
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 26 May 2021
Sec. Drugs Outcomes Research and Policies
Volume 12 - 2021 | https://doi.org/10.3389/fphar.2021.581293
 Nan-Nan Shen1,2†
Nan-Nan Shen1,2† Chi Zhang2,3†
Chi Zhang2,3† Ying Hang4†
Ying Hang4† Zheng Li5
Zheng Li5 Ling-Cong Kong5
Ling-Cong Kong5 Na Wang6
Na Wang6 Jia-Liang Wang1
Jia-Liang Wang1 Zhi-Chun Gu2,7,8*
Zhi-Chun Gu2,7,8*Background: The use of direct oral anticoagulant (DOAC) off-label doses in atrial fibrillation (AF) patients may result in poor clinical outcomes. However, the true prevalence remains scarce. This study aims at estimating the prevalence of DOAC off-label doses in AF patients.
Methods: Databases of MEDLINE, EMBASE, and COCHRANE were searched from inception through February 2020 for real-world studies that reported the off-label definition and prevalence data of AF patients using DOACs. The primacy outcomes were the overall prevalence of DOAC off-label doses and the corresponding underdose and overdose. The random-effects model was used for data synthesis. Variations on individual DOAC and different regions were examined by subgroup analyses.
Results: A total of 23 studies involving 162,474 AF patients were finally included. The overall prevalence of DOAC off-label doses was 24% (95% CI, 19–28%), with 18% for dabigatran, 27% for rivaroxaban, 24% for apixaban, and 26% for edoxaban. The prevalence of underdosed DOACs was 20% (95% CI, 16–24%) with significant difference among individual anticoagulants (13% for dabigatran, 22% for rivaroxaban, 22% for apixaban, and 18% for edoxaban; Pinteraction=0.02). The prevalence of overdosed DOACs was 5% (95% CI, 3–7%), with the lowest prevalence observed in apixaban (2%). Subgroup analyses by regions demonstrated that the prevalence of DOAC off-label doses was higher in Asia (32%) than in North America (14%) and in Europe (22%), with underdose being predominant. Regardless of different regions, the prevalence of overdose was relatively low (4–6%).
Conclusion: This study provides an estimation of DOAC off-label doses in the real-world setting. The prevalence rate of DOAC off-label doses in AF patients was relatively high, with underdose being predominant. Clinicians in Asia preferred to prescribe underdose of DOACs to AF patients. More evidence about the appropriateness of DOAC off-label doses in AF patients is urgently needed. Education programs concerning the appropriate prescription of DOACs within the drug labels and accepted guidelines are necessary to DOAC prescribers to ensure the safety and effectiveness of anticoagulation therapy for patients with AF.
Atrial fibrillation (AF) is the most prevalent arrhythmia, estimated to affect more than 33 million people worldwide (Cohen et al., 2018). Stroke is the most feared complication of AF, and oral anticoagulation is the principal priority of AF management. Although dose-adjusted warfarin was commonly used for decades, direct oral anticoagulants (DOACs) are now recommended as the treatment of choice for a majority of nonvalvular AF patients. Based on the vital trials of DOACs (Connolly et al., 2009; Granger et al., 2011; Patel et al., 2011; Giugliano et al., 2013), dose adjustment for each DOAC was approved by the National Food and Drug Administration according to patient characteristics (e.g., age, body weight, renal function) and concomitant medications (Camm et al., 2012; Lehr et al., 2012; January et al., 2014; Steinberg et al., 2016; Martin et al., 2018; De Caterina et al., 2019). Nevertheless, when the first DOAC dabigatran was launched, concerns about the fixed dose and possibility of overdosing were raised, as there was no need for dose titration or monitoring of blood levels, unlike older treatments such as using warfarin (Malmstrom et al., 2013; Cohen, 2014). These resulted in an extensive range of activities, among health authorities in many countries and regions, such as Europe and New Zealand, to improve the quality and efficiency of prescribing dabigatran (Godman et al., 2014). Despite the explicitness of specific recommended dose adjustment for each DOAC, the off-label dose of DOACs in AF patients was not uncommon in the real-world setting. One recent prospective cohort study concerning label adherence for DOACs in Asian patients revealed that more than one-third of patients with DOAC prescriptions received an off-label underdose (Lee et al., 2019b). In fact, concerns have currently been raised regarding the off-label dose of DOACs in AF patients (Garcia Rodriguez et al., 2019a; Lee et al., 2019b), which is classified as underdose and overdose. Some studies have now focused on the clinical outcomes of nonstandard dosing of DOACs. A previous U.S. national registry study reported that overdose of DOACs was closely related to increased all-cause mortality, whereas underdose was associated with increased cardiovascular disease-related hospitalization (Steinberg et al., 2016). It was also reported that dose adherence to the guideline is associated with improved clinical outcomes in Asian AF patients compared with under- or overtreatment (Krittayaphong et al., 2020).
Nevertheless, the present criteria for DOAC doses are similar but slightly different between Europe, the USA, and Asia, and physicians in different regions have different dosage adjustment styles, as patients of different races and ethnicities have different characteristics. Until now, many studies assessing the prevalence of DOAC off-label dose have been published, and the rate varied with different regions and different DOACs (Steinberg et al., 2016; McAlister et al., 2018; Garcia Rodriguez et al., 2019b; Lee et al., 2019a; Murata et al., 2019). Regretfully, reliable estimates of the prevalence of off-label dose for DOACs at the global level have rarely been obtained, which could serve as the basis for rational anticoagulation for AF patients. To fill the gaps of this knowledge, we summarized all available evidence to conduct a comprehensive systematic review.
This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (Moher et al., 2009). The protocol for this study was prospectively registered in PROSPERO (CRD42020170600). All the supporting data are available within the article and the Supplement.
A literature search in MEDLINE, EMBASE, and COCHRANE databases was conducted from inception to February 19, 2020, using the combination of search terms related to DOACs and label. The detailed search strategy is outlined in Supplementary eTable S1. In addition, manual search was also performed to screen relevant articles from the reference lists of all included studies and relevant reviews.
The articles were eligible for inclusion if they met the following criteria: included AF patients, involved more than 500 patients, and reported off-label dose data of DOACs (dabigatran, rivaroxaban, apixaban, and edoxaban). The studies with a small sample size (<500 patients), or performed in a single center, or in the form of a conference abstract or letter were excluded. If the same data source or overlapped data were reported by several studies, the most comprehensive data were included. For different subgroups of the same data source separately reported in different studies, all studies were included. The primacy outcomes were the global prevalence of DOAC off-label doses, classified as underdose and overdose in reference to the recommended criteria (Table 1). Two researchers (N. S. and C. Z.) independently screened titles and abstracts of retrieved records and obtained the potentially relevant full-text for further assessment. Consensus was achieved for any disagreement discussed with the corresponding investigator (Z.C.).
Two investigators (N. S. and C. Z.) independently extracted data from the included full-text studies. The following data were extracted: study characteristics (study name, countries or regions, data source, follow-up duration, proportion of each DOAC in the study, total patient number, risk factors associated with off-label doses, and definition of DOAC off-label doses); demographics and clinical characteristics (mean age, gender ratio, comorbidities, CHA2DS2-VASc score, HAS-BLED score, etc.); conflicts of interest; article funding, author–industry financial ties, and author employment. The regions of included studies were classified as North America, Asia, and Europe.
The methodological quality of each included studies was assessed according to the revised Newcastle-Ottawa Scale (NOS), which consists of 5 dimensions: sample population, sample size, participation rate, outcome assessment, and analytical methods to control for bias (Cota et al., 2013). Each item could receive a maximum of 2 points, and the total score ranged from 0 to 10 points (Supplementary eTable S2).
A random-effects meta-analysis was used to calculate the pooled prevalence rate and the corresponding 95% confidence intervals (95% CIs) of DOAC off-label doses (overall, underdose and overdose) in AF patients. Heterogeneity of prevalence estimates among studies was assessed using I2 statistic, with I2 > 50% representing considerable heterogeneity. Subgroup analyses were performed by individual DOAC (dabigatran, rivaroxaban, apixaban, and edoxaban) in different regions (North America, Asia, and Europe). The interaction analyses (P for interaction) were calculated to assess the comparability in each subgroup. A leave-1-out sensitivity analysis was performed for each subgroup to explore whether a single study had an influence on the prevalence of off-label doses. Furthermore, additional sensitivity analysis was conducted by excluding studies with off-label definition only based on renal function. Meta-regression analysis was conducted to assess the potential association between patient characteristics and the estimates of prevalence. Publication bias was explored qualitatively by funnel plots and quantitatively by Begg’s test and Egger’s test (Liberati et al., 2009). Trim and fill method was performed to deal with publication bias (Duval and Tweedie, 2000). Statistical analyses were performed using STATA version 13.0 (Statacorp, College Station, Texas, United States).
A total of 2188 records were identified in the initial database search, 279 duplicates were removed and 1849 records were excluded by screening titles and abstracts. Afterward, 60 full-text studies were retained for further review, and 37 studies were excluded with the detailed reasons outlined in Supplementary eTable S3. Ultimately, 23 studies involving 162,474 patients met the criteria for inclusion. Of these, 9 articles reported off-label data about DOACs; 6 about dabigatran, rivaroxaban, and apixaban; 3 about dabigatran and rivaroxaban; 3 about rivaroxaban; 1 about apixaban; and 1 about edoxaban (Figure 1). Seven studies were performed in North America (5 in the United States and 2 in Canada), 10 studies in Asia (1 in Taiwan, 5 in Japan, 3 in Korea, and 1 in Israel), and 6 studies in Europe (2 in the United Kingdom, 1 in France, 1 in the Netherlands, 1 in Turkey, and 1 in Spain). Other study characteristics and risk factors related to DOAC off-label doses are presented in Table 2. The detailed definition of DOAC off-label doses in each included study is represented in Supplementary eTable S4. Of the 23 studies, 6 were funded by DOAC pharmaceutical companies (3 founded by Bayer, 2 founded by Bristol-Myers Squibb, and 1 founded by Daiichi Sankyo). Authors in 3 articles received consultant fees from multiple companies (Supplementary eTable S5).
The mean age of patients was 72.4 years, and 42.6% of patients were female. The mean body mass index (BMI) was 25.4 kg/m2, and the rate of concomitant aspirin use was 32.3%. The major comorbidities were hypertension (75.9%), heart failure (28.3%), diabetes mellitus (27.9%), and myocardial infarction (9.8%) (Table 3). All included studies satisfied the following risk bias items: sample population, sample size, and participation rate. 18 studies (70%) reported detailed analytical methods to control bias, and all 23 studies were rated as relatively good quality (Supplementary eTable S6).
As outlined in Figure 2, the estimated global prevalence of DOAC off-label doses in AF patients was 24% (95% CI, 19–28%; I2, 99.8%) (Supplementary eFigure S1). The highest prevalence for off-label doses was found in rivaroxaban (27%; 95% CI, 21–32%; I2, 99.7%), followed by edoxaban (26%; 95% CI, 15–37%; I2, 93.9%), apixaban (24%; 95% CI, 18–29%; I2, 98.9%), and dabigatran (18%; 95% CI, 12%–24%; I2, 99.7%; Pinteraction =0.28) (Supplementary eFigures S2–S5). Regarding the underdose use of DOACs in AF, the pooled prevalence was 20% (95% CI, 16–24%; I2, 99.8%) (Figure 2; Supplementary eFigure S1). The prevalence rates varied from different DOACs, with the estimated value being 22%, 22%, 18%, and 13% for rivaroxaban, apixaban, edoxaban, and dabigatran, respectively (Supplementary eFigures S2–S5). Moreover, the pooled prevalence of overdose use for DOACs was 5% (95% CI, 3–7%; I2, 99.8%), with the lowest prevalence found in apixaban (2%; 95% CI, 1–3%; I2, 97.9%). The overdose rates were observed 5% for dabigatran, 7% for rivaroxaban, and 9% for edoxaban (Supplementary eFigures S2–S5).
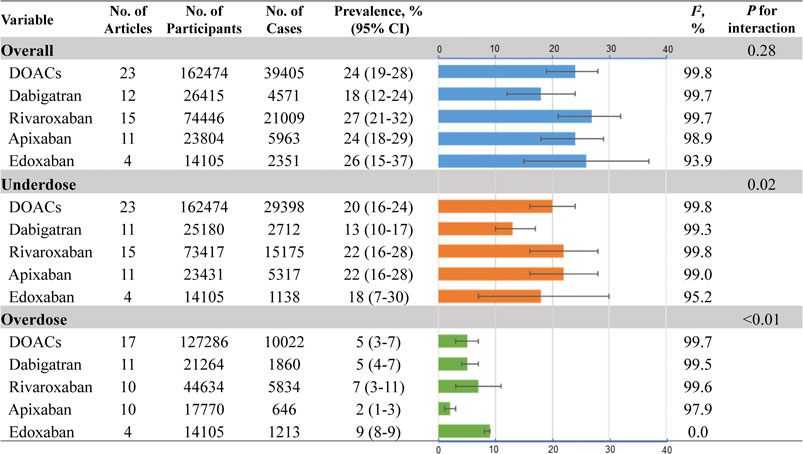
FIGURE 2. Pooled prevalence of DOAC off-label doses No.: number of included studies; DOACs: direct oral anticoagulants.
Figure 3 gives the regional picture of DOAC off-label doses in AF. The prevalence in Asia (32%; 95% CI, 28–36%; I2, 98.6%) was higher than that in Europe (22%; 95% CI, 17–27%; I2, 99.4%) and North America (14%; 95% CI, 6–21%; I2, 99.9%; Pinteraction<0.01) (Supplementary eFigures S6–S8). Similar trends were also found in the situation of underdose use [Asia: 31% (95% CI, 26–36%; I2, 99.2%)]; [Europe: 16% (95% CI, 11–21%; I2, 99.6%)]; North America: 9% [95% CI, 6–11%; I2, 99.1%]; Pinteraction<0.01) (Supplementary eFigures S6–S8). For overdose use, no significant difference was detected among different regions, with the prevalence being 4, 6, and 5% for Asia, Europe, and North America, respectively (Supplementary eFigures S6–S8).
Sensitivity analyses confirmed the robustness of primacy results, by removing a single study at one time or excluding studies with off-label definition only based on renal function (Supplementary eTables S7–S9). Meta-regression analyses failed to detect any potential patient characteristics associated with the prevalence of DOAC off-label dose (p > 0.05 for each variable; Supplementary eTable S10).
Majority of funnel plots was relatively symmetrical on visual inspection, with the exception of DOAC underdose (P for Begg’s test = 0.080; P for Begg’s test =0.022) and dabigatran overdose (P for Begg’s test =0.013; P for Begg’s test = 0.025) (Supplementary eFigures S9–S12). The trim and fill method was used to deal with publication bias, resulting in 11% (95% CI, 7–16%) for DOAC underdose and 1% (95% CI, 1–3%) for dabigatran overdose (Supplementary eTable S11). Because of the limited study number in edoxaban (<8 studies), the funnel plot was not performed.
This systematic review and meta-analysis first provided a comprehensive overview on the global prevalence of DOAC off-label dose based on 23 studies involving 162,474 AF patients. The major findings were as follows: (1) 24% of AF patients were treated with DOAC off-label doses in the real world, with the majority being underdosed (20%); (2) higher prevalence of off-label underdose use was found in rivaroxaban (22%) and apixaban (22%) than that of dabigatran (13%); (3) regionally, the prevalence of both off-label doses and underdose use on DOAC was high in Asia (32 and 31%, respectively), which was observed to be relatively lower than in North America (14 and 9%, respectively) and Europe (22 and 16%, respectively).
DOACs are currently the optimal anticoagulation choice for most AF patients (January et al., 2014). According to the clinical guidelines (Lip et al., 2018; Steffel et al., 2018; January et al., 2019), dose adjustment of DOAC should be made based on patient’s characteristics, for example, age, renal function, and body weight. Although the criteria of dose adjustment of each DOAC have been well established, inappropriate off-label dose of DOACs is not uncommon (Nielsen et al., 2017; Ellis et al., 2018; Staerk et al., 2018). In the ORBIT-AF II registry, almost 1 in 8 patients received DOAC doses inconsistent with labeling (Steinberg et al., 2016). In another observational study based on the U.S. claims database, 43.0% of the patients with renal indication for dose reduction were overdosed, while 13.3% of the patients with no renal indication were underdosed (Yao et al., 2017). However, the investigation for the global prevalence rate of appropriate dosage of DOACs in the real-world setting is scarce. Our study found that the overall prevalence of DOAC off-label doses was estimated to be 24% in real-world AF population. Underdose use was predominant, with the prevalence nearly fourfold higher than overdose use.
Actually, a considerable proportion of patients received off-label doses of DOACs according to the preference of clinicians instead of the FDA-approved label (Steinberg et al., 2018). It was reported that patients receiving off-label doses, mainly underdoses, were older (≥75 years), more likely female, with lower body weight (≤60 kg), or with high CHA2DS2-VASc (≥2 scores), than those receiving recommended doses (Cheng et al., 2019; Lee et al., 2019b). Besides, renal dysfunction (CrCl ≤ 50 ml/min), previous stroke, history of bleeding, hypertension, and concomitant use of antiplatelet were also risk factors of the off-label dose of DOACs (Cheng et al., 2019; Lee et al., 2019b). Accordingly, underdosing DOACs are more likely to be prescribed to frail patients. Interestingly, contrary to the above expectation, patients prescribed underdosed DOACs were found to be younger and had lower scores of bleeding risk than those appropriate users (Steinberg et al., 2018). Admittedly, risk factors related to the off-label dose of DOACs need further exploration.
In our results, the prevalence of underdose use in rivaroxaban was relatively high. The data mainly originated from the extensive use of underdosed rivaroxaban in the Asian population, especially in the Japanese population. It is worth noting that 15 mg of rivaroxaban has been approved as a standard dose for patients without renal dysfunction in Japan, based on the J-ROCEKT-AF trial (Hori et al., 2012), which is different from the recommended doses in other countries and regions. Nevertheless, because of the overconsidering high bleeding risk, elderly age, and renal impairment of AF patients (Ikeda et al., 2019), clinicians in Japan still tend to prescribe underdosed rivaroxaban of 10 mg to patients irrespective of the label (Chan et al., 2016; Cha et al., 2017; Kim et al., 2018). Different from rivaroxaban, dabigatran was observed with the lowest prevalence of underdose use in our study. The underlying cause is unclear. It is estimated that the FDA-recommended dose of dabigatran being 150 mg instead of 110 mg according to the RE-LY trial might be one factor attributed to the on-label prescribing pattern (Beasley et al., 2011).
Obviously, regional difference in the prevalence of off-label doses in DOACs was detected in our study, with higher rate value observed in the Asian population than other regions. Many Asian clinicians held deep-rooted opinions of conservative anticoagulation for AF patients since the warfarin era. It is speculated that the higher rate of warfarin-induced hemorrhage, especially intracranial hemorrhage, in Asian patients might be one of the reasons leading to the underdosing behavior (Shen et al., 2007). Therefore, it is understandable that low-dose of DOACs are widely used in Asia in the same way. Based on nationwide database studies of Taiwan and Korea, almost 90% and 40–60% of the total patients were prescribed underdose DOACs, respectively (Cha et al., 2017; Kim et al., 2018). Furthermore, 38% of Korean AF patients were considered as DOAC off-label underdose in a cohort study (Lee et al., 2019a). Conversely, the lowest prevalence of underdose use was observed in North America with the estimated rate of 9%, while the estimated rate remains relatively high of 16% in Europe.
Inappropriate use of DOACs in AF patients might result in poor clinical consequences. Patients with underdose DOACs could not receive the benefits of recommended dose in preventing stroke and systemic embolism. On the contrary, patients might suffer higher bleeding risk when taking overdose DOACs. Data from the ORBIT-AF-II registry revealed that patients taking underdose DOACs had less favorable outcomes in terms of thromboembolic events and death (Saunders et al., 2019). Similarly, higher risk of ischemic stroke without risk reduction in intracranial bleeding was detected in patients with underdose DOACs (Cheng et al., 2019). Meanwhile, a registry in Japan (SAKURA AF Registry) suggested that overdose users were at higher risk for all clinical events, including thromboembolism, major bleeding, and all-cause death, and needed careful follow-up (Murata et al., 2019). Therefore, to ensure the effectiveness and safety of anticoagulation, the off-label dose of DOACs should be cautious in the real-world setting. The awareness of appropriate use should be strengthened in clinicians who could prescribe anticoagulants.
According to this study, two kinds of DOAC off-label doses, including overdose and underdose, were commonly found in the clinical practice. Differences in DOAC off-label doses were also detected in different DOACs and different regions. Once understanding the current situation, we realize that it would be significant to further investigate the possible influencing factors and the possible clinical outcomes of DOAC off-label doses in the future studies. Therefore, more evidence would be obtained to determine whether off-label doses of DOACs would be appropriate or not for AF patients.
Strengths of this study mainly included the systematic and rigorous approach to estimate the prevalence of DOAC off-label doses in AF patients. We performed a comprehensive search of databases; applied the revised NOS tool to assess the inclusive study quality; conducted the subgroup analyses by individual DOAC and different regions; performed serial sensitivity analyses to strengthen the robustness of results; used meta-regression to explore the risk factors associated with off-label prevalence; and applied trim and fill method to deal with the potential publication bias. However, several limitations should be noted in this study. First, the definitions of DOAC off-label doses were not completely consistent according to different region-approved dosing criteria. We therefore conducted subgroup analysis by different regions to account for this issue. Second, there is high heterogeneity among the included studies, possibly due to different sample sizes and regions, thus we conducted sensitivity analyses and subgroup analyses, and used random-effects model to pool prevalence estimates. Third, due to the lack of participants’ characteristics, the subgroup analysis by age stratification was not performed. Similarly, due to the lack of studies, we did not assess the off-label prevalence in other regions except for Asia, Europe, and North America.
In this meta-analysis, the overall prevalence of DOAC off-label doses was 24% in AF patients, with underdose being predominant (20%). Clinicians in Asia preferred to prescribe underdose of DOACs (31%) to AF patients. More evidence about the appropriateness of DOAC off-label doses in AF patients is urgently needed. Education programs concerning the appropriate prescription of DOACs within the drug labels and accepted guidelines are necessary to DOAC prescribers to ensure the safety and effectiveness of anticoagulation therapy for patients with AF.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Z-CG is the guarantor of the entire manuscript. Z-CG, N-NS, and CZ contributed to the study conception and design, critical revision of the manuscript for important intellectual content, and final approval of the version to be published. YH, ZL, L-CK, and NW contributed to the data acquisition, analysis, and interpretation.
This study was supported by the Program of General Scientific Project of Zhejiang Education Department (Y201941020), Research Funds of Shanghai Health and Family Planning Commission (20184Y0022), Cultivation Fund of Clinical Research of Renji Hospital (PY2018-III-06), Clinical Pharmacy Innovation Research Institute of Shanghai Jiao Tong University School of Medicine (CXYJY2019ZD001), and Shanghai “Rising Stars of Medical Talent” Youth Development Program—Youth Medical Talents—Clinical Pharmacist Program (SHWJRS (2019) _072).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.581293/full#supplementary-material
Başaran, Ö., Dogan, V., Beton, O., Tekinalp, M., Aykan, A. C., Kalaycioğlu, E., et al. (2016). Suboptimal Use of Non-vitamin K Antagonist Oral Anticoagulants: Results from the RAMSES Study. Medicine (Baltimore) 95 (35), e4672. doi:10.1097/md.0000000000004672
Beasley, B. N., Unger, E. F., and Temple, R. (2011). Anticoagulant Options - Why the FDA Approved a Higher but Not a Lower Dose of Dabigatran. N. Engl. J. Med. 364 (19), 1788–1790. doi:10.1056/NEJMp1103050
Bell, A. D., Gross, P., Heffernan, M., Deschaintre, Y., Roux, J.-F., Purdham, D. M., et al. (2016). Appropriate Use of Antithrombotic Medication in Canadian Patients With Nonvalvular Atrial Fibrillation. Am. J. Cardiol. 117 (7), 1107–1111. doi:10.1016/j.amjcard.2015.12.055
Briasoulis, A., Gao, Y., Inampudi, C., Alvarez, P., Asleh, R., Chrischilles, E., et al. (2020). Characteristics and Outcomes in Patients with Atrial Fibrillation Receiving Direct Oral Anticoagulants in Off-Label Doses. BMC Cardiovasc. Disord. 20 (1), 42. doi:10.1055/a-0952-638510.1186/s12872-020-01340-4
Camm, A. J., Lip, G. Y., De Caterina, R., Savelieva, I., Atar, D., Hohnloser, S. H., et al. (2012). 2012 Focused Update of the ESC Guidelines for the Management of Atrial Fibrillation: an Update of the 2010 ESC Guidelines for the Management of Atrial Fibrillation. Developed with the Special Contribution of the European Heart Rhythm Association. Eur. Heart J. 33 (21), 2719–2747. doi:10.1093/eurheartj/ehs253
Cha, M.-J., Choi, E.-K., Han, K.-D., Lee, S.-R., Lim, W.-H., Oh, S., et al. (2017). Effectiveness and Safety of Non-Vitamin K Antagonist Oral Anticoagulants in Asian Patients With Atrial Fibrillation. Stroke 48 (11), 3040–3048. doi:10.1161/STROKEAHA.117.018773
Chan, Y.-H., Kuo, C.-T., Yeh, Y.-H., Chang, S.-H., Wu, L.-S., Lee, H.-F., et al. (2016). Thromboembolic, Bleeding, and Mortality Risks of Rivaroxaban and Dabigatran in Asians With Nonvalvular Atrial Fibrillation. J. Am. Coll. Cardiol. 68 (13), 1389–1401. doi:10.1016/j.jacc.2016.06.062
Cheng, W.-H., Chao, T.-F., Lin, Y.-J., Chang, S.-L., Lo, L.-W., Hu, Y.-F., et al. (2019). Low-Dose Rivaroxaban and Risks of Adverse Events in Patients With Atrial Fibrillation. Stroke 50 (9), 2574–2577. doi:10.1002/joa3.1218410.1161/strokeaha.119.025623
Cohen, A. T., Hill, N. R., Luo, X., Masseria, C., Abariga, S. A., and Ashaye, A. O. (2018). A Systematic Review of Network Meta-Analyses Among Patients with Nonvalvular Atrial Fibrillation: A Comparison of Efficacy and Safety Following Treatment with Direct Oral Anticoagulants. Int. J. Cardiol. 269, 174–181. doi:10.1016/j.ijcard.2018.06.114
Cohen, D. (2014). Dabigatran: How the Drug Company Withheld Important Analyses. BMJ 349, g4670. doi:10.1136/bmj.g4670
Connolly, S. J., Ezekowitz, M. D., Yusuf, S., Eikelboom, J., Oldgren, J., Parekh, A., et al. (2009). Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 361 (12), 1139–1151. doi:10.1056/NEJMoa0905561
Cota, G. F., de Sousa, M. R., Fereguetti, T. O., and Rabello, A. (2013). Efficacy of Anti-leishmania Therapy in Visceral Leishmaniasis Among HIV Infected Patients: a Systematic Review with Indirect Comparison. Plos Negl. Trop. Dis. 7 (5), e2195. doi:10.1371/journal.pntd.0002195
De Caterina, R., Kelly, P., Kelly, P., Monteiro, P., Deharo, J. C., de Asmundis, C., et al. (2019). Characteristics of Patients Initiated on Edoxaban in Europe: Baseline Data from Edoxaban Treatment in Routine Clinical Practice for Patients with Atrial Fibrillation (AF) in Europe (ETNA-AF-Europe). BMC Cardiovasc. Disord. 19 (1), 165. doi:10.1186/s12872-019-1144-x
Draper, E., Parkhurst, B., Carley, B., Krueger, K., Larson, T., and Griesbach, S. (2017). Comparison of Prescribing Practices with Direct Acting Oral Anticoagulant Protocols. Am. J. Cardiovasc. Drugs 17 (6), 475–479. doi:10.1007/s40256-017-0243-2
Duval, S., and Tweedie, R. (2000). Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 56 (2), 455–463. doi:10.1111/j.0006-341x.2000.00455.x
Ellis, M. H., Dotan, S. G., Hammerman, A., Battat, E., Derazne, E., and Avnery, O. (2018). Appropriateness of Non-vitamin K Antagonist Oral Anticoagulant Dose in Patients with Atrial Fibrillation in Israel: A Population-Based Study. Thromb. Res. 169, 140–142. doi:10.1016/j.thromres.2018.07.024
Falissard, B., Picard, F., Mahe, I., Hanon, O., Touze, E., Danchin, N., et al. (2019). Apixaban for Prevention of Stroke and Systemic Embolism in Patients with Non-valvular Atrial Fibrillation in France: The PAROS Cross-Sectional Study of Routine Clinical Practice. JAMA Cardiol. 112 (6-7), 400–409. doi:10.1001/jamacardio.2019.104910.1016/j.acvd.2019.02.003
García Rodríguez, L. A., Martín-Pérez, M., Vora, P., Roberts, L., Balabanova, Y., Brobert, G., et al. (2019a). Appropriateness of Initial Dose of Non-vitamin K Antagonist Oral Anticoagulants in Patients with Non-valvular Atrial Fibrillation in the UK. BMJ Open 9 (9), e031341. doi:10.1136/bmjopen-2019-031341
Garcia Rodriguez, L. A., Martin-Perez, M., Vora, P., Roberts, L., Balabanova, Y., Brobert, G., et al. (2019b). Appropriateness of Initial Dose of Non-vitamin K Antagonist Oral Anticoagulants in Patients with Non-valvular Atrial Fibrillation in the UK. Intern. Med. J. 9 (9), e031341. doi:10.1111/imj.1464010.1136/bmjopen-2019-031341
Giugliano, R. P., Ruff, C. T., Braunwald, E., Murphy, S. A., Wiviott, S. D., Halperin, J. L., et al. (2013). Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 369 (22), 2093–2104. doi:10.1056/NEJMoa1310907
Godman, B., Malmstrom, R. E., Diogene, E., Jayathissa, S., McTaggart, S., Cars, T., et al. (2014). Dabigatran - a Continuing Exemplar Case History Demonstrating the Need for Comprehensive Models to Optimize the Utilization of New Drugs. Front. Pharmacol. 5, 109. doi:10.3389/fphar.2014.00109
Granger, C. B., Alexander, J. H., McMurray, J. J. V., Lopes, R. D., Hylek, E. M., Hanna, M., et al. (2011). Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 365 (11), 981–992. doi:10.1056/NEJMoa1107039
Hori, M., Matsumoto, M., Tanahashi, N., Momomura, S.-i., Uchiyama, S., Goto, S., et al. (2012). Rivaroxaban vs. Warfarin in Japanese Patients With Atrial Fibrillation. Circ. J. 76 (9), 2104–2111. doi:10.1253/circj.cj-12-0454
Ikeda, T., Ogawa, S., Kitazono, T., Nakagawara, J., Minematsu, K., Miyamoto, S., et al. (2019). Outcomes Associated with Under-dosing of Rivaroxaban for Management of Non-valvular Atrial Fibrillation in Real-World Japanese Clinical Settings. J. Thromb. Thrombolysis 48 (4), 653–660. doi:10.1007/s11239-019-01934-6
Inoue, H., Umeyama, M., Yamada, T., Hashimoto, H., Komoto, A., and Yasaka, M. (2020). Safety and Effectiveness of Reduced-Dose Apixaban in Japanese Patients with Nonvalvular Atrial Fibrillation in Clinical Practice: A Sub-analysis of the STANDARD Study. J. Cardiol. 75 (2), 208–215. doi:10.1016/j.jjcc.2019.07.007
Jacobs, M. S., van Hulst, M., Campmans, Z., and Tieleman, R. G. (2019). Inappropriate Non-vitamin K Antagonist Oral Anticoagulants Prescriptions: Be Cautious with Dose Reductions. Neth. Heart J. 27 (7-8), 371–377. doi:10.1007/s12471-019-1267-9
January, C. T., Wann, L. S., Alpert, J. S., Calkins, H., Cigarroa, J. E., Cleveland, J. C., et al. (2014). 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 64 (21), e1–e76. doi:10.1016/j.jacc.2014.03.022
January, C. T., Wann, L. S., Calkins, H., Chen, L. Y., Cigarroa, J. E., Cleveland, J. C., et al. (2019). 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 74 (1), 104–132. doi:10.1016/j.jacc.2019.01.011
Kakkar, A. K., Mueller, I., Bassand, J.-P., Fitzmaurice, D. A., Goldhaber, S. Z., Goto, S., et al. (2013). Risk Profiles and Antithrombotic Treatment of Patients Newly Diagnosed with Atrial Fibrillation at Risk of Stroke: Perspectives from the International, Observational, Prospective GARFIELD Registry. PLoS One 8 (5), e63479. doi:10.1371/journal.pone.0063479
Kim, Y. H., Shim, J., Tsai, C. T., Wang, C. C., Vilela, G., Muengtaweepongsa, S., et al. (2018). XANAP: A Real‐world, Prospective, Observational Study of Patients Treated with Rivaroxaban for Stroke Prevention in Atrial Fibrillation in Asia. J. Arrhythmia 34 (4), 418–427. doi:10.1002/joa3.12073
Krittayaphong, R., Winijkul, A., Kunjara-Na-Ayudhya, R., Apiyasawat, S., Siriwattana, K., Kanjanarutjawiwat, W., et al. (2020). Adherence to Anticoagulant Guideline for Atrial Fibrillation Improves Outcomes in Asian Population. Stroke 51 (6), 1772–1780. doi:10.1161/STROKEAHA.120.029295
Lee, K.-N., Choi, J.-I., Boo, K. Y., Kim, D. Y., Kim, Y. G., Oh, S.-K., et al. (2020). Effectiveness and Safety of Off-Label Dosing of Non-vitamin K Antagonist Anticoagulant for Atrial Fibrillation in Asian Patients. Sci. Rep. 10 (1), 1801. doi:10.1038/s41598-020-58665-5
Lee, S.-R., Choi, E.-K., Han, K.-D., Jung, J.-H., Oh, S., and Lip, G. Y. H. (2019a). Optimal Rivaroxaban Dose in Asian Patients With Atrial Fibrillation and Normal or Mildly Impaired Renal Function. Stroke 50 (5), 1140–1148. doi:10.1161/strokeaha.118.024210
Lee, S.-R., Lee, Y. S., Park, J.-S., Cha, M.-J., Kim, T.-H., Park, J., et al. (2019b). Label Adherence for Non-Vitamin K Antagonist Oral Anticoagulants in a Prospective Cohort of Asian Patients with Atrial Fibrillation. Yonsei Med. J. 60 (3), 277–284. doi:10.3349/ymj.2019.60.3.277
Leef, G. C., Perino, A. C., Askari, M., Fan, J., Ho, P. M., Olivier, C. B., et al. (2019). Appropriateness of Direct Oral Anticoagulant Dosing in Patients With Atrial Fibrillation: Insights From the Veterans Health Administration. J. Pharm. Pract. 33, 647–653. doi:10.5603/KP.a2019.0033
Lehr, T., Haertter, S., Liesenfeld, K.-H., Staab, A., Clemens, A., Reilly, P. A., et al. (2012). Dabigatran Etexilate in Atrial Fibrillation Patients with Severe Renal Impairment: Dose Identification Using Pharmacokinetic Modeling and Simulation. J. Clin. Pharmacol. 52 (9), 1373–1378. doi:10.1177/0091270011417716
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P. A., et al. (2009). The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Health Care Interventions: Explanation and Elaboration. J. Clin. Epidemiol. 62 (10), e1–e34. doi:10.1016/j.jclinepi.2009.06.006
Lip, G. Y. H., Banerjee, A., Boriani, G., Chiang, C. e., Fargo, R., Freedman, B., et al. (2018). Antithrombotic Therapy for Atrial Fibrillation. Chest 154 (5), 1121–1201. doi:10.1016/j.chest.2018.07.040
Malmström, R. E., Godman, B. B., Diogene, E., Baumgärtel, C., Bennie, M., Bishop, I., et al. (2013). Dabigatran - a Case History Demonstrating the Need for Comprehensive Approaches to Optimize the Use of New Drugs. Front. Pharmacol. 4, 39. doi:10.3389/fphar.2013.00039
Martin, J. L., Esmaeili, H., Manuel, R. C., Petrini, M., Wiebe, S., and Maas, H. (2018). Pharmacokinetics/Pharmacodynamics of Dabigatran 75 Mg Twice Daily in Patients With Nonvalvular Atrial Fibrillation and Severely Impaired Renal Function. J. Cardiovasc. Pharmacol. Ther. 23 (5), 399–406. doi:10.1177/1074248418769167
McAlister, F. A., Garrison, S., Kosowan, L., Ezekowitz, J. A., and Singer, A. (2018). Use of Direct Oral Anticoagulants in Canadian Primary Care Practice 2010-2015: A Cohort Study From the Canadian Primary Care Sentinel Surveillance Network. J. Am. Heart Assoc. 7 (3). doi:10.1161/jaha.117.007603
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. Ann. Intern. Med. 151 (4), 264–269. doi:10.7326/0003-4819-151-4-200908180-00135
Morris, R., Sergienko, R., Hammerman, A., Greenberg-Dotan, S., Batat, E., Avnery, O., et al. (2019). Effectiveness and Safety of Off-Label Dose-Reduced Direct Oral Anticoagulants in Atrial Fibrillation. Am. J. Med. 132 (7), 847–855. doi:10.1111/bjh.1580810.1016/j.amjmed.2019.01.025
Murata, N., Okumura, Y., Yokoyama, K., Matsumoto, N., Tachibana, E., Kuronuma, K., et al. (2019). Clinical Outcomes of Off-Label Dosing of Direct Oral Anticoagulant Therapy Among Japanese Patients With Atrial Fibrillation Identified From the SAKURA AF Registry. Circ. J. 83 (4), 727–735. doi:10.1253/circj.cj-18-0991
Navarro-Almenzar, B., Cerezo-Manchado, J. J., Caro-Martinez, C., García-Candel, F., Flores Blanco, P. J., Ruiz, G. E., et al. (2019). Real-life Behaviour of Direct Oral Anticoagulants in a Spanish Cohort with Non-valvular Atrial Fibrillation: Refase Registry. Curr. Med. Res. Opin. 35 (12), 2035–2041. doi:10.1016/j.jjcc.2019.06.00210.1080/03007995.2019.1647735
Nielsen, P. B., Skjøth, F., Søgaard, M., Kjældgaard, J. N., Lip, G. Y. H., and Larsen, T. B. (2017). Effectiveness and Safety of Reduced Dose Non-vitamin K Antagonist Oral Anticoagulants and Warfarin in Patients with Atrial Fibrillation: Propensity Weighted Nationwide Cohort Study. Bmj 356, j510. doi:10.1136/bmj.j510
Patel, M. R., Mahaffey, K. W., Garg, J., Pan, G., Singer, D. E., Hacke, W., et al. (2011). Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 365 (10), 883–891. doi:10.1056/NEJMoa1009638
Saunders, J. A., Gustafson, W. L., Vazquez, S. R., Jones, A. E., and Witt, D. M. (2019). Real-world Assessment of Off-Label Direct Oral Anticoagulant Dosing for Venous Thromboembolism. J. Thromb. Thrombolysis 48 (3), 506–510. doi:10.1007/s11239-019-01904-y
Shen, A. Y.-J., Yao, J. F., Brar, S. S., Jorgensen, M. B., and Chen, W. (2007). Racial/ethnic Differences in the Risk of Intracranial Hemorrhage Among Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 50 (4), 309–315. doi:10.1016/j.jacc.2007.01.098
Staerk, L., Gerds, T. A., Lip, G. Y. H., Ozenne, B., Bonde, A. N., Lamberts, M., et al. (2018). Standard and Reduced Doses of Dabigatran, Rivaroxaban and Apixaban for Stroke Prevention in Atrial Fibrillation: a Nationwide Cohort Study. J. Intern. Med. 283 (1), 45–55. doi:10.1111/joim.12683
Steffel, J., Verhamme, P., Potpara, T. S., Albaladejo, P., Antz, M., Desteghe, L., et al. (2018). The 2018 European Heart Rhythm Association Practical Guide on the Use of Non-vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Eur. Heart J. 39 (16), 1330–1393. doi:10.1093/eurheartj/ehy136
Steinberg, B. A., Shrader, P., Thomas, L., Ansell, J., Fonarow, G. C., Gersh, B. J., et al. (2016). Off-Label Dosing of Non-Vitamin K Antagonist Oral Anticoagulants and Adverse Outcomes. J. Am. Coll. Cardiol. 68 (24), 2597–2604. doi:10.1016/j.jacc.2016.09.966
Steinberg, B. A., Shrader, P., Pieper, K., Thomas, L., Allen, L. A., Ansell, J., et al. (2018). Frequency and Outcomes of Reduced Dose Non-Vitamin K Antagonist Anticoagulants: Results From ORBIT‐AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II). Jaha 7 (4), e007633. doi:10.1161/JAHA.117.007633
Yamaji, H., Murakami, T., Hina, K., Higashiya, S., Kawamura, H., Murakami, M., et al. (2017). Safety and Efficacy of Underdosing Non-vitamin K Antagonist Oral Anticoagulants in Patients Undergoing Catheter Ablation for Atrial Fibrillation. J. Cardiovasc. Pharmacol. 69 (2), 118–126. doi:10.1097/fjc.0000000000000448
Keywords: atrial fibrillation, direct oral anticoagulants, off-label doses, prevalence, dabigatran, rivaroxaban (Bay-59-7939), apixaban
Citation: Shen N-N, Zhang C, Hang Y, Li Z, Kong L-C, Wang N, Wang J-L and Gu Z-C (2021) Real-World Prevalence of Direct Oral Anticoagulant Off-Label Doses in Atrial Fibrillation: An Epidemiological Meta-Analysis. Front. Pharmacol. 12:581293. doi: 10.3389/fphar.2021.581293
Received: 06 October 2020; Accepted: 04 May 2021;
Published: 26 May 2021.
Edited by:
Michael Thiede, IUBH University of Applied Sciences, GermanyReviewed by:
Yohei Sotomi, Osaka University, JapanCopyright © 2021 Shen, Zhang, Hang, Li, Kong, Wang, Wang and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Chun Gu, Z3V6aGljaHVuMjEzQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.