
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 12 April 2021
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.629607
This article is part of the Research TopicMetabolomics and Ethnopharmacology in the Development of Herbal and Traditional MedicineView all 34 articles
Aims: Scientific biological evaluation of standardized extracts is becoming one of the central needs for the globalization of customary medication in current times. And to validate the presence of active constituents in crude medicinal extracts, analytical techniques like HPLC and HPTLC are the most suitable authentication systems. In the current study we aimed to standardize and evaluate Clerodendrum serratum (L.) Moon (Verbenaceae). For its unique anti-inflammatory and anti-arthritic properties. Evaluation and analysis of the plant, therefore, offers a new platform for the development of the herbal drug and could prove to be a safe and cost effective treatment for arthritis management.
Methods: The aqueous extract of C. serratum, a common plant in the Southeastern Asian region, was used for phytochemical investigation and standardization by HPTLC and HPLC. The standardized HPLC method was further validated by using ICH guidelines. The standardized extract was investigated for anti-inflammatory and anti-arthritic activity. Complete Freund’s adjuvant (CFA) model was performed to evaluate the activity. Paw diameter, joint diameter, arthritic score, and body weight was accepted as a parameter for the evaluation of biological activity.
Results: HPTLC method revealed the presence of ursolic acid with an Rf value of 0.38 and the amount quantified was 0.03% w/w. The presence of the bioactive phytochemical was further analyzed and confirmed by HPLC for which the validation was done successfully in accordance with ICH guidelines. The assay content for ursolic acid was found to be 0.059% with relative standard deviation (RSD) <2.5% for specificity and precision with spike recovery between 95–110%. The anti-arthritic activity of aqueous extract exhibited COX-2 and TNF-α inhibition as observed in various parameters like paw edema, arthritic index, and joint diameter. Plant extract showed reclamation of arthritis in regard to body weight, arthritic score, paw edema, and joint diameter. The extract showed significant results for TNF-α and COX-2(p < 0.0001). The plant extract also exhibited in-vitro anti-inflammatory activity.
Conclusion: The current study established the scientific basis of ethnomedicinal use of the plant for anti-inflammatory purposes and the management of arthritis and can also be used for quality control purposes.
Arthritis and its associated musculoskeletal imbalances is one of the common afflictions, affecting millions of people and greatly restricting their activities of daily living (Baranwal et al., 2012b). It is a common term used by medical practitioners to describe the progressive inflammatory responses to one or more joint structures due to various causes ranging from traumatic, rheumatic, to degenerative concerns resulting in muscular stiffness and restricted physical movements. Further, arthritis commonly affects persons from all age groups, race, sex, and global regions with more than 100 different types, such as juvenile arthritis, rheumatoid arthritis, ankylosing spondylitis, gout, psoriatic arthritis, and osteoarthritis, the last of which is degenerative. Its clinical features in suffering patients may vary from person to person and range from mild pain and swelling to intense forms like complete/partial joint immobility, muscular atrophy, and contractures (Rho et al., 2009). Subsequently, the drug regime includes administration of non-steroidal anti-inflammatory drugs (NSAIDs) to patients as a first line of treatment but its long term usage has led to certain potential adverse reactions, such as gastroduodenal diseases and renal insufficiency (Harirforoosh and Jamil, 2009), that are probably induced by the inhibition of cyclo-oxygenase for reduction in prostaglandin content. Although various types of treatments are available today, like NSAIDs, corticosteroids, and DMARDs, they are mainly used to treat the symptomatic concerns and do not target the pathological origin like membrane stabilizing, protein denaturation, etc. Moreover, treatment by the above available modes can cause serious hepatic damage, gastric bleeding (Craig and Cappelli, 2019), Hospitalization, and death (Rostom et al., 2005).
Therefore, to surpass all these issues and find a more harmless and equally efficacious therapeutic option, researchers are considering plants as a source of medicine. Initially, these plant-based medicinal systems have formed the foundation of folk or ethno medicines, practiced in India and some other parts of the world like China, Africa, and South America. Later a considerable part of this indigenous knowledge was formulated, documented, and eventually passed into organized systems of medicine such as Ayurveda, Unani Siddha, etc. Traditionally plants were used for various therapeutic claims by tribal communities throughout India (Jain and Singh, 2010; Balasankar et al., 2013; Nayak and Dhal, 2013). In our study we have selected the plant Clerodendrum serratum (L.) Moon belonging to the family Verbenaceae. This is a deciduous shrub widely distributed in the Western Ghats (Manjunatha, 2004) of India. In Ayurveda, the plant is popularly known as Bharangi (Sanskrit) and customarily called Blue Glory (English). As per the traditional claims, the roots of this plant are a potential source of medicine for ailments such as allergic disease, body soreness, respiratory illness, infectious disease, dropsy, eye diseases, fever, inflammation, malaria, opthalmia, rheumatism, snakebite, tuberculosis, ulcers, and wounds (Keshavamurthy, 1994). Studies reported various chemical constituents from the plant, like stigmasterol, bis(2-ethylhexyl) phthalate, hispulidin, serratumin A, acteoside, martynoside, serratumoside-A,myricoside, ursolic acid, spinasterol, spinasteryl-β-D-glucopyranoside etc., in various parts of plant including the stem, root, and erial part (Singh et al., 2012; Kumar and Nishteswar, 2013; Padal et al., 2013). The current study was focused on its in-vitro anti-inflamatory activity and in-vivo arthritis activity by using Complete Freund’s adjuvant (CFA) induced arthritis model.
All the chemicals used in the current study were procured from Sigma Aldrich Co., St. Louis, United States. All other chemicals/solvent adopted were of analytical grade.
Roots of C. serratum L. were collected from Faizabad, Uttar Pradesh, India and taxonomical identification was done at the Department of Agronomy, Aacharya Narendra Dev Agriculture Technical University, Dist. Faizabad, Uttar Pradesh. The prepared brown colored powdered root (100 g) was extracted (decoction) with water (500 ml) at a temperature not exceeding 110°C for 2 h. The extract was strained and lyophilized. The yield of obtained dried extract was 10% w/w.
The presence of ursolic acid was identified by performing High-performance thin layer chromatography (HPTLC). The solvent system used for the analysis was toluene: ethyl acetate (8:2 v/v) as a binary mobile phase system. For spot detection, anisaldehyde sulfuric acid was used as the spraying reagent. Standard analytical grade of ursolic acid was used as a reference by dissolving in HPLC grade methanol. The obtained concentration was 1,070 µg/ml, which was further used for preparing the subsequent working standards. Camag Linomat V HPTLC system (Switzerland) equipped with 100 µl Camag syringe and scanner III was used for the current qualitative estimation. Sample and standard as narrow bands of a width of 3 mm were applied to precoated silica gel aluminum plate 60F-254.
An isocratic HPLC method was developed and validated for the identification of ursolic acid. A prepacked column, C18 (25 cm × 4.6 mm) 5 µm, with UV detector (210 nm) was used for the current study. The injection volume was 20 µl and its flow rate was maintained at 0.6 ml/min. Run time for standard and sample were 45 min and the data acquisition was done. For HPLC analytical development studies, methanol and acetonitrile (30:70, v/v) were selected as the binary mobile phase system. Before using, it was filtered and degassed. Thereafter, the standard solution was prepared by adding 10 mg of ursolic acid in methanol (10 ml) and subjected for sonication. The solution was settled to room temperature and diluted further with the help of diluent up to the mark.
Then the sample solution was prepared by taking 1.5 g of the test sample in iodine flask and adding 25 ml of water followed by 20 min of sonication. Reflux was done for about 30 min in reflux assembly and filtered. The process was repeated twice and the filtrate was evaporated to dryness. The obtained residue was dissolved in methanol and the volume was made up of 10 ml methanol. A further 0.5 ml of this solution was taken into a 10 ml volumetric flask and diluted with methanol. Injection of equal volumes of the standard solution was done and chromatograms were recorded along with measurement of peak area. The percentage of ursolic acid was calculated by using the formula:
At= Peak area of a test sample, As = Peak area of reference standard, Cs = Concentration of reference standard, Cu = Concentration of test sample, P = Potency of ursolic acid working standard
The developed method was validated according to ICH guidelines (Baranwal et al., 2012a). The parameters adopted for its validation are Linearity, Specificity, Accuracy, Range, Precision, Repeatability, Intermediate precision, Robustness, Limit of Detection, and Limit of Quantification (Manukumar and Umesha, 2015).
The evaluation test was performed as per the method reported by Manukumar and Umesha (2015). The extracted sample contains 0.03 ml stock erythrocytes suspension infused with 5 ml hypotonic solution prepared with 154 mM NaCl in 10 mM Sodium Phosphate Buffer at pH 7.4, along with the test sample ranging from 100–500 μg/ml. The blank (without the test samples) and the standard (acetylsalicylic drug) was also treated accordingly. This mixture was incubated at room temperature for 10 min, followed by centrifugation for 10 min at 3,000 rpm. The absorbance of the supernatant was observed spectrophotometrically at 540 nm. The experiment was performed in triplicate. The percentage inhibition of haemolysis was computed by the following formula:
where: A1 = absorbance of blank; A2 = Absorbance of test and standard sample
The effect of protein denaturation was performed as per the procedure mentioned below. The concentrations ranging from 100–500 μg/ml for both test sample and standard acetylsalicylic sample with 1 ml of 1 mM egg albumin solution were incubated at 27°C for 15 min. The mixture was further denatured at 70°C in a water bath for 10 min. The samples were allowed to cool down and observed spectrophotometrically at 660 nm. This experiment was performed in triplicate. Percentage inhibition of denaturation was calculated from the control without sample and standard (Badii and Howell, 2002; Chung et al., 2012). The percentage inhibition of denaturation was done by the below-mentioned formula:
For the in-vivo anti-arthritic activity, the Complete Freund’s Adjuvant (CFA) model was adopted. For this model, Albino Wistar male rats weighing 200 ± 25 g were considered. The oral route of administration was given to rats placed in different cage groups under a controlled temperature of 22 ± 2°C conditions with administration of golden feed diet and water to all the animals regularly. The dose adopted was 100 and 200 mg/kg body weight (b.w.). Paw diameter, joint diameter, arthritic score, and body weight were used as parameters for the activity. The histopathological estimation of TNF-α and COX-2 were also performed as confirmative studies. Institutional Animal Ethics Committee (IAEC) approval (Reg No. 1824/PO/ERe/S/15/CPCSEA) as well as Protocol Approval (Reference No. IAEC/PN-16045) were taken before performing the experiments.
In the current study, animals (6 nos.) were divided into five different groups. Group 1 received vehicle normal saline (10 ml/kg) orally before the 30 min waiting period. To group 2, 0.1 ml of CFA (0.05% Mycobacterium butyricum in mineral oil; 10 ml/kg b.w.) and vehicle (10 ml/kg) was injected into the left hind paw (subplantar surface) with the help of a 26 gauge needle. Group 3 received CFA and indomethacin (3 mg/kg b.w.) whereas group 4 and 5 administered samples at a dose of 100 mg/kg b.w. and 200 mg/kg b.w. respectively. For the arthritic evaluations, the paw diameter, joint diameter, arthritic score, and body weight measurements were carried out on the third, seventh, 14th, and 21st days. After completion of the protocol on the 21st day, the blood (terminal) samples were collected and the inflammatory mediators, like TNF-α and COX-2 levels, were estimated from the collected serum using ELISA kit assay for all the assigned groups. The ankle joint tissues were fixed with formalin and preserved for further histopathological studies. All obtained results were compared and evaluated with the standard (Pincus et al., 2003; Kamal and Baldi, 2015; Ghosh et al., 2016). The statistical analysis was subjected to mean ± Standard Error Mean (SEM). Differences were considered significant at p < 0.001, or p < 0.01, or p < 0.05 when compared test group vs. control (−ve) group. For numerical results, a one-way analysis of variance (ANOVA) (compare all vs. control) was performed using Graph Pad InStat Version 3 (GraphPad Software).
The presence of ursolic acid was confirmed by performing the HPTLC from the aqueous extract of C. serratum L. using standard ursolic acid. The stabilized HPTLC system produced a compact spot of standard ursolic acid with an Rf value of 0.38. HPTLC chromatogram of ursolic acid standard and plant extract are shown in Figure 1. UV scanning for sample (A) and standard (B,C) were graphically presented in Figures 2A–C.
The statistical analysis was performed by plotting the standard ursolic acid concentration and the peak responded with a straight line of correlation coefficient 0.998. The observed results showed good recovery within 95–110% when spiked with the standard sample at four different concentrations levels. The developed HPLC method was found to provide repeatable results at various concentrations; thus, the developed protocol was found to be reliable. The sample peak purity was confirmed by comparing with the standards of retention time and peak area. The LOD and LOQ were quantified at 33 µg/ml and 0.059% respectively for the ursolic acid of the test sample (extract). The performed analytical development and validation was assured as per the ICH guidelines and graphically presented in Figure 3 for sample (A) and standard (B).
Membrane stabilizing activity (8%) at a concentration level of 100 µg/ml was shown in Figure 4.
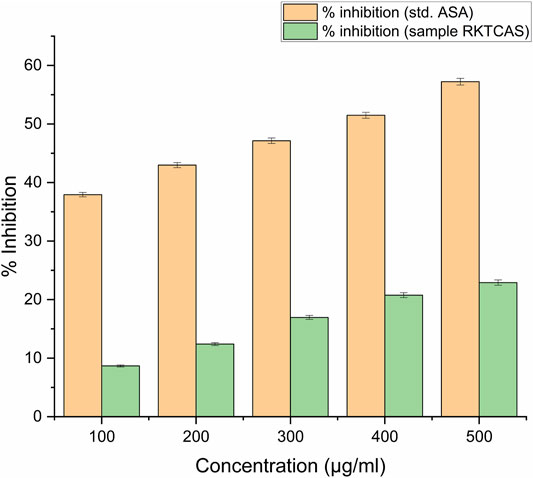
FIGURE 4. In-vitro membrane stabilizing activity showing the possible mechanism of action for the anti-inflammatory activity of aqueous extract of Clerodendrum serratum L.
Around 40% of protein denaturation with 100 μg/ml concentration was shown in Figure 5.
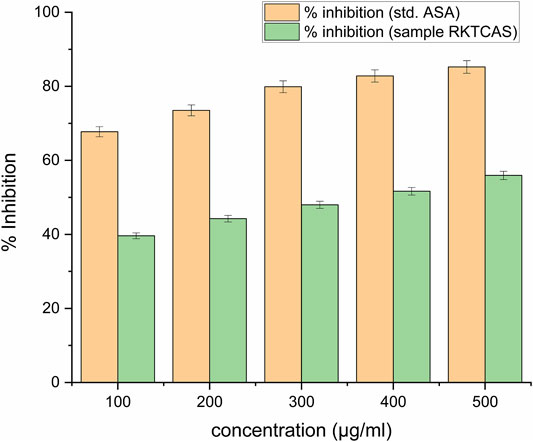
FIGURE 5. In-vitro protein denaturation activity of aqueous extract of Clerodendrum serratum L. against protein denaturation using egg albumin.
The measured arthritic parameters like paw diameter, joint diameter, arthritic score, and body weight were presented in Figures 6–9. Assessment of the blood sample was collected from the terminal parts of the animals. The level of TNF-α and COX-2 was estimated by using ELISA assay (Figure 10). The histopathological analysis of ankle joints after 21 days at a dose of 200 mg/kg was shown in Figure 11.
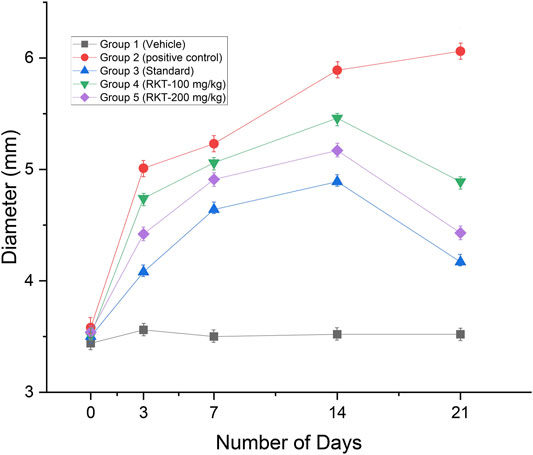
FIGURE 6. In-vivo anti-arthritic activity (Paw diameter in mm) n = 6; Data = Mean ± SEM; **p < 01 (G2 Vs G4); ***p < 0.001 (G2 vs. G3, G4 and G5).
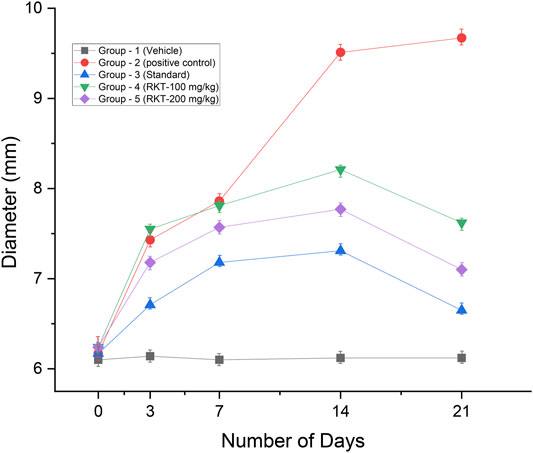
FIGURE 7. In-vivo anti-arthritic activity (joint diameter in mm). All the values are expressed as Mean ± SEM (n = 6) **p < 0.01 (G2 vs. G4); ***p < 0.001 (G2 vs. G3, G4 and G5).
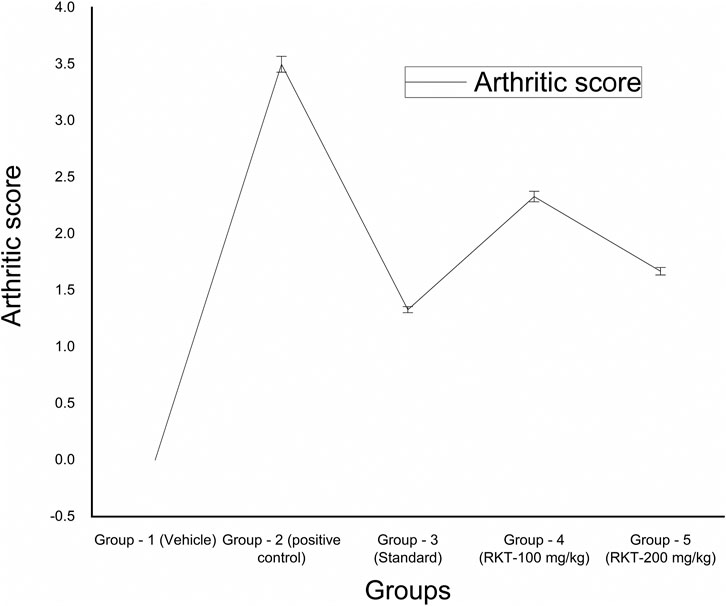
FIGURE 8. In-vivo anti-arthritic activity (arthritic score). All the values are expressed as Mean ± SEM (n = 6) *p < 0.05 (G2 vs. G4); ***p < 0.001 (G2 vs. G3 and G5).
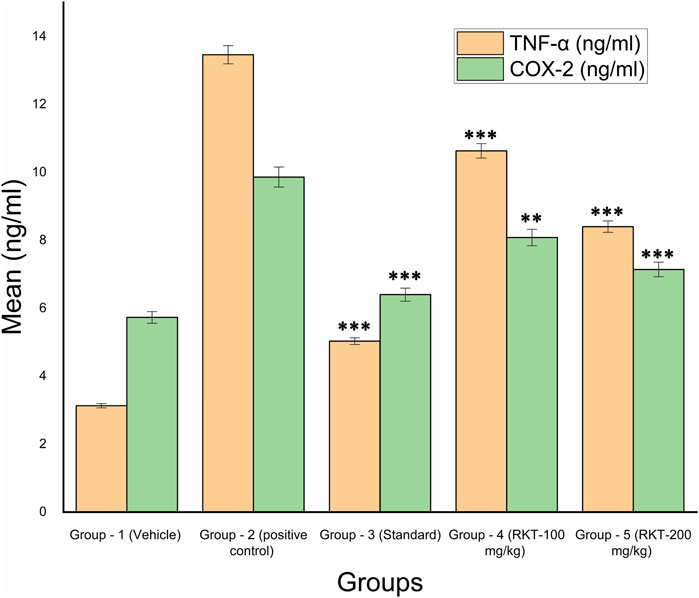
FIGURE 10. In-vivo TNF-α and COX-2 Estimation. All the values are expressed as Mean ± SEM (n = 6) ***p < 0.001 (G2 vs. G3, G4 and G5) For COX 2: **p < 0.01 (G2 vs. G4); ***p < 0.001 (G2 Vs G3 and G5).
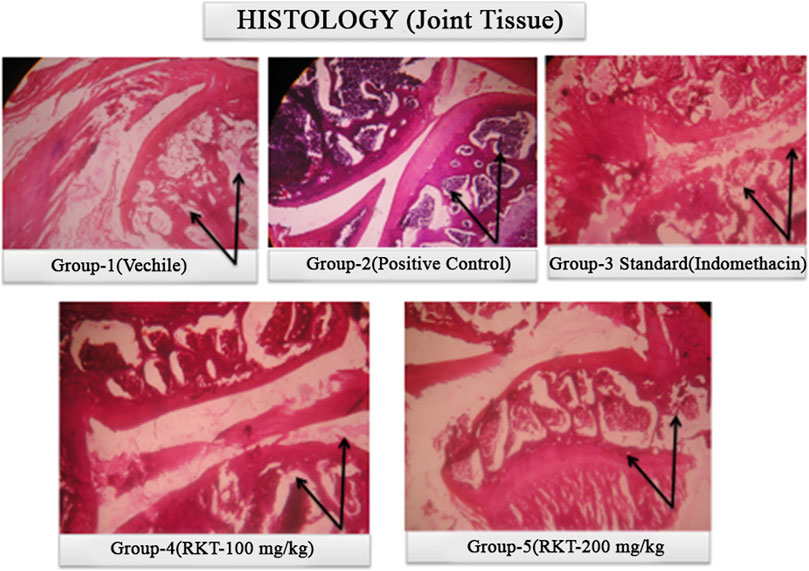
FIGURE 11. Histopathological study of ankle joint at 21 days of treatment after Complete Freund’s adjuvant (CFA) injection.
Arthritis is a persistent disease that equally affects adults and the elderly. Although the prevalence rate ranges between 0.3 and 1% (World Health Organization, 2020), women are mostly affected. As per Sharon et al., (2017), the high arthritis prevalence rate in low to middle-income countries leads to an inability to meet the daily personal as well as social needs. Fundamental endeavors toward prevention and management of arthritis should be prioritzed. The majority of the manufactured medicines, like NSAIDs (Ghosh et al., 2016), Glucocorticoids (Joseph et al., 2016), DMARDs (Pincus et al., 2003; Donahue et al., 2008; Luo et al., 2013; Kapoor et al., 2014) and certain biologicals (Banse et al., 2015; Brady et al., 2015; Zengin et al., 2017), target the symptoms but not the etiology of the disease with extreme adverse drug effects, whereas the surgical treatments (Krause and Matteson, 2014) can lead to post-operative complexities with mental unacceptance.
The natural product has a significant impact with fewer side effects and co-morbidities. Natural cures could be used to combat the constraints related with current accessible synthetic treatments. The plant Bharangi is a popular folk medicine used for inflammation, rheumatism, and pain. Specialists are considering utilizing the Bharangu plant to improve the symptoms of arthritis. The identification of metabolites with proper integration of modern scientific techniques in herbal research is an essential step for the global market and also for reproducible results. Thus, both HPLC and HPTLC analytical methods were used in the current study as the most applicable tools for identification and standardization.
In the current study, we focused on the root as a biological source in Ayurvedic Pharmacopoeia of India. Ursolic acid was identified and quantified by HPTLC and HPLC in the aqueous extract of the root. The developed HPLC method was also validated as per ICH guidelines. Shanmuga et al. (2017) likewise quantified the metabolites apigenin and luteolin by reverse phase high-performance liquid chromatography (RP-HPLC) from its leaf extract (Shanmuga et al., 2017). Followed by its analytical analysis, our study focused on its anti-inflammatory and anti-arthritic activity. In our study, it has been found that the presence of secondary metabolites enhances the stability of the biological membranes when stressed under lysis conditions, considered to be the preliminary investigation involved during the screening of anti-inflammatory activity. The RBC membrane structurally resembles the lysosomal membrane. The protein denaturation of the anti-arthritic property was successfully evaluated using in-vitro assessments. Literature (Krause and Matteson, 2014) also supported the use of ursolic acid in inhibition of the release of proinflammatory cytokines TNF-α, IL-2, and IFN-γ. Studies also revealed the presence of β-sitosterol as an anti-arthric potential in ethanolic extract of the leaf of the plant. The methanolic extract demonstrated dual inhibitory effects on arachidonic acid metabolism which is responsible for the inhibition of the synthesis of inflammatory mediators released through cyclooxygenase and lipoxygenase pathways (Cargnin and Gnoatto, 2017). Patel et al. (2014) revealed the presence of Apigenin-7-glucoside which further confirmed its anti-inflammatory effects and also claimed pain relief to knee osteoarthritis patients in a randomized controlled clinical trial study (Shoara et al., 2015). Thus the presence of phytochemicals like ursolic acid, flavonoid, apigenin, and β-sitosterol strongly support the traditional use and potentiality of C. Serratum as an anti-inflammatory and anti-arthritic.
The current study showed that the extract possesses both anti-inflammatory and anti-arthritic activities. The possible mode of action of the anti-arthritic activity of the aqueous extract of C. serratum may be mediated by the inhibition of COX-2 and TNF-α. Based on the results, anti-arthritic activity may be due to shielding of a synovial membrane, obstruction of cartilage destruction, and inhibition of vascular permeability. Through our intensive efforts and thorough research, the results confirmed to show favorable arthritis restoration using arthritis parameters and histopathological observations from the selected plant C. serratum. The developed HPTLC and HPLC method could be used as an analytical tool for its quality control purpose.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Institutional Animal Ethics Committee (IAEC) (Reg No. 1824/PO/ERe/S/15/CPCSEA; Protocol Approval Reference No. IAEC/PN-16045).
Conceived and design the experiment : RT and UM Conduct experiment : RT, SC, MS and SA Writing of Manuscript : RT, SC, UM and MS Analysis the statistical data and interpretation : RT, SC, UM Manuscript Approval : All authors approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Badii, F., and Howell, N. K. (2002). Effect of antioxidants, citrate, and cryoprotectants on protein denaturation and texture of frozen cod (Gadus morhua). J. Agric. Food Chem. 50 (7), 2053–2061. doi:10.1021/jf010824f
Balasankar, D., Vanilarasu, K., Preetha, P. S., Umadevi, S. R. M., and Bhowmik, D. (2013). Journal of medicinal plants studies. J. Med. Plants 1 (3), 191–200.
Banse, C., Benhamou, Y., Lequerré, T., Le Cam-Duchez, V., Lévesque, H., and Vittecoq, O. (2015). Acquired hemophilia possibly induced by etanercept in a patient with rheumatoid arthritis. Joint Bone Spine 82 (3), 200–202. doi:10.1016/j.jbspin.2014.12.003
Baranwal, V. K., Irchhaiya, R., and Alok, S. (2012a). Anti-arthritic activity of some indigenous plants: a review. Int. J. Pharm. Sci. Res. 3 (4), 981. doi:10.13040/IJPSR.0975-8232.3(4).981-86
Baranwal, V. K., Irchhaiya, R., and Alok, S. (2012b). Antiarthritic activity of some indigenous plants. Res. J. Pharmacogn. Phytochem. 4 (3), 152–157.
Brady, B. L., Tkacz, J. P., Lofland, J., Meyer, R., and Bolge, S. C. (2015). Prescribing patterns of intravenous golimumab for rheumatoid arthritis. Clin. Ther. 37 (9), 2028–2036. doi:10.1016/j.clinthera.2015.06.017
Cargnin, S. T., and Gnoatto, S. B. (2017). Ursolic acid from apple pomace and traditional plants: a valuable triterpenoid with functional properties. Food Chem. 220, 477–489. doi:10.1016/j.foodchem.2016.10.029
Chung, J.-I., Barua, S., Choi, B. H., Min, B.-H., Han, H. C., and Baik, E. J. (2012). Anti-inflammatory effect of low intensity ultrasound (LIUS) on complete Freund’s adjuvant-induced arthritis synovium. Osteoarthr. cartil. 20 (4), 314–322. doi:10.1016/j.joca.2012.01.005
Craig, E., and Cappelli, L. C. (2019). Gastrointestinal and hepatic disease in rheumatoid arthritis. Rheum. Dis. Clin. North. Am. 44, 89–111. doi:10.1016/j.rdc.2017.09.005
Donahue, K. E., Gartlehner, G., Jonas, D. E., Lux, L. J., Thieda, P., Jonas, B. L., et al. (2008). Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann. Intern. Med. 148 (2), 124–134. doi:10.7326/0003-4819-148-2-200801150-00192
Ghosh, R., Hwang, S. M., Cui, Z., Gilda, J. E., and Gomes, A. V. (2016). Different effects of the nonsteroidal anti-inflammatory drugs meclofenamate sodium and naproxen sodium on proteasome activity in cardiac cells. J. Mol. Cell. Cardiol. 94, 131–144. doi:10.1016/j.yjmcc.2016.03.016
Harirforoosh, S., and Jamali, F. (2009). Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin. Drug Saf. 8 (6), 669–681. doi:10.1517/14740330903311023
Jain, S., and Singh, J. (2010). Traditional medicinal practices among the tribal people of Raigarh (Chhatisgarh), India. Indian J. Nat. Prod. Resour. 1 (1), 109–115.
Joseph, R. M., Hunter, A. L., Ray, D. W., and Dixon, W. G. (2016). Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin. Arthritis Rheum. 46, 133–141. doi:10.1016/j.semarthrit.2016.03.001
Kamal, J., and Baldi, A. (2015). Approaches to overcome NSAID induced ulceration in arthritic pain management: perspectives and prospects. J. Drug Deliv. Ther. 5 (2), 9–16. doi:10.22270/jddt.v5i2.1069
Kapoor, B., Singh, S. K., Gulati, M., Gupta, R., and Vaidya, Y. (2014). Application of liposomes in treatment of rheumatoid arthritis: quo vadis. Sci. World J. 2014, 1–17. doi:10.1155/2014/978351
Keshavamurthy, K. (1994). Medicinal plants of Karnataka. Bangalore, India: Karnataka Forest Department, 223–224.
Krause, M. L., and Matteson, E. L. (2014). Perioperative management of the patient with rheumatoid arthritis. WJO 5 (3), 283. doi:10.5312/wjo.v5.i3.283
Kumar, P., and Nishteswar, K. (2013). Phytochemical and pharmacological profiles of Clerodendrum serratum linn. (bharngi): a review. Int. J. Res. Ayurveda Pharm. 4 (2), 276–278. doi:10.7897/2277-4343.04239
Luo, Q., Sun, Y., Liu, W., Qian, C., Jin, B., Tao, F., et al. (2013). A novel disease-modifying antirheumatic drug, iguratimod, ameliorates murine arthritis by blocking IL-17 signaling, distinct from methotrexate and leflunomide. J. Immunol. 191 (10), 4969–4978. doi:10.4049/jimmunol.1300832
Manukumar, H. M. G., and Umesha, S. (2015). Assessment of membrane stabilizing activity from honey. an in-vitro approach. Acta Sci. Pol. Technol. Aliment. 14 (1), 85–90. doi:10.17306/j.afs.2015.1.10
Nayak, C. S. R., and Dhal, N. (2013). 2. Ethnomedicine and magico-religious beliefs of the kondh tribe in boudh district of Odisha India by CR Sahu, RK Nayak and NK Dhal. Life Sci. leaflets 45, 10–22.
Padal, S., Raju, J. B., and Chandrasekhar, P. (2013). Traditional knowledge of konda dora tribes, visakhapatnam district, Andhra Pradesh, India. IOSR J. Pharm. 3 (4), 22–28. doi:10.9790/3013-034202228
Patel, J. J., Acharya, S. R., and Acharya, N. S. (2014). Clerodendrum serratum (L.) Moon.—a review on traditional uses, phytochemistry and pharmacological activities. J. ethnopharmacol. 154 (2), 268–285. doi:10.1016/j.jep.2014.03.071
Pincus, T., Yazici, Y., Sokka, T., Aletaha, D., and Smolen, J. S. (2003). Methotrexate as the “anchor drug” for the treatment of early rheumatoid arthritis. Clin. Exp. Rheumatol. 21 (5), S179–S185.
Rho, Y. H., Oeser, A., Chung, C. P., Milne, G. L., and Stein, C. M. (2009). Drugs used in the treatment of rheumatoid arthritis: relationship between current use and cardiovascular risk factors. Arch. Drug Inf. 2 (2), 34–40. doi:10.1111/j.1753-5174.2009.00019.x
Rostom, A., Goldkind, L., and Laine, L. (2005). Nonsteroidal anti-inflammatory drugs and hepatic toxicity: a systematic review of randomized controlled trials in arthritis patients. Clin. Gastroenterol. Hepatol. 3 (5), 489–498. doi:10.1016/s1542-3565(04)00777-3
Shanmuga, S. R., Babitha, K. V., Varghese, Li., and Mahadevan, N. (2017). Development and validation of RP-HPLC method for simultaneous determination of apigenin and luteolin in ethanol extract of Clerodendrum serratum (Linn.) leaves. Asian J. Appl. Sci. 5, 1–5. doi:10.1155/2015/503139
Sharon, S. L., Cook, S., Leech, M. T., Bowe, S. J., Kowal, P., Naidoo, N., et al. (2017). Prevalence of arthritis according to age, sex and socioeconomic status in six low and middle income countries: analysis of data from the World Health Organization study on global AGEing and adult health (SAGE) wave 1. BMC Musculoskelet. Disord. 18, 271. doi:10.1186/s12891-017-1624-z
Shoara, R., Hashempur, M. H., Ashraf, A., Salehi, A., Dehshahri, S., and Habibagahi, Z. (2015). Efficacy and safety of topical Matricaria chamomilla L. (chamomile) oil for knee osteoarthritis: a randomized controlled clinical trial. Complement. Ther. Clin. Pract. 21 (3), 181–187. doi:10.1016/j.ctcp.2015.06.003
Singh, M. K., Khare, G., Iyer, S. K., Sharwan, G., and Tripathi, D. (2012). Clerodendrum serratum: a clinical approach. J. Appl. Pharm. Sci. 2 (2), 11–15. doi:10.7324/japs.2012.2513
World Health Organization (2020). Chronic disease and health promotion. Available at: https://www.who.int/chp/topics/rheumatic/en/#:∼:text=Worldwide%20estimates%20are%20that%209.6,major%20daily%20activities%20of%20life (Accessed November 15, 2020).
Keywords: anti-inflammatory, ursolic acid, standardization, HPTLC, arthritic, Clerodendrum serratum
Citation: Tiwari RK, Chanda S, M U, Singh M and Agarwal S (2021) Anti-Inflammatory and Anti-Arthritic Potential of Standardized Extract of Clerodendrum serratum (L.) Moon. Front. Pharmacol. 12:629607. doi: 10.3389/fphar.2021.629607
Received: 15 November 2020; Accepted: 17 February 2021;
Published: 12 April 2021.
Edited by:
Sayeed Ahmad, Jamia Hamdard University, IndiaReviewed by:
Armando Caceres, Universidad de San Carlos de Guatemala, GuatemalaCopyright © 2021 Tiwari, Chanda, M, Singh and Agarwal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raj Kumar Tiwari, cmFqX3QzNjVAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.