- 1Department of Ophthalmology, School of Pharmacy, College of Medical Technology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Department of Respiratory, School of Pharmacy, College of Medical Technology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Oridonin, as a natural terpenoids found in traditional Chinese herbal medicine Isodon rubescens (Hemsl.) H.Hara, is widely present in numerous Chinese medicine preparations. The purpose of this review focuses on providing the latest and comprehensive information on the pharmacology, pharmacokinetics and toxicity of oridonin, to excavate the therapeutic potential and explore promising ways to balance toxicity and efficacy of this natural compound. Information concerning oridonin was systematically collected from the authoritative internet database of PubMed, Elsevier, Web of Science, Wiley Online Library and Europe PMC applying a combination of keywords involving “pharmacology,” “pharmacokinetics,” and “toxicology”. New evidence shows that oridonin possesses a wide range of pharmacological properties, including anticancer, anti-inflammatory, hepatorenal activities as well as cardioprotective protective activities and so on. Although significant advancement has been witnessed in this field, some basic and intricate issues still exist such as the specific mechanism of oridonin against related diseases not being clear. Moreover, several lines of evidence indicated that oridonin may exhibit adverse effects, even toxicity under specific circumstances, which sparked intense debate and concern about security of oridonin. Based on the current progress, future research directions should emphasize on 1) investigating the interrelationship between concentration and pharmacological effects as well as toxicity, 2) reducing pharmacological toxicity, and 3) modifying the structure of oridonin—one of the pivotal approaches to strengthen pharmacological activity and bioavailability. We hope that this review can provide some inspiration for the research of oridonin in the future.
Introduction
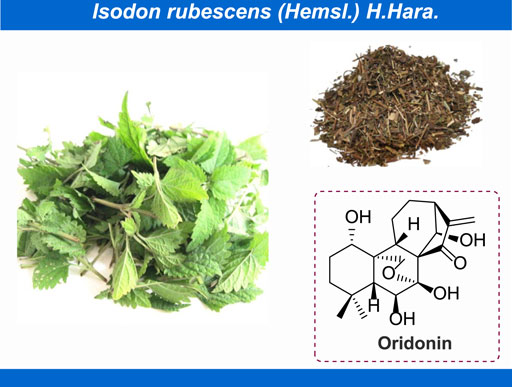
Oridonin, (PubChem CID: 5321010, CAS No: 28957-04-2, MW: 364.4 g/mol), with the molecular formula of C20H28O6 (Cheng et al., 2019), is a naturally occurring terpenoids that mainly exists in Isodon rubescens (Hemsl.) H.Hara (Figure 1; Yang I.-H. et al., 2017; Jian et al., 2019; Meng et al., 2019). In thousands of years of clinical practice, the Isodon rubescens (Hemsl.) H.Hara has been widely applied as central agent in classic traditional Chinese medicine (TCM) formulas with its efficacy of clearing away heat and detoxifying, boosting blood circulation and alleviating pain. Generally, I. rubescens (Hemsl.) H.Hara is frequently utilized in the treatment of acute and chronic pharyngitis, tonsillitis and bronchitis in clinic (Zhang et al., 2020). As the main bioactive chemical component of I. rubescens (Hemsl.) H.Hara, in recent years, numerous achievements have been witnessed on the exploration of pharmacological effects of oridonin, such as anti-inflammatory (Cummins et al., 2019; He et al., 2019), anti-cancer (Vasaturo et al., 2018; Jeon et al., 2019; Hu et al., 2020), anti-microbial (Li D. et al., 2016), anti-sepsis (Zhao et al., 2016), neuroprotection (Lin et al., 2019), immunoregulation (Guo et al., 2013) and so on. Consequently, to some extent, these rapid advancements in the discovery of the pharmacological activity of oridonin have provided extensive opportunities for the development of innovative disease strategies. On the other hand, there have been mounting reports concentrated on the adverse reactions of oridonin. Recent studies have shown that oridonin can cause suicidal erythrocyte death, induce the expression and activation of CYP2C and CYP3A family, and interfere with the early embryonic development of zebrafish. Under this background, thereby motivated, we herein to summarize the latest and comprehensive information on the pharmacology, toxicity and pharmacokinetics of oridonin, to excavate the potential of this natural active ingredient in the treatment of various diseases and furnish basic information for the rational and secure utilization of oridonin.
Pharmacology
Anti-Inflammatory Activity
According to the literature, oridonin can markedly inhibit experimental autoimmune neuritis (EAN) by lessening local inflammatory reaction and increasing the proportion of immune regulating macrophages in the peripheral nerves possibly by the pathway of Notch, which indicates that it can be developed as a potential therapeutic agent for human Guillain-Barre syndrome (GBS) and neuropathies (Xu L. et al., 2019). Moreover, the employment of oridonin enables to relieve carrageenan-induced pleurisy through activating the KEAP-1/Nrf2 pathway and suppressing the TXNIP/NLRP3 and NF-κB pathway in the model of BALB/c mice. These specific manifestations includes reducing lung injury scores, releasing of cytokines, neutrophil infiltration, exudating volume and the exudate protein concentration, decreasing the levels of oxidative stress markers (Yang et al., 2020). Recently, researcher relies on the fact that oridonin itself can act as a protective agent against LPS-induced inflammatory response, which the specific mechanisms involve in ROS accumulation, JNK activation, nuclear translocation of NF-κB (Huang et al., 2020). Oridonin also inhibits autophagy and survival in rheumatoid arthritis fibroblast-like synoviocytes (He et al., 2020). In addition, oridonin can also resist a series of inflammatory reactions including LPS-induced inflammation in human gingival fibroblasts (Yu et al., 2019), IL-1β-induced inflammation in human osteoarthritis chondrocytes (Jia et al., 2019) and LPS-induced endometritis (Zhou et al., 2019). These findings indicate that oridonin may be served as a potential therapeutic agent for a variety of inflammatory related diseases. A great deal of immune cells including T cells plays an important role in the process of inflammation. In recent years, studies on anti-inflammatory effect of oridonin based on immune response have gradually increased. Research showed that it alleviated the colitis induced by trinitrobenzene sulfonic acid as represented by a decrease in colonic interferon-/inteleukin-17 secretion and a consumption in splenic Th1/Th17 cells and effector memory CD4(+) T cells (Wang et al., 2015). In addition, oridonin inhibited inflammatory graft rejection by depleting a great number of T cells in spleen and peripheral blood (Guo et al., 2013).
Anticancer Activity
The efficacy of mainstay cancer therapies such as cytotoxics and radiation, has reached a plateau in the treatment of multiple cancers. In this regard, there is an urgent sense that ameliorations must now come from fresh approaches. In recent years, continuous attention is also shifting to the development of natural anti-tumor agents. Oridonin has a variety of documented anti-cancer activities such as its ability to against gastric cancer (He et al., 2017), oral cancer (Yang Y.-C. et al., 2017), nasopharyngeal carcinoma (Liu et al., 2021), esophageal cancer (Jiang et al., 2019), ovarian cancer (Dong et al., 2018), leukemia (Li and Ma, 2019; Zhang D. et al., 2019), and myeloma (Wu et al., 2020), etc. Its main mechanism involves in inhibiting proliferation (Hao et al., 2016), inducing apoptosis (Gu et al., 2015; Clayton et al., 2016; Qing et al., 2016; Xu et al., 2016) and autophagy (Tiwari et al., 2015; Yao et al., 2017), suppressing migration and invasion (Li Y.-C. et al., 2016), reversing drug resistance (Kadioglu et al., 2018)] and so on.
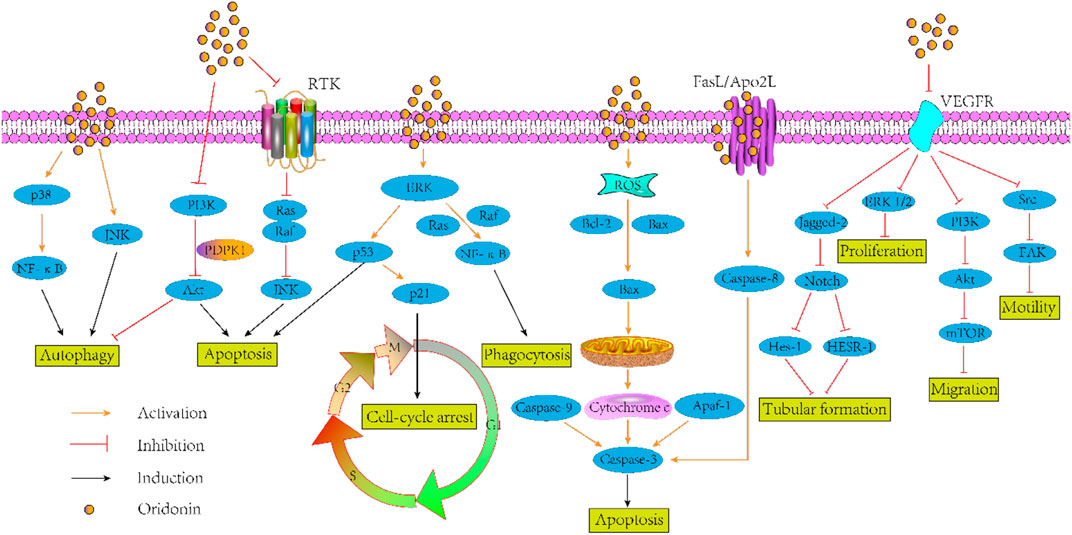
As documented in literature, utilization of oridonin increased the level of E-cadherin and ALP, reduced the vimentin, phospho-FAK levels, snail, slug, and LDH in human small cell lung cancer cell line H1688 with concentration of 2.5, 5, 10, 20, and 40 µM for 24 and 48 h in vitro. Of course, the author also confirmed the anti-lung cancer effect of oridonin in the model of BALB/c nude mice (Xu et al., 2020). Another study on the anti-lung cancer of oridonin proved that, oridonin sensitized cisplatin-induced apoptosis via AMPK/Akt/mTOR-dependent autophagosome accumulation in A549 Cells (Yang et al., 2019a). Moreover, it augmented the radiosensitivity of lung cancer cells by up-regulating Bax and down-regulating Bcl-2 (Li C. et al., 2018), underpinned radiation-induced cell death by accelerating DNA damage in non-small cell lung cells (Park et al., 2018) and promoted G2/M arrest in A549 cells by facilitating ATM (Zheng et al., 2017). In the aspect of anti-breast cancer, oridonin could synergistically enhance the anti-tumor effect of doxorubicin on aggressive breast cancer by promoting apoptosis and anti-angiogenesis (Li et al., 2019). Besides, this compound could inhibit angiogenesis and EMT related to VEGF-A (Li C. Y. et al., 2018), block Notch signaling pathway to inhibit the growth and metastasis of breast cancer (Xia et al., 2017), and induce autophagy to promote apoptosis (Li and Yang, 2015). In addition to its above anti-tumor effects, there is growing evidences that oridonin exhibits other anti-tumor activities such as colorectal cancer (Bu H. et al., 2019), pancreatic cancer (Liu D. et al., 2020), gallbladder cancer (Chen, et al., 2019), prostate cancer (Lu et al., 2017) and so on. Given that pathway defects have been recognized by most chemotherapies, oridonin may be a logical botanical for future researches of tumor adjuvant therapy. Figure 2 gives the antitumor mechanism of oridonin.
Hepatorenal Protective Activity
With the deepening of the research, the hepatorenal protective activity of oridonin has been gradually recognized. In a report on the research of LPS/D-galactosamine-induced acute liver injury in mice, oridonin was used as a compound known to be effective at improving the survival rate, alleviating histopathological abnormalities, and suppressing plasma aminotransferases, which the mechanisms may involve in the suppression of pro-apoptotic cytokine TNF-α and JNK-associated pro-apoptotic signaling (Deng et al., 2017). Oridonin also ameliorated carbon tetrachloride-induced liver fibrosis in mice through inhibiting the NLRP3 inflammasome (Liu D.-L. et al., 2020). Mouse immortalized stellate cell line JS1 treated with oridonin at the concentration of 5, 10, and 15 µM showed that it significantly impede posttranslational modifications of IRAK4 in the TLR4 signaling pathway (Shi et al., 2019). In addition, the inhibition of LPS induced apoptosis promoting cytokines IL-1 β, IL-6, and MCP-1, as well as ICAM-1 and VCAM-1 observed in LX-2 cells also appear to be able to validate the protective effect of oridonin on liver (Cummins et al., 2018). In terms of kidney protection, oridonin alleviated IRI-induced kidney injury by suppressing inflammatory response of macrophages through AKT-related pathways (Yan et al., 2020). Furthermore, oridonin at the concentrations of 2.5, 5, 10, and 20 µM managed to alleviate albuminuria, improve renal function and attenuate renal histopathological injury, hinder inflammatory cytokine production, down-regulate TLR4 expression and inhibit NF-κB and p38-MAPK activation, with the effects augmented as the dose increased (Li J. et al., 2018). These studies may provide a new recognition of natural medicine for the treatment of liver and kidney diseases.
Cardioprotective Activity
Diseases associated with cardiovascular diseases are an increasing problem in most parts of the world and, as with many other problems of today, are becoming more and more urgent for people all over the world. Therefore, a reasonable and effective strategy and approach is now essential to fight against this malady. As reported by researches in recent years, oridonin exhibited beneficial influences on cardiovascular disease. In a myocardial ischemia-reperfusion injury mouse models, down-regulation of oxidative stress and NLRP3 inflammation has been shown to mitigative effect of oridonin to myocardial ischemia reperfusion injury (Lu et al., 2020). Similar results have been verified by researchers from the perspective of metabonomics (Zhang J. et al., 2019). Oxidative stress, which has a critical link with the development of cardiac hypertrophy and heart failure, can reportedly be inhibited by oridonin via mitigating pressure overload-induced cardiac hypertrophy and fibrosis, preserving heart function, enhancing myocardial autophagy in pressure-overloaded hearts and angiotensin II-stimulated cardiomyocytes (Xu M. et al., 2019). In the respect of inhibition for vascular inflammation, oridonin could reduce the endothelial-leukocyte adhesion and leukocyte transmigration, inhibit the expression of TNF-α-induced endothelial adhesion molecules, suppress the penetration of the leukocyte, suppress the TNF-α-activated MAPK and Nuclear factor kappa B (NF-κB) activation, as described in the literature (Huang et al., 2018).
Lung Protective Activity
In recent years, oridonin, isolated from the plants of the genus rubescens, has shown great potential in lung protection due to its antioxidant and anti-inflammatory effects. Oxidative stress and the resulting inflammation are significant pathological processes in acute lung injury (ALI). According to the literature, oridonin can exert protective effects on LPS-induced ALI through Nrf2-independent anti-inflammatory and Nrf2-dependent anti-oxidative activities (Yang et al., 2019b). It also protects against chemical induced pulmonary fibrosis. Research shows that it could markedly suppress the mRNA and protein expression of α-SMA and COL1A1 in TGF-b1-induced MRC-5 cells as well as undermine pathological changes, such as alveolar space collapse, emphysema, and infiltration of inflammatory cells induced by BLM (Fu et al., 2018). Immune regulation disorder and persistent inflammatory injury are important mechanisms of ventilator-induced lung injury (VILI). As research has shown, oridonin can reduce VILI by blocking the interaction between NEK7 and NLRP3 and halting the activation of NLRP3 inflammatory bodies (Liu H. et al., 2020). In addition, post-exposure treatment with oridonin was able to ameliorate lung pathology, attenuate lung edema, abate MDA and TNF-α, and elevate GSH and IL-10 in the lung, which indicate that it can defend the lung against hyperoxia-induced injury in the model of mice (Liu et al., 2017).
Neuroprotective Activity
Oridonin produced a conspicuous effect of neuroprotective in PC12 and N2a cells by rescuing IR, reducing the autophagosome formation and synaptic loss and ameliorating cognitive dysfunction, halting IR-induced synaptic deficits (Wen et al., 2020). In the Aβ1–42-induced mouse model of Alzheimer’s disease (AD), oridonin sharply rescues synaptic loss induced by Aβ1–42, lessens the alterations in dendritic structure and spine density, augment PSD-95 and promotes mitochondrial activity (Wang J. et al., 2016). The neuropathological characteristics of AD are amyloid aggregation, tau phosphorylation, and neuroinflammation. A study indicates that different routes of administration of oridonin severely attenuated-amyloid deposition, plaque-associated APP expression and microglial activation, which suggest that this natural terpenoid might be considered a prospective therapeutic agent for human neurodegenerative diseases such as AD (Zhang et al., 2013). Furthermore, available data suggest the potentiality of oridonin to attenuate Aβ1–42-induced neuroinflammation and inhibit NF-κB pathway (Wang et al., 2014).
Other Pharmacological Activities
Several lines of evidence suggest oridonin exerts its potential role of amelioration lupus-like symptoms through suppressing BAFF expression, improving serological and clinical manifestations of SLE, lessening proteinuria levels, diminishing production of specific auto-antibodies (Zhou et al., 2013). Besides, oridonin exerted its protective effects against hydrogen peroxide-induced damage by altering the profiles of mRNA in human dermal fibroblasts (Lee et al., 2013). In the treatment of respiratory diseases, oridonin could lessen protein quantification in bronchoalveolar lavage fluid and the lung W/D ratio, mitigate inflammation and suppress the injuries, as well as abate the TNF-α, IL-6 (Jiang et al., 2017). Oridonin could also decrease the OVA-induced airway hyper-responsiveness and eosinophil number, and suppress the eosinophilia and mucus production, which confirms its great prospect in the treatment of asthma (Wang S. et al., 2016). In addition, oridonin could effectively ameliorate inflammation-induced bone loss in the model of mice by inhibiting DC-STAMP expression (Zou et al., 2020), halt the growth of methicillin-resistant Staphylococcus aureus (MRSA) (Yuan et al., 2019), mitigate visceral hyperalgesia in a rat model of postinflammatory irritable bowel syndrome (Zang et al., 2016), and augment gamma-globin expression in erythroid precursors from patients (Guo et al., 2020).
Due to the extensive biological effects of oridonin, its application in aquaculture has been gradually discovered in recent years. As reported in the literature, oridonin could improve the antioxidant capacity of arbor acres broilers liver, as evidenced by the decrease in MDA and the increase in total SOD activities and mRNA expression levels of the liver antioxidant genes (Zheng, et al., 2016). Adding oridonin to the diet of arbor acres broilers could significantly improve the immune response induced by Salmonella and protect the intestinal health (Wu et al., 2018a), increase the relative weights of spleen and bursa, number of proliferation peripheral blood T and B lymphocytes, the phagocytic rate of neutrophils, as well as the IL-2, IL-4, and TNF-α (Wu et al., 2018a). In addition, oridonin could also interfere with the effects of Salmonella pullorum on immune cells and Th1/Th2 balance of spleen in arbor acres broilers (Wu et al., 2018b). As discussed above, oridonin is a natural active compound with therapeutic potential for dozens of diseases. Additional details on the pharmacological activities of oridonin were depicted as in Table 1.
Pharmacokinetics
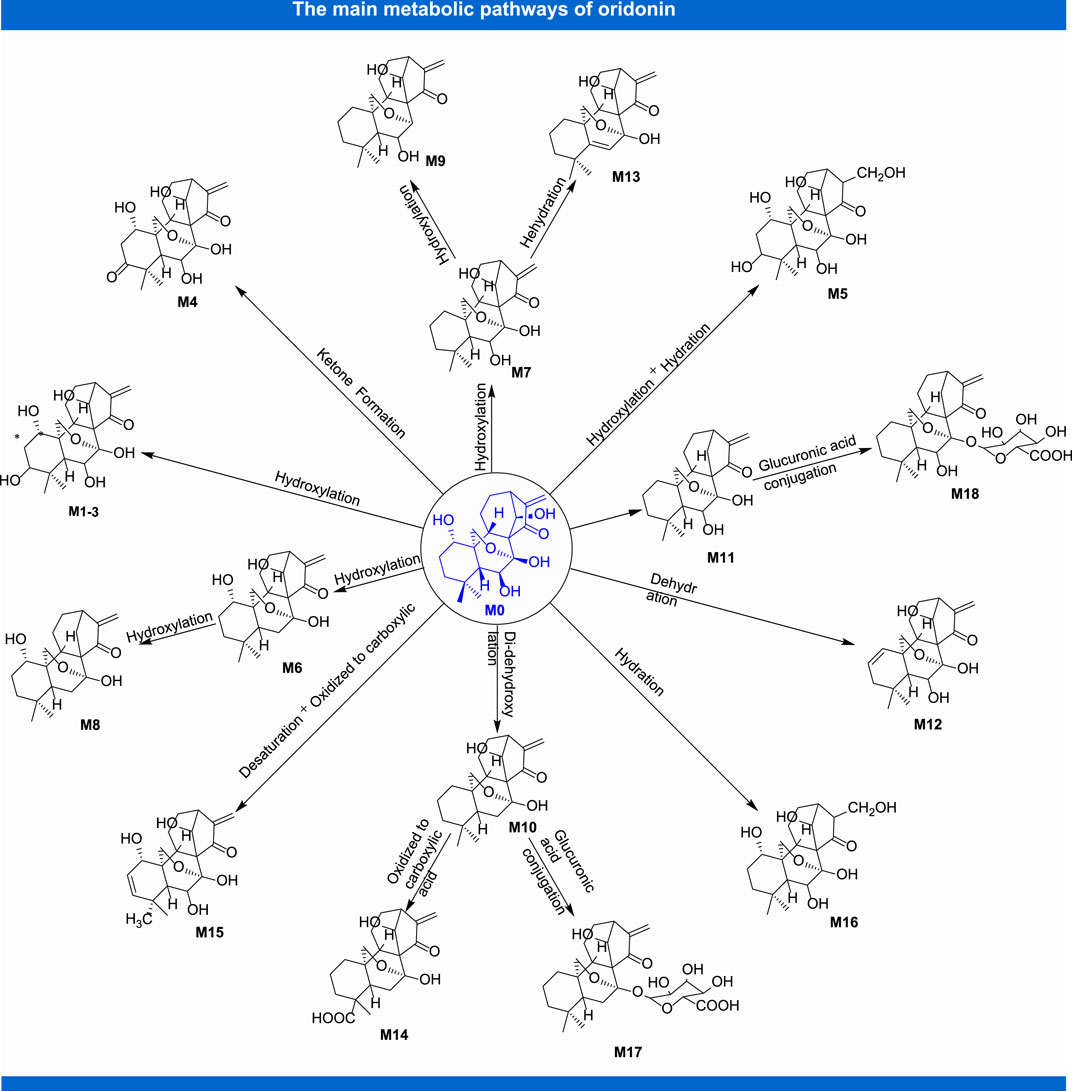
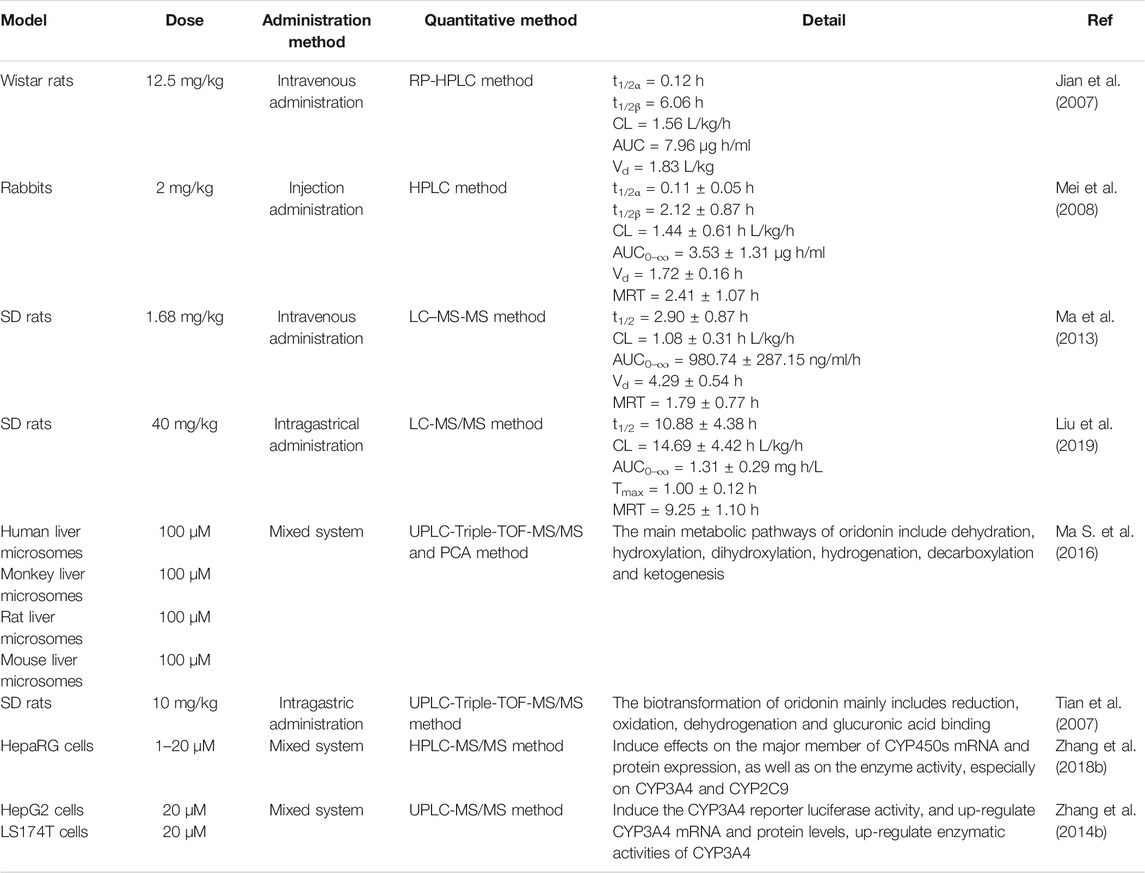
In the process of innovative agent development, pharmacokinetic research has become a pivotal part of preclinical and clinical research of drugs. It not only plays a supporting role in drug toxicity or clinical research, but also contributes to optimize the screening of candidate agents, which provides a novel approach to study modern pharmacotherapy (Sun et al., 2020a). Up to now, benefited from the continuous emergence of novel analytical techniques, researchers have investigated the pharmacokinetic parameters of oridonin in vivo by means of MS-MS (Jin et al., 2010), LC-MS-MS (Du et al., 2010; Jin et al., 2015) and other analytical methods with rats (Jian et al., 2007) and rabbits (Mei et al., 2008), which partially interpreted the kinds of events related to the efficacy and toxicity of relevant herbal preparations in which this constituent is used. Following rat oral administration of Herba Isodi Rubescentis extract containing oridonin (1.68 mg/kg), the pharmacokinetic parameters in rat plasma were obtained with the method of LC-MS-MS, revealing AUC0-t at 78.45 ± 33.83 ng/ml/h and AUC(0-infinity) at 79.29 ± 34.26 ng/ml/h, t1/2 at 0.19 ± 0.05 h, Tmax at 0.69 ± 0.13 h, Cmax at 164.51 ± 58.42 ng/ml (Ma et al., 2013). Determination of oridonin (40 mg/kg) in rat plasma after intragastrical administration with determination of LC-MS-MS suggested that it mainly metabolized in liver, and acquired main pharmacokinetic parameters, such as t1/2 at 10.88 ± 4.38 h, Tmax at 1.00 ± 0.12 h, Cmax at 146.9 ± 10.17 ng/ml, AUC(0–t) at 1.31 ± 0.29 mg h/L. At the same time, this project also told us that verapamil could substantially alter the pharmacokinetic profile of oridonin in rats, as well as it might exert these effects via elevating the absorption of this terpenoid compound by suppressing the activity of P-gp, or through hindering the metabolism of it in rat liver (Liu et al., 2019). Figure 3 shows the main metabolites of oridonin.
A strategy of using ultra-high-performance liquid chromatography-Triple/time-of-flight mass spectrometry (UPLC-Triple-TOF-MS/MS) to identify metabolites and evaluate the in vitro metabolic profile of oridonin corroborate that, oridonin is universally metabolized in vitro, which the metabolic pathway mainly consists of dehydration, hydroxylation, di-hydroxylation, hydrogenation, decarboxylation, and ketone formation. Meanwhile, 16 metabolites of I- and II-phase were identified (Ma Y. et al., 2016). Another similar study also indicated that 16 phase I and 2 phase II metabolites were detected after oral administration of oridonin in rats, and the main biotransformation pathways of oridonin were reduction, oxidation, dehydroxylation and glucuronic acid coupling (Tian et al., 2015). In addition, the treatment of HepaRG cells with oridonin at concentration of 1, 5, 10, and 20 µM demonstrated that oridonin induced the mRNA and protein expression and enzyme activity of CYP450s, especially on the CYP3A4 and CYP2C9 (Zhang Y. W. et al., 2018). Besides, studies have also shown that oridonin could induce the expression of human CYP3A4 mRNA and protein through pregnane X receptor-mediated (PXR) pathway. Notably, there is no effect on the expression of PXR-nnRNA and protein (Zhang Y.-w. et al., 2014). In the aspect of interaction between oridonin and blood protein, it could bind to human serum albumin (HAS) through hydrogen bonding and van der Waals force, and induce conformational changes of HSA, thus affecting its biological function as carrier protein. The research provides an accurate and full basic data for clarifying the binding mechanism of oridonin with HSA and is beneficial for comprehending its activity on protein function and biological activity in vivo during blood transportation process (Li et al., 2015). Other pharmacokinetic studies on oridonin are shown in Table 2.
Toxicity
When evaluating the efficacy of ingredients, the toxicity and safety of them should be considered particularly (Sun et al., 2020b). For a long time, traditional Chinese medicine (TCM) is well known for its safety. But in recent years, the adverse reactions have been reported frequently. Being a diterpenoids compound broadly distributed in medicinal plants, oridonin has an extensive range of pharmacological activities. However, several lines of evidence indicated that oridonin may exhibit adverse effects, even toxicity under specific circumstances, which sparked intense debate and concern about security of oridonin. As discussed above, it was discovered that oridonin showed antitumor activity on small cell lung cancer (SCLC), but at the same time, HE staining revealed a certain degree of cytotoxicity in hepatic tissue after treatment with oridonin (10 mg/kg) (Xu et al., 2020). In addition, intervention of oridonin induced abnormalities in zebrafish, such as uninflated swim bladder and pericardial congestion at an EC50 of 411.94 mg/L in vitro, as well as it also decreased the body length of zebrafish. In this article, researcher relied on the fact that the downregulation of VEGFR3 gene expression probably be related to the occurrence of abnormalities following oridonin exposure during embryonic development (Tian et al., 2019). A 48 h exposure to oridonin (⩾25 µM) sharply augmented cytosolic Ca2+ concentration, potentiated formation of ceramide, and then triggered suicidal death of erythrocytes (Jilani et al., 2011).
On the other hand, some reports suggested that oridonin could induce the expression and activation of CYP2C and CYP3A family (Zhang Y. W. et al., 2018), and appeared to be a potential risk to herb-drug interactions as a result of its induction effects on drug processing genes expression and activation (Zhang Y.-w. et al., 2014). Therefore, these reports suggested that we should pay attention to the safety issues caused by the combination of oridonin in clinical practice. Generally speaking, there are few adverse reports on the safety of oridonin, but the lack of reports does not mean that there are no such potential risks. In view of this, it is particularly important to explore the mechanisms responsible for the adverse risk of oridonin under particular circumstances. Other toxicity researches of oridonin are shown in Table 3.
Summary and Outlooks
Oridonin exists in considerable number of traditional herbal medicines and possesses salient medicinal value. Numerous researches have exhibited that it can regulate a variety of gene and protein expression such as ALP, IL-6, TNF-α, Bcl-2, caspase-3, PGE2, etc. It also shows extensive effects in the regulation of NF-κB, PI3K/Akt/mTOR, and ERK1/2 signaling pathways. This review summarized the mechanism by which oridonin is utilized to treat related diseases (as shown in Table 1) and the related parameters of the pharmacokinetics (as shown in Table 2), as well as security problems in clinical practice (as shown in Table 3). However, there are some issues that need further clarification in future research.
Although oridonin has been proved to possess assorted pharmacological activities in vivo and in vitro, the specific mechanism of its biological activity has not been fully expounded. Hence, it is severely significant to further excavate the mechanism of pharmacological activity at molecular level.
Additionally, as described herein, it has shown prominent adverse effects, even toxicity under specific circumstances in vitro and in vivo. Hence, the conduction of essential investigations and comprehensive strategies to strike the balance between toxicological safety and therapeutic efficacy, as well as the establishment of an all-round research on the effect of dosage on pharmacological activity and toxicity, is highly demanded in this field.
As described herein, oridonin has shown prominent adverse effects, even toxicity under specific circumstances in vitro and in vivo. It showed hepatotoxicity and hepatoprotective effects, which the pair of pharmacological activities seems to be a paradox. However, through the analysis, it is found that this is mainly related to the concentration of oridonin and the time of administration. Long-term administration and high dose administration may cause liver damage. Therefore, it is necessary to further investigate the effects of the concentration of oridonin on pharmacological effects and toxicity. On the other hand, according to the chemical structure of oridonin, it may react covalently with the sulfhydryl group of some proteins, which can partly explain the reason of adverse reactions even toxicity of oridonin in specific environment. In addition, based on the analysis of the existing literatures, we think that the current researches are focus more on the toxicity of oridonin itself. Nevertheless, the toxic process of oridonin metabolites is still unknown. These aspects can be further interpreted in future. Therefore, in view of the above reasons for the safety of oridonin, we suggest that the conduction of essential investigations and comprehensive strategies to strike the balance between toxicological safety and therapeutic efficacy are necessary, as well as the establishment of an all-round research on the effect of dosage on pharmacological activity and toxicity, is highly demanded in this field.
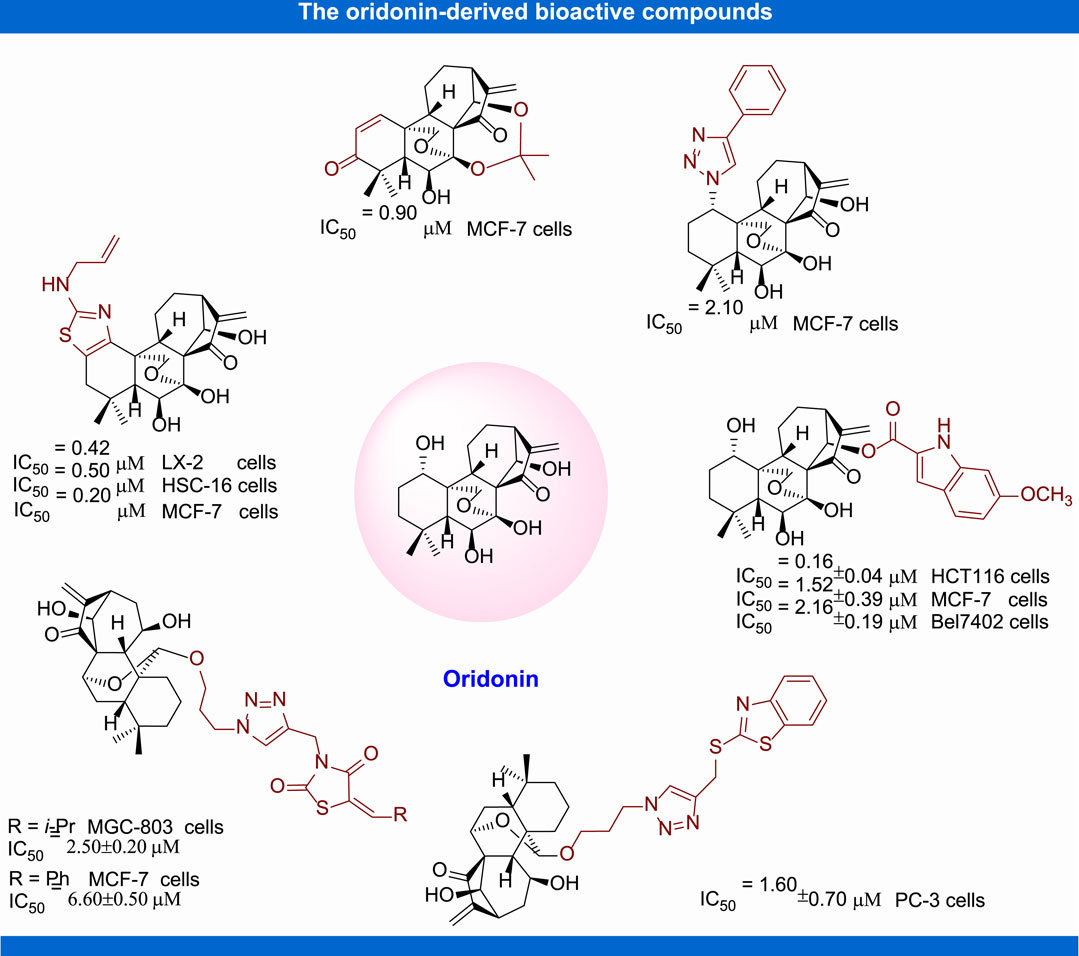
In recent years, structural modification of oridonin, including 1) the derivatization of hydroxyl groups, 2) modification of A-ring, 3) modification of the enone system, and 4) the transformation and derivatization of the framework structure, has been conducted in order to ameliorate the activity and amplify their application scope (Zhang et al., 2020). In the past decades, great progress has been made in structure activity relationship and mechanism of action studies of oridonin for the treatment of malignant tumor and other diseases (Figure 4). The structure and activity relation studies based on these new derivatives have tremendously contributed to the comprehension of their mechanism of actions and molecular targets.
According to the above literatures, we deeply realized that an increasing number of reports indicate that oridonin has miscellaneous positive pharmacological activities. However, on the whole, the oridonin’s specific mechanism related various diseases still remain to be clarified. On the other hand, although this natural active ingredient can positively influence the disease process by regulating multiple signal pathways or targets, it is only utilized as adjuvant agents in clinical practice, and rarely applied in the treatment of specific diseases. Therefore, in consideration of the current scattered research, detailed mechanism of oridonin in the treatment of specific diseases should be systematically integrated in the future.
Author Contributions
XL and QH contributed to the conception and design of the study. XL, WM, and C-TZ organized the database, performed the statistical analysis, and wrote the first draft of the manuscript. XX and QH contributed to the manuscript revision. All authors read and approved the submitted version.
Funding
Financial support was provided by the China Postdoctoral Science Foundation, Grant/Award Number: 2020M673295, 2020T130273; Project of the Open Research Fund of Chengdu University of Traditional Chinese Medicine Key Laboratory of Systematic Research of Distinctive Chinese Medicine Resources in Southwest China, Grant/Award Number: 2020BSH008; Xinglin Scholar Research Promotion Project of Chengdu University of TCM, Grant/Award Number: YXRC2019004, ZRYY1922, BSH2020018; Sichuan Science and technology program, Grant/Award Number: 2020JDRC0114, 2020YFH0164; Special Project of Science and Technology Research of Sichuan Administration of Traditional Chinese Medicine, Grant/Award Number: 2021MS093, 2021MS539; “100 Talent Plan” Project of Hospital of Chengdu University of Traditional Chinese Medicine, Grant/Award Number: Hospital office (2020) 42; Science and technology development fund of Hospital of Chengdu University of traditional Chinese Medicine, Grant/Award Number: 19SX01, 19YY10, 20YY12, 19LW19.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bae, S., Lee, E.-J., Lee, J. H., Park, I.-C., Lee, S.-J., Hahn, H. J., et al. (2014). Oridonin Protects Hacat Keratinocytes against Hydrogen Peroxide-Induced Oxidative Stress by Altering Microrna Expression. Int. J. Mol. Med. 33, 185–193. doi:10.3892/ijmm.2013.1561
Bi, E., Liu, D., Li, Y., Mao, X., Wang, A., and Wang, J. (2018). Oridonin Induces Growth Inhibition and Apoptosis in Human Gastric Carcinoma Cells by Enhancement of P53 Expression and Function. Braz. J. Med. Biol. Res. 51, e7599. doi:10.1590/1414-431X20187599
Bohanon, F. J., Wang, X., Ding, C., Ding, Y., Radhakrishnan, G. L., Rastellini, C., et al. (2014). Oridonin Inhibits Hepatic Stellate Cell Proliferation and Fibrogenesis. J. Surg. Res. 190, 55–63. doi:10.1016/j.jss.2014.03.036
Bu H.-Q., H.-Q., Shen, F., and Cui, J. (2019). The Inhibitory Effect of Oridonin on colon Cancer Was Mediated by Deactivation of TGF-β1/Smads-PAI-1 Signaling Pathway In Vitro and Vivo. Onco Targets Ther. 12, 7467–7476. doi:10.2147/Ott.S220401
Bu H., H., Liu, D., Cui, J., Cai, K., and Shen, F. (2019). Wnt/β-catenin Signaling Pathway Is Involved in Induction of Apoptosis by Oridonin in colon Cancer COLO205 Cells. Transl. Cancer Res. 8, 1782–1794. doi:10.21037/tcr.2019.08.25
Cao, S., Huang, Y., Zhang, Q., Lu, F., Donkor, P. O., Zhu, Y., et al. (2019). Molecular Mechanisms of Apoptosis and Autophagy Elicited by Combined Treatment with Oridonin and Cetuximab in Laryngeal Squamous Cell Carcinoma. Apoptosis 24, 33–45. doi:10.1007/s10495-018-1497-0
Chen, K., Ye, J., Qi, L., Liao, Y., Li, R., Song, S., et al. (2019). Oridonin Inhibits Hypoxia-Induced Epithelial-Mesenchymal Transition and Cell Migration by the Hypoxia-Inducible Factor-1α/matrix Metallopeptidase-9 Signal Pathway in Gallbladder Cancer. Anti-Cancer Drug 30, 925–932. doi:10.1097/Cad.0000000000000797
Cheng, W., Huang, C., Ma, W., Tian, X., and Zhang, X. (2018). Recent Development of Oridonin Derivatives with Diverse Pharmacological Activities. Mini Rev Med Chem. 19, 114–124. doi:10.2174/1389557517666170417170609
Clayton, J. E., Held, P. G., Larson, B., and Banks, P. (2016). Quantification of Oridonin-Induced Apoptosis and Cytotoxicity in Cancer Cells Using Noninvasive Live-Cell Imaging. Mol. Biol. Cel 27, P1088.
Cummins, C. B., Wang, X., Sommerhalder, C., Bohanon, F. J., Nunez Lopez, O., Tie, H.-Y., et al. (2018). Natural Compound Oridonin Inhibits Endotoxin-Induced Inflammatory Response of Activated Hepatic Stellate Cells. Biomed. Res. Int. 2018, 1–10. doi:10.1155/2018/6137420
Cummins, C., Wang, X., Gu, Y., Fang, X., and Radhakrishnan, R. (2019). Protective Effects of Oridonin on Intestinal Epithelial Cells by Suppressing TNFα-Induced Inflammation and Epithelial-Mesenchymal Transition. J. Am. Coll. Surgeons 229, e239–e240. doi:10.1016/j.jamcollsurg.2019.08.1390
Deng, Y., Chen, C., Yu, H., Diao, H., Shi, C., Wang, Y., et al. (2017). Oridonin Ameliorates Lipopolysaccharide/d-Galactosamine-Induced Acute Liver Injury in Mice via Inhibition of Apoptosis. Am. J. Transl Res. 9, 4271–4279.
Dong, X., Liu, F., and Li, M. (2016). Inhibition of Nuclear Factor κB Transcription Activity Drives a Synergistic Effect of Cisplatin and Oridonin on HepG2 Human Hepatocellular Carcinoma Cells. Anti-Cancer Drug 27, 286–299. doi:10.1097/Cad.0000000000000329
Dong, Y. L., Huang, C. P., and Li, J. (2018). The Inhibitive Effects of Oridonin on Cisplatin-Resistant Ovarian Cancer Cells via Inducing Cell Apoptosis and Inhibiting Adam17. Acta Med. Mediterr 34, 819–825. doi:10.19193/0393-6384_2018_3_125
Du, Y., Liu, P., Shi, X., Jin, Y., Wang, Q., Zhang, X., et al. (2010). A Novel Analysis Method for Diterpenoids in Rat Plasma by Liquid Chromatography-Electrospray Ionization Mass Spectrometry. Anal. Biochem. 407, 111–119. doi:10.1016/j.ab.2010.07.009
Fu, Y., Zhao, P., Xie, Z., Wang, L., and Chen, S. (2018). Oridonin Inhibits Myofibroblast Differentiation and Bleomycin-Induced Pulmonary Fibrosis by Regulating Transforming Growth Factor β (TGFβ)/Smad Pathway. Med. Sci. Monit. 24, 7548–7555. doi:10.12659/Msm.912740
Gao, S., Tan, H., Zhu, N., Gao, H., Lv, C., Gang, J., et al. (2016). Oridonin Induces Apoptosis through the Mitochondrial Pathway in Human Gastric Cancer Sgc-7901 Cells. Int. J. Oncol. 48, 2453–2460. doi:10.3892/ijo.2016.3479
Gong, L., Xu, H., Zhang, X., Zhang, T., Shi, J., and Chang, H. (2019). Oridonin Relieves Hypoxia-Evoked Apoptosis and Autophagy via Modulating Microrna-214 in H9c2 Cells. Artif. Cell Nanomedicine, Biotechnol. 47, 2585–2592. doi:10.1080/21691401.2019.1628037
Gu, Z., Wang, X., Qi, R., Wei, L., Huo, Y., Ma, Y., et al. (2015). Oridonin Induces Apoptosis in Uveal Melanoma Cells by Upregulation of Bim and Downregulation of Fatty Acid Synthase. Biochem. Biophysical Res. Commun. 457, 187–193. doi:10.1016/j.bbrc.2014.12.086
Gui, Z., Li, S., Liu, X., Xu, B., and Xu, J. (2015). Oridonin Alters the Expression Profiles of Micrornas in Bxpc-3 Human Pancreatic Cancer Cells. BMC Complement. Altern. Med. 15, 117. doi:10.1186/s12906-015-0640-5
Gui, Z., Luo, F., Yang, Y., Shen, C., Li, S., and Xu, J. (2017). Oridonin Inhibition and Mir-200b-3p/zeb1 axis in Human Pancreatic Cancer. Int. J. Oncol. 50, 111–120. doi:10.3892/ijo.2016.3772
Guo, L., Chen, J., Wang, Q., Zhang, J., and Huang, W. (2020). Oridonin Enhances γ-globin Expression in Erythroid Precursors from Patients with β-thalassemia via Activation of P38 MAPK Signaling. Mol. Med. Rep. 21, 909–917. doi:10.3892/mmr.2019.10848
Guo, W., Zheng, P., Zhang, J., Ming, L., Zhou, C., and Zhang, S. (2013). Oridonin Suppresses Transplant Rejection by Depleting T Cells from the Periphery. Int. Immunopharmacology 17, 1148–1154. doi:10.1016/j.intimp.2013.10.023
Han, J. M., Hong, K. O., Yang, I. H., Ahn, C. H., Jin, B., Lee, W., et al. (2020). Oridonin Induces the Apoptosis of Mucoepidermoid Carcinoma Cell Lines in a Myeloid Cell Leukemia-1-dependent Manner. Int. J. Oncol. 57, 377–385. doi:10.3892/ijo.2020.5061
Hao, Y., Li, J., Luo, Y. Y., Zhang, M., and Li, S. S. (2016). Proteomic Research on Honeybee. J. Tradit Chin. Med. 36, 225–252. doi:10.1007/978-3-319-43275-5_12
He, H. B., Jiang, H., Chen, Y., Deng, X., Jiang, W., and Zhou, R. (2019). Oridonin Is a Covalent Nlrp3 Inhibitor with strong Anti-inflammasome Activity. Eur. J. Immunol. 49, 192.
He, S.-D., Huang, S.-G., Zhu, H.-J., Luo, X.-G., Liao, K.-H., Zhang, J.-Y., et al. (2020). Oridonin Suppresses Autophagy and Survival in Rheumatoid Arthritis Fibroblast-like Synoviocytes. Pharm. Biol. 58, 146–151. doi:10.1080/13880209.2020.1711783
He, Z., Xiao, X., Li, S., Guo, Y., Huang, Q., Shi, X., et al. (2017). Oridonin Induces Apoptosis and Reverses Drug Resistance in Cisplatin Resistant Human Gastric Cancer Cells. Oncol. Lett. 14, 2499–2504. doi:10.3892/ol.2017.6421
Hu, X., Wang, Y., Gao, X., Xu, S., Zang, L., Xiao, Y., et al. (2020). Recent Progress of Oridonin and its Derivatives for the Treatment of Acute Myelogenous Leukemia. Mini Rev Med Chem 20, 483–497. doi:10.2174/1389557519666191029121809
Huang, J.-H., Lan, C.-C., Hsu, Y.-T., Tsai, C.-L., Tzeng, I.-S., Wang, P., et al. (2020). Oridonin Attenuates Lipopolysaccharide-Induced Ros Accumulation and Inflammation in Hk-2 Cells. Evidence-Based Complement. Altern. Med. 2020, 1–8. doi:10.1155/2020/9724520
Huang, W., Huang, M., Ouyang, H., Peng, J., and Liang, J. (2018). Oridonin Inhibits Vascular Inflammation by Blocking NF-κB and MAPK Activation. Eur. J. Pharmacol. 826, 133–139. doi:10.1016/j.ejphar.2018.02.044
Jeon, M.-Y., Seo, S. U., Woo, S. M., Min, K.-j., Byun, H. S., Hur, G. M., et al. (2019). Oridonin Enhances Trail-Induced Apoptosis through Galnt14-Mediated Dr5 Glycosylation. Biochimie 165, 108–114. doi:10.1016/j.biochi.2019.07.015
Jia, T., Cai, M., Ma, X., Li, M., Qiao, J., and Chen, T. (2019). Oridonin Inhibits IL-1β-induced Inflammation in Human Osteoarthritis Chondrocytes by Activating PPAR-γ. Int. Immunopharmacology 69, 382–388. doi:10.1016/j.intimp.2019.01.049
Jian, G., Wang, Y.-W., Lu, X.-C., and Cao, J.-Y. (2007). Determination of Oridonin in Rat Plasma by Reverse-phase High-Performance Liquid Chromatography. J. Pharm. Biomed. Anal. 43, 793–797. doi:10.1016/j.prp.2020.153031
Jian, Z. Y., Xu, G. F., and Dai, L. (2019). Analysis on the Accumulation of Oridonin in Different Portions of Isodon Rubescens (Hemsley) H. Hara. Bangladesh J. Bot. 48, 1193–1197. doi:10.3329/bjb.v48i4.49075
Jiang, J.-H., Pi, J., and Cai, J.-Y. (2020). Oridonin Exhibits Anti-angiogenic Activity in Human Umbilical Vein Endothelial Cells by Inhibiting Vegf-Induced Vegfr-2 Signaling Pathway. Pathol. - Res. Pract. 216, 153031. doi:10.1016/j.prp.2020.153031
Jiang, J. H., Pi, J., Jin, H., and Cai, J. Y. (2019). Oridonin‐induced Mitochondria‐dependent Apoptosis in Esophageal Cancer Cells by Inhibiting PI3K/AKT/mTOR and Ras/Raf Pathways. J. Cel Biochem 120, 3736–3746. doi:10.1002/jcb.27654
Jiang, J., Shan, X. X., and Zhu, L. (2017). Effects and Mechanisms of Oridonin in the Treatment of Acute Respiratory Distress Syndrome Mice. Int. J. Clin. Exp. Med. 10, 6191–6197.
Jilani, K., Qadri, S. M., Zelenak, C., and Lang, F. (2011). Stimulation of Suicidal Erythrocyte Death by Oridonin. Arch. Biochem. Biophys. 511, 14–20. doi:10.1016/j.abb.2011.05.001
Jin, Y., Du, Y., Shi, X., and Liu, P. (2010). Simultaneous Quantification of 19 Diterpenoids in Isodon Amethystoides by High-Performance Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 53, 403–411. doi:10.1016/j.jpba.2010.04.030
Jin, Y., Tian, T., Ma, Y., Xu, H., and Du, Y. (2015). Simultaneous Determination of Ginsenoside Rb1, Naringin, Ginsenoside Rb2 and Oridonin in Rat Plasma by LC-MS/MS and its Application to a Pharmacokinetic Study after Oral Administration of Weifuchun Tablet. J. Chromatogr. B 1000, 112–119. doi:10.1016/j.jchromb.2015.06.027
Kadioglu, O., Saeed, M., Kuete, V., Greten, H. J., and Efferth, T. (2018). Oridonin Targets Multiple Drug-Resistant Tumor Cells as Determined by In Silico and In Vitro Analyses. Front. Pharmacol. 9, 355. doi:10.3389/fphar.2018.00355
Kang, N., Cao, S.-J., Zhou, Y., He, H., Tashiro, S.-I., Onodera, S., et al. (2015). Inhibition of Caspase-9 by Oridonin, a Diterpenoid Isolated from Rabdosia Rubescens, Augments Apoptosis in Human Laryngeal Cancer Cells. Int. J. Oncol. 47, 2045–2056. doi:10.3892/ijo.2015.3186
Kang, N., Cao, S., Jiang, B., Zhang, Q., Donkor, P. O., Zhu, Y., et al. (2020). Cetuximab Enhances Oridonin-Induced Apoptosis through Mitochondrial Pathway and Endoplasmic Reticulum Stress in Laryngeal Squamous Cell Carcinoma Cells. Toxicol. Vitro 67, 104885. doi:10.1016/j.tiv.2020.104885
Lee, E.-J., Cha, H. J., Ahn, K. J., An, I.-S., An, S., and Bae, S. (2013). Oridonin Exerts Protective Effects against Hydrogen Peroxide-Induced Damage by Altering Microrna Expression Profiles in Human Dermal Fibroblasts. Int. J. Mol. Med. 32, 1345–1354. doi:10.3892/ijmm.2013.1533
Li, C., Wang, Q., Shen, S., Wei, X., and Li, G. (2018). Oridonin Inhibits VEGF-A-associated Angiogenesis and Epithelial-mesenchymal Transition of Breast Cancer In Vitro and In Vivo. Oncol. Lett. 16, 2289–2298. doi:10.3892/ol.2018.8943
Li, C. Y., Wang, Q., Shen, S., Wei, X. L., and Li, G. X. (2018). Oridonin Inhibits Migration, Invasion, Adhesion and TGF-β1-induced Epithelial-mesenchymal Transition of Melanoma Cells by Inhibiting the Activity of PI3K/Akt/GSK-3β Signaling Pathway. Oncol. Lett. 15, 1362–1372. doi:10.3892/ol.2017.7421
Li, D., Han, T., Xu, S., Zhou, T., Tian, K., Hu, X., et al. (2016). Antitumor and Antibacterial Derivatives of Oridonin: A Main Composition of Dong-Ling-Cao. Molecules 21, 575. doi:10.3390/molecules21050575
Li, J., Bao, L., Zha, D., Zhang, L., Gao, P., Zhang, J., et al. (2018). Oridonin Protects against the Inflammatory Response in Diabetic Nephropathy by Inhibiting the TLR4/p38-MAPK and TLR4/NF-κB Signaling Pathways. Int. Immunopharmacology 55, 9–19. doi:10.1016/j.intimp.2017.11.040
Li, J., Wu, Y., Wang, D., Zou, L., Fu, C., Zhang, J., et al. (2019). Oridonin Synergistically Enhances the Anti-tumor Efficacy of Doxorubicin against Aggressive Breast Cancer via Pro-apoptotic and Anti-angiogenic Effects. Pharmacol. Res. 146, 104313. doi:10.1016/j.phrs.2019.104313
Li, S., Shi, D., Zhang, L., Yang, F., and Cheng, G. (2018). Oridonin Enhances the Radiosensitivity of Lung Cancer Cells by Upregulating Bax and Downregulating Bcl-2. Exp. Ther. Med. 16, 4859–4864. doi:10.3892/etm.2018.6803
Li, W., and Ma, L. (2019). Synergistic Antitumor Activity of Oridonin and Valproic Acid on HL‐60 Leukemia Cells. J. Cel Biochem 120, 5620–5627. doi:10.1002/jcb.27845
Li, X., and Yang, Z. (2015). Interaction of Oridonin with Human Serum Albumin by Isothermal Titration Calorimetry and Spectroscopic Techniques. Chemico-Biological Interactions 232, 77–84. doi:10.1016/j.cbi.2015.03.012
Li, Y.-C., Sun, M.-R., Zhao, Y.-H., Fu, X.-Z., Xu, H.-W., and Liu, J.-F. (2016). Oridonin Suppress Cell Migration via Regulation of Nonmuscle Myosin Iia. Cytotechnology 68, 389–397. doi:10.1007/s10616-014-9790-4
Li, Y., Wang, Y., Wang, S., Gao, Y., Zhang, X., Lu, C. H., et al. (2015). Oridonin Phosphate-Induced Autophagy Effectively Enhances Cell Apoptosis of Human Breast Cancer Cells. Med. Oncol. 32, 365. doi:10.1007/s12032-014-0365-1
Liang, J., Wang, W., Wei, L., Gao, S., and Wang, Y. (2018). Oridonin Inhibits Growth and Induces Apoptosis of Human Neurocytoma Cells via the Wnt/β-catenin Pathway. Oncol. Lett. 16, 3333–3340. doi:10.3892/ol.2018.8977
Liermann, J., Naumann, P., Fortunato, F., Schmid, T. E., Weber, K.-J., Debus, J., et al. (2017). Phytotherapeutics Oridonin and Ponicidin Show Additive Effects Combined with Irradiation in Pancreatic Cancer In Vitro. Radiol. Oncol. 51, 407–414. doi:10.1515/raon-2017-0048
Lin, K.-H., Li, C.-Y., Hsu, Y.-M., Tsai, C.-H., Tsai, F.-J., Tang, C.-H., et al. (2019). Oridonin, A Natural Diterpenoid, Protected NGF-Differentiated PC12 Cells against MPP+- and Kainic Acid-Induced Injury. Food Chem. Toxicol. 133, 110765. doi:10.1016/j.fct.2019.110765
Liu, D.-L., Bu, H.-Q., Jin, H.-M., Zhao, J.-F., Li, Y., and Huang, H. (2014). Enhancement of the Effects of Gemcitabine against Pancreatic Cancer by Oridonin via the Mitochondrial Caspase-dependent Signaling Pathway. Mol. Med. Rep. 10, 3027–3034. doi:10.3892/mmr.2014.2584
Liu, D.-L., Bu, H.-Q., Wang, W.-L., Luo, H., and Cheng, B.-N. (2020). Oridonin Enhances the Anti-tumor Activity of Gemcitabine towards Pancreatic Cancer by Stimulating Bax- and Smac-dependent Apoptosis. Transl Cancer Res. TCR 9, 4148–4161. doi:10.21037/tcr-19-3000
Liu, D., Qin, H., Yang, B., Du, B., and Yun, X. (2020). Oridonin Ameliorates Carbon Tetrachloride‐induced Liver Fibrosis in Mice through Inhibition of the NLRP3 Inflammasome. Drug Dev. Res. 81, 526–533. doi:10.1002/ddr.21649
Liu, H., Gu, C., Liu, M., Liu, G., and Wang, Y. (2020). Nek7 Mediated Assembly and Activation of Nlrp3 Inflammasome Downstream of Potassium Efflux in Ventilator-Induced Lung Injury. Biochem. Pharmacol. 177, 113998. doi:10.1016/j.bcp.2020.113998
Liu, J., Zhang, N., Li, N., Fan, X., Li, Y., and Li, Y. (2019). Influence of Verapamil on the Pharmacokinetics of Oridonin in Rats. Pharm. Biol. 57, 787–791. doi:10.1080/13880209.2019.1688844
Liu, R.-X., Ma, Y., Hu, X.-L., Ren, W.-Y., Liao, Y.-P., Wang, H., et al. (2018). Anticancer Effects of Oridonin on colon Cancer Are Mediated via Bmp7/p38 Mapk/p53 Signaling. Int. J. Oncol. 53, 2091–2101. doi:10.3892/ijo.2018.4527
Liu, W., Huang, G., Yang, Y., Gao, R., Zhang, S., and Kou, B. (2021). Oridonin Inhibits Epithelial-Mesenchymal Transition of Human Nasopharyngeal Carcinoma Cells by Negatively Regulating Akt/stat3 Signaling Pathway. Int. J. Med. Sci. 18, 81–87. doi:10.7150/ijms.48552
Liu, X., Kang, J., Wang, H., Huang, T., and Huang, T. (2018). Mitochondrial Ros Contribute to Oridonin-Induced Hepg2 Apoptosis through Parp Activation. Oncol. Lett. 15, 2881–2888. doi:10.3892/ol.2017.7665
Liu, Y., Zhang, P.-x., Han, C.-h., Wei, D., Qiao, T., Peng, B., et al. (2017). Oridonin Protects the Lung against Hyperoxia-Induced Injury in a Mouse Model. Undersea Hyperb Med 44, 33–38. doi:10.22462/1.2.2017.6
Lou, S., Xu, J., Wang, B., Li, S., Ren, J., Hu, Z., et al. (2019). Downregulation of Lncrna Afap1-As1 by Oridonin Inhibits the Epithelial-To-Mesenchymal Transition and Proliferation of Pancreatic Cancer Cells. Acta Bioch Bioph Sin 51, 814–825. doi:10.1093/abbs/gmz071
Lu, C., Chen, C., Chen, A., Wu, Y., Wen, J., Huang, F., et al. (2020). Oridonin Attenuates Myocardial Ischemia/reperfusion Injury via Downregulating Oxidative Stress and Nlrp3 Inflammasome Pathway in Mice. Evidence-Based Complement. Altern. Med. 2020, 1–9. doi:10.1155/2020/7395187
Lu, J., Chen, X., Qu, S., Yao, B., Xu, Y., Wu, J., et al. (2017). Oridonin Induces G2/M Cell Cycle Arrest and Apoptosis via the PI3K/Akt Signaling Pathway in Hormone-independent Prostate Cancer Cells. Oncol. Lett. 13, 2838–2846. doi:10.3892/ol.2017.5751
Ma, B., Wang, Y., Zhang, Q., Liu, Y., Li, J., Xu, Q., et al. (2013). Simultaneous Determination of Oridonin, Ponicidin and Rosmarinic Acid from Herba Isodi Rubescentis Extract by Lc-Ms-Ms in Rat Plasma. J. Chromatogr. Sci. 51, 910–918. doi:10.1093/chromsci/bms189
Ma S., S., Tan, W., Du, B., Liu, W., Li, W., Che, D., et al. (2016). Oridonin Effectively Reverses Cisplatin Drug Resistance in Human Ovarian Cancer Cells via Induction of Cell Apoptosis and Inhibition of Matrix Metalloproteinase Expression. Mol. Med. Rep. 13, 3342–3348. doi:10.3892/mmr.2016.4897
Ma Y., Y., Xie, W., Tian, T., Jin, Y., Xu, H., Zhang, K., et al. (2016). Identification and Comparative Oridonin Metabolism in Different Species Liver Microsomes by Using Uplc-Triple-Tof-Ms/ms and Pca. Anal. Biochem. 511, 61–73. doi:10.1016/j.ab.2016.08.004
Mei, Y., Xu, J., Zhao, J., Feng, N., Liu, Y., and Wei, L. (2008). An Hplc Method for Determination of Oridonin in Rabbits Using Isopsoralen as an Internal Standard and its Application to Pharmacokinetic Studies for Oridonin-Loaded Nanoparticles. J. Chromatogr. B 869, 138–141. doi:10.1016/j.jchromb.2008.05.005
Meng, L., Gui, X., and Yun, Z. (2019). A New Method to Extract Oridonin and Rosmarinic Acid Simultaneously from Rabdosia Rubescens. Int. J. Food Eng. 15, 1–11. doi:10.1515/ijfe-2019-0013
Oh, H.-N., Seo, J.-H., Lee, M.-H., Yoon, G., Cho, S.-S., Liu, K., et al. (2018). Oridonin Induces Apoptosis in Oral Squamous Cell Carcinoma Probably through the Generation of Reactive Oxygen Species and the P38/jnk Mapk Pathway. Int. J. Oncol. 52, 1749–1759. doi:10.3892/ijo.2018.4319
Park, H., Jeong, Y., Han, N.-K., Kim, J., and Lee, H.-J. (2018). Oridonin Enhances Radiation-Induced Cell Death by Promoting DNA Damage in Non-small Cell Lung Cancer Cells. Int J Mol Sci. 19, 2378. doi:10.3390/ijms19082378
Pi, J., Cai, H., Jin, H., Yang, F., Jiang, J., Wu, A., et al. (2015). Qualitative and Quantitative Analysis of Ros-Mediated Oridonin-Induced Oesophageal Cancer Kyse-150 Cell Apoptosis by Atomic Force Microscopy. Plos One 10, e0140935. doi:10.1371/journal.pone.0140935
Qing, K., Jin, Z., Fu, W., Wang, W., Liu, Z., Li, X., et al. (2016). Synergistic Effect of Oridonin and a Pi3k/mtor Inhibitor on the Non-germinal center B Cell-like Subtype of Diffuse Large B Cell Lymphoma. J. Hematol. Oncol. 9, 72. doi:10.1186/s13045-016-0303-0
Ren, C.-M., Li, Y., Chen, Q.-Z., Zeng, Y.-H., Shao, Y., Wu, Q.-X., et al. (2016). Oridonin Inhibits the Proliferation of Human colon Cancer Cells by Upregulating Bmp7 to Activate P38 Mapk. Oncol. Rep. 35, 2691–2698. doi:10.3892/or.2016.4654
Ren, D. L., Ghoorun, R., Wu, X. H., Chen, H. L., Zhou, Q., and Wu, X. B. (2020). Oridonin Induces Apoptosis in HGC-27 Cells by Activating the JNK Signaling Pathway. Oncol. Lett. 19, 255–260. doi:10.3892/ol.2019.11104
Shang, C.-h., Zhang, Q.-q., and Zhou, J.-h. (2016). Oridonin Inhibits Cell Proliferation and Induces Apoptosis in Rheumatoid Arthritis Fibroblast-like Synoviocytes. Inflammation 39, 873–880. doi:10.1007/s10753-016-0318-2
Shi, M., Deng, Y., Yu, H., Xu, L., Shi, C., Chen, J., et al. (2019). Protective Effects of Oridonin on Acute Liver Injury via Impeding Posttranslational Modifications of Interleukin-1 Receptor-Associated Kinase 4 (Irak4) in the Toll-like Receptor 4 (Tlr4) Signaling Pathway. Mediators Inflamm. 2019, 1–11. doi:10.1155/2019/7634761
Shi, M., Lu, X.-J., Zhang, J., Diao, H., Li, G., Xu, L., et al. (2016). Oridonin, a Novel Lysine Acetyltransferases Inhibitor, Inhibits Proliferation and Induces Apoptosis in Gastric Cancer Cells through P53- and Caspase-3-Mediated Mechanisms. Oncotarget 7, 22623–22631. doi:10.18632/oncotarget.8033
Spirin, P., Lebedev, T., Orlova, N., Morozov, A., Poymenova, N., Dmitriev, S. E., et al. (2017). Synergistic Suppression of T(8;21)-Positive Leukemia Cell Growth by Combining Oridonin and Mapk1/erk2 Inhibitors. Oncotarget 8, 56991–57002. doi:10.18632/oncotarget.18503
Sun, Q., He, M., Zhang, M., Zeng, S., Chen, L., Zhou, L., et al. (2020a). Ursolic Acid: A Systematic Review of its Pharmacology, Toxicity and Rethink on its Pharmacokinetics Based on PK-PD Model. Fitoterapia 147, 104735. doi:10.1016/j.fitote.2020.104735
Sun, Q., Xie, L., Song, J., and Li, X. (2020b). Evodiamine: A Review of its Pharmacology, Toxicity, Pharmacokinetics and Preparation Researches. J. Ethnopharmacology 262, 113164. doi:10.1016/j.jep.2020.113164
Sun, Y., Jiang, X., Lu, Y., Zhu, J., Yu, L., Ma, B., et al. (2018). Oridonin Prevents Epithelial-Mesenchymal Transition and TGF-β1-Induced Epithelial-Mesenchymal Transition by Inhibiting TGF-β1/Smad2/3 in Osteosarcoma. Chemico-Biological Interactions 296, 57–64. doi:10.1016/j.cbi.2018.09.013
Sun, Z., Han, Q., Duan, L., Yuan, Q., and Wang, H. (2018). Oridonin Increases Anticancer Effects of Lentinan in Hepg2 Human Hepatoblastoma Cells. Oncol. Lett. 15, 1999–2005. doi:10.3892/ol.2017.7485
Tian, L., Sheng, D., Li, Q., Guo, C., and Zhu, G. (2019). Preliminary Safety Assessment of Oridonin in Zebrafish. Pharm. Biol. 57, 632–640. doi:10.1080/13880209.2019.1662457
Tian, L., Xie, K., Sheng, D., Wan, X., and Zhu, G. (2017). Antiangiogenic Effects of Oridonin. BMC Complement. Altern. Med. 17, 192. doi:10.1186/s12906-017-1706-3
Tian, T., Jin, Y., Ma, Y., Xie, W., Xu, H., Zhang, K., et al. (2015). Identification of Metabolites of Oridonin in Rats with a Single Run on Uplc-Triple-Tof-Ms/ms System Based on Multiple Mass Defect Filter Data Acquisition and Multiple Data Processing Techniques. J. Chromatogr. B 1006, 80–92. doi:10.1016/j.jchromb.2015.10.006
Tiwari, R. V., Parajuli, P., and Sylvester, P. W. (2015). Synergistic Anticancer Effects of Combined γ-tocotrienol and Oridonin Treatment Is Associated with the Induction of Autophagy. Mol. Cel Biochem 408, 123–137. doi:10.1007/s11010-015-2488-x
Vasaturo, M., Cotugno, R., Fiengo, L., Vinegoni, C., Dal Piaz, F., and De Tommasi, N. (2018). The Anti-tumor Diterpene Oridonin Is a Direct Inhibitor of Nucleolin in Cancer Cells. Sci. Rep. 8, 16735. doi:10.1038/s41598-018-35088-x
Wang, B., Shen, C., Li, Y., Zhang, T., Huang, H., Ren, J., et al. (2019). Oridonin Overcomes the Gemcitabine Resistant Panc-1/gem Cells by Regulating Gst Pi and Lrp/1 Erk/jnk Signalling. Onco Targets Ther. 12, 5751–5765. doi:10.2147/Ott.S208924
Wang, J., Li, F., Ding, J., Tian, G., Jiang, M., Gao, Z., et al. (2016). Investigation of the Anti-asthmatic Activity of Oridonin on a Mouse Model of Asthma. Mol. Med. Rep. 14, 2000–2006. doi:10.3892/mmr.2016.5485
Wang, S., Yang, H., Yu, L., Jin, J., Qian, L., Zhao, H., et al. (2014). Oridonin Attenuates Aβ1-42-Induced Neuroinflammation and Inhibits NF-κB Pathway. Plos One 9, e104745. doi:10.1371/journal.pone.0104745
Wang, S., Yu, L., Yang, H., Li, C., Hui, Z., Xu, Y., et al. (2016). Oridonin Attenuates Synaptic Loss and Cognitive Deficits in an Aβ1-42-Induced Mouse Model of Alzheimer's Disease. Plos One 11, e0151397–16. doi:10.1371/journal.pone.0151397
Wang, S., Zhang, Y., Saas, P., Wang, H., Xu, Y., Chen, K., et al. (2015). Oridonin's Therapeutic Effect: Suppressing Th1/Th17 Simultaneously in a Mouse Model of Crohn's Disease. J. Gastroenterol. Hepatol. 30, 504–512. doi:10.1111/jgh.12710
Wang, Y., and Zhu, Z. (2019). Oridonin Inhibits Metastasis of Human Ovarian Cancer Cells by Suppressing the Mtor Pathway. aoms 15, 1017–1027. doi:10.5114/aoms.2018.77068
Wen, F., Zhuge, W., Wang, J., Lu, X., You, R., Liu, L., et al. (2020). Oridonin Prevents Insulin Resistance-Mediated Cognitive Disorder through Pten/akt Pathway and Autophagy in Minimal Hepatic Encephalopathy. J. Cel Mol Med 24, 61–78. doi:10.1111/jcmm.14546
Wu, H., Zhu, G. H., and Su, Y. Z. (2020). Oridonin Improves the Sensitivity of Multiple Myeloma Cells to Bortezomib through the Pten/pi3k/akt Pathway. Curr. Top. Nutraceut R. 18, 292–296. doi:10.37290/ctnr2641-452X.18:292-296
Wu, Q.-X., Yuan, S.-X., Ren, C.-M., Yu, Y., Sun, W.-J., He, B.-C., et al. (2016). Oridonin Upregulates Pten through Activating P38 Mapk and Inhibits Proliferation in Human colon Cancer Cells. Oncol. Rep. 35, 3341–3348. doi:10.3892/or.2016.4735
Wu, Q. J., Zheng, X. C., Wang, T., and Zhang, T. Y. (2018a). Effect of Dietary Oridonin Supplementation on Growth Performance, Gut Health, and Immune Response of Broilers Infected with salmonella Pullorum. Ir Vet. J. 71, 1–6. doi:10.1186/s13620-018-0128-y
Wu, Q. J., Zheng, X. C., Wang, T., and Zhang, T. Y. (2018b). Effects of Dietary Supplementation with Oridonin on the Growth Performance, Relative Organ Weight, Lymphocyte Proliferation, and Cytokine Concentration in Broiler Chickens. Bmc Vet. Res. 14, 34. doi:10.1186/s12917-018-1359-6
Wu, Q. J., Zheng, X. C., Wang, T., and Zhang, T. Y. (2018c). Effects of Oridonin on Immune Cells, Th1/th2 Balance and the Expression of Blys in the Spleens of Broiler Chickens Challenged with salmonella Pullorum. Res. Vet. Sci. 119, 262–267. doi:10.1016/j.rvsc.2018.07.008
Xia, S., Zhang, X., Li, C., and Guan, H. (2017). Oridonin Inhibits Breast Cancer Growth and Metastasis through Blocking the Notch Signaling. Saudi Pharm. J. 25, 638–643. doi:10.1016/j.jsps.2017.04.037
Xiao, X., He, Z., Cao, W., Cai, F., Zhang, L., Huang, Q., et al. (2016). Oridonin Inhibits Gefitinib-Resistant Lung Cancer Cells by Suppressing Egfr/erk/mmp-12 and Cip2a/akt Signaling Pathways. Int. J. Oncol. 48, 2608–2618. doi:10.3892/ijo.2016.3488
Xu, L., Bi, Y., Xu, Y., Zhang, Z., Xu, W., Zhang, S., et al. (2020). Oridonin Inhibits the Migration and Epithelial‐to‐mesenchymal Transition of Small Cell Lung Cancer Cells by Suppressing FAK‐ERK1/2 Signalling Pathway. J. Cel Mol Med 24, 4480–4493. doi:10.1111/jcmm.15106
Xu, L., Li, L., Zhang, C.-Y., Schluesener, H., and Zhang, Z.-Y. (2019). Natural Diterpenoid Oridonin Ameliorates Experimental Autoimmune Neuritis by Promoting Anti-inflammatory Macrophages through Blocking Notch Pathway. Front. Neurosci. 13, 272. doi:10.3389/fnins.2019.00272
Xu, M., Wan, C.-x., Huang, S.-h., Wang, H.-b., Fan, D., Wu, H.-M., et al. (2019). Oridonin Protects against Cardiac Hypertrophy by Promoting P21-Related Autophagy. Cell Death Dis 10, 403. doi:10.1038/s41419-019-1617-y
Xu, T., Jin, F., Wu, K., Ye, Z., Li, N., and Li, N. (2017). Oridonin Enhances In Vitro Anticancer Effects of Lentinan in SMMC-7721 Human Hepatoma Cells through Apoptotic Genes. Exp. Ther. Med. 14, 5129–5134. doi:10.3892/etm.2017.5168
Xu, Z.-Z., Fu, W.-B., Jin, Z., Guo, P., Wang, W.-F., and Li, J.-M. (2016). Reactive Oxygen Species Mediate Oridonin-Induced Apoptosis through DNA Damage Response and Activation of Jnk Pathway in Diffuse Large B Cell Lymphoma. Leuk. Lymphoma 57, 888–898. doi:10.3109/10428194.2015.1061127
Yan, Y., Tan, R.-z., Liu, P., Li, J.-c., Zhong, X., Liao, Y., et al. (2020). Oridonin Alleviates Iri-Induced Kidney Injury by Inhibiting Inflammatory Response of Macrophages via Akt-Related Pathways. Med. Sci. Monit. 26, e921114. doi:10.12659/MSM.921114
Yang, H., Gao, Y., Fan, X., Liu, X., Peng, L., Ci, X. X., et al. (2019a). Oridonin Sensitizes Cisplatin-Induced Apoptosis via Ampk/akt/mtor-dependent Autophagosome Accumulation in A549 Cells. Front. Oncol. 9, 769. doi:10.3389/fonc.2019.00769
Yang, H., Huang, J., Gao, Y., Wen, Z., Peng, L., and Ci, X. (2020). Oridonin Attenuates Carrageenan-Induced Pleurisy via Activation of the KEAP-1/Nrf2 Pathway and Inhibition of the TXNIP/NLRP3 and NF-κB Pathway in Mice. Inflammopharmacol 28, 513–523. doi:10.1007/s10787-019-00644-y
Yang, H., Lv, H., Li, H., Ci, X., and Peng, L. (2019b). Oridonin Protects LPS-Induced Acute Lung Injury by Modulating Nrf2-Mediated Oxidative Stress and Nrf2-independent NLRP3 and NF-κB Pathways. Cell Commun Signal 17, 62. doi:10.1186/s12964-019-0366-y
Yang, I.-H., Shin, J.-A., Lee, K.-E., Kim, J., Cho, N.-P., and Cho, S.-D. (2017). Oridonin Induces Apoptosis in Human Oral Cancer Cells via Phosphorylation of Histone H2ax. Eur. J. Oral Sci. 125, 438–443. doi:10.1111/eos.12387
Yang, Y.-C., Lin, P.-H., and Wei, M.-C. (2017). Production of Oridonin-Rich Extracts fromRabdosia Rubescensusing Hyphenated Ultrasound-Assisted Supercritical Carbon Dioxide Extraction. J. Sci. Food Agric. 97, 3323–3332. doi:10.1002/jsfa.8182
Yao, Z., Xie, F., Li, M., Liang, Z., Xu, W., Yang, J., et al. (2017). Oridonin Induces Autophagy via Inhibition of Glucose Metabolism in P53-Mutated Colorectal Cancer Cells. Cel Death Dis 8, e2633. doi:10.1038/cddis.2017.35
Yu, T., Xie, W., and Sun, Y. (2019). Oridonin Inhibits LPS-Induced Inflammation in Human Gingival Fibroblasts by Activating PPARγ. Int. Immunopharmacology 72, 301–307. doi:10.1016/j.intimp.2019.04.006
Yuan, Z., Ouyang, P., Gu, K., Rehman, T., Zhang, T., Yin, Z., et al. (2019). The Antibacterial Mechanism of Oridonin against Methicillin-Resistant staphylococcus Aureus (Mrsa). Pharm. Biol. 57, 710–716. doi:10.1080/13880209.2019.1674342
Zang, K.-h., Shao, Y.-y., Zuo, X., Rao, Z., and Qin, H.-y. (2016). Oridonin Alleviates Visceral Hyperalgesia in a Rat Model of Postinflammatory Irritable Bowel Syndrome: Role of Colonic Enterochromaffin Cell and Serotonin Availability. J. Med. Food 19, 586–592. doi:10.1089/jmf.2015.3595
Zhang D., D., Zhou, Q., Huang, D., He, L., Zhang, H., Hu, B., et al. (2019). Ros/jnk/c-jun axis Is Involved in Oridonin-Induced Caspase-dependent Apoptosis in Human Colorectal Cancer Cells. Biochem. Biophysical Res. Commun. 513, 594–601. doi:10.1016/j.bbrc.2019.04.011
Zhang J., J., Zhou, Y., Sun, Y., Yan, H., Han, W., Wang, X., et al. (2019). Beneficial Effects of Oridonin on Myocardial Ischemia/reperfusion Injury: Insight Gained by Metabolomic Approaches. Eur. J. Pharmacol. 861, 172587. doi:10.1016/j.ejphar.2019.172587
Zhang W., W., Lu, Y., Zhen, T., Chen, X., Zhang, M., Liu, P., et al. (2019). Homoharringtonine Synergy with Oridonin in Treatment of T(8; 21) Acute Myeloid Leukemia. Front. Med. 13, 388–397. doi:10.1007/s11684-018-0624-1
Zhang, Y.-w., Bao, M.-h., Hu, L., Qu, Q., and Zhou, H.-h. (2014a). Dose-response of Oridonin on Hepatic Cytochromes P450 Mrna Expression and Activities in Mice. J. Ethnopharmacology 155, 714–720. doi:10.1016/j.jep.2014.06.009
Zhang, Y.-w., Zheng, X.-w., Liu, Y.-j., Fang, L., Pan, Z.-f., Bao, M.-h., et al. (2018b). Effect of Oridonin on Cytochrome P450 Expression and Activities in Heparg Cell. Pharmacology 101, 246–254. doi:10.1159/000486600
Zhang, Y. W., Bao, M. H., Wang, G., Qu, Q., and Zhou, H. H. (2014b). Induction of Human Cyp3a4 by Huperzine a, Ligustrazine and Oridonin through Pregnane X Receptor-Mediated Pathways. Pharmazie 69, 532–536. doi:10.1691/ph.2014.3950
Zhang, Y., Wang, S., Dai, M., Nai, J., Zhu, L., and Sheng, H. (2020). Solubility and Bioavailability Enhancement of Oridonin: A Review. Molecules 25, 332. doi:10.3390/molecules25020332
Zhang, Y. W., Bao, M. H., Lou, X. Y., Cheng, Y., Yu, J., and Zhou, H. H. (2018a). Effects of Oridonin on Hepatic Cytochrome P450 Expression and Activities in Pxr-Humanized Mice. Biol. Pharm. Bull. 41, 707–712.
Zhang, Z. Y., Daniels, R., and Schluesener, H. J. (2013). Oridonin Ameliorates Neuropathological Changes and Behavioural Deficits in a Mouse Model of Cerebral Amyloidosis. J. Cel. Mol. Med. 17, 1566–1576. doi:10.1111/jcmm.12124
Zhao, G., Zhang, T., Ma, X., Jiang, K., Wu, H., Qiu, C., et al. (2017). Oridonin Attenuates the Release of Pro-inflammatory Cytokines in Lipopolysaccharide-Induced raw264.7 Cells and Acute Lung Injury. Oncotarget 8, 68153–68164. doi:10.18632/oncotarget.19249
Zhao, J., Zhang, M., He, P., Zhao, J., Chen, Y., Qi, J., et al. (2017). Proteomic Analysis of Oridonin-Induced Apoptosis in Multiple Myeloma Cells. Mol. Med. Rep. 15, 1807–1815. doi:10.3892/mmr.2017.6213
Zhao, Y.-J., Lv, H., Xu, P.-B., Zhu, M.-M., Liu, Y., Miao, C.-H., et al. (2016). Protective Effects of Oridonin on the Sepsis in Mice. Kaohsiung J. Med. Sci. 32, 452–457. doi:10.1016/j.kjms.2016.07.013
Zhao, Y., and Xia, H. (2019). Oridonin Elevates Sensitivity of Ovarian Carcinoma Cells to Cisplatin via Suppressing Cisplatin-Mediated Autophagy. Life Sci. 233, 116709. doi:10.1016/j.lfs.2019.116709
Zheng, M., Zhu, Z., Zhao, Y., Yao, D., Wu, M., and Sun, G. (2017). Oridonin Promotes G2/m Arrest in A549 Cells by Facilitating Atm Activation. Mol. Med. Rep. 15, 375–379. doi:10.3892/mmr.2016.6008
Zheng, W., Zhou, C.-Y., Zhu, X.-Q., Wang, X.-J., Li, Z.-Y., Chen, X.-C., et al. (2018). Oridonin Enhances the Cytotoxicity of 5-fu in Renal Carcinoma Cells by Inducting Necroptotic Death. Biomed. Pharmacother. 106, 175–182. doi:10.1016/j.biopha.2018.06.111
Zheng, X. C., Wu, Q. J., Song, Z. H., Zhang, H., Zhang, J. F., Zhang, L. L., et al. (2016). Effects of Oridonin on Growth Performance and Oxidative Stress in Broilers Challenged with Lipopolysaccharide. Poult. Sci. 95, 2281–2289. doi:10.3382/ps/pew161
Zhou, L., Sun, L., Wu, H., Zhang, L., Chen, M., Liu, J., et al. (2013). Oridonin Ameliorates Lupus-like Symptoms of Mrllpr/lpr Mice by Inhibition of B-Cell Activating Factor (Baff). Eur. J. Pharmacol. 715, 230–237. doi:10.1016/j.ejphar.2013.05.016
Zhou, M., Yi, Y., and Hong, L. (2019). Oridonin Ameliorates Lipopolysaccharide-Induced Endometritis in Mice via Inhibition of the TLR-4/NF-κBpathway. Inflammation 42, 81–90. doi:10.1007/s10753-018-0874-8
Zhu, H. Q., Zhang, C., Guo, Z. Y., Yang, J. M., Guo, J. H., Chen, C., et al. (2019). Oridonin Induces Mdm2‐p60 to Promote P53‐mediated Apoptosis and Cell Cycle Arrest in Neuroblastoma. Cancer Med. 8, 5313–5326. doi:10.1002/cam4.2393
Keywords: oridonin, pharmacology, pharmacokinetics, toxicity, Isodon rubescens (Hemsl.) H.Hara
Citation: Li X, Zhang C-T, Ma W, Xie X and Huang Q (2021) Oridonin: A Review of Its Pharmacology, Pharmacokinetics and Toxicity. Front. Pharmacol. 12:645824. doi: 10.3389/fphar.2021.645824
Received: 24 December 2020; Accepted: 18 June 2021;
Published: 05 July 2021.
Edited by:
Thomas Efferth, Johannes Gutenberg University Mainz, GermanyReviewed by:
Ling Yang, Shanghai University of Traditional Chinese Medicine, ChinaChia-Hsin Lin, China Medical University, Taiwan
Copyright © 2021 Li, Zhang, Ma, Xie and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan-Tao Zhang, emhhbmdjaHVhbnRhb0BjZHV0Y20uZWR1LmNu; Xin Xie, eGlleGluQGNkdXRjbS5lZHUuY24=; Qun Huang, c2tpcnRoQDE2My5jb20=
 Xiang Li
Xiang Li Chuan-Tao Zhang
Chuan-Tao Zhang Wei Ma
Wei Ma Xin Xie
Xin Xie Qun Huang
Qun Huang