
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 11 June 2021
Sec. Inflammation Pharmacology
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.614753
Background: Osteoarthritis (OA) high disability rate will increase as people getting older, and is the most prevalent form of arthritis in the future. This study identified the clinical effects of optimum doses of tanezumab for patients with OA.
Method: Three electronic databases were searched up until January 15, 2021. The mean difference (MD) or odds ratio (OR) was considered an effect measure. The design-by-treatment interaction model was adopted for network meta-analyses. Analyses were conducted using WinBUGS 1.4.3 and R 4.0.5 software.
Results: nine publications with 10 studies were included. Compared with placebo in network meta-analysis, except the outcomes of Western Ontario and McMaster Universities Osteoarthritis (WOMAC) stiffness subscale and joints replaced, all dosages of tanezumab in the other effectiveness outcome were superior to placebo, and the difference was statistically significant. However, there was no statistical difference among all different doses of tanezumab. Compared with placebo, except the outcomes of adverse events (AEs) and AEs of abnormal peripheral sensation, all different dosages of tanezumab weren’t superior to placebo in the other effectiveness outcome, and the difference was statistically significant. The 10 mg of tanezumab with highest SUCRA had the best effect, but it was associated with a higher safety event. Compared with placebo, except the outcomes of WOMAC stiffness subscale and joints replaced, all dosages of tanezumab in the other effectiveness outcome were superior to placebo, and the difference was statistically significant. Compared with placebo, except for the outcomes of AEs and AEs of abnormal peripheral sensation, all dosages of tanezumab in the other effectiveness outcome were superior to placebo, and the difference was statistically significant. Other direct comparisons showed no statistical difference.
Conclusion: This study recommended that clinicians should give priority to the treatment of OA patients with a low dose of 2.5 mg according to the patient’s condition and actual situation. If the effect using tanezumab with 2.5 mg is not satisfactory, the increase up to 10 mg should be carefully pondered, because of a more unbalanced risk/benefit ratio.
Osteoarthritis (OA) is unpredictable chronic joint disease, which usually lies dormant for a long time (Ekman et al., 2014; Karsdal et al., 2019; Berenbaum et al., 2020). Patients with OA will increase as people getting older. Once elderly OA patients combined with diabetes, these are more prone to experience adverse effects (Ekman et al., 2014; Kan et al., 2016; Tive et al., 2019).
At present, drug therapy is still the most important intervention method and challenge (Kan et al., 2016). Non-steroidal anti-inflammatory drugs (NSAIDs) and opioids are representative drugs improving and relieving the pain of OA, but they may increase adverse events (AEs). According to relevant studies, based on the mechanism that anti NGF may reduce OA related pain, nerve growth factor (NGF) antagonists may become candidate drugs (Chevalier et al., 2013). Subsequently, related studies (Brown et al., 2012; Brown et al., 2013; Schnitzer et al., 2015; Berenbaum et al., 2020) reported the clinical efficacy and safety of tanezumab. Based on some studies (Karsdal et al., 2019; Schnitzer et al., 2019; Tive et al., 2019; Patel et al., 2020), transient cutaneous paresthesia was reported in some patients, while subsequent studies minimized the risk and provided a comprehensive summary of joint and nervous system AEs. Tanezumab has high affinity and specificity for NGF, which is an important carrier to transmit pain signals (Ekman et al., 2014). In adults, reducing NGF sensitivity results in decreased peripheral receptor sensitivity and decreased neuropeptide levels (Tive et al., 2019). Related evidences suggested that NGF injection into the skin can cause pain (Ekman et al., 2014; Schnitzer and Marks, 2015; Tive et al., 2019). Based on the results of the latest randomized controlled trials (RCT) from Berenbaum’s phase III study in 2020 (Berenbaum et al., 2020), the tanezumab 5 mg statistically significantly improved pain, physical function and Patient’s Global Assessment of OA, while tanezumab 2.5 mg only achieved two co-primary end points. However, rapidly progressive osteoarthritis occurred more frequently with tanezumab 5 mg than tanezumab 2.5 mg. Moreover, the results of stratified meta-analysis in 2020 (Yu et al., 2020) showed that there was no difference in benefit between 2.5 and 5 mg of tanezumab, except for the outcome of rapidly progressive osteoarthritis. In addition, no complementary analysis of different doses was performed in other meta-analyses about tanezumab for OA (Kan et al., 2016; Chen et al., 2017; Fan et al., 2020), leading to a lack of evidence of a dose-response relationship to guide clinical application. A conclusion of RCT from Nagashima (Nagashima et al., (2011)) showed that at doses of 10 and 50 mg/kg, the effect of tanezumab on these efficacy endpoints was not substantially different from placebo. A previous study in patients with knee OA from the United States (Lane et al., 2010) showed tanezumab to be effective in reducing pain at doses of 10 and 50 mg/kg. The reason for the lack of efficacy of tanezumab at these doses in this study is unclear. By contrast, we observed a generally dose-related incidence of AEs of abnormal peripheral sensation (Lane et al., 2010; Brown et al., 2012). To demonstrate the clinical effects of optimum dosages of tanezumab for patients with OA, this research investigated the clinical efficacy and safety of tanezumab of clinical outcomes to guide clinicians to make the best decisions based on network meta-analysis.
Three electronic academic databases, including the Medline and EMbase from Ovid, the Cochrane Library, and Web of Science were searched up until January 15, 2021, using “Osteoarthritis,” “Osteoarthrosis,” “Arthritis, Degenerative,” “Degenerative Arthritides,” “Arthrosis,” “Osteoarthrosis Deformans,” and “Tanezumab”.
The following inclusion criteria for clinical trials were adopted: 1) Adult patients with OA of knee or hip; 2) The intervention group was treated with tanezumab, which must be fixed dosage in order to avoid differences or inconsistences in dose changes; 3) The control group was placebo; 4) Effective outcomes, including pain subscale, physical function subscale, stiffness subscale and pain reduction (≥30, ≥50, ≥70, and ≥90%) based on the Western Ontario and McMaster Universities Osteoarthritis (WOMAC), patient’s global assessment of OA, and joints replaced; Safety outcomes, including AEs, serious AEs, discontinued due to AEs, treatment-related AEs, AEs of abnormal peripheral sensation, and new or worsened abnormalities. 4) The included study must be RCT.
Studies were excluded based on the following criteria: 1) Duplicate studies 2) The data could not be extracted or obtained through contact with the author; 3) Studies with insufficient data for statistical analysis; 4) Studies without available full text.
The study design included: patient characteristics, interventions, controls, and outcomes. The data acquisition was done independently by two authors. The methodological quality was assessed using the Cochrane Collaboration’s tool (Higgins and Green, 2011).
The weighted mean difference (MD) was considered an effect size for continuous outcomes (Higgins and Green, 2011), and odds ratio (OR) was employed for other dichotomous outcomes (Higgins and Green, 2011). The statistical test for heterogeneity was performed, and I2 > 40% and p < 0.1 were considered as heterogeneity as well. For outcomes with high heterogeneity, meta-regression analysis was used to explore confounding factors in order to identify potential sources of heterogeneity (Higgins and Green, 2011).
The design-by-treatment interaction model (Günhan et al., 2018) was adopted for network meta-analyses. By using non-informative priors with vague normal (mean 0, variance 10,000) and uniform (0–5) prior distributions for parameters such as the means and standard deviations (Lu and Ades, 2004). First, 10,000 simulations were performed, and then we generated an additional 60,000 simulations with three sets of different initial values and sheared the first 10,000 simulations as the burn-in period in our model. We used the Brooks-Gelman-Rubin statistical method for assessing model convergence. Based on 50,000 simulations with 10 thin, the point estimate adopted the median of the posterior distribution, and the corresponding 95% credible interval (CrIs) used the 2.5th and 97.5th percentiles of the posterior distributions, which were interpreted in a similar fashion as conventional 95% confidence intervals.
We assessed loop inconsistency in the network meta-analysis (Salanti, 2012). To summarize probabilities, we used the surface under the cumulative ranking curve (SUCRA) to provide a summary statistic for the cumulative ranking (Salanti et al., 2011). All data analyses were conducted using WinBUGS 1.4.3 and R 4.0.5 software. The latest Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement (Hutton et al., 2015) for the reporting of systematic reviews and network meta-analysis was used.
1,726 individual studies were searched. After inclusion and exclusion criteria, 45 full texts were assessed for eligibility. Finally, nine RCTs (Brown et al., 2012; Brown et al., 2013; Spierings et al., 2013; Balanescu et al., 2014; Brown et al., 2014; Ekman et al., 2014; Schnitzer et al., 2015; Schnitzer et al., 2019; Berenbaum et al., 2020) with 10 studies involving 7,004 patients were involved in the meta-analysis (Figure 1). Table 1 displayed the essential characteristic of included 10 studies. The result of methodological quality is shown in Table 2.
Figure 2 indicates the network of eligible studies with different dosages of tanezumab and placebo from all efficiency outcomes. Supplementary Figure S1 shows the results of loop consistency for all efficiency outcomes, showing no inconsistency. Compared with placebo, except the outcomes of WOMAC stiffness subscale and joints replaced, all dosages of tanezumab in the other effectiveness outcome were superior to placebo, and the difference weren’t statistically significant (Table 3). In particular, tanezumab with 10 mg (MD = −0.83, 95%CrIs = −1.04, −0.61), 5 mg (MD = −0.81, 95%CrIs = −1.01, −0.61), and 2.5 mg (MD = −0.68, 95%CrIs = −0.93, −0.41) were superior to placebo for WOMAC pain subscale. In WOMAC physical function subscale, tanezumab with 10 mg (MD = −0.93, 95%CrIs = −1.12, −0.74), 5 mg (MD = −0.92, 95%CrIs = −1.10, −0.73), and 2.5 mg (MD = −0.69, 95%CrIs = −0.93, −0.44) were superior to placebo. In patient’s global assessment of OA, tanezumab with 10 mg (MD = −0.25, 95%CrIs = −0.33, −0.17), 5 mg (MD = −0.25, 95%CrIs = −0.33, −0.18), and 2.5 mg (MD = −0.20, 95%CrIs = −0.30, −0.10) were superior to placebo. In WOMAC pain reduction ≥30%, tanezumab with 10 mg (OR = 1.57, 95%CrIs = 1.26, 1.95), 5 mg (OR = 1.58, 95%CrIs = 1.31, 1.90), and 2.5 mg (OR = 1.37, 95%CrIs = 1.07, 1.74) were superior to placebo. In WOMAC pain reduction ≥50%, tanezumab with 10 mg (OR = 1.90, 95%CrIs = 1.51, 2.39), 5 mg (OR = 1.73, 95%CrIs = 1.42, 2.09), and 2.5 mg (OR = 1.54, 95%CrIs = 1.21, 1.96) were superior to placebo. In WOMAC pain reduction ≥70%, tanezumab with 10 mg (OR = 1.96, 95%CrIs = 1.47, 2.64), 5 mg (OR = 1.85, 95%CrIs = 1.47, 2.37), and 2.5 mg (OR = 1.65, 95%CrIs = 1.22, 2.22) were superior to placebo. In WOMAC pain reduction ≥90%, tanezumab with 10 mg (OR = 1.98, 95%CrIs = 1.24, 3.26), 5 mg (OR = 2.35, 95%CrIs = 1.60, 3.56), and 2.5 mg (OR = 1.97, 95%CrIs = 1.21, 3.24) were superior to placebo. There was no statistical difference among all doses of tanezumab.
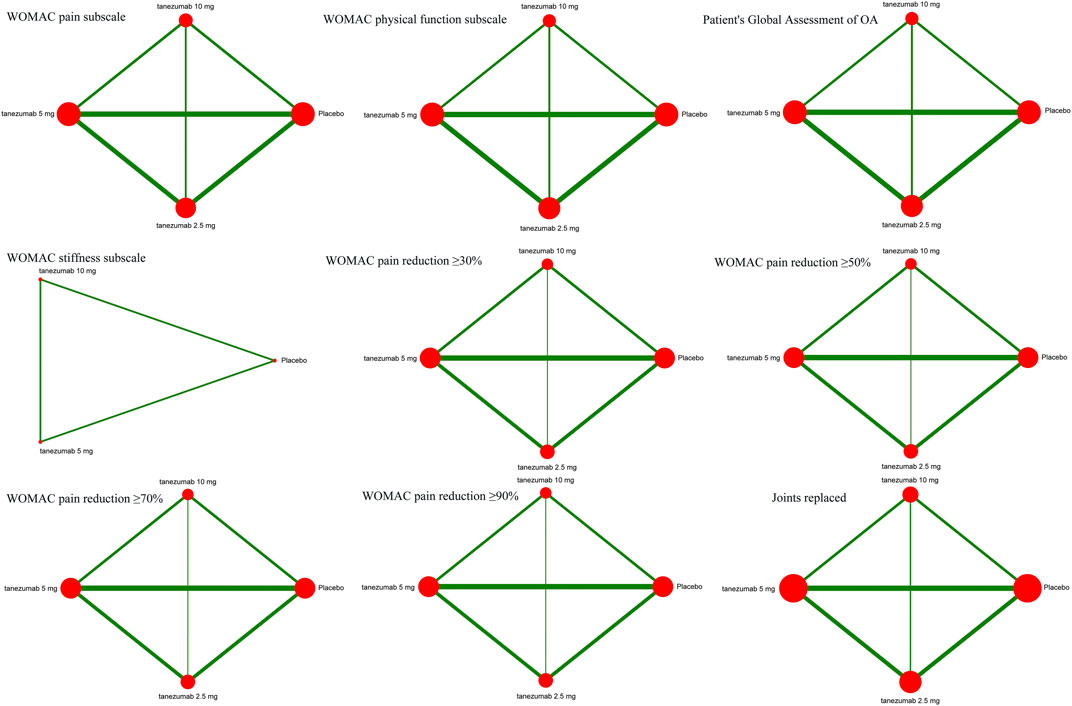
FIGURE 2. The network of eligible studies in efficiency outcomes. The node sizes correspond to the sample size that investigated the treatments. Directly comparable treatments are linked with a line, and the thickness of the line corresponds to the sum of the sample size in each pairwise treatment comparison.

TABLE 3. Results of network meta-analysis and direct comparison meta-analysis for efficiency outcomes.
Figure 3 indicates the network of eligible studies with different dosages of tanezumab and placebo from all safety outcomes. Compared with placebo, except the outcomes of serious AEs, treatment-related AEs and new or worsened abnormalities, all the active drugs of tanezumab in the other effectiveness outcomes weren’t superior to placebo, and it were statistically significant in other outcomes (Table 4). In outcomes of AEs, the incidence of tanezumab with 10 mg (OR = 1.41, 95%CrIs = 1.22, 1.64), tanezumab with 5 mg (OR = 1.27, 95%CrIs = 1.11, 1.45), and tanezumab with 2.5 mg (OR = 1.44, 95%CrIs = 1.20, 1.73) was significantly higher than with placebo. In outcomes of AEs of abnormal peripheral sensation, the incidence of tanezumab with 10 mg (OR = 3.97, 95%CrIs = 2.34, 6.86), tanezumab with 5 mg (OR = 2.71, 95%CrIs = 1.58, 4.66), and 2.5 mg (OR = 3.23, 95%CrIs = 1.67, 6.37) was significantly higher than with placebo. In discontinued due to AEs, compared with the other groups, tanezumab with 10 mg (OR = 2.01, 95%CrIs = 1.41, 3.03) had a higher discontinuation rate. There was no statistical difference among all doses of tanezumab.
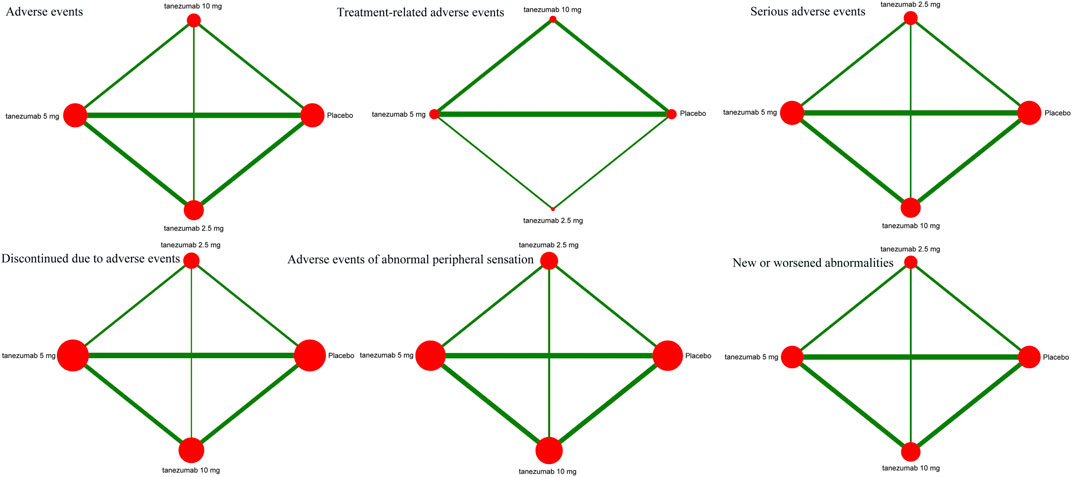
FIGURE 3. The network of eligible studies in safety outcomes. The node sizes correspond to the sample size that investigated the treatments. Directly comparable treatments are linked with a line, and the thickness of the line corresponds to the sum of the sample size in each pairwise treatment comparison.
Compared with placebo, except for the outcomes of WOMAC stiffness subscale and joints replaced, all dosages of tanezumab in the other effectiveness outcome were superior to placebo, and the difference was statistically significant (Table 3). Compared with tanezumab with 2.5 mg, tanezumab with 10 mg were more effective in outcomes of WOMAC pain subscale (MD = −0.28, 95%CrIs = −0.50, −0.05), WOMAC physical function subscale (MD = −0.29, 95%CrIs = −0.49, −0.06), patient’s global assessment of OA (MD = −0.10, 95%CrIs = −0.17, −0.04), WOMAC pain reduction ≥30% (OR = 1.62, 95%CrIs = 1.01, 2.58), and WOMAC pain reduction ≥50% (OR = 1.73, 95%CrIs = 1.09, 2.74). However, the tanezumab with 5 mg (MD = 0.19, 95%CrIs = 0.14, 0.24) was superior to tanezumab with 10 mg in WOMAC stiffness subscale. Other direct comparisons showed no statistical difference.
Compared with placebo, except for the outcomes of AEs and AEs of abnormal peripheral sensation, all dosages of tanezumab in the other effectiveness outcome were superior to placebo, and the difference was statistically significant (Table 4). Compared with tanezumab with 10 mg, tanezumab with 5 mg (OR = 1.44, 95%CrIs = 1.10, 1.89) and placebo (OR = 1.95, 95%CrIs = 1.46, 2.62) have high incidences in outcomes for discontinuation based on AEs. However, other direct comparisons showed no statistical difference.
As for the efficiency outcomes, Figure 4 indicates the ranking of tanezumab with the three doses under study. With the exception of joints replacement, all other efficacy outcomes showed that the 10 mg dose of tanezumab with highest SUCRA had the best effect, and the placebo with lowest SUCRA had the worst.
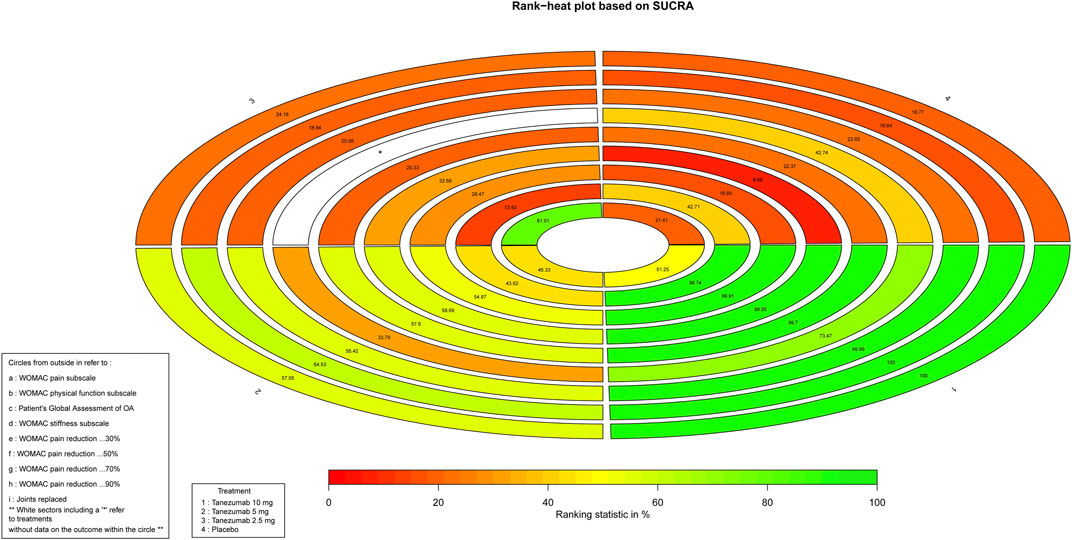
FIGURE 4. The Rank-heat plot of SUCRA for efficiency outcomes. Each sector is colored according to the SUCRA value of the corresponding treatment and outcome. The scale consists of the transformation of three colors red (0%), yellow (50%), and green (100%), and each color is associated with a different pattern. Uncolored sectors show that the underlying treatment was not included in the network meta-analysis for the particular outcome. SUCRA: Surface under the cumulative ranking.
As for safety outcomes, Figure 5 indicates the ranking of tanezumab with 10 mg, tanezumab with the three doses under study. With the exception of new or worsened abnormalities, all other safety outcomes showed that the 10 mg dose of tanezumab with highest SUCRA was associated with a higher safety event.
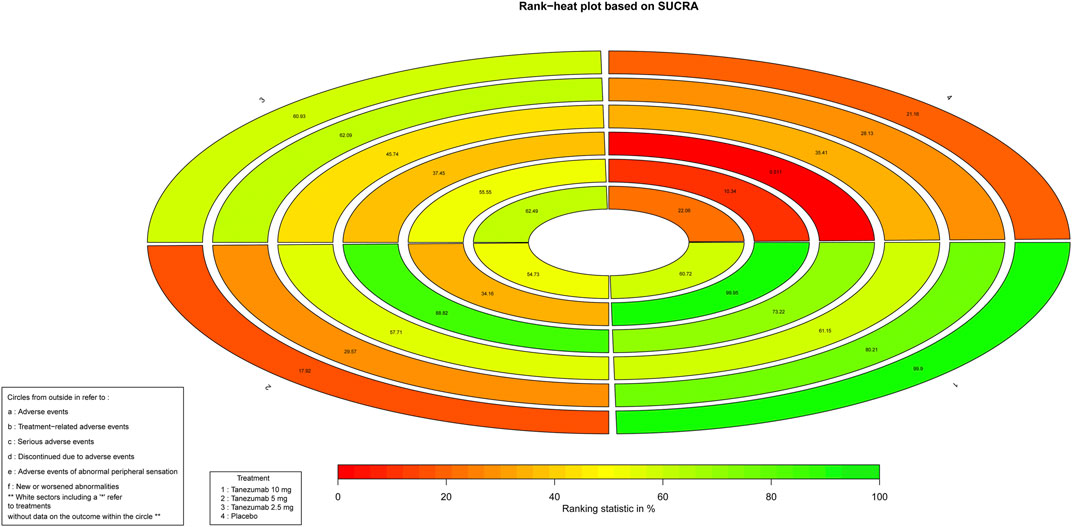
FIGURE 5. The Rank-heat plot of SUCRA for safety outcomes. Each sector is colored according to the SUCRA value of the corresponding treatment and outcome. The scale consists of the transformation of three colors red (0%), yellow (50%), and green (100%), and each color is associated with a different pattern. Uncolored sectors show that the underlying treatment was not included in the network meta-analysis for the particular outcome. SUCRA: Surface under the cumulative ranking.
Analysis of the heterogeneity in all direct comparisons revealed that significant heterogeneity was mainly distributed in efficiency outcomes, including WOMAC pain subscale, WOMAC physical function subscale, and patient’s global assessment of OA. Regression meta-analysis of variables, including age, sample, percentage of different Kellgren-Lawrence grade, average value of WOMAC Pain subscale score, average value of WOMAC Physical Function subscale score, and duration of joint disease, showed no significant difference (p > 0.05) in outcomes with high heterogeneity. It’s worth noting that, for the inconsistency assessment in network meta-analysis, Supplementary Figures S1, S2 reprot the results of loop consistency for all efficiency and safety outcomes, showing no inconsistency.
NGF is a neurotrophic factor involved in pain signal transduction and gene expression of nociceptors (Zhu and Oxford, 2007). NGF has been shown to contribute to the clinical symptoms of pain hypersensitivity, which is commonly seen in inflammatory and chronic pain states (Ghilardi et al., 2012). NGF is expressed in subchondral bone in patients with OA, and is consistent with the role of symptomatic OA pain (Walsh et al., 2010). Tanezumab inhibs the binding of NGF to its receptors and is studied to treat chronic pain, such as OA and chronic low back pain (Bélanger et al., 2018). Since some patients in previous tanezumab studies reported transient cutaneous paresthesia, subsequent studies implemented an overall risk minimization strategy and evaluated joint and nervous system AEs in a comprehensive way (International Conference, 1997; Hochberg et al., 2016).
The present meta-analysis evaluated the clinical outcomes of tanezumab for OA. As for WOMAC indicators, our study showed that tanezumab with different dosages significantly reduced the pain, stiffness subscale, physical punction, and pain reduction of WOMAC and patient’s global assessment of OA. Although our meta-analysis did not directly compare tanezumab with other active pharmaceuticals, other studies (Spierings et al., 2013) showed that compared with NSAIDs or oxycodone, tanezumab treatment showed higher efficacy rates. Joint replacement is the treatment for joint failure. Notably, the present study showes that different doses of tanezumab do not increase joint replacement.
In line with a previous meta analysis (Yu et al., 2020), our study also found that different doses of tanezumab did not increase joint replacement. This also confirmed that the treatment with tanezumab does not increase the risk of osteonecrosis. However, a systemic review and meta-analysis of randomized phase III clinical trials (Yu et al., 2020) showed that tanezumab had a higher rate of rapidly progressive OA (RPOA) than the NSAIDs and opioids group, and 10 mg tanezumab combined with NSAIDs had the highest estimated rate of RPOA, which is also a contributor to joint replacement. It has been suggested that the reason why tanezumab causes RPOA may be that pain relief promotes an increase in joint motion, which may inadvertently lead to joint overload (Dimitroulas et al., 2017; Watt and Gulati, 2017). In conclusion, the mechanism is still unknown, and more studies are needed to pay attention to this, so as to provide reference for clinical use.
At the same time, we also found that the higher the dose, the more significant the target efficacy of WOMAC. This conclusion has also been reported by other studies (Brown et al., 2014; Ekman et al., 2014; Schnitzer and Marks, 2015). However, no differences in the benefits were found in the benefits of all drug doses, including 2.5, 5, and 10 mg. This also makes us to believe that the high dose investment does not bring a high profit return. In the previous meta-analyses, except the study published by Chen in 2016 (Chen et al., 2017), which recommended 2.5 mg as the optimal dose, the other three meta-analyses (Kan et al., 2016; Fan et al., 2020; Yu et al., 2020) did not provide the conclusion of the optimal dose.
On the contrary, other researches (Spierings et al., 2013; Balanescu et al., 2014) reported that tanezumab had certain AEs and leads to an increase in the withdrawal rate associated with AEs. In our results, tanezumab had a higher proportion of AEs than placebo. However, there was no statistical difference in the incidence of adverse events between different doses. As for treatment-related AEs and serious AEs, no obvious significant increase was found in tanezumab with all dosages, but the tanezumab with 10 mg significantly increased treatment discontinuation due to AEs. This may be due to the fact that the number of studies and sample sizes included in the outcome of treatment-related adverse events were too small to meet expectations for the statistical power of the current results. Of concern, tanezumab, has high rates of AEs of abnormal peripheral sensation, but did not increase other rate of new or worsened abnormalities.
Heterogeneity tests based on 10 RCT studies showed that the dominant heterogeneity was in the effectiveness outcome, including WOMAC pain subscale, WOMAC physical function subscale, and patient’s global assessment of OA. Although relevant confounders may exist and affect the accuracy of the results, no significant confounders were found in this study based on the regression analysis. The confounding factors cannot be strictly broken down and can only be analyzed “symbolically” in the form of percentages, such as Kellgren-Lawrence grade, or the average value of WOMAC pain subscale score, which may also affect the results of regression. However, no inconsistency was found in the loop-based inconsistency detection network meta-analysis, which further ensures the reliability of results meta-analysis.
The biggest advantage of network meta-analysis is that it can comprehensively rank the effectiveness and safety of all current interventions, so as to provide a basis for clinicians to make better decisions. In order to better use relevant evidence to patients with OA, it has been a focus to minimize the occurrence of safety events while ensuring maximum effectiveness. Based on the analysis of the ranking results of this study, tanezumab with 10 mg was ranked first in both effectiveness and AE outcomes. This also brings about some contradictions and conflicts in clinical decision-making. Therefore, this study suggested that clinicians should give priority to the treatment of OA patients with a low dose of 2.5 mg according to the patient’s condition and actual situation. If the effect using tanezumab with 2.5 mg is not satisfactory, its dose can be increased to 5 or 10 mg, but the relevant safety events must be monitored more intensively.
The clinical outcomes of tanezumab in the management of OA were comprehensively evaluated while including as many qualified studies and sample sizes as possible in this study. This study also has some limitations. First, there are too few studies on different dosages of drugs in all outcomes, which may lead to unstable results. Secondly, unlawfully controlled confounding factors were mixed into this study, resulting in greater direct heterogeneity. However, the results of network meta-analysis prompted the existence of consistencies.
Overall, this study confirmed that tanezumab with 10 mg has a powerful effect on the treatment of OA. However, it also increases the risk of AE. Therefore, we recommend that clinicians should give priority to the treatment of OA patients with a low dose of 2.5 mg according to the patient’s condition and actual situation. If the effect using tanezumab with 2.5 mg is not satisfactory, the increase up to 10 mg should be carefully pondered, because of a more unbalanced risk/benefit ratio.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
BT had full access to all of the data in the study, and took responsibility for the integrity of the data and the accuracy of the data analysis. RH and YFS designed the study. RH and YFS developed and tested the data collection forms. RH, YFS, and ZYY acquired the data. ZYY and CZ conducted the analysis and interpreted the data. RH and YFS drafted the article. All authors critically revised the article. BT had guarantor. All authors read and approved the final article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.614753/full#supplementary-material
Balanescu, A. R., Feist, E., Wolfram, G., Davignon, I., Smith, M. D., Brown, M. T., et al. (2014). Efficacy and Safety of Tanezumab Added on to Diclofenac Sustained Release in Patients with Knee or Hip Osteoarthritis: a Double-Blind, Placebo-Controlled, Parallel-Group, Multicentre Phase III Randomised Clinical Trial. Ann. Rheum. Dis. 73 (9), 1665–1672. doi:10.1136/annrheumdis-2012-203164
Bélanger, P., West, C. R., and Brown, M. T. (2018). Development of Pain Therapies Targeting Nerve Growth Factor Signal Transduction and the Strategies Used to Resolve Safety Issues. J. Toxicol. Sci. 43 (1), 1–10. doi:10.2131/jts.43.1
Berenbaum, F., Blanco, F. J., Guermazi, A., Miki, K., Yamabe, T., Viktrup, L., et al. (2020). Subcutaneous Tanezumab for Osteoarthritis of the Hip or Knee: Efficacy and Safety Results from a 24-week Randomised Phase III Study with a 24-week Follow-Up Period. Ann. Rheum. Dis. 79 (6), 800–810. doi:10.1136/annrheumdis-2019-216296
Brown, M. T., Herrmann, D. N., Goldstein, M., Burr, A. M., Smith, M. D., West, C. R., et al. (2014). Nerve Safety of Tanezumab, a Nerve Growth Factor Inhibitor for Pain Treatment. J. Neurol. Sci. 345 (1-2), 139–147. doi:10.1016/j.jns.2014.07.028
Brown, M. T., Murphy, F. T., Radin, D. M., Davignon, I., Smith, M. D., and West, C. R. (2013). Tanezumab Reduces Osteoarthritic Hip Pain: Results of a Randomized, Double-Blind, Placebo-Controlled Phase III Trial. Arthritis Rheum. 65 (7), 1795–1803. doi:10.1002/art.37950
Brown, M. T., Murphy, F. T., Radin, D. M., Davignon, I., Smith, M. D., and West, C. R. (2012). Tanezumab Reduces Osteoarthritic Knee Pain: Results of a Randomized, Double-Blind, Placebo-Controlled Phase III Trial. The J. Pain 13 (8), 790–798. doi:10.1016/j.jpain.2012.05.006
Chen, J., Li, J., Li, R., Wang, H., Yang, J., Xu, J., et al. (2017). Efficacy and Safety of Tanezumab on Osteoarthritis Knee and Hip Pains: A Meta-Analysis of Randomized Controlled Trials. Pain Med. 18 (2), 374–385. doi:10.1093/pm/pnw262
Chevalier, X., Eymard, F., and Richette, P. (2013). Biologic Agents in Osteoarthritis: Hopes and Disappointments. Nat. Rev. Rheumatol. 9 (7), 400–410. doi:10.1038/nrrheum.2013.44
Dimitroulas, T., Lambe, T., Klocke, R., Kitas, G. D., and Duarte, R. V. (2017). Biologic Drugs as Analgesics for the Management of Osteoarthritis. Semin. Arthritis Rheum. 46 (6), 687–691. doi:10.1016/j.semarthrit.2016.12.001
Ekman, E. F., Gimbel, J. S., Bello, A. E., Smith, M. D., Keller, D. S., Annis, K. M., et al. (2014). Efficacy and Safety of Intravenous Tanezumab for the Symptomatic Treatment of Osteoarthritis: 2 Randomized Controlled Trials versus Naproxen. J. Rheumatol. 41 (11), 2249–2259. doi:10.3899/jrheum.131294
Fan, Z. R., Ma, J. X., Wang, Y., Chen, H. T., Lang, S., and Ma, X. L. (2020). Efficacy and Safety of Tanezumab Administered as a Fixed Dosing Regimen in Patients with Knee or Hip Osteoarthritis: a Meta-Analysis of Randomized Controlled Phase III Trials. Clin. Rheumatol. 40, 2155. doi:10.1007/s10067-020-05488-4
Ghilardi, J. R., Freeman, K. T., Jimenez-Andrade, J. M., Coughlin, K. A., Kaczmarska, M. J., Castaneda-Corral, G., et al. (2012). Neuroplasticity of Sensory and Sympathetic Nerve Fibers in a Mouse Model of a Painful Arthritic Joint. Arthritis Rheum. 64 (7), 2223–2232. doi:10.1002/art.34385
Günhan, B. K., Friede, T., and Held, L. (2018). A Design-By-Treatment Interaction Model for Network Meta-Analysis and Meta-Regression with Integrated Nested Laplace Approximations. Res. Syn Meth 9 (2), 179–194. doi:10.1002/jrsm.1285
Higgins, J., and Green, S. (2011). Cochrane Handbook for Systematic Reviews of Interventions. v.5.1. Available at: http://www.cochrane-handbook.org. (Accessed 2011 Mar 05).
Hochberg, M. C., Tive, L. A., Abramson, S. B., Vignon, E., Verburg, K. M., West, C. R., et al. (2016). When Is Osteonecrosis Not Osteonecrosis?: Adjudication of Reported Serious Adverse Joint Events in the Tanezumab Clinical Development Program. Arthritis Rheumatol. 68 (2), 382–391. doi:10.1002/art.39492
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/m14-2385
Author Anonymous, (1997). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Adopts Consolidated Guideline on Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use. Int. Dig. Health Legis. 48 (2) 231–234.
Kan, S. L., Li, Y., Ning, G. Z., Yuan, Z. F., Chen, L. X., Bi, M. C., et al. (2016). Tanezumab for Patients with Osteoarthritis of the Knee: A Meta-Analysis. PLoS One 11 (6), e0157105. doi:10.1371/journal.pone.0157105
Karsdal, M. A., Verburg, K. M., West, C. R., Bay-Jensen, A. C., Keller, D. S., and Arends, R. H. G. P. (2019). Serological Biomarker Profiles of Rapidly Progressive Osteoarthritis in Tanezumab-Treated Patients. Osteoarthritis and Cartilage 27 (3), 484–492. doi:10.1016/j.joca.2018.12.001
Lane, N. E., Schnitzer, T. J., Birbara, C. A., Mokhtarani, M., Shelton, D. L., Smith, M. D., et al. (2010). Tanezumab for the Treatment of Pain from Osteoarthritis of the Knee. N. Engl. J. Med. 363 (16), 1521–1531. doi:10.1056/nejmoa0901510
Lu, G., and Ades, A. E. (2004). Combination of Direct and Indirect Evidence in Mixed Treatment Comparisons. Statist. Med. 23 (20), 3105–3124. doi:10.1002/sim.1875
Nagashima, H., Suzuki, M., Araki, S., Yamabe, T., and Muto, C. (2011). Preliminary Assessment of the Safety and Efficacy of Tanezumab in Japanese Patients with Moderate to Severe Osteoarthritis of the Knee: a Randomized, Double-Blind, Dose-Escalation, Placebo-Controlled Study. Osteoarthritis and Cartilage 19 (12), 1405–1412. doi:10.1016/j.joca.2011.09.006
Patel, F., Hess, D. K., and Maher, D. P. (2020). Anti-nerve Growth Factor Antibodies for the Treatment of Low Back Pain. Expert Rev. Clin. Pharmacol. 13 (6), 631–639. doi:10.1080/17512433.2020.1772052
Salanti, G., Ades, A. E., and Ioannidis, J. P. A. (2011). Graphical Methods and Numerical Summaries for Presenting Results from Multiple-Treatment Meta-Analysis: an Overview and Tutorial. J. Clin. Epidemiol. 64 (2), 163–171. doi:10.1016/j.jclinepi.2010.03.016
Salanti, G. (2012). Indirect and Mixed-Treatment Comparison, Network, or Multiple-Treatments Meta-Analysis: many Names, many Benefits, many Concerns for the Next Generation Evidence Synthesis Tool. Res. Syn. Meth. 3 (2), 80–97. doi:10.1002/jrsm.1037
Schnitzer, T. J., Easton, R., Pang, S., Levinson, D. J., Pixton, G., Viktrup, L., et al. (2019). Effect of Tanezumab on Joint Pain, Physical Function, and Patient Global Assessment of Osteoarthritis Among Patients with Osteoarthritis of the Hip or Knee. JAMA 322 (1), 37–48. doi:10.1001/jama.2019.8044
Schnitzer, T. J., Ekman, E. F., Spierings, E. L. H., Greenberg, H. S., Smith, M. D., Brown, M. T., et al. (2015). Efficacy and Safety of Tanezumab Monotherapy or Combined with Non-steroidal Anti-inflammatory Drugs in the Treatment of Knee or Hip Osteoarthritis Pain. Ann. Rheum. Dis. 74 (6), 1202–1211. doi:10.1136/annrheumdis-2013-204905
Schnitzer, T. J., and Marks, J. A. (2015). A Systematic Review of the Efficacy and General Safety of Antibodies to NGF in the Treatment of OA of the Hip or Knee. Osteoarthritis and Cartilage 23 (Suppl. 1), S8–S17. doi:10.1016/j.joca.2014.10.003
Spierings, E. L. H., Fidelholtz, J., Wolfram, G., Smith, M. D., Brown, M. T., and West, C. R. (2013). A Phase III Placebo- and Oxycodone-Controlled Study of Tanezumab in Adults with Osteoarthritis Pain of the Hip or Knee. Pain 154 (9), 1603–1612. doi:10.1016/j.pain.2013.04.035
Tive, L., Bello, A. E., Radin, D., Schnitzer, T. J., Nguyen, H., Brown, M. T., et al. (2019). Pooled Analysis of Tanezumab Efficacy and Safety with Subgroup Analyses of Phase III Clinical Trials in Patients with Osteoarthritis Pain of the Knee or Hip. J. Pain. Res. Vol. 12, 975–995. doi:10.2147/jpr.s191297
Walsh, D. A., McWilliams, D. F., Turley, M. J., Dixon, M. R., Fransès, R. E., Mapp, P. I., et al. (2010). Angiogenesis and Nerve Growth Factor at the Osteochondral junction in Rheumatoid Arthritis and Osteoarthritis. Rheumatology 49 (10), 1852–1861. doi:10.1093/rheumatology/keq188
Watt, F. E., and Gulati, M. (2017). New Drug Treatments for Osteoarthritis: What Is on the Horizon? Eur. Med. J. Rheumatol. 2 (1), 50–58.
Yu, Y., Lu, S. T., Sun, J. P., and Zhou, W. (2020). Safety of Low-Dose Tanezumab in the Treatment of Hip or Knee Osteoarthritis: A Systemic Review and Meta-Analysis of Randomized Phase III Clinical Trials. Pain Med. 18, 585. doi:10.1093/pm/pnaa260
Keywords: osteoarthritis, tanezumab, WOMAC, clinical outcomes, systematic review
Citation: Hu R, Song Y-F, Yang Z-Y, Zhang C and Tan B (2021) Clinical Outcomes of Tanezumab With Different Dosages for Patient With Osteoarthritis: Network Meta-Analysis. Front. Pharmacol. 12:614753. doi: 10.3389/fphar.2021.614753
Received: 07 October 2020; Accepted: 01 June 2021;
Published: 11 June 2021.
Edited by:
Annalisa Bruno, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Giuseppe Tridente, University of Verona, ItalyCopyright © 2021 Hu, Song, Yang, Zhang and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Tan, dGFuYm9vOEAxNjMuY29t
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.