- Key Laboratory of Chinese Materia Medica, Ministry of Education, Heilongjiang University of Chinese Medicine, Harbin, China
On January 2, 2020, The National Health Commission and the State Administration for Market Regulation listed Panacis Quinquefolii Radix (PQR) as a medicinal and food homologous product. PQR is the dry root of Panax quinquefolium L., which has the functions of replenishing qi and nourishing Yin, clearing heat and producing body fluid. It is often used for qi deficiency and Yin deficiency, heat exhaustion, asthma and phlegm, dry mouth and pharynx. PQR is sweet, slightly bitter and cool in nature, and enter the heart, lung and kidney meridian exerts the remedial and hygienical effect. At present, active components such as saponins, flavonoids, fatty acids, polyalkynes, volatile oils and other nutrients such as amino acids, carbohydrates, vitamins and trace elements have been isolated from PQR. Modern pharmacological studies have shown that PQR has the effects of hypoglycemic, antihypertensive, anti fatigue, anti-oxidation, anti-tumor, immunomodulatory, neuroprotective and so on. In addition, PQR is recognized as a health care product to strengthen the body and dispel diseases. It is not only the raw material of Traditional Chinese medicine preparations, but also the treasure of dietary therapy and herbal cuisine. This study not only reviewed the botany, phytochemistry and pharmacology of PQR, but also summarized its quality control, toxicity and industrial applications for the first time. This paper not only summarizes the development status of PQR, but also analyzes the shortcomings of the current research on PQR, and puts forward the corresponding solutions, in order to provide reference for future scholars to study PQR.
Introduction
Panacis Quinquefolii Radix (PQR), has a Chinese name of “Xiyangshen,” (Figure 1), derives from the dry root of Panax quinquefolium L., which is sweet, slightly bitter and cool in nature and has the functions of replenishing qi and nourishing Yin, clearing heat and producing body fluid. PQR was listed as a medicinal and food homologous product by the National Health Commission and the State Administration for Market Regulation on January 2, 2020. PQR is native to Montreal, Quebec and Vancouver mountains in Canada, and Wisconsin, Missouri and New York in the eastern United States and West Virginia, Oregon and California, etc. (Qu, 2009).

FIGURE 1. Panax quinquefolium L. stem-leaves (A), Panax quinquefolium L. fruit (B), Panax quinquefolium L. roots (C) and Panax quinquefolium L. slices (D).
PQR was first published in “ New Compilation of Materia Medica” (本草从新), which can prove that PQR has been used in China for more than 200 years (Chen, 1995). For a long time, China has been dependent on the import of PQR, and most of the medicines are limited to nourishing and strengthening or used as a placebo for patients with advanced cancer, which limits the medicinal range of PQR and makes this precious medicinal material fail to play its full role. PQR was introduced and planted in China in 1970s, now it is widely cultivated in Jilin, Heilongjiang, Shaanxi, Henan, Jiangxi, Liaoning, Beijing, Fujian and other places (Jin and Fan, 1992; Ma and Rui, 2019). At present, China has developed into the third largest PQR producing country in the world after the United States and Canada, and the world’s largest PQR consumer country.
In recent years, scholars at home and abroad have studied PQR. Chemical studies have shown that PQR contains active components such as saponins (Wang M. Y. et al., 2020), polysaccharides (Zhai et al., 2015), flavonoids (Zhen, 2012), fatty acids, polyalkynes (Fujimoto et al., 1991), sterols, volatile oils (Liu X. F. et al., 2016) and nutrients such as amino acids, carbohydrates, vitamins (Wang Y. et al., 2013), trace elements (Zhao et al., 2012). Modern pharmacological studies have shown that PQR has the effects of hypoglycemia (Jenkins et al., 2017), hypotension (Stavro et al., 2006), antioxidant (Liu et al., 2019), anti-tumor (Chang et al., 2018), immune regulation (Jiang et al., 2020), neuroprotection (Shin et al., 2016) and so on. It is commonly used in the treatment of cancer (Chen et al., 2016), hypertension (Jovanovski et al., 2020), diabetes (Vuksan et al., 2019), myocardial ischemia (Yu et al., 2020), gastrointestinal tract and other diseases (Liu et al., 2020). Furthermore, PQR is also widely used in health care medicines, health food, health care cosmetics and other fields, such as drinks, biscuits, medicated meals, facial mask, etc. (Li et al., 2015; Yim et al., 2015), they have the functions of enhancing immunity, relieving physical fatigue (Qi et al., 2014), delaying aging (Song et al., 2019),anti-radiation (Luo et al., 2014) auxiliary protective function for chemical liver injury (Jiang et al., 2020)
PQR is a valuable medicinal material, which has been favored by people for its unique medical and health care functions. In this study, 1,742 literatures about PQR from 1982 to now were collected and consulted, in addition to repetitive and irrelevant literatures, 172 articles were reviewed in this study on botany, phytochemistry, quality control, pharmacology, toxicology and industrial applications of PQR. In this study, the ingredients in PQR were displayed in detail, and their biological activities were summarized. For the first time, the research on quality control, toxicity and industrial applications were reviewed. Not only the development status of PQR was briefly reviewed, but also the shortcomings of the current research were discussed and the corresponding solutions were proposed, so as to provide important information for future scholars to study, development and utilization of PQR. In a word, this review has important reference value for the further study of PQR.
Botany
According to the Chinese Pharmacopoeia (2020 edition), PQR is spindle shaped, cylindrical or conical, with a length of 3–12 cm and a diameter of 0.8–2 cm. Its surface is light yellow-brown or yellow-white, with transverse annular lines and linear lenticel like protuberances, and have fine light longitudinal wrinkles and fibrous root marks. There are one or several lateral roots in the middle and lower part of the main root of PQR, most of which have been broken. Some PQR have rhizomes at the upper end, and the links are obvious. The stem marks are round or semicircular, and most of them have been broken.PQR is heavy in quality, solid in texture and not easy to be broken. The cross section of PQR is flat, pale yellow and white, with slightly powdery appearance. There are yellow-brown dot resin channels in the skin, cambium ring with brownish yellow color, and wood part with slightly radial texture. PQR has a small and unique smell and a slightly bitter and sweet taste. As the species of PQR are precious and wild resources are scarce, the majority of PQR in the market are cultivated products, and the difference between its traits and wild products is shown in Table 1.
“Chinese Materia Medica” (中华本草) records: PQR likes humid environment, should avoid strong light irradiation. Therefore, the cultivation of PQR needs to build a shading shed, the light transmittance of the shed is 20–25%. The light transmittance should be higher in spring (about 22%) and lower in summer, which can reduce the disease. And the soil for PQR cultivation should be sandy loam with good water permeability and fertility, mixed with large coarse sand, rather than clay or low-lying areas with poor drainage and easy to accumulate water.
Nutritional and Physiochemical Composition
Nutriment
At present, the world medicine is developing toward the combination of curative effect and nutrition. Modern research has confirmed that PQR contains protein, carbohydrate, vitamin, fat and other essential nutrients and macro elements, trace elements. Undoubtedly, they are valuable resources for nourishment, fitness, nutrition and medicine, and should be fully utilized.
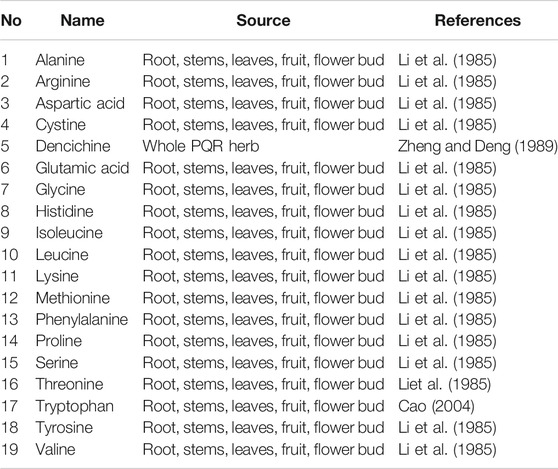
Amino Acid
PQR is rich in nutrients and contains a variety of amino acids, (As shown in Table 2), which have a positive effect on the human body. Zhang et al. determined that there were 18 kinds of amino acids (12.838 g/L) in PQR berries, among which 10 kinds were essential and semi-essential amino acids for human body (Cao, 2004). Zheng et al. isolated a special amino acid from PQR, denciemne, which is a major component for promoting blood circulation (Zheng and Deng, 1989). The amino acid contents in roots, stems, leaves, buds and seeds of PQR at different ages were analyzed. The results showed that the amino acid content of PQR decreased with the increase of PQR age. The contents of total amino acids in four-year-old PQR were as follows: flower bud (13.922%) > leaf (11.723%) > root (5.932%) > seed (5.006%) > stem (3.372%) (Li et al., 1985). Therefore, the flower buds and leaves of PQR contain a lot of nutrients. However, at present, the flower bud and leaf of PQR are rarely used, and it has not been deeply applied in nutrition and health care. Most of the flower bud and leaf resources are abandoned and cannot be fully utilized. In the future, the flower buds and leaves can be developed into PQR tea with health functions, or the flower buds of PQR can be made into PQR flower-cakes and biscuits, so that the resources of PQR can be better developed and utilized.
Polysaccharides
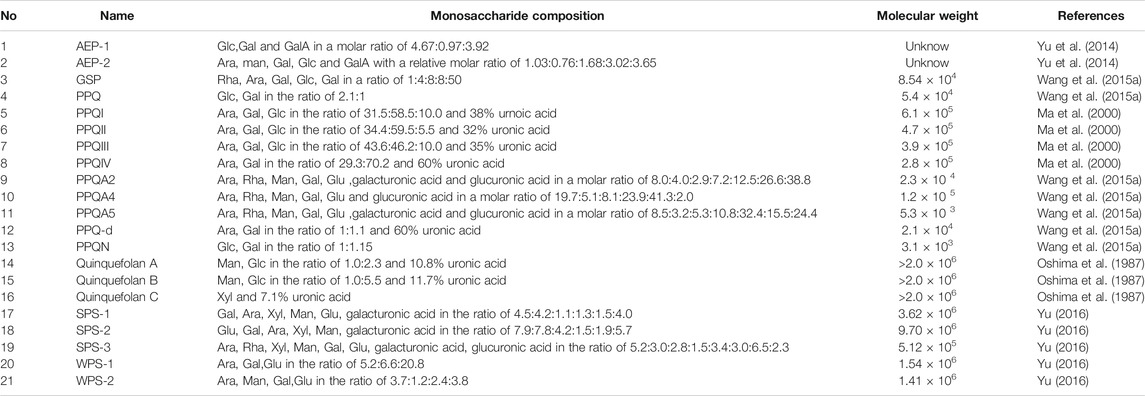
The proportion of polysaccharides in PQR is higher, and monosaccharides include glucose, fructose and sorbose, and the oligosaccharides include ginseng trisaccharide, maltose and sucrose (Chen and Cui, 2002). Zhang’s study showed that the average carbohydrate content of PQR from different regions was: reducing sugar 3.70%, oligosaccharide 15.40%, polysaccharide 49.74%, total sugar 68.84%. The results showed that 11.74–19.86% of water-soluble pectin could be extracted from PQR. The water-soluble pectin was composed of galacturonic acid, galactose (Gal), glucose (Glu), arabinose (Ara), xylose (Xyl), rhamnose (Rha) and a few unknown sugars (Zhang and Li, 1989). Liang et al. showed that the total sugar in PQR include starch, pectin, oligosaccharide and monosaccharide. The total amount is 68.2%–74.3% (Liang et al., 1989). In addition, some scholars also isolated a variety of polysaccharides from PQR, as shown in Table 3. However, there are few studies on the isolation and identification of polysaccharides in PQR. The possible reason is that the components of polysaccharides are complex and it is difficult to identify the polysaccharides by modern analytical methods. Polysaccharides from PQR have many pharmacological functions, such as immune regulation, anti-oxidation, anti-virus, anti-inflammatory, etc. In the future, researchers can develop more new instruments and methods, improve the identification system of polysaccharides, and develop monosaccharide drugs to facilitate clinical use. At the same time, the development of PQR polysaccharides into health food can also be used as a dietary supplement for human body, which can obtain a higher degree of acceptance than drugs.
Fatty Acid
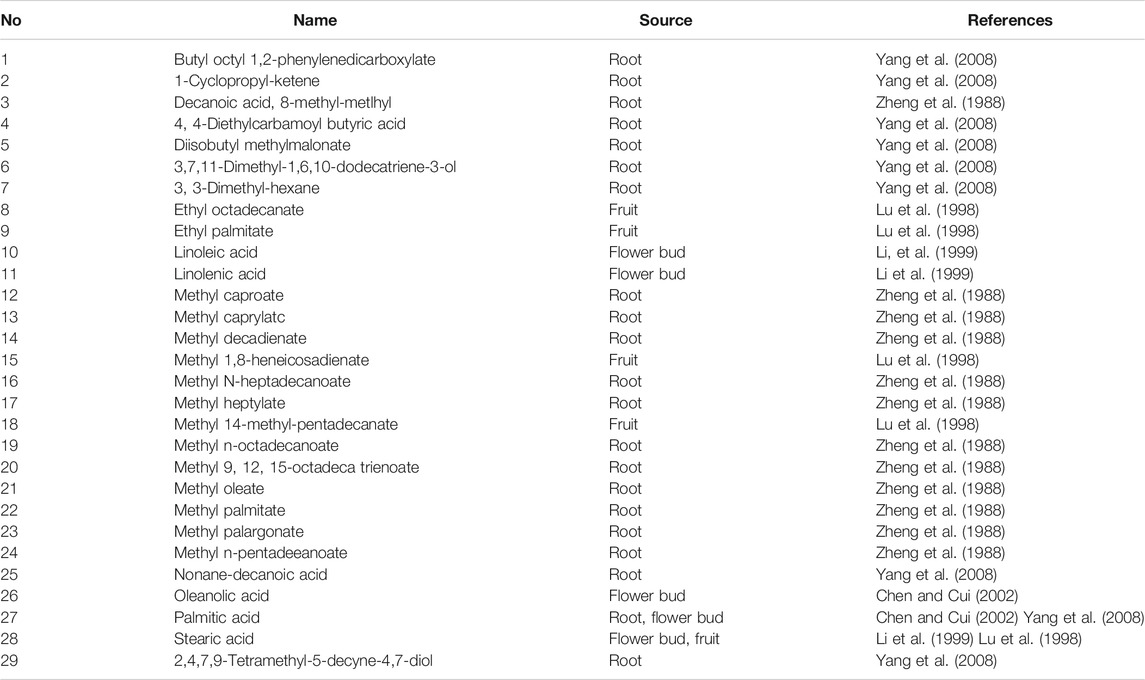
PQR is rich in fatty acids. (As shown in Table 4). Li et al. showed that the saturated fatty acid with the highest content in the flower bud of PQR was palmitic acid, and the unsaturated fatty acid with the highest content was linoleic acid (Li et al., 1999). Yang et al. showed that the fatty acid with the highest content in the root of PQR was methyl linoleate (59.56%) (Yang et al., 2008). Linoleic acid is an essential fatty acid for human body. Linoleic acid is very important for cholesterol metabolism. Only when cholesterol is combined with linoleic acid, can it run in the body and metabolize normally. If linoleic acid is deficient, cholesterol will combine with saturated fatty acid and deposit in human body. Moreover, linoleic acid can protect skin from radiation damage. In addition, linoleic acid also has a strong effect on reducing the concentration of LDL cholesterol. In Chinese Pharmacopoeia, ethyl linoleic acid pills and drops are still used to prevent and treat hypertension, atherosclerosis and coronary heart disease. However, most of the articles on anti-radiation, anti-hypertension and anti-cardiovascular diseases of PQR mainly focus on the study of the crude PQR and ginseng saponin, and few studies have conducted in-depth discussion on the composition, pharmacological effect and mechanism of fatty acid compounds in PQR. In the future, scholars can try to extract linoleic acid from PQR, and then apply it to the treatment of some diseases. Future scholars should not only study the saponins, they should pay more attention to other active components and analyze the overall characteristics of PQR.
Mineral Elements
PQR contains macroelement, including Ca, K, Na, Mg. The essential microelements include: V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Se, Mo, Sn, etc. Rare earth elements include: Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, etc. Toxic elements such As, Pb, Hg and Th radioactive elements also contain Li, Be, B, Al, Ga, Rb,As, Sr, Ag, Cd, Cs, Ba, Ti, Bi, U, I, P, Ti, Si and other elements (Liu et al., 1987; Zhang et al., 1987) (Zhang et al., 1988a; Feng et al., 2018). Zhang’s research showed that the content of mineral elements in the aboveground part (stem, leaf and seed) of PQR was higher than that in the underground part. And the content of mineral elements in the stems and leaves which had not been used as medicine in the past was much higher than that in the taproot. Moreover, various mineral elements in PQR can promote the growth of children and other therapeutic effects, on the other hand, they can play a role in disease prevention and health care.
In addition, PQR also contains vitamins A, B, B2, B6 (Chen and Cui, 2002), superoxide dismutase (SOD), peroxidase (POD), esterase (ES) (Wu and Lin, 1991), nitrate reductase (NR), catalase (CAT), polyphenol oxidase (PPO), ascorbate peroxidase (APX) (Wang S. et al., 2016), crude protein, crude fiber and other components (Sun and Liu, 1993)
Physiochemical Composition
Saponins
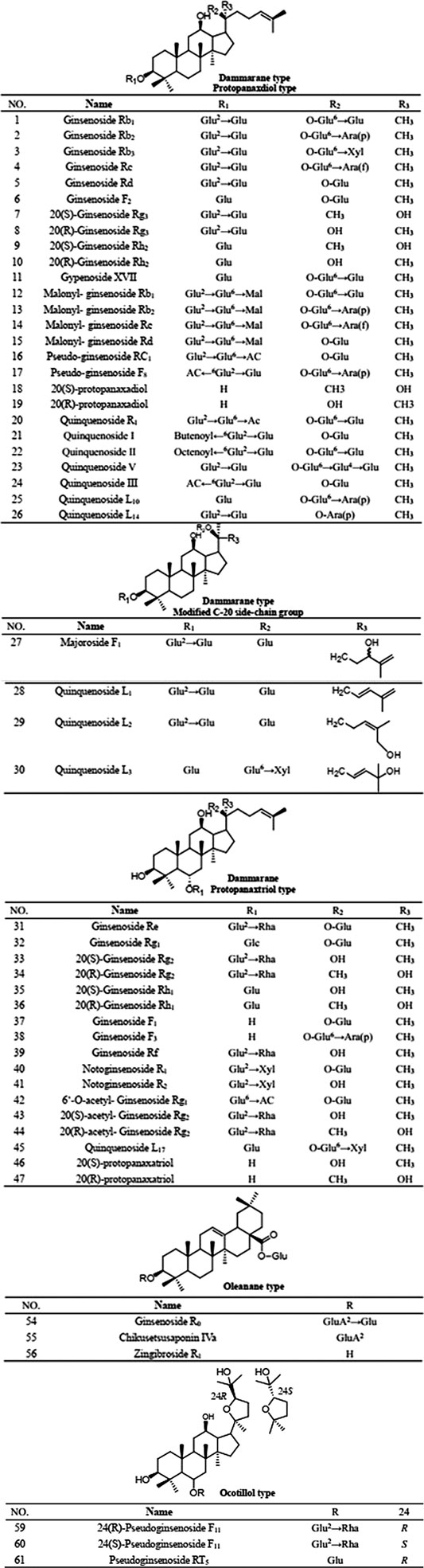
PQR mainly contains ginsenosides, which can be divided into three types according to their parent structure: dammarane, oleanane and oceanillol. Studies have confirmed that ginsenosides are contained in roots, stems, leaves, flowers, fruits and seeds of PQR. Now more than 70 ginsenosides have been isolated from different parts of PQR (as shown in Table 5; Figure 2). Meng et al. studies showed that the total saponins content in different parts of PQR was as follows: flower bud > flower stalk > pulp > root > stem and leaf, the highest content of saponins in flower bud was 14.87%, and the lowest was 5.79% in stem and leaf (Meng et al., 2000). Wang et al. showed that the stem, leaves and taproot of PQR contain saponins, and the content of ginsenosides in the stem was 1/3-1/2 of that in the taproot, while the content of ginsenosides in the leaves was 1.5–4 times of that in the taproot (Wang and Dong, 1995). In other words, because the leaves of PQR are not exploited and utilized, a lot of the medicinal ingredients are lost, which results in a great waste of resources. Future scholars can focus on the stems and leaves of PQR in order to make full use of its resources. As mentioned above, PQR is expensive and the resources are in short supply. If the root of PQR is used as medicine, abandoning the buds of stems and leaves will cause a great waste of resources. The above studies also showed that the content of saponins in the flower bud was the highest, followed by the fruit. In Pharmacopoeia, the plants with dried flower buds and fruits as medicinal parts were not rare. Future scholars can study the pharmacological effects or edible value of the flower buds and fruits of PQR. On the one hand, it can make full use of PQR resources and alleviate the pressure of supply exceeding demand of the market of PQR. On the other hand, it can develop new medicinal parts of PQR and enrich its clinical application and food health application.
Volatile Oil
Shen et al. identified 37 compounds from the volatile oil of PQR cultivated in Jilin province, among which 26 sesquiterpenes accounted for 75% of the total volatile oil, the content of trans-β-farnesene was as high as 36%, and the content of bisabolene was as high as 10%, which were the main components of the volatile in PQR (Shen and Wang, 1991). Yang and Meng et al. isolated 47 and 39 volatile oil components from the root of PQR, which also indicated that the content of β-farnesene was the highest (Meng et al., 2001; Yanget al., 2008). Zheng et al. isolated and identified the volatile oil components of PQR cultivated in Heilongjiang province. The basic components contained ketones, alcohols, phenols and aldehydes. The strong acidic components were dominated by organic acids, and also contained aldehydes and ketones. The weak acidic components were mainly contain alkene, and also contained ketones and esters.The neutral components were dominated by esters and also contain alkenes and phenols (Zheng and Zhang, 1993).
Polyacetylene
Polyacetylene is an unsaturated compound with a carbon-carbon triple bond. Polyacetylene is one of the active ingredients of PQR. So far, a total of 15 polyacetylene have been reported in PQR, such as acetylpan axydol, (6R, 7S)-6, 7-epoxyte-tradeca-1,3-diyne, falcalinol, ginsenoyne G, heptadeca-1,8-diene-4,6-diyne-3,10-diol, panaxydol, panaxytriol, panaquinquecols I1, panaquinquecols I2, panaquinquecols I3, PQ-1, PQ-2, PQ-3, PQ-7, PQ-1, PQ-8 (Fujimoto et al., 1991; Fujimoto et al., 1994; Satoh et al., 1997; Bao et al., 1998).
Sterol
Sterol has many functions such as anti-tumor, promoting metabolism, regulating hormone level and preventing cardiovascular disease. At present, a variety of sterols have been isolated from PQR, daucosterol has been isolated from root and stem of PQR. β-sitosterol (Zhang, 1997), stigmasterol and stigmasterol-3,5-dien-7-one were obtained from the roots of PQR (Zhang et al., 1988b). Stigmast- 5- en- 3- ol was identified from the fruit of PQR (Wang et al., 2007).
Flavone
Wei et al. isolated two flavonoid monomers, kaempferol and panasenoside, from the leaves of PQR. Meng et al. first discovered the existence of panasenoside in the root and fruit of PQR (Meng et al., 2002). And Liu (2008). isolated panasenoside II and trifolin from PQR. Flavonoid is one of the natural active ingredients which has been widely concerned in recent years. It has the functions of anti-oxidation and sterilization At present, there are few studies on the flavonoids in PQR, and it is hoped that future scholars could pay more attention to this compound with strong pharmacological activity.
Other
In addition, PQR contains nucleoside compounds: deoxyuridine, deoxythymidine, deoxyadenosine and adenine. Lignan compounds such as pinoresinol and 4,4′, 8′ - trihydroxy-3,3′- dimethoxy-9′ – lignanolide. And Panaxynol compounds include panaxynol, panaxytriol, panaxynol A and panaxynol D. PQR also contains polyphenols compounds such as vanillic acid, p-coumaric acid, cinnamic acid (Jing et al., 2011), caffeic acid and chlorogenic acid (Wang et al., 2007).
With the deepening of the research on PQR, the ministry of health has approved it as a medicinal and food homologous product. The successful introduction and planting of PQR in China provides good materials for clinical, medical and other fields.
At present, the research on the chemical composition of PQR mainly focuses on the determination of its saponins, amino acids and trace elements.There are relatively few studies on the analysis of proteins, fatty acids and other organic compounds and their functions, which may be due to the complex components of proteins and polysaccharides, which are difficult to separate and purify. Moreover, in the process of extraction of PQR and the determination of substance content and types in the residue after production, it was found that a large amount of crude protein and crude polysaccharide could not be effectively used and could only be discarded with the discharge of waste residue. PQR as a high-grade health care products, the price is expensive, and the demand exceeds supply. According to the determination, the content of saponins and polysaccharides in the leaves of PQR is as high as more than 10%, which is twice as much as that in the root of PQR. In other words, because the leaves of Panax quinquefolium are not exploited and utilized, 2/3 of the medicinal ingredients are lost. And modern pharmacological studies have shown that PQR polysaccharides have good immunomodulatory and antioxidant activities. Due to the high price of PQR, future scholars can focus on the stems and leaves of PQR. Therefore, how to make full use of PQR remains to be further studied.
Quality Control
With the increasing demand for PQR, PQR is in short supply and expensive. In order to seek excessive profits, the adulterants of PQR increase accordingly, and the phenomenon of shoddy products frequently occurs in the market. Moreover, plant resources are easily affected by different factors, such as provenance, growth environment, harvesting time, processing methods and storage conditions, which makes the quality of PQR from different regions uneven. In addition, PQR has complex chemical components and many chemical substances have pharmacological effects. The quality control of medicinal materials is the most problematic link in the production of medicinal materials. At present, the key problem in the quality evaluation of PQR is that the evaluation index is too one-sided and unitary.In Chinese pharmacopoeia (2020 edition), only Rg1, Re and Rb1 were used as the quality control indexes of PQR. However, medicine materials contains many kinds of ingredients, different ingredients work together, it is not scientific and objective to evaluate the quality of medicine materials only by using one or several effective components as indicators. Therefore, in order to ensure the safety and effectiveness of PQR, it is necessary to establish a rapid, accurate and sensitive detection method to simultaneously measure a variety of chemical components, evaluate the quality of medicinal materials, and improve the quality control methods.
Some qualitative and quantitative methods have been used to evaluate the quality of PQR. In the past, scholars mostly used thin-layer chromatography to qualitatively identify PQR (Liu Y. et al., 2016), or established fingerprint of PQR by high-performance liquid chromatography for the qualitative identification (Tang et al., 2015).In the past, colorimetry (Yin et al., 2010), near infrared spectroscopy (Huang et al., 2011) nuclear magnetic resonance (NMR) spectroscopy (Nabuurs et al., 2017) inductively coupled plasma method (Jiang et al., 2020)gas chromatography (Lin et al., 2018), high performance liquid chromatography in series with various detectors (Chen et al., 2020, Xiong et al., 2020), and immunoassay (Morinaga et al., 2010) were used for multi-component determination and quality control of PQR. However, there are some defects in the design of these methods.
These studies did not clarify the quality markers of PQR. They only determined and analyzed the saponins and mineral elements commonly existing in the PQR, and did not show that these components are the main active substances of PQR. The future research of quality control should consider integrating quality overall evaluation technology and Internet analysis technology. In order to promote the sustainable development of PQR industry, the pharmacological effects of active ingredients and the efficacy of Chinese medicine can be studied in an integrated way.
Secondly, previous methods lack of studies on other compounds in PQR except saponins, volatile substances and mineral elements. Chinese medicine has the characteristics of multi-component and multi-target joint effect. Only one or two kinds of compounds as the main research direction is far from meeting the needs of the development of traditional Chinese medicine. Besides, PQR, as a medicinal and food homologous product, should pay more attention to the study of the effective active components such as carbohydrate, fatty acid and amino acid in future quality control research.
Finally, most of the methods have some disadvantages, such as complex sample pretreatment, using toxic organic solvents, polluting the soil and not green enough. Future scholars should not only focus on the existing methods, but also try to adopt other methods to control the multi-component quality of PQR. For example, Hou et al. used UPLC-QDA method to analyze the spectral effect of Angelicae Pubescentis. Compared with other mass spectrometry methods, this method is cheaper and more cost-effective (Houet al., 2020).Yang et al. established the fingerprint of Angelica Pubescens by UPCC method, and analyzed the overall information of Angelica Pubescens. The method uses CO2 as mobile phase, which is environmentally friendly and green (Yang et al., 2020). At the same time, it is also hoped that with the rapid development of science and technology, future engineers will develop more and more inexpensive, efficient instruments and green reagents.
Pharmacology
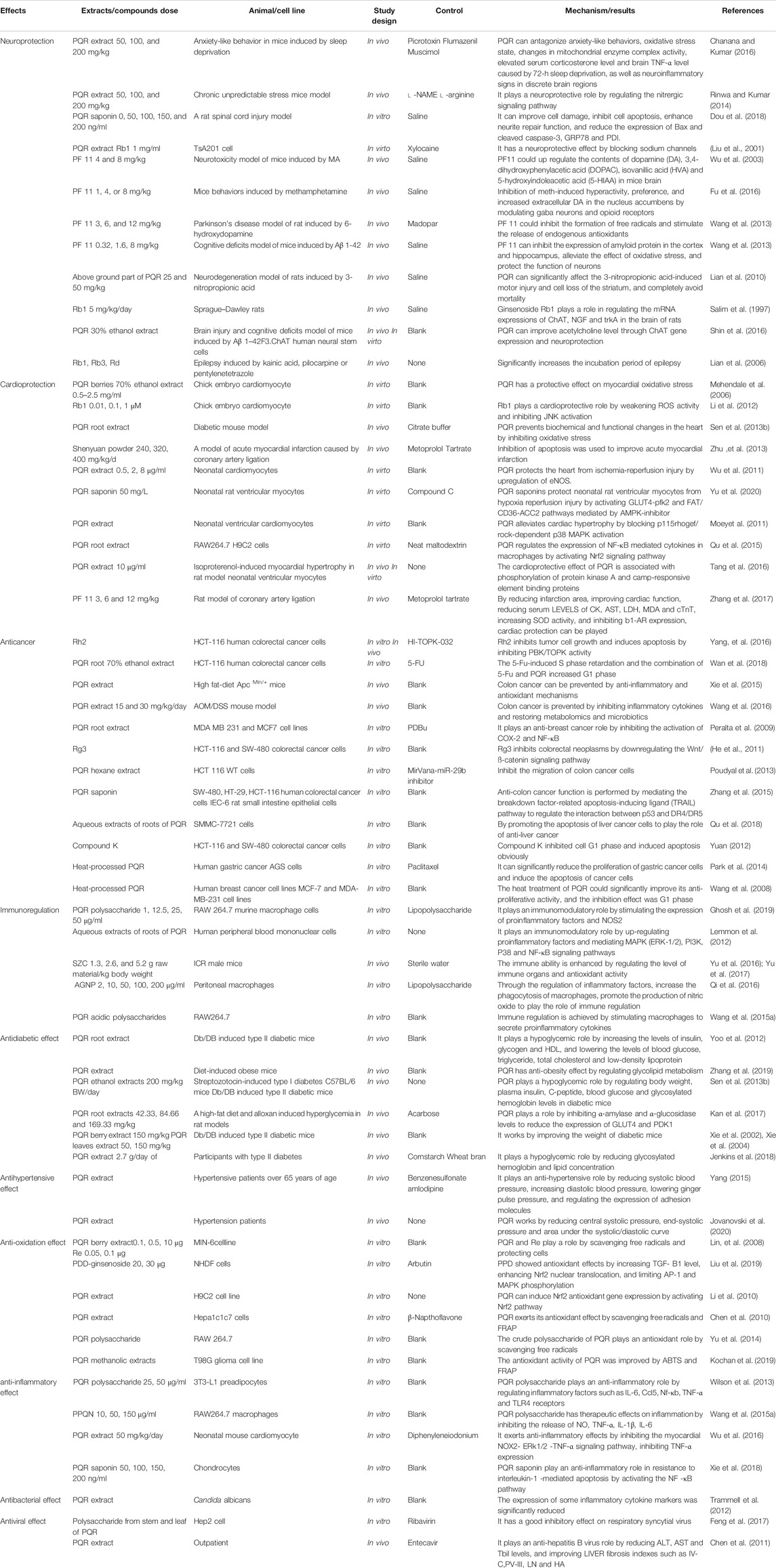
Modern pharmacological studies have shown that American ginseng has neuroprotective (Chanana and Kumar. 2016), anti-cardiovascular disease (Mehendale et al., 2006), anti-tumor (He et al., 2011), immunomodulatory (Qi et al., 2016), anti-hypertension (Wu et al., 2007), anti-diabetes (Sievenpiper et al., 2003), anti-oxidation (Hu and Kitts, 2001), anti-inflammatory (Xie et al., 2018), antibacterial, antiviral (Mousa, 2015), anti-radiation and other effects (Liao et al., 2019). These pharmacological effects are summarized in Table 6 and discussed in detail below.
Centralsystem Protection
PQR can resist anxiety like behavior caused by 72-h sleep deprivation, oxidative stress state, changes in mitochondrial enzyme complex activity, elevated serum corticosterone level, increased brain TNF-α level, and neuroinflammatory like signs in discrete brain regions Pretreatment with GABA agonist could enhance the protective effect of PQR, and the protective effect is more significant than itself (p < 0.05) (Chanana and Kumar, 2016). PQR can significantly improve cognitive impairment caused by chronic unpredictable stress (CUS), regulate oxidative stress markers in the hippocampus, mitochondrial enzyme complex activity, and the level of proinflammatory cytokines (TNF-α), acetylcholinesterase, and serum corticosterone. l-arginine could reverse the protective effect of PQR. And l-NAME could enhance the function of PQR (Rinwa and Kumar, 2014). In addition, PQR could also be used as a drug for the treatment of acute central nervous system injury. Studies have shown that PQR could improve endoplasmic reticulum stress and neuronal cultural-related cell apoptosis after acute spinal cord injury induced by triglyceride (TG), and promote functional recovery after spinal cord injury (Dou et al., 2018). Other studies have shown that the extract of PQR enhances and reversibly blocks the Na+ channel in the way of concentration and voltage dependence. And PQR changed the voltage dependence of inactivation and delayed the recovery after inactivation to protect the ischemic nerve (Liu et al., 2001).
Studies have shown that pseudoginsenoside-F11 (PF11) in PQR may be a potential drug candidate for the prevention and treatment of neurologic disorders caused by methamphetamine (MA) abuse. PF11 could significantly shorten the resting time of mice induced by MA, (p < 0.05) and significantly shorten the incubation period of MA, (p < 0.05) antagonize the decrease of dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA) and 5-hydroxyindoleacetic acid (5-HIAA) induced by MA (Wu et al., 2003). In addition, PF11 can inhibit methamphetamine induced hyperactivity, preference and the increase of extracellular DA by regulating GABA neurons and opioid receptors. It has effective neuroprotection, promotes learning and memory, and antagonizes the pharmacological effects of morphine (Fu et al., 2016). Wang et al. showed that PF11 in PQR could significantly improve the motor ability, motor balance and coordination ability of Parkinson rats. PF11 could also increase the expression of tyrosine hydroxylase (TH) in substantia nigra and the content of extracellular DA in striatum, decrease the levels of extracellular hydroxyl radical (∙OH), 2,3 - and 2,5-dihydroxybenzoic acid (2,3 - and 2,5-DHBA), and increase the level of extracellular ascorbic acid (AA) (Wang J. Y. et al., 2013). Other studies have shown that PF11 could significantly inhibit the expression of amyloid protein (APP) and Aβ 1-42 in the cortex and hippocampus, restore the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX), and reduce the generation of malondialdehyde (MDA) in the cortex. And the expressions of JNK2, p53, and cleaved caspase 3 in the hippocampus were down-regulated by PF11. These results suggest that PF11 may be a potential drug for the treatment of AD (Wanget al., 2013).
The root of PQR has no protective effect on 3-nitropropionic acid (3-NP)-induced neurodegeneration, but the stems and leaves of its aerial part could reduce the volume of striatal lesions induced by 3-NP. The main reason is that the aerial part of PQR contains a higher level of ginsenoside, among which Rb1, Rb3 and Rd play a key role. Since the neurodegeneration induced by 3-NP is similar to the neuron loss in Huntington’s disease, PQR may have clinical application value in the prevention of neurodegeneration in Huntington’s disease or other neurological diseases (Lian et al., 2010). Chen et al. showed that Rb1, Rg1 and other important ginsenosides in PQR are well-known components to improve human cognitive function (Chen and Hui, 2012). In vivo studies have shown that it may play a role by increasing the expression of choline acetyltransferase and trkA mRNA in the basal forebrain and the expression of nerve growth factor mRNA in the hippocampus through the cholinergic system (Salim et al., 1997). In vitro studies have shown that PQR could protect against F3 cytotoxicity induced by Aβ 1-42 by enhancing the expression of CHAT gene. It can also restore the cognitive function of mice by regulating the concentration of microtubule-associated proteins 2, synaptophysin and acetylcholine in the brain (Shin et al., 2016). Shi’s study showed that daily oral consumption of PQR could enhance the neurocognitive function of aging mice, which may be related to the up-regulation of insulin and adrenaline gene expression in the brain (Shi et al., 2012). Moreover, 52 healthy volunteers were tested, and according to the double-blind, placebo-controlled, balanced, and cross-design principles, the study showed that PQR significantly improved working memory in middle-aged people (Ossoukhova et al., 2015). In addition, Rb1,Rb3 and Rd in PQR have a good neuroprotective effect on the epilepsy induced by heneic acid, pilocarpine or pentaerythrazol (Lian et al., 2006).
The above studies have shown that PQR has a good neuroprotective effect on neurological disorders such as Huntington’s disease, epilepsy, acute central nervous system injury, neurodegenerative and other diseases. However, there are common problems in the above experiments, such as the lack of a comparative study between positive control drugs and PQR, and the study only on related biochemical factors, and the lack of in-depth study on the target pathways, target proteins and relevant neuroprotective mechanisms. Previous literature indicated that rapamycin, sodium valproate, donepezil hydrochloride and other drugs could be used as positive drugs to explore neuroprotective effects. ALOX5, ADORA2A, CDK2, GSK3B and TLR4/JNK/caspase-3, AMPK/Mtor/ULK1 are the key targets and pathways of neuroprotective effect. Whether future scholars can use this as a starting point to explore the in-depth mechanism of the neuroprotective effect of PQR. In addition, attention to insulin/IGF signaling, mitochondrial function and glucose utilization in neurodegenerative models may contribute to a better understanding of diseases such as Alzheimer’s disease.
Cardioprotective Effect
Previous studies have shown that H2O2 is an important reactive oxygen species (ROS) which could induce oxidative damage through the interaction with iron. The berry extract and ginsenoside Re of PQR have high cardioprotective effects against oxidative stress. Phenolic acids such as caffeic acid and chlorogenic acid in PQR fruit could up-regulate the ability of myocardial cells to metabolize H2O2 in a dose-dependent manner and reduce the oxidative damage of cardiomyocytes induced by H2O2. It has protective effect on myocardial oxidative stress. However, the specific mechanism is not clarified (Mehendale et al., 2006). Rb1 has the activity of scavenging DPPH and hydroxyl radical, and can inhibit the increase of JNK phosphorylation (p < 0.01), reduce the expression of P-JNK, and improve the cell survival rate after H2O2 exposure to play a protective role on myocardial oxidative stress (Li et al., 2012). PQR could also significantly inhibit the upregulation of extracellular matrix protein and vasoactive factor caused by oxidative stress, and reduce the mRNA expression of cardiac natriuretic factor and brain natriuretic factor, so as to prevent the biochemical and functional changes of heart caused by diabetes (Sen et al., 2013b). Zhu et al. have shown that Panax quinquefolium can significantly inhibit oxidative stress, balance the ratio of Bcl-2/Bax, and inhibit the activation of Caspase-3 (Zhu et al., 2013). In addition, it could also significantly increase the phosphorylation of Akt and eNOS protein levels in ischemia-reperfusion myocardial cells, and significantly reduce the infarct area and myocardial cell apoptosis in mice to improve myocardial infarction (Wu et al., 2011). And PQR saponin could significantly increase the survival rate of neonatal rat ventricular myocytes induced by hypoxia reperfusion, down regulate lactate dehydrogenase (LDH) leakage, slow down early cell apoptosis, increase energy production (p < 0.05), and increase the expression of glucose transporter 4 (GLUT4) and fatty acid translocation enzyme/cluster 36 differentiation (FAT/CD36) on cell membrane (p < 0.05) (Yu et al., 2020).
As a potential dietary supplement, PQR has been proved to be effective in preventing cardiomyocyte hypertrophy and heart failure. It could reduce the occurrence of myocardial hypertrophy by blocking leptin stimulating RhoA/ROCK and significantly reducing the expression of p115RhoGEF gene and protein (Moey et al., 2011). It also activates Nrf2 signaling pathway by inhibiting Kelch-like ECH-associated protein (Keap) 1, and significantly inhibits inflammatory macrophage-mediated cardiac cell death and hypertrophy to play a cardiac protective role (Qu et al., 2015). PQR could inhibit the harmful effects of adrenaline agonist isoproterenol on cardiac hypertrophy and function. In vivo experiments, PQR could attenuate the effect of isoproterenol on left ventricular function, increase heart quality, and decrease the up-regulation of fetal gene expression. In vitro studies, PQR completely inhibited isoprenaline induced hypertrophy on the surface area of myocytes (Tang et al., 2016). In addition, PF11, the characteristic component of PQR, could reduce the levels of MDA, aspartate aminotransferase (AST), creatine kinase (CK), LDH, cardiac troponin T (cTnT), increase the activity of SOD, and significantly improve cardiac function and reduce the area of myocardial infarction. PF11 could be used as a natural anti-myocardial ischemia drug to improve cardiac function (Zhang et al., 2017).
In summary, PQR has anti-myocardial ischemia, anti-myocardial infarction, anti-atherosclerosis and other effects. In recent years, more and more studies have preliminarily confirmed the effect of PQR on myocardial ischemia-reperfusion injury. In addition, PQR also has the function of anti-arrhythmia, protecting the damaged myocardium and anti-hemorrhagic shock. Although the current research on heart protection of PQR is more and more in-depth, however, there are still some problems to be further explored. For example, the key components that play a cardiac protective effect on PQR are mainly concentrated in the PQR crude extract and PF11, and there is a lack of research on the mechanism of effect on other components. Studies have shown that panaxadiol saponins and panaxatriol saponins also have cardioprotective effects (Wang and Shi, 2011). Then, apoptosis is the key link of myocardial injury. In addition to the known mitochondrial apoptosis and death receptor pathway (Liu et al., 2010), whether there are other apoptotic pathways involved in cardiomyocyte apoptosis, such as the endoplasmic reticulum associated apoptosis pathway located upstream of mitochondrial apoptosis and death receptor pathway. These problems can be explored in future studies.
Anti-Tumor Effect
After cardiovascular and cerebrovascular diseases become the first major killer of human health, the incidence rate of tumors is increasing, and has become the second major killer of human life and health, and the incidence rate is increasing year by year.At the same time, more and more attention has been paid to the search for new anticancer drugs with high selectivity, strong activity, little toxic and side effects and wide application from medicinal and food homologous plants. PQR is a medicinal and food homologous plant, widely used as dietary additives, has many benefits to human health, can effectively prevent and treat cancer diseases (Yu et al., 2015). Studies have shown that the anticancer active ingredients in PQR can be used as anticancer drugs alone (Yang et al., 2016), and can also be used in combination with other chemotherapy drugs to improve the efficacy or reduce the toxic and side effects (Wan et al., 2018).
Inflammation or excessive intake of animal fat are often risk factors for colorectal cancer. The preventive effect of PQR on cancer is related to the reduction of damaged amino acid, organic acid, fatty acid and carbohydrate metabolism. The metabolites that PQR can significantly change include arachidonic acid, linolenic acid, glutamic acid, docosahexaenoic acid, tryptophan and fructose, all of which are related to inflammation and oxidation (Xie et al., 2015). Oral administration of PQR could significantly inhibit the increase of IL-1 α, IL-1 β, IL-6, G-CSF, GM-CSF and other inflammatory cytokines in inflammatory mice, and inhibit the obvious changes of intestinal microbial community to prevent colorectal cancer (Wang C. Z. et al., 2016). In addition, PQR can inhibit COX-2 and NF-κB and reduce the proliferation of human breast cancer cells through anti-inflammatory effects (Peralta et al., 2009).
He et al. showed that Rg 3 could inhibite colorectal cancer by down-regulating the Wnt/-catenin signaling pathway (He et al., 2011). Poudyal study showed that PQR could induce the expression of miR-29b with matrix metalloproteinase-2 (MMP-2) as a target, thus inhibiting the migration of colon cancer cells (Poudyal et al., 2013). And protopanaxadiol, as a promising colon cancer inhibitor, it plays an anti colon cancer effect by mediating the interaction between p53 and DR4/DR5 by mediating the necrosis factor-related apoptosis-inducing ligand (TRAIL) pathway (Zhang et al., 2015). In vitro studies showed that ginsenoside Rh2 significantly induced colon cancer cell death by regulating phosphorylation levels of protein kinases 1/2 (ERK1/2) and H3. In vivo studies showed that ginsenoside Rh2 could inhibite the growth of tumor cells (Yang et al., 2016). In addition, the water extract of PQR root could play an anti hepatoma effect by promoting the apoptosis of SMMC-7721 cells (Qu et al., 2018).
Intestinal bacteria are fully involved in ginsenoside metabolism, and after oral administration of PQR, intestinal bacteria can gradually hydrolyze the sugar chain of ginsenoside. Under the action of intestinal bacteria, ginsenosides Rb1, Rc and Rd were first transformed into ginsenoside F2 and then converted into compound K (as shown in Figure 2). Therefore, it is not the ginsenoside Rb1, the main component of PQR, that plays an anti-cancer role, but the intestinal bacterial metabolite Compound K has significant inhibitory activity on the growth of colorectal cancer cells (Yuan, 2012). In addition, in the treatment of colorectal cancer, when 5-FU combined with PQR, the cell proliferation inhibition rate was significantly increased. The ability of PQR to enhance the anticancer effect of 5-FU was related to apoptosis (Wan et al., 2018). PQR could significantly enhance its anti-proliferation effect on a variety of cancer cells after being processed at high temperature, because the structure and composition of saponins were changed at high temperature, and more low-polarity saponins were produced, such as ginsenosides Rg3, 20R-Rg3, Rk1 and Rg5, etc. These components can enhance the enzyme activity of caspase-3 and caspase-9, significantly reduce the proliferation of gastric cancer cells and induce the apoptosis of cancer cells (Park et al., 2014). Other studies have shown that the contents of ginsenoside Rb1, Re, Rc and Rd are reduced, the contents of Rg2 and Rg3 are increased after processing at high temperature, the expressions of cyclin A and cyclin D1 are significantly reduced, and the antiproliferative activity of PQR is significantly improved (Wang et al., 2008). In addition, PQR can significantly reduce the nausea and vomiting caused by cisplatin without affecting its anticancer properties. And studies have shown that the PQR extract can significantly increase NK cells and prolong the life span of leukemia mice in a dose-specific manner (Miller et al., 2011).
The above studies show that PQR has significant effect in the prevention and treatment of colorectal cancer, gastric cancer, liver cancer, leukemia and so on. In addition to its potential anticancer effect, PQR is often used as an adjuvant drug in cancer treatment to alleviate the toxic and side effects caused by chemotherapy drugs. However, the above studies are lack of research on monomer compounds, as well as the validation of in vivo experiments and clinical studies. It is difficult to provide scientific basis for clinical development of reasonable and reliable new anticancer drugs. In the future, scholars can further study the anticancer effect and mechanism of compounds such as Rg2, Rg3 and intestinal metabolite compound K in PQR, and add corresponding animal in vivo and clinical trials to verify their efficacy. It is believed that based on clinical practice, combined with modern pharmaceutical theories and technologies, the development and application of anticancer TCM will make new and greater progress.
Immunomodulatory Effects
It is understood that polysaccharides in PQR could stimulate the immune system. PQR polysaccharide is a weaker immune stimulant than lipopolysaccharide, which has the characteristics of immune regulation. Its main action pathway is to promote cell proliferation and regulate the expression of proinflammatory mediators IFN-g, IL-23A, IL-6, TNF - α, MCP-1 and GM-CSF. Stimulation of NOS2 gene expression leads to increased levels of iNOS and downstream the level of NO. Furthermore, studies have shown that its immunoregulatory effects are also related to MAPK (ERK-1/2), PI3K, P38 and NF-κB pathways. And PQR could also be used as an adjuvant or as a nutritional drug, or in combination with other therapies (Lemmon et al., 2012; Ghosh et al., 2019). The health tea containing PQR and PQR polysaccharide can significantly improve the spleen, thymus index and T lymphocyte proliferation ability of immune mice, and improve the NK cell activity and hemolytic activity to improve the immune ability of mice. In addition, the activities of antioxidant enzymes (superoxide dismutase, catalase and glutathione peroxidase) which inhibit oxidative stress are also one of the functions of enhancing immune function (Yu et al., 2016; Yu et al., 2017). A new protein (AGNP) isolated from Panaxanthus quinquefolium can also significantly increase the phagocytosis of macrophages, promote the production of nitric oxide, promote the inactivation of TNF-α and the production of IL-6 to play an immunomodulatory role (Qi et al., 2016).
The above studies showed that PQR polysaccharide was the most effective material basis for immune regulation in PQR. The immunoregulation mechanism is mainly to regulate the expression of pro-inflammatory factors and the level of immune organs. And the dosage forms of PQR immunomodulator are mainly granule wrapped by nanometer biomaterials (Akhter et al., 2019). However, the present study has several limitations, such as the lack of research on the optimal extraction technology of polysaccharide compounds from PQR and the lack of research on the optimal effective dose of immune regulation of PQR. Because the content of polysaccharides extracted by different extraction methods is different, it is difficult to have an accurate index to measure the clinical dosage. Secondly, the mechanism of immunoregulation of PQR only stays at the level of inflammatory factors and immune organs. Whether it can exert a pharmacological effects in other ways remains to be further studied by future scholars. Finally, there are only studies on the gastrointestinal permeability, dissolution rate and other factors of PQR polysaccharide encapsulated with nano biomaterials. In the future, scholars can also consider developing cheaper, environmentally friendly, safe and effective materials to replace it, and develop more new dosage forms of Chinese medicine to facilitate clinical application.
Antidiabetic Effect
Diabetes has become a worldwide epidemic disease, and is a chronic progressive disease. The main drugs for the treatment of diabetes include sulfonylureas, biguanides, insulin, etc. (Liu et al., 2018). However, these drugs have a lot of side effects, which often require combined medication and poor patient compliance. In recent years, more and more people began to use dietary supplements and herbal supplements. Both clinical and experimental studies have shown that PQR, as a food supplement, has significant and well-tolerated effects in regulating glycolipid metabolism, anti-diabetes and obesity.
Studies have shown that PQR can improve cellular immune response and mitochondrial function, increase insulin production, reduce pancreatic cell death, and improve postprandial blood glucose in patients with type II diabetes mellitus. However, the active components of PQR to improve the function of pancreatic cells have not been found (Wu et al., 2007). Arginine, cinnamic acid, ferulic acid and other bioactive substances in the root of PQR can increase the levels of insulin, glycogen and high-density lipoprotein (HDL) in the mice with type II diabetes, and meanwhile reduce the levels of blood glucose, triglyceride, total cholesterol and low-density lipoprotein (LDL) to play a hypoglycemic role (Yoo et al., 2012). Ginsenosides Rb 1, Rg1, Rb2, Rc may also play a role in hypoglycemic effect (Sievenpiper et al., 2003). In addition, protopanaxdiol (PDG) and Protopanaxatriol (PTG) types of ginsenosides in PQR leaves can prevent and treat obesity, fatty liver and hypertriglyceridemia in mice fed with high-fat diet (Liu et al., 2010). The mechanism may be mainly through the regulation of linoleic acid metabolism, cysteine and methionine metabolism, and the biosynthesis of unsaturated fatty acids (Zhang et al., 2019). PQR could significantly reduced body weight and plasma insulin and C-peptide levels of type II diabetic animals, while increased body weight and plasma insulin and C-peptide levels of type I diabetic animals. However, both of them could decrease the blood glucose and glycosylated hemoglobin levels and increase the area of islets. The mechanism of action may be to promote the regeneration of B cells, leading to increased insulin secretion. In addition, the effect of PQR on weight may also be one of the mechanisms of hypoglycemia in type II diabetes mellitus (Sen et al., 2013a). PQR could also dose-dependent inhibit α-amylase (IC50 = 73.2 μg/ml) and α-glucosidase (IC50 = 111.8 ng/ml), improve insulin sensitivity and glucose uptake in human fat cells, improve fasting glucose level, and reduce the expression of GLUT4 and PDK1 in fatty tissue induced by high-fat diet and alloxan (Kan et al., 2017). And PQR berry and leaf extract could also reduce the weight of ob/ob mice, with anti-hyperglycemia and anti-obesity activity, and have important clinical significance for the prevention and treatment of type II diabetes (Xie et al., 2002; Xie et al., 2004).
Traditional Chinese medicine in the treatment of diabetes can prevent some complications, but also significantly reduce the side effects of western medicine.Studies show that Konjac-mannan (KJM) can regulate the absorption rate of nutrients in the small intestine, and PQR has post-absorptive effects. Therefore, it can be seen that KJM plays a role of hypoglycemic effect by increasing insulin sensitivity, while PQR may play a role by increasing insulin secretion, which plays a different and complementary role when combined PQR with KJM (Vuksan et al., 2001). And PQR combined with konjac-based fiber blend could reduce glycosylated hemoglobin and lipid concentrations in patients with type II diabetes, improve the effectiveness of routine treatment (Jenkins et al., 2018). Furthermore, PQR has a good preventive effect on diabetic complications, such as kidney injury, arteriosclerosis, retinopathy, etc.
The above studies show that PQR has a good therapeutic effect on type II diabetes. The hypoglycemic activity of PQR is mostly studied by animal in vivo experiments and clinical double-blind control. In the future, more large sample size and longer-term controlled trials are needed to provide reliable data support for the wide application of PQR. Moreover, the composition and content of active ingredients in PQR are complex, which are often caused by varieties, medicinal parts (roots, leaves, fruits), extraction methods, harvest years, growing places and so on. Therefore, although the international community has made positive comments on the role of PQR in the treatment of diabetes and thinks that it is likely to be widely used in the future, but it is pointed out that the current PQR as a drug has not been standardized and can not be widely used. In the future, it is necessary to conduct in-depth study on different effective parts and monomer components of PQR, and to clarify the exact components and main mechanism of its anti diabetes and obesity, so as to reduce the risk of drug use in clinical patients.
Antihypertensive Effect
At present, the number of hypertension patients in China has exceeded 330 million, and the antihypertensive drugs currently used in reducing systolic blood pressure are often accompanied by the decline of diastolic blood pressure. Some patients often cause angina pectoris or even myocardial infarction or ischemic stroke because of low diastolic blood pressure.
Some research show that PQR combined with antihypertensive drugs can reduce systolic blood pressure, increase diastolic blood pressure, and decrease pulse pressure significantly. It can inhibit inflammatory reaction by regulating the expression of adhesion molecules and reducing the levels of sICAM-1 and sE-selectin, so as to protect and repair vascular endothelial cells and resist vascular sclerosis (Yang, 2015). PQR, rich in Ginsenoside Rg3, can improve central systolic blood pressure and pulse wave components, reduce late systolic blood pressure and myocardial oxygen consumption, and reduce peripheral wave reflex to play a role in lowering blood pressure (Jovanovski et al., 2020). However, although some studies have shown that PQR has a significant effect on the treatment of hypertension, there is a lack of in-depth research on its related mechanism.The pathogenesis of hypertension is very complex, and regulating inflammatory response may be only one of the important links. PQR may also play its role by regulating immunity, regulating the levels of endogenous substances Na+ and Ca2+ in vascular wall cells, and regulating cardiomyocytes, etc., which need further study and discussion. Moreover, most of the antihypertensive models of PQR are clinical subjects with small sample size, large individual differences, single pathological models and various external influencing factors. In the future, scholars can use chemical reagents to induce different types of hypertension animal models, study the mechanism of action of PQR one by one, so as to improve the clinical treatment of various types of hypertension with PQR.
Antioxidation Effect
Studies have shown that PQR can delay the time of lipid peroxidation by scavenging DPPH free radicals, peroxidation (LOO•) and hydroxyl (•OH) free radicals, and protect human low-density lipoprotein from the influence of Fe2+, Cu2+ and Fe3+ mediated oxidation. It also has a similar protective effect on superhelical DNA breaking induced by hydrogen peroxide free radicals. And the antioxidant activity may be related to the content of Ginsenoside Rb1/Rb2 (Hu and Kitts, 2001). PQR berry extract and ginsenoside Re can reduce the oxidative damage of pancreatic cells induced by hydrogen peroxide, which can directly scavenge free radicals, prevent pancreatic cells from oxidative stress damage, and better regulate the blood glucose level in human body (Lin et al., 2008). PDD-ginsenoside is isolated from PQR and can be used as a potential natural antioxidant. PDD-ginsenoside can block the production of ROS under ultraviolet exposure, limit the production of MMP-1, and promote the synthesis of type I procollagen. Inhibition of vascular endothelial growth factor and TNF-α by NFAT signaling pathway. PDD-ginsenoside showed antioxidant effects by increasing TGF-β1 level, enhancing Nrf2 nuclear translocation, and limiting AP-1 and MAPK phosphorylation. PDD ginsenoside could inhibit melanin secretion and tyrosinase activity, reduce the content of Melan-A and zebrafish embryos, protect skin from light damage caused by ultraviolet rays, and prevent skin pigmentation (Liu et al., 2019). Other studies have shown that PQR could induce Nrf2 antioxidant gene expression by activating Nrf2 pathway (Li et al., 2010). In addition, ginsengsaponin g1 has a high oxygen free radical absorption capacity (Chen et al., 2010). And PQR crude polysaccharide also has obvious free radical scavenging effect (Yu et al., 2014). And the antioxidant activities of ginsenosides and phenolic acids in PQR could be improved by ABTS (scaling of free radicals) and ferric reducing antioxidant power (FRAP) (Kochan et al., 2019).
The above studies showed that PQR has good antioxidant activity. However, the current experimental research is limited to the cell level in vitro. In the future, scholars can consider using animal experiments and epidemiological methods of the relationship between antioxidant capacity in human serum and certain diseases to study the antioxidant mechanism and capacity of PQR in vivo. Moreover, at present, few studies have explored the monomer compounds that play the antioxidant role in PQR. In the future, scholars can use the spectrum–effect relationship method to screen the active substances that play the antioxidant role in PQR. Synthetic antioxidants often have some toxic and side effects, and PQR is expected to become a low-toxic and highly effective food antioxidants. In addition, the study showed that phenolic acids and flavonoids had strong antioxidant effect, phenolic acids and flavonoids accounted for a high proportion in the leaves and fruits of PQR, but the root was the medicinal part specified in the Pharmacopoeia. In the future, scholars can conduct in-depth study on the pharmacological effects of the stems, leaves and fruits of PQR, so as to provide the basis for the rational development and utilization of PQR.
Anti-Inflammatory Effect
PQR is a popular natural health care product with anti-inflammatory properties. Wilson’s study showed that PQR polysaccharide could play an anti-inflammatory effect by regulating IL-6, NF-κB (Nuclear factor-Kappab), Ccl5 [Chemokine (C-C motif) ligand 5], TNF-α and other inflammatory factors, and TLR4 receptor could reduce the up-regulation of inflammatory gene expression, and also plays an important role in the regulation of inflammation (Wilson et al., 2013). Subsequently, it was found that the neutral polysaccharide PPQN in PQR has a therapeutic effect on inflammation and inflammation-related diseases by inhibiting the release of inflammatory mediators (NO) and cytokines (TNF-α, IL-1β, IL-6) (Wang et al., 2015b). The water extract of PQR could significantly inhibit the expression of NOX2, superoxide generation, ERK1/2 phosphorylation and TNF-α in the heart during endotoxemia by inhibiting the signaling pathway of NOX2-ERK1/2-TNF-α, and improve the level of inflammatory factors to improve cardiac function (Wu et al., 2016). PQR saponins could inhibit endoplasmic reticulum stress and associated chondrocytes' inflammatory response by activating the NF-κB pathway, and protect chondrocytes against triglycerides-induced endoplasmic reticulum stress and interleukin-1 -mediated apoptosis, thereby eliminating cartilage degeneration in rats (Xie et al., 2018).
PQR has good therapeutic effect on various diseases caused by inflammation, including chondroarthritis, endotoxemia, enteritis, myocardial injury and other diseases. However, the research on the anti-inflammatory mechanism of PQR is limited to the level of inflammatory factors, lacking the in-depth exploration of its anti-inflammatory mechanism. And there is a lack of research on effective anti-inflammatory ingredients. Future scholars can establish the network pharmacology of the anti-inflammatory effects of PQR to screen its effective anti-inflammatory components, potential targets and action pathways, and verify it through animal and clinical studies, so as to improve the anti-inflammatory mechanism of PQR.
Antibacterial and Antiviral Effect
The active components of PQR, such as ginsenoside, polysaccharide, volatile oil, protein and panaxatriol, have antibacterial activities against pseudomonas aeruginosa, helicobacter pylori, staphylococcus aureus, escherichia coli, propionibacterium spinosum, Candida albicans and Fusarium oxysporum. The antibacterial mechanism includes 1) Affecting the formation of biofilm and destroying the mature biofilm. 2) Induction of apoptosis. 3) Stimulate the immune system and reduce microbial-induced cell apoptosis, inflammation and DNA damage. 4) Significantly reduced the expression of inflammatory factors. 5) Inhibition of antibiotic efflux (Wang and Ng, 2000; Trammell et al., 2012; Wang L. et al., 2020).
Frequent changes in the antigen structure of respiratory viruses have hindered the development of new vaccines. Society uses traditional herbal medicine to prevent and treat viral respiratory diseases. Studies have shown that the mechanism of PQR in preventing or treating influenza and influenza-like diseases may be related to the presence of substances that prevent the virus from replicating in human body (Mousa, 2015). Polysaccharide extracts from stem and leaf of PQR had good inhibitory effect on respiratory syncytial virus, and its therapeutic index TI was 32.57 (Feng et al., 2017). And PQR combined with bezoar has a good inhibitory effect on hepatitis B virus, could significantly reduce the levels of ALT, AST, Tbil, improveIV-C, PV-III, LN, HA and other indicators of liver fibrosis, improve liver function of patients, and have no side effects. It provides a new choice for clinical treatment of hepatitis B, which is worthy of further promotion and research (Chen et al., 2011). In addition, PQR has protective effects on both viral myocarditis and herpes simplex virus infected cells.
The above studies have shown that PQR has antibacterial and antiviral effects. Due to its unique natural, edible, low toxicity and high efficiency characteristics, as well as broad-spectrum antibacterial and antiviral activity and immune-enhancing effects, it is expected to become an important screening target for new antibacterial and antiviral drugs. The corona virus disease 2019 (COVID-19) outbreak at the end of 2019 has yet to develop a suitable vaccine or specific medicine. Studies have shown that traditional herbal medicine has a significant effect in the prevention and treatment of viral respiratory diseases. Ginseng Toxin-Vanquishing Powder (人身败毒散) combined with western medicine has made certain progress in the treatment of COVID-19, while PQR is more applicable to a wider range of people than ginseng. PQR is cold and tonic in nature, it is more suitable for patients with lung fever and physical weakness. As a kind of health care food, PQR is rich in protein, polysaccharide and other nutrients, and has anti-inflammatory, antiviral, immune enhancing and other pharmacological effects. Future scholars should explore the role of PQR in the prevention or treatment of COVID-19, or the possibility of using it as a post-COVID-19 dietary supplement.
Other
PQR could also prevent premature ovarian failure by regulating prostaglandin biosynthesis and ovulation (Zhu et al., 2015), and promote penile erection by directly inducing dilation and relaxation of penile cavernous vessels (Murphy and Lee, 2002). In addition, PQR has the effects of anti-radiation (Liao et al., 2019), protecting liver and kidney (Jiang et al., 2020), strengthening the function of spleen and stomach (Gao et al., 1998; Long et al., 2019), anti-fatigue (Chang et al., 2018),and so on. However, previous scholars have seldom studied these pharmacological effects of PQR, and future scholars can further study its pharmacodynamic components, action targets and mechanism, so as to enrich the clinical practice of PQR.
PQR and its active ingredients not only have obvious effects on central nervous system and cardiovascular protection, but also have their own characteristics in other pharmacological effects. For example, ginsenoside Rg3 has anticancer activity, Re has antioxidant and antiemetic effects, and PF11, a unique saponin of PQR, has neuroprotective effect, caffeic acid and chlorogenic acid could help to protect the myocardial cells. Therefore, it is very important to study the biological activity of monomer, because it can exclude the existence of other components, and it is easy to clarify its exact pharmacological mechanism. However, there is a lack of in-depth study on the pharmacodynamic mechanism monomer compounds of PQR, and the mechanism of PQR in animals or human is not comprehensive enough. Moreover, there is a lack of investigation on the clinical optimal effective dose and safe dose. Other pharmacological effects such as immune regulation, anti-inflammatory, antihypertensive and so on are less studied, and the mechanism is not fully described. This provides a broad space for future scholars to study PQR. The purpose of this part is to summarize the modern pharmacological research of PQR, find out the shortcomings, and put forward the relevant solutions, so as to provide reference and direction for future scholars to study PQR.
Toxicity
Toxicity
1) There were two males and one female, aged 24, 28 and 45 y, respectively, with no history of disease, who chewed fresh PQR rhizomes planted at home with a chewing amount of about 0.5–1 g. After chewing about 5–10 min, they were sudden chest tightness, palpitation, abdominal distension, nausea and mild dizziness. Two cases were accompanied by obvious vomiting and cold sweat due to a large dosage. The results showed that the blood pressure was low and the heart rate was fast, and the electrocardiogram examination showed no abnormality. And there was no skin flush, rash, pruritus and other allergic reactions in all three patients. In this case, a little or even a small amount of fresh PQR root by oral administration cause poisoning, which is very rare (Ye and Wang, 2001). Therefore, people must be very careful not to taste the fresh PQR rhizome, so as not to induce similar poisoning. As for the toxic mechanism, it needs to be further explored and studied by futurologist.
2) Male, 3 g of PQR was taken 4 h after birth, and on the second day he developed symptoms of food refusal and convulsion. Physical examination: the face and the whole skin were obviously flushed, the muscle tension of limbs was slightly increased, and the skin was irritated with twitching, which was relieved automatically after 3–4 s. CT diagnosis: extensive cerebral edema and subarachnoid hemorrhage, consistent with the changes of brain CT caused by PQR poisoning. There are many causes of intracranial hemorrhage in newborns, and intracranial hemorrhage caused by PQR poisoning is rare (Zhao et al., 1999). However, it can be cured through blood transfusion, vitamin K supplementation and hemostatic agent.
Sensitization
1) Female, 6-year-old, with a history of food allergy, atopic dermatitis and long-term asthma. A few minutes after inhalation of powdered PQR, she was referred to the emergency department for anaphylaxis (urticaria and respiratory symptoms). The skin punctum test (SPT) showed that she was allergic to PQR, and the basophil activation test showed that she was allergic to PQR. After treatment with salbutamol, dexamethasone and diphenhydramine, the symptoms were relieved quickly (Stephanie et al., 2018).
2) Male, 3-year-old, with a history of asthma, atopic dermatitis and conjunctivitis allergy. The symptoms of conjunctivitis appeared after contact with atomized powder PQR. After leaving the allergen, symptoms disappeared within 24 h. The SPT showed that he was allergic to PQR, but no IgE mediated allergic reaction was observed after oral administration of PQR (Stephanie et al., 2018).
The above studies indicated that the toxicity and sensitization cases of PQR were rare. “New Compilation of Materia Medica” recorded that PQR, sweet and spicy, cool nature, non-toxic. In addition to the above studies, the rest of the literature on the toxicity and safety of PQR and its preparations showed that PQR belongs to non-toxic substances with no acute toxicity, subacute toxicity or genetic toxicity, and can be used as a toxicological safe functional food (Shi et al., 2014). However, PQR unlike traditional Chinese medicine, has only been used in China for more than 200 years. The toxicological study of PQR has not been carried out in depth, and its safety remains to be studied. In the future, a large amount of clinical and animal data will be needed to verify its safety and make it better used as a medicinal and food homologous product in the clinical and health care field.
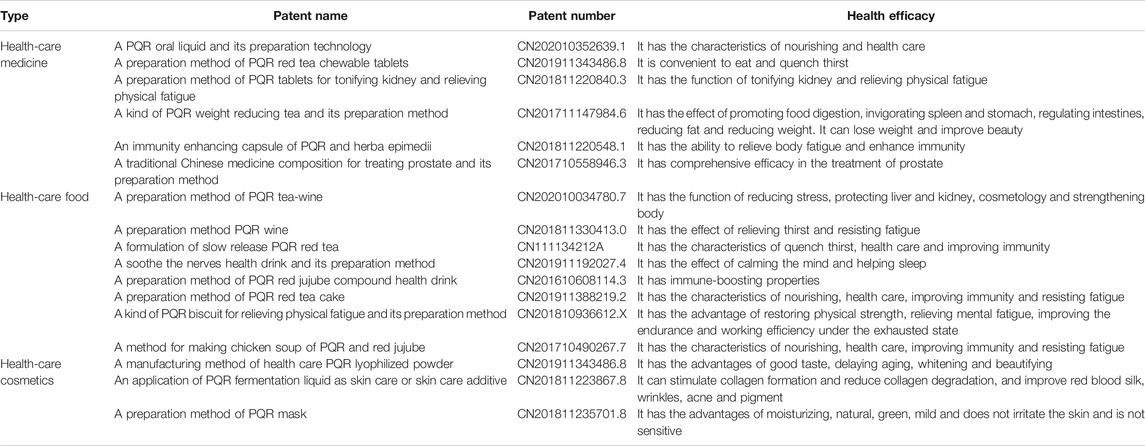
Industrial Applications
In recent years, with the improvement of people’s living standard and the enhancement of health care awareness, it is a project beneficial to human health to develop active natural products with medical health care functions into industrial products with market value. PQR could eliminate fatigue, enhance memory, anti-arrhythmia, anti-myocardial ischemia, improve immunity, promote blood circulation, protect liver and kidney and other health functions. In recent decades, a number of patents have reported the potential development value of PQR. (As shown in Table 7).
In the field of health care, the buccal tablets, oral liquid and capsule of PQR can tonify the kidney, strengthen the spleen and benefit the stomach, moisten the intestines and defecate, relieve fatigue and enhance immunity (Jin, 2002; Wang, 2005). On January 2, 2020, The National Health Commission and the State Administration for Market Regulation listed PQR as a medicinal and food homologous product. In the application of food field,PQR provides people with some optional health food types, such as tea, wine, beverage, biscuit, medicated diet, etc. (Ma et al., 2010). PQR contains some potential antioxidant capacity, which can reduce the oxidation substances in the body and improve the oxygen absorption ability of skin. PQR also shows its potential as a health cosmetics, such as PQR lyophilized powder and PQR mask. PQR has been proved to have the functions of stimulating collagen formation and reducing collagen degradation, delaying aging, whitening and freckle removing. (Liu, 2018) At present, the industrial application of PQR involves many aspects, and a variety of products have been developed. These products better retain the nutritional, medicinal and health value of PQR, so that people’s daily consumption of food and the natural health care function of PQR achieve organic integration, in line with the current people’s requirements for healthy diet. Moreover, PQR is applied to cosmetic industry, which has great social and economic benefits.
Health care products industry has also played an important role in China’s economic development. PQR has been transformed from a medicinal plant into a medicinal and food homologous product, expanding the consumer population. Moreover, the positive development of health food industry can also promote the development of health industry and traditional Chinese medicine in the new era. Consumers also hope that through the use of health food, they can enhance their health, improve their physical functions and even prevent diseases. In addition, PQR is recognized as a kind of nutritive and health medicine and food homology product. In the processing of PQR, the stems and leaves of PQR are often treated as garbage, which can not be used reasonably. According to the data, the stems and leaves of PQR contain a large number of saponins, polysaccharides, flavonoids, which can play a good role in immune regulation and antioxidant. In the process of processing PQR, if the stems, leaves and berries are fully utilized and squeezed into juice, and then concentrated into PQR juice, the waste can be turned into treasure, which can avoid the waste of resources and increase the economic income. It is hoped that this part can provide ideas and directions for future scholars to fully develop and utilize the resources of PQR.
Summary and Prospect
On January 2, 2020, The National Health Commission and the State Administration for Market Regulation listed PQR as a medicinal and food homologous product. This study summarized the botany, chemical composition, quality control, pharmacological, toxicity and industrial development of PQR. At present, the components isolated from PQR include saponins, volatile oils, flavonoids, sterols, polyacetylene, phenolic acids and other chemical components as well as proteins, carbohydrates, vitamins, fatty acid and other essential nutrients for human body. Pharmacological studies have shown that PQR has pharmacological effects such as neuroprotection, cardiovascular protection, immune regulation, anti-tumor, anti-diabetes, anti-hypertension and so on. Studies on the safety of PQR showed that PQR was a non-toxic substance and could be used as a toxicologically safe functional food, but it also had rare toxicity and sensitization. This paper summarizes the industrial development of PQR and finds that PQR has relatively high economic and social benefits, but there are still some important gaps in the scientific literature:
First, PQR as a high-grade health care products, the price is expensive, although there are artificial cultivation products, but still in short supply. Currently, the medicinal part of PQR is dried root. According to the determination, the content of saponins and polysaccharides in the leaves of PQR is more than 10%, which is twice as much as that in the root of PQR. As the price of Panax quinquefolium remains high and its resources are in short supply, future scholars can focus their research on the stems and leaves of PQR with lower price, so as to fully develop and utilize them and prevent resource waste.
Second, a large number of compounds have been isolated from PQR, but the current research on these compounds may be only the tip of the iceberg. The research on the chemical composition of PQR mainly focuses on the saponins, amino acids and trace elements while the analysis and function of organic compounds such as protein and fatty acid are relatively less. Moreover, it was found that a large amount of crude protein and crude polysaccharide could not be effectively used with the discharge of waste residue after extraction. In the future, scholars can try to develop new separation and determination methods to comprehensively measure and analyze PQR, so as to prevent its effective components from not being fully utilized.
Third, at present, the determination of compounds in PQR is limited to saponins, volatile oil and amino acids, and the quality markers of PQR were not elucidated. The analytical methods have the disadvantages of complex sample preparation, toxic organic solvents, soil pollution, and not green enough. In the future, scholars can consider introducing integrated quality evaluation method and Internet analysis technology to find quality markers of PQR. Instead of being limited to the existing methods, they can try to adopt the UPCC and other environment-friendly methods to carry out the multi-component quality control of PQR.
Fourth, as a health medicine, the pharmacological activity of PQR has been verified by scholars at home and abroad. However, there is a lack of in-depth study on the mechanism of action of PQR monomeric compounds, and the mechanism of PQR in animals is not comprehensive enough. Future scholars should combine the biological activity research and clinical application research of PQR to explore the material basis of its therapeutic effect. At the same time, the clinical optimal effective dose and safe dose of PQR also need to be investigated.
Fifth, although PQR has been widely used as a medicinal and food homologous product, there is still a lack of in-depth study on its safety. Although most studies show that PQR is a non-toxic substance and can be used as a safe functional food. However, there are still some cases showing that PQR has certain toxicity and sensitization. In the future, a large number of clinical and animal experiments are needed to verify its safety, so that it can be better used as a medicinal and food homologous product in clinical and health care fields.
In general, PQR has good biological activity and has developed a variety of products in health care medicine, health food, health cosmetics and other industrial fields. It is a valuable edible and medicinal resource worthy of attention. PQR has changed from medicinal plant to medicine and food homologous product, which has expanded consumer groups. The positive development of health care industry can also promote the development of health industry and traditional Chinese medicine in the new era. Consumers also hope that they can enhance their health, improve their physical functions and even prevent diseases through the use of health food. Further development of health products of PQR will undoubtedly be the focus of future research. However, the available health-related data on PQR are insufficient, and its clinical health value needs to be further verified.
In this study, the botany, phytochemistry, quality control methods, pharmacological effects, toxicity, industrial development of PQR were reviewed, and its current situation was analyzed, and its future development direction was prospected, so as to provide valuable reference for future scholars to develop and utilize PQR.
Author Contributions
HJ, SL conceived of and designed the review; AH, WM, SW, JZ, HY, SZ, and XW searched the literature and downloaded the documents and made classification; AH wrote the paper; and LY and AH checked the chemical structures and formula and contributed comments for version of the manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 81973604, 81803690, and 81703684), Special Funds from the Central Finance to Support the Development of Local Universities, the National Natural Science Foundation Matching Project (No. 2018PT02), the Innovative Talents Funding of Heilongjiang University of Chinese Medicine (NO. 2018RCD25), the Postdoctoral Initial Fund of Heilongjiang Province (UNPYSCT 2017219), the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (No. UNPYSCT-2017215), the National Natural Science Foundation Matching Project (No. 2017PT01), the Natural Science Foundation of Heilongjiang Province (No. H2015037), the Heilongjiang University of Chinese Medicine Doctoral Innovation Foundation (No. 2014bs05), the Application Technology Research and Development Projects of Harbin Technology Bureau (No. 2014RFQXJ149), the Heilongjiang Postdoctoral Scientific Research Developmental Fund (No. LBH-Q16210 and LBH-Q17161), the Heilongjiang University of Chinese Medicine Doctoral Innovation Foundation (NO: 2013bs04).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Abbreviations
AA, ascorbic acid; APP, amyloid protein; APX, ascorbate peroxidase; Ara, arabinose; AST, aminotransferase; CAT, catalase; CK, creatine kinase; cTnT, cardiac troponin T; CUS, chronic unpredictable stress; DA, dopamine; 2,3- and 2,5-DHBA, 2,3- and 2,5-dihydroxybenzoic acid; DOPAC, 3,4-dihydroxyphenylacetic acid; ERK1/2, kinases 1/2; ES, esterase; FAT/CD36, fatty acid translocation enzyme/cluster differentiation 36; Gal, galactose; Glu, glucose; GLUT4, glucose transporter four; GSH-PX, glutathione peroxidase; HDL, high-density lipoprotein; 5-HIAA, 5-hydroxyindoleacetic acid; HVA, homovanillic acid; Keap 1, Kelch-like ECH-associated protein one; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; MA, methamphetamine; MDA, malondialdehyde; MMP-2, metalloproteinase-2; 3-NP, 3-nitropropionic acid; NR, nitrate reductase; PDG, protopanaxdiol; PF11, pseudoginsenoside-F11; POD, peroxidase; PPO, polyphenol oxidase; PQR, Panacis Quinquefolii Radix; PTG, Protopanaxatriol; Rha, rhamnose; ROS, reactive oxygen species; SOD, superoxide dismutase; TG, triglyceride; TH, tyrosine hydroxylase; TRAIL, necrosis factor-related apoptosis-inducing ligand; Xyl, xylose.
References
Akhter, K. F., Mumin, M. A., Lui, E. M. K., and Charpentier, P. A. (2019). Immunoengineering with ginseng polysaccharide nanobiomaterials through oral administration in mice. ACS Biomat. Eng. 5 (6), 2916–2925. doi:10.1021/acsbiomaterials.9b00348
Bao, W., Li, H., and Yang, B. (1998). Advances in the study of chemical constituents of Panax quinquefolium L. J. Shenyang Pharm. Univ. 15 (2), 149–153.
Cao, M., (2004). The recent progress of chemical composition research of Panax quinquefolium was summarized. Pharm. Clin. Res. 12 (2), 25–26.
Chanana, P., and Kumar, A. (2016). GABA-BZD receptor modulating mechanism of Panax quinquefolius against 72-h sleep deprivation induced anxiety like behavior: possible roles of oxidative stress, mitochondrial dysfunction and neuroinflammation. Front. Neurosci. 10, 84. doi:10.3389/fnins.2016.00084
Chang, Y. D., Smith, J., Portman, D., Kim, R., Ritika, O. J., Rajasekhara, S., et al. (2018). Single institute experience with methylphenidate and American ginseng in cancer-related fatigue. Am. J. Hospice Palliat. Med. 35 (1), 144–150. doi:10.1177/1049909117695733
Chen, C. Y. O., Ribaya, M. J. D., Mckay, D. L., Croom, E., and Blumberg, J. B. (2010). Differential antioxidant and quinone reductase inducing activity of American, Asian, and Siberian ginseng. Food Chem. 119 (2), 445–451. doi:10.1016/j.foodchem.2009.06.049
Chen, E. Y. H., and Hui, C. L. M. (2012). HT1001, a proprietary North American ginseng extract, improves working memory in schizophrenia: a double-blind, placebo-controlled study. Phytother. Res. 26 (8), 1166–1172. doi:10.1002/ptr.3700
Chen, L. F., Li, L. Q., and Zhao, Y. Q. (2011). Clinical study on chronic hepatitis B treated by “ginseng and calculus bovis”. J. Hubei Univ. Chin. Med. 13 (6), 15–17.
Chen, L. L., and Cui, N. (2002). Progress in the research on chemical composition of ginseng. Lishizhen Med. Mater. Medi. Res. 13 (10), 59–60.
Chen, X. M., (1995). Research progress of domestic Panax quinquefolium. Primary J. Chin. Materia Medica 3, 37–38.
Chen, X. J., Zhang, X. J., Shui, Y. M., Wan, J. B., and Gao, J. L. (2016). Anticancer activities of protopanaxadiol- and protopanaxatriol-type ginsenosides and their metabolites. Evi. Based Compl. Alternat. Med. 2016, 1–19. doi:10.1155/2016/5738694
Chen, W., Balan, P., and Popovich, D. G. (2020). Comparison of ginsenoside components of various tissues of New Zealand forest-grown Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolium L.). Biomolecules 10 (3). 372. doi:10.3390/biom10030372
Dou, H. C., Chen, J. Y., Ran, T. F., and Jiang, W. M. (2018). Panax quinquefolius saponin inhibits endoplasmic reticulum stress-mediated apoptosis and neurite injury and improves functional recovery in a rat spinal cord injury model. Biomed. Pharmacother. 102, 212–220. doi:10.1016/j.biopha.2018.03.074
Erdle, S. C., Chan, S. E., Yang, H., Vallance, A. B., and Mill, C. (2018). First-reported pediatric cases of American ginseng anaphylaxis and allergy. Allergy Asthma Clin. Immunol. 14, 79. doi:10.1186/s13223-018-0304-3
Feng, K. M., Meng, H. T., Zhang, Q., Yan, J. Y., Bi, Y., Cun, Y. T., et al. (2017). Optimization of extraction of polysaccharide from Panax quinquefolius stems and leaves and its antiviral activity. J. Liaoning Univ. Trad. Chin. Med. 19 (4), 52–55.
Feng, K. R., Zhang, Y. T., Wang, G. L., and Song, Y. (2018). Forty-seven elements in the extracts of Panax ginseng and Panax quinquefolium were determined simultaneously by ICP-MS. Anal. Instrum. No 219 (4), 40–47.
Fu, K. Q., Lin, H. Y., Miyamoto, Y., Wu, C. F., Yang, J. Y., Uno, K., et al. (2016). Pseudoginsenoside-F11 inhibits methamphetamine-induced behaviors by regulating dopaminergic and GABAergic neurons in the nucleus accumbens. Psychopharmacology 233 (5), 831–840. doi:10.1007/s00213-015-4159-8
Fujimoto, Y., Satoh, M., Takeuchi, N., and Kirisawa, M. (1991). Cytotoxic acetylenes from Panax quinquefolium. Chem. Pharmaceut. Bull. 39 (2), 521–523. doi:10.1248/cpb.39.521
Fujimoto, Y., Wang, H., Satoh, M., and Takeuchi, N. (1994). Polyacetylenes from Panax quinquefolium. Phytochemistry 35 (5), 1255–1257. doi:10.1016/S0031-9422(00)94831-3
Gao, Y., Chen, Y., and Wang, B. (1998). Research on the mechanism of Yin-enriching and Qi-reinforcing, strengthening body and tonification deficiency syndrome action of radix Panacis quinquefolii. J. Chin. Med. Mater. 12, 3–5 [in Chinese].
Ghosh, R., Smith, S. A., Nwangwa, E. E., Arivett, B. A., Bryant, D. L., Fuller, M. L., et al. (2019). Panax quinquefolius (North American ginseng) cell suspension culture as a source of bioactive polysaccharides: immunostimulatory activity and characterization of a neutral polysaccharide AGC1. Int. J. Biol. Macromol. 139, 221–232. doi:10.1016/j.ijbiomac.2019.07.215
Guo, N., Rui, F. U., and Dou, D. Q. (2006). Chemical constituents from Panax quinquefolium. Chin. J. Med. Chem. 3, 172–174+187.
Hao, X. H., Li, P. Y., Li, X., and Liu, Y. Q. (2000). Saponins from the fruit of Panax quinquefolius collected in Canada. Chin. Herbal Med. 31 (11), 801–803.
He, B. C., Gao, J. L., Luo, X. J., Luo, J. Y., and Zhang, B. Q. (2011). Ginsenoside Rg3 inhibits colorectal tumor growth through the down-regulation of Wnt/-catenin signaling. Int. J. Oncol. 38 (2), 437–445. doi:10.3892/ijo.2010.858
Hou, A. J., Yang, L., Zhang, J., Wang, S., Man, W. J., Guo, X., et al. (2020). A strategy for qualitative and quantitative profiling of Angelicae Pubescentis Radix and detection of its analgesic and anti-inflammatory components by spectrum-effect relationship and multivariate statistical analysis. Biomed. Chromatogr. 34, e4910. doi:10.1002/bmc.4910
Hu, C., and Kitts, D. D. (2001). Free radical scavenging capacity as related to antioxidant activity and ginsenoside composition of Asian and North American ginseng extracts. J. Am. Oil Chem. Soc. 78 (3), 249–255. doi:10.1007/s11746-001-0253-8
Hui, X., Zhang, A. H., Zhao, Q. Q., Yan, G. L., Sun, H., and Wang, X. J. (2020). Discovery of quality-marker ingredients of Panax quinquefolius driven by high-throughput chinmedomics approach. Phytomedicine 74, 152928. doi:10.1016/j.phymed.2019.152928
Huang, B., Chen, T., Xiao, S., Zha, Q., Luo, P., Wang, Y., et al. (2017). A new approach for authentication of four ginseng herbs and their related products based on the simultaneous quantification of 19 ginseng saponins by UHPLC-TOF/MS coupled with OPLS-DA. RSC Adv. 7 (74), 46839–46851. doi:10.1039/C7RA06812C
Huang, Y. W., Wang, J. H., Jshan, J., Lei, L., and Han, D. T. (2011). Determination of total main ginsenosides contents in American ginseng and Chinese ginseng using near infrared spectroscopy. Chin. J. Anal. Chem. 39 (3), 377–381.
Jenkins, A. L., Morgan, L. M., Bishop, J., Jovanovski, E., Jenkins, D. J. A., and Vuksan, V. (2017). Co-administration of a konjac-based fibre blend and American ginseng (Panax quinquefolius L.) on glycaemic control and serum lipids in type 2 diabetes: a randomized controlled, cross-over clinical trial. Eur. J. Nutr. 57 (6), 2217–2225. doi:10.1007/s00394-017-1496-x
Jenkins, A. L., Morgan, L. M., Bishop, J., Jovanovski, E., Jenkins, D. J. A., and Vuksan, V. (2018). Co-administration of a konjac-based fibre blend and American ginseng (Panax quinquefolius L.) on glycaemic control and serum lipids in type 2 diabetes: a randomized controlled, cross-over clinical trial. Eur. J. Nutr. 57 (6), 2217–2225. doi:10.1007/s00394-017-1496-x
Jiang, J., Xiao, S., Yan, S., Zhang, J., and Xu, X. (2020). The effects of sulfur fumigation processing on Panacis quinquefolii Radix in chemical profile, immunoregulation and liver and kidney injury. J. Ethnopharmacol. 249, 112377. doi:10.1016/j.jep.2019.112377
Jin, Q. W. (2002). The application of American ginseng in health food. Chin. J. Viral Dis. (2), 101–103.
Jin, Y., and Fan, Y. L. (1992). A review of the chemical and pharmacological research of domestic Panax quinquefolium. Jilin J. Trad. Chin. Med. (4), 40–42.
Jing, D., Yang, J., Jiao, X., and Gao, W. (2011). Effect of nitrogen, phosphorus and potassium deficiency on content of phenolic compounds in exudation of American ginseng. Zhongguo Zhongyao Zazhi 36 (3), 326–329. doi:10.4268/cjcmm20110321
Jing, Y., and Zhao, Y. Q. (2007). Study on chemical composition of American ginseng fruit. Mod. Chin. Med. 9 (6), 7–9.
Jovanovski, E., Lea, D. S., Komishon, A., Au, Y. F., Zurbau, A., Jenkins, A. L., et al. (2020). Vascular effects of combined enriched Korean Red ginseng (Panax ginseng) and American ginseng (Panax quinquefolius) administration in individuals with hypertension and type 2 diabetes: a randomized controlled trial. Compl. Ther. Med. 49, 102338. doi:10.1016/j.ctim.2020.102338
Kan, J., Velliquette, R. A., Grann, K., Burns, C. R., Scholten, J., Tian, F., et al. (2017). A novel botanical formula prevents diabetes by improving insulin resistance. BMC Compl. Alternat. Med. 17 (1), 352. 10.1186/s12906-017-1848-3
Kochan, E., Szymańska, S., Wielanek, M., Anna, W. O., and Izabela, G. K. (2019). The content of triterpene saponins and phenolic compounds in American ginseng hairy root extracts and their antioxidant and cytotoxic properties. Plant Cell Tissue Organ Cult. 138 (2), 353–362. doi:10.1007/s11240-019-01633-3
Lemmon, H. R., Sham, J., Chau, L. A., and Madrenas, J. (2012). High molecular weight polysaccharides are key immunomodulators in North American ginseng extracts: characterization of the ginseng genetic signature in primary human immune cells. J. Ethnopharmacol. 142 (1), 1–13. doi:10.1016/j.jep.2012.04.004
Li, J., Ichikawa, T., Jin, Y., Hofseth, L. J., Nagarkatti, P., Nagarkatti, M., et al. (2010). An essential role of Nrf2 in American ginseng-mediated anti-oxidative actions in cardiomyocytes. J. Ethnopharmacol. 130 (2), 222–230. doi:10.1016/j.jep.2010.03.040
Li, J., Shao, Z. H., Xie, J. T., Wang, C. Z., Ramachandran, S., Yin, J. Y., et al. (2012). The effects of ginsenoside Rb1 on JNK in oxidative injury in cardiomyocytes. Arch Pharm. Res. (Seoul) 35 (7), 1259–1267. doi:10.1007/s12272-012-0717-3
Li, J., Tang, J. L., Yin, J. Y., and Wei, Y. D. (1999). Determination of fatty acids in flower buds of American ginseng and ginseng by GC - MS. Ginseng Res. (2), 31–32.
Li, J. L., Lu, S. W., Ren, C. Y., Gao, Y., Hong, B., Wang, K., et al. (2015). Processing technology of fresh American ginseng beverage. China Brew. 34 (8), 82–85.
Li, X., Wang, J. H., and Zhang, G. F. (1997). Phytochemical study on Panax quinquefolium from Canada. China Acad. J. Elect. Publ. House.
Li, X. G., Lu, Q., Fu, L., and Liu, M. X. (1998). Chemical composition from fruit of Panax quinquefolium. J. Jilin Agric. Univ. 20 (2), 5–10.
Li, X. G., Zheng, Y. L., and Jia, J. H. (1985). Analysis of amino acid composition of Panax quinquefolium L. China J. Chin. Mater. Med. 10 (5), 33.
Lian, X. Y., Zhang, Z. Z., and Stringer, J. L. (2006). Anticonvulsant and neuroprotective effects of ginsenosides in rats. Epilepsy Res. 70, 244–256. doi:10.1016/j.eplepsyres.2006.05.010
Lian, X. Y., Zhang, Z. Z., and Stringer, J. L. (2010). Protective effects of ginseng components in a rodent model of neurodegeneration. Ann. Neurol. 57 (5), 642–648. doi:10.1002/ana.20450
Liang, Z. Y., Zhang, Y. S., and Miao, C. Y. (1989). The comparative study of total sugar composition and content of ginseng was conducted. Chin. Pharmaceut. J. 24 (4), 207–209.
Liao, D., Jia, C., Sun, P., Qi, J., and Li, X. E. (2019). Quality evaluation of Panax quinquefolium from different cultivation regions based on their ginsenoside content and radioprotective effects on irradiated mice. Sci. Rep. 9 (1), 1079. doi:10.1038/s41598-018-37959-9
Lin, E., Wang, Y., Mehendale, S., Sun, S., Wang, C. Z., Xie, J. T., et al. (2008). Antioxidant protection by American ginseng in pancreatic beta-cells. Am. J. Chin. Med. 36 (5), 981–988. doi:10.1142/S0192415X08006399
Lin, H. Q., Tan, J., Wang, H., Wu, F. L., Dong, Q. H., Li, P. Y., et al. (2018). Identification of volatile constituents in mountain-cultivated and garden-cultivated American ginseng. Spec. Res. 40, 31–37.
Liu, C. D. (2008). Research on the Chemical Constituents of Flower Buds of Panax quinquefolium L. Shenyang Pharmaceutical University.
Liu, C. Z., Chen, W., Wang, M., Wang, Y., and Chen, H. (2020). Dendrobium officinale Kimura et Migo and American ginseng mixture: a Chinese herbal formulation for gut microbiota modulation. Chin. J. Nat. Med. 18 (6), 446–459. doi:10.1016/S1875-5364(20)30052-2
Liu, D., Chu, S., Li, H., Zhao, H., Liu, X., Qu, X., et al. (2018). Relationship between free fatty acid spectrum, blood stasis score, and macroangiopathy in patients with type 2 diabetes. World J. Trad. Chin. Med. 4 (1), 28. doi:10.4103/wjtcm.wjtcm_5_18
Liu, D. M., Li, B., Liu, Y., Attele, A. S., Kyle, J. W., and Yuan, C. S. (2001). Voltage-dependent inhibition of brain Na(+) channels by American ginseng. Eur. J. Pharmacol. 413 (1), 47–54. doi:10.1016/s0014-2999(01)00735-x
Liu, J. P., Li, P. Y., Li, Y. J., and Lu, D. (2010). “Study on saponins in Panax ginseng fruit,” in 2010 Proceedings of shihuida Cup 10th national young pharmaceutical workers’ latest scientific research achievements exchange meeting, (Chinese Pharmaceutical Association) 6, 318–323.
Liu, R., Zhang, J. Z., Liu, W. C., Kimura, Y., and Zheng, Y. N. (2010). Anti-obesity effects of protopanaxdiol types of ginsenosides isolated from the leaves of American ginseng (Panax quinquefolius L.) in mice fed with a high-fat diet. Fitoterapia 81 (8), 1079–1087. doi:10.1016/j.fitote.2010.07.002
Liu, T. C., Liu, H., Zhao, Y. J., Fu, J. G., and Hu, B. Y. (1987). Trace elements in root of American ginseng. China J. Chin. Mater. Med. 12 (9), 17–18.
Liu, X. F., Hao, J. Y., Tang, Y., Li, J. K., Yan, Z. H., Chen, H. F., et al. (2016). Analysis of liposoluble constituents of white ginseng, red ginseng and American ginseng by GC-MS. Mod. Chin. Med. 18 (1), 76–81.
Liu, X.-Y., Xiao, Y.-K., Hwang, E., Haeng, J. J., and Yi, T.-H. (2019). Antiphotoaging and antimelanogenesis properties of ginsenoside C-Y, a ginsenoside Rb2 metabolite from American ginseng PDD-ginsenoside. Photochem. Photobiol. 95 (6), 1412–1423. doi:10.1111/php.13116
Liu, Y., Meng, H. T., Song, Y., Zhang, Q., and Han, C. C. (2016). Extraction and purification of ginsenoside-Re from the stem and leaf of Radix Panacis Quinquefolii based on the concept of environmental protection. Central South Pharm. 14 (3), 243–246.
Liu, Z., Zhang, F., Wang, P., and Yang, Y. E. (2010). Effect of PQS on cardiomyocyte apoptosis induced by ischemia-reperfusion in new borne mice. Chin. J. Vet. Med. 46 (7), 14–16.
Long, H. Q., Zhao, W. H., Nie, X. J., Ma, J. Z., Wu, Y., Chen, X., et al. (2019). Reversal effect of American ginseng alcohol extracts on rats with chronic atrophic gastritis. Chin. J. Mod. Appl. Pharm. 36 (17), 2131–2135.
Lu, Q., Fu, L., Li, X. G., and Meng, X. Y. (1998). Structure identification and antitumor activity of fatty acid components of Panax quiculatum L. Chin. Soc. Nat. Res. 4, 204–207.
Luo, Y. P., Ma, H.-R., Chen, J. W., Li, J. J., and Li, C. X. (2014). Effect of American ginseng capsule on the liver oxidative injury and the Nrf2 protein expression in rats exposed by electromagnetic radiation of frequency of cell phone. Chin. J. Integr. Trad. Western Med. 34 (5), 575–580.
Ma, J., and Rui, H. B. (2019). Analysis of main chemical composition of Panax quinquefolium from different habitats. Northwest Pharm. J. 34 (5), 601–604.
Ma, W. G., Huang, Z. P., You, W. L., You, W. X., Zeng, G. Y., and Liu, Y. Q. (2010). Anti-fatigue function of compound Pu’er tea with American ginseng in mice. Food Sci. 31 (1), 224–226.
Ma, X. L., Zhao, D. C., Sun, X. X., Liu, J. Z., Hao, C. Y., and Liu, S. Y. (2000). Isolation and characterization of four polysaccharides from American ginseng (Panax quiquefolium). Chin. Herbal Med. 31 (3), 165–167.
Mehendale, S. R., Wang, C. Z., Shao, Z. H., Li, C. Q., Xie, J. T., Han, H. A., et al. (2006). Chronic pretreatment with American ginseng berry and its polyphenolic constituents attenuate oxidant stress in cardiomyocytes. Eur. J. Pharmacol. 553 (1–3), 209–214. doi:10.1016/j.ejphar.2006.09.051
Meng, X., Li, X., and Yang, Y. U. (2000). Chemical constituents of flowerbuds of Panax quinquefolium L. cultivated in China. Isolation, identification and content determination of saponins. J. Jilin Agric. Univ. 22 (3), 1–8.
Meng, X. Y., Li, X. G., and Liu, D. Y. (2002). Determination of flavonoids in different parts of Panax quinquefolium L. J. Changchun Coll. Trad. Chin. Med. 18 (6), 45–46.
Meng, X. Y., Li, X. G., Zhang, H., and Han, Y. (2001). Analysis of the volatile oils in the flowerbuds of Panax quinquefolium L. Chin. J. Anal. Chem. 29 (5), 544–545.
Miller, S. C., Delorme, D., and Shan, J. J. (2011). Extract of North American ginseng (Panax quinquefolius), administered to leukemic, juvenile mice extends their life span. J. Compl. Integr. Med. 8 (1), 1–10. doi:10.2202/1553-3840.1315
Moey, M., Rajapurohitam, V., Zeidan, A., and Karmazyn, M. (2011). Ginseng (Panax quinquefolius) attenuates leptin-induced cardiac hypertrophy through inhibition of p115Rho guanine nucleotide exchange factor-RhoA/Rho-associated, coiled-coil containing protein kinase-dependent mitogen-activated protein kinase pathway activ. J. Pharmacol. Exp. Therapeut. 339 (3), 746–756. doi:10.1124/jpet.111.182600
Morinaga, O., Uto, T., Yuan, C. S., Tanaka, H., and Shoyama, Y. (2010). Evaluation of a new eastern blotting technique for the analysis of ginsenoside Re in American ginseng berry pulp extracts. Fitoterapia 81 (4), 284–288. doi:10.1016/j.fitote.2009.10.005
Mousa, A. L. (2015). Herbal therapies for prevention and treatment of influenza and influenza-like illness. J. Infec. Dis. Ther. 3. (3), 1–3. doi:10.4172/2332-0877.1000215
Murphy, L. L., and Lee, T. J. F. (2002). Ginseng, sex behavior, and nitric oxide. Ann. N. Y. Acad. Sci. 962 (1), 372–377. doi:10.1111/j.1749-6632.2002.tb04081.x
Nabuurs, M. H., Mccallum, J. L., Brown, D. C., and Kirby, C. W. (2017). NMR characterization of novel pyranoanthocyanins derived from the pulp of Panax quinquefolius L. (North American ginseng). Magn. Reson. Chem. 55 (3), 177–182. doi:10.1002/mrc.4366
Oshima, Y., Sato, K., and Hikino, H. (1987). Isolation and hypoglycemic activity of quinquefolans A, B, and C, glycans of Panax quinquefolium roots. J. Nat. Prod. 50 (2), 188–190. doi:10.1021/np50050a010
Ossoukhova, A., Owen, L., Savage, K., Meyer, M., Ibarra, A., Roller, M., et al. (2015). Improved working memory performance following administration of a single dose of American ginseng (Panax quinquefolius L.) to healthy middle-age adults. Hum. Psychopharmacol. 30 (2), 108–122. doi:10.1002/hup.2463
Park, E. H., Kim, Y. J., Yamabe, N., Park, S. H., Kim, H. K., Jang, H. J., et al. (2014). Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J. Ginseng Res. 38 (1), 22–27. doi:10.1016/j.jgr.2013.11.007
Peralta, E. A., Murphy, L. L., Minnis, J., Louis, S., and Dunnington, G. L. (2009). American ginseng inhibits induced COX-2 and NFKB activation in breast cancer cells. J. Surg. Res. 157 (2), 261–267. doi:10.1016/j.jss.2009.05.011
Poudyal, D., Cui, X., Le, P. M., Hofseth, A. B., Windust, A., Nagarkatti, M., et al. (2013). A key role of microRNA-29b for the suppression of colon cancer cell migration by American ginseng. PLoS One 8 (10), e75034. doi:10.1371/journal.pone.0075034
Qi, B., Liu, L., Zhang, H., Zhou, G. X., Wang, S., Duan, X. Z., et al. (2014). Anti-fatigue effects of proteins isolated from Panax quinquefolium. J. Ethnopharmacol. 153 (2), 430–434. doi:10.1016/j.jep.2014.02.045
Qi, B., Wang, S., Wang, Q., Zhang, H., Bai, X. Y., He, H. N., et al. (2016). Characterization and immunostimulating effects on murine peritoneal macrophages of a novel protein isolated from Panax quinquefolius L. J. Ethnopharmacol. 193, 700–705. doi:10.1016/j.jep.2016.10.034
Qu, C., Li, B., Lai, Y., Li, H., Windust, A., Hofseth, L. J., et al. (2015). Identifying panaxynol, a natural activator of nuclear factor erythroid-2 related factor 2 (Nrf2) from American ginseng as a suppressor of inflamed macrophage-induced cardiomyocyte hypertrophy. J. Ethnopharmacol. 168, 326–336.
Qu, S. L. (2009). Study on chemical composition of Panax quinquefolium L. Jilin, China: Jilin University.
Qu, Y., Wang, Z., Zhao, F., Liu, J., Zhang, W., Li, J., et al. (2018). AFM-detected apoptosis of hepatocellular carcinoma cells induced by American ginseng root water extract. Micron Internat. Res. Rev. J. Microsc. 104, 1–7. doi:10.1016/j.micron.2017.10.003
Rinwa, P., and Kumar, A. (2014). Modulation of nitrergic signalling pathway by American ginseng attenuates chronic unpredictable stress-induced cognitive impairment, neuroinflammation, and biochemical alterations. Naunyn Schmiedebergs Arch. Pharmacol. 387 (2), 129–141. doi:10.1007/s00210-013-0925-5
Salim, K. N., Mcewen, B. S., and Chao, H. M. (1997). Ginsenoside Rb1 regulates ChAT, NGF and trkA mRNA expression in the rat brain. Mol. Brain Res. 47 (1–2), 177–182. doi:10.1007/s00210-013-0925-5
Satoh, M., Takeuchi, N., and Fujimoto, Y. (1997). Synthesis and the absolute configuration of PQ-8, a C14-polyacetylene compound isolated from Panax quinquefolium. Heterocycles 45 (1), 177.
Sen, S., Querques, M. A., and Chakrabarti, S. (2013a). North American ginseng (Panax quinquefolius) prevents hyperglycemia and associated pancreatic abnormalities in diabetes. J. Med. Food 16 (7), 587–592. doi:10.1089/jmf.2012.0192
Sen, S., Chen, S., Wu, Y., Feng, B., and Chakrabarti, S. (2013b). Preventive effects of North American ginseng (Panax quinquefolius) on diabetic retinopathy and Cardiomyopathy. Phytother. Res. 27 (2), 290–298. doi:10.3987/COM-96-7665
Shen, N., and Wang, S. S. (1991). A recent study on the constituents of essential oil from western China cultivated in Jilin, China. J. Shenyang Pharm. Univ. 8 (3), 175–181.
Shi, A. H., Li, E. D., Zhai, P. G., Zhao, J. Y., Hong, X. Y., and Selenium, O. (2014). “Toxicological assessment of American ginseng extract”. Chin. Arch. Trad. Chin. Med. 32 (8), 2002–2004.
Shi, S., Shi, R., and Hashizume, K. (2012). American ginseng improves neurocognitive function in senescence-accelerated mice: possible role of the upregulated insulin and choline acetyltransferase gene expression. Geriatr. Gerontol. Int. 12 (1), 123–130. doi:10.1111/j.1447-0594.2011.00719.x
Shin, K., Guo, H., Cha, Y., Ban, Y. H., Seo, D. W., Choi, Y., et al. (2016). Cereboost, an American ginseng extract, improves cognitive function via up-regulation of choline acetyltransferase expression and neuroprotection. Regul. Toxicol. Pharmacol. 78, 53–58. doi:10.1016/j.yrtph.2016.04.006
Sievenpiper, J. L., Arnason, J. T., Leiter, L. A., and Vuksan, V. (2003). Variable effects of American ginseng: a batch of American ginseng (Panax quinquefolius L.) with a depressed ginsenoside profile does not affect postprandial glycemia. Eur. J. Clin. Nutr. 57 (2), 243.
Song, L. Q., Liu, R., and Zhou, Y. (2019). Protective effect of American ginseng-acorus tatarinowii on brain of aging mice. J. Harbin Univ. Comm. 35 (3), 269–273.
Stavro, P. M., Woo, M., Leiter, L. A., Heim, T. F., Sievenpiper, J. L., and Vuksan, V. (2006). Long-term intake of North American ginseng has no effect on 24-hour blood pressure and renal function. Hypertension 47 (4), 791–796. doi:10.1161/01.HYP.0000205150.43169.2c
Sun, F., and Liu, L. X. (1993). Comparison of isozyme spectra between Panax ginseng and Panax quinquefolium l. Chin. Herbal Med. 24 (3), 148–149.
Tang, C. C., Qiu, Z. D., Yang, J., and Dong, J. X. (2015). Study on quality standard and fingerprint of American ginseng granule. Ence Technol. Food Industry 36 (24), 71–75+81.
Tang, J. L., Li, J., and Wei, Y. D. (2000). Isolation and identification of saponins from flower buds of Panax quinquefolium L. Chin. Herbal Med. 31 (7), 18–20.
Tang, X., Gan, X. T., Rajapurohitam, V., Huang, C. X., Xue, J., Lui, E. M. K., et al. (2016). North American ginseng (P. quinquefolius) suppresses β adrenergic-dependent signalling, hypertrophy and cardiac dysfunction. Can. J. Physiol. Pharmacol. 94, 1325–1335. doi:10.1139/cjpp-2016-0337
Trammell, R. A., Cox, L., Pikora, J., Murphy, L. L., and Toth, L. A. (2012). Evaluation of an extract of North American ginseng (Panax quinquefolius L.) in Candida albicans-infected complement-deficient mice. J. Ethnopharmacol. 139 (2), 414–421. doi:10.1016/j.jep.2011.11.026
Vuksan, V., Sievenpiper, J. L., Xu, Z. Z., Wong, E. Y. Y., Jenkins, A. L., Beljan, Z. U., et al. (2001). Konjac-Mannan and American ginsing: emerging alternative therapies for type 2 diabetes mellitus. J. Am. Coll. Nutr. 20 (Suppl. 5), 370S–380S. doi:10.1080/07315724.2001.10719170
Vuksan, V., Xu, Z. Z., Jovanovski, E., Jenkins, A. L., Beljan-Zdravkovic, U., Sievenpiper, J. L., et al. (2019). Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: a double-blind, randomized, cross-over clinical trial. Eur. J. Nutr. 58 (3), 1237–1245. doi:10.1007/s00394-018-1642-0
Wan, J. Y., Yao, H. Q., Zhang, C. F., Huang, W. H., Zhang, Q. H., Liu, Z., et al. (2018). Red American ginseng enhances the effect of fluorouracil on human colon cancer cells via both paraptosis and apoptosis pathways. J. Appl. Biomed. 16 (16), 311–319.
Wang, C., and Shi, D. Z. (2011). Research of cardiovascular effects and mechanism of Panax quinquefolius saponin. Chin. J. Integ. Trad. Western Med. 31 (6), 825–831.
Wang, C. M., Liu, M. Y., Wang, F., Wei, M. J., Wang, S., Wu, C. F., et al. (2013). Anti-amnesic effect of pseudoginsenoside-F11 in two mouse models of Alzheimer’s disease. Pharmacol. Biochem. Behav. 106, 57–67. doi:10.1016/j.pbb.2013.03.010
Wang, C. Z., Aung, H. H., Zhang, B. Q., Sun, S., and Yuan, C. S. (2008). Chemopreventive effects of heat-processed Panax quinquefolius root on human breast cancer cells. Anticancer Res. 28 (5A), 2545–2551.
Wang, C. Z., Yu, C., Wen, X. D., Chen, L., Zhang, C. F., Calway, T., et al. (2016). American ginseng attenuates colitis associated colon carcinogenesis in mice: impact on gut microbiota and metabolomics. Canc. Prev. Res. 9 (10), 803–811. doi:10.1158/1940-6207.CAPR-15-0372
Wang, H. X., and Ng, T. B. (2000). Quinqueginsin, a novel protein with anti-human immunodeficiency virus, antifungal, ribonuclease and cell-free translation-inhibitory activities from American ginseng roots. Biochem. Biophys. Res. Commun. 269 (1), 203–208. doi:10.1006/bbrc.2000.2114
Wang, J., and Dong, G. H. (1995). Analysis of the saponins in stem and leaves of Panax quinquefolium l. Chin. Trad. Patent Med. 17 (1), 36–38.
Wang, J., Xian, L., and Wang, Y. (1997). A new triterpenoid in the leaves and stems of Panax quinquefolium L.from Canada. J. Shenyang Pharm. Univ. (2), 60–61.
Wang, J. Y., Yang, J. Y., Wang, F., Fu, S. Y., Hou, Y., Jiang, B., et al. (2013). Neuroprotective effect of pseudoginsenoside-f11 on a rat model of Parkinson’s disease induced by 6-hydroxydopamine. Evid. Based Compl. Alternat. Med. 2013, 152798. doi:10.1155/2013/152798
Wang, L., Huang, Y. F., Yin, G., Wang, J., Wang, P., Chen, Z. Y., et al. (2020). Antimicrobial activities of Asian ginseng, American ginseng, and notoginseng. Phytother. Res. 34 (6), 1226–1236. doi:10.1002/ptr.6605
Wang, L., Wang, Y. P., Xu, S. Q., Sun, C. H., Nian, S., Liu, J. Y., et al. (2007). A review on studies of the components and pharmacological activity of Panax quinquefolium L. Special Wild Econ. Anim. Plant Res. 3, 73–77.
Wang, L., Yao, Y., Sang, W., Yang, X. S., and Ren, G. X. (2015a). Structural features and immunostimulating effects of three acidic polysaccharides isolated from Panax quinquefolius. Internat. J. Biol. Macromol. Struct. Func. Interact. 80, 77–86. doi:10.1016/j.ijbiomac.2015.06.007
Wang, L., Yu, X., Yang, X., Li, Y., Yao, Y., Lui, E. M. K., et al. (2015b). Structural and anti-inflammatory characterization of a novel neutral polysaccharide from North American ginseng (Panax quinquefolius). Int. J. Biol. Macromol. 74, 12–17. doi:10.1016/j.ijbiomac.2014.10.062
Wang, L. J., Li, P. Y., Zhao, C. F., and Li, X. (2000). Studies on the chemical constituents in the fruit of Panax quinquefolius. Chin. Tradit. Herb. Drugs 10, 5–6.
Wang, M. Y., Li, H. N., Liu, W. W., Cao, H., and Li, D. H. (2020). Dammarane-type leads panaxadiol and protopanaxadiol for drug discovery: biological activity and structural modification. Eur. J. Med. Chem. 189, 112087. doi:10.1016/j.ejmech.2020.112087
Wang, S., Bin, Q. I., Hu, N., Wang, Q., An, X. H., Sun, W. J., et al. (2016). Comparison on activity of oxidation-reduction enzyme in different parts of American ginseng with different growth years. China J. Trad. Chin. Med. Pharm. 31 (3), 1049–1051.
Wang, X. B., (2005). Application of HACCP in the manufacture of the tradition health food American ginseng liquid. Food Res. Dev. 26 (2), 25–29.
Wang, Y., and Su, C. (2011). Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry 72 (8), 689–699.
Wang, Y., Wang, Y., Guo, Z. Y., Zhong, Z. L., and Ye, G. Q. (2013). Content determination of ginsenosides and vitamin C in compound effervescent tablets of radix Panacis quinquefolii by HPLC. Chin. J. Exp. Trad. Med. Formulae 19 (1), 122–125.
Wilson, S. A. F., Wong, M. H. T., Stryjecki, C., De Boer, A., Lui, E. M. K., and Mutch, D. M. (2013). Unraveling the adipocyte inflammomodulatory pathways activated by North American ginseng. Int. J. Obes. 37 (3), 350–356. doi:10.1038/ijo.2012.50
Wu, J. Z., and Lin, R. H. (1991). Comparison of carbohydrate content in four plants of Panax ginseng genus. J. Guizhou Med. Univ. 16 (2), 184–186.
Wu, C. F., Liu, Y. L., Song, M., Liu, W., Wang, J. H., Li, X., et al. (2003). Protective effects of pseudoginsenoside-F11 on methamphetamine-induced neurotoxicity in mice. Pharmacol. Biochem. Behav. 76 (1), 103–109. doi:10.1016/s0091-3057(03)00215-6
Wu, Y., Lu, X. R., Xiang, F. L., Lui, E. M. K., and Feng, Q. P. (2011). North American ginseng protects the heart from ischemia and reperfusion injury via upregulation of endothelial nitric oxide synthase. Pharmacol. Res. 64 (3), 195–202. doi:10.1016/j.phrs.2011.05.006
Wu, Y., Qin, C., Lu, X. R., Marchiori, J., and Feng, Q. (2016). North American ginseng inhibits myocardial NOX2-ERK1/2 signaling and tumor necrosis factor-α expression in endotoxemia. Pharmacol. Res. 111, 217–225. doi:10.1016/j.phrs.2016.06.010
Wu, Z. P., Luo, J. Z., and Luo, L. (2007). American ginseng modulates pancreatic beta cell activities. Chin. Med. 2 (1), 11. doi:10.1186/1749-8546-2-11
Xie, G., Wang, C. Z., Yu, C., Qiu, Y., and Jia, W. (2015). Metabonomic profiling reveals cancer chemopreventive effects of American ginseng on colon carcinogenesis in ApcMin/+ mice. J. Proteome Res. 14 (8), 3336–3347. doi:10.1021/acs.jproteome.5b00388
Xie, J. J., Chen, J., Guo, S. K., Gu, Y. T., Yan, Y. Z., Guo, W. J., et al. (2018). Panax quinquefolium saponin inhibits endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and attenuates the progression of osteoarthritis in rat. Biomed. Pharmacother. 97, 886–894. doi:10.1016/j.biopha.2017.10.068
Xie, J. T., Aung, H. H., Wu, J. A., Attel, A. S., and Yuan, C. S. (2002). Effects of American ginseng berry extract on blood glucose levels in ob/ob mice. Am. J. Chin. Med. 30, 187–194.
Xie, J. T., Mehendale, S. R., Wang, A., Han, A. H., Wu, J. A., Osinski, J., et al. (2004). American ginseng leaf: ginsenoside analysis and hypoglycemic activity. Pharmacol. Res. 49 (2), 113–117. doi:10.1142/S0192415X02000442
Xiong, H., Zhang, A. H., Zhao, Q. Q., Yan, G. L., Sun, H., and Wang, X. J. (2020). Discovery of quality-marker ingredients of Panax quinquefolius driven by highthroughput chinmedomics approach. Phytomedicine. 74, 152928. doi:10.1016/j.phymed.2019.152928
Yang, J., Yuan, D., Xing, T., Su, H., Zhang, S., Wen, J., et al. (2016). Ginsenoside Rh2 inhibiting HCT116 colon cancer cell proliferation through blocking PDZbinding kinase/T‐LAK cell‐originated protein kinase. J. Ginseng Res. 40 (4), 400–408. doi:10.1016/j.jgr.2016.03.007
Yang, J. L., Qiu, C. R., Lv, S. L., and Yang, X. (2008). Analysis of fatty acids and essential oil in Panax quinquefolium by gas chromatography-mass spectrometry. Shanxi Med. J. 37 (23), 1134–1136.
Yang, L., Hou, A. J., Sun, Y. P., Wang, S., Zhang, J. X., and Jiang, H. (2020). Screening and quantifying the quality markers of DuHuo by fingerprint modeling. J. Liq. Chromatogr. Relat. Technol. 43, 604–614. doi:10.1080/10826076.2020.1772287
Yang, Y. Y. (2015). Effect of American ginseng on the serum E-selectin and soluble ICAM-1 levels in elderly hypertensive patients with low diastolic blood pressure. World Chin. Med. 10 (10), 1503–1505+1508.
Ye, H. N., and Wang, T. (2001). Analysis of 3 cases of fresh American ginseng poisoning by chewing. Tianjin J. Trad. Chin. Med. 18 (1), 41.
Yim, T. S., Yuk, S. P. S., Sok, C. P., and Wouter, K. (2015). American ginseng tea protects cellular DNA within 2 h from consumption: results of a pilot study in healthy human volunteers. Int. J. Food Sci. Nutr. 66 (7), 815–818. doi:10.3109/09637486.2015.1088937
Yin, C. M., Li, X. C., Sun, J. M., Tang, S., and Chen, X. T. (2010). Cluster analysis of content of total saponin in Panax quinquefolium. J. Jilin Agric. Univ. 32 (3), 289–292.
Yoo, K. M., Lee, C., Lo, Y. M., and Moon, B. K. (2012). The hypoglycemic effects of American red ginseng (Panax quinquefolius L.) on a diabetic mouse model. J. Food Sci. 77 (7–9), H147–H152. doi:10.1111/j.1750-3841.2012.02748.x
Yu, C. H., Wen, X. D., Zhang, Z. Y., Zhang, C. F., Wu, X. H., He, X., et al. (2015). American ginseng significantly reduced the progression of high-fat-diet-enhanced colon carcinogenesis in ApcMin/+mice. J. Ginseng Res. 39 (3), 230–237. doi:10.1016/j.jgr.2014.12.004
Yu, H. H., Zhang, P., Wang, C. L., Liu, J. G., and Zhang, D. W. (2020). Panax quinquefolium saponin optimizes energy homeostasis by modulating AMPK-activated metabolic pathways in hypoxia-reperfusion induced cardiomyocytes. Chin. J. Integr. Med. 1–8. doi:10.1007/s11655-020-3194-4
Yu, X. H. (2016). Study on structure characterization and immune activity of Polysaccharide of Panax quinquefolium L. Harbin University of Science and Technology.
Yu, X. H., Liu, Y., Wu, X., Liu, L., Fu, W., and Song, D. (2017). Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng. Carbohydr. Polym. 156, 9–18.
Yu, X. N., Yang, X. S., Cui, B., Wang, L. J., and Ren, G. Q. (2014). Antioxidant and immunoregulatory activity of alkali-extractable polysaccharides from North American ginseng. Int. J. Biol. Macromol. 65, 357–361. doi:10.1016/j.ijbiomac.2014.01.046
Yu, Z. P., Xu, D. D., Lu, L. F., Zheng, X. D., and Chen, W. (2016). Immunomodulatory effect of a formula developed from American ginseng and Chinese jujube extracts in mice. J. Zhejiang Univ. Series B 17 (2), 147–157. doi:10.1631/jzus.B1500170
Zhai, X., Ren, Y., Teng, Y., Zhao, W., Wang, J. F., Han, Y. T., et al. (2015). The plant morphology and total polysaccharides content in the leaves of Panax quinquefolius L. grown in Canada. Res. Med. Plants 6 (1), 25–27+31.
Zhang, C. X., and Li, X. G. (1989). Analysis of polysaccharide composition in imported Panax quinquefolium from China. Chin. Tradit. Patent Med. 11 (2), 32–33.
Zhang, C. X., Zheng, Y. L., and Li, X. G. (1988a). Comparative study on domestic and imported American ginseng. Chin. Tradit. Patent Med. (6), 39–42.
Zhang, C. X., Zheng, Y. L., and Li, X. G. (1988b). Study on chemical composition of Panax quinquefolium l. Chin. Tradit. Patent Med. (7), 32–33.
Zhang, G. F. (1997). Study on the chemical composition of root of Canadian ginseng. J. Shenyang Pharm. Univ. 14 (2), 114.
Zhang, J. S., Ye, H. G., An, R. G., Pu, F. Q., Shi, Y., Li, W. X., et al. (1987). Determination of trace elements in various parts of Chinese Panax quinquefolium. J. Jilin Univ.(Med. Ed.) 13 (6), 503–504.
Zhang, N. Q., Wang, C. Z., Wang, Z. Z., Li, Z., Sai, J. Y., Meng, Y., et al. (2017). Anti-myocardial ischaemic effect of pseudoginsenoside F11 by inhibiting expression of beta1-adrenoceptor in rats with coronary artery ligation. J. Func. Foods 36, 224–232. doi:10.1016/j.jff.2017.06.053
Zhang, W. S., Pan, A., Yang, L., Cai, Y. Y., and Liu, Q. (2019). American ginseng and Asian ginseng intervention in diet-induced obese mice: metabolomics reveals distinct metabolic profiles. Am. J. Chin. Med. 47 (4), 787–801. doi:10.1142/S0192415X19500411
Zhang, Z., Li, Z., Wu, X., Zhang, C. F., Calway, T., He, T. C., et al. (2015). TRAIL pathway is associated with inhibition of colon cancer by protopanaxadiol. J. Pharmacol. Sci. 127 (1), 83–91. doi:10.1016/j.jphs.2014.11.003
Zhao, X. Y., Liu, Y. H., Xu, Y. Z., Sun, M., Wang, J. X., Zuo, X., et al. (2012). ICP-OES determination of inorganic elements in Panax quinquefolium L.and its processed products. Med. Plant 3 (12), 14–18.
Zhao, Z. G., Qing, K., Xuan, D., and Yang, S. Y. (1999). A case report of brain CT changes caused by Panax quiculatum poisoning in neonates. J. Prac. Radiol. 15 (10), 593.
Zhen, Z. H. (2012). Study on extraction of total flavonoids from Panax quinquefolium and its antioxidation effects. J. Anhui Agric. Sci. 40 (32), 15903–15904.
Zheng, Y. L., and Zhang, C. X. (1993). Isolation and identification of oil in Panax quinquefolium from Heilongjiang province. Chin. Herbal Med. 24 (11), 570–571.
Zheng, Y. L., Zhang, C. X., Li, X. G., and Guo, S. Z. (1988c). Study on chemical composition of American ginseng–separation and identification of fatty acids in fats and oils. Chin. Tradit. Patent Med. (8), 32–33.
Zheng, Y. N., and Deng, Q. H. (1989). Comparative analysis of hemostatic components of Panax ginseng. J. Jilin Agric. Univ. 011 (1), 24–27.
Zhu, L., Li, J., Xing, N. N., Han, D. W., Kuang, H. X., and Ge, P. L. (2015). American ginseng regulates gene expression to protect against premature ovarian failure in rats. BioMed Res. Int. 2015, 767124. doi:10.1155/2015/767124
Zhu, X. Y., Zhang, Z. L., Li, P., Liang, W. Y., Feng, X., and Liu, M. L. (2013). Shenyuan, an extract of American ginseng and corydalis tuber formula, attenuates cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and oxidative stress in a porcine model of acute myocardial infarction. J. Ethnopharmacol. 150 (2), 672–681. doi:10.1016/j.jep.2013.09.044
Keywords: Panacis Quinquefolii Radix, phytochemistry, quality control, pharmacology, toxicology, industrial applications
Citation: Yang L, Hou A, Zhang J, Wang S, Man W, Yu H, Zheng S, Wang X, Liu S and Jiang H (2020) Panacis Quinquefolii Radix: A Review of the Botany, Phytochemistry, Quality Control, Pharmacology, Toxicology and Industrial Applications Research Progress. Front. Pharmacol. 11:602092. doi: 10.3389/fphar.2020.602092
Received: 02 September 2020; Accepted: 19 October 2020;
Published: 08 December 2020.
Edited by:
Karl Tsim, Hong Kong University of Science and Technology, Hong KongReviewed by:
Tao Yi, Hong Kong Baptist University, Hong KongHubiao Chen, Hong Kong Baptist University, Hong Kong
Copyright © 2020 Yang, Hou, Zhang, Wang, Man, Yu, Zheng, Wang, Liu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songtao Liu, c29uZ3Rhb2xpdV84ODhAMTYzLmNvbQ==; Hai Jiang, amlhbmdoYWlfNzc3QDEyNi5jb20=
 Liu Yang
Liu Yang Ajiao Hou
Ajiao Hou