
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 22 April 2021
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.652926
This article is part of the Research TopicTherapeutic Effects of Herbal Medicines: How Can We Best Investigate Bioactive Metabolites?View all 19 articles
Sarcandra glabra (Thunb.) Nakai is a folk medicine with a long history in China, which has been applied to treat sore throat, abscess, even tumor and so on. Meanwhile, it is also used as tea in some areas. At present, more than 200 chemical compounds have been isolated and identified from it, such as, sesquiterpenes, flavonoids, phenolic acids, coumarins and so on. Pharmacological studies have already confirmed that the extracts of S. glabra have many effects, such as antibacterial, antiviral, anti-inflammatory, anti-tumor, and anti-thrombocytopenia, especially the effects of anti-tumor and anti-thrombocytopenia are confirmed in clinic. Therefore, this paper systematically summarized the traditional uses, botany, phytochemistry, pharmacology, and toxicity of S. glabra, in order to provide a beneficial reference of its further research.
S. glabra is a perennial evergreen plant belonging to the Chloranthaceae family, and its resources are widely distributed throughout China, Japan, Korea, and Southeast Asia (Xu et al., 2011). S. glabra is commonly called Zhong Jie Feng in Chinese, because its ripe fruits resemble shiny red coral beads, it is also known as Cao Shan Hu. Meanwhile, after soaking S. glabra in hot water for a period of time, it will emit attractive aroma and taste delicious. Therefore, it is also regarded as tea in some areas (Yang, 1992; Han et al., 2013), also known as Jiu Jie Cha.
S. glabra has high medicinal value. S. glabra has been used as a folk medicine since the Qing Dynasty (Chen and Li, 2015), commonly applied by numerous ethnic groups in clinical practice in China, such as Han, Miao, Dong, Yao, Zhuang, etc., which has been officially listed in Chinese Pharmacopoeia since 1977. Traditionally, S. glabra is widely used to treat traumatic fracture, joint swelling and pain, sore throat, abscess, bleeding, and other diseases (Jia and Li, 2005). In modern clinical practice, it also has been applied to treat upper respiratory tract infection (Li, 2003), pneumonia (He et al., 2003), gastritis (Chen et al., 2012), viral myocarditis (Li, 2004), tumor (Cong et al., 2005; Song, 2017), and thrombocytopenia (Jiang and Zhou, 2003; Su and Luo, 2009), with significantly clinical therapeutic effect. Owing to the advantages of definite clinical effect, good safety, and abundant resources, many Chinese patent medicines with S. glabra as primary ingredient have been developed in modern times, 38 kinds of which have been approved for marketing by the State Food and Drug Administration of China (Figure 1).
In recent decades, considerable work has been done on pharmacology and phytochemistry of S. glabra. Many studies have proved that S. glabra exhibits a plenty of pharmacological effects, such as anti-inflammatory (Tsai et al., 2017), antibacterial (Jiang et al., 2000), antiviral (Cao et al., 2012), anti-tumor (Zhang et al., 2014), antioxidant (Liu et al., 2016), and anti-thrombocytopenic effects (Lu et al., 2018b). So far, over 200 chemical compounds have been isolated from S. glabra, including sesquiterpenes, flavonoids, coumarins, phenolic acids, lignans, anthraquinones and steroids. Among them, flavonoids are considered to be important bioactive components in S. glabra, which are also closely related to anti-thrombocytopenic activity of S. glabra (Xu et al., 2005). However, findings on pharmacology and phytochemistry are still difficult to comprehensively reflect its pharmacological effects and mechanisms, most pharmacological studies are still focused on exploring the activity of crude extracts, and the correlation between pharmacological effects and chemical components has yet to be fully established. Thus, there are many issues that deserve further investigation.
At present, reviews on S. glabra are not comprehensive enough (Han and Wu, 2017; Yang, 2017), and the chemical constituents and mechanism of pharmacological effects are deficiency, which impedes further research of S. glabra. In this paper, we used “Sarcandra glabra” as the keywords to collect information related to S. glabra from Web of Science, Science Direct, Springer, Google Scholar, PubMed, China National Knowledge Infrastructure (CNKI), and other professional websites, as well as classic books of herbal medicine. This paper intended to make a comprehensive and systematic review about S. glabra, so as to enhance further understanding of its traditional uses, botany, phytochemistry, pharmacology, and toxicity. This paper would also provide a beneficial reference for its in-depth research, development and utilization.
The genus Sarcandra comprises three accepted species worldwide (Chen and Cheng, 1994). Sarcandra glabra (Thunberg) Nakai is a species of the genus Sarcandra, widely distributed in the south of the Yangtze River in China, as well as other Asian countries, including Korea, Japan, Malaysia, Philippines, Vietnam, India, etc. (Zhou, 1993; Chen and Cheng, 1994). It is a semi-shade plant, prefers a warm and humid environment, but avoids direct sunlight, thus, it usually grows in ravines, slopes, valleys, and wet places under forests.
S. glabra derives from the dried whole plant of Sarcandra glabra (Thunb.) Nakai (synonym: Chloranthus glaber (Thunb.) Makino), which belongs to the genus Sarcandra of the Chloranthaceae family. It is a perennial evergreen subshrub with a height of approximately 50–120 cm. Its stem is erect, usually branched, and the nodes of the stem and branches are obviously swollen, which also have obvious longitudinal grooves and ridges between the nodes. Its leaves are opposite, leathery or papery, and glabrous on both surfaces. The shape of leaves is ovate or oval, about 6–17 cm long and 2–6 cm in wide. Its leaves are similar to tea leaves, the apex is acuminate, the base is wedge-shaped, the edges are serrated, and the marginal teeth are hard bone. Its petiole is approximately 1 cm in length. The stipule is small, like a sheath. There are small yellow-green flowers on the top of the branches, with a fragrant smell, no perianth, and cluster into spikes. S. glabra is monoecious, in which the stamens are clubbed to cylindrical, while the pistil is globose. Its fruit looks like pearl, which turns into shiny red at maturity, about 3–4 mm in diam. The florescence ranges from June to July, and the fruit period is from August to October. The whole plant of S. glabra is shown in Figure 2 [(Cheng, 1982), http://ppbc.iplant.cn/sp/15108].

FIGURE 2. Sarcandra glabra.(A–C) represent the whole plants (A), inflorescence (B) and fruits (C) of S. glabra.
S. glabra was first found in the Tang Dynasty’s medical book “Ben Cao Shi Yi” (AD 741) under the name of Jie gu mu, and then it was recorded in the Ming Dynasty’s Plant book “Ru Nan Pu Shi” (AD 1620) in the name of Shan hu (Chen and Li, 2015). However, its medicinal value was first appeared in “Sheng Cao Yao Xing Bei Yao” (AD 1711) in the Qing Dynasty: “Boiling it in water to drink, reducing fever”. According to “Ben Cao Gang Mu Shi Yi” (AD 1765), S. glabra could treat traumatic injury and fracture. In traditional clinical practice, S. glabra was effective in the treatment of joint swelling and pain, sore throat, carbuncle, tumor, trauma, bleeding, etc. In particular, the production technology of Miao nationality using S. glabra to treat traumatic fracture has been included in the list of National Intangible Cultural Heritage Protection at present (http://www.ihchina.cn/). Furthermore, in both ancient and modern times, S. glabra has been commonly used by Miao nationality to treat postpartum abdominal pain and dizziness; Dong nationality to treat appendicitis; the nationality of Yao and Zhuang to treat stomachache, dysentery, and influenza; Dai nationality to treat gastric ulcer; the nationality of Jinpo and Lahu to treat many gynecological diseases such as irregular menstruation, dysmenorrhea, and puerperal metrorrhagia (Jia and Li, 2005).
S. glabra has long been regarded as an edible plant in some areas. According to the records of Xingan County Chronicles in Jiangxi Province, people grind S. glabra with salt, rice, sesame and houttuynia in pottery bowls, then mix it with well water to drink, which is locally called Lei Cha. Lei Cha has been popular in the region since the Ming and Qing Dynasties, at present, Lei Cha in Gannan of Jiangxi Province has been included in the list of National Intangible Cultural Heritage Protection (http://www.ihchina.cn/). The Dong, Miao, Shui, Buyi and other ethnic groups in Guizhou province use S. glabra to make tea instead of ordinary tea in daily life. Especially, the Dong people prefer to make camellia oleifera for consumption through mixing S. glabra tea with glutinous rice, peanuts, soybeans and other condiments they like. Hence, the above records illustrate the safety of S. glabra as a medicine from another point of view.
Since the 1970s, the chemical constituents of S. glabra have gained the interest of the scholars at home and abroad. Up to now, over 200 compounds have been isolated and identified from S. glabra, including sesquiterpenes, flavonoids, phenolic acids, coumarins, lignans, anthraquinones, volatile oil, a small quantity of amino acids, trace elements, polysaccharides and proteoglycans. Among them, flavonoids are considered to be the main active components in S. glabra. The chemical constituents reported are listed in Table 1 and their corresponding structures are shown in Figures 3–7.
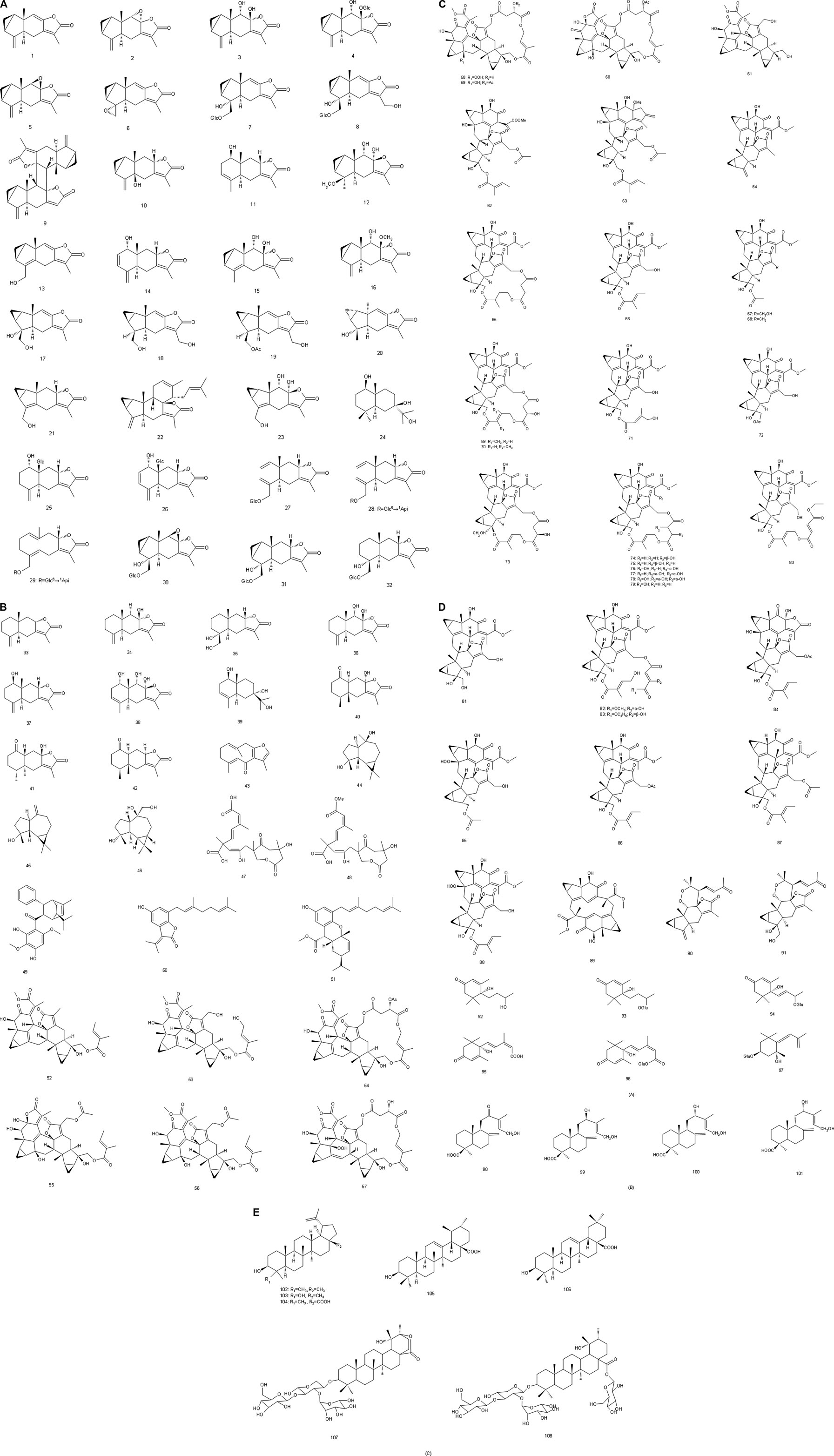
FIGURE 3. (A) Chemical structures of sesquiterpenes (A) identified in S. glabra extract. (B) Chemical structures of sesquiterpenes (A) identified in S. glabra extract. (C) Chemical structures of sesquiterpenes (A) identified in S. glabra extract. (D) Chemical structures of sesquiterpenes (A), and diterpenes (B) identified in S. glabra extract. (E) Chemical structures of triterpenes (C) identified in S. glabra extract.
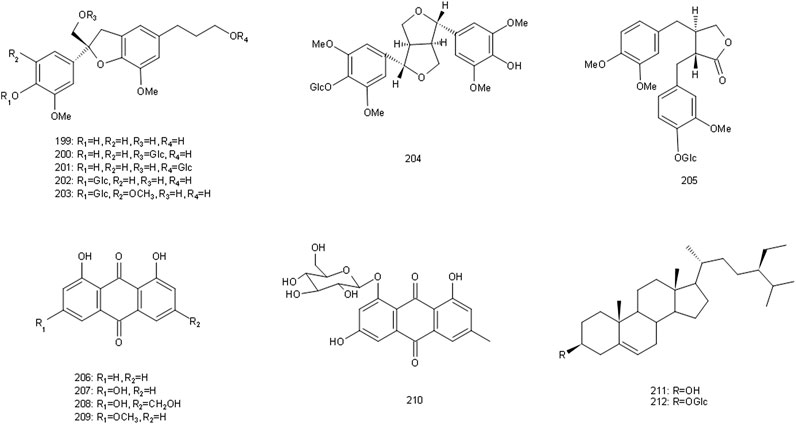
FIGURE 7. Chemical structures of lignins, anthraquinones and steroids identified in S. glabra extract.
There are sesquiterpenes (1–97), diterpenes (98–101) and triterpenes (102–108) in S. glabra, among them, sesquiterpenes are the most abundant substances, including the characteristic components such as chloranthalactone, chloranoside, sarcandralactone, shizukaol, and sarglabolide. Sesquiterpenes isolated and identified from S. glabra have been reported to possess anti-inflammatory, antibacterial and antitumor effects, etc. (He et al., 2010; Wang P. et al., 2015, Wang et al., 2016). For instance, chloranthalactone E (3), atractylenolide III (34) and sarcandrolides A-C (52–54) exhibited reportedly antitumor effects (Wang et al., 2007; He et al., 2010), while shizukaol B (65), shizukaol G (69) and sarglabolide A (73) showed anti-inflammatory activities (Wang P. et al., 2015). Sarglaperoxide A (90) possessed anti-inflammatory and antibacterial effects, inhibiting 53.6% nitric oxide (NO) production at 25 μM and 64.5% Staphylococcus aureus growth at 25 μg/ml (Wang et al., 2016).
So far, over 40 flavonoids have been found in S. glabra (109–148). Flavonoids are the main components within S. glabra, and now are considered to be the main bioactive components in the treatment of thrombocytopenia. Flavonoids are also often used as important indicators to control the quality of S. glabra. Astilbin (119), as one of the active components of S. glabra, was reported to play an anti-thrombocytopenic role in rat bone marrow megakaryocytes by up-regulating transforming growth factor beta (TGF-β1) content and down-regulating thermoplastic polyolefin (TPO) content, which may be the effective component against thrombocytopenia (Tang et al., 2014). Besides, there were differences in the content of total flavonoids in different parts of S. glabra. The content of total flavonoids in leaves reached 3.17%, which was higher than that in roots (2.38%) and stems (2.11%) (Li et al., 2007). The results suggested that the medicinal part could be selected according to the clinical needs, which was beneficial to the sustainable utilization of S. glabra.
At present, more than 20 organic acids have been isolated from S. glabra (149–182), which can be divided into phenolic acids and fatty acids. Phenolic acids are important components in S. glabra, containing rosmarinic acid (149), caffeic acid (153), chlorogenic acid (157), neochlorogenic acid (158), cryptochlorogenic acid (159), and other components with significant pharmacological activities. They might be the bioactive components of S. glabra to exert antibacterial, anti-inflammatory, and antioxidant effects, etc. Among them, rosmarinic acid (149) possessed various pharmacological effects including anti-inflammatory, antibacterial, antiviral, antioxidant, and anti-tumor effects, its anti-inflammatory and antioxidant effects were particularly significant (Nunes et al., 2017). Rosmarinic acid is also one of the phenolic acids with the highest content within S. glabra, serving as a marker in Chinese pharmacopoeia for controlling the quality of S. glabra.
Currently, more than a dozen coumarins have been isolated from S. glabra (183–198). As the most representative coumarin with strong pharmacological activity, isofraxidin (187) is used as an index for controlling the quality of S. glabra and its preparations by Chinese Pharmacopoeia. Studies have shown that isofraxidin has a wide range of pharmacological effects (Li et al., 2014; Liu et al., 2015; Jin et al., 2020), including anti-inflammatory, anti-viral, and anti-tumor effects, as well as inhibition of platelet aggregation. Furthermore, 3,3′-biisofraxidin (194) had been reported to induce gastric cancer cells apoptosis by activating the mitochondrial-mediated apoptosis pathway (Wu et al., 2015).
There are lignans (199–205), anthraquinones (206–210) and steroids (211–212) in S. glabra. Furthermore, there are abundant volatile components in S. glabra (Yang R. et al., 2008), mainly including α-pinene, β-phellandrene, and α-thujene. It also contains 16 kinds of amino acids, such as aspartic acid, glutamic acid, leucine and so on, six kinds of which are essential amino acids for human body, as well as trace elements including iron, zinc, calcium, magnesium and so on (Yang B. et al., 2008). In addition, acidic polysaccharide and proteoglycan are also isolated from S. glabra (Liu W. et al., 2017; Sun et al., 2020).
Pharmacological studies have indicated that S. glabra has a wide range of pharmacological effects, including antibacterial, antiviral, anti-inflammatory, anti-tumor, anti-oxidant, anti-thrombocytopenic effects, etc. Pharmacological effects of S. glabra and its preparations as well as monomeric compounds were summarized in Table 2, which were described in the following sections as well.
Studies had shown that S. glabra possessed a broad spectrum of antibacterial effects, which had inhibitory effects on Staphylococcus aureus and its drug resistant bacteria, Pseudomonas eruginosa, Escherichia coli, Streptococcus pneumoniae, Dysentery bacilli, Typhoid and Paratyphoid bacilli, especially on S. aureus and P. aeruginosa, it showed strong antibacterial activity (Jiang et al., 2000; Wang and Du, 2008). In vitro experiment demonstrated that S. glabra showed antibacterial effects through inhibiting the growth of Streptococcus mutans along with the activity of its glucosyltransferase (Huang and He, 2001). Besides, the aqueous extract of S. glabra could significantly promote the exosmosis of glucose and aspartate amino transferase in Helicobacter pylori and its drug-resistant bacteria at the concentration of 95 μg/ml, indicating that its antibacterial mechanism may be related to the damage of the outer membrane barrier (Guo, 2015). Some phenolic acids, coumarins and flavonoids isolated from the antibacterial fraction of S. glabra also showed good antibacterial activity (Wang and Ma, 1979b; Xu et al., 2008; Yuan et al., 2008). Fumaric acid and succinic acid had been proved to have excellent antibacterial effects on S. aureus and P. aeruginosa (Wang and Ma, 1979b). Isofraxidin (187) and 4,4′-bisofraxidin (195) showed good antibacterial effects on Porphyromonas gingivalis and Streptococcus transglucosans respectively, and their corresponding MIC values were 0.078 mg/ml and 0.125 mg/ml (Xu et al., 2008). Also, Kaempferol-3-O-β-D-glucuronide (110) exhibited a strong inhibitory effect on S. aureus, and its diameter of bacteriostasis circle was 14.67 ± 0.08 mm (Yuan et al., 2008). However, the current pharmacological studies mainly concentrate on in vitro models, and lack of discussion on the bioactive components and mechanism of antibacterial effect. Therefore, it is necessary to further evaluate the antibacterial effect and specific mechanism of S. glabra on in vivo models.
S. glabra extract (250 mg/kg) could reduce the incidence rate and mortality of restraint stress mice caused by H1N1 influenza virus via reducing the pathological changes and the amount of virus in lung tissue, as well as regulating susceptibility genes and inhibiting the expression of pro-inflammatory factors (Cao et al., 2012). However, the dose used in this study was too high, and it could be considered to be reduced in future studies. What’s more, the ethanol extract of S. glabra could reduce pulmonary edema, inhibit viral replication in lung tissue and alleviate oxidative stress level in mice infected with H1N1 virus, and its mechanism may be related to activating nuclear factor-erythroid 2-related factor 2 (Nrf-2)/heme oxygenase-1 (HO-1) pathway to regulate superoxide dismutase (SOD), malondialdehyde (MDA) and NO to reduce oxidative stress injury (Huo et al., 2020). In recent years, it has been found that some components from S. glabra exhibit antiviral effects (Liu J.-x. et al., 2017; Wang et al., 2017). Rosmarinic acid-4-O-β-D-glucoside (150) could reduce the mortality of mice with pneumonia caused by A/FM/1/47 H1N1 virus at the concentration of 20 and 50 mg/kg (Liu J.-x. et al., 2017). Eleutheroside B1 (193) could inhibit the influenza virus ribonucleoprotein and the expression of RN mRNA (Wang et al., 2017). These results indicated that S. glabra has the potential to be developed as new drugs for the treatment of viral infectious diseases. Thus, in-depth research on active components and mechanism of antiviral activity should be taken into consideration.
S. glabra showed significant anti-inflammatory activity, which had a certain degree of inhibitory effect on various inflammation models. In vitro, Xie et al. confirmed that polysaccharide and ethyl acetate extracts from S. glabra could inhibit RAW264.7 cell proliferation and NO expression (Xie et al., 2010). Besides, studies have proved that sesquiterpenes, phenolic compounds and coumarins from S. glabra may be the bioactive components of its anti-inflammatory effect (Liu et al., 2015; Tsai et al., 2017; Wei et al., 2019). Wei et al. isolated ten sesquiterpenes from the anti-inflammatory fraction of S. glabra and found that all of them could inhibit NO production in RAW264.7 cells induced by LPS (Wei et al., 2019). Among them, shizukaol D (67: 5, 10, 15, and 20 μM) showed the most significant anti-inflammatory effect with IC50 values of 8.13 ± 0.37 μM, and its mechanism may be related to activating protein kinase B (AKT) to regulate Nrf2/HO-1 signaling pathway, thus down-regulating inducible nitric oxide synthase (iNOS) expression, inhibiting phosphorylated nuclear factor kappa B (NF-κB) expression along with nuclear translocation and regulating the activity of oxidation indexes (Wei et al., 2019). Furthermore, isofraxidin (187: 1, 5, and 15 mg/kg) had also been proven to improve the survival rate of mice induced by LPS via inhibiting the production of pro-inflammatory cytokines such as NF-κB, NO, interleukin-6 (IL-6) along with tumor necrosis factor alpha (TNF-α) and reducing the damage of inflammatory factors to organs. The mechanism may be related to the inhibition of TNF-α expression by regulating NF-κB signaling pathway (Liu et al., 2015). Therefore, S. glabra may play its anti-inflammatory effect mainly by regulating the expression of inflammatory factors such as NF-κB, NO, IL-6, TNF-α and the signal pathways related to inflammation, but how to regulate them is not completely clear and needs to be further explored.
S. glabra had been reported to inhibit the growth of gastric cancer, leukemia, liver cancer, lung cancer and other malignant tumors, which played an anti-tumor role by regulating cell cycle and inducing cell apoptosis. Zhongjiefeng injection, a Chinese patent medicine made from S. glabra, was reported to have a strong cytotoxicity on human lung cancer A-549, colon cancer HCT-29 and gastric cancer BGC-823, with IC50 values less than 50 μg/ml (Zhao et al., 2008). Zhongjiefeng tablets, made from S. glabra, could induce p21 expression by up-regulating TGF-β pathway, and arrested A549 and H1299 cells in G0/G1 phase, thus inducing cell apoptosis and inhibiting cell proliferation (Chen et al., 2018). The total flavonoids extract from S. glabra (25, 50 and 100 μg/ml) also showed significant inhibitory effect on leukemic K562 cells, which could promote cell apoptosis by decreasing the expression of Bcl-2 and caspase-3, and increasing expression of Cleaved caspase-3 (Sun et al., 2019). The polysaccharide from S. glabra (SGP-2) could inhibit human osteosarcoma cells U2OS proliferation and promote U2OS cells apoptosis at the concentration of 31.25, 62.5, and 125 nM, through down-regulating extracellular regulated protein kinases (ERK)/eIF4F/Bcl-XL signaling pathway to promote the release of cytochromes C and activate caspase protein (Zhang et al., 2014). Moreover, in S-180 cell-derived tumor mice model, it was further confirmed that SGP-2 (25, 50, 100 mg/kg) could inhibit the growth of transplanted tumor and activate endogenous apoptosis pathway through down regulating ERK-eIF4F pathway (Zhang et al., 2014).
Jiang et al. reported that S. glabra could enhance the clearance index of macrophages in mice, but it had no obvious effect on specific humoral immunity, indicating that S. glabra mainly acted on the non-specific immunity of the body (Jiang et al., 2001). Meanwhile, S. glabra polysaccharide extract played an immune role through promoting the expression of membrane protein-related immune molecules and regulating the expression of pro-inflammatory and anti-inflammatory cytokines in RAW264.7 macrophages (Jiang et al., 2014). Furthermore, S. glabra also ameliorated immunodepression caused by stress. In restraint stress model mice, it was found that S. glabra extract (125 mg/kg) not only increased the number of lymphocytes, natural killer cells and natural killer T cells, normalized the ratio of T lymphocyte subsets, but also significantly reduced the lipid peroxidation level in spleen cells and increased the activity of oxygen free radicals, which partly through improving the ability of antioxidant to enhance immunity (He R. R. et al., 2009; He R. et al., 2009).
S. glabra extract exhibited strong free radical scavenging ability. Aqueous extract of S. glabra could scavenge hydroxy free radical in a concentration-dependent manner, at the concentration of 1.2 mg/ml, the scavenging rate on hydroxy free radical reached 89.89% (Qin et al., 2007). Aqueous extract of S. glabra also had a significant scavenging effect on DPPH radical, with half scavenging concentration of 13.49 mg/l (Li et al., 2009). S. glabra polysaccharide had obvious scavenging effect on hydroxy, superoxide anion, DPPH, and ABTS radicals (Jin et al., 2012). The active components of S. glabra also had the ability of scavenging free radicals. It was found that phenolic acids isolated from antioxidant active sites, such as rosmarinic acid (149), chlorogenic acid (157), and cryptochlorogenic acid (159), as well as flavonoids, such as quercetin-3-O-α-D-glucuronide (114) and neoastibin (120), showed antioxidant activity with strong ability of DPPH radical scavenging (Li et al., 2009, Li et al., 2010). In addition, ethanol extract, astilbin (119) and rosmarinic acid (149) from S. glabra had been reported to exhibit significant antioxidant activities, which could directly or indirectly scavenge reactive oxygen species (ROS) to protect mesenchymal stem cells from oxidative stress at the concentration of 10–100 μg/ml and hydroxy free radical mediated DNA damage at the concentration of 20–110 μg/ml. More importantly, the antioxidant capacity of ethanol extract from S. glabra may be related to the presence of total phenolics, especially astilbin and rosmarinic acid (Liu et al., 2016). These studies implied that S. glabra had the potential to treat a variety of diseases associated with oxidative stress. But, the current studies on antioxidant activity mainly focus on in vitro models, and a variety of in vivo models should be established to further evaluate its antioxidant activities, and to explore the relevant targets and pathways.
Nowadays, S. glabra is commonly used to treat hemorrhagic diseases caused by thrombocytopenia, and its extract has been made into a Chinese patent medicine in China that are used to increase the platelets. Experimental studies had shown that S. glabra extract and its single drug preparation--Xuekang oral liquid could increase the number of peripheral blood platelets in mice with immune thrombocytopenic purpura, and the experimental results also showed that the total flavonoids from S. glabra (TFSG) was better than positive control (prednisone) in increasing the platelets (Xu et al., 2005). Besides, in bone marrow stromal cells-megakaryocyte co-culture system, TFSG (1.95, 3.9, and 7.8 μg/ml) promoted the differentiation and maturation of megakaryocytes in the co-culture system, which may be related to decreasing the rate of stromal cell apoptosis, regulating the content of cytokines that promote megakaryocyte differentiation including TPO, stromal cell derived factor-1 (SDF-1), TGF-β1, and vascular cell adhesion molecule-1 (VCAM-1), thereby affecting the state of stromal cells and secretion function. And the experimental results also suggested that this may be one of the mechanisms of S. glabra in the treatment of immune thrombocytopenia (Lu et al., 2019).
At present, most chemotherapeutic drugs can cause bone marrow suppression and lead to thrombocytopenia, S. glabra can significantly resist these side effects. Studies had shown that S. glabra significantly improved thrombocytopenia induced by 5-FU (Zhong et al., 2005). Based on this, Lu et al. established thrombocytopenia mice to explore the mechanism of TFSG on improving thrombocytopenia induced by chemotherapy (Lu et al., 2018a). The results demonstrated that TFSG (31.5, 63, and 94.5 mg/kg) could promote the secretion of TPO from stromal cells in the bone marrow microenvironment and the corresponding receptor C-mpl expression in megakaryocytes, then promote megakaryocyte to release mature platelets by regulating the TPO-C-mpl pathway. In addition, TFSG (31.5, 63, and 94.5 mg/kg) could also promote the proliferation, differentiation and maturation of megakaryocytes by promoting SDF-1 in bone marrow and the corresponding receptor CXCR-4 expression in megakaryocytes, thereby accelerating megakaryocyte to produce platelets (Lu et al., 2018b). These experimental results indicate that TFSG can promote megakaryocyte proliferation through multiple pathways and multiple targets, thereby increasing the number of platelets, but how does the TFSG promote the secretion of TPO or SDF-1 from stromal cells in the bone marrow microenvironment and regulate their corresponding receptors in megakaryocytes are still unclear, and further studies are needed to clarify.
S. glabra had significant protective effects on various liver injury models. In rat with liver injury induced by dimethylnitrosamine, S. glabra could significantly improve the pathological changes of liver tissue, and it not only normalized the serum protein index, but also enhanced the level of antioxidant index (Jin and Li, 1998). In mice with liver injury caused by P. acnes-LPS, the plasma alanine aminotransferase (ALT) activity increased, however, S. glabra extract could significantly reduce this trend, and the inhibition rate of high dose of the extract was up to 78.5% (Li et al., 2008). Meanwhile, 70% ethanol extract of S. glabra and seven sesquiterpenes from the extract showed significant hepatoprotective activity in hepatic epithelial stem cells from WB-F344 rats induced by D-galactosamine, among them, chloranoside A (7) and sarcaglaboside A-C (25–27) showed stronger liver protection activity than the positive drug dicyclool (Li et al., 2006a). Besides, S. glabra also had a good inhibitory effect on liver fibrosis. It was found that S. glabra extract reduced the serum liver function indexes (ALT and aspartate aminotransferase (AST)), liver fibrosis indexes (hyaluronic acid (HA), procollagen type III (PC-III), procollagen type IV (C-IV) and laminin (LN)) and tissue inhibitor of metalloproteinase-1 (TIMP-1) in rats with hepatic fibrosis induced by CCl4, as well as increasing the level of albumin (ALB). In particular, it could reduce the content of TIMP-1 to the normal level, and the related research indicated that the decrease of TIMP-1 expression contributed to the degradation of liver fibrosis, so its mechanism may be related to decreasing the expression of TIMP-1 (Xiong et al., 2015).
In vitro and in vivo experiments, the polysaccharide from S. glabra showed excellent hypoglycemic effect. In vitro, the inhibitory effect of S. glabra polysaccharide (SEPR1) on α-glucosidase (IC50 = 49.01 μg/ml) was significantly stronger than that of acarbose (IC50 = 148.3 μg/ml). While in diabetic mice induced by HFD/STZ, SEPR1 (100 and 200 mg/kg) showed hypoglycemic effect by reducing fasting blood glucose levels and relieving the insulin resistance, which was better than that of positive control Acarbose (10 mg/kg) and Metformin (200 mg/kg). And the experimental results also indicated that SERP1 could increase the activity of antioxidant enzymes and decrease MDA level (Liu W. et al., 2017). In addition, total flavonoids from S. glabra reduced the levels of triglyceride (TG), total cholesterol (TC) and low-density lipoprotein (LDL-C) in serum of mice with hyperlipidemia, and the hypolipidemic effect of the high-dose total flavonoids was similar to that of positive control (Ji, 2012).
S. glabra also exhibited other pharmacological effects. Aqueous extract, ethanol extract, and essential oil from S. glabra could shorten the healing time of experimental fracture in rabbits, among which aqueous extract had the most significant effect in promoting fracture healing (Shi et al., 1980). This pharmacological study was consistent with the traditional use of S. glabra in the treatment of fractures, but the specific mechanism and effective components were still unclear. In addition, S. glabra had a protective effect on sport-injured skeletal muscle cells. In exercise-induced injury rats, it could be observed that the levels of SOD, catalase (CAT) and total antioxidant capacity (T-AOC) in the skeletal muscle and tissues of the rats decreased, the levels of MDA, creatine kinase (CK) and lactate dehydrogenase (LDH) increased, meanwhile, related inflammatory factors such as TNF-α, interleukin-18 (IL-18) and IL-1β levels increased. After the intervention of S. glabra polysaccharide, these indexes were significantly improved, suggesting that S. glabra polysaccharide could promote the repair and remodeling process of skeletal muscle structure after injury (Liu, 2015; Wang et al., 2020).
From the long-term medicinal and edible history, it can be found that S. glabra is a kind of medicine food homology herb with good safety. Zhang et al. indicated that the maximum tolerance dose of aqueous extract of S. glabra in mice was more than 20 g kg−1·bw, without obvious genetic toxic effect, and there was no pathological damage in rats fed with the extract for 90 days at a dosage of 1.67, 3.33, and 5 g kg−1·bw (Zhang et al., 2016). These results were consistent with the findings of Xia et al.Xia et al. (1996) and Sun et al.Sun et al. (1998). In their studies, the results of the acute toxicity test, genetic toxicity test and teratogenicity test of aqueous extract of S. glabra were negative, suggesting that S. glabra had almost no obvious toxicity. However, these studies have only evaluated the toxicology of aqueous extract of S. glabra, and have not yet systematically evaluated the toxicology of its ethanol extract or other extracts. Therefore, future toxicological studies need more abundant experimental models, multiple types of S. glabra extracts or its active ingredients for further evaluation.
As a traditional Chinese medicine, S. glabra has a long history of medicinal use and definite clinical curative effect. It is traditionally used to treat many diseases, including joint swelling and pain, sore throat, carbuncle, traumatic fracture, tumor, bleeding, etc. Because of its significant pharmacological effects, such as antibacterial, antiviral, anti-inflammatory, anti-tumor and anti-thrombocytopenic effects that are found in modern studies, S. glabra has attracted extensive attention. After decades of efforts by scholars, research on S. glabra has achieved certain results on chemical constituents and pharmacological effects. However, there is still a lot of work needs to be further explored. The future research of S. glabra can be considered from the following aspects:
Firstly, S. glabra has used as a folk medicine in China for more than 300 years, and a great quantity of folk empirical prescriptions with remarkable therapeutic effect also have appeared. Among them, the production technology of Miao nationality using S. glabra to treat traumatic fracture has been included in the list of National Intangible Cultural Heritage Protection. Nevertheless, research on the relationship between the traditional efficacy and its modern pharmacological activity has not yet been thoroughly investigated. Therefore, we should look for the potential pharmacological effects of S. glabra on the basis of its traditional application. For instance, “Fen Lei Cao Yao Xing”, an herbal medicine book written in the Qing Dynasty (AD 1906), recorded that S. glabra was used to treat rheumatic numbness, arthralgia and myalgia. Nevertheless, there is currently a lack of modern pharmacological studies of S. glabra on rheumatic arthritis. S. glabra has the effect of clearing heat and detoxification, which has a good reputation as “natural antibiotics” in folk, and is often used to treat infective inflammation caused by bacteria and virus in clinic, showing remarkable therapeutic effects. Modern pharmacological research has found that S. glabra possesses significant antibacterial, antiviral, and anti-inflammatory effects, which scientifically explains its heat-clearing and detoxifying effects. However, studies on antibacterial, antiviral, and anti-inflammatory effects of S. glabra are still in its infancy. Thus, more experiments are urgently needed to clarify its bioactive components and mechanism of action, in order to further establish the correlation between the traditional application and the modern pharmacological activity of S. glabra.
Secondly, more than 200 chemical constituents have been isolated from S. glabra, such as sesquiterpenes, flavonoids, phenolic acids, coumarins, lignans, anthraquinones, etc. However, related research on the pharmacological effects and targets of these components are still insufficient. There are relatively more studies on isofraxidin and rosmarinic acid, which have been used as markers to control the quality of S. glabra, but they are not only the characteristic chemical components in S. glabra (Alagawany et al., 2017; Majnooni et al., 2020), and whether they are the main active components of S. glabra remains to be confirmed. Therefore, the chemical constituents of S. glabra need to be further excavated in order to find more potentially active and specific compounds.
Thirdly, S. glabra has a good inhibitory effect on leukemia, gastric cancer, liver cancer and other malignant tumors. Ji et al. reviewed that S. glabra mainly played an anti-tumor role by inhibiting proliferation, inducing apoptosis, inhibiting telomerase activity and improving immune function (Ji et al., 2016). However, the active components, related targets and signaling pathways of its antitumor effects are still unclear. This suggests that the active components of antitumor effect may be polysaccharide, flavonoids, rosmarinic acid, isofraxidin, 3,3′-biisofraxidin, as well as atractylenolide Ⅲ, and the mechanism may be related to regulating ERK-eIF4F signaling pathway, along with apoptosis-related protein including Bcl-2, Bax and caspase-3. Nevertheless, the anti-tumor research on S. glabra is not comprehensive enough, its effective anti-tumor components and related mechanism still need to be further studied in the future.
Finally, S. glabra possesses an excellent anti-thrombocytopenic effect. In 2013, Dong et al. summarized the research advance of S. glabra on thrombocytopenia diseases, and found that the effective part of S. glabra against thrombocytopenia was total flavonoids, which could promote megakaryocytes proliferation to increase the platelets (Dong et al., 2013). However, how S. glabra regulated megakaryocytes proliferation was not discussed in their review. In this paper, we summarized the mechanism of S. glabra against thrombocytopenia, and found that total flavonoids of S. glabra could promote megakaryocytes proliferation through regulating the content of cytokines promoted megakaryocyte differentiation including TPO, SDF-1, TGF-β1 along with VCAM-1 and promoting the expression of SDF-1 and TPO in bone marrow microenvironment as well as their corresponding receptors CXCR-4 and C-mpl in megakaryocytes. Furthermore, Xuekang oral liquid, a single plant-based drug extracted from S. glabra, has a remarkable curative effect on primary and secondary thrombocytopenic purpura, thrombocytopenia caused by chemotherapy and radiotherapy, without side effects, which is a unique Chinese patent medicine for increasing the platelets in China (Xu et al., 1997; Shi, 2009). At present, research on Xuekang oral liquid mainly focuses on clinical trials, and there are few studies on its active components and mechanism. In addition, isofraxidin rather than flavonoids is stipulated as a marker by Chinese Pharmacopoeia to control the quality of Xuekang oral liquid, thus, the components of anti-thrombocytopenic effect still need to be further studied.
In summary, S. glabra with a long history and widely distributed resources, has been widely used for anti-bacterial, anti-viral, anti-inflammatory, anti-tumor, and anti-thrombocytopenia in clinic. S. glabra as an excellent traditional medicine for the sufficient experience in traditional medicine as well as remarkable curative effect, is also a kind of medicine and food homologous plant with great development potential, which is worthy of in-depth research and exploration in the field of medicine.
SJ and XQ conceived the original idea. YZ wrote the manuscript with help from JL and XQ. QZ gave some suggestions. ZL and GS provided the professional guidance and the financial support. All authors read and approved the final manuscript.
This work was funded by the construction project of Inheritance Studio for National Famous Chinese Medicine Specialists (Grant No. 003111001025) and Sichuan Science and Technology Department Project (Grant No. 2019YFS0412).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Akt, protein kinase B; ALB, albumin; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; C-IV, procollagen type IV; CAT, catalase; CK, creatine kinase; ERK, extracellular regulated protein kinases; HA, hyaluronia acid; HO-1, heme oxygenase one; IL-6, interleukin-6; IL-18, interleukin-18; iNOS, inducible nitric oxide synthase; MDA, malondialdehyde; NF-ҡB, nuclear factor kappa B; NO, nitric oxide; Nrf2, nuclear factor erythroid 2-related factor 2; PC-III, procollagen type III; ROS, reactive oxygen species; SOD, superoxide dismutase; SDF-1, stromal cell derived factor-1; TGF-β, transforming growth factor beta; TPO, thermoplastic polyolefin; TNF-α, Tumor necrosis factor alpha; VCAM-1, vascular cell adhesion molecule-1; LN, laminin; TIMP-1, tissue inhibitor of metalloproteinase-1; TG, triglyceride; TC, total cholesterol; LDL-C, low density lipoprotein; T-AOC, total antioxidant capacity; LDH, lactate dehydrogenase.
Alagawany, M., Abd El-Hack, M. E., Farag, M. R., Gopi, M., Karthik, K., Malik, Y. S., et al. (2017). Rosmarinic Acid: Modes of Action, Medicinal Values and Health Benefits. Anim. Health Res. Rev. 18, 167–176. doi:10.1017/s1466252317000081
Cao, H.-J., Tan, R.-R., He, R.-R., Tang, L.-P., Wang, X.-L., Yao, N., et al. (2012). Sarcandra glabra Extract Reduces the Susceptibility and Severity of Influenza in Restraint-Stressed Mice. Evidence-Based Complement. Altern. Med. 2012, 1. doi:10.1155/2012/236539
Chen, C., and Li, S. (2015). Herbal Textual Research on Sarcandra glabra. J. Chin. Med. Mater. 38, 2628–2631. doi:10.13863/j.issn1001-4454.2015.12.043
Chen, H., Chen, P., Bao, Y., and Ao, J. (2012). Treatment of Varioliform Gastritis with Zhongjiefeng Capsule. Chin. J. Integr. Tradit. West. Med. Dig. 20, 519–520.
Chen, H., and Cheng, Y. (1994). The Origin, Differentiation and Geography of Chrolanthaceae. J. Trop. Subtrop. Bot. 2, 31–44.
Chen, Y., Xie, Q., Li, Z., and Wu, Z. (2018). Study on the Influence of Zhongjiefeng Dispersible Tablet on the Proliferation of Non-small Cell Lung Cancer and its Molecular Mechanism. J. Qiqihar Med. Univ. 39, 2626–2630.
Cong, S., Bi, W., and Jiang, L. (2005). Zhongjiefeng Injection Combined with Chemotherapy in the Treatment of Advanced Non-small Cell Lung Cancer. Chin. J. Cancer Prev. Treat. 12, 156.
Dong, W., Xu, G., Zhang, Q., Shang, G., Li, B., and Tang, X. (2013). Pharmacological Effects of Sarcand Glabra and its Application in Thrombocytopenia. Pharmacol. Clin. Chin. Mater. Med. 29, 176–178.
Duan, Y., Dai, Y., Gao, H., Ye, W., and Yao, X. (2010). Chemical Constituents of Sarcandra glabra. Chin. Tradit. Herb. Drugs 41, 29–32.
Feng, S., Xu, L., Wu, M., Hao, J., Qiu, S. X., and Wei, X. (2010). A New Coumarin from Sarcandra glabra. Fitoterapia 81, 472–474. doi:10.1016/j.fitote.2009.12.009
Fu, J., and Liang, J. (2013). Studies on the Chemical Constituents of Sarcandra glabra. Strait Pharm. J. 25, 46–50.
Guo, M. (2015). The Efficacy of Sarcandra glabra Extract Alone or Combined with Antibiotics against helicobacter Pylori in vitro. Master’s Thesis. (China): Nanchang University.
Han, B., Peng, Y., and Xiao, P. (2013). Systematic Research on Chinese Non-camellia Tea. Mod. Chin. Med. 15, 259–269.
Han, Q., and Wu, X. (2017). Advances on Chemical Constituents and Pharmacological Activities of Sarcandra glabra. Agriculture of Jilin, 63–64.
He, R., Wang, M., Li, Y., Dai, Y., Duan, Y., Yao, X., et al. (2009). [Effects of Sarcandra glabra Extract on Immune Activity in Restraint Stress Mice]. Zhongguo Zhong Yao Za Zhi 34, 100–103.
He, R. R., Yao, X. S., Li, H. Y., Dai, Y., Duan, Y. H., Li, Y. F., et al. (2009). The Anti-stress Effects of Sarcandra glabra Extract on Restraint-Evoked Immunocompromise. Biol. Pharm. Bull. 32, 247–252. doi:10.1248/bpb.32.247
He, W., Dong, F., Li, A., and Wang, S. (2003). Comparison of Curative Effect between Sarcandra glabra and Ribavirin in Treating Children Viral Pneumonia. Mod. J. Integr. Tradit. Chin. West. Med. 12, 605.
He, X.-F., Yin, S., Ji, Y.-C., Su, Z.-S., Geng, M.-Y., and Yue, J.-M. (2010). Sesquiterpenes and Dimeric Sesquiterpenoids from Sarcandra glabra. J. Nat. Prod. 73, 45–50. doi:10.1021/np9006469
Hu, X.-R., Wu, H.-F., Zhang, X.-P., Yang, J.-S., Dai, Z., Lin, R.-C., et al. (2013). A New Sesquiterpene Lactone from Sarcandra glabra. Nat. Product. Res. 27, 1197–1201. doi:10.1080/14786419.2012.722084
Hu, X.-r., Yang, J.-s., and Xu, X.-d. (2009). Three Novel Sesquiterpene Glycosides of Sarcandra glabra. Chem. Pharm. Bull. 57, 418–420. doi:10.1248/cpb.57.418
Huang, F., and He, H. (2001). Effect of Sarcandra on Cariogenicity of streptococcus Mutans on Vitro. Med. J. Chin. Civ. Adm. 13, 201–203.
Huang, M. J., Zeng, G. Y., Tan, J. B., Li, Y. L., Tan, G. S., and Zhou, Y. J. (2008). [Studies on Flavonoid Glycosides of Sarcandra glabra]. Zhongguo Zhong Yao Za Zhi 33, 1700–1702.
Huang, M., Li, Y., Zeng, G., Yuan, W., Tan, J., Tan, G., et al. (2007). Chemical Constituents of Sarcandra glabra. Cent. South. Pharm. 5, 459–461.
Huo, Y., Zhang, Y., An, M., Li, X., Lai, X., Liu, X., et al. (2020). Effects of Different Parts of Sarcandra glabra Extract on Oxidative Stress in Mice with Viral Lung Injury. J. Chin. Med. Mater. 43, 2555–2559.
Ishikura, N. (1971). Pelargonidin Glycosides in Fruits. Experientia 27, 1006. doi:10.1007/bf02138844
Ji, N. (2012). Effects of Total Flavonoids of Sarcandrae glabra on Mouse Blood-Fat Content. J. Mt. Agric. Biol. 31, 268–270.
Ji, Y., Zhu, X., and Wu, J. (2016). The Progress in the Antitumor Effect and Mechanism of Zhongjiefeng (Sarcandra glabra). Guid. J. Tradit. Chin. Med. Pharmacol. 22, 44–46.55
Jiang, A., and Zhou, H. (2003). Clinical Observation of Xuekang Capsules on Thrombocytopenic Purpura in 52 Cases. Pract. Clin. J. Integr. Tradit. Chin. West. Med. 3, 43–44.
Jiang, W., Kong, X., Huang, R., Lin, J., and Dai, M. (2000). Studies on Anti-inflammatory and Antibacterial Effects of Tabellae Sarcandrae. J. Guangxi Univ. Chin. Med. 17, 50–52.
Jiang, W., Kong, X., Liang, G., Huang, Z., Chen, J., and Huang, R. (2001). Effects of Tabellae Sarcandrae on Malignant Tumour and Immunity. J. Guangxi Med. Univ. 18, 39–41.
Jiang, Z., Chen, Z., Li, X., Zhao, J., Li, S., Hu, J., et al. (2014). Immunomodulatory Effects of Sarcandra glabra Polysaccharides on Macrophage RAW264.7. Chin. J. Exp. Tradit. Med. Formulae 20, 178–182.
Jin, L., Guan, X., Liu, W., Zhang, X., Yan, W., Yao, W., et al. (2012). Characterization and Antioxidant Activity of a Polysaccharide Extracted from Sarcandra glabra. Carbohydr. Polym. 90, 524–532. doi:10.1016/j.carbpol.2012.05.074
Jin, L., Ying, Z.-H., Yu, C.-H., Zhang, H.-H., Yu, W.-Y., and Wu, X.-N. (2020). Isofraxidin Ameliorated Influenza Viral Inflammation in Rodents via Inhibiting Platelet Aggregation. Int. Immunopharmacology 84, 106521. doi:10.1016/j.intimp.2020.106521
Jin, S., and Li, Z. (1998). Experimental Study on Intervention Effect of Sarcandra glabra on Rats with Liver Injury Induced by Dimethylnitrosamine. Shanghai J. Tradit. Chin. Med., 43–45.
Li, B., Huang, M., Li, Y., Zeng, G., Tan, J., and Zhou, Y. (2009). Antioxidant Constituents of Sarcandra glabra (Thunb.)Nakai. J. Shenyang Pharm. Univ. 26, 900–903.
Li, H., He, R., Liang, T., Ye, W., Yao, X., and Kurihara, H. (2008). Effect of Sarcandra glabra (Thunb.) Nakai Extract on Mice Model of Immunological Hepatitis and Acute Inflammation. Chin. Pharmacol. Bull. 24, 244–250.
Li, L. (2004). Treatment of 53 Cases of Acute Viral Myocarditis with Zhongjiefeng Injection. Tradit. Chin. Med. Res. 17, 48–49.
Li, N., Yang, R., Wang, B., and Zhang, X. (2007). Determination of Total Flavonoids in Different Parts of Sarcandra glabra. Chongqing J. Res. Chin. Drugs Herbs 16, 16–17.
Li, P., Zhao, Q.-L., Wu, L.-H., Jawaid, P., Jiao, Y.-F., Kadowaki, M., et al. (2014). Isofraxidin, a Potent Reactive Oxygen Species (ROS) Scavenger, Protects Human Leukemia Cells from Radiation-Induced Apoptosis via ROS/mitochondria Pathway in P53-independent Manner. Apoptosis 19, 1043–1053. doi:10.1007/s10495-014-0984-1
Li, W. (2003). Clinical Observation on 120 Cases of Acute Upper Respiratory Tract Infection in Children Treated with Zhongjiefeng Injection. Med. J. Liaoning 17, 186.
Li, X., Zhang, Y. F., Yang, L., Feng, Y., Liu, Y. M., and Zeng, X. (2011). [Sesquiterpenoids from the Whole Plant of Sarcandra glabra]. Yao Xue Xue Bao 46, 1349–1351. doi:10.16438/j.0513-4870.2011.11.003
Li, X., Huang, M., Li, Y., Zeng, G., Tan, J., Liang, J., et al. (2010). Study on Antioxidant Constituents from Sarcandra glabra (Thunb.) Nakai. Chin. J. Med. Chem. 20, 57–60.
Li, X., Zhang, Y.-F., Zeng, X., Liu, Y.-M., and Feng, Y. (2012a). Two New-Skeleton Compounds from Sarcandra glabra. Hca 95, 998–1002. doi:10.1002/hlca.201100352
Li, X., Zhang, Y., Yang, L., Yi, Feng., Liu, Y., and Zeng, X. (2012b). Studies of Phenolic Acid Constituents from the Whole Plant of Sarcandra glabra. Tradit. Chin. Drug Res. Clin. Pharmacol. 23, 295–298. doi:10.3969/j.issn.1003-9783.2012.03.015
Li, Y. (2006). Studies on the Chemical Constituents and Bioactivities of Sarcandra glabra, Cercis Chinensis, and Photinia Parvifolia. Master’s Thesis. (China): Chinese Academy of Medical Science, Peking Union Medical College.
Li, Y., Zhang, D.-M., Li, J.-B., Yu, S.-S., Li, Y., and Luo, Y.-M. (2006a). Hepatoprotective Sesquiterpene Glycosides from Sarcandra glabra. J. Nat. Prod. 69, 616–620. doi:10.1021/np050480d
Li, Y., Zhang, D. M., Yu, S. S., Li, J. B., and Luo, Y. M. (2006b). A Novel Phenylpropanoid-Substituted Catechin Glycoside and a New Dihydrochalcone from Sarcandra Glabra. Chin. Chem. Lett. 17, 207–210.
Lian, X. (2006). Medical Composition Containing Polyphenols of Sarcandra glabra and its Application. Chin. Patent ZL200610050821 1.
Liu, J.-x., Zhang, Y., Hu, Q.-P., Liqiang, J.-q. Liu., Liu, Y.-t. Wu, Q. guang., Wu, Q.-g., et al. (2017a). Anti-inflammatory Effects of Rosmarinic Acid-4-O-β-D-Glucoside in Reducing Acute Lung Injury in Mice Infected with Influenza Virus. Antiviral Res. 144, 34–43. doi:10.1016/j.antiviral.2017.04.010
Liu, W., Lu, W., Chai, Y., Liu, Y., Yao, W., and Gao, X. (2017b). Preliminary Structural Characterization and Hypoglycemic Effects of an Acidic Polysaccharide SERP1 from the Residue of Sarcandra glabra. Carbohydr. Polym. 176, 140–151. doi:10.1016/j.carbpol.2017.08.071
Liu, J., Li, X., Lin, J., Li, Y., Wang, T., Jiang, Q., et al. (2016). Sarcandra glabra (Caoshanhu) Protects Mesenchymal Stem Cells from Oxidative Stress: A Bioevaluation and Mechanistic Chemistry. BMC Complement. Altern. Med. 16, 423. doi:10.1186/s12906-016-1383-7
Liu, L., Mu, Q., Li, W., Xing, W., Zhang, H., Fan, T., et al. (2015). Isofraxidin Protects Mice from LPS Challenge by Inhibiting Pro-inflammatory Cytokines and Alleviating Histopathological Changes. Immunobiology 220, 406–413. doi:10.1016/j.imbio.2014.10.007
Liu, Y. (2015). Sarcandra glabra Polysaccharides on High-Intensity Exercise in Rat Skeletal Muscle Microinjury under a Protective Mechanism. J. Sanming Univ. 32, 97–100.
Lu, X., Sun, H., Zhu, J., Hu, X., Yan, X., and Shang, G. (2019). Effects of Flavonoids from Sarcandrae glabra on Differentiation and Maturation of Megakaryocytes in Cell Co-culture System. Tradit. Chin. Drug Res. Clin. Pharmacol. 30, 1277–1283. doi:10.1016/j.cclet.2019.09.012
Lu, X., Zhang, J., Peng, W., Wu, Q., Xu, G., Yan, X., et al. (2018a). Effects of Flavonoids Sarcandrae on Bone Marrow Stromal Cells and Mcgakaryocytcs of Mice with Cytarabine-Induced Thrombocytopenia. Pharmacol. Clin. Chin. Mater. Med. 34, 32–35.
Lu, X., Zhang, J., Yan, X., Xu, G., and Shang, G. (2018b). Effects of Flavonoids from Sarcandra Herba on Expression of SDF-1 and CXCR-4 in the Bone Marrow of Chemotherapy-Induced Thrombocytopenia Model Mice. Tradit. Chin. Drug Res. Clin. Pharmacol. 29, 433–437.
Luo, Y., Liu, A., Yu, B., Kang, L., and Huang, L. (2005a). Studies on Chemical Constituents of Sarcandra glabra. Chin. Pharm. J. 40, 1296–1298.
Luo, Y.-M., Liu, A.-H., Zhang, D.-M., and Huang, L.-Q. (2005b). Two New Triterpenoid Saponins from Sarcandra glabra. J. Asian Nat. Prod. Res. 7, 829–834. doi:10.1080/10286020410001721104
Luo, Y. (2004). Study on Jiangxi Characteristic Chinese Medicinal Materials Sarcandra glabra and Cinnamomum Camphora. Master’s Thesis. (China): Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences.
Majnooni, M. B., Fakhri, S., Shokoohinia, Y., Mojarrab, M., Kazemi-Afrakoti, S., and Farzaei, M. H. (2020). Isofraxidin: Synthesis, Biosynthesis, Isolation, Pharmacokinetic and Pharmacological Properties. Molecules 25, 2040. doi:10.3390/molecules25092040
Ni, G., Zhang, H., Liu, H.-C., Yang, S.-P., Geng, M.-Y., and Yue, J.-M. (2013). Cytotoxic Sesquiterpenoids from Sarcandra glabra. Tetrahedron 69, 564–569. doi:10.1016/j.tet.2012.11.023
Nunes, S., Madureira, R., Campos, D., Sarmento, B., Gomes, A. M., Pintado, M., et al. (2017). Therapeutic and Nutraceutical Potential of Rosmarinic Acid - Cytoprotective Properties and Pharmacokinetic Profile. Crit. Rev. Food Sci. Nutr. 57, 1799–1806. doi:10.1080/10408398.2015.1006768
Oanh, D. T., Ky, P. T., Hang, N. T. B., Yen, P. H., Hanh, T. H., Cuong, N. X., et al. (2010). Two New Sesquiterpenes from Sarcandra glabra. Nat. Prod. Commun. 5, 1717–1720. doi:10.1177/1934578x1000501102
Okamura, H., Iwagawa, T., and Nakatani, M. (1995). A Revised Structure of Chloranthalactone F and Chloranthalactone A Photodimer. Bcsj 68, 3465–3467. doi:10.1246/bcsj.68.3465
Qin, J., Wang, R., Teng, J., Chen, J., and Huang, R. (2007). The Effect of Sarcandra glabra Extracts on Oxygen Free Radical. Lishizhen Med. Mater. Med. Res. 18, 2945–2946.
Shi, G., Zeng, S., and Chen, J. (1980). Effects of Three Extracts of Sarcandra glabra on Experimental Fracture Healing (Animal Experiment Report). J. Guiyang Coll. Tradit. Chin. Med., 59–66.
Shi, J. (2009). Clinical Observation of Xuekang Oral Liquid on Thrombocytopenia. Chin. J. Mod. Drug Appl. 3, 110–111.
Song, C. (2017). Clinical Study on Zhongjiefeng Injection Combined with Apatinib in Treatment of Advanced Gastric Cancer. Drugs Clin. 32, 1114–1117. doi:10.7501/j.issn.1674-5515.2017.06.036
Su, M., and Luo, C. (2009). Clinical Effect of Sarcandra glabra on Abdominal Incision Infection. China Mod. Med. 16, 88.
Sun, H., Lu, X., Hu, X., Chen, Z., Zhu, J., Lu, L., et al. (2019). Effect and Mechanism of Total Flavonoids from Sarcandrae Herba on the Apoptosis in Human Leukemia K562 Cells. Pharmacol. Clin. Chin. Mater. Med. 35, 54–57. doi:10.13412/j.cnki.zyyl.2019.06.012
Sun, J., Sun, X., Wang, H., Zhan, G., and Zhan, J. (1998). Toxicity Study of Sarcandra glabra. Joural Guiyang Med. Coll. 23, 43–44.
Sun, X., Zhao, Q., Si, Y., Li, K., Zhu, J., Gao, X., et al. (2020). Bioactive Structural Basis of Proteoglycans from Sarcandra glabra Based on Spectrum-Effect Relationship. J. Ethnopharmacology 259, 112941. doi:10.1016/j.jep.2020.112941
Takeda, Y., Yamashita, H., Matsumoto, T., and Terao, H. (1993). Chloranthalactone F, A Sesquiterpenoid from the Leaves of Chloranthus Glaber. Phytochemistry 33, 713–715. doi:10.1016/0031-9422(93)85480-F
Tang, X., Liao, Q., Bao, T., Shang, G., Li, J., Zhu, F., et al. (2014). Effects of Flavonoids Sarcandrae and its Ingredients Rosmarinic Acid and Astilbin on Proliferation of Rat Bone Marrow Megakaryocytes. Pharmacol. Clin. Chin. Mater. Med. 30, 47–49. doi:10.13412/j.cnki.zyyl.2014.02.016
Tong, S., Huang, J., Wang, B., and Yan, J. (2010). Study on the Chemical Constituents of Sarcandra glabra. Chin. Tradit. Herb. Drugs 41, 198–201.
Tsai, Y.-C., Chen, S.-H., Lin, L.-C., and Fu, S.-L. (2017). Anti-inflammatory Principles from Sarcandra glabra. J. Agric. Food Chem. 65, 6497–6505. doi:10.1021/acs.jafc.6b05125
Tsui, W.-Y., and Brown, G. D. (1996). Cycloeudesmanolides from Sarcandra glabra. Phytochemistry 43, 819–821. doi:10.1016/j.nuclphysb.2004.12.02510.1016/0031-9422(96)00352-4
Wang, A., and Ma, X. (1979a). Preliminary Study on the Active Constituents of Sarcandra glabra. Chin. Tradit. Herb. Drugs 8–9.
Wang, A., and Ma, X. (1979b). Study on the Effective Components of Sarcandra glabra. Chin. Tradit. Herb. Drugs 8–9.
Wang, C., Li, Y., Li, C. J., Yu, S. S., and Zhang, D. M. (2012). Three New Compounds from Sarcandra glabra. Chin. Chem. Lett. 23, 823–826. doi:10.1016/j.cclet.2012.05.007
Wang, C., Li, Y., Yang, J., Li, C., and Zhang, D. D. (2010a). Study on the Chemical Constituents of Sarcandra glabra (Abstract). 10th Natl. Symp. Traditional Chin. Med. Nat. Med., 35, 714–717. doi:10.4268/cjcmm20100612
Wang, C., Zhu, L., Yang, J., Li, C., and Zhang, D. (2010b). [Chemical Constituents from Sarcandra glabra]. Zhongguo Zhong Yao Za Zhi 35, 714–717.
Wang, F., Yuan, S.-T., and Zhu, D.-N. (2007). Active Components of Antitumor Fraction from Sarcandra glabra. Chin. J. Nat. Med. 5, 174–178.
Wang, J., and Du, M. (2008). Comparison of Antibacterial Effect of Zhongjiefeng Tablets with Chaiyin, Shuanghuanglian and Qutan Oral Liquid In Vitro. Shanghai Med. Pharm. J. 29, 80–82. doi:10.1080/01932690701783499
Wang, M., Zhao, J., Zhao, Y., Huang, R.-Y., Li, G., Zeng, X., et al. (2015a). A New Coumarin Isolated from Sarcandra glabra as Potential Anti-inflammatory Agent. Nat. Product. Res. 30, 1796–1801. doi:10.1080/14786419.2015.1079186
Wang, P., Luo, J., Zhang, Y.-M., and Kong, L.-Y. (2015b). Sesquiterpene Dimers Esterified with Diverse Small Organic Acids from the Seeds of Sarcandra glabra. Tetrahedron 71, 5362–5370. doi:10.1016/j.tet.2015.05.112
Wang, P., Li, R.-J., Liu, R.-H., Jian, K.-L., Yang, M.-H., Yang, L., et al. (2016). Sarglaperoxides A and B, Sesquiterpene-Normonoterpene Conjugates with a Peroxide Bridge from the Seeds of Sarcandra glabra. Org. Lett. 18, 832–835. doi:10.1021/acs.orglett.6b00112
Wang, Y., Hou, G., and Liu, Y. (2020). Intervention Effect and Mechanism of Polysaccharides of Sarcandra glabra on Exercise-Induced Muscle Damage. J. Sanming Univ. 37, 1–9.
Wang, Y., Yan, W., Chen, Q., Huang, W., Yang, Z., Li, X., et al. (2017). Inhibition Viral RNP and Anti-inflammatory Activity of Coumarins against Influenza Virus. Biomed. Pharmacother. 87, 583–588. doi:10.1016/j.biopha.2016.12.117
Wei, S., Chi, J., Zhou, M., Li, R., Li, Y., Luo, J., et al. (2019). Anti-inflammatory Lindenane Sesquiterpeniods and Dimers from Sarcandra glabra and its Upregulating AKT/Nrf2/HO-1 Signaling Mechanism. Ind. Crops Prod. 137, 367–376. doi:10.1016/j.indcrop.2019.05.041
Wu, H., Hu, X., Zhang, X., Chen, S., Yang, J., and Xu, X. (2012a). Benzyl 2-β-Glucopyranosyloxybenzoate, a New Phenolic Acid Glycoside from Sarcandra glabra. Molecules 17, 5212–5218. doi:10.3390/molecules17055212
Wu, H., Hu, X., Zhang, X., Chen, S., Yang, J., and Xu, X. (2012b). Isolation and Chemotaxonomic Significance of Megastigmane-type Sesquiterpenoids from Sarcandra glabra. J. Med. Plant Res. 6, 4501–4504. doi:10.5897/JMPR12.523
Wu, J.-T., Lv, S.-M., Lu, C.-H., Gong, J., and An, J.-B. (2015). Effect of 3,3′-Biisofraxidin on Apoptosis of Human Gastric Cancer BGC-823 Cells. Trop. J. Pharm. Res. 14, 1803–1811. doi:10.4314/tjpr.v14i10.10
Xia, Y., Fu, J., Xu, C., and Wu, H. (1996). Study on Acute Toxicity and Mutagenicity of Sarcandra glabra. J. Zhejiang Coll. TCM 20, 36–37.
Xie, Y., Zeng, J., Zheng, Y., Lin, P., and Liang, Y. (2010). Study on Anti-inflammatory Active Sites of Sarcandra glabra (Thunb.) Nakai Produced in Fujian Province. J. Fujian Univ. TCM 20, 35–38.
Xiong, Y., Ying, F., Yu, H., Zi, X., and Yang, H. (2015). Effects of Liquid Extract of Sarcandra glabra to Anti-hepatic Fibrosis in Rats. J. Jiangxi Univ. TCM 27, 76–78.
Xu, G., Xiao, B., Chen, Q., Xu, Y., and Bi, M. (2005). Effect of Sarcand glabra and its Separated Parts on Amount of Blood Platelet of Immunologic Thrombocytopenic Purpura in Mice. Chin. J. Exp. Tradit. Med. Formulae 11, 33–36.
Xu, T., Hu, B., and Zhuang, J. (1997). Clinical Observation of Xuekang in the Treatment of Thrombocytopenia Caused by Chemotherapy. Acta Univ. Med. Anhui 663.
Xu, X. D., Hu, X. R., Yuan, J. Q., and Yang, J. S. (2008). [Studies on Chemical Constituents of Sarcandra glabra]. Zhongguo Zhong Yao Za Zhi 33, 900–902.
Xu, Y., Liu, X., Huang, X., and Ge, F. (2011). Status and Prospect of Studies on Sarcandra glabra. Chin. Tradit. Herb. Drugs 42, 2552–2559.
Yang, B., Sun, W., Xun, D., and Teng, W. (2008a). Determination of Twelve Elements in Herba Sarcandrae by ICP-MS. Chin. J. Spectrosc. Lab. 25, 502–504.
Yang, R., Wang, B., Li, N., and Zhang, X. (2008b). Analysis of Chemical Constituents of Essential Oil from the Leaves of Sarcandra glabra by GC-MS. Chin. Tradit. Pat. Med. 30, 1703–1704.
Yang, C. (1992). Development and Utilization of Folk Tea Plant Resources of Dong Nationality in Tongdao. Hunan For. Sci. Technol. 19, 58–61.
Yang, W.-q., Hai, P., Xiao, H., Gao, Y., Tao, Y.-h., Miao, D.-r., et al. (2018). Glabralides A - C, Three Novel Meroterpenoids from Sarcandra glabra. Tetrahedron 74, 341–347. doi:10.1016/j.tet.2017.12.001
Yang, X.-R., Tanaka, N., Tsuji, D., Lu, F.-L., Yan, X.-J., Itoh, K., et al. (2020). Sarcaglabrin A, a Conjugate of C15 and C10 Terpenes from the Aerial Parts of Sarcandra glabra. Tetrahedron Lett. 61, 151916. doi:10.1016/j.tetlet.2020.151916
Yang, X. (2017). Bioactive Material Basis of Medicinal Plants in Genre Sarcandra. Mod. Chin. Med. 19, 155–164.
Yu, F., Fu, J., and Liang, J. (2012). Chemical Constituents of Sarcandra glabra. Biotech. World 5-6, 8.
Yuan, K., Zhu, J. X., Si, J. P., Cai, H. K., Ding, X. D., and Pan, Y. J. (2008). [Studies on Chemical Constituents and Antibacterial Activity from N-Butanol Extract of Sarcandra glabra]. Zhongguo Zhong Yao Za Zhi 33, 1843–1846.
Zeng, A., and Luo, Y. (2005). Chemical Constituents of Sarcandra glabra. J. Chin. Med. Mater. 28, 292–293.
Zhang, L., Zou, S., Hu, X., Yu, Z., and Fan, Q. (2016). Toxicology Safety Evaluation of New Food Resources of Sarcandra glabra. J. Nanchang Univ. Sci. 40, 478–482. doi:10.13764/j.cnki.ncdl.2016.05.014
Zhang, Z., Zheng, Y., Zhu, R., Zhu, Y., Yao, W., Liu, W., et al. (2014). The ERK/eIF4F/Bcl-XL Pathway Mediates SGP-2 Induced Osteosarcoma Cells Apoptosis In Vitro and In Vivo. Cancer Lett. 352, 203–213. doi:10.1016/j.canlet.2014.06.015
Zhao, Y., Sun, Y., and Chen, Q. (2008). Studies on the Antitumor Activity of Zhong Jiefeng Injection In Vitro. Chin. J. ethnomedieine ethnopharmacy 8-9, 36.
Zheng, X., Liu, H.-Y., and Zhong, H. (2014). Chemical Constituents from Sarcandra glabra. Nat. Prod. Res. Dev. 26, 1221–1224.
Zheng, Y., Xu, X., Zou, X., Guan, L., Zhang, D., Liu, J., et al. (2016). Chemical Constituents with the Antioxidant Activity in Sarcandra glabra. J. Fujian Norm. Univ. Sci. Ed. 32, 98–102.
Zhong, L., Liu, T., Chen, Y., Zhong, X., Du, X., Lu, Z., et al. (2005). [The Study on Effect of Sarcandra glabra on Prevention and Treatment of Thrombocytopenia by Chemotherapy]. Zhong Yao Cai 28, 35–38.
Zhou, Z. (1993). Origin, Systematics and Distribution of Chloranthaceae. Acta Bot. Yunnanica 15, 321–331.
Zhu, L., Li, Y., Yang, J., Zuo, L., and Zhang, D. (2008a). Studies on Chemical Constituents of Sarcandra glabra. China J. Chin. Mater. Med. 33, 155–157.
Zhu, L.-P., Li, Y., Yang, J.-Z., Zuo, L., and Zhang, D.-M. (2008b). Two New Sesquiterpene Lactones from Sarcandra glabra. J. Asian Nat. Prod. Res. 10, 541–545. doi:10.1080/10286020801966773
Keywords: Sarcandra glabra (Thunb.) Nakai, traditional uses, phytochemistry, pharmacology, toxicity
Citation: Zeng Y, Liu J, Zhang Q, Qin X, Li Z, Sun G and Jin S (2021) The Traditional Uses, Phytochemistry and Pharmacology of Sarcandra glabra (Thunb.) Nakai, a Chinese Herb With Potential for Development: Review. Front. Pharmacol. 12:652926. doi: 10.3389/fphar.2021.652926
Received: 13 January 2021; Accepted: 01 April 2021;
Published: 22 April 2021.
Edited by:
Shuai Ji, Xuzhou Medical University, ChinaReviewed by:
Taoufiq Benali, Cadi Ayyad University, MoroccoCopyright © 2021 Zeng, Liu, Zhang, Qin, Li, Sun and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guojuan Sun, c3VuZ3VvanVhbkAxMjYuY29t; Shenrui Jin, SjE1MTg0NDM2NTM5QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.