- 1Cancer Institute, Xuzhou Medical University, Xuzhou, China
- 2Center of Clinical Oncology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, China
The kidney is vital in maintaining fluid, electrolyte, and acid–base balance. Kidney-related diseases, which are an increasing public health issue, can happen to people of any age and at any time. Circular RNAs (circRNAs) are endogenous RNA that are produced by selective RNA splicing and are involved in progression of various diseases. Studies have shown that various kidney diseases, including renal cell carcinoma, acute kidney injury, and chronic kidney disease, are linked to circRNAs. This review outlines the characteristics and biological functions of circRNAs and discusses specific studies that provide insights into the function and potential of circRNAs for application in the diagnosis and treatment of kidney-related diseases.
Background
Circular RNAs (circRNAs) were first discovered in RNA viruses in the 1970s. They were used to be considered byproducts of mis-splicing and much rare (Sanger et al., 1976). With the development of next-generation sequencing and bioinformatic analysis, circRNAs have been recognized to be widely found, and have their own biological functions in the pathogenesis of various diseases (Hansen et al., 2013; Zhang et al., 2020).
The kidney plays an important role in maintaining water and electrolyte balance, and regulating homeostasis. Renal diseases, including renal cell carcinoma (RCC), acute kidney injury (AKI), chronic kidney disease (CKD), are major causes of kidney failure, which leads to a poor quality of life of patients and poses great burden and loss to the society (Lowenstein and Grantham, 2017).
circRNAs are dynamically expressed and spatiotemporally regulated in kidney-related diseases, this review summarizes the formation and characteristics of circRNAs and discusses how they are involved in the progression of these disorders to propose circRNAs as optional strategies for regulating disease progression and improving therapeutic outcomes.
Formation and Characteristics of Circular RNAs
RNAs can be classified into protein-coding or nonprotein-coding molecules according to their size, location and function. Only about 2% of RNAs are protein coding, and most RNAs belong to noncoding RNAs (ncRNAs), which are a kind of RNA with large amount and diverse functions. These RNA molecules can be sorted in terms of their sizes, and 200 nucleotides can separate small ncRNAs from long ncRNAs (lncRNAs). MicroRNAs (miRNAs), which have 20–22 nucleotides and can downregulate the expression of target protein-coding genes, are among the most well-researched small ncRNAs. lncRNAs are generally divided into linear lncRNAs (acquiesced as lncRNAs) and circular RNAs (circRNAs) (Brandenburger et al., 2018).
Most precursor messenger RNAs (pre-mRNAs) are spliced into linear RNA molecules through canonical splicing. However, pre-mRNAs can also be spliced into circRNAs through back splicing. A circRNA is a closed loop structure formed through the covalent bonding of 5′-cap structures and 3′-poly A tails. According to different splicing sources, circRNAs can be divided into exonic circRNAs (ecircRNAs), circular intron circRNAs (ciRNAs), and exon- and intron-derived or retained intron circRNAs (EIciRNA) (Han et al., 2017). Three circularization mechanisms, namely, intron pairing-driven, RNA-binding protein-driven, and lariat-driven mechanisms, are known to achieve the formation of circRNAs (Aufiero et al., 2019) (Figure 1).
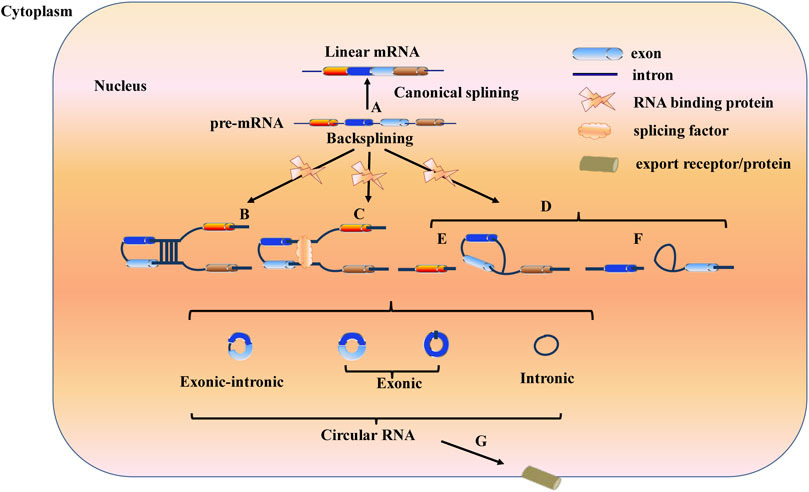
FIGURE 1. Three mechanisms of circRNAs biogenesis Canonical splicing results in the formation of mature mRNA after the removal of introns (A); While back-splicing contributes to the formation of circRNAs. Intron pairing-driven circularization, RNA-binding protein (RBP)-driven circularization and lariat-driven circularization are three mechanisms to generate circRNAs. In intron pairing-driven circularization the back-splicing event is guided by base pairing of complementary sequences located in the introns flanking the back-spliced exons (B). In RNA-binding protein (RBP)-driven circularization, RBPs play a dominant role, they recognize and binding to specific motifs located in the introns flanking the circularized exons (C). In lariat-driven circularization, including intronic lariats and exon-containing lariats circularization, circRNAs are formed during linear splicing (D). Intronic lariats circularization: circRNAs are formed when an intron is removed during precursor mRNA (pre-mRNA) splicing (F). Exon-containing lariats: circRNAs are generated during exon-skipping (E). Those circRNAs then are exported to cytoplasm through export receptor or export protein including Hel25E in drosophila and UAP56/URH49 in human (G).
circRNAs have the following highlighted characteristics. 1) They are widespread and stable. Many circRNAs are highly conserved in different species, including humans, mice, Drosophila, and yeasts (Cocquerelle et al., 1993; Jeck et al., 2013; Wang et al., 2014). The expression patterns of circRNAs vary in different tissues and conditions. They are enriched in the brain and kidneys, and are abnormally expressed when diseases occur. They can be detected in the blood, urine, saliva, and also in exosomes (Salzman et al., 2012; Li et al., 2015). circRNAs are resistant to RNase, benefiting from their covalently closed loop structures. This special structure gives circRNAs higher expression and longer average half-life than that of their linear isomers (Yan et al., 2019). This feature lays the foundation of circRNAs as biomarkers and regulators. 2) circRNAs have specific location and expression. ecircRNAs, which account for the majority of circRNAs, are mostly located in the cytoplasm. ecircRNAs can act as miRNA sponges, which prevent miRNAs from forming a complementary pair with target mRNA 3ʹ-UTRs; as a result, the expression of target mRNAs increases. ecircRNAs also can stabilize or activate the functions of miRNAs, decreasing the expression of mRNAs, which are called miRNA reservoirs (Hansen et al., 2013; Memczak et al., 2013; Qu et al., 2015; Zheng et al., 2017; Wang et al., 2018). Conversely, ciRNAs and EIciRNAs are mainly located in the nucleus and may be involved in the regulation of gene expression at transcriptional or post-transcriptional levels (Jeck et al., 2013; Zhang et al., 2013; Han et al., 2017).
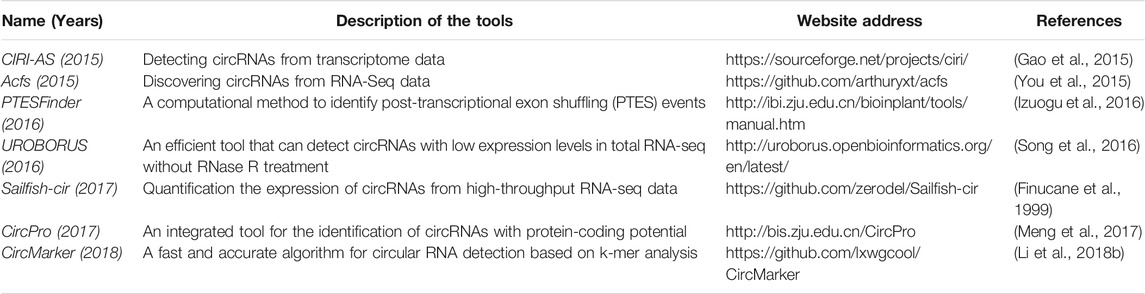
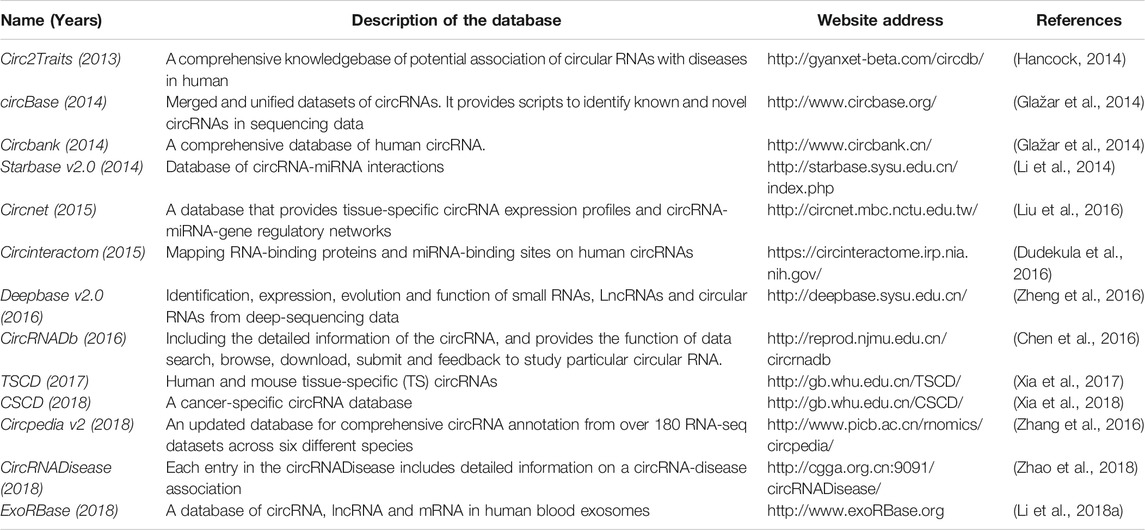
There are some computational tools and databases are designed to identify and analyze circRNAs (Tables 1, 2). These predictive tools can be utilized to identify different kinds of circRNAs based on different identification strategies. Appropriate tools should be selected on the basis of the purposes of a particular research, and multiple predictive tools are recommended to reduce the likelihood of losing target circRNAs (Aufiero et al., 2019; Shang et al., 2019).
Circular RNAs in Kidney Development
The role of circRNAs in the development of the brain and neurodegenerative disorders have already been expounded (Westholm et al., 2014; Huang et al., 2018; Mehta et al., 2020), while theirs role in kidney development and diseases still need to be clarified. In 1996, the first kidney-related circRNA known as cytochrome P450 2C24 gene was found in rat kidney; it is a transcript containing exons 2 and 4 spliced at the correct sites, but the donor site of exon 4 is directly joined to the acceptor site of exon 2 (exon scrambling) (Zaphiropoulos, 1996). After 2 years, a circular formin mRNA transcript was found in the brain and kidney of mice; the blunting of circRNA formation leads to renal aplasia in gene-targeted mutant mice, suggesting that circRNAs may also play a critical role in the development of the kidney of mice (Chao et al., 1998). RNA sequencing analysis in humans has verified that the expression of 1,664 circRNAs in fetal kidney samples is higher than that in the corresponding adult kidney tissues. Further analysis has shown that about 474 circRNAs are more enriched in the kidney than in other organs, indicating the potentially critical roles of circRNAs in the development of the kidney of humans (Xu et al., 2017). Existing studies only focus on the changes of circRNAs expressions, further studies should pay attention to the specific mechanisms of how circRNAs work during kidney development.
Circular RNAs in Renal Cell Carcinoma
RCC is one of the most common malignant cancers in the world, although the 5-years survival rates have shown some considerable improvements, the overall prognosis is still far from satisfactory (Siegel et al., 2017). The possible mechanism on how RCC deteriorates and some specific biomarkers should be defined to optimize treatment strategies.
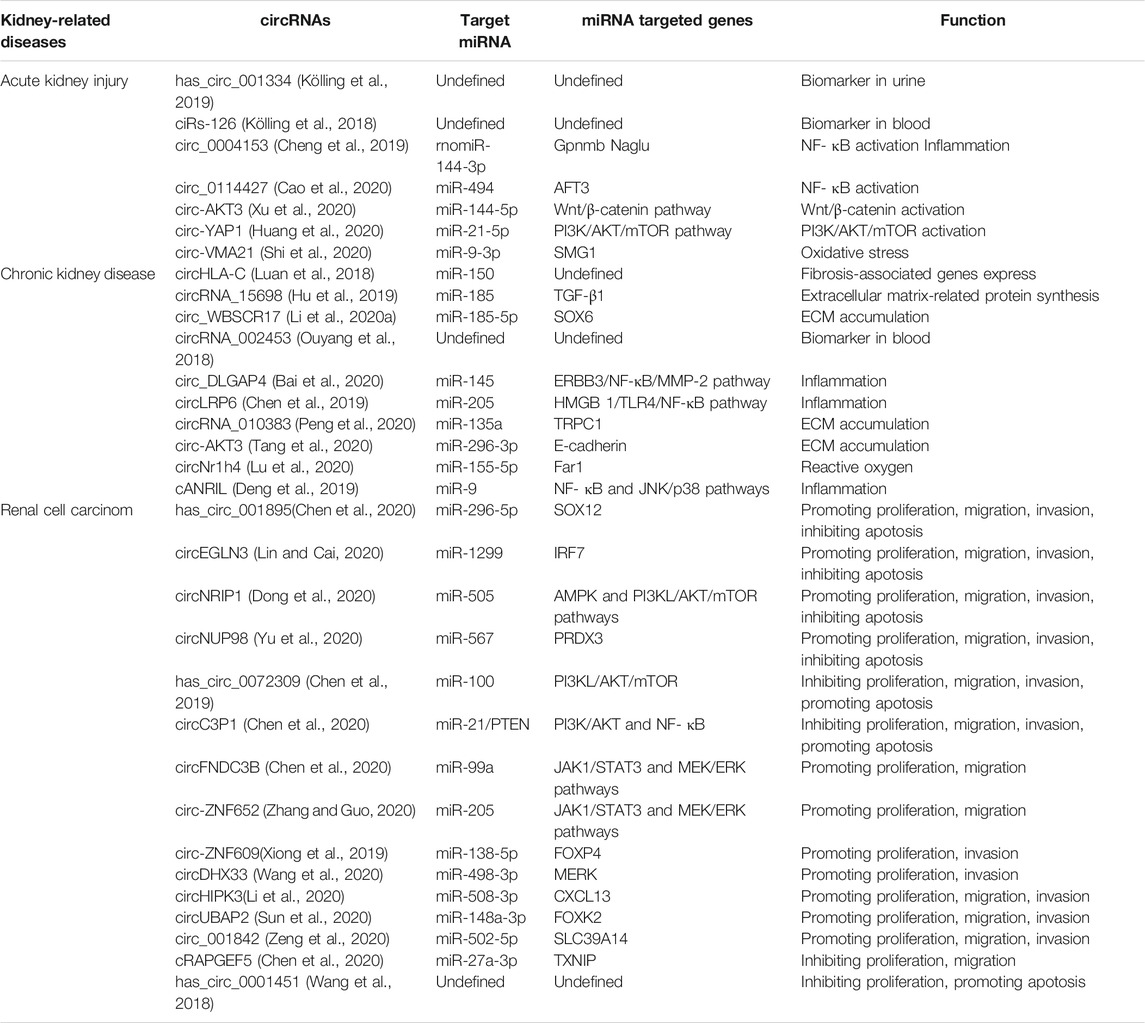
The motility of tumor cells is often enhanced, with remodeling of tumor microenvironments when tumor develops. Dysregulated circRNAs are involved in RCC progression by altering tumor cell dynamics, participating in the remodeling of tumor microenvironments including matrix remodeling, hypoxia and immunesuppression and so on ((Figure 2; Table 3).
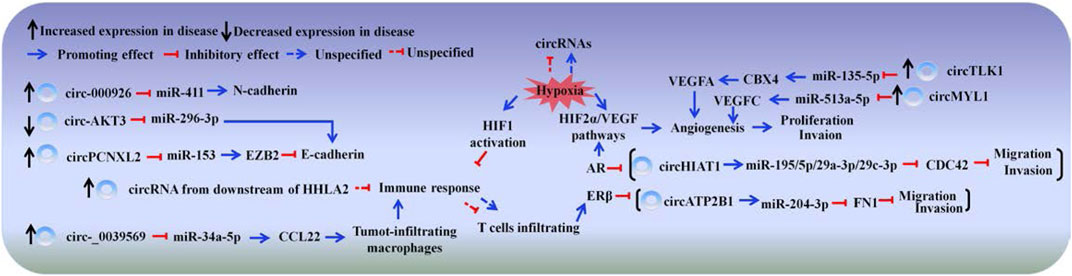
FIGURE 2. CircRNAs participate in renal cell carcinoma. The schematic diagram depicts some of circRNAs in renal cell carcinoma progression.
Tumorigenesis-Related Circular RNAs
circRNAs are widely involved in many biological processes including proliferation, invasion and apoptosis of RCC cells.
circRNAs can act as an miRNA sponge to accelerate RCC progression. hsa_circ_0002286/has-mir-222-5p/TRIM2 axis had been identified to play a critical role in the progression of RCC via database analysis (Wei et al., 2020). Hsa_circ_001895 is upregulated in RCC specimens; knocked down hsa_circ_001895 can inhibit RCC progression and promote apoptosis by reducing the adsorption of miR-296-5p and decreasing the expression of sex-determining region Y (SRY)-box 12 (SOX12) (Chen et al., 2020). SOX12 participates in cell differentiation during embryonic development, and its high expression predicts poor prognosis (Gu et al., 2018). Similarly, the high expression of interferon regulatory factor 7(IRF7) is related to a poor survival rate. circEGLN3 promotes the proliferation and aggressiveness of RCC via miR-1299-mediated IRF7 activation (Lan et al., 2019; Lin and Cai, 2020) The up-regulated circDHX33 can promote the proliferation and invasion of RCC, by sponging with miR-489-3p and increasing mitogen-activated protein kinase (MEK) expression (Wang et al., 2020). Likewise, forkhead-box P4 (FOXP4) highly contributes to cancer cell growth and invasion (Yang et al., 2015), circ-ZNF609 remarkably increases in RCC tissues and consequently promotes the expression of FOXP4 by sponging with miR-138-5p (Xiong et al., 2019). The high expressed circ_001842 was found to elevate SLC39A14 expression by binding to miR-502-5p, thereby promoting invasion, metastasis and inflammation of RCC (Zeng et al., 2020). Forkhead box K2 (FOXK2) belongs to the forkhead box transcription factor family and plays essential roles in cellular proliferation and survival (van der Heide et al., 2015). The decreased circUBAP2 sponges with miR-148a-3p to increasing FOXK2 expression to promote RCC progression (Sun et al., 2020). Additionally, methyltransferase-like 14 (METTL14) is positively correlated with the tumor suppressor gene PTEN. Bioinformatic analysis has demonstrated that circRNAs may act as an miRNA sponge that decreases the mRNA expression of METTL14. The mRNA of METTL14 likely regulates the mRNA expression of PTEN by changing its m6A RNA modification level; leading to RCC progresses (Wang et al., 2019).
circRNAs are involved in some signaling pathways to regulate RCC progression. circRNA nuclear receptor-interacting protein 1 (circNRIP1) is overexpressed in RCC tissues, and si-circNRIP1 in ACHN and CAKI-1 cells deactivates AMPK and PI3K/AKT/mTOR pathways, which are mediated by miR-505 (Dong et al., 2020). circFNDC3B is highly expressed in RCC tissues and attends to the modulation of RCC growth and metastasis by activating the pathways of JAK1/STAT3 and MEK/ERK(Chen et al., 2020). The increased circ-ZNF652 has the similar effect by sponging with miR-205 (Zhang and Guo, 2020). It is also found that circNUP98 is under the regulation of STAT3, and it functions as a sponge of miR-567 and promotes the expression of PRDX3 (an antioxidant protein, belongs to the peroxiredoxins), leading to RCC progression (Cox et al., 2009; Yu et al., 2020).
circRNAs also act as a suppressor gene. cRAPGEF5 is downregulated and can inhibit the progression of RCC by acting as a sponge of miR-27a-3p to upregulate TXNIP(Chen et al., 2020). The TXNIP gene serves as a suppressor during oxidative stress-induced renal carcinogenesis (Jiao et al., 2019). Likewise, hsa_circ_0001451 is downregulated in RCC, and its inhibition leads to OSRC-2 and 786-O cell proliferation and decreases apoptosis (Wang et al., 2018). Similarly, hsa-circ-0072309 is poorly expressed, and plays antitumor roles by blocking the PI3K/AKT/mTOR cascades in CAKI-1 and ACHN cell by targeting miR-100 (Chen et al., 2019). Another circRNA called circC3P1 is downregulated, and its overexpression in ACHN restrains NF-κB pathways (Zhong et al., 2018; Chen et al., 2020).
EMT-Related Circular RNAs
The distinguishing feature of EMT is the functional loss of cell adherens junctions (Nieto et al., 2016; Xue et al., 2019). Adherens junctions are mainly composed of a transmembrane calcium-dependent glycoprotein named E-cadherin (also known as CDH1), which is considered a tumor suppressor maintaining integrity at local and tissue levels (Lecuit and Yap, 2015). Abnormal E-cadherin expression is often observed in RCC samples, and the loss of E-cadherin is considered as early carcinogenic event (Evans et al., 2007). circPCNXL2 is increased in RCC tissues, it sponges with miR-153 to up-regulate the expression of Zinc finger E-box-binding homeobox 2 (ZEB2), which can inhibit the expression of E-cadherin (Nam et al., 2012; Zhou et al., 2018). While, circ-AKT3 is stably downregulated in RCC tissues and negatively related to metastasis. circAKT3 functions as an miR-296-3p sponge to increase E-cadherin expression (Xue et al., 2019).
In addition to E-cadherin, N-cadherin (also known as cadherin 2, CDH2), which is a mesenchymal marker, is differently expressed in RCC and EMT progression (Behnes et al., 2012; Alimperti and Andreadis, 2015). circ_000926 is highly expressed and may function as an miR-411 sponge to upregulate CDH2 expression, thereby facilitating EMT progression and leading to poor prognosis (Zhang et al., 2019).
EMT is a complicated process that involves multiple transcription factors, and several important signaling pathways (Shang et al., 2019). Changes in the expression of CDH1 and CDH2 elicit a domino effect, and other factors are needed in EMT progression. Therefore, further studies should focus on whether other circRNAs are involved and determine how they cooperate with EMT transcription factors or pathways to accelerate or impede EMT in renal cancer.
Hormone Receptor-Related Circular RNA
RCC is 1.7 times more common in men than in women (Lee et al., 2012). Gender difference in the development and prognosis of renal cancer has been considered in numerous investigations (Aron et al., 2008; Marchioni et al., 2017; Lughezzani et al., 2019).
Androgen receptor (AR) is a transcriptional regulator involved in many cellular functions in men and women (Heemers and Tindall, 2007). The expression of AR can be detected in 14.8–42% of RCCs, which have short lifetimes (Langner et al., 2004; Noh et al., 2013). CDC42, as a member of the Rho family, is overexpressed in a number of human cancers and can be activated in response to extracellular matrix; it functions as a molecular switch for cell migration and invasion (Stengel and Zheng, 2011; Ni et al., 2013). Wang et al. (2017) found that the 3ʹ-UTR of miR-195-5p/29a-3p/29c-3p targets CDC42 to suppress its protein expression. circHIAT1, which can be suppressed by AR, increases miR-195-5p/29a-3p/29c-3p stability by acting as an miRNA reservoir to partly reverse AR-enhanced RCC migration and invasion.
Besides AR, estrogen receptor beta (ERβ) is involved in RCC progression. Yu et al. (2013) reported that ERβ expression is much higher in RCC cell lines than in breast cancer cell lines, and estrogen-activated ERβ acts as a tumor suppressor in RCC. However, clinical data from the TCGA database confirmed that higher ERβ expression was related to poorer prognosis in patients with RCC; these data serve as powerful evidence to prove that ERβ can be an oncogene in RCC progression (Song et al., 2015; Yeh et al., 2015). Fibronectin 1 (FN1) is highly expressed in vascular endothelial cells and vascular smooth muscles and promotes angiogenesis and endothelial cell migration, thereby aggravating RCC (Waalkes et al., 2010; Steffens et al., 2012). It is reported that ERβ-suppressed circATP2B1 functions as an miR-204-3p reservoir, it leads to miR-204-3p reduction, which increases FN1 expression and enhances RCC cell invasion (Han et al., 2018).
Hypoxia- and Immune-Related Circular RNAs
Mutations in the VHL gene and abnormal angiogenesis in RCC can lead to an increased activity of hypoxia-induced factors (HIFs), which can activate the transcription of downstream oncogenes that contain anoxic response elements (HREs) and signal pathways to affect the proliferation and metastasis of cancer cells (Min et al., 2002; Hsieh et al., 2017; Shan et al., 2018).
Vascular endothelial growth factor (VEGF) is needed for angiogenesis (Carmeliet and Jain, 2000). circTLK1 is found over-expressed in RCC and correlated with poor prognosis. circTLK1 sponges with miR-136-5p to increase CBX4 (a small ubiquitin-related modifier E3 ligase) expression, which promoting the expression of VEGFA (Ismail et al., 2012; Li et al., 2020). The increased circMYLK also can capture miR-513a-5p to facilitate VEGFC expression (Li et al., 2020). The uncontrolled expression of VEGF fails to abnormal vascular structure, resulting in hypoxia and may participate in RCC metastasis progression further. For example, the loss of E-cadherin in the EMT of RCC is mainly due to HIF-1 activation, and AR promotes RCC progression mainly through the HIF2a/VEGF pathway (He et al., 2014).
RCC has been well recognized as a disease that can evade the immune system and strongly respond to immunotherapy (Perez-Ruiz et al., 2019). T cells and natural killer cells are the most common types of immune cells in RCC tumors; infiltrating T cells can promote RCC cell invasion by increasing ERβ expression (Van den Hove et al., 1997; Yeh et al., 2015). Likewise, tumor cells and tumor-infiltrating macrophages (TAMs) produce the chemokine CCL22, which attracts regulatory T cells (Tregs) to create an immune-suppressive microenvironment, thereby impairing anticancer immunity (Martinenaite et al., 2016). circ_0039569 is upregulated, it can promote RCC progression by upregulating CCL22 expression though sponging with miR-34a-5p (Jin et al., 2019). Human endogenous retrovirus-H long terminal repeat-associating protein 2 (HHLA2) can interact with PD-1 and CTLA-4, resulting in T cell suppression. A high HHLA2 expression in RCC tissues is associated with poor prognosis. Comprehensive microarray analysis identify that thousands of circRNAs, which are considered downstream of HHLA2 may function in immune response to participate in RCC progress (Minárik et al., 2013; Chen et al., 2019).
These results suggest that different circRNAs play multifaceted roles in RCC. Whether other hypoxic- and immune-related circRNAs are formed in RCC is still unknown, future studies may focus on finding new related circRNAs and functionally investigate how circRNAs work in a systematic network of deterioration in RCC.
Circular RNAs in Acute Kidney Injury
AKI is characteristic as abrupt or rapid decline in the glomerular filtration rate, and mainly caused by ischemia/reperfusion (I/R), nephrotoxicity, and sepsis (Mehta et al., 2007; Barrantes et al., 2008). AKI is often diagnosed on the basis of creatinine levels, but creatinine assessments sometimes cannot accurately identify kidney function and it is insufficient for detecting early renal injury (Sanjeevani et al., 2014). Thus, novel biomarkers combining clinical sensitivity, specificity, and noninvasion are desired. circRNAs have been gradually employed in this field because of their universality and stability.
Inflammatory response including NF-κB pathways activation, interleukin 6 (IL-6) production are involved in the progression of AKI (Bonventre and Yang, 2011). ATF3 can block the activation of the NF-κB pathway and inhibit the release of IL-6 in AKI (Li et al., 2010). The expression of circ-0114427 is upregulated and can resist the early inflammatory state, by binding to miR-494 as an miRNA sponge to decrease ATF3 expression and further increase IL-6 expression (Cao et al., 2020). Similar, circVMA21 alleviated sepsis-associated AKI via regulating miR-9-3p/SMG1/inflammation and oxidative stress (Shi et al., 2020).
I/R induces the pathological damage and death of renal cells (Wang et al., 2017). Several circRNAs have been shown to be altered in IR–AKI model (Zhou et al., 2017). The increased circ-AKT3 promotes I/R injury progression via sponging to miR-144-5p to activate Wnt/β-catenin signal and regulating oxidative stress (Xu et al., 2020). While circYAP1 activates PI3K/AKT/mTOR pathway and secures HK-2 cells from I/R injury via sponging miR-21-5p (Huang et al., 2020). AKI also occurs in transplanted kidneys during or after the transplantation procedure itself (Munshi et al., 2011; Lameire et al., 2013). hsa_circ_0001334 is upregulated in patients who have acute kidney rejection, but it can return to normal levels when patients are successfully treated with an anti-rejection therapy (Kölling et al., 2019). A high ciRs-126 expression is connected with poor prognosis and an independent predictor of the 28 days survival of patients with AKI (Kölling et al., 2018).
Nephrotoxicity often occurs during disease diagnosis and treatment, and it accounts for approximately 20% of AKI cases (Uchino et al., 2005). Contrast-induced (CI) AKI is an acute renal insufficiency during disease diagnosis (Kellum and Lameire, 2013; Luo et al., 2017). Naglu is used to predict the prerenal development of AKI, and Gpnmb plays a protective role against AKI. It is reported that circ_0004153-rnomiR-144-3p-Gpnmb or Naglu, are validated in a CI-AKI rat model, and they are likely related to oxidative stress, drug metabolism through GO and KEGG pathway analyses (Cheng et al., 2019). Cisplatin chemotherapy is also a frequent cause of nephrotoxicity leading to AKI during disease treatment. A total of 224 upregulated circRNAs and 144 downregulated circRNAs, which are predominantly implicated in nucleic acid binding transcription and metabolic pathways, have been detected in cisplatin-treated mice through RNA sequencing analysis and bioinformatic analysis (Li et al., 2019).
Benefit from their structural stability and tissue specificity, circRNAs have great potential in the diagnosis and treatment of AKI at an early stage by changing the contents of urine and blood. More studies are needed to identify circRNAs specifically expressed during AKI development, and in-depth basic studies are warranted to assess their functions and improve AKI diagnosis and treatments.
Circular RNAs in Chronic Kidney Diseases
CKD is characterized by a reduced glomerular filtration rate and increased urinary albumin excretion; it is an important cause of low life quality and death. Hypertension and diabetes, glomerulonephritis and unknown causes are common causes of CKD (Jha et al., 2013).
In a mouse model of hypertension-related kidney disease, circNr1h4 is significantly downregulated in kidneys. circNr1h4 sponges with miR-155-5p to decreases the expression of fatty acid reductase 1 (Far1), increasing reactive oxygen species, thereby causing damage to renal epithelial cells (Buchert et al., 2014; Lu et al., 2020). These findings may help develop new therapeutic strategies of targeting circRNAs for hypertension-related kidney diseases.
Diabetic nephropathy (DN) is characterized by the proliferation of mesangial cells and the accumulation of the extracellular matrix (Wang et al., 2018; Lu et al., 2019), and there is also increasing evidence of the role of the inflammatory response in developing DN (Yaribeygi et al., 2019). circ-AKT3 inhibited the extracellular matrix accumulation through modulating miR-296-3p/E-cadherin signals in diabetic nephropathy mesangial cells (Tang et al., 2020). circRNA_15698 is upregulated in db/db mice, and acts as an miR-185 sponge to regulate TGF-β1 expression, which promoting extracellular matrix-related protein synthesis (Hu et al., 2019). Likewise, circRNA_010383 expression is markedly downregulated, it promotes proteinuria and the accumulation ECM proteins and down-regulate the expression of transient receptor potential cation channel, subfamily C, member (TRPC1) leading to the aggravation of renal fibrosis in DN by acting as a sponge for miRNA-135a (Peng et al., 2020). circ_WBSCR17 is highly expressed in DN mice, it triggers fibrosis and in ammation through increasing the expression of SOX6 by targeting miR-185-5p (Li et al., 2020). What’s more, it has been reported that silencing cANRIL (circular antisense noncoding RNA in the INK4 locus) alleviates inflammatory responses and blocks NF-κB and JNK/p38 pathways by positively regulating miR-9 in LPS-induced CKD modle (Deng et al., 2019). While The increased circ_DLGAP4 sponges with miR-143 and activates ERBB3/NF-κB/MMP-2 to promote fibrosis of mesangial cells (Bai et al., 2020). circLRP6 was found to be upregulated in high glucose (HG)-treated mesangial cells, regulated HG-induced cell injure via sponging miR-205, upregulating HMGB1 and activating TLR4/NF-κB pathway (Chen et al., 2019). The regulatory effect of circRNAs in DN should be verified in human tissues further.
Idiopathic membranous nephropathy (IMN) and lupus nephritis (LN) belong to autoimmune diseases that have a long disease course and an impaired kidney function (Almaani et al., 2017; Cattran and Brenchley, 2017). circRNAs serve as a potential biomarker for the diagnosis of IMN and LN. Some intron-derived circRNAs are reduced in serum and urine exosomes of patients with IMN and may be involved in IMN pathogenesis (Ma et al., 2019). Plasma circRNA_002453 is considered to be a potential biomarker to assess the severity of renal involvement in patients with LN (Ouyang et al., 2018). Bioinformatic analysis has predicted that several circRNAs, which are significantly upregulated in LN, participate in regulating dendritic cell differentiation and MHC protein complex. circHLA-C is found to be significantly increased, and may serve as a sponge of miR-150, which promotes renal fibrosis by regulating fibrosis-associated genes (Zhou et al., 2013; Luan et al., 2018). These data suggest the possible roles of circRNAs in immune-related CKD development. However, large cohorts and in vitro and in vivo experiments are needed to clarify the detailed mechanism.
Kidney stone often manifests as urinary tract infection and pain, and is also a cause of CKD (Zeng et al., 2017). 58 upregulated and 87 downregulated circRNAs have been identified to reveal the significant differential expression in the pathogenesis of kidney stones (Cao et al., 2018). Further studies are also needed to determine the detailed mechanism on how circRNAs work in kidney stone growth and CKD development.
With multiple pathogenic factors, CKD is still a global public health issue. Even though circRNAs have attracted much attention in the development of CKDs, the specific mechanism and function of circRNAs are still needed to provide a precise and new perspective for diagnosing and treating CKD.
Conclusion and Perspectives
In this review, multiple potential roles of circRNAs in RCC, AKI and CKD are summarized (Figure 2; Table 3), which enriching our understanding of the abundant circRNAs.
However, studies on the role of circRNAs in kidney-related diseases are still in the fledging period, most studies have elucidated biological phenomena mainly dependent on bioinformatic analyses but have not further systematically and experimentally explored the mechanisms of how circRNAs work in kidney-related diseases. There are some questions need to be discussed.
First, circRNAs are more stable, and they exist in specific tissues, giving them the potential of being ideal biomarkers for disease diagnosis. Current studies on circRNAs in RCC mainly focus on their effects on the biological function of RCC cells, and the expression of circRNAs was mainly detected in tumor tissues. But in AKI and CKD, circRNAs have been gradually identified as biomarkers combining clinical sensitivity, specificity and non-invasion to assess kidney function. The expression of circRNAs in blood or urine of patients with RCC may be a potential research topic to be used as a diagnostic basis for evaluating therapeutic efficacy. Even though most circRNAs are reported as biomarker, the correlation between the circRNAs levels and the degree of disease severity is not well analyzed. It needs more studies to confirm that whether circRNAs can return to normal level when patients with kidney-related diseases receive treatments.
Second, circRNAs can act as biomarkers in blood or urine, they settle in the cytoplasm once they are generated, how they are exported from the nucleus to the cytoplasm remains unclear. DExH/D-box helicase Hel25E participates in the extranuclear transport of circRNAs (>800 nt). The two human homologs of Hel25E are reported to participate the localization of circular RNA: UAP56 (DDX39B) contributes to the exportation of long (>1300 nt) circular RNAs, whereas URH49 (DDX39A) is necessary for the export of short (<400 nt) circular RNAs (Huang et al., 2018). Yet, how circRNAs between 400 and 800 nt are exported in humans is unknown.
Third, when circRNAs are exported, how they are degraded after they perform their functions is still ambiguous. Some circRNAs can be cleared by packaging into extracellular vesicles, such as exosomes (Lasda and Parker, 2016), which still act as biomarkers. CDR1as/ciRS-7 can be cleaved by Argonaute-2 (Ago2) to trigger transcript degradation (Hansen et al., 2013). circRNAs can be enriched in synapses and implicated in the development of neuronal differentiations (Rybak-Wolf et al., 2015; You et al., 2015), indicating the possibility of directional transmissions, some RNA endonucleases specifically exist in tissues to degrade certain circRNAs and cause their tissue specificity.
Furthermore, kidney-related diseases can interevolve to each other. Nephrectomy due to RCC may impair kidney function, AKI likely contributes to the development and progression of CKD, AKI is also one of the major complications in CKD (Uchino et al., 2005; He et al., 2017; Hassan et al., 2018). And cir-ATK3 takes part in RCC and AKI, whether exists a particular circRNA that regulates the progression of multiple kidney-related diseases simultaneously need to be confirmed.
Through continuous biological technology development and further circRNA exploration, circRNAs will eventually provide a new theoretical basis for conducting disease diagnosis, treatment in the coming years.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Author Contributions
XT-C, ZW-L, and XZ were major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82072649, 81872304 and 81802637), the Outstanding Youth Foundation of Jiangsu Province (BK20200046), the Education Department of Jiangsu Province (No. 19KJA130001), the Jiangsu Provincial Key Medical Discipline, and the Project of Invigorating Health Care through Science, Technology and Education (NO. ZDXKA2016014, ZDRCC2016009), the Natural Science Foundation of Jiangsu Province (BK20180989) and the Qing Lan Project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
AKI acute kidney injury;
AR Androgen receptor;
CCRCC clear cell renal cell carcinoma;
CDH1 cadherin 1, E-cadherin;
CDH2 cadherin 2, N-cadherin;
CI Contrast-induced;
circRNAs Circular RNAs;
ciRNAs circular intron circRNAs;
CKD chronic kidney disease;
DN Diabetic nephropathy;
ecircRNAs exonic circRNAs;
ECM extracellular matrix;
EIciRNA exon- and intron-derived or retained intron circRNAs;
EMT Epithelial-to-mesenchymal transition;
ERBB3 Erb-b2 receptor tyrosine kinase 3;
ERβ estrogen receptor beta;
Far1 fatty acid reductase 1;
FN1 Fibronectin 1;
FOXP4 forkhead-box P4;
HHLA2 Human endogenous retrovirus-H long terminal repeat-associating protein 2;
HIFs hypoxia-induced factors;
HREs hypoxia-responsive elements;
I/R Ischemia/reperfusion;
IMN Idiopathic membranous nephropathy;
IRF7 interferon regulatory factor 7;
LN Lupus nephritis;
lncRNAs long ncRNAs;
METTL14 methyltransferase-like 14;
miRNAs MicroRNAs;
ncRNAs noncoding RNAs;
RCC renal cell carcinoma;
SMG1 serine/threonine-protein kinase 1;
SOX12 sex-determining region Y (SRY)-box 12;
TGF-β Transforming growth factor β;
Tregs regulatory T cells;
TRPC1 transient receptor potential cation channel 1;
ZEB2 Zinc finger E-box-binding homeobox 2.
References
Alimperti, S., and Andreadis, S. T. (2015). CDH2 and CDH11 act as regulators of stem cell fate decisions. Stem Cell Res. 14, 270–282. doi:10.1016/j.scr.2015.02.002
Almaani, S., Meara, A., and Rovin, B. H. (2017). Update on lupus nephritis. Clin. J. Am. Soc. Nephrol. 12, 825–835. doi:10.2215/CJN.05780616
Aron, M., Nguyen, M. M., Stein, R. J., and Gill, I. S. (2008). Impact of gender in renal cell carcinoma: an analysis of the SEER database. Eur. Urol. 54, 133–140. doi:10.1016/j.eururo.2007.12.001
Aufiero, S., Reckman, Y. J., Pinto, Y. M., and Creemers, E. E. (2019). Circular RNAs open a new chapter in cardiovascular biology. Nat. Rev. Cardiol. 16, 503–514. doi:10.1038/s41569-019-0185-2
Bai, S., Xiong, X., Tang, B., Ji, T., Li, X., Qu, X., et al. (2020). Exosomal circ_DLGAP4 promotes diabetic kidney disease progression by sponging miR-143 and targeting ERBB3/NF-κB/MMP-2 axis. Cell Death Dis. 11, 1008. doi:10.1038/s41419-020-03169-3
Barrantes, F., Tian, J., Vazquez, R., Amoateng-Adjepong, Y., and Manthous, C. A. (2008). Acute kidney injury criteria predict outcomes of critically ill patients. Crit. Care Med. 36, 1397–1403. doi:10.1097/CCM.0b013e318168fbe0
Behnes, C. L., Hemmerlein, B., Strauss, A., Radzun, H. J., and Bremmer, F. (2012). N-cadherin is differentially expressed in histological subtypes of papillary renal cell carcinoma. Diagn. Pathol. 7, 95. doi:10.1186/1746-1596-7-95
Bonventre, J. V., and Yang, L. (2011). Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Invest. 121, 4210–4221. doi:10.1172/JCI45161
Brandenburger, T., Salgado Somoza, A., Devaux, Y., and Lorenzen, J. M. (2018). Noncoding RNAs in acute kidney injury. Kidney Int. 94, 870–881. doi:10.1016/j.kint.2018.06.033
Buchert, R., Tawamie, H., Smith, C., Uebe, S., Innes, A. M., Al Hallak, B., et al. (2014). A peroxisomal disorder of severe intellectual disability, epilepsy, and cataracts due to fatty acyl-CoA reductase 1 deficiency. Am. J. Hum. Genet. 95, 602–610. doi:10.1016/j.ajhg.2014.10.003
Cao, Y., Gao, X., Yang, Y., Ye, Z., Wang, E., and Dong, Z. (2018). Changing expression profiles of long non-coding RNAs, mRNAs and circular RNAs in ethylene glycol-induced kidney calculi rats. BMC Genomics 19, 660. doi:10.1186/s12864-018-5052-8
Cao, Y., Mi, X., Zhang, D., Wang, Z., Zuo, Y., and Tang, W. (2020). Transcriptome sequencing of circular RNA reveals a novel circular RNA-has_circ_0114427 in the regulation of inflammation in acute kidney injury. Clin. Sci. 134, 139–154. doi:10.1042/CS20190990
Carmeliet, P., and Jain, R. K. (2000). Angiogenesis in cancer and other diseases. Nature 407, 249–257. doi:10.1038/35025220
Cattran, D., and Brenchley, P. (2017). Membranous nephropathy: thinking through the therapeutic options. Nephrol. Dial. Transplant. 32, i22–i29. doi:10.1093/ndt/gfw404
Chao, C. W., Chan, D. C., Kuo, A., and Leder, P. (1998). The mouse formin (Fmn) gene: abundant circular RNA transcripts and gene-targeted deletion analysis. Mol. Med. 4, 614–628. doi:10.1007/bf03401761
Chen, B., Li, Y., Liu, Y., and Xu, Z. (2019a). circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J. Cell. Physiol. 234, 21249–21259. doi:10.1002/jcp.28730
Chen, D., Chen, W., Xu, Y., Zhu, M., Xiao, Y., Shen, Y., et al. (2019b). Upregulated immune checkpoint HHLA2 in clear cell renal cell carcinoma: a novel prognostic biomarker and potential therapeutic target. J. Med. Genet. 56, 43–49. doi:10.1136/jmedgenet-2018-105454
Chen, Q., Liu, T., Bao, Y., Zhao, T., Wang, J., Wang, H., et al. (2020a). CircRNA cRAPGEF5 inhibits the growth and metastasis of renal cell carcinoma via the miR-27a-3p/TXNIP pathway. Cancer Lett. 469, 68–77. doi:10.1016/j.canlet.2019.10.017
Chen, T., Shao, S., Li, W., Liu, Y., and Cao, Y. (2019c). The circular RNA hsa-circ-0072309 plays anti-tumour roles by sponging miR-100 through the deactivation of PI3K/AKT and mTOR pathways in the renal carcinoma cell lines. Artif. Cells Nanomed. Biotechnol. 47, 3638–3648. doi:10.1080/21691401.2019.1657873
Chen, T., Yu, Q., Shao, S., and Guo, L. (2020b). Circular RNA circFNDC3B protects renal carcinoma by miR-99a downregulation. J. Cell. Physiol. 235, 4399–4406. doi:10.1002/jcp.29316
Chen, T., Yu, Q., Xin, L., and Guo, L. (2020c). Circular RNA circC3P1 restrains kidney cancer cell activity by regulating miR-21/PTEN axis and inactivating PI3K/AKT and NF- kB pathways. J. Cell. Physiol. 235, 4001–4010. doi:10.1002/jcp.29296
Chen, X., Han, P., Zhou, T., Guo, X., Song, X., and Li, Y. (2016). circRNADb: a comprehensive database for human circular RNAs with protein-coding annotations. Sci. Rep. 6, 34985. doi:10.1038/srep34985
Chen, Z., Xiao, K., Chen, S., Huang, Z., Ye, Y., and Chen, T. (2020d). Circular RNA hsa_circ_001895 serves as a sponge of microRNA-296-5p to promote clear cell renal cell carcinoma progression by regulating SOX12. Cancer Sci. 111, 713–726. doi:10.1111/cas.14261
Cheng, W., Li, X. W., Xiao, Y. Q., and Duan, S. B. (2019). Non-coding RNA-associated ceRNA networks in a new contrast-induced acute kidney injury rat model. Mol. Ther. Nucleic Acids 17, 102–112. doi:10.1016/j.omtn.2019.05.011
Cocquerelle, C., Mascrez, B., Hétuin, D., and Bailleul, B. (1993). Mis-splicing yields circular RNA molecules. FASEB J. 7, 155–160. doi:10.1096/fasebj.7.1.7678559
Cox, A. G., Winterbourn, C. C., and Hampton, M. B. (2009). Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J. 425, 313–325. doi:10.1042/BJ20091541
Deng, W., Chen, K., Liu, S., and Wang, Y. (2019). Silencing circular ANRIL protects HK-2 cells from lipopolysaccharide-induced inflammatory injury through up-regulating microRNA-9. Artif. Cells Nanomed. Biotechnol. 47, 3478–3484. doi:10.1080/21691401.2019.1652187
Dong, Z., Liu, Y., Wang, Q., Wang, H., Ji, J., Huang, T., et al. (2020). The circular RNA-NRIP1 plays oncogenic roles by targeting microRNA-505 in the renal carcinoma cell lines. J. Cell. Biochem. 121, 2236–2246. doi:10.1002/jcb.29446
Dudekula, D. B., Panda, A. C., Grammatikakis, I., De, S., Abdelmohsen, K., and Gorospe, M. (2016). CircInteractome: a web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol. 13, 34–42. doi:10.1080/15476286.2015.1128065
Evans, A. J., Russell, R. C., Roche, O., Burry, T. N., Fish, J. E., Chow, V. W., et al. (2007). VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail. Mol. Cell. Biol. 27, 157–169. doi:10.1128/MCB.00892-06
Finucane, M. D., Tuna, M., Lees, J. H., and Woolfson, D. N. (1999). Core-directed protein design. I. An experimental method for selecting stable proteins from combinatorial libraries. Biochemistry 38, 11604–11612. doi:10.1021/bi990765n
Gao, Y., Wang, J., and Zhao, F. (2015). CIRI: an efficient and unbiased algorithm for de novo circular RNA identification. Genome Biol. 16, 4. doi:10.1186/s13059-014-0571-3
Glažar, P., Papavasileiou, P., and Rajewsky, N. (2014). circBase: a database for circular RNAs. RNA 20, 1666–1670. doi:10.1261/rna.043687.113
Gu, W., Wang, B., Wan, F., Wu, J., Lu, X., Wang, H., et al. (2018). SOX2 and SOX12 are predictive of prognosis in patients with clear cell renal cell carcinoma. Oncol. Lett. 15, 4564–4570. doi:10.3892/ol.2018.7828
Han, Y. N., Xia, S. Q., Zhang, Y. Y., Zheng, J. H., and Li, W. (2017). Circular RNAs: a novel type of biomarker and genetic tools in cancer. Oncotarget 8, 64551–64563. doi:10.18632/oncotarget.18350
Han, Z., Zhang, Y., Sun, Y., Chen, J., Chang, C., Wang, X., et al. (2018). Erβ-mediated alteration of circATP2B1 and miR-204-3p signaling promotes invasion of clear cell renal cell carcinoma. Cancer Res. 78, 2550–2563. doi:10.1158/0008-5472.CAN-17-1575
Hancock, J. M. (2014). Circles within circles: commentary on Ghosal et al. (Circ2Traits: a comprehensive database for circular RNA potentially associated with disease and traits. Front. Genet. 5, 459. doi:10.3389/fgene.2014.00459
Hansen, T. B., Jensen, T. I., Clausen, B. H., Bramsen, J. B., Finsen, B., Damgaard, C. K., et al. (2013a). Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388. doi:10.1038/nature11993
Hansen, T. B., Kjems, J., and Damgaard, C. K. (2013b). Circular RNA and miR-7 in cancer. Cancer Res. 73, 5609–5612. doi:10.1158/0008-5472.CAN-13-1568
Hassan, M. O., Duarte, R., Dix-Peek, T., Dickens, C., Naidoo, S., Vachiat, A., et al. (2018). Transforming growth factor-β protects against inflammation-related atherosclerosis in South African CKD patients. Int. J. Nephrol. 2018, 8702372. doi:10.1155/2018/8702372
He, D., Li, L., Zhu, G., Liang, L., Guan, Z., Chang, L., et al. (2014). ASC-J9 suppresses renal cell carcinoma progression by targeting an androgen receptor-dependent HIF2α/VEGF signaling pathway. Cancer Res. 74, 4420–4430. doi:10.1158/0008-5472.CAN-13-2681
He, L., Wei, Q., Liu, J., Yi, M., Liu, Y., Liu, H., et al. (2017). AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 92, 1071–1083. doi:10.1016/j.kint.2017.06.030
Heemers, H. V., and Tindall, D. J. (2007). Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr. Rev. 28, 778–808. doi:10.1210/er.2007-0019
Hsieh, J. J., Purdue, M. P., Signoretti, S., Swanton, C., Albiges, L., Schmidinger, M., et al. (2017). Renal cell carcinoma. Nat. Rev. Dis. Primers 3, 17009. doi:10.1038/nrdp.2017.9
Hu, W., Han, Q., Zhao, L., and Wang, L. (2019). Circular RNA circRNA_15698 aggravates the extracellular matrix of diabetic nephropathy mesangial cells via miR-185/TGF-β1. J. Cell. Physiol. 234, 1469–1476. doi:10.1002/jcp.26959
Huang, C., Liang, D., Tatomer, D. C., and Wilusz, J. E. (2018a). A length-dependent evolutionarily conserved pathway controls nuclear export of circular RNAs. Genes Dev. 32, 639–644. doi:10.1101/gad.314856.118
Huang, J. L., Qin, M. C., Zhou, Y., Xu, Z. H., Yang, S. M., Zhang, F., et al. (2018b). Comprehensive analysis of differentially expressed profiles of Alzheimer's disease associated circular RNAs in an Alzheimer's disease mouse model. Aging 10, 253–265. doi:10.18632/aging.101387
Huang, T., Cao, Y., Wang, H., Wang, Q., Ji, J., Sun, X., et al. (2020). Circular RNA YAP1 acts as the sponge of microRNA-21-5p to secure HK-2 cells from ischaemia/reperfusion-induced injury. J. Cell. Mol. Med. 24, 4707–4715. doi:10.1111/jcmm.15142
Ismail, I. H., Gagné, J. P., Caron, M. C., McDonald, D., Xu, Z., Masson, J. Y., et al. (2012). CBX4-mediated SUMO modification regulates BMI1 recruitment at sites of DNA damage. Nucleic Acids Res. 40, 5497–5510. doi:10.1093/nar/gks222
Izuogu, O. G., Alhasan, A. A., Alafghani, H. M., Santibanez-Koref, M., Elliott, D. J., and Jackson, M. S. (2016). PTESFinder: a computational method to identify post-transcriptional exon shuffling (PTES) events. BMC Bioinformatics 17, 31. doi:10.1186/s12859-016-0949-1
Jeck, W. R., Sorrentino, J. A., Wang, K., Slevin, M. K., Burd, C. E., Liu, J., et al. (2013). Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19, 141–157. doi:10.1261/rna.035667.112
Jha, V., Garcia-Garcia, G., Iseki, K., Li, Z., Naicker, S., Plattner, B., et al. (2013). Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272. doi:10.1016/S0140-6736(13)60687-X
Jiao, D., Huan, Y., Zheng, J., Wei, M., Zheng, G., Han, D., et al. (2019). UHRF1 promotes renal cell carcinoma progression through epigenetic regulation of TXNIP. Oncogene 38, 5686–5699. doi:10.1038/s41388-019-0822-6
Jin, C., Shi, L., Li, Z., Liu, W., Zhao, B., Qiu, Y., et al. (2019). Circ_0039569 promotes renal cell carcinoma growth and metastasis by regulating miR-34a-5p/CCL22. Am. J. translational Res. 11, 4935–4945.
Kellum, J. A., and Lameire, N.KDIGO AKI Guideline Work Group (2013). Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit. Care 17, 204. doi:10.1186/cc11454
Kölling, M., Haddad, G., Wegmann, U., Kistler, A., Bosakova, A., Seeger, H., et al. (2019). Circular RNAs in urine of kidney transplant patients with acute T cell-mediated allograft rejection. Clin. Chem. 65, 1287–1294. doi:10.1373/clinchem.2019.305854
Kölling, M., Seeger, H., Haddad, G., Kistler, A., Nowak, A., Faulhaber-Walter, R., et al. (2018). The circular RNA ciRs-126 predicts survival in critically ill patients with acute kidney injury. Kidney Int. Rep. 3, 1144–1152. doi:10.1016/j.ekir.2018.05.012
Lameire, N. H., Bagga, A., Cruz, D., De Maeseneer, J., Endre, Z., Kellum, J. A., et al. (2013). Acute kidney injury: an increasing global concern. Lancet 382, 170–179. doi:10.1016/S0140-6736(13)60647-9
Lan, Q., Peyvandi, S., Duffey, N., Huang, Y. T., Barras, D., Held, W., et al. (2019). Type I interferon/IRF7 axis instigates chemotherapy-induced immunological dormancy in breast cancer. Oncogene 38, 2814–2829. doi:10.1038/s41388-018-0624-2
Langner, C., Ratschek, M., Rehak, P., Schips, L., and Zigeuner, R. (2004). Steroid hormone receptor expression in renal cell carcinoma: an immunohistochemical analysis of 182 tumors. J. Urol. 171, 611–614. doi:10.1097/01.ju.0000108040.14303.c2
Lasda, E., and Parker, R. (2016). Circular RNAs co-precipitate with extracellular vesicles: a possible mechanism for circRNA clearance. PLoS One 11, e0148407. doi:10.1371/journal.pone.0148407
Lecuit, T., and Yap, A. S. (2015). E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol. 17, 533–539. doi:10.1038/ncb3136
Lee, S., Jeon, H. G., Kwak, C., Kim, H. H., Byun, S. S., Lee, S. E., et al. (2012). Gender-specific clinicopathological features and survival in patients with renal cell carcinoma (RCC). BJU Int. 110, E28–E33. doi:10.1111/j.1464-410X.2011.10667.x
Li, C. M., Li, M., Ye, Z. C., Huang, J. Y., Li, Y., Yao, Z. Y., et al. (2019). Circular RNA expression profiles in cisplatin-induced acute kidney injury in mice. Epigenomics 11, 1191–1207. doi:10.2217/epi-2018-0167
Li, G., Qin, Y., Qin, S., Zhou, X., Zhao, W., and Zhang, D. (2020a). Circ_WBSCR17 aggravates inflammatory responses and fibrosis by targeting miR-185-5p/SOX6 regulatory axis in high glucose-induced human kidney tubular cells. Life Sci. 259, 118269. doi:10.1016/j.lfs.2020.118269
Li, H. F., Cheng, C. F., Liao, W. J., Lin, H., and Yang, R. B. (2010). ATF3-mediated epigenetic regulation protects against acute kidney injury. J. Am. Soc. Nephrol. 21, 1003–1013. doi:10.1681/ASN.2009070690
Li, H., Heng, B., Ouyang, P., Xie, X., Zhang, T., Chen, G., et al. (2020b). Comprehensive profiling of circRNAs and the tumor suppressor function of circHIPK3 in clear cell renal carcinoma. J. Mol. Histol. 51, 317–327. doi:10.1007/s10735-020-09882-9
Li, J. H., Liu, S., Zhou, H., Qu, L. H., and Yang, J. H. (2014). starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 42, D92–D97. doi:10.1093/nar/gkt1248
Li, J., Huang, C., Zou, Y., Ye, J., Yu, J., and Gui, Y. (2020c). CircTLK1 promotes the proliferation and metastasis of renal cell carcinoma by sponging miR-136-5p. Mol. Cancer 19, 103. doi:10.1186/s12943-020-01225-2
Li, J., Huang, C., Zou, Y., Yu, J., and Gui, Y. (2020d). Circular RNA MYLK promotes tumour growth and metastasis via modulating miR-513a-5p/VEGFC signalling in renal cell carcinoma. J. Cell. Mol. Med. 24, 6609–6621. doi:10.1111/jcmm.15308
Li, S., Li, Y., Chen, B., Zhao, J., Yu, S., Tang, Y., et al. (2018a). exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 46, D106–D112. doi:10.1093/nar/gkx891
Li, X., Chu, C., Pei, J., Măndoiu, I., and Wu, Y. (2018b). CircMarker: a fast and accurate algorithm for circular RNA detection. BMC Genomics 19, 572. doi:10.1186/s12864-018-4926-0
Li, Y., Zheng, Q., Bao, C., Li, S., Guo, W., Zhao, J., et al. (2015). Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 25, 981–984. doi:10.1038/cr.2015.82
Lin, L., and Cai, J. (2020). Circular RNA circ-EGLN3 promotes renal cell carcinoma proliferation and aggressiveness via miR-1299-mediated IRF7 activation. J. Cell. Biochem. 121, 4377–4385. doi:10.1002/jcb.29620
Liu, Y. C., Li, J. R., Sun, C. H., Andrews, E., Chao, R. F., Lin, F. M., et al. (2016). CircNet: a database of circular RNAs derived from transcriptome sequencing data. Nucleic Acids Res. 44, D209–D215. doi:10.1093/nar/gkv940
Lowenstein, J., and Grantham, J. J. (2017). Residual renal function: a paradigm shift. Kidney Int. 91, 561–565. doi:10.1016/j.kint.2016.09.052
Lu, C., Chen, B., Chen, C., Li, H., Wang, D., Tan, Y., et al. (2020). CircNr1h4 regulates the pathological process of renal injury in salt-sensitive hypertensive mice by targeting miR-155-5p. J. Cell. Mol. Med. 24, 1700–1712. doi:10.1111/jcmm.14863
Lu, Q., Chen, Y. B., Yang, H., Wang, W. W., Li, C. C., Wang, L., et al. (2019). Inactivation of TSC1 promotes epithelial-mesenchymal transition of renal tubular epithelial cells in mouse diabetic nephropathy. Acta Pharmacol. Sin. 40, 1555–1567. doi:10.1038/s41401-019-0244-6
Luan, J., Jiao, C., Kong, W., Fu, J., Qu, W., Chen, Y., et al. (2018). circHLA-C plays an important role in lupus nephritis by sponging miR-150. Mol. Ther. Nucleic Acids 10, 245–253. doi:10.1016/j.omtn.2017.12.006
Lughezzani, G., Paciotti, M., Fasulo, V., Casale, P., and Saita, A. (2019). Gender-specific risk factors for renal cell carcinoma: a systematic review. Curr. Opin. Urol. 29, 272–278. doi:10.1097/MOU.0000000000000603
Luo, M., Yang, Y., Xu, J., Cheng, W., Li, X. W., Tang, M. M., et al. (2017). A new scoring model for the prediction of mortality in patients with acute kidney injury. Sci. Rep. 7, 7862. doi:10.1038/s41598-017-08440-w
Ma, H., Xu, Y., Zhang, R., Guo, B., Zhang, S., and Zhang, X. (2019). Differential expression study of circular RNAs in exosomes from serum and urine in patients with idiopathic membranous nephropathy. Arch. Med. Sci. 15, 738–753. doi:10.5114/aoms.2019.84690
Marchioni, M., Martel, T., Bandini, M., Pompe, R. S., Tian, Z., Kapoor, A., et al. (2017). Marital status and gender affect stage, tumor grade, treatment type and cancer specific mortality in T1-2 N0 M0 renal cell carcinoma. World J. Urol. 35, 1899–1905. doi:10.1007/s00345-017-2082-9
Martinenaite, E., Munir Ahmad, S., Hansen, M., Met, Ö., Westergaard, M. W., Larsen, S. K., et al. (2016). CCL22-specific T Cells: modulating the immunosuppressive tumor microenvironment. Oncoimmunology 5, e1238541. doi:10.1080/2162402X.2016.1238541
Mehta, R. L., Kellum, J. A., Shah, S. V., Molitoris, B. A., Ronco, C., Warnock, D. G., et al. (2007). Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care 11, R31. doi:10.1186/cc5713
Mehta, S. L., Dempsey, R. J., and Vemuganti, R. (2020). Role of circular RNAs in brain development and CNS diseases. Prog. Neurobiol. 186, 101746. doi:10.1016/j.pneurobio.2020.101746
Memczak, S., Jens, M., Elefsinioti, A., Torti, F., Krueger, J., Rybak, A., et al. (2013). Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338. doi:10.1038/nature11928
Meng, X., Chen, Q., Zhang, P., and Chen, M. (2017). CircPro: an integrated tool for the identification of circRNAs with protein-coding potential. Bioinformatics 33, 3314–3316. doi:10.1093/bioinformatics/btx446
Min, J. H., Yang, H., Ivan, M., Gertler, F., Kaelin, W. G., and Pavletich, N. P. (2002). Structure of an HIF-1alpha -pVHL complex: hydroxyproline recognition in signaling. Science 296, 1886–1889. doi:10.1126/science.1073440
Minárik, I., Lašťovička, J., Budinský, V., Kayserová, J., Spíšek, R., Jarolím, L., et al. (2013). Regulatory T cells, dendritic cells and neutrophils in patients with renal cell carcinoma. Immunol. Lett. 152, 144–150. doi:10.1016/j.imlet.2013.05.010
Munshi, R., Hsu, C., and Himmelfarb, J. (2011). Advances in understanding ischemic acute kidney injury. BMC Med. 9, 11. doi:10.1186/1741-7015-9-11
Nam, E. H., Lee, Y., Park, Y. K., Lee, J. W., and Kim, S. (2012). ZEB2 upregulates integrin α5 expression through cooperation with Sp1 to induce invasion during epithelial-mesenchymal transition of human cancer cells. Carcinogenesis 33, 563–571. doi:10.1093/carcin/bgs005
Ni, S., Hu, J., Duan, Y., Shi, S., Li, R., Wu, H., et al. (2013). Down expression of LRP1B promotes cell migration via RhoA/Cdc42 pathway and actin cytoskeleton remodeling in renal cell cancer. Cancer Sci. 104, 817–825. doi:10.1111/cas.12157
Nieto, M. A., Huang, R. Y., Jackson, R. A., and Thiery, J. P. (2016). EMT: 2016. Cell 166, 21–45. doi:10.1016/j.cell.2016.06.028
Noh, S. J., Kang, M. J., Kim, K. M., Bae, J. S., Park, H. S., Moon, W. S., et al. (2013). Acetylation status of P53 and the expression of DBC1, SIRT1, and androgen receptor are associated with survival in clear cell renal cell carcinoma patients. Pathology 45, 574–580. doi:10.1097/PAT.0b013e3283652c7a
Ouyang, Q., Huang, Q., Jiang, Z., Zhao, J., Shi, G. P., and Yang, M. (2018). Using plasma circRNA_002453 as a novel biomarker in the diagnosis of lupus nephritis. Mol. Immunol. 101, 531–538. doi:10.1016/j.molimm.2018.07.029
Peng, F., Gong, W., Li, S., Yin, B., Zhao, C., Liu, W., et al. (2020). circRNA_010383 acts as a sponge for miR-135a and its downregulated expression contributes to renal fibrosis in diabetic nephropathy. Diabetes [Epub ahead of print]. doi:10.2337/db200203
Perez-Ruiz, E., Minute, L., Otano, I., Alvarez, M., Ochoa, M. C., Belsue, V., et al. (2019). Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 569, 428–432. doi:10.1038/s41586-019-1162-y
Qu, S., Yang, X., Li, X., Wang, J., Gao, Y., Shang, R., et al. (2015). Circular RNA: a new star of noncoding RNAs. Cancer Lett. 365, 141–148. doi:10.1016/j.canlet.2015.06.003
Rybak-Wolf, A., Stottmeister, C., Glažar, P., Jens, M., Pino, N., Giusti, S., et al. (2015). Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell 58, 870–885. doi:10.1016/j.molcel.2015.03.027
Salzman, J., Gawad, C., Wang, P. L., Lacayo, N., and Brown, P. O. (2012). Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One 7, e30733. doi:10.1371/journal.pone.0030733
Sanger, H. L., Klotz, G., Riesner, D., Gross, H. J., and Kleinschmidt, A. K. (1976). Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA 73, 3852–3856. doi:10.1073/pnas.73.11.3852
Sanjeevani, S., Pruthi, S., Kalra, S., Goel, A., and Kalra, O. P. (2014). Role of neutrophil gelatinase-associated lipocalin for early detection of acute kidney injury. Int. J. Crit. Illn Inj. Sci. 4, 223–228. doi:10.4103/2229-5151.141420
Shan, H., Hou, P., Zhang, M., Li, L., Pan, Y., Chen, F., et al. (2018). PTBP1 knockdown in renal cell carcinoma inhibits cell migration, invasion and angiogenesis in vitro and metastasis in vivo via the hypoxia inducible factor-1α pathway. Int. J. Oncol. 52, 1613–1622. doi:10.3892/ijo.2018.4296
Shang, B. Q., Li, M. L., Quan, H. Y., Hou, P. F., Li, Z. W., Chu, S. F., et al. (2019). Functional roles of circular RNAs during epithelial-to-mesenchymal transition. Mol. Cancer 18, 138. doi:10.1186/s12943-019-1071-6
Shi, Y., Sun, C. F., Ge, W. H., Du, Y. P., and Hu, N. B. (2020). Circular RNA VMA21 ameliorates sepsis-associated acute kidney injury by regulating miR-9-3p/SMG1/inflammation axis and oxidative stress. J. Cell. Mol. Med. 24, 11397–11408. doi:10.1111/jcmm.15741
Siegel, R. L., Miller, K. D., and Jemal, A. (2017). Cancer statistics, 2017. CA Cancer J. Clin. 67, 7–30. doi:10.3322/caac.21387
Song, W., Yeh, C. R., He, D., Wang, Y., Xie, H., Pang, S. T., et al. (2015). Infiltrating neutrophils promote renal cell carcinoma progression via VEGFa/HIF2α and estrogen receptor β signals. Oncotarget 6, 19290–19304. doi:10.18632/oncotarget.4478
Song, X., Zhang, N., Han, P., Moon, B. S., Lai, R. K., Wang, K., et al. (2016). Circular RNA profile in gliomas revealed by identification tool UROBORUS. Nucleic Acids Res. 44, e87. doi:10.1093/nar/gkw075
Steffens, S., Schrader, A. J., Vetter, G., Eggers, H., Blasig, H., Becker, J., et al. (2012). Fibronectin 1 protein expression in clear cell renal cell carcinoma. Oncol. Lett. 3, 787–790. doi:10.3892/ol.2012.566
Stengel, K., and Zheng, Y. (2011). Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell. Signal. 23, 1415–1423. doi:10.1016/j.cellsig.2011.04.001
Sun, J., Yin, A., Zhang, W., Lv, J., Liang, Y., Li, H., et al. (2020). CircUBAP2 inhibits proliferation and metastasis of clear cell renal cell carcinoma via targeting miR-148a-3p/FOXK2 pathway. Cell Transplant. 29, 963689720925751. doi:10.1177/0963689720925751
Tang, B., Li, W., Ji, T. T., Li, X. Y., Qu, X., Feng, L., et al. (2020). Circ-AKT3 inhibits the accumulation of extracellular matrix of mesangial cells in diabetic nephropathy via modulating miR-296-3p/E-cadherin signals. J. Cell. Mol. Med. 24, 8779–8788. doi:10.1111/jcmm.15513
Uchino, S., Kellum, J. A., Bellomo, R., Doig, G. S., Morimatsu, H., Morgera, S., et al. (2005). Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294, 813–818. doi:10.1001/jama.294.7.813
Van den Hove, L. E., Van Gool, S. W., Van Poppel, H., Baert, L., Coorevits, L., Van Damme, B., et al. (1997). Phenotype, cytokine production and cytolytic capacity of fresh (uncultured) tumour-infiltrating T lymphocytes in human renal cell carcinoma. Clin. Exp. Immunol. 109, 501–509. doi:10.1046/j.1365-2249.1997.4771375.x
van der Heide, L. P., Wijchers, P. J., von Oerthel, L., Burbach, J. P., Hoekman, M. F., and Smidt, M. P. (2015). FoxK2 is required for cellular proliferation and survival. J. Cell. Physiol. 230, 1013–1023. doi:10.1002/jcp.24828
Waalkes, S., Atschekzei, F., Kramer, M. W., Hennenlotter, J., Vetter, G., Becker, J. U., et al. (2010). Fibronectin 1 mRNA expression correlates with advanced disease in renal cancer. BMC Cancer 10, 503. doi:10.1186/1471-2407-10-503
Wang, G., Xue, W., Jian, W., Liu, P., Wang, Z., Wang, C., et al. (2018a). The effect of Hsa_circ_0001451 in clear cell renal cell carcinoma cells and its relationship with clinicopathological features. J. Cancer 9, 3269–3277. doi:10.7150/jca.25902
Wang, J., Zhang, J. Q., Zhao, X. L., Lu, J. Y., Weng, Z. M., Ding, Z. M., et al. (2020). Circular RNA DHX33 promotes malignant behavior in ccRCC by targeting miR-489-3p/MEK1 axis. Aging 12, 14885–14896. doi:10.18632/aging.103550
Wang, K., Sun, Y., Tao, W., Fei, X., and Chang, C. (2017a). Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett. 394, 1–12. doi:10.1016/j.canlet.2016.12.036
Wang, P. L., Bao, Y., Yee, M. C., Barrett, S. P., Hogan, G. J., Olsen, M. N., et al. (2014). Circular RNA is expressed across the eukaryotic tree of life. PLoS One 9, e90859. doi:10.1371/journal.pone.0090859
Wang, Q., Zhang, H., Chen, Q., Wan, Z., Gao, X., and Qian, W. (2019). Identification of METTL14 in kidney renal clear cell carcinoma using bioinformatics analysis. Dis. Markers 2019, 5648783. doi:10.1155/2019/5648783
Wang, R., Zhang, S., Chen, X., Li, N., Li, J., Jia, R., et al. (2018b). CircNT5E acts as a sponge of miR-422a to promote glioblastoma tumorigenesis. Cancer Res. 78, 4812–4825. doi:10.1158/0008-5472.CAN-18-0532
Wang, X. B., Zhu, H., Song, W., and Su, J. H. (2018c). Gremlin regulates podocyte apoptosis via transforming growth factor-β (TGF-β) pathway in diabetic nephropathy. Med. Sci. Monit. 24, 183–189. doi:10.12659/msm.905758
Wang, Y., Mu, Y., Zhou, X., Ji, H., Gao, X., Cai, W. W., et al. (2017b). SIRT2-mediated FOXO3a deacetylation drives its nuclear translocation triggering FasL-induced cell apoptosis during renal ischemia reperfusion. Apoptosis 22, 519–530. doi:10.1007/s10495-016-1341-3
Wei, X., Dong, Y., Chen, X., Ren, X., Li, G., Wang, Y., et al. (2020). Construction of circRNA-based ceRNA network to reveal the role of circRNAs in the progression and prognosis of metastatic clear cell renal cell carcinoma. Aging 12, 24184–24207. doi:10.18632/aging.104107
Westholm, J. O., Miura, P., Olson, S., Shenker, S., Joseph, B., Sanfilippo, P., et al. (2014). Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep. 9, 1966–1980. doi:10.1016/j.celrep.2014.10.062
Xia, S., Feng, J., Chen, K., Ma, Y., Gong, J., Cai, F., et al. (2018). CSCD: a database for cancer-specific circular RNAs. Nucleic Acids Res. 46, D925–D929. doi:10.1093/nar/gkx863
Xia, S., Feng, J., Lei, L., Hu, J., Xia, L., Wang, J., et al. (2017). Comprehensive characterization of tissue-specific circular RNAs in the human and mouse genomes. Brief. Bioinformatics 18, 984–992. doi:10.1093/bib/bbw081
Xiong, Y., Zhang, J., and Song, C. (2019). CircRNA ZNF609 functions as a competitive endogenous RNA to regulate FOXP4 expression by sponging miR-138-5p in renal carcinoma. J. Cell. Physiol. 234, 10646–10654. doi:10.1002/jcp.27744
Xu, T., Wu, J., Han, P., Zhao, Z., and Song, X. (2017). Circular RNA expression profiles and features in human tissues: a study using RNA-seq data. BMC Genomics 18, 680. doi:10.1186/s12864-017-4029-3
Xu, Y., Jiang, W., Zhong, L., Li, H., Bai, L., Chen, X., et al. (2020). circ-AKT3 aggravates renal ischaemia-reperfusion injury via regulating miR-144-5p/Wnt/β-catenin pathway and oxidative stress. J. Cell. Mol. Med. [Epub ahead of print]. doi:10.1111/jcmm.16072
Xue, D., Wang, H., Chen, Y., Shen, D., Lu, J., Wang, M., et al. (2019). Circ-AKT3 inhibits clear cell renal cell carcinoma metastasis via altering miR-296-3p/E-cadherin signals. Mol. Cancer 18, 151. doi:10.1186/s12943-019-1072-5
Yan, L., Liu, G., Cao, H., Zhang, H., and Shao, F. (2019). Hsa_circ_0035483 sponges hsa-miR-335 to promote the gemcitabine-resistance of human renal cancer cells by autophagy regulation. Biochem. Biophys. Res. Commun. 519, 172–178. doi:10.1016/j.bbrc.2019.08.093
Yang, T., Li, H., Thakur, A., Chen, T., Xue, J., Li, D., et al. (2015). FOXP4 modulates tumor growth and independently associates with miR-138 in non-small cell lung cancer cells. Tumour Biol. 36, 8185–8191. doi:10.1007/s13277-015-3498-8
Yaribeygi, H., Atkin, S. L., Simental-Mendía, L. E., Barreto, G. E., and Sahebkar, A. (2019). Anti-inflammatory effects of resolvins in diabetic nephropathy: mechanistic pathways. J. Cell. Physiol. [Epub ahead of print]. doi:10.1002/jcp.28315
Yeh, C. R., Ou, Z. Y., Xiao, G. Q., Guancial, E., and Yeh, S. (2015). Infiltrating T cells promote renal cell carcinoma (RCC) progression via altering the estrogen receptor β-DAB2IP signals. Oncotarget 6, 44346–44359. doi:10.18632/oncotarget.5884
You, X., Vlatkovic, I., Babic, A., Will, T., Epstein, I., Tushev, G., et al. (2015). Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat. Neurosci. 18, 603–610. doi:10.1038/nn.3975
Yu, C. P., Ho, J. Y., Huang, Y. T., Cha, T. L., Sun, G. H., Yu, D. S., et al. (2013). Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-β activation. PLoS One 8, e56667. doi:10.1371/journal.pone.0056667
Yu, R., Yao, J., and Ren, Y. (2020). A novel circRNA, circNUP98, a potential biomarker, acted as an oncogene via the miR-567/PRDX3 axis in renal cell carcinoma. J. Cell. Mol. Med. 24, 10177–10188. doi:10.1111/jcmm.15629
Zaphiropoulos, P. G. (1996). Circular RNAs from transcripts of the rat cytochrome P450 2C24 gene: correlation with exon skipping. Proc. Natl. Acad. Sci. USA 93, 6536–6541. doi:10.1073/pnas.93.13.6536
Zeng, G., Mai, Z., Xia, S., Wang, Z., Zhang, K., Wang, L., et al. (2017). Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int. 120, 109–116. doi:10.1111/bju.13828
Zeng, J., Feng, Q., Wang, Y., Xie, G., Li, Y., Yang, Y., et al. (2020). Circular RNA circ_001842 plays an oncogenic role in renal cell carcinoma by disrupting microRNA-502-5p-mediated inhibition of SLC39A14. J. Cell. Mol. Med. doi:10.1111/jcmm.15529
Zhang, D., Yang, X. J., Luo, Q. D., Fu, D. L., Li, Z. L., Zhang, P., et al. (2019). Down-regulation of circular RNA_000926 attenuates renal cell carcinoma progression through miRNA-411-dependent CDH2 inhibition. Am. J. Pathol. 189, 2469–2486. doi:10.1016/j.ajpath.2019.06.016
Zhang, L., and Guo, Y. (2020). Silencing circular RNA-ZNF652 represses proliferation and EMT process of renal carcinoma cells via raising miR-205. Artif. Cells Nanomed. Biotechnol. 48, 648–655. doi:10.1080/21691401.2020.1725532
Zhang, Q., Wang, W., Zhou, Q., Chen, C., Yuan, W., Liu, J., et al. (2020). Roles of circRNAs in the tumour microenvironment. Mol. Cancer 19, 14. doi:10.1186/s12943-019-1125-9
Zhang, X. O., Dong, R., Zhang, Y., Zhang, J. L., Luo, Z., Zhang, J., et al. (2016). Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res. 26, 1277–1287. doi:10.1101/gr.202895.115
Zhang, Y., Zhang, X. O., Chen, T., Xiang, J. F., Yin, Q. F., Xing, Y. H., et al. (2013). Circular intronic long noncoding RNAs. Mol. Cell 51, 792–806. doi:10.1016/j.molcel.2013.08.017
Zhao, Z., Wang, K., Wu, F., Wang, W., Zhang, K., Hu, H., et al. (2018). circRNA disease: a manually curated database of experimentally supported circRNA-disease associations. Cell Death Dis. 9, 475. doi:10.1038/s41419-018-0503-3
Zheng, L. L., Li, J. H., Wu, J., Sun, W. J., Liu, S., Wang, Z. L., et al. (2016). deepBase v2.0: identification, expression, evolution and function of small RNAs, LncRNAs and circular RNAs from deep-sequencing data. Nucleic Acids Res. 44, D196–D202. doi:10.1093/nar/gkv1273
Zheng, X. B., Zhang, M., and Xu, M. Q. (2017). Detection and characterization of ciRS-7: a potential promoter of the development of cancer. Neoplasma 64, 321–328. doi:10.4149/neo_2017_301
Zhong, L., Wang, Y., Cheng, Y., Wang, W., Lu, B., Zhu, L., et al. (2018). Circular RNA circC3P1 suppresses hepatocellular carcinoma growth and metastasis through miR-4641/PCK1 pathway. Biochem. Biophys. Res. Commun. 499, 1044–1049. doi:10.1016/j.bbrc.2018.03.221
Zhou, B., Zheng, P., Li, Z., Li, H., Wang, X., Shi, Z., et al. (2018). CircPCNXL2 sponges miR-153 to promote the proliferation and invasion of renal cancer cells through upregulating ZEB2. Cell Cycle 17, 2644–2654. doi:10.1080/15384101.2018.1553354
Zhou, H., Hasni, S. A., Perez, P., Tandon, M., Jang, S. I., Zheng, C., et al. (2013). miR-150 promotes renal fibrosis in lupus nephritis by downregulating SOCS1. J. Am. Soc. Nephrol. 24, 1073–1087. doi:10.1681/ASN.2012080849
Keywords: circRNAs, renal cell carcinoma, acute kidney injury, chronic kidney diseases, EMT
Citation: Chen X-T, Li Z-W, Zhao X, Li M-L, Hou P-F, Chu S-F, Zheng J-N and Bai J (2021) Role of Circular RNA in Kidney-Related Diseases. Front. Pharmacol. 12:615882. doi: 10.3389/fphar.2021.615882
Received: 10 October 2020; Accepted: 02 February 2021;
Published: 11 March 2021.
Edited by:
Shrikant R Mulay, Central Drug Research Institute (CSIR), IndiaReviewed by:
Harshal Waghulde, Leidos Biomedical Research, Inc., United StatesAnton Jan Van Zonneveld, Leiden University Medical Center, Netherlands
Copyright © 2021 Chen, Li, Zhao, Li, Hou, Chu, Zheng and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Nian Zheng, am56aGVuZ0B4emhtdS5lZHUuY24=; Jin Bai, YmpAeHpobXUuZWR1LmNu
†These authors have contributed equally to this work
 Xin-Tian Chen
Xin-Tian Chen Zhong-Wei Li
Zhong-Wei Li Xue Zhao1†
Xue Zhao1† Jun-Nian Zheng
Jun-Nian Zheng Jin Bai
Jin Bai