
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol. , 01 February 2021
Sec. Drug Metabolism and Transport
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.630500
Effective, safe, and pharmacokinetically suitable drugs are urgently needed to curb the ongoing COVID-19 pandemic. The main protease or 3C-like protease (Mpro or 3CLpro) of SARS-CoV-2 is considered an important target to formulate potent drugs corresponding to its crucial role in virus replication and maturation in addition to its relatively conserved active site. Promising baseline data on the potency and safety of drugs targeting SARS-CoV-2 Mpro are currently available. However, preclinical and clinical data on the pharmacokinetic profiles of these drugs are very limited. This review discusses the potency, safety, and pharmacokinetic profiles of potential inhibitors of SARS-CoV-2 Mpro and forward directions on the development of future studies focusing on COVID-19 therapeutics.
Coronavirus disease 19 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is causing significant social, economic, and political disturbances worldwide. The number of cases is above seventy-nine million with a toll of death surpassing 1.74 million (https://www.worldometers.info/coronavirus/) as of December 24, 2020. The presence of asymptomatic carriers, various modes of transmission, limitation of point-of-care diagnostic facilities especially in resource-limited countries, and lack of globally approved vaccines and antiviral drugs (Cascella et al., 2020; Covid et al., 2020; Mekonnen et al., 2020; Patel et al., 2020; Wang et al., 2020b; Zhang et al., 2020d) are among others worsening the challenge.
Although remdesivir is currently approved by the FDA of the USA for COVID-19 treatment (Beigel et al., 2020), conflicting clinical results have been reported. Remdesivir helps fast recovery of moderate and severely affected patients but its clinical effect on nonmechanically ventilated severely affected patients is optimal (Elsawah et al., 2020). This indicates that the treatment of COVID-19 is still medically unmet requiring further efforts. Currently, patient management is primarily dependent on symptomatic treatment and respiratory support including intensive care in case of complicated disease (Cascella et al., 2020; Chen et al., 2020; Gattinoni et al., 2020). Fifteen drugs (chloroquine, hydroxychloroquine, lopinavir, ritonavir, nafamostat, camostat, famotidine, umifenovir, nitazoxanide, ivermectin, corticosteroids, tocilizumab, sarilumab, bevacizumab, and fluvoxamine) are under clinical trial (Shaffer, 2020) for COVID-19 treatment. In addition, several antivirals (bemcentinib, chloroquine and hydroxychloroquine, lopinavir boosted with ritonavir and remdesivir) and immune modulators (anakinra and canakinumab, azithromycin, brensocatib, convalescent plasma, corticosteroids, interferon beta, ruxolitinib, mesenchymal stromal cells and sarilumab and tocilizumab) are also being considered for clinical use (Connelly, 2020).
Promising drugs targeting SARS-CoV-2 Mpro have been under investigation demonstrating efficient binding and potential antiviral activities. The main protease is a key enzyme important for viral replication and maturation (Ziebuhr et al., 2000; Jain and Mujwar, 2020). Besides, the Mpro has enhanced enzymatic activity and there are no human proteases reported yet having similar specificity with it (Hilgenfeld, 2014; Zhang et al., 2020a, Zhang et al., 2020b, Zhang et al., 2020c) strengthening its preference of being a potential drug target. Several compounds including new drugs, known antivirals, and repurposed broad-spectrum drugs showed effective inhibition of SARS-CoV-2 Mpro with promising antiviral activities.
So far, peptidomimetic alpha ketoamide inhibitors (13a, 13b) (Zhang et al., 2020a, Zhang et al., 2020b; Mengist et al., 2020), Michael acceptor N3 (Jin et al., 2020a), carmofur (Jin et al., 2020a; Jin et al., 2020b), ebselen (Jin et al., 2020a; Sies and Parnham, 2020), aldehyde-based compounds 11a and 11b (Dai et al., 2020), and 6e (Rathnayake and Zheng, 2020), clinically approved anti-Human immunodeficiency virus (HIV) drugs lopinavir/ritonavir (Liu and Wang, 2020), antiplatelet drug dipyridamole (Li et al., 2020c; Liu et al., 2020), anti-Hepatitis C virus (HCV) drug boceprevir, GC-376, calpain inhibitors (II, XII), and GC-373 (Choy et al., 2020; Ma et al., 2020; Vuong and Khan, 2020) are among the most promising drugs reported exhibiting effective in vitro and in vivo antiviral activity. These drugs bind on the substrate-binding cleft of the Mpro and inhibit its activity with the subsequent halting of virus replication and infection (Figure 1). However, the clinical outcome of these drugs in humans is not determined yet. Further, the antiviral activity and safety of several drugs are heterogeneous and the results of various studies are not collated together yet. Summarizing the potency, safety, and pharmacokinetic profiles of these drugs could be crucial to recommend the best ones for further investigation. Therefore, this review aims to evaluate potential inhibitors of SARS-CoV-2 Mpro concerning their antiviral activity (potency), safety, and pharmacokinetic profiles summarized in Table 1.
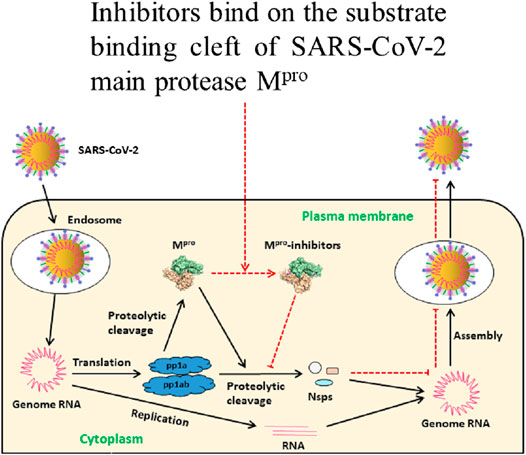
FIGURE 1. Schematic illustration of inhibitors of Mpro preventing SARS-CoV-2 replication. After entering into the host cell, SARS-CoV-2 releases its genomic RNA. Translation produces polyproteins pp1a and pp1ab which are cleaved to Mpro and nonstructural proteins (nsps). Mpro is involved in the production of nsps and virion maturation. These proteins are essential for assembling the viral replication transcription complex (RTC) to engage in RNA synthesis. Mpro inhibitors bind on its substrate-binding cleft resulting in inactivation with subsequent failure of virion assembly. Eventually, host cells fail to release the new intact virions and thus new infection is inhibited, modified from Mengist et al. (2020).
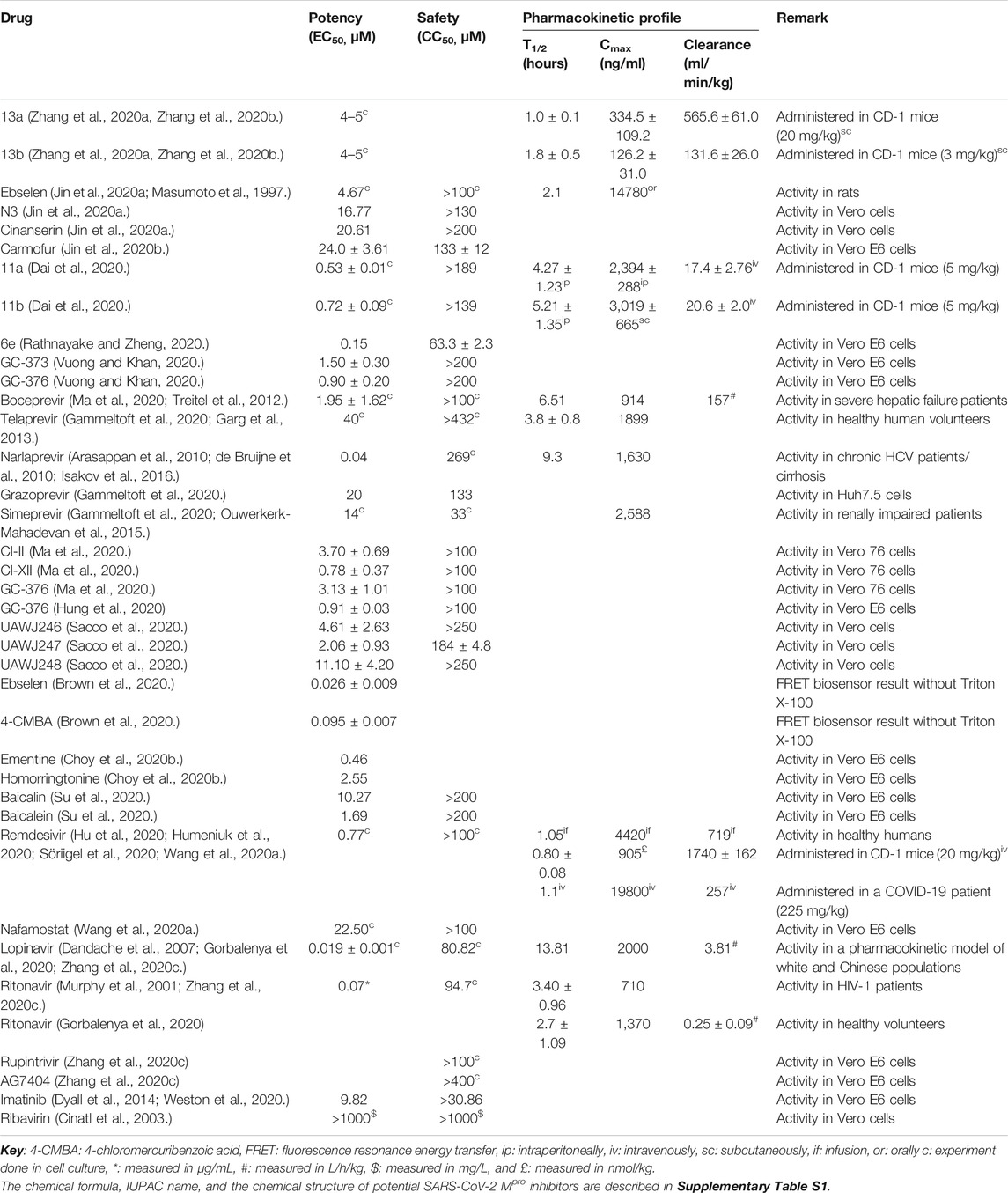
TABLE 1. Description of the potency, safety, and pharmacokinetic profiles of drugs targeting SARS-CoV-2 Mpro.
Many potential drugs have been showing effective antiviral activity in vitro and in vivo (Ullrich and Nitsche, 2020). Pyridones containing peptidomimetic alpha-ketoamide inhibitors 13a and 13b are amongst the promising drugs that exhibited strong SARS-CoV-2 inhibition. In human Calu-3 lung cells infected with SARS-CoV-2, 13b showed good inhibitory activity with a half-maximal effective concentration (EC50) value of 4–5 µM (Zhang et al., 2020a, Zhang et al., 2020b). However, the in vivo potency and safety of 13a and 13b are not reported yet, which needs further investigation. Ebselen and N3 are other promising drugs targeting Mpro. In Vero cells, ebselen and N3 displayed effective inhibition of SARS-CoV-2 replication and infection. Ebselen demonstrated more potency (EC50 < 5 µM) over N3 as the antiviral activity of N3 was moderate with a relatively higher EC50 value >16 µM (Jin et al., 2020a).
Aldehyde-based drugs 11a and 11b were synthesized being suitable to bind and inhibit SARS-CoV-2 Mpro with respective 100% and 96% in vitro inhibition of the Mpro at 1 µM. Regarding their antiviral activity, plaque assay in cell culture showed that 11a and 11b demonstrated excellent anti-SARS-COV-2 infection activity with very low EC50 values <1 µM (Dai et al., 2020). Carmofur is an antineoplastic drug currently considered for COVID-19 treatment. Carmofur is reported to moderately inhibit SARS-CoV-2 infection in Vero E6 cells with an EC50 value of >20 µM (Jin et al., 2020b).
Rathnayake et al. (Rathnayake and Zheng, 2020) demonstrated the potency of compounds in SARS-CoV-2 infected Vero E6 cells via targeting the main protease. Accordingly, the synthesized compounds showed effective inhibition of virus replication with EC50 values between 0.15 and 0.9 µM where compound 6e exhibited the most potent activity. The activity of synthesized compounds was also confirmed by the significant difference in the virus plaque-forming units (PFU) observed in the presence and absence of Mpro inhibitors in cell culture. Plaque assays and virus reduction assays indicated that GC-373 and GC-376 demonstrated effective inhibition and reduction of SARS-CoV-2 RNA copies in Vero E6 cells with EC50 values between 0.9 and 1.5 μM. Comparatively, GC-376 showed stronger inhibitory activity over GC-373 evidenced by a very low EC50 value below 1 μM (Vuong and Khan, 2020).
According to Ma et al. (2020), boceprevir, GC-376, and calpain inhibitors II and XII also demonstrated effective inhibition of SARS-CoV-2 replication in Vero 76 cells. The FDA approved HCV drug boceprevir that showed an effective viral reduction with an EC50 value below 2 µM while calpain inhibitor II and GC-376 demonstrated a higher EC50 value above 3 µM. Among these, calpain inhibitor XII exhibited the most potent antiviral activity against SARS-CoV-2 with a very low EC50 value below 1 µM. Further, GC-376 and boceprevir showed effective inhibition of SARS-CoV-2 replication in Vero cells. GC-376 exhibited a strong inhibition potency more than boceprevir (average EC50 values: 0.70 µM for GC-376 and 15.57 µM for boceprevir). The authors reported that a combination of 1 µM GC-376 and 1 µM remdesivir can completely inhibit SARS-CoV-2 in vitro replication (Choy et al., 2020).
Besides, GC-376 was also reported to effectively inhibit SARS-CoV-2 infection in Vero E6 cells (Hung et al., 2020) where a plaque assay stated a 0.49 ± 0.35 μM EC50 value of GC-376. GC-376 analogs (UAWJ246, UAWJ247, and UAWJ248) also produced effective inhibition of SARS-CoV-2 in Vero cells where UAWJ247 demonstrated the strongest inhibition (Sacco et al., 2020). Another study also reported excellent potency of GC-376 against SARS-CoV-2 with an EC50 value of 0.91 ± 0.03 μM in Vero E6 cells (Hung et al., 2020). This drug (GC-376) is known to exhibit a strong potency against several other coronaviruses in cell lines (Kim et al., 2012). Brown et al. (2020) used a fluorescence resonance energy transfer (FRET) biosensor to evaluate the potency of 65 compounds against SARS-CoV-2 Mpro. Among these, ebselen and 4-chloromercuribenzoic acid demonstrated the strongest virus inhibition in the presence and absence of Triton X-100. Baicalin and baicalein are noncovalent nonpeptidomimetic compounds exhibiting effective binding and inhibition of SARS-CoV-2 Mpro with baicalein showing the strongest potency (EC50 value <2 µM) close to chloroquine and remdesivir (Su et al., 2020). An in silico study predicted remdesivir and nafamostat bind on the catalytic dyad of the Mpro (Chakraborti et al., 2020) with potent antiviral activities in cells (Wang et al., 2020a).
The in vivo safety of proposed drugs for COVID-19D targeting the Mpro is not explicitly reported. But the in vitro half cytotoxic concentration (CC50) values of some drugs are reported. Drugs 11a and 11b showed good safety to cells with a CC50 value of >100 µM in vitro. Specifically, 11a showed no obvious toxicity in rats and dogs given at different doses for seven days (Dai et al., 2020). Studies reported that ebselen has very low toxicity in rats (Renson et al., 1982) and is safe for humans in clinical trials (Lynch and Kil, 2009; Masaki et al., 2016; Kil et al., 2017). N3 and cinanserin are also reported to be safe to Vero cells with a CC50 value of >100 µM with cinanserin exhibiting comparatively low toxicity (CC50 value > 200 µM) (Jin et al., 2020a). Carmofur also demonstrated low toxicity in Vero E6 cells with an average CC50 value of >133 µM (Jin et al., 2020b). In cell culture, although reported to have high potency, 6e exhibited relatively higher toxicity to cells with a CC50 value below 100 µM. On the contrary, other compounds (6c, 6h, and 6j) demonstrated an acceptable level of toxicity with CC50 values > 100 µM (Rathnayake and Zheng, 2020).
Feline coronavirus drugs targeting SARS-CoV-2 Mpro (GC-373 and GC-376) demonstrated very low toxicity in Vero E6 cells with CC50 values above 200 µM (Vuong and Khan, 2020). Boceprevir, GC-376, and calpain inhibitors II and XII also demonstrated acceptable level of toxicity with CC50 values above 100 µM in cell culture (Ma et al., 2020). A study showed that boceprevir and GC-376 did not cause obvious in vitro toxicity to Vero cells (Choy et al., 2020). Interestingly the CC50 value of GC-376 is higher in Vero E6 cells indicating its low toxicity (Hung et al., 2020). GC-376 analogs UAWJ246, UAWJ247, and UAWJ248 also demonstrated very low toxicity to Vero cells where UAWJ246 and UAWJ248 displayed a CC50 value > 250 µM while the CC50 value of UAWJ247 was between 179 and 189 µM (Sacco et al., 2020). The safety of the anticipated drugs should be elaborately investigated for a better understanding of their toxicity properties.
Baicalin and baicalein demonstrated very low cytotoxicity in Vero E6 cells with CC50 values > 200 µM (Su et al., 2020). Thimerosal, phenylmercuric acetate, hematoporphyrin, chloranil, plumbagin, Evans blue, and Chicago sky blue showed effective inhibition against SARS-CoV-2 Mpro (Coelho et al., 2020). Earlier, the safety of plumbagin, Evans blue, and Chicago sky blue measured by median lethal dose (LD50) was reported to be 16, 340, and 2,260 mg/kg administered through different routes in mice/rats (Weinberg et al., 1951; Krishnaswamy and Purushothaman, 1980; Balzarini et al., 1986). Known drugs, remdesivir and nafamostat, also showed acceptable cytotoxicity in cell culture (Wang et al., 2020a; Wang et al., 2020b); however, with increasing clinical application, remdesivir is showing adverse effects in COVID-19 patients (Fan et al., 2020). Lopinavir/ritonavir monotherapy was also found to be toxic with poor clinical effects in mild/moderate COVID-19 patients (Li et al., 2020b).
Studies reporting the in vivo pharmacokinetic properties of prospective COVID-19 drugs targeting SARS-CoV-2 Mpro are scarce. Alpha-ketoamide drug 13a demonstrated good metabolic stability with low intrinsic clearance rates in mouse and human microsomes. When administered subcutaneously in CD-1 mice with different doses, 13b showed higher plasma half-life (T1/2) and a lower clearance rate than 13a. On the other side, 13a was better concerning the average amount in plasma with a higher plasma maximal concentration (Cmax) value above 334 ng/ml (Zhang et al., 2020a, Zhang et al., 2020b).
More data are available for drugs 11a and 11b which exhibited different pharmacokinetic properties when administered in different routes in CD-1 mice. 11a showed better plasma T1/2 when administered to mice intraperitoneally than intravenously (5 mg/kg). Comparatively, 11a displayed a high Cmax and a good bioavailability when administered intraperitoneally. Its metabolic stability, measured by the rate of clearance (ml/min/kg), was also good. 11b also showed good pharmacokinetic properties when administered intraperitoneally (20 mg/kg), subcutaneously (5 mg/kg), and intravenously (5 mg/kg). More specifically, 11b showed good bioavailability when given both intraperitoneally and subcutaneously (Dai et al., 2020).
When administered intravenously, 11b showed faster clearance and shorter half-life indicating the suitability of 11a through this route. Further pharmacokinetic assessment of 11a showed, when administered intravenously (10 mg/kg) to SD rat, that it demonstrated low clearance (4.01 ml/min/kg), long T1/2 (7.6 h), and high 3-min maximum concentration (81,500 ng/ml). Conversely, 11a, when administered intravenously (5 mg/kg) to beagle dog, exhibited higher clearance (5.80 ml/min/kg), shorter T1/2 (5.5 h), and lower 3-min maximum concentration (21,900 ng/ml) (Dai et al., 2020) indicating better pharmacokinetic profiles in SD rat administered at high dose than beagle dog. Further, the authors also reported that 11a exhibited no obvious toxicity in rats and dogs administered intravenously at appropriate doses. It is considered, due to safety issues, that intravenous administration is more appropriate where 11a exhibited interesting pharmacokinetic properties. In a single-ascending-dose randomized controlled study, remdesivir showed different pharmacokinetic profiles. When administered as a 2-h infusion (225 mg), remdesivir showed a low clearance rate, good half-life, and high Cmax (Humeniuk et al., 2020).
Several clinically approved drugs showed effective binding on SARS-CoV-2 Mpro with possible antiviral activities. Among these, HCV NS3/4A protease inhibitors (sovaprevir, vaniprevir, glecaprevir, boceprevir, simeprevir, paritaprevir, danoprevir, and grazoprevir) (Bafna et al., 2020), HIV protease inhibitors [nelfinavir (Xu et al., 2020) and lopinavir/ritonavir (Nukoolkarn et al., 2008)], immune modulators (vinflunine, vindesine, and topotecan) (Chakraborti et al., 2020), and other drugs including colistin (antibiotic), valrubicin (antitumor), icatibant (indicated for hereditary angioedema), bepotastine (prescribe for rhinitis), caspofungin (antifungal), perphenazine (antipsychotic) (Liu and Wang, 2020), bromocriptine (a dopamine antagonist), ergotamine (antimigraine), bictegravir (antiviral), antibacterial agents (oxytetracycline, tigecycline, and ceftolozane) (Chakraborti et al., 2020), viz. D2 receptor antagonist, HMG-CoA inhibitors, HIV reverse transcriptase and protease inhibitors, anticancer agents, folate inhibitors, and imatinib (Balaramnavar et al., 2020) showed effective binding on the Mpro. Although their suitability in COVID-19 patients is to be determined, the potency, safety, and/or pharmacokinetic profiles of known drugs inhibiting SARS-CoV-2 Mpro are reported before, which is briefly described in Table 1.
Determining the potency, safety, and pharmacokinetic profiles of drugs and applying it into clinical practice is the ultimate aim of drug discovery. Determining the binding affinity and efficiency of the anticipated drugs with the target, evaluating its target inhibitory activity, and assessing its role in curbing infection in vitro are all early stages of the process of drug discovery. The drug should be evaluated in model organisms in vivo and should be tested in a cohort of humans under clinical trials which is the most challenging step to achieve. The physiological process in humans is quite complex which affects the pharmacokinetic and pharmacodynamic properties of the anticipated drugs. The final goal in therapeutic medical research is to find an effective, safe, and pharmacokinetically suitable drug with minimum side effects on human tissues. This is quite challenging and that is why, although a couple of months passed, there is no globally approved specific antiviral drug yet to treat the COVID-19 pandemic.
Scientists have been investigating the potency and safety of old and new drugs through studying the ability of the drugs to specifically bind and inhibit target proteins and control virus replication. The emergence of tremendous publications on COVID-19 therapy is proof of the ongoing efforts in discovering potential drugs (Bein et al., 2020; Li et al., 2020a; McCreary and Pogue, 2020; Sanders et al., 2020). However, only a small fraction of studies presented data on the preclinical and clinical potency, safety, and pharmacokinetic properties of drugs where the progress is more infant in drugs targeting SARS-CoV-2 Mpro as most studies lack experimental validation where only scientific simulation data are available.
Urgent COVID-19 therapeutic options are desired by the community (de Almeida et al., 2020). In this regard, based on previous therapeutic experience with SARS-CoV and MERS-CoV, there has been a substantial inquisitiveness in the repurposing of approved antiviral drugs (for example, drugs used to treat HIV, HBV, HCV, filoviruses, and influenza) and development of new drugs for COVID-19 (Artese et al., 2020; Liu and Wang, 2020). Apart from the ongoing struggles in searching for effective drugs for COVID-19, challenges are facing these efforts (Ghaebi et al., 2020). Among these, urgency is of significant factor which is exacerbated by the time-consuming and expensive nature of the data acquisition process in physical experiments. Intriguingly, the application of computational simulations and drug repurposing programs significantly alleviates the problem through providing basic data; however, whether these drugs pass clinical trials is another headache to the scientific world which puts the progress of finding clinically applicable COVID-19 drugs at its early stage. A mutant coronavirus was reported on November 5, 2020 in mink populations in Denmark which can spread to humans (Lesté-Lasserre, 2020). Besides, a recent study by Hou et al. (Wu et al., 2020) reported that spike protein D614G SARS-CoV-2 variant demonstrates more efficient infection, replication, and competitive fitness than the wild type indicating that the evolution of the virus could make the drug and vaccine discovery efforts more challenging.
Here, we discussed the potency, safety, and pharmacokinetic profiles of drugs halting SARS-CoV-2 infection through targeting the Mpro. Several drugs including alpha-ketoamide inhibitors, aldehyde-based inhibitors, N3, ebselen, carmofur, Feline coronavirus inhibitors (GC-373 and GC-376), GC-376 analogs, calpain inhibitors II and XII, and clinically approved anti-HCV and HIV drugs have been investigated for their potential anti-SARS-CoV-2 activity. Here we observed that most studies report only the in vitro potency and safety results while data on the in vivo pharmacokinetic profiles of potential drugs are very limited. Drugs 13a, 13b, ebselen, 11a and 11b, GC-376, GC-373, 6e, boceprevir, narlaprevir, baicalein, remdesivir, calpain inhibitors II and XII, and UAWJ247 showed a very low EC50 value and a high CC50 value above 100 µM (except 6e with a CC50 value below 65 µM) indicating their potency and safety. However, data on the in vivo pharmacokinetic profiles of new drugs were reported only for 13a, 13b, 11a, and 11b. Accordingly, although the currently available data are limited to decide the best new drug for further investigation, 11a demonstrated better potency, safety, and in vivo pharmacokinetic activity (Table 1). Lack of sufficient data especially on new drugs hampered us to discuss the potency, safety, and pharmacokinetic characteristics of potential drugs impeding SARS-CoV-2 infection through inhibiting the Mpro in detail. Drug repurposing and the use of previously known drugs are very important to speed up the discovery of putative therapeutic options for new diseases during urgent times. As data on the pharmacokinetic profiles of known drugs are comparatively available, trying this option could be ultimately helpful provided that their suitability for COVID-19 patients should be determined. Despite limited data on the pharmacokinetic profiles of drugs, this review provides a glimpse into choosing the best new and/or repurposed drugs for further investigation.
Generally, current therapeutic options proposed to treat COVID-19 are mostly based on the results of in vitro studies, observational studies, and clinical trials (Fernandes et al., 2020); perhaps, computational predictions also account for a big proportion of these studies. Specifically, most studies on drugs targeting the main protease of SARS-CoV-2 present only data related to the in vitro potency and safety while in vivo pharmacokinetic profiling is very limited. The main protease is a crucial enzyme for virus replication and maturation (Ziebuhr et al., 2000; Jain and Mujwar, 2020) and has a relatively conserved active site (Stoermer, 2020; Ullrich and Nitsche, 2020) which makes it considered as a potential drug target (Naqvi et al., 2020). Remarkably, there are promising baseline data on potential inhibitors of SARS-CoV-2 Mpro. Therefore, future research on drugs targeting SARS-CoV-2 Mpro should escape from preliminary computational, in vitro, and in vivo studies and advance to preclinical and clinical applications. Besides, cautious use of known broad-spectrum drugs in terms of potency, safety, selectivity, suitability, and binding affinity is also recommended. More importantly, COVID-19 therapeutic studies should consider the emergence of new SARS-CoV-2 variants due to virus evolution as the occurrence of 1-2 mutations every month is estimated (Duchene et al., 2020).
HM conceived the topic and wrote the original draft. All authors read and approved the final draft.
TJ is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB29030104), the National Natural Science Fund (Grant Nos.: 31870731 and 31971129), the Fundamental Research Funds for the Central Universities, and the 100 Talents Program of the Chinese Academy of Sciences. HM is supported by the University of Science and Technology of China scholarship program. DM is supported by ANSO scholarship. AM is supported with CSC scholarship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.630500/full#supplementary-material.
Arasappan, A., Bennett, F., Bogen, S. L., Venkatraman, S., Blackman, M., Chen, K. X., et al. (2010). Discovery of narlaprevir (SCH 900518): a potent, second generation HCV NS3 serine protease inhibitor. ACS Med. Chem. Lett. 1 (2), 64–69. doi:10.1021/ml9000276
Artese, A., Svicher, V., Costa, G., Salpini, R., Di Maio, V. C., Alkhatib, M., et al. (2020). Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer Chemother. 53, 100721. doi:10.1016/j.drup.2020.100721
Bafna, K., Krug, R. M., and Montelione, G. T. (2020). Structural similarity of SARS-CoV2 Mpro and HCV NS3/4A proteases suggests new approaches for identifying existing drugs useful as COVID-19 therapeutics. ChemRxiv. doi:10.26434/chemrxiv.12153615
Balaramnavar, V. M., Ahmad, K., Saeed, M., Ahmad, I., Kamal, M., and Jawaid, T. (2020). Correction: pharmacophore-based approaches in the rational repurposing technique for FDA approved drugs targeting SARS-CoV-2 Mpro. RSC Adv. 10 (70), 40264–40275. doi:10.1039/D0RA06038K
Balzarini, J., Mitsuya, H., De Clercq, E., and Broder, S. (1986). Aurintricarboxylic acid and Evans Blue represent two different classes of anionic compounds which selectively inhibit the cytopathogenicity of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus. Biochem. Biophys. Res. Commun. 136 (1), 64–71. doi:10.1016/0006-291x(86)90877-6
Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., et al. (2020). Remdesivir for the treatment of Covid-19—final report. N. Engl. J. Med. 383 (19), 1813–1826. doi:10.1056/NEJMoa2007764
Bein, B., Bachmann, M., Huggett, S., and Wegermann, P. (2020). SARS CoV-2/COVID-19: evidence-based recommendation on diagnosis and therapy. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 55 (4), 257–265. doi:10.1055/a-1146-8674
Brown, A. S., Ackerley, D. F., and Calcott, M. J. (2020). High-throughput screening for inhibitors of the SARS-CoV-2 protease using a FRET-biosensor. Molecules 25 (20), 4666. doi:10.3390/molecules25204666
Cascella, M., Rajnik, M., Cuomo, A., Dulebohn, S. C., and Di Napoli, R. (2020). Features, evaluation, and treatment of Coronavirus Statpearls. Treasure Island (FL): StatPearls Publishing.
Chakraborti, S., Bheemireddy, S., and Srinivasan, N. (2020). Repurposing drugs against the main protease of SARS-CoV-2: mechanism-based insights supported by available laboratory and clinical data. Mol. Omics. 16 (5), 474–491. doi:10.1039/D0MO00057D
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 395 (10223), 507–513. doi:10.1016/s0140-6736(20)30211-7
Choy, K.-T., Wong, A. Y.-L., Kaewpreedee, P., Sia, S. F., Chen, D., Hui, K. P. Y., et al. (2020). Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 178, 104786. doi:10.1016/j.antiviral.2020.104786
Cinatl, J., Morgenstern, B., Bauer, G., Chandra, P., Rabenau, H., and Doerr, H. (2003). Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. The Lancet 361 (9374), 2045–2046. doi:10.1016/S0140-6736(03)13615-X
Coelho, C., Gallo, G., Campos, C. B., Hardy, L., and Würtele, M. (2020). Biochemical screening for SARS-CoV-2 main protease inhibitors. PloS One 15 (10), e0240079. doi:10.1371/journal.pone.0240079
Connelly, D. (2020). Targeting COVID-19: the drugs being fast-tracked through clinical trials and how they work. Pharm. J. 304 (7937), 312–313. doi:10.1211/PJ.2020.20207949
Covid, C., Chow, N., Fleming-Dutra, K., and Gierke, R. (2020). Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. Morbid. Mortal. Week. Rep. 69 (13), 382. doi:10.15585/mmwr.mm6913e2
Dai, W., Zhang, B., Jiang, X.-M., Su, H., Li, J., Zhao, Y., et al. (2020). Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 368 (6497), 1331–1335. doi:10.1126/science.abb4489
Dandache, S., Sévigny, G., Yelle, J., Stranix, B. R., Parkin, N., Schapiro, J. M., et al. (2007). In vitro antiviral activity and cross-resistance profile of PL-100, a novel protease inhibitor of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 51 (11), 4036–4043. doi:10.1128/AAC.00149-07
de Almeida, S. M. V., Soares, J. C. S., dos Santos, K. L., Alves, J. E. F., Ribeiro, A. G., Jacob, Í. T. T., et al. (2020). COVID-19 therapy: what weapons do we bring into battle?. Bioorg. Med. Chem. 28 (23), 115757.
de Bruijne, J., Bergmann, J. F., Reesink, H. W., Weegink, C. J., Molenkamp, R., Schinkel, J., et al. (2010). Antiviral activity of narlaprevir combined with ritonavir and pegylated interferon in chronic hepatitis C patients. Hepatology 52 (5), 1590–1599. doi:10.1002/hep.23899
Duchene, S., Featherstone, L., Haritopoulou-Sinanidou, M., Rambaut, A., and Lemey, P. (2020). Temporal signal and the phylodynamic threshold of SARS-CoV-2. Virus Evol. 6 (2), 061. doi:10.1093/ve/veaa061
Dyall, J., Coleman, C. M., Hart, B. J., Venkataraman, T., Holbrook, M. R., Kindrachuk, J., et al. (2014). Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 58 (8), 4885–4893. doi:10.1128/aac.03036-14
Elsawah, H. K., Elsokary, M. A., Abdallah, M. S., and ElShafie, A. H. (2020). Efficacy and safety of remdesivir in hospitalized Covid-19 patients: systematic review and meta-analysis including network meta-analysis. Rev. Med. Virol. 20, e2187. doi:10.1002/rmv.2187
Fan, Q., Zhang, B., Ma, J., and Zhang, S. (2020). Safety profile of the antiviral drug remdesivir: an update. Biomed. Pharmacother. 130, 110532.
Fernandes, A. C. L., Vale, A. J. M., Guzen, F. P., Pinheiro, F. I., Cobucci, R. N., and de Azevedo, E. P. (2020). Therapeutic options against the new coronavirus: updated clinical and laboratory evidences. Front. Med. 7, 546. doi:10.3389/fmed.2020.00546
Gammeltoft, K. A., Zhou, Y., Galli, A., Offersgaard, A., Pham, L. V., Fahnøe, U., et al. (2020). Hepatitis C virus protease inhibitors show differential efficacy and interactions with remdesivir for treatment of SARS-CoV-2 in Vitro. bioRxiv. Preprint. doi:10.1101/2020.12.02.408112
Garg, V., Chandorkar, G., Yang, Y., Adda, N., McNair, L., Alves, K., et al. (2013). The effect of CYP3A inhibitors and inducers on the pharmacokinetics of telaprevir in healthy volunteers. Br. J. Clin. Pharmacol. 75 (2), 431–439. doi:10.1111/j.1365-2125.2012.04345.x
Gattinoni, L., Coppola, S., and Cressoni, M. (2020). COVID-19 does not lead to a "typical" acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 201 (10), 1299–1300. doi:10.1164/rccm.202003-0817LE
Ghaebi, M., Osali, A., Valizadeh, H., Roshangar, L., and Ahmadi, M. (2020). Vaccine development and therapeutic design for 2019-nCoV/SARS-CoV-2: challenges and chances. J. Cell. Physiol. 235 (12), 9098–9109. doi:10.1002/jcp.29771
Gorbalenya, A., Baker, S., Baric, R., de Groot, R., Drosten, C., Gulyaeva, A., et al. (2020). The species severe acute respiratory syndrome related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 5, 536–544
Hilgenfeld, R. (2014). From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 281 (18), 4085–4096. doi:10.1111/febs.12936
Hu, C. L., Yang, Y., Wang, X., Xie, Y. C., Shen, J. S., Tan, B., et al. (2020). Pharmacokinetics and tissue distribution of remdesivir and its metabolites nucleotide monophosphate, nucleotide triphosphate, and nucleoside in mice. Acta Pharmacol. Sin. 41 (4), 1–6. doi:10.1038/s41401-41020-00537-41409
Humeniuk, R., Mathias, A., Cao, H., Osinusi, A., Shen, G., Chng, E., et al. (2020). Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clinical and Translational Science 13 (5), 896–906. doi:10.1111/cts.12840
Hung, H.-C., Ke, Y.-Y., Huang, S. Y., Huang, P.-N., Kung, Y.-A., Chang, T.-Y., et al. (2020). Discovery of M Protease inhibitors encoded by SARS-CoV-2. Antimicrob. Agents Chemother. 64 (9), e00872. doi:10.1128/aac.00872-20
Isakov, V., Koloda, D., Tikhonova, N., Kikalishvili, T., Krasavina, E., Lekishvili, K., et al. (2016). Pharmacokinetics of the new hepatitis C virus NS3 protease inhibitor narlaprevir following single-dose use with or without ritonavir in patients with liver cirrhosis. Antimicrob. Agents Chemother. 60 (12), 7098–7104. doi:10.1128/AAC.01044-16
Jain, R., and Mujwar, S. (2020). Repurposing metocurine as main protease inhibitor to develop novel antiviral therapy for COVID-19. Struct. Chem. 31 (6), 2487–2499. doi:10.1007/s11224-020-01605-w
Jin, Z., Du, X., and Xu, Y. (2020a). Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 582 (7811), 289–293. doi:10.1038/s41586-020-2223-y
Jin, Z., Zhao, Y., and Sun, Y. (2020b). Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat. Struct. Mol. Biol. 27 (6), 529–532. doi:10.1038/s41594-020-0440-6
Kil, J., Lobarinas, E., Spankovich, C., Griffiths, S. K., Antonelli, P. J., Lynch, E. D., et al. (2017). Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. The lancet. 390 (10098), 969–979
Kim, Y., Lovell, S., Tiew, K. C., Mandadapu, S. R., Alliston, K. R., Battaile, K. P., et al. (2012). Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. J. Virol. 86 (21), 11754–11762. doi:10.1128/jvi.01348-12
Krishnaswamy, M., and Purushothaman, K. K. (1980). Plumbagin: a study of its anticancer, antibacterial & antifungal properties. Indian J. Exp. Biol. 18 (8), 876–877.
Lesté-Lasserre, C. (2020). Mutant coronaviruses found in mink spark massive culls and doom a Danish group’s research. New York, N.Y.: Science. doi:10.1126/science.abf6565
Li, H., Zhou, Y., Zhang, M., Wang, H., Zhao, Q., and Liu, J. (2020a). Updated approaches against SARS-CoV-2. Antimicrob. Agents Chemother. 64 (6), e00483. doi:10.1128/aac.00483-20
Li, Y., Xie, Z., Lin, W., Cai, W., Wen, C., Guan, Y., et al. (2020b). Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial. MedRxiv. 1 (1), 105–113. doi:10.1016/j.medj.2020.04.001
Li, Z., Li, X., Huang, Y.-Y., Wu, Y., Liu, R., Zhou, L., et al. (2020c). Identify potent SARS-CoV-2 main protease inhibitors via accelerated free energy perturbation-based virtual screening of existing drugs. Proc. Natl. Acad. Sci. USA. 117 (44), 27381–27387. doi:10.1073/pnas.2010470117
Liu, X., Li, Z., Liu, S., Sun, J., Chen, Z., Jiang, M., et al. (2020). Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm. Sin. B. 10 (7), 1205–1215. doi:10.1016/j.apsb.2020.04.008
Liu, X., and Wang, X.-J. (2020). Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J.Genet. Genom. 47 (2), 119–121. doi:10.1016/j.jgg.2020.02.001
Lynch, E., and Kil, J. (2009). Development of ebselen, a glutathione peroxidase mimic, for the prevention and treatment of noise-induced hearing loss. Semin. Hearing 30 (1), 47–55. doi:10.1055/s-0028-1111106
Ma, C., Sacco, M. D., Hurst, B., Townsend, J. A., Hu, Y., Szeto, T., et al. (2020). Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. bioRxiv. Preprint. doi:10.1101/2020.04.20.051581
Masaki, C., Sharpley, A. L., Cooper, C. M., Godlewska, B. R., Singh, N., Vasudevan, S. R., et al. (2016). Effects of the potential lithium-mimetic, ebselen, on impulsivity and emotional processing. Psychopharmacology 233 (14), 2655–2661.
Masumoto, H., Hashimoto, K., Hakusui, H., Takaichi, M., Yokota, T., Honda, T., et al. (1997). Studies on the pharmacokinetics of ebselen in rats (1): absorption, distribution, metabolism and excretion after single oral administration. Drug Metabol. Pharmacokinet. 12 (6), 596–609.
McCreary, E. K., and Pogue, J. M. (2020). Coronavirus disease 2019 treatment: a review of early and emerging options. Open Forum Infect Dis. 7 (4), 105. doi:10.1093/ofid/ofaa105
Mekonnen, D., Mengist, H. M., Derbie, A., Nibret, E., Munshea, A., He, H., et al. (2020). Diagnostic accuracy of serological tests and kinetics of severe acute respiratory syndrome coronavirus 2 antibody: a systematic review and meta-analysis. Rev. Med. Virol. 21 (1), 2181. doi:10.1002/rmv.2181
Mengist, H. M., Fan, X., and Jin, T. (2020). Designing of improved drugs for COVID-19: crystal structure of SARS-CoV-2 main protease Mpro. Signal Transduc. Target. Therapy 5 (1), 67. doi:10.1038/s41392-020-0178-y
Murphy, R. L., Brun, S., Hicks, C., Eron, J. J., Gulick, R., King, M., et al. (2001). ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. AIDS. 15 (1), F1–F9.
Naqvi, A. A. T., Fatima, K., Mohammad, T., Fatima, U., Singh, I. K., Singh, A., et al. (2020). Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 1866 (10), 165878. doi:10.1016/j.bbadis.2020.165878
Nukoolkarn, V., Lee, V. S., Malaisree, M., Aruksakulwong, O., and Hannongbua, S. (2008). Molecular dynamic simulations analysis of ritronavir and lopinavir as SARS-CoV 3CLpro inhibitors. J. Theor. Biol. 254 (4), 861–867.
Ouwerkerk-Mahadevan, S., Beumont-Mauviel, M., Mortier, S., Peeters, M., Verloes, R., Truyers, C., et al. (2015). Evaluation of the pharmacokinetics and renal excretion of simeprevir in subjects with renal impairment. Drugs 15 (3), 261–270. doi:10.1007/s40268-015-0101-0
Patel, K. P., Vunnam, S. R., Patel, P. A., Krill, K. L., Korbitz, P. M., Gallagher, J. P., et al. (2020). Transmission of SARS-CoV-2: an update of current literature. Eur. J. Clin. Microbiol. Infect. Dis. 39 (11), 2005–2011. doi:10.1007/s10096-020-03961-1
Rathnayake, A. D., and Zheng, J. (2020). 3C-like protease inhibitors block coronavirus replication in vitro and improve survival in MERS-CoV-infected mice. Sci. Transl. Med. 12 (557), 5332. doi:10.1126/scitranslmed.abc5332
Renson, M., Etschenberg, E., and Winkelmann, J. (1982). 2-Phenyl-1, 2-benzisoselenazol-3 (2H)-one containing pharmaceutical preparations and process for the treatment of rheumatic diseases, United States patent US Google Patents. 7, 352.
Sacco, M. D., Ma, C., Lagarias, P., Gao, A., Townsend, J. A., Meng, X., et al. (2020). Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against Mpro and cathepsin L. Science Adv. 6 (50), 751. doi:10.1126/sciadv.abe0751
Sanders, J. M., Monogue, M. L., Jodlowski, T. Z., and Cutrell, J. B. (2020). Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 323 (18), 1824–1836. doi:10.1001/jama.2020.6019
Shaffer, L. (2020). 15 drugs being tested to treat COVID-19 and how they would work. Nat. Med. 19, 415. doi:10.1038/d41591-020-00019-9
Sies, H., and Parnham, M. J. (2020). Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Rad. Biol. Med. 156, 107–112. doi:10.1016/j.freeradbiomed.2020.06.032
Sörgel, F., Malin, J. J., Hagmann, H., Kinzig, M., Bilal, M., Eichenauer, D. A., et al. (2020). Pharmacokinetics of remdesivir in a COVID-19 patient with end-stage renal disease on intermittent haemodialysis. J. Antimicrob. Chemother. 93 (2), dkaa500. doi:10.1093/jac/dkaa500
Stoermer, M. (2020). Homology models of Coronavirus 2019-nCoV 3CLpro protease. chemrxiv. Preprint. doi:10.26434/chemrxiv.11637294.v3
Su, H., Yao, S., Zhao, W., Li, M., Liu, J., Shang, W., et al. (2020). Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. bioRxiv. Preprint. doi:10.1101/2020.04.13.038687
Treitel, M., Marbury, T., Preston, R. A., Triantafyllou, I., Feely, W., O'Mara, E., et al. (2012). Single-dose pharmacokinetics of boceprevir in subjects with impaired hepatic or renal function. Clin. Pharmacokinet. 51 (9), 619–628. doi:10.1007/bf03261935
Ullrich, S., and Nitsche, C. (2020). The SARS-CoV-2 main protease as drug target. Bioorg. Med. Chem. Lett. 12, 7377.
Vuong, W., and Khan, M. B. (2020). Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat. Commun. 11 (1), 4282. doi:10.1038/s41467-020-18096-2
Wang, M., Cao, R., Zhang, L., Yang, X., Liu, J., Xu, M., et al. (2020a). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30 (3), 269–271. doi:10.1038/s41422-020-0282-0
Wang, Y., Wang, Y., Chen, Y., and Qin, Q. (2020b). Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 92 (6), 568–576. doi:10.1002/jmv.25748
Weinberg, J., Greaney, E. M., Rawlings, B., and Haley, T. J. (1951). The use and toxicity of pontamine sky blue. Science 114 (2950), 41–42. doi:10.1126/science.114.2950.41
Weston, S., Coleman, C. M., Haupt, R., Logue, J., Matthews, K., Li, Y., et al. (2020). Broad anti-coronavirus activity of food and drug administration-approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo. J. Virol. 94 (21), e01218. doi:10.1128/jvi.01218-20
Wu, C., Liu, Y., Yang, Y., Zhang, P., Zhong, W., Wang, Y., et al. (2020). Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 10 (5), 766–788.
Xu, Z., Peng, C., Shi, Y., Zhu, Z., Mu, K., Wang, X., et al. (2020). Nelfinavir was predicted to be a potential inhibitor of 2019-nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation. bioRxiv. Preprint. doi:10.1101/2020.01.27.921627
Zhang, L., Lin, D., Kusov, Y., Nian, Y., Ma, Q., Wang, J., et al. (2020a). α-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment. J. Med. Chem. 63 (9), 4562–4578. doi:10.1021/acs.jmedchem.9b01828
Zhang, L., Lin, D., Sun, X., Curth, U., Drosten, C., Sauerhering, L., et al. (2020b). Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 368 (6489), 409–412. doi:10.1126/science.abb3405
Zhang, L., Liu, J., Cao, R., Xu, M., Wu, Y., Shang, W., et al. (2020c). Comparative antiviral efficacy of viral protease inhibitors against the novel SARS-CoV-2 in vitro. Virol. Sin. 12, 1–9. doi:10.1007/s12250-020-00288-1
Zhang, W., Du, R. H., Li, B., Zheng, X. S., Yang, X. L., Hu, B., et al. (2020d). Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microb. Infect. 9 (1), 386–389. doi:10.1080/22221751.2020.1729071
Keywords: potency, safety, pharmacokinetics, inhibitors, SARS-CoV-2, main protease, COVID-19
Citation: Mengist HM, Mekonnen D, Mohammed A, Shi R and Jin T (2021) Potency, Safety, and Pharmacokinetic Profiles of Potential Inhibitors Targeting SARS-CoV-2 Main Protease. Front. Pharmacol. 11:630500. doi: 10.3389/fphar.2020.630500
Received: 17 November 2020; Accepted: 30 December 2020;
Published: 01 February 2021.
Edited by:
Petr Pavek, Charles University, CzechiaReviewed by:
Guozheng Huang, Anhui University of Technology, ChinaCopyright © 2021 Mengist, Mekonnen, Mohammed, Shi and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronghua Shi, cmhzaEB1c3RjLmVkdS5jbg== Tengchuan Jin, amludEB1c3RjLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.