
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
GENERAL COMMENTARY article
Front. Pharmacol., 20 April 2021
Sec. Inflammation Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.619344
This article is a commentary on:
The Efficacy of Nerve Growth Factor Antibody for the Treatment of Osteoarthritis Pain and Chronic Low-Back Pain: A Meta-Analysis
A Commentary on
The Efficacy of Nerve Growth Factor Antibody for the Treatment of Osteoarthritis Pain and Chronic Low-Back Pain: A Meta-analysis
by Yang, S., Huang, Y., Ye, Z., Li, L. and Zhang, Y. (2020). Front Pharmacol. 11:817. doi:10.3389/fphar.2020.00817
The recent article by Yang et al. (2020) is timely. Nerve growth factor (NGF) inhibitors may have benefits for chronic pain, and the U. S. Food and Drug Administration has recently accepted regulatory submission of tanezumab (an anti-NGF agent) for osteoarthritis. However, we have two major concerns with the review that question the validity of their results: 1) missing studies and 2) incorrect data extraction/analysis.
First, Yang et al. (2020) have missed several osteoarthritis trials that were eligible for inclusion in their review, for example, Schnitzer et al. (2015), Brown et al. (2013), and Brown et al. (2012). These trials are indexed in both Embase and PubMed (two of the databases searched by Yang et al. (2020)) and meet all inclusion criteria for the review. Brown et al. (2013) and Brown et al. (2012) are also found in the reference list of one of the included trials (Kivitz et al., 2013) indicating that Yang et al. (2020) failed to include these studies since they stated in the review that “The references of the articles included were also searched in case of any additional studies not previously identified in the initial literature search.”
In regard to trials for chronic low back pain, we have concerns about the decision by Yang et al. (2020) to restrict inclusion criteria to only studies that assessed pain intensity using the Western Ontario and McMaster Universities Arthritis Index (WOMAC). Justification for this decision is unclear; the WOMAC was designed to assess individuals with osteoarthritis (Bellamy, 2002) and it is rarely used in low back pain clinical trials (Chiarotto et al., 2018). By excluding studies that assessed pain intensity using measurement tools that are more common in back pain trials (e.g. visual analogue scale, numerical rating scale), several relevant trials have likely been missed. Probably, this was also the reason that Yang et al. (2020) only identified one study for low back pain. Interestingly, the one low back pain trial (Kivitz et al., 2013) included in the Yang et al. (2020) review did not actually use the WOMAC to measure pain intensity, despite the authors stating that it did. Kivitz et al. (2013), used the 11-point numerical rating scale, leading us to believe other eligible trials with similar outcomes should be included (Katz et al., 2011; Sanga et al., 2016).
Our second concern is that Yang et al. (2020) have incorrectly used the standard error (SE) in place of standard deviation (SD) as the arm-level measure of variance for each included trial. In Figure 4 of their article, anti-NGF interventions produced a substantial decrease in pain intensity compared to placebo: standardized mean difference −2.22 (95% confidence interval −3.44 to −0.99). Substantial heterogeneity and inconsistency were reported (tau2 = 2.3248, I2= 99%). We noted that only Tiseo et al. (2014) had reported SD; all other studies reported SE but had not been converted to SD by Yang et al. (2020) before analysis. We re-extracted the data of each trial in Figure 4 of the authors’ review, transformed SE to SD for each arm in five studies, then lumped the intervention arms together using recommendations from the Cochrane Handbook, emulating the process undertaken by Yang et al. (2020). We then reproduced the meta-analysis with the correct data using the metafor package in R which resulted in a markedly smaller effect size: standardized mean difference −0.27 (95% confidence interval −0.35 to −0.19) (Figure 1 of this letter). We detected no heterogeneity or inconsistency (tau2 = 0.0, I2= 0%). There is a substantial discrepancy between the results reported by Yang et al. (2020) and our re-analysis. The American College of Physicians Clinical Practice Guideline by Chou et al. (2017) suggests a large/substantial reduction in pain intensity corresponds to a standardized mean difference >0.8 (approximately >2 points on a 0 to 10-point scale). The results from Yang et al. (2020) show evidence for an effect magnitude (−2.22) that is extremely large based on recommended thresholds for clinically important differences (Chou et al., 2017), which infers that anti-NGF drugs are a game-changing treatment for reducing pain. Our re-analysis with the appropriate data shows the correct result is −0.27, eight times smaller than the original effect and one that is unlikely to be considered slight/small (<0.5), and therefore meaningful, by Chou et al. (2017). We highlight the importance of checking data extraction carefully and scrutinizing the results of meta-analyses. Data and code of our reanalysis are available at https://osf.io/tp4e6/.
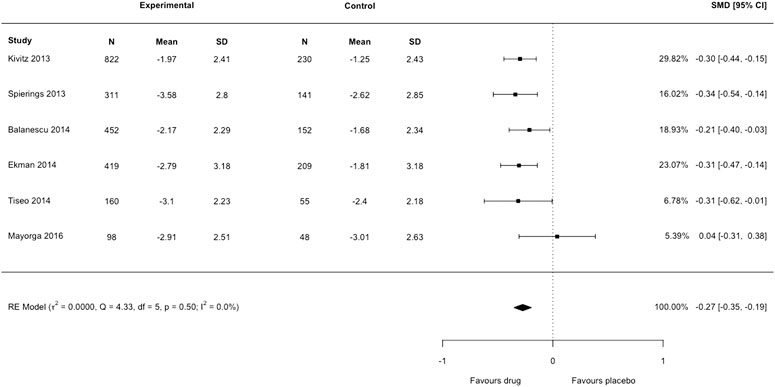
FIGURE 1. Forest plot of changes from baseline to checkpoint for pain. This is a re-analysis in which we transformed the standard error reported in individual studies to standard deviation. SMD = Standardised Mean Difference. CI = confidence Interval. N = number of participants.
In conclusion, we urge readers to be cautious of the quality and validity of Yang et al. (2020)’s review. The review misses several important trials and contains extraction/synthesis errors in the meta-analysis which resulted in authors overstating the efficacy of anti-NGF for patients with osteoarthritis and chronic low back pain.
RRNR, MAW, HBL conceived the idea and wrote the manuscript. RRNR and MAW analyzed the data. All authors reviewed the final manuscript.
RRNR was supported by the University of New South Wales School of Medical Sciences Postgraduate Research Scholarship and a NeuRA PhD Candidature Supplementary Scholarship. MAW was supported by a Postgraduate Scholarship from the National Health and Medical Research Council of Australia and a School of Medical Sciences Top-Up Scholarship from the University of New South Wales. HBL was supported by an Australian Government Research Training Program Scholarship. JHM receives project funding support from the National Health and Medical Research Council and the Medical Research Future Fund of Australia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.619344/full#supplementary-material.
Bellamy, N. (2002). WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J. Rheumatol. 29, 2473–2476.
Brown, M. T., Murphy, F. T., Radin, D. M., Davignon, I., Smith, M. D., and West, C. R. (2013). Tanezumab reduces osteoarthritic hip pain: results of a randomized, double-blind, placebo-controlled phase III trial. Arthritis Rheum. 65, 1795–1803. doi:10.1002/art.37950
Brown, M. T., Murphy, F. T., Radin, D. M., Davignon, I., Smith, M. D., and West, C. R. (2012). Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. The J. Pain 13, 790–798. doi:10.1016/j.jpain.2012.05.006
Chiarotto, A., Boers, M., Deyo, R. A., Buchbinder, R., Corbin, T. P., Costa, L. O. P., et al. (2018). Core outcome measurement instruments for clinical trials in nonspecific low back pain. Pain 159, 481–495. doi:10.1097/j.pain.0000000000001117
Chou, R., Deyo, R., Friedly, J., Skelly, A., Weimer, M., Fu, R., et al. (2017). Systemic pharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical Practice guideline. Ann. Intern. Med. 166, 480–492. doi:10.7326/m16-2458
Katz, N., Borenstein, D. G., Birbara, C., Bramson, C., Nemeth, M. A., Smith, M. D., et al. (2011). Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 152, 2248–2258. doi:10.1016/j.pain.2011.05.003
Kivitz, A. J., Gimbel, J. S., Bramson, C., Nemeth, M. A., Keller, D. S., Brown, M. T., et al. (2013). Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 154, 1009–1021. doi:10.1016/j.pain.2013.03.006
Sanga, P., Polverejan, E., Wang, S., Kelly, K. M., and Thipphawong, J. (2016). Efficacy, safety, and tolerability of fulranumab as an adjunctive therapy in patients with inadequately controlled, moderate-to-severe chronic low back pain: a randomized, double-blind, placebo-controlled, dose-ranging, dose-loading phase II study. Clin. Ther. 38, 1435–1450. doi:10.1016/j.clinthera.2016.03.030
Schnitzer, T. J., Ekman, E. F., Spierings, E. L. H., Greenberg, H. S., Smith, M. D., Brown, M. T., et al. (2015). Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann. Rheum. Dis. 74, 1202–1211. doi:10.1136/annrheumdis-2013-204905
Tiseo, P. J., Kivitz, A. J., Ervin, J. E., Ren, H., and Mellis, S. J. (2014). Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, placebo-controlled exploratory study in osteoarthritis of the knee. Pain 155, 1245–1252. doi:10.1016/j.pain.2014.03.018
Keywords: anti-nerve growth factor antibody, osteoarthritis pain, chronic low-back pain, meta-analysis, systematic review
Citation: Rizzo RRN, Wewege MA, Leake HB and McAuley JH (2021) Commentary: The Efficacy of Nerve Growth Factor Antibody for the Treatment of Osteoarthritis Pain and Chronic Low-Back Pain: A Meta-analysis. Front. Pharmacol. 12:619344. doi: 10.3389/fphar.2021.619344
Received: 20 October 2020; Accepted: 23 March 2021;
Published: 20 April 2021.
Edited by:
Domenico Criscuolo, Italian Society of Pharmaceutical Medicine, ItalyReviewed by:
Kurt Neumann, Independent researcher, Kerékteleki, HungaryCopyright © 2021 Rizzo, Wewege, Leake and McAuley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rodrigo R. N. Rizzo, ci5yaXp6b0BuZXVyYS5lZHUuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.