
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 22 April 2021
Sec. Drug Metabolism and Transport
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.662535
This article is part of the Research Topic Organic Cation Transporter 1 (OCT1): Not Vital for Life, but of Substantial Biomedical Relevance View all 11 articles
Organic Cation Transporter 1 (OCT1, gene symbol: SLC22A1) is predominately expressed in human liver, localized in the basolateral membrane of hepatocytes and facilitates the uptake of endogenous compounds (e.g. serotonin, acetylcholine, thiamine), and widely prescribed drugs (e.g. metformin, fenoterol, morphine). Furthermore, exogenous compounds such as MPP+, ASP+ and Tetraethylammonium can be used as prototypic substrates to study the OCT1-mediated transport in vitro. Single-transfected cell lines recombinantly overexpressing OCT1 (e.g., HEK-OCT1) were established to study OCT1-mediated uptake and to evaluate transporter-mediated drug-drug interactions in vitro. Furthermore, double-transfected cell models simultaneously overexpressing basolaterally localized OCT1 together with an apically localized export protein have been established. Most of these cell models are based on polarized grown MDCK cells and can be used to analyze transcellular transport, mimicking the transport processes e.g. during the hepatobiliary elimination of drugs. Multidrug and toxin extrusion protein 1 (MATE1, gene symbol: SLC47A1) and the ATP-driven efflux pump P-glycoprotein (P-gp, gene symbol: ABCB1) are both expressed in the canalicular membrane of human hepatocytes and are described as transporters of organic cations. OCT1 and MATE1 have an overlapping substrate spectrum, indicating an important interplay of both transport proteins during the hepatobiliary elimination of drugs. Due to the important role of OCT1 for the transport of endogenous compounds and drugs, in vitro cell systems are important for the determination of the substrate spectrum of OCT1, the understanding of the molecular mechanisms of polarized transport, and the investigation of potential drug-drug interactions. Therefore, the aim of this review article is to summarize the current knowledge on cell systems recombinantly overexpressing human OCT1.
Transport proteins located in different membrane domains are important for the uptake, distribution and excretion of endogenous substances and drugs (International Transporter Consortium et al., 2010; König et al., 2013; Müller et al., 2018a; Koepsell, 2020). Whereas members of the SLC (Solute Carrier) transporter superfamily generally mediate the uptake of substances from the extracellular space into cells, members of the ABC (ATP-binding cassette) transporter superfamily are export proteins responsible for the energy-dependent export of substrates out of cells. SLC and ABC family members are important for the transport of a variety of approved drugs. Therefore, it is important to characterize drugs or drug metabolites as substrates or transport inhibitors. In vitro cell models are useful tools for this characterization. The importance of in vitro cell models is also highlighted by the fact that they are recommended as tools to study transporter-mediated drug interactions in the guideline/guidance of FDA Food and Drug Administration (2020) and EMA European Medicines Agency (2012).
This article focuses on transport data of the SLC22 family member OCT1 (gene symbol SLC22A1) generated by different in vitro cell models. OCT1 is predominantly expressed in liver and localized in the basolateral membrane of human hepatocytes (Gorboulev et al., 1997; Nies et al., 2008). It mediates the uptake of several endogenous and exogenous compounds and drugs (Table 1). Single-transfected cell models (e.g., HEK-OCT1 cells) recombinantly overexpressing OCT1 were established to study OCT1-mediated transport, to calculate transport parameters (e.g., Km values), to investigate the impact of genetic variations and to evaluate OCT1-mediated drug-drug interactions in vitro (Figure 1A; Table 1). Since OCT1 has an overlapping substrate spectrum with the apically localized export proteins MATE1 [gene symbol SLC47A1 (Nies et al., 2011)] and P-glycoprotein [P-gp, MDR1; gene symbol ABCB1 (Nies et al., 2008; Misaka et al., 2016)], double-transfected cell models have been established (MDCK-OCT1-MATE1 or MDCK-OCT1-P-gp) for investigating the vectorial transport mediated by both proteins (Table 2). MATE1 and P-glycoprotein are both localized in the apical (canalicular) membrane of human hepatocytes and responsible for the export of substances out of the cells into bile (Thiebaut et al., 1987; Otsuka et al., 2005). When expressed together with OCT1 in MDCK cells grown as a monolayer, OCT1 localizes in the basolateral and MATE1 or P-gp in the apical membrane (Figure 1B). In this experimental setup, substrates of OCT1 and MATE1/P-gp applied to the basolateral compartment will be first taken up into the cells mediated by OCT1 and subsequently exported via MATE1 or P-gp into the apical compartment (Figure 1B). Therefore, these cell models can be used to study not only OCT1-mediated uptake into the cells, but also the vectorial transport of substances from the basolateral into the apical compartment mimicking the transport processes during the hepatobiliary elimination e.g. of drugs (Taghikhani et al., 2017). Moreover, the importance of uptake and efflux transporters for perpetrator disposition can be assessed (Müller et al., 2018b). In this review, we summarize transport data related to the hepatocellular uptake transporter OCT1 obtained by studies in different cell models. Furthermore, the advantages and disadvantages of these cell models will be addressed.
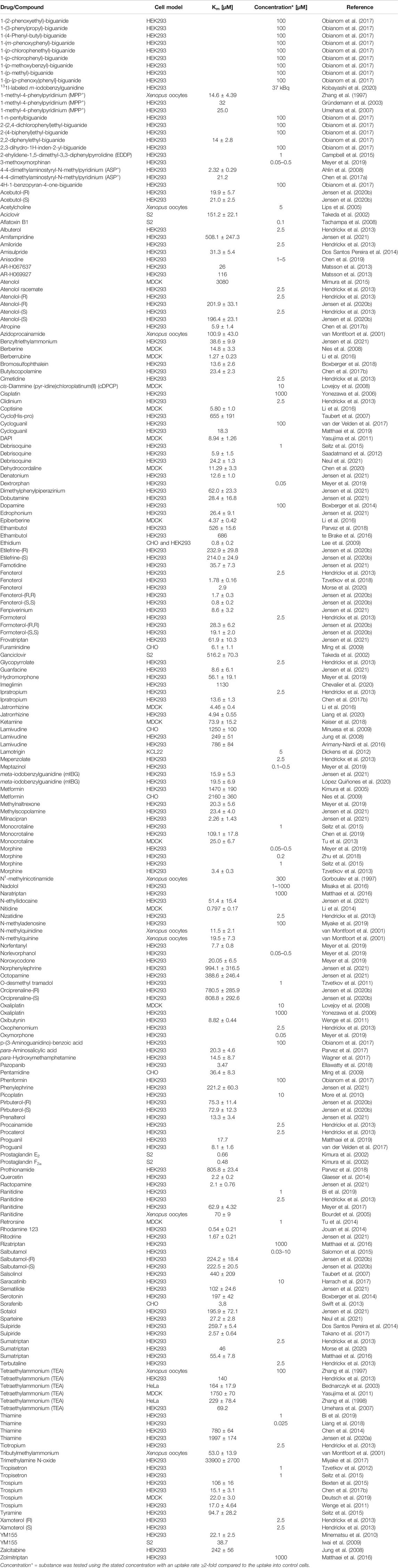
TABLE 1. Substrates of OCT1 (drugs, drug metabolites, endogenous molecules, chemicals) studied in single-transfected cell lines.
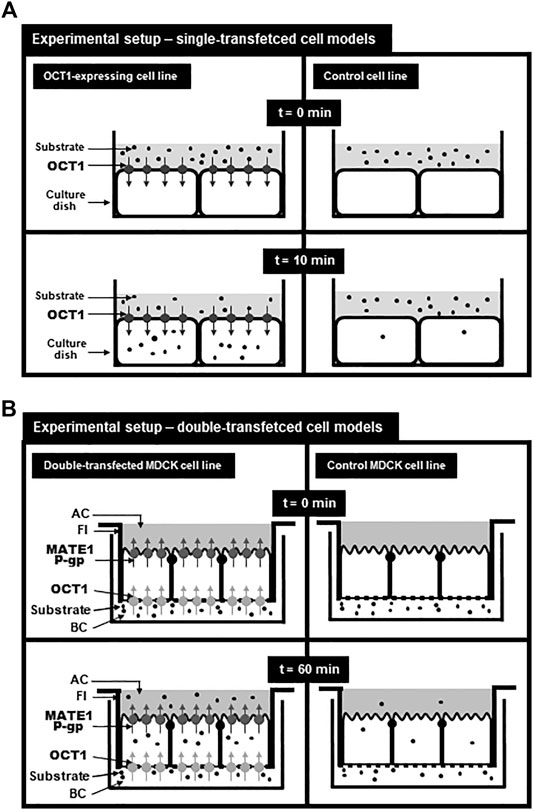
FIGURE 1. Experimental setup for using single-transfected (A) and double-transfected (B) cell models modified from Taghikhani et al. (Taghikhani et al., 2017). (A): Setup for analyzing the transport function of OCT1 in single-transfected cell lines. At time point 0 min, the donor solution containing the substrate is applied onto the cell layer and after 10 min, the uptake of the substrate into OCT1-expressing cells and into control cells can be determined. By subtracting the uptake into the control cell line from the uptake into the OCT1-expressing cell line, the so called net uptake can be calculated referring to the uptake mediated by recombinantly expressed OCT1. (B): Setup for vectorial transport assays using double-transfected MDCK cell lines expressing OCT1 in the basolateral membrane and MATE1 or P-glycoprotein in the apical membrane. MDCK cells were cultured on filter inserts (FI) separating a basolateral (BC) from an apical (AC) compartment. The substrate was added to the basolateral compartment and after 60 min the substrate concentration in the cells (uptake) or in the apical compartment (vectorial transport) can be calculated and compared to the uptake or the vectorial transport of the control MDCK cell line. Net intracellular substrate concentrations reflects OCT1-mediated substrate uptake and the net substrate concentration in the apical compartment reflects the vectorial transport mediated by OCT1-mediated uptake and MATE1-or P-gp-mediated export.
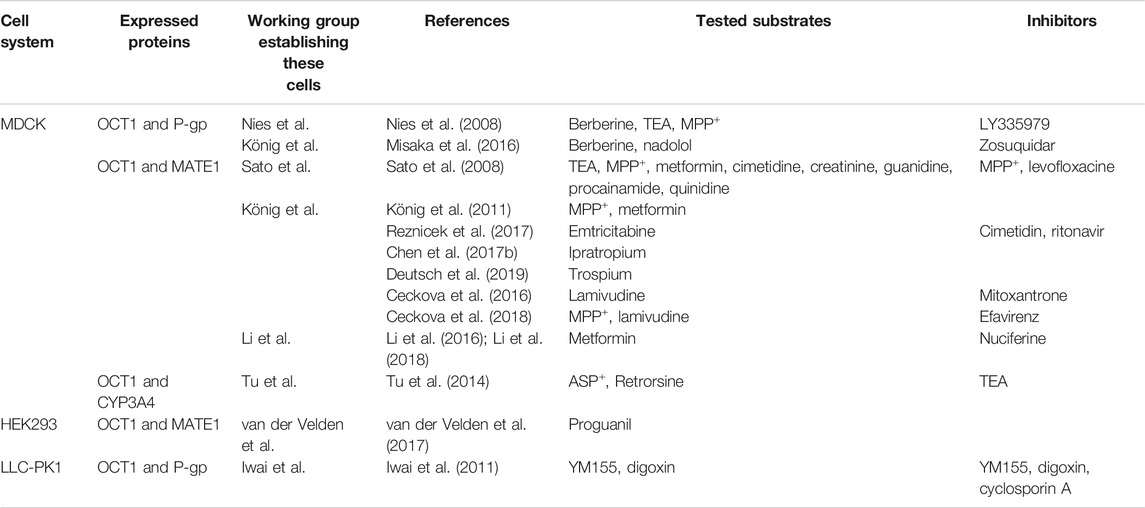
TABLE 2. OCT1 expressing, double-transfectant cell lines and investigated substrates and inhibitors.
The rodent orthologue of human OCT1 (rOct1) was first isolated from a rat kidney library and expressed in Xenopus oocytes. This rOct1 transporter showed inhibitable and potential-dependent Tetraethylammonium (TEA) and 1-methyl-4-phenylpyridinium (MPP+) uptake (Gründemann et al., 1994). Additionally, in situ hybridization and northern blotting analysis demonstrated Oct1 expression in rat hepatocytes and enterocytes. In 1997, human OCT1 (gene symbol: SLC22A1) was cloned and characterized by two independent working groups (Gorboulev et al., 1997; Zhang et al., 1997). Although Gorbulev et al. amplified hOCT1 using kidney cDNA, northern blot analysis demonstrated OCT1 expression mainly in the liver (Gorboulev et al., 1997), which was in line with the findings of Zhang et al. using liver cDNA (Zhang et al., 1997). Later, OCT1 was localized at the basolateral membrane of human hepatocytes (Nies et al., 2008). OCT1 facilitates the uptake of organic cations or weak bases (Table 1), which comprises approximately 40–67.5% of all drugs (Comer and Tam, 2001; Neuhoff et al., 2003; Manallack, 2007), into human hepatocytes. In the 2018 recommendations of the International Transporter Consortium (ITC), the investigation of OCT1-mediated transport during drug development was added, based on clinically important OCT1-mediated drug-drug interactions (Zamek-Gliszczynski et al., 2018a; Zamek-Gliszczynski et al., 2018b).
The existence of an organic cation-H+ antiporter was already postulated back in 1985 by studying the transport of N1-methylnicotinamide by the use of membrane vesicles, derived from the brush border membrane of rabbit kidney (Wright, 1985; Inui et al., 2000). The multidrug and toxic compound extrusion family (MATE) was first characterized in bacteria (Pallen, 1999) and Otsuka et al. (Otsuka et al., 2005) identified human and mouse orthologues of the bacterial MATE protein by genomic databank screening. The human MATE family consists of two members, the more widely expressed MATE1 protein and the kidney-specific member MATE2-K. The MATE1 protein is localized in the apical membrane of kidney proximal tubule epithelial cells and in the canalicular membrane of human hepatocytes (Otsuka et al., 2005; Masuda et al., 2006). MATE1 substrates are cations or have a positively charge at physiological pH (Nies et al., 2016). MATE proteins have a strong substrate overlap with the SLC22 family members OCT1, OCT2 and OCT3, indicating an interplay between these transporters in the hepatobiliary and renal elimination of drugs and endogenous compounds. The ITC recommends in vitro uptake studies using MATE-transfected cells, if the new molecular entity (NME) shows renal secretion as route of elimination or if the NME is an inhibitor of MATE1/2-K or OCT2 (Hillgren et al., 2013). So far, no criteria are defined for the evaluation of hepatic elimination of drugs mediated by MATE1. Detailed lists of substrates and inhibitors are available in several reviews (Terada and Inui, 2008; Damme et al., 2011; Nies et al., 2011; Motohashi and Inui, 2013; Nies et al., 2016; Koepsell, 2020).
P-glycoprotein (P-gp) is an ABC transporter and acts as an efflux pump for a variety of drugs such as digoxin, dabigatran etexilate and indinavir. P-gp is due to its ability of extruding drugs an limiting factor for drug bioavailability (Fromm, 2004). The substrate spectrum shows a strong overlap with the substrates of the Cytochrome P450 enzyme CYP3A4 and both proteins together protect the organism from xenobiotics (Kivistö et al., 2004; von Richter et al., 2004). P-gp is expressed in the apical membrane of several tissues such as small intestine, liver and kidney (Thiebaut et al., 1987). Additionally, P-gp plays an important role at blood-tissue barriers such as the blood-brain barrier and placenta, protecting the central nervous system or the unborn child from drugs or other xenobiotics (Fromm, 2004). Furthermore, P-gp is overexpressed in several cancer tissues, leading to multidrug resistance (Gottesman et al., 2002; Leopoldo et al., 2019). Wang et al. (Wang et al., 2003) analyzed by structure activity relationship analysis (SAR) several substrates and inhibitors of P-gp. They postulated that a tertiary nitrogen atom could be beneficial for the binding to P-gp due to the stronger interaction of the formed cation with the binding sites of P-gp. These cationic properties of some P-gp substrates already indicate that there might be an interplay between the OCT1-mediated uptake and the P-gp-mediated efflux during hepatobiliary elimination. Based on the recommendations of the ITC and FDA (International Transporter Consortium et al., 2010; Food and Drug Administration, 2020), a NME should be tested as P-gp substrate using inside-out oriented membrane vesicles or by vectorial transport assays using polarized grown cell lines such as Caco-2 cells or cell lines (MDCK, LLC-PK1) recombinantly overexpressing P-gp.
Use of single-transfected cell models expressing the transporter of interest is often the first step to gain insights into the substrate spectrum. The transporter is either transiently or stably transfected into a suitable cell line. The most commonly used cell lines for uptake studies are Human Embryonic Kidney 293 cells (HEK293). HEK293 cells are easy to culture and have, due to their human origin, comparable posttranslational protein modification to human tissues (Hu et al., 2018). Additionally, after transfection HEK293 cells are capable of expressing a variety of different proteins (Thomas and Smart, 2005). To study transport proteins, uptake assays can be used to determine transport parameters (Km or Cmax values) of the selected substrate (Figure 1A) or to perform drug-interaction studies. One limitation of using HEK293 cells is the lack of polarized growth, which excludes them for the analysis of transcellular transport studies. Other frequently used cell lines for establishing single-transfected cell models with the expression of one transport protein are Madin-Darby Canine Kidney cells (MDCK), Chinese Hamster Ovary cells (CHO), Drosophila Schneider 2 cells (S2), HeLa cells and Xenopus oocytes. Xenopus oocytes are a robust cell model, which is derived from Xenopus laevis (Zeng et al., 2020). The exogenous mRNA encoding the transport protein of interest is injected into oocytes leading to a functional expression of the protein. However, because of their limited longevity Xenopus oocytes cannot be used to generate stable transfectants.
Pioneering work on the characterization of OCT1 was done by Zhang et al. (1997). They were the first to clone OCT1 from human liver and they used Xenopus oocytes to analyze OCT1-mediated transport. They calculated the first transport Km and Vmax parameters for the uptake of the organic cation MPP+ and measured the IC50 values for the inhibition of OCT1-mediated transport of MPP+ by the cations decynium-22, vecuronium and TEA (Zhang et al., 1997). Furthermore, they extended their research by using transiently transfected HeLa cells and characterized the transport of TEA and obtained IC50 values for 15 different compounds (Zhang et al., 1998). The first inhibitor analysis using a wide range of compounds was done by Bednarczyk et al. (Bednarczyk et al., 2003). They used OCT1-transfected HeLa cells and calculated IC50 values of 30 structurally diverse organic cations and established a model of inhibitor/OCT1 interaction (Bednarczyk et al., 2003). These findings of structural requirements for OCT1 inhibition were extended by Ahlin and coworkers and their analysis of the inhibitory effect of 191 compounds on the OCT1-mediated uptake of ASP+ (Ahlin et al., 2008). ASP+ [4-(4-(dimethylamino)styryl)-N-methylpyridinium] is a fluorescent cationic model substrate for OCT1, which enables the fast screening of drugs as inhibitors of OCT1-mediated transport by analyzing fluorescence uptake. They identified 62 of the investigated compounds as inhibitors (cutoff value ≥50% inhibition) of which 66% were cations, 32% were neutral and repaglinide was the only anionic compound. Therefore, they estimated that high lipophilicity and a cationic character are the two main physicochemical properties of potent OCT1 inhibitors (Ahlin et al., 2008). A detailed analysis of the ‘structure-transport relationship’ was missing until Hendrickx et al. analyzed the uptake of 354 (with 83 marketed drugs) compounds into stably transfected HEK293 cells expressing OCT1 using a LC-MS/MS approach (Hendrickx et al., 2013). TEA and ipratropium served as reference compounds. In this study, the molecular volume of a compound was identified as the best descriptor for OCT1 substrates and lipophilicity was identified to be not important (Hendrickx et al., 2013). Recent publications emphasized the use of in silico predictions and machine learning approaches for the identification of new OCT1 substrates and their molecular characteristics (Baidya et al., 2020; Jensen et al., 2021). The OCT1 substrate and/or inhibitor spectrum has intensively been studied by various groups [e.g., (Gorboulev et al., 1997; Ciarimboli et al., 2005; Wenge et al., 2011; Tzvetkov et al., 2013; Knop et al., 2015; Otter et al., 2017; Meyer et al., 2019; Jensen et al., 2020b; Koepsell, 2020)].
Single-transfected cell models have also been extensively used to study the influence of genetic polymorphisms in the SLC22A1 gene on kinetic parameters of the OCT1-mediated transport (Kerb et al., 2002; Shu et al., 2003; Tzvetkov et al., 2011; Tzvetkov et al., 2013; Dos Santos Pereira et al., 2014; Matthaei et al., 2016; Meyer et al., 2017; Jensen et al., 2020b). A detailed list about the in vitro analyzed effects of genetic polymorphisms in the SLC22A1 gene has been published by Koepsell (2020). Furthermore, comparisons of human OCT1 with the orthologues of rat or mouse Oct1 has been performed using single-transfected cell models to gain insights into our understanding of potential substrate binding sites or protein regions involved in substrate recognition (Egenberger et al., 2012; Floerl et al., 2020; Koepsell, 2020; Meyer et al., 2020).
Table 1 summarizes currently known OCT1 substrates. We included all data where a Km-value was determined or where the uptake was ≥2-fold higher in the OCT1-expressing cells compared to the uptake into the respective control cell line. Potential substrates with uptake ratios between 1.5 and 2 are shown in Supplementary Table S1, together with publications that were not able to reproduce uptake experiments with controversial substrates (e.g., imatinib). OCT1 inhibitors are shown in Supplementary Table S2. We also included inhibition experiments, where no IC50 values were calculated, if the inhibitor was able to reduce the uptake of the substrate to ≤50%. Nevertheless, these lists are not exhaustive.
In contrast to HEK293 cells, MDCK cells form confluent monolayers when seeded on permeable membranes, such as microplate thinserts, separating a basolateral from an apical compartment (Figure 1B). These cells can be transfected with two cDNAs, for example one cDNA encoding for a basolaterally localized uptake transporter and one cDNA for an apically localized export protein. This allows a more versatile experimental setup, because these culture conditions enable transcellular transport measurements in combination with the measurement of the intracellular accumulation of the substrates. Furthermore, substrates can be applied either to the basolateral or apical compartment mimicking both routes of substrate transport, the route of excretion with the uptake of substrates from blood across the basolateral membrane and the export across the apical membrane into bile or urine (basal to apical transport) or the route of reuptake of substances across the apical membrane and the export into the blood (apical to basolateral transport e.g., during renal reabsorption). Limitations of this cell line are the expression of endogenous canine transporters such as canine Mdr1, Mrp2 and Oct2, which may affect the transport studies. Additionally, it is absolutely necessary to investigate the tightness of the cell monolayer to avoid paracellular transport of substances (Volpe, 2011).
The first double-transfected MDCK cell line expressing human OCT1 as uptake transporter together with P-gp in the apical membrane was established by Nies et al. [MDCK-OCT1-P-gp, Table 2 (Nies et al., 2008)]. The protein expression was investigated by immunoblot and immunofluorescence analysis and for the functional testing, TEA and MPP+ served as prototypic substrates for OCT1. Subsequent to the identification of berberine, a quaternary isoquinoline alkaloid, as an OCT1 and OCT2 substrate, the authors used the MDCK-OCT1-P-gp cell line to analyze the transcellular transport of this substance. The transport of berberine from the basal to the apical compartment was 3-fold, 5-fold and 1-fold higher in MDCK-OCT1-P-gp cells compared to the vectorial transport measured with MDCK-OCT1 and MDCK-P-gp single-transfected cells and MCDK control cells, respectively. Furthermore, the addition of the P-gp inhibitor LY335979 resulted in a decrease of the transcellular transport to the level measured in MCDK control cells. Even though the transcellular transport could be inhibited, an increase of the intracellular berberine amount was observed in MDCK-OCT-P-gp cells, indicating that LY335979 specifically inhibits the P-gp mediated export. Misaka et al. also established a MDCK-OCT1-P-gp double-transfectant and this cell line also showed a significant basal to apical transcellular transport of berberine, which could not be measured in the respective single-transfectants (Misaka et al., 2016). They also investigated the transcellular transport of nadolol (10 µM) with and without the addition of 1 µM zosuquidar, a known P-gp inhibitor, demonstrating that zosuquidar was able to significantly inhibit the basal to apical transport of nadolol (Misaka et al., 2016).
Sato et al. (Sato et al., 2008) established an OCT1-MATE1 double-transfected MDCK cell line and investigated the expression and localization by immunofluorescence microscopy. They used TEA as prototypic substrate and measured the transcellular transport from the basolateral to apical (b→a) and from the apical to basolateral (a→b) compartment demonstrating that the cellular accumulation was 66-fold higher, when TEA was applied to the basolateral compartment. Additionally, they were able to reproduce the pH-dependency of MATE1-mediated transport by varying the apical pH and demonstrated that the transcellular transport showed maximal transport rates at extracellular pH 6.5. The addition of 10 mM MPP+ or 1 mM levofloxacin significantly decreased the basolateral to apical transport of TEA. To further analyze the transport of organic cations, Sato and coworkers measured the transcellular transport and cellular accumulation of MPP+, metfomin, cimetidine, creatinine, guanidine, procainamide and quinidine and found significant vectorial transport rates for all substances, applied to the basolateral compartment. Unfortunately, they did not show a comparison between transcellular transport rates and the cellular uptake of substances into the MDCK-OCT1-MATE1 double-transfectant and into the corresponding single-transfectants (MDCK-OCT1 or MDCK-MATE1). The importance of the interplay of OCT1 and MATE1, studied in double-transfected cell lines could also be demonstrated by Sato et al. (Sato et al., 2008). Experiments using HEK293 cells transfected with OCT1 only showed slightly higher uptake rates of quinidine and procainamide (<2 fold) and the HEK-MATE1 cell line showed small uptake rates for quinidine (<2 fold) compared to the uptake into the vector control cell lines. This is contradictory to in vivo data that had already shown that quinidine (Notterman et al., 1986) and procainamide (Somogyi et al., 1983) are secreted renally. This underestimation of the role of OCT1 and MATE1 for the transport of both substrates was abolished by the use of double-transfected cell lines where significant transcellular transport rates could be measured for procainamide as well as for quinidine (Sato et al., 2008).
Our working group extended the investigations of Sato et al. by also establishing a MDCK-OCT1-MATE1 double-transfectant (König et al., 2011). The corresponding single-transfected cell lines (MDCK-OCT1 and MDCK-MATE1) were also used for transport assays. The cellular accumulation of MPP+ (10 and 50 µM) and metformin (10 and 50 µM) was highest in MDCK-OCT1 single-transfected cells. Interestingly, the lowest intracellular accumulation was measured in the MDCK-MATE1 single-transfected cells and not in the MDCK control cells. This can be explained by MATE1-mediated efflux of MPP+ or metformin taken up by an endogenous transporter or diffused passively into the cells when applied to the basolateral compartment. Intracellular accumulation in the MDCK-OCT1-MATE1 double-tranfected cell line was also significantly higher compared to the accumulation in the MDCK control cell line demonstrating OCT1-mediated uptake. As expected, there was no significant difference in the transcellular transport of the single-transfected cell lines and the MDCK control cells. In contrast, the MDCK-OCT1-MATE1 double-transfectant showed significantly higher transcellular transport rates for both substrates (10-fold basal to apical over apical to basal transcellular transport of metformin after 60 min). In the following years, several publications used double-transfected OCT1-MATE1 cell models to gain more insights into vectorial transport of organic cations. Reznicek et al. (Reznicek et al., 2017) used emtricitabine as substrate for vectorial transport studies and demonstrated that the transcellular transport is independent of OCT1-mediated uptake. This transport was saturable at very high concentrations (1 mM), temperature- and pH-dependent (decreasing the apical pH significantly increased the b→a transcellular transport). Furthermore, the addition of cimetidine and ritonavir, both known MATE1 inhibitors, resulted in an inhibition of the transcellular transport of emtricitabine by 43 and 35% in the double-transfectant, whereas the intracellular accumulation increased to 143 and 135%, respectively.
Chen et al. (Chen et al., 2017b) demonstrated that the basal to apical transcellular transport of ipratropium (0.5 µM) was 9.9-fold higher in MDCK-OCT1-MATE1 double-transfected cells compared to control cells and Deutsch and colleagues (Deutsch et al., 2019) identified trospium as substrate for both transporters using the same transporter combination. The vectorial basal to apical transport of trospium (1 µM) was 24.5-fold higher compared to the vectorial transport in the control cell line. As expected, the transcellular transport was highest at extracellular pH 6.5, whereas intracellular accumulation was lowest at this pH, demonstrating that OCT1 and MATE1 play an important role in the transcellular transport of trospium.
Ceckova et al. (Ceckova et al., 2016) analyzed the transcellular transport and intracellular accumulation of lamivudine in MDCK-OCT1-MATE1 double-transfected cells and their respective control and single-transfected cell lines. The transcellular transport (b→a) measured in the MDCK-MATE1 and MDCK-OCT1-MATE1 cells was significantly higher in comparison to the MDCK control cells and to the MDCK-OCT1 single-transfectant, whereas the intracellular accumulation of lamivudine was the highest in the MDCK-OCT1 cell line. This transcellular transport could be inhibited by the simultaneous application of lamivudine and mitoxantrone (2 µM) to the basolateral compartment and was reduced to a level which was not significantly different to the MDCK control cells. The fact, that mitoxantrone inhibition led to an increase of the intracellular accumulation of lamivudine, underlines the importance of MATE1 on the transport of lamivudine. Later, Ceckova et al. (Ceckova et al., 2018) used the MDCK-OCT1-MATE1 double-transfectant to study the inhibition of the transcellular transport of 2 nM MPP+ and 10 nM lamivudine by adding efavirenz. In both cases, the presence of 10 µM efavirenz in the basolateral compartment reduces the basolateral to apical transport in all single- and double-transfected cell lines, except in the MDCK control cells. The intracellular accumulation of both substrates was decreased in the MDCK-OCT1 cells but increased in the MDCK-MATE1 cells, confirming the potential of efavirenz as an in vitro inhibitor of both transport proteins (Ceckova et al., 2018). Li et al. (Li et al., 2018) addressed a potential drug-drug interaction between metformin and nuciferine, the active ingredient of lotus leafs (Folium Nelumbinis). This herbal drug is used as tea or food supplement for the elderly population suffering from hyperlipidemia and therefore a concomitant use of these herbs with antidiabetic drugs seems quite likely. After the evaluation of nuciferine inhibition (0.01–100 µM) on the OCT1-and MATE1-mediated uptake of metformin (10 µM) in single-transfected cells, they verified these findings by measuring the intracellular accumulation and transcellular transport of 10 µM metformin alone and in the presence of nuciferine (5–80 µM) in the double-transfected cell line. At all investigated time points the basolateral to apical transport of metformin was significantly higher in the MDCK-OCT1-MATE1 double-transfectant, compared to the transport in the MDCK-OCT1 single-transfectant. This transport could be inhibited by adding nuciferine in a concentration-dependent manner. Furthermore, nuciferine also reduced the intracellular accumulation of metformin. In contrast, transcellular transport from the apical to the basolateral compartment was unaltered by the addition of nuciferine. This demonstrates that nuciferine is an inhibitor of both OCT1 and MATE1. Remarkably, when applying the same experimental setup to the MDCK-OCT2-MATE1 double-tranfectant, the transcellular transport of metformin was also decreased but the intracellular accumulation of metformin significantly increased in a concentration-dependent manner after addition of nuciferine. This indicates, that the inhibition of MATE1 is responsible for this effect and nuciferine inhibits OCT1, but not OCT2 (Li et al., 2018).
In an interesting experimental setup van der Velden et al. (van der Velden et al., 2017) were not using MDCK cells to establish double-tranfectants. Instead, they used single-transfected HEK293 cells expressing OCT1 and cotransfected them with MATE1 or with MATE2-K and analyzed proguanil uptake. Because of the lack of polarized growth, vectorial transport studies cannot be performed with the double-transfected HEK293 cells. There was no significant difference in the uptake rate of HEK-OCT1 cells compared to HEK-OCT1-MATE1 cells, but the HEK-OCT1-MATE2-K cells showed a significant lower intracellular accumulation of proguanil, indicating an interplay between OCT1-mediated uptake and MATE2-K-mediated export (van der Velden et al., 2017).
Double-transfected cell models cannot only be used to study the interplay of uptake and efflux transporters, but also to investigate the interplay between transport proteins and metabolizing enzymes. To investigate this, Tu et al. established a double-transfected MDCK cell line, expressing OCT1 together with the phase I drug metabolizing enzyme CYP3A4 (Tu et al., 2014). This CYP enzyme is responsible for the metabolism of approx. 50% of all marketed drugs (Zhou, 2008). They validated the mRNA expression by RT-qPCR and confirmed the OCT1-mediated uptake by using the prototypical substrate ASP+ with or without the presence of TEA as transport inhibitor. The MDCK-OCT1 single-transfectant as well as the MDCK-OCT1-CYP3A4 double-transfectant showed significantly higher ASP+ uptake rates compared to the control cell line, which was strongly reduced by the addition of TEA. The CYP3A4 function in the MDCK-OCT1-CYP3A4 cells was confirmed by a CYP3A4 metabolism activity assay and was comparable to the values determined in MDCK-CYP3A4 single-transfected cells. Subsequently, they tested the cytotoxic activity of retrorsine, a hepatotoxic pyrrolizidine alkaloid, using all established MDCK cell lines. Prior experiments showed that the uptake of retrorsine is significantly higher in MDCK-OCT1 cells compared to the uptake into the MDCK control cells. Furthermore, Fu et al. demonstrated that pyrrolizidine alkaloids exhibit cytotoxicity only after bioactivation, which is mainly mediated by CYP3A4 (Fu et al., 2004). In line with these findings, the cytotoxicity of retrorsine was highest in the MDCK-OCT1-CYP3A4 cell line because of both uptake and bioactivation. There was no difference in the cytotoxicity between control cells and MDCK-OCT1 cells, due to the missing CYP-mediated activation. The MDCK-CYP3A4 single-transfectant also exhibit significantly higher retrorsine sensitivity, but still significantly lower compared to the double-transfectant (Tu et al., 2014).
Instead of MDCK cells, Iwai et al. used Lilly Laboratory Cancer Porcine Kidney 1 cells (LLC-PK1) to establish an OCT1-P-gp double-transfected cell line (Iwai et al., 2011). LLC-PK1 cells form tight monolayers and LLC-P-gp cells are recommended by the FDA as bidirectional transcellular transport system for identifying P-gp substrates and inhibitors Food and Drug Administration (2020). OCT1 function in these double-transfected cells was confirmed by using MPP+ as prototypical substrate and the transport function of P-gp was verified by using digoxin as substrate. The basal to apical transcellular transport of 1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-dihydro-1H-naphtho [2,3-d]imidazolium bromide (YM155, 1 µM), a survivin suppressant and known substrate of OCT1 (Iwai et al., 2009), was much higher in the LLC-OCT1-P-gp double-transfectant compared to LLC-control, LLC-OCT1 and LLC-P-gp single-tranfected cell lines, demonstrated by the high basal to apical flux ratio of 16.6. This transcellular transport decreased by adding cyclosporine A or 1 mM MPP+, respectively, indicating that YM155 is a substrate of both OCT1 and P-gp. The relatively high basal to apical transcellular transport of 1 µM digoxin was unaffected by the addition of 100 µM YM155 but was reduced to the level of the apical to basal transport by adding 10 µM cyclosporine A, demonstrating that YM155 has a low inhibitory effect on P-gp-mediated transport even at higher concentrations. Table 2 gives an overview about the studies using OCT1 expressing double-transfected cell lines.
In vitro cell models expressing transport proteins are useful tools for studies of transporter function and for the identification of transporter substrates and/or inhibitors. Therefore, the FDA and EMA recommend the usage of such cell lines during preclinical drug development. The FDA considers an investigational drug as an in vitro substrate for hepatic or renal transporters, ‘if uptake is ≥ 2-fold of the drug uptake in empty vector-transfected cells and if a known inhibitor can decrease the drug uptake to ≤50% at a concentration at least 10 times that of the Ki or IC50’. To test whether a drug is an inhibitor it is recommended to ‘determine the inhibition potency (Ki or IC50) of the drug on the uptake of a known substrate’ Food and Drug Administration (2020). In this review we describe cell models for the investigation of the SLC22 family member OCT1. Using single-transfected cell lines expressing OCT1, several drugs could be identified as substrates and inhibitors of this transporter (Table 1; Supplementary Table S2). Interestingly, it has been demonstrated that OCT1 transport inhibition is substrate-dependent. For example, Boxberger et al. detected substrate-dependent inhibition for several drug (e.g., ranitidine and fluoxetine) by using MPP+, serotonin and TEA as probe substrates in competitive counterflow experiments (Boxberger et al., 2018). Therefore, the use of multiple probe substrates for in vitro testings of OCT1 seems reasonable and the use of substrates for the inhibition analysis in vitro that can also be used in the subsequent clinical studies as recommended by the FDA Food and Drug Administration (2020).
Despite the frequent use of single- and double-transfected cell lines, in vitro-in vivo extrapolations (IVIVE) have still limitations. Many drugs listed in Supplementary Table S2 only inhibit the transport of substrates at concentrations above their therapeutic plasma concentration or environmentally exposed concentration so that the inhibitory potential is more theoretically relevant (Chedik et al., 2019). In vitro studies that analyzed opioids as inhibitors of OCT1, Meyer et al. showed that the calculated maximal unbound plasma concentrations for most of the tested opioids are lower than the obtained IC50 values for OCT1 mediated transport (Meyer et al., 2019). Only the maximal portal vein concentration of tapentadol was comparable to the obtained IC50 value, indicating a potential drug-drug interaction in vivo (Meyer et al., 2019). Furthermore, the influence of endogenous expression of transport proteins in the different cell lines, the use of different cell models (e.g., Table 1: Km TEA determined in MDCK cells, HEK293 cells and HeLa cells) and the independent establishment of several stable transfectants by different working groups lead to interlaboratory variability in the gained Km and IC50 values and to a limited IVIVE. The use of primary human hepatocytes after the in vitro validation of drugs as substrates or inhibitors of OCT1, as recommended by Bi et al., could be helpful to gain better predictions of the hepatic clearance or to identify potential DDIs and could help to evaluate the contribution of the OCT1-mediated transport of potential substrates by using selective inhibitors (Bi et al., 2019; Jensen et al., 2020a). Interestingly, strong variations in the uptake of OCT1 substrates (MPP+ and ASP+) were detected comparing human hepatocytes from different donors (De Bruyn et al., 2011; Fattah et al., 2017) and the genetic characterization revealed strong genetic variabilities between the tested batches, where 13 of 27 tested hepatocyte batches showed at least 1 nonfunctional allele of the SLC22A1 gene (Fattah et al., 2017).
The identification of OCT1 as rate-limiting transporter in the hepatic uptake of clinical important drugs together with in vivo data on reported genetic effects led to the update of the ITC recommendations, where OCT1 is now mentioned as transporter of emerging clinical importance (Zamek-Gliszczynski et al., 2018b).
Double-transfected cell lines could lead to an even better understanding of vectorial transport processes during hepatobiliary and renal elimination. They allow the simultaneous measurement of more parameters and are helpful to identify the individual transport protein underlying clinically observed drug-drug interactions and to study the impact of the respective transporters on perpetrator disposition (Müller et al., 2018b). Important double-transfected cell models for investigating the role of OCT1 in the hepatobiliary elimination of drugs are MDCK-OCT1-MATE1 cells expressing OCT1 together with the apically localized export protein MATE1. Both proteins share an overlapping substrate spectrum (Nies et al., 2011) and the vectorial transport of drugs mediated by both transporters has been described (Table 2). Interestingly, only by using double-transfected cell models the direction of the MATE1-mediated transport in the double-transfected cell lines resembles the physiological direction (efflux of substrates into the apical compartment), whereas the use of MATE1-transfected HEK293 cells only allows uptake measurements into the cell. In the recent years, several working groups established double-transfected cell lines to analyze the molecular mechanisms underlying polarized transport of endogenous compounds and drugs. Moreover, they are very useful tools for the understanding of the molecular mechanisms underlying clinically relevant drug-drug interactions (Table 2).
BH reviewed the literature, BH and JK drafted the manuscript, MFF revised the manuscript.
This work was supported by the Open Access Publishing Fund of the FAU.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the Wilhelm Sander-Stiftung (2019.050.1).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.662535/full#supplementary-material
Ahlin, G., Karlsson, J., Pedersen, J. M., Gustavsson, L., Larsson, R., Matsson, P., et al. (2008). Structural requirements for drug inhibition of the liver specific human organic cation transport protein 1. J. Med. Chem. 51, 5932–5942. doi:10.1021/jm8003152
Arimany-Nardi, C., Minuesa, G., Keller, T., Erkizia, I., Koepsell, H., Martinez-Picado, J., et al. (2016). Role of human organic cation transporter 1 (hOCT1) polymorphisms in lamivudine (3TC) uptake and drug-drug interactions. Front. Pharmacol. 7, 175. doi:10.3389/fphar.2016.00175
Baidya, A. T. K., Ghosh, K., Amin, S. A., Adhikari, N., Nirmal, J., Jha, T., et al. (2020). In silico modelling, identification of crucial molecular fingerprints, and prediction of new possible substrates of human organic cationic transporters 1 and 2. New J. Chem. 44, 4129–4143. doi:10.1039/C9NJ05825G
Bednarczyk, D., Ekins, S., Wikel, J. H., and Wright, S. H. (2003). Influence of molecular structure on substrate binding to the human organic cation transporter, hOCT1. Mol. Pharmacol. 63, 489–498. doi:10.1124/mol.63.3.489
Bexten, M., Oswald, S., Grube, M., Jia, J., Graf, T., Zimmermann, U., et al. (2015). Expression of drug transporters and drug metabolizing enzymes in the bladder urothelium in man and affinity of the bladder spasmolytic trospium chloride to transporters likely involved in its pharmacokinetics. Mol. Pharmaceutics 12, 171–178. doi:10.1021/mp500532x
Bi, Y. A., Costales, C., Mathialagan, S., West, M., Eatemadpour, S., Lazzaro, S., et al. (2019). Quantitative contribution of six major transporters to the hepatic uptake of drugs: "SLC-Phenotyping" using primary human hepatocytes. J. Pharmacol. Exp. Ther. 370, 72–83. doi:10.1124/jpet.119.257600
Bourdet, D. L., Pritchard, J. B., and Thakker, D. R. (2005). Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3). J. Pharmacol. Exp. Ther. 315, 1288–1297. doi:10.1124/jpet.105.091223
Boxberger, K. H., Hagenbuch, B., and Lampe, J. N. (2014). Common drugs inhibit human organic cation transporter 1 (OCT1)-mediated neurotransmitter uptake. Drug Metab. Dispos. 42, 990–995. doi:10.1124/dmd.113.055095
Boxberger, K. H., Hagenbuch, B., and Lampe, J. N. (2018). Ligand-dependent modulation of hOCT1 transport reveals discrete ligand binding sites within the substrate translocation channel. Biochem. Pharmacol. 156, 371–384. doi:10.1016/j.bcp.2018.08.028
Campbell, S. D., Gadel, S., Friedel, C., Crafford, A., Regina, K. J., and Kharasch, E. D. (2015). Influence of HIV antiretrovirals on methadone N-demethylation and transport. Biochem. Pharmacol. 95, 115–125. doi:10.1016/j.bcp.2015.03.007
Ceckova, M., Reznicek, J., Deutsch, B., Fromm, M. F., and Staud, F. (2018). Efavirenz reduces renal excretion of lamivudine in rats by inhibiting organic cation transporters (OCT, Oct) and multidrug and toxin extrusion proteins (MATE, Mate). PLoS One 13, e0202706. doi:10.1371/journal.pone.0202706
Ceckova, M., Reznicek, J., Ptackova, Z., Cerveny, L., Müller, F., Kacerovsky, M., et al. (2016). Role of ABC and solute carrier transporters in the placental transport of lamivudine. Antimicrob. Agents Chemother. 60, 5563–5572. doi:10.1128/AAC.00648-16
Chedik, L., Bruyère, A., and Fardel, O. (2019). Interactions of organophosphorus pesticides with solute carrier (SLC) drug transporters. Xenobiotica 49, 363–374. doi:10.1080/00498254.2018.1442030
Chen, L., Shu, Y., Liang, X., Chen, E. C., Yee, S. W., Zur, A. A., et al. (2014). OCT1 is a high-capacity thiamine transporter that regulates hepatic steatosis and is a target of metformin. Proc. Natl. Acad. Sci. USA 111, 9983–9988. doi:10.1073/pnas.1314939111
Chen, E. C., Khuri, N., Liang, X., Stecula, A., Chien, H.-C., Yee, S. W., et al. (2017a). Discovery of competitive and noncompetitive ligands of the organic cation transporter 1 (OCT1; SLC22A1). J. Med. Chem. 60, 2685–2696. doi:10.1021/acs.jmedchem.6b01317
Chen, J., Brockmöller, J., Seitz, T., König, J., Tzvetkov, M. V., and Chen, X. (2017b). Tropane alkaloids as substrates and inhibitors of human organic cation transporters of the SLC22 (OCT) and the SLC47 (MATE) families. Biol. Chem. 398, 237–249. doi:10.1515/hsz-2016-0236
Chen, J. Y., Brockmöller, J., Tzvetkov, M. V., Wang, L. J., and Chen, X. J. (2019). An in vitro study on interaction of anisodine and monocrotaline with organic cation transporters of the SLC22 and SLC47 families. Chin. J. Nat. Med. 17, 490–497. doi:10.1016/S1875-5364(19)30070-6
Chen, M., Neul, C., Schaeffeler, E., Frisch, F., Winter, S., Schwab, M., et al. (2020). Sorafenib activity and disposition in liver cancer does not depend on organic cation transporter 1. Clin. Pharmacol. Ther. 107, 227–237. doi:10.1002/cpt.1588
Chevalier, C., Fouqueray, P., and Bolze, S. (2020). In vitro investigation, pharmacokinetics and disposition of imeglimin, a novel oral antidiabetic drug, in preclinical species and humans. Drug Metab. Dispos. doi:10.1124/dmd.120.00015410.1007/s40262-020-00948-1
Ciarimboli, G., Koepsell, H., Iordanova, M., Gorboulev, V., Dürner, B., Lang, D., et al. (2005). Individual PKC-phosphorylation sites in organic cation transporter 1 determine substrate selectivity and transport regulation. Jasn 16, 1562–1570. doi:10.1681/ASN.2004040256
Comer, J., and Tam, K. (2001). “Lipophilicity profiles: theory and measurement,” in Pharmacokinetic optimization in drug research. Editors B. Testa, H. Van De Waterbeemd, G. Folkers, and R. Guy (Zürich, Switzerland: Verlag Helvetica Chimica Acta), 275–304. doi:10.1002/9783906390437.ch17
Damme, K., Nies, A. T., Schaeffeler, E., and Schwab, M. (2011). Mammalian MATE (SLC47A) transport proteins: impact on efflux of endogenous substrates and xenobiotics. Drug Metab. Rev. 43, 499–523. doi:10.3109/03602532.2011.602687
De Bruyn, T., Ye, Z. W., Peeters, A., Sahi, J., Baes, M., Augustijns, P. F., et al. (2011). Determination of OATP-, NTCP- and OCT-mediated substrate uptake activities in individual and pooled batches of cryopreserved human hepatocytes. Eur. J. Pharm. Sci. 43, 297–307. doi:10.1016/j.ejps.2011.05.002
Deutsch, B., Neumeister, C., Schwantes, U., Fromm, M. F., and König, J. (2019). Interplay of the organic cation transporters OCT1 and OCT2 with the apically localized export protein MATE1 for the polarized transport of trospium. Mol. Pharmaceutics 16, 510–517. doi:10.1021/acs.molpharmaceut.8b00779
Dickens, D., Owen, A., Alfirevic, A., Giannoudis, A., Davies, A., Weksler, B., et al. (2012). Lamotrigine is a substrate for OCT1 in brain endothelial cells. Biochem. Pharmacol. 83, 805–814. doi:10.1016/j.bcp.2011.12.032
Dos Santos Pereira, J. N., Tadjerpisheh, S., Abed, M. A., Saadatmand, A. R., Weksler, B., Romero, I. A., et al. (2014). The poorly membrane permeable antipsychotic drugs amisulpride and sulpiride are substrates of the organic cation transporters from the SLC22 family. AAPS J. 16, 1247–1258. doi:10.1208/s12248-014-9649-9
Egenberger, B., Gorboulev, V., Keller, T., Gorbunov, D., Gottlieb, N., Geiger, D., et al. (2012). A substrate binding hinge domain is critical for transport-related structural changes of organic cation transporter 1*. J. Biol. Chem. 287, 31561–31573. doi:10.1074/jbc.M112.388793
Ellawatty, W. E. A., Masuo, Y., Fujita, K.-I., Yamazaki, E., Ishida, H., Arakawa, H., et al. (2018). Organic cation transporter 1 is responsible for hepatocellular uptake of the tyrosine kinase inhibitor pazopanib. Drug Metab. Dispos 46, 33–40. doi:10.1124/dmd.117.076554
European Medicines Agency (2012). Guideline on the investigation of drug interactions. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions-revision-1_en.pdf (Accessed November 23, 2020)
Fattah, S., Shinde, A. B., Matic, M., Baes, M., Van Schaik, R. H. N., Allegaert, K., et al. (2017). Inter-subject variability in OCT1 activity in 27 batches of cryopreserved human hepatocytes and association with OCT1 mRNA expression and genotype. Pharm. Res. 34, 1309–1319. doi:10.1007/s11095-017-2148-9
Floerl, S., Kuehne, A., and Hagos, Y. (2020). Functional and pharmacological comparison of human, mouse, and rat organic cation transporter 1 toward drug and pesticide interaction. Int. J. Mol. Sci 21, 6871. doi:10.3390/ijms21186871
Food and Drug Administration (2020). In vitro drug interaction studies-cytochrome P450 enzyme- and transporter-mediated drug interactions; guidance for industry. Available at: https://www.fda.gov/media/134582/download (Accessed November 23, 2020)
Fromm, M. F. (2004). Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol. Sci. 25, 423–429. doi:10.1016/j.tips.2004.06.002
Fu, P. P., Xia, Q., Lin, G., and Chou, M. W. (2004). Pyrrolizidine alkaloids-genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab. Rev. 36, 1–55. doi:10.1081/dmr-120028426
Glaeser, H., Bujok, K., Schmidt, I., Fromm, M. F., and Mandery, K. (2014). Organic anion transporting polypeptides and organic cation transporter 1 contribute to the cellular uptake of the flavonoid quercetin. Naunyn-schmiedeberg's Arch. Pharmacol. 387, 883–891. doi:10.1007/s00210-014-1000-6
Gorboulev, V., Ulzheimer, J. C., Akhoundova, A., Ulzheimer-Teuber, I., Karbach, U., Quester, S., et al. (1997). Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 16, 871–881. doi:10.1089/dna.1997.16.871
Gottesman, M. M., Fojo, T., and Bates, S. E. (2002). Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer 2, 48–58. doi:10.1038/nrc706
Gründemann, D., Gorboulev, V., Gambaryan, S., Veyhl, M., and Koepsell, H. (1994). Drug excretion mediated by a new prototype of polyspecific transporter. Nature 372, 549–552. doi:10.1038/372549a0
Gründemann, D., Hahne, C., Berkels, R., and Schömig, E. (2003). Agmatine is efficiently transported by non-neuronal monoamine transporters extraneuronal monoamine transporter (EMT) and organic cation transporter 2 (OCT2). J. Pharmacol. Exp. Ther. 304, 810–817. doi:10.1124/jpet.102.044404
Harrach, S., Edemir, B., Schmidt-Lauber, C., Pap, T., Bertrand, J., and Ciarimboli, G. (2017). Importance of the novel organic cation transporter 1 for tyrosine kinase inhibition by saracatinib in rheumatoid arthritis synovial fibroblasts. Sci. Rep. 7, 1258. doi:10.1038/s41598-017-01438-4
Hendrickx, R., Johansson, J. G., Lohmann, C., Jenvert, R.-M., Blomgren, A., Börjesson, L., et al. (2013). Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J. Med. Chem. 56, 7232–7242. doi:10.1021/jm400966v
Hillgren, K. M., Keppler, D., Zur, A. A., Giacomini, K. M., Stieger, B., Cass, C. E., et al. (2013). Emerging transporters of clinical importance: an update from the International Transporter Consortium. Clin. Pharmacol. Ther. 94, 52–63. doi:10.1038/clpt.2013.74
Hu, J., Han, J., Li, H., Zhang, X., Liu, L. l., Chen, F., et al. (2018). Human embryonic kidney 293 cells: a vehicle for biopharmaceutical manufacturing, structural biology, and electrophysiology. Cells Tissues Organs 205, 1–8. doi:10.1159/000485501
International Transporter Consortium Giacomini, K. M., Huang, S. M., Tweedie, D. J., Benet, L. Z., Brouwer, K. L., et al. (2010). Membrane transporters in drug development. Nat. Rev. Drug Discov. 9, 215–236. doi:10.1038/nrd3028
Inui, K.-I., Masuda, S., and Saito, H. (2000). Cellular and molecular aspects of drug transport in the kidney. Kidney Int. 58, 944–958. doi:10.1046/j.1523-1755.2000.00251.x
Iwai, M., Minematsu, T., Li, Q., Iwatsubo, T., and Usui, T. (2011). Utility of P-glycoprotein and organic cation transporter 1 double-transfected LLC-PK1 cells for studying the interaction of YM155 monobromide, novel small-molecule survivin suppressant, with P-glycoprotein. Drug Metab. Dispos. 39, 2314–2320. doi:10.1124/dmd.111.040733
Iwai, M., Minematsu, T., Narikawa, S., Usui, T., and Kamimura, H. (2009). Involvement of human organic cation transporter 1 in the hepatic uptake of 1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-dihydro-1H-naphtho[2,3-d]imidazolium bromide (YM155 monobromide), a novel, small molecule survivin suppressant. Drug Metab. Dispos 37, 1856–1863. doi:10.1124/dmd.109.027359
Jensen, O., Brockmöller, J., and Dücker, C. (2021). Identification of novel high-affinity substrates of OCT1 using machine learning-guided virtual screening and experimental validation. J. Med. Chem. 64, 2762. doi:10.1021/acs.jmedchem.0c02047
Jensen, O., Matthaei, J., Blome, F., Schwab, M., Tzvetkov, M. V., and Brockmöller, J. (2020a). Variability and heritability of thiamine pharmacokinetics with focus on OCT1 effects on membrane transport and pharmacokinetics in humans. Clin. Pharmacol. Ther. 107, 628–638. doi:10.1002/cpt.1666
Jensen, O., Rafehi, M., Tzvetkov, M. V., and Brockmöller, J. (2020b). Stereoselective cell uptake of adrenergic agonists and antagonists by organic cation transporters. Biochem. Pharmacol. 171, 113731. doi:10.1016/j.bcp.2019.113731
Jouan, E., Le Vee, M., Denizot, C., Da Violante, G., and Fardel, O. (2014). The mitochondrial fluorescent dye rhodamine 123 is a high-affinity substrate for organic cation transporters (OCTs) 1 and 2. Fundam. Clin. Pharmacol. 28, 65–77. doi:10.1111/j.1472-8206.2012.01071.x
Jung, N., Lehmann, C., Rubbert, A., Knispel, M., Hartmann, P., Van Lunzen, J., et al. (2008). Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab. Dispos 36, 1616–1623. doi:10.1124/dmd.108.020826
Keiser, M., Hasan, M., and Oswald, S. (2018). Affinity of ketamine to clinically relevant transporters. Mol. Pharmaceutics 15, 326–331. doi:10.1021/acs.molpharmaceut.7b00627
Kerb, R., Brinkmann, U., Chatskaia, N., Gorbunov, D., Gorboulev, V., Mornhinweg, E., et al. (2002). Identification of genetic variations of the human organic cation transporter hOCT1 and their functional consequences. Pharmacogenetics 12, 591–595. doi:10.1097/00008571-200211000-00002
Kimura, H., Takeda, M., Narikawa, S., Enomoto, A., Ichida, K., and Endou, H. (2002). Human organic anion transporters and human organic cation transporters mediate renal transport of prostaglandins. J. Pharmacol. Exp. Ther. 301, 293–298. doi:10.1124/jpet.301.1.293
Kimura, N., Masuda, S., Tanihara, Y., Ueo, H., Okuda, M., Katsura, T., et al. (2005). Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab. Pharmacokinet. 20, 379–386. doi:10.2133/dmpk.20.379
Kivistö, K. T., Niemi, M., and Fromm, M. F. (2004). Functional interaction of intestinal CYP3A4 and P-glycoprotein. Fundam. Clin. Pharmacol. 18, 621–626. doi:10.1111/j.1472-8206.2004.00291.x
Knop, J., Misaka, S., Singer, K., Hoier, E., Müller, F., Glaeser, H., et al. (2015). Inhibitory effects of green tea and (-)-epigallocatechin gallate on transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-Glycoprotein. PLoS One 10, e0139370. doi:10.1371/journal.pone.0139370
Kobayashi, M., Mizutani, A., Nishi, K., Muranaka, Y., Nishii, R., Shikano, N., et al. (2020). [131I]MIBG exports via MRP transporters and inhibition of the MRP transporters improves accumulation of [131I]MIBG in neuroblastoma. Nucl. Med. Biol. 90-91, 49–54. doi:10.1016/j.nucmedbio.2020.09.004
Koepsell, H. (2020). Organic cation transporters in health and disease. Pharmacol. Rev. 72, 253–319. doi:10.1124/pr.118.015578
König, J., Müller, F., and Fromm, M. F. (2013). Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol. Rev. 65, 944–966. doi:10.1124/pr.113.007518
König, J., Zolk, O., Singer, K., Hoffmann, C., and Fromm, M. (2011). Double-transfected MDCK cells expressing human OCT1/MATE1 or OCT2/MATE1: determinants of uptake and transcellular translocation of organic cations. Br. J. Pharmacol. 163, 546–555. doi:10.1111/j.1476-5381.2010.01052.x
Lee, W.-K., Reichold, M., Edemir, B., Ciarimboli, G., Warth, R., Koepsell, H., et al. (2009). Organic cation transporters OCT1, 2, and 3 mediate high-affinity transport of the mutagenic vital dye ethidium in the kidney proximal tubule. Am. J. Physiol.-Renal Physiol. 296, F1504–F1513. doi:10.1152/ajprenal.90754.2008
Leopoldo, M., Nardulli, P., Contino, M., Leonetti, F., Luurtsema, G., and Colabufo, N. A. (2019). An updated patent review on P-glycoprotein inhibitors (2011-2018). Expert Opin. Ther. Patents 29, 455–461. doi:10.1080/13543776.2019.1618273
Li, L., Tu, M., Yang, X., Sun, S., Wu, X., Zhou, H., et al. (2014). The contribution of human OCT1, OCT3, and CYP3A4 to nitidine chloride-induced hepatocellular toxicity. Drug Metab. Dispos 42, 1227–1234. doi:10.1124/dmd.113.056689
Li, L., Sun, S., Weng, Y., Song, F., Zhou, S., Bai, M., et al. (2016). Interaction of six protoberberine alkaloids with human organic cation transporters 1, 2 and 3. Xenobiotica 46, 175–183. doi:10.3109/00498254.2015.1056283
Li, L., Lei, H., Wang, W., Du, W., Yuan, J., Tu, M., et al. (2018). Co-administration of nuciferine reduces the concentration of metformin in liver via differential inhibition of hepatic drug transporter OCT1 and MATE1. Biopharm. Drug Dispos 39, 411–419. doi:10.1002/bdd.2158
Liang, X., Yee, S. W., Chien, H. C., Chen, E. C., Luo, Q., Zou, L., et al. (2018). Organic cation transporter 1 (OCT1) modulates multiple cardiometabolic traits through effects on hepatic thiamine content. PLoS Biol. 16, e2002907. doi:10.1371/journal.pbio.2002907
Liang, R. F., Ge, W. J., Song, X. M., Zhang, J. P., Cui, W. F., Zhang, S. F., et al. (2020). Involvement of organic anion-transporting polypeptides and organic cation transporter in the hepatic uptake of jatrorrhizine. Xenobiotica 50, 479–487. doi:10.1080/00498254.2019.1651921
Lips, K. S., Volk, C., Schmitt, B. M., Pfeil, U., Arndt, P., Miska, D., et al. (2005). Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am. J. Respir. Cell Mol Biol 33, 79–88. doi:10.1165/rcmb.2004-0363OC
López Quiñones, A. J., Wagner, D. J., and Wang, J. (2020). Characterization of meta-iodobenzylguanidine (mIBG) transport by polyspecific organic cation transporters: implication for mIBG therapy. Mol. Pharmacol. 98, 109–119. doi:10.1124/mol.120.119495
Lovejoy, K. S., Todd, R. C., Zhang, S., Mccormick, M. S., D'aquino, J. A., Reardon, J. T., et al. (2008). cis-Diammine(pyridine)chloroplatinum(II), a monofunctional platinum(II) antitumor agent: uptake, structure, function, and prospects. Proc. Natl. Acad. Sci. 105, 8902–8907. doi:10.1073/pnas.0803441105
Manallack, D. T. (2007). The pK(a) distribution of drugs: application to drug discovery. Perspect. Med. Chem. 1, 25–38. doi:10.1177/1177391x0700100003
Masuda, S., Terada, T., Yonezawa, A., Tanihara, Y., Kishimoto, K., Katsura, T., et al. (2006). Identification and functional characterization of a new human kidney-specific H+/Organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J. Am. Soc. Nephrol. 17, 2127–2135. doi:10.1681/ASN.2006030205
Matsson, E. M., Eriksson, U. G., Palm, J. E., Artursson, P., Karlgren, M., Lazorova, L., et al. (2013). Combined in vitro-in vivo approach to assess the hepatobiliary disposition of a novel oral thrombin inhibitor. Mol. Pharmaceutics 10, 4252–4262. doi:10.1021/mp400341t
Matthaei, J., Kuron, D., Faltraco, F., Knoch, T., Dos Santos Pereira, J., Abu Abed, M., et al. (2016). OCT1 mediates hepatic uptake of sumatriptan and loss-of-functionOCT1polymorphisms affect sumatriptan pharmacokinetics. Clin. Pharmacol. Ther. 99, 633–641. doi:10.1002/cpt.317
Matthaei, J., Seitz, T., Jensen, O., Tann, A., Prukop, T., Tadjerpisheh, S., et al. (2019). OCT 1 deficiency affects hepatocellular concentrations and pharmacokinetics of cycloguanil, the active metabolite of the antimalarial drug proguanil. Clin. Pharmacol. Ther. 105, 190–200. doi:10.1002/cpt.1128
Meyer, M. J., Neumann, V. E., Friesacher, H. R., Zdrazil, B., Brockmöller, J., and Tzvetkov, M. V. (2019). Opioids as substrates and inhibitors of the genetically highly variable organic cation transporter OCT1. J. Med. Chem. 62, 9890–9905. doi:10.1021/acs.jmedchem.9b01301
Meyer, M. J., Seitz, T., Brockmöller, J., and Tzvetkov, M. V. (2017). Effects of genetic polymorphisms on the OCT1 and OCT2-mediated uptake of ranitidine. PLoS One 12, e0189521. doi:10.1371/journal.pone.0189521
Meyer, M. J., Tuerkova, A., Römer, S., Wenzel, C., Seitz, T., Gaedcke, J., et al. (2020). Differences in metformin and thiamine uptake between human and mouse organic cation transporter 1: structural determinants and potential consequences for intrahepatic concentrations. Drug Metab. Dispos 48, 1380. doi:10.1124/dmd.120.000170
Mimura, Y., Yasujima, T., Ohta, K., Inoue, K., and Yuasa, H. (2015). Functional identification of organic cation transporter 1 as an atenolol transporter sensitive to flavonoids. Biochem. Biophys. Rep. 2, 166–171. doi:10.1016/j.bbrep.2015.06.005
Minematsu, T., Iwai, M., Umehara, K.-i., Usui, T., and Kamimura, H. (2010). Characterization of human organic cation transporter 1 (OCT1/SLC22A1)- and OCT2 (SLC22A2)-mediated transport of 1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-dihydro-1H-naphtho[2,3-d]imidazolium bromide (YM155 monobromide), a novel small molecule survivin suppressant. Drug Metab. Dispos. 38, 1–4. doi:10.1124/dmd.109.028142
Ming, X., Ju, W., Wu, H., Tidwell, R. R., Hall, J. E., and Thakker, D. R. (2009). Transport of dicationic drugs pentamidine and furamidine by human organic cation transporters. Drug Metab. Dispos. 37, 424–430. doi:10.1124/dmd.108.024083
Minuesa, G., Volk, C., Molina-Arcas, M., Gorboulev, V., Erkizia, I., Arndt, P., et al. (2009). Transport of lamivudine [(-)-β-l-2′,3′-dideoxy-3′-thiacytidine] and high-affinity interaction of nucleoside reverse transcriptase inhibitors with human organic cation transporters 1, 2, and 3. J. Pharmacol. Exp. Ther. 329, 252–261. doi:10.1124/jpet.108.146225
Misaka, S., Knop, J., Singer, K., Hoier, E., Keiser, M., Müller, F., et al. (2016). The nonmetabolized β-blocker nadolol is a substrate of OCT1, OCT2, MATE1, MATE2-K, and P-glycoprotein, but not of OATP1B1 and OATP1B3. Mol. Pharm. 13, 512–519. doi:10.1021/acs.molpharmaceut.5b00733
Miyake, T., Mizuno, T., Mochizuki, T., Kimura, M., Matsuki, S., Irie, S., et al. (2017). Involvement of organic cation transporters in the kinetics of trimethylamine N-oxide. J. Pharm. Sci. 106, 2542–2550. doi:10.1016/j.xphs.2017.04.067
Miyake, T., Mizuno, T., Takehara, I., Mochizuki, T., Kimura, M., Matsuki, S., et al. (2019). Elucidation of N1-methyladenosine as a potential surrogate biomarker for drug interaction studies involving renal organic cation transporters. Drug Metab. Dispos. 47, 1270–1280. doi:10.1124/dmd.119.087262
More, S. S., Li, S., Yee, S. W., Chen, L., Xu, Z., Jablons, D. M., et al. (2010). Organic cation transporters modulate the uptake and cytotoxicity of picoplatin, a third-generation platinum analogue. Mol. Cancer Ther. 9, 1058–1069. doi:10.1158/1535-7163.MCT-09-1084
Morse, B. L., Kolur, A., Hudson, L. R., Hogan, A. T., Chen, L. H., Brackman, R. M., et al. (2020). Pharmacokinetics of organic cation transporter 1 (OCT1) substrates in Oct1/2 knockout mice and species difference in hepatic OCT1-mediated uptake. Drug Metab. Dispos 48, 93–105. doi:10.1124/dmd.119.088781
Motohashi, H., and Inui, K.-i. (2013). Multidrug and toxin extrusion family SLC47: physiological, pharmacokinetic and toxicokinetic importance of MATE1 and MATE2-K. Mol. Aspects Med. 34, 661–668. doi:10.1016/j.mam.2012.11.004
Müller, F., Sharma, A., König, J., and Fromm, M. F. (2018a). Biomarkers for in vivo assessment of transporter function. Pharmacol. Rev. 70, 246–277. doi:10.1124/pr.116.013326
Müller, F., Weitz, D., Mertsch, K., König, J., and Fromm, M. F. (2018b). Importance of OCT2 and MATE1 for the cimetidine-metformin interaction: insights from investigations of polarized transport in single- and double-transfected MDCK cells with a focus on perpetrator disposition. Mol. Pharmaceutics 15, 3425–3433. doi:10.1021/acs.molpharmaceut.8b00416
Neuhoff, S., Ungell, A. L., Zamora, I., and Artursson, P. (2003). pH-dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: implications for drug-drug interactions. Pharm. Res. 20, 1141–1148. doi:10.1023/a:1025032511040
Neul, C., Hofmann, U., Schaeffeler, E., Winter, S., Klein, K., Giacomini, K. M., et al. (2021). Characterization of cytochrome P450 (CYP) 2D6 drugs as substrates of human organic cation transporters and multidrug and toxin extrusion proteins. Br. J. Pharmacol. 178, 1459. doi:10.1111/bph.15370
Nies, A. T., Koepsell, H., Damme, K., and Schwab, M. (2011). “Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy,” in Handbook of Exprimental Pharmacol 201, drug transporters. Editors M. F. Fromm, and R. B. Kim (Berlin-Heidelberg: Springer-Verlag), 105–167. doi:10.1007/978-3-642-14541-4_3
Nies, A. T., Damme, K., Kruck, S., Schaeffeler, E., and Schwab, M. (2016). Structure and function of multidrug and toxin extrusion proteins (MATEs) and their relevance to drug therapy and personalized medicine. Arch. Toxicol. 90, 1555–1584. doi:10.1007/s00204-016-1728-5
Nies, A. T., Herrmann, E., Brom, M., and Keppler, D. (2008). Vectorial transport of the plant alkaloid berberine by double-transfected cells expressing the human organic cation transporter 1 (OCT1, SLC22A1) and the efflux pump MDR1 P-glycoprotein (ABCB1). Naunyn-schmied Arch. Pharmacol. 376, 449–461. doi:10.1007/s00210-007-0219-x
Nies, A. T., Koepsell, H., Winter, S., Burk, O., Klein, K., Kerb, R., et al. (2009). Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology 50, 1227–1240. doi:10.1002/hep.23103
Notterman, D. A., Drayer, D. E., Metakis, L., and Reidenberg, M. M. (1986). Stereoselective renal tubular secretion of quinidine and quinine. Clin. Pharmacol. Ther. 40, 511–517. doi:10.1038/clpt.1986.216
Obianom, O. N., Coutinho, A. L., Yang, W., Yang, H., Xue, F., and Shu, Y. (2017). Incorporation of a biguanide scaffold enhances drug uptake by organic cation transporters 1 and 2. Mol. Pharm. 14, 2726–2739. doi:10.1021/acs.molpharmaceut.7b00285
Otsuka, M., Matsumoto, T., Morimoto, R., Arioka, S., Omote, H., and Moriyama, Y. (2005). A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. 102, 17923–17928. doi:10.1073/pnas.0506483102
Otter, M., Oswald, S., Siegmund, W., and Keiser, M. (2017). Effects of frequently used pharmaceutical excipients on the organic cation transporters 1-3 and peptide transporters 1/2 stably expressed in MDCKII cells. Eur. J. Pharm. Biopharm. 112, 187–195. doi:10.1016/j.ejpb.2016.11.028
Pallen, M. (1999). RpoN-dependent transcription of rpoH? Mol. Microbiol. 31, 393. doi:10.1046/j.1365-2958.1999.01148.x
Parvez, M. M., Kaisar, N., Shin, H. J., Lee, Y. J., and Shin, J. G. (2018). Comprehensive substrate characterization of 22 antituberculosis drugs for multiple solute carrier (SLC) uptake Transporters in vitro. Antimicrob. Agents Chemother. 62, e00512-18. doi:10.1128/AAC.00512-18
Parvez, M. M., Shin, H. J., Jung, J. A., and Shin, J. G. (2017). Evaluation of para-aminosalicylic acid as a substrate of multiple solute carrier uptake transporters and possible drug interactions with nonsteroidal anti-inflammatory drugs in vitro. Antimicrob. Agents Chemother. 61, e02392-16. doi:10.1128/AAC.02392-16
Reznicek, J., Ceckova, M., Cerveny, L., Müller, F., and Staud, F. (2017). Emtricitabine is a substrate of MATE1 but not of OCT1, OCT2, P-gp, BCRP or MRP2 transporters. Xenobiotica 47, 77–85. doi:10.3109/00498254.2016.1158886
Saadatmand, A. R., Tadjerpisheh, S., Brockmöller, J., and Tzvetkov, M. V. (2012). The prototypic pharmacogenetic drug debrisoquine is a substrate of the genetically polymorphic organic cation transporter OCT1. Biochem. Pharmacol. 83, 1427–1434. doi:10.1016/j.bcp.2012.01.032
Salomon, J. J., Hagos, Y., Petzke, S., Kühne, A., Gausterer, J. C., Hosoya, K.-i., et al. (2015). Beta-2 adrenergic agonists are substrates and inhibitors of human organic cation transporter 1. Mol. Pharm. 12, 2633–2641. doi:10.1021/mp500854e
Sato, T., Masuda, S., Yonezawa, A., Tanihara, Y., Katsura, T., and Inui, K. I. (2008). Transcellular transport of organic cations in double-transfected MDCK cells expressing human organic cation transporters hOCT1/hMATE1 and hOCT2/hMATE1. Biochem. Pharmacol. 76, 894–903. doi:10.1016/j.bcp.2008.07.005
Seitz, T., Stalmann, R., Dalila, N., Chen, J., Pojar, S., Dos Santos Pereira, J. N., et al. (2015). Global genetic analyses reveal strong inter-ethnic variability in the loss of activity of the organic cation transporter OCT1. Genome Med. 7, 56. doi:10.1186/s13073-015-0172-0
Shu, Y., Leabman, M. K., Feng, B., Mangravite, L. M., Huang, C. C., Stryke, D., et al. (2003). Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc. Natl. Acad. Sci. 100, 5902–5907. doi:10.1073/pnas.0730858100
Somogyi, A., Mclean, A., and Heinzow, B. (1983). Cimetidine-procainamide pharmacokinetic interaction in man: evidence of competition for tubular secretion of basic drugs. Eur. J. Clin. Pharmacol. 25, 339–345. doi:10.1007/bf01037945
Swift, B., Nebot, N., Lee, J. K., Han, T., Proctor, W. R., Thakker, D. R., et al. (2013). Sorafenib hepatobiliary disposition: mechanisms of hepatic uptake and disposition of generated metabolites. Drug Metab. Dispos. 41, 1179–1186. doi:10.1124/dmd.112.048181
Tachampa, K., Takeda, M., Khamdang, S., Noshiro-Kofuji, R., Tsuda, M., Jariyawat, S., et al. (2008). Interactions of organic anion transporters and organic cation transporters with mycotoxins. J. Pharmacol. Sci. 106, 435–443. doi:10.1254/jphs.fp0070911
Taghikhani, E., Fromm, M. F., and König, J. (2017). “Assays for analyzing the role of transport proteins in the uptake and the vectorial transport of substances affecting cell viability,” in Methods in molecular biology. Cell viability assays. Editors D. F. Gilbert, and O. Friedrich (New York, NY: Humana Press), 1601, 123–135. doi:10.1007/978-1-4939-6960-9_11
Takano, H., Ito, S., Zhang, X., Ito, H., Zhang, M. R., Suzuki, H., et al. (2017). Possible role of organic cation transporters in the distribution of [11C]sulpiride, a dopamine D2 receptor antagonist. J. Pharm. Sci. 106, 2558–2565. doi:10.1016/j.xphs.2017.05.006
Takeda, M., Khamdang, S., Narikawa, S., Kimura, H., Kobayashi, Y., Yamamoto, T., et al. (2002). Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J. Pharmacol. Exp. Ther. 300, 918–924. doi:10.1124/jpet.300.3.918
Taubert, D., Grimberg, G., Stenzel, W., and Schömig, E. (2007). Identification of the endogenous key substrates of the human organic cation transporter OCT2 and their implication in function of dopaminergic neurons. PLoS One 2, e385. doi:10.1371/journal.pone.0000385
Te Brake, L. H. M., Van Den Heuvel, J. J. M. W., Buaben, A. O., Van Crevel, R., Bilos, A., Russel, F. G., et al. (2016). Moxifloxacin is a potent in vitro inhibitor of OCT‐ and MATE‐mediated transport of metformin and ethambutol. Antimicrob. Agents Chemother. 60, 7105–7114. doi:10.1128/AAC.01471-16
Terada, T., and Inui, K. I. (2008). Physiological and pharmacokinetic roles of H+/organic cation antiporters (MATE/SLC47A). Biochem. Pharmacol. 75, 1689–1696. doi:10.1016/j.bcp.2007.12.008
Thiebaut, F., Tsuruo, T., Hamada, H., Gottesman, M. M., Pastan, I., and Willingham, M. C. (1987). Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. 84, 7735–7738. doi:10.1073/pnas.84.21.7735
Thomas, P., and Smart, T. G. (2005). HEK293 cell line: a vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods 51, 187–200. doi:10.1016/j.vascn.2004.08.014
Tu, M., Li, L., Lei, H., Ma, Z., Chen, Z., Sun, S., et al. (2014). Involvement of organic cation transporter 1 and CYP3A4 in retrorsine-induced toxicity. Toxicology 322, 34–42. doi:10.1016/j.tox.2014.04.007
Tu, M., Sun, S., Wang, K., Peng, X., Wang, R., Li, L., et al. (2013). Organic cation transporter 1 mediates the uptake of monocrotaline and plays an important role in its hepatotoxicity. Toxicology 311, 225–230. doi:10.1016/j.tox.2013.06.009
Tzvetkov, M. V., Dos Santos Pereira, J. N., Meineke, I., Saadatmand, A. R., Stingl, J. C., and Brockmöller, J. (2013). Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in OCT1 gene affect morphine pharmacokinetics after codeine administration. Biochem. Pharmacol. 86, 666–678. doi:10.1016/j.bcp.2013.06.019
Tzvetkov, M. V., Matthaei, J., Pojar, S., Faltraco, F., Vogler, S., Prukop, T., et al. (2018). Increased systemic exposure and stronger cardiovascular and metabolic adverse reactions to fenoterol in individuals with heritable OCT1 deficiency. Clin. Pharmacol. Ther. 103, 868–878. doi:10.1002/cpt.812
Tzvetkov, M. V., Saadatmand, A. R., Bokelmann, K., Meineke, I., Kaiser, R., and Brockmöller, J. (2012). Effects of OCT1 polymorphisms on the cellular uptake, plasma concentrations and efficacy of the 5-HT3 antagonists tropisetron and ondansetron. Pharmacogenomics J. 12, 22–29. doi:10.1038/tpj.2010.75
Tzvetkov, M. V., Saadatmand, A. R., Lötsch, J., Tegeder, I., Stingl, J. C., and Brockmöller, J. (2011). Genetically polymorphic OCT1: another piece in the puzzle of the variable pharmacokinetics and pharmacodynamics of the opioidergic drug tramadol. Clin. Pharmacol. Ther. 90, 143–150. doi:10.1038/clpt.2011.56
Umehara, K.-I., Iwatsubo, T., Noguchi, K., and Kamimura, H. (2007). Comparison of the kinetic characteristics of inhibitory effects exerted by biguanides and H2-blockers on human and rat organic cation transporter-mediated transport: insight into the development of drug candidates. Xenobiotica 37, 618–634. doi:10.1080/00498250701397705
van der Velden, M., Bilos, A., Van Den Heuvel, J. J. M. W., Rijpma, S. R., Hurkmans, E. G. E., Sauerwein, R. W., et al. (2017). Proguanil and cycloguanil are organic cation transporter and multidrug and toxin extrusion substrates. Malar. J. 16, 422. doi:10.1186/s12936-017-2062-y
van Montfoort, J. E., Müller, M., Groothuis, G. M., Meijer, D. K., Koepsell, H., and Meier, P. J. (2001). Comparison of "type I" and "type II" organic cation transport by organic cation transporters and organic anion-transporting polypeptides. J. Pharmacol. Exp. Ther. 298, 110–115.
Volpe, D. A. (2011). Drug-permeability and transporter assays in Caco-2 and MDCK cell lines. Future Med. Chem. 3, 2063–2077. doi:10.4155/fmc.11.149
von Richter, O., Burk, O., Fromm, M., Thon, K., Eichelbaum, M., and Kivistö, K. (2004). Cytochrome P450 3A4 and P-glycoprotein expression in human small intestinal enterocytes and hepatocytes: a comparative analysis in paired tissue specimens. Clin. Pharmacol. Ther. 75, 172–183. doi:10.1016/j.clpt.2003.10.008
Wagner, D. J., Sager, J. E., Duan, H., Isoherranen, N., and Wang, J. (2017). Interaction and transport of methamphetamine and its primary metabolites by organic cation and multidrug and toxin extrusion transporters. Drug Metab. Dispos. 45, 770–778. doi:10.1124/dmd.116.074708
Wang, R. B., Kuo, C. L., Lien, L. L., and Lien, E. J. (2003). Structure-activity relationship: analyses of p-glycoprotein substrates and inhibitors. J. Clin. Pharm. Ther. 28, 203–228. doi:10.1046/j.1365-2710.2003.00487.x
Wenge, B., Geyer, J., and Bönisch, H. (2011). Oxybutynin and trospium are substrates of the human organic cation transporters. Naunyn-schmied Arch. Pharmacol. 383, 203–208. doi:10.1007/s00210-010-0590-x
Wright, S. H. (1985). Transport of N1-methylnicotinamide across brush border membrane vesicles from rabbit kidney. Am. J. Physiol.-Renal Physiol. 249, F903–F911. doi:10.1152/ajprenal.1985.249.6.F903
Yasujima, T., Ohta, K., Inoue, K., and Yuasa, H. (2011). Characterization of human OCT1-mediated transport of DAPI as a fluorescent probe substrate. J. Pharm. Sci. 100, 4006–4012. doi:10.1002/jps.22548
Yonezawa, A., Masuda, S., Yokoo, S., Katsura, T., and Inui, K. I. (2006). Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family). J. Pharmacol. Exp. Ther. 319, 879–886. doi:10.1124/jpet.106.110346
Zamek-Gliszczynski, M. J., Giacomini, K. M., and Zhang, L. (2018a). Emerging clinical importance of hepatic organic cation transporter 1 (OCT1) in drug pharmacokinetics, dynamics, pharmacogenetic variability, and drug interactions. Clin. Pharmacol. Ther. 103, 758–760. doi:10.1002/cpt.941
Zamek-Gliszczynski, M. J., Taub, M. E., Chothe, P. P., Chu, X., Giacomini, K. M., Kim, R. B., et al. (2018b). Transporters in drug development: 2018 ITC recommendations for transporters of emerging clinical importance. Clin. Pharmacol. Ther. 104, 890–899. doi:10.1002/cpt.1112
Zeng, S. L., Sudlow, L. C., and Berezin, M. Y. (2020). Using xenopus oocytes in neurological disease drug discovery. Expert Opin. Drug Discov. 15, 39–52. doi:10.1080/17460441.2020.1682993
Zhang, L., Schaner, M. E., and Giacomini, K. M. (1998). Functional characterization of an organic cation transporter (hOCT1) in a transiently transfected human cell line (HeLa). J. Pharmacol. Exp. Ther. 286, 354–361.
Zhang, L., Dresser, M. J., Gray, A. T., Yost, S. C., Terashita, S., and Giacomini, K. M. (1997). Cloning and functional expression of a human liver organic cation transporter. Mol. Pharmacol. 51, 913–921. doi:10.1124/mol.51.6.913
Zhou, S.-F. (2008). Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr. Drug. Metab. 9, 310–322. doi:10.2174/138920008784220664
Keywords: HEK 293, double-transfected cell line, single-transfected cell line, P-glycoprotein, MATE1, OCT1, SLC22A1 (OCT1), MDCK cell line
Citation: Haberkorn B, Fromm MF and König J (2021) Transport of Drugs and Endogenous Compounds Mediated by Human OCT1: Studies in Single- and Double-Transfected Cell Models. Front. Pharmacol. 12:662535. doi: 10.3389/fphar.2021.662535
Received: 01 February 2021; Accepted: 19 March 2021;
Published: 22 April 2021.
Edited by:
Mladen Vassilev Tzvetkov, University of Greifswald, GermanyReviewed by:
Michele Visentin, University Hospital Zürich, SwitzerlandCopyright © 2021 Haberkorn, Fromm and König. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jörg König, am9lcmcua29lbmlnQGZhdS5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.