- 1Department of Intensive Care Unit, The First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, China
- 2Department of Respiratory Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, The Second Clinical College of Guangzhou University of Chinese Medicine and Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
- 3Guangdong-Hong Kong-Macau Joint Lab on Chinese Medicine and Immune Disease Research, Guangzhou, China
Aims: The occurrence of vascular permeability pulmonary edema in acute lung injury (ALI) is related to the imbalance of alveolar fluid transport. Regulating the active transport of alveolar fluid by aquaporins (AQPs), epithelial sodium channels (ENaCs), and Na+-K+-ATPase can effectively reduce the edema fluid in the alveolar cavity and protect against ALI. We evaluated the therapeutic effects of total flavonoids, extracted from Nervilia fordii (TFENF), and investigated its potential mechanisms of alveolar fluid transport in a rat ALI model.
Materials and methods: A model of lipopolysaccharide (LPS, 5 mg/kg)-induced ALI was established in Sprague-Dawley (SD) rats through the arteriae dorsalis penis. SD rats were divided into six groups, including the vehicle, LPS model, TFENF (6 mg/kg, 12 mg/kg, 24 mg/kg), and dexamethasone group (DEX group, 5 mg/kg). The wet-to-dry (W/D) lung weight ratio, oxygenation index, and histopathological observation were used to evaluate the therapeutic effect of TFENF. The mRNA expression of AQPs, ENaCs, and pro-inflammatory cytokines was determined using real-time polymerase chain reaction, whereas protein expression was determined using immunohistochemistry. The Na+-K+-ATPase activity was assessed using enzyme-linked immunosorbent assay.
Results: LPS significantly stimulated the production of inflammatory mediators including tumor necrosis factor (TNF)-α and interleukin (IL)-1β, and disrupted the water transport balance in the alveolar cavity by inhibiting AQPs/ENaCs/Na+-K+-ATPase. Pretreatment with TFENF reduced the pathological damage and W/D ratio of the lungs and ameliorated the arterial blood oxygen partial pressure (PaO2) and oxygenation index. TFENF further decreased the mRNA level of TNF-α and IL-1β; increased the expression of AQP-1, AQP-5, αENaC, and βENaC; and increased Na+-K+-ATPase activity. Moreover, the regulation of AQPs, βENaC, and Na+-K+-ATPase and the inhibition of TNF-α and IL-1β by TFENF were found to be dose dependent.
Conclusion: TFENF protects against LPS-induced ALI, at least in part, through the suppression of inflammatory cytokines and regulation of the active transport capacity of AQPs/ENaCs/Na+-K+-ATPase. These findings suggest the therapeutic potential of TFENF as phytomedicine to treat inflammation and pulmonary edema in ALI.
Introduction
Acute lung injury (ALI) is a life-threatening respiratory disease characterized by increased permeability of alveolar capillaries, effusion of protein fluid in the pulmonary alveoli, and hyaline membrane formation. A clinical epidemiology investigation revealed that the incidence of ALI in patients over 15 years of age was 86.2 cases per 100,000 person-years (Rubenfeld et al., 2005). ALI has a worrisome high mortality rate and is 40% in the United States (Johnson et al., 2010) and 50% in Europe (Brun-Buisson et al., 2004). There are no specific pharmacological strategies to treat ALI (Diamond et al., 2020; Matera et al., 2020), and current therapies are mainly based on the support of lung-protective mechanical ventilation (Fan et al., 2018; Chen et al., 2019). Therefore, the discovery of drugs for the management of ALI is challenging and should be actively pursued.
Inflammatory cytokine storm and the resultant impairment in gas exchange are the major causes of death in ALI. Pulmonary edema is the most important pathological characteristic in the progression of ALI (Matthay et al., 2005). Reabsorption of alveolar fluid ensures normal pulmonary gas exchange, which relies on the coordinated control of epithelial sodium channels (ENaCs), aquaporins (AQPs), and Na+-K+-ATPase (Ma et al., 2020). Na+ transport across epithelial cells is driven by Na+-K+-ATPase, which is present on the basolateral surface of epithelial cells. Na+-K+-ATPase provides 10 times the concentration of Na+ in the pulmonary interstitium than in epithelial cells. This Na+ gradient accelerates Na+ along with the transport of water from the alveolar spaces to the pulmonary interstitium and capillaries. ENaCs on the apical surface of alveolar epithelial cells and AQPs are the main channels for Na+ and water on epithelial and endothelial cells. It has been reported that ENaCs contribute to 40–60% of Na+ transport in rat lungs and up to 90% in mouse lungs (Mutlu et al., 2005). Briefly, ENaCs, AQPs, and Na+-K+-ATPase play pivotal roles in alleviating pulmonary alveolar edema (Wang et al., 2018). Regulation of the expression of AQPs/ENaCs/Na + -K + -ATPase has great significance in water transport in the lungs.
Inflammatory cytokine storm is a characteristic of ALI and one of the main factors interfering with the reabsorption of lung fluid during ALI, although ENaC is known to be directly regulated by the renin-angiotensin-aldosterone system (Zaika et al., 2013). Excessive release of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, destroying the sodium and chloride transport on epithelial barriers is reported to be the main reason for the inhibition of reabsorption of the lung fluid (Eisenhut et al., 2020). It has been reported that TNF-α induces alveolar epithelium apoptosis by p55-related death pathway and downregulates Na+-K+-ATPase in the rat colon via prostaglandin (PG) E2 (Markossian and Kreydiyyeh, 2005; Patel et al., 2013). Moreover, interleukin (IL)-6 is known to upregulate the expression of angiotensin and aldosterone or their receptors, which may indirectly increase ENaC expression (Wassmann et al., 2004). Therefore, anti-inflammation is an important therapeutic strategy in the management of ALI.
Traditional Chinese medicine, including Chinese herbal decoction and Chinese medicine injection, has potential advantages in the prevention and treatment of ALI and has been widely used in China. Evidence from systematic evaluation shows that Chinese medicine injection combined with conventional therapy can significantly reduce the inflammatory score and improve clinical efficacy (Chen et al., 2019). N. fordii (Hance) Schltr. a Chinese herbal medicine, is widely used to treat infectious diseases in China. In traditional Chinese medicine, it is used for clearing heat toxins, inducing diuresis for edema removal, and moistening the lung to prevent coughing. It also exerts antiviral, antibacterial, and anti-inflammatory effects (Chen et al., 2013). Flavonoids are found in many fruits, vegetables, and herbs, and are known to possess antiaging, anti-inflammatory, and anti-cancer properties (Zhou et al., 2019). Flavonoids are the main components of N. fordii. To date, more than 20 flavonoids have been identified in N. fordii. (Qiu et al., 2013).
We previously showed that pretreatment with N. fordii water decoction could regulate aquaporin and reduce pulmonary edema caused by ALI (Xu et al., 2010). The protective effect of a N. fordii injection, containing total flavonoids and amino acids, against ALI through the inhibition of inflammation has been confirmed (Xu et al., 2014). However, the efficacy of the total flavonoids from N. fordii (TFENF), which are the main components of N. fordii injection, on ALI and the molecular mechanism underlying their effects are currently unknown. Thus, we assessed the therapeutic effects of TFENF using an LPS-induced ALI model. We evaluated the effects of TFENF on the release of inflammatory cytokines and expression of AQPs/ENaCs/Na+-K+-ATPase, and investigated its potential mechanisms in alveolar fluid transport.
Materials and Methods
Plant Materials and Preparation of TFENF
Wild N. fordii was collected from Guangxi Province, China, and was confirmed as the whole herbal medicine, N. fordii (Hance) Schltr, by the Chinese pharmacist Yunfeng Huang from the Guangxi Institute of Traditional Chinese Medicine and Pharmaceutical Science. N. fordii was cut into pieces, placed in a leakage cylinder, and soaked in 95% ethanol. The ethanol extract was collected and concentrated to remove the alcohol. The resultant N. fordii extract was poured into glass columns containing AB-8 porous resin, washed with pure water until the outflowing liquid was colorless, and was then washed with 70% ethanol until the eluent was colorless. The ethanol eluate was collected, concentrated to remove alcohol, and then diluted with pure water. The residual liquid was added into the polyamide column, washed until colorless, and eluted with 70% ethanol until colorless. The eluent was collected and concentrated into TFENF powder.
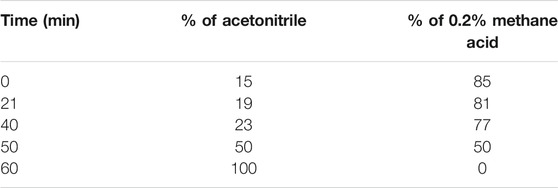
TFENF composition was determined by high-performance liquid chromatography (HPLC) using an Agilent-1260 HPLC System equipped with a PhenomenexLuna 5 μ PFP column (4.6 × 250 mm, 5 μm). A mobile phase of acetonitrile and 0.2% methane acid was used for the analysis and the elution profile for TFENF is shown in Table 1. Two mixed standard reference substances were selected and identified by Dr. Zhu Chenchen from the Institute of Clinical Pharmacology of Guangzhou University of Chinese Medicine to have a purity >98.5%. The standard reference compounds are shown in Table 2 and their chemical structural formula is depicted in Figure 1.
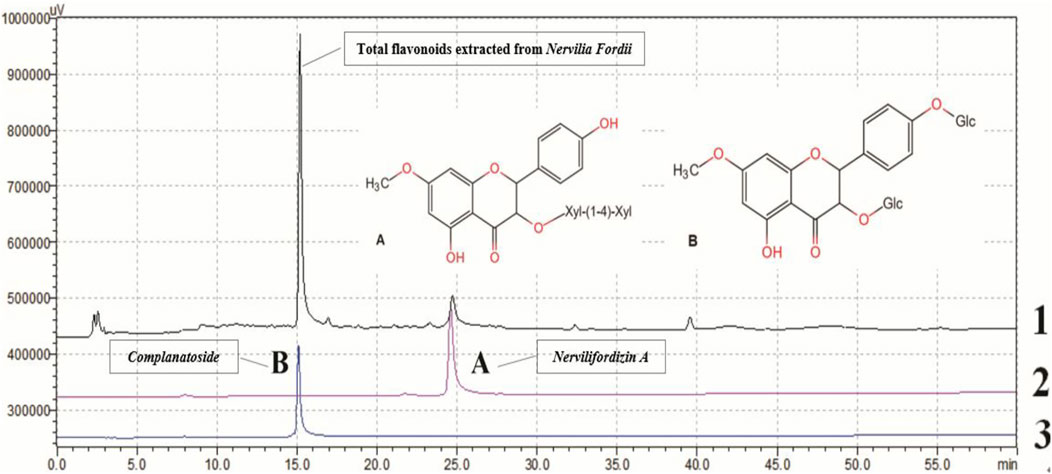
FIGURE 1. Chemical profile of TFENF analyzed using HPLC. 1. Total flavonoids extracted from Nervilia fordii; 2 (A) Nervilifordizin A; 3 (B) Complanatoside.
Animals and Experimental Design
Male, 5–7 week-old, SD rats weighing 150–200 g were purchased from the Animal Laboratory Center of the Guangzhou University of Chinese Medicine. All the animals were allowed to adapt to the environment for 5 days prior to experiments. They were fed food and water under sterile pathogen-free conditions and maintained at 23–26°C at a relative humidity of 40–60% and subjected to a standard 12 h/12 h light/dark cycle. Anesthetic drugs and other necessary measures were used to reduce animal suffering during all experimental procedures. All standardized processes were carried out in accordance with the Regulations of Experimental Animal Administration issued by the Ministry of Science and Technology of the People’s Republic of China.
Rats were divided into six groups, with six rats per group as follows: Vehicle, LPS model, Low dose (6 mg/kg) of TFENF (TFENF-L group), Middle dose (12 mg/kg) of TFENF (TFENF-M group), High dose (24 mg/kg) of TFENF (TFENF-H group), and dexamethasone (DEX group, 5 mg/kg). TFENF and dexamethasone dosages were based on our previous research (Yinji et al., 2014) and preliminary experiments. Rats in the drug-intervention groups were pretreated with TFENF or DEX through intraperitoneal injection for 7 days, whereas those in the vehicle and LPS model groups were injected with an equal amount of vehicle. One hour after the last drug pretreatment, rats in the experimental groups were injected with 5 mg/kg LPS or 0.1 ml normal saline through the vena dorsalis penis, while those in the Vehicle group were injected with an equal volume of vehicle. Rats were sacrificed 6 h after the LPS injection.
Histopathological Evaluation
The right middle lung tissue was removed and fixed with 10% formaldehyde solution for 3 days. The lung tissue-biopsy material was dehydrated using a serial alcohol gradient, embedded in paraffin, and prepared into 4 μm-thick sections. After dewaxing with xylene and hydration with ethanol, lung tissue sections were washed with phosphate-buffered saline (PBS) and the sections were stained with hematoxylin-eosin (H&E). Next, the sections were sealed with neutral balsam and histopathological changes were observed using a light microscope (Olympus, Japan). The lung injury scores of each rat were calculated in five random fields (×400) for histopathological evaluation and graded as follows: 0 = no injury, 1 = slight injury (25%), 2 = moderate injury (50%), 3 = severe injury (75%), and 4 = very severe injury (almost 100%) (Deng et al., 2012).
Blood Gas Analysis
Blood gas analysis including the oxygenation index (OI) and partial pressure of oxygen in the artery (PaO2) was obtained using a blood gas analyzer (Siemens RAP500, Germany). Samples for blood gas analysis were drawn from the aorta abdominals and immediately sent for evaluation. The OI was calculated using the following equation: OI = PaO2/21%.
Wet-To-Dry (W/D) Lung Weight Ratio
The W/D ratio was used to evaluate lung edema in ALI. The left lung tissue was weighed to obtain the wet lung weight, placed in an oven at 65°C for 72 h, and reweighed to obtain the dry lung weight. The W/D ratio was calculated using the following equation: W/D = Wet lung weight/Dry lung weight.
Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from the lung tissues using NucleoZOL reagent (MN, Duren, Germany) and a reverse transcription kit (Lot#AI20778A, TaKaRa, Japan) was used to transcribe the RNA to cDNA. Reverse-transcription PCR expression analysis was performed using an ABI 7500 PT-PCR System (Applied Biosystems, United States) and SYBR Premix Ex Taq (Lot#RR820A, TaKaRa, Japan). Primers used for RT-PCR are shown in Table 3.
Immunohistochemistry
The right middle lung sections from each group were deparaffinized with xylene, rehydrated using gradient ethanol, and incubated in 3% H2O2 for 15 min at 37°C. The sections were rinsed 3 times (5 min per rinse) in PBS, blocked with bovine serum albumin for 30 min, and incubated with primary antibodies at 4°C for 24 h. After washing 3 times (5 min per rinse) with PBS, rabbit IgG was added to the sections and incubated for 30 min, followed by staining with DBA, counterstaining with hematoxylin, dehydration with gradient ethanol, vitrification with xylene, and lastly, sealing with neutral balsam.
Na+-K+-ATPase Activity Assay
Lung tissue homogenate was prepared using normal saline according to the weight-to-volume ratio. After centrifugation, the supernatant was removed, and the lung protein concentration was determined using an enzyme-labeled meter. ATP enzyme test kits (Jiancheng Company, Nanjing, China) were used to assay Na+-K+-ATPase activity following the manufacturer’s instructions.
Statistical Analysis
Data are presented as the mean ± standard error of the mean (S.E.M.). One-way analysis of variance (ANOVA) was used to analyze differences between groups. A 5% level of probability was considered significant for all analyses. SPSS 20.0 was used to analyze data and the graphs were generated using GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA, United States). p < 0.05 was considered statistically significant.
Results
Qualitative Analysis of TFENF Using HPLC
TFENF was analyzed using HPLC with two different reference standard compounds. As shown in Figure 1, over three peaks were generated within 40 min. At 15 min, the first chromatographic peak appeared in the sample and was found to correspond to that of complanatoside. The second peak appearing in the sample at 25 min was found to correspond with that of nervilifordizin A. In brief, a comparison of individual peak retention times with those of the reference standard compounds resulted in the identification of the two compounds, nervilifordizin A and complanatoside.
TFENF Reduced the Severity of Pathological Injury of Lung Tissue
As shown in Figure 2, no histopathological changes were observed in the vehicle group (Figure 2A), the alveolar morphology was uniform, and there was no alveolar collapse or neutrophil infiltration. The bronchial wall was smooth and no hyperemia and edema were observed in the interstitial edema. However, in the LPS model group, a series of pathological changes were observed including the pulmonary interstitial thickening and alveolar collapse, and large numbers of neutrophils infiltrating the pulmonary interstitium (Figure 2A). After pretreatment with TFENF and dexamethasone, pulmonary interstitial thickening, alveolar collapse, and neutrophil invasion were significantly reduced, the lung tissue morphology was closer to that of the Vehicle group, and the edema of lung interstitium significantly decreased (Figure 2A). The lung injury score showed that the model group scored higher than the Vehicle group after pretreatment with TFENF and dexamethasone, and the lung injury score decreased significantly compared with that of the model group (Figure 2B).
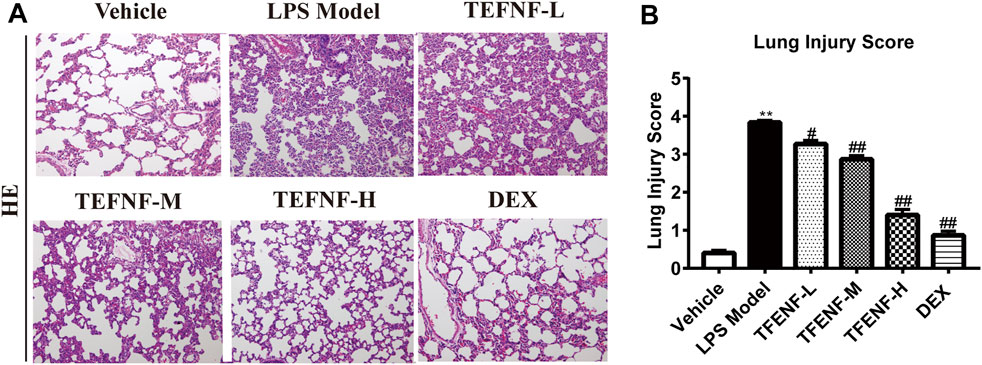
FIGURE 2. TFENF protected against pulmonary pathological damage and inflammatory cell infiltration in LPS-induced ALI. Effect of TFENF on LPS-induced changes in lung histology (A) and lung injury score (B) 6 h after LPS challenge or saline treatment (n = 6 per group). Lung sections stained using H&E (×200). Lung injury scores are presented as mean ± S.E.M. **p < 0.01 compared with the vehicle group; #p < 0.05 compared with the LPS model group; ##p < 0.01 compared with the LPS model group.
TFENF Improved PaO2 and OI in LPS-Induced ALI
As shown in Figure 2A, stimulation with LPS destroyed the alveolar integrity, caused pulmonary edema, increased pulmonary capillary permeability, and, consequently, led to a reduction in pulmonary ventilation and ventilation function. The decrease in OI is one of the main parameters when diagnosing ALI. Arterial blood gas analysis was performed to confirm the role of TFENF oxygenation improvement in LPS-induced ALI. PaO2 and OI were decreased in the LPS model group; however, the degree of PaO2 and O/I were ameliorated in the TFENF and DEX groups compared with those in the LPS model group (p < 0.01) (Figure 3).
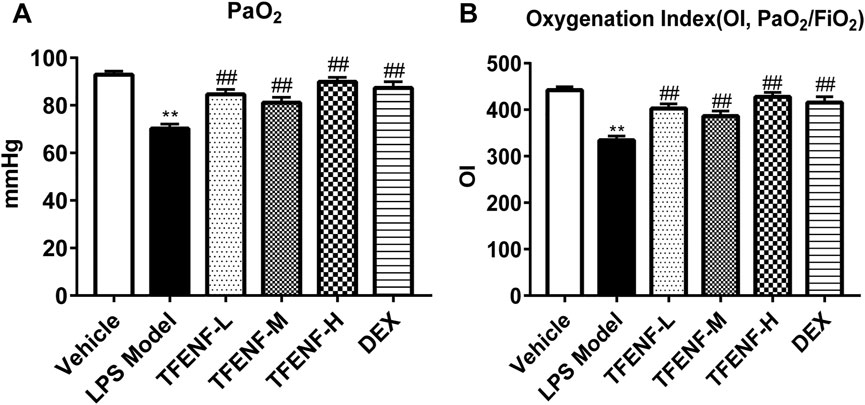
FIGURE 3. TFENF ameliorated PaO2 and IO of arterial blood gas analysis in LPS-induced ALI. Rat arterial blood gas analysis to determine PaO2 (A) and IO (B) 6 h after LPS-induced ALI or saline treatment (n = 6 per group). Data are presented as mean ± S.E.M. **p < 0.01compared with the vehicle group; #p < 0.05 compared with the LPS model group; ##p < 0.01 compared with the LPS model group.
TFENF Reduced Lung W/D Ratio in LPS-Induced ALI
The lung W/D ratio was calculated to evaluate the potential protective effect of TFENF on pulmonary edema. As shown in Figure 4, compared with that in the vehicle group, stimulation with LPS caused a significant increase in the lung W/D ratio in the LPS model group (p < 0.01). However, compared with that in the LPS model group, the lung W/D ratio declined significantly after TFENF intervention (p < 0.01), similar to that in the DEX group. Moreover, with an increased dosage of TFENF in the intervention group, the W/D ratio was determined to be closer to that of the vehicle group.
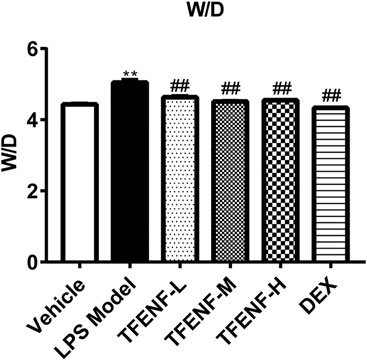
FIGURE 4. Effects of TFENF on the W/D ratio in LPS-induced ALI. Effects of TEFNF on the W/D ratio in rat lungs 6 h after LPS challenge (n = 6 per group). Data are presented as mean ± S.E.M. **p < 0.01 compared with the vehicle group; ##p < 0.01 compared with the LPS model group.
TFENF Decreased the mRNA Expression Level of TNF-α and IL-1β
To determine the anti-inflammatory effect of TFENF pretreatment on LPS-induced ALI, we evaluated the gene expression of TNF-α and IL-1β in lung tissue samples from different groups. TNF-α and IL-1β mRNA expression increased significantly in the LPS model group (Figure 5). Compared with the LPS model group, the gene expression of TNF-α and IL-1β decreased after TFENF pretreatment, especially in the TFENF-M and TFENF-H groups (p < 0.01). Similarly, a significant decline was observed in the DEX group.

FIGURE 5. TFENF suppressed the expression of pro-inflammatory cytokines in LPS-induced ALI. Measurement of mRNA levels of TNF-α (A) and IL-1β (B) in lung tissue 6 h after LPS-induced ALI or saline treatment (n = 6 per group). Data are presented as mean ± S.E.M. **p < 0.01 compared with the vehicle group; #p < 0.05 compared with the LPS model group; ##p < 0.01 compared with the LPS model group.
TFENF Increased the mRNA Expression Level of AQPs and ENaCs
AQPs and ENaCs play key roles in LPS-induced ALI lung edema pathogenesis. Compared with that in the vehicle group, LPS stimulation suppressed the mRNA expression of AQP-1, AQP-5, αENaC, βENaC, and γENaC in the LPS model group (Figure 6). The mRNA expression of AQP-1, AQP-5, and βENaC increased in the TFENF pretreatment groups (Figures 6A,B,D). With an increase in TFENF dosage, the gene expression in the three groups (TFENF-L, TFENF-M, and TFENF-H) exhibited an increasing trend. This trend was not observed in terms of αENaC gene expression (Figure 6C). In the AQP-1 group, the TFENF-M and TFENF-H groups exhibited differences compared with the LPS model group, but in the AQP-5 and ENaC groups, the differences were only observed in the TFENF-H group when compared with the LPS model group. However, TFENF pretreatment had no obvious effect on γENaC (Figure 6E). In the DEX group, the mRNA levels of AQP-1, AQP-5, αENaC, βENaC, and γENaC increased significantly compared with those of the LPS model group.
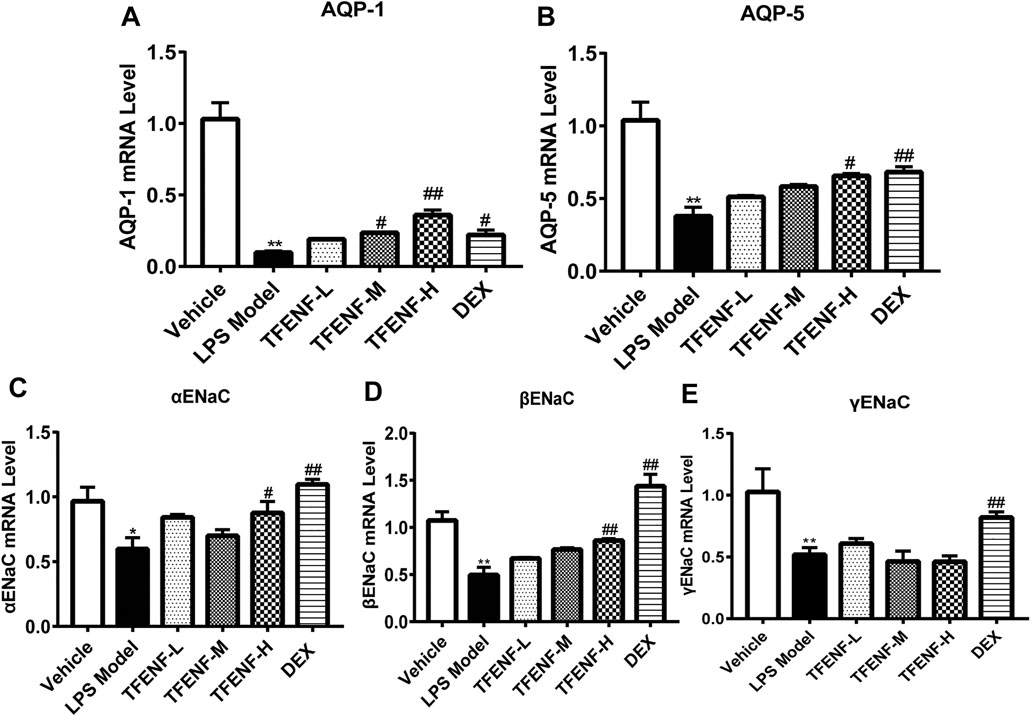
FIGURE 6. TFENF increased the mRNA expression of AQPs and ENaC in LPS-induced ALI. mRNA expression of AQP-1 (A), AQP-5 (B), αENaC (C), βENaC (D), and γENaC (E) in lung tissue 6 h after LPS-induced ALI or saline treatment (n = 6 per group). Data are presented as mean ± S.E.M. *p < 0.05 compared with the vehicle group; **p < 0.01 compared with the vehicle group; #p < 0.05 compared with the LPS model group; ##p < 0.01 compared with the LPS model group.
TFENF Promoted the Protein Expression of AQPs and ENaCs
Immunohistochemical analysis was used to determine the effect of pretreatment with TFENF on the subcellular distribution of AQP-1, AQP-5, αENaC, βENaC, and γENaC. Positively immunostained cells appeared brown. As shown in Figure 7, compared with that in the LPS model group, AQP-1, AQP-5, αENaC, and βENaC protein expression increased after treatment with different doses of TFENF, and this difference was especially prominent in the TFENF-H group (p < 0.01). However, the expression of γENaC in the TFENF-H group was significantly lower than that in the LPS model group (p < 0.05). The protein expression levels of AQP-5, αENaC, and βENaC in the DEX group were different compared with those in the LPS model group (p < 0.01), except for AQP-1 protein expression.
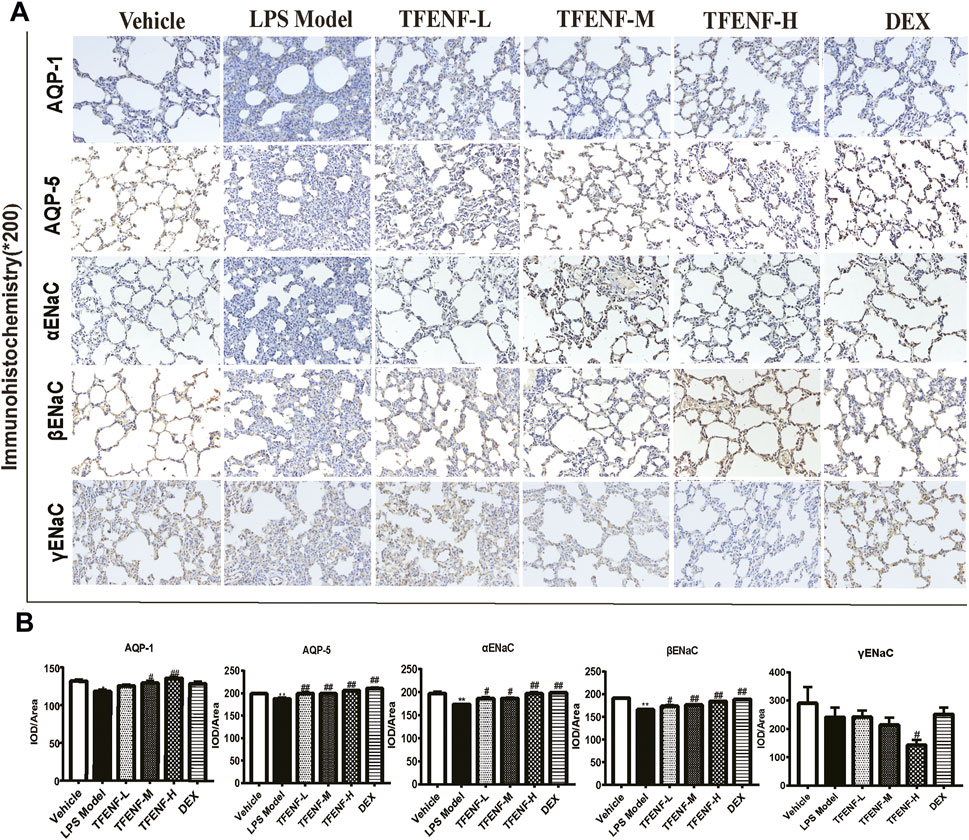
FIGURE 7. TFENF promoted the protein expression of AQPs and ENaCs in LPS-induced ALI. Effect of TFENF on the protein expression of AQP-1, AQP-5, αENaC, βENaC, and γENaC in rat lung tissue 6 h after LPS-induced ALI or saline treatment (n = 6 per group) (immunohistochemistry staining, magnification ×200). Representative photographs of immunohistochemistry assay (A) and quantitative analysis of immunohistochemistry results (B). Data are presented as mean ± S.E.M. The numbers of cells expressing AQP-1, AQP-5, αENaC, and βENaC are significantly decreased in LPS-induced ALI compared with those in the vehicle group. Meanwhile, the numbers of cells expressing AQP-1, AQP-5, αENaC, and βENaC are significantly increased after TFENF treatment. Brown immunostained cells are positive. *p < 0.05 compared with the vehicle group; **p < 0.01 compared with the vehicle group; #p < 0.05 compared with the LPS model group; ##p < 0.01 compared with the LPS model group.
TFENF Increased the Enzymatic Activity of Na+-K+-ATPase
To determine whether TFENF affected the enzymatic activity of Na+-K+-ATPase, we measured Na+-K+-ATPase activity in lung tissue. The results showed that LPS inhibited Na+-K+-ATPase activity whereas Na+-K+-ATPase activity in the TFENF-administered groups increased, and a significant difference was observed between the TFENF-L and LPS model groups. However, compared with the LPS model group, TFENF-M, TFENF-H, and DEX groups showed a significant increase in Na+-K+-ATPase activity (p < 0.01) (Figure 8).
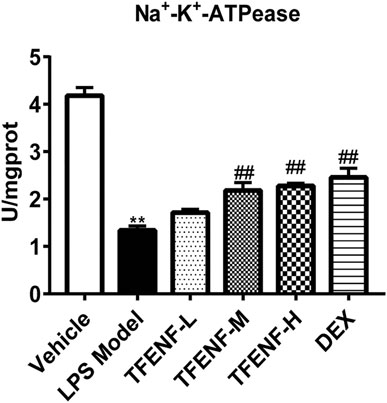
FIGURE 8. TFENF increased the enzymatic activity of Na+-K+-ATPase in LPS-induced ALI. Increase in the enzymatic activity of Na+-K+-ATPase by TFENF in rat lung tissue 6 h after LPS-induced ALI or saline treatment (n = 6 per group). Data are presented as mean ± S.E.M. **p < 0.01 compared with the vehicle group; ##p < 0.01 compared with the LPS model group.
Discussion
Various factors, within or outside the lung, likely induce ALI. Damage to the pulmonary capillary endothelial and alveolar epithelial cells can lead to increased permeability of the capillary wall, imbalance in alveolar fluid transport, influx of a large amount of protein-rich fluid into the alveoli, and acute pulmonary edema, which cannot be explained by cardiogenic pulmonary edema (Jiang et al., 2015). In ALI, pulmonary ventilation and air-exchange functions are severely impaired, and the ventilation/blood flow ratio is imbalanced as alveoli are filled with a large amount of fluid. The blood oxygen saturation cannot be improved despite the increased inhaled oxygen. Therefore, the OI decreases in ALI, and the decrease is positively related to disease severity. In addition, large numbers of inflammatory factors accumulate in the lung and trigger an inflammatory waterfall effect in ALI, leading to acute pulmonary edema, the main pathological feature of ALI.
ALI has an acute onset, complex mechanism, rapid progression, and high mortality, which is challenging in critical illness in a clinical setting. As a traditional Chinese herbal medicine, N. fordii has been widely used to treat pneumonia, sepsis, and other infectious diseases. Recently, some studies have confirmed the antibacterial (Xiong et al., 2014), antioxidant (Zhou et al., 2018), anti-inflammatory (Qiu et al., 2013; Jiao et al., 2014), antipyretic, and analgesic effects of N. fordii (Xie, 2008; Mei et al., 2011). Our previous study has reported the protective effect of N. fordii against ALI. The N. fordii water decoction promotes the expression of AQP-1 and AQP-5 in ALI and increases the clearance and transfer of lung water, improves water metabolism, and reduces pulmonary edema (Xu et al., 2010). Additionally, the total flavonoids and amino acids have been extracted as an injectable, which have been shown to exhibit protective effects against lung injury by inhibiting the overexpression of TNF-α and IL-6 (Xu et al., 2014). Furthermore, in vivo studies, N. fordii injection has demonstrated significant inhibition of the proliferation of J774 macrophages and production of TNF-α and IL-6 in the cell supernatant (Xu et al., 2014). However, there are only a few reports on the effect of N. fordii or its main components on the regulation of alveolar fluid transport.
In this study, a rat model of LPS-induced ALI was established to elucidate the possible mechanism of TFENF in the inflammatory response and alveolar fluid transport. We showed that LPS significantly stimulated the production of inflammatory mediators and disrupted the balance of water transport in the alveolar cavity. To our knowledge, this is the first study to report changes in different key links (AQPs/ENaCs/Na+-K+-ATPase) in the imbalance of the active transport of alveolar fluid in an ALI model. Moreover, pretreatment with TFENF reduced the pathological damage and W/D ratio and improved PaO2 and OI. TFENF also decreased the mRNA levels of TNF-α and IL-1β in a dose-dependent manner, increased both the mRNA and protein levels of AQP-1, AQP-5, αENaC, and βENaC (but not γENaC), and upregulated Na+-K+-ATPase activity. All of the above results demonstrated that TFENF reduced the transcription of pro-inflammatory cytokines in the lungs and alleviated lung edema induced hypoxima by increasing the expression of aquaporins and ENaCs, and Na+-K+-ATPase activity. Meanwhile, both mRNA and protein levels of AQP-1, AQP-5, αENaC, and βENaC were upregulated by TFENF, which indicated that TFENF upregulated the expression of AQPs and ENaCs at the transcriptional level. Moreover, our results indicated that TFENF selectively increased αENa and βENaC expression but not that of γENaC, demonstrating the possibility that α and β but not the γ subunit are the regulation targets of TFENF.
However, why TFENF is unable to increase γENaC expression is an interesting topic. Studies have demonstrated that the three subunits are usually synthesized in a differential fashion in cells that express endogenous ENaC, with one or two subunits expressed constitutively (Weisz et al., 2003; Peters et al., 2014). This finding suggests that ENaC subunits are regulated in a non-coordinated manner (Kim et al., 2018). On the other hand, whether TFENF is selective in the regulation of ENaC subunits needs further discussion.
LPS, the main component of the outer membrane of Gram-negative bacteria (Maldonado et al., 2016), stimulates neutrophils, macrophages, and other immune cells to produce different mediators including pro-inflammatory cytokines such as TNF-α and IL-1β, and the anti-inflammatory cytokines IL-10 and TGF-β (Fligiel et al., 2006). Excessive release of these cytokines recruits polymorphonuclear neutrophils to the injury site and leads to ALI. Therefore, it is crucial to suppress the excessive production of pro-inflammatory factors to control ALI progression (Idell, 2001; Toews, 2001).
Intravenous LPS-induced ALI is a commonly used modeling method. The pathological features of ALI are acute pulmonary edema, accompanied by a decrease in OI, and pathological damage to the lung tissue. W/D is a common indicator to evaluate the degree of pulmonary edema in an ALI model. In our study, an increase in the W/D ratio and a decrease in OI were observed in the LPS model group, in addition to significant pathological damage. Furthermore, a large increase in pro-inflammatory cytokines including TNF-α and IL-1β were observed, which indicated ALI. Our results are consistent with those of previous studies that demonstrated that pro-inflammatory cytokines play an important role in LPS-induced inflammatory responses (Yang et al., 2018; Ding et al., 2019). TFENF intervention could reduce pathological damage and W/D ratio, and improve PaO2 and OI in ALI. The main mechanism is the inhibition of the production of pro-inflammatory cytokines (TNF-α and IL-1β).
The mechanism of active transport of alveolar fluid in pulmonary edema is complex and involves multiple key links or pathways. Currently, it is clear that the regulation of AQPs, ENaC, and Na+-K+-ATPase is a typical representation and plays a vital role in active transport. Previous studies have shown that the expression of AQPs and ENaCs was decreased and the activity of Na+-K+-ATPase was suppressed in LPS-induced models of ALI (Hasan et al., 2014; He et al., 2015; Jiang et al., 2015). Our study also confirmed that an imbalance in alveolar fluid transport was related to the inhibition of AQP-1, AQP-5, αENaC, βENaC, γENaC, and Na+-K+-ATPase.
Some studies have reported the association between pro-inflammatory cytokines and active transport of alveolar fluid. TNF-α induces a decrease in AQP-1 and AQP-5 (Mezzasoma et al., 2013), whereas IL-1β inhibition can reverse the decrease of AQP-1 and AQP-5 (Yu et al., 2018). Previous evidence has also confirmed that TNF-α and IL-1β directly downregulate ENaC levels and expression in alveolar epithelial cells and their profound influence on the capacity of alveolar epithelial cells to transport sodium (Dagenais et al., 2004; Matalon et al., 2015; Wynne et al., 2017). Moreover, TNF-α is known to downregulate Na+-K+-ATPase and Na+-K+2Cl- cotransporter in the renal cortex and medulla and in the colon of rats via PGE2 (Markossian and Kreydiyyeh, 2005; keydiyyeh and Markossian, 2006), whereas IL-1β has been shown to inhibit Na+-K+-ATPase activity and protein expression in cardiac myocytes (Kreydiyyeh et al., 2004). However, there are only a few studies that focus on pulmonary edema. In brief, stimulation of inflammatory cytokines may inhibit the expression and activity of AQPs, ENaCs, and Na+-K+-ATPase in alveolar epithelial cells. In the current study, different doses of TFENF were found to promote alveolar fluid transport by regulating AQP-1, AQP-5, αENaC, βENaC, and Na+-K+-ATPase to reduce pulmonary edema. The underlying mechanism may be associated with the inhibition of the pro-inflammatory cytokines, TNF-α and IL-1β.
We determined the composition of TFENF using HPLC. Two standard reference compounds nervilifordizin A and complanatoside were used. The composition of the N. fordii is complex and the identification of total flavonoids is a useful approach to provide an objective and accurate basis for future animal- or cell-based experiments.
We found that N. fordii not only inhibited the expression of inflammatory factors but also promoted the expression of AQPs, ENaCs, and Na+-K+-ATPase in LPS-induced ALI in rats. Thus, N. fordii demonstrates the potential for multi-target effects in ALI treatment. Our findings may provide new avenues for the discovery of effective drugs to treat ALI.
Conclusion
Using in vivo experiments, we demonstrated that pretreatment with TFENF can protect against pulmonary edema caused by LPS-induced ALI in rats. The protective mechanism is associated with the regulation of AQPs/ENaCs/Na+-K+-ATPase, which may be associated with the inhibition of pro-inflammatory cytokines. These findings demonstrate that TFENF could be a potential therapeutic phytomedicine to treat inflammation and pulmonary edema in ALI.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Guangdong Provincial Academy of Chinese Medical Sciences.
Author Contributions
YC and LL conceived and designed the animal study. SY and MD drafted the manuscript, YC and LL participated in the revision of the manuscript. SY, MD, XY, LF, ZL, and LW performed experiments and analyzed data. All authors read and approved the final manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (Grant number 81503511), State Key Laboratory of Dampness Syndrome of Chinese Medicine (Grant number SZ2021ZZ42), the 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong-Macau Joint Lab, Grant number 2020B1212030006), double first-class and high-level university discipline collaborative innovation team project of Guangzhou University of Chinese Medicine (Grant number 2021XK10 and 2021XK27), and Zhongying Zhou Famous Doctors’ Workshop of Guangdong Provincial Hospital of Chinese Medicine.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Brun-Buisson, C., Minelli, C., Bertolini, G., Brazzi, L., Pimentel, J., Lewandowski, K., et al. (2004). Epidemiology and Outcome of Acute Lung Injury in European Intensive Care Units. Results from the ALIVE Study. Intensive Care Med. 30, 51–61. doi:10.1007/s00134-003-2022-6
Chen, J. M., Wei, L. B., Lu, C. L., and Zhou, G. X. (2013). A Flavonoid 8-C-Glycoside and a Triterpenoid Cinnamate from Nervilia Fordii. J. Asian Nat. Prod. Res. 15 (10), 1088–1093. doi:10.1080/10286020.2013.814107
Chen, Y. B., Liu, Q., Xie, H., Yin, S. M., Wu, L., Yu, X. H., et al. (2019). Is Chinese Medicine Injection Applicable for Treating Acute Lung Injury and Acute Respiratory Distress Syndrome? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Chin. J. Integr. Med. 26 (11), 857–866. doi:10.1007/s11655-019-3078-7
Dagenais, A., Fréchette, R., Yamagata, Y., Yamagata, T., Carmel, J. F., Clermont, M. E., et al. (2004). Downregulation of ENaC Activity and Expression by TNF-Alpha in Alveolar Epithelial Cells. Am. J. Physiol. Lung Cel Mol Physiol 286 (2), L301–L311. doi:10.1152/ajplung.00326.2002
Deng, W., Li, C. Y., Tong, J., Zhang, W., and Wang, D. X. (2012). Regulation of ENaC-Mediated Alveolar Fluid Clearance by Insulin via PI3K/Akt Pathway in LPS-Induced Acute Lung Injury. Respir. Res. 13 (1), 29. doi:10.1186/1465-9921-13-29
Diamond, M., Peniston Feliciano, H. L., Sanghavi, D., and Mahapatra, S. (2020). Acute Respiratory Distress Syndrome (ARDS), StatPearls. Treasure Island (FL): StatPearls Publishing LLC.
Ding, Y. H., Song, Y. D., Wu, Y. X., He, H. Q., Yu, T. H., Hu, Y. D., et al. (2019). Isoalantolactone Suppresses LPS-Induced Inflammation by Inhibiting TRAF6 Ubiquitination and Alleviates Acute Lung Injury. Acta Pharmacol. Sin 40 (1), 64–74. doi:10.1038/s41401-018-0061-3
Eisenhut, M., and Shin, J. I. (2020). Pathways in the Pathophysiology of Coronavirus 19 Lung Disease Accessible to Prevention and Treatment. Front. Physiol. 11, 872. doi:10.3389/fphys.2020.00872
Fan, E., Brodie, D., and Slutsky, A. S. (2018). Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 319 (7), 698–710. doi:10.1001/jama.2017.21907
Fligiel, S. E., Standiford, T., Fligiel, H. M., Tashkin, D., Strieter, R. M., Warner, R. L., et al. (2006). Matrix Metalloproteinases and Matrix Metalloproteinase Inhibitors in Acute Lung Injury. Hum. Pathol. 37 (4), 422–430. doi:10.1016/j.humpath.2005.11.023
Hasan, B., Li, F. S., Siyit, A., Tuyghun, E., Luo, J. H., Upur, H., et al. (2014). Expression of Aquaporins in the Lungs of Mice with Acute Injury Caused by LPS Treatment. Respir. Physiol. Neurobiol. 200, 40–45. doi:10.1016/j.resp.2014.05.008
He, J., Qi, D., Wang, D. X., Deng, W., Ye, Y., Feng, L. H., et al. (2015). Insulin Upregulates the Expression of Epithelial Sodium Channel In Vitro and in a Mouse Model of Acute Lung Injury: Role of mTORC2/SGK1 Pathway. Exp. Cel Res 331 (1), 164–175. doi:10.1016/j.yexcr.2014.09.024
Idell, S. (2001). Anticoagulants for Acute Respiratory Distress Syndrome: Can They Work. Am. J. Respir. Crit. Care Med. 164 (4), 517–520. doi:10.1164/ajrccm.164.4.2102095
Jiang, Y. X., Dai, Z. L., Zhang, X. P., Zhao, W., Huang, Q., and Gao, L. K. (2015). Dexmedetomidine Alleviates Pulmonary Edema by Upregulating AQP1 and AQP5 Expression in Rats with Acute Lung Injury Induced by Lipopolysaccharide. J. Huazhong Univ. Sci. Technolog Med. Sci. 35 (5), 684–688. doi:10.1007/s11596-015-1490-6
Jiao, Y., Qiu, L., Xie, J. Z., Tang, S. Y., and Zhang, R. H. (2014). Anti-inflammatory Effect of Nervilifordin F'. Chin. J. Pharmaccuticals 45 (02), 143–146.
Johnson, E. R., and Matthay, M. A. (2010). Acute Lung Injury: Epidemiology, Pathogenesis, and Treatment. J. Aerosol Med. Pulm. Drug Deliv. 23, 243–252. doi:10.1089/jamp.2009.0775
Kim, C. S., Ahmad, S., Wu, T., Walton, W. G., Redinbo, M. R., and Tarran, R. (2018). SPLUNC1 Is an Allosteric Modulator of the Epithelial Sodium Channel. FASEB J. 32 (5), 2478–2491. doi:10.1096/fj.201701126R
Kreydiyyeh, S. I., Abou-Chahine, C., and Hilal-Dandan, R. (2004). Interleukin-1 Beta Inhibits Na+-K+ ATPase Activity and Protein Expression in Cardiac Myocytes. Cytokine 26 (1), 1–8. doi:10.1016/j.cyto.2003.11.014
Kreydiyyeh, S. I., and Markossian, S. (2006). Tumor Necrosis Factor Alpha Down-Regulates the Na+-K+ ATPase and the Na+-K+2Cl- Cotransporter in the Kidney Cortex and Medulla. Cytokine 33 (3), 138–144. doi:10.1016/j.cyto.2005.12.007
Ma, C., Dong, L., Li, M., and Cai, W. (2020). Qidonghuoxue Decoction Ameliorates Pulmonary Edema in Acute Lung Injury Mice through the Upregulation of Epithelial Sodium Channel and Aquaporin-1. Evid. Based Complement. Alternat Med. 2020, 2492304. doi:10.1155/2020/2492304
Maldonado, R. F., Sá-Correia, I., and Valvano, M. A. (2016). Lipopolysaccharide Modification in Gram-Negative Bacteria during Chronic Infection. FEMS Microbiol. Rev. 40 (4), 480–493. doi:10.1093/femsre/fuw007
Markossian, S., and Kreydiyyeh, S. I. (2005). TNF-α Down-Regulates the Na+-K+ ATPase and the Na+-K+-2Cl−cotransporter in the Rat colon via PGE2. Cytokine 30 (6), 319–327. doi:10.1016/j.cyto.2004.11.009
Matalon, S., Bartoszewski, R., and Collawn, J. F. (2015). Role of Epithelial Sodium Channels in the Regulation of Lung Fluid Homeostasis. Am. J. Physiol. Lung Cel Mol Physiol 309 (11), L1229–L1238. doi:10.1152/ajplung.00319.2015
Matera, M. G., Rogliani, P., Bianco, A., and Cazzola, M. (2020). Pharmacological Management of Adult Patients with Acute Respiratory Distress Syndrome. Expert Opin. Pharmacother. 21 (17), 2169–2183. doi:10.1080/14656566.2020.1801636
Matthay, M. A., and Zimmerman, G. A. (2005). Acute Lung Injury and the Acute Respiratory Distress Syndrome: Four Decades of Inquiry into Pathogenesis and Rational Management. Am. J. Respir. Cel Mol Biol 33 (4), 319–327. doi:10.1165/rcmb.F305
Mei, Q. X., Gao, Y. Q., Zhong, X. W., Dai, W. B., and Liang, Y. (2011). Effect of 12 Chinese Herbs for Clearing Heat and Detoxifying on the Expression of EB Virus Shell Antigen and its Cytotoxic Effect. J. Chin. Med. Mater. 34 (11), 1760–1762. doi:10.13863/j.issn1001-4454.2011.11.041
Mezzasoma, L., Cagini, L., Antognelli, C., Puma, F., Pacifico, E., and Talesa, V. N. (2013). TNF-α Regulates Natriuretic Peptides and Aquaporins in Human Bronchial Epithelial Cells BEAS-2B. Mediators Inflamm. 2013, 159349. doi:10.1155/2013/159349
Mutlu, G. M., and Sznajder, J. I. (2005). Mechanisms of Pulmonary Edema Clearance. Am. J. Physiol. Lung Cel Mol Physiol 289 (5), L685–L695. doi:10.1152/ajplung.00247.2005
Patel, B. V., Wilson, M. R., O'Dea, K. P., and Takata, M. (2013). TNF-induced Death Signaling Triggers Alveolar Epithelial Dysfunction in Acute Lung Injury. J. Immunol. 190 (8), 4274–4282. doi:10.4049/jimmunol.1202437
Peters, D. M., Vadász, I., Wujak, L., Wygrecka, M., Olschewski, A., Becker, C., et al. (2014). TGF-β Directs Trafficking of the Epithelial Sodium Channel ENaC Which Has Implications for Ion and Fluid Transport in Acute Lung Injury. Proc. Natl. Acad. Sci. U S A. 111 (3), E374–E383. doi:10.1073/pnas.1306798111
Qiu, L., Jiao, Y., Xie, J. Z., Huang, G. K., Qiu, S. L., Miao, J. H., et al. (2013a). Five New Flavonoid Glycosides from Nervilia Fordii. J. Asian Nat. Prod. Res. 15 (6), 589–599. doi:10.1080/10286020.2013.790377
Qiu, S. L., Jiao, Y., Xie, J. Z., Qiu, L., Huang, G. K., Guo, X. B., et al. (2013b). Anti-inflammatory Effect of the Extractive from Nervilia Fordii (Hance) Schltr. Shizhen guoyi guoyao 24 (05), 1033–1034. doi:10.3969/j.issn.1008-0805.2013.05.004
Rubenfeld, G. D., Caldwell, E., Peabody, E., Weaver, J., Martin, D. P., Neff, M., et al. (2005). Incidence and Outcomes of Acute Lung Injury. N. Engl. J. Med. 353 (16), 1685–1693. doi:10.1056/NEJMoa050333
Toews, G. B. (2001). Cytokines and the Lung. Eur. Respir. J. Suppl. 34, 3s–17s. doi:10.1183/09031936.01.00266001
Wang, Q., Yan, S. F., Hao, Y., and Jin, S. W. (2018). Specialized Pro-resolving Mediators Regulate Alveolar Fluid Clearance during Acute Respiratory Distress Syndrome. Chin. Med. J. (Engl) 131 (8), 982–989. doi:10.4103/0366-6999.229890
Wassmann, S., Stumpf, M., Strehlow, K., Schmid, A., Schieffer, B., Böhm, M., et al. (2004). Interleukin-6 Induces Oxidative Stress and Endothelial Dysfunction by Overexpression of the Angiotensin II Type 1 Receptor. Circ. Res. 94 (4), 534–541. doi:10.1161/01.RES.0000115557.25127.8D
Weisz, O. A., and Johnson, J. P. (2003). Noncoordinate Regulation of ENaC: Paradigm Lost. Am. J. Physiol. Ren. Physiol 285 (5), F833–F842. doi:10.1152/ajprenal.00088.2003
Wynne, B. M., Zou, L., Linck, V., Hoover, R. S., Ma, H. P., and Eaton, D. C. (2017). Regulation of Lung Epithelial Sodium Channels by Cytokines and Chemokines. Front. Immunol. 8, 766. doi:10.3389/fimmu.2017.00766
Xie, Y. L. (2008). The Mechanism of Anti-inflammation and Study of Quality Control of Southern Chinese Material Medica of Herba Nerviliae Fordii. Doctor. Guangzhou, China: Guangzhou Univ Chin Med.
Xiong, M., Jiang, Yu., Liang, D. F., Zhang, Y. Z., and Liu, J. (2014). Antibacterial Activity of Folium Nerviliae In Vitro. Prog. Mod. Biomed 14 (14), 2753–2756. doi:10.13241/j.cnki.pmb.2014.14.038
Xu, Y. J., Chen, Y. B., W, L., and Lin, L. (2010). Effect of Nervilia Fordii on Lung Aquaporin 1 and 5 Expression in Endotoxin-Induced Acute Lung Injury Rat. Chin. J. Integr. Med. 30 (08), 861–866.
Xu, Y. J., Lin, J. Y., Chen, Y. B., W, L., and Lin, L. (2014). Regulatory Effect of Nervilia Fordii Injection on Inflammatory Cytokines in Rats with Lipopolysaccharide-Induced Acute Lung Injury. J. Guangzhou Univ. Chin. Med. 31 (05), 772–775. doi:10.13359/j.cnki.gzxbtcm.2014.05.019
Yang, J., Li, S., Wang, L., Du, F., Zhou, X., Song, Q., et al. (2018). Ginsenoside Rg3 Attenuates Lipopolysaccharide-Induced Acute Lung Injury via MerTK-dependent Activation of the PI3K/AKT/mTOR Pathway. Front. Pharmacol. 9, 850. doi:10.3389/fphar.2018.00850
Yu, G., Liu, Q., Dong, X., Tang, K., Li, B., Liu, C., et al. (2018). Inhibition of Inflammation Using Diacerein Markedly Improved Renal Function in Endotoxemic Acute Kidney Injured Mice. Cell Mol Biol Lett 23, 38. doi:10.1186/s11658-018-0107-z
Zaika, O., Mamenko, M., Staruschenko, A., and Pochynyuk, O. (2013). Direct Activation of ENaC by Angiotensin II: Recent Advances and New Insights. Curr. Hypertens. Rep. 15, 17–24. doi:10.1007/s11906-012-0316-1
Zhou, Y., Lv, L., Liu, Q., and Song, J. (2019). Total Flavonoids Extracted from Nervilia Fordii Function in Polycystic Ovary Syndrome through IL-6 Mediated JAK2/STAT3 Signaling Pathway. Biosci. Rep. 39 (1), 39. doi:10.1042/BSR20181380
Keywords: acute lung injury, nervilia fordii, flavonoids, lipopolysaccharide, aquaporins, epithelial sodium channel, Na+-K+-ATPase
Citation: Yin S, Ding M, Fan L, Yu X, Liang Z, Wu L, Gao Z, Lin L and Chen Y (2021) Inhibition of Inflammation and Regulation of AQPs/ENaCs/Na+-K+-ATPase Mediated Alveolar Fluid Transport by Total Flavonoids Extracted From Nervilia fordii in Lipopolysaccharide-induced Acute Lung Injury. Front. Pharmacol. 12:603863. doi: 10.3389/fphar.2021.603863
Received: 08 September 2020; Accepted: 07 October 2021;
Published: 23 November 2021.
Edited by:
Xiuli Zhang, Dalian Institute of Chemical Physics (CAS), ChinaCopyright © 2021 Yin, Ding, Fan, Yu, Liang, Wu, Gao, Lin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Lin, ZHJsaW5saW42MjBAMTYzLmNvbQ==; Yuanbin Chen, Y2hlbnl1YW5iaW4xMTBAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship.
 Shuomiao Yin
Shuomiao Yin Meizhu Ding2†
Meizhu Ding2† Lei Wu
Lei Wu Yuanbin Chen
Yuanbin Chen