
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 02 March 2021
Sec. Ethnopharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.578970
This article is part of the Research Topic Ethnopharmacological Responses to the Coronavirus Disease 2019 (COVID-19) Pandemic View all 38 articles
 Sayeed Ahmad1†*
Sayeed Ahmad1†* Sultan Zahiruddin1†
Sultan Zahiruddin1† Bushra Parveen1†
Bushra Parveen1† Parakh Basist1
Parakh Basist1 Abida Parveen2
Abida Parveen2 Gaurav1
Gaurav1 Rabea Parveen3
Rabea Parveen3 Minhaj Ahmad4
Minhaj Ahmad4The cases of COVID-19 are still increasing day-by-day worldwide, even after a year of its first occurrence in Wuhan city of China. The spreading of SARS-CoV-2 infection is very fast and different from other SARS-CoV infections possibly due to structural differences in S proteins. The patients with severe diseases may die due to acute respiratory distress syndrome (ARDS) caused by systemic inflammatory reactions due to the excessive release of pro-inflammatory cytokines and chemokines by the immune effector cells. In India too, it is spreading very rapidly, although the case fatality rate is below 1.50% (https://www.statista.com), which is markedly less than in other countries, despite the dense population and minimal health infrastructure in rural areas. This may be due to the routine use of many immunomodulator medicinal plants and traditional AYUSH formulations by the Indian people. This communication reviews the AYUSH recommended formulations and their ingredients, routinely used medicinal plants and formulations by Indian population as well as other promising Indian medicinal plants, which can be tested against COVID-19. Special emphasis is placed on Indian medicinal plants reported for antiviral, immunomodulatory and anti-allergic/anti-inflammatory activities and they are categorized for prioritization in research on the basis of earlier reports. The traditional AYUSH medicines currently under clinical trials against COVID-19 are also discussed as well as furtherance of pre-clinical and clinical testing of the potential traditional medicines against COVID-19 and SARS-CoV-2. The results of the clinical studies on AYUSH drugs will guide the policymakers from the AYUSH systems of medicines to maneuver their policies for public health, provide information to the global scientific community and could form a platform for collaborative studies at national and global levels. It is thereby suggested that promising AYUSH formulations and Indian medicinal plants must be investigated on a priority basis to solve the current crisis.
A novel coronavirus-induced pneumonia, which was later called coronavirus disease 2019 (COVID-19), has rapidly increased to an epidemic scale and affected whole human population globally (WHO, 2020a). In India, the first case of COVID-19 was an imported case from Wuhan, China on January 30, 2020 traced in Kerala (Sahasranaman and Kumar, 2020) and the death rate of COVID-19 in India is 1.45%, as of 12th December, 2020 (Worldometers, 2020). Severe acute respiratory syndrome-related coronavirus (SARS-CoV-2) has become a pandemic hazard to global public health worldwide.
Coronaviruses (CoVs) are large viruses comprising of four genera, namely alpha, beta, gamma, and delta. The beta-coronavirus class includes severe acute respiratory syndrome (SARS) virus (SARS-CoV), Middle East respiratory syndrome (MERS) virus (MERS-CoV), and the COVID-19 causative agent SARS-CoV-2. (Li G. et al., 2020). The novel SARS-CoV-2 is a beta CoV that shows 88% similarity to two bat-derived SARS-like CoVs (bat-SL-CoVZC45 and bat-SL-CoVZXC21), about 50% identical to the sequence of MERSCoV, and 70% similarity in genetic sequence to SARS-CoV (Cheng and Shan, 2020). Although there is an extremely high resemblance between SARS-CoV and the novel SARS-CoV-2, the SARS-CoV-2 is spreading rapidly as compared to the SARS-CoV, which may be explained by structural differences in the S proteins (Rabaan et al., 2020).
The SARS-CoV-2 S protein has been found as a significant determinant of virus entry into host cells using angiotensin converting enzyme 2 (ACE2) receptor similar to SARS-CoV. Whereas the binding affinity of virion S glycoprotein and ACE2 is reported to be 10–20 folds higher in SARS-CoV-2 as compared to that of SARS-CoV (Song et al., 2018).
Severe cases of COVID-19 are reported to have increased plasma concentrations of pro-inflammatory cytokines, including interleukins (IL-6 and IL-10), tumor necrosis factor (TNF)-α granulocyte-colony stimulating factor (G-CSF), monocyte chemoattractant protein 1 (MCP1), and macrophage inflammatory protein (MIP)1α (Yuki et al., 2020). Akin to the common viral infections, the antibody profile against the SARS-CoV virus manifests a typical pattern of IgM and IgG antibody production. The IgG antibody is believed to play a protective role, as the SARS-specific IgG antibodies last for a longer time while IgM antibodies disappear at the end of 12 weeks. The latest reports show a significant reduction in the number of CD4+ and CD8+ T cells in the peripheral blood of SARS-CoV-2-infected patients, besides activation of other pro-inflammatory cytokines such as nuclear factor-κB (NF-κB), interferon regulatory factor 3 (IRF3) and type I Interferons (IFN-α/β) (Li G. et al., 2020). A recent report shows that many patients died from acute respiratory distress syndrome (ARDS) caused by the cytokine storm, which is a deadly uncontrolled systemic inflammatory response resulting from the release of large amounts of pro-inflammatory cytokines and chemokines by immune effector cells in SARS-CoV infection (Guo et al., 2020).
Although the pathogenesis of COVID-19 is still not clear, patients with COVID-19 show non-specific symptoms ranging from no symptoms (asymptomatic) to severe pneumonia and death. However, the most common symptoms include fever, non-productive cough, dyspnea, myalgia, fatigue, diarrhea, lung damage, normal or decreased leukocyte counts, and radiographic evidence of pneumonia, which are similar to the symptoms of SARS-CoV and MERS-CoV infections (WHO, 2020b; Rothan and Byrareddy, 2020). Complications include ARDS, acute heart injury, and secondary infections (Guo et al., 2020).
The present conventional strategy of the disease control includes isolation of cases and tracing their contacts, providing optimal care to these infected cases, reducing chances of secondary infections by early diagnosis, and rapid development of effective diagnostic, preventive and therapeutic strategies, including vaccines (WHO, 2020b). The treatment approach for COVID-19 is supportive care, which is supplemented by the combination of broad-spectrum antibiotics, antivirals, corticosteroids and convalescent plasma (Yang et al., 2020).
Scientists are working hard to develop effective treatments. As of October 18, 2020, more than 3611 clinical trials (with more than 100 complementary medicines) on COVID-19 are either ongoing or enrolling patients, and new ones are being added every day, as the case count skyrockets globally. The drugs being tested range from repurposed flu treatments to failed ebola drugs, to malaria treatments that were first developed decades ago (Lythgoe and Middleton, 2020). There is scale-up development of vaccines across the world by many pharmaceutical companies as well as research organizations. These treatments undergoing trials may require months or years to develop and hit the market, meaning that an immediate treatment or control mechanism should be found, if possible (Table 1).
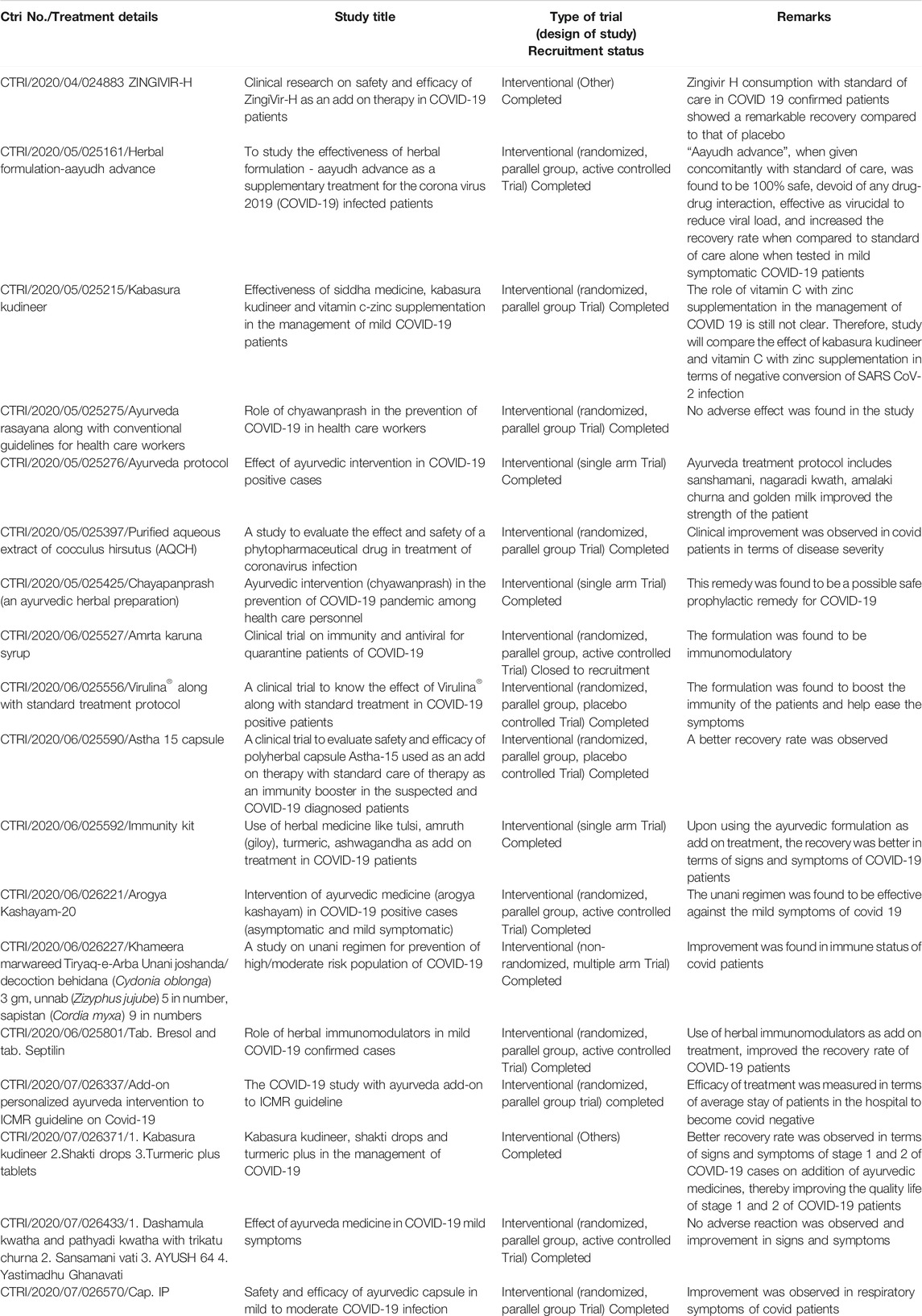
TABLE 1. Details of clinical trials completed on AYUSH drugs for COVID-19 (Source: www.ctri.nic.in).
Considering the current situation, various treatment modalities have been well-thought-out, including traditional medicine, which has been widely used during the past epidemic outbreaks, including SARS and H1N1 influenza (Luo et al., 2020). Until now three countries including India, China, and South Korea, have issued guidelines on traditional regimens for the prevention and management of COVID-19 (Ang et al., 2020).
The Indian Traditional System of Medicine is one of the oldest systems of medical practice in the world and has played an essential role in providing health care service to human civilization, right from its inception. India has the exclusive distinction of its own recognized traditional medicine; Ayurveda, Yoga, Unani, Siddha, and Homoeopathy (AYUSH) (Adhikari and Paul, 2018). These systems are based on definite medical philosophies and represent a way of achieving a healthy lifestyle with conventional and established ideas on the prevention of diseases and the promotion of health. The basic treatment approach of all these systems is holistic and the pharmacological modalities are based on natural products of plants, animals, or mineral origin. Given this, there is a resurgence of interest in AYUSH systems, which have helped the nation in the pandemic crisis due to plague, cholera, Spanish flu, etc. in the past. Hence, by repurposing the traditional uses of Indian medicinal plants and formulations, new treatment options can be identified to combat the current deadly pandemic. In view of the COVID-19 outbreak, the entire human race across the globe is perturbed. While there is no medicine for COVID-19 as of now, it is imperative to take preventive measures such as practicing self-hygiene, social distancing and boosting immunity. Many safe traditional formulations of AYUSH, which are well known immunity modulators, have been used for centuries in respiratory disorders and in allergic conditions. The Ministry of AYUSH (Govt of India) has listed out such formulations and recommended their use as a prophylactic measure in red zones, containment zones, as well as for corona warriors. Many of them are now under clinical trial in COVID-19 patients (Table 1).
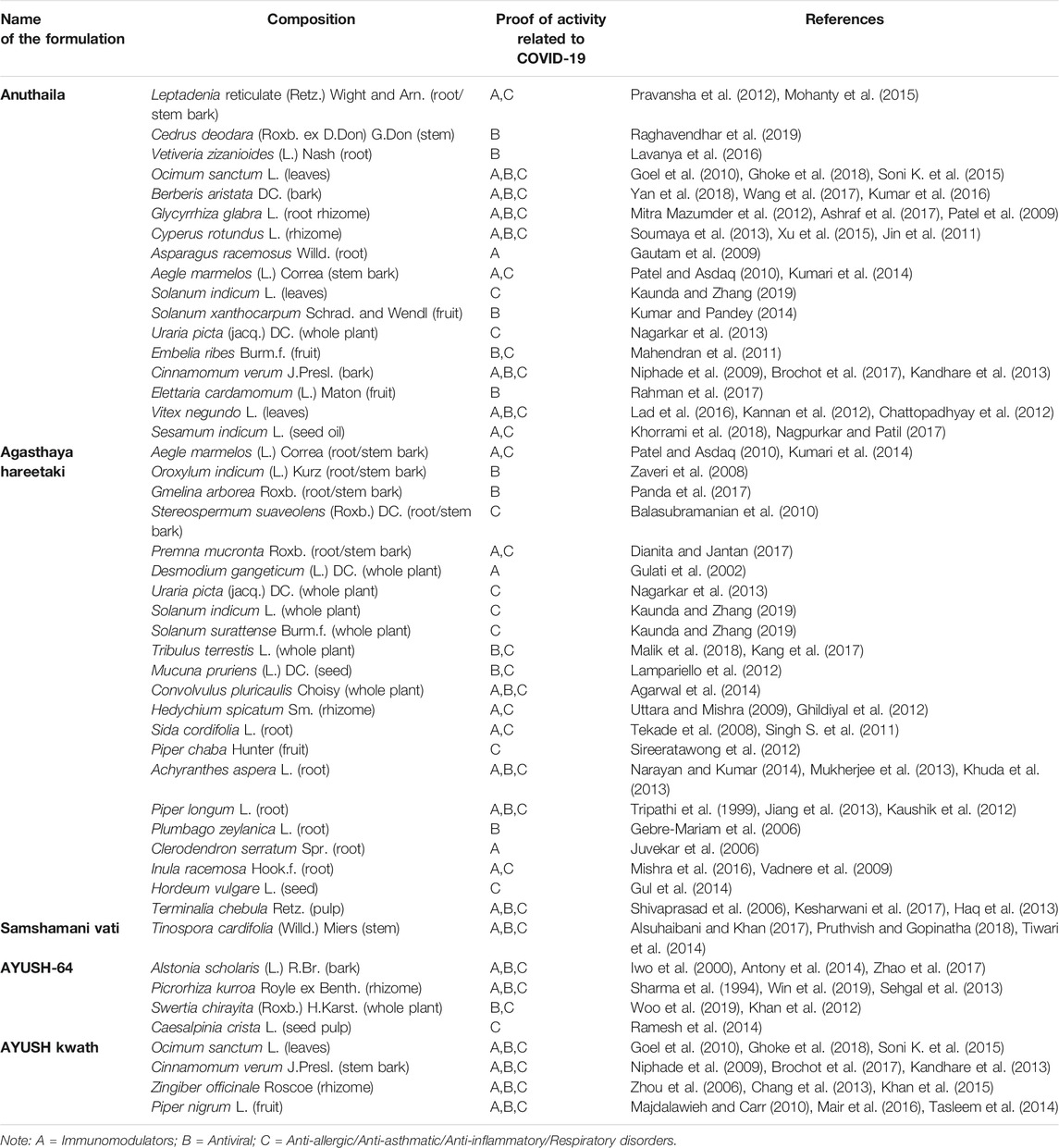
TABLE 2. AYUSH recommended prophylactic approach through Ayurvedic formulations. Ref: AYUSH Ministry of Health Corona Advisory-D.O. No. S. 16030/18/2019- NAM; dated: 06th March, 2020. Ref: AYUSH Ministry of Health Corona Advisory -F.No. Z 25.23/09/2018–2020-DCC (AYUSH); dated: 24th April, 2020.
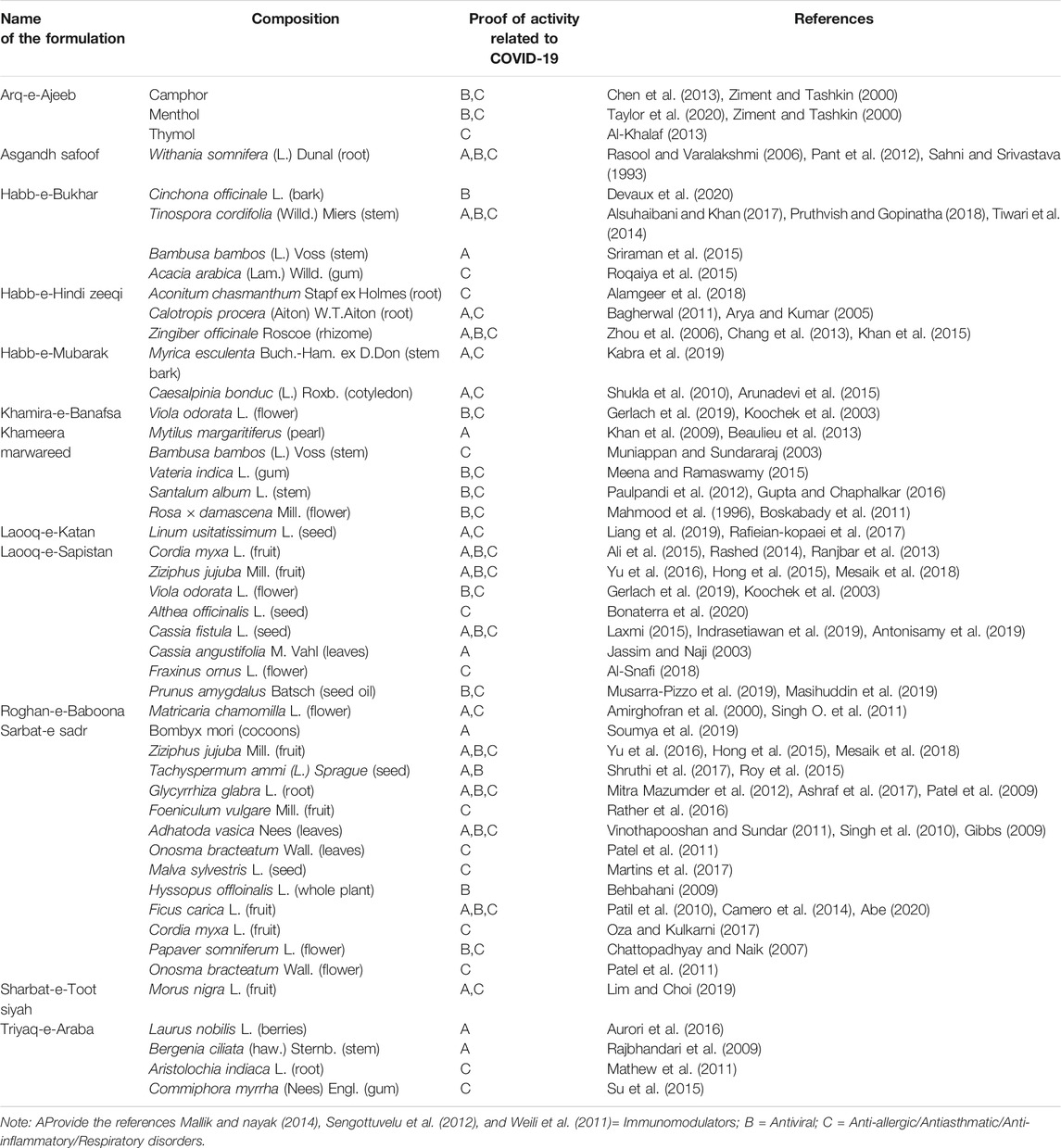
TABLE 3. AYUSH recommended prophylactic approach through Unani formulation. Ref: AYUSH Ministry of Health Corona Advisory–D.O. No. S. 16030/18/2019- NAM; dated: 06th March, 2020.
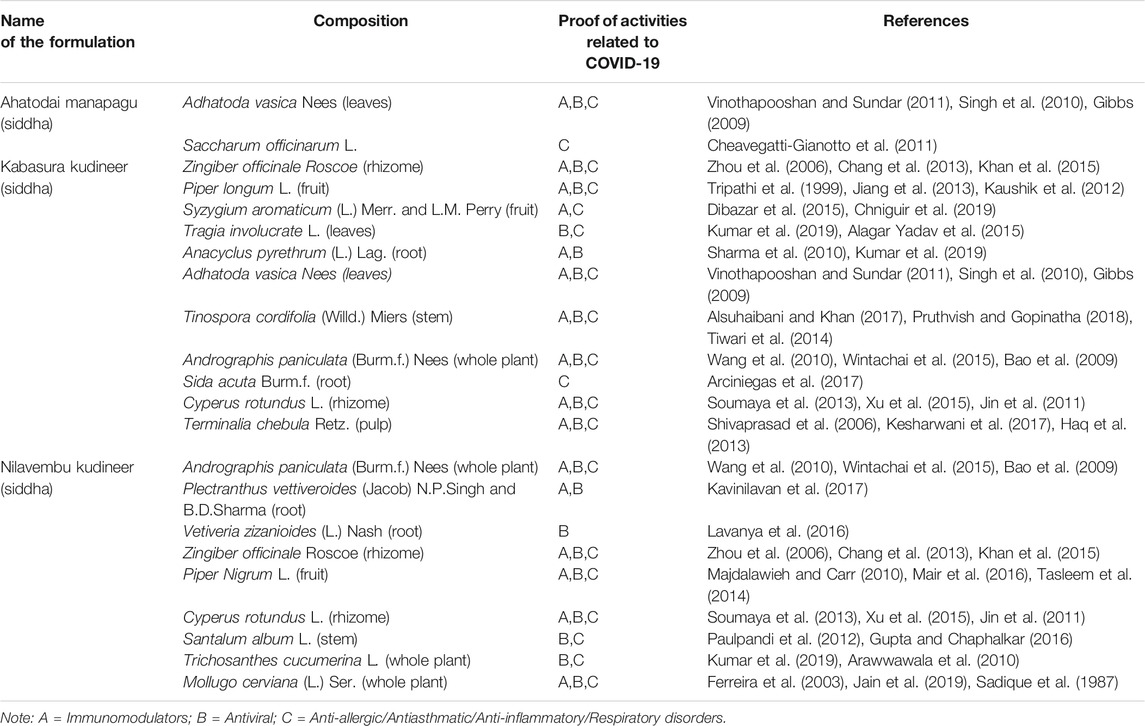
TABLE 4. AYUSH recommended prophylactic approach through formulations of Siddha system of medicine. Ref: AYUSH Ministry of Health Corona Advisory–D.O. No. S. 16030/18/2019-NAM; dated: 06th March, 2020.
Similarly, there are many medicinal plants indigenous to India and used in the Indian Systems of Medicine which have been reported as potent antiviral with immunomodulatory and anti-allergic/anti asthmatic activities. Many of these medicinal plants are also an integral part of several traditional formulations that have been in use for a long time.
This review discusses the possible alternative strategies for the management of the SARS-CoV-2 infection by reducing its morbidity in patients as an adjuvant to modern therapy and also by providing prophylactic management. Further, potential testing targets of botanicals from Indian medicinal plants need to be explored against SARS-CoV-2 infection and categorized on a priority basis in view of their reported antiviral, immunomodulatory and other related activities.
There is plenty of data supporting the effectiveness of herbs in treating the viral infection. For instance, in controlling the contagious disease spread in the Guangdong Province of China during the 2003 SARS outbreak (Zhang et al., 2020). There are convincing pieces of evidence to establish that traditional Chinese medicine (TCM) has favorable effect in the treatment or prevention of SARS (Yang et al., 2020). A combination of modern and traditional therapy might reduce the severity of the disease, intensity of symptoms, death rate, and side effects. Similar are the observations for Shuanghuanglian (A Chinese medicine) a liquid composed of a blend of honeysuckle, Chinese skullcap, and forsythia, which is claimed to have antiviral, antibacterial, and immunomodulatory effects (https://www.bioworld.com/). Since AYUSH encompasses five different systems of medicine, rich in a variety of traditional formulations, it is likely to have a better chance than other systems to come up with a satisfactory solution to the COVID-19 crisis.
Ayurveda means ‘Science of life’. It provides a complete system to have a long and healthy life. It is derived from the concepts of “Dinacharya” - daily regimes and “Ritucharya” - seasonal regimes to maintain a healthy life. Uplifting and maintaining the immunity is duly emphasized across the Ayurveda’s classical scriptures.
The Unani system of medicine, known as Greco-Arab Medicine, is built on the four conditions of living (hot, sodden, frosty, and dry) and four humors of Hippocratic hypothesis namely, blood, yellow bile, dark bile, and mucus. Epidemics, referred to as waba in the Unani system of medicine, are thought to occur if any contagion or ajsam-i -khabitha, finds a place in air and water. Furthering the view, Ibn-e-Sina (980–1035 CE) stated that epidemics spread from one person to another, and one city to another ‘like a message’ (Sina, 1878).
AYUSH systems of medicine propagate general preventive measures aimed at preventing the spread of infection such as social distancing, hygiene and anti-septic measures (sanitization of surroundings), improvement of immunity, and promotion of general health (dietary modifications and herbal drugs). The present article elucidates some traditional Indian AYUSH formulations with proven antiviral, anti-asthmatic, and immunomodulatory activities, however their role in combating COVID-19 needs to be established. Clinical trials of AYUSH medicines like Ashwagandha, Yashtimadhu, Guduchi, Pippali, and AYUSH-64 on patients, health workers, and those working in high-risk areas have been initiated in India by the Ministry of AYUSH, Ministry of Health and Family Welfares, and the Council of Scientific and Industrial Research (CSIR) with the technical support of Indian Council of Medical Research (ICMR) (Table 1).
Based on the different systems of Indian Medicine, separate recommendations have been issued from time to time from the Ministry of AYUSH (Government of India) for the management of COVID-19. These different approaches are being followed by the Hospitals as per their specialization, mainly as adjuvants to modern medicine, which could be potentially relevant for COVID 19 treatment. Details of recommended formulations are described below and depicted in Table 2 (Ayurveda), Table 3 (Unani) and Table 4 (Siddha).
Ministry of AYUSH promotes the use of AYUSH kwath, which is a ready-made formulation for health promotion of the masses. The formulation is made of four herbs Ocimum sanctum L. leaves, Cinnamomum verum J. Presl. stem barks, Zingiber officinale Roscoe rhizomes and Piper nigrum L. fruits. The formulation is sold in the market with different names like ‘AYUSH Kwath’, ‘AYUSH Kudineer’ or ‘AYUSH Joshanda’. It is available in powder and tablet forms in the market. These herbs are reported to boost immunity (Carrasco et al., 2009; Niphade et al., 2009; Alsuhaibani and Khan, 2017; Bhalla et al., 2017) and are active remedies to various viral diseases (Mair et al., 2016; Ghoke et al., 2018; Pruthvish and Gopinatha, 2018).
Samshamani vati (Guduchi ghana vati) is an ayurvedic formulation used in all types of fevers. It is also used as an antipyretic and anti-inflammatory remedy (Patgiri et al., 2014). Samshamani vati is made of aqueous extract of Tinospora cordifolia (Willd.) Miers (family Menispermaceae), and reported to be an immunomodulator (More and Pai, 2011) due to the synergistic effect of the various compounds present. It is also effective in various viral diseases (Sachan et al., 2019).
AYUSH-64 tablet is composed of Alstonia scholaris (L.) R. Br. bark, Picrorhiza kurroa Royle ex Benth. rhizomes, Swertia chirayita (Roxb.) H. Karst. whole plant, and Caesalpinia crista L. seed pulp. Because of its antimalarial activity, AYUSH-64 is considered to be effective among the high-risk coronavirus population. Researchers have reported that each of its constituents is effectively antiviral, anti-asthmatic, and immunoboosting (Sharma et al., 1994; Siddiqui et al., 2012; Sehgal et al., 2013; Panda et al., 2017; Win et al., 2019; Woo et al., 2019).
Agastya Haritaki Rasayana is a popular ‘Avaleha kalpana’, used in the management of various respiratory infection and comprises more than 15 herbal ingredients. Most of its ingredients showed antiviral, anti-asthmatic, anti-inflammatory, and immunomodulatory activities (Mouhajir et al., 2001; Tripathi and Upadhyay, 2001; Balasubramanian et al., 2007; Vadnere et al., 2009; Patel and Asdaq, 2010; Pathak et al., 2010; Jain et al., 2011; Kumar et al., 2011; Lampariello et al., 2012; Jiang et al., 2013). The above literature suggests the symptomatic management of COVID-19 by Agastya Haritaki.
Anuthaila consists of about twenty ingredients and out of them Leptadenia reticulate (Retz.) Wight and Arn. has been reported in allergic response, treatment of asthma, bronchitis, and throat trouble (Mohanty et al., 2017). Similarly, Ocimum sanctum L. is recommended for a wide range of conditions including, cough, asthma, fever, and malaria (Cohen, 2014) and Sesamum indicum L. oil for dry cough, asthma, migraine, and respiratory infections (Nagpurkar and Patil, 2017). There are reports on S. indicum seeds with Tachyspermum ammi (L.) Sprague seeds for dry cough, asthma, lung diseases, and common cold (Patil et al., 2008). On the basis of above literature, Anuthaila justifies its use in corona virus pandemic condition (Table 2).
Triyaq-e-Araba is an important Unani formulation used as a detoxifying agent. It contains Laurus nobilis L. berries, Bergenia ciliate (Haw.) Sternb. stem, Aristolochia indica L. roots and Commiphora myrrha (Nees) Engl. It has been reported by several authors as a potent antiviral agent (Aurori et al., 2016), including against SARS-CoV (Loizzo et al., 2008). Further, B. ciliata is found to be effective against the influenza virus-A and herpes simplex virus-1 (HSV-1) (Rajbhandari et al., 2003), whereas its active principal, bergenin, has been found to be effective against hepatitis C virus (HCV) and HIV virus (Ahmad et al., 2018). On the basis of this literature, Triyaq-e-Araba could be one of the effective antiviral medicine and certifies its use against COVID-19.
Roghan-e-Baboona is an Unani remedy utilized as an anti-asthmatic and for the treatment of inflammatory complaints. Flowers of Matricaria chamomilla L. are the main ingredient of Roghan-e-Baboona. It is composed of the flowers of M. chamomilla, which is found effective for acute viral nasopharyngitis (Srivastava et al., 2010), as well as for sore throat (Kyokong et al., 2002).
Arq-e-Ajeeb is a liquid preparation that contains thymol, menthol, and camphor. Thymol is a promising candidate for topical application as an antiviral agent for herpetic infections (Lai et al., 2012; Sharifi-Rad et al., 2017). Menthol has been reported as an anti-inflammatory agent (Zaia et al., 2016). The Unani physicians have a very successful history of treating Nazla wabai (Swine flu) using Arq -e-Ajeeb. These studies support the use of Arq-e-Ajeeb for COVID-19.
Khamira-e-Banafsha is a semi-solid Unani formulation prepared by adding decoction of flowers of Viola odorata L. to a base of sugar or sugar with honey and used for cold-cough as expectorant and for the treatment of ailments of respiratory system and chest diseases, bronchitis, whooping cough, fever, expectorant, antipyretic etc. Further, V. odorata has been reported to suppress the viral load and increase antiretroviral drug efficacy (Gerlach et al., 2019), decrease the thickness of the alveolar wall, hemorrhage area, and alter the epithelial lining of bronchioles of the lungs (Koochek et al., 2003). The above literature supports its use for the management of COVID-19.
Laooq-e-Sapistan is a semisolid sugar-based polyherbal Unani formulation extensively used by the masses in India for the treatment of cold and cough, whooping cough, and phlegm. It reduces inflammation of the pharynx, tonsils, and irritation or infection. The jelly like sticky mass of ripe fruit of Cordia myxa L. is the main ingredient, which has been reported as antiviral and antitussive (Jamkhande et al., 2013). Another important constituent is Ziziphus fruit, which contains betulinic acid. Literature showed the down-regulation of IFN-γ level by betulinic acid in mouse lung, thus enhancing immunity and suggested as potential therapeutic agent for viral infections (Hong et al., 2015). Aqueous extract also reported increasing thymus and spleen indices as well as enhance the T-lymphocyte proliferation, hemolytic activity, and natural killer (NK) cell activity (Yu et al., 2016). Viola odorata L., one of its ingredients, suppresses the viral load (Gerlach et al., 2019). Hence, the literature supports the use of AYUSH formulation Laooq-e-Sapistan in COVID-19.
Sharbat-e- Sadar is an Unani polyherbal syrup formulation and is widely used for common cold, cough and respiratory diseases. Trachyspermum ammi (L.) Sprague, an important ingredient, reported to neutralize antibodies for Japanese encephalitis virus (Roy et al., 2015), and a glycoprotein was found to proliferate B-cells (Shruthi et al., 2017). Adhatoda vasica Nees inhibits HIV-Protease (Singh et al., 2010), Bombyx mori was reported to increase immune responses against viral infection (Lü et al., 2018). Other ingredients such as Glycyrrhiza glabra L., Ficus carica L., Onosma bracteatum Wall., and Ziziphus jujuba Mill. also possess the antiviral and immunomodulatory activities, as summarized in Table 5.
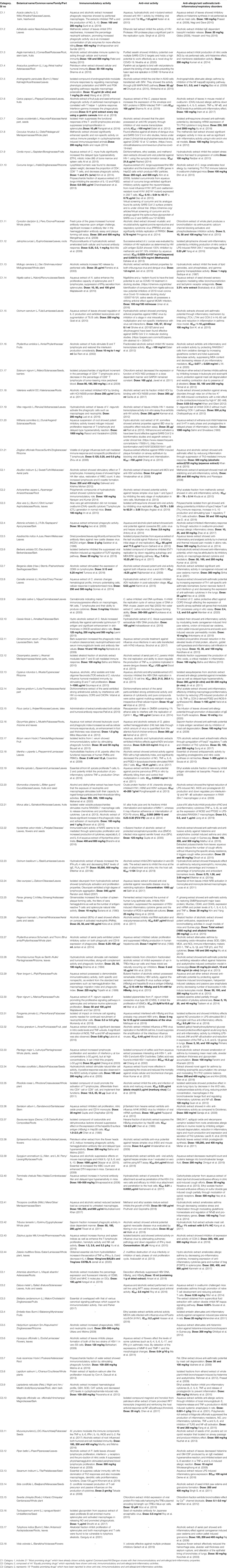
TABLE 5. List of Indian Medicinal Plants/AYUSH drugs with proven immunomodulatory, antiviral and anti-allergic/anti-inflammatory/anti-asthmatic activity having potential for exploring against COVID 19 categorized for prioritization on the basis of their earlier reports.
Khameera marwareed is a compound, sugar-based, semisolid Unani formulation used as an immunomodulator. It has been reported to stimulate the immune system through T helper 1 (Th1) type cytokine response and maintains the body in a healthier position to fight against viral infections (Khan et al., 2009). Its ingredients showed powerful antiviral activities by inhibiting replication (Benencia and Courrèges, 1999).
Asgand (Withania somnifera (L.) Dunal) is a very popular Indian medicinal plant. The root powder is used in the Unani system of medicine as an immunomodulator. It is reported that the root’s extract significantly increases the CD4+ and CD8+ counts (Bani et al., 2006) and blood profile, especially WBC and platelet counts (Agarwal et al., 1999). Aqueous suspension showed potent inhibitory activity toward mitogen-induced proliferative response of T-lymphocytes and prevent SARS-CoV-2 entry by disturbing connections between viral S-protein receptor binding domain and host ACE2 receptor (Balkrishna et al., 2020). The above literature supports the preventive use of Asgandh safoof against COVID-19.
Habb-e-Bukhar is a polyherbal tablet formulation of Unani system of medicine, prescribed in elephantiasis and malarial fever. The main ingredient of Habb-e-Bukhar is cinchona bark. Its active constituent quinine is being used by some countries as either experimental treatment or suggested as a drug with a promising profile against COVID-19 (Devaux et al., 2020). Another constituent, Tinospora cordifolia (Willd.) Miers is reported as potent antiviral agent against HSV (Pruthvish and Gopinatha, 2018) as well as suggested for immune-enhancing activity (Rastogi et al., 2020). Thus, literature supports Habb-e-Bukhar in the treatment of COVID-19.
Sharbat-e-Toot Siyah is composed of the juice of Morus nigra L. in a sugar base and is used to treat tonsillitis and sore throat. It has been reported as anti-inflammatory and analgesic and inhibits the pro-inflammatory cytokines (Chen et al., 2016). Very recently, it has been reported to enhance immunomodulatory activity (Lim and Choi, 2019).
Laook-e-Katan is a sugar-based semisolid Unani formulation composed of Linum usitatissimum L. seed, which contains alpha linolenic acid and has been reported to have antiviral, anti-inflammatory, and immunomodulatory activities (Leu et al., 2004; Erdinest et al., 2012; Miccadei et al., 2016). In Unani, it is recommended for respiratory disorders (Table 3).
Nilavembu Kudineer is a polyherbal Siddha formulation prescribed for the prevention and management of viral infections and fevers. It acts as an immunomodulator and plays a defending role against dengue fever and chikungunya. Recent studies showed that formulation has antiviral and antimicrobial actions, which makes it suitable for viral fevers, malaria, and typhoid fever (Mahadevan and Palraj, 2016). Previously, studies proved that most of its constituents are effective as antiviral, anti-asthmatic, and immunobooster agents (Carrasco et al., 2009; Wang et al., 2010; Jin et al., 2011; Chang et al., 2013; Wintachai et al., 2015; Mair et al., 2016).
Ahatodai Manapagu is composed of Adathoda vasica Nees leaves, which contains alkaloids like vasicine, the active ingredient in various cough syrups. A. vasica has been used in the Indian medicinal system for thousands of years, to treat various types of respiratory disorders (Sampath Kumar et al., 2010). Vinothapooshan et al. suggested that its extract positively modulates the immunity of the host (Vinothapooshan and Sundar, 2011).
Kabasura Kudineer is a traditional formulation used in the Siddha system of medicine for managing common respiratory complaints such as flu and cold. Siddha practitioners also recommended this formulation for severe phlegm, dry cough, and fever. It is made up of more than ten herbal ingredients, and each ingredient has a unique pharmacological activity in respiratory disorders. Hence, the ministry of AYUSH recommends its use for symptomatic management in COVID-19 (Sampath Kumar et al., 2010; Jin et al., 2011; Vinothapooshan and Sundar, 2011; Chang et al., 2013).
In addition to Ayurvedic, Unani, and Siddha formulations recommended by AYUSH there are some homeopathic formulations such as Arsenium album, Brayonia alba, and Rhus toxicodendrum have been recommended which have not been included due to controversies over the use of homeopathic medicine. These formulations are prepared by dilutions in such a way so that no single detectable molecule is present in the final formulation, which results in controversy (Ernst, 2010). The criticism is due to nonevidential rationale to determine the biological effects of solutions containing unmeasurable starting material (Kaur, 2013).
Further, advancements in pathogenesis and understanding of diseases provide a wider platform to report the pharmacological limitations and opportunities of these highly diluted homeopathic medicines. Day by day, it is becoming more challenging for a pharmacologist to validate the therapeutic claims of homeopathic medicines through experiments. Low acceptance of homeopathic formulations is due to the absence of standardized protocols to justify their pharmacological potential. A major concern is to develop evidence-based validated methods and advancements in the homeopathic system to justify its measurable dilutions, which will help in understanding the mechanism of action and acceptability of homeopathic medicine (Table 4).
Ashwagandha, giloe, ginger, cinnamon, tulsi, black pepper, black cumin, amla, turmeric, garlic, and flax seeds have been traditionally used as herbal remedies for multiple diseases since ancient times. These herbs have been utilized in food preparations and traditional medicines in several countries. However, in India, their culinary use is very common and they are a part of kitchen in every house. Similarly, there are some traditional Indian formulations such as Chyawanprash, Triphala, and Rooh Afza etc. that are very commonly used in Indian territory as a part of daily used nutritional supplements. These plants and formulations are very common and at least one of them is being used daily by every Indian, irrespective of religion/community/financial status. The above-mentioned herbs and formulations have been proved potent scientifically for their immunomodulatory, antioxidant, and anti-infective properties, which might be one of the reasons behind the lower death rate of Indians per million of population due to COVID-19 even with minimum health infrastructure.
Various research has been conducted in vivo to highlight the effect of A. sativum in immunomodulation using garlic oil extract. The results showed reduction in serum TNF-α, ICAM-1 and immunoglobulin (G and M) levels confirming the enhancement in immune system activity (Kamel and El-Shinnawy, 2015). Pre-treatment with aqueous garlic extract showed notable antiviral effects mainly by reduction in infectivity and titer of virus against the velogenic strain of Newcastle disease virus in embryonated chicken eggs (Arify et al., 2018). A. sativum also showed antiviral effect against avian influenza virus H9N2 on Vero cells (Rasool et al., 2017). Its defensive effect on allergen-induced airway inflammation in rodent model showed significant reduction in inflammatory cell count, eosinophil infiltration and serum IgE modulation of Th1, Th2, and Th3 cytokines, upregulation of Th-1, Th-3 and simultaneous down-regulation of Th-2 expression. (Hsieh et al., 2019). Old extract of A. sativum showed modulation of airway inflammation established in BALB/c mice by reduction in percentage of eosinophil, lavage and serum IgG1 levels, and perivascular inflammation. The study suggested the attenuation of allergic airway inflammation by aged garlic extract (Zare et al., 2008). It has been found that fresh raw garlic extract showed anti-inflammatory effects by decreasing production of prostaglandin E2 (PGE2), IL-6, IL-1β, nitric oxide (NO), and leukotrienes (LT D4 and E4) in lipopolysaccharide activated RAW264.7 cells (Jeong et al., 2016).
C. verum essential oil and powder exhibited anti-oxidant, immunostimulant, and antiviral activity in Newcastle disease virus in chickens mainly by modulating total protein, globulin, total antioxidant capacity, and lysozyme activity, and significantly increased phagocytic activity (Islam et al., 2017). Another study reported that C. zeylanicum essential oil when blended with other essential oils showed effective antiviral potential against H1N1 and HSV1 viruses. Reduction in virus infectivity has been observed with 99% at 60-min contact time and more than 99.99% after 60 min for both H1N1 and HSV1 viruses (Brochot et al., 2017). Its bark extract exhibited immunomodulatory activity and significantly increased serum immunoglobulins, phagocytic index, neutrophil adhesion, and antibody titer (Niphade et al., 2009). Procyanidine polyphenols (Type A) extracted from C. zeylanicum bark showed anti-inflammatory potential in edema induced by carrageenan (Vetal et al., 2013). Alcoholic extract of bark suppressed intracellular release of TNF-α (murine neutrophils) and leukocytes (pleural fluid) as well as inhibition of TNF-α gene expression in lipopolysaccharide-stimulated human peripheral blood mononuclear cells (Joshi et al., 2010).
Aqueous extract of C. longa decreased relative spleen weight and modulation in hematological changes indicating the potential of C. longa as an immunomodulator in cyclophosphamide-immunosuppressed in vivo model. The study observed promising effects of turmeric as an immunomodulator by representing spleen cells in younger mice (Mustafa and Blumenthal, 2017). C. longa extract also showed antiviral potential against dengue virus in in vitro and in vivo studies on Huh7it-1 cells and a remarkable reduction in viral load has been observed by in in vivo model (Ichsyani et al., 2017). Water and ethanolic crude extracts have been found to be antiviral in H5N1 also showed upregulated TNF-α as well as IFN-β mRNA expression, highlighting its promising role in the inhibition of the replication of viruses (Sornpet et al., 2017). Turmeric extract has been found to be anti-allergic in mice immunized with ovalbumin and alum. Attenuation of food allergy by maintaining balance of Th1/Th2 has been reported. Extract has been found to cause reduction in Th2 and increase in Th1 cell-related cytokines. Further, increased levels of IgE, IgG1 and mMCP-1 levels were also decreased proving effects of turmeric in allergic disorders mainly, asthma and food allergies (Shin et al., 2015). Various other studies also reported anti-inflammatory effects of C. longa either alone or in combination (Lee et al., 2020).
Heteropolysaccharide, extracted from flax seed hull possessed immunomodulatory activity and anti-hepatitis B virus potential. It significantly stimulated mRNA expression of TNF-α, NO and IL exhibiting immune responses in murine macrophages. Antiviral activity has been reported through inhibition of expression of surface antigen as well as envelop antigen and also interfered with DNA replication. The study suggested its promising potential as an immunostimulant and vaccine adjuvant (Liang et al., 2019). It showed anti-inflammatory and immunomodulatory potential in obesity-associated insulin resistance. Its oil in co-culture with 3T3-L1 adipocytes-RAW 264.7 macrophages of C57BL/6 mice reported shifting the cytokines toward anti-inflammatory with a decrement in TNF-α. Immunomodulation has been observed through an increase in levels of Th2-related cytokine (IL-4), serum anti-ova IgG1, and IgE, and a decrease in Th-1 related cytokines (TNF-α and IFN-γ) and anti-ova IgG levels (Palla et al., 2015). Another study reported the immunomodulatory activity of phenolic components of flax seed mainly through reduction in cell-mediated immune responses (Kasote et al., 2012).
Nigella sativa L.’s bioactive compounds have been observed as potential inhibitors of COVID-19 in molecular docking studies. Nigellidine gave energy complex at active site (6LU7) with energy scores closest to chloroquine and better than hydroxychloroquine and favipiravir whereas α-hederin gave energy complex at the active site (2GTB) with energy scores better than chloroquine, hydroxychloroquine, and favipiravir (Salim and Noureddine, 2020). The alcoholic seed extract has shown immunosuppressive activity on a phytohemagglutinin and immunostimulating effect on non-phytohemagglutinin (PHA) stimulated proliferation (Alshatwi, 2014). The thymoquinone-rich oil showed suppression of cytokine signaling molecules, and PGE2 in T-lymphocytes as well as enhanced PGE2 release in adrenocarcinomic human alveolar basal epithelial A549 cells (Koshak et al., 2018).
Hydro-alcoholic extract of Ocimum sanctum inhibited intracellular multiplication of virus. It also inhibits non-specific interference with virus-cell interactions in H9N2 viruses. (Ghoke et al., 2018). The immunomodulatory potential of alcoholic leaves extracts at IC50 value of 73.3 μg/ml showed reduction in hepatic parasite and, skewing of the humoral response toward Th1 type (Bhalla et al., 2017). O. sanctum inhibits leukotriene-C4-synthase, leukotriene-A4-hydrolase and cyclooxygenase-2 activities in cultured HL-60 cells and causes a significant reduction in OVA-induced lung inflammation (Soni et al., 2015).
Amla has been reported to significantly relieve chromium-induced immunosuppressive effect on lymphocyte proliferation and led to restoration in production of IL-2 and INFγ (Sai Ram et al., 2002). Phenolics from emblica has been found to increase splenocytes proliferation. Geraniin and isocorilagin showed significant immunostimulatory effects (Liu et al., 2012). Ethanolic extract of amla strongly reduced levels of pro-inflammatory cytokines and increased levels of anti-inflammatory cytokine (Bandyopadhyay et al., 2011). An isolated compound (1, 2, 4, 6-tetra-O-galloyl-β-d-glucose) of P. emblica showed antiviral potential against HSV by HSV-1 inactivation, which leads to inhibition of early infection indulging attachment and penetration of virus, suppression of intracellular growth and inhibited gene expression of HSV-1 E and L along with DNA replication (Xiang et al., 2011).
Piperamides isolated from P. nigrum fruits showed significant inhibition of coxsackie virus type B3 in a cytopathic effect inhibition assay (Mair et al., 2016). Aqueous extract of P. nigrum acted as a potent modulator of the macrophages and significantly enhanced splenocyte proliferation in a dose-dependent manner (Majdalawieh and Carr, 2010). The isolated alkaloid from P. nigrum exhibited anti-inflammatory effect in RAW 264.7 cells stimulated by LPS and significant inhibition in iNOS-mediated NO and IL-1β, IL-6, and TNF-α. It also demonstrated anti-inflammatory activity in edema induced by carrageenan (Pei et al., 2020). Reports have confirmed the improvement of ovalbumin-induced nasal epithelial barrier dysfunction in allergic rhinitis mouse model. Further, protection of epithelium integrity, enhancement in E-cadherin tight junction protein as well as inhibition of the degraded levels of zonula occludens-1 and occluding in the nasal passage have been reported. Additionally, enhancing the activation of Nrf2/HO-1 signaling showed anti-allergic and anti-asthma activities (Bui et al., 2020).
In vitro screening of T. cordifolia silver nanoparticles against chikungunya virus cell showed significant antiviral potential (Sharma V. et al., 2019). Alcoholic leaves extract of T. cordifolia significantly decreases intracellular reactive oxygen species (ROS) in chikungunya patients with high levels of intracellular ROS in persisting polyarthralgia by ex vivo treatment (Banerjee et al., 2018). An in vitro study revealed the antiviral potential of crude stem extract of T. cordifolia against HSV in Vero cell lines by inhibiting the growth of HSV (Pruthvish and Gopinatha, 2018). Aqueous extract of T. cordifolia stem significantly increase INFγ and IL levels (IL-1, IL-2, IL-4) in isolated chicken peripheral blood mononuclear cells (PBMCs) against infectious bursal disease virus. Further, immunomodulatory potential via the toll like receptor (TLR)-mediated pathway was also concluded (Sachan et al., 2019). The hydro-alcoholic extract of T. cordifolia stem in drinking water caused enhancement of cellular immunity as well as humoral immunity in broiler chicks (Nety et al., 2017). Chloroform extract significantly prevented pro-inflammatory biomarkers (IL-6, IL-1β and PGE2) and decreased paw oedema (p ≤ 0.05) with no toxicity reported when conducted in RAW264.7 macrophages (Philip et al., 2018).
Multiple studies have proved that Ashwagandha has antiviral and immunomodulatory potential. Very recently, an in silico study concluded that Withaferin-A exhibits antiviral potential against SARS-CoV-2 through inhibiting RNA polymerase with higher binding energy than hydroxychloroquine and other drugs used against SARS-CoV-2. Another study on withanone showed blockage of SARS-CoV–2 entry and also its subsequent infection by interrupting electrostatic interactions between the RBD and ACE2 (Balkrishna et al., 2020). Grover and colleagues through molecular docking reported the potential of withaferin A against HSV through inhibition of DNA polymerase enzyme (Grover et al., 2011). W. somnifera molecular mechanism has been elucidated by using network ethnopharmacological technique and reported that withanolide-phytosterol combination is a good immunomodulator (Chandran and Patwardhan, 2017). W. somnifera formulation (supplemented with minerals) has been reported to improve both cellular and humoral immunity as well as hematological profile in addition to the significant inhibition in mouse splenocytes (Trivedi et al., 2017). Aqueous root extract of W. somnifera attenuates production of pro-inflammatory cytokines and transcription factor in collagen-induced arthritis (Khan et al., 2018). A study in 2018 showed that W. somnifera significantly inhibited mRNA expression of inflammatory cytokines and promotes the mRNA expression of the anti-inflammatory cytokine in HaCaT cells (Sikandan et al., 2018).
Fresh ginger aqueous extract showed antiviral activity against human respiratory syncytial virus in human respiratory tract cell lines (HEp-2 and A549) and decreased the plaque counts in a dose-dependent manner. It also stimulated the secretion of IFN-β that contributes to counteracting against viral infection (Chang et al., 2013). It also showed antiviral potential against avian influenza virus H9N2 on Vero cells in a dose-dependent manner (Rasool et al., 2017). Oral administration of Soft gel capsules containing a Z. officinale in combination showed immunomodulatory and anti-inflammatory properties parallel to those exerted by positive control, and gene expression data highlighted overall same transcriptional remodeling (Dall’Acqua et al., 2019). A study on essential oil of ginger reported immunomodulatory effects by improving the humoral immunity in cyclophosphamide-immunosuppressed mice in a dose-dependent manner (Carrasco et al., 2009). Oral administration of alcoholic ginger extract to allergic rhinitis patients showed significant reduction in total nasal symptom scores (TNSS), with overall improvement in rhino conjunctivitis quality of life questionnaire (Yamprasert et al., 2020). The aqueous and alcoholic extracts of rhizome decreased goblet cell hyperplasia, infiltration of inflammatory cells in airways with reduced total and differential counts of eosinophils and neutrophils in mouse model (Khan et al., 2015) (Table 5).
Chyawanprash is an Ayurvedic polyherbal health supplement, which is made up of concentrated extracts of nutrient-rich herbs and minerals. Chyawanprash comes under Awaleha (electuaries/herbal jams) due to its consistency, and composed of Amla fruit as a base, which is considered as the most active Rasayana to improve strength, stamina, and vitality.
Although several types of research have been published on Chyawanprash to report its health benefits against various ailments, the study reports antioxidant (Anil and Suresh, 2011) free radical scavenging (Bhattacharya et al., 2002) antibacterial, antiviral, anti-inflammatory, antiallergic, and antithrombotic effects (Gupta et al., 2017). In a randomized controlled trial, it was found effective for pulmonary tuberculosis as an adjunct to antitubercular drugs. (Debnath et al., 2012; Sharma R. et al., 2019). An experimental study showed that Chyawanprash pre-treatment reduced plasma histamine levels and IgE release when rats and mice were challenged with allergen- and ovalbumin-induced allergy, suggesting its anti-allergic potential. NK cell activity was significantly increased by Chyawanprash treatment. On treating dendritic cells with Chyawanprash, there was a significant increase in immunity marker levels as well as phagocytic activity that proves its immunomodulatory activity (Sastry et al., 2011).
Triphala is a well-known polyherbal Ayurvedic medicine consisting of equal proportions of fruits of Phyllanthus emblica L., Terminalia bellerica (Gaertn.) Roxb. and Terminalia chebula Retz. in the form of powder for digestive and refreshing action. Triphala is associated with many of the therapeutic potentials such as antioxidants, antiinflammatory, antineoplastic, antimicrobial, antidiabetic, etc. (Peterson et al., 2017). Alcoholic extract of Triphala showed specific antimicrobial activity (Tambekar and Dahikar, 2011), broad-spectrum antimicrobial activity against antibiotic-resistant bacteria isolated from humans (Peterson et al., 2017).
Triphala extract was found more active than the NSAID drug, indomethacin, in improving arthritic and inflammatory effects and reduced expression of inflammatory mediators through inhibition of NF-κB activation (Kalaiselvan and Rasool, 2015). In LPS-stimulated macrophages, Triphala inhibited the production of inflammatory mediators, intracellular free radicals, and inflammatory enzymes (Reddy et al., 2009; Kalaiselvan and Rasool, 2016). It has been shown to reduce multiple cell signaling pathways of inflammation and oxidative stress and prevented the noise-stress induced changes in rats thereby strengthening the cell-mediated immune response (Prasad and Srivastava, 2020). A clinical study of Triphala showed immunostimulatory properties on T cells and NK cells, however did not change the cytokine levels in healthy volunteers (Phetkate et al., 2012). The individual constituents of Triphala have also showed immunomodulatory activity (Aher and Wahi, 2011). The stated data on Triphala reveals that it is a powerful polyherbal formulation with countless therapeutic uses for maintaining homeostasis as well as the cure and management of various disease.
Rooh Afza is a well-known refreshing formulation with global acceptance. It is a concentrated squash prepared as sugar syrup with distillates of numerous medicinal plants including seeds of khurfa (Portulaca oleracea L.), kasni (Cichorium intybus L.), angoor (Vitis vinifera L.), nilofar (Nymphaea alba L.), Neel Kamal (Nymphaea nouchali Burm. f.), kamal (Nelumbo nucifera Gaertn.), gaozaban (Borago officinalis L.), badiyan (Coriandrum sativum L.), fruits/juices of santara (Citrus × sinensis (L.) Osbeck), ananas (Ananas comosus (L.) Merr.), seb (Malus domestica (Suckow) Borkh.), berries (Rubus fruticosus L.), vegetables like palak (Spinacia oleracea L.), gazar (Daucus carota L.), and pudina (Mentha arvensis L.). Rooh Afza boosts the energy system of the body by naturally refreshing. Although there is no evidence on Rooh Afza revealing its therapeutic value, its constituents have been reported as potently antiviral, immunomodulatory, and antiallergic against respiratory disorders.
The flower extract of P. oleracea possessed significant antioxidant and protective effects against DNA damage induced by necrotic effects (Dogan and Anuk, 2019). V. vinifera fruits exhibit anti-asthmatic activity by inhibiting cellular response and subsequent production of inflammatory cytokines (Arora et al., 2016). A study on N. alba flower has been reported against inflammatory activity in Swiss Albino mice using acute inflammatory models in a dose-dependent manners (RS et al., 2013). The immunoregulatory and anti-HIV-1 enzyme activities of N. nucifera suggest that it could be potentially important against virus development (Jiang et al., 2011).
Thus, it can be perceived that Rooh Afza not only provides natural refreshness to the body but also has antioxidant, immunomodulatory, and anti-inflammatory/antiviral activities. However, to validate the scientific data on the therapeutic value of Rooh Afza, experimental research should be undertaken to prove its role in health benefits therapeutically.
The above studies encourage further investigations of traditional medicinal plants for their preventive use against coronavirus infection. The herbs could be taken individually or synergistically at appropriate concentrations as candidates for developing potential therapeutic tools against COVID-19.
There are many other Indian medicinal plants, which are either part of AYUSH recommendations as such or as ingredients of formulations or are known for improving immunity with antiviral and anti-allergic/anti-inflammatory potential and can offer potential leads against COVID-19. Table 5 provides a list of 83 medicinal plants categorized on a priority basis as per their reported properties. Category 1 (C1) includes 21 “Most promising drugs” which have already shown activity against Coronaviruses/HIV/Dengue viruses with their immunomodulatory and anti-allergic/anti-inflammatory properties. Category 2 (C2) is composed of 44 “Equally promising drugs” which reportedly have shown anti-viral, immunomodulatory, and anti-allergic/anti-inflammatory activities. Category 3 (C3) represents 18 “Possibly promising drugs” which have been reported to show anti-viral/immunomodulatory and/or anti-allergic/anti-inflammatory activities.
Listed medicinal plants and AYUSH recommended formulations could help as the potential alternate therapeutics for management and cure of COVID-19. However, this needs scientific explorations and validation of their preclinical and clinical studies. Since there is such a rich diversity, many other medicinal plants and their bioactive fractions need the attention of the scientific community to be explored against COVID-19.
The SARS-CoV-2 has become a threat to human population due to non-availability of approved vaccines or drugs for its treatment. Many herbs that have been reported to work as an immunity booster against other viral infections, and to possess anti-allergic/anti-inflammatory activities, need to be tested against COVID-19. Indian Traditional Medicines have a wide potential for being used in these tough times either for prophylaxis or as adjuvant, owing to their longstanding use in community, ancient references and scientific evidence about their safety and clinical efficacy. The AYUSH ministry, Govt of India has issued several advisories from time to time, considering the strength and evidence of these systems of medicines and making considerable efforts to encourage researchers to explore herbal products for COVID-19. Interventions and herbal formulations from different AYUSH systems have the support of evidence for their immunity-enhancing, anti-inflammatory and antiviral effects. These herbal remedies may, therefore, provide some respite until the availability of trial-tested drug or vaccine to combat the COVID-19 menace. Further, it was noted that a major portion of public and private funding were dedicated to AYUSH trials. More than 50% of these trials were sponsored by the government and various stakeholders associated with the Ministry of AYUSH. It is expected that the results of these clinical studies will be disseminated soon at the public platform so that the policymakers from the AYUSH systems of medicines may reframe their policies for public health and provide information to the global scientific community, which could form a platform for collaborative studies at the national and global levels. The medicinal plant species discussed in this review and categorized for their preclinical and clinical investigation may be taken up by research organizations on priority basis, as this may result in the development of lead molecule against SARS-CoV-2 and COVID-19. Keeping in view the potential of AYUSH medicines and medicinal plants of India, the herbal drug, manufacturers, and the national and global research organizations should develop necessary strategies for furtherance of preclinical and clinical research on these promising therapeutic leads.
SA: conceptualization, methodology, writing - reviewing and editing; SZ and BP: data curation, writing - original draft preparation; PB, GG, and AP: visualization, investigation; RP and MA: software, validation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The corresponding author is thankful to Ministry of AYUSH, Govt of India and DBT for providing funding at Bioactive Natural Product Laboratory, Jamia Hamdard for Research on AYUSH drugs and Medicinal Plants of India.
Abd-Alla, H. I., Moharram, F. A., Gaara, A. H., and El-Safty, M. M. (2009). Phytoconstituents of Jatropha curcas L. Leaves and their immunomodulatory activity on humoral and cell-mediated immune response in chicks. Zeitschrift fur Naturforsch C. 64 (7-8), 495–501. doi:10.1515/znc-2009-7-805
Abe, T. (2020). Fig (Ficus carica L.) leaf tea suppresses allergy by acceleration disassembly of IgE-receptor complexes. Biosci. Biotechnol. Biochem. 84 (5), 1013–1022. doi:10.1080/09168451.2020.1722608
Adhikari, P. P., and Paul, S. B. (2018). History of Indian traditional medicine: a medical inheritance. Asian J. Pharm. Clin. Res. 11 (1), 421. doi:10.22159/ajpcr.2018.v11i1.21893
Agarwal, P., Sharma, B., Fatima, A., and Jain, S. K. (2014). An update on ayurvedic herb convolvulus pluricaulis choisy. Asian Pac. J. Trop. Biomed. 4 (3), 245–252. doi:10.1016/S2221-1691(14)60240-9
Agarwal, R., Diwanay, S., Patki, P., and Patwardhan, B. (1999). Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extracts in experimental immune inflammation. J. Ethnopharmacol. 67 (1), 27–35. doi:10.1016/S0378-8741(99)00065-3
Aher, V., and Wahi, A. K. (2011). Immunomodulatory activity of alcohol extract of Terminalia chebula retz combretaceae. Trop. J. Pharm. Res. 10 (5), 567–575. doi:10.4314/tjpr.v10i5.5
Ahmad, M., Butt, M. A., Zhang, G., Sultana, S., Tariq, A., and Zafar, M. (2018). Bergenia ciliata: a comprehensive review of its traditional uses, phytochemistry, pharmacology and safety. Biomed. Pharmacother. 97, 708–721. doi:10.1016/j.biopha.2017.10.141
Al-Khalaf, M. I. (2013). Thyme and thymol effects on induced bronchial asthma in mice. Life Sci. J. 10 (2), 693–699.
Al-Snafi, A. E. (2018). Chemical constituents and pharmacological effects of fraxinus ornus-A review. Indo Am. J. Pharm. Sci. 5 (3), 1721–1727. doi:10.5281/zenodo.1210511
Alagar Yadav, S., Ramalingam, S., Jabamalai Raj, A., and Subban, R. (2015). Antihistamine from tragia involucrata L. leaves. J. Complementary Integr. Med. 12 (3), 217–226. doi:10.1515/jcim-2015-0015
Alamgeer, , Younis, W., Asif, H., Sharif, A., Riaz, H., Bukhari, I. A., et al. (2018). Traditional medicinal plants used for respiratory disorders in Pakistan: a review of the ethno-medicinal and pharmacological evidence. Chin. Med. 13 (1), 48. doi:10.1186/s13020-018-0204-y
Alhajj, M. S., Qasem, M. A., and Al-Mufarrej, S. I. (2020). Inhibitory activity of illicium verum extracts against avian viruses. Adv. Virol. 2020, 1–8. doi:10.1155/2020/4594635
Ali, W. R., Al-Asady, Z. T., and Ibrahim, A. A. J. (2015). Immunomodulatory of Cordia myxa (L.) aqueous extract fruit in immunized mice with hydatid cyst fluid. J. Nat. Sci. Res. 5 (10), 75–72.
Alshatwi, A. A. (2014). Bioactivity-guided identification to delineate the immunomodulatory effects of methanolic extract of Nigella sativa seed on human peripheral blood mononuclear cells. Chin. J. Integr. Med. [Epub ahead of print]. doi:10.1007/s11655-013-1534-3
Alsuhaibani, S., and Khan, M. A. (2017). Immune-stimulatory and therapeutic activity of tinospora cordifolia: double-edged sword against salmonellosis. J. Immunol. Res. 2017, 1–9. doi:10.1155/2017/1787803
Amin, A. H., Bughdadi, F. A., Abo-Zaid, M. A., Ismail, A. H., El-Agamy, S. A., Alqahtani, A., et al. (2019). Immunomodulatory effect of papaya (Carica papaya) pulp and seed extracts as a potential natural treatment for bacterial stress. J. Food Biochem. 43 (12), e13050. doi:10.1111/jfbc.13050
Amirghofran, Z., Azadbakht, M., and Karimi, M. H. (2000). Evaluation of the immunomodulatory effects of five herbal plants. J. Ethnopharmacol. 72 (1-2), 167–172. doi:10.1016/S0378-8741(00)00234-8
Ang, L., Lee, H. W., Choi, J. Y., Zhang, J., and Lee, M. S. (2020). Herbal medicine and pattern identification for treating COVID-19: a rapid review of guidelines. Integr. Med. Res. 9 (2), 100407. doi:10.1016/j.imr.2020.100407
Anil, M., and Suresh, P. (2011). Determination of free radical scavenging activity in herbal supplement: chyawanprash. Int. J. Drug Dev. Res. 3 (1), 328–333.
Ansari, S., Siddiqui, M. A., Malhotra, S., and Maaz, M. (2018). Antiviral efficacy of qust (Saussurea lappa) and afsanteen (Artemisia absinthium) for chronic Hepatitis B: a prospective single-arm pilot clinical trial. Pharmacogn. Res. 10 (3), 282. doi:10.4103/pr.pr-157-17
Antonisamy, P., Agastian, P., Kang, C. W., Kim, N. S., and Kim, J. H. (2019). Anti-inflammatory activity of rhein isolated from the flowers of Cassia fistula L. and possible underlying mechanisms. Saudi J. Biol. Sci. 26 (1), 96–104. doi:10.1016/j.sjbs.2017.04.011
Antony, M., Misra, C. S., and Thankamani, V. (2014). Evaluation of active fraction from plant extracts of Alstonia scholaris for its in-vitro and in-vivo antiviral activity. Int. J. Pharm. Pharm. Sci. 6 (Suppl 2), 775–781.
Arabzadeh, A. M., Ansari-Dogaheh, M., Sharififar, F., Shakibaie, M., and Heidarbeigi, M. (2013). Anti herpes simplex-1 activity of a standard extract of Zataria multiflora Boiss. Pak. J. Biol. Sci. 16 (4), 180–184. doi:10.3923/pjbs.2013.180.184
Arawwawala, M., Thabrew, I., Arambewela, L., and Handunnetti, S. (2010). Anti-inflammatory activity of Trichosanthes cucumerina Linn. in rats. J. Ethnopharmacol. 131 (3), 538–543. doi:10.1016/j.jep.2010.07.028
Arciniegas, A., Pérez-Castorena, A. L., Nieto-Camacho, A., Kita, Y., and De Vivar, A. R. (2017). Anti-hyperglycemic, antioxidant, and anti-inflammatory activities of extracts and metabolites from Sida acuta and Sida rhombifolia. Quim. Nova 40 (2), 176–181. doi:10.21577/0100-4042.20160182
Arify, T., Jaisree, S., Manimaran, K., Valavan, S., and Sundaresan, A. (2018). Antiviral effects of garlic (allium sativum) and nilavembu (andrographis paniculata) against velogenic strain of newcastle disease virus- an in ovo study. Int. J. Livest. Res. 8 (5), 157. doi:10.5455/ijlr.20170814051902
Arora, P., Ansari, S. H., Najmi, A. K., Anjum, V., and Ahmad, S. (2016). Investigation of anti-asthmatic potential of dried fruits of Vitis vinifera L. in animal model of bronchial asthma. Allergy Asthma Clin. Immunol. 12 (1), 42. doi:10.1186/s13223-016-0145-x
Arunadevi, R., Murugammal, S., Kumar, D., and Tandan, S. (2015). Evaluation of Caesalpinia bonducella flower extract for anti-inflammatory action in rats and its high performance thin layer chromatography chemical fingerprinting. Indian J. Pharmacol. 47 (6), 638–643. doi:10.4103/0253-7613.169582
Arya, S., and Kumar, V. L. (2005). Antiinflammatory efficacy of extracts of latex of Calotropis procera against different mediators of inflammation. Mediat. Inflamm. 2005 (4), 228–232. doi:10.1155/MI.2005.228
Ashraf, A., Ashraf, M. M., Rafiqe, A., Aslam, B., Galani, S., Zafar, S., et al. (2017). In vivo antiviral potential of Glycyrrhiza glabra extract against Newcastle disease virus. Pak. J. Pharm. Sci. 30 (Suppl 2), 567–572.
Aurori, A. C., Bobiş, O., Dezmirean, D. S., Mărghitaş, L. A., and Erler, S. (2016). Bay laurel (Laurus nobilis) as potential antiviral treatment in naturally BQCV infected honeybees. Virus Res. 222, 29–33. doi:10.1016/j.virusres.2016.05.024
Azeguli, H., Xia, L., Wei, X., and Li, J. (2018). Effects of Artemisia absinthium L. extracts on the maturation and function of dendritic cells. Chin. J. Microbiol. Immunol. (Beijing) 38 (9), 673–682. doi:10.3760/cma.j.issn.0254-5101.2018.09.005
Bafna, A., and Mishra, S. (2010). Antioxidant and immunomodulatory activity of the alkaloidal fraction of Cissampelos pareira Linn. Sci. Pharm. 78 (1), 21–31. doi:10.3797/scipharm.0904-16
Bafna, A. R., and Mishra, S. H. (2007). Immunomodulatory activity of petroleum ether extract of flower heads of Sphaeranthus indicus linn. J. Herb. Pharmacother. 7 (1), 25–37. doi:10.1300/J157v07n01_03
Bagherwal, P. (2011). Immunomodulatory activities of the nondialyzable latex fraction (NDL) from calotropis procera(Ait.) R. Br. Int. J. PharmTech Res. 3 (3), 1843–1849.
Balasubramanian, G., Sarathi, M., Kumar, S. R., and Hameed, A. S. S. (2007). Screening the antiviral activity of Indian medicinal plants against white spot syndrome virus in shrimp. Aquaculture 263 (1-4), 15–19. doi:10.1016/j.aquaculture.2006.09.037
Balasubramanian, T., Chatterjee, T. K., Sarkar, M., and Meena, S. L. (2010). Anti-inflammatory effect of Stereospermum suaveolens ethanol extract in rats. Pharm. Biol. 48 (10), 318–323. doi:10.3109/13880200903127383
Balkrishna, A., Pokhrel, S., Singh, J., and Varshney, A. (2020). Withanone from withania somnifera may inhibit novel coronavirus (COVID-19) entry by disrupting interactions between viral S-protein receptor binding domain and host ACE2 receptor. Virol. J. [Epub ahead of print]. doi:10.21203/RS.3.RS-17806/V1
Bandyopadhyay, S. K., Chatterjee, A., and Chattopadhyay, S. (2011). Biphasic effect of Phyllanthus emblica L. extract on NSAID-induced ulcer: an antioxidative trail weaved with immunomodulatory effect. Evidence Based Complementary Altern. Med. 2011, 1–13. doi:10.1155/2011/146808
Banerjee, N., Saha, B., and Mukhopadhyay, S. (2018). Intracellular ROS generated in chikungunya patients with persisting polyarthralgia can be reduced by Tinospora cordifolia leaf extract. Virus Dis. 29 (3), 375–379. doi:10.1007/s13337-018-0465-1
Bani, S., Gautam, M., Sheikh, F. A., Khan, B., Satti, N. K., Suri, K. A., et al. (2006). Selective Th1 up-regulating activity of Withania somnifera aqueous extract in an experimental system using flow cytometry. J. Ethnopharmacol. 107 (1), 107–115. doi:10.1016/j.jep.2006.02.016
Bao, Z., Guan, S., Cheng, C., Wu, S., Wong, S. H., Michael Kemeny, D., et al. (2009). A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-κb pathway. Am. J. Respir. Crit. Care Med. 179 (8), 657–665. doi:10.1164/rccm.200809-1516OC
Beaulieu, L., Thibodeau, J., Bonnet, C., Bryl, P., and Carbonneau, M. E. (2013). Evidence of anti-proliferative activities in blue mussel (Mytilus edulis) by-products. Mar. Drugs 11 (12), 975–990. doi:10.3390/md11040975
Behbahani, M. (2009). Anti-viral activity of the methanolic leaf extract of an Iranian medicinal plant “Hyssopus officinalis” against herpes simplex virus. J. Med. Plants Res. 3, 1118–1125.
Benencia, F., and Courrèges, M. C. (1999). Antiviral activity of sandalwood oil against Herpes simplex viruses-1 and -2. Phytomedicine 6 (2), 119–123. doi:10.1016/S0944-7113(99)80046-4
Bhalla, G., Kaur, S., Kaur, J., Kaur, R., and Raina, P. (2017). Antileishmanial and immunomodulatory potential of Ocimum sanctum Linn. and Cocos nucifera Linn. in murine visceral leishmaniasis. J. Parasit. Dis. 41 (1), 76–85. doi:10.1007/s12639-016-0753-x
Bharani, S. E. R., Asad, M., Dhamanigi, S. S., and Chandrakala, G. K. (2010). Immunomodulatory activity of methanolic extract of Morus alba Linn. (mulberry) leaves. Pak. J. Pharm. Sci. 23 (1), 63–68.
Bharshiv, C. K., Garg, S. K., and Bhatia, A. K. (2016). Immunomodulatory activity of aqueous extract of Nyctanthes arbor-tristis flowers with particular reference to splenocytes proliferation and cytokines induction. Indian J. Pharmacol. 48 (4), 412–417. doi:10.4103/0253-7613.186210
Bhattacharya, S. K., Bhattacharya, D., Sairam, K., and Ghosal, S. (2002). Effect of bioactive tannoid principles of Emblica officinalis on ischemia-reperfusion-induced oxidative stress in rat heart. Phytomed. 9 (2), 171–174. doi:10.1078/0944-7113-00090
Bonaterra, G. A., Bronischewski, K., Hunold, P., Schwarzbach, H., Heinrich, E. U., Fink, C., et al. (2020). Anti-inflammatory and anti-oxidative effects of Phytohustil® and root extract of althaea officinalis L. On macrophages in vitro. Front. Pharmacol. 11, 290. doi:10.3389/fphar.2020.00290
Borges-Argáez, R., Chan-Balan, R., Cetina-Montejo, L., Ayora-Talavera, G., Sansores-Peraza, P., Gómez-Carballo, J., et al. (2019). In vitro evaluation of anthraquinones from Aloe vera (Aloe barbadensis Miller) roots and several derivatives against strains of influenza virus. Ind. Crop. Prod. 132, 468–475. doi:10.1016/j.indcrop.2019.02.056
Boskabady, M. H., Kiani, S., Jandaghi, P., Ziaei, T., and Zarei, A. (2003). Comparison of antitussive effect of Nigella sativa with codeine in guinea pig. Iran. J. Med. Sci. 28 (3), 111–115.
Boskabady, M. H., Shafei, M. N., Saberi, Z., and Amini, S. (2011). Pharmacological effects of Rosa damascena. Iran. J. Basic Med. Sci. 14 (4), 295–307. doi:10.22038/ijbms.2011.5018
Bouadi, H., Necib, Y., and Bahi, A. (2015). Immunomodulatory activity of lectins extracted from illicium Verum. Int. J. Pharm. Sci. Rev. Res. 31 (1), 129–131.
Brochot, A., Guilbot, A., Haddioui, L., and Roques, C. (2017). Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiologyopen 6 (4), e00459. doi:10.1002/mbo3.459
Buchineni, M., Rajesh Kumar, M., Kudagi, B. L., Pathapati, R. M., Salmakamal, , Haritha, M., et al. (2014). Evaluation of anti-inflammatory and analgesic activity of azadirachta indica (leaf) extract in chemical and thermal induced pain models in rats. Int. J. Toxicol. Pharmacol. Res. 4 (6), 144–147.
Bui, T. T., Fan, Y., Piao, C. H., Nguyen, T. V., Shin, D. U., Jung, S. Y., et al. (2020). Piper Nigrum extract improves OVA-induced nasal epithelial barrier dysfunction via activating Nrf2/HO-1 signaling. Cell. Immunol. 351, 104035. doi:10.1016/j.cellimm.2019.104035
Buriana, K., Hristo, N., Christina, H., and Petkov, V. D. (1990). Immunomodulating activity of ginsenoside Rg1 from panax ginseng. Jpn. J. Pharmacol. 54 (4), 447–454. doi:10.1254/jjp.54.447
Camero, M., Marinaro, M., Lovero, A., Elia, G., Losurdo, M., Buonavoglia, C., et al. (2014). In vitro antiviral activity of Ficus carica latex against caprine herpesvirus-1. Nat. Prod. Res. 28 (22), 2031–2035. doi:10.1080/14786419.2014.918120
Carrasco, F. R., Schmidt, G., Romero, A. L., Sartoretto, J. L., Caparroz-Assef, S. M., Bersani-Amado, C. A., et al. (2009). Immunomodulatory activity of zingiber officinale Roscoe, salvia officinalis L. And syzygium aromaticum L. Essential oils: evidence for humor- and cell-mediated responses. J. Pharm. Pharmacol. 61 (7), 961–967. doi:10.1211/jpp/61.07.0017
Chandran, U., and Patwardhan, B. (2017). Network ethnopharmacological evaluation of the immunomodulatory activity of Withania somnifera. J. Ethnopharmacol. 197, 250–256. doi:10.1016/j.jep.2016.07.080
Chandrasekaran, C., Sundarajan, K., Edwin, J., Gururaja, G., Mundkinajeddu, D., and Agarwal, A. (2013). Immune-stimulatory and anti-inflammatory activities of Curcuma longa extract and its polysaccharide fraction. Pharmacogn. Res. 5 (2), 71–79. doi:10.4103/0974-8490.110527
Chang, J. S., Wang, K. C., Yeh, C. F., Shieh, D. E., and Chiang, L. C. (2013). Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 145 (1), 146–151. doi:10.1016/j.jep.2012.10.043
Chattopadhyay, D., and Naik, T. (2007). Antivirals of ethnomedicinal origin: structure-activity relationship and scope. Mini Rev. Med. Chem. 7 (3), 275–301. doi:10.2174/138955707780059844
Chattopadhyay, P., Hazarika, S., Dhiman, S., Upadhyay, A., Pandey, A., Karmakar, S., et al. (2012). Vitex negundo inhibits cyclooxygenase-2 inflammatory cytokine-mediated inflammation on carrageenan-induced rat hind paw edema. Pharmacogn. Res. 4 (3), 134–137. doi:10.4103/0974-8490.99072
Cheavegatti-Gianotto, A., de Abreu, H. M. C., Arruda, P., Bespalhok Filho, J. C., Burnquist, W. L., Creste, S., et al. (2011). Sugarcane (saccharum X officinarum): a reference study for the regulation of genetically modified cultivars in Brazil. Trop. Plant Biol. 4 (1), 62–89. doi:10.1007/s12042-011-9068-3
Chen, H. C., Chou, C. K., Lee, S. D., Wang, J. C., and Yeh, S. F. (1995). Active compounds from Saussurea lappa Clarks that suppress hepatitis B virus surface antigen gene expression in human hepatoma cells. Antiviral Res. 27 (1-2), 99–109. doi:10.1016/0166-3542(94)00083-K
Chen, H., Pu, J., Liu, D., Yu, W., Shao, Y., Yang, G., et al. (2016). Anti-inflammatory and antinociceptive properties of flavonoids from the fruits of black mulberry (Morus nigra L). PLoS One 11 (4), e0153080. doi:10.1371/journal.pone.0153080
Chen, W., Vermaak, I., and Viljoen, A. (2013). Camphor-A fumigant during the black death and a coveted fragrant wood in ancient Egypt and babylon-A review. Molecules 18 (5), 5434–5454. doi:10.3390/molecules18055434
Chen, X., Hu, Y., Shan, L., Yu, X., Hao, K., and Wang, G. X. (2017). Magnolol and honokiol from Magnolia officinalis enhanced antiviral immune responses against grass carp reovirus in Ctenopharyngodon idella kidney cells. Fish Shellfish Immunol. 63, 245–254. doi:10.1016/j.fsi.2017.02.020
Cheng, Z. J., and Shan, J. (2020). 2019 Novel coronavirus: where we are and what we know. Infection 48 (2), 155–163. doi:10.1007/s15010-020-01401-y
Chiang, L. C., Chiang, W., Chang, M. Y., and Lin, C. C. (2003). In vitro cytotoxic, antiviral and immunomodulatory effects of Plantago major and Plantago asiatica. Am. J. Chin. Med. 31 (02), 225–234. doi:10.1142/S0192415X03000874
Chiang, L. C., Chiang, W., Chang, M. Y., Ng, L. T., and Lin, C. C. (2002). Antiviral activity of Plantago major extracts and related compounds in vitro. Antiviral Res. 55 (1), 53–62. doi:10.1016/S0166-3542(02)00007-4
Chniguir, A., Zioud, F., Marzaioli, V., El-Benna, J., and Bachoual, R. (2019). Syzygium aromaticum aqueous extract inhibits human neutrophils myeloperoxidase and protects mice from LPS-induced lung inflammation. Pharm. Biol. 57 (1), 55–63. doi:10.1080/13880209.2018.1557697
Churiyah, , Pongtuluran, O. B., Rofaani, E., and Tarwadi, (2015). Antiviral and immunostimulant activities of andrographis paniculata. Hayati J. Biosci. 22 (2), 67–72. doi:10.4308/hjb.22.2.67
Cohen, M. M. (2014). Tulsi - Ocimum sanctum: a herb for all reasons. J. Ayurveda Integr. Med. 5 (4), 251–259. doi:10.4103/0975-9476.146554
Cosentino, M., Bombelli, R., Conti, A., Colombo, M. L., Azzetti, A., Bergamaschi, A., et al. (2009). Antioxidant properties and in vitro immunomodulatory effects of peppermint (Mentha x piperita l.) essential oils in human leukocytes. J. Pharm. Sci. Res. 1 (3), 33–43.
Cui, Q., Du, R., Anantpadma, M., Schafer, A., Hou, L., Tian, J., et al. (2018). Identification of ellagic acid from plant rhodiola rosea l. as an anti-ebola virus entry inhibitor. Viruses 10 (4), 152. doi:10.3390/v10040152
Dahake, R., Roy, S., Patil, D., Rajopadhye, S., Chowdhary, A., and Deshmukh, R. A. (2013). Potential Anti-HIV activity of jatropha curcas Linn. Leaf extracts. J. Antivirals Antiretrovirals 5 (7), 160–165. doi:10.4172/jaa.1000082
Dall’Acqua, S., Grabnar, I., Verardo, R., Klaric, E., Marchionni, L., Luidy-Imada, E., et al. (2019). Combined extracts of Echinacea angustifolia DC. and Zingiber officinale Roscoe in softgel capsules: pharmacokinetics and immunomodulatory effects assessed by gene expression profiling. Phytomedicine 65, 153090. doi:10.1016/j.phymed.2019.153090
Dao, T. T., Nguyen, P. H., Won, H. K., Kim, E. H., Park, J., Won, B. Y., et al. (2012). Curcuminoids from Curcuma longa and their inhibitory activities on influenza A neuraminidases. Food Chem. 134 (1), 21–28. doi:10.1016/j.foodchem.2012.02.015
Daoudi, A., Aarab, L., and Abdel-Sattar, E. (2013). Screening of immunomodulatory activity of total and protein extracts of some Moroccan medicinal plants. Toxicol. Ind. Health 29 (3), 245–253. doi:10.1177/0748233711430972
de Sousa, A. A. S., Soares, P. M. G., de Almeida, A. N. S., Maia, A. R., de Souza, E. P., and Assreuy, A. M. S. (2010). Antispasmodic effect of Mentha piperita essential oil on tracheal smooth muscle of rats. J. Ethnopharmacol. 130 (2), 433–436. doi:10.1016/j.jep.2010.05.012
Debnath, P. K., Chattopadhyay, J., Mitra, A., Adhikari, A., Alam, M. S., Bandopadhyay, S. K., et al. (2012). Adjunct therapy of Ayurvedic medicine with anti tubercular drugs on the therapeutic management of pulmonary tuberculosis. J. Ayurveda Integr. Med. 3 (3), 141–149. doi:10.4103/0975-9476.100180
Deme, P., Narasimhulu, C. A., and Parthasarathy, S. (2018). Identification and evaluation of anti-inflammatory properties of aqueous components extracted from sesame (Sesamum indicum) oil. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1087-1088, 61–69. doi:10.1016/j.jchromb.2018.04.029
Devaux, C. A., Rolain, J. M., Colson, P., and Raoult, D. (2020). New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?. Int. J. Antimicrob. Agents 55 (5), 105938. doi:10.1016/j.ijantimicag.2020.105938
Dianita, R., and Jantan, I. (2017). Ethnomedicinal uses, phytochemistry and pharmacological aspects of the genus Premna: a review. Pharm. Biol. 55 (1), 1715–1739. doi:10.1080/13880209.2017.1323225
Dibazar, S. P., Fateh, S., and Daneshmandi, S. (2015). Immunomodulatory effects of clove (Syzygium aromaticum) constituents on macrophages: in vitro evaluations of aqueous and ethanolic components. J. Immunotoxicol. 12 (2), 124–131. doi:10.3109/1547691X.2014.912698
Dogan, A., and Anuk, O. O. (2019). Investigation of the phytochemical composition and antioxidant properties of chinar (Platanus orientalis L.) leaf infusion against ethanol-induced oxidative stress in rats. Mol. Biol. Rep. 46 (3), 3049–3061. doi:10.1007/s11033-019-04741-7
Eftekhar, N., Moghimi, A., Hossein Boskabady, M., Kaveh, M., and Shakeri, F. (2019a). Ocimum basilicum affects tracheal responsiveness, lung inflammatory cells and oxidant–antioxidant biomarkers in sensitized rats. Drug Chem. Toxicol. 42 (3), 286–294. doi:10.1080/01480545.2018.1459672
Eftekhar, N., Moghimi, A., Mohammadian Roshan, N., Saadat, S., and Boskabady, M. H. (2019b). Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimum basilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma. BMC Complementary Altern. Med. 19 (1), 349. doi:10.1186/s12906-019-2765-4
Erdinest, N., Shmueli, O., Grossman, Y., Ovadia, H., and Solomon, A. (2012). Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Invest. Ophthalmol. Visual Sci. 53 (8), 4396–4406. doi:10.1167/iovs.12-9724
Ernst, E. (2010). In search of the later hahnemann. Focus Altern. Complementary. Ther. 2 (4), 175. doi:10.1111/j.2042-7166.1997.tb00694.x
Estari, M., Venkanna, L., Sripriya, D., and Lalitha, R. (2012). Human immunodeficiency virus (HIV-1) reverse transcriptase inhibitory activity of phyllanthus emblica plant extract. Biol. Med. 4 (4), 178–182. doi:10.4172/0974-8369.1000175
Faccin-Galhardi, L. C., Aimi Yamamoto, K., Ray, S., Ray, B., Carvalho Linhares, R. E., and Nozawa, C. (2012). The in vitro antiviral property of Azadirachta indica polysaccharides for poliovirus. J. Ethnopharmacol. 142 (1), 86–90. doi:10.1016/j.jep.2012.04.018
Fang, C. Y., Chen, S. J., Wu, H. N., Ping, Y. H., Lin, C. Y., Shiuan, D., et al. (2015). Honokiol, a lignan biphenol derived from the Magnolia tree, inhibits dengue virus type 2 infection. Viruses 7 (9), 4894–4910. doi:10.3390/v7092852
Farokhi, F., and Khaneshi, F. (2013). Histopathologic changes of lung in asthmatic male rats treated with hydro-alcoholic extract of Plantago major and theophylline. Avicenna J. phytomed. 3 (2), 143–151. doi:10.22038/ajp.2013.4
Ferreira, A. P., Soares, G. L. G., Salgado, C. A., Gonçalves, L. S., Teixeira, F. M., Teixeira, H. C., et al. (2003). Immunomodulatory activity of Mollugo verticillata L. Phytomed. 10 (2-3), 154–158. doi:10.1078/094471103321659861
Gaikwad, S. B., and Krishna Mohan, G. (2012). Investigation of immunomodulatory potential of Abutilon indicum Linn. Leaves. Int. J. Pharmacol. Res.
Ganguly, T., Badheka, L. P., and Sainis, K. B. (2001). Immunomodulatory effect of Tylophora indica on Con A induced lymphoproliferation. Phytomedicine 8 (6), 431–437. doi:10.1078/S0944-7113(04)70061-6
Ganta, K. K., Mandal, A., Debnath, S., Hazra, B., and Chaubey, B. (2017). Anti-HCV activity from semi-purified methanolic root extracts of valeriana wallichii. Phytother. Res. 31 (3), 433–440. doi:10.1002/ptr.5765
Gautam, M., Saha, S., Bani, S., Kaul, A., Mishra, S., Patil, D., et al. (2009). Immunomodulatory activity of Asparagus racemosus on systemic Th1/Th2 immunity: implications for immunoadjuvant potential. J. Ethnopharmacol. 121 (2), 241–247. doi:10.1016/j.jep.2008.10.028
Gebre-Mariam, T., Neubert, R., Schmidt, P. C., Wutzler, P., and Schmidtke, M. (2006). Antiviral activities of some Ethiopian medicinal plants used for the treatment of dermatological disorders. J. Ethnopharmacol. 104 (1-2), 182–187. doi:10.1016/j.jep.2005.08.071
Gerlach, S., Chandra, P., Roy, U., Gunasekera, S., Göransson, U., Wimley, W., et al. (2019). The membrane-active phytopeptide cycloviolacin O2 simultaneously targets HIV-1-infected cells and infectious viral particles to potentiate the efficacy of antiretroviral drugs. Medicines 6 (1), 33. doi:10.3390/medicines6010033
Ghildiyal, S., Gautam, M. K., Joshi, V. K., and Goel, R. K. (2012). Pharmacological evaluation of extracts of Hedychium spicatum (Ham-ex-Smith) rhizome. Ancient Sci. Life 31 (3), 117–122. doi:10.4103/0257-7941.103189
Ghoke, S. S., Sood, R., Kumar, N., Pateriya, A. K., Bhatia, S., Mishra, A., et al. (2018). Evaluation of antiviral activity of Ocimum sanctum and Acacia arabica leaves extracts against H9N2 virus using embryonated chicken egg model. BMC Complementary Altern. Med. 18 (1), 174. doi:10.1186/s12906-018-2238-1
Ghosh, K., Nosalova, G., Ray, S., Sivova, V., Nosal, S., and Ray, B. (2015). Extracted polysaccharide from Nyctanthes arbor-tristis leaves: chemical and antitussive properties. Int. J. Biol. Macromol. 75, 128–132. doi:10.1016/j.ijbiomac.2015.01.021
Gibbs, B. F. (2009). Differential modulation of IgE-dependent activation of human basophils by ambroxol and related secretolytic analogues. Int. J. Immunopathol. Pharmacol. 22 (4), 919–927. doi:10.1177/039463200902200407
Goel, A., Singh, D. K., Kumar, S., and Bhatia, A. K. (2010). Immunomodulating property of Ocimum sanctum by regulating the IL-2 production and its mRNA expression using rat’s splenocytes. Asian Pac. J. Trop. Med. 3 (1), 8–12. doi:10.1016/S1995-7645(10)60021-1
Gomes, A., Datta, P., Sarkar, A., Dasgupta, S. C., and Gomes, A. (2014). Black tea (Camellia sinensis) extract as an immunomodulator against immunocompetent and immunodeficient experimental rodents. Orient. Pharm. Exp. Med. 14 (1), 37–45. doi:10.1007/s13596-013-0134-2
Grover, A., Agrawal, V., Shandilya, A., Bisaria, V. S., and Sundar, D. (2011). Non-nucleosidic inhibition of Herpes simplex virus DNA polymerase: mechanistic insights into the anti-herpetic mode of action of herbal drug withaferin A. BMC Bioinf. 12 (Suppl 13), S22. doi:10.1186/1471-2105-12-S13-S22
Guan, S., He, J., Guo, W., Wei, J., Lu, J., and Deng, X. (2011). Adjuvant effects of salidroside from Rhodiola rosea L. on the immune responses to ovalbumin in mice. Immunopharmacol. Immunotoxicol. 33 (4), 738–743. doi:10.3109/08923973.2011.567988
Guan, S., Xiong, Y., Song, B., Song, Y., Wang, D., Chu, X., et al. (2012). Protective effects of salidroside from Rhodiola rosea on LPS-induced acute lung injury in mice. Immunopharmacol. Immunotoxicol. 34 (4), 667–672. doi:10.3109/08923973.2011.650175
Gul, S., Ahmed, S., Kifli, N., Uddin, Q. T., Tahir, N. B., Hussain, A., et al. (2014). Multiple pathways are responsible for Anti-inflammatory and Cardiovascular activities of Hordeum vulgare L. J. Transl. Med. 12 (1), 316. doi:10.1186/s12967-014-0316-9
Gulati, K., Ray, A., Debnath, P. K., and Bhattacharya, S. K. (2002). Immunomodulatory Indian medicinal plants. J. Nat. Remedies 2 (2), 121–131. doi:10.18311/jnr/2002/142
Guo, Y. R., Cao, Q. D., Hong, Z. S., Tan, Y. Y., Chen, S. D., Jin, H. J., et al. (2020). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- A n update on the status. Mil. Med. Res. 7 (1), 11. doi:10.1186/s40779-020-00240-0
Gupta, A., and Chaphalkar, S. R. (2016). Immunopharmacological screening of aqueous root extract of Santalum album. J. HerbMed Pharmacol. 5 (1), 7–11.
Gupta, A., Kumar, S., Dole, S., Deshpande, S., Deshpande, V., Singh, S., et al. (2017). Evaluation of Cyavanaprāśa on health and immunity related parameters in healthy children: a two arm, randomized, open labeled, prospective, multicenter, clinical study. Ancient Sci. Life 36 (3), 141–150. doi:10.4103/asl.asl_8_17
Gupta, P., Bajpai, S. K., Chandra, K., Singh, K. L., and Tandon, J. S. (2005). Antiviral profile of Nyctanthes arbortristis L. against encephalitis causing viruses. Indian J. Exp. Biol. 43 (12), 1156–1160.
Han, X., and Parker, T. L. (2017). Cardamom (Elettaria cardamomum) essential oil significantly inhibits vascular cell adhesion molecule 1 and impacts genome-wide gene expression in human dermal fibroblasts. Cogent Med. 4 (1), 1308066. doi:10.1080/2331205x.2017.1308066
Haq, R. U., Wahab, A., Ayub, K., Mehmood, K., Sherkheli, M. A., Khan, R. A., et al. (2013). Antitussive efficacy and safety profile of ethyl acetate fraction of Terminalia chebula. ISRN Pharmacol. 2013, 1–7. doi:10.1155/2013/256934
Harizi, H., Chaabane, F., Ghedira, K., and Chekir-Ghedira, L. (2011). Inhibition of proinflammatory macrophage responses and lymphocyte proliferation in vitro by ethyl acetate leaf extract from Daphne gnidium. Cell. Immunol. 267 (2), 94–101. doi:10.1016/j.cellimm.2010.12.002
Heo, J. C., Rho, J. R., Kim, T. H., Kim, S. Y., and Lee, S. H. (2008). An aqueous extract of green tea Camellia sinensis increases expression of Th1 cell-specific anti-asthmatic markers. Int. J. Mol. Med. 22 (6), 763–767. doi:10.3892/ijmm_00000083
Hong, E. H., Song, J. H., Kang, K. B., Sung, S. H., Ko, H. J., and Yang, H. (2015). Anti-influenza activity of betulinic acid from Zizyphus Jujuba on influenza A/PR/8 virus. Biomol. Ther. 23 (4), 345–349. doi:10.4062/biomolther.2015.019
Hossain, M. T., and Hoq, M. O. (2016). Therapeutic use of Adhatoda vasica. Asian J. Med. Biol. Res. 2 (2), 156–163. doi:10.3329/ajmbr.v2i2.29005
Hossan, M. S., Fatima, A., Rahmatullah, M., Khoo, T. J., Nissapatorn, V., Galochkina, A. V., et al. (2018). Antiviral activity of Embelia ribes Burm. f. against influenza virus in vitro. Arch. Virol. 163 (8), 2121–2131. doi:10.1007/s00705-018-3842-6
Hsieh, C. C., Peng, W. H., Tseng, H. H., Liang, S. Y., Chen, L. J., and Tsai, J. C. (2019). The protective role of garlic on allergen-induced airway inflammation in mice. Am. J. Chin. Med. 47 (5), 1099–1112. doi:10.1142/S0192415X19500563
Ichsyani, M., Ridhanya, A., Risanti, M., Desti, H., Ceria, R., Putri, D. H., et al. (2017). Antiviral effects of Curcuma longa L. against dengue virus in vitro and in vivo. IOP Conf. Ser. Earth Environ. Sci. 101, 012005. doi:10.1088/1755-1315/101/1/012005
Im, S. A., Lee, Y. R., Lee, Y. H., Lee, M. K., Park, Y. I., Lee, S., et al. (2010). In vivo evidence of the immunomodulatory activity of orally administered Aloe vera gel. Arch Pharm. Res. (Seoul) 33 (3), 451–456. doi:10.1007/s12272-010-0315-1
Inam, A., Shahzad, M., Shabbir, A., Shahid, H., Shahid, K., and Javeed, A. (2017). Carica papaya ameliorates allergic asthma via down regulation of IL-4, IL-5, eotaxin, TNF-α, NF-ĸB, and iNOS levels. Phytomed. 32, 1–7. doi:10.1016/j.phymed.2017.04.009
Indrasetiawan, P., Aoki-Utsubo, C., Hanafi, M., Hartati, S. R. I., Wahyuni, T. S., Kameoka, M., et al. (2019). Antiviral activity of cananga odorata against hepatitis B virus. Kobe J. Med. Sci. 65 (2), E71–E79.
Islam, M. R., Oomah, D. B., and Diarra, M. S. (2017). Potential immunomodulatory effects of non-dialyzable materials of cranberry extract in poultry production. Poultry Sci. 96 (2), 341–350. doi:10.3382/ps/pew302
Iwo, M. I., Soemardji, A. A., Retnoningrum, D. S., Sukrasno, , and Ua, U. M. (2000). Immunostimulating effect of Pule (Alstonia scholaris L. R.Br., Apocynaceae) bark extracts. Clin. Hemorheol. Microcirc. 23 (2-4), 177–183.
Jain, A., Choubev, S., Singour, P. K., Rajak, H., and Pawar, R. S. (2011). Sida cordifolia (Linn) - an overview. J. Appl. Pharm. Sci. 1 (2), 23–31.
Jain, J., Kumar, A., Narayanan, V., Ramaswamy, R. S., Sathiyarajeswaran, P., Shree Devi, M. S., et al. (2019). Antiviral activity of ethanolic extract of Nilavembu Kudineer against dengue and chikungunya virus through in vitro evaluation. J. Ayurveda Integr. Med. 11 (3), 329–335. doi:10.1016/j.jaim.2018.05.006
Jamali, T., Kavoosi, G., and Ardestani, S. K. (2020). In-vitro and in-vivo anti-breast cancer activity of OEO (Oliveria decumbens vent essential oil) through promoting the apoptosis and immunomodulatory effects. J. Ethnopharmacol. 248, 112313. doi:10.1016/j.jep.2019.112313
Jamkhande, P. G., Barde, S. R., Patwekar, S. L., and Tidke, P. S. (2013). Plant profile, phytochemistry and pharmacology of Cordia dichotoma (Indian cherry): a review. Asian Pac. J. Trop. Biomed. 3 (12), 1009–1012. doi:10.1016/S2221-1691(13)60194-X
Jantan, I., Ilangkovan, M., Yuandani, , and Mohamad, H. F. (2014). Correlation between the major components of Phyllanthus amarus and Phyllanthus urinaria and their inhibitory effects on phagocytic activity of human neutrophils. BMC Complementary Altern. Med. 14 (1), 429. doi:10.1186/1472-6882-14-429
Jassim, S. A. A., and Naji, M. A. (2003). Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol. 95 (3), 412–427. doi:10.1046/j.1365-2672.2003.02026.x
Javed, T., Ashfaq, U. A., Riaz, S., Rehman, S., and Riazuddin, S. (2011). In-vitro antiviral activity of Solanum nigrum against Hepatitis C Virus. Virol. J. 8 (1), 26. doi:10.1186/1743-422X-8-26
Jeong, Y. Y., Ryu, J. H., Shin, J. H., Kang, M. J., Kang, J. R., Han, J., et al. (2016). Comparison of anti-oxidant and anti-inflammatory effects between fresh and aged black garlic extracts. Molecules 21 (4), 430. doi:10.3390/molecules21040430
Jiang, Y., Tzi Bun, N. G., Zhaokun, L. I. U., Wang, C., Ning, L. I., Qiao, W., et al. (2011). Immunoregulatory and anti-HIV-1 enzyme activities of antioxidant components from lotus (nelumbo nucifera gaertn.) rhizome. Biosci. Rep. 31 (5), 381–390. doi:10.1042/BSR20100062
Jiang, Z. Y., Liu, W. F., Zhang, X. M., Luo, J., Ma, Y. B., and Chen, J. J. (2013). Anti-HBV active constituents from Piper longum. Bioorg. Med. Chem. Lett. 23 (7), 2123–2127. doi:10.1016/j.bmcl.2013.01.118
Jin, J. H., Lee, D. U., Kim, Y. S., and Kim, H. P. (2011). Anti-allergic activity of sesquiterpenes from the rhizomes of Cyperus rotundus. Arch. Pharmacal Res. 34 (2), 223–228. doi:10.1007/s12272-011-0207-z
Joshi, K., Awte, S., Bhatnagar, P., Walunj, S., Gupta, R., Joshi, S., et al. (2010). Cinnamomum zeylanicum extract inhibits proinflammatory cytokine TNFµ: in vitro and in vivo studies. Res. Pharmaceut. Biotechnol. 2, 014–021. doi:10.5897/RPB.9000004
Jung, S., Lee, M. S., Choi, A. J., Kim, C. T., and Kim, Y. (2019). Anti-inflammatory effects of high hydrostatic pressure extract of mulberry (morus alba) fruit on lps-stimulated RAW264.7 Cells. Molecules 24 (7), 1425. doi:10.3390/molecules24071425
Juvekar, A., Nachankar, R., Hole, R., Wakade, A., Kulkarni, M., and Ambaye, R. (2006). In vitro and in vivo immunomodulatory activity of aqueous extract of Clerodendrum serratum L. roots. Planta Med. 72 (11), 87. doi:10.1055/s-2006-949887
Kabra, A., Martins, N., Sharma, R., Kabra, R., and Baghel, U. S. (2019). Myrica esculenta Buch.-Ham. ex D. Don: a natural source for health promotion and disease prevention. Plants 8 (6), 149. doi:10.3390/plants8060149
Kalaiselvan, S., and Rasool, M. K. (2015). The anti-inflammatory effect of triphala in arthritic-induced rats. Pharm. Biol. 53 (1), 51–60. doi:10.3109/13880209.2014.910237
Kalaiselvan, S., and Rasool, M. K. (2016). Triphala herbal extract suppresses inflammatory responses in LPS-stimulated RAW 264.7 macrophages and adjuvant-induced arthritic rats via inhibition of NF-κB pathway. J. Immunotoxical. 13 (4), 509–525. doi:10.3109/1547691X.2015.1136010
Kamel, R. O. A., and El-Shinnawy, N. A. (2015). Immunomodulatory effect of garlic oil extract on Schistosoma mansoni infected mice. Asian Pac. J. Trop. Med. 8 (12), 999–1005. doi:10.1016/j.apjtm.2015.11.016
Kandhare, A. D., Bodhankar, S. L., Singh, V., Mohan, V., and Thakurdesai, P. A. (2013). Anti-asthmatic effects of type-A procyanidine polyphenols from cinnamon bark in ovalbumin-induced airway hyperresponsiveness in laboratory animals. Biomed. Aging Pathol. 3 (1), 23–30. doi:10.1016/j.biomag.2013.01.003
Kang, S. Y., Jung, H. W., Nam, J. H., Kim, W. K., Kang, J. S., Kim, Y. H., et al. (2017). Effects of the fruit extract of tribulus terrestris on skin inflammation in mice with oxazolone-induced atopic dermatitis through regulation of calcium channels, orai-1 and TRPV3, and mast cell activation. Evidence-based Complementary Altern. Med. 2017, 1–12. doi:10.1155/2017/8312946
Kanjwani, D. G., Marathe, T. P., Chiplunkar, S. V., and Sathaye, S. S. (2008). Evaluation of immunomodulatory activity of methanolic extract of Piper betel. Scand. J. Immunol. 67 (6), 589–593. doi:10.1111/j.1365-3083.2008.02110.x
Kannan, M., Rajendran, P., Vedha, V., Ashok, G., Anushka, S., and Chandran Ramachandran Nair, P. (2012). HIV-1 reverse transcriptase inhibition by Vitex negundo L. leaf extract and quantification of flavonoids in relation to anti-HIV activity. J. Cell Mol. Biol. 10 (2), 53–59.
Karimi, A., Moradi, M. T., Alidadi, S., and Hashemi, L. (2016). Anti-adenovirus activity, antioxidant potential, and phenolic content of black tea (Camellia sinensis Kuntze) extract. J. Complementary Integr. Med. 13 (4), 357–363. doi:10.1515/jcim-2016-0050
Kasote, D. M., Zanwar, A. A., Devkar, S. T., Hegde, M. V., and Deshmukh, K. K. (2012). Immunomodulatory activity of ether insoluble phenolic components of n-butanol fraction (EPC-BF) of flaxseed in rat. Asian Pac. J. Trop. Biomed. 2 (2), S623–S626. doi:10.1016/S2221-1691(12)60285-8
Kaunda, J. S., and Zhang, Y. J. (2019). The genus solanum: an ethnopharmacological, phytochemical and biological properties review. Nat. Prod. Bioprospect. 9, 77–137. doi:10.1007/s13659-019-0201-6
Kaur, H. (2013). Homoeopathic research-understanding the challenges. J. Homeopathy Ayurvedic Med. 02 (01), 118. doi:10.4172/2167-1206.1000118
Kaushik, D., Rani, R., Kaushik, P., Sacher, D., and Yadav, J. (2012). In vivo and in vitro antiasthmatic studies of plant Piper longum Linn. Int. J. Pharmacol. 8 (3), 192–197. doi:10.3923/ijp.2012.192.197
Kavinilavan, R., Mekala, P., Raja, M., Eswaran, M. A., and Thirumalaisamy, G. (2017). Exploration of immunomodulatory effect of nilavembu kudineer chooranam against newcastle disease virus in backyard chicken. J. Pharmacogn. Phytochem. 6 (6), 749–751.
Kesharwani, A., Polachira, S. K., Nair, R., Agarwal, A., Mishra, N. N., and Gupta, S. K. (2017). Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Complementary Altern. Med. 17 (1), 110. doi:10.1186/s12906-017-1620-8
Khan, A. M., Shahzad, M., Raza Asim, M. B., Imran, M., and Shabbir, A. (2015). Zingiber officinale ameliorates allergic asthma via suppression of Th2-mediated immune response. Pharm. Biol. 53 (3), 359–367. doi:10.3109/13880209.2014.920396
Khan, A. U., and Gilani, A. H. (2012). Antidiarrhoeal and bronchodilatory potential of Valeriana wallichii. Nat. Prod. Res. 26 (11), 1045–1049. doi:10.1080/14786419.2010.551754
Khan, A. U., Rahim, A., Iqbal, Z., and Gilani, A. H. (2012). Insights into mechanisms underlying the gut and airways modulatory effects of Swertia chirata. J. Nat. Med. 66 (1), 140–148. doi:10.1007/s11418-011-0566-2
Khan, F., Ali, S., Ganie, B. A., and Rubab, I. (2009). Immunopotentiating effect of khamira marwarid, an herbo-mineral preparation. Methods Find. Exp. Clin. Pharmacol. 31 (8), 513–522. doi:10.1358/mf.2009.31.8.1419719
Khan, M. A., Ahmed, R. S., Chandra, N., Arora, V. K., and Ali, A. (2018). In vivo, extract from withania somnifera root ameliorates arthritis via regulation of key immune mediators of inflammation in experimental model of arthritis. Anti Inflammatory Anti Allergy. Agents Med. Chem. 18 (1), 55–70. doi:10.2174/1871523017666181116092934
Khorrami, S., Daneshmandi, S., and Mosayebi, G. (2018). Sesame seeds essential oil and Sesamol modulate the pro-inflammatory function of macrophages and dendritic cells and promote Th2 response. Med. J. Islam. Repub. Iran 32 (1), 566–573. doi:10.14196/mjiri.32.98
Khuda, F., Iqbal, Z., Khan, A., Zakiullah, , Nasir, F., and Shah, Y. (2013). Anti-inflammatory activity of the topical preparation of Valeriana wallichii and Achyranthes aspera leaves. Pak. J. Pharm. Sci. 26 (3), 451–454.
Kianmehr, M., Haghmorad, D., Nosratabadi, R., Rezaei, A., Alavinezhad, A., and Boskabady, M. H. (2017). The effect of zataria multiflora on Th1/Th2 and Th17/T regulatory in a mouse model of Allergic Asthma. Front. Pharmacol. 8, 458. doi:10.3389/fphar.2017.00458
Killestein, J., Hoogervorst, E. L. J., Reif, M., Blauw, B., Smits, M., Uitdehaag, B. M. J., et al. (2003). Immunomodulatory effects of orally administered cannabinoids in multiple sclerosis. J. Neuroimmunol. 137 (1-2), 140–143. doi:10.1016/S0165-5728(03)00045-6
Kim, D. Y., and Yang, W. M. (2011). Panax ginseng ameliorates airway inflammation in an ovalbumin-sensitized mouse allergic asthma model. J. Ethnopharmacol. 136 (1), 230–235. doi:10.1016/j.jep.2011.04.048
Kim, H. J., Yoo, H. S., Kim, J. C., Park, C. S., Choi, M. S., Kim, M., et al. (2009). Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication. J. Ethnopharmacol. 124 (2), 189–196. doi:10.1016/j.jep.2009.04.046
Kim, Y. M., Lee, J. A., Kim, T.-H., Jung, B. G., and Lee, B. J. (2014). Dietary turmeric presents mild immune suppressing and anti-inflammatory effects. J. Prev. Vet. Med. 38 (1), 7–14. doi:10.13041/jpvm.2014.38.1.7
Kiran Kumar, K. P., Singh, G. P., Sinha, A. K., Madhusudhan, K. N., and Prasad, B. C. (2012). Antiviral action of certain medicinal plants against AmCPV and their effect on cellular and biochemical changes in tasar silkworm, antheraea mylitta D. Res. J. Med. Plant 6 (1), 92–99. doi:10.3923/rjmp.2012.92.99
Koko, W. S., Mesaik, M. A., Yousaf, S., Galal, M., and Choudhary, M. I. (2008). In vitro immunomodulating properties of selected Sudanese medicinal plants. J. Ethnopharmacol. 118 (1), 26–34. doi:10.1016/j.jep.2008.03.007
Koochek, M. H., Pipelzadeh, M. H., and Mardani, H. (2003). The effectiveness of Viola odorata in the prevention and treatment of formalin-induced lung damage in the rat. J. Herbs Spices Med. Plants 10 (2), 95–103. doi:10.1300/J044v10n02_11
Koshak, A. E., Yousif, N. M., Fiebich, B. L., Koshak, E. A., and Heinrich, M. (2018). Comparative immunomodulatory activity of Nigella sativa L. preparations on proinflammatory mediators: a focus on asthma. Front. Pharmacol. 9, 1075. doi:10.3389/fphar.2018.01075
Kumar, P. M., Meenakshi Sundaram, K., and Ramasamy, M. S. (2019). Coronavirus spike (S) glycoprotein (2019-ncov) targeted Siddha medicines kabasura kudineer and thonthasura kudineer-in silico evidence for corona viral drug. Asian J. Pharm. Res. Health Care 12 (1), 20–27. doi:10.18311/ajprhc/2019/25103
Kumar, R., Gupta, Y. K., and Singh, S. (2016). Anti-inflammatory and anti-granuloma activity of Berberis aristata DCexperimental models of inflammation. Indian J. Pharmacol. 48 (2), 155–161. doi:10.4103/0253-7613.178831
Kumar, S., Kamboj, J., Suman, , and Sharma, S. (2011). Overview for various aspects of the health benefits of piper longum linn. Fruit. J. Acupunct. Meridian Stud. 4 (2), 134–140. doi:10.1016/S2005-2901(11)60020-4
Kumar, S., and Pandey, A. K. (2014). Medicinal attributes of Solanum xanthocarpum fruit consumed by several tribal communities as food: an in vitro antioxidant, anticancer and anti HIV perspective. BMC Complementary Altern. Med. 14 (1), 112. doi:10.1186/1472-6882-14-112
Kumari, K. D. K. P., Weerakoon, T. C. S., Handunnetti, S. M., Samarasinghe, K., and Suresh, T. S. (2014). Anti-inflammatory activity of dried flower extracts of Aegle marmelos in Wistar rats. J. Ethnopharmacol. 151 (3), 1202–1208. doi:10.1016/j.jep.2013.12.043
Kurokawa, M., Ochiai, H., Nagasaka, K., Neki, M., Xu, H., Kadota, S., et al. (1993). Antiviral traditional medicines against herpes simplex virus (HSV-1), poliovirus, and measles virus in vitro and their therapeutic efficacies for HSV-1 infection in mice. Antiviral Res. 22 (2-3), 175–188. doi:10.1016/0166-3542(93)90094-Y
Kyokong, O., Charuluxananan, S., Muangmingsuk, V., Rodanant, O., Subornsug, K., and Punyasang, W. (2002). Efficacy of chamomile-extract spray for prevention of post-operative sore throat. J. Med. Assoc. Thailand 85 (Suppl 1), S180–S185.
Labsi, M., Khelifi, L., Mezioug, D., Soufli, I., and Touil-Boukoffa, C. (2016). Antihydatic and immunomodulatory effects of Punica granatum peel aqueous extract in a murine model of echinococcosis. Asian Pac. J. Trop. Med. 9 (3), 211–220. doi:10.1016/j.apjtm.2016.01.038
Lad, H., Joshi, A., Dixit, D., Sharma, H., and Bhatnagar, D. (2016). Antioxidant, genoprotective and immunomodulatory potential of Vitex negundo leaves in experimental arthritis. Orient. Pharm. Exp. Med. 16 (3), 217–224. doi:10.1007/s13596-016-0234-x
Lai, W. L., Chuang, H. S., Lee, M. H., Wei, C. L., Lin, C. F., and Tsai, Y. C. (2012). Inhibition of herpes simplex virus type 1 by thymol-related monoterpenoids. Planta Med. 78 (15), 1636–1638. doi:10.1055/s-0032-1315208
Lampariello, L., Cortelazzo, A., Guerranti, R., Sticozzi, C., and Valacchi, G. (2012). The magic velvet bean of mucuna pruriens. J. Tradit. Complementary Med. 2 (4), 331–339. doi:10.1016/S2225-4110(16)30119-5
Latha, P. G., Evans, D. A., Panikkar, K. R., and Jayavardhanan, K. K. (2000). Immunomodulatory and antitumour properties of Psoralea corylifolia seeds. Fitoterapia 71 (3), 223–231. doi:10.1016/S0367-326X(99)00151-3
Lavanya, P., Ramaiah, S., and Anbarasu, A. (2016). Ethyl 4-(4-methylphenyl)-4-pentenoate from vetiveria zizanioides inhibits dengue NS2B–NS3 protease and prevents viral assembly: a computational molecular dynamics and docking study. Cell Biochem. Biophys. 74 (3), 337–351. doi:10.1007/s12013-016-0741-x
Laxmi, V. (2015). Investigating the immunomodulatory effect of Cassia fistula on albino rats. Adv. Pharm. Ethnomed. 3 (1), 1–5. doi:10.14737/journal.ape/2015/3.1.1.5
Lee, B. K., Park, S. J., Nam, S. Y., Kang, S., Hwang, J., Lee, S. J., et al. (2018). Anti-allergic effects of sesquiterpene lactones from Saussurea costus (Falc.) Lipsch. determined using in vivo and in vitro experiments. J. Ethnopharmacol. 213, 256–261. doi:10.1016/j.jep.2017.11.018
Lee, D., Kim, H. S., Shin, E., Do, S. G., Lee, C. K., Kim, Y. M., et al. (2018). Polysaccharide isolated from Aloe vera gel suppresses ovalbumin-induced food allergy through inhibition of Th2 immunity in mice. Biomed. Pharmacother. 101, 201–210. doi:10.1016/j.biopha.2018.02.061
Lee, J. H., Bae, S. Y., Oh, M., Kim, K. H., and Chung, M. S. (2014). Antiviral effects of mulberry (Morus alba) juice and its fractions on foodborne viral surrogates. Foodborne Pathog. Dis. 11 (3), 224–229. doi:10.1089/fpd.2013.1633
Lee, J. S., Synytsya, A., Kim, H. B., Choi, D. J., Lee, S., Lee, J., et al. (2013). Purification, characterization and immunomodulating activity of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Int. Immunopharmcol. 17 (3), 858–866. doi:10.1016/j.intimp.2013.09.019
Lee, S. Y., Cho, S. S., Li, Y. C., Bae, C. S., Park, K. M., and Park, D. H. (2020). Anti-inflammatory effect of curcuma longa and allium hookeri Co-treatment via NF-κB and COX-2 pathways. Sci. Rep. 10 (1), 5718. doi:10.1038/s41598-020-62749-7
Lee, Y. C., and Kim, S. H. (2008). Immunomodulatory effect of Juglans sinensis, Psoralea corylifolia, Cheong-a-hwan extract and cyclosporine A on Th1(IFN-γ)/Th2(IL-4) cytokine balance, eosinophil accumulation in a murine model of asthma. Phytochem. Lett. 1 (1), 6–10. doi:10.1016/j.phytol.2007.11.002
Leu, G. Z., Lin, T. Y., and Hsu, J. T. A. (2004). Anti-HCV activities of selective polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 318 (1), 275–280. doi:10.1016/j.bbrc.2004.04.019
Li, G., Fan, Y., Lai, Y., Han, T., Li, Z., Zhou, P., et al. (2020). Coronavirus infections and immune responses. J. Med. Virol. 92 (4), 424–432. doi:10.1002/jmv.25685
Li, W., Zhang, X., Chen, R., Li, Y., Miao, J., Liu, G., et al. (2020). HPLC fingerprint analysis of Phyllanthus emblica ethanol extract and their antioxidant and anti-inflammatory properties. J. Ethnopharmacol. 254, 112740. doi:10.1016/j.jep.2020.112740
Li, J., Li, Q. W., Gao, D. W., Han, Z. S., and Lu, W. Z. (2009). Antitumor and immunomodulating effects of polysaccharides isolated from Solanum nigrum Linne. Phyther. Res. 23 (11), 1524–1530. doi:10.1002/ptr.2769
Li, Y. X., Liu, Y. B., Ma, A. Q., Bao, Y., Wang, M., and Sun, Z. L. (2017). In vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 26 (6), 1675–1683. doi:10.1007/s10068-017-0217-9
Liang, S., Li, X., Ma, X., Li, A., Wang, Y., Reaney, M. J. T., et al. (2019). A flaxseed heteropolysaccharide stimulates immune responses and inhibits hepatitis B virus. Int. J. Biol. Macromol. 136, 230–240. doi:10.1016/j.ijbiomac.2019.06.076
Lii, C. K., Chen, H. W., Yun, W. T., and Liu, K. L. (2009). Suppressive effects of wild bitter gourd (Momordica charantia Linn. var. abbreviata ser.) fruit extracts on inflammatory responses in RAW 264.7 macrophages. J. Ethnopharmacol. 122 (2), 227–233. doi:10.1016/j.jep.2009.01.028
Lim, S. H., and Choi, C. I. (2019). Pharmacological properties of morus nigra L. (Black Mulberry) as a promising nutraceutical resource. Nutrients 11 (2), 437. doi:10.3390/nu11020437
Liu, W., Cheng, X., Wang, Y., Li, S., Zheng, T., Gao, Y., et al. (2015). In vivo evaluation of the antitussive, expectorant and bronchodilating effects of extract and fractions from aerial parts of Peganum harmala linn. J. Ethnopharmacol. 162, 79–86. doi:10.1016/j.jep.2014.12.046
Liu, X., Zhao, M., Wu, K., Chai, X., Yu, H., Tao, Z., et al. (2012). Immunomodulatory and anticancer activities of phenolics from emblica fruit (Phyllanthus emblica L.). Food Chem. 131 (2), 685–690. doi:10.1016/j.foodchem.2011.09.063
Loizzo, M. R., Saab, A. M., Tundis, R., Statti, G. A., Menichimi, F., Lampronti, D., et al. (2008). Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodiversity 5 (3), 461–470. doi:10.1002/cbdv.200890045
Lü, P., Pan, Y., Yang, Y., Zhu, F., Li, C., Yao, Q., et al. (2018). Discovery of anti-viral molecules and their vital functions in Bombyx mori. J. Invertebr. Pathol. 154, 12–18. doi:10.1016/j.jip.2018.02.012
Lucas, L., Russell, A., and Keast, R. (2011). Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 17 (8), 754–768. doi:10.2174/138161211795428911
Luo, H., Tang, Q. ling., Shang, Y. xi., Liang, S. bing., Yang, M., Robinson, N., et al. (2020). Can Chinese medicine Be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 26 (4), 243–250. doi:10.1007/s11655-020-3192-6
Lythgoe, M. P., and Middleton, P. (2020). Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol. Sci. 41 (6), 363–382. doi:10.1016/j.tips.2020.03.006
Ma, X., Ma, X., Ma, Z., Sun, Z., Yu, W., Wang, J., et al. (2014). The effects of uygur herb hyssopus officinalis L. On the process of airway remodeling in asthmatic mice. Evidence based complementary Altern. Med. 2014, 1–7. doi:10.1155/2014/710870
Mahadevan, H., and Palraj, V. (2016). Literature review on Siddha herbal formulations (kudineer) available for the management of dengue. Int. J. Pharmacol. Clin. Sci. 5 (3), 90–96. doi:10.5530/ijpcs.5.3.5
Mahamat, O., Flora, H., Tume, C., and Kamanyi, A. (2020). Immunomodulatory activity of momordica charantia L. (Cucurbitaceae) leaf diethyl ether and methanol extracts on Salmonella typhi -infected mice and LPS-induced phagocytic activities of macrophages and neutrophils. Evidence based complementary Altern. Med. 2020, 1–11. doi:10.1155/2020/5248346
Mahendran, S., Badami, S., Ravi, S., Thippeswamy, B. S., and Veerapur, V. P. (2011). Synthesis and evaluation of analgesic and anti-inflammatory activities of most active free radical scavenging derivatives of embelin - a Structure-activity relationship. Chem. Pharm. Bull. 59 (8), 913–919. doi:10.1248/cpb.59.913
Mahmood, N., Piacente, S., Pizza, C., Burke, A., Khan, A. I., and Hayt, A. J. (1996). The anti-HIV activity and mechanisms of action of pure compounds isolated from Rosa damascena. Biochem. Biophys. Res. Commun. 229 (1), 73–79. doi:10.1006/bbrc.1996.1759
Mair, C., Liu, R., Atanasov, A., Schmidtke, M., Dirsch, V., and Rollinger, J. (2016). Antiviral and anti-proliferative in vitro activities of piperamides from black pepper. Planta Med. 81 (S 01), S1–S381. doi:10.1055/s-0036-1596830
Majdalawieh, A. F., and Carr, R. I. (2010). In vitro investigation of the potential immunomodulatory and anti-cancer activities of black pepper (Piper nigrum) and cardamom (Elettaria cardamomum). J. Med. Food 13 (2), 371–381. doi:10.1089/jmf.2009.1131
Majdalawieh, A. F., Hmaidan, R., and Carr, R. I. (2010). Nigella sativa modulates splenocyte proliferation, Th1/Th2 cytokine profile, macrophage function and NK anti-tumor activity. J. Ethnopharmacol. 131 (2), 268–275. doi:10.1016/j.jep.2010.06.030
Malik, A., Mehmood, M. D., Anwar, H., Noreen, S., and Sultan, U. (2018). In-vivo antiviral potential of crude extracts derived from Tribulus terrestris against newcastle disease virus. J. Drug Delivery. Ther. 8 (6), 149–154. doi:10.22270/jddt.v8i6.2114
Mallik, A., and Nayak, S. (2014). Comparative study of methanolic and aqueous extracts of Cocculus hirsutus leaves on specific and non specific immune responses. Int. J. Phytomed. 6 (2), 316.
Mamber, S. W., Gurel, V., Lins, J., Ferri, F., Beseme, S., and McMichael, J. (2020). Effects of cannabis oil extract on immune response gene expression in human small airway epithelial cells (HSAEpC): implications for chronic obstructive pulmonary disease (COPD). J. Cannabis Res. 2 (1), 5. doi:10.1186/s42238-019-0014-9
Mangathayaru, K., Umadevi, M., and Reddy, C. U. (2009). Evaluation of the immunomodulatory and DNA protective activities of the shoots of Cynodon dactylon. J. Ethnopharmacol. 123 (1), 181–184. doi:10.1016/j.jep.2009.02.036
Martins, C. A. F., Campos, M. L., Irioda, A. C., Stremel, D. P., Trindade, A. C. L. B., and Pontarolo, R. (2017). Anti-inflammatory effect of malva sylvestris, Sida cordifolia, and Pelargonium graveolens is related to inhibition of prostanoid production. Molecules 22 (11), 1883. doi:10.3390/molecules22111883
Masihuddin, M., Ma, J., Siddiqui, A., and Chaudhary, S. (2019). Traditional uses, phytochemistry, and pharmacological activities of Amla with special reference of Unani medicine - an updated review. Asian J. Pharm. Clin. Res. 12 (2), 70–74. doi:10.22159/ajpcr.2019.v12i2.29178
Mathayan, M., Jayaraman, S., Kulanthaivel, L., and Suresh, A. (2019). Inhibition studies of HBV DNA polymerase using seed extracts of Pongamia pinnata. Bioinformation 15 (7), 506–512. doi:10.6026/97320630015506
Mathew, J. E., Kaitheri, S. K., Dinakaranvachala, S., and Jose, M. (2011). Anti-inflammatory, antipruritic and mast cell stabilizing activity of Aristolochia indica. Iran J. Basic Med. Sci. 14 (5), 422–427. doi:10.22038/ijbms.2011.5037
Mathur, K., Mathur, A. K., Issrani, R., Ambani, S. R., and Goyal, M. (2016). Anti-asthmatic and anti-tussive activity of ethanolic extract of leaves of Nyctanthes arbortristis Linn. Int. J. Appl. Pharm. Biol. Res.
Meena, R., and Ramaswamy, R. S. (2015). Therapeutic potency of a Siddha formulation kandhaga rasayanam: a review. Int. J. Res. Ayurveda Pharm. 6 (1), 58–64. doi:10.7897/2277-4343.06114
Meher, B. R., Rath, B. G., and Biswal, S. (2011). Evaluation of anti-inflammatory activity of ethanolic extract of Sphaeranthus indicus. J. Chem. Pharm. Res. 3, 831–834.
Mehta, A. A., and Paranjape, A. N. (2008). Investigation into the mechanism of action of abutilon indicum in the treatment of bronchial asthma. Global J. Pharmacol. 2 (2), 23–30.
Mesaik, A. M., Poh, H. W., Bin, O. Y., Elawad, I., and Alsayed, B. (2018). In vivo anti-inflammatory, anti-bacterial and anti-diarrhoeal activity of Ziziphus Jujuba fruit extract. Open Access Maced. J. Med. Sci. 6 (5), 757–766. doi:10.3889/oamjms.2018.168
Miccadei, S., Masella, R., Mileo, A. M., and Gessani, S. (2016). ω3 polyunsaturated fatty acids as immunomodulators in colorectal cancer: new potential role in adjuvant therapies. Front. Immunol. 7, 486. doi:10.3389/fimmu.2016.00486
Mishra, A., Thakur, M., and Alok, S. (2016). Evaluation of immunomodulatory activity of polysacchride fraction of Inula racemosa, Bombax ceiba and Allium sativum. Int. J. Pharm. Sci. Res. 7 (9), 3749–3755. doi:10.13040/IJPSR.0975-8232
Mitra Mazumder, P., Pattnayak, S., Parvani, H., Sasmal, D., and Rathinavelusamy, P. (2012). Evaluation of immunomodulatory activity of Glycyrhiza glabra L roots in combination with zing. Asian Pac. J. Trop. Biomed. 2 (1), S15–S20. doi:10.1016/S2221-1691(12)60122-1
Mohanty, S. K., Swamy, M. K., Middha, S. K., Prakash, L., Subbanarashiman, B., and Maniyam, A. (2015). Analgesic, anti- inflammatory, anti- lipoxygenase activity and characterization of three bioactive compounds in the most active fraction of Leptadenia reticulata (Retz.)wight & arn.–a valuable medicinal plant. Iran. J. Pharm. Res. 14 (3), 933–942. doi:10.22037/ijpr.2015.1701
Mohanty, S. K., Swamy, M. K., Sinniah, U. R., and Anuradha, M. (2017). Leptadenia reticulata (Retz.) Wight & Arn. (Jivanti): botanical, agronomical, phytochemical, pharmacological, and biotechnological aspects. Molecules 22 (6), 1019. doi:10.3390/molecules22061019
Moradi, M. T., Karimi, A., Alidadi, S., and Hashemi, L. (2018). In vitro anti-herpes simplex virus activity, antioxidant potential and total phenolic compounds of selected iranian medicinal plant extracts. Indian J. Tradit. Knowl. 17 (2), 255–262.
Moradi, M. T., Karimi, A., Rafieian-Kopaei, M., and Fotouhi, F. (2017). In vitro antiviral effects of Peganum harmala seed extract and its total alkaloids against Influenza virus. Microb. Pathog. 110, 42–49. doi:10.1016/j.micpath.2017.06.014
Moradi, M. T., Karimi, A., Shahrani, M., Hashemi, L., and Ghaffari-Goosheh, M. S. (2019). Anti-influenza virus activity and phenolic content of pomegranate (punica granatum l.) peel extract and fractions. Avicenna J. Med. Biotechnol. 11 (4), 285–291.
More, P., and Pai, K. (2011). Immunomodulatory effects of Tinospora cordifolia (Guduchi) on macrophage activation. Biol. Med. 3 (2), 134–140.
Mouhajir, F., Hudson, J. B., Rejdali, M., and Towers, G. H. N. (2001). Multiple antiviral activities of endemic medicinal plants used by Berber peoples of Morocco. Pharm. Biol. 39 (5), 364–374. doi:10.1076/phbi.39.5.364.5892
Mukherjee, H., Ojha, D., Bag, P., Chandel, H. S., Bhattacharyya, S., Chatterjee, T. K., et al. (2013). Anti-herpes virus activities of Achyranthes aspera: an Indian ethnomedicine, and its triterpene acid. Microbiol. Res. 168 (4), 238–244. doi:10.1016/j.micres.2012.11.002
Muniandy, K., Gothai, S., Badran, K. M. H., Kumar, S. S., Esa, N. M., and Arulselvan, P. (2018). Suppression of proinflammatory cytokines and mediators in LPS-Induced RAW 264.7 macrophages by stem extract of alternanthera sessilis via the inhibition of the NF-κB pathway. J. Immunol. Res. 2018, 1–12. doi:10.1155/2018/3430684
Muniappan, M., and Sundararaj, T. (2003). Antiinflammatory and antiulcer activities of Bambusa arundinacea. J. Ethnopharmacol. 88 (2-3), 161–167. doi:10.1016/S0378-8741(03)00183-1
Murthy, K., and Mishra, S. H. (2016). Velvet bean roots stimulates humoral and cell mediated immunity and offers protection against cyclophosphamide induced myelosuppression. Int. J. Phytomed. 8 (1), 69–79.
Musarra-Pizzo, M., Ginestra, G., Smeriglio, A., Pennisi, R., Sciortino, M. T., and Mandalari, G. (2019). The antimicrobial and antiviral activity of polyphenols from almond (prunus dulcis L.) skin. Nutrients 11 (10), 2355. doi:10.3390/nu11102355
Mustafa, R., and Blumenthal, E. (2017). Immunomodulatory effects of turmeric: proliferation of spleen cells in mice. J. Immunoassay Immunochem. 38 (2), 140–146. doi:10.1080/15321819.2016.1227835
Nagarkar, B., Nirmal, P., Narkhede, A., Kuvalekar, A., Kulkarni, O., Harsulkar, A., et al. (2013). Comparative evaluation of anti-inflammatory potential of medicinally important plants. Int. J. Pharm. Pharm. Sci. 5 (3), 239–243.
Nagpurkar, M., and Patil, N. M. (2017). A review on sesame–an ethno medicinally significant oil crop. Int. J. Life Sci. Pharma Res. 7 (2), L58–L63.
Narayan, C., and Kumar, A. (2014). Antineoplastic and immunomodulatory effect of polyphenolic components of Achyranthes aspera (PCA) extract on urethane induced lung cancer in vivo. Mol. Biol. Rep. 41 (1), 179–191. doi:10.1007/s11033-013-2850-6
Negi, B. S., and Dave, B. P. (2010). In Vitro antimicrobial activity of Acacia catechu and its phytochemical analysis. Indian J. Microbiol. 50 (4), 369–374. doi:10.1007/s12088-011-0061-1
Nety, S., Koley, K. M., Choudhary, M., Chourasia, D., and Kumar, V. (2017). Comparative study of immunomodulatory effect of tinospora cordifolia stem and azadirachta indica leaf extract in broiler chicks. Vet. Pract. 18 (2), 286–288.
Niphade, S. R., Asad, M., Chandrakala, G. K., Toppo, E., and Deshmukh, P. (2009). Immunomodulatory activity of Cinnamomum zeylanicum bark. Pharm. Biol. 47 (12), 1168–1173. doi:10.3109/13880200903019234
Nirmal, S. A., Patel, A. P., Bhawar, S. B., and Pattan, S. R. (2012). Antihistaminic and antiallergic actions of extracts of Solanum nigrum berries: possible role in the treatment of asthma. J. Ethnopharmacol. 142 (1), 91–97. doi:10.1016/j.jep.2012.04.019
Nivetha, R., Bhuvaragavan, S., and Janarthanan, S. (2020).Inhibition of multiple SARS-CoV-2 proteins by an antiviral biomolecule, seselin from Aegle marmelos deciphered using molecular docking analysis. Res. Square [Epub ahead of print]. doi:10.21203/rs.3.rs-31134/v1
Nosalova, G., Jurecek, L., Chatterjee, U. R., Majee, S. K., Nosal, S., and Ray, B. (2013). Antitussive activity of the water-extracted carbohydrate polymer from terminalia chebula on citric acid-induced cough. Evidence Based Complementary Altern. Med. 2013, 1–7. doi:10.1155/2013/650134
Nutan, S. K., Modi, M., Dezzutti, C. S., Kulshreshtha, S., Rawat, A. K. S., Srivastava, S. K., et al. (2013). Extracts from Acacia catechu suppress HIV-1 replication by inhibiting the activities of the viral protease and Tat. Virol. J. 10 (1), 309. doi:10.1186/1743-422X-10-309
Nuzhat, S., Khan, R. A., and Iqbal, A. (2013). Anti-tussive effect and gross toxicities of methanol extract of mucuna pruriens (l) DC. in comparison of codeine phosphate. Int. Res. J. Pharm. 4 (6), 62–65. doi:10.7897/2230-8407.04614
Orhan, I. E., Mesaik, M. A., Jabeen, A., and Kan, Y. (2016). Immunomodulatory properties of various natural compounds and essential oils through modulation of human cellular immune response. Ind. Crop. Prod. 8 (1), 117–122. doi:10.1016/j.indcrop.2015.11.088
Oza, M. J., and Kulkarni, Y. A. (2017). Traditional uses, phytochemistry and pharmacology of the medicinal species of the genus Cordia (Boraginaceae). J. Pharm. Pharmacol. 69 (7), 755–789. doi:10.1111/jphp.12715
Palla, A. H., Khan, N. A., Bashir, S., Ur-Rehman, N., Iqbal, J., and Gilani, A. H. (2015). Pharmacological basis for the medicinal use of Linum usitatissimum (Flaxseed) in infectious and non-infectious diarrhea. J. Ethnopharmacol. 160, 61–68. doi:10.1016/j.jep.2014.11.030
Panda, S. K., Padhi, L., Leyssen, P., Liu, M., Neyts, J., and Luyten, W. (2017). Antimicrobial, anthelmintic, and antiviral activity of plants traditionally used for treating infectious disease in the Similipal Biosphere Reserve, Odisha, India. Front. Pharmacol. 8, 658. doi:10.3389/fphar.2017.00658
Panigrahi, G. K., Yadav, A., Mandal, P., Tripathi, A., and Das, M. (2016). Immunomodulatory potential of Rhein, an anthraquinone moiety of Cassia occidentalis seeds. Toxicol. Lett. 245, 15–23. doi:10.1016/j.toxlet.2016.01.006
Pant, M., Ambwani, T., and Umapathi, V. (2012). Antiviral activity of Ashwagandha extract on infectious bursal disease virus replication. Indian J. Sci. Technol. 5 (5), 1–2. doi:10.17485/ijst/2012/v5i5.20
Patel, K. G., Detroja, J. R., Shah, T. A., Patel, K. V., and Gandhi, T. R. (2011). Evaluation of the effect of Onosma bracteatum, wall (Boraginaceae) using experimental allergic and inflammatory models. Global J. Pharmacol. 5 (1), 40–49.
Patel, M. R., Bhalodia, Y. S., Pathak, N. L., Patel, M. S., Suthar, K., Patel, N., et al. (2013). Study on the mechanism of the bronchodilatory effects of Cynodon dactylon (Linn.) and identification of the active ingredient. J. Ethnopharmacol. 150 (3), 946–952. doi:10.1016/j.jep.2013.09.053
Patel, P., and Asdaq, S. M. B. (2010). Immunomodulatory activity of methanolic fruit extract of Aegle marmelos in experimental animals. Saudi Pharm. J. 18 (3), 161–165. doi:10.1016/j.jsps.2010.05.006
Patel, S., Saxena, N., Saxena, R. C., Arya, N., Saxena, R., and Tharani, M. (2009). Evaluation of anti-asthmatic activity of Glycyrrhiza glabra. Biosci. Biotechnol. Res. Asia 6 (2), 761–766.
Patgiri, B., Umretia, B., Vaishnav, P., Prajapati, P., Shukla, V., and Ravishankar, B. (2014). Anti-inflammatory activity of Guduchi Ghana (aqueous extract of tinospora cordifolia Miers. AYU (An Int. Q. J. Res. Ayurveda) 35 (1), 108–110. doi:10.4103/0974-8520.141958
Pathak, M., Vyas, H., and Vyas, M. (2010). A clinical trial of Pippali (Piper longum Linn.) with special reference to Abheshaja. AYU (An Int. Q. J. Res. Ayurveda) 31 (4), 442–446. doi:10.4103/0974-8520.82038
Patil, G. G., Mali, P. Y., and Bhadane, V. V. (2008). Folk remedies used against respiratory disorders in Jalgaon district, Maharashtra. Indian J. Nat. Prod. Resour. 7 (4), 354–358.
Patil, V. V., Bhangale, S. C., and Patil, V. R. (2010). Studies on immunomodulatory activity of ficus carica. Int. J. Pharm. Pharm. Sci. 2 (4), 97–99.
Paulpandi, M., Kannan, S., Thangam, R., Kaveri, K., Gunasekaran, P., and Rejeeth, C. (2012). In vitro anti-viral effect of β-santalol against influenza viral replication. Phytomed. 19 (3-4), 231–235. doi:10.1016/j.phymed.2011.11.006
Pei, H., Xue, L., Tang, M., Tang, H., Kuang, S., Wang, L., et al. (2020). Alkaloids from black pepper (piper nigrum L.) exhibit anti-inflammatory activity in murine macrophages by inhibiting activation of NF-κB pathway. J. Agric. Food Chem. 68 (8), 2406–2417. doi:10.1021/acs.jafc.9b07754
Peterson, C. T., Denniston, K., and Chopra, D. (2017). Therapeutic uses of triphala in ayurvedic medicine. J. Altern. Complementary Med. 23 (8), 607–614. doi:10.1089/acm.2017.0083
Phetkate, P., Kummalue, T., U-Pratya, Y., and Kietinun, S. (2012). Significant increase in cytotoxic T lymphocytes and natural killer cells by triphala: a clinical phase I study. Evidence Based Complementary Altern. Med. 2012, 1–6. doi:10.1155/2012/239856
Philip, S., Tom, G., and Vasumathi, A. V. (2018). Evaluation of the anti-inflammatory activity of Tinospora cordifolia (Willd.) Miers chloroform extract – a preclinical study. J. Pharm. Pharmacol. 70 (8), 1113–1125. doi:10.1111/jphp.12932
Pinheiro, A., Mendes, A., Neves, M., Prado, C. M., Bittencourt-Mernak, M. I., Santana, F., et al. (2019). Galloyl-hexahydroxydiphenoyl (HHDP)-Glucose isolated from punica granatum L. Leaves protects against lipopolysaccharide (LPS)-Induced acute lung injury in BALB/c mice. Front. Immunol. 10, 1978. doi:10.3389/fimmu.2019.01978
Pongthanapisith, V., Ikuta, K., Puthavathana, P., and Leelamanit, W. (2013). Antiviral protein of Momordica charantia L. inhibits different subtypes of influenza A. Evidence Based Complement. Altern. Med. 2013, 729081. doi:10.1155/2013/729081
Prasad, R., Lawania, R. D., Manvi, , and Gupta, R. (2009). Role of herbs in the management of asthma. Pharm. Rev. 3 (6), 247–258.
Prasad, S., and Srivastava, S. K. (2020). Oxidative stress and cancer: chemopreventive and therapeutic role of triphala. Antioxidants 9 (1), 72. doi:10.3390/antiox9010072
Pravansha, S., Thippeswamy, B. S., and Veerapur, V. P. (2012). Immunomodulatory and antioxidant effect of Leptadenia reticulata leaf extract in rodents: possible modulation of cell and humoral immune response. Immunopharmacol. Immunotoxicol. 34 (6), 1010–1019. doi:10.3109/08923973.2012.689767
Pringproa, K., Khonghiran, O., Kunanoppadol, S., Potha, T., and Chuammitri, P. (2014). In vitro virucidal and virustatic properties of the crude extract of cynodon dactylon against porcine reproductive and respiratory syndrome virus. Vet. Med. Int. 2014, 1–5. doi:10.1155/2014/947589
Pruthvish, R., and Gopinatha, S. M. (2018). Antiviral prospective of Tinospora cordifolia on HSV-1. Int. J. Curr. Microbiol. Appl. Sci. 7 (1), 3617–3624. doi:10.20546/ijcmas.2018.701.425
Rabaan, A. A., Al-Ahmed, S. H., Haque, S., Sah, R., Tiwari, R., Malik, Y. S., et al. (2020). SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Le Infez. Med. 28 (2), 174–184.
Rafieian-kopaei, M., Shakiba, A., Sedighi, M., and Bahmani, M. (2017). The analgesic and anti-inflammatory activity of Linum usitatissimum in balb/c mice. J. Evidence Based complementary Altern. Med. 22 (4), 892–896. doi:10.1177/2156587217717416
Raghavendhar, S., Tripati, P. K., Ray, P., and Patel, A. K. (2019). Evaluation of medicinal herbs for Anti-CHIKV activity. Virology 533, 45–49. doi:10.1016/j.virol.2019.04.007
Rahman, M. M., Alam, M. N., Ulla, A., Sumi, F. A., Subhan, N., Khan, T., et al. (2017). Cardamom powder supplementation prevents obesity, improves glucose intolerance, inflammation and oxidative stress in liver of high carbohydrate high fat diet induced obese rats. Lipids Health Dis. 16 (1), 151. doi:10.1186/s12944-017-0539-x
Rai, S. N., Birla, H., Zahra, W., Singh, S. Sen., and Singh, S. P. (2017). Immunomodulation of Parkinson’s disease using Mucuna pruriens (Mp). J. Chem. Neuroanat. 85, 27–35. doi:10.1016/j.jchemneu.2017.06.005
Raj, C. D., Shabi, M. M., Brahatheeswaran, D,, and Mahesh, N. (2006). Anti-inflammatory activity of Tylophora indica in albino rats. J. Pharmacol. Toxicol. 1 (5), 490–492. doi:10.3923/jpt.2006.490.492
Rajbhandari, M., Mentel, R., Jha, P. K., Chaudhary, R. P., Bhattarai, S., Gewali, M. B., et al. (2009). Antiviral activity of some plants used in Nepalese traditional medicine. Evidence Based complementary Altern. Med. 6 (4), 517–522. doi:10.1093/ecam/nem156
Rajbhandari, M., Wegner, U., Schöpke, T., Lindequist, U., and Mentel, R. (2003). Inhibitory effect of Bergenia ligulata on influenza virus A. Pharmazie 58 (4), 268–271.
Ramesh, B. N., Girish, T. K., Raghavendra, R. H., Naidu, K. A., Prasada Rao, U. J. S., and Rao, K. S. (2014). Comparative study on anti-oxidant and anti-inflammatory activities of Caesalpinia crista and Centella asiatica leaf extracts. J. Pharm. BioAllied Sci. 6 (2), 86–91. doi:10.4103/0975-7406.129172
Randon, A. M., and Attard, E. (2007). The in vitro immunomodulatory activity of oleuropein, a secoiridoid glycoside from olea europaea L. Nat. Prod. Commun. 2 (5), 1934578x0700200501. doi:10.1177/1934578x0700200501
Ranjbar, M., Varzi, H. N., Sabbagh, A., Bolooki, A., and Sazmand, A. (2013). Study on analgesic and anti-inflammatory properties of Cordia myxa fruit Hydro-alcoholic extract. Pak. J. Biol. Sci. 16 (24), 2066–2069. doi:10.3923/pjbs.2013.2066.2069
Rashed, K. (2014). Evaluation of anti–hiv–1 activity of cordia myxa l. And phytochemical profile. Banat's. J. Biotechnol. 5 (9), 51–56. doi:10.7904/2068-4738-v(9)-51
Rasool, A., Khan, M. U. R., Ali, M. A., Anjum, A. A., Ahmed, I., Aslam, A., et al. (2017). Anti-Avian influenza virus H9N2 activity of aqueous extracts of Zingiber officinalis (Ginger) and Allium sativum (Garlic) in chick embryos. Pak. J. Pharm. Sci. 30 (4), 1341–1344.
Rasool, M., and Varalakshmi, P. (2006). Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: an in vivo and in vitro study. Vasc. Pharmacol. 44 (6), 406–410. doi:10.1016/j.vph.2006.01.015
Rastogi, S., Pandey, D. N., and Singh, R. H. (2020). COVID-19 pandemic: a pragmatic plan for Ayurveda intervention. J. Ayurveda Integr. Med. [Epub ahead of print]. doi:10.1016/j.jaim.2020.04.002
Rather, M. A., Dar, B. A., Sofi, S. N., Bhat, B. A., and Qurishi, M. A. (2016). Foeniculum vulgare: a comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 9, S1574–S1583. doi:10.1016/j.arabjc.2012.04.011
Reddy, D. B., Reddy, T. C. M., Jyotsna, G., Sharan, S., Priya, N., Lakshmipathi, V., et al. (2009). Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J. Ethnopharmacol. 124 (3), 506–512. doi:10.1016/j.jep.2009.05.022
Rehman, N. U., Khan, A. U., Alkharfy, K. M., and Gilani, A. H. (2012). Pharmacological basis for the medicinal use of Lepidium sativum in airways disorders. Evidence Based Complementary Altern. Med. 2012, 1–8. doi:10.1155/2012/596524
Rifa’i, M., Satwika, D., and Aulanni, A. M. (2014). Datura Metel linn ameliorates asthma symptoms in BALB/c mice. J. Bio. Sci. 22, 1–8. doi:10.3329/jbs.v22i0.30002
Roqaiya, M., Begum, W., and Jahufer, R. (2015). Acacia arabica (Babool) - a review on ethnobotanical and unani traditional uses as well as phytochemical and pharmacological properties. Int. J. Pharm. Phytopharmacol. Res. 4 (6), 315–321.
Rothan, H. A., and Byrareddy, S. N. (2020). The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 109, 102433. doi:10.1016/j.jaut.2020.102433
Roy, S., Chaurvedi, P., and Chowdhary, A. (2015). Evaluation of antiviral activity of essential oil of Trachyspermum Ammi against Japanese encephalitis virus. Pharmacogn. Res. 7 (3), 263–267. doi:10.4103/0974-8490.157977
Roy, S., Mukherjee, S., Pawar, S., and Chowdhary, A. (2016). Evaluation of in vitro antiviral activity of Datura metel Linn. against rabies virus. Pharmacogn. Res. 8 (4), 265–269. doi:10.4103/0974-8490.188874
RS, J. J., Jagadeesh, S., Ganesan, S., K, V. R., and Eerike, M. (2013). Anti inflammatory activity of ethanolic extract of Nymphaea Alba flower in Swiss albino mice. Int. J. Med. Res. Health Sci. 2 (3), 474–478. doi:10.5958/j.2319-5886.2.3.082
Sachan, S., Dhama, K., Latheef, S. K., Samad, H. A., Mariappan, A. K., Munuswamy, P., et al. (2019). Immunomodulatory potential of tinospora cordifolia and CpG ODN (TLR21 agonist) against the very virulent, infectious bursal disease virus in SPF chicks. Vaccines 7 (3), 106. doi:10.3390/vaccines7030106
Sadekar, R. D., Kolte, A. Y., Barmase, B. S., and Desai, V. F. (1998). Immunopotentiating effects of Azadirachta indica (Neem) dry leaves powder in broilers, naturally infected with IBD virus. Indian J. Exp. Biol. 36 (11), 1151–1153.
Sadique, J., Chandra, T., Thenmozhi, V., and Elango, V. (1987). The anti-inflammatory activity of Enicostemma littorale and Mollugo cerviana. Biochem. Med. Metab. Biol. 37 (2), 167–176. doi:10.1016/0885-4505(87)90023-5
Sahasranaman, A., and Kumar, N. (2020). Network structure of COVID-19 spread and the lacuna in India’s testing strategy. arXiv:10.2139/ssrn.3558548.
Sahni, Y. P., and Srivastava, D. N. (1993). Anti-inflammatory activity of Withania somnifera: possible mode of action. J. Appl. Anim. Res. 3 (2), 129–136. doi:10.1080/09712119.1993.9705964
Sai Ram, M., Neetu, D., Yogesh, B., Anju, B., Dipti, P., Pauline, T., et al. (2002). Cyto-protective and immunomodulating properties of Amla (Emblica officinalis) on lymphocytes: an in-vitro study. J. Ethnopharmacol. 81 (1), 5–10. doi:10.1016/S0378-8741(01)00421-4
Salih, R. H., Odisho, S. M., Al-Shammari, A. M., and Ibrahim, O. M. S. (2017). Antiviral effects of Olea europaea leaves extract and interferon-beta on gene expression of newcastle disease virus. Adv. Anim. Vet. Sci. 5 (11), 436–445. doi:10.17582/journal.aavs/2017/5.11.436.445
Salim, B., and Noureddine, M. (2020). Identification of compounds from nigella sativa as new potential inhibitors of 2019 novel coronasvirus (Covid-19): molecular docking study. chemRxiv:10.26434/chemrxiv.12055716.v1.
Sampath Kumar, K. P., Bhowmik, D., Tiwari, P., and Kharel, R. (2010). Indian traditional herbs Adhatoda vasica and its Medicinal application. J. Chem. Pharm. Res. 2 (1), 240–245.
Saneja, A., Kaushik, P., Kaushik, D., Kumar, S., and Kumar, D. (2009). Antioxidant, analgesic and anti-inflammatory activities of santalum album linn. Planta Med. 75 (04), 102. doi:10.1055/s-2009-1216540
Sastry, J. L. N., Gupta, A., Brindavanam, N. B., Kanjilal, S., Kumar, S., Setia, M., et al. (2011). Quantification of immunity status of dabur Chyawanprash - a review part- 2 (clinical studies). Indian J. Appl. Res. 4 (3), 205–211. doi:10.15373/2249555x/mar2014/61
Sehgal, R., Chauhan, A., Gilhotra, U. K., and Gilhotra, A. (2013). In vitro and in vivo evaluation of antiasthmatic activity of picrorhiza kurroa plant. Int. J. Pharm. Sci. Res. 4 (9), 3440–3443. doi:10.13040/IJPSR.0975-8232
Sengottuvelu, S., Rajesh, K., Sherief, S. H., Duraisami, R., Vasudevan, M., Nandhakumar, J., et al. (2012). Evaluation of analgesic and anti-inflammatory activity of methanolic extract of Cocculus hirsutus leaves. J. Res. Educ. Indian Med. 18, 175–182.
Sharifi-Rad, J., Salehi, B., Schnitzler, P., Ayatollahi, S. A., Kobarfard, F., Fathi, M., et al. (2017). Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn. Cell. Mol. Biol. 63 (8), 42–47. doi:10.14715/cmb/2017.63.8.10
Sharma, M. L., Rao, C. S., and Duda, P. L. (1994). Immunostimulatory activity of Picrorhiza kurroa leaf extract. J. Ethnopharmacol. 41 (3), 185–192. doi:10.1016/0378-8741(94)90031-0
Sharma N., N., Mishra, K. P., Chanda, S., Bhardwaj, V., Tanwar, H., Ganju, L., et al. (2019). Evaluation of anti-dengue activity of Carica papaya aqueous leaf extract and its role in platelet augmentation. Arch. Virol. 164 (4), 1095–1110. doi:10.1007/s00705-019-04179-z
Sharma, R., Martins, N., Kuca, K., Chaudhary, A., Kabra, A., Rao, M. M., et al. (2019). Chyawanprash: a traditional indian bioactive health supplement. Biomolecules 9 (5), 161. doi:10.3390/biom9050161
Sharma, V., Kaushik, S., Pandit, P., Dhull, D., Yadav, J. P., and Kaushik, S. (2019). Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl. Microbiol. Biotechnol. 103 (2), 881–891. doi:10.1007/s00253-018-9488-1
Sharma, V., Thakur, M., Chauhan, N. S., and Dixit, V. K. (2010). Immunomodulatory activity of petroleum ether extract of Anacyclus pyrethrum. Pharm. Biol. 48 (11), 1247–1254. doi:10.3109/13880201003730642
Shin, H. S., See, H. J., Jung, S. Y., Choi, D. W., Kwon, D. A., Bae, M. J., et al. (2015). Turmeric (Curcuma longa) attenuates food allergy symptoms by regulating type 1/type 2 helper T cells (Th1/Th2) balance in a mouse model of food allergy. J. Ethnopharmacol. 175, 21–29. doi:10.1016/j.jep.2015.08.038
Shin, T. Y., Kim, D. K., Chae, B. S., and Lee, E. J. (2001). Antiallergic action of magnolia officinalis on immediate hypersensitivity reaction. Arch Pharm. Res. 24 (3), 249–255. doi:10.1007/BF02978266
Shivaprasad, H. N., Kharya, M. D., Rana, A. C., and Mohan, S. (2006). Preliminary immunomodulatory activities of the aqueous extract of Terminalia chebula. Pharm. Biol. 44 (1), 32–34. doi:10.1080/13880200500530542
Shruthi, R. R., Venkatesh, Y. P., and Muralikrishna, G. (2017). Structural and functional characterization of a novel immunomodulatory glycoprotein isolated from ajowan (Trachyspermum ammi L.). Glycoconjugate J. 34 (4), 499–514. doi:10.1007/s10719-017-9771-x
Shukla, S., Mehta, A., Mehta, P., Vyas, S. P., and Shivaprasad, H. N. (2010). In vivo immunomodulatory activities of the aqueous extract of bonduc nut Caesalpinia bonducella seeds. Pharm. Biol. 48 (2), 227–230. doi:10.3109/13880200903085474
Siddiqui, N. A., Singh, S., Siddiquei, M. M., and Khan, T. H. (2012). Immunomodulatory effect of withania somnifera, asparagus racemosus and picrorhiza kurroa roots. Int. J. Pharmacol. 8 (2), 108–114. doi:10.3923/ijp.2012.108.114
Sikandan, A., Shinomiya, T., and Nagahara, Y. (2018). Ashwagandha root extract exerts anti-inflammatory effects in HaCaT cells by inhibiting the MAPK/NF-κB pathways and by regulating cytokines. Int. J. Mol. Med. 42 (1), 425–434. doi:10.3892/ijmm.2018.3608
Singh, K. P., Upadhyay, R., and Kumar, A. (2010). Screening of adhatoda vasica needs as a putative HIV-protease inhibitor. J. Phytol. 2 (4), 78–82.
Singh, P., Chakraborty, P., He, D. H., and Mergia, A. (2019). Extract prepared from the leaves of Ocimum basilicum inhibits the entry of Zika virus. Acta Virol. 63 (03), 316–321. doi:10.4149/av_2019_307
Singh, S., Panchaksharimath, P., and Devaru, S. (2011). Evaluation of anti-inflammatory activities of Sida cordifolia Linn in albino rats. J. Chem. Pharmaceut. Res. 3 (6), 136–142.
Singh, O., Khanam, Z., Misra, N., and Srivastava, M. K. (2011). Chamomile (Matricaria chamomilla L.): an overview. Pharm. Rev. 5 (9), 82–95. doi:10.4103/0973-7847.79103
Sinha, S., Murugesan, T., Maiti, K., Gayen, J. R., Pal, M., and Saha, B. P. (2001). Evaluation of anti-inflammatory potential of Bergenia ciliata Sternb. rhizome extract in rats. J. Pharm. Pharmacol. 53 (2), 193–196. doi:10.1211/0022357011775398
Sireeratawong, S., Itharat, A., Lerdvuthisopon, N., Piyabhan, P., Khonsung, P., Boonraeng, S., et al. (2012). Anti-inflammatory, analgesic, and antipyretic activities of the ethanol extract of piper interruptum opiz. and piper chaba linn. ISRN Pharmacol. 2012, 1–6. doi:10.5402/2012/480265
Song, W., Gui, M., Wang, X., and Xiang, Y. (2018). Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 14 (8), e1007236. doi:10.1371/journal.ppat.1007236
Soni, K., Lawal, T., Wicks, S., Patel, U., and Mahady, G. (2015). Boswellia serrata and Ocimum sanctum extracts reduce inflammation in an ova-induced asthma model of BALB/c mice. Planta Med. 81 (11), B4. doi:10.1055/s-0035-1556201
Sood, R., Raut, R., Tyagi, P., Pareek, P. K., Barman, T. K., Singhal, S., et al. (2015). Cissampelos pareira linn: natural source of potent antiviral activity against all four dengue virus serotypes. PLoS Neglected Trop. Dis. 9 (12), e0004255. doi:10.1371/journal.pntd.0004255
Sornpet, B., Potha, T., Tragoolpua, Y., and Pringproa, K. (2017). Antiviral activity of five Asian medicinal pant crude extracts against highly pathogenic H5N1 avian influenza virus. Asian Pac. J. Trop. Med. 10 (9), 871–876. doi:10.1016/j.apjtm.2017.08.010
Souissi, M., Azelmat, J., Chaieb, K., and Grenier, D. (2020). Antibacterial and anti-inflammatory activities of cardamom (Elettaria cardamomum) extracts: potential therapeutic benefits for periodontal infections. Anaerobe 61, 102089. doi:10.1016/j.anaerobe.2019.102089
Soumaya, K. J., Dhekra, M., Fadwa, C., Zied, G., Ilef, L., Kamel, G., et al. (2013). Pharmacological, antioxidant, genotoxic studies and modulation of rat splenocyte functions by Cyperus rotundus extracts. BMC Complementary Altern. Med. 13 (1), 28. doi:10.1186/1472-6882-13-28
Soumya, M., Reddy, H., and NageswariG, , and Venkatappa, (2019). Evaluation of immunomodulatory effects of silkworm (bombyxmori L.) cocoon extracts 0n methylprednisolone induced rats. Int. J. Sci. Res. Biol. Sci. 5 (6), 80–88. doi:10.26438/ijsrbs/v5i6.8088
Sriraman, S., Ramanujam, G. M., Ramasamy, M. K., and Dubey, G. P. (2015). Identification of beta-sitosterol and stigmasterol in Bambusa bambos (L.) Voss leaf extract using HPLC and its estrogenic effect in vitro. J. Pharmaceut. Biomed. Anal. 115, 55–61. doi:10.1016/j.jpba.2015.06.024
Srivastava, J. K., Shankar, E., and Gupta, S. (2010). Chamomile: a herbal medicine of the past with a bright future (review). Mol. Med. Rep. 3 (6), 895–901. doi:10.3892/mmr.2010.377
Su, S., Duan, J., Chen, T., Huang, X., Shang, E., Yu, L., et al. (2015). Frankincense and myrrh suppress inflammation via regulation of the metabolic profiling and the MAPK signaling pathway. Sci. Rep. 5 (1), 13668. doi:10.1038/srep13668
Sung, Y. Y., Kim, S. H., Kim, D. S., Lee, J. E., and Kim, H. K. (2017). Illicium verum extract and trans-anethole attenuate ovalbumin-induced airway inflammation via enhancement of Foxp3+ regulatory T cells and inhibition of Th2 cytokines in mice. Mediat. Inflamm. 2017, 1–12. doi:10.1155/2017/7506808
Sunil, M. A., Sunitha, V. S., Radhakrishnan, E. K., and Jyothis, M. (2019). Immunomodulatory activities of Acacia catechu, a traditional thirst quencher of South India. J. Ayurveda Integr. Med. 10 (3), 185–191. doi:10.1016/j.jaim.2017.10.010
Tambekar, D. H., and Dahikar, S. B. (2011). Antibacterial activity of some Indian ayurvedic preparations against enteric bacterial pathogens. J. Adv. Pharm. Technol. Res. 2 (1), 24–29. doi:10.4103/2231-4040.79801
Tasleem, F., Azhar, I., Ali, S. N., Perveen, S., and Mahmood, Z. A. (2014). Analgesic and anti-inflammatory activities of Piper nigrum L. Asian Pac. J. Trop. Med. 7 (Suppl 1), S461–S468. doi:10.1016/S1995-7645(14)60275-3
Taylor, D. J. R., Hamid, S. M., Andres, A. M., Saadaeijahromi, H., Piplani, H., Germano, J. F., et al. (2020). Antiviral effects of menthol on coxsackievirus B. Viruses 12 (4), 373. doi:10.3390/v12040373
Tekade, S. H., Mode, S. G., and Waghmare, S. P. (2008). Effect of Asparagus racemosus, Sida cordifolia and levamisole on immunological parameters in experimentally induced immunosuppressed broilers. Vet. World 1 (2), 49–50.
Tilwari, A., Shukla, N. P., and Uma Devi, P. (2011). Effect of five medicinal plants used in Indian system of medicines on immune function in Wistar rats. Afr. J. Biotechnol. 10 (73), 16637–16645. doi:10.5897/AJB10.2168
Tiwari, M., Dwivedi, U. N., and Kakkar, P. (2014). Tinospora cordifolia extract modulates COX-2, iNOS, ICAM-1, pro-inflammatory cytokines and redox status in murine model of asthma. J. Ethnopharmacol. 153 (2), 326–337. doi:10.1016/j.jep.2014.01.031
Tripathi, D. M., Gupta, N., Lakshmi, V., Saxena, K. C., and Agrawal, A. K. (1999). Antigiardial and immunostimulatory effect of Piper longum on giardiasis due to Giardia lamblia. Phyther. Res. 13 (7), 561–565. doi:10.1002/(SICI)1099-1573(199911)13:7<561::AID-PTR479>3.0.CO;2-W
Tripathi, Y. B., and Upadhyay, A. K. (2001). Antioxidant property of mucuna pruriens linn. Curr. Sci. 80 (11), 1377–1378.
Trivedi, M. K., Mondal, S. C., Gangwar, M., and Jana, S. (2017). Effect of a novel ashwagandha-based herbomineral formulation on pro-inflammatory cytokines expression in mouse splenocyte cells: a potential immunomodulator. Pharmacog. Mag. 13 (49), 90–94. doi:10.4103/0973-1296.197709
Tumova, L., Endrychová, H., and Vokurková, D. (2018). Immunostimulant activity of Bergenia extracts. Pharmacog. Mag. 14 (56), 328–332. doi:10.4103/pm.pm_423_17
Umar, S., Rehman, A., Younus, M., Qamar-Un-Nisa, , Ali, A., Shahzad, M., et al. (2016). Effects of Nigella sativa on immune responses and pathogenesis of avian influenza (H9N2) virus in turkeys. J. Appl. Poultry Res. 25 (1), 95. doi:10.3382/japr/pfv070
Uttara, J., and Mishra, S. H. (2009). Preliminary evaluation of immunomodulatory and antistress activity of methanol extract of Hedychium spicatum. Pharmacologyonline 1, 1057–1071.
Vadnere, G. P., Gaud, R. S., Singhai, A. K., and Somani, R. S. (2009). Effect of Inula racemosa root extract on various aspects of asthma. Pharmacologyonline 2, 84–94.
Vetal, S., Bodhankar, S. L., Mohan, V., and Thakurdesai, P. A. (2013). Anti-inflammatory and anti-arthritic activity of type-A procyanidine polyphenols from bark of Cinnamomum zeylanicum in rats. Food Sci. Hum. Wellness 2 (2), 59–67. doi:10.1016/j.fshw.2013.03.003
Vidal, V., Potterat, O., Louvel, S., Hamy, F., Mojarrab, M., Sanglier, J. J., et al. (2012). Library-based discovery and characterization of daphnane diterpenes as potent and selective hiv inhibitors in daphne gnidium. J. Nat. Prod. 75 (3), 414–419. doi:10.1021/np200855d
Vimalanathan, S., Ignacimuthu, S., and Hudson, J. B. (2009). Medicinal plants of Tamil Nadu (Southern India) are a rich source of antiviral activities. Pharm. Biol. 47 (5), 422–429. doi:10.1080/13880200902800196
Vinothapooshan, G., and Sundar, K. (2011). Immunomodulatory activity of various extracts of Adhatoda vasica Linnexperimental rats. African J. Pharm. Pharmacol. 5 (3), 306–310. doi:10.5897/AJPP10.126
Wang, H., Li, K., Ma, L., Wu, S., Hu, J., Yan, H., et al. (2017). Berberine inhibits enterovirus 71 replication by downregulating the MEK/ERK signaling pathway and autophagy. Virol. J. 14 (1), 2. doi:10.1186/s12985-016-0674-4
Wang, W., Wang, J., Dong, S. F., Liu, C. H., Italiani, P., Sun, S. H., et al. (2010). Immunomodulatory activity of andrographolide on macrophage activation and specific antibody response. Acta Pharmacol. Sin. 31 (2), 191–201. doi:10.1038/aps.2009.205
Wang, Y., Jung, Y. J., Kim, K. H., Kwon, Y., Kim, Y. J., Zhang, Z., et al. (2018). Antiviral activity of fermented ginseng extracts against a broad range of influenza viruses. Viruses 10 (9), 471. doi:10.3390/v10090471
Weili, H., Baoan, C., HongYing, Z., XueBing, W., DuanHong, X., and RuiLiang, C. (2011). Antiviral activity of Mentha spicata Linn. extracts against porcine parvovirus in vitro. Jiangsu J. Agric. Sci. 27 (3), 556–560.
Wen, R., Lv, H., Jiang, Y., and Tu, P. (2018). Anti-inflammatory isoflavones and isoflavanones from the roots of Pongamia pinnata (L.) Pierre. Bioorg. Med. Chem. Lett. 28 (6), 1050–1055. doi:10.1016/j.bmcl.2018.02.026
WHO (2020a). Coronavirus disease (COVID-19) outbreak. Emergencies Dis. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed December 19, 2020).
WHO (2020b). Rolling updates on coronavirus disease (COVID-19). Events as they happen. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (Accessed December 19, 2020).
Win, N. N., Kodama, T., Lae, K. Z. W., Win, Y. Y., Ngwe, H., Abe, I., et al. (2019). Bis-iridoid and iridoid glycosides: viral protein R inhibitors from Picrorhiza kurroa collected in Myanmar. Fitoterapia 134, 101–107. doi:10.1016/j.fitote.2019.02.016
Wintachai, P., Kaur, P., Lee, R. C. H., Ramphan, S., Kuadkitkan, A., Wikan, N., et al. (2015). Activity of andrographolide against chikungunya virus infection. Sci. Rep. 5 (1), 14179. doi:10.1038/srep14179
Wirotesangthong, M., Inagaki, N., Tanaka, H., Thanakijcharoenpath, W., and Nagai, H. (2008). Inhibitory effects of Piper betle on production of allergic mediators by bone marrow-derived mast cells and lung epithelial cells. Int. Immunopharmacol. 8 (3), 453–457. doi:10.1016/j.intimp.2007.11.005
Woo, S. Y., Win, N. N., Noe Oo, W. M., Ngwe, H., Ito, T., Abe, I., et al. (2019). Viral protein R inhibitors from Swertia chirata of Myanmar. J. Biosci. Bioeng. 128 (4), 445–449. doi:10.1016/j.jbiosc.2019.04.006
Worldometers (2020). COVID-19 coronavirus pandemic. Available at: https://www.worldometers.info/coronavirus/ (Accessed December 19, 2020).
Wu, W., Li, Y., Jiao, Z., Zhang, L., Wang, X., and Qin, R. (2019). Phyllanthin and hypophyllanthin from Phyllanthus amarus ameliorates immune-inflammatory response in ovalbumin-induced asthma: role of IgE, Nrf2, iNOs, TNF-α, and IL’s. Immunopharmacol. Immunotoxicol. 41 (1), 55–67. doi:10.1080/08923973.2018.1545788
Xiang, Y., Pei, Y., Qu, C., Lai, Z., Ren, Z., Yang, K., et al. (2011). In vitro anti-herpes simplex virus activity of 1,2,4,6-tetra-O-galloyl- β-D-glucose from Phyllanthus emblica L. (Euphorbiaceae). Phyther. Res 25 (7), 975–982. doi:10.1002/ptr.3368
Xu, H. B., Ma, Y. B., Huang, X. Y., Geng, C. A., Wang, H., Zhao, Y., et al. (2015). Bioactivity-guided isolation of anti-hepatitis B virus active sesquiterpenoids from the traditional Chinese medicine: rhizomes of Cyperus rotundus. J. Ethnopharmacol. 171, 131–140. doi:10.1016/j.jep.2015.05.040
Xu, W., Hu, M., Zhang, Q., Yu, J., and Su, W. (2018). Effects of anthraquinones from Cassia occidentalis L. on ovalbumin-induced airways inflammation in a mouse model of allergic asthma. J. Ethnopharmacol. 221, 1–9. doi:10.1016/j.jep.2018.04.012
Yamprasert, R., Chanvimalueng, W., Mukkasombut, N., and Itharat, A. (2020). Ginger extract versus Loratadine in the treatment of allergic rhinitis: a randomized controlled trial. BMC Complementary Med. Ther. 20 (1), 119. doi:10.1186/s12906-020-2875-z
Yan, Y. Q., Fu, Y. J., Wu, S., Qin, H. Q., Zhen, X., Song, B. M., et al. (2018). Anti-influenza activity of berberine improves prognosis by reducing viral replication in mice. Phyther. Res. 32 (12), 2560–2567. doi:10.1002/ptr.6196
Yang, H., Huimin, Z., Aiping, Y., Qi, L., Ning, D., Tu-Lin, L., et al. (2019). Jatrophacine, a 4,5-seco-rhamnofolane diterpenoid with potent anti-inflammatory activity from Jatropha curcas. Nat. Prod. Res. 1–5. doi:10.1080/14786419.2019.1660656
Yang, Y., Islam, M. S., Wang, J., Li, Y., and Chen, X. (2020). Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 16 (10), 1708–1717. doi:10.7150/ijbs.45538
Yeh, S. F., Hong, C. Y., Huang, Y. L., Liu, T. Y., Choo, K. B., and Chou, C. K. (1993). Effect of an extract from Phyllanthus amarus on hepatitis B surface antigen gene expression in human hepatoma cells. Antiviral Res. 20 (3), 185–192. doi:10.1016/0166-3542(93)90019-F
Yu, Z. p., Xu, D. D., Lu, L. F., Zheng, X. D., and Chen, W. (2016). Immunomodulatory effect of a formula developed from American ginseng and Chinese jujube extracts in mice. J. Zhejiang Univ. Sci. B. 17 (2), 147–157. doi:10.1631/jzus.B1500170
Yuki, K., Fujiogi, M., and Koutsogiannaki, S. (2020). COVID-19 pathophysiology: a review. Clin. Immunol. 215, 108427. doi:10.1016/j.clim.2020.108427
Zaia, M. G., Cagnazzo, T., di, O., Feitosa, K. A., Soares, E. G., Faccioli, L. H., et al. (2016). Anti-inflammatory properties of menthol and menthone in Schistosoma mansoni infection. Front. Pharmacol. 7, 170. doi:10.3389/fphar.2016.00170
Zare, A., Farzaneh, P., Pourpak, Z., Zahedi, F., Moin, M., Shahabi, S., et al. (2008). Purified aged garlic extract modulates allergic airway inflammation in Balb/c mice. Iran. J. Allergy Asthma Immunol. 7 (3), 133–141.
Zaveri, M., Khandhar, A., and Jain, S. (2008). Quantification of baicalein, chrysin, biochanin-A and ellagic acid in root bark of Oroxylum indicum by RP-HPLC with UV detection. Eurasian J. Anal.Chem.
Zhang, T., Wu, Q., and Zhang, Z. (2020). Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 30 (7), 1346–1351.e2. doi:10.1016/j.cub.2020.03.022
Zhao, Y. L., Cao, J., Shang, J. H., Liu, Y. P., Khan, A., Wang, H. S., et al. (2017). Airways antiallergic effect and pharmacokinetics of alkaloids from Alstonia scholaris. Phytomed. 27, 63–72. doi:10.1016/j.phymed.2017.02.002
Zhou, H. L., Deng, Y. M., and Xie, Q. M. (2006). The modulatory effects of the volatile oil of ginger on the cellular immune response in vitro and in vivo in mice. J. Ethnopharmacol. 105 (1-2), 301–305. doi:10.1016/j.jep.2005.10.022
Keywords: COVID-19, AYUSH medicine, indian medicinal plants, indian traditional medicine, immunomodulators, antiviral agents
Citation: Ahmad S, Zahiruddin S, Parveen B, Basist P, Parveen A, Gaurav , Parveen R and Ahmad M (2021) Indian Medicinal Plants and Formulations and Their Potential Against COVID-19–Preclinical and Clinical Research. Front. Pharmacol. 11:578970. doi: 10.3389/fphar.2020.578970
Received: 01 July 2020; Accepted: 29 December 2020;
Published: 02 March 2021.
Edited by:
Michael Heinrich, UCL School of Pharmacy, United KingdomReviewed by:
Shailendra Shivaji Gurav, Goa College of Pharmacy, IndiaCopyright © 2021 Ahmad, Zahiruddin, Parveen, Basist, Parveen, Gaurav, Parveen and Ahmad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sayeed Ahmad, c2FobWFkX2poQHlhaG9vLmNvLmlu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.