
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 15 April 2021
Sec. Drugs Outcomes Research and Policies
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.637667
This article is part of the Research TopicNew Horizons in Health-Promoting: From Methods to Implementation ScienceView all 24 articles
Aims: To compare the efficacy of five kinds of antiangiogenic drugs in the treatment of diabetic macular edema
Methods: A comprehensive search of seven databases without language restrictions includes PubMed, EMBASE, Web of Science, CBM, the Cochrane Library, CNKI, and WanFang date. All literature used was published before October 2020. Eligible randomized trials were screened for inclusion in this study, and Bayesian framework was used to perform a network meta-analysis (NMA). Data on the mean change of best-corrected visual acuity (BCVA), central macular thickness (CMT) and intraocular pressure (IOP) at 6 months were extracted.
Results: 25 randomized controlled trials (RCTs) that covered 2214 eyes, which received treatment of more than 3 months durations were included. In the pooled pair-wise meta-analysis, there was no statistically significant difference between all treatments. The same result was observed in the network meta-analysis with 0–37.82% Global I-squared. For BCVA at 6 months, conbercept and ranibizumab may be favorable than bevacizumab, aflibercept, triamcinolone acetonide and sham injections according to the ranking probabilities. As for CMT at 6 months, ranibizumab may be the most effective compared to bevacizumab, aflibercept and triamcinolone acetonide. In terms of IOP at 6 months, ranibizumab have better effect than bevacizumab, triamcinolone acetonide and sham injections. The results of sensitivity analysis also confirm it.
Conclusion: The analysis confirms that ranibizumab may be the most favorable for BCVA improvement and have a stronger efficacy in decreasing CMT and IOP than other drugs when taking all the indicators into consideration. This conclusion may provide clinical evidence to guide treatment decisions. However, more high-quality randomized controlled trials will be necessary to further confirm this.
Diabetic macular edema is one of the most common and serious complications of diabetes (Sayin et al., 2015). It is due to the unstable state of long-term high blood glucose levels that causes damage to the retinal vasculature, resulting in enhanced permeability of the retinal blood vessels and accumulation of fluid in the macular area, causing diabetic macular edema (Shin et al., 2014). Once diabetic macular edema occurs, it can significantly reduce vision. Data shows that about one-third of people with diabetes will develop retinopathy, and about 2.6% of blind syndromes worldwide can be contributed to diabetes (Leasher et al., 2016). With the development of a large number of randomized clinical trials, intravitreal injection has gradually replaced the method of grid laser photocoagulation, and has significantly improved the treatment efficacy of macular edema. Diabetic macular edema treatment guidelines issued by European Retina Specialist Association (EURETINA) in 2017 pointed out that anti-vascular growth factor is a safe and effective first-line treatment for diabetic macular edema (Schmidt-Erfurth et al., 2017).
There are many types of antiangiogenic drugs, the effectiveness of various drugs is different, and there is a lack of direct comparison evidence for these drugs, making it difficult to evaluate the effectiveness of multiple drugs. Moreover, there are few studies comparing the efficacy of drugs alone, and the number of included literatures is not enough. This is the first study which has estimated and compared the effectiveness of all triple antiangiogenic therapy that has been studied in randomized trials. Compared to same type meta-analysis, this study included a full range of antiangiogenic drugs and compared the differences in efficacy between single drugs. In addition, this study is of a higher standard, due to accurate experimental types of randomized trials, identifying interventions outside of laser interference, unity of follow-up time and outcome indicators. We aimed to evaluate the efficacy of five kinds of antiangiogenic drugs in the treatment of diabetic macular edema. This method can provide clinical evidence to guide treatment decisions.
The study was conducted in accordance with the guidelines of the Cochrane Multiple Interventions Methods Group (Chaimani et al., 2017).
PubMed, EMBASE, Web of Science, CBM, the Cochrane Library, CNKI, and WanFang data were used to identify relevant studies published before October 2020. The search terms (diabetic macular edema) and (ranibizumab or bevacizumab or aflibercept or conbercept or pegaptanib or triamcinolone acetonide or dexamethasone or fluocinolone) and (randomized controlled trial or RCT or random) were used. All available literatures were considered for review. Manual search was also performed to ensure complete retrieval using references of key articles.
All investigators independently assessed all trials for eligibility and extracted data. After the initial extraction was completed, the results were checked by each investigator. In the case of disagreements, consensus was reached through discussion. The full text of all the articles were obtained and the same eligibility criteria was used to determine which, if any, to exclude at this stage. The follow-up time of each randomized trial, the average age and sex ratio of patients, grouping, intervention measures, and outcome indicators were recorded. Each reviewer independently read each article, assessed the completeness of the data extraction, and confirmed the quality rating.
The included literature needs to meet the following criteria:1)Meets the relevant diagnostic criteria of type Ⅰ or Ⅱ diabetes complicated with diabetic macular edema; 2) Interventions can only be antiangiogenic drugs, including ranibizumab or bevacizumab or aflibercept or conbercept or triamcinolone acetonide or pegaptanib or dexamethasone or fluocinolone; 3) Outcome measures included BCVA or CMT or IOP more than 3 months; 4) Study design must be randomized controlled trials; 5) Only included patients who had not received laser treatment for at least 3 months before trials. Trials that included other drugs, trials that had incomplete or incalculable data, trials that did not have baseline data and trials that had left and right eyes compared to each other were excluded.
Four independent reviewers (MR W, WT Z, R Z and YF W) reviewed all the included studies. Any disagreements were discussed and rechecked together. The Cochrane Collaboration using the Cochrane risk of bias tool of Review Manager were used for assessing risk of bias method in randomized controlled trials. After all the outcomes were evaluated, a summary of confidence examining the effectiveness of a comparison between ranibizumab and other drugs was created using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (Nikolakopoulou et al., 2020).
BCVA, CMT at 6 months were set as primary visual outcomes. In addition, IOP at 6 months was also used as important outcome indicator.
In order to make the results more reliable and reduce the heterogeneity between data, the mean changes before and after treatment were calculated. This was an intention-to-treat analysis based on random effect model. Summarized effect size was calculated as mean differences (MD). First, a direct meta-analysis of trials that compared different treatments was done. Then, the Bayesian network meta-analysis was conducted to compare between different treatment that no head-to-head trials.
Pairwise meta-analyses were conducted beforehand for every treatment comparison. Heterogeneity across individual studies was assessed by the Cochran Q test (Chi-squared) and Higgins I-squared inconsistency statistic. If there was no significant heterogeneity (p > 0.05, I-squared <50%), a fixed effects model was used. Otherwise, a random effects model was used. Given the low heterogeneity detected across all direct meta-analyses, fixed-effects estimates were reported.
This Bayesian network meta-analysis was done by R with gemtc package. Within the Bayesian hierarchical model frameworks, the number of chains was 3; thinning interval, 10; tuning iterations, 10,000; simulation iterations, 60,000; and initial values scaling, 2.5. Moreover, all the treatments were ranked based on the analysis of ranking probabilities.
Furthermore, the analysis at 6 months is star-shaped and does not contain a closed loop, so local inconsistency of the network cannot be assessed. Global inconsistency was judged by using the value of deviance information criterion (DIC) between the consistency and inconsistency model. If the DIC difference was within five, the data was generally considered consistent. To demonstrate the robustness of the results, a sensitivity analysis was performed. According to the follow-up time, the efficacy evaluation was brought forward to 3 months and later to 12 months, to see whether the conclusion was consistent with the follow-up time of 6 months. Inconsistency as well as statistical disagreement of direct and indirect evidence was assessed by using node-split method in sensitivity analysis. Moreover, a p > 0.05 was considered as no significant inconsistency. All data was analyzed by using R 3.6.3.
Figure 1 shows the detailed steps of the literature selection process. Through searching the literature databases, 2538 relevant literatures were found, and 2424 duplications and unrelated literatures were excluded. Then, literatures were further selected by reading the full text, excluding substandard samples, substandard interventions and substandard research design, and finally included 25 articles.
In total, 25 trials were included in the network meta-analysis, the basic characteristics of them were presented in Table 1. A network of eligible comparisons for the multiple-treatment meta-analysis was constructed (Figure 2). Five treatments were analyzed: ranibizumab, bevacizumab, aflibercept, conbercept, triamcinolone acetonide. Most trials (22 of 25) were two-grouped studies (Jonas et al., 2006; Paccola et al., 2007; Wang et al., 2009; Ma, 2010; Massin et al., 2010; Marey and Ellakwa, 2011; Lim et al., 2012; Wan et al., 2013; Ekinci et al., 2014; Sonoda et al., 2014; Pan, 2016; Xue et al., 2016; Yan et al., 2016; Zhang et al., 2016b; Zhou, 2016; Fouda and Bahgat, 2017; Neto et al., 2017; Li et al., 2018; Ji et al., 2019; Ren et al., 2019; Schlingemann et al., 2019; Rodrigues et al., 2020) and the rest were three-grouped studies (Genentech, Inc., 2007; Baghi et al., 2017; Jabbarpoor et al., 2018). Overall, 2214 eyes were randomly assigned to one or two of the five antiangiogenic treatments or to the sham injection and were included in the network meta-analysis.
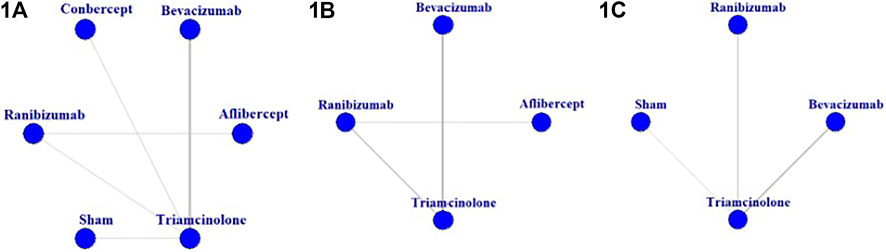
FIGURE 2. Network of eligible comparisons for the meta-analysis of (1A) best-corrected visual acuity at 6 months; (1B) central macular thickness at 6 months; (1C) intraocular pressure at 6 months.
The mean duration of the studies was 6 months (nine studies lasted 3 months, eleven lasted 6 months, and ten lasted 12 months or more), and the mean sample size was 43 eyes per group (minimum/maximum 12/127). In the sample included in the study, the gender ratio was balanced (males accounted for 55.0%) with an average age of about 60.62 years. In terms of clinical characteristics, most included studies recruited patients classified as having type Ⅰ or Ⅱ diabetes complicated with diabetic macular edema. The overall quality of studies was rated as good, despite some studies not disclosing details regarding randomize and allocation concealment.
The quality of the studies included in this network meta-analysis is shown in Supplementary Figure S1. In relation to the random sequence generation, allocation concealment, blinding of participants and personnel and outcome assessment, one trial was rated as “high risk of bias” (1 of 25 trials), whereas for attrition bias and reporting bias, the majority of trials were rated as “low risk of bias”, because they reported the complete outcome data (24 and 25 trials respectively). Those rated at “unclear risk of bias” had issues relating to random sequence generation, and allocation concealment (13 and 21 trials, respectively) and masking of participants and personnel (16 trials/64.0%).
The results of the GRADE evaluation of interventions for ranibizumab were presented in Supplementary Table S1. All the reasons for downgrading were labeled. Due to the network analysis at 6 months is star-shaped and does not contain a closed loop, local inconsistency of the network cannot be assessed. Therefore, the level of evidence was all downgraded.
In pooled pair-wise meta-analysis, there was no statistically significant difference between ranibizumab, bevacizumab, aflibercept, conbercept, triamcinolone acetonide and sham injections in BCVA at 6 months. As for CMT at 6 months, ranibizumab, bevacizumab, aflibercept and triamcinolone acetonide also did not show a statistically significant difference. In terms of IOP at 6 months, the same conclusion was observed in ranibizumab, bevacizumab, triamcinolone acetonide and sham injections.
Figure 3 and Supplementary Table S2 shows the BCVA comparison results at 6 months. Non-significant results were found when comparing between all treatments. As for ranking the results, the efficacy of antiangiogenic drugs in order of most to least are as follows: conbercept, ranibizumab, aflibercept, triamcinolone acetonide, bevacizumab and sham injections (Figure 4).
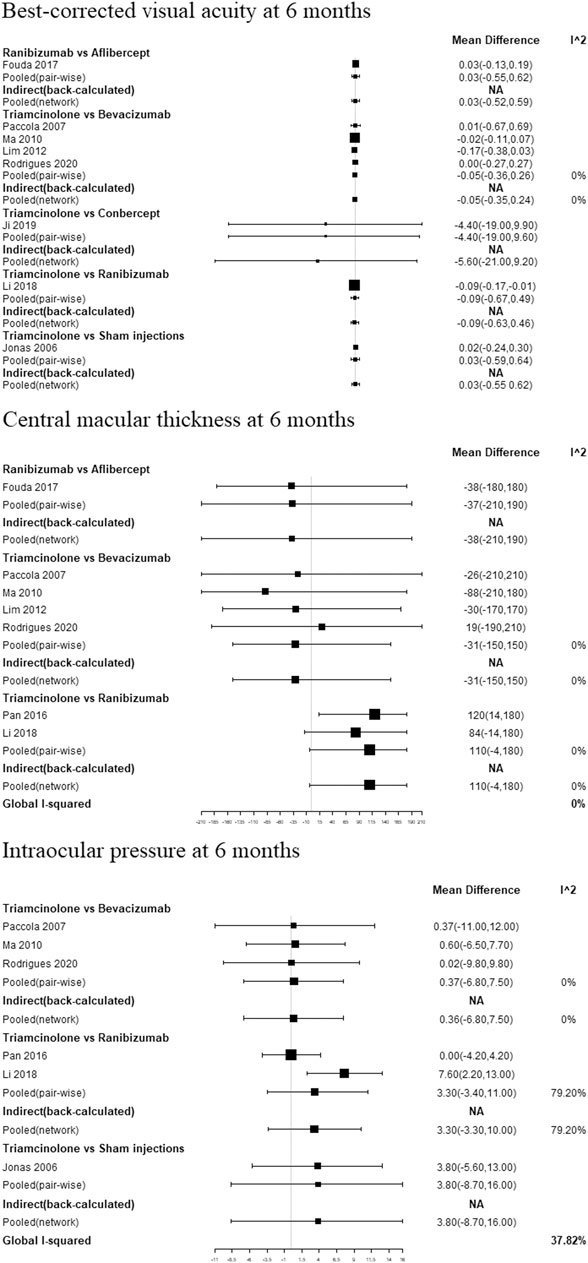
FIGURE 3. Pair-wise meta-analysis and network meta-analysis of different pharmacological interventions on effect of diabetic macular edema at 6 months.
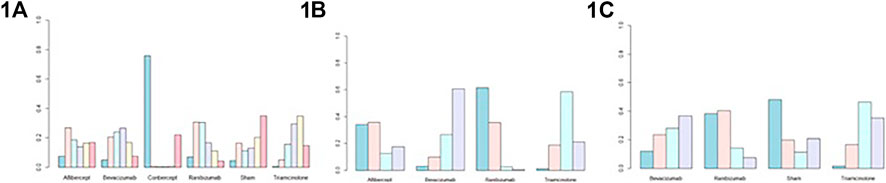
FIGURE 4. Probability plots of (1A) best-corrected visual acuity at 6 months; (1B) central macular thickness at 6 months; (1C) intraocular pressure at 6 months.
Figure 3 and Supplementary Table S2 show the CMT comparison results at 6 months. The NMA comparison using Bayesian framework indicated that ranibizumab, bevacizumab, aflibercept and triamcinolone acetonide also did not a show statistically significant difference. According to ranking probabilities, ranibizumab may decrease CMT more significantly than aflibercept, bevacizumab and triamcinolone acetonide, with aflibercept being probably the second most effective (Figure 4).
IOP comparison results at 6 months are show in Figure 3 and Supplementary Table S2. There were no significant differences between pairs of treatment between ranibizumab, bevacizumab, sham injections and triamcinolone acetonide. As for ranking the results, ranibizumab may decrease IOP more significantly than bevacizumab, sham injections and triamcinolone acetonide (Figure 4). Meanwhile, bevacizumab may be the second most effective.
Global inconsistency of the network meta-analysis is summarized under consistency and inconsistency assumption (Supplementary Table S3). The difference of DIC between two models was no more than five, which means the results are generally considered consistent.
To demonstrate the robustness of our results, a sensitivity analysis was conducted by dividing different follow-up periods. After 3 months, both pooled pair-wise meta-analysis and network meta-analysis showed no statistically significant difference between all treatments (Supplementary Figure S2), with ranibizumab being the most favorable for BCVA improvement, decreasing CMT and IOP according to the ranking probabilities (Supplementary Figure S3). After 12 months, the same conclusion was observed in both pooled pair-wise meta-analysis and network meta-analysis (Supplementary Figure S4), with ranibizumab being the most favorable for BCVA improvement and decreasing CMT (Supplementary Figure S3). After sensitivity analysis, the main result revealed no significant change in NMA results, indicating the robustness and reliability of the statistical analysis.
This is the first study which has estimated and compared the effectiveness of all triple antiangiogenic therapy that has been studied in randomized trials. Compared to previous meta-analysis, this study included a full range of antiangiogenic drugs and compared the differences from three perspectives between single drugs. In addition, this study has a higher standard, due to accurate experimental types of randomized trials, identifying interventions outside of laser interference, unity of follow-up time and inclusion of trials published in non-English publications. A sufficient number of literatures, a total of 25 articles, was selected and included in this study.
According to the statistical results, it is shown that no matter which outcome indicator is compared or which follow-up period is observed, ranibizumab may have the best curative effect. A randomized controlled trial showed that the relative effect of intravitreous aflibercept, bevacizumab, or ranibizumab depended on baseline visual acuity, and that these drugs are equally effective in patients who initially had mild visual impairment (Wells et al., 2015), which is consistent with the comparison of the network meta-analysis. From a head-to-head trial, ranibizumab improves vision quickly and steadily compared with placebo and reduced the risk of long-term visual loss (Quan et al., 2012). Another study confirmed that ranibizumab significantly improved vision and is well tolerated by patients.
A recent review (Zhang et al., 2016a) published in 2016 that compared 21 trials (4703 eyes), suggested that intravitreal aflibercept is most favorable, with both BCVA improvement and CMT reduction within 12 months compared with other current therapies in the management of diabetic macular edema. In addition, another study (Pham et al., 2019) evaluated head-to-head randomized controlled trials comparing aflibercept, bevacizumab, and ranibizumab (or any combination of head-to-head comparisons) in adult patients with diabetic macular edema. The study found that patients with diabetic macular edema treated with aflibercept experienced significantly higher vision gain at 12 months than patients receiving ranibizumab or bevacizumab. This is slightly different from the conclusion reached in this study, which indicated aflibercept is second only to ranibizumab in both BCVA improvement and CMT reduction according to the ranking probabilities.
With respect to the efficacy of the various drugs, short-term and long-term efficacy were compared, and data from three periods (3, 6, and 12 months). As for the safety of antiangiogenic drugs in the treatment of diabetic macular edema, IOP was chosen to quantitatively evaluate this. Because the amount of data available regarding IOP at 12 months is limited, only data from 3 to 6 months were compared. There was no statistical difference in the efficacy of all drugs at 6 months.
This study has limitations to some extent, because the present literature is not comprehensive enough, random test sample size is not big enough, and it incorporated literature of variable quality. In addition, the research results were impacted by using selected trials from different regions, ages of the patients, and levels of medical treatment. These factors may have a potential impact on the results, although it was found that the heterogeneity of the study is not high. Moreover, measurements that are not easily affected by patients' baseline conditions, including changes in visual acuity, central macular thickness and intraocular pressure before and after treatment, thus the statistical analysis and conclusions are relatively reliable. 3, 6, and 12 months after the operation were chosen as times of result analysis. This choice was guided by the availability of various trial data, which also helped to compare the short-term and long-term effects of using the drugs.
This systematic review and network meta-analysis determined the efficacy of different anti-angiogenic drugs on diabetic macular edema. The analysis confirms that ranibizumab may be most favorable for BCVA improvement and have a stronger efficacy in decreasing CMT and IOP than using other drugs when taking all the indicators into consideration. This conclusion may help doctors evaluate the balance of pros and cons of various drugs and adjust their treatment accordingly.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Conception and design: QL and MW; Collection and assembly of data: XZ and YL; Data analysis and interpretation: All authors; Manuscript writing: All authors; Final approval of manuscript: All authors; Accountable for all aspects of the work: All authors.
This work is supported by funds from the 01 National Science and Technology Major Project (No. 2018ZX01031201).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.637667/full#supplementary-material.
Baghi, A., Jabbarpoor Bonyadi, M. H., Ramezani, A., Azarmina, M., Moradian, S., Dehghan, M. H., et al. (2017). Two doses of intravitreal ziv-aflibercept versus bevacizumab in treatment of diabetic macular edema: a three-armed, double-blind randomized trial. Ophthalmol. Retina 1 (2), 103–110. doi:10.1016/j.oret.2016.08.007
Chaimani, A., Caldwell, D. M., Li, T., Higgins, J. P. T., and Salanti, G. (2017). Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. J. Clin. Epidemiol. 83, 65–74. doi:10.1016/j.jclinepi.2016.11.015
Ekinci, M., Ceylan, E., Çakıcı, Ö., Tanyıldız, B., Olcaysu, O., and Çağatay, H. H. (2014). Treatment of macular edema in diabetic retinopathy: comparison of the efficacy of intravitreal bevacizumab and ranibizumab injections. Expert Rev. Ophthalmol. 9 (2), 139–143. doi:10.1586/17469899.2014.900439
Fouda, S. M., and Bahgat, A. M. (2017). Intravitreal aflibercept versus intravitreal ranibizumab for the treatment of diabetic macular edema. Clin. Ophthalmol. 11, 567–571. doi:10.2147/OPTH.S131381
Genentech, Inc. (2007). A study of ranibizumab injection in subjects with clinically significant macular edema (ME) with center involvement secondary to diabetes mellitus (RISE). Available at: https://clinicaltrialsgov/show/NCT00473330 2007 (Accessed May 15, 2007).
Jabbarpoor, B. M., Baghi, A., Ramezani, A., Yaseri, M., and Soheilian, M. (2018). One-year results of a trial comparing 2 doses of intravitreal ziv-aflibercept versus bevacizumab for treatment of diabetic macular edema. Ophthalmol. Retina. 2 (5), 428–440. doi:10.1016/j.oret.2017.09.010
Ji, X. Y., Sun, H. L., Li, C. W., and Chen, X. Y. (2019). Comparison of different drugs in the treatment of retinopathy caused by type 2 diabetes. J. Pract. Med. 35 (4), 598–601. doi:10.3969/j.issn.1006-5725.2019.04.022
Jonas, J. B., Kamppeter, B. A., Harder, B., Vossmerbaeumer, U., Sauder, G., and Spandau, U. H. M. (2006). Intravitreal triamcinolone acetonide for diabetic macular edema: a prospective, randomized study. J. Ocul. Pharmacol. Ther. 22 (3), 200–207. doi:10.1089/jop.2006.22.200
Leasher, J. L., Bourne, R. R. A., Flaxman, S. R., Jonas, J. B., Keeffe, J., Naidoo, K., et al. (2016). Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care 39 (9), 1643–1649. doi:10.2337/dc15-2171
Li, Y. X., Hu, J., and Zhang, W. (2018). Effect of rezumab and triamcinolone acetonide on the treatment effect of diabetic macular edema. Contemp. Med. 24 (14), 19–21. doi:10.3969/j.issn.1009-4393.2018.14.007
Lim, J. W., Lee, H. K., and Shin, M. C. (2012). Comparison of intravitreal bevacizumab alone or combined with triamcinolone versus triamcinolone in diabetic macular edema: a randomized clinical trial. Ophthalmologica 227 (2), 100–106. doi:10.1159/000331935
Ma, J. (2010). Intravitreal triamcinolone acetonide versus bevacizumab for the treatment of diabetic macular edema. Available at: http://www.hebmu.edu.cn/.
Marey, H., and Ellakwa, A. F. (2011). Intravitreal bevacizumab alone or combined with triamcinolone acetonide as the primary treatment for diabetic macular edema. Clin. Ophthalmol. 5 (1), 1011–1016. doi:10.2147/OPTH.S22103
Massin, P., Bandello, F., Garweg, J. G., Hansen, L. L., Harding, S. P., Larsen, M., et al. (2010). Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes care 33 (11), 2399–2405. doi:10.2337/dc10-0493
Neto, H. O., Regatieri, C. V., Nobrega, M. J., Muccioli, C., Casella, A. M., Andrade, R. E., et al. (2017). Multicenter, randomized clinical trial to assess the effectiveness of intravitreal injections of bevacizumab, triamcinolone, or their combination in the treatment of diabetic macular edema. Ophthalmic Surg. Lasers Imaging Retina. 48 (9), 734–740. doi:10.3928/23258160-20170829-08
Nikolakopoulou, A., Higgins, J. P. T., Papakonstantinou, T., Chaimani, A., Del Giovane, C., Egger, M., et al. (2020). CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 17 (4), e1003082. doi:10.1371/journal.pmed.1003082
Paccola, L., Costa, R. A., Folgosa, M. S., Barbosa, J. C., Scott, I. U., and Jorge, R. (2007). Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME study). Br. J. Ophthalmol. 92 (1), 76–80. doi:10.1136/bjo.2007.129122
Pan, Z. J. (2016). The effect of intravitreal injection of ranibizumab and triamcinolone acetonide on diabetic macular edema. Mod. Diagn. Treat. 27 (12), 2214–2215.
Pham, B., Thomas, S. M., Lillie, E., Lee, T., Hamid, J., and Richter, T.et al. (2019). Anti-vascular endothelial growth factor treatment for retinal conditions: a systematic review and meta-analysis. BMJ Open 9 (5), e022031. doi:10.1136/bmjopen-2018-022031
Quan, D. N., David, M. B., Dennis, M. M., David, S. B., Sunil, P., and Leonard, F.et al. (2012). Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119 (4), 789–801. doi:10.1016/j.ophtha.2011.12.039
Ren, Z., Zhang, W., Huang, W., Zeng, J., Zhao, X., and Zhang, X. (2019). Clinical efficacy and safety of intra-vitreous injection of Lucentis combined with triamcinolone acetonide in treatment of diffuse diabetic macular edema. Chin. J. Clinicians 13 (6), 409–418. doi:10.3877/cma.j.issn.1674-0785.2019.06.002
Rodrigues, M. W., Cardillo, J. A., Messias, A., Siqueira, R. C., Scott, I. U., and Jorge, R. (2020). Bevacizumab versus triamcinolone for persistent diabetic macular edema: a randomized clinical trial. Graefes Arch. Clin. Exp. Ophthalmol. 258 (3), 479–490. doi:10.1007/s00417-019-04564-z
Sayin, N., Kara, N., and Pekel, G. (2015). Ocular complications of diabetes mellitus. WJD. 6 (1), 92–108. doi:10.4239/wjd.v6.i1.92
Schlingemann, R. O., Vader, M., Schauwvlieghe, A., Dijkman, G., Hooymans, J., Los, L., et al. (2019). Comparing the efficacy of bevacizumab to ranibizumab in patients with diabetic macular oedema: the brdme study. Eur. J. Ophthalmol. 29 (3), Np18-NP9. doi:10.1016/j.oret.2020.02.008
Schmidt-Erfurth, U., Garcia-Arumi, J., Bandello, F., Berg, K., Chakravarthy, U., Gerendas, B. S., et al. (2017). Guidelines for the management of diabetic macular edema by the European society of Retina specialists (EURETINA). Ophthalmologica 237 (4), 185–222. doi:10.1159/000458539
Shin, E. S., Sorenson, C. M., and Sheibani, N. (2014). Diabetes and retinal vascular dysfunction. J. Ophthalmic Vis. Res. 9 (3), 362–373. doi:10.1371/journal.pone.0103148
Sonoda, S., Sakamoto, T., Yamashita, T., Otsuka, H., Shirasawa, M., Kakiuchi, N., et al. (2014). Effect of intravitreal triamcinolone acetonide or bevacizumab on choroidal thickness in eyes with diabetic macular edema. Invest. Ophthalmol. Vis. Sci. 55 (6), 3979–3985. doi:10.1167/iovs.14-14188
Wan, L., Wang, R., and Zhong, J. (2013). Investigation on lucentis inhibition to macular edema in diabetic retinopathy. J. Chin. Ophthalmol. Otorhinolaryngol. 3 (4), 204–207. doi:10.3969/j.issn.1674-9006.2013.04.010
Wang, X. J., Kuang, G. Y., Chen, Y. B., Zhai, N., Wang, W., Qin, W., et al. (2009). Intravitreal injection of triamcinolone acetonide in the treatment of diabetic retinopathy and macular edema. Youjiang Med. J. 37 (04), 425–426.
Wells, J. A., Wells, J. A., Glassman, A. R., Ayala, A. R., Jampol, L. M., Aiello, L. P., et al. (2015). Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 372 (13), 1193–1203. doi:10.1056/NEJMoa1414264
Xue, S., Chen, P., Liu, W., and Yuan, J. (2016). Intravitreal injection of ranibizumab versus triamcinolone acetonide for diabetic macular edema. Int. Med. Health Guidance News 22 (20), 3149–3151. doi:10.3760/cma.j.issn.1007-1245.2016.20.033
Yan, J., Jiang, W. H., and Peng, X. F. (2016). Observation of clinical effect of lucentis in treatment of diabetic macular edema. China Med. Pharm. 6 (5), 109–111.
Zhang, L., Wang, W., Gao, Y., Lan, J., and Xie, L. (2016a). The efficacy and safety of current treatments in diabetic macular edema: a systematic review and network meta-analysis. PLoS One 11 (7), e0159553. doi:10.1371/journal.pone.0159553
Zhang, Z. G., Ma, W. X., and Zhang, W. G. (2016b). Clinical effect of treating diabetic macular edema by vitreous cavity injection of ranibizumab and triamcinolone acetonide. World Latest Med. Inf. 16 (94), 11. doi:10.3969/j.issn.1671-3141.2016.94.007
Keywords: antiangiogenic drugs, diabetic macular edema, network meta-analysis, best-corrected visual acuity, central macular thickness, intraocular pressure
Citation: Zhang X, Liu Y, Wang M, Li Q, Zhang W, Zhang R and Wu Y (2021) Efficacy of Antiangiogenic Drugs in the Treatment of Diabetic Macular Edema: A Bayesian Network Analysis. Front. Pharmacol. 12:637667. doi: 10.3389/fphar.2021.637667
Received: 04 December 2020; Accepted: 11 March 2021;
Published: 15 April 2021.
Edited by:
Cristiane De Cássia Bergamaschi, University of Sorocaba, BrazilReviewed by:
Yusra Habib Khan, Universiti Sains Malaysia (USM), MalaysiaCopyright © 2021 Zhang, Liu, Wang, Li, Zhang, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miaoran Wang, d2FuZ21pYW9yYW4xMjNAMTYzLmNvbQ==; Qiuyan Li, bGlxaXV5YW4xOTY4QHNvaHUuY29t
†These authors have contributed equally to this work and share first authorship.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.