
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 29 July 2021
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.694507
This article is part of the Research TopicNetwork Pharmacology and Traditional Medicine: Setting the New Standards by Combining In silico and Experimental WorkView all 40 articles
 Weibo Dai1†
Weibo Dai1† Jing Yang1†
Jing Yang1† Haili Cao1,2†
Haili Cao1,2† Zhuqiang Wang1
Zhuqiang Wang1 Guangru Li1
Guangru Li1 Xiwen Zhong1
Xiwen Zhong1 Weiwen Peng1
Weiwen Peng1 Chang Chen1
Chang Chen1 Xin Liu1
Xin Liu1 Congyan Zeng1*
Congyan Zeng1* Xianjing Hu1,3,4*
Xianjing Hu1,3,4*Background: Rheumatoid arthritis (RA) is a kind of chronic autoimmune disease with several tissues damaged. Shuji tablet (SJT) is a prescription approved for treating lumbago and leg pain in the clinic. However, the efficacy of SJT against RA is still unknown. This study aims to evaluate the therapeutic effect of SJT on adjuvant-induced arthritis (AIA) rats and explore the mechanism via a network pharmacological approach.
Methods: AIA rats were treated with SJT for 30 days at the dosages of 3.6, 1.8, and 0.9 g/kg, respectively, and the anti-RA effect was determined by measuring paw swelling, systemic symptoms score, arthritis index, and histopathological change. ELISA assay was used to evaluate the level of inflammatory cytokines in serum. The mechanism exploration and target prediction of SJT against RA were performed via a network pharmacological approach.
Results: SJT showed excellent alleviation on AIA rats, with evidence of reducing paws swelling, decreasing systemic symptoms score, and arthritis index. Furthermore, SJT significantly reduced the serum cytokines of IL-6, IL-1β, TNF-α in AIA rats. Histopathological examination showed SJT remarkably reduced synovial hyperplasia, cartilage damage, and inflammatory infiltration in the secondary-side paws. According to network pharmacological analysis, 208 candidate compounds and 445 potential targets of SJT were identified, and 4465 RA therapy-related targets were searched out. Subsequently, 292 target genes of SJT were speculated to be associated with RA treatment, among which the top 5 “response values” targets were STAT3, AKT1, JUN, HSP90AA1, TNF. GO and KEGG enrichment analysis suggested that 45 signaling pathways were associating with SJT treating RA. The top 10 signaling pathways were PI3K-Akt, MAPK, AGE-RAGE pathway in diabetic complications, Ras, HIF-1, TNF, Chemokine, IL-17, FoxO, and Rap1.
Conclusion: Our experimental study showed that SJT significantly alleviated rheumatoid arthritis of AIA rats. Network pharmacology showed that the key targets of SJT against RA probably were STAT3, AKT1, JUN, HSP90AA1, TNF, and the potential mechanism was associated with modulation on the signaling pathways of PI3K-Akt, MAPK, Ras, AGE-RAGE, HIF-1, TNF, chemokine, IL-17, FoxO, Rap 1. Our study strongly provides evidence for Shuji tablet in RA therapy and would enlarge its application in the clinic.
Rheumatoid arthritis (RA) is a systemic disease with several tissues damaged, such as synovial membrane, cartilage, and bone. The incidence of RA increases along with age (Tuncer et al., 2017). In North America and Europe, 0.5–1% of adults are suffering from RA (Bao et al., 2019). RA patients are usually suffering from a chronic syndrome of pain, joints swelling and morning stiffness, even deformity, causing disability (Pan et al., 2017). Scientists worldwide are seeking effective remedies to treat RA, including developing new drugs and employing traditional ethnologic therapies. Currently, drugs used for RA treatment include glucocorticoids, non-steroidal anti-inflammatory drugs (NSAIDs), and disease-modifying antirheumatic drugs (DMARDs). Series of anti-RA drugs used in the clinic, such as methotrexate, inhibitors of Janus kinase (JAK), and tumor necrosis factor (TNF), would induce nonnegligible side-effects such as cytopenia, transaminase elevation, cardiovascular disease (CV), and gastrointestinal (GI) events (Walsem et al., 2015; Burmester et al., 2017; Chatzidionysiou et al., 2017). Therefore, it is urgently demanded to develop other new drugs with more efficacy and fewer side effects in RA therapy.
RA is a kind of disease induced by multifactorial factors, including genetic, epigenetic, and environmental factors (Scherer et al., 2020). Pathologically, RA patients would harbor multi-features in the clinic, such as disordered body-immunity, dysregulated cytokine networks, and activated osteoclast and chondrocyte (Firestein et al., 2017). Series of signaling pathways were reported to be referred to in the occurrence and development of RA, such as the JAK/STAT pathway which regulates the gene expression of matrix metalloproteinases of inflamed synovial tissues (Malemud et al., 2018), PI3K/AKT/mTOR pathway which regulates the cell cycle, cellular quiescence and proliferation (King et al., 2015; Feng et al., 2018), MAPK pathway which regulates the production of proinflammatory cytokines (Yang et al., 2018; Thalhamer, et al., 2008), and so on. Hence, a good remedy used in RA therapy should involve the modulation of multiple targets and signaling pathways.
Network pharmacology is a newly emerged analytical method via integrating information network and pharmacological approach to identify drugs or disease targets from series of databases, and predict the signaling pathways those the drugs probably modulate (Ao et al., 2020). It is a useful method in the complicated mechanism study for multicomponent drugs, by which the “drug-active ingredient-target-disease network” would be clarified, and the multiple-biological processes, -mechanisms, and -signaling pathways would be comprehensively analyzed (Zhou et al., 2020). Traditional Chinese medicine (TCM) has been used for thousands of years in China. It has the feature of synergistic effect combining with several different kinds of herbal medicine (named “Jun-Chen-Zuo-Shi” in Chinese medicine). Understanding how the multiple ingredients in a herbal formula act in synergy, and the regulation of the active ingredients on multiple targets of diseases is an important approach to develop traditional Chinese medicine. Combining the network science with ancient TCM may potentially explore the scientific evidence of herbal formulae on the basis of complex biological systems (Li et al., 2013). “TCM network pharmacology” methodology is integrating Chinese medicine, network science, information science, and experimental science to study TCM systematically and predictably, and is usually considered to be a highly effective approach for predicting potential pathways and targets of drugs, which is now widely used in investigating the pharmacological mechanism and predicting the candidate targets of TCM (Wang et al., 2017).
Shuji tablet (SJT), a Chinese medicine prescription, has been approved for treating lumbago and leg pain in the clinic by the Preparation Specification of Medical Institutions in Guangdong Province (approval number Z20130008) (Zeng et al., 2016). SJT formula is composed of 14 medicinal herbs, including Paeonia lactiflora Pall. (PL, Baishao), Ligusticum striatum DC. (LS, Chuanxiong), Angelica sinensis (Oliv.) Diels (AS, Danggui), Angelica pubescens Maxim. (AP, Duhuo), Stephania tetrandra S. Moore (ST, Fangji), Smilax glabra Roxb. (SG, Fuling), Glycyrrhiza uralensis Fisch. ex DC. (GU, Gancao), Spatholobus suberectus Dunn (SS, Jixueteng), Callerya speciosa (Champ. ex Benth.) Schot (CS, Niudali), Achyranthes bidentata Blume (AB, Niuxi), Flemingia prostrata Roxb. Junior ex Roxb. (FP, Qianjinba), Taxillus chinensis (DC.) Danser (TC, Sangjisheng), Clematis chinensis Osbeck (CC, Weilingxian), Tinospora sinensis (Lour.) Merr. (TS, Kuanjinteng), among which TS can relieve pain induced by chemicals (Cao et al., 2019), and CS can suppress inflammation by decreasing COX-2 (Cao et al., 2019a). According to some literature, the herbs of PL, LS, AS, AP, SG, AB, TC, the major constitutes of Duhuo-Jisheng decoction, were commonly used for RA treatment in the clinic (Zhang et al., 2020), and tetrandrine, one of the main ingredients of ST, was reported to alleviate symptoms of RA through regulating NF-κB and MAPK signaling pathways (Wu et al., 2020). Our previous study had verified the anti-inflammatory and analgesic effects of SJT in rats (Wu et al., 2016). Furthermore, during the clinical practice of treating patients with lumbago or leg pain, SJT was also discovered to relieve RA symptoms in patients. However, the experimental evidence about the efficacy of SJT against RA is still limited, and the mechanism remains unclarified. In our present study, the therapeutic effect of SJT on RA was evaluated via setting up adjuvant-induced arthritis (AIA) rat model, and the underlying mechanisms were investigated via a network pharmacological approach. The whole study procedure is shown in Figure 1.
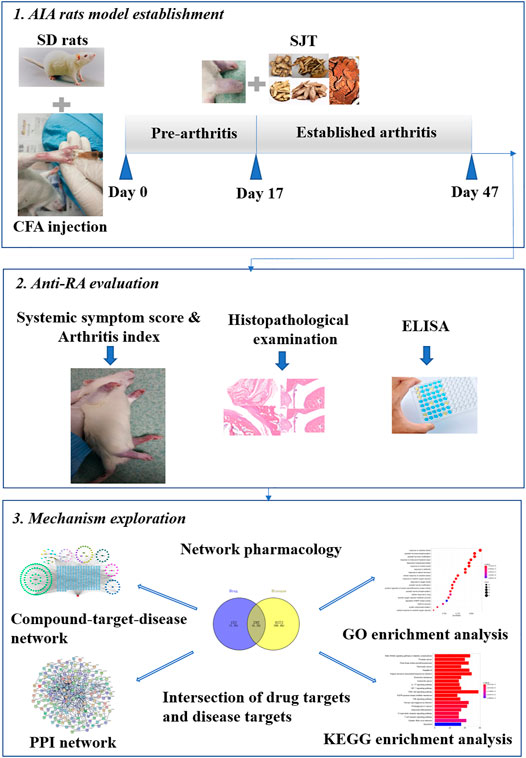
FIGURE 1. Workflow for the study of SJT on anti-rheumatoid arthritis effect and mechanism exploration via network pharmacological approach.
All the 14 herbal medicines (PL: 50100; LS: 50078; AS: 50077; AP: 50086; ST:50082; SG: 54201; GU:50012; SS: 54117; CS: 50026; AB: 50090; FP: 50113; TC: 54106; CC: 50029; TS: 54112) contained in SJT were purchased by Kang Sheng Pharmaceutical Company (Guangzhou, China). Tripterygium glycoside (TG) was selected as the positive control and purchased from Huangshi Feiyun Pharmaceutical Co., Ltd. (Huangshi, China). Freeze-dried Bacillus Calmette-Guerin vaccine (BCG) powder used as the complete Freund’s adjuvant (CFA) was purchased from National Vaccine and Serum Institute (Beijing, China). Enzyme-linked immunosorbent assay (ELISA) kits of tumor necrosis factor-α (TNF-α, #RK00029), interleukin-6 (IL-6, #RK00020), interleukin-1β (IL-1β, #RK00009) were purchased from ABclonal Biotech Co., Ltd. (Boston, United States).
The preparation of SJT in our study was performed following the “SJT Production Standard” recorded in the approved document. Briefly, the powders of PL (45 g), SG (54 g), GU (22.5 g), SS (90 g), CS (135 g), AB (45 g), FP (135 g), TC (67.5 g), CC (45 g), TS (67.5 g) were mixed and decocted twice with deionized water (1:10, w/v) for 2 h each time, then the decoction was filtered. The filtrate was combined and concentrated via a rotary evaporator at 60°C. Subsequently, the extract was dried to obtain the powder via the spray drying method. The powder was finally mixed completely with the other four pulverized herbals consisting of AP (54 g), LS (45 g), AS (45 g), ST (45 g), to form an SJT prescription. The extraction yield of SJT is 16%, complying with the criterion of Chinese Pharmacopoeia (State Pharmacopoeia Commission., 2020). The SJT powder was stored at 4°C and suspended in distilled water before use.
SJT powder (0.2 g) was extracted in 8 ml methanol/water (1:1, v/v) for 40 min ultrasonically at room temperature. The extract solution was then centrifuged at 13,000 rpm for 10 min and the supernatant was collected, filtered with a filter (0.22 µm). The sample obtained was applied for components identification through UHPLC-QTOF-MS analysis. Chromatographic separation was performed on a Shimadzu LC-30A liquid chromatograph (Shimadzu, Japan), and the LC conditions were: C18 column (100 mm × 2.1 mm i.d., 1.7 µm; Waters). Water containing 0.1% formic acid (solvent system A) and acetonitrile (solvent system B) served as the mobile phase. The gradient elution program was 0.01–15 min, 5–30% B; 15–35 min, 30–95% B; 35–37 min, 95–95% B; 37–37.1 min, 95–5% B; 37.1–40 min, 5% B. Flow rate: 0.4 ml/min; temperature: 45°C; injection volume: 5 μl. Mass detection was performed using a TripleTOF 5600 (AB Sciex, United States) operating in both positive and negative mode electrospray ionization with the following operating parameters: ion spray voltages for positive and negative modes were 5.5 and −4.5 kV; temperature: 500°C; declustering potential (DP): 100 V; collision energy (CE): ±35 eV. Ion spray and curtain gases were set at 50 and 40 psi, respectively. MS spectra were recorded over the m/z range of 50–1,000 to determine the top six most intense ions for QTOF-MS analysis. All data was processed by Analyst software, version 1.6 TF (Sciex).
Sprague Dawley rats (SD, ♂, aged 5–6 weeks) were supplied by Guangdong Medical Laboratory Animal Center [license no. SYXK (Y) 2018-0002]. Rats were maintained in a specific-pathogen-free (SPF) grade animal house with environmental conditions of 23 ± 2°C, 60–70% humidity, 12 h light/dark cycle, and allowed free access to food and water. The animal experiment was performed according to the Laboratory Animal-Guideline for “Ethical Review of Animal Welfare” and approved by the Experimental Animal Management and Ethics Committee of Guangzhou University of Chinese Medicine.
CFA was used to set up the AIA model as previously described (Feng et al., 2004). Briefly, CFA was prepared using 150 g freeze-dried BCG powder emulsified in 12 ml light liquid paraffin. SD rats, except for the normal group rats, were inoculated with CFA (0.1 ml/rat) in the right hind paw after receiving inhalational anesthesia via a tabletop anesthesia machine (Harvard, United States). The normal group rats were injected with 0.1 ml of normal saline. After 17 days, the rats with systemic symptom scores being more than six were randomly divided into six groups (12 rats/group), including normal saline (control) group, AIA (model) group, positive control (TG, 9.5 mg/kg) group, SJT high dosage (3.6 g/kg) group, SJT middle dosage (1.8 g/kg) group, and SJT low dosage (0.9 g/kg) group. From day 17th onward, SJT and TG were orally administered for 30 days continuously. The normal appearances of rats were monitored and body weights were regularly recorded. The rats were sacrificed after treatment, and the organs including the thymus, spleen were removed and weighed. Organ indexes were calculated according to the formula: organ index (mg/g) = organ weight (mg)/body weight (g).
Paw thicknesses were measured via a vernier caliper purchased from Jingjiang Measuring Tools Co., Ltd. (Jingjiang, China). The thicknesses were measured on day 0 (before CFA injection) and day 17th (before treatment), and regularly monitored (once/6 days) during drugs treatment.
A systemic symptom score table (Wang et al., 2011) and a 5-point ordinal scale scoring system (Zhang et al., 2008) were used to quantitatively evaluate the severity of arthritis in two dimensions. The systemic symptom score reflected the inflammatory response of the whole body, and each rat could receive 0–8 points. The arthritis index was used to measure the inflammation of three paws (except the right hind paw), and it was graded from 0 to 4, with a maximum score of 12 points per rat.
After drug administration, the blood was collected and serum was isolated after centrifugation at 4,000 rpm for 10 min. Levels of TNF-α, IL-6, and IL-1β in serum were determined by commercial ELISA assay kits. The procedure was conducted according to the manufacturer’s instructions.
The left hind paws (the secondary side) were removed and fixed in 10% (v/v) neutral formalin after the rats were sacrificed. The ankle joint tissues were put into 10% (w/v) ethylene diamine tetraacetic acid to decalcify for 60 days (Pan et al., 2017). After that, tissue samples were embedded in paraffin and cut into 4 μm sections. Slices were stained with hematoxylin-eosin (HE) to observe the histopathological changes under a light microscope.
The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (https://tcmspw.com/tcmsp.php), which recorded 499 common traditional Chinese medicines, was used to collect the chemical composition of SJT. The potential active ingredients in the herbals of SJT were determined according to the information of drug-like (DL) and oral bioavailability (OB), with the selected criteria of DL ≥ 0.18 and OB ≥ 30% in this study. After that, the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) was used to check the compound names and molecular structures. Concurrently, target genes of these active ingredients were predicted by searching the TCMSP database and corrected by the Uniprot database (https://www.uniprot.org/).
The “rheumatoid arthritis” was used as a keyword in searching Genecards database (https://www.genecards.org/) and OMIM database (https://omim.org/) to obtain target genes related to RA disease.
Candidate targets of SJT in RA therapy were predicted by integrating the drug-target genes of SJT and disease-targets of RA. Venn diagram was drawn by Venn diagram package and the overlapping genes were screened out, which stand for the candidate targets of SJT against RA.
The protein-protein interaction (PPI) regulation network was constructed through the String database (https://string-db.org/) to explain the complicated association among the compounds and target genes. The minimum required interaction score was set to 0.97. A drug-active ingredient-target network was constructed using Cytoscape 3.7.2 software (http://www.cytoscape.org) by integrating the target genes for RA disease, the active ingredients in SJT, and their corresponding targets.
The target genes identified were input into David v 6.8 Database for Annotation and Visualization, Integrated Discovery (https://david.ncifcrf.gov) for Gene Ontology (GO) biological process enrichment, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. The analysis of GO and KEGG was to demonstrate the major biological processes and potential molecular mechanisms of SJT against RA.
Data were analyzed with SPSS 22.0 software and results were expressed as mean ± S.D. Differences between the two groups were assessed by one-way analysis of variance (ANOVA), with p < 0.05 being considered to be significantly different.
In our study, the compounds of SJT were rapidly identified by UHPLC-QTOF-MS analysis. As a result, a total of 17 major compounds were identified, including betaine, albiflorin, D-tetrandrine, ononin, bergapten, ligustilide, 3-butyl-1(3H)-isobenzofuranone, 3-butylidene-1(3H)-isobenzofuranone, columbianetin, levistilide A, stigmasterol, glycerin fatty acid ester, dioctyl phthalate, rutin, liquiritin, quercitrin, glycyrrhizic acid (Figures 2A,B).
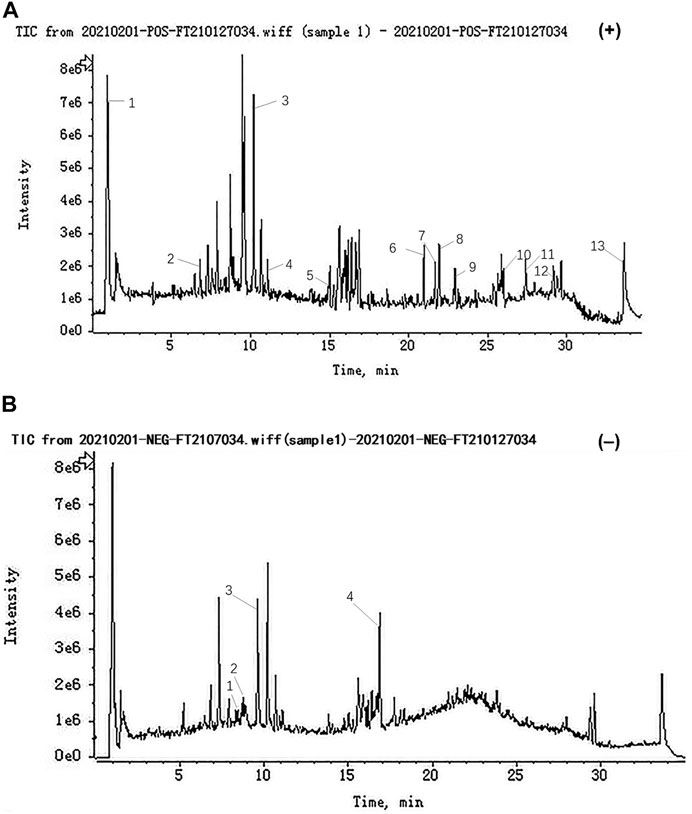
FIGURE 2. Representative base peak chromatogram (BPC) of SJT in the positive and negative ions mode, respectively. (A) Positive ions mode: (1) Betaine, (2) Albflorin, (3) D-Tetrandrine, (4) Ononin, (5) Bergapten, (6) Ligustilide, (7) 3-butyl-1(3H)-Isobenzofuranone, (8) 3-butylidene-1(3H)-Isobenzofuranone, (9) Columbianetin, (10) Levistilide A, (11) Stigmasterol, (12) Glycerin fatty acid ester, (13) Dioctyl phthalate, and (B) negative ions mode: (1) Rutin, (2) Liquiritin, (3) Quercitrin, (4) Glycyrrhizic acid.
The arthritis index and systemic symptoms score are two important parameters for determining the anti-RA function of drugs, of which the arthritis index is reflecting the inflammatory response of joints, while the systemic symptoms score is referring to the inflammatory response of the whole body. In our study, SJT (3.6, 1.8, 0.9 g/kg) and TG (9.5 mg/kg) significantly reduced the systemic symptoms score (Figure 3A) and arthritis index (Figure 3B) in a dose-dependent and time-dependent manner. In the clinic, the SJT dosage of 0.071 g/kg/day is commonly recommended used in humans, which converted into rats is 0.45 g/kg. Considering the higher rate of metabolism in rodents, we chose 0.9, 1.8, 3.6 g/kg as our testing dosages in rats. As a matter of fact, according to the extraction yield of SJT, the dosages of extract were 144, 288, 576 mg/kg, which were partly consistent with the referring dose range of 100–200 mg/kg recommended in “best practice in research” (Heinrich et al., 2020).

FIGURE 3. SJT suppressed rheumatoid arthritis in AIA rats. AIA rat model was established by inoculating with the complete Freund’s adjuvant (CFA) in the right hind paw. From the 17th day onward, rats were grouped and treated with or without SJT (0.9, 1.8, 3.6 g/kg) or tripterygium glycoside (TG, 9.5 mg/kg) for 30 days continuously. The rats were regularly analyzed for body weight and paw swelling, and sacrificed after drug treatment. (A) Arthritis index was evaluated by the 5-point ordinal scale scoring system. (B) Systemic symptoms scores were evaluated according to the systemic symptom score table. (C) Paw swelling evaluation in the primary side of AIA rats. (D) Paw swelling evaluation in the secondary side of AIA rats. The paw swelling was calculated by subtracting the initial (day 0) paw thickness. (E) Representation for paws in the secondary side of AIA rats with or without SJT treatment for 30 days (F) Body weights of AIA rats. Organ indexes of the spleen (G) and thymus (H) of AIA rats with or without SJT treatment for 30 days. All data were expressed as mean ± S.D. vs. model, *p < 0.05, **p < 0.01; vs. normal, #p < 0.05, ##p < 0.01.
Paw swelling is a classic character in the AIA model, and reducing paw-swelling is an important parameter to determine a remedy possessing an anti-RA effect. In our study, SJT suppressed paw swelling of AIA rats on the primary (Figure 3C) and secondary sides (Figure 3D) in a dose-dependent and time-dependent manner, showing that SJT had the capability of anti-RA effect. The paws photos also showed the strong suppression of SJT against arthritis (Figure 3E).
RA is a kind of chronic systemic disease with a long time lasting. Patients harboring RA disease always experience extraarticular changes and over-response of immunity, resulting in body weight loss and immune organ augment (Summers et al., 2008; Lemmey et al., 2016). In this study, the effects of SJT on body weight and immune organ index were measured accordingly. As shown in Figure 3F, compared with the normal rats, the model rats (CFA-injected only) got a significant bodyweight loss. However, after treatment with SJT or TG, the bodyweight losses of rats were significantly alleviated, in a good time-dependent manner. Furthermore, SJT showed good attenuation on the augment of immune organs induced by CFA (Figures 3G,H).
Furthermore, the effect of SJT on the inflammation and bone lesion (secondary side) induced by CFA was evaluated by hematoxylin-eosin staining. As shown in Figure 4, comparing with the normal group, pathological changes of ankle joints were observable in model group rats. CFA induced massive inflammatory cells infiltrating into the joint cavity, which resulted in synovial hyperplasia, cartilage, and bone being eroded severely. After SJT treatment for 30 days, the inflammatory cells infiltrating into the joint cavity significantly decreased, and cartilage surface, bone erosion, joints degradation were reduced, indicating that SJT strongly inhibited inflammation and bone lesion in the joints of AIA rats.
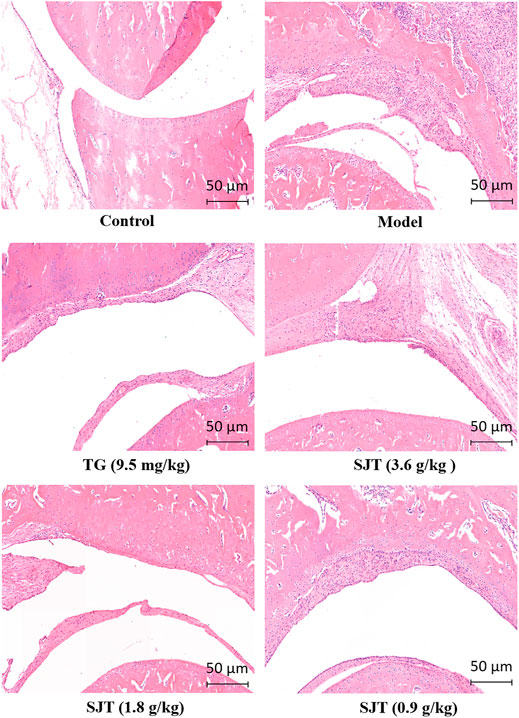
FIGURE 4. Effect of SJT on histopathological change of paws in AIA rats. Hematoxylin-eosin (HE) staining was used to detect the histopathological change of the second joints of AIA rats after SJT treatment for 30 days. The histopathological change was determined under a light microscope and images were taken. Magnification, 100 ×.
ELISA assay was used to detect the effect of SJT on the production of proinflammatory cytokines in the serum of AIA rats, including TNF-α, IL-1β, and IL-6. The results showed that CFA induced high levels of TNF-α, IL-1β, and IL-6, while SJT (3.6, 1.8, 0.9 g/kg) treatment remarkably reduced the high levels of these cytokines, suggesting that SJT significantly alleviated the inflammation response in AIA rats (Figure 5).
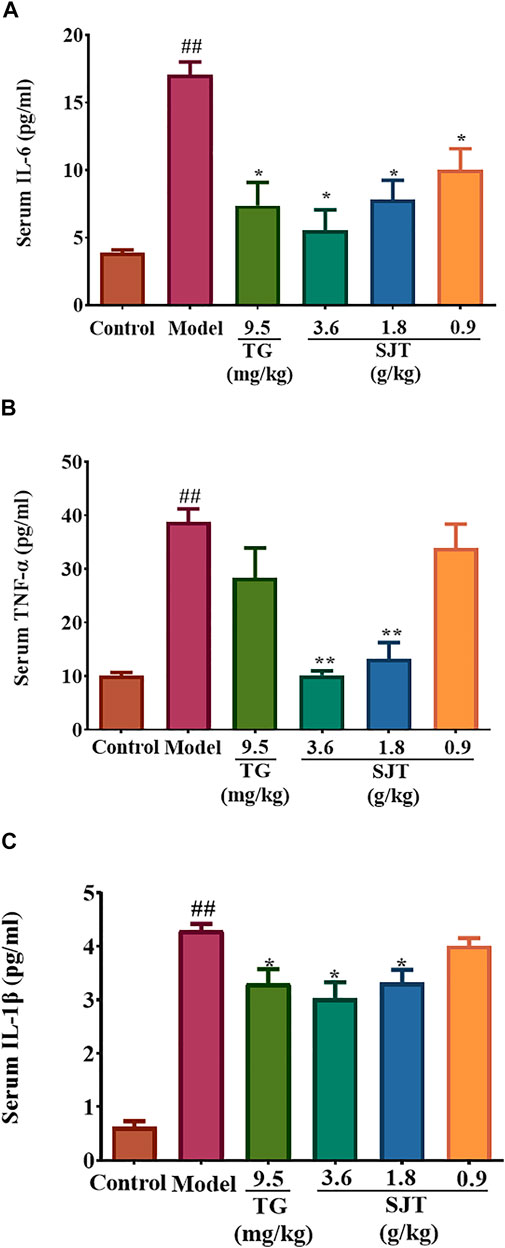
FIGURE 5. Effect of SJT on pro-inflammatory cytokines in the serum of AIA rats. The levels of IL-6 (A), TNF-α (B), and IL-1β (C) were determined by ELISA assay. Data were expressed as mean ± S.D. vs. model, *p < 0.05, **p < 0.01; vs. normal, #p < 0.05, ##p < 0.01.
SJT was composed of PL, LS, AS, AP, ST, SG, GU, SS, CS, AB, FP, TC, CC, TS. A total of 208 SJT active ingredients were obtained by searching the TCMSP database, among which the ingredients of TSM were collected by literature searching. All of the active ingredients conformed to the ADME standard (OB ≥ 30%, DL ≥ 0.18). The numbers of compounds from PL, LS, AS, AP, ST, SG, GU, SS, CS, AB, FP, TC, CC, TS were 13, 7, 2, 9, 3, 15, 92, 15, 10, 20, 1, 2, 7, 12, respectively. Based on the results, a total of 2,748 targets related to the bioactive components were picked out by searching the TCMSP database. Then, the Uniprot database was used to correct the target names. The numbers of targets for PL, LS, AS, AP, ST, SG, GU, SS, CS, AB, FP, TC, CC, TS were 95, 31, 52, 55, 31, 22, 1,257, 177, 48, 363, 13, 139, 54, 411, respectively. The reappeared targets were deleted, and a total of 445 targets was obtained finally. Bioactive compounds and related target information in each herb are shown in Supplementary Table S1.
A total of 4,465 genes related to RA disease were obtained by searching Genecards and OMIM database. According to the score of relevance, the top 30 genes were screen out. As shown in Table 1, the top 10 “high response” genes were IL6, HLA-DRB1, IL10, PTPN22, TNF, STAT4, MIF, CTLA4, PADI4, TNFRSF1A, and so on.
The SJT target genes were intersected with RA disease targets to obtain the potential targets of SJT in RA therapy. The result showed that 292 overlapped genes were identified by matching the target genes of SJT with the therapeutic target genes of RA (Figure 6 and Supplementary Table S2), suggesting that SJT probably performs the function of anti-RA via modulating the 292 genes. The numbers of overlapping targets of PL, LS, AS, AP, ST, SG, GU, SS, CS, AB, FP, TC, CC, TS were 49, 15, 30, 19,22, 4, 145, 43, 24, 131, 2,104, 16, 6, respectively. The distribution of overlapping genes in each herb is shown in Supplementary Table S3.
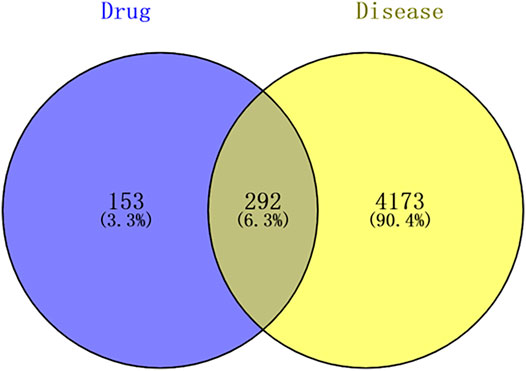
FIGURE 6. Venn diagram for the predicted targets of SJT against RA. The 292 candidate targets of SJT in RA therapy were predicted by integrating the 445 drug-target genes of SJT and 4,465 disease targets of RA.
Cytoscape 3.7.2 software was employed to build up the “drug-component-target-disease” network. The potential active components and overlapped targets of SJT and RA were input into the system, and the “SJT-component-target-RA” network was constructed by connecting to the predicted targets. The results are shown in Figure 7.
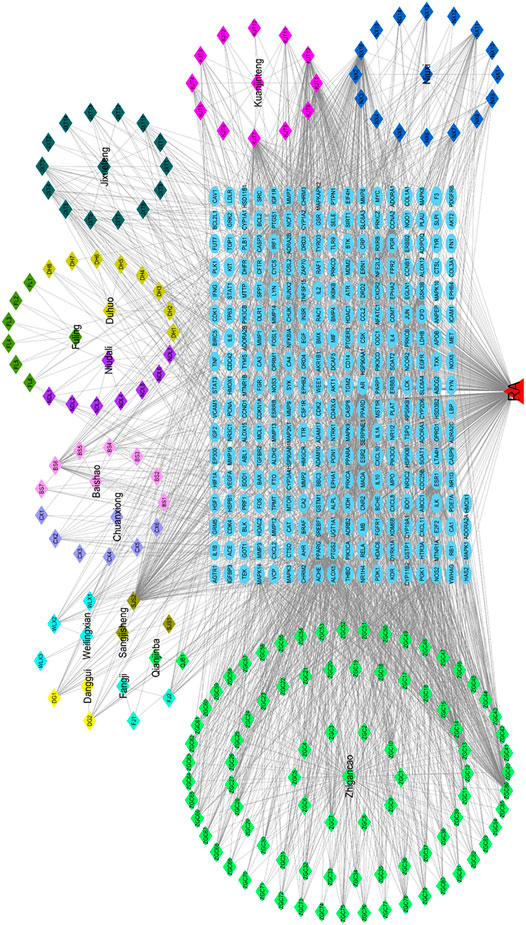
FIGURE 7. Drug-active ingredient-target network diagram. The polygon with different colors represented herbs and corresponding ingredients in the prescription of Shuji tablet. The blue ovals in the middle were the 292 collective target genes.
Basing on the overlapped targets of SJT and RA, the protein-protein interaction (PPI) network was established by the String database (http://www.string-db.org). As shown in Figure 8A, there were 292 nodes and 467 edges in the network diagram and the average node degree was 3.2. The top 30 core genes were selected out, which had a node degree greater than 9 (Figure 8B). The results showed that key targets probably were STAT3, AKT1, JUN, HSP90AA1, TNF, which possessed more connections than other genes.
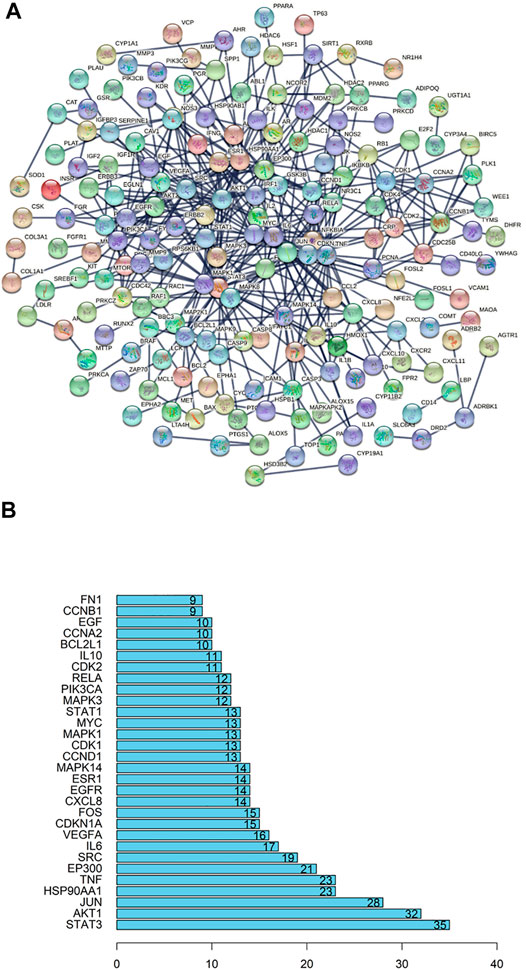
FIGURE 8. PPI (protein-protein interaction) network. (A) The collective genes were screened out and combined for constructing a PPI network. The nodes represented targets, and the connected lines represented interactions between different target proteins. (B) Bar chart for numbers of target proteins connecting with other target proteins. Data were showing the top 30 targets harboring the most quantity of connections with other targets.
The David v 6.8 Database (https://david.ncifcrf.gov) was applied for GO and KEGG enrichment analysis, aiming to understand the biological process (BP), cellular component (CC), molecular function (MF), and potential signaling pathways, which were involved in the anti-RA function of SJT. As a result, the numbers of BP, MF, and CC of SJT against RA were 3,053, 210, and 115 (Supplementary Table S4), respectively, and the top 20 GO analysis of BP (Figure 9A), CC (Figure 9B), MF (Figure 9C) were represented as graphical bubbles. Furthermore, 45 signaling pathways were identified through KEGG pathway enrichment analysis (Supplementary Table S5), and the top 20 signaling pathways were represented as a bar graph (Figure 9D), among which, PI3K-Akt pathway, MAPK pathway, Ras pathway, HIF-1 pathway, TNF pathway, Chemokine pathway, IL-17 pathway, FoxO pathway, Rap1 pathway, AGE-RAGE signaling pathway in diabetic complications had been proved associated with the treatment of RA.
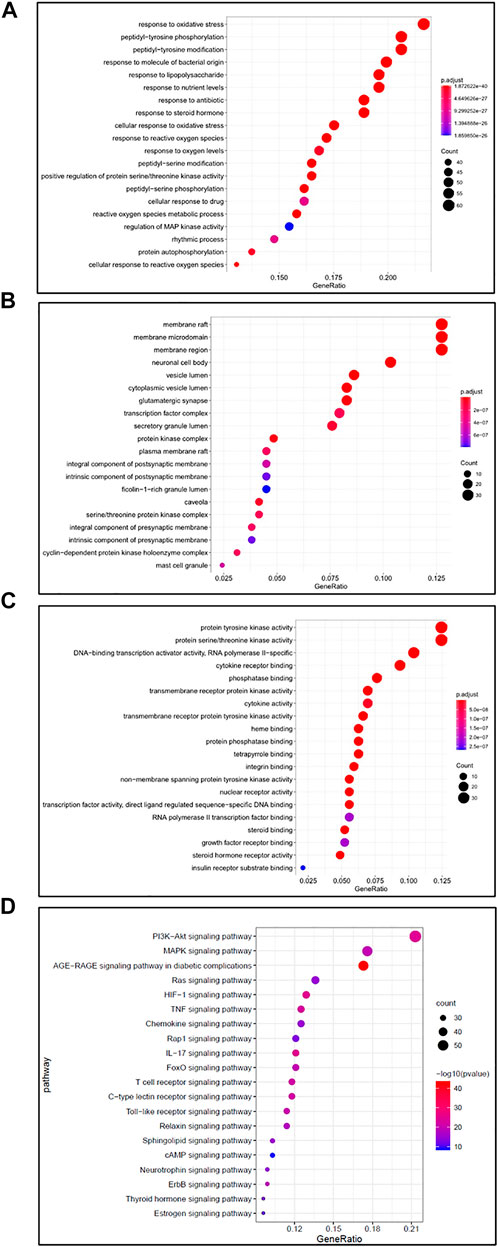
FIGURE 9. GO and KEGG pathway enrichment analysis. (A–C) Dot plot showed the GO analysis for SJT in anti-RA, including (A) biological process (BP), (B) cellular component (CC), (C) molecular function (MF). (D) KEGG pathway enrichment analysis for SJT in anti-RA. Dot plot showed the top 20 signaling pathways related to SJT in RA treatment.
RA is a kind of chronic disease lasting for a long course, and there is no drug used in the clinic that can cure RA successfully. RA patients would not only experience arthrophlogosis such as joints swelling and stiffness, but also other diseases with extraarticular symptoms or systemic manifestations, such as rheumatoid nodules, vasculitis, cardiovascular disease, and so on (Hochberg et al., 2008). Early diagnosis and early treatment are the key strategies to improve the outcome of RA therapy. However, patients mostly are diagnosed with RA in the advanced phase, so they miss the best chance for therapy. RA is a systemic disease owing to the feature of disordered multiple-biological processes, -signaling pathways, and -target proteins. Hence, a remedy with comprehensive treatment is demanded by targeting multiple biological processes, -mechanisms, and -targets. It is a good strategy to develop a new therapeutic method basing on traditional remedies or ethnological medicine to overcome RA disease. Furthermore, ameliorating systemic manifestations by inhibiting inflammation response can reduce the mortality of RA, and is recognized to be a common therapeutic approach in the clinic.
Traditional Chinese medicine (TCM) possesses a long history of application and shows good potency of RA therapy in the clinic. Precious studies showed that several traditional Chinese herbal medicines such as Wangbi tablet (Wang et al., 2020) and Wutou decoction (Guo et al., 2017) had a good effect in treating RA. The prescriptions were discovered to modulate the imbalance of the whole body and ameliorate local symptoms accordingly. Especially, Qing-Luo-Yin not only shows an anti-rheumatic effect but also antagonizes the toxicity and side effect induced by methotrexate (Zuo et al., 2018). SJT, a traditional Chinese medicine prescription, was approved using in the clinic for treating lumbago and leg pain. SJT was also found to alleviate the symptom of RA patients during clinical practice. However, the experimental data against RA disease is still limited. In this study, we evaluated the anti-RA effect of SJT on AIA rats, and the results showed that SJT could dose-dependently and time-dependently ameliorate arthritis symptoms, whole-body inflammation response, and also reduce the levels of serum cytokines relating with pro-inflammation, all of which were consistent with what we have observed in clinical practice. The results of UHPLC-QTOF-MS showed that Betaine, Albflorin, D-Tetrandrine, Ononin, Bergapten, Ligustilide, 3-butyl-1(3H)-Isobenzofuranone, 3-butylidene-1(3H)-Isobenzofuranone, Columbianetin, Levistilide A, Stigmasterol, Glycerin fatty acid ester, Dioctyl phthalate, Rutin, Liquiritin, Quercitrin, Glycyrrhizic acid were the major ingredients in SJT. Among them, D-Tetrandrine (Li et al., 2018), Ononin (Meng et al., 2021), Stigmasterol (Ahmad et al., 2020), Rutin (Sun et al., 2017), Liquiritin (Zhai et al., 2019) were reported to have an anti-RA function in previous research, which suggested that SJT had the potential property of treating RA.
With the rapid development of bioinformatics technology, network pharmacology is usually employed to speed up the progress of drug research and has become a promising approach for drug discovery and development, especially for TCM study due to its complicated ingredients. According to the new guidance, the network pharmacology evaluation should be conducted in three aspects: reliability, standardization, and rationality (Li et al., 2021). Actually, in our study, to ensure the reliability of TCM network pharmacology, all the data were collected from the TCMSP database, which recorded 499 common traditional Chinese medicines. A total of 208 active ingredients were obtained from 14 herbs of SJT. After that, the databases of PubChem and Uniprot were used to correct the compound names and molecular structures to make sure that the relevant data can be traced based on our description. Simultaneously, setting parameters of “DL ≥ 0.18 and OB ≥ 30%”, “The minimum required interaction score being 0.97”, and “hiding disconnected nodes in the network” to make sure that data were completed and consistent with our research objective. The top five herbs which harbored the most quantity of ingredients were PL, SG, GU, AB, TS, with compound numbers of 13, 15, 92, 20, 12, respectively. However, the distribution of overlapping genes in each herb showed a different result. PL, GU, SS, AB, TC were the top five herbs possessing the most quantity of overlapping genes, with gene numbers of 49, 145, 43, 131, 104, respectively. Probably, the reason was that some compounds from the herbs which contained few ingredients would have more targets associated with RA therapy. For instance, TC had only two active compounds but 104 genes related to RA.
KEGG pathway enrichment analysis showed that there were 45 signaling pathways directly linked to RA, indicating that SJT might modulate these signaling pathways against RA. The top 10 signaling pathways firmly related to RA therapy were PI3K-Akt pathway, MAPK pathway, AGE-RAGE signaling pathway in diabetic complications, Ras pathway, HIF-1 pathway, TNF pathway, Chemokine pathway, IL-17 pathway, FoxO pathway, Rap1 pathway. Most of these signaling pathways had been proved associated with the occurrence and development of RA. PI3K-AKT signaling pathway is an intracellular signaling pathway that regulates the cell proliferation and cell cycle process and resists apoptosis, angiogenesis, autophagy (Johnson et al., 2010; Xia et al., 2015; Manfredi et al., 2015). Regulating the metabolism of chondrocytes via PI3K/AKT pathway would affect the disease progression in RA rats (Feng et al., 2018). Mitogen-activated protein kinase (MAPK) signaling is a fundamental pathway in cell biology and plays an important role in the pathophysiological process of human diseases (Yuan et al., 2020). Activation of p38 MAPK contributes to almost all RA-related pathologies, including synovial inflammation, damage of cartilage and bone, and angiogenesis (Schett et al., 2008). Inhibiting MAPK signaling pathways would reduce RA-associated ROS accumulation, leading to suppression of inflammation and cell proliferation of synovial cells, as well as mitigation of angiogenesis (Yang et al., 2018). Ras superfamily of GTPases plays an important role in the immune system of the human body (Zayoud et al., 2017). T cells highly expressing K-Ras would induce autoimmunity, which was mediated by citrullinated vimentin-derived peptide, a pathogenic autoantigen in RA (Weaver et al., 2007; Singh et al., 2009). Blocking the Ras signaling pathway had been proved to be a promising therapeutic approach for RA (Zayoud et al., 2017). Hypoxia is one of the major characteristics of RA synovium. Hypoxia-inducible factors (HIFs) are transcription factors that can promote glycolysis to produce energy via enhancing gene expressions of glucose transporters and glycolytic enzymes (Fearon et al., 2016). RA synovium cells within the hypoxic environment can survive by an adaptive mechanism mediated by the HIF-1 pathway (Harris et al., 2002). HIF-1ɑ enhances the catalytic activity of lactate dehydrogenase A (LDHA), resulting in the hyper-acidic microenvironment, which would promote synovium cell proliferation and invasion (Fearon et al., 2016). Overexpression of HIF-1ɑ increases the production of IFN-γ and IL-17 (Larsen et al., 2012), and enhances the effects of IL-1β and TNF on angiogenesis and invasion in RA (Li et al., 2013). Tumor necrosis factor (TNF) and its receptors, two kinds of transmembrane proteins of immune cells, play a key role in the inflammation response and the development of RA (Blüml et al., 2012). It is a kind of pleiotropic cytokine that enhances synovial proliferation, produces prostaglandins and metalloproteinases, as well as regulates other proinflammatory cytokines (Taylor et al., 2009). Hence, TNF is considered a useful therapeutic target for RA therapy. Agents targeting TNF have been proved to be effective in treating RA in the clinic (Taylor et al., 2009). Rheumatoid synovial fibroblasts secrete a lot of matrix-degrading metalloproteinases (MMPs), which lead to tissue damage by proteolytic degradation of collagens and proteoglycans. IL-17 can modulate MMP-1 and its inhibitor TIMP-1 to regulate the progression of RA (Chabaud et al., 2000). Cysteine-rich protein 61 (CYR-61) plays a key role in the pathogenesis of RA, and activation of Fox signaling would induce the secretion of CYR-61 in rheumatoid synovial fibroblasts (Kok et al., 2013). Therefore, targeting Fox signaling pathway would be also an important therapeutic regimen for RA disease. The chemokine signaling pathway is involved in the progression of RA by mediating leukocyte extravasation and is considered to be a good target pathway for RA therapy. As reported, blocking C-C motif chemokine receptor 1 (CCR 1) was proved to be a promising therapeutic approach against RA (Szekanecz et al., 2010). As a small G protein in the Ras superfamily, Ras-proximate-1 (Rap 1) plays an important role in modulating intracellular signaling pathways and stimulating T cells (Remans et al., 2006). Rap1 signaling regulates the production of reactive oxygen species (ROS) and is associated with oxidative stress of T cells infiltrated in the RA synovium (Remans et al., 2004). Advanced glycation end products (AGEs) are generated as a result of oxidative stress during chronic inflammation and sever as a measurement of cumulative inflammation in RA patients (de Groot et al., 2011).
As expected in our study, SJT reduced the levels of IL-1β, IL-6, and TNF-α in the serum of AIA rats, indicating SJT could suppress RA by modulating the inflammation-related pathways, which were also predicted in the follow-up study by network pharmacological approach. However, the detailed modulated mechanism of SJT against RA needs further investigation, including the modulation on more detailed inflammatory pathways, metabolism pathways, and immune pathways related to RA. Furthermore, the network pharmacology analysis should be done by more a rational and normative process, and the parameters of accuracy, precision, recall should be considered sufficiently. Employing a professional platform such as the integrative TCM network pharmacology platform (Zhang et al., 2013) is necessary to ensure all data can be traced easily.
Our findings demonstrated that SJT strongly alleviated rheumatoid arthritis and reduced the secretion of IL-6, IL-1β, TNF-α of AIA rats. Network pharmacological analysis showed that the key targets of SJT in the treating RA probably were STAT3, AKT1, JUN, HSP90AA1, TNF, and the potential mechanism would be associated with modulation on PI3K-Akt, MAPK, Ras, AGE-RAGE, HIF-1, TNF, chemokine, IL-17, FoxO, Rap 1 signaling pathways. Our study provides evidence for Shuji tablet in RA therapy and would expand the usage of Shuji tablet in the clinic.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation, to any qualified researcher.
The animal study was reviewed and approved by the Ethics Committee of Guangzhou University of Chinese Medicine.
XH and CZ contributed to the design, concepts of the whole study, and revision of the manuscript. WD, JY, HC and ZW performed the study and drafted the manuscript. GL, XZ, and WP helped with data acquisition and analysis. CC and XL helped with network pharmacological analysis. All authors have read and approved the final version of the manuscript.
This research was financially supported by the National Natural Science Foundation of China (81503303), and Natural Science Foundation of Guangdong Province of China (2015A030310226, 2016A030313825), the Special Project for Medical-Health of Science and Technology Plan of Zhongshan, Guangdong, China (2017B1082), and Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (20182165).
Author HC was employed by the company Guangzhou Xiangxue Pharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.694507/full#supplementary-material
Supplementary Table S1 | Compounds from the herbals of Shuji tablet (SJT) based on the network approach.
Supplementary Table S2 | The overlapping genes of Shuji tablet (SJT) and rheumatoid arthritis (RA).
Supplementary Table S3 | The distribution of overlapping genes in each herb.
Supplementary Table S4 | GO analysis for biological process (BP), molecular function (MF), cellular component (CC).
Supplementary Table S5 | Signaling pathways identified via KEGG pathway enrichment analysis.
Ahmad Khan, M., Sarwar, A. H. M. G., Rahat, R., Ahmed, R. S., and Umar, S. (2020). Stigmasterol Protects Rats from Collagen Induced Arthritis by Inhibiting Proinflammatory Cytokines. Int. Immunopharmacol 85, 106642. doi:10.1016/j.intimp.2020.106642
Ao, Y. W., Xiao, Y., Xu, T., Wang, X. Y., Lu, L., and Wei, C. (2020). Study on the Mechanism of Action of "Sangzhi-Guizhi" on Rheumatoid Arthritis Based on Network Pharmacology. J. Hainan Med. Univ. doi:10.13210/j.cnki.jhmu.20200609.002
Bao, Y. K., Weide, L. G., Ganesan, V. C., Jakhar, I., McGill, J. B., Sahil, S., et al. (2019). High Prevalence of Comorbid Autoimmune Diseases in Adults with Type 1 Diabetes from the HealthFacts Database. J. Diabetes 11 (4), 273–279. doi:10.1111/1753-0407.12856
Blüml, S., Scheinecker, C., Smolen, J. S., and Redlich, K. (2012). Targeting TNF Receptors in Rheumatoid Arthritis. Int. Immunol. 24 (5), 275–281. doi:10.1093/intimm/dxs047
Burmester, G. R., and Pope, J. E. (2017). Novel Treatment Strategies in Rheumatoid Arthritis. The Lancet 389 (10086), 2338–2348. doi:10.1016/S0140-6736(17)31491-5
Cao, H. L., Zeng, C. Y., Dai, W. B., and Mei, Q. X. (2019a). Research Progress on Chemical Constituents and Pharmacological Effects of Millettia Spliueciosa Champ. Guiding J. Tradit. Chin. Med. Pharm. 11, 135–137+141. doi:10.13862/j.cnki.cn43-1446/r.2019.11.038
Cao, H. L., Zeng, C. Y., Hu, Y., Dai, W. B., Gao, Y. Q., and Zheng, Y. L. (2019). Experimental Study on the Anti-inflammatory and Analgesic Effects of Shuji Tablet. Lishizhen Med. Mater. Med. Res. 11, 2579–2581.
Chabaud, M., Garnero, P., Dayer, J.-M., Guerne, P.-A., Fossiez, F., and Miossec, P. (2000). Contribution of Interleukin 17 to Synovium Matrix Destruction in Rheumatoid Arthritis. Cytokine 12 (7), 1092–1099. doi:10.1006/cyto.2000.0681
Chatzidionysiou, K., Emamikia, S., Nam, J., Ramiro, S., Smolen, J., van der Heijde, D., et al. (2017). Efficacy of Glucocorticoids, Conventional and Targeted Synthetic Disease-Modifying Antirheumatic Drugs: a Systematic Literature Review Informing the 2016 Update of the EULAR Recommendations for the Management of Rheumatoid Arthritis. Ann. Rheum. Dis. 76 (6), 1102–1107. doi:10.1136/annrheumdis-2016-210711
de Groot, L., Hinkema, H., Westra, J., Smit, A. J., Kallenberg, C. G., Bijl, M., et al. (2011). Advanced Glycation Endproducts Are Increased in Rheumatoid Arthritis Patients with Controlled Disease. Arthritis Res. Ther. 13 (6), R205. doi:10.1186/ar3538
Fearon, U., Canavan, M., Biniecka, M., and Veale, D. J. (2016). Hypoxia, Mitochondrial Dysfunction and Synovial Invasiveness in Rheumatoid Arthritis. Nat. Rev. Rheumatol. 12 (7), 385–397. doi:10.1038/nrrheum.2016.69
Feng, F.-B., and Qiu, H.-Y. (2018). Effects of Artesunate on Chondrocyte Proliferation, Apoptosis and Autophagy through the PI3K/AKT/mTOR Signaling Pathway in Rat Models with Rheumatoid Arthritis. Biomed. Pharmacother. 102, 1209–1220. doi:10.1016/j.biopha.2018.03.142
Feng, F., Ding, Z, J., and Liu, J. (2004). Experimental Study on Adjuvant Arthritis Rat Model. Tianjin Pharm. 02, 1–4.
Firestein, G. S., and McInnes, I. B. (2017). Immunopathogenesis of Rheumatoid Arthritis. Immunity 46 (2), 183–196. doi:10.1016/j.immuni.2017.02.006
Guo, Q., Zheng, K., Fan, D., Zhao, Y., Li, L., Bian, Y., et al. (2017). Wu-tou Decoction in Rheumatoid Arthritis: Integrating Network Pharmacology and In Vivo Pharmacological Evaluation. Front. Pharmacol. 8, 230. doi:10.3389/fphar.2017.00230
Harris, A. L. (2002). Hypoxia - a Key Regulatory Factor in Tumour Growth. Nat. Rev. Cancer 2 (1), 38–47. doi:10.1038/nrc704
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best Practice in Research - Overcoming Common Challenges in Phytopharmacological Research. J. Ethnopharmacology 246, 112230. doi:10.1016/j.jep.2019.112230
Hochberg, M. C., Johnston, S. S., and John, A. K. (2008). The Incidence and Prevalence of Extra-articular and Systemic Manifestations in a Cohort of Newly-Diagnosed Patients with Rheumatoid Arthritis between 1999 and 2006. Curr. Med. Res. Opin. 24 (2), 469–480. doi:10.1185/030079908x261177
Johnson, S. M., Gulhati, P., Rampy, B. A., Han, Y., Rychahou, P. G., Doan, H. Q., et al. (2010). Novel Expression Patterns of PI3K/Akt/mTOR Signaling Pathway Components in Colorectal Cancer. J. Am. Coll. Surgeons 210 (5), 767–776. doi:10.1016/j.jamcollsurg.2009.12.008
King, D., Yeomanson, D., and Bryant, H. E. (2015). PI3King the Lock. J. Pediatr. Hematol. Oncol. 37 (4), 245–251. doi:10.1097/MPH.0000000000000329
Kok, S.-H., Lin, L.-D., Hou, K.-L., Hong, C.-Y., Chang, C.-C., Hsiao, M., et al. (2013). Simvastatin Inhibits Cysteine-Rich Protein 61 Expression in Rheumatoid Arthritis Synovial Fibroblasts through the Regulation of sirtuin-1/FoxO3a Signaling. Arthritis Rheum. 65 (3), 639–649. doi:10.1002/art.37807
Larsen, H., Muz, B., Khong, T. L., Feldmann, M., and Paleolog, E. M. (2012). Differential Effects of Th1 versus Th2 Cytokines in Combination with Hypoxia on HIFs and Angiogenesis in RA. Arthritis Res. Ther. 14 (4), R180. doi:10.1186/ar3934
Lemmey, A. B. (2016). Rheumatoid Cachexia: the Undiagnosed, Untreated Key to Restoring Physical Function in Rheumatoid Arthritis Patients?. Rheumatology 55 (7), 1149–1150. doi:10.1093/rheumatology/kev412
Li, G., Zhang, Y., Qian, Y., Zhang, H., Guo, S., Sunagawa, M., et al. (2013). Interleukin-17A Promotes Rheumatoid Arthritis Synoviocytes Migration and Invasion under Hypoxia by Increasing MMP2 and MMP9 Expression through NF-Κb/hif-1α Pathway. Mol. Immunol. 53 (3), 227–236. doi:10.1016/j.molimm.2012.08.018
Li, S., Chen, Y, T., Ding, Q, Y., Dai, J, Y., Duan, X, C., Hu, Y, J., et al. (2021). Network Pharmacology Evaluation Method Guidance - Draft. World J. Tradit Chin. Med. 7 (1), 146–154. doi:10.4103/wjtcm.wjtcm_11_21
Li, S., and Zhang, B. (2013). Traditional Chinese Medicine Network Pharmacology: Theory, Methodology and Application. Chin. J. Nat. Medicines 11 (2), 110–120. doi:10.1016/S1875-5364(13)60037-0
Li, X., Wu, Z., He, B., and Zhong, W. (2018). Tetrandrine Alleviates Symptoms of Rheumatoid Arthritis in Rats by Regulating the Expression of Cyclooxygenase-2 and I-nflammatory F-actors. Exp. Ther. Med. 16 (3), 2670–2676. doi:10.3892/etm.2018.6498
Malemud, C. J. (2018). The Role of the JAK/STAT Signal Pathway in Rheumatoid Arthritis. Ther. Adv. Musculoskelet. 10 (5-6), 117–127. doi:10.1177/1759720X18776224
Manfredi, G. I., Dicitore, A., Gaudenzi, G., Caraglia, M., Persani, L., and Vitale, G. (2015). PI3K/Akt/mTOR Signaling in Medullary Thyroid Cancer: a Promising Molecular Target for Cancer Therapy. Endocrine 48 (2), 363–370. doi:10.1007/s12020-014-0380-1
Meng, Y., Ji, J., Xiao, X., Li, M., Niu, S., He, Y., et al. (2021). Ononin Induces Cell Apoptosis and Reduces Inflammation in Rheumatoid Arthritis Fibroblast-like Synoviocytes by Alleviating MAPK and NF-Κb Signaling Pathways. Acta Biochim. Pol. 68 (2), 239–245. doi:10.18388/abp.2020_5528
Pan, T., Cheng, T.-f., Jia, Y.-r., Li, P., and Li, F. (2017). Anti-rheumatoid Arthritis Effects of Traditional Chinese Herb Couple in Adjuvant-Induced Arthritis in Rats. J. Ethnopharmacology 205, 1–7. doi:10.1016/j.jep.2017.04.020
Remans, P. H. J., Gringhuis, S. I., van Laar, J. M., Sanders, M. E., Papendrecht-van der Voort, E. A. M., Zwartkruis, F. J. T., et al. (2004). Rap1 Signaling Is Required for Suppression of Ras-Generated Reactive Oxygen Species and protection against Oxidative Stress in T Lymphocytes. J. Immunol. 173 (2), 920–931. doi:10.4049/jimmunol.173.2.920
Remans, P. H. J., Wijbrandts, C. A., Sanders, M. E., Toes, R. E., Breedveld, F. C., Tak, P. P., et al. (2006). CTLA-4IG Suppresses Reactive Oxygen Species by Preventing Synovial Adherent Cell-Induced Inactivation of Rap1, a Ras Family GTPASE Mediator of Oxidative Stress in Rheumatoid Arthritis T Cells. Arthritis Rheum. 54 (10), 3135–3143. doi:10.1002/art.22139
Scherer, H. U., Häupl, T., and Burmester, G. R. (2020). The Etiology of Rheumatoid Arthritis. J. Autoimmun. 110, 102400. doi:10.1016/j.jaut.2019.102400
Schett, G., Zwerina, J., and Firestein, G. (2008). The P38 Mitogen-Activated Protein Kinase (MAPK) Pathway in Rheumatoid Arthritis. Ann. Rheum. Dis. 67 (7), 909–916. doi:10.1136/ard.2007.074278
Singh, K., Deshpande, P., Pryshchep, S., Colmegna, I., Liarski, V., Weyand, C. M., et al. (2009). ERK-dependent T Cell Receptor Threshold Calibration in Rheumatoid Arthritis. J. Immunol. 183 (12), 8258–8267. doi:10.4049/jimmunol.0901784
State Pharmacopoeia Commission, (2020). “Hot Dip Process” in Chinese Pharmacopoeia. Beijing, China: China Medical Science and Technology Press), 232.
Summers, G. D., Deighton, C. M., Rennie, M. J., and Booth, A. H. (2008). Rheumatoid Cachexia: a Clinical Perspective. Rheumatology 47 (8), 1124–1131. doi:10.1093/rheumatology/ken146
Sun, C.-l., Wei, J., and Bi, L.-q. (2017). Rutin Attenuates Oxidative Stress and Proinflammatory Cytokine Level in Adjuvant Induced Rheumatoid Arthritis via Inhibition of NF-Κb. Pharmacology 100 (1-2), 40–49. doi:10.1159/000451027
Szekanecz, Z., Vegvari, A., Szabo, Z., and Koch, A. E. (2010). Chemokines and Chemokine Receptors in Arthritis. Front. Biosci. S2, 153–167. doi:10.2741/s53
Taylor, P. C., and Feldmann, M. (2009). Anti-TNF Biologic Agents: Still the Therapy of Choice for Rheumatoid Arthritis. Nat. Rev. Rheumatol. 5 (10), 578–582. doi:10.1038/nrrheum.2009.181
Thalhamer, T., McGrath, M. A., and Harnett, M. M. (2008). MAPKs and Their Relevance to Arthritis and Inflammation. Rheumatology 47 (4), 409–414. doi:10.1093/rheumatology/kem297
Tuncer, T., Gilgil, E., Kaçar, C., Kurtaiş, Y., Kutlay, Ş., Bütün, B., et al. (2018). Prevalence of Rheumatoid Arthritis and Spondyloarthritis in Turkey: a Nationwide Study. Arch. Rheumatol. 33 (2), 128–136. doi:10.5606/ArchRheumatol.2018.6480
van Walsem, A., Pandhi, S., Nixon, R. M., Guyot, P., Karabis, A., and Moore, R. A. (2015). Relative Benefit-Risk Comparing Diclofenac to Other Traditional Non-steroidal Anti-inflammatory Drugs and Cyclooxygenase-2 Inhibitors in Patients with Osteoarthritis or Rheumatoid Arthritis: a Network Meta-Analysis. Arthritis Res. Ther. 17 (1), 66. doi:10.1186/s13075-015-0554-0
Wang, D., Chang, Y., Wu, Y., Zhang, L., Yan, S., Xie, G., et al. (2011). Therapeutic Effects of TACI-Ig on Rat with Adjuvant Arthritis. Clin. Exp. Immunol. 163 (2), 225–234. doi:10.1111/j.1365-2249.2010.04293.x
Wang, J., Luo, J., Xu, Y., Yan, Z., Qu, X., Chen, M., et al. (2020). “Wang-Bi Tablet, a Patented Chinese Medicine, Maintains the Balance of Th1/Th2 in Mice with Collagen-Induced Arthritis”. J. Tradit. Chin. Med. 40 (3), 401–406. doi:10.19852/j.cnki.jtcm.2020.03.006
Wang, X., Shen, Y., Wang, S., Li, S., Zhang, W., Liu, X., et al. (2017). PharmMapper 2017 Update: a Web Server for Potential Drug Target Identification with a Comprehensive Target Pharmacophore Database. Nucleic Acids Res. 45 (W1), W356–W360. doi:10.1093/nar/gkx374
Weaver, C. T., Hatton, R. D., Mangan, P. R., and Harrington, L. E. (2007). IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu. Rev. Immunol. 25, 821–852. doi:10.1146/annurev.immunol.25.022106.141557
Wu, F. R., Zeng, C. Y., Dai, W. B., Gao, Y. Q., Hu, Y., and Lin, H. (2016). Experimental Study on Analgesic and Anti-inflammatory Effects of Aqueous Extract of Tinospora Sinensi. Chin. Arch. Tradit. Chin. Med. 07, 37–39+47. doi:10.13862/j.cnki.cn43-1446/r.2018.07.013
Wu, X. Y., and Zhang, G. R. (2020). The Effect of Tetrandrine on Human Synovial Cell Inflammation and its Therapeutic Effect on Rheumatoid Arthritis through NF-Kappa B and MAPK Signaling Pathway. J. Guangxi Med. Univ. (09), 1636–1641. doi:10.16190/j.cnki.45-1211/r.2020.09.009
Xia, P., and Xu, X. Y. (2015). PI3K/Akt/mTOR Signaling Pathway in Cancer Stem Cells: from Basic Research to Clinical Application. Am. J. Cancer Res. 5 (5), 1602–1609.
Yang, G., Chang, C.-C., Yang, Y., Yuan, L., Xu, L., Ho, C.-T., et al. (2018). Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 66 (49), 12953–12960. doi:10.1021/acs.jafc.8b05047
Yuan, J., Dong, X., Yap, J., and Hu, J. (2020). The MAPK and AMPK Signalings: Interplay and Implication in Targeted Cancer Therapy. J. Hematol. Oncol. 13 (1), 113. doi:10.1186/s13045-020-00949-4
Zayoud, M., Marcu-Malina, V., Vax, E., Jacob-Hirsch, J., Elad-Sfadia, G., Barshack, I., et al. (2017). Ras Signaling Inhibitors Attenuate Disease in Adjuvant-Induced Arthritis via Targeting Pathogenic Antigen-specific Th17-type Cells. Front. Immunol. 8, 799. doi:10.3389/fimmu.2017.00799
Zeng, C. Y., and Chen, Y. L. (2016). Identification of Spatholobus Suberectus in Shuji Tablet by TLC. J. North. Pharm. 12, 2–3.
Zhai, K.-f., Duan, H., Cui, C.-y., Cao, Y.-y., Si, J.-l., Yang, H.-j., et al. (2019). Liquiritin from Glycyrrhiza Uralensis Attenuating Rheumatoid Arthritis via Reducing Inflammation, Suppressing Angiogenesis, and Inhibiting MAPK Signaling Pathway. J. Agric. Food Chem. 67 (10), 2856–2864. doi:10.1021/acs.jafc.9b00185
Zhang, B., Wang, X., and Li, S. (2013). An Integrative Platform of TCM Network Pharmacology and its Application on a Herbal Formula,Qing-Luo-Yin. Evidence-Based Complement. Altern. Med. 2013, 1–12. doi:10.1155/2013/456747
Zhang, H. T., Du, X., Guo, D. D., Tang, P., and Zhao, H. Y. (2020). Research Progress on Pharmacodynamics and Mechanism of Duhuo Jisheng Decoction in Treating Rheumatoid Arthritis. Acta Chin. Med. Pharm. (09), 77–80. doi:10.19664/j.cnki.1002-2392.200167
Zhang, L. L., Chen, Y., Chen, J. Y., Wang, Q. T., Wu, H. X., and Wei, W. (2008). Effect of Paeoniflorin on the Expression of Ras and Raf-1 in Synoviocytes of Rats with Collagen Induced Arthritis. Chin. J. New Drugs 05, 375–379.
Zhou, Z., Chen, B., Chen, S., Lin, M., Chen, Y., Jin, S., et al. (2020). Applications of Network Pharmacology in Traditional Chinese Medicine Research. Evidence-Based Complement. Altern. Med. 2020, 1–7. doi:10.1155/2020/1646905
Keywords: Shuji tablet, rheumatoid arthritis, network pharmacology, traditional Chinese medicine, adjuvant-induced arthritis, mechanism
Citation: Dai W, Yang J, Cao H, Wang Z, Li G, Zhong X, Peng W, Chen C, Liu X, Zeng C and Hu X (2021) Clinical Evidence-Guided Anti-rheumatoid Arthritis Study of Shuji Tablet in Adjuvant-Induced Arthritis Rats and Mechanism Exploration via Network Pharmacological Approach. Front. Pharmacol. 12:694507. doi: 10.3389/fphar.2021.694507
Received: 13 April 2021; Accepted: 16 July 2021;
Published: 29 July 2021.
Edited by:
Shao Li, Tsinghua University, ChinaCopyright © 2021 Dai, Yang, Cao, Wang, Li, Zhong, Peng, Chen, Liu, Zeng and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Congyan Zeng, enN6Y3lAMTI2LmNvbQ==; Xianjing Hu, aHV4ajIwMDNAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.