- 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Department of Endocrinology, Guang’anmen Hospital of China, China Academy of Chinese Medical Sciences, Beijing, China
Type 2 diabetes mellitus (T2DM) is a chronic disease that has become a global public health problem. Studies on T2DM prevention and treatment mostly focus on discovering therapeutic drugs. Artemisinin and its derivatives were originally used as antimalarial treatments. In recent years, the roles of artemisinins in T2DM have attracted much attention. Artemisinin treatments not only attenuate insulin resistance and restore islet ß-cell function in T2DM but also have potential therapeutic effects on diabetic complications, including diabetic kidney disease, cognitive impairment, diabetic retinopathy, and diabetic cardiovascular disease. Many in vitro and in vivo experiments have confirmed the therapeutic utility of artemisinin and its derivatives on T2DM, but no article has systematically demonstrated the specific role artemisinin plays in the treatment of T2DM. This review summarizes the potential therapeutic effects and mechanism of artemisinin and its derivatives in T2DM and associated complications, providing a reference for subsequent related research.
Introduction
Diabetes mellitus is a group of metabolic disorders characterized by prolonged elevated blood glucose levels (Punthakee et al., 2018). Currently, the number of patients with diabetes mellitus has reached more than 422 million worldwide, and this figure is predicted to increase to 693 million by 2045, which illustrates the severity of this public health problem (Zimmet et al., 2016; Cho et al., 2018). In general, the majority of diabetes mellitus cases can be divided into type 1 diabetes and type 2 diabetes (T2DM) according to different pathological characteristics, and T2DM cases account for more than 95% of the total diabetes population (Elkhidir et al., 2017). The main pathological basis of T2DM is insulin resistance (IR) and relatively insufficient insulin secretion. However, prolonged hyperglycemia may cause irreversible damage to the function of pancreatic islet ß-cells, resulting in an absolute decrease in insulin secretion (Tan and Cheah, 1990; Johnson and Luciani, 2010). Without good control, hyperglycemia will cause a series of severe complications, including renal failure, heart attack, vision loss, unhealed wounds, cognitive impairment, and increased risk of premature death (Schena and Gesualdo, 2005; Tang et al., 2013; Saedi et al., 2016; Stitt et al., 2016; Jia et al., 2018). Unfortunately, to date, the pathogenesis of T2DM has not been fully elucidated; therefore, symptomatic treatments, such as those that lower blood sugar, are still the main treatments. However, these hypoglycemic agents are neither effective for the overall improvement of patients’ condition nor completely prevent the progression of T2DM. Therefore, it is urgent to find a better drug to treat T2DM.
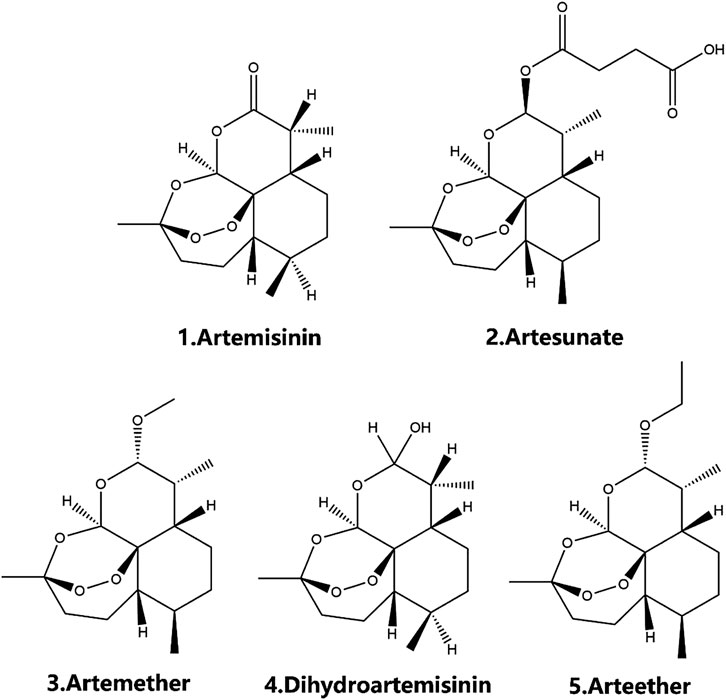
Traditional Chinese medicine (TCM) has been applied in the clinic for thousands of years and has been a substantial frontline treatment for treating various diseases. TCM has received increasing attention since it plays a huge role as a source of new drugs in modern drug discovery. In 2015, Youyou Tu was awarded the Nobel Prize in Physiology or Medicine for her discovery of the natural antimalarial drug artemisinin, which is extracted from Artemisia annua (A. annua) (Klayman, 1985; Youyou et al., 2015). Since then, semisynthetic derivatives of artemisinin have been gradually developed, including artesunate, artemether, dihydroartemisinin, artelinic acid, and arteether, all of which show great promise in the treatment of malaria (Klayman, 1985; Teja-Isavadharm et al., 2010; Morris et al., 2011; Morris et al., 2013) (Figure 1). As research has progressed, the effects of artemisinin and its derivatives have been greatly extended, and these compounds are widely used in antitumor (Verma et al., 2017; Wang et al., 2017; Wei and Liu, 2017), antifibrosis (Cao et al., 2016; Wang et al., 2019a), immunosuppressive (Li et al., 2013; Hou and Huang, 2016), antivirus (Sharma et al., 2014), antiatherosclerosis (Du et al., 2019; Jiang et al., 2020), antiobesity (Lu et al., 2016), and antidiabetes (Ho et al., 2014) treatments. Recently, increasing evidence has shown that artemisinins have significant therapeutic effects on metabolic diseases, especially diabetes, obesity, and hypercholesterolemia. Although much evidence suggests a positive role for artemisinins in the treatment of T2DM and its complications, no article has systematically demonstrated the specific roles artemisinins have played in the treatment of T2DM. As shown in Tables 1,2 and Figure 2, in this review, we summarize the roles of artemisinin and its derivatives in T2DM, including a variety of diabetes complications, expecting to provide new ideas for future therapy development.
Overview of Artemisinin and Its Derivatives
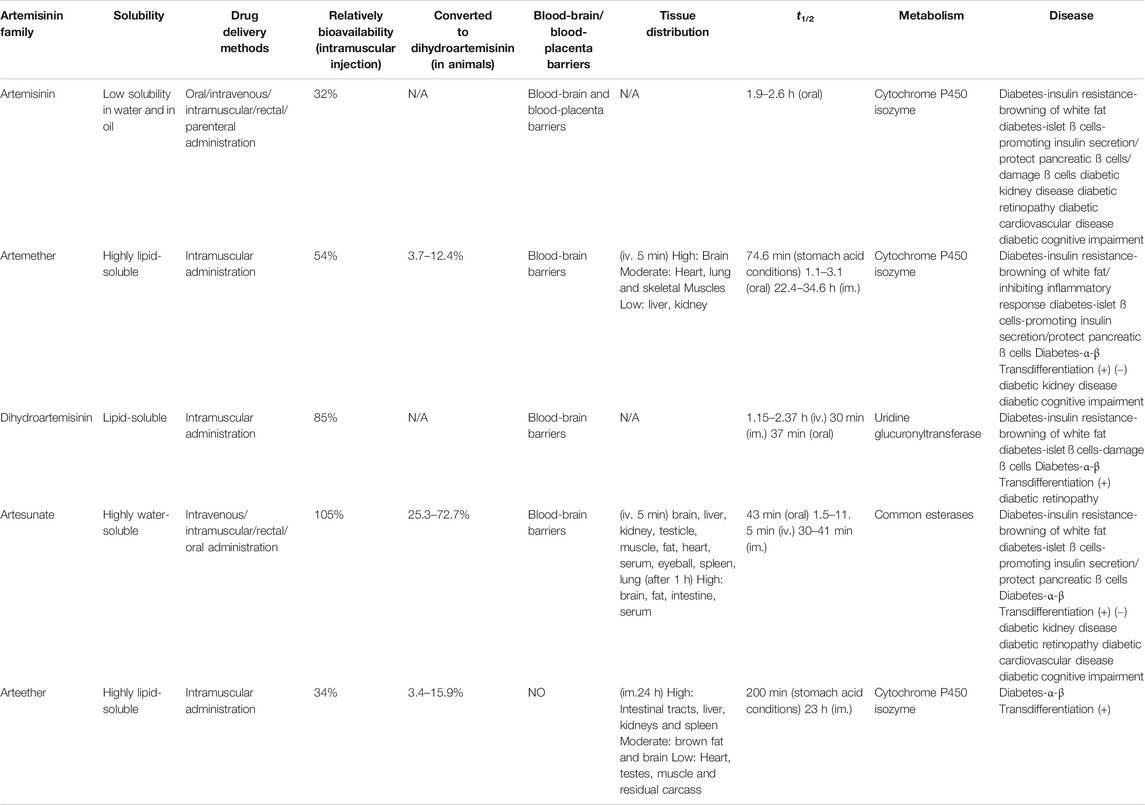
The properties of artemisinins determine their roles. Artemisinin is insoluble in oil and water and is the starting material of semisynthetic derivatives such as artemether, dihydroartemisinin, artesunate, and arteether (O’Neill and Posner, 2004; Gautam et al., 2009). These compounds are characterized by a short half-life, fast onset of effects, and low oral bioavailability (19%–35%) (Thomas et al., 1992; Navaratnam et al., 2000). The artemisinin compounds mentioned above are lipophilic, with the exception of artesunate, which is the only artemisinin derivative for which an intravenous formulation is available; dihydroartemisinin, artemether and arteether are currently administered intramuscularly in various oil formulations (Navaratnam et al., 2000; Krishna et al., 2001). Compared with other artemisinin derivatives, intramuscular artemether and arteether have a longer half-life, which may be attributed to the “depot” effect and/or the local blood supply and the slow and prolonged absorption of the sesame oil preparations at the site of injection (Ashton et al., 1998; Li et al., 1999; Visser et al., 2014). After either parenteral or gastrointestinal administration, artemisinin derivatives are mainly converted into dihydroartemisinin, a bioactive metabolite. The conversion rate of artemether is the lowest (3.7–12.4%), while that of water-soluble artesunate is the highest (25.3–72.7%) (Maggs et al., 1997; Li and Weina, 2010). The transformation of artemisinin and its derivatives into the primary metabolite dihydroartemisinin mostly depends on the action of the liver cytochrome P450 isozyme family, except for artesunate, which depends on the action of common esterases (de Waziers et al., 1990; van Agtmael et al., 1998; van Agtmael et al., 1999; White et al., 1999). Different artemisinin derivatives have different distribution characteristics (Niu et al., 1985). Artemisinin can cross the blood-brain and blood-placenta barriers after intravenous administration (Niu et al., 1985). Similarly, artemether has the ability to cross the blood-brain barrier, and the highest concentration of artemether is in the brain, followed by the heart, lung, skeletal muscles, liver, and kidney after intravenous administration (Jiang et al., 1989; Maggs et al., 1997; Maggs et al., 2000). For artesunate, the highest concentrations were found in rat intestine 10 min after intravenous administration, followed by the brain, liver, kidney, testicle, muscle, fat, heart, serum, eyeball, spleen, and lung in decreasing order (Zhao and Song, 1989). Arteether is the only derivative that cannot directly pass the blood-brain barrier. Twenty-four hours after intramuscular administration, the highest concentration is found in the intestinal tract, followed by the liver, kidneys, spleen, brown fat and brain, and concentrations in the heart, testes, muscle and residual carcass are very low (Navaratnam et al., 2000) (Table 3). In addition, sex (Ashton et al., 1999), diet (Dien et al., 1997; White et al., 1999) and disease state (Batty et al., 1998) have been recognized to contribute significantly to the metabolism of artemisinin and its semisynthetic derivatives. Details on the pharmacokinetics of artemisinin and its derivatives have been described in the relevant literature and will not be described here.
Type 2 Diabetes
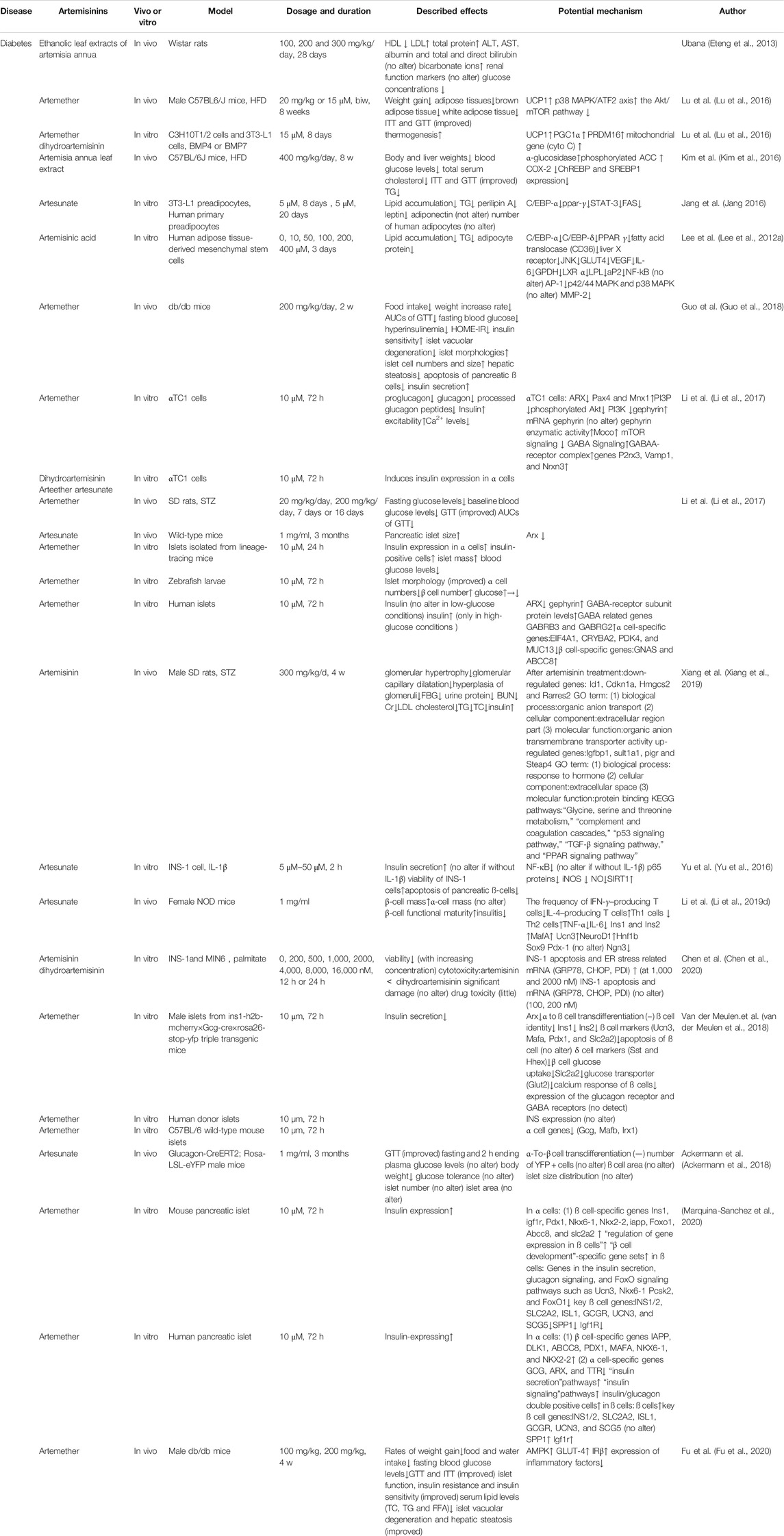
It is now generally accepted that IR and progressive damage to islet cell function are the fundamental pathological mechanisms of T2DM (Li et al., 2014). IR is often the first pathological manifestation of T2DM and accompanies the entire disease process (Kim et al., 2019; Wang et al., 2020). Before the diagnosis of T2DM, patients often have long-term IR, and the body compensates by secreting more insulin. As the disease progresses, islet ß-cell function is impaired, while glucose metabolism is severely decompensated, and eventually T2DM develops. Therefore, the crucial goals for treating T2DM are mainly to improve IR and islet cell function. In Fu’s research, islet function and IR were significantly improved in male db/db mice treated for 4 weeks with 100 mg/kg or 200 mg/kg artemether (Fu et al., 2020). Many studies have shown that artemisinin and its derivatives have great potential in the treatment of T2DM, whether in the early stage or late stage of the disease.
Insulin Resistance
IR is a precursor of T2DM caused by decreased glucose metabolism and utilization efficiency of liver, adipose, skeletal muscle, and other tissues for various reasons (Czech, 2017; Yazıcı and Sezer, 2017; Petersen and Shulman, 2018). Many conditions can induce IR, with obesity being one of the most common and main precursor conditions. Obesity can induce disorders of adipose metabolism, followed by free fatty acid accumulation and chronic inflammation, consequently leading to IR and accelerating the progression of T2DM. Naturally, weight loss can effectively improve adipose metabolism, increase insulin sensitivity, and improve the basic condition of T2DM patients. According to clinical observations, weight loss-induced improvements in glycemia are most likely to occur early in the natural history of T2DM, when obesity-associated IR has caused reversible ß-cell dysfunction, but insulin secretory capacity remains relatively preserved (Schauer et al., 2016; Steven et al., 2016).
The effects of artemisinin and its derivatives on IR have attracted increasing attention. In 2010, Goto et al. found that eating terpenoids daily might be useful for the management of obesity-induced metabolic disorders, such as T2DM, hyperlipidemia, and IR (Goto et al., 2010). In subsequent studies, artemisinin-induced improvements in glucose tolerance test (GTT) and insulin tolerance test (ITT) results and a decrease in the IR index (HOME-IR) have been observed simultaneously (Kim et al., 2016; Lu et al., 2016; Li et al., 2017; Guo et al., 2018). These results confirm the important role of artemisinins in improving IR. Notably, many studies have reported that artemisinin and its derivatives can increase insulin sensitivity and improve IR. Artemisinin and its derivatives can also reduce food intake and the rate of body weight increases caused by a high-fat diet (HFD) (Lu et al., 2016; Guo et al., 2018; Fu et al., 2020). It seems that the antiobesity effect of artemisinins may be one of the most important ways they alleviate IR.
Acting on Adipocyte Production and Differentiation
Reducing adipogenesis and altering the direction of adipocyte differentiation can effectively alleviate obesity and the IR caused by obesity. Currently, strong anti-adipogenesis effects of artemisinins have been reported. In both 3T3-L1 adipocytes and human primary adipocytes, artemisinin inhibited the generation of intracellular lipids, reduced triglyceride (TG) levels, and lowered glyceraldehyde-3-phosphate dehydrogenase activity in a dose-dependent manner, indicating that artemisinin affects adipocyte differentiation but does not change the number of human adipocytes (Lee et al., 2012a; Jang, 2016). Glucose transporter-4 (GLUT4) is a marker gene of late-stage preadipocyte differentiation, and vascular endothelial growth factor (VEGF) is abundantly expressed in mature adipocytes but not in preadipocytes, and these markers are widely used to evaluate the development of adipocytes (Miyazawa-Hoshimoto et al., 2005). Investigators confirmed that the antiadipogenic effects of artemisinic acid alter human adipose tissue-derived mesenchymal stem cell differentiation by reducing GLUT4 and VEGF levels. In addition, the overinduction of hepatic sterol regulatory element-binding protein 1 (SREBP1) (Tu et al., 2012; Han et al., 2015) and carbohydrate-responsive element-binding protein (ChREBP) in both ob/ob mice and HFD mice indicated the overproduction of glucose (Benhamed et al., 2012; Abdul-Wahed et al., 2017). Interestingly, the administration of A. annua extract decreased the nuclear levels of SREBP1 and ChREBP and increased the phosphorylation of acetyl-CoA carboxylase (ACC), and it ameliorated hepatic steatosis and IR. These findings indicate the reversal of hepatic de novo lipogenesis and lipid accumulation (Kim et al., 2016).
CCAAT/enhancer-binding proteins (C/EBPs) and peroxisome proliferator-activated receptors (PPARs) are widely valued as dietary lipid sensors that control energy homeostasis (Dubois et al., 2017; Gross et al., 2017; Blüher, 2019). Knocking out or reducing the expression/activity of C/EBP-α or PPAR-γ by the pharmacological inhibitor of each or by siRNA transfection inhibits lipid accumulation during adipocyte differentiation (Linhart et al., 2001; Lehrke and Lazar, 2005; Rosen and MacDougald, 2006). Jang et al. demonstrated that artesunate at 5 μM precisely reduced the expression of C/EBP-α and PPAR-γ during adipocyte differentiation (Jang, 2016). Notably, another study attempted to elucidate the possible mechanism underlying the artemisinic acid-mediated effects by incubating artemisinic acid or artesunate with human adipose tissue-derived mesenchymal stem cells for 15 days. The results show that the development and differentiation of adipocytes were inhibited by the suppression of the master regulators C/EBP δ and PPARγ during adipogenesis (Lee et al., 2012a). Furthermore, decreased expression of the C/EBP δ gene was related to the inhibition of Jun N-terminal kinase (JNK) and activator protein 1 (AP-1) upon artemisinic administration when PPARγ was suppressed and the lower expression of genes that participate in controlling adipocyte fatty acid metabolism, including lipoprotein lipase (LPL), fatty acid translocase (CD36) and liver X receptor α (LXR α) (Liu et al., 2007; Lee et al., 2012a). Artemisinic acid also inhibits the expression and activity of gelatinase matrix metalloproteinase (MMP)-2, which is important to the development of adipose tissue (Croissandeau et al., 2002; Lee et al., 2012a). These results show that artemisinins inhibit adipocyte formation and differentiation by suppressing the master regulators C/EBPs and PPAR γ and related molecules to improve IR.
IR is also attributed to differentiated adipocytes, which synthesize and release an array of adipokines, including fatty acid synthase (FAS), leptin, perilipin A, and the phosphorylation levels of signal transducer and activator of transcription-3 (STAT-3) (Morrison and Farmer, 2000; Fève, 2005; Jang, 2016). Increasing evidence suggests that adiponectin and leptin are involved in the endocrine control of energy homeostasis, and the leptin/adiponectin ratio is often used as a surrogate marker for insulin sensitivity (Drevon, 2005; Trujillo and Scherer, 2005; Finucane et al., 2009; Yadav et al., 2013). RT-PCR analysis further revealed that artesunate reduced the insulin- and FBS-induced mRNA expression of FAS and leptin and enhanced the mRNA levels of adiponectin (Jang, 2016). These findings may suggest that artemisinins lower adipokine levels and attenuate IR by affecting the leptin/adiponectin ratio.
Activation of Brown Fat and Browning of White Fat
Adipose tissue is metabolically active and can be classified as white, brown and beige adipose tissue based on the morphology, physiology, and function (Boss and Farmer, 2012; Xu et al., 2018). The imbalance of brown fat and white fat leads to abnormal adipose accumulation, which in turn produces a series of metabolic diseases. The activation of brown fat and browning of white fat are the two main sources of adaptive heat generation and important output of energy expenditure (Cypess and Kahn, 2010; Boss and Farmer, 2012). Interestingly, some studies reported that artemisinin and its derivatives can activate brown adipose tissue and brown white adipose tissue, effectively inhibiting abnormal adipose accumulation and ameliorating IR. Lu et al. have identified artemether as an activator of browning and thermogenesis in vitro, which significantly enhances the metabolism of mice, as indicated by the insulin tolerance test (ITT) and glucose tolerance test (GTT) results (Lu et al., 2016). To further evaluate the pharmacological potential, Lu et al. found that artemether and other artemisinin derivatives induce C3H10T1/2 cell browning by activating the p38 mitogen-activated protein kinase (MAPK)/activating transcription factor-2 (ATF2) axis and deactivating the Akt/mTOR pathway, which has suggested to be involved in various anabolic and catabolic processes (Cai et al., 2016; Lu et al., 2016; Fischer et al., 2020; Toda et al., 2020). Moreover, one-step qPCR suggested that the relative mRNA levels of browning-related genes, such as PR domain containing 16 (PRDM16), uncoupling protein 1 (UCP1) and peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), were elevated after treatment with artemether, indicating that the increased thermogenesis in brown fat may also cause weight loss and enhance the metabolism in artemether-treated mice (Lu et al., 2016).
Inhibiting the Inflammatory Response
IR is closely associated with chronic inflammation (Brenachot et al., 2017; Caprio et al., 2017; Reilly and Saltiel, 2017; Saltiel and Olefsky, 2017; Wu and Ballantyne, 2020). Abnormal accumulation of adipose and the increased release of free fatty acids, which can be internalized by hepatocytes, consequently leading to chronic inflammation (Kamari et al., 2011; Saltiel and Olefsky, 2017; Shimobayashi et al., 2018). Moreover, preadipocytes and macrophages release proinflammatory cytokines, including tumor necrosis factor α (TNF-α) (Hotamisligil et al., 1993), interleukin 6 (IL-6) (Rotter et al., 2003; Sopasakis et al., 2004), interleukin 1β (IL-1β) (Tateya et al., 2013), and monocyte chemoattractant protein 1 (MCP-1), and induce IR in target cells (Wellen and Hotamisligil, 2003; Bastard et al., 2006; Olefsky and Glass, 2010). Due to the interference of artemisinic acid with adipogenesis, artemisinic acid significantly attenuated the increased secretion of TNF-α and IL-6 in undifferentiated human adipose tissue-derived mesenchymal stem cells, influencing the inflammatory state (Lee et al., 2012a). In addition, cyclooxygenase-2 (COX-2) is an inducible enzyme that is expressed at low levels in normal tissues but is highly expressed when cells are stimulated by inflammation (Ferrer et al., 2019). Kim’s team found that A. annua leaf extract significantly improved ITT and GTT results and decreased COX-2 levels in HFD-fed mice (Kim et al., 2016). Recently, investigators also found that artemether can promote AMP-activated protein kinase (AMPK) activity and downregulate the expression of inflammatory factors to reverse the pathological state in db/db mice (Fu et al., 2020). NF-κB plays a key role. Many studies have shown that inhibiting the abnormal activation of the NF-κB signaling pathway by various stimuli, such as endogenous advanced glycation end products (AGEs), can reduce the inflammatory response, which has been proven to contribute to anti-IR effects (Shoelson et al., 2006). In 2001, researchers understood that the anti-inflammatory drug salicylic salicylate greatly ameliorates IR and the symptoms of diabetes via the IKKβ/NF-κB axis (Yuan et al., 2001). Surprisingly, many studies have shown that artemisinin and its derivatives exhibit a strong anti-inflammatory effect by targeting the NF-κB signaling pathway, which is a molecular mechanism critical for the state of chronic inflammation in IR and T2DM; however, direct evidence showing that artemisinins reverse IR through NF-κB is lacking (Baumgart and Sandborn, 2007; Xu et al., 2007; Duckworth et al., 2009; Chen et al., 2010; Lee et al., 2012b; Yazıcı and Sezer, 2017). In summary, through a literature review, we found that artemisinins have anti-inflammatory effects and relieve IR. Based on the common pathological state of chronic inflammation in diabetes and IR, it is speculated that the anti-inflammatory effects of artemisinins have a causal relationship with attenuated IR (Fu et al., 2020).
Restoring Islet ß-Cell Function
Obesity-related IR causes reversible ß-cell dysfunction, which in turn affects the secretion of insulin (Steven et al., 2016). However, long-term IR or hyperglycemia can cause irreversible damage to islet cells and lead to a complete failure of insulin secretion, which is an important feature of T2DM progression. Most agree that protecting ß cells is beneficial to insulin secretion and glucose control and slows the T2DM process (Marrano et al., 2020). Increasing evidence indicates that artemisinin and its derivatives have the potential to alleviate T2DM by restoring islet ß-cell function.
On the one hand, the effect of artemisinins on promoting insulin secretion is gradually recognized (Kim et al., 2016; Li et al., 2017; Guo et al., 2018; Xiang et al., 2019). In 2012, after treatment with ethanolic leaf extracts (100 and 200 mg/kg) of A. annua, Wistar rats showed a significantly reduced glucose concentration, and there were no adverse effects on liver function, hematological indices, or testosterone levels (Eteng et al., 2013). Kim and the team proved that A. annua inhibited α-glucosidase activity in a dose-dependent manner even more effectively than acarbose, a known antidiabetic drug, in HFD mouse models (Kim et al., 2016). It has also been reported that artemisinin and its derivatives attenuate diabetic hyperglycemia by increasing insulin secretion, which has been observed not only in rats and mice but also in human islets (Li et al., 2017). Moreover, the unbalanced ratio of insulin, glucagon, and somatostatin content was also reversed by the administration of artemether in islets (Guo et al., 2018). The increase in insulin concentration was accompanied by a decrease in proglucagon, glucagon, and processed glucagon peptides in αTC1 cells treated with artemether and its analogs, namely, dihydroartemisinin, arteether, and artesunate, for 72 h, except for the deoxyarteether-treated group, in which the insulin expression did not change (Li et al., 2017). These findings demonstrated that, although their physical and chemical properties are very similar, artemisinin and its derivatives show differences in pharmacological effects and/or effectiveness. The applications of different kinds of artemisinins require further study. After in rats injected with streptozotocin (STZ) received 4 weeks of 300 mg/kg/d artemisinin treatment, upregulated insulin levels and the insulin-like growth factor binding protein one gene (Igfbp1) were observed (Xiang et al., 2019). Furthermore, artemisinin has long-term effects on diabetes treatment, which is reflected by the improved hemoglobin A1c (HbA1c) levels (Han et al., 2019). As for the specific mechanism of artemether, through pull-down assays, artemether was found to interact with the protein gephyrin, and it strengthens gephyrin expression, increasing the expression of P2rx3, Vamp1, and Nrxn3 genes, resulting in the activation of the GABAA receptor complex and GABA signaling, subsequently increasing insulin secretion and inhibiting glucagon secretion (Soltani et al., 2011; Purwana et al., 2014; Li et al., 2017). When gephyrin was knocked down, single-cell analysis of the image data revealed a high correlation between increases in gephyrin and insulin, which was basically abolished, in artemether-treated cells (Li et al., 2017). Yu et al. reported that artesunate reversed the suppressed state of insulin secretion caused by IL-1β in rat islets after stimulation with 16.7 mmol/L glucose, while artesunate alone did not affect insulin secretion in normal rat islets (Yu et al., 2016). This finding indicates that artesunate may play the role of “balancer” and thus helping to achieve homeostasis. Moreover, in ß-cells, artesunate upregulated the expression of SIRT1, which plays a key role in glucose/lipid metabolism, and deacetylated lysine residues on various transcription factors, such as FOXO and PGC-1α, ultimately stimulating insulin secretion (Monteiro and Cano, 2011; Lai et al., 2012; Yu et al., 2016; Han et al., 2019).
On the other hand, studies have shown that artemisinin and its derivatives play roles in protecting islet ß cells. Db/db mice were treated with 200 mg/kg artemether for 2 weeks, and Guo’s team observed that artemether significantly reversed pancreatic ß-cell damage, which was reflected in improved islet morphologies, ameliorated islet vacuolar degeneration, a reduced apoptosis rate of pancreatic ß cells and increased islet cell numbers and size (Guo et al., 2018). Hence, artemether has generated intense interest for use in strategies designed to regenerate functional ß cells toward a cure for diabetes (Thorel et al., 2010; Chera et al., 2014). Upon further research, it was reported that artemisinins triggers ß-cell-like induction of neogenesis following streptozotocin (STZ)-induced ß-cell death by indirectly activating the GABA signaling pathway in cell, zebrafish larva, and wild-type mouse models (Ben-Othman et al., 2017; Li et al., 2017). Moreover, artesunate can stimulate SIRT1 expression, which not only improves insulin levels but also protects pancreatic ß cells (Kitada and Koya, 2013). An abundance of evidence confirms that artesunate can block the NF-κB pathway, inhibit inducible nitric oxide synthase (iNOS) expression and decrease nitric oxide (NO) production, conferring a protective effect on ß cells exposed to IL-1β (Bordone et al., 2006; Lee et al., 2009; Yang et al., 2012; Yu et al., 2016). The inhibition of the NF-κB signaling pathway may be an important target for artemisinin to protect islet cells. Artesunate can inhibit NF-κB nuclear translocation and reduce its transcriptional activity by promoting the deacetylation of p65, reducing the activity of IKK, and preventing IkB phosphorylation (Huxford et al., 1998; Dejardin, 2006; Xu et al., 2007; Yu et al., 2016). In addition, the protective effects of artemisinin on islet ß cells is also reflected by the reversed suppression of cell proliferation at the same time that inhibitor of DNA binding 1 (ID1) and cyclin-dependent kinase inhibitor 1A (CDKN1A) levels are increased by STZ (Xiang et al., 2019).
However, some subsequent papers failed to replicate these findings. van der Meulen et al. found that artemether-treated islets showed an obvious pattern of speckles or fragmentation in the red channel after 72 h, which indicated that ß-cell health had declined in male islets from Ins1-H2B-mCherry × Gcg-Cre × Rosa26-stop-YFP triple transgenic mice (van der Meulen et al., 2018). In contrast to the roles they play upon inflammatory factor-induced ß-cell damage, artemisinins may participate in the ß-cell damage specifically induced by palmitate. In contrast to Yu’s conclusion, Chen et al. found that artemisinin and dihydroartemisinin cause the deterioration following pancreatic ß-cell damage in palmitate-induced INS-1 and MIN6 cells by triggering ER stress. The expression levels of ER stress-related mRNA (GRP78, CHOP, PDI) in the artemisinin and dihydroartemisinin groups were both increased in a dose-dependent manner (Chen et al., 2020). The researchers of this study concluded that relatively high concentrations of artemisinin and dihydroartemisinin may cause damage to pancreatic cells in obese patients but not in healthy individuals.
A Controversial Mechanism: α-Cell to ß-Cell Transdifferentiation
Although T1DM and T2DM are fundamentally different diseases, both are associated with a deficiency in functional ß cells. It has been suggested that the defect in the quality of ß cells in diabetes is not due to the death of ß cells but to the dedifferentiation or transdifferentiation of ß cells (Talchai et al., 2012; Spijker et al., 2013). In addition, phenotypic changes of ß cells promote transdifferentiation to other pancreatic endocrine cells (mainly α cells and δ cells) (Spijker et al., 2013; Cinti et al., 2016). The possibility of curing T2DM by reversing the phenotypic changes that cause ß-cell identity loss or by promoting other cells to transdifferentiate into ß cells is gradually being recognized and considered. Specifically, α cells are attractive starting points for transdifferentiation protocols, as they are developmentally closely related to ß cells (Thorel et al., 2010; Unger and Orci, 2010; Ye et al., 2015). Over the past decade, multiple studies have shown that pancreatic α cells can transdifferentiate into ß cells or ß-like cells after deletion of the α cell-specific transcription factor Arx (Courtney et al., 2013; Wilcox et al., 2013; Chakravarthy et al., 2017), overexpression of transcription factors necessary for ß-cell differentiation, such as Pax4 (Collombat et al., 2009) or Pdx1 and Mafa (Matsuoka et al., 2017), or after extreme ß-cell loss (Thorel et al., 2010). Li et al. found that artemisinins were able to convert glucagon-producing α cells into insulin-producing ß cells (Li et al., 2017). The expression of Arx was significantly downregulated, while Pax4 and Mnx1 were elevated in the artemisinins (artemether, dihydroartemisinin, arteether, and artesunate) groups. They found that antimalarial drugs from the artemisinin family (particularly artemether) induced the conversion of α ells into ß-like cells by enhancing GABA signaling not only in mouse cell lines but also in mice (using lineage tracking), rats, and zebrafish in vivo. Vieira et al. suggested that artemether acts through its interaction with gephyrin, which potentiates GABA signaling and induces Arx translocation from the nucleus to the cytoplasm, thereby leading to its inactivation and the consequent conversion of α cells into ß-like cells (Vieira et al., 2017). Reducing the abundance of both proglucagon and processed glucagon peptides inhibited glucagon secretion under low-glucose conditions, thereby triggering the loss of α-cell identity. After artemether treatment with key ß cell-specific genes, such as GNAS and ABCC8, were profoundly upregulated, whereas α cell-specific genes, including EIF4A1, CRYBA2, PDK4, and MUC13, were significantly downregulated in human islets (Li et al., 2017).
However, some researchers have questioned this phenomenon. Although alterations in the identity of α cells and/or ß cells have been observed, and there no direct evidence of α cell to ß cell transdifferentiation has been observed in subsequent experiments. Li’s study explored whether the identity and functional maturation of ß cells are influenced by 1 mg/ml artesunate in female NOD mice. ß-cell mass was significantly increased, whereas α-cell mass was not altered after artesunate treatment when Ins1, Ins2, MafA, Ucn3, and NeuroD1, which are essential for maintaining the identity and functional maturation of ß cells, were dramatically increased (Nishimura et al., 2015; Li et al., 2019d). Interestingly, the expression of the endocrine progenitor marker Ngn3 was decreased in artesunate-treated islets (Li et al., 2019d). Ackermann et al. used Glucagon-CreERT2; Rosa-LSL-eYFP male mice as models, in which >90% of α-cells were labeled, which enabled the accurate quantification of mature α cell to ß cell transdifferentiation. The investigators treated the mice for 3 months with 1 mg/ml artesunate. At the end of this period, the results revealed no changes in the fractions of insulin+/YFP + cells, ß-cell area, islet number, or proportion of pancreatic area composed of islets between the treatment and control groups. Hence, there were no indications of a naturally occurring, slow transdifferentiation process in vivo (Ackermann et al., 2018). van der Meulen et al. reported that a 3 days artemether treatment of islets from Ins1-H2B-mCherry × Gcg-Cre × Rosa26-stop-YFP triple transgenic reporter male mice caused the sustained loss of identity across all islet endocrine cell types. The expression of other α-cell genes, including Gcg, Mafb, and Irx1, was also downregulated, suggesting a general loss of α-cell identity, and some mature ß-cell markers, including Ucn3, Mafa, Pdx1, and Slc2a2, and two δ-cell markers, somatostatin (Sst) and Hhex, were significantly inhibited. Indeed, the expression of Ins1 and Ins2 was downregulated by >10- and >100-fold, respectively. These results confirmed that artemether does not selectively inhibit Arx but causes broad inhibition of α cell, ß cell, and δ cell-specific transcription factors (van der Meulen et al., 2018). Brenda Marquina-Sanchez et al. pointed out that any sample carryover or cross-contamination that occurs in droplet-based single-cell RNA-seq greatly affects the results of the experiment, and therefore, the results need to be effectively corrected. They developed a method that combined standardized reference cells as spike-in controls with a computational decontamination algorithm to eliminate differences and obtain more accurate conclusions. With this method, in addition to increased insulin expression, the downregulation of α cell-specific genes and upregulation of key ß-cell genes were observed in the α cells of mouse and human islets. In subsequent experiments, they also found that the effects of artemether on ß cells in pancreatic islets were species-specific, causing more species-dependent gene expression changes (Marquina-Sanchez et al., 2020). In contrast to the decrease in ß cells in the mouse model, human islet ß cells showed a slight increase. Similarly, the expression of key ß-cell genes, such as INS1/2, SLC2A2, ISL1, GCGR, UCN3, and SCG5, decreased in the mouse models but increased in the human islets. In addition, SPP1 and Igf1r have also undergone inconsistent changes. These data suggest that drug effects are species-dependent.
In summary, it can be seen from these results that most of the experiments did not directly lead to the conclusion that artemisinin can achieve α-cell to β-cell transdifferentiation, but many experiments revealed the emergence of functional β cells or/and the restoration of β-cell identity. Unfortunately, some experimental results showed that artemisinin does not lead to transdifferentiation and may further damage β cells. Combined with the results of studies on the characteristics of artemisinin and its derivatives and comparing the relevant experimental process and results, we speculate that sex, food intake, and objective pollutants may be the reasons for the differences in the experimental results. Although there is no direct evidence that sex plays a role in artemisinin-promoting transdifferentiation, females have higher bioavailability than males after administration of artemisinins. Since most experiments use single-sex animal models or cells, it would be interesting and is necessary to study and compare the effects of sex (Navaratnam et al., 2000). On the one hand, food intake can affect the absorption and metabolism of artemisinin (Ashton et al., 1999); on the other hand, it can affect the intestinal flora, and artemisinins may affect the intestinal flora and thereby modulate the state of the overall internal environment. Brenda et al suggested that species differences and the presence of pollutants in general methods are also noteworthy (Marquina-Sanchez et al., 2020), which shows that we may be able to obtain more precise results and obtain more accurate conclusions by improving experimental technology.
Type 2 Diabetes Mellitus-Related Complications
Diabetic Kidney Disease
Diabetic kidney disease (DKD) is a serious complication of diabetes (Sharaf El Din et al., 2017; Tanabe et al., 2017; Tesch, 2017; Uwaezuoke, 2017; Wu et al., 2018b). The early clinical manifestations of DKD are decreased glomerular filtration, followed by increased arterial blood pressure, proteinuria, and fluid retention, which ultimately lead to renal failure (Egido et al., 2017; Tanabe et al., 2017; Wu et al., 2018b). Glomerular hypertrophy, thickening of the glomerular and tubular basement membrane, and accumulation of extracellular matrix in the mesangial area can be observed in the early stages of DKD. The late pathological features are glomerular and tubular interstitial fibrosis (Dai et al., 2017; Kitada et al., 2017). Recently, it was reported that artemisinin and its derivatives may be a promising therapy for DKD.
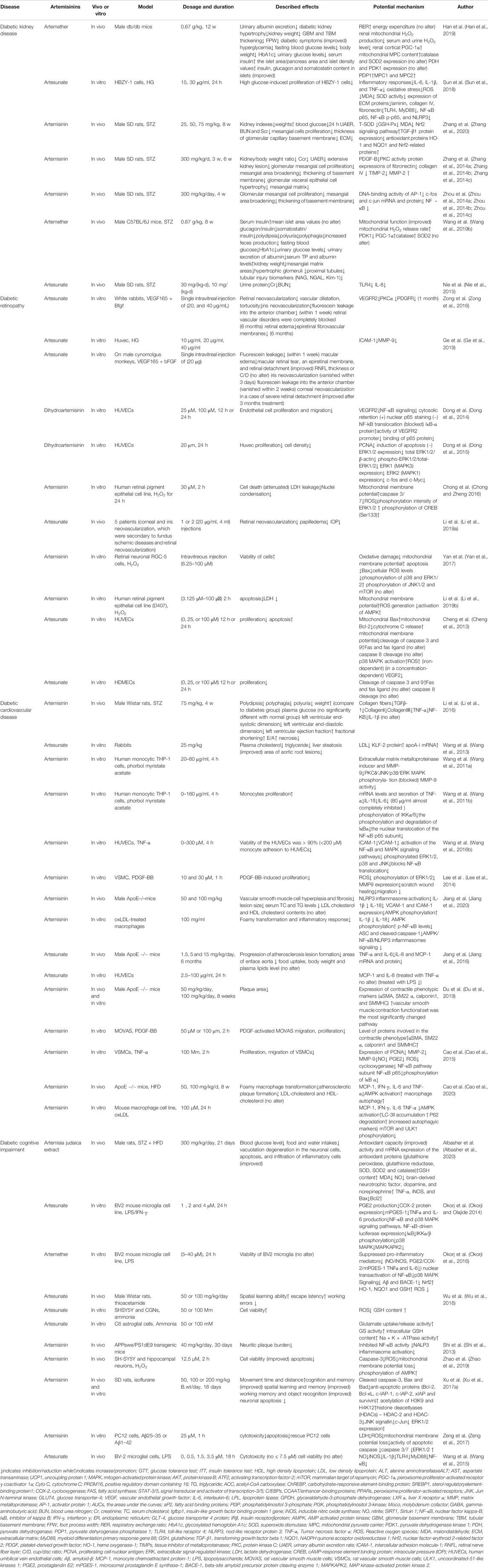
The symptoms, signs, and pathological changes of diabetic nephropathy were attenuated in artemisinin-treated rats. Urinary albumin excretion was significantly decreased, and serum total protein (TP) and albumin (ALB) levels were restored in the artemisinin group compared to the levels in the diabetic without treatment group (Han et al., 2019; Wang et al., 2019b; Xiang et al., 2019). In addition, tubular injury biomarkers also show a downward trend, and the decreased N-acetyl-β-D-glucosaminidase (NAG), neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (Kim-1) levels explained the effect of artemether in proteinuria reduction (Wang et al., 2019b). Notably, blood urea nitrogen (BUN), creatinine (Cr), creatinine clearance rate (Ccr) and urinary albumin excretion rate (UAER) were downregulated in the artemisinin and its derivatives treatment group, and glomerular hypertrophy and hyperplasia, glomerular basement membrane (GBM) and tubular basement membrane (TBM) thickening, glomerular capillary dilatation, foot process width (FPW) broadening and extracellular matrix accumulation, which are characteristics of mice with DKD, were all attenuated (Zhang et al., 2014a; Zhang et al., 2014b; Zhang et al., 2014c; Zhou et al., 2014a; Zhou et al., 2014b; Zhou et al., 2014c; Nie et al., 2015; Xiang et al., 2019; Zhang et al., 2020). In addition, diabetic symptoms such as polydipsia, polyuria, urinary glucose, increased food intake and weight loss, were all reversed by artemisinins (Han et al., 2019; Wang et al., 2019b; Zhang et al., 2020).
Recent studies have demonstrated that metabolic alterations and mitochondrial dysfunction play critical roles in DKD initiation and progression (Forbes and Thorburn, 2018). The diabetic kidney is characterized by incomplete glucose oxidation and enhanced fatty acid utilization. Artemether treatment increased the respiratory exchange ratio (RER) but did not affect total energy expenditure, indicating that artemether shifted the energy metabolic substrate from lipids and proteins to glucose (Choi et al., 2015). To further address the role of artemether on mitochondrial function, treating male db/db mice with 0.67 g/kg artemether for 12 weeks, Han et al. reported first that artemether inhibited the production of renal mitochondrial hydrogen peroxide (H2O2), reduced serum and urine H2O2 levels, and regulated the expression of renal cortex- and mitochondrial-related proteins. Specifically, PGC-1α, which can stimulate MPC1 transcription, MPC1, and MPC2, which are both carriers that facilitate pyruvate transport into mitochondria, are all enhanced with artemether therapy (Choi et al., 2014; Rabinovitch et al., 2017; Han et al., 2019). Similar results were replicated in another experiment. Apart from restoring PGC-1α, MPC1, and MPC2 levels, the expression of pyruvate dehydrogenase kinase 1 (PDK1) also decreased in the artemether group compared to the levels in the diabetic STZ mice, indicating enhanced pyruvate oxidation in mitochondria (Wang et al., 2019b). Oxidative stress is a crucial pathogenesis of DKD. Artemisinin attenuates renal damage in DKD rats by suppressing transforming growth factor-β1 (TGF-β1) regulation, increasing antioxidant proteins heme oxygenase-1 (HO-1) and NADPH quinone acceptor oxidoreductase 1 (NQO1) and activating the nuclear factor-erythroid 2-related factor 2 (Nrf2) signaling pathway (Jha et al., 2016; Akash et al., 2018; Zhang et al., 2020).
Amelioration of DKD by inhibiting the inflammatory response and preventing the inflammatory pathway is gradually being recognized and appreciated. A high-glucose environment and oxidative stress state induce the transfer of protein kinase C (PKC) from the cytoplasm to the cell membrane in nephrocytes; that is, the translocation of PKC is induced, resulting in the phosphorylation of the transcription factors AP-1 and NF-κB, which can upregulate the expression of downstream genes, thereby increasing the production of a large number of cytokines and growth factors, causing renal cell hypertrophy and proliferation, glomerular basement membrane thickening and cell-matrix accumulation. Zhang et al. proved that intraperitoneal injection of artemisinin can inhibit the activation of PKC, downregulate AP-1 synthesis-related genes c-jun and c-fos expression, and further decrease the expression of AP-1 and NF-κB, ultimately alleviating renal pathological changes induced by high glucose and reactive oxide (Zhang et al., 2014b; Zhou et al., 2014a; Zhou et al., 2014b; Zhou et al., 2014c). Nie et al. speculated that the efficacy of 30 mg/(kgd) artesunate might be similar to that of 10 mg/(kgd) enalapril in protecting renal function by lowering the expression of TLR4 and IL-8 (Nie et al., 2015). Sun et al. reported that artesunate conferred protective effects on HG-induced HBZY-1 cells through the toll-like receptor 4 (TLR4)/NF-κB/nod-like receptor protein 3 (NLRP3) inflammasome pathway (Sun et al., 2018). The results suggested that artemisinins might be potential therapeutic agents for DKD treatment.
During the onset and progression of diabetic nephropathy, the renin-angiotensin system (RAS) in kidney tissue plays an important role. In the hyperglycemic state, RAS in renal tissue can be activated, causing an increase in angiotensin II (AngII) levels in renal tissue, leading to the accumulation of platelet-derived growth factor (PDGF) and an imbalance of matrix metallopeptidases (MMPs)/tissue inhibitor of metalloproteinases (TIMPs). In the artemisinin-treated group, decreased MMP-2 protein levels and increased expression of PDGF-B and TIMP-2 in the glomeruli were obviously reversed. In addition, artemisinin significantly downregulated the expression of fibronectin (FN) and collagen IV significantly reducing the accumulation of the extracellular matrix in glomeruli and enhancing renal function (Zhang et al., 2014a; Zhang et al., 2014c).
Next-generation sequencing for ditag genome scanning (DGS) was applied to study the effect of artemisinins on DKD (Kelly et al., 2013; Rudnicki et al., 2015). Using a microarray, Brennan et al. found that Tgfbi and Ark5 were induced by TGF-β1 and were also upregulated in human DKD (Brennan et al., 2012). Using RNA sequencing, Xiang et al. examined the profile of differentially expressed genes following the administration of artemisinin. They found that 69 gene expression levels were different between the normal samples, STZ samples, and artemisinin treatment samples. Specifically, 38 genes, including insulin-like growth factor binding protein 1 (Igfbp1), sulfotransferase 1A1 (Sult1a1) and six-transmembrane epithelial antigen of prostate 4 (Steap4), were increased after artemisinin treatment, and 31 genes, including 3-hydroxy-3-methylglutaryl-CoA synthase 2 (Hmgcs2), ID1, and CDKN1A showed a downtrend in the artemisinin group compared to the STZ group. These identified genes were also related to a list of Gene Ontology (GO) terms and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. For example, the pathways involve “complement and coagulation cascades,” which have been reported to play important roles in the progression of DKD (Wang et al., 2016c), and the “p53 signaling pathway” and “TGF-β signaling pathway,” which are closely associated with DKD (Zhang et al., 2011; Wang et al., 2016a; Mukhi et al., 2017; Xu et al., 2017a; Shelbaya et al., 2018; Sheng et al., 2018; Xiang et al., 2019). These results indicate promising targets in the treatment of DKD with artemisinins.
Cognitive Impairment Related to Type 2 Diabetes Mellitus
There is increasing evidence that diabetes predisposes individuals to cognitive decline, even leading to dementia (Biessels et al., 2006; Grünblatt et al., 2011; Wong et al., 2014). It has been postulated that Alzheimer’s disease (AD) may represent the consequence of a distinct form of brain-specific IR and impaired glucose regulation (Xu et al., 2010; Akter et al., 2011; de la Monte, 2012; Shuba and Karan, 2012). However, the effectiveness of the current treatment for T2DM-related cognitive dysfunction is far from sufficient. Moreover, the insulin treatment itself can exacerbate cognitive impairment (Ott et al., 1999). In the progression of T2DM, the inflammatory response and oxidative stress caused by high glucose are the bridges between diabetes and cognitive impairment (Dinel et al., 2011). Recently, the great potential of artemisinin and its derivatives in reversing diabetic cognitive impairment has gradually received attention.
HFD/STZ administration induces a state of neuroinflammation, as indicated by the infiltration of inflammatory cells and elevated protein and mRNA expression levels of TNF-α, IL-6, and iNOS, which can be reversed by artemisia extract (Gaspar et al., 2016; Albasher et al., 2020). Through a literature review, we found more possible but unproven mechanisms. The activation of the NF-κB signaling pathway is closely related to the release of proinflammatory factors, while inhibiting inhibitor of kappa B (IκB) α can enhance the learning ability and memory of diabetic rats (Datusalia and Sharma, 2016). In Shi’s study, artemisinin decreased the neuritic plaque burden and improved AD symptoms by inhibiting NF-κB activity and NALP3 inflammasome activation in APPswe/PS1dE9 double transgenic mice but not diabetic rats (Shi et al., 2013). In vitro experiments were performed with lipopolysaccharide (LPS), which is an essential component of the Gram-negative bacterial cell wall and is most commonly used to activate astrocytes (Chen et al., 2015; Zhang et al., 2016). Artemisinins (artemisinin, artesunate, and artemether) showed an anti-inflammatory action in an LPS-induced BV-2 mouse microglial cell line by interfering with IκB/NF-κB signaling, releasing fewer pro-inflammatory mediators, such as NO, iNOS, prostaglandin E2 (PGE2), COX-2, microsomal prostaglandin E synthase-1 (mPGES-1), TNFα, IL-6, IL-1β, and the NLRP3 inflammasome complex, making them good candidates for decreasing the neuritic plaque burden and alleviating neurological inflammation disorders (Zhu et al., 2012; Shi et al., 2013; Badshah et al., 2015; Wang et al., 2015; Badshah et al., 2016; Okorji et al., 2016; Zuo et al., 2016; Irrera et al., 2017; Gugliandolo et al., 2018). In addition, suppressing the NF-κB pathway and inhibiting p38 phosphorylation and downstream kinase MAP kinase-activated protein kinase 2 (MAPKAPK2) are crucial ways for the anti-neuroinflammatory activity of artesunate (Okorji and Olajide, 2014). Another study discovered that dihydroartemisinin alleviates learning and memory and depression-like behavior and prevents neuronal degeneration in the hippocampal CA1, CA2, CA3, and DG regions by inhibiting the production of LPS-induced inflammatory mediators via the PI3K/AKT signaling pathway (Gao et al., 2020). These experiments point us in the direction of progress, but need to be validated in diabetic mice.
The interaction between inflammation and oxidative stress has been confirmed in T2DM (Teodoro et al., 2018). Hyperglycemia promotes oxidative stress, which is thought to play an important role in the progression of diabetes-related complications (Abdelfattah et al., 2020). According to the sensitivity of the brain to peroxide reagents, antioxidants have a place in diabetes-related cognitive impairment (Asmat et al. 2016). A. annua extract, which is an antioxidant similar to the antidiabetic standard drug metformin, upregulated cortical antioxidant enzymes (glutathione peroxidase, glutathione peroxidase 1, glutathione reductase, superoxide dismutase, superoxide dismutase 2, catalase) and downregulated lipoperoxidation levels [malondialdehyde (MDA), NO] in STZ diabetic rat models (Albasher et al., 2020). In other cognitive impairment diseases, artesunate enhanced the spatial learning ability of thioacetamide-induced male Wistar rats and shortened the escape latency and working errors (Wu et al., 2016). In addition to animal experiments, astrocytes and microglial cells have been studied because they play crucial roles in T2DM-mediated chronic inflammation and oxidative stress, maintaining brain homeostasis (Shi et al., 2018). Artemisinins significantly suppressed ROS release and played a protective role in Aβ25-35-induced PC12 cells and ammonia-treated SH-SY5Y cells (Okorji and Olajide, 2014; Badshah et al., 2015; Badshah et al., 2016; Wu et al., 2016). Furthermore, the protective effects of artemisinin on H2O2-induced SH-SY5Y and hippocampal neurons can also be achieved by activating AMPK signaling (Zhao et al., 2019). Another study confirmed that the antioxidant protection mechanism of artemether was dependent on Nrf2 in BV2 microglia and showed a downtrend in the production of ROS and elevated levels of the antioxidant protein NQO1 (Okorji et al., 2016). Artesunate showed a protective effect on ammonia-treated C6 astroglial cells by reversing oxidative stress, which subsequently led to elevated glutamate uptake/release activity, glutamine synthetase activity, intracellular glutathione (GSH) content, and Na+K+-ATPase activity (Wu et al., 2016). Treatment of cerebrovascular diseases with artesunate suppressed ROS production in CIRI mice by restoring Nrf2 protein expression and downregulating ROS-dependent p38 MAPK in these mice. In addition, the misfolding and aggregation of human islet amyloid polypeptide (hIAPP) and amyloid-β (Aβ) protein are closely related to T2DM and AD. In Xu’s study, while four compounds of artemisinins (artemisinin, dihydroartemisinin, artesunate, and artemether) regulated the glucose homeostasis of diabetes, they also had great inhibition and disaggregation effects against hIAPP (Xu et al., 2019). There is abundant evidence showing that artemisinins decrease the level of amyloid-β protein (Aβ), which accumulates in AD and attenuate neurocognitive deficits by inhibiting c-Jun N-terminal kinase (JNK) and activating the extracellular signal-regulated kinase (ERK) 1/2 pathway (Chong and Zheng, 2016; Xu et al., 2017b; Zeng et al., 2017). These findings provide new perspectives on the use artemisinins as inhibitors against amyloidosis-related diseases. Although we currently lack enough direct evidence to prove the specific effects of artemisinins on diabetic cognitive diseases, through a systematic assessment of the relationship between diabetes and cognitive impairment and the effects of artemisinin on other cognitive disorders, we speculate that artemisinin and its derivatives may prevent and treat diabetes-related cognitive impairment by relieving oxidative stress and suppressing inflammation status and propose some potential action points of the oxidative stress pathway and inflammatory pathway that deserve further exploration.
At high concentrations of glucose, A. annua extract significantly attenuated the vacuolation degeneration of neurocytes and prevented cortical apoptosis by enhancing the expression of anti-apoptotic marker Bcl2 and inhibiting the expression of proapoptotic marker Bax. In addition, the increased brain-derived neurotrophic factor norepinephrine and dopamine levels indicated neuroprotective efficiency in diabetes (Albasher et al., 2020). Interestingly, the protective results were replicated in an artesunate-treated traumatic brain injury (TBI) group, suggesting that artemisinins of broad spectrum protection of nerve cells (Gugliandolo et al., 2018).
Evidence indicates that the gut microbiome is a potential new target for Chinese herbal medicines in treating diabetes mellitus (Wang et al., 2018; Wu et al., 2019; Zhang et al., 2019). Currently, Liu et al. investigated the effects of dihydroartemisinin on the intestinal microbiome in mice. Dihydroartemisinin downregulates the “neurodegenerative diseases” and “infectious diseases” signaling pathways while upregulating “energy metabolism” and “nucleotide metabolism” as indicated by a KEGG signaling pathway enrichment analysis (Liu et al., 2020). However, the role of artemisinins in the intestinal flora of T2DM is still unclear and needs to be further explored.
Diabetic Retinopathy (DR)
Diabetic retinopathy (DR) is a common complication of advanced T2DM and is largely related to two late-stage conditions: proliferative diabetic retinopathy and diabetic macular edema (Wong et al., 2016; Tan et al., 2017). Many factors regulate the proliferation and migration of endothelial cells through a series of molecular mediators (Amadio et al., 2016). Ge et al. directly proved that artesunate can inhibit retinal neovascularization and leakage by decreasing the increase in intercellular adhesion molecule-1 (ICAM-1) and MMP-9 protein in human umbilical vein endothelial cells (HUVECs) in a concentration-dependent manner under high-glucose conditions (Ge et al., 2019). In addition, VEGF can also destroy the blood-retinal barrier and lead to an increase in vascular permeability such that exuded fluid accumulates in the macula, which worsens retinal ischemia and hypoxia, seriously threatening vision (Crawford et al., 2009; Wong et al., 2016; Tan et al., 2017; Jiang et al., 2018). The current DR treatment strategies aim to control microvascular complications; therefore, the administration of intravitreal anti-VEGF drugs is currently the main means of therapy for early and advanced stages of DR (Cheung, Wong, and Wong, 2014; Wang and Lo, 2018). Interestingly, clinical and experimental studies demonstrated that artemisinins can reverse pathological angiogenesis and pathological exudation, which accelerates disease progression (Chen et al., 2003). Li et al. injected 80 μg of artesunate into five patients with retinal neovascularization, and upon following up at 52 weeks, found that retinal neovascularization, papilledema, and high intraocular pressure (IOP) were significantly relieved (Li et al., 2019a). A single intravitreal dose of 20 μg of artesunate was injected in rabbit and monkey models and 6 months later the artesunate had reversed retinal and iris neovascularization, vascular dilatation and tortuosity, macular edema, and fluorescein leakage. Current clinical trials have suggested that anti-VEGF therapy may represent a first-line therapy for proliferative DR treatment. In the present study, artesunate targeted VEGF and had greater anterior chamber penetrability and more durable efficacy than Avastin, which is an anti-VEGF protein drug. This result indirectly proves the effect of artemisinin in DR: VEGF receptor 2 (VEGFR2) was significantly changed, and several proangiogenic cytokines, including protein kinase C α isoenzyme (PKCα) and platelet-derived growth factor receptor (PDGFR), were also reduced, indicating that the multitarget action of artemisinins may resolve the limitations and adverse reactions of anti-VEGF drugs (Zong et al., 2016). Moreover, Dong et al. also found that endothelial cell proliferation and migration were inhibited by dihydroartemisinin, which targets VEGFR2, which is realized by blocking NF-κB signaling (Dong et al., 2014). Additionally, suppressing ERK1/2 expression and its downstream effectors c-fos and c-myc may be another pathway by which dihydroartemisinin inhibits HUVEC proliferation (Dong et al., 2015). Cheng et al. observed that artesunate therapy induced reactive oxygen species (ROS) generation, and these ROS promote the apoptosis of HUVECs by activating p38 MAPK (Cheng et al., 2013). Extensive evidence indicates that diabetes can cause oxidative stress in the retina and capillary cells, and the increase in reactive oxygen species also damages the structure and function of mitochondria (Duraisamy et al., 2018; Volpe et al., 2018; Wu et al., 2018a).
In terms of antioxidant stress, Yan et al. also demonstrated that artemisinin can prevent retinal pigment epithelial cells growing in high-glucose from oxidative stress via the MAPK/CREB pathway (Yan et al., 2017). Chong and Zheng demonstrated that artemisinin was able to suppress H2O2-induced oxidative stress in D407 retinal pigment epithelial cells, which are first damaged in retinal diseases through the activation of ERK/cAMP-response element-binding protein (CREB) signaling (Chong and Zheng, 2016). In Li’s research, artemisinin protection of H2O2-induced human retinal pigmented (D407) cells relied on decreased ROS generation via the activation of AMPK. In addition, artemisinin recovered mitochondrial function, restoring the mitochondrial membrane potential that had been decreased by H2O2 (Li et al., 2019b). These results illustrated that artemisinin induces the generation of ROS to promote endothelial cell apoptosis in diabetic proliferative retinal diseases, but under oxidative stress conditions, when reactive oxygen species accumulate, artemisinins can protect cells from damage. Therefore, we speculate that artemisinins act as a “balancer,” playing a bidirectional regulatory role in the treatment of Dr. Kowluru et al. suggested that the regulation of mitochondrial homeostasis through antioxidants may provide a treatment modality for the treatment of diabetic retinopathy (Kowluru et al., 2015). Both direct and indirect evidence suggests that artemisinins have great potential in the treatment of DR as antiangiogenic drugs, balancers of oxidative stress, and regulators of mitochondrial function.
Potential Benefits of Treating Diabetic Cardiovascular Disease With Artemisinins
Because of its high mortality, diabetic cardiovascular disease is one of the most concerning diabetic complications, including macrovascular disease, which mainly involves the coronary artery and aorta, and microvascular disease, which involves diabetic cardiomyopathy (Balakumar et al., 2016; Henning, 2018). Metformin, as a first-line medication for diabetic patients, also shows benefits, including a reduction in cardiovascular events (Maruthur et al., 2016). Cardiovascular benefits are among the most important standards for evaluating diabetes drugs in the clinic.
Diabetic cardiomyopathy (DCM) is an important factor affecting the survival rate of diabetic patients (Kannel et al., 1974; Nichols et al., 2004). The early pathological manifestations of DCM are inflammation and fibrosis of cardiomyocytes, followed by apoptosis and necrosis of cardiomyocytes. Li et al. surprisingly found that artemisinin not only relieved symptoms of T2DM, such as polydipsia, polyphagia, and polyuria, but also ameliorated the general states of DCM in rats. Specifically, it lowered plasma glucose levels and improved cardiac function, such as the left ventricular end-systolic dimension, left ventricular end-diastolic dimension, and left ventricular ejection fraction, by inhibiting high glucose-induced early inflammatory responses, especially by decreasing TNF-α and NF-κB levels, reducing the deposition of collagen fibers and inhibiting myocardial fibrosis in terms of the downregulated expression of TGFβ-1, Collagen Ⅰ, and Collagen Ⅲ (Li et al., 2016).
Abundant evidence shows that after artemisinin therapy, the area of aortic root lesions shrunk, vascular smooth muscle cell hyperplasia and fibrosis were attenuated, and the progression of atherosclerosis lesion formation was diminished, indicating the potential therapeutic effects on atherosclerosis, which is one of the most common manifestations of diabetic cardiovascular disease. Thus, we deduced that artemisinins may alleviate diabetic cardiovascular disease by inhibiting the occurrence and development of atherosclerosis (Wang et al., 2013; Jiang et al., 2016; Du et al., 2019; Cao et al., 2020; Jiang et al., 2020).
Atherosclerosis is a chronic inflammatory disease, and macrophages are the main immune cells involved in atherosclerotic inflammation (Bories and Leitinger, 2017; Moore et al., 2018). In protecting the cardiovascular process, AMPK seems to be the target for artemisinin to reverse pathological changes in activated macrophages. On the one hand, artemisinin significantly promotes phosphorylation of AMPK, followed by suppressing the NF-κB pathway (Cacicedo et al., 2004; Okayasu et al., 2008). Subsequently, the NF-κB network is considered to be the basis of NLRP3 inflammasome activation, which induces the generation of the inflammatory cytokines IL-1β and IL-18 (Bauernfeind et al., 2009). The artemisinin and artesunate groups showed not only an almost complete reversal of elevated NLRP3 inflammasome-related protein expression but also the downregulation of the proinflammatory cytokines TNF-α and IL-6 and the inflammatory chemokines IL-8 and MCP-1 in phorbol 12-myristate 13-acetate (PMA)-induced human monocytic THP-1 cells (Wang et al., 2011a; Jiang et al., 2016; Wang et al., 2016b). Moreover, artemisinin can induce AMPK phosphorylation and suppress NF-κB translocation, inhibiting the expression of VCAM-1 and ICAM-1, which are considered the main adhesion mediators, blocking monocyte-endothelial cell interactions and attachment (Ouchi et al., 2000; Wang et al., 2016b; Jiang et al., 2020). Similarly, the mitogen-activated protein kinase (MAPK) signaling pathway, which is downstream of the NF-κB signal transduction pathway in TNF-α-stimulated HUVECs, also participates in the pathogenesis of these features (Wang et al., 2016b). In the artemisinin group, the expression of phosphorylated ERK1/2, p38, and JNK downregulated and EMMPRIN and MMP-9 activity were decreased (Wang et al., 2011a; Lee et al., 2014; Cao et al., 2015).
On the other hand, a deficiency of macrophage autophagy accelerates foamy macrophage transformation (Shao et al., 2016). Treating HFD-fed ApoE-/-mice with 50, 100 mg/kg/d artemisinin for 8 weeks successfully attenuated foamy macrophage transformation and enhanced macrophage autophagy (Cao et al., 2020). Similar results were obtained in the experiments performed in vitro. Mammalian target of rapamycin (mTOR) and uncoordinated-51-like kinase 1 (ULK1) phosphorylation was inhibited upon artemisinin administration, and some autophagic markers were increased, such as LC-3II, which accumulated, and P62 was degraded, showing the enhancement of macrophage autophagy in the oxLDL-treated mouse macrophage cell line (Cao et al., 2020). Furthermore, inflammation disturbs the normal function of vascular endothelial cells and smooth muscle cells (Reglero-Real et al., 2016; Li et al., 2018). Artemisinin decreases the expression of proliferating cell nuclear antigen (PCNA) and the proliferation and migration of rat vascular smooth muscle cells (VSMCs) (Cao et al., 2015). Du et al. provided in vivo and in vitro evidence demonstrating that artemisinin can decrease PDGF-activated MOVAS migration and proliferation and elevate the expression of contractile phenotypic markers (αSMA, SM22 α, calponin 1, and SMMHC), partly by inhibiting the phenotype switching that leads to a dedifferentiated phenotype (Du et al., 2019). Wang’s team first found that artesunate can significantly increase the expression of KLF2 protein, which regulates the expression of multiple endothelial vascular protection genes (Wang et al., 2013).
Overall, many studies have provided evidence that artemisinins have definitive benefits on the cardiovascular system, but the effects of artemisinin on diabetes-related cardiovascular diseases are still in the preliminary stage. Thus, we still need more evidence to confirm the role of artemisinins in diabetic cardiovascular disorders.
Artemisinin-Related Side Effects
The limited evidence of drug resistance and low toxicity of artemisinin has been recognized by researchers, whereas the side effects of artemisinin have gradually been recognized with the continuous in-depth study of artemisinin in recent years. As we mentioned earlier, the effects of artemisinin and dihydroartemisinin on INS-1 cell viability gradually increased with increasing concentration, indicating that the toxicity of the two drugs to cells gradually increased with increasing concentration (Chen et al., 2020). Notably, Efferth et al. claimed that protein alkylation is the cause of artesunate-induced toxicity (Efferth and Kaina, 2010). Sun and Zhou also reported that long-term and low-dose exposure to artemisinin might induce free-radical scavengers such as the antioxidant enzyme SOD (Sun and Zhou, 2017), which can destroy the fragile internal peroxide bridge structure in artemisinin, resulting in artemisinin’s reduced treatment efficiency and a dilemma similar to the unrestricted use of antibiotics. Importantly, because of the wide spectrum and nonspecific features of artemisinin, neurotoxicity (Li and Hickman, 2011; Li et al., 2019c), reproductive toxicity (Farombi et al., 2015; Luo et al., 2018), genotoxicity (Singh et al., 2015), etc., have been gradually reported. For example, Singh et al. reported that some unexpected metabolic dysfunctions or abnormalities, including genotoxicity due to sperm DNA damage, might emerge upon excessive artemisinin use. Considering the broad application prospects of artemisinin and its derivatives, we presume that different artemisinin concentrations may be suitable for different pathological conditions. Once the concentration is excessively high or low, artemisinin treatments may cause a number of side effects. Looking for the proper concentration of artemisinin analogs that are suitable in the pathological state of interest may be the next step to recognize the maximum potential of artemisinin, which will be beneficial to clinical applications and will ultimately achieve the goal of precision medicine.
Discussion
As a range of drugs with huge potential for the treatment of metabolic diseases, artemisinin and its derivatives play critical roles in the therapy of T2DM as well as its related complications. Firstly, through horizontally comparisons of the properties and tissue distribution of artemisinins, it is helpful for us to understand the therapeutic effects and mechanism of artemisinins on diabetes and its complications and provide directions and ideas for future research. For example, artemisinins, except arteether, can pass through the blood-brain barrier; thus, we can infer from the experimental results of Albasher and Zeng that artemisinin may attenuate diabetic cognitive impairment (Zeng et al., 2017; Albasher et al., 2020). Because of these characteristics, artemether, dihydroartemisinin, and artesunate deserve further study to determine their effects on diabetic cognitive impairment. Moreover, artesunate and artemether are distributed in skeletal muscles and liver, which may align with their function of ameliorating IR. Similarly, artesunate distributed in the eyeball has also been confirmed to alleviate eye diseases (Cheng et al., 2013; Ge et al., 2019). Furthermore, artemisinin can pass through blood-placenta barriers (Niu et al., 1985), and artesunate can be distributed in testicular tissue; therefore, we need to pay attention to the reproductive toxicity of these two drugs. The pharmacological understanding of artemisinin and its derivatives is incomplete, and there are still many gaps that need to be filled (Karbwang et al., 1997; Navaratnam et al., 2000; Gautam et al., 2009).
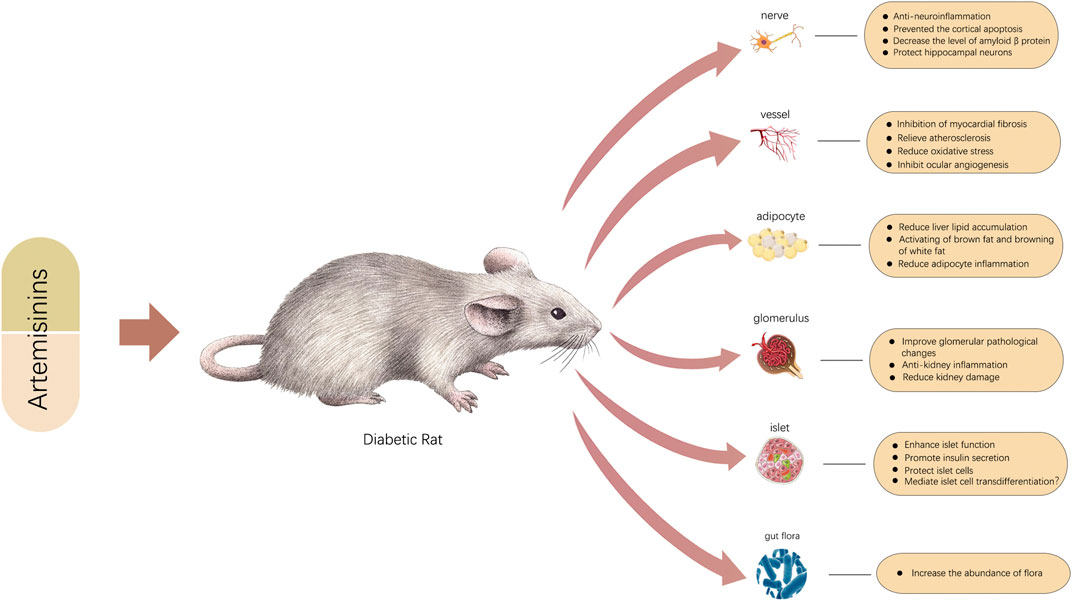
Attenuating IR and restoring islet cell function are two pathways through which artemisinins can alleviate T2DM. Because of their close relationship, obesity, IR, and inflammation often exert cross-influences on each other and continuously promote the development of diseases. Accumulating evidence confirms that artemisinin and its derivatives can break any step of a vicious cycle by modulating adipose production, differentiation, and consumption and inhibiting the inflammatory response to reverse metabolic dysfunction. Artemisinin and its derivatives also act on islet cells by promoting insulin secretion, protecting pancreatic islet ß cells, and achieving islet α-cell to ß-cell transdifferentiation by reversing a number of abnormal proteins and RNAs, thereby inhibiting the development and progression of T2DM.
In addition to playing a crucial role in treating T2DM, artemisinins can also participate in the therapy of diabetic complications through a series of molecular pathways. Notably, in numerous studies, inhibiting the expression of key inflammatory pathways or inflammatory factors and attenuating the chronic inflammation state will significantly improve the functions of related organs. Attenuating inflammation seems to be an important therapeutic mechanism, even for diabetes and all the complications that have been studied. In addition, artemisinin and its derivatives have demonstrated great promise as regulators of oxidative stress, especially in diabetic nephropathy, diabetic retinopathy, and diabetic cognitive impairment. Specifically, artemisinins ameliorate DKD by regulating metabolism, restoring mitochondrial function, modulating RAS, and altering a range of related abnormal molecules. The technology of next-generation sequencing can be applied to identify related genes and pathways influenced by artemisinin. For DR, according to one study, artesunate is more effective than the anti-VEGF drug Avastin and can also effectively prevent the occurrence of retinal detachment (Zong et al., 2016). On the one hand, artesunate can induce apoptosis of epithelial cells and reduce neovascularization by inducing oxidative stress; on the other hand, artemisinin can protect retinal epithelial cells from damage caused by the oxidative stress state of diabetes (Zong et al., 2016; Yan et al., 2017). Therefore, we speculate that the effects of artemisinin are different based on the different pathological conditions of epithelial cells. Generally, artemisinins act as balancing agents and thus can reverse the pathological state and stabilize the intracellular environment. The protective effects of artemisinins on nerve cells are also significant. In addition to anti-inflammatory and antioxidative effects, artemisinins also decrease the level of Aβ, upregulate neurotrophic factors, modulate the apoptosis of neurons, and change the composition of the gut microbiota to e reverse cognitive impairment. Cardiovascular benefits are among the criteria for evaluating diabetes drugs. In addition to reducing early inflammation, reducing fiber formation can also help prevent the occurrence and development of diabetic cardiomyopathy. It is also worth noting artemisinin’s cardiovascular protective function. Metformin also has a positive role in protecting the cardiovascular system, and artemisinin shows certain advantages that are similar to metformin to some degree. Artemisinins play protective roles in atherosclerosis by inhibiting inflammation, promoting macrophage autophagy, and improving the expression of endothelial vascular protection genes. However, the toxicity of artemisinin and its derivatives has gradually been recognized. The side effects of artemisinin and its derivatives have become an obstacle to the treatment of diabetes. In addition to inducing possible damage to islet ß cells (Chen et al., 2020), artemisinins also have adverse effects upon its inappropriate use in the course of treatment and doses, which limit clinical application and need to be addressed seriously (Farombi et al., 2015; Singh et al., 2015; Cao et al., 2020). Although artemisinins have not shown obvious toxicity in various experiments, ways to reduce side effects to the greatest extent possible while retaining the maximum therapeutic effect may be the next issues to be addressed.
The hypoglycemic effects of artemisinin have been verified in most experiments, but the differences in the experimental results and some questions are still worth further study. 1) Different animal models should be considered. Sex has been considered to play an important role in the metabolism of artemisinin and its semisynthetic derivatives. The free fraction of artemisinin in the plasma of male rats was significantly lower than that of female rats (Ashton et al., 1999). Most studies use a single-sex model, and the effects of sex on the results were not studied, which is a limitation to clinical application. Therefore, it is crucial to perform further studies to assess the general applicability of artemisinin and its derivatives for the treatment of diverse patients with diabetes. The use of different mouse strains may be one of the most likely reasons for the discrepant results. In Ins1-H2B-mCherry × Gcg-Cre × Rosa26-stop-YFP triple transgenic mice and Glucagon-CreERT2; Rosa-LSL-eYFP male mice, α-cell to ß-cell transdifferentiation was not observed; however, they were observed in the αTC1 and Min6 cell lines and transgenic (Gcga: GFP)ia1 − (ins: NTR-mcherry)ml10 zebrafish. Designing a model that better fits the simulated situation may lead to a conclusion more in line with the true situation. 2) Different mediators should be considered. Different media can cause the same result from different processes; for example, palmitate, high glucose levels, and proinflammatory cytokines can cause ß-cell failure through different pathways. Artemisinin and its derivatives have been shown to respond to reverse inflammatory factor-induced ß cell damage but not to palmitate-induced ß cell damage. It has been demonstrated that artemisinins exert effects in an inflammatory environment rather than in a state where free fatty acids are abundant. Therefore, assessing the disease states of the body in which artemisinin functions may expand the scope for the application of artemisinins. 3) Dose and duration should be considered. Side effects are different based on the dose and duration of artemisinin treatments in different diseases. By reviewing the adverse effects, we found that much attention should be paid to suitable artemisinins, reasonable doses and courses of treatment. Hence, artemisinin analogs can be better exploited for therapeutic interventions. 4) Relatively unknown areas should be explored. Traditional Chinese medicine, such as berberine, is potent in modulating gut microbiota (Zhang et al., 2012; Zheng et al., 2018). Different artemisinin derivatives might have differential roles in changing the gut microbiota. In addition, different dietary and living conditions of the mice may lead to different outcomes through changes in the gut microbiota. 5) Without improved technology, efficacy may be affected by contaminating proteins and RNAs; the original droplet-based single-cell transcriptome contains up to 20% contaminating transcripts, indicating that there are still some defects in the detection and extraction methods at this stage. The development of a greater number of efficient separation and purification methods and techniques will greatly improve the accuracy and the precise understanding of the drug effects. 6) Species-dependent effects need to be considered. Marquina-Sanchez’s report demonstrated that the efficacy of artemisinin is species-dependent. Although abundant evidence shows the great potential of artemisinins in the treatment of T2DM and related complications in diabetic models, there is still reasonable doubt about its efficacy in humans. 7) Multitargeted effects need to be explored. From reviewing various experiments, we suggest that artemisinins are multitargeted in the treatment of diabetes and its related complications. However, there are many unresearched but very valuable targets for the reversion of diabetic pathological changes. Further research will contribute to a comprehensive understanding of the roles of artemisinins in T2DM.
In summary, artemisinin and its derivatives play vital roles in the treatment of T2DM, while the clinical application of artemisinin is still challenging. It is essential to further study the interaction between artemisinins and T2DM and then provide clear reasons to use artemisinin as a potential treatment for T2DM and its complications, paving the way for the future cure of diabetes in patients.
Author Contributions
RS-Y is the corresponding author of the study. YY-J is the first author and responsible for collecting materials and writing the paper. JC-S helped organizing the information and edited the article pictures. BX-Z and JW-C are responsible for the second check. All authors read and approved the final article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks for all the help from everyone in our lab. We thank the members of our lab for providing some discussions.
References
Abdelfattah, M. S., Badr, S. E. A., Lotfy, S. A., Attia, G. H., Aref, A. M., Abdel Moneim, A. E., et al. (2020). Rutin and selenium co-administration reverse 3-nitropropionic acid-induced neurochemical and molecular impairments in a mouse model of huntington’s disease. Neurotox. Res. 37 (1), 77–92. doi:10.1007/s12640-019-00086-y
Abdul-Wahed, A., Guilmeau, S., and Postic, C. (2017). Sweet sixteenth for ChREBP: established roles and future goals. Cell Metabol. 26 (2), 324–341. doi:10.1016/j.cmet.2017.07.004
Ackermann, A. M., Moss, N. G., and Kaestner, K. H. (2018). GABA and artesunate do not induce pancreatic α-to-β cell transdifferentiation in vivo. Cell Metabol. 28 (5), 787–792.e3. doi:10.1016/j.cmet.2018.07.002
Akash, M. S. H., Rehman, K., and Liaqat, A. (2018). Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 119 (1), 105–110. doi:10.1002/jcb.26174
Akter, K., Lanza, E. A., Martin, S. A., Myronyuk, N., Rua, M., and Raffa, R. B. (2011). Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment?. Br. J. Clin. Pharmacol. 71 (3), 365–376. doi:10.1111/j.1365-2125.2010.03830.x
Albasher, G., Aljarba, N., Al Sultan, N., Alqahtani, W. S., and Alkahtani, S. (2020). Evaluation of the neuro-protective effect of Artemisia judaica extract in a murine diabetic model. J. Food Biochem. 44 (8), e13337. doi:10.1111/jfbc.13337
Amadio, M., Govoni, S., and Pascale, A. (2016). Targeting VEGF in eye neovascularization: what’s new?: a comprehensive review on current therapies and oligonucleotide-based interventions under development. Pharmacol. Res. 103, 253–269. doi:10.1016/j.phrs.2015.11.027
American Diabetes Association, (2019). Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes-2019. Diabetes Care 42 (Suppl. 1), S81–s89. doi:10.2337/dc19-S008
Ashton, M., Gordi, T., Trinh, N. H., Nguyen, V. H., Nguyen, D. S., Nguyen, T. N., et al. (1998). Artemisinin pharmacokinetics in healthy adults after 250, 500 and 1000 mg single oral doses. Biopharm Drug Dispos. 19 (4), 245–250. doi:10.1002/(sici)1099-081x(199805)19:4<245::aid-bdd99>3.0.co;2-z
Ashton, M., Johansson, L., Thornqvist, A. S., and Svensson, U. S. (1999). Quantitative in vivo and in vitro sex differences in artemisinin metabolism in rat. Xenobiotica 29 (2), 195–204. doi:10.1080/004982599238740
Asmat, U., Abad, K., and Ismail, K. (2016). Diabetes mellitus and oxidative stress-a concise review. Saudi Pharmaceut. J. 24 (5), 547–553. doi:10.1016/j.jsps.2015.03.013
Badshah, H., Ali, T., Ahmad, A., Kim, M. J., Abid, N. B., Shah, S. A., et al. (2015). Co-treatment with anthocyanins and vitamin C ameliorates ethanol- induced neurodegeneration via modulation of GABAB receptor signaling in the adult rat brain. CNS Neurol. Disord. - Drug Targets 14 (6), 791–803. doi:10.2174/1871527314666150225142919
Badshah, H., Ali, T., and Kim, M. O. (2016). Osmotin attenuates LPS-induced neuroinflammation and memory impairments via the TLR4/NFκB signaling pathway. Sci. Rep. 6, 24493. doi:10.1038/srep24493
Balakumar, P., Maung, U. K., and Jagadeesh, G. (2016). Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 113 (Pt A), 600–609. doi:10.1016/j.phrs.2016.09.040
Bastard, J. P., Maachi, M., Lagathu, C., Kim, M. J., Caron, M., Vidal, H., et al. (2006). Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 17 (1), 4–12.
Batty, K. T., Le, A. T., Ilett, K. F., Nguyen, P. T., Powell, S. M., Nguyen, C. H., et al. (1998). A pharmacokinetic and pharmacodynamic study of artesunate for vivax malaria. Am. J. Trop. Med. Hyg. 59 (5), 823–827. doi:10.4269/ajtmh.1998.59.823
Bauernfeind, F. G., Horvath, G., Stutz, A., Alnemri, E. S., MacDonald, K., Speert, D., et al. (2009). Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183 (2), 787–791. doi:10.4049/jimmunol.0901363
Baumgart, D. C., and Sandborn, W. J. (2007). Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369 (9573), 1641–1657. doi:10.1016/s0140-6736(07)60751-x
Ben-Othman, N., Vieira, A., Courtney, M., Record, F., Gjernes, E., Avolio, F., et al. (2017). Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 168 (1-2), 73–85.e11. doi:10.1016/j.cell.2016.11.002
Benhamed, F., Denechaud, P. D., Lemoine, M., Robichon, C., Moldes, M., Bertrand-Michel, J., et al. (2012). The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Invest. 122 (6), 2176–2194. doi:10.1172/jci41636
Biessels, G. J., Staekenborg, S., Brunner, E., Brayne, C., and Scheltens, P. (2006). Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 5 (1), 64–74. doi:10.1016/s1474-4422(05)70284-2
Blüher, M. (2019). Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15 (5), 288–298. doi:10.1038/s41574-019-0176-8
Bordone, L., Motta, M. C., Picard, F., Robinson, A., Jhala, U. S., Apfeld, J., et al. (2006). Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 4 (2), e31. doi:10.1371/journal.pbio.0040031
Bories, G. F. P., and Leitinger, N. (2017). Macrophage metabolism in atherosclerosis. FEBS Lett. 591 (19), 3042–3060. doi:10.1002/1873-3468.12786
Boss, O., and Farmer, S. R. (2012). Recruitment of brown adipose tissue as a therapy for obesity-associated diseases. Front. Endocrinol. 3, 14. doi:10.3389/fendo.2012.00014
Brenachot, X., Ramadori, G., Ioris, R. M., Veyrat-Durebex, C., Altirriba, J., Aras, E., et al. (2017). Hepatic protein tyrosine phosphatase receptor gamma links obesity-induced inflammation to insulin resistance. Nat. Commun. 8 (1), 1820. doi:10.1038/s41467-017-02074-2
Brennan, E. P., Morine, M. J., Walsh, D. W., Roxburgh, S. A., Lindenmeyer, M. T., Brazil, D. P., et al. (2012). Next-generation sequencing identifies TGF-β1-associated gene expression profiles in renal epithelial cells reiterated in human diabetic nephropathy. Biochim. Biophys. Acta 1822 (4), 589–599. doi:10.1016/j.bbadis.2012.01.008
Cacicedo, J. M., Yagihashi, N., Keaney, J. F., Ruderman, N. B., and Ido, Y. (2004). AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 324 (4), 1204–1209. doi:10.1016/j.bbrc.2004.09.177
Cai, H., Dong, L. Q., and Liu, F. (2016). Recent advances in adipose mTOR signaling and function: therapeutic prospects. Trends Pharmacol. Sci. 37 (4), 303–317. doi:10.1016/j.tips.2015.11.011
Cao, J., Wang, W., Li, Y., Xia, J., Peng, Y., Zhang, Y., et al. (2016). Artesunate attenuates unilateral ureteral obstruction-induced renal fibrosis by regulating the expressions of bone morphogenetic protein-7 and uterine sensitization-associated gene-1 in rats. Int. Urol. Nephrol. 48 (4), 619–629. doi:10.1007/s11255-016-1232-0
Cao, Q., Du, H., Fu, X., Duan, N., Liu, C., and Li, X. (2020). Artemisinin attenuated atherosclerosis in high-fat diet-fed ApoE-/- mice by promoting macrophage autophagy through the AMPK/mTOR/ULK1 pathway. J. Cardiovasc. Pharmacol. 75 (4), 321–332. doi:10.1097/fjc.0000000000000794
Cao, Q., Jiang, Y., Shi, J., Xu, C., Liu, X., Yang, T., et al. (2015). Artemisinin inhibits the proliferation, migration, and inflammatory reaction induced by tumor necrosis factor-α in vascular smooth muscle cells through nuclear factor kappa B pathway. J. Surg. Res. 194 (2), 667–678. doi:10.1016/j.jss.2014.12.013
Caprio, S., Perry, R., and Kursawe, R. (2017). Adolescent obesity and insulin resistance: roles of ectopic fat accumulation and adipose inflammation. Gastroenterology 152 (7), 1638–1646. doi:10.1053/j.gastro.2016.12.051
Chakravarthy, H., Gu, X., Enge, M., Dai, X., Wang, Y., Damond, N., et al. (2017). Converting adult pancreatic islet α cells into β cells by targeting both Dnmt1 and Arx. Cell Metabol. 25 (3), 622–634. doi:10.1016/j.cmet.2017.01.009
Chen, H. H., Zhou, H. J., and Fang, X. (2003). Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol. Res. 48 (3), 231–236. doi:10.1016/s1043-6618(03)00107-5
Chen, H., Sun, B., Wang, S., Pan, S., Gao, Y., Bai, X., et al. (2010). Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: involvement of cell cycle arrest and inactivation of nuclear factor-kappaB. J. Canc. Res. Clin. Oncol. 136 (6), 897–903. doi:10.1007/s00432-009-0731-0
Chen, K., Hua, H., Zhu, Z., Wu, T., Jia, Z., and Liu, Q. (2020). Artemisinin and dihydroartemisinin promote β-cell apoptosis induced by palmitate via enhancing ER stress. Apoptosis 25 (3-4), 192–204. doi:10.1007/s10495-019-01587-z
Chen, T., Guo, Q., Wang, H., Zhang, H., Wang, C., Zhang, P., et al. (2015). Effects of esculetin on lipopolysaccharide (LPS)-induced acute lung injury via regulation of RhoA/Rho Kinase/NF-кB pathways in vivo and in vitro. Free Radic. Res. 49 (12), 1459–1468. doi:10.3109/10715762.2015.1087643
Cheng, R., Li, C., Li, C., Wei, L., Li, L., Zhang, Y., et al. (2013). The artemisinin derivative artesunate inhibits corneal neovascularization by inducing ROS-dependent apoptosis in vascular endothelial cells. Invest. Ophthalmol. Vis. Sci. 54 (5), 3400–3409. doi:10.1167/iovs.12-11068
Chera, S., Baronnier, D., Ghila, L., Cigliola, V., Jensen, J. N., Gu, G., et al. (2014). Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 514 (7523), 503–507. doi:10.1038/nature13633
Cheung, N., Wong, I. Y., and Wong, T. Y. (2014). Ocular anti-VEGF therapy for diabetic retinopathy: overview of clinical efficacy and evolving applications. Diabetes Care 37 (4), 900–905. doi:10.2337/dc13-1990
Cho, N. H., Shaw, J. E., Karuranga, S., Huang, Y., da Rocha Fernandes, J. D., Ohlrogge, A. W., et al. (2018). IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281. doi:10.1016/j.diabres.2018.02.023
Choi, H. M., Kim, H. R., Kim, E. K., Byun, Y. S., Won, Y. S., Yoon, W. K., et al. (2015). An age-dependent alteration of the respiratory exchange ratio in the db/db mouse. Lab Anim Res 31 (1), 1–6. doi:10.5625/lar.2015.31.1.1
Choi, J., Chandrasekaran, K., Inoue, T., Muragundla, A., and Russell, J. W. (2014). PGC-1α regulation of mitochondrial degeneration in experimental diabetic neuropathy. Neurobiol. Dis. 64, 118–130. doi:10.1016/j.nbd.2014.01.001
Chong, C. M., and Zheng, W. (2016). Artemisinin protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of ERK/CREB signaling. Redox Biol 9, 50–56. doi:10.1016/j.redox.2016.06.002
Cinti, F., Bouchi, R., Kim-Muller, J. Y., Ohmura, Y., Sandoval, P. R., Masini, M., et al. (2016). Evidence of β-cell dedifferentiation in human type 2 diabetes. J. Clin. Endocrinol. Metab. 101 (3), 1044–1054. doi:10.1210/jc.2015-2860
Collombat, P., Xu, X., Ravassard, P., Sosa-Pineda, B., Dussaud, S., Billestrup, N., et al. (2009). The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 138 (3), 449–462. doi:10.1016/j.cell.2009.05.035
Courtney, M., Gjernes, E., Druelle, N., Ravaud, C., Vieira, A., Ben-Othman, N., et al. (2013). The inactivation of Arx in pancreatic α-cells triggers their neogenesis and conversion into functional β-like cells. PLoS Genet. 9 (10), e1003934. doi:10.1371/journal.pgen.1003934
Crawford, T. N., Alfaro, D. V., Kerrison, J. B., and Jablon, E. P. (2009). Diabetic retinopathy and angiogenesis. Curr. Diabetes Rev. 5 (1), 8–13. doi:10.2174/157339909787314149
Croissandeau, G., Chrétien, M., and Mbikay, M. (2002). Involvement of matrix metalloproteinases in the adipose conversion of 3T3-L1 preadipocytes. Biochem. J. 364 (Pt 3), 739–746. doi:10.1042/bj20011158
Cypess, A. M., and Kahn, C. R. (2010). Brown fat as a therapy for obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 17 (2), 143–149. doi:10.1097/MED.0b013e328337a81f
Czech, M. P. (2017). Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 23 (7), 804–814. doi:10.1038/nm.4350
Dai, H., Liu, Q., and Liu, B. (2017). Research progress on mechanism of podocyte depletion in diabetic nephropathy. J Diabetes Res 2017, 2615286. doi:10.1155/2017/2615286
Datusalia, A. K., and Sharma, S. S. (2016). NF-κB inhibition resolves cognitive deficits in experimental type 2 diabetes mellitus through CREB and glutamate/GABA neurotransmitters pathway. Curr. Neurovascular Res. 13 (1), 22–32. doi:10.2174/1567202612666151030104810
de la Monte, S. M. (2012). Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr. Alzheimer Res. 9 (1), 35–66. doi:10.2174/156720512799015037
de Waziers, I., Cugnenc, P. H., Yang, C. S., Leroux, J. P., and Beaune, P. H. (1990). Cytochrome P 450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J. Pharmacol. Exp. Therapeut. 253 (1), 387–394.
Dejardin, E. (2006). The alternative NF-kappaB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem. Pharmacol. 72 (9), 1161–1179. doi:10.1016/j.bcp.2006.08.007
Dien, T. K., de Vries, P. J., Khanh, N. X., Koopmans, R., Binh, L. N., Duc, D. D., et al. (1997). Effect of food intake on pharmacokinetics of oral artemisinin in healthy Vietnamese subjects. Antimicrob. Agents Chemother. 41 (5), 1069–1072. doi:10.1128/aac.41.5.1069
Dinel, A. L., André, C., Aubert, A., Ferreira, G., Layé, S., and Castanon, N. (2011). Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One 6 (9), e24325. doi:10.1371/journal.pone.0024325
Dong, F., Tian, H., Yan, S., Li, L., Dong, X., Wang, F., et al. (2015). Dihydroartemisinin inhibits endothelial cell proliferation through the suppression of the ERK signaling pathway. Int. J. Mol. Med. 35 (5), 1381–1387. doi:10.3892/ijmm.2015.2140
Dong, F., Zhou, X., Li, C., Yan, S., Deng, X., Cao, Z., et al. (2014). Dihydroartemisinin targets VEGFR2 via the NF-κB pathway in endothelial cells to inhibit angiogenesis. Canc. Biol. Ther. 15 (11), 1479–1488. doi:10.4161/15384047.2014.955728
Drevon, C. A. (2005). Fatty acids and expression of adipokines. Biochim. Biophys. Acta 1740 (2), 287–292. doi:10.1016/j.bbadis.2004.11.019
Du, H., Zhao, Q., Zang, H., Chang, C., and Li, X. (2019). Artemisinin attenuates the development of atherosclerotic lesions by the regulation of vascular smooth muscle cell phenotype switching. Life Sci. 237, 116943. doi:10.1016/j.lfs.2019.116943
Dubois, V., Eeckhoute, J., Lefebvre, P., and Staels, B. (2017). Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J. Clin. Invest. 127 (4), 1202–1214. doi:10.1172/jci88894
Duckworth, W., Abraira, C., Moritz, T., Reda, D., Emanuele, N., Reaven, P. D., et al. (2009). Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360 (2), 129–139. doi:10.1056/NEJMoa0808431
Duraisamy, A. J., Mishra, M., Kowluru, A., and Kowluru, R. A. (2018). Epigenetics and regulation of oxidative stress in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 59 (12), 4831–4840. doi:10.1167/iovs.18-24548
Efferth, T., and Kaina, B. (2010). Toxicity of the antimalarial artemisinin and its dervatives. Crit. Rev. Toxicol. 40 (5), 405–421. doi:10.3109/10408441003610571
Egido, J., Rojas-Rivera, J., Mas, S., Ruiz-Ortega, M., Sanz, A. B., Gonzalez Parra, E., et al. (2017). Atrasentan for the treatment of diabetic nephropathy. Expet Opin. Invest. Drugs 26 (6), 741–750. doi:10.1080/13543784.2017.1325872
Elkhidir, A. E., Eltaher, H. B., and Mohamed, A. O. (2017). Association of lipocalin-2 level, glycemic status and obesity in type 2 diabetes mellitus. BMC Res. Notes 10 (1), 285. doi:10.1186/s13104-017-2604-y
Eteng, M. U., Abolaji, A. O., Ebong, P. E., Brisibe, E. A., Dar, A., Kabir, N., et al. (2013). Biochemical and haematological evaluation of repeated dose exposure of male Wistar rats to an ethanolic extract of Artemisia annua. Phytother Res. 27 (4), 602–609. doi:10.1002/ptr.4758
Farombi, E. O., Abolaji, A. O., Adedara, I. A., Maduako, I., and Omodanisi, I. (2015). Artemisinin induces hormonal imbalance and oxidative damage in the erythrocytes and uterus but not in the ovary of rats. Hum. Exp. Toxicol. 34 (1), 83–92. doi:10.1177/0960327114532385
Ferrer, M. D., Busquets-Cortés, C., Capó, X., Tejada, S., Tur, J. A., Pons, A., et al. (2019). Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr. Med. Chem. 26 (18), 3225–3241. doi:10.2174/0929867325666180514112124
Fève, B. (2005). Adipogenesis: cellular and molecular aspects. Best Pract. Res. Clin. Endocrinol. Metabol. 19 (4), 483–499. doi:10.1016/j.beem.2005.07.007
Finucane, F. M., Luan, J., Wareham, N. J., Sharp, S. J., O’Rahilly, S., Balkau, B., et al. (2009). Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia 52 (11), 2345–2349. doi:10.1007/s00125-009-1508-3
Fischer, K., Fenzl, A., Liu, D., Dyar, K. A., Kleinert, M., Brielmeier, M., et al. (2020). The scaffold protein p62 regulates adaptive thermogenesis through ATF2 nuclear target activation. Nat. Commun. 11 (1), 2306. doi:10.1038/s41467-020-16230-8
Forbes, J. M., and Thorburn, D. R. (2018). Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 14 (5), 291–312. doi:10.1038/nrneph.2018.9
Fu, W., Ma, Y., Li, L., Liu, J., Fu, L., Guo, Y., et al. (2020). Artemether regulates metaflammation to improve glycolipid metabolism in db/db mice. Diabetes Metab. Syndr. Obes. 13, 1703–1713. doi:10.2147/dmso.S240786
Gao, Y., Cui, M., Zhong, S., Feng, C., Nwobodo, A. K., Chen, B., et al. (2020). Dihydroartemisinin ameliorates LPS-induced neuroinflammation by inhibiting the PI3K/AKT pathway. Metab. Brain Dis. 35 (4), 661–672. doi:10.1007/s11011-020-00533-2
Gaspar, J. M., Baptista, F. I., Macedo, M. P., and Ambrósio, A. F. (2016). Inside the diabetic brain: role of different players involved in cognitive decline. ACS Chem. Neurosci. 7 (2), 131–142. doi:10.1021/acschemneuro.5b00240
Gautam, A., Ahmed, T., Batra, V., and Paliwal, J. (2009). Pharmacokinetics and pharmacodynamics of endoperoxide antimalarials. Curr. Drug Metabol. 10 (3), 289–306. doi:10.2174/138920009787846323
Ge, P. F., Jiang, T., Zong, Y., Yang, X. J., Ma, Y. N., Wang, Y. X., et al. (2019). Effect of artesunate on the expression of ICAM-1 and MMP-9 in vascular endothelial cells under high glucose condition. Hans J. Ophthalmol. 8, 41–51. doi:10.12677/HJO.2019.81008
Goto, T., Takahashi, N., Hirai, S., and Kawada, T. (2010). Various terpenoids derived from herbal and dietary plants function as PPAR modulators and regulate carbohydrate and lipid metabolism. PPAR Res. 2010, 483958. doi:10.1155/2010/483958
Gross, B., Pawlak, M., Lefebvre, P., and Staels, B. (2017). PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 13 (1), 36–49. doi:10.1038/nrendo.2016.135
Grünblatt, E., Bartl, J., and Riederer, P. (2011). The link between iron, metabolic syndrome, and Alzheimer’s disease. J. Neural. Transm. 118 (3), 371–379. doi:10.1007/s00702-010-0426-3
Gugliandolo, E., D’Amico, R., Cordaro, M., Fusco, R., Siracusa, R., Crupi, R., et al. (2018). Neuroprotective effect of artesunate in experimental model of traumatic brain injury. Front. Neurol. 9, 590. doi:10.3389/fneur.2018.00590
Guo, Y., Fu, W., Xin, Y., Bai, J., Peng, H., Fu, L., et al. (2018). Antidiabetic and antiobesity effects of artemether in db/db mice. BioMed Res. Int. 2018, 8639523. doi:10.1155/2018/8639523
Han, J., Li, E., Chen, L., Zhang, Y., Wei, F., Liu, J., et al. (2015). The CREB coactivator CRTC2 controls hepatic lipid metabolism by regulating SREBP1. Nature 524 (7564), 243–246. doi:10.1038/natre14557
Han, P., Wang, Y., Zhan, H., Weng, W., Yu, X., Ge, N., et al. (2019). Artemether ameliorates type 2 diabetic kidney disease by increasing mitochondrial pyruvate carrier content in db/db mice. Am. J. Transl. Res. 11 (3), 1389–1402.
Henning, R. J. (2018). Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 14 (6), 491–509. doi:10.2217/fca-2018-0045
Ho, W. E., Peh, H. Y., Chan, T. K., and Wong, W. S. (2014). Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol. Ther. 142 (1), 126–139. doi:10.1016/j.pharmthera.2013.12.001
Hotamisligil, G. S., Shargill, N. S., and Spiegelman, B. M. (1993). Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259 (5091), 87–91. doi:10.1126/science.7678183
Hou, L., and Huang, H. (2016). Immune suppressive properties of artemisinin family drugs. Pharmacol. Ther. 166, 123–127. doi:10.1016/j.pharmthera.2016.07.002
Huxford, T., Huang, D. B., Malek, S., and Ghosh, G. (1998). The crystal structure of the IkappaBalpha/NF-kappaB complex reveals mechanisms of NF-kappaB inactivation. Cell 95 (6), 759–770. doi:10.1016/s0092-8674(00)81699-2
Irrera, N., Pizzino, G., Calò, M., Pallio, G., Mannino, F., Famà, F., et al. (2017). Lack of the Nlrp3 inflammasome improves mice recovery following traumatic brain injury. Front. Pharmacol. 8, 459. doi:10.3389/fphar.2017.00459
Jang, B. C. (2016). Artesunate inhibits adipogeneis in 3T3-L1 preadipocytes by reducing the expression and/or phosphorylation levels of C/EBP-α, PPAR-γ, FAS, perilipin A, and STAT-3. Biochem. Biophys. Res. Commun. 474 (1), 220–225. doi:10.1016/j.bbrc.2016.04.109
Jha, J. C., Banal, C., Chow, B. S., Cooper, M. E., and Jandeleit-Dahm, K. (2016). Diabetes and kidney disease: role of oxidative stress. Antioxidants. Redox. Signal. 25 (12), 657–684. doi:10.1089/ars.2016.6664
Jia, G., Whaley-Connell, A., and Sowers, J. R. (2018). Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 61 (1), 21–28. doi:10.1007/s00125-017-4390-4
Jiang, J. R., Zou, C. D., Shu, H. L., and Zeng, Y. L. (1989). Assessment of absorption and distribution of artemether in rats using a thin layer chromatography scanning technique. Zhongguo Yaoli Xuebao 10 (5), 431–434.
Jiang, W., Cen, Y., Song, Y., Li, P., Qin, R., Liu, C., et al. (2016). Artesunate attenuated progression of atherosclerosis lesion formation alone or combined with rosuvastatin through inhibition of pro-inflammatory cytokines and pro-inflammatory chemokines. Phytomedicine 23 (11), 1259–1266. doi:10.1016/j.phymed.2016.06.004
Jiang, Y. C., Wang, X. F., Xu, Y. Y., Qiao, Y. H., Guo, X., Wang, D. F., et al. (2018). Polycaprolactone nanofibers containing vascular endothelial growth factor-encapsulated gelatin particles enhance mesenchymal stem cell differentiation and angiogenesis of endothelial cells. Biomacromolecules 19 (9), 3747–3753. doi:10.1021/acs.biomac.8b00870
Jiang, Y., Du, H., Liu, X., Fu, X., Li, X., and Cao, Q. (2020). Artemisinin alleviates atherosclerotic lesion by reducing macrophage inflammation via regulation of AMPK/NF-κB/NLRP3 inflammasomes pathway. J. Drug Target. 28 (1), 70–79. doi:10.1080/1061186x.2019.1616296
Johnson, J. D., and Luciani, D. S. (2010). Mechanisms of pancreatic beta-cell apoptosis in diabetes and its therapies. Adv. Exp. Med. Biol. 654, 447–462. doi:10.1007/978-90-481-3271-3_19
Kamari, Y., Shaish, A., Vax, E., Shemesh, S., Kandel-Kfir, M., Arel, Y., et al. (2011). Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J. Hepatol. 55 (5), 1086–1094. doi:10.1016/j.jhep.2011.01.048
Kannel, W. B., Hjortland, M., and Castelli, W. P. (1974). Role of diabetes in congestive heart failure: the Framingham study. Am. J. Cardiol. 34 (1), 29–34. doi:10.1016/0002-9149(74)90089-7
Karbwang, J., Na-Bangchang, K., Congpuong, K., Molunto, P., and Thanavibul, A. (1997). Pharmacokinetics and bioavailability of oral and intramuscular artemether. Eur. J. Clin. Pharmacol. 52 (4), 307–310. doi:10.1007/s002280050295
Kelly, K. J., Liu, Y., Zhang, J., Goswami, C., Lin, H., and Dominguez, J. H. (2013). Comprehensive genomic profiling in diabetic nephropathy reveals the predominance of proinflammatory pathways. Physiol. Genom. 45 (16), 710–719. doi:10.1152/physiolgenomics.00028.2013
Kim, J. Y., Bacha, F., Tfayli, H., Michaliszyn, S. F., Yousuf, S., and Arslanian, S. (2019). Adipose tissue insulin resistance in youth on the spectrum from normal weight to obese and from normal glucose tolerance to impaired glucose tolerance to type 2 diabetes. Diabetes Care 42 (2), 265–272. doi:10.2337/dc18-1178
Kim, K. E., Ko, K. H., Heo, R. W., Yi, C. O., Shin, H. J., Kim, J. Y., et al. (2016). Artemisia annua leaf extract attenuates hepatic steatosis and inflammation in high-fat diet-fed mice. J. Med. Food. 19 (3), 290–299. doi:10.1089/jmf.2015.3527
Kitada, M., and Koya, D. (2013). SIRT1 in type 2 diabetes: mechanisms and therapeutic potential. Diabetes Metab. J 37 (5), 315–325. doi:10.4093/dmj.2013.37.5.315
Kitada, M., Ogura, Y., Monno, I., and Koya, D. (2017). Regulating autophagy as a therapeutic target for diabetic nephropathy. Curr. Diabetes Rep. 17 (7), 53. doi:10.1007/s11892-017-0879-y
Klayman, D. L. (1985). Qinghaosu (artemisinin): an antimalarial drug from China. Science 228 (4703), 1049–1055. doi:10.1126/science.3887571
Kowluru, R. A., Kowluru, A., Mishra, M., and Kumar, B. (2015). Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 48, 40–61. doi:10.1016/j.preteyeres.2015.05.001
Krishna, S., Planche, T., Agbenyega, T., Woodrow, C., Agranoff, D., Bedu-Addo, G., et al. (2001). Bioavailability and preliminary clinical efficacy of intrarectal artesunate in Ghanaian children with moderate malaria. Antimicrob. Agents Chemother. 45 (2), 509–516. doi:10.1128/aac.45.2.509-516.2001
Lai, C. S., Tsai, M. L., Badmaev, V., Jimenez, M., Ho, C. T., and Pan, M. H. (2012). Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARγ and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. J. Agric. Food Chem. 60 (4), 1094–1101. doi:10.1021/jf204862d
Lee, J. H., Song, M. Y., Song, E. K., Kim, E. K., Moon, W. S., Han, M. K., et al. (2009). Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes 58 (2), 344–351. doi:10.2337/db07-1795
Lee, J., Kim, M. H., Lee, J. H., Jung, E., Yoo, E. S., and Park, D. (2012a). Artemisinic acid is a regulator of adipocyte differentiation and C/EBP δ expression. J. Cell. Biochem. 113 (7), 2488–2499. doi:10.1002/jcb.24124
Lee, J., Zhang, G., Wu, X., Xu, F., Zhou, J., and Zhang, X. (2012b). Growth inhibitory effect of dihydroartemisinin on Bcr/Abl+ chronic myeloid leukemia K562 cells involve AKT, ERK and NF-κB modulation. J. Canc. Res. Clin. Oncol. 138 (12), 2095–2102. doi:10.1007/s00432-012-1292-1
Lee, K. P., Park, E. S., Kim, D. E., Park, I. S., Kim, J. T., and Hong, H. (2014). Artemisinin attenuates platelet-derived growth factor BB-induced migration of vascular smooth muscle cells. Nutr Res Pract 8 (5), 521–525. doi:10.4162/nrp.2014.8.5.521
Lehrke, M., and Lazar, M. A. (2005). The many faces of PPARgamma. Cell 123 (6), 993–999. doi:10.1016/j.cell.2005.11.026
Li, C., Feng, X., Wen, X., Li, Y., Liu, B., Hu, J., et al. (2019a). A pilot clinical study of intravitreal injection of artesunate for ocular neovascularization. J. Ocul. Pharmacol. Therapeut. 35 (5), 283–290. doi:10.1089/jop.2018.0097
Li, J., Casteels, T., Frogne, T., Ingvorsen, C., Honoré, C., Courtney, M., et al. (2017). Artemisinins target GABA(A) receptor signaling and impair α cell identity. Cell 168 (1-2), 86–100.e15. doi:10.1016/j.cell.2016.11.010
Li, J. L., Cao, X. R., Wang, Y., Chen, Z. X., Hei, N. H., and Dong, B. (2016). Effects of artemisinin on cardiac function and fibrosis in diabetic cardiomyopathy rats. Shanghai J. Tradit. Chin. Med. 50, 45–48.
Li, M., van Esch, B. C. A. M., Wagenaar, G. T. M., Garssen, J., Folkerts, G., and Henricks, P. A. J. (2018). Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 831, 52–59. doi:10.1016/j.ejphar.2018.05.003
Li, Q. G., Brueckner, R. P., Peggins, J. O., Trotman, K. M., and Brewer, T. G. (1999). Arteether toxicokinetics and pharmacokinetics in rats after 25 mg/kg/day single and multiple doses. Eur. J. Drug Metab. Pharmacokinet. 24 (3), 213–223. doi:10.1007/bf03190023
Li, Q., and Hickman, M. (2011). Toxicokinetic and toxicodynamic (TK/TD) evaluation to determine and predict the neurotoxicity of artemisinins. Toxicology 279 (1–3), 1–9. doi:10.1016/j.tox.2010.09.005
Li, Q., and Weina, P. (2010). Artesunate: the best drug in the treatment of severe and complicated malaria. Pharmaceuticals 3 (7), 2322–2332. doi:10.3390/ph3072322
Li, S., Chaudhary, S. C., Zhao, X., Gaur, U., Fang, J., Yan, F., et al. (2019b). Artemisinin protects human retinal pigmented epithelial cells against hydrogen peroxide-induced oxidative damage by enhancing the activation of AMP-active protein kinase. Int. J. Biol. Sci. 15 (9), 2016–2028. doi:10.7150/ijbs.30536
Li, S., Zhao, X., Lazarovici, P., and Zheng, W. (2019c). Artemether activation of AMPK/GSK3β(ser9)/Nrf2 signaling confers neuroprotection towards β-amyloid-induced neurotoxicity in 3xTg Alzheimer’s mouse model. Oxid. Med. Cell Longev. 2019, 1862437. doi:10.1155/2019/1862437
Li, W., Zhang, S., Liu, H., Wang, L., Zhang, C., Leng, J., et al. (2014). Different associations of diabetes with β-cell dysfunction and insulin resistance among obese and nonobese Chinese women with prior gestational diabetes mellitus. Diabetes Care 37 (9), 2533–2539. doi:10.2337/dc14-0573
Li, X., Li, T. T., Zhang, X. H., Hou, L. F., Yang, X. Q., Zhu, F. H., et al. (2013). Artemisinin analogue SM934 ameliorates murine experimental autoimmune encephalomyelitis through enhancing the expansion and functions of regulatory T cell. PLoS One 8 (8), e74108. doi:10.1371/journal.pone.0074108
Li, Z., Shi, X., Liu, J., Shao, F., Huang, G., Zhou, Z., et al. (2019d). Artesunate prevents type 1 diabetes in NOD mice mainly by inducing protective IL-4-producing T cells and regulatory T cells. Faseb. J. 33 (7), 8241–8248. doi:10.1096/fj.201900146R
Linhart, H. G., Ishimura-Oka, K., DeMayo, F., Kibe, T., Repka, D., Poindexter, B., et al. (2001). C/EBPalpha is required for differentiation of white, but not brown, adipose tissue. Proc. Natl. Acad. Sci. U. S. A. 98 (22), 12532–12537. doi:10.1073/pnas.211416898
Liu, Y. W., Chen, C. C., Wang, J. M., Chang, W. C., Huang, Y. C., Chung, S. Y., et al. (2007). Role of transcriptional factors Sp1, c-Rel, and c-Jun in LPS-induced C/EBPdelta gene expression of mouse macrophages. Cell. Mol. Life Sci. 64 (24), 3282–3294. doi:10.1007/s00018-007-7375-5
Liu, Y., Yang, Y., Lei, Y., Yang, L., Zhang, X., Yuan, J., et al. (2020). Effects of dihydroartemisinin on the gut microbiome of mice. Mol. Med. Rep. 22 (2), 707–714. doi:10.3892/mmr.2020.11165
Lu, P., Zhang, F. C., Qian, S. W., Li, X., Cui, Z. M., Dang, Y. J., et al. (2016). Artemisinin derivatives prevent obesity by inducing browning of WAT and enhancing BAT function. Cell Res. 26 (10), 1169–1172. doi:10.1038/cr.2016.108
Luo, Y., Che, M. J., Liu, C., Liu, H. G., Fu, X. W., and Hou, Y. P. (2018). Toxicity and related mechanisms of dihydroartemisinin on porcine oocyte maturation in vitro. Toxicol. Appl. Pharmacol. 341, 8–15. doi:10.1016/j.taap.2018.01.002
Maggs, J. L., Bishop, L. P., Edwards, G., O’Neill, P. M., Ward, S. A., Winstanley, P. A., et al. (2000). Biliary metabolites of beta-artemether in rats: biotransformations of an antimalarial endoperoxide. Drug Metab. Dispos. 28 (2), 209–217
Maggs, J. L., Madden, S., Bishop, L. P., O’Neill, P. M., and Park, B. K. (1997). The rat biliary metabolites of dihydroartemisinin, an antimalarial endoperoxide. Drug Metab. Dispos. 25 (10), 1200–1204.
Marquina-Sanchez, B., Fortelny, N., Farlik, M., Vieira, A., Collombat, P., Bock, C., et al. (2020). Single-cell RNA-seq with spike-in cells enables accurate quantification of cell-specific drug effects in pancreatic islets. Genome Biol. 21 (1), 106. doi:10.1186/s13059-020-02006-2
Marrano, N., Biondi, G., Cignarelli, A., Perrini, S., Laviola, L., Giorgino, F., et al. (2020). Functional loss of pancreatic islets in type 2 diabetes: how can we halt it?. Metabolism 110, 154304. doi:10.1016/j.metabol.2020.154304
Maruthur, N. M., Tseng, E., Hutfless, S., Wilson, L. M., Suarez-Cuervo, C., Berger, Z., et al. (2016). Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann. Intern. Med. 164 (11), 740–751. doi:10.7326/m15-2650
Matsuoka, T. A., Kawashima, S., Miyatsuka, T., Sasaki, S., Shimo, N., Katakami, N., et al. (2017). Mafa enables Pdx1 to effectively convert pancreatic islet progenitors and committed islet α-cells into β-cells in vivo. Diabetes 66 (5), 1293–1300. doi:10.2337/db16-0887
Miyazawa-Hoshimoto, S., Takahashi, K., Bujo, H., Hashimoto, N., Yagui, K., and Saito, Y. (2005). Roles of degree of fat deposition and its localization on VEGF expression in adipocytes. Am. J. Physiol. Endocrinol. Metab. 288 (6), E1128–E1136. doi:10.1152/ajpendo.00003.2004
Monteiro, J. P., and Cano, M. I. (2011). SIRT1 deacetylase activity and the maintenance of protein homeostasis in response to stress: an overview. Protein Pept. Lett. 18 (2), 167–173. doi:10.2174/092986611794475039
Moore, K. J., Koplev, S., Fisher, E. A., Tabas, I., Björkegren, J. L. M., Doran, A. C., et al. (2018). Macrophage trafficking, inflammatory resolution, and genomics in atherosclerosis: JACC macrophage in CVD series (Part 2). J. Am. Coll. Cardiol. 72 (18), 2181–2197. doi:10.1016/j.jacc.2018.08.2147
Morris, C. A., Duparc, S., Borghini-Fuhrer, I., Jung, D., Shin, C. S., and Fleckenstein, L. (2011). Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar. J. 10, 263. doi:10.1186/1475-2875-10-263
Morris, C. A., Tan, B., Duparc, S., Borghini-Fuhrer, I., Jung, D., Shin, C. S., et al. (2013). Effects of body size and gender on the population pharmacokinetics of artesunate and its active metabolite dihydroartemisinin in pediatric malaria patients. Antimicrob. Agents Chemother. 57 (12), 5889–5900. doi:10.1128/aac.00635-13
Morrison, R. F., and Farmer, S. R. (2000). Hormonal signaling and transcriptional control of adipocyte differentiation. J. Nutr. 130 (12), 3116s–3121s. doi:10.1093/jn/130.12.3116S
Mukhi, D., Nishad, R., Menon, R. K., and Pasupulati, A. K. (2017). Novel actions of growth hormone in podocytes: implications for diabetic nephropathy. Front. Med. 4, 102. doi:10.3389/fmed.2017.00102
Shuba, N., and Karan, (2012). Assessment of the cognitive status in diabetes mellitus. J. Clin. Diagn. Res. 6 (10), 1658–1662. doi:10.7860/jcdr/2012/4837.2649
Navaratnam, V., Mansor, S. M., Sit, N. W., Grace, J., Li, Q., and Olliaro, P. (2000). Pharmacokinetics of artemisinin-type compounds. Clin. Pharmacokinet. 39 (4), 255–270. doi:10.2165/00003088-200039040-00002
Nichols, G. A., Gullion, C. M., Koro, C. E., Ephross, S. A., and Brown, J. B. (2004). The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 27 (8), 1879–1884. doi:10.2337/diacare.27.8.1879
Nie, H., Su, K., Long, Y., Jiang, S. S., and Sun, Q. (2015). Effect of artesunate on expressions of Toll-like receptor 4 and interleukin-8 in renal tissues of diabetic nephropathy rats. Tianjin Med. J. 43, 356–360. doi:10.3892/mmr.2017.7362
Nishimura, W., Takahashi, S., and Yasuda, K. (2015). MafA is critical for maintenance of the mature beta cell phenotype in mice. Diabetologia 58 (3), 566–574. doi:10.1007/s00125-014-3464-9
Niu, X. Y., Ho, L. Y., Ren, Z. H., and Song, Z. Y. (1985). Metabolic fate of Qinghaosu in rats; a new TLC densitometric method for its determination in biological material. Eur. J. Drug Metab. Pharmacokinet. 10 (1), 55–59. doi:10.1007/bf03189697
O’Neill, P. M., and Posner, G. H. (2004). A medicinal chemistry perspective on artemisinin and related endoperoxides. J. Med. Chem. 47 (12), 2945–2964. doi:10.1021/jm030571c
Okayasu, T., Tomizawa, A., Suzuki, K., Manaka, K., and Hattori, Y. (2008). PPARalpha activators upregulate eNOS activity and inhibit cytokine-induced NF-kappaB activation through AMP-activated protein kinase activation. Life Sci. 82 (15–16), 884–891. doi:10.1016/j.lfs.2008.02.002
Okorji, U. P., and Olajide, O. A. (2014). A semi-synthetic derivative of artemisinin, artesunate inhibits prostaglandin E2 production in LPS/IFNγ-activated BV2 microglia. Bioorg. Med. Chem. 22 (17), 4726–4734. doi:10.1016/j.bmc.2014.07.007
Okorji, U. P., Velagapudi, R., El-Bakoush, A., Fiebich, B. L., and Olajide, O. A. (2016). Antimalarial drug artemether inhibits neuroinflammation in BV2 microglia through nrf2-dependent mechanisms. Mol. Neurobiol. 53 (9), 6426–6443. doi:10.1007/s12035-015-9543-1
Olefsky, J. M., and Glass, C. K. (2010). Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246. doi:10.1146/annurev-physiol-021909-135846
Ott, A., Stolk, R. P., van Harskamp, F., Pols, H. A., Hofman, A., and Breteler, M. M. (1999). Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology 53 (9), 1937–1942. doi:10.1212/wnl.53.9.1937
Ouchi, N., Kihara, S., Arita, Y., Okamoto, Y., Maeda, K., Kuriyama, H., et al. (2000). Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 102 (11), 1296–1301. doi:10.1161/01.cir.102.11.1296
Petersen, M. C., and Shulman, G. I. (2018). Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98 (4), 2133–2223. doi:10.1152/physrev.00063.2017
Punthakee, Z., Goldenberg, R., and Katz, P. (2018). Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabetes 42 (1), S10–s15. doi:10.1016/j.jcjd.2017.10.003
Purwana, I., Zheng, J., Li, X., Deurloo, M., Son, D. O., Zhang, Z., et al. (2014). GABA promotes human β-cell proliferation and modulates glucose homeostasis. Diabetes 63 (12), 4197–4205. doi:10.2337/db14-0153
Rabinovitch, R. C., Samborska, B., Faubert, B., Ma, E. H., Gravel, S. P., Andrzejewski, S., et al. (2017). AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 21 (1), 1–9. doi:10.1016/j.celrep.2017.09.026
Reglero-Real, N., Colom, B., Bodkin, J. V., and Nourshargh, S. (2016). Endothelial cell junctional adhesion molecules: role and regulation of expression in inflammation. Arterioscler. Thromb. Vasc. Biol. 36 (10), 2048–2057. doi:10.1161/atvbaha.116.307610
Reilly, S. M., and Saltiel, A. R. (2017). Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 13 (11), 633–643. doi:10.1038/nrendo.2017.90
Rosen, E. D., and MacDougald, O. A. (2006). Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7 (12), 885–896. doi:10.1038/nrm2066
Rotter, V., Nagaev, I., and Smith, U. (2003). Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 278 (46), 45777–45784. doi:10.1074/jbc.M301977200
Rudnicki, M., Beckers, A., Neuwirt, H., and Vandesompele, J. (2015). RNA expression signatures and posttranscriptional regulation in diabetic nephropathy. Nephrol. Dial. Transplant. 30 (4), 35–42. doi:10.1093/ndt/gfv079
Saedi, E., Gheini, M. R., Faiz, F., and Arami, M. A. (2016). Diabetes mellitus and cognitive impairments. World J. Diabetes 7 (17), 412–422. doi:10.4239/wjd.v7.i17.412
Saltiel, A. R., and Olefsky, J. M. (2017). Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 127 (1), 1–4. doi:10.1172/jci92035
Schauer, P. R., Mingrone, G., Ikramuddin, S., and Wolfe, B. (2016). Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 39 (6), 902–911. doi:10.2337/dc16-0382
Schena, F. P., and Gesualdo, L. (2005). Pathogenetic mechanisms of diabetic nephropathy. J. Am. Soc. Nephrol. 16 (1), S30–S33. doi:10.1681/asn.2004110970
Shao, B. Z., Han, B. Z., Zeng, Y. X., Su, D. F., and Liu, C. (2016). The roles of macrophage autophagy in atherosclerosis. Acta Pharmacol. Sin. 37 (2), 150–156. doi:10.1038/aps.2015.87
Sharaf El Din, U. A. A., Salem, M. M., and Abdulazim, D. O. (2017). Diabetic nephropathy: time to withhold development and progression—a review. J. Adv. Res. 8 (4), 363–373. doi:10.1016/j.jare.2017.04.004
Sharma, B. N., Marschall, M., and Rinaldo, C. H. (2014). Antiviral effects of artesunate on JC polyomavirus replication in COS-7 cells. Antimicrob. Agents Chemother. 58 (11), 6724–6734. doi:10.1128/aac.03714-14
Shelbaya, S., Abu Shady, M. M., Nasr, M. S., Bekhet, M. M., Mageed, Y. A., and Abbas, M. (2018). Study of irisin hormone level in type 2 diabetic patients and patients with diabetic nephropathy. Curr. Diabetes Rev. 14 (5), 481–486. doi:10.2174/1573399813666170829163442
Sheng, J., Li, H., Dai, Q., Lu, C., Xu, M., Zhang, J., et al. (2018). NR4A1 promotes diabetic nephropathy by activating mff-mediated mitochondrial fission and suppressing parkin-mediated mitophagy. Cell. Physiol. Biochem. 48 (4), 1675–1693. doi:10.1159/000492292
Shi, J. Q., Zhang, C. C., Sun, X. L., Cheng, X. X., Wang, J. B., Zhang, Y. D., et al. (2013). Antimalarial drug artemisinin extenuates amyloidogenesis and neuroinflammation in APPswe/PS1dE9 transgenic mice via inhibition of nuclear factor-κB and NLRP3 inflammasome activation. CNS Neurosci. Ther. 19 (4), 262–268. doi:10.1111/cns.12066
Shi, Z., Chen, Y., Lu, C., Dong, L. M., Lv, J. W., Tuo, Q. H., et al. (2018). Resolving neuroinflammation, the therapeutic potential of the anti-malaria drug family of artemisinin. Pharmacol. Res. 136, 172–180. doi:10.1016/j.phrs.2018.09.002
Shimobayashi, M., Albert, V., Woelnerhanssen, B., Frei, I. C., Weissenberger, D., Meyer-Gerspach, A. C., et al. (2018). Insulin resistance causes inflammation in adipose tissue. J. Clin. Invest. 128 (4), 1538–1550. doi:10.1172/jci96139
Shoelson, S. E., Lee, J., and Goldfine, A. B. (2006). Inflammation and insulin resistance. J. Clin. Invest. 116 (7), 1793–1801. doi:10.1172/jci29069
Singh, S., Giri, A., and Giri, S. (2015). The antimalarial agent artesunate causes sperm DNA damage and hepatic antioxidant defense in mice. Mutat. Res. Genet. Toxicol. Environ. Mutagen 777, 1–6. doi:10.1016/j.mrgentox.2014.11.001
Soltani, N., Qiu, H., Aleksic, M., Glinka, Y., Zhao, F., Liu, R., et al. (2011). GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. U. S. A. 108 (28), 11692–11697. doi:10.1073/pnas.1102715108
Sopasakis, V. R., Sandqvist, M., Gustafson, B., Hammarstedt, A., Schmelz, M., Yang, X., et al. (2004). High local concentrations and effects on differentiation implicate interleukin-6 as a paracrine regulator. Obes. Res. 12 (3), 454–460. doi:10.1038/oby.2004.51
Spijker, H. S., Ravelli, R. B., Mommaas-Kienhuis, A. M., van Apeldoorn, A. A., Engelse, M. A., Zaldumbide, A., et al. (2013). Conversion of mature human β-cells into glucagon-producing α-cells. Diabetes 62 (7), 2471–2480. doi:10.2337/db12-1001
Steven, S., Hollingsworth, K. G., Al-Mrabeh, A., Avery, L., Aribisala, B., Caslake, M., et al. (2016). Very low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders. Diabetes Care 39 (5), 808–815. doi:10.2337/dc15-1942
Stitt, A. W., Curtis, T. M., Chen, M., Medina, R. J., McKay, G. J., Jenkins, A., et al. (2016). The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 51, 156–186. doi:10.1016/j.preteyeres.2015.08.001
Sun, C., and Zhou, B. (2017). The antimalarial drug artemisinin induces an additional, Sod1-supressible anti-mitochondrial action in yeast. Biochim. Biophys. Acta Mol. Cell Res. 1864 (7), 1285–1294. doi:10.1016/j.bbamcr.2017.04.014
Sun, Z., Ma, Y., Chen, F., Wang, S., Chen, B., and Shi, J. (2018). Artesunate ameliorates high glucose-induced rat glomerular mesangial cell injury by suppressing the TLR4/NF-κB/NLRP3 inflammasome pathway. Chem. Biol. Interact. 293, 11–19. doi:10.1016/j.cbi.2018.07.011
Talchai, C., Xuan, S., Lin, H. V., Sussel, L., and Accili, D. (2012). Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150 (6), 1223–1234. doi:10.1016/j.cell.2012.07.029
Tan, G. S., Cheung, N., Simó, R., Cheung, G. C., and Wong, T. Y. (2017). Diabetic macular oedema. Lancet Diabetes Endocrinol 5 (2), 143–155. doi:10.1016/s2213-8587(16)30052-3
Tan, K. T., and Cheah, J. S. (1990). Pathogenesis of type 1 and type 2 diabetes mellitus. Ann. Acad. Med. Singapore 19 (4), 506–511
Tanabe, K., Maeshima, Y., Sato, Y., and Wada, J. (2017). Antiangiogenic therapy for diabetic nephropathy. BioMed Res. Int. 2017, 5724069. doi:10.1155/2017/5724069
Tang, Y., Zhang, M. J., Hellmann, J., Kosuri, M., Bhatnagar, A., and Spite, M. (2013). Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes 62 (2), 618–627. doi:10.2337/db12-0684
Tateya, S., Kim, F., and Tamori, Y. (2013). Recent advances in obesity-induced inflammation and insulin resistance. Front. Endocrinol. 4, 93. doi:10.3389/fendo.2013.00093
Teja-Isavadharm, P., Siriyanonda, D., Siripokasupkul, R., Apinan, R., Chanarat, N., Lim, A., et al. (2010). A simplified liquid chromatography-mass spectrometry assay for artesunate and dihydroartemisinin, its metabolite, in human plasma. Molecules 15 (12), 8747–8768. doi:10.3390/molecules15128747
Teodoro, J. S., Nunes, S., Rolo, A. P., Reis, F., and Palmeira, C. M. (2018). Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front. Physiol. 9, 1857. doi:10.3389/fphys.2018.01857
Tesch, G. H. (2017). Diabetic nephropathy - is this an immune disorder?. Clin. Sci. (Lond.) 131 (16), 2183–2199. doi:10.1042/cs20160636
Thomas, C. G., Ward, S. A., and Edwards, G. (1992). Selective determination, in plasma, of artemether and its major metabolite, dihydroartemisinin, by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr 583 (1), 131–136. doi:10.1016/0378-4347(92)80355-t
Thorel, F., Népote, V., Avril, I., Kohno, K., Desgraz, R., Chera, S., et al. (2010). Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 464 (7292), 1149–1154. doi:10.1038/nature08894
Toda, G., Soeda, K., Okazaki, Y., Kobayashi, N., Masuda, Y., Arakawa, N., et al. (2020). Insulin- and lipopolysaccharide-mediated signaling in adipose tissue macrophages regulates postprandial glycemia through akt-mTOR activation. Mol. Cell 79, 43–53.e4, doi:10.1016/j.molcel.2020.04.033
Trujillo, M. E., and Scherer, P. E. (2005). Adiponectin—journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J. Intern. Med. 257 (2), 167–175. doi:10.1111/j.1365-2796.2004.01426.x
Tu, K., Zheng, X., Yin, G., Zan, X., Yao, Y., and Liu, Q. (2012). Evaluation of Fbxw7 expression and its correlation with expression of SREBP-1 in a mouse model of NAFLD. Mol. Med. Rep. 6 (3), 525–530. doi:10.3892/mmr.2012.953
Unger, R. H., and Orci, L. (2010). Paracrinology of islets and the paracrinopathy of diabetes. Proc. Natl. Acad. Sci. U. S. A. 107 (37), 16009–16012. doi:10.1073/pnas.1006639107
Uwaezuoke, S. N. (2017). The role of novel biomarkers in predicting diabetic nephropathy: a review. Int J Nephrol Renovasc Dis 10, 221–231. doi:10.2147/ijnrd.S143186
van Agtmael, M. A., Gupta, V., van der Wösten, T. H., Rutten, J. P., and van Boxtel, C. J. (1999). Grapefruit juice increases the bioavailability of artemether. Eur. J. Clin. Pharmacol. 55 (5), 405–410. doi:10.1007/s002280050648
van Agtmael, M. A., Van Der Graaf, C. A., Dien, T. K., Koopmans, R. P., and van Boxtel, C. J. (1998). The contribution of the enzymes CYP2D6 and CYP2C19 in the demethylation of artemether in healthy subjects. Eur. J. Drug Metab. Pharmacokinet. 23 (3), 429–436. doi:10.1007/bf03192305
van der Meulen, T., Lee, S., Noordeloos, E., Donaldson, C. J., Adams, M. W., Noguchi, G. M., et al. (2018). Artemether does not turn α cells into β cells. Cell Metabol. 27 (1), 218–225.e4. doi:10.1016/j.cmet.2017.10.002
Verma, S., Das, P., and Kumar, V. L. (2017). Chemoprevention by artesunate in a preclinical model of colorectal cancer involves down regulation of β-catenin, suppression of angiogenesis, cellular proliferation and induction of apoptosis. Chem. Biol. Interact. 278, 84–91. doi:10.1016/j.cbi.2017.10.011
Vieira, A., Ben-Othman, N., and Collombat, P. (2017). GABA triggers pancreatic β-like cell neogenesis. Cell Cycle 16 (8), 727–728. doi:10.1080/15384101.2017.1302212
Visser, B. J., Wieten, R. W., Kroon, D., Nagel, I. M., Bélard, S., van Vugt, M., et al. (2014). Efficacy and safety of artemisinin combination therapy (ACT) for non-falciparum malaria: a systematic review. Malar. J. 13, 463. doi:10.1186/1475-2875-13-463
Volpe, C. M. O., Villar-Delfino, P. H., Dos Anjos, P. M. F., and Nogueira-Machado, J. A. (2018). Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 9 (2), 119. doi:10.1038/s41419-017-0135-z
Wang, D., Shi, J., Lv, S., Xu, W., Li, J., Ge, W., et al. (2015). Artesunate attenuates lipopolysaccharide-stimulated proinflammatory responses by suppressing TLR4, MyD88 expression, and NF-κB activation in microglial cells. Inflammation 38 (5), 1925–1932. doi:10.1007/s10753-015-0172-7
Wang, J., Zhang, J., Shi, Y., Xu, C., Zhang, C., Wong, Y. K., et al. (2017). Mechanistic investigation of the specific anticancer property of artemisinin and its combination with aminolevulinic acid for enhanced anticolorectal cancer activity. ACS Cent. Sci. 3 (7), 743–750. doi:10.1021/acscentsci.7b00156
Wang, L., Zhang, Z., Li, M., Wang, F., Jia, Y., Zhang, F., et al. (2019a). P53-dependent induction of ferroptosis is required for artemether to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. IUBMB Life 71 (1), 45–56. doi:10.1002/iub.1895
Wang, R., Zang, P., Chen, J., Wu, F., Zheng, Z., Ma, J., et al. (2018). Gut microbiota play an essential role in the antidiabetic effects of rhein. Evid Based Complement Alternat Med 2018, 6093282. doi:10.1155/2018/6093282
Wang, T., Lu, J., Shi, L., Chen, G., Xu, M., Xu, Y., et al. (2020). Association of insulin resistance and β-cell dysfunction with incident diabetes among adults in China: a nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol 8 (2), 115–124. doi:10.1016/s2213-8587(19)30425-5
Wang, W., and Lo, A. C. Y. (2018). Diabetic retinopathy: pathophysiology and treatments. Int. J. Mol. Sci. 19 (6), 1816. doi:10.3390/ijms19061816
Wang, W. N., Zhang, W. L., Zhou, G. Y., Ma, F. Z., Sun, T., Su, S. S., et al. (2016a). Prediction of the molecular mechanisms and potential therapeutic targets for diabetic nephropathy by bioinformatics methods. Int. J. Mol. Med. 37 (5), 1181–1188. doi:10.3892/ijmm.2016.2527
Wang, Y., Cao, J., Fan, Y., Xie, Y., Xu, Z., Yin, Z., et al. (2016b). Artemisinin inhibits monocyte adhesion to HUVECs through the NF-κB and MAPK pathways in vitro. Int. J. Mol. Med. 37 (6), 1567–1575. doi:10.3892/ijmm.2016.2579
Wang, Y., Han, P., Wang, M., Weng, W., Zhan, H., Yu, X., et al. (2019b). Artemether improves type 1 diabetic kidney disease by regulating mitochondrial function. Am J Transl Res 11 (6), 3879–3889
Wang, Y., Huang, Z. Q., Wang, C. Q., Wang, L. S., Meng, S., Zhang, Y. C., et al. (2011a). Artemisinin inhibits extracellular matrix metalloproteinase inducer (EMMPRIN) and matrix metalloproteinase-9 expression via a protein kinase Cδ/p38/extracellular signal-regulated kinase pathway in phorbol myristate acetate-induced THP-1 macrophages. Clin. Exp. Pharmacol. Physiol. 38 (1), 11–18. doi:10.1111/j.1440-1681.2010.05454.x
Wang, Y., Huang, Z., Wang, L., Meng, S., Fan, Y., Chen, T., et al. (2011b). The anti-malarial artemisinin inhibits pro-inflammatory cytokines via the NF-κB canonical signaling pathway in PMA-induced THP-1 monocytes. Int. J. Mol. Med. 27 (2), 233–241. doi:10.3892/ijmm.2010.580
Wang, Y. L., Wang, Z. J., Shen, H. L., Yin, M., and Tang, K. X. (2013). Effects of artesunate and ursolic acid on hyperlipidemia and its complications in rabbit. Eur. J. Pharmaceut. Sci. 50 (3-4), 366–371. doi:10.1016/j.ejps.2013.08.003
Wang, Z., Wang, Z., Zhou, Z., and Ren, Y. (2016c). Crucial genes associated with diabetic nephropathy explored by microarray analysis. BMC Nephrol. 17 (1), 128. doi:10.1186/s12882-016-0343-2
Wei, T., and Liu, J. (2017). Anti-angiogenic properties of artemisinin derivatives (review). Int. J. Mol. Med. 40 (4), 972–978. doi:10.3892/ijmm.2017.3085
Wellen, K. E., and Hotamisligil, G. S. (2003). Obesity-induced inflammatory changes in adipose tissue. J. Clin. Invest. 112 (12), 1785–1788. doi:10.1172/jci20514
White, N. J., van Vugt, M., and Ezzet, F. (1999). Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clin. Pharmacokinet. 37 (2), 105–125. doi:10.2165/00003088-199937020-00002
Wilcox, C. L., Terry, N. A., Walp, E. R., Lee, R. A., and May, C. L. (2013). Pancreatic α-cell specific deletion of mouse Arx leads to α-cell identity loss. PLoS One 8 (6), e66214. doi:10.1371/journal.pone.0066214
Wong, R. H., Scholey, A., and Howe, P. R. (2014). Assessing premorbid cognitive ability in adults with type 2 diabetes mellitus—a review with implications for future intervention studies. Curr. Diabetes Rep. 14 (11), 547. doi:10.1007/s11892-014-0547-4
Wong, T. Y., Cheung, C. M., Larsen, M., Sharma, S., and Simó, R. (2016). Diabetic retinopathy. Nat Rev Dis Primers 2, 16012. doi:10.1038/nrdp.2016.12
Wu, H., and Ballantyne, C. M. (2020). Metabolic inflammation and insulin resistance in obesity. Circ. Res. 126 (11), 1549–1564. doi:10.1161/circresaha.119.315896
Wu, M. Y., Yiang, G. T., Lai, T. T., and Li, C. J. (2018a). The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy. Oxid. Med. Cell Longev. 2018, 3420187. doi:10.1155/2018/3420187
Wu, R., Zhao, D., An, R., Wang, Z., Li, Y., Shi, B., et al. (2019). Linggui zhugan formula improves glucose and lipid levels and alters gut microbiota in high-fat diet-induced diabetic mice. Front. Physiol. 10, 918. doi:10.3389/fphys.2019.00918
Wu, T., Qiao, S., Shi, C., Wang, S., and Ji, G. (2018b). Metabolomics window into diabetic complications. J Diabetes Investig 9 (2), 244–255. doi:10.1111/jdi.12723
Wu, Y. B., Zhang, L., Li, W. T., Yang, Y., and Zhao, J. M. (2016). Artesunate restores spatial learning of rats with hepatic encephalopathy by inhibiting ammonia-induced oxidative damage in neurons and dysfunction of glutamate signaling in astroglial cells. Biomed. Pharmacother. 84, 972–978. doi:10.1016/j.biopha.2016.09.104
Xiang, M., Chen, Z., He, L., Xiong, G., and Lu, J. (2019). Transcription profiling of artemisinin-treated diabetic nephropathy rats using high-throughput sequencing. Life Sci. 219, 353–363. doi:10.1016/j.lfs.2019.01.032
Xu, G., Huang, Y. L., Li, P. L., Guo, H. M., and Han, X. P. (2017a). Neuroprotective effects of artemisinin against isoflurane-induced cognitive impairments and neuronal cell death involve JNK/ERK1/2 signalling and improved hippocampal histone acetylation in neonatal rats. J. Pharm. Pharmacol. 69 (6), 684–697. doi:10.1111/jphp.12704
Xu, G., Huang, Y. L., Li, P. L., Guo, H. M., and Han, X. P. (2017b). Neuroprotective effects of artemisinin against isoflurane-induced cognitive impairments and neuronal cell death involve JNK/ERK1/2 signalling and improved hippocampal histone acetylation in neonatal rats. Pharmacol 69, 684–697. doi:10.1111/jphp.12704
Xu, H., He, Y., Yang, X., Liang, L., Zhan, Z., Ye, Y., et al. (2007). Anti-malarial agent artesunate inhibits TNF-alpha-induced production of proinflammatory cytokines via inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology 46 (6), 920–926. doi:10.1093/rheumatology/kem014
Xu, J., Zhao, C., Huang, X., and Du, W. (2019). Regulation of artemisinin and its derivatives on the assembly behavior and cytotoxicity of amyloid polypeptides hIAPP and Aβ. ACS Chem. Neurosci. 10 (11), 4522–4534. doi:10.1021/acschemneuro.9b00385
Xu, L., Ma, X., Verma, N. K., Wang, D., Gavrilova, O., Proia, R. L., et al. (2018). Ablation of PPARγ in subcutaneous fat exacerbates age-associated obesity and metabolic decline. Aging Cell 17 (2), 12721. doi:10.1111/acel.12721
Xu, W., Caracciolo, B., Wang, H. X., Winblad, B., Bäckman, L., Qiu, C., et al. (2010). Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes 59 (11), 2928–2935. doi:10.2337/db10-0539
Yadav, A., Kataria, M. A., Saini, V., and Yadav, A. (2013). Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta 417, 80–84. doi:10.1016/j.cca.2012.12.007
Yan, F., Wang, H., Gao, Y., Xu, J., and Zheng, W. (2017). Artemisinin protects retinal neuronal cells against oxidative stress and restores rat retinal physiological function from light exposed damage. ACS Chem. Neurosci. 8 (8), 1713–1723. doi:10.1021/acschemneuro.7b00021
Yang, H., Zhang, W., Pan, H., Feldser, H. G., Lainez, E., Miller, C., et al. (2012). SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS One 7 (9), e46364. doi:10.1371/journal.pone.0046364
Yazıcı, D., and Sezer, H. (2017). Insulin resistance, obesity and lipotoxicity. Adv. Exp. Med. Biol. 960, 277–304. doi:10.1007/978-3-319-48382-5_12
Ye, L., Robertson, M. A., Hesselson, D., Stainier, D. Y., and Anderson, R. M. (2015). Glucagon is essential for alpha cell transdifferentiation and beta cell neogenesis. Development 142 (8), 1407–1417. doi:10.1242/dev.117911
Youyou, T., Muyun, N., Yurong, Z., Lanna, L., Shulian, G., Muqun, Z., et al. (2015). Studies on the constituents of artemisia annua L. Yao Xue Xue Bao 50 (10), 366–370.
Yu, L., Chen, J. F., Shuai, X., Xu, Y., Ding, Y., Zhang, J., et al. (2016). Artesunate protects pancreatic beta cells against cytokine-induced damage via SIRT1 inhibiting NF-κB activation. J. Endocrinol. Invest. 39 (1), 83–91. doi:10.1007/s40618-015-0328-1
Yuan, M., Konstantopoulos, N., Lee, J., Hansen, L., Li, Z. W., Karin, M., et al. (2001). Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293 (5535), 1673–1677. doi:10.1126/science.1061620
Zeng, Z., Xu, J., and Zheng, W. (2017). Artemisinin protects PC12 cells against β-amyloid-induced apoptosis through activation of the ERK1/2 signaling pathway. Redox Biol 12, 625–633. doi:10.1016/j.redox.2017.04.003
Zhang, B., Yue, R., Chen, Y., Yang, M., Huang, X., Shui, J., et al. (2019). Gut microbiota, a potential new target for Chinese herbal medicines in treating diabetes mellitus. Evid. Based Complement. Alternat Med. 2019, 2634898. doi:10.1155/2019/2634898
Zhang, D., Yang, H., Kong, X., Wang, K., Mao, X., Yan, X., et al. (2011). Proteomics analysis reveals diabetic kidney as a ketogenic organ in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 300 (2), E287–E295. doi:10.1152/ajpendo.00308.2010
Zhang, H., Qi, S., Song, Y., and Ling, C. (2020). Artemisinin attenuates early renal damage on diabetic nephropathy rats through suppressing TGF-β1 regulator and activating the Nrf2 signaling pathway. Life Sci. 256, 117966. doi:10.1016/j.lfs.2020.117966
Zhang, K., Liu, J., You, X., Kong, P., Song, Y., Cao, L., et al. (2016). P2X7 as a new target for chrysophanol to treat lipopolysaccharide-induced depression in mice. Neurosci. Lett. 613, 60–65. doi:10.1016/j.neulet.2015.12.043
Zhang, L., Su, Y., Zhou, F., Zhang, J., and An, Z. (2014a). Effect of Artemisinin on the upregulation of PDGF B protein expression in the kidney of experimental diabetic rats. Modern Journal of Integrated Traditional Chinese and Western Medicine 23, 1392–1393. doi:10. 3969/j. issn.1008 - 8849. 2014. 13. 009
Zhang, L., Zhang, J., Zhou, F., Su, Y., and An, Z. (2014b). Inhibitory effect of artemisinin on the spatiotemporal dynamics activation of protein kinase C in the kidney of experimental diabetic rats. Modern Journal of Integrated Traditional Chinese and Western Medicine 23, 1964–1966. doi:10.3969/j.issn.1008-8849.2014.18.010
Zhang, L., Zhou, F., Su, Y., Zhang, J., and An, Z. (2014c). Study on the mechanism of renoprotective effects of artemisinin in diabetic rats. Modern Journal of Integrated Traditional Chinese and Western Medicine 23, 2862–2863. doi:10.3969/j.issn.1008-8849.2014.26.005
Zhang, X., Zhao, Y., Zhang, M., Pang, X., Xu, J., Kang, C., et al. (2012). Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One 7 (8), e42529. doi:10.1371/journal.pone.0042529
Zhao, K. C., and Song, Z. Y. (1989). Distribution and excretion of artesunate in rats. Proc. Chin. Acad. Med. Sci. Peking Union Med. Coll. 4 (4), 186–188.
Zhao, X., Fang, J., Li, S., Gaur, U., Xing, X., Wang, H., et al. (2019). Artemisinin attenuated hydrogen peroxide (H2O2)-induced oxidative injury in SH-SY5Y and hippocampal neurons via the activation of AMPK pathway. Int. J. Mol. Sci. 20 (11), 2680. doi:10.3390/ijms20112680
Zheng, P., Li, Z., and Zhou, Z. (2018). Gut microbiome in type 1 diabetes: a comprehensive review. Diabetes Metab Res Rev 34 (7), e3043. doi:10.1002/dmrr.3043
Zhou, F., Su, Y., Zhang, L., Zhang, J., and An, Z. (2014a). Inhibitory effect of artemisinin on the upregulation of DNA-binding activity of NF-κB in kidney tissue of diabetic rats. Modern Journal of Integrated Traditional Chinese and Western Medicine 23, 2075–2076. doi:10.3969/j.issn.1008-8849.2014.19.009
Zhou, F., Zhang, J., Zhang, L., Su, Y., and An, Z. (2014b). Inhibitory effect of artemisinin on the upregulation of the DNA-binding activity of AP-1 in the kidney tissue of experimental diabetic rats. Modern Journal of Integrated Traditional Chinese and Western Medicine 23, 2651–2652. doi:10.3969/j.issn.1008-8849.2014.24.009
Zhou, F., Zhang, L., Su, Y., Zhang, J., and An, Z. (2014c). Inhibitory effect of artemisinin on the upregulation of c fos and c jun gene expression in kidney tissue of diabetic rats. Modern Journal of Integrated Traditional Chinese and Western Medicine 23, 2294–2295. doi:10.3969/j.issn.1008-8849.2014.21.005
Zhu, C., Xiong, Z., Chen, X., Peng, F., Hu, X., Chen, Y., et al. (2012). Artemisinin attenuates lipopolysaccharide-stimulated proinflammatory responses by inhibiting NF-κB pathway in microglia cells. PLoS One 7 (4), e35125. doi:10.1371/journal.pone.0035125
Zimmet, P., Alberti, K. G., Magliano, D. J., and Bennett, P. H. (2016). Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat. Rev. Endocrinol. 12 (10), 616–622. doi:10.1038/nrendo.2016.105
Zong, Y., Yuan, Y., Qian, X., Huang, Z., Yang, W., Lin, L., et al. (2016). Small molecular-sized artesunate attenuates ocular neovascularization via VEGFR2, PKCα, and PDGFR targets. Sci. Rep. 6, 30843. doi:10.1038/srep30843
Keywords: Artemisinin, artemisinin derivatives, type 2 diabetes, complications, therapy, pharmacology
Citation: Jiang Y-y, Shui J-c, Zhang B-x, Chin J-w and Yue R-s (2020) The Potential Roles of Artemisinin and Its Derivatives in the Treatment of Type 2 Diabetes Mellitus. Front. Pharmacol. 11:585487. doi: 10.3389/fphar.2020.585487
Received: 22 July 2020; Accepted: 13 October 2020;
Published: 26 November 2020.
Edited by:
Paula Gomes, University of Porto, PortugalReviewed by:
Zhihua Liao, Southwest University, ChinaPeilin Zheng, Shenzhen People’s Hospital, Jinan University, China
Copyright © 2020 Jiang, Shui, Zhang, Chin and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ren-song Yue, songrenyue@cdutcm.edu.cn
 Ya-yi Jiang
Ya-yi Jiang