- 1Department of Medicine I, Division of Infectious Diseases and Tropical Medicine, Medical University of Vienna, Vienna, Austria
- 2Department of Clinical Pharmacology, Medical University Vienna, Vienna, Austria
Objectives: This study investigated the synergistic in vitro and in vivo activity of cefazolin plus fosfomycin against methicillin-susceptible and methicillin-resistant S. aureus (MSSA and MRSA) to provide the basis for a potential treatment alternative.
Methods: Antimicrobial susceptibility and in vitro synergy tests were performed with five MSSA and five MRSA isolates using the broth microdilution and chequerboard assays, respectively. The in vivo efficacy of cefazolin plus fosfomycin for the treatment of MRSA infections was assessed using the Galleria mellonella survival assay.
Results: Using fractional inhibitory concentration index (FICI), the evaluated combination of cefazolin plus fosfomycin showed synergistic in vitro activity against all MSSA and MRSA isolates tested. In addition, cefazolin susceptibility was recovered in all MRSA isolates except one fosfomycin-resistant strain when combined with fosfomycin at readily achievable concentrations. The G. mellonella survival assay demonstrated highly synergistic in vivo activity of cefazolin plus fosfomycin, resulting in a 44–52% reduction in mortality when compared to cefazolin-alone and fosfomycin-alone, respectively.
Conclusion: If susceptibility to fosfomycin is either confirmed or can be assumed based on local resistance patterns, combination therapy with cefazolin plus fosfomycin could be a valuable treatment option for empirical as well as targeted therapy of S. aureus and MRSA infections. Future studies proving the clinical significance of this combination therapy are therefore warranted.
Introduction
Over recent years, fosfomycin has raised considerable interest due to its potent activity against a wide spectrum of problematic pathogens including Staphylococcus aureus, the leading cause of bacteremia and infective endocarditis (IE). According to current guidelines vancomycin or daptomycin are recommended for treatment of bacteremia and IE caused by methicillin-resistant Staphylococcus aureus (MRSA), although bactericidal activity of glycopeptides is poorer than that of beta-lactams and their penetration into endocardial vegetations is markedly lower (Stevens, 2006; Habib et al., 2015). Daptomycin shows rapid bactericidal activity and is therefore a reliable alternative for severe MRSA infections (Richter et al., 2003; Habib et al., 2015). However, it possesses some shortcomings, including a strong inoculum effect and notable rates of emergent resistance in patients with left-sided IE, highlighting the need for alternative regimens and rescue therapies (Fowler et al., 2006; Moise et al., 2009; Morrisette et al., 2020). Due to its broad antimicrobial activity against Gram-positive and Gram-negative organisms, fosfomycin has been studied in combination with various beta-lactam antibiotics because of their wide therapeutic range and strong clinical efficacy. These combinations have shown highly synergistic activity against MRSA, especially when fosfomycin was studied together with imipenem (Grif et al., 2001; del Río et al., 2016). A multicenter clinical trial investigating the efficacy and safety of fosfomycin plus imipenem as rescue therapy for complicated MRSA bacteremia and IE showed that this combination therapy is a safe and effective alternative and should be further investigated (del Río et al., 2014). However, given the global public-healthcare issue posed by the emergence and rapid spread of carbapenem resistance, the restrained use of carbapenems, especially for treatment of Gram-positive infections for which reliable alternatives are still available, is of utmost importance (Meletis, 2016). Therefore, we selected the narrow-spectrum beta-lactam cefazolin for combination with fosfomycin, which has shown good clinical efficacy and tolerability in the treatment of MSSA infections (Loubet et al., 2018).
Methods
Bacterial Strains
Ten S. aureus isolates were tested in this study: five methicillin- and fosfomycin-susceptible (ATCC-29213 and four clinical isolates), one methicillin- and fosfomycin-resistant (DSMZ-23622) and four methicillin-resistant and fosfomycin-susceptible (ATCC-33592 and three clinical isolates) isolates. All clinical isolates were routinely obtained from positive blood cultures and identified by routine microbiological methods including Matrix-Assisted Laser Desorption/ionization Time-Of-Flight Mass Spectrometry (MALDI-TOF MS, MALDI Biotyper smart with the Compass IVD software v4.2, Bruker Daltonics GmbH, Germany) (Supplementary Table S1). In addition, all isolates were tested by polymerase chain reaction (PCR) for the presence of the methicillin-resistance gene mecA as previously described by Terpstra et al. (Terpstra et al., 1999).
Antimicrobial Susceptibility and Synergy Testing
Minimum inhibitory concentrations (MICs) for cefazolin and fosfomycin were determined by broth microdilution method in cation-adjusted Mueller-Hinton broth (CA-MHB) supplemented with glucose-6-phosphate (G6P) at a final concentration of 25 mg/L which was also used for synergy testing.
Synergy-testing was performed using a chequerboard assay as previously described (Li et al., 2018). Briefly, serial dilutions of cefazolin and fosfomycin were made in u-bottomed 96-well microtiter plates with a final inoculum of approximately 5 × 105 CFU/ml and a final volume of 200 µL per well. Plates were read after an incubation of 18–24 h at 36°C (±1°C). After calculation of the fractional inhibitory concentration indices (FICI) results were interpreted as synergism ≤0.5, >0.5–4 = no interaction and >4 antagonism. The susceptible breakpoint index (SBPI) was calculated according to the following formula: SBPI = (susceptible breakpoint of antimicrobial A/combined MIC of antimicrobial A) + (susceptible breakpoint of antimicrobial B/combined MIC of antimicrobial B), using the clinical breakpoint of 32 mg/L for fosfomycin and the pharmacokinetic/pharmacodynamic breakpoint of 2 mg/L for cefazolin (Milne and Gould, 2010; The European Committee on Antimicrobial Susceptibility Testing, 2021. Breakpoint tables for interpretation of MICs and zone diameters. Version 11.0, 2021. http://www.eucast.org). An SBPI ≥2 indicates that the combined MICs of the tested antimicrobials are equally or lower than their respective breakpoints. It follows that the greater the SBPI value, the more effective the antimicrobial combination is. All experiments were performed in duplicates.
Penicillin-Binding Protein Expression Analysis
Relative gene expression of penicillin-binding protein 1 (PBP1), PBP2, PBP2′ (also called PBP2a), PBP3, and PBP4 was determined for one fosfomycin-susceptible (ATCC-33592) and one fosfomycin-resistant (DSMZ-23622) MRSA after a 4 h incubation with either fosfomycin or cefazolin at 0.25xMIC or without antibiotics as control. Bacterial inocula were prepared by diluting overnight cultures with fresh tryptic soy broth (TSB) followed by an incubation period on an orbital shaker at 36°C (±1°C) to achieve exponential growth. RNA was extracted using lysing matrix tubes (MP Biomedicals) and the FavorPrep-Tissue Total RNA Mini-Kit (Favorgen Biotech Corp, Taiwan). Copy-DNA was obtained using the Onescript cDNA Synthesis-Kit (ABMgood, Canada) and RT-PCR was performed with low-ROX BrightGreen qPCR Mastermix (ABMgood, Canada) using previously described primers for PBPs and gap, which encodes for the glyceraldehyde-3-phosphate dehydrogenase, as housekeeping gene (Supplementary Table S2) (Navratna et al., 2010). All experiments were performed in quadruplicates and relative expression values (±SD) were calculated by ΔΔCt using no treatment controls as references.
In vivo Galleria mellonella Survival Assay
A fosfomycin-susceptible MRSA (ATCC-33592) was used for the in vivo G. mellonella survival assay. Bacterial inocula were prepared by diluting overnight cultures with fresh TSB followed by incubation of 4 h on an orbital shaker at 36°C (±1°C) to obtain bacteria in exponential growth phase and with a cell density causing a mortality rate of ≥80% within 5 days post infection. G. mellonella larvae were originally obtained from TruLarv™ (Biosystems Technology), further bred in our laboratory and used at a weight between 220 and 280 mg, after a 24-h fasting period. After random distribution into four treatment groups: infected control, cefazolin-alone, fosfomycin-alone or cefazolin plus fosfomycin, infection of the larvae was performed by injecting 10 µL (∼7 × 108 CFU/ml) of the bacterial inoculum into one of the last prolegs using a 50 µL Hamilton syringe (Merck, Darmstadt, Germany). One hour after infection, a single dose of antibiotics was administered into another proleg to minimize leakage of the hemolymph. For cefazolin the human dose of 100 mg/kg was used whereas the fosfomycin dose (0.8 mg/kg) was determined in preliminary experiments to achieve mortality rates of 60–90% (Supplementary Figure S1). For the entire experiment, larvae were incubated at 37°C for five days and survival was measured every 24 h. The first experiment contained 20–25 larvae per treatment group, while the duplicate experiment was performed with 10–15 larvae from a different batch on a different day (n per treatment group = 36–40). Both infected as well as uninfected larvae, which only received sterile PBS, served as controls. In addition, drug toxicity was ruled out by tracking the survival of 10 larvae each after a single dose of 200 mg/kg fosfomycin and 100 mg/kg cefazolin. Survival curves were plotted using GraphPad Prism v6.01 (GraphPad Software Inc. San Diego) and analyzed using the log-rank test.
Results
Antimicrobial Susceptibility and Synergy Testing
Five of the ten S. aureus isolates tested positive for the presence of mecA, namely ATCC-33592.
DSMZ-23622, 874/19, 845/19, 563/18 (Supplementary Figure S2).
The results of the in vitro susceptibility and synergy testing are summarized in Table 1. All isolates showed a FICI ≤0.5 for the combination of cefazolin plus fosfomycin indicating synergism. In addition, all isolates except the fosfomycin-resistant MRSA demonstrated a SBPI >2.
In vivo G.mellonella Survival Assay
The control group infected with MRSA (ATCC-33592) showed a mortality of 89% within 5 days. Fosfomycin-alone (200 mg/kg) was highly effective and resulted in a survival rate of 100% (Supplementary Figure S1), whereas cefazolin-alone (100 mg/kg) resulted in a mortality rate of 65%. When cefazolin was combined with low-dose fosfomycin (0.8 mg/kg), which achieved 73% mortality alone, mortality decreased to 21% (p = 0.0002 for combination vs. cefazolin-alone; p < 0.0001 for combination vs fosfomycin-alone), as shown in Figure 1A.
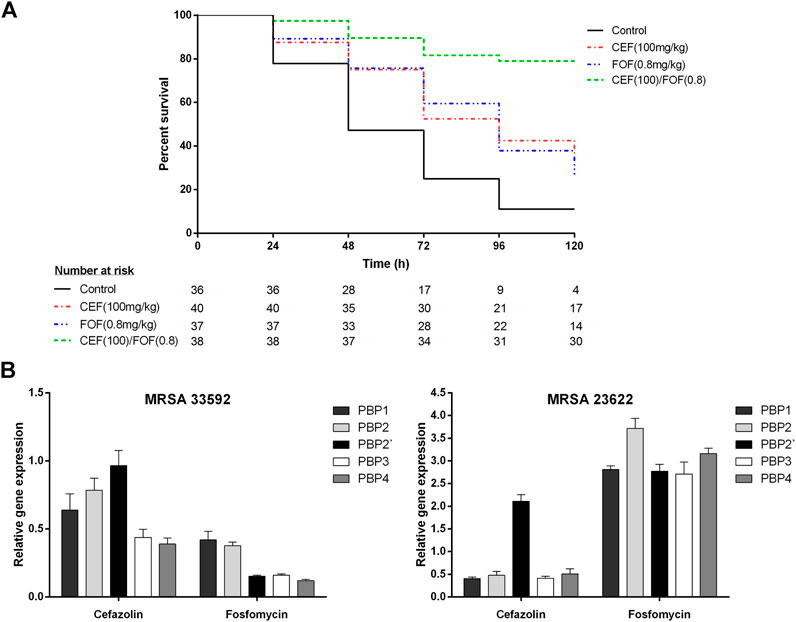
FIGURE 1. (A) Survival curves of G. mellonella larvae infected with methicillin-resistant Staphylococcus aureus (ATCC-33592) followed by treatment with cefazolin (100 mg/kg), fosfomycin (0.8 mg/kg) or the combination of both (cefazolin 100 mg/kg plus fosfomycin 0.8 mg/kg). Curves represent the pooled data of two experiments performed on separate days. (B) Relative gene expression of penicillin binding proteins (PBP1, PBP2, PBP2′, PBP3, and PBP4) determined for one fosfomycin-susceptible (ATCC-33592) and one fosfomycin-resistant (DSMZ-23622) methicillin-resistant Staphylococcus aureus (MRSA) isolate using RT-PCR with gap as housekeeping gene and a no treatment control as reference. Bacteria were exposed to either cefazolin or fosfomycin at a concentration corresponding to 0.25 times of their respective minimum inhibitory concentrations for a time period of 4 h. Data is stated as mean (±SD) relative quantification values. *CEF, cefazolin; FOF, fosfomycin; MRSA, methicillin-resistant Staphylococcus aureus; PBP, penicillin-binding protein.
Penicillin-Binding Protein Expression Analysis
Data of the PBP expression analysis is demonstrated in Figure 1B; Supplementary Table S3. After exposure to cefazolin (0.25xMIC), both MRSA showed reduced expression of PBP1, PBP2, PBP3 and PBP4, while PBP2′ remained unchanged in the fosfomycin-susceptible MRSA (ATCC-33592) and was even overexpressed in the fosfomycin-resistant isolate (DSMZ-23622). Exposure to fosfomycin (0.25xMIC) reduced the expression of all PBPs and most significantly PBP2′, PBP3, and PBP4 in the fosfomycin-susceptible isolate while overexpression of all PBPs was observed for the fosfomycin-resistant strain.
Discussion
This study demonstrated the highly synergistic activity of cefazolin plus fosfomycin against both, MSSA and MRSA. With regard to the FICI, all isolates showed synergistic activity, which was even more pronounced in fosfomycin-susceptible MRSA. When combined with fosfomycin at readily achievable concentrations, all of these isolates regained susceptibility to cefazolin and demonstrated SBPIs ≥40. Furthermore, cefazolin susceptibility of an MRSA was recovered in vivo by combination with low-dose fosfomycin, resulting in significantly reduced mortality of at least 44% (Figure 1A). In contrast, the fosfomycin-resistant MRSA showed synergistic activity with respect to the FICI but failed to recover its susceptibility to cefazolin and consequently achieved only an SBPI of 1.1, highlighting the importance of additional parameters to evaluate in vitro synergies regarding their potential clinical relevance (Table 1) (Milne and Gould, 2010). The data obtained in the present study are consistent with a previous study by Grif et al. that demonstrated synergistic in vitro activity of fosfomycin plus cefazolin and fosfomycin plus meropenem against five S. aureus strains, including a glycopeptide-intermediate S. aureus and an MRSA (Grif et al., 2001). However, in this previous contribution to the field no fosfomycin-resistant isolate was tested and neither individual nor combination MICs of the antimicrobials tested are reported, so the extent of synergistic activity cannot be compared (Grif et al., 2001).
The differences between the fosfomycin-susceptible and resistant MRSA isolates were also observed in PBP expression analysis when both strains were exposed to fosfomycin. The fosfomycin-susceptible strain showed an overall reduction in PBP expression with a shift toward PBP1 and PBP2 whereas the fosfomycin-resistant strain overexpressed all PBPs with a slight shift toward PBP2 (Figure 1B). This is consistent with the overall reduction of PBPs, including PBP2′, determined fluorographically by Utsui et al. In a more recent study only a reduction of PBP1 and PBP2, but not PBP2′, was observed using SDS-PAGE electrophoresis, although fosfomycin-susceptible MRSA were studied at comparable concentrations (Utsui et al., 1986; del Río et al., 2016). Thus, given the reduced but still synergistic activity of cefazolin plus fosfomycin, observed in the present study in a fosfomycin-resistant MRSA without a shift or reduction in PBP expression, the previously hypothesized mechanisms of synergy can only partially explain these findings (Utsui et al., 1986; Najioullah et al., 1992; del Río et al., 2016).
Despite efforts to optimize management, MRSA bloodstream infections still demonstrate high mortality rates >30% regardless of the antimicrobial therapy used (Gasch et al., 2013; Veganzones et al., 2019). In addition, the increasing prevalence of MRSA worldwide was associated to epidemiological changes such as older age and the increased presence of comorbidities, highlighting the need for safe and effective treatment alternatives (Gasch et al., 2013; Veganzones et al., 2019). In light of these facts, del Rio et al. investigated imipenem plus fosfomycin for treatment of MRSA bacteremia and IE after failure of vancomycin or daptomycin, demonstrating its safety and efficacy (del Río et al., 2014).
However, based on the data obtained in the present study, cefazolin plus fosfomycin may represent an effective therapeutic option for MRSA and MSSA infections, including settings of unknown beta-lactam susceptibility and may help to maintain carbapenems as reliable last-resort treatment. Thus, further studies proving its clinical significance are warranted.
Transparency Declaration
Dr Vossen reports personal fees from Astro Pharma, other from Infectopharm, outside the submitted work.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
MKu, MV, and LT conceived this study and participated in its design and coordination. MKu, MO, MKa, and LH designed the various experiments. MKu, MO, LH, RK, and LS carried out the G. mellonella experiments. R-YC, LH, MKa, RK, and LS carried out the broth microdilution, the checkerboard assay and qPCR. MKu drafted the manuscript and MV and LT participated in the data analysis and revision of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.685807/full#supplementary-material
References
del Río, A., García-de-la-Mària, C., Entenza, J. M., Gasch, O., Armero, Y., Soy, D., et al. (2016). Fosfomycin Plus β-Lactams as Synergistic Bactericidal Combinations for Experimental Endocarditis Due to Methicillin-Resistant and Glycopeptide-Intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 60, 478–486. doi:10.1128/AAC.02139-15
del Río, A., Gasch, O., Moreno, A., Peña, C., Cuquet, J., Soy, D., et al. (2014). Efficacy and Safety of Fosfomycin Plus Imipenem as Rescue Therapy for Complicated Bacteremia and Endocarditis Due to Methicillin-Resistant Staphylococcus aureus: A Multicenter Clinical Trial. Clin. Infect. Dis. 59, 1105–1112. doi:10.1093/cid/ciu580
Fowler, V. G., Boucher, H. W., Corey, G. R., Abrutyn, E., Karchmer, A. W., Rupp, M. E., et al. (2006). Daptomycin versus Standard Therapy for Bacteremia and Endocarditis Caused byStaphylococcus Aureus. N. Engl. J. Med. 355, 653–665. doi:10.1056/NEJMoa053783
Gasch, O., Camoez, M., Dominguez, M. A., Padilla, B., Pintado, V., Almirante, B., et al. (2013). Predictive Factors for Mortality in Patients with Methicillin-Resistant Staphylococcus aureus Bloodstream Infection: Impact on Outcome of Host, Microorganism and Therapy. Clin. Microbiol. Infect. 19, 1049–1057. doi:10.1111/1469-0691.12108
Grif, K., Dierich, M. P., Pfaller, K., Miglioli, P. A., and Allerberger, F. (2001). In vitro activity of Fosfomycin in Combination with Various Antistaphylococcal Substances. J. Antimicrob. Chemother. 48, 209–217. doi:10.1093/jac/48.2.209
Habib, G., Lancellotti, P., Antunes, M. J., Bongiorni, M. G., Casalta, J.-P., Del Zotti, F., et al. (2015). 2015 ESC Guidelines for the Management of Infective Endocarditis. Eur. Heart J. 36, 3075–3128. doi:10.1093/eurheartj/ehv319
Li, L., Chen, H., Liu, Y., Xu, S., Wu, M., Liu, Z., et al. (2020). Synergistic Effect of Linezolid with Fosfomycin against Staphylococcus aureus In Vitro and in an Experimental Galleria Mellonella Model. J. Microbiol. Immunol. Infect. 53, 731–738. doi:10.1016/j.jmii.2018.12.007
Loubet, P., Burdet, C., Vindrios, W., Grall, N., Wolff, M., Yazdanpanah, Y., et al. (2018). Cefazolin versus Anti-staphylococcal Penicillins for Treatment of Methicillin-Susceptible Staphylococcus aureus Bacteraemia: a Narrative Review. Clin. Microbiol. Infect. 24, 125–132. doi:10.1016/j.cmi.2017.07.003
Meletis, G. (2016). Carbapenem Resistance: Overview of the Problem and Future Perspectives. Ther. Adv. Infect. 3, 15–21. doi:10.1177/2049936115621709
Milne, K. E. N., and Gould, I. M. (2010). Combination Testing of Multidrug-Resistant Cystic Fibrosis Isolates of Pseudomonas aeruginosa: Use of a New Parameter, the Susceptible Breakpoint Index. J. Antimicrob. Chemother. 65, 82–90. doi:10.1093/jac/dkp384
Moise, P. A., North, D., Steenbergen, J. N., and Sakoulas, G. (2009). Susceptibility Relationship between Vancomycin and Daptomycin in Staphylococcus aureus: Facts and Assumptions. Lancet Infect. Dis. 9, 617–624. doi:10.1016/S1473-3099(09)70200-2
Morrisette, T., Alosaimy, S., Abdul-Mutakabbir, J. C., Kebriaei, R., and Rybak, M. J. (2020). The Evolving Reduction of Vancomycin and Daptomycin Susceptibility in MRSA-Salvaging the Gold Standards with Combination Therapy. Antibiotics 9, 762. doi:10.3390/antibiotics9110762
Najioullah, F., Pellon, G. r., Freney, J., Michel, G., and Fleurette, J. (1992). Fosfomycin Enhances the Expression of Penicillin-Binding Protein 2 in Methicillin-Sensitive and Methicillin-resistantStaphylococcus Aureusstrains. FEMS Microbiol. Lett. 97, 221–226. doi:10.1111/j.1574-6968.1992.tb05467.x
Navratna, V., Nadig, S., Sood, V., Prasad, K., Arakere, G., and Gopal, B. (2010). Molecular Basis for the Role of Staphylococcus aureus Penicillin Binding Protein 4 in Antimicrobial Resistance. Jb 192, 134–144. doi:10.1128/JB.00822-09
Richter, S. S., Kealey, D. E., Murray, C. T., Heilmann, K. P., Coffman, S. L., and Doern, G. V. (2003). The In Vitro Activity of Daptomycin against Staphylococcus aureus and Enterococcus Species. J. Antimicrob. Chemother. 52, 123–127. doi:10.1093/jac/dkg288
Stevens, D. L. (2006). The Role of Vancomycin in the Treatment Paradigm. Clin. Infect. Dis. 42 (Suppl. 1), S51–S57. doi:10.1086/491714
Terpstra, S., Noordhoek, G. T., Voesten, H. G. J., Hendriks, B., and Degener, J. E. (1999). Rapid Emergence of Resistant Coagulase-Negative Staphylococci on the Skin after Antibiotic Prophylaxis. J. Hosp. Infect. 43, 195–202. doi:10.1053/JHIN.1999.0636
The European Committee on Antimicrobial Susceptibility Testing (2021). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. http://www.eucast.org.
Utsui, Y., Ohya, S., Magaribuchi, T., Tajima, M., and Yokota, T. (1986). Antibacterial Activity of Cefmetazole Alone and in Combination with Fosfomycin against Methicillin- and Cephem-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 30, 917–922. doi:10.1128/aac.30.6.917
Keywords: methicillin-resistant Staphylococcus aureus, synergy, combination therapy, antibiotic resistance, narrow-spectrum beta-lactam, rescue therapy
Citation: Kussmann M, Obermueller M, Karer M, Kriz R, Chen R-Y, Hohl L, Schneider L, Burgmann H, Traby L and Vossen MG (2021) Synergistic Effect of Cefazolin Plus Fosfomycin Against Staphylococcus aureus in vitro and in vivo in an Experimental Galleria mellonella Model. Front. Pharmacol. 12:685807. doi: 10.3389/fphar.2021.685807
Received: 25 March 2021; Accepted: 26 April 2021;
Published: 11 May 2021.
Edited by:
Priyia Pusparajah, Monash University Malaysia, MalaysiaReviewed by:
Mélanie Roch, Université de Genève, SwitzerlandLifen Hu, First Affiliated Hospital of Anhui Medical University, China
Copyright © 2021 Kussmann, Obermueller, Karer, Kriz, Chen, Hohl, Schneider, Burgmann, Traby and Vossen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias G. Vossen, bWF0dGhpYXMudm9zc2VuQG1lZHVuaXdpZW4uYWMuYXQ=; Ludwig Traby, bHVkd2lnLnRyYWJ5QG1lZHVuaXdpZW4uYWMuYXQ=
†These authors have contributed equally to this work
 Manuel Kussmann
Manuel Kussmann Markus Obermueller1
Markus Obermueller1 Matthias Karer
Matthias Karer Rui-Yang Chen
Rui-Yang Chen Lena Hohl
Lena Hohl Ludwig Traby
Ludwig Traby Matthias G. Vossen
Matthias G. Vossen