficiency Virus Long Terminal Repeat-Directed Gene Expression and Virus Replication. Proc. Natl. Acad. Sci. U S A. 90, 1839–1842. doi:10.1073/pnas.90.5.1839
Li, H. C., Yashiki, S., Sonoda, J., Lou, H., Ghosh, S. K., Byrnes, J. J., et al. (2000). Green tea Polyphenols Induce Apoptosis In Vitro in Peripheral Blood T Lymphocytes of Adult T-Cell Leukemia Patients. Jpn. J. Cancer Res. 91, 34–40. doi:10.1111/j.1349-7006.2000.tb00857.x
Li, L. K., Rola, A. S., Kaid, F. A., Ali, A. M., and Alabsi, A. M. (2016). Goniothalamin Induces Cell Cycle Arrest and Apoptosis in H400 Human Oral Squamous Cell Carcinoma: A Caspase-dependent Mitochondrial-Mediated Pathway with Downregulation of NF-Κβ. Arch. Oral Biol. 64, 28–38. doi:10.1016/j.archoralbio.2015.12.002
Li, N., Sun, Z., Han, C., and Chen, J. (1999). The Chemopreventive Effects of tea on Human Oral Precancerous Mucosa Lesions. Proc. Soc. Exp. Biol. Med. 220, 218–224. doi:10.1046/j.1525-1373.1999.d01-37.x
Liang, Y. C., Chen, Y. C., Lin, Y. L., Lin-Shiau, S. Y., Ho, C. T., and Lin, J. K. (1999a). Suppression of Extracellular Signals and Cell Proliferation by the Black tea Polyphenol, Theaflavin-3,3'-Digallate. Carcinogenesis 20, 733–736. doi:10.1093/carcin/20.4.733
Liang, Y. C., Lin-Shiau, S. Y., Chen, C. F., and Lin, J. K. (1999b). Inhibition of Cyclin-dependent Kinases 2 and 4 Activities as Well as Induction of Cdk Inhibitors P21 and P27 during Growth Arrest of Human Breast Carcinoma Cells by (-)-Epigallocatechin-3-Gallate. J. Cel Biochem 75, 1–12. doi:10.1002/(sici)1097-4644(19991001)75:1<1::aid-jcb1>3.0.co;2-n
Liang, Y. C., Lin-Shiau, S. Y., Chen, C. F., and Lin, J. K. (1997). Suppression of Extracellular Signals and Cell Proliferation through EGF Receptor Binding by (-)-epigallocatechin Gallate in Human A431 Epidermoid Carcinoma Cells. J. Cel Biochem 67, 55–65. doi:10.1002/(sici)1097-4644(19971001)67:1<55::aid-jcb6>3.0.co;2-v
Liao, M. Y., Chuang, C. Y., Hsieh, M. J., Chou, Y. E., Lin, C. W., Chen, W. R., et al. (2018). Antimetastatic Effects of Eclipta Prostrata Extract on Oral Cancer Cells. Environ. Toxicol. 33, 923–930. doi:10.1002/tox.22577
Liberto, M., and Cobrinik, D. (2000). Growth Factor-dependent Induction of p21(CIP1) by the green tea Polyphenol, Epigallocatechin Gallate. Cancer Lett. 154, 151–161. doi:10.1016/s0304-3835(00)00378-5
Licitra, L., Perrone, F., Bossi, P., Suardi, S., Mariani, L., Artusi, R., et al. (2006). High-risk Human Papillomavirus Affects Prognosis in Patients with Surgically Treated Oropharyngeal Squamous Cell Carcinoma. J. Clin. Oncol. 24, 5630–5636. doi:10.1200/JCO.2005.04.6136
Lin, C. C., Yang, J. S., Chen, J. T., Fan, S., Yu, F. S., Yang, J. L., et al. (2007). Berberine Induces Apoptosis in Human HSC-3 Oral Cancer Cells via Simultaneous Activation of the Death Receptor-Mediated and Mitochondrial Pathway. Anticancer Res. 27, 3371–3378.
Lin, C. W., Chin, H. K., Lee, S. L., Chiu, C. F., Chung, J. G., Lin, Z. Y., et al. (2019). Ursolic Acid Induces Apoptosis and Autophagy in Oral Cancer Cells. Environ. Toxicol. 34, 983–991. doi:10.1002/tox.22769
Lin, F. Y., Hsieh, Y. H., Yang, S. F., Chen, C. T., Tang, C. H., Chou, M. Y., et al. (2015). Resveratrol Suppresses TPA-Induced Matrix Metalloproteinase-9 Expression through the Inhibition of MAPK Pathways in Oral Cancer Cells. J. Oral Pathol. Med. 44, 699–706. doi:10.1111/jop.12288
Lind, P. O. (1987). Malignant Transformation in Oral Leukoplakia. Scand. J. Dent Res. 95, 449–455. doi:10.1111/j.1600-0722.1987.tb01959.x
Lippman, S. M., Batsakis, J. G., Toth, B. B., Weber, R. S., Lee, J. J., Martin, J. W., et al. (1993). Comparison of Low-Dose Isotretinoin with Beta Carotene to Prevent Oral Carcinogenesis. N. Engl. J. Med. 328, 15–20. doi:10.1056/NEJM199301073280103
Lippman, S. M., Kessler, J. F., Al-Sarraf, M., Alberts, D. S., Itri, L. M., Mattox, D., et al. (1988). Treatment of Advanced Squamous Cell Carcinoma of the Head and Neck with Isotretinoin: a Phase II Randomized Trial. Invest. New Drugs 6, 51–56. doi:10.1007/BF00170781
Liu, G., Wu, D., Liang, X., Yue, H., and Cui, Y. (2015). Mechanisms and In Vitro Effects of Cepharanthine Hydrochloride: Classification Analysis of the Drug-Induced Differentially-Expressed Genes of Human Nasopharyngeal Carcinoma Cells. Oncol. Rep. 34, 2002–2010. doi:10.3892/or.2015.4193
Liu, T., Sun, X., and Cao, Z. (2019). Shikonin-induced Necroptosis in Nasopharyngeal Carcinoma Cells via ROS Overproduction and Upregulation of RIPK1/RIPK3/MLKL Expression. Onco Targets Ther. 12, 2605–2614. doi:10.2147/OTT.S200740
Liu, T. B., Zheng, Z. H., Pan, J., Pan, L. L., and Chen, L. H. (2017). Prognostic Role of Plasma Epstein-Barr Virus DNA Load for Nasopharyngeal Carcinoma: a Meta-Analysis. Clin. Invest. Med. 40, E1–E12. doi:10.25011/cim.v40i1.28049
Louvanto, K., Sarkola, M., Rintala, M., Syrjänen, K., Grenman, S., and Syrjänen, S. (2017). Breast Milk Is a Potential Vehicle for Human Papillomavirus Transmission to Oral Mucosa of the Spouse. Pediatr. Infect. Dis. J. 36, 627–630. doi:10.1097/INF.0000000000001546
Lu, K. W., Chen, J. C., Lai, T. Y., Yang, J. S., Weng, S. W., Ma, Y. S., et al. (2011). Gypenosides Inhibits Migration and Invasion of Human Oral Cancer SAS Cells through the Inhibition of Matrix Metalloproteinase-2 -9 and Urokinase-Plasminogen by ERK1/2 and NF-Kappa B Signaling Pathways. Hum. Exp. Toxicol. 30, 406–415. doi:10.1177/0960327110372405
Lubin, J. H., Purdue, M., Kelsey, K., Zhang, Z. F., Winn, D., Wei, Q., et al. (2009). Total Exposure and Exposure Rate Effects for Alcohol and Smoking and Risk of Head and Neck Cancer: a Pooled Analysis of Case-Control Studies. Am. J. Epidemiol. 170, 937–947. doi:10.1093/aje/kwp222
Macha, M. A., Matta, A., Chauhan, S. S., Siu, K. W., and Ralhan, R. (2011). Guggulsterone (GS) Inhibits Smokeless Tobacco and Nicotine-Induced NF-Κb and STAT3 Pathways in Head and Neck Cancer Cells. Carcinogenesis 32, 368–380. doi:10.1093/carcin/bgq278
Maitland, N. J., Bromidge, T., Cox, M. F., Crane, I. J., Prime, S. S., and Scully, C. (1989). Detection of Human Papillomavirus Genes in Human Oral Tissue Biopsies and Cultures by Polymerase Chain Reaction. Br. J. Cancer 59, 698–703. doi:10.1038/bjc.1989.146
Mallery, S. R., Tong, M., Shumway, B. S., Curran, A. E., Larsen, P. E., Ness, G. M., et al. (2014). Topical Application of a Mucoadhesive Freeze-Dried Black Raspberry Gel Induces Clinical and Histologic Regression and Reduces Loss of Heterozygosity Events in Premalignant Oral Intraepithelial Lesions: Results from a Multicentered, Placebo-Controlled Clinical Trial. Clin. Cancer Res. 20, 1910–1924. doi:10.1158/1078-0432.CCR-13-3159
Marier, J. F., Vachon, P., Gritsas, A., Zhang, J., Moreau, J. P., and Ducharme, M. P. (2002). Metabolism and Disposition of Resveratrol in Rats: Extent of Absorption, Glucuronidation, and Enterohepatic Recirculation Evidenced by a Linked-Rat Model. J. Pharmacol. Exp. Ther. 302, 369–373. doi:10.1124/jpet.102.033340
Marquard, F. E., and Jücker, M. (2020). PI3K/AKT/mTOR Signaling as a Molecular Target in Head and Neck Cancer. Biochem. Pharmacol. 172, 113729. doi:10.1016/j.bcp.2019.113729
Marur, S., and Forastiere, A. A. (2008). Head and Neck Cancer: Changing Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 83, 489–501. doi:10.4065/83.4.489
Marur, S., and Forastiere, A. A. (2016). Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 91, 386–396. doi:10.1016/j.mayocp.2015.12.017
Marur, S., Li, S., Cmelak, A. J., Gillison, M. L., Zhao, W. J., Ferris, R. L., et al. (2017). E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients with HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group. J. Clin. Oncol. 35, 490–497. doi:10.1200/JCO.2016.68.3300
Mashberg, A., and Samit, A. (1995). Early Diagnosis of Asymptomatic Oral and Oropharyngeal Squamous Cancers. CA Cancer J. Clin. 45, 328–351. doi:10.3322/canjclin.45.6.328
Masterson, L., Sorgeloos, F., Winder, D., Lechner, M., Marker, A., Malhotra, S., et al. (2015). Deregulation of SYCP2 Predicts Early Stage Human Papillomavirus-Positive Oropharyngeal Carcinoma: A Prospective Whole Transcriptome Analysis. Cancer Sci. 106, 1568–1575. doi:10.1111/cas.12809
Masuda, M., Suzui, M., and Weinstein, I. B. (2001). Effects of Epigallocatechin-3-Gallate on Growth, Epidermal Growth Factor Receptor Signaling Pathways, Gene Expression, and Chemosensitivity in Human Head and Neck Squamous Cell Carcinoma Cell Lines. Clin. Cancer Res. 7, 4220–4229.
Mathew, B., Sankaranarayanan, R., Nair, P. P., Varghese, C., Somanathan, T., Amma, B. P., et al. (1995). Evaluation of Chemoprevention of Oral Cancer with Spirulina Fusiformis. Nutr. Cancer 24, 197–202. doi:10.1080/01635589509514407
Mckaig, R. G., Baric, R. S., and Olshan, A. F. (1998). Human Papillomavirus and Head and Neck Cancer: Epidemiology and Molecular Biology. Head Neck 20, 250–265. doi:10.1002/(sici)1097-0347(199805)20:3<250::aid-hed11>3.0.co;2-o
Mehanna, H., Beech, T., Nicholson, T., El-Hariry, I., Mcconkey, C., Paleri, V., et al. (2013). Prevalence of Human Papillomavirus in Oropharyngeal and Nonoropharyngeal Head and Neck Cancer-Ssystematic Review and Meta-Analysis of Trends by Time and Region. Head Neck 35, 747–755. doi:10.1002/hed.22015
Mehra, R., Seiwert, T. Y., Gupta, S., Weiss, J., Gluck, I., Eder, J. P., et al. (2018). Efficacy and Safety of Pembrolizumab in Recurrent/metastatic Head and Neck Squamous Cell Carcinoma: Pooled Analyses after Long-Term Follow-Up in KEYNOTE-012. Br. J. Cancer 119, 153–159. doi:10.1038/s41416-018-0131-9
Meisels, A., and Fortin, R. (1976). Condylomatous Lesions of the Cervix and Vagina. I. Cytologic Patterns. Acta Cytol. 20, 505–509.
Mishra, A., Bharti, A. C., Varghese, P., Saluja, D., and Das, B. C. (2006). Differential Expression and Activation of NF-kappaB Family Proteins during Oral Carcinogenesis: Role of High Risk Human Papillomavirus Infection. Int. J. Cancer 119, 2840–2850. doi:10.1002/ijc.22262
Mishra, A., Kumar, R., Tyagi, A., Kohaar, I., Hedau, S., Bharti, A. C., et al. (2015). Curcumin Modulates Cellular AP-1, NF-kB, and HPV16 E6 Proteins in Oral Cancer. Ecancermedicalscience 9, 525. doi:10.3332/ecancer.2015.525
Monisha, J., Roy, N. K., Bordoloi, D., Kumar, A., Golla, R., Kotoky, J., et al. (2017). Nuclear Factor Kappa B: A Potential Target to Persecute Head and Neck Cancer. Curr. Drug Targets 18, 232–253. doi:10.2174/1389450117666160201112330
Mork, J., Lie, A. K., Glattre, E., Hallmans, G., Jellum, E., Koskela, P., et al. (2001). Human Papillomavirus Infection as a Risk Factor for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 344, 1125–1131. doi:10.1056/NEJM200104123441503
Müller, S. (2018). Oral Epithelial Dysplasia, Atypical Verrucous Lesions and Oral Potentially Malignant Disorders: Focus on Histopathology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 125, 591–602. doi:10.1016/j.oooo.2018.02.012
Muzaffar, J., Bari, S., Kirtane, K., and Chung, C. H. (2021). Recent Advances and Future Directions in Clinical Management of Head and Neck Squamous Cell Carcinoma. Cancers (Basel) 13. doi:10.3390/cancers13020338
Myoung, H., Hong, S. P., Yun, P. Y., Lee, J. H., and Kim, M. J. (2003). Anti-cancer Effect of Genistein in Oral Squamous Cell Carcinoma with Respect to Angiogenesis and In Vitro Invasion. Cancer Sci. 94, 215–220. doi:10.1111/j.1349-7006.2003.tb01422.x
Ndiaye, C., Mena, M., Alemany, L., Arbyn, M., Castellsagué, X., Laporte, L., et al. (2014). HPV DNA, E6/E7 mRNA, and p16INK4a Detection in Head and Neck Cancers: a Systematic Review and Meta-Analysis. Lancet Oncol. 15, 1319–1331. doi:10.1016/S1470-2045(14)70471-1
Neville, B. W., and Day, T. A. (2002). Oral Cancer and Precancerous Lesions. CA Cancer J. Clin. 52, 195–215. doi:10.3322/canjclin.52.4.195
Nikolaou, M., Pavlopoulou, A., Georgakilas, A. G., and Kyrodimos, E. (2018). The challenge of Drug Resistance in Cancer Treatment: a Current Overview. Clin. Exp. Metastasis 35, 309–318. doi:10.1007/s10585-018-9903-0
Oeckinghaus, A., and Ghosh, S. (2009). The NF-kappaB Family of Transcription Factors and its Regulation. Cold Spring Harb Perspect. Biol. 1, a000034. doi:10.1101/cshperspect.a000034
Ologe, F. E., Adeniji, K. A., and Segun-Busari, S. (2005). Clinicopathological Study of Head and Neck Cancers in Ilorin, Nigeria. Trop. Doct 35, 2–4. doi:10.1258/0049475053001949
Oreggia, F., De Stefani, E., Correa, P., and Fierro, L. (1991). Risk Factors for Cancer of the Tongue in Uruguay. Cancer 67, 180–183. doi:10.1002/1097-0142(19910101)67:1<180::aid-cncr2820670130>3.0.co;2-r
Owen, J. H., Graham, M. P., Chinn, S. B., Darr, O. F., Chepeha, D. B., Wolf, G. T., et al. (2016). Novel Method of Cell Line Establishment Utilizing Fluorescence-Activated Cell Sorting Resulting in 6 New Head and Neck Squamous Cell Carcinoma Lines. Head Neck 38 Suppl 1 Suppl. 1, E459–E467. doi:10.1002/hed.24019
Pang, J. S., Yen, J. H., Wu, H. T., and Huang, S. T. (2017). Gallic Acid Inhibited Matrix Invasion and AP-1/ets-1-Mediated MMP-1 Transcription in Human Nasopharyngeal Carcinoma Cells. Int. J. Mol. Sci. 18. doi:10.3390/ijms18071354
Pardoll, D. M. (2012). The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 12, 252–264. doi:10.1038/nrc3239
Parsons, R. J., and Kidd, J. G. (1943). Oral Papillomatosis of Rabbits: A Virus Disease. J. Exp. Med. 77, 233–250. doi:10.1084/jem.77.3.233
Partlová, S., Bouček, J., Kloudová, K., Lukešová, E., Zábrodský, M., Grega, M., et al. (2015). Distinct Patterns of Intratumoral Immune Cell Infiltrates in Patients with HPV-Associated Compared to Non-virally Induced Head and Neck Squamous Cell Carcinoma. Oncoimmunology 4, e965570. doi:10.4161/21624011.2014.965570
Paschka, A. G., Butler, R., and Young, C. Y. (1998). Induction of Apoptosis in Prostate Cancer Cell Lines by the green tea Component, (-)-Epigallocatechin-3-Gallate. Cancer Lett. 130, 1–7. doi:10.1016/s0304-3835(98)00084-6
Patel, R. G. (2017). Nasal Anatomy and Function. Facial Plast. Surg. 33, 3–8. doi:10.1055/s-0036-1597950
Payne, A. (1891). On the Contagious Rise of Common Warts. Br. J. Dermatol. 3, 185. doi:10.1086/120258
Peng, X., Zhang, Q., Zeng, Y., Li, J., Wang, L., and Ai, P. (2015). Evodiamine Inhibits the Migration and Invasion of Nasopharyngeal Carcinoma Cells In Vitro via Repressing MMP-2 Expression. Cancer Chemother. Pharmacol. 76, 1173–1184. doi:10.1007/s00280-015-2902-9
Pintos, J., Franco, E. L., Black, M. J., Bergeron, J., and Arella, M. (1999). Human Papillomavirus and Prognoses of Patients with Cancers of the Upper Aerodigestive Tract. Cancer 85, 1903–1909. doi:10.1002/(sici)1097-0142(19990501)85:9<1903::aid-cncr4>3.0.co;2-6
Pollock, N. I., Wang, L., Wallweber, G., Gooding, W. E., Huang, W., Chenna, A., et al. (2015). Increased Expression of HER2, HER3, and HER2:HER3 Heterodimers in HPV-Positive HNSCC Using a Novel Proximity-Based Assay: Implications for Targeted Therapies. Clin. Cancer Res. 21, 4597–4606. doi:10.1158/1078-0432.CCR-14-3338
Porceddu, S. V., Campbell, B., Rischin, D., Corry, J., Weih, L., Guerrieri, M., et al. (2004). Postoperative Chemoradiotherapy for High-Risk Head-And-Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 60, 365–373. doi:10.1016/j.ijrobp.2004.03.011
Posner, M. R., Lorch, J. H., Goloubeva, O., Tan, M., Schumaker, L. M., Sarlis, N. J., et al. (2011). Survival and Human Papillomavirus in Oropharynx Cancer in TAX 324: a Subset Analysis from an International Phase III Trial. Ann. Oncol. 22, 1071–1077. doi:10.1093/annonc/mdr006
Prasad, R., and Katiyar, S. K. (2012). Bioactive Phytochemical Proanthocyanidins Inhibit Growth of Head and Neck Squamous Cell Carcinoma Cells by Targeting Multiple Signaling Molecules. PLoS One 7, e46404. doi:10.1371/journal.pone.0046404
Price, K. A., and Cohen, E. E. (2012). Current Treatment Options for Metastatic Head and Neck Cancer. Curr. Treat. Options. Oncol. 13, 35–46. doi:10.1007/s11864-011-0176-y
Prusty, B. K., and Das, B. C. (2005). Constitutive Activation of Transcription Factor AP-1 in Cervical Cancer and Suppression of Human Papillomavirus (HPV) Transcription and AP-1 Activity in HeLa Cells by Curcumin. Int. J. Cancer 113, 951–960. doi:10.1002/ijc.20668
Purola, E., and Savia, E. (1977). Cytology of Gynecologic Condyloma Acuminatum. Acta Cytol. 21, 26–31.
Pyrhönen, S., and Neuvonen, E. (1978). The Occurrence of Human Wart-Virus Antibodies in Dogs, Pigs and Cattle. Arch. Virol. 57, 297–305. doi:10.1007/BF01320069
Qiao, X. W., Jiang, J., Pang, X., Huang, M. C., Tang, Y. J., Liang, X. H., et al. (2020). The Evolving Landscape of PD-1/pd-L1 Pathway in Head and Neck Cancer. Front. Immunol. 11, 1721. doi:10.3389/fimmu.2020.01721
Qiu, Y., Li, C., Wang, Q., Zeng, X., and Ji, P. (2018). Tanshinone IIA Induces Cell Death via Beclin-1-dependent Autophagy in Oral Squamous Cell Carcinoma SCC-9 Cell Line. Cancer Med. 7, 397–407. doi:10.1002/cam4.1281
Quick, C. A., Faras, A., and Krzysek, R. (1978). The Etiology of Laryngeal Papillomatosis. Laryngoscope 88, 1789–1795. doi:10.1288/00005537-197811000-00009
Ragin, C. C., and Taioli, E. (2007). Survival of Squamous Cell Carcinoma of the Head and Neck in Relation to Human Papillomavirus Infection: Review and Meta-Analysis. Int. J. Cancer 121, 1813–1820. doi:10.1002/ijc.22851
Rajamoorthi, A., Shrivastava, S., Steele, R., Nerurkar, P., Gonzalez, J. G., Crawford, S., et al. (2013). Bitter Melon Reduces Head and Neck Squamous Cell Carcinoma Growth by Targeting C-Met Signaling. PLoS One 8, e78006. doi:10.1371/journal.pone.0078006
Ranganathan, K., Devi, M. U., Joshua, E., Kirankumar, K., and Saraswathi, T. R. (2004). Oral Submucous Fibrosis: a Case-Control Study in Chennai, South India. J. Oral Pathol. Med. 33, 274–277. doi:10.1111/j.0904-2512.2004.00116.x
Ravindranath, V., and Chandrasekhara, N. (1980). Absorption and Tissue Distribution of Curcumin in Rats. Toxicology 16, 259–265. doi:10.1016/0300-483x(80)90122-5
Recchia, F., Lelli, S., Di Matteo, G., Rea, S., and Frati, L. (1993). [5-fluorouracil, Cisplatin and Retinol Palmitate in the Management of Advanced Cancer of the Oral Cavity. Phase II Study]. Clin. Ter 142, 403–409.
Reichart, P. A., and Philipsen, H. P. (2005). Oral Erythroplakia-Aa Review. Oral Oncol. 41, 551–561. doi:10.1016/j.oraloncology.2004.12.003
Ren, S., Gaykalova, D., Wang, J., Guo, T., Danilova, L., Favorov, A., et al. (2018). Discovery and Development of Differentially Methylated Regions in Human Papillomavirus-Related Oropharyngeal Squamous Cell Carcinoma. Int. J. Cancer 143, 2425–2436. doi:10.1002/ijc.31778
Rieckmann, T., Tribius, S., Grob, T. J., Meyer, F., Busch, C. J., Petersen, C., et al. (2013). HNSCC Cell Lines Positive for HPV and P16 Possess Higher Cellular Radiosensitivity Due to an Impaired DSB Repair Capacity. Radiother. Oncol. 107, 242–246. doi:10.1016/j.radonc.2013.03.013
Rischin, D., Young, R. J., Fisher, R., Fox, S. B., Le, Q. T., Peters, L. J., et al. (2010). Prognostic Significance of p16INK4A and Human Papillomavirus in Patients with Oropharyngeal Cancer Treated on TROG 02.02 Phase III Trial. J. Clin. Oncol. 28, 4142–4148. doi:10.1200/JCO.2010.29.2904
Ryerson, A. B., Peters, E. S., Coughlin, S. S., Chen, V. W., Gillison, M. L., Reichman, M. E., et al. (2008). Burden of Potentially Human Papillomavirus-Associated Cancers of the Oropharynx and Oral Cavity in the US, Cancer 113, 2901–2909. doi:10.1002/cncr.23745
Sabeena, S., Bhat, P., Kamath, V., and Arunkumar, G. (2017). Possible Non-sexual Modes of Transmission of Human Papilloma Virus. J. Obstet. Gynaecol. Res. 43, 429–435. doi:10.1111/jog.13248
Salazar-Ruales, C., Arguello, J. V., López-Cortés, A., Cabrera-Andrade, A., García-Cárdenas, J. M., Guevara-Ramírez, P., et al. (2018). Salivary MicroRNAs for Early Detection of Head and Neck Squamous Cell Carcinoma: A Case-Control Study in the High Altitude Mestizo Ecuadorian Population. Biomed. Res. Int. 2018, 9792730. doi:10.1155/2018/9792730
Salmani, J. M. M., Zhang, X. P., Jacob, J. A., and Chen, B. A. (2017). Apigenin's Anticancer Properties and Molecular Mechanisms of Action: Recent Advances and Future Prospectives. Chin. J. Nat. Med. 15, 321–329. doi:10.1016/S1875-5364(17)30052-3
Sankaranarayanan, R., Mathew, B., Varghese, C., Sudhakaran, P. R., Menon, V., Jayadeep, A., et al. (1997). Chemoprevention of Oral Leukoplakia with Vitamin A and Beta Carotene: an Assessment. Oral Oncol. 33, 231–236. doi:10.1016/s0964-1955(97)00010-9
Sarkola, M. E., Grénman, S. E., Rintala, M. A., Syrjänen, K. J., and Syrjänen, S. M. (2008). Human Papillomavirus in the Placenta and Umbilical Cord Blood. Acta Obstet. Gynecol. Scand. 87, 1181–1188. doi:10.1080/00016340802468308
Schmidt, M., Polednik, C., Roller, J., and Hagen, R. (2014). Galium Verum Aqueous Extract Strongly Inhibits the Motility of Head and Neck Cancer Cell Lines and Protects Mucosal Keratinocytes against Toxic DNA Damage. Oncol. Rep. 32, 1296–1302. doi:10.3892/or.2014.3316
Schmidt, M., Skaf, J., Gavril, G., Polednik, C., Roller, J., Kessler, M., et al. (2017). The Influence of Osmunda Regalis Root Extract on Head and Neck Cancer Cell Proliferation, Invasion and Gene Expression. BMC Complement. Altern. Med. 17, 518. doi:10.1186/s12906-017-2009-4
Schröck, A., Bode, M., Göke, F. J., Bareiss, P. M., Schairer, R., Wang, H., et al. (2014). Expression and Role of the Embryonic Protein SOX2 in Head and Neck Squamous Cell Carcinoma. Carcinogenesis 35, 1636–1642. doi:10.1093/carcin/bgu094
Schwartz, D. L., Bankson, J., Bidaut, L., He, Y., Williams, R., Lemos, R., et al. (2011). HIF-1-dependent Stromal Adaptation to Ischemia Mediates In Vivo Tumor Radiation Resistance. Mol. Cancer Res. 9, 259–270. doi:10.1158/1541-7786.MCR-10-0469
Seiwert, T. Y., Zuo, Z., Keck, M. K., Khattri, A., Pedamallu, C. S., Stricker, T., et al. (2015). Integrative and Comparative Genomic Analysis of HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinomas. Clin. Cancer Res. 21, 632–641. doi:10.1158/1078-0432.CCR-13-3310
Seixas-Silva, J. A., Richards, T., Khuri, F. R., Wieand, H. S., Kim, E., Murphy, B., et al. (2005). Phase 2 Bioadjuvant Study of Interferon Alfa-2a, Isotretinoin, and Vitamin E in Locally Advanced Squamous Cell Carcinoma of the Head and Neck: Long-Term Follow-Up. Arch. Otolaryngol. Head Neck Surg. 131, 304–307. doi:10.1001/archotol.131.4.304
Seo, Y. S., Yim, M. J., Kim, B. H., Kang, K. R., Lee, S. Y., Oh, J. S., et al. (2015). Berberine-induced Anticancer Activities in FaDu Head and Neck Squamous Cell Carcinoma Cells. Oncol. Rep. 34, 3025–3034. doi:10.3892/or.2015.4312
Shaikh, J., Ankola, D. D., Beniwal, V., Singh, D., and Kumar, M. N. (2009). Nanoparticle Encapsulation Improves Oral Bioavailability of Curcumin by at Least 9-fold when Compared to Curcumin Administered with Piperine as Absorption Enhancer. Eur. J. Pharm. Sci. 37, 223–230. doi:10.1016/j.ejps.2009.02.019
Shi, X., Wang, L., Li, X., Bai, J., Li, J., Li, S., et al. (2017). Dihydroartemisinin Induces Autophagy-dependent Death in Human Tongue Squamous Cell Carcinoma Cells through DNA Double-Strand Break-Mediated Oxidative Stress. Oncotarget 8, 45981–45993. doi:10.18632/oncotarget.17520
Shin, D. M., Khuri, F. R., Murphy, B., Garden, A. S., Clayman, G., Francisco, M., et al. (2001). Combined Interferon-Alfa, 13-Cis-Retinoic Acid, and Alpha-Tocopherol in Locally Advanced Head and Neck Squamous Cell Carcinoma: Novel Bioadjuvant Phase II Trial. Jco 19, 3010–3017. doi:10.1200/jco.2001.19.12.3010
Siddiqui, I. A., Adhami, V. M., Bharali, D. J., Hafeez, B. B., Asim, M., Khwaja, S. I., et al. (2009). Introducing Nanochemoprevention as a Novel Approach for Cancer Control: Proof of Principle with green tea Polyphenol Epigallocatechin-3-Gallate. Cancer Res. 69, 1712–1716. doi:10.1158/0008-5472.CAN-08-3978
Siddiqui, S., Ahamad, M. S., Jafri, A., Afzal, M., and Arshad, M. (2017). Piperine Triggers Apoptosis of Human Oral Squamous Carcinoma through Cell Cycle Arrest and Mitochondrial Oxidative Stress. Nutr. Cancer 69, 791–799. doi:10.1080/01635581.2017.1310260
Silverman, S., Gorsky, M., and Lozada, F. (1984). Oral Leukoplakia and Malignant Transformation. A Follow-Up Study of 257 Patients. Cancer 53, 563–568. doi:10.1002/1097-0142(19840201)53:3<563::aid-cncr2820530332>3.0.co;2-f
Singh, M., and Bagewadi, A. (2017). Comparison of Effectiveness of Calendula officinalis Extract Gel with Lycopene Gel for Treatment of Tobacco-Induced Homogeneous Leukoplakia: A Randomized Clinical Trial. Int. J. Pharm. Investig. 7, 88–93. doi:10.4103/jphi.JPHI_19_17
Singh, M., Krishanappa, R., Bagewadi, A., and Keluskar, V. (2004). Efficacy of Oral Lycopene in the Treatment of Oral Leukoplakia. Oral Oncol. 40, 591–596. doi:10.1016/j.oraloncology.2003.12.011
Singh, T., Gupta, N. A., Xu, S., Prasad, R., Velu, S. E., and Katiyar, S. K. (2015). Honokiol Inhibits the Growth of Head and Neck Squamous Cell Carcinoma by Targeting Epidermal Growth Factor Receptor. Oncotarget 6, 21268–21282. doi:10.18632/oncotarget.4178
Sivanantham, B., Sethuraman, S., and Krishnan, U. M. (2016). Combinatorial Effects of Curcumin with an Anti-neoplastic Agent on Head and Neck Squamous Cell Carcinoma through the Regulation of EGFR-Erk1/2 and Apoptotic Signaling Pathways. ACS Comb. Sci. 18, 22–35. doi:10.1021/acscombsci.5b00043
Smith, E. M., Ritchie, J. M., Summersgill, K. F., Klussmann, J. P., Lee, J. H., Wang, D., et al. (2004). Age, Sexual Behavior and Human Papillomavirus Infection in Oral Cavity and Oropharyngeal Cancers. Int. J. Cancer 108, 766–772. doi:10.1002/ijc.11633
Snijders, P. J., Cromme, F. V., Van Den Brule, A. J., Schrijnemakers, H. F., Snow, G. B., Meijer, C. J., et al. (1992). Prevalence and Expression of Human Papillomavirus in Tonsillar Carcinomas, Indicating a Possible Viral Etiology. Int. J. Cancer 51, 845–850. doi:10.1002/ijc.2910510602
Sogno, I., Vannini, N., Lorusso, G., Cammarota, R., Noonan, D. M., Generoso, L., et al. (2009). Anti-Angiogenic Activity of a Novel Class of Chemopreventive Compounds: Oleanic Acid Terpenoids. Recent Results Canc. Res. 181, 209–212.
Song, J. I., and Grandis, J. R. (2000). STAT Signaling in Head and Neck Cancer. Oncogene 19, 2489–2495. doi:10.1038/sj.onc.1203483
Steenbergen, R. D., Hermsen, M. A., Walboomers, J. M., Joenje, H., Arwert, F., Meijer, C. J., et al. (1995). Integrated Human Papillomavirus Type 16 and Loss of Heterozygosity at 11q22 and 18q21 in an Oral Carcinoma and its Derivative Cell Line. Cancer Res. 55, 5465–5471.
Stich, H. F., Hornby, A. P., Mathew, B., Sankaranarayanan, R., and Nair, M. K. (1988a). Response of Oral Leukoplakias to the Administration of Vitamin A. Cancer Lett. 40, 93–101. doi:10.1016/0304-3835(88)90266-2
Stich, H. F., Rosin, M. P., Hornby, A. P., Mathew, B., Sankaranarayanan, R., and Nair, M. K. (1988b). Remission of Oral Leukoplakias and Micronuclei in Tobacco/betel Quid Chewers Treated with Beta-Carotene and with Beta-Carotene Plus Vitamin A. Int. J. Cancer 42, 195–199. doi:10.1002/ijc.2910420209
Sturgis, E. M., and Cinciripini, P. M. (2007). Trends in Head and Neck Cancer Incidence in Relation to Smoking Prevalence: an Emerging Epidemic of Human Papillomavirus-Associated Cancers? Cancer 110, 1429–1435. doi:10.1002/cncr.22963
Sun, Z., Guan, X., Li, N., Liu, X., and Chen, X. (2010). Chemoprevention of Oral Cancer in Animal Models, and Effect on Leukoplakias in Human Patients with ZengShengPing, a Mixture of Medicinal Herbs. Oral Oncol. 46, 105–110. doi:10.1016/j.oraloncology.2009.06.004
Sur, S., Steele, R., Aurora, R., Varvares, M., Schwetye, K. E., and Ray, R. B. (2018). Bitter Melon Prevents the Development of 4-NQO-Induced Oral Squamous Cell Carcinoma in an Immunocompetent Mouse Model by Modulating Immune Signaling. Cancer Prev. Res. (Phila) 11, 191–202. doi:10.1158/1940-6207.CAPR-17-0237
Syrjänen, K., Syrjänen, S., Lamberg, M., Pyrhönen, S., and Nuutinen, J. (1983a). Morphological and Immunohistochemical Evidence Suggesting Human Papillomavirus (HPV) Involvement in Oral Squamous Cell Carcinogenesis. Int. J. Oral Surg. 12, 418–424. doi:10.1016/s0300-9785(83)80033-7
Syrjänen, K., Syrjänen, S., and Pyrhönen, S. (1982). Human Papilloma Virus (HPV) Antigens in Lesions of Laryngeal Squamous Cell Carcinomas. ORL J. Otorhinolaryngol. Relat. Spec. 44, 323–334. doi:10.1159/000275612
Syrjänen, K. J., Pyrhönen, S., and Syrjänen, S. M. (1983b). Evidence Suggesting Human Papillomavirus (HPV) Etiology for the Squamous Cell Papilloma of the Paranasal Sinus. Arch. Geschwulstforsch 53, 77–82.
Syrjänen, S., Happonen, R. P., Virolainen, E., Siivonen, L., and Syrjänen, K. (1987). Detection of Human Papillomavirus (HPV) Structural Antigens and DNA Types in Inverted Papillomas and Squamous Cell Carcinomas of the Nasal Cavities and Paranasal Sinuses. Acta Otolaryngol. 104, 334–341. doi:10.3109/00016488709107337
Syrjänen, S. (2018). Oral Manifestations of Human Papillomavirus Infections. Eur. J. Oral Sci. 126 Suppl 1 Suppl. 1, 49–66. doi:10.1111/eos.12538
Syrjänen, S., Rautava, J., and Syrjänen, K. (2017). HPV in Head and Neck Cancer-30 Years of History. Recent Results Cancer Res. 206, 3–25. doi:10.1007/978-3-319-43580-0_1
Syrjanen, S., Andersson, B., Juntunen, L., and Syrjanen, K. (1992). Polymerase Chain Reaction for Producing Biotinylated Human Papillomavirus DNA Probes for In Situ Hybridization. Sex. Transm. Dis. 19, 140–145. doi:10.1097/00007435-199205000-00006
Tang, A. L., Hauff, S. J., Owen, J. H., Graham, M. P., Czerwinski, M. J., Park, J. J., et al. (2012). UM-SCC-104: a New Human Papillomavirus-16-Positive Cancer Stem Cell-Containing Head and Neck Squamous Cell Carcinoma Cell Line. Head Neck 34, 1480–1491. doi:10.1002/hed.21962
Thomas, G. T., Lewis, M. P., and Speight, P. M. (1999). Matrix Metalloproteinases and Oral Cancer. Oral Oncol. 35, 227–233. doi:10.1016/s1368-8375(99)00004-4
Tilakaratne, W. M., Jayasooriya, P. R., Jayasuriya, N. S., and De Silva, R. K. (2019). Oral Epithelial Dysplasia: Causes, Quantification, Prognosis, and Management Challenges. Periodontol. 2000 80, 126–147. doi:10.1111/prd.12259
Toporcov, T. N., Znaor, A., Zhang, Z. F., Yu, G. P., Winn, D. M., Wei, Q., et al. (2015). Risk Factors for Head and Neck Cancer in Young Adults: a Pooled Analysis in the INHANCE Consortium. Int. J. Epidemiol. 44, 169–185. doi:10.1093/ije/dyu255
Tosetti, F., Ferrari, N., De Flora, S., and Albini, A. (2002). Angioprevention': Angiogenesis Is a Common and Key Target for Cancer Chemopreventive Agents. FASEB J. 16, 2–14. doi:10.1096/fj.01-0300rev
Tsao, A. S., Liu, D., Martin, J., Tang, X. M., Lee, J. J., El-Naggar, A. K., et al. (2009). Phase II Randomized, Placebo-Controlled Trial of green tea Extract in Patients with High-Risk Oral Premalignant Lesions. Cancer Prev. Res. (Phila) 2, 931–941. doi:10.1158/1940-6207.CAPR-09-0121
Tsukishiro, T., Donnenberg, A. D., and Whiteside, T. L. (2003). Rapid Turnover of the CD8(+)CD28(-) T-Cell Subset of Effector Cells in the Circulation of Patients with Head and Neck Cancer. Cancer Immunol. Immunother. 52, 599–607. doi:10.1007/s00262-003-0395-6
Van Houten, V. M., Snijders, P. J., Van Den Brekel, M. W., Kummer, J. A., Meijer, C. J., Van Leeuwen, B., et al. (2001). Biological Evidence that Human Papillomaviruses Are Etiologically Involved in a Subgroup of Head and Neck Squamous Cell Carcinomas. Int. J. Cancer 93, 232–235. doi:10.1002/ijc.1313
Verma, G., Vishnoi, K., Tyagi, A., Jadli, M., Singh, T., Goel, A., et al. (2017). Characterization of Key Transcription Factors as Molecular Signatures of HPV-Positive and HPV-Negative Oral Cancers. Cancer Med. 6, 591–604. doi:10.1002/cam4.983
Verma, V., Sprave, T., Haque, W., Simone, C. B., Chang, J. Y., Welsh, J. W., et al. (2018). A Systematic Review of the Cost and Cost-Effectiveness Studies of Immune Checkpoint Inhibitors. J. Immunother. Cancer 6, 128. doi:10.1186/s40425-018-0442-7
Vermorken, J. B., Psyrri, A., Mesía, R., Peyrade, F., Beier, F., De Blas, B., et al. (2014). Impact of Tumor HPV Status on Outcome in Patients with Recurrent And/or Metastatic Squamous Cell Carcinoma of the Head and Neck Receiving Chemotherapy with or without Cetuximab: Retrospective Analysis of the Phase III EXTREME Trial. Ann. Oncol. 25, 801–807. doi:10.1093/annonc/mdt574
Visalli, G., Currò, M., Facciolà, A., Riso, R., Mondello, P., Laganà, P., et al. (2016). Prevalence of Human Papillomavirus in Saliva of Women with HPV Genital Lesions. Infect. Agent Cancer 11, 48. doi:10.1186/s13027-016-0096-3
Vizcaíno, C., Mansilla, S., and Portugal, J. (2015). Sp1 Transcription Factor: A Long-Standing Target in Cancer Chemotherapy. Pharmacol. Ther. 152, 111–124. doi:10.1016/j.pharmthera.2015.05.008
Voskens, C. J., Sewell, D., Hertzano, R., Desanto, J., Rollins, S., Lee, M., et al. (2012). Induction of MAGE-A3 and HPV-16 Immunity by Trojan Vaccines in Patients with Head and Neck Carcinoma. Head Neck 34, 1734–1746. doi:10.1002/hed.22004
Wang, W. C., Chen, C. Y., Hsu, H. K., Lin, L. M., and Chen, Y. K. (2016). Chemopreventive Effect of Toona Sinensis Leaf Extract on 7,12-Dimethylbenz[a]anthracene-Induced Hamster Buccal Pouch Squamous Cell Carcinogenesis. Arch. Oral Biol. 70, 130–142. doi:10.1016/j.archoralbio.2016.06.015
Wang, Y., Hu, Y., Chen, L., Wu, J., Wu, K., Du, J., et al. (2020). Molecular Mechanisms and Prognostic Markers in Head and Neck Squamous Cell Carcinoma: a Bioinformatic Analysis. Int. J. Clin. Exp. Pathol. 13, 371–381.
Warnakulasuriya, S., and Ariyawardana, A. (2016). Malignant Transformation of Oral Leukoplakia: a Systematic Review of Observational Studies. J. Oral Pathol. Med. 45, 155–166. doi:10.1111/jop.12339
Wei, W. I., and Sham, J. S. (2000). Nasopharyngeal Carcinoma. Lancet 365, 2041–2054. doi:10.1016/S0140-6736(05)66698-6
Weisburg, J. H., Schuck, A. G., Reiss, S. E., Wolf, B. J., Fertel, S. R., Zuckerbraun, H. L., et al. (2013). Ellagic Acid, a Dietary Polyphenol, Selectively Cytotoxic to HSC-2 Oral Carcinoma Cells. Anticancer Res. 33, 1829–1836.
Weisman, R. A., Christen, R., Los, G., Jones, V., Kerber, C., Seagren, S., et al. (1998). Phase I Trial of Retinoic Acid and Cis-Platinum for Advanced Squamous Cell Cancer of the Head and Neck Based on Experimental Evidence of Drug Synergism. Otolaryngol. Head Neck Surg. 118, 597–602. doi:10.1177/019459989811800506
Wendt, T. G., Grabenbauer, G. G., Rödel, C. M., Thiel, H. J., Aydin, H., Rohloff, R., et al. (1998). Simultaneous Radiochemotherapy versus Radiotherapy Alone in Advanced Head and Neck Cancer: a Randomized Multicenter Study. J. Clin. Oncol. 16, 1318–1324. doi:10.1200/JCO.1998.16.4.1318
White, J. S., Weissfeld, J. L., Ragin, C. C., Rossie, K. M., Martin, C. L., Shuster, M., et al. (2007). The Influence of Clinical and Demographic Risk Factors on the Establishment of Head and Neck Squamous Cell Carcinoma Cell Lines. Oral Oncol. 43, 701–712. doi:10.1016/j.oraloncology.2006.09.001
Wierzbicka, M., Klussmann, J. P., San Giorgi, M. R., Wuerdemann, N., and Dikkers, F. G. (2021). Oral and Laryngeal HPV Infection: Incidence, Prevalence and Risk Factors, with Special Regard to Concurrent Infection in Head, Neck and Genitals. Vaccine.39(17), 2344-2350. doi:10.1016/j.vaccine.2021.03.047
Wils, L. J., Poell, J. B., Evren, I., Koopman, M. S., Brouns, E. R. E. A., De Visscher, J. G. A. M., et al. (2020). Incorporation of Differentiated Dysplasia Improves Prediction of Oral Leukoplakia at Increased Risk of Malignant Progression. Mod. Pathol. 33, 1033–1040. doi:10.1038/s41379-019-0444-0
Windon, M. J., D'souza, G., Rettig, E. M., Westra, W. H., Van Zante, A., Wang, S. J., et al. (2018). Increasing Prevalence of Human Papillomavirus-Positive Oropharyngeal Cancers Among Older Adults. Cancer 124, 2993–2999. doi:10.1002/cncr.31385
Winning, T. A., and Townsend, G. C. (2000). Oral Mucosal Embryology and Histology. Clin. Dermatol. 18, 499–511. doi:10.1016/s0738-081x(00)00140-1
Wolter, K. G., Wang, S. J., Henson, B. S., Wang, S., Griffith, K. A., Kumar, B., et al. (2006). (-)-gossypol Inhibits Growth and Promotes Apoptosis of Human Head and Neck Squamous Cell Carcinoma In Vivo. Neoplasia 8, 163–172. doi:10.1593/neo.05691
Wookey, V. B., Appiah, A. K., Kallam, A., Ernani, V., Smith, L. M., and Ganti, A. K. (2019). HPV Status and Survival in Non-oropharyngeal Squamous Cell Carcinoma of the Head and Neck. Anticancer Res. 39, 1907–1914. doi:10.21873/anticanres.13299
Wu, X., Fan, Z., Masui, H., Rosen, N., and Mendelsohn, J. (1995). Apoptosis Induced by an Anti-epidermal Growth Factor Receptor Monoclonal Antibody in a Human Colorectal Carcinoma Cell Line and its Delay by Insulin. J. Clin. Invest. 95, 1897–1905. doi:10.1172/JCI117871
Xiao, C., Beitler, J. J., Higgins, K. A., Glazer, T., Huynh, L. K., Paul, S., et al. (2018). Associations Among Human Papillomavirus, Inflammation, and Fatigue in Patients with Head and Neck Cancer. Cancer 124, 3163–3170. doi:10.1002/cncr.31537
Yang, G., Liao, J., Kim, K., Yurkow, E. J., and Yang, C. S. (1998). Inhibition of Growth and Induction of Apoptosis in Human Cancer Cell Lines by tea Polyphenols. Carcinogenesis 19, 611–616. doi:10.1093/carcin/19.4.611
Yang, J., Pi, C., and Wang, G. (2018a). Inhibition of PI3K/Akt/mTOR Pathway by Apigenin Induces Apoptosis and Autophagy in Hepatocellular Carcinoma Cells. Biomed. Pharmacother. 103, 699–707. doi:10.1016/j.biopha.2018.04.072
Yang, J., Ren, X., Zhang, L., Li, Y., Cheng, B., and Xia, J. (2018b). Oridonin Inhibits Oral Cancer Growth and PI3K/Akt Signaling Pathway. Biomed. Pharmacother. 100, 226–232. doi:10.1016/j.biopha.2018.02.011
Yang, J.-S., Lin, C.-W., Hsin, C.-H., Hsieh, M.-J., and Chang, Y.-C. (2013). Selaginella Tamariscina Attenuates Metastasis via Akt Pathways in Oral Cancer Cells. PLoS One 8, e68035. doi:10.1371/journal.pone.0068035
Yang, K. Y., Lin, L. C., Tseng, T. Y., Wang, S. C., and Tsai, T. H. (2007). Oral Bioavailability of Curcumin in Rat and the Herbal Analysis from Curcuma Longa by LC-MS/MS. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 853, 183–189. doi:10.1016/j.jchromb.2007.03.010
Yang, W. E., Ho, Y. C., Tang, C. M., Hsieh, Y. S., Chen, P. N., Lai, C. T., et al. (2019). Duchesnea Indica Extract Attenuates Oral Cancer Cells Metastatic Potential through the Inhibition of the Matrix Metalloproteinase-2 Activity by Down-Regulating the MEK/ERK Pathway. Phytomedicine 63, 152960. doi:10.1016/j.phymed.2019.152960
Ye, D., Zhou, X., Pan, H., Jiang, Q., Zhong, L., Chen, W., et al. (2011). Establishment and Characterization of an HPV16 E6/E7-Expressing Oral Squamous Cell Carcinoma Cell Line with Enhanced Tumorigenicity. Med. Oncol. 28, 1331–1337. doi:10.1007/s12032-010-9558-4
Ye, M., Wu, Q., Zhang, M., and Huang, J. (2016). Lycopene Inhibits the Cell Proliferation and Invasion of Human Head and Neck Squamous Cell Carcinoma. Mol. Med. Rep. 14, 2953–2958. doi:10.3892/mmr.2016.5597
Yi, J., Yang, J., He, R., Gao, F., Sang, H., Tang, X., et al. (2004). Emodin Enhances Arsenic Trioxide-Induced Apoptosis via Generation of Reactive Oxygen Species and Inhibition of Survival Signaling. Cancer Res. 64, 108–116. doi:10.1158/0008-5472.can-2820-2
Yin, F., Zhao, L., Zhang, L., Chen, Y., Sun, G., Li, J., et al. (2020). Chemopreventive Role of Apigenin against the Synergistic Carcinogenesis of Human Papillomavirus and 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone. Biomedicines 8. doi:10.3390/biomedicines8110472
Yoshimura, H., Yoshida, H., Matsuda, S., Ryoke, T., Ohta, K., Ohmori, M., et al. (2019). The Therapeutic Potential of Epigallocatechin‑3‑gallate against Human Oral Squamous Cell Carcinoma through Inhibition of Cell Proliferation and Induction of Apoptosis: In Vitro and In Vivo Murine Xenograft Study. Mol. Med. Rep. 20, 1139–1148. doi:10.3892/mmr.2019.10331
Yu, X. D., Yang, J. L., Zhang, W. L., and Liu, D. X. (2016). Resveratrol Inhibits Oral Squamous Cell Carcinoma through Induction of Apoptosis and G2/M Phase Cell Cycle Arrest. Tumour Biol. 37, 2871–2877. doi:10.1007/s13277-015-3793-4
Zaridze, D., Evstifeeva, T., and Boyle, P. (1993). Chemoprevention of Oral Leukoplakia and Chronic Esophagitis in an Area of High Incidence of Oral and Esophageal Cancer. Ann. Epidemiol. 3, 225–234. doi:10.1016/1047-2797(93)90023-w
Zeka, A., Gore, R., and Kriebel, D. (2003). Effects of Alcohol and Tobacco on Aerodigestive Cancer Risks: a Meta-Regression Analysis. Cancer Causes Control 14, 897–906. doi:10.1023/b:caco.0000003854.34221.a8
Zhang, C., Hao, Y., Sun, Y., and Liu, P. (2019). Quercetin Suppresses the Tumorigenesis of Oral Squamous Cell Carcinoma by Regulating microRNA-22/wnt1/β-Catenin axis. J. Pharmacol. Sci. 140, 128–136. doi:10.1016/j.jphs.2019.03.005
Zhang, W., Kang, M., Zhang, T., Li, B., Liao, X., and Wang, R. (2016). Triptolide Combined with Radiotherapy for the Treatment of Nasopharyngeal Carcinoma via NF-Κb-Related Mechanism. Int. J. Mol. Sci. 17. doi:10.3390/ijms17122139
Zhang, W., Yin, G., Dai, J., Sun, Y. U., Hoffman, R. M., Yang, Z., et al. (2017). Chemoprevention by Quercetin of Oral Squamous Cell Carcinoma by Suppression of the NF-Κb Signaling Pathway in DMBA-Treated Hamsters. Anticancer Res. 37, 4041–4049. doi:10.21873/anticanres.11789
Zhang, Y., Sturgis, E. M., Dahlstrom, K. R., Wen, J., Liu, H., Wei, Q., et al. (2013). Telomere Length in Peripheral Blood Lymphocytes Contributes to the Development of HPV-Associated Oropharyngeal Carcinoma. Cancer Res. 73, 5996–6003. doi:10.1158/0008-5472.CAN-13-0881
Zhao, M., Ma, J., Zhu, H. Y., Zhang, X. H., Du, Z. Y., Xu, Y. J., et al. (2011). Apigenin Inhibits Proliferation and Induces Apoptosis in Human Multiple Myeloma Cells through Targeting the trinity of CK2, Cdc37 and Hsp90. Mol. Cancer 10, 104. doi:10.1186/1476-4598-10-104
Zhu, X., Sui, M., and Fan, W. (2005). In Vitro and In Vivo Characterizations of Tetrandrine on the Reversal of P-Glycoprotein-Mediated Drug Resistance to Paclitaxel. Anticancer Res. 25, 1953–1962.
Zhu, Y., Mao, Y., Chen, H., Lin, Y., Hu, Z., Wu, J., et al. (2013). Apigenin Promotes Apoptosis, Inhibits Invasion and Induces Cell Cycle Arrest of T24 Human Bladder Cancer Cells. Cancer Cel Int 13, 54. doi:10.1186/1475-2867-13-54
Zou, Y., Cheng, C., Omura-Minamisawa, M., Kang, Y., Hara, T., Guan, X., et al. (2010). The Suppression of Hypoxia-Inducible Factor and Vascular Endothelial Growth Factor by siRNA Does Not Affect the Radiation Sensitivity of Multicellular Tumor Spheroids. Jrr 51, 47–55. doi:10.1269/jrr.09070
Zur Hausen, H., Gissmann, L., Steiner, W., Dippold, W., and Dreger, I. (1975). Human Papilloma Viruses and Cancer. Bibl Haematol., 569–571. doi:10.1159/000399220
4NQO 4-nitroquinoline 1-oxide
5-FU 5-fluorouracil
Akt protein kinase B
AP-1 activator protein 1
Bax Bcl-2–associated X protein
Bcl-2 B-cell lymphoma 2
Bcl-xL Bcl-2 homolog B-cell lymphoma-extra large
BH3 Bcl-2 homology 3
Bim Bcl-2–interacting mediator of cell death
BRB black raspberry
CAPE caffeic acid phenethyl ester
Cdc2 cell division control 2
CDK2/4 cyclin-dependent kinase 2/4
CDKN2A cyclin-dependent kinase inhibitor 2A
c-Fos cellular oncogene Fos
CIAP2 calf intestinal alkaline phosphatase
Cip1 CDK interacting protein 1
c-Jun cellular Jun
COX-2 cyclooxygenase-2
c-Raf c-Rapidly accelerated fibrosarcoma
CT concurrent chemotherapy
E6/7 Early protein 6/7
EBV Epstein–Barr virus
ECGC (-)-Epigallocatechin-3-gallate
EGFR epidermal growth factor receptor
ERK extracellular signal–regulated kinase
FOXO1 forkhead box protein O1
Fra-2 Fos-related antigen 2
GSK3 glycogen synthase kinase 3
SK3β glycogen synthase kinase 3 beta
HN head and neck
HER2 human epidermal growth factor receptor 2
HIF-1 α hypoxia-inducible factor-1 α
HNC head and neck cancer
HNSCC head and neck squamous cell carcinoma
HO1 heme oxygenase 1
HPV human papillomavirus
IARC international agency for research on cancer
IC50 half maximal inhibitory concentration
IgE immunoglobulin E
IKK inhibitor of nuclear factor-κB kinase
IL-6 interleukin 6
iNOS inducible nitric oxide synthase
IRS-1 insulin receptor substrate 1
IκB inhibitor of nuclear factor-κB
IκBα I-kappa-B-alpha
JAK2 janus kinase2
JNK c-Jun N-terminal kinase
Kip2 kinase inhibitor 1
LC3 microtubule-associated protein 1A/1B-light chain 3
LCN2 lipocalin-2
LSCC laryngeal squamous cell carcinoma
M1 type macrophages activated macrophages
MAPK mitogen-activated protein kinase
MDM2 mouse double minute 2 homolog
miR micro RNA
MLKL mixed lineage kinase domain-like pseudokinase
MMPs metalloproteinases
mTOR mammalian target of rapamycin
mTORC2 mTOR Complex 2
NF-κB nuclear factor kappa light chain enhancer of activated B cells
NPC nasopharyngeal carcinoma
NPSCC nasopharyngeal squamous cell carcinoma
OED oral epithelial dysplasia
OPC oropharyngeal carcinoma
OPMDs oral potentially malignant disorders
OPSCC oropharyngeal squamous cell carcinoma
OSCC oral squamous cell carcinoma
P70S6K 70-kDa ribosomal protein S6 kinase
PCNA proliferating cell nuclear antigen
PD1 programmed cell death protein 1
PI3K phosphatidylinositol 3-kinase
PIP2 phosphatidylinositol-4,5-bisphosphate
PIP3 phosphatidylinositol-3,4,5-bisphosphate
PKA-AMPK protein kinase A–AMP-activated protein kinase
PPARδ peroxisome proliferator–activated receptor delta
pRB phosphorylated retinoblastoma protein
PRR5 proline rich protein 5
pSTAT3 phosphorylated signal transducer and activator of transcription 3
PTEN phosphatase and tensin homolog
Raf rapidly accelerated fibrosarcoma
RBP RNA-binding protein
RICTOR rapamycin-insensitive companion of mammalian target of rapamycin
RIPK1 receptor-interacting serine/threonine-protein kinase 1
ROS reactive oxygen species
RT radiation therapy
RTK receptor tyrosine kinase
S6K1 ribosomal protein S6 kinase beta-1
SCC squamous cell carcinoma
Sin1 stress-activated protein kinase-interacting protein
SLT smokeless tobacco
SOX2 SRY (sex determining region Y)-box 2
STAT3 signal transducer and activator of transcription 3
TFs transcription factors
TIMPs tissue inhibitor of metalloproteinases
TNFα tumor necrosis factor alpha
Topo IIα topoisomerase II- alpha
TRPA1 transient receptor potential ankyrin 1
VEGF vascular endothelial growth factor
WHO World Health Organization
XIAP X-linked inhibitor of apoptosis protein
ZSP ZengShengPing
Keywords: head and neck cancer, human pappillomavirus, tobacco, smoking, phytochemicals, therapeutics, prevention
Citation: Aggarwal N, Yadav J, Chhakara S, Janjua D, Tripathi T, Chaudhary A, Chhokar A, Thakur K, Singh T and Bharti AC (2021) Phytochemicals as Potential Chemopreventive and Chemotherapeutic Agents for Emerging Human Papillomavirus–Driven Head and Neck Cancer: Current Evidence and Future Prospects. Front. Pharmacol. 12:699044. doi: 10.3389/fphar.2021.699044
Received: 22 April 2021; Accepted: 17 June 2021;
Published: 20 July 2021.
Edited by:
Farrukh Aqil, University of Louisville, United StatesReviewed by:
Lalit Batra, University of Louisville, United StatesCopyright © 2021 Aggarwal, Yadav, Chhakara, Janjua, Tripathi, Chaudhary, Chhokar, Thakur, Singh and Bharti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alok Chandra Bharti, YWxva2NoYW5kcmFiQHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.