- 1 Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 2 Department of Pharmacy, The First Affiliated Hospital of University of Science and Technology of China, Hefei, China
- 3 Medical Decision and Economic Group, Department of Pharmacy, Ren Ji Hospital, South Campus, School of Medicine, Shanghai Jiaotong University, Shanghai, China
Purpose: The effectiveness of nivolumab plus ipilimumab for advanced non-small cell lung cancer (NSCLC) has been demonstrated. Decisions have to be made about allocating healthcare resources. Economic evidence could support policy decisions to fund expensive interventions. The current analysis evaluated the cost-effectiveness of nivolumab plus ipilimumab in advanced NSCLC harboring no EGFR or ALK mutations. It is set in the context of the US and China, representing developed and resource-constrained settings, respectively.
Patients and Methods: A Markov model consisting of three discrete health states was used to assess the cost-effectiveness of nivolumab plus ipilimumab vs. chemotherapy. The key clinical data were derived from the CheckMate-227 trial, and the cost and health preference data were derived from the literature. Costs, quality-adjusted life-years (QALYs), incremental cost-effectiveness ratios (ICERs) and incremental net health benefits (INHBs) were calculated for the two strategies. Subgroup, one-way and probabilistic sensitivity analyses were performed.
Results: In the United States, nivolumab plus ipilimumab increased by 1.260 QALYs with an additional cost of $95,617 compared with the features of chemotherapy, which led to an ICER of $75,871 per QALY gained. INHB indicated that nivolumab plus ipilimumab treatment had a 99% probability of being cost-effective at the ICER threshold of $100,000/QALY in all subgroups.
The results of sensitivity analyses revealed that the model outcomes were robust. In China, the ICER of nivolumab plus ipilimumab vs. chemotherapy was $59,773/QALY, and the INHB was -1.972 QALY at the threshold of $27,351/QALY.
Conclusion: Nivolumab plus ipilimumab treatment is a cost-effective option compared with chemotherapy for patients with advanced NSCLC harboring no EGFR or ALK mutations in the United States. However, nivolumab plus ipilimumab is not a preferred option in China.
Introduction
The Global Burden of disease Study revealed that lung cancer is one of the leading causes of non-communicable disease worldwide (The Global Burden of Disease Study, 2019). Approximately 85–90% of lung cancers are non-small-cell lung cancer (NSCLC). Platinum-based chemotherapy has been the standard of care for the first-line treatment of metastatic NSCLC without EGFR or ALK mutations (Griesinger et al., 2019). However, the overall survival (OS) and progression-free survival (PFS) of chemotherapy are unsatisfactory with metastatic NSCLC.
Recently, the use of immune checkpoint inhibitors (ICIs) as a treatment for blocking the programmed cell death one ligand 1 (PD-L1) and programmed cell death 1 (PD-1) pathways has become standard as a replacement for chemotherapy (Zhou et al., 2018; Gubens and Davies, 2019; Peters et al., 2019). Nivolumab, a fully human anti-PD-1 antibody, and ipilimumab, a fully human anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody, are immune checkpoint inhibitors with distinct but complementary mechanisms of action. The phase three CheckMate-227 trial showed that first-line treatment with nivolumab plus ipilimumab prolonged the median overall survival of 3.2 months in comparison with chemotherapy in patients with advanced NSCLC (Hellmann et al., 2019). However, due to the prohibitive cost of implementing nivolumab plus ipilimumab in the first-line setting, the cost-effectiveness of nivolumab plus ipilimumab needs to be evaluated. The present analysis investigated the economic outcomes of implementing nivolumab plus ipilimumab regimens for treating newly diagnosed advanced NSCLC in the first-line setting from the United States third-party payer and Chinese health care perspectives, representing developed and resource-constrained settings, respectively.
Materials and Methods
Model Structure
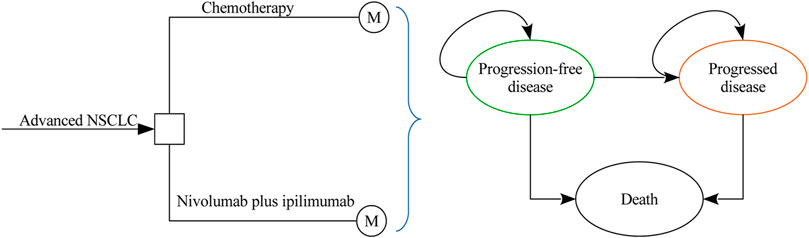
A Markov model was developed to evaluate the costs and health outcomes of treating advanced NSCLC with chemotherapy and nivolumab plus ipilimumab. The model included three discrete health states reflecting different characteristics of the disease: progression-free disease (PFD), progressed disease (PD), and death (Figure 1). The cycle length of the Markov model was one week with a 10 years time horizon, and the initial health state for all of the patients was PFD. The 10 years time horizon was adopted because the long-term survival of patients with advanced NSCLC is still uncertain in current clinical practice. During each one-week cycle, the patients either remained in their assigned health state or progressed to a new health state. The hypothetical patient demographics when entering the model matched those of the patients in the CheckMate-227 trial (Hellmann et al., 2019): previously untreated squamous or nonsquamous stage IV or recurrent NSCLC without EGFR or ALK mutations. Model development and data analysis were performed in the R statistical environment (version 3.4.2; R Development Core Team, Vienna, Austria). The current analysis was carried out from the US third-party payer and Chinese health care perspectives, which means that the two scenarios shared the same clinical and utility inputs except the local cost estimates and life table data.
Clinical Model Inputs
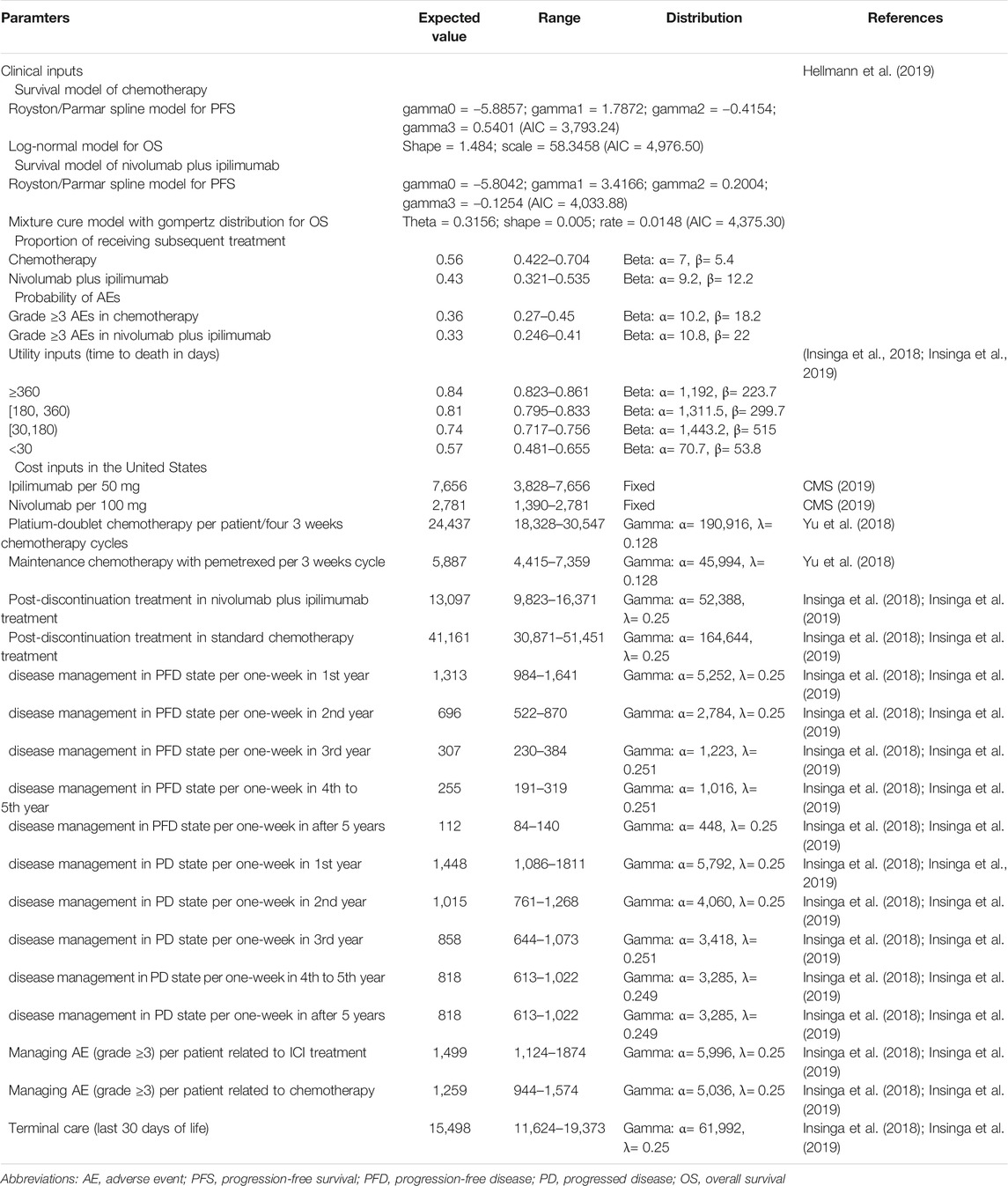
The PFS and OS data for chemotherapy and nivolumab plus ipilimumab were informed by the results of the CheckMate-227 trial (Hellmann et al., 2019). The virtual patient-level data were reconstructed by using standard statistical analyses described by Guyot et al. (2012). The digitized R package (https://github.com/tpoisot/digitize/) was used to gather the data points from the PFS and OS curves, and these data points were then used to fit the following parametric survival functions for exploring the survival probabilities over the model time horizon: Weibull, log-normal, log-logistic, exponential, Gompertz, Royston/Parmar spline model, mixture and non-mixture cure models. The Akaike information criterion was used to measure the goodness of fit. In the full cohort with unknown PD-L1 status, the Royston/Parmar spline and log-logistic models were found to be the most rational models to extrapolate the long-term PFS and OS of chemotherapy, and the Royston/Parmar spline model and the mixture cure model with a Gompertz distribution were used for nivolumab plus ipilimumab, respectively. The model parameters are shown in Table 1. The constructed patient-level data were generated from Kaplan–Meier curves by using event and censor times, which closely matched the reported Kaplan–Meier curves of the CheckMate-227 trial (Hellmann et al., 2019). The PFS and OS Kaplan–Meier graphs generated by using the constructed data and the predicted curves by adopting the selected parametric survival models are presented in Supplementary Appendix Figures S1, S2. By using the fitted PFS and OS parametric survival models, denoted as P(t) and S(t), the transition probability Prob (PFS→PD) and cancer-specific mortality Prob (PD→Death) at cycle t were computed as Prob (PFS→PD) = (P[t] − P[t+1])/P(t) and Prob (PD→Death) = (S[t] − S[t+1])/(S[t] − P[t]), respectively. After the cancer progressed, the proportions of patients who received subsequent active therapy were extracted from the CheckMate-227 trial (Hellmann et al., 2019). By considering long-term survival, all-cause mortality estimated from United States Life Tables (2015) was adopted beyond the observation period of the CheckMate-227 trial (Hellmann et al., 2019). The Chinese lifetable was extracted from the Global Health Observatory data (WHO Life tables, 2020).
Cost and Utility Model Inputs
The current analysis was carried out from Unites States third-party payer and Chinese health care perspectives. Therefore, only direct medical costs were considered in this analysis, including the drug costs, laboratory costs, follow-up costs, adverse event (AE) costs, and costs of end-of-life care. The costs related to healthcare services in the Unites States were inflated to 2018 values based on the Unites States consumer price index (US Department of Labor, 2019). In China, the costs were translated into 2018 Unites States dollars (annual average rate: $1 = CNY 6.8) (National Bureau of Statistics of China, 2019), which were not inflated because the Chinese cost of health remained stable. The Unites States and Chinese cost estimates are shown in Table 1 and Supplementary Appendix Table S1, respectively.
According to the CheckMate-227 trial (Hellmann et al., 2019), nivolumab is given at a dose of 3 mg/kg of body weight every two weeks plus ipilimumab at a dose of 1 mg/kg every six weeks. The assumed mean body weights in the Unites States and China were 70 and 65 kg, respectively (Wu et al., 2017; Wu and Shi, 2020). Treatment continued until disease progression or unacceptable toxicity or, for the immunotherapy regimens, until two years of follow-up (Hellmann et al., 2019). The prices of ipilimumab and nivolumab in the Unites States (average wholesale price) were derived from CMS (CMS, 2019). Because the wholesale price was lower than the retail price (Curtiss et al., 2010), the vial price of nivolumab and ipilimumab was decreased by 17% to account for Unites States contract pricing, as reported in a previous study by Hornberger and others (Hornberger et al., 2015). We also checked its impact in the sensitivity analysis. The cost related to cytotoxic chemotherapy for untreated metastatic NSCLC was $24,437 per patient regardless of histology (Yu et al., 2018. For nonsquamous NSCLC, the cost related to maintenance chemotherapy was $5,887 per three-week chemotherapy cycle (Yu et al., 2018). The costs of chemotherapy infusion in the first hour and additional hour were $148 and $33, and the subsequent infusion per hour was $70 (Insinga et al., 2018; Insinga et al., 2019). The average one-week costs of disease management (excluding drug, drug administration, and AE-related costs) in the PFD and PD states were stratified by survival years following the initiation of first-line treatment (Insinga et al., 2018; Insinga et al., 2019). The average weekly costs of disease management (excluding drug, drug administration, and AE related costs) in the PFD and PD states were estimated from an analysis of 2013 SEER Medicare data for Stage 4 non-squamous NSCLC patients. The costs related to subsequent therapies applied following the discontinuation of initial trial treatments, managing grade ≥3 AEs, disease management, and terminal care during the last 30 days of life were extracted from the literature (Insinga et al., 2018; Insinga et al., 2019). The Chinese cost data were collected from our previous reports (Chai et al., 2020; Li et al., 2020). We assumed that vial wastage is not permitted. This assumption was examined in the sensitivity analysis.
As previous studies have done (Insinga et al., 2018; Insinga et al., 2019), a time-to-death approach, reflecting the decline in cancer patients’ quality of life, was used for modeling utilities. The utility scores for the ≥360, 180 to <360, 30 to <180, and <30 days time-to-death categories were estimated on the basis of EuroQOL-5D (EQ-5D) 3-level utility data (Table 1).
Analysis
The main endpoint in the base-case analysis was the incremental cost-effectiveness ratio (ICER), which was estimated as the incremental cost per additional quality-adjusted life-year (QALY) gained between the two alternatives. Cost and QALYs were discounted at an annual rate of 3% in the United States and 5% in China (Sanders et al., 2016; 2011), respectively. We also estimated the incremental net-health benefit (INHB) based on the following formula: INHB(λ) = (μE1 - μE0) - (μC1- μC0)/λ = ΔE - ΔC/λ, where μCi and μEi are the cost and effectiveness of the new option (i = 1) or old option (i = 0), respectively, and λ is the willingness-to-pay threshold in the United States ($100,000/QALY) and China ($27,351/QALY) (Craig and Black, 2001; Stinnett and Mullahy, 1998; 2011), respectively. Subgroup analyses were conducted in the subgroups as implied in the CheckMate-227 trial, where the INHBs of nivolumab plus ipilimumab vs. chemotherapy were calculated at the lower, mean and upper estimates of the HRs of OS. In the subgroup analysis, we assumed that only the HRs of OS changed.
One-way sensitivity analyses were performed where varied values of each parameter within its range at a specific time were used to examine the effect of these parameters on the ICER. The ranges were derived from the reported or estimated 95% confidence intervals; when reported data were not available, a range of ±25% of the base-case value was used (Table 1). In the one-way sensitivity analysis, we fixed the survival distributions of chemotherapy and adjusted the PFS and OS of the intervention arm by adopting the hazard ratios between nivolumab plus ipilimumab and chemotherapy. Probabilistic sensitivity analyses were carried out where 1,000 Monte Carlo repetitions were generated by sampling all parameters simultaneously during each repetition from the following distributions: gamma distribution for the cost parameters, log-normal distribution for hazard ratios, and beta distribution for the probability, proportion, and preference value parameters. In the probabilistic sensitivity analysis, each parameter in the survival distribution was first sampled based on their expected values, and a variance-covariance matrix was used to calculate the survival probabilities. A cost-effectiveness acceptability curve (CEAC) was constructed, which represents the probability that a strategy is cost-effective compared to the alternative at a range of willingness-to-pay thresholds.
Results
Base-Case and Subgroup Analyses
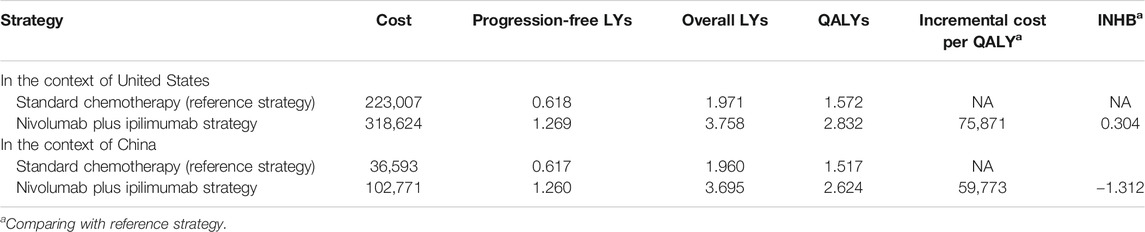
In comparison with chemotherapy, nivolumab plus ipilimumab produced an incremental 1.260 QALYs and 1.787 expected overall life years with an incremental cost of $95,617, which led to an ICER of $75,871/QALY and INHB of 0.304 QALY at the threshold of $100,000/QALY in the United States setting (Table 2). In China, the ICER of nivolumab plus ipilimumab vs. chemotherapy was $59,773/QALY, and the INHB was -1.312 QALY at the threshold of $27,351/QALY.
Subgroup analysis by varying the HRs of OS in the United States found that nivolumab plus ipilimumab presented positive INHBs in all subgroups (Figure 2). The INHBs in the subgroups for the health benefit varied from 0.06 (range: 0.14 to 0.23, probabilities of cost-effectiveness: 62%) in patients with squamous tumors to 0.57 (range: 0.16 to 0.9, probabilities of cost-effectiveness: 100%) in cancer with PD-L1 <1% and tumor mutational burden ≥10 mut/Mb. In the Chinese context, all subgroups resulted in negative INHBs (Supplementary Appendix Figure S3).
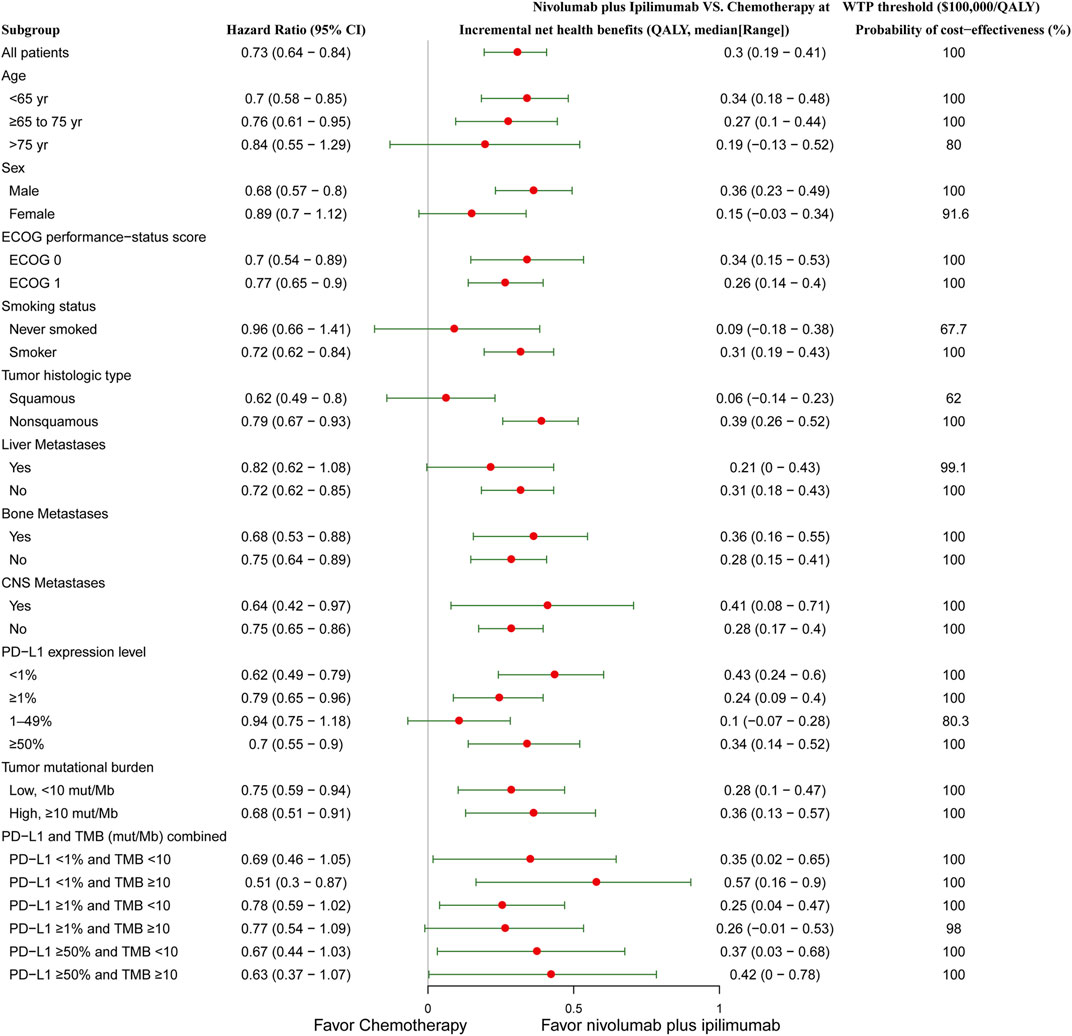
FIGURE 2. Subgroup analysis of incremental net health benefits (INHB) and probabilities of cost-effectiveness by varying the hazard ratios (HRs) of OS in the context of the United States. The vertical line indicates the point of no effect (INHB = 0), the red circle indicates the median INHB, and the green bar indicates the ranges of INHB adjusted by the HRs.
Sensitivity Analyses
The results of the one-way sensitivity analysis are presented in the Tornado diagram (Figure 3), which indicated that the discount of the prices of nivolumab plus ipilimumab treatment played a vital role in model outcomes in the United States. When its values used the lower and upper boundaries, the ICERs of nivolumab plus ipilimumab adjusted from reflected $33,257/QALY to $97,823/QALY respectively. Other considerable parameters that the model was sensitive to included the body weight and the cost of nivolumab and ipilimumab. The rest of the parameters, such as the cost and disutilities associated with managing ADRs, had a medium and small impact on the outcome. In general, the model outcomes were robust to the adjustment of parameters. With the long time horizon, the nivolumab plus ipilimumab therapy would become more cost-effective.
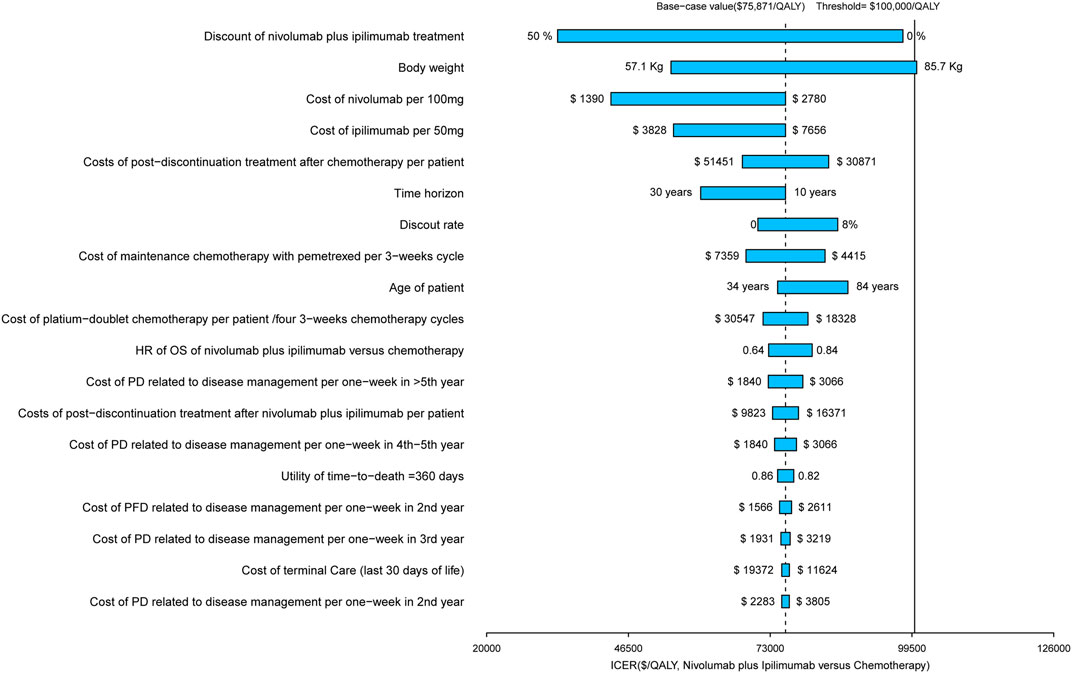
FIGURE 3. Tornado diagram of one-way sensitivity analyses of nivolumab plus ipilimumab vs. chemotherapy in the context of United States.
The CEAC showed a nearly 99% probability of nivolumab plus ipilimumab and a 1% probability of chemotherapy being a cost-effective strategy at the threshold of $100,000/QALY in the United States setting (Figure 4A). However, nivolumab plus ipilimumab achieved only a nearly 1% probability of cost-effectiveness in the Chinese context (Figure 4B).
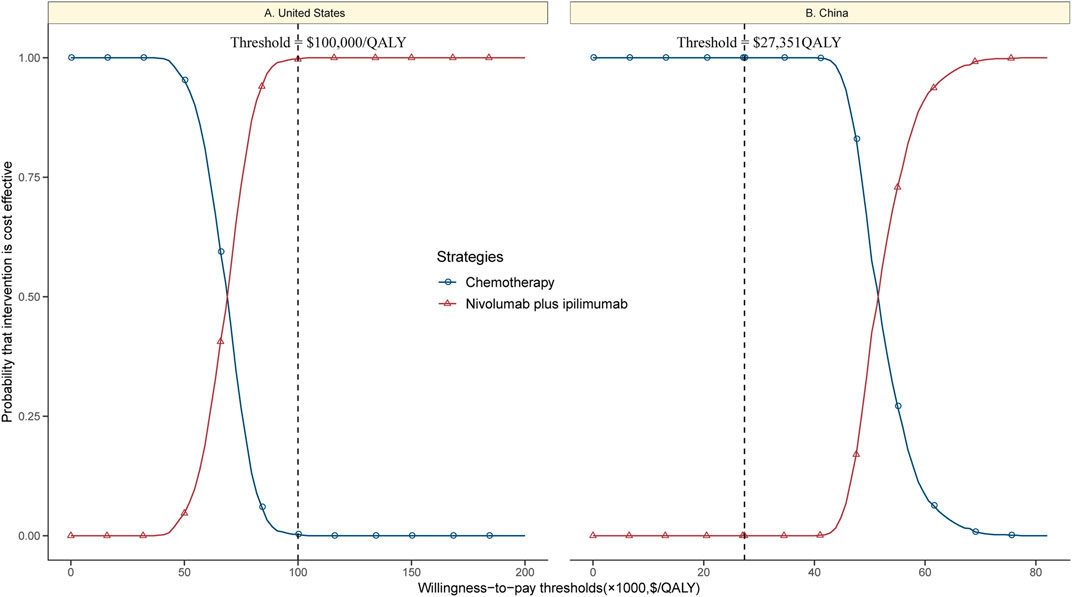
FIGURE 4. Cost-effectiveness acceptability curves of nivolumab plus ipilimumab vs. chemotherapy in the United States (A) and China (B).
In the scenario in which vial wastage was not permitted, the incremental cost of nivolumab plus ipilimumab vs. chemotherapy was $187,137, which led to an ICER of $148,491/QALY.
Discussion
While oncologists and patients are interested in the clinical benefit of nivolumab plus ipilimumab in the CheckMate-227 trial due to the increasing incidence of lung cancer, the high cost of an anticancer drug can limit its widespread use. Health policymakers and payers assess the economic outcomes of expensive drugs to ensure the ability of patients to access the drug and its sustainability for both national healthcare and reimbursement systems and pharmaceutical companies (Uyl-De and Lowenberg, 2018). Our study addresses this emergent need for the economic assessment of nivolumab plus ipilimumab. Based on the results of the CheckMate-227 trial, our analysis demonstrated nivolumab plus ipilimumab for advanced NSCLC to be preferred for WTP thresholds higher than $75,871 per QALY.
This result is generally robust, as shown by the results of both the one-way and probabilistic sensitivity analyses. At a threshold of $100,000/QALY, all subgroups were favored for nivolumab plus ipilimumab because of its positive trend of gaining incremental net health benefits compared to chemotherapy. It should be noted that nivolumab plus ipilimumab showed more favorable economic outcomes in non-squamous tumors than in squamous tumors, although immunotherapy had a better trend of prognosis. The potential reason is that maintenance chemotherapy is considered in nonsquamous tumors, which substantially augments the cost of chemotherapy in nonsquamous tumors compared with squamous tumors. The recent two economic analyses showed the opposite results (Hu et al., 2020; Courtney et al., 2021), which might be led by the different gained health outcomes. However, nivolumab plus ipilimumab is not a cost-effective option in the Chinese context because its ICER exceeded the local threshold of $27,351/QALY. A potential reason for this could be the relatively lower costs related to chemotherapy and the higher costs related to nivolumab plus ipilimumab treatment. Based on our estimation for achieving the Chinese cost-effectiveness threshold, a 64% discount of the cost of nivolumab plus ipilimumab in the deterministic sensitivity analysis could push this regimen to be cost-effective in the Chinese context.
The findings of the one-way sensitivity analysis indicated that body weight is a substantial model input because nivolumab and ipilimumab are administered based on body weight. This result suggested that nivolumab plus ipilimumab would become unfavorable in overweight patients because more nivolumab and ipilimumab would be needed. Because significant wastage has been associated with body size dosing of monoclonal antibodies, dosing strategies without compromising exposure and efficacy should be adopted to reduce the wastage (Ogungbenro et al., 2018). This finding could also be supported by the costs of nivolumab and ipilimumab, which were also found to be two important influential factors. When the unit costs of nivolumab and ipilimumab are discounted by 50%, the ICER for nivolumab plus ipilimumab would be lower than $50,000/QALY in the United States. To help bring down their relatively high prices, the United States and Chinese governments have considered referencing the prices in other countries (Dyer, 2018). Once it is enacted or implemented, this initiative might lead to a reduction in the prices of nivolumab and ipilimumab and achieve more favorable economic outcomes.
This study has several strengths. First, to the best of our knowledge, this is the first study to assess the cost-effectiveness of nivolumab plus ipilimumab therapy in advanced NSCLC by incorporating the latest clinical data through a modeling technique. Monotherapy blockade of PD-1 or such treatment in combination with chemotherapy is becoming popular in advanced NSCLC. However, the economic outcome of the ICI combination of an anti-PD-1 antibody and a CTLA-4 antibody for advanced NSCLC is death. Second, the current analysis examined the economic outcomes of 29 subgroups prespecified by the CheckMate-227 trial. Economic information on each of the subgroups would be helpful for physicians and patients when they have to make a treatment decision the patient will be covering out of pocket. Third, the current analysis examined the economic outcomes in both the United States and China, which are representative of high-income and middle-income countries, respectively. Our findings could be transferred to other high-income and middle-income regions.
There are several limitations in this analysis. First, due to the absence of a head-to-head study, we did not include other ICIs as first-line treatments, such as pembrolizumab and atezolizumab, which have shown favorable health benefits as first-line treatments in combination with chemotherapy or monotherapy (Reck et al., 2016; Mok et al., 2019; Reck et al., 2019; West et al., 2019). The present study needs to be revised when direct comparison data becomes available. Second, health outcomes beyond the observation time of the CheckMate-227 study were assumed through the fitting of parametric survival functions to the PFS and OS data of the trial, which could introduce uncertainty into the results, although we validated the predicted and observed survival data. Third, this analysis did not consider the budget impact of using nivolumab and ipilimumab on society. Because nearly 64,901 new NSCLC patients annually would be eligible for 17.9 first-line treatment cycles of ICI treatment (Goldstein et al., 2017), the first-line prescription of nivolumab plus ipilimumab might substantially increase the financial burden on society. Finally, the costs of managing grade 1/2 AEs were not included in this study, which might overestimate the economic results of nivolumab plus ipilimumab. This weakness may not be a major one, as implied by the findings in the one-way sensitivity analysis, which indicated that the costs related to AEs only have a tiny impact.
In summary, this evaluation demonstrated that nivolumab plus ipilimumab was a cost-effective option for patients with advanced NSCLC harboring no EGFR or ALK mutations from a United States payer perspective. However, in a middle-income country, such as China, nivolumab plus ipilimumab should be considered only when its costs can be substantially reduced. These findings might be helpful for physicians and decision-makers.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
BW was involved in the design of the study. AS and XH collected the data and performed the economic analysis. BW and XH wrote the first draft of the manuscript, which was critically revised by BW.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.573852/full#supplementary-material
References
Chai, Q., Shen, Y., Du, J., Zhu, J., and Wu, B. (2020). Economic burden of Patients with Advanced Non-small-cell Lung Cancer Receiving Nivolumab versus Chemotherapy in China. Immunotherapy 12 (4), 245–254. doi:10.2217/imt-2020-0030
CMS: Centers for Medicare & Medicaid Services (2019). ASP Drug Pricing Files. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2019ASPFiles (Accessed October 30, 2019).
Courtney, P. T., Yip, A. T., Cherry, D. R., Salans, M. A., Kumar, A., and Murphy, J. D. (2021). Cost-effectiveness of Nivolumab-Ipilimumab Combination Therapy for the Treatment of Advanced Non-small Cell Lung Cancer. JAMA Netw. Open 4 (5), e218787. doi:10.1001/jamanetworkopen.2021.8787
Craig, B. A., and Black, M. A. (2001). Incremental Cost-Effectiveness Ratio and Incremental Net-Health Benefit: Two Sides of the Same coin. Expert Rev. Pharmacoeconomics Outcomes Res. 1 (1), 37–46. doi:10.1586/14737167.1.1.37
Curtiss, F. R., Lettrich, P., and Fairman, K. A. (2010). What Is the price Benchmark to Replace Average Wholesale price (AWP)? J. Manag. Care.Pharm. 16 (7), 492–501. doi:10.18553/jmcp.2010.16.7.492
Dyer, O. (2018). US Drug Prices Should Be Tied to Foreign Prices to Tackle "global Freeloading," Says Trump. BMJ 363, k4542. doi:10.1136/bmj.k4542
Goldstein, D. A., Gordon, N., Davidescu, M., Leshno, M., Steuer, C. E., Patel, N., et al. (2017). A Phamacoeconomic Analysis of Personalized Dosing vs Fixed Dosing of Pembrolizumab in Firstline PD-L1-Positive Non-small Cell Lung Cancer. JNCI: J. Natl. Cancer Inst. 109 (11), 1–6. doi:10.1093/jnci/djx063
Griesinger, F., Korol, E. E., Kayaniyil, S., Varol, N., Ebner, T., and Goring, S. M. (2019). Efficacy and Safety of First-Line Carboplatin-Versus Cisplatin-Based Chemotherapy for Non-small Cell Lung Cancer: A Meta-Analysis. Lung Cancer 135, 196–204. doi:10.1016/j.lungcan.2019.07.010
Gubens, M. A., and Davies, M. (2019). NCCN Guidelines Updates: New Immunotherapy Strategies for Improving Outcomes in Non-small Cell Lung Cancer. J. Natl. Compr. Canc Netw. 17 (5.5), 574–578. doi:10.6004/jnccn.2019.5005
Guyot, P., Ades, A., Ouwens, M. J., and Welton, N. J. (2012). Enhanced Secondary Analysis of Survival Data: Reconstructing the Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 12, 9. doi:10.1186/1471-2288-12-9
Hellmann, M. D., Paz-Ares, L., Bernabe Caro, R., Zurawski, B., Kim, S.-W., Carcereny Costa, E., et al. (2019). Nivolumab Plus Ipilimumab in Advanced Non-small-cell Lung Cancer. N. Engl. J. Med. 381 (21), 2020–2031. doi:10.1056/NEJMoa1910231
Hornberger, J., Hirsch, F. R., Li, Q., and Page, R. D. (2015). Outcome and Economic Implications of Proteomic Test-Guided Second- or Third-Line Treatment for Advanced Non-small Cell Lung Cancer: Extended Analysis of the PROSE Trial. Lung Cancer 88 (2), 223–230. doi:10.1016/j.lungcan.2015.03.006
Hu, H., She, L., Liao, M., Shi, Y., Yao, L., Ding, D., et al. (2020). Cost-Effectiveness Analysis of Nivolumab Plus Ipilimumab vs. Chemotherapy as First-Line Therapy in Advanced Non-small Cell Lung Cancer. Front. Oncol. 10, 1649. doi:10.3389/fonc.2020.01649
Insinga, R. P., Vanness, D. J., Feliciano, J. L., Vandormael, K., Traore, S., and Burke, T. (2018). Cost-effectiveness of Pembrolizumab in Combination with Chemotherapy in the 1st Line Treatment of Non-squamous NSCLC in the US. J. Med. Econ. 21 (12), 1191–1205. doi:10.1080/13696998.2018.1521416
Insinga, R. P., Vanness, D. J., Feliciano, J. L., Vandormael, K., Traore, S., Ejzykowicz, F., et al. (2019). Cost-effectiveness of Pembrolizumab in Combination with Chemotherapy versus Chemotherapy and Pembrolizumab Monotherapy in the First-Line Treatment of Squamous Non-small-cell Lung Cancer in the US. Curr. Med. Res. Opin. 35 (7), 1241–1256. doi:10.1080/03007995.2019.1571297
Li, H., Lai, L., and Wu, B. (2020). Cost Effectiveness of Ceritinib and Alectinib versus Crizotinib in First-Line Anaplastic Lymphoma Kinase-Positive Advanced Non-small-cell Lung Cancer. Clin. Drug Investig. 40 (2), 183–189. doi:10.1007/s40261-019-00880-8
Mok, T. S. K., Wu, Y., Kudaba, I., Kowalski, D. M., Cho, B. C., Turna, H. Z., et al. (2019). Pembrolizumab versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-small-cell Lung Cancer (KEYNOTE-042): a Randomised, Open-Label, Controlled, Phase 3 Trial. The Lancet 393 (10183), 1819–1830. doi:10.1016/S0140-6736(18)32409-7
National Bureau of Statistics of China (2019). Exchange Rate Data. Available at: http://www.stats.gov.cn/english/ (Accessed June 5, 2019).
Ogungbenro, K., Patel, A., Duncombe, R., Nuttall, R., Clark, J., and Lorigan, P. (2018). Dose Rationalization of Pembrolizumab and Nivolumab Using Pharmacokinetic Modeling and Simulation and Cost Analysis. Clin. Pharmacol. Ther. 103 (4), 582–590. doi:10.1002/cpt.875
Peters, S., Reck, M., Smit, E. F., Mok, T., and Hellmann, M. D. (2019). How to Make the Best Use of Immunotherapy as First-Line Treatment of Advanced/metastatic Non-small-cell Lung Cancer. Ann. Oncol. 30 (6), 884–896. doi:10.1093/annonc/mdz109
Reck, M., Rodríguez-Abreu, D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A., et al. (2019). Updated Analysis of KEYNOTE-024: Pembrolizumab versus Platinum-Based Chemotherapy for Advanced Non-small-cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 37 (7), 537–546. doi:10.1200/JCO.18.00149
Reck, M., Rodríguez-Abreu, D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A., et al. (2016). Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-small-cell Lung Cancer. N. Engl. J. Med. 375 (19), 1823–1833. doi:10.1056/NEJMoa1606774
Sanders, G. D., Neumann, P. J., Basu, A., Brock, D. W., Feeny, D., Krahn, M., et al. (2016). Recommendations for Conduct, Methodological Practices, and Reporting of Cost-Effectiveness Analyses. JAMA 316 (10), 1093–1103. doi:10.1001/jama.2016.12195
Stinnett, A. A., and Mullahy, J. (1998). Net Health Benefits. Med. Decis. Making 18 (2 Suppl. l), S68–S80. doi:10.1177/0272989X98018002S09
The Global Burden of Disease Study (2019). Regional, and National Disability-Adjusted Life-Years (DALYs) for 359 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990-2017: a Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 392 (10159), 1859–1922. doi:10.1016/S0140-6736(18)32335-3
US Department of Labor (2019). Calculators. Available at: https://www.bls.gov/data/inflation_calculator.htm (Accessed January 5, 2019).
Uyl-De, G. C., and Lowenberg, B. (2018). Sustainability and Affordability of Cancer Drugs: a Novel Pricing Model. Nat. Rev. Clin. Oncol. 15 (7), 405–406. doi:10.1038/s41571-018-0027-x
West, H., Mccleod, M., Hussein, M., Morabito, A., Rittmeyer, A., Conter, H. J., et al. (2019). Atezolizumab in Combination with Carboplatin Plus Nab-Paclitaxel Chemotherapy Compared with Chemotherapy Alone as First-Line Treatment for Metastatic Non-squamous Non-small-cell Lung Cancer (IMpower130): a Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 20 (7), 924–937. doi:10.1016/S1470-2045(19)30167-6
WHO Life tables (2020). Global Health Observatory (GHO) Data. Available at: http://www.who.int/gho/mortality_burden_disease/life_tables/life_tables/en/ (Accessed July 26, 2020).
Wu, B., and Shi, L. (2020). FrontlineBRAFTesting-Guided Treatment for Advanced Melanoma in the Era of Immunotherapies. JAMA Dermatol. 156, 1177. doi:10.1001/jamadermatol.2020.2398
Wu, B., Yao, Y., Zhang, K., and Ma, X. (2017). RAS Testing and Cetuximab Treatment for Metastatic Colorectal Cancer: a Cost-Effectiveness Analysis in a Setting with Limited Health Resources. Oncotarget 8 (41), 71164–71172. doi:10.18632/oncotarget.17029
Yu, T. M., Morrison, C., Gold, E. J., Tradonsky, A., and Arnold, R. J. G. (2018). Budget Impact of Next-Generation Sequencing for Molecular Assessment of Advanced Non-small Cell Lung Cancer. Value Health 21 (11), 1278–1285. doi:10.1016/j.jval.2018.04.1372
Zhou, Y., Chen, C., Zhang, X., Fu, S., Xue, C., Ma, Y., et al. (2018). Immune-checkpoint Inhibitor Plus Chemotherapy versus Conventional Chemotherapy for First-Line Treatment in Advanced Non-small Cell Lung Carcinoma: a Systematic Review and Meta-Analysis. J. Immunotherapy Cancer 6 (1), 155. doi:10.1186/s40425-018-0477-9
Keywords: ipilimumab, nivolumab, advanced non-small-cell lung cancer, chemotherapy, cost-effectiveness
Citation: Hao X, Shen A and Wu B (2021) Cost-Effectiveness of Nivolumab Plus Ipilimumab as First-Line Therapy in Advanced Non–small-cell Lung Cancer. Front. Pharmacol. 12:573852. doi: 10.3389/fphar.2021.573852
Received: 15 January 2021; Accepted: 22 June 2021;
Published: 05 July 2021.
Edited by:
Ileana Mardare, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Jacqui Miot, University of the Witwatersrand, South AfricaAdina Turcu-Stiolica, University of Medicine and Pharmacy of Craiova, Romania
Copyright © 2021 Hao, Shen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Wu, c2NpbHdzanR1LXdiQHlhaG9vLmNvbQ==
 Xuezhi Hao1
Xuezhi Hao1 Bin Wu
Bin Wu