
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 11 December 2020
Sec. Ethnopharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.584849
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease without effective and beneficial drugs. Many traditional folk medicines have been proven to be effective in treating RA. Among these, the root of Pterospermum heterophyllum Hance has been widely used as a traditional remedy against RA in China, but there is no scientific basis yet. The aim of this study was to investigate for the first time the chemical compositions and therapeutic effect of P. heterophyllum on adjuvant-induced arthritis (AIA) model in rats. 73 compounds were identified from P. heterophyllum based on ultra-performance liquid chromatography-quadrupole time-of-flight tandem mass spectrometry (UPLC-qTOF-MS/MS), and flavonoids may be partly responsible for the major anti-arthritic effect. In parallel, the P. heterophyllum extract at 160, 320, and 640 mg/kg/day were orally administered to rats for 22 days after post-administration adjuvant. The results showed that P. heterophyllum remarkably ameliorated histological lesions of the knee joint, increased body weight growth, decreased arthritis score, reduced thymus and spleen indices in model rats. Moreover, P. heterophyllum treatment persuasively downregulated the levels of rheumatoid factor (RF), C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, IL-17, cyclooxygenase-2 (COX-2), 5-lipoxygenase (5-LOX) and matrix metalloproteinase-2 (MMP-2), and observably upregulated IL-4 and IL-10 levels in model rats. These findings suggest that P. heterophyllum has a prominent anti-RA effect on AIA rats by modulating the inflammatory responses, and supports the traditional folk use of this plant.
Rheumatoid arthritis (RA) is a chronic, systematic and autoimmune inflammatory disease that results in progressive synovitis, joint swelling and damage, synovial hyperplasia, and bone and cartilage erosion (Yousefi et al., 2014; Saleem et al., 2020; Zhu et al., 2020). Although the etiology of RA is intricate and vague, inflammatory factors, including pro-inflammatory cytokines, anti-inflammatory cytokines and inflammatory mediators, are responsible for bone and cartilage erosions, and play a crucial role in this disease (Wang et al., 2017a; Rui et al., 2019; Saleem et al., 2020). Additionally, the serum levels of these inflammatory mediators were determined by enzyme-linked immunosorbent assay (ELISA) kits (Lin et al., 2013; Wang et al., 2017a). Currently, immunosuppressants, biological agents and disease-modifying anti-rheumatic drugs (DMARDs), and steroidal and non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used for the treatment of RA, but most of them display long-term adverse effects, toxicity and comorbidities (Dai et al., 2020; Li et al., 2019a, Li et al., 2019b; Rui et al., 2019). As a result of this, exploring effective and safe anti-RA drug candidates from natural products, especially traditional folk medicines, could be a momentous breakthrough.
Traditional Chinese medicines (TCMs) are decisive complementary and alternative medicines, which have been verified to be effective treating RA for centuries with more safety and little side-effects in China and other Southeast Asian countries (Bao et al., 2018; Li et al., 2019a, Li et al., 2019b; Lin et al., 2013; Jing et al., 2019; Yang et al., 2020). Pterospermum heterophyllum Hance is native only to China and widely distributed in Fujian, Guangdong, Guangxi and Hainan provinces, belonging to the Sterculiaceae family (Editorial Committee of Traditional Chinese Medicine 1999; Yang et al., 2016, 2019a). The root of P. heterophyllum is a vital TCM and has been used for centuries as an empiric treatment for RA and other inflammation-related diseases (Editorial Committee of Traditional Chinese Medicine 1999; Yang et al., 2016; Yang et al., 2019a). Despite good clinical practice and good clinical effects, the phytochemical profiling and anti-RA efficacy of P. heterophyllum are still unknown, leading to numerous obstacles in the clinical application and reasonable development of this plant.
Therefore, in this study, the AIA rat model was adopted to evaluate the therapeutic efficacy and underlying mechanisms of P. heterophyllum. Following this step, ultra-performance liquid chromatography-quadrupole time-of-flight tandem mass spectrometry (UPLC-qTOF-MS/MS) analysis was performed to explore the phytochemicals present in this plant. Our findings will provide adequate scientific evidence for the development and clinical application of P. heterophyllum.
Pentobarbital sodium (Shanghai Rongbai Biological Technology Co., Ltd., Shanghai, China), Complete Freund’s adjuvant (CFA) and Histopaque 1,083 (Sigma Co., USA), MTX (Shanghai Xingyi Pharmaceutical Co., China), TNF-α, IL-1β, IL-4, IL-6, IL-10, IL-17, COX-2, 5-LOX and MMP-2 ELISA kits (Chuzhou Shinuoda Biological Technology Co., China) were used in this experiment.
Plant materials of P. heterophyllum roots were collected from the town of Pulu, Lipu Country, Guilin City, Guangxi, China (GPS location: 110.51682262,911,989, 24.576018798,987,043), in October 2017, and was authenticated by professor Ronghua Liu. A voucher specimen (No. PH20171024) for P. heterophyllum root was deposited in the author’s laboratory.
The dried and powdered roots of P. heterophyllum (1.0 kg) were extracted with 95% EtOH (5 L × 3) and subsequently with 50% EtOH (5 L × 3) by maceration at room temperature for 7 days. The ethanol crude extract of P. heterophyllum roots was filtrated and evaporated to obtain a black residue (PH, 160 g), with a yield of 16.0%.
According to the TCM clinical practice (9–30 g/day) (Editorial Committee of Traditional Chinese Medicine 1999), the dosage of P. heterophyllum roots for rat was 0.8–2.7 g/kg/day (body weight). Thus, the dosages of PH for rat were 1.0 g/kg (equivalent to 160 mg/kg crude extract, low-dose), 2.0 g/kg (320 mg/kg, medium-dose) and 4.0 g/kg (640 mg/kg, high-dose) in this experiment. All these extracts were dissolved in 0.3% sodium carboxymethyl cellulose (CMC-Na) for oral administration.
The identification of phytochemicals in the ethanol crude extract of PH was carried out using UPLC-qTOF-MS/MS in a Shimadzu UHPLC System (Kyoto, Japan) coupled with an AB SCIEX Triple TOF™ 5600 + system (Foster City, CA, USA) (Yang et al., 2019b). The chromatographic separation was conducted in an ACQUITY UPLC®BEH C18 (100 × 2.1 mm, 1.7 μm) maintained at 35°C. 0.1% aqueous formic acid (v/v, A) and acetonitrile (B) were used as mobile phases. The gradient elution with the flow rate of 0.3 ml/min was performed as follows: 0–8 min 5–8% B; 8–12 min 8–8% B; 12–17 min 8–12% B; 17–28 min 12–35% B; 28–35 min 55–55% B; 35–45 min 55–95% B; 45–47 min 95–95% B; 47–47.1 min 95–5% B; 47.1–50.0 min 5–5% B. The sample inject volume was 3 μL.
Sprague-Dawley rats (weighing 160–180 g) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and housed in cages at a room temperature of 21–25°C with 12 light/dark reverse cycles.
After adaptive feeding, the rats were randomly assigned to six groups (n = 8): normal control (Control), AIA model (AIA), AIA model + MTX (AIA + MTX, 0.35 mg/kg), AIA model + PH low-dose (AIA + PH-L, 160 mg/kg), AIA model + PH medium-dose (AIA + PH-M, 320 mg/kg), and AIA model + PH high-dose (AIA + PH-H, 640 mg/kg). In accordance with the previous method, the AIA rat model was induced by a single intradermal injection of 100 μL CFA into the rat’s left hind footpad (day 1) (Yang et al., 2016; Pan et al., 2017). After the establishment of the AIA model, all PH crude extracts were administered orally once a day from day 7 to day 28. MTX was used as a positive drug and administered intragastrically (i.g.) twice a week. Meanwhile, the rats in the normal control group and the AIA model group were treated with an equal volume of 0.3% CMC-Na. The experimental protocol of PH effect on CFA-induced RA in rats was shown in Figure 1.
The body weight and arthritis score of rats were measured every 4 days. The arthritis scores of rat paws were evaluated using a 5-point scale (Yang et al., 2016; Jing et al., 2019): 0 = no erythema or swelling; 1 = erythema or toe joints swelling; 2 = toes and joints swelling; 3 = toes swelling and ankle joints swelling; 4 = the entire paw swelling and ankle joints swelling. The maximum arthritis score of each rat was set at 16 (4 points ×4 paws).
On day 29th day after immunization, all rats were killed after anesthesia (1% pentobarbital sodium, 40 mg/kg), and the immune organs including thymus and spleen were harvested and weighed. The index of thymus or spleen (mg/g) = thymus or spleen wet weight/body weight (Lin et al., 2013; Jing et al., 2019).
Blood was collected from the carotid artery of rats after being anesthetized. The peripheral blood mononuclear cells (PBMC) isolation process was performed according to the previous method (Lin et al., 2013). The serum levels of RF, CRP, TNF-α, IL-1β, IL-4, IL-6, IL-10, and IL-17, and the PBMC levels of COX-2, 5-LOX and MMP-2, were quantified by commercially available ELISA kits based on the manufacturer’s instructions (Chuzhou Shinuoda Biological Technology Co., China).
The ankle joints of the rats were removed and fixed in 4% (w/v) paraformaldehyde, decalcified in 10% ethylene-diamine-tetraacetic acid (EDTA) at 4°C for 30 days. Tissues were embedded in paraffin and 4 µm joint sections were obtained. Subsequently, the sections were deparaffinized, dehydrated and stained with hematoxylin and eosin (HE). These sections were examined with a DS-F12 microscope (magnification, ×100, Nikon Corporation, Japan) for histopathological analysis.
Graphpad Prism6 was used for statistical analysis, and the data were presented as the means ± standard deviation (SD). One-way analysis of variance (ANOVA) and Tukey’s test were used for comparison differences groups. Differences with p < 0.05 indicated statistical significance.
All the experiments were carried out in adherence with the guidelines of the Institutional Animal Care and Use Committee of China and were approved by the Animal Care and Research Committee of Jiangxi University of Traditional Chinese Medicine. All surgical procedures were performed under sodium pentobarbital anesthesia to minimize suffering.
The chemical constituents corresponding to the chromatographic peaks were determined by MS/MS analysis using negative- and positive-ion modes based on literature and databases (Yang et al., 2019b) (Figure 2).
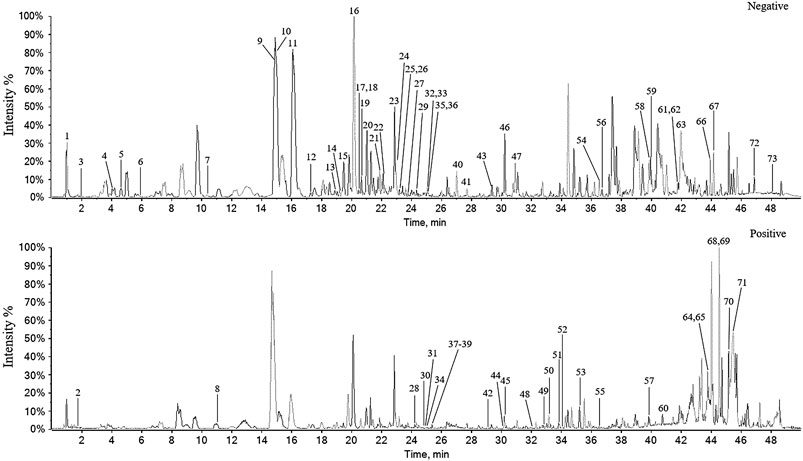
FIGURE 2. The base peak chromatogram of P. heterophyllum by UPLC-Q-TOF-MS/MS in negative and positive-ion modes.
As a result, a total of 73 compounds, including 34 flavonoids, eight fatty acids, seven triterpenoids, six steroids, six alkaloids, five phenylpropanoids, and seven others were identified from P. heterophyllum based on UPLC-qTOF-MS/MS (Table 1). Therefore, this study has greatly enriched the chemical constituents and diversity. Among them, 15 flavonoids, including procyanidin B2 (peak 9) (Wang et al., 2017b), dihydromyricetin (peak 10) (Chu et al., 2018), (-)-epicatechin (peak 11) (Osman et al., 2019), puerarin (peak 12) (Wang et al., 2016), rutin (peak 24) (Sun et al., 2017), naringin (peak 28) (Ahmad et al., 2014), hesperidin (peak 32) (Li et al., 2019a), myricetin (peak 35) (Yuan et al., 2015), eriodictyol (peak 40) (Lei et al., 2020), quercetin (peak 41) (Saccol et al., 2019), naringenin (peak 43) (Zhu et al., 2015), kaempferol (peak 44) (Pan et al., 2018), diosmetin (peak 46) (Chen et al., 2019), nobiletin (peak 51) (Li et al., 2019a), and tangeretin (peak 53) (Li et al., 2019c), have been reported to have arthritis inhibitory effect in rats. Additionally, cinnamaldehyde (peak 8, phenylpropanoid) (Mateen et al., 2019), ursolic acid (peak 65, triterpenoid) (Kim et al., 2018), linoleic acid (peak 67, fatty acid) (Wang et al., 2011) and emodin (peak 54, other) (Zhu et al., 2013) were also exhibited anti-arthritis activities in vivo. Consequently, flavonoids may be responsible for the major active constituents in the roots of P. heterophyllum against RA.
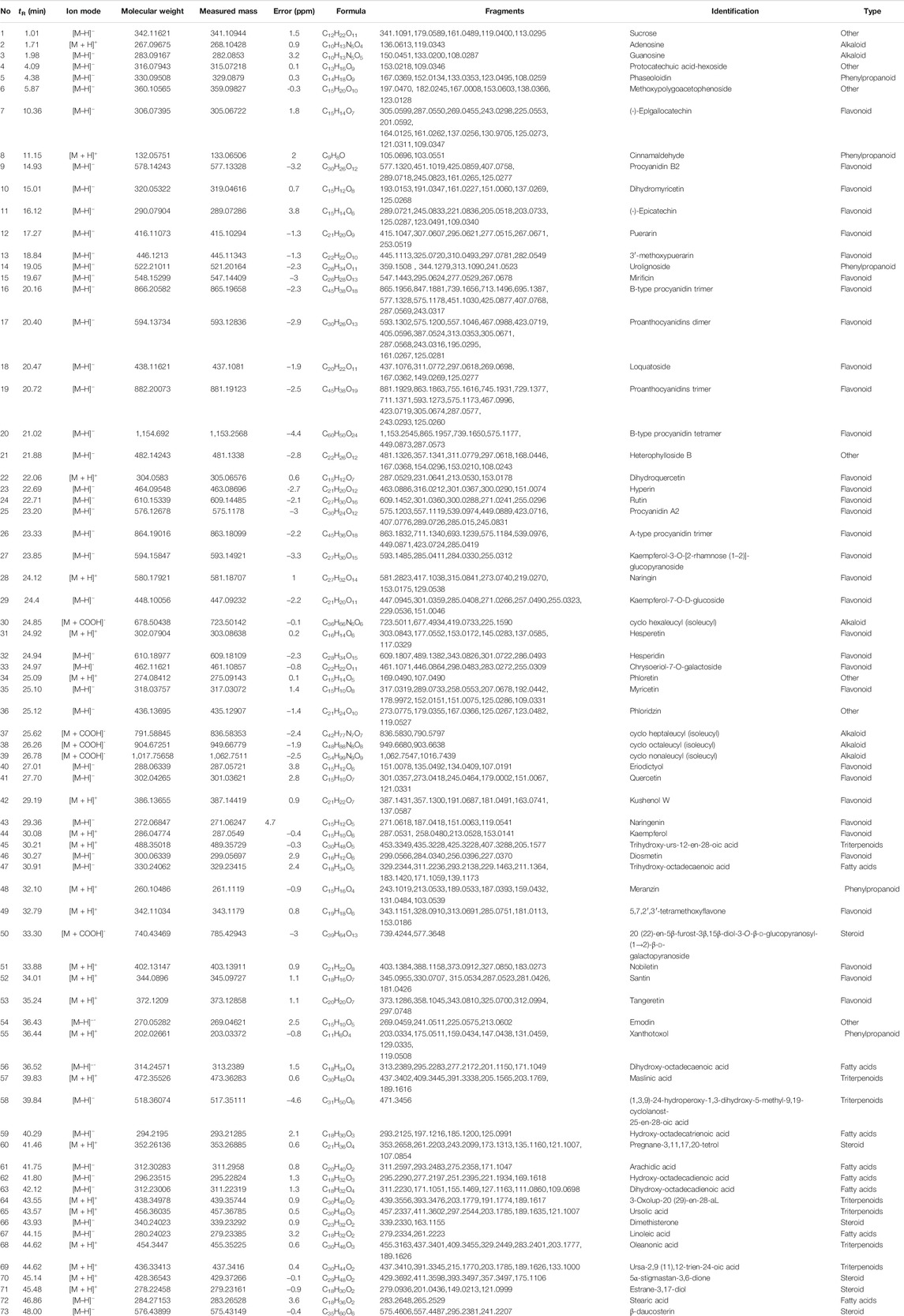
TABLE 1. Chemical constituents of P. heterophyllum identified by UPLC-qTOF-MS/MS in negative- and positive-ion modes.
The body weight and arthritis score of the rats in this experiment were evaluated at 4-day intervals from day 0 to day 28. As shown in Figure 3A, the body weight of the normal control rats increased steadily throughout the process, whereas the body weight slowly increased in AIA model rats. Importantly, P. heterophyllum treatment in three doses (160, 320, and 640 mg/kg) ameliorated the body weight loss of the model rats to some extent.
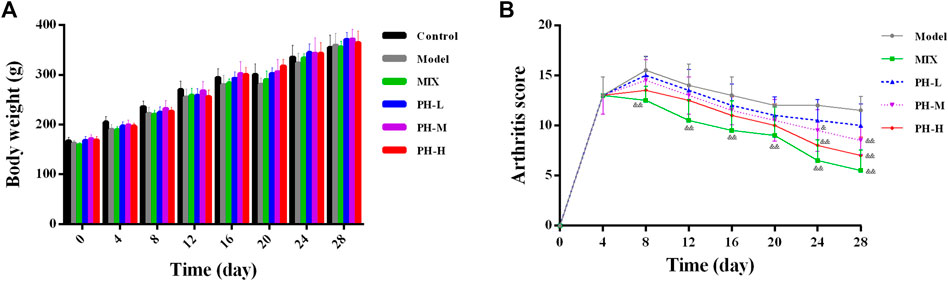
FIGURE 3. Effect of P. heterophyllum on the body weight (A) and arthritis score (B) in the AIA rats. Data are shown as mean ± SD (n = 8). Differences were analyzed using ANOVA by Tukey’s test. &p < 0.05 and &&p < 0.01 compared with the model group.
As presented in Figure 3B, the rats in the model group had markedly higher arthritis scores compared to the normal control group (arthritis scores = 0, p < 0.01). After drug treatment, the positive drug methotrexate (MTX) showed prominently decreased arthritis scores compared to the model group from day 8 (p < 0.01). Similar to the MTX treatment, after administration of PH-M (320 mg/kg) and PH-H (640 mg/kg), the arthritis scores values decreased significantly from day 24 (p < 0.01 or p < 0.05). These results indicate that P. heterophyllum possesses a potent anti-RA effect in AIA model rats.
Histopathological examination is the most informative and intuitive technique for exploring the manifestations of RA disease. Compared to the normal control rats, histopathological changes of the ankle joint in AIA model rats were characterized by massive inflammatory cell infiltration into synovial tissue, pannus formation, synovial hyperplasia, and bone and cartilage erosions (Figure 4). These abnormal histopathological changes were prominently alleviated in AIA model rats after treatment with MTX and P. heterophyllum, especially P. heterophyllum at a dosage of 640 mg/kg.
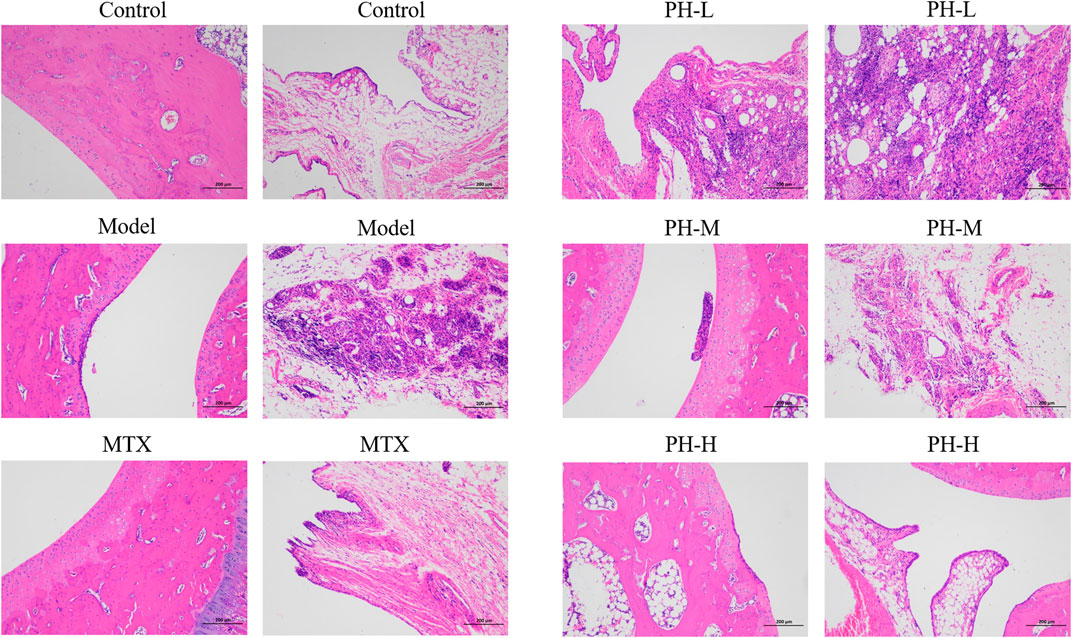
FIGURE 4. Effect of P. heterophyllum on histopathological changes of ankle joints in the AIA rats (×100, HE staining).
The results summarized in Figure 5 indicate that the weights of the thymus (Figure 5A) and spleen (Figure 5B) increased remarkably in the rats of the AIA model group in contrast to the rats of the normal control group (p < 0.01). After treatment with three crude extracts of P. heterophyllum and MTX, the weight of the thymus and spleen persuasively decreased (p < 0.01 or p < 0.05) compared to the model group. The results showed that rats in the AIA model group had hyperimmune response after intradermal injection of CFA, while P. heterophyllum could suppress abnormal immune function.
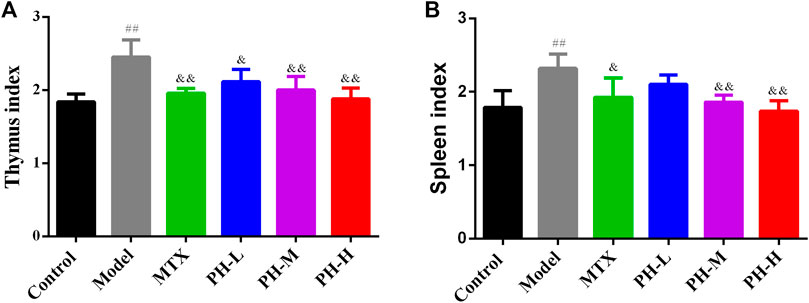
FIGURE 5. Effect of P. heterophyllum on the indexes of thymus (A) and spleen (B) of AIA rats. Data are shown as mean ± SD (n = 8). Differences were analyzed using ANOVA by Tukey’s test. ##p < 0.01 compared with the control group, &p < 0.05 and &&p < 0.01 compared with the model group.
As summarized in Figure 6, the serum levels of RF and CRP in AIA model rats were significantly higher than those of rats in normal control group (p < 0.01). The three crude extracts from treatment with P. heterophyllum and MTX observably downregulated the levels of RF and CRP in serum (p < 0.01).
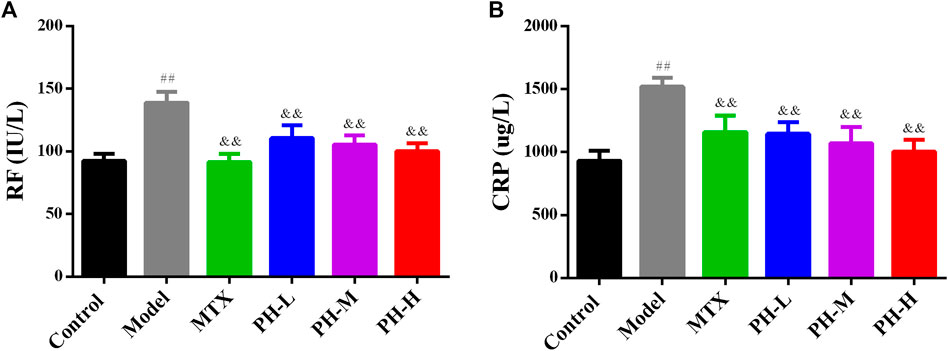
FIGURE 6. Effect of P. heterophyllum on the serum levels of RF (A) and CRP (B) in AIA rats. Data are shown as mean ± SD (n = 8). Differences were analyzed using ANOVA by Tukey’s test. ##p < 0.01 compared with the control group, &&p < 0.01 compared with the model group.
The results showed that serum concentrations of TNF-α, IL-1β, IL-6 and IL-17 increased prominently (p < 0.01) in the AIA model group compared to the normal control group. Treatment with P. heterophyllum markedly decreased (p < 0.01) the serum levels of all the above-mentioned anti-inflammatory cytokines (Figure 7).
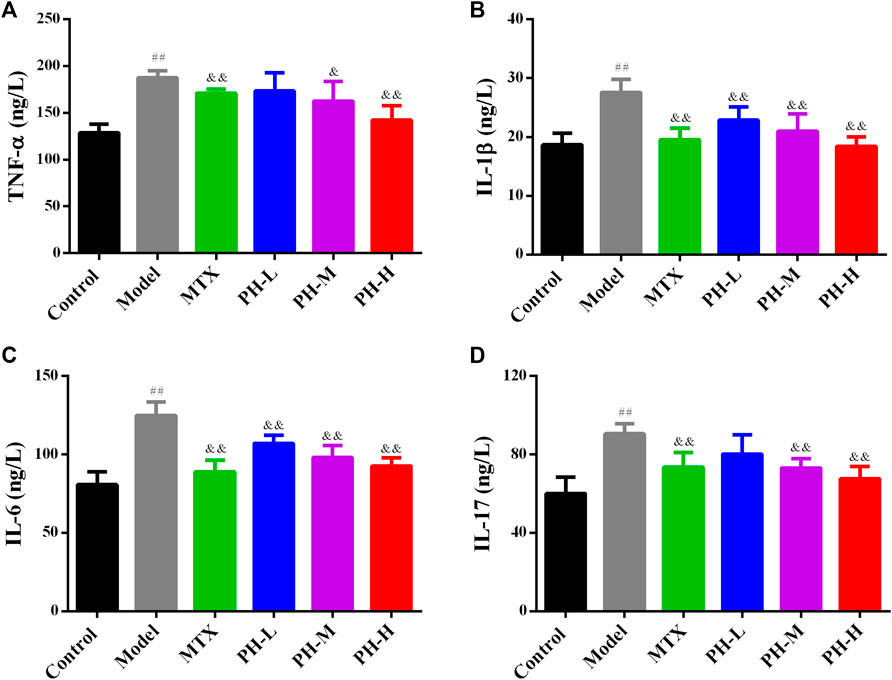
FIGURE 7. Effect of P. heterophyllum on the serum levels of TNF-α (A), IL-1β (B), IL-6 (C) and IL-17 (D) in AIA rats. Data are shown as mean ± SD (n = 8). Differences were analyzed using ANOVA by Tukey’s test. ##p < 0.01 compared with the control group, &p < 0.05 and &&p < 0.01 compared with the model group.
Compared to rats in the normal control group, the levels of anti-inflammatory cytokines including IL-4 and IL-10 in the serum of AIA model group rats were significantly up-regulated (p < 0.01, Figure 8). Treatment with 640 mg/kg of P. heterophyllum remarkably down-regulated the levels of IL-4 and IL-10 in the serum of AIA model rats.
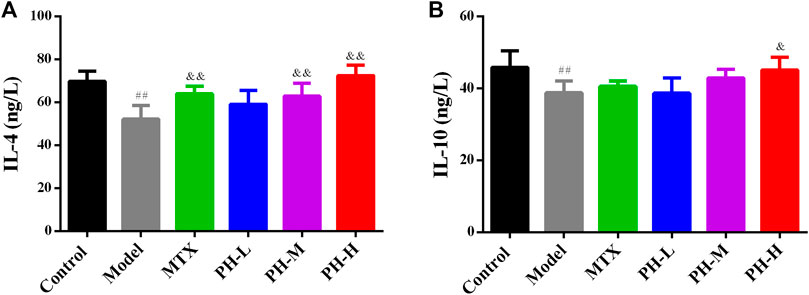
FIGURE 8. Effect of P. heterophyllum on the serum levels of IL-4 (A) and IL-10 (B) in AIA rats. Data are shown as mean ± SD (n = 8). Differences were analyzed using ANOVA by Tukey’s test. ##p < 0.01 compared with the control group, &p < 0.05 and &&p < 0.01 compared with the model group.
The levels of inflammatory mediators (COX-2 and 5-LOX) and MMP-2 in the rat PBMC were also evaluated by ELISA kits (Figure 9). The results showed that the levels of COX-2, 5-LOX and MMP-2 in PBMC of model rats were remarkably reduced than those of normal control rats (p < 0.01). After treatment with P. heterophyllum and MTX, the levels of COX-2, 5-LOX and MMP-2 were significantly elevated (p < 0.01 or p < 0.05) compared to those of the model group.
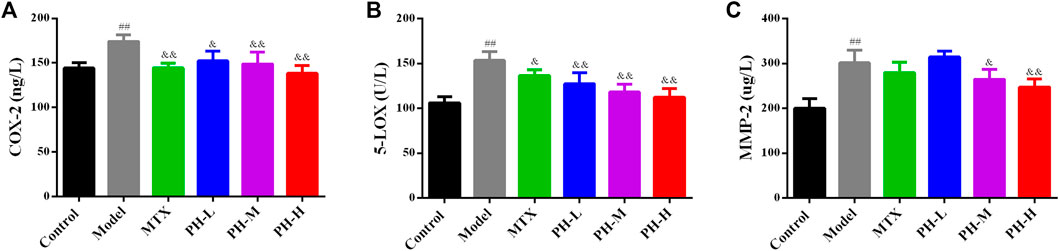
FIGURE 9. Effect of P. heterophyllum on the PBMC levels of COX-2 (A), 5-LOX (B) and MMP-2 (C) in AIA rats. Data are shown as mean ± SD (n = 8). Differences were analyzed using ANOVA by Tukey’s test. ##p < 0.01 compared with the control group, &p < 0.05 and &&p < 0.01 compared with the model group.
RA is the most prevalent chronic and long-term autoimmune inflammatory disease (Wang et al., 2017a; Li et al., 2019a; Saleem et al., 2020; Zhu et al., 2020). Although there are many anti-RA drugs in clinic, such as immunosuppressants, biological agents, DMARDs, steroidal drugs, and NSAIDs, most of them are associated with long-term adverse effects and costs (Li et al., 2019b; Dai et al., 2020; Zhu et al., 2020). In addition, the CFA-induced arthritis (AIA) model and the collagen-induced arthritis model are two typical preclinical experimental animal models of RA, and the former is a classic, easy-to-measure, short duration, reliable and reproducible test animal model, which is extensively used for the preclinical evaluation of anti-RA drugs since its pathological and morphological characteristics were similar to those of human RA (Yang et al., 2016; Pan et al., 2017; Voon et al., 2017; Zhang et al., 2019; Saleem et al., 2020).
The roots of P. heterophyllum have been widely used to treat RA as a vital TCM for centuries (Editorial Committee of Traditional Chinese Medicine 1999; Yang et al., 2016), but their anti-RA effect and chemical profiling have not been reported so far. Previous phytochemical studies have found that only 42 secondary metabolites, including six phenylpropanoids, eight triterpenoids, four flavonoids, 14 phenols and 10 others, were reported. In parallel, only 5-hydroxy-2-methoxy-1,4-naphtoquinone and taraxer-14-ene-1α,3β-diol exhibited antitumor effects in vitro (Yang et al., 2019a). In this work, we reported for the first time the anti-RA effect and chemical profiling of P. heterophyllum, thereby identifying 34 flavonoids and 39 others, while 15 flavonoids, including procyanidin B2, dihydromyricetin (-)-epicatechin, puerarin, rutin, naringin, hesperidin, myricetin, eriodictyol, quercetin, naringenin, kaempferol, diosmetin, nobiletin, and tangeretin, have been reported to have anti-RA effects in rats. Consequently, flavonoids may be responsible for the major active constituents in the roots of P. heterophyllum against RA as traditional folk medicine in China for centuries; however, further studies are needed to isolate and identify the bio-constituents directly related to anti-RA activity and its probable mechanism in vivo and in vitro of this plant.
In the animal model of AIA, histopathological lesions were aggravated due to massive inflammatory cell infiltration into synovial tissue, synovial hyperplasia, pannus formation, and bone and cartilage erosion (Lin et al., 2013; Voon et al., 2017; Rui et al., 2019; Yang et al., 2020). In the present research, P. heterophyllum exhibited the possible anti-RA effect, which prominently alleviates the above-mentioned abnormal histopathological changes in AIA model rats, accompanied by the reduction of inflammatory cytokines. Moreover, there is a straightforward relationship between weight loss/slow gain in rats and the massive inflammatory cell infiltration into synovial tissue (Lin et al., 2013; Pan et al., 2017; Jing et al., 2019). In this study, with P. heterophyllum treatment, body weight rose continuously in AIA model rats compared to rats in the model group. In addition, the arthritis score is a vital index to measure the anti-RA effect of drugs (Lin et al., 2013; Pan et al., 2017; Saleem et al., 2020) and is employed here to evaluate the possible therapeutic effect of P. heterophyllum, which was significantly decreased from day 24 compared to the model group. Finally, the spleen and thymus are two important immune organs, and their hyperfunction is closely related to the stimulation of the immune system in the AIA model rat (Lin et al., 2013; Zuo et al., 2018; Xiong et al., 2019), and the simultaneous decrease of the thymus and spleen indices by P. heterophyllum indicate the conceivable immunosuppressive effect.
In RA, serum RF and CRP are considered to be two important biomarkers of systemic inflammation in RA, indicating an active inflammatory response and are used to assess arthritic activity in rats with RA (Arjumand et al., 2019). This study shows that the expression of RF and CRP in serum of AIA model rat is remarkably increased, and the significant deduction after treatment with P. heterophyllum also suggests the feasible immunosuppressive activity.
A large number of studies have demonstrated that inflammation is a primary mechanism and a crucial role in rats with RA (Jing et al., 2019; Li et al., 2019c; Lin et al., 2013; Pan et al., 2017). Moreover, infiltration of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6 and IL-17, inflammatory mediators augment like COX-2 and 5-LOX, reduction of anti-inflammatory factors such as IL-4 and IL-10, which have been positively related to RA, causes synovial inflammation and cartilage damage (Jing et al., 2019; Li et al., 2019c; Lin et al., 2013; Pan et al., 2017). In RA, TNF-α, IL-1β, IL-6 and IL-17 play a decisive and synergistic role in synovial inflammation and cartilage damage (Jing et al., 2019; Yu et al., 2019; Saleem et al., 2020). In addition, the overproduction of TNF-α elevates the levels of IL-1β and IL-6, and generates matrix degrading enzymes (Jing et al., 2019). Likewise, IL-1β promotes osteoclast activation and MMP generation, just like increasing the expression of MMP-1, which ultimately leads to bone injury (Jing et al., 2019). On the other hand, IL-6 incites immunological reaction, MMP overproduction, and osteoclast differentiation and formation (Jing et al., 2019). IL-17 also plays a pivotal role in RA, which promotes the overproduction of pro-inflammatory cytokines and MMPs, as well as the activation of the osteoclasts and angiogenesis (Jing et al., 2019). Based on the above, therapeutic substances that particularly impede the production of TNF-α, IL-1β, IL-6 and IL-17 distinguish a crucial target for RA treatment (Jing et al., 2019; Rui et al., 2019; Yu et al., 2019). IL-4 and IL-10 by contrast, are two pivotal anti-inflammatory cytokines, which also play an important role in regulating the levels of endogenous pro-inflammatory cytokines during RA (Jing et al., 2019; Saleem et al., 2020). Our results indicate that treatment of P. heterophyllum obviously reduces the levels of TNF-α, IL-1β, IL-6 and IL-17, and increases the expression of IL-4 and IL-10, implying that the anti-RA effect of P. heterophyllum is achieved to a certain extent via the inhibition of pro-inflammatory cytokines and the elevation of anti-inflammatory cytokines in AIA model rats.
COX-2 is an overexpression of inflammatory tissues such as rheumatoid disease, and is a pivotal enzyme involved in the production of pro-inflammatory cytokines and cartilage destruction (Lin et al., 2013, Lin et al., 2014; Jing et al., 2019; Rui et al., 2019). Moreover, 5-LOX is the decisive enzyme involved in the synthesis of leukotriene, which is directly responsible for RA diseases (Lin et al., 2013, Lin et al., 2014). In addition, MMPs belong to the family of proteolytic enzymes, which play a crucial role during RA and are primarily responsible for bone and cartilage erosion (Jing et al., 2019; Yu et al., 2019). Our results demonstrate that PBMC levels of COX-2, 5-LOX and MMP-2 are highly expressed in AIA model rats, while a significant decrease is observed in PH-treated rats.
In summary, the chemical profiling and anti-RA effects of P. heterophyllum on AIA in rats were studied for the first time. The results demonstrate that flavonoids may be partly responsible for the major anti-RA effect of P. heterophyllum, which can ameliorate joint damage and suppress the hyperimmune response via downregulation of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6 and IL-17), inflammatory mediators (COX-2 and 5-LOX) and MMP-2, and upregulation of anti-inflammatory cytokines (IL-4 and IL-10). Our findings suggest that P. heterophyllum possesses the therapeutic effect of RA and supports the claim that it is a vital folk medicine in TCM for treating RA and inflammation-related diseases for centuries.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by JZLLSC2018-0701.
JH designed the project and wrote the manuscript. LY, JH, RL, AF, JZ and YZ performed the experiments and analyzed the data. JH and LY discussed the data.
This work was financially supported by the National Natural Science Foundation of China (NSFC) (No. 81760705), the Natural Science Foundation of Jiangxi Province (Nos. 20192BBHL80008 and 20192BAB215059), and the Jiangxi University of Traditional Chinese Medicine (no. JXSYLXK-ZHYA0031).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Modern Manuscript Editing Services (http://www.mmanuscripteditserv.com) for providing linguistic assistance during the preparation of this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.584849/full#supplementary-material.
Abd-Allah, S., Shahzad, M., Shabbir, A., and Yousaf, M. Z. (2019). Thymoquinone attenuates rheumatoid arthritis by downregulating TLR2, TLR4, TNF-α, IL-1, and NFκB expression levels. Biomed. Pharmacother. 111, 958–963. doi:10.1016/j.biopha.2019.01.006
Ahmad, S. F., Zoheir, K. M. A., Abdel-Hamied, H. E., Ashour, A. E., Bakheet, S. A., Attia, S. M., et al. (2014). Amelioration of autoimmune arthritis by naringin through modulation of T regulatory cells and Th1/Th2 cytokines. Cell. Immunol. 287, 112–120. doi:10.1016/j.cellimm.2014.01.001
Bao, Y., Li, H., Li, Q.-Y., Li, Y., Li, F., Zhang, C.-F., et al. (2018). Therapeutic effects of Smilax glabra and Bolbostemma paniculatum on rheumatoid arthritis using a rat paw edema model. Biomed. Pharmacother. 108, 309–315. doi:10.1016/j.biopha.2018.09.004
Chen, Y., Wang, Y., Liu, M., Zhou, B., and Yang, G. (2019). Diosmetin exhibits anti‐proliferative and anti‐inflammatory effects on TNF‐α‐stimulated human rheumatoid arthritis fibroblast‐like synoviocytes through regulating the Akt and NF‐κB signaling pathways. Phytother Res. 34, 1310–1319. doi:10.1002/ptr.6596
Chu, J., Wang, X., Bi, H., Li, L., Ren, M., and Wang, J. (2018). Dihydromyricetin relieves rheumatoid arthritis symptoms and suppresses expression of pro-inflammatory cytokines via the activation of Nrf2 pathway in rheumatoid arthritis model. Int. Immunopharm. 59, 174–180. doi:10.1016/j.intimp.2018.04.001
Dai, X., Yang, D., Bao, J., Zhang, Q., Ding, J., au, M., et al. (2020). Er Miao San, a traditional Chinese herbal formula, attenuates complete Freund's adjuvant-induced arthritis in rats by regulating Th17/Treg cells. Pharmaceut. Biol. 58, 157–164. doi:10.1080/13880209.2020.1720745
Editorial Committee of Traditional Chinese Medicine (1999). Chinese materia medica. Shanghai, CN: Zhonghua Bencao, 3, 751–752.
Jing, R., Ban, Y., Xu, W., Nian, H., Guo, Y., Geng, Y., et al. (2019). Therapeutic effects of the total lignans from Vitex negundo seeds on collagen-induced arthritis in rats. Phytomedicine 58, 152825. doi:10.1016/j.phymed.2019.152825
Kim, E. Y., Sudini, K., Singh, A. K., Haque, M., Leaman, D., Khuder, S., et al. (2018). Ursolic acid facilitates apoptosis in rheumatoid arthritis synovial fibroblasts by inducing SP1-mediated Noxa expression and proteasomal degradation of Mcl-1. Feaeb. J. 32, 6174–6185. doi:10.1096/fj.201800425r
Lei, Z., Ouyang, L., Gong, Y., Wang, Z., and Yu, B. (2020). Effect of eriodictyol on collagen-induced arthritis in rats by Akt/HIF-1 alpha pathway. Drug Des. Dev. Ther. 14, 1633–1639. doi:10.2147/dddt.s239662
Li, H. J., Zhang, C. T., Du, H., Xu, T., Li, Q., Wang, P., et al. (2019a). Chemical composition of Bawei Longzuan granule and its anti-arthritic activity on collagen-induced arthritis in rats by inhibiting inflammatory responses. Chem. Biodivers. 16, e1900294. doi:10.1002/cbdv.201900294
Li, T. P., Zhang, A. H., Miao, J. H., Sun, H., Yan, G. L., et al. (2019b). Applications and potential mechanisms of herbal medicines for rheumatoid arthritis treatment: a systematic review. RSC Adv. 9, 26381. doi:10.1039/c9ra04737a
Li, X., Xie, P. G., Hou, Y., Chen, S. D., He, P. H., Xiao, Z. F., et al. (2019c). Tangeretin inhibits oxidative stress and inflammation via upregulating Nrf-2 signaling pathway in collagen-induced arthritic rats. Pharmacology 104, 187–195. doi:10.1159/000501163
Lin, B., Zhang, H., Zhao, X. X., Rahman, K., Wang, Y., Ma, X. Q., et al. (2013). Inhibitory effects of the root extract of Litsea cubeba (lour.) pers. on adjuvant arthritis in rats. J. Ethnopharmacol. 147, 327–334. doi:10.1016/j.jep.2013.03.011
Lin, H. C., Lin, T. H., Wu, M. Y., Chiu, Y. C., Tang, C. H., Hour, M. J., et al. (2014). 5-lipoxygenase inhibitors attenuate TNF-α-induced inflammation in human synovial fibroblasts. PloS One 9, e107890. doi:10.1371/journal.pone.0107890
Liu, J., Zeng, L., Wei, R., Zhong, G., Zhu, Y., Xu, T., et al. (2019). Lagopsis supina exerts its diuretic effect via inhibition of aquaporin-1, 2 and 3 expression in a rat model of traumatic blood stasis. J. Ethnopharmacol. 231, 446–452. doi:10.1016/j.jep.2018.10.034
Mateen, S., Shahzad, S., Ahmad, S., Naeem, S. S., Khalid, S., Akhtar, K., et al. (2019). Cinnamaldehyde and eugenol attenuates collagen induced arthritis via reduction of free radicals and pro-inflammatory cytokines. Phytomedicine 53, 70–78. doi:10.1016/j.phymed.2018.09.004
Osman, W. N. W., Tantowi, N. A. C. A., Lau, S. F., and Mohamed, S. (2019). Epicatechin and scopoletin rich Morinda citrifolia (Noni) leaf extract supplementation, mitigated osteoarthritis via anti-inflammatory, anti-oxidative, and anti-protease pathways. J. Food Biochem. 43, e12755.
Pan, D. M., Li, N., Liu, Y. Y., Xu, Q., Liu, Q. P., You, Y. T., et al. (2018). Kaempferol inhibits the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes by blocking activation of the MAPK pathway. Int. Immunopharm. 555, 174–182. doi:10.1016/j.intimp.2017.12.011
Pan, T., Cheng, T. F., Jia, Y. R., Li, P., and Li, F. (2017). Anti-rheumatoid arthritis effects of traditional Chinese herb couple in adjuvant-induced arthritis in rats. J. Ethnopharmacol. 205, 1–7. doi:10.1016/j.jep.2017.04.020
Rui, J., Ban, Y. F., Xu, W. H., Nian, H., Guo, Y. L., Geng, Y. Y., et al. (2019). Therapeutic effects of the total lignans from Vitex negundo seeds on collagen-induced arthritis in rats. Phytomedicine 58, 152825. doi:10.1016/j.phymed.2019.152825
Saccol, R. D. P., Silveira, K. L., Adefegha, S. A., Manoni, A. G., sa Silveira, L. L., Coelho, A. P. V., et al. (2019). Effect of quercetin on E-NTPDase/E-ADA activities and cytokine secretion of complete Freund adjuvant-induced arthritic rats. Cell Biochem. Funct. 37, 474–485. doi:10.1002/cbf.3413
Saleem, A., Saleem, M., Akhtar, M. F., Shahzad, M., and Jahan, S. (2020). Moringa rivae leaf extracts attenuate complete Freund’s adjuvant-induced arthritis in wistar rats via modulation of inflammatory and oxidative stress biomarkers. Inflammopharmacology 28, 139–151. doi:10.1007/s10787-019-00596-3
Sun, C. L., Wei, J., and Bi, L. Q. (2017). Rutin attenuates oxidative stress and proinflammatory cytokine level in adjuvant induced rheumatoid arthritis via inhibition of NF-kappa B. Pharmacology 100, 40–49. doi:10.1159/000451027
Tong, Z. W., Cheng, L., Song, J. Z., Wang, M., Yuan, J. Z., Li, X. Z., et al. (2018). Therapeutic effexts of Caesalpinia minax Hance on complete Frund’s adjuvant (CFA)-induced arthritis and the anti-inflammatory activity of cassane diterpenes as main active components. J. Ethnopharmacol. 226, 90–96. doi:10.1016/j.jep.2018.08.011
Voon, F. L., Sulaiman, M. R., Akhtar, M. N., Idris, M. F., Akira, A., Perimal, E. K., et al. (2017). Cardamonin (2',4'-dihydroxy-6'-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibitits CFA-induced rheumatoid arthritis in rats. Eur. J. Pharmacol. 794, 127–134. doi:10.1016/j.ejphar.2016.11.009
Wang, A., Leong, D. J., He, Z. Y., Xu, L., Liu, L. D., Kim, S. J., et al. (2017b). Procyanidins mitigate osteoarthritis pathogenesis by, at Least in part, suppressing vascular endothelial growth factor signaling. Int. J. Mol. Sci. 17, 2065. doi:10.3390/ijms17122065
Wang, C., Meriwether, D., Lee, Y. Y., and Reddy, S. T. (2011). Oxidation products of arachidonic acid and linoleic acid are increased in high density lipoprotein and low density lipoprotein from patients with active rheumatoid arthritis. Arthritis Rheum-US 63, s299.
Wang, C. X., Wang, W. D., and Jin, X. P. (2016). Puerarin attenuates inflammation and oxidation in mice with collagen antibody-induced arthritis viaTLR4/NF-kappa B signaling. Mol. Med. Rep. 14, 1365–1370. doi:10.3892/mmr.2016.5357
Wang, X., He, X., Zhang, C. F., Guo, C. R., Wang, C. Z., and Yuan, C. S. (2017a). Anti-arthritic effect of berberine on adjuvant-induced rheumatoid arthritis in rats. Biomed. Pharmacother. 89, 887–893. doi:10.1016/j.biopha.2017.02.099
Xiong, H., Ding, X., Wang, H., Jiang, H. Q., Wu, X. Y., Tu, C. Y., et al. (2019). Tibetan medicine Kuan-Jin-Teng exerts anti-artitic effects on collagen-induced arthritis rats vvia inhibition the production of pro-inflammatory cytokines and down-regulation of MAPK signaling pathway. Phytomedicine 57, 271–281. doi:10.1016/j.phymed.2018.12.023
Yang, L., He, J. W., Wang, S. F., Wang, L. F., and Liu, R. H. (2019a). Research progress of chemical constituents, pharmacological activities and quality control of Pterospermum heterophyllum Hance. J. Jiangxi Univi. TCM. 31, 113–116.
Yang, L., Liu, R. H., and He, J. W. (2019b). Rapid analysis of the chemical compositions in Semiliquidambar cathayensis roots by ultra-high-performance liquid chromatography and quadrupole time-of-flight tandem mass spectrometry. Molecules 24, 4098. doi:10.3390/molecules24224098
Yang, L., Wang, Y. Q., Liu, S. C., and He, J. W. (2016). Research progress of chemical constituents and pharmacological activities from three commonly used Ban-feng-he medicinal plants. Chin. J. Exp. Tradit. Med. Form. 22, 191–196.
Yang, P., Chen, G., Qin, W.-Y., Zhong, Y., Yang, J., and Rong, X.-F. (2016). Xitong Wan attenuates inflammation development through inhibiting the activation of nuclear factor-κB in rats with adjuvant-induced arthritis. J. Ethnopharmacol. 193, 266–271. doi:10.1016/j.jep.2016.08.006
Yang, P., Qian, F. Y., Zhang, M. F., Xu, A. L., Wang, X., Jiang, B. P., et al. (2020). Zishen Tongluo formula ameliorates collagen-induced arthritis in mice by modulation of Th17/Treg balance. J. Ethnopharmacol. 250, 112428. doi:10.1016/j.jep.2019.112428
Yousefi, B., Jadidi-Niaragh, F., Azizi, G., Hajighasemi, F., and Mirshafiey, A. (2014). The role of leukotrienes in immunopathogenesis of rheumatoid arthritis. Mod. Rheumatol. 24, 225–235. doi:10.3109/14397595.2013.854056
Yu, H. H., Zeng, R., Lin, Y., Li, X,, Shumaila, T., Yang, Z., et al. (2019). Kadsura heteroclita stem suppresses the onset and progression of adjuvant-induced arthritis in rats. Phytomedicine 58, 152876. doi:10.1016/j.phymed.2019.152876
Yuan, X. L., Liu, Y. G., Hua, X., Deng, X. X., Sun, P. J., Yu, C. M., et al. (2015). Myricetin ameliorates the symptoms of collagen-induced arthritis in mice by inhibiting cathepsin Kactivity. Immunopharmacol. Immunotoxicol. 37, 513–519. doi:10.3109/08923973.2015.1096942
Zhang, C. F., Zhang, W. F., Shi, R. Y., Tang, B. Y., and Xie, S. C. (2019). Coix lachrymal-jobi extract ameliorates inflammation and oxidative stress in a complete Freund’s adjuvant-induced rheumatoid arthritis model. Pharm. Biol. 57, 792–798. doi:10.1080/13880209.2019.1687526
Zhu, L. J., Zhang, Z. S., Xia, N. N., Zhang, W. F., Wei, Y. L., Huang, J. S., et al. (2020). Anti-arthritic activity of ferulic acid in complete Freund’s adjuvant (CFA)-induced arthritis in rats: JAK2 inhibition. Inflammopharmacology 28, 463–473. doi:10.1007/s10787-019-00642-0
Zhu, L. P., Wang, J., Wei, T. T., Gao, J., He, H., Chang, X. Y., et al. (2015). Effects of naringenin on inflammation in complete Freund's adjuvant-induced arthritis by regulating Bax/Bcl-2 balance. Inflammation 38, 245–251. doi:10.1007/s10753-014-0027-7
Zhu, X. F., Zeng, K., Qiu, Y., Yan, F. H., and Lin, C. Z. (2013). Therapeutic effect of emodin on collagen-induced arthritis in mice. Inflammation 36, 1253–1259. doi:10.1007/s10753-013-9663-6
Zuo, J., Yin, Q., Wang, Y. W., Lu, L. M., Xiao, Z. G., Wang, G. D., et al. (2018). Inhibition of NF-Kb pathway in fibroblast-like synoviocytes by α-magostin implicated in protective effects on joints in rats suffering from adjuvant-induced arthritis. Int. Immunopharm. 56, 78–89. doi:10.1016/j.intimp.2018.01.016
Keywords: Pterospermum heterophyllum root, rheumatoid arthritis, chemical composition, flavonoid, inflammatory response
Citation: Yang L, Liu R, Fan A, Zhao J, Zhang Y and He J (2020) Chemical Composition of Pterospermum heterophyllum Root and its Anti-Arthritis Effect on Adjuvant-Induced Arthritis in Rats via Modulation of Inflammatory Responses. Front. Pharmacol. 11:584849. doi: 10.3389/fphar.2020.584849
Received: 18 July 2020; Accepted: 14 October 2020;
Published: 11 December 2020.
Edited by:
Lyndy Joy McGaw, University of Pretoria, South AfricaReviewed by:
Sadiq Umar, University of Illinois at Chicago, United StatesCopyright © 2020 Yang, Liu, Fan, Zhao, Zhang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junwei He, aGp3am4yMDA4QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.