- 1Department of Nephrology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
- 2Department of Critical Care Medicine, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 3Department of Respirology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 4Department of Emergency Medicine, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 5Department of Endocrinology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 6Department of Gastroenterology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 7Department of Anesthesiology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 8Department of Psychiatry, Renmin Hospital of Wuhan University, Wuhan, China
- 9Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan, China
- 10Department of Quality Control, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 11Department of Nephropathy, Shandong Provincial Third Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 12Department of Nephropathy, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
Background: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may persist in patients with coronavirus disease 2019 (COVID-19) despite receiving standard care.
Methods: In this pilot study of hospitalized adult patients (≥18 years of age), with radiologically confirmed pneumonia who were SARS-CoV-2 positive for more than 28 days despite standard care, were assigned to receive standard of care (SOC, grp I) or leflunomide + SOC (grp 2). After 2 weeks, grp 1 and grp 2 patients who continued to be SARS-CoV-2-positive received leflunomide for 14 days while continuing SOC. The primary outcomes were the rate of and time to SARS-CoV-2 clearance and the 14-day and 30-day hospital discharge rate.
Results: 12 patients were enrolled in grp 1 and 15 patients were in grp 2. The 14 days SARS-CoV-2 viral clearance rate was 80.0% (12/15) for grp 2 patients receiving leflunomide vs. 16.7% for grp 1 patients (2/12) (p = 0.002). By day 14, the median time to SARS-CoV-2 clearance was 6.0 days (range 1–12, IQR 1–12) for grp 2 patients. In grp 1, two patients converted to viral negative on days 1 and 6 (p = 0.002). The 14-day discharge rate was 73.3% (11/15) for the grp 2 vs. 8.3% (1/12) for grp 1 (p = 0.001). The 30 days discharge rate was 100% (15/15) for the grp 2 vs. 66.7% (8/12) for grp 1. No severe adverse events or deaths were reported.
Conclusion: Leflunomide may improve the SARS-CoV-2 clearance rate and discharge rate in patients with refractory COVID-19. The tolerability of the 14–28 days course of treatment with leflunomide is acceptable. These preliminary observations need to be verified by a large sample size and randomized controlled trial.
Clinical Trial Registration: www.chictr.org, identifier ChiCTR2000033372.
Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The SARS-CoV-2 pandemic has so far spread to 210 countries and infected over 174 million people globally and caused over 3.7 million deaths (World Health Organization, 2020a). Even though the majority of patients with COVID-19 are clear of SARS-CoV-2 within 14–20 days, our observations indicate that SARS-CoV-2 persists in 2–5% of infected hospitalized patients for up to 4 weeks despite the current best available care (Borba et al., 2020; Cao et al., 2020; Magagnoli et al., 2020; Sanders et al., 2020). These patients are defined as having refractory or chronic COVID-19, and are not discharged from the hospital due to the risk of shedding the virus in communities.
Although the dexamethasone and remdesivir have showed effect on treating COVID-19, repurposing existing drugs to treat COVID-19 still offers a rational option to tackle this global public health emergency (Grein et al., 2020; Horby et al., 2021). Leflunomide is an isoxazole derivate with immunosuppressive activities and has been used as disease-modifying anti-rheumatic drugs in rheumatoid arthritis and psoriatic arthritis (Mladenovic et al., 1995; Cohen et al., 2001; Bao et al., 2003; Nash et al., 2006).1 The drug also possesses anti-viral activities against BK polyomavirus and cytomegalovirus (Waldman et al., 1999; Williams et al., 2005; Avery et al., 2010; Bernhoff et al., 2010; Chacko and John, 2012; Jaw et al., 2017). Schläpfer et al. reported that leflunomide decreased HIV replication by approximately 75% at concentrations that can be obtained with conventional dosing (Schläpfer et al., 2003). Martin et al. observed a strong dose-dependent decrease in replication and transcription of the Ebola virus in the presence of teriflunomide (the active metabolite of leflunomide) (Martin et al., 2018). Upon oral administration, over 80% of leflunomide is converted to teriflunomide via hepatic metabolism in the first pass through the liver (Bar-Or et al., 2014).2 At standard doses for rheumatoid arthritis, teriflunomide inhibits de novo pyrimidine synthesis via dihydroorotate dehydrogenase (DHODH), and at higher doses suppresses tyrosine and serine kinase activities (Xu et al., 1996). Leflunomide is safe and well tolerated9-10. A recent study suggested that leflunomide/teriflunomide could be repurposed for SARS-CoV-2 therapy (Xiong et al., 2020) with a therapeutic range between 6 and 26 μM of teriflunomide for SARS-CoV-2, which is within the recognized therapeutic level for rheumatoid arthritis (Mladenovic et al., 1995).
In this non-randomized pilot study, we evaluated the safety and efficacy of leflunomide for refractory COVID-19 in adult patients who were hospitalized between March 13 and April 17 of 2020 at Hu Bei Renmin Hospital in Wu Han City, China. Crossover of control patients to leflunomide treatment was allowed if control patients were still SARS CoV-2 positive after 14 days in the control arm.
Methods
Study Design
This pilot study of leflunomide as adjunctive therapy to SOC was designed as a pilot study in anticipation for a randomized controlled trial (ChiCTR2000030058) and enrolled hospitalized adult patients (≥18 years of age) with radiologically confirmed COVID-19 pneumonia who were reverse transcriptase-polymerase chain reaction (RT-PCR)-positive for SARS-CoV-2 for more than 28 days despite standard care. Eligible patients had pneumonia confirmed by chest imaging and had an oxygen saturation (SaO2) of 94% or higher on room air PaO2/FiO2 ratio ≥300 mg Hg [ratio of the partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2)]. Exclusion criteria were pregnancy, a history of liver disease, an alanine aminotransferase level five times higher than the upper normal limit (50 U/L), and stage 4 chronic kidney disease.
The study protocol adhered to the SPIRIT statement (Chan et al., 2013) and was conducted under the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki and the reporting of the study adhered to the CONSORT statement (Schulz et al., 2010). The corresponding author was responsible for the study design. All the authors contributed to the analysis of the data. All patients provided written informed consent to the study.
Study Intervention
Patients were assigned to the standard care group (grp 1) or the leflunomide group (grp 2) via patient choice. Standard care was provided to all patients according to the guideline (National Health Commission of the People’s Republic of China, 2020) including supplemental oxygen and supportive care, as well as concurrent therapy with hydroxychloroquine, interferon-α, anti-human immunodeficiency virus drugs (lopinavir/ritonavir), or anti-influenza drugs (arbidol, oseltamivir) was allowed. Leflunomide (Airuohua, manufactured by Changzheng-Cinkate) was given at 30 mg/day to patients who were less than 64 years old, and 20 mg/day to patients who were ≥65 years old. The treatment lasted for 14 days during the first phase. All patients who continued to be RT-PCR-positive for SARS-CoV-2 by day 14 received leflunomide for 14 days as the second phase. Once RT-PCR for SARS-CoV-2 tests were negative for two consecutive assays over 24 h apart, the physician could terminate the treatment and were observed for 2 days before discharge.
SARS-CoV-2 Testing
Throat swap samples were obtained by skilled nurses one day before and daily after the start of leflunomide therapy until discharge from the hospital. RNA was extracted using the MagNA Pure 96 system and semiquantitative real-time RT-PCR was performed on an ABI 7500 PCR analyzer (Thermofisher) by using LightMix Modular SARS-CoV-2 (COVID-19) assays (TIB MOBIOL) with primers targeting the nucleocapsid protein (NP) gene and open reading frame (ORF) of SARS-CoV-2. A cycle threshold (Ct) ≤ 38.0 was considered positive. The report was presented as a grade scale of 1+∼3+ to indicate increasing viral load (Cao et al., 2020). Two consecutive negative RT-PCR results 24 h apart for SARS-CoV-2 indicated SARS-CoV-2 clearance.
Study Outcomes
The primary outcomes included the rate of and time to SARS-CoV-2 clearance, and the 14-day and 30-day hospital discharge rate. Secondary outcomes included the incidence of flares, defined as the event of conversion to being RT-PCR-positive for SARS-CoV-2 after turning negative for SARS-CoV-2 in the course of treatment, and adverse events. Patients were discharged when clinical symptoms and radiographic images of COVID-19 were significantly improved and they were RT-PCR-negative for SARS-CoV-2 with two consecutive assays over 24 h apart.
Adverse events (AEs) were graded and recorded according to NCI-CTCAE version 4.03. Safety events included AEs and severe adverse events (SAEs). SAEs included any untoward medical occurrence that resulted in death, was life-threatening, required hospitalization or prolongation of hospitalization, or caused significant or persistent disability or incapacity, or birth defects. AEs were coded to a preferred term using the Medical Dictionary for Regulatory Activities (MedDRA) 22.0. Safety assessments were based mainly on the occurrence, frequency, and severity of AE and analyzed mainly using descriptive statistics.
Statistical Analysis
The analysis population included all patients. Analyses were descriptive in nature. Summary tabulations included the number of observations; mean, standard deviation, median, interquartile range (IQR), minimum and maximum for continuous variables; number and percentage per category for categorical data. The rate of SARS-CoV-2 clearance and discharge were analyzed by Fisher’s Exact Test. Time to SARS-CoV-2 clearance and time to discharge from the hospital were described with Kaplan-Meier analysis. All analyses were conducted with SPSS software, version 25 (IBM Corp.). p < 0.05 indicated a statistically significant difference.
Results
Patient Demographic and Baseline Characteristics
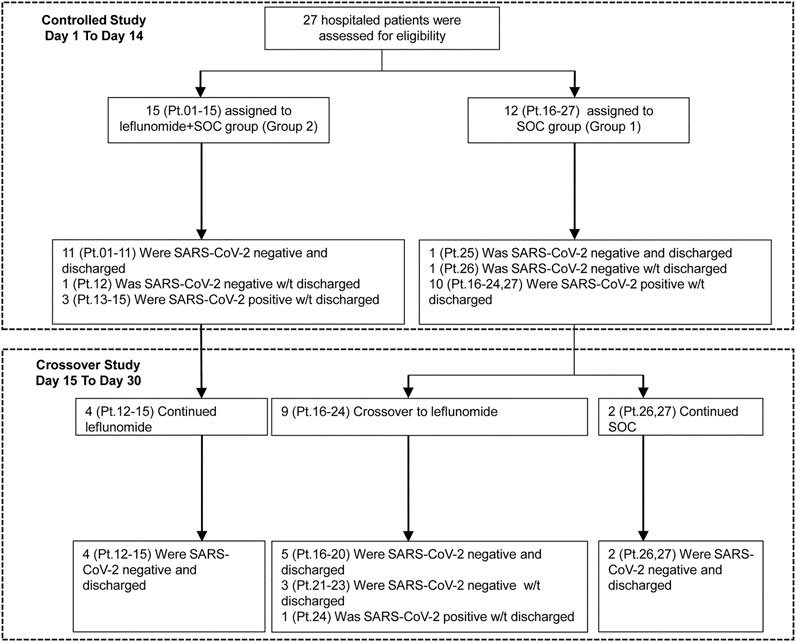
The study flowchart is shown in Figure 1. 27 patients were enrolled in the study. Their median age was 62 (IQR 43–70), and 52% of the patients were men. Patient demographic and baseline characteristics are shown in Table 1. The median duration of symptomatic onset or positive SARS-CoV-2 was 45 days (IQR 41–50). Twelve (44%) patients on admission had severe COVID-19 pneumonia (World Health Organization, 2020b). Sixty-seven percent of the patients had comorbidities. Upon enrollment, 15 patients were assigned to receive leflunomide (grp 2) and 12 patients to receive standard care only (grp 1). In grp 2, 10 patients received 20 mg/day leflunomide and five patients received 30 mg/day leflunomide. Except for gender, the two groups were comparable in the demographic and baseline characteristics. The median number of medicines received was 6 (IQR 5–8) in the leflunomide group and 7.5 (IQR 7–9) in the standard care group. Patient pharmacotherapy data are shown in Supplementary Table S1.
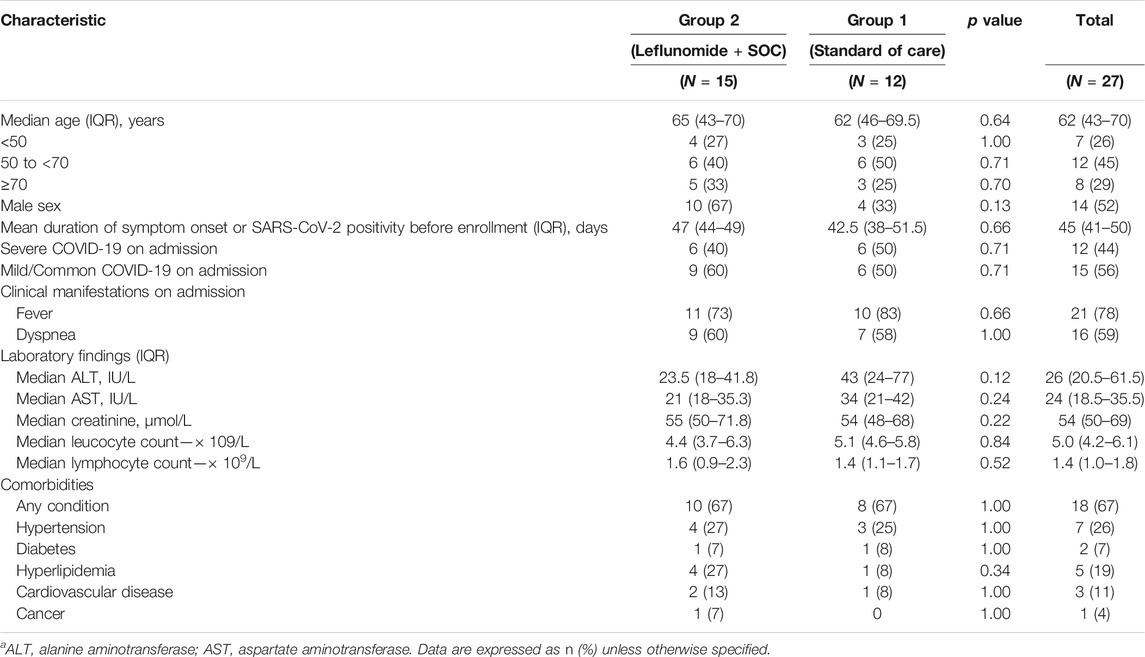
TABLE 1. Patient demographic and baseline characteristics.a
Primary Outcomes
SARS-CoV-2 Clearance
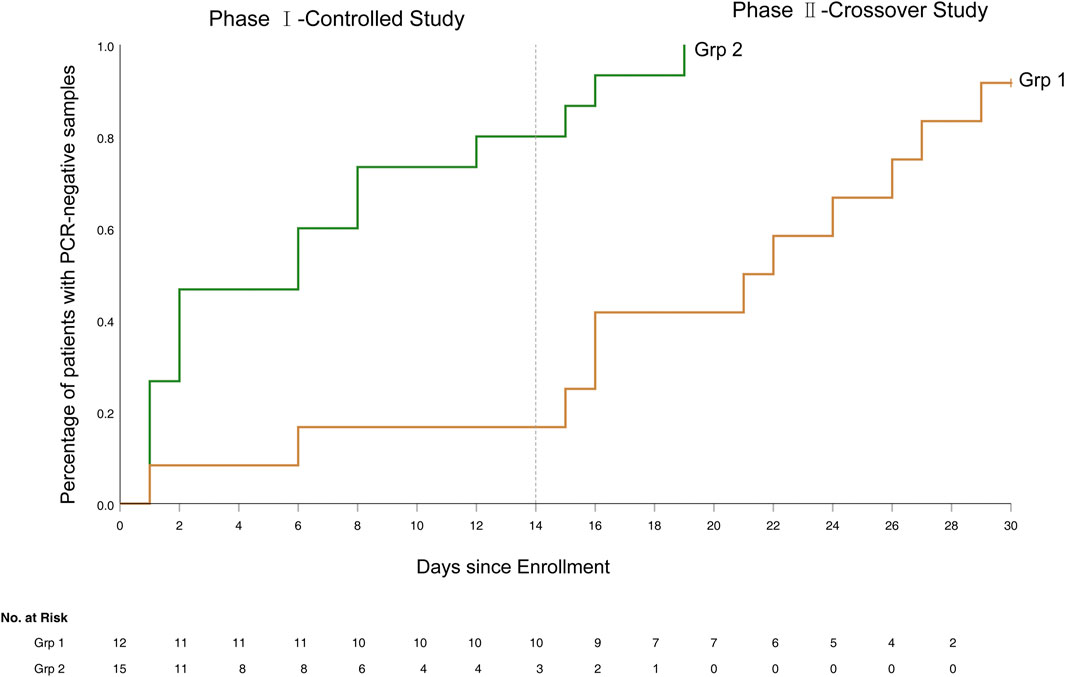
The 14-day SARS-CoV-2 clearance rate was 80.0% (12/15) for patients in grp 2 vs. 16.7% (2/12) for patients in grp 1 (p = 0.002). Grp 2 patients had a more rapid clearance of SARS-CoV-2 than in grp 1 (Figure 2). The median time to SARS-CoV-2 clearance in all grp 2 patients that cleared (n = 12) was 6.0 days (range 1–12, IQR 1–12), vs. only two patients clearing the virus in grp 1 (statistical analysis not performed secondary to the small number of clearances in grp 1).
Three patients who remained RT-PCR positive for SARS-CoV-2 despite 2 weeks of leflunomide therapy continued to receive leflunomide and they achieved SARS-CoV-2 clearance on days 15, 16, and 19, respectively. Nine patients receiving standard care remained RT-PCR positive for SARS-CoV-2 on day 14 post-enrollment and were crossed over to receive leflunomide. Eight patients achieved SARS-CoV-2 clearance in a median duration of 9 days (range 0–14, IQR 1–13) from the crossover. (Figure 3). By day 30, 15/15 (100%) of patients in grp 2 and 11/12 (91.7%) patients in grp 1 cleared virus. One patient (Pt. 24) in grp 1 initiated leflunomide treatment on day 14 when SARS-CoV-2 tests continued to be positive. After 6 days of leflunomide treatment, SARS-CoV-2 assay converted to negative twice on days 20 and 21. This patient decided to stop leflunomide on day 21. The test of SARS-CoV-2 was positive on day 23 and persisted in positive to day 30 without being discharged.
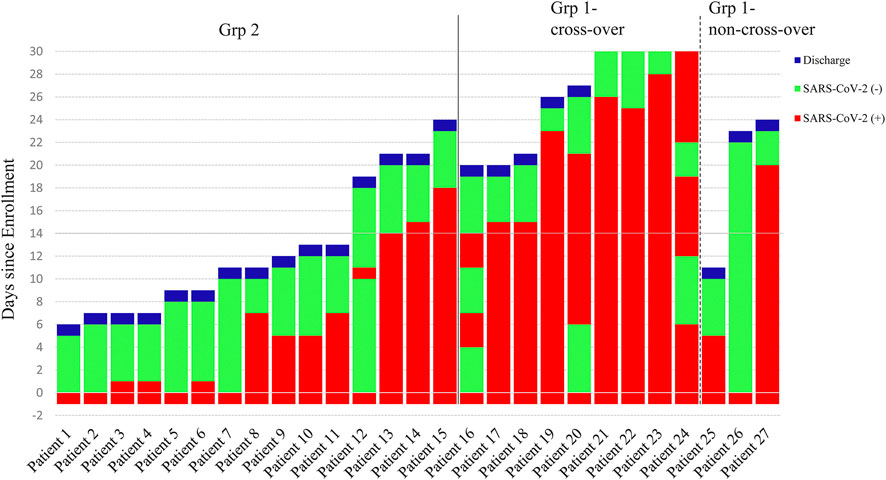
FIGURE 3. Time course of the status of the SARS-CoV-2 Test and End Points. Patients 1 to 15 were in the leflunomide group and patients 16 to 27 were in the standard care group.
Discharge
The 14-day discharge rate was 73.3% (11/15) for grp 2 vs. 8.3% (1/12) for grp 1 (p = 0.001), while the 30-day discharge rate was 100% (15/15) for grp 2 vs. 66.7% (8/12) patients for grp 1. The median hospital stay was 11 days (IQR 7–19) for grp 2 vs. 24.0 days for grp 1 (p<0.001) (Figure 4).
Sensitivity Analysis
In order to further confirm the efficacy of leflunomide in enhancing the clearance of SARS-CoV-2, a sensitivity analysis was performed with the elimination of the patients whose viruses were cleared on day one after the enrollments. Based on this principle, four patients in the leflunomide group (Grp 2) and one patient in standard care (grp 1) were eliminated. Therefore, 11 patients were left in each group for analysis. The results of the sensitivity analysis showed in Supplementary Figures S1–S3. The sensitivity analysis results are consistent with the overall primary endpoint results.
Secondary Outcomes
Flares (SARS-CoV-2 Recurrence)
Flares occurred in one patient in group 2 (Figure 3). The patient (Pt. 12) became viral negative on day 1. On day 11, the patient tested positive SARS-CoV-2. The patient tested negative on day 12 and onward, and he was discharged on day 19.
Safety
AEs were observed in 73.3% (11/15) patients in grp 2 and 83.3% (10/12) patients in group 1. Treatment Emergent Adverse Event (TEAEs) were observed in 40% (6/15) patients in grp 2 and 25% (3/12) patients in group 1 (Supplementary Table S2). The most frequent TEAEs were hyperlipidemia (20%), leukopenia (20%), and neutropenia (13.3%) in the grp 2 and hyperlipidemia (16.7%) and hypoalbuminemia (8.3%) in grp 1. No SAEs or deaths were reported.
Discussion
The pilot study demonstrated that leflunomide may shorten the time to SARS-CoV-2 clearance, improve the SARS-CoV-2 clearance rate and the 14-day hospital discharge rate of COVID-19 patients refractory to standard care, suggesting that the approved drug may be able to treat COVID-19. Although not the typical viral course, we observed that 2–5% of COVID-19 patients fail to clear SARS-CoV-2 in as long as 28 days, indicating that a significant proportion of COVID-19 patients may harbor SARS-CoV-2 for an extended period of time (Wang et al., 2020). This phenomenon of chronic viral shedding may be a manifestation of relative immune-incompetence related to the SARS-Cov-2 infection, severe initial infection depleting the host’s immune capacity, or underlying immune-dysregulation. The clinical manifestations are usually stabilized with standard care but show persistent SARS-CoV-2 positivity. The clinical and infectious consequences of the continued viral positivity is currently unknown, but we assume that at least a portion of these patients are still contagious and could redevelop clinical symptoms. As they may pose an infectious risk to the community, these patients could not be discharged from the hospital. Recently Remdesivir was granted emergency use authorization from the FDA (2019), however, full approval from the FDA is still several months away. Accelerating the development of vaccines and new drugs is warranted; however, new drug development is time-consuming and cannot meet the immediate and urgent needs. Therefore, repurposing approved medicine is a rational option (Grein et al., 2020). Several investigators have looked to currently approved drugs to impact COVID-19 and many have not lived up to expectations (Cao et al., 2020; Magagnoli et al., 2020; Borba et al., 2020; Sanders et al., 2020; Horby et al., 2021).
To our knowledge, our study is the first clinical study evaluating a DHODH inhibitor, leflunomide, for the treatment of COVID-19. Leflunomide is the prodrug to the active drug teriflunomide. It has been approved for the treatment of rheumatoid arthritis (Cohen et al., 2001) by the USA Food and Drug Administration (FDA), lupus nephritis (Wang et al., 2008) in China, and psoriatic arthritis (Nash et al., 2006) in the EU. Leflunomide is well tolerated9-10. Anti-viral activities have been described for leflunomide in CMV (Avery et al., 2010) and BK polyomavirus (Williams et al., 2005; Jaw et al., 2017). Both the anti-viral and immunosuppressive activities of leflunomide have been attributed to its inhibition of de novo pyrimidine synthesis at concentrations active for rheumatoid arthritis (Mladenovic et al., 1995)1, and inhibition of tyrosine and serine kinase activities at higher teriflunomide concentrations (Xu et al., 1996). Additionally, previous studies (Liacini et al., 2010; Jaw et al., 2017) reported that leflunomide inhibits the PI3K-AKT-mTOR pathway, and the release of viral genetic material from capsids depends on this signaling pathway. More recently, in vitro studies (Xiong et al., 2020) indicated that teriflunomide has anti-SARS-CoV-2 activities at a range of 6–26 μM that fall within the recognized therapeutic levels for rheumatoid arthritis. Their data suggested that SARS-CoV-2 was sensitive to teriflunomide via inhibition of DHODH (Xiong et al., 2020), a rate-limiting enzyme in pyrimidine de novo synthesis. DHODH catalyzes the dehydrogenation of dihydroorotate to orotic acid to generate uridine and cytosine nucleotides. The authors further speculated that under normal conditions, nucleotides are supplied via both de novo biosynthesis and the salvage pathway that recycles pre-existing nucleotides from food or other nutrients. The salvage pathway is sufficient for supplying pyrimidines in non-proliferating quiescent cells, but in virus-infected cells, the de novo nucleotides biosynthesis is critical to supply the larger intracellular nucleotide pool required for viral replication. Finally, Xiong et al. reported that influenza A virus replication was reduced in cells deficient in DHODH-/- cells, even though cell growth is not affected (Xiong et al., 2020).
In the current study, we investigated leflunomide as additional pharmacologic therapy for hospitalized patients who were RT-PCR-positive for SARS-CoV-2 for more than 28 days despite receiving standard of care. The study showed that leflunomide noticeably increased the 14 days SARS-CoV-2 clearance rate of refractory COVID-19 patients (80.0% vs. 16.7% for standard care). In addition, leflunomide shortened the median time to SARS-CoV-2 clearance, which was 6.0 days for patients receiving leflunomide, whereas in the standard care group, only 2 of 12 patients converted to SARS-CoV-2 RT-PCR negative on days 1 and 6. Notably, nine COVID-19 patients who remained SARS-CoV-2 positive crossed over to leflunomide treatment after 2 weeks of standard care, eight patients achieved SARS-CoV-2 clearance approximately 9 days from crossover. In addition, leflunomide significantly increased the 14-day discharge rate (73.3% vs. 8.3% for standard care) and greatly shortened the length of hospital stay. In this study, we applied leflunomide at 20–30 mg/day, matching the dose range for rheumatoid arthritis (Cohen et al., 2001; Bao et al., 2003) or systemic lupus erythematosus (Wang et al., 2008). We found that leflunomide showed good tolerability and efficacy in COVID-19 patients, with an acceptable safety profile of leflunomide, with no SAEs or deaths reported.
Despite the demonstration of promising efficacy of leflunomiude, this study has several limitations. This pilot study was not randomized, the sample size of refractory COVID-19 patients was small, and all patients came from a single center. The RT-PCR assay utilized was semi-quantitative, and no plasma was saved to assay for serum teriflunomide levels.
In conclusion, this pilot study has demonstrated that leflunomide has an acceptable safety profile and may be effective in enhancing SARS-CoV-2 clearance in refractory COVID-19 patients. Based on the efficacy and safety profiles of leflunomide on COVID-19 from this pilot study, a large sample size and randomized controlled trial is warranted.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of Qilu Hospital, Shandong University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZH is the chief investigator. QW, HG, YL (3rd author), and XJ were responsible for the design, analyzing, and writing of the manuscript. XH, NZ, JF, DS, ZB, and YZ were responsible for recruitment and clinical care of the patients. YH, YS, XY, YL (3rd author), BJ, and YG were responsible for the data analyses. YL (16th author), FQ, and YW were responsible for the sample collection and laboratory analysis. All authors reviewed and approved the manuscript.
Funding
This work was supported by the COVID-19 Emergency Tackling Research Project of Shandong University (Grant No. 2020XGA 01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript has been released as a pre-print at medRxiv. https://www.medrxiv.org/content/10.1101/2020.05.29.20114223v1 (Qiang et al., 2020).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.581833/full#supplementary-material
Footnotes
1ARAVA (LEFLUNOMIDE), Labels for NDA 020905.
2AUBAGIO (TERIFLUNOMIDE) Labels for NDA 202992.
References
Avery, R. K., Mossad, S. B., Poggio, E., Lard, M., Budev, M., Bolwell, B., et al. (2010). Utility of Leflunomide in the Treatment of Complex Cytomegalovirus Syndromes. Transplantation 90, 419–426. doi:10.1097/tp.0b013e3181e94106
Bao, C., Chen, S., Gu, Y., Lao, Z., Ni, L., Yu, Q., et al. (2003). Leflunomide, a New Disease-Modifying Drug for Treating Active Rheumatoid Arthritis in Methotrexate-Controlled Phase II Clinical Trial. Chin. Med. J. (Engl) 116, 1228–1234.
Bar-Or, A., Pachner, A., Menguy-Vacheron, F., Kaplan, J., and Wiendl, H. (2014). Teriflunomide and its Mechanism of Action in Multiple Sclerosis. Drugs 74, 659–674. doi:10.1007/s40265-014-0212-x
Bernhoff, E., Tylden, G. D., Kjerpeseth, L. J., Gutteberg, T. J., Hirsch, H. H., and Rinaldo, C. H. (2010). Leflunomide Inhibition of BK Virus Replication in Renal Tubular Epithelial Cells. Jvi 84, 2150–2156. doi:10.1128/jvi.01737-09
Borba, M. G. S., Val, F. F. A., Sampaio, V. S., et al. (2020). Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw. Open 3 (4), e208857. doi:10.1001/jamanetworkopen.2020.8857
Cao, B., Wang, Y., Wen, D., Liu, W., Wang, J., Fan, G., et al. (2020). A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 382 (19), 1787–1799. doi:10.1056/NEJMoa2001282
Chacko, B., and John, G. T. (2012). Leflunomide for Cytomegalovirus: Bench to Bedside. Transpl. Infect. Dis. 14, 111–120. doi:10.1111/j.1399-3062.2011.00682.x
Chan, A.-W., Tetzlaff, J. M., Altman, D. G., Laupacis, A., Gøtzsche, P. C., Krleža-Jerić, K., et al. (2013). SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann. Intern. Med. 158, 200–207. doi:10.7326/0003-4819-158-3-201302050-00583
Cohen, S., Cannon, G. W., Schiff, M., Weaver, A., Fox, R., Olsen, N., et al. (2001). Utilization of Leflunomide in the Treatment of Rheumatoid Arthritis Trial Investigator Group. Two-Year, Blinded, Randomized, Controlled Trial of Treatment of Active Rheumatoid Arthritis with Leflunomide Compared with Methotrexate. Arthritis Rheum. 44, 1984–1992. doi:10.1002/1529-0131(200109)44:9<1984::AID-ART346>3.0.CO;2-B
FDA (2019). Emergency Use Authorization (EUA) of Remdesivir for Coronavirus Disease (COVID-19). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (Accessed October 22, 2020).
Grein, J., Ohmagari, N., Shin, D., Diaz, G., Asperges, E., Castagna, A., et al. (2020). Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 382 (24), 2327–2336. doi:10.1056/NEJMoa2007016
Jaw, J., Hill, P., and Goodman, D. (2017). Combination of Leflunomide and Everolimus for Treatment of BK Virus Nephropathy. Nephrology 22, 326–329. doi:10.1111/nep.12948
Horby, P., Lim, W. S., Emberson, J. R., Mafham, M., Bell, J. L., Linsell, L., et al. (2021). Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 384 (8), 693–704. doi:10.1056/NEJMoa2021436
Liacini, A., Seamone, M. E., Muruve, D. A., and Tibbles, L. A. (2010). Anti-BK Virus Mechanisms of Sirolimus and Leflunomide Alone and in Combination: Toward a New Therapy for BK Virus Infection. Transplantation 90, 1450–1457. doi:10.1097/tp.0b013e3182007be2
Magagnoli, J., Narendran, S., Pereira, F., Cummings, T., Hardin, J. W., Sutton, S. S., et al. (2020). Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with Covid-19. medRxiv 04, 20065920. doi:10.1101/2020.04.16.20065920
Martin, S., Chiramel, A. I., Schmidt, M. L., et al. (2018). A Genome-wide siRNA Screen Identifies a Druggable Host Pathway Essential for the Ebola Virus Life Cycle. Genome Med. 10, 58. doi:10.1186/s13073-018-0570-1
Mladenovic, V., Domljan, Z., Rozman, B., Jajic, I., Mihajlovic, D., Dordevic, J., et al. (1995). Safety and Effectiveness of Leflunomide in the Treatment of Patients with Active Rheumatoid Arthritis. Arthritis Rheum. 38, 1595–1603. doi:10.1002/art.1780381111
Nash, P., Thaçi, D., Behrens, F., Falk, F., and Kaltwasser, J. P. (2006). Leflunomide Improves Psoriasis in Patients with Psoriatic Arthritis: An In-Depth Analysis of Data from the TOPAS Study. Dermatology 212, 238–249. doi:10.1159/000091251
National Health Commission of the People’s Republic of China (2020). Guidelines for the Diagnosis and Treatment of Novel Coronavirus Pneumonia (Trial Version Sixth). Chin. J. Viral. Dis. 10 (2), 81–85. doi:10.16505/j.2095-0136.2020.0016
Qiang, W., Haipeng, G., Yu, L., Jian, X., Hou, X., Zhong, N., et al. (2020). Efficacy and Safety of Leflunomide for Refractory COVID-19: An Open-Label Controlled Study. medRiv [Epub ahead of print]. doi:10.1101/2020.05.29.20114223
Sanders, J. M., Monogue, M. L., Jodlowski, T. Z., and Cutrell, J. B. (2020). Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19). JAMA 323 (18), 1824–1836. doi:10.1001/jama.2020.6019
Schläpfer, E., Fischer, M., Ott, P., and Speck, R. F. (2003). Anti-HIV-1 Activity of Leflunomide. AIDS 17, 1613–1620. doi:10.1097/00002030-200307250-00005
Schulz, K. F., Altman, D. G., and Moher, D.CONSORT Group (2010). CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMJ 340, c332. doi:10.1136/bmj.c332
Waldman, W. J., Knight, D. A., Blinder, L., Shen, J., Lurain, N. S., Miller, D. M., et al. (1999). Inhibition of Cytomegalovirus In Vitro and In Vivo by the Experimental Immunosuppressive Agent Leflunomide. Intervirology 42, 412–418. doi:10.1159/000053979
Wang, B., Wang, L., Kong, X., Geng, J., Xiao, D., Ma, C., et al. (2020). Long‐term Coexistence of SARS‐CoV‐2 with Antibody Response in COVID‐19 Patients. J. Med. Virol. 92, 1684–1689. doi:10.1002/jmv.25946
Wang, H., Cui, T., Hou, F., Ni, Z., Chen, X., Lu, F., et al. (2008). Induction Treatment of Proliferative Lupus Nephritis with Leflunomide Combined with Prednisone: a Prospective Multi-centre Observational Study. Lupus 17, 638–644. doi:10.1177/0961203308089408
Williams, J. W., Javaid, B., Kadambi, P. V., Gillen, D., Harland, R., Thistlewaite, J. R., et al. (2005). Leflunomide for Polyomavirus Type BK Nephropathy. N. Engl. J. Med. 352, 1157–1158. doi:10.1056/nejm200503173521125
World Health Organization (2020a). Coronavirus Disease (COVID-2019) Situation Reports on July 7. Availabel at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (Accessed 11 June 2021).
World Health Organization (2020b). Clinical Management of Severe Acute Respiratory Infection (SARI) when COVID-19 Disease Is Suspected. Available at: https://apps.who.int/iris/handle/10665/331446 (Accessed March 13, 2020).
Xiong, R., Zhang, L., Li, S., Sun, Y., Ding, M., Wang, Y., et al. (2020). Novel and Potent Inhibitors Targeting DHODH, a Rate-Limiting Enzyme in De Novo Pyrimidine Biosynthesis, Are Broad-Spectrum Antiviral against RNA Viruses Including Newly Emerged Coronavirus SARS-CoV-2. bioRxiv 11, 983056. doi:10.1101/2020.03.11.983056
Keywords: COVID-19, leflunomide, refractory, pilot study, safety
Citation: Wang Q, Guo H, Li Y, Jian X, Hou X, Zhong N, Fei J, Su D, Bian Z, Zhang Y, Hu Y, Sun Y, Yu X, Li Y, Jiang B, Li Y, Qin F, Wu Y, Gao Y and Hu Z (2021) Efficacy and Safety of Leflunomide for Refractory COVID-19: A Pilot Study. Front. Pharmacol. 12:581833. doi: 10.3389/fphar.2021.581833
Received: 16 July 2020; Accepted: 04 May 2021;
Published: 02 July 2021.
Edited by:
Alastair George Stewart, The University of Melbourne, AustraliaReviewed by:
Daniel F. B. Wright, University of Otago, New ZealandClaire Gordon, The University of Melbourne, Australia
Copyright © 2021 Wang, Guo, Li, Jian, Hou, Zhong, Fei, Su, Bian, Zhang, Hu, Sun, Yu, Li, Jiang, Li, Qin, Wu, Gao and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhao Hu, c2RodXpoYW9AdmlwLjEyNi5jb20=
†These authors share first authorship
 Qiang Wang
Qiang Wang Haipeng Guo
Haipeng Guo Yu Li
Yu Li Xiangdong Jian4†
Xiangdong Jian4† Xinguo Hou
Xinguo Hou Zhouyan Bian
Zhouyan Bian Yi Zhang
Yi Zhang Zhao Hu
Zhao Hu