- 1Department of Cardiology, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, China
- 2Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing Institute of Traditional Chinese Medicine, Beijing, China
- 3School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China
Background: Shensong Yangxin capsule (SSYX) is a well-known traditional Chinese patent medicine for treating arrhythmia. Recently, a flurry of randomized controlled trials (RCTs) of SSYX combined with amiodarone (SSYX-amiodarone) was reported in the treatment of heart failure (HF) complicated by ventricular arrhythmia (VA) in China. However, these RCTs have not been systematically evaluated. Therefore, this study aimed to evaluate the efficacy and safety of SSYX-amiodarone in the treatment of heart failure complicated by ventricular arrhythmia (HF-VA).
Methods: Seven electronic literature databases (the Cochrane Library, PubMed, EMBASE, China Biomedical database web, China National Knowledge Infrastructure Databases, Chinese Scientific Journal database and Wanfang database) were searched from their inceptions to June 1, 2020 to identify RCTs of SSYX-amiodarone in the treatment of HF-VA. The primary outcomes included the total effective rate and adverse events (ADRs). The secondary outcomes included the frequency of ventricular premature complexes, left ventricular ejection fraction, N terminal pro Btype natriuretic peptide (NT-proBNP), and QT dispersion (QTd). The quality of the included RCTs was assessed using the Cochrane risk-of-bias tool. All data was analyzed using RevMan 5.3 software. The registration number of this protocol is PROSPERO CRD42020196689.
Results: There are Eighteen trials involving 1,697 patients were included in this study. Meta-analysis showed that SSYX-amiodarone group was superior to the amiodarone group in improving the total effective rate [RR = 1.21; 95%CI (1.16, 1.27); p < 0.01], meanwhile reducing the ADRs [RR = 0.65; 95%CI (0.45, 0.95); p = 0.03], VPCs [MD = 170.96; 95%CI (159.88, 182.04); p < 0.01] and QTd [MD = 8.39; 95%CI (6.91, 9.87); p < 0.01]. No significant difference of enhancing LVEF [MD = 4.32; 95%CI (−0.56, 9.20); p = 0.08] and reducing NT-proBNP [SMD = 0.17; 95%CI (−0.81, 1.14); p = 0.73] was observed between SSYX-amiodarone and amiodarone group.
Conclusions: Despite the apparent positive findings reported, the evidence provided by this meta-analysis was still insufficient to support the routine use of SSYX-amiodarone for HF-VA due to the poor methodological quality of included studies. The overall effect should to be verified in further through more well-design clinical studies with reasonable sample and good methodological quality.
Introduction
Heart failure (HF) is the end stage of various heart diseases, affecting the total 1–2% population in United States (Mozaffarian et al., 2016). Currently, the HF incidence rate is 0.9% in China (Chen et al., 2017), and 50% of HF patients have sudden cardiac death, which is closely related to ventricular arrhythmia (VA) (Gust et al., 2005). HF and VA often occur at the same time, and almost all patients with HF can record VA in the dynamic electrocardiogram monitoring (Santangeli and Marchlinski, 2015). HF and VA promote each other, HF will increase the incidence of VA, and VA can also enhance the severity of HF and increase the risk of HF-induced death (Leslie et al., 2006; Goldberger et al., 2011).
Amiodarone, which exerted anti-arrhythmic effect through prolonging action potential duration of cardiomyocytes, played an extremely important role in treating HF complicated by VA (HF-VA) (Li et al., 2019). While amiodarone had many adverse reactions (ADRs), including bradycardia, atrioventricular block, QT interval prolongation, pulmonary toxicity, hepatotoxicity, thyroid dysfunction, skin changes, and so on (Jaworski et al., 2014; Danzi and Klein, 2015), which have limited the wide usage of amiodarone in clinic. Nowadays, although Non-drug therapies such as implantable cardioverter defibrillator implantation, radiofrequency catheter ablation, and cardiac resynchronization therapy have been widely used in the treatment of HF-VA, a considerable number of HF-VA patients were still unable to get effective treatments (Sardu et al., 2017). Hence, it was much-needed to explore some other effective interventions for treating HF-VA. Some studies (Ju et al., 2019; Peng et al., 2020) indicated that, as a kind of ethnic medicine, traditional Chinese medicine has potential advantages in the treatment of HF and VA.
Shensong Yangxin capsule (SSYX) is a kind of Chinese patent medicine, widely using in the treatment of arrhythmia in China (Li, 2020). It composed of Ginseng Radix et Rhizoma, Radix Ophiopogonis, Corni Fructus, Taxilli Herba, Eupolyphaga, Paeoniae Radix Rubra, Coptidis Rhizoma, Schisandrae Sphenantherae, Os Draconis. SSYX has the functions of tonifying Qi and Yin, promoting blood circulation to remove meridian obstruction, clearing away heart fire, and calming the mind. The overall ingredient of SSYX was listed in Table 1.
In recent 10 years, a flurry of randomized controlled trials (RCTs) of SSYX combined with amiodarone (SSYX-amiodarone) in the treatment of HF-VA was reported in China. These RCTs showed that SSYX-amiodarone could not only improve the efficacy in the treatment of HF-VA compared with amiodarone alone, but also reduce the incidence of ADRs (Zhao, 2018; He, 2019). However, there was insufficient evidence to support the conclusion. Therefore, we conducted a meta-analysis to evaluate the efficacy and safety of SSYX-amiodarone in the treatment of HF-VA.
Material and Methods
This study was conducted following the registered protocol with PROSPERO (Protocol number: CRD42020196689) (Cao et al., 2020b). This meta-analysis was performed in accordance with The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (Moher et al., 2015). A completed PRISMA checklist is included as an additional file (Supplementary File 1).
Literature Search
Literature searching was performed in several databases of Cochrane Library, PubMed, EMBASE, China Biomedical database web, China National Knowledge Infrastructure Databases, Chinese Scientific Journal database and Wanfang database from their inceptions to June 1, 2020. A list of Chinese and English journals that might publish potentially eligible studies were also searched manually. The details of search terms and strategies are described in the Supplementary Files 2.
Study Selection
The criteria about literature inclusion are as follows: 1) Study design: RCTs. 2) Participants: All the enrolled participants were required to meet the current or past definitions of HF and VA. Trials without a description of the detailed diagnostic criteria but which reported patients with definite HF-VA were also included. 3) Interventions: The control group was treated with amiodarone alone. While, the experimental group was treated with SSYX and amiodarone. The basic therapies in two groups were similar, including angiote nsin II receptor antagonists, angiotensin-converting enzyme inhibitors, beta-blockers, diuretics, aldosterone receptor antagonists, nitrates, et al. SSYX was the only intervention difference between the control group and the experimental group in all studies. 4) Outcomes: The primary outcomes included the total effective rate and ADRs. The secondary outcomes included the frequency of ventricular premature complexes (VPCs), left ventricular ejection fraction (LVEF), N terminal pro Btype natriuretic peptide (NT-proBNP), and QT dispersion (QTd). The included studies reported at least one of the above outcomes.
The criteria of literature exclusion were as follows: 1) For duplicate publications, only those with the earliest publication time were selected. 2) Interventions in the control group included other traditional Chinese medicine therapies, such as acupuncture or Chinese herbs. 3) The criteria of efficiency evaluation did not meet the following definitions: Markedly effective: Based on 24 h electrocardiogram, VPCs disappeared or decreased by 70% or more; Ventricular tachycardias (VTs) disappeared or decreased by 80% or more; The improvement of New York Heart Association functional class (NYHA) was more than two grades or reached grade I, and there was an obvious improvement in the clinical symptom. Effective: VPCs decreased by 50–70%; VTs decreased by 50–80%; The improvement of NYHA was more than one grade or reached grade I, and the clinical symptom partially improved partly. Ineffective: It did not reach the above standards of efficiency, and even exacerbation. The total effective rate = markedly effective rate + effective rate.
Data Extraction and Risk of Bias Assessment
Data extraction and quality assessment were independently performed by two researchers and disagreements were resolved by consensus. We also tried to contact the original authors for missing information about the studies by emails, telephone, or fax whenever possible. The data consisted of the following items: 1) Basic information of the eligibility: The first author, nationality, publication year, and study design. 2) Basic characteristics of patients: Sample size, sex composition, average age, course of treatment, and the causes of HF of participants. 3) Details of interventions; 4) Details of outcomes. 5) Information of quality assessment of RCTs.
The methodological quality of included RCTs was assessed using the Cochrane risk-of-bias tool. The missing data was obtained from contacting the corresponding author via telephone, email, or fax. The quality assessment items of Cochrane tools included the following: 1) Selection bias: Random sequence generation and allocation concealment; 2) Performance bias: Blinding of the participants and personnel; 3) Detection bias: Blinding of the outcome assessment; 4) Attrition bias: Incomplete outcome data; 5) Reporting bias: Selective reporting; 6) Other bias. Each aspect was categorized into three levels: high risk, unclear risk, and low risk. Any disagreements were resolved by a third researcher.
Data Analysis
Statistical analysis was performed using Cochrane Review Manager 5.3 (Copenhagen, The Nordic Cochrane Center, The Cochrane Collaboration, 2014). For dichotomous outcomes, the combined results were calculated as risk ratios (RRs) with 95% confidence intervals (95% CIs). For continuous outcomes, the Change Score was used to conduct the meta-analysis, and the Change Score was estimated according to the standard deviation (SD) of the pre-intervention data and post-intervention data by using the following formula provided by the Cochrane Handbook (16.1.3.2 Imputing standard deviations for changes from baseline):
The Change Scores were expressed as mean differences (MD) or standard mean differences (SMD) with 95% CIs. In this meta-analysis, the Change Scores of VPCs, LVEF, NT-proBNP, and QTd were presented as MD or SMD, while the total effective rate and ADRs were presented as RR. Heterogeneity across studies were assessed using the Cochrane I2 statistic. Data with low heterogeneity (I2 < 50%) was assessed as a fixed-effects model whereas others were assessed as a random-effects model. When data with high heterogeneity (I2>50%), sensitivity analysis was used to find the source of heterogeneity. The potential publication bias of outcomes was assessed by using Begg’s test. If the p-value of Begg’s test was lower than 0.05, there was publication bias among the studies.
Results
Study Selection
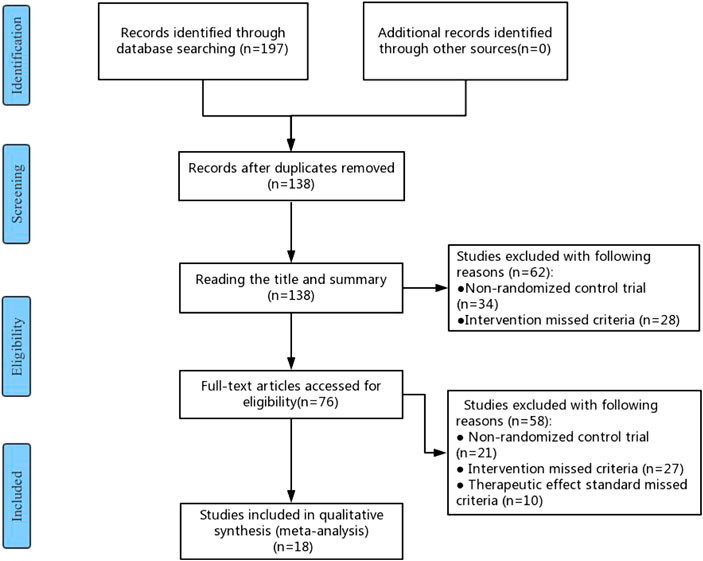
A total of 197 studies were identified in the initial literature search. After removing duplicates, 138 studies were retained for further examination. After screening the titles and abstracts, 62 studies were excluded because they were not RCTs (n = 34) or their interventions missed the criteria (n = 28). Further, 76 studies were eligible and examined, of which 58 were excluded due to the following reasons: 1) not RCTs (n = 21); 2) The interventions of RCTs did not meet the eligibility criteria (n = 27); 3) The effect standard did not meet the eligibility criteria (n = 10). Finally, 18 RCTs were eligible for this meta-analysis, which were carried out in China between 2010 and 2020 (He, 2010; Chen et al., 2011; Yang et al., 2012; Zhang et al., 2012; Song et al., 2014; Wan, 2014; Fang, 2016; Xue, 2016; Fang, 2017; Xu et al., 2017; Zeng et al., 2017; Hou, 2018; Wang, 2018; Zhao, 2018; He, 2019; Jing and Liu, 2019; Zhang, 2019; Li and Tan, 2020). The flow chart of the search strategy is shown in Figure 1.
Study Characteristics
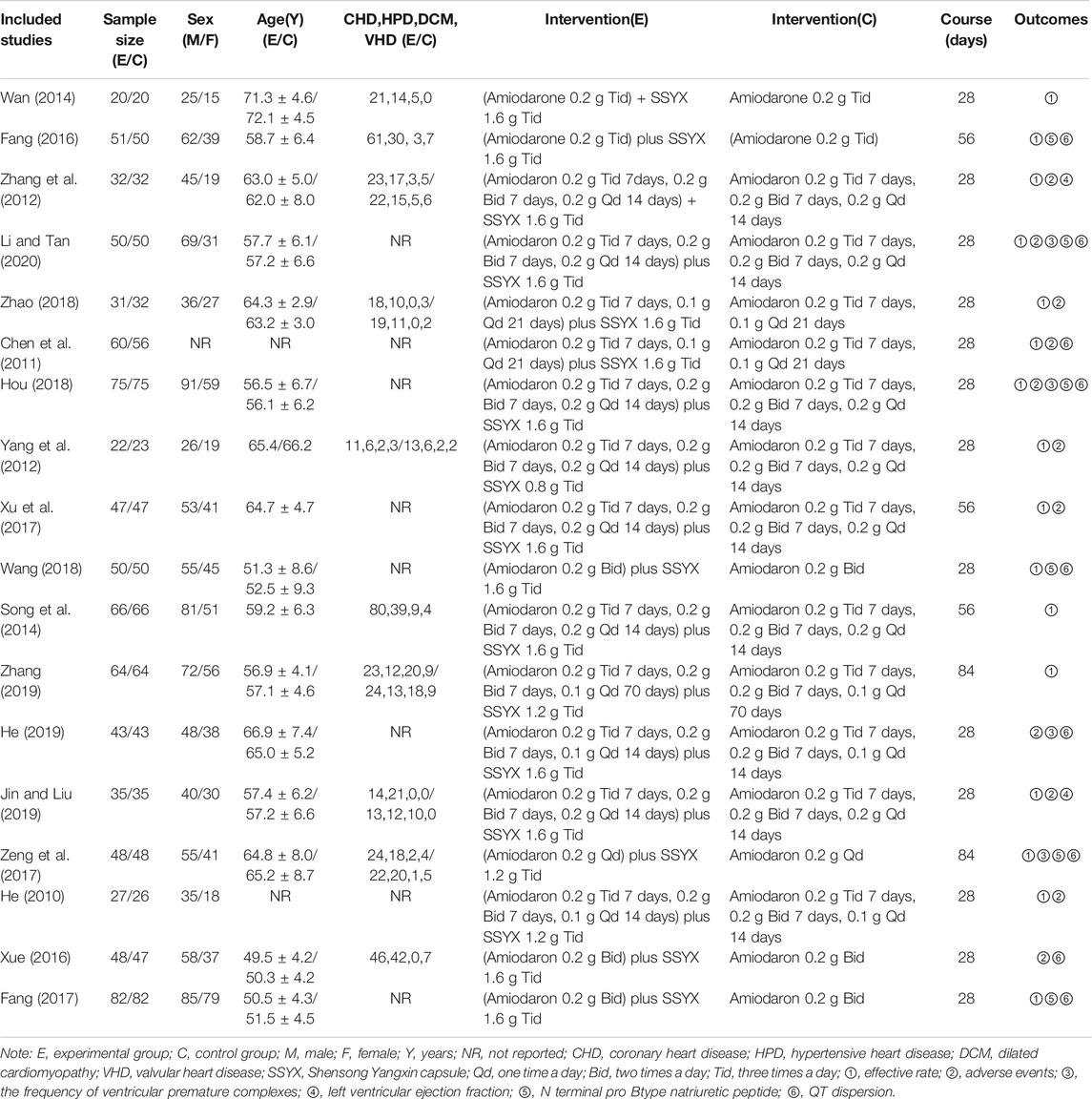
Eighteen RCTs with 1697 HF-VA patients accorded with the eligibility criteria, including 851 patients in the experimental groups and 846 patients in the control groups. 59.5% of the participants were male, and the majority was middle-aged and elderly. The causes of HF included coronary heart disease, hypertensive heart disease, dilated cardiomyopathy and valvular heart disease, of which coronary heart disease was the most common cause, accounting for 50.64%, followed by hypertensive heart disease (33.37%). There were no significant differences on general information between two groups. The patients in 55.6% of RCTs treated with amiodarone 0.2 g/time 3 times/day for 7–14 days, 0.2 g/time 2 times/day for next 7–14 days, and 0.1–0.2 g/time 1 time/day for final 7 days. The patients in 77.8% of RCTs treated with SSYX 1.6 g/time three times a day for the whole treatment course. The treatment course was 28 days in 77.8% of RCTs. The details of the study characteristics are depicted in Table 2. The detailed information of Chinese patent medicines of all the included studies are described in Supplementary File 3.
Quality Evaluation
The Cochrane risk-of-bias assessment tool was used to evaluate the quality of each study. Further information of these studies was tried to obtain through contacting the correspondence authors via mail, telephone, or fax, however, none of them replied to our questions. 1) Selection bias (random sequence generation and allocation concealment): In one RCTs (Zeng et al., 2017), the patients were grouped according to the time of admission. Therefore, the risk of selection bias was considered “high”. In four RCTs (Xu et al., 2017; Hou, 2018; Wang, 2018; He, 2019), randomization was generated via random number table, therefore, the risk of selection bias was considered low. The remaining RCTs referred to only random grouping, and the risk of selection bias was considered “unclear”. Moreover, the information on allocation concealment was not observed in all included RCTs, so risk of selection bias was considered “unclear”. 2) Performance bias: All studies did not provide information on blinding, so the performance bias was evaluated as “unclear risk.” 3) Detection bias: The risk of detection bias was considered “low”, because the measurements of related results of the included RCTs were not affected by the blinding toward the outcome assessors. 4) Attrition bias: None of the included RCTs had incomplete data, so the risk of attrition bias was considered “low”. 5) Reporting bias: Considering that the complete implementation scheme could not be acquired, the risk of reporting bias was considered “unclear”. 6) Other bias: The risk of this bias was considered “low”, because no other obvious bias was observed in all studies. The quality assessment of the included RCTs is shown in Figure 2.
Outcomes
The Total Effective Rate
Sixteen studies involving 1,516 participants adopted the effective rate to assess the clinical improvement (He, 2010; Chen et al., 2011; Yang et al., 2012; Zhang et al., 2012; Song et al., 2014; Wan, 2014; Fang, 2016; Fang, 2017; Xu et al., 2017; Zeng et al., 2017; Hou, 2018; Wang, 2018; Zhao, 2018; Jing and Liu, 2019; Zhang, 2019; Li and Tan, 2020). No statistically significant heterogeneity was found in analyses (I2 = 0%), and a fixed-effect model was used for statistical analysis. The meta-analysis results showed that SSYX-amiodarone could improve the total effective rate in patients with HF-VA compared with amiodarone alone (RR = 1.21; 95% CI (1.16, 1.27); p < 0.01), (Figure 3).
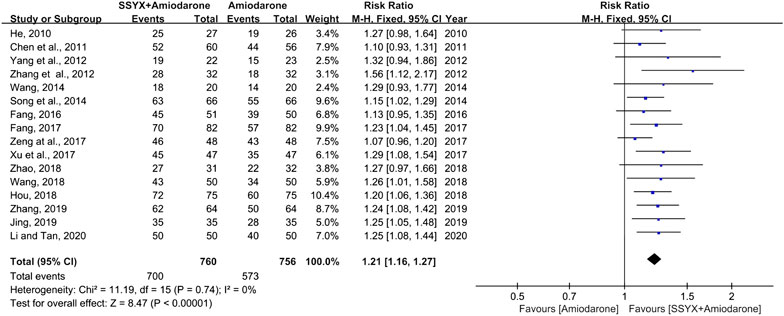
FIGURE 3. Forest plot of comparison: SSYX-amiodarone vs. amiodarone alone: The total effective rate.
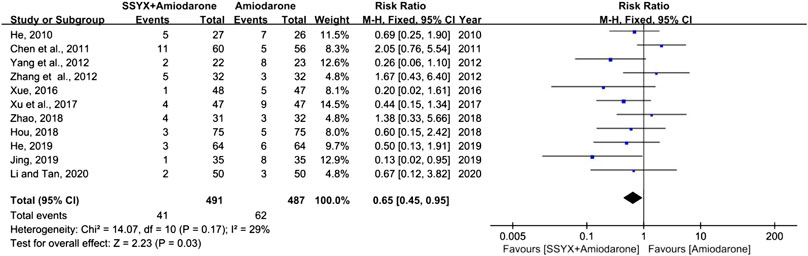
ADRs
Eleven studies involving 978 patients reported the ADRs (Chen et al., 2011; Hou, 2018; He, 2019; He, 2010; Jing and Liu, 2019; Li and Tan, 2020; Xu et al., 2017; Xue, 2016; Yang et al., 2012; Zhang et al., 2012; Zhao, 2018). Chest distress, nausea, dizziness, and sinus bradycardia were the common ADRs, while QT interval prolongation and liver damage were the rare ADRs. Low heterogeneity was found in analyses (I2 = 29%), thus a fixed-effect model was used for statistical analysis. The results showed that SSYX-amiodarone was safer than amiodarone alone in the treatment of HF-VA (RR = 0.65; 95% CI (0.45, 0.95); p = 0.03), (Figure 4).
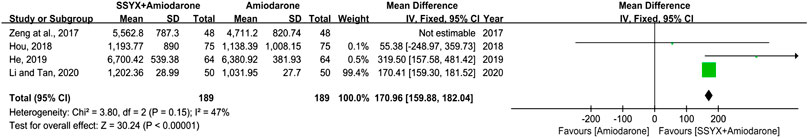
VPCs
Four studies involving 474 participants reported the VPCs (Li and Tan, 2020; Hou, 2018; He, 2019; Zeng et al., 2017). There was significant heterogeneity in the four studies (I2 = 86%), and a sensitivity analysis was conducted by excluding the studies one by one. The heterogeneity between the studies was significantly reduced after removing the study reported by Zeng et al. (I2 = 47%) (Zeng et al., 2017). As shown in Table 2, the amiodarone dose in the study “Zeng et al., 2017” was different from the other three studies, which might contribute to the heterogeneity. A fix-effect model was used for meta-analysis after removing the study “Zeng et al., 2017”. The results showed that SSYX-amiodarone was more effective in reducing the VPCs compared with amiodarone alone (MD = 170.96; 95% CI (159.88, 182.04); p < 0.01), (Figure 5).
LVEF
Two studies involving 134 participants reported the LVEF (Zhang et al., 2012; Jing and Liu, 2019). Due to the high heterogeneity (I2 = 94%), the random-effect model was used for statistical analysis. The meta-analysis showed no significant difference between SSYX-amiodarone and amiodarone groups in enhancing LVEF (MD = 4.32; 95% CI (−0.56, 9.20); p = 0.08), (Figure 6).
NT-proBNP
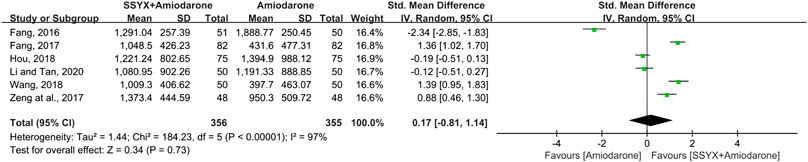
Six studies involving 711 patients reported the NT-proBNP (Fang, 2016; Fang, 2017; Zeng et al., 2017; Hou, 2018; Wang, 2018; Li and Tan, 2020). Significant heterogeneity was founded among the four studies (I2 = 97%) and the sensitivity analysis was performed by excluding the studies one by one. However, the heterogeneity could not be eliminated, thus a random-model was used for statistical analysis. The cause of high heterogeneity might be related tothe difference of usage and dosage of amiodarone among the six studies (Table 2). The results showed no significant difference between SSYX-amiodarone and amiodarone group in reducing the NT-proBNP (SMD = 0.17; 95% CI (-0.81, 1.14); p = 0.73), (Figure 7).
QTd
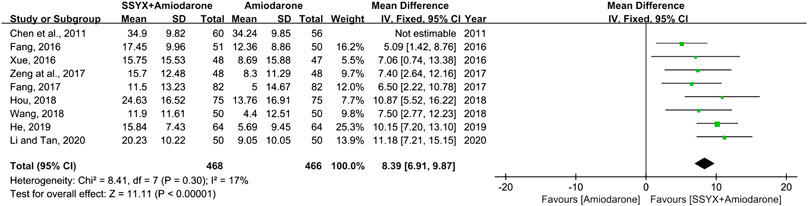
Nine studies involving 934 participants reported the QTd (Fang, 2016; Li and Tan, 2020; Chen et al., 2011; Hou, 2018; Wang, 2018; He, 2019; Zeng et al., 2017; Xue, 2016; Fang, 2017). There was significant heterogeneity among the nine studies (I2 = 66%), and the sensitivity analysis was carried out by excluding the studies one by one. The heterogeneity between the studies was significantly reduced after removing the study reported Chen et al. (I2 = 17%) (Chen et al., 2011). As shown in Table 2, the study “Chen et al., 2011” have not reported the gender and age of participants, while all the other studies have mentioned, and indicated there was not significant difference in above information between groups. Therefore, the cause of the heterogeneity might be related to the difference of gender and age of participants in the study “Chen et al., 2011”. A fix-effect model was used for meta-analysis after removing the study “Chen et al., 2011”. The results showed that SSYX-amiodarone was more effective in reducing the QTd compared with amiodarone alone (MD = 8.39; 95% CI (6.91, 9.87); p < 0.01), (Figure 8).
Publication Bias of Outcomes
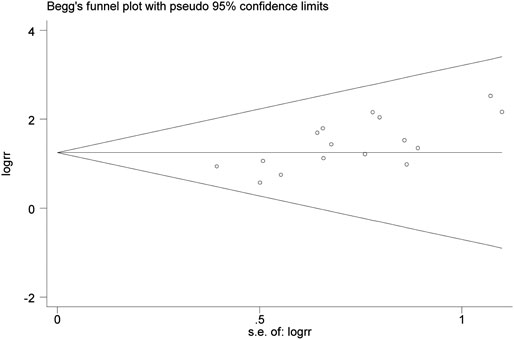
The publication bias was evaluated by Begg’s test. The p-value of Begg’s test of the total effective rate was 0.017 (p < 0.05), indicating publication bias among the studies. Besides, as shown in Figure 9, the Begg’s funnel plot of the total effective rate was not visually symmetrical, also indicating the existence of publication bias. The reason of the publication bias among studies might be that the sample size of the included RCTs was too small, and the lack of negative results might also be contributed to the bias.
Discussion
Summary of Evidences
This meta-analysis included 18 studies involving 1,697 participants. The results showed that SSYX-amiodarone had a better performance in improving the total effective rate, and reducing the ADRs, VPCs and QTd compared with amiodarone alone. Nevertheless, no significant differences of enhancing LVEF and reducing NT-proBNP were observed between SSYX-amiodarone and amiodarone groups. The main findings suggested that SSYX-amiodarone was better in improving VA compared with amiodarone alone, while there was no superiority in the improvement of HF.
The safety of traditional Chinese medicine has always been concerned. A total of 11 studies in this meta-analysis reported safety, and no serious ADRs were observed. The common side effects were chest tightness, nausea, dizziness and sinus bradycardia. QT interval prolongation and liver damage were occasionally reported. These discomforts could be effectively relieved after symptomatic treatment. The results of meta-analysis also showed that the safety of SSYX-amiodarone was better than those amiodarone alone. However, the end-point events including all-cause death and cardiovascular events in both SSYX-amiodarone and amiodarone alone groups were not observed and reported in all the included studies, which made it impossible to evaluate the long-term efficacy and safety of SSYX-amiodarone in the treatment of HF-VA.
Patient's conditions with HF-VA were often serious, while western medicine including Drug therapies and Non-drug therapies had limited efficacy. Amiodarone was a western medicine recommended by several guidelines for the treatment of HF-VA (Pedersen et al., 2014; Cao et al., 2020a). The innovative combination between amiodarone and traditional Chinese medicine in the treatment of HF-VA was a positive attempt, and some studies have shown that this combination had some advantages. For example, some studies (Chai et al., 2009a; Yang et al., 2013) showed that SSYX-amiodarone could normalize heart rate variability and heart rate turbulence, and reduced the incidence of ventricular tachycardia, suggesting that SSYX might provide electrophysiological benefits to patients with HF. SSYX was a new anti-arrhythmia drug developed under the guidance of the theory of venation, with “improving qi and nourishing Yin, activating blood and collaterals, clearing the heart and calming the spirit” as the treatment methods and “temperature, clarity, communication and tonic” as the prescription principles (Ma et al., 2016). Basic studies (Li et al., 2007a; Shi et al., 2009) have confirmed that SSYX could affect cardiac action potentials by blocking many ion channels, such as L-type calcium channel current, Na channel current, inward rectified potassium current and delayed rectified current, thus playing a broad-spectrum anti-arrhythmia effect. SSYX could inhibit Na channel current and L-type calcium channel current at different membrane potential levels, showing the effect of class I and IV antiarrhythmic drugs (Li et al., 2007b); At the same time, SSYX could reduce calcium overload in myocardial cells and played a protective role in myocardium (Gao and Li, 2014); SSYX could slow down the reactivation of the inactivated Kv1.4ΔN channel by suppressing the peak current (Wang et al., 2009); SSYX had a significant inhibitory effect on hHCN4 current, thus playing a role in the treatment of ventricular premature beats (Sun et al., 2010). At the same time, SSYX also had a non-ion channel regulation effect. For example, SSYX could improve the myocardial conduction function by promoting the pulse conduction in the sinoatrial node, atrium and atrioventricular node (Jin et al., 2009); Meanwhile, SSYX could improve the electrophysiological matrix of the ventricle by inhibiting ventricular remodeling, and reduce the dispersion of action potential duration in different parts of the ventricle, which was beneficial to eliminate reentrant and reduce the occurrence of arrhythmia (Chai et al., 2009b; Feng et al., 2009; Shen et al., 2014); Additionally, researchers also found the regulatory effect of SSYX on the cardiac autonomic nerve function by inhibiting the neural remodeling after myocardial infarction, thus playing an anti-arrhythmia role (Jiang et al., 2014).
Recently, a meta-analysis similar to our study was evaluated the efficacy and safety of SSYX-amiodarone in the treatment of HF with arrhythmia (Tian, et al., 2020). Tian's research included patients with HF complicated by atrial premature beat, atrial fibrillation, sinus arrhythmia and ventricular premature beat, while our research focused on patients with HF-VA. The deadline of Tian's research search was October 2018, while the deadline of our research was June 2020. Four studies included in our meta-analysis were published from October 2018 to June 2020. In addition, there were some other differences in methodology and selection of outcome indicators between our study and Tian's study. In conclusion, our study was different from the previous published meta-analysis in the topic and content of research. Through our study, the efficacy and safety of SSYX-amiodarone in the treatment of HF-VA could be comprehensively evaluated.
Limitations
Several potential limitations should be mentioned in this meta-analysis. First, the quality of the included RCTs was poor. Despite all patients reported in the trials were randomly assigned into different groups, only four RCTs described the methods of generating random sequences, such as a random number table. No studies mentioned blinding for researchers or participants. These contributed to an exaggerated curative effect and decreased reliability of the evidence. Second, the risk of publication bias was so high that it might affect the strength of the results. Most of the trials were small sample studies with positive findings. All included trials were published in Chinese. Finally, the International Committee of Medical Journal Editors published a statement in September 2004 requiring that all clinical trials must be registered to be considered for publication (DeAngelis et al., 2006). However, all included studies of this meta-analysis have not been registered.
Implication
The significance of this meta-analysis might be more to identify current problems and areas worthy of improvement. Due to the poor methodological quality of the included studies, the evidence provided by this meta-analysis was still insufficient to support the routine use of SSYX-amiodarone for HF-VA. The validity of the conclusion of meta-analysis was highly dependent on the quality of the RCTs included, thus the importance of the quality assessment of individual RCT must be emphasized again. There were some recommendations for further studies on SSYX-amiodarone in the treatment of HF-VA: 1) Clinical trials should be prospectively registered in international clinical trials registry platform. 2) The quality of study designs including randomization, allocation concealment, and blinding should be improved. 3) Consistency in outcome measures should be paid more attention. 4) The end-point events of SSYX-amiodarone for the treatment of HF-VA should be observed.
Conclusion
The findings of this meta-analysis suggested SSYX-amiodarone seemed to be more effective and safer than amiodarone alone in the treatment of HF-VA. However, because of the high risk of bias and low quality of the included trials, we might be unable to draw any conclusions about its routine use. The overall effect still needs to be verified through more well-design clinical studies with reasonable sample and good methodological quality in further.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
Conception and design: XFC, HXL; Collection and assembly of data: XFC, XL, SHJ, XFC; Data analysis and interpretation: XFC, MXZ; Manuscript writing: All authors; Final approval of manuscript: All authors.
Funding
This work was funded by key medical professional development program fund of Beijing Municipal Hospital Administration (ZYLX201817), and fund for evidence-based capacity building project of Chinese medicine administration of the people's Republic of China (2019XZZX-XXG001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.613922/full#supplementary-material.
References
Cao, K. J., Chen, K. P., Chen, M. L., Hong, K., Hua, W., Huang, C. X., et al. (2020a). Chinese expert consensus on ventricular arrhythmia (2016 consensus upgrade version). Chin. J. Card. Pac. Electrophys. 34, 189–253. doi:10.13333/j.cnki.cjcpe.2020.03.001
Cao, X. F., Zhou, M. X., Liu, H. X., Chen, X. F., Li, X., and Jia, S. H. (2020b). Clinical efficacy and safety of Shensong Yangxin capsule-amiodarone combination on heart failure complicated by ventricular arrhythmia: a meta-analysis of randomized controlled trials. Prospero 2020 CRD42020196689 1-5. Available at: https://www.crd.york.ac.uk/PROSPERO/#recordDetails (Accessed August 2, 2020).
Chai, S., Wang, S., Yao, L., Wu, A., Liu, Y., and Rao, C. (2009a). Effect of shensongyangxin capsule on myocardial remodeling and ventricular fibrillation threshold value in rat with coronary artery ligation. J. Chin. Mat. Med. 34, 2101–2104. doi:10.3321/j.issn:1001-5302
Chai, S. B., Wang, S. R., Yao, L. F., and Wu, A. M. (2009b). Effect of Shensong Yangxin Capsule on ventricular remodeling and action potential of isolated heart after myocardial infarction in rats. Beijing. Trad. Chin. Med. 28, 967–971.
Chen, W. W., Gao, R. L., Liu, L. S., Zhu, M. L., Wang, W., Wang, Y. J., et al. (2017). Summary of China cardiovascular disease report 2017. Chin. Circul. J. 33, 1–8.
Chen, Y., Xiang, L., Hu, Y. H., and Wu, W. N. (2011). The efficacy and safety of Shensongyangxin capsule combined with amiodarone in the treatment of congestive heart failure with ventricular arrhythmia. J. Int. Chin. West. Med. 9, 1047–1048.
Danzi, S., and Klein, I. (2015). Amiodarone-induced thyroid dysfunction. J Intensive Care Med 30, 179–185. doi:10.1177/0885066613503278
DeAngelis, C., Drazen, J. M., Frizelle, F. A., Haug, C., Hoey, J., Horton, R., et al. (2006). Clinical trial registration: a statement from the International committee of medical journal editors. Med. J. Aust. 172, 1700–1702. doi:10.1001/jama.292.11.1363
Fang, P. (2017). Effect evaluation of Shensong Yangxin capsule combined with amiodarone in the treatment of heart failure complicated with ventricular arrhythmia. J. Clin. Med. Res. 2, 104–105. doi:10.19347/j.cnki.2096-1413.201701056
Fang, Y. (2016). Protective effect of Shensong Yangxin capsule on target organ damage in patients with heart failure and ventricular arrhythmia. Nei. Mongol. Trad. Chin. Med. 35, 6–7. doi:10.16040/j.cnki.cn15-1101.2016.07.005
Feng, L., Gong, J., Jin, Z. Y., Li, N., Sun, L. P., Wu, Y. L., et al. (2009). Electrophysiological effects of Chinese medicine Shen song Yang xin (SSYX) on Chinese miniature swine heart and isolated Guinea pig ventricular myocytes. Chin. Med. J. 122, 1539–1543.
Gao, H. Y., and Li, X. W. (2014). Inhibitory effect of Shensong Yangxin Capsule on L-type calcium current and transient outward potassium current in rat ventricular myocytes. Chin. Pharm. 23, 2128–2130. doi:10.6039/j.issn.1001-0408.2014.23.06
Goldberger, J. J., Buxton, A. E., Cain, M., Costantini, O., Exner, D. V., Knight, B. P., et al. (2011). Risk stratification for arrhythmic sudden cardiac death: identifying the roadblocks. Circulation. 123, 2423–2430. doi:10.1161/CIRCULATIONAHA.110.959734
Gust, H. B., Kerry, L. L., Daniel, B. M., Jeanne, E. P., Douglas, L. P., Robin, B., et al. (2005). Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 352, 225–237. doi:10.1056/NEJMoa043399
He, J. X. (2019). Clinical effect of Shensongyangxin capsule combined with western medicine in the treatment of heart failure complicated with ventricular arrhythmia. J. Taiwan. Pharm. 31, 192–193.
He, L. P. (2010). Shensong Yangxin combined with amiodarone in the treatment of senile heart failure complicated with ventricular arrhythmia. J. Med. Inf. 23, 4002–4003.
Hou, W. Q. (2018). Clinical study on Shensong Yangxin capsule combined with amiodarone in the treatment of chronic heart failure with ventricular arrhythmia. Chin. J. Rat. Drug.Use. 15, 44–46.
Jaworski, K., Walecka, I., Rudnicka, L., Gnatow, M., and Kosior, D. A. 2014). Cutaneous adverse reactions of amiodarone. Med. Sci. Monit. 20, 2369–2372. doi:10.12659/MSM.890881
Jiang, X. B., Huang, C. X., Huang, H., Wang, X., and Xiong, L. (2014). Effect of Shensong Yangxin capsule on nerve remodeling after myocardial infarction in rabbits. Chin. J. Cardiol. Pac. Elect. Phys. 28, 59–62. doi:10.13333/j.cnki.cjcpe.2014.01.015
Jin, Z. Y., Gong, Q., Pu, J. L., and Sun, Y. (2009). Effect of Shensong Yangxin Capsule on cardiac electrophysiology of Chinese miniature pigs. Chin. Pat. Med. 31, 471–473.
Jing, G., and Liu, Z. Z. (2019). Effect of Shensong Yangxin capsule on ventricular arrhythmia in patients with chronic heart failure. J. Mod. Med. Heal. Res. 3, 29–30+32.
Ju, L. Z., Fan, H., and Wang, Y. H. (2019). Research progress of traditional Chinese medicine on tachyarrhythmia. Chin. J. Int. Med. Cardiol/Cereb. Dis. 17, 1642–1644.
Leslie, A. S., Michael, R. B., John, B., Steven, K., David, A. K., Teresa, D. M., et al. (2006). Predictors of sudden cardiac death and appropriate shock in the comparison of medical therapy, pacing, and defibrillation in heart failure (COMPANION) trial. Circulation. 114, 2766–2772. doi:10.1161/CIRCULA-TIONAHA.106.642892
Li, F., and Tan, Y. J. (2020). Study on the therapeutic effect of Shensong Yangxin capsule combined with amiodarone on heart failure with ventricular arrhythmia. Prev. Treat. Cardiol. Dis. 10, 32–34.
Li, M. H. (2020). Research progress of Shensong Yangxin capsule in the treatment of arrhythmia. Nei. Mongol. Trad. Chin. Med. 39, 166–167. doi:10.16040/j.cnki.cn.15-1101.2020.02.097
Li, N., Ma, K. J., Wu, X. F., Sun, Q., Zhang, Y. H., and Pu, J. L. (2007a). Effects of Chinese herbs on multiple ion channels in isolated ventricular myocytes. Chin. Med. J. 120, 1068–1074.
Li, N., Huo, Y. P., Ma, K. J., Sun, Q., and Pu, J. L. (2007b). Effects of Tongluo drugs on sodium calcium channels in cardiomyocytes. Chin. Med. J. 87,995–998.
Li, X. Y., Liu, J., and Fu, L. (2019). Research and treatment progress of heart failure combined with ventricular arrhythmia. Progr. Cardiol. 40, 400–403. doi:10.16806/j.cnki.issn.1004-3934.2019.03.024
Ma, L. Y., Yin, Y. J., Liu, H., Zhang, J. F., and Jia, Z. H. (2016). Overview of pharmacological mechanism of Shensong Yangxin Capsule in treating arrhythmia. J. Trad. Chin. Med. 57, 794–797. doi:10.13288/j.11-2166/r.2016.09.020
Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., Shekele, P., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4, 1. doi:10.1186/2046-4053-4-1
Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., Cushman, M., et al. (2016). Heart disease and stroke statistics-2016 update. CircIllmion. 133, e38–360. doi:10.1161/CIR.0000000000000350
Pedersen, C. T., Kay, G. N., Kalman, J., Kalman, J., Borggrefe, M., Bella, P. D., et al. (2014). EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart. Rhythm. 11, e166–96. doi:10.1016/j.hrthm.2014.07.024
Peng, Y. X., Lu, G. L., and Zhang, B. X. (2020). Research progress in the treatment of chronic heart failure by traditional Chinese medicine. Chin. J. Emer. Med. 29, 373–376.
Santangeli, P., and Marchlinski, F. E. (2015). Ventricular ectopy as a modifiable risk factor for heart failure and death: “éjà vu all over again” may be a good thing. J. Am. Coll. Cardiol. 66, 110–112. doi:10.1016/j.jacc.2015.05.031
Sardu, C., Marfella, R., Santulli, G., and Paolisso, G. (2017). Multipolar pacing by cardia resynchr -onization therapy with a defibrillators treatment in type 2 diabetes mellitus failing heart patients: impact on responders rate, and clinical outcomes. Cardiol. Diab. 16, 75. doi:10.1186/s12933-017-0554-2
Shen, N., Li, X., Zhou, T., Bilal, M. U., Du, N., and Hu, Y. (2014). Shensong Yangxin capsule prevents diabetic myocardial fibrosis by inhibiting TGF-β1/Smad signaling. J. Ethnopharmacol. 157, 161–170. doi:10.1016/j.jep.2014.09.035
Shi, L., Yang, X. C., Liu, X. L., Zong, M., and Wu, Y. L. (2009). Effects of ShenSongYangXin on action potential and some current channels in isolated rabbit pulmonary vein cardiomyocytes. Chin. Med. J. 89, 2142–2146. doi:10.3760/cma.j.issn.0376-2491.2009.30.017
Song, S. F., Xu, T., and Shi, P. (2014). Observation on the effect of Shensong Yangxin capsule combined with amiodarone in the treatment of heart failure with ventricular arrhythmia. Shandong. Med. J. 54, 59–60. doi:10.3969/j.issn.1002-266X.2014.30.022
Sun, L. p., Li, N., Zhang, S. L., and Pu, J. L. (2010). Effect of Shensong Yangxin Capsule on hHCN4 pacing current channel. J. Card. Cereb. Dise. Int. Trad. Chin. West. Med. 8, 530–531.
Tian, Y., Cui, M., Song, Q. Q., Yang, R. L., and Guo, L. (2020). Meta analysis on the efficacy and safety of amiodarone combined with Shensong Yangxin Capsule in the treatment of heart failure complicated with arrhythmia. J. card. Cereb. Dise. Int. Trad. Chin. West. Med. 18, 1852–1857. doi:10.12102/j.issn.1672-1349.2020.12.003
Wan, F. X. (2014). Clinical observation of amiodarone combined with Shensong Yangxin capsule in the treatment of chronic heart failure complicated with arrhythmia. J. Henan. Med. Res. 23, 46–47.
Wang, J. H. (2018). Effective analysis of Shensong Yangxin capsule combined with amiodarone in the treatment of heart failure complicated with ventricular arrhythmia. J. Henan. Med. Res. 27, 1832–1833. doi:10.3969/j.issn.1004-437X.2018.10.050
Wang, Z. Q., Jiang, X. J., Chao, S. P., Ren, J. J., Cao, M. Y., and Zhang, D. (2009). Effect of Shensong Yangxin capsule on potassium current characteristics of Kv1.4. J. Heart. 21, 782–785+789. doi:10.13191/j.chj.2009.06.32.wangzhq.003
Xu, Q. H., Feng, H. Y., and Li, J. (2017). Effect observation of Shensong Yangxin capsule combined with amiodarone in the treatment of heart failure and arrhythmia. Electr. J. Int. Chin. West. Med. Cardiol. Dis. 5, 34–35. doi:10.16282/j.cnki.cn11-9336/r.2017.01.023
Xue, T. T. (2016). Curative effect observation of Shensong Yangxin capsule on chronic heart failure complicated with ventricular arrhythmia. J. Chin. Med. Guid. 14, 210–211. doi:10.15912/j.cnki.gocm.2016.05.173
Yang, X. G., Mao, Z. M., and Tian, L. X. (2012). Clinical observation of Shensong Yangxin capsule combined with amiodarone in the treatment of heart failure and ventricular arrhythmia. Chin. For. Med. Res. 10, 28–29. doi:10.14033/j.cnki.cfmr.2012.03.064
Yang, Z., Yu, X., and Yu, M. L. (2013). Effects of shensongyangxin capsule on heart rate turbulence and heart rate variability in chronic heart failure. Chin. Med. J. 126, 4389–4391. doi:10.3760/cma.j.issn.0366-6999.20130135
Zeng, W. J., Li, H. T., and Gao, Y. W. (2017). Effect of Shensongyangxin capsule on QTd and plasma NT-proBNP level in patients with heart failure complicated with ventricular arrhythmia. J. World.Trad. Chin. Med. 12, 829–832. doi:10.3969/j.issn.1673-7202.2017.04.031
Zhang, N. (2019). Shensong Yangxin capsule combined with western medicine in the treatment of heart failure with ventricular arrhythmia. J. Changzhi. Med.Col. 33, 59–61.
Zhang, W., Xu, L. M., He, H., Wang, W., Lin, Y. X., and Huang, P. L. (2012). The effect of Shensong Yangxin capsule combined with amiodarone on the quality of life of patients with chronic heart failure and ventricular premature beats. J. New. Chin. Med. 44, 10–12. doi:10.13457/j.cnki.jncm.2012.10.068
Keywords: Shensong Yangxin capsule, traditional Chinese medicine, amiodarone, heart failure, Ventricular arrhythmia, meta-analysis
Citation: Cao X, Zhou M, Liu H, Chen X, Li X and Jia S (2021) Clinical Efficacy and Safety of Shensong Yangxin Capsule-Amiodarone Combination on Heart Failure Complicated by Ventricular Arrhythmia: A Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 12:613922. doi: 10.3389/fphar.2021.613922
Received: 04 October 2020; Accepted: 20 January 2021;
Published: 22 February 2021.
Edited by:
Linlin Lu, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Jia Liu, China Academy of Chinese Medical Sciences, ChinaShaonan Liu, Guangdong Provincial Hospital of Chinese Medicine, China
Copyright © 2021 Cao, Zhou, Liu, Chen, Li and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongxu Liu, bGh4X0AyNjMubmV0
 Xinfu Cao
Xinfu Cao Mingxue Zhou
Mingxue Zhou Hongxu Liu1*
Hongxu Liu1*