- 1Department of Traditional Chinese Medicine, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
- 2Master Program in Clinical Pharmacy, School of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan
- 3Department of Internal Medicine, Chi Mei Medical Center, Chiali, Tainan, Taiwan
- 4Department of Pharmacy, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
- 5Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
- 6Center for Big Data Research, Kaohsiung Medical University, Kaohsiung, Taiwan
Purpose: Many comorbidities, including depression, anxiety, and insomnia, occur in patients with chronic obstructive pulmonary disease (COPD). These patients may be prescribed benzodiazepines (BZDs). However, there are some concerns that benzodiazepines increase the risk of drug overdose, hypercapnic respiratory failure, acute exacerbation and increased mortality. The aim of our study was to evaluate the drug safety of BZDs in patients with COPD.
Methods: We used the National Health Insurance Research database in Taiwan from 2002 to 2016 to perform a retrospective cohort study. We enrolled patients who were exposed to the first prescription of BZDs, non-BZDs or a combination (mix user) after COPD diagnosis. We performed 1:1:1 propensity score matching in three groups. The outcomes were COPD with acute exacerbation and all-cause mortality. Poisson regression analysis was performed to evaluate the incidence rate ratios for the outcomes in the groups.
Results: After propensity score matching, there were 2,856 patients in each group. After adjusting for confounding factors, we found that compared to BZD users, non-BZD and mix users had nonsignificant differences in outpatient management of acute exacerbations, hospitalization management of acute exacerbations, emergency department management of acute exacerbations and all-cause mortality. BZD and mix groups showed significantly increased admission for acute exacerbation of COPD compared with that of the nonuser group, with IRRs of 2.52 (95% CI, 1.52–4.18; p = 0.0004) and 2.63 (95% CI, 1.57–4.40; p = 0.0002), respectively.
Conclusion: BZD, non-BZD, and mix users showed increased COPD-related respiratory events compared to nonusers in Asian subjects.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic lung disease that is not fully reversible and is characterized by airflow obstruction. COPD is associated with a substantial health burden worldwide (Soriano and Rodriguez-Roisin, 2011; World Health Organization 2017; Terzano et al., 2010; Decramer et al., 2012). Comorbidities such as insomnia, anxiety, sleep disorder, depression, and psychological disorders are commonly reported in patients with COPD (Light et al., 1985; Kunik et al., 2005; Solano et al., 2006; Cheng et al., 2008; Maurer et al., 2008; Biswas et al., 2017). Benzodiazepines (BZDs) are the most widely prescribed class of sedative-anxiolytic drugs; BZDs cause sedation and muscle relaxation, can lower anxiety levels and are commonly used for the treatment of insomnia, anxiety and sleep disorder. However, previous studies reported that BZDs may have considerable risks or adverse effects and can be fatal in patients with COPD; these adverse effects include hypoxemia, hypercapnia (Block et al., 1984; Beaupre et al., 1988), decreased respiratory muscle strength (Jolly et al., 1996) and respiratory failure (Chen et al., 2015). The risk may increase as the plasma half-life of BZDs increases (Greenblatt et al., 1991; Fisher, 1999).
Benzodiazepine receptor agonists (BZRAs) include traditional BZDs and newer generation non-benzodiazepine receptor drugs that preferentially bind to the ω1-benzodiazepine receptor of the GABAA receptor complex. BZDs nonselectively bind to the receptor (Ebert et al., 2006), whereas non-BZD drugs selectively bind to the benzodiazepine receptor and have a lower affinity for the GABAA receptor; therefore, they lack significant muscle relaxant, anxiolytic, and anticonvulsant activities of traditional BZDs, resulting in fewer pulmonary adverse effects and fewer adverse effects on other systems (Ranlov and Nielsen, 1987; Cohn, 1993; Dämgen and Lüddens, 1999; George, 2000; Roth, 2009).
The Joint American Thoracic Society/European Respiratory Society guidelines do not recommend the use of BZDs for COPD patients, especially when COPD is severe (Celli and MacNee, 2004). Nevertheless, BZRAs are widely used for patients with COPD, with estimates that 44.7%–69% of patients receive these drugs (Halvorsen and Martinussen, 2015; Park et al., 2017). Elderly COPD patients increase the prevalence, exposure duration and dose of BZRAs because of the patient’s illness, psychological dependence or other factors (Steinman et al., 2017).
A concern in clinical practice is whether COPD patients using BZDs or non-BZD drugs, or a combination of the two, are potentially at increased risk of respiratory problems. Some studies are available for BZRAs used in patients with COPD, and these studies were limited by a small number of subjects and short-term effects (lasting a few hours) (Murciano et al., 1990; Berry et al., 1992; Murciano et al., 1993).
Therefore, the aim of the study was to evaluate the risk of acute exacerbation in patients with COPD after receiving BZRAs or non-BZRAs and in nonusers by performing a large, population-based cohort study in an Asian population.
Methods
Study Design and Data Source
This retrospective cohort study included data for patients with COPD aged between 40 and 90 years from the Taiwan National Health Insurance Research database in Health and Welfare Data Science Center January 1, 2002 to December 31, 2016. National Health Insurance Research database was established in 1995 and covers 99% of the 23 million inhabitants in Taiwan under compulsory national health insurance. The database includes personal information, codes for diagnoses and procedures from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and 10th Revision. The patient’s medication was classified according to the Anatomical Therapeutic Chemical and National Health Insurance codes. These codes are widely accepted drug classification systems coordinated by the World Health Organization Collaborating Center for Drug Statistics Methodology. The study was approved by the Institutional Review Board of Kaohsiung Medical University.
Identification of the COPD Cohort
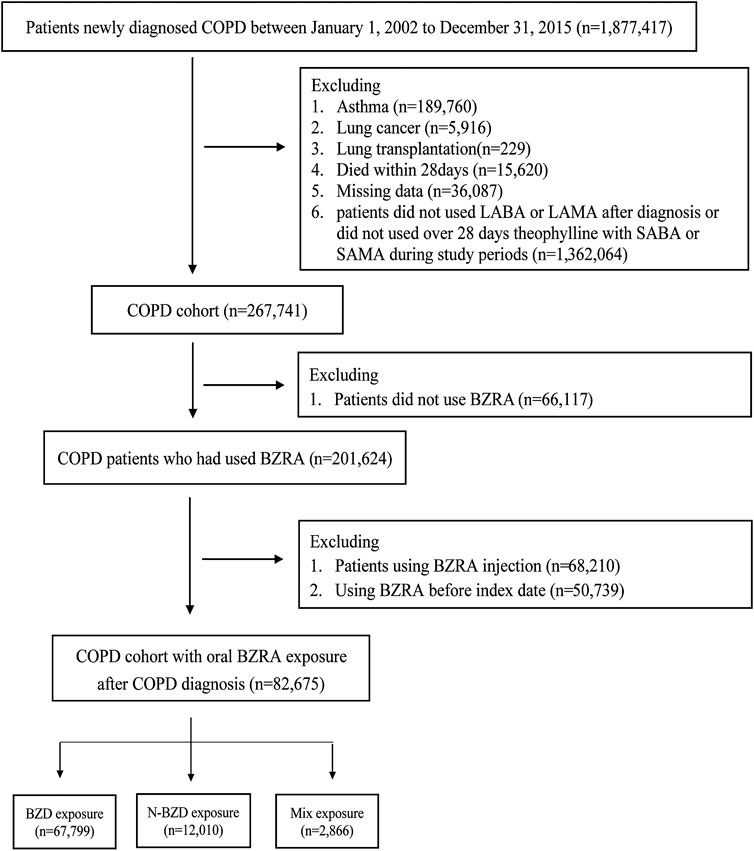
Using the database, we identified adults aged between 40 and 90 years with newly diagnosed COPD (ICD-9-CM codes 490, 491, 492, and 496) who had received more than one inpatient diagnosis or two or more outpatient diagnoses between January 1, 2002, and December 31, 2015. Patients with COPD should receive one of the following medications for more than one month: long-acting β adrenergic agonists (LABAs); long-acting anti-muscarinic agonists (LAMAs); or oral methylxanthines combined with short-acting β2-agonists (SABAs) or short-acting muscarinic antagonists (SAMAs) (McKay et al., 1993; Ram et al., 2002; Wang et al., 2018). Patients were excluded if they met any of the following conditions: asthma, lung transplantation, lung cancer or death within one month after diagnosis of COPD (Ekstrom et al., 2013). Moderate COPD exacerbation was defined as a requirement for treatment with antibiotics or systemic corticosteroids or both. Severe exacerbations of COPD was defined as those leading to hospitalization or emergency department visit.
Identification of the Benzodiazepine Receptor Agonists Cohort
The BZRA cohort was identified from COPD patients newly exposed to BZRAs and divided into an oral BZD group and an oral non-BZD BZRA group. Patients were required to continue using the BZRA without change for 30 days. Patients were excluded if they received BZRA injections during the study period or died before the index date. Each patient was followed until an outcome occurred: mortality or the end of the study (December 31, 2016). The BZRA cohort was divided into three groups based on the initial prescription: those taking BZDs (the BZD cohort), those taking non-BZD BZRAs (the non-BZD cohort), and those prescribed both BZDs and non-BZD drugs simultaneously in the initial prescription (the mix cohort). A cohort of patients with COPD who were not taking BZRAs (the nonuser cohort) was also identified.
Exposure and Index Date Assessment
A new prescription for BZRAs was defined as the patient receiving his or her first prescription for BZRAs during the study period following entry into the cohort. We excluded patients who received their first BZRA prescription prior to the diagnosis of COPD. When patients received more than prescription during the study period, only the first was included in the analysis. The index date was the date of the first BZD, non-BZD or Mixed-use prescription after COPD diagnosis. For each patient, we collected any of the following outcomes that occurred during the 30 days following the index date (The index date was the date of the first BZD, non-BZD or Mixed-use prescription after COPD diagnosis and patients were required to continue using the medication without change for 30 days): outpatient visits for respiratory exacerbations, hospital admission for COPD acute exacerbation or for respiratory exacerbations, emergency department attendance for COPD or pneumonia and all-cause mortality. The observation period was short (only 30 days) and only the first prescription was included in the analysis.
Covariates
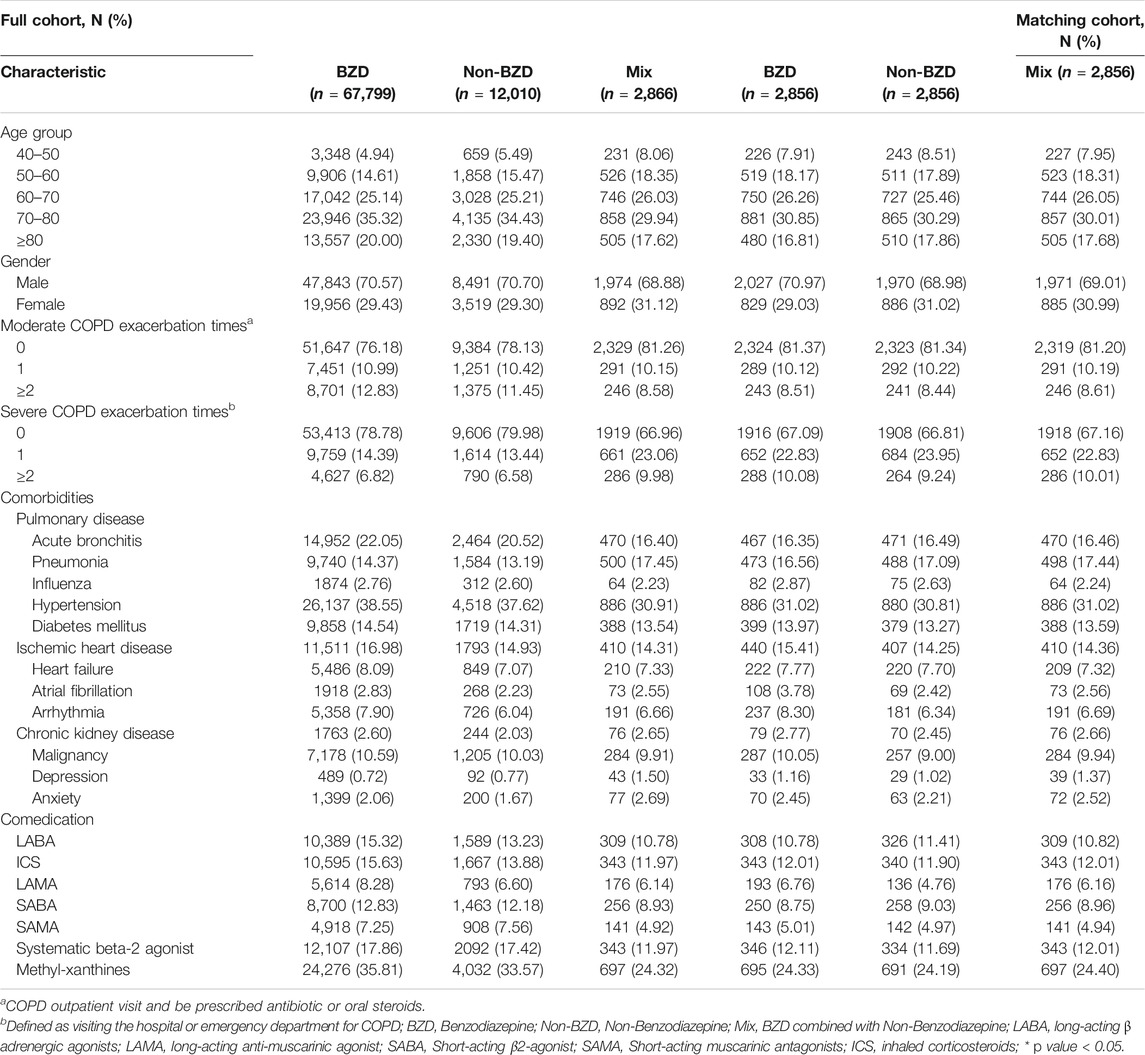
The baseline characteristics and comorbidities of each patient were collected within one year prior to the index date. These included COPD severity, hypertension, diabetes mellitus, ischemic heart disease, heart failure, atrial fibrillation, arrhythmia, chronic kidney disease, malignancy, depression and anxiety. In addition, the frequency of COPD acute exacerbations was recorded. Severe acute exacerbation was defined as patients requiring admission or visits to the emergency department. Moderate acute exacerbation was defined as a patent prescription for either antibiotics or oral corticosteroids from an outpatient department. Other COPD treatment medications, including LABAs, LAMAs, inhaled corticosteroids, SABAs, SAMAs, systematic beta-2 agonists and methylxanthines, were recorded for analysis.
Propensity Score Matching to Create the Final Groups
Propensity score matching was applied on a 1:1:1 basis to the BZD, non-BZD and mix cohorts to create three final groups (the BZD, non-BZD and mix groups) with balanced baseline characteristics. The matching factors included age group, sex, comorbidities and COPD medication (Lori, 2004). Propensity score matching was also used to match the patients in the three BZRA groups and the patients in the nonuser cohort 1:1 to perform sensitivity analyses.
Outcome Assessment
For each patient, we collected any of the following outcomes that occurred during the 30 days following the index date: outpatient visits for respiratory exacerbations, hospital admission for acute COPD exacerbation or for respiratory exacerbations, emergency department attendance for COPD or pneumonia and all-cause mortality.
Statistical Analysis
Differences in baseline characteristics were evaluated by the chi-square test (for categorical variables). Univariate and multivariate Poisson regression with robust error variance analysis was used to generate crude and adjusted incidence rate ratios (IRRs) with 95% CIs for each type of outcome. Consequently, the IRRs for all the study outcomes and further sensitivity analyses were evaluated by the Poisson regression model with a robust error variance (Zou, 2004) between the BZD, non-BZD and mix groups. Time-to-event analyses were performed using Kaplan–Meier plots and the log-rank test. Various sensitivity analyses were performed. First, we used several different follow-up periods (60, 90, 180, and 365 days) to ensure that the results were consistent with those obtained for the 30-days follow-up. Second, we compared risks between the three BZRA groups and the nonuser group. Third, because BZDs with a longer half-life would be expected to cause more serious side effects than those with shorter half-lives (Greenblatt et al., 1989; Greenblatt et al., 1991), we stratified the BZD group according to half-life to reduce any pharmacokinetic effects. SAS statistical software (Version 9.4; SAS Institute, Inc., Cary, NC) was used to perform all the analyses. A two-sided p value < 0.05 was considered statistically significant.
Results
There are 267,741 patients in COPD cohort. There are 201,624 patients used BZRA and 66,117 (24.69%) patients did not have BZRA. In total, 267,741 patients were included in the COPD cohort (Figure 1), of whom 82,675 were first prescribed BZRA drugs after their COPD diagnosis. After propensity score matching, there were 2,856 patients in each of the three groups. All of the baseline characteristics were balanced after propensity score matching (Table 1). According to our insurance regulation, benzodiazepines are used for a range of some health issues, including anxiety, sleep disorders, and epilepsy. Most of our study cohort (70%) were used BZRA for sleep disorders.
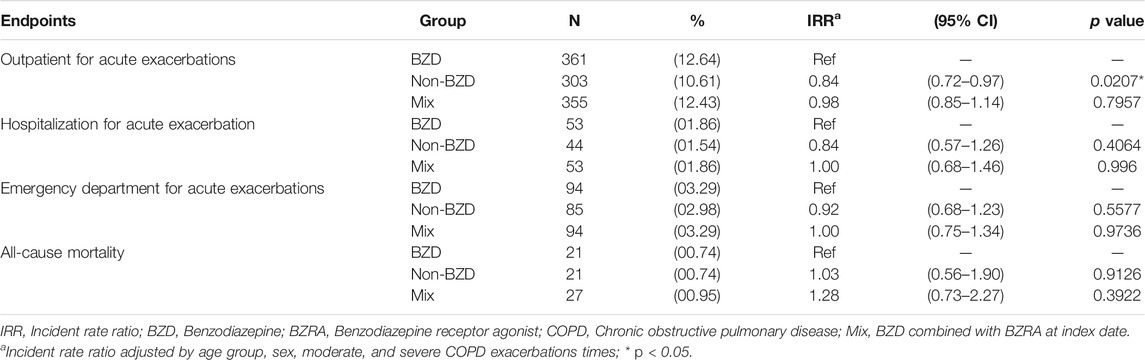
Table 2 summarizes the IRRs. The non-BZD groups experienced significantly fewer outpatient visits for acute exacerbation than the BZD group (IRR = 0.84, 95% CI = 0.72–0.97, p = 0.0207). However, compared with the BZD group, the non-BZD group was not associated with significant decreases in admission for COPD acute exacerbation (p = 0.4064), emergency department attendance for COPD acute exacerbation (p = 0.5577), or all-cause mortality (p = 0.9126). Similarly, compared with the BZD group, the mix group was not associated with significant IRRs for outpatient visits for acute exacerbation (p = 0.7957), admission for COPD acute exacerbation (p = 0.9660), emergency department visit for COPD acute exacerbation (p = 0.9736), or all-cause mortality (p = 0.3922).
The clinical outcomes were evaluated further by three sensitivity analyses (Table 3). The analysis using various follow-up periods showed two inconsistencies with the main findings for 30 days. At 90 days, there was no longer a decrease in outpatient visits for respiratory exacerbation in the non-BZD group compared to that in the BZD group, and at 180 days, admission for the acute exacerbation of COPD was inconsistent with the 30-days finding. All the other results in the follow-up sensitivity analysis were consistent with the main findings.
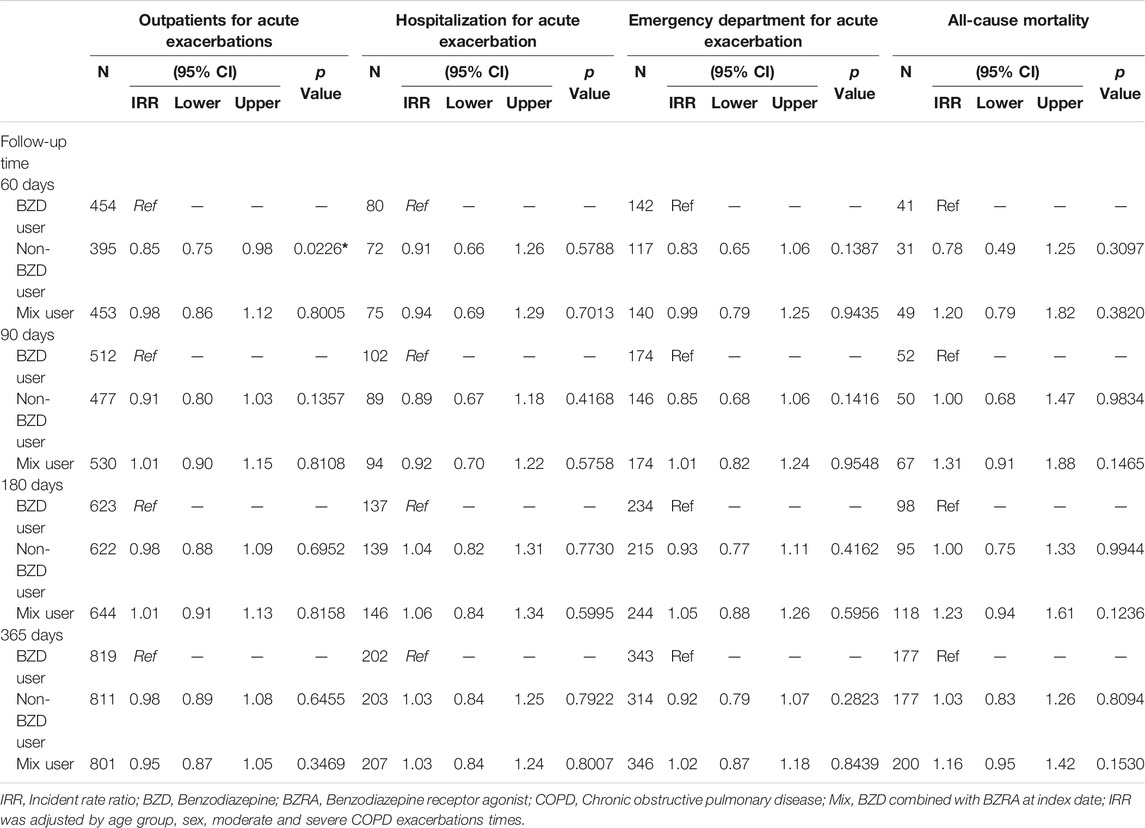
TABLE 3. Incident rate ratio of study outcomes for matched cohorts with different types of follow-up.
The second sensitivity analysis compared the outcomes between the three BZRA groups and the nonuser group of patients with COPD. Compared to the nonuser group, the BZD, non-BZD and mix groups experienced significantly more outpatient visits because of respiratory exacerbation, with IRRs of 2.57 (95% CI, 2.13–3.10; p < 0.0001), 2.40 (95% CI, 1.97–2.94; p < 0.0001) and 3.38 (95% CI, 2.74–4.17; p < 0.0001), respectively. The three groups also experienced increased emergency department attendance for COPD acute exacerbation, with IRRs of 2.11 (95% CI, 1.48–3.02; p < 0.0001), 2.12 (95% CI, 1.46–3.09; p < 0.0001) and 1.87 (95% CI, 1.33–2.64; p = 0.0003), respectively, compared to the nonuser group, as well as significantly decreased all-cause mortality, with IRRs of 0.43 (95% CI, 0.26–0.72; p = 0.0013), 0.56 (95% CI, 0.33–0.96; p = 0.0356) and 0.46 (95% CI, 0.29–0.73; p = 0.0010). In addition, the BZD and mix groups showed significantly increased admission for acute exacerbation of COPD compared with that of the nonuser group, with IRRs of 2.52 (95% CI, 1.52–4.18; p = 0.0004) and 2.63 (95% CI, 1.57–4.40; p = 0.0002), respectively, as well as increased admission for respiratory exacerbation, with IRRs of 1.46 (95% CI, 1.05–2.03; p = 0.0259) and 2.26 (95% CI, 1.57–3.25; p < 0.0001). The third sensitivity analysis stratified the BZD group according to the half-life of the drug. This did not reveal any significant differences in outcome (Table 4).
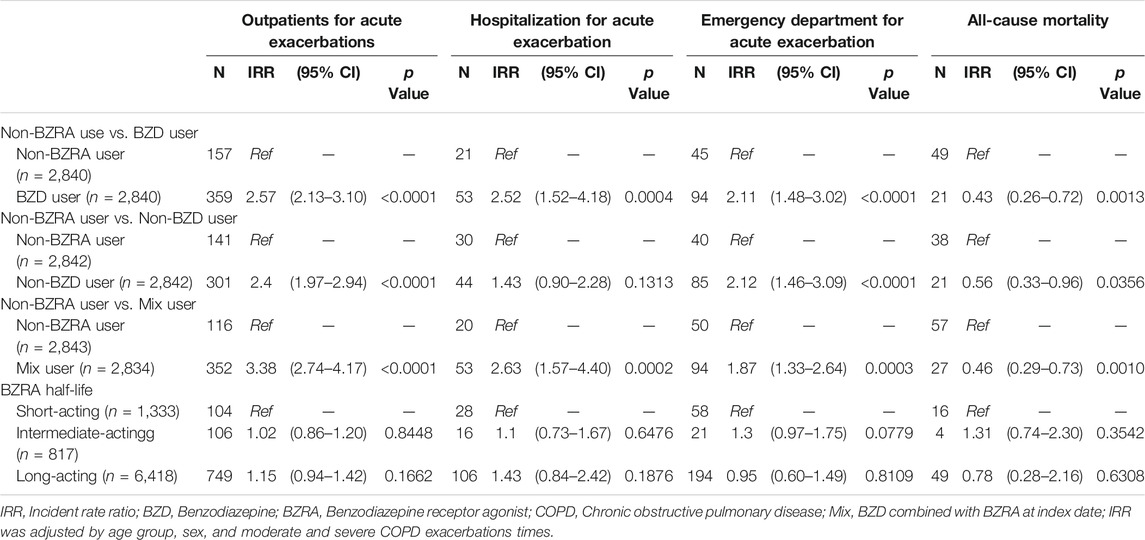
TABLE 4. The incident rate ratio for study outcomes in poisson regression of different groups and half-life sensitivity analysis.
Discussion
This retrospective observational cohort study examined data for 267,741 patients with COPD, of whom 82,675 used BZRAs. The comparison of the outcomes among the three types of BZRAs found that the patients administered the non-BZD drugs experienced 0.84-fold fewer outpatient visits for respiratory exacerbation in the first 30 days than did those who received BZDs. This was not influenced by baseline comorbidity or the severity of COPD. The other results showed no statistically significant differences among the use of BZDs, non-BZD BZRA drugs or a combination of these drugs. Previous studies of patients with COPD have shown inconsistent results, with some finding that BZDs did not influence respiratory effects (Beaupre et al., 1988; Murciano et al., 1990), whereas others found that it did (Murciano et al., 1993; Chen et al., 2015). However, none of these studies was designed as a cohort study with a 30-days outcome period, and all of the studies focused on differences in lung parameters rather than on COPD-related outcomes. Therefore, the present study provides new evidence that patients using BZD and non-BZD drugs or a combination were at equal risk of COPD-related exacerbation. This finding allows for flexibility in selecting a BZRA to treat COPD. However, although there were no statistically significant results for most respiratory-related outcomes, the non-BZD group experienced fewer outpatient visits for acute exacerbation.
A sensitivity analysis checked whether a longer follow-up period affected the clinical outcome. Although two of the main outcomes changed at particular follow-up periods, most of the outcomes remained consistent with the main outcomes at 30 days. Any side effects of BZRAs would be expected to be observed soon after the drugs were administered, so the 30-day period of follow-up in this study is acceptable. The results of our second sensitivity analysis that compared the outcomes with those of COPD patients who did not receive BZRA drugs revealed an association with increased respiratory exacerbation, with the BZD and mix groups experiencing greater risk of the occurrence of a clinical outcome than the nonuser group. This was consistent with the results of a cohort study by Vohoris et al., who found that COPD patients using BZDs had a higher association with emergency and outpatient visits for exacerbation than the patients who did not take BZRAs (Vozoris et al., 2014). Other previous studies have also observed an increased risk of respiratory exacerbation in COPD patients administered BZRA compared to those who were not (Beaupre et al., 1988; Berry et al., 1992; Cohn et al., 1992; Berry et al., 1995; Jolly et al., 1996).
Our study examined population-based, real-world data, adjusted according to COPD severity, and provided evidence related to various follow-up periods and for BZRAs with different half-lives. The non-BZD group showed a risk equal to that of the other groups regarding admission for acute exacerbation or emergency department for respiratory exacerbation but a lower risk of outpatient visits for exacerbation. No previous study has considered the risk of using BZRAs for patients with COPD. Some experimental studies have shown that non-BZD drugs do not affect the lung function parameters of COPD patients in the short term (Murciano et al., 1990; Murciano et al., 1993; Girault et al., 1996). However, one study showed that the non-BZD group showed fewer acute exacerbation events (Chen et al., 2015).
Our study compared the non-BZD and BZD groups based on propensity score matching for age group, sex, COPD severity, comorbidities and COPD medication.
Compared with the BZD group, the non-BZD group underwent fewer outpatient visits for acute exacerbation; however, other clinical COPD-related exacerbation outcomes were similar for the non-BZD, BZD and mix groups, including admission for acute COPD exacerbation, emergency department attendance for COPD exacerbation, and all-cause mortality. In a sensitivity analysis, we examined various follow-up times to check our outcomes and reduce selection bias. Compared to the nonuser group, all three BZRA groups were associated with an increased risk of COPD-related exacerbation. Our results provide treatment information in clinical practice and provide potentially useful data about BZRA risk.
Strengths and Limitations
This study and its findings had some limitations. First, we were unable to obtain direct information about COPD severity from the database. Instead, two surrogate values were used to estimate the severity. There was no information regarding the severity of COPD. We need to evaluate how this correlates with the increased risks. Second, we were unable to establish an indication for using BZRA, which requires a prospective study design. The individual indication of BZRA might have provided information related to underlying diseases of the COPD patients and about particular clinical situations or procedures. Consequently, we performed propensity score matching to balance the severity of COPD between the groups and reduce the bias. Third, the study was based on dispensed prescriptions; therefore, we could not establish the patients’ drug adherence. Despite the limitations, our study had some important strengths. The findings were derived from a population-based database. This study evaluated COPD patients divided into BZD, non-BZD and mix cohorts. The observation period is short, therefore the study provides information on short-term effects only, so further studies are needed. Although we used a short period to compare the risk between the groups, we found the same trends in the longer-term sensitivity analysis. We found no differences among the three BZRA groups; however, compared to the nonuser group, the patients taking BZRAs were at increased risk of respiratory events.
Conclusions
This study has provided clues that the risk of short-term COPD acute exacerbation is similar when using BZDs, non-BZD drugs or a combination of these drugs. In addition, the use of BZDs and non-BZD BZRAs by patients with COPD increased their risk of acute exacerbation compared with that in COPD patients who did not take BZRAs in Asian subjects. Therefore, COPD patients prescribed BZRAs should be observed for potential risk of acute exacerbation in Asian subjects.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The study was approval by KMUHIRB-EXEMPT-20180043.
Author Contributions
Conceived and designed the experiments: C-YC. Performed the analysis: L-YC. Analyzed the data: L-YC and C-YC. Wrote the paper: Y-HL, L-YC, and K-ML. Provided constructive opinions and suggestions and study supervision: C-YC. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study is based partly on secondary data from the National Health Insurance Research database provided by the Bureau of National Health Insurance, Department of Health, Taiwan. The interpretations and conclusions herein do not represent the views of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes. We are grateful to Kaohsiung Medical University for providing administrative and funding support. This manuscript has been released as a pre-print at Research Square Y-HL
Abbreviations
COPD, Chronic obstructive pulmonary disease; BZD, Benzodiazepines; IRR, Incidence rate ratios; BZRAs, Benzodiazepine receptor agonists; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; LABAs, Long-acting β adrenergic agonists; LAMAs, Long-acting anti-muscarinic agonists; SABAs, Short-acting β2-agonists; SAMAs, Short-acting muscarinic antagonists.
References
Beaupré, A., Soucy, R., Phillips, R., and Bourgouin, J. (1988). Respiratory center output following zopiclone or diazepam administration in patients with pulmonary disease. Respiration 54, 235–240. doi:10.1159/000195530
Berry, R. B., McCasland, C. R., and Light, R. W. (1992). The effect of triazolam on the arousal response to airway occlusion during sleep in normal subjects. Am. Rev. Respir. Dis. 146, 1256–1260. doi:10.1164/ajrccm/146.5_pt_1.1256
Berry, R. B., Kouchi, K., Bower, J., Prosise, G., and Light, R. W. (1995). Triazolam in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 151, 450–454. doi:10.1164/ajrccm.151.2.7842205
Biswas, D., Mukherjee, S., Chakroborty, R., Chatterjee, S., Rath, S., Das, R., et al. (2017). Occurrence of anxiety and depression among stable COPD patients and its impact on functional capability. J. Clin. Diagn. Res. 11, OC24–OC27. doi:10.7860/JCDR/2017/24203.9393
Block, A. J., Dolly, F. R., and Slayton, P. C. (1984). Does flurazepam ingestion affect breathing and oxygenation during sleep in patients with chronic obstructive lung disease?. Am. Rev. Respir. Dis. 129, 230–233. .
Celli, B. R., MacNee, W., Agusti, A., Anzueto, A., Berg, B., Buist, A. S., et al. 2004). Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur. Respir. J. 23, 932–946. doi:10.1183/09031936.04.00014304
Chen, S.-J., Yeh, C.-M., Chao, T.-F., Liu, C.-J., Wang, K.-L., Chen, T.-J., et al. (2015). The use of benzodiazepine receptor agonists and risk of respiratory failure in patients with chronic obstructive pulmonary disease: a nationwide population-based case-control study. Sleep 38, 1045–1050. doi:10.5665/sleep.4808
Cheng, J.-S., Huang, W.-F., Lin, K.-M., and Shih, Y.-T. (2008). Characteristics associated with benzodiazepine usage in elderly outpatients in Taiwan. Int. J. Geriatr. Psychiatr. 23, 618–624. doi:10.1002/gps.1950
Cohn, M. A. (1993). Effects of zolpidem, codeine phosphate and placebo on respiration. Drug Saf. 9, 312–319. doi:10.2165/00002018-199309040-00009
Cohn, M. A., Morris, D. D., and Juan, D. (1992). Effects of estazolam and flurazepam on cardiopulmonary function in patients with chronic obstructive pulmonary disease. Drug Saf. 7, 152–158. doi:10.2165/00002018-199207020-00006
Dämgen, K., and Lüddens, H. (1999). Zaleplon displays a selectivity to recombinant GABAA receptors different from zolipdem, zopiclone and benzodiazepines. Neurosci. Res. Commun. 25, 139–148
Decramer, M., Janssens, W., and Miravitlles, M. (2012). Chronic obstructive pulmonary disease. Lancet 379, 1341–1351. doi:10.1016/s0140-6736(11)60968-9
Ebert, B., Wafford, K. A., and Deacon, S. (2006). Treating insomnia: current and investigational pharmacological approaches. Pharmacol. Ther. 112, 612–629. doi:10.1016/j.pharmthera.2005.04.014
Ekström, M. P., Hermansson, A. B., and Ström, K. E. (2013). Effects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 187, 715–720. doi:10.1164/rccm.201208-1565oc
Fisher, D. M. (1999). Clinical pharmacology of neuromuscular blocking agents. Am. J. Health Syst. Pharm. 56 (11 Suppl. 1), S4–S9. doi:10.1093/ajhp/56.suppl_1.s4
George, C. F. (2000). Perspectives on the management of insomnia in patients with chronic respiratory disorders. Sleep 23 (Suppl. 1), S31–S38, discussion S6-8.
Girault, C., Muir, J.-F., Mihaltan, F., Borderies, P., De La Giclais, B., Verdure, A., et al. (1996). Effects of repeated administration of zolpidem on sleep, diurnal and nocturnal respiratory function, vigilance, and physical performance in patients with COPD. Chest 110, 1203–1211. doi:10.1378/chest.110.5.1203
Greenblatt, D. J., Shader, R. I., and Harmatz, J. S. (1989). Implications of altered drug disposition in the elderly: studies of benzodiazepines. J. Clin. Pharmacol. 29, 866–872. doi:10.1002/j.1552-4604.1989.tb03246.x
Greenblatt, D. J., Harmatz, J. S., and Shader, R. I. (1991). Clinical pharmacokinetics of anxiolytics and hypnotics in the elderly. Clin. Pharmacokinet. 21, 165–177. doi:10.2165/00003088-199121030-00002
Halvorsen, T., and Martinussen, P. E. (2015). Benzodiazepine use in COPD: empirical evidence from Norway. Int. J. Chronic Obstr. Pulm. Dis. 10, 1695–1702. doi:10.2147/COPD.S83107
Jolly, E., Aguirre, L., Jorge, E., and Luna, C. (1996). [Acute effect of lorazepam on respiratory muscles in stable patients with chronic obstructive pulmonary disease]. Medicina 56, 472–478.
Kunik, M. E., Roundy, K., Veazey, C., Souchek, J., Richardson, P., Wray, N. P., et al. (2005). Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest 127, 1205–1211. doi:10.1016/s0012-3692(15)34468-8
Light, R. W., Merrill, E. J., Despars, J. A., Gordon, G. H., and Mutalipassi, L. R. (1985). Prevalence of depression and anxiety in patients with COPD. Chest 87, 35–38. doi:10.1378/chest.87.1.35
Lori, S. (2004). Parsons ORGSUGI 29. Seattle, Washington. Lori S. ParsonsSeattle, Washington: Ovation Research Group.
Maurer, J., Rebbapragada, V., Borson, S., Goldstein, R., Kunik, M. E., Yohannes, A. M., et al. (2008). Anxiety and depression in COPD. Chest 134, 43s–56s. doi:10.1378/chest.08-0342
McKay, S. E., Howie, C. A., Thomson, A. H., Whiting, B., and Addis, G. J. (1993). Value of theophylline treatment in patients handicapped by chronic obstructive lung disease. Thorax 48, 227–232. doi:10.1136/thx.48.3.227
Murciano, D., Aubier, M., Palacios, S., and Pariente, R. (1990). Comparison of zolpidem (Z), triazolam (T), and flunitrazepam (F) effects on arterial blood gases and control of breathing in patients with severe chronic obstructive pulmonary disease (COPD). Chest 97, 51s–52s. doi:10.1378/chest.97.3_supplement.51s
Murciano, D., Armengaud, M. H., Cramer, P. H., Neveux, E., L’Héritier, C., Pariente, R., et al. (1993). Acute effects of zolpidem, triazolam and flunitrazepam on arterial blood gases and control of breathing in severe COPD. Eur. Respir. J. 6, 625–629.
Park, S. Y., Bae, S., and Shin, J.-Y. (2017). Real-world prescribing patterns of long-acting benzodiazepines for elderly Koreans in 2013. Cp 55, 472–479. doi:10.5414/cp202974
Ram, F. S., Jones, P. W., Castro, A. A., De Brito, J. A., Atallah, A. N., Lacasse, Y., et al. (2002). Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 4, Cd003902. doi:10.1002/14651858.CD003902
Ranløv, P. J., and Nielsen, S. P. (1987). Effect of zopiclone and diazepam on ventilatory response in normal human subjects. Sleep 10 Suppl 1 (Suppl. 1), 40–47. doi:10.1093/sleep/10.suppl_1.40
Roth, T. (2009). Hypnotic use for insomnia management in chronic obstructive pulmonary disease. Sleep Med. 10, 19–25. doi:10.1016/j.sleep.2008.06.005
Solano, J. P., Gomes, B., and Higginson, I. J. (2006). A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J. Pain Symptom Manag. 31, 58–69. doi:10.1016/j.jpainsymman.2005.06.007
Soriano, J. B., and Rodriguez-Roisin, R. (2011). Chronic obstructive pulmonary disease overview: epidemiology, risk factors, and clinical presentation. Proc. Am. Thorac. Soc. 8, 363–367. doi:10.1513/pats.201102-017rm
Steinman, M. A., Low, M., Balicer, R. D., and Shadmi, E. (2017). Epidemic use of benzodiazepines among older adults in Israel: epidemiology and leverage points for improvement. J. Gen. Intern. Med. 32, 891–899. doi:10.1007/s11606-017-4059-1
Terzano, C., Conti, V., Di Stefano, F., Petroianni, A., Ceccarelli, D., Graziani, E., et al. (2010). Comorbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung 188, 321–329. doi:10.1007/s00408-009-9222-y
Vozoris, N. T., Fischer, H. D., Wang, X., Stephenson, A. L., Gershon, A. S., Gruneir, A., et al. (2014). Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur. Respir. J. 44, 332–340. doi:10.1183/09031936.00008014
Wang, M.-T., Liou, J.-T., Lin, C. W., Tsai, C.-L., Wang, Y.-H., Hsu, Y.-J., et al. (2018). Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease. JAMA Intern. Med. 178, 229–238. doi:10.1001/jamainternmed.2017.7720
World Health Organization. (2017). Chronic obstructive pulmonary disease (COPD). Available at: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd).
Keywords: benzodiazepines, chronic obstrucive pulmonary disease, safety, national health insurance research database, acute exacerbation
Citation: Liao Y-H, Chen L-Y, Liao K-M and Chen C-Y (2020) Drug Safety of Benzodiazepines in Asian Patients With Chronic Obstructive Pulmonary Disease. Front. Pharmacol. 11:592910. doi: 10.3389/fphar.2020.592910
Received: 08 August 2020; Accepted: 27 October 2020;
Published: 09 December 2020.
Edited by:
Nadia Mores, Catholic University of the Sacred Heart, ItalyReviewed by:
Vaidehi Thanawala, Vir Biotechnology, Inc., United StatesAntonio Molino, University of Naples Federico II, Italy
Copyright © 2020 Liao, Chen, Liao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chung-Yu Chen, amsyOTc1NTI1QGhvdG1haWwuY29t; Kuang-Ming Liao, YWJjODg3MEB5YWhvby5jb20udHc=
 Yi-Hsiang Liao1
Yi-Hsiang Liao1 Liang-Yu Chen
Liang-Yu Chen Kuang-Ming Liao
Kuang-Ming Liao