
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 20 May 2021
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.644103
This article is part of the Research TopicNatural Bioactive Compounds and Neurodegenerative Disorders: From Molecular Mechanisms Towards Clinical TherapiesView all 7 articles
 Valentina Echeverria1,2*
Valentina Echeverria1,2* Florencia Echeverria1
Florencia Echeverria1 George E. Barreto3,4
George E. Barreto3,4 Javier Echeverría5
Javier Echeverría5 Cristhian Mendoza1
Cristhian Mendoza1In mammals, sexual hormones such as estrogens play an essential role in maintaining brain homeostasis and function. Estrogen deficit in the brain induces many undesirable symptoms such as learning and memory impairment, sleep and mood disorders, hot flushes, and fatigue. These symptoms are frequent in women who reached menopausal age or have had ovariectomy and in men and women subjected to anti-estrogen therapy. Hormone replacement therapy alleviates menopause symptoms; however, it can increase cardiovascular and cancer diseases. In the search for therapeutic alternatives, medicinal plants and specific synthetic and natural molecules with estrogenic effects have attracted widespread attention between the public and the scientific community. Various plants have been used for centuries to alleviate menstrual and menopause symptoms, such as Cranberry, Ginger, Hops, Milk Thistle, Red clover, Salvia officinalis, Soy, Black cohosh, Turnera diffusa, Ushuva, and Vitex. This review aims to highlight current evidence about estrogenic medicinal plants and their pharmacological effects on cognitive deficits induced by estrogen deficiency during menopause and aging.
Sex hormones such as androgens and estrogens significantly influence behavior (Hamson et al., 2016) and support learning and memory (Andreano and Cahill, 2009; Munro et al., 2012; Colciago et al., 2015; Kerschbaum et al., 2017). The effects of sex hormones on cognition may explain the differences in cognitive abilities among sexes. For example, typically, women have superior verbal memory and are better at recalling and associating words from a spoken list than men (Munro et al., 2012).
Experimental and epidemiological studies suggest that female sex hormones are neuroprotective, preventing cognitive decline during aging. The discovery that brain regions involved in learning and memory, such as the hippocampus and prefrontal cortex, differed in structure and function between sexes suggested that sex hormones played a role in cognition (Kapur et al., 1995; Gasbarri et al., 2012; Pompili et al., 2012). Sex hormones influence aging (Boss et al., 2014) and the incidence, progression, and severity of symptoms in psychiatric disorders (Xing et al., 2013). One interesting potential beneficial effect of estrogens in the brain is to prevent the deterioration of cognitive abilities during aging. Likewise, estrogenic compounds' study revealed a central role of the cerebral estrogen receptors (ER)α and ERβ signaling on cognitive abilities (Baez-Jurado et al., 2019). These receptors have differential expression in the body's tissues and cells. They can have selective effects on different tissues. Most phytoestrogens are full ERβ agonists and weak ligands of the ERα (Gencel et al., 2012; Martinkovich et al., 2014). Estrogenic compounds support cognitive abilities by a mechanism involving the ERα (Tang S.-S. et al., 2018; Mitchnick et al., 2019). This receptor, acting as a transcription factor, controls gene expression in brain regions playing a vital role in learning and memory, such as the hippocampus (Spencer-Segal et al., 2012; Frick et al., 2018; Tang et al., 2019). Also, ERs promote neuroregeneration after traumatic brain injury by stimulating astrocytes' neuroprotective effects (Martin-Jimenez et al., 2019).
There is abundant evidence on the therapeutic impact of estrogenic compounds and the molecular mechanisms underlying their beneficial effects diminishing inflammation, oxidative stress, and neuronal loss after menopause. These benefits may also account for their positive impact alleviating the cognitive deficits in animal models of two leading types of dementia; Alzheimer’s disease (AD) and Parkinson’s disease (PD) (Figure 1). Tibolone (7-α, 17- α-17-hydroxy-7-methyl-19-norpregn-5 [10]-en-20-ψn-3-one) an estrogenic synthetic drug used as Hormone replacement therapy (HRT), is neuroprotective against the neurotoxic effects of palmitic acid in the brain by protecting astrocytes from inflammation by a mechanism involving the ERβ In experimental models of inflammation induced by a high-fat diet (González-Giraldo et al., 2018; Hidalgo-Lanussa et al., 2018; González-Giraldo et al., 2019; Martin-Jimenez et al., 2019; Hidalgo-Lanussa et al., 2020). On the other hand, the phytoestrogens, also called natural selective estrogen receptor modulators (SERMs), act as agonists or antagonists for ERs. They have different effects on different tissues and cells. The phytoestrogens have structural similarity with 17-β estradiol, showing both agonist and antagonist effects on the ERα and ERβ (Rietjens et al., 2017).
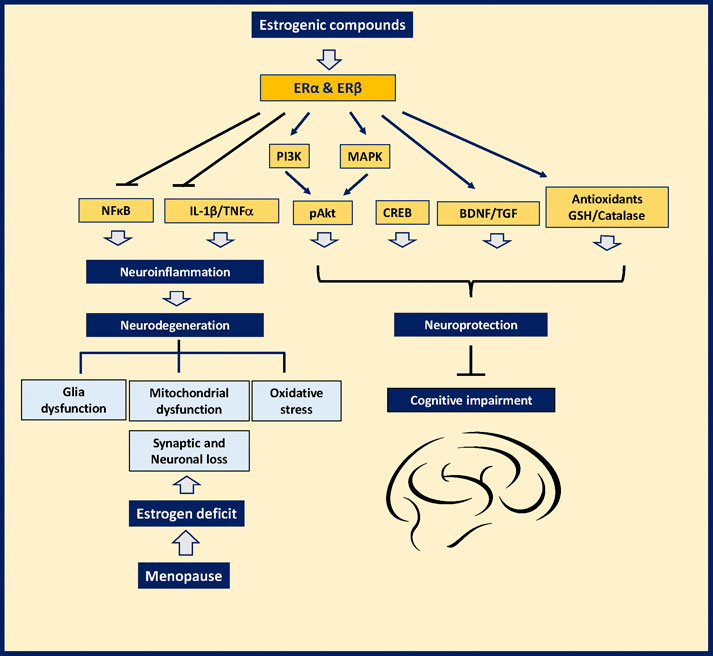
FIGURE 1. Mechanism of action of estrogenic plants to prevent neurodegeneration and memory loss and other symptoms in women after menopause.
A comprehensive review of pertinent published literature on estrogenic plants using the scientific databases SCOPUS, PubMed, and Science Direct was done. The revision emphasized plants and compounds with beneficial effects against oxidative stress, inflammation, cell viability, and memory loss in cell and in vivo studies of AD and PD. Only plants with scientific evidence of estrogenic activity were included.
It is estimated that by 2030, the world population of menopausal and postmenopausal women will be 1.2 billion (Borrelli and Ernst, 2008). A systematic review of the literature and random-effect meta-analyses of several clinical studies showed that surgical menopause at any age was associated with a decline in verbal and semantic memory and information processing speed (Georgakis et al., 2019). As discussed in previous reports, the role of estrogen over cognition became more relevant due to the worldwide increase in female life expectancy.
Different ER ligands, including phytoestrogens and synthetic compounds such as tibolone, are under investigation to alleviate or prevent these cognitive deficits. Tibolone has many beneficial effects on the central nervous system (Pinto-Almazan et al., 2017) by mechanisms counteracting the effects of neuroinflammation, oxidative stress, and mitochondrial dysfunction induced by different insults acting on glial cells, including microglia and astrocytes (González-Giraldo et al., 2018; Hidalgo-Lanussa et al., 2018; González-Giraldo et al., 2019; Martin-Jimenez et al., 2019; Osorio et al., 2019; Hidalgo-Lanussa et al., 2020) Tibolone binds to the ERs and has positive effects diminishing the negative consequences of estrogen deficiency on cognition in ovariectomized (OVX) Sprague–Dawley rats by a mechanism involving the enhancement of both the serotoninergic and cholinergic systems (Espinosa-Raya et al., 2012; Pinto-Almazan et al., 2017).
Clinical studies: Considering that menopause arrives on average at 50 years of age, women without intervention can spend more than 30 years in a hypoestrogenic state with several global consequences on cognition and mood (Slaven and Lee, 1997; Shilling et al., 2001). Sex hormones modulate brain plasticity processes involved in high-order cognitive functions such as learning and memory (Frick, 2009; Henderson and Popat, 2011; Frick, 2012; Pompili et al., 2012; Hamson et al., 2016; Korol and Wang, 2018). Clinical studies investigated the correlation between the adult levels of sex hormones and the documented differences in spatial and verbal memory abilities among male and female subjects (Hugdahl et al., 2006).
HRT with estrogens and progestins reduces postmenopausal symptoms but increases the risk of mammary carcinomas and cardiovascular events. For this reason, plant-derived estrogenic compounds mimicking the effect of sex hormones with a better safety profile are highly regarded (Wuttke and Seidlova-Wuttke, 2014).
Although no consensus has been reached (Kocoska-Maras et al., 2011), most clinical studies show that HRT improves cognitive abilities in menopausal women (Sherwin and Grigorova, 2011). One of these studies included 96 non-menopausal control women, 60 women treated with HRT, 56 postmenopausal women, and 41 men. Participants were requested to learn two-word lists. When they learned list 1, they were given either a forget or a remember list 1 cue. When participants had learned list 2 and finished an interference task, they need to write down all items they recalled. Data analysis revealed an effect of sex hormones on learning abilities as follows. Remember-cued control women recalled more list 1 items than HRT users, young men, and postmenopausal women. In forget-cued women, salivary progesterone correlated positively with the recall of items. Salivary 17 β-estradiol did not correlate with the number of recalled items from list one or two in either remember- or forget-cued control women. However, salivary 17 beta-estradiol correlated with item recall in remember-cued postmenopausal women. They concluded that sex hormones do not affect general verbal memory acquisition or consolidation but modulated cue-specific learning and memory recall (Kerschbaum et al., 2017). Another study showed that HRT with estrogen plus progesterone improved speed cognitive processing in women carrying the Apolipoprotein E (APOE) polymorphisms epsilon (ε) 2/ε3, and ε4. However, women receiving HRT but carrying e3/e3 alleles showed worse verbal memory scores in the Montreal Cognitive Assessment test than control subjects (Bojar et al., 2013). The study of the relationship between endogenous levels of sex hormones (estradiol, estrone, progesterone, and free testosterone) and cognitive abilities (verbal episodic memory, executive functions) showed that progesterone concentrations positively correlated with verbal memory and global cognition (Henderson et al., 2013). They also found that the level of sex hormone-binding globulin positively correlated with verbal memory performance (Henderson et al., 2013).
Various studies revealed that estrogen and progesterone positively influence verbal memory in females, and this and other cognitive skills in postmenopausal women (Keenan et al., 2001; Maki and Hogervorst, 2003; Hogervorst et al., 2009; Brown, 2013; Doty et al., 2015; Pinto-Almazan et al., 2017; Moradi et al., 2018). Nevertheless, clinical studies by the Women's Health Initiative showed that women who initiated estrogen treatment alone or combined with progestin at older ages (65–79 years of age) presented a higher risk of cognitive decline and dementia (Rocca et al., 2011). This discrepancy can be explained by the window of opportunity hypothesis, suggesting that estrogen's effects will vary depending on age, cause, and stage of menopause. It seems that in women, estrogen treatment is neuroprotective when started early after menopause (50–60 years of age). The authors recommend that women younger than 51 years of age with premature natural menopause should benefit from HRT (Rocca et al., 2011). Based on this evidence, it is reasonable to postulate that estrogen deficiency can be a crucial factor affecting brain function after menopause.
Estrogenic plants have been used from ancient times to diminish the symptoms of menopause, such as hot flushes, cognitive impairment, and depression. The most commonly used are the Black cohosh (Actaea racemosa L) (Wuttke et al., 2014), Ginger (Zingiber officinale Roscoe), Hops (Humulus lupulus L.), Milk thistle (Silybum marianum), golden root (Rhodiola rosea L.), Red clover (Trifolium pratense L), Sage (Salvia officinalis L), Soy (Glycine max L), Damiana (Turnera diffusa Willd. ex Schult), Ushuva (Physalis peruviana L), and vitex (Vitex agnus-castus L). However, many more have been investigated (Table 1).
The positive effects of sex hormones on cognition encouraged the study of phytoestrogens present in medicinal plants to alleviate the cognitive deficits during menopause. A modern study used molecular docking investigated more than 500 phytochemicals from 17 herbal supplements sold in the United States to identify potential estrogenic or anti-estrogens herbal compounds. Each compound was docked with the known structures of the ERα and ERβ. The results revealed numerous compounds that strongly docked with the ER, including Echinacea, milk thistle (Silybum marianum), GB, Sambucus nigra, chaste tree (Vitex agnus-castus), fenugreek (Trigonella foenum-graecum), Rhodiola rosea, Licorice (Glycyrrhiza glabra), wild yam (Dioscorea villosa), black cohosh (Actaea racemosa), muira puama (Ptychopetalum olacoides or P. uncinatum), red clover (Trifolium pratense), damiana (Turnera aphrodisiaca or T. diffusa), and dong quai (Angelica sinensis). Also, some men's herbal supplements showed robust docking to the ER: GB, gotu kola (Centella asiatica), muira puama (Ptychopetalum olacoides or P. uncinatum), and Tribulus terrestris. The authors concluded that almost all popular herbal supplements contain phytochemical components that may bind and modulate hER, causing unwanted effects (Powers and Setzer, 2015).
Phytoestrogens including flavonoids, isoflavonoids, stilbenes, lignans, ginsenosides, tetrahydrofurandiol, chalcones, and coumestans, and are commonly found in fruits and other parts of various medicinal plants (Slavin and Lloyd, 2012). Various traditional plants to alleviate the effects of menopause contain phytoestrogens (Lorand et al., 2010). In addition to their use to counteract menopause symptoms (Rowe and Baber, 2021), phytoestrogens for their antioxidant and anti-inflammatory properties may have many other therapeutic uses including being considered anti-cancer drugs (Ardito et al., 2018; Basu and Maier, 2018; Petrine and Del Bianco-Borges, 2021), regulators of cholesterol metabolism, hepatoprotective (Alipour and Karimi-Sales, 2020) and cardioprotective compounds (Asokan Shibu et al., 2017). These properties have a broad impact on human health under normal and pathological states in aging, including beneficial effects on cognitive abilities (Thaung Zaw et al., 2017; Dominguez and Barbagallo, 2018; Hussain et al., 2018). Phytoestrogens at low doses have little or no effects on climacteric complaints, but they mimic estrogen effects at high doses.
Phytoestrogens display higher selectivity for specific ERs permitting more organ-specific actions than estrogen. For example, the flavonoids catechin, rutin, daidzein, luteolin, naringenin, and genistein are present in powder extracts of Lespedeza bicolor. In behavioral experiments, L. bicolor’s extracts (25 and 50 mg/kg) improved the cognitive impairment induced by intracerebroventricular injection of Aβ25-35 in several cognitive tests, including the novel object recognition. These plant extracts enhanced the endogenous antioxidant system increasing the glutathione (GSH) expression and inhibiting the hippocampus’s acetylcholinesterase activities. Also, L. bicolor increased the expression of the brain-derived neurotrophic factor (BDNF), and phospho-Akt, extracellular signal-regulated kinase (ERK), and cAMP response element (CRE) expression that is reduced in the hippocampus by Aβ. In conclusion, L. bicolor exerts a memory-enhancing effect in an Aβ-induced mouse model of AD-like pathology (Ko et al., 2019). Phytoestrogen-primarily the isoflavones genistein, daidzein and coumestrol, stemming from soy (Glycine max) or red clover (Trifolium pratense)—were suggested to have the desired but not the undesired effects of estrogens.
However, the results of the most recent placebo-controlled studies question the beneficial effects of phytoestrogens. Nevertheless, when taken at the time of puberty, phytoestrogens seem to prevent mammary cancer in adulthood (Seidlova-Wuttke et al., 2013).
Astragalus mongholicus Bunge. (syn. Astragalus membranaceus) (Fabaceae), commonly named Mongolian milk, is used as a constituent of the traditional Chinese medicine (TCM) named Danggui Buxue Tang (DBT). DBT is an herbal decoction containing A. mongholicus and Angelica sinensis roots (5:1 w/w) that is employed to diminish menopausal symptoms. Interestingly, calycosin, the main flavonoid in A. mongholicus, has a structure similar to β-estradiol. Based on this evidence, Gong investigated DBT and Calycosin's ability to activate the estrogen-responsive element (ERE) in cultured MCF-7 cells (Gong et al., 2016). DBT has an EA that can be enhanced 2–5 times by Calycosin that did not show EA by itself. A derivative from DBT, the herbal recipe-RRF that is composed of Radix Astragali (root of A. mongholicus), Radix Angelicae sinensis (root of A. sinensis), and Folium Epimedii (leaves of Epimedium brevicomum Maxim.). RRF has shown a remarkable protective effect in OVX rats and represents a promising candidate for treating perimenopausal disorders (Xie et al., 2012). The experiments consisted of giving daily oral treatments with vehicle (Sham and OVX group), RRF (141, 282, and 564 mg/kg), and conjugated equine estrogens (0.1 mg/kg) for 16 weeks. They analyzed bone mineral density (BMD) assay and oxidative stress. The results showed that repeated administration of RRF attenuated osteoporosis by elevating the BMD levels of the total body and arrest bone trabeculae degradation. 3) RRF exposure decreased serum levels of constituent MDA and increased endogenous Superoxide dismutase (SOD) activity (Xie et al., 2012).
Cynomorium coccineum subsp. songaricum (Rupr.) J.Léonard (Cynomoriaceae) is a estrogenic medicinal plant is commonly known as Hongo de malta, and Jopo de Lobo grows in Mediterranean countries. Ethanolic C. coccineum has an estrogenic RP of 1.78 × 10–4 (Zhang et al., 2005). Recent studies reported that C. coccineum was antidepressant in perimenopausal rats (Miao et al., 2017) and reduced memory impairment in OVX rats (Tian et al., 2019). In this plant, phytoestrogen- and phytoandrogen-like effects have been detected (Wang et al., 2017; Tao et al., 2019) and various type of compounds, including eight triterpenoids, six flavonoids, four fatty acids, eight phenolic acids, one anthraquinone, one nucleoside, and one sterol have been identified (Li X. et al., 2020).
Vitex agnus-castus L. (Lamiaceae), also known as chaste-tree berry, is used to treat menopause symptoms and menstrual dysphoria. The estrogenic effect of extracts of chaste-tree berry has been investigated using cultured cells (Liu et al., 2001; Liu et al., 2004). These studies reported that methanol extracts of this plant increased the mRNA expression of ERβ and PR in the ER + hormone-dependent T47D: A18 cells (breast cancer cell line) and Ishikawa cells (endometrial cancer cell line). The analysis of the extracts using an ER binding bioassay revealed the presence of linoleic acid, a lipid with presumed EA, in the fruits. Vitex agnus-castus L (Liu et al., 2001; Liu et al., 2004). Chasteberry, like other estrogenic plant, induced the expression of the estrogen-inducible gene pS2 in the breast cancer cell line S30 (Liu et al., 2001).
Epimedium brevicornu Maxim. (Berberidaceae) is a TCM commonly known as Horney goat weed. A study investigating the effect of Ethanolic extract of E. brevicornu showed an EA (RP = 2.30 × 10–4) (Zhang et al., 2005). Besides, the investigation of the effect of different plants of the TCM on BMD found that treatment with extracts of E. brevicornu stimulated cyclic AMP response element (CRE) and the expression of osteoblastic markers such as Runx2 and Bmp4 in MC3T3-E1 cells (Rebhun et al., 2019). Also, Astragalus onobrychis extracts inhibited the Interleukin (IL)-1β-induced activation of the nuclear factor kappa B (NFκB) and a mix of these plants prevented the loss of BMD with efficacy comparable to estradiol in OVX rats (Rebhun et al., 2019).
Glycine max (L.) Merr (Fabaceae) generally identified as soybean, contains several phenolic compounds with EA, including biochanin A, genistein, daidzein, formononetin, and glycitein. These compounds activate both the ERα and ERβ and bind to the progesterone receptor (PR) and androgen receptor (AR). For example, Biochanin A, daidzein, genistein, and formononetin bind to the PR and AR at concentrations of 0.39–110 mM (Beck et al., 2003). A study showed that Soybean extracts exhibited selectivity for the ERβ and induced a higher cell proliferation level than estradiol (Boue et al., 2003). The increase in cell proliferation was suppressed by the estrogen antagonist, ICI 182,780, suggesting that the ER was involved (Boue et al., 2003). A double-blind, randomized, placebo-controlled, 12 weeks trial evaluated the effect of high-dose isoflavones on quality of life (QOL), cognition, plasma levels of lipoproteins, and androgens in postmenopausal women attended at a tertiary care center in the United States (Basaria et al., 2009). Ninety-three healthy, ambulatory, and postmenopausal women (mean age 56 years) were randomly assigned to receive soy protein (20g-160 mg of total isoflavones) or taste-matched placebo (20 g whole-milk protein), with 84 women (90%) completed the study. QOL was judged by the Menopause-specific Quality of Life (MENQOL) questionnaire. Total, free, and bioavailable testosterone, gonadotropins, Sex Hormone Binding Globulin (SHBG), and fasting lipids were measured. The study found significant improvements in all QOL subscales (vasomotor, psychosexual, physical, and sexual) among the women treated with isoflavones, while no changes were seen in the placebo group (Basaria et al., 2009). No significant changes in cognition, serum androgens, or plasma lipids induced by isoflavones were observed. Total testosterone and HDL levels were significantly lower in the people treated with isoflavones when compared to the placebo group. The timing of isoflavone supplementation with regards to the onset of menopause appears to be important. The authors concluded that high doses of isoflavones are associated with improved QOL among women at the early stages of menopause, indicating that isoflavones as alternatives to estrogen therapy may be useful and safe to relieve menopausal symptoms (Basaria et al., 2009).
Angelica sinensis (Oliv.) (Apiaceae) commonly known as Dong quai, and it is one of the most used medicinal plants. It is prescribed for gastrointestinal, vascular, mental, and gynecological disorders (Giacomelli et al., 2017). The primary components identified in the plant extracts, Ferulic acid, Z-Ligustilide, and polysaccharides, showed weak ER binding and pS2 mRNA induction in the cells (Liu et al., 2001). The administration of standardized ethanol extracts of A. senensis to OVX rats stimulated the uterine histoarchitecture, induced cornification of the vaginal epithelium establishing an epidermal barrier, and reduced luteinizing hormone (LH) concentration in serum. Moreover, plant extracts' administration provoked a significant modification of the vaginal smear in 67% of female control rats. Treated rats showed a prolonged estrus stage with a transient interruption of their regular cyclicity (Circosta et al., 2006). All this evidence suggests estrogenic effects for this plant.
Rhodiola rosea L. (Crassulaceae) is also known as golden root, rose root, roseroot, Aaron's rod, Arctic root, king's crown, lignum rhodium, and orpin rosea. R. rosea has been employed in Russia for a half-century, and ethanolic extract of this plant (40% v/v) are used to relieve fatigue and increase attention, memory, and work productivity. The typical dose recommended is 5–40 drops 2–3 times a day, 15–30 min before eating for 10–60 days. In Denmark, R. rosea is registered as a botanical drug and widely used in Scandinavian countries to increase cognitive abilities under stressful conditions. A phytochemistry analysis of R. rosea has revealed six distinct groups of compounds present in the root: Phenylpropanoids: rosavin, rosin, rosarin (specific to R. rosea); Phenylethanol derivatives: salidroside (rhodioloside), tyrosol; Flavonoids: rodiolin, rodionin, rhodiosin, acetylrodalgin, tricin; Monoterpernes: rosiridol, rosaridin; Triterpenes: daucosterol, beta-sitosterol; Phenolic acids: chlorogenic and hydroxycinnamic, and gallic acids (Elameen et al., 2010; Elameen et al., 2020; Shikov et al., 2020). R. rosea is a selective ER modulator, anti-inflammatory, and NFκB inhibitor, as well as an inducer of the endothelial nitric oxide synthase (NOS) that protects osteoblasts from hydrogen peroxide toxicity (Gerbarg and Brown, 2016).
Anemarrhena asphodeloides Bunge (Asparagaceae), commonly named zhi mu and rhizome, is native to China, Korea, and Mongolia is a TCM. In Korea, A. asphodeloides B is employed as antipyretic, diuretic, sedative, and antitussive medicine (Lee et al., 2018). The study of ethanol extracts of A. asphodeloides B revealed anti-osteoporotic effects, inhibiting osteoclastogenesis and reducing inflammation (Kim et al., 2017). Dichloromethane fractions of A. asphodeloides B showed EA with a RP of 1.9 × 10–4 (RP of Estradiol (E2) was 1.0) (Kim et al., 2008). Methanolic extract (0.1 mg/ml) showed moderate EA (Kang et al., 2006).
Salvia officinalis L. (Lamiaceae), also known as sage, is one of the most abundant species of Salvia (>500 species). These flowering plants, native to the Mediterranean countries, have been used as traditional medicines for centuries. S. officinalis contains phenolic diterpenoids with antioxidants and anti-inflammatory activities such as carnosic acid and carnosol (Abdellatif et al., 2020). S. officinalis has EA and is used to control hot flashes. A study investigating an aqueous-ethanolic extract of this plant discovered EA in the ERLUX assay with an EC50 = 64 mg/ml (Rahte et al., 2013). Fractionation of the EA in the extracts revealed that luteolin-7-O-glucuronide (EC50 = 159 mg/ml) was the active estrogenic compound. The authors concluded that the positive effect of S. officinalis on hot flashes must be due to the effect of estrogenic flavonoids present in Salvia (Rahte et al., 2013).
Scutellaria baicalensis Georgi (Lamiaceae) is also known as Baickal Skullcap and Huang Qin. An early study found that ethanolic extracts of S. baicalensis have EA (RP of 8.77 × 10–5) (Zhang et al., 2005). Additional studies have investigated S. baicalensis alone or as part of herbal mixtures to treat health conditions related to hormone dysregulation such as tumors (Murashima et al., 2009), endometriosis (Ferella et al., 2018), and prostate cancer (Hsieh and Wu, 2002). In a prostate cancer study, LNCaP cells were treated with ethanol extracts of the herbal mix PC-SPES or their components. Cells treated with PC-SPES showed a 70–80% reduction in cell growth and viability. S. baicalensis extracts halted cell growth by a 66.5% (Hsieh and Wu, 2002).
Actaea racemosa L. (syn. Cimicifuga racemosa (L.) Nutt.) (Ranunculaceae) is also known as black cohosh. Extracts of the rhizome of black cohosh have shown no having estrogenic effects on mammary cancer cells in vitro and on mammary gland and uterine histology in OVX rats. However, in those rats, the extract A. racemosa L. BNO1055 reduced hot flushes and the occurrence of osteoporosis (Seidlova-Wuttke et al., 2003). Further reports from same author suggested that Actaea racemosa L. extracts at low doses are adequate to ameliorate climacteric complaints without adverse estrogenic effects (Wuttke et al., 2014).
Clinical studies: In postmenopausal women, compared to placebo Actaea racemosa L. BNO 1055 in a meaningful manner reduced menopause symptoms similarly to conjugated estrogens. Similar data were published for other European Actaea racemosa L. preparations, whereas 2 US American preparations were ineffective, most likely due to the doses or purity of the preparations used. The European studies did not report Actaea racemosa L. effects in the uterus nor mammary glands.
A recent study evaluated the efficacy on climacteric problems and safety of a combination of plant extracts (soy isoflavone, black cohosh, chasteberry, and evening primrose oil extracts) in post-menopausal women (Rattanatantikul et al., 2020). The study consisted of a randomized, double-blinded, placebo-controlled trial. Post-menopausal women of 45–60 years old were randomly assigned to either treatment (n = 50) or placebo group (n = 51). Herb extracts and placebo were administered to participants, and at baseline and after 6 and 12 weeks of treatments, symptoms of menopause, endocrine profile, and blood chemistry were assessed. Herbs' combination significantly reduced hot flushes and sweating, sleep problems, depressed mood, and irritability symptoms compared with the placebo group. There were no significant differences in hormonal levels between the test and placebo groups; however, the C-reactive protein levels were found significantly decreased. Moreover, serum LDL-C and triglyceride levels were significantly lower than baseline levels in the treatment group at 6- and 12 weeks timepoints. No adverse effects were reported during the trial. These data indicate that this combination of four medicinal herbs effectively and safely improves menopausal symptoms, as well as general health indicators, in post-menopausal women (Rattanatantikul et al., 2020).
Some estrogenic plants have been investigated as therapeutic tools to prevent memory loss after menopause and reducing the risk of developing the most frequent neurodegenerative conditions. AD is a neurodegenerative disease characterized by cholinergic deficits, neuronal loss, glial dysfunction (Echeverria et al., 2009), intracellular and extracellular accumulation of aggregated forms of Aβ, and the appearance of intraneuronal neurofibrillary tangles of hyperphosphorylated Tau in the brain (McGeer, 1986; Mann, 1989; Masdeu et al., 2005). The main risk factors involve traumatic brain injury (Gardner and Yaffe, 2015; Daulatzai, 2017), posttraumatic stress disorder (PTSD) (Tsolaki et al., 2009; Meziab et al., 2014; Ritchie et al., 2019), diabetes (Chen and Zhong, 2013; Boccardi et al., 2019; Jash et al., 2020), and age (Simpson et al., 2010; Bartzokis, 2011; Bove et al., 2014). These abnormalities occur with neuroinflammation and oxidative stress that orchestrate a cascade of events leading to cognitive decline, gliosis, and synaptic loss. This idea is endorsed by studies showing that inhibition of pro-inflammatory cascades improves cognitive abilities in transgenic AD mice. Indeed, the posttreatment of AD mice with the Raf inhibitor Sorafenib inhibited the expression of several downstream inflammatory factors such as NFkB and nitric oxide synthase (Echeverria et al., 2009). Medicinal plants having anti-inflammatory effects can be of use in preventing AD after menopause. Women are more affected by AD, indicating that sex hormones are involved in the disease progression (Vina and Lloret, 2010). Conditions affecting estrogen production, such as premature ovarian insufficiency in women less than 40 years of age, are associated with neurological dysfunction and an increased risk of dementia (Soni and Hogervorst, 2014).
Preclinical studies suggest a role for estrogenic compounds preventing AD (Saunders-Pullman, 2003) (Morale et al., 2006) (Morissette et al., 2008) (Siani et al., 2017) and other neurological conditions (Hugdahl et al., 2006; Pompili et al., 2012). The putative role of female hormones in the development of dementia may explain the finding that women with a late menopausal arrival (>53 years of age) have a decreased risk of AD. It has been proposed that brain differences in the cholinergic system among males and females may underlie the differences in response to acetylcholinesterase inhibitors (AChEI) and the rate of progression of AD between sexes, with a higher benefit of AChEI treatment delaying the progression of the disease in men than women (Giacobini and Pepeu, 2018). Estrogenic compounds are neuroprotective against Aβ toxicity in vitro (Svensson and Nordberg, 1999; Szego et al., 2011; Xing et al., 2011; Bagheri et al., 2012; Wu et al., 2012; Napolitano et al., 2014; Costa et al., 2016; Li et al., 2017; You et al., 2017; Yang et al., 2019) as well as in vivo models of AD (Bagheri et al., 2011; Limon et al., 2012; Pompili et al., 2012; Zhao et al., 2013). In one of these preclinical studies, to investigate the effect of estrogen on the cholinergic deficit in AD, different Aβ assemblies were injected in the nucleus basalis magnocellular (NBM) of sham control and OVX mice, and the viability of cholinergic cells was measured (Szego et al., 2011). The results showed that Aβ oligomers were the most neurotoxic aggregated species and estrogen treatment before Aβo infusion prevented cholinergic neuronal loss (Szego et al., 2011). Furthermore, a proteomic analysis of the NBM revealed significant differences among Aβ-treated mice and control mice in the expression of the mitochondrial factors DJ-1, NADH ubiquinone oxidoreductase, ATP synthase, phosphatidyl-ethanolamine-binding protein 1, protein phosphatase (PP) 2A, and dimethyl-arginine dimethyl-aminohydrolase 1 (Szego et al., 2011). Besides, Aβ inhibited MAPK signaling and stimulated NOS activity. The observed increase in the activities of PP1 and PP2A may explain the inhibition of MAPK signaling in the neocortex. Impressively, pretreatment with estrogen prevented most of these proteome changes (Szego et al., 2011). A few years ago, the role of the ERα, a transcriptional factor involved in mitochondrial and energy metabolism, was investigated in a transgenic (Tg) model of AD expressing the amyloid β precursor protein (APP) and presenilin 1 (PS1) containing familial AD (FAD) mutations (Tg APP/PS1 mice). They found that the expression of the ERα was progressively reduced in the brain of AD mice. More importantly, HEK293 cells stably expressing the human APP, and ERα showed reduced production of Aβ peptides, decreased Tau phosphorylation, and reduced activity of a critical Tau kinase in vivo, the glycogen synthase kinase 3β (GSK3β) (Tang Y. et al., 2018). Coherent with the preclinical evidence, the results of several clinical studies suggest that estrogen may prevent cognitive decline and improve cognitive function in postmenopausal women (Sherwin, 2012; Brown, 2013; Doty et al., 2015). Estrogen or HRT would be an adequate preventative treatment to reduce the risk of dementia in women with a low risk of cardiovascular disease and a heightened risk of developing dementia, For example, a person with a family history of AD (Loucks and Berga, 2009). However, some studies indicated that the effect of estrogen could also depend on the age of the person treated as conjugated equine estrogens plus medroxyprogesterone acetate seems to augment the risk of dementia in women over 65 years of age (Sano et al., 2008; Maki and Henderson, 2012).
Medicinal plants have been used throughout the history of humanity to improve mental abilities. The connection between the estrogenic neuroprotective effect of medicinal plants and their role against neurodegeneration has recently been investigated. One of these investigations by Zhao et al. described a phytoestrogen formulation named Phyto-β-SERM, which exhibited a higher binding selectivity (83 times) for ERβ than ERα. Phyto-β-SERM has central estrogenic neuroprotective effects without inducing feminizing effects over the body. Furthermore, chronic exposure to Phyto-β-SERM prevented menopause symptoms in mice. Additionally, nine-month treatment with Phyto-β-SERM prolonged survival improved spatial recognition memory and reduced cerebral amyloid-β plaque deposition in female triple-transgenic AD mice. A gene expression analysis of AD mouse brains showed that phytoestrogens were neuroprotective by a mechanism involving the crosstalk between the ERβ and glycogen synthase kinase 3 (GSK3β). This evidence supports the therapeutic value of phytoestrogen for preventing AD (Zhao et al., 2013). Various studies investigated whether there were changes in estrogen/ERα expression on AD. Since ERα gene expression is regulated by methylation (Frick, 2015), a recent clinical study examined whether the methylation level of the promoter of the ERα gene and its protein expression correlated with changes in cognitive function and quality of daily living in AD. The QOL of the participants was tested with the QOL- AD scale and their cognitive abilities were assessed using both the Mini-Mental State Examination (MMSE) and the activities of daily living (ADL) tests. The methylation status and level of expression of the ERα gene were determined in the peripheral blood of 132 patients with AD and 135 healthy control subjects (Li et al., 2019). This study showed that a higher level of methylation of the ERα gene promoter correlated with a decreased ERα expression and reduced cognitive abilities in AD patients (Li et al., 2019). This evidence supports the view that lower estrogenic signaling correlates with cognitive impairment in AD. While most phytoestrogens act through ERα and ERβ, some of their biological actions may also be mediated by other effectors affecting these receptors' signaling.
Kummerowia striata (Thunb.) Schindl. (Fabaceae) is a flowering plant generally named Japanese clover. The ethanolic extracts have anti-inflammatory effects as tested in LPS-treated RAW264.7 cells. In these cells, K striata downregulated the expression of interleukin (IL)-1β, IL-6, NO, Tumour Necrosis Factor alpha (TNF-α), cyclooxygenase 2 (COX-2), upregulated IL-10 and the antioxidant factor heme oxygenase-1 (HO-1), and inhibited the production of NFκB when compared to LPS treated cells. This evidence suggests that the anti-inflammatory effects of K. striata’s ethanol extract were due to the downregulation of IL-1β, IL-6, nitric oxide, TNF-α, and COX-2 via the suppression of NFκB activation and upregulation of IL-10 and HO-1. The methanolic extracts of whole plant extracts of K. striata also showed high EA with EC50 values of 7.7 μg/ml (Yoo et al., 2005).
Lespedeza bicolor Turcz (Fabaceae) is a flowering plant known as shrubby bush clover, bicolor lespedeza, and shrub lespedeza. L. bicolor is broadly used in Australia, North America, and Eastern Asia to reduce inflammation, nephritis, hyperpigmentation, and diuresis (Ko et al., 2019). Catechin, genistein daidzein, luteolin, naringenin, and rutin were identified in the powdered extract of L. bicolor. The root extracts contain polyphenolic compounds that delay prostate cancer progression (Dyshlovoy et al., 2020). Also, the effects of L. bicolor on cognitive function were investigated in a mice model of AD using a cerebral injection of Aβ. In this study, analyzed in the following section, they identified Catechin, rutin, daidzein, luteolin, naringenin, and genistein in the powdered extract of L. bicolor. The methanolic extract of the stem of L. bicolor showed high EA with EC50 values of 8.6 μg/ml (Yoo et al., 2005).
Salvia lavandulaefolia Vahl. (Laminaceae), also known as Spanish Sage, is originally from Spain and its extracts have anti-oxidant, estrogenic, anti-inflammatory properties, and anti-butyryl and anti-acetyl-cholinesterase activities (Kennedy and Scholey, 2006). The essential oil and some of its monoterpenoid components have acetylcholinesterase inhibitor (AChEI) activity. Antioxidant activity was found in the ethanolic extract of the herb (5 mg/ml) and the monoterpenoids (0.1 M) alpha- and beta-pinene and 1,8-cineole. These ethanolic extracts (50 mg/ml) and alpha-pinene and geraniol (0.2 mM), showed anti-inflammatory effects in vitro. The essential oil of Spanish sage (0.01 mg/ml) and its monoterpenoid component geraniol (0.1–2 mM) showed a potential EA as evidenced by the induction of β-galactosidase activity in yeast cells carrying a promoter stimulated by estrogenic compounds (Perry et al., 2001). These properties can be useful in AD, a condition characterized by a deficiency of the cholinergic system, oxidative stress, and inflammation in the brain.
Elettaria cardamomum (L.) Maton (Zingiberaceae), also known as Cardamom, is used to treat menstrual disorders in India (Bhatia et al., 2015). A study tested the EA of E. cardamomum, Plantago ovata seeds, and Gingko biloba (GBL) and found that GBL and E. cardamomum seed extracts showed both EA and anti-EA (Real et al., 2015). Interestingly, another study showed that perinatal exposure to E. cardamomum extracts positively affected learning and memory in mice (Abu-Taweel, 2018), suggesting that it may also be helpful in AD.
Glycyrrhiza uralensis Fisch. (F. Fabaceae) is also known as Chinese Liquorice, has potent EA. Screening of plant extracts showed that G. uralensis induced a 300% increase in EA over basal levels (Kang et al., 2006). In addition, Liquorice is used in the Gegen Qinlian Decoction (GQD), a TCM prescribed to treat diabetes mellitus. GQD is composed of Pueraria lobata (Willd.) Ohwi (gen), Scutellaria baicalensis Georgi (huang qin), Coptidis chinensis Franch (huang lian), and Glycyrrhiza uralensis Fisch (gan cao). GQD markedly decreased blood glucose and increased serum insulin levels in type II diabetic mice (Xu et al., 2020). Since diabetes is a risk factor for AD (Jash et al., 2020), this plant's extracts could be useful to diminish the risk of AD in persons having a propensity to develop diabetes due to genetic background (Jash et al., 2020).
Lycopus cavaleriei H.Lév. (syn. Lycopus ramosissimus (Makino) Makino) (Lamiaceae) is an estrogenic plant that in Japan is known as ko-shiro-ne, a term that means tiny white roots. Dichloromethane extraction of L. cavaleriei yield extracts showing EA with a RP to E2 of 0.8 × 10–4 (RP of E2 was 1.0) (Kim et al., 2008). The methanolic extracts of other plant of the same family, Lycopus lucidus Turcz. ex Benth. showed moderate EA (Kang et al., 2006).
In a recent study, the inhibitory effect of three phenylpropanoids isolated from L. lycidus, schizotenuin A, and lycopic acids A and B-on Aβ fibrillization was investigated using thioflavin-T assay and transmission electron microscopy (Sun et al., 2020). These phenylpropanoids, containing three cathecol groups, inhibited Aβ aggregation into amyloid plaques and the authors suggested that the catechol moieties may be crucial in reducing Aβ plaque formation (Sun et al., 2020).
A further study investigated the therapeutic potential of the prenylflavonoid xanthohumol obtained from Humulus lupulus L for AD in neuroblastoma N2a cells stably expressing human Swedish mutant amyloid precursor protein (N2a/APP), considered a cellular model of AD and HEK293/Tau cells, showing tau hyperphosphorylation. The protein analysis revealed that xanthohumol inhibited APP amyloidogenic processing to Aβ the peptide acumulation, and decreased tau hyperphosphorylation in the HEK293/Tau cells as well as in the N2a/APP by promoting protein phosphatase 2 A PP2A, and inhibiting GSK3β cells. Proteomic analysis by mass spectrometry revealed 30 proteins differentially expressed in the N2a/APP when compared to wild-type (WT) N2a cells (N2a/WT), and a total of 21 differentially expressed proteins in lysates of N2a/APP cells treated with xanthohumol. This treatment reduced AD-like changes in the proteome of N2a/APP cells, affecting proteins involved in endoplasmic reticulum stress, cytoskeleton, oxidative stress, and proteasome therapeutic potential of xanthohumol for treatment of AD and/or other tauopathies (Huang et al., 2018). Colupulone, a prenylated phloroglucinol obtained from Humulus lupulus, activates the pregnane-X-receptor (PXR), a nuclear receptor controlling the expression P-gp a protein that controls the transport troghout the Blood-brain barrier (BBB). Various colupulone analogs have been synthesized, and their effect on Aβ uptake and transport has been evaluated. Among all tested compounds, diprenylated acyl phloroglucinol displayed a significant increase (29%) in Aβ transport across bEnd3 cells grown on inserts as a BBB model. The results suggest the potential of Colupulone to enhance the clearance of Aβ across the BBB (Bharate et al., 2015).
Glycyrrhiza glabra L. (Fabaceae), also named licorice, is a well-known estrogenic plant (Zayed et al., 1964; Simons et al., 2011). An evaluation of the EA in extracts of licorice showed only weak ER and PR binding and estrogen-induced transcription of pS2 (presenilin-2), an estrogen-inducible gene present in S30 breast cancer cells (Liu et al., 2001).
Other studies investigated the effect of Glabridin a compound isolated from the roots of Glycyrrhiza glabra on cognitive functions and cholinesterase activity in mice. Mice were treated daily with Glabridin (1, 2 and 4 mg/kg.) for 3 consecutive days. The higher doses of glabridin significantly reduced the amnesia induced by scopolamine (0.5 mg/kg) as tested in the passive avoidance test. Furthermore, glabridin remarkably reduced the brain cholinesterase activity in mice compared to the control group. Based on the evidence they postulated that glabridin could be a promising candidate for treating memory impairment in AD patients (Cui et al., 2008).
Trifolium pratense L. (Fabaceae) known as red clover contain several polycyclic phenolic compounds, including biochanin A, genistein, daidzein, formononetin, and glycitein. Biochanin A, daidzein, genistein, and formononetin bound to the PR and AR in the range of 0.39–110 mM (Beck et al., 2003). Compounds isolated from methanolic extracts of red clover bound to the ERα and ERβ and increased alkaline phosphatase activity and the expression of PR in endometrial cells (Boue et al., 2003). Also, red clover extracts upregulated estrogen-inducible gene presenelin-2 (pS2) in S30 cells. Isolation of estrogenic components in fractionated extracts using liquid chromatography, mass spectrometry, and an EA screening bioassay showed that the isoflavone genistein was the most active phytoestrogen in the red clover extracts (Liu et al., 2001).
Preclinical studies have shown that components isolated from this estrogenic plant can be helpful in treating neuroinflammation and cognitive disorders induced by a high-fat diet in mice (Fu et al., 2019) and Aβ-induced cognitive deficits in rats (Wei et al., 2015). In this latter study, they tested the cognitive effects of an isoflavone isolated from T. pratense named pretensein. Cognitive impairment was induced in the experimental rats via bilateral injection of Aβ peptide into their hippocampi. The results showed that treatment with pratensein significantly decreased Aβ-induced cognitive impairment and hippocampal neurodegeneration observed in the vehicle-treated rats. Pratensein show anti-inflammatory effects significantly decreasing inflammatory biomarkers such as MDA, nitric oxide (NO), neuronal nitric oxide synthase (nNOS), IL-1β and TNF-α. Pratensein also reduced Ab levels by decreasing the expression of amyloid precursor protein (APP), and the Aβ-synthetizing enzyme β-secretase (BACE1), cathepsin B (CatB), neprilysin 2 (NEP-2), and the insulin-degrading enzyme (IDE). Moreover, pratensein significantly increased the expressions of synapse plasticity-related proteins, cAMP-response element binding protein (CREB), the brain derived neurotrophic factor (BDNF), Ca2+/calmodulin kinase II (CaMKII), N-methyl-D-aspartate receptor 1 (NMDAR1), postsynaptic density protein 95 (PSD-95), protein kinase A beta subunit (PKACβ), and protein kinase C gamma (PKCγ). Furthermore, pratensein significantly decreased the activity of acetylcholinesterase. The authors concluded that pratensein represents an anti-AD active compound that enhances synaptic plasticity and increases cholinesterase activity in the brain (Wei et al., 2015).
Sophora flavescens Aiton (Fabaceae) is commonly known as Ku shen. Methanolic root extracts of S. flavescens induced a 400% increase of EA in a yeast ER activity assay (Kang et al., 2006). Similarly, methanolic extracts of whole plants showed high EA (EC50 values of 3.2 μg/ml) (Yoo et al., 2005). In another study, the ability of both Prenylflavonoids and lavandulyl flavonoids obtained from the roots of S. flavescens, to displace 17 beta-estradiol (E2) from rat uterine ER was investigated. The flavonoids investigated included: 8-prenylkaempferol, leachianone A, maackiain, norkurarinone, kushenol C, kushenol X. The results showed low binding affinities of prenylated flavonoids; However, lavandulyl or prenyl groups at position 8 enhanced their binding to rat uterine ER (Hillerns and Wink, 2005). Anti-AD activities have been characterized in compounds isolated from S. flavescens, such as sophoflavescenol, a prenylated flavonol (Jung et al., 2011). Prenylated flavonols from S. flavescens inhibit the generation of advanced glycation endproducts (AGE), as well as BACE1 and the ACh degrading cholinesterases (ChE). Isolation of the active principles from S. flavescens by an activity-guided isolation protocol permitted to identify compound sophoflavescenol possessing high inhibitory activities against AGE, BACE1, and ChEs.
Besides, another study reported noncompetitive BACE1 inhibitory effects of lavandulyl flavanones from S. flavescens. Coherent with a BACE1 inhibition, these flavanones decreased Aβ release by human embryonic kidney (HEK-293) cells (Hwang et al., 2008).
Panax ginseng C.A. Meyer and Panax quinquefolius L. (Araliaceae) known as Asian ginseng and North American ginseng, respectively. Intriguingly, extracts of P. ginseng and P. quinquefolius increased the transcription of pS2 mRNA in S30 cells; however, no significant binding to the ER or induction of PR expression was detected in the endometrial adenocarcinoma Ishikawa cells that express both types of receptors (Liu et al., 2001).
Many recent studies have shown its antioxidant and anti-inflammatory effects of Ginseng in the brain affected by aging-associated diseases and toxic insults such as sodium fluoride and aluminum chloride (AlCl3) (Mahaboob Basha and Saumya, 2013; Adam et al., 2019; Choi et al., 2021). One of these studies investigated potencial pro-cognitive and neuroprotective effects of compound K, a metabolite of ginsenosides, present in the enzymatically hydrolyzed red ginseng extracts (HRGE). Amnesic mice were treated with scopolamine and tested in the passive avoidance Morris water maze and Y-maze tests. After euthanasia, the mouse’s brains were dissected and analyzed for the expression of antioxidant proteins analyzed by Western blot. The results showed that oral HRGE (300 mg/kg body weight) reversed learning and memory deficits in the scopolamine-treated mice. In the hippocampus, HRGE upregulated the nuclear-factor-E2-related factor 2 and its downstream antioxidant enzymes NAD(P)H: quinone oxidoreductase and heme oxygenase-1(HO). Treatment with HRGE also decreased glutamate excitotoxicity and ROS levels in HT22 mouse hippocampal neuronal cells. The authors proposed that HRGE alleviates cognitive impairment by preventing neuronal apoptosis triggered by oxidative stress via the upregulation of antioxidant factors such as HO (Ju et al., 2021).
Coherent with its antioxidant effects, P . ginseng has proven to be neuroprotective effects against Aβ toxicity in cellular and animal models of AD-like neurodegeneration in rats treated with a combination of D-Galactose and AlCl3. The results showed that GP (10–100 μg/ml) increased neuronal survival the mRNA expression of apoptotic proteins such as caspase-3 and Bax/Bcl-2. Also, P. ginseng decreased Aβ and p-tau, iNOS, MDA, and NO, and increased the cAMP levels, and the expression of SOD, phospho-PKA and phospho-CREB. The authors concluded that the neuroprotective effects of P . ginseng were due to the activation of the cAMP/PKA/CREB pathway.
A preparation containing ginseng known as Jangwonhwan, a boiled extract of 7 medicinal plants/mushrooms, including Korean red ginseng, has been investigated. In a preclinical study, 4.5 month-old transgenic (Tg)-APPswe/PS1dE9 AD mice (Seo et al., 2010a; Seo et al., 2010b). In one of these studies Tg mice were treated with a revised formula of Jangwonhwan 300 mg/kg/day for 3 months. LMK02-Jangwonhwan notably reduced Aβ1-42/1-40 levels, and plaque deposition, in the brain of Tg-APPswe/PS1dE9 mice. LMK02-Jangwonhwan moderately diminished oxidative stress and prevented the down-regulation of phospho-CREB and calbindin in the hippocampus. Also, when added to SH-SY5Y neuroblastoma cells, Jangwonhwan inhibited oxidative stress and Aβ-induced neurotoxicity (Seo et al., 2010b).
Rheum palmatum L. (Polygonaceae), is a plant native to southern Siberia and North and Central China. Commonly known as rhubarb, and is used as traditional medicine (Staglich, 1948). Ethanolic extracts of R. palmatum showed an RP of 3.85 × 10–4 (Zhang et al., 2005). The screening of different fractions of methanolic extracts for EA using the recombinant yeast system, consisting of a hER expression plasmid and a reporter plasmid, showed high EA levels (EC50 = 0.093 mg/ml) in the dichloromethane fraction. An activity-guided analysis of this fraction revealed five anthraquinones: chrysophanol, physcion, emodin, aloe-emodin, and rhein. Emodin showed the highest estrogenic RP (6.3 × 10–2) to 17 b estradiol RP = 1.0), and strong cytotoxicity for ER (+) MCF-7 and ER (-) MDA-MB-231 breast cancer cells (Kang et al., 2008). Rhubarb also has protective effects against Aβ toxicity in IMR-32 cells (Misiti et al., 2006).
Parkinson's disease (PD) is a neurodegenerative disease and the second cause of dementia in the elderly. The physical examination reveals resting tremor, bradykinesia/akinesia, cogwheel rigidity, and gate impairment. The neuropathological hallmarks of PD are the appearance of intracellular aggregated forms of α-synuclein and the loss of dopaminergic neurons in the midbrain (Iarkov et al., 2020). Because there are no effective long-term treatments against PD, new therapeutic avenues are intensely searched (Danielyan et al., 2011; Danielyan et al., 2014; Jurado-Coronel et al., 2016; Mazo et al., 2017).
Some clues about potential new therapeutic avenues could arise from the epidemiological studies showing a clear difference in the PD incidence among sexes, with men being more affected than women (Jurado-Coronel et al., 2018). Estrogenic hormones such as estradiol seem to exert some protective effects against this condition; unfortunately, in addition to its beneficial effects in the brain, it may promote tumor growth in the ovary and breast. For this reason, new ER modulators, or estrogen analogs with lower activity on reproductive tissues, are being actively investigated as preventative and curative drugs for PD (Hidalgo-Lanussa et al., 2018).
Vicia faba L. (Fabaceae) also known as broad bean is a plant of the genus Vicia which members are considered to have many health benefits against PD that are attributed to several biological activities, including estrogenic, anticholinesterase, antidepressant, antioxidant, anti-inflammatory and antinociceptive, antidiabetic, anticoagulant, and antihypoxic activities (Rabey et al., 1992; Apaydin et al., 2000; Raguthu et al., 2009; Ramirez-Moreno et al., 2015; Abdel-Sattar et al., 2021; Salehi et al., 2021).
Clinical studies: After discovering the deficiency o dopamine in the striatum of patients with PD by Ehringer and Hornykiewicz in 1960, and the motor improvement with levodopa by Cotzias et al. in patients with PD, levodopa became the gold standard for treating PD. Nevertheless, this treatment resulted in motor fluctuations and dyskinesias in most patients. Many years later, a clinical study showed that Broad bean (Vicia faba)-a natural source of L-dopa-prolonged the "on" periods in patients with PD who have "on-off" fluctuations and shortened the “off (Apaydin et al., 2000). The study reported eight patients that benefited from broad bean meals. The patients were administered high doses of levodopa up to 800–1,000 mg per day, benefits increasing “on” time without the appearance of dyskinesias. One of the patients (Patient 1) showed a persistent response, even when they ingested broad beans only every other day. Astoundingly, in Patient 3, broad bean treatment was accompanied by a decrease of dyskinesias and a reduction of carbidopa/levodopa (L-DOPA) therapy. The authors considered that broad bean’s effects are because the patients ingested their broad bean meals garnished with yogurt rich in proteins. The authors proposed that because amino acids from dietary proteins compete with L-DOPA in crossing the BBB, they could reduce the motor effects of L-DOPA. Unfortunately, this was an unblinded trial, and a placebo effect cannot be discarded. A previous study by Rabek et al. described the acute responses following a single administration of broad beans to six patients with PD. They reported an increase in plasma L-DOPA levels after broad bean administration. This increase mirrored the motor improvements achieved by single doses of carbidopa/levodopa (Rabey et al., 1992). A similar rise of L-DOPA in plasma induced by broad bean administration was described by Vered et al. that reported that 40 g of freshly chopped broad bean provided 120–130 mg of L-DOPA (Vered et al., 1997).
Mucuna pruriens (L.) DC. (Fabaceae) or velvet beans (Atmagupta, Sanskrit) contain L-DOPA and have been used to treat PD in India for many years (Vaidya et al., 1978; Manyam, 1990). Several animal studies in rodents (Dhanasekaran et al., 2008) and monkeys (Lieu et al., 2012) have shown the beneficial effects of M. pruriens against PD-like pathology. More importantly, the therapeutic advantage compared to synthetic L-DOPA is the reduced risk of developing dyskinesias (Lieu et al., 2010).
One of the studies used a hemiparkinsonian rat model of PD to compare the effects of chronic parenteral administration of a water extract of M. pruriens seed powder (MPE) alone, with MPE combined with the peripheral dopa-decarboxylase inhibitor benserazide (BZ), L-DOPA alone and L-DOPA + BZ. The results were encouraging and chronic treatment with MPE alone showed significant anti-parkinsonian effects without causing dyskinesias (Lieu et al., 2010).
In a recent multicenter open trial in India, M. pruriens, formulated as HP-200 (Manyam et al., 2004), was administered orally to 60 patients with PD for 3 months. It was extracted as a powder from the plant seeds and administered mixed with water. The authors found that consumption of dry seeds of broad beans did not experience any clinical benefit. However, they found a significant improvement of motor abilities in the patients treated with HP-200, and hypothesized that this extract might contain L-DOPA and other antiparkinsonian compounds (Manyam et al., 2004).
Estrogenic plants contain estrogenic compounds that, according to many studies including from our research groups, have antioxidant and anti-inflammatory activities that can be useful in many conditions that characterize for inflammation and oxidative stress, including neurodegenerative diseases as well as other neurological conditions (Lanussa et al., 2016; Hidalgo-Lanussa et al., 2018; Baez-Jurado et al., 2019; González-Giraldo et al., 2019; Martin-Jimenez et al., 2019; Vesga-Jimenez et al., 2019; Hidalgo-Lanussa et al., 2020).
Zingiber officinale Roscoe (Zingiberaceae), commonly known as ginger, is used as a food, spice, herbal supplement for its antioxidant, anti-inflammatory and pharmacological activities (Kiyama, 2020). Epidemiological studies indicated that Ginger has beneficial effects on diabetes, metabolic syndrome, hypercholesterolemia, and inflammatory disorders (Kiyama, 2020). Ginger components include monoterpenes (cineole, citral, limonene, and alpha/beta-pinenes), sesquiterpenes (beta-elemene, farnesene, and zerumbone), phenolics compounds (gingerols, [6]-shogaol, [6]-paradol and zingerone), and diarylheptanoids (curcumin). These compounds prevented cell apoptosis, DNA damage, and inflammation by regulating cell signaling, controlling autophagy and cellular metabolism. Ginger-derived phytoestrogens are important bioactive active components modulating essential brain functions. In a previous study, ginger showed EA (RP of 3.7 × 10–1) (Kim et al., 2008). A methanolic extract of the plant effectively induced EA to more than 50% (Kang et al., 2006).
An analysis of a raw extract of the aerial parts of Achillea millefolium L. (Asteraceae) has EA when tested in MCF-7 cells transiently expressing ERs. After fractionating the extract, they found EA in a methanol/water fraction. From the phytoestrogens present in this extract, apigenin and luteolin activated ERs, but luteolin activated the ERβ but not the ERα (Innocenti et al., 2007). Female rats treated with acetaminophen and aqueous extracts of A. millefollium (2 g/kg/day) showed increased plasma levels of the toxicity marker lactate dehydrogenase. However, the extracts enhanced the activity of the antioxidant proteins reduced GSH and glutathione S-transferase (GST) in the uterus (Baggio et al., 2016). Furthermore, treatment with A. millefollium extracts inhibited autoimmune encephalomyelitis in mice (Vazirinejad et al., 2014).
A clinical study showed that A. millefolium extracts (250–500 mg/day), when administered to 75 patients with multiple sclerosis (MS) for a year, significantly delayed the time to first relapse, reduced the number of lesions, and improved their cognitive abilities (Ayoobi et al., 2019).
Reynoutria japonica Houtt. (Polygonaceae), known as Huzhang in China, is an herbaceous perennial plant natural to Eastern Asia, China, and Russia. However, several species also find in Europe and North America (Peng et al., 2013). Reynoutria japonica Houtt. (Polygonaceae) has been used in clinical practice for the treatment of cancer, endotoxic shock, favus, hyperlipemia, infection, inflammation, jaundice, and scald in China and Japan (Peng et al., 2013). More than 67 compounds, including quinones, stilbenes, flavonoids, coumarins, and ligands have been isolated from this plant. Ethanolic extract of R. japonica showed an estrogenic RP (3.28 × 10–3) (Zhang et al., 2005).
This plant, known as damiana, oreganillo, or Mexican tea, grows in Bolivia, Brazil, Mexico, and North America. The leaves of T. diffusa contain the monoterpenes 1,8-cineole, p-cymene, alpha and beta-pinene, tetrafilin B, and caffeine. In the branches, also it has the flavonoid gonzalistosin, steroid beta-sitosterol, and the xacosanol alkanes n-triacontane and tricosan-2-one. The extracts of T. diffusa show antibacterial and antioxidant properties (Soriano-Melgar Lde et al., 2014). Methanolic extract demonstrated a dose-dependent inhibitory effect on aromatase activity (IC50 = 63.1 mg/ml). From 24 compounds tested, pinocembrin and acacetin were the most potent aromatase inhibitors having IC50 values of 10.8 and 18.7 mM, respectively. In addition, three compounds from the extracts apigenin 7-glucoside, Z-echinacin, and pinocembrin showed EA (Zhao et al., 2008). Previous studies have shown that T. diffusa has components such as arbutin that inhibit lipid peroxidation and has immunomodulatory and anti-oxidant properties that have proven beneficial for stomach ulcers (Taha et al., 2012). Anti-inflammatory activity has been found for leaf extracts of Turnera subulata found in the Northeastern regions of Brazil (Souza et al., 2016).
In the TCM, Cullen corylifolium (L.) Medik. (Fabaceae) is also known as Buguzhi, and is used to reduce inflammatory processes. The study of ethanolic extracts of C. corylifolium, revealed a estrogenic RP of (1.90 × 10–4) (Zhang et al., 2005). The ethanolic extracts of C. corylifolium contained bakuchiol, psoralen, isobavachalcone, isobavachromene, and bavachinin. Also, hexane and chloroform extracts showed EA when tested in a yeast estrogen screen assay. In this assay, ethanol, hexane, chloroform extracts of CC showed EA, and bakuchiol showed similar EA than genistein (10–6 M). In an ER binding assay, bakuchiol displayed the strongest ER-binding affinity and five times higher affinity for ERα than for ERβ (Lim et al., 2011).
Psoralen is the main bioactive compound in the fruits of C. corylifolium. It is used to treat inflammation, bacterial infections (destroying the formation of biofilm), viral infections, and conditions associated with menopause such as osteoporosis (Ren et al., 2020). Psoralen’s anti-osteoporotic effect involve different proteins factors that participate in the bone remodeling such as bone morphogenetic proteins (BMPs), matrix metalloproteinases, peroxisome proliferators-activated receptor-gamma (PPARgamma), as well as signaling cascades including the inositol-requiring enzyme 1 (IRE1)/apoptosis signaling kinase 1 (ASK1)/c-jun N-terminal kinase (JNK), wnt/β-catenin, and the Protein kinase B (Akt)/activator protein-1 (AP-1) pathways (Ren et al., 2020). These signaling factors may help to treat osteoporosis by activating the osteoblast/osteoclast/chondrocyte differentiation process. The anti-inflammatory and neuroprotective effects of psoralen seem to be the result of its interaction with viral polymerase (Pol), and regulating the activation of TNF-α, transforming growth factor β (TGF-β), interleukin (IL) -4/5/6/8/12/13, GATA-3, and AChE (Ren et al., 2020).
Curcuma aromatica Salisb. (Zingiberaceae) also known as wild turmeric. An early study found EA in rhizome extracts of this plant with RP of 8.5 × 10–4 (Kang et al., 2006). Another study investigated the putative estrogenic and/or antiestrogenic activities of several Asiatic medicinal plants. They found that the highest EA (relative to 17 β -estradiol, RP = 1) were in this order: flowers extracts of Pueraria lobata (RP, 7.75 × 10–3), Amomum xanthioides (1.25 × 10–3), Glycyrrhiza uralensis, Zingiber officinale, Rheum undulatum, Curcuma aromatica, Eriobotrya japonica, Sophora flavescens, Anemarrhena asphodeloides, Polygonum multiflorum, and Pueraria lobata roots (ranging from 9.5 × 10–4 to 1.0 × 10–4). Prunus persica, Lycoppus lucidus, and Adenophora stricta had lower RP (9.0 × 1 0–5 to 8.0 × 10–5). Cinnamomum cassia and Prunus persica, showed antiestrogenic effects with RP of 1.14 × 10–3 and 7.4 × 10–4, respectively (tamoxifen RP = 1) (Kim et al., 2008).
In addition, Curcuminoids from C. aromatica have neuroprotective activities against oxidative stress. Curcuminoids present in the radix of C. aromatica are neuroprotective against the toxic effects of 24 h-treatment with H2O2 in PC12 cells. The curcumoids increased cell survival, decreased the release of LDH (a measure of cell death), the cellular Ca2+, and ROS levels, enhanced the activities of antioxidant proteins such as SOD, catalase (CAT) and GSH, stabilized the mitochondrial membrane potential, and improved PC12 morphology. The chemical analysis showed that diarylheptanoids and sesquiterpenoids are the primary active chemicals in the extracts tested. On the other hand, Curcumin is the main component of Curcuma longa, a Chinese medicinal plant used to alleviate brain disorders, which is neuroprotective and increases the survival of cultured rodent cortical neurons.
The protective effects of curcumin were blocked when neurons were pretreated with a tyrosine kinase B (TrkB) antibody, known to inhibit the activity of the brain-derived neurotrophic factor (BDNF). Conversely, treatment with curcumin increased BDNF and phospho-TrkB effects that, in turn, were blocked by ERK and PI3K inhibitors. Curcumin activated ERK, CREB, and AKT, by mechanisms involving MAPK and PI3K. The authors concluded that curcumin beneficial effects might be mediated via the BDNF/TrkB-MAPK/PI-3K-CREB pathway (Wang et al., 2010).
Eriobotrya japonica (Thunb.) Lindl. (Rosaceae) commonly known as the Japanese Medlar or loquat. A study reported that methanolic extracts of E. japonica induced an increase of EA (>50%) (Kang et al., 2006). Ethyl acetate fractions of E. japonica showed EA with an RP of 6.1 × 10–2 (RP E2 = 1.0) (Kim et al., 2008). However, it my be used with caution as seeds, or pips, and young leaves are slightly poisonous because contain little amounts of cyanogenic glycosides such as amygdalin which release cyanide if eaten. However, numerous studies have shown powerful anti-inflammatory effects in different disease conditions. Silver nanoparticles of Eriobotrya japonica AgNPs stimulated phagocytosis and prevent allergies and the authors concluded that E. japonica could be a promising therapy for preventing inflammation, and bacterial infection by enhancing of phagocytosis (Jabir et al., 2021), arthritis (Kuraoka-Oliveira et al., 2020), asthma (Kim T.-M. et al., 2020), intestinal problems, hepatic damage induced by acetaminophen (Jiang et al., 2017), and metabolic syndrome induced by a high fat diet in C57BL/6J mice (Li F. et al., 2020). In the most recent study male mice 7–9 weeks of age were randomly divided into three groups (n = 6 mice/group). The first treatment group received 250 µl of saline by IP injection, the second received 50 µg LPS via IP and group 3 was pre-treated with AgNPs (1 mg/kg) and then received 50 µg LPS. After 10 h, mice were sacrificed, and blood and peritoneal fluid was taken for analysis of IL-1β and IL-6 levels. The results showed that the AgNPs synthesized using E. japonica leaf extract inhibited the LPS-induced synthesis and the levels of cytokines such as IL-1β and IL-6 (Jabir et al., 2021).
Vigna radiata (L.) R.Wilczek (Fabaceae) is known as mung bean sprout, common in the tropics and warm temperature zones and its extracts have shown to increase cell proliferation above the levels induced by estradiol. This proliferation was suppressed by the ER antagonist, ICI 182.780, suggesting that ER signaling pathways are mediating the proliferative effects of V. radiata (Boue et al., 2003).
Preclinical studies have investigated anti-inflammatory and antiarthritic activity of V. radiata sprouts in rats. Ethanolic extracts of the sprouts were used to evaluate its antiarthritic activity using rats treated with complete Freund's adjuvant as a model of arthritis. Treatment with ethanolic extracts of V. radiata significantly reduced the biochemical changes induced by the Freund's adjuvant, such as lipid peroxidation, total reduced glutathione, myeloperoxidase and lysosomal enzymes like cathepsin-D, N-acetyl beta-D-glucosaminase and beta-D-glucuronidase. The findings of this study suggest that V. radiata can reduce inflammation in arthritis (Venkateshwarlu et al., 2016). Further studies are required to investigate its effects in other inflammatory conditions.
Wurfbainia villosa var. Xanthioides (Wall. ex Baker) Skornick. and A.D.Poulsen, commonly known as bastard cardamom and Malabar Cardamom, is a medicinal plant native to Indonesia that is used to treat spleen and stomach diseases in China (An et al., 2020). Butanolic fractions of W. villosa var. xanthioides showed EA with a relative potency of 6.6 × 10–2 (RP of E2 = 1.0) (Kim et al., 2008). Also, methanolic extract of this plant effectively induced a 50% increase in EA (Kang et al., 2006).
The herbal mix named CG (plus) contains Artemisia gmelinii Weber ex Stechm. (syn, Artemisia iwayomogi Kitamura), W. villosa and Salvia miltiorrhiza Bunge. The effect of CG (plus) on stress for immobilization (IS) was recently assessed in mice. Male BALB/c mice were daily treated with different doses of aqueous extracts of CG (plus) (0–200 mg/kg) for 5 days. On the last day, mice were subjected to IS for 6 h. Stress increased corticosterone levels, adrenaline, cytokines, and the enzyme markers of hepatic injury (ALT and AST) in serum. The mix CG (plus) significantly prevented the effects of stress (Kim H.-G. et al., 2020).
Iris domestica (L.) Goldblatt & Mabb. syn Belamcanda chinensis (Iridaceae) commonly known as Blackberry Lilly. The ethanolic extract of I. domestica showed EA with a RP of (1.26 × 10–4) (Zhang et al., 2005). Isoflavonoids obtained from a methanolic extract of I. domestica rhizomes inhibited mutagenesis when tested in the Ames test and have antioxidant effects. The isoflavonoids reduced transition-metal ions and prevented the peroxidation of polyunsaturated fatty acids. The isoflavones, identified included the glycosides tectoridin and iridin and the aglycones irigenin, tectorigenin, and 5,6,7,3′-tetrahydroxy-4′-methoxyisoflavone (Wozniak et al., 2010).
Senna obtusifolia (L.) H.S.Irwin and Barneby (syn. Cassia obtusifolia L.) (Fabaceae) is commonly known as American sicklepod. S. obtusifolia is a weed that grows in the world's tropical and subtropical regions, even when the environmental conditions are unfavorable. This plant has been investigated as a laxative and sexual stimulant in male rats (Ajayi et al., 2014). Ethanolic extracts of S. obtusifolia have shown estrogenic RP of 3.49 × 10–4 (Zhang et al., 2005). However, its toxic effects observed in cattle and mice discouraged its use as a medicinal plant (Furlan et al., 2014; Ana Maria et al., 2019).
A different study investigated the effects of Aurantio-obtusin, an anthraquinone isolated from dried seeds of S. obtusifolia and Cassia tora L. (syn. Senna tora). Aurantio-obtusin was tested as an anti-inflammatory compound on lipopolysaccharide- (LPS)-induced inflammation in RAW264.7 cells. ELISA and quantitative real-time PCR (qRT-PCR) assays revealed that this compound significantly decreased the production of NO and PGE2 by inhibiting the expression of COX-2 and iNOS, as well as the cytokines TNF-α, and IL-6. Aurantio-obtusin showed anti-inflammatory effects via the inhibition of NFκB in RAW264.7 cells. These results suggested that this compound could have therapeutic value against inflammatory diseases.
Medicago sativa L. (Fabaceae) is commonly known as alfalfa sprout. M. sativa extracts induce more cell proliferative effects than estradiol. The estrogen receptor antagonist ICI 182,780 (Fulvestrant) suppressed the cell proliferation induced by the extracts, suggesting that ERs were involved (Boue et al., 2003). Moreover, the phytoestrogens present in these extracts exhibited selectivity toward ERβ (Boue et al., 2003). Also a recent study showed that Alfalfa extracts have dose-dependent beneficial effect on nicotine-induced oxidative damage and neuroinflammation in the brain as well as reducing anxiety (Raeeszadeh et al., 2021). In this study the authors investigated the effect of the hydroalcoholic extract of M. sativa on brain damage and anxiety behavior in male Wistar rats exposed to nicotine. Rats were randomly divided into six treatment (T) groups: T1 and T2 were subcutaneously injected with 250 and 500 mg/kg alfalfa extract, respectively, T3 and T4 groups where animals were injected subcutaneously with 0.2 mg/kg nicotine and 250 and 500 mg/kg alfalfa extract, and T5 group was injected with 0.2 mg/kg nicotine. After treatments, the serum levels of the cytokines TCA, IL-1, and TNFα and oxidative stress-defensive factors such as gluthation (GPx, SOD, total antioxidant capacity (TAC), and MDA) in the brain were assayed. The time and number of entrances in the open arm significantly decreased in the treated groups in a dose-depended manner. Oxidative stress parameters were higher in the nicotine -treated rats and lower in the rats treated with 500 mg/kg alfalfa extracts (T2). Nicotine increased the levels of the cytokines TNFα and IL-1 in the T5 group when compared to the other groups. More importantly, treatment with the alfalfa extracts increased the levels of the antioxidant factors GPx and SOD and decreased the number of necrotic neurons and gliosis in the nicotine-treated rats (Raeeszadeh et al., 2021).
Phaseolus vulgaris L.(Fabaceae) is also known as green bean or red kidney bean (Boue et al., 2011; de Lima et al., 2014). P. vulgaris produces phytoalexins (isoflavones) under stress conditions such as wounding and/or insect damage. Thus to induce the expression of flavonoids, the components of methanolic extracts of red kidney bean that were treated with the fungus Aspergillus sojae, and untreated controls plants were tested for estrogenic activity using an ERE luciferase reporter assay (Boue et al., 2011). The estrogenic activities of the phytoalexins kievitone and phaseollin were isolated from fungus-treated red kidney bean extracts and analyzed for EA using ERα and ERβ binding and ERE luciferase and cell proliferation assays in MCF-7 and HEK 293 cells. These phytoalexins stimulated MCF-7 cell proliferation and Kievitone showed a higher binding affinity to ERα and Phaseollin to ERβ. Although phaseollin displayed attenuation of ER transactivation in the ERE luciferase assay in MCF-7 cells, both phytoalexins attenuated the effects of E2 in an MCF-7 cell survival assay showing estrogenic and antiestrogenic activities (Boue et al., 2011). The green bean extracts were more effective in inducing cell proliferation than estradiol and exhibited a preferential affinity for ERβ. The proliferative effects were due to its estrogenic effects as it was suppressed by ICI 182,780 (Boue et al., 2011). P. vulgaris has shown antioxidant activity in both cell and animal studies (Venkateswaran et al., 2002; Venkateswaran and Pari, 2002; Ampofo and Ngadi, 2020). One recent study, investigated phenolic compounds, and antioxidant activity, of different species of the genus Phaseolus. The species closest to each other in terms of essential amino acid content were P. polyanthus with P. vulgaris and P. lunatus with P. coccineus. Previous studies show a positive correlation between antioxidant activity and flavonoids, anthocyanins, and lectins. Significant differences in the content of phenolic compounds have been found among the bean species. No obstant, in addition to P. vulgaris, other species such as P. coccineus and P. lunatus have high antioxidant potential (Alcazar-Valle et al., 2020).
Pueraria montana var. lobata (Willd.) Maesen and S.M.Almeida ex Sanjappa and Predeep (Fabaceae), also known as kudzu, is a perennial leguminous vine used as an herbal medicine in Asia, containing flavonoids that are concentrated in its root (Eom et al., 2018). Methanolic extract of flowers of P. lobata increased the EA > 50%, but roots only showed moderate EA (Kang et al., 2006). Extracts of kudzu roots induced cell proliferation by a mechanism involving ER as it was suppressed by the antagonist, ICI 182,780 (Boue et al., 2003). Ethanolic extracts of Pueraria lobata showed an estrogenic RP of (6.17 × 10–5) (Zhang et al., 2005). In another study, 17β-estradiol showed EA with an EC50 = 0.205 ± 0.025 ng/ml (RP = 100) and methanolic extracts of flowers and stem of P. montana var. lobata showed EA with EC50 values of 8.6–1.0 μg/ml, respectively (Yoo et al., 2005).
A study that used peritoneal macrophages isolated from BALB/c mice and cells activation with lipopolysaccharide (LPS) or LPS plus interferon (IFN)-gamma revealed that kudzu leaf and its principal active compound robinin (kaempferol-3-O-robinoside-7-O-rhanmoside) have anti-inflammatory activity. The extract inhibited the macrophage synthesis of the inflammatory factors iNOS, COX-2, TNFα, and IL-6. Kudzu extracts decreased LPS-induced activation of (JNK and TANK-binding kinase 1 (TBK1). Also, kudzu extract inhibited LPS/IFN-gamma-induced expression of the signal transducer and activator of transcription 1 (STAT1). Robinin decreased iNOS protein involving modulation of JNK and STAT1 activation. But without any impact on other inflammatory markers such as NFκB (Eom et al., 2018).
Many other plants with demonstrated EA require further characterization include: Adenophora stricta Miq. (Campanulaceae), native to East Asia (China), also known as Sha Shen and Ladybells (Kim et al., 2008); Artemisia vulgaris L. (Asteraceae), often named mugwort, contains the flavonoids eriodictyol and apigenin (Lee et al., 1998); Asplenium trichomanes L. (Aspleniaceae) known as maidenhair spleenwort, it is used to control irregular menses and as an emmenagogue, aborticide, expectorant, anti-cough remedy, and laxative (Dall'Acqua et al., 2009); Hylodesmum podocarpum subsp. oxyphyllum (DC) H. Ohashi and R.R.Mill. syn. Desmodium oxyphyllum (Fabaceae). This plant, also called ticktrefoils, is a medicinal plant from Asia, that is used for breast cancer (Yoo et al., 2005); Maackia amurensis-Rupr (Fabaceae), is a tree also known as Amur maackia (Yoo et al., 2005; Grishchenko et al., 2016); Humulus lupulus L. (Cannabaceae) Methanolic plant extracts bind to ERα and ERβ. And it has EA. Numerous studies of the estrogenic properties for hops are attributed to the component 8-prenylnarigenin (Liu et al., 2001). Another study compared the estrogenic effects of hops to other plants of the licorice species (Glycyrrhiza glabra, G. inflata, and G. uralensis) (Hajirahimkhan et al., 2013). All extracts showed EA when tested in cultured endometrial cancer cells, MCF-7 cells expressing an ERE directing the expression of the fluorescent reporter luciferase. The authors ordered the plant extracts by decremental EA as follows: H. lupulus > G. uralensis > G. inflata > G. glabra. Liquiritigenin was selective for ERβ, and isoliquiritigenin was equipotent for both ERα/β. 8-Prenylnaringenin showed nanomolar estrogenic RP without ER selectivity (Hajirahimkhan et al., 2013). A recent study identified 8-Prenylapigenin from Glycyrrhiza inflata as an ERβ agonist (Hajirahimkhan et al., 2018).
The deficit of estrogen is involved in the development or progression of various types of autoimmune diseases, cancer (breast, colorectal, endometrium, ovary, prostate), cardiovascular diseases, osteoporosis, obesity, insulin resistance, and neurodegenerative diseases and mood disorders. The deficit in sex hormones such as estrogen negatively impacts the cognitive abilities, general health, and consequently the quality of life of millions of persons around the world. HRT positively impacts cognitive abilities and immune function, but its benefits seem to be restricted to the early stages of menopause. After menopause, HRT with estrogens combined with progestins has shown beneficial effects on climacteric symptoms and osteoporosis in the bone and on body weight. However, it increases the risk of mammary carcinomas and cardiovascular diseases. Consequently, plant-derived alternatives are currently investigated. The advancement of our understanding of the molecular mechanisms underlying the effects of plant-derived estrogenic compounds will facilitate the discovery of new therapies to counteract the adverse effects of a deficit of estrogen in the brain. Interestingly, many plants used in traditional medicine have shown estrogenic activity that is concurrent with antioxidant, antiproliferative, and anti-inflammatory activities.
The discovery of estrogenic compounds from medicinal plants with lower side effects should permit to improve the cognitive abilities in women affected by menopause and other conditions inducing oxidative stress and neurodegeneration without the side effects elicited by actual HRT.
Recently encouraging clinical results with a combination of herbs have shown to be effective and safe in decreasing menopause symptoms (Rattanatantikul et al., 2020). Also, phytoestrogens and their metabolites from medicinal plants have been shown to mimic the benefits of endogenous estrogens, and estrogen-based therapies are considered promising approaches for preventing the onset and progression of neurodegenerative disorders (Bustamante-Barrientos et al., 2021). Though, xenoestrogens derived from plastic polycarbonates, such as water bottles and varnish coating in food cans, can alter estrogen-mediated signaling pathways inducing a negative effect on health. It may be required to counteract the estrogen deficit in a more holistic approach comprising an adequate diet containing natural estrogenic compounds, body and mental exercise practices that promote neurogenesis, a more ecologically friendly way of life, and adequate social and spiritual support systems to decrease the effects of stress. On the other hand, the explosive development of new genetic therapies may permit the modulation of specific estrogenic receptors in the brain, bones, and other tissues without increasing the risk of disease while preventing the pathological effects of lower sex hormones' levels.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This work was supported by the National Commission for Scientific and Technological Research in Chile (ANID) (Grant No. FONDECYT 1190264), and the Universidad San Sebastian, Chile.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This material is the result of work supported with resources and the use of facilities from the Universidad San Sebastian (Chile). The contents do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.644103/full#supplementary-material
AAB Anemarrhena asphodeloides Bunge
Aβ Amyloid beta petide
AChEI acetylcholinesterase inhibitors
AD Alzheimer's disease
AGE advanced glycation endproducts
APOE Apolipoprotein E
APP Aβ precursor protein
AR androgen receptor
BDNF brain-derived neurotrophic factor
BMD bone mineral density
BMP bone morphogenetic protein
CAT catalase
ChE cholinesterases
COX-2 cyclooxygenase 2
CRE cAMP response element
DBT Danggui Buxue Tang
ER estrogen receptor
ERE estrogen-responsive element
ERK extracellular-regulated kinase
GBL Gingko biloba
GFAP glial fibrillar acidic protein
GQD Gegen Qinlian Decoction
GSH reduced glutathione
GST glutathione S-transferase activity
hER human ER
HRT hormone replacement therapy
IL Interleukin
l-DOPA levodopa
LH Luteinizing hormone
MENQOL menopause-specific QOL
MMSE Mini-Mental State Examination
MS multiple sclerosi
NFκB nuclear factor kappa B
NO nitric oxide
NOS nitric oxide synthase
OVX ovariectomized
PKA protein kinase A
PKB protein kinase B
PKC protein kinase C
PS1 presenilin 1
PR progesterone receptor
QOL quality of life
RP relative potency
SHBG sex hormone binding globulin
Tg transgenic
TNF-α tumor necrosis factor alpha
YCJ young coconut juice
Abdel-Sattar, E., Mahrous, E. A., Thabet, M. M., Elnaggar, D. M. Y., Youssef, A. M., Elhawary, R., et al. (2021). Methanolic Extracts of a Selected Egyptian Vicia faba Cultivar Mitigate the Oxidative/inflammatory Burden and Afford Neuroprotection in a Mouse Model of Parkinson's Disease. Inflammopharmacol 29 (1), 221–235. doi:10.1007/s10787-020-00768-6
Abdellatif, A. A. H., Elgayed, S. H., Afify, E. A., and Amin, H. A. (2020). Estrogenic Effect of Salvia Officinalis Extract on Reproductive Function of Female Mice and Identification of its Phenolic Content. Cchts 23. doi:10.2174/1386207323666200811095527
Abu-Taweel, G. M. (2018). Cardamom ( Elettaria Cardamomum ) Perinatal Exposure Effects on the Development, Behavior and Biochemical Parameters in Mice Offspring. Saudi J. Biol. Sci. 25 (1), 186–193. doi:10.1016/j.sjbs.2017.08.012
Adam, G. O., Kim, G.-B., Lee, S.-J., Lee, H., Kang, H.-S., and Kim, S.-J. (2019). Red Ginseng Reduces Inflammatory Response via Suppression MAPK/P38 Signaling and P65 Nuclear Proteins Translocation in Rats and Raw 264.7 Macrophage. Am. J. Chin. Med. 47 (7), 1589–1609. doi:10.1142/S0192415X19500812
Ajayi, C., Funso-Babarimisa, F., and Elujoba, A. (2014). Laxative Activities of Cassia Sieberiana and Senna obtusifolia. Afr. J. Trad. Compl. Alt. Med. 11 (4), 44–47. doi:10.4314/ajtcam.v11i4.7
Alcázar-Valle, M., Lugo-Cervantes, E., Mojica, L., Morales-Hernández, N., Reyes-Ramírez, H., Enríquez-Vara, J. N., et al. (2020). Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species. Molecules 25 (15), 3528. doi:10.3390/molecules25153528
Alipour, M. R., and Karimi-Sales, E. (2020). Molecular Mechanisms of Protective Roles of Isoflavones against Chemicals-Induced Liver Injuries. Chemico-Biological Interactions 329, 109213. doi:10.1016/j.cbi.2020.109213
Ampofo, J. O., and Ngadi, M. (2020). Ultrasonic Assisted Phenolic Elicitation and Antioxidant Potential of Common Bean (Phaseolus vulgaris) Sprouts. Ultrason. Sonochem. 64, 104974. doi:10.1016/j.ultsonch.2020.104974
An, W., Li, J., Yang, Z., Huang, Y., Huang, S., and Zheng, X. (2020). Characteristics Analysis of the Complete Wurfbainia Villosa Chloroplast Genome. Physiol. Mol. Biol. Plants 26 (4), 747–758. doi:10.1007/s12298-019-00748-3
Ana María, D.-B., Rosa María, V. V., Lilian, M.-N., Lucía, M.-M., Oscar, G.-P., and Rosa, E.-R. (2019). Neurobehavioral and Toxicological Effects of an Aqueous Extract of Turnera Diffusa Willd (Turneraceae) in Mice. J. Ethnopharmacology 236, 50–62. doi:10.1016/j.jep.2019.02.036
Andreano, J. M., and Cahill, L. (2009). Sex Influences on the Neurobiology of Learning and Memory. Learn. Mem. 16 (4), 248–266. doi:10.1101/lm.918309
Apaydin, H. l., Ertan, S., and Ozekmekci, S. (2000). Broad Bean (Vicia faba)?A Natural Source ofL-dopa?Prolongs ?on? Periods in Patients with Parkinson's Disease Who Have ?on-Off? Fluctuations. Mov. Disord. 15 (1), 164–166. doi:10.1002/1531-825710.1002/1531-8257(200001)15:1<164::aid-mds1028>3.0.co;2-e
Ardito, F., Di Gioia, G., Pellegrino, M. R., and Muzio, L. L. (2018). Genistein as a Potential Anticancer Agent against Head and Neck Squamous Cell Carcinoma. Ctmc 18 (3), 174–181. doi:10.2174/1568026618666180116122650
Asokan Shibu, M., Kuo, W.-W., Kuo, C.-H., Day, C.-H., Shen, C.-Y., Chung, L.-C., et al. (2017). Potential Phytoestrogen Alternatives Exert Cardio-Protective Mechanismsviaestrogen Receptors. Biomedicine (Taipei) 7 (2), 11. doi:10.1051/bmdcn/2017070204
Ayoobi, F., Moghadam-Ahmadi, A., Amiri, H., Vakilian, A., Heidari, M., Farahmand, H., et al. (2019). Achillea millefolium Is Beneficial as an Add-On Therapy in Patients with Multiple Sclerosis: A Randomized Placebo-Controlled Clinical Trial. Phytomedicine 52, 89–97. doi:10.1016/j.phymed.2018.06.017
Baez-Jurado, E., Rincón-Benavides, M. A., Hidalgo-Lanussa, O., Guio-Vega, G., Ashraf, G. M., Sahebkar, A., et al. (2019). Molecular Mechanisms Involved in the Protective Actions of Selective Estrogen Receptor Modulators in Brain Cells. Front. Neuroendocrinology 52, 44–64. doi:10.1016/j.yfrne.2018.09.001
Baggio, C. H., Otofuji, G. d. M., Freitas, C. S., Mayer, B., Marques, M. C. A., and Mesia-Vela, S. (2016). Modulation of Antioxidant Systems by Subchronic Exposure to the Aqueous Extract of Leaves fromAchillea millefoliumL. In Rats. Nat. Product. Res. 30 (5), 613–615. doi:10.1080/14786419.2015.1030738
Bagheri, M., Joghataei, M.-T., Mohseni, S., and Roghani, M. (2011). Genistein Ameliorates Learning and Memory Deficits in Amyloid β(1-40) Rat Model of Alzheimer's Disease. Neurobiol. Learn. Mem. 95 (3), 270–276. doi:10.1016/j.nlm.2010.12.001
Bagheri, M., Roghani, M., Joghataei, M.-T., and Mohseni, S. (2012). Genistein Inhibits Aggregation of Exogenous Amyloid-Beta1-40 and Alleviates Astrogliosis in the hippocampus of Rats. Brain Res. 1429, 145–154. doi:10.1016/j.brainres.2011.10.020
Bartzokis, G. (2011). Alzheimer's Disease as Homeostatic Responses to Age-Related Myelin Breakdown. Neurobiol. Aging 32 (8), 1341–1371. doi:10.1016/j.neurobiolaging.2009.08.007
Basaria, S., Wisniewski, A., Dupree, K., Bruno, T., Song, M. Y., Yao, F., et al. (2009). Effect of High-Dose Isoflavones on Cognition, Quality of Life, Androgens, and Lipoprotein in Post-menopausal Women. J. Endocrinol. Invest. 32 (2), 150–155. doi:10.1007/BF03345705
Basu, P., and Maier, C. (2018). Phytoestrogens and Breast Cancer: In vitro Anticancer Activities of Isoflavones, Lignans, Coumestans, Stilbenes and Their Analogs and Derivatives. Biomed. Pharmacother. 107, 1648–1666. doi:10.1016/j.biopha.2018.08.100
Beck, V., Unterrieder, E., Krenn, L., Kubelka, W., and Jungbauer, A. (2003). Comparison of Hormonal Activity (Estrogen, Androgen and Progestin) of Standardized Plant Extracts for Large Scale Use in Hormone Replacement Therapy. J. Steroid Biochem. Mol. Biol. 84 (2-3), 259–268. doi:10.1016/s0960-0760(03)00034-7
Bharate, J. B., Batarseh, Y. S., Wani, A., Sharma, S., Vishwakarma, R. A., Kaddoumi, A., et al. (2015). Synthesis and P-Glycoprotein Induction Activity of Colupulone Analogs. Org. Biomol. Chem. 13 (19), 5488–5496. doi:10.1039/c5ob00554j
Bhatia, H., Pal Sharma, Y., Manhas, R. K., and Kumar, K. (2015). Traditional Phytoremedies for the Treatment of Menstrual Disorders in District Udhampur, J&K, India. J. Ethnopharmacology 160, 202–210. doi:10.1016/j.jep.2014.11.041
Boccardi, V., Murasecco, I., and Mecocci, P. (2019). Diabetes Drugs in the Fight against Alzheimer's Disease. Ageing Res. Rev. 54, 100936. doi:10.1016/j.arr.2019.100936
Bojar, I., Gujski, M., Raczkiewicz, D., and Rothenberg, K. G. (2013). Cognitive Functions, Apolipoprotein E Genotype and Hormonal Replacement Therapy of Postmenopausal Women. Neuro Endocrinol. Lett. 34 (7), 635–642.
Borrelli, F., and Ernst, E. (2008). Black Cohosh (Cimicifuga Racemosa): a Systematic Review of Adverse Events. Am. J. Obstet. Gynecol. 199 (5), 455–466. doi:10.1016/j.ajog.2008.05.007
Boss, L., Kang, D.-H., Marcus, M., and Bergstrom, N. (2014). Endogenous Sex Hormones and Cognitive Function in Older Adults. West. J. Nurs. Res. 36 (3), 388–426. doi:10.1177/0193945913500566
Boué, S. M., Burow, M. E., Wiese, T. E., Shih, B. Y., Elliott, S., Carter-Wientjes, C. H., et al. (2011). Estrogenic and Antiestrogenic Activities of Phytoalexins from Red Kidney Bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 59 (1), 112–120. doi:10.1021/jf102255u
Boué, S. M., Wiese, T. E., Nehls, S., Burow, M. E., Elliott, S., Carter-Wientjes, C. H., et al. (2003). Evaluation of the Estrogenic Effects of Legume Extracts Containing Phytoestrogens. J. Agric. Food Chem. 51 (8), 2193–2199. doi:10.1021/jf021114s
Bove, R., Secor, E., Chibnik, L. B., Barnes, L. L., Schneider, J. A., Bennett, D. A., et al. (2014). Age at Surgical Menopause Influences Cognitive Decline and Alzheimer Pathology in Older Women. Neurology 82 (3), 222–229. doi:10.1212/WNL.0000000000000033
Brown, S. (2013). No Adverse-Oor Beneficial-Eeffect of HRT on Cognitive Function in Younger Postmenopausal Women. Menopause Int. 19 (3), 104–105.
Bustamante-Barrientos, F. A., Méndez-Ruette, M., Ortloff, A., Luz-Crawford, P., Rivera, F. J., Figueroa, C. D., et al. (2021). The Impact of Estrogen and Estrogen-like Molecules in Neurogenesis and Neurodegeneration: Beneficial or Harmful?. Front. Cel. Neurosci. 15, 636176. doi:10.3389/fncel.2021.636176
Chen, Z., and Zhong, C. (2013). Decoding Alzheimer's Disease from Perturbed Cerebral Glucose Metabolism: Implications for Diagnostic and Therapeutic Strategies. Prog. Neurobiol. 108, 21–43. doi:10.1016/j.pneurobio.2013.06.004
Choi, S.-H., Lee, R., Nam, S. M., Kim, D.-G., Cho, I.-H., Kim, H.-C., et al. (2021). Ginseng Gintonin, Aging Societies, and Geriatric Brain Diseases. Integr. Med. Res. 10 (1), 100450. doi:10.1016/j.imr.2020.100450
Circosta, C., Pasquale, R. D., Palumbo, D. R., Samperi, S., and Occhiuto, F. (2006). Estrogenic Activity of Standardized Extract ofAngelica Sinensis. Phytother. Res. 20 (8), 665–669. doi:10.1002/ptr.1928
Colciago, A., Casati, L., Negri-Cesi, P., and Celotti, F. (2015). Learning and Memory: Steroids and Epigenetics. J. Steroid Biochem. Mol. Biol. 150, 64–85. doi:10.1016/j.jsbmb.2015.02.008
Costa, A. R., Marcelino, H., Gonçalves, I., Quintela, T., Tomás, J., Duarte, A. C., et al. (2016). Sex Hormones Protect against Amyloid-β Induced Oxidative Stress in the Choroid Plexus Cell Line Z310. J. Neuroendocrinol 28 (9). doi:10.1111/jne.12404
Cui, Y.-M., Ao, M.-Z., Li, W., and Yu, L.-J. (2008). Effect of Glabridin from Glycyrrhiza Glabra on Learning and Memory in Mice. Planta Med. 74 (4), 377–380. doi:10.1055/s-2008-1034319
Dall'Acqua, S., Tomè, F., Vitalini, S., Agradi, E., and Innocenti, G. (2009). In vitro estrogenic Activity of Asplenium trichomanes L. Extracts and Isolated Compounds. J. Ethnopharmacol 122 (3), 424–429. doi:10.1016/j.jep.2009.02.012
Danielyan, L., Beer-Hammer, S., Stolzing, A., Schäfer, R., Siegel, G., Fabian, C., et al. (2014). Intranasal Delivery of Bone Marrow-Derived Mesenchymal Stem Cells, Macrophages, and Microglia to the Brain in Mouse Models of Alzheimer's and Parkinson's Disease. Cel Transpl. 23 (Suppl. 1), 123–139. doi:10.3727/096368914X684970
Danielyan, L., Schäfer, R., von Ameln-Mayerhofer, A., Bernhard, F., Verleysdonk, S., Buadze, M., et al. (2011). Therapeutic Efficacy of Intranasally Delivered Mesenchymal Stem Cells in a Rat Model of Parkinson Disease. Rejuvenation Res. 14 (1), 3–16. doi:10.1089/rej.2010.1130
Daulatzai, M. A. (2017). Cerebral Hypoperfusion and Glucose Hypometabolism: Key Pathophysiological Modulators Promote Neurodegeneration, Cognitive Impairment, and Alzheimer's Disease. J. Neurosci. Res. 95 (4), 943–972. doi:10.1002/jnr.23777
de Lima, P. F., Colombo, C. A., Chiorato, A. F., Yamaguchi, L. F., Kato, M. J., and Carbonell, S. A. M. (2014). Occurrence of Isoflavonoids in Brazilian Common Bean Germplasm (Phaseolus vulgarisL.). J. Agric. Food Chem. 62 (40), 9699–9704. doi:10.1021/jf5033312
Dhanasekaran, M., Tharakan, B., and Manyam, B. V. (2008). Antiparkinson Drug - Mucuna Pruriens Shows Antioxidant and Metal Chelating Activity. Phytother. Res. 22 (1), 6–11. doi:10.1002/ptr.2109
Dominguez, L. J., and Barbagallo, M. (2018). Nutritional Prevention of Cognitive Decline and Dementia. Acta Biomed. 89 (2), 276–290. doi:10.23750/abm.v89i2.7401
Doty, R. L., Tourbier, I., Ng, V., Neff, J., Armstrong, D., Battistini, M., et al. (2015). Influences of Hormone Replacement Therapy on Olfactory and Cognitive Function in Postmenopausal Women. Neurobiol. Aging 36 (6), 2053–2059. doi:10.1016/j.neurobiolaging.2015.02.028
Dyshlovoy, S. A., Tarbeeva, D., Fedoreyev, S., Busenbender, T., Kaune, M., Veselova, M., et al. (2020). Polyphenolic Compounds from Lespedeza Bicolor Root Bark Inhibit Progression of Human Prostate Cancer Cells via Induction of Apoptosis and Cell Cycle Arrest. Biomolecules 10 (3), 451. doi:10.3390/biom10030451
Echeverria, V., Burgess, S., Gamble-George, J., Zeitlin, R., Lin, X., Cao, C., et al. (2009). Sorafenib Inhibits Nuclear Factor Kappa B, Decreases Inducible Nitric Oxide Synthase and Cyclooxygenase-2 Expression, and Restores Working Memory in APPswe Mice. Neuroscience 162 (4), 1220–1231. doi:10.1016/j.neuroscience.2009.05.019
Elameen, A., Dragland, S., and Klemsdal, S. S. (2010). Bioactive Compounds Produced by Clones of Rhodiola Rosea Maintained in the Norwegian Germplasm Collection. Pharmazie 65 (8), 618–623.
Elameen, A., Kosman, V. M., Thomsen, M., Pozharitskaya, O. N., and Shikov, A. N. (2020). Variability of Major Phenyletanes and Phenylpropanoids in 16-Year-Old Rhodiola Rosea L. Clones in Norway. Molecules 25 (15), 3463. doi:10.3390/molecules25153463
Eom, S., Jin, S.-J., Jeong, H.-Y., Song, Y., Lim, Y., Kim, J.-I., et al. (2018). Kudzu Leaf Extract Suppresses the Production of Inducible Nitric Oxide Synthase, Cyclooxygenase-2, Tumor Necrosis Factor-Alpha, and Interleukin-6 via Inhibition of JNK, TBK1 and STAT1 in Inflammatory Macrophages. Ijms 19 (5), 1536. doi:10.3390/ijms19051536
Espinosa-Raya, J., Neri-Gómez, T., Orozco-Suárez, S., Campos, M. G., and Guerra-Araiza, C. (2012). Chronic Administration of Tibolone Modulates Anxiety-like Behavior and Enhances Cognitive Performance in Ovariectomized Rats. Horm. Behav. 61 (1), 76–83. doi:10.1016/j.yhbeh.2011.10.005
Ferella, L., Bastón, J. I., Bilotas, M. A., Singla, J. J., González, A. M., Olivares, C. N., et al. (2018). Active Compounds Present inRosmarinus Officinalis Leaves andScutellaria Baicalensis Root Evaluated as New Therapeutic Agents for Endometriosis. Reprod. BioMedicine Online 37 (6), 769–782. doi:10.1016/j.rbmo.2018.09.018
Frick, K. M. (2012). Building a Better Hormone Therapy? How Understanding the Rapid Effects of Sex Steroid Hormones Could Lead to New Therapeutics for Age-Related Memory Decline. Behav. Neurosci. 126 (1), 29–53. doi:10.1037/a0026660
Frick, K. M. (2009). Estrogens and Age-Related Memory Decline in Rodents: what Have We Learned and where Do We Go from Here?. Horm. Behav. 55 (1), 2–23. doi:10.1016/j.yhbeh.2008.08.015
Frick, K. M., Kim, J., and Koss, W. A. (2018). Estradiol and Hippocampal Memory in Female and Male Rodents. Curr. Opin. Behav. Sci. 23, 65–74. doi:10.1016/j.cobeha.2018.03.011
Frick, K. M. (2015). Molecular Mechanisms Underlying the Memory-Enhancing Effects of Estradiol. Horm. Behav. 74, 4–18. doi:10.1016/j.yhbeh.2015.05.001
Fu, X., Qin, T., Yu, J., Jiao, J., Ma, Z., Fu, Q., et al. (2019). Formononetin Ameliorates Cognitive Disorder via PGC-1α Pathway in Neuroinflammation Conditions in High-Fat Diet-Induced Mice. Cnsnddt 18 (7), 566–577. doi:10.2174/1871527318666190807160137
Furlan, F. H., Zanata, C., Damasceno, E. d. S., de Oliveira, L. P., da Silva, L. A., Colodel, E. M., et al. (2014). Toxic Myopathy and Acute Hepatic Necrosis in Cattle Caused by Ingestion of Senna obtusifolia (Sicklepod; Coffee senna) in Brazil. Toxicon 92, 24–30. doi:10.1016/j.toxicon.2014.09.007
Gardner, R. C., and Yaffe, K. (2015). Epidemiology of Mild Traumatic Brain Injury and Neurodegenerative Disease. Mol. Cell Neurosci. 66 (Pt B), 75–80. doi:10.1016/j.mcn.2015.03.001
Gasbarri, A., Tavares, M. C. H., Rodrigues, R. C., Tomaz, C., and Pompili, A. (2012). Estrogen, Cognitive Functions and Emotion: an Overview on Humans, Non-human Primates and Rodents in Reproductive Years. Rev. Neurosci. 23 (5-6), 587–606. doi:10.1515/revneuro-2012-0051
Gencel, V. B., Benjamin, M. M., Bahou, S. N., and Khalil, R. A. (2012). Vascular Effects of Phytoestrogens and Alternative Menopausal Hormone Therapy in Cardiovascular Disease. Mini Rev. Med. Chem. 12 (2), 149–174. doi:10.2174/138955712798995020
Georgakis, M. K., Beskou-Kontou, T., Theodoridis, I., Skalkidou, A., and Petridou, E. T. (2019). Surgical Menopause in Association with Cognitive Function and Risk of Dementia: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 106, 9–19. doi:10.1016/j.psyneuen.2019.03.013
Gerbarg, P. L., and Brown, R. P. (2016). Pause Menopause with Rhodiola Rosea, a Natural Selective Estrogen Receptor Modulator. Phytomedicine 23 (7), 763–769. doi:10.1016/j.phymed.2015.11.013
Giacobini, E., and Pepeu, G. (2018). Sex and Gender Differences in the Brain Cholinergic System and in the Response to Therapy of Alzheimer Disease with Cholinesterase Inhibitors. Car 15 (11), 1077–1084. doi:10.2174/1567205015666180613111504
Giacomelli, N., Yongping, Y., Huber, F., Ankli, A., and Weckerle, C. (2017). Angelica Sinensis (Oliv.) Diels: Influence of Value Chain on Quality Criteria and Marker Compounds Ferulic Acid and Z-Ligustilide. Medicines 4 (1), 14. doi:10.3390/medicines4010014
Gong, A. G. W., Lau, K. M., Xu, M. L., Lin, H. Q., Dong, T. T. X., Zheng, K. Y. Z., et al. (2016). The Estrogenic Properties of Danggui Buxue Tang, a Chinese Herbal Decoction, Are Triggered Predominantly by Calycosin in MCF-7 Cells. J. Ethnopharmacology 189, 81–89. doi:10.1016/j.jep.2016.05.035
González-Giraldo, Y., Forero, D. A., Echeverria, V., Garcia-Segura, L. M., and Barreto, G. E. (2019). Tibolone Attenuates Inflammatory Response by Palmitic Acid and Preserves Mitochondrial Membrane Potential in Astrocytic Cells through Estrogen Receptor Beta. Mol. Cell Endocrinol. 486, 65–78. doi:10.1016/j.mce.2019.02.017
González-Giraldo, Y., Garcia-Segura, L. M., Echeverria, V., and Barreto, G. E. (2018). Tibolone Preserves Mitochondrial Functionality and Cell Morphology in Astrocytic Cells Treated with Palmitic Acid. Mol. Neurobiol. 55 (5), 4453–4462. doi:10.1007/s12035-017-0667-3
Grishchenko, O. V., Kiselev, K. V., Tchernoded, G. K., Fedoreyev, S. A., Veselova, M. V., Bulgakov, V. P., et al. (2016). RolB Gene-Induced Production of Isoflavonoids in Transformed Maackia Amurensis Cells. Appl. Microbiol. Biotechnol. 100 (17), 7479–7489. doi:10.1007/s00253-016-7483-y
Hajirahimkhan, A., Mbachu, O., Simmler, C., Ellis, S. G., Dong, H., Nikolic, D., et al. (2018). Estrogen Receptor (ER) Subtype Selectivity Identifies 8-Prenylapigenin as an ERβ Agonist from Glycyrrhiza Inflata and Highlights the Importance of Chemical and Biological Authentication. J. Nat. Prod. 81 (4), 966–975. doi:10.1021/acs.jnatprod.7b01070
Hajirahimkhan, A., Simmler, C., Yuan, Y., Anderson, J. R., Chen, S.-N., Nikolić, D., et al. (2013). Evaluation of Estrogenic Activity of Licorice Species in Comparison with Hops Used in Botanicals for Menopausal Symptoms. PLoS One 8 (7), e67947. doi:10.1371/journal.pone.0067947
Hamson, D. K., Roes, M. M., and Galea, L. A. M. (2016). Sex Hormones and Cognition: Neuroendocrine Influences on Memory and Learning. Compr. Physiol. 6 (3), 1295–1337. doi:10.1002/cphy.c150031
Henderson, V. W., and Popat, R. A. (2011). Effects of Endogenous and Exogenous Estrogen Exposures in Midlife and Late-Life Women on Episodic Memory and Executive Functions. Neuroscience 191, 129–138. doi:10.1016/j.neuroscience.2011.05.059
Henderson, V. W., St. John, J. A., Hodis, H. N., McCleary, C. A., Stanczyk, F. Z., Karim, R., et al. (2013). Cognition, Mood, and Physiological Concentrations of Sex Hormones in the Early and Late Postmenopause. Proc. Natl. Acad. Sci. 110 (50), 20290–20295. doi:10.1073/pnas.1312353110
Hidalgo-Lanussa, O., Baez-Jurado, E., Echeverria, V., Ashraf, G. M., Sahebkar, A., Garcia-Segura, L. M., et al. (2020). Lipotoxicity, Neuroinflammation, Glial Cells and Oestrogenic Compounds. J. Neuroendocrinol 32 (1), e12776. doi:10.1111/jne.12776
Hidalgo-Lanussa, O., Ávila-Rodriguez, M., Baez-Jurado, E., Zamudio, J., Echeverria, V., Garcia-Segura, L. M., et al. (2018). Tibolone Reduces Oxidative Damage and Inflammation in Microglia Stimulated with Palmitic Acid through Mechanisms Involving Estrogen Receptor Beta. Mol. Neurobiol. 55 (7), 5462–5477. doi:10.1007/s12035-017-0777-y
Hillerns, P. I., and Wink, M. (2005). Binding of Flavonoids fromSophora Flavescensto the Rat Uterine Estrogen Receptor. Planta Med. 71 (11), 1065–1068. doi:10.1055/s-2005-871302
Hogervorst, E., Yaffe, K., Richards, M., and Huppert, F. A. (2009). Hormone Replacement Therapy to Maintain Cognitive Function in Women with Dementia. Cochrane Database Syst. Rev. 1, CD003799. doi:10.1002/14651858.CD003799.pub2
Hsieh, T. C., and Wu, J. M. (2002). Mechanism of Action of Herbal Supplement PC-SPES: Elucidation of Effects of Individual Herbs of PC-SPES on Proliferation and Prostate Specific Gene Expression in Androgen-dependent LNCaP Cells. Int. J. Oncol. 20 (3), 583–588. doi:10.3892/ijo.20.3.583
Huang, X., Wang, J., Chen, X., Liu, P., Wang, S., Song, F., et al. (2018). The Prenylflavonoid Xanthohumol Reduces Alzheimer-like Changes and Modulates Multiple Pathogenic Molecular Pathways in the Neuro2a/APPswe Cell Model of AD. Front. Pharmacol. 9, 199. doi:10.3389/fphar.2018.00199
Hugdahl, K., Thomsen, T., and Ersland, L. (2006). Sex Differences in Visuo-Spatial Processing: an fMRI Study of Mental Rotation. Neuropsychologia 44 (9), 1575–1583. doi:10.1016/j.neuropsychologia.2006.01.026
Hussain, A., Tabrez, E. S., Muhammad, A., and Peela, J. R. (2018). The Mechanisms of Dietary Phytoestrogen as a Potential Treatment and Prevention Agent against Alzheimer's Disease. Crit. Rev. Eukaryot. Gene Expr. 28 (4), 321–327. doi:10.1615/CritRevEukaryotGeneExpr.2018025847
Hwang, E. M., Ryu, Y. B., Kim, H. Y., Kim, D.-G., Hong, S.-G., Lee, J. H., et al. (2008). BACE1 Inhibitory Effects of Lavandulyl Flavanones from Sophora Flavescens. Bioorg. Med. Chem. 16 (14), 6669–6674. doi:10.1016/j.bmc.2008.05.080
Iarkov, A., Barreto, G. E., Grizzell, J. A., and Echeverria, V. (2020). Strategies for the Treatment of Parkinson's Disease: Beyond Dopamine. Front. Aging Neurosci. 12, 4. doi:10.3389/fnagi.2020.00004
Innocenti, G., Vegeto, E., Dall’Acqua, S., Ciana, P., Giorgetti, M., Agradi, E., et al. (2007). In vitro estrogenic Activity of Achillea millefolium L. Phytomedicine 14 (2-3), 147–152. doi:10.1016/j.phymed.2006.05.005
Jabir, M. S., Hussien, A. A., Sulaiman, G. M., Yaseen, N. Y., Dewir, Y. H., Alwahibi, M. S., et al. (2021). Green Synthesis of Silver Nanoparticles from Eriobotrya Japonica Extract: a Promising Approach against Cancer Cells Proliferation, Inflammation, Allergic Disorders and Phagocytosis Induction. Artif. Cell Nanomedicine, Biotechnol. 49 (1), 48–60. doi:10.1080/21691401.2020.1867152
Jash, K., Gondaliya, P., Kirave, P., Kulkarni, B., Sunkaria, A., and Kalia, K. (2020). Cognitive Dysfunction: A Growing Link between Diabetes and Alzheimer's Disease. Drug Dev. Res. 81 (2), 144–164. doi:10.1002/ddr.21579
Jiang, W.-P., Huang, S.-S., Matsuda, Y., Saito, H., Uramaru, N., Ho, H.-Y., et al. (2017). Protective Effects of Tormentic Acid, a Major Component of Suspension Cultures of Eriobotrya Japonica Cells, on Acetaminophen-Induced Hepatotoxicity in Mice. Molecules 22 (5), 830. doi:10.3390/molecules22050830
Ju, S., Seo, J. Y., Lee, S. K., Oh, J., and Kim, J.-S. (2021). Oral Administration of Hydrolyzed Red Ginseng Extract Improves Learning and Memory Capability of Scopolamine-Treated C57BL/6J Mice via Upregulation of Nrf2-Mediated Antioxidant Mechanism. J. Ginseng Res. 45 (1), 108–118. doi:10.1016/j.jgr.2019.12.005
Jung, H. A., Jin, S. E., Park, J.-S., and Choi, J. S. (2011). Antidiabetic Complications and Anti-alzheimer Activities of Sophoflavescenol, a Prenylated Flavonol fromSophora Flavescens, and its Structure-Activity Relationship. Phytother. Res. 25 (5), 709–715. doi:10.1002/ptr.3326
Jurado-Coronel, J. C., Ávila-Rodriguez, M., Echeverria, V., Hidalgo, O. A., Gonzalez, J., Aliev, G., et al. (2016). Implication of Green Tea as a Possible Therapeutic Approach for Parkinson Disease. CNS Neurol. Disord. Drug Targets 15 (3), 292–300. doi:10.2174/1871527315666160202125519
Jurado-Coronel, J. C., Cabezas, R., Ávila Rodríguez, M. F., Echeverria, V., García-Segura, L. M., and Barreto, G. E. (2018). Sex Differences in Parkinson's Disease: Features on Clinical Symptoms, Treatment Outcome, Sexual Hormones and Genetics. Front. Neuroendocrinology 50, 18–30. doi:10.1016/j.yfrne.2017.09.002
Kang, S. C., Lee, C. M., Choi, H., Lee, J. H., Oh, J. S., Kwak, J. H., et al. (2006). Evaluation of Oriental Medicinal Herbs for Estrogenic and Antiproliferative Activities. Phytother. Res. 20 (11), 1017–1019. doi:10.1002/ptr.1987
Kang, S. C., Lee, C. M., Choung, E. S., Bak, J. P., Bae, J. J., Yoo, H. S., et al. (2008). Anti-proliferative Effects of Estrogen Receptor-Modulating Compounds Isolated from Rheum Palmatum. Arch. Pharm. Res. 31 (6), 722–726. doi:10.1007/s12272-001-1218-1
Kapur, S., Craik, F. I. M., Jones, C., Brown, G. M., Houle, S., and Tulving, E. (1995). Functional Role of the Prefrontal Cortex in Retrieval of Memories: a PET Study. Neuroreport 6 (14), 1880–1884. doi:10.1097/00001756-199510020-00014
Keenan, P. A., Ezzat, W. H., Ginsburg, K., and Moore, G. J. (2001). Prefrontal Cortex as the Site of Estrogen's Effect on Cognition. Psychoneuroendocrinology 26 (6), 577–590. doi:10.1016/s0306-4530(01)00013-0
Kennedy, D., and Scholey, A. (2006). The Psychopharmacology of European Herbs with Cognition-Enhancing Properties. Cpd 12 (35), 4613–4623. doi:10.2174/138161206779010387
Kerschbaum, H. H., Hofbauer, I., Gföllner, A., Ebner, B., Bresgen, N., and Bäuml, K.-H. T. (2017). Sex, Age, and Sex Hormones Affect Recall of Words in a Directed Forgetting Paradigm. J. Neurosci. Res. 95 (1-2), 251–259. doi:10.1002/jnr.23973
Kim, B. R., Cho, Y.-C., Le, H. T. T., Vuong, H. L., Lee, S., and Cho, S. (2017). Suppression of Inflammation by the Rhizome of Anemarrhena Asphodeloides via Regulation of Nuclear Factor-Κb and P38 Signal Transduction Pathways in Macrophages. Biomed. Rep. 6 (6), 691–697. doi:10.3892/br.2017.895
Kim, H.-G., Kim, Y.-H., Lee, S.-B., Lee, J.-S., Chae, S.-W., Kim, D.-G., et al. (2020). An Herbal Formula CGplus Ameliorates Stress-Induced Hepatic Injury in a BALB/c Mouse Model. Front. Pharmacol. 11, 447. doi:10.3389/fphar.2020.00447
Kim, I. G., Kang, S. C., Kim, K. C., Choung, E. S., and Zee, O. P. (2008). Screening of Estrogenic and Antiestrogenic Activities from Medicinal Plants. Environ. Toxicol. Pharmacol. 25 (1), 75–82. doi:10.1016/j.etap.2007.09.002
Kim, T.-M., Paudel, K. R., and Kim, D.-W. (2020). Eriobotrya Japonica Leaf Extract Attenuates Airway Inflammation in Ovalbumin-Induced Mice Model of Asthma. J. Ethnopharmacology 253, 112082. doi:10.1016/j.jep.2019.112082
Kiyama, R. (2020). Nutritional Implications of Ginger: Chemistry, Biological Activities and Signaling Pathways. J. Nutr. Biochem. 86, 108486. doi:10.1016/j.jnutbio.2020.108486
Ko, Y.-H., Shim, K.-Y., Kim, S.-K., Seo, J.-Y., Lee, B.-R., Hur, K.-H., et al. (2019). Lespedeza Bicolor Extract Improves Amyloid Beta25 - 35-Induced Memory Impairments by Upregulating BDNF and Activating Akt, ERK, and CREB Signaling in Mice. Planta Med. 85 (17), 1363–1373. doi:10.1055/a-1018-5402
Kocoska-Maras, L., Zethraeus, N., Rådestad, A. F., Ellingsen, T., von Schoultz, B., Johannesson, M., et al. (2011). A Randomized Trial of the Effect of Testosterone and Estrogen on Verbal Fluency, Verbal Memory, and Spatial Ability in Healthy Postmenopausal Women. Fertil. Sterility 95 (1), 152–157. doi:10.1016/j.fertnstert.2010.05.062
Korol, D. L., and Wang, W. (2018). Using a Memory Systems Lens to View the Effects of Estrogens on Cognition: Implications for Human Health. Physiol. Behav. 187, 67–78. doi:10.1016/j.physbeh.2017.11.022
Kuraoka-Oliveira, Â. M., Radai, J. A. S., Leitão, M. M., Lima Cardoso, C. A., Silva-Filho, S. E., and Leite Kassuya, C. A. (2020). Anti-inflammatory and Anti-arthritic Activity in Extract from the Leaves of Eriobotrya Japonica. J. Ethnopharmacology 249, 112418. doi:10.1016/j.jep.2019.112418
Lanussa, O. H., Ávila-Rodriguez, M., García-Segura, L. M., González, J., Echeverria, V., Aliev, G., et al. (2016). Microglial Dependent Protective Effects of Neuroactive Steroids. CNS Neurol. Disord. Drug Targets 15 (2), 242–249. doi:10.2174/1871527315666160202122032
Lee, J., Kim, M., Lee, H., and Yang, W. (2018). Anemarrhena asphodeloides Bunge Ameliorates Osteoporosis by Suppressing Osteoclastogenesis. Int. J. Mol. Med. 42 (6), 3613–3621. doi:10.3892/ijmm.2018.3908
Lee, S.-J., Chung, H.-Y., Maier, C. G.-A., Wood, A. R., Dixon, R. A., and Mabry, T. J. (1998). Estrogenic Flavonoids fromArtemisia vulgarisL. J. Agric. Food Chem. 46 (8), 3325–3329. doi:10.1021/jf9801264
Li, F., Li, Y., Li, Q., and Shi, X. (2020). Eriobotrya Japonica Leaf Triterpenoid Acids Ameliorate Metabolic Syndrome in C57BL/6J Mice Fed with High-Fat Diet. Biomed. Pharmacother. 132, 110866. doi:10.1016/j.biopha.2020.110866
Li, K.-X., Sun, Q., Wei, L.-L., Du, G.-H., Huang, X., and Wang, J.-K. (2019). ERα Gene Promoter Methylation in Cognitive Function and Quality of Life of Patients with Alzheimer Disease. J. Geriatr. Psychiatry Neurol. 32 (4), 221–228. doi:10.1177/0891988719835325
Li, L., Xue, Z., Chen, L., Chen, X., Wang, H., and Wang, X. (2017). Puerarin Suppression of Aβ 1-42 -induced Primary Cortical Neuron Death Is Largely Dependent on ERβ. Brain Res. 1657, 87–94. doi:10.1016/j.brainres.2016.11.023
Li, X., Sdiri, M., Peng, J., Xie, Y., and Yang, B. B. (2020). Identification and Characterization of Chemical Components in the Bioactive Fractions of Cynomorium Coccineum that Possess Anticancer Activity. Int. J. Biol. Sci. 16 (1), 61–73. doi:10.7150/ijbs.38475
Lieu, C. A., Kunselman, A. R., Manyam, B. V., Venkiteswaran, K., and Subramanian, T. (2010). A Water Extract of Mucuna Pruriens Provides Long-Term Amelioration of Parkinsonism with Reduced Risk for Dyskinesias. Parkinsonism Relat. Disord. 16 (7), 458–465. doi:10.1016/j.parkreldis.2010.04.015
Lieu, C. A., Venkiteswaran, K., Gilmour, T. P., Rao, A. N., Petticoffer, A. C., Gilbert, E. V., et al. (2012). The Antiparkinsonian and Antidyskinetic Mechanisms ofMucuna Pruriensin the MPTP-Treated Nonhuman Primate. Evidence-Based Complement. Altern. Med. 2012, 1–10. doi:10.1155/2012/840247
Lim, S.-H., Ha, T.-Y., Ahn, J., and Kim, S. (2011). Estrogenic Activities of Psoralea Corylifolia L. Seed Extracts and Main Constituents. Phytomedicine 18 (5), 425–430. doi:10.1016/j.phymed.2011.02.002
Limón, D., Díaz, A., Hernandez, M., Fernandez-G, J. M., Torres-Martínez, A. C., Pérez-Severiano, F., et al. (2012). Neuroprotective Effect of the Aminoestrogen Prolame against Impairment of Learning and Memory Skills in Rats Injected with Amyloid-β-25-35 into the hippocampus. Eur. J. Pharmacol. 685 (1-3), 74–80. doi:10.1016/j.ejphar.2012.04.020
Liu, J., Burdette, J. E., Sun, Y., Deng, S., Schlecht, S. M., Zheng, W., et al. (2004). Isolation of Linoleic Acid as an Estrogenic Compound from the Fruits of Vitex Agnus-Castus L. (Chaste-berry). Phytomedicine 11 (1), 18–23. doi:10.1078/0944-7113-00331
Liu, J., Burdette, J. E., Xu, H., Gu, C., van Breemen, R. B., Bhat, K. P. L., et al. (2001). Evaluation of Estrogenic Activity of Plant Extracts for the Potential Treatment of Menopausal Symptoms. J. Agric. Food Chem. 49 (5), 2472–2479. doi:10.1021/jf0014157
Lorand, T., Vigh, E., and Garai, J. (2010). Hormonal Action of Plant Derived and Anthropogenic Non-steroidal Estrogenic Compounds: Phytoestrogens and Xenoestrogens. Cmc 17 (30), 3542–3574. doi:10.2174/092986710792927813
Loucks, T., and Berga, S. (2009). Does Postmenopausal Estrogen Use Confer Neuroprotection?. Semin. Reprod. Med. 27 (3), 260–274. doi:10.1055/s-0029-1216279
Mahaboob Basha, P., and Saumya, S. M. (2013). Suppression of Mitochondrial Oxidative Phosphorylation and TCA Enzymes in Discrete Brain Regions of Mice Exposed to High Fluoride: Amelioration by Panax Ginseng (Ginseng) and Lagerstroemia Speciosa (Banaba) Extracts. Cell Mol Neurobiol 33 (3), 453–464. doi:10.1007/s10571-013-9912-0
Maki, P., and Hogervorst, E. (2003). HRT and Cognitive Decline. Best Pract. Res. Clin. Endocrinol. Metab. 17 (1), 105–122. doi:10.1016/s1521-690x(02)00082-9
Maki, P. M., and Henderson, V. W. (2012). Hormone Therapy, Dementia, and Cognition: the Women's Health Initiative 10 Years on. Climacteric 15 (3), 256–262. doi:10.3109/13697137.2012.660613
Mann, D. M. A. (1989). The Pathogenesis and Progression of the Pathological Changes of Alzheimer's Disease. Ann. Med. 21 (2), 133–136. doi:10.3109/07853898909149200
Manyam, B. V., Dhanasekaran, M., and Hare, T. A. (2004). Effect of Antiparkinson Drug HP-200 (Mucuna Pruriens ) on the Central Monoaminergic Neurotransmitters. Phytother. Res. 18 (2), 97–101. doi:10.1002/ptr.1407
Manyam, B. V. (1990). Paralysis Agitans and Levodopa in ?Ayurveda?: Ancient Indian Medical Treatise. Mov Disord. 5 (1), 47–48. doi:10.1002/mds.870050112
Martin-Jiménez, C., Gaitán-Vaca, D. M., Areiza, N., Echeverria, V., Ashraf, G. M., González, J., et al. (2019). Astrocytes Mediate Protective Actions of Estrogenic Compounds after Traumatic Brain Injury. Neuroendocrinology 108 (2), 142–160. doi:10.1159/000495078
Martinkovich, S., Shah, D., Planey, S. L., and Arnott, J. A. (2014). Selective Estrogen Receptor Modulators: Tissue Specificity and Clinical Utility. Clin. Interv. Aging 9, 1437–1452. doi:10.2147/CIA.S66690
Masdeu, J. C., Zubieta, J. L., and Arbizu, J. (2005). Neuroimaging as a Marker of the Onset and Progression of Alzheimer's Disease. J. Neurol. Sci. 236 (1-2), 55–64. doi:10.1016/j.jns.2005.05.001
Mazo, N. A., Echeverria, V., Cabezas, R., Avila-Rodriguez, M., Tarasov, V. V., Yarla, N. S., et al. (2017). Medicinal Plants as Protective Strategies against Parkinson's Disease. Cpd 23 (28), 4180–4188. doi:10.2174/1381612823666170316142803
McGeer, P. L. (1986). Brain Imaging in Alzheimer's Disease. Br. Med. Bull. 42 (1), 24–28. doi:10.1093/oxfordjournals.bmb.a072093
Meziab, O., Kirby, K. A., Williams, B., Yaffe, K., Byers, A. L., and Barnes, D. E. (2014). Prisoner of War Status, Posttraumatic Stress Disorder, and Dementia in Older Veterans. Alzheimer's Demen. 10 (3 Suppl. l), S236–S241. doi:10.1016/j.jalz.2014.04.004
Miao, M., Yan, X., Guo, L., and Li, P. (2017). Effect of Cynomorium Total Flavone on Depression Model of Perimenopausal Rat. Saudi J. Biol. Sci. 24 (1), 139–148. doi:10.1016/j.sjbs.2016.09.009
Misiti, F., Sampaolese, B., Mezzogori, D., Orsini, F., Pezzotti, M., Giardina, B., et al. (2006). Protective Effect of Rhubarb Derivatives on Amyloid Beta (1-42) Peptide-Induced Apoptosis in IMR-32 Cells: a Case of Nutrigenomic. Brain Res. Bull. 71 (1-3), 29–36. doi:10.1016/j.brainresbull.2006.07.012
Mitchnick, K. A., Mendell, A. L., Wideman, C. E., Jardine, K. H., Creighton, S. D., Muller, A.-M., et al. (2019). Dissociable Involvement of Estrogen Receptors in Perirhinal Cortex-Mediated Object-Place Memory in Male Rats. Psychoneuroendocrinology 107, 98–108. doi:10.1016/j.psyneuen.2019.05.005
Moradi, F., Jahanian Sadatmahalleh, S., and Ziaei, S. (2018). The Effect of Hormone Replacement Therapy on Cognitive Function in Postmenopausal Women: An RCT. Ijrm 16 (12), 767. doi:10.18502/ijrm.v16i12.3682
Morale, M. C., Serra, P. A., L’Episcopo, F., Tirolo, C., Caniglia, S., Testa, N., et al. (2006). Estrogen, Neuroinflammation and Neuroprotection in Parkinson's Disease: Glia Dictates Resistance versus Vulnerability to Neurodegeneration. Neuroscience 138 (3), 869–878. doi:10.1016/j.neuroscience.2005.07.060
Morissette, M., Sweidi, S. A., Callier, S., and Di Paolo, T. (2008). Estrogen and SERM Neuroprotection in Animal Models of Parkinson's Disease. Mol. Cell Endocrinol. 290 (1-2), 60–69. doi:10.1016/j.mce.2008.04.008
Munro, C. A., Winicki, J. M., Schretlen, D. J., Gower, E. W., Turano, K. A., Muñoz, B., et al. (2012). Sex Differences in Cognition in Healthy Elderly Individuals. Aging Neuropsychol. Cogn. 19 (6), 759–768. doi:10.1080/13825585.2012.690366
Murashima, T., Yamasaki, M., Nishizawa, Y., Katayama, H., Tanigaki, Y., Saeki, Y., et al. (2009). Proliferation of Estrogen-Responsive Mouse Tumor Cell Line B-1F Stimulated by Saiboku-To, but Inhibited by Scutellaria Baicalensis, a Component of Saiboku-To. Oncol. Rep. 22 (2), 257–264. doi:10.3892/or_00000432
Napolitano, M., Costa, L., Piacentini, R., Grassi, C., Lanzone, A., and Gulino, A. (2014). 17β-Estradiol Protects Cerebellar Granule Cells against β-amyloid-induced Toxicity via the Apoptotic Mitochondrial Pathway. Neurosci. Lett. 561, 134–139. doi:10.1016/j.neulet.2013.11.030
Osorio, D., Pinzón, A., Martín-Jiménez, C., Barreto, G. E., and González, J. (2019). Multiple Pathways Involved in Palmitic Acid-Induced Toxicity: A System Biology Approach. Front. Neurosci. 13, 1410. doi:10.3389/fnins.2019.01410
Peng, W., Qin, R., Li, X., and Zhou, H. (2013). Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: a review. J. Ethnopharmacology 148 (3), 729–745. doi:10.1016/j.jep.2013.05.007
Perry, N. S. L., Houghton, P. J., Sampson, J., Theobald, A. E., Hart, S., Lis-Balchin, M., et al. (2010). In-vitro Activity of S. Lavandulaefolia (Spanish Sage) Relevant to Treatment of Alzheimer's Disease. J. Pharm. Pharmacol. 53 (10), 1347–1356. doi:10.1211/0022357011777846
Petrine, J. C. P., and Del Bianco‐Borges, B. (2021). The Influence of Phytoestrogens on Different Physiological and Pathological Processes: An Overview. Phytotherapy Res. 35 (1), 180–197. doi:10.1002/ptr.6816
Pinto-Almazán, R., Segura-Uribe, J. J., Farfán-García, E. D., and Guerra-Araiza, C. (2017). Effects of Tibolone on the Central Nervous System: Clinical and Experimental Approaches. Biomed. Res. Int. 2017, 1–9. doi:10.1155/2017/8630764
Pompili, A., Arnone, B., and Gasbarri, A. (2012). Estrogens and Memory in Physiological and Neuropathological Conditions. Psychoneuroendocrinology 37 (9), 1379–1396. doi:10.1016/j.psyneuen.2012.01.007
Powers, C. N., and Setzer, W. N. (2015). A Molecular Docking Study of Phytochemical Estrogen Mimics from Dietary Herbal Supplements. Silico Pharmacol. 3, 4. doi:10.1186/s40203-015-0008-z
Rabey, J. M., Vered, Y., Shabtai, H., Graff, E., and Korczyn, A. D. (1992). Improvement of Parkinsonian Features Correlate with High Plasma Levodopa Values after Broad Bean (Vicia faba) Consumption. J. Neurol. Neurosurg. Psychiatry 55 (8), 725–727. doi:10.1136/jnnp.55.8.725
Radenahmad, N., Saleh, F., Sawangjaroen, K., Rundorn, W., Withyachumnarnkul, B., and Connor, J. R. (2009). Young Coconut Juice Significantly Reduces Histopathological Changes in the Brain that Are Induced by Hormonal Imbalance: a Possible Implication to Postmenopausal Women. Histol. Histopathol 24 (6), 667–674. doi:10.14670/HH-24.667
Raeeszadeh, M., Mortazavi, P., and Atashin-Sadafi, R. (2021). The Antioxidant, Anti-inflammatory, Pathological, and Behavioural Effects of Medicago Sativa L. (Alfalfa) Extract on Brain Injury Caused by Nicotine in Male Rats. Evidence-Based Complement. Altern. Med. 2021, 1–9. doi:10.1155/2021/6694629
Raguthu, L., Varanese, S., Flancbaum, L., Tayler, E., and Di Rocco, A. (2009). Fava Beans and Parkinson's Disease: Useful 'natural Supplement' or Useless Risk?. Eur. J. Neurol. 16 (10), e171. doi:10.1111/j.1468-1331.2009.02766.x
Rahte, S., Evans, R., Eugster, P., Marcourt, L., Wolfender, J.-L., Kortenkamp, A., et al. (2013). Salvia Officinalis for Hot Flushes: towards Determination of Mechanism of Activity and Active Principles. Planta Med. 79 (9), 753–760. doi:10.1055/s-0032-1328552
Ramírez-Moreno, J. M., Salguero Bodes, I., Romaskevych, O., and Duran-Herrera, M. C. (2015). Consumo de habas (Vicia faba) y enfermedad de Parkinson: una fuente natural de L-dopa a tener en cuenta. Neurología 30 (6), 375–376. doi:10.1016/j.nrl.2013.08.006
Rattanatantikul, T., Maiprasert, M., Sugkraroek, P., and Bumrungpert, A. (2020). Efficacy and Safety of Nutraceutical on Menopausal Symptoms in Post-Menopausal Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Dietary Supplements Suppl, 1–15. doi:10.1080/19390211.2020.1853648
Real, M., Molina-Molina, J.-M., Jimenez, J., Diéguez, H. R., Fernández, M. F., and Olea, N. (2015). Assessment of Hormone-like Activities inGinkgo biloba,Elettaria cardamomumandPlantago Ovataextracts Usingin Vitroreceptor-specific Bioassays. Food Additives & Contaminants: A 32 (9), 1531–1541. doi:10.1080/19440049.2015.1071922
Rebhun, J. F., Du, Q., Hood, M., Guo, H., Glynn, K. M., Cen, H., et al. (2019). Evaluation of Selected Traditional Chinese Medical Extracts for Bone Mineral Density Maintenance: A Mechanistic Study. J. Traditional Complement. Med. 9 (3), 227–235. doi:10.1016/j.jtcme.2017.07.004
Ren, Y., Song, X., Tan, L., Guo, C., Wang, M., Liu, H., et al. (2020). A Review of the Pharmacological Properties of Psoralen. Front. Pharmacol. 11, 571535. doi:10.3389/fphar.2020.571535
Rietjens, I. M. C. M., Louisse, J., and Beekmann, K. (2017). The Potential Health Effects of Dietary Phytoestrogens. Br. J. Pharmacol. 174 (11), 1263–1280. doi:10.1111/bph.13622
Ritchie, K., Cramm, H., Aiken, A., Donnelly, C., and Goldie, K. (2019). Post‐traumatic Stress Disorder and Dementia in Veterans: A Scoping Literature Review. Int. J. Ment. Health Nurs 28 (5), 1020–1034. doi:10.1111/inm.12601
Rocca, W. A., Grossardt, B. R., and Shuster, L. T. (2011). Oophorectomy, Menopause, Estrogen Treatment, and Cognitive Aging: Clinical Evidence for a Window of Opportunity. Brain Res. 1379, 188–198. doi:10.1016/j.brainres.2010.10.031
Rowe, I. J., and Baber, R. J. (2021). The Effects of Phytoestrogens on Postmenopausal Health. Climacteric 24 (1), 57–63. doi:10.1080/13697137.2020.1863356
Salehi, B., Abu‐Reidah, I. M., Sharopov, F., Karazhan, N., Sharifi‐Rad, J., Akram, M., et al. (2021). Vicia Plan Ts-A Comprehensive Review on Chemical Composition and Phytopharmacology. Phytotherapy Res. 35 (2), 790–809. doi:10.1002/ptr.6863
Sano, M., Jacobs, D., Andrews, H., Bell, K., Graff-Radford, N., Lucas, J., et al. (2008). A Multi-Center, Randomized, Double Blind Placebo-Controlled Trial of Estrogens to Prevent Alzheimer's Disease and Loss of Memory in Women: Design and Baseline Characteristics. Clin. Trials 5 (5), 523–533. doi:10.1177/1740774508096313
Saunders-Pullman, R. (2003). Estrogens and Parkinson Disease: Neuroprotective, Symptomatic, Neither, or Both?. Endo 21 (1), 81–88. doi:10.1385/ENDO:21:1:81
Seidlová-Wuttke, D., Jarry, H., Becker, T., Christoffel, V., and Wuttke, W. (2003). Pharmacology of Cimicifuga Racemosa Extract BNO 1055 in Rats: Bone, Fat and Uterus. Maturitas 44 (Suppl. 1), S39–S50. doi:10.1016/s0378-5122(02)00347-x
Seidlova-Wuttke, D., Jarry, H., and Wuttke, W. (2013). Plant Derived Alternatives for Hormone Replacement Therapy (HRT). Horm. Mol. Biol. Clin. Investig. 16 (1), 35–45. doi:10.1515/hmbci-2013-0024
Seo, J.-S., Jung, E.-Y., Kim, J.-H., Lyu, Y.-S., Han, P.-L., and Kang, H.-W. (2010a). A Modified Preparation (LMK03) of the Oriental Medicine Jangwonhwan Reduces Aβ1-42 Level in the Brain of Tg-APPswe/PS1dE9 Mouse Model of Alzheimer Disease. J. Ethnopharmacology 130 (3), 578–585. doi:10.1016/j.jep.2010.05.055
Seo, J.-S., Yun, J.-H., Baek, I.-S., Leem, Y.-H., Kang, H.-W., Cho, H. K., et al. (2010b). Oriental Medicine Jangwonhwan Reduces Aβ(1-42) Level and β-amyloid Deposition in the Brain of Tg-APPswe/PS1dE9 Mouse Model of Alzheimer Disease. J. Ethnopharmacology 128 (1), 206–212. doi:10.1016/j.jep.2010.01.014
Sherwin, B. B. (2012). Estrogen and Cognitive Functioning in Women: Lessons We Have Learned. Behav. Neurosci. 126 (1), 123–127. doi:10.1037/a0025539
Sherwin, B. B., and Grigorova, M. (2011). Differential Effects of Estrogen and Micronized Progesterone or Medroxyprogesterone Acetate on Cognition in Postmenopausal Women. Fertil. Sterility 96 (2), 399–403. doi:10.1016/j.fertnstert.2011.05.079
Shikov, A. N., Kosman, V. M., Flissyuk, E. V., Smekhova, I. E., Elameen, A., and Pozharitskaya, O. N. (2020). Natural Deep Eutectic Solvents for the Extraction of Phenyletanes and Phenylpropanoids of Rhodiola Rosea L. Molecules 25 (8), 1826. doi:10.3390/molecules25081826
Shilling, V., Jenkins, V., Fallowfield, L., and Howell, A. (2001). The Effects of Oestrogens and Anti-oestrogens on Cognition. The Breast 10 (6), 484–491. doi:10.1054/brst.2001.0311
Siani, F., Greco, R., Levandis, G., Ghezzi, C., Daviddi, F., Demartini, C., et al. (2017). Influence of Estrogen Modulation on Glia Activation in a Murine Model of Parkinson's Disease. Front. Neurosci. 11, 306. doi:10.3389/fnins.2017.00306
Simons, R., Vincken, J.-P., Mol, L. A. M., The, S. A. M., Bovee, T. F. H., Luijendijk, T. J. C., et al. (2011). Agonistic and Antagonistic Estrogens in Licorice Root (Glycyrrhiza Glabra). Anal. Bioanal. Chem. 401 (1), 305–313. doi:10.1007/s00216-011-5061-9
Simpson, J. E., Ince, P. G., Lace, G., Forster, G., Shaw, P. J., Matthews, F., et al. (2010). Astrocyte Phenotype in Relation to Alzheimer-type Pathology in the Ageing Brain. Neurobiol. Aging 31 (4), 578–590. doi:10.1016/j.neurobiolaging.2008.05.015
Slaven, L., and Lee, C. (1997). Mood and Symptom Reporting Among Middle-Aged Women: the Relationship between Menopausal Status, Hormone Replacement Therapy, and Exercise Participation. Health Psychol. 16 (3), 203–208. doi:10.1037//0278-6133.16.3.20310.1037/0278-6133.16.3.203
Slavin, J. L., and Lloyd, B. (2012). Health Benefits of Fruits and Vegetables. Adv. Nutr. 3 (4), 506–516. doi:10.3945/an.112.002154
Soni, M., and Hogervorst, E. (2014). Premature Ovarian Insufficiency and Neurological Function. Minerva Endocrinol. 39 (3), 189–199.
Soriano-Melgar, Lde. A., Alcaraz-Meléndez, L., Méndez-Rodríguez, L. C., Puente, M. E., Rivera-Cabrera, F., and Zenteno-Savín, T. (2014). Antioxidant Responses of Damiana (Turnera Diffusa Willd) to Exposure to Artificial Ultraviolet (UV) Radiation in an In Vitro Model; Part Ii; UV-B Radiation. Nutr. Hosp. 29 (5), 1116–1122. doi:10.3305/nh.2014.29.5.7092
Souza, N. C., de Oliveira, J. M., Morrone, M. d. S., Albanus, R. D. O., Amarante, M. d. S. M., Camillo, C. d. S., et al. (2016). Turnera Subulata Anti-inflammatory Properties in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. J. Med. Food 19 (10), 922–930. doi:10.1089/jmf.2016.0047
Spencer-Segal, J. L., Tsuda, M. C., Mattei, L., Waters, E. M., Romeo, R. D., Milner, T. A., et al. (2012). Estradiol Acts via Estrogen Receptors Alpha and Beta on Pathways Important for Synaptic Plasticity in the Mouse Hippocampal Formation. Neuroscience 202, 131–146. doi:10.1016/j.neuroscience.2011.11.035
Staglich, H. (1948). Possible Uses of Rheum Palmatum and RH. Undulatum. Suddeutsch Apoth Ztg 88 (12), 332.
Sun, J., Murata, T., and Shigemori, H. (2020). Inhibitory Activities of Phenylpropanoids from Lycopus Lucidus on Amyloid Aggregation Related to Alzheimer's Disease and Type 2 Diabetes. J. Nat. Med. 74 (3), 579–583. doi:10.1007/s11418-020-01398-6
Svensson, A.-L., and Nordberg, A. (1999). β-Estradiol Attenuate Amyloid β-peptide Toxicity via Nicotinic Receptors. Neuroreport 10 (17), 3485–3489. doi:10.1097/00001756-199911260-00004
Szego, E. M., Csorba, A., Janáky, T., Kékesi, K. A., Abrahám, I. M., Mórotz, G. M., et al. (2011). Effects of Estrogen on Beta-Amyloid-Induced Cholinergic Cell Death in the Nucleus Basalis Magnocellularis. Neuroendocrinology 93 (2), 90–105. doi:10.1159/000321119
Taha, M. M. E., Salga, M. S., Ali, H. M., Abdulla, M. A., Abdelwahab, S. I., and Hadi, A. H. A. (2012). Gastroprotective Activities of Turnera Diffusa Willd. Ex Schult. Revisited: Role of Arbutin. J. Ethnopharmacology 141 (1), 273–281. doi:10.1016/j.jep.2012.02.030
Tang, S.-S., Ren, Y., Ren, X.-Q., Cao, J.-R., Hong, H., Ji, H., et al. (2019). ERα And/or ERβ Activation Ameliorates Cognitive Impairment, Neurogenesis and Apoptosis in Type 2 Diabetes Mellitus Mice. Exp. Neurol. 311, 33–43. doi:10.1016/j.expneurol.2018.09.002
Tang, S.-S., Ren, Y., Xu, L.-J., Cao, J.-R., Hong, H., Ji, H., et al. (2018). Activation of ERα And/or ERβ Ameliorates Cognitive Impairment and Apoptosis in Streptozotocin-Induced Diabetic Mice. Horm. Behav. 105, 95–103. doi:10.1016/j.yhbeh.2018.08.002
Tang, Y., Min, Z., Xiang, X.-J., Liu, L., Ma, Y.-L., Zhu, B.-L., et al. (2018). Estrogen-related Receptor Alpha Is Involved in Alzheimer's Disease-like Pathology. Exp. Neurol. 305, 89–96. doi:10.1016/j.expneurol.2018.04.003
Tao, R., Miao, L., Yu, X., Orgah, J. O., Barnabas, O., Chang, Y., et al. (2019). Cynomorium Songaricum Rupr Demonstrates Phytoestrogenic or Phytoandrogenic like Activities that Attenuates Benign Prostatic Hyperplasia via Regulating Steroid 5-α-Reductase. J. Ethnopharmacology 235, 65–74. doi:10.1016/j.jep.2019.01.038
Thaung Zaw, J. J., Howe, P. R. C., and Wong, R. H. X. (2017). Does Phytoestrogen Supplementation Improve Cognition in Humans? A Systematic Review. Ann. N.Y. Acad. Sci. 1403 (1), 150–163. doi:10.1111/nyas.13459
Tian, F.-z., Chang, H.-s., Liu, J.-x., Zheng, J., Cheng, D., and Lu, Y. (2019). Cynomorium Songaricum Extract Alleviates Memory Impairment through Increasing CREB/BDNF via Suppression of p38MAPK/ERK Pathway in Ovariectomized Rats. Evidence-Based Complement. Altern. Med. 2019, 1–10. doi:10.1155/2019/9689325
Tsolaki, M., Eleftheriou, M., and Karavida, N. (2009). Alzheimer's Dementia and Post-traumatic Stress Disorder Differences and Similarities in Neuroimaging. Hell J. Nucl. Med. 12 (1), 41–46.
Vaidya, A. B., Rajagopalan, T. G., Mankodi, N. A., Antarkar, D. S., Tathed, P. S., Purohit, A. V., et al. (1978). Treatment of Parkinson's Disease with the Cowhage Plant-Mucuna Pruriens Bak. Neurol. India 26 (4), 171–176.
Vazirinejad, R., Ayoobi, F., Arababadi, M. K., Eftekharian, M. M., Darekordi, A., Goudarzvand, M., et al. (2014). Effect of Aqueous Extract of Achillea millefolium on the Development of Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice. Indian J. Pharmacol. 46 (3), 303–308. doi:10.4103/0253-7613.132168
Venkateshwarlu, E., Reddy, K. P., and Dilip, D. (2016). Potential of Vigna Radiata (L.) Sprouts in the Management of Inflammation and Arthritis in Rats: Possible Biochemical Alterations. Indian J. Exp. Biol. 54 (1), 37–43.
Venkateswaran, S., and Pari, L. (2002). Antioxidant Effect of Phaseolus vulgaris in Streptozotocin-Induced Diabetic Rats. Asia Pac. J. Clin. Nutr. 11 (3), 206–209. doi:10.1046/j.1440-6047.2002.00292.x
Venkateswaran, S., Pari, L., and Saravanan, G. (2002). Effect ofPhaseolus Vulgarison Circulatory Antioxidants and Lipids in Rats with Streptozotocin-Induced Diabetes. J. Med. Food 5 (2), 97–103. doi:10.1089/109662002760178186
Vered, Y., Grosskopf, I., Palevitch, D., Harsat, A., Charach, G., Weintraub, M., et al. (1997). The Influence ofVicia faba(Broad Bean) Seedlings on Urinary Sodium Excretion. Planta Med. 63 (3), 237–240. doi:10.1055/s-2006-957661
Vesga-Jiménez, D. J., Hidalgo-Lanussa, O., Baez-Jurado, E., Echeverria, V., Ashraf, G. M., Sahebkar, A., et al. (2019). Raloxifene Attenuates Oxidative Stress and Preserves Mitochondrial Function in Astrocytic Cells upon Glucose Deprivation. J. Cel Physiol 234 (3), 2051–2057. doi:10.1002/jcp.27481
Viña, J., and Lloret, A. (2010). Why Women Have More Alzheimer's Disease Than Men: Gender and Mitochondrial Toxicity of Amyloid-β Peptide. Jad 20 (Suppl. 2), S527–S533. doi:10.3233/JAD-2010-100501
Wang, R., Li, Y. H., Xu, Y., Li, Y. B., Wu, H. L., Guo, H., et al. (2010). Curcumin Produces Neuroprotective Effects via Activating Brain-Derived Neurotrophic factor/TrkB-dependent MAPK and PI-3K Cascades in Rodent Cortical Neurons. Prog. Neuropsychopharmacol. Biol. Psychiatry 34 (1), 147–153. doi:10.1016/j.pnpbp.2009.10.016
Wang, X., Tao, R., Yang, J., Miao, L., Wang, Y., Munyangaju, J. E., et al. (2017). Compounds from Cynomorium Songaricum with Estrogenic and Androgenic Activities Suppress the Oestrogen/Androgen-Induced BPH Process. Evidence-Based Complement. Altern. Med. 2017, 1–12. doi:10.1155/2017/6438013
Wei, L., Lv, S., Huang, Q., Wei, J., Zhang, S., Huang, R., et al. (2015). Pratensein Attenuates Aβ-Induced Cognitive Deficits in Rats: Enhancement of Synaptic Plasticity and Cholinergic Function. Fitoterapia 101, 208–217. doi:10.1016/j.fitote.2015.01.017
Wozniak, D., Janda, B., Kapusta, I., Oleszek, W., and Matkowski, A. (2010). Antimutagenic and Anti-oxidant Activities of Isoflavonoids from Belamcanda Chinensis (L.) DC. Mutat. Research/Genetic Toxicol. Environ. Mutagenesis 696 (2), 148–153. doi:10.1016/j.mrgentox.2010.01.004
Wu, J., Pan, Z., Wang, Z., Zhu, W., Shen, Y., Cui, R., et al. (2012). Ginsenoside Rg1 Protection against β-amyloid Peptide-Induced Neuronal Apoptosis via Estrogen Receptor α and Glucocorticoid Receptor-dependent Anti-protein Nitration Pathway. Neuropharmacology 63 (3), 349–361. doi:10.1016/j.neuropharm.2012.04.005
Wuttke, W., Jarry, H., Haunschild, J., Stecher, G., Schuh, M., and Seidlova-Wuttke, D. (2014). The Non-estrogenic Alternative for the Treatment of Climacteric Complaints: Black Cohosh (Cimicifuga or Actaea Racemosa). J. Steroid Biochem. Mol. Biol. 139, 302–310. doi:10.1016/j.jsbmb.2013.02.007
Wuttke, W., and Seidlova-Wuttke, D. (2014). Foreword to the Special Issue on Phytoestrogens and beyond. J. Steroid Biochem. Mol. Biol. 139, 223–224. doi:10.1016/j.jsbmb.2012.11.004
Xie, Q.-F., Xie, J.-H., Dong, T. T. X., Su, J.-Y., Cai, D.-K., Chen, J.-P., et al. (2012). Effect of a Derived Herbal Recipe from an Ancient Chinese Formula, Danggui Buxue Tang, on Ovariectomized Rats. J. Ethnopharmacology 144 (3), 567–575. doi:10.1016/j.jep.2012.09.041
Xing, G., Dong, M., Li, X., Zou, Y., Fan, L., Wang, X., et al. (2011). Neuroprotective Effects of Puerarin against Beta-Amyloid-Induced Neurotoxicity in PC12 Cells via a PI3K-dependent Signaling Pathway. Brain Res. Bull. 85 (3-4), 212–218. doi:10.1016/j.brainresbull.2011.03.024
Xing, Y., Qin, W., Li, F., Jia, X.-F., and Jia, J. (2013). Associations between Sex Hormones and Cognitive and Neuropsychiatric Manifestations in Vascular Dementia (VaD). Arch. Gerontol. Geriatr. 56 (1), 85–90. doi:10.1016/j.archger.2012.10.003
Xu, X., Niu, L., Liu, Y., Pang, M., Lu, W., Xia, C., et al. (2020). Study on the Mechanism of Gegen Qinlian Decoction for Treating Type II Diabetes Mellitus by Integrating Network Pharmacology and Pharmacological Evaluation. J. Ethnopharmacology 262, 113129. doi:10.1016/j.jep.2020.113129
Yang, J., Sun, Y., Xu, F., Liu, W., Hayashi, T., Hattori, S., et al. (2019). Silibinin Protects Rat Pancreatic β‐cell through Up‐regulation of Estrogen Receptors' Signaling against Amylin‐ or Aβ 1-42 ‐induced Reactive Oxygen Species/reactive Nitrogen Species Generation. Phytotherapy Res. 33 (4), 998–1009. doi:10.1002/ptr.6293
Yoo, H. H., Kim, T., Ahn, S., Kim, Y. J., Kim, H. Y., Piao, X. L., et al. (2005). Evaluation of the Estrogenic Activity of Leguminosae Plants. Biol. Pharm. Bull. 28 (3), 538–540. doi:10.1248/bpb.28.538
You, F., Li, Q., Jin, G., Zheng, Y., Chen, J., and Yang, H. (2017). Genistein Protects against Aβ25-35 Induced Apoptosis of PC12 Cells through JNK Signaling and Modulation of Bcl-2 Family Messengers. BMC Neurosci. 18 (1), 12. doi:10.1186/s12868-016-0329-9
Zayed, S. M., Hassan, A., and Elghamry, M. I. (1964). Estrogenic Substances from Egyptian Glycyrrhiza Glabra. II. Beta-Sitosterol as an Estrogenic Principle. Zentralbl Veterinarmed A 11 (5), 476–482.
Zhang, C. Z., Wang, S. X., Zhang, Y., Chen, J. P., and Liang, X. M. (2005). In vitro estrogenic Activities of Chinese Medicinal Plants Traditionally Used for the Management of Menopausal Symptoms. J. Ethnopharmacology 98 (3), 295–300. doi:10.1016/j.jep.2005.01.033
Zhao, J., Dasmahapatra, A. K., Khan, S. I., and Khan, I. A. (2008). Anti-aromatase Activity of the Constituents from Damiana (Turnera Diffusa). J. Ethnopharmacology 120 (3), 387–393. doi:10.1016/j.jep.2008.09.016
Zhao, L., Mao, Z., Chen, S., Schneider, L. S., and Brinton, R. D. (2013). Early Intervention with an Estrogen Receptor β-Selective Phytoestrogenic Formulation Prolongs Survival, Improves Spatial Recognition Memory, and Slows Progression of Amyloid Pathology in a Female Mouse Model of Alzheimer's Disease. Jad 37 (2), 403–419. doi:10.3233/jad-122341
Keywords: Alzheimer's disease, Parkinson's disease, estrogenic plants, neurodegeneration, neuroprotection, menopause, cognitive impairment
Citation: Echeverria V, Echeverria F, Barreto GE, Echeverría J and Mendoza C (2021) Estrogenic Plants: to Prevent Neurodegeneration and Memory Loss and Other Symptoms in Women After Menopause. Front. Pharmacol. 12:644103. doi: 10.3389/fphar.2021.644103
Received: 19 December 2020; Accepted: 15 April 2021;
Published: 20 May 2021.
Edited by:
Javad Sharifi-Rad, Shahid Beheshti University of Medical Sciences, IranReviewed by:
Sefirin Djiogue, University of Yaounde I, CameroonCopyright © 2021 Echeverria, Echeverria, Barreto, Echeverría and Mendoza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentina Echeverria, ZWNoZXZlcnJpYS52YWxlbnRpbmFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.