- 1Section of Neurosurgery, Department of Surgery, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
- 2Department of Human Anatomy and Cell Science, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
- 3Biomedical Engineering, Faculty of Engineering, University of Manitoba, Winnipeg, MB, Canada
- 4Centre on Aging, University of Manitoba, Winnipeg, MB, Canada
- 5Division of Anaesthesia, Department of Medicine, Addenbrooke’s Hospital, University of Cambridge, Cambridge, United Kingdom
Background: Disruption in cerebrovascular reactivity following traumatic brain injury (TBI) is a known phenomenon that may hold prognostic value and clinical relevance. Ultimately, improved knowledge of this process and more robust means of continuous assessment may lead to advances in precision medicine following TBI. One such method is transcranial Doppler (TCD), which has been employed to evaluate cerebrovascular reactivity following injury utilizing a continuous time-series approach.
Objective: The present study undertakes a scoping review of the literature on the association of continuous time-domain TCD based indices of cerebrovascular reactivity, with global functional outcomes, cerebral physiologic correlates, and imaging evidence of lesion change.
Design: Multiple databases were searched from inception to November 2020 for articles relevant to the association of continuous time-domain TCD based indices of cerebrovascular reactivity with global functional outcomes, cerebral physiologic correlates, and imaging evidence of lesion change.
Results: Thirty-six relevant articles were identified. There was significant evidence supporting an association with continuous time-domain TCD based indices and functional outcomes following TBI. Indices based on mean flow velocity, as measured by TCD, were most numerous while more recent studies point to systolic flow velocity-based indices encoding more prognostic utility. Physiologic parameters such as intracranial pressure, cerebral perfusion pressure, Carbon Dioxide (CO2) reactivity as well as more established indices of cerebrovascular reactivity have all been associated with these TCD based indices. The literature has been concentrated in a few centres and is further limited by the lack of multivariate analysis.
Conclusions: This systematic scoping review of the literature identifies that there is a substantial body of evidence that cerebrovascular reactivity as measured by time-domain TCD based indices have prognostic utility following TBI. Indices based on mean flow velocities have the largest body of literature for their support. However, recent studies indicate that indices based on systolic flow velocities may contain the most prognostic utility and more closely follow more established measures of cerebrovascular reactivity. To a lesser extent, the literature supports some associations between these indices and cerebral physiologic parameters. These indices provide a more complete picture of the patient’s physiome following TBI and may ultimately lead to personalized and precise clinical care. Further validation in multi-institution studies is required before these indices can be widely adopted clinically.
Introduction
The disruption of cerebral autoregulation (CA) following traumatic brain injury (TBI) has been known since the 1970s (Overgaard and Tweed, 1974; Cold and Jensen, 1978). At that time, evaluation of CA was cumbersome and involved perturbation of the patient’s blood pressure while low frequency measurement of cerebral blood flow (CBF) were obtained. This meant that assessment of dynamic changes in CA was limited and thus its utility in precision medicine limited.
Aaslid and colleagues first described transcranial doppler ultrasound (TCD) in 1982 as a non-invasive means of evaluating CBF through insonation of flow velocities (FV) in the basal arteries of the brain (Aaslid et al., 1982). Since then, the role of TCD in the management of TBI patients has grown substantially with widespread adoption in the neurocritical care setting. It has become one of the most commonly utilized methods for intracranial monitoring in the critically ill TBI patient, outside of ICP monitoring, and the most popular non-invasive cerebral monitoring modality for this population. While direct TCD measures, such as FV, have been measured for their association with secondary neurologic decline and global outcomes, derived TCD metrics have been developed to non-invasively estimate intracranial pressure (ICP), carbon dioxide (CO2) reactivity and even CA (Gomez et al., 2021). Unfortunately, early methods of evaluating CA using TCD, such as the Thigh Cuff Deflation Technique (TCDT) and Orthostatic Hypotension Test (OHT), were intermittent in nature since they still depended on induced changes in arterial blood pressure (ABP) (Aaslid, 1986; Aaslid et al., 1989; Steinmeier et al., 2002).
TCD was first described as a tool to continuously evaluate CA following TBI by Czosnyka and colleagues in 1996 (Czosnyka et al., 1996). In this study, they described a time-domain based mean flow index (Mx), a continuously updating Pearson correlation coefficient between the natural fluctuations in cerebral perfusion pressure (CPP), equal to the difference between ABP and ICP, and mean FV through the middle cerebral artery (MCA) as measured by TCD. This method used mean FV as a surrogate measure for CBF with CPP used as the driving force in order to continuously interrogate cerebrovascular reactivity. It should be noted that cerebrovascular reactivity and CA are not synonymous as vascular reactivity can occur outside the limits of autoregulation (Varsos et al., 2014). Being a correlation coefficient, Mx ranged from +1 to −1 with a more negative correlation being associated with intact cerebrovascular reactivity and a more positive coefficient being associated with disrupted reactivity. In their study of 82 moderate and severe TBI patients, they found a correlation between the state of cerebrovascular reactivity, as measured by Mx, and 6-month outcomes (Czosnyka et al., 1996). Of note, for metrics to be designated as a CA measure, it must have some pre-clinical validation it its ability to measure aspects of the Lassen autoregulatory curve. To date, TCD-based metrics have not received such validation. As such, such measures are referred to as cerebrovascular reactivity metrics, as opposed to CA measures, as the provide surrogate assessments of cerebral vessel vasomotion, but have yet to be validated as CA measures. Subsequently, through the remainder of this article, such TCD-based measures will be referred to as cerebrovascular reactivity metrics.
Since then, a significant amount of research has been undertaken to examine the association between continuous TCD based indices of cerebrovascular reactivity and outcomes following TBI (It should be noted that cerebrovascular reactivity and CA are not entirely interchangeable as cerebrovascular reactivity can occur outside the limits of autoregulation. Cerebrovascular reactivity is the broader term that describes the physiologic process that is measured by these indices). Slightly modified indices that used the diastolic and systolic FV were examined (Dx and Sx, respectively) along with indices that utilized ABP, as opposed to CPP, as the driving force (Mx_a, Dx_a, and Sx_a). Table 1 summarizes these indices and their derivation. Notably, those that utilize ABP instead of CPP open the door to the entirely non-invasive measurement of cerebrovascular reactivity (Zeiler et al., 2018b; Zeiler et al.,2019b; Zeiler and Smielewski, 2018; Gomez et al., 2020). This has the potential to expand their application to the neurocritical care of patient populations that do not typically have ICP monitoring as well as to the outpatient setting. While not yet adopted widely in clinical practice, these indices have an ever-growing body of evidence supporting their association with outcomes following TBI. The development of these indices has renewed the intertest in leveraging measures of cerebrovascular reactivity in the development of personalized treatments following TBI. Further to this, adoption of this continuous non-invasive cerebrovascular reactivity assessment has expanded outside of TBI, including recent work in subarachnoid haemorrhage and general operative populations (Budohoski et al., 2012b; Klein et al., 2019). Thus, to aid with the development of further prospective studies in both TBI and non-TBI cohorts, a comprehensive understanding of the association between TCD based continuous time-domain cerebrovascular reactivity indices with patient-oriented outcomes is warranted. Given that the majority of the literature to date is focused in the TBI populations, the natural first step is to provide a comprehensive scoping overview of the association between TCD based cerebrovascular reactivity indices with: A. global patient outcomes, B. other cerebral physiologic correlates, and C. lesion change/progression on serial imaging. Thus, the aim of this study was to perform a systematically conducted review of the literature to evaluate the association between these continuous time-domain TCD based indices of cerebrovascular reactivity and the above outcomes, in adult moderate/severe TBI. In doing so, a better understanding of the role these indices play in describing the post-TBI physiome may be developed.

TABLE 1. Various transcranial Doppler based Indices of cerebrovascular reactivity with their component signals and derivation.
Methods
A systematically conducted scoping review of the available literature was conducted based on the methodological framework described by Arksey and O’Malley (2005). The data was reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR; Tricco et al., 2018). The search strategy and methodology used here is similar to other systematically conducted scoping reviews published by our group (Froese et al., 2020a, Froese et al.,2020b, Froese et al.,2020c; Hasen et al., 2020).
The review questions and search strategy were decided upon by the senior author (FAZ) and primary author (AG)
Search Questions, Populations, and Inclusion/Exclusion Criteria
The question of this systematically conducted scoping review was: What is the association of continuous time-domain TCD based indices of cerebrovascular reactivity, with: A. global functional outcomes, B. cerebral physiologic correlates, and C. imaging evidence of lesion change following moderate-to-severe TBI?
All English language studies, either prospective or retrospective, with 20 or more adult (age 18 years or older) moderate and severe TBI patients, were included. Moderate TBI is defined as an admission Glasgow coma scale (GCS) of 9–12 while a GCS of 3–8 defines severe TBI.
Only continuous time-domain TCD based cerebrovascular reactivity metrics were of interest, excluding both intermittent techniques (Zeiler et al., 2017a) and those based in frequency-domain (i.e., transfer-function techniques; Czosnyka et al., 2008). The eligible time-domain TCD based cerebrovascular reactivity indices of interest were Mx, Sx, Dx, Mx_a, Sx_a, and Dx_a. Table 1 outlines the components of these indices and their derivation.
The primary outcome of interest was the statistically significant association between these indices and morbidity/mortality following TBI. Secondary outcomes included statistically significant associations with severity of injury and age. Additionally, associations with various cerebral physiologic parameters such as ICP, CPP, brain tissue oxygenation (PbtO2), and CO2 reactivity were also examined. Give that the pressure reactivity index (PRx) has become a widely accepted continuous measures of cerebrovascular reactivity (Czosnyka et al., 1997; Zeiler et al., 2018d), its associations with TCD based indices were also collected. Associations between TCD based indices and more novel ICP based indices of cerebrovascular reactivity, such as PAx and RAC, were excluded as these indices are not as well established despite their evidence of their prognostic utility in TBI (Zeiler et al., 2017b). Table 2 outlines the cerebral physiologic parameters that were examined for their association with TCD based indices. All parameters selected as secondary outcomes are known to be associated with global outcomes and/or are physiologic targets in guideline based management following moderate to severe TBI (Carney et al., 2017; Zeiler et al., 2018d; Hawryluk et al., 2019).
Studies relating to TCD time-domain based cerebrovascular reactivity measures and imaging changes were searched for. Specifically, studies examining the association of these indices with changes in CT score (Marshall, Rotterdam, Helsinki, or Stockholm), midline shift, and hematoma volume as well as the development on new lesions were all considered relevant.
Exclusion criteria for studies were the following: non-English, non-human, non-TBI, mild TBI, paediatric cohorts, non-time-domain TCD based indices, non-continuous TCD metrics, cohort < 20 TBI patients, or no relevant outcome (functional or physiologic) association. Review studies and meta-analysis were also excluded from consideration.
Search Strategy
BIOSIS, Cochrane Library, EMBASE, MEDLINE, and SCOPUS were searched from inception to November 2020 using individualized search strategies for each database. The search strategy for SCOPUS can be seen in Supplementary Appendix A with similar strategies used for each of the other databases. Finally, the reference lists of each article were reviewed to ensure no studies were missed. Search results were then combined, and deduplication was performed.
Study Selections
Using two reviewers (AG and LF) a two-step review of all articles returned by our search strategies was performed. In the first filter phase, each reviewer independently screened all studies identified using the above-described search strategy and determined if they met the inclusion criteria based on their title and abstract. The resulting list of studies was then passed through the second filter phase where once again each reviewer independently determined if the studies met the inclusion criteria, but this time based on the full text. Any discrepancies between the two reviewers were resolved by a third party (FAZ).
Data Collection
Data was extracted from the selected articles and compiled into various data fields. These fields included the following: number of patients, study design, institution, mean age, mean GCS on presentation, number of male patients, additional patient characteristics, goals of the study, indices examined, duration of insonation, outcomes evaluated, key results, and conclusions.
Bias Assessment
Given the goal of this review was to provide a comprehensive scoping overview of the available literature, a formal bias assessment was not conducted.
Statistical Analysis
Due to the heterogeneity of the results/study design no meta-analysis was performed.
Results
Search Results and Study Characteristics
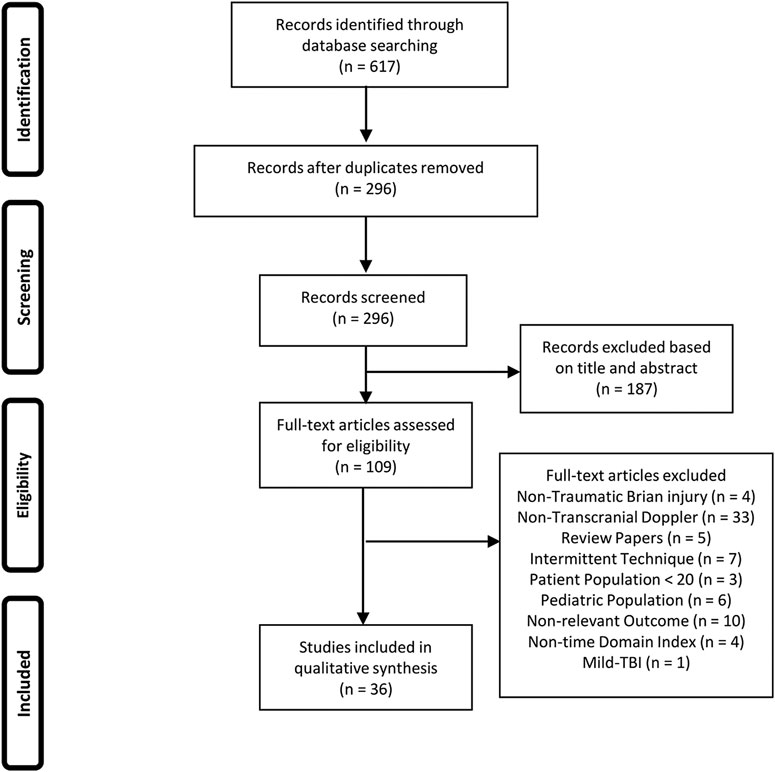
The results of the search and filtration strategy can be seen in Figure 1. Overall, the search strategy identified 617 articles with 296 remaining following deduplication. Following the first filtration stage, based on article title and abstract, 187 articles were removed for not fitting into the inclusion/exclusion criteria. Full text documents were reviewed on the remaining 109 articles in the second filtration phase with 73 articles being found to not meet the inclusion/exclusion criteria for this study. This left 36 articles to be included in this scoping review. Figure 1 provides the PRISMA flow-diagram.
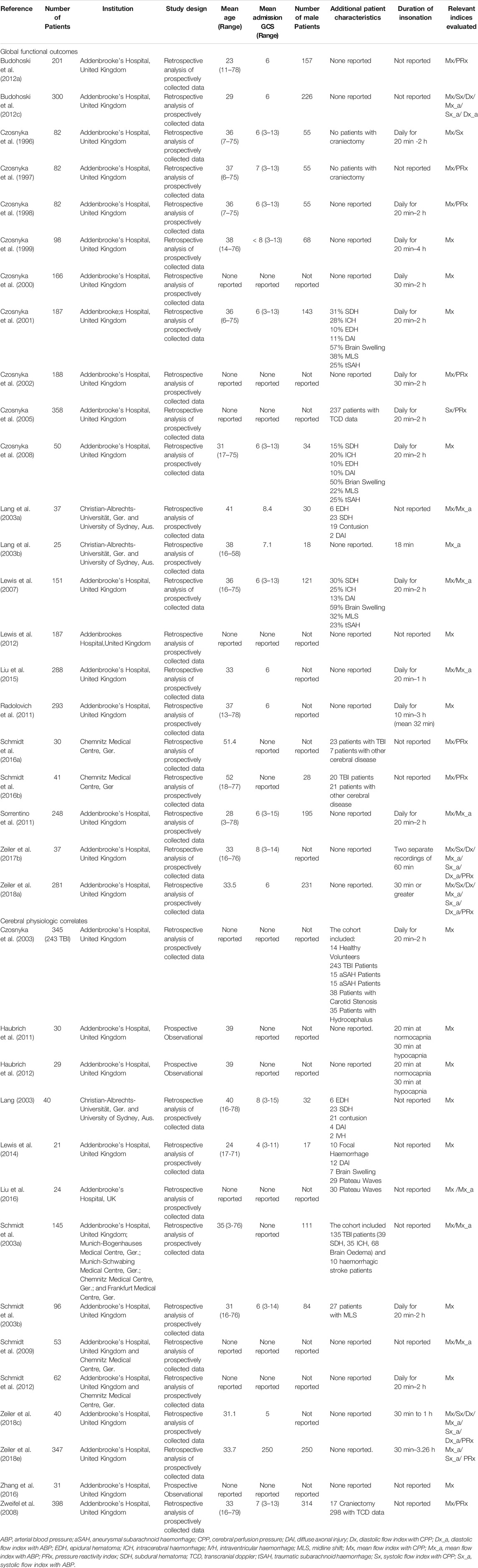
Table 3 summarizes general characteristics of each study while Table 4 outlines the key results, conclusions, and limitations of each study. Mx was the most common time-domain TCD index that was studied, with all but one study reporting on it (Czosnyka et al., 2005). It was also the only index with evidence showing that it worsened with injury severity (Czosnyka et al., 1996; Czosnyka et al., 1999; Czosnyka et al., 2000) and with advanced age (Czosnyka et al., 2005; Czosnyka et al., 2008). The next most common index was Mx_a with it being measure in nine studies (Lang et al., 2003a; Lang et al., 2003b; Lewis et al., 2007; Sorrentino et al., 2011; Budohoski et al., 2012c; Liu et al., 2015; Zeiler et al., 2017b; Zeiler et al., 2018a; Zeiler et al., 2018c). Notably, Mx, and Mx_a have been found to be strongly associated with one another (Schmidt et al., 2003a; Lewis et al., 2007; Sorrentino et al., 2011). The remainder of time-domain TCD based indices were examined only in a minority of studies. The number of patients with TCD recordings included in each study varied from as few as 20 TBI patients to as many as 347 (Schmidt et al., 2016b; Zeiler et al., 2018e). It should be noted that only five of the 36 articles did not report on data from patients at the Addenbrooke’s Hospital in Cambridge, United Kingdom with the largest of these studies only including 40 TBI patients (Lang, 2003; Lang et al., 2003a; Lang et al., 2003b; Schmidt et al., 2016a; Schmidt et al., 2016b).
Mortality
There is strong evidence of an association between dysfunctional cerebrovascular reactivity, as measured by continuous time-domain TCD based indices, and mortality following TBI, with numerous studies finding a higher Mx correlating with mortality (Czosnyka et al., 2002; Schmidt. et al., 2003b; Schmidt. et al., 2016a; Budohoski et al., 2012a; Budohoski et al., 2012c). A notable exceptions to this was a 2016 study by Schmidt and colleagues which contained a mixed cohort of 20 TBI patients and 21 non-TBI patients (Schmidt et al., 2016b).
The evidence for the association between other TCD based indices and mortality is not as clear with a 2018 study by Zeiler and colleagues having most comprehensively examined this relationship in a cohort of 281 TBI patients (Zeiler et al., 2018a). Sx and Sx_a were found to have the strongest associations with mortality for indices with CPP and ABP as inputs, respectively, while Mx and Mx_a were also found to have some association. Neither Dx nor Dx_a were found to have any correlation with mortality.
Global Functional Outcomes
Most studies chose to use Glasgow Outcome Scale (GOS) as their outcome metric of choice, while opting to dichotomize outcome into favourable/unfavourable or good/poor (with favourable typically denoted as GOS of 5 or above and unfavourable as GOS 4 or less). Follow up was usually collected at 6 months post-injury. Once again, Mx seems to have the strongest body of evidence supporting its association with outcomes with studies consistently finding that a higher Mx was associated with poor or unfavourable outcome at follow up (Czosnyka et al., 1996; Czosnyka et al., 1997; Czosnyka et al., 1999; Czosnyka et al., 2000; Czosnyka et al., 2001; Czosnyka et al., 2002; Czosnyka et al., 2008; Lang et al., 2003a; Lewis et al., 2007; Lewis et al., 2012; Radolovich et al., 2011; Sorrentino et al., 2011; Budohoski et al., 2012a; Budohoski et al., 2012c; Liu et al., 2015; Schmidt et al., 2016a). When Mx was found to not be associated with outcomes, it was often attributed to small sample sizes (Schmidt et al., 2016b; Zeiler et al., 2017b).
Once again, the evidence supporting other TCD based indices is not as prevalent. Mx_a was found to be associated with global functional outcomes in four different articles with higher values being associated with worse outcomes (Lang et al., 2003a; Sorrentino et al., 2011; Liu et al., 2015; Zeiler et al., 2018a). Dx and Dx_a have been more recently examined with Dx having a weak association with functional outcomes and Dx_a failing to demonstrate any predictive value. In the original 1996 study by Czosnyka and colleagues Sx was correlated with 6-month GOS (Czosnyka et al., 1996). More recently, Sx and Sx_a have been found to be the most strongly correlated with outcome of those indices with CPP and ABP as inputs, respectively, (Zeiler et al., 2018a). Of note, a 2005 study by Czosnyka and colleagues found that Sx was not associated with functional outcome when utilizing a multiple regression model including ICP and age (Czosnyka et al., 2005).
Thresholds
Thresholds values of indices, where outcomes or mortality significantly increases, was the focus of 4 articles. In a 2002 study by Czonyka and colleagues they found a threshold for Mx of 0.23, above which mortality went from 11 to 47% (Czosnyka et al., 2002). A similar threshold for mortality, Mx = 0.3, was found in a follow up study by Sorrentino and colleagues in 2011. In that same study, a threshold of 0.05 for Mx was found to be where functional outcomes most drastically worsened while Schmidt and colleagues found a threshold for unfavourable outcomes closer to that of the mortality threshold at Mx = 0.2 (Sorrentino et al., 2011; Schmidt et al., 2016b).
In the 2011 study by Sorrentino and colleagues, Mx_a was found to have a threshold at 0.3 where both mortality and functional outcome both worsened in their cohort (Sorrentino et al., 2011). Zeiler and colleagues found in 2018 that in their cohort of 281 TBI patients, Sx had thresholds of −0.15 and −0.20 while Sx_a had thresholds of −0.10 and 0.05 for functional outcome and mortality, respectively. They also identified a threshold of −0.10 for Dx where outcomes worsened (Zeiler et al., 2018a).
Hemispheric Asymmetry
TCD can be utilized to insonate the left and right MCA of the patient without any increased risk to the patient and so a number of studies examined the effect of hemispheric asymmetry of TCD based indices of cerebrovascular reactivity. Czosnyka and colleagues noted in their 2003 study that Mx was worse on the side of contusion or expansion if the patient had midline shift. They also noted that a hemispheric asymmetry was significantly more common in patients that died than those that survived (Czosnyka et al., 2003). In a follow up study Schmidt and colleagues found that the magnitude of hemispheric asymmetry, as determined by Mx, was higher in patients that died compared to those that survived and that hemispheric asymmetry was independently associated with functional outcome by multiple regression analysis (Schmidt et al., 2003b). Interestingly, hemispheric asymmetry, as determined by Mx_a was not found to be associated with outcomes by Lang and colleagues in their 2003 study (Lang et al., 2003b).
Established Measure of Cerebrovascular Reactivity
Cerebrovascular reactivity is often measured by the fluctuation of ICP in response to changes in ABP in a more established index known as the pressure reactivity index (PRx). There has been a building body of evidence supporting cerebrovascular reactivity, in the form of PRx, being an important physiologic measure in TBI and so several studies have assessed the covariance of PRx and TCD based indices (Czosnyka et al., 1997; Czosnyka et al., 2002; Lang, 2003; Zweifel et al., 2008; Budohoski et al., 2012a; Schmidt et al., 2016a; Zeiler et al., 2017b; Zeiler et al., 2018a; Zeiler et al., 2018c). Once again, Mx has been the most well examined TCD based index with eight studies demonstrating some degree of correlation between Mx and PRx (Czosnyka et al., 1997; Czosnyka et al., 2002; Lang, 2003; Zweifel et al., 2008; Budohoski et al., 2012a; Schmidt et al., 2016a; Zeiler et al., 2017b; Zeiler et al., 2018c). Despite this, recent work comparing various TCD based indices found that Sx had the strongest correlation with PRx (Zeiler et al., 2017b; Zeiler et al., 2018a; Zeiler et al., 2018c), and both Sx and Sx_a appear to closely approximate PRx through advanced time-series modeling (Zeiler et al., 2018e). Finally, utilizing complex time-series analysis, PRx has been found to be estimated well with Mx_a and Sx_a based models (Zeiler et al., 2018e).
Cerebral Perfusion Pressure and Intracranial Pressure
The relationship between TCD based indices and ICP seems to be somewhat independent with Mx being found to be positively correlated with ICP in early studies and a notable increased in Mx with ICPs above 20 mm Hg (Czosnyka et al., 1999; Czosnyka et al., 2001; Czosnyka et al., 2003; Czosnyka et al., 2008). Notably, during measurements of plateau waves of ICP, Mx was greater than the threshold of 0.2 and significantly more positive than pre-plateau and post-plateau periods (Czosnyka et al., 2003; Lewis et al., 2014). This difference during plateau waves was not found in Mx_a (Liu et al., 2016). The association between TCD indices and CPP is slightly more complex. While early studies showed a negative correlation between Mx and CPP(Czosnyka et al., 1999; Czosnyka et al., 2000) subsequent studies have found that when plotting Mx vs. CPP a U-shaped distribution is observed (Czosnyka et al., 2001; Czosnyka et al., 2003; Lewis et al., 2012). This may indicate that an optimal CPP (CPPopt) may exist where Mx is at a minimum, and therefore cerebrovascular reactivity may be best preserved, for individual patients. Cerebrovascular reactivity may then be disrupted both when CPP is inadequate or insufficient and this has been postulated as a means of identifying individual CPP targets based on a CPPopt derived from Mx. Interestingly, some studies have also found that Mx was significantly lower when CPP was increasing compared to when it was decreasing and that the correlation between Mx and CPP is stronger during increases in CPP than decreases in CPP (Schmidt et al., 2009; Schmidt et al., 2012). This may indicate that current management generally places patients at a CPP less than their CPPopt.
CO2 Reactivity
A number of smaller studies have explored the relationship between TCD based indices and hypocapnia as well as CO2 reactivity (Haubrich et al., 2011; Haubrich et al., 2012; Zhang et al., 2016). In general, those with disrupted cerebrovascular reactivity (Mx ≥ 0.25), moderate hypocapnia seemed to significantly decreased Mx while those with intact reactivity (Mx ≤ 0.25) saw no significant change with hypocapnia (Haubrich et al., 2011, Haubrich et al., 2012). Of note, while less than half of the patients examined had an identifiable CPPopt at normocapnia, nearly all of them had one with hypocapnia (Haubrich et al., 2011). In a similar study, Zhang and colleagues found that CO2 reactivity was correlated with Mx but did not find any significant changes in Mx with hyperventilation (Zhang et al., 2016).
Radiographic Evolution of Injury
No study was identified that examined the association between time-domain TCD based indices of cerebrovascular reactivity and the evolution of imaging findings following TBI.
Discussion
A strong relationship between time-domain TCD based indices of cerebrovascular reactivity and mortality/functional outcome following TBI has been demonstrated through this scoping review of the literature (Czosnyka et al., 1996; Czosnyka et al., 1997; Czosnyka et al., 1999; Czosnyka et al., 2000; Czosnyka et al., 2001; Czosnyka et al., 2002; Lang et al., 2003a; Schmidt et al., 2003b; Lewis et al., 2007; Radolovich et al., 2011; Sorrentino et al., 2011; Budohoski et al., 2012a; Budohoski et al., 2012c; Lewis et al., 2012; Schmidt et al., 2016a). The prognostic utility of TCD based indices has been emphasized with the identification of thresholds for most indices at which outcomes worsen and mortality increases (Czosnyka et al., 2002; Sorrentino et al., 2011; Schmidt et al., 2016b; Zeiler et al., 2018a). Hemispheric asymmetry of cerebrovascular reactivity, as measured by TCD based indices, also seems to pertain a poor prognosis following TBI (Czosnyka et al., 2003; Lang et al., 2003b; Schmidt et al., 2003b). There also seems to be a reasonable degree of covariance between TCD based indices of cerebrovascular reactivity and more established measures, such as PRx (Czosnyka et al., 1997; Czosnyka et al., 2002; Lang, 2003; Zweifel et al., 2008; Budohoski et al., 2012a; Schmidt et al., 2016a; Zeiler et al., 2017b, Zeiler et al., 2018c). Elevations in ICP seem to be associated with the disruption of cerebrovascular reactivity (Czosnyka et al., 1999; Czosnyka et al., 2001; Czosnyka et al., 2003; Czosnyka et al., 2008) while a U-shape relationship exist between CPP and Mx indicating the possibility of a CPPopt where cerebrovascular reactivity is the least disrupted (Czosnyka et al., 2001; Czosnyka et al., 2003; Lewis et al., 2012). Finally, hyperventilation seems to improve cerebrovascular reactivity when it is already disrupted but does not significantly change it when already intact (Haubrich et al., 2011; Haubrich et al., 2012; Zhang et al., 2016). These findings highlight the utility of these indices in not only provided a greater understanding of the post-TBI physiome but also indicate a role for these measure of cerebrovascular reactivity in precision medicine by aiding in developing personalized targets for physiologic parameters following injury.
This review has also identified some major limitations to this body of literature. Perhaps, most obvious, is that the predominance of data supporting these findings comes from a single institution. This limits the confidence in the generalizability of these findings. A more subtle corollary of this is that there is significant overlap in cohorts used over various articles and so while there may be numerous publications finding prognostic utility in these indices, the strength of these finding may be somewhat overstated as the data is not wholly unique between them.
There are also limitations to the assessment of global outcomes measured at follow up. None of the studies identified in this scoping review utilized pathology specific, detailed quality of life measures such as the Quality of Life after Brain Injury (QOLIBRI) instrument. Assessments such as these would have provided a more comprehensive understanding of a patient’s health-related quality of life following their injury (von Steinbüchel et al., 2020). Additionally, trends in functional outcome, identified through serial measurements collected over multiple timepoints, were not investigated by any of the studies. As a result, no comments can be made about the utility of TCD based indices of cerebrovascular reactivity to stratify various trajectories of functional recovery.
Another weakness is in the analysis performed in these studies. We have seen that these indices are related to age, severity of injury and ICP (Czosnyka et al., 2000; Czosnyka et al., 2003; Czosnyka et al., 2005), however, what is unclear is if these indices provide prognostic utility independent of these associated variables as no multivariate analysis was performed. Without multivariate analysis, the role of TCD based indices as an independent prognostic tool remains unclear. Further to this, the studies identified reduced measures of TCD indices to grand averages over the recording period or over the length of stay with no study performing time-series analytics to evaluate causal relationships. While this simplifies analysis, it is at the cost information encoded in the fluctuations of these indices over a recording period or over the entire length of stay.
The literature also seems to mainly focus on Mx as the TCD based index most studied. This is likely due to it being the first TCD based index described (Czosnyka et al., 1996). While this means there is a large volume of evidence supporting its use, studies exploring other indices are limited. Of note, systolic TCD based indices (Sx and Sx_a) were only examined in seven studies (Czosnyka et al., 1996; Czosnyka et al., 2005; Budohoski et al., 2012c; Zeiler et al., 2017b; Zeiler et al., 2018a; Zeiler et al., 2018c; Zeiler et al., 2018e). This is especially unfortunate given recent studies finding systolic based indices to closest association with more established ICP based indices of cerebrovascular reactivity and provide better prognostic utility than mean flow (Mx and Mx_a) and diastolic (Dx and Dx_a) TCD based indices(Zeiler et al., 2018a). Diastolic TCD based indices (Dx and Dx_a) were only examined in four studies and were found to have the weakest outcome associations of all modalities (Budohoski et al., 2012c; Zeiler et al., 2017b; Zeiler et al., 2018a; Zeiler et al., 2018c). However, generalization of the findings of diastolic (Dx and Dx_a) and systolic (Sx and Sx_a) modalities are limited not only by the small number of studies but also due to the fact that all of the identified studies evaluating them draw from a single institution’s database (Addenbrooke’s Hospital, United Kingdom).
There are also some limitations inherent to TCD that become apparent when reviewing the literature. Due to the difficulty in obtaining prolonged recordings of TCD data, most studies report only data collection during a small proportion of time spent in ICU with some studies reporting as little as 10 min of insonation a day (Radolovich et al., 2011) and no study reporting more than 4 h per day. Given the dynamic nature of cerebrovascular reactivity, as demonstrated by the PRx literature (Adams et al., 2017), it is hard to believe that prognostic utility would not benefit from longer periods of insonation. In addition to the variable duration of insonation, there is also inconsistent timing and rate of measurements with some studies performing serial daily measurements (Czosnyka et al., 1996; Czosnyka et al., 1998; Czosnyka et al., 1999; Czosnyka et al., 2000; Czosnyka et al., 2001; Czosnyka et al., 2002; Czosnyka et al., 2003; Schmidt et al., 2003b; Czosnyka et al., 2005; Lewis et al., 2007; Czosnyka et al., 2008; Radolovich et al., 2011; Sorrentino et al., 2011; Schmidt et al., 2012; Liu et al., 2015) while others reported only performing one recording session over the course of admission (Lang et al., 2003b; Zeiler et al., 2018a; Zeiler et al., 2018c; Zeiler et al., 2018e). A number of studies also failed to report any details around duration and frequency of insonation as well as timing of measurement following injury(Czosnyka et al., 1997; Lang et al., 2003a; Lang, 2003; Schmidt et al., 2003a; Zweifel et al., 2008; Schmidt et al., 2009; Budohoski et al., 2012a; Budohoski et al., 2012c; Lewis et al., 2012; Lewis et al., 2014; Schmidt et al., 2016a; Schmidt et al., 2016b; Liu et al., 2016; Zhang et al., 2016). In those studies, in which serial assessments were performed, no comment was made on the progression of cerebrovascular reactivity over time.
Finally, there seems to be an absence of any literature evaluating the link between cerebrovascular reactivity, as measured by time-domain TCD based indices, and the evolution or progression of imaging findings in the setting of TBI. Similar reports have been published with regards to PRx and have found a link between cerebrovascular reactivity and lesion progression following TBI (Mathieu et al., 2020a; Mathieu et al., 2020b; Zeiler et al., 2020). A link between similar TCD based indices may provide a less invasive means of predicting radiographic progression and should be explored further.
Limitations
This scoping review does, in and of itself, has some limitations. Articles included in this study were limited to those that contained cohorts of greater than 20 TBI patients. This was done in order to limit the prevalence of small case series and case reports, however, this may have limited the diversity of institutions included in this review. Additionally, this review was limited to continuous time-domain TCD based indices at the exclusion of frequency domain based TCD indices. While this was done to avoid excessive heterogeneity in indices examined, there is evidence that these indices do associate with outcomes following TBI, but to a lesser degree (Liu et al., 2015). Additionally, the inclusion articles in journal supplements resulted in some very similar reports being included in this review (Haubrich et al., 2011; Haubrich et al., 2012). Finally, given that TBI has a global burden of disease, the exclusion of non-English language articles may have further narrowed the institutional diversity as well as likely skewed the ethnocultural diversity of the patient cohorts included in this review.
Future Directions
This review highlights some key areas for further development. First, before TCD based indices are adopted clinically, validation of these findings will need to occur on more diverse datasets. Large multi-institutional/multi-national collaborative networks such as the ones found in the CENTER-TBI study and CAnadian High-Resolution TBI (CAHR-TBI) Research Collaborative are ideal for such validation studies (Bernard et al., 2020). In order to identify the true prognostic utility of TCD based indices, these studies should ideally examine the unique contributions of these indices in and above ICP, age and injury severity through multivariant analysis.
The clinically applicability of these indices may also be expanded past prognostication. Further exploration of utilizing TCD based indices to identify personalized CPP targets in critically ill patients following TBI must be undertaken. Precision medicine with personalized targets derived from these indices may provide the means to significantly alter outcomes following TBI and is already being explored utilizing more established measure of cerebrovascular reactivity, such as PRx (Zeiler et al., 2019a).
Further investigation of TCD based indices, aside from Mx, should also be done given the evidence of the strength of correlation with outcome Sx has demonstrated. Additionally, ABP based indices are of particular interest given the recent description of entirely non-invasive methods of collecting the requisite physiologic data (Zeiler et al., 2018b; Zeiler et al., 2019b; Zeiler and Smielewski, 2018; Gomez et al., 2020).
Finally, advances in TCD technology, such as robotic TCD has allowed for prolonged recording in and above 4 h due to the continuous robotic optimization of probe position. This has already been described as a means of assessing cerebrovascular reactivity for extended periods of time and may extend the percentage of time monitored while in ICU (Zeiler et al., 2018b; Zeiler and Smielewski, 2018). This may prove to further improve the association of these indices with outcomes following TBI while providing a more complete picture of the post-TBI physiome.
Conclusion
This systematic scoping review of the literature identifies that there is a substantial body of evidence that cerebrovascular reactivity as measured by time-domain TCD based indices have prognostic utility following TBI. To a lesser extent, the literature has also explored some associations between these indices and cerebral physiologic parameters in this patient population. Notably, there is lack of evidence evaluating the correlation between these indices and radiographic progression following injury. Further research needs to be done to expand the generalizability of these results and identify optimal inputs and collection methods for these indices before they can be widely clinically adopted. However, their role in precision medicine following traumatic brain injury is promising.
Author Contributions
AG: Designed the search strategy and search terms, collected articles for review, undertook review and screening of the literature, and prepared the manuscript. LF: Undertook review and screening of the literature as well as aided in preparing the manuscript. AS: Aided in preparation of the manuscript. CB: Aided in preparation of the manuscript. FZ: Responsible for conceptual development of this article, development of search strategy, undertook screening of the literature, and aided in preparation of the manuscript.
Funding
FZ receives research support from the Manitoba Public Insurance (MPI) Neuroscience/TBI Research Endowment, the Health Sciences Center Foundation Winnipeg, the United States National Institutes of Health (NIH) through the National Institute of Neurological Disorders and Stroke (NINDS) (Grant #: R03NS114335-01), the Canadian Institutes of Health Research (CIHR) (Grant #: 432061), the Canada Foundation for Innovation (CFI) (Project #: 38583), Research Manitoba (Grant #: 3906), the University of Manitoba VPRI Research Investment Fund (RIF), the University of Manitoba Centre on Aging, and the University of Manitoba Rudy Falk Clinician-Scientist Professorship. AG is supported through the University of Manitoba Clinician Investigator Program. LF is supported through the University of Manitoba—Department of Surgery GFT Research Grant, and the University of Manitoba Office of Research Services (ORS)—University Research Grant Program (URGP). CB is support through the Center on Aging at the University of Manitoba.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.690921/full#supplementary-material
References
Aaslid, R., Lindegaard, K. F., Sorteberg, W., and Nornes, H. (1989). Cerebral Autoregulation Dynamics in Humans. Stroke 20, 45–52. doi:10.1161/01.STR.20.1.45
Aaslid, R., Markwalder, T.-M., and Nornes, H. (1982). Noninvasive Transcranial Doppler Ultrasound Recording of Flow Velocity in Basal Cerebral Arteries. J. Neurosurg. 57, 769–774. doi:10.3171/jns.1982.57.6.0769
Aaslid, R. (1986). “The Doppler Principle Applied to Measurement of Blood Flow Velocity in Cerebral Arteries,” in Transcranial Doppler Sonography. Editor R. Aaslid (Vienna: Springer Vienna), 22–38. doi:10.1007/978-3-7091-8864-4_3
Adams, H., Donnelly, J., Czosnyka, M., Kolias, A. G., Helmy, A., Menon, D. K., et al. (2017). Temporal Profile of Intracranial Pressure and Cerebrovascular Reactivity in Severe Traumatic Brain Injury and Association with Fatal Outcome: An Observational Study. Plos Med. 14, e1002353. doi:10.1371/journal.pmed.1002353
Arksey, H., and O'Malley, L. (2005). Scoping Studies: towards a Methodological Framework. Int. J. Soc. Res. Methodol. 8, 19–32. doi:10.1080/1364557032000119616
Bernard, F., Gallagher, C., Griesdale, D., Kramer, A., Sekhon, M., and Zeiler, F. A. (2020). The CAnadian High-Resolution Traumatic Brain Injury (CAHR-TBI) Research Collaborative. Can. J. Neurol. Sci. 47, 551–556. doi:10.1017/cjn.2020.54
Budohoski, K. P., Czosnyka, M., de Riva, N., Smielewski, P., Pickard, J. D., Menon, D. K., et al. (2012a). The Relationship between Cerebral Blood Flow Autoregulation and Cerebrovascular Pressure Reactivity after Traumatic Brain Injury. Neurosurgery 71, 652–661. doi:10.1227/NEU.0b013e318260feb1
Budohoski, K. P., Czosnyka, M., Smielewski, P., Kasprowicz, M., Helmy, A., Bulters, D., et al. (2012b). Impairment of Cerebral Autoregulation Predicts Delayed Cerebral Ischemia after Subarachnoid Hemorrhage. Stroke 43, 3230–3237. doi:10.1161/STROKEAHA.112.669788
Budohoski, K. P., Reinhard, M., Aries, M. J. H., Czosnyka, Z., Smielewski, P., Pickard, J. D., et al. (2012c). Monitoring Cerebral Autoregulation after Head Injury. Which Component of Transcranial Doppler Flow Velocity Is Optimal? Neurocrit. Care 17, 211–218. doi:10.1007/s12028-011-9572-1
Carney, N., Totten, A. M., O'Reilly, C., Ullman, J. S., Hawryluk, G. W. J., Bell, M. J., et al. (2017). Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 80, 6–15. doi:10.1227/NEU.0000000000001432
Cold, G. E., and Jensen, F. T. (1978). Cerebral Autoregulation in Unconscious Patients with Brain Injury. Acta Anaesthesiologica Scand. 22, 270–280. doi:10.1111/j.1399-6576.1978.tb01301.x
Czosnyka, M., Balestreri, M., Steiner, L., Smielewski, P., Hutchinson, P. J., Matta, B., et al. (2005). Age, Intracranial Pressure, Autoregulation, and Outcome after Brain Trauma. J. Neurosurg. 102, 450–454. doi:10.3171/jns.2005.102.3.0450
Czosnyka, M., Smielewski, P., Czosnyka, Z., Piechnik, S., Steiner, L. A., Schmidt, E., et al. (2003). Continuous Assessment of Cerebral Autoregulation: Clinical and Laboratory Experience. Acta Neurochirurgica. Suppl. 86, 581–585. doi:10.1007/978-3-7091-0651-8_118
Czosnyka, M., Smielewski, P., Kirkpatrick, P., Laing, R. J., Menon, D., and Pickard, J. D. (1997). Continuous Assessment of the Cerebral Vasomotor Reactivity in Head Injury. Neurosurgery 41, 11–19. doi:10.1097/00006123-199707000-00005
Czosnyka, M., Smielewski, P., Kirkpatrick, P., Menon, D. K., and Pickard, J. D. (1996). Monitoring of Cerebral Autoregulation in Head-Injured Patients. Stroke 27, 1829–1834. doi:10.1161/01.STR.27.10.1829
Czosnyka, M., Smielewski, P., Kirkpatrick, P., Piechnik, S., Laing, R., and Pickard, J. D. (1998). Continuous Monitoring of Cerebrovascular Pressure-Reactivity in Head Injury. Acta Neurochir. Suppl. 71, 74–77. doi:10.1007/978-3-7091-6475-4_23
Czosnyka, M., Smielewski, P., Lavinio, A., Pickard, J. D., and Panerai, R. (2008). An Assessment of Dynamic Autoregulation from Spontaneous Fluctuations of Cerebral Blood Flow Velocity: A Comparison of Two Models, Index of Autoregulation and Mean Flow Index. Anesth. Analgesia 106, 234–239. doi:10.1213/01.ane.0000295802.89962.13
Czosnyka, M., Smielewski, P., Piechnik, S., Al-Rawi, P. G., Kirkpatrick, P. J., Matta, B. F., et al. (1999). Critical Closing Pressure in Cerebrovascular Circulation. J. Neurol. Neurosurg. Psychiatry 66, 606–611. doi:10.1136/jnnp.66.5.606
Czosnyka, M., Smielewski, P., Piechnik, S., and Pickard, J. D. (2002). Clinical Significance of Cerebral Autoregulation. Acta Neurochirurgica. Suppl. 81, 117–119. doi:10.1007/978-3-7091-6738-0_30
Czosnyka, M., Smielewski, P., Piechnik, S., Schmidt, E. A., Seeley, H., al-Rawi, P., et al. (2000). Continuous Assessment of Cerebral Autoregulation - Clinical Verification of the Method in Head Injured Patients. Acta Neurochirurgica. Suppl. 76, 483–484. doi:10.1007/978-3-7091-6346-7_101
Czosnyka, M., Smielewski, P., Piechnik, S., Steiner, L. A., and Pickard, J. D. (2001). Cerebral Autoregulation Following Head Injury. J. Neurosurg. 95, 756–763. doi:10.3171/jns.2001.95.5.0756
Froese, L., Dian, J., Batson, C., Gomez, A., Unger, B., and Zeiler, F. A. (2020a). Cerebrovascular Response to Propofol, Fentanyl, and Midazolam in Moderate/Severe Traumatic Brain Injury: A Scoping Systematic Review of the Human and Animal Literature. Neurotrauma Rep. 1, 100–112. doi:10.1089/neur.2020.0040
Froese, L., Dian, J., Gomez, A., Unger, B., and Zeiler, F. A. (2020b). Cerebrovascular Response to Phenylephrine in Traumatic Brain Injury: A Scoping Systematic Review of the Human and Animal Literature. Neurotrauma Rep. 1, 46–62. doi:10.1089/neur.2020.0008
Froese, L., Dian, J., Gomez, A., Unger, B., and Zeiler, F. A. (2020c). The Cerebrovascular Response to Norepinephrine: A Scoping Systematic Review of the Animal and Human Literature. Pharmacol. Res. Perspect. 8, e00655. doi:10.1002/prp2.655
Gomez, A., Batson, C., Froese, L., Sainbhi, A. S., and Zeiler, F. A. (2021). Utility of Transcranial Doppler in Moderate and Severe Traumatic Brain Injury: A Narrative Review of Cerebral Physiologic Metrics. J. Neurotrauma [Epub ahead of print]. doi:10.1089/neu.2020.7523
Gomez, A., Dian, J., and Zeiler, F. A. (2020). Continuous and Entirely Non-invasive Method for Cerebrovascular Reactivity Assessment: Technique and Implications. J. Clin. Monit. Comput. 35, 307–315. doi:10.1007/s10877-020-00472-4
Hasen, M., Gomez, A., Froese, L., Dian, J., Raj, R., Thelin, E. P., et al. (2020). Alternative Continuous Intracranial Pressure-Derived Cerebrovascular Reactivity Metrics in Traumatic Brain Injury: a Scoping Overview. Acta Neurochir 162, 1647–1662. doi:10.1007/s00701-020-04378-7
Haubrich, C., Steiner, L., Kasprowicz, M., Diedler, J., Carrera, E., Diehl, R. R., et al. (2011). Short-term Moderate Hypocapnia Augments Detection of Optimal Cerebral Perfusion Pressure. J. Neurotrauma 28, 1133–1137. doi:10.1089/neu.2010.1577
Haubrich, C., Steiner, L., Kim, D. J., Kasprowicz, M., Smielewski, P., Diehl, R. R., et al. (2012). How Does Moderate Hypocapnia Affect Cerebral Autoregulation in Response to Changes in Perfusion Pressure in TBI Patients? Acta Neurochir Suppl. 114, 153–156. doi:10.1007/978-3-7091-0956-4_28
Hawryluk, G. W. J., Aguilera, S., Buki, A., Bulger, E., Citerio, G., Cooper, D. J., et al. (2019). A Management Algorithm for Patients with Intracranial Pressure Monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 45, 1783–1794. doi:10.1007/s00134-019-05805-9
Klein, S. P., Depreitere, B., and Meyfroidt, G. (2019). How I Monitor Cerebral Autoregulation. Crit. Care 23, 160. doi:10.1186/s13054-019-2454-1
Lang, E. W. (2003). Cerebral Vasomotor Reactivity Testing in Head Injury: the Link between Pressure and Flow. J. Neurol. Neurosurg. Psychiatry 74, 1053–1059. doi:10.1136/jnnp.74.8.1053
Lang, E. W., Lagopoulos, J., Griffith, J., Yip, K., Mudaliar, Y., Mehdorn, H. M., et al. (2003a). Noninvasive Cerebrovascular Autoregulation Assessment in Traumatic Brain Injury: Validation and Utility. J. Neurotrauma 20, 69–75. doi:10.1089/08977150360517191
Lang, E. W., Yip, K., Griffith, J., Lagopoulos, J., Mudaliar, Y., and Dorsch, N. W. (2003b). Hemispheric Asymmetry and Temporal Profiles of Cerebral Pressure Autoregulation in Head Injury. J. Clin. Neurosci. 10, 670–673. doi:10.1016/s0967-5868(03)00197-8
Lewis, P. M., Smielewski, P., Pickard, J. D., and Czosnyka, M. (2007). Dynamic Cerebral Autoregulation: Should Intracranial Pressure Be Taken into Account? Acta Neurochir (Wien) 149, 549–555. doi:10.1007/s00701-007-1160-y
Lewis, P. M., Smielewski, P., Rosenfeld, J. V., Pickard, J. D., and Czosnyka, M. (2014). A Continuous Correlation between Intracranial Pressure and Cerebral Blood Flow Velocity Reflects Cerebral Autoregulation Impairment during Intracranial Pressure Plateau Waves. Neurocrit. Care 21, 514–525. doi:10.1007/s12028-014-9994-7
Lewis, P. M., Smielewski, P., Rosenfeld, J. V., Pickard, J. D., and Czosnyka, M. (2012). Monitoring of the Association between Cerebral Blood Flow Velocity and Intracranial Pressure. Acta Neurochirurgica. Suppl. 114, 147–151. doi:10.1007/978-3-7091-0956-4_27
Liu, X., Czosnyka, M., Donnelly, J., Budohoski, K. P., Varsos, G. V., Nasr, N., et al. (2015). Comparison of Frequency and Time Domain Methods of Assessment of Cerebral Autoregulation in Traumatic Brain Injury. J. Cereb. Blood Flow Metab. 35, 248–256. doi:10.1038/jcbfm.2014.192
Liu, X., Czosnyka, M., Pickard, J. D., Varsos, G. V., Nasr, N., and Smielewski, P. (2016). Derangement of Cerebral Blood Flow Autoregulation during Intracranial Pressure Plateau Waves as Detected by Time and Frequency-Based Methods. Acta Neurochir. Suppl. 122, 233–238. doi:10.1007/978-3-319-22533-3_47
Mathieu, F., Zeiler, F. A., Ercole, A., Monteiro, M., Kamnitsas, K., Glocker, B., et al. (2020a). Relationship between Measures of Cerebrovascular Reactivity and Intracranial Lesion Progression in Acute Traumatic Brain Injury Patients: A CENTER-TBI Study. J. Neurotrauma 37, 1556–1565. doi:10.1089/neu.2019.6814
Mathieu, F., Zeiler, F. A., Whitehouse, D. P., Das, T., Ercole, A., Smielewski, P., et al. (2020b). Relationship between Measures of Cerebrovascular Reactivity and Intracranial Lesion Progression in Acute TBI Patients: an Exploratory Analysis. Neurocrit. Care 32, 373–382. doi:10.1007/s12028-019-00885-3
Overgaard, J., and Tweed, W. A. (1974). Cerebral Circulation after Head Injury. J. Neurosurg. 41, 531–541. doi:10.3171/jns.1974.41.5.0531
Radolovich, D. K., Aries, M. J. H., Castellani, G., Corona, A., Lavinio, A., Smielewski, P., et al. (2011). Pulsatile Intracranial Pressure and Cerebral Autoregulation after Traumatic Brain Injury. Neurocrit. Care 15, 379–386. doi:10.1007/s12028-011-9553-4
Schmidt, B., Czosnyka, M., and Klingelhöfer, J. (2012). Asymmetry of Cerebral Autoregulation Does Not Correspond to Asymmetry of Cerebrovascular Pressure Reactivity. Perspect. Med. 1, 285–289. doi:10.1016/j.permed.2012.02.026
Schmidt, B., Czosnyka, M., Raabe, A., Yahya, H., Schwarze, J. J., Sackerer, D., et al. (2003a). Adaptive Noninvasive Assessment of Intracranial Pressure and Cerebral Autoregulation. Stroke 34, 84–89. doi:10.1161/01.str.0000047849.01376.ae
Schmidt, B., Klingelhöfer, J., Perkes, I., and Czosnyka, M. (2009). Cerebral Autoregulatory Response Depends on the Direction of Change in Perfusion Pressure. J. Neurotrauma 26, 651–656. doi:10.1089/neu.2008.0784
Schmidt, B., Lezaic, V., Weinhold, M., Plontke, R., Schwarze, J., and Klingelhöfer, J. (2016a). Is Impaired Autoregulation Associated with Mortality in Patients with Severe Cerebral Diseases? Acta Neurochir Suppl. 122, 181–185. doi:10.1007/978-3-319-22533-3_37
Schmidt, B., Reinhard, M., Lezaic, V., McLeod, D. D., Weinhold, M., Mattes, H., et al. (2016b). Autoregulation Monitoring and Outcome Prediction in Neurocritical Care Patients: Does One index Fit All? J. Clin. Monit. Comput. 30, 367–375. doi:10.1007/s10877-015-9726-3
Schmidt, E. A., Czosnyka, M., Steiner, L. A., Balestreri, M., Smielewski, P., Piechnik, S. K., et al. (2003b). Asymmetry of Pressure Autoregulation after Traumatic Brain Injury. J. Neurosurg. 99, 991–998. doi:10.3171/jns.2003.99.6.0991
Sorrentino, E., Budohoski, K. P., Kasprowicz, M., Smielewski, P., Matta, B., Pickard, J. D., et al. (2011). Critical Thresholds for Transcranial Doppler Indices of Cerebral Autoregulation in Traumatic Brain Injury. Neurocrit. Care 14, 188–193. doi:10.1007/s12028-010-9492-5
Steinmeier, R., Hofmann, R. P., Bauhuf, C., Hübner, U., and Fahlbusch, R. (2002). Continuous Cerebral Autoregulation Monitoring by Cross-Correlation Analysis. J. Neurotrauma 19, 1127–1138. doi:10.1089/08977150260337949
Tricco, A. C., Lillie, E., Zarin, W., O'Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 169, 467–473. doi:10.7326/M18-0850
Varsos, G. V., Budohoski, K. P., Kolias, A. G., Liu, X., Smielewski, P., Varsos, V. G., et al. (2014). Relationship of Vascular wall Tension and Autoregulation Following Traumatic Brain Injury. Neurocrit. Care 21, 266–274. doi:10.1007/s12028-014-9971-1
von Steinbüchel, N., Meeuwsen, M., Zeldovich, M., Vester, J. C., Maas, A., Koskinen, S., et al. (2020). Differences in Health-Related Quality of Life after Traumatic Brain Injury between Varying Patient Groups: Sensitivity of a Disease-specific (QOLIBRI) and a Generic (SF-36) Instrument. J. Neurotrauma 37, 1242–1254. doi:10.1089/neu.2019.6627
Zeiler, F. A., Cardim, D., Donnelly, J., Menon, D. K., Czosnyka, M., and Smielewski, P. (2018a). Transcranial Doppler Systolic Flow Index and ICP-Derived Cerebrovascular Reactivity Indices in Traumatic Brain Injury. J. Neurotrauma 35, 314–322. doi:10.1089/neu.2017.5364
Zeiler, F. A., Czosnyka, M., and Smielewski, P. (2018b). Optimal Cerebral Perfusion Pressure via Transcranial Doppler in TBI: Application of Robotic Technology. Acta Neurochir 160, 2149–2157. doi:10.1007/s00701-018-3687-5
Zeiler, F. A., Donnelly, J., Calviello, L., Menon, D. K., Smielewski, P., and Czosnyka, M. (2017a). Pressure Autoregulation Measurement Techniques in Adult Traumatic Brain Injury, Part I: A Scoping Review of Intermittent/Semi-Intermittent Methods. J. Neurotrauma 34, 3207–3223. doi:10.1089/neu.2017.5085
Zeiler, F. A., Donnelly, J., Cardim, D., Menon, D. K., Smielewski, P., and Czosnyka, M. (2018c). ICP versus Laser Doppler Cerebrovascular Reactivity Indices to Assess Brain Autoregulatory Capacity. Neurocrit. Care 28, 194–202. doi:10.1007/s12028-017-0472-x
Zeiler, F. A., Donnelly, J., Menon, D. K., Smielewski, P., Zweifel, C., Brady, K., et al. (2017b). Continuous Autoregulatory Indices Derived from Multi-Modal Monitoring: Each One Is Not like the Other. J. Neurotrauma 34, 3070–3080. doi:10.1089/neu.2017.5129
Zeiler, F. A., Donnelly, J., Smielewski, P., Menon, D. K., Hutchinson, P. J., and Czosnyka, M. (2018d). Critical Thresholds of Intracranial Pressure-Derived Continuous Cerebrovascular Reactivity Indices for Outcome Prediction in Noncraniectomized Patients with Traumatic Brain Injury. J. Neurotrauma 35, 1107–1115. doi:10.1089/neu.2017.5472
Zeiler, F. A., Ercole, A., Cabeleira, M., Carbonara, M., Stocchetti, N., Menon, D. K., et al. (2019a). Comparison of Performance of Different Optimal Cerebral Perfusion Pressure Parameters for Outcome Prediction in Adult Traumatic Brain Injury: A Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) Study. J. Neurotrauma 36, 1505–1517. doi:10.1089/neu.2018.6182
Zeiler, F. A., Mathieu, F., Monteiro, M., Glocker, B., Ercole, A., Beqiri, E., et al. (2020). Diffuse Intracranial Injury Patterns Are Associated with Impaired Cerebrovascular Reactivity in Adult Traumatic Brain Injury: A CENTER-TBI Validation Study. J. Neurotrauma 37, 1597–1608. doi:10.1089/neu.2019.6959
Zeiler, F. A., and Smielewski, P. (2018). Application of Robotic Transcranial Doppler for Extended Duration Recording in Moderate/severe Traumatic Brain Injury: First Experiences. Crit. Ultrasound J. 10, 16. doi:10.1186/s13089-018-0097-0
Zeiler, F. A., Smielewski, P., Donnelly, J., Czosnyka, M., Menon, D. K., and Ercole, A. (2018e). Estimating Pressure Reactivity Using Noninvasive Doppler-Based Systolic Flow Index. J. Neurotrauma 35, 1559–1568. doi:10.1089/neu.2017.5596
Zeiler, F. A., Smielewski, P., Stevens, A., Czosnyka, M., Menon, D. K., and Ercole, A. (2019b). Non-Invasive Pressure Reactivity Index Using Doppler Systolic Flow Parameters: A Pilot Analysis. J. Neurotrauma 36, 713–720. doi:10.1089/neu.2018.5987
Zhang, Y., Liu, X., Steiner, L., Smielewski, P., Feen, E., Pickard, J. D., et al. (2016). Correlation between Cerebral Autoregulation and Carbon Dioxide Reactivity in Patients with Traumatic Brain Injury. Acta Neurochir. Suppl. 122, 205–209. doi:10.1007/978-3-319-22533-3_41
Keywords: cerebral autoregulation, cerebrovascular reactivity, scoping review, traumatic brain injury, transcranial Doppler, precision medicine
Citation: Gomez A, Froese L, Sainbhi AS, Batson C and Zeiler FA (2021) Transcranial Doppler Based Cerebrovascular Reactivity Indices in Adult Traumatic Brain Injury: A Scoping Review of Associations With Patient Oriented Outcomes. Front. Pharmacol. 12:690921. doi: 10.3389/fphar.2021.690921
Received: 04 April 2021; Accepted: 25 June 2021;
Published: 06 July 2021.
Edited by:
Danielle K. Sandsmark, University of Pennsylvania, United StatesReviewed by:
Haiping Zhao, Capital Medical University, ChinaKimbra Kenney, Uniformed Services University of the Health Sciences, United States
Copyright © 2021 Gomez, Froese, Sainbhi, Batson and Zeiler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alwyn Gomez, Z29tZXphMzVAbXl1bWFuaXRvYmEuY2E=
 Alwyn Gomez
Alwyn Gomez Logan Froese
Logan Froese Amanjyot Singh Sainbhi
Amanjyot Singh Sainbhi Carleen Batson
Carleen Batson Frederick A. Zeiler
Frederick A. Zeiler