
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 30 April 2021
Sec. Ethnopharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.628198
This article is part of the Research Topic Medicinal Plants as a Source of Novel Autoimmune-Modulating and Anti-Inflammatory Drug Products View all 14 articles
 Salinee Jantrapirom1,2
Salinee Jantrapirom1,2 Pannaphak Hirunsatitpron1,3
Pannaphak Hirunsatitpron1,3 Saranyapin Potikanond1
Saranyapin Potikanond1 Wutigri Nimlamool1
Wutigri Nimlamool1 Nutthiya Hanprasertpong1*
Nutthiya Hanprasertpong1*Allergic rhinitis (AR) is considered a major nasal condition impacting a large number of people around the world, and it is now becoming a global health problem. Because the underlying mechanisms of AR are complex, the development of single-drug treatment might not be enough to treat a wide spectrum of the disease. Although the standard guidelines classify and provide suitable diagnosis and treatment, the vast majority of people with AR are still without any means of controlling it. Moreover, the benefits of AR drugs are sometimes accompanied by undesirable side effects. Thus, it is becoming a significant challenge to find effective therapies with limited undesirable side effects for a majority of patients suffering from uncontrolled AR. Aller-7/NR-A2, a polyherbal formulation, has revealed promising results in patients by reducing nasal symptoms and eosinophil counts without serious adverse effects. Interestingly, three out of seven of the herbals in the Aller-7/NR-A2 formulation are also found in an Ayurvedic polyherbal formulation known as “Triphala,” which is a potential candidate for the treatment of AR. However, there are no current studies that have examined the effects of Triphala on the disease. This review aims to describe the complexity of AR pathophysiology, currently available treatments, and the effects of Triphala on AR in order to help develop it as a promising alternative treatment in the future.
Although allergic rhinitis (AR), commonly known as hay fever, is not considered a serious condition or one with the potential for mortality, it might become a clinical burden if it were to become uncontrollable in a majority of patients. Epidemiological studies have revealed that patients with AR have a high probability of subsequently developing asthma, and a large proportion of asthmatic patients also have AR as a concomitant condition (Simons, 1999; Thomas, 2006; Vujnovic and Domuz, 2018). Moreover, prolonged AR symptoms, particularly nasal congestion, can lead to the development of obstructive sleep apnea (Young et al., 1997). Currently, three acceptable strategies for managing AR are employed: 1) avoiding sensitized allergenic exposure, 2) starting pharmacotherapy, and 3) starting immunotherapy (Klimek et al., 2019). However, to achieve final outcomes, it is often suggested to patients that they use long-term multiple therapies, which sometimes come with problems of compliance, leading to failures in treatment (Small et al., 2018). Therefore, combinations of two drugs in one device, such as antihistamine/steroid or anticholinergic/steroid, are becoming novel strategic treatments that have been found to be more effective than using a monotherapy (Seresirikachorn et al., 2018).
Several studies have highlighted the beneficial effects of phytochemicals in attenuating allergic responses and the overall symptoms of AR. Curcumin, ginger, ginseng, resveratrol, and quercetin compounds have all shown effective results in relieving nasal symptoms and suppressing AR-related mediators in animal models by controlling the main anti-inflammatory mitogen-activated protein kinase/nuclear factor κB (MAPK/NF-κB) pathway (Jung et al., 2011, 2013; Zhang et al., 2015; Kashiwabara et al., 2016; Kawamoto et al., 2016; Lv et al., 2018). A major component of ginger, 6-gingerol, possesses the ability to suppress T helper 2 (Th2) cytokines by attenuating the activation of MAPK/NF-κB signaling in ovalbumin (OVA)-sensitized spleen cells (Kawamoto et al., 2016). In addition, resveratrol nasal spray has recently been developed to overcome the problem of drug absorption, and specifically to retain bioactive activities in the affected area, such as the nasal mucosa. An intranasal resveratrol formulation has been shown to be successful in alleviating allergic symptoms and reducing eosinophils and mast cell infiltration in the nasal mucosa of both animals and AR patients (Lv et al., 2018). Moreover, children with AR have significantly reduced nasal symptoms following administration of a combined resveratrol and carboxymethyl β-glucan nasal spray (Miraglia Del Giudice et al., 2014).
Triphala possesses several activities that could be beneficial for respiratory and immune systems. However, there is no study directly evidencing the potential benefits of Triphala on AR, even though its constituent phytochemicals have been suggested to serve many aspects of AR therapeutic strategies. Therefore, it is important to review and clarify the possible mechanism of action of Triphala and its active constituents on AR. This review focuses mainly on the antioxidative, anti-inflammatory, immunomodulatory, and other effects of Triphala on the AR-affected respiratory system. The quality of the articles relevant to the scope of AR has been evaluated according to the guideline reported by Heinrich et al. (2020) and the Good Research for Comparative Effectiveness (GRACE) tool (Dreyer et al., 2016) (Supplementary Table S1).
AR is a condition of nasal hypersensitivity caused by an immunologically mediated inflammation by non-infectious stimulants or environmental allergens, such as pollen, dust, fungi, cockroaches, etc. AR is becoming a global health problem affecting approximately 10–40% of the global population (Björkstén et al., 2008; World Allergy Organization, 2013; Bernstein et al., 2016). Although it is not a condition that is associated with high morbidity and mortality, it generates a heavy burden and has a negative impact on the patients’ quality of life (QoL) by reducing performance at work and disturbing sleep. In some cases, the condition can persist throughout the patient’s life. The common symptoms of AR include nasal congestion, rhinorrhea, nasal itchiness and/or itchy eyes, sneezing, and postnasal drip (Klimek et al., 2019). Uncontrolled moderate or severe AR may also impact the control of asthma (Thomas, 2006).
Currently, AR is classified according to the duration (intermittent or persistent) and severity (mild, moderate/severe) of symptoms. Intermittent AR is diagnosed if the frequency of symptoms is fewer than four times per week or a month per year. A frequency greater than this is classified as persistent. Moreover, the impact of the disease on a patient’s QoL is also used to evaluate the severity of the symptoms. Symptoms are considered to be mild if normal daily activities, such as sleep, study, and work, can be undertaken without troublesome or interfering symptoms. On the other hand, if a patient presents at least one symptomatic difficulty, then the symptoms are defined as moderate/severe (Figure 1) (Klimek et al., 2019). Therefore, consideration of both duration and severity is an important guide to choosing the most suitable treatment for each patient.
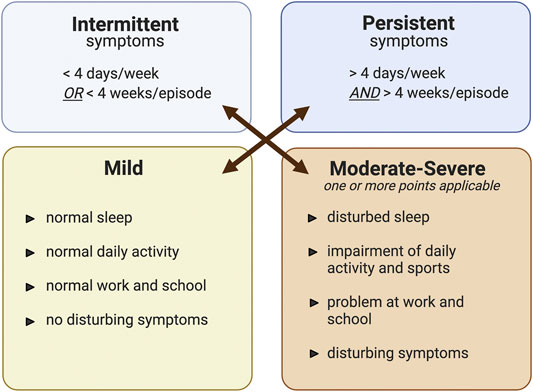
FIGURE 1. The classification of allergic rhinitis modified from ARIA guideline (Klimek et al., 2019). Classification of allergic rhinitis by the level of severity (mild or moderate-severe) and episode of symptom occurrence (intermittent or persistent). The figure was created by BioRender.com.
The key elements for AR management are as follows: 1) avoidance of suspected allergens in sensitized individuals, 2) providing appropriate therapeutic agents to reduce nasal symptoms and improve patients’ QoL, and/or 3) considering allergen-specific immunotherapy (AIT) (Varshney and Varshney, 2015). The list of medications used for AR, their mechanism of action, and their known adverse effects are listed in Table 1 (Bousquet et al., 2008; Klimek et al., 2019; Hossenbaccus et al., 2020). Although these treatments alleviate many AR-causing symptoms effectively, their use may also cause additional adverse effects.
The pathophysiology of AR is quite complex (Figure 2), beginning with sensitization and early and late-phase responses (Min, 2010; Sin and Togias, 2011). Allergens can enter the submucosal area guided by antigen-presenting cells (APCs), which are mainly dendritic cells and macrophages, or pass through disturbed epithelial cells (Bharadwaj et al., 2007; Lambrecht and Hammad, 2010). Some allergens may have an additional protease-related activity to help them cleave the tight junctions between epithelial cells (Reithofer and Jahn-Schmid, 2017). After recognizing the antigen, the activated APCs then undergo maturation, migrate to regional lymph nodes, and present allergen peptides to naïve T cells (Th0). The presentation of peptides requires the involvement of major histocompatibility complex (MHC) class II molecules expressed on the APC surface, together with T cell receptors located on naïve T lymphocyte (Th0) surfaces (Bharadwaj et al., 2007). Depending on the cytokine-stimulated pattern, the Th0 cells can be differentiated into T helper type 1 (Th1) and T helper type 2 (Th2) cells (Zhu et al., 2010). Recognition of the antigenic peptides, together with activation by interleukin-4 (IL-4) derived from various resident cells, drives the Th0 cells to preferentially acquire the differentiated form of Th2 cells (Zhu et al., 2010). Furthermore, the release of IL-4, IL-5, and IL-13 by Th2 cells induces B-cell immunoglobulin class-switch recombination (Poulsen and Hummelshoj, 2007). The gene segments responsible for encoding the immunoglobulin heavy chain are rearranged in order to produce allergen-specific IgE antibodies (Gadermaier et al., 2014).
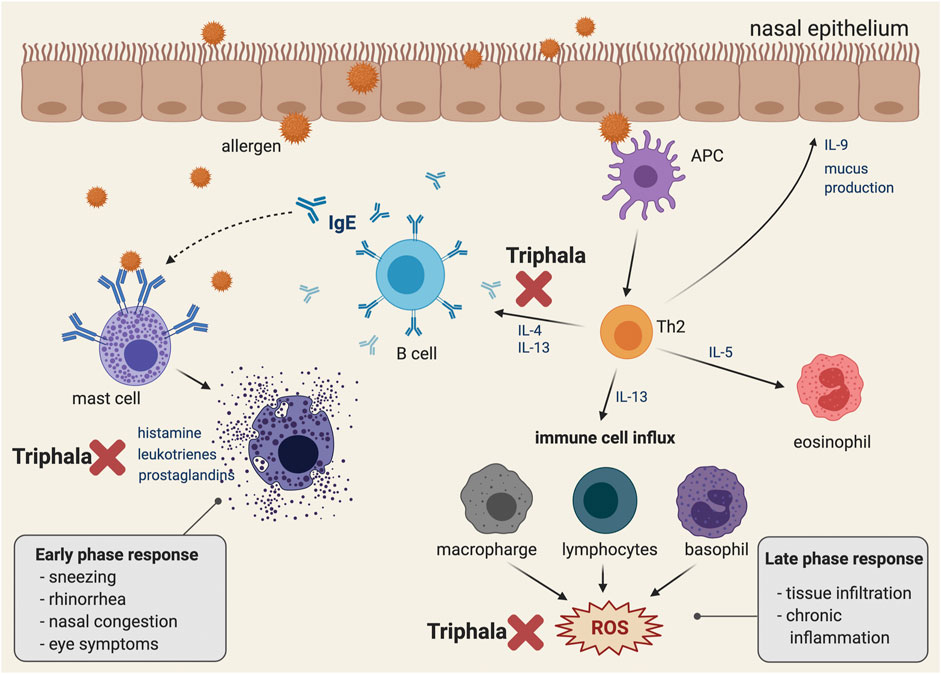
FIGURE 2. Pathophysiology of allergic rhinitis and the conceptual representative of a possible mechanism of action of Triphala in suppressing IgE dependent allergic reaction. A schematic illustration representing the pathophysiology of the IgE-associated allergic reaction. Immediately following exposure to the allergen, the latter is bound to the IgE on the surface of the mast cell and eventually activates mast cell degranulation, which releases inflammatory mediators. In addition, late phase and chronic allergic inflammation occur as a result of eosinophil, macrophage, lymphocyte, and basophil activation. Eventually, following these cell activations, the intracellular ROS are generated. Triphala may play an inhibitory role at the early phase response by suppressing the release of autacoids (including histamine, leukotrienes, and prostaglandins) and by down-regulating the production and secretion of IL-4 and IL-13, which are key AR mediators for enhancing inflammation and IgE production in B cells. Moreover, Triphala may help modulate the late phase response by scavenging the oxidative stress molecules and products. The figure was created by BioRender.com.
The early phase occurs in sensitized individuals within minutes of exposure to allergens. Soluble IgE antibodies, which are produced by B cells in the sensitization phase, locally diffuse and systematically enter the body fluids via lymphatic vessels (Burton and Oettgen, 2011). IgE can strongly bind to the tetrameric (αβγ2) high-affinity surface receptors for IgE (FcεRI) on tissue mast cells and basophils (Turner and Kinet, 1999). Any cells with surface-bound IgE antibodies are called sensitized cells, and they are prompted to respond to specific antigens through the membrane-bound IgE antibodies. The crosslinking of adjacent IgE antibodies by allergens induces aggregation of FcεRI and then triggers a rapid degranulation and secretion of various mediators, including histamine and tryptase (Turner and Kinet, 1999). Mast cells also release newly synthesized lipid mediators, including leukotrienes (LTC4, LTD4, and LTE4) and prostaglandins (PGD2) to help sustain inflammation by neutrophil and eosinophil infiltration (Fanning and Boyce, 2013).
There are many signs and symptoms associated with the early phase of AR, such as nasal pruritus, sneezing, and coughing caused by the histamine-stimulated sensory nerve endings of the trigeminal nerves (Taylor-Clark et al., 2005; Rosa and Fantozzi, 2013). The activation of sympathetics and parasymphathetics by histamines can also stimulate the mucous glands to produce a watery discharge that is presented as rhinorrhea (Al Suleimani and Walker, 2007). Histamines, together with leukotrienes and prostaglandins, act on blood vessels, causing vasodilation and nasal congestion (White, 1990).
The late phase response is characterized by recruitment of T cells, eosinophils, basophils, and neutrophils following a release of their various mediators, which leads to a continuation of the symptoms and inflammatory induction. This phase usually begins around 4–5 h following exposure to allergens and can last for 24 h. Some cytokines, such as IL-4, IL-13, and tumor necrosis factor-α (TNF-α) released from mast cells, have the ability to upregulate two adhesion molecules, which are vascular cell adhesion molecule-1 (VCAM-1) and intracellular cell adhesion molecule-1 (ICAM-1) (Lee et al., 1994). These adhesion molecules subsequently induce migration of basophils, eosinophils, neutrophils, and T cells to the nasal mucosa. Moreover, an activated form of group 2 innate lymphoid cells (ILC2), which are induced by mast cell-related cytokines (leukotrienes, prostaglandins, and platelet-activating factor (PAF)) play a role in continuing the inflammation by releasing large amounts of Th2 cytokines (Li and Hendriks, 2013). Neutrophils that are recruited into the nasal mucosa can harm the epithelium by inducing reactive oxygen species (ROS) and enzyme (protease, matrix metallopeptidase 9 (MMP-9), and myeloperoxidase) production (Lacy, 2006; Ventura et al., 2014). Persistent inflammation in the late phase is associated with tissue remodeling, which might also lead to alteration of organ function and nasal hyperresponsiveness (Galli et al., 2008).
Triphala, one of the polyherbal formulations used in Ayurvedic medicine, comprises the dried fruits of three plants, namely, Terminalia chebula Retz (Family Combretaceae R. Br), Terminalia bellirica (Gaertn.) Roxb (Family Combretaceae R. Br), and Phyllanthus emblica L (Family Phyllanthaceae Martinov). The composition of Triphala is derived from a rational combination of T. chebula Retz., T. bellirica (Gaertn.) Roxb., and P. emblica L., which are combined to balance the three pillars of life or elemental substances, known in Sanskrit as the three doshas, namely, Vata (elements of space and air), Kappha (elements of earth and water), and Pitta (elements of fire and water) (Peterson et al., 2017). Normally, the herbs are mixed in equal proportions (1:1:1), but sometimes the ratios can be modified to be one part T. chebula Retz., two parts T. bellirica (Gaertn.) Roxb., and three parts P. emblica L (1:2:3) (Gajendra et al., 2016) or one part each of T. chebula Retz. and T. bellirica (Gaertn.) Roxb. and two parts P. emblica L (1:1:2) (Ginsburg et al., 2009). According to a traditional Thai medicine textbook, Triphala is used to provide relief for different disease conditions related to the imbalance of water element (Kappha); specifically, the difficulty of breathing caused by excessive water-soluble element and mucus retention in the lungs. Although there is no specific definition of AR in Traditional Thai medicine, Triphala is used to reduce runny nose, cough, the viscosity of phlegm, sore and dry throat, and thirst. These symptoms are somehow related to AR symptoms.
Several phytochemicals have been detected in the fruits of these three plants. These include phenolic compounds (gallic acid, ellagic acid, chebulinic acid, chebulagic acid, and emblicanin A and B), flavonoids (quercetin and kaempferol), alkaloids (phyllantidine and phyllantin), ascorbic acid, carbohydrates, proteins, etc. (Parveen et al., 2018). The amount of each phytochemical varies depending on the methods used to extract it, the solvents used, and the region in which the parent plant was grown (Parveen et al., 2018). One study by Liu et al. found that samples collected from different regions in China contained a range of phenolic contents in P. emblica L. methanolic extracts (Liu et al., 2008). Moreover, methanolic extraction appeared to yield more phytochemicals than aqueous extraction (Parveen et al., 2018). Therefore, characterization of the compound to be extracted should be a mandatory procedure before undertaking extraction experiments.
The phytochemicals contained in Triphala may be beneficial for the treatment of AR. Gallic acid and quercetin are prominent phenolic compounds and are well-known antioxidants in AR-related studies. Gallic acid was shown to alleviate nasal inflammation by shifting the immune response toward Th1 in a mouse model of OVA-induced AR (Fan et al., 2019). The increment of Th2 cytokines, including IL-4, IL-5, IL-13, and IL-17 was attenuated, whereas Th1-related cytokines, including interferon-γ (IFN-γ) and IL-12, were upregulated in nasal larval fluid (NALF) upon treatment with gallic acid (Fan et al., 2019). Moreover, histopathological improvements such as nasal mucosa thickening, goblet cell hyperplasia, and eosinophil infiltration, have been observed in a gallic acid-treated group of patients (Fan et al., 2019). One study also found that the AR-positive effects of gallic acid were caused by inhibition of pro-inflammatory cytokines and histamine release via the modulation of cyclic adenosine monophosphate (cAMP), intracellular calcium regulation, NF-κB), and a p38 mitogen-activated protein kinase (p38 MAPK)-dependent mechanism (Kim et al., 2006).
Quercetin, a flavonoid aglycone, functions extensively as a major active compound in anti-allergic supplements. Both transcriptional and translational levels of AR-related mediators can be suppressed by quercetin both in vitro and in vivo. In IL-4-induced human nasal epithelial cells (HNEpCs), nitric oxide (NO) production, inducible nitric oxide synthase (iNOS) mRNA expression, and signal transducer and activator of transcription 6 (STAT6) activation are all attenuated (Ebihara et al., 2018; Edo et al., 2018). Quercetin has the ability to suppress pro-inflammatory cytokines and inflammatory inducers such as cyclooxygenase-2 (COX-2), vasoactive intestinal peptide (VIP), substance P, calcitonin gene-related peptide (CGRP), nerve growth factor (NGF), and HIR gene upregulation in animal models of antigen-induced AR (Mlcek et al., 2016). In addition, periostin, a novel marker of respiratory inflammation, and thioredoxin, an antioxidant enzyme, were found to be moderated by quercetin, resulting in an improvement in AR clinical condition (Irie et al., 2016; Edo et al., 2018). The effects of quercetin were also revealed in the clinical setting in which the combination of herbs [quercetin, Perilla frutescens (L.) Britton, and vitamin D3] known as “Lertal®” was effective in relieving the overall symptoms of AR and reducing the use of anti-allergic drugs without additional adverse effects (Thornhill and Kelly, 2000).
Other active compounds in Triphala are ascorbic acid (vitamin C) and kaempferol. In particular, the plasma level of ascorbic acid has been found to be reduced in allergic-related diseases in which patients with allergy-related respiratory and cutaneous symptoms can derive benefits after receiving an exogenous vitamin C treatment (Vollbracht et al., 2018). One study found that vitamin C in combination with exercise potentially reduced inflammatory cytokines in nasal secretion, reduced malondialdehyde (MDA), an important oxidative stress marker, and exhibited improvement in physiological function in rhinitis patients (Seo et al., 2013; Tongtako et al., 2018). Kaempferol moderates several kinds of cytokines and inflammatory markers in both the eosinophil cell line and the mouse model of OVA induction, leading to a reduction in AR-related inflammation (Oh et al., 2013).
Considering the beneficial aspect of Triphala for manipulating the pathophysiology of AR, specific convincing compounds (shown in Table 2.) are likely to be major responsible molecules since many previous studies have revealed their pharmacological activities specifically for physiological processes related to airway inflammation and AR development.
All living cells of organisms are a major source of ROS. Moreover, other exogenous sources of oxidative stress can also induce an exacerbation of AR symptoms. For example, 1) urban pollutants aggravate nasal inflammation and AR-related symptoms, 2) pollen grains that contain endogenous reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase damage airway epithelial cells and trigger granulocyte recruitment (Boldogh et al., 2005). However, our bodies have sophisticated antioxidant defense mechanisms, for example, enzymatic mechanisms, including glutathione-S-transferase (GST), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), as well as non-enzymatic mechanisms involving glutathione, ascorbate, urate, a-tocopherol, bilirubin, lipoic acid, transferrin, and albumin, in order to prevent overwhelming by and accumulation of oxidative stress (Frei et al., 1988; Adwas et al., 2019). The imbalance between ROS production and endogenous antioxidant defense leads to an exhaustion of oxidative stress markers such as NO, MDA, and nitrite/nitrate (Birben et al., 2012; Ayala et al., 2014; Nadif et al., 2014).
Oxidative stress is now considered to be one of the markers of the pathogenesis of AR, asthma, and chronic obstructive pulmonary disease (COPD). Epidemiological and clinical studies of AR have found that eroallergen and pollutant co-exposure can induce immunological effects, leading to recruitment of inflammatory cells, cytokines, chemokines, and finally AR-related symptoms (Naclerio et al., 2020). Ozone exposure was also found to exacerbate antigen-induced rhinitis, nasal symptoms such as rhinorrhea, sneezing, nasal hyperresponsiveness, and eosinophil infiltration in guinea pigs (Iijima et al., 2001). An increase in plasma total oxidant status (TOS) and a decrease in the plasma antioxidative enzyme paraoxonase (PON-1) have both been detected in children with persistent AR, and these two factors were correlated with their nasal symptoms. TOS and PON-1 may serve as disease markers in children with AR (Ozkaya et al., 2013). Therefore, any treatments that can attenuate this increase in oxidants and prevent the decrease in endogenous antioxidants may help improve AR-related symptoms and can be considered a new strategy for treating AR.
In recent decades, several studies have examined and described the antioxidative properties of Triphala, as shown in Table 3. Even with different extraction methods, Triphala retains its ability to scavenge free radicals in both in vitro and in vivo models. For this aspect, several studies reported the activity of Triphala by using chemical assays in which the results do not directly represent the antioxidative properties of Triphala in the actual physiological condition and cannot guarantee its intracellular antioxidant activities (Naik et al., 2006; Liu et al., 2008; Mehrotra et al., 2011; Babu et al., 2013; Gajendra et al., 2016; Varma et al., 2016) However, screening by using such in vitro chemical assays provide beneficial information related to potential antioxidant activities of Triphala, which may be further verified in the cell models or animal models. An enrichment of polyphenolic compounds such as phenolic acids, flavonoids, and tannins is considered to be one of the most important oxidant defensive mechanisms of Triphala (Rice-Evans et al., 1997; Francenia Santos-Sánchez et al., 2019). Thus, quantification of total phenolic content using gallic acid as a standard is necessary (Chandra et al., 2014). Parveen et al. discovered that the methanolic extract of Triphala and its constituents showed a more intense chromogenic reaction than aqueous extracts, and this result supports its greater free radical scavenging ability (Parveen et al., 2018). Interestingly, Triphala possesses radical scavenging effects by reducing the concentrations of oxidative stress molecules and products, and it also works to increase the number of antioxidant enzymes present (Figure 2) (Gupta et al., 2010). Giving Triphala in dimethylhydrazine (DMH)-induced endoplasmic reticulum (ER)-stressed mice for 35 days can prevent an increase in hepatic oxidative products, including MDA and lactate dehydrogenase (LDH), and it also helps to maintain the levels of antioxidative enzymes (GST and GSH) (Sharma and Sharma, 2011). These effects have also been found in a noise-stress induction model, with additional benefits for the restoration of plasma vitamin C and corticosterone levels (Srikumar et al., 2006). Because Triphala contains three different herbs, some researchers have tried to compare the overall ability of Triphala in terms of the potency of different herb extracts. Hazra et al. found that each herb contained individual polyphenolic profiles. The methanolic extract of P. emblica L. expresses higher phenolic and ascorbic contents than those of T. chebula Retz. and T. bellirica (Gaertn.) Roxb., and its scavenging ability for 2,2-diphenyl-1-picrylhydrazyl (DPPH) and peroxynitrite radicals seems to be superior. On the other hand, although T. bellirica (Gaertn.) Roxb. contains fewer polyphenolic compounds than the other two herbs, it still has a greater effect in reducing the number of superoxide, nitric oxide, and hydroxyl radicals (Hazra et al., 2010). However, different sample sources, herbal proportions, and extraction methods can cause variations in the medicinal profiles (Liu et al., 2008; Parveen et al., 2018). Therefore, it is important to carry out herb qualification and characterization before examining the effects of these herbs or permitting their consumption by humans.
Inflammation plays an important role during AR progression (Gelfand, 2004). Several kinds of inflammatory cytokines have been found to be increased in AR patients in both the early and late phases of allergen-induced inflammation. IgE-mediated mast cell degranulation in the early phase is an initial factor in inducing Th2 and T helper cell 17 (Th17) differentiation (Iwakura et al., 2008; Mukai et al., 2018). Cytokines, such as IL-4, IL-13, and IL-17, released by these T cells possess an ability to aggravate nasal mucosa inflammation and disturb the intact mucosal barrier (Wise et al., 2014; Ramezanpour et al., 2016). Many studies in both in vitro and in vivo models have revealed the marked benefits of inflammatory modulation of AR conditions by using various anti-inflammatory approaches (Holgate et al., 2005; Canonica and Baiardini, 2006). An in vitro IL-4 and IL-13 antagonizing treatment and an in vivo anti-IL-4 treatment in mice were found to prevent epithelial barrier disruption (Steelant et al., 2018). Suppression of IL-4, TNF-α, IgE, and eosinophil levels in serum significantly alleviated symptoms and improved QoL in AR patients (Lv et al., 2018). Additionally, the strategy of upregulating anti-inflammatory signaling pathways, nuclear factor erythoid 2-related factor 2/heme oxygenase-1 (Nrf2/HO-1), and suppression of toll-like receptor-myeloid differentiation primary response 88-nuclear factor kappa B (TLR-MyD88-NF- κB) are all promising strategies for relieving nasal inflammation and improving airway epithelial barrier integrity (Bui et al., 2020).
Regardless of the prominent effects of a single herb, the polyherbal Triphala formulation possesses anti-inflammatory activity by acting through several mechanisms (Figure 2; Table 4). Using different extraction methodologies, the herbs retain their ability to reduce both acute and chronic inflammation. For example, at a dose of 100–200 mg/kg, Triphala was shown to be effective in reducing inflammation in carrageenan- and ethyl phenylpropiolate (EPP)-induced paw edema, which are both used as acute inflammatory models (Prabu et al., 2008). Similarly, chronic inflammation in a granuloma formation induced by a cotton pellet was also reduced by a Triphala pretreatment (Prabu et al., 2008). Sireeratawong et al. reported that even though the animal received a high dose of Triphala water extracted at 1,200 mg/kg, the granuloma formation was not reduced by the cotton pellet induction model (Sireeratawong et al., 2013). On the other hand, another study using a similar induction model found that the methanolic extract was beneficial, especially at lower doses (100–200 mg/kg) (Prabu et al., 2008). These results suggest that the methanolic extract might possess higher potency than the water extract, hence further comparisons of the active compounds identified in each extract are needed in order to address this point.
Most of the anti-inflammatory properties of Triphala have been reported in arthritis models. Pretreatment with the herbs significantly alleviated the inflammatory markers and enzymes associated with arthritis (Rasool and Sabina, 2007). Moreover, over-activation and overexpression of the NF-κB pathway and the production of cytokines, chemokines, and growth factors, such as TNF-α, IL-17, TNF-α, IL-1β, IL-6, monocyte chemoattractant protein-1 (MCP-1), vascular endothelial growth factor (VEGF), prostaglandin E2 (PGE2), COX-2, NO, and iNOS, were attenuated when patients were pretreated with Triphala compared with the untreated groups (Kalaiselvan and Rasool, 2016). Compound identification was partially accomplished by Shanmuganathan et al. by focusing on the TNF-α modulatory effect in which chebulinic acid, gallic acid, and the whole Triphala extract showed inhibitory characteristics on inflammation markers. These effects were mediated through the MAPK and NF-κB signaling pathways. Moreover, the authors confirmed the binding capability of these active compounds to TNF-α-receptor-1 by using an in silico (i.e., computer simulated) approach (Shanmuganathan and Angayarkanni, 2018).
Although the effect of Triphala on AR has not yet been investigated, this polyherbal recipe counteracts various kinds of inflammation, suggesting the possibility of using these herbs to treat many inflammation-related diseases, including AR. High penetrating capacity and high toxic threshold are considered to be the advantages of Triphala for developing a preferential therapeutic formulation in the future. Its highly penetrative ability is evident in different parts of various body systems, such as the effects of P. emblica L. in attenuating brain inflammation in epileptic models and the reduction in psoriasis-related skin inflammation by T. chebula Retz (Golechha et al., 2011; An et al., 2016).
Activation and translocation of NF-κB are fundamental steps for increasing inflammation-related gene expression and maintaining airway inflammation (Hart et al., 1998; Schuliga, 2015). Although no study has reported a direct effect of Triphala on airway inflammation, there is some evidence that suggests that this polyherbal recipe might be a good candidate for use in AR treatment. Triphala and its active constituents (T. bellirica (Gaertn.) Roxb. and T. chebula Retz.) exhibited an ability to decrease NF-κB activation and nuclear translocation by suppressing the phosphorylation of p38, c-Jun N-terminal kinase (JNK), and ERK and activating the Akt/AMP-activated protein kinase/nuclear factor erythoid 2-related factor (Akt/AMPK/Nrf) signaling pathway (Tanaka et al., 2018). Therefore, further studies need to be undertaken in order to explore the effects of Triphala in AR models.
A balance between immunostimulation and immunosuppression is important for helping our bodies maintain physiological function. In both autoimmune and inflammatory diseases, immunological imbalance occurs mainly in helper and regulatory T cells (Dejaco et al., 2006; Noack and Miossec, 2014). Especially in AR, which is known as a type I hypersensitivity condition, several immune cells are prone to activation and to be aberrant in both the sensitization and response phases (Kiboneka and Kibuule, 2019). A shift predominantly to Th2 cell responses or a lowering of the Th1/Th2 ratio has been found in several allergic diseases (Romagnani, 2004). Normally, Th1 responses result in IL-2, IFN-gamma, IgG2a, IgG2b, and IgG3 production, whereas Th2 responses lead to IL-3, IL-4, IL-5, IL-13, and IgG1 production (Gelfand, 2004; Galli et al., 2008). Most of the Th2 cytokines act as enhancers of the inflammatory process. For example, IL-3 and IL-5 affect mast cells and eosinophil proliferation and differentiation (Denburg et al., 1994; Kouro and Takatsu, 2009). IL-13 participates in the recruitment of inflammatory cells to inflammation sites by enhancing VCAM-1 expression (De Vries, 1998). An increment in IL-4, which is considered to be a major mediator in AR, can activate IgE production in B cells (Poulsen and Hummelshoj, 2007).
In herbal medicine, quercetin is a well-known candidate compound for AR-related cytokine modulation. This compound is one of the essential elements in the Triphala formulation. Quercetin has been reported to inhibit cytokines such as IL-5, RANTES, and eotaxin released by eosinophils and mast cells (Sakai-Kashiwabara and Asano, 2013). Moreover, Th1 and Th2 markers including IFN-gamma and IL-4, which are aberrantly expressed in an asthmatic model, were found to be modulated by quercetin (Park et al., 2009).
Researchers have found that Triphala and its active constituents have dual activities in relation to both immunostimulant and immunosuppressive effects that might be beneficial in AR treatment (Figure 2; Table 5). Pretreatment with Triphala (1 g/kg/day) improved neutrophil function and reduced Pan T and CD4+/CD8+ along with an increase in corticosteroid levels in a noise-induced stress model (Srikumar et al., 2007). Interestingly, these herbs also reduced IL-4 (Th2 cytokine) and increased IL-2 and IFN-gamma (Th1 cytokines), suggesting an influence on Th1 shifting (Srikumar et al., 2005, 2007). In nonspecific mitogen-induced T lymphocyte proliferation, the application of Triphala reduced T cell proliferation, together with a reduced complement, antibody titer, and a delayed-type hypersensitivity (DTH) response following CFA and sheep red blood cell (SRBC) induction (Sabina et al., 2009). Similar results have also been observed in a single constituent, especially with T. chebula Retz. Atopic lesions induced by 2,4-dinitrofluorobenzene (DNFB) were relieved following topical application of T. chebula Retz. extract. As well as lesion improvement, the extract attenuated atopic markers (MMP-9 and IL-31) and eosinophil infiltration in affected and adjacent areas, respectively (Nam et al., 2011). Another interesting finding was that this extract was able to shift forward the Th1 response by attenuating IL-4 and increasing IFN-gamma in an OVA-induced allergic model (Rubab and Ali, 2016). The results strongly suggest the benefits of using T. chebula Retz. extract for treating AR because the shifting of immunity from Th2 to Th1 is also used to define a successful response following treatment with allergen-specific immunotherapy (AIT) (Lam et al., 2020), and alteration of Th2 cytokines by reducing the Th2 response may offer a kind of protective effect in treating allergic diseases (Bosnjak et al., 2011). Moreover, upregulation of IFN-gamma in T cells is correlated with a reduction in nasal symptoms following exposure to allergens (Durham et al., 1999).
In theory, it is accepted that a balance of the numbers and ratios of Th cells and Tc cells is considered to be a homeostasis marker of the intrinsic immune system. Significant increments in Th cells, Tc cells, and natural killer cells following exposure to Triphala and its active constituents could confirm an additional improvement in the general cellular-mediated immunity of these agents in order to deal with harsh environments and exogenous microorganisms.
Although intranasal corticosteroids are now considered to be the most effective treatment for AR, their mechanism of action acts mainly preferentially through immunosuppression rather than immunomodulation (Bousquet et al., 2008; Hossenbaccus et al., 2020). It will be of interest to find any alternative treatments having dual immunostimulatory and immunosuppressive effects. Luckily, Triphala and its active constituents possess these properties. Thus, this polyherbal formulation might be an important potential candidate in the area of AR therapeutic development.
Following exposure to allergens or the start of the pollen season, some AR patients may have symptoms of cough (dry cough or a cough containing phlegm that is loose in character) together with nasal symptoms (Galway and Shields, 2019). Chronic cough and bronchoconstriction may be caused by bronchial hyper-responsiveness (BHR), which has been reported in AR patients even without asthma (Eggleston, 1988; Polosa et al., 2000). The relationship between AR and BHR may be explained by the following: 1) evidence of the neuronal connection between the upper and lower airways in which the exposure of nasal mucosal to certain stimuli, such as allergens and histamine, has been found to be associated with bronchospasm, and 2) direct contact with local topical nasal inflammatory or systemic inflammatory circulating cytokines from the upper airways might cause inflammation of the lower airways (Braunstahl, 2005; Taylor-Clark et al., 2005; Undem and Taylor-Clark, 2014). IL-13, in particular, which has been reported to not only mediate airway inflammation but is also considered to be a compounding factor driving BHR, may play a role in both upper and lower airway communication (Townley et al., 2009).
The effects of Triphala and its constituents on the lower respiratory system have been reported mainly in animal models. P. emblica L. and T. chebula Retz. extracts have the ability to alleviate coughs induced by mechanical stimulation and citric acid, respectively (Table 6) (Nosál’Ováx et al., 2003; Nosalova et al., 2013). T. bellirica (Gaertn.) Roxb. itself can dose dependently reduce BHR induced by carbachol (Gilani et al., 2008). One study showed that Triphala alleviated bronchial hyper-reactivity via immunomodulation and anti-oxidative pathways in OVA-induced asthma in murines (Horani et al., 2012). Interestingly, some of the airway hypersensitivity endpoints using a whole-body plethysmograph, such as improvement of the pulmonary Penh value, have revealed that Triphala is superior to classical treatments such as the corticosteroid budesonide (Horani et al., 2012). However, although the immunomodulatory effects of these two treatments seem to play an important role in relieving bronchial hyper-reactivity, there are some differences in the immune response. Budesonide acts mainly through an anti-humoral mechanism by inhibiting antibody production. On the other hand, Triphala has no effect on antibody production, but its effects are selective toward lymphocyte distribution in both intra- and extrapulmonary organs (Horani et al., 2012).
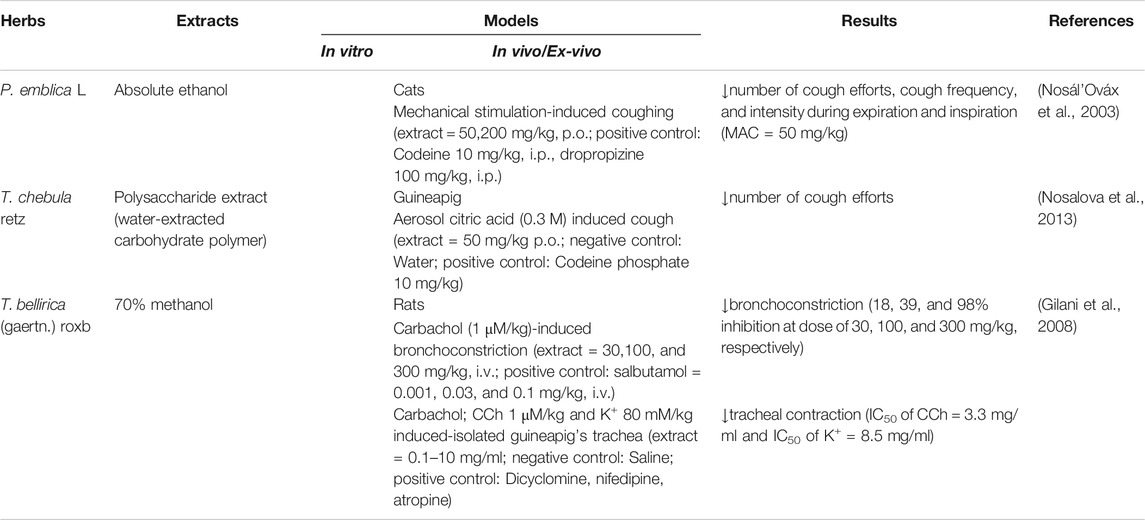
TABLE 6. Triphala and its constituents in the respiratory system (Antitussive effect + Bronchodilator).
AR inflammation is a complex disease that involves several different types of cells and mediators. Specific treatments that aim to manipulate only a single mediator or one type of cell are less likely to be effective. On this basis, it is necessary to employ agents with a broader spectrum of action that can alleviate the complex symptoms of this disease. The realm of complementary and alternative medicine has gained greater attention since much evidence from different study models for specific pharmacological properties (as well as the results from clinical trials) has been accumulated, and that increases the reliability and confidence of using this lineage of medicine. Recently, several plants and their constituents are approved to be safe, effective, and less expensive for managing the development and progression of various diseases, and many of them are already on the WHO’s list of essential medicines. Therefore, Triphala which contains three different plants may be one of the effective remedies to alleviate AR-related symptoms. Experimental data from previous in vitro and in vivo studies of Triphala or individual plants in this remedy have demonstrated potential natural compounds that may be the main acting molecules able to promote the attenuation of AR symptoms. The current review (as shown in Table 2) helps reveal phytochemicals in Triphala that exhibit the promising pharmacological activities that may provide potential benefits in alleviating AR symptoms. Specifically, Triphala contains flavonoids like quercetin. The benefits of quercetin on AR may be through its ability to decrease histamine release from mast cells, and that may help reduce the process of allergic inflammation at the early phase. Moreover, its effects on suppressing the production of cytokines and eosinophil chemoattractants may help reduce the late phase inflammation and eventually the AR symptoms. However, in the clinical view, it may be challenging to utilize quercetin as a monotherapy since the pathophysiology of AR is complex. It would be more convincing to incorporate this compound in the standard regimen if there is more scientific data identifying its pharmacological targets that explain the specific effects of quercetin on alleviating AR. Besides quercetin, kaempferol is another flavonoid that shows interesting inhibitory effects on inflammation in the airways. This compound can suppress mast cell stimulation, production, and release of inflammatory cytokines and chemokines. Triphala also contains ellagic acid which has been shown to reduce histamine release and inflammation in the lung via suppressing the activity of NF-κB, and these effects help speed up the resolution of allergic airway inflammation. Another functional compound found in Triphala is gallic acid which has been reported to suppress nasal histamine release and inflammation. This compound is one of the active components that may help reduce nasal symptoms caused by autacoids and inflammatory cytokines. Similarly, chebulagic acid has been shown to possess anti-inflammatory properties by reducing cytokine production and inflammatory enzymes including COX-2 and LOX. Moreover, Triphala contains various natural compounds that may help suppress allergic responses through their anti-oxidant potential. Those compounds include vitamin C, chebulinic acid, and emblicanin. These compounds function mainly in scavenging free radicals and reducing oxidative stress; therefore, they may be the responsible compounds to help suppress exacerbation of AR-related symptoms. In short, Triphala is explored to be an important source of bioactive compounds able to be used for AR treatments. The orchestration of these compounds may create synergistic effects to decrease the exacerbation of inflammatory response and oxidative stress that lead to AR development.
The anti-inflammatory, immunomodulatory, antioxidant, and antibacterial activities of Triphala are similar to those of other herbal formulas clinically proven to treat AR effectively, such as Shi-Bi-Lin (a Chinese herbal formula), butterbur, and Nigella sativa (black caraway or black cumin). However, there have not been any in vivo studies that utilize a direct model for AR to test the effects of Triphala. The ovalbumin-induced guinea pig model of AR could well be a proper assay to definitively evaluate if Triphala can reduce AR-associated inflammation. However, there is a limitation to these types of study models because it is not a direct AR model to give us enough reliable information specific to AR. It would provide strong evidence if in the future there are direct experimental designs specifically developed for animal AR models to verify the effectiveness of Triphala.
During allergic inflammation, the nasal epithelial barriers of AR patients appear to become impaired. Consequently, this condition leads to the development of sinusitis and otitis media, with effusion caused by bacteria, viruses, and fungi. Although Triphala shows promise in being an effective bacteriostatic/bactericidal agent, most studies have focused only on oral streptococci. Thus, further studies are needed to address whether Triphala is effective in killing other types of bacteria, as well as viruses and fungi that are related to allergic rhinosinusitis. Additionally, clinical studies should be conducted to verify the efficacy of this herbal formula. As a step toward the clinical investigation of Triphala for its efficacy and safety, our research team has created the study design/clinical trial, and the study protocol has already been registered in Thai Clinical Trials Registry (TCTR20191008005). Moreover, study models for pharmacokinetics are crucial to fulfill all aspects of the pharmacology of Triphala and to obtain valuable information about the active compound profiles. If the accumulated data clearly show that Triphala can be used effectively to inhibit inflammation, modulate immunity, alleviate unwanted symptoms in patients with AR, and prevent AR-related infections, this will provide a novel alternative treatment for patients. For example, Triphala could be used as a monotherapy in patients with mild AR. For patients with moderate-to-severe AR (for which intranasal corticosteroids are usually prescribed) but who do not want to use steroidal drugs, Triphala may be used in combination with an antihistamine. For patients using steroids who have inadequate control of AR, Triphala may be added to the treatment plan to reduce the use of high-dose steroids. Similarly, for patients who require immunotherapy, a Triphala supplement may offer a faster and better response to immunotherapy.
In conclusion, Triphala exhibits beneficial effects if used as an alternative treatment for AR. However, extensive investigations in both animals and humans are first needed to be certain about its definitive mechanisms of action and the efficacy/safety of treatments. Although most studies reported the pharmacological effects of Triphala in a reasonable range of concentrations, some studies evaluated the effects of Triphala at relatively high doses. This may create concerns about its low efficacy and toxicity. With this, specifying the efficacy and adverse effects of Triphala is a vital base to ensure its safe use. Triphala should be carefully examined further to gain adequate data about toxic target organs, safe dose range, safety window of effective dose, and minimum toxic dose. Considering the actual applications, appropriate dosage and course of treatment are required to be precisely adjusted to ensure that Triphala is safe and effective. The accumulated evidence obtained from such studies will be beneficial to properly prescribe Triphala to individual AR patients with different clinical symptoms and varying degrees of severity in order to achieve the highest efficacy with the fewest side effects.
Conceptualization, NH and SP; writing—original draft preparation, SJ, NH, SP, PH and WN; writing—review and editing, SJ, NH, SP, and WN. All authors have read and agreed to the published version of the manuscript.
This work was supported by the Faculty of Medicine, Chiang Mai University (Grant Number 061–2563).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
PH was supported in part by Grants from the Teaching Assistant and Research Assistant. (TA/RA) scholarships, Graduate School, Chiang Mai University, Chiang Mai, Thailand.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.628198/full#supplementary-material.
Adwas, A. A., Sedik, A., Elsayed, I., Azab, A. E., and Quwaydir, F. A. (2019). Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 11, 23. doi:10.15406/jabb.2019.06.00173
Aher, V. D., Kumar, A., and Wahi, (2010). Immunomodulatory effect of alcoholic extract of Terminalia chebula ripe fruits. J. Pharm. Sci. Res. 6, 121. doi:10.21276/ap.2017.6.2.15
Aher, V., and Wahi, A. (2011). Immunomodulatory activity of alcohol extract of Terminalia chebula retz combretaceae. Trop. J. Pharm. Res. 10 (5), 37. doi:10.4314/tjpr.v10i5.5
Alsuleimani, Y. M, and Walker, M.J A. (2007). Allergic rhinitis and its pharmacology. Pharmacol. Ther. 114 (3), 233–260. doi:10.1016/j.pharmthera.2007.01.012
An, J., Li, T., Dong, Y., Li, Z., and Huo, J. (2016). Terminalia chebulanin attenuates psoriatic skin lesion via regulation of heme oxygenase-1. Cell. Physiol. Biochem 39 (2), 531–43. doi:10.1159/000445645
Athira, A. P., Helen, A., Saja, K., Reddanna, P., and Sudhakaran, P. R. (2013). Inhibition of AngiogenesisIn vitroby chebulagic acid: a COX-LOX dual inhibitor. Int. J. Vasc. Med. 13, 1–8. doi:10.1155/2013/843897
Ayala, A., Muñoz, M. F., and Argüelles, S. (2014). Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell Longevity 2014, 360438. doi:10.1155/2014/360438
Babu, D., Gurumurthy, P., Borra, S. K., and Cherian, K. M. (2013). Falling through the safety net: Americans without health insurance. Choice Reviews Online 7, 2898–2905. doi:10.5897/JMPR2013
Bag, A., Kumar Bhattacharyya, S., Kumar Pal, N., and Ranjan Chattopadhyay, R. (2013). Anti-inflammatory, anti-lipid peroxidative, antioxidant and membrane stabilizing activities of hydroalcoholic extract ofTerminalia chebulafruits. Pharm. Biol. 51 (12), 1515–1520. doi:10.3109/13880209.2013.799709
Bernstein, D. I., Schwartz, G., and Bernstein, J. A. (2016). Allergic rhinitis: mechanisms and treatment. Immunol. Allergy Clin. North America 36 (2), 261–78. doi:10.1016/j.iac.2015.12.004
Bharadwaj, A. S., Bewtra, A. K., and Agrawal, D. K. (2007). Dendritic cells in allergic airway inflammationThis article is one of a selection of papers published in the Special Issue on Recent Advances in Asthma Research. Can. J. Physiol. Pharmacol. 85 (7), 686–699. doi:10.1139/Y07-062
Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S., and Kalayci, O. (2012). Oxidative stress and antioxidant defense. World Allergy Organ. J. 5, 9. doi:10.1097/WOX.0b013e3182439613
Björkstén, B., Clayton, T., Ellwood, P., Stewart, A., Strachan, D., Phase III Study Group, t. I., et al. (2008). Worldwide time trends for symptoms of rhinitis and conjunctivitis: phase III of the international study of asthma and allergies in childhood. Pediatr. Allergy Immunol. 19 (2), 110–124. doi:10.1111/j.1399-3038.2007.00601.x
Boldogh, I., Bacsi, A., Choudhury, B. K., Dharajiya, N., Alam, R., Hazra, T. K., et al. (2005). ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Invest. 115 (8), 2169–2179. doi:10.1172/JCI24422
Bosnjak, B., Stelzmueller, B., Erb, K. J., and Epstein, M. M. (2011). Treatment of allergic asthma: modulation of Th2 cells and their responses. Respir. Res. 12(1), 114. doi:10.1186/1465-9921-12-114
Bousquet, J., Khaltaev, N., Cruz, A. A., Denburg, J., Fokkens, W. J., Togias, A., et al. (2008). Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the world health organization, GA(2)len and AllerGen). Allergy 63 Suppl 86, 8–160. doi:10.1111/j.1398-9995.2007.01620.x
Braunstahl, G.-J. (2005). The unified immune system: respiratory tract-nasobronchial interaction mechanisms in allergic airway disease. J. Allergy Clin. Immunol. 115 (1), 142–148. doi:10.1016/j.jaci.2004.10.041
Bui, T. T., Fan, Y., Piao, C. H., Nguyen, T. V., Shin, D.-u., Jung, S. Y., et al. (2020). Piper Nigrum extract improves OVA-induced nasal epithelial barrier dysfunction via activating Nrf2/HO-1 signaling. Cell Immunol. 351, 104035. doi:10.1016/j.cellimm.2019.104035
Burton, O. T., and Oettgen, H. C. (2011). Beyond immediate hypersensitivity: evolving roles for IgE antibodies in immune homeostasis and allergic diseases. Immunol. Rev. 242 (1), 128–183. doi:10.1111/j.1600-065X.2011.01024.x
Canonica, G., and Baiardini, I. (2006). Minimal persistent inflammation in allergic rhinitis: Its clinical implications. Allergy Clin. Immunol. Int. - J. World Allergy Organ. 18, 169. doi:10.1027/0838-1925.18.4.169
Cao, J., Li, C., Ma, P., Ding, Y., Gao, J., Jia, Q., et al. (2020). Effect of kaempferol on IgE-mediated anaphylaxis in C57BL/6 mice and LAD2 cells. Phytomedicine 79, 153346. doi:10.1016/j.phymed.2020.153346
Chandra, S., Khan, S., Avula, B., Lata, H., Yang, M. H., Elsohly, M. A., et al. (2014). Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evidence-Based Complement. Altern. Med. 2014, 253875. doi:10.1155/2014/253875
Chang, H.-H., Chen, C.-S., and Lin, J.-Y. (2009). High dose vitamin C supplementation increases the Th1/Th2 cytokine secretion ratio, but decreases eosinophilic infiltration in bronchoalveolar lavage fluid of ovalbumin-sensitized and challenged mice. J. Agric. Food Chem. 57 (21), 10471–10476. doi:10.1021/jf902403p
Charoenteeraboon, J., Ngamkitidechakul, C., Soonthornchareonnon, N., Jaijoy, K., and Sireeratawong, S. (2010). Antioxidant activities of the standardized water extract from fruit of phyllanthus emblica linn. Songklanakarin J. Sci. Technol. 77, 547. doi:10.1055/s-0031-1282780
Chauhan, P., Singh, S., Gupta, Y., and Kumar, U. (2018). Evaluation of toxicity studies and anti-inflammatory activity of Terminalia Bellerica in carrageenan-induced paw edema in experimental rats. J. Nat. Sci. Biol. Med. 9 (2), 169–174. doi:10.4103/jnsbm.JNSBM_159_17
Choi, Y. H., and Yan, G. H. (2009). Ellagic acid attenuates immunoglobulin e-mediated allergic response in mast cells. Biol. Pharm. Bull. 32 (6), 1118–1121. doi:10.1248/bpb.32.1118
Cornélio Favarin, D., Martins Teixeira, M., Lemos De Andrade, E., De Freitas Alves, C., Lazo Chica, J. E., Artério Sorgi, C., et al. (2013). Anti-inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediators Inflamm. 2013, 1. doi:10.1155/2013/164202
Cortes, J. R., Perez-G, M., Rivas, M. D., and Zamorano, J. (2007). Kaempferol inhibits IL-4-induced STAT6 activation by specifically targeting JAK3. J. Immunol. 179 (6), 3881–3887. doi:10.4049/jimmunol.179.6.3881
De Freitas Alves, C., Angeli, G. N., Favarin, D. C., Lemos De Andrade, E., Lazo Chica, J. E., Faccioli, L. H., et al. (2013). The effects of proresolution of ellagic acid in an experimental model of allergic airway inflammation. Mediators Inflamm. 2013, 1. doi:10.1155/2013/863198
De Vries, J. E. (1998). The role of IL-13 and its receptor in allergy and inflammatory responses. J. Allergy Clin. Immunol. 102 (9), 165–169. doi:10.1016/S0091-6749(98)70080-6
Dejaco, C., Duftner, C., Grubeck-Loebenstein, B., and Schirmer, M. (2006). Imbalance of regulatory T cells in human autoimmune diseases. Immunology 117 (3), 289–300. doi:10.1111/j.1365-2567.2005.02317.x
Denburg, J. A., Woolley, M., Leber, B., Linden, M., and O'Byrne, P. (1994). Basophil and eosinophil differentiation in allergic reactions. J. Allergy Clin. Immunol. 94 (6 pt 2), 1135–1141. doi:10.1016/0091-6749(94)90321-2
Deshmukh, C. D., Veeresh, B., and Pawar, A. T. (2010). Protective effect OF emblica officinalis fruit extract ON acetic acid induced colitis IN rats. J. Herb. Med. Toxicol. 7, 127. doi:10.18535/jmscr/v7i7.122
Dreyer, N. A., Bryant, A., and Velentgas, P. (2016). The GRACE checklist: a validated assessment tool for high quality observational studies of comparative effectiveness. Jmcp 22 (10), 1107–1113. doi:10.18553/jmcp.2016.22.10.1107
Durham, S. R., Walker, S. M., Varga, E.-M., Jacobson, M. R., O'Brien, F., Noble, W., et al. (1999). Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 341 (7), 468–475. doi:10.1056/NEJM199908123410702
Ebihara, N., Takahashi, K., Takemura, H., Akanuma, Y., Asano, K., and Sunagawa, M. (2018). Suppressive effect of quercetin on nitric oxide production from nasal epithelial cells in vitro. Evid-Based Complement. Altern. Med. 2018, 1. doi:10.1155/2018/6097625
Edo, Y., Otaki, A., and Asano, K. (2018). Quercetin enhances the thioredoxin production of nasal epithelial cells in vitro and in vivo. Medicines 5 (4), 124. doi:10.3390/medicines5040124
Eggleston, P. A. (1988). Upper airway inflammatory diseases and bronchial hyperresponsiveness. J. Allergy Clin. Immunol. 81 (5 pt 2), 1036–1041. doi:10.1016/0091-6749(88)90176-5
Fan, Y., Piao, C. H., Hyeon, E., Jung, S. Y., Eom, J.-E., Shin, H. S., et al. (2019). Gallic acid alleviates nasal inflammation via activation of Th1 and inhibition of Th2 and Th17 in a mouse model of allergic rhinitis. Int. Immunopharmacol. 70, 512–519. doi:10.1016/j.intimp.2019.02.025
Fanning, L. B., and Boyce, J. A. (2013). Lipid mediators and allergic diseases. Ann. Allergy Asthma Immunol. 111 (3), 155–162. doi:10.1016/j.anai.2013.06.031
Forte, G. C., da Silva, D. T. R., Hennemann, M. L., Sarmento, R. A., Almeida, J. C., and de Tarso Roth Dalcin, P. (2018). Diet effects in the asthma treatment: a systematic review. Crit. Rev. Food Sci. Nutr. 58 (11), 1878–1887. doi:10.1080/10408398.2017.1289893
Francenia Santos-Sánchez, N., Salas-Coronado, R., Villanueva-Cañongo, C., and Hernández-Carlos, B. (2019). Antioxidant compounds and their antioxidant mechanism. Antioxidation 7, 347. doi:10.5772/intechopen.85270
Frei, B., Stocker, R., and Ames, B. N. (1988). Antioxidant defenses and lipid peroxidation in human blood plasma. Proc. Natl. Acad. Sci. 85 (24), 9748–9752. doi:10.1073/pnas.85.24.9748
Gadermaier, E., Levin, M., Flicker, S., and Ohlin, M. (2014). The human IgE repertoire. Int. Arch. Allergy Immunol. 163 (2), 77–91. doi:10.1159/000355947
Gajendra, S., Pushkar, C., Syed, A. Y., Rajveer, S. R., and Bhanwar, L. J. (2016). Research article antibacterial and antioxidant activity of triphala extracts. Int. J. Curr. Res. 8, 38335–38348. doi:10.7176/cmr/11-6-01
Galli, S. J., Tsai, M., and Piliponsky, A. M. (2008). The development of allergic inflammation. Nature 454 (7203), 445–454. doi:10.1038/nature07204
Galway, N. C., and Shields, M. D. (2019). The child with an incessant dry cough. Paediatric Respir. Rev. 30, 58–64. doi:10.1016/j.prrv.2018.08.002
Gelfand, E. (2004). Inflammatory mediators in allergic rhinitis. J. Allergy Clin. Immunol. 114 (5 Suppl), S135–S138. doi:10.1016/j.jaci.2004.08.043
Gilani, A. H., Khan, A.-u., Ali, T., and Ajmal, S. (2008). Mechanisms underlying the antispasmodic and bronchodilatory properties of Terminalia bellerica fruit. J. Ethnopharmacol. 116 (3), 528–538. doi:10.1016/j.jep.2008.01.006
Ginsburg, I., Koren, E., Horani, A., Mahamid, M., Doron, S., Muhanna, N., et al. (2009). Amelioration of hepatic fibrosis via Padma Hepaten is associated with altered natural killer T lymphocytes. Clin. Exp. Immunol. 157 (1), 155–164. doi:10.1111/j.1365-2249.2009.03936.x
Golechha, M., Bhatia, J., Ojha, S., and Arya, D. S. (2011). Hydroalcoholic extract of Emblica officinalis protects against kainic acid-induced status epilepticus in rats: evidence for an antioxidant, anti-inflammatory, and neuroprotective intervention. Pharm. Biol. 49 (11), 1128–1136. doi:10.3109/13880209.2011.571264
Golechha, M., Sarangal, V., Ojha, S., Bhatia, J., and Arya, D. S. (2014). Anti-inflammatory effect of Emblica officinalis in rodent models of acute and chronic inflammation: involvement of possible mechanisms. Int. J. Inflamm. 2014, 1. doi:10.1155/2014/178408
Gong, J.-H., Cho, I.-H., Shin, D., Han, S.-Y., Park, S.-H., and Kang, Y.-H. (2014). Inhibition of airway epithelial-to-mesenchymal transition and fibrosis by kaempferol in endotoxin-induced epithelial cells and ovalbumin-sensitized mice. Lab. Invest. 94 (3), 297–308. doi:10.1038/labinvest.2013.137
Gong, J.-H., Shin, D., Han, S.-Y., Kim, J.-L., and Kang, Y.-H. (2012). Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J. Nutr. 142 (1), 47–56. doi:10.3945/jn.111.150748
Gupta, S., Kalaiselvan, V., Srivastava, S., Agrawal, S., and Saxena, R. (2010). Evaluation of anticataract potential of Triphala in selenite-induced cataract: in vitro and in vivo studies. J. Ayurveda Integr. Med. 1 (4), 280–286. doi:10.4103/0975-9476.74425
Hart, L. A., Krishnan, V. L., Adcock, I. M., Barnes, P. J., and Chung, K. F. (1998). Activation and localization of transcription factor, nuclear factor- κ B, in asthma. Am. J. Respir. Crit. Care Med. 158, 1585. doi:10.1164/ajrccm.158.5.9706116
Hattori, M., Mizuguchi, H., Baba, Y., Ono, S., Nakano, T., Zhang, Q., et al. (2013). Quercetin inhibits transcriptional up-regulation of histamine H1 receptor via suppressing protein kinase C-δ/extracellular signal-regulated kinase/poly(ADP-ribose) polymerase-1 signaling pathway in HeLa cells. Int. Immunopharmacology 15 (2), 232–239. doi:10.1016/j.intimp.2012.12.030
Hazra, B., Sarkar, R., Biswas, S., and Mandal, N. (2010). Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalis. BMC Complement. Altern. Med. 10, 20. doi:10.1186/1472-6882-10-20
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best practice in research - overcoming common challenges in phytopharmacological research. J. Ethnopharmacology 246, 112230. doi:10.1016/j.jep.2019.112230
Holgate, S., Casale, T., Wenzel, S., Bousquet, J., Deniz, Y., and Reisner, C. (2005). The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J. Allergy Clin. Immunol. 115 (3), 459–465. doi:10.1016/j.jaci.2004.11.053
Horani, A., Shoseyov, D., Ginsburg, I., Mruwat, R., Doron, S., Amer, J., et al. (2012). Triphala (PADMA) extract alleviates bronchial hyperreactivity in a mouse model through liver and spleen immune modulation and increased anti-oxidative effects. Ther. Adv. Respir. 6 (4), 199–210. doi:10.1177/1753465812452194
Hossenbaccus, L., Linton, S., Garvey, S., and Ellis, A. K. (2020). Towards definitive management of allergic rhinitis: best use of new and established therapies. Allergy Asthma Clin. Immunol. 16, 39. doi:10.1186/s13223-020-00436-y
Huang, C.-H., Jan, R.-L., Kuo, C.-H., Chu, Y.-T. W. L., Wang, W.-L., Lee, M.-S., et al. (2010). Natural flavone kaempferol suppresses chemokines expression in human monocyte THP-1 cells through MAPK pathways. J. Food Sci. 75 (8), H254–H259. doi:10.1111/j.1750-3841.2010.01812.x
Ibne Jami, M. S., Sultana, Z., Ali, M. E., Begum, M. M., and and Haque, M. M. (2014). Evaluation of analgesic and anti-inflammatory activities on ethanolic extract of Terminalia chebula fruits in experimental animal models. Am. J. Plant Sci 05 (01), 63–69. doi:10.4236/ajps.2014.51010
Iijima, M. K., Kobayashi, T., Kamada, H., and Shimojo, N. (2001). Exposure to ozone aggravates nasal allergy-like symptoms in Guinea pigs. Toxicol. Lett. 123 (1), 77–85. doi:10.1016/S0378-4274(01)00392-7
Irie, S., Kashiwabara, M., Yamada, A., and Asano, K. (2016). Suppressive activity of quercetin on periostin functions in vitro. In Vivo 30 (1), 17–25. doi:10.4172/2155-6121.1000253
Iwakura, Y., Nakae, S., Saijo, S., and Ishigame, H. (2008). The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol. Rev. 226, 57–59. doi:10.1111/j.1600-065X.2008.00699.x
Jayesh, K., Karishma, R., Vysakh, A., Gopika, P., and Latha, M. S. (2018). Terminalia bellirica (Gaertn.) Roxb fruit exerts anti-inflammatory effect via regulating arachidonic acid pathway and pro-inflammatory cytokines in lipopolysaccharide-induced RAW 264.7 macrophages. Inflammopharmacol 28, 265. doi:10.1007/s10787-018-0513-x
Jung, J.-W., Kang, H.-R., Ji, G.-E., Park, M.-S., Song, W.-J., Kim, M.-H., et al. (2011). Therapeutic effects of fermented red ginseng in allergic rhinitis: a randomized, double-blind, placebo-controlled study. Allergy Asthma Immunol. Res. 3 (2), 103–110. doi:10.4168/aair.2011.3.2.103
Jung, J. H., Kang, I. G., Kim, D. Y., Hwang, Y. J., and Kim, S. T. (2013). The effect of Korean red ginseng on allergic inflammation in a murine model of allergic rhinitis. J. Ginseng Res. 37 (2), 167–175. doi:10.5142/jgr.2013.37.167
Kalaiselvan, S., and Rasool, M. K. (2015). The anti-inflammatory effect oftriphalain arthritic-induced rats. Pharm. Biol. 53, 51. doi:10.3109/13880209.2014.910237
Kalaiselvan, S., and Rasool, M. K. (2016). Triphala herbal extract suppresses inflammatory responses in LPS-stimulated RAW 264.7 macrophages and adjuvant-induced arthritic rats via inhibition of NF-κB pathway. J. Immunotoxicol. 13 (4), 509–525. doi:10.3109/1547691X.2015.1136010
Kang, D. R., Belal, S. A., Choe, H. S., Shin, D. K., and Shim, K. S. (2018). Effect of kaempferol on cyclooxygenase 2 (Cox2) and cytosolic phospholipase A2 (cPLA2) protein expression in BALB/c mice. Iran J Allergy Asthma Immunol 17 (5), 428–435. doi:10.18502/ijaai.v17i5.301
Kashiwabara, M., Asano, K., Mizuyoshi, T., and Kobayashi, H. (2016). Suppression of neuropeptide production by quercetin in allergic rhinitis model rats. BMC Complement. Altern. Med. 16, 132. doi:10.1186/s12906-016-1123-z
Kawamoto, Y., Ueno, Y., Nakahashi, E., Obayashi, M., Sugihara, K., Qiao, S., et al. (2016). Prevention of allergic rhinitis by ginger and the molecular basis of immunosuppression by 6-gingerol through T cell inactivation. J. Nutr. Biochem. 27, 112–122. doi:10.1016/j.jnutbio.2015.08.025
Khan, T., Sankhe, K., Suvarna, V., Sherje, A., Patel, K., and Dravyakar, B. (2018). DNA gyrase inhibitors: progress and synthesis of potent compounds as antibacterial agents. Biomed. Pharmacother. 103, 923–938. doi:10.1016/j.biopha.2018.04.021
Kiboneka, A., and Kibuule, D. (2019). The immunology of asthma and allergic rhinitis. Rhinosinusitis 12, 77. doi:10.5772/intechopen.86964
Kim, M., Lim, S. J., Kang, S. W., Um, B.-H., and Nho, C. W. (2014). Aceriphyllum rossiiExtract and its active compounds, quercetin and kaempferol inhibit IgE-mediated mast cell activation and passive cutaneous anaphylaxis. J. Agric. Food Chem. 62 (17), 3750–3758. doi:10.1021/jf405486c
Kim, S.-H., Jun, C.-D., Suk, K., Choi, B.-J., Lim, H., Park, S., et al. (2006). Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol. Sci. 91 (1), 123–131. doi:10.1093/toxsci/kfj063
Kimata, M., Shichijo, M., Miura, T., Serizawa, I., Inagaki, N., and Nagai, H. (2000). Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin. Exp. Allergy 30 (4), 501–508. doi:10.1046/j.1365-2222.2000.00768.x
Kirubanandan, S., Ravi, B., and Renganathan, S. (2015). Anti-inflammatory and analgesic activities of methanol extract of Terminalia chebula fruits. Int. J. Pharm. Chem. Sci. 4, 400–404. doi:10.4236/pp.2015.612056
Klimek, L., Bachert, C., Pfaar, O., Becker, S., Bieber, T., Brehler, R., et al. (2019). ARIA guideline 2019: treatment of allergic rhinitis in the German health system. Allergo J. Int. 28, 255. doi:10.1007/s40629-019-00110-9
Kouro, T., and Takatsu, K. (2009). IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int. Immunol. 21, 1303–1309. doi:10.1093/intimm/dxp102
Lacy, P. (2006). Mechanisms of degranulation in neutrophils. Allergy Asthma Clin. Immunol. 2 (3), 98–108. doi:10.1186/1710-1492-2-3-98
Lam, H. Y., Tergaonkar, V., and Ahn, K. S. (2020). Mechanisms of allergen-specific immunotherapy for allergic rhinitis and food allergies. Biosci. Rep. 40 (4). BSR20200256. doi:10.1042/BSR20200256
Lambrecht, B. N., and Hammad, H. (2010). The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet 376 (9743), 835–843. doi:10.1016/S0140-6736(10)61226-3
Lee, B. J., Naclerio, R. M., Bochner, B. S., Taylor, R. M., Lim, M. C., and Baroody, F. M. (1994). Nasal challenge with allergen upregulates the local expression of vascular endothelial adhesion molecules. J. Allergy Clin. Immunol. 94 (6 pt 1), 1006–1016. doi:10.1016/0091-6749(94)90119-8
Lee, E.-J., Ji, G.-E., and Sung, M.-K. (2010). Quercetin and kaempferol suppress immunoglobulin E-mediated allergic inflammation in RBL-2H3 and Caco-2 cells. Inflamm. Res. 59 (10), 847–854. doi:10.1007/s00011-010-0196-2
Lee, H.-S., Jung, S.-H., Yun, B.-S., and Lee, K.-W. (2007). Isolation of chebulic acid from Terminalia chebula Retz. and its antioxidant effect in isolated rat hepatocytes. Arch. Toxicol. 81, 211. doi:10.1007/s00204-006-0139-4
Lee, S.-I., Hyun, P.-M., Kim, S.-H., Kim, K.-S., Lee, S.-K., Kim, B.-S., et al. (2005). Suppression of the onset and progression of collagen-induced arthritis by chebulagic acid screened from a natural product library. Arthritis Rheum. 52, 345. doi:10.1002/art.20715
Li, B. W. S., and Hendriks, R. W. (2013). Group 2 innate lymphoid cells in lung inflammation. Immunology. 140 (3), 281–287. doi:10.1111/imm.12153
Li, P., Du, R., Wang, Y., Hou, X., Wang, L., Zhao, X., et al. (2020). Identification of chebulinic acid and chebulagic acid as novel influenza viral neuraminidase inhibitors. Front. Microbiol. 11, 182. doi:10.3389/fmicb.2020.00182
Liu, X., Zhao, M., Wang, J., Yang, B., and Jiang, Y. (2008). Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L.) from six regions in China. J. Food Compost. Anal. 21, 219. doi:10.1016/j.jfca.2007.10.001
Liu, Y., Bao, L., Xuan, L., Song, B., Lin, L., and Han, H. (2015). Chebulagic acid inhibits the LPS-induced expression of TNF-α and IL-1β in endothelial cells by suppressing MAPK activation. Exp. Ther. Med. 10 (1), 263–268. doi:10.3892/etm.2015.2447
Lv, C., Zhang, Y., and Shen, L. (2018). Preliminary clinical effect evaluation of resveratrol in adults with allergic rhinitis. Int. Arch. Allergy Immunol. 175, 231. doi:10.1159/000486959
Mahesh, R., Bhuvana, S., and Hazeena Begum, V. M. (2009). Effect ofTerminalia chebulaaqueous extract on oxidative stress and antioxidant status in the liver and kidney of young and aged rats. Cell Biochem. Funct. 27 (6), 358–363. doi:10.1002/cbf.1581
Mehrotra, S., Jamwal, R., Shyam, R., Meena, D. K., Mishra, K., Patra, R., et al. (2011). Anti-helicobacter pylori and antioxidant properties of Emblica officinalis pulp extract: a potential source for therapeutic use against gastric ulcer. J. Med. Plants Res. 14, 203–209. doi:10.1046/j.1365-2036.2000.00679.x
Middha, S. K., Goyal, A. K., Lokesh, P., Yardi, V., Mojamdar, L., Keni, D. S., et al. (2015). Toxicological evaluation of emblica officinalis fruit extract and its anti-inflammatory and free radical scavenging properties. Pharmacogn. Mag. 11, S427. doi:10.4103/0973-1296.168982
Min, Y.-D., Choi, C.-H., Bark, H., Son, H.-Y., Park, H.-H., Lee, S., et al. (2007). Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 56 (5), 210. doi:10.1007/s00011-007-6172-9
Min, Y.-G. (2010). The pathophysiology, diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol. Res. 2, 65. doi:10.4168/aair.2010.2.2.65
Miraglia Del Giudice, M., Maiello, N., Decimo, F., Capasso, M., Campana, G., Leonardi, S., et al. (2014). Resveratrol plus carboxymethyl-β-glucan may affect respiratory infections in children with allergic rhinitis. Pediatr. Allergy Immunol. 25 (7), 724–728. doi:10.1111/pai.12279
Mlcek, J., Jurikova, T., Skrovankova, S., and Sochor, J. (2016). Quercetin and its anti-allergic immune response. Molecules 21 (5), 623. doi:10.3390/molecules21050623
Mukai, K., Tsai, M., Saito, H., and Galli, S. J. (2018). Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 282 (1), 121–150. doi:10.1111/imr.12634
Murdock, N. (2015). Evaluation of Terminalia chebula extract for anti-arthritic efficacy and safety in osteoarthritic dogs. J. Veterinar Sci. Technol. 7, 28. doi:10.4172/2157-7579.1000290
Muthuraman, A., Sood, S., and Singla, S. K. (2011). The antiinflammatory potential of phenolic compounds from Emblica officinalis L. in rat. Inflammopharmacol 19 (6), 327–334. doi:10.1007/s10787-010-0041-9
Naclerio, R., Ansotegui, I. J., Bousquet, J., Canonica, G. W., D'Amato, G., Rosario, N., et al. (2020). International expert consensus on the management of allergic rhinitis (AR) aggravated by air pollutants. World Allergy Organ. J. 13 (3), 100106. doi:10.1016/j.waojou.2020.100106
Nadif, R., Rava, M., Decoster, B., Huyvaert, H., Le Moual, N., Bousquet, J., et al. (2014). Exhaled nitric oxide, nitrite/nitrate levels, allergy, rhinitis and asthma in the EGEA study. Eur. Respir. J. 44 (2), 351–360. doi:10.1183/09031936.00202413
Naik, G. H., Priyadarsini, K. I., and Mohan, H. (2006). Free radical scavenging reactions and phytochemical analysis of triphala, an ayurvedic formulation. Curr. Sci. 90 (8), 49.
Nair, V., Kumar, R., Singh, S., and Gupta, Y. K. (2012). Anti-granuloma activity ofTerminalia ChebulaRetz. In wistar RatsWistar rats. Eur. J. Inflamm. 10 (2), 185–192. doi:10.1177/1721727X1201000203
Nair, V., Singh, S., and Gupta, Y. K. (2010). Anti-arthritic and disease modifying activity of Terminalia chebula Retz. in experimental modelsexperimental models. J. Pharm. Pharmacol. 62 (12), 1801–1811. doi:10.1111/j.2042-7158.2010.01193.x
Nam, D. Y., Lee, J. M., Heo, J. C., and Lee, S. H. (2011). Mitigation of 2,4-dinitrofluorobenzene-induced atopic dermatitis-related symptoms by Terminalia chebula Retzius. Int. J. Mol. Med. 28, 1013–1018. doi:10.3892/ijmm.2011.792
Noack, M., and Miossec, P. (2014). Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 13 (6), 668–677. doi:10.1016/j.autrev.2013.12.004
Nosalova, G., Jurecek, L., Chatterjee, U. R., Majee, S. K., Nosal, S., and Ray, B. (2013). Antitussive activity of the water-extracted carbohydrate polymer fromTerminalia chebulaon citric acid-induced cough. Evidence-Based Complement. Altern. Med. 2013, 1. doi:10.1155/2013/650134
Nosál’Ováx, G., Mokrý, J., and Hassan, K. M. T. (2003). Antitussive activity of the fruit extract of Emblica officinalis Gaertn. (Euphorbiaceae). Phytomedicine. 10 (6-7), 583–589. doi:10.1078/094471103322331872
Oh, H.-A., Han, N.-R., Kim, M.-J., Kim, H.-M., and Jeong, H.-J. (2013). Evaluation of the effect of kaempferol in a murine allergic rhinitis model. Eur. J. Pharmacol. 718 (1-3), 48–56. doi:10.1016/j.ejphar.2013.08.045
Ozkaya, E., Akduman, H., Erenberk, U., Demir, A., and Dundaroz, M. R. (2013). Plasma paraoxonase activity and oxidative stress and their relationship to disease severity in children with allergic rhinitis. Am. J. Rhinol Allergy 27, 13. doi:10.2500/ajra.2013.27.3837
Park, H.-j., Lee, C.-M., Jung, I. D., Lee, J. S., Jeong, Y.-i., Chang, J. H., et al. (2009). Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int. Immunopharmacology 9 (3), 261–267. doi:10.1016/j.intimp.2008.10.021
Parveen, R., Shamsi, T. N., Singh, G., Athar, T., and Fatima, S. (2018). Phytochemical analysis and In-vitro Biochemical Characterization of aqueous and methanolic extract of Triphala, a conventional herbal remedy. Biotechnol. Rep. 17, 126–136. doi:10.1016/j.btre.2018.02.003
Peterson, C. T., Denniston, K., and Chopra, D. (2017). Therapeutic uses of triphala in ayurvedic medicine. J. Altern. Complement. Med. 23, 607–614. doi:10.1089/acm.2017.0083
Phetkate, P., Kummalue, T., U-Pratya, Y., and Kietinun, S. (2012). Significant increase in cytotoxic T lymphocytes and natural killer cells by triphala: a clinical phase I study. Evidence-Based Complement. Altern. Med. 2012, 239856. doi:10.1155/2012/239856
Polosa, R., Ciamarra, I., Mangano, G., Prosperini, G., Pistorio, M. P., Vancheri, C., et al. (2000). Bronchial hyperresponsiveness and airway inflammation markers in nonasthmatics with allergic rhinitis. Eur. Respir. J. 15 (1), 30–35. doi:10.1034/j.1399-3003.2000.15a07.x
Poulsen, L. K., and Hummelshoj, L. (2007). Triggers of IgE class switching and allergy development. Ann. Med. 39 (6), 440–456. doi:10.1080/07853890701449354
Pozharitskaya, O. N., Ivanova, S. A., Shikov, A. N., and Makarov, V. G. (2007). Separation and evaluation of free radical-scavenging activity of phenol components ofEmblica officinalis extract by using an HPTLC-DPPH method. J. Sep. Sci. 30 (9), 1250–1254. doi:10.1002/jssc.200600532
Prabu, D., Kirubanandan, S., Ponnudurai, K., Nappinnai, M., Jinu, A. J. S., and Renganathan, S. (2008). Anti-inflammatory and analgesic activities of methanol extract of Triphala - a poly herbal formulation. Oriental Pharm. Exp. MedicinePharm. Exp. Med 8, 423. doi:10.3742/opem.2008.8.4.423
Pradyumna Rao, T., Okamoto, T., Akita, N., Hayashi, T., Kato-Yasuda, N., and Suzuki, K. (2013). Amla (Emblica officinalis Gaertn.) extract inhibits lipopolysaccharide-induced procoagulant and pro-inflammatory factors in cultured vascular endothelial cells. Br. J. Nutr. 110 (12), 2201–2206. doi:10.1017/S0007114513001669
Rahimi, V. B., Askari, V. R., Shirazinia, R., Soheili-Far, S., Askari, N., Rahmanian-Devin, P., et al. (2018). Protective effects of hydro-ethanolic extract of Terminalia chebula on primary microglia cells and their polarization (M1/M2 balance). Mult. Scler. Relat. Disord. 25, 5–3. doi:10.1016/j.msard.2018.07.015
Ramezanpour, M., Moraitis, S., Smith, J. L. P., Wormald, P. J., and Vreugde, S. (2016). Th17 cytokines disrupt the airway mucosal barrier in chronic rhinosinusitis. Mediators Inflamm. 2016, 9798206. doi:10.1155/2016/9798206
Rasool, M., and Sabina, E. P. (2007). Antiinflammatory effect of the Indian ayurvedic herbal formulation Triphala on adjuvant-induced arthritis in mice. Phytother. Res. 21 (9), 889–894. doi:10.1002/ptr.2183
Reddy, D. B., Reddy, T. C. M., Jyotsna, G., Sharan, S., Priya, N., Lakshmipathi, V., et al. (2009). Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J. Ethnopharmacology 124 (3), 506–512. doi:10.1016/j.jep.2009.05.022
Reithofer, M., and Jahn-Schmid, B. (2017). Allergens with protease activity from house dust mites. Int. J. Mol. Sci. 18 (7), 1368. doi:10.3390/ijms18071368
Rice-Evans, C. A., Miller, N. J., and Paganga, G. (1997). Antioxidant properties of phenolic compounds. Trends Plant Sci.2 (4), 152–159. doi:10.1016/S1360-1385(97)01018-2
Ríos, J.-L., Giner, R., Marín, M., and Recio, M. (2018). A pharmacological update of ellagic acid. Planta Med. 84 (15), 1068–1093. doi:10.1055/a-0633-9492
Roidoung, S., Dolan, K. D., and Siddiq, M. (2016). Gallic acid as a protective antioxidant against anthocyanin degradation and color loss in vitamin-C fortified cranberry juice. Food Chem. 210, 422–427. doi:10.1016/j.foodchem.2016.04.133
Romagnani, S. (2004). Immunologic influences on allergy and the TH1/TH2 balance. J. Allergy Clin. Immunol. 113 (3), 395–400. doi:10.1016/j.jaci.2003.11.025
Rosa, A. C., and Fantozzi, R. (2013). The role of histamine in neurogenic inflammation. Br. J. Pharmacol. 170 (1), 38–45. doi:10.1111/bph.12266
Rubab, I., and Ali, S. (2016). Dried fruit extract ofTerminalia chebulamodulates the immune response in mice. Food Agric. Immunol. 27, 1. doi:10.1080/09540105.2015.1055554
Sabina, E. P., Rasool, M. K., and Mathew, L. (2009). Vivo and in vitro immunomodulatory effects of Indian ayurvedic herbal formulation triphala on experimental induced inflammation. London: Pharmacologyonline.
Sagit, M., Polat, H., Gurgen, S. G., Berk, E., Guler, S., and Yasar, M. (2017). Effectiveness of quercetin in an experimental rat model of allergic rhinitis. Eur. Arch. Otorhinolaryngol. 274 (8), 3087–3095. doi:10.1007/s00405-017-4602-z
Sai Ram, M., Neetu, D., Yogesh, B., Anju, B., Dipti, P., Pauline, T., et al. (2002). Cyto-protective and immunomodulating properties of Amla (Emblica officinalis) on lymphocytes: an in-vitro study. J. Ethnopharmacol. 81 (1), 5–10. doi:10.1016/S0378-8741(01)00421-4
Sakai-Kashiwabara, M., and Asano, K. (2013). Inhibitory action of quercetin on eosinophil Activation in vitro. Evidence-Based Complement. Altern. Med. 2013, 1. doi:10.1155/2013/127105
Schuliga, M. (2015). NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules 5 (3), 1266–1283. doi:10.3390/biom5031266
Seo, J.-H., Kwon, S.-O., Lee, S.-Y., Kim, H. Y., Kwon, J.-W., Kim, B.-J., et al. (2013). Association of antioxidants with allergic rhinitis in children from Seoul. Allergy Asthma Immunol. Res. 5 (2), 81–87. doi:10.4168/aair.2013.5.2.81
Seresirikachorn, K., Chitsuthipakorn, W., Kanjanawasee, D., Khattiyawittayakun, L., and Snidvongs, K. (2018). Effects of H1 antihistamine addition to intranasal corticosteroid for allergic rhinitis: a systematic review and meta-analysis. Int. Forum Allergy Rhinol. 8 (10), 1083–1092. doi:10.1002/alr.22166
Shanmuganathan, S., and Angayarkanni, N. (2018). Chebulagic acid Chebulinic acid and Gallic acid, the active principles of Triphala, inhibit TNFα induced pro-angiogenic and pro-inflammatory activities in retinal capillary endothelial cells by inhibiting p38, ERK and NFkB phosphorylation. Vasc. Pharmacol. 108, 23–35. doi:10.1016/j.vph.2018.04.005
Sharma, A., and Sharma, K. K. (2011). Chemoprotective role of triphala against 1,2-dimethylhydrazine dihydrochloride induced carcinogenic damage to mouse liver. Ind. J. Clin. Biochem. 26 (3), 290–295. doi:10.1007/s12291-011-0138-y
Shin, D., Park, S.-H., Choi, Y.-J., Kim, Y.-H., Antika, L., Habibah, N., et al. (2015). Dietary compound kaempferol inhibits airway thickening induced by allergic reaction in a bovine serum albumin-induced model of asthma. Ijms 16 (12), 29980–2995. doi:10.3390/ijms161226218
Shin, T. Y., Jeong, H. J., Kim, D. K., Kim, S. H., Lee, J. K., Kim, D. K., et al. (2001). Inhibitory action of water soluble fraction of Terminalia chebula on systemic and local anaphylaxis. J. Ethnopharmacol. 74 (2), 133–140. doi:10.1016/S0378-8741(00)00360-3
Simons, F. E. R. (1999). Allergic rhinobronchitis: the asthma-allergic rhinitis link. J. Allergy Clin. Immunol. 104 (3 Pt 1), 534–540. doi:10.1016/s0091-6749(99)70320-9
Sin, B., and Togias, A. (2011). Pathophysiology of allergic and nonallergic rhinitis. Proc. Am. Thorac. Soc. 8 (1), 106–114. doi:10.1513/pats.201008-057RN
Singh, M. K., Yadav, S. S., Yadav, R. S., Chauhan, A., Katiyar, D., and Khattri, S. (2015). Protective effect of Emblica-officinalis in arsenic induced biochemical alteration and inflammation in mice. Springerplus 4, 438. doi:10.1186/s40064-015-1227-9
Sireeratawong, S., Jaijoy, K., Khonsung, P., and Soonthornchareonnon, N. (2014). Analgesic and anti-inflammatory activities of the water extract from terminalia chebula rezt. Afr. J. Trad. Compl. Alt. Med. 11, 77. doi:10.4314/ajtcam.v11i6.8
Sireeratawong, S., Jaijoy, K., and Soonthornchareonnon, N. (2013). Evaluation of anti-inflammatory and antinociceptive activity of Triphala recipe. Afr. J. Trad. Compl. Alt. Med. 10 (2), 246–250. doi:10.4314/ajtcam.v10i2.8
Small, P., Keith, P. K., and Kim, H. (2018). Allergic rhinitis. Allergy Asthma Clin. Immunol. 14, 139. doi:10.1186/s13223-018-0280-7
Srikumar, R., Jeya Parthasarathy, N., and Sheela Devi, R. (2005). Immunomodulatory activity of triphala on neutrophil functions. Biol. Pharm. Bull. 28 (8), 1398–403. doi:10.1248/bpb.28.1398
Srikumar, R., Parthasarathy, N. J., Manikandan, S., Muthuvel, A., Rajamani, R., and Sheeladevi, R. (2007). Immunomodulatory effect of Triphala during experimentally induced noise stress in albino rats. J. Health Sci. 53, 142. doi:10.1248/jhs.53.142
Srikumar, R., Parthasarathy, N. J., Manikandan, S., Narayanan, G. S., and Sheeladevi, R. (2006). Effect of Triphala on oxidative stress and on cell-mediated immune response against noise stress in rats. Mol. Cel. Biochem. 283 (1–2), 67–74. doi:10.1007/s11010-006-2271-0
Steelant, B., Seys, S. F., Van Gerven, L., Van Woensel, M., Farré, R., Wawrzyniak, P., et al. (2018). Histamine and T helper cytokine-driven epithelial barrier dysfunction in allergic rhinitis. J. Allergy Clin. Immunol. 141 (3), 951–963. doi:10.1016/j.jaci.2017.08.039
Sukakul, T., Kettawan, A., Chompoopong, S., and Rungruang, T. (2013). Topical application of Terminalia chebula extract helps croton oil-induced dermatitis in mice. Int. Food Res. J. 20 (5), 2269–2272.
Takauji, Y., Miki, K., Mita, J., Hossain, M. N., Yamauchi, M., Kioi, M., et al. (2016). Triphala, a formulation of traditional Ayurvedic medicine, shows protective effect against X-radiation in HeLa cells. J. Biosci. 41 (4), 569–575. doi:10.1007/s12038-016-9639-4
Tanaka, M., Kishimoto, Y., Sasaki, M., Sato, A., Kamiya, T., Kondo, K., et al. (2018). Terminalia bellirica (gaertn.) Roxb. Extract and gallic acid attenuate LPS-induced inflammation and oxidative stress via MAPK/NF-κB and Akt/AMPK/Nrf2 pathways. Oxidative Med. Cell Longevity 2018, 9364364. doi:10.1155/2018/9364364
Tanaka, Y., Atsuko, F., Asano, K., and Koyabashi, H. (2020). Modulation of Th1/Th2 cytokine balance by. Medicines 7, 1–9. doi:10.3390/medicines7080046
Taylorclark, T., Kollarik, M., Macglashanjr, D., and Undem, B. (2005). Nasal sensory nerve populations responding to histamine and capsaicin. J. Allergy Clin. Immunol. 116 (6), 1282–1288. doi:10.1016/j.jaci.2005.08.043
Thomas, M. (2006). Allergic rhinitis: evidence for impact on asthma. BMC Pulm. Med. 6 Suppl 1 (Suppl 1), S4. doi:10.1186/1471-2466-6-S1-S4
Thornhill, S. M., and Kelly, A. M. (2000). Natural treatment of perennial allergic rhinitis. Altern. Med. Rev. 5 (5), 448–454. doi:10.1007/bf03297230
Tongtako, W., Klaewsongkram, J., Mickleborough, T. D., and Suksom, D. (2018). Effects of aerobic exercise and vitamin C supplementation on rhinitis symptoms in allergic rhinitis patients. Asian Pac. J. Allergy Immunol. 36 (4), 222–231. doi:10.12932/AP-040417-0066
Townley, R. G., Gendapodi, P. R., Qutna, N., Evans, J., Romero, F. A., and Abel, P. (2009). Effect of interleukin 13 on bronchial hyperresponsiveness and the bronchoprotective effect of beta-adrenergic bronchodilators and corticosteroids. Ann. Allergy Asthma Immunol. 102 (3), 190–197. doi:10.1016/S1081-1206(10)60080-4
Turner, H., and Kinet, J.-P. (1999). Signalling through the high-affinity IgE receptor FcεRI. Nature 402 (S6760), 24–30. doi:10.1038/35037021
Undem, B. J., and Taylor-Clark, T. (2014). Mechanisms underlying the neuronal-based symptoms of allergy. J. Allergy Clin. Immunol. 133 (6), 1521–1534. doi:10.1016/j.jaci.2013.11.027
Varma, S. R., Sivaprakasam, T. O., Mishra, A., Kumar, L. M. S., Prakash, N. S., Prabhu, S., et al. (2016). Protective effects of triphala on dermal fibroblasts and human keratinocytes. PLoS One 11 (1), e0145921. doi:10.1371/journal.pone.0145921
Varshney, J., and Varshney, H. (2015). Allergic rhinitis: an overview. Indian J. Otolaryngol. Head Neck Surg. 67, 143. doi:10.1007/s12070-015-0828-5
Ventura, I., Vega, A., Chacón, P., Chamorro, C., Aroca, R., Gómez, E., et al. (2014). Neutrophils from allergic asthmatic patients produce and release metalloproteinase-9 upon direct exposure to allergens. Allergy 69 (7), 898–905. doi:10.1111/all.12414
Vollbracht, C., Raithel, M., Krick, B., Kraft, K., and Hagel, A. F. (2018). Intravenous vitamin C in the treatment of allergies: an interim subgroup analysis of a long-term observational study. J. Int. Med. Res. 46 (9), 3640–3655. doi:10.1177/0300060518777044
Vujnovic, S. D., and Domuz, A. (2018). Epidemiological aspects of rhinitis and asthma: comorbidity or united airway disease in asthma diagnosis and management—approach based on phenotype and endotype. Asthma Diag. Manage. Approach Based Phenotype Endotype 33, 237. doi:10.5772/intechopen.76773
Wang, X., Zhao, H., Ma, C., Lv, L., Feng, J., and Han, S. (2018). Gallic acid attenuates allergic airway inflammation via suppressed interleukin-33 and group 2 innate lymphoid cells in ovalbumin-induced asthma in mice. Int. Forum Allergy Rhinol. 8 (11), 1284–1290. doi:10.1002/alr.22207
White, M. V. (1990). The role of histamine in allergic diseases. J. Allergy Clin. Immunol. 86 (4 Pt 2), 599–605. doi:10.1016/S0091-6749(05)80223-4
Wise, S. K., Laury, A. M., Katz, E. H., Den Beste, K. A., Parkos, C. A., and Nusrat, A. (2014). Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. Int. Forum Allergy Rhinology 4 (5), 361–370. doi:10.1002/alr.21298
World Allergy Organization, (2013). White book on allergy: update 2013, executive summary. World Allergy Organ. 12, 49. doi:10.1080/14693062.2006.9685626
Yang, M. H., Ali, Z., Khan, I. A., and Khan, S. I. (2014). Anti-inflammatory activity of constituents isolated from Terminalia chebula. Nat. Product. Commun. 9 (7), 965–968. doi:10.1177/1934578x1400900721
Yang, Y., Xiu, J., Liu, J., Zhang, L., Li, X., Xu, Y., et al. (2013). Chebulagic acid, a hydrolyzable tannin, exhibited antiviral activity in vitro and in vivo against human enterovirus 71. Ijms 14 (5), 9618–9627. doi:10.3390/ijms14059618
Young, T., Finn, L., and Kim, H. (1997). Nasal obstruction as a risk factor for sleep-disordered breathing. The university of Wisconsin sleep and respiratory research GroupThe university of Wisconsin sleep and respiratory research group. J. Allergy Clin. Immunol. 99, S757.
Zhang, N., Li, H., Jia, J., and He, M. (2015). Anti-inflammatory effect of curcumin on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse. Cell Immunol. 298 (1–2), 88–95. doi:10.1016/j.cellimm.2015.09.010
Keywords: allergic rhinitis, triphala, antioxidant effect, anti-inflamamtion, immunomodulaion
Citation: Jantrapirom S, Hirunsatitpron P, Potikanond S, Nimlamool W and Hanprasertpong N (2021) Pharmacological Benefits of Triphala: A Perspective for Allergic Rhinitis. Front. Pharmacol. 12:628198. doi: 10.3389/fphar.2021.628198
Received: 11 November 2020; Accepted: 16 March 2021;
Published: 30 April 2021.
Edited by:
Michael Heinrich, UCL School of Pharmacy, United KingdomReviewed by:
Jen-Tsung Chen, National University of Kaohsiung, TaiwanCopyright © 2021 Jantrapirom, Hirunsatitpron, Potikanond, Nimlamool and Hanprasertpong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nutthiya Hanprasertpong, bnV0dGhpeWEuaEBjbXUuYWMudGg=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.