
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Pharmacol. , 27 January 2021
Sec. Experimental Pharmacology and Drug Discovery
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.629618
This article is part of the Research Topic Purinergic Signaling 2020: the State-of-The-Art Commented by the Members of the Italian Purine Club View all 41 articles
Multiple sclerosis (MS) is an inflammatory immune-mediated disease of the central nervous system (CNS) characterized by damage of myelin-forming oligodendrocytes and destruction of myelin itself, leaving denuded axons without trophic and metabolic support and prone to degeneration (Nave, 2010). Clinically, this results in neurological disability and progression from the relapsing-remitting form of the disease to the irreversible chronic progressive one (Franklin and ffrench-Constant, 2008). Current therapies are immunomodulatory drugs that efficiently reduce the number and severity of debilitating immune-mediated attacks at initial stages (Comi et al., 2017), but are not effective in the presence of extensive axonal degeneration. Therefore, early therapeutic strategies promoting the formation of new oligodendrocytes and myelin sheaths around demyelinated axons are highly needed (Franklin and ffrench-Constant, 2017). Importantly, the major source of new myelinating oligodendrocytes, i.e., NG2-glia, traditionally defined as oligodendrocyte precursor cells (OPCs) (Nishiyama et al., 2009), do persist and slowly proliferate in the adult CNS (Dawson et al., 2003). Spontaneous remyelination occurs in MS patients but eventually fails due to several reasons (Franklin and ffrench-Constant, 2008). First, for remyelination to occur, it is important to have functionally healthy axons (Stangel et al., 2017) likely releasing myelination signals. Second, a remyelination supportive environment is required, i.e., a correct balance between different cell types (astrocytes, microglia, macrophages, and other immune cells) (Lombardi et al., 2019; Molina-Gonzalez and Miron, 2019) and mechanisms, including debris phagocytosis and secretion of growth signals or inhibitory molecules, from transcription factors to extracellular (ECM) proteins (Marangon et al., 2020). Finally, successful differentiation of OPCs also depends on their intrinsic potential which is, in turn, strictly dependent on regional heterogeneity (Marques et al., 2016).
Continuous communication between OPCs, neurons, and other glial cells is crucial to regulate both developmental myelination and myelin dynamics during adulthood. In this respect, ATP emerges as an important signaling molecule profoundly influencing OPCs, and functional purinoceptors are found to be expressed on these cells (Fields and Stevens, 2000). Extracellular ATP can be degraded to ADP or adenosine by ectonucleotidases expressed on the cell surface and differently activate purinergic membrane P1 and P2 receptors: the former are G protein-coupled receptors selectively activated by adenosine, whereas the latter are further divided into P2X ionotropic receptors, exclusively activated by ATP and G protein-coupled P2Y receptors with very specific pharmacological profiles (Alexander et al., 2019). For example, P2Y1 is primarily activated by ADP and only partially by ATP. This complexity highlights how a single molecule can produce a wide variety of downstream signaling in terms of proliferation, differentiation, migration, response to damage, and cell death, based on the actors involved at cellular and molecular levels. Of note, the expression of some purinoceptors is strictly dependent on specific differentiation stages, indicating critical roles in OPC maturation and myelination. Some of these receptors are also altered under demyelinating conditions, which can itself contribute to disease development (Fumagalli et al., 2016).
In this opinion, we discuss key questions that still remain unclear regarding the involvement of purinergic signaling in NG-glia response. First, what is the function of purinoceptors in OPCs, and do they exert peculiar roles in their behavioral heterogeneity? Second, how do purinoceptors control OPC reactive response to myelin injury? Are their expression and roles altered during the course of MS? Third, would purinergic strategies to promote remyelination by NG-glia be of therapeutic interest?
Confocal calcium imaging on OPCs from optic nerve demonstrated that ATP evokes rapid and transient increases in [Ca2+]i, mainly through P2Y1 (Hamilton et al., 2010). P2Y1 activation also promoted OPC chemotaxis in both isolated OPCs and cerebellar slices (Agresti et al., 2005). ATP-evoked Ca2+ signals in both OPCs and mature oligodendrocytes are also mediated by P2X7. However, P2Y1 may play a greater role under physiological conditions, being activated at nanomolar ATP concentrations, whereas only upon cell rupture do ATP extracellular levels become millimolar, i.e., high enough to activate P2X7 (Hamilton et al., 2010). In a similar way to NMDA receptors, after prolonged activation, P2X7 promotes the formation of a large nonselective pore enabling leakage of ions, metabolites, and ATP itself, ultimately causing cell death (Matute et al., 2007) and fostering a vicious cycle that activates P2X7 in nearby cells.
The very efficient degradation of extracellular ATP by ectonucleotidases prevents the activation of P2X7-mediated danger pathways and enables a further level of signaling through P1 receptors. Adenosine was initially described to inhibit OPC proliferation and promote OPC differentiation and myelin formation (Stevens et al., 2002). However, the presence of all four P1 receptors, whose expression is timely regulated during differentiation, makes adenosine a more complex modulator.
Adenosine regulates the transition from proliferating OPCs to mature cells in a cAMP-regulated manner. Outward K+ currents, essential in early OPCs, are abolished by the selective A2A receptor agonist CGS21680 that blocks their differentiation by stimulating adenylyl-cyclase activity (Coppi et al., 2013). Similarly, the A2B selective agonist BAY60-6583 stimulates cAMP production and inhibits K+ currents, thus depolarizing OPC membranes and blocking cell maturation. A2B stimulation also elevates levels of sphingosine-1-phosphate (S1P), a bioactive lipid mediator, contributing to delayed maturation (Coppi et al., 2020). The cross-talk between adenosine signaling and S1P may be clinically relevant because receptors for S1P are targeted by fingolimod, a widely used drug for MS, and whose direct action on the CNS remains unclear (Soliven et al., 2011). In contrast, both the prodifferentiating and promyelinating effect of adenosine on OPCs are likely due to activation of A1 receptors inhibiting cAMP (Coppi et al., 2015). The physiological role of A3 receptors has not been described, but in optic nerve-derived OPCs, its stimulation with the specific agonist 2-CI-IB-MECA induced apoptosis (González-Fernández et al., 2014).
Although uracil-nucleotides are produced and released in brain tissues, and their receptors are present on both neurons and glia, the contribution of P2Y2, P2Y4, P2Y6, and P2Y14 in OPCs has been poorly investigated. In 2006, we described the G protein-coupled receptor GPR17 as a P2Y-like receptor, based on its pharmacological response to UDP, UDP-glucose, and UDP-galactose (Ciana et al., 2006). In physiological conditions, GPR17 is almost exclusively expressed in oligodendrocytes, with a clearly characterized transient pattern: it starts to be expressed in early OPCs, reaches its peak in immature oligodendrocytes, and then disappears in myelinating oligodendrocytes (Fumagalli et al., 2011). Of note, transcriptome analyses revealed that GPR17 clearly characterizes a population of differentiation-committed precursors (Marques et al., 2016) and predominantly labels OPCs within axodentritic area, potentially able to myelinate axons (Marisca et al., 2020).
In OPCs, UDP-glucose promoted maturation to MBP-positive cells, whereas cangrelor, a nonselective GPR17 antagonist, maintained cells at an undifferentiated stage (Fumagalli et al., 2011). The prodifferentiative effect of GPR17 agonists may be due to receptor desensitization and internalization: prolonged activation of GPR17 promotes its removal from the membrane, thus enabling terminal maturation (Fratangeli et al., 2013). In purified primary OPCs, UDP-glucose stimulated cell migration and enhanced outward K+ currents (Coppi et al., 2013). In both transfected cell lines and primary OPCs, uracil-ligand-evoked responses were antagonized by Cangrelor and MRS2179, two purinergic antagonists (for review, see Lecca et al., 2020).
In human MS and animal models, P2X7, GPR17, and adenosine receptors undergo significant changes. Specifically, postmortem analysis of human MS specimens revealed P2X7 increases in optic nerve oligodendrocytes and in activated microglia and astrocytes in both spinal cord and brain (Amadio et al., 2017). In experimental autoimmune encephalomyelitis (EAE) mice, a model reproducing several features of human MS, upregulated P2X7 was described in both activated microglia and astrocytes already during the asymptomatic phase and in oligodendrocytes and neurons after disease onset (Matute et al., 2007; Grygorowicz et al., 2010). The adenosine produced after ATP breakdown may exhibit anti-inflammatory and immunosuppressive actions by inhibiting T-cell proliferation and cytokines secretion (Saze et al., 2013), but ATP signaling is overwhelming and generates an amplification cascade that eventually kills oligodendrocytes. After disease onset, administration of oxATP reduced clinical outcomes and demyelination extent (Matute et al., 2007), likely acting on astroglial and microglial P2X7, supporting the hypothesis that P2X7 receptor blockers could have a role in preventing/improving MS symptoms. Due to its peculiar pharmacology, P2X7 acts as a “silent” receptor whose activation takes place only under pathological conditions (Bhattacharya and Biber, 2016). Thus, P2X7 inhibitors are not expected to significantly affect physiological receptor activity and may become ideal candidate drugs in treating inflammation in a pleiotropic manner. However, despite several specific P2X7 antagonists have been developed so far, improving pharmacokinetics and blood–brain barrier (BBB) permeability, only a few of them entered clinical trials for peripheral diseases, such as rheumatoid arthritis and chronic obstructive pulmonary disease. The safety on humans was confirmed, but the efficacy was disappointing in most cases, potentially due to different factors, including the limits of the animal models, the presence of several P2X7 haplotypes in humans, and the lack of knowledge about their actual contribution in disease pathogenesis. Moreover, some of the candidate molecules were noncompetitive allosteric modulators that may not be sufficient to inhibit massive P2X7 activation. (for review, see Di Virgilio et al., 2017; Calzaferri et al., 2020).
GPR17 is a relatively novel receptor. Its almost exclusive expression in OPCs gives a new opportunity to target these cells and enhance their remyelination capabilities in damage conditions. In chronic damage and inflammation, GPR17 becomes pathologically overexpressed, which prevents cells’ terminal maturation. Lack of GPR17 timely downregulation was described in several animal models, resulting in impaired myelination (Lecca et al., 2008; Chen et al., 2009; Coppolino et al., 2018). Antagonists were indeed effective in preventing acute damage in a model of brain ischemia (Ciana et al., 2006; Lecca et al., 2008), but chronic administration of receptor antagonists in MS should be carefully evaluated to avoid unexpected side effects in oligodendrocyte functions in terms of metabolic support to neurons or interaction with other cells (Lecca et al., 2020). Conversely, receptor internalization mediated by agonists may promote GPR17 removal from the membrane, thus enabling OPCs to resume maturation. In this respect, promising results have come from in vivo study where the selective GPR17 agonist galinex was proved to significantly retard EAE induction (Parravicini et al., 2020). Since inflammation contributes to sustaining GPR17 expression (Coppolino et al., 2018), cotreatment with anti-inflammatory agents may efficiently favor remyelination.
As mentioned, adenosine receptors play crucial roles in OPC differentiation, and their pharmacological modulation may foster remyelination. Despite completely different etiology compared to MS, Niemann–Pick type C 1 (NPC1), a genetic disease characterized by lysosomal accumulation of cholesterol and sphyngolipids, is also characterized by neurodegeneration, neuroinflammation, and dysmyelination. As in MS, in NPC1, OPCs are blocked in immature stages and do not undergo maturation. In a mouse model of NPC1, administration of CGS21680, a selective A2A agonist, rescued OPC maturation (De Nuccio et al., 2019), apparently in contrast to the in vitro results described above. Further studies should be encouraged to interpret these conflicting data.
Data discussed above suggest that P2X7, GPR17, and adenosine receptors could be valuable targets to stimulate myelin repair, preventing chronic axonal degeneration. For all these receptors, promising molecules have been tested in preclinical studies, but further studies are needed to prove their efficacy in a context close to human paradigms, with minimal or absent interferences with physiologically essential pathways (Figure 1). To reach cells inside the CNS, these compounds should also be able to cross BBB. Although MRI-based gadolinium studies showed leaky BBB during acute MS relapses, evidence indicates that BBB is then re-established. Thus, besides developing brain permeable molecules (as already done for P2X7 antagonist), other approaches should be considered. While virus-based CNS drug delivery to maximize tropism for oligodendroglia (McCall et al., 2014) can still bear some problems, bioengineered extracellular vesicles are emerging as delivery vehicles for such therapeutic agents (Wiklander et al., 2019). Another challenge is to identify the right therapeutic intervention window. Regenerative agents delivered too late during disease course could be useless since axons might be already irreversibly committed to degeneration. Other issues are the proper design of clinical trials (e.g., correct stratification of patients) and the development of appropriate outcome measures, which are both critical to demonstrate the efficacy of a remyelinating drug (Ontaneda et al., 2015). Importantly, the complex pathophysiology of progressive MS suggests that combination therapies targeting different processes would represent the “ideal” therapeutic approach, as anticipated for GPR17 (Coppolino et al., 2018; Lecca et al., 2020). Although several issues still remain to be addressed, the emerging and promising developments in clinical remyelination therapy (Plemel et al., 2017) raise hope for having soon new therapeutic options for progressive MS.
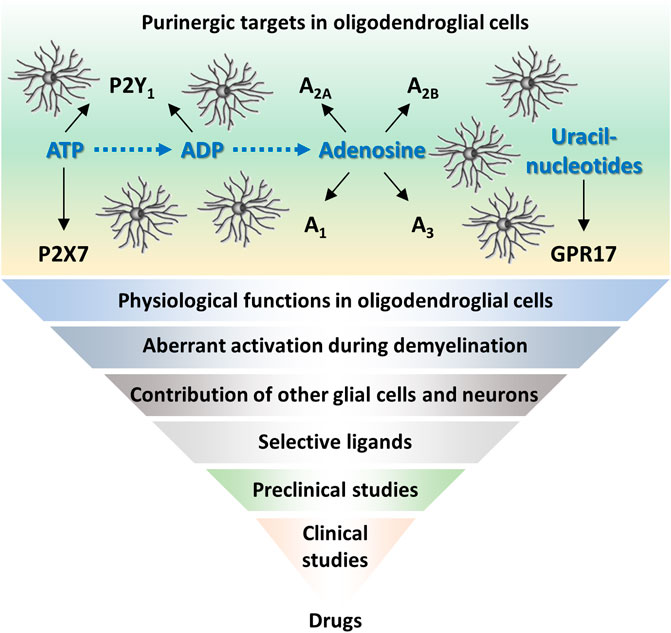
FIGURE 1. Purinergic receptors in oligodendrocyte progenitors as promising targets in multiple sclerosis. Schematic representation of purinergic receptors expressed in oligodendroglial cells and their ligands. To develop new purinergic-based drugs for remyelination, further efforts should be done in basic research, to provide robust data to be translated into clinics.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Supported by FISM—Fondazione Italiana Sclerosi Multipla—cod. 2017/R/1 to MPA and financed or co-financed with the ‘5 per mille’ public funding.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This commentary is dedicated to Geoffrey Burnstock, the founder of the purinergic field, who died on June 2, 2020, aged 91. Without his continuous and passionate intellectual support to the field none of the progresses described here would have been possible.
Agresti, C., Meomartini, M. E., Amadio, S., Ambrosini, E., Volonté, C., Aloisi, F., et al. (2005). ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res. Brain Res. Rev. 48 (2), 157–165. doi:10.1016/j.brainresrev.2004.12.005
Alexander, S. P. H., Christopoulos, A., Davenport, A. P., Kelly, E., Mathie, A., Peters, J. A., et al. (2019). The concise guide to pharmacology 2019/20: G protein-coupled receptors. Br. J. Pharmacol. 176 (Suppl. 1), S21–S141. doi:10.1111/bph.14748
Amadio, S., Parisi, C., Piras, E., Fabbrizio, P., Apolloni, S., Montilli, C., et al. (2017). Modulation of P2X7 receptor during inflammation in multiple sclerosis. Front. Immunol. 8, 1529. doi:10.3389/fimmu.2017.01529
Bhattacharya, A., and Biber, K. (2016). The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 64 (10), 1772–1787. doi:10.1002/glia.23001
Calzaferri, F., Ruiz-Ruiz, C., de Diego, A. M. G., de Pascual, R., Méndez-López, I., Cano-Abad, M. F., et al. (2020). The purinergic P2X7 receptor as a potential drug target to combat neuroinflammation in neurodegenerative diseases. Med. Res. Rev. 40 (6), 2427–2465. doi:10.1002/med.21710
Chen, Y., Wu, H., Wang, S., Koito, H., Li, J., Ye, F., et al. (2009). The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci. 12 (11), 1398–1406. doi:10.1038/nn.2410
Ciana, P., Fumagalli, M., Trincavelli, M. L., Verderio, C., Rosa, P., Lecca, D., et al. (2006). The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 25 (19), 4615–4627. doi:10.1038/sj.emboj.7601341
Comi, G., Radaelli, M., and Soelberg Sørensen, P. (2017). Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 389 (10076), 1347–1356. doi:10.1016/S0140-6736(16)32388-1
Coppi, E., Maraula, G., Fumagalli, M., Failli, P., Cellai, L., Bonfanti, E., et al. (2013). UDP-glucose enhances outward K(+) currents necessary for cell differentiation and stimulates cell migration by activating the GPR17 receptor in oligodendrocyte precursors. Glia 61 (7), 1155–1171. doi:10.1002/glia.22506
Coppi, E., Cellai, L., Maraula, G., Dettori, I., Melani, A., Pugliese, A. M., et al. (2015). Role of adenosine in oligodendrocyte precursor maturation. Front. Cell. Neurosci. 9, 155. doi:10.3389/fncel.2015.00155
Coppi, E., Cherchi, F., Fusco, I., Dettori, I., Gaviano, L., Magni, G., et al. (2020). Adenosine A2B receptors inhibit K+ currents and cell differentiation in cultured oligodendrocyte precursor cells and modulate sphingosine-1-phosphate signaling pathway. Biochem. Pharmacol. 177, 113956. doi:10.1016/j.bcp.2020.113956
Coppolino, G. T., Marangon, D., Negri, C., Menichetti, G., Fumagalli, M., Gelosa, P., et al. (2018). Differential local tissue permissiveness influences the final fate of GPR17-expressing oligodendrocyte precursors in two distinct models of demyelination. Glia 66 (5), 1118–1130. doi:10.1002/glia.23305
Dawson, M. R., Polito, A., Levine, J. M., and Reynolds, R. (2003). NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 24 (2), 476–488. doi:10.1016/s1044-7431(03)00210-0
De Nuccio, C., Bernardo, A., Ferrante, A., Pepponi, R., Martire, A., Falchi, M., et al. (2019). Adenosine A2A receptor stimulation restores cell functions and differentiation in Niemann-Pick type C-like oligodendrocytes. Sci. Rep. 9 (1), 9782. doi:10.1038/s41598-019-46268-8
Di Virgilio, F., Dal Ben, D., Sarti, A. C., Giuliani, A. L., and Falzoni, S. (2017). The P2X7 receptor in infection and inflammation. Immunity 47 (1), 15–31. doi:10.1016/j.immuni.2017.06.020
Fields, R. D., and Stevens, B. (2000). ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci. 23 (12), 625–633. doi:10.1016/s0166-2236(00)01674-x
Franklin, R. J., and ffrench-Constant, C. (2017). Regenerating CNS myelin - from mechanisms to experimental medicines. Nat. Rev. Neurosci. 18 (12), 753–769. doi:10.1038/nrn.2017.136
Franklin, R. J., and ffrench-Constant, C. (2008). Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9 (11), 839–855. doi:10.1038/nrn2480
Fratangeli, A., Parmigiani, E., Fumagalli, M., Lecca, D., Benfante, R., Passafaro, M., et al. (2013). The regulated expression, intracellular trafficking, and membrane recycling of the P2Y-like receptor GPR17 in Oli-neu oligodendroglial cells. J. Biol. Chem. 288 (7), 5241–5256. doi:10.1074/jbc.M112.404996
Fumagalli, M., Daniele, S., Lecca, D., Lee, P. R., Parravicini, C., Fields, R. D., et al. (2011). Phenotypic changes, signaling pathway, and functional correlates of GPR17-expressing neural precursor cells during oligodendrocyte differentiation. J. Biol. Chem. 286 (12), 10593–10604. doi:10.1074/jbc.M110.162867
Fumagalli, M., Lecca, D., and Abbracchio, M. P. (2016). CNS remyelination as a novel reparative approach to neurodegenerative diseases: the roles of purinergic signaling and the P2Y-like receptor GPR17. Neuropharmacology 104, 82–93. doi:10.1016/j.neuropharm.2015.10.005
González-Fernández, E., Sánchez-Gómez, M. V., Pérez-Samartín, A., Arellano, R. O., and Matute, C. (2014). A3 Adenosine receptors mediate oligodendrocyte death and ischemic damage to optic nerve. Glia 62 (2), 199–216. doi:10.1002/glia.22599
Grygorowicz, T., Struzyńska, L., Sulkowski, G., Chalimoniuk, M., and Sulejczak, D. (2010). Temporal expression of P2X7 purinergic receptor during the course of experimental autoimmune encephalomyelitis. Neurochem. Int. 57 (7), 823–829. doi:10.1016/j.neuint.2010.08.021
Hamilton, N., Vayro, S., Wigley, R., and Butt, A. M. (2010). Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia 58 (1), 66–79. doi:10.1002/glia.20902
Lecca, D., Raffaele, S., Abbracchio, M. P., and Fumagalli, M. (2020). Regulation and signaling of the GPR17 receptor in oligodendroglial cells. Glia 68 (10), 1957–1967. doi:10.1002/glia.23807
Lecca, D., Trincavelli, M. L., Gelosa, P., Sironi, L., Ciana, P., Fumagalli, M., et al. (2008). The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS One 3 (10), e3579. doi:10.1371/journal.pone.0003579
Lombardi, M., Parolisi, R., Scaroni, F., Bonfanti, E., Gualerzi, A., Gabrielli, M., et al. (2019). Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta Neuropathol. 138 (6), 987–1012. doi:10.1007/s00401-019-02049-1
Marangon, D., Boccazzi, M., Lecca, D., and Fumagalli, M. (2020). Regulation of oligodendrocyte functions: targeting lipid metabolism and extracellular matrix for myelin repair. J. Clin. Med. 9 (2), 470. doi:10.3390/jcm9020470
Marisca, R., Hoche, T., Agirre, E., Hoodless, L. J., Barkey, W., Auer, F., et al. (2020). Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat. Neurosci. 23 (3), 363–374. doi:10.1038/s41593-019-0581-2
Marques, S., Zeisel, A., Codeluppi, S., van Bruggen, D., Falcão, A. M., Xiao, L., et al. (2016). Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352 (6291), 1326–1329. doi:10.1126/science.aaf6463
Matute, C., Torre, I., Pérez-Cerdá, F., Pérez-Samartín, A., Alberdi, E., Etxebarria, E., et al. (2007). P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. 27 (35), 9525–9533. doi:10.1523/JNEUROSCI.0579-07.2007
McCall, R. L., Cacaccio, J., Wrabel, E., Schwartz, M. E., Coleman, T. P., and Sirianni, R. W. (2014). Pathogen-inspired drug delivery to the central nervous system. Tissue Barriers 2 (4), e944449. doi:10.4161/21688362.2014.944449eCollection 2014
Molina-Gonzalez, I., and Miron, V. E. (2019). Astrocytes in myelination and remyelination. Neurosci. Lett. 713, 134532. doi:10.1016/j.neulet.2019.134532
Nave, K. A. (2010). Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 11 (4), 275–283. doi:10.1038/nrn2797
Nishiyama, A., Komitova, M., Suzuki, R., and Zhu, X. (2009). Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 10 (1), 9–22. doi:10.1038/nrn2495
Ontaneda, D., Fox, R. J., and Chataway, J. (2015). Clinical trials in progressive multiple sclerosis: lessons learned and future perspectives. Lancet Neurol. 14 (2), 208–223. doi:10.1016/S1474-4422(14)70264-9
Parravicini, C., Lecca, D., Marangon, D., Coppolino, G. T., Daniele, S., Bonfanti, E., et al. (2020). Development of the first in vivo GPR17 ligand through an iterative drug discovery pipeline: a novel disease-modifying strategy for multiple sclerosis. PLoS One 15 (4), e0231483. doi:10.1371/journal.pone.0231483
Plemel, J. R., Liu, W. Q., and Yong, V. W. (2017). Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat. Rev. Drug Discov. 16 (9), 617–634. doi:10.1038/nrd.2017.115
Saze, Z., Schuler, P. J., Hong, C. S., Cheng, D., Jackson, E. K., and Whiteside, T. L. (2013). Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood 122 (1), 9–18. doi:10.1182/blood-2013-02-482406
Soliven, B., Miron, V., and Chun, J. (2011). The neurobiology of sphingosine 1-phosphate signaling and sphingosine 1-phosphate receptor modulators. Neurology 76 (8, Suppl. 3), S9–S14. doi:10.1212/WNL.0b013e31820d9507
Stangel, M., Kuhlmann, T., Matthews, P. M., and Kilpatrick, T. J. (2017). Achievements and obstacles of remyelinating therapies in multiple sclerosis. Nat. Rev. Neurol. 13 (12), 742–754. doi:10.1038/nrneurol.2017.139
Stevens, B., Porta, S., Haak, L. L., Gallo, V., and Fields, R. D. (2002). Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36 (5), 855–868. doi:10.1016/s0896-6273(02)01067-x
Keywords: purinoceptors, GPR17, oligodendrocyte progenitors, myelin repair, multiple sclerosis
Citation: Lecca D, Abbracchio MP and Fumagalli M (2021) Purinergic Receptors on Oligodendrocyte Progenitors: Promising Targets for Myelin Repair in Multiple Sclerosis?. Front. Pharmacol. 11:629618. doi: 10.3389/fphar.2020.629618
Received: 15 November 2020; Accepted: 17 December 2020;
Published: 27 January 2021.
Edited by:
Francesco Caciagli, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Christian Lohr, University of Hamburg, GermanyCopyright © 2021 Lecca, Abbracchio and Fumagalli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta Fumagalli, bWFydGEuZnVtYWdhbGxpQHVuaW1pLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.